Abstract
Crohn’s disease is complicated by the development of fibrosis and stricture in ~30–50% of patients over time. The pathogenesis of fibrostenotic disease is multifactorial involving the activation of mesenchymal cells by cytokines, growth factors and other mediators released by immune cells, epithelial cells and mesenchymal cells themselves. Transforming growth factor-β (TGF-β), a key activator of mesenchymal cells, is central to the process of fibrosis and regulates numerous genes involved in the disordered wound healing including collagens, and other extracellular matrix proteins, connective tissue growth factor (CTGF), and insulin-like growth factors (IGFs). The activated mesenchymal compartment is expanded by recruitment of new mesenchymal cells via epithelial to mesenchymal transition, endothelial to mesenchymal transition and invasion of circulating fibrocytes. Cellular hyperplasia and increased extracellular matrix (ECM) production, particularly collagens, from fibroblasts, myofibroblasts and smooth muscle cells add to the disturbed architecture and scarring on the intestine. ECM homeostasis is further disrupted by alterations in the expression of matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinase (TIMPs) in the gut. Among the 163 susceptibility genes identified that contribute to susceptibility in IBD mutations in NOD2/CARD15, innate immune system components, and autophagy jointly contribute to the activation of mesenchymal cells and pathogenesis of fibrosis in this polygenic disorder. Numerous growth factorscytokinesand other mediators. The review focuses on the molecular mechanism that regulate mesenchymal cell function, particularly smooth muscle cells, the largest compartment of mesenchyme in the intestine, that lead to fibrosis in Crohn’s disease.
INTRODUCTION
Intestinal fibrosis and stricture formation in Crohn’s disease is the result of the dysregulated wound healing that follows the initial injury and inflammatory response in a susceptible patient. Fibrosis is progressive and characterized by excessive net extracellular matrix deposition, architectural distortion and bowel obstruction. While many patients express a Montreal Class B1 inflammatory phenotype at initial presentation, the natural history of patients with Crohn’s disease is commonly an evolution to a Montreal Class B2 fibrostenotic or Montreal B3 penetrating phenotype1, 2. Critical intestinal fibrosis and stricture formation necessitating surgery will occur in 30–50% of patients within 10 years of disease onset 1–3. More than half of the patients who undergo surgical intervention will have recurrent strictures 2. Despite advances in the treatment of Crohn’s disease, the incidence of strictures and the resulting morbidity and mortality has changed little over time as the available pharmacologic therapies which target inflammation have little or no effect on fibrosis. Fibrosis, once initiated, is self-perpetuating and can proceed in the absence of inflammation in the susceptible patient. A number of GI disorders in addition to Crohn’s disease share intestinal fibrosis as a complication of disease including ulcerative colitis, radiation enteritis, diverticulitis, ischemic colitis and graft-vs-host disease to name a few.
Stricture development in Crohn’s disease is transmural, initiated by injury and immune-mediated inflammation, and culminates in excess extracellular matrix production and deposition. Fibrosis may follow injury and associated inflammation in any portion of the GI tract but the ileum is most commonly affected. In normal individuals, intestinal injury followed by acute inflammatory response activates a physiologic wound healing response that results in restitution of damaged cells and regulated fibrogenesis with balanced extracellular matrix deposition. Intestinal fibrosis in the susceptible patient is the result of failure of physiologic wound healing characterized by the abnormal regulation and function of activated mesenchymal cells, the main source of extracellular matrix (ECM) in the intestine. Three main types of mesenchymal cells reside in the intestine: smooth muscle cells, fibroblasts and myofibroblasts. The mesenchymal cell population can be expanded through recruitment of cells from the circulation into the intestine, and transition to mesenchyme of epithelial and endothelial cells 4, 5.
Mesenchymal cells are activated in response to paracrine signals from luminal microbiota, epithelial and immune cells and by autocrine mechanisms as well. Activation elicits plasticity and the ability to undergo trans-differentiation, development of a synthetic phenotype with increased ECM production, cellular proliferation and altered cell mobility.
Production of excess ECM is a pathogenic feature of fibrosis. Once excess ECM is produced it is regulated by the balance between matrix metalloproteinases (MMPs) that degrade ECM and tissue inhibitors of metalloproteinases (TIMPs) that regulate MMPs activity. This balance is also perturbed in the intestine of patients with fibrostenotic phenotype Crohn’s disease6–9.
The currently available therapy for Crohn’s disease, including aminosalicylates, corticosteroids, immunomodulators and biologic therapies, can be effective in limiting the inflammatory component of the disease process. However, these therapies are not able to significantly influence the development of fibrosis in the susceptible patient 10, 11. A greater understanding the mechanisms that underlie the development of fibrosis in the susceptible patient has potential to identify new avenues for therapeutic intervention.
INJURY, INFLAMMATION AND FIBROSIS
Wound healing is the normal biologic process to resolve tissue injury and the effects of inflammation 12. Damaged cells are replaced by cells of similar type and by the deposition of ECM. The fibrotic process is terminated and tissue remodeling occurs through the maintained balance between ECM production and factors that control degradation. During chronic inflammation in Crohn’s disease the epithelial and endothelial barrier is disrupted resulting in activation of the innate and adaptive immune systems with release of cytokines and chemokines, elaboration of reactive oxygen species, and release of pro-fibrotic cytokines and growth factors that result in activation of mesenchymal cells. In susceptible patients there is failure of the negative feedback mechanisms and excess ECM production by activated mesenchymal cells. Once mesenchymal cells become activated their largely autocrine, pro-fibrotic program can continue even in the absence of continued inflammation resulting in excess ECM deposition in the intestine, architectural distortion and stricture formation.
ECM proteins in the intestine include structural proteins particularly collagens (predominantly Types I, III and V), specialized glycoproteins including vitronectin, fibronectin and laminin, and matricellular proteins including thrombospondin and osteopontin. The ECM protein compartment is a complex dynamic environment that is highly regulated by ECM protein production by mesenchymal cell, and by MMPs and TIMPs produced by mesenchymal and other cells. The ECM compartment can in turn regulate the function of mesenchymal cells 13.
Genetic factors, the intestinal microbiome and the innate immune system are tightly interlinked in the pathogenesis of fibrosis and have been implicated in the chronic inflammation that is triggered by exposure to luminal bacterial products and environmental factors. Bacterial PAMPs like muramyl dipeptide, DNA and dsRNA bind to and activate pattern recognition receptors including Toll-like receptors (TLRs) and intracellular nucleotide oligomerization domain/caspase recruitment domain (NOD/CARD) receptors of the innate immune system. A strong innate immune response to bacterial antigens, manifesting as increased expression of serologic markers in patients, has been linked to more aggressive disease including fibrosis14.
Loss of epithelial barrier function exposes subepithelial cells to bacterial ligands that can activate mesenchymal cells. The presence of mRNA encoding Toll-like receptor (TLR) 1–9 has been documented on the intestinal mesenchymal cells. Activation of TLRs by PAMPs is linked to mesenchymal cell activation and correlates with progressive fibrosis. This may be the result on upregulation of TLR2 and TLR4 on these cells. Exposure of human myofibroblasts to TLR ligands also resulted in increased expression of the ECM protein, fibronectin, and α-smooth muscle actin 15
The intracellular NOD2 receptor for muramyl dipeptide plays a key role in activation of the innate immune system in the development of Crohn’s disease. Polymorphisms in NOD2/CARD15 are associated with persistent intracellular infection and chronic stimulation of immune responses. Evidence suggests an interrelation between common NOD2/CARD15 gene polymorphisms and susceptibility to ileal disease both alone or in combination with mutations in the TLR4 gene or the ATG16L1 autophagy gene. Cells carrying NOD2 variants develop a stronger immune response, result in more TGF-β1 production, collagen production and are associated with fibrostenotic phenotype Crohn’s disease 15
During injury or damage-induced responses a number of endogenous molecules, including DNA, reactive oxygen species (ROS), laminins or S100 proteins, act as damage-associated molecular patterns (DAMPs) or alarmins that bind TLRs, integrins and other receptors to stimulate the immune system, activate dendritic cells and recruit neutrophils 16. The role of DAMPs in the response to injury and the initiation of inflammation in Crohn’s disease is increasingly recognized and investigated 17.
GENETIC BASIS OF FIBROSIS
Unlike single gene diseases, IBD including Crohn’s disease is polygenic with 163 loci identified that confer a risk of susceptibility or modify disease course18–21. While the development of fibrosis is preceded by a period of initial inflammation, not all patients with Crohn’s disease express a fibrostenotic phenotype or develop strictures. This highlights the essential difference between susceptibility genes and clinical disease. Commonly carried polymorphisms, NOD2/CARD15, ATG16L1 and IL-23R, can occur with other risk variants where they act in concert to promote disease 22.
The most consistently and robustly associated genes linked to Crohn’s disease susceptibility include NOD2 (polymorphisms are associated with disturbed surveillance of bacteria microflora); IL-23 receptor (IL23R), a member of the Th17/IL-23 pathway (polymorphisms linked to regulation of adaptive immunity and protection from development of Crohn’s disease); and ATG16LI and IRGM (lead to deficient autophagy) 21. Individuals with NOD2/CARD15 mutations have a 10-fold greater risk of developing aggressive disease 23. Moreover, different variants of this gene show correlation with distinct phenotypes, such as fibrostenosis, and the need for surgery23, 24. However, efforts to link Crohn’s disease genetics to clinical phenotypes have not been overly successful25. Recent attempts using Latent Class analysis, a statistical method to look for a genetic basis for disease phenotype subgroups, identified six genetic-based clusters 26. Although modest differences in prevalence of disease behavior were identified among clusters, Random Forest analysis in this study showed that patients could not be allocated to genetic-based subgroups based on clinical parameters alone.
A number of polymorphisms that lie in the Th17 pathway genes are important pathogenic participants in the Th1/Th17 patterned response in Crohn’s disease such as Janus kinase 2 (Jak2), Signal transducers and activator of transcription 3 (STAT3), IL12B, and the chemokine receptor CCR6 18. Deep re-sequencing of the loci identified by GWAS has led to identification of new variants associated with IBD. Smad3, a signaling intermediate of TGF-β is one such recently identified gene 27. Associations between genetic variants of MMPs and TIMPs and risk of developing stenotic complications in Crohn’s disease as well as with Crohn’s disease in general have been identified in MMP-1, MMP2, MMP-3, MMP-9, TIMP-1 and TIMP2 28.
CELLULAR BASIS OF FIBROSIS
The development of fibrosis depends not only on the recruitment of immune cells and inflammatory cells, but also on the exposure of mesenchymal cells to bacterial products from the microbiome, paracrine cytokines and inflammatory mediators that results in their activation. An activated mesenchymal cell is synthetic and produces increased amounts of extracellular matrix proteins. Mesenchymal cells are immune competent and can produce a number of cytokines and growth factors in response to activation. They are also capable of undergoing de-differentiation and trans-differentiation between smooth muscle cells, myofibroblasts and fibroblasts 29. Four features of mesenchymal cells that are hallmark to the pathology of fibrosis are excess extracellular matrix production, increased proliferation and decreased apoptosis, cellular hypertrophy and effacement of the muscularis mucosa in the affected segment of strictured intestine.
The mesenchymal compartment of the intestine is also expanded by the addition of non-mesenchymal cells. Myofibroblasts can result from transition of epithelial cells (EMT) or transition of endothelial cells (Endo-MT) 5, 30. Bone marrow-derived circulating fibrocytes can be progenitors of mesenchymal cells after invasion of the intestine during the process of fibrosis 31. Each of these originally non-mesenchymal cells can ultimately contribute to excess extracellular matrix production and fibrosis.
Smooth Muscle Cells
Intestinal smooth muscle cells are found in the muscularis propria, which is intimately involved in the development of fibrosis, where there is marked thickening with cellular hyperplasia, hypertrophy and collagen deposition that contribute to fibrosis and stricture formation. Muscularis propria represents the largest mesenchymal cell compartment in the intestine. The muscularis mucosa is also comprised of smooth muscle cells, but this layer becomes effaced in the region of strictured intestine. Smooth muscle cells are characteristically α-smooth muscle actin, desmin and collagen I positive32.
Smooth muscle cells contribute to fibrosis by their ability to produce large amounts of extracellular matrix proteins like collagen, particularly collagen I and III, and fibronectin and vitronectin33, 34.They are highly synthetic and produce large amounts of both cytokines and growth factors including TGF-β1, IL-6, IGF-I, PDGF, and CTGF. In the muscularis propria the master fibrosis cytokine, TGF-β1, can stimulate the expression of all of these factors through autocrine mechanisms.
Myofibroblasts
Myofibroblasts have a phenotype between smooth muscle cells and fibroblasts. Both intestine-resident myofibroblasts including subepithelial myofibroblasts and Interstitial cell of Cajal (ICC) and cells transitioned to myofibroblasts are involved in the process 35. They are α-SMA, vimentin and collagen I positive32. ICCs are also characterized by c-kit positivity. All can be abundant sources of collagen production and other ECM proteins including vitronectin.
Myofibroblasts in patients with Montreal B2 fibrostenotic disease are distinctly phenotypically different than those in patients with non-B2 disease. This is highlighted by distinctly different mobility of B2 myofibroblasts from those in B3 disease. Myofibroblasts from patients with B3 disease have faster rates of mobility in wounding assays than do myofibroblasts isolated from stricturing disease. Mesenchymal stem cells (MSCs) migrate to sites of injury based on homing signals provided by chemotactic factors. Migration is dependent on the interaction between MSCs and ECM 36, 37. Myofibroblast activation and migration are regulated by both autocrine (e.g. TGF-β1, and IGF-I) and paracrine (e.g. TNF-α, IL-6 and IL-13, IFN-γ, and TGF-β1) factors37, 38. Expression of the TNF superfamily member, TL1A (TNSF15) by lymphoid and myeloid cells results in development of mild inflammation that is accompanied by the development of fibrosis in the intestine 39.
The migratory potential of activated myofibroblasts illustrates, on the one hand, a key feature of Crohn’s disease phenotype, as well as our lack of understanding of the process, on the other. The migratory potential of subepithelial myofibroblasts from patients with Crohn’s disease is dependent on the underlying disease phenotype: myofibroblasts from patients with fibrostenotic disease exhibit decreased migration whereas those from patients with penetrating disease exhibit increased migration 36. This correlates with a difference in the levels of the non-receptor protein kinase, focal adhesion kinase (FAK; a regulator of cell migration), the phosphorylation of which increases fibroblast migration 40.
Subepithelial myofibroblasts can play a role not only in the development of fibrosis but also in the regulation of the immune response as well. In the normal intestine, myofibroblasts can regulate mucosal tolerance by their ability to support a FoxP3+ Treg response in the intestine 41. In the intestine of patients with Crohn’s disease, myofibroblasts are activated by numerous paracrine mediators in their environment that promote their production of ECM and proliferation including TGF-β1, PDGF, CTGF, IGFI/II, bFGF and various interleukins: IL-1β, IL:-6 and IL-13. Activated myofibroblasts are highly synthetic and produce not only ECM but are also rich autocrine sources of many of these mediators. This has important implications on the crosstalk between subepithelial myofibroblasts and epithelial cells that regulate each other’s function. Epithelial cells respond to the paracrine stimuli from myofibroblasts to increase their TGF-β1 and TIMP-1 expression 42.
Fibroblasts
Fibroblasts are a more heterogeneous group of cells in the intestine than myofibroblasts or smooth muscle cells. They are scattered throughout the wall of the intestine where they are involved in the response to injury and participate in either normal wound healing of the development of fibrosis. They are characteristically vimentin positive, collagen I positive and N-cadherin positive, but α-SMA negative32. In the setting of fibrostenotic Crohn’s disease their expression of N-cadherin is upregulated compared to non-fibrostenotic Crohn’s disease. Increased N-cadherin expression is upregulated in response to TGF-β1 and increases their migratory potential. Mice expressing a dominant-negative N-cadherin develop a spontaneous Crohn’s-like inflammation suggesting this mutation confers defective wound healing in these animal 43, 44. The reduced migration observed in subepithelial myofibroblasts derived from patients with fibrostenotic disease is in contrast with the increased migratory potential of fibroblasts allowing for enhanced invasion of fibroblasts into the injured intestinal wall 43, 44, which suggests that dysfunctional wound healing process may play a role in delayed recovery of physiologic intestine structure and function.
Fibroblasts respond to activation by TGF-β1 and other growth factors such as IGF, CTGF, PDGF and bFGF and cytokines such as IL-1β, IL-6 and TNF-α, with increased cellular proliferation and ECM production. Autocrine production of the ECM protein fibronectin also influences motility of fibroblasts.
Recent evidence suggests that matrix stiffness itself, the product of excess ECM production and fibrosis, is capable of further activation of intestinal fibroblasts 45.
Other Mesenchymal cells
The mesenchymal cells compartment can also be expanded by the products of Epithelial-Mesenchymal cell Transition (EMT), Endothelial-Mesenchymal cell Transition (Endo-MT) and invasion of bone-marrow derived circulating fibrocytes31, 46. Each of these processes described results in activated fibroblasts and myofibroblasts within the intestinal wall at sites in injury.
Injury and resultant loss of barrier results in the reprogramming of pluripotent epithelial and endothelial cells. Cells lose epithelial or endothelial markers and initiate synthesis of α-smooth muscle actin. They undergo cytoskeletal rearrangement and assume a spindle shape and migration through basement membrane from the compartment of origin to the interstitial space. Production of ECM proteins, including collagen and fibronectin, by cells following EMT or EndoMT is a significant contributor to the development of fibrosis5, 30.
Circulating fibrocytes derived from bone marrow express markers of hematopoietic cells, but also express collagen I and α-smooth muscle actin characteristic of mesenchymal cells. Once recruited to sites of injury and inflammation they undergo differentiation into fibroblasts and myofibroblasts. They not only respond to local mediators such as TGF-β1, IL-1β, but also are sources of TGF-β1, CTGF which act in paracrine and autocrine fashion on intestinal mesenchymal cells 46.
Myofibroblasts in fibrotic tissues can be derived from various sources. These include from resident tissue fibroblasts that evolve from quiescent fibroblasts to cells expressing a myofibroblast phenotype, via transition of epithelial cells into mesenchymal cells (EMT), from tissue migration of bone marrow-derived circulating fibrocytes, and via the recently discovered endothelial to mesenchymal transition (EndoMT) 5, 31, 47. EMT, which is induced by transforming growth factor-β (TGF-β), is not unique to the intestine and occurs in other organs as well 48. Fibrocytes are capable of producing fibroblastic proteins and migrating to diseased areas 47, 49. EndoMT is a process in which endothelial cells lose their endothelial cell markers and acquire a mesenchymal phenotype with expression of α-smooth muscle actin (α-SMA), vimentin, and type I collagen 5, 50. In vitro studies have shown that EndoMT can be induced by TGF-β1 via a “non-canonical” (non-Smad) signaling pathway 50.
MOLECULAR MECHANISMS OF FIBROSIS
Activated mesenchymal cells are key regulators of the fibrosis that occurs in stricturing Crohn’s disease. Mesenchymal cells respond to the mediators elaborated in the injured and inflamed intestine similarly and synergistically leading to the development of fibrosis rather than orderly wound healing in the susceptible patient. The mediators which activate all types of mesenchymal cells and initiate a pro-fibrotic phenotype are similar including growth factors: TGF-β1, CTGF, IGF, PDGF, ET; cytokines: IL-1, IL-6, IL-13, IL-17, IL-23, TNF-α; and other mediators including ROS, MMPs and TIMPs 51. It is worth noting that the active mesenchymal phenotype is highly synthetic in addition to proliferative. Once activated mesenchymal cells themselves produce many of the mediators that elicit their activation. Thus an autocrine pathway leading to fibrosis is initiated with their activation and can progress even in the absence of continued inflammation.
TGF-β
TGF-β is the central regulator in the injured and inflamed intestine. It has both wound healing and Treg properties but its increased expression and activation is a central mediator of fibrosis. Three isoforms of TGFβ exist: TGF-β1, TGF-β, and TGF-β3. Expression of TGF-β1 and TGF-β3 is specifically increased in smooth muscle cells, myofibroblasts, and fibroblasts of strictures as compared with normal intestine in the same patient undergoing resection 52. Fibroblasts in the mucosal layer also display increased expression of TGF-β2 53.
Overexpression of TGF-β1 in transgenic animals is associated with development of fibrosis. As a therapeutic target, however, TGF-β1 and its SMAD signaling pathways are problematic. TGF-βRI/II receptors expressed by mesenchymal cells are linked to canonical Smad2/3 signaling.54 Binding of active TGF-β1 to TGF-βRI/II receptors in human intestinal muscle activates canonical Smad signaling, r-Smad2/3 phosphorylation and increased collagen I production which accounts for ~70% of collagen present in the intestine33, 55. With the exception of Smad3 deletion, deletion of TGF-β protein, its receptors or other Smad proteins are generally lethal mutations or a feature of neoplastic cells. In two of the three Smad3 deletion mutants developed, a lethal wasting syndrome develops early in association with immunodeficiency56, 57. In the third Smad3 mutant developed, while the effects of TNBS-induced colitis were reduced, metastatic adenocarcinoma of the colon developed within 4–6 months of age58. Overall these observations suggest that pharmacologic targeting of the TGF-β system in Crohn’s disease patients to decrease the development of fibrosis could have untoward effects.
Activation of latent TGF-β1 by smooth muscle and intestinal fibrosis
In our laboratory we have taken an approach to examine paired samples from affected regions of patients not only with Montreal Class B2 fibrostenotic disease but also from Montreal Class B1 inflammatory and Montreal B3 penetrating disease using non-Crohn’s intestine as control to determine the factors involved in the development and progression of fibrosis. Expression and production of TGF-β1, is increased specifically in smooth muscle cells of ileal strictures compared to histologically normal proximal resection margin.34, 59 This is in contrast to patients expressing a B1 or B3 phenotype where TGF-β1 expression is unchanged from non-Crohn’s subjects in either affected regions or normal resection margin. TGF-β1, in addition, stimulates expression of other fibrogenic factors including: fibronectin, CTGF and IGF-I.33, 60
Levels of active TGF-β1 associated with muscle cells is increased in strictured regions in comparison to normal intestine in the same patient. Activation of TGF-β1 occurs by the binding of the RGD sequence in Latent TGF-β1 to the RGD binding domain in the extracellular region of αVβ3 integrin. Latent TGF-β3 also possesses and RGD sequence but transcript levels are only slightly increased in regions of stricture; in contrast latent TGF-β2 possesses an SGD sequence that does not bind to and cannot be activated by αVβ3 integrin. The intracellular domain of αVβ3 integrin binds Src kinase via β3(Ser752) 61. Phosphorylation of β3(Ser752) in response to binding of ligands of the RGD domain (vitronectin, fibronectin or Latent TGF-β1) provides linkage to the actin cytoskeletal62. Integrins expressed on smooth muscle cells are key elements of mechano-transduction pathways that communicate with and are regulated by the focal adhesion complex that includes FAK, c-Src, and paxillin, and proteins mediating cytoskeletal remodeling and motility.63 Once bound to αVβ3 integrin, the mechanical activity of the muscle cells, contraction and relaxation, provides for the excess non-proteolytic activation of latent TGF-β1. The increased phasic motor activity in the intestine and colon leads to increased active TGF-β1 liberation 64. When binding of latent TGF-β1 to αVβ3 integrin is blocked by use of an RGD inhibitor, Cilengitide, activation of latent TGF-β1 is blocked along with the production of Collagen I, and the development of fibrosis in the TNBS model of Crohn’s disease 65.
A unique relationship exists between αVβ3 integrin, not only with respect to activation of TGF-β1 but also the activity of the profibrotic growth factor IGF-I and its binding proteins, IGFBP-3 and IGFBP-5. The expression of all three are increased in muscle cells of strictured intestine 34, 59. Production of fibronectin and vitronectin, activating ligands of αVβ3 integrin (the cognate vitronectin receptor), is increased in stricturing Crohn’s disease. Binding of vitronectin and fibronectin to αVβ3 integrin elicits maximal activation of IGF-I and expression of IGFBP-5. These two factors, in turn, increase expression of collagen IαI. IGFBP-3, on the other hand, binds to TGF-βRI/II receptors and activates canonical Smad signaling via receptor-based Smad2/3 and recruitment of co-Smad4. Translocation of the active r-Smad2/3-co-Smad4 complex to the nucleus regulates expression of numerous genes that contribute to the development of fibrosis including additional fibronectin, IGF-I and CTGF in addition to Collagen I and III. Inhibitory-Smad7 (i-Smad7) is upregulated as the normal counter regulatory signal that inhibits activity of the r-Smad2/3 activity. Increased expression of i-Smad7 has been demonstrated in the epithelium and T-cells of patients with Crohn’s disease where its increase results in inappropriate suppression of the Treg response mediated by TGF-β166. Knockdown of i-Smad7 in animal models of Crohn’s disease and in Phase I clinical trials has demonstrated efficacy to decrease inflammation. In the later study patients expressed exclusively a Montreal B1 inflammatory phenotype67. In contrast, our studies have demonstrated a decrease in i-Smad7 in patients with Montreal B2 phenotype Crohn’s disease that contributes to excess TGF-β1 signaling and effects that result in excess collagen I production and fibrosis. These two scenarios with disparate roles for i-Smad7 also illustrate the problematic nature of targeting TGF-β1 and its signaling pathways in Crohn’s disease.
A variety of other cytokines are released by immune cells in Crohn’s disease and in animal models of Crohn’s disease that stimulate fibrosis. In the TNBS-induced model of chronic colitis in BALB/c mice, IL-13 (via IL-13Rα2) increases expression of TGF-β1 and IGF-I and results in fibrosis. Fibrosis was decreased by disruption of IL-13Rα2 signaling68, 69.
IGF-I in development of intestinal fibrosis
The central role of IGF-I in the development of fibrosis in Crohn’s disease was shown by the increased expression of IGF-I, IGFBP-3 and IGFBP-5 in smooth muscle cells of strictured intestine34, 59, 70–72. In the muscularis propria of human intestine, increased autocrine IGF-I production results increased smooth muscle cell proliferation, inhibition of apoptosis as well a increased ECM production including collagen I, fibronectin and vitronectin. The importance of TGF-β1 regulated increased IGF-I expression on the development of fibrosis was confirmed using the Crohn’s disease model, chronic TNBS-colitis, in IGF-I heterozygous mice 73. TGF-β1 also stimulates the expression of IGFBP-3, which further activates TGF-β1 receptors, additionally increases collagen production and also contributes to fibrosis59. In the mucosa, IGF-I also stimulates collagen production from subepithelial myofibroblasts 74.
Innate immunity and intestinal fibrosis
The innate immune system plays a role both in the pathogenesis of Crohn’s disease and also in initiation of fibrosis. Similarly to immune cells, myofibroblasts express Toll-like receptors (TLRs) that bind bacterial products via pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs) 4. Activation of TLRs on immune cells activates mitogen-activated protein (MAP) kinases, causes translocation of nuclear factor-κB (NF-κB) to the nucleus, and ultimately results in the secretion of proinflammatory cytokines and chemokines that activate and attract myofibroblasts. Activation of TLRs also stimulates myofibroblast proliferation and collagen production 75.
The important role of bacteria in the development of fibrosis in susceptible hosts was examined in the IL-10 mouse model of spontaneous inflammation 76. IL10-null mice raised in either conventional housing or under germ-free conditions were randomized to ileocecal resection, intestinal transection without resection, or to no treatment. The post-surgical inflammation and initiation of fibrosis that occurred in genetically susceptible IL10-null mice did not occur in the absence of intestinal bacteria 76. The ability of the gut microbiota to alter the expression of profibrotic genes and influence inflammation-associated fibrosis was also demonstrated using the Salmonella typhimurium model of intestinal fibrosis 76. Delayed eradication of infection repressed but did not prevent fibrosis. Early eradication of infection and inflammation diminished but did not eradicate the development of subsequent fibrosis.
Development of fibrosis is also a reflection of an abnormal immune response to luminal bacteria. The bacterial product, flagellin, antigen for a commonly observed antibody in patients with Crohn’s disease, is a TLR-5 ligand. Activation of TLR-5 by flagellin in human intestinal fibroblasts in conjunction with the inflammasome leads to increased fibrosis that is mediated directly via hypoxia inducible factor (HIF) and indirectly by eliciting TGF-β1 release from monocytes 77.
Adaptive immunity and intestinal fibrosis
Cytokines are integral in the response to inflammation. The immunologic responses in Crohn’s disease include a pro-inflammatory Th1 response involving IL-1β and TNF-α. The Th17 response is characteristic of autoimmunity. TGF-β1 is needed to induce differentiation of this lineage. Th17 and Treg responses are important mediators of the fibrosis observed in Crohn’s disease. While a Th2 response involving IL-4 and IL-13 is most characteristic of ulcerative colitis it can be seen later in the course of Crohn’s disease. Key cytokines in the Th17 pathway include IL-17, IL-23, IL-6 and TNF-α. IL-23, IL-17 and IL-6 induce and activate the transcription factors, STAT3 and RORc.
A general theme emerges from the actions of the cytokines involved in Th1, Th17, Th2 and Treg. They are able to activate mesenchymal cells, stimulate their proliferation and increase collagen production. Il-6, Il-1β, IL-13 and IL-17a are direct mediators of increased latent TGF-β expression in mesenchymal cells. In the proper setting this broad array of cytokines can promote the development of fibrosis in the susceptible patient.
Dysregulation of ECM homeostasis
In the intestine, the components of ECM include structural proteins such as collagen, particularly collagen I, III, and V; specialized proteins, such as vitronectin and fibronectin; and matricellular proteins, such as osteopontin and thrombospondin. These all exert regulatory functions by interacting with cell-surface receptors, including integrins. Within regions of stricturing, expression of collagen, fibronectin and vitronectin, and thrombospondin are all increased [34]. However, regulation of the ECM is a dynamic process involving not only its expression and production, but also its regulated turnover. Turnover of ECM is regulated by the production of 23 human MMPs; these are zinc- and calcium-dependent proteases that are produced by epithelial cells, endothelial cells, MSCs, and macrophages, and activated by proteolytic cleavage. MMPs comprise collagenases, gelatinases, stromolysins, matrilysins, and membrane-type MMPs (MT-MMPs), and thus possess broad substrate specificity6, 7, 78, 79. Increased expression of MMPs, such as the collagenase MMP-8 and the gelatinase MMP-9, is observed subjacent to the intestinal ulcerations of active disease. MMPs, in turn, are regulated by four tissue inhibitors of metalloproteinases (TIMPs) that can be produced by the same cells that produce MMPs.
Cytokines and growth factors, such as TNF-α and TGF-β can regulate MMP expression and promote fibrosis. In intestinal myofibroblasts, for example, TGF-β downregulates MMP-1 and MMP-3 expression and enhances TIMP-1 expression, while TNF-α decreases MMP-2 activity and increases TIMP-1 expression 7. IL-21, another T-cell derived cytokine that is increased in IBD, can increase expression of MMPs by intestinal myofibroblasts, contributing to mucosal ulcer formation 80. Increased expression of MMP-1, MMP-3, and TIMP-1 has also been demonstrated in the muscularis propria of the intestine in active Crohn’s disease 81.
The action of MT-MMP-1 exemplifies a unique mechanism of ECM regulation. Through interaction with αVβ8 integrin, MT-MMP-1 regulates the activation of TGF-β1, particularly in the epithelium, thereby increasing the availability of this pro-fibrotic cytokine 82.
CONCLUSION ON THE IMPACT ON EXPERIMENTAL THEAPEUTICS
The identification of TGF-β1 as a central mediator of fibrosis in the Crohn’s has important therapeutic implications for stricturing Crohn’s disease. 83 Mesenchymal cells express TGF-βRI/II receptors linked to canonical Smad2/3 signaling and increased expression of other genes mediating e.g. fibrosis, IGF-I and CTGF.54 Based on outcomes in animals with deletion mutations in TGF-β signaling, pharmacologic targeting of the TGF-β system with the goal of targeting fibrosis may have untoward effects56–58. The development of better therapeutic targets depends on progression of our understanding of complexity of intestinal fibrosis in CD from in vitro and in vivo models. In the present study, we have explored an alternative approach utilizing cilengitide to decrease TGF-β1 activation and thereby decrease collagen production and fibrosis. Cilengitide also possesses anti-angiogenic effects which have also been implicated in the pathogenesis of Crohn’s disease and could have combined beneficial effects 84.
MMPs or TIMPs could be considered alternative targets for anti-fibrotic therapy. The effects of increased MMP (e.g. collagenase) expression would be expected to decrease net ECM levels that derive from activated mesenchymal cells. However, increasing MMP levels are associated with development of mucosal ulcerations in Crohn’s disease6, 7, 9, 80, 81, 85.
The mediators initiating and regulating fibrosis are present throughout the intestine not only in regions of strictured intestine but also in normal intestine. However, once inflamed, the expression of specific profibrotic mediators in regions of strictured intestine is significantly increased compared with that in regions of adjacent normal intestine. While some of these mediators are elaborated by activated immune cells such as specific subsets of T cells and macrophages, others are autocrine and paracrine mediators produced by MSCs themselves
The wound healing process triggered by intestinal injury and inflammation fails to undergo resolution with restoration of normal intestinal architecture. Excess net ECM production from activated mesenchymal cells and expansion of the mesenchymal compartment contribute to this process. Intestinal fibrosis remains a common complication of Crohn’s disease. Rates of fibrosis-related complications and surgery have remained the same despite advances in anti-inflammatory therapies. While currently available interventions can ameliorate inflammation, once fibrosis is initiated in the susceptible patient, fibrosis is self-perpetuating and can proceed even in the absence of active inflammation.
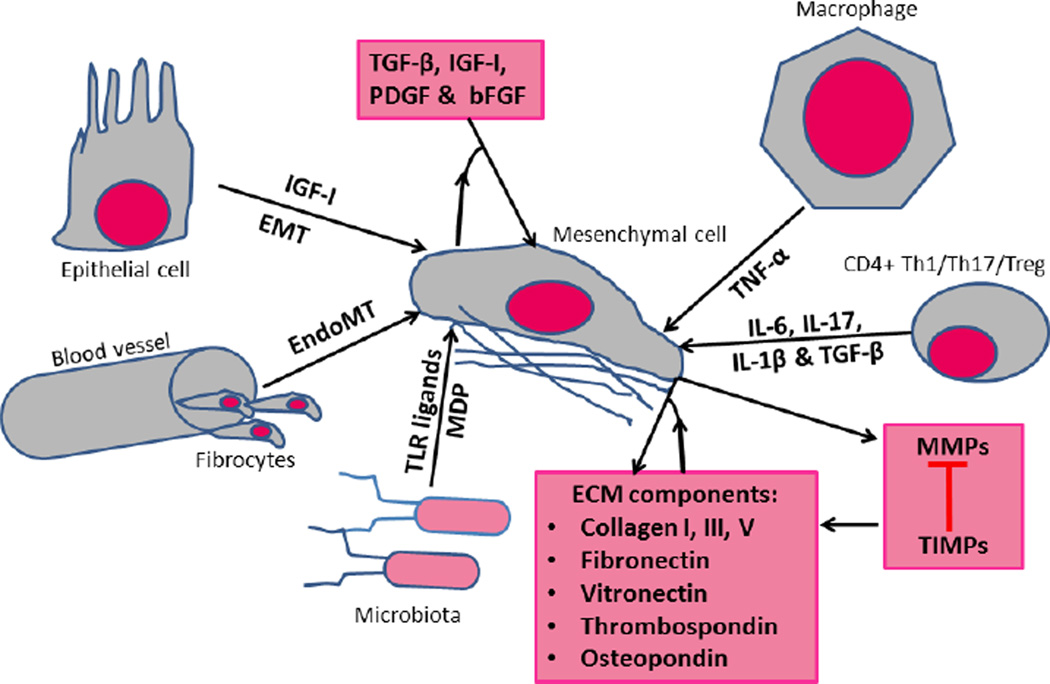
Figure 1. Activation of intestinal mesesnchymal cells leading to fibrosis.
After tissue injury in a susceptible patient, mesenchymal cells of the intestine are activated and expanded via the interaction of both paracrine and autocrine mediators. The mediators involved represent the participation of the innate and adaptive immune systems, interactions between intestinal microbiota, epithelial cells and ECM with mesenchymal cells and mesenchymal cells themselves. The mesenchymal cell compartment is expanded during this process by EMT, EndoMT and invasion of circulating fibrocytes. The fibrogenic cytokine/growth factor, TGF-β1, is a central regulator of fibrosis. ECM components are further regulated by the interaction of MMPs and TIMPs some of which are also mesenchymal cells products.
ECM: extracellular matrix; EMT: epithelial-to-mesenchymal transition; IGF-I: insulin-like growth factor-I; TGF- β: transforming growth factor- β; TNF-α: tumor necrosis factor-α; TLR: Toll-like receptors; CTGF: Connective tissue growth factor; MDP: Muramyl dipeptide; PDGF: Platelet-derived growth factor; bFGF: Basic fibroblast growth factor; IL-6: Interleukin-6; IL-23: Interleukin-23; IL-1β: Interleukin-1β; IFN-γ: Interferon-γ.
Table 1.
Factors that initiate or perpetuate fibrosis in Crohn’s disease
| Cytokines | IL-1, IL-4, IL-6, IL-13, IL-17, IL-12, IL-23, IL-33, TNF-α, MCP-1, IFN-γ |
| Growth Factors | TGF-β, CTGF, bFGF, IGF-I/II, IGFBP-5, PDGF, Endothelin |
| Matrix proteins | MMPs, TIMPs, Collagens (Types I, III and V), Vitronectin, Fibronectin, Osteopontin, Thrombospondin |
| Bacterial products | Muramyl dipeptide (MDP, NOD2 ligand), Flagellin (TLR5 ligand), PAMPs |
| Mediators | Endothelin, ROS, PPAR |
Acknowledgements
The authors apologize to those researchers whose work could not be included in this review due to space limitation.
Supported by DK49691 (JFK) from the National Institute of Diabetes, Digestive and Kidney Diseases
Footnotes
Disclosure:
The authors have no competing of conflicts of interest.
REFERENCES
- 1.Cosnes J, Cattan S, Blain A, et al. Long-Term Evolution of Disease Behavior of Crohn's Disease. Inflamm Bowel Dis. 2002;8:244–250. doi: 10.1097/00054725-200207000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Louis E, Collard A, Oger AF, et al. Behaviour of Crohn's disease according to the Vienna classification: changing pattern over the course of the disease. Gut. 2001;49:777–782. doi: 10.1136/gut.49.6.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van Assche G, Geboes K, Rutgeerts P. Medical therapy for Crohn's disease strictures. Inflamm Bowel Dis. 2004;10:55–60. doi: 10.1097/00054725-200401000-00009. [DOI] [PubMed] [Google Scholar]
- 4.Rieder F, Fiocchi C. Intestinal fibrosis in IBDa dynamic, multifactorial process. Nat Rev Gastroenterol Hepatol. 2009;6:228–235. doi: 10.1038/nrgastro.2009.31. [DOI] [PubMed] [Google Scholar]
- 5.Rieder F, Kessler SP, West GA, et al. Inflammation-Induced Endothelial-to-Mesenchymal Transition: A Novel Mechanism of Intestinal Fibrosis. Am J Pathol. 2011;179:2660–2673. doi: 10.1016/j.ajpath.2011.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mäkitalo L, Sipponen T, Kärkkäinen P, et al. Changes in matrix metalloproteinase (MMP) and tissue inhibitors of metalloproteinases (TIMP) expression profile in Crohn's disease after immunosuppressive treatment correlate with histological score and calprotectin values. Int J Colorectal Dis. 2009;24:1157–1167. doi: 10.1007/s00384-009-0756-5. [DOI] [PubMed] [Google Scholar]
- 7.McKaig BC, McWilliams D, Watson SA, et al. Expression and Regulation of Tissue Inhibitor of Metalloproteinase-1 and Matrix Metalloproteinases by Intestinal Myofibroblasts in Inflammatory Bowel Disease. Am J Pathol. 2003;162:1355–1360. doi: 10.1016/S0002-9440(10)63931-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murphy G, Nagase H. Progress in matrix metalloproteinase research. Mol Aspects Med. 2008;29:290–308. doi: 10.1016/j.mam.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nienke W, Hofker HS, Mark HJM, et al. Matrix metalloproteinases as profibrotic factors in terminal ileum in Crohn's disease. Inflamm Bowel Dis. 2006;12:863–869. doi: 10.1097/01.mib.0000231568.43065.ed. [DOI] [PubMed] [Google Scholar]
- 10.Faubion WA, Jr, Loftus EV, Jr, Harmsen WS, et al. The natural history of corticosteroid therapy for inflammatory bowel disease: a population-based study. Gastroenterology. 2001;121:255–260. doi: 10.1053/gast.2001.26279. [DOI] [PubMed] [Google Scholar]
- 11.Peyrin-Biroulet L, Loftus EV, Jr, Colombel JF, et al. The natural history of adult Crohn's disease in population-based cohorts. Am J Gastroenterol. 2010;105:289–297. doi: 10.1038/ajg.2009.579. [DOI] [PubMed] [Google Scholar]
- 12.Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol. 2008;214:199–210. doi: 10.1002/path.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schultz GS, Wysocki A. Interactions between extracellular matrix and growth factors in wound healing. Wound Repair Regen. 2009;17:153–162. doi: 10.1111/j.1524-475X.2009.00466.x. [DOI] [PubMed] [Google Scholar]
- 14.Dubinsky MC, Kugathasan S, Mei L, et al. Increased immune reactivity predicts aggressive complicating Crohn's disease in children. Clin Gastroenterol Hepatol. 2008;6:1105–1111. doi: 10.1016/j.cgh.2008.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martinon F, Tschopp J. NLRs join TLRs as innate sensors of pathogens. Trends Immunol. 2005;26:447–454. doi: 10.1016/j.it.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 16.Ojcius D, Saïd-Sadier N. Alarmins, inflammasomes and immunity. Biomed J. 2012;35(6):437–449. doi: 10.4103/2319-4170.104408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mueller C. Danger-Associated Molecular Patterns and Inflammatory Bowel Disease: Is There a Connection? DigDis. 2012;30(suppl 3):40–46. doi: 10.1159/000342600. [DOI] [PubMed] [Google Scholar]
- 18.Barrett JC, Hansoul S, Nicolae DL, et al. Genome-wide association defines more than 30 distinct susceptibility loci for Crohn's disease. Nat Genet. 2008;40:955–962. doi: 10.1038/NG.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brant SR. Promises, Delivery, and Challenges of Inflammatory Bowel Disease Risk Gene Discovery. Clin Gastroenterol Hepatol. 2013;11:22–26. doi: 10.1016/j.cgh.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 20.Cho JH, Brant SR. Recent Insights Into the Genetics of Inflammatory Bowel Disease. Gastroenterology. 2011;140:1704.e2–1712.e2. doi: 10.1053/j.gastro.2011.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jostins L, Ripke S, Weersma RK, et al. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature. 2012;491:119–124. doi: 10.1038/nature11582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Franke A, McGovern DPB, Barrett JC, et al. Genome-wide meta-analysis increases to 71 the number of confirmed Crohn's disease susceptibility loci. Nat Genet. 2010;42:1118–1125. doi: 10.1038/ng.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burke JP, Mulsow JJ, O'Keane C, et al. Fibrogenesis in Crohn's disease. Am J Gastroenterol. 2007;102:439–448. doi: 10.1111/j.1572-0241.2006.01010.x. [DOI] [PubMed] [Google Scholar]
- 24.Hugot JP. Genetic origin of IBD. Inflamm Bowel Dis. 2004;10(Suppl 1):S11–S15. doi: 10.1097/00054725-200402001-00003. [DOI] [PubMed] [Google Scholar]
- 25.Waterman M, Xu W, Stempak JM, et al. Distinct and overlapping genetic loci in Crohn's disease and ulcerative colitis: correlations with pathogenesis. Inflamm Bowel Dis. 2011;17:1936–1942. doi: 10.1002/ibd.21579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cleynen I, Mahachie John JM, Henckaerts L, et al. Molecular Reclassification of Crohn's Disease by Cluster Analysis of Genetic Variants. PLoS ONE. 2010;5:e12952. doi: 10.1371/journal.pone.0012952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rivas MA, Beaudoin M, Gardet A, et al. Deep resequencing of GWAS loci identifies independent rare variants associated with inflammatory bowel disease. Nat Genet. 2011;43:1066–1073. doi: 10.1038/ng.952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meijer MJM, Mieremet-Ooms MACB, Sier CFMP, et al. Matrix metalloproteinases and their tissue inhibitors as prognostic indicators for diagnostic and surgical recurrence in Crohn's disease. Inflamm Bowel Dis. 2009;15:84–92. doi: 10.1002/ibd.20581. [DOI] [PubMed] [Google Scholar]
- 29.Blennerhassett MG, Bovell FM, Lourenssen S, et al. Characteristics of inflammation-induced hypertrophy of rat intestinal smooth muscle cell. Dig Dis Sci. 1999;44:1265–1272. doi: 10.1023/a:1026669409229. [DOI] [PubMed] [Google Scholar]
- 30.Flier SN, Tanjore H, Kokkotou EG, et al. Identification of Epithelial to Mesenchymal Transition as a Novel Source of Fibroblasts in Intestinal Fibrosis. J Biol Chem. 2010;285:20202–20212. doi: 10.1074/jbc.M110.102012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sahebally SM, Burke JP, Chang KH, et al. Circulating fibrocytes and Crohn's disease. Br J Surg. 2013;100:1549–1556. doi: 10.1002/bjs.9302. [DOI] [PubMed] [Google Scholar]
- 32.Pinchuk IV, Mifflin RC, Saada JI, et al. Intestinal Mesenchymal Cells. Curr Gastroenterol Rep. 2010;12:310–318. doi: 10.1007/s11894-010-0135-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Graham MF, Diegelmann RF, Elson CO, et al. Collagen content and types in the intestinal strictures of Crohn's disease. Gastroenterology. 1988;94:257–265. doi: 10.1016/0016-5085(88)90411-8. [DOI] [PubMed] [Google Scholar]
- 34.Flynn RS, Murthy KS, Grider JR, et al. Endogenous IGF-I and [alpha]V[beta]3 Integrin Ligands Regulate Increased Smooth Muscle Hyperplasia in Stricturing Crohn's Disease. Gastroenterology. 2010;138:285–293. doi: 10.1053/j.gastro.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Powell DW, Pinchuk IV, Saada JI, et al. Mesenchymal Cells of the Intestinal Lamina Propria. Annu Rev Physiol. 2011;73:213–237. doi: 10.1146/annurev.physiol.70.113006.100646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meier JKH, Scharl M, Miller SN, et al. Specific differences in migratory function of myofibroblasts isolated from Crohn's disease fistulae and strictures. Inflamm Bowel Dis. 2011;17:202–212. doi: 10.1002/ibd.21344. [DOI] [PubMed] [Google Scholar]
- 37.Leeb SN, Vogl D, Grossmann J, et al. Autocrine Fibronectin-Induced Migration of Human Colonic Fibroblasts. Am J Gastroenterol. 2004;99:335–340. doi: 10.1111/j.1572-0241.2004.04044.x. [DOI] [PubMed] [Google Scholar]
- 38.Leeb SN, Vogl D, Gunckel M, et al. Reduced migration of fibroblasts in inflammatory bowel disease: role of inflammatory mediators and focal adhesion kinase. Gastroenterology. 2003;125:1341–1354. doi: 10.1016/j.gastro.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 39.Shih DQ, Barrett R, Zhang X, et al. Constitutive TL1A (TNFSF15) Expression on Lymphoid or Myeloid Cells Leads to Mild Intestinal Inflammation and Fibrosis. PLoS ONE. 2011;6:e16090. doi: 10.1371/journal.pone.0016090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sieg DJ, Hauck CR, Schlaepfer DD. Required role of focal adhesion kinase (FAK) for integrin-stimulated cell migration. J Cell Sci. 1999;112:2677–2691. doi: 10.1242/jcs.112.16.2677. [DOI] [PubMed] [Google Scholar]
- 41.Pinchuk IV, Beswick EJ, Saada JI, et al. Human Colonic Myofibroblasts Promote Expansion of CD4+ CD25high Foxp3+ Regulatory T Cells. Gastroenterology. 2011;140:2019–2030. doi: 10.1053/j.gastro.2011.02.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Drygiannakis I, Valatas V, Sfakianaki O, et al. Proinflammatory cytokines induce crosstalk between colonic epithelial cells and subepithelial myofibroblasts: Implication in intestinal fibrosis. J Crohns Colitis. 2013;7:286–300. doi: 10.1016/j.crohns.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 43.Hermiston ML, Gordon J. Inflammatory bowel disease and adenomas in mice expressing a dominant negative N-cadherin. Science. 1995;270:1203–1207. doi: 10.1126/science.270.5239.1203. [DOI] [PubMed] [Google Scholar]
- 44.Burke JPPM, Cunningham MFMB, Sweeney CMMB, et al. N-cadherin is overexpressed in Crohn's stricture fibroblasts and promotes intestinal fibroblast migration. Inflamm Bowel Dis. 2011;17:1665–1673. doi: 10.1002/ibd.21543. [DOI] [PubMed] [Google Scholar]
- 45.Johnson LABS, Rodansky ESMS, Sauder KLBS, et al. Matrix Stiffness Corresponding to Strictured Bowel Induces a Fibrogenic Response in Human Colonic Fibroblasts. Inflamm Bowel Dis. 2013;19:891–903. doi: 10.1097/MIB.0b013e3182813297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Quan T, Cowper S, Bucala R. The role of circulating fibrocytes in fibrosis. Curr Rheumatol Rep. 2006;8:145–150. doi: 10.1007/s11926-006-0055-x. [DOI] [PubMed] [Google Scholar]
- 47.Bellini A, Mattoli S. The role of the fibrocyte, a bone marrow-derived mesenchymal progenitor, in reactive and reparative fibroses. Lab Invest. 2007;87:858–870. doi: 10.1038/labinvest.3700654. [DOI] [PubMed] [Google Scholar]
- 48.Willis BC, Borok Z. TGF-beta-induced EMT: mechanisms and implications for fibrotic lung disease. Am J Physiol Lung Cell Mol Physiol. 2007;293:L525–L534. doi: 10.1152/ajplung.00163.2007. [DOI] [PubMed] [Google Scholar]
- 49.Rovedatti L, Di Sabatino A, Knowles CH, et al. Fibroblast activation protein expression in Crohn's disease strictures. Inflamm Bowel Dis. 2011;17:1251–1253. doi: 10.1002/ibd.21446. [DOI] [PubMed] [Google Scholar]
- 50.Piera-Velazquez S, Li Z, Jimenez SA. Role of endothelial-mesenchymal transition (EndoMT) in the pathogenesis of fibrotic disorders. Am J Pathol. 2011;179:1074–1080. doi: 10.1016/j.ajpath.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Speca S, Giusti I, Rieder F, et al. Cellular and molecular mechanisms of intestinal fibrosis. World J Gastrointest. 2012;18:3635–3661. doi: 10.3748/wjg.v18.i28.3635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McKaig BC, Hughes K, Tighe PJ, et al. Differential expression of TGF-β isoforms by normal and inflammatory bowel disease intestinal myofibroblasts. Am J Physiol Cell Physiol. 2002;282:C172–C182. doi: 10.1152/ajpcell.00048.2001. [DOI] [PubMed] [Google Scholar]
- 53.Yan X, Liu Z, Chen Y. Regulation of TGF-β signaling by Smad7. Acta Biochim Biophys Sin (Shanghai) 2009;41:263–272. doi: 10.1093/abbs/gmp018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kuemmerle JF, Murthy KS, Bowers JG. IGFBP-3 activates TGF-beta receptors and directly inhibits growth in human intestinal smooth muscle cells. Am J Physiol Gastrointest Liver Physiol. 2004;287:G795–G802. doi: 10.1152/ajpgi.00009.2004. [DOI] [PubMed] [Google Scholar]
- 55.Flynn RS, Mahavadi S, Murthy KS, et al. Endogenous IGFBP-3 Regulates Excess Collagen Expression in Intestinal Smooth Muscle Cells of Crohn's Disease Strictures. Inflamm Bowel Dis. 2010;17(1):193–201. doi: 10.1002/ibd.21351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Datto MB, Frederick JP, Pan L, et al. Targeted disruption of Smad3 reveals an essential role in transforming growth factor beta-mediated signal transduction. Mol Cell Biol. 1999;19:2495–2504. doi: 10.1128/mcb.19.4.2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yang X, Letterio JJ, Lechleider RJ, et al. Targeted disruption of SMAD3 results in impaired mucosal immunity and diminished T cell responsiveness to TGF-beta. EMBO J. 1999;18:1280–1291. doi: 10.1093/emboj/18.5.1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Latella G, Vetuschi A, Sferra R, et al. Smad3 loss confers resistance to the development of trinitrobenzene sulfonic acidinduced colorectal fibrosis. Eur J Clin Invest. 2009;39:145–156. doi: 10.1111/j.1365-2362.2008.02076.x. [DOI] [PubMed] [Google Scholar]
- 59.Flynn RS, Mahavadi S, Murthy KS, et al. Endogenous IGFBP-3 regulates excess collagen expression in intestinal smooth muscle cells of Crohn's disease strictures. Inflamm Bowel Dis. 2011;17:193–201. doi: 10.1002/ibd.21351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.John PB, Marc F, Karen D, et al. Transcriptomic analysis of intestinal fibrosis-associated gene expression in response to medical therapy in Crohn's disease. Inflamm Bowel Dis. 2008;14:1197–1204. doi: 10.1002/ibd.20482. [DOI] [PubMed] [Google Scholar]
- 61.Huveneers S, Danen EHJ. The Interaction of Src Kinase with beta 3 Integrin Tails: A Potential Therapeutic Target in Thrombosis and Cancer. ScientificWorldJournal. 2010;10:1100–1106. doi: 10.1100/tsw.2010.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Clemmons DR, Maile LA. Minireview: Integral membrane proteins that function coordinately with the insulin-like growth factor I receptor to regulate intracellular signaling. Endocrinology. 2003;144:1664–1670. doi: 10.1210/en.2002-221102. [DOI] [PubMed] [Google Scholar]
- 63.Gerthoffer WT, Gunst SJ. Invited Review: Focal adhesion and small heat shock proteins in the regulation of actin remodeling and contractility in smooth muscle. J Appl Physiol. 2001;91:963–972. doi: 10.1152/jappl.2001.91.2.963. [DOI] [PubMed] [Google Scholar]
- 64.Li C, Kendig DM, Grider JR, et al. Non-proteolytic activation of Latent TGF-β1 by colonic muscle motor activity in rat. Neurogastroenterol Motil 2. 1013;25:44. [Google Scholar]
- 65.Li C, Flynn RS, Grider JRP, et al. Increased Activation of Latent TGF-[beta]1 by [alpha]V[beta]3 in Human Crohn's Disease and Fibrosis in TNBS Colitis Can Be Prevented by Cilengitide. Inflamm Bowel Dis. 2013;19:2829–2839. doi: 10.1097/MIB.0b013e3182a8452e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Monteleone G, Del Vecchio Blanco G, Monteleone I, et al. Post-transcriptional Regulation of Smad7 in the Gut of Patients With Inflammatory Bowel Disease. Gastroenterology. 2005;129:1420–1429. doi: 10.1053/j.gastro.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 67.Monteleone G, Fantini MC, Onali S, et al. Phase I Clinical Trial of Smad7 Knockdown Using Antisense Oligonucleotide in Patients With Active Crohn's Disease. Mol Ther. 2012;20:870–876. doi: 10.1038/mt.2011.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fichtner-Feigl S, Fuss IJ, Young CA, et al. Induction of IL-13 triggers TGF-beta1-dependent tissue fibrosis in chronic 2,4,6-trinitrobenzene sulfonic acid colitis. J Immunol. 2007;178:5859–5870. doi: 10.4049/jimmunol.178.9.5859. [DOI] [PubMed] [Google Scholar]
- 69.Fichtner-Feigl S, Young CA, Kitani A, et al. IL-13 Signaling via IL-13R[alpha]2 Induces Major Downstream Fibrogenic Factors Mediating Fibrosis in Chronic TNBS Colitis. Gastroenterology. 2008;135:2003–2013. doi: 10.1053/j.gastro.2008.08.055. [DOI] [PubMed] [Google Scholar]
- 70.Pucilowska JB, McNaughton KK, Mohapatra NK, et al. IGF-I and procollagen alpha1(I) are coexpressed in a subset of mesenchymal cells in active Crohn's disease. Am J Physiol Gastrointest Liver Physiol. 2000;279:G1307–G1322. doi: 10.1152/ajpgi.2000.279.6.G1307. [DOI] [PubMed] [Google Scholar]
- 71.Zimmermann EM, Li L, Hou YT, et al. Insulin-like growth factor I and insulin-like growth factor binding protein 5 in Crohn's disease. Am J Physiol Gastrointest Liver Physiol. 2001;280:G1022–G1029. doi: 10.1152/ajpgi.2001.280.5.G1022. [DOI] [PubMed] [Google Scholar]
- 72.Bushman TL, Kuemmerle JF. IGFBP-3 and IGFBP-5 production by human intestinal muscle: reciprocal regulation by endogenous TGF-beta1. Am J Physiol. 1998;275:G1282–G1290. doi: 10.1152/ajpgi.1998.275.6.G1282. [DOI] [PubMed] [Google Scholar]
- 73.Mahavadi S, Flynn RS, Grider JR, et al. Amelioration of excess collagen IαI, fibrosis, and smooth muscle growth in TNBS-induced colitis in IGF-I(+/−) mice. Inflamm Bowel Dis. 2011;17:711–719. doi: 10.1002/ibd.21437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Simmons JG, Pucilowska JB, Keku TO, et al. IGF-I and TGF-beta 1 have distinct effects on phenotype and proliferation of intestinal fibroblasts. Am J Physiol Gastrointest Liver Physiol. 2002;283:G809–G818. doi: 10.1152/ajpgi.00057.2002. [DOI] [PubMed] [Google Scholar]
- 75.Fiocchi C, Lund PK. Themes in fibrosis and gastrointestinal inflammation. Am J Physiol Gastrointest Liver Physiol. 2011;300:G677–G683. doi: 10.1152/ajpgi.00104.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rigby RJ, Hunt MR, Scull BP, et al. A new animal model of postsurgical bowel inflammation and fibrosis: the effect of commensal microflora. Gut. 2009;58:1104–1112. doi: 10.1136/gut.2008.157636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rieder F, Bhilocha S, Anja S, et al. Toll-like Recptor (TLR) 5 Promotes a Pro Fibrogenic Phenotype in Human Intestinal Fibroblasts (HIF) via MyD88, Caspase-1 and Posttransciptional Regulation. Inflamm Bowel Dis. 2013;19:S17. [Google Scholar]
- 78.Di Sabatino A, Jackson CL, Pickard KM, et al. Transforming growth factor {beta} signalling and matrix metalloproteinases in the mucosa overlying Crohn's disease strictures. Gut. 2009;58:777–789. doi: 10.1136/gut.2008.149096. [DOI] [PubMed] [Google Scholar]
- 79.Nagase H, Visse R, Murphy G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc Res. 2006;69:562–573. doi: 10.1016/j.cardiores.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 80.Monteleone G, Caruso R, Fina D, et al. Control of matrix metalloproteinase production in human intestinal fibroblasts by interleukin 21. Gut. 2006;55:1774–1780. doi: 10.1136/gut.2006.093187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Warnaar N, Hofker HS, Maathuis MH, et al. Matrix metalloproteinases as profibrotic factors in terminal ileum in Crohn's disease. Inflamm Bowel Dis. 2006;12:863–869. doi: 10.1097/01.mib.0000231568.43065.ed. [DOI] [PubMed] [Google Scholar]
- 82.Mu D, Cambier S, Fjellbirkeland L, et al. The integrin alpha(v)beta8 mediates epithelial homeostasis through MT1-MMP-dependent activation of TGF-beta1. J Cell Biol. 2002;157:493–507. doi: 10.1083/jcb.200109100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gasche C, Scholmerich J, Brynskov J, et al. A simple classification of Crohn's disease: report of the Working Party for the World Congresses of Gastroenterology, Vienna 1998. Inflamm Bowel Dis. 2000;6:8–15. doi: 10.1097/00054725-200002000-00002. [DOI] [PubMed] [Google Scholar]
- 84.Danese S, Sans M, de la Motte C, et al. Angiogenesis as a novel component of inflammatory bowel disease pathogenesis. Gastroenterology. 2006;130:2060–2073. doi: 10.1053/j.gastro.2006.03.054. [DOI] [PubMed] [Google Scholar]
- 85.Hall M-C, Young DA, Waters JG, et al. The Comparative Role of Activator Protein 1 and Smad Factors in the Regulation of Timp-1 and MMP-1 Gene Expression by Transforming Growth Factor-β1. J Biol Chem. 2003;278:10304–10313. doi: 10.1074/jbc.M212334200. [DOI] [PubMed] [Google Scholar]