Abstract
Sorting nexin 1 (SNX1) and SNX2, homologues of the yeast vacuolar protein-sorting (Vps)5p, contain a phospholipid-binding motif termed the phox homology (PX) domain and a carboxyl terminal coiled-coil region. A role for SNX1 in trafficking of cell surface receptors from endosomes to lysosomes has been proposed; however, the function of SNX2 remains unknown. Toward understanding the function of SNX2, we first examined the distribution of endogenous protein in HeLa cells. We show that SNX2 resides primarily in early endosomes, whereas SNX1 is found partially in early endosomes and in tubulovesicular-like structures distributed throughout the cytoplasm. We also demonstrate that SNX1 interacts with the mammalian retromer complex through its amino terminal domain, whereas SNX2 does not. Moreover, activated endogenous epidermal growth factor receptor (EGFR) colocalizes markedly with SNX2-positive endosomes, but minimally with SNX1-containing vesicles. To assess SNX2 function, we examined the effect of a PX domain-mutated SNX2 that is defective in vesicle localization on EGFR trafficking. Mutant SNX2 markedly inhibited agonist-induced EGFR degradation, whereas internalization remained intact. In contrast, SNX1 PX domain mutants failed to effect EGFR degradation, whereas a SNX1 deletion mutant significantly inhibited receptor down-regulation. Interestingly, knockdown of SNX1 and SNX2 expression by RNA interference failed to alter agonist-induced EGFR down-regulation. Together, these findings suggest that both SNX1 and SNX2 are involved in regulating lysosomal sorting of internalized EGFR, but neither protein is essential for this process. These studies are the first to demonstrate a function for SNX2 in protein trafficking.
INTRODUCTION
Sorting nexins (SNXs) are a diverse group of cellular proteins defined by the presence of a phospholipid-binding motif termed the phox homology (PX) domain (Worby and Dixon, 2002). The PX domain of SNXs bind to various phosphatidylinositol phosphates (PtdInsPs), including PtdIns-3-P, which is highly enriched in endosomal membranes (Lemmon and Traub, 2000). SNX1 and SNX2 are the mammalian homologues of the yeast vacuole protein-sorting molecule Vps5p (Haft et al., 1998). Vps5p is part of a multimeric protein complex termed the “retromer” that functions in retrograde trafficking from prevacuolar endosomes to the Golgi (Horazdovsky et al., 1997; Nothwehr and Hindes, 1997; Seaman et al., 1998). Many proteins require Vps5p function for proper localization, including the hydrolase Vps10p receptor that cycles between the Golgi and endosomes. This process is required for efficient sorting of newly synthesized hydrolases to the vacuole, an organelle analogous to the mammalian lysosome. In addition, Vps5p retains the resident Golgi enzymes dipeptidyl amino peptidase and Kex2p by retrieving misplaced molecules from prevacuolar endosomes.
Because many membrane trafficking events are conserved throughout evolution, the mammalian SNX1 and SNX2 are likely to have similar functions in protein trafficking. Indeed, SNX1 was originally identified as a protein that interacts with the epidermal growth factor receptor (EGFR) (Kurten et al., 1996), a receptor tyrosine kinase that is efficiently sorted to lysosomes and degraded. EGFR degradation is enhanced in cells overexpressing SNX1, and deletion mutants of SNX1 block EGFR degradation but fail to inhibit receptor endocytosis (Kurten et al., 1996; Zhong et al., 2002). These findings suggest a role for SNX1 in endosome-to-lysosome trafficking. We also recently found that SNX1 associates with protease-activated receptor-1, a G protein-coupled receptor for thrombin, and deletion mutants of SNX1 inhibit agonist-promoted lysosomal degradation of the receptor (Wang et al., 2002). However, the mechanism by which SNX1 regulates lysosomal sorting of cell surface receptors is unknown. Moreover, the function of SNX2 in the regulation of receptor trafficking has not been determined.
SNX1 and SNX2 are ∼68-kDa proteins that contain a PX domain and a carboxyl terminal coiled-coil region (Haft et al., 1998). SNX1 and SNX2 can self-assemble as homodimers or assemble with each other to form heterodimers both in vitro and in vivo (Haft et al., 1998; Kurten et al., 2001). The ability of SNX1 and SNX2 to heterodimerize or self-associate suggests they may act together as a heterocomplex or separately as a homodimer. The recent report of mice with targeted null alleles for SNX1 and SNX2 have provided insight regarding the function of SNXs in mammals (Schwarz et al., 2002). Mice that lack either SNX1 or SNX2 are viable and fertile, whereas embryos deficient in both SNX1 and SNX2 arrest at midgestation. These results suggest that SNX1 and SNX2 perform essential functions in mammalian development. However, the viability of mice lacking only SNX1 or SNX2 suggests that it is not necessary that both proteins act together. In addition, phenotypic differences between SNX1 and SNX2 were demonstrated by reducing the dosage of the paralogous gene by using Snx1+/–;Snx2–/– and Snx1–/–; Snx2+/– mice. Snx1+/–;Snx2–/– mice are runted and underrepresented in litter frequencies, whereas Snx1–/–; Snx2+/– mice are phenotypically normal (Schwarz et al., 2002). These findings raise the distinct possibility that SNX1 and SNX2 also have unique functions in mammalian cells.
In support of some distinct roles for SNX1 and SNX2, it has been previously demonstrated that SNX1 and SNX2 differentially interact with certain cell surface receptors when ectopically overexpressed (Haft et al., 1998). Moreover, SNX1 and SNX2 display distinct PtdInsPs binding specificities (Cozier et al., 2002; Zhong et al., 2002). Interestingly, SNX1 binds to the plasma membrane-enriched PtdIns-3,4,5-P as well as PtdIns-3,5-P and PtdIns-3-P. In contrast, SNX2 seems to bind preferentially to PtdIns-3-P, a phospholipid highly enriched in endosomes (Zhong et al., 2002). Thus, in the present study we sought to determine whether SNX1 and SNX2 differentially regulate protein trafficking in mammalian cells. We report that endogenous SNX1 and SNX2 largely reside in distinct intracellular compartments and differentially bind to the mammalian retromer complex. Interestingly, both SNX1 and SNX2 are involved in regulating agonist-induced EGFR degradation, perhaps through different mechanisms, but neither protein is essential. These studies also reveal for the first time a function for SNX2 in protein trafficking.
MATERIALS AND METHODS
Antibodies and Reagents
The rabbit polyclonal anti-SNX antibodies were generated against full-length SNX1 or SNX2 expressed as a glutathione S-transferase fusion protein (Haft et al., 1998) or raised against specific SNX1 and SNX2 amino terminal peptide sequences (Schwarz et al., 2002). Mouse anti-early endosome antigen-1 (EEA1) and trans-Golgi network (TGN) 230 antibodies were purchased from BD Transduction Laboratories (Lexington, KY). Anti-lysosome-associated membrane protein-1 (LAMP1) H4A3 mouse antibody was obtained from the Developmental Studies Hybridoma Bank maintained by the University of Iowa (Iowa City, IA). Anti-actin antibody was obtained from Sigma-Aldrich (St. Louis, MO). A mouse monoclonal anti-human transferrin receptor (TfnR) antibody B3/25 was from Roche Diagnostics (Indianapolis, IN). Mouse monoclonal LA22 anti-EGFR antibody was from Upstate Biotechnology (Lake Placid, NY). Rabbit anti-myc (A-14) and mouse anti-myc (9E10) antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA). Chicken anti-myc IgY antibody was from Molecular Probes (Eugene, OR). The rabbit polyclonal anti-Vps35 antibody was generated as described previously (Haft et al., 2000). Monoclonal 3F10 anti-hemagglutinin (HA)-peroxidase conjugated antibody was purchased from Roche Diagnostics. The secondary antibodies goat anti-mouse– and anti-rabbit–conjugated to horseradish peroxidase were from Bio-Rad (Hercules, CA). Donkey anti-chicken secondary antibody conjugated to peroxidase was from Jackson ImmunoResearch Laboratories (West Grove, PA). Alexa488- and Alexa594-conjugated goat anti-mouse and anti-rabbit antibodies were obtained from Molecular Probes. The epidermal growth factor (EGF) ligand and LY294002 compound were purchased from Sigma-Aldrich.
Cell Lines and cDNAs
HeLa cells were maintained as described previously (Trejo et al., 2000). LNCaP prostate carcinoma cells were grown in RPMI 1640 medium with 2 mM l-glutamine, 1.5 g/l sodium bicarbonate, 4.5 g/l glucose, 10 mM HEPES, 1.0 mM sodium pyruvate, and 10% fetal bovine serum.
N-terminal myc- and HA-tagged SNXs were described previously (Haft et al., 1998). SNX1 deletion mutants were generated as described (Wang et al., 2002). The N-terminal myc-tagged Vps cDNA constructs were gifts from C. R. Haft (National Institutes of Diabetes and Digestive and Kidney Diseases, National Institutes of Health) and have been described previously (Haft et al., 2000). H. Radhakrishna (Georgia Institute of Technology, Atlanta, GA) provided green fluorescent protein (GFP)-tagged Rab5 wild-type and mutant Q79L plasmids. The PX domain mutants of SNX1 and SNX2 were generated using QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, CA), and specific mutations were confirmed by dideoxy sequencing. The cDNA plasmids encoding wild-type and GFP-tagged EGFR were kindly provided by A. Sorkin (University of Colorado Health Science Center, Denver, CO) and described previously (Carter and Sorkin, 1998).
Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR)
Total RNA was isolated from HeLa cells by using TRIzol reagent (Invitrogen, Carlsbad, CA), treated with DNase, and quantified by OD260. Reverse transcriptase (RT) reactions were performed using 2 μg of total cellular RNA, 100 ng of random primers, and 1 μl of SuperScript II RT (Invitrogen) in 20-μl reaction volume, according to the manufacturer's instructions. Mock RT reactions of total cellular RNA were performed with no reverse transcriptase added. RT product (1 μl) was amplified by PCR in a 50-μl volume containing a final 0.2 μM specific primers (see below) and 5 U of Taq polymerase (Invitrogen). Reaction conditions were one cycle of 95°C for 5 min, followed by 30 cycles of 95°C for 30 s, 55°C for 30 s, 72°C for 1 min, and then one cycle of 72°C for 10 min. The following primer sets were used for PCR where nucleotide numbering is such that 1 equals the A of the start ATG. The primer set for the first SNX1 PCR reaction included the forward SNX1-F21 CTGTAGCGCTTCGGAGAGAC and reverse SNX1-R233 TGGATCCCATTTTCTTTGGA primers with the resulting product of 212 base pairs. The second SNX1 PCR reaction used forward SNX1-F151 GCGGTGGTCAGTAAACATCAG and reverse SNX1-R376 TCGAAGAATTTGTGGCTTCC primers, resulting in a product of 225 base pairs. The first primer set for SNX2 PCR reaction included the forward SNX2-F67 GACGGAGAGGACCTGTTCAC and reverse SNX2-R307 CAGGTGTGACTGCAGGAGAA that generated a 240-base pair product. For the second SNX2 PCR reaction, we used SNX2-F356 CTGCTCCCGTGATCTTTGAT and SNX2-R577 TGTGCAAACCAAGAAAGTCG, resulting in a 221-base pair product. The EGFR PCR reaction was performed with the forward EGFR-F169 AACCTGTGAGGTGGTCCTTGG and reverse EGFR-R404 AGCTCCTTCAGTCCGGTTTT primers that generated a 235-base pair product.
Transient Transfections
Cells were transiently transfected with a total of 2 μg of plasmid DNA per well of a six-well dish (Falcon Plastics, Oxnard, CA) or 0.8 μg of plasmid DNA per well of a 12-well dish (Falcon Plastics) by using LipofectAMINE reagent according to the manufacturer's instructions (Invitrogen). All experiments were performed 48 h posttransfection.
Small Interfering RNAs (siRNAs) Transient Transfections
HeLa cells plated at 1.25 × 105 cells in 12-well dishes were grown overnight. Cells were then transiently transfected with 100 nM specific siRNAs by using LipofectAMINE 2000 according to the manufacturer's instructions (Invitrogen), and experiments were performed 48 h after transfections. SNX siRNAs were from Dharmacon (Lafayette, CO) and used to target the following specific mRNA sequences: SNX1 siRNA2 (5′-ACUCUAGUCAACCAUAGGA-3′), SNX1 siRNA3 (5′-CCACGUGAUCAAGUACCUU-3′), SNX2 siRNA1 (5′-AGUGCUGCCAUGUUAGGUA-3′), and SNX2 siRNA2 (5′-GAUA GACCAGUUACAUCAA-3′)
Immunoblotting and Coimmunoprecipitation
Cell lysates were prepared and processed from cells plated at 2 × 105 cells per well of a 12-well dish (Trejo et al., 2000). Lysates were resolved by SDS-PAGE, transferred, and membranes were then incubated overnight at 4°C with anti-SNX1 or SNX2 antibodies (1:2000) or with other antibodies at 1:1000 dilution. Membranes were washed, incubated with species-specific secondary antibodies-conjugated to horseradish peroxidase at (1:10,000), and washed again. Immunoblots were then developed using enhanced chemiluminescence (Amersham Biosciences, Piscataway, NJ), imaged by autoradiography, and quantitated using a Fluor-S Imager (Bio-Rad).
Transiently transfected HeLa cells plated at 5 × 105 cells per well in a six-well dish were grown for 48 h. Cell were lysed and immunoprecipitated as we described previously (Wang et al., 2002).
Immunofluorescence Confocal Microscopy
HeLa cells were plated at 2.5 × 105 on fibronectin-coated glass coverslips (22 × 22 mm) in six-well dishes and grown for 48 h. Cells were fixed and processed for microscopy as described previously (Trejo et al., 2000). Endogenous SNXs, EEA1, LAMP1, and TGN230 were detected by incubation with appropriate antibodies for 1 h at 25°C. To detect the subcellular localization of ectopically expressed SNX2 wild-type and mutant proteins, cells were prepermeabilized with 80 mM PIPES, pH 6.8, 5 mM EGTA, 1 mM MgCl2, 0.1% bovine serum albumin, and 0.01% saponin for 5 min at 4°C. Cells were then washed, fixed in 4% paraformaldehyde, and processed as described previously (Leprince et al., 2003). Colocalization of endogenous EGFR with either SNX1 or SNX2 was detected in cells that were serum-starved overnight. Cells were then incubated with anti-EGFR antibody for 1 h at 4°C, washed, and then incubated in the absence or presence of 100 ng/ml EGF ligand for 20 min at 37°C. Cells were then fixed and processed for microscopy. The extent of EGFR colocalization with either SNX1 or SNX2 was quantitated by counting the number puncta in cells that costained for EGFR and either SNX1 or SNX2 in the merged image after various times of agonist exposure. The values shown represent puncta counted in at least three different cells in three independent experiments; sample conditions were blindly coded and counted by two individuals. The data (mean ± SEM) are expressed as the percentage of EGFR-positive puncta that costained for either SNX1 or SNX2.
TfnR internalization was examined in serum-starved cells incubated with anti-TfnR antibody for 1 h at 4°C; under these conditions, only receptor present on the cell surface are labeled with antibody. Cells were washed and then warmed at 37°C for various times, fixed, and processed for microscopy. Confocal images were acquired using a Fluoview 300 laser scanning confocal imaging system (Olympus, Tokyo, Japan) configured with an IX70 fluorescence microscope fitted with a PlanApo 60× oil objective (Olympus). Fluorescent images, X-Y section at 0.28 μm, were collected sequentially at 800 × 600 resolution with 2× optical zoom. The final composite image was created using Adobe Photoshop 6.0 (Adobe Systems, Mountain View, CA).
RESULTS
Endogenous SNX1 and SNX2 Expression in HeLa Cells
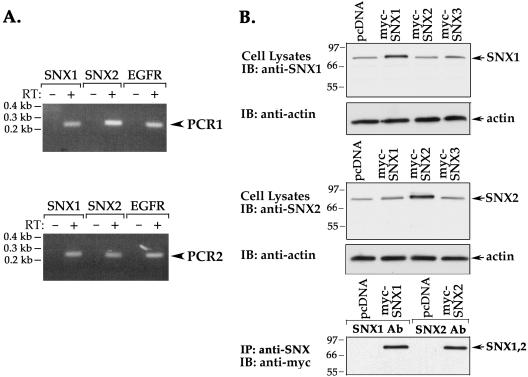
HeLa cells were used to examine the function of SNX1 and SNX2 in protein trafficking. SNX1 is expressed in HeLa cells endogenously (Kurten et al., 1996; Cozier et al., 2002); however, the expression of SNX2 in these cells had not previously been determined. We first used a RT-PCR assay to detect SNX1 and SNX2 mRNA in HeLa cells. The RT-PCR revealed the presence SNX1 and SNX2 mRNA transcripts (Figure 1A), suggesting that these proteins are endogenously expressed in HeLa cells. EGFR mRNA was also detected by RT-PCR in these cells (Figure 1A). To confirm whether SNX proteins were endogenously expressed, we performed immunoblot analysis by using purified antibodies generated against recombinant SNX proteins (Figure 1B) (Haft et al., 1998). The specificity of anti-SNX antibodies was determined by immunoblotting lysates from HeLa cells transiently expressing myc-tagged SNX1, SNX2, or SNX3. A single ∼68-kDa band was detected with anti-SNX1 antibody in cell lysates, and the intensity of this band was increased in lysates prepared from myc-SNX1–transfected cells (Figure 1B, top). The anti-SNX2 antibody also detected a prominent ∼68-kDa protein that seems more abundant in lysates from myc-SNX2–expressing cells (Figure 1B, middle). The anti-SNX antibodies were also capable of immunoprecipitating the specific recombinant myc-tagged SNX proteins (Figure 1B, bottom). Thus, antibodies generated against SNX1 and SNX2 recognize both endogenous and recombinant SNX proteins, respectively, in HeLa cells.
Figure 1.
SNX1 and SNX2 are expressed endogenously in HeLa cells. (A) Expression of SNX1, SNX2, and EGFR mRNAs in HeLa cells was detected by RT-PCR. Total cellular RNA (2 μg) was reverse-transcribed and PCR amplified. Mock RT-PCR of total cellular RNA with no reverse transcriptase added is shown in lanes marked as RT: –. SNX1 and SNX2 mRNA was analyzed twice by RT-PCR by using two distinct sets of primers and revealed the presence of mRNA transcript in HeLa cells. (B) HeLa cells transiently transfected with either myc-tagged SNX1, SNX2, SNX3, or pcDNA vector were lysed and immunoblotted with rabbit polyclonal anti-SNX1 or anti-SNX2 antibodies (top and middle, respectively). Actin expression was also detected as a loading control. HeLa cells transiently expressing either myc-SNX1, SNX2, or pcDNA vector were lysed and immunoprecipitated with anti-SNX polyclonal antibodies, respectively. Immunoprecipitates were immunoblotted for SNXs by using chicken anti-myc antibody (bottom).
Endogenous SNX2 Is Localized to an Early Endocytic Compartment
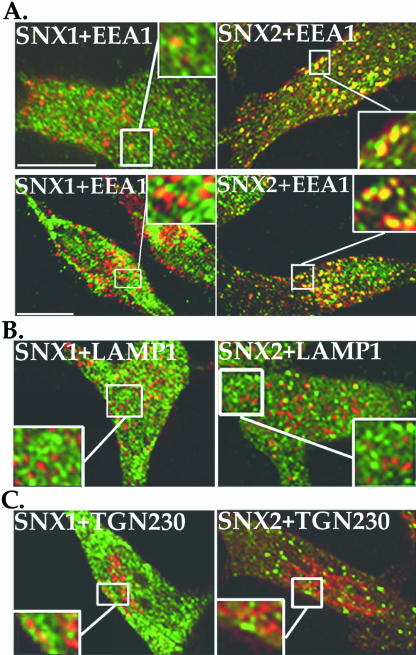
The localization of endogenous SNX1 and SNX2 to specific intracellular compartments has not previously been determined. We initially examined the localization of endogenous SNXs with EEA1, a specific marker of early endosomes (Christoforidis et al., 1999). Confocal microscopy studies revealed that endogenous SNX1 is localized primarily to vesicles that show partial overlap with EEA1 and to tubulovesicular-like structures (Figure 2A, top left), consistent with published studies of ectopically expressed SNX1 (Cozier et al., 2002; Zhong et al., 2002). A similar intracellular distribution of endogenous SNX1 was observed using an antibody generated against a SNX1 amino terminal peptide sequence (Schwarz et al., 2002) (Figure 2A, bottom left). In contrast, endogenous SNX2 is found largely in discrete vesicles that markedly colocalize with EEA1 detected using two different antibodies (Figure 2A, top and bottom right). These results suggest that SNX2 is localized primarily to the early endocytic pathway. Interestingly, neither endogenous SNX1 nor SNX2 show significant colocalization with late endosomes/lysosomes nor the TGN (Figure 2, B and C), as assessed by colocalization with LAMP1 and the vesicular-TGN230 localized protein (Kjer-Nielsen et al., 1999), respectively. These findings suggest that endogenous SNX1 and SNX2 largely reside in distinct intracellular compartments.
Figure 2.
Endogenous SNX1 and SNX2 localize largely to distinct intracellular compartments. (A–C) HeLa cells were fixed, permeabilized, and immunostained for endogenous SNX1 or SNX2 (green) and either EEA1, LAMP1, or TGN230 (shown in red) and examined by confocal microscopy. These images are representative of many cells examined in at least five independent experiments. Note the prominent colocalization (yellow) of endogenous SNX2 and EEA1 shown in the merged image (A), and the partial colocalization between SNX1 and EEA1. Neither endogenous SNX1 nor SNX2 showed significant colocalization with LAMP1 or TGN230 (B and C). The insets are magnifications of boxed areas. Bar, 10 μm.
Phosphatidylinositol 3-Kinase (PI-3 Kinase) Activity and the Small GTPase Rab5 Regulate Subcellular Localization of Endogenous SNX2
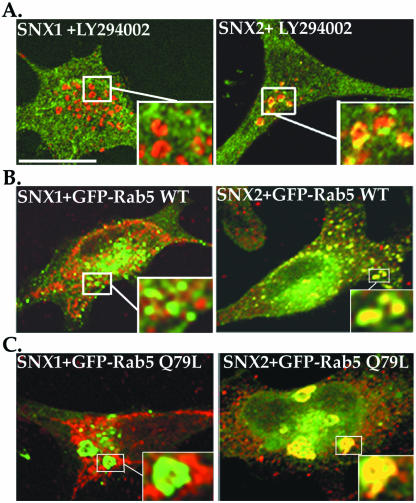
The PX domain of ectopically expressed SNX1 is involved in recruitment of the protein to endosomal membranes by binding to various PtdInsPs (Cozier et al., 2002; Zhong et al., 2002), including the PI-3 kinase product PtdIns-3-P, which is highly enriched in endosomal membranes. Inhibition of PI-3 kinase activity blocks homotypic fusion of endosomes in vitro and perturbs membrane trafficking in vivo (Brown et al., 1995; Shpetner et al., 1996). To determine whether PI-3 kinase activity and PtdIns-3-P are involved in endogenous SNX1 and SNX2 localization, we examined the distribution of these proteins in cells treated with LY294002, an inhibitor of PI-3 kinase. The distribution of endogenous SNX1 was unperturbed in LY294002-treated HeLa cells (Figure 3A), compared with the localization of EEA1, a FYVE domain containing and PtdIns-3-P binding protein, to enlarged endosomes. In striking contrast, endogenous SNX2 showed significant colocalization with EEA1 in expanded endosomes in LY294002-treated cells (Figure 3A), suggesting that SNX2 resides primarily in a PtdIns-3-P–sensitive endosomal compartment.
Figure 3.
Endogenous SNX2 localization is altered by inhibition of PI-3 kinase activity and expression of Rab5 Q79L mutant. (A) HeLa cells were incubated in the absence (our unpublished data) or presence of 50 μM LY294002 compound for 1 h at 37°C, fixed, permeabilized, and immunostained for endogenous SNX1 or SNX2 (green) and EEA1 (red). Colocalization was assessed by confocal microscopy. Note the localization of endogenous SNX2 in enlarged endosomes observed in LY294002 PI-3 kinase inhibitor treated cells. (B and C) HeLa cells were transiently transfected with GFP-tagged wild-type (WT) or mutant Q79L Rab5, fixed, permeabilized, and immunostained for endogenous SNX1 or SNX2 (red). Insets are magnifications of boxed areas. Note the prominent colocalization (yellow) of endogenous SNX2 with both wild-type and Q79L mutant Rab5 as detected by confocal microscopy. These images are representative of many cells observed in three independent experiments. Bar, 10 μm.
To confirm the differential distribution of SNXs in the endocytic compartment, we examined the regulation of localization by the small GTPase Rab5. Rab5 is involved in transport and fusion of endocytic vesicles, and the constitutively active Rab5 Q79L mutant causes enlargement of these endosomes (Stenmark et al., 1994). In transiently transfected HeLa cells, endogenous SNX1 showed minimal colocalization with wild-type Rab5 tagged with GFP (Figure 3B), whereas endogenous SNX2-positive vesicles markedly colocalized with wild-type GFP-Rab5 (Figure 3B). Surprisingly, the enlarged endosomes caused by constitutively active Rab5 Q79L did not significantly alter endogenous SNX1 subcellular distribution (Figure 3C). However, we observed a marked disruption in endogenous SNX2 localization, which showed significant colocalization with GFP-tagged Rab5 Q79L in enlarged endosomes (Figure 3C). Together, these observations strongly suggest that endogenous SNX2 is localized primarily to an early endocytic EEA1-positive compartment, whereas endogenous SNX1 resides partially in early endocytic vesicles and tubulovesicular-like structures distributed throughout the cytoplasm.
SNX1 Forms a Stable Complex with the Vps35 Retromer Subunit
To further investigate the differences between SNX1 and SNX2, we determined whether these proteins associate with components of the mammalian retromer complex in HeLa cells by using coimmunoprecipitation. The human homologues of three of the retromer subunits, Vps35, Vps29, and Vps26, have been identified and shown to form a complex with SNX1 in transfected COS-7 cells (Haft et al., 2000). However, the association of SNX2 with the retromer complex in mammalian cells remains unknown. We attempted to express the retromer Vps35, Vps29, and Vps26 subunits individually in HeLa cells; however, Vps35 was not efficiently expressed compared with Vps26 and Vps29 expression detected by immunoblot (data not shown). However, increased expression of Vps35 could be achieved by coexpressing Vps29, whereas coexpression of Vps26 failed to stabilize Vps35 expression. These findings are consistent with yeast studies in which Vps35 and Vps29 have been shown to assemble into a stable subcomplex that is linked to Vps5/Vps17 through an interaction involving Vps26 (Horazdovsky et al., 1997; Nothwehr and Hindes, 1997; Seaman et al., 1998).
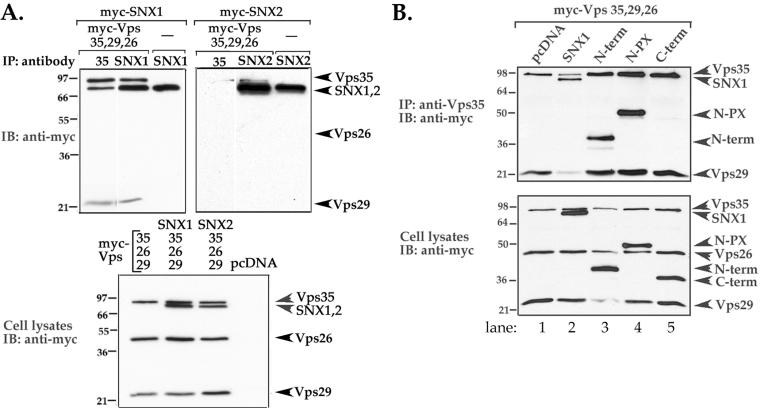
Thus, we next transiently expressed SNX1 or SNX2 together with all the retromer Vps subunits and assessed association by coimmunoprecipitation. Immunoprecipitates from cells expressing myc-SNX1 and the myc-tagged Vps retromer subunits revealed a robust association between Vps35 and SNX1 (Figure 4A, left), suggesting that these proteins form a stable complex in HeLa cells. However, only a small amount of Vps29 was coimmunoprecipitated with the Vps35/SNX1 complex (Figure 4A), indicating that either Vps29 weakly associates with the subcomplex or associates with less than a 1:1 stoichiometry. In contrast, immunoprecipitates from cells expressing myc-SNX2 and the Vps retromer subunits revealed virtually no association between these proteins (Figure 4A, right). Immunoblot analysis of total cell lysates revealed that both SNXs and Vps retromer subunits were expressed equally in these experiments (Figure 4A, bottom). These findings raise the distinct possibility that SNX1 and the Vps35 retromer subunit act together in mammalian cells, whereas SNX2 may function independently.
Figure 4.
SNX1 amino-terminal domain mediates interaction with the retromer complex in mammalian cells. (A) HeLa cells transiently expressing the retromer subunits (myc-tagged Vps35, Vps29, and Vps26) together with either myc-tagged SNX1 or SNX2 were lysed and immunoprecipitated with either anti-Vps35, -SNX1, or -SNX2 rabbit polyclonal antibodies. Immunoprecipitates were resolved by SDS-PAGE and then immunoblotted with chicken anti-myc antibody (top panels). The expression of SNXs and Vps subunits in total cell lysates was detected with anti-myc antibody (bottom). Note the robust association between SNX1 and Vps35 and the lack of association with SNX2 (top right). Similar findings were observed in three separate experiments. (B) HeLa cells transiently transfected with myc-tagged Vps35, Vps29, Vps26, and either full-length SNX1, deletion mutants of SNX1, or pcDNA vector alone were lysed, immunoprecipitated with anti-Vps35 antibody, resolved by SDS-PAGE, and immunoblotted with anti-myc antibody to detect protein expression (top). The expression of SNX1, deletion mutants, and Vps subunits in total cell lysates was detected with anti-myc antibody (bottom). Note that the amino terminus alone is sufficient to interact with the retromer complex (lane 3).
To define the SNX1 domain that interacts with the retromer complex we coexpressed various deletion mutants of SNX1 together with the retromer subunits and assessed association by coimmunoprecipitation. As shown above, SNX1 associated robustly with the complex, whereas the C-terminal domain failed to interact with the retromer subunits (Figure 4B, lanes 2 and 5). Interestingly, the N-terminal region containing the PX domain strongly associated with the retromer complex (Figure 4B, lane 4). However, the N-terminal domain alone also associated with the retromer complex (Figure 4B, lane 3), suggesting that the N-terminal region is sufficient for SNX1 interaction with retromer complex in mammalian cells. These findings are also consistent with the idea that the amino terminus specifies distinct SNX functions because SNX1 and SNX2 are most divergent in their amino terminal domains, which share only 26% amino acid identity, whereas the PX domain and carboxyl terminal regions are 70% homologous in amino acid composition.
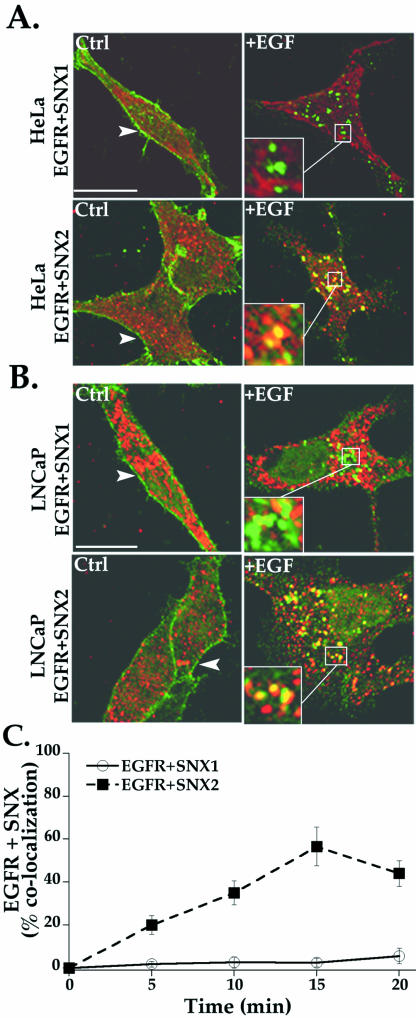
Activated Endogenous EGFR Colocalizes with Endogenous SNX2-Positive Vesicles
SNX1 has been shown to regulate EGFR receptor trafficking in a variety of cell types (Kurten et al., 1996; Cozier et al., 2002; Zhong et al., 2002); however, the function of SNX2 in the regulation of EGFR trafficking has not previously been investigated. We initially used confocal microscopy to determine whether agonist induced colocalization of endogenous EGFR with either endogenous SNX1 or SNX2 in HeLa cells. In untreated cells, EGFR is localized primarily to the cell surface (Figure 5A, left), whereas SNX1 and SNX2 were distributed in vesicles throughout the cytoplasm (Figure 5A). The addition of agonist for 20 min caused a marked redistribution of EGFR into distinct vesicles that failed to show significant colocalization with SNX1 in HeLa cells (Figure 5A, top). A lack of robust EGFR and SNX1 colocalization was similarly observed in LNCaP prostate carcinoma cells (Figure 5B, top), a cell line that also expresses EGFR endogenously. In striking contrast, activated EGFR showed substantial colocalization with SNX2-positive endosomes in both cell types after 20 min of agonist exposure (Figure 5, A and B, bottom). An analysis of EGFR and SNX colocalization at various times is consistent with internalized EGFR distribution mainly to a SNX2- but not SNX1-positive compartment (Figure 5C). Thus, after agonist-induced internalization, endogenous EGFR markedly colocalized with endogenous SNX2 in an endosomal compartment but failed to significantly redistribute to SNX1-containing endosomes.
Figure 5.
Colocalization of activated endogenous EGFR with SNX2. (A) Serum-starved HeLa and (B) LNCaP cells were incubated in the absence or presence of 100 ng/ml EGF for 20 min at 37°C. Cells were fixed, permeabilized, and immunostained for endogenous EGFR (green) and SNXs (red) and examined by confocal microscopy. The colocalization of activated EGFR with SNX2 was easily detected in HeLa and LNCaP cells, whereas EGFR and SNX1 showed minimal overlap in either cell type. Similar findings were observed in three separate experiments. Insets are magnifications of boxed areas. Bar, 10 μm. (C) Results of quantitative analysis are expressed as a percentage of HeLa cells that showed EGFR-positive vesicles and costained for either SNX1 or SNX2 after various times of agonist treatment. The data (mean ± SEM) are representative of three separate experiments.
SNX2 PX Domain Mutant Self-Associates but Fails to Localize to Vesicles
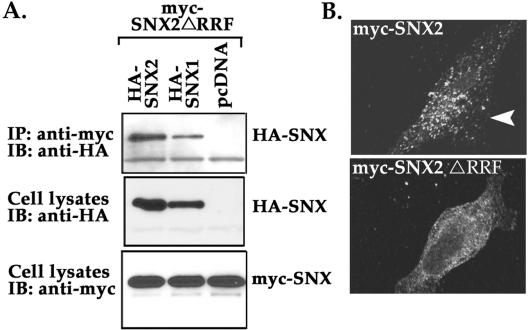
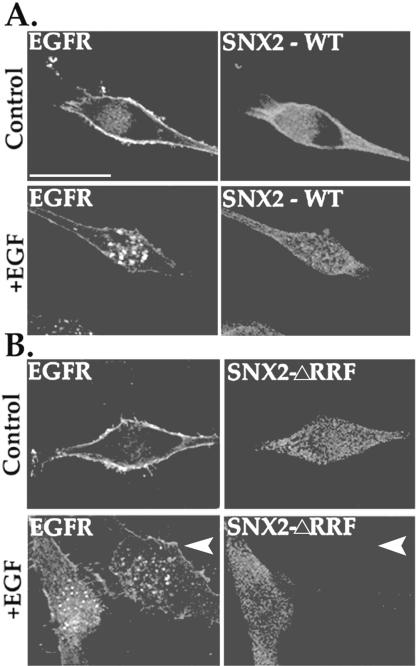
To begin to understand the function of SNX2 in EGFR trafficking, we generated mutants of critical residues important for binding to PtdInsPs. A region of highly conserved residues [Arg-Arg-(Tyr, Phe)-Ser-(Asp, Glu)-Phe] is present in the PX domain of most SNXs and forms part of the positively charged pocket in which the second Arg exhibits extensive interactions with the D3 phosphate of bound PtdIns-3-P (Bravo et al., 2001). Other conserved residues such as the Tyr/Phe seem to stabilize the binding pocket by forming hydrogen bonds with the 3-phosphate and the main chain NH groups of Tyr. It was recently shown that mutation of SNX1 PX domain residues R185R186→G185S186 (RR185/6GS) and SNX3 PX domain residues R69RY71→A69AA71 interferes with binding to PtdInsPs and endosomal localization (Xu et al., 2001; Zhong et al., 2002). Therefore, we made similar mutations in the PX domain of SNX2. Interestingly, we observed that point mutants of the first and second conserved Arg residues in SNX2 led to protein instability, consistent with a naturally occurring mutation in the first Arg of p47-phox PX domain protein that destabilizes the protein and causes chronic granulomatous disease (Noack et al., 2001). However, a triple mutant R182RF184→A182AA184 (ΔRRF) of SNX2 was expressed in HeLa cells (Figure 6).
Figure 6.
SNX2 mutant can self-associate but fails to localize to an endosomal compartment. (A) HeLa cells transiently cotransfected with myc-SNX2 ΔRRF mutant together with wild-type HA-SNX2, HA-SNX1, or pcDNA vector were lysed and immunoprecipitated with anti-myc antibody. Immunoprecipitates were immunoblotted for HA-SNXs by using anti-HA peroxidase conjugated antibody (top). The expression of HA-SNXs and myc-SNX2 mutant in total cell lysates is shown in the middle and bottom panels, respectively. (B) HeLa cells transiently overexpressing wild-type and SNX2 mutant protein were permeabilized with saponin and endosomal localization detected by immunofluorescence microscopy. Note the loss of endosomal localization of SNX2 ΔRRF mutant compared with wild-type SNX2.
SNX1 and SNX2 can self-assemble both in vitro and in vivo (Haft et al., 1998; Kurten et al., 2001). Thus, to exclude the possibility that the mutated SNX2 protein was globally dysfunctional, we examined the association of SNX2 mutant with wild-type SNX1 and SNX2 proteins by coimmunoprecipitation. Mutant SNX2 ΔRRF protein retained the ability to self-associate and to associate with SNX1, suggesting that the protein retained conformation and was not globally disrupted (Figure 6A). Interestingly, we found that SNX2 ΔRRF mutant protein failed to localize to discrete vesicles like the wild-type version of this protein (Figure 6B). These findings suggest that mutation of RRF residues in the PX domain of SNX2 sufficiently disrupts lipid binding and therefore is no longer localized to vesicles, consistent with that previously reported for SNX1 and SNX3 (Xu et al., 2001; Cozier et al., 2002; Zhong et al., 2002).
SNX2 PX Domain Mutant Inhibits Agonist-Induced EGFR Degradation
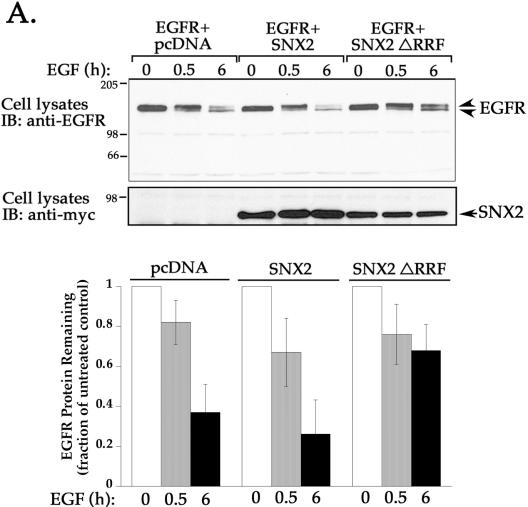
To assess SNX2 function in EGFR trafficking, we examined whether the SNX2 ΔRRF mutant would block agonist-induced EGFR degradation. HeLa cells transiently cotransfected with EGFR and either SNX2, SNX2 ΔRRF mutant, or pcDNA vector were treated with the EGF ligand for 0, 0.5, or 6 h at 37°C. Cell lysates were prepared and the amount of EGFR protein remaining was examined by immunoblotting with anti-EGFR antibody. Immunoblot of lysates from untreated transfected cells revealed one major band migrating at ∼175 kDa (Figure 7). A short 0.5-h exposure to EGF ligand caused a decrease in mobility of this band, probably due to receptor phosphorylation, and reduction in the level of receptor protein by ∼25% in all transfection conditions (Figure 7). In cells cotransfected with wild-type SNX2 or empty pcDNA vector, exposure to agonist for 6 h decreased EGFR protein by ∼65 and 75%, respectively (Figure 7). These findings are consistent with the extent of EGFR degradation reported previously in other cell types (Carter and Sorkin, 1998). In contrast, agonist-induced degradation of EGFR was markedly inhibited in cells cotransfected with SNX2 ΔRRF mutant; only a 32% decrease in EGFR protein was detected after 6 h of agonist exposure (Figure 7). Thus, the observation that mutant SNX2 inhibits EGFR degradation suggests that SNX2 is involved in regulating trafficking of activated EGFR to a degradative pathway.
Figure 7.
Overexpression of SNX2 mutant markedly inhibits agonist-induced EGFR degradation. HeLa cells transiently cotransfected with EGFR and either pcDNA vector, SNX2, or SNX2 ΔRRF mutant were serum-starved and incubated in the absence or presence of EGF ligand (100 ng/ml) for 0, 0.5, or 6 h at 37°C. Cells were then lysed, and equivalent amounts of lysates were resolved by SDS-PAGE. The amount of EGFR remaining was detected with anti-EGFR antibody (top) and quantitated by Fluor-S Imager. The expression of SNX2 wild type and ΔRRF mutant in cell lysates was detected with anti-myc antibody (bottom). Results in the bar graph are expressed as a fraction of EGFR measured in untreated control lysates and was determined for each transfection condition. The data are represented as the mean ± SEM of three separate experiments. Note the marked inhibition of agonist-induced EGFR degradation in cells expressing SNX2 ΔRRF mutant.
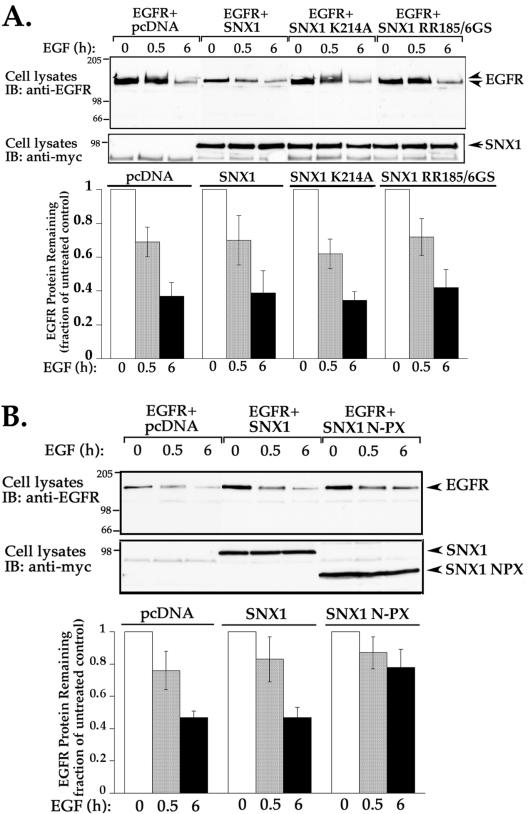
We next examined whether EGFR trafficking is regulated by SNX1 PX domain mutants K214A and RR185/6GS, shown to be defective in lipid binding and vesicle localization (Cozier et al., 2002; Zhong et al., 2002). HeLa cells transiently transfected with EGFR and either SNX1, SNX1 PX domain mutants (K214A and RR185/6GS), or pcDNA were treated and processed as described above. The extent of EGFR degradation stimulated by agonist at 0.5 and 6 h was similar in cells transfected with wild-type SNX1 and vector control (Figure 8A). Interestingly, neither K214A nor RR185/6GS SNX1 PX domain mutants significantly inhibited agonist-induced EGFR degradation (Figure 8A), suggesting that disruption of SNX1 vesicle localization does not perturb EGFR trafficking. In contrast, coexpression of EGFR with SNX1 N-PX domain deletion mutant encompassing the amino terminal 273 residues caused a significant inhibition of agonist-induced EGFR degradation (Figure 8B), consistent with that reported previously (Zhong et al., 2002). In contrast, neither the SNX1 N-terminal nor C-terminal domains affected EGFR trafficking (data not shown). Together, these findings suggest the possibility that SNX1 and SNX2 use distinct mechanisms for the regulation of EGFR trafficking.
Figure 8.
Effect of SNX1 PX domain and deletion mutants on agonist-stimulated EGFR degradation. (A and B) Serum-deprived HeLa cells transiently cotransfected with EGFR and either wild-type or mutant SNX1, or vector only were treated and processed as described in Figure 7. The expression of wild type and SNX1 mutants in cell lysates was detected with anti-myc antibody. The data are shown as the mean ± SEM of at least three independent experiments. Note that SNX1 PX domain mutants fail to inhibit agonist-induced EGFR degradation, whereas SNX1 N-PX deletion mutant (1–273) blocked receptor degradation.
Effect of SNX2 PX Domain Mutant on Agonist-induced EGFR Internalization
To determine whether the SNX2 ΔRRF mutant blocked EGFR degradation by inhibiting receptor endocytosis, we examined agonist-induced internalization by using immunofluorescence microscopy. HeLa cells were transiently cotransfected with GFP-tagged EGFR and either wild-type SNX2 or SNX2 ΔRRF mutant, and treated with agonist for 20 min at 37°C. Cells were fixed and immunostained for SNX2 expression and examined by confocal microscopy. In cells transfected with wild-type SNX2, agonist treatment induced substantial redistribution of EGFR into endocytic vesicles (Figure 9A). Activated EGFR was similarly internalized into endosomes in SNX2 ΔRRF mutant-expressing cells (Figure 9B). An adjacent cell that lacked SNX2 expression also showed substantial agonist-induced EGFR internalization (Figure 9B, arrow). Thus, the marked inhibitory effect of SNX2 ΔRRF mutant on EGFR degradation is unlikely to involve regulation at the level of receptor internalization.
Figure 9.
Agonist-induced EGFR internalization in SNX2 wild-type and mutant expressing cells. HeLa cells transiently cotransfected with GFP-tagged EGFR and either (A) SNX2 wild-type (WT) or (B) ΔRRF mutant were serum deprived and then treated in the absence (control) or presence of 100 ng/ml EGF for 20 min at 37°C. Cells were fixed, immunostained for SNX2 expression, and imaged by confocal microscopy. Note the presence of EGFR-positive endosomes in both SNX2 wild-type and ΔRRF mutant-transfected cells. These results are representative of many cells examined in at least three individual experiments. Bar, 10 μm.
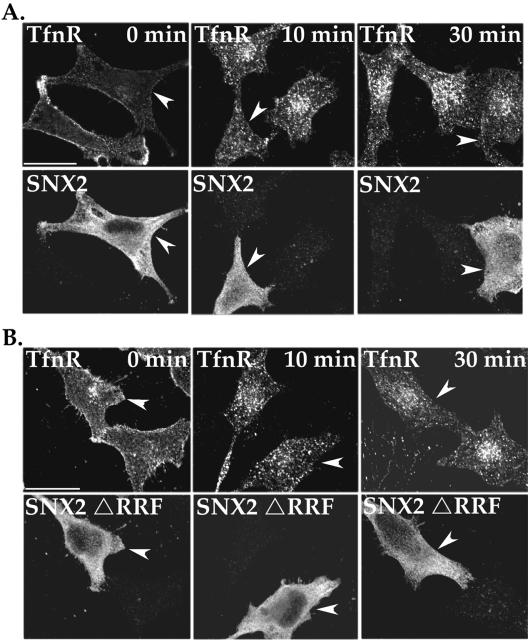
To exclude the possibility that SNX2 ΔRRF mutant has nonspecific inhibitory effects on protein trafficking, we examined the steady-state distribution of TfnR. HeLa cells transiently transfected with SNX2 wild-type or ΔRRF mutant were incubated with anti-TfnR antibody for 1 h at 4°C; under these conditions, only receptors residing on the cell surface bind antibody. In control cells (0 min), the extent of TfnR localized to the cell surface was not significantly altered in either wild-type and mutant SNX2-expressing cells compared with adjacent untransfected cells (Figure 10, A and B). After 10 and 30 min of incubation, TfnR was found distributed in vesicles dispersed throughout the cytoplasm in wild-type and mutant SNX2-expressing cells similar to that observed in untransfected control cells (Figure 10, A and B). Together, these findings suggest that overexpression of wild-type or mutant SNX2 does not alter endocytosis and recycling of TfnR.
Figure 10.
Effect of wild-type and mutant SNX2 on TfnR trafficking. (A and B) HeLa cells transiently transfected with either SNX2 wild type or mutant were serum starved and incubated with anti-TfnR antibody for 1 h at 4°C. Cells were washed to remove unbound antibody and then incubated in medium for 0, 10, or 30 min at 37°C. Cells were then fixed, permeabilized, and immunostained for TfnR and SNX2 and processed for microscopy. The arrowheads indicate cells transfected with either wild-type or mutant SNX2. The findings are representative of many cells examined in two independent experiments. Bar, 10 μm.
siRNA Knockdown of SNX1 and SNX2 Fails to Affect Agonist-induced EGFR degradation
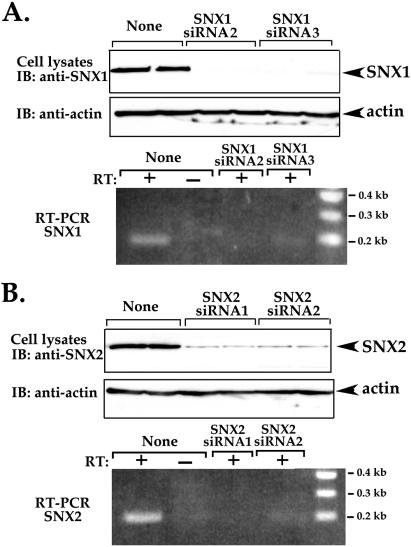
We first determined whether siRNAs targeted against specific SNX1 and SNX2 mRNA sequences were effective at reducing expression of these proteins in HeLa cells. HeLa cells were transiently transfected with SNX1 or SNX2 siRNAs, and the amounts of SNX1 and SNX2 protein and mRNA levels were then determined. After 48 h of siRNA transfection, cells lysates were prepared and immunoblotted for either SNX1, SNX2, or actin protein expression. The expression of SNX1 was virtually abolished in cells transfected with two different siRNAs directed against SNX1 mRNA, whereas actin and SNX2 expression was not effected (Figure 11A; our unpublished data). Consistent with loss of SNX1 protein, siRNAs caused a significant decrease in SNX1 mRNA transcripts as detected by RT-PCR (Figure 11A). Small interfering RNAs directed against SNX2 mRNA sequences also caused a significant 80–90% decrease in SNX2 protein, whereas SNX2 mRNA transcripts were undetectable (Figure 11B). Thus, both SNX1 and SNX2 siRNAs caused almost complete loss of protein expression in HeLa cells.
Figure 11.
SNX1 and SNX2 knockdown by siRNA in HeLa cells. (A and B) HeLa cells were transiently transfected with 100 nM siRNA targeted to either SNX1 or SNX2 mRNA sequences or mock transfected indicated as “None.” Cell lysates were prepared and immunoblotted with either anti-SNX1 or anti-SNX2 antibodies (top panels). Actin expression was also detected as a loading control. Total cellular RNA was also prepared from siRNA transfected cells and reverse-transcribed and PCR amplified. A mock RT-PCR of total cellular RNA with no reverse transcriptase added is shown in lanes marked as RT: –.
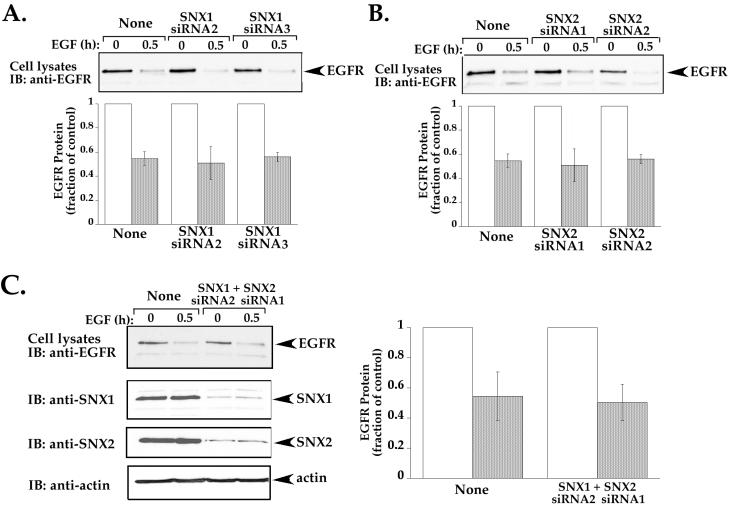
We next examined whether SNX1 and SNX2 proteins are necessary for agonist-induced EGFR degradation. HeLa cells transiently transfected with siRNAs targeted against either SNX1 or SNX2 mRNA sequences were incubated in the absence or presence of EGF for 0.5 h and the amount of endogenous EGFR protein remaining was determined by immunoblot. The extent of SNX1 and SNX2 knockdown of protein expression in these experiments is shown in Figure 11. In mock-transfected cells, an ∼50% decrease in EGFR protein was detected following 0.5 h of agonist incubation (Figure 12). In cells transfected with either SNX1 or SNX2 siRNAs, a similar ∼50% decrease in EGFR protein was induced after 0.5 h of agonist exposure (Figure 12, A and B), suggesting that neither protein is essential for EGFR trafficking. It is possible that SNX1 and SNX2 have redundant functions (Schwarz et al., 2002) and are capable of compensating for each other in critical cellular processes. Therefore, we examined EGFR down-regulation in cells in which both SNX1 and SNX2 protein expression has been substantially diminished by siRNA (Figure 12C). Surprisingly, in cells lacking both SNX1 and SNX2 expression, the extent of agonist-induced EGFR degradation was not significantly different than that observed in mock-transfected control cells, ∼50% decrease in receptor protein was observed in all conditions (Figure 12C). Together, these findings suggest that both SNX1 and SNX2 are involved in regulating lysosomal sorting of EGFR but that neither protein is essential for this process.
Figure 12.
Knockdown of SNX1 and SNX2 protein expression fails to affect agonist-induced EGFR degradation. (A and B) HeLa cells were transiently transfected with either 100 nM SNX1 or SNX2-specific siRNAs as described above and then incubated in the absence or presence of EGF (100 ng/ml) for 0.5 h at 37°C. “None” indicates cells that were mock transfected. Cell lysates were prepared and the amount of endogenous EGFR remaining was detected by immunoblot and quantitated as described above. The data shown (mean ± SEM) are representative of three independent experiments performed in duplicate. (C) HeLa cells were transiently transfected with 100 nM SNX1 and SNX2 siRNAs to achieve knockdown of both proteins. Cells were then incubated with 100 ng/ml EGF for 0.5 h at 37°C, lysates were prepared and immunblotted as described. The extent of EGFR degradation was quantitated and shown as fraction of control. Note that neither SNX1 nor SNX2 seems to be essential for agonist-induced EGFR down-regulation.
DISCUSSION
The function of SNX1 and SNX2 in intracellular protein trafficking in mammalian cells is not clearly understood. To begin to elucidate the function of these proteins, we first examined the intracellular distribution of endogenous SNX1 and SNX2 in HeLa cells. Our confocal microscopy studies indicate that endogenous SNX2 is markedly colocalized with the early endosome marker EEA1, whereas endogenous SNX1 is found partially in EEA1-positive endosomes and in tubulovesicular-like structures distributed throughout the cytoplasm. Consistent with the largely distinct subcellular localization of SNX1 and SNX2, a robust association was found between SNX1 and the retromer Vps35 and Vps29 subunits, a complex thought to function in retrograde trafficking between endosomes and the Golgi. By contrast, SNX2 failed to interact with any of the retromer Vps subunits in mammalian cells. These results are consistent with the direct binding observed between Vps35 and SNX1, but not SNX2, assessed using the yeast-two hybrid system (Haft et al., 2000). Together, these findings raise the distinct possibility that SNX1 and SNX2 can have unique functions in mammalian cells.
Several recent studies using both genetic and biochemical approaches suggest the possibility of distinct functions for SNX1 and SNX2 in mammalian cells. Mice deficient in both SNX1 and SNX2 result in embryonic lethality (Schwarz et al., 2002). These results indicate that both SNX1 and SNX2 are essential for mouse development. Interestingly, however, mice lacking only SNX1 or SNX2 are viable and fertile, suggesting that these proteins do not have to act together as a heterocomplex in embryonic development. Moreover, phenotypic differences between SNX1 and SNX2 were noted in mice by reducing the dosage of the paralogous gene in Snx1+/–;Snx2–/– and Snx1–/–;Snx2+/– mice. These differences could be simply due to differences in protein expression during development or attributed to unique functional properties of SNX1 and SNX2 proteins. Indeed, SNX1 and SNX2 bind to some common and also to distinct cell surface receptors (Haft et al., 1998). Consistent with these results, we demonstrate differential binding of SNX1 and SNX2 to the retromer complex in HeLa cells (Figure 4). Intriguingly, the interaction of SNX1 with the retromer complex is mediated via its amino-terminal domain, the most divergent portion of the molecule that shares only 26% amino acid homology with SNX2. In addition, SNX1 binds to a variety of membrane phospholipids including PtdIns-3,4,5-P3, PtdIns-3,5-P2, PtdIns-3-P, whereas SNX2 binds preferentially to PtdIns-3-P (Cozier et al., 2002; Zhong et al., 2002). It is possible that the different interacting partners of SNX1 and SNX2 could influence the specificity of PtdInsPs binding and hence distinct membrane localization and function.
A function for SNX1 in endosome-to-lysosome sorting of EGFR and protease-activated receptor-1 has been suggested based on studies using overexpression of SNX1 deletion mutants (Kurten et al., 1996; Wang et al., 2002; Zhong et al., 2002). SNX1 can associate with SNX2, SNX4, SNX6, and SNX15, suggesting that other sorting nexins besides SNX1 can regulate receptor trafficking. Indeed, we found that a PX domain mutant of SNX2 markedly inhibits agonist-induced EGFR degradation, whereas EGFR internalization remained intact. The colocalization of activated endogenous EGFR and SNX2 provides further evidence in support of a role for SNX2 in trafficking of EGFR. Our studies also indicate that endogenous SNX2 is localized primarily to early endosomes, suggesting that SNX2 regulates EGFR trafficking at an early endosomal stage. Consistent with this idea, SNX2 has been shown to bind to PtdIns-3-P, a phospholipid highly enriched in endosomal membranes, and mutation of critical residues important for lipid binding results in the loss of SNX2 vesicle localization (Figure 6B). Interestingly, endogenous SNX2 localization was also severely altered by inhibition of PI-3 kinase activity and a mutant Rab5 Q79L, both of which have previously been shown to disrupt membrane trafficking to the lysosome (Brown et al., 1995; Shpetner et al., 1996). Thus, the localization of SNX2 to early endosomes and the ability of mutant SNX2 to inhibit internalized EGFR degradation strongly suggest that SNX2 is involved in mediating lysosomal sorting of EGFR.
Both SNX1 and SNX2 have been shown to associate with EGFR by coimmunoprecipitation in transiently transfected COS-7 cells (Haft et al., 1998). However, the relative contribution of SNX1 versus SNX2 in regulation of EGFR trafficking remains unclear. We show that PX domain-mutated SNX2 retains the ability to dimerize with both wild-type SNX1 and SNX2. Thus, it is conceivable that mutant SNX2 functions by sequestering either endogenous SNX1 or SNX2 and thereby inhibits sorting of internalized EGFR from endosomes to lysosomes. However, neither SNX1 nor SNX2 seem to be essential for EGFR down-regulation based on our studies with siRNA-mediated knockdown of SNX expression (Figures 11 and 12). Interestingly, SNX1 PX domain mutants K214A and RR185/6GS defective in PtdInsP-binding fail to inhibit EGFR degradation in HeLa cells (Figure 8) (Cozier et al., 2002; Zhong et al., 2002). In contrast, a SNX1 N-PX deletion mutant significantly affected EGFR degradation, consistent with that reported previously (Zhong et al., 2002). These observations raise the possibility that although not essential, SNX1 and SNX2 are involved in regulating trafficking of EGFR perhaps via distinct mechanisms.
The mechanism by which SNX1 and SNX2 regulate endocytic sorting of EGFR is not known. It is possible that mutant SNXs sequester other endogenous proteins whose function is critical for sorting of internalized EGFR from endosomes to lysosomes, because neither SNX1 nor SNX2 is essential for this process. Both tumor susceptibility gene 101 (tsg101) and hepatocyte growth factor-regulated tyrosine kinase substrate (HRS) are critically important for endosomal sorting of EGFR (Bishop et al., 2002; Lu et al., 2003). The TSG101/HRS complex has been shown to interact with a variety of other proteins implicated in endosomal trafficking, including SNX1 (Chin et al., 2001). TSG101/HRS interaction is thought to coordinate functions of macromolecular complexes involved in early and late endosomal sorting. It is not known whether SNX2 interacts with TSG101/HRS and thereby contributes to the regulation of EGFR trafficking.
In summary, we used HeLa cells as a model system to investigate the function of SNX1 and SNX2 in protein trafficking. SNX1 was discovered as a protein that interacted with the EGFR cytoplasmic tail and promoted lysosomal degradation (Kurten et al., 1996). In these studies, we demonstrate that SNX2 is also involved in regulating lysosomal sorting of EGFR. These studies are the first to describe a function for SNX2 in protein trafficking. We also demonstrate that endogenous SNX2 is localized primarily to early endosomes, whereas endogenous SNX1 is found partially in early endosomes and tubulovesicular-like structures. In contrast to SNX2, SNX1 robustly associates with the retromer complex, suggesting a function in endosome-to-Golgi trafficking. These findings bring new insight into how SNX1 and SNX2 might serve differential functions in mammalian cells. The challenge now becomes to elucidate the mechanism by which SNX1 and SNX2 regulate sorting of proteins in the same or distinct intracellular compartments.
Acknowledgments
We thank Drs. Sharon Milgram and David P. Siderovski and members of our laboratory for comments and helpful discussions. National Institutes of Health Grants HL67697 and HL073328 (to J.T.) supported this work. M.M. P. is supported by an American Heart Association Predoctoral Fellowship.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E03–09–0711. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E03–09–0711.
Abbreviations used: EEA1, early endosome antigen-1; EGFR, epidermal growth factor receptor; GFP, green fluorescent protein; LAMP1, lysosome-associated protein-1; PI, phosphoinositide; PX, phox homology; siRNA, small interfering RNA; SNX, sorting nexin; TfnR, transferrin receptor; TGN, trans-Golgi network, Vps, vacuolar protein-sorting.
References
- Bishop, N., Horman, A., and Woodman, P. (2002). Mammalian class E vps proteins recognize ubiquitin and act in the removal of endosomal protein-ubiquitin conjugates. J. Cell Biol. 157, 91–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo, J., et al. (2001). The crystal structure of the PX domain from p40phox bound to phosphatidylinositol 3-phosphate. Mol. Cell. 8, 829–839. [DOI] [PubMed] [Google Scholar]
- Brown, W.J., DeWald, D.B., Emr, S.D., Plutner, H., and Balch, W.E. (1995). Role for phosphatidylinositol 3-kinase in the sorting and transport of newly synthesized lysosomal enzymes in mammalian cells. J. Cell Biol. 130, 781–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter, R.E., and Sorkin, A. (1998). Endocytosis of functional epidermal growth factor receptor-green fluorescent protein chimera. J. Biol. Chem. 273, 35000–35007. [DOI] [PubMed] [Google Scholar]
- Chin, L.S., Raynor, M.C., Wei, X., Chen, H.Q., and Li, L. (2001). Hrs interacts with sorting nexin 1 and regulates degradation of epidermal growth factor receptor. J. Biol. Chem. 276, 7069–7078. [DOI] [PubMed] [Google Scholar]
- Christoforidis, S., McBride, H., Burgoyne, R., and Zerial, M. (1999). The Rab5 effector EEA1 is a core component of endosome docking. Nature 397, 621–625. [DOI] [PubMed] [Google Scholar]
- Cozier, G.E., Carlton, J., McGregor, A.H., Gleeson, P.A., Teasdale, R.D., Mellor, H., and Cullen, P.J. (2002). The phox homology (PX) domain-dependent, 3-phosphoinositide-mediated association of sorting nexin-1 with an early sorting endosomal compartment is required for its ability to regulate epidermal growth factor receptor degradation. J. Biol. Chem. 277, 48730–48736. [DOI] [PubMed] [Google Scholar]
- Haft, C.R., Sierra, M., Bafford, R., Lesniak, M.A., Barr, V.A., and Taylor, S.I. (2000). Human orthologs of yeast vacuolar protein sorting proteins Vps26, 29, and 35: assembly into multimeric complexes. Mol. Biol. Cell. 11, 4105–4116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haft, C.R., Sierra, M., Barr, V.A., Haft, D.H., and Taylor, S.I. (1998). Identification of a family of sorting nexin molecules and characterization of their association with receptors. Mol. Cell. Biol. 18, 7278–7287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horazdovsky, B.F., Davies, B.A., Seaman, M.N.J., McLaughin, S.A., Yoon, S.-H., and Emr, S.D. (1997). A sorting nexin-1 homologue, Vps5p, forms a complex with Vps17p and is required for recycling the vacuolar protein-sorting receptor. Mol. Biol. Cell. 8, 1529–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjer-Nielsen, L., van Vliet, C., Erlich, R., Toh, B.-H., and Gleeson, P.A. (1999). The Golgi-targeting sequence of the peripheral membrane protein p230. J. Cell Sci. 112, 1645–1654. [DOI] [PubMed] [Google Scholar]
- Kurten, R.C., Cadena, D.L., and Gill, G.N. (1996). Enhanced degradation of EGF receptors by a sorting nexin, SNX1. Science 272, 1008–1010. [DOI] [PubMed] [Google Scholar]
- Kurten, R.C., Eddington, A.D., Chowdhury, P., Smith, R.D., Davidson, A.D., and Shank, B.B. (2001). Self-assembly and binding of a sorting nexin to sorting endosomes. J. Cell Sci. 114, 1743–1756. [DOI] [PubMed] [Google Scholar]
- Lemmon, S.K., and Traub, L.M. (2000). Sorting in the endosomal system in yeast and animal cells. Curr. Opin. Cell Biol. 12, 457–466. [DOI] [PubMed] [Google Scholar]
- Leprince, C., Le Scolan, E., Meunier, B., Fraiser, V., Brandon, N., De Gunzburg, J., and Camonis, J. (2003). Sorting nexin 4 and amphiphysin 2, a new partnership between endocytosis and intracellular trafficking. J. Cell Sci. 116, 1937–1948. [DOI] [PubMed] [Google Scholar]
- Lu, Q., Hope, L.W., Brasch, M., Reinhard, C., and Cohen, S.N. (2003). TSG101 interaction with HRS mediates endosomal trafficking and receptor down-regulation. Proc. Natl. Acad. Sci. USA 100, 7626–7631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noack, D., Rae, J., Cross, A.R., Ellis, B.A., Newburger, P.E., Curnutte, J.T., and Heyworth, P.G. (2001). Autosomal recessive chronic granulomatous disease caused by defects in NCF-1, the gene encoding the phagocyte p47-phox: mutations not arising in the NCF-1 pseudogenes. Blood 97, 305–311. [DOI] [PubMed] [Google Scholar]
- Nothwehr, S.F., and Hindes, A.E. (1997). The yeast VPS5/GRD2 gene encodes a sorting nexin-1-like protein required for localizing membrane proteins to the late Golgi. J. Cell Sci. 110, 1063–1072. [DOI] [PubMed] [Google Scholar]
- Schwarz, D.G., Griffin, C.T., Schneider, E.A., Yee, D., and Magnuson, T. (2002). Genetic analysis of sorting nexins 1 and 2 reveals a redundant and essential function in mice. Mol. Biol. Cell. 13, 3588–3600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaman, M.N.J., McCaffery, J.M., and Emr, S.D. (1998). A membrane coat complex essential for endosome-to-Golgi retrograde transport in yeast. J. Cell Biol. 142, 665–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shpetner, H., Joly, M., Hartley, D., and Corvera, S. (1996). Potential sites of PI-3 kinase function in the endocytic pathway revealed by the PI-3 kinase inhibitor, wortmannin. J. Cell Biol. 132, 595–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenmark, H., Parton, R.G., Steele-Mortimer, O., Lutcke, A., Gruenberg, J., and Zerial, M. (1994). Inhibition of rab5 GTPase activity stimulates membrane fusion in endocytosis. EMBO J. 13, 1287–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trejo, J., Altschuler, Y., Fu, H.-W., Mostov, K.E., and Coughlin, S.R. (2000). Protease-activated receptor downregulation: a mutant HeLa cell line suggests novel requirements for PAR1 phosphorylation and recruitment to clathrin-coated pits. J. Biol. Chem. 275, 31255–31265. [DOI] [PubMed] [Google Scholar]
- Trejo, J., and Coughlin, S.R. (1999). The cytoplasmic tails of protease-activated receptor-1 and substance P receptor specify sorting to lysosomes versus recycling. J. Biol. Chem. 274, 2216–2224. [DOI] [PubMed] [Google Scholar]
- Wang, Y., Zhou, Y., Szabo, K., Haft, C.R., and Trejo, J. (2002). Down-regulation of protease-activated receptor-1 is regulated by sorting nexin 1. Mol. Biol. Cell. 13, 1965–1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worby, C.A., and Dixon, J.E. (2002). Sorting out the cellular functions of sorting nexins. Nat. Rev. 3, 919–931. [DOI] [PubMed] [Google Scholar]
- Xu, Y., Hortsman, H., Seet, S., Wong, S.H., and Hong, W. (2001). SNX3 regulates endosomal function through its PX-domain-mediated interaction with PtdIns(3)P. Nat. Cell Biol. 3, 658–666. [DOI] [PubMed] [Google Scholar]
- Zhong, Q., Lasar, C.S., Tronchere, H., Sato, T., Meerloo, T., Yeo, M., Songyang, Z., Emr, S.D., and Gill, G.N. (2002). Endosomal localization and function of sorting nexin 1. Proc. Natl. Acad. Sci. USA 99, 6767–6772. [DOI] [PMC free article] [PubMed] [Google Scholar]