Abstract
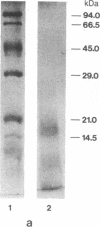
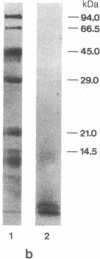
We have purified and characterized the cerebral amyloid protein that forms the plaque core in Alzheimer disease and in aged individuals with Down syndrome. The protein consists of multimeric aggregates of a polypeptide of about 40 residues (4 kDa). The amino acid composition, molecular mass, and NH2-terminal sequence of this amyloid protein are almost identical to those described for the amyloid deposited in the congophilic angiopathy of Alzheimer disease and Down syndrome, but the plaque core proteins have ragged NH2 termini. The shared 4-kDa subunit indicates a common origin for the amyloids of the plaque core and of the congophilic angiopathy. There are superficial resemblances between the solubility characteristics of the plaque core and some of the properties of scrapie infectivity, but there are no similarities in amino acid sequences between the plaque core and scrapie polypeptides.
Full text
PDF

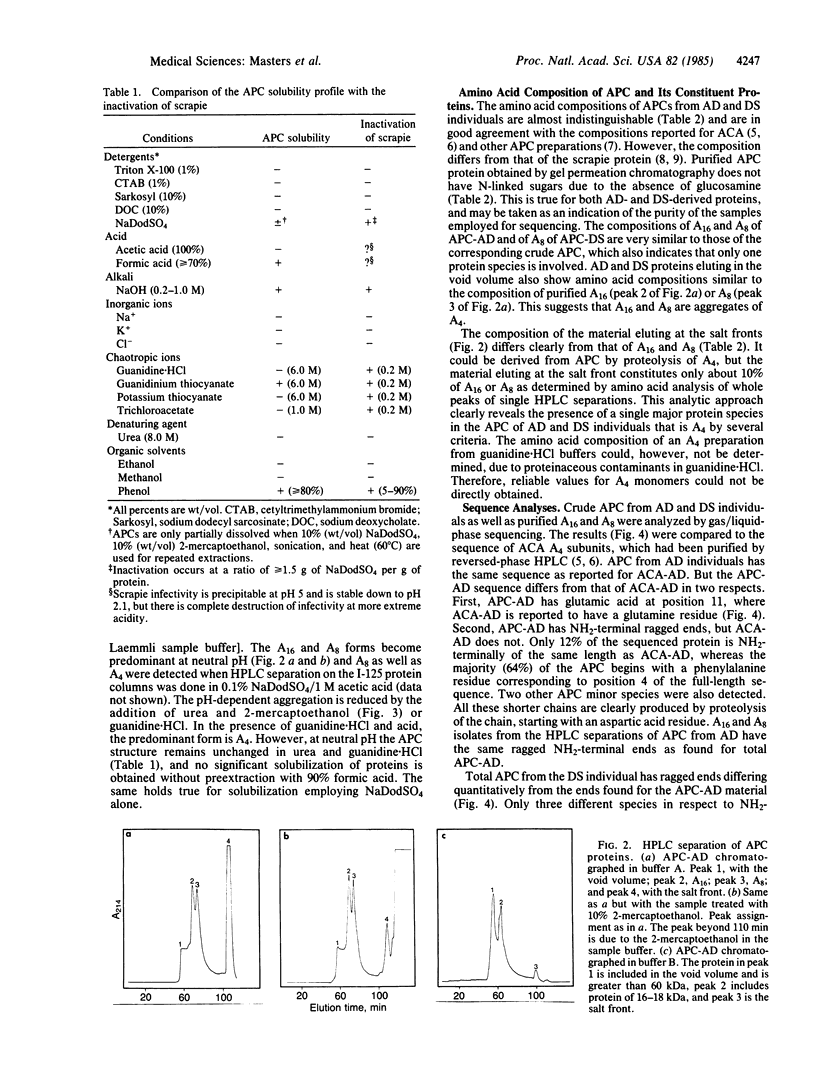

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allsop D., Landon M., Kidd M. The isolation and amino acid composition of senile plaque core protein. Brain Res. 1983 Jan 24;259(2):348–352. doi: 10.1016/0006-8993(83)91273-8. [DOI] [PubMed] [Google Scholar]
- Glenner G. G., Wong C. W. Alzheimer's disease and Down's syndrome: sharing of a unique cerebrovascular amyloid fibril protein. Biochem Biophys Res Commun. 1984 Aug 16;122(3):1131–1135. doi: 10.1016/0006-291x(84)91209-9. [DOI] [PubMed] [Google Scholar]
- Glenner G. G., Wong C. W. Alzheimer's disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Commun. 1984 May 16;120(3):885–890. doi: 10.1016/s0006-291x(84)80190-4. [DOI] [PubMed] [Google Scholar]
- Masters C. L., Gajdusek D. C., Gibbs C. J., Jr Creutzfeldt-Jakob disease virus isolations from the Gerstmann-Sträussler syndrome with an analysis of the various forms of amyloid plaque deposition in the virus-induced spongiform encephalopathies. Brain. 1981 Sep;104(3):559–588. doi: 10.1093/brain/104.3.559. [DOI] [PubMed] [Google Scholar]
- Merz P. A., Somerville R. A., Wisniewski H. M., Iqbal K. Abnormal fibrils from scrapie-infected brain. Acta Neuropathol. 1981;54(1):63–74. doi: 10.1007/BF00691333. [DOI] [PubMed] [Google Scholar]
- Prusiner S. B., Groth D. F., Bolton D. C., Kent S. B., Hood L. E. Purification and structural studies of a major scrapie prion protein. Cell. 1984 Aug;38(1):127–134. doi: 10.1016/0092-8674(84)90533-6. [DOI] [PubMed] [Google Scholar]
- Selkoe D. J., Ihara Y., Salazar F. J. Alzheimer's disease: insolubility of partially purified paired helical filaments in sodium dodecyl sulfate and urea. Science. 1982 Mar 5;215(4537):1243–1245. doi: 10.1126/science.6120571. [DOI] [PubMed] [Google Scholar]