Abstract
Neurotransmitter stimulation of plasma membrane receptors stimulates salivary gland fluid secretion via a complex process that is determined by coordinated temporal and spatial regulation of several Ca2+ signaling processes as well as ion flux systems. Studies over the past four decades have demonstrated that Ca2+ is a critical factor in the control of salivary gland function. Importantly, critical components of this process have now been identified, including plasma membrane receptors, calcium channels, and regulatory proteins. The key event in activation of fluid secretion is an increase in intracellular [Ca2+] ([Ca2+]i) triggered by IP3-induced the release of Ca2+ from ER via the IP3R. This increase regulates the ion fluxes required to drive vectorial fluid secretion. IP3Rs determine the site of initiation and the pattern of [Ca2+]i signal in the cell. However, Ca2+ entry into the cell is required to sustain the elevation of [Ca2+]i and fluid secretion. This Ca2+ influx pathway, store-operated calcium influx pathway (SOCE), has been studied in great detail and the regulatory mechanisms as well as key molecular components have now been identified. Orai1, TRPC1, and STIM1 are critical components of SOCE and among these, Ca2+ entry via TRPC1 is a major determinant of fluid secretion. The receptor-evoked Ca2+ signal in salivary gland acinar cells is unique in that it starts at the apical pole and then rapidly increases across the cell. The basis for the polarized Ca2+ signal can be ascribed to the polarized arrangement of the Ca2+ channels, transporters, and signaling proteins. Distinct localization of these proteins in the cell suggests compartmentalization of Ca2+ signals during regulation of fluid secretion. This chapter will discuss new concepts and findings regarding the polarization and control of Ca2+ signals in the regulation of fluid secretion.
Introduction
Neurotransmitter-generated Ca2+ signals in exocrine gland cells, such as pancreatic and salivary gland acinar cells, are critical for the regulation of their secretory functions; fluid secretion in salivary acinar cells and protein secretion in pancreatic acinar cells (1-6). Under normal physiological conditions, salivary glands maintain a continuous low level of saliva flow, often referred to as “resting” or “basal” secretion, which is dramatically increased upon demand. Upregulation of fluid secretion in salivary glands is achieved by autonomic sympathetic and parasympathetic stimuli which activate a coordinated sequence of signal transduction and intracellular signaling events, including activation of membrane receptors, generation of intracellular second messengers, calcium mobilization, and stimulation of ion transport pathways. The primary site of fluid secretion in salivary glands is the acinar cell. Although some salivary gland ducts might also serve a secretory function, this has not yet been well characterized. The key trigger for stimulation of fluid secretion is an increase in cytosolic [Ca2+] ([Ca2+]i) which activates and maintains vectorial fluid secretion. The regulation of fluid secretion is a temporally and spatially coordinated process involving several ion channels and transporters, water channels, as well as polarized calcium signaling events that is determined by Ca2+ channels and transporters. The primary role of the [Ca2+]i increase in acinar cells is to regulate ion channel activity in various cellular domains in order to generate the appropriate osmotic gradient required to drive fluid secretion across the apical membrane (7,8).
Physiologically, an increase in [Ca2+]i in salivary acinar cells is initiated in response to activation of plasma membrane receptors that are coupled to PIP2 hydrolysis. Stimulation of receptors, e.g. muscarinic (M1 and M3), alpha-adrenergic (α1A), or purinergic leads to G-protein-mediated activation of phosphatidylinositol 4,5, bisphosphate (PIP2)-specific phospholipase C (PLC), hydrolysis of PIP2 which results in the generation of inositol 1,4,5, trisphosphate (IP3) and diacylglycerol. IP3 diffuses into the cytosol and binds to the IP3 receptor (IP3R) localized on the endoplasmic reticulum (ER) membrane and induces release of Ca2+ from the ER Ca2+ store(s) via the IP3R, a well characterized intracellular Ca2+-release channel [Figure 1]. Three subtypes of the IP3 receptors have been described (IP3R1, IP3R2 and IP3R3), of which IP3R2 and 3 are the major subtypes found in exocrine gland cells and these are concentrated in the apical pole of the cells [3,4,6]. This localization of IP3Rs has important functional consequences and can be correlated with the initial increase [Ca2+]i is detected in the apical region of cells following stimulation. Subsequently, the [Ca2+]i increase spreads to the basal pole (2,4) and [Ca2+]i is maintained at level above resting for the period of stimulation. This spread of Ca2+ within the cell achieves activation of various ion channels and transporters that coordinately regulate fluid secretion (2,7,8). A number of these are located in the apical membrane, some in the lateral region close to the apical pole while others are more basally located. Thus, in addition to the magnitude, temporal and spatial control of [Ca2+]i increase is critical in the stimulation and maintenance of fluid secretion. Neurotransmitter induced [Ca2+]i increase in acinar cells involves two components; Ca2+ release from ER-Ca2+ stores induced by IP3 and Ca2+ entry via plasma membrane Ca2+ channels (7,8,9). These two components account for the biphasic changes in cytosolic [Ca2+]i levels that have been measured in stimulated cells using fluorescent Ca2+ indicator dyes; a rapid initial increase primarily due to intracellular release and a subsequent sustained elevation due to Ca2+ entry. In the absence of extracellular Ca2+, only a transient increase in [Ca2+]i is seen, reflecting only the intracellular Ca2+ release component, while in the presence of extracellular Ca2+ a sustained [Ca2+]i increase is detected. Earlier studies have shown that sustained K+ efflux from cells which is correlated with fluid secretion, requires presence of external Ca2+ (1,2,7,8,10). Thus, it has been well established for more than three decades that this sustained increase in [Ca2+]i in stimulated acinar cells is critical for maintaining prolonged fluid secretion.
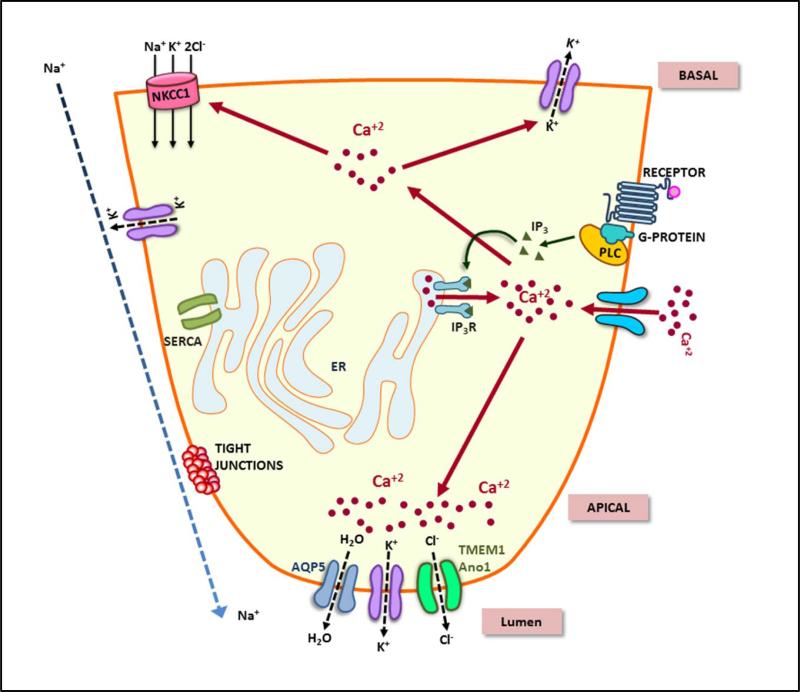
Figure 1. Ca2+ signaling and regulation of fluid secretion in salivary gland acinar cells.
This figure illustrates the key signaling events and components involved in regulation of vectorial fluid secretion in salivary acinar cells. Increase in cytosolic calcium as a consequence of neurotransmitter stimulation, intracellular Ca2+ release and Ca2+ entry, leads to regulation of ion transport, generation of an osmotic gradient, and water flow via the apical membrane of the cell.
While intracellular Ca2+ release mechanisms were identified relatively early and characterized in detail, the exact nature of the Ca2+ entry channels remained a puzzle for a long time. Data reported thus far provide strong evidence that the primary mechanism involved in regulating salivary fluid secretion is store-operated Ca2+ entry (SOCE) (11). Ca2+ influx via store-operated Ca2+ channels (SOCs) is activated in response to Ca2+ release from the ER, i.e. depletion of Ca2+ within these stores, and is terminated when the stimulus is removed and the stores are refilled. It is clear that some members of the Transient Receptor Canonical (TRPC) channels (12,13), including TRPC1 and TRPC3 (14-16), contribute to SOCE in salivary glands cells and significantly impact fluid secretion. TRPC channels function as Ca2+ permeable non-selective cation channels and notably all members are activated in response to neurotransmitter-stimulated PIP2 hydrolysis (12,13). The primary mechanism involved in regulating these channels, and SOCE per se, was only elucidated fairly recently. STIM1, a Ca2+ binding protein, functions as the ER Ca2+ sensor and relays the status of ER-[Ca2+] to the plasma membrane channels (17,18). When [Ca2+] within the ER is lowered, Ca2+ dissociates from the luminal EF hand of STIM1, resulting in conformational changes in the protein, aggregation of STIM1 monomers, and translocation of the aggregated STIM1 complexes to the periphery of the cells where it accumulates in specific ER/PM junctional domains (17,19). While the exact nature of these domains has not yet been described, it is now established that at these sites STIM1 regulates two types of plasma membrane Ca2+ entry channels; Orai (20,21) and TRPC (22-25). Orai1, the most studied and well characterized member of the Orai channel family, is a four transmembrane membrane domain channel that when activated by STIM1 generates a highly Ca2+-selective, inwardly rectifying Ca2+ current, ICRAC (20,21,23). This current is the primary current detected in lymphocytes and mast cells, but has not yet been measured in salivary acinar cells, although Orai1 is present in these cells and is required for SOCE (25, 26). In contrast salivary gland cells display a relatively non-selective cation current in response to store-depletion, more consistent with the properties of TRPC channels (14). It has been shown that STIM1 gates both Orai1 and TRPC channels via different domains in the C terminus (22-24). Importantly, these channels are localized in distinct regions of acinar cells. Thus, their relative contributions to the regulation of [Ca2+]i responses and ion channel activities involved in fluid secretion is an interesting question that is currently receiving much interest. It appears that Ca2+ entry via these channels can generate local microdomains of Ca2+ near the respective channels and also contribute to more global Ca2+ changes in the cells (27). These [Ca2+]i signals are sensed and decoded differently by the cells and utilized for regulation of distinct cellular functions. These and other recent developments in the regulation of Ca2+ signaling as well as its role in fluid secretion will be discussed in the following sections.
Role of cytosolic [Ca2+] in fluid secretion
The initial response to receptor activation is release of intracellular Ca2+ stores and activation of SOCE. Very early studies have shown that fluid secretion is initiated when the [Ca2+]i increase activates K+ and Cl− channels located in acinar cells and is maintained as long as [Ca2+]i elevation is sustained. Removal of external Ca2+ decreases [Ca2+]i together with inhibition K+ and Cl− efflux as well as fluid secretion (1,2,7-10). A primary requirement for fluid secretion is the transepithelial transport of Cl− from the basolateral to the apical side of the cell, while Na+ flux via the tight junctions results in NaCl accumulation in the lumen (2). This generates the osmotic gradient to drive water from the cells via the AQP5 channels that are localized in the apical membrane. The exact channel involved in influx of water in the basolateral regions of acinar cells is not yet established. Efflux of K+ from the cells via both apical and basolateral membranes maintains the cells in a hyperpolarized state which further supports fluid secretion. The key ion flux mechanisms involved achieving transepithelial Cl− flux are Na+/K+ adenosine triphosphatase (ATPase) and Na++K+-2Cl− cotransporter in the basolateral membrane, Ca2+-activated K+ channels in the apical and basolateral membrane, and a Ca2+-activated Cl− channel (TMEM16A) in the apical membrane (28). Na+/K+ ATPase maintains low [Na+] and high [K+], respectively, in the cell by mediating exchange of 3Na+ for 2K+ at the expense of ATP while Na++K+-2Cl− transporter mediates Cl− influx into the cell coupled to Na+ and K+ influx which leads to an increase in [Cl−] in the cells. Most salivary gland acinar cells possess paired basolateral Cl−/HCO3− and Na+/H+ exchangers which also contribute to concentrating Cl− in the cell (2).
[Ca2+]i plays a central and essential role in regulation of K+, Na+, and Cl− flux in salivary acinar cells. In the resting cells [Ca2+]i is low and maintained around 50-100 nM, which is lower than the threshold required to activate the Ca2+ activate the K+ and Cl− channels. [Ca2+]i increase following stimulation is primarily dependent on the magnitude of the stimulus. In vitro assays have demonstrated stimulation of fluid secretion with relatively low (<300nM) carbachol. Further, in the absence of external Ca2+ (i.e. without the Ca2+ influx component), the K+ and Cl− channels are only transiently activated as is fluid secretion. Thus, while intracellular Ca2+ release is sufficient for triggering activation of the K+ and Cl− channels, Ca2+ entry is required for sustained ion channel activation. In the continued presence of the secretagogue, Ca2+ entry, transepithelial Cl− flux, as well as fluid secretion are sustained. Additionally, [Ca2+]i increase stimulates Na+/H+ exchangers (29), which raises the intracellular pH of acinar cells by 0.1–0.3 units and increases the sensitivity of Ca2+-activated K+ and Cl− channels to Ca2+. This increase in [Ca2+] also activates the Na+/K+/2Cl− cotransporter activity by about 20-fold relative to the basal flux rate (30) as well as the Na+/H+ and Cl−/HCO −3 exchangers (31,32). Together, the concerted regulation of these transporters by [Ca2+]i maintains intracellular Cl− concentration above its electrochemical gradient. Furthermore, the rise in [Ca2+]i regulates the insertion of AQP5 water channels into the apical plasma membrane thus substantially increasing the fluid secretion capacity as per demand (33).
Determinants of [Ca2+]i increase in salivary gland acinar cells
The major mechanisms that are involved in generating and regulating [Ca2+]i signals are the receptor-signaling complex in the basolateral plasma membrane, Ca2+ channels and pumps in the intracellular membranes and in the plasma membrane (1,5,34) as well as the mitochondria. The role of mitochondria in controlling cytosolic [Ca2+] changes has been studied in greater detail in pancreatic acinar cells (4,6) but has not yet been clearly assessed in salivary gland cells (35). There is also relatively less information regarding the exact contributions of SERCA and PMCA pumps to fluid secretion despite early evidence for their role in accumulation of Ca2+ into the ER and efflux of Ca2+ from the cells, respectively. It is known that SERCA activity affects the spread of waves from the apical to the basal region of the cells (9). Here we will discuss the major mechanisms involved in generation and maintenance of [Ca2+]i signals in response to neurotransmitter stimulation.
Receptor-Signaling
Salivary gland function is controlled by parasympathetic and sympathetic stimuli (1,2,10). The parasympathetic neurons release acetylcholine which activates muscarinic receptors in the basolateral plasma membrane of acinar and duct cells. The sympathetic nervous system activates β-adrenergic receptors in acinar and duct cells resulting in an increase in cAMP which is the main pathway for stimulated enzyme secretion in salivary gland acinar cells. α-Adrenergic receptor stimulation at the basal plasma membrane also primarily elicits a Ca2+− mobilization response via activation of PLC, similar to that seen in response to muscarinic receptor stimulation. A conclusive physiological role for muscarinic receptors in salivary fluid secretion was demonstrated by studies using mice lacking M1, or M3 or both receptors (36, 37). While no secretion was induced in M1/M3−/− mice following muscarinic stimulation, relatively high dose of pilocarpine caused fluid secretion comparable to that of wild-type mice in M(3)−/− mice, although the mice displayed very little or no secretion at relatively low pilocarpine levels. Notably, M3-/-, but not M1-/-, mice, demonstrated problems with chewing and ingesting dry food. Consistent with this, carbachol-induced [Ca2+]i increase was markedly impaired in submandibular gland cells from mice lacking M3 receptors and completely absent in those lacking both M1 and M3 receptors. This demonstrated that M3 and M1 are required for regulation of physiological [Ca2+]i signaling required for fluid secretion. Other key components of receptor-coupled signaling events in the plasma membrane are Gαq/11 and PLCβ3(5,34). They are recruited to, and co-localize with, receptors in the basolateral plasma membranes where they are assembled in a complex with key Ca2+ signaling proteins such as IP3R, PMCA, SERCA as well as plasma membrane channels such as TRPC channels. Cytosolic proteins such as calmodulin, Homer, and ezrin, and RACK1 have also been identified in this complex. Thus, there is a signaling hub in the plasma membrane which includes proteins upstream in the signaling process as well as downstream effectors. The net result of stimulation of plasma membrane receptors is the activation of PIP2 hydrolysis, resulting in the generation of IP3 and DAG. An interesting question that arises is why the initial [Ca2+]i elevation seen following cell stimulation is detected in the apical region of the cells although the receptors and other signaling proteins are located the basal membrane. One simple explanation is that receptors, G proteins, as well as PLC are localized all along the basal as well as lateral membranes (till the tight junction) and so can be relatively close to the apical region of the cell, thus making it feasible for IP3 released from the complex to elicit Ca2+ release in this region of the cell. Indeed some tight junction proteins have been shown to co-immunoprecipitate with the receptor-Ca2+ signaling protein complex (9, 34). The spatial arrangement of ER and IP3Rs in these cells also determines the location of the initial Ca2+ signal.
Intracellular Ca2+ release
One of the best characterized and most important Ca2+ channels in all cell types is the IP3-sensitive Ca2+ channel in the ER which binds, and is activated by, IP3, resulting in rapid release of Ca2+ from the ER Ca2+ stores [3-6,38]. IP3R is regulated both by IP3 as well as Ca2+. Ca2+ stimulates IP3-mediated Ca2+ release at lower concentrations while inhibiting it at relatively higher concentrations (> 300nM). Furthermore, as the IP3 concentration is increased IP3R is more sensitive to lower [Ca2+]. This dual regulation by IP3 and [Ca2+] ensures that the channel is relatively more active when [Ca2+]i is low and less active when [Ca2+] is high, thus protecting ER Ca2+ stores and regulating [Ca2+]i within the physiological range required for the cell function [38]. [Ca2+] in the ER lumen has also been suggested to regulate IP3R function, but this has not yet been fully elucidated. This feed-forward and feedback regulation of IP3R can also explain the oscillatory changes in [Ca2+]i, namely activation of IP3R resulting in Ca2+ release, inhibition of IP3R which supports re-uptake into the store, and subsequently re-release from the ER. While sustained [Ca2+]i oscillations are not detected in salivary gland acinar cells, release and reuptake from the store regulates the spread of the Ca2+ increase from the apical to the basolateral regions of the cell (2,4,9).
Two major isoforms IP3R2 and IP3R3 are found in salivary gland acinar cells (2,3,4). Mice lacking either IP3R2 or IP3R3 do not display significant decreases in muscarinic-receptor stimulated secretion or [Ca2+]i increases. However, mice lacking both receptors fail to survive past weaning and also show loss of measurable agonist-stimulated [Ca2+]i increases [39]. More importantly, these data suggested that minimal Ca2+ signaling, including Ca2+ entry, occurs in the absence of IP3Rs, i.e. supported by PIP2 hydrolysis, per se. Together this established that IP3R-activation is the critical initial step in the regulation of fluid secretion. Several cellular factors and proteins modulate IP3R function including ATP, cAMP, RACK1, and the IP3R-binding protein called IRBIT (3). Among these, IRBIT appears to have an active role in regulation secretory function (40). Binding of IP3 to the receptor releases IRBIT which then binds to several other signaling proteins. It has been shown to increase HCO3− transport across ductal cells and thus increase secretion of this anion into the salivary fluid (3,8,41). In bovine parotid gland it has been reported to increase Na+-HCO3− co-transporter activity (42). Whether it also affects other critical acinar ion flux mechanisms such as TMEM16, maxi K+ channels, or NKCC1 is still to be determined.
Another main intracellular Ca2+ release channel is the ryanodine receptor, RyR (43). This channel is the major channel involved in muscle function, but is also present in non-excitable cells where it appears to amplify the signal generated by IP3. The expression of functional ryanodine receptors (RyR) in salivary acinar cells has been reported (2, 43). RyR receptors are gated by elevations in cytoplasmic Ca2+ via a mechanism termed calcium-induced calcium release (CICR) and contribute to the spread of [Ca2+]i increase in acinar cells following stimulation. It has been suggested that Ca2+ release via IP3R can activate neighboring RyR to release Ca2+ from sites in the ER where IP3Rs are sparse or those at a distance from the site of IP3 generation. Interestingly RyR, unlike IP3R, have been reported to be found at a relatively higher level in the basal region of acinar cells and thus could play a role in the globalization of the Ca2+ signal. However, their exact contribution to fluid secretion has not been directly demonstrated.
Ca2+ influx pathway
Ca2+ influx has been long recognized as the primary factor regulating sustained fluid secretion from salivary acinar cells (1,2,7,8,14). Earlier studies established that Ca2+ influx into cells is increased several fold following stimulation of the cells with an agonist suggesting that it is dependent on receptor activation (1,10). The suggested modes for the influx were either via a receptor-operated channel, i.e. the receptor itself functions as a channel, or via a second messenger operated channel, where intracellular signals generated in response to receptor stimulation regulate a plasma membrane channel (10,17). Critical insight into the mechanism involved in Ca2+ entry was revealed by the use of the SERCA inhibitor, thapsigargin, which by blocking Ca2+ uptake into the ER causes slow depletion of internal Ca2+ stores via an as yet uncharacterized leak pathway. Importantly, similar Ca2+ influx was activated by this maneuver as seen in response to agonist stimulation. These data conclusively established that internal Ca2+ store depletion, and not receptor-coupled mechanisms per se, was the signal for activation of Ca2+ influx while inactivation was triggered by refilling of ER Ca2+ stores once the agonist had been removed. This Ca2+ influx was termed capacitative Ca2+ entry (CCE) and currently is referred to as store-operated Ca2+ entry (SOCE) (11). Physiologically SOCE can be activated by any stimuli that lead to IP3 generation and depletion of ER-Ca2+. SOCE is the primary mode of Ca2+ influx in salivary gland following receptor stimulation and is critical for salivary fluid secretion (8,9,14). It also regulates key physiological functions in other cell types; e.g. secretion in pancreatic acinar cells, platelet aggregation, endothelial cell permeability and migration, cell proliferation, T-lymphocyte activation, and mast cell degranulation among others (44).
Members of the transient receptor potential canonical (TRPC) channels were proposed as molecular components of the SOCE-channel (12,13,15,25). Among these, strongest and most consistent data have been reported for TRPC1 in different cell types, including salivary gland cells (13-15,45,46). Confirmation of its physiological function in salivary acinar cells came from studies with TRPC1−/− mice. These mice display severe decrease in neurotransmitter-stimulated fluid secretion and acinar cells isolated from the mice show significant decrease in SOCE stimulated either by agonist- or thapsigargin (Tg) (14). Furthermore, deletion of TRPC1 eliminates sustained KCa channel activity in acinar cells, a critical Ca2+-dependent process required for fluid secretion. These findings are consistent with data showing that inhibitors of SOCE, 1μM Gd3+ as well as 20 μM 2APB, completely block SOCE in agonist and Tg-stimulated submandibular gland acini and demonstrate that SOCE is the primary Ca2+ entry pathway into these cells. This is further corroborated by the lack of fluid secretion in acini from IP3R2+IP3R3−/− mice (discussed above). Together, these data conclusively established that TRPC1 is a non-redundant, channel component of SOCE in salivary gland acinar cells and required for neurotransmitter-regulation of fluid secretion.
STIM1 and Orai1 were identified as critical components of the SOCE mechanism (20,21,24,25). STIM1 is a single transmembrane domain protein with an N-terminal Ca2+- binding domain located in the ER lumen and a cytosolic C-terminus. Depletion of ER Ca2+ results in dissociation of Ca2+ from the EF hand domain and this triggers molecular and spatial reorganization of STIM1. The C and N terminal domains of the protein undergo substantial conformational changes that result in aggregation of STIM1 and its translocation and clustering at ER-PM junctional domains at the cell periphery. At these locations, STIM1 interacts with and activates Orai1 and TRPC1 (20-24). Orai1 has been established as the main pore-forming component of store-operated CRAC (calcium release activated Ca2+) channels that are found in T lymphocytes and other hematopoietic cells (20,21). Importantly, STIM1 also binds and activate TRPC channels, including TRPC1 and TRPC3 (22-24, 46, 47) and co-expression of STIM1 and TRPC1 results in generation of non-selective cation channels. Studies reported by Muallem and colleagues finally established that distinct C-terminal domains of STIM1 are involved in gating Orai1 and TRPC1 (22,23). While the STIM1-SOAR domain of STIM1 interacts with Orai1 to gate the CRAC channel, the C-terminal polybasic (639KK640) motif of STIM1 activates TRPC1 by an electrostatic gating mechanism which results in channel activation.
Both STIM1 and Orai1 are required for SOCE in salivary gland cells lines (25,45,46). Knockdown of either decreases SOCE to levels lower than that achieved by suppression of TRPC1 expression. Studies in salivary gland cells demonstrate that although TRPC1 is gated by STIM1, its function is dependent on Orai1 (47-49). When endogenous Orai1 expression is suppressed or pore-deficient Orai1 is expressed, TRPC1+STIM1 function is eliminated. The critical mechanism underlying the functional interaction between TRPC1 and Orai1 has also been revealed in studies using human salivary gland cell line (49). This study demonstrated that Orai1 is likely the first channel that is activated by STIM1 in response to store depletion. Orai1-mediated Ca2+ entry then triggers recruitment of TRPC1 to the plasma membrane where it is gated by STIM1. Thus, at least two channels are activated by STIM1 following neurotransmitter simulation of salivary gland cells and both contribute to the [Ca2+]i increase seen in stimulated cells. An interesting observation that has been made is that Ca2+ entry via TRPC1, or Orai1, contributes to regulation of distinct Ca2+-dependent cell functions. TRPC1 is required for NFκB and KCa channel activation while Orai1 function is sufficient for supporting NFAT activation. These channels have also been shown to induce different pattern of Ca2+ signals in salivary gland cells lines (27). Studies with TRPC1−/− mice suggest that the residual Orai1 in the TRPC1−/− glands cannot sustain the activation of the ion channel mechanisms required for salivary secretion; e.g. KCa or Cl− channels (14, 26). Together these findings support the suggestion that Orai1 and TRPC1 lead to generation of functionally distinct Ca2+ signaling microdomains and differentially contribute to salivary gland fluid secretion. The exact nature of the Ca2+ signals generated by them in salivary gland acinar cells as well as the Ca2+ sensors and effector proteins in the vicinity of the channels that determine the specificity of functional regulation remains to be elucidated. Furthermore, the physiological regulation of TRPC1 by Orai1 has also not been demonstrated as yet within the intact gland.
Spatial patterns of Ca2+ signals and polarized fluid secretion
Fluid secretion in salivary gland acinar cells is regulated by the magnitude, as well as temporal and spatial characteristics, of the [Ca2+]i signal generated by neurotransmitter stimulation (2,4,7-8). As discussed above, fluid secretion is a polarized process and components that regulate the mechanism are localized in specific domains of the cell. Thus, the appropriate [Ca2+]i signal has to reach these microdomains. [Ca2+]i increase in salivary gland acinar cells is initiated at the apical pole and rapidly rises across the cell (5,9). In addition to the location of the proteins in the cell, this dynamic [Ca2+]i signal is shaped and determined by the local architecture of the microdomain where the proteins are located as well as the signaling components associated with them. For example the ER-SERCA pump is localized close to the site of Ca2+ entry and thus Ca2+ entering the cells can be rapidly accumulated into the ER, with minimal change in cytosolic [Ca2+]. Further, IP3 release from the plasma membrane can bind to IP3Rs in the vicinity and activate them. Importantly, there is dynamic regulation of the components within the signaling complex which is triggered by stimulation of cells and in turn regulates the downstream physiological processes (50,51). Removal of the signal could reset the protein complex to its resting status or some components could remain in an activated status, thus endowing a “memory” process that is utilized for long-term events. Example of this is rapid activation and translocation of NFAT into the nucleus (acute effect) in response to Ca2+ entry while exit of NFAT from the nucleus is a slow process as required for gene expression (delayed, long-term effect) (27). In salivary acinar cells the acute effect of the Ca2+ signal is stimulation of fluid secretion. More long-term effects on gene expression have not been studied as yet.
Regulation and polarized localization of intracellular Ca2+ release channels
The initial signal has been attributed to release from IP3R localized in the apical region of the cell induced by the generation of IP3. IP3R modulating proteins, e.g. RACK1 and IRBIT, also undergo dynamic regulation following stimulation, RACK1 interaction with IP3R increases, while IRBIT interaction with IP3R decreases, upon stimulation (3,40,41,51). Such dynamic interactions and reassembly of IP3R signaling complex exerts fine regulation of this important channel. Also of interest is the fact that cAMP can regulate the IP3R function (2,4). Thus, since both cAMP and IP3 generating systems can be stimulated simultaneously in acinar cells, cAMP and IP3 can exert synergistic effects on IP3R function. This becomes more critical at low levels of neurotransmitter stimulation where the [IP3] generated is low. Under such conditions, cAMP dependent phosphorylation of IP3R can increase IP3R function by increasing its sensitivity to IP3 (52) This appears to be sufficient to cause dissociation of IRBIT (40).
Although the physiological significance of polarized Ca2+ signaling can be predicted and has been experimentally validated to some extent, little is known about the mechanism of targeting, assembly, and retention of Ca2+ signaling complexes. Immunolocalization and biochemical studies have revealed the co-localization of proteins in various cellular domains, suggesting that they are associated. Further, immunoprecipitation experiments show that there is dynamic remodeling of the signaling complexes in response to stimulation. Cytoskeletal and other scaffolding proteins have been suggested to be involved in retaining and regulating the protein complexes within specific microdomains in the cell. An example is RACK1 which binds to IP3R and also to various other proteins including TRPC channels (51). Evidence is provided by immunoprecipitation data which show that IP3Rs can associate with plasma membrane as well as ER proteins (5,50). Increased association of IP3R with TRP channels has been reported in stimulated cells. Such data indicate close proximity of IP3R to the plasma membrane. Measurement of [Ca2+]i in the sub-plasma membrane domain using TIRF microscopy (53) demonstrates that Ca2+ release occurs close to the plasma membrane but when SOCE is activated, there is high and sustained [Ca2+]i elevation in the microdomain underneath the plasma membrane (PM). More importantly, the [Ca2+]i in this region has been calculated to be considerably higher than that measured in the cytosol. Such local compartmentalization of [Ca2+]i microdomains serve to regulate cellular functions within these regions that might require be relatively high [Ca2+]. There is increasing evidence that Ca2+ entry channels, such as TRPC1 and TRPC3, can be recruited to the plasma membrane within these microdomains and thus amplify and modulate the initial [Ca2+]i signals.
The initial Ca2+ signal evoked by physiological agonist concentrations is in the form of Ca2+ oscillations, where the Ca2+ signal is periodically repeated due to repetitive uptake and release from the ER Ca2+ store. In the absence of Ca2+ entry, this response is not sustained as ultimately the ER Ca2+ stores are fully depleted. The frequency and amplitude of the oscillation is determined by the intensity of receptor stimulation and the regulation of IP3R as well as the SOCE channels (2,4,6,27). This is achieved by repetitive release and uptake into Ca2+ stores with both IP3R and RyR-mediated Ca2+ release contributing to the global spread of the [Ca2+]i in salivary gland acinar cells. It can be suggested that at low more physiological levels of stimulation, the stores will be relatively filled compared to higher levels of stimuli. The extent of Ca2+ entry will in turn will be determined by the state of refilling of the store as well as rapid removal of Ca2+ that enters via the channel to minimize feedback inhibition of the channel. SERCA pump in the ER serves this function by actively pumping the Ca2+ entering via the plasma membrane into the ER. This has led to the suggestion that Ca2+ entering the cell is rapidly taken up into the ER from where it is released at specific cellular sites via the IP3R. This “tunnel hypothesis” has not yet been examined in salivary gland acinar cells (6). These cells are capable of maintaining a relatively high, sustained, [Ca2+]i for prolonged time periods, likely due to the continuous stimulus exerted by chewing and eating a meal. Conversely, prolonged salivary secretion is an essential requirement for mastication and swallowing of food. Studies in salivary gland cells demonstrate that Ca2+ signaling complexes might be concentrated in the lateral regions of the cells near tight junctions (2,5,26,50). Further, invaginations of the apical membrane likely position IP3R very close to both lateral membrane regions and ion channels localized in the apical membrane. It will be important in future studies to resolve the spatial arrangement of STIM1-Orai1-TRPC1 complexes in relation to IP3Rs.
Assembly and regulation of plasma membrane Ca2+ channels
Since both TRPC1 and Orai1 are present in salivary gland acinar cells, the question arises as to how exactly the two channels contribute to salivary fluid secretion. To understand this, it is important to consider the localization of the channels in relation to the key Ca2+-dependent ion channels involved in fluid secretion. NKCC1 is localized in the basolateral region of acinar cells while TMEM16A, and AQP5 (which is recruited to the apical membrane in response to [Ca2+]i increase) are localized in the luminal membrane, KCa channels are found in both membrane regions (2). TRPC1 is primarily localized in the basal and lateral regions of acinar cells (14,15) while Orai1 appears to be localized in the lateral membrane towards the luminal side and possibly at very low levels in the basal membrane (26,49). Following cell stimulation, STIM1 moves to the lateral and basal region of the cells and co-localizes with Orai1 and TRPC channels. While the exact local and global [Ca2+]i increases contributed by Orai1 and TRPC1 need to be determined, their localization suggests that they can contribute to apical and basal [Ca2+]i signals and thus regulate different ion flux mechanisms residing in the respective cellular domains[Figure 2]. However, as noted above, TRPC1 appears to be the primary Ca2+ entry channel involved in regulating fluid secretion since the residual Orai1 channel in TRPC1−/− mice is unable to compensate for the loss of function resulting from the deletion of TRPC1 or regulate apically localized KCa channels, which are localized quite close to Orai1 (14). Furthermore, sustained elevation is also much reduced in cells from TRPC1−/− mice. Thus, how TRPC1 function results in increasing [Ca2+]i in the apical region needs to be determined. Further, the physiological function of Orai1 in salivary gland acinar cells, whether it is required for plasma membrane insertion of TRPC1 or Ca2+-dependent gene expression, needs to be determined.
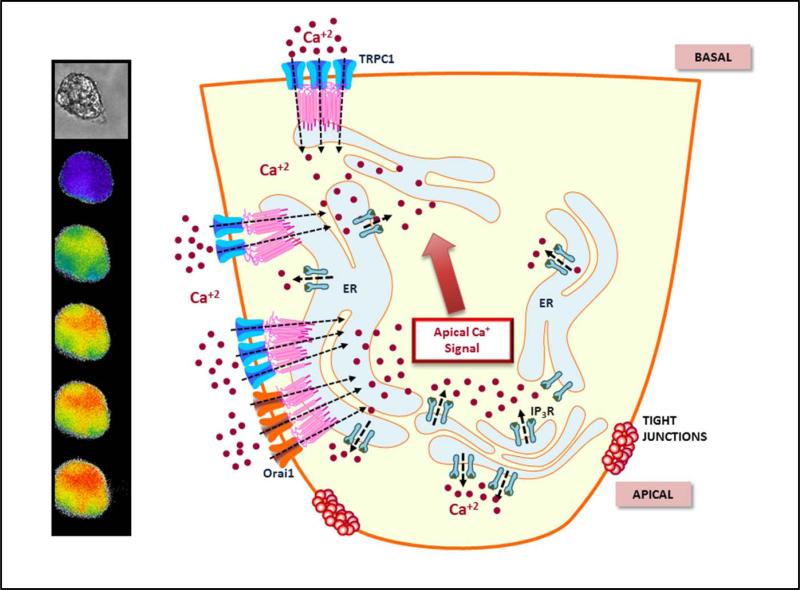
Figure 2. Spatiotemporal regulation of Ca2+ signals in salivary gland acinar cells.
Left: images show Ca2+ changes in an acinar cell following stimulation. The first increase is detected in the acinar region which then spreads to the basal region, although apical [Ca2+] is relatively higher. Right: The illustration depicts the underlying Ca2+ fluxes that determine [Ca2+]i changes in the cell. The initial increase occurs by release of Ca2+ via the IP3R at the apical pole. Ca2+ entry channels Orai1 and TRPC1 contribute to the global spread of Ca2+ in the cell, with TRPC1 being the primary component of this increase. While the apical membrane in acinar cells in the illustration is presented in a simplified manner, it is likely to be highly invaginated and thus could extend within the cell to areas close to or even past the tight junction region. Such an arrangement would bring apical components relatively close to the ion channels in the lateral region and facilitate Ca2+ sensing by the latter. Further studies will be required to establish this.
Interaction of STIM1 with TRPC1 has been demonstrated by co-immunoprecipitation of TRPC1 and STIM1 following store depletion and attenuation of TRPC1-mediated Ca2+ entry in response to store depletion by knockdown of STIM1 expression (26,49). A lysine-rich region (referred to as polybasic tail or K domain, aa 671-685) present at the C-terminal end of STIM1 has been proposed to anchor STIM1 to PIP2 in the plasma membrane. Following stimulation, STIM1 interacts with TRPC1 and Orai1 in the basolateral regions of the acinar cell. Notably, regions can be distinguished where STIM1 associates with TRPC1 alone, TRPC1-STIM1 are co-localized towards basal end of the lateral membrane, and where all three proteins are detected, Orai1-TRPC1-STIM1 overlap in the lateral membrane towards the apical end. As discussed above, in salivary gland acinar cells, at both low and high levels of stimuli, the initial apical Ca2+ increase rapidly changes to a more global increase during which time the various ion channels and transporters involved in fluid secretion are activated. Based on the data obtained with salivary gland cells lines, it can be speculated that the initial Ca2+ release via IP3R at the apical pole will deplete local Ca2+ stores and induce Ca2+ entry via Orai1 localized in the apical region of the cell. TRPC1 localized in close proximity to Orai1 will be recruited to the plasma membrane, activated by STIM1, and contribute to further [Ca2+]i increase. As [Ca2+]i increases in the basolateral membrane region, additional TRPC1 (not located near Orai1) can be recruited along the lateral membrane, and possibly in the basal membrane as well, resulting in amplifying a global [Ca2+]i increase [Figure 3]. Recruitment of TRPC1 into the membrane and its assembly into a stable complex with STIM1 can provide the sustained [Ca2+]i increase required to drive fluid secretion.
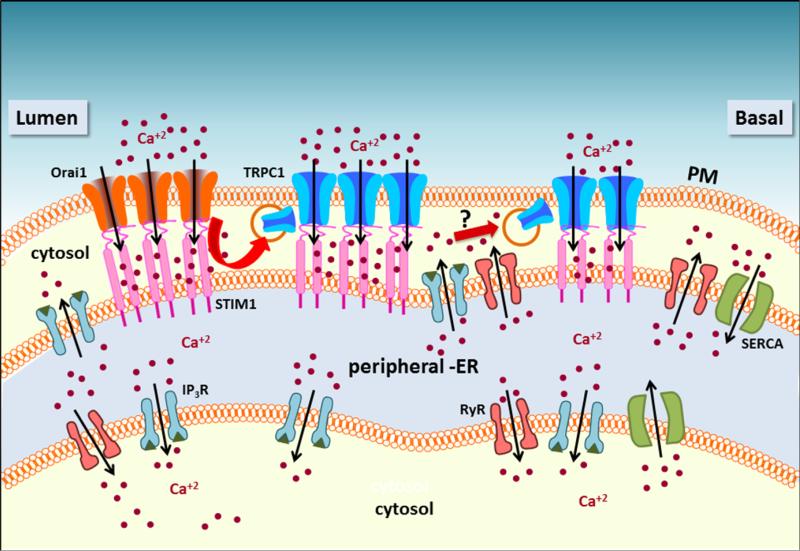
Figure 3. Regulation of TRPC1 and Orai1 in cellular microdomains.
The figure illustrates regulation of the calcium entry channels in various cellular domains. Release of intracellular Ca2+ in the apical region via IP3R activates STIM1 which translocates towards the plasma membrane binds to Orai1 and activates it. Ca2+ entry via Orai1 recruits TRPC1 to the membrane where it is also activated by STIM1. Further rise in [Ca2+]i along the basolateral region by the activites of the IP3R and RyR could drive recruitment of additional TRPC1 in these regions of the cell and generate sustained increases in [Ca2+]i. This latter trafficking of TRPC1, i.e. Ca2+ dependent but Orai1 independent, has yet to be experimentally validated.
Plasma membrane microdomains and fluid secretion
Lipid raft domains (LRDs) are biochemically distinct plasma membrane lipid domains that are enriched in cholesterol, sphingolipids, PIP2, PIP3. LRD have been shown to serve as a platform for assembly of signaling complexes, including key calcium signaling proteins (e.g. Cav1, G-proteins, PMCA, TRP channels, STIM1, and Orai1) (54-57). SOCE has been proposed to occur within LRDs, as disruption of these domains attenuates SOCE. The dependence of TRPC1 channel function on intact LRDs has been shown in many cell types, such as HSG cells, C2C12 skeletal myoblasts, polymorphonuclear neutrophils, endothelial cells and human platelets (55,57). Further evidence for the involvement of LRD in assembly of functional TRPC1 channels is provided by data demonstrating an increase in the partitioning of TRPC1 and STIM1 into lipid rafts following stimulation of cells and Ca2+ store depletion (58,59). When these domains are disrupted by cholesterol depletion, co-immunoprecipitation of TRPC1 and STIM1 as well as SOCE are attenuated. The polybasic tail of STIM1, and STIM2, contains a consensus poly-lysine sequence that can potentially mediate its binding to PIP2 in the plasma membrane (60-62). This has been confirmed in experiments showing that deletion of the polybasic tail results in loss of SOCE as well as STIM1 puncta formation in the ER-plasma membrane (PM) junctional regions. The exact interactions between STIM1 and plasma membrane proteins or lipids have not yet been resolved. It also unclear whether other scaffolding proteins are involved in targeting the STIM1 clusters to specific plasma membrane regions. A number of proteins have been identified which modify STIM1 activity. Some of them involve cytoskeletal and phospholipid modifying proteins, such as POST, septin, synaptotagmins, ESyt1 and Nir2. It has been proposed that remodeling of the PIP2 and cytoskeleton in the SOCE microdomain stabilizes the STIM1-channel complex (63-66). It is likely that these regions have specific biochemical, structural and spatial characteristics since ion channels and possibly other effector proteins regulated by SOCE, such as CaM and calcineurin, are recruited and regulated within this domain.
Caveolin1 (Cav1), a cholesterol binding protein that is localized within LRD, has been proposed to be involved in the regulation of SOCE and serve as a scaffold for recruitment of various proteins into LRD domains. A number of studies published demonstrate a role for Cav1 in the regulation of TRPC1 in salivary gland cell lines (55,57). An N-terminal Cav1-binding motif (aa 322 and 349 of TRPC) binds to the scaffolding domain in Cav1 (aa 82 and 101) and serves to scaffold TRPC1 in the plasma membrane region and determines its activation by store depletion. Loss of this binding results in mislocalization of TRPC1 (67). Further, Cav1 and STIM1 appear to concertedly regulate TRPC1 (68). In resting cells, Cav1 interacts with the inactive TRPC1 channel while following ER-Ca2+ store depletion binding of STIM1 to TRPC1 induces dissociation of TRPC1-Cav1 complex, resulting in channel activation. Refilling of the ER-Ca2+ stores leads to dissociation of STIM1 from TRPC1 and re-association of TRPC1 with Cav1. Caveolin also serves a critical role in the regulation of TRPC1 function in salivary gland acinar cells. Abrogation of caveolin1 in mice significantly attenuated Ca2+ influx in acinar cells and consequently led to loss of saliva secretion (69). This was due to disruption of plasma membrane localization of TRPC1, its association with lipid raft microdomains, and interaction with STIM1 after stimulation, while Orai1-STIM1 interactions were not affected. These findings suggest that by controlling TRPC1 localization in specific ER-PM junctional domains where it can be activated by STIM1, Cav1 has a crucial physiological role in regulating salivary gland function. The molecular components of the trafficking vesicles that carry TRPC1 to the plasma membrane, as well as the Ca2+-sensor and vesicle fusion mechanisms involved in TRPC1 insertion into the plasma membrane are not yet known. Resolving these components will provide complete understanding of how TRPC1 is regulated in salivary gland cells and provide novel tools to modify the function of this key Ca2+ entry pathway in the gland.
Conclusions
Salivary gland fluid secretion is initiated by neurotransmitter stimulation of plasma membrane receptors and mediated via a complex process that is determined by coordinated temporal and spatial regulation of several Ca2+ signaling processes as well as ion flux systems. The key events in fluid secretion are an increase in [Ca2+]i in the apical region of the cell triggered by IP3-induced the release of Ca2+ from ER via the IP3R and a more global sustained elevation of [Ca2+] due to Ca2+i entry via SOCE. The mechanism regulating SOCE is now well understood and the key molecular components have been identified. Orai1, TRPC1, and STIM1 are the critical components of SOCE in salivary gland cells. Among these TRPC1 is a major determinant of fluid secretion. Distinct localization of these channels and other signaling proteins in the cell suggests compartmentalization of Ca2+ signals. Important insights into the organization, composition, and functional significance of Ca2+ signaling complexes are now emerging. For example, concentration of IP3Rs in the luminal region of the cells is consistent with the initial increase in [Ca2+]i in this region of the cell. Similarly, the localization of TRPC1, Orai1, and STIM1 in acinar cells following stimulation is consistent with their role in SOCE as well as the functional interactions between the three proteins. Further studies should address how specific Ca2+ signals are detected in cells and then decoded for regulation of function. in this context more studies are required to resolve the spatial architecture of the apical and basolateral membranes. For example, the apical membrane has been shown to be highly invaginated. Thus components in the apical membrane could be localized close to the lateral regions of the cells where the two membranes could be apposed. This would facilitate local detection of Ca2+ signals generated by ion channels in the lateral membrane region of the cells by components localized in the apical membrane. Another important area that needs further understanding is the targeting and assembly of signaling complexes to various cellular locations. Salivary gland dysfunction in Sjogren's Syndrome or following radiation treatment likely reflects defects in calcium signaling and/or ion channel function. Therefore, more detailed understanding of the exact regulation and function of these components can define new clinical targets and therapeutic strategies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ambudkar IS. Regulation of calcium in salivary gland secretion. Crit Rev Oral Biol Med. 2000;11:4–25. doi: 10.1177/10454411000110010301. [DOI] [PubMed] [Google Scholar]
- 2.Melvin JE, Yule D, Shuttleworth T, Begenisich T. Regulation of fluid and electrolyte secretion in salivary gland acinar cells. Annu Rev Physiol. 2005;67:445–469. doi: 10.1146/annurev.physiol.67.041703.084745. [DOI] [PubMed] [Google Scholar]
- 3.Mikoshiba K, Hisatsune C, Futatsugi A, Mizutani A, Nakamura T, Miyachi K. The role of Ca2+ signaling in cell function with special reference to exocrine secretion. Cornea. 2008;27(Suppl 1):S3–8. doi: 10.1097/ICO.0b013e31817f246e. [DOI] [PubMed] [Google Scholar]
- 4.Yule DI. Subtype-specific regulation of inositol1,4,5-trisphosphate receptors: controlling calcium signals in time and space. J Gen Physiol. 117:431–434. doi: 10.1085/jgp.117.5.431. 20010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kiselyov K, Wang X, Shin DM, Zang W, Muallem S. Calcium signaling complexes in microdomains of polarized secretory cells. Cell Calcium. 2006;40:451–459. doi: 10.1016/j.ceca.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 6.Petersen OH, Tepikin AV. Polarized calcium signaling in exocrine gland cells. Annu Rev Physiol. 2008;70:273–299. doi: 10.1146/annurev.physiol.70.113006.100618. [DOI] [PubMed] [Google Scholar]
- 7.Ambudkar IS. Polarization of calcium signaling and fluid secretion in salivary gland cells. Curr Med Chem. 2012;19:5774–5781. doi: 10.2174/092986712804143321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ambudkar IS. Dissection of calcium signaling events in exocrine secretion. Neurochem Res. 2011;36:1212–1221. doi: 10.1007/s11064-011-0465-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee MG, Xu X, Zeng W, et al. Polarized expression of Ca2+ channels in pancreatic and salivary gland cells. Correlation with initiation and propagation of [Ca2+]i waves. J Biol Chem. 1997;272:15765–15770. doi: 10.1074/jbc.272.25.15765. [DOI] [PubMed] [Google Scholar]
- 10.Putney JW. Identification of cellular activation mechanisms associated with salivary secretion. Annu Rev Physiol. 1986;48:75–88. doi: 10.1146/annurev.ph.48.030186.000451. [DOI] [PubMed] [Google Scholar]
- 11.Putney JW. Capacitative calcium entry revisited. Cell Calcium. 1990;10:611–624. doi: 10.1016/0143-4160(90)90016-n. [DOI] [PubMed] [Google Scholar]
- 12.Birnbaumer L, Zhu X, Jiang M, et al. On the molecular basis and regulation of cellular capacitative calcium entry: roles for Trp proteins. Proc Natl Acad Sci U S A. 1996;93:15195–151202. doi: 10.1073/pnas.93.26.15195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Montell C. The TRP superfamily of cation channels. Sci STKE. 2005;272:re3. doi: 10.1126/stke.2722005re3. [DOI] [PubMed] [Google Scholar]
- 14.Liu X, Cheng KT, Bandyopadhyay BC, et al. Attenuation of store-operated Ca2+ current impairs salivary gland fluid secretion in TRPC1(−/−) mice. Proc Natl Acad Sci U S A. 2007;104:17542–17547. doi: 10.1073/pnas.0701254104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu X, Wang W, Singh BB, et al. Trp1, a candidate protein for the store-operated Ca(2+) influx mechanism in salivary gland cells. J Biol Chem. 2000;275:3403–3411. doi: 10.1074/jbc.275.5.3403. [DOI] [PubMed] [Google Scholar]
- 16.Kim MS, Lee KP, Yang D. Genetic and pharmacologic inhibition of the Ca2+ influx channel TRPC3 protects secretory epithelia from Ca2+-dependent toxicity. Gastroenterology. 2011;140:2107–2115. doi: 10.1053/j.gastro.2011.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liou J, Kim ML, Heo WD, et al. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr Biol. 2005;15:1235–1241. doi: 10.1016/j.cub.2005.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang SL, Yu Y, Roos J, et al. STIM1 is a Ca2+ sensor that activates CRAC channels and migrates from the Ca2+ store to the plasma membrane. Nature. 2005;437:902–905. doi: 10.1038/nature04147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu MM, Buchanan J, Luik RM, Lewis RS. Ca2+ store depletion causes STIM1 to accumulate in ER regions closely associated with the plasma membrane. J Cell Biol. 2006;174:803–813. doi: 10.1083/jcb.200604014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hogan PG, Lewis RS, Rao A. Molecular basis of calcium signaling in lymphocytes: STIM and ORAI. Annu Rev Immunol. 2010;28:491–533. doi: 10.1146/annurev.immunol.021908.132550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prakriya M. The molecular physiology of CRAC channels. Immunol Rev. 2009;231:88–98. doi: 10.1111/j.1600-065X.2009.00820.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zeng W, Yuan JP, Kim MS, et al. STIM1 gates TRPC channels, but not Orai1, by electrostatic interaction. Mol Cell. 2008;32:439–444. doi: 10.1016/j.molcel.2008.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yuan JP, Zeng W, Dorwart MR, Choi YJ, Worley PF, Muallem S. SOAR and the polybasic STIM1 domains gate and regulate Orai channels. Nat Cell Biol. 2009;11:337–343. doi: 10.1038/ncb1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee KP, Yuan JP, Hong JH, So I, Worley PF, Muallem S. An endoplasmic reticulum/plasma membrane junction: STIM1/Orai1/TRPCs. FEBS Lett. 2010;584:2022–2027. doi: 10.1016/j.febslet.2009.11.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheng KT, Ong HL, Liu X, Ambudkar IS. Contribution and regulation of TRPC channels in store-operated Ca2+ entry. Curr Top Membr. 2013;71:149–179. doi: 10.1016/B978-0-12-407870-3.00007-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hong JH, Li Q, Kim MS, et al. Polarized but Differential Localization and Recruitment of STIM1, Orai1 and TRPC Channels in Secretory Cells. Traffic. 2011;12:232–245. doi: 10.1111/j.1600-0854.2010.01138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ong HL, Jang SI, Ambudkar IS. Distinct contributions of Orai1 and TRPC1 to agonist-induced [Ca(2+)](i) signals determine specificity of Ca(2+)-dependent gene expression. PLoS One. 2012;7:e47146. doi: 10.1371/journal.pone.0047146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang Yd, Cho h, Koo JY, et al. TMEM16A confers receptor-activated calcium-dependent chloride conductance. Nature. 2008;455:1210–1215. doi: 10.1038/nature07313. [DOI] [PubMed] [Google Scholar]
- 29.Evans RL, Bell SM, Schultheis PJ, Shull GE, Melvin JE. Targeted disruption of the Nhe1 gene prevents muscarinic agonist-induced up-regulation of Na(+)/H(+) exchange in mouse parotid acinar cells. J Biol Chem. 1999;274:29025–29030. doi: 10.1074/jbc.274.41.29025. [DOI] [PubMed] [Google Scholar]
- 30.Evans RL, Turner RJ. Upregulation of Na(+)-K(+)-2Cl-cotransporter activity in rat parotid acinar cells by muscarinic stimulation. J Physiol. 1997;499:351–359. doi: 10.1113/jphysiol.1997.sp021932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nguyen HV, Stuart-Tilley A, Alper SL, Melvin JE. Cl(−)/HCO(3)(−) exchange is acetazolamide sensitive and activated by a muscarinic receptor-induced [Ca(2+)](i) increase in salivary acinar cells. Am J Physiol Gastrointest Liver Physiol. 2004;286:312–20. doi: 10.1152/ajpgi.00158.2003. [DOI] [PubMed] [Google Scholar]
- 32.Manganel M, Turner RJ. Agonist-induced activation of Na+/H+ exchange in rat parotid acinar cells is dependent on calcium but not on protein kinase C. J Biol Chem. 1990;265:4284–4289. [PubMed] [Google Scholar]
- 33.Ishikawa Y, Eguchi T, Skowronski MT, Ishida H. Acetylcholine acts on M3 muscarinic receptors and induces the translocation of aquaporin5 water channel via cytosolic Ca2+ elevation in rat parotid glands. Biochem Biophys Res Commun. 1998;245:835–84033. doi: 10.1006/bbrc.1998.8395. [DOI] [PubMed] [Google Scholar]
- 34.Ambudkar IS. Ca2+ signaling microdomains:platforms for the assembly and regulation of TRPC channels. Trends Pharmacol Sci. 2006;27:25–32. doi: 10.1016/j.tips.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 35.Bruce JI, Giovanucci DR, Blinder G, Shuttleworth TJ, Yule DI. Modulation of [ca2+]I signaling dynamics and metabolism by perinuclear mitochondria in mouse parotid acinar cells. J Biol Chem. 2004;279:1209–1217. doi: 10.1074/jbc.M309070200. [DOI] [PubMed] [Google Scholar]
- 36.Nakamura T, Matsui M, Uchida K, et al. M(3) muscarinic acetylcholine receptor plays a critical role in parasympathetic control of salivation in mice. J Physiol. 2004;558:561–575. doi: 10.1113/jphysiol.2004.064626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gautam D, Heard TS, Cui Y, Miller G, Bloodworth L, Wess J. Cholinergic stimulation of salivary secretion studied with M1 and M3 muscarinic receptor single- and double-knockout mice. Mol Pharmacol. 2004;66:260–267. doi: 10.1124/mol.66.2.260. [DOI] [PubMed] [Google Scholar]
- 38.Mikoshiba K. The IP3 receptor/Ca2+ channel and its cellular function. Biochem Soc Symp. 2007;74:9–22. doi: 10.1042/BSS0740009. [DOI] [PubMed] [Google Scholar]
- 39.Futatsugi A, Nakamura T, Yamada MK, et al. IP3 receptor types 2 and 3 mediate exocrine secretion underlying energy metabolism. Science. 2005;309:2232–2234. doi: 10.1126/science.1114110. [DOI] [PubMed] [Google Scholar]
- 40.Park S, Shcheynikov N, Hong JH. Irbit mediates synergy between ca(2+) and cAMP signaling pathways during epithelial transport in mice. Gastroenterology. 2013;145:232–241. doi: 10.1053/j.gastro.2013.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang D, Shcheynikov N, Zeng W. IRBIT coordinates epithelial fluid and HCO3-secretion by stimulating the transporters pNBC1 and CFTR in the murine pancreatic duct. J Clin Invest. 2009;119:193–202. doi: 10.1172/JCI36983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yamaguchi S, Ishikawa T. IRBIT reduces the apparent affinity for intracellular Mg2+ in inhibition of the electrogenic Na+-HCO −3 cotransporter NBCe1-B. Biochem Biophys Res Commun. 2012;424:433–438. doi: 10.1016/j.bbrc.2012.06.127. [DOI] [PubMed] [Google Scholar]
- 43.Zhang X, Wen J, Bidasee KR, et al. Ryanodine and inositol trisphosphate receptors are differentially distributed and expressed in rat parotid gland. Biochem J. 1999;340:519–527. [PMC free article] [PubMed] [Google Scholar]
- 44.Putney JW. The physiological function of store-operated calcium entry. Neurochem Res. 2011;36:1157–1165. doi: 10.1007/s11064-010-0383-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ambudkar IS. TRPC1: a core component of store-operated calcium channels. Biochem Soc Trans. 2007;35:96–100. doi: 10.1042/BST0350096. [DOI] [PubMed] [Google Scholar]
- 46.Ambudkar IS, Ong HL, Liu X, Bandyopadhyay BC, Cheng KT. TRPC1: the link between functionally distinct store-operated calcium channels. Cell Calcium. 2007;42:213–223. doi: 10.1016/j.ceca.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 47.Ong HL, Cheng KT, Liu X, et al. Dynamic assembly of TRPC1-STIM1-Orai1 ternary complex is involved in store-operated calcium influx. Evidence for similarities in store-operated and calcium release-activated calcium channel components. J Biol Chem. 2007;282:9105–9116. doi: 10.1074/jbc.M608942200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cheng KT, Liu X, Ong HL, Ambudkar IS. Functional requirement for Orai1 in store operated TRPC1-STIM1 channels. J Biol Chem. 2008;2008;283:12935–12940. doi: 10.1074/jbc.C800008200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cheng KT, Liu X, Ong HL, Swaim W, Ambudkar IS. Local Ca2+ entry via Orai1 regulates plasma membrane recruitment of TRPC1 and controls cytosolic Ca2+ signals required for specific cell functions. PLoS Biol. 2011;9:e1001025. doi: 10.1371/journal.pbio.1001025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bandyopadhyay BC, Swaim WD, Liu X, Redman RS, Patterson RL, Ambudkar IS. Apical localization of a functional TRPC3/TRPC6-Ca2+-signaling complex in polarized epithelial cells. Role in apical Ca2+ influx. J Biol Chem. 2005;280:12908–12916. doi: 10.1074/jbc.M410013200. [DOI] [PubMed] [Google Scholar]
- 51.Bandyopadhyay BC, Ong HL, Lockwich TP, et al. TRPC3 controls agonist-stimulated intracellular Ca2+ release by mediating the interaction between inositol 1,4,5-trisphosphate receptor and RACK1. J Biol Chem. 2008;283:32821–32830. doi: 10.1074/jbc.M805382200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bruce JI, Shuttleworth TJ, Giovannucci DR, Yule DI. Phosphorylation of inositol 1,4,5-trisphosphate receptors in parotid acinar cells. A mechanism for synergistic effects of cAMP on Ca2+ signaling. J Biol Chem. 2002;277:1340–1348. doi: 10.1074/jbc.M106609200. [DOI] [PubMed] [Google Scholar]
- 53.Won JH, Yule DI. Measurement of Ca2+ signaling dynamics in exocrine cells with total internal reflection microscopy. Am J Physiol Gastrointest Liver Physiol. 2006;291:146–155. doi: 10.1152/ajpgi.00003.2006. [DOI] [PubMed] [Google Scholar]
- 54.Ong HL, Ambudkar IS. The dynamic complexity of the TRPC1 channelosome. Channels (Austin) 2011;5:424–431. doi: 10.4161/chan.5.5.16471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ong HL, Ambudkar IS. Role of lipid rafts in regulation of store-operated Ca2+ channels. In: Levitan I, Barrantes FJ, editors. Cholesterol regulation of Ion Channels and Receptors. pub. Wiley; 2012. pp. 69–90. [Google Scholar]
- 56.Lingwood D, Simons K. Lipid rafts as a membrane-organizing principle. Science. 2010;327:46–50. doi: 10.1126/science.1174621. [DOI] [PubMed] [Google Scholar]
- 57.Pani B, Singh BB. Lipid rafts/caveolae as microdomains of calcium signaling. Cell Calcium. 2009;45:625–633. doi: 10.1016/j.ceca.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lockwich TP, Liu X, Singh BB, et al. Assembly of Trp1 in a signaling complex associated with caveolin-scaffolding lipid raft domains. J Biol Chem. 2000;275:11934–11942. doi: 10.1074/jbc.275.16.11934. [DOI] [PubMed] [Google Scholar]
- 59.Pani B, Ong HL, Liu X, Rauser K, Ambudkar IS, Singh BB. Lipid rafts determine clustering of STIM1 in endoplasmic reticulum-plasma membrane junctions and regulation of store-operated Ca2+ entry (SOCE). J Biol Chem. 2008;283:17333–17340. doi: 10.1074/jbc.M800107200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Carrasco S, Meyer T. STIM proteins and the endoplasmic reticulum-plasma membrane junctions. Annu Rev Biochem. 2011;80:973–1000. doi: 10.1146/annurev-biochem-061609-165311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bhardwaj R, Müller HM, Nickel W, Seedorf M. Oligomerization and Ca2+/Calmodulin control binding of the ER Ca2+-sensors STIM1 and STIM2 to plasma membrane lipids. Biosci Rep. 2013 Sep 17; doi: 10.1042/BSR20130089. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Calloway N, Owens T, Corwith K, Rodgers W, Holowka D, Baird B. Stimulated association of STIM1 and Orai1 is regulated by the balance of PtdIns(4,5)P2 between distinct membrane pools. J Cell Sci. 2011;124:2602–2610. doi: 10.1242/jcs.084178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Giordano F, Saheki Y, Idevall-Hagren O, et al. membrane pools. PI(4,5)P(2)-dependent and Ca(2+)-regulated ER-PM interactions mediated by the extended synaptotagmins. Cell. 2013;153:1494–1509. doi: 10.1016/j.cell.2013.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chang CL, Hsieh TS, Yang TT. Feedback regulation of receptor-induced Ca2+ signaling mediated by E-Syt1 and Nir2 at endoplasmic reticulum-plasma membrane junctions. Cell Rep. 2013;5:813–825. doi: 10.1016/j.celrep.2013.09.038. [DOI] [PubMed] [Google Scholar]
- 65.Sharma S, Quintana A, Findlay GM, et al. An siRNA screen for NFAT activation identifies septins as coordinators of store-operated Ca2+ entry. Nature. 2013;499:238–242. doi: 10.1038/nature12229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Krapivinsky G, Krapivinsky L, Stotz SC, Manasian Y, Clapham DE. Proc Natl Acad Sci U S A. POST, partner of stromal interaction molecule 1 (STIM1), targets STIM1 to multiple transporters. Proc Nat Acad Sci (USA) 2011;108:19234–19239. doi: 10.1073/pnas.1117231108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Brazer SC, Singh BB, Liu X, Swaim I.S. W, Ambudkar IS. Caveolin-1 contributes to assembly of store-operated Ca2+ influx channels by regulating plasma membrane localization of TRPC1. J Biol Chem. 2003;278:27208–27215. doi: 10.1074/jbc.M301118200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pani B, Ong HL, Brazer SC. SC Activation of TRPC1 by STIM1 in ER-PM microdomains involves release of the channel from its scaffold caveolin-1. Proc Natl Acad Sci U S A. 2009;106:20087–20092. doi: 10.1073/pnas.0905002106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pani B, Liu X, Bollimuntha S, et al. Impairment of TRPC1-STIM1 channel assembly and AQP5 translocation compromise agonist-stimulated fluid secretion in mice lacking caveolin1. J Cell Sci. 2013;126:667–675. doi: 10.1242/jcs.118943. [DOI] [PMC free article] [PubMed] [Google Scholar]