Abstract
Current seasonal influenza vaccines have reduced immunogenicity and are of suboptimal efficacy in older adults. We have previously shown that the novel candidate vaccine MVA-NP+M1 is able to boost memory T cell responses in adults aged 50–85 years. Preclinical studies have demonstrated that viral vectored vaccines can act as adjuvants when coadministered with protein-based vaccines. We have conducted a phase I clinical trial to compare the coadministration of seasonal influenza vaccine and MVA-NP+M1 with seasonal influenza vaccine alone in adults aged 50 years and above. This combination of vaccines was safe and well tolerated. T cell responses to internal influenza proteins were boosted to significantly higher levels in the group receiving MVA-NP+M1 compared with the group receiving seasonal influenza vaccine alone. Rates of seroprotection and seroconversion against the three vaccine strains were similar in both groups; however, there was a significant increase in the geometric mean titer ratio for the H3N2 component of seasonal influenza vaccine in the coadministration group. While some vaccine combinations result in immune interference, the coadministration of MVA-NP+M1 alongside seasonal influenza vaccine is shown here to increase some influenza strain-specific antibody responses and boost memory T cells capable of recognizing a range of influenza A subtypes.
Introduction
Influenza is a globally important pathogen accounting for approximately 250,000–500,000 worldwide deaths per year.1 Vaccination programs are the most effective interventions available to reduce influenza-associated mortality and lessen the pressures exerted by influenza epidemics on healthcare systems and the economy. The trivalent-inactivated influenza vaccine (TIV), currently used to protect against seasonal epidemics, induces neutralizing antibodies to the influenza surface glycoproteins, hemagglutinin (HA), and neuraminidase.
Older adults are more likely to develop severe complications and require hospitalization following influenza infection and therefore represent a critical target population in vaccination campaigns. Unfortunately standard doses of TIV are less immunogenic in the elderly. A recent quantitative review found rates of seroprotection and seroconversion in adults ≥65 years to be 2–4 times lower (dependent on the strain) than the responses observed in younger adults.2 The lack of high-quality randomized controlled trial data means that the true rate of vaccine efficacy in the elderly is unknown;3,4 however, the largest randomized controlled trial suggested a far lower rate of vaccine efficacy in those aged 70 years and above when the results were stratified by age.5
Several strategies have been proposed to overcome the observed reduction in immunogenicity, including the administration of high-dose formulations of TIV,6 combining live and killed vaccine formulations,7 or the use of adjuvants. Adjuvants act in a nonspecific manner to enhance the specific immune response to an antigen.8 For influenza vaccines, oil-in-water adjuvants have been well studied, having been administered to more than 30 million individuals over the last 15 years.9 Such adjuvants enhance immunity through TLR-independent pathways and can induce higher titers of functional antibodies, produce greater antibody cross-reactivity, and permit antigen dose sparing.10
Replication defective viral vectors are highly effective tools for inducing immunity to vaccine antigens. Infected cells express high levels of correctly folded protein, which can then be released following apoptosis or necrosis.11 Viral vectored vaccines activate the innate immune system via multiple MyD88-dependent TLR signaling pathways and stimulate both humoral and cellular arms of the adaptive immune system.12,13
MVA-NP+M1 is a viral vectored vaccine comprising modified vaccinia virus Ankara (MVA), expressing a fusion protein of influenza A nucleoprotein (NP) and matrix protein 1 (M1).14 We have recently demonstrated it to be safe and highly immunogenic in a group of healthy adults aged 50–85 years.15 Because of the intrinsic adjuvant capacity of viral vectored vaccines, we hypothesized that the administration of MVA-NP+M1 alongside a HA protein-based vaccine may result in enhanced antibody responses to the protein antigens. The adjuvant effect of poxviral vectors in a murine model has been described previously for Hepatitis B surface antigen16 and more recently within our group for influenza in three distinct animal species.17
The coadministration of these two vaccines could potentially stimulate both high frequencies of cross-reactive influenza-specific T cells and increased antibody responses to influenza HA proteins. Here, we describe the results of a clinical trial comparing the safety and immunogenicity of vaccine coadministration or vaccination with TIV alone in adults aged 50 years and above.
Results
Demographics
There were no significant differences between the two treatment groups. Group 1 (MVA-NP+M1 and TIV) had a mean age of 63.8 years (SD = ±8.2 years) and group 2 (TIV and placebo) had a mean age of 59.6 years (SD = ±4.7 years). Group 1 comprised 44.4% female volunteers (four out of nine) versus 62.5% female volunteers (five out of eight) in group 2.
The safety and reactogenicity of vaccine coadministration
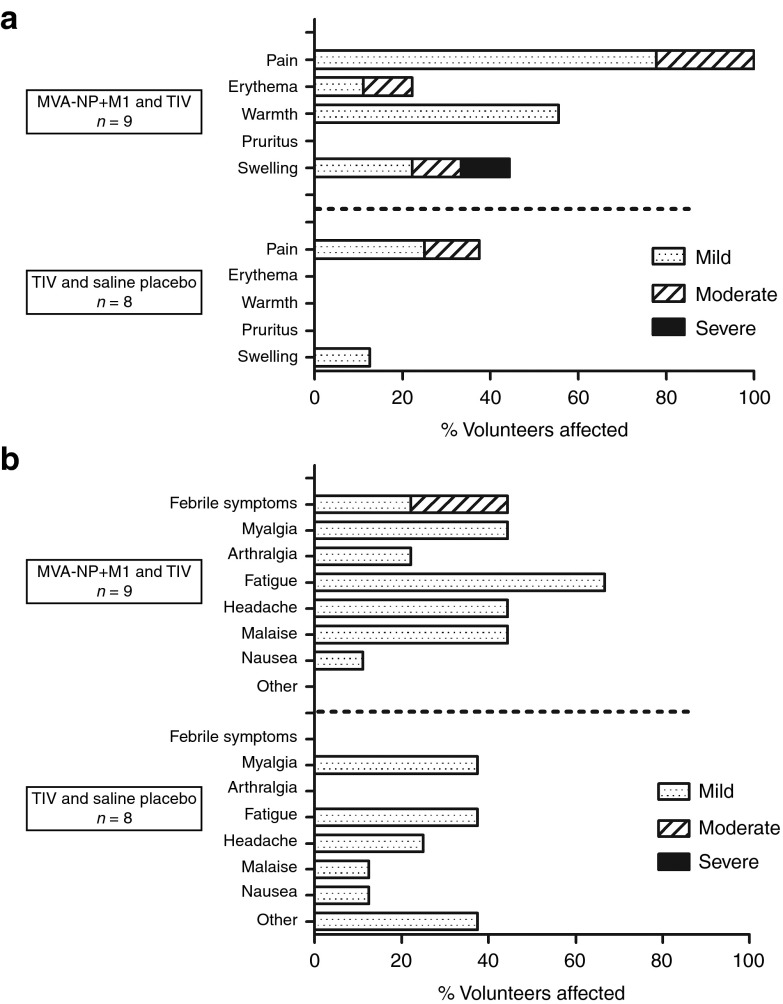
The coadministration of MVA-NP+M1 and TIV was well tolerated (Figure 1), with the majority of adverse events being mild in nature. One adverse event was classified as severe, and there were no serious clinical or laboratory adverse events.
Figure 1.
Vaccine safety and reactogenicity. The proportion of volunteers experiencing local (a) and systemic (b) adverse events. Only adverse events with possible, probable or definite causal relationships are shown.
Adverse events were reported more frequently in the group receiving the viral vectored vaccine in comparison with the control group. This observation was expected from the investigators brochure and summary of product characteristics from the two vaccines. The reactogenicity profile of vaccine coadministration was similar to that observed when the same dose of MVA-NP+M1 was given alone in a previous phase I study.15
T cell responses to NP and M1 are boosted to higher levels following vaccine coadministration
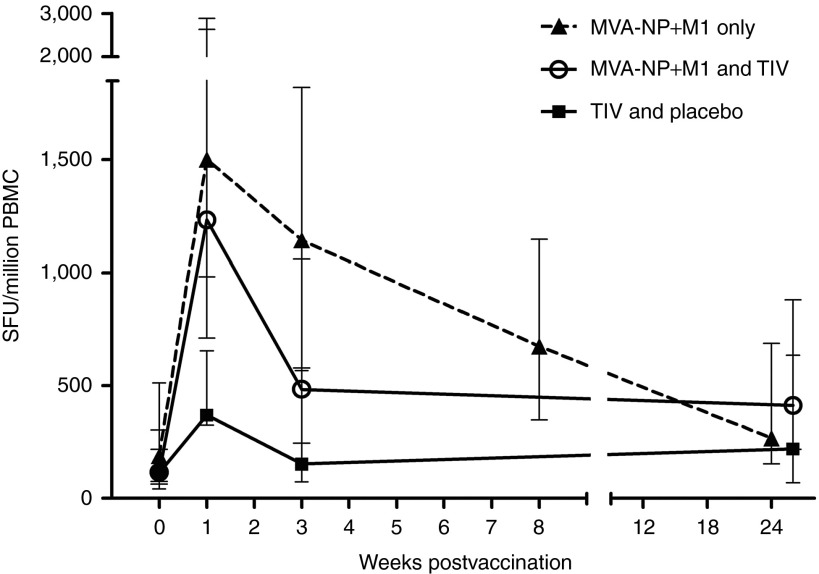
Median interferon-γ enzyme-linked immunosorbent spot (IFN-γ ELISpot) responses to NP and M1 antigens are shown in Figure 2. Prior to vaccination, these responses did not significantly differ between the coadministration and the control groups (115 vs 114 spot-forming units [SFU]). At 7-day postvaccination, median ELISpot responses had increased more than tenfold compared with baseline in the coadministration group (115 vs 1233 SFU, P = 0.008). These responses were also significantly higher than those observed 7 days after vaccination with TIV alone (1233 vs 369 SFU, P = 0.027).
Figure 2.
T cell responses to NP and M1 as determined by IFN-γ ELISpot. Median responses are shown with error bars indicating IQR. Volunteers in group 1 (open circles) demonstrate a greater peak T cell response than the volunteers in group 2 (closed squares). Historical data from 30 volunteers above the age of 50 receiving MVA-NP+M1 alone15 are also shown for comparison (dashed lines). This historical data had comparable population demographics (mean age = 65.8 years and proportion of females = 57%). IFN-γ ELISpot, interferon-γ enzyme-linked immunosorbent spot; IQR, interquartile range; M1, matrix protein 1; MVA, modified vaccinia virus Ankara; NP, nucleoprotein.15
At 3- and 26-week postvaccination, ELISpot responses in the coadministration group remained significantly raised in comparison with baseline (P = 0.027); however, the increases in these T cell responses relative to the control group do not reach statistical significance.
We then compared data with the results from a previous phase I study where MVA-NP+M1 had been given intramuscularly, at the same dose, to 30 individuals aged 50 years and above (Figure 2).15 The magnitude of ELISpot responses to NP and M1 in our coadministration group (compared with the MVA-NP+M1 only group) was not significantly different either at the peak of response 1-week postvaccination (P = 0.83) or at the final comparable time point 24–26-week postvaccination (P = 0.29).
Humoral immune responses measured by ELISA
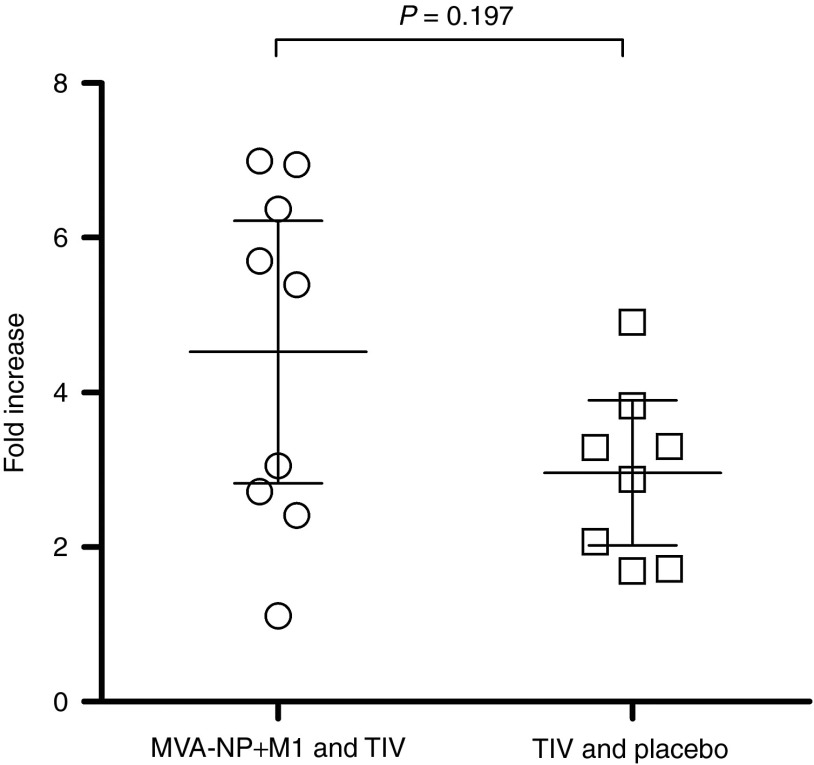
In preclinical studies of the coadministration of these vaccines, an anti-TIV enzyme-linked immunosorbent assay (ELISA) method was used to identify significant differences between experimental groups.17 We therefore used this method to evaluate antibody responses to TIV in our two groups, hypothesizing that the coadministration group would demonstrate higher responses if MVA-NP+M1 was acting as an adjuvant. Paired samples from day 0 and day 21 postvaccination were assayed in parallel for all subjects. We found that while the fold increase in anti-TIV ELISA units was indeed greater in the coadministration group, these differences were not statistically significant (Figure 3).
Figure 3.
Antibody responses to TIV as measured by anti-TIV ELISA. Lines with error bars indicate group means and 95% confidence intervals. Fold change in ELISA units in volunteers receiving TIV coadministered with MVA-NP+M1 (open circles) and volunteers receiving TIV and placebo (open squares). ELISA, enzyme-linked immunosorbent assay; M1, matrix protein 1; MVA, modified vaccinia virus Ankara; NP, nucleoprotein; TIV, trivalent-inactivated influenza vaccine.
Humoral immune responses measured by HAI assay
Antibody responses to the three vaccine strains of influenza were then measured by Hemagglutination inhibition (HAI) assay. In addition to the vaccine strains, we also determined hemagglutination inhibition (HI) titers to four drifted influenza strains and one H5N1 strain to examine the potential breadth of the humoral immune response in all volunteers.
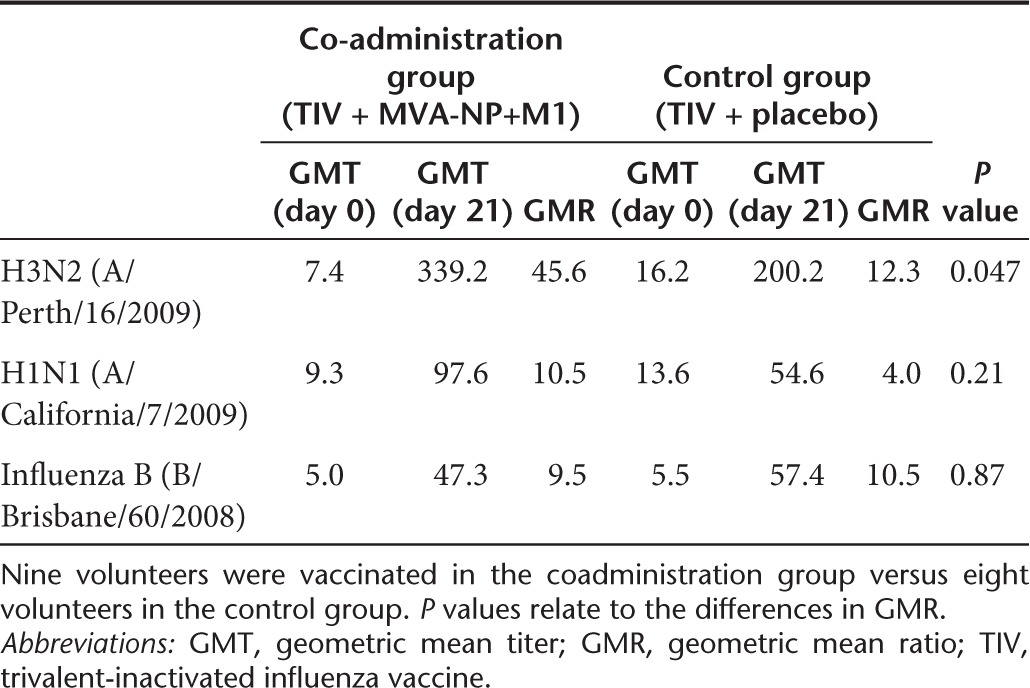
Geometric mean ratios (GMR) were calculated as the fold change in geometric mean titer (GMT) between baseline and 21-day postvaccination. A comparison of GMR is therefore able to account for baseline differences in GMT between the two groups. We found a significant increase in GMR for the vaccine strain of H3N2 in our coadministration group compared with the control group (Table 1). For the H1N1 strain, the GMR was more than doubled, but this difference did not reach statistical significance. In contrast for Influenza B and in the five nonvaccine strains tested differences between groups in GMR were generally small and nonsignificant (Table 1 and Supplementary Table S1). HI titer data expressed as either seroprotection (postvaccination HI titer ≥40) or seroconversion (fourfold increase in HI titer postvaccination) is shown in Supplementary Tables S2 and S3.
Table 1. HI titres for TIV strains of influenza.
Discussion
Here, we report that the coadministration of TIV and the novel candidate vaccine MVA-NP+M1 is both safe and immunogenic in adults aged 50 years and above. Age-related decline of the immune system, or immunosenescence, contributes to a heightened susceptibility to infectious disease as well as to the reduced efficacy of vaccines in the elderly. Indeed, approximately 90% of annual mortality from seasonal influenza infections is in individuals aged 65 years and above.18 Therefore, it is critical that we assess the potential for novel vaccines that improve immunogenicity in this target population cohort.
TIV provides protection against seasonal influenza by stimulating neutralizing antibodies to surface-exposed antigens (predominately HA). MVA-NP+M1 functions to boost T cell immunity to the internal influenza antigens NP and M1. By directing T cell memory toward internal and highly conserved influenza antigens, these vaccines have the potential to boost broadly heterosubtypic immunity and are therefore thought to have the potential to be “universal” influenza vaccines. It is well documented in the literature that vaccine-induced T cell responses to influenza are protective against influenza challenge in animal models.19 Furthermore, evidence from human influenza challenge studies demonstrates a negative correlation between influenza-specific T cell responses and influenza disease and virus shedding.20,21 In a preliminary assessment of the efficacy of MVA-NP+M1, vaccinees who developed influenza were shown to shed virus for fewer days.22
Ideally, influenza vaccines should combine approaches to induce both T cell- and B cell-mediated immunity. In this study, we have demonstrated the feasibility of such an approach and have shown that coadministration of MVA-NP+M1 with TIV can boost T cell responses to conserved influenza antigens as well as augment humoral immune responses to TIV. Although cooperativity between T cells recognizing internal influenza antigens and B cells recognizing HA has been reported,23 this requires the influenza antigens to be present on the same viral particle. This is not true of the split trivalent vaccine tested here, although could potentially result in a greater enhancement of the humoral response if an inactivated whole virion vaccine was to be used.
Increased reactogenicity is often a concern for coadministration vaccination regimes.24 However, we found the reactogenicity profile of MVA-NP+M1 when coadministered with TIV to be very similar to that observed when MVA-NP+M1 is administered alone.15 Minor increases to circulating influenza-specific T cells can be observed following TIV, most likely due to increases in the CD4+ T cell subset.25 We found that median NP- and M1-specific T cells as measured by IFN-γ ELISpot increased threefold following vaccination with TIV, compared with over 10-fold in the group also receiving MVA-NP+M1. The ELISpot assay does not allow the relative contribution of CD4+ and CD8+ T cells to be determined; however, previous studies have shown that MVA-NP+M1 stimulates both populations.15 Importantly, we found that the boosting effect of MVA-NP+M1 is not significantly impaired by coadministration with the seasonal influenza vaccine.
We measured serum antibody responses in volunteers both by ELISA and using the HAI assay. The latter method has the advantage of measuring functional antibodies capable of blocking binding of the HA protein to host receptors, rather than antibodies recognizing the denatured virus that may not be neutralizing. We observed a significant increase in GMR for the H3N2 strain and an increase in GMR for H1N1, but not for influenza B. Further studies will be required to establish whether or not such differences are found consistently; however, large studies have shown strain-specific variations in the ability of adjuvants to enhance immunogenicity.26 Indeed, Lopez et al.27 have observed a H3N2-specific increase in GMT when TIV was mixed with an MF59-adjuvanted H5N1 vaccine.
We attempted to determine the breadth of an antibody response by measuring HI titers to four drifted strains and one heterosubtypic strain of influenza. We were unable to show any significant increases in the breadth of responses; however, the sample size in this study may not have been large enough for underlying differences to become apparent. Alternative techniques for measuring antibody diversity such as phage display libraries or massively parallel gene sequencing28 may detect more subtle increases in diversity.
In this study, we coadministered MVA-NP+M1 with a trivalent influenza vaccine; however, this strategy could also be utilized in the context of monovalent pandemic influenza vaccine. Protein-based H5N1 vaccines are poorly immunogenic and require formulation with adjuvants to achieve adequate rates of seroconversion.29 We have already demonstrated the adjuvant effect of MVA-NP+M1 on recombinant H5HA vaccines in preclinical studies.17 The results presented here suggest that coadministration of MVA-NP+M1 with inactivated or recombinant H5 HA vaccines has the potential to increase humoral responses while simultaneously generating a population of influenza-specific T cells that could result in significantly improved vaccine efficacy in humans.
Materials and Methods
Study design and participants. Seventeen volunteers aged 50 years or above were enrolled into one of two groups, between November 2011 and May 2012. Group 1 received an intramuscular injection of TIV (0.5 ml) followed by an intramuscular injection of MVA-NP+M1 (1.15 ml) at a dose of 1.5 × 108 plaque forming units. Group 2 received an intramuscular injection of TIV followed by an intramuscular injection of 0.9% saline of equal volume (1.15 ml) to the MVA-NP+M1 administered in group 1. Vaccines were not mixed prior to administration, and they were delivered within a minute of each another.
Allocation of volunteers to the two groups was randomized, and volunteers were blinded as to which group they had been allocated to. The randomization list was generated using a computer program by independent colleagues at the Centre for Statistics in Medicine, University of Oxford, UK. Allocation results were placed into sequentially numbered opaque sealed envelopes, and only opened on the day of vaccination.
Volunteers were recruited and progressed according to a protocol approved within the UK by the Medicines and Healthcare products Regulatory Agency and the Regional Ethics Committee (www.clinicaltrials.gov, identifier: NCT00942071). All volunteers were healthy adults, resident in the Oxford area, with negative pre-vaccination tests for HIV antibodies, hepatitis B surface antigen and hepatitis C antibodies. Written informed consent was obtained in all cases and the trial was performed according to the principals of the Declaration of Helsinki.
MVA-NP+M1 vaccine. The vaccine was described previously14 and consists of MVA expressing the NP and M1 antigens from influenza A/Panama/2007/99 as a single fusion protein. The degree of sequence identity and divergence of NP and M1 from other influenza A viruses has also been previously described.14
TIV. Influenza vaccine (split viron) was manufactured by Sanofi Pasteur MSD (Lyon, France). Each 0.5 ml dose comprised 15 µg HA of H1N1 (A/California/7/2009), H3N2 (A/Perth/16/2009), and Influenza B (B/Brisbane/60/2008).
Procedures. On the day of enrolment, blood samples were taken before vaccination. In all cases, TIV was administered first, followed by either MVA-NP+M1 or the saline placebo. Coadministration took place in the same muscle with injection sites being approximately 1 cm apart.
Volunteers were contacted by telephone 2 days later to collect information regarding potential adverse events. Volunteers were reassessed and blood samples were taken at subsequent visits, which occurred at 1-, 3-, and 26-week postvaccination.
Interferon-γ ELISpot. Ex vivo IFN-γ ELISpot assays were performed by individuals blinded with regard to the volunteer group allocation. Samples were processed within 4 hours of blood samples being taken. Fresh peripheral blood mononuclear cells (PBMC) were washed and resuspended in R10 medium, which comprised RPMI 1640 containing 10% fetal calf serum, 100 IU/ml penicillin, 100 μg/ml streptomycin (all Sigma, Poole, UK), and 2 mmol/l L-glutamine (Life Technologies, Paisley, UK). For all samples peptides of 15–20 amino acids in length, overlapping by 10 amino acids and spanning the whole of the vaccine's NP+M1 insert, were used to stimulate PBMC at a final concentration of 10 μg/ml in eight pools of 10 peptides. R10 medium alone was used as a negative control, and a mixture of phytohemagglutinin (10 μg/ml) and staphylococcal enterotoxin B (1 μg/ml) was used as a positive control. Each condition was assayed in triplicate using 2 × 105 PBMC in a final volume of 100 μl per well. ELISpot plates were incubated for 18–20 hours at 37°C. Developed and dried ELISpot plates were counted with an AID ELISpot reader (AID Diagnostika, Strassberg, Germany). Results are expressed as SFU per million PBMC, calculated by subtracting the mean negative control response from the mean of each peptide pool response and then summing the response for the eight peptide pools. Plates were excluded if responses were >100 SFU/million PBMC in the R10 wells or <1,000 SFU/million PBMC in the phytohemagglutinin/staphylococcal enterotoxin B wells.
Standardized ELISA. To detect total IgG to TIV, NuncMaxisorp plates were coated at a concentration of 0.75 μg/ml TIV (Sanofi Pasteur MSD) and left overnight at 4°C. Lot numbers for the TIV were identical to those used in vaccination of the trial subjects. The following day, plates were washed 6× with PBS containing 0.05% Tween 20 (PBS/T). Plates were blocked with 100 µl/well of Casein block solution (Pierce) for 2 hours at room temperature followed by another wash step. Dilution series of serum samples (twofold from 1:1,000) were added to the plates for 2 hour at room temperature. A reference serum (comprising pooled samples from high-responding volunteers) was used to generate a standard curve. Volunteer sera from a previous clinical trial (and known to have low anti-TIV ELISA responses), was used as a negative control. Plates underwent a further wash step before the addition of the either alkaline phosphatase conjugated goat anti-human IgG (1:5,000, Sigma), for 1 hour at room temperature. Bound antibodies were detected by adding p-nitrophenylphosphate substrate (Sigma) diluted in diethanolamine buffer (Fisher Scientific). Plates were allowed to develop until the fifth dilution of standard reference serum (1:8,100) reached an OD405 of 1 using an ELx800 microplate reader (Biotek, Winooski, VT). This point was defined as 1 relative antibody unit and units were read off of the standard curve similar to published methodology.30 Samples were diluted to fall on the linear part of the standard curve. Fold changes were calculated to compensate for differences in baseline anti-TIV ELISA response.
HAI assays. HAI assays were performed at the laboratory of the Public Health England (London, UK) according to standardized methods.31 Those individuals performing the assays were blinded with regard to which vaccines the subjects had received. Briefly, serum samples obtained from subjects at day 0 and day 21 were tested using the following egg-grown viruses: H1N1-like virus (A/California/7/2009), H3N2-like virus (A/Perth/16/2009), and B-like virus (B/Brisbane/60/2008) viruses (as included in the trivalent inactivated vaccine composition for the Northern Hemisphere in 2011/12) as well as A/Brisbane/10/2007(H3N2), A/Victoria/36/2011(H3N2), B/Wisconsin/1/2010, A/Brisbane/59/2007(H1N1), and NIBRG23 (rgA/turkey/Turkey/1/2005 (H5N1)). Sera were treated prior to analysis with receptor destroying enzyme (RDEII, Denka Seiken Co Ltd, Japan) according to manufacturer's recommendation and tested in duplicate using twofold serial dilutions. Negative values (titers <10) were expressed as nominal values of 5, and the results for percentage seroprotection, seroconversion, and GMT were calculated and reported as per European Agency for the Evaluation of Medicinal Products guidelines.32
Statistical analysis. Statistical analysis was carried out using GraphPad Prism software version 5.04. The nonparametric Mann-Whitney U-test was used to test the significant differences between groups of volunteers, and the nonparametric Wilcoxon-signed rank test was used to test for significant differences between time points within the same group of volunteers. Fisher's exact test was used to compare group differences in seroconversion and seroprotection. HI titer data were log transformed and tested for normality with the Shapiro Wilk test. For normally distributed data, an analysis of covariance was performed on the log transformed data and adjusted for baseline value. Where data were not normally distributed, nonparametric tests were applied.
SUPPLEMENTARY MATERIAL Table S1. HI titer data for nonvaccine strains of influenza. Table S2. HI titers expressed as seroprotection. Table S3. HI titers expressed as seroconversion at day 21.
Acknowledgments
We acknowledge Rachel Roberts, Ian Poulton, Raquel Lopez Ramon, and Mary Smith (CCVTM, Oxford) for their assistance with management and conduct of the clinical trial. We acknowledge Claudia Rosenow, Surita Gangar, and Janice Baldevarona (Respiratory Virus Unit, PHE) for their participation in the laboratory analysis of the sera and Pooja Mange (The Jenner Institute, Oxford) for assistance with ELISpots and Nicola Williams (Centre for Statistics in Medicine, Oxford) for assistance with data analysis. R.D.A. is supported by the NIHR Biomedical Research Centre, Oxford. S.C.G and A.V.S.H are Jenner Investigators. S.C.G and A.V.S.H are named as inventors on patents relating to methods of vaccination, including influenza vaccines.
Supplementary Material
References
- World Health Organization 2009Influenza (seasonal) fact sheet. http://www.who.int/mediacentre/factsheets/fs211/en/
- Goodwin K, Viboud C, Simonsen L. Antibody response to influenza vaccination in the elderly: a quantitative review. Vaccine. 2006;24:1159–1169. doi: 10.1016/j.vaccine.2005.08.105. [DOI] [PubMed] [Google Scholar]
- Jefferson T, Di Pietrantonj C, Al-Ansary LA, Ferroni E, Thorning S, Thomas RE. Vaccines for preventing influenza in the elderly. Cochrane Database Syst Rev. 2010. p. CD004876. [DOI] [PubMed]
- Osterholm MT, Kelley NS, Sommer A, Belongia EA. Efficacy and effectiveness of influenza vaccines: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12:36–44. doi: 10.1016/S1473-3099(11)70295-X. [DOI] [PubMed] [Google Scholar]
- Simonsen L, Taylor RJ, Viboud C, Miller MA, Jackson LA. Mortality benefits of influenza vaccination in elderly people: an ongoing controversy. Lancet Infect Dis. 2007;7:658–666. doi: 10.1016/S1473-3099(07)70236-0. [DOI] [PubMed] [Google Scholar]
- Falsey AR, Treanor JJ, Tornieporth N, Capellan J, Gorse GJ. Randomized, double-blind controlled phase 3 trial comparing the immunogenicity of high-dose and standard-dose influenza vaccine in adults 65 years of age and older. J Infect Dis. 2009;200:172–180. doi: 10.1086/599790. [DOI] [PubMed] [Google Scholar]
- Treanor JJ, Mattison HR, Dumyati G, Yinnon A, Erb S, O'Brien D, et al. Protective efficacy of combined live intranasal and inactivated influenza A virus vaccines in the elderly. Ann Intern Med. 1992;117:625–633. doi: 10.7326/0003-4819-117-8-625. [DOI] [PubMed] [Google Scholar]
- Warren HS, Leclerc C.1998Adjuvants. Peter JD.ed). Encyclopedia of Immunology2nd edn. Elsevier: Oxford; 36–39. [Google Scholar]
- O'Hagan DT. MF59 is a safe and potent vaccine adjuvant that enhances protection against influenza virus infection. Expert Rev Vaccines. 2007;6:699–710. doi: 10.1586/14760584.6.5.699. [DOI] [PubMed] [Google Scholar]
- Dormitzer PR, Galli G, Castellino F, Golding H, Khurana S, Del Giudice G, et al. Influenza vaccine immunology. Immunol Rev. 2011;239:167–177. doi: 10.1111/j.1600-065X.2010.00974.x. [DOI] [PubMed] [Google Scholar]
- Lambe T. Novel viral vectored vaccines for the prevention of influenza. Mol Med. 2012;18:1153–1160. doi: 10.2119/molmed.2012.00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee EG, Blattman JN, Kasturi SP, Kelley RP, Kaufman DR, Lynch DM, et al. Multiple innate immune pathways contribute to the immunogenicity of recombinant adenovirus vaccine vectors. J Virol. 2011;85:315–323. doi: 10.1128/JVI.01597-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaloye J, Roger T, Steiner-Tardivel QG, Le Roy D, Knaup Reymond M, Akira S, et al. Innate immune sensing of modified vaccinia virus Ankara (MVA) is mediated by TLR2-TLR6, MDA-5 and the NALP3 inflammasome. PLoS Pathog. 2009;5:e1000480. doi: 10.1371/journal.ppat.1000480. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Berthoud TK, Hamill M, Lillie PJ, Hwenda L, Collins KA, Ewer KJ, et al. Potent CD8+ T-cell immunogenicity in humans of a novel heterosubtypic influenza A vaccine, MVA-NP+M1. Clin Infect Dis. 2011;52:1–7. doi: 10.1093/cid/ciq015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antrobus RD, Lillie PJ, Berthoud TK, Spencer AJ, McLaren JE, Ladell K, et al. A T cell-inducing influenza vaccine for the elderly: safety and immunogenicity of MVA-NP+M1 in adults aged over 50 years. PLoS ONE. 2012;7:e48322. doi: 10.1371/journal.pone.0048322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchings CL, Gilbert SC, Hill AV, Moore AC. Novel protein and poxvirus-based vaccine combinations for simultaneous induction of humoral and cell-mediated immunity. J Immunol. 2005;175:599–606. doi: 10.4049/jimmunol.175.1.599. [DOI] [PubMed] [Google Scholar]
- Mullarkey CE, Boyd A, van Laarhoven A, Lefevre EA, Veronica Carr B, Baratelli M, et al. Improved adjuvanting of seasonal influenza vaccines: Preclinical studies of MVA-NP+M1 coadministration with inactivated influenza vaccine. Eur J Immunol. 2013;43:1940–1952. doi: 10.1002/eji.201242922. [DOI] [PubMed] [Google Scholar]
- Thompson WW, Shay DK, Weintraub E, Brammer L, Cox N, Anderson LJ, et al. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA. 2003;289:179–186. doi: 10.1001/jama.289.2.179. [DOI] [PubMed] [Google Scholar]
- Ulmer JB, Donnelly JJ, Parker SE, Rhodes GH, Felgner PL, Dwarki VJ, et al. Heterologous protection against influenza by injection of DNA encoding a viral protein. Science. 1993;259:1745–1749. doi: 10.1126/science.8456302. [DOI] [PubMed] [Google Scholar]
- McMichael AJ, Gotch FM, Noble GR, Beare PA. Cytotoxic T-cell immunity to influenza. N Engl J Med. 1983;309:13–17. doi: 10.1056/NEJM198307073090103. [DOI] [PubMed] [Google Scholar]
- Wilkinson TM, Li CK, Chui CS, Huang AK, Perkins M, Liebner JC, et al. Preexisting influenza-specific CD4+ T cells correlate with disease protection against influenza challenge in humans. Nat Med. 2012;18:274–280. doi: 10.1038/nm.2612. [DOI] [PubMed] [Google Scholar]
- Lillie PJ, Berthoud TK, Powell TJ, Lambe T, Mullarkey C, Spencer AJ, et al. Preliminary assessment of the efficacy of a T-cell-based influenza vaccine, MVA-NP+M1, in humans. Clin Infect Dis. 2012;55:19–25. doi: 10.1093/cid/cis327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell SM, Liew FY. Cell cooperation in antibody responses to influenza virus. I. priming of helper t cells by internal components of virion. Eur J Immunol. 1980;10:791–796. doi: 10.1002/eji.1830101013. [DOI] [PubMed] [Google Scholar]
- Bar-On ES, Goldberg E, Hellmann S, Leibovici L. Combined DTP-HBV-HIB vaccine versus separately administered DTP-HBV and HIB vaccines for primary prevention of diphtheria, tetanus, pertussis, hepatitis B and Haemophilus influenzae B (HIB). Cochrane Database Syst Rev. 2012;4:CD005530. doi: 10.1002/14651858.CD005530.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WH, Cross AS, Edelman R, Sztein MB, Blackwelder WC, Pasetti MF. Antibody and Th1-type cell-mediated immune responses in elderly and young adults immunized with the standard or a high dose influenza vaccine. Vaccine. 2011;29:2865–2873. doi: 10.1016/j.vaccine.2011.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruf BR, Colberg K, Frick M, Preusche A. Open, randomized study to compare the immunogenicity and reactogenicity of an influenza split vaccine with an MF59-adjuvanted subunit vaccine and a virosome-based subunit vaccine in elderly. Infection. 2004;32:191–198. doi: 10.1007/s15010-004-3204-z. [DOI] [PubMed] [Google Scholar]
- Lopez P, Caicedo Y, Sierra A, Tilman S, Banzhoff A, Clemens R. Combined, concurrent, and sequential administration of seasonal influenza and MF59-adjuvanted A/H5N1 vaccines: a phase II randomized, controlled trial of immunogenicity and safety in healthy adults. J Infect Dis. 2011;203:1719–1728. doi: 10.1093/infdis/jir191. [DOI] [PubMed] [Google Scholar]
- Pollard AJ, Hill AV. Antibody repertoire: embracing diversity. Sci Transl Med. 2011;3:93ps32. doi: 10.1126/scitranslmed.3002694. [DOI] [PubMed] [Google Scholar]
- Nicholson KG, Colegate AE, Podda A, Stephenson I, Wood J, Ypma E, et al. Safety and antigenicity of non-adjuvanted and MF59-adjuvanted influenza A/Duck/Singapore/97 (H5N3) vaccine: a randomised trial of two potential vaccines against H5N1 influenza. Lancet. 2001;357:1937–1943. doi: 10.1016/S0140-6736(00)05066-2. [DOI] [PubMed] [Google Scholar]
- Miura K, Orcutt AC, Muratova OV, Miller LH, Saul A, Long CA. Development and characterization of a standardized ELISA including a reference serum on each plate to detect antibodies induced by experimental malaria vaccines. Vaccine. 2008;26:193–200. doi: 10.1016/j.vaccine.2007.10.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis JS, Zambon MC. Molecular analysis of an outbreak of influenza in the United Kingdom. Eur J Epidemiol. 1997;13:369–372. doi: 10.1023/A:1007391222905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The European Agency for the Evaluation of Medicinal Products 1997Note for guidance on harmonisation of requirements for influenza vaccines. http://www.ema.europa.eu/docs/en_GB/ document_library/Scientific_guideline/2009/09/WC500003945.pdf
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.