Abstract
Bringing together topic-related European Union (EU)-funded projects, the so-called “NanoSafety Cluster” aims at identifying key areas for further research on risk assessment procedures for nanomaterials (NM). The outcome of NanoSafety Cluster Working Group 10, this commentary presents a vision for concern-driven integrated approaches for the (eco-)toxicological testing and assessment (IATA) of NM. Such approaches should start out by determining concerns, i.e., specific information needs for a given NM based on realistic exposure scenarios. Recognised concerns can be addressed in a set of tiers using standardised protocols for NM preparation and testing. Tier 1 includes determining physico-chemical properties, non-testing (e.g., structure–activity relationships) and evaluating existing data. In tier 2, a limited set of in vitro and in vivo tests are performed that can either indicate that the risk of the specific concern is sufficiently known or indicate the need for further testing, including details for such testing. Ecotoxicological testing begins with representative test organisms followed by complex test systems. After each tier, it is evaluated whether the information gained permits assessing the safety of the NM so that further testing can be waived. By effectively exploiting all available information, IATA allow accelerating the risk assessment process and reducing testing costs and animal use (in line with the 3Rs principle implemented in EU Directive 2010/63/EU). Combining material properties, exposure, biokinetics and hazard data, information gained with IATA can be used to recognise groups of NM based upon similar modes of action. Grouping of substances in return should form integral part of the IATA themselves.
Keywords: nanotoxicology, 3Rs principle, human health hazard assessment, environmental hazard assessment, grouping of substances
Background
Recognising nanotechnology as an enabling technology contributing to innovation, economic growth, employment and competitiveness, the European Union’s (EU) Commission, in its second regulatory review on nanomaterials, has reinforced its continuous commitment to promoting research and development in this area (Anon 2012a). Warranting the safety of nanotechnological products is seen as a crucial element in ensuring that the benefits of the new technology can be fully exploited. Therefore, up until the end of 2012, the Commission has funded a total of 46 nanosafety projects representing a total EU investment of 130 million EUR (Anon 2012b). To facilitate the formation of consensus on nanotoxicology, the Commission has requested EU-funded nanosafety projects to join forces through the so-called NanoSafety Cluster (Anon 2012b). The members of this initiative have assigned themselves the goal to identify key areas of nanosafety research which are likely to be of special significance in the coming years (NanoSafety Cluster 2011). Covering all aspects of nanomaterial characterisation, exposure and hazard assessment, research challenges relating to these key areas are further specified in topic-specific Working Groups. In delineating a timeframe for meeting such challenges, the NanoSafety Cluster has taken on the deadline of 2020, a year that has been spelled out by the Commission both in the Europe 2020 strategy for smart, sustainable and inclusive growth (Anon 2010a) and for the upcoming Research Framework Programme Horizon 2020 (Anon 2011a).
The present commentary summarises the outcome of the discussions of NanoSafety Cluster Working Group (WG) 10 on integrated approaches to testing and assessment (IATA) of nanomaterials (NM). Such approaches, in the literature also referred to as integrated testing strategies, are required for an adequate assessment of the impact of NM on human health and the environment. Whereas WG 10 has pursued its deliberations on IATA independently of existing regulations, they do stand in line with current EU guidance on NM safety testing. In the context of REACH Regulation 1907/2006 (Registration, Evaluation, Authorisation of Chemicals; Anon 2006), a testing strategy for NM should consider the procedure established for conventional chemicals expanded to address the specific peculiarities of NM (RIP-oN 2 2011).
One prominent trait of NM is the fact that, during the lifetime of a given NM, humans and the environment can be exposed to different forms of the material, for example due to agglomeration or aggregation, corona formation or interaction with surrounding organic material, or dissolution. Hence, it is of paramount importance for adequate testing to ensure that the testing conditions applied (including NM characteristics and exposure conditions) are appropriate to assess the risk under relevant real-life exposure situations. One aspect is that the physico-chemical properties of the nanomaterial during testing are known, either by analytical techniques or standardised techniques when suspending or dispersing NM for toxicity testing. One way or another, this issue needs to be addressed in the risk assessment strategy for NM.
Since a multitude of different NM in different exposure scenarios is expected, it will not be possible to perform all-embracing testing of all NM in all relevant scenarios. Instead, testing must be targeted to the actual concerns for a given NM making use of realistic exposure scenarios. Moreover, a testing strategy should include possibilities for the grouping of NM (e.g., by applying a “read-across” methodology, some tests could be waived based on a categorisation of NM), and should also aid the grouping concept itself (e.g., the testing strategy should provide information that is relevant for grouping).
The actual concerns associated with a given NM should be determined in relation to material properties, specific exposure situations, biokinetic data and/or markers of early biological effects. They should be used to define the crucial human health and environmental end points to be tested in focused studies, including the test designs of these studies. All of these issues should be considered for the grouping of NM which, in return, should form integral part of the IATA. The integrated NM toxicity and ecotoxicity testing approaches proposed in this commentary are based on these considerations.
State-of-the-art
Multiple toxicity studies with NM have been carried out in the last decade. However, most of them used non-standardised testing protocols leading to sometimes not reproducible and hardly comparable results, which therefore are insufficient for univocal hazard and risk assessment. In addition, the unavailability of consistent physico-chemical characterisation data in the same studies makes it difficult to identify which (combinations of) material characteristics determine the documented toxic effects. One reason behind the lack of convincing patterns could be that characterisation has been performed ex situ, since NM are generally difficult to characterise in situ (Tiede et al. 2008, Card & Magnuson 2010). Nevertheless, it has been shown that a variety of factors such as ionic strength, pH and other media-specific properties cause changes to the primary NM once they enter media (e.g., related to NM agglomeration, aggregation and surface modification), which affects both their bioavailability and toxicity (Hassellov et al. 2008). Besides, the results are derived using a variety of dispersion and analytical protocols and here again standard methods require to be developed.
Current toxicological approaches to assess hazards of NM are either based on methods adopted from classical toxicology or on alternative methods. These approaches do not fully consider the unique aspects of NM, for example, 1) different forms of a given NM in different biological media; 2) uptake/absorption, distribution, corona formation and elimination/deposition (ADCE, used by analogy to ADME, absorption, distribution, metabolism, excretion; since, for most NM, metabolism, unlike corona formation, does not play a major role); 3) functional impacts at the organ and cellular levels.
Slow elimination and persistence are main drivers for bioaccumulation. Similar to conventional hydrophobic persistent chemicals, such as DDT, that are resistant to environmental and biological degradation, NM have the potential to accumulate in humans and biota (food chain) when exposure takes place on, for example, a daily basis. Presently, the structure and dynamics of protein corona are considered to be key to the nanoparticle’s rate of uptake and transport into cells and final subcellular localisation (Nel et al. 2009; Lundqvist et al. 2011). A number of proteomics methods to identify the nature, composition and dynamics of the biomolecules associated with NM have been developed (Lai et al. 2012). Without suitable information on the potential for NM to bioaccumulate, it is not possible to carry out higher-tier human health or environmental risk assessment or to derive environmental quality standards (Anon 2008).
While the risk assessment of conventional substances is based on the notion that their chemical identity governs the biological effects of a substance, there is general agreement that the toxicity of NM is determined by a set of characteristics, for example, size, shape, surface area, charge. Given the substantial diversity within each group of nanomaterials and the complexity of nanosystems (e.g., stability of dispersions under different conditions), a large number of property combinations need to be considered in order to assess the overall hazard of a single material type. Currently, it is widely accepted among scientists and regulators that the hazard/risk assessment of NM can only be addressed on a case-by-case basis. However, considering the large number of existing and emerging nanoformulations, this would be a time and resource intensive task, conflicting with the 3Rs principle to replace, reduce and refine animal testing (Russell & Burch 1959) implemented in EU Directive 2010/63/EU on the protection of animals used for scientific purposes (Anon 2010b). In this context, IATA have become particularly relevant since they are intended to speed up the risk assessment process, while at the same time reducing testing costs and animal use by effectively exploiting all existing data (van Leeuwen et al. 2007; Hartung 2009). Integrated processes consolidate all available information (including both exposure and toxicity information) to identify relevant concerns and to determine the information that best addresses these concerns (focusing on toxicity studies and/or exposure information).
The need for standardisation and new approaches in the nanosafety area has been recognised. In parallel to ongoing scientific research, a number of international initiatives have been launched, such as the OECD Working Party on Manufactured Nanomaterials (OECD WPMN; see: http://www.oecd.org/env/ehs/nanosafety/ note: all websites were accessed in February and March 2013) and the ISO Technical Committee 229 (see: http://www.iso.org/iso/iso_technical_ committee?commid=381983).
Of particular importance for the above discussions are the OECD WPMN Steering Groups (SG) 6 and 7. SG 6 deals with risk assessment approaches, such as “read-across” methodologies, and SG 7 addresses the use of alternative test methods and integrated testing strategies for NM hazard assessment. This SG has proposed a new short-term inhalation study (STIS) for NM testing (OECD 2011) and has compiled a list of in vitro methods that might be used for NM human hazard identification. Furthermore, it has initiated a similar discussion for environmental impacts. Comparable structures of the human and environmental hazard identification frameworks aim at allowing a better integration of human health and ecological risk assessment into one coherent strategy and at facilitating its implementation for regulatory purposes.
Vision beyond the state-of-the-art
The NanoSafety Cluster vision 2020 foresees the development of a concern-driven guidance for investigating potential risks of NM. This will enable focused research on NM that may be of particular concern based on (expected) exposure levels and exposure routes, material properties as well as in silico, hazard and biokinetic data. By advocating that animals are only used for crucial and focused studies minimising numbers of animals used and the distress inflicted upon the animals, this approach further meets the provisions of EU Directive 2010/63/EU (Anon 2010b).
Based on these considerations, IATA will be developed allowing for different sequences of testing depending on the types of NM. Such IATA will start out by identifying relevant concerns, based on NM exposure and use scenarios and taking into account already available toxicological information and basic information assessed by non-testing (structure–activity relationships (SAR), generic physiologically based pharmacokinetic (PBPK) modelling based on in vitro data, and grouping), proceeding with tests using acellular systems and then cellular systems, up until in vivo, long-term testing approaches, if deemed necessary (Johnston et al. 2013). These strategies will be based on a thorough understanding of the biokinetics of a given NM in exposure-relevant media and matrixes (e.g., medium-dependent agglomeration, dispersion, etc.) enabled by a continuous sampling, analysis and characterisation paradigm. They will be designed both for human health and environmental safety/risk assessment, for example, by mimicking realistic exposure scenarios and by using appropriate dose levels for different purposes and in different situations. Finally, these strategies will be based on validated methods with established predictive power.
Such guidance, to be fully developed and effective by 2020 and beyond, will require less testing whenever the available information is sufficient and adequate for decision-making. For instance, if an NM rapidly dissolves, the substance should be treated as a conventional chemical, although the assessment of dissolution, and under which conditions and in which media, could be the actual, underlying challenge (remaining to be addressed in future research projects, such as NanoREG; see: http://ihcp.jrc.ec.europa.eu/our_activities/nanotechnology/symp2012/doc/van_Teunenbroek_Research_Reg_needs_s2.pdf). At the same time, it will be possible to obtain more information from the selected tests, for example, on the particles’ mechanism of action (Rossini & Hartung 2012; Nel et al. 2013). Nevertheless, the guidance might also imply requiring more information if it is necessary to address specific concerns (e.g., immunotoxicity, cardiovascular toxicity, neurotoxicity, developmental toxicity). Eventually, it will be inevitable to discuss which level of certainty on the potential risks of a given NM in a specific application should be required.
Furthermore, by 2020 and beyond, guidance will be developed and effective on when NM (based on physico-chemical characteristics, exposure and early biological effects) can be grouped, how these groupings should be constructed and what kind of information is necessary to be able to group. In addition to avoiding extensive hazard testing of NM, this will also provide insight into the conditions under which information on exposure and hazard for NM can be used for risk assessment purposes (Som et al. 2012).
This vision 2020 of the NanoSafety Cluster should be realised in the context of existing international and national chemical regulatory frameworks taking into account existing OECD Test Guidelines (TG) and adapted as appropriate to take into account the specific properties of NM, as recommended by the OECD Chemicals Committee (OECD 2013). Following on a mid-term evaluation of the OECD’s nanosafety programme in 2012, the Chemicals Committee, a parent body to the WPMN, additionally recommended updating existing OECD TG or including new ones in the light of experience with NM, to apply the OECD principles of Good Laboratory Practice to NM safety testing, and to make safety data related to NM available to the public (OECD 2013).
NM dispersion and in situ characterisation for toxicity testing
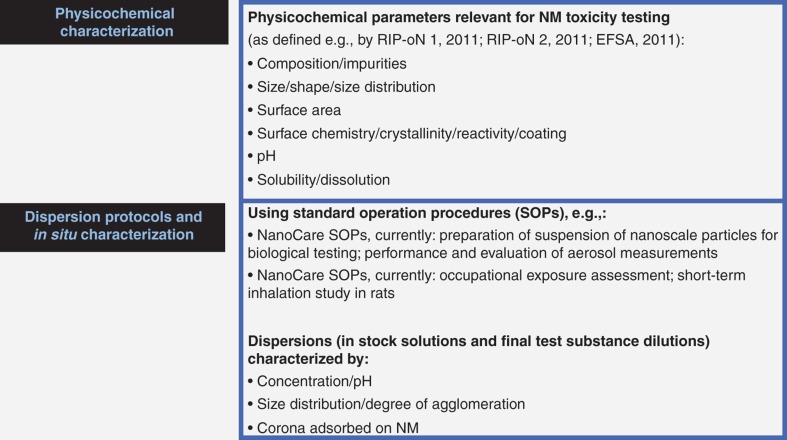
On the one hand, meaningful exposures allowing to understand and monitor the important characteristics of real-life exposure and to correctly interpret the test results cannot be mimicked in all toxicological test systems (Hristozov et al. 2013). On the other hand, the form of the NM (agglomeration state, corona, etc.) can be largely dependent on the exposure scenario. Therefore, test sample preparation by dispersion of the respective NM is a critical step for toxicity testing, and it must be well considered and appropriate (Rothen-Rutishauser et al. 2010). Likewise, the resulting test sample dispersions (both stock and test solutions) have to be characterised. Over the past years, multiple dispersion protocols have been used and an extensive list of parameters for in situ characterisation has been proposed (Schulze et al. 2008; Card & Magnuson 2009; SCENIHR 2009; OECD 2010). Figure 1 lists characterisation parameters currently considered relevant for NM preparation and safety testing.
Figure 1.
Physico-chemical characterisation, dispersion and in situ characterisation of NM for toxicity testing.
NanoCare SOPs http://www.nanopartikel.info/cms/lang/de/Projekte/NanoCare/NanoCare-Publikationen.
NanoGem SOPs http://www.nanogem.de/cms/nanogem/front_content.php?idcat=159&lang=10.
Testing strategies to assess NM biopersistence and biokinetics
NM may potentially cross portals of entry into the body, such as the gills, the gastrointestinal tract, lung epithelium or skin, and also internal barriers, such as the blood–brain barrier, placenta and blood–testis barrier. NM with different characteristics (size, coating, shape) may differ considerably in the extent of their transportation across these barriers. The types of particles that can be transferred across barriers have not been systematically investigated, and to date very little is known about the parameters that influence differences in the extent of transportation (Oberdörster 2009; Landsiedel et al. 2012). However, such information will be relevant to determine if NM have reached the systemic circulation, and if so, to what extent bioavailability is increased, and whether, for instance, systemic effects, including cardiovascular or immunological toxicity, should be considered.
In contrast to many soluble chemicals, NM generally tend to disappear rapidly from the blood by being taken up into tissues, mainly those containing phagocytic cells. The apparent very short blood-plasma half-life stands in sharp contrast with the apparent long whole body or tissue half-life. Once filtered from the blood by macrophages, NM tend to stay in these cells and in similar cell types, and they undergo whole-body elimination only to a fairly limited extent. Strong indications for this are already available for a number of NM, such as silver, SiO2 and cerium nanoparticles (Lankveld et al. 2010; Dan et al. 2012).
Coatings as intentional modifications of the NM surface impact the effect on organisms by for example, increasing the blood-plasma half-life (such as in the case of polyethylene glycol (PEG)-coated NM). Likewise, unintentional surface modifications may have an impact on the tissue distribution of NM, as well as on the rate in which distribution occurs (Liu et al. 2012). A “protein corona” is likely to be formed around the NM when they enter the body or even already before, for example, when they are added to food products (Nel et al. 2009; Lundqvist et al. 2011; Johnston et al. 2012). It should be noted that the corona of biological molecules covering the NM surface is most likely composed not only of proteins, but also of lipids and carbohydrates (Gasser et al. 2010). This “bio-corona” surrounding NM is dynamic, since the types of molecules forming it may change in time. Additionally, proteins and other molecules in the body fluids (mucus, blood, etc.) can differ significantly depending on the individual’s health conditions, thereby affecting the composition of the corona (Nel et al. 2009; Johnston et al. 2012). Similar to surface modifications of the NM, the corona affects the distribution pattern and the rate of distribution. This also implies that the uptake into cells is driven by the corona (Nel et al. 2009; Landsiedel et al. 2010). Concerning ecotoxicity, NM coating with organic molecules, such as humic acids or with minerals, is of relevance. More evidence is becoming available in regard to such issues, but this is still far too little to obtain a clear overview on the influence of NM surface modification and corona on bioavailability, biokinetics and cell entry.
Effects of NM may be related to 1) the particles themselves and their coatings (particle effects); 2) ions or molecules released from the particles (chemical effects) and 3) molecules formed by the catalytic surface of the particle (nanorelated effects) (Landsiedel et al. 2010; Nel et al. 2013). In vitro dissolution tests in physiologically relevant media (e.g., lysosomal fluid, gastrointestinal fluid, lung lining fluid) may give indications on the time frame in which mainly particles, both particles and ions, and mainly ions are present (Dekkers et al. 2011, 2012; EFSA 2011). If particles are unlikely to dissolve, efforts should focus on long-term particle-related effects, since the particles are likely to accumulate over time and exert their particle effects accordingly (Dekkers et al. 2012). If particles dissolve, both particle and ion-related toxicity should be considered. In that case, accumulation of particles is to be expected to a smaller extent, due to elimination by dissolution of particles to ions. The ion-related effects may be different from the effects of the dispersed particles. If NM distribution and cellular uptake occurs in the particulate form, the ions may be released at different sites than when the NM are distributed as dispersed particles. Hence, the resulting toxicity may be different.
Uptake by cells without significant clearance from those cells, as known for some NM, may pose a potential risk that up to now has not sufficiently been taken into account in the context of risk assessment. In order to prevent problems from arising, such as those encountered for persistent organic pollutants, information on long-term effects of NM should be addressed.
In conclusion, the biokinetics of NM is certainly a research area of high priority. By 2020, the understanding of the mechanisms and significance of NM absorption via different routes, including de-agglomeration, and their distribution and translocation throughout the body, as well as cell type-specific NM uptake, breakdown and excretion will have markedly improved. Furthermore, the significance of biological barriers will be better understood and can be estimated based on simple tools (in silico, in vitro), allowing for a high-throughput and reliable assessment of the possible risks posed by NM to human health and environmental species. Kinetic data and kinetic modelling should become tools to evaluate whether a specific NM behaves differently from another NM or from the corresponding bulk material. This should also allow assessment of whether NM can be grouped for risk assessment purposes. Based on such mechanistic understanding, it may also become possible to predict under which circumstances overload of various cell types is likely to occur.
Testing strategies to assess NM human health hazards (i.e., toxicity)
To date, no new type of toxic effect has been described for NM (i.e., no effects which have not been observed with any other substance or particle before). However, as has been discussed above, the uptake, tissue distribution and clearance of NM may be different from dissolved molecules or larger particles. Nevertheless, the toxicity of NM can generally be tested by standard testing methods used for conventional chemicals, adapted to take into account, for example, the NM-specific requirements for test sample preparation and characterisation as well as test interference controls. Further adaptations of standard test methods may be necessary. It should be noted that these adaptations might not only be relevant for NM testing, but that they might be challenges for the modernisation of safety testing approaches as such.
Traditional toxicity testing is largely restricted to the mere observation of apical effects (i.e., clinical or histopathological manifestation of effects) and was performed according to an extensive list rather than a targeted strategy. A targeted testing strategy would be an efficient approach in line with the 3Rs principle: fewer animals might be needed for testing, or more – and more relevant – information would become available with the reduced numbers of animals used (Silbergeld et al. 2011). A modern and efficient approach would address specific concerns for the respective NM in its given area of use (Nel et al. 2013).
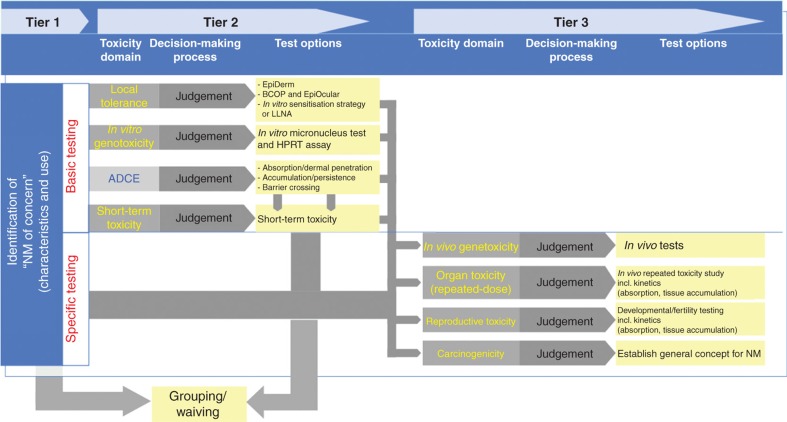
A concern-driven NM toxicity testing scheme consisting of three main tiers is presented in Figure 2. Tier 1 includes a concern assessment based on the physico-chemical characteristics of the specific NM and relevant scenarios for potential exposure, depending on its envisaged use. Such relevant exposure scenarios should take into account realistic dose levels that for example, workers or consumers might be exposed to and whether nanomaterial exposure is likely to be in the aggregated or agglomerated states. For NM of concern identified in tier 1, tier 2 focuses on identifying their basic toxicological concerns, while tier 3 provides options to study specific end points of concern in more detail. The need for the performance of specific tests in the last tier is determined by the combined results of the first two tiers (Zuin et al. 2011; Cockburn et al. 2012).
Figure 2.
Targeted integrated approach to NM toxicity testing and assessment addressing the specific concerns for an individual NM.
Tiers 2 and 3 each consist of three parts, the so-called toxicity domain, the decision-making process and the description of options for testing within each domain. Each toxicity domain reflects a specific toxicity end point or type of testing (e.g., repeated dose, biokinetics) to be addressed and contains a number of options for testing or non-testing (e.g., grouping/waiving) that can be selected in the decision-making process based on the concerns identified in the preceding tier(s).
These three tiers of the concern-driven toxicity testing strategy for NM are discussed below.
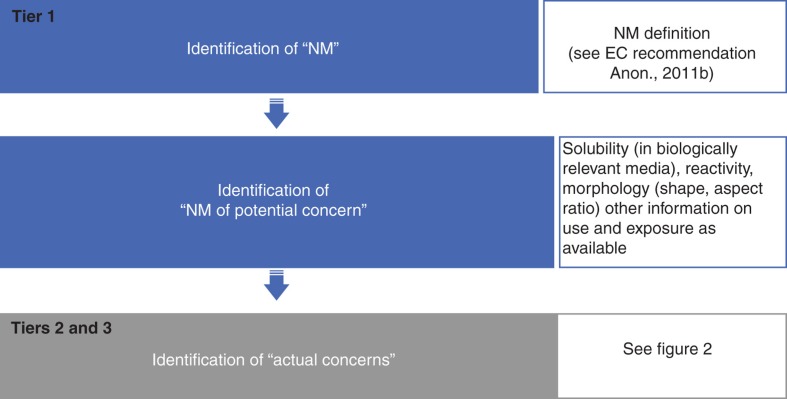
Tier 1: Identification of NM of concern
The first step of tier 1 is the identification of the NM (Figures 2 and 3). This will depend on the definition of NM used (Bleeker et al. 2013). In the EU, the respective Commission Recommendation (Anon 2011b) defines a “nanomaterial” to be a “natural, incidental or manufactured material containing particles, in an unbound state or as an aggregate or as an agglomerate and where, for 50% or more of the particles in the number size distribution, one or more external dimensions is in the size range 1 nm–100 nm”. The Commission recommendation provides for flexibility in the number size distribution threshold of 50% and lays down that “fullerenes, graphene flakes, and single wall carbon nanotubes with one or more external dimensions below 1 nm should [also] be considered as nanomaterials”.
Figure 3.
Identification of potential health concerns of an individual NM based on its physico-chemical properties and relevant exposure scenarios (numberings of tiers relate to Figure 2).
Many NM will not raise specific concerns, but will readily dissolve or form other, larger, particles (e.g., granular, biopersistent NM with low surface area and reactivity). However, other NM are likely to possess properties raising concerns for toxic effects (Figure 3, step 2, identification of NM of potential concern). These concerns may be general or linked to specific uses and exposures (Figure 3, step 3, identification of actual, relevant concerns). For instance, NM solely used in non-spray cosmetic sunscreen lotions may be of low concern for inhalation toxicity for consumers. The output of tier 1 might be a prioritisation list for the testing of an individual NM in tier 2.
Tier 2: Basic testing
Concerns identified for an NM in its handling and use scenario can be addressed by (modified) standard test methods in a targeted strategy (Figure 2). At present, four toxicity domains are defined in tier 2, that is, biokinetics, local effects at the point of entry or primary contact, genotoxicity and short-term toxicity (including inflammation and necrosis/cytotoxicity). Within each domain, a number of options for testing will be available, from which a selection for further testing can be made taking into account the concerns identified in tier 1. For instance, the most relevant route of exposure or a potential for persistence identified in tier 1 will determine choices for testing, including test designs (e.g., parameters to be addressed, appropriate routes of exposure). Similarly, the order of testing can be decided upon to ensure that tests are combined meaningfully leading to best possible test designs and test results. During this decision-making phase, all available information will be considered. Grouping of substances (see below) is seen as an integral part of the decision-making process: based on grouping, it can be decided to waive specific tests or to confirm the necessity of a further test. The outcome of grouping can either be negative (no concern) or positive (a concern is confirmed based on the grouping). The latter will not automatically lead to further testing, but may lead to a decision, such as to stop research and development of the given substance or to the recommendation and application of adequate risk management measures.
Tier 3: Specific testing
The outcome of tier 2 tests will allow identification of the need for additional information and determining appropriate toxicity domain(s) to be addressed in tier 3 (Figure 3). The selection and design of tests for tier 3 will thus be based on the information obtained during tiers 1 and 2.
Considerations for decision criteria and testing options
The options and decision criteria for testing within each toxicity domain of the presented testing strategy remain to be determined. Possible options for testing in tiers 2 and 3, as illustrated in Figure 2, require completion. Of paramount importance, such criteria should ensure an overarching testing strategy and an integrated combination of tests rather than a set of separate tests for individual domains. The set of decision criteria should aim at obtaining the best possible data set, making use of the minimum number of tests possible. However, the criteria should not only relate to the identification of testing requirements, but also include options for waiving specific tests and provide guidance for grouping. If the need for specific additional tests is identified in the decision step of a given tier or is considered obligatory for grouping purposes, this will be described and included. An example for this may be a test for confirmation of a comparable mode of action.
For several concerns, the expected toxic effect will be obvious from the composition (or shape or modification) of the NM and may thus be related to the bulk material (e.g., NM releasing zinc ions will show zinc toxicity). Differences in toxicity profiles may, however, arise from different distributions of a given NM. Once more, these considerations underline the importance of biokinetics and material properties (such as surface effects and persistence of NM in different biological media) in the context of NM toxicity testing (Krug & Wick 2011). Therefore, the biokinetics (ADCE) of NM are to be specifically addressed in any NM testing strategy.
Information on potential routes of exposure combined with indications for absorption across portals of entry determines the relevant route(s) of exposure to be chosen for testing systemic toxicity. Transportation across further barriers (placenta, blood–brain, blood–testis) will provide indications for the choice of (end-point-)specific testing (such as testing for reproduction toxicity or neurotoxicity). During physico-chemical characterisation, solubility in physiologically relevant media should be addressed to determine potential concern for long-lasting particle-related effects. If an NM, for which oral exposure is a relevant exposure route, dissolves in the gastrointestinal tract, only particle-related local effects on the gastrointestinal are anticipated (Dekkers et al. 2011; EFSA 2011; Peters et al. 2012). If an NM dissolves within a couple of days in the macrophage/lysosome environment, equilibrium between intake and degradation will be reached within weeks, even on daily exposure. Therefore, long-term particle-related effects are unlikely. The potential use of information on dissolution for the risk assessment strategy should be investigated in more detail. Indications for biopersistence (in contrast to dissolution) and accumulation (to be investigated by a short-term kinetic study) of NM point to the need for long-term testing.
So far, carcinogenic properties of NM in the lung have not been studied extensively (Becker et al. 2011), and very limited data on long-term studies by inhalation are available (Aschberger et al. 2011). Long-term inhalation studies with selected NM are required, also allowing recognising pathways by which NM can cause lung tumours (e.g., inflammation, overload, genotoxicity). As soon as such general principles will be understood, less demanding tests can be applied in the toxicity testing strategies (e.g., short-term inhalation tests, genotoxicity and cell transformation tests). The same concept of test selection could be used to address further possible concerns, such as detrimental cardiovascular effects. These considerations clearly indicate priorities for further research in nanosafety.
Proposals for future testing strategies
The testing strategy presented in Figure 2 is largely based on modified OECD standard methods extended with selected new methods, which partly were specifically developed for NM, such as short-term nanotoxicity studies (Ma-Hock et al. 2009; Klein et al. 2012). Further development of toxicological methods and increasing knowledge on NM adverse effects, their mechanism of action and the relevance of test results for the situation in humans will also alter the choice of test methods and improve NM toxicity testing strategies.
Toxicity testing of NM should make use of realistic models that represent different exposure scenarios. Silbergeld et al. (2011) discuss a potential modern testing battery for NM consisting of four elements. In recognition that rodent models oftentimes do not reproduce the reactivity found in humans, emphasis should be placed on in vitro models of human primary cells in tissue-like organisation (Silbergeld et al. 2011). These may include barrier models (microvasculature endothelial cells, colon vs. small intestine, upper respiratory tract vs. alveolar space, skin) and models of the blood (in the case of intravenous delivery of nanomedicines).
Complex models reproducing tissue architecture and conditions would provide scenarios much closer to the human situation than when using continuous transformed cell lines in vitro or animals in vivo. An example for such a complex model is colon tissue composed of primary enterocytes with M cells cultured on a thin porous support, with underlying lamina propria macrophages and dendritic cells, overlaid with mucus and enterobacteria (Leonard et al. 2012). Determination of NM effects in such models will allow a more comprehensive analysis of NM kinetics of interaction with the various extra-epithelial components of the biological system (mucus, bacteria, soluble factors), features of uptake by different mucosal cell types, intracellular trafficking, translocation between cells or compartments and also biological effects, such as cell viability/damage (stress/autophagy, apoptosis, necrosis) and inflammatory cell activation (induction of inflammatory cytokines, inflammasome activation, down-regulation of inflammation inhibitors). In addition, such models can be adapted to reproducing conditions of disease (e.g., chronic gut inflammation, increased permeability, leukocyte influx, enhanced tissue destruction, impaired barrier function). This will allow testing different NM exposure scenarios, including those that are likely to be more relevant to safety assessment, for example, in pathological or frailty conditions.
In the following, taking the example of genotoxicity testing, the choice of test methods, prevailing research needs and the potential to improve existing methodologies shall be discussed.
Based on a comprehensive literature review, Landsiedel et al. (2009) have spelled out the following seven recommendations for genotoxicity testing of NM:
know the NM to be tested and its form;
recognise that NM are not all the same;
consider uptake and distribution of the NM;
take the NM-specific properties into account;
use standardised methods;
use in vivo studies and assess correlations with in vitro results;
use this information to compile the likely mechanisms of genotoxic effects.
For many NM, both direct and indirect primary genotoxic effects as well as secondary ones can be partially explained as the result of enhanced production of oxidative stress which in return can lead to the induction of oxidative DNA damage (Bhattacharya et al. 2012; Kühnel et al. 2012). Thus, one of the main end points to be included in a range of assays addressing genotoxicity is oxidative DNA damage (Fenech 2007). However, no specific assay for oxidative damage detection is available as OECD TG, and the already widely used Comet assay remains under evaluation (Burlinson 2012).
Data obtained from general toxicity tests are of fundamental importance for studying genotoxic properties, since a comparison of genotoxicity with general toxicity is critical for any assessment of genotoxic effects. Efforts should be made to identify assays that can yield multiple results by analysing many different end points simultaneously, such as both cytotoxicity and genotoxicity, and that can be applied to many cell types.
Recently, there has been growing interest in epigenetic changes in gene expression. Several epigenetic mechanisms, including DNA methylation, histone modifications and microRNA expression, can change genome function under exogenous influence (Koturbash et al. 2012). Many metals possibly act by epigenetic mechanisms. Therefore, nanosized metal particles might also act through an epigenetic mechanism, for instance, by directly interacting with subcellular components of the mitotic machineries, giving rise to aneuploidy (Colognato et al. 2007). Investigations making use of in vitro, animal and human studies have identified air pollutants (particulate matter, black carbon and benzene) modifying epigenetic markers (Holloway et al. 2012). Most studies conducted to date have addressed DNA methylation (Szyf 2011), whereas only a few investigations have studied histone modifications or microRNA expression (Cheng & Cho 2012). Indeed, an increasing number of nanosized compounds has been found to induce changes in the acetylation and methylation of histone tails as well as microRNA deregulated expression (Stoccoro et al. 2012). A comprehensive testing battery, however, should cover end points detectable at the gene, chromosome and epigenetic levels and make use of a range of target cells (Lynch et al. 2011).
As a further important end point of concern, the impact of NM on the immune system should be a major component of NM safety assessment. Immune responses protect an organism from damage and disease. Interference with the normal course of a protective immune response can have detrimental effects. Thus, assessing the capacity of NM to alter the normal course of immune responses is of particular importance for hazard assessment (Boraschi et al. 2012). Interactions of NM with the immune system should be tested at the exposure site. Complex human primary models that include local immune cells can be used for this purpose, and several types of interactions should be assessed (Oostingh et al. 2012).
When interacting with immune cells, NM may induce a reaction, for instance, inflammation in the case of sentinel innate immune cells. During NM safety assessment, the type of inflammatory reaction requires further distinction: Inflammation can be a normal defence reaction. In this case, the inflammatory reaction is short-lived and beneficial in maintaining homeostasis of tissues, such as the barrier tissues, exposed to a stressful environment. Prolonged inflammation, however, is detrimental since it induces pathological tissue damage. The capacity of NM to induce protective versus pathological inflammation should be evaluated in kinetic models using complex tissue-like human cell cultures. Profiling of the evolution of the response under normal conditions (successful elimination of the potentially dangerous agent and re-establishment of tissue homeostasis) will allow identification of possible immune-related derangements in the presence of NM (Oostingh et al. 2012).
The majority of NM entering the body and not being immediately excreted ends up in the reticuloendothelial system (RES) and are sequestered there. While RES cells have the task of destroying foreign materials, possible toxic effects of NM on these cells, in particular the mononuclear phagocytes of the different anatomical structures, is a major safety issue and must be carefully addressed (Boraschi et al. 2012), especially since elimination from the RES tissues may be very slow for some NM.
Testing strategy to assess NM impact on the environment (i.e., ecotoxicity)
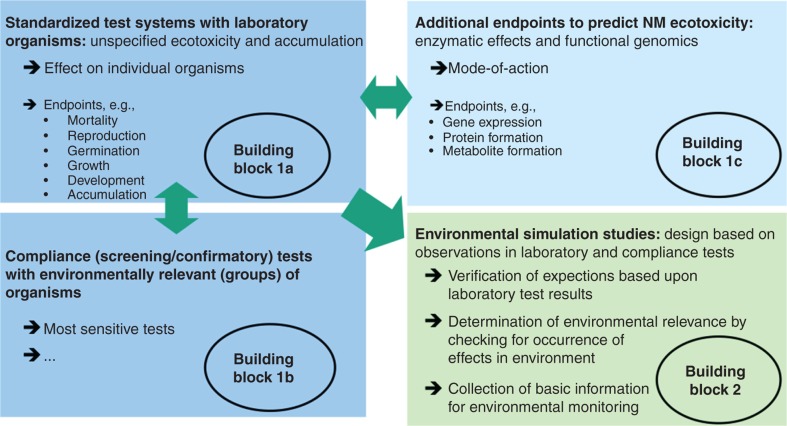
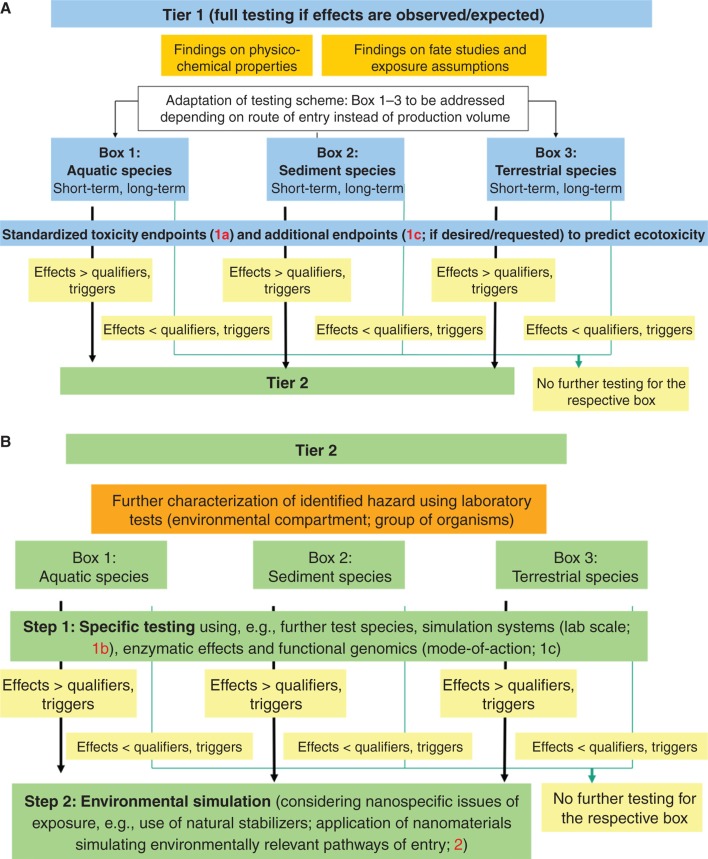
A variety of methods and approaches for assessing the ecotoxicity and bioaccumulation of NM is already available, and further ones are under development. Underlining the fundamental difference between human health hazard and environmental hazard assessment, Figure 4 illustrates the main building blocks (i.e., different types of test methods and purposes of testing) of ecotoxicity testing strategies. These consist of laboratory tests, on the one hand (Figure 4; building blocks 1a, 1b and 1c; in blue, with building block 1c in light blue, underlining that relevant test methods for this building block remain to be developed), and environmental simulation studies (Figure 2; building block 2; in green). Subsequently, Figure 5A and B present a proposal on how to integrate these building blocks into a tiered ecotoxicity testing strategy for NM.
Figure 4.
Building blocks for integrated approaches to ecotoxicity testing and assessment, that is, different types of test methods and purposes for ecotoxicity testing.
Figure 5.
(A). Proposal for tier 1 of an integrated approach to ecotoxicity testing and assessment (numberings 1a and 1c refer to the respective building blocks presented in Figure 4). (B). Proposal for tier 2 of an integrated approach to ecotoxicity testing and assessment (numberings 1b, 1c and 2 refer to the respective building blocks presented in Figure 4).
For assessing the impact of NM on the environment, both ecotoxicity and bioaccumulation will be determined making use of standardised laboratory test systems (Figure 4, building block 1a), similar to the standardised procedures established for conventional chemicals (e.g., according to OECD TG). In these test systems, standardised, and often artificial, test media are employed. Therefore, they only allow a general characterisation of NM effects, and verification of the results from such tests taking into consideration environmentally relevant groups of organisms (building block 1b) and realistic environmental exposure scenarios may be necessary (building block 2) (von der Kammer et al. 2012). The complex interactions between NM, test media and organisms may require amendment of existing TG. Furthermore, the information obtained in standardised test systems is limited with regard to determining modes of action of the NM. This information (Figure 4, building block 1c) may, however, be required for classifying NM, and it is likely to be a prerequisite for reduced, efficient testing aimed at a practical assessment of NM environmental hazard. Such suitable test systems beyond standardised testing, however, remain to be defined (Klaine et al. 2008; Handy et al. 2012a, 2012b).
Likewise, further research is required to define which test systems are applicable for screening or confirmatory testing of the compliance of standardised laboratory tests using more specific tests with environmentally relevant organisms (Figure 4, building block 1b). Depending on the potential environmental concerns identified in basic laboratory testing, further test species, end points and food webs (interspecies transfer) might be studied in environmental simulation studies applying more realistic environmental exposure scenarios (Figure 4, building block 2).
Since NM bioavailability is highly dependent on the chosen test conditions, care has to be taken when simulating environmentally relevant exposure. In this context, sedimentation and (hetero-)agglomeration of the NM or application of natural stabilisers, such as humic acids, and their variability under different environmental conditions have to be taken into account (Handy et al. 2012a, 2012b). Apart from providing data for environmental risk assessment, the results of environmental simulation studies performed under environmentally relevant exposure conditions can be used as basic information for environmental monitoring. Compartments, habitats, feeding types or organisms identified as potentially endangered can trigger the need to collect specific environmental samples. Due to methodological limitations, however, such deliberations are currently speculative.
Due to the diversity of NM in regard to type of material, shape, size, surface modification, crystalline structure, etc., for efficient ecotoxicological testing it is of special importance to be able to define groups of NM. Specific groups may comprise NM with comparable fate, behaviour and effects in the environment, as determined with the applied testing strategy (Stone et al. 2010). To obtain comprehensive information on the properties and effects of a given group, a sufficient number of materials belonging to it should be studied in detail. For further NM belonging to the same group, only recognised key parameters, such as the most sensitive end point making use of environmentally relevant organisms, remain to be addressed (Figure 4, building block 1b).
Based on these building blocks describing different purposes of ecotoxicity test methods, a tiered testing strategy to assess environmental hazards is proposed in Figure 5A and B. Testing begins with a selection of those tests considered necessary for the given NM (Figure 5A; tier 1). (In this context, it remains a matter of further investigation to determine whether information from human toxicity tests is useful in supporting the selection of ecotoxicological end points). Depending on the main exposure path (i.e., water, sediment, soil), testing should be performed in aquatic, sediment and/or terrestrial species. Furthermore, it is advisable to include long-term testing alongside short-term testing from the beginning due to the persistent nature of many nanoparticle types.
In the context of REACH, the choice of test systems and test organisms to be used for ecotoxicological testing of conventional chemicals depends on the production volume of the respective substance. In the case of NM, however, it seems more appropriate to base the testing scheme on expected concerns based on routes of exposure, findings on physico-chemical properties and on fate studies, rather than production volume. Such an adaptation of the testing scheme seems permissible since the REACH guidance documents (ECHA 2012) allow adapting the testing strategy in specific cases. Exposure route-driven selections of test systems are considered to be most appropriate, if exposure in soil and sediment is more likely than in pelagic systems. This may not always be the case, although one might intuitively assume that NM tend to agglomerate/aggregate and sink. Furthermore, higher exposure does not always equate with higher risks.
All test results are compared with triggers (qualifiers). For conventional chemicals, such triggers are based on a comparison of the expected environmental concentration to the effect on mass basis corrected by assessment factors. It remains to be determined whether triggers comparable with those used for the testing of conventional chemicals can be applied for ecotoxicity testing of NM or whether modifications are required. For NM, expression of the environmental concentration on mass basis may be less suitable, and particle number concentration instead of mass concentration may be an appropriate alternative. To address these issues, further knowledge on NM exposure and fate are necessary. If such triggers are exceeded in tier 1 test results, tier 2 testing has to follow.
In tier 2 of the ecotoxicity testing strategy (Figure 5B), only the specific group of organisms or the environmental compartment has to be considered for which a potential unacceptable risk was identified in tier 1. In step 1 of tier 2, further tests with further species, laboratory-scale simulation systems and mode-of-action tests are performed as appropriate. In the second step of tier 2, nanospecific aspects of particle exposure have to be taken into account: Since the testing conditions can affect surface functionalisation, agglomeration behaviour and bioavailability, it might be advisable to consider using natural stabilisers, such as humic acids, or to apply NM by simulating environmentally relevant pathways of entry. Analytical verification of the physico-chemical characteristics of the NM during testing may be necessary. Since environmental conditions are highly variable, such simulations can only cover a limited number of different scenarios.
Due to prevailing knowledge gaps in the area of environmental hazard assessment of NM, further research is needed to confirm and complete the proposed ecotoxicity testing strategy. Such research should address the following topics (Aschberger et al. 2011):
NM solubility, agglomeration and heteroagglomeration processes in environmentally relevant media.
Selection of test organisms and end points for full NM testing (in standardised laboratory test systems): based on concern, related for example, to route of entry, rather than production volume?
NM modes of action: elaboration of suitable methods.
Criteria for classifying NM (particle characteristics vs. mode of action).
Confirmatory tests: Which organisms and end points should be considered? Are the test methods that proved most sensitive for the NM respective category sufficient?
Definition of triggers and qualifiers: Are the procedures applied for the testing of conventional chemicals suitable for NM?
Grouping of NM for human health and environmental hazard assessment
Numerous NM are likely to find widespread applications in the near future, and a much greater number of modifications and uses of these NM is expected. It will be impossible to perform a full testing program on all NM with all modifications and in all use scenarios, notwithstanding that modifications or use/release scenarios may alter their biological effects (Som et al. 2012). Alternatively, it seems possible to assign certain biological effects to specific material properties (physical properties and chemical and sterical composition) and group NM based on these material characteristics (Zuin et al. 2011). Even though many NM have been shown to interact with cellular systems in vitro (inducing inflammation, ion release, catalysis of the formation of reactive species or direct mechanical interaction), grouping of NM based on material characteristics has only been partly successful to date. So far, no single material property – be it surface, volume or generation of reactive oxygen species – perfectly correlates with the observed biological effects for various types of NM. Therefore, grouping of NM should be a high research priority for the next years.
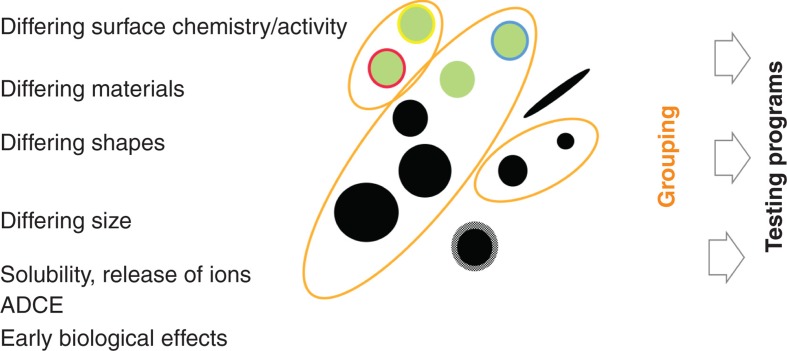
As illustrated in Figure 6, alternative or complementary groupings of NM may be based on:
Figure 6.
Illustration of grouping of NM based on material properties and/or biological effects. This schematic example shows three groups of NM and also NM not assignable to any group.
Similar biopersistence and biokinetic characteristics. Fate and kinetics may also give a measure for grouping/summing nanomaterials, for example, if the distribution and mode of action are the same, but the bioavailability is different (due to differences in size). Identical distribution patterns in tissues or environmental compartments might also be a criterion for grouping different NM. If a nanomaterial shows a deviating distribution pattern, this may result in different effects and/or different target species for environmental exposure.
Similar or common biological effects, including early effects and NM cell structure interactions. The latter approach can be served by cellular assays as well as short-term in vivo tests.
These specifications reveal that a given NM could belong to more than one group. Furthermore, grouping based on early biological effects is tightly linked to the adverse outcome pathways concept (Ankley et al. 2010; OECD 2012) and the so-called Tox21c Initiative in the USA (Firestone et al. 2010). Hence, grouping of NM by concern is considered a potential route.
Grouping of substances should form an integral part of any NM testing strategy. In the course of NM toxicity and ecotoxicity testing, it seems an effective and efficient approach to combine exposure, fate, biokinetics and hazard data for risk assessment and grouping purposes. This document envisions a guidance regarding the integration of material properties, exposure, biokinetics and hazard data, which therefore should be a high priority for future research on the safety assessment of nanomaterials.
Recommendations for the human health and environmental hazard assessment of NM
Testing strategies
Find an approach to deal with changing characteristics, and thus hazard, of NM during their life cycle.
Integrate exposure, material properties, biopersistence, biokinetics (ADME/ADCE) as well as primary effect and apical effect testing into a concern-driven testing strategy that can be applied to an individual NM but also includes guidance for grouping of NM. Use all available information to identify relevant concerns and to choose the right studies to be performed. Fill in the criteria for the decision-making process of the concern-driven tiered testing strategy.
Use grouping as an integral part of the testing strategy.
Define adverse outcome pathways for different NM.
Use data obtained in A.2, A.3 and A.4 to fill in criteria for grouping of NM.
Define triggers for tier 2 and 3 for human health risk assessment.
-
Define trigger values for environmental risk assessment
Provide guidance on the extent of ecotoxicity testing (terrestrial vs. aquatic tests)
Provide guidance on the consideration of accumulation (which criteria trigger accumulation testing in individual organisms or in the food web)
Provide guidance on the selection of ecotoxicological tests for compliance testing.
Testing methods
Designate potential testing methods applied to NM to be of use for each concern
Define a list of NM as performance standards for testing methods
(ideally covering different toxic effects and including positive and negative controls)
-
Update and amend existing testing methods for specific needs of NM testing
Provide guidance on NM dispersion and in situ characterisation in the test system
Provide guidance on methods to study biokinetics (ADME/ADCE) and biopersistence, and on application of these data
Provide guidance on development and application of testing methods as part of a testing strategy rather than as stand-alone tests
Provide guidance for the simulation of increased environmentally relevant exposure; for example, application of natural stabilisers
-
Update and amend existing testing methods in general (for NM and chemicals alike)
Bronchoalveolar lavage in inhalation studies
Extended histopathology (e.g., lung)
Aquatic and terrestrial ecotoxicity tests
-
Establish general concepts for NM (and other particles) effects
Carcinogenicity
Cardiovascular effects
Epigenetic effects
Immunological effects
Reproductive toxicity, developmental toxicity
Establish concepts for environmental monitoring to verify the effects detected in laboratory tests or simulation tests
Grouping of NM
For grouping to be used as an integral part of the testing strategy, define and validate scientifically sound grouping criteria based on available data and material properties (metrology), biopersistence, fate, ADME/ADCE as well as primary and apical effects.
Use quantitative structure–activity relationship (QSAR), if applicable.
Acknowledgements
The authors would like to thank Dr Ursula G. Sauer, Scientific Consultancy – Animal Welfare, Neubiberg, Germany, for editing and proofreading the manuscript.
Declaration of interest
A.G. Oomen and P.M.J. Bos are employees of the Dutch National Institute for Public Health and the Environment (RIVM); their contribution was funded by the EU FP7 project MARINA (grant agreement no. 263215) and the Dutch government. T.F. Fernandes is employee of the School of Life Sciences of the Heriot-Watt University, Edinburgh, UK. K. Hund-Rinke is an employee of the Fraunhofer Institute for Molecular Biology and Applied Ecology, IME, an institute of the Fraunhofer-Gesellschaft, a non-profit organisation performing applied research; she received funding of her nanosafety research from the EU (FP7), and the German government (BMBF, BMU). D. Boraschi is research director at the Italian National Research Council, the governmental institution of research; for research in nanoparticles she receives funding from the EU Commission (FP7), the Italian Government (MIUR) and a bank foundation (Fondazione Cariplo); she chairs the Immunosafety Task Force within the NanoSafety Cluster Working Group 2 – Hazard. H.J. Byrne is Head of the Focas Research Institute, Dublin Institute of Technology, Ireland. He has received support for research through the “Integrated NanoScience Platform for Ireland” (INSPIRE), funded by the Irish Government Platform for Research in Third Level Institutions, Cycle 4, and in part by the EU Structural Development Funds. K. Aschberger is employee and S. Gottardo a post-doctoral research fellow at the Joint Research Centre (JRC) of the European Commission. They both received funding for their nanosafety research from JRC and EU (FP7) projects. F. von der Kammer is employee of the University of Vienna. He receives funding for his nanosafety research from the Austrian Ministry for Education, the EU (FP7) and the Austrian Research Promotion Agency (FFG; Forschungsförderungsgesellschaft). D. Kühnel is an employee of UFZ, a research center within the Helmholtz-Association. She received funding from the EU (NanoValid, grant agreement no. 263147) and the German government (BMBF). L. Migliore is a full Professor in Medical Genetics of the University of Pisa; she received funding of her nanosafety research from the EU (FP7: NanoReTox No. CP-FP 214478-2 and Sanowork No. 280716) and the Italian Ministry of University, Research and Education. D. Hristozov and A. Marcomini are employees of the University of Venice, Italy; and J. Scott-Fordsmand of Aarhus University, Denmark. P. Wick is an employee of the Empa, Swiss Federal Laboratories for Materials Science and Technology, a national research institute of the ETH domain and is financed by Empa, Swiss government (SNSF) as well as the EU (FP7). R. Landsiedel is an employee of BASF SE, a chemical company producing and marketing nanomaterials; he received funding of his nanosafety research from BASF, EU (FP7), and the German government (BMBF). R. Landsiedel chaired the NanoSafety Cluster Working Group 10 without specific funding. Likewise all co-authors contributed to the NanoSafety Cluster Working Group 10 without specific funding. A detailed risk assessment strategy for nanomaterials is being developed within the FP7 project MARINA (Managing Risks of Nanoparticles). Input from the body of thought of MARINA was provided by Work Packages 12 and 13 chaired by A. Oomen and J. Scott-Fordsmand, respectively.
References
- Ankley GT, Bennett RS, Erickson RJ, Hoff DJ, Hornung MW, Johnson RD, et al. Adverse outcome pathways: A conceptual framework to support ecotoxicology research and risk assessment. Env Toxicol Chem. 2010;29:730–741. doi: 10.1002/etc.34. [DOI] [PubMed] [Google Scholar]
- Anon 2006. Regulation (EC) No 1907/2006 of the European Parliament and of the Council of 18 December 2006 concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH), establishing a European Chemicals Agency, amending Directive 1999/45/EC and repealing Council Regulation (EEC) No 793/93 and Commission Regulation (EC) No 1488/94 as well as Council Directive 76/769/EEC and Commission Directives 91/155/EEC, 93/67/EEC, 93/105/EC and 2000/21/EC. OJ L 396/1, 30 December 2006.
- Anon 2008. Directive 2008/105/EC of the European Parliament and of the Council of 16 December 2008 on environmental quality standards in the field of water policy, amending and subsequently repealing Council Directives 82/176/EEC, 83/513/EEC, 84/156/EEC, 84/491/EEC, 86/280/EEC and amending Directive 2000/60/EC of the European Parliament and of the Council. OJ L 348/84, 24 December 2008.
- Anon Communication from the Commission. Europe 2020 – A European strategy for smart, sustainable and inclusive growth. COM(2010)2020. Brussels, 3 March 2010. http://ec.europa.eu/research/era/docs/en/investing-in-research-european-commission-europe-2020-2010.pdf 2010a:37. [Google Scholar]
- Anon 2010b. Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes. O.J. L 276/33, 20 October 2010.
- Anon Proposal for a regulation of the European Parliament and of the Council establishing Horizon 2020 – The Framework Programme for Research and Innovation (2014 – 2020). COM(2011)809 final, Brussels, 30 November 2011 . http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=COM:2011:0809:FIN:en:PDF 2011a [Google Scholar]
- Anon 2011b. Commission recommendation on the definition of nanomaterial. OJ L 275/38, 18 October 2011.
- Anon Communication from the Commission to the European Parliament, the Council and the European Economic and Social Committee. Second regulatory review on nanomaterials. COM(2012)572 final. Brussels, 3 October 2012 . http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=COM:2012:0572:FIN:en:PDF 2012a [Google Scholar]
- Anon Commission staff working paper. Types and uses of nanomaterials, including safety aspects; accompanying the Communication from the Commission to the European Parliament, the Council and the European Economic and Social Committee on the Second Regulatory Review on Nanomaterials (COM(2012) 572 final. SWD(2012) 288 final. Brussels, 3 October 2012. http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=SWD:2012:0288:FIN:EN:PDF 2012b:111. [Google Scholar]
- Aschberger K, Micheletti C, Sokull-Klüttgen B, Christensen FM. Analysis of currently available data for characterising the risk of engineered nanomaterials to the environment and human health - lessons learned from four case studies . Environ Int. 2011;37(6):1143–1156. doi: 10.1016/j.envint.2011.02.005. [DOI] [PubMed] [Google Scholar]
- Becker H, Herzberg F, Schulte A, Kolossa-Gehring M. The carcinogenic potential of nanomaterials, their release from products and options for regulating them. Int J Hygiene Environ Health. 2011;214(3):231–238. doi: 10.1016/j.ijheh.2010.11.004. [DOI] [PubMed] [Google Scholar]
- Bhattacharya K, Naha PC, Naydenova I, Mintova S, Byrne HJ. Reactive oxygen species mediated DNA damage in human lung alveolar epithelial (A549) cells from exposure to non-cytotoxic MFI-type zeolite nanoparticles . Toxicol Lett. 2012;215(3):151–160. doi: 10.1016/j.toxlet.2012.10.007. [DOI] [PubMed] [Google Scholar]
- Bleeker EA, de Jong WH, Geertsma RE, Groenewold M, Heugens EH, Koers-Jacquemijns M, et al. Considerations on the EU definition of a nanomaterial: science to support policy making . Regul Toxicol Pharmacol. 2013;65(1):119–125. doi: 10.1016/j.yrtph.2012.11.007. [DOI] [PubMed] [Google Scholar]
- Boraschi D, Costantino L, Italiani P. Interaction of nanoparticles with immunocompetent cells: nanosafety considerations. Nanomed. 2012;7(1):121–131. doi: 10.2217/nnm.11.169. [DOI] [PubMed] [Google Scholar]
- Burlinson B. The in vitro and in vivo Comet assays . Methods Mol Biol. 2012;817:143–163. doi: 10.1007/978-1-61779-421-6_8. [DOI] [PubMed] [Google Scholar]
- Card JW, Magnuson BA. Letter to the Editor . J Food Sci. 2009;74:vi–vii. doi: 10.1111/j.1750-3841.2009.01366.x. doi: 10.1111/j.1750-3841.2009.01366.x. [DOI] [PubMed] [Google Scholar]
- Card JW, Magnuson BA. A method to assess the quality of studies that examine the toxicity of engineered nanomaterials . Int J Toxicol. 2010;29(4):402–410. doi: 10.1177/1091581810370720. [DOI] [PubMed] [Google Scholar]
- Cheng JB, Cho RJ. Genetics and epigenetics of the skin meet deep sequence . J Invest Dermatol. 2012;132(3 Pt 2):923–932. doi: 10.1038/jid.2011.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockburn A, Bradford R, Buck N, Constable A, Edwards G, Haber B, et al. Approaches to the safety assessment of engineered nanomaterials (ENM) in food . Food Chem Toxicol. 2012;50(6):2224–2242. doi: 10.1016/j.fct.2011.12.029. [DOI] [PubMed] [Google Scholar]
- Colognato R, Coppedè F, Ponti J, Sabbioni E, Migliore L. Genotoxicity induced by arsenic compounds in peripheral human lymphocytes analysed by cytokinesis-block micronucleus assay . Mutagenesis. 2007;22(4):255–261. doi: 10.1093/mutage/gem010. [DOI] [PubMed] [Google Scholar]
- Dan M, Wu P, Grulke EA, Graham UM, Unrine JM, Yokel RA. Ceria engineered nanomaterial distribution in and clearance from blood: size matters. Nanomed. 2012;7(1):95–110. doi: 10.2217/nnm.11.103. [DOI] [PubMed] [Google Scholar]
- Dekkers S, Bouwmeester H, Bos PMJ, Peters RJB, Rietveld AG, Oomen AG. Knowledge gaps in risk assessment of nanosilica in food: evaluation of the dissolution and toxicity of different forms of silica. Nanotoxicology. 2012 doi: 10.3109/17435390.2012.662250. Epub ahead of print. doi: 10.3109,17435390.2012.662250. [DOI] [PubMed] [Google Scholar]
- Dekkers S, Krystek P, Peters RJB, Lankveld DPK, Bokkers BGH, van Hoeven-Arentzen PH, et al. Presence and risks of nanosilica in food products . Nanotoxicology. 2011;5(3):393–405. doi: 10.3109/17435390.2010.519836. [DOI] [PubMed] [Google Scholar]
- ECHA European Chemicals Agency – Guidance on information requirements and chemical safety assessment. Appendix R7-1 Recommendations for nanomaterials applicable to Chapter R7a Endpoint specific guidance. ECHA. 2012 12-G-03-EN. [Google Scholar]
- EFSA European Food Safety Authority - Guidance on the risk assessment of the application of nanoscience and nanotechnologies in the food and feed chain. EFSA J. 2011;9(5):2140. doi: 10.2903/j.efsa.2018.5327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenech M. Cytokinesis-block micronucleus cytome assay . Nat Protoc. 2007;2(5):1084–1104. doi: 10.1038/nprot.2007.77. [DOI] [PubMed] [Google Scholar]
- Firestone M, Kavlock R, Zenick H, Kramer M, US Environmental Protection Agency Working Group on the Future of Toxicity Testing The U.S. Environmental Protection Agency strategic plan for evaluating the toxicity of chemicals . J Toxicol Environ Health B Crit Rev. 2010;13(2-4):139–162. doi: 10.1080/10937404.2010.483178. [DOI] [PubMed] [Google Scholar]
- Gasser M, Rothen-Rutishauser B, Krug HF, Gehr P, Nelle M, Yan B, et al. Lipids of the pulmonary surfactant and functional groups on multi-walled carbon nanotubes influence blood plasma proteins adsorption differently. J Nanobiotech. 2010;8:31. doi: 10.1186/1477-3155-8-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handy RD, Cornelis G, Fernandes TF, Tsyusko O, Decho A, Sabo-Attwood T, et al. Ecotoxicity test methods for engineered nanomaterials: Practical experiences and recommendations from the bench . Environ Toxicol Chem. 2012b;31:15–31. doi: 10.1002/etc.706. [DOI] [PubMed] [Google Scholar]
- Handy RD, van den Brink N, Chappell M, Mühling M, Behra R, Dušinská M, et al. Practical considerations for conducting ecotoxicity test methods with manufactured nanomaterials: what have we learnt so far? . Ecotoxicology. 2012a;21(4):933–972. doi: 10.1007/s10646-012-0862-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartung T. Toxicology for the twenty first century . Nature. 2009;460(7252):208–212. doi: 10.1038/460208a. [DOI] [PubMed] [Google Scholar]
- Hassellov M, Readman J, Rabville J, Tiede K. Nanoparticle analysis and characterization methodologies in environmental risk assessment of engineered nanoparticles . Ecotoxicology. 2008;17:344–361. doi: 10.1007/s10646-008-0225-x. [DOI] [PubMed] [Google Scholar]
- Holloway JW, Savarimuthu Francis S, Fong KM, Yang IA. Genomics and the respiratory effects of air pollution exposure . Respirology. 2012;17(4):590–600. doi: 10.1111/j.1440-1843.2012.02164.x. [DOI] [PubMed] [Google Scholar]
- Hristozov DR, Gottardo S, Cinelli M, Isigonis P, Zabeo A, Critto A, et al. Application of a quantitative weight of evidence approach for ranking and prioritising occupational exposure scenarios for titanium dioxide and carbon nanomaterials. Nanotoxicology. 2013 doi: 10.3109/17435390.2012.760013. Epub ahead of print. doi:10.3109/17435390.2012.760013. [DOI] [PubMed] [Google Scholar]
- Johnston H, Brown D, Kermanizadeh A, Gubbins E, Stone V. Investigating the relationship between nanomaterial hazard and physicochemical properties: Informing the exploitation of nanomaterials within therapeutic and diagnostic applications. J Controll Rel. 2012;164(3):307–313. doi: 10.1016/j.jconrel.2012.08.018. [DOI] [PubMed] [Google Scholar]
- Johnston H, Pojana G, Zuin S, Jacobsen NR, Møller P, Loft S, et al. Engineered nanomaterial risk. Lessons learnt from completed nanotoxicology studies: potential solutions to current and future challenges . Crit Rev Toxicol. 2013;43(1):1–20. doi: 10.3109/10408444.2012.738187. [DOI] [PubMed] [Google Scholar]
- Klaine SJ, Alvarez PJ, Batley GE, Fernandes TF, Handy RD, Lyon DY, et al. Nanomaterials in the environment: behavior, fate, bioavailability, and effects . Environ Toxicol Chem. 2008;27(9):1825–1851. doi: 10.1897/08-090.1. [DOI] [PubMed] [Google Scholar]
- Klein CL, Wiench K, Wiemann M, Ma-Hock L, van Ravenzwaay B, Landsiedel R. Hazard identification of inhaled nanomaterials: making use of short-term inhalation studies . Arch Toxicol. 2012;86(7):1137–1151. doi: 10.1007/s00204-012-0834-2. [DOI] [PubMed] [Google Scholar]
- Koturbash I, Simpson NE, Beland FA, Pogribny IP. Alterations in histone H4 lysine 20 methylation: implications for cancer detection and prevention . Antioxid Redox Signal. 2012;17(2):365–374. doi: 10.1089/ars.2011.4370. [DOI] [PubMed] [Google Scholar]
- Krug HF, Wick P. Nanotoxicology: An interdisciplinary challenge . Angew Chem Int Ed Engl. 2011;50:1260–1278. doi: 10.1002/anie.201001037. [DOI] [PubMed] [Google Scholar]
- Kühnel D, Scheffler K, Wellner P, Meißner T, Potthoff A, Busch W, et al. Comparative evaluation of particle properties, formation of reactive oxygen species and genotoxic potential of tungsten carbide based nanoparticles in vitro. J Hazard Mat. 2012;227-228:418–426. doi: 10.1016/j.jhazmat.2012.04.070. [DOI] [PubMed] [Google Scholar]
- Lai ZW, Yan Y, Caruso F, Nice EC. Emerging techniques in proteomics for probing nano-bio interactions . ACS Nano. 2012;6(12):10438–10448. doi: 10.1021/nn3052499. [DOI] [PubMed] [Google Scholar]
- Landsiedel R, Fabian E, Ma-Hock L, Wohlleben W, Wiench K, Oesch F, et al. Toxico-/biokinetics of nanomaterials . Arch Toxicol. 2012;86(7):1021–1060. doi: 10.1007/s00204-012-0858-7. [DOI] [PubMed] [Google Scholar]
- Landsiedel R, Kapp MD, Schulz M, Wiench K, Oesch F. Genotoxicity investigations on nanomaterials: methods, preparation and characterization of test material, potential artifacts and limitations - many questions, some answers . Mutat Res. 2009;681(2-3):241–258. doi: 10.1016/j.mrrev.2008.10.002. [DOI] [PubMed] [Google Scholar]
- Landsiedel R, Ma-Hock L, Kroll A, Hahn D, Schnekenburger J, Wiench K, et al. Testing metal-oxide nanomaterials for human safety. Adv Mat. 2010;22:2601–2627. doi: 10.1002/adma.200902658. [DOI] [PubMed] [Google Scholar]
- Lankveld DP, Oomen AG, Krystek P, Neigh A, Troost-de Jong A, Noorlander CW, et al. The kinetics of the tissue distribution of silver nanoparticles of different sizes . Biomaterials. 2010;31:8350–8361. doi: 10.1016/j.biomaterials.2010.07.045. [DOI] [PubMed] [Google Scholar]
- Leonard F, Ali H, Collnot E.-M, Crielaard BJ, Lammers T, Storm G, et al. Screening of budesonide nanoformulations for treatment of inflammatory bowel disease in an inflamed 3D cell-culture model . ALTEX. 2012;29(3):275–285. doi: 10.14573/altex.2012.3.275. [DOI] [PubMed] [Google Scholar]
- Liu J, Legros S, Ma G, Veinot JG, von der Kammer F, Hofmann T. Influence of surface functionalization and particle size on the aggregation kinetics of engineered nanoparticles . Chemosphere. 2012;87(8):918–924. doi: 10.1016/j.chemosphere.2012.01.045. [DOI] [PubMed] [Google Scholar]
- Lundqvist M, Stigler J, Cedervall T, Berggård T, Flanagan MB, Lynch I, et al. The evolution of the protein corona around nanoparticles: a test study . ACS Nano. 2011;5:7503–7509. doi: 10.1021/nn202458g. [DOI] [PubMed] [Google Scholar]
- Lynch AM, Sasaki JC, Elespuru R, Jacobson-Kram D, Thybaud V, De Boeck M, et al. 2011. New and emerging technologies for genetic toxicity testing . Environ Mol Mutagen. 52(3):205–223. doi: 10.1002/em.20614. [DOI] [PubMed] [Google Scholar]
- Ma-Hock L, Burkhardt S, Strauss V, Gamer AO, Wiench K, van Ravenzwaay B, et al. Development of a short-term inhalation test in the rat using nano titanium dioxide as a model substance . Inhal Toxicol. 2009;21:102–118. doi: 10.1080/08958370802361057. [DOI] [PubMed] [Google Scholar]
- NanoSafety Cluster Nanosafety 2015-2020: A Strategic Research Agenda towards safe and sustainable nanomaterial and nanotechnology innovations. Identified key-areas of nanosafety research and expected and necessary achievements in these areas by 2015-2020. http://www.nanosafetycluster.eu/intranet/intra_strategic-research-agenda.html 2011:37.
- Nel AE, Mädler L, Velegol D, Xia T, Hoek EMV, Somasundaran P, et al. Understanding biophysicochemical interactions at the nano- bio interface . Nat Mater. 2009;8(7):543–557. doi: 10.1038/nmat2442. [DOI] [PubMed] [Google Scholar]
- Nel AE, Xia T, Meng H, Wang X, Lin S, Ji Z, et al. Nanomaterial toxicity testing in the 21st century: use of a predictive toxicological approach and high-throughput screening. Acc Chem Res. 2013;46(3):607–621. doi: 10.1021/ar300022h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberdörster G. Safety assessment for nanotechnology and nanomedicine: concepts of nanotoxicology. J Int Med. 2009;267(1):89–105. doi: 10.1111/j.1365-2796.2009.02187.x. [DOI] [PubMed] [Google Scholar]
- OECD Guidance manual for the testing of manufactured nanomaterials: OECD’s sponsorship programme; first revision. ENV/JM-MONO(2009)20/REV, OECD; 2 June 2010; Paris, France. 2010. [Google Scholar]
- OECD A steering group 7 case-study for hazard identification of inhaled nanomaterials: an integrated approach with short-term inhalation studies. ENV/CHEM/NANO(2011)6/REV1. 10th Meeting of the Working Party on Manufactured Nanomaterials, OECD; 27-29 June 2012; Paris, France. 2011. [Google Scholar]
- OECD Proposal for a template, and guidance on developing and assessing the completeness of adverse outcome pathways. http://www.oecd.org/env/ehs/testing/49963554.pdf 2012:17.
- OECD Revised Draft Recommendation of the Council on the safety testing and assessment of manufactured nanomaterials. ENV/CHEM/NANO(2013)3/REV1 Working Party on Manufactured Nanomaterials. OECD; 25 February 2013; Paris, France. 2013. [Google Scholar]
- Oostingh GJ, Casals E, Italiani P, Colognato R, Stritzinger R, Ponti J, et al. Problems and challenges in the development and validation of human cell-based assays to determine nanoparticle-induced immunomodulatory effects . Part Fibre Toxicol. 2012;8(1):8. doi: 10.1186/1743-8977-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters R, Kramer E, Oomen AG, Rivera ZE, Oegema G, Tromp PC, et al. Presence of nano-sized silica during in vitro digestion of foods containing silica as a food additive . ACS Nano. 2012;6(3):2441–2451. doi: 10.1021/nn204728k. [DOI] [PubMed] [Google Scholar]
- RIP-oN 1 European Commission REACH Implementation Project. Substance Identification of Nanomaterials. AA N°070307/2009/DI/534733 between DG ENV and JRC. Advisory report, March 2011. http://ec.europa.eu/environment/chemicals/nanotech/pdf/report_ripon1.pdf 2011
- RIP-oN 2. 2011 Hankin SM, Peters SAK, Poland CA, Foss Hansen S, Holmqvist J, Ross BL, et al. Specific advice on fulfilling information requirements for nanomaterials under Reach (RIP-oN 2) – final project report. Reach-Nano consultation. RNC/RIP-oN2/FPR/1/Final. http://ec.europa.eu/environment/chemicals/nanotech/pdf/report_ripon2.pdf 2011
- Rossini GP, Hartung T. Food for thought. Towards tailored assays for cell based approaches to toxicity testing . ALTEX. 2012;29:359–372. doi: 10.14573/altex.2012.4.359. [DOI] [PubMed] [Google Scholar]
- Rothen-Rutishauser B, Brown DM, Piallier-Boyles M, Kinloch IA, Windle AH, Gehr P, et al. Relating the physicochemical characteristics and dispersion of multiwalled carbon nanotubes in different suspension media to their oxidative reactivity in vitro and inflammation in vivo. Nanotoxicol. 2010;4(3):331–342. doi: 10.3109/17435390.2010.489161. [DOI] [PubMed] [Google Scholar]
- Russell WMS, Burch RL. The principles of humane experimental technique. London, UK: Methuen; 1959. Reprinted by UFAW, 1992, 238 pp., 8 Hamilton Close, South Mimms, Potters Bar, Herts EN6 3QD England. [Google Scholar]
- SCENIHR The Scientific committee on emerging and newly identified health risks opinion on risk assessment of products of nanotechnologies. http://ec.europa.eu/health/archive/ph_risk/committees/04_scenihr/docs/scenihr_o_023.pdf 200919 January 2009
- Schulze C, Kroll A, Lehr C, Schäfer U.F, Becker K, Schnekenburger J, et al. Not ready to use - overcoming pitfalls when dispersing nanoparticles in physiological media. Nanotoxicol. 2008;2(2):51–61. [Google Scholar]
- Silbergeld EK, Contreras EQ, Hartung T, Hirsch C, Hogberg H, Jachak AC, et al. tτ workshop report Nanotoxicology: "the end of the beginning" - signs on the roadmap to a strategy for assuring the safe application and use of nanomaterials . ALTEX. 2011;28(3):236–241. doi: 10.14573/altex.2011.3.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Som C, Nowack B, Krug HF, Wick P. Toward the development of decision supporting tools that can be used for safe production and use of nanomaterials . Acc Chem Res. 2012 doi: 10.1021/ar3000458. epub ahead of print. doi: 10.1021/ar3000458. [DOI] [PubMed] [Google Scholar]
- Stoccoro A, Karlsson HL, Coppedè F, Migliore L. Epigenetic effects of nano-sized materials. Toxicol. 2012 doi: 10.1016/j.tox.2012.12.002. Epub ahead of print. doi:pii: S0300-483X(12)00421-0. [DOI] [PubMed] [Google Scholar]
- Stone V, Nowack B, Baun A, van den Brink N, von der Kammer F, Dusinska M, et al. Nanomaterials for environmental studies: classification, reference material issues, and strategies for physico- chemical characterisation. Sci Total Env. 2010;408(7):1745–1754. doi: 10.1016/j.scitotenv.2009.10.035. [DOI] [PubMed] [Google Scholar]
- Szyf M. The implications of DNA methylation for toxicology: toward toxicomethylomics, the toxicology of DNA methylation . Toxicol Sci. 2011;120(2):235–255. doi: 10.1093/toxsci/kfr024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiede K, Boxal A, Tear A, Lewis J, David H, Hassellov M. Detection and characterization of engineered nanoparticles in food and the environment. Food Addit Contam. 2008;25:795–821. doi: 10.1080/02652030802007553. [DOI] [PubMed] [Google Scholar]
- van Leeuwen CJ, Patlewicz GY, Worth AP. Intelligent testing strategies. In: van Leeuwen CJ, Vermeire TG, Vermeire T, editors. Risk assessment of chemicals: an introduction. Heidelberg, Germany: Springer; 2007. pp. 467–509. [Google Scholar]
- von der Kammer F, Ferguson PL, Holden PA, Masion A, Rogers KR, Klaine SJ, et al. Analysis of engineered nanomaterials in complex matrices (environment and biota): general considerations and conceptual case studies . Environ Toxicol Chem. 2012;31(1):32–49. doi: 10.1002/etc.723. [DOI] [PubMed] [Google Scholar]
- Zuin S, Micheletti C, Critto A, Pojana G, Johnston H, Stone V, et al. Weight of evidence approach for the relative hazard ranking of nanomaterials. Nanotoxicol. 2011;5(3):445–458. doi: 10.3109/17435390.2010.512986. [DOI] [PubMed] [Google Scholar]