Abstract
Modulation of a specific prostanoid synthase or receptor provides therapeutic alternatives to non-steroidal anti-inflammatory drugs (NSAIDs) for treating cyclooxygenase-2 (COX-2 or PTGS2)-governed pathological conditions. Among the COX-2 downstream signaling pathways, the prostaglandin E2 (PGE2) receptor EP2 subtype (PTGER2) is emerging as a crucial mediator of many physiological and pathological events. Genetic ablation strategies and recent advances in chemical biology provide tools for a better understanding of EP2 signaling. In the brain, the EP2 receptor modulates some beneficial effects including neuroprotection in acute models of excitotoxicity, neuroplasticity, and spatial learning via cAMP/PKA signaling. Conversely, EP2 activation accentuates chronic inflammation mainly through the cAMP/Epac pathway, likely contributing to delayed neurotoxicity. EP2 receptor activation also engages β-arrestin in a G protein-independent pathway that promotes tumor cell growth and migration. Understanding the conditions under which multiple EP2 signaling pathways are engaged might suggest novel therapeutic strategies targeting this key inflammatory prostaglandin receptor.
Keywords: cyclooxygenase-2, tumorigenesis, innate immunity, epilepsy, neurotoxicity, neuroinflammation
Overview
Cyclooxygenase (COX) is the rate-limiting enzyme to synthesize biological mediators termed prostanoids, consisting of prostaglandin PGD2, PGE2, PGF2α, prostacyclin PGI2, and thromboxane TXA2. Prostanoids function via activation of nine G protein-coupled receptors (GPCRs): DP1 and DP2 receptors for PGD2, EP1, EP2, EP3 and EP4 for PGE2, FP for PGF2α, IP for PGI2, and TP for TXA2 (Figure 1). As the inducible COX isoform, COX-2 is generally regarded as a pro-inflammatory enzyme and contributes to tissue injury [1, 2]. However, the deleterious cardiovascular and cerebrovascular side effects from sustained inhibition of COX-2 point to beneficial actions of some COX-2 downstream prostanoid signaling [3]. The Jekyll and Hyde nature of COX-2 signaling pathways suggests that modulation of a specific prostanoid synthase or receptor could be a superior therapeutic strategy compared with generic block of the entire COX-2 cascade. The rapid induction of COX-2 by cell injury or excessive neuronal activity is often associated with induction of membrane-associated PGE synthase-1 (mPGES-1 or PTGES), which produces PGE2 from COX-2-derived PGH2 [4]. Among the multiple COX-2 downstream signaling pathways, prostaglandin PGE2 signaling via its EP2 receptor subtype appears to be a major mediator of inflammatory and anaphylactic reactions within both the periphery and brain. EP2 signaling pathways engage protein kinase A (PKA), the exchange protein activated by cAMP (Epac), and β-arrestin. Here, we highlight our current understanding of EP2 receptor signaling and summarize its pathophysiological roles in disparate disease conditions involving inflammation such as chronic pain, cancer and brain injury with an emphasis, where possible, on recent in vivo experimental data.
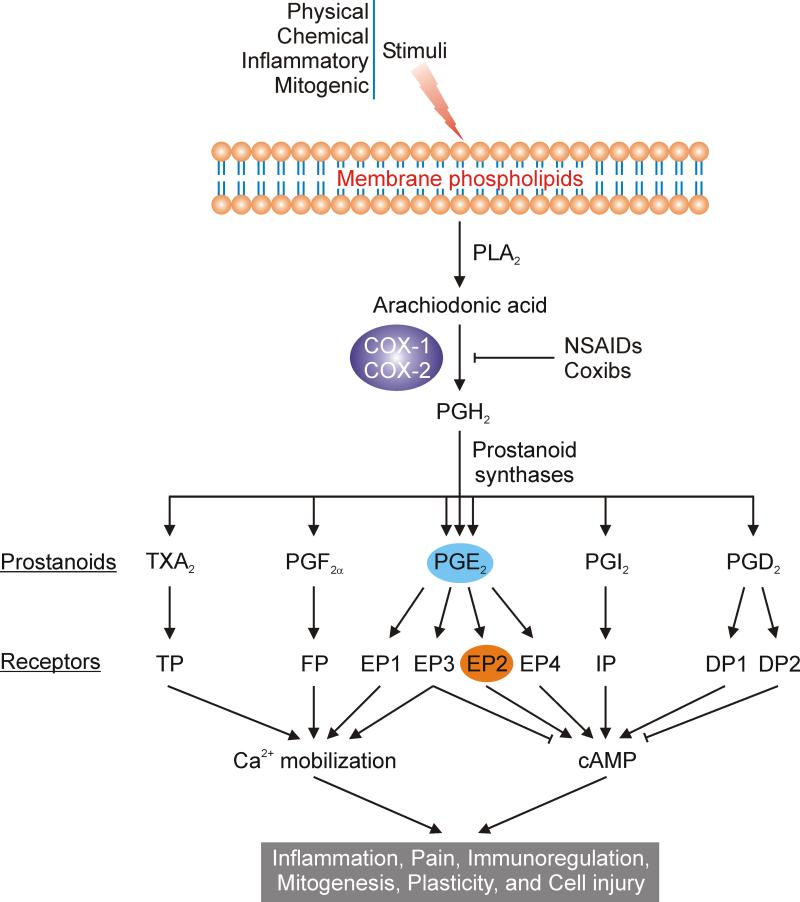
Figure 1.
COX signaling cascade regulates multiple physiological and pathological events. In response to a variety of stimuli, arachiodonic acid (AA), a 20-carbon fatty acid, is freed from membrane phospholipids by phospholipase A2 (PLA2), and then converted in a dual enzymatic reaction to unstable intermediate prostaglandin H2 (PGH2) by cyclooxygenase (COX), which has two forms: COX-1 and COX-2. The COX-1 isozyme is constitutively expressed in most mammalian cells to maintain normal homeostasis, while COX-2 is usually undetectable in most normal tissues but strongly induced by excessive neuronal activity, growth factors, or pro-inflammatory stimuli in activated macrophages and other cells at sites of inflammation. Most non-steroidal anti-inflammatory drugs (NSAIDs) such as aspirin, ibuprofen and naproxen act as nonselective COX inhibitors, whereas the coxibs selectively inhibit the COX-2 isoform. Short-lived PGH2 is then quickly converted to five prostanoids: PGD2, PGE2, PGF2α, PGI2 and TXA2, by tissue-specific prostanoid synthases. Prostanoids exert their functions by activating a suite of G protein coupled receptors (GPCRs). Two GPCRs (DP1 and DP2) are activated by PGD2, and four by PGE2 (EP1, EP2, EP3 and EP4), whereas each of the other three prostanoids activates a single receptor (FP, IP, TP). Prostanoids mediate multiple physiological and pathological effects including inflammation, pain, immunoregulation, mitogenesis, plasticity, and cell injury. Only the major pathways are shown.
PGE2/EP2 signaling
As a stimulatory G protein (Gs)-coupled receptor, EP2 activation by PGE2 stimulates adenylate cyclase (AC), resulting in elevation of cytoplasmic cAMP level to initiate multiple downstream events via its prototypical effector–PKA. PKA directly phosphorylates and activates transcription factors such as the cAMP responsive element binding protein (CREB), which in the brain mediates neuronal plasticity, long-term memory formation, neuronal survival and neurogenesis (Figure 2) [5]. In the past decade, Epac has emerged as an alternative cAMP sensor [6]. Two Epac isoforms are identified so far: Epac1, known as Rap guanine nucleotide exchange factor 3 (RAPGEF3), and Epac2, Rap guanine nucleotide exchange factor 4 (RAPGEF4). They only differ in that Epac2 has an extra cAMP binding site and a Ras-association domain for subcellular localization [6]. In response to cAMP binding, Epac activates the downstream effectors Rap1/2 to mediate a wide range of biological processes. In the central nervous system (CNS), Epac can regulate learning and memory [7], axon growth, guidance and regeneration [8], neuronal differentiation [9], neuronal excitability [10], learning and social Interactions [11], brain oxidative stress [12], neuronal apoptosis [13], and inflammatory hyperalgesia [14, 15]. PKA and Epac are often involved in the same biological process, in which they function either synergistically or oppositely [6]. For example, like PKA, Epac can also activate CREB directly [9]. Interestingly, PKA signaling is often related to neuronal survival [5, 12, 16], whereas Epac activation can lead to oxidative stress and neuronal injury [12, 13] (Figure 2). The differential regulation of PKA and Epac by cAMP could be related to the gradient of cytoplasmic cAMP because cAMP has a lower affinity for Epac than for PKA [17], (i.e., cAMP initially stimulates PKA signaling at the beginning of EP2 receptor activation; with sustained EP2 activation the Epac pathway dominates as the cytoplasmic cAMP level continues rising).
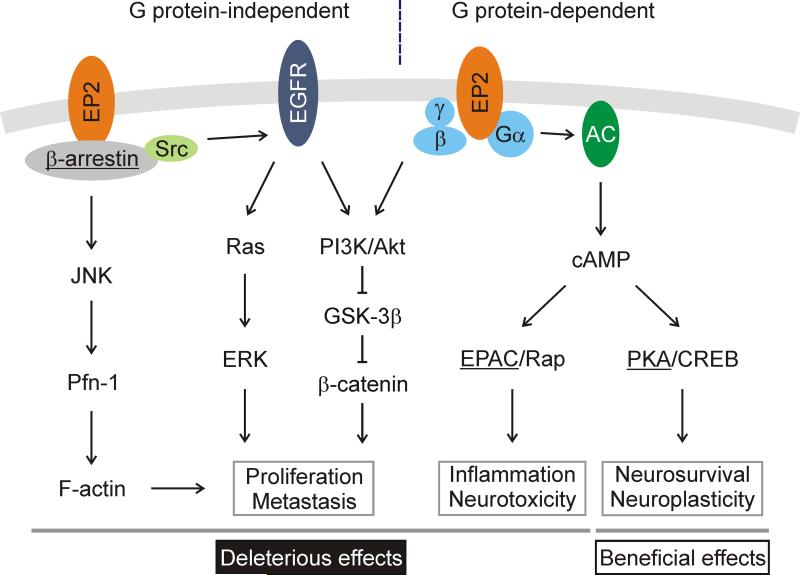
Figure 2.
Signal transduction by prostaglandin receptor EP2. In response to PGE2, EP2 receptor mediates both G protein-dependent and -independent signaling pathways to conduct multiple beneficial and deleterious actions. We hypothesize that the EP2 receptor mediates cellular survival and neuroplasticity mainly via cAMP/PKA/CREB pathway, but inflammation and neurotoxicity via cAMP/Epac/Rap signaling, and cell proliferation and migration via β-arrestin. Signaling cross-talk occurs among these three EP2 downstream pathways, but only the major pathways and effects are indicated.
Activated GPCRs can be phosphorylated by G protein-coupled receptor kinases (GRKs) and recruit β-arrestin to modify subsequent G protein-dependent signaling by initiating receptor desensitization, internalization and resensitization. β-arrestin also serves as adaptor and scaffold to switch signaling to G protein-independent pathways. An EP4 receptor/β-arrestin signaling complex has been well characterized, whereas the EP2 receptor was recently recognized to regulate β-arrestin signaling to initiate phosphoinositide 3-kinase (PI3K)/Akt, Ras/extracellular-signal-regulated kinase (ERK) and c-Jun N-terminal kinase (JNK) pathways, which are particularly important for cell proliferation and migration (Figure 2) [18-20]. Like EP4, EP2 promotes T helper (Th1) cell differentiation also through PI3K/Akt rather than its conventional cAMP signaling [21].
The EP2 receptor has been shown to regulate synaptic transmission and cognitive function. RNA interference for the EP2 receptor can decrease long-term potentiation (LTP) in rat visual cortex [22]. In response to theta-burst stimulation, Gs-coupled EP2 receptor translocates from cytosol to postsynaptic membrane, while Gi-coupled EP3 receptor moves oppositely, resulting in enhanced postsynaptic cAMP/PKA signaling [22], which in turn activates CREB, a well-documented transcription factor for the late stage of LTP and memory (Figure 2) [5]. Thus, EP2 receptor trafficking mimics that of α-amino-3-hydroxy-5-methyl-4-isoxazole propionate (AMPA)-type glutamate receptor during LTP expression. AMPA receptor trafficking to and away from postsynaptic surface modulates synaptic strength [23-25], thus it would be very interesting to examine whether EP2 signaling regulates synaptic transmission through regulating AMPA receptor trafficking in the postsynaptic sites. However, presynaptic EP2 receptors might also be involved in synaptic transmission if postsynaptic PGE2 acts as a retrograde messenger [26]. A contribution of EP2 receptor to synaptic plasticity and cognitive functions is further recognized by findings of impaired hippocampal LTP, long-term depression, and cognitive functions in EP2−/− mice [27, 28]. Application of PGE2 or butaprost enhances synaptic transmission in wild-type mice, which can be attenuated by PKA inhibitor H-89, suggesting that PGE2 modulates long-term synaptic plasticity and cognitive functions mainly through EP2/Gs/cAMP/PKA/CREB signaling cascade (Figure 2), although ERK and IP3 pathways might also be involved [28].
Advances in chemical biology
Mice lacking EP2 receptors (EP2−/−) have been independently developed in at least three groups [29-31]. Genetic ablation of prostanoid receptors has been useful but complicated by the developmental and other homeostatic adjustments that result in reduced litter size and hypertension [29-31]. As a complement to the genetic strategy, a number of small molecule ligands targeted to EP2 receptor have been developed. EP2 is activated by its natural agonist, PGE2, and also by a number of PGE2 analogs–butaprost, CAY10399 and ONO-AE1-259– and compounds with non-prostanoid structures such as CP-533536 and compound 9 (Figure 3). These agonists and the non-selective EP receptor antagonist AH6809 have been widely used to explore the roles of PGE2/EP2 signaling under normal or pathological conditions. However, butaprost is only about 18-fold selective for EP2 over EP3 in binding studies [32]; CAY10399 and ONO-AE1-259 are highly EP2 selective but have a prostanoid-like structure; CP-533536 is only about 64-fold selective over the EP4 receptor [33]; compound 9 is quite selective against other PGE2 receptors but less than 4-fold selective over the TP receptor [34]; AH6809 is neither selective nor potent, and unsuitable for in vivo study [32]. Recently reported allosteric potentiators and selective antagonists with non-prostanoid structures for EP2 receptor provide alternative probes to elucidate the physiological functions of this key prostaglandin receptor [35-37] (Figure 3). These EP2 small molecule modulators make it possible to functionally differentiate EP2 from other prostanoid receptors, particularly EP4, in COX-2-mediated physiological and pathological events. As our tools for studying the EP2 receptor expand, so too will our understanding of its role in health and disease conditions.
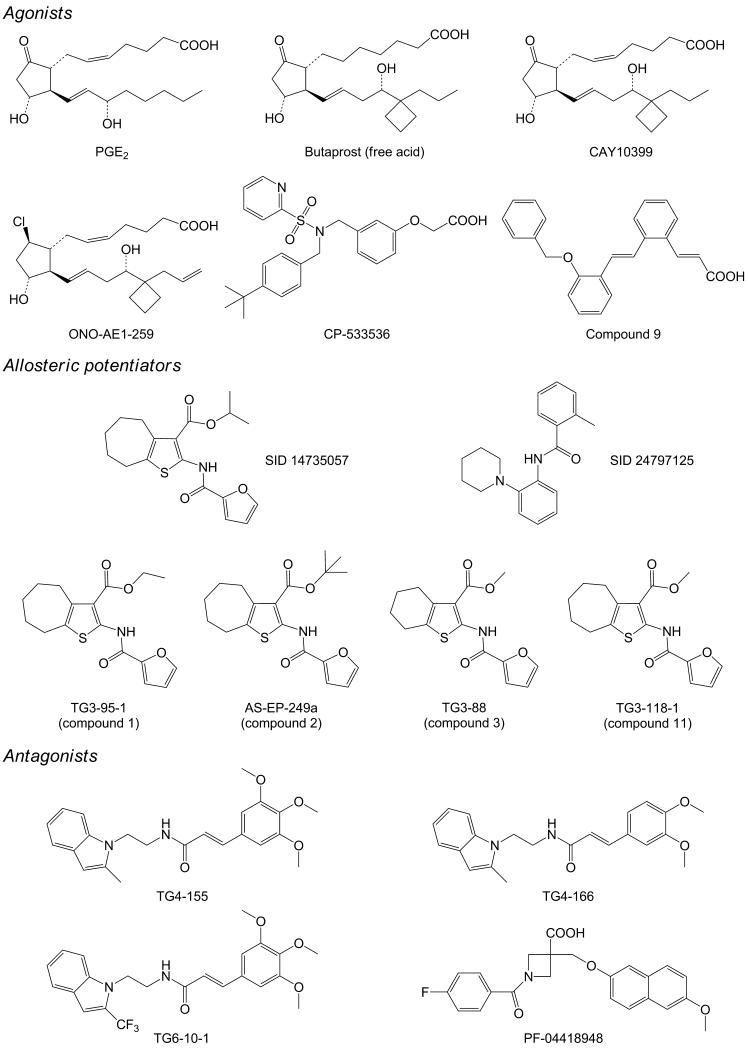
Figure 3.
Chemical structures of selective small molecule modulators of the EP2 receptor. Agonists: PGE2, butaprost, CAY10399, ONO-AE1-259, CP-533536 and compound 9; allosteric potentiators: substance identification number (SID) 14735057, SID 24797125, TG3-95-1 (referred to as compound 1 in Ref. [35]), AS-EP-249a (referred to as compound 2 in Ref. [35]), TG3-88 (referred to as compound 3 in Ref. [35]) and TG3-118-1 (referred to as compound 11 in Ref. [35]); and antagonists: TG4-155, TG4-166, TG6-10-1 and PF-04418948. These EP2 ligands are well characterized for their potency and selectivity. Some of them have been evaluated for pharmacokinetics and tested in animal disease models.
Inflammation and pain
Acute inflammation is initiated by tissue-resident immune cells such as macrophages and microglia, which undergo activation in response to signals released by injured tissue. Activated macrophages and microglia rapidly synthesize and release primary inflammatory mediators such as bradykinin, histamine, cytokines and chemokines. These mediators dilate local blood vessels and increase their permeability leading to leakage of plasma proteins into the tissue. They also increase pain sensitivity in tissues innervated by sensory nerve endings, and attract leukocytes that migrate along a chemotactic path from blood into the tissue. Certain inflammatory cytokines induce COX-2 expression via an NF-κB pathway [38], which in turn synthesizes PGE2, a secondary mediator of inflammation that promotes local vasodilation and attraction and activation of neutrophils, macrophages and mast cells during acute inflammation [39, 40]. However, PGE2 also induces anti-inflammatory cytokines including IL-10, as well as suppresses production of pro-inflammatory cytokines. Thus PGE2 acts as an immune modulator rather than a simple pro-inflammatory molecule [41].
Often inflammation resolves rather quickly, but chronic inflammation appears to contribute to the pathophysiology of many chronic conditions including rheumatoid arthritis, pain, cancer and neurological disorders [39, 40, 42]. Experiments with mice deficient in each of the four subtypes of the PGE2 receptor demonstrate that PGE2/EP2 or EP4 together with PGI2/IP signaling play crucial roles in the development of collagen-induced arthritis (CIA) [43]. PGE2 signaling through EP2 or EP4 exacerbates symptoms of inflammation by increasing IL-23 expression and reducing IL-12/IL-27, which together cause T cells to differentiate to Th17 effectors in inflammatory bowel disease (colitis) and CIA [42, 44]. PGE2, together with IL-1β and IL-23, facilitates Th17 cell differentiation and cytokine expression mainly through EP2 and cAMP signaling; whereas PGE2 acts on the EP4 receptor to downregulate IFN-γ and IL-10 produced in Th17 cells [45]. In addition, PGE2 signaling via EP2 or EP4 receptors can regulate UV-induced acute skin inflammation by increasing skin microenviromental blood flow [46]. Furthermore, EP2 activation by oxidized 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphorylcholine (OxPAPC), an oxidized phospholipid species accumulated in atherosclerotic lesions and other sites of chronic inflammation, can activate β1 integrin and stimulate monocyte binding to endothelial cells, accompanied by differential modification of TNF-α and IL-10 expression in monocytes and macrophages, which may contribute to vascular inflammation and thus accelerate atherosclerotic lesion formation [47]. This monocytic vascular inflammation via OxPAPC-mediated EP2 activation is independent of COX-2. EP2 signaling via cAMP also suppresses phagocytosis and antimicrobial function by pulmonary macrophages thus dampening innate antibacterial responses, which would be expected to exacerbate inflammation [48].
As a prominent sign of acute inflammation, pain is triggered by stimulation of nerve endings by bradykinin and other inflammatory molecules. PGE2 was initially identified as a pain modulator due to its role in the generation of exaggerated pain sensations in inflamed tissues. In a model of peripheral inflammation, EP2−/− mice show normal early peripheral hyperalgesia, but lack the chronic hyperalgesic phase of spinal origin that might be mediated by dorsal horn nociceptive neurons [49]. It appears that PGE2 mediates inflammatory pain sensitization induced by spinal PGE2 injection or peripheral inflammation via inhibiting the glycine receptor α3 subtype, and thus blocking inhibitory glycinergic neurotransmission [50]. However, this EP2 receptor-dependent inhibition of glycinergic neurotransmission does not contribute to pain sensitization in the chronic constriction injury model of neuropathic pain and chemical-induced pain [51]. This finding indicates that additional mechanisms of central sensitization are involved in inflammatory and neuropathic pain. For example, a recent study demonstrated that EP1-mediated NO production along with EP2 is involved in the maintenance of neuropathic pain by retaining activated microglia among the central terminals of primary afferent fibers [52]. Thus, blockade of EP2 and EP1 receptor signaling together is expected to afford better pain relief than either one individually. A challenge for the future is to uncover the signaling mechanisms involved.
Tumorigenesis and angiogenesis
Tumorigenesis, the process by which normal cells are converted to cancer cells, involves a progressive disruption of the balance between cell division and apoptosis leading to a state of uncontrolled proliferation. As a solid tumor grows it typically requires an augmented blood supply, which involves angiogenesis. COX-2 and its derived prostanoids have attracted substantial attention for their possible roles in tumor progression and angiogenesis [53-57]. For example, genetic ablation of COX-2 reduces colorectal polyp formation by 86% in a mouse model of human familial adenomatous polyposis (FAP), which can be recapitulated by administration of COX inhibitors [58]. Epidemiological and experimental data suggest a positive correlation between taking COX-2 inhibitor drugs regularly and reduced rates of certain cancers and cancer-related deaths [59]. Multiple downstream prostanoid signaling pathways appear to be involved in tumorigenesis. Upregulation of COX-2 in tumor tissues is usually accompanied by high levels of PGE2 [59], and administration of PGE2 can enhance colon carcinogenesis in an azoxymethane-induced colon tumor model [60]. Although the underlying mechanisms are unclear, a growing body of evidence supports PGE2 as the predominant COX-2-derived prostaglandin that facilitates tumor activities, including tumor cell proliferation, migration, angiogenesis and immunosuppression [55, 57]. Intriguingly, genetic ablation of EP2, but not EP1 or EP3, reduces the number and size of intestinal polyps in the mouse FAP model [61], mimicking the effect of COX-2 gene disruption or COX inhibitors in the same model [58]. In addition, PGE2 signaling through EP2 can in turn boost expression of COX-2 and vascular endothelial growth factor (VEGF) in polyp tissues [61]. Deletion of EP2 receptors was further demonstrated to attenuate tumor growth and prolong survival in syngeneic mouse tumor models, possibly because EP2 plays an essential role in PGE2-induced inhibition of dendritic cell differentiation and function and cancer-associated immunodeficiency [62]. EP2 ablation also suppresses skin tumor development by limiting angiogenesis and promoting apoptosis [63]. By contrast, EP2 overexpression facilitates skin tumor development [54]. The EP2 agonist butaprost promotes growth and invasion of prostate tumor cells, and this effect is blocked by the EP2 antagonist TG4-155 [64]. PGE2 signaling via EP2 in mammary epithelial cells triggers hyperplasia of mammary glands and regulates VEGF induction in mouse mammary tumor cells [65, 66]. Additionally, EP2 signaling directly regulates tumor angiogenesis in endothelium by enhancing endothelial cell motility and cell survival, mediates epidermal hypertrophy and tumor aggression in response to UV-irradiation, and induces skin carcinogenesis [54, 67, 68].
EP2 receptor appears to regulate tumor development by multiple mechanisms. For example, EP2 receptor activation can promote squamous cell carcinoma growth by activating iNOS/guanylate cyclase (GC) and ERK1/2 via transactivation of the epidermal growth factor receptor (EGFR) [69]. In response to PGE2 stimulation, the EP2 receptor recruits β-arrestin 1 to phosphorylate tyrosine-protein kinase Src, which in turn activates EGFR, leading to activation of PI3K/Akt and Ras/ERK pathways, which together promote tumor cell activities (Figure 2) [18, 19]. Alternatively, the βγ subunits liberated upon Gsα subunit activation by EP2 receptor can directly stimulate PI3K/Akt signaling, leading to phosphorylation and inactivation of glycogen synthase kinase-3β (GSK-3β), which eventually causes nuclear translocation of β-catenin to initiate growth-promoting gene expression, and thereby growth of colorectal cancer [70]. Furthermore, β-arrestin 1 also phosphorylates JNK, which upregulates Profilin-1 (Pfn-1) to increase F-actin expression and organization, thus promoting tumor cell migration and proliferation (Figure 2) [20]. The involvement of G protein-dependent signaling by EP2 in tumor progression cannot be excluded. For example, aromatase-dependent estrogen synthesis is associated with hormone-dependent breast carcinogenesis, and EP2 can regulate cytochrome P450 aromatase via the cAMP/PKA/CREB pathway [71]. In addition, PGE2 facilitates tube formation via EP2/PKA signaling in rat luteal endothelial cells, indicating the involvement of EP2 receptor in luteal angiogenesis and progression of ovarian cancer [72]. EP2 activation promotes growth of human umbilical cord blood-derived mesenchymal stem cells (hUCB-MSCs), by increasing β-catenin-mediated c-Myc and VEGF expression, in which both Epac and PKA pathways are engaged [73].
Chronic inflammation promoted by EP2 signaling might underlie its role in tumor progression, given that inflammation has long been associated with tumorigenesis [56, 74]. Inflammatory events create a local microenvironment that fosters genomic alterations and tumor initiation. Some tumor cells release cytokines and chemokines to attract monocytes and macrophages. The infiltrating macrophages in turn secrete growth factors that promote tumor progression, and recruit secondary leukocytes to enhance and maintain this mutual promotion between inflammation and tumor. As a major inflammatory mediator derived from COX-2, PGE2 via EP2 can induce many pro-inflammatory mediators including cytokines, chemokines, iNOS, and COX-2 itself, which then facilitate cell proliferation, cell survival, angiogenesis, invasion, and metastasis [64, 74]. In addition, EP2 activation also downregulates IFN-γ and TNF-α expression in immune cells such as natural killer T cells, neutrophils and macrophages [47, 75-78], impairing the ability of these immune cells to induce apoptotic death and restrain tumorigenesis. EP2 activation converts TGF-β, often considered an anti-inflammatory cytokine, from a tumor suppressor to a tumor promoter by altering oncogenic TGF-β signaling, thus promoting breast tumor growth, angiogenesis and pulmonary metastasis [79]. Therefore, attenuation of PGE2/EP2 signaling by small molecule antagonists might mitigate chronic inflammation in tumor tissues and thereby provide an alternative strategy for cancer treatment via an anti-inflammatory mechanism [64].
Innate immunity in the brain and neurotoxicity
The EP2 receptor can promote chronic neuroinflammation in both in vitro and in vivo models. EP2 expression is substantially induced during systemic inflammation in models of innate immunity produced by LPS, IL-1β or turpentine [80]. EP2 upregulation by LPS contributes to cerebral oxidative damage and secondary neurotoxicity, usually accompanied by induction of NOS and COX activities [81-83]. As the resident macrophage of the brain, microglia are the major executor of innate immunity in the CNS and their activities are highly regulated by PGE2/EP2 signaling [52, 84-86]. It is now clear that microglia are major cellular culprits of EP2-mediated chronic inflammation and neuronal damage [82], because only glia, particularly microglia, express TLR4 [87], which is activated by LPS or proteins released from nearby injured neurons to initiate an innate immune response via CD14 [88]. PGE2 signaling via either EP1 or EP2 leads to TLR4-dependent degeneration of intermediate progenitor cells in the hippocampal subgranular zone [89]. TLR4 activates the NFκB pathway and all three mitogen-activated protein kinase (MAPK) pathways: ERK, stress-activated protein kinase (SAPK)/JNK, and p38 MAPK, which in turn induce transcription of a series of pro-inflammatory genes such as COX-2, inducible NOS (iNOS), and NADPH oxidase (NOX). Other inflammatory mediators regulated by PGE2/EP2 signaling include IL-6 [75, 77, 78, 86], IL-10 [47, 78, 86], IFN-γ [76, 86], TNF-α [47, 75, 77, 78, 86], CCL-2 (MCP-1) [86, 90], and intercellular adhesion molecule-1 (ICAM-1) [91], although the EP4 receptor might also potentially contribute. EP2 activation in microglia induces inflammatory mediators such as COX-2, iNOS and a host of inflammatory cytokines, which can be enhanced by the EP2 allosteric potentiator TG3-95-1 (referred to as compound 1 in Ref. [35]) and substantially blunted by antagonist TG4-155 [37, 86]. The inflammation regulated by microglial EP2 appears to be mediated largely via cAMP/Epac signaling (Figure 2) [86]. Interestingly, EP2 can also upregulate iNOS in activated astrocytes via potentiating the response to inflammatory cytokines like TNF-α and IFN-γ [92].
Sustained inhibition of COX-2 can exert beneficial effects in neurodegenerative disease models such as Alzheimer's disease (AD) [93], Parkinson's disease (PD) [94], and amyotrophic lateral sclerosis (ALS) [95], and is accompanied by reduced number of activated microglia [96], suggesting that downstream prostanoid signaling pathways in microglia are involved in disease progression. Given that EP2 has an immunomodulatory function [82, 86], activation of EP2 in microglia could promote chronic neurodegeneration and neuroinflammation by regulating innate immunity. PGE2 via its EP2 receptor increases the expression of amyloid precursor protein (APP) in cultured rat microglia [97]. Furthermore, EP2 is involved in PGE2-stimulated production of amyloid-β (Aβ) peptides in both cell and mouse APP models, produced by β- and γ-secretases from APP and most commonly known in association with AD [98]. Interestingly, EP2 receptor ablation enhances microglia-mediated phagocytosis of Aβ and totally eliminates Aβ-triggered paracrine neurotoxicity mediated by microglia [82, 99]. EP2 receptor activation by PGE2 and butaprost can reduce Aβ-induced phagocytosis in cultured rat microglia [100], identifying microglial EP2 as a possible therapeutic target for AD. Consistently, EP2 activation suppresses phagocytosis of alveolar macrophages, the essential components of lung innate immunity [101]. Genetic ablation of EP2 reduces oxidative stress in the APPSwe-PS1ΔE9 mouse model of familial AD, accompanied by reduced levels of Aβ peptides and APP C-terminal fragments, possibly via regulating β-secretase [102].
Deletion of EP2 also enhances microglia-mediated clearance of α-synuclein aggregates in the mesocortex of patients with Lewy body disease [84]. Conversely, EP2-regulated microglial activation contributes to neurotoxicity induced by aggregated α-synuclein in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) model of PD, in which NOX appears to play a critical role [84]. As a major regulator of inflammatory oxidative injury in innate immunity, the EP2 receptor is induced in microglia and astrocytes and regulates inflammatory neurodegeneration in the G93A superoxide dismutase (SOD) model of familial amyotrophic lateral sclerosis by inducing pro-inflammatory effectors such as COX-2, iNOS and NOX [103]. More recently, pharmacological inhibition of EP2 receptor by antagonists TG4-155 and TG6-10-1 (Figure 3), after pilocarpine-induced status epilepticus in mice, was shown to produce a number of beneficial effects–reducing delayed mortality, accelerating weight regain, blunting the inflammatory reaction and gliosis in hippocampus, maintaining blood-brain barrier integrity, and reducing delayed neurodegeneration in hippocampus [37, 104]. These results strengthen the value of the EP2 receptor as a potential therapeutic target in the treatment of inflammation-related neurological disorders. Future study using EP2 antagonists in AD and PD models will help determine whether inflammatory EP2 signaling is a common pathogenic mechanism in other chronic neurologic conditions.
Neuroprotection and other beneficial effects
PGE2 is a major COX-2 product in the brain, and the EP2 receptor is widely expressed in both neurons and glia [16, 103]. PGE2/EP2 signaling is involved in a variety of physiological and pathological events in the nervous system as discussed above, but EP2 activation can also be beneficial in excitotoxicity models. EP2 activation by the selective agonist butaprost, or EP2 allosteric potentiators, can protect neurons from N-methyl-D-aspartate (NMDA) receptor-mediated excitotoxicity and oxygen-glucose deprivation(OGD) induced-anoxia in cultured neurons and hippocampal organotypic slices [16, 35, 105, 106]. Activation of EP2 by PGE2 can rescue postnatal motor neurons from chronic glutamate toxicity induced by glutamate transport inhibitors in organotypic spinal cord slices [107]. In addition, butaprost can protect cultured dopaminergic neurons from 6-hydroxydopamine-induced neurotoxicity [108]. An in vivo study shows that the EP2 agonist ONO-AE1-259 protects the ganglion cell layer and the inner plexiform layer in rat retina from NMDA-induced neurotoxicity, which suggests that EP2 activation might provide a therapy for retinal injuries involving glutamate excitotoxicity, such as diabetic retinopathy and glaucoma [109]. EP2 receptor-mediated neuroprotection is likely mediated by cAMP/PKA signaling (Figure 2),as both H-89 and KT-5720, PKA specific inhibitors, can abolish, whereas the adenylate cyclase activator forskolin can mimic, these beneficial effects [16, 107, 108].
Neuroprotection by EP2 activation has been well investigated in models of ischemic stroke. Genetic ablation of EP2 increases cerebral infarction in cerebral cortex, subcortical structures, and stroke volume in both the transient [16] and permanent [105] middle cerebral artery occlusion (MCAO) models of forebrain ischemia. Administration of misoprostol, an anti-ulcer agent and agonist for EP2, EP3, and EP4 receptors, reduces the infarct volume in the transient MCAO model. EP3 receptor should be excluded from involvement of the misoprostol-mediated neuroprotection because EP3 deficiency does not change the infarct volume and EP3+/+ and EP3−/− mice treated with misoprostol exhibit similar levels of infarct rescue [110]. More to the point, EP2 agonist ONO-AE1-259 reduces infarct volume and neurologic dysfunction in the transient MCAO model [111]. The finding that EP2 agonists and allosteric potentiators can be neuroprotective, and that ischemic damage is increased in EP2−/− mice, raise the possibility that pharmacological activation or potentiation of EP2 could be beneficial in ischemic stroke therapy.
In the periphery, EP2 activation can improve survival of gastrointestinal epithelial cells after radiation injury, via transactivation of EGFR and enhancement of PI3K/Akt signaling cascade, which suppresses translocation of the proapoptotic protein bax to the mitochondrial membrane, thus abrogating a prominent apoptotic pathway in these cells [112]. Among all four subtypes of PGE2 receptor, EP2 dominantly promotes bone biomechanical strength properties. EP2 activation by agonist CP-533536 stimulates local bone formation and enhances fracture healing, suggesting that EP2 activation restricted to the injured area could be a therapeutic alternative for the treatment of fractures and bone defects [113, 114]. EP2 also can protect cystic epithelial cells from apoptosis and promote cystogenesis through cAMP signaling in autosomal-dominant polycystic kidney disease [115]. Interestingly, EP2 and EP4 receptors synergistically exert beneficial actions under some pathological circumstances possibly because of their similarity in signal transduction profiles, (i.e., they are both Gs-coupled and are activated by PGE2). For example, both EP2 and EP4 receptors play important roles in slowing the progression of chronic kidney failure, although only EP4 provides protection in an acute kidney failure model [116]. In addition, both EP2 and EP4 receptors can improve survival of cardiac transplants in mice by inhibiting the alloimmune response, whereas EP4 activation appears to be more effective than EP2 in suppressing the acute allograft rejection [117]. Similarly, PGE2 dampens thromboxane-induced platelet aggregation via both EP2 and EP4 receptors [118], which demonstrates their value as targets for anti-platelet therapy. Both EP2 and EP4 receptors mediate the anabolic functions of PGE2 on bone formation, but via p38- and ERK-dependent MAPK signaling pathways, respectively [119]. Finally, PGE2 signaling via EP2 and EP4 receptors promotes survival of human endometriotic cells by transactivating cell survival pathways including ERK, Akt, NFκB and β-catenin, suggesting that inhibition of EP2 and EP4 might represent a nonestrogen-targeted therapy for endometriosis [120].
Concluding remarks
The EP2 receptor exerts both beneficial and deleterious effects depending on types of injury and responding components (Figure 2). This dichotomy of EP2 functions is particularly conspicuous in the CNS. For example, intracerebroventricular administration of EP2 agonist– butaprost immediately after termination of pilocarpine-induced status epilepticus affords moderate neuroprotection in a rat [2]. This finding is seemingly incongruent with the broad benefits from delayed systemic administration of EP2 antagonists in a similar model [37, 104], but might reflect the complexity of inflammatory signaling in the brain, and indicate a dual consequence of EP2 activation–early neuroprotection followed by later neurotoxicity involving chronic inflammation. It appears that neuronal EP2 activation promotes acute neuroprotection, neuronal survival and neuronal plasticity clearly through cAMP/PKA signaling. Conversely, glial– especially microglial–EP2 activation often leads to secondary neurotoxicity and neuronal injury via upregulation of inflammatory mediators such as COX-2, iNOS and NOX in chronic brain inflammation [121]. More studies are needed to clarify whether the cAMP/Epac or β-arrestin signaling pathway is dominant in microglial EP2-mediated deleterious actions, although cAMP/Epac has already been demonstrated to promote oxidative stress [12], neuronal apoptosis [13], inflammatory hyperalgesia [14, 15], and microglia-produced pro-inflammatory mediators [86]. Epac1 is up-regulated in AD and after PGE2-mediated inflammation [122, 123]. Interestingly, the EP2 receptor mediates neuroprotection in rat pure neuronal cultures treated with NMDA through a PKA-dependent pathway [16], whereas EP2 activation exacerbates NMDA receptor-mediated neurotoxicity in rat cortical cultures with glia present through a cAMP-but not PKA-dependent pathway, suggesting the involvement of Epac in EP2-regulated neurotoxicity [124]. In addition, the EP2-regulated protein dedicator of cytokinesis 2 (DOCK2), another family member of GEFs, contributes to Aβ plaque burden via regulation of microglial innate immune function [125]. Taken together, the net effect of EP2 receptor activation might be determined by a yin and yang balance of the receptor in neurons and glia, and possibly by the cytoplasmic cAMP level, which is spatiotemporally regulated by the receptor to favor either PKA or Epac pathway (Figure 4). Future studies using neuron- or monocyte-specific conditional EP2−/− mice might definitively distinguish the roles of EP2 receptor in neurons or microglia.
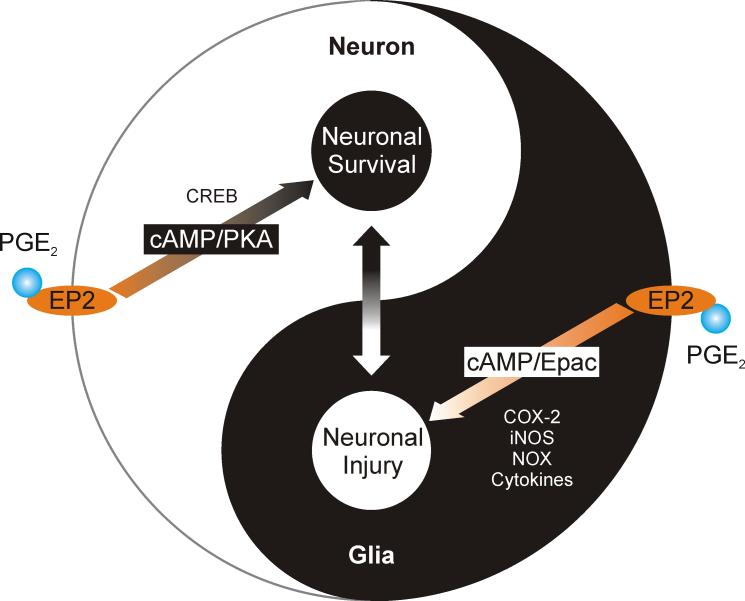
Figure 4.
The yin and yang of prostaglandin receptor EP2 in the brain. EP2 receptors in neurons are hypothesized to promote cAMP/PKA-dependent neuroprotection. In contrast, glial EP2 activation leads to neurotoxicity and neurodegeneration partly via cAMP/Epac signaling-mediated upregulation of inflammatory mediators including iNOS, COX-2, NOX and pro-inflammatory cytokines. The net effect of EP2 activation is determined by injury types and is spatiotemporally regulated by responding cells and molecules.
The past decade has witnessed growing recognition of adverse effects of selective COX-2 inhibitors [3], suggesting that the downstream prostanoid synthases or receptors should be explored as next-generation therapeutic targets. PGE2/EP2 signaling plays multiple essential roles in inflammation, tumorigenesis, cytoprotection and neurodegeneration, which renders EP2 a therapeutic target candidate for a broad range of peripheral and CNS diseases [126]. Emerging allosteric potentiators and antagonists have already proved valuable tools to explore the roles of EP2 receptor under normal or disease conditions [35-37, 64, 86, 104]; however, development of EP2-targeted drugs for therapeutic use will require careful attention to temporal and probably spatial extent of drug action to avoid widespread effects. For example, transient and early delivery of EP2 allosteric potentiators might provide neuroprotection in acute neuronal injuries from excitotoxic conditions such as ischemic and hemorrhagic strokes [127], whereas delayed inhibition of EP2 via selective antagonists would be expected to reduce brain inflammation and injury in chronic inflammation-associated neurological disorders such as epilepsy, Alzheimer's disease, Parkinson's disease and amyotrophic lateral sclerosis. Targeted block of EP2 might also be useful to combat peripheral inflammation and pain, or to slow tumor progression.
The multiplicity of signal transduction engaged by EP2, involving both G protein-dependent and -independent pathways, endow this receptor with diverse physiologic and pathological functions. Biased agonists toward G protein-independent signaling pathway have been recently reported for β1- and β2-adrenergic receptors (β1/β2-AR) [128, 129]. These small molecules preferably engage β-arrestin-dependent effects rather than the canonical G protein-dependent signaling by changing the GRK-dependent phosphorylation pattern of the receptor cytoplasmic regions to regulate the conformational states of the receptor [129, 130]. Small molecules that are biased toward EP2-coupled cAMP/PKA, cAMP/Epac, or β-arrestin signaling pathway could form the next generation of EP2 modulators that would exert pharmacological effects targeting one signaling pathway. For example, a biased EP2 agonist toward cAMP/PKA might provide neuroprotection and other beneficial effects without triggering neurotoxicity and other deleterious effects that are mediated by cAMP/Epac or β-arrestin, and vice versa.
Acknowledgments
J.J. is supported by the National Institute of Neurological Disorders and Stroke (NINDS) grant K99NS082379 and the Epilepsy Foundation; R.D. is supported by the NINDS grants U01NS058158, U01NS074509 and R21NS074169.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authors declare no conflict of interest.
References
- 1.Iadecola C, et al. Reduced susceptibility to ischemic brain injury and N-methyl-D-aspartate-mediated neurotoxicity in cyclooxygenase-2-deficient mice. Proc. Natl. Acad. Sci. U.S.A. 2001;98:1294–1299. doi: 10.1073/pnas.98.3.1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Serrano GE, et al. Ablation of cyclooxygenase-2 in forebrain neurons is neuroprotective and dampens brain inflammation after status epilepticus. J Neurosci. 2011;31:14850–14860. doi: 10.1523/JNEUROSCI.3922-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grosser T, et al. Emotion recollected in tranquility: lessons learned from the COX-2 saga. Annual review of medicine. 2010;61:17–33. doi: 10.1146/annurev-med-011209-153129. [DOI] [PubMed] [Google Scholar]
- 4.Samuelsson B, et al. Membrane prostaglandin E synthase-1: a novel therapeutic target. Pharmacol Rev. 2007;59:207–224. doi: 10.1124/pr.59.3.1. [DOI] [PubMed] [Google Scholar]
- 5.Barco A, Kandel ER. The Role of CREB and CBP in Brain Function. In: Thiel G, editor. Transcription Factors in the Nervous System. Wiley-VCH Verlag GmbH & Co.; 2006. pp. 207–241. [Google Scholar]
- 6.Bos JL. Epac proteins: multi-purpose cAMP targets. Trends Biochem Sci. 2006;31:680–686. doi: 10.1016/j.tibs.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 7.Ouyang M, et al. Epac signaling is required for hippocampus-dependent memory retrieval. Proc Natl Acad Sci U S A. 2008;105:11993–11997. doi: 10.1073/pnas.0804172105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murray AJ, Shewan DA. Epac mediates cyclic AMP-dependent axon growth, guidance and regeneration. Mol Cell Neurosci. 2008;38:578–588. doi: 10.1016/j.mcn.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 9.Shi GX, et al. A novel cyclic AMP-dependent Epac-Rit signaling pathway contributes to PACAP38-mediated neuronal differentiation. Mol Cell Biol. 2006;26:9136–9147. doi: 10.1128/MCB.00332-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ster J, et al. Exchange protein activated by cAMP (Epac) mediates cAMP activation of p38 MAPK and modulation of Ca2+-dependent K+ channels in cerebellar neurons. Proc Natl Acad Sci U S A. 2007;104:2519–2524. doi: 10.1073/pnas.0611031104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang Y, et al. EPAC null mutation impairs learning and social interactions via aberrant regulation of miR-124 and Zif268 translation. Neuron. 2012;73:774–788. doi: 10.1016/j.neuron.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hara S, et al. Dual contradictory roles of cAMP signaling pathways in hydroxyl radical production in the rat striatum. Free radical biology & medicine. 2012;52:1086–1092. doi: 10.1016/j.freeradbiomed.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 13.Suzuki S, et al. Differential roles of Epac in regulating cell death in neuronal and myocardial cells. J Biol Chem. 2010;285:24248–24259. doi: 10.1074/jbc.M109.094581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eijkelkamp N, et al. Low nociceptor GRK2 prolongs prostaglandin E2 hyperalgesia via biased cAMP signaling to Epac/Rap1, protein kinase Cepsilon, and MEK/ERK. J Neurosci. 2010;30:12806–12815. doi: 10.1523/JNEUROSCI.3142-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hucho TB, et al. Epac mediates a cAMP-to-PKC signaling in inflammatory pain: an isolectin B4(+) neuron-specific mechanism. J Neurosci. 2005;25:6119–6126. doi: 10.1523/JNEUROSCI.0285-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McCullough L, et al. Neuroprotective function of the PGE2 EP2 receptor in cerebral ischemia. J. Neurosci. 2004;24:257–268. doi: 10.1523/JNEUROSCI.4485-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Poppe H, et al. Cyclic nucleotide analogs as probes of signaling pathways. Nature methods. 2008;5:277–278. doi: 10.1038/nmeth0408-277. [DOI] [PubMed] [Google Scholar]
- 18.Chun KS, et al. The prostaglandin receptor EP2 activates multiple signaling pathways and beta-arrestin1 complex formation during mouse skin papilloma development. Carcinogenesis. 2009;30:1620–1627. doi: 10.1093/carcin/bgp168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chun KS, et al. The prostaglandin E2 receptor, EP2, stimulates keratinocyte proliferation in mouse skin by G protein-dependent and {beta}-arrestin1-dependent signaling pathways. J Biol Chem. 2010;285:39672–39681. doi: 10.1074/jbc.M110.117689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yun SP, et al. Interaction of profilin-1 and F-actin via a beta-arrestin-1/JNK signaling pathway involved in prostaglandin E(2)-induced human mesenchymal stem cells migration and proliferation. J Cell Physiol. 2011;226:559–571. doi: 10.1002/jcp.22366. [DOI] [PubMed] [Google Scholar]
- 21.Yao C, et al. Prostaglandin E2-EP4 signaling promotes immune inflammation through Th1 cell differentiation and Th17 cell expansion. Nat Med. 2009;15:633–640. doi: 10.1038/nm.1968. [DOI] [PubMed] [Google Scholar]
- 22.Akaneya Y, Tsumoto T. Bidirectional trafficking of prostaglandin E2 receptors involved in long-term potentiation in visual cortex. J Neurosci. 2006;26:10209–10221. doi: 10.1523/JNEUROSCI.3028-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang J, et al. Posttranslational modifications and receptor-associated proteins in AMPA receptor trafficking and synaptic plasticity. Neurosignals. 2006;15:266–282. doi: 10.1159/000105517. [DOI] [PubMed] [Google Scholar]
- 24.Song I, Huganir RL. Regulation of AMPA receptors during synaptic plasticity. Trends in neurosciences. 2002;25:578–588. doi: 10.1016/s0166-2236(02)02270-1. [DOI] [PubMed] [Google Scholar]
- 25.Jiang J, et al. AMPA receptor trafficking and synaptic plasticity require SQSTM1/p62. Hippocampus. 2009;19:392–406. doi: 10.1002/hipo.20528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sang N, et al. Postsynaptically synthesized prostaglandin E2 (PGE2) modulates hippocampal synaptic transmission via a presynaptic PGE2 EP2 receptor. J Neurosci. 2005;25:9858–9870. doi: 10.1523/JNEUROSCI.2392-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Savonenko A, et al. Impaired cognition, sensorimotor gating, and hippocampal long-term depression in mice lacking the prostaglandin E2 EP2 receptor. Exp Neurol. 2009;217:63–73. doi: 10.1016/j.expneurol.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang H, et al. Altered hippocampal long-term synaptic plasticity in mice deficient in the PGE2 EP2 receptor. J. Neurochem. 2009;108:295–304. doi: 10.1111/j.1471-4159.2008.05766.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hizaki H, et al. Abortive expansion of the cumulus and impaired fertility in mice lacking the prostaglandin E receptor subtype EP(2). Proc Natl Acad Sci U S A. 1999;96:10501–10506. doi: 10.1073/pnas.96.18.10501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kennedy CR, et al. Salt-sensitive hypertension and reduced fertility in mice lacking the prostaglandin EP2 receptor. Nat Med. 1999;5:217–220. doi: 10.1038/5583. [DOI] [PubMed] [Google Scholar]
- 31.Tilley SL, et al. Reproductive failure and reduced blood pressure in mice lacking the EP2 prostaglandin E2 receptor. J Clin Invest. 1999;103:1539–1545. doi: 10.1172/JCI6579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abramovitz M, et al. The utilization of recombinant prostanoid receptors to determine the affinities and selectivities of prostaglandins and related analogs. Biochim Biophys Acta. 2000;1483:285–293. doi: 10.1016/s1388-1981(99)00164-x. [DOI] [PubMed] [Google Scholar]
- 33.Cameron KO, et al. Discovery of CP-533536: an EP2 receptor selective prostaglandin E2 (PGE2) agonist that induces local bone formation. Bioorg Med Chem Lett. 2009;19:2075–2078. doi: 10.1016/j.bmcl.2009.01.059. [DOI] [PubMed] [Google Scholar]
- 34.Belley M, et al. Structure-activity relationship studies on ortho-substituted cinnamic acids, a new class of selective EP(3) antagonists. Bioorg Med Chem Lett. 2005;15:527–530. doi: 10.1016/j.bmcl.2004.11.051. [DOI] [PubMed] [Google Scholar]
- 35.Jiang J, et al. Neuroprotection by selective allosteric potentiators of the EP2 prostaglandin receptor. Proc Natl Acad Sci U S A. 2010;107:2307–2312. doi: 10.1073/pnas.0909310107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.af Forselles KJ, et al. In vitro and in vivo characterization of PF-04418948, a novel, potent and selective prostaglandin EP(2) receptor antagonist. British journal of pharmacology. 2011;164:1847–1856. doi: 10.1111/j.1476-5381.2011.01495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jiang J, et al. Small molecule antagonist reveals seizure-induced mediation of neuronal injury by prostaglandin E2 receptor subtype EP2. Proc Natl Acad Sci U S A. 2012;109:3149–3154. doi: 10.1073/pnas.1120195109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee KM, et al. Spinal NF-kB activation induces COX-2 upregulation and contributes to inflammatory pain hypersensitivity. Eur J Neurosci. 2004;19:3375–3381. doi: 10.1111/j.0953-816X.2004.03441.x. [DOI] [PubMed] [Google Scholar]
- 39.Trebino CE, et al. Impaired inflammatory and pain responses in mice lacking an inducible prostaglandin E synthase. Proc Natl Acad Sci U S A. 2003;100:9044–9049. doi: 10.1073/pnas.1332766100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aoki T, Narumiya S. Prostaglandins and chronic inflammation. Trends in pharmacological sciences. 2012;33:304–311. doi: 10.1016/j.tips.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 41.Cheon H, et al. Prostaglandin E2 augments IL-10 signaling and function. J Immunol. 2006;177:1092–1100. doi: 10.4049/jimmunol.177.2.1092. [DOI] [PubMed] [Google Scholar]
- 42.Sheibanie AF, et al. Prostaglandin E2 exacerbates collagen-induced arthritis in mice through the inflammatory interleukin-23/interleukin-17 axis. Arthritis Rheum. 2007;56:2608–2619. doi: 10.1002/art.22794. [DOI] [PubMed] [Google Scholar]
- 43.Honda T, et al. Prostacyclin-IP signaling and prostaglandin E2-EP2/EP4 signaling both mediate joint inflammation in mouse collagen-induced arthritis. J Exp Med. 2006;203:325–335. doi: 10.1084/jem.20051310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sheibanie AF, et al. The proinflammatory effect of prostaglandin E2 in experimental inflammatory bowel disease is mediated through the IL-23-->IL-17 axis. J Immunol. 2007;178:8138–8147. doi: 10.4049/jimmunol.178.12.8138. [DOI] [PubMed] [Google Scholar]
- 45.Boniface K, et al. Prostaglandin E2 regulates Th17 cell differentiation and function through cyclic AMP and EP2/EP4 receptor signaling. J Exp Med. 2009;206:535–548. doi: 10.1084/jem.20082293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kabashima K, et al. Prostaglandin E2 is required for ultraviolet B-induced skin inflammation via EP2 and EP4 receptors. Lab Invest. 2007;87:49–55. doi: 10.1038/labinvest.3700491. [DOI] [PubMed] [Google Scholar]
- 47.Li R, et al. Identification of prostaglandin E2 receptor subtype 2 as a receptor activated by OxPAPC. Circ Res. 2006;98:642–650. doi: 10.1161/01.RES.0000207394.39249.fc. [DOI] [PubMed] [Google Scholar]
- 48.Medeiros AI, et al. Efferocytosis impairs pulmonary macrophage and lung antibacterial function via PGE2/EP2 signaling. J Exp Med. 2009;206:61–68. doi: 10.1084/jem.20082058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reinold H, et al. Spinal inflammatory hyperalgesia is mediated by prostaglandin E receptors of the EP2 subtype. J Clin Invest. 2005;115:673–679. doi: 10.1172/JCI200523618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Harvey RJ, et al. GlyR alpha3: an essential target for spinal PGE2-mediated inflammatory pain sensitization. Science. 2004;304:884–887. doi: 10.1126/science.1094925. [DOI] [PubMed] [Google Scholar]
- 51.Hosl K, et al. Spinal prostaglandin E receptors of the EP2 subtype and the glycine receptor alpha3 subunit, which mediate central inflammatory hyperalgesia, do not contribute to pain after peripheral nerve injury or formalin injection. Pain. 2006;126:46–53. doi: 10.1016/j.pain.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 52.Kunori S, et al. A novel role of prostaglandin E2 in neuropathic pain: blockade of microglial migration in the spinal cord. Glia. 2011;59:208–218. doi: 10.1002/glia.21090. [DOI] [PubMed] [Google Scholar]
- 53.Soslow RA, et al. COX-2 is expressed in human pulmonary, colonic, and mammary tumors. Cancer. 2000;89:2637–2645. doi: 10.1002/1097-0142(20001215)89:12<2637::aid-cncr17>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 54.Sung YM, et al. Overexpression of the prostaglandin E2 receptor EP2 results in enhanced skin tumor development. Oncogene. 2006;25:5507–5516. doi: 10.1038/sj.onc.1209538. [DOI] [PubMed] [Google Scholar]
- 55.Wang D, Dubois RN. Prostaglandins and cancer. Gut. 2006;55:115–122. doi: 10.1136/gut.2004.047100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Grivennikov SI, et al. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Howe LR, et al. Genetic Deletion of Microsomal Prostaglandin E Synthase-1 Suppresses Mouse Mammary Tumor Growth and Angiogenesis. Prostaglandins Other Lipid Mediat. 2013 doi: 10.1016/j.prostaglandins.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Oshima M, et al. Suppression of intestinal polyposis in Apc delta716 knockout mice by inhibition of cyclooxygenase 2 (COX-2). Cell. 1996;87:803–809. doi: 10.1016/s0092-8674(00)81988-1. [DOI] [PubMed] [Google Scholar]
- 59.Greenhough A, et al. The COX-2/PGE2 pathway: key roles in the hallmarks of cancer and adaptation to the tumour microenvironment. Carcinogenesis. 2009;30:377–386. doi: 10.1093/carcin/bgp014. [DOI] [PubMed] [Google Scholar]
- 60.Kawamori T, et al. Enhancement of colon carcinogenesis by prostaglandin E2 administration. Carcinogenesis. 2003;24:985–990. doi: 10.1093/carcin/bgg033. [DOI] [PubMed] [Google Scholar]
- 61.Sonoshita M, et al. Acceleration of intestinal polyposis through prostaglandin receptor EP2 in Apc(Delta 716) knockout mice. Nat Med. 2001;7:1048–1051. doi: 10.1038/nm0901-1048. [DOI] [PubMed] [Google Scholar]
- 62.Yang L, et al. Cancer-associated immunodeficiency and dendritic cell abnormalities mediated by the prostaglandin EP2 receptor. J Clin Invest. 2003;111:727–735. doi: 10.1172/JCI16492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sung YM, et al. Lack of expression of the EP2 but not EP3 receptor for prostaglandin E2 results in suppression of skin tumor development. Cancer Res. 2005;65:9304–9311. doi: 10.1158/0008-5472.CAN-05-1015. [DOI] [PubMed] [Google Scholar]
- 64.Jiang J, Dingledine R. Role of prostaglandin receptor EP2 in the regulations of cancer cell proliferation, invasion, and inflammation. The Journal of pharmacology and experimental therapeutics. 2013;344:360–367. doi: 10.1124/jpet.112.200444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chang SH, et al. The prostaglandin E2 receptor EP2 is required for cyclooxygenase 2-mediated mammary hyperplasia. Cancer Res. 2005;65:4496–4499. doi: 10.1158/0008-5472.CAN-05-0129. [DOI] [PubMed] [Google Scholar]
- 66.Chang SH, et al. Regulation of vascular endothelial cell growth factor expression in mouse mammary tumor cells by the EP2 subtype of the prostaglandin E2 receptor. Prostaglandins Other Lipid Mediat. 2005;76:48–58. doi: 10.1016/j.prostaglandins.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 67.Kamiyama M, et al. EP2, a receptor for PGE2, regulates tumor angiogenesis through direct effects on endothelial cell motility and survival. Oncogene. 2006;25:7019–7028. doi: 10.1038/sj.onc.1209694. [DOI] [PubMed] [Google Scholar]
- 68.Brouxhon S, et al. Deletion of prostaglandin E2 EP2 receptor protects against ultraviolet-induced carcinogenesis, but increases tumor aggressiveness. J Invest Dermatol. 2007;127:439–446. doi: 10.1038/sj.jid.5700547. [DOI] [PubMed] [Google Scholar]
- 69.Donnini S, et al. EP2 prostanoid receptor promotes squamous cell carcinoma growth through epidermal growth factor receptor transactivation and iNOS and ERK1/2 pathways. FASEB J. 2007;21:2418–2430. doi: 10.1096/fj.06-7581com. [DOI] [PubMed] [Google Scholar]
- 70.Castellone MD, et al. Prostaglandin E2 promotes colon cancer cell growth through a Gs-axin-beta-catenin signaling axis. Science. 2005;310:1504–1510. doi: 10.1126/science.1116221. [DOI] [PubMed] [Google Scholar]
- 71.Subbaramaiah K, et al. EP2 and EP4 receptors regulate aromatase expression in human adipocytes and breast cancer cells. Evidence of a BRCA1 and p300 exchange. J Biol Chem. 2008;283:3433–3444. doi: 10.1074/jbc.M705409200. [DOI] [PubMed] [Google Scholar]
- 72.Sakurai T, et al. Stimulation of tube formation mediated through the prostaglandin EP2 receptor in rat luteal endothelial cells. The Journal of endocrinology. 2011;209:33–43. doi: 10.1530/JOE-10-0357. [DOI] [PubMed] [Google Scholar]
- 73.Jang MW, et al. Cooperation of Epac1/Rap1/Akt and PKA in prostaglandin E(2) - induced proliferation of human umbilical cord blood derived mesenchymal stem cells: involvement of c-Myc and VEGF expression. J Cell Physiol. 2012;227:3756–3767. doi: 10.1002/jcp.24084. [DOI] [PubMed] [Google Scholar]
- 74.Aggarwal BB, et al. Inflammation and cancer: how hot is the link? Biochem Pharmacol. 2006;72:1605–1621. doi: 10.1016/j.bcp.2006.06.029. [DOI] [PubMed] [Google Scholar]
- 75.Yamane H, et al. Prostaglandin E(2) receptors, EP2 and EP4, differentially modulate TNF-alpha and IL-6 production induced by lipopolysaccharide in mouse peritoneal neutrophils. Biochem Biophys Res Commun. 2000;278:224–228. doi: 10.1006/bbrc.2000.3779. [DOI] [PubMed] [Google Scholar]
- 76.Walker W, Rotondo D. Prostaglandin E2 is a potent regulator of interleukin-12- and interleukin-18-induced natural killer cell interferon-gamma synthesis. Immunology. 2004;111:298–305. doi: 10.1111/j.1365-2567.2004.01810.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Akaogi J, et al. Prostaglandin E2 receptors EP2 and EP4 are up-regulated in peritoneal macrophages and joints of pristane-treated mice and modulate TNF-alpha and IL-6 production. J Leukoc Biol. 2004;76:227–236. doi: 10.1189/jlb.1203627. [DOI] [PubMed] [Google Scholar]
- 78.Treffkorn L, et al. PGE2 exerts its effect on the LPS-induced release of TNF-alpha, ET-1, IL-1alpha, IL-6 and IL-10 via the EP2 and EP4 receptor in rat liver macrophages. Prostaglandins Other Lipid Mediat. 2004;74:113–123. doi: 10.1016/j.prostaglandins.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 79.Tian M, Schiemann WP. PGE2 receptor EP2 mediates the antagonistic effect of COX-2 on TGF-beta signaling during mammary tumorigenesis. FASEB J. 2010;24:1105–1116. doi: 10.1096/fj.09-141341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang J, Rivest S. Distribution, regulation and colocalization of the genes encoding the EP2- and EP4-PGE2 receptors in the rat brain and neuronal responses to systemic inflammation. Eur J Neurosci. 1999;11:2651–2668. doi: 10.1046/j.1460-9568.1999.00682.x. [DOI] [PubMed] [Google Scholar]
- 81.Montine TJ, et al. Neuronal oxidative damage from activated innate immunity is EP2 receptor-dependent. J Neurochem. 2002;83:463–470. doi: 10.1046/j.1471-4159.2002.01157.x. [DOI] [PubMed] [Google Scholar]
- 82.Shie FS, et al. Microglial EP2 is critical to neurotoxicity from activated cerebral innate immunity. Glia. 2005;52:70–77. doi: 10.1002/glia.20220. [DOI] [PubMed] [Google Scholar]
- 83.Wu L, et al. Divergent effects of prostaglandin receptor signaling on neuronal survival. Neurosci Lett. 2007;421:253–258. doi: 10.1016/j.neulet.2007.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jin J, et al. Prostaglandin E2 receptor subtype 2 (EP2) regulates microglial activation and associated neurotoxicity induced by aggregated alpha-synuclein. J Neuroinflammation. 2007;4:2. doi: 10.1186/1742-2094-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nagano T, et al. Prostaglandin E2 reduces extracellular ATP-induced migration in cultured rat microglia. Brain Res. 2008;1221:1–5. doi: 10.1016/j.brainres.2008.05.018. [DOI] [PubMed] [Google Scholar]
- 86.Quan Y, et al. EP2 Receptor Signaling Pathways Regulate Classical Activation of Microglia. J Biol Chem. 2013;288:9293–9302. doi: 10.1074/jbc.M113.455816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Saijo K, et al. A Nurr1/CoREST pathway in microglia and astrocytes protects dopaminergic neurons from inflammation-induced death. Cell. 2009;137:47–59. doi: 10.1016/j.cell.2009.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lehnardt S, et al. Activation of innate immunity in the CNS triggers neurodegeneration through a Toll-like receptor 4-dependent pathway. Proc Natl Acad Sci U S A. 2003;100:8514–8519. doi: 10.1073/pnas.1432609100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Keene CD, et al. Protection of hippocampal neurogenesis from toll-like receptor 4-dependent innate immune activation by ablation of prostaglandin E2 receptor subtype EP1 or EP2. Am J Pathol. 2009;174:2300–2309. doi: 10.2353/ajpath.2009.081153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zahner G, et al. Prostaglandin EP2 and EP4 receptors modulate expression of the chemokine CCL2 (MCP-1) in response to LPS-induced renal glomerular inflammation. Biochem J. 2009;422:563–570. doi: 10.1042/BJ20090420. [DOI] [PubMed] [Google Scholar]
- 91.Takahashi HK, et al. Prostaglandin E(2) inhibits IL-18-induced ICAM-1 and B7.2 expression through EP2/EP4 receptors in human peripheral blood mononuclear cells. J Immunol. 2002;168:4446–4454. doi: 10.4049/jimmunol.168.9.4446. [DOI] [PubMed] [Google Scholar]
- 92.Hsiao HY, et al. TNF-alpha/IFN-gamma-induced iNOS expression increased by prostaglandin E2 in rat primary astrocytes via EP2-evoked cAMP/PKA and intracellular calcium signaling. Glia. 2007;55:214–223. doi: 10.1002/glia.20453. [DOI] [PubMed] [Google Scholar]
- 93.in t' Veld BA, et al. Nonsteroidal antiinflammatory drugs and the risk of Alzheimer's disease. N Engl J Med. 2001;345:1515–1521. doi: 10.1056/NEJMoa010178. [DOI] [PubMed] [Google Scholar]
- 94.Teismann P, et al. Cyclooxygenase-2 is instrumental in Parkinson's disease neurodegeneration. Proc Natl Acad Sci U S A. 2003;100:5473–5478. doi: 10.1073/pnas.0837397100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Drachman DB, et al. Cyclooxygenase 2 inhibition protects motor neurons and prolongs survival in a transgenic mouse model of ALS. Ann Neurol. 2002;52:771–778. doi: 10.1002/ana.10374. [DOI] [PubMed] [Google Scholar]
- 96.Varvel NH, et al. NSAIDs prevent, but do not reverse, neuronal cell cycle reentry in a mouse model of Alzheimer disease. J Clin Invest. 2009;119:3692–3702. doi: 10.1172/JCI39716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pooler AM, et al. Prostaglandin E2 regulates amyloid precursor protein expression via the EP2 receptor in cultured rat microglia. Neurosci Lett. 2004;362:127–130. doi: 10.1016/j.neulet.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 98.Hoshino T, et al. Involvement of prostaglandin E2 in production of amyloid-beta peptides both in vitro and in vivo. J Biol Chem. 2007;282:32676–32688. doi: 10.1074/jbc.M703087200. [DOI] [PubMed] [Google Scholar]
- 99.Shie FS, et al. Microglia lacking E Prostanoid Receptor subtype 2 have enhanced Abeta phagocytosis yet lack Abeta-activated neurotoxicity. Am J Pathol. 2005;166:1163–1172. doi: 10.1016/s0002-9440(10)62336-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nagano T, et al. Prostaglandin E2 reduces amyloid beta-induced phagocytosis in cultured rat microglia. Brain Res. 2010;1323:11–17. doi: 10.1016/j.brainres.2010.01.086. [DOI] [PubMed] [Google Scholar]
- 101.Aronoff DM, et al. Prostaglandin E2 inhibits alveolar macrophage phagocytosis through an E-prostanoid 2 receptor-mediated increase in intracellular cyclic AMP. J Immunol. 2004;173:559–565. doi: 10.4049/jimmunol.173.1.559. [DOI] [PubMed] [Google Scholar]
- 102.Liang X, et al. Deletion of the prostaglandin E2 EP2 receptor reduces oxidative damage and amyloid burden in a model of Alzheimer's disease. J Neurosci. 2005;25:10180–10187. doi: 10.1523/JNEUROSCI.3591-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Liang X, et al. The prostaglandin E2 EP2 receptor accelerates disease progression and inflammation in a model of amyotrophic lateral sclerosis. Ann Neurol. 2008;64:304–314. doi: 10.1002/ana.21437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jiang J, et al. Inhibition of the prostaglandin receptor EP2 following status epilepticus reduces delayed mortality and brain inflammation. Proc Natl Acad Sci U S A. 2013;110:3591–3596. doi: 10.1073/pnas.1218498110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Liu D, et al. Neuroprotection by the PGE2 EP2 receptor in permanent focal cerebral ischemia. Ann. Neurol. 2005;57:758–761. doi: 10.1002/ana.20461. [DOI] [PubMed] [Google Scholar]
- 106.Ahmad AS, et al. Stimulation of prostaglandin EP2 receptors prevents NMDA-induced excitotoxicity. J. Neurotrauma. 2006;23:1895–1903. doi: 10.1089/neu.2006.23.1895. [DOI] [PubMed] [Google Scholar]
- 107.Bilak M, et al. PGE2 receptors rescue motor neurons in a model of amyotrophic lateral sclerosis. Ann Neurol. 2004;56:240–248. doi: 10.1002/ana.20179. [DOI] [PubMed] [Google Scholar]
- 108.Carrasco E, et al. Prostaglandin receptor EP2 protects dopaminergic neurons against 6-OHDA-mediated low oxidative stress. Neurosci Lett. 2008;441:44–49. doi: 10.1016/j.neulet.2008.05.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mori A, et al. The prostanoid EP(2) receptor agonist ONO-AE1-259-01 protects against glutamate-induced neurotoxicity in rat retina. Eur J Pharmacol. 2009;616:64–67. doi: 10.1016/j.ejphar.2009.04.051. [DOI] [PubMed] [Google Scholar]
- 110.Li J, et al. Misoprostol, an anti-ulcer agent and PGE2 receptor agonist, protects against cerebral ischemia. Neurosci Lett. 2008;438:210–215. doi: 10.1016/j.neulet.2008.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ahmad M, et al. The PGE2 EP2 receptor and its selective activation are beneficial against ischemic stroke. Exp Transl Stroke Med. 2010;2:12. doi: 10.1186/2040-7378-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tessner TG, et al. Prostaglandin E2 reduces radiation-induced epithelial apoptosis through a mechanism involving AKT activation and bax translocation. J Clin Invest. 2004;114:1676–1685. doi: 10.1172/JCI22218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Li M, et al. A novel, non-prostanoid EP2 receptor-selective prostaglandin E2 agonist stimulates local bone formation and enhances fracture healing. J Bone Miner Res. 2003;18:2033–2042. doi: 10.1359/jbmr.2003.18.11.2033. [DOI] [PubMed] [Google Scholar]
- 114.Paralkar VM, et al. An EP2 receptor-selective prostaglandin E2 agonist induces bone healing. Proc Natl Acad Sci U S A. 2003;100:6736–6740. doi: 10.1073/pnas.1037343100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Elberg G, et al. EP2 receptor mediates PGE2-induced cystogenesis of human renal epithelial cells. Am J Physiol Renal Physiol. 2007;293:F1622–1632. doi: 10.1152/ajprenal.00036.2007. [DOI] [PubMed] [Google Scholar]
- 116.Vukicevic S, et al. Role of EP2 and EP4 receptor-selective agonists of prostaglandin E(2) in acute and chronic kidney failure. Kidney Int. 2006;70:1099–1106. doi: 10.1038/sj.ki.5001715. [DOI] [PubMed] [Google Scholar]
- 117.Nomi T, et al. Protective effect of prostaglandin E2 receptors EP2 and EP4 in alloimmune response in vivo. Transplant Proc. 2006;38:3209–3210. doi: 10.1016/j.transproceed.2006.10.118. [DOI] [PubMed] [Google Scholar]
- 118.Kuriyama S, et al. Selective activation of the prostaglandin E2 receptor subtype EP2 or EP4 leads to inhibition of platelet aggregation. Thrombosis and haemostasis. 2010;104:796–803. doi: 10.1160/TH10-01-0043. [DOI] [PubMed] [Google Scholar]
- 119.Minamizaki T, et al. EP2 and EP4 receptors differentially mediate MAPK pathways underlying anabolic actions of prostaglandin E2 on bone formation in rat calvaria cell cultures. Bone. 2009;44:1177–1185. doi: 10.1016/j.bone.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 120.Banu SK, et al. Selective inhibition of prostaglandin E2 receptors EP2 and EP4 induces apoptosis of human endometriotic cells through suppression of ERK1/2, AKT, NFkappaB, and beta-catenin pathways and activation of intrinsic apoptotic mechanisms. Mol Endocrinol. 2009;23:1291–1305. doi: 10.1210/me.2009-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Andreasson K. Emerging roles of PGE2 receptors in models of neurological disease. Prostaglandins Other Lipid Mediat. 2010;91:104–112. doi: 10.1016/j.prostaglandins.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.McPhee I, et al. Cyclic nucleotide signalling: a molecular approach to drug discovery for Alzheimer's disease. Biochem Soc Trans. 2005;33:1330–1332. doi: 10.1042/BST0331330. [DOI] [PubMed] [Google Scholar]
- 123.Wang C, et al. A critical role of the cAMP sensor Epac in switching protein kinase signalling in prostaglandin E2-induced potentiation of P2X3 receptor currents in inflamed rats. J Physiol. 2007;584:191–203. doi: 10.1113/jphysiol.2007.135616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Takadera T, Ohyashiki T. Prostaglandin E2 deteriorates N-methyl-D-aspartate receptor-mediated cytotoxicity possibly by activating EP2 receptors in cultured cortical neurons. Life sciences. 2006;78:1878–1883. doi: 10.1016/j.lfs.2005.08.026. [DOI] [PubMed] [Google Scholar]
- 125.Cimino PJ, et al. Ablation of the Microglial Protein DOCK2 Reduces Amyloid Burden in a Mouse Model of Alzheimer's Disease. Experimental and molecular pathology. 2013 doi: 10.1016/j.yexmp.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Cimino PJ, et al. Therapeutic targets in prostaglandin E2 signaling for neurologic disease. Curr Med Chem. 2008;15:1863–1869. doi: 10.2174/092986708785132915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Mohan S, et al. Putative role of prostaglandin receptor in intracerebral hemorrhage. Frontiers in neurology. 2012;3:145. doi: 10.3389/fneur.2012.00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Galandrin S, et al. Conformational rearrangements and signaling cascades involved in ligand-biased mitogen-activated protein kinase signaling through the beta1-adrenergic receptor. Mol Pharmacol. 2008;74:162–172. doi: 10.1124/mol.107.043893. [DOI] [PubMed] [Google Scholar]
- 129.Nobles KN, et al. Distinct phosphorylation sites on the beta(2)-adrenergic receptor establish a barcode that encodes differential functions of beta-arrestin. Science signaling. 2011;4:ra51. doi: 10.1126/scisignal.2001707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Liu JJ, et al. Biased signaling pathways in beta2-adrenergic receptor characterized by 19F-NMR. Science. 2012;335:1106–1110. doi: 10.1126/science.1215802. [DOI] [PMC free article] [PubMed] [Google Scholar]