Abstract
Evidence from C57BL/6 mice suggests that CD8+ T cells, specific to the immunodominant HSV-1 glycoprotein B (gB) H-2b–restricted epitope (gB498–505), protect against ocular herpes infection and disease. However, the possible role of CD8+ T cells, specific to HLA-restricted gB epitopes, in protective immunity seen in HSV-1–seropositive asymptomatic (ASYMP) healthy individuals (who have never had clinical herpes) remains to be determined. In this study, we used multiple prediction algorithms to identify 10 potential HLA-A*02:01–restricted CD8+ T cell epitopes from the HSV-1 gB amino acid sequence. Six of these epitopes exhibited high-affinity binding to HLA-A*02:01 molecules. In 10 sequentially studied HLA-A*02:01–positive, HSV-1–seropositive ASYMP individuals, the most frequent, robust, and polyfunctional CD8+ T cell responses, as assessed by a combination of tetramer, IFN-γ-ELISPOT, CFSE proliferation, CD107a/b cytotoxic degranulation, and multiplex cytokine assays, were directed mainly against epitopes gB342–350 and gB561–569. In contrast, in 10 HLA-A*02:01–positive, HSV-1–seropositive symptomatic (SYMP) individuals (with a history of numerous episodes of recurrent clinical herpes disease) frequent, but less robust, CD8+ T cell responses were directed mainly against nonoverlapping epitopes (gB183–191 and gB441–449). ASYMP individuals had a significantly higher proportion of HSV-gB–specific CD8+ T cells expressing CD107a/b degranulation marker and producing effector cytokines IL-2, IFN-γ, and TNF-α than did SYMP individuals. Moreover, immunization of a novel herpes-susceptible HLA-A*02:01 transgenic mouse model with ASYMP epitopes, but not with SYMP epitopes, induced strong CD8+ T cell–dependent protective immunity against ocular herpes infection and disease. These findings should guide the development of a safe and effective T cell–based herpes vaccine.
A staggering number of individuals carry HSV-1 and/or HSV-2 that cause a wide range of diseases throughout their life (1–5). Most HSV-infected individuals are asymptomatic (ASYMP). They do not experience any recurrent herpetic disease (e.g., cold sore, ocular and genital herpes) even though spontaneously reactivated virus is surreptitiously shed in their body fluids (e.g., saliva, tears, and vaginal secretions) multiple times each year (1–3, 6, 7). In contrast, a small proportion of HSV-seropositive individuals are symptomatic (SYMP) and experience endless recurrences of herpetic disease, usually multiple times a year (8, 9), often requiring continuous antiviral therapy (i.e., acyclovir and derivatives). Additionally, in some HSV-1–seropositive SYMP individuals, sporadic reactivation of the virus from latency and corneal reinfection can cause blinding recurrent herpetic stromal keratitis (rHSK), a T cell–mediated immunopathological lesion of the cornea (10–12). Understanding the immune mechanisms by which ASYMP individuals, who spontaneously shed virus at the same frequency as SYMP individuals, control herpetic disease should prove informative for the design of future therapeutic vaccines. However, the human epitope specificity of T cells and the nature of SYMP and ASYMP T cells remain to be determined. We hypothesize that 1) although both SYMP and ASYMP patients recognize most HSV T cell epitopes, there are distinct human T cell epitopes that are recognized mainly by ASYMP individuals or mainly by SYMP patients (9, 13–15); and 2) T cell responses to SYMP epitopes may cause, or at least not protect against, immunopathological recurrent herpetic disease that leads to substantial morbidity, whereas T cell responses to ASYMP epitopes prevent/reduce recurrent herpes disease or cause it to remain subclinical (9, 13–17).
The clinical spectrum of HSV-1 and HSV-2 infections, ranging from asymptomatic to frequently distressing symptomatic outbreaks, are associated with HLA class I molecules (18–20). These associations suggest that a CD8+ T cell–mediated immune mechanism may influence the outcome of recurrent herpes infection (8). CD8+ T cells are found in the vicinity of latently infected sensory neurons during subclinical reactivation in mice (21–23) and in humans (24, 25). Of many adaptive immune responses explored as correlates of protection against herpes in mice, an overwhelming majority of data suggests that HSV-gB–specific CD8+ T cells contribute to protection (1–5). CD8+ T cells, specific to the immunodominant H-2b–restricted gB498–505 epitope, achieve at least partial control of herpetic ocular disease in C57BL/6 mice (8, 12, 26, 27). We recently reported a negative correlation between dysfunctional HSV-gB498–505–specific CD8+ T cells that reside within sensory trigeminal ganglia (i.e., the site of latent infection) and control of HSV-1 reactivation (21, 23). However, in clinical trials, therapeutic vaccination with a recombinant gB protein, which presumably contains both ASYMP and SYMP epitopes, led only to moderate and transient protection (6). Considering the wealth of data addressing the mechanism of CD8+ T cell antiviral activity in mice, it is surprising how few reports exist exploring the immune mechanisms of SYMP and ASYMP infection in humans. The immune mechanisms by which HSV-specific asymptomatic CD8+ T cells control herpes disease and HSV-specific symptomatic CD8+ T cells do not remain to be fully elucidated in humans. Identifying these mechanisms, or at least the viral epitopes involved, is critical for a rational design of an effective herpes vaccine. The present study was undertaken to characterize the population size, specificity, and function of CD8+ T cells in HSV-1–seropositive ASYMP versus SYMP patients. Because of the obvious ethical and practical considerations in obtaining tissue-resident CD8+ T cells (i.e., from the cornea or trigeminal ganglia), our investigation was limited to peripheral blood-derived CD8+ T cells.
We found that CD8+ T cells from HLA-A*02:01–positive, HSV-seropositive ASYMP individuals preferentially recognize naturally processed specific epitopes from HSV-1 gB, whereas CD8+ T cells from HLA-A*02:01–positive, HSV-seropositive SYMP patients preferentially recognized other nonoverlapping gB epitopes. The ASYMP epitope-primed CD8+ T cells displayed polyfunctional activity (p < 0.005). They concurrently produced three to five functions, including cytotoxic activity, ability to proliferate, and ability to produce the effector cytokines IL-2, IFN-γ and TNF-α. In contrast, SYMP epitope-primed CD8+ T cells were primarily monofunctional and produced IL-6, IL-8, and IL-17 inflammatory cytokines. Furthermore, immunization of a novel susceptible “humanized” HLA-A*02:01 transgenic mouse model (i.e., on BALB/c genetic background) with ASYMP epitopes, but not with SYMP epitopes, induced a strong CD8+ T cell–dependent protective immunity against ocular herpes infection and disease. Altogether, using a combination of several immunological assays, the results presented in this study characterize the breadth, function, and specificity of a virus-specific CD8+ T cell population that segregates with immunologic control of herpes disease in both ASYMP individuals and in “humanized” HLA-A*02:01 transgenic mice. These preclinical findings 1) further corroborate our hypothesis that although both SYMP and ASYMP patients recognize most HSV T cell epitopes, there are distinct human T cell epitopes that are strongly recognized mainly by ASYMP individuals or mainly by SYMP patients (9, 13–17); and 2) should help guide the development of the next generation of safe and effective T cell–based herpes vaccine in humans.
Materials and Methods
Human study population
During the last decade (i.e., January 2003–January 2013) we have screened a total of 525 individuals for HSV-1 and HSV-2 seropositivity. Among these, a cohort of 207 immunocompetent individuals, with an age range of 18–65 y (median, 32 y), who were seropositive for HSV-1 were enrolled in the current study. Three hundred eighty-five individuals were white, 140 were non-white (African, Asian, Hispanic, and others), 274 were females, and 251 were males. All patients were negative for HIV, hepatitis B virus, and had no history of immunodeficiency. Two hundred eighteen patients were HSV-1– or HSV-1/HSV-2–seropositive, among whom 208 patients were healthy and ASYMP (individuals who have never had any recurrent herpes disease, ocular, genital, or elsewhere, based on their self-report and physician examination; even a single episode of any herpetic disease in their life will exclude the individual from this group). The other 10 remaining patients were defined as HSV-1–seropositive SYMP who suffered frequent and severe recurrent oral and/or orofacial lesions, with two patients having had clinically well-documented HSK. Signs of recurrent disease in SYMP patients were defined as herpetic lid lesions, herpetic conjunctivitis, dendritic or geographic keratitis, stromal keratitis, and iritis consistent with HSK, with one or more episodes per year for the past 2 y. However, at the time of blood collection, SYMP patients have no recurrent disease (other than corneal scarring) and have had no recurrences during the past 30 d. They have no ocular disease other than HSK, have no history of recurrent genital herpes, are HSV-1–seropositive and HSV-2–seronegative. Because the spectrum of recurrent ocular herpes disease is wide, our emphasis is mainly on the number of recurrent episodes, not on the severity of the recurrent disease. For simplicity, just the extreme cases of SYMP and ASYMP patients are used. Thus, no attempt was made to assign specific T cell epitopes to specific severity of recurrent lesions. Patients are also excluded when they 1) have an active ocular (or elsewhere) herpetic lesion, or had one in the past 30 d; 2) are seropositive for HSV-2; 3) are pregnant or breastfeeding; or 4) ever took acyclovir or related antiviral drugs or any immunosuppressive drugs. SYMP and ASYMP groups were matched for age, gender, serological status, and race. Sixty-nine healthy control individuals were seronegative for both HSV-1 and HSV-2 and had no history of ocular HSK, genital lesions, or orofacial herpes disease. All subjects were enrolled at the University of California Irvine under approved Institutional Review Board–approved protocols (nos. 2003–3111 and 2009–6963). Written informed consent was received from all participants prior to inclusion in the study.
Bioinformatics analyses
HSV-1 gB open reading frames used in this study were from strain 17 (National Center for Biotechnology Information, accession no. NC-001806). Candidate HLA-A*02:01–restricted epitopes were identified using previously described software from the National Institutes of Health Bioinformatics and Molecular Analysis Section (Washington, DC; http://bimas.dcrt.nih.gov/molbio/hla_bind/) and the SYFPEITHI algorithm (http://www.syfpeithi.de/) (3). Potential cleavage sites for human proteasome were identified using NetChop 3.0 (http://www.cbs.dtu.dk/services/NetChop/) (3). MHC Pathway (http://www.mhc-pathway.net) was also employed in this screening (3).
Peptide synthesis
Based on the bioinformatics analysis, 10 putative HLA-A*02:01–binding peptides from gB with high estimated t1/2 of dissociation were synthesized by Magenex (San Diego, CA) on a 9050 Pep Synthesizer using solid-phase peptide synthesis and standard 9-fluorenylmethoxycarbonyl technology (PE Applied Biosystems, Foster City, CA). The purity of peptides was between 75 and 96%, as determined by reversed-phase HPLC (Vydac C18) and mass spectroscopy (Voyager MALDI-TOF system). Stock solutions were made at 1 mg/ml in 10% DMSO in PBS. All peptides were aliquoted and stored at −20°C until assayed.
Binding with soluble HLA-A*02:01 molecules
Quantitative assays to measure binding of peptides to soluble HLA-A*02:01 molecules are based on inhibition of binding of a radiolabeled standard peptide, as recently described (28). Briefly, 1–10 nM radiolabeled peptide was coincubated with 1 M–1 nM purified MHC and 1–3 µM human β2-microglobulin. After 2 d, binding of radiolabeled peptide to MHC class I molecules was determined by capturing MHC/peptide complexes on Greiner Lumitrac 600 microplates coated with W6/32 Ab and measuring bound counts per minutes using a TopCount microscintillation counter. Concentration of peptide yielding 50% inhibition of binding of radiolabeled probe peptide (IC50) was then calculated.
Stabilization of HLA-A*02:01 on class I-HLA–transfected B × T hybrid cell lines (T2 cell line)
To determine whether synthetic peptides could stabilize HLA-A*02:01 molecule expression on the T2 cell surface, peptide-inducing HLA-A*02:01 upregulation on T2 cells was examined according to a previously described protocol (3). T2 cells (3 × 105/well) were incubated with different concentrations of individual gB peptide (as indicated in Fig. 2B) in 48-well plates for 18 h at 26°C. Cells were then incubated at 37°C for 3 h in the presence of human β2-microglobulin (1 µg/ml) and BD GolgiStop (5 µg/ml) to block cell surface expression of newly synthesized HLA-A*02:01 molecules. The cells were washed with FACS buffer (1% BSA and 0.1% sodium azide in PBS) and stained with anti-HLA-A2.1–specific mAb BB7.2 (BD Pharmingen, San Diego, CA) at 4°C for 30 min. After incubation, the cells were washed with FACS buffer, fixed with 1% paraformaldehyde in PBS, and analyzed by flow cytometry using a BD LSR II (Becton Dickinson, Mountain View, CA). The acquired data, including mean fluorescence intensity (MFI), were analyzed with a FlowJo software version 9.5.2 (Tree Star). Percentage MFI increase was calculated as [(MFI with the given peptide − MFI without peptide)/(MFI without peptide)] × 100. Each experiment was performed three times, and means ± SD were calculated.
FIGURE 2.
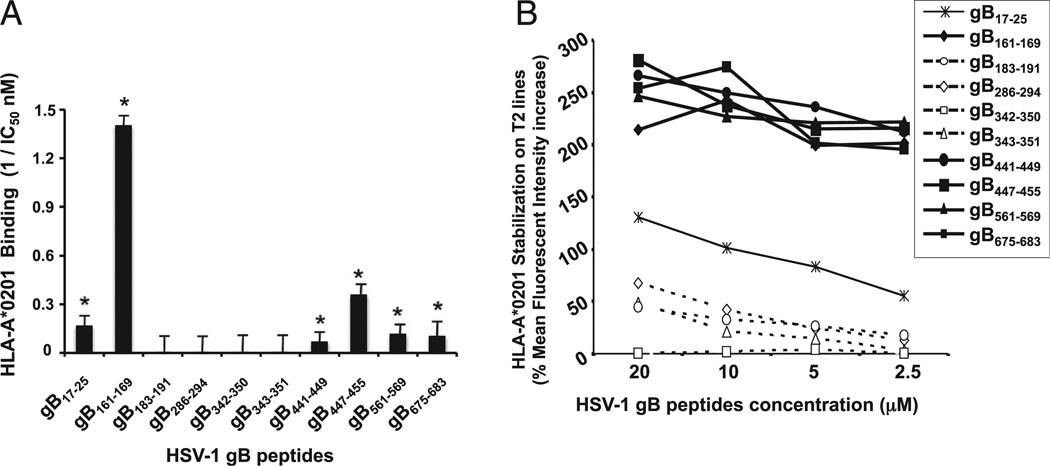
Binding capacities of HSV-1 gB-derived epitope peptides to HLA-A*0201 molecules. (A) In vitro binding capacities of HSV-1 gB-derived epitope peptides to soluble HLA-A*0201 molecules. HSV-1 gB-derived peptides were tested by an ELISA binding assay specific for HLA-A*0201 molecules as described in Materials and Methods. A reference nonherpes peptide was used to validate each assay. Data are expressed as relative activity (ratio of the IC50 of the test peptide to the IC50 of the reference peptide) and are the means of two experiments. Six peptide epitopes with high-affinity binding to HLA-A*0201 molecules (IC50 < 100) are indicated by an asterisk. The columns show four peptide epitopes that failed to bind HLA-A*0201 molecules. (B) Stabilization of HLA-A*02:01 molecules on the surface of T2 cells by gB peptides. T2 cells (3 × 105) were incubated with individual gB peptides at various concentrations (20, 10, 5, and 2.5 µM), as described in Materials and Methods. T2 cells were then stained with FITC-conjugated anti–HLA-A2 mAb (BB7.2). The graph represents the percentage of MFI of HLA-A*02:01 molecules on the surface of T2 cells following incubation with peptides. MFI was calculated as follows: percentage of MFI increase = [(MFI with the given peptide − MFI without peptide)/(MFI without peptide)] × 100. Error bars show SD for three independent experiments. Solid lines represent high levels of HLA-A*02:01 expression on the surface of T2 cells incubated with gB peptides. Broken lines represent low levels of stabilized HLA-A*02:01 expression.
HLA typing
HLA-A2 subtyping was performed using a commercial sequence-specific primer kit (SSPR1-A2; One Lambda, Canoga Park, CA) following the manufacturer’s instructions (29). Briefly, genomic DNA extracted from PBMCs of HSV-seropositive SYMP and ASYMP individuals was analyzed using a Tecan DNA workstation from a 96-well plate with 2 µl volume per well, as previously described (29). The yield and purity of each DNA sample were tested using a UV spectrophotometer. The integrity of DNA samples was ascertained by electrophoresis on agarose gel. Each DNA sample was then subjected to multiple small volume PCR reactions using primers specific to areas of the genome surrounding the single point mutations associated with each allele. Only primers that matched the specific sequence of a particular allele would amplify a product. The PCR products were subsequently electrophoresed on a 2.5% agarose gel with ethidium bromide, and the pattern of amplicon generation was analyzed using HLA Fusion software (One Lambda). Additionally, the HLA-A2 status was confirmed by staining PBMCs with 2 µl anti–HLA-A2 mAb BB7.2 (BD Pharmingen) at 4°C for 30 min. The cells were washed, acquired on a BD LSR II, and analyzed using FlowJo software version 9.5.2 (Tree Star).
PBMC isolation
Healthy individuals (negative for HIV, hepatitis B virus, and with or without any HSV infection history) were recruited at the University of California Irvine Institute for Clinical and Translational Science. Between 40 and 100 ml blood was drawn into yellow-top Vacutainer tubes (Becton Dickinson). The serum was isolated and stored at −80°C for detection of anti–HSV-1 and anti–HSV-2 Abs, as we previously described (9). PBMCs were isolated by gradient centrifugation using leukocyte separation medium (Cellgro, Manassas, VA). The cells were washed in PBS and resuspended in complete culture medium consisting of RPMI 1640 medium containing 10% FBS (Gemini Bio-Products, Woodland, CA) supplemented with 1× penicillin/l-glutamine/streptomycin, 1× sodium pyruvate, 1× nonessential amino acids, and 50 µM 2-ME (Life Technologies, Rockville, MD). Aliquots of freshly isolated PBMCs were also cryopreserved in 90% FCS and 10% DMSO in liquid nitrogen for future testing.
T cell proliferation assay
CD8+ T cell proliferation was measured using a CFSE assay as we recently described (2). Briefly, PBMCs were labeled with CFSE (2 µM) and incubated for 5 d with or without individual gB peptide (10 µg/ml). As a positive control, 2 µg/ml PHA was used to stimulate T cells for 3 d. The cells were then washed and stained with PE-conjugated mAbs specific to human CD8 molecules (clone HIT8A; BD Pharmingen). The numbers of dividing CD8+ T cells per 300,000 total cells were analyzed by FACS. Their absolute number was calculated using the following formula: number of events in CD8+/CFSE+ cells × number of events in gated lymphocytes/number of total events acquired.
Flow cytometry analysis
For each stimulation condition, at least 500,000 total events were acquired on a BD LSR II, and data analysis was performed using FlowJo version 9.5.2 (Tree Star). PBMCs were analyzed by flow cytometry after staining with fluorochrome-conjugated human specific mAbs. FITC-conjugated CD8 (clone HIT8A) and FITC-conjugated HLA-A2 (clone BB7.2) were purchased from BD Pharmingen. PE-conjugated gB peptide/tetramer complexes were gifted by the National Institutes of Health Tetramer Facility. A total of 106 PBMCs were stained in PBS containing 1% BSA and 0.1% sodium azide (FACS buffer) for 45 min at 4°C followed by three washes in FACS buffer and fixed in 1% paraformaldehyde. The gating strategy was similar to that used in our previous work (3). Briefly, we gated on single cells, dump− cells, viable cells (aqua blue−), lymphocytes, CD3+ cells, and CD8+ cells before finally gating on functional cells. Reported data have been corrected for background based on the negative (no peptide) control where appropriate, and only responses with a total frequency >0.10% of total CD8+ T cells (after background subtraction) were considered to be positive responses.
Tetramer/gB peptide complexes staining
Fresh PBMCs were analyzed for the frequency of CD8+ T cells recognizing the gB peptide/tetramer complexes, as we previously described (12, 30). The cells were incubated with gB peptide/tetramer complex for 30–45 min at 37°C. The cell preparations were then washed with FACS buffer and stained with FITC-conjugated anti-human CD8 mAb (BD Pharmingen). The cells were washed and fixed with 1% paraformaldehyde in PBS. The cells were then acquired on a BD LSR II and data were analyzed using FlowJo version 9.5.2 (Tree Star).
IFN-γ-ELISPOT assays
gB-specific IFN-γ–producing CD8+ T cells were characterized using ELISPOT. T cell stimulation was measured by IFN-γ production in peptide-stimulated PBMCs using a BD IFN-γ-ELISPOT kit (BD Pharmingen). Briefly, 5 × 105 PBMCs were stimulated with 20 µM individual gB peptides for 5 d. Then, activated PBMCs were harvested, washed, and restimulated with the gB peptides for 24 h in IFN-γ-ELISPOT plates (Millipore) that had been previously coated with anti-human IFN-γ capture Ab in a humidified incubator at 37°C with 5% CO2. The spot-forming cells were developed as described by the manufacturer (BD IFN-γ-ELISPOT kit; BD Pharmingen) and counted under stereoscopic microscope. Average spot counts for duplicate wells were calculated and background from wells with cells in medium only was subtracted.
Multiplex cytokine array
Fresh PBMCs (5 × 105 cells) were stimulated in a 96-well round-bottom plate with or without gB peptides for 24, 48, or 96 h. Supernatants were collected after 24, 48, or 96 h of stimulation. Production of six different cytokines (IL-2, IL-6, IL-8, IL-17, IFN-γ, and TNF-α) was assayed using multiplex cytokine arrays (BioLegend) per the manufacturer’s protocols. Samples were acquired on a Labscan 100 analyzer (Luminex) using Bio-Plex manager 6.0 software (Bio-Rad). Background levels were determined from nonstimulated PBMCs.
CD107 cytotoxicity assay
To detect cytolytic CD8+ T cells recognizing gB peptides in freshly activated and in vitro–activated PBMCs, we performed CD107a/b cytotoxicity assay. Betts and colleagues (31, 32) have described the CD107 assay as an alternative cytotoxicity assay that is able to address some of the shortcomings of the 51Cr-release assay. The CD107 assay was performed as described (33, 34) with a few modifications. On the day of the assay, nonstimulated or in vitro gB peptide-stimulated PBMCs were incubated at 37°C for 6 h in a 96-well plate with BD GolgiStop (BD Biosciences), costimulatory anti-CD28 and anti-CD49d Abs (1 µg/ml), and 10 µl CD107a-FITC and CD107b-FITC. At the end of the incubation period the cells were harvested into separate tubes and washed twice with FACS buffer then stained with PE-conjugated anti-human CD8 for 30 min at 4°C. The cells were then washed again, fixed, and 500,000 total events were acquired on a BD LSR II, and data analysis was performed using FlowJo version 9.5.2 (Tree Star).
HLA-A*02:01 transgenic mice
HLA-A*02:01 transgenic mice provided by Dr. Lemonier (Pasteur Institute) were bred at the University of California Irvine. These mice represent the F1 generation resulting from a cross between HLA-A*02:01/Kb transgenic mice (expressing a chimeric gene consisting of the 1 and 2 domains of HLA-A*02:01 and the 3 domain of H-2Kb) created on the BALB/c genetic background. Genotype of the HLA transgenic mice used in this study was confirmed as HLA-A*02:01, the most common A*02 subtype, supporting that the immunogenic SYMP versus ASYM peptide epitopes reported in this study are likely presented by the HLA-A*02:01 molecule. All animal studies were conducted in facilities approved by the Association for Assessment and Accreditation of Laboratory Animal Care and according to Institutional Animal Care and Use Committee–approved animal protocol (no. 202–2372). All studies have been approved by the University of California Irvine review Institutional Animal Care and Use Committee.
Virus production
HSV-1 (strain McKrae) was used in this study (3) and was grown and titrated on rabbit skin (RS) cells. UV-inactivated HSV-1 was generated as we previously described (1). HSV inactivation was confirmed by the inability to produce plaques when tested on RS cells.
Immunization of “humanized” HLA transgenic mice with SYMP and ASYMP peptide epitopes and ocular herpes challenge
Three groups of age-matched female HLA-A*02:01 transgenic mice (n = 10 each) were immunized s.c. with the ASYMP CD8+ T cell human epitopes (gB342–350 and gB561–569) delivered with the CD4+ T cell PADRE epitope emulsified in CpG1826 adjuvant (ASYMP), with the SYMP CD8+ T cell human epitopes (gB183–191 and gB441–449) delivered with the CD4+ T cell PADRE epitope emulsified in CpG1826 adjuvant (SYMP), or with the CpG1826 adjuvant alone (mock) on days 0 and day 21. All immunizations were carried out with 100 µM each peptide. A preliminary experiment was conducted to determine the LD50 of strain McKrae in naive HLA-A*02:01 transgenic mice following ocular challenge. Two × LD50 was then used in peptide- and mock-immunized mice to determine the protective efficacy of SYMP and ASYMP epitopes against lethal ocular herpes infection and disease. Two weeks after the final immunization, mice received an ocular HSV-1 challenge with 2 × 105 PFU (strain McKrae). The corneas were inoculated, without scarification, with the virus in a 4 µl tissue culture medium placed gently and topically on the corneas of immunized and control mice, as previously described (3). Control mice were inoculated using mock samples of virus.
Monitoring of ocular herpes infection and disease
Animals were examined for signs of ocular disease by slit lamp. Clinical assessments were made immediately before inoculation and on days 1, 4, 7, 10, 14, and 21 thereafter. The examination was performed by investigators blinded to the treatment regimen of the mice and scored according to a standard 0–4 scale: 0, no disease; 1, 25%; 2, 50%; 3, 75%; and 4, 100% staining, as previously described (3). To quantify replication and clearance of HSV-1 from the eyes, mice were swabbed daily with moist, type 1 calcium alginate swabs. Swabs were placed in 1.0 ml titration media (Media 199, 2% penicillin/streptomycin, 2% newborn calf serum) and frozen at −80°C until titrated on RS cell monolayers as described previously (3). Mice were also examined for survival in a window of 30 d after challenge, as we previously described (3).
Statistical analyses
Data for each assay were compared by ANOVA and a Student t test using GraphPad Prism 5 software (GraphPad Software, San Diego, CA). Differences between the groups were identified by ANOVA and multiple comparison procedures, as we previously described (35). Data are expressed as the means ± SD. Results were considered to be statistically significant at p < 0.05.
Results
In silico prediction of potential HLA-A*02:01–restricted T cell epitopes from HSV-1 gB Ag
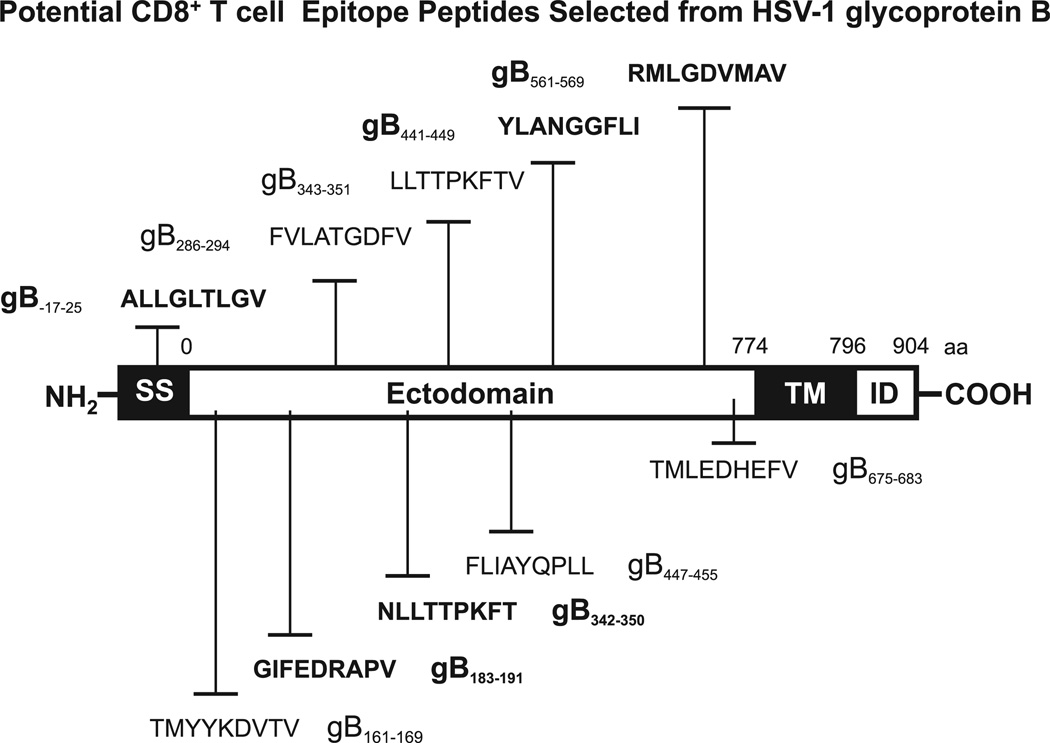
We have searched the deduced HSV-1 (strain 17) gB amino acid sequence for potential HLA-A*02:01–binding regions using BIMAS, SYFPEITHI, and MAPPP predictive computational algorithms. Based on these analyses, we selected 10 potential peptide epitopes with high predicted affinity to the HLA-A*02:01 molecule, a haplotype that is represented in >50% of the world human population, irrespective of gender and ethnicity (2) (Table I). Nine of the 10 gB peptide epitopes shared the HLA-A*02:01–binding motifs: leucine, isoleucine, or methionine at the second position and a valine, leucine, isoleucine, or threonine at the ninth position. As confirmed by the MHCPred computational algorithm, all 10 gB peptides that bear putative antigenic and immunogenic HLA-A*02:01–binding CD8+ T cell epitopes are more readily accessible to proteolysis, an event that precedes T cell epitope presentation in association with HLA molecules (2, 3). The 10 potential epitopes are not confined to a particular region of gB (Fig. 1). One of the 10 predicted epitopes, gB17–25, is located within the gB signal sequence. Seven belong to the external N-terminal ectodomain portion of gB (gB161–169, gB183–191, gB286–294, gB342–350, gB343–351, gB441–449, and gB447–455). Two are adjacent to the hydrophobic membrane transmembrane anchor domain (gB561–569 and gB675–683). None of the predicted peptides is within the transmembrane or the C-terminal intracellular domain and none belongs to known glycosylated regions of gB protein.
Table I.
Potential HLA-A*0201–restricted epitopes selected from HSV gB
| Peptide | Sequence | Molecular Mass (kDa) | Amino Acids (No.) | BIMAS | SYFPEITHI | MAPPP | MHCPred |
|---|---|---|---|---|---|---|---|
| gB17–25 | ALLGLTLGV | 856.0 | 9 | 257.342 | 31 | 0.5241 | 0.27 |
| gB161–169 | TMYYKDVTV | 1119.3 | 9 | 160.742 | 23 | 0.8421 | 0.34 |
| gB183–191 | GIFEDRAPV | 1003.1 | 9 | 145.077 | 24 | 0.5003 | 0.24 |
| gB286–294 | FVLATGDFV | 968.1 | 9 | 279.149 | 17 | — | 0.09 |
| gB342–350 | NLLTTPKFT | 1034.2 | 9 | 151.648 | 15 | — | — |
| gB343–351 | LLTTPKFTV | 1019.2 | 9 | 685.783 | 24 | 1 | 0.19 |
| gB441–449 | YLANGGFLI | 967.1 | 9 | 278.347 | 24 | — | 0.2 |
| gB447–455 | FLIAYQPLL | 1077.3 | 9 | 98.267 | 25 | 0.8300 | 0.4 |
| gB561–569 | RMLGDVMAV | 991.2 | 9 | 427.474 | 27 | 0.7222 | 0.37 |
| gB675–683 | TMLEDHEFV | 1120.2 | 9 | 2053.642 | 22 | 0.9942 | 0.19 |
The sequence of HSV-1 glycoprotein B was submitted to screening of potential HLA-A*0201 epitopes using several computer algorithms. Ten peptides were selected on the basis of HLA-A*0201 binding motif sequence from HSV-1 gB. The numbers in the four right columns show predicted IC50 as calculated by BIMAS (http://www-bimas.cit.nih.gov/molbio/hla_bind), SYFPEITHI http://www.syfpeithi.de), MAPPP (http://www.mpiib-berlin.mpg.de/MAPPP), and MHCPred (http://www.mhc-pathway.net). The sequences of synthesized peptides are based on the HSV-1 strain 17.
FIGURE 1.
Schematic representation showing the relative location within HSV-1 gB of the potential CD8+ T cell epitopes studied. HSV-1 (strain 17) gB regions carrying potential HLA A*0201 (HLA-A*0201)–restricted T cell epitopes were predicted using computer-assisted algorithms based on known HLA/peptide/TCR interactions, as described in Materials and Methods. The amino acid sequence, in a single letter code, and the peptide positions based on the 904-aa sequence of gB are shown. The high-affinity immunodominant epitopes identified in this study are shown in bold. ID, Intracellular domain; SS, signal sequence; TM, transmembrane domain.
Six of 10 potential gB epitope peptides bind with high affinity to HLA-A*02:01 and stabilize its expression on the surface of target cells
Peptides corresponding to the 10 highest probability gB epitopes, described above, were synthesized and the binding affinity of each peptide to soluble purified HLA-A*02:01 molecules was tested using a quantitative assay based on competitive inhibition of the binding of a radiolabeled reference peptide (2, 3). Six of 10 gB peptide epitopes bound with high affinity to the soluble HLA-A*02:01 molecules with a Kd of <100 nM (Fig. 2A). Specifically, gB17–25, gB161–169, gB441–449, gB447–455, and gB561–569 peptides bound to HLA-A*02:01 molecules with a Kd of <25 nM. The gB675–683 peptide bound to HLA-A*02:01 with a Kd of ~95 nM. In contrast, two peptides, gB286–294 and gB343–351, bound to HLA-A*02:01 with 100 nM > Kd < 500 nM (intermediate binding affinities). The remaining two peptides, gB183–191 and gB342–350, bound to HLAA*02:01 with a Kd of >500 nM (low binding affinities).
In a subsequent experiment, peptides corresponding to the 10 gB epitopes were used to perform a stabilization assay of HLA-A*02:01 molecules on the cell membrane of T2 cells (a class I-HLA–transfected B × T hybrid cell line). This assay used a mAb specific to a folded structure of HLA-A*02:01 to estimate in a FACS assay the relative amount of HLA-A*02:01 molecules retained on the surface of T2 cells following incubation with a potential peptide epitope (3). The amount of empty HLA-A*02:01 molecules retained on the surface of T2 cells is normally at a low level. Each gB peptide was tested individually at four descending concentrations: 20, 10, 5, and 2.5 µM. As shown in Fig. 2B, 5 of 10 peptides, gB161–169, gB441–449, gB447–455, gB561–569, and gB675–683, significantly increased the level of HLA-A*02:01 molecules detectable by FACS on the surface of T2 cells in a dose-dependent manner, suggesting a high affinity of these peptide to HLA-A*02:01 molecules (p < 0.005). The gB17–25 peptide demonstrated a moderate stabilization ability of HLA-A*02:01 molecules on the surface of T2 cells, indicating only a medium affinity of this peptide for HLA-A*02:01 molecules. In contrast, the remaining four peptides produced no significant stabilization of HLA-A*02:01 molecules on the surface of T2 cells. Overall, the six high binding affinity peptides found in Fig. 2A appeared to stabilize HLA-A*02:01 molecules on the cell surface of T2 cells, as shown in Fig. 2B.
Collectively, these results indicate that, under these conditions, gB17–25, gB161–169, gB441–449, gB447–455, gB561–569, and gB675–683 bind with high affinity to HLA-A*02:01 and stabilize its expression on the surface of target cells. Because binding affinity of an epitope peptide may not always reflect its efficient presentation to T cells or predict its ability to stimulate T cells, we next used a combination of several immunological assays to assess the ability of each of the 10 gB peptides to recall functional HSV-specific CD8+ T cells in HSV-1–seropositive individuals.
Frequent IFN-γ–producing CD8+ T cells, specific to gB17–25, gB183–191, gB342–350, gB441–449, and gB561–569 epitopes, detected in HLA-A*02:01–positive, HSV-seropositive individuals
CD8+ T cell responses specific to each gB peptide epitope described above were studied in HLA-A*02:01–positive, HSV-seropositive individuals. HLA-A*02:01–positive, HSV-seronegative individuals were used as controls. The characteristics of our study population with respect to gender, age, HSV-1 and HSV-2 seropositivity, and HLA-A*02:01 frequency distribution are detailed in Materials and Methods. Briefly, during a period of 10 y, 525 immunocompetent individuals, with an age range of 18–65 y (median, 32 y) who were seropositive or seronegative for HSV-1 and/or HSV-2, were enrolled in the study. Among HSV-seropositive individuals, 207 were HSV-1–seropositive and HSV-2–seronegative, 238 were HSV-2–seropositive and HSV-1–seronegative, and 11 individuals were both HSV-1– and HSV-2–seropositive. Because HSV-1 is the principal cause of ocular herpetic disease, this study focused solely on individuals who are HSV-1–seropositive and HSV-2–seronegative.
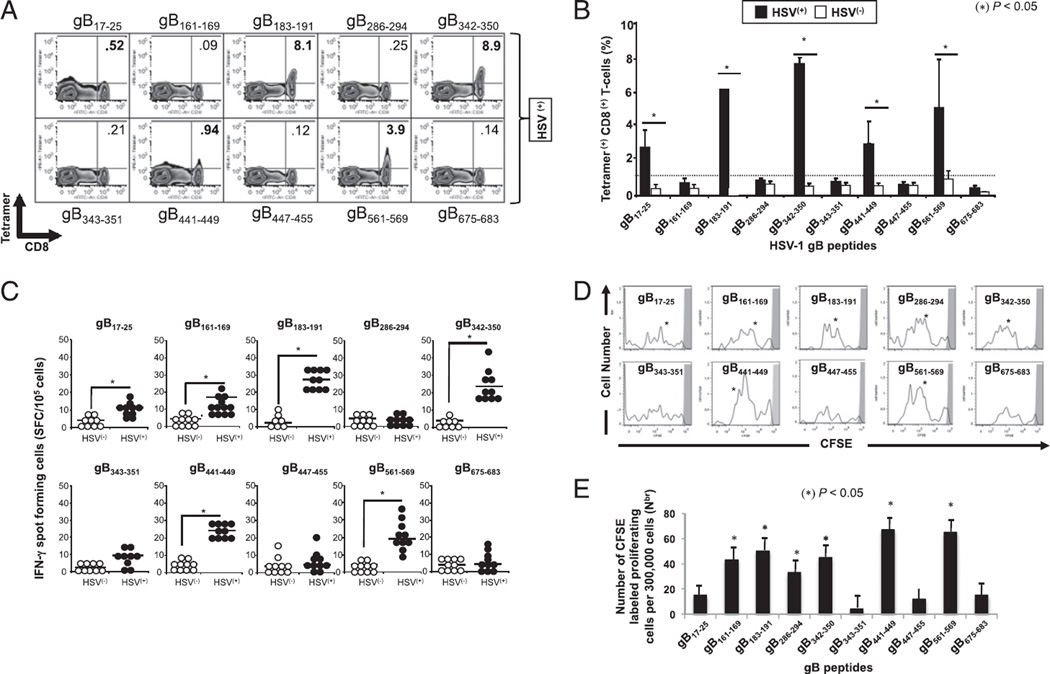
We first determined the frequency of CD8+ T cells specific to each of the 10 gB peptides in HSV-1–seropositive individuals using peptide/PE-labeled HLA-A*02:01 tetramers together with FITC-conjugated mAb specific to human CD8+ T cells (Fig. 3A). After peptide in vitro stimulation, we detected significantly higher percentages of tetramer+ CD8+ T cells specific to 5 of 10 peptides: gB17–25, gB183–191, gB342–350, gB441–449, and gB561–569 in PBMCs of 10 HLA-A*02:01–positive, HSV-1–seropositive individuals compared with PBMCs of 10 HLA-A*02:01–positive, HSV-seronegative individuals (p < 0.005; Fig. 3A, 3B). The highest frequencies of tetramer+ CD8+ T cells, detected with or without in vitro expansion, were recorded against gB183–191, gB342–350, and gB561–569 peptide epitopes (5.5–8.9%). Despite repeated attempts, with and without in vitro expansions, the remaining five gB peptide/HLA-A*02:01 tetramers consistently detected a low and nonsignificant frequency of CD8+ T cells in HLA-A*02:01–positive, HSV-seropositive individuals.
FIGURE 3.
High frequency of IFN-γ–producing CD8+ T cells specific to gB17–25, gB183–191, gB342–350, gB441–449, and gB561–569 epitopes detected in HLA-A*02:01–positive, HSV-seropositive individuals. PBMC-derived CD8+ T cells were analyzed ex vivo, after in vitro expansion, for the frequency of CD8+ T cells specific to 10 gB peptide/tetramer complexes representing each of the 10 potential gB epitopes shown in Fig. 1. (A) Representative FACS data of the frequencies of CD8+ T cells in an HSV-1–seropositive individual following a 5-d expansion with the indicated peptide. (B) Average frequency of PBMC-derived CD8+ T cells that recognize each of the indicated gB epitopes from 10 HLA-A*02:01–positive, HSV-1–seropositive individuals (HSV+) compared with 10 HLA-A*02:01–positive, HSV-seronegative individuals (HSV−). (C) gB epitope-specific IFN-γ–producing CD8+ T cells detected in healthy HLA-A*02:01–positive, HSV-seropositive individuals. PBMC-derived CD8+ T cells, either from HSV+ or from HSV− individuals, were stimulated with individual gB peptides. The number of gB epitope-specific, IFN-γ–producing T cells was determined by ELISPOT assay. Tests were performed in duplicates for each experiment. Spots were developed as described in Materials and Methods and calculated as spot-forming cells = mean number of spots in the presence of Ag − mean number of spots in the absence of stimulation. Open circles represent HSV− individuals whereas filled circles represent HSV+ individuals. (D) Profile of T cell proliferation with individual gB peptides. The graphs represent the proliferation of CD8+ T cells in the presence of 10 µM of each individual gB peptide detected from HSV+ individuals using a CFSE assay. (E) Absolute numbers of dividing CD8+ T cells per 300,000 total cells after 5 d stimulation. The results are representative of two independent experiments. *p < 0.005, comparing HSV+ to HSV− individuals using one-way ANOVA test.
Because the frequency of CD8+ T cells specific to a given epitope may not always reflect their functionality, we next determined the ability of each of the 10 gB epitope peptides to recall proliferative and IFN-γ–producing epitope-specific CD8+ T cells in HLA-A*02:01–positive, HSV-1–seropositive individuals. HLA-A*02:01–positive, HSV-seronegative individuals were used as negative controls. CD8+ T cells were isolated from fresh peripheral blood of each group and stimulated for 5 d with individual gB peptides, and the number of epitope-specific IFN-γ–producing CD8+ T cells was determined by a 24-h ELISPOT assay. Our previous analysis revealed that 10 or more spots per 105 CD8+ T cells gave a 99.5% probability for defining a positive response and 10 spot-forming cells per 105 CD8+ T cells was therefore used as the cutoff point (1, 9). Significant IFN-γ–producing CD8+ T cell responses specific to 6 of 10 peptides, gB17–25, gB161–169, gB183–191, gB342–350, gB441–449, and gB561–569 were detected in HLA-A*02:01–positive, HSV-1–seropositive individuals (p < 0.005; Fig. 3C, 3E). In comparison, <10% of individuals showed positive T cell responses to the remaining four peptides (gB286–294, gB343–351, gB447–455, and gB675–683). Of note, significant IFN-γ–producing CD8+ T cell response specific to the gB161–169 epitope was detected regardless of the low frequency of CD8+ T cells detected in HLA-A*02:01–positive, HSV-seropositive individuals (Fig. 3A, 3B). No significant IFN-γ–producing CD8+ T cell responses specific to any of the 10 gB epitopes were detected in HLA-A*02:01–positive, HSV-seronegative healthy controls.
Because lack of IFN-γ production by T cells may not always reflect lack of a T cell response (12, 35), we also studied the proliferative responses of CD8+ T cells from HSV-1–seropositive individuals labeled with CFSE and restimulated in vitro with each of the 10 gB peptides. The cells were stained with anti-human CD8-PE, and the number of dividing CD8+ T cells among a total number of 300,000 cells was determined by FACS, as we previously described (3). Briefly, gated CFSE+CD8+ T cells from HLAA*02:01–positive, HSV-1–seropositive individuals were examined for proliferation. As shown in Fig. 3D, gB17–25, gB161–169, gB183–191, gB286–294, gB342–350, gB441–449, and gB561–569 peptide epitopes all induced significant CD8+ T cell proliferative responses in HLA-A*02:01–positive, HSV-1–seropositive individuals (p < 0.005). All but gB286–294 epitopes were positive in the tetramer and ELISPOT assay above. As expected, no proliferative HSV-gB epitope-specific CD8+ T cell responses were detected in HLA-A*02:01–positive, HSV-seronegative controls (not shown).
Altogether, the HLA binding and the three immunological assays described above (tetramer frequency, IFN-γ production, and CFSE proliferation) point to gB17–25, gB183–191, gB342–350, gB441–449, and gB561–569 as functional immunodominant gB epitopes that specifically recall frequent CD8+ T cells in HLA-A*02:01–positive, HSV-1–seropositive individuals.
Frequent IFN-γ–producing CD8+ T cells specific to immunodominant gB342–350 and gB561–569 epitopes detected in asymptomatic individuals
We next asked whether there was a difference in the frequency and function of CD8+ T cells specific to gB17–25, gB183–191, gB342–350, gB441–449, and gB561–569 epitopes between HSV-seropositive SYMP and ASYMP individuals. The characteristics of the SYMP and ASYMP study populations used in this study with regard to HSV seropositivity and disease are described in detail in Materials and Methods. Briefly, among the HSV-1–seropositive population, 208 individuals are clinically healthy with no apparent, or history of, ocular, genital, or labial herpes lesions, and these are designated as ASYMP (no history of recurrent HSV disease). Ten patients were identified as HSV-1–seropositive, HSV-2–seronegative SYMP who had a clinically well-documented history of HSV-1 recurrent ocular disease (rHSK) such as herpetic lid lesions, herpetic conjunctivitis, dendritic or geographic keratitis, stromal keratitis, and iritis consistent with rHSK, with one or more episodes per year for the past 2 y.
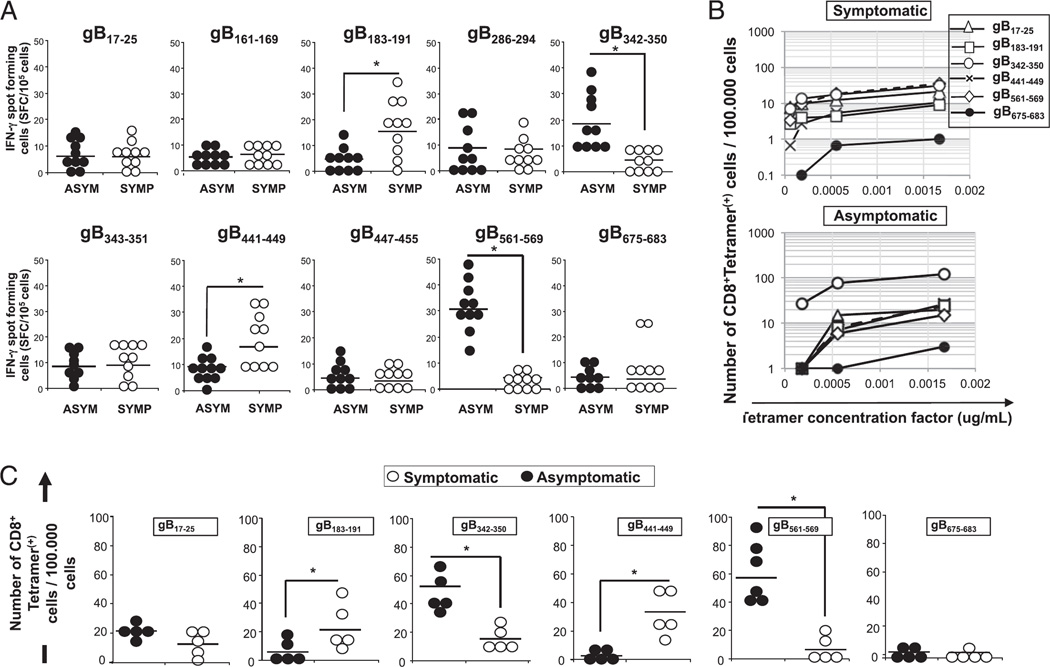
We first compared the ability of all 10 potential gB epitopes to recall functional CD8+ T cells in 10 HLA-A*02:01–positive, HSV-1–seropositive ASYMP and 10 HLA-A*02:01–positive, HSV-1–seropositive SYMP individuals. CD8+ T cells, isolated from fresh peripheral blood in each group, were stimulated for 5 d with individual gB peptides and the number of gB-epitope–specific IFN-γ–producing CD8+ T cells was determined in a 24-h ELISPOT assay. As shown in Fig. 4A, 2 of 10 peptides, gB342–350 and gB561–569, induced significantly higher IFN-γ–producing CD8+ T cells from 10 sequentially studied HLA-A*02:01–positive, HSV-1–seropositive ASYMP healthy individuals as compared with 10 SYMP individuals, suggesting that those two epitopes are ASYMP epitopes (p < 0.005). In contrast, gB183–191 and gB441–449 peptides appeared to induce significantly higher IFN-γ–producing CD8+ T cells preferentially from SYMP individuals compared with ASYMP individuals, suggesting that those two epitopes are SYMP epitopes (p < 0.005). The gB17–25 and gB286–294 peptides appeared to induce significantly higher IFN-γ–producing CD8+ T cells in both SYMP and ASYMP individuals.
FIGURE 4.
Frequent and strong IFN-γ–producing CD8+ T cells preferentially induced by gB342–350 and gB561–569 epitopes in asymptomatic individuals. (A) PBMC-derived CD8+ T cells, either from 10 ASYMP or from 10 SYMP individuals, were stimulated with individual gB peptides. The number of gB epitope-specific, IFN-γ–producing T cells was determined by ELISPOT assay, as in Fig. 4. (B) Representative data from one SYMP and one ASYMP individual showing the number of gB epitope-specific CD8+ T cells per 100,000 cells detected ex vivo using several concentrations of tetramer. (C) The average number of gB epitope-specific, PBMC-derived CD8+ T cells per 100,000 cells from five HLA-A*02:01–positive, HSV-1–seropositive SYMP individuals versus five ASYMP individuals. The results are representative of two independent experiments. *p < 0.005, comparing ASYMP to SYMP individuals using one-way ANOVA test.
We then extended our analysis to assess the frequency of circulating CD8+ T cells, specific to each of the five immunodominant gB epitopes described above (gB17–25, gB183–191, gB342–350, gB441–449, and gB561–569), in PBMCs isolated from five ASYMP and five SYMP individuals. The subdominant epitope gB675–683 was used as control. To obtain an objective enumeration of gB epitope-specific CD8+ T cells, each tetramer was tested at three or four dilutions and the numbers (instead of percentage) of epitope-specific CD8+ T cells per 100,000 T cells were determined. Significantly more tetramer+CD8+ T cells specific to gB342–350 and gB561–569 epitopes were detected in ASYMP individuals, confirming these as ASYMP epitopes (p < 0.005; Fig. 4B, 4C). In contrast, significantly higher numbers of tetramer+CD8+ T cells specific to gB183–191 and gB441–449 epitopes were detected in SYMP individuals (p < 0.005; Fig. 4B, 4C), suggesting these as SYMP epitopes.
Asymptomatic gB epitope-specific CD8+ T cells displayed concurrent polyfunctional activities and were capable of recognizing naturally processed epitopes on HSV-1–infected target cells
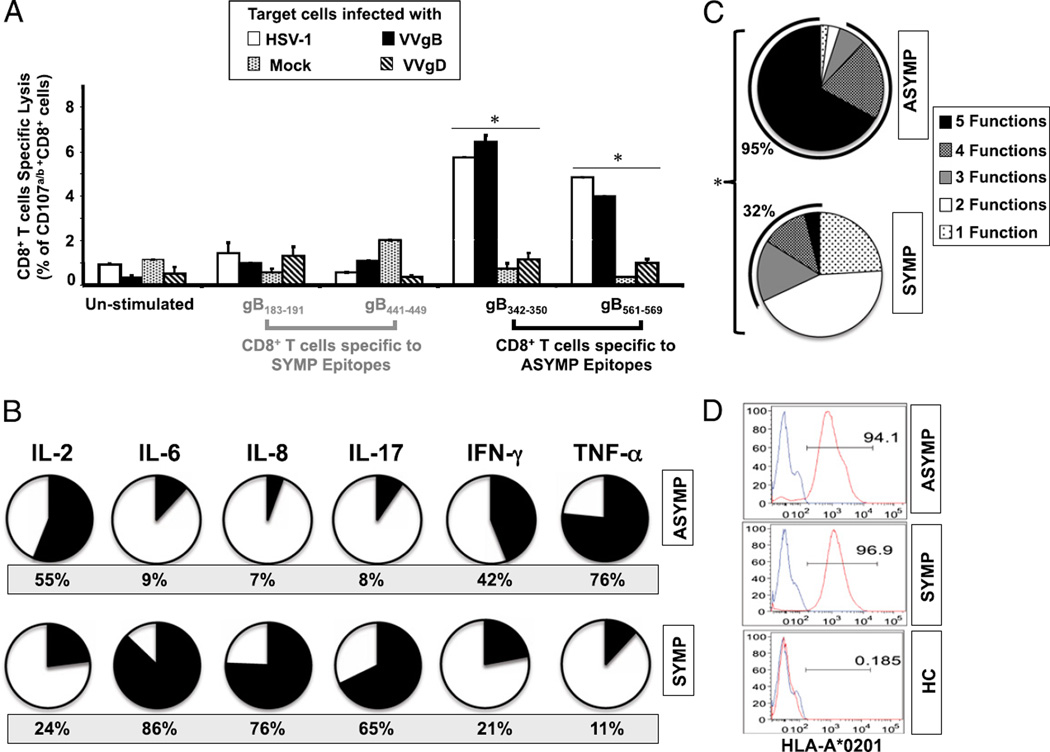
We examined the cytotoxic function of CD8+ T cells from SYMP and ASYMP individuals. CD107a and CD107b are lysosomal-associated membrane glycoproteins that surround the core of the lytic granules in cytotoxic T cells (CTLs) (31, 32). Upon TCR engagement and stimulation by Ags in association with MHC molecules, CD107a/b are exposed on the cell membrane of cytotoxic T cells. Thus, the level of CD107a/b expression on the surface of CTLs is used as a direct assay for the epitope-specific CTL response (31). To assess whether gB epitope-specific CD8+ T cells display CTL activity, fresh PBMC-derived CD8+ T cell lines were generated from HLA-A*02:01–positive ASYMP and HLA-A*02:01–positive SYMP individuals following stimulation in vitro with individual ASYMP (gB342–350 and gB561–569) or SYMP (gB183–191 and gB441–449) peptides. The cytotoxicity of each of the four CD8+ T cell lines was measured against autologous target monocyte-derived dendritic cells either left uninfected (mock) or infected with UV-inactivated HSV-1, with a vaccinia virus expressing gB (VVgB), or with a control vaccinia virus expressing glycoprotein D (VVgD) by detecting the level of CD107a/b expression by FACS on gated CD8+ T cells. As shown in Fig. 5A, a high percentage of ASYMP gB342–350 and gB561–569 epitope-specific CD8+ T cells from healthy HLA-A*02:01–positive, HSV-seropositive ASYMP individuals expressed significant levels of CD107a/b (percentage of CD107a/b/CD8+ T cells) following incubation with either HSV-1– or VVgB-infected target monocyte-derived dendritic cells. In contrast, very few SYMP gB183–191 and gB441–449 epitope-specific CD8+ T cells upregulated CD107a/b after incubation with HSV-1– or VVgB-infected target cells. As expected, no significant percentage of SYMP or ASYMP epitope-specific CD8+ T cells upregulated CD107a/b after incubation with mock-infected or VVgD-infected target cells. The results indicate that ASYMP epitope-induced CD8+ T cells have cytotoxic activity against HSV-1–infected cells and are able to specifically recognize endogenously processed gB epitopes from both HSV-1– and VVgB-infected target cells. There was no CTL response against any peptides in individuals that were seronegative for HSV regardless of whether they were HLA-A*02:01–positive or HLA-A*02:01–negative (data not shown).
FIGURE 5.
Asymptomatic epitopes preferentially induced polyfunctional CD8+ T cells. (A) ASYMP gB epitope-primed CD8+ T cells displayed lytic activity. CD8+ T cell lines specific to gB342–350 and gB561–569, gB183–191 and gB441–449 epitopes were derived from HLA-A*02:01–positive ASYMP (n = 5) and SYMP (n = 5) individuals. Each CD8+ T cell line was incubated with HSV-1– or VVgB-infected autologous target monocyte-derived dendritic cells (moDCs) in the presence of anti-CD28/49d, FITC-conjugated anti-CD107a and CD107b, and GolgiStop for 6 h. Uninfected target cells were used as control. FACS was used to analyze CD107 expression, as described in Materials and Methods. The graph represents the means ± SD of the percentage of CD107a/b and CD8+ T cells in the presence of uninfected (mock)-, HSV-1–, VVgB-, or control VVgD-infected target cells (moDCs). Samples were acquired on a BD LSR II, and data analysis was performed using FlowJo. *p < 0.005, comparing ASYMP to SYMP individuals using one-way ANOVA test. (B) Summary pie charts showing the average amount of each cytokine produced by CD8+ T cells from ASYMP patients (n = 10, top row) and SYMP patients (n = 8, bottom row), as detected by Luminex assay. The average frequency of different cytokines producing CD8+ T cells is shown under each pie chart. (C) Each pie chart represents the overall mean of CD8+ T cell functions from five HLA-A*02:01–positive ASYMP and five SYMP individuals in responses to stimulation with either SYMP or ASYMP gB peptides. Each sector of the pie chart represents the number of CD8+ T cell functions produced. (D) Representative data showing the expression of HLA-A0201 molecule by dendritic cells from an HLA-A*02:01–positive, HSV-1–seropositive SYMP individual versus an ASYMP individual and by dendritic cells from an HLA-A*02:01–negative, HSV-1–seronegative healthy control (HC).
The levels of six different inflammatory cytokines (IL-2, IL-6, IL-8, IL-17, IFN-γ, and TNF-α) produced by CD8+ T cells from ASYMP versus SYMP individuals following in vitro restimulation with UV-inactivated HSV-1 (strain McKrae) were compared by the Luminex microbeads system. As shown in Fig. 5B, CD8+ T cells from SYMP patients produced high levels of IL-6, IL-8, and IL-17 (black-filled portion of the pie chart), consistent with differentiated inflammatory T cells. In contrast, T cells from ASYMP individuals produced more of the effector cytokines IL-2, IFN-γ, and TNF-α (black-filled portion of the pie chart). Fig. 5C summarizes the percentage of ASYMP and SYMP individuals who showed positive results for one or several CD8+ T cell functions following in vitro restimulation with either ASYMP or SYMP gB peptides. Overall, 95% of ASYMP individuals had HSV-specific CD8+ T cells with three to five functions, indicating their ability to maintain greater frequencies and display concurrent polyfunctional activities: 1) production of high levels of lytic granules upon TCR engagement (CD107a/b cytotoxic activity); 2) expression of IFN-γ (ELISPOT); 3) high proliferation (CFSE); 4) production of high levels of IL-2, IFN-γ, and TNF-α effector cytokines (or proinflammatory cytokines in the case of SYMP individuals); and 5) tetramer frequency (p < 0.005). In contrast, only 32% of SYMP individuals had HSV-specific CD8+ T cells with three to five functions whereas most had just one function, suggesting that CD8+ T cells from SYMP patients tended to be monofunctional and produced more inflammatory cytokines (p < 0.005). Finally, similar levels of HLA-A*02:01 expression were detected by FACS on the surface of APCs (both dendritic cells and macrophages) derived from SYMP and ASYMP individuals (Fig. 5D). This result suggests that the apparent polyfunctionality of CD8+ T cells detected in ASYMP individuals is not a result of a high level of expression of HLA-A0201 molecules on their APCs, but rather to an intrinsic multifunctionality of CD8+ T cells specific to ASYMP epitopes.
Collectively, the results indicate that 1) a particular breadth and specificity of virus-specific CD8+ T cell responses is associated with control of herpes disease, and 2) HSV-specific CD8+ T cells from ASYMP individuals displayed concurrent polyfunctional activities whereas CD8+ T cells from SYMP patients tended to be monofunctional.
Immunization with asymptomatic epitopes induced a CD8+ T cell–dependent protective immunity against ocular herpes in “humanized” HLA-A*02:01 transgenic mice
Because the in vitro antigenicity of CD8+ T cell epitopes may not always translate into functional in vivo immunogenicity, we next set up a preclinical vaccine trial using SYMP and ASYMP epitopes in a novel humanized HLA-A*02:01 transgenic mouse model.
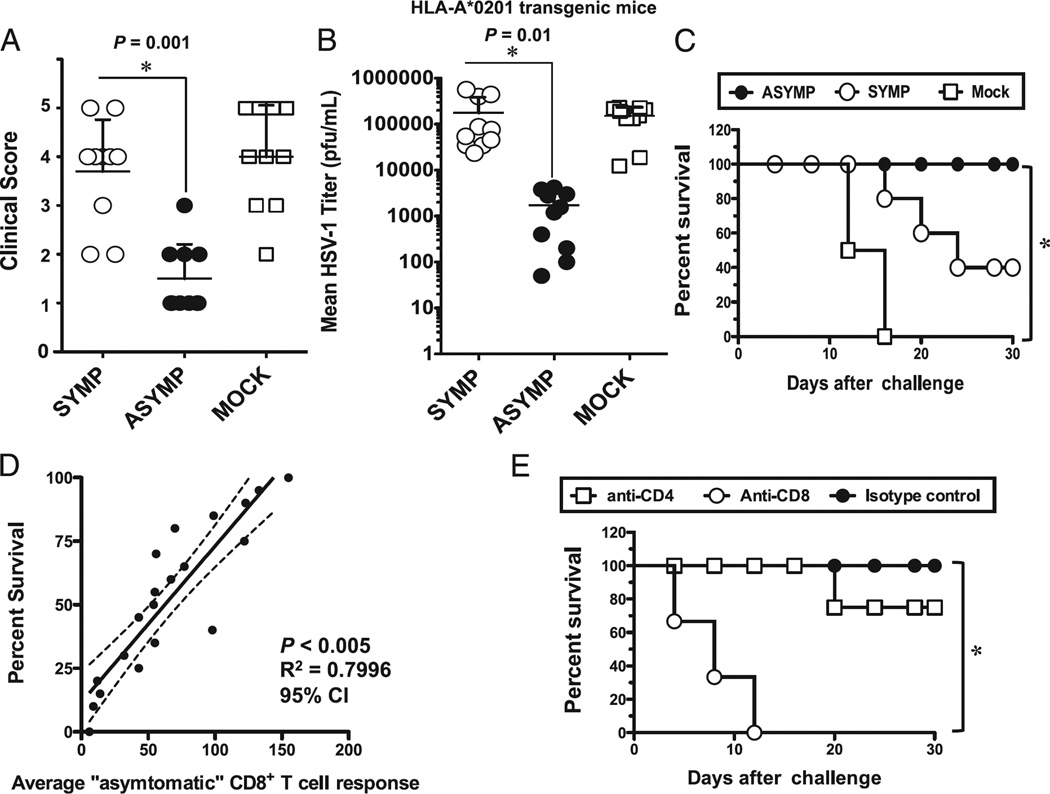
To evaluate whether immunization with ASYMP CD8+ T cell epitopes confer any protection against ocular herpes, groups of susceptible HLA-A*02:01 transgenic humanized mice (n = 10 mice/group, BALB/c genetic background) were immunized s.c. twice, 21 d apart with the two ASYMP CD8+ T cell epitopes gB342–350 and gB561–569 (group 1) or with SYMP epitopes, gB183–191 and gB441–449 (group 2). These were delivered together with the CD4+ T helper PADRE epitope and emulsified in CpG1826 adjuvant. As negative control, mock-immunized mice received adjuvant alone (group 3). Two weeks after the second and final immunization, animals from all groups received an ocular HSV-1 challenge (2 × 105 PFU, McKrae strain). Of note, the sequences of both SYMP and ASYMP epitopes are highly conserved between HSV-1 and HSV-2 strains (Table II); however, no significant homology exists between the amino acid sequences of the 10 HSV-1 gB T cell epitopes studied and the gB amino acid sequences of varicella zoster virus, EBV, and CMV (Table II). The pathology clinical scores observed in the ASYMP group were significantly lower than those observed in the SYMP group and the mock-immunized group (p = 0.001 for all; Fig. 6A). Furthermore, significantly lower viral loads were detected on day 7 postinfection in eye swabs of the ASYMP group compared with the SYMP and mock-immunized groups (p = 0.01; Fig. 6B). All animals in the ASYMP group survived lethal infection (100%) compared with only 40% survival in the SYMP group and 0% in the mock-immunized group (p < 0.005; Fig. 6C). Overall, there was a positive correlation between survival and the number of ASYMP CD8+ T cells detected in the draining lymph node (Fig. 6D, p < 0.005, r2 = 0.7996, 95% confidence interval). Similar to what has been observed in ASYMP individuals, cultured CD8+ T cells from ASYMP-immunized mice had significantly higher proportions of cells with simultaneous expression of three to five functions, whereas SYMP-immunized mice had few or no detectable cells expressing more than three functions simultaneously (75.5 and 17.5%, respectively; p = 0.005; not shown). To verify the involvement of CD8+ T cells in the observed protection induced following immunization with the ASYMP CD8+ T cell epitopes, we performed an in vivo depletion of either CD4+ or CD8+ T cells in immunized mice before virus challenge using specific mAbs. As shown in Fig. 6E, depletion of CD8+ T cells, but not of CD4+ T cells, significantly abrogated protection against death induced by immunization with the ASYMP CD8+ T cell epitopes (p < 0.005), suggesting that CD8+ T cells, but not CD4+ T cells, were required for protection against lethal ocular herpes.
Table II.
Comparative analysis of the sequences of HSV-1 SYMP and ASYMP CD8+ T cell gB epitopes between the strains of HSV-1 and HSV-2 and across other human herpes viruses
| Virus | Strain | gB17–25 | gB161–169 | gB183–191 | gB286–294 | gB342–350 | gB343–351 | gB441–449 | gB447–455 | gB561–569 | gB675–683 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| HSV-1 | 17 | ALLGLTLGV | TMYYKDVTV | GIFEDRAPV | FVLATGDFV | NLLTTPKFT | LLTTPKFTV | YLANGGFLI | FLIAYQPLL | RMLGDVMAV | TMLEDHEFV |
| HSV-2 | 333 | ALVVGALVA | TMYYKDVTV | GIFEDRAPV | FVLATGDFV | NLLTTPKFT | LLTTPKFTV | YLATGGFLI | FLIAYQPLL | RMLGDVMAV | TMLEDHEFV |
| VZV | Dumas | YRRNLRFRR | TVYYKDVIV | NRYADRVPI | FGLSTGDII | NFLVTPHLT | FLVTPHLTV | YLARGGFVV | FVVVFQPLL | RILGDVISV | TLLKDREFM |
| EBV | B95-8 | VVLLAALAC | RSYTKIVTN | NRHEEKFSV | FVTTTGQTV | AFLDKGTYT | FLDKGTYTL | FITSGGLLL | LLLAWLPLT | KRLGDVISV | SLIENIDFA |
| CMV | AD169 | VCVNLCIVC | RVYQKVLTF | GSNTEYVAP | FATSTGDVV | AFLERADSV | FLERADSVI | FETSGGLVV | LVVFWQGIK | RFMGDVLGL | DPLENTDFR |
The amino acid residues that are conserved across other human herpesviruses are shown in bold.
FIGURE 6.
CD8+ T cell–dependent protective immunity against ocular herpes induced by ASYMP epitopes in humanized HLA transgenic mice. Three groups of age-matched female HLA-A*02:01 transgenic mice (n = 10 each) were immunized s.c. with the ASYMP CD8+ T cell human epitopes (gB342–350 and gB561–569) delivered with the CD4+ T cell PADRE epitope emulsified in CpG1826 adjuvant (ASYMP), with the SYMP CD8+ T cell human epitopes (gB183–191 and gB441–449) delivered with the CD4+ T cell PADRE epitope emulsified in CpG1826 adjuvant (SYMP), or with the CpG1826 adjuvant alone (mock) on days 0 and 21. Two weeks after the final immunization, all animals were challenged ocularly with 2 × 105 PFU of HSV-1 (strain McKrae). (A) The eye disease was detected and scored 2 wk after immunization as described in Materials and Methods. (B) Viral loads were detected on day 7 postinfection in eye swabs, as described in Materials and Methods. (C) Immunized and infected mice were examined for survival in a window of 30 d postchallenge, as described in Materials and Methods. (D) Scattergram and linear regression analysis of mouse survival (%) and HSV-specific CD8+ T cell responses after challenge with HSV-1. Correlation was performed using the Pearson test with two-tailed p value analysis (r2 = 0.7996; p < 0.0001). (E) The protective immunity against ocular herpes induced by the ASYMP CD8+ T cell human epitope immunization is abrogated following depletion of CD8+ T cells, but not of CD4+ T cells. Following the second immunization, and before challenge with HSV-1, mice were injected i.p. with six doses (i.e., 1 every other day) of 100 µl saline containing anti-CD4, anti-CD8, or isotype control. Flow cytometry analysis confirmed a decrease in spleen CD4+ and CD8+ T cells in treated mice to consistently <2% after mAb treatment. The p values compare protection achieved in mAb treated versus untreated mice using the ANOVA test. Immunized, mAb-treated, and infected mice were examined for survival in a window of 30 d after challenge. Results are representative of two independent experiments.
Altogether, these results indicate that immunization with ASYMP CD8+ T cell epitopes, but not with SYMP epitopes, decreased ocular herpes disease, decreased virus replication, and protected against lethal ocular herpes in susceptible HLA transgenic mice.
Discussion
In the present study, we compared the population size, specificity, and function of HSV-specific CD8+ T cells from ASYMP and SYMP patients (i.e., those who did not show recurrent HSV disease episodes versus those that did, respectively). Striking quantitative and qualitative differences were found between CD8+ T cells from HSV-seropositive ASYMP individuals, who manage to immunologically control herpetic disease to clinically undetectable levels, and CD8+ T cells from SYMP patients, who failed to prevent recurrent herpetic disease. We identified two ASYMP CD8+ epitopes from HSV-1 gB that were preferentially recognized by CD8+ T cells from “protected” ASYMP individuals. Immunization with these ASYMP epitopes, gB342–350 and gB561–569, induced high protection against herpes infection and disease in humanized HLA transgenic mice. In contrast, two nonoverlapping epitopes, gB183–191 and gB441–449, that were preferentially recognized by CD8+ T cells from SYMP individuals produced little protection.
Rather than just using a few in vitro predictive assays to map CD8+ epitopes, we used several computational algorithms followed by both in vitro and in vivo functional immunological assays. High-performing predictive computational algorithms have been available for more than a decade; however, their value in terms of actual epitope binding has only been extensively studied more recently (28, 36, 37). Many immunological assays have been developed to validate computationally predicted human CD8+ T cell epitopes, including binding affinity to purified HLA molecules, tetramer assays, cell membrane HLA stabilization assays, CD8+ T cell cytokine analysis, and CFSE proliferation assays (28, 38, 39). When used individually, each screen is not sufficient to conclusively identify functional CD8+ T cell epitopes (27, 28, 38, 40). However, combinations of these screens can provide strong evidence of functional epitopes (3, 28, 33). In this study, we supported our predictive computational algorithms with immunological results, including in vitro assays of cell surface stabilization of HLA-A*02:01 molecules on T2 cells, functional ex vivo and in vitro CD8+ T cells assays, and tetramer staining of CD8+ T cells. Using these multiple screens we identified four high-affinity gB epitopes that were naturally processed and able to recall frequent functional human CD8+ T cells in vitro. Of interest is that the gB183–191 and gB342–350 peptides that appeared to bind with only low affinity to HLA-A*02:01 molecules in vitro still induced a strong expansion of CD8+ T cell response in vitro. Although the reason for this is not completely understood, this result highlights the importance of screening potential peptide epitopes using multiple immunological assays.
The in vivo immunogenicity and protective efficacy of human T cell epitopes found to be antigenic in vitro is crucial in vaccine design (34, 41–43). Only two of four immunodominant gB epitopes were found in this study to be immunogenic and protective in humanized HLA transgenic mice. This suggests that up to 50% of human CD8+ epitopes recently reported as recognized by human CD8+ T cells in vitro may not be as functionally immunogenic and protective as might be expected (8, 9, 24, 44–46). Additionally, some of the HSV-1 human T cell epitopes may actually be pathogenic and contribute to exacerbation of disease as we recently found for an HLA-A*02:01–restricted CD8+ T cell epitope from HSV-1 gK (47). Because our approach to epitope identification in this study was tailored specifically for HLA-A*02:01–restricted epitopes, the results reported are not to imply that these are the only human CD8+ T cell epitopes present on gB. However, this study describes a model system that could be applied to any HLA class I haplotype and to any other herpes structural or regulatory proteins to identify symptomatic versus asymptomatic epitopes.
To gain more insight into the nature of CD8+ T cell populations that are predominant in SYMP versus ASYMP individuals, we used several immunological assays to determine the population size, as well as the specificity and the function of CD8+ T cells obtained from peripheral blood. Although we are aware that information gained from peripheral blood T cells may not be completely reflective of tissue-resident T cells, because of the obvious ethical and practical considerations of obtaining tissue-resident CD8+ T cells (i.e., from the cornea or from trigeminal ganglia, the site of latent infection), our investigations were limited to PBMC-derived CD8+ T cells. A comparison of CD8+ T cell responses in vitro versus ex vivo points to a similar pattern and hierarchy of immunodominance of HSV-1 gB epitope-specific CD8+ T cell responses in ASYMP and SYMP individuals: 1) the most frequent, robust, and polyfunctional CD8+ T cell responses in ASYMP individuals were directed mainly against gB342–350 and gB561–569 epitopes; 2) in contrast, the most frequent, robust CD8+ T cell responses in SYMP individuals were directed mainly against gB183–191 and gB441–449 epitopes. Hence, peptide enrichment of PBMC-derived CD8+ T cells did not appear to introduce an in vitro culture bias or to change the main conclusions of the present study. Besides, it is well known that peptide epitope boosting of T cells in vitro, in situations of low CD8+ T cell frequencies such as during latent herpes infection, may potentially enrich PBMC-derived T cells (3, 24, 45). Thus, as expected, the magnitudes of HSV-specific CD8+ T cell responses were much higher than ex vivo responses, when PBMC-derived T cells were restimulated in vitro with gB peptide epitopes. Under the above circumstances, HSV-specific CD8+ T cells from ASYMP patients had a greater frequency of “polyfunctionality”. Of ASYMP individuals tested, 95.5% had CD8+ T cells able to produce three to five functions concurrently—namely 1) CD107a/b cytotoxic activity; 1) expression of IFN-γ (ELISPOT); 3) high proliferation (CFSE); 4) high levels of IL-2, IFN-γ, and TNF-α effector cytokines (or proinflammatory cytokines in the case of SYMP individuals); and 5) tetramer frequency—compared with only 32% of SYMP individuals. Thus, CD8+ T cells from ASYMP individuals tended to be polyfunctional, whereas those from SYMP individuals tended to be monofunctional.
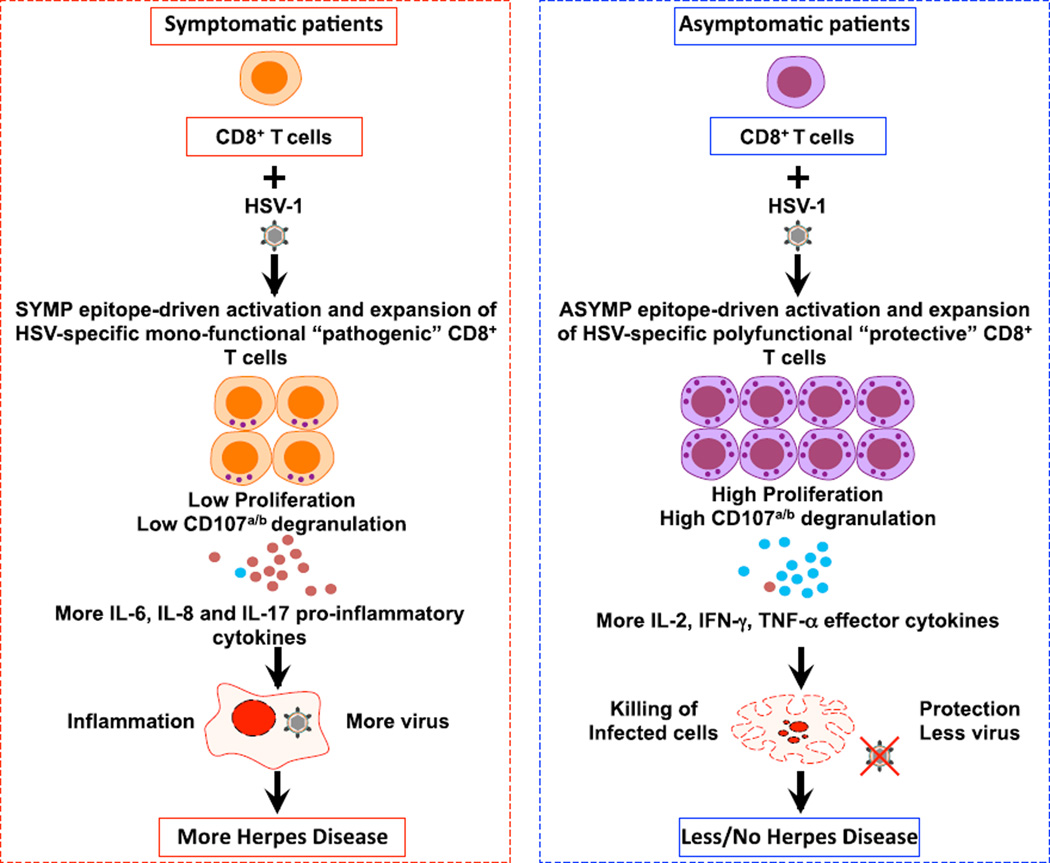
Phenotypic and functional characterization of various subsets of effector and central memory CD8+ T cells in SYMP and ASYMP individuals is certainly an important goal, but it remains beyond the scope of the present study, which focuses mainly on comparing epitope specificities and function of CD8+ T cells in SYMP versus ASYMP individuals. Studies of characterization of HSV-specific memory CD8+ T cell subsets in SYMP and ASYMP individuals, using tetramers and CD44, CCR7, and CD62L markers, are currently being conducted in our laboratory. Detailed results of those studies, which overall point to SYMP epitope-specific CD8+ T cells are mainly of the central memory phenotype, whereas ASYMP epitope-specific CD8+ T cells are mainly of the effector memory phenotype, will be the subject of future studies. Nevertheless, as illustrated in Fig. 7, both direct and indirect mechanisms might be involved in ASYMP CD8+ T cell–mediated protection and in SYMP CD8+ T cell–mediated susceptibility to recurrent herpetic disease. The polyfunctionality of ASYMP CD8+ T cells segregated with immunologic control of herpes infection and disease in both ASYMP individuals and humanized HLA transgenic mice. Thus, polyfunctionality of HSV-specific CD8+ T cells is likely to be an important mechanism accounting for the immunologic control of herpes. Symptomatic CD8+ T cells may be directly involved in the appearance and/or exacerbation of recurrent immunopathologic corneal disease. This could occur through inflammatory cytokines. This mechanism is supported by the functional characterization of the CD8+ T cells from SYMP patients that produced inflammatory cytokines (IL-6, IL-8, and IL-17) compared with those from ASYMP individuals. ASYMP epitope-specific CD8+ T cell lines from ASYMP individuals appeared to recognize naturally processed epitopes on HSV-1–infected cells and had strong virus inhibitory cytotoxic activity. The ultimate underlying molecular mechanism by which CD8+ T cells contribute to the maintenance of the asymptomatic state is not defined by the present study. However, the findings are consistent with a potential role of CD8+ T cells specific to ASYMP epitopes as “protective” and of CD8+ T cells specific to SYMP epitopes as “nonprotective” or even as “pathogenic.” A formal demonstration that ASYMP CD8+ T cells, with these functions intact, cause immunologic control of recurrent herpes infection and disease would require passive-transfer studies in humans or experimental animals that develop spontaneous recurrent disease (2). Unfortunately, mice do not develop spontaneous recurrent disease (reviewed in Ref. 8). It is likely that other host or viral factors, either alone or in concert with cellular immune responses, also play an important role in determining the course of herpes infection and disease.
FIGURE 7.
A proposed model of phenotypic and functional characteristics of HSV-specific CD8+ T cells in HSV-seropositive SYMP versus ASYMP individuals. After stimulation with HSV-1 ASYMP epitopes, CD8+ T cells (purple) from HSV-seropositive ASYMP individuals develop more protective CD8+ T cells that prevent herpes disease. In contrast, after stimulation with HSV-1 SYMP epitopes, CD8+ T cells (orange) from SYMP individuals develop more pathogenic CD8+ T cells that lead to inflammation. CD8+ T cells from SYMP individuals secrete substantial amounts of proinflammatory cytokines IL-6, IL-8, and IL-17 (small red circles) and have low proliferation and low lytic granules. In contrast, CD8+ T cells from ASYMP individuals have efficient proliferation and clonal expansion, and they produce more lytic granules and more IL-2, IFN-γ, and TNF-α (small blue circles), resulting in decreased or subclinical disease. In SYMP individuals, lower amounts of lytic granules likely account for inefficient killing of HSV-infected target cells.
Although several factors are likely involved in determining the outcome of herpes infection and disease, accumulating direct evidence in animal models and indirect observations in humans suggest that virus-specific CD8+ T cells mediate control of herpes, although the mechanisms by which this occurs remain unknown (reviewed in Ref. 8). A recent study by Carr and colleagues (48) evaluated the role of mouse CD8+ T cells in ocular immunity using gBT-I.1 transgenic mice in which >98% of CD8+ T cells are specific for the immunodominant mouse H-2Kb–restricted HSV-1 epitope (gB498–505) and found a significant reduction in virus, elevation in HSV-specific CD8+ T cell influx, and more CD8+ T cells expressing CXCR3 in the cornea of transgenic mice compared with those in the cornea of wild-type controls yet similar acute corneal pathology. However, by day 30 postinfection, wild-type mice had drastically more blood and lymphatic vessel projections into the cornea compared with gBT-I.1 transgenic mice, in which lymphatic vessel growth occurred only in response to vascular endothelial growth factor-C. The study concludes that CD8+ T cells are required to eliminate virus more efficiently from the cornea but play a minimal role in immunopathology as a source of vascular endothelial growth factor-C. The translation of these findings to the human situation, where SYMP and ASYMP CD8+ T cells may be in play, remains to be determined. We recently proposed a novel “symptomatic/asymptomatic concept,” in which inflammatory corneal lesions are not a direct consequence of damage caused by the virus or by autoreactive or bystander T cells, but rather result from the balance between immunopathological T cell responses specific to symptomatic HSV-1 epitopes and immunoprotective T cell responses specific to asymptomatic HSV-1 epitopes (as illustrated in Fig. 7). Regardless of the mechanism, if SYMP individuals tend to generate CD8+ T cells that recognize SYMP epitopes that differ from ASYMP epitopes, it would be logical to exclude such SYMP epitopes from future herpes vaccines on the grounds that they may exacerbate rather than cure recurrent herpetic disease.
To our knowledge, the current study is the first to report tha 1) CD8+ T cells specific to naturally processed ASYMP HSV-1 CD8+ gB epitopes positively correlate with protection against disease in healthy individuals, whereas 2) CD8+ T cells specific to nonoverlapping SYMP HSV-1 CD8+ epitopes positively correlate with frequently distressing symptomatic outbreaks in SYMP patients. Previous reports indicate that HSV-1 gB, which produces transient protective immunity in humans (6, 12, 49, 50), is a major target for T cells in seropositive humans (6, 7, 51). It is likely that the breadth of T cell response may be a critical factor in the outcome of herpes infection and disease. The simultaneous recognition of multiple ASYMP CD8+ T cell epitopes, restricted to HLA-A*02:01 and other HLA class I haplotypes, may confer an advantage on the host, as we previously suggested using murine models of ocular and genital herpes infection (35, 52). In contrast, simultaneous recognition of multiple SYMP CD8+ T cell epitopes may confer a disadvantage on the host.
Humans are not immunologically naive and often carry multiple pathogens and hence can develop T cells that cross-react between different viruses, members of the same family (53–55). However, this is unlikely for the gB epitopes analyzed in this study because 1) alignment of the sequences of the 10 HSV-1 gB T cell epitopes with published sequences of gB proteins from other human herpesviruses (i.e., varicella zoster virus, EBV, and CMV) did not reveal significant sequence homologies that might lead to cross-reactive T cells (Table II), and 2) the four HLA-A*02:01–restricted immunodominant epitopes reported in this study (gB342–350 and gB561–569, gB183–191, and gB441–449) as recognized by CD8+ T cells from HLA-A*02:01–positive, HSV-1 seropositive individuals are similarly recognized by CD8+ T cells induced in “pathogen-free” HLA-A*02:01 transgenic mice following HSV-1 ocular infection. A similar pattern of CD8+ T cell responses was detected against the 10 HSV-1 gB epitopes in HLA-A*02:01–positive, HSV-1 seropositive humans and HSV-1–infected, pathogen-free HLA-A*02:01 transgenic mice. These mouse results suggest that CD8+ T cells detected in humans are likely induced by HSV-1 gB epitopes. Thus, although the sequence of gB appears the most highly conserved within the herpesvirus family (56–58), no significant sequence similarities were found for the epitopes studied. However, one cannot definitely exclude a cross-reactivity of these HSV-1 gB epitopes with epitopes from other pathogens outside the herpes family.
HLA-A*02 has become an important target for T cell–based immunotherapy reflecting its high prevalence in most populations worldwide, regardless of race and ethnicity (59–62). In this study, we performed HLA-A*02 subtyping of SYMP versus ASYM individuals using sequence-specific primer genotyping (29). The results showed that all but one of the A*02 individuals in our cohort are of HLA-A*02:01 subtype, with the remaining (SYMP) individual being HLA-A*02:06. Although each HLA-A*02 subtype molecule can be expected to have a unique specificity, large overlaps in the repertoires of A*02 molecules have been reported (62). Indeed, >70% of the peptides that bind HLA-A*02:01, the most common A*02 subtype, with high affinity were found to also bind at least two other of the four next most common A*02-subtypes, including A*02:06 (62). Thus, it is possible that the epitopes characterized in this study may be useful in studies with individuals expressing other A*02 alleles. At the same time, however, although cross-reactivity at the binding level appears to be particularly high in the case of HLA-A*02, also note that differences between the molecules may encompass residues at positions involved in TCR recognition, and thus may be associated with differences that lead to divergence in T cell repertoires.
Although developing an effective herpes vaccine is scientifically feasible, progress has been miserably slow because correlation between the effector arm of the immune system and the infection/disease process continues to puzzle virologists and immunologists (63). Recently, we have advanced the hypothesis that the ultimate development of an effective herpes vaccine can be drawn from HSV-seropositive ASYMP individuals who manage to “naturally” control recurrent herpetic disease to clinically undetectable levels (64, 65). The present study showed that ASYMP gB epitopes used as a vaccine protected HLA transgenic mice against ocular herpes infection and disease. In contrast, SYMP gB epitopes provided no protection and might be harmful. We now plan to construct candidate epitope-based vaccines exclusively incorporating ASYMP CD8+ T cell epitopes together with ASYMP human CD4+ T cells epitopes that we recently identified (9). This should produce the next generation of safe and powerful ASYMP CD4-CD8 vaccines. Additionally, a vaccine covering a wide range of strains of HSV-1 and HSV-2 is highly desirable. In this context, it is important to emphasize that the two HSV-1 CD8+ ASYMP epitopes identified in this study are sequence-identical within, and between, HSV-1 and HSV-2 strains and are appropriate for candidate-type common vaccines. Interestingly, none of the four immunodominant gB epitopes reported in this study was detected in a recent ORFome study by Koelle and colleagues (46) that reported HLA-A*02:01–restricted epitopes from HSV-1. This may be due to differences in the immunological screens, the epitope mapping strategies, and selection of HSV-seropositive individuals used in the two studies.
In conclusion, the current study provides several new insights about the breadth, function, and specificity of a virus-specific CD8+ T cell population that segregates with immunologic control of herpes infection and disease in both ASYMP individuals and in humanized HLA-A*02:01 transgenic mice. We report two previously unknown HLA-A*02:01–restricted ASYMP epitopes derived from HSV-1 gB that tended to recall polyfunctional CD8+ T cell responses in HLA-A*02:01–positive, HSV-seropositive ASYMP humans and protected humanized HLA-A*02:01 transgenic mice from ocular herpes infection and disease. Understanding the detailed mechanisms by which HLA-A*02:01–restricted ASYMP CD8+ T cells are associated with protection against disease will reveal underlying principles of immune control of herpes, which is critical for the rational design of a T cell–based vaccine strategy. The findings demonstrate quantitative and qualitative features of an effective HSV-specific CD8+ T cell response that should be considered in the testing of the next generation of herpes vaccines.
Acknowledgments
We thank Dale Long from the National Institutes of Health Tetramer Facility for providing the tetramers used in this study. We also thank Barbara Bodenhoefer from the University of California Irvine Institute for Clinical and Translational Science for helping with drawing blood.
This work was supported by Public Health Service Research Grants EY14900 and EY019896 from the National Institutes of Health, as well as by the Discovery Eye Foundation.
Abbreviations used in this article
- ASYMP
asymptomatic
- gB
glycoprotein B
- MFI
mean fluorescence intensity
- rHSK
recurrent herpetic stromal keratitis
- RS
rabbit skin
- SYMP
symptomatic
- VVgB
vaccinia virus expressing glycoprotein B
- VVgD
vaccinia virus expressing glycoprotein D
Footnotes
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Zhang X, Dervillez X, Chentoufi AA, Badakhshan T, Bettahi I, BenMohamed L. Targeting the genital tract mucosa with a lipopeptide/recombinant adenovirus prime/boost vaccine induces potent and long-lasting CD8+ T cell immunity against herpes: importance of MyD88. J. Immunol. 2012;189:4496–4509. doi: 10.4049/jimmunol.1201121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chentoufi AA, Dasgupta G, Christensen ND, Hu J, Choudhury ZS, Azeem A, Jester JV, Nesburn AB, Wechsler SL, BenMohamed L. A novel HLA (HLA-A*0201) transgenic rabbit model for preclinical evaluation of human CD8+ T cell epitope-based vaccines against ocular herpes. J. Immunol. 2010;184:2561–2571. doi: 10.4049/jimmunol.0902322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chentoufi AA, Zhang X, Lamberth K, Dasgupta G, Bettahi I, Nguyen A, Wu M, Zhu X, Mohebbi A, Buus S, et al. HLA-A*0201-restricted CD8+ cytotoxic T lymphocyte epitopes identified from herpes simplex virus glycoprotein D. J. Immunol. 2008;180:426–437. doi: 10.4049/jimmunol.180.1.426. [DOI] [PubMed] [Google Scholar]
- 4.Chentoufi AA, BenMohamed L, Van De Perre P, Ashkar AA. Immunity to ocular and genital herpes simplex viruses infections. Clin. Dev. Immunol. 2012;2012:732546. doi: 10.1155/2012/732546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chentoufi AA, BenMohamed L. Mucosal herpes immunity and immunopathology to ocular and genital herpes simplex virus infections. Clin. Dev. Immunol. 2012;2012:149135. doi: 10.1155/2012/149135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Corey L, Langenberg AG, Ashley R, Sekulovich RE, Izu AE, Douglas JM, Jr, Handsfield HH, Warren T, Marr L, Tyring S, et al. Chiron HSV Vaccine Study Group. Recombinant glycoprotein vaccine for the prevention of genital HSV-2 infection: two randomized controlled trials. JAMA. 1999;282:331–340. doi: 10.1001/jama.282.4.331. [DOI] [PubMed] [Google Scholar]
- 7.Langenberg AG, Corey L, Ashley RL, Leong WP, Straus SE Chiron HSV Vaccine Study Group. A prospective study of new infections with herpes simplex virus type 1 and type 2. N. Engl. J. Med. 1999;341:1432–1438. doi: 10.1056/NEJM199911043411904. [DOI] [PubMed] [Google Scholar]
- 8.Dasgupta G, BenMohamed L. Of mice and not humans: how reliable are animal models for evaluation of herpes CD8+-T cell-epitopes-based immunotherapeutic vaccine candidates? Vaccine. 2011;29:5824–5836. doi: 10.1016/j.vaccine.2011.06.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chentoufi AA, Binder NR, Berka N, Durand G, Nguyen A, Bettahi I, Maillère B, BenMohamed L. Asymptomatic human CD4+ cytotoxic T-cell epitopes identified from herpes simplex virus glycoprotein B. J. Virol. 2008;82:11792–11802. doi: 10.1128/JVI.00692-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.BenMohamed L, Bertrand G, McNamara CD, Gras-Masse H, Hammer J, Wechsler SL, Nesburn AB. Identification of novel immunodominant CD4+ Th1-type T-cell peptide epitopes from herpes simplex virus glycoprotein D that confer protective immunity. J. Virol. 2003;77:9463–9473. doi: 10.1128/JVI.77.17.9463-9473.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang X, Issagholian A, Berg EA, Fishman JB, Nesburn AB, BenMohamed L. Th-cytotoxic T-lymphocyte chimeric epitopes extended by Nε-palmitoyl lysines induce herpes simplex virus type 1-specific effector CD8+ Tc1 responses and protect against ocular infection. J. Virol. 2005;79:15289–15301. doi: 10.1128/JVI.79.24.15289-15301.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nesburn AB, Bettahi I, Zhang X, Zhu X, Chamberlain W, Afifi RE, Wechsler SL, BenMohamed L. Topical/mucosal delivery of sub-unit vaccines that stimulate the ocular mucosal immune system. Ocul. Surf. 2006;4:178–187. doi: 10.1016/s1542-0124(12)70164-7. [DOI] [PubMed] [Google Scholar]
- 13.Chentoufi AA, Kritzer E, Yu DM, Nesburn AB, BenMohamed L. Towards a rational design of an asymptomatic clinical herpes vaccine: the old, the new, and the unknown. Clin. Dev. Immunol. 2012;2012:187585. doi: 10.1155/2012/187585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dasgupta G, Nesburn AB, Wechsler SL, BenMohamed L. Developing an asymptomatic mucosal herpes vaccine: the present and the future. Future Microbiol. 2010;5:1–4. doi: 10.2217/fmb.09.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chentoufi AA, BenMohamed L. Future viral vectors for the delivery of asymptomatic herpes epitope-based immunotherapeutic vaccines. Future Virol. 2010;5:525–528. doi: 10.2217/fvl.10.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dervillez X, Gottimukkala C, Kabbara KW, Nguyen C, Badakhshan T, Kim SM, Nesburn AB, Wechsler SL, BenMohamed L. Future of an “asymptomatic” T-cell epitope-based therapeutic herpes simplex vaccine. Future Virol. 2012;7:371–378. doi: 10.2217/fvl.12.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dasgupta G, Chentoufi AA, Kalantari M, Falatoonzadeh P, Chun S, Lim CH, Felgner PL, Davies DH, BenMohamed L. Immunodominant “asymptomatic” herpes simplex virus 1 and 2 protein antigens identified by probing whole-ORFome microarrays with serum antibodies from seropositive asymptomatic versus symptomatic individuals. J. Virol. 2012;86:4358–4369. doi: 10.1128/JVI.07107-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moraru M, Cisneros E, Gómez-Lozano N, de Pablo R, Portero F, Cañizares M, Vaquero M, Roustán G, Millán I, López-Botet M, Vilches C. Host genetic factors in susceptibility to herpes simplex type 1 virus infection: contribution of polymorphic genes at the interface of innate and adaptive immunity. J. Immunol. 2012;188:4412–4420. doi: 10.4049/jimmunol.1103434. [DOI] [PubMed] [Google Scholar]
- 19.Lekstrom-Himes JA, Hohman P, Warren T, Wald A, Nam JM, Simonis T, Corey L, Straus SE. Association of major histocompatibility complex determinants with the development of symptomatic and asymptomatic genital herpes simplex virus type 2 infections. J. Infect. Dis. 1999;179:1077–1085. doi: 10.1086/314729. [DOI] [PubMed] [Google Scholar]
- 20.Böhringer D, Sundmacher R, Reinhard T. HLA B27 seems to promote graft failure following penetrating keratoplasties for herpetic corneal scars. Ophthalmologe. 2007;104:705–708. doi: 10.1007/s00347-007-1550-9. [DOI] [PubMed] [Google Scholar]
- 21.Chentoufi AA, Dervillez X, Dasgupta G, Nguyen C, Kabbara KW, Jiang X, Nesburn AB, Wechsler SL, BenMohamed L. The herpes simplex virus type 1 latency-associated transcript inhibits phenotypic and functional maturation of dendritic cells. Viral Immunol. 2012;25:204–215. doi: 10.1089/vim.2011.0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiang X, Chentoufi AA, Hsiang C, Carpenter D, Osorio N, BenMohamed L, Fraser NW, Jones C, Wechsler SL. The herpes simplex virus type 1 latency-associated transcript can protect neuron-derived C1300 and Neuro2A cells from granzyme B-induced apoptosis and CD8 T-cell killing. J. Virol. 2011;85:2325–2332. doi: 10.1128/JVI.01791-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chentoufi AA, Kritzer E, Tran MV, Dasgupta G, Lim CH, Yu DC, Afifi RE, Jiang X, Carpenter D, Osorio N, et al. The herpes simplex virus 1 latency-associated transcript promotes functional exhaustion of virus-specific CD8+ T cells in latently infected trigeminal ganglia: a novel immune evasion mechanism. J. Virol. 2011;85:9127–9138. doi: 10.1128/JVI.00587-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhu J, Koelle DM, Cao J, Vazquez J, Huang ML, Hladik F, Wald A, Corey L. Virus-specific CD8+ T cells accumulate near sensory nerve endings in genital skin during subclinical HSV-2 reactivation. J. Exp. Med. 2007;204:595–603. doi: 10.1084/jem.20061792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhu J, Peng T, Johnston C, Phasouk K, Kask AS, Klock A, Jin L, Diem K, Koelle DM, Wald A, et al. Immune surveillance by CD8αα+ skin-resident T cells in human herpes virus infection. Nature. 2013;497:494–497. doi: 10.1038/nature12110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blaney JE, Jr, Nobusawa E, Brehm MA, Bonneau RH, Mylin LM, Fu TM, Kawaoka Y, Tevethia SS. Immunization with a single major histocompatibility complex class I-restricted cytotoxic T-lymphocyte recognition epitope of herpes simplex virus type 2 confers protective immunity. J. Virol. 1998;72:9567–9574. doi: 10.1128/jvi.72.12.9567-9574.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.St Leger AJ, Peters B, Sidney J, Sette A, Hendricks RL. Defining the herpes simplex virus-specific CD8+ T cell repertoire in C57BL/6 mice. J. Immunol. 2011;186:3927–3933. doi: 10.4049/jimmunol.1003735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sidney J, Southwood S, Moore C, Oseroff C, Pinilla C, Grey HM, Sette A. Measurement of MHC/peptide interactions by gel filtration or monoclonal antibody capture. Curr. Protoc. Immunol. 2013;18:18.3. doi: 10.1002/0471142735.im1803s100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bunce M. PCR-sequence-specific primer typing of HLA class I and class II alleles. Methods Mol. Biol. 2003;210:143–171. doi: 10.1385/1-59259-291-0:143. [DOI] [PubMed] [Google Scholar]
- 30.BenMohamed L, Wechsler SL, Nesburn AB. Lipopeptide vaccines: yesterday, today, and tomorrow. Lancet Infect. Dis. 2002;2:425–431. doi: 10.1016/s1473-3099(02)00318-3. [DOI] [PubMed] [Google Scholar]
- 31.Betts MR, Brenchley JM, Price DA, De Rosa SC, Douek DC, Roederer M, Koup RA. Sensitive and viable identification of antigen-specific CD8+ T cells by a flow cytometric assay for degranulation. J. Immunol. Methods. 2003;281:65–78. doi: 10.1016/s0022-1759(03)00265-5. [DOI] [PubMed] [Google Scholar]
- 32.Ferrari G, Neal W, Ottinger J, Jones AM, Edwards BH, Goepfert P, Betts MR, Koup RA, Buchbinder S, McElrath MJ, et al. Absence of immunodominant anti-Gag p17 (SL9) responses among Gag CTL-positive, HIV-uninfected vaccine recipients expressing the HLA-A*0201 allele. J. Immunol. 2004;173:2126–2133. doi: 10.4049/jimmunol.173.3.2126. [DOI] [PubMed] [Google Scholar]
- 33.Gilchuk P, Spencer CT, Conant SB, Hill T, Gray JJ, Niu X, Zheng M, Erickson JJ, Boyd KL, McAfee KJ, et al. Discovering naturally processed antigenic determinants that confer protective T cell immunity. J. Clin. Invest. 2013;123:1976–1987. doi: 10.1172/JCI67388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moutaftsi M, Salek-Ardakani S, Croft M, Peters B, Sidney J, Grey H, Sette A. Correlates of protection efficacy induced by vaccinia virus-specific CD8+ T-cell epitopes in the murine intranasal challenge model. Eur. J. Immunol. 2009;39:717–722. doi: 10.1002/eji.200838815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang X, Chentoufi AA, Dasgupta G, Nesburn AB, Wu M, Zhu X, Carpenter D, Wechsler SL, You S, BenMohamed L. A genital tract peptide epitope vaccine targeting TLR-2 efficiently induces local and systemic CD8+ T cells and protects against herpes simplex virus type 2 challenge. Mucosal Immunol. 2009;2:129–143. doi: 10.1038/mi.2008.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McKinney DM, Skvoretz R, Livingston BD, Wilson CC, Anders M, Chesnut RW, Sette A, Essex M, Novitsky V, Newman MJ. Recognition of variant HIV-1 epitopes from diverse viral subtypes by vaccine-induced CTL. J. Immunol. 2004;173:1941–1950. doi: 10.4049/jimmunol.173.3.1941. [DOI] [PubMed] [Google Scholar]
- 37.Pelte C, Cherepnev G, Wang Y, Schoenemann C, Volk HD, Kern F. Random screening of proteins for HLA-A*0201-binding nine-amino acid peptides is not sufficient for identifying CD8 T cell epitopes recognized in the context of HLA-A*0201. J. Immunol. 2004;172:6783–6789. doi: 10.4049/jimmunol.172.11.6783. [DOI] [PubMed] [Google Scholar]
- 38.Hertz T, Yanover C. Identifying HLA supertypes by learning distance functions. Bioinformatics. 2007;23:e148–e155. doi: 10.1093/Bioinformatics/btl324. [DOI] [PubMed] [Google Scholar]
- 39.Botten J, Alexander J, Pasquetto V, Sidney J, Barrowman P, Ting J, Peters B, Southwood S, Stewart B, Rodriguez-Carreno MP, et al. Identification of protective Lassa virus epitopes that are restricted by HLA-A2. J. Virol. 2006;80:8351–8361. doi: 10.1128/JVI.00896-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harndahl M, Rasmussen M, Roder G, Dalgaard Pedersen I, Sørensen M, Nielsen M, Buus S. Peptide-MHC class I stability is a better predictor than peptide affinity of CTL immunogenicity. Eur. J. Immunol. 2012;42:1405–1416. doi: 10.1002/eji.201141774. [DOI] [PubMed] [Google Scholar]
- 41.Remakus S, Rubio D, Ma X, Sette A, Sigal LJ. Memory CD8+ T cells specific for a single immunodominant or subdominant determinant induced by peptide-dendritic cell immunization protect from an acute lethal viral disease. J. Virol. 2012;86:9748–9759. doi: 10.1128/JVI.00981-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pulendran B, Ahmed R. Immunological mechanisms of vaccination. Nat. Immunol. 2011;12:509–517. doi: 10.1038/ni.2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bevan MJ. Understand memory, design better vaccines. Nat. Immunol. 2011;12:463–465. doi: 10.1038/ni.2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Koelle DM, Chen HB, McClurkan CM, Petersdorf EW. Herpes simplex virus type 2-specific CD8 cytotoxic T lymphocyte cross-reactivity against prevalent HLA class I alleles. Blood. 2002;99:3844–3847. doi: 10.1182/blood.v99.10.3844. [DOI] [PubMed] [Google Scholar]
- 45.Laing KJ, Magaret AS, Mueller DE, Zhao L, Johnston C, De Rosa SC, Koelle DM, Wald A, Corey L. Diversity in CD8+ T cell function and epitope breadth among persons with genital herpes. J. Clin. Immunol. 2010;30:703–722. doi: 10.1007/s10875-010-9441-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jing L, Haas J, Chong TM, Bruckner JJ, Dann GC, Dong L, Marshak JO, McClurkan CL, Yamamoto TN, Bailer SM, et al. Cross-presentation and genome-wide screening reveal candidate T cells antigens for a herpes simplex virus type 1 vaccine. J. Clin. Invest. 2012;122:654–673. doi: 10.1172/JCI60556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mott KR, Chentoufi AA, Carpenter D, BenMohamed L, Wechsler SL, Ghiasi H. The role of a glycoprotein K (gK) CD8+ T-cell epitope of herpes simplex virus on virus replication and pathogenicity. Invest. Ophthalmol. Vis. Sci. 2009;50:2903–2912. doi: 10.1167/iovs.08-2957. [DOI] [PubMed] [Google Scholar]
- 48.Conrady CD, Zheng M, Stone DU, Carr DJ. CD8+ T cells suppress viral replication in the cornea but contribute to VEGF-C-induced lymphatic vessel genesis. J. Immunol. 2012;189:425–432. doi: 10.4049/jimmunol.1200063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stanberry LR, Spruance SL, Cunningham AL, Bernstein DI, Mindel A, Sacks S, Tyring S, Aoki FY, Slaoui M, Denis M, et al. GlaxoSmithKline Herpes Vaccine Efficacy Study Group. Glycoprotein-D-adjuvant vaccine to prevent genital herpes. N. Engl. J. Med. 2002;347:1652–1661. doi: 10.1056/NEJMoa011915. [DOI] [PubMed] [Google Scholar]
- 50.Zhu X, Ramos TV, Gras-Masse H, Kaplan BE, BenMohamed L. Lipopeptide epitopes extended by an Nε-palmitoyl-lysine moiety increase uptake and maturation of dendritic cells through a Toll-like receptor-2 pathway and trigger a Th1-dependent protective immunity. Eur. J. Immunol. 2004;34:3102–3114. doi: 10.1002/eji.200425166. [DOI] [PubMed] [Google Scholar]
- 51.Zarling JM, Moran PA, Brewer L, Ashley R, Corey L. Herpes simplex virus (HSV)-specific proliferative and cytotoxic T-cell responses in humans immunized with an HSV type 2 glycoprotein subunit vaccine. J. Virol. 1988;62:4481–4485. doi: 10.1128/jvi.62.12.4481-4485.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bettahi I, Nesburn AB, Yoon S, Zhang X, Mohebbi A, Sue V, Vanderberg A, Wechsler SL, BenMohamed L. Protective immunity against ocular herpes infection and disease induced by highly immunogenic self-adjuvanting glycoprotein D lipopeptide vaccines. Invest. Ophthalmol. Vis. Sci. 2007;48:4643–4653. doi: 10.1167/iovs.07-0356. [DOI] [PubMed] [Google Scholar]
- 53.Cornberg M, Clute SC, Watkin LB, Saccoccio FM, Kim SK, Naumov YN, Brehm MA, Aslan N, Welsh RM, Selin LK. CD8 T cell cross-reactivity networks mediate heterologous immunity in human EBV and murine vaccinia virus infections. J. Immunol. 2010;184:2825–2838. doi: 10.4049/jimmunol.0902168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Selin LK, Brehm MA, Naumov YN, Cornberg M, Kim SK, Clute SC, Welsh RM. Memory of mice and men: CD8+ T-cell cross-reactivity and heterologous immunity. Immunol. Rev. 2006;211:164–181. doi: 10.1111/j.0105-2896.2006.00394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Selin LK, Cornberg M, Brehm MA, Kim SK, Calcagno C, Ghersi D, Puzone R, Celada F, Welsh RM. CD8 memory T cells: cross-reactivity and heterologous immunity. Semin. Immunol. 2004;16:335–347. doi: 10.1016/j.smim.2004.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Eberle R, Black D. Sequence analysis of herpes simplex virus gB gene homologs of two platyrrhine monkey alpha-herpesviruses. Arch. Virol. 1993;129:167–182. doi: 10.1007/BF01316893. [DOI] [PubMed] [Google Scholar]
- 57.Hannah BP, Heldwein EE, Bender FC, Cohen GH, Eisenberg RJ. Mutational evidence of internal fusion loops in herpes simplex virus glycoprotein B. J. Virol. 2007;81:4858–4865. doi: 10.1128/JVI.02755-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Heldwein EE, Lou H, Bender FC, Cohen GH, Eisenberg RJ, Harrison SC. Crystal structure of glycoprotein B from herpes simplex virus 1. Science. 2006;313:217–220. doi: 10.1126/science.1126548. [DOI] [PubMed] [Google Scholar]
- 59.El-Awar N, Lee JH, Tarsitani C, Terasaki PI. HLA class I epitopes: recognition of binding sites by mAbs or eluted alloantibody confirmed with single recombinant antigens. Hum. Immunol. 2007;68:170–180. doi: 10.1016/j.humimm.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 60.Pekiner FN, Aytugar E, Demirel GY, Borahan MO. HLA-A, B (class I) and HLA-DR, DQ (class II) antigens in Turkish patients with recurrent aphthous ulceration and Behçet’s disease. Med. Princ. Pract. 2013;22:464–468. doi: 10.1159/000348366. PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Castelli EC, Gil DS, Veiga LC, de Camargo JL. Typing class I HLA-A gene using a nested PCR-RFLP procedure. Braz. J. Med. Biol. Res. 2005;38:837–842. doi: 10.1590/s0100-879x2005000600004. [DOI] [PubMed] [Google Scholar]
- 62.Sidney J, Southwood S, Mann DL, Fernandez-Vina MA, Newman MJ, Sette A. Majority of peptides binding HLA-A*0201 with high affinity crossreact with other A2-supertype molecules. Hum. Immunol. 2001;62:1200–1216. doi: 10.1016/s0198-8859(01)00319-6. [DOI] [PubMed] [Google Scholar]
- 63.Cohen J. Immunology. Painful failure of promising genital herpes vaccine. Science. 2010;330:304. doi: 10.1126/science.330.6002.304. [DOI] [PubMed] [Google Scholar]
- 64.Wald A, Zeh J, Selke S, Warren T, Ryncarz AJ, Ashley R, Krieger JN, Corey L. Reactivation of genital herpes simplex virus type 2 infection in asymptomatic seropositive persons. N. Engl. J. Med. 2000;342:844–850. doi: 10.1056/NEJM200003233421203. [DOI] [PubMed] [Google Scholar]
- 65.Tronstein E, Johnston C, Huang ML, Selke S, Magaret A, Warren T, Corey L, Wald A. Genital shedding of herpes simplex virus among symptomatic and asymptomatic persons with HSV-2 infection. JAMA. 2011;305:1441–1449. doi: 10.1001/jama.2011.420. [DOI] [PMC free article] [PubMed] [Google Scholar]