Significance
Asthmatic inflammation is orchestrated by T-lymphocyte cell trafficking network within lungs, blood circulation, secondary lymphoid organ, and peripheral tissue. Here, we demonstrated that T cell and following eosinophil recruitment was substantially reduced in our recently generated mouse model, where heparan sulfate synthase exostoses-1 (Ext1) is knockout in an inducible manner. Moreover, we discovered that even a monosaccharide, 2,4-disulfated iduronic acid (Di-S-IdoA), bound to chemokine CCL20 and significantly inhibited CCL20 binding to heparan sulfate and to endothelial cell surface. We found that Di-S-IdoA attenuated asthmatic reaction, measured by T cell, eosinophil, and CCL20 recruitment in asthmatic mice. These findings show for the first time (to our knowledge) that sulfate monosaccharide can be developed into a potent therapeutic agent for treating asthma.
Keywords: sulfated monosaccharide, lymphocyte recruitment
Abstract
Identification of carbohydrate sequences that determine affinity to specific chemokines is a critical step for strategies to interfere with chemokine-mediated leukocyte trafficking. Here, we first characterized the development of allergic asthma in Tie2-dependent and inducible Ext1-knockout (Tie2-Ext1iKO) mice. We showed that heparan sulfate is essential for leukocyte recruitment in the peribronchial region and bronchoalveolar lavage fluid (BALF), and is crucial for induction of airway hyperresponsiveness. Our glycan microarray showed a unique affinity profile of chemokine CCL20 to substructures of heparin and heparin-like oligo/di/monosaccharides. Among them, we identified a synthetic and not naturally occurring monosaccharide, 2,4-O-di-sulfated iduronic acid (Di-S-IdoA), as a potential inhibitor for CCL20–heparan sulfate interaction. Mice injected with Di-S-IdoA via tail vain or nasal inhalation showed attenuated leukocyte recruitment into inflammatory sites and BALF. These results demonstrate a critical role of chemokine–heparan sulfate interaction in the asthma development and Di-S-IdoA as a potential drug for asthma treatment.
Asthma is a common allergic disease characterized by chronic airway inflammation, mucus hypersecretion, and airway hyperreactivity to inhaled allergens (1). Despite the importance of T lymphocytes in adaptive immunity and host defense, their accumulation in airway in allergic asthma causes Th2-mediated pulmonary inflammation. The asthmatic inflammatory response is orchestrated by T-cell trafficking network among lung, blood circulation, secondary lymphoid organ, and peripheral tissue (2). Of note, the significant increase of T cells in the airway in asthma is mostly due to T-cell recruitment from regional lymph nodes rather than their proliferation at the inflamed site (3). Therefore, a therapeutic approach that shuts off the trafficking pathway of pathogenic T cells should significantly inhibit the Th2-mediated inflammation in allergic asthma.
It is well known that the destination of T-cell trafficking pathway is tightly restricted by the profile of chemokines, lipid chemoattractants, and T-cell chemokine receptors. As a part of immune surveillance, naïve T cells and central memory T cells constantly access secondary lymphoid organs from blood circulation via specialized high endothelial venules (HEVs). The interaction between T cells and HEV cells includes in a stepwise manner (4, 5), L-selectin–dependent tethering and rolling, activation, firm arrest, and transendothelial migration. Besides 6-sulfo sialyl Lewis X as a L-selectin ligand, HEVs constitutively express chemokine CCL21 and CCL19 and attract T cells that express its cognate receptor CCR7 (5). In contrast to this homeostatic homing, circulating T cells interact with inflamed blood vessels in lung after asthmatic exposure to an inhaled allergen. Among numerous combinations of chemokines and their receptors, there is considerable evidence that CCL20 and its cognate receptor CCR6 may contribute to the pathogenesis of asthma (6). CCL20-CCR6 plays a key role in the recruitment of Th17 (7) cells and Th2 cells (8). Indeed, CCL20 is highly enriched on inflammatory epithelium (9) and CCR6 is expressed on memory T cells infiltrated in the lung during allergic inflammation (7). In addition, CCR6-deficient mice have decreased airway responsiveness, and reduced recruitment of eosinophils into lung (10, 11). These findings suggest that CCL20-CCR6 axis is a putative target for the treatment of asthma.
Cumulative evidence in vivo and in vitro indicates that chemokines cannot be functionally active in HEVs and inflamed sites without their interaction with heparan sulfate (12). Heparan sulfate protects chemokines from proteolysis, immobilizes them on the endothelium surface and produces chemokine gradients in the vasculature. Heparan sulfate is composed of repeating disaccharide units of uronic acid [glucuronic acid (GlcA) or iduronic acid (IdoA)] and N-acetylglucosamine (GlcNAc) carbohydrates. Some of GlcA (or IdoA) carbohydrates are subsequently O-sulfated, and GlcNAc carbohydrates are partially modified with N-deacetylation and N-sulfation (13). Previous reports have indicated that the sulfation patterns in heparan sulfate are more restricted than expected (14), and the sulfation is associated with respiratory distress (15) and asthma (16). It is believed that there are specific interactions between heparan sulfate and chemokines (17). Nevertheless, the nontemplate nature of long carbohydrate chains (<25,000 disaccharide units) and conformational plasticity still make it difficult to identify the common sequences of heparan sulfate that display affinity to specific chemokines. In this regard, our previously established glycan microarray system (18, 19) is a powerful tool to define the selectivity in heparan sulfate–chemokine interactions.
Recently, we established the exostoses-1 (Ext1) gene conditional knockout Tie2-Ext1iKO mouse model, in which GlcA/IdoA-GlcNAc repeat of heparan sulfate can be abrogated in endothelial cells in a tetracycline-inducible manner (20). In this study, Tie2-Ext1iKO mouse showed significant reduction of both leukocyte recruitment to lung tissues and of airway hyperresponsiveness in ovalbumin (OVA) asthma model. Moreover, glycan microarray analysis surprisingly identified that an unnatural and synthetic monosaccharide, 2,4-O-di-sulfated iduronic acid (Di-S-IdoA) has a high affinity to recombinant CCL20. Intravenous and inhalation challenges of Di-S-IdoA significantly inhibited the leukocyte infiltration in bronchoalveolar lavage fluid (BALF). Our finding that even a monosaccharide can attenuate airway inflammation suggests its potential use as an antiasthma therapy that can be administered by inhalation.
Results
Tie2-Ext1iKO Mice Show Diminished T-Cell Accumulation and Inflammatory Response in the Lung in a Mouse Model of OVA-Induced Asthma.
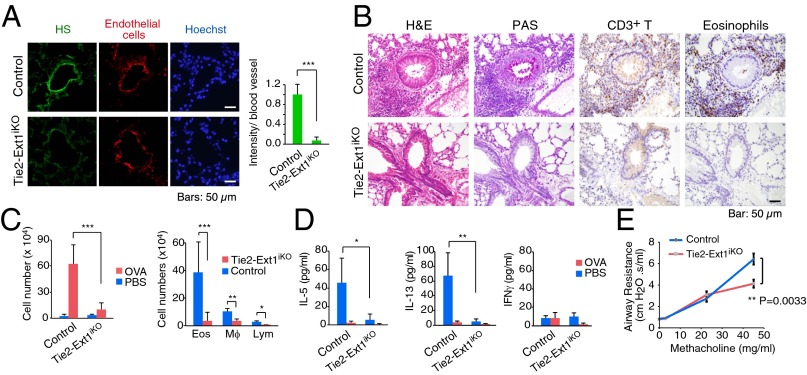
Recently, we have developed Tie2-Ext1iKO mouse line in which embryonic lethality of homozygous Ext1−/− mouse is avoidable. Indeed, there was little expression of endothelial heparan sulfate in the lungs of Tie2-Ext1iKO mice (Fig. 1A). To determine the role of endothelial heparan sulfate in asthma, we sensitized and challenged these mice with OVA allergen. In OVA-challenged control mice, periodic acid–Schiff (PAS) staining showed significant mucus accumulation in airway epithelium. In addition, a large number of CD3+ T cells and eosinophils were detected in the peribronchial regions (Fig. 1B). In contrast, there was little infiltration of CD3+ T cells and eosinophil cells, as well as mucus secretion in Tie2-Ext1iKO mice. There was also a significant reduction of total cell number including lymphocytes, macrophages, and eosinophils in BALF in Tie2-Ext1iKO mice (Fig. 1C). Moreover, whereas BALF in control mice contained high levels of Th2 cytokines IL-5 and IL-13 but not the Th1 cytokine IFN-γ, only low levels of IL-5 and IL-13 could be detected in that of Tie2-Ext1iKO (Fig. 1D), suggesting that OVA-triggered Th2 activation was abolished in Tie2-Ext1iKO mice.
Fig. 1.
Reduced OVA-induced airway inflammation and airway responsiveness in endothelial bheparan sulfate-deficient mice. (A) Immunofluorescent staining of heparan sulfate in the lung endothelial cells from WT and endothelial heparan sulfate-deficient mice (Tie2-Ext1iKO). Quantification of fluorescent intensity is shown at Right. (B) Histological and immunohistochemical staining of the mouse lung sections from WT and Tie2-Ext1iKO mice challenged with OVA. Representative images of the infiltrating eosinophils and T cells near airway lumens are shown. (C) Quantification of leukocyte infiltration in the bronchoalveolar lavage fluid (BALF) from the OVA-challenged mice with the indicated genotypes. (D) Cytokine measurement in the lung lavage from OVA-challenged mice. (E) Reduced airway responsiveness in Tie2-Ext1iKO mice in OVA-challenged mice (n = 8 mice per group). The degree of airway responsiveness to PBS or methacholine (MCh) at 0, 3, 24, and 48 mg/mL was determined. Error bars indicate SD. Unpaired two-tailed Student t test was used for statistical analysis. P value less than 0.05 was considered significant (*). *P < 0.05, **P < 0.01, and ***P < 0.001.
To determine the effect of deficiency in heparan sulfate on airway responsiveness, methacholine (MCh) responsiveness was assessed in OVA-challenged Tie2-Ext1iKO mice. Compared with OVA-challenged control mice, OVA-challenged Tie2-Ext1iKO mice showed significantly less response to MCh at 48 mg/mL (P = 0.0033) (Fig. 1E). Taken together, these results indicate that endothelial heparan sulfate is essential for lymphocyte accumulation at sites of allergic inflammation in the lung, and that the reduced accumulation is associated with less airway responsiveness.
Interruption of Lymphocyte Trafficking Pathway Attenuates Asthmatic Response.
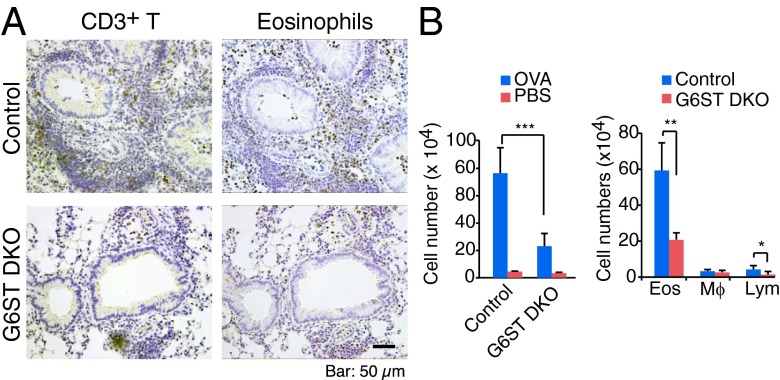
We have previously reported that endothelial heparan sulfate is required for lymphocyte recruitment to secondary lymph nodes. Here, we hypothesized that the attenuation of the OVA induced asthmatic response in Tie2-Ext1iKO mice is due to blocking of lymphocyte recruitment from regional lymph nodes. Previous studies have demonstrated that the homeostatic lymphocyte trafficking pathway was impaired in L-selectin ligand-deficient [GlcNAc6ST-1/2 double knockout (G6ST DKO)] mice (21, 22). To evaluate the contribution of homeostatic lymphocyte trafficking to the lung, G6ST DKO mice were challenged with OVA. Similar to Tie2-Ext1iKO mice, G6ST DKO mice had significantly fewer CD3+ T cells and eosinophil cells around the airway walls compared with control mice (Fig. 2A). Moreover, G6ST DKO mice BALF contained significantly fewer eosinophils and lymphocytes (Fig. 2B). These data support that accumulation of lymphocytes in lung tissue is mainly contributed by their recruitment from regional lymph nodes.
Fig. 2.
Reduced OVA-induced airway inflammation in L-selectin ligand-deficient mice. (A) Immunohistochemical staining of control or GlcNAc6ST-1/2 double-knockout (G6ST DKO) mice challenged with OVA. (B) Number of infiltrating cells in BALF recovered from control or G6ST DKO mice. Quantification of total BALF cells (Left) and each cell type (Right) are shown. Error bars indicate SD. *P < 0.05, **P < 0.01, and ***P < 0.001.
Glycan Microarray Showed Chemokine CCL20 Binds Short Heparin-Like Sulfated Oligosaccharide.
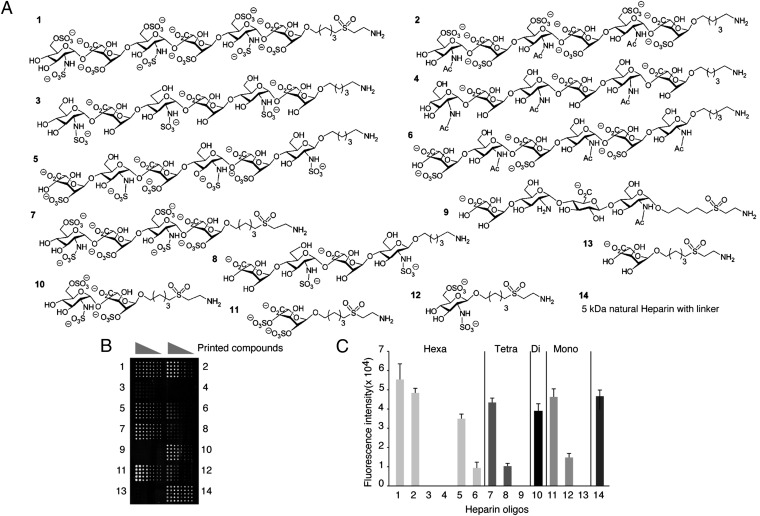
Our previous studies have indicated that heparan sulfate is important to maintain morphologic localization of the homeostatic chemokine CCL21 (20). Here, the results from OVA-challenged Tie2-Ext1iKO asthmatic mice led us suggest that heparan sulfate also interacts with inflammation-related chemokines such as CCL20. Thus, we analyzed the binding profile of CCL20 to a heparin-like carbohydrate library, which contains carbohydrates with various sulfation patterns ranging from monosaccharides to hexasaccharides (Fig. 3A) by our glycan microarray system (18, 19). The screening results showed that CCL20 strongly bound to hexasaccharides 1, 2, and moderately bound to hexasaccharide 5 and tetrasaccharide 7. This indicates that the affinity of CCL20 toward heparan sulfate becomes higher with increasing sulfations levels (Fig. 3 B and C). Notably, CCL20 exhibited high affinity to disaccharide 10 and even monosaccharide 11. Observed fluorescent intensities of those carbohydrates were comparable to 5-kDa natural heparin.
Fig. 3.
Binding of CCL20 to heparin-like oligosaccharides on a glycan microarray. (A) Compounds tested for glycan microarray. (B) Binding of recombinant human CCL20 to heparin oligosaccharide-like glycans on a microarray. Numbers 1–14 donate different glycan structures. Each sugar was printed on the slide at four different concentrations ranging from 1,000, 250, 63, and 16 μM in 10 replicas. (C) Quantification of the binding of CCL20 to the heparin oligosaccharide-like glycans shown in B (n = 10; concentration of printed compounds, 250 µM).
A Unique and Unnatural Monosaccharide Di-S-IdoA Inhibits Heparan Sulfate–CCL20 Interaction.
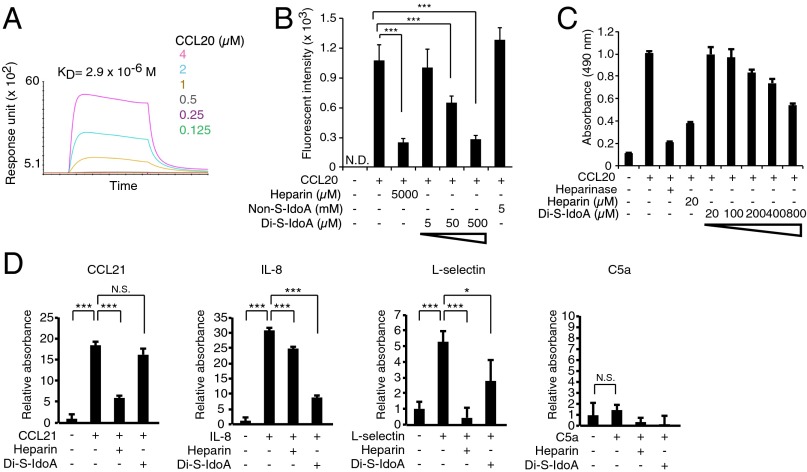
Monosaccharide 11 is Di-S-IdoA, which contains two axial sulfate groups. Because Di-S-IdoA is an unnatural synthetic monosaccharide that has never been isolated from a natural source, we were prompted to further characterize its interaction with CCL20 in more detail. The data from surface plasmon resonance (SPR) showed that CCL20 binds to immobilized Di-S-IdoA in the micromolar range (KD = 2.9 × 10−6 M) (Fig. 4A). We next analyzed the inhibitory effect of Di-S-IdoA. Interaction between immobilized hexasaccharides 1 and CCL20 was blocked by Di-S-IdoA in a concentration-dependent manner and 500 µM Di-S-IdoA inhibited at a comparable level of 5,000 µM heparin (Fig. 4B), whereas nonsulfated IdoA showed no such inhibitory activity at 5 mM. We next synthesized Di-S-IdoA with aminopentyl linker (Figs. S1 and S2). Interestingly, in the cultured F2 cell, which is known to express endogenous heparan sulfate, Di-S-IdoA also interfered with the CCL20–heparan sulfate interactions in a dose-dependent manner (Fig. 4C). These results suggest that Di-S-IdoA is an effective as a functional inhibitor of CCL20 chemokine activity. To next study the specificity of Di-S-IdoA, the inhibitory effect of Di-S-IdoA on the bindings between the various proteins and endothelial cells was assayed. It is known that CCL21 (23, 24), IL-8 (23, 25), L-selectin (26, 27), and complement component 5a (C5a) (28, 29) are involved both in the binding to heparin/heparan sulfate in vitro and in the asthma pathogenesis. The result showed that Di-S-IdoA did not block the attachment of CCL21 to mouse endothelial F2 cells, whereas heparin efficiently blocked (Fig. 4D). Di-S-IdoA significantly blocked the binding of L-electin to F2 cells. However, Di-S-IdoA showed even higher inhibitory effect than heparin in IL-8 binding. In this experimental model, C5a did not show any bindings to F2 cells. Those results indicate Di-S-IdoA has unique binding preferences distinct from heparin.
Fig. 4.
An affinity of unnatural 2,4-di-sulfated Iduronic acid (Di-S-IdoA) to CCL20 and its inhibitory effect on CCL20-heparan sulfate interaction. (A) Binding kinetics of CCL20 to immobilized Di-S-IdoA (monosaccharide 11) by SPR. (B) Inhibition assay with heparin oligosaccharide-immobilized plate. Binding of CCL20 to heparin-coated plate was assayed in the presence of soluble heparin, nonsulfated IdoA (Non-S-IdoA), and various concentration of Di-S-IdoA (n = 30). (C) Inhibitory activity of Di-S-IdoA in the binding of CCL20 to F2 endothelial cells. Heparin was included as a control. Heparinase treatment preceded the addition to the cells. The data are representative of two experiments with similar results. (D) Inhibitory activity of Di-S-IdoA in the binding of various proteins to F2 endothelial cells. Di-S-IdoA (800 µM) or heparin (20 µM) was used for the inhibition. Error bars indicate SD. *P < 0.05 and ***P < 0.001.
Inhibitory Effect of Di-S-IdoA in OVA-Challenged WT Mice.
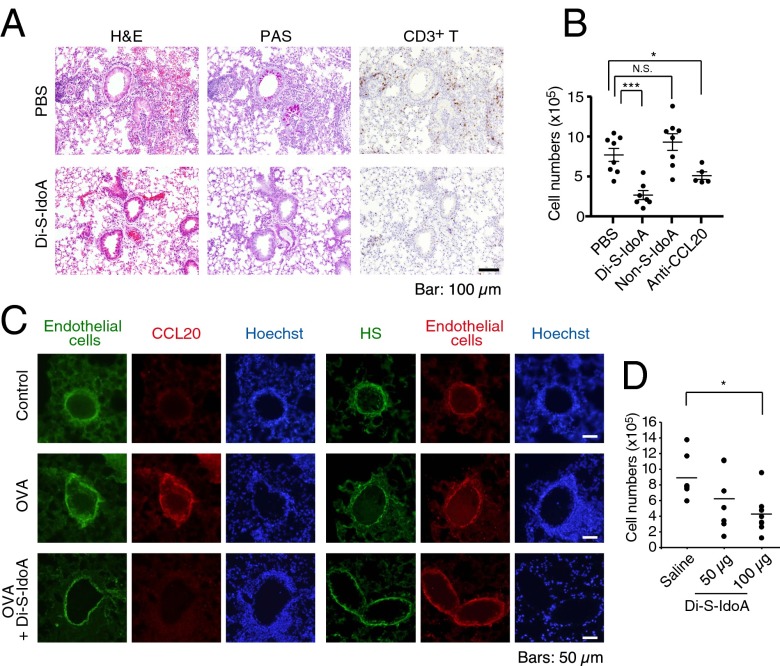
To test the possibility of Di-S-IdoA as an inhibitor of CCL20 activity in vivo, we injected either PBS or Di-S-IdoA into tail vein right before the reexposure of OVA in WT mice. Pretreatment of Di-S-IdoA decreased the mucus secretion and recruitment of CD3+ T cells in the lung tissues (Fig. 5A). We also observed a reduced total leukocyte number in Di-S-IdoA–treated and neutralizing anti-CCL20 antibody-treated mice but not nonsulfated IdoA (Non-S-IdoA)-treated mice (Fig. 5B). These results suggest that i.v. administered Di-S-IdoA can access at the inflamed postcapillary venule, where it inhibits the activity of chemoattractants.
Fig. 5.
Di-S-IdoA administration attenuated OVA-induced airway inflammation. (A) Histology and immunohistochemical staining of lungs from mice receiving either PBS or Di-S-IdoA. (B) Quantification of infiltrating T cells in the airway of mice receiving PBS, Di-S-IdoA, Non-S-IdoA, or neutralizing anti-CCL20 antibody (n = 5–8 mice per group). Result are shown as mean ± SEM. *P < 0.05 and ***P < 0.001. (C) Immunofluorescent staining of CCL20, heparan sulfate, and endothelial cells in the lung from pulmonary Di-S-IdoA–inhaled mice. Images are representatives from mice treated with PBS or Di-S-IdoA (100 µg), respectively. Note that the primary antibody against CCL20 was injected through the tail vein before the collection of lung tissue. (D) Pulmonary administration of Di-S-IdoA decreases OVA-induced airway inflammation. Leukocyte infiltration into BALF following inhalation pretreatment with either saline or different concentration of Di-S-IdoA (50 and 100 µg) (n = 5–7 mice per group). *P < 0.05.
Because CCL20 has been demonstrated to be mainly synthesized from airway epithelial cells, we hypothesized that administration of Di-S-IdoA through intranasal inhalation would interfere the localization and presentation on the vascular endothelial cell surface in inflamed lung. To test the accessibility of Di-S-IdoA through the lung alveoli, we administered Di-S-IdoA by inhalation before OVA challenge in WT mice. Indeed, we found robust expression of CCL20 on the vascular endothelial cell wall in the OVA-challenged WT mice. In contrast, there was little expression of CCL20 in the Di-S-IdoA–pretreated mice, although heparan sulfate is present on vascular surface (Fig. 5C). These data suggest that CCL20 presentation on the endothelial cells at the lung inflammatory site was blocked by Di-S-IdoA administration. Indeed, the number of total leukocytes in BALF was dramatically decreased in 100 µg of Di-S-IdoA treatment (P < 0.05) compared with saline-treated mice (Fig. 5D). Blood tests from the WT mice treated with Di-S-IdoA showed no side effect in liver function, kidney function, and blood cell count (Table S1). Taken together, our results demonstrated that inhalation administration of Di-S-IdoA was effective in reducing airway inflammation in allergen-challenged WT mice.
Discussion
Despite extensive research efforts in the past decades, the prevalence and severity of asthma are still increasing in the developed countries. The pathology of allergic asthma is directly linked to the consequence of long-term chronic Th2-mediated inflammation in the lung at the site of continuous exposure to allergens (30). Previous report has showed that a partial loss of N-sulfate in heparan sulfate contributes to attenuation of airway inflammation (16). However, the role of the main chain of heparan sulfate in the pathogenesis of asthma had not been addressed because of the embryonic lethality of homozygous Ext1−/− mouse. In this study, we used our established inducible gene knockout system to circumvent this lethality (20). In contrast to WT mice, Th2-mediated allergic inflammation in the lung in OVA-challenged Tie2-Ext1iKO mice was abolished as assessed by eosinophil recruitment to the lung, and eosinophil, macrophage, and lymphocyte infiltration into BALF. Moreover, Tie2-Ext1iKO mice showed lower airway hyperresponsiveness to MCh than control mice did, indicating that GlcA/IdoA-GlcNAc repeat of endothelial heparan sulfate is not only essential for lymphocyte, eosinophil, and macrophage recruitment, but also for airway responsiveness a cardinal feature of asthma. Importantly, we have shown that the proliferation and rolling ability of lymphocytes in Tie2-Ext1iKO mice do not change during homing (20). In another report, lymphocyte-specific deletion of heparan sulfate has little effects on their differentiation (31). Our results also showed that selectin ligand-deficient G6ST DKO mice had decreased lymphocyte recruitment into the lung. These results not only reemphasize the importance of T-cell trafficking in the pathogenesis of asthma, but also indicate the potential importance of blocking of this T-cell trafficking pathway as an effective therapeutic strategy for asthma.
Among the asthma-associated chemokines, the role of CCL20 in asthma is only beginning to be understood and a large part of its physiological significance remains to be elucidated. Given that affinity between chemokine and heparan sulfate also varies depending on type of chemokines (18), we hypothesized there are specific oligosaccharide patterns preferable for binding to CCL20. In this study, a glycan microarray identified unnatural and synthetic monosaccharide Di-S-IdoA, which has two axial O-sulfations at C2 and C4 positions, as a potent inhibitor of CCL20. This finding was surprising because previously seven other chemokines including CCL21 did not show high affinities for Di-S-IdoA as equivalent to sulfated hexasaccharides (18). In addition, the data that nonsulfated IdoA did not show any binding to CCL20 suggests that two sulfate groups are important to form a pool of negative charge on one side and this ionic charge contributed to interaction with CCL20. Furthermore, inhalation as well as i.v. administration of this compound significantly decreased lymphocyte recruitment into the allergen-challenged lung inflammatory site, suggesting that CCL20-mediated Th17 and Th2 cell recruitments in early phase is critical for following disease development including eosinophil, macrophage recruitments.
Heparin, a soluble analog of heparan sulfate, and its derivatives have an antiinflammatory activity as well as an anticoagulant effect. A role for heparin in improving symptoms in inflammatory disease was demonstrated in neonatally immunized rabbits (32), naturally sensitized sheep (33), and asthma patients in clinical trials (34, 35). The mechanism of its antiinflammatory effect is likely due to the interference with the chemokine retention on vascular heparan sulfate, blockage of interleukins, selectins, and complement components. In this regard, a therapeutic approach using heparin-derived low–molecular-weight saccharides would be beneficial (12). However, so far, X-ray crystallography of natural heparin revealed that a disaccharide unit of heparin is not sufficient and at least tetrasaccharide is necessary to inhibit activity of chemokine RANTES in vivo (36). In this context, our result that a monosaccharide with unusual sulfation pattern can interfere with the chemokine–heparan sulfate interaction is a novel finding. Taken together, our findings strongly suggest that Di-S-IdoA binds to CCL20, resulting in inhibiting CCL20 binding to heparan sulfate and to the cellular receptor, CCR6.
As a whole, our results have demonstrated an essential role of endothelial heparan sulfate in T-cell trafficking into lung and thus in the pathogenesis of allergic asthma. We identified a novel monosaccharide Di-S-IdoA as a potent inhibitor of chemokine CCL20. With the establishment of this new tool for CCL20, we determined the effect of Di-S-IdoA in lymphocyte recruitment into the allergen-challenged lung inflammation site. Pulmonary inhalation drug delivery of this compound may help to attenuate asthmatic symptoms by suppressing chemokine-mediated inflammatory responses, mucus production, and airway responsiveness.
Materials and Methods
Reagents and Animals.
Tie2-Ext1iKO mice were generated and maintained as reported (20). To induce the deletion of endothelial heparan sulfate, mice of 4-wk age were treated with doxycycline for 3 consecutive weeks before experiments. All protocols for animal experiments were approved by the Institutional Animal Care and Use Committee (IACUC) of the Sanford-Burnham Medical Research Institute, and were conducted in accordance with National Institutes of Health guidelines. OVA was purchased from Sigma, heparan sulfate antibody 3G10 from US Biological, recombinant human CCL20 from R&D and Shenandoah Biotech, recombinant human CCL21 from Sino Biological, recombinant human IL-8 from Cell Sciences, recombinant mouse L-selectin from Life Technologies, recombinant human C5a from BioVision, eosinophil anti-MBP antibody (Dr. James Lee, Mayo Clinic, Scottsdale, AZ) (37), anti-CD3ε antibody from Santa Cruz, anti-macrophage inflammatory protein 3α (CCL20) antibody from Abcam, neutralizing anti-CCL20 antibody and anti-CCL21 antibody from R&D, anti-His antibody from Clontech, and anti-von Willebrand factor antibody from Dako.
OVA-Induced Model of Asthma in Mice.
For sensitization, mice were intraperitoneally administrated a suspension containing 50 µg of OVA and 0.5 mg of aluminum hydroxide (Sigma) in 200 µL of PBS on day 0 and day 12. On days 23, 25, and 27, the mice were briefly anesthetized with isoflurane and challenged intranasally with 20 µg of OVA in 50 µL of PBS through the nose. For studies with a therapeutic intervention, 100 µg of Di-S-IdoA or Non-S-IdoA, or 50 µg of neutralizing anti-CCL20 antibody was administered either i.v. or inhalationally 30 min before each OVA challenge. Twenty-four hours after the last OVA challenge, the mice were killed and the lung bronchoalveolar lavage was performed by infusion of 1 mL of 0.1% PBS through the trachea with Surflash IV catheters (Terumo). The lung lavage was subjected to leukocyte counting by a cytometer, and each subpopulation was analyzed by cytospin followed by a stain with Hema-3 (Fisher).
Immunohistochemistry.
Mouse lung lobes were fixed with 4% (wt/vol) PFA followed by paraffin embedding and histological examination by H&E staining and PAS staining. For evaluation of immune infiltration into lung inflammatory sites, tissues were stained by anti-CD3ε antibody and anti-MBP antibody described above.
Airway Responsiveness to MCh.
Airway responsiveness to MCh was assayed 24 h after the final OVA challenge in anesthetized, intubated, and ventilated mice as previously described (38). Intubated and ventilated mice were anesthetized intraperitoneally. The dynamic airway resistance was determined in mice exposed to nebulized PBS or MCh at 0, 3, 24, and 48 mg/mL.
Glycan Microarray.
Microarrays were fabricated as previously reported (39). Microarray slide was incubated with 100 µL (5 µg) of rhCCL20 (R&D Systems) in HBS-N containing 0.01% Tween 20 for 1 h at room temperature under mild shaking, washed three times with HBS-N, and dried by centrifugation. The slide was then reacted with 100 µL (2 µg) of anti-hCCL20 goat IgG (R&D Systems) for 1 h, followed by incubation with anti-goat Alexa Fluor 594 antibody (Invitrogen) for 1 h. Slides were scanned with a GenePix 4300A microarray scanner and analyzed by GenePix Pro software. Binding of CCL20 to immobilized heparin-like oligosaccharide 1 in the presence of Non-S-IdoA and Di-S-IdoA was observed in the same manner. The amino group of each monosaccharide sample was acylated before use by treatment with a solution of acetic anhydride–triethylamine–methanol (2:3:5, vol/vol/vol) followed by condensation and lyophilization. The inhibition solution contained 5 mM 5-kDa heparin (Santa Cruz Biotechnology), 5 mM Non-S-IdoA, or 5, 50, and 500 µM Di-S-IdoA.
Immunohistochemistry.
For heparan sulfate staining, tissue sections were treated with heparatinase at 37 °C for 1 h before incubation with anti-heparan sulfate 3G10 antibody. Alexa Fluor 568 anti-mouse IgG2b was used for secondary reaction. Vascular endothelial cells were visualized by anti-von Willebrand factor antibody (1:100 dilution). Lung endothelial CCL20 could not be visualized by the general fluorescent staining protocol. Instead, localization of blood vessel CCL20 was examined by i.v. injection of anti-CCL20 antibody (25 µg per mouse) through mouse tail vein. Thirty minutes after the injection, lungs were collected and frozen in OCT compound (Tissue Tek). Frozen tissue sections were then incubated with Alexa Fluor 568 anti-rabbit IgG (Invitrogen).
To avoid the cross-reaction in double staining with CCL20, sections were blocked with anti-rabbit IgG antibody and protein A. Then the sections were incubated with anti-von Willebrand factor antibody, followed by incubation with FITC–anti-rabbit IgG-Fc antibody (Jackson ImmunoResearch).
Statistical Analyses.
All data are showed as means ± SD. Unpaired two-tailed Student t test was used for statistical analyses. We considered P values of less than 0.05 as statistically significant. Degrees of statistical significance are presented as *P < 0.05, **P < 0.01, and ***P < 0.001.
Supplementary Material
Acknowledgments
We thank Dr. S. Rosen (University of California, San Francisco) for helpful suggestions for lung CCL20 staining, Dr. P. Rosenthal (University of California, San Diego) for help with assay of methacholine responsiveness, and Dr. M. N. Hecht for help with the microarray and SPR experiments. This work was supported by National Institutes of Health Grants P01 CA71932 (to M.F.) and AI 107779, AI 38425, AI 70535, and AI 72115 (to D.H.B.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1319870111/-/DCSupplemental.
References
- 1.Busse WW, Lemanske RF., Jr Asthma. N Engl J Med. 2001;344(5):350–362. doi: 10.1056/NEJM200102013440507. [DOI] [PubMed] [Google Scholar]
- 2.Medoff BD, Thomas SY, Luster AD. T cell trafficking in allergic asthma: The ins and outs. Annu Rev Immunol. 2008;26:205–232. doi: 10.1146/annurev.immunol.26.021607.090312. [DOI] [PubMed] [Google Scholar]
- 3.Harris NL, Watt V, Ronchese F, Le Gros G. Differential T cell function and fate in lymph node and nonlymphoid tissues. J Exp Med. 2002;195(3):317–326. doi: 10.1084/jem.20011558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McEver RP, Cummings RD. Role of PSGL-1 binding to selectins in leukocyte recruitment. J Clin Invest. 1997;100(11) Suppl:S97–S103. [PubMed] [Google Scholar]
- 5.Förster R, Davalos-Misslitz AC, Rot A. CCR7 and its ligands: Balancing immunity and tolerance. Nat Rev Immunol. 2008;8(5):362–371. doi: 10.1038/nri2297. [DOI] [PubMed] [Google Scholar]
- 6.Thomas SY, Banerji A, Medoff BD, Lilly CM, Luster AD. Multiple chemokine receptors, including CCR6 and CXCR3, regulate antigen-induced T cell homing to the human asthmatic airway. J Immunol. 2007;179(3):1901–1912. doi: 10.4049/jimmunol.179.3.1901. [DOI] [PubMed] [Google Scholar]
- 7.Liao F, et al. CC-chemokine receptor 6 is expressed on diverse memory subsets of T cells and determines responsiveness to macrophage inflammatory protein 3 alpha. J Immunol. 1999;162(1):186–194. [PubMed] [Google Scholar]
- 8.Weckmann M, et al. Critical link between TRAIL and CCL20 for the activation of TH2 cells and the expression of allergic airway disease. Nat Med. 2007;13(11):1308–1315. doi: 10.1038/nm1660. [DOI] [PubMed] [Google Scholar]
- 9.Dieu-Nosjean MC, et al. Macrophage inflammatory protein 3alpha is expressed at inflamed epithelial surfaces and is the most potent chemokine known in attracting Langerhans cell precursors. J Exp Med. 2000;192(5):705–718. doi: 10.1084/jem.192.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lukacs NW, Prosser DM, Wiekowski M, Lira SA, Cook DN. Requirement for the chemokine receptor CCR6 in allergic pulmonary inflammation. J Exp Med. 2001;194(4):551–555. doi: 10.1084/jem.194.4.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lundy SK, et al. Attenuation of allergen-induced responses in CCR6−/− mice is dependent upon altered pulmonary T lymphocyte activation. J Immunol. 2005;174(4):2054–2060. doi: 10.4049/jimmunol.174.4.2054. [DOI] [PubMed] [Google Scholar]
- 12.Handel TM, Johnson Z, Crown SE, Lau EK, Proudfoot AE. Regulation of protein function by glycosaminoglycans—as exemplified by chemokines. Annu Rev Biochem. 2005;74:385–410. doi: 10.1146/annurev.biochem.72.121801.161747. [DOI] [PubMed] [Google Scholar]
- 13.Esko JD, Selleck SB. Order out of chaos: Assembly of ligand binding sites in heparan sulfate. Annu Rev Biochem. 2002;71:435–471. doi: 10.1146/annurev.biochem.71.110601.135458. [DOI] [PubMed] [Google Scholar]
- 14.Gallagher JT. Heparan sulfate: Growth control with a restricted sequence menu. J Clin Invest. 2001;108(3):357–361. doi: 10.1172/JCI13713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ringvall M, et al. Defective heparan sulfate biosynthesis and neonatal lethality in mice lacking N-deacetylase/N-sulfotransferase-1. J Biol Chem. 2000;275(34):25926–25930. doi: 10.1074/jbc.C000359200. [DOI] [PubMed] [Google Scholar]
- 16.Zuberi RI, et al. Deficiency of endothelial heparan sulfates attenuates allergic airway inflammation. J Immunol. 2009;183(6):3971–3979. doi: 10.4049/jimmunol.0901604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lortat-Jacob H, Grosdidier A, Imberty A. Structural diversity of heparan sulfate binding domains in chemokines. Proc Natl Acad Sci USA. 2002;99(3):1229–1234. doi: 10.1073/pnas.032497699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Paz JL, et al. Profiling heparin-chemokine interactions using synthetic tools. ACS Chem Biol. 2007;2(11):735–744. doi: 10.1021/cb700159m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Paz JL, Noti C, Böhm F, Werner S, Seeberger PH. Potentiation of fibroblast growth factor activity by synthetic heparin oligosaccharide glycodendrimers. Chem Biol. 2007;14(8):879–887. doi: 10.1016/j.chembiol.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 20.Bao X, et al. Endothelial heparan sulfate controls chemokine presentation in recruitment of lymphocytes and dendritic cells to lymph nodes. Immunity. 2010;33(5):817–829. doi: 10.1016/j.immuni.2010.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kawashima H, et al. N-Acetylglucosamine-6-O-sulfotransferases 1 and 2 cooperatively control lymphocyte homing through L-selectin ligand biosynthesis in high endothelial venules. Nat Immunol. 2005;6(11):1096–1104. doi: 10.1038/ni1259. [DOI] [PubMed] [Google Scholar]
- 22.Uchimura K, et al. A major class of L-selectin ligands is eliminated in mice deficient in two sulfotransferases expressed in high endothelial venules. Nat Immunol. 2005;6(11):1105–1113. doi: 10.1038/ni1258. [DOI] [PubMed] [Google Scholar]
- 23.Uchimura K, et al. HSulf-2, an extracellular endoglucosamine-6-sulfatase, selectively mobilizes heparin-bound growth factors and chemokines: Effects on VEGF, FGF-1, and SDF-1. BMC Biochem. 2006;7:2. doi: 10.1186/1471-2091-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Flaishon L, et al. Anti-inflammatory effects of an inflammatory chemokine: CCL2 inhibits lymphocyte homing by modulation of CCL21-triggered integrin-mediated adhesions. Blood. 2008;112(13):5016–5025. doi: 10.1182/blood-2007-12-129122. [DOI] [PubMed] [Google Scholar]
- 25.Govindaraju V, et al. Interleukin-8: Novel roles in human airway smooth muscle cell contraction and migration. Am J Physiol Cell Physiol. 2006;291(5):C957–C965. doi: 10.1152/ajpcell.00451.2005. [DOI] [PubMed] [Google Scholar]
- 26.Nelson RM, et al. Heparin oligosaccharides bind L- and P-selectin and inhibit acute inflammation. Blood. 1993;82(11):3253–3258. [PubMed] [Google Scholar]
- 27.Uchimura K, Rosen SD. Sulfated L-selectin ligands as a therapeutic target in chronic inflammation. Trends Immunol. 2006;27(12):559–565. doi: 10.1016/j.it.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 28.Sinitsyn VV, et al. C3a and C5a anaphylatoxins bind to heparin-based sorbent in low density lipoprotein apheresis: In vitro and in vivo investigations. Artif Organs. 1992;16(3):291–293. doi: 10.1111/j.1525-1594.1992.tb00312.x. [DOI] [PubMed] [Google Scholar]
- 29.Lajoie S, et al. Complement-mediated regulation of the IL-17A axis is a central genetic determinant of the severity of experimental allergic asthma. Nat Immunol. 2010;11(10):928–935. doi: 10.1038/ni.1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kay AB. Allergy and allergic diseases. First of two parts. N Engl J Med. 2001;344(1):30–37. doi: 10.1056/NEJM200101043440106. [DOI] [PubMed] [Google Scholar]
- 31.Garner OB, Yamaguchi Y, Esko JD, Videm V. Small changes in lymphocyte development and activation in mice through tissue-specific alteration of heparan sulphate. Immunology. 2008;125(3):420–429. doi: 10.1111/j.1365-2567.2008.02856.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Preuss JM, Page CP. Effect of heparin on antigen-induced airway responses and pulmonary leukocyte accumulation in neonatally immunized rabbits. Br J Pharmacol. 2000;129(8):1585–1596. doi: 10.1038/sj.bjp.0703247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ahmed T, Ungo J, Zhou M, Campo C. Inhibition of allergic late airway responses by inhaled heparin-derived oligosaccharides. J Appl Physiol (1985) 2000;88(5):1721–1729. doi: 10.1152/jappl.2000.88.5.1721. [DOI] [PubMed] [Google Scholar]
- 34.Bowler SD, Smith SM, Lavercombe PS. Heparin inhibits the immediate response to antigen in the skin and lungs of allergic subjects. Am Rev Respir Dis. 1993;147(1):160–163. doi: 10.1164/ajrccm/147.1.160. [DOI] [PubMed] [Google Scholar]
- 35.Diamant Z, et al. Effect of inhaled heparin on allergen-induced early and late asthmatic responses in patients with atopic asthma. Am J Respir Crit Care Med. 1996;153(6 Pt 1):1790–1795. doi: 10.1164/ajrccm.153.6.8665036. [DOI] [PubMed] [Google Scholar]
- 36.Shaw JP, et al. The X-ray structure of RANTES: Heparin-derived disaccharides allows the rational design of chemokine inhibitors. Structure. 2004;12(11):2081–2093. doi: 10.1016/j.str.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 37.Jacobsen EA, et al. Allergic pulmonary inflammation in mice is dependent on eosinophil-induced recruitment of effector T cells. J Exp Med. 2008;205(3):699–710. doi: 10.1084/jem.20071840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cho JY, et al. Chronic OVA allergen challenged Siglec-F deficient mice have increased mucus, remodeling, and epithelial Siglec-F ligands which are up-regulated by IL-4 and IL-13. Respir Res. 2010;11:154. doi: 10.1186/1465-9921-11-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hecht ML, et al. Natural cytotoxicity receptors NKp30, NKp44 and NKp46 bind to different heparan sulfate/heparin sequences. J Proteome Res. 2009;8(2):712–720. doi: 10.1021/pr800747c. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.