Abstract
Radiation therapy is a part of the standard treatment for brain tumor patients, often resulting in irreversible neuropsychological deficits. These deficits may be due to permanent damage to the neural stem cell (NSC) niche, damage to local neural progenitors, or neurotoxicity. Using a CT-guided localized radiation technique, we studied the effects of radiation on NSC proliferation and neuroblast migration in the mouse brain. Localized irradiation of the subventricular zone (SVZ) eliminated the proliferating neural precursor cells (NPCs) and migrating neuroblasts. After irradiation, type B cells in the SVZ lacked the ability to generate migrating neuroblasts. Neuroblasts from the unirradiated posterior SVZ did not follow their normal migratory path through the irradiated anterior SVZ. Our results indicate that the migrating neuroblasts were not replenished, despite the presence of type B cells in the SVZ post-irradiation. This study provides novel insights into the effects of localized SVZ radiation on neurogenesis and cell migration that may potentially lead to the development of new radiotherapy strategies to minimize damage to NSCs and neuroblast migration.
Keywords: Radiation therapy, neural stem cells, migrating neuroblasts, subventricular zone
INTRODUCTION
Brain irradiation is an integral component of the treatment of patients with brain tumors [1, 2]. Despite its effectiveness in controlling the progression of some cancers, radiation is often associated with neuropsychological deficits [3–6]. These deficits may be partly due to radiation-induced damage to neurogenic niches, as shown in animal models [7–11]. In the adult mammalian brain, these niches are localized in the subventricular zone (SVZ) and the subgranular zone of the hippocampal dentate gyrus [12–14]. The neural stem cells (NSCs) in these two regions are of great interest for therapeutic applications in the brain because of their ability to proliferate and produce new cells. These newly generated cells are able to migrate and differentiate into any of the main cell types of the central nervous system, such as neurons, oligodendrocytes or astrocytes [12–15]. Damaging NSCs during radiation therapy can have detrimental effects [4, 7]. Mitigating the neurotoxic effects of radiation is important particularly in the context of brain tumor patients who survive longer due to new combination therapies [1, 2, 6]. Further, NSCs have the potential to be used to treat brain cancer in two different ways. First, endogenous NSCs in the brain can participate in the repair of normal tissue and/or inhibit tumor growth [16]. Second, engineered stem cells may be used to deliver cytotoxic agents and prodrug-activating enzymes to the tumor [17–21]. Both of these possibilities require that NSCs generate neuroblasts to migrate toward the tumor site, a process that is not well understood in the context of radiation therapy.
The SVZ is the largest source of NSCs in the adult brain, located underneath the layer of ependymal cells lining the ventricles [13, 22–24]. Neural stem cells, or type B1 cells in the SVZ, are astrocyte-like GFAP+ cells that divide and give rise to transit amplifying neural precursor cells (NPCs), or type C cells, which in turn give rise to migrating neuroblasts, or type A cells [25]. Neuroblasts migrate long distances through the rostral migratory stream (RMS) before differentiating into olfactory bulb (OB) interneurons [26, 27]. The mouse SVZ-RMS-OB migration route represents a well-characterized system of endogenous neuroblast migration that serves as a model to study migration in humans receiving radiotherapy [27, 28].
Current radiotherapy treatments, such as intensity-modulated radiation therapy (IMRT), are intended to deliver a maximum dose of radiation to the tumor while reducing radiation exposure to the surrounding normal tissue [29, 30]. To more accurately reproduce human radiotherapy, we used a small animal radiation research platform (SARRP) developed in-house [31]. Using this technology, we recently reported that local irradiation of hippocampus significantly decreased the number of proliferating cells in the irradiated dentate gyrus compared to the unirradiated side [32]. Further, the number of newborn cells differentiating into mature neurons two months after irradiation was significantly reduced [32]. In this study, we examined the effect of SVZ-restricted irradiation on NSC and NPC proliferation and neuroblast migration in the adult mouse SVZ-RMS-OB system. The two main findings from this study are that type B cells lacked the ability to regenerate NPCs and neuroblasts even a month after irradiating SVZ, and that neuroblasts failed to migrate through the irradiated regions. These results will help inform future radiotherapy protocols to minimize damage to neurogenic niches in the human brain.
MATERIALS & METHODS
Subjects
The subjects were 6–8-week- old male C57BL6/J mice from the National Cancer Institute maintained on a 12:12 hour light: dark cycle. Food and water were available ad libitum. All experiments described were performed with the approval of the Johns Hopkins Animal Care and Use Committee under standard protocols.
CT-based Localized Brain Irradiation
Mice were anesthetized with the injection of 100mg/kg ketamine+10mg/kg xylazine intraperitoneally. To directly visualize the ventricles and target the SVZ, 70µL of iodine contrast was injected intrathecally and CT images were obtained as described previously [32]. A single dose of 10Gy was delivered using CT-based tissue visualization. Previous studies have demonstrated that the overall geometric targeting accuracy of this technique is 0.2mm [32]. A radiation beam of 3×3mm was used to target right SVZ while left brain structures served as controls. For migration studies, a radiation beam of 1 mm diameter was used to target the anterior dorsal region of the SVZ. Additionally, a radiation beam of 5×9mm was used to target right olfactory bulb (OB), rostral migratory stream (RMS), and/or anterior SVZ (aSVZ). For sham irradiation, control animals were anesthetized, brought into the treatment room, and handled and positioned identically to irradiated animals without radiation delivery.
Immunohistochemistry
Mice (n=5/group) were deeply anesthetized and perfused transcardially with 0.9% saline followed by 4% paraformaldehyde (PFA) in 0.1M phosphate-buffered saline (PBS). Brains were removed from the skull and post-fixed in 4% PFA overnight at 4°C. The brains were then equilibrated in 30% sucrose and were frozen in Tissue-Tek OCT Compound. 10µm thick coronal slices were sectioned using a cryostat. Sections were treated with 0.01M sodium citrate at 95°C for 10 min for antigen retrieval. Sections were incubated in PBS containing 0.1% Triton-X-100, and 10% normal goat serum (NGS) for 30min, and then incubated with primary antibodies for 16hrs at 4°C. Primary antibodies used in this study were mouse anti-rH2Ax (Ser139) (1:700), rabbit anti-Ki67 (1:200), mouse anti-GFAP (1:500), rabbit anti-doublecortin (DCx) (1:500), mouse anti-nestin (1:100), mouse anti Mash-1 (1:100), cleaved caspase-3 (1:100) and mouse anti-CD31 (1:200). More details on the primary antibodies are summarized in Supplementary Table 1. The sections were washed with PBS and incubated with secondary antibodies conjugated with fluorophores for 1 hour at room temperature (RT). Anti-mouse, anti-rat and anti-rabbit Alexa 488 and/or 594 secondary antibodies (1:500, Invitrogen) were used. Nuclei were stained with DAPI (1:500). All the sections were air-dried and coverslipped with Aquamount mounting media (Vector Labs). Fluorescent images were taken with an ORCA II camera (Hamamatsu) connected to an Olympus IX81 inverted microscope.
SVZ Whole Mount Dissection and Immunostaining
Mice (n=3/group) were sacrificed by cervical dislocation and their brains were immediately extracted into L-15 Leibovitz medium (Gibco). A whole mount of SVZ tissue (2–4mm) with the lateral ventricle and striatum was dissected under microscope, and the hippocampus and septum were removed as described [24, 33]. The SVZ whole mounts were fixed in 4% PFA/0.1% Triton-X-100 overnight at 4° C. They were then washed with PBS and blocked with 10% NGS/0.5% Triton for 30min at RT. The tissues were incubated in primary and secondary antibodies diluted in blocking buffer for 48hr at 4°C. After staining, the SVZ whole mounts were further dissected from underlying striatum to 200–300µm thick tissue, transferred to a glass slide and coverslipped with Aquamount mounting media for imaging. A Nikon C1si True Spectral Imaging Confocal Laser Scanning Microscope was used to image the SVZ whole mounts.
Semi-Thin sections, Electron microscopy and Ultrastructural Analysis
Mice (n=5/group) were anesthetized and perfused with 0.9% saline, followed by 2% paraformaldehyde and 2.5% glutaraldehyde. Heads were removed and post-fixed in the same fixative overnight. After post-fixation, brains were dissected and washed in 0.1M phosphate buffer (PB) (pH 7.4), cut into 200µm coronal sections with a VT 1000M vibratome (Leica, Germany), and treated with 2% osmium tetraoxide in 0.1M PB for 2 hr. Sections were then rinsed, dehydrated through increasing ethanol solutions and stained with 2% uranyl acetate in 70% ethanol. Following dehydration, slices were embedded in araldite (Durcupan, Fluka BioChemika). To study the cellular organization of the SVZ and RMS, we cut serial 1.5µm coronal semithin sections with a diamond knife and stained them with 1% Toluidine blue. Sections were visualized under E200 light microscope (NIKON, Japan). To identify cell types, 60–70nm ultrathin sections were cut with a diamond knife, stained with lead citrate, and examined under a Spirit transmission electron microscope (FEI Tecnai, USA). The different cell types in the SVZ and RMS were identified based on their ultrastructural characteristics as described previously [25].
SVZ Neurosphere Cultures
Adult mouse brains were removed into sterile cold Neurocult basal medium (Stem Cell Technologies). The SVZ was dissected out from each hemisphere as described in Ferron S.R. et al. [34]. The left and right SVZ were collected separately and pooled from four different mice. The tissue was triturated gently until all the pieces were dissociated and cell suspension was homogenous. The cells were washed in the complete mouse Neurocult proliferation medium with Neurocult proliferation supplement, recombinant growth factors (10µg/mL epidermal growth factor [EGF] and 10µg/mL fibroblast growth factor [FGF]) and 2µg/mL heparin (Stem Cell Technologies). Viable cells were counted and plated at 2×104 cells/cm2. The cultures were incubated at 37°C in a 5% CO2 humidified incubator adding fresh medium every 4 days. Neurospheres of 100–200µm size were passaged and replated.
Retrovirus Preparation and Stereotactic Injections
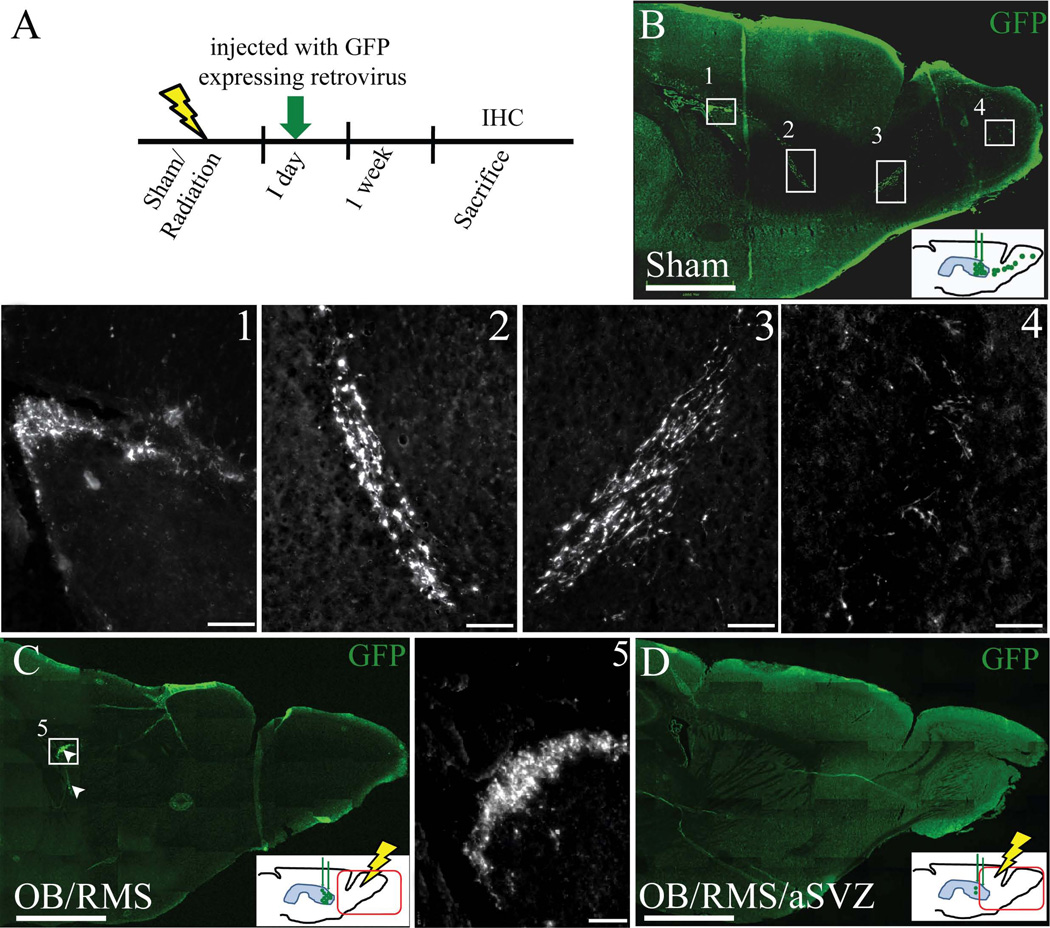
Engineered self-inactivating murine oncoretrovirus expressing green fluorescent protein (GFP), with the Woodchuck hepatitis virus post-transcriptional regulatory element under the Ubiquitin-C promoter, was produced as previously described [35]. A day after irradiation, mice (n=5/group) were anesthetized with ketamine (100mg/kg)/xylazine (10mg/kg) anesthesia and injected with buprenorphine (0.02mg/kg) for post-operative analgesia. The head was secured to a stereotaxic frame (David Kopf). The skull surface was exposed and retrovirus was injected (1µl per site) into the SVZ using a glass needle at the following two different coordinates bilaterally relative to bregma: anterior=1.0mm, lateral=±1.0mm, ventral=2.2mm; anterior =0. 0mm, lateral=±1.8mm, ventral=1.6mm. The scalp was sutured and the mice were returned to their standard housing. Mice were sacrificed a week after retrovirus injections, the brains were fixed, sectioned sagittally and immunostained against Ki67. Sections were visualized under a microscope for GFP-expressing migrating neuroblasts and Ki67+ cells. The experimental paradigm is shown in Figure 7A.
Figure 7.
Neuroblasts failed to migrate through the irradiated RMS to the OB. A) Experimental paradigm showing the time line for radiation, GFP expressing retroviral injections (green arrow), and sacrifice. B) Sagittal section of a non-irradiated mouse brain injected with GFP expressing retrovirus into the SVZ a week after injections, insets show the GFP labeled cells (green) in SVZ (1), descending limb of RMS (2), ascending limb of RMS (3), and olfactory bulb (4) at higher magnification. C) Sagittal section of a RMS/OB irradiated mouse brain with GFP labeled cells (green, shown in arrowheads) in SVZ (inset 5 at higher magnification) and no GFP labeled cells in the RMS+OB. The GFP+ cells in the SVZ appeared to be similar to the sham irradiated mice (inset 1 and 5). D) Sagittal section of a aSVZ/RMS/OB irradiated mouse brain with no GFP labeled cells in aSVZ/RMS/OB. Hypothetical presence of GFP+ cells in the SVZ, RMS and OB, a week after retroviral injections, in three different groups of mice that received sham or RMS/OB or aSVZ/RMS/OB radiation are shown in the insets of B, C, and D. Scale bars: 1mm (B–D), 100µm (1–5).
Cell Quantification and Statistical Analysis
In the coronal sections of the mouse brain, the quantification of Ki67+ cells in the SVZ, RMS and OB was performed on every 20th section evenly spaced along the entire rostral-caudal length of the lateral ventricle of the SVZ using a fluorescent microscope. A total of 7, 9, and 10 sections were counted for SVZ, RMS and OB respectively for each animal. The cell counts for all mice in a given group were averaged per section and the standard error of the mean (SEM) was calculated. To quantify the number of cells within the SVZ under electron microscope, we considered the area comprised of the first 20µm adjacent to the ventricle lumen (0–1mm anterior to bregma). The counts were performed on 3 different levels per animal, and the average was shown in cells/mm. Blood vessel analysis was performed using semithin sections. We considered the area comprised of the first 100µm adjacent to the ventricle lumen (0–1mm anterior to bregma). The number and size of blood vessels were measured with Image Tool software (Evans Technology). Changes in RMS size were determined by measuring the area occupied by RMS in semithin sections using a light microscope, at 3 different levels per animal. The analysis was performed with Image Tool software. An unpaired t-test was performed using SigmaPlot 11.0 software to determine the difference between the left and right SVZ for all our data quantified. The differences were considered significant at a p-value less than 0.05.
RESULTS
Localized radiation eliminates actively proliferating cells in the SVZ
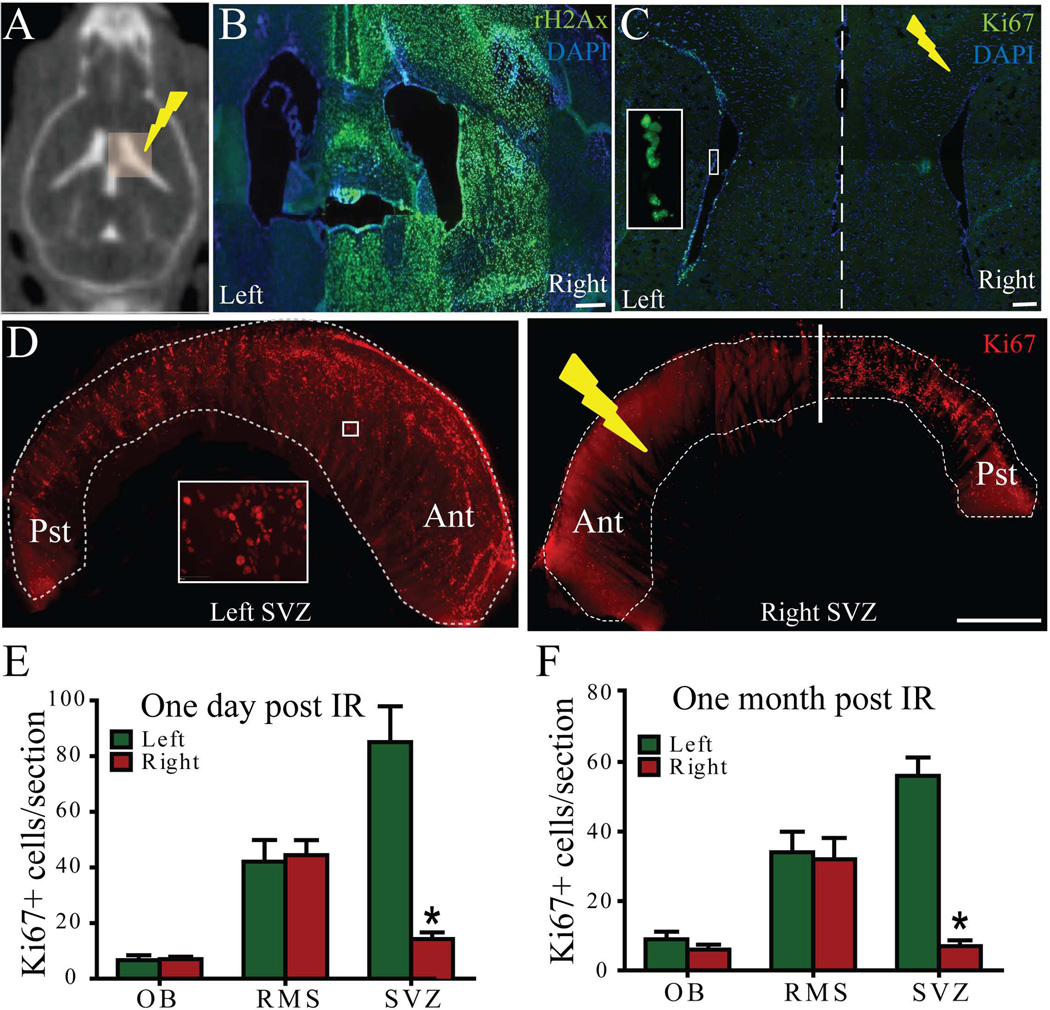
A series of pilot experiments was performed to validate the localized nature of the radiation beams from the SARRP. A 10-Gy, 3×3mm field of localized radiation was delivered to the right ventricle while the non-irradiated left ventricle served as a control (Figure 1A). The accuracy of localized radiation delivery in the targeted right SVZ region was validated by immunohistochemical staining of γH2Ax, a marker of DNA double strand breaks, one hour post irradiation. The irradiated right ventricle of the mouse brain showed restricted expression of γH2Ax (Figure 1B). Cell proliferation in the SVZ was assessed by immunostaining against Ki67 in response to the localized radiation. A strong lateral asymmetry in cell proliferation was observed on every coronal section one-day post irradiation (Figure 1C) and on SVZ whole mounts one-month post irradiation (Figure 1D). There was a significant decrease in the number of Ki67+ cells in the right SVZ at one day (left SVZ: 85.05±12.84 cells/section vs. right SVZ: 14.27±2.35 cells/section) (Figure 1E) and one month (left SVZ: 56±5.26 cells/section vs. right SVZ: 7±1.67cells/section) (Figure 1F) after irradiation (p<0.001). Remarkably, the number of proliferating cells in the RMS and OB was not affected by localized radiation of the SVZ at either one day or one month after irradiation (Figure 1E–F). These results indicate that the vast majority of proliferating cells in the mouse SVZ were permanently eliminated in a highly localized fashion using SARRP technology.
Figure 1.
Localized radiation of the right SVZ disrupts proliferation as compared to the left (control) side. A) Computed tomography (CT) image of a mouse after intrathecal iodine contrast showing ventricles and radiation plan with 3×3 mm beam covering the right ventricle. B) Immunohistochemistry (IHC) against γH2Ax (green) one hour after radiation confirmed that the 3×3 mm beam irradiated only the right lateral ventricle. C) IHC against Ki67 of a coronal section of the mouse brain one day post irradiation. Inset shows the Ki67+ cells (green) at higher magnification. D) Whole mount staining of SVZ against Ki67 one month post irradiation. Inset shows the Ki67+ cells (red) at higher magnification. The number of Ki67+ cells in the irradiated SVZ decreased significantly compared to the control SVZ after one day (E) and one month (F). Ant-Anterior, Pst-Posterior. Scale bars: 100µm (B–C), 1mm(D). *p<0.001.
Localized radiation of SVZ decreases NPCs and neuroblasts
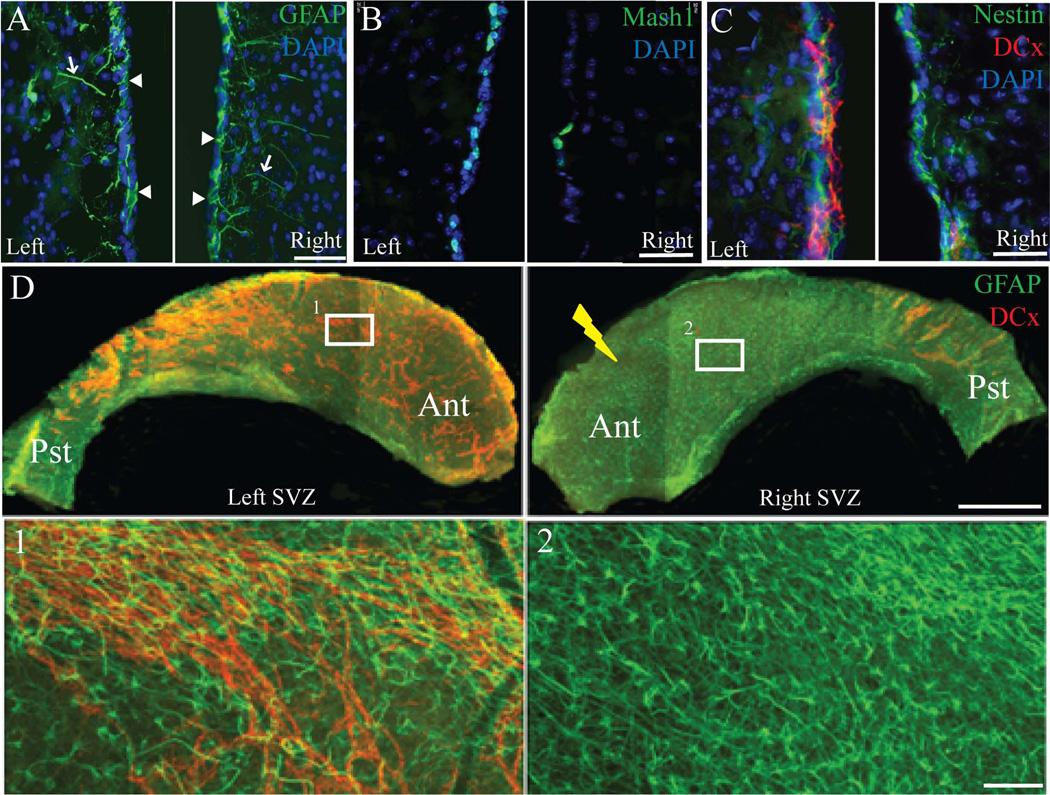
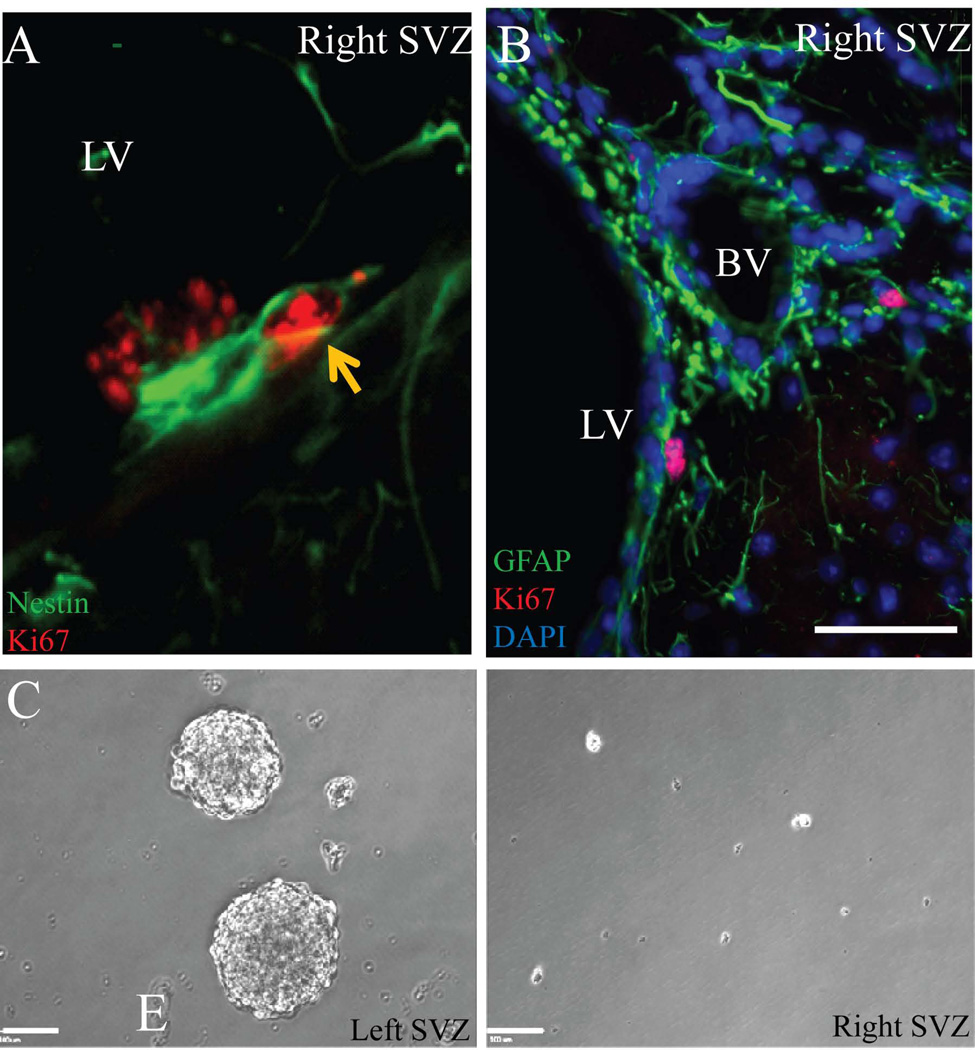
We then explored the effect of localized radiation on different cell types in the SVZ, such as NSCs/type B1 cells, NPCs/type C cells, and migrating neuroblasts/type A cells, using cell specific markers and electron microscopy one-month post irradiation. Nestin and GFAP immunostaining was used to label type B cells, Mash-1 for type C cells, and doublecortin (DCx) for migrating neuroblasts [24]. While GFAP and nestin expression appeared to remain stable (Figure 2A, and 2C), there was a strong qualitative reduction of Mash-1+ cells (Figure 2B) and DCx+ cells (Figure 2C). The GFAP+ type B1 cells in the coronal sections were distinguished by their contact with the ventricular surface (arrowheads in Figure 2A) and long distal processes (arrows in Figure 2A) from the parenchymal astrocytes or type B2 cells that form glial tubes and are also positive for GFAP [24]. GFAP and DCx co-staining of lateral ventricle whole mounts further confirmed the presence of GFAP+ cells and the absence of DCx+ cells in the irradiated SVZ (Figure 2D). These findings from SVZ immunostaining were further confirmed with electron microscopy, and quantitative analysis of different cell types was performed using electron microscopy (Figure 3).
Figure 2.
Localized irradiation of the right SVZ eliminates the NPCs and migrating neuroblasts but not type B cells after one month. A) GFAP+ cells (green) in a coronal section of the mouse brain appeared to show no difference between the left and right SVZ. Type B cells were identified by their long GFAP processes (arrows) and their contact with the lateral ventricle (arrowheads). B) Fewer Mash-1+ NPCs (green) in the right SVZ with localized radiation compared to the control left SVZ. C) Nestin (green) and DCx (red) double labeling showed the presence of type- B cells but not migrating chains of neuroblasts upon localized radiation. D) GFAP (green) and DCx (red) immunohistochemistry of whole mount SVZ also confirmed the ablation of neuroblasts after localized radiation (right SVZ) after one month. Higher magnification images of left and right whole mount SVZ (insets) are shown in 1 and 2. Ant-Anterior, Pst-Posterior. Scale bars: 50µm (A–C), 1mm (D), 100µm (insets 1–2).
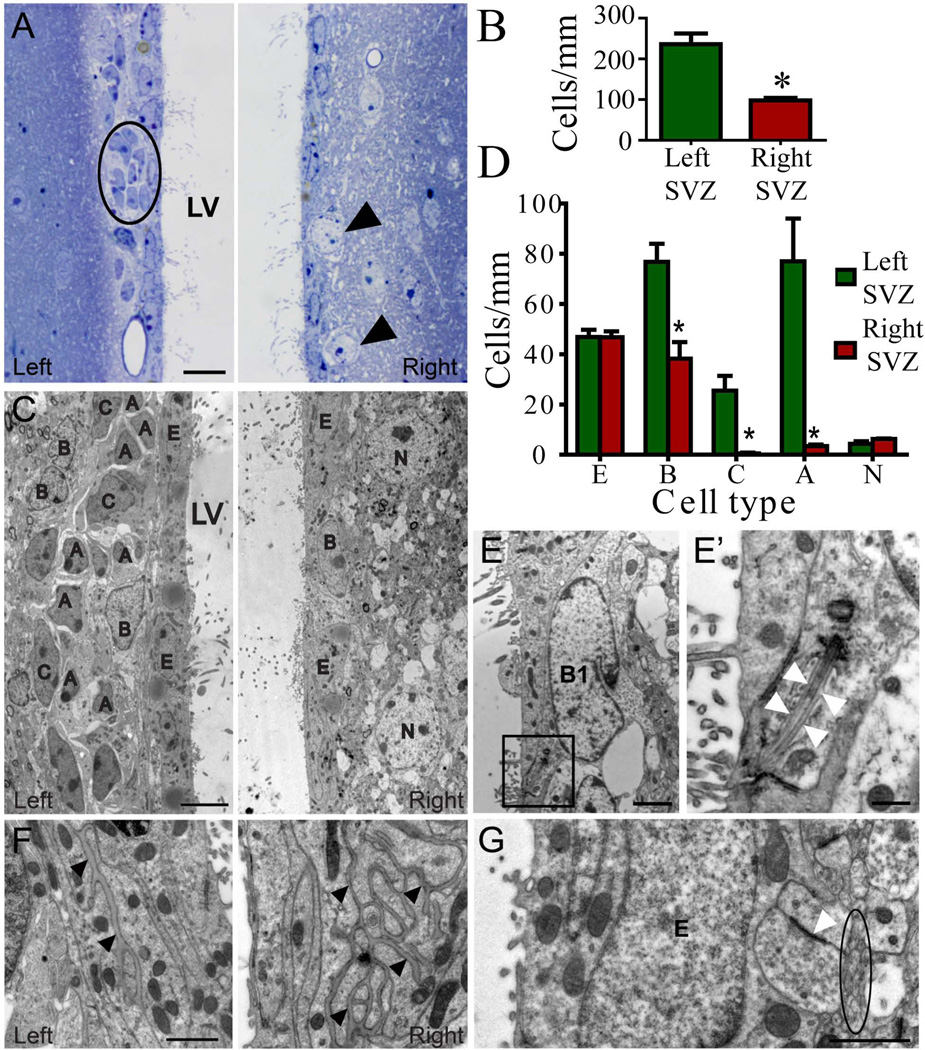
Figure 3.
Cell organization of the non-irradiated left and irradiated right SVZ one month post-irradiation. A) Semithin sections of the left SVZ reveal typical cell organization with dark cells forming the migratory chains (circle) surrounded by expansions of astrocytes (light cells), located beneath the ependymal layer, while the irradiated SVZ lacks the proliferative populations, showing a monolayer of ependymal cells with neurons located close to the ventricle (arrow). B) Bar graph representing the number of cells/mm for the total cells in the SVZ, resulting in a cell reduction in the irradiated hemisphere. C) Electron micrographs depict details of each cell type remained in the left and right SVZ. Irradiated SVZ was reduced to a monolayer of ependymal cells with dispersed astrocytes and neurons without NPCs or neuroblasts. D) Bar graph representing the number of cells/mm for each SVZ cell type. E) Electron micrograph of a B1 cell contacting the lumen of the lateral ventricle of irradiated SVZ. Details of the primary cilia are shown at higher magnifications (E' arrowheads). F) Basal lamina in the right SVZ was more extensive after irradiation compared to the left SVZ (arrowheads). G) Amyelenic axons (circle) and synaptic contacts (arrowheads) next to ependymal cells in contact with ependymal cells in the irradiated SVZ. A-neuroblast, B-astrocyte, C-type C cell, N-neuron, LV-lateral ventricle, BV-blood vessel. Scale bars: 10µm (A), 5µm (C), 2µm (E), 500nm (E’), 1µm (F, G). * p<0.001
Light and electron microscope examination of the SVZ showed a clear disruption in both the antero-posterior and dorso-ventral axes in irradiated hemisphere one month after irradiation. While the cell organization in non-irradiated SVZ appeared normal, with a monolayer of ependymal cells and typical chains of migratory neuroblasts (dark cells) surrounded by type B (light cells), SVZ in irradiated hemisphere was reduced to ependymal cells with dispersed astrocytes and neuronal bodies close to the ventricle (Figure 3A and 3C). There were no migratory chains after irradiation (Figure 3A and 3C), but dispersed type A cells were occasionally observed. Consequently, cell density was drastically decreased in irradiated SVZ (left SVZ: 236.1±26.2 cells/mm vs. right SVZ: 97.9±6 cells/mm; p<0.005) (Figure 3B). This loss of cell density was attributed to a decrease in the number of type B cells (left SVZ: 76.8±7.2 cells/mm vs. right SVZ: 38.2±6.6 cells/mm; p=0.004), type C cells (left SVZ: 25.5±5.9 cells/mm vs. right SVZ: 0.5±0.3 cells/mm; p=0.01) and type A cells (left SVZ: 77.0±17.0 cells/mm vs. right SVZ: 3.5±0.6 cells/mm; p=0.01), while ependymal cells remained unaltered (left SVZ: 46.9±2.9 cells/mm vs. right SVZ: 46.8±2.3 cells/mm) (Figure 3D and Supplementary Table 2). Most importantly, type B1 cells, identified by their apical process extending into the lateral ventricle and primary cilia, were observed in the right SVZ one month after irradiation (Figure 3E-E’, and Table 2). Extensive portions of basal lamina were found frequently between astrocytes and/or ependymal cells in the irradiated SVZ, compare to non-irradiated hemisphere (Figure 3F). As a result of the decreased cell density, neuropil structures were shifted medially towards the ependymal layer in the right SVZ following irradiation. Mylenic and amylenic axons, including synaptic contacts, were observed adjacent to the ependymal layer, establishing direct contact with ependymal and/or astrocytic cells in some cases (Figure 3G). Together, these results indicate that the migrating neuroblasts were not replenished even one month after irradiation, despite the presence of type B cells in the SVZ post-irradiation (Figure 2 and 3E).
Localized radiation affects the generation of NPCs and migrating neuroblasts from NSCs
The presence of type B1 cells after irradiation raised the question if their proliferation was intact. Double labeling of the SVZ against nestin and Ki67 one month after radiation showed proliferation in few of the residual type B cells (Figure 4A). These proliferating type B cells were further found to be in close proximity to blood vessels (Figure 4B). CD31 immunostaining for endothelial cells in the SVZ appeared to be no different between the control and irradiated SVZ (Supplementary Figure 1A). These results were also confirmed by electron microscopy, where the basal lamina and endothelial cells were no different between the left (unirradiated) SVZ and the right (irradiated) SVZ (Supplementary Figure 1B). There was no difference in the number of blood vessels (left SVZ: 66±4.93 vs. right SVZ: 51±7.57; p=0.172) and in the size of the blood vessels (left SVZ: 50.93±11.6 vs. right SVZ: 45.09±7.29; p=0.692) as quantified in the semithin section (Supplementary Figure 1C–D). Interestingly, when the dissected SVZ tissue was dissociated and cultured in vitro, the cells from the left SVZ gave rise to neurospheres whereas the cells from the irradiated right SVZ failed to form neurospheres (Figure 4C). Taken together, these results indicate that the residual type B cells are able to proliferate after irradiation but lack the ability to generate NPCs and migrating neuroblasts.
Figure 4.
Residual type B cells in the irradiated right SVZ one month after radiation. A) Nestin (green) and Ki67 (red) double labeling show proliferating type B cells in the right SVZ (arrow). B) GFAP (green) and Ki67 (red) show proliferating type B cells (arrow) in close proximity to blood vessels. C) Neurospheres were formed from unirradiated control SVZ (left), while no neurospheres were formed from the 10-Gy irradiated SVZ (right). LV-lateral ventricle, BV-blood vessel. Scale bars: 50µm (B), 100µm (C)
Localized radiation of SVZ affected the size of the RMS
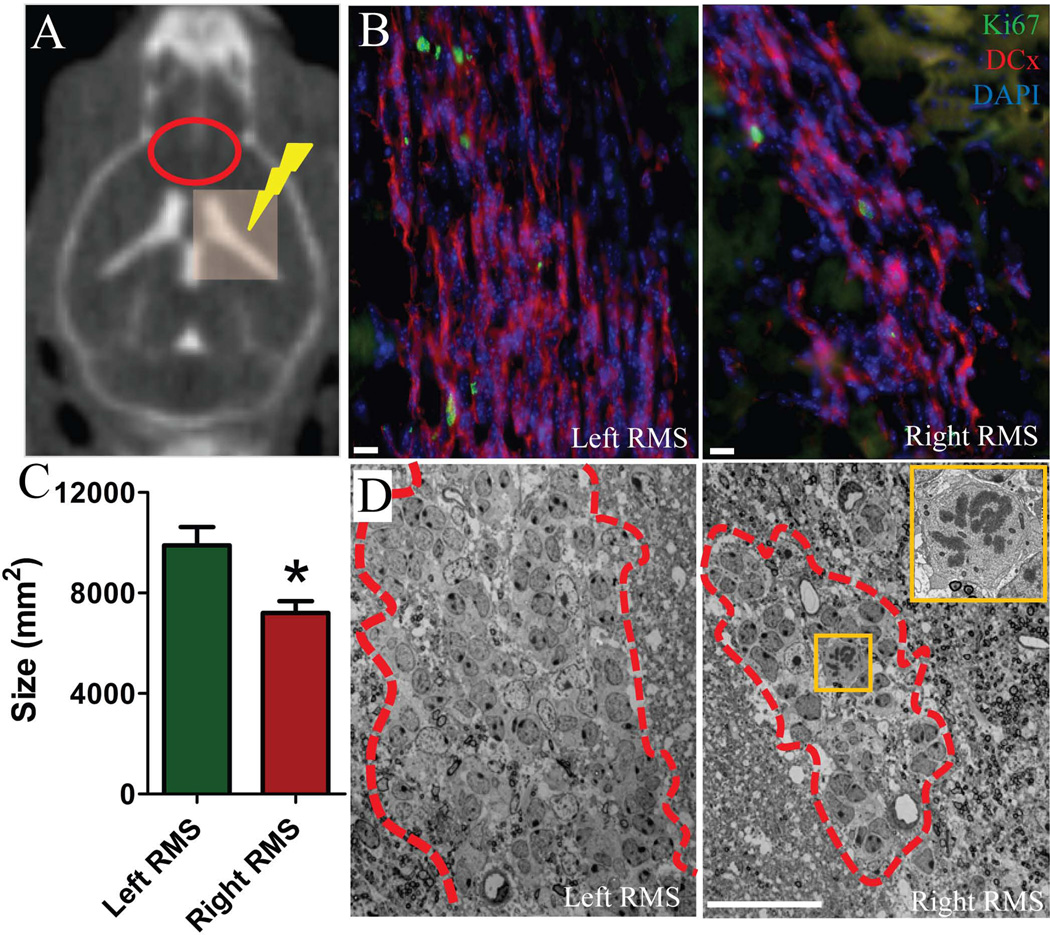
Although there was no significant difference in the number of proliferating cells in the RMS (Figure 1F), there were fewer DCx+ migrating neuroblasts in the right RMS one month after localized right SVZ radiation as compared to the left RMS (Figure 5B). In addition, there was a significant reduction in cross-sectional size of the RMS (left RMS: 9892±737mm2 vs. right RMS: 7202±474mm2) (Figure 5C). Ultrastructural analysis with electron microscopy revealed no morphological alterations in cell populations of the RMS despite the reduction in size (Figure 5D). Further, we noticed few cells in the irradiated RMS in their mitotic phase, which based on their ultrastructural features, were identified as neuroblasts (Figure 5D, inset).
Figure 5.
Fewer migrating neuroblasts were observed in the RMS of the irradiated hemisphere after one month. A) Computed tomography (CT) image of a mouse after intrathecal iodine contrast showing ventricles and radiation plan with 3×3 mm beam covering the right ventricle. Red oval shows the region of RMS on CT. B) Ki67 (green) and DCx (red) immunolabeling in the RMS. Note a smaller number of migrating neuroblasts (DCx+/red cells) in the right RMS compared to the left RMS. C) Bar graph showing a significant reduction in the size of the RMS in the irradiated right hemisphere. D) Panoramic images of migratory chains and astrocytes forming the RMS (outlined with dashed line) under electron microscope. Note the difference of the RMS size. Inset shows a neuroblast in mitotic phase at higher magnification in the right RMS. Scale bars: 10µm (B), 20nm (D). * p<0.001.
Localized radiation disrupts the migration of neuroblasts
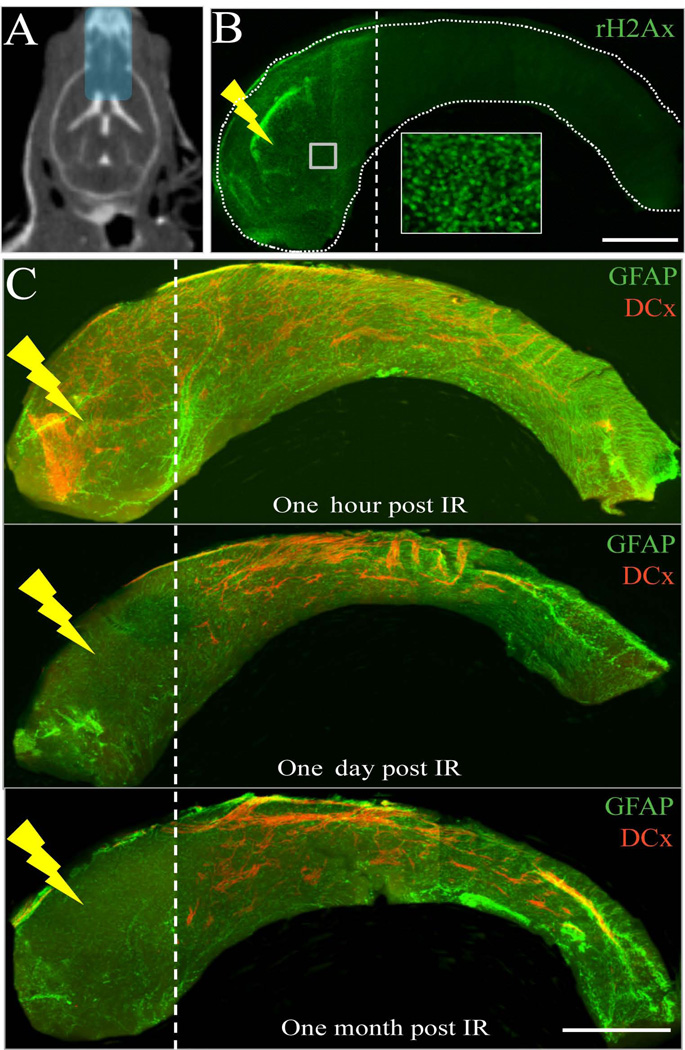
The anterior dorsal SVZ is one of the regions with the largest NSC population [24]. In this experiment, we irradiated the anterior dorsal region of the SVZ with a single 10-Gy lateral beam of 1 mm diameter (Supplementary Figure 2A–B). Whole mount staining of SVZ against GFAP and DCx showed no neuroblasts in the irradiated region after one week (Supplementary Figure 2C). Additionally, no neuroblasts from the posterior dorsal SVZ were found to migrate into the irradiated region indicating that radiation may have affected the migration of neuroblasts in the SVZ (Supplementary Figure 2C’-C”). We then examined the effect of irradiation on anterior SVZ (aSVZ) while sparing the posterior SVZ. A 5×9mm beam of 10Gy was used to deliver radiation to the aSVZ, RMS and OB (Figure 6A). Immunostaining of SVZ whole mount against γH2Ax, one-hour post irradiation, showed a precise radiation field confined only to the aSVZ, sparing the posterior SVZ (Figure 6B). DCx+ neuroblasts were seen one hour post-irradiation, but were absent after one day and one month post-irradiation (Figure 6C). The neuroblasts from the posterior SVZ did not migrate through the irradiated aSVZ, consistent with our results in Supplementary Figure 2. Further, there were no apoptotic cells observed one week after localized irradiation of the anterior SVZ, as assessed by immunohistochemistry against activated Caspase-3 (data not shown).
Figure 6.
Neuroblasts from the posterior SVZ do not migrate into the irradiated anterior SVZ. A) CT image of a mouse after intrathecal iodine contrast showing ventricles and treatment plan of 5×9 mm beam to irradiate anterior SVZ, RMS and OB. B) γH2Ax staining (green) of whole mount SVZ one hour post-irradiation validates the localized radiation treatment plan. Inset shows the γH2Ax+ cells at higher magnification. C) GFAP (green) and DCx (red) double labeling of SVZ whole mounts one hour, one day and one month after irradiation. Scale bars: 1mm (B–C).
To evaluate if the migratory properties of neuroblasts were affected by irradiation, we injected GFP-expressing retrovirus into the SVZ to label NSCs and track the migratory pattern of newly generated neuroblasts, one day after radiation treatment. Three separate groups of mice received either no radiation or a single 10-Gy dose to their RMS/OB (sparing the entire SVZ) or to their aSVZ/RMS/OB. One day after irradiation, mice were injected with GFP-expressing retrovirus in the SVZ to label dividing NSCs and track the migration of neuroblasts (Figure 7A). As predicted, sham-irradiated mice had GFP+ cells in the SVZ, RMS and OB one week after injection (Figure 7B, insets 1, 2, 3 and 4). Mice that received radiation to the RMS/OB showed GFP+ cells in their SVZ but not in RMS and OB (Figure 7C, inset 5). Mice that received radiation to the aSVZ/RMS/OB showed no GFP+ cells (Figure 7D). Quantification of Ki67+ cells further revealed that the RMS/OB irradiation did not affect the SVZ proliferation (Supplementary Figure 3). These results indicate that irradiation of the RMS alone (sparing SVZ) can inhibit the migration of neuroblasts. Our findings suggest that irradiation did not disrupt the intrinsic mechanism of neuroblast migration, but the local microenvironment permissive of cell migration.
DISCUSSION
The effect of radiation on NSC/NPC proliferation and neuroblast migration cannot be disregarded, given that radiation therapy is a first-line treatment for many brain tumors, including glioblastoma [2]. Numerous studies have examined the decrease in proliferating cells in SVZ and subgranular zone after radiation [8, 9, 11, 36–38]. The anti-proliferative effects last for several months after a single radiation treatment and are dose-dependent [9, 11, 37–39]. Most studies to date have been restricted to irradiating relatively large areas of the brain [7, 10, 39]. Very few studies used localized brain irradiation using custom-designed radiation shields or the Gamma Knife unit (Elekta Inc., Sweden) to irradiate sub-regions of the brain [9, 40–42]. We used SARRP technology, capable of delivering millimeter-scale beams to the mouse brain under CT guidance. This novel technology accurately mimics clinical radiation treatment, on the murine scale [31]. The localized radiation treatment used in our study is validated by immunohistochemistry against γH2Ax, a marker for DNA strand breaks [43]. Localized irradiation of right SVZ affected the proliferation in that region but not in the RMS and OB, further confirming the precise nature of the SARRP technology. There was a significant decrease in the proliferative population of cells, including NPCs and neuroblasts [24, 44], in the SVZ one day after localized radiation. The same effect was noticed even one month after localized radiation of SVZ (Figure 1D–E). The decrease in the proliferative population after irradiation appeared to be due to the loss of NPCs and neuroblasts as shown by immunohistochemistry (Figure 2B–C). To determine if this was not due to changes in antibody binding efficiency, we employed electron microscopy. The ultrastructural analyses from electron microscopy confirmed the elimination of NPCs and neuroblasts and the presence of NSCs in the SVZ after localized radiation. This could be explained by the quiescent nature of NSCs (cell cycle time ~15 days) [44, 45]. Proliferating cells such as NPCs (cell cycle time ~13 hours) and migrating neuroblasts are highly susceptible to ionizing radiation [9–11, 44, 45]. Our results on the depletion of NPCs and neuroblasts after radiation treatment are similar to the effects of anti-mitotic drugs such as cytosine-beta-D-arabinofuranoside (AraC) [44] or the alkylating reagent N-ethyl-N-nitrosourea (ENU) on SVZ [46, 47]. The NPCs and neuroblasts regenerate from NSCs and replenish the entire SVZ in approximately four days after AraC treatment [44, 45]. In case of ENU-exposure, residual NSCs allowed a partial recovery after treatment [46, 47]. On the contrary, we did not observe the regeneration of NPCs and neuroblasts from NSCs even one month after localized radiation of SVZ in our study (Figure 2 and 3). This suggests that irradiation might have caused permanent damage to the NSCs and/or the microenvironment that supports the proliferation and maintenance of the NSCs [10, 48–52].
NSCs are known to associate closely with the vascular niche in the SVZ [50–52]. At higher doses of irradiation, the endothelial cells that support NSCs undergo apoptosis, resulting in vascular damage and decreased neurogenesis [53–55]. We did not notice any morphological changes in endothelial cells after localized irradiation, as shown by CD31 immunohistochemistry and electron microscopy. There was no difference in the number or size of blood vessels in the irradiated SVZ as compared to the unirradiated SVZ (Supplementary Figure 1). Further, the radiation dose used in our study is less than has been shown to cause such vascular damage [49, 56]. Therefore, the inability of NSCs to give rise to NPCs cannot be attributed to vascular niche damage. However, we cannot rule out the possibility that, even though the gross morphology of endothelial cells is intact, the molecular signals that trigger NSCs to proliferate and give rise to NPCs could be affected by irradiation [57–59]. Basal lamina in the neurogenic niche is known to play a role in the proliferation of NSCs [52]. There were no differences in the ultrastructural features of basal lamina between the irradiated and non-irradiated hemispheres. However, we found it more often and extensive in the irradiated right SVZ compared to left SVZ (Figure 3F). The extensive basal lamina observed in our animals could be a response due to the cell loss post-irradiation. Cranial irradiation is known to disrupt the microenvironment and to activate reactive microglia, affecting neurogenesis [10, 48, 49, 60]. When NSCs isolated from irradiated brain were transplanted into normal brain, NSCs regained the ability to proliferate and gave rise to neuronal and glial cell lineages [10]. In our study, we isolated cells from the irradiated and unirradiated SVZ and cultured them in NSC growth medium. While cells from irradiated SVZ remained as single cells, the cells from unirradiated SVZ divided and gave rise to neurospheres, nonadherent clusters of cells generated from NSCs in vitro [61] (Figure 4C). Though the specific phenomena are not completely understood, our interpretation of these data is that immediately following irradiation, the rapidly dividing NPCs and migrating neuroblasts are eliminated and the NSC compartment becomes unable to fully repopulate. To better understand the effects of localized radiation on SVZ neurogenesis, detailed analysis of gene expression and/or molecular signaling pathways is warranted [62].
The ability of NPCs and neuroblasts to migrate to the site of damage and to participate in repair is well documented [63–65]. Radiation may compromise this ability, since radiation kills NPCs and neuroblasts in the SVZ, and also inflicts long-term damage to the NSCs in this region, as shown in this and previous studies [9–11, 37–39, 41, 60, 66]. Further, we show that there are fewer neuroblasts in the right RMS one month after irradiating the right SVZ (Figure 5). The few neuroblasts observed in the right RMS could have been generated locally from the multipotent, self-renewing NSCs present in the RMS that were not affected by SVZ irradiation (Figure 1F) [67–69]. The depletion of migrating neuroblasts in the right SVZ after radiation also resulted in the reduction in the size of the right RMS, suggesting that the NSCs within the RMS does not compensate for the loss of migrating neuroblasts from the SVZ and also the number of neuroblasts generated in the SVZ can affect the size of the RMS [70, 71].
With recent advancements in clinical radiotherapy planning and delivery, neurogenic niches can be spared from radiation without compromising dose coverage of the tumor [29, 30]. We have recently shown that it is possible to spare the NSC niche during radiation therapy in a retrospective clinical radiation treatment planning study [30]. NSCs may be used either to inhibit tumors by causing apoptosis or to repair tissue damage that results from radiation treatment [16–19, 65, 72]. Both uses rely on the migration of the neuroblasts to locations where they are needed, but very little is known about the effect of radiation on the migration of neuroblasts.
The anterior SVZ is one of the hotspots in the adult mouse brain with the highest number of NSC clusters [24]. When the anterior SVZ was irradiated, we noticed that neuroblasts from the posterior SVZ did not migrate through the irradiated area (Figure 6). Even when a part of the anterior dorsal SVZ received a radiation beam as small as one millimeter in diameter, neuroblasts did not migrate through the irradiated region of the SVZ (Supplementary Figure 2). This is important in the context of emerging studies on the transplantation of modified stem cells into the mouse brain to migrate and undergo repair at the site of damage [17, 19, 65, 70]. To further confirm these findings, we labeled NSCs locally in the SVZ with GFP-expressing retrovirus one day after irradiation of RMS/OB or SVZ/RMS/OB. The inclusion of RMS/OB irradiation allowed us to study the effect of radiation on the migrating neuroblasts when the SVZ is spared from irradiation. The GFP-expressing cells from the SVZ failed to migrate through the RMS in the RMS/OB irradiated mice, indicating that neuroblasts did not follow their normal migratory path through the irradiated SVZ-RMS-OB route (Figure 7). This migratory alteration is probably not due to an intrinsic disruption in the migratory machinery of new-formed neuroblasts, but rather due to a disturbance of parenchymal migratory signaling in situ [59, 73–78]. To our knowledge, this is the first report showing a strong inhibitory effect on the migration of neuroblasts after localized radiation. The mechanisms involved in this effect are unknown. At this point, we cannot predict if this inhibitory effect on neuroblast migration after irradiation will be seen outside the SVZ-RMS-OB migration route. Also, we cannot disregard the possibility that the effect of RMS/OB irradiation would have functional impact on the SVZ, despite the proliferation in the SVZ was not compromised in these mice compared to sham mice. Though our data on the effects of localized radiation on SVZ NSCs, NPCs and neuroblast migration are striking, much more work is needed before the mechanisms are fully understood and the clinical implications of the use of NSCs for damage repair in brain tumor patients can be fully appreciated.
CONCLUSIONS
In summary, we show that (i) SVZ-restricted irradiation affects the proliferation of type C and A cells in a highly localized manner; (ii) the residual type B cells lack the ability to regenerate NPCs, which in turn give rise to migrating neuroblasts; (iii) SVZ-restricted irradiation reduces the size of the RMS; and (iv) neuroblasts failed to migrate through the irradiated regions of the SVZ-RMS-OB route. Future studies are indicated to investigate the effects of localized radiation not only on the molecular signals involved in the proliferation of NSCs/NPCs but also on those involved in neuroblast migration. This will allow for a thorough understanding of the effects of localized irradiation on the neurogenic regions of the brain both at the cellular and molecular levels to better improve radiotherapy protocols in the clinical setting.
Supplementary Material
ACKNOWLEDGMENTS
This research is supported by the Maryland Stem Cell Research Fund (AQH), The Robert Wood Johnson Foundation (AQH), The Howard Hughes Medical Institute (AQH), and The National Institutes of Health (AQH). Dr. Oscar Gonzalez-Perez was supported in part by the Consejo Nacional de Ciencia y Tecnologia’s grant (CONACyT, CB-2008-101476)) and Dr. Jose Manuel Garcia-Verdugo by the Prometeo grant (GVPROMETEO-2009/011). We thank Dr. Zaman Mirzadeh for technical help with whole mount dissections. We thank Dr. Michael Bonaguidi for fruitful discussions on the experimental design and data interpretation. We thank Dr. Hugo Guerrero-Cazares for helpful technical hints and critical reading of manuscript.
Footnotes
Author Contributions: PA: Conception and design of experiments, data collection, assembly, analysis and interpretation, manuscript writing, and final approval of manuscript; DP: data collection, assembly, and analysis; JR: data collection; VCG & JMGV: data collection, assembly, analysis and interpretation from electron microscopy; KS & HS: provided the GFP expressing retrovirus, and assisted with stereotactic injections; OGP: experimental design and data interpretation; EF: conception and design of experiments, SARPP technology, and data interpretation; AQH: conception and design of experiments, final approval of manuscript and financial support. All authors contributed to the manuscript editing.
DISCLOSURES: The authors declare no conflict of interest and no financial disclosures.
REFERENCES
- 1.Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–466. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 2.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 3.Calabrese P, Schlegel U. Neurotoxicity of treatment. Recent Results Cancer Res. 2009;171:165–174. doi: 10.1007/978-3-540-31206-2_10. [DOI] [PubMed] [Google Scholar]
- 4.Marazziti D, Baroni S, Catena-Dell'osso M, et al. Cognitive, Psychological and Psychiatric Effects of Ionizing Radiation Exposure. Curr Med Chem. 2012 doi: 10.2174/092986712800099776. [DOI] [PubMed] [Google Scholar]
- 5.Silber JH, Radcliffe J, Peckham V, et al. Whole-brain irradiation and decline in intelligence: the influence of dose and age on IQ score. J Clin Oncol. 1992;10:1390–1396. doi: 10.1200/JCO.1992.10.9.1390. [DOI] [PubMed] [Google Scholar]
- 6.Walter AW, Mulhern RK, Gajjar A, et al. Survival and neurodevelopmental outcome of young children with medulloblastoma at St Jude Children's Research Hospital. J Clin Oncol. 1999;17:3720–3728. doi: 10.1200/JCO.1999.17.12.3720. [DOI] [PubMed] [Google Scholar]
- 7.Achanta P, Fuss M, Martinez JL., Jr Ionizing radiation impairs the formation of trace fear memories and reduces hippocampal neurogenesis. Behav Neurosci. 2009;123:1036–1045. doi: 10.1037/a0016870. [DOI] [PubMed] [Google Scholar]
- 8.Fike JR, Rola R, Limoli CL. Radiation response of neural precursor cells. Neurosurg Clin N Am. 2007;18:115–127. doi: 10.1016/j.nec.2006.10.010. x. [DOI] [PubMed] [Google Scholar]
- 9.McGinn MJ, Sun D, Colello RJ. Utilizing X-irradiation to selectively eliminate neural stem/progenitor cells from neurogenic regions of the mammalian brain. J Neurosci Methods. 2008;170:9–15. doi: 10.1016/j.jneumeth.2007.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Monje ML, Mizumatsu S, Fike JR, et al. Irradiation induces neural precursor-cell dysfunction. Nat Med. 2002;8:955–962. doi: 10.1038/nm749. [DOI] [PubMed] [Google Scholar]
- 11.Tada E, Yang C, Gobbel GT, et al. Long-term impairment of subependymal repopulation following damage by ionizing irradiation. Exp Neurol. 1999;160:66–77. doi: 10.1006/exnr.1999.7172. [DOI] [PubMed] [Google Scholar]
- 12.Abrous DN, Koehl M, Le Moal M. Adult neurogenesis: from precursors to network and physiology. Physiol Rev. 2005;85:523–569. doi: 10.1152/physrev.00055.2003. [DOI] [PubMed] [Google Scholar]
- 13.Alvarez-Buylla A, Garcia-Verdugo JM. Neurogenesis in adult subventricular zone. J Neurosci. 2002;22:629–634. doi: 10.1523/JNEUROSCI.22-03-00629.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ming GL, Song H. Adult neurogenesis in the mammalian central nervous system. Annu Rev Neurosci. 2005;28:223–250. doi: 10.1146/annurev.neuro.28.051804.101459. [DOI] [PubMed] [Google Scholar]
- 15.Menn B, Garcia-Verdugo JM, Yaschine C, et al. Origin of oligodendrocytes in the subventricular zone of the adult brain. J Neurosci. 2006;26:7907–7918. doi: 10.1523/JNEUROSCI.1299-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Glass R, Synowitz M, Kronenberg G, et al. Glioblastoma-induced attraction of endogenous neural precursor cells is associated with improved survival. J Neurosci. 2005;25:2637–2646. doi: 10.1523/JNEUROSCI.5118-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aboody KS, Brown A, Rainov NG, et al. Neural stem cells display extensive tropism for pathology in adult brain: evidence from intracranial gliomas. Proc Natl Acad Sci USA. 2000;97:12846–12851. doi: 10.1073/pnas.97.23.12846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Achanta P, Sedora Roman NI, Quinones-Hinojosa A. Gliomagenesis and the use of neural stem cells in brain tumor treatment. Anticancer Agents Med Chem. 2010;10:121–130. doi: 10.2174/187152010790909290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahmed AU, Thaci B, Alexiades NG, et al. Neural stem cell-based cell carriers enhance therapeutic efficacy of an oncolytic adenovirus in an orthotopic mouse model of human glioblastoma. Mol Ther. 2011;19:1714–1726. doi: 10.1038/mt.2011.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frank RT, Edmiston M, Kendall SE, et al. Neural stem cells as a novel platform for tumor-specific delivery of therapeutic antibodies. PloS One. 2009;4:e8314. doi: 10.1371/journal.pone.0008314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee EX, Lam DH, Wu C, et al. Glioma gene therapy using induced pluripotent stem cell derived neural stem cells. Mol. Pharm. 2011;8:1515–1524. doi: 10.1021/mp200127u. [DOI] [PubMed] [Google Scholar]
- 22.Quinones-Hinojosa A, Sanai N, Soriano-Navarro M, et al. Cellular composition and cytoarchitecture of the adult human subventricular zone: a niche of neural stem cells. J Comparative Neurol. 2006;494:415–434. doi: 10.1002/cne.20798. [DOI] [PubMed] [Google Scholar]
- 23.Sanai N, Tramontin AD, Quinones-Hinojosa A, et al. Unique astrocyte ribbon in adult human brain contains neural stem cells but lacks chain migration. Nature. 2004;427:740–744. doi: 10.1038/nature02301. [DOI] [PubMed] [Google Scholar]
- 24.Mirzadeh Z, Merkle FT, Soriano-Navarro M, et al. Neural stem cells confer unique pinwheel architecture to the ventricular surface in neurogenic regions of the adult brain. Cell Stem Cell. 2008;3:265–278. doi: 10.1016/j.stem.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Doetsch F, Garcia-Verdugo JM, Alvarez-Buylla A. Cellular composition and three-dimensional organization of the subventricular germinal zone in the adult mammalian brain. J Neurosci. 1997;17:5046–5061. doi: 10.1523/JNEUROSCI.17-13-05046.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lois C, Alvarez-Buylla A. Proliferating subventricular zone cells in the adult mammalian forebrain can differentiate into neurons and glia. Proc Natl Acad Sci USA. 1993;90:2074–2077. doi: 10.1073/pnas.90.5.2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lois C, Garcia-Verdugo JM, Alvarez-Buylla A. Chain migration of neuronal precursors. Science. 1996;271:978–981. doi: 10.1126/science.271.5251.978. [DOI] [PubMed] [Google Scholar]
- 28.Curtis MA, Kam M, Nannmark U, et al. Human neuroblasts migrate to the olfactory bulb via a lateral ventricular extension. Science. 2007;315:1243–1249. doi: 10.1126/science.1136281. [DOI] [PubMed] [Google Scholar]
- 29.Marsh JC, Godbole R, Diaz AZ, et al. Sparing of the hippocampus, limbic circuit and neural stem cell compartment during partial brain radiotherapy for glioma: a dosimetric feasibility study. J Med Imaging Radiat Oncol. 2011;55:442–449. doi: 10.1111/j.1754-9485.2011.02282.x. [DOI] [PubMed] [Google Scholar]
- 30.Redmond KJ, Achanta P, Grossman SA, et al. A radiotherapy technique to limit dose to neural progenitor cell niches without compromising tumor coverage. J Neurooncol. 2011;104:579–587. doi: 10.1007/s11060-011-0530-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wong J, Armour E, Kazanzides P, et al. High-resolution, small animal radiation research platform with x-ray tomographic guidance capabilities. Int J Radiat Oncol Biol Phys. 2008;71:1591–1599. doi: 10.1016/j.ijrobp.2008.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ford EC, Achanta P, Purger D, et al. Localized CT-guided irradiation inhibits neurogenesis in specific regions of the adult mouse brain. Radiat Res. 2011;175:774–783. doi: 10.1667/RR2214.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mirzadeh Z, Doetsch F, Sawamoto K, et al. The subventricular zone en-face: wholemount staining and ependymal flow. J Vis Exp. 2010 doi: 10.3791/1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ferron SR, Andreu-Agullo C, Mira H, et al. A combined ex/in vivo assay to detect effects of exogenously added factors in neural stem cells. Nat Protoc. 2007;2:849–859. doi: 10.1038/nprot.2007.104. [DOI] [PubMed] [Google Scholar]
- 35.Ge S, Goh EL, Sailor KA, et al. GABA regulates synaptic integration of newly generated neurons in the adult brain. Nature. 2006;439:589–593. doi: 10.1038/nature04404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Belvindrah R, Lazarini F, Lledo PM. Postnatal neurogenesis: from neuroblast migration to neuronal integration. Rev Neurosci. 2009;20:331–346. doi: 10.1515/revneuro.2009.20.5-6.331. [DOI] [PubMed] [Google Scholar]
- 37.Hellstrom NA, Bjork-Eriksson T, Blomgren K, et al. Differential recovery of neural stem cells in the subventricular zone and dentate gyrus after ionizing radiation. Stem Cells. 2009;27:634–641. doi: 10.1634/stemcells.2008-0732. [DOI] [PubMed] [Google Scholar]
- 38.Panagiotakos G, Alshamy G, Chan B, et al. Long-term impact of radiation on the stem cell and oligodendrocyte precursors in the brain. PloS One. 2007;2:e588. doi: 10.1371/journal.pone.0000588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tada E, Parent JM, Lowenstein DH, et al. X-irradiation causes a prolonged reduction in cell proliferation in the dentate gyrus of adult rats. Neuroscience. 2000;99:33–41. doi: 10.1016/s0306-4522(00)00151-2. [DOI] [PubMed] [Google Scholar]
- 40.Shi L, Molina DP, Robbins ME, et al. Hippocampal neuron number is unchanged 1 year after fractionated whole-brain irradiation at middle age. Int J Radiat Oncol Biol Phys. 2008;71:526–532. doi: 10.1016/j.ijrobp.2008.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jirak D, Namestkova K, Herynek V, et al. Lesion evolution after gamma knife irradiation observed by magnetic resonance imaging. Int J Radiat Biol. 2007;83:237–244. doi: 10.1080/09553000601169792. [DOI] [PubMed] [Google Scholar]
- 42.Santarelli L, Saxe M, Gross C, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- 43.Saxe MD, Battaglia F, Wang JW, et al. Ablation of hippocampal neurogenesis impairs contextual fear conditioning and synaptic plasticity in the dentate gyrus. Proc Natl Acad Sci USA. 2006;103:17501–17506. doi: 10.1073/pnas.0607207103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rogakou EP, Nieves-Neira W, Boon C, et al. Initiation of DNA fragmentation during apoptosis induces phosphorylation of H2AX histone at serine 139. J Biol Chem. 2000;275:9390–9395. doi: 10.1074/jbc.275.13.9390. [DOI] [PubMed] [Google Scholar]
- 45.Doetsch F, Caille I, Lim DA, et al. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97:703–716. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- 46.Morshead CM, Craig CG, van der Kooy D. In vivo clonal analyses reveal the properties of endogenous neural stem cell proliferation in the adult mammalian forebrain. Development. 1998;125:2251–2261. doi: 10.1242/dev.125.12.2251. [DOI] [PubMed] [Google Scholar]
- 47.Capilla-Gonzalez V, Gil-Perotin S, Ferragud A, et al. Exposure to N-ethyl-N-nitrosourea in adult mice alters structural and functional integrity of neurogenic sites. PloS one. 2012;7:e29891. doi: 10.1371/journal.pone.0029891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Capilla-Gonzalez V, Gil-Perotin S, Garcia-Verdugo JM. Postnatal exposure to N-ethyl-N-nitrosurea disrupts the subventricular zone in adult rodents. Eur J Neurosci. 2010;32:1789–1799. doi: 10.1111/j.1460-9568.2010.07450.x. [DOI] [PubMed] [Google Scholar]
- 49.Snyder EY, Park KI. Limitations in brain repair. Nat Med. 2002;8:928–930. doi: 10.1038/nm0902-928. [DOI] [PubMed] [Google Scholar]
- 50.Monje ML, Palmer T. Radiation injury and neurogenesis. Curr Opin Neurol. 2003;16:129–134. doi: 10.1097/01.wco.0000063772.81810.b7. [DOI] [PubMed] [Google Scholar]
- 51.Shen Q, Goderie SK, Jin L, et al. Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science. 2004;304:1338–1340. doi: 10.1126/science.1095505. [DOI] [PubMed] [Google Scholar]
- 52.Shen Q, Wang Y, Kokovay E, et al. Adult SVZ stem cells lie in a vascular niche: a quantitative analysis of niche cell-cell interactions. Cell Stem Cell. 2008;3:289–300. doi: 10.1016/j.stem.2008.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tavazoie M, Van der Veken L, Silva-Vargas V, et al. A specialized vascular niche for adult neural stem cells. Cell Stem Cell. 2008;3:279–288. doi: 10.1016/j.stem.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brown WR, Thore CR, Moody DM, et al. Vascular damage after fractionated whole-brain irradiation in rats. Radiat Res. 2005;164:662–668. doi: 10.1667/rr3453.1. [DOI] [PubMed] [Google Scholar]
- 55.Li YQ, Chen P, Haimovitz-Friedman A, et al. Endothelial apoptosis initiates acute blood-brain barrier disruption after ionizing radiation. Cancer Res. 2003;63:5950–5956. [PubMed] [Google Scholar]
- 56.Ljubimova NV, Levitman MK, Plotnikova ED, et al. Endothelial cell population dynamics in rat brain after local irradiation. Br J Radiol. 1991;64:934–940. doi: 10.1259/0007-1285-64-766-934. [DOI] [PubMed] [Google Scholar]
- 57.Otsuka S, Coderre JA, Micca PL, et al. Depletion of neural precursor cells after local brain irradiation is due to radiation dose to the parenchyma, not the vasculature. Radiat Res. 2006;165:582–591. doi: 10.1667/RR3539.1. [DOI] [PubMed] [Google Scholar]
- 58.Andreu-Agullo C, Morante-Redolat JM, Delgado AC, et al. Vascular niche factor PEDF modulates Notch-dependent stemness in the adult subependymal zone. Nat Neurosci. 2009;12:1514–1523. doi: 10.1038/nn.2437. [DOI] [PubMed] [Google Scholar]
- 59.Ramirez-Castillejo C, Sanchez-Sanchez F, Andreu-Agullo C, et al. Pigment epithelium-derived factor is a niche signal for neural stem cell renewal. Nat Neurosci. 2006;9:331–339. doi: 10.1038/nn1657. [DOI] [PubMed] [Google Scholar]
- 60.Wittko IM, Schanzer A, Kuzmichev A, et al. VEGFR-1 regulates adult olfactory bulb neurogenesis and migration of neural progenitors in the rostral migratory stream in vivo. J Neurosci. 2009;29:8704–8714. doi: 10.1523/JNEUROSCI.5527-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Monje ML, Toda H, Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302:1760–1765. doi: 10.1126/science.1088417. [DOI] [PubMed] [Google Scholar]
- 62.Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255:1707–1710. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- 63.Mu Y, Lee SW, Gage FH. Signaling in adult neurogenesis. Curr Opin Neurol. 2010;20:416–423. doi: 10.1016/j.conb.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Arvidsson A, Collin T, Kirik D, et al. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002;8:963–970. doi: 10.1038/nm747. [DOI] [PubMed] [Google Scholar]
- 65.Goings GE, Sahni V, Szele FG. Migration patterns of subventricular zone cells in adult mice change after cerebral cortex injury. Brain Res. 2004;996:213–226. doi: 10.1016/j.brainres.2003.10.034. [DOI] [PubMed] [Google Scholar]
- 66.Aboody K, Capela A, Niazi N, et al. Translating stem cell studies to the clinic for CNS repair: current state of the art and the need for a Rosetta Stone. Neuron. 2011;70:597–613. doi: 10.1016/j.neuron.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 67.Rola R, Raber J, Rizk A, et al. Radiation-induced impairment of hippocampal neurogenesis is associated with cognitive deficits in young mice. Exp Neurol. 2004;188:316–330. doi: 10.1016/j.expneurol.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 68.Craig CG, D'sa R, Morshead CM, et al. Migrational analysis of the constitutively proliferating subependyma population in adult mouse forebrain. Neuroscience. 1999;93:1197–1206. doi: 10.1016/s0306-4522(99)00232-8. [DOI] [PubMed] [Google Scholar]
- 69.Gritti A, Bonfanti L, Doetsch F, et al. Multipotent neural stem cells reside into the rostral extension and olfactory bulb of adult rodents. J Neurosci. 2002;22:437–445. doi: 10.1523/JNEUROSCI.22-02-00437.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hack MA, Saghatelyan A, de Chevigny A, et al. Neuronal fate determinants of adult olfactory bulb neurogenesis. Nat Neurosci. 2005;8:865–872. doi: 10.1038/nn1479. [DOI] [PubMed] [Google Scholar]
- 71.Diaz D, Recio JS, Baltanas FC, et al. Long-lasting changes in the anatomy of the olfactory bulb after ionizing irradiation and bone marrow transplantation. Neuroscience. 2011;173:190–205. doi: 10.1016/j.neuroscience.2010.10.082. [DOI] [PubMed] [Google Scholar]
- 72.Martoncikova M, Racekova E, Orendacova J. The number of proliferating cells in the rostral migratory stream of rat during the first postnatal month. Cell Mol Neurobiol. 2006;26:1453–1461. doi: 10.1007/s10571-006-9039-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hallbergson AF, Gnatenco C, Peterson DA. Neurogenesis and brain injury: managing a renewable resource for repair. J Clin Invest. 2003;112:1128–1133. doi: 10.1172/JCI20098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Catchpole T, Henkemeyer M. EphB2 tyrosine kinase-dependent forward signaling in migration of neuronal progenitors that populate and form a distinct region of the dentate niche. J Neurosci. 2011;31:11472–11483. doi: 10.1523/JNEUROSCI.6349-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Connor B, Gordon RJ, Jones KS, et al. Deviating from the well travelled path: precursor cell migration in the pathological adult mammalian brain. J Cell Biochem. 2011;112:1467–1474. doi: 10.1002/jcb.23086. [DOI] [PubMed] [Google Scholar]
- 76.Kaneko N, Marin O, Koike M, et al. New neurons clear the path of astrocytic processes for their rapid migration in the adult brain. Neuron. 2010;67:213–223. doi: 10.1016/j.neuron.2010.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nie K, Molnar Z, Szele FG. Proliferation but not migration is associated with blood vessels during development of the rostral migratory stream. Dev Neurosci. 2010;32:163–172. doi: 10.1159/000301135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang Y, Kaneko N, Asai N, et al. Girdin is an intrinsic regulator of neuroblast chain migration in the rostral migratory stream of the postnatal brain. J Neurosci. 2011;31:8109–8122. doi: 10.1523/JNEUROSCI.1130-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.