Abstract
Programmed cell death is an integral part of host defense against invading intracellular pathogens. Apoptosis, programmed necrosis, and pyroptosis each serve to limit pathogen replication in infected cells, while simultaneously promoting the inflammatory and innate responses that shape effective long-term host immunity. The importance of carefully regulated cell death is evident in the spectrum of inflammatory and autoimmune disorders caused by defects in these pathways. Moreover, many viruses encode inhibitors of programmed cell death to subvert these host responses during infection, thereby facilitating their own replication and persistence. Thus, as both virus and cell vie for control of these pathways, the battle for survival has shaped a complex host-pathogen interaction. This review will discuss the multifaceted role programmed cell death plays in maintaining the immune system and its critical function in host defense, with a special emphasis on viral infections.
Keywords: apoptosis, necrosis, necroptosis, pyroptosis, caspase, RIP1, RIP3, MCMV, vaccinia virus, LCMV, Fas, TNF, granzyme
The mammalian immune system is tasked to defend the organism against foreign pathogens. Inflammatory cytokines such as interferons (IFNs) and TNF often serve as the first line of defense during infections. Both Type I IFNs and TNF are induced by pathogen associated molecular patterns (PAMPs) that stimulate toll-like receptors (TLRs) and intracellular pattern recognition receptors (PRRs). TLRs, intracellular PRRs and TNFR are potent inducers of inflammatory cytokines and chemokines that promote recruitment of leukocytes into the infected tissue milieu. Their proinflammatory functions critically depend on the latent transcription factors NF-κB, IRF3/7 and MAP kinase induced AP-1. In addition, both TNF and type I IFNs can directly signal for cell death. Moreover, type I IFNs stimulate NK cell cytolytic function and induce expression of MHC molecules. These innate immune defense mechanisms serve to limit pathogen proliferation and propagation before a more robust and targeted adaptive immune response is launched. As such, the host environment can be a dangerous abode for pathogens. For pathogens with a limited genome, this is an especially daunting challenge. To survive and thrive in this hostile environment, viruses have developed strategies to neutralize or co-opt the host immune responses to its advantage. Here, we will discuss the role of cell death in pathogen infection with an emphasis on viral infections.
An overview of major cell death pathways in immunity
Programmed cell death is important for development, immune homeostasis and protection against microbial infections in multicellular organisms. Since its initial description, apoptosis has been considered the main cell death mechanism used in these processes. Apoptosis can be triggered by extrinsic signals such as death cytokines, or via intrinsic stimuli that impinge on the integrity of mitochondria. Both pathways rely on the activation of cysteine aspartyl proteases known as caspases. Caspases exist as inactive zymogens that can be autoactivated for apical caspases such as caspase 8, 9 and 10, or activated as part of a proteolytic caspase cascasde for effector caspases such as caspase 3, 6 and 7. Apoptosis has a characteristic morphology that includes cell shrinkage, mitochondrial permeabilization, nuclear and chromatin condensation, DNA fragmentation, membrane blebbing and eventual breakdown into apoptotic bodies (Kerr et al., 1972). These characteristics ensure the ordered demise of unwanted or infected cells, enabling the immune system to effectively remove apoptotic cells.
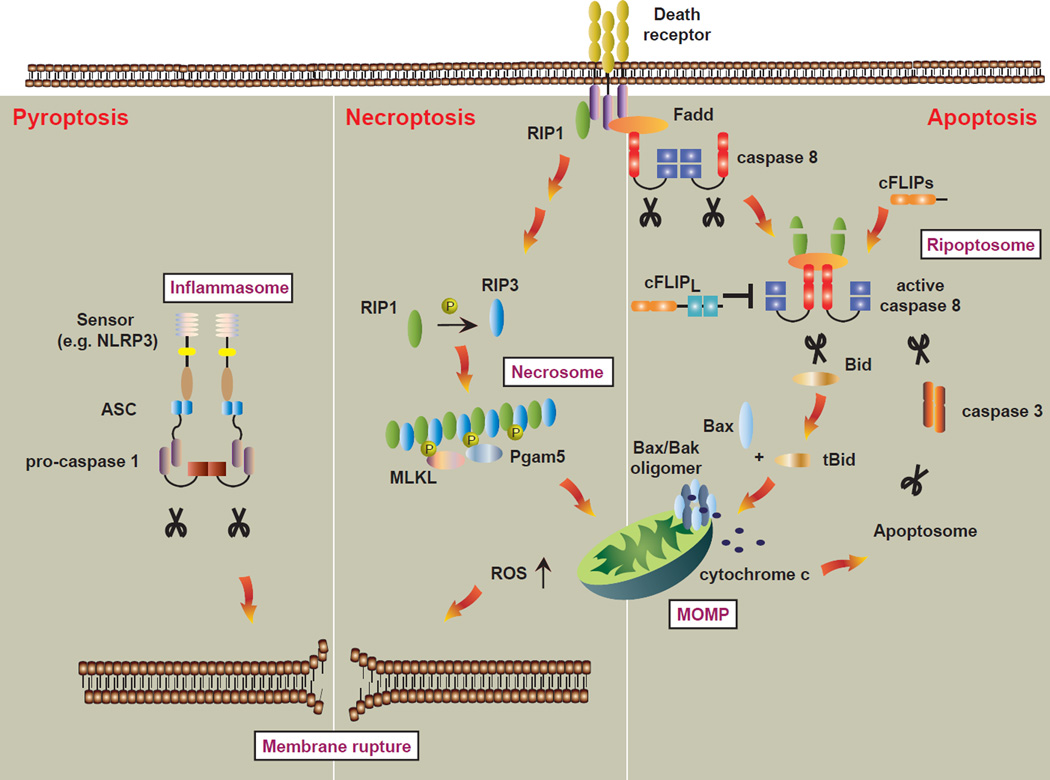
The extrinsic apoptotic pathway is stimulated by death ligands in the TNF superfamily such as TNF, Fas ligand (FasL), and TNF-related apoptosis-inducing ligand (TRAIL) and their cognate cell surface receptors. Ligand binding promotes conformational change of the preassembled receptor trimer and recruitment of adaptor and effector molecules to the cytoplasmic death domains (DD) of the receptors (Chan et al., 2000). This membrane complex eventually gives rise to a second, late-forming signaling complex in the cytosol containing Fas-associated death domain (FADD), receptor interacting protein kinase (RIP) 1 and pro-caspase 8 (Micheau and Tschopp, 2003). Activation of caspase 8 in this complex, often referred to as “Complex II”, initiates the death signal by one of two different pathways. The first is executed by direct cleavage and activation of effector caspases 3, 6, and 7. Caspase activity is tightly regulated by cellular FLICE-like inhibitor protein (cFLIP) and X-linked inhibitor of apoptosis protein (XIAP). The second pathway involves an intermediate step mediated by cleavage of the pro-apoptotic Bcl-2 family member Bid (Li et al., 1998; Luo et al., 1998), triggering Bcl-2 family members Bax and Bak to orchestrate mitochondrial outer membrane permeabilization (MOMP) to initiate the intrinsic apoptotic pathway (Figure 1).
Figure 1.
The major cell death pathways at a glance.
The extrinsic (death receptors) and intrinsic (mitochondria) apoptosis pathways intersect at the mitochondria and cumulate in the formation and activation of the apoptosome. In some cell types, death receptors can bypass the mitochondria to directly engage downstream effector caspases. When caspase 8 is inactivated, RIP1 forms an amyloid-like signaling complex with RIP3 to recruit downstream effectors such as MLKL and PGAM5. ROS may act upstream to strengthen necrosis signaling, or downstream to induce cellular damages such as membrane rupture for the execution of necrosis. Pyroptosis, which is triggered by the inflammasome, is also marked by rapid membrane rupture. Whereas apoptotic cells are rapidly engulfed and cleared by phagocytes such as macrophages, necrosis and pyroptosis promote inflammation through leakage of cellular “danger signals”.
The Bcl-2 family of proteins is comprised of both pro-and anti-apoptotic members that represent an important regulatory network mediating MOMP. They are categorized by domain architecture and fall into three basic categories: pro-apoptotic effector members such as Bak and Bax that directly initiate MOMP, anti-apoptotic members such as Bcl-2, Bcl-Xl, and Mcl-1 that inhibit apoptosis by sequestering pro-apoptotic family members, and accessory BH3-only proteins including Bid, Bad, and Bim that promote MOMP by inhibiting anti-apoptotic family members, or activating pro-apoptotic members (Tait and Green, 2010). Together, this family serves as gatekeepers for mitochondrial integrity and the initiation of intrinsic apoptosis.
The intrinsic pathway can be induced by a variety of intracellular stimuli that promote MOMP and the release of pro-apoptotic mitochondrial proteins. Among them, cytochrome c promotes the formation of the apoptosome, an oligomeric complex comprised of Apaf-1 and caspase 9. The apoptosome stimulates auto-activation of caspase 9, which cleaves and activates downstream effector caspases (Cain et al., 2000), resulting in a sequential proteolytic cascade that dismantles the cell in an ordered fashion. Additionally, mitochondrial release of second mitochondrial-derived activator of caspase (SMAC), or small molecular mimetics, inhibits or promotes the degradation of IAPs, thereby relieving inhibition of effector caspases to promote cell death (Wu et al., 2007).
Unlike apoptosis, necrosis has historically been considered an accidental from of death due to physical injury or chemical insult. However, accumulating evidence unequivocally demonstrates that necrosis can be tightly regulated, initiated by specific signals and controlled by specific genes. Diverse stimuli, including death and pathogen receptor activation, DNA damage, perturbations to intracellular calcium levels, ATP depletion, or excessive production of reactive oxygen species (ROS), can result in necrotic cell death (Moriwaki and Chan, 2013). These signals induce discrete signaling pathways leading to organelle distension and disruption, cell swelling leading to the loss of plasma membrane integrity, and the release of cytoplasmic contents. While sharing morphological characteristics with accidental or traumatic cell lysis, these regulated pathways are more commonly referred to as programmed necrosis or necroptosis.
It has long been known that the TNF can induce apoptosis in some cases, and necrosis in others (Laster et al., 1988). Studies of the TNF signaling pathway has provided significant insight into the molecular machinery leading to necrosis and the overlapping nature between apoptosis and programmed necrosis. Discovery that RIP1 is involved in death receptor-induced necrosis established the idea of necrosis as a controlled cellular response (Holler et al., 2000). A key determinant in the choice between apoptosis and necrosis lies with the activity of caspase 8 within the ripoptosome, a Complex II-like heterotypic multi-protein complex comprised of FADD, RIP1, and caspase 8. Additional recruitment of cFLIPL protects cells from death, while cFLIPs promotes apoptosis downstream of the ripoptosome (Feoktistova et al., 2011; Tenev et al., 2011). RIP3 incorporation into the ripoptosome results in formation an amyloid-like signaling complex termed the necrosome (Figure 1). Active caspase 8 homodimer or caspase 8-cFLIPL heterodimer regulate programmed necrosis, likely by cleaving RIP1, RIP3 and the deubiquitinase CYLD. In support of caspase 8’s role in necrosis, cleavage resistant mutants of RIP1, RIP3 and CYLD promote necrosis without an obligate need to inhibit caspases (Moquin et al., 2013; O'Donnell et al., 2011; Zhang et al., 2009).
RIP1 and RIP3 are members of a family of kinases involved in innate immune signaling. Both contain an amino terminal kinase domain, but unlike RIP1, RIP3 lacks a DD. However, both share another crucial protein-protein interaction motif called the RIP homotypic interaction motif (RHIM). The RHIM is a small motif with a highly conserved core of hydrophobic amino acid residues that mediates binding between RHIM containing proteins (Sun et al., 2002). The RHIM is essential for necrosis-specific interaction between RIP3 and RIP1. Two other innate immune signaling adaptors contain RHIMs; the TIR-domain-containing adapter-inducing interferon-β (TRIF) (Meylan et al., 2004) and the DNA-dependent activator of interferon regulatory factor (DAI/ZBP1) (Kaiser et al., 2008). In addition to activating transcription of NF-κB and Type I IFNs in response to pathogens, both TRIF and DAI can mediate RHIM-dependent necrosis with RIP3 independent of RIP1 kinase activity (He et al., 2011; Kaiser et al., 2013; Upton et al., 2012). Whether TRIF or DAI recruitment and activation of RIP3 results in assembly of a necrosome-like complex is currently an area of active investigation. Regardless of its binding partner, RIP3 kinase activity and RHIM-mediated interaction are crucial for various pathway-specific necrosis.
Studies using chemical inhibitors of RIP1 and RIP3 or site-directed mutagenesis have established an essential role for protein phosphorylation in TNF-induced necrosis (Degterev et al., 2013; Kaiser et al., 2013). RIP1 kinase activity is required for association with and activation of RIP3 (Cho et al., 2009). Site specific phosphorylation of RIP3 was proposed to be key for recruitment of mixed lineage kinase-like (MLKL), a downstream effector of RIP3 (Chen et al., 2013; Sun et al., 2012; Zhao et al., 2012). However, phospho-mimetic mutant analysis suggests multiple regulatory phosphorylation sites on RIP1 and RIP3 mediate TNF-induced necrosis (McQuade et al., 2013). MLKL is a catalytically inactive pseudokinase that binds ATP (Murphy et al., 2013), and while required for necrosis, the precise mechanism by which MLKL functions remains unclear. One recent study suggests that MLKL recruits phosphoglycerate mutase 5 (PGAM5), a mitochondrial phosphatase that promotes mitochondrial fission and ROS production (Wang et al., 2012). However, ROS scavengers do not inhibit TNF-induced necrosis in all cell types (F.K.C, unpublished observation), and the role of PGAM5 in necrosis has been recently challenged (Murphy et al., 2013). Therefore, the relevance of PGAM5 in diverse RIP3-dependent necrosis pathways will require further experimental validation.
Another distinct mode of caspase-dependent cell death that is important for control of infectious microorganisms is pyroptosis. Initially described as caspase 1-dependent apoptosis of macrophages, it is now clear that apoptosis and pyroptosis differ significantly in morphology and mechanism. Pyroptosis is mediated by an oligomeric signaling platform called the inflammasome, which contains a cytosolic pathogen-sensing Nod-like receptor (NLR), the adaptor ASC and caspase 1 (Rathinam et al., 2012). The inflammasome is widely known to promote proteolytic maturation and release of the pro-inflammatory cytokines IL-1β and IL-18 through caspase 1. However, inflammasome activation often leads to disruption of the plasma membrane and cell death (Figure 1). The combination of inflammatory cytokines and release of “danger-associated molecular patterns” from cell rupture can act in synergy to maximize protective immunity against invading pathogens.
Autophagy is a cytoprotective process whereby cells self-cannibalize cytoplasmic materials and organelles in response to nutritional, metabolic, or infection stresses. It is an important pathway in immunity against pathogens (Deretic et al., 2013). Normally, autophagy represents a desperate effort for cells facing metabolic catastrophe to ward off cell death. However, it can promote cell death under certain situations. It is noteworthy that there is extensive crosstalk between apoptosis, programmed necrosis, pyroptosis and autophagy. For instance, pharmacologic caspase inhibitors or genetic ablation of FADD or caspase 8 leads to RIP3-dependent necrosis. FADD/caspase 8-mediated inhibition of necrosis tampers damaging necrotic cell death during embryonic development (Kaiser et al., 2011; Oberst et al., 2011; Zhang et al., 2011). Additionally, pro-IL-1β can be processed by caspase 8 in a RIP3 dependent manner (Antonopoulos et al., 2013; Vince et al., 2012). These findings emphasize the complex interplay and coordinated regulation that is necessary to control host cell death pathways in the context of infection and immunity.
Apoptotic mechanisms in anti-viral immunity
Because viruses are intracellular pathogens, neutralizing antibodies alone may not provide sufficient protection against infection. This does not mean that they enjoy a free ride inside the host, since immune effector cells are adept at detecting minute changes associated with virus infection. For example, many viruses down-regulate class I MHC as one strategy to avoid detection by the host immune system. However, this “missing self” is actually a signal that activates cytotoxic natural killer (NK) cells. In addition, viral RNAs and DNAs are recognized by intracellular PRRs such as RIG-I and MDA5 (Dixit and Kagan, 2013). These intracellular receptors are strong inducers of type I interferons, which upregulate expression of class I MHC and sensitize infected cells to TNF-like death cytokines. By presenting viral peptide epitopes on class I MHC, infected cells can be further contained by conventional cytotoxic lymphocytes.
NK cells and conventional cytotoxic T lymphocytes rely heavily on the perforin-granzyme system to eliminate virus-infected cells. Both perforin and granyzmes are released to the target cells from cytotoxic granules. Perforin oligomerizes to form a macromolecular “membrane attack complex (MAC)” through which the proteolytic granzymes are released into the cytosol of the target cell. There are 5 human granzymes, while mice encode 10 functional granzymes. Granzyme B (GzmB) is the best characterized of all granzymes. Purified perforin and GzmB induce cell death characterized by cytochrome c release, loss of mitochondrial membrane potential and membrane blebbing (Heibein et al., 1999; MacDonald et al., 1999). GzmB is unique among serine proteases in that it cleaves substrates after an aspartic acid residue (Odake et al., 1991). As such, caspase 3, 6, 8 and 10 have all been shown to be direct substrates of GzmB . In addition, GzmB can cleave Bid to activate mitochondrial release of SMAC, which binds XIAP to relieve caspase 3 from inhibition (reviewed in (Ewen et al., 2012).
Although the evidence supporting a caspase-dependent mechanism of cell killing by GzmB is quite compelling, many other granzymes are insensitive to inhibition by pan-caspase inhibitors. For example, GzmA enters the mitochondria to cleave NDUFS3 in Complex I, increasing ROS production and driving the ER-associated SET complex to the nucleus (Martinvalet et al., 2008). Endonucleases in the SET complex then induce single-stranded DNA damage. In addition, GzmA cleaves histones, lamins and heterogeneous nuclear ribonucleoproteins (hnRNPs) to breakdown the nuclear architecture (Rajani et al., 2012). Despite differences in molecular mechanisms and targets, most granzymes ultimately elicit apoptotic features such as exposure of phosphatidyl serine, chromatin condensation and DNA fragmentation (Ewen et al., 2012). However, because caspases are not required for the action of many granzymes, the possibility that granzymes may induce non-apoptotic cell death should not be ignored.
At the organismal level, granzymes have overlapping and redundant functions in anti-viral immunity. For example, GzmA−/− and GzmM−/− mice exhibited mild defects in clearance of ectromelia virus and murine cytomegalovirus (MCMV) respectively (Mullbacher et al., 1996; Pao et al., 2005). However, the mildly increased susceptibility of GzmB−/− mice to gammaherpevirus was further exacerbated by deficiency in GzmA or GzmC and GzmF (Loh et al., 2004). GzmA−/− B−/− mice were much more susceptible to ectromelia virus infection than single knock-out mice (Mullbacher et al., 1999). Hence, despite their distinct mechanisms of action, granzymes appear to have somewhat redundant functions in vivo.
Besides perforin/granzymes, cytotoxic lymphocytes also use Fas ligand (FasL) to eradicate virus-infected cells. Residual cytolytic activity in perforin deficient T cells was abolished when Fas was also inactivated (Kagi et al., 1994; Lowin et al., 1994). However, lpr or gld mice, which harbor mutations in Fas and FasL respectively, cleared lymphocytic choriomenigitis virus (LCMV) infection normally. This is in contrast to perforin deficient mice, which failed to clear the virus (Kagi et al., 1995). Similarly, Fas was dispensable for control of MCMV induced retinitis (Dix et al., 2004). In fact, lpr mice were more efficient at controlling vaccinia virus replication (Seedhom et al., 2012). Hence, although cytotoxic lymphocytes can use perforin/granzyme or Fas/FasL pathways to eliminate target cells, in vivo evidence indicates that Fas-FasL pathway has a modest role in cytolysis of virus-infected cells.
To evade this barrage of attack from the host, viruses often encode apoptosis inhibitors in their genomes. One group of well known inhibitors are those that target caspases, such as the cowpox virus cytokine modifier A (CrmA). CrmA was originally identified as an inhibitor of the IL-1 converting enzyme (ICE), now more commonly known as caspase 1 (Ray et al., 1992). CrmA is unique among serpins as it inhibits serine as well as cysteine proteases. The first hint that CrmA also functions as an apoptosis inhibitor came from the realization that IL-1 converting enzyme (ICE)/caspase 1, a homolog of the C. elegans cell death effector CED3, caused apoptosis when over-expressed in fibroblasts (Miura et al., 1993). Later, CrmA was shown to be a high affinity inhibitor for caspase 8 and GzmB (Quan et al., 1995; Zhou et al., 1997). Since then, other viral caspase inhibitors have been identified, especially in herpeviruses (Brune, 2011).
Besides caspase inhibitors, many viruses encode Bcl-2 homologs that are direct inhibitors of Bax and Bak (Galluzzi et al., 2008). These include the adenovirus E1B-19K, and Epstein Barr virus BALF1 and BHRF1. However, the gamma-herpevirus 68 Bcl-2 homolog M11 does not inhibit Bax and Bak, but instead interferes with autophay via binding to Beclin-1 (Sinha et al., 2008). On the other hand, viral mitochondria inhibitor of apoptosis (vMIA) from cytomegalovirus does not share sequence homology with Bcl-2, but is nonetheless a potent inhibitor of Bax and Bak (Goldmacher, 2005). Thus, a common theme is emerging that viruses often interfere with multiple host cell signaling pathways to maximize their chance for survival and successful dissemination.
Necrosis in anti-viral immunity
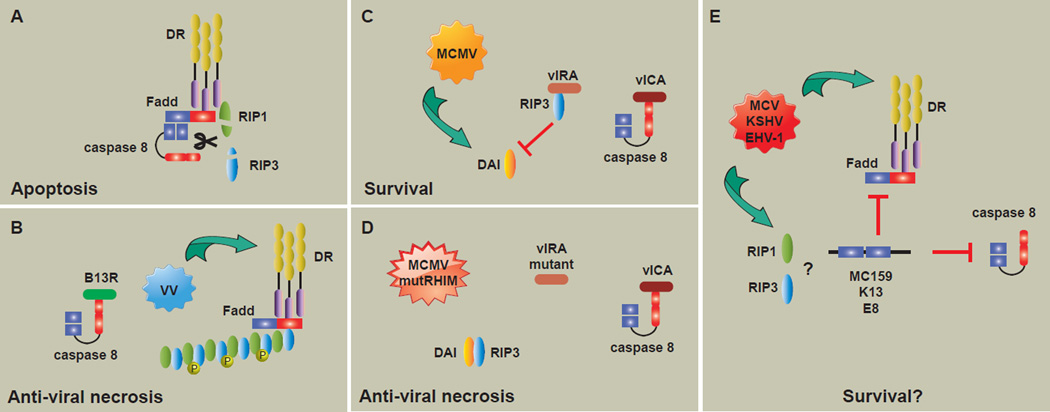
Inhibitors of apoptosis are critical tools in the arsenal of viral pathogens. However, apoptosis inhibition can trigger another host defense response, anti-viral necrosis. Vaccinia virus encodes a CrmA ortholog, B13R, whose expression is required for pathogenesis in mouse infection (Legrand et al., 2004). While B13R protects infected cells from apoptosis (Dobbelstein and Shenk, 1996; Kettle et al., 1997), its expression during infection actually sensitizes infected cells to TNF-mediated, RIP1-RIP3 dependent necrosis (Chan et al., 2003; Cho et al., 2009; Li and Beg, 2000) (Figure 2A-B). As a result, RIP3−/− mice show reduced innate inflammation and succumb to the infection due to overwhelming viral replication (Cho et al., 2009). Thus, programmed necrosis is a potent cellular response that limits viral replication when apoptosis is blocked by viral caspase inhibitors.
Figure 2.
Interaction of viral inhibitors with host cell death machineries.
(A) Ligation of death receptors (DRs) induces recruitment and association of FADD, RIP1 and caspase 8. The activity of caspase 8 negatively regulates necrotic signaling by cleavage of RIP1 and RIP3 as well as initiates the proteolytic cascade of apoptosis. (B) Infection of cells with vaccinia virus (VV) leads to expression and release of DR cytokines. VV encodes the B13R gene product, which potently inhibits caspase 8. Signaling via the DR proceeds in the absence of caspase 8 activity results in necrosome formation and anti-viral necrosis. (C) Murine cytomegalovirus (MCMV) encodes inhibitors of apoptosis and necrosis to promote survival and viral replication. MCMV expresses the viral Inhibitor of Caspase Activity (vICA), which binds and inhibits capsase 8. This normally sensitizes cells to necrosis through DAI, a RIP3 interacting partner. However, MCMV also expresses the viral Inhibitor of RIP Activation (vIRA) that directly targets RIP3 to prevent association with DAI and initiation of necrotic signaling. (D) Functional inactivation of the vIRA RHIM releases RIP3 from inhibition, resulting in antiviral necrosis. (E) Some poxvirus- and herpesviruses-encoded vFLIPs (e.g. MC159, K13, E8) are strong inhibitors of apoptosis and necrosis. Although the underlying mechanism remains unclear, the net result is dual-functioning cell death inhibitors capable of preventing both apoptosis and necrosis to promote cell survival and viral persistence.
In many regards, viral inhibition of caspase 8 may stimulate anti-viral necrosis (Figure 2). As such, some viruses encoding inhibitors targeting caspase 8 must concurrently have a strategy for dealing with host cell necrosis. MCMV encodes multiple inhibitors of apoptosis, including the viral inhibitor of caspase activation (vICA), which directly targets pro-caspase 8 to prevent its association with FADD (McCormick et al., 2003; Menard et al., 2003; Skaletskaya et al., 2001). Indeed, vICA expression alone was sufficient to cause necrosis in L929 cells (Kaiser et al., 2011). However, unlike vaccinia virus, MCMV infection does not sensitize or induce necrosis (Mack et al., 2008). Instead, MCMV prevents RIP3-dependent necrosis by the expression of the viral inhibitor of RIP activation (vIRA), a product of the M45 gene. Interestingly, vIRA is a RHIM-containing inhibitor that directly targets RIP3 to prevent its association with and activation by DAI (Upton et al., 2008, 2010, 2012). Recombinant virus encoding a mutant vIRA lacking its RHIM was severely attenuated and failed to establish productive infection even in immuno-compromised mice. However, productive infection by the mutant virus was restored in RIP3−/− mice (Upton et al., 2010). This indicates that suppression of host cell necrosis is a vital survival strategy of the virus (Figure 2C-D).
Many other viruses, particularly the poxvirus and herpesvirus families, encode viral FLIP-like molecules that, like cFLIP, contain two tandem death effector domains (DED) and are strong apoptosis inhibitors (Mocarski et al., 2012). However, a subset of these vFLIP molecules including MC159 from Mollulscum contagiosum virus, E8 from equine herpesvirus 1, and K13 from Kaposi’s sarcoma associated herpesvirus can also inhibit TNF-induced programmed necrosis (Chan et al., 2003) (Figure 2E). It is noteworthy that in addition to cell death inhibition, vFLIPs and vIRA can also modulate NF-κB signaling, (Challa et al., 2010; Fliss et al., 2012) although it is unclear whether these activities influence inhibition of necrosis. It will be interesting to see if inhibition of necrosis is a common strategy employed by viruses to evade the host immune system.
Lymphocyte cell death in anti-viral responses
While killing of infected cells is no doubt a critical defense mechanism against viruses, cell death of uninfected cells, especially lymphocytes, can also greatly impact the overall quality of immune responses against viruses. In response to antigenic stimulation, T and B cells undergo massive clonal expansion and functional differentiation. Prior to this expansion, there is a transient contraction of CD8+ T cells that is thought to create “space” to accommodate the ensuing clonal expansion (Welsh et al., 2012). Studies with LCMV indicate that this early wave of cell death affects mostly memory cells and is dependent on type I interferons, but not TNF or FasL (Bahl et al., 2006).
Rapidly dividing lymphocytes are prone to death signals. When restimulated through the antigen receptor, they undergo activation-induced cell death through autocrine expression of TNF and FasL. Failure to do so can result in immunopathology or worse yet, autoimmunity. In human, mutations in Fas or FasL cause a systemic autoimmune disease called the ALPS (Autoimmune Lymphoproliferative Syndromes) (Su and Lenardo, 2008), which is recapitulated in lpr and gld mice. As discussed above, lpr and gld mice are generally competent in controlling different viral pathogens, however the persistence of activated lymphocytes in lpr and gld mice often led to severe immunopathology (Balkow et al., 2001; Olson and Varga, 2009; Zhang et al., 2000). Thus, while perforin and granzymes are more important for cytolysis of virus-infected cells, it appears the Fas-FasL pathway serves to minimize the debilitating immunopathology from virus infections.
Mice deficient in TNF, TNF receptor 1 (TNFR-1) or TNFR-2 do not develop systemic autoimmune disease. On face value, it appears to suggest a subsidiary role for TNF in lymphocyte homeostasis. However, TNF−/− mice developed higher number of CD8+ memory T cells against LCMV (Singh and Suresh, 2007), suggesting that TNF and TNFR interaction also has a minor role in lymphocyte cell death. Thus, while innate production of TNF can have direct cytotoxic effects on virus-infected cells, TNF has a modest role in lymphocyte cell death during the adaptive phase of immune reactions. These results highlight another important concept in anti-viral immunity: that the biological consequence of cytokine signaling is temporally controlled and context-dependent.
After clearance of a pathogen, the absence of antigen reduces production of the trophic factor IL-2. This cytokine withdrawal primes lymphocytes for “intrinsic” cell death controlled by Bim and related Bcl-2 family members. The majority of the expanded effector lymphocytes are eliminated through this process. Consistent with this, Bim−/− mice developed lymphoproliferative disease, and effector T cells from Bim−/− mice are refractory to cell death (Bouillet et al., 1999). Bim−/− lpr mice exhibited much higher effector CD8+ T cells responses to LCMV (Weant et al., 2008), indicating that Bim collaborates with Fas to control lymphocyte homeostasis. Effector T cells from Mcl-1−/− mice undergo rapid Bax/Bak-dependent cell death in response to LCMV infection (Tripathi et al., 2013). Thus, pro-survival and pro-apoptotic Bcl-2 family members have important functions in maintaining clonal expansion and immunologic memory.
Besides T and B cells, cell death of other immune effectors can also impact the efficacy of anti-viral immune responses. Dendritic cells are powerful antigen presenting cells that prime and activate antigen-specific lymphocytes. Human patients with mutations in perforin developed a fatal disease called hemophagocytic lymphohistiocytosis (HLH). LCMV and MCMV infection of perforin-deficient mice led to a similarly fatal reaction due to failure of cytotoxic T cells to eliminate dendritic cells, which in turn caused sustained T cell activation and the associated pathology (Terrell and Jordan, 2013; van Dommelen et al., 2006).
Concluding remarks
As we have illustrated here, different cell death mechanisms can have distinct effects on the quality of immune responses against viruses. While apoptosis is usually non-immunogenic, it has a vital role in eliminating virus-infected cells. For viruses that successfully resist apoptosis, programmed necrosis and pyroptosis may be vital to eliminate the viral factory before adaptive immunity is engaged. It is striking that many innate immune signaling pathways can promote inflammatory cytokine expression and cell death. However, the signaling outcome is dependent on many factors such as when the cytokines are induced, whether the cells are infected, and the presence of other synergizing or inhibitory cytokines. This reinforces the notion that these signaling pathways can synergize with each other to achieve containment of the pathogen and to shape the outcome of host-pathogen interactions.
Acknowledgment
We apologize to our colleagues whose work we could not reference due to space limitation. This work is supported by NIH grant AI083497 (F.K-M.C.) and by Cancer Prevention & Research Institute of Texas (CPRIT) Scholar award R1202 (J.W.U).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature cited
- Antonopoulos C, El Sanadi C, Kaiser WJ, Mocarski ES, Dubyak GR. Proapoptotic Chemotherapeutic Drugs Induce Noncanonical Processing and Release of IL-1beta via Caspase-8 in Dendritic Cells. J Immunol. 2013;191:4789–4803. doi: 10.4049/jimmunol.1300645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahl K, Kim SK, Calcagno C, Ghersi D, Puzone R, Celada F, Selin LK, Welsh RM. IFN-induced attrition of CD8 T cells in the presence or absence of cognate antigen during the early stages of viral infections. J Immunol. 2006;176:4284–4295. doi: 10.4049/jimmunol.176.7.4284. [DOI] [PubMed] [Google Scholar]
- Balkow S, Kersten A, Tran TT, Stehle T, Grosse P, Museteanu C, Utermohlen O, Pircher H, von Weizsacker F, Wallich R, et al. Concerted action of the FasL/Fas and perforin/granzyme A and B pathways is mandatory for the development of early viral hepatitis but not for recovery from viral infection. Journal of virology. 2001;75:8781–8791. doi: 10.1128/JVI.75.18.8781-8791.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouillet P, Metcalf D, Huang DC, Tarlinton DM, Kay TW, Kontgen F, Adams JM, Strasser A. Proapoptotic Bcl-2 relative Bim required for certain apoptotic responses, leukocyte homeostasis, and to preclude autoimmunity. Science. 1999;286:1735–1738. doi: 10.1126/science.286.5445.1735. [DOI] [PubMed] [Google Scholar]
- Brune W. Inhibition of programmed cell death by cytomegaloviruses. Virus research. 2011;157:144–150. doi: 10.1016/j.virusres.2010.10.012. [DOI] [PubMed] [Google Scholar]
- Cain K, Bratton SB, Langlais C, Walker G, Brown DG, Sun XM, Cohen GM. Apaf-1 oligomerizes into biologically active approximately 700-kDa and inactive approximately 1.4-MDa apoptosome complexes. J Biol Chem. 2000;275:6067–6070. doi: 10.1074/jbc.275.9.6067. [DOI] [PubMed] [Google Scholar]
- Challa S, Woelfel M, Guildford M, Moquin D, Chan FK. Viral cell death inhibitor MC159 enhances innate immunity against vaccinia virus infection. Journal of virology. 2010;84:10467–10476. doi: 10.1128/JVI.00983-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan FK, Chun HJ, Zheng L, Siegel RM, Bui KL, Lenardo MJ. A domain in TNF receptors that mediates ligand-independent receptor assembly and signaling. Science. 2000;288:2351–2354. doi: 10.1126/science.288.5475.2351. [DOI] [PubMed] [Google Scholar]
- Chan FK, Shisler J, Bixby JG, Felices M, Zheng L, Appel M, Orenstein J, Moss B, Lenardo MJ. A role for tumor necrosis factor receptor-2 and receptor-interacting protein in programmed necrosis and antiviral responses. J Biol Chem. 2003;278:51613–51621. doi: 10.1074/jbc.M305633200. [DOI] [PubMed] [Google Scholar]
- Chen W, Zhou Z, Li L, Zhong CQ, Zheng X, Wu X, Zhang Y, Ma H, Huang D, Li W, et al. Diverse Sequence Determinants Control Human and Mouse Receptor Interacting Protein 3 (RIP3) and Mixed Lineage Kinase domain-Like (MLKL) Interaction in Necroptotic Signaling. J Biol Chem. 2013 doi: 10.1074/jbc.M112.435545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho YS, Challa S, Moquin D, Genga R, Ray TD, Guildford M, Chan FK. Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell. 2009;137:1112–1123. doi: 10.1016/j.cell.2009.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degterev A, Maki JL, Yuan J. Activity and specificity of necrostatin-1, small-molecule inhibitor of RIP1 kinase. Cell Death Differ. 2013;20:366. doi: 10.1038/cdd.2012.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deretic V, Saitoh T, Akira S. Autophagy in infection, inflammation and immunity. Nature reviews Immunology. 2013;13:722–737. doi: 10.1038/nri3532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dix RD, Ekworomadu CO, Hernandez E, Cousins SW. Perforin knockout mice, but not mice with MAIDS, show protection against experimental cytomegalovirus retinitis after adoptive transfer of immune cells with a functional perforin cytotoxic pathway. Archives of virology. 2004;149:2235–2244. doi: 10.1007/s00705-004-0370-3. [DOI] [PubMed] [Google Scholar]
- Dixit E, Kagan JC. Intracellular pathogen detection by RIG-I-like receptors. Advances in immunology. 2013;117:99–125. doi: 10.1016/B978-0-12-410524-9.00004-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbelstein M, Shenk T. Protection against apoptosis by the vaccinia virus SPI-2 (B13R) gene product. Journal of virology. 1996;70:6479–6485. doi: 10.1128/jvi.70.9.6479-6485.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewen CL, Kane KP, Bleackley RC. A quarter century of granzymes. Cell Death Differ. 2012;19:28–35. doi: 10.1038/cdd.2011.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feoktistova M, Geserick P, Kellert B, Dimitrova DP, Langlais C, Hupe M, Cain K, MacFarlane M, Hacker G, Leverkus M. cIAPs block Ripoptosome formation, a RIP1/caspase-8 containing intracellular cell death complex differentially regulated by cFLIP isoforms. Mol Cell. 2011;43:449–463. doi: 10.1016/j.molcel.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fliss PM, Jowers TP, Brinkmann MM, Holstermann B, Mack C, Dickinson P, Hohenberg H, Ghazal P, Brune W. Viral mediated redirection of NEMO/IKKgamma to autophagosomes curtails the inflammatory cascade. PLoS Pathog. 2012;8:e1002517. doi: 10.1371/journal.ppat.1002517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galluzzi L, Brenner C, Morselli E, Touat Z, Kroemer G. Viral control of mitochondrial apoptosis. PLoS Pathog. 2008;4:e1000018. doi: 10.1371/journal.ppat.1000018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldmacher VS. Cell death suppression by cytomegaloviruses. Apoptosis : an international journal on programmed cell death. 2005;10:251–265. doi: 10.1007/s10495-005-0800-z. [DOI] [PubMed] [Google Scholar]
- He S, Liang Y, Shao F, Wang X. Toll-like receptors activate programmed necrosis in macrophages through a receptor-interacting kinase-3-mediated pathway. Proc Natl Acad Sci U S A. 2011;108:20054–20059. doi: 10.1073/pnas.1116302108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heibein JA, Barry M, Motyka B, Bleackley RC. Granzyme B-induced loss of mitochondrial inner membrane potential (Delta Psi m) and cytochrome c release are caspase independent. J Immunol. 1999;163:4683–4693. [PubMed] [Google Scholar]
- Holler N, Zaru R, Micheau O, Thome M, Attinger A, Valitutti S, Bodmer JL, Schneider P, Seed B, Tschopp J. Fas triggers an alternative, caspase-8-independent cell death pathway using the kinase RIP as effector molecule. Nat Immunol. 2000;1:489–495. doi: 10.1038/82732. [DOI] [PubMed] [Google Scholar]
- Kagi D, Seiler P, Pavlovic J, Ledermann B, Burki K, Zinkernagel RM, Hengartner H. The roles of perforin- and Fas-dependent cytotoxicity in protection against cytopathic and noncytopathic viruses. European journal of immunology. 1995;25:3256–3262. doi: 10.1002/eji.1830251209. [DOI] [PubMed] [Google Scholar]
- Kagi D, Vignaux F, Ledermann B, Burki K, Depraetere V, Nagata S, Hengartner H, Golstein P. Fas and perforin pathways as major mechanisms of T cell-mediated cytotoxicity. Science. 1994;265:528–530. doi: 10.1126/science.7518614. [DOI] [PubMed] [Google Scholar]
- Kaiser WJ, Sridharan H, Huang C, Mandal P, Upton JW, Gough PJ, Sehon CA, Marquis RW, Bertin J, Mocarski ES. Toll-like Receptor 3-mediated Necrosis via TRIF, RIP3, and MLKL. J Biol Chem. 2013;288:31268–31279. doi: 10.1074/jbc.M113.462341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser WJ, Upton JW, Long AB, Livingston-Rosanoff D, Daley-Bauer LP, Hakem R, Caspary T, Mocarski ES. RIP3 mediates the embryonic lethality of caspase-8-deficient mice. Nature. 2011;471:368–372. doi: 10.1038/nature09857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser WJ, Upton JW, Mocarski ES. Receptor-interacting protein homotypic interaction motif-dependent control of NF-kappa B activation via the DNA-dependent activator of IFN regulatory factors. J Immunol. 2008;181:6427–6434. doi: 10.4049/jimmunol.181.9.6427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettle S, Alcami A, Khanna A, Ehret R, Jassoy C, Smith GL. Vaccinia virus serpin B13R (SPI-2) inhibits interleukin-1beta-converting enzyme and protects virus-infected cells from TNF- and Fas-mediated apoptosis, but does not prevent IL-1beta-induced fever. The Journal of general virology. 1997;78(Pt 3):677–685. doi: 10.1099/0022-1317-78-3-677. [DOI] [PubMed] [Google Scholar]
- Laster SM, Wood JG, Gooding LR. Tumor necrosis factor can induce both apoptic and necrotic forms of cell lysis. J Immunol. 1988;141:2629–2634. [PubMed] [Google Scholar]
- Legrand FA, Verardi PH, Jones LA, Chan KS, Peng Y, Yilma TD. Induction of potent humoral and cell-mediated immune responses by attenuated vaccinia virus vectors with deleted serpin genes. Journal of virology. 2004;78:2770–2779. doi: 10.1128/JVI.78.6.2770-2779.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Zhu H, Xu CJ, Yuan J. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell. 1998;94:491–501. doi: 10.1016/s0092-8674(00)81590-1. [DOI] [PubMed] [Google Scholar]
- Li M, Beg AA. Induction of necrotic-like cell death by tumor necrosis factor alpha and caspase inhibitors: novel mechanism for killing virus-infected cells. J Virol. 2000;74:7470–7477. doi: 10.1128/jvi.74.16.7470-7477.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh J, Thomas DA, Revell PA, Ley TJ, Virgin HWt. Granzymes and caspase 3 play important roles in control of gammaherpesvirus latency. Journal of virology. 2004;78:12519–12528. doi: 10.1128/JVI.78.22.12519-12528.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowin B, Hahne M, Mattmann C, Tschopp J. Cytolytic T-cell cytotoxicity is mediated through perforin and Fas lytic pathways. Nature. 1994;370:650–652. doi: 10.1038/370650a0. [DOI] [PubMed] [Google Scholar]
- Luo X, Budihardjo I, Zou H, Slaughter C, Wang X. Bid, a Bcl2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell. 1998;94:481–490. doi: 10.1016/s0092-8674(00)81589-5. [DOI] [PubMed] [Google Scholar]
- MacDonald G, Shi L, Vande Velde C, Lieberman J, Greenberg AH. Mitochondria-dependent and -independent regulation of Granzyme B-induced apoptosis. J Exp Med. 1999;189:131–144. doi: 10.1084/jem.189.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mack C, Sickmann A, Lembo D, Brune W. Inhibition of proinflammatory and innate immune signaling pathways by a cytomegalovirus RIP1-interacting protein. Proc Natl Acad Sci U S A. 2008;105:3094–3099. doi: 10.1073/pnas.0800168105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinvalet D, Dykxhoorn DM, Ferrini R, Lieberman J. Granzyme A cleaves a mitochondrial complex I protein to initiate caspase-independent cell death. Cell. 2008;133:681–692. doi: 10.1016/j.cell.2008.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick AL, Skaletskaya A, Barry PA, Mocarski ES, Goldmacher VS. Differential function and expression of the viral inhibitor of caspase 8-induced apoptosis (vICA) and the viral mitochondria-localized inhibitor of apoptosis (vMIA) cell death suppressors conserved in primate and rodent cytomegaloviruses. Virology. 2003;316:221–233. doi: 10.1016/j.virol.2003.07.003. [DOI] [PubMed] [Google Scholar]
- McQuade T, Cho Y, Chan FK. Positive and Negative Phosphorylation Regulates RIP1 and RIP3-Induced Programmed Necrosis. The Biochemical journal. 2013;456:409–415. doi: 10.1042/BJ20130860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menard C, Wagner M, Ruzsics Z, Holak K, Brune W, Campbell AE, Koszinowski UH. Role of murine cytomegalovirus US22 gene family members in replication in macrophages. Journal of virology. 2003;77:5557–5570. doi: 10.1128/JVI.77.10.5557-5570.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meylan E, Burns K, Hofmann K, Blancheteau V, Martinon F, Kelliher M, Tschopp J. RIP1 is an essential mediator of Toll-like receptor 3-induced NF-kappa B activation. Nat Immunol. 2004;5:503–507. doi: 10.1038/ni1061. [DOI] [PubMed] [Google Scholar]
- Micheau O, Tschopp J. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell. 2003;114:181–190. doi: 10.1016/s0092-8674(03)00521-x. [DOI] [PubMed] [Google Scholar]
- Miura M, Zhu H, Rotello R, Hartwieg EA, Yuan J. Induction of apoptosis in fibroblasts by IL-1 beta-converting enzyme, a mammalian homolog of the C. elegans cell death gene ced-3. Cell. 1993;75:653–660. doi: 10.1016/0092-8674(93)90486-a. [DOI] [PubMed] [Google Scholar]
- Mocarski ES, Upton JW, Kaiser WJ. Viral infection and the evolution of caspase 8-regulated apoptotic and necrotic death pathways. Nat Rev Immunol. 2012;12:79–88. doi: 10.1038/nri3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moquin DM, McQuade T, Chan FK. CYLD Deubiquitinates RIP1 in the TNFalpha-Induced Necrosome to Facilitate Kinase Activation and Programmed Necrosis. Plos One. 2013;8:e76841. doi: 10.1371/journal.pone.0076841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriwaki K, Chan FK. RIP3: a molecular switch for necrosis and inflammation. Genes Dev. 2013;27:1640–1649. doi: 10.1101/gad.223321.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullbacher A, Ebnet K, Blanden RV, Hla RT, Stehle T, Museteanu C, Simon MM. Granzyme A is critical for recovery of mice from infection with the natural cytopathic viral pathogen, ectromelia. Proc Natl Acad Sci U S A. 1996;93:5783–5787. doi: 10.1073/pnas.93.12.5783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullbacher A, Waring P, Tha Hla R, Tran T, Chin S, Stehle T, Museteanu C, Simon MM. Granzymes are the essential downstream effector molecules for the control of primary virus infections by cytolytic leukocytes. Proc Natl Acad Sci U S A. 1999;96:13950–13955. doi: 10.1073/pnas.96.24.13950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy JM, Czabotar PE, Hildebrand JM, Lucet IS, Zhang JG, Alvarez-Diaz S, Lewis R, Lalaoui N, Metcalf D, Webb AI, et al. The Pseudokinase MLKL Mediates Necroptosis via a Molecular Switch Mechanism. Immunity. 2013;39:443–453. doi: 10.1016/j.immuni.2013.06.018. [DOI] [PubMed] [Google Scholar]
- O'Donnell MA, Perez-Jimenez E, Oberst A, Ng A, Massoumi R, Xavier R, Green DR, Ting AT. Caspase 8 inhibits programmed necrosis by processing CYLD. Nat Cell Biol. 2011;13:1437–1442. doi: 10.1038/ncb2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberst A, Dillon CP, Weinlich R, McCormick LL, Fitzgerald P, Pop C, Hakem R, Salvesen GS, Green DR. Catalytic activity of the caspase-8-FLIP(L) complex inhibits RIPK3-dependent necrosis. Nature. 2011;471:363–367. doi: 10.1038/nature09852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odake S, Kam CM, Narasimhan L, Poe M, Blake JT, Krahenbuhl O, Tschopp J, Powers JC. Human and murine cytotoxic T lymphocyte serine proteases: subsite mapping with peptide thioester substrates and inhibition of enzyme activity and cytolysis by isocoumarins. Biochemistry. 1991;30:2217–2227. doi: 10.1021/bi00222a027. [DOI] [PubMed] [Google Scholar]
- Olson MR, Varga SM. Fas ligand is required for the development of respiratory syncytial virus vaccine-enhanced disease. J Immunol. 2009;182:3024–3031. doi: 10.4049/jimmunol.0803585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pao LI, Sumaria N, Kelly JM, van Dommelen S, Cretney E, Wallace ME, Anthony DA, Uldrich AP, Godfrey DI, Papadimitriou JM, et al. Functional analysis of granzyme M and its role in immunity to infection. J Immunol. 2005;175:3235–3243. doi: 10.4049/jimmunol.175.5.3235. [DOI] [PubMed] [Google Scholar]
- Quan LT, Caputo A, Bleackley RC, Pickup DJ, Salvesen GS. Granzyme B is inhibited by the cowpox virus serpin cytokine response modifier A. J Biol Chem. 1995;270:10377–10379. doi: 10.1074/jbc.270.18.10377. [DOI] [PubMed] [Google Scholar]
- Rajani DK, Walch M, Martinvalet D, Thomas MP, Lieberman J. Alterations in RNA processing during immune-mediated programmed cell death. Proc Natl Acad Sci U S A. 2012;109:8688–8693. doi: 10.1073/pnas.1201327109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathinam VA, Vanaja SK, Fitzgerald KA. Regulation of inflammasome signaling. Nat Immunol. 2012;13:333–332. doi: 10.1038/ni.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray CA, Black RA, Kronheim SR, Greenstreet TA, Sleath PR, Salvesen GS, Pickup DJ. Viral inhibition of inflammation: cowpox virus encodes an inhibitor of the interleukin-1 beta converting enzyme. Cell. 1992;69:597–604. doi: 10.1016/0092-8674(92)90223-y. [DOI] [PubMed] [Google Scholar]
- Seedhom MO, Mathurin KS, Kim SK, Welsh RM. Increased protection from vaccinia virus infection in mice genetically prone to lymphoproliferative disorders. Journal of virology. 2012;86:6010–6022. doi: 10.1128/JVI.07176-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A, Suresh M. A role for TNF in limiting the duration of CTL effector phase and magnitude of CD8 T cell memory. J Leukoc Biol. 2007;82:1201–1211. doi: 10.1189/jlb.0407240. [DOI] [PubMed] [Google Scholar]
- Sinha S, Colbert CL, Becker N, Wei Y, Levine B. Molecular basis of the regulation of Beclin 1-dependent autophagy by the gamma-herpesvirus 68 Bcl-2 homolog M11. Autophagy. 2008;4:989–997. doi: 10.4161/auto.6803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skaletskaya A, Bartle LM, Chittenden T, McCormick AL, Mocarski ES, Goldmacher VS. A cytomegalovirus-encoded inhibitor of apoptosis that suppresses caspase-8 activation. Proc Natl Acad Sci U S A. 2001;98:7829–7834. doi: 10.1073/pnas.141108798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su HC, Lenardo MJ. Genetic defects of apoptosis and primary immunodeficiency. Immunology and allergy clinics of North America. 2008;28:329–351. ix. doi: 10.1016/j.iac.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L, Wang H, Wang Z, He S, Chen S, Liao D, Wang L, Yan J, Liu W, Lei X, et al. Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell. 2012;148:213–227. doi: 10.1016/j.cell.2011.11.031. [DOI] [PubMed] [Google Scholar]
- Sun X, Yin J, Starovasnik MA, Fairbrother WJ, Dixit VM. Identification of a novel homotypic interaction motif required for the phosphorylation of receptor-interacting protein (RIP) by RIP3. J Biol Chem. 2002;277:9505–9511. doi: 10.1074/jbc.M109488200. [DOI] [PubMed] [Google Scholar]
- Tait SW, Green DR. Mitochondria and cell death: outer membrane permeabilization and beyond. Nat Rev Mol Cell Biol. 2010;11:621–632. doi: 10.1038/nrm2952. [DOI] [PubMed] [Google Scholar]
- Tenev T, Bianchi K, Darding M, Broemer M, Langlais C, Wallberg F, Zachariou A, Lopez J, MacFarlane M, Cain K, et al. The Ripoptosome, a signaling platform that assembles in response to genotoxic stress and loss of IAPs. Mol Cell. 2011;43:432–448. doi: 10.1016/j.molcel.2011.06.006. [DOI] [PubMed] [Google Scholar]
- Terrell CE, Jordan MB. Perforin deficiency impairs a critical immunoregulatory loop involving murine CD8(+) T cells and dendritic cells. Blood. 2013;121:5184–5191. doi: 10.1182/blood-2013-04-495309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi P, Koss B, Opferman JT, Hildeman DA. Mcl-1 antagonizes Bax/Bak to promote effector CD4(+) and CD8(+) T-cell responses. Cell Death Differ. 2013;20:998–1007. doi: 10.1038/cdd.2013.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upton JW, Kaiser WJ, Mocarski ES. Cytomegalovirus M45 cell death suppression requires receptor-interacting protein (RIP) homotypic interaction motif (RHIM)-dependent interaction with RIP1. J Biol Chem. 2008;283:16966–16970. doi: 10.1074/jbc.C800051200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upton JW, Kaiser WJ, Mocarski ES. Virus inhibition of RIP3-dependent necrosis. Cell Host Microbe. 2010;7:302–313. doi: 10.1016/j.chom.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upton JW, Kaiser WJ, Mocarski ES. DAI/ZBP1/DLM-1 complexes with RIP3 to mediate virus-induced programmed necrosis that is targeted by murine cytomegalovirus vIRA. Cell Host Microbe. 2012;11:290–297. doi: 10.1016/j.chom.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dommelen SL, Sumaria N, Schreiber RD, Scalzo AA, Smyth MJ, Degli-Esposti MA. Perforin and granzymes have distinct roles in defensive immunity and immunopathology. Immunity. 2006;25:835–848. doi: 10.1016/j.immuni.2006.09.010. [DOI] [PubMed] [Google Scholar]
- Vince JE, Wong WW, Gentle I, Lawlor KE, Allam R, O'Reilly L, Mason K, Gross O, Ma S, Guarda G, et al. Inhibitor of apoptosis proteins limit RIP3 kinase-dependent interleukin-1 activation. Immunity. 2012;36:215–227. doi: 10.1016/j.immuni.2012.01.012. [DOI] [PubMed] [Google Scholar]
- Wang Z, Jiang H, Chen S, Du F, Wang X. The mitochondrial phosphatase PGAM5 functions at the convergence point of multiple necrotic death pathways. Cell. 2012;148:228–243. doi: 10.1016/j.cell.2011.11.030. [DOI] [PubMed] [Google Scholar]
- Weant AE, Michalek RD, Khan IU, Holbrook BC, Willingham MC, Grayson JM. Apoptosis regulators Bim and Fas function concurrently to control autoimmunity and CD8+ T cell contraction. Immunity. 2008;28:218–230. doi: 10.1016/j.immuni.2007.12.014. [DOI] [PubMed] [Google Scholar]
- Welsh RM, Bahl K, Marshall HD, Urban SL. Type 1 interferons and antiviral CD8 T-cell responses. PLoS Pathog. 2012;8:e1002352. doi: 10.1371/journal.ppat.1002352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Tschopp J, Lin SC. Smac mimetics and TNFalpha: a dangerous liaison? Cell. 2007;131:655–658. doi: 10.1016/j.cell.2007.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang DW, Shao J, Lin J, Zhang N, Lu BJ, Lin SC, Dong MQ, Han J. RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science. 2009;325:332–336. doi: 10.1126/science.1172308. [DOI] [PubMed] [Google Scholar]
- Zhang H, Zhou X, McQuade T, Li J, Chan FK, Zhang J. Functional complementation between FADD and RIP1 in embryos and lymphocytes. Nature. 2011;471:373–376. doi: 10.1038/nature09878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang HG, Fleck M, Kern ER, Liu D, Wang Y, Hsu HC, Yang P, Wang Z, Curiel DT, Zhou T, et al. Antigen presenting cells expressing Fas ligand down-modulate chronic inflammatory disease in Fas ligand-deficient mice. The Journal of clinical investigation. 2000;105:813–821. doi: 10.1172/JCI8236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Jitkaew S, Cai Z, Choksi S, Li Q, Luo J, Liu ZG. Mixed lineage kinase domain-like is a key receptor interacting protein 3 downstream component of TNF-induced necrosis. Proc Natl Acad Sci U S A. 2012;109:5322–5327. doi: 10.1073/pnas.1200012109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Snipas S, Orth K, Muzio M, Dixit VM, Salvesen GS. Target protease specificity of the viral serpin CrmA. Analysis of five caspases. J Biol Chem. 1997;272:7797–7800. doi: 10.1074/jbc.272.12.7797. [DOI] [PubMed] [Google Scholar]