Abstract
Background
Although CMV infection after allogeneic stem cell transplantation (SCT) is only rarely fatal, the management of CMV by preemptive medication for viral reactivation has toxicity and carries a financial burden. New strategies to prevent CMV reactivation with vaccines and antiviral T cells may represent an advance over preemptive strategies, but have yet to be justified in terms of transplant outcome and cost.
Patients and methods
We compared outcomes and post-transplant treatment cost in 44 patients who never required preemptive CMV treatment with 90 treated patients undergoing SCT at our institute between 2006–2012. 81 subjects received CD34+ selected myeloablative SCT, 12 umbilical cord blood transplants, and 41 T-replete nonmyeloablative SCT. 119 (89%) patients were at risk for CMV because either the donor or recipient was seropositive. Of these, 90 (75.6%) reactivated CMV at a median of 30 (range 8–105) days post transplant and received antivirals.
Results
There was no difference in standard transplant risk factors between the two groups. In multivariate modeling, CMV reactivation >250 copies/ml (OR=3, P<0.048), total duration of inpatient IV antiviral therapy (OR = 1.04, P<0.001), type of transplant (T-deplete vs. T-replete) (OR=4.65, P<0.017) were found to be significantly associated with increased non-relapse mortality. The treated group incurred an additional cost of antiviral medication and longer hospitalization within the first 6 months post SCT of $58,000 to $74,000/patient.
Discussion
Our findings suggest that to prevent CMV reactivation treatment should be given within 1 week of SCT. Preventative treatment may improve outcome and have significant cost savings.
Keywords: antiviral cellular therapy, CMV reactivation, economic cost, preemptive therapy
Introduction
Early detection of cytomegalovirus (CMV) reactivation, followed by preemptive antiviral treatment, has transformed the outcome of allogeneic stem cell transplantation (SCT). Introduced into standard transplant practice over 20 years ago, the preemptive treatment approach has been optimized by the introduction of sensitive CMV DNA testing (1–3). Such testing is usually performed weekly in the first 3–4 months after SCT and results determine preemptive treatment of CMV antigenemia with antiviral medications (4–7). With preemptive treatment, the incidence of fatal CMV disease after SCT has been dramatically reduced (8–10). Nevertheless, CMV disease remains a significant problem for CMV seropositive recipients of transplants from CMV naïve donors (notably in the context of umbilical cord blood transplant [UCBT])(11–14). Furthermore, preemptive treatment of CMV uses costly antiviral agents which incur toxicities such as renal damage and cytopenia, requiring further treatment and hospitalizations (7, 15). The use of ganciclovir enhances immunosuppression in vitro (16) and multiple episodes of CMV reactivation are associated with late relapse and treatment failure (17). Prevention of CMV reactivation rather than its preemptive management may be advantageous by avoiding toxicity from antiviral treatment and reducing the economic burden of post-transplant care.
Under development are strategies using CMV vaccines and CMV specific T cell infusions to prevent rather than pre-empt CMV disease after SCT. Since the first proof of principle that CMV specific T cells can treat CMV reactivation by Riddell et al and Walter et al (18, 19), many successful approaches to selecting and generating CMV specific T cells for infusion have been developed (20–23). Infusion of CMV specific T cells is safe and shows high efficacy in treating antigenemia and established CMV disease (24, 25). CMV vaccines also show promise in reducing the frequency of CMV reactivation after SCT(12, 23, 26–28).
Effective prevention of CMV reactivation with vaccines or T cells could reduce morbidity and mortality from CMV and reduce the cost of post-transplant care. However, a global change in practice from pre-emptive to preventative treatment of CMV would only occur if several conditions are met: First, clinical trials with preventative treatment would need to show nearly 100% efficiency to convince transplant physicians to change. Second, the cost of the alternative treatment strategy would have to be less than the current expense of antiviral medications and related post-transplant hospitalizations incurred by CMV reactivation. No studies have yet explored the implications of CMV reactivation from this viewpoint. We therefore analyzed CMV reactivation in patients undergoing SCT at our institute, comparing outcomes and cost after viral reactivation with a contemporaneous group of patients who did not require antiviral treatment. We show for the first time that CMV reactivation requiring antiviral medications incurs a worse outcome and a significant cost that might be avoided if CMV preventative treatment were available.
PATIENTS AND METHODS
Patient study design
We analyzed CMV reactivation in the first six months post transplant in 134 patients who underwent SCT at the Hematology Branch of the NHLBI at the National Institutes of Health between January 2006 and April 2012 on institutional review board- approved protocols (94-H-0010, 99-H-0050, 06-H-0248, 07-H-0136, 08-H-0046). Written informed consent, consistent with the Helsinki Declaration, was obtained from all patients and donors.
Definitions
CMV reactivation and disease
As previously defined by Ljungman et al, CMV DNAemia was defined as the detection of DNA in the samples of plasma, whole blood, and isolated peripheral blood leukocytes or in buffy-coat specimens by PCR-based techniques, hybrid capture, and branched-chain DNA analysis (29). CMV pneumonia was defined by the presence of signs and/or symptoms of pulmonary disease combined with the detection of CMV in bronchoalveolar lavage or lung tissue samples by culture, histopathological testing, immunohistochemical analysis, or in situ hybridization but not by PCR alone (29). CMV gastrointestinal (GI) disease was defined by the identification of a combination of clinical symptoms from upper or lower GI, findings of macroscopic mucosal lesions on endoscopy, and demonstration of CMV infection (by culture, histopathological testing, immunohistochemical analysis, or in situ hybridization but not PCR) in a GI biopsy specimen (29). Central nervous system (CNS) disease was defined by identification of CMV in cerebrospinal fluid samples, by culture or PCR, or in brain biopsy specimens, by culture, histopathological testing, immunohistochemical analysis, or in situ hybridization (29). In peripheral blood or bone marrow SCT, we used >250 CMV copies/ml as the cut off for as the threshold for treatment in most of our patients. Only a minority of patients had threshold copy numbers of 500/ml or higher. Because of the high risk of CMV disease in UCBT, any level of CMV reactivation was an indication for preemptive therapy (26, 30, 31).
CMV treatment groups
For comparison, we defined two groups: “Antiviral medication group” consisting of the 90 subjects who had had received CMV antiviral medications either for reactivation or organ disease; and a “No antiviral group” consisting of 44 subjects (29 at risk subjects who did not require antiviral treatment, and 15 who were not at risk).
Antiviral medication
Antiviral drugs including foscarnet, ganciclovir, valganciclovir were administered promptly after CMV reactivation to prevent CMV organ disease (7, 15, 32).
Cost calculations
We used the mean cost per day of inpatient hospitalization in matched related donor transplants, and in UCBT of about $3582, and $4700 respectively from Majhail et al (33, 34). Pharmacy costs were based on 2013 purchases: intravenous (IV) foscarnet $277.37/infusion; IV ganciclovir $64.64/infusion; oral valganciclovir $83.70 per tablet. We calculated the additional cost for a patient in the antiviral treatment group to be the sum of the cost of the mean number of extra days in hospital and the mean cost of drugs (IV ganciclovir + IV foscarnet + oral valganciclovir tablets) (supplementary table Ia and Ib). Although some patients also received intravenous cidofovir, we did not include it in these cost calculations as most of these had concomitant adenovirus infection.
Statistical Analysis
Summary statistics such as sample proportions, medians, standard deviations, and 95% confidence intervals, were used to describe patient characteristics. Categorical data was analyzed using the Fisher exact test. CMV reactivation >250 copies/ml, total duration of inpatient IV antiviral therapy, type of transplant (T-deplete vs. T-replete), age, sex, diagnosis (malignant vs. non malignant) and CMV risk status were entered into the multivariate model (forward-stepwise multiple regression). Statistical significance was considered when p<0.05. All statistical analyses were performed using IBM SPSS v19 (SPSS Inc., Chicago, IL). Graphs were created using Prism 5.03 (GraphPad Software, Inc. La Jolla, CA, USA).
RESULTS
Patient characteristics
Median age at transplant was 40 years and the median follow-up was 4.25 years. One hundred and two subjects were transplanted for malignant diseases and 32 for non-malignant diseases. Eighty-one subjects received CD34+ selected myeloablative SCT, 12 received combined haploidentical plus UCBT, and 41 had T-replete nonmyeloablative transplants. Ninety patients were allocated to the antiviral treatment group and 44 to the no therapy group (as defined above). Patient and transplant characteristics are shown in Table I.
Table I.
Patient characteristics of antiviral therapy and no therapy groups.
| Antiviral group (N=90) | No therapy group (N=44) | P-value | ||
|---|---|---|---|---|
| Sex | Male | 43 (47.8%) | 29 (66%) | 0.064 |
| Female | 47 (52.2%) | 15 (34%) | ||
| Age at BMT | Median (range) | 33 (9–69) years | 46 (8–68) years | |
| Diagnosis | ALL | 17 (18.7%) | 4 (9.3%) | 0.528 |
| AML | 28 | 10(16.3%) | ||
| CML/CMML | 3 (3.3%) | 1 (2.3%) | ||
| HL | 0 | 1 (2.3%) | ||
| MDS/MPD | 10 (9.9%) | 7 (18.6%) | ||
| MM/NHL | 10 (9.9%) | 9 (47.4%) | ||
| SAA/PNH | 22 (24.2%) | 12 (27.9%) | ||
| Disease risk | Standard risk | 59 (65.5%) | 23 (52.3%) | 0.186 |
| High risk | 31(34.4%) | 21(47.7%) | ||
| Type of transplant | T-cell deplete (CD34+ selected) | 59 (65.6%) | 22 (50%) | 0.03 |
| UCBT + haploidentical | 10 (11.1%) | 2 (4.5%) | ||
| T-replete | 21 (23.3%) | 20 (45.5%) | ||
| CMV D/R status | D−/R+ | 13 (14.3%) | 5 (11.6%) | < 0.0001 |
| D+/R− | 5 (5.4%) | 7(16.3%) | ||
| D+/R+ | 72(77%) | 17(39.5%) | ||
| D−/R− | 0 | 15 (34.9%) | ||
| aGvHD (Grade II–IV) | Yes | 44 (48.9%) | 17 (38.6%) | 0.275 |
| No | 46 (51.1%) | 27 (61.4%) |
Abbreviations: AML, Acute Myeloid Leukemia; ALL, Acute Lymphoid Leukemia; BMT, Bone Marrow Transplant; CML, Chronic Myeloid Leukemia; CLL, Chronic Lymphoid Leukemia; CMML, Chronic Myelomonocytic Leukemia; D/R, Donor/recipient; HL, Hodgkin’s Lymphoma; MDS, Myelodysplastic Syndrome; MM, Multiple Myeloma; MPD, Myeloproliferative Disease; NHL, Non Hodgkin’s Lymphoma; PNH, Paroxysmal Nocturnal Hematuria; SAA, Severe Aplastic Anemia; UCBT, Umbilical Cord Blood Transplant
CMV reactivation
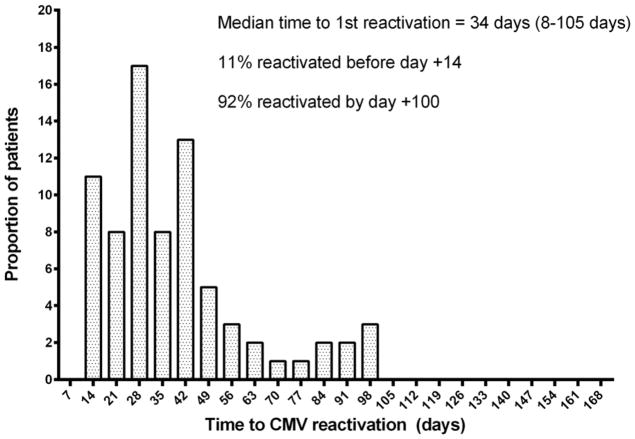
One hundred and nineteen (89%) patients were at risk for CMV by virtue of either the donor or recipient being seropositive prior to transplant. Of these, 90 (75.6%) had CMV reactivation in the blood (>250 copies/ml by PCR) including 4 (3.4%) who had CMV organ disease (involving the GI tract=3, lung=2 and CNS=1) and all received antivirals. The median time to first CMV reactivation for all patients was 34 days (range: 8–105 days). Eleven percent reactivated before day+14 and 92% by day+100. Time to reactivation differed by protocol: Median time to first CMV reactivation for CD34+ selected transplant patients was 28 days (range: 8–100 days); while the median time to first reactivation in the T-replete transplant recipients was 43 days (range: 14–105 days) and 24 days (24 – 94 days) for UCB recipients (Figure 1).
Figure 1.
Proportion of patients reactivating CMV for the first time post transplant.
Survivor outcomes
Of the 134 patients, 54 had died at the time of analysis (31 non-relapse mortality, and 23 from relapse). In multivariate modeling, any CMV reactivation >250 copies/ml (OR=3, P<0.048), total duration of inpatient IV antiviral medications (OR = 1.04, P<0.001), and type of transplant (T-deplete vs. T-replete) (OR=4.65, P<0.017), carried a significantly greater risk of NRM. There was no impact of CMV risk on overall survival. The model excluded age, sex, diagnosis (malignant vs. non malignant), and CMV risk status. Given the heterogeneity of transplant disease indications between the two groups, we did not evaluate the relapse mortality.
Cost of preemptive therapy
The antiviral treatment group had an average of 12.6 days of IV antiviral therapy per patient; and 13.9 additional days of hospitalization per patient (Figure 2). The number of inpatient days that the patients received IV antiviral therapy for CMV (which included ganciclovir and foscarnet) corresponded closely to the average additional days of hospitalization per patient in the antiviral treatment group. We calculated the average additional cost for preemptive antiviral treatment to be between $58,000 to $74,000 per patient (calculations based on type of transplant – see methods).
Figure 2.
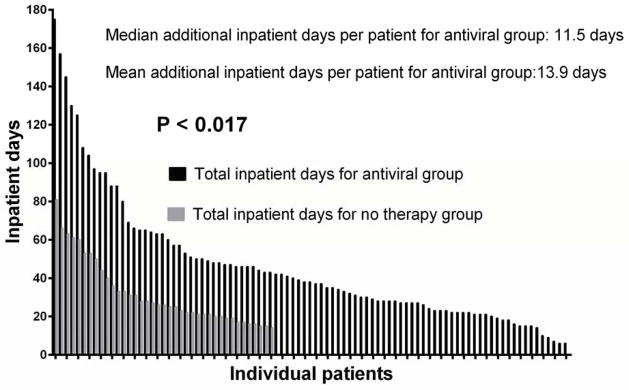
Comparison of total inpatient days for antiviral therapy group vs. total inpatient days for no therapy group.
DISCUSSION
Since its introduction in the 1980’s pre-emptive treatment of CMV reactivating after SCT has been adopted as the most effective means of minimizing morbidity and mortality from this virus (8–10). Treatment failure is more common in individuals with no effective antiviral T cell immunity (35, 36). Against these unwanted complications there is some evidence that CMV reactivation can protect against relapse of myeloid malignancies (37–39). The mechanism is unclear but may be more directly associated with the immune response to the reactivating virus that the reactivation itself. Here we show that, while preemptive therapy for CMV across a variety of SCT conditions is efficient in controlling and preventing major complications from CMV disease, there is a cost incurred in terms of in-patient stay (and the associated expense of in-patient hospital care), and a small negative effect on transplant-related mortality. There was no difference in overall survival between the two groups, and it is possible that any impact on OS may have been negated by improvement in relapse free survival in myeloid malignancies. However our study group was too small and diverse to make meaningful evaluations of a protective effect of CMV reactivation on disease relapse.
Our comparison groups comprised of 90 transplant recipients requiring antiviral medications for CMV and of 44 recipients who did not reactivate CMV or reactivated to a level below that required to trigger antiviral medications. This group included 15 obligate non reactivators where the patient and donor were both seronegative. T-replete transplant subjects were less likely to receive antiviral therapy. Importantly there were no differences in standard risk factors for transplant outcome between the groups that might have biased our results. Notably the proportion of SCT for malignant vs. non malignant disease, disease risk status for malignant disease, age, conditioning regimen, and occurrence of acute GvHD did not differ statistically between the two groups. However, we cannot exclude the possibility that differences in quality of immune reconstitution, rather than CMV reactivation itself might have contributed to the longer hospital stay of the antiviral treated group. Nevertheless, our findings suggest that prevention of CMV reactivation above a threshold requiring antiviral medications, could result in fewer post-reactivation events, and a reduction in in-patient management (and its associated expenses).
Our study is limited by a small population size which prevents a detailed analysis of CMV associated outcomes within defined transplant categories. For example, recipients of UCBT and T-cell depleted SCT would be expected to have a greater risk of CMV reactivation rather than the recipients of unmanipulated SCT, who might not derive benefit from preventative antiviral therapy. Cost estimates were based on United States data and may not apply elsewhere. Furthermore, other transplant centers may deliver more treatment in a day-care facility, thus reducing the duration of in-patient days post transplant in CMV reactivators. Finally, although there were no differences in factors that might have affected NRM and post-transplant hospitalization (Table I), it remains possible that factors other than the need for CMV treatment, that we could not identify, may have led to the different outcomes in two groups. Studies in a larger patient population, where there are sufficient numbers of obligate CMV non-reacting patients (recipient and donor CMV seronegative) serving as a control group for treated CMV reactivators, would be required to better define the financial, therapeutic, and outcome burden of CMV reactivation.
Preventive therapy for CMV is now a possibility. The ability to obtain potent CMV specific T cells from healthy donors, whether prior exposed or naïve to CMV, and the introduction of CMV vaccines, opens the way to clinical trials aimed at boosting cellular immunity to CMV to a level that avoids the need for antiviral chemotherapy (21, 40–44). To date, clinical trials with CMV specific T cells have focused on treatment of established viral reactivation or administration of cytotoxic T- lymphocytes (CTL) beyond the time of the first reactivation (43, 45–47). In our cohort, we found that CMV reactivation occurs before day+14 in 11% of the subjects (Figure 1), hence the preventative use of antiviral CTL would be required within a week of transplant to avoid the need for pre-emptive antiviral therapy. Alternatively preemptive treatment might be avoided by administering CMV vaccines to the donor to boost antiviral immunity in the graft (27, 28, 48–50).
Antiviral CTL treatment has proven efficacy in CMV reactivation with over 90% complete responses reported in recent series (51). Nevertheless, such cellular therapy is faced with significant obstacles to its general introduction, associated with its sophisticated good manufacturing practice for manufacture and general acceptance in the stem cell transplant community. Our study provides an important platform for future antiviral cell therapy and vaccine approaches because it identifies both a potential therapeutic and economic benefit of preventative anti-CMV management. Current cost estimates for manufacture of donor derived CTL in good manufacturing practice conditions are in the order of $10,000. Given the cost of antiviral treatment and associated hospital care of over $50,000 per patient, our findings suggest that even if CTL prevention is only 50% effective at avoiding the need for antiviral treatment, the preventative approach would remain the less expensive option. Economics aside, the opportunity to prevent the need for antiviral treatment and its consequences should contribute significantly to the development of complication free allogeneic stem cell transplants.
Supplementary Material
Supplementary table Ia. Comparison of average hospitalization days between antiviral group and no therapy group.
Supplementary table Ib. Calculation of average cost per patient in the antiviral therapy group (N=90).
Acknowledgments
This work was supported by the intramural research program of the NHLBI, NIH.
Abbreviations
- CMV
Cytomegalovirus
- CTL
Cytotoxic T-lymphocytes
- DNA
Deoxyribonucleic acid
- GI
Gastrointestinal
- GvHD
Graft versus host disease
- IV
Intravenous
- NRM
Non relapse mortality
- PCR
Polymerase chain reaction
- SCT
Allogeneic stem cell transplant
- UCBT
Umbilical cord blood transplant
Footnotes
Authorship Contributions:
N.A.J, A.J.B. and M.B. designed the study; N.A.J, L.C, J.H, P.D.C, E.C, and C.R collected data; N.A.J, A.J.B. and M.B. analyzed and interpreted the data; P.M, S.I., K.L, R.C, A.J.B and M.B. took care of patients; N.A.J, A.J.B., K.L. and M.B. wrote the manuscript. C.S.H critically revised the manuscript.
Conflict-of-interest disclosure: The authors have no conflicts of interest to disclose
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nichols WG, Corey L, Gooley T, Drew WL, Miner R, Huang M, et al. Rising pp65 antigenemia during preemptive anticytomegalovirus therapy after allogeneic hematopoietic stem cell transplantation: risk factors, correlation with DNA load, and outcomes. Blood. 2001 Feb 15;97(4):867–74. doi: 10.1182/blood.v97.4.867. [DOI] [PubMed] [Google Scholar]
- 2.Nichols WG, Corey L, Gooley T, Davis C, Boeckh M. High risk of death due to bacterial and fungal infection among cytomegalovirus (CMV)-seronegative recipients of stem cell transplants from seropositive donors: evidence for indirect effects of primary CMV infection. The Journal of infectious diseases. 2002 Feb 1;185(3):273–82. doi: 10.1086/338624. [DOI] [PubMed] [Google Scholar]
- 3.Boeckh M, Nichols WG. The impact of cytomegalovirus serostatus of donor and recipient before hematopoietic stem cell transplantation in the era of antiviral prophylaxis and preemptive therapy. Blood. 2004 Mar 15;103(6):2003–8. doi: 10.1182/blood-2003-10-3616. [DOI] [PubMed] [Google Scholar]
- 4.Boeckh M, Gooley TA, Myerson D, Cunningham T, Schoch G, Bowden RA. Cytomegalovirus pp65 antigenemia-guided early treatment with ganciclovir versus ganciclovir at engraftment after allogeneic marrow transplantation: a randomized double-blind study. Blood. 1996 Nov 15;88(10):4063–71. [PubMed] [Google Scholar]
- 5.Griffiths P, Whitley R, Snydman DR, Singh N, Boeckh M. Contemporary management of cytomegalovirus infection in transplant recipients: guidelines from an IHMF workshop, 2007. Herpes: the journal of the IHMF. 2008 Oct;15(1):4–12. [PubMed] [Google Scholar]
- 6.Bacigalupo A, Bregante S, Tedone E, Isaza A, Van Lint MT, Moro F, et al. Combined foscarnet -ganciclovir treatment for cytomegalovirus infections after allogeneic hemopoietic stem cell transplantation (Hsct) Bone marrow transplantation. 1996 Nov;18(Suppl 2):110–4. [PubMed] [Google Scholar]
- 7.Bregante S, Bertilson S, Tedone E, Van Lint MT, Trespi G, Mordini N, et al. Foscarnet prophylaxis of cytomegalovirus infections in patients undergoing allogeneic bone marrow transplantation (BMT): a dose-finding study. Bone marrow transplantation. 2000 Jul;26(1):23–9. doi: 10.1038/sj.bmt.1702450. [DOI] [PubMed] [Google Scholar]
- 8.Boeckh M, Bowden RA, Gooley T, Myerson D, Corey L. Successful modification of a pp65 antigenemia-based early treatment strategy for prevention of cytomegalovirus disease in allogeneic marrow transplant recipients. Blood. 1999 Mar 1;93(5):1781–2. [PubMed] [Google Scholar]
- 9.Einsele H, Ehninger G, Hebart H, Wittkowski KM, Schuler U, Jahn G, et al. Polymerase chain reaction monitoring reduces the incidence of cytomegalovirus disease and the duration and side effects of antiviral therapy after bone marrow transplantation. Blood. 1995 Oct 1;86(7):2815–20. [PubMed] [Google Scholar]
- 10.Ljungman P, Engelhard D, Link H, Biron P, Brandt L, Brunet S, et al. Treatment of interstitial pneumonitis due to cytomegalovirus with ganciclovir and intravenous immune globulin: experience of European Bone Marrow Transplant Group. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 1992 Apr;14(4):831–5. doi: 10.1093/clinids/14.4.831. [DOI] [PubMed] [Google Scholar]
- 11.Junghanss C, Storb R, Maris MB, Carter RA, Sandmaier BM, Maloney DG, et al. Impact of unrelated donor status on the incidence and outcome of cytomegalovirus infections after non-myeloablative allogeneic stem cell transplantation. British journal of haematology. 2003 Nov;123(4):662–70. doi: 10.1046/j.1365-2141.2003.04671.x. [DOI] [PubMed] [Google Scholar]
- 12.Ljungman P, Brand R, Einsele H, Frassoni F, Niederwieser D, Cordonnier C. Donor CMV serologic status and outcome of CMV-seropositive recipients after unrelated donor stem cell transplantation: an EBMT megafile analysis. Blood. 2003 Dec 15;102(13):4255–60. doi: 10.1182/blood-2002-10-3263. [DOI] [PubMed] [Google Scholar]
- 13.Craddock C, Szydlo RM, Dazzi F, Olavarria E, Cwynarski K, Yong A, et al. Cytomegalovirus seropositivity adversely influences outcome after T-depleted unrelated donor transplant in patients with chronic myeloid leukaemia: the case for tailored graft-versus-host disease prophylaxis. British journal of haematology. 2001 Jan;112(1):228–36. doi: 10.1046/j.1365-2141.2001.02519.x. [DOI] [PubMed] [Google Scholar]
- 14.Meijer E, Dekker AW, Rozenberg-Arska M, Weersink AJ, Verdonck LF. Influence of cytomegalovirus seropositivity on outcome after T cell-depleted bone marrow transplantation: contrasting results between recipients of grafts from related and unrelated donors. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2002 Sep 15;35(6):703–12. doi: 10.1086/342332. [DOI] [PubMed] [Google Scholar]
- 15.Salzberger B, Bowden RA, Hackman RC, Davis C, Boeckh M. Neutropenia in allogeneic marrow transplant recipients receiving ganciclovir for prevention of cytomegalovirus disease: risk factors and outcome. Blood. 1997 Sep 15;90(6):2502–8. [PubMed] [Google Scholar]
- 16.Battiwalla M, Wu Y, Bajwa RP, Radovic M, Almyroudis NG, Segal BH, et al. Ganciclovir inhibits lymphocyte proliferation by impairing DNA synthesis. Biology of blood and marrow transplantation: journal of the American Society for Blood and Marrow Transplantation. 2007 Jul;13(7):765–70. doi: 10.1016/j.bbmt.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 17.Nakamura R, Battiwalla M, Solomon S, Follmann D, Chakrabarti S, Cortez K, et al. Persisting posttransplantation cytomegalovirus antigenemia correlates with poor lymphocyte proliferation to cytomegalovirus antigen and predicts for increased late relapse and treatment failure. Biology of blood and marrow transplantation: journal of the American Society for Blood and Marrow Transplantation. 2004 Jan;10(1):49–57. doi: 10.1016/j.bbmt.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 18.Riddell SR, Watanabe KS, Goodrich JM, Li CR, Agha ME, Greenberg PD. Restoration of viral immunity in immunodeficient humans by the adoptive transfer of T cell clones. Science. 1992 Jul 10;257(5067):238–41. doi: 10.1126/science.1352912. [DOI] [PubMed] [Google Scholar]
- 19.Walter EA, Greenberg PD, Gilbert MJ, Finch RJ, Watanabe KS, Thomas ED, et al. Reconstitution of cellular immunity against cytomegalovirus in recipients of allogeneic bone marrow by transfer of T-cell clones from the donor. N Engl J Med. 1995 Oct 19;333(16):1038–44. doi: 10.1056/NEJM199510193331603. [DOI] [PubMed] [Google Scholar]
- 20.Micklethwaite K, Hansen A, Foster A, Snape E, Antonenas V, Sartor M, et al. Ex vivo expansion and prophylactic infusion of CMV-pp65 peptide-specific cytotoxic T-lymphocytes following allogeneic hematopoietic stem cell transplantation. Biology of blood and marrow transplantation: journal of the American Society for Blood and Marrow Transplantation. 2007 Jun;13(6):707–14. doi: 10.1016/j.bbmt.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 21.Cobbold M, Khan N, Pourgheysari B, Tauro S, McDonald D, Osman H, et al. Adoptive transfer of cytomegalovirus-specific CTL to stem cell transplant patients after selection by HLA-peptide tetramers. The Journal of experimental medicine. 2005 Aug 1;202(3):379–86. doi: 10.1084/jem.20040613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leen AM, Myers GD, Sili U, Huls MH, Weiss H, Leung KS, et al. Monoculture-derived T lymphocytes specific for multiple viruses expand and produce clinically relevant effects in immunocompromised individuals. Nat Med. 2006 Oct;12(10):1160–6. doi: 10.1038/nm1475. [DOI] [PubMed] [Google Scholar]
- 23.Park KD, Marti L, Kurtzberg J, Szabolcs P. In vitro priming and expansion of cytomegalovirus-specific Th1 and Tc1 T cells from naive cord blood lymphocytes. Blood. 2006 Sep 1;108(5):1770–3. doi: 10.1182/blood-2005-10-006536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Einsele H, Roosnek E, Rufer N, Sinzger C, Riegler S, Loffler J, et al. Infusion of cytomegalovirus (CMV)-specific T cells for the treatment of CMV infection not responding to antiviral chemotherapy. Blood. 2002 Jun 1;99(11):3916–22. doi: 10.1182/blood.v99.11.3916. [DOI] [PubMed] [Google Scholar]
- 25.Green ML, Leisenring W, Stachel D, Pergam SA, Sandmaier BM, Wald A, et al. Efficacy of a viral load-based, risk-adapted, preemptive treatment strategy for prevention of cytomegalovirus disease after hematopoietic cell transplantation. Biology of blood and marrow transplantation: journal of the American Society for Blood and Marrow Transplantation. 2012 Nov;18(11):1687–99. doi: 10.1016/j.bbmt.2012.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matsumura T, Narimatsu H, Kami M, Yuji K, Kusumi E, Hori A, et al. Cytomegalovirus infections following umbilical cord blood transplantation using reduced intensity conditioning regimens for adult patients. Biology of blood and marrow transplantation: journal of the American Society for Blood and Marrow Transplantation. 2007 May;13(5):577–83. doi: 10.1016/j.bbmt.2006.12.454. [DOI] [PubMed] [Google Scholar]
- 27.Saglio F, Hanley PJ, Bollard CM. The time is now: moving toward virus-specific T cells after allogeneic hematopoietic stem cell transplantation as the standard of care. Cytotherapy. 2014 Feb;16(2):149–59. doi: 10.1016/j.jcyt.2013.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.La Rosa C, Longmate J, Lacey SF, Kaltcheva T, Sharan R, Marsano D, et al. Clinical evaluation of safety and immunogenicity of PADRE-cytomegalovirus (CMV) and tetanus-CMV fusion peptide vaccines with or without PF03512676 adjuvant. The Journal of infectious diseases. 2012 Apr 15;205(8):1294–304. doi: 10.1093/infdis/jis107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ljungman P, Griffiths P, Paya C. Definitions of cytomegalovirus infection and disease in transplant recipients. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2002 Apr 15;34(8):1094–7. doi: 10.1086/339329. [DOI] [PubMed] [Google Scholar]
- 30.Takami A, Mochizuki K, Asakura H, Yamazaki H, Okumura H, Nakao S. High incidence of cytomegalovirus reactivation in adult recipients of an unrelated cord blood transplant. Haematologica. 2005 Sep;90(9):1290–2. [PubMed] [Google Scholar]
- 31.Tomonari A, Takahashi S, Ooi J, Tsukada N, Konuma T, Kato S, et al. Impact of cytomegalovirus serostatus on outcome of unrelated cord blood transplantation for adults: a single-institute experience in Japan. European journal of haematology. 2008 Mar;80(3):251–7. doi: 10.1111/j.1600-0609.2007.01006.x. [DOI] [PubMed] [Google Scholar]
- 32.Vij R, Khoury H, Brown R, Goodnough LT, Devine SM, Blum W, et al. Low-dose short-course intravenous ganciclovir as pre-emptive therapy for CMV viremia post allo-PBSC transplantation. Bone marrow transplantation. 2003 Oct;32(7):703–7. doi: 10.1038/sj.bmt.1704216. [DOI] [PubMed] [Google Scholar]
- 33.Khera N, Zeliadt SB, Lee SJ. Economics of hematopoietic cell transplantation. Blood. 2012 Aug 23;120(8):1545–51. doi: 10.1182/blood-2012-05-426783. [DOI] [PubMed] [Google Scholar]
- 34.Majhail NS, Mothukuri JM, Brunstein CG, Weisdorf DJ. Costs of hematopoietic cell transplantation: comparison of umbilical cord blood and matched related donor transplantation and the impact of posttransplant complications. Biology of blood and marrow transplantation: journal of the American Society for Blood and Marrow Transplantation. 2009 May;15(5):564–73. doi: 10.1016/j.bbmt.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 35.Gratama JW, Boeckh M, Nakamura R, Cornelissen JJ, Brooimans RA, Zaia JA, et al. Immune monitoring with iTAg MHC Tetramers for prediction of recurrent or persistent cytomegalovirus infection or disease in allogeneic hematopoietic stem cell transplant recipients: a prospective multicenter study. Blood. 2010 Sep 9;116(10):1655–62. doi: 10.1182/blood-2010-03-273508. [DOI] [PubMed] [Google Scholar]
- 36.Einsele H, Hebart H, Kauffmann-Schneider C, Sinzger C, Jahn G, Bader P, et al. Risk factors for treatment failures in patients receiving PCR-based preemptive therapy for CMV infection. Bone marrow transplantation. 2000 Apr;25(7):757–63. doi: 10.1038/sj.bmt.1702226. [DOI] [PubMed] [Google Scholar]
- 37.Ito S, Pophali P, Co W, Koklanaris EK, Superata J, Fahle GA, et al. CMV reactivation is associated with a lower incidence of relapse after allo-SCT for CML. Bone marrow transplantation. 2013 Oct;48(10):1313–6. doi: 10.1038/bmt.2013.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Elmaagacli AH, Steckel NK, Koldehoff M, Hegerfeldt Y, Trenschel R, Ditschkowski M, et al. Early human cytomegalovirus replication after transplantation is associated with a decreased relapse risk: evidence for a putative virus-versus-leukemia effect in acute myeloid leukemia patients. Blood. 2011 Aug 4;118(5):1402–12. doi: 10.1182/blood-2010-08-304121. [DOI] [PubMed] [Google Scholar]
- 39.Green ML, Leisenring WM, Xie H, Walter RB, Mielcarek M, Sandmaier BM, et al. CMV reactivation after allogeneic HCT and relapse risk: evidence for early protection in acute myeloid leukemia. Blood. 2013 Aug 15;122(7):1316–24. doi: 10.1182/blood-2013-02-487074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mackinnon S, Thomson K, Verfuerth S, Peggs K, Lowdell M. Adoptive cellular therapy for cytomegalovirus infection following allogeneic stem cell transplantation using virus-specific T cells. Blood cells, molecules & diseases. 2008 Jan-Feb;40(1):63–7. doi: 10.1016/j.bcmd.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 41.Peggs KS, Thomson K, Samuel E, Dyer G, Armoogum J, Chakraverty R, et al. Directly selected cytomegalovirus-reactive donor T cells confer rapid and safe systemic reconstitution of virus-specific immunity following stem cell transplantation. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2011 Jan 1;52(1):49–57. doi: 10.1093/cid/ciq042. [DOI] [PubMed] [Google Scholar]
- 42.Heslop HE, Leen AM. T-cell therapy for viral infections. Hematology/the Education Program of the American Society of Hematology American Society of Hematology Education Program. 2013;2013:342–7. doi: 10.1182/asheducation-2013.1.342. [DOI] [PubMed] [Google Scholar]
- 43.Gerdemann U, Katari UL, Papadopoulou A, Keirnan JM, Craddock JA, Liu H, et al. Safety and clinical efficacy of rapidly-generated trivirus-directed T cells as treatment for adenovirus, EBV, and CMV infections after allogeneic hematopoietic stem cell transplant. Molecular therapy: the journal of the American Society of Gene Therapy. 2013 Nov;21(11):2113–21. doi: 10.1038/mt.2013.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gerdemann U, Keirnan JM, Katari UL, Yanagisawa R, Christin AS, Huye LE, et al. Rapidly generated multivirus-specific cytotoxic T lymphocytes for the prophylaxis and treatment of viral infections. Molecular therapy: the journal of the American Society of Gene Therapy. 2012 Aug;20(8):1622–32. doi: 10.1038/mt.2012.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Blyth E, Clancy L, Simms R, Ma CK, Burgess J, Deo S, et al. Donor-derived CMV-specific T cells reduce the requirement for CMV-directed pharmacotherapy after allogeneic stem cell transplantation. Blood. 2013 May 2;121(18):3745–58. doi: 10.1182/blood-2012-08-448977. [DOI] [PubMed] [Google Scholar]
- 46.Meij P, Jedema I, Zandvliet ML, van der Heiden PL, van de Meent M, van Egmond HM, et al. Effective treatment of refractory CMV reactivation after allogeneic stem cell transplantation with in vitro-generated CMV pp65-specific CD8+ T-cell lines. Journal of immunotherapy (Hagerstown, Md: 1997) 2012 Oct;35(8):621–8. doi: 10.1097/CJI.0b013e31826e35f6. [DOI] [PubMed] [Google Scholar]
- 47.Leen AM, Bollard CM, Mendizabal AM, Shpall EJ, Szabolcs P, Antin JH, et al. Multicenter study of banked third-party virus-specific T cells to treat severe viral infections after hematopoietic stem cell transplantation. Blood. 2013 Jun 27;121(26):5113–23. doi: 10.1182/blood-2013-02-486324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kharfan-Dabaja MA, Boeckh M, Wilck MB, Langston AA, Chu AH, Wloch MK, et al. A novel therapeutic cytomegalovirus DNA vaccine in allogeneic haemopoietic stem-cell transplantation: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Infect Dis. 2012 Apr;12(4):290–9. doi: 10.1016/S1473-3099(11)70344-9. [DOI] [PubMed] [Google Scholar]
- 49.Schleiss MR. Cytomegalovirus vaccines: at last, a major step forward. Herpes: the journal of the IHMF. 2009 Jan;15(3):44–5. [PMC free article] [PubMed] [Google Scholar]
- 50.Grigoleit GU, Kapp M, Hebart H, Fick K, Beck R, Jahn G, et al. Dendritic cell vaccination in allogeneic stem cell recipients: induction of human cytomegalovirus (HCMV)-specific cytotoxic T lymphocyte responses even in patients receiving a transplant from an HCMV-seronegative donor. The Journal of infectious diseases. 2007 Sep 1;196(5):699–704. doi: 10.1086/520538. [DOI] [PubMed] [Google Scholar]
- 51.Peggs KS, Verfuerth S, Pizzey A, Khan N, Guiver M, Moss PA, et al. Adoptive cellular therapy for early cytomegalovirus infection after allogeneic stem-cell transplantation with virus-specific T-cell lines. Lancet. 2003 Oct 25;362(9393):1375–7. doi: 10.1016/S0140-6736(03)14634-X. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary table Ia. Comparison of average hospitalization days between antiviral group and no therapy group.
Supplementary table Ib. Calculation of average cost per patient in the antiviral therapy group (N=90).