Significance
The nucleotide-binding oligomerization domain-like receptor (NLR) family members, NLRC4 and NLRP3, activate the inflammasome to provide host defenses against infection. The precise molecular constituents of an inflammasome are unknown; however, it is believed that receptor-specific complexes containing apoptosis-associated speck-like protein containing a caspase activation and recruitment domain (ASC) and caspase-1 are formed. Here, we used confocal and superresolution microscopy to show that in macrophages infected with Salmonella Typhimurium, a pathogen that activates two distinct NLRs, ASC forms an outer ring-like structure that comprises NLRC4, NLRP3, caspase-1, caspase-8, and pro–IL-1β within the same macromolecular complex. These results suggest that the inflammasome is a highly dynamic macromolecular protein complex capable of recruiting different NLRs and effectors to coordinate inflammasome responses to infection.
Keywords: innate immunity, bacteria, caspase-1, caspase-8, ASC
Abstract
Pathogen recognition by nucleotide-binding oligomerization domain-like receptor (NLR) results in the formation of a macromolecular protein complex (inflammasome) that drives protective inflammatory responses in the host. It is thought that the number of inflammasome complexes forming in a cell is determined by the number of NLRs being activated, with each NLR initiating its own inflammasome assembly independent of one another; however, we show here that the important foodborne pathogen Salmonella enterica serovar Typhimurium (S. Typhimurium) simultaneously activates at least two NLRs, whereas only a single inflammasome complex is formed in a macrophage. Both nucleotide-binding domain and leucine-rich repeat caspase recruitment domain 4 and nucleotide-binding domain and leucine-rich repeat pyrin domain 3 are simultaneously present in the same inflammasome, where both NLRs are required to drive IL-1β processing within the Salmonella-infected cell and to regulate the bacterial burden in mice. Superresolution imaging of Salmonella-infected macrophages revealed a macromolecular complex with an outer ring of apoptosis-associated speck-like protein containing a caspase activation and recruitment domain and an inner ring of NLRs, with active caspase effectors containing the pro–IL-1β substrate localized internal to the ring structure. Our data reveal the spatial localization of different components of the inflammasome and how different members of the NLR family cooperate to drive robust IL-1β processing during Salmonella infection.
Inflammasomes are cytosolic multimeric protein complexes formed in the host cell in response to the detection of pathogen-associated molecular patterns (PAMPs) or danger-associated molecular patterns (DAMPs). Formation of the inflammasome in response to PAMPs is critical for host defense because it facilitates processing of the proinflammatory cytokines pro–IL-1β and pro–IL-18 into their mature forms (1). The inflammasome also initiates host cell death in the form of pyroptosis, releasing macrophage-resident microbes to be killed by other immune mechanisms (2). The current paradigm is that there are individual, receptor-specific inflammasomes consisting of one nucleotide-binding oligomerization domain-like receptor (NLR; leucine-rich repeat–containing) or PYHIN [pyrin domain and hematopoietic expression, interferon-inducible nature, and nuclear localization (HIN) domain-containing] receptor, the adaptor protein apoptosis-associated speck-like protein containing a caspase activation and recruitment domain (CARD; ASC), and caspase-1 (3). How the protein constituents of the inflammasome are spatially orientated is unclear.
Nucleotide-binding domain and leucine-rich repeat caspase recruitment domain 4 (NLRC4) and nucleotide-binding domain and leucine-rich repeat pyrin domain 3 (NLRP3) are the best-characterized inflammasomes, especially with respect to their responses to pathogenic bacteria. The NLRC4 inflammasome is activated primarily by bacteria, including Aeromonas veronii (4), Escherichia coli (5), Listeria monocytogenes (6, 7), Pseudomonas aeruginosa (5), Salmonella enterica serovar Typhimurium (S. Typhimurium) (5, 8–10), and Yersinia species (11). In mouse macrophages, the NLRC4 inflammasome responds to flagellin and type III secretion system-associated needle or rod proteins (5, 8, 9) after their detection by NLR family, apoptosis inhibitory protein (NAIP) 5 or NAIP6 and NAIP1 or NAIP2, respectively (12–15). Phosphorylation of NLRC4 at a single, evolutionarily conserved residue, Ser 533, by PKCδ kinase is required for NLRC4 inflammasome assembly (16). The NLRP3 inflammasome is activated by a large repertoire of DAMPs, including ATP, nigericin, maitotoxin, uric acid crystals, silica, aluminum hydroxide, and muramyl dipeptide (17–20). NLRP3 is also activated by bacterial PAMPs from many species, including Aeromonas species (4, 21), L. monocytogenes (6, 7, 22), Neisseria gonorrhoeae (23), S. Typhimurium (10), Streptococcus pneumoniae (24), and Yersinia species (11). The mechanisms by which NLRC4 and NLRP3 inflammasomes contribute to host defense against bacterial pathogens are emerging; however, little is known about the dynamics governing inflammasome assembly in infections caused by bacteria that activate multiple NLRs, such as S. Typhimurium (10), A. veronii (4), and Yersinia (11).
NLRP3 does not have a CARD and requires ASC to interact with the CARD of procaspase-1. This interaction requires a charged interface around Asp27 of the procaspase-1 CARD (25). Whether ASC is also required for the assembly of the NLRC4 inflammasome is less clear. NLRC4 contains a CARD that can interact directly with the CARD of procaspase-1 (26); however, ASC is required for some of the responses driven by NLRC4 (27). Macrophages infected with S. Typhimurium or other pathogens exhibit formation of a distinct cytoplasmic ASC focus or speck, which can be visualized under the microscope and is indicative of inflammasome activation (10, 28, 29). Our laboratory and others have shown that only one ASC speck is formed per cell irrespective of the stimulus used (29–32). However, many bacteria activate two or more NLRs, and it is unclear whether a singular inflammasome is formed at a time or if multiple inflammasomes are formed independent of each other, with each inflammasome containing one member of the NLR family.
In this study, we describe the endogenous molecular constituents of the Salmonella-induced inflammasome and their spatial orientation. In cross-section, ASC forms a large external ring with the NLRs and caspases located internally. Critically, NLRC4, NLRP3, caspase-1, and caspase-8 coexist in the same ASC speck to coordinate pro–IL-1β processing. All ASC specks observed contained both NLRC4 and NLRP3. These results suggest that Salmonella infection induces a single inflammasome protein complex containing different NLRs and recruiting multiple caspases to coordinate a multifaceted inflammatory response to infection.
Results
NLR and Caspase-1 Are Both Spatially Organized Within the Salmonella-Induced ASC Speck.
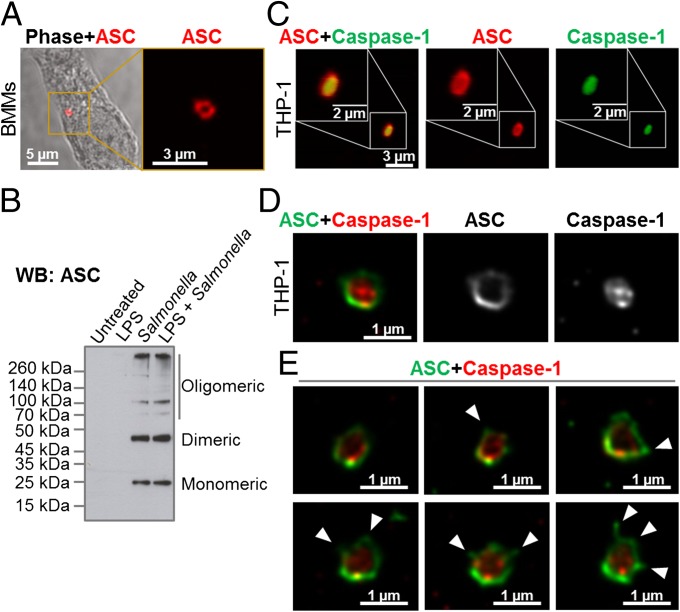
The inflammasome adaptor protein ASC aggregates and forms a large speck in response to different inflammasome stimuli. Using confocal imaging, we immunolocalized ASC after infection of primary bone marrow-derived macrophages (BMMs) with S. Typhimurium [multiplicity of infection (MOI) of 10] for 1 h. ASC redistributed to form a focus or speck in BMMs infected with S. Typhimurium (Fig. 1A), whereas endogenous ASC predominately distributed throughout the cytosol of unstimulated BMMs (Fig. S1A). Each infected cell contained one ASC speck with a diameter of 0.6–0.7 μm after infection with S. Typhimurium (Fig. 1A). Protein oligomerization assays confirmed that ASC formed oligomers following Salmonella infection (Fig. 1B). Specks that appeared in a cross-section orientation had an apparent hole in the middle (Fig. 1A). A similar ASC “ring” structure was observed in human THP-1 macrophages (Fig. 1C and Fig. S1B). We used Bayesian localization superresolution microscopy to obtain higher magnification images of the specks in cross-section (33). After infection of THP-1 cells or BMMs with S. Typhimurium (MOI of 10) for 30 min, immunolabeled ASC specks from different cells were visualized and the images were compared with each other. Analysis of multiple cross-sections of an ASC speck revealed that it forms a ring-like structure (Fig. 1D). Immunolabeling of ASC using two different antibodies revealed the same ring-like structure (Fig. S1C), suggesting that this structure is not due to the effect of a particular antibody. Further analysis of the ASC specks showed the presence of side filaments frequently decorating the external region of the ASC ring structure (Fig. 1E).
Fig. 1.
Spatial organization of the ASC speck. (A) Immunolabeling of endogenous ASC in primary BMMs infected with S. Typhimurium. (B) Endogenous ASC oligomerized in unprimed or LPS-primed BMMs following S. Typhimurium infection. (C) Confocal microscopy imaging of endogenous immunolabeled ASC and fluorescent-labeled inhibitor of caspases (FLICA) staining of caspase-1 in human THP-1 macrophages infected with S. Typhimurium. (D) Bayesian localization superresolution microscopy of endogenous ASC and caspase-1 in C. (E) Multiple cross-sections of the ASC–caspase-1 speck. Arrowheads indicate side filaments of ASC coming off the external region of the ring-like structure.
How other endogenous proteins of the inflammasome associate with and spatially distribute relative to ASC is unclear. We used superresolution microscopy to resolve the spatial orientation of ASC, caspase-1, and either NLRP3 or NLRC4 in macrophages infected with S. Typhimurium. Confocal imaging using caspase-1–specific fluorescent-labeled inhibitor of caspases labeling in THP-1 cells or mouse BMMs identified caspase-1 specks of a smaller diameter [0.426 ± 0.047 μm (n = 8)] than the ASC specks (Fig. 1 C–E and Fig. S1D). Superresolution imaging of ASC and caspase-1 colocalization showed that the ASC ring surrounded caspase-1, with the caspase-1 filling the central “hole” of the ASC ring (Fig. 1 C–E and Fig. S1D). We attempted to generate a 3D reconstruction of the superresolution ASC structure; however, the point spread function of the microscope is symmetrical, which means that it is not possible to discriminate between point spread functions that are out of focus above the plane of focus and those that are an equal distance below the plane of focus. However, we were able to discriminate between in-focus and out-of-focus point spread functions in the superresolution data. These images show the in-focus fluorophores tending to be localized to the edge of the ring, with the out-of-focus fluorophores being more spread out (Fig. S2). This analysis suggests that the outer ring structure is reasonably well defined in one plane, with some degree of narrowing occurring above or below.
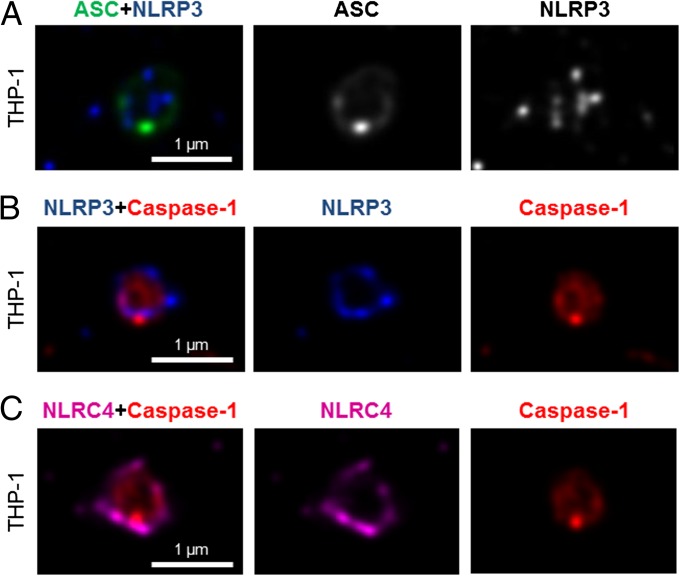
We next wondered how individual NLRs would spatially associate with ASC. LPS-primed THP-1 cells were infected with S. Typhimurium for 30 min at an MOI of 10 and immunolabeled for endogenous NLRP3 and ASC. Confocal imaging of NLRP3 showed, like those seen for caspase-1, specks that were smaller (0.4 μm) than those seen with ASC immunolabeling (Fig. 2A). Superresolution imaging of the colabeled ASC and NLRP3 showed that NLRP3 formed a smaller ring-like structure within the ASC ring (Fig. 2A). This technique also showed that diffused caspase-1 predominantly localized within the NLRP3 ring-like speck in infected BMMs (Fig. 2B). Superresolution microscopy of immunolabeled NLRC4 also revealed smaller specks [0.578 ± 0.079 μm (n = 8)] than those formed by ASC (Fig. 2C and Fig. S3). Caspase-1 predominantly localized in an NLRC4 ring-like speck (Fig. 2C). Immunolocalization of NLRP3 and ASC in the nigericin-stimulated canonical NLRP3 inflammasome produced a similar pattern to that observed in the Salmonella-induced inflammasome structure (Fig. S4). We confirmed our colocalization findings in HEK 293 cells transfected with DNA constructs encoding different inflammasome proteins (Figs. S5 and S6). Taken together, we provide evidence to show that following Salmonella infection of macrophages, endogenous NLRC4 or NLRP3 resides in the ASC speck, within which caspase-1 is located to form an inflammasome complex.
Fig. 2.
NLRs reside in the ASC speck following S. Typhimurium infection of macrophages. Bayesian localization superresolution microscopy of endogenous immunolabeled ASC and NLRP3 (A), endogenous immunolabeled NLRP3 and FLICA-stained caspase-1 (B), and endogenous immunolabeled NLRC4 and FLICA-stained caspase-1 in human THP-1 macrophages infected with S. Typhimurium (C) are shown.
Coordinated Activation of NLRs Sustains Infection-Induced IL-1β Production.
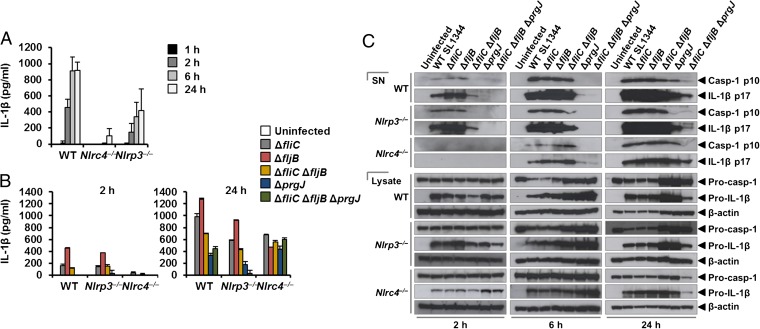
NLRC4 and NLRP3 can both be activated by Salmonella (10), but the dynamics of how NLRC4 and NLRP3 activation is coordinated with respect to their localization within the ASC speck during infection is unclear. Infection of BMMs from WT and Asc−/− mice with log phase-grown WT S. Typhimurium for 2 h showed that Salmonella induction of caspase-1 proteolysis, pro–IL-1β processing, and IL-1β release required ASC (Fig. S7). Both NLRC4 and NLRP3 were required to drive robust IL-1β production (Fig. 3A). To investigate the dynamics of NLRC4- and NLRP3-mediated IL-1β production further, we infected primary BMMs from WT, Nlrc4−/−, or Nlrp3−/− mice with S. Typhimurium lacking flagellin (ΔfliCΔfljB), PrgJ (ΔprgJ), or all known activators of the NLRC4 inflammasome (ΔfliCΔfljBΔprgJ) (14, 15). We found that NLRC4 predominantly drove caspase-1 proteolysis, pro–IL-1β processing, and IL-1β production in response to all Salmonella mutants after 2 h of infection, whereas NLRP3 responded to all mutants following 24 h of infection (Fig. 3 B and C). Our results suggest that NLRC4 and NLRP3 cooperate to induce robust inflammasome activation following infection with S. Typhimurium.
Fig. 3.
Coordinated activation of NLRs is required for IL-1β production induced by S. Typhimurium infection. Unprimed WT, Nlrc4−/−, and Nlrp3−/− primary BMMs were infected with S. Typhimurium SL1344 (A) or its isogenic mutants ΔfliC, ΔfljB, ΔfliCΔfljB, ΔprgJ, and ΔfliCΔfljBΔprgJ (B) (MOI of 1) for the indicated times. (A and B) Supernatant was collected, and the levels of IL-1β were measured using ELISA. (C) Procaspase-1 (Pro–casp-1), cleaved caspase-1 (Casp-1) p10, pro–IL-1β, and cleaved IL-1β p17 were detected in the supernatant (SN) or cell lysate using Western blotting. β-actin was used as a loading control. Data shown are representatives of two (C) and three (A and B) experiments.
Our in vitro data suggest a role for both NLRC4 and NLRP3 in the host response to infection with S. Typhimurium. In lethal salmonellosis, NLRP3 and NLRC4 are required to control the infection on day 5 postinfection (10), but the relative contributions of these NLRs in regulating the bacterial burden over time are unknown. We therefore used a sublethal model of murine salmonellosis, where the mice control bacterial growth and eventually clear the infection over time, which allows us to monitor the impact of NLRs on Salmonella growth over a longer time course. Mice were infected with S. Typhimurium and killed at days 1, 7, and 13 postinfection. A similar bacterial burden was found in all mice at day 1 (Fig. 4). At day 7, both Nlrc4−/− and Nlrp3−/− mice had higher bacterial counts than WT mice (Fig. 4). Casp-1−/−(casp-11−/−) mice had the highest bacterial burden compared with other mice on day 7, suggesting that both NLRs contribute to the host defense against Salmonella infection 7 d postinfection. On day 13, we observed elevated bacterial burden in Nlrc4−/− or Nlrp3−/− mice compared with WT mice (Fig. 4). Unlike day 7, substantially higher bacterial numbers were found in Nlrc4−/− mice compared with Nlrp3−/− mice on day 13. Casp-1−/−(casp-11−/−) mice still contained the highest bacterial burden compared with both Nlrc4−/− and Nlrp3−/− mice. These results suggest that the relative contribution between NLRC4 and NLRP3 in the host control of sublethal salmonellosis is comparable on day 7, whereas on day 13, NLRC4 exerts a greater antibacterial effect than NLRP3. The reason why we see distinct roles for NLRC4 and NLRP3, in comparison to Broz et al. (10), is simply because the differences in bacterial growth only become apparent as the infection progresses over time. This effect can only be seen if the animals can ultimately control bacterial growth. These data support the concept that NLRC4 and NLRP3 coordinate suppression of bacterial growth in vivo during Salmonella infection.
Fig. 4.
NLRC4 and NLRP3 have nonredundant roles in the host defense against sublethal murine S. Typhimurium infection. WT, Nlrc4−/−, Nlrp3−/−, and casp-1−/−(casp-11−/−) mice were infected i.v. with 1.33 × 104 cfu of S. Typhimurium strain M525P (a strain that establishes sublethal murine salmonellosis), and the mean bacterial load was determined in the spleen and liver after 1, 7, and 13 d of infection. Three to four animals of each genotype were used per group per time point. Data are from one set of experiments representative of two. ns, no statistical significance; **P < 0.01; ***P < 0.001.
Multiple NLRs and Caspases Can Be Recruited to the Same Salmonella-Induced Inflammasome.
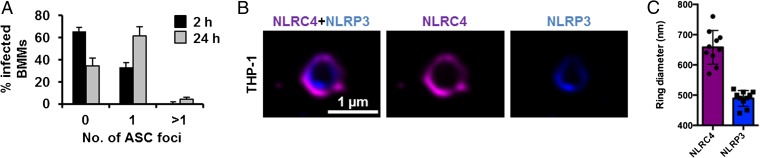
Activation of multiple NLRs in response to Salmonella infection occurs in vitro and in vivo, yet how is this activation achieved if cells only form a single inflammasome per cell? Infection of BMMs with S. Typhimurium for 24 h to induce the activation of both NLRC4 and NLRP3 still resulted in the formation of a single ASC speck per macrophage (Fig. 5A). A possible explanation for the presence of a single speck is that triggering speck formation by multiple NLRs could result in more than one NLR being present in the same inflammasome complex. Rather than having separate NLRP3 or NLRC4 inflammasomes occurring, we hypothesized that the inflammasome could be a single dynamic protein complex that is formed in response to different NLR inputs. NAIPs and NLRC4 are recruited to the same inflammasome, so it is feasible that NLRP3 and NLRC4 may also be present in a single inflammasome complex (14, 15). To investigate this hypothesis, we used superresolution microscopy to visualize immunolabeled endogenous NLRC4 and NLRP3 in LPS-primed THP-1 macrophages infected with S. Typhimurium. All of the ASC specks observed contained both NLRC4 and NLRP3, and image analysis of these ASC foci showed colocalization of both NLRs in the same speck (Fig. 5B). NLRC4 localized in a ring-like structure [ring diameter of 0.658 ± 0.056 μm (n = 10)], whereas NLRP3 formed a smaller and more centralized ring-like structure [ring diameter of 0.489 ± 0.029 μm (n = 10)] (Fig. 5C). We confirmed these findings by coexpressing DNA constructs encoding human NLRC4 and NLRP3 in HEK 293 cells and found that both receptors redistributed and colocalized into a single complex (Fig. S8). Our data suggest that NLRC4 and NLRP3 can be located in the same inflammasome.
Fig. 5.
NLRC4 and NLRP3 are recruited to the same Salmonella-induced ASC speck. (A) Unprimed WT primary BMMs were infected with S. Typhimurium expressing GFP for 2 or 24 h, and were stained for ASC and DNA. The number of ASC foci per infected BMM was counted. At least 180 infected BMMs (indicated by the presence of GFP-expressing S. Typhimurium) were counted in each of the four independent experiments. (B) Bayesian localization superresolution microscopy of endogenous NLRC4 and NLRP3 in THP-1 macrophages infected with S. Typhimurium. (C) Image analysis of the ring diameter of the NLRC4 and NLRP3 structures in B (n = 10).
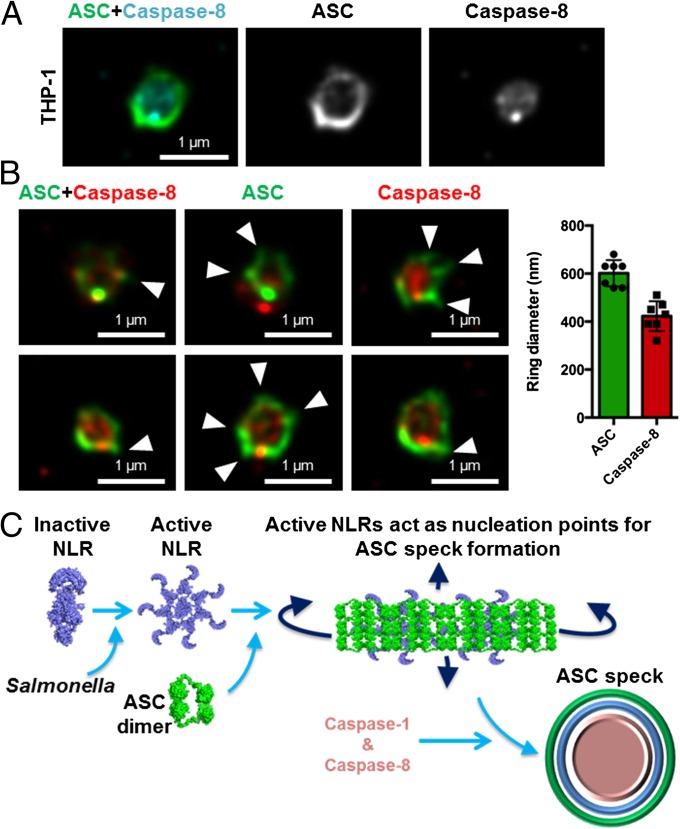
Given that NLRs are oriented in a distinct manner within the speck, we wondered whether inflammasome-associated caspases have a similar spatial orientation. We have previously shown that the ASC speck is required to recruit the inflammasome effectors caspase-1 and caspase-8 into a single complex during Salmonella infection (32) (Fig. S9A). In THP-1 cells, superresolution analysis of the ASC–caspase-8 speck showed a similar distribution to that seen for caspase-1 distribution within the ASC ring, with the diameter of the caspase-8 core measured at 0.422 ± 62 μm (n = 7) (Fig. 6 A and B). We performed additional cross-sectional analysis of the ASC–caspase-8 specks (Fig. 6B) and confirmed these findings in primary BMMs (Fig. S9B). Taken together, we hypothesized that a single inflammasome platform provides a major proteolytic cleavage site within the cell where pro–IL-1β must be recruited for processing. We stained pro–IL-1β, ASC, and caspase-1 and found that pro–IL-1β formed a smaller speck-like structure (0.4 μm) that colocalized within the ASC–caspase-1 speck (Fig. S10), suggesting that pro–IL-1β may be required to enter the inflammasome “pore” for proteolytic processing. It is not unreasonable to speculate that the ASC speck is a substrate-specific entity whereby only specific substrates, such as pro–IL-1β and pro–IL-18, are able to enter for caspase-1– and caspase-8–dependent processing while providing a controlled environment to prevent unregulated caspase-1 and caspase-8 activity within the cell. The concentric organization of the speck suggests that ligand-mediated NLR activation provides a nucleation point for the rapid recruitment of ASC dimers and the subsequent assembly of the ASC speck, concomitant with the recruitment of caspase-1 and caspase-8 (Fig. 6C). This process results in the formation of an inflammasome platform containing more than one NLR family member, which drives the recruitment of multiple effectors to the same protein complex to process pro–IL-1β and pro–IL-18.
Fig. 6.
Caspase-8 is recruited to the same Salmonella-induced ASC speck. (A and B) Bayesian localization superresolution microscopy of endogenous immunolabeled ASC and FLICA-stained caspase-8 in THP-1 macrophages infected with S. Typhimurium. (B) Multiple cross-sections of the ASC–Caspase-8 speck. Arrowheads indicate side filaments of ASC. Image analysis of the ring diameter of the ASC and caspase-8 structures (n = 7). (C) Schematic of ASC speck assembly in response to Salmonella infection in a macrophage.
Discussion
Bacterial infection activates different NLRs to drive inflammasome production of IL-1β and IL-18 in the host tissue to help control the spread of the organism. Here, we provide an answer to a central question in inflammasome biology. That is, in response to pathogens like Salmonella, which activate multiple NLRs, why does only a single ASC speck form? Our combined confocal and superresolution imaging approach along with functional analysis shows, for the first time to our knowledge, that endogenous NLRC4 and NLRP3 colocalize in the same speck. Different NLRs can therefore be present in the same ASC speck to coordinate inflammasome functions after Salmonella infection. A single ASC speck can also contain multiple caspase effectors (caspase-1 and caspase-8) at the same time, suggesting that the inflammasome platform is a dynamic protein complex that contains multiple NLRs and recruits more than one effector caspase to control the production of IL-1β.
Our work provides a clear picture of the gross organization of the endogenous ASC speck. The concentric organization of an external ASC layer surrounding the NLR proteins, which, in turn, surround the effector caspases, provides important insight into the function of the inflammasome. This arrangement permits the dynamic recruitment and movement of speck constituents following activation, thereby ensuring the inflammasome remains functionally active. For example, NLRP3 is critical for sustained pro–IL-1β processing and is recruited to the ASC speck at a later time point than NLRC4, which is instead crucial during early infection. Hence, NLRP3 is positioned inside NLRC4 (Fig. 5B). The formation of an outer shell of ASC, rather than a solid or closed speck, is also consistent with continual dynamic recruitment and with the presence of a functional core consisting of active caspases and pro–IL-1β (Fig. S10), which would be inaccessible if ASC formed a closed shell. The outer ASC shell may also serve to stabilize and protect the internal structure. It is interesting to note that side filaments are often found decorating ASC shell, although the functional significance of these filaments is currently unclear.
The nature of the protein–protein interactions within the speck remains elusive at this resolution, and it is intriguing that direct colocalization of components that reportedly interact is not always observed. This apparent discrepancy is likely to be a result of both the experimental reagents available and the functional organization of the speck. We predominantly costain ASC and active caspase-1 or caspase-8 in our study, resulting in detection of active caspase-1 or caspase-8 in the functional center of the speck, presumably following its release from ASC (Fig. 6). Whereas when we stained ASC and caspase-8 using an antibody that detects both cleaved and proforms, more of the caspase is localized with the ASC ring (compare Fig. 6 and Fig. S9B).
Our results show that the spatial orientation of NLRP3 and ASC in the nigericin-stimulated canonical inflammasome resembles a pattern similar to that observed in the more complex Salmonella-induced inflammasome structure (Fig. S4). We speculate that the composition of the absent in melanoma 2 (AIM2) inflammasome would have a structure similar to that of the multi-NLR inflammasome. Indeed, analysis of the crystal structure of the HIN200 domain of AIM2 and dsDNA revealed that the positively charged HIN domain embraces the dsDNA, whereas the AIM2 pyrin domain located peripheral to the HIN-dsDNA structure is thought to facilitate the recruitment of ASC (34). This proposed structure composed of dsDNA-AIM2 internal to ASC is similar to our observed structure comprising NLRC4 and NLRP3 within an ASC shell. In contrast to our concentric ring structure, where caspase-1 lies within the ASC ring, a recent study using cryo-EM analysis suggests that caspase-1 forms filamentous structures external to ASC following nucleation by either NLRP3 or AIM2 (35). This difference is most likely due to the altered protein levels between an exogenous overexpression system and our own visualization of the endogenous inflammasome. Earlier EM staining of endogenous ASC also reports a hollow, ring-like organization (36). The cryo-EM analysis of the AIM2 ternary complex and the NLRP3 inflammasome represents a snapshot of inflammasome formation and may correlate with the starting point of full speck formation. This step would be followed by additional NLR and ASC recruitment leading, ultimately, to the ring-like structure we observe.
Salmonella activates both NLRC4 and NLRP3, which results in ASC focus formation and recruitment of caspases to the inflammasome (10, 32). Our data show that in vitro NLRP3 contributes to the macrophage response against Salmonella at a later stage than NLRC4 in unprimed conditions. This finding is unsurprising, because NLRP3 expression in BMMs requires induction by LPS or cytokines, such as TNF-α (37). In experiments using unprimed BMMs infected with Salmonella (Fig. 3), there will be a delay before NLRP3 is expressed, whereas NLRC4, which is constitutively expressed, will be activated immediately upon cellular infection. In vivo, as infection progresses, the effect of NLRC4 is slightly more important at day 13 in comparison to days 1 and 7. There are many factors that could explain the differences, including time-dependent effects of NLRC4 and NLRP3 in vitro and in vivo. These factors include the changing profile of cytokines; the effects of NLRC4, but not NLRP3, in driving Salmonella-induced pyroptosis; and the switch in cell type dominance over time during infection. Macrophages, for example, play a key role at day 7, the time point at which NLRC4 and NLRP3 have a similar effect in vivo, whereas by day 13, other cells, such as natural killer cells and CD4+ T cells, are starting to play a major role in controlling the infection.
If NLRC4 and NLRP3 both contribute to the host defense against Salmonella infection, then mice lacking either of these receptors should show a greater susceptibility to Salmonella infection compared with WT mice. Our work supports this hypothesis because mice lacking NLRC4 or NLRP3 have higher bacterial loads than the WT mice at days 7 and 13 postinfection. Broz et al. (10) previously reported that mice lacking NLRC4 or NLRP3 harbor similar bacterial numbers in the spleen, liver, and mesenteric lymph nodes 5 d after Salmonella infection; however, consistent with our observations, they showed that mice lacking both NLRP3 and NLRC4 harbor a higher bacterial load compared with WT controls. We observed similar bacterial numbers in the spleen and liver between WT mice and any of the KO strains at an early time point (day 1), but as the infection progresses (days 7 and 13), we see a clear increase in the bacterial burden in mice lacking NLRC4 or NLRP3. This observation highlights an important role for each of these receptors over the course of Salmonella infection, which is consistent with our in vitro data.
Our data lead us to propose that the inflammasome is a dynamic multiprotein complex, where NLRC4, NLRP3, caspase-1, caspase-8, and pro–IL-1β colocalize to the same ASC inflammasome in macrophages infected with Salmonella. Whether caspase-11 might associate with ASC in the noncanonical inflammasome is unclear, and the spatial distribution of these proteins will be an interesting area for future research. In conclusion, our data reveal cooperative interactions between distinct members of the NLR and caspase families that form a dynamic inflammasome during Salmonella infection.
Materials and Methods
Detailed information is presented in SI Materials and Methods. We used Bayesian localization microscopy to obtain superresolution images of the ASC inflammasome structure (33). Superresolution experiments were performed on a Nikon TI Eclipse inverted microscope using a Nikon Intensilight light source for fluorescence.
Supplementary Material
Acknowledgments
We thank K. A. Fitzgerald (University of Massachusetts Medical School) for critical review of our manuscript and for supplying the KO mouse strains; G. Núñez (University of Michigan Medical School) for the anti-NLRC4 antibody; E. Creagh (Trinity College Dublin) for providing technical advice and the ASC construct; and F. Morgan, S. Achouri, and I. Kazanis (University of Cambridge) for providing assistance in microscopy. S.M.M. was supported by a Cambridge International Scholarship. T.P.M. was supported by Wellcome Trust Research Career Development Fellowship WT085090MA. This study was supported by Biotechnology and Biological Sciences Research Council (BBSRC) Grants BB/H003916/1 and BB/K006436/1 and by BBSRC Research Development Fellowship BB/H021930/1 (to C.E.B.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1402911111/-/DCSupplemental.
References
- 1.Franchi L, Muñoz-Planillo R, Núñez G. Sensing and reacting to microbes through the inflammasomes. Nat Immunol. 2012;13(4):325–332. doi: 10.1038/ni.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miao EA, et al. Caspase-1-induced pyroptosis is an innate immune effector mechanism against intracellular bacteria. Nat Immunol. 2010;11(12):1136–1142. doi: 10.1038/ni.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rathinam VAK, Vanaja SK, Fitzgerald KA. Regulation of inflammasome signaling. Nat Immunol. 2012;13(4):333–342. doi: 10.1038/ni.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McCoy AJ, Koizumi Y, Higa N, Suzuki T. Differential regulation of caspase-1 activation via NLRP3/NLRC4 inflammasomes mediated by aerolysin and type III secretion system during Aeromonas veronii infection. J Immunol. 2010;185(11):7077–7084. doi: 10.4049/jimmunol.1002165. [DOI] [PubMed] [Google Scholar]
- 5.Miao EA, et al. Innate immune detection of the type III secretion apparatus through the NLRC4 inflammasome. Proc Natl Acad Sci USA. 2010;107(7):3076–3080. doi: 10.1073/pnas.0913087107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu J, Fernandes-Alnemri T, Alnemri ES. Involvement of the AIM2, NLRC4, and NLRP3 inflammasomes in caspase-1 activation by Listeria monocytogenes. J Clin Immunol. 2010;30(5):693–702. doi: 10.1007/s10875-010-9425-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meixenberger K, et al. Listeria monocytogenes-infected human peripheral blood mononuclear cells produce IL-1beta, depending on listeriolysin O and NLRP3. J Immunol. 2010;184(2):922–930. doi: 10.4049/jimmunol.0901346. [DOI] [PubMed] [Google Scholar]
- 8.Miao EA, et al. Cytoplasmic flagellin activates caspase-1 and secretion of interleukin 1beta via Ipaf. Nat Immunol. 2006;7(6):569–575. doi: 10.1038/ni1344. [DOI] [PubMed] [Google Scholar]
- 9.Franchi L, et al. Cytosolic flagellin requires Ipaf for activation of caspase-1 and interleukin 1beta in salmonella-infected macrophages. Nat Immunol. 2006;7(6):576–582. doi: 10.1038/ni1346. [DOI] [PubMed] [Google Scholar]
- 10.Broz P, et al. Redundant roles for inflammasome receptors NLRP3 and NLRC4 in host defense against Salmonella. J Exp Med. 2010;207(8):1745–1755. doi: 10.1084/jem.20100257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brodsky IE, et al. A Yersinia effector protein promotes virulence by preventing inflammasome recognition of the type III secretion system. Cell Host Microbe. 2010;7(5):376–387. doi: 10.1016/j.chom.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang J, Zhao Y, Shi J, Shao F. Human NAIP and mouse NAIP1 recognize bacterial type III secretion needle protein for inflammasome activation. Proc Natl Acad Sci USA. 2013;110(35):14408–14413. doi: 10.1073/pnas.1306376110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rayamajhi M, Zak DE, Chavarria-Smith J, Vance RE, Miao EA. Cutting edge: Mouse NAIP1 detects the type III secretion system needle protein. J Immunol. 2013;191(8):3986–3989. doi: 10.4049/jimmunol.1301549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kofoed EM, Vance RE. Innate immune recognition of bacterial ligands by NAIPs determines inflammasome specificity. Nature. 2011;477(7366):592–595. doi: 10.1038/nature10394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao Y, et al. The NLRC4 inflammasome receptors for bacterial flagellin and type III secretion apparatus. Nature. 2011;477(7366):596–600. doi: 10.1038/nature10510. [DOI] [PubMed] [Google Scholar]
- 16.Qu Y, et al. Phosphorylation of NLRC4 is critical for inflammasome activation. Nature. 2012;490(7421):539–542. doi: 10.1038/nature11429. [DOI] [PubMed] [Google Scholar]
- 17.Li H, Willingham SB, Ting JP, Re F. Cutting edge: Inflammasome activation by alum and alum’s adjuvant effect are mediated by NLRP3. J Immunol. 2008;181(1):17–21. doi: 10.4049/jimmunol.181.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mariathasan S, et al. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440(7081):228–232. doi: 10.1038/nature04515. [DOI] [PubMed] [Google Scholar]
- 19.Dostert C, et al. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science. 2008;320(5876):674–677. doi: 10.1126/science.1156995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hornung V, et al. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat Immunol. 2008;9(8):847–856. doi: 10.1038/ni.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McCoy AJ, et al. Cytotoxins of the human pathogen Aeromonas hydrophila trigger, via the NLRP3 inflammasome, caspase-1 activation in macrophages. Eur J Immunol. 2010;40(10):2797–2803. doi: 10.1002/eji.201040490. [DOI] [PubMed] [Google Scholar]
- 22.Kim S, et al. Listeria monocytogenes is sensed by the NLRP3 and AIM2 inflammasome. Eur J Immunol. 2010;40(6):1545–1551. doi: 10.1002/eji.201040425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duncan JA, et al. Neisseria gonorrhoeae activates the proteinase cathepsin B to mediate the signaling activities of the NLRP3 and ASC-containing inflammasome. J Immunol. 2009;182(10):6460–6469. doi: 10.4049/jimmunol.0802696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McNeela EA, et al. Pneumolysin activates the NLRP3 inflammasome and promotes proinflammatory cytokines independently of TLR4. PLoS Pathog. 2010;6(11):e1001191. doi: 10.1371/journal.ppat.1001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kersse K, Lamkanfi M, Bertrand MJ, Vanden Berghe T, Vandenabeele P. Interaction patches of procaspase-1 caspase recruitment domains (CARDs) are differently involved in procaspase-1 activation and receptor-interacting protein 2 (RIP2)-dependent nuclear factor κB signaling. J Biol Chem. 2011;286(41):35874–35882. doi: 10.1074/jbc.M111.242321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Poyet JL, et al. Identification of Ipaf, a human caspase-1-activating protein related to Apaf-1. J Biol Chem. 2001;276(30):28309–28313. doi: 10.1074/jbc.C100250200. [DOI] [PubMed] [Google Scholar]
- 27.Proell M, Gerlic M, Mace PD, Reed JC, Riedl SJ. The CARD plays a critical role in ASC foci formation and inflammasome signalling. Biochem J. 2013;449(3):613–621. doi: 10.1042/BJ20121198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jones JW, et al. Absent in melanoma 2 is required for innate immune recognition of Francisella tularensis. Proc Natl Acad Sci USA. 2010;107(21):9771–9776. doi: 10.1073/pnas.1003738107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang MT, et al. Critical role of apoptotic speck protein containing a caspase recruitment domain (ASC) and NLRP3 in causing necrosis and ASC speck formation induced by Porphyromonas gingivalis in human cells. J Immunol. 2009;182(4):2395–2404. doi: 10.4049/jimmunol.0800909. [DOI] [PubMed] [Google Scholar]
- 30.Fernandes-Alnemri T, et al. The pyroptosome: A supramolecular assembly of ASC dimers mediating inflammatory cell death via caspase-1 activation. Cell Death Differ. 2007;14(9):1590–1604. doi: 10.1038/sj.cdd.4402194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Broz P, von Moltke J, Jones JW, Vance RE, Monack DM. Differential requirement for Caspase-1 autoproteolysis in pathogen-induced cell death and cytokine processing. Cell Host Microbe. 2010;8(6):471–483. doi: 10.1016/j.chom.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Man SM, et al. Salmonella infection induces recruitment of Caspase-8 to the inflammasome to modulate IL-1β production. J Immunol. 2013;191(10):5239–5246. doi: 10.4049/jimmunol.1301581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cox S, et al. Bayesian localization microscopy reveals nanoscale podosome dynamics. Nat Methods. 2012;9(2):195–200. doi: 10.1038/nmeth.1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jin T, et al. Structures of the HIN domain:DNA complexes reveal ligand binding and activation mechanisms of the AIM2 inflammasome and IFI16 receptor. Immunity. 2012;36(4):561–571. doi: 10.1016/j.immuni.2012.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lu A, et al. Unified Polymerization Mechanism for the Assembly of ASC-Dependent Inflammasomes. Cell. 2014;156(6):1193–1206. doi: 10.1016/j.cell.2014.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Masumoto J, et al. ASC, a novel 22-kDa protein, aggregates during apoptosis of human promyelocytic leukemia HL-60 cells. J Biol Chem. 1999;274(48):33835–33838. doi: 10.1074/jbc.274.48.33835. [DOI] [PubMed] [Google Scholar]
- 37.Bauernfeind FG, et al. Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J Immunol. 2009;183(2):787–791. doi: 10.4049/jimmunol.0901363. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.