Abstract
The neonatal Fc receptor (FcRn) is a major regulator of IgG and albumin homeostasis systemically and in the kidneys. We investigated the role of FcRn in the development of immune complex–mediated glomerular disease in mice. C57Bl/6 mice immunized with the noncollagenous domain of the α3 chain of type IV collagen (α3NC1) developed albuminuria associated with granular capillary loop deposition of exogenous antigen, mouse IgG, C3 and C5b-9, and podocyte injury. High-resolution imaging showed abundant IgG deposition in the expanded glomerular basement membrane, especially in regions corresponding to subepithelial electron dense deposits. FcRn-null and -humanized mice immunized with α3NC1 developed no albuminuria and had lower levels of serum IgG anti-α3NC1 antibodies and reduced glomerular deposition of IgG, antigen, and complement. Our results show that FcRn promotes the formation of subepithelial immune complexes and subsequent glomerular pathology leading to proteinuria, potentially by maintaining higher serum levels of pathogenic IgG antibodies. Therefore, reducing pathogenic IgG levels by pharmacologic inhibition of FcRn may provide a novel approach for the treatment of immune complex–mediated glomerular diseases. As proof of concept, we showed that a peptide inhibiting the interaction between human FcRn and human IgG accelerated the degradation of human IgG anti-α3NC1 autoantibodies injected into FCRN-humanized mice as effectively as genetic ablation of FcRn, thus preventing the glomerular deposition of immune complexes containing human IgG.
The MHC class I–like neonatal Fc receptor (FcRn), a heterodimer comprising a heavy chain and β2-microglobulin light chain, is the major regulator of IgG and albumin homeostasis.1 Perinatally, FcRn mediates the transfer of IgG from mother to offspring, across the placenta in primates and trans-intestinally in suckling rodents. Throughout life, FcRn protects IgG and albumin from catabolism, explaining the unusually long t1/2 and high serum levels of these proteins. IgG and albumin taken up by cells by pinocytosis bind strongly to FcRn at pH 6.0–6.5 in endosomes. FcRn-bound ligands are then recycled to the plasma membrane, where they dissociate at pH 7.4, whereas IgG and albumin not bound to FcRn are targeted to lysosomes for degradation. FcRn is thought to promote some autoimmune diseases because it protects pathogenic IgG from degradation. For instance, Fcrn−/− mice are resistant to passive transfer of arthritis by K/BxN sera and autoimmune skin pathology induced by antibodies targeting autoantigens at the dermal–epidermal junction, although this protection can be overcome by excess autoantibodies.2–4
In kidneys, FcRn is expressed in podocytes and proximal tubular epithelial cells.5 Overall, renal FcRn reclaims albumin but facilitates elimination of IgG.6 Tubular FcRn mediates IgG transcytosis.7 Podocytes use FcRn to clear IgG from the glomerular basement membrane (GBM).8 IgG accumulates in the glomeruli of aged Fcrn−/− mice due to impaired clearance of IgG from the GBM, and saturating this clearance mechanism by excess ligand potentiates the pathogenicity of nephrotoxic sera in wild-type mice. Podocyte FcRn has been postulated to be involved in the clearance of immune complexes (ICs) present in pathologic conditions such as membranous nephropathy.5 Expression of FcRn in human podocytes is increased in various immune-mediated glomerular diseases.9 Given its role in IgG and albumin handling in the kidneys and systemically, FcRn can be expected to influence the development of immune-mediated kidney diseases at multiple levels. This conjecture awaits experimental verification.
To determine the role of FcRn in IgG-mediated glomerular disease, we asked how FcRn deficiency alters the course of disease in mice immunized with the NC1 domain of α3 type IV collagen (α3NC1). We chose this antigen because of its reported ability to induce disease in C57Bl/6 (B6) mice,10 corroborated in pilot studies (Supplemental Figure 1). Fcrn−/− mice are hypoalbuminemic due to impaired albumin recycling,11 and also exhibit reduced urinary albumin excretion.12 As a control for this potential confounder, we used FCRN-humanized mice, which have normal serum albumin because human FcRn recycles mouse albumin but not mouse IgG.13
All mice immunized with α3NC1 developed circulating mouse IgG anti-α3NC1 antibodies, which reached the maximum titer about 6 weeks later and gradually declined thereafter. At all times, the levels of mouse IgG anti-α3NC1 antibodies in sera from Fcrn−/− mice and FCRN-humanized mice were approximately 50%–70% lower than those in wild-type mouse sera (Figure 1A). The results were similar for mouse IgG1, IgG2b, and IgG2c anti-α3NC1 antibodies (Supplemental Figure 2). Wild-type B6 mice immunized with α3NC1 started developing progressive albuminuria 8–10 weeks later (Figure 1B). By week 14, the urinary albumin creatinine ratio increased approximately 100-fold, and hypoalbuminemia developed (Figure 1C). Urinary albumin excretion in Fcrn−/− mice and FCRN-humanized mice immunized with α3NC1 was not significantly higher than in adjuvant-immunized control mice. No mice developed renal failure (Supplemental Figure 3).
Figure 1.
FcRn ablation reduces serum levels of mouse IgG anti-α3NC1 antibodies and prevents the development of albuminuria in α3NC1-immunized mice. (A) The left panel shows circulating mIgG anti-α3NC1 antibodies from C57Bl6 wild-type mice (○), Fcrn−/− mice (□), FCRN-humanized (hFCRN) mice (◇), and the control CFA group (△), which are assayed by indirect ELISA in plates coated with α3NC1 (100 ng/well). Mouse sera are diluted 1:5000. The right panel shows the significance of circulating mIgG anti-α3NC1 antibody differences among groups at week 12, as assessed by one-way ANOVA followed by Bonferroni post tests for pairwise comparisons. (B) The left panel shows that the urinary albumin creatinine ratio (mean±SEM) time course is monitored in C57Bl6 wild-type mice (○), Fcrn−/− mice (□), and hFCRN mice (◇) immunized with α3NC1 (n=5–8 mice in each group, from two separate experiments). Mice in the control group (△) are immunized with adjuvant alone (n=9). The right panel shows the urinary albumin creatinine ratio (mean±SEM) at 14 weeks, when mice are euthanized. The significance of differences among groups is assessed by one-way ANOVA followed by Bonferroni post tests for pairwise comparisons. (C) The left panel shows SDS-PAGE analysis of serum (0.5 µl/lane) and urine samples (2 µl/lane) from CFA-immunized control mice (a) and α3NC1-immunized wild-type mice (b), Fcrn−/− mice (c), and hFCRN mice (d) collected at week 14. The right panel presents a densitometric analysis of the relative levels of albumin in mouse serum samples showing that α3NC1-immunized wild-type mice developed hypoalbuminemia. *P<0.05 by two-tailed t test versus CFA-immunized wild-type mice; **P<0.01; ***P<0.001. ns, not significant; WT, wild type.
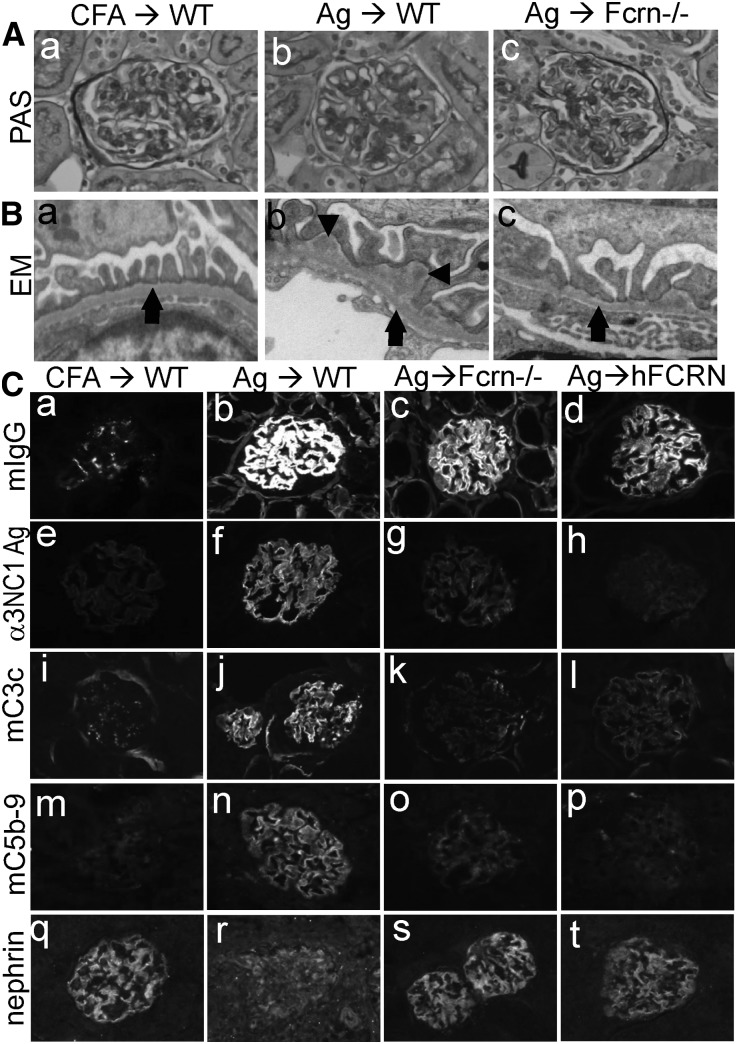
At 14 weeks after α3NC1 immunization, kidneys examined by light microscopy showed mild glomerular pathology, with few crescents and relatively little inflammation (Figure 2A), similar to α3NC1-immunized DBA/1 mice with comparable albuminuria.14,15 Electron microscopy showed extensive subepithelial IC deposits surrounded by an expanded GBM and effacement of podocyte foot processes in α3NC1-immunized B6 mice, whereas Fcrn−/− mice had fewer subepithelial deposits (Figure 2B, Supplemental Figure 4). Immunofluorescence staining showed granular capillary loop deposition of mouse IgG, exogenous antigen, C3, and C5b-9, more intense in wild-type mice than in Fcrn−/− mice and FCRN-humanized mice (Figure 2, Ca–Cp, Supplemental Figure 5). A loss of nephrin staining, indicative of podocyte injury, occurred in α3NC1-immunized B6 mice but not in Fcrn−/− mice or FCRN-humanized mice (Figure 2, Cq–Ct).
Figure 2.
FcRn deficiency reduces formation of pathogenic subepithelial ICs. (A) Light microscopic evaluation of kidneys from adjuvant-immunized control mice (a) and α3NC1-immunized wild-type mice (b) and Fcrn−/− mice (c) revealed few pathogenic changes and the absence of glomerular inflammation (periodic acid–Schiff staining). (B) Transmission electron microscopy shows normal GBM (arrow) and podocyte foot processes in control mice (a), extensive subepithelial electron dense deposits (arrowhead), thickened GBM, and podocyte foot process effacement in α3NC1-immunized wild-type mice (b), and fewer IC deposits in the Fcrn−/− mice (c). (C) Immunofluorescence analysis of kidneys from adjuvant-immunized control mice (a, e, i, m, and q) and α3NC1-immunized wild-type mice (b, f, j, n, and r), FcRn−/− mice (c, g, k, o, and s), and hFCRN mice (d, h, l, p, and t) evaluate the deposition of mouse IgG (a–d), exogenous α3NC1 antigen stained by mAb RH34 (e–h), mouse C3c (i–l), C5b-9 (m–p), and nephrin staining (q–t) at 14 weeks. Wild-type mice exhibit linear-granular GBM deposition of mouse IgG and granular GBM deposition of exogenous antigen, C3, and C5b-9, which are attenuated in Fcrn−/− mice and hFCRN mice and essentially absent in control mice. Compared with control mice, α3NC1-immunized wild-type mice but not Fcrn−/− or hFCRN mice exhibit a loss of nephrin staining, indicative of podocyte injury. WT, wild type; EM, electron microscopy, PAS, periodic acid–Schiff. Original magnification, ×400 in A; ×2850 in B; ×200 in C.
Because B6 mice immunized with bovine GBM NC1 hexamers have normal kidney function and histology despite linear GBM deposition of IgG autoantibodies binding to mouse α345(IV) collagen (Supplemental Figure 1), the question arises as to what causes proteinuria in α3NC1-immunized mice. Because the clinical presentation, morphology, and effector mechanisms depend on where ICs are localized in the capillary wall, we compared IgG distribution in α3NC1-immunized mice and mice injected with anti-α3NC1 antibodies modeling anti-GBM autoantibodies. The distribution and relative abundance of mouse IgG, as imaged by immunoperoxidase immunoelectron microscopy and stochastic optical reconstruction microscopy (STORM), a method for super-resolution fluorescence microscopy, were concordant. In α3NC1-immunized mice, IgG deposition was abundant in the areas of expanded GBM and especially in regions corresponding to the subepithelial dense deposits seen by routine electron microscopy. By contrast, in mice injected with α3NC1-specific anti-GBM mAb, the IgG was confined to an ultrastructurally normal GBM that lacked subepithelial deposits (Figure 3).
Figure 3.
Localization of IgG by high-resolution imaging. The localization of mouse IgG in glomerular capillary walls of wild-type mice immunized with α3NC1 (A, C–E), or intravenously injected with anti-mouse α3NC1 IgG mAb 8D1 (B, F–H) is determined by immunoperoxidase electron microscopy (A and B) and STORM imaging (C–H). In A, the GBM is irregularly thickened, and abundant electron dense peroxidase reaction product is present in discontinuous, subepithelial patterns beneath broadly effaced podocyte foot processes (arrows). In B, the peroxidase reaction product is diffusely present throughout the GBM (arrowhead), but less abundant compared with A. Electron dense deposits are absent, and podocyte foot process architecture appears normal. (C–E) By STORM imaging, anti-agrin (blue) identifies both normal and thickened areas of the GBM, both of which contain dense accumulations of mouse IgG throughout (red). The electron microscopy correlation in E shows GBM staining with respect to the podocytes and endothelial cells. (F–H) IgG mAb 8D1 (red) is present in the GBM, which shows no evidence of thickening. CL, capillary lumen; EM, electron microscopy En, endothelium;Po, podocyte.
Subepithelial ICs, a hallmark of human membranous nephropathy (MN), form when IgG antibodies bind to podocyte antigens, such as phospholipase A2 receptor (PLA2R) and neutral endopeptidase (NEP), or to planted antigens, such as cationic BSA.16–18 Subsequent expansion of the GBM, complement activation, and podocyte injury by C5b-9 cause proteinuria. Although it is unexpected, formation of subepithelial ICs in α3NC1-immunized mice may be explained by exogenous α3NC1 deposited in glomeruli acting as a planted antigen.19 Alternatively, anti-α3NC1 antibodies in complex with α3NC1 antigen may act as surrogate antipodocyte antibodies, because α3NC1-containing ICs bind to podocytes.20 After four immunizations with α3NC1 monomers, B6 mice and DBA/1 mice eventually develop crescentic GN by 26 and 10 weeks, respectively.10,14 The combination of subepithelial ICs and crescentic anti-GBM antibody GN was most recently described in a series of eight patients with circulating anti-α3NC1 autoantibodies but undetectable anti-PLA2R autoantibodies.21
In contrast to wild-type B6 mice, congenic Fcrn−/− mice and FCRN-humanized mice did not develop albuminuria after α3NC1 immunization. Their resistance to proteinuria was associated with lower serum titers of anti-α3NC1 IgG antibodies and reduced glomerular deposition of IgG, antigen, C3, and C5b-9. Because C5b-9 is an essential mediator of podocyte damage and proteinuria by subepithelial ICs,22,23 reduced complement activation potentially explains the attenuated glomerular pathology in FcRn-deficient mice. The resistance of FCRN-humanized mice indicates that FcRn promotes IC-mediated glomerular disease due to its interaction with IgG rather than albumin. We propose that FcRn promotes the development of subepithelial ICs and subsequent glomerular injury primarily by maintaining higher serum levels of pathogenic IgG (Supplemental Figure 6). However, we cannot formally exclude a possible pathogenic role of podocyte FcRn, whose stimulation by ICs may induce maladaptive signaling.9 Future studies in mice with podocyte-specific ablation of FcRn would address this possibility.
Our findings identify FcRn as a potential target for therapeutic intervention in IC-mediated glomerular diseases, typically treated with nonspecific immunosuppressants that are toxic and sometimes ineffective. More specific therapies include ablation of B cells by rituximab. In patients with idiopathic MN who respond to rituximab therapy, serum levels of anti-PLA2R IgG autoantibodies decline over a period of many months, and their disappearance is followed by resolution of proteinuria.24 The slow decline in proteinuria is problematic for patients already suffering from complications of nephrotic syndrome, who would benefit from ancillary therapies that reduce pathogenic IgG antibodies more rapidly. This may be achieved by inhibiting FcRn.
One implementation of this concept is therapy with high-dose intravenous Ig (HD-IVIG). HD-IVIG accelerates the degradation of IgG by saturating FcRn,25 one of the mechanisms that explain the beneficial effects of HD-IVIG therapy in some autoimmune diseases.3 In pregnant women with circulating anti-NEP alloantibodies mediating antenatal MN, treatment with HD-IVIG reduces the titers of IgG alloantibodies by approximately 30% within 2–3 weeks.26 However, HD-IVIG is inefficient, because large amounts of IgG (1–2 g/kg) cause relatively modest reductions in pathogenic IgG titers. Specific FcRn inhibitors recapitulate this activity of HD-IVIG more effectively at lower doses. By reducing pathogenic IgG levels, function-blocking anti-FcRn mAbs ameliorate experimental myasthenia gravis in rats,27 and engineered IgG “Abdegs” that bind with high affinity to FcRn ameliorate arthritis transferred by K/BxN serum.28
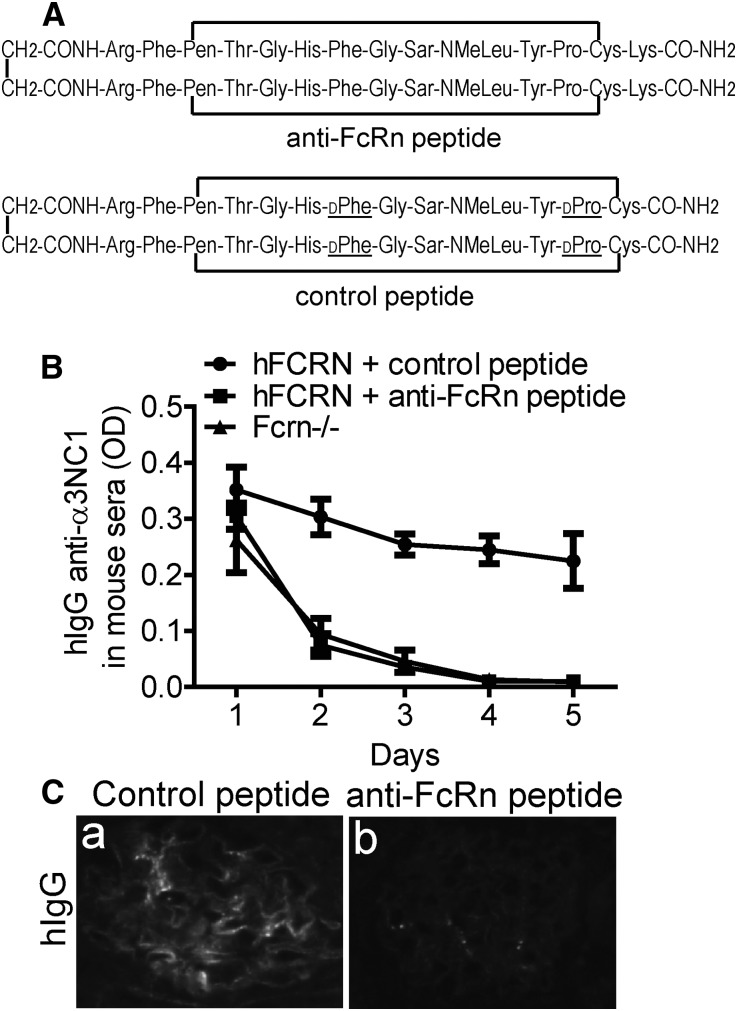
To assess the translational potential of our findings, we asked whether pharmacologic blockade of human FcRn can reproduce the effects of genetic FcRn deficiency. To this end, FCRN-humanized and Fcrn−/− mice were passively immunized with human IgG containing anti-α3NC1 (Goodpasture) autoantibodies. To inhibit human FcRn, we used a lysine analog of SYN1436 (Figure 4A),29 a peptide that binds with subnanomolar affinity to human FcRn, thus preventing IgG binding.30 In vivo, SYN1436 reduces IgG levels in cynomolgus monkeys by 80%.30 Serum anti-α3NC1 autoantibodies in FCRN-humanized mice treated with anti-FcRn peptide, but not with control peptide, sharply decreased to the same levels as in Fcrn−/− mice (Figure 4B), and were no longer detected after 4 days. In mice, human IgG elicits murine anti-human IgG antibodies, forming ICs that can deposit in glomeruli, as shown in active serum sickness models. Glomerular deposition of ICs containing human IgG was abolished in mice treated with anti-FcRn peptide, but not with control peptide (Figure 4C). Linear GBM deposition of human anti-GBM IgG was not observed, because the epitopes recognized by Goodpasture autoantibodies are completely inaccessible in the mouse GBM.31 These results provide proof of concept that therapies targeting human FcRn effectively lower serum levels of pathogenic human IgG autoantibodies, which could be beneficial in patients with IgG-mediated kidney diseases. Because FcRn also mediates the trans-placental transfer of IgG from mother to the fetus, FcRn inhibition may be particularly attractive for preventing antenatal MN caused by maternal anti-NEP alloantibodies.
Figure 4.
Pharmacologic blockade of human FcRn accelerates the catabolism of human IgG autoantibodies in FCRN-humanized mice. (A) Structure of a peptide that binds with high affinity to human FcRn, competitively inhibiting its interaction with human IgG (top). The control peptide (bottom) containing D-amino acids does not bind to human FcRn. Pen, Sar, and NMeLeu denote penicillamine, sarcosine, and N-methyl-leucine, respectively. (B) Serum level of human IgG anti-α3NC1 antibodies in FCRN-humanized mice treated with anti-FcRn peptide (▪) or control peptide (●) and in Fcrn−/− (▲) mice sera (n=3 in each group) is analyzed by indirect ELISA in plates coated with α3NC1 (100 ng/well). Mouse sera are diluted 1:500. (C) Kidney deposition of human IgG (a and b) and mouse IgG (c and d) in FCRN-humanized mice treated with control peptide (a and c) or anti-FcRn peptide (b and d) is evaluated by direct immunofluorescence staining. Treatment with anti-FcRn peptide prevents the glomerular deposition of ICs containing human IgG.
Concise Methods
Materials
Recombinant NC1 monomers of human α3NC1 were expressed in human embryonic kidney 293 cells and purified as described.32 Human IgG was purified from plasma exchange fluid of a patient with Goodpasture disease.31 A peptide antagonist of human FcRn, described in detail as “peptide 3” in a previous publication,29 and a control peptide (Figure 4A), were generously provided by Biogen Idec Hemophilia (Waltham, MA).
Animal Experiments
C57Bl/6J, B6.129×1-Fcgrt<tm1Dcr>/Dcr (Fcrn−/−), and B6.Cg-Fcgrt<tm1Dcr> Tg(FCGRT)32Dcr (FCRN-humanized) mice were purchased from The Jackson Laboratory (Bar Harbor, ME). Fcrn−/− mice have lower IgG and serum albumin levels than wild-type mice. In contrast, FCRN-humanized mice have lower IgG levels but similar serum albumin levels compared with wild-type mice.13 Mice were housed in a specific pathogen-free facility with free access to food and water. All procedures were approved by the Institutional Animal Care and Use Committee and conducted in accordance with the Guidelines for Animal Care and Use Program of Vanderbilt University.
Male mice (aged 6–10 weeks) were used for experiments. For active immunizations, α3NC1 (30 μg in 50 µl PBS) was emulsified in an equal volume of CFA (Difco, Detroit, MI), and then injected subcutaneously at two sites on the back. Mice were boosted three times with the same amount of α3NC1 in IFA (Sigma-Aldrich, St. Louis, MO). In control mice, the antigen was replaced by PBS. Mice were regularly checked for signs of disease. Blood and spot urine were collected as described.15,33 Mice were euthanized at 14 weeks after immunization. Blood and kidneys were collected for further analyses.
For IgG imaging studies, DBA/1J mice immunized with α3NC1, as described,15 were euthanized 8 weeks later. To model the glomerular deposition of anti-GBM IgG autoantibodies, DBA/1 mice were injected intravenously with mouse IgG anti-α3NC1 mAb 8D1 (200 μg in sterile PBS) and euthanized the next day.
For passive immunization experiments, FCRN-humanized and FcRn−/− mice were injected intravenously in the tail vein with 1 mg of human IgG containing anti-α3NC1 autoantibodies at day 0. FCRN-humanized mice were treated with anti-FCRN peptide or control peptide (10 mg/kg daily injected in the tail vein) on days 1–4. After 14 days, mice were euthanized and the deposition of human IgG in kidneys was analyzed.
Evaluation of Kidney Function and Renal Histopathology
Urine albumin, urine creatinine, and BUN were measured using commercial kits as described.15,33 Albuminuria was expressed as the urinary albumin to creatinine ratio. The proteins in serum and urine samples were separated by SDS-PAGE under nonreducing conditions and stained with Coomassie Brilliant Blue. For light microscopy, portions of mouse kidneys were fixed in 10% buffered formalin, dehydrated through graded ethanols, embedded in paraffin, and 2 μm–thick sections were stained with periodic acid–Schiff. To evaluate ultrastructural changes, transmission electron microscopy was performed as described.15
Direct and Indirect Immunofluorescence
Portions of snap-frozen mouse kidneys or lung embedded in Tissue Tek O.C.T. Compound (Electron Microscopy Sciences, Fort Washington, PA) were cryosectioned (5 µm), fixed in acetone for 10 minutes at −20°C, and blocked with 1% BSA. For direct immunofluorescence, frozen sections were stained with Alexa Fluor 488–conjugated goat anti-mouse IgG (Invitrogen, Carlsbad, CA) or FITC-conjugated goat anti-mouse C3c (Nordic Immunology, Tilburg, Netherlands). Primary antibodies were rat IgG mAbs RH34, specific for human α3NC1 (generously provided by Dr. Yoshikazu Sado), rabbit anti–C5b-9 (Abcam, Cambridge, MA), and guinea pig anti-nephrin (Progen, Heidelberg, Germany). Secondary antibodies were Alexa Fluor 488–conjugated goat anti-rat and anti-rabbit IgG (Invitrogen). Sections were examined under Nikon Eclipse E800 epifluorescence microscope. Photomicrographs were recorded with a charge-coupled device digital camera, using the same exposure settings for each primary antibody.
STORM Imaging
Kidneys were taken from mice after perfusion with 4% paraformaldehyde (PFA). After multiple washes, kidney pieces were impregnated overnight at 4°C in a cryoprotectant solution of 2.3 M sucrose and 10% polyvinylpyrrolidone in 0.1 M PIPES (pH 7.2). Cryoprotected tissues were mounted on a metal sectioning pin and frozen by immersion in liquid nitrogen. Sections were collected on carbon coating and fresh glow discharge coverglass. Frozen tissues were sectioned at approximately 200 nm in thickness on a Leica EM-FC6 ultracryomicrotome equipped with a diamond knife. Sections were refixed for 20 minutes at room temperature with 4% PFA, followed by three washes in PBS, quenched using 50 mM glycine in PBS, and blocked overnight at 4°C using 3% BSA in PBS. Primary antibody diluted in 3% BSA-PBS was applied on the sections and incubated overnight at 4°C. The next day, sections were washed 3×20 minutes with PBS at room temperature and then incubated with the secondary antibodies diluted in 3% BSA-PBS at room temperature for 2 hours. After washing the samples 3×20 minutes with PBS at room temperature, immunolabeled sections were postfixed using 3% PFA and 0.1% glutaraldehyde (Electron Microscopy Sciences) in PBS and prepared for STORM imaging. For orientation, tissues were stained with hamster anti-agrin C terminus as a primary antibody.34 Anti-total mouse IgG as well as donkey anti-hamster secondary antibodies (Jackson ImmunoResearch, West Grove, PA) were using for STORM imaging. The secondary antibodies were custom conjugated to Alexa647 reporter dye and either Alexa405 or Cy3 activator dyes.
STORM image acquisition was performed using a custom-made setup as described.35,36 Coverglass with the sections on it was inverted onto a slide containing a drop of imaging buffer containing mercaptoethylamine along with an oxygen scavenger system, and coverglass edges were sealed with nail polish. Approximately 10,000 images per channel were captured and analyzed using custom software. For quick-freeze deep-etch electron microscopy, the nail polish from the coverglass was removed after STORM imaging and the tissue sections were fixed in 2% glutaraldehyde. Areas of the coverglass containing STORM-imaged sections were cut, rinsed in dH2O, and deep frozen, and then etched and replicated with approximately 2 nm platinum deposition. After dissolving the glass in concentrated hydrofluoric acid, replicas were rinsed with distilled water, picked up on Luxel grids (Friday Harbor, WA), and photographed on a JEOL 1400 microscope with attached AMT digital camera. The glomeruli imaged by electron microscopy were matched with the corresponding STORM images, and the two images were superimposed using Adobe Photoshop.
Immunoelectron Microscopy
Strips of kidney cortex from mice that were perfusion-fixed with 4% PFA were cryoprotected with 30% sucrose in PBS. Tissue was covered with Tissue Tek O.C.T. Compound (Electron Microscopy Sciences) and snap-frozen in isopentane chilled in a dry ice-acetone bath. Cryostat sections, 30 µm thick, were cut at −20°C, picked up on Thermanox coverslips (Nalgene Nunc International, Rochester, NY), and air dried at room temperature for 1 hour. Coverslips were blocked with 0.5 M ammonium chloride in PBS for 30 minutes at room temperature in a humid chamber, and then incubated overnight at 4°C with rabbit anti-mouse IgG conjugated to horseradish peroxidase (1:200 in PBS; Sigma-Aldrich). The next day, coverslips were washed three times with PBS and then refixed with 2% glutaraldehyde in 0.1 M sodium phosphate buffer (pH 7.3) for 30 minutes at room temperature. Coverslips were washed three times with buffer, and the peroxidase histochemistry reaction was carried out for 1 hour at room temperature. After washing the sections three times with buffer, coverslips were postfixed in 2% osmium tetroxide in 0.1 M sodium phosphate buffer in a humid chamber for 2 hours at room temperature. Slips were then dehydrated through a graded series of increasing concentrations of ethanol and then 100% propylene oxide. Samples were then infiltrated overnight in a 1:1 mixture of propylene oxide and Polybed 812 embedding resin, including DMP-30 (Polysciences, Warrington, PA). The next day, pieces of coverslips were flat-embedded in molds containing fresh Polybed 812 and DMP-30 and polymerized overnight at 60°C. Ultrathin sections were stained briefly with lead citrate and then viewed in a JEOL JEM-1400 transmission electron microscope.
Analysis of Circulating IgG Antibodies
Circulating mouse IgG or human IgG anti-α3NC1 antibodies were analyzed by ELISA in plates were coated overnight with α3NC1 (100 ng/well) and blocked with 1% BSA. Mouse sera were diluted as indicated. Secondary antibodies were alkaline phosphatase–conjugated goat anti-mouse IgG or anti-human IgG (Rockland Immunochemicals, Gilbertsville, PA) and horseradish peroxidase–conjugated goat anti-mouse IgG1, IgG2b, and IgG2c (Bethyl Laboratories, Montgomery, TX).
Statistical Analyses
Data are shown as the mean±SD. Statistical analyses were performed using GraphPad Prism software (version 5.01). The significance of differences between groups was evaluated by the t test (for two groups) or by one-way ANOVA, followed by post hoc tests for pairwise comparisons. A P value<0.05 was considered to be statistically significant.
Disclosures
None.
Acknowledgments
We thank Ms. Jeanette Cunningham for performing the electron microscopy and Dr. Shreeram Akilesh (University of Washington) for helpful comments and discussions.
This work was supported by the National Institutes of Health (NIH) (Grants R01-DK080799 to D.B.B. and R01-DK078314 to J.H.M.), Satellite Healthcare (Norman S. Coplon Extramural Grant to D.B.B.), and the American Heart Association (Grant-in-Aid 12GRNT11480005to D.B.B.). F.O. has been supported, in part, by a postdoctoral fellowship (11POST7300008) from the American Heart Association South-East Affiliate. Electron microscopy was performed by the Washington University O’Brien Center for Kidney Disease Research supported by NIH Grant P30-DK079333. The Electron Microscope Research Laboratory at the University of Kansas Medical Center is supported, in part, by NIH Grant P20-GM104936. The JEOL JEM transmission electron microscope used for immunoelectron microscopy was purchased with funds from NIH Grant S10-RR027564.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Present address: Dr. Adam R. Mezo, Eli Lilly and Company, Indianapolis, Indiana.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2013050498/-/DCSupplemental.
References
- 1.Roopenian DC, Akilesh S: FcRn: The neonatal Fc receptor comes of age. Nat Rev Immunol 7: 715–725, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Akilesh S, Petkova S, Sproule TJ, Shaffer DJ, Christianson GJ, Roopenian D: The MHC class I-like Fc receptor promotes humorally mediated autoimmune disease. J Clin Invest 113: 1328–1333, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li N, Zhao M, Hilario-Vargas J, Prisayanh P, Warren S, Diaz LA, Roopenian DC, Liu Z: Complete FcRn dependence for intravenous Ig therapy in autoimmune skin blistering diseases. J Clin Invest 115: 3440–3450, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sesarman A, Sitaru AG, Olaru F, Zillikens D, Sitaru C: Neonatal Fc receptor deficiency protects from tissue injury in experimental epidermolysis bullosa acquisita. J Mol Med (Berl) 86: 951–959, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Haymann JP, Levraud JP, Bouet S, Kappes V, Hagège J, Nguyen G, Xu Y, Rondeau E, Sraer JD: Characterization and localization of the neonatal Fc receptor in adult human kidney. J Am Soc Nephrol 11: 632–639, 2000 [DOI] [PubMed] [Google Scholar]
- 6.Sarav M, Wang Y, Hack BK, Chang A, Jensen M, Bao L, Quigg RJ: Renal FcRn reclaims albumin but facilitates elimination of IgG. J Am Soc Nephrol 20: 1941–1952, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kobayashi N, Suzuki Y, Tsuge T, Okumura K, Ra C, Tomino Y: FcRn-mediated transcytosis of immunoglobulin G in human renal proximal tubular epithelial cells. Am J Physiol Renal Physiol 282: F358–F365, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Akilesh S, Huber TB, Wu H, Wang G, Hartleben B, Kopp JB, Miner JH, Roopenian DC, Unanue ER, Shaw AS: Podocytes use FcRn to clear IgG from the glomerular basement membrane. Proc Natl Acad Sci U S A 105: 967–972, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gan H, Feng S, Wu H, Sun Y, Hu R, Zhao Z, Zhang Z: Neonatal Fc receptor stimulation induces ubiquitin c-terminal hydrolase-1 overexpression in podocytes through activation of p38 mitogen-activated protein kinase. Hum Pathol 43: 1482–1490, 2012 [DOI] [PubMed] [Google Scholar]
- 10.Hopfer H, Maron R, Butzmann U, Helmchen U, Weiner HL, Kalluri R: The importance of cell-mediated immunity in the course and severity of autoimmune anti-glomerular basement membrane disease in mice. FASEB J 17: 860–868, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Chaudhury C, Mehnaz S, Robinson JM, Hayton WL, Pearl DK, Roopenian DC, Anderson CL: The major histocompatibility complex-related Fc receptor for IgG (FcRn) binds albumin and prolongs its lifespan. J Exp Med 197: 315–322, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koltun M, Nikolovski J, Strong K, Nikolic-Paterson D, Comper WD: Mechanism of hypoalbuminemia in rodents. Am J Physiol Heart Circ Physiol 288: H1604–H1610, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Stein C, Kling L, Proetzel G, Roopenian DC, de Angelis MH, Wolf E, Rathkolb B: Clinical chemistry of human FcRn transgenic mice. Mamm Genome 23: 259–269, 2012 [DOI] [PubMed] [Google Scholar]
- 14.Hopfer H, Holzer J, Hünemörder S, Paust HJ, Sachs M, Meyer-Schwesinger C, Turner JE, Panzer U, Mittrücker HW: Characterization of the renal CD4+ T-cell response in experimental autoimmune glomerulonephritis. Kidney Int 82: 60–71, 2012 [DOI] [PubMed] [Google Scholar]
- 15.Zhang JJ, Malekpour M, Luo W, Ge L, Olaru F, Wang XP, Bah M, Sado Y, Heidet L, Kleinau S, Fogo AB, Borza DB: Murine membranous nephropathy: Immunization with α3(IV) collagen fragment induces subepithelial immune complexes and FcγR-independent nephrotic syndrome. J Immunol 188: 3268–3277, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Debiec H, Guigonis V, Mougenot B, Decobert F, Haymann JP, Bensman A, Deschênes G, Ronco PM: Antenatal membranous glomerulonephritis due to anti-neutral endopeptidase antibodies. N Engl J Med 346: 2053–2060, 2002 [DOI] [PubMed] [Google Scholar]
- 17.Beck LH, Jr, Bonegio RG, Lambeau G, Beck DM, Powell DW, Cummins TD, Klein JB, Salant DJ: M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med 361: 11–21, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Debiec H, Lefeu F, Kemper MJ, Niaudet P, Deschênes G, Remuzzi G, Ulinski T, Ronco P: Early-childhood membranous nephropathy due to cationic bovine serum albumin. N Engl J Med 364: 2101–2110, 2011 [DOI] [PubMed] [Google Scholar]
- 19.Borza DB, Zhang JJ, Beck LH, Jr, Meyer-Schwesinger C, Luo W: Mouse models of membranous nephropathy: The road less travelled by. Am J Clin Exp Immunol 2: 135–145, 2013 [PMC free article] [PubMed] [Google Scholar]
- 20.Borza CM, Borza DB, Pedchenko V, Saleem MA, Mathieson PW, Sado Y, Hudson HM, Pozzi A, Saus J, Abrahamson DR, Zent R, Hudson BG: Human podocytes adhere to the KRGDS motif of the alpha3alpha4alpha5 collagen IV network. J Am Soc Nephrol 19: 677–684, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jia XY, Hu SY, Chen JL, Qu Z, Liu G, Cui Z, Zhao MH: The clinical and immunological features of patients with combined anti-glomerular basement membrane disease and membranous nephropathy [published online ahead of print September 18, 2013]. Kidney Int 10.1038/ki.2013.364 [DOI] [PubMed] [Google Scholar]
- 22.Cybulsky AV, Rennke HG, Feintzeig ID, Salant DJ: Complement-induced glomerular epithelial cell injury. Role of the membrane attack complex in rat membranous nephropathy. J Clin Invest 77: 1096–1107, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cunningham PN, Quigg RJ: Contrasting roles of complement activation and its regulation in membranous nephropathy. J Am Soc Nephrol 16: 1214–1222, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Beck LH, Jr, Fervenza FC, Beck DM, Bonegio RG, Malik FA, Erickson SB, Cosio FG, Cattran DC, Salant DJ: Rituximab-induced depletion of anti-PLA2R autoantibodies predicts response in membranous nephropathy. J Am Soc Nephrol 22: 1543–1550, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bleeker WK, Teeling JL, Hack CE: Accelerated autoantibody clearance by intravenous immunoglobulin therapy: Studies in experimental models to determine the magnitude and time course of the effect. Blood 98: 3136–3142, 2001 [DOI] [PubMed] [Google Scholar]
- 26.Nortier JL, Debiec H, Tournay Y, Mougenot B, Nöel JC, Deschodt-Lanckman MM, Janssen F, Ronco P: Neonatal disease in neutral endopeptidase alloimmunization: Lessons for immunological monitoring. Pediatr Nephrol 21: 1399–1405, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Liu L, Garcia AM, Santoro H, Zhang Y, McDonnell K, Dumont J, Bitonti A: Amelioration of experimental autoimmune myasthenia gravis in rats by neonatal FcR blockade. J Immunol 178: 5390–5398, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Patel DA, Puig-Canto A, Challa DK, Perez Montoyo H, Ober RJ, Ward ES: Neonatal Fc receptor blockade by Fc engineering ameliorates arthritis in a murine model. J Immunol 187: 1015–1022, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mezo AR, Sridhar V, Badger J, Sakorafas P, Nienaber V: X-ray crystal structures of monomeric and dimeric peptide inhibitors in complex with the human neonatal Fc receptor, FcRn. J Biol Chem 285: 27694–27701, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mezo AR, McDonnell KA, Hehir CA, Low SC, Palombella VJ, Stattel JM, Kamphaus GD, Fraley C, Zhang Y, Dumont JA, Bitonti AJ: Reduction of IgG in nonhuman primates by a peptide antagonist of the neonatal Fc receptor FcRn. Proc Natl Acad Sci U S A 105: 2337–2342, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luo W, Wang XP, Kashtan CE, Borza DB: Alport alloantibodies but not Goodpasture autoantibodies induce murine glomerulonephritis: Protection by quinary crosslinks locking cryptic α3(IV) collagen autoepitopes in vivo. J Immunol 185: 3520–3528, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kang JS, Colon S, Hellmark T, Sado Y, Hudson BG, Borza DB: Identification of noncollagenous sites encoding specific interactions and quaternary assembly of alpha 3 alpha 4 alpha 5(IV) collagen: Implications for Alport gene therapy. J Biol Chem 283: 35070–35077, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Olaru F, Wang XP, Luo W, Ge L, Miner JH, Kleinau S, Geiger XJ, Wasiluk A, Heidet L, Kitching AR, Borza DB: Proteolysis breaks tolerance toward intact α345(IV) collagen, eliciting novel anti-glomerular basement membrane autoantibodies specific for α345NC1 hexamers. J Immunol 190: 1424–1432, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harvey SJ, Jarad G, Cunningham J, Rops AL, van der Vlag J, Berden JH, Moeller MJ, Holzman LB, Burgess RW, Miner JH: Disruption of glomerular basement membrane charge through podocyte-specific mutation of agrin does not alter glomerular permselectivity. Am J Pathol 171: 139–152, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dani A, Huang B, Bergan J, Dulac C, Zhuang X: Superresolution imaging of chemical synapses in the brain. Neuron 68: 843–856, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Suleiman H, Zhang L, Roth R, Heuser JE, Miner JH, Shaw AS, Dani A: Nanoscale protein architecture of the kidney glomerular basement membrane. Elife 2: e01149, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]