Abstract
The endoscopic finding of a gastric polyp and the histopathologic report that follows may leave clinicians with questions that have not been addressed in formal guidelines: do all polyps need to be excised, or can they just be sampled for biopsy? If so, which ones and how many should be sampled? What follow-up evaluation is needed, if any? This review relies on the existing literature and our collective experience to provide practical answers to these questions. Fundic gland polyps, now the most frequent gastric polyps in Western countries because of widespread use of proton pump inhibitors, and hyperplastic polyps, the second most common polyps notable for their association with gastritis and their low but important potential for harboring dysplastic or neoplastic foci, are discussed in greater detail. Adenomas have had their name changed to raised intraepithelial neoplasia and are decreasing in parallel with Helicobacter pylori infection; however, they do retain their importance as harbingers of gastric cancer, particularly in East Asia. Gastrointestinal stromal tumors have low incidence and no known associations, but their malignant potential is high; early diagnosis and proper management are crucial. Although rare and benign, inflammatory fibroid polyps need to recognized, particularly by pathologists, to avoid misdiagnosis. Gastric neuroendocrine tumors (carcinoids) are important because of their association with either atrophic gastritis or the multiple endocrine neoplasia syndromes; those that do not arise in these backgrounds have high malignant potential and require aggressive management. The review concludes with some practical suggestions on how to approach gastric polyps detected at endoscopy.
Keywords: Gastric Polyps, Endoscopic Management
Agastric polyp is an abnormal growth of tissue projecting from the gastric mucosal membrane. Encountering a polyp in the stomach prompts concerns regarding its histology, cause, natural history, and whether specific therapy is required. During the past few decades, North America and much of the world have experienced a marked decrease of Helicobacter pylori–related gastroduodenal diseases; during the same period, the use of proton pump inhibitors (PPIs) has become widespread. Furthermore, the indications for esophagogastroduodenoscopy (EGD) have undergone a shift, with a greater emphasis on the evaluation of gastroesophageal reflux disease and the prevention of esophageal adenocarcinoma related to Barrett’s esophagus. As a result of these new paradigms, the findings encountered at EGD have changed substantially.
In North America and the industrialized West these changes have affected both the incidence and the types of gastric polyps. The overall incidence of polyps appears to have increased, as indicated by a higher prevalence in large series.1 There also has been a shift in the relative proportion of the different types of polyps: the clinically inconsequential fundic gland polyps have become the dominant type, while growths traditionally associated with H pylori gastritis (eg, hyperplastic and adenomatous polyps) have become less common. In contrast, in East Asian, Latin American, and possibly African populations, where H pylori infection and chronic gastritis remain common, larger proportions of gastric polyps are related to the underlying inflammatory process and are either hyperplastic or neoplastic. Despite these geographic differences, the finding of gastric polyps, particularly when numerous, will make clinicians in all regions face similar quandaries: which polyps need to be excised? Which ones and how many should be sampled for histologic evaluation? Also, what follow-up evaluation is needed?
This review attempts to provide practical answers to these questions. Although it relies largely on prevalence data derived from North American and European populations, its recommendations regarding natural history, clinical approach, and follow-up evaluation are based on the natural history of each type of polyp, which is determined largely by its histology and the gastric mucosal background on which it arises. Such features are independent of prevalence and, therefore, have universal validity.
Polyps that reveal a malignancy upon histopathologic examination lose their polyp status, irrespective of their initial endoscopic appearance, and we have excluded them from this review. Furthermore, because it is impossible to be simultaneously practical and comprehensive, we also had to neglect lesions (eg, lipomas, heterotopias, and leiomyomas) because they are unlikely to cause clinical dilemmas.
Fundic Gland Polyps
Fundic gland polyps are the most common type of polyps detected at EGD in Western countries. In a large recent pathologic study, fundic gland polyps were diagnosed in approximately 6% of patients who had an EGD and represented 74% of all gastric polyps submitted for histopathologic evaluation. 1 Endoscopically, fundic gland polyps are usually multiple, small (<1 cm), and appear smooth, glassy, and sessile. By narrow band imaging they have a honeycomb appearance with dense vasculature, a nonspecific pattern that also can be seen in hyperplastic polyps.2
When first discovered, fundic gland polyps were believed to be hamartomatous.3 However, their association with PPI use, confirmed in a number of studies, suggests that mechanisms related to the suppression of acid secretion by proton pump inhibition may be involved in their pathogenesis.4, 5
Histopathologic Features and Diagnostic Criteria
Histologically, fundic gland polyps consist of one or more dilated oxyntic glands, lined by flattened parietal and mucous cells (Figure 1). Fundic gland polyps are among the most characteristic lesions of the stomach: the recognition of the dilated oxyntic glands with flattened parietal and mucous cells in slides stained with H&E is immediate and unequivocal (Figure 1B and C). One caveat is that when the surface of a polyp is eroded the regenerative appearance may be misinterpreted as dysplasia. True dysplasia, particularly high grade, is exceedingly rare and is virtually limited to fundic gland polyps found in patients with polyposis syndromes. No special stains or molecular studies are warranted.
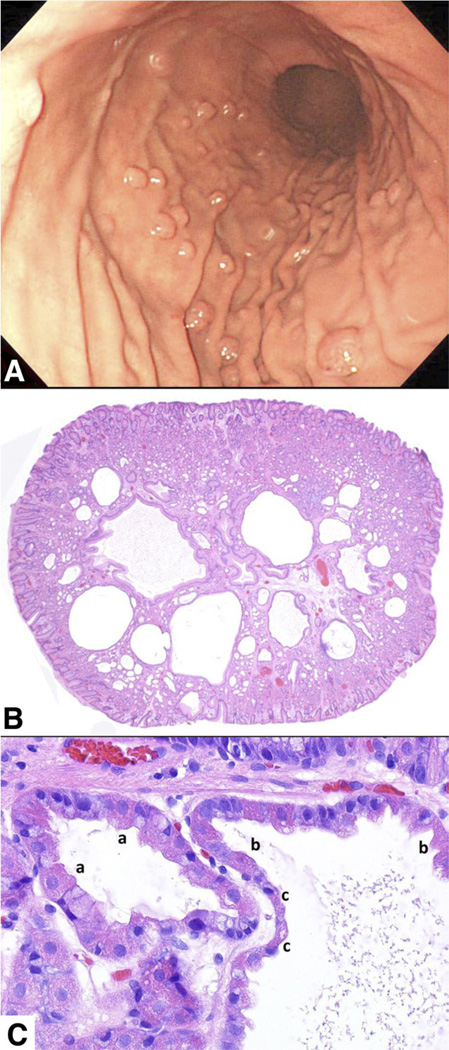
Figure 1.
Fundic gland polyp. (A) Endoscopic view of multiple fundic gland polyps in the body of the stomach in a patient taking PPIs. (B) Low-power photomicrograph showing the characteristic dilatations of oxyntic glands. There is only minimal stroma, and the surface foveolar epithelium is either normal or focally flattened. (C) High-power photomicrograph showing a dilated oxyntic gland lined by cuboidal parietal cells (a); as the gland dilate more (right), both mucous and parietal cells progressively flatten (b and c).
Clinical Approach
The finding of multiple characteristic polyps in the oxyntic portion of the stomach in a patient taking PPIs is essentially diagnostic of fundic gland polyps. Generally, when first encountered, one or more representative polyps should undergo a biopsy examination to confirm the diagnosis. Large polyps (>1 cm in diameter) should be removed entirely to confirm the diagnosis because fundic gland polyps rarely exceed this size.6 A biopsy specimen from the polyp in these cases is not adequate because if the polyp is not of the fundic gland type, biopsy sampling may not include crucial areas of possible dysplasia or neoplasia. In addition, a thorough visual inspection of the remaining polyps should be made; any lesion that appears significantly different from the others should be undergo a biopsy examination, or, if possible, be removed. Specifically, size larger than 1 cm, ulceration, and unusual location such as the antrum should prompt a more aggressive approach. When fundic gland polyps are found in a young patient, especially if numerous (eg, ≥20), the possibility of a polyposis syndrome should be considered. Individuals with familial polyposis syndromes typically are younger than the average patient with fundic gland polyps (mean age, 40 y),7–9 and occasionally have polyps in the antrum.10 When gastric polyps are associated with duodenal adenomas a familial polyposis syndrome strongly should be considered and colonoscopy should be recommended.
Fundic gland polyps rarely are found in stomachs affected by H pylori infection and, therefore, in the absence of a familial polyposis syndrome, concerns about gastric cancer are moot.11 Nonetheless, when polyps are innumerable or large (>1 cm) there may be cause for concern regarding eventual outcome. Although no guidelines exist, we suggest that when either more than 20 polyps are present or their size is larger than 1 cm one should consider reducing or preferably stopping the medication to assess whether this will result in regression of the polyps.12 If regression occurs, it is unknown whether PPIs can be reinstituted. Practically, if surgical therapy is not an option, one might consider a different PPI and at the minimally effective dose. Although there does not seem to be a correlation between serum gastrin levels and the presence of fundic gland polyps, it may be worthwhile to measure gastrin levels in these patients.13 A high level (>400 pg/mL) suggests profound acid suppression. If the patient is not an intrinsic hypersecretor and does not have a gastrinoma or Zollinger–Ellison syndrome, PPIs should be withdrawn, and, if gastroesophageal reflux disease symptoms persist, replaced with an H2-receptor antagonist. Recent reports of gastric carcinoids associated with profound PPI-induced acid suppression14, 15 and the increasing awareness of other potential adverse effects (eg, interference with the absorption of a variety of other medications, possible hip fracture, and Clostridium difficile infection) have provided further impetus to using PPIs less often and at the minimal effective dose.15–22
Hyperplastic Polyps
Hyperplastic polyps are inflammatory proliferations of the gastric foveolar cells (the mucin-producing epithelial cells that line the gastric surface and the gastric pits). When the inflammatory infiltrates are prominent, they may be referred to as inflammatory polyps. When hyperproliferation of foveolar cells is the most salient characteristic, gastric pits (also known as foveolae, from the Latin word for “small pit”) become elongated, tortuous, and generate elevations of the mucosa; these lesions are known as polypoid foveolar hyperplasia. In patients with post-Billroth I and II gastric stumps the gastric mucosa adjacent to the anastomosis continuously is exposed to bile reflux and significant degrees of foveolar hyperplasia may occur. In some cases there also may be cystic dilatation of the foveolae, with a resulting polypoid lesion consisting of cysts, tortuous pits, and often an eroded surface epithelium. This is sometimes referred to as gastritis cystica polyposa. Because all these are expressions of the same basic lesion we suggest that confusion be avoided by referring to all variants as hyperplastic polyps.
The classic association of gastric hyperplastic polyps has been with mucosal atrophy, whether caused by H pylori infection or autoimmune gastritis.23, 24 However, in recent years we have seen an increase in the proportion of such polyps in the background of a normal or reactive gastric mucosa with no evidence of current or prior H pylori infection. In Western countries, hyperplastic polyps have slipped from being the most common type of gastric polyp encountered at endoscopy to less than 20% of them.1 Hyperplastic polyps are equally common in men and women and typically occur in the sixth and seventh decades (median age, 66 y). Endoscopically, they are found most frequently in the antrum and often are multiple. They are usually smooth, dome-shaped, and measure between 0.5 and 1.5 cm in diameter (Figure 2), although they may be much larger. Large hyperplastic polyps often become lobulated and pedunculated,25 and the surface epithelium typically is eroded (Figure 2), which may result in chronic blood loss and irondeficiency anemia. Rarely, patients with large hyperplastic polyps present with gastric outlet obstruction because the polyp may obstruct or prolapse through the pylorus.26, 27 Hyperplastic polyps are believed to arise as a hyperproliferative response to tissue injury (erosions or ulcers) accompanied by increased cellular exfoliation.28 The resulting foveolar hyperplasia, long recognized as a prominent feature in chemical gastropathy and, to a lesser extent in H pylori gastritis, may be the initial step in their genesis. This could explain why they increasingly are seen arising in a background of reactive gastropathy.29
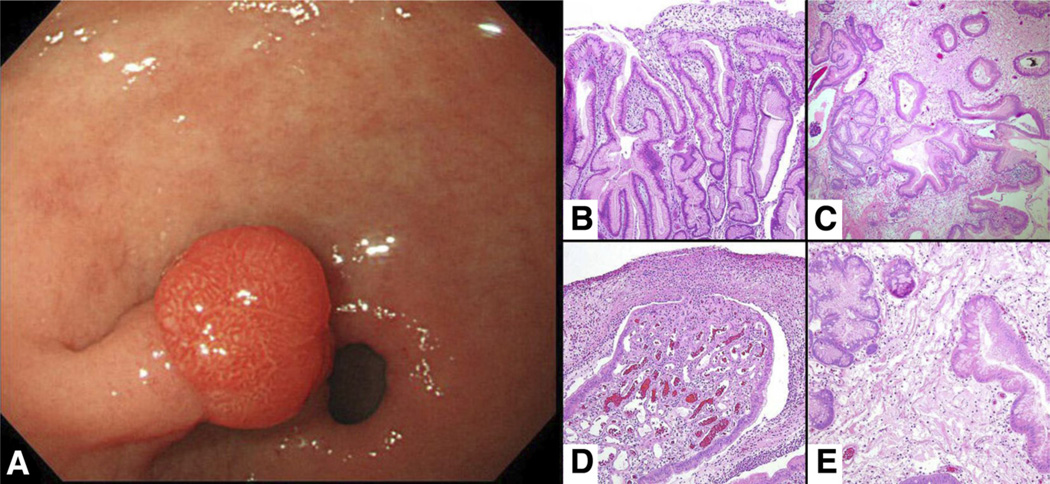
Figure 2.
Hyperplastic polyp. (A) Endoscopic view of a hyperplastic polyp on a stalk in the antrum. Histologically, (B) hyperplastic polyps are characterized by marked foveolar hyperplasia, and (C) a mixoid stroma are characterized with dilated tortuous glands lined by normal or reactive foveolar epithelium. (D) Larger polyps have prominent erosions covered with fibrinopurulent material with underlying granulation tissue, (E) often with areas of edematous stroma and oddly shaped glands.
Histopathologic Features and Diagnostic Criteria
The typical features of hyperplastic polyps include elongated, grossly distorted, branching, and dilated hyperplastic foveolae lying in an edematous stroma rich in vasculature, and small haphazardly distributed smooth muscle bundles with varying degrees of chronic and active inflammation (Figure 2). Between 1% and 20% of hyperplastic polyps have been reported to harbor foci of dysplasia (Figure 3). This wide range is more likely a reflection of the different criteria used in the assessment of dysplasia than because of real geographic or biological variability. Mutations of the p53 gene, chromosomal aberrations, and microsatellite instability all have been detected in these polyps.30–32 The overall prevalence of carcinoma in hyperplastic polyps is less than 2%, and it is more frequent in polyps larger than 2 cm.33–35
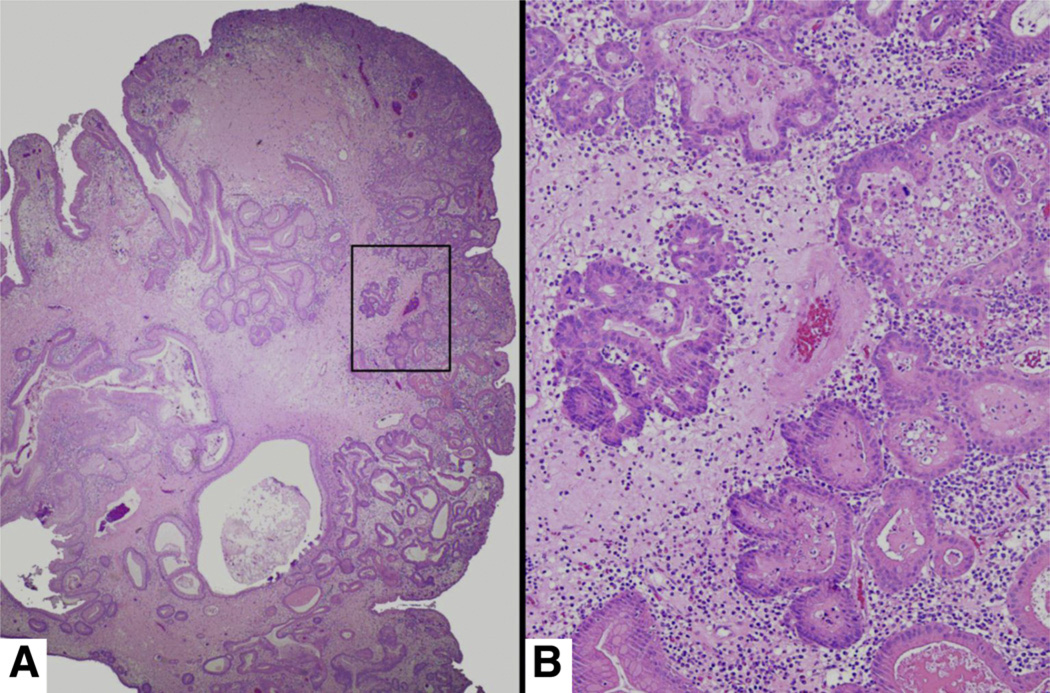
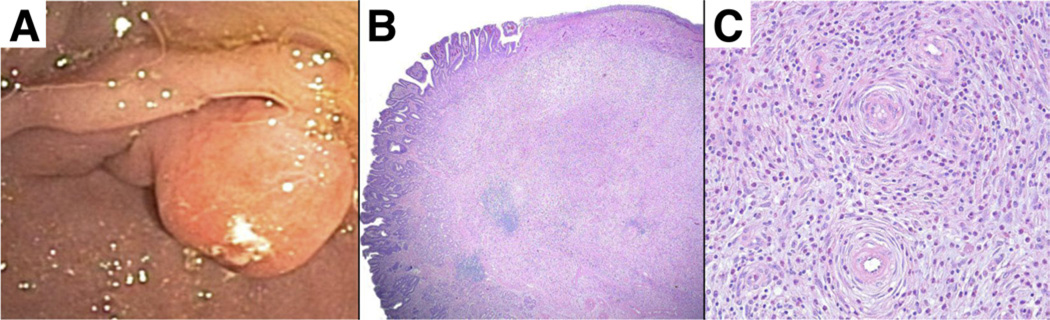
Figure 3.
(A) Hyperplastic polyp with focus of high-grade dysplasia. (B) Multiple sections from this 3-cm hyperplastic polyp revealed an area with dysplastic epithelial cells forming complex glandular structures. These represent high-grade dysplasia or possibly a focus of intramucosal carcinoma.
When foci of dysplasia or carcinoma are diagnosed within a hyperplastic polyp, clinicians often inquire whether the lesion was in fact an adenoma or a carcinoma erroneously identified as a hyperplastic polyp. This is almost never the case. Adenomas (as detailed later) consist entirely of dysplastic epithelium; in contrast, hyperplastic polyps with dysplastic foci are inflammatory lesions formed by long distorted branching, and dilated hyperplastic foveolae lying in a vascular edematous stroma. Finding foci of dysplastic (ie, neoplastic) epithelium within these lesions does not call for a reconsideration of their original pathogenesis or classification, although it does change their management.
The histopathologic diagnosis rests on the detection of the earlier-mentioned features in traditional H&E-stained slides. Because erosions, ulcerations, and inflammation, even extensive, are expected features of these polyps, pathologists are encouraged to avoid adding the word “inflammatory” to the diagnostic line. This invariably results in calls from puzzled clinicians who want to know whether hyperplastic and inflammatory gastric polyps are different or, as one put it, one and the same. They are one and the same.
When inflammatory polyps measure less than 1 cm, the routine examination of representative sections from each polyp is considered adequate. Larger polyps (>1 cm) may harbor dysplasia and even carcinoma. Therefore, they should be sectioned in 2- to 3-mm slices, and sections should be prepared from each slice. This practice will allow the detection of otherwise easily missed dysplastic or neoplastic lesions (Figure 3). Outside the research arena, no special stains or molecular studies are necessary. If no specimens from other parts of the stomach are included, an immunohistochemical stain for H pylori can be helpful.
Clinical Approach
In view of the potential cancer risk, all hyperplastic polyps larger than 1 cm should be excised completely. If dysplasia or intramucosal carcinoma is found, but the stalk is not affected, the lesion can be considered completely removed and most likely cured.36 The excision of the polypoid lesion always should be accompanied by additional sampling of the unaffected mucosa to obtain reliable information about the topography and severity of the background gastritis and atrophy.
When hyperplastic polyps arise in a background of chronic atrophic gastritis (a precursor lesion for gastric adenocarcinoma) the severity and extent of the atrophic gastritis should be evaluated. Risk stratification for gastric cancer can be assessed histologically using the Operative Link for Gastritis Assessment (OLGA) or the Operative Link on Gastritis/Intestinal Metaplasia Assessment staging systems.37, 38 Both require histologic grading of adequate samples from the antrum and corpus; grades then are combined to provide a risk stratification category. One can use the 5-biopsy specimen protocol recommended by the updated Sydney system.39 We prefer an extended 7-biopsy protocol consisting of 3 specimens from the antrum (which can be submitted in a single formalin container), 2 from the lesser curvature of the corpus, and 2 from the greater curvature of the corpus, with each set in a separate container.40 The topographic separation of specimens from the antrum and corpus is crucial because pseudopyloric metaplasia (a feature of corpus atrophy) mimics the antral mucosa histologically. Although it has not been established whether the additional information obtained by this extended sampling protocol substantially improves risk assessment, we prefer it because it allows a more detailed evaluation of the extent and distribution of atrophy.
If present, H pylori should be eradicated and an endoscopic follow-up evaluation should be scheduled between 3 and 6 months after therapy to confirm successful eradication. Alternatively, a noninvasive test such as the urea breath test may be used.41 In many instances any remaining small hyperplastic polyps will have regressed or disappeared.42, 43 Patients with OLGA stages III and IV (moderate diffuse atrophy or severe atrophy in either the corpus or antrum, usually accompanied by extensive intestinal metaplasia) should be considered for longterm endoscopic surveillance. The best intervals for such surveillance are unclear (eg, annual, bi-annual, or some other interval) as is the number of years for which it should be continued. No evidence-based guidelines exist; therefore, local recommendations should be followed when available.
Gastric Adenomas (Raised Intraepithelial Neoplasia)
The most common gastric neoplastic polyp is an epithelial dysplastic growth still commonly referred to as an adenoma, despite the new nomenclature (raised intraepithelial neoplasia) suggested by the World Health Organization.44, 45 In the Western industrialized world, H pylori–related sporadic gastric adenomas have become rare, accounting for less than 1% of all gastric polyps. This contrasts markedly with some East Asian regions, where the incidence of gastric cancer remains high and gastric adenomas still constitute approximately a quarter of all gastric polyps.46, 47 Similar to hyperplastic polyps, gastric adenomas occur with similar frequency in men and women, most commonly in the sixth and seventh decades. Endoscopically, they have a velvety lobulated appearance and are usually solitary (Figure 4). Although they can be found anywhere in the stomach, they are located more often in the antrum. The narrow band imaging features of gastric adenomas are not yet well defined.2
Figure 4.
Adenoma. (A) Flat gastric adenoma with a velvety appearance in the distal body of the stomach. (B) Gastric adenomas consist of dysplastic columnar epithelium indistinguishable from colonic adenoma. In resected specimens, the only clue to their gastric origin is often a small remnant of gastric tissue from which they originate (arrow).
Gastric adenomas consist of dysplastic epithelial cells that often arise in a background of atrophy and intestinal metaplasia typically associated with H pylori infection. As in the colon, gastric adenomas can be viewed as part of a sequence leading from dysplasia to carcinoma. The larger an adenomatous polyp, the greater the probability it contains foci of adenocarcinoma. Synchronous adenocarcinomas, in other areas of the stomach, have been reported in up to 30% of patients with adenomas containing foci of adenocarcinoma.48–50
Clinical Approach
Gastric adenomas frequently arise in a background of chronic atrophic gastritis. Because this is a precursor lesion for gastric adenocarcinoma, in addition to completely excising all adenomas, the severity and extent of the atrophic gastritis should be evaluated. The same biopsy protocols39, 40 and the use of the OLGA or Operative Link on Gastritis/Intestinal Metaplasia Assessment system suggested in the section “Hyperplastic Polyps” should be followed.37, 38 It must be emphasized that adenomas are neoplastic lesions (ie, past the stage of preneoplastic) and, therefore, all patients with a diagnosis of gastric adenoma need to be placed in a surveillance program irrespective of their atrophy stage. Eradication of H pylori followed by confirmation of the cure by biopsy examination or urea breath test is necessary in these patients.
Histopathologic Diagnostic Tips: Special Stains, Immunohistochemistry, and Molecular Studies
Gastric adenomas are neoplastic lesions with malignant potential. Therefore, multiple sections from each lesion must be examined to exclude invasion. Outside the research arena, neither special stains nor molecular studies are necessary.
Gastrointestinal Stromal Tumors
Gastrointestinal stromal tumors (GISTs) are neoplastic proliferations of the interstitial cells of Cajal (or their precursors) that can arise in any segment of the digestive tract as well as, rarely, in the abdominal and pelvic cavity.51 Of the estimated 4000 GISTs newly diagnosed each year in the United States,52–55 40% to 60% originate in the stomach, where they represent approximately 2% of all tumors.56 GISTs are more common in men and in the gastric fundus, although they can be found in other regions of the stomach.57 No predisposing factors are known; thus, the gastric mucosa overlying these tumors may be normal or display any type of gastritis. Interestingly, microscopic GISTs have been found to be common in the upper stomach of Japanese patients who underwent gastric resections for gastric cancer, suggesting that only infrequently does a GIST enlarge and develop malignant potential.58
Endoscopically, GISTs are well-circumscribed submucosal lesions; the overlying gastric mucosa is usually normal, but it may have an eroded or ulcerated center (Figure 5A). Biopsy sampling of these tumors is often met with frustration. Because the mucosa tends to slide over benign submucosal tumors, the biopsy forceps often fail to grab an adequate fragment of GIST tissue. In these cases the pathology report often will state that the gastric mucosa is normal, sometimes inducing the clinician to believe that either there was no lesion or the quality of the pathology report was suboptimal. Therefore, the best way to obtain diagnostic tissue is to perform an endosonographic fine-needle aspiration or a tru-cut needle biopsy.
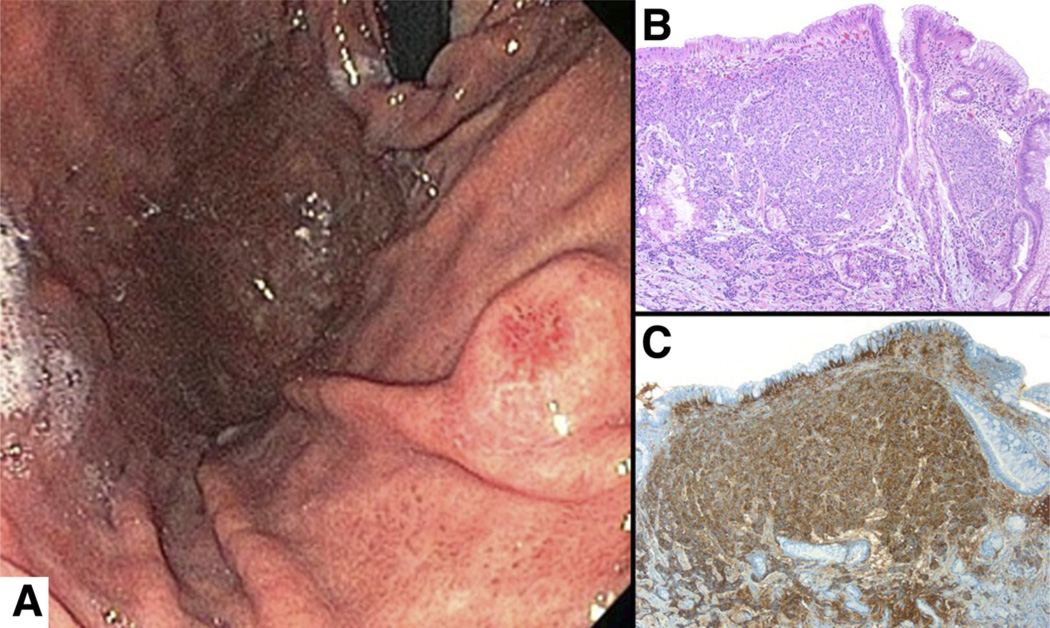
Figure 5.
GIST. (A) Endoscopic view of an ulcerated submucosal mass in the body of the stomach in a patient who presented with upper-gastrointestinal bleeding. (B) The stroma consists of compact bundles of spindle cells, (C) which stain uniformly with CD117. Staining with DOG-1 (not shown) would have an identical appearance to the staining in panel B.
Histologically, GISTs are composed by dense aggregates of fusiform cells (spindle cells) arranged in bundles aligned in different directions (Figure 5B). Two main types of GISTs are recognized in the stomach (spindled and epithelioid), and within each type a number of histologic characteristics (presence of perinuclear vacuoles, numbers of mitoses, necrosis, and tissue invasion) help predict their behavior.57
Clinical Approach
The vast majority of GISTs that measure less than 1 cm are asymptomatic and are detected incidentally during an endoscopic examination scheduled for other indications. As GISTs grow, they may cause erosions or ulcerations in the overlaying mucosa or compress adjacent structures; consequently, the 2 more common manifestations are bleeding (occult or overt) and pain.
All GISTs must be considered as having malignant potential: up to 50% of patients with larger GISTs (>2 cm) have metastatic disease at presentation, usually to the liver.52 In practice, there is good correlation between size, mitotic activity, and clinical behavior of GISTs. Surgical resection is recommended for lesions greater than 2 cm; endoscopic enucleation followed by surveillance is an option for smaller GISTs. Endoscopic removal is controversial, however, because of reports of positive resection margins and tumor spillage.55 Tyrosine kinase inhibitors are used as targeted therapy in cases of metastasis and surgically unresectable GISTs.59 Neoadjuvant therapy with use of tyrosine kinase inhibitors after surgical resection of high-risk GISTs deters recurrence, but the optimal duration of therapy has not been determined.60
Histopathologic Diagnostic Tips: Special Stains, Immunohistochemistry, and Molecular Studies
Immunohistochemical stains are central to the diagnosis of GISTs. The c-kit proto-oncogene mutation (most often occurring in exon 11) is the key molecular event in gastric GISTs and can be detected by an immunohistochemical stain directed against the KIT protein. The antibody used for the staining, designated as CD117, stains approximately 95% of GISTs (Figure 5C); the remaining 5% (which typically have a more epithelioid morphology) stain with antibodies to the DOG-1 or platelet-derived growth factor α.56, 57, 61 If none of these 3 stains is positive on a tumor with morphologic characteristics suggestive of GIST, tumors of smooth muscle (leiomyomas) or neural (neuromas, schwannomas) origin should be considered and immunostaining for actin and S-100 should be performed.
Predictors of behavior that pathologists must evaluate in a GIST include tumor size and mitotic counts. In general, it can be stated that the larger a tumor the greater the likelihood that it has metastasized. Figures most often cited indicate that 15% of GISTs smaller than 2.5 cm metastasize, in contrast to more than 80% of GISTs larger than 6 cm.57 These data, reproduced in many subsequent studies, emphasize the malignant potential of even small GISTs. Most studies have found that higher mitotic counts are associated with decreased survival; however, the way mitotic counts traditionally are reported (per high-power field) are imprecise, not standardized, and subject to many technical and interpretational variables. Only unequivocally high mitotic counts (eg, >5 mitoses/50 high-power field) obtained under rigorously controlled conditions (details of the methods used to find and count 50 separate fields) and precise information on the exact area of a high-power field (which varies 3-fold in different types of commonly used microscopes) can be used to make a prognostic assessment.61 Therefore, when clinicians receive a report indicating a high mitotic count in a GIST, they should interpret it cautiously and question the pathologist about the methods used to reach that conclusion.
Inflammatory Fibroid Polyps
Inflammatory fibroid polyps (also known as Vanek tumors) are extremely rare lesions that represent less than 0.1% of all gastric polyps.62 Endoscopically, they are usually firm, solitary, sessile or pedunculated, and often ulcerated (Figure 6). The histologic features of these polyps are distinctive: they consist of submucosal proliferations of spindle cells, small vessels, and a conspicuous inflammatory infiltrate with a predominance of eosinophils. Hence, these polyps are occasionally (and inaccurately) referred to as eosinophilic granulomas. The adjacent mucosa usually is unremarkable. The pathogenesis of these lesions is unknown, although a familial tendency has been documented in one family.63 Immunohistochemical staining suggests these polyps have a dendritic cell origin.64 A recent study found that 70% of inflammatory fibroid polyps contain gain-of-function mutations in the platelet-derived growth factor receptor α polypeptide gene, similar to those found in CD117-negative GISTs, suggesting the possibility of a neoplastic process.65
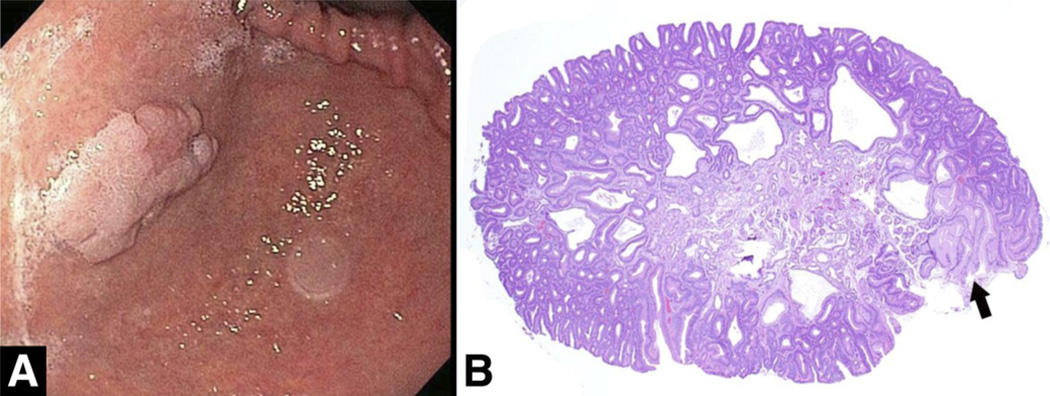
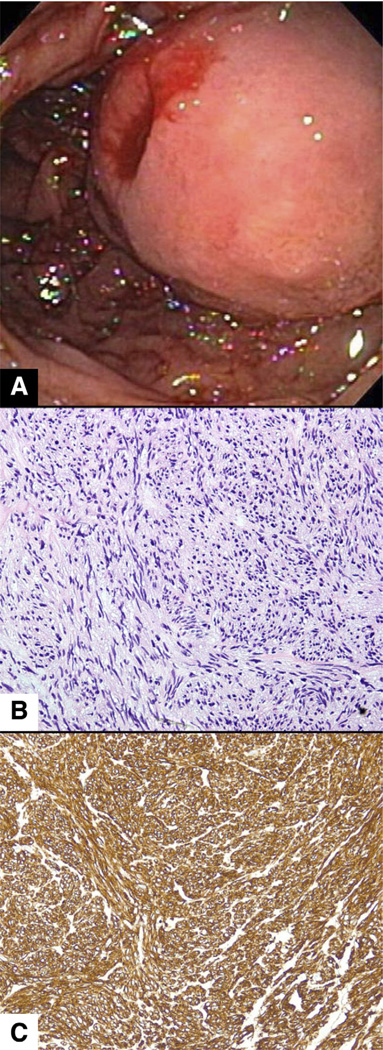
Figure 6.
Inflammatory fibroid polyp. (A) Endoscopic view of an inflammatory fibroid polyp in the antrum showing a firm, well-circumscribed submucosal lesion. (B) Histologically, a flattened, often eroded, gastric epithelium lines a compact aggregate of fibrous tissue mixed with inflammatory cells. (C) Vessels usually are surrounded by a characteristic circumferential deposition of fibroblasts (onion skin), and the stroma contains myriad eosinophils.
Clinical Approach
Most inflammatory fibroid polyps are asymptomatic, but larger polyps have been reported to cause abdominal pain, early satiety, anemia, and gastric outlet obstruction.66 The endoscopic ultrasound appearance, characterized by an indistinct margin, a hypoechoic homogeneous lesion, and location within the second or third layer with an intact fourth layer, may be helpful in establishing the diagnosis.67–69
Histopathologic Diagnostic Tips: Special Stains, Immunohistochemistry, and Molecular Studies
Because fibroepithelial polyps are extremely rare, general pathologists may spend their entire career without ever seeing (or at least recognizing) one. However, once familiar with their unique morphology they can be diagnosed instantly (Figure 6). Immunohistochemical staining for CD31 (an endothelial marker) is strongly positive, but it is almost never necessary for the diagnosis and should be used only as a teaching tool.
Gastric Neuroendocrine Tumors (Carcinoids)
Carcinoids are neuroendocrine tumors derived from enterochromaffin-like (ECL) cells.70 The term carcinoid was discarded in the most recent (2010) World Health Organization classification of tumors in favor of neuroendocrine tumor.71 In 2 large studies (Germany in 1994 and the United States in 2008) gastric neuroendocrine tumors comprised less than 2% of gastric polypoid lesions.1, 46
Gastric neuroendocrine tumors are classified in 3 distinct types. Type I tumors represent 70% to 80% of all gastric endocrine tumors. They are associated with hypergastrinemia resulting from autoimmune (corpus-restricted) atrophic gastritis and, therefore, are found more commonly in elderly patients, particularly women, with atrophic gastritis and often are associated with pernicious anemia.72–74 These tumors (Figure 7) are small (<1 cm), confined to the oxyntic mucosa, and tend to be multiple and usually co-exist with multifocal ECL cell hyperplasia. Gastric neuroendocrine tumors tend to be found incidentally, often in patients undergoing EGD as part of an evaluation for anemia. Histologically, they consist of nests or ribbons of endocrine cells (small polygonal cells with round nuclei featuring salt-and-pepper chromatin) with a very low proliferation index.
Figure 7.
Gastric neuroendocrine tumor. (A) A small gastric carcinoid with surface ulceration seen on retroflexion in the distal body. (B) Merging nests of ECL cells arranged in cords in the deeper part of carcinoids are characteristic of carcinoid tumors. (C) The neuroendocrine origin of their cells can be confirmed by a positive synaptophysin immunohistochemical stain.
Type II gastric neuroendocrine tumors are associated with hypergastrinemia resulting from a gastrin-secreting tumor. They frequently are detected as part of the work-up for MEN-1 syndrome or for Zollinger–Ellison syndrome.75 In both instances the tumors are usually small (<1 cm) and show neither infiltrating nor pleomorphic features. In patients with MEN-1 syndrome the gastric mucosa is normal or mildly inflamed, but not atrophic. The fundic mucosa of patients with Zollinger–Ellison syndrome often is hypertrophic, with long densely packed oxyntic glands and no significant inflammation. This type of neuroendocrine tumor is the most uncommon, representing only 5% to 8% of gastric neuroendocrine tumors.76, 77 Type III (sporadic) neuroendocrine tumors are not associated with hypergastrinemia, are generally solitary, arise in otherwise healthy gastric mucosa, and are not accompanied by ECL cell hyperplasia. These tumors, which represent approximately 20% of all gastric neuroendocrine tumors, usually are detected when they become symptomatic, either secondary to mucosal erosion and blood loss or metastasis. Because these events tend to occur only after the tumors reach a certain size, these tumors are usually larger than 1.5 cm, display infiltrating growth patterns with areas of necrosis, and show various degrees of pleomorphism. Their proliferation index is high (mitotic count >20 per high-power field or a Ki-67 index > 20%). Type III neuroendocrine tumors have a generally poor prognosis with a mean survival of 28 months.73, 78
Clinical Approach
Type I and II tumors often can be removed endoscopically. In select patient with numerous and recurrent type I neuroendocrine tumors, antral resection could be a reasonable option. Antrectomy works by reducing the gastrin-producing cell mass in the stomach, thus removing the stimulus (ie, hypergastrinemia) for ECL cell proliferation.79 Newer treatments such as the gastrin receptor antagonist netazepide are being investigated and could represent an alternative new medical treatment for type I gastric carcinoids.80 Patients with sporadic carcinoids (type III) may present with anemia, epigastric pain, or signs and symptoms caused by metastases, including the rare carcinoid syndrome (characterized by cutaneous flushing, diarrhea, bronchospasm, and cardiac valvular lesions). Surgery followed with chemotherapy is the treatment of choice.
Histopathologic Diagnostic Tips: Special Stains, Immunohistochemistry, and Molecular Studies
Neuroendocrine tumors are best diagnosed with the help of an immunohistochemical stain (synaptophysin, chromogranin A, or CD56). These 3 stains are essentially equivalent in their ability to highlight neuroendocrine markers: their specific use is mostly a matter of preference for each pathologist. In addition, a Ki-67 stain should be performed to count the percentage of proliferating cells and to determine the grade of the tumor (Figure 8).
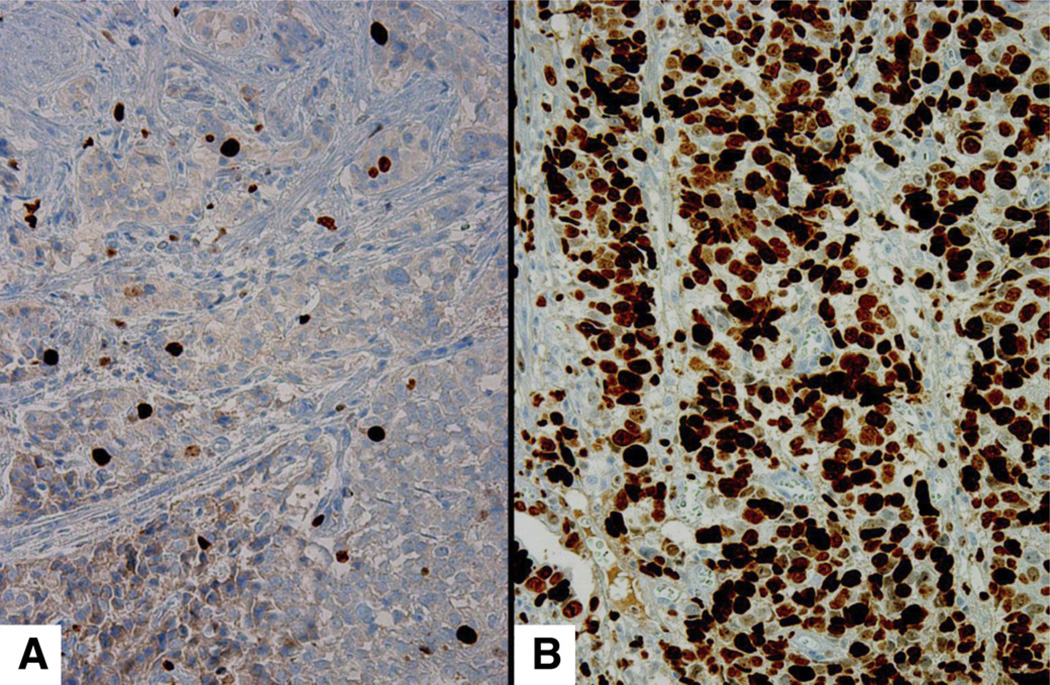
Figure 8.
Ki-67 for carcinoids. When stained with Ki-67 (an immunohistochemical stain that selectively highlights proliferating cells), the indolent carcinoids type I neuroendocrine tumors) found in patients with atrophic gastritis show that less than 2% of the neuroendocrine cells are in a proliferative status (A). In contrast, the usually malignant sporadic carcinoids (type III) show a proliferation index of a 20% or higher (B).
Approach to Gastric Polyps Found at Endoscopy
Because most polyps are found incidentally during upper endoscopies, it is crucial that the endoscopist be prepared to acquire as much information as possible during the procedure to help with the future management of the polyp.
If the appearance strongly suggests fundic gland polyps, biopsy specimens from 1 or more polyps should be taken; polyps larger than 1 cm should be resected. In the setting of fundic gland polyps special attention should be given to atypical-looking lesions, all of which should undergo a biopsy examination because they may represent other, more clinically relevant, lesions. If the appearance is not suggestive of fundic gland polyps, the endoscopist should consider complete removal of all polyps that measure 1 cm or more; if not removed such polyps should be adequately sampled. In the case of larger polyps, after the histopathologic diagnosis is received, a decision needs to be made regarding whether polypectomy is needed and, if it is, should it be endoscopic or surgical. Several factors should be considered when making that decision: (1) risk of missing more serious pathology in the large polyps,81 (2) the presence of symptoms, (3) the patient’s overall health status and preferences, and (4) local expertise. Considering that many endoscopists are not experienced with the resection of large polyps and the risk of complications is not insignificant, the biopsy-first approach is reasonable because it allows definitive treatment to be planned according to pathology results and after consultation with the patient. If resection is planned, the endoscopist should be prepared to deal with potential complications. Many of these lesions are highly vascular and tend to bleed; some (inflammatory fibroid polyps, carcinoids, and GISTs) have submucosal components that increase the risk of perforation.
The conscientious endoscopist should be guided by the principle that no polyp is an island unto itself. Thus, after polyps are removed or sampled, the nonaffected gastric mucosa should be inspected and a minimum of 3 biopsy specimens from the antrum (including one from the incisura angularis) and 2 to 4 from the corpus, sampling both the greater and lesser curvature, should be submitted for pathologic examination, ideally in separate containers. Putting them into separate containers makes possible a more precise topographic definition of any abnormalities. The information so acquired will allow determining, for example, whether the patient has H pylori infection, atrophic gastritis, possibly with diffuse neuroendocrine hyperplasia, or a normal mucosa. Each of these findings would point to different management directions.
Follow-up Evaluation
There is a dearth of data on both the short- and the long-term follow-up evaluation of gastric polyps; therefore, no evidence-based guidelines exist.82 A surveillance endoscopy on nonfundic gland polyps within 1 year is a reasonable approach to evaluate the site for recurrence and to assess for new polyps. Follow-up evaluation after resection of polyps with high-grade dysplasia or early cancer should be individualized, but (at least for the first 2–3 years) short intervals (eg, 6 mo) would seem desirable.83 Gastric carcinoids managed endoscopically (usually type 1) should be followed up with endoscopy every 1 to 2 years.
Abbreviations used in this paper
- ECL
enterochromaffin-like
- EGD
esophagogastroduodenoscopy
- GIST
gastrointestinal stromal tumors
- OLGA
Operative Link for Gastritis Assessment
- PPI
proton pump inhibitor
Footnotes
Conflicts of interest
The authors disclose no conflicts.
References
- 1.Carmack SW, Genta RM, Schuler CM, et al. The current spectrum of gastric polyps: a 1-year national study of over 120,000 patients. Am J Gastroenterol. 2009;104:1524–1532. doi: 10.1038/ajg.2009.139. [DOI] [PubMed] [Google Scholar]
- 2.Omori T, Kamiya Y, Tahara T, et al. Correlation between magnifying narrow band imaging and histopathology in gastric protruding/or polypoid lesions: a pilot feasibility trial. BMC Gastroenterol. 2012;12:17. doi: 10.1186/1471-230X-12-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elster K. Histologic classification of gastric polyps. Curr Top Pathol. 1976;63:77–93. doi: 10.1007/978-3-642-66481-6_3. [DOI] [PubMed] [Google Scholar]
- 4.el-Zimaity HM, Jackson FW, Graham DY. Fundic gland polyps developing during omeprazole therapy. Am J Gastroenterol. 1997;92:1858–1860. [PubMed] [Google Scholar]
- 5.Raghunath AS, O’Morain C, McLoughlin RC. Review article: the long-term use of proton-pump inhibitors. Aliment Pharmacol Ther. 2005;22(Suppl 1):55–63. doi: 10.1111/j.1365-2036.2005.02611.x. [DOI] [PubMed] [Google Scholar]
- 6.Carmack SW, Genta RM, Graham DY, et al. Management of gastric polyps: a pathology-based guide for gastroenterologists. Nat Rev Gastroenterol Hepatol. 2009;6:331–341. doi: 10.1038/nrgastro.2009.70. [DOI] [PubMed] [Google Scholar]
- 7.Bertoni G, Sassatelli R, Nigrisoli E, et al. Dysplastic changes in gastric fundic gland polyps of patients with familial adenomatous polyposis. Ital J Gastroenterol Hepatol. 1999;31:192–197. [PubMed] [Google Scholar]
- 8.Bianchi LK, Burke CA, Bennett AE, et al. Fundic gland polyp dysplasia is common in familial adenomatous polyposis. Clin Gastroenterol Hepatol. 2008;6:180–185. doi: 10.1016/j.cgh.2007.11.018. [DOI] [PubMed] [Google Scholar]
- 9.Attard TM, Cuffari C, Tajouri T, et al. Multicenter experience with upper gastrointestinal polyps in pediatric patients with familial adenomatous polyposis. Am J Gastroenterol. 2004;99:681–686. doi: 10.1111/j.1572-0241.2004.04115.x. [DOI] [PubMed] [Google Scholar]
- 10.Domizio P, Talbot IC, Spigelman AD, et al. Upper gastrointestinal pathology in familial adenomatous polyposis: results from a prospective study of 102 patients. J Clin Pathol. 1990;43:738–743. doi: 10.1136/jcp.43.9.738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Genta RM, Schuler CM, Robiou CI, et al. No association between gastric fundic gland polyps and gastrointestinal neoplasia in a study of over 100,000 patients. Clin Gastroenterol Hepatol. 2009;7:849–854. doi: 10.1016/j.cgh.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 12.Kim JS, Chae HS, Kim HK, et al. Spontaneous resolution of multiple fundic gland polyps after cessation of treatment with omeprazole. Korean J Gastroenterol. 2008;51:305–308. [PubMed] [Google Scholar]
- 13.Hongo M, Fujimoto K. Gastric Polyps Study Group. Incidence and risk factor of fundic gland polyp and hyperplastic polyp in long-term proton pump inhibitor therapy: a prospective study in Japan. J Gastroenterol. 2010;45:618–624. doi: 10.1007/s00535-010-0207-7. [DOI] [PubMed] [Google Scholar]
- 14.Jianu CS, Fossmark R, Viset T, et al. Gastric carcinoids after long-term use of a proton pump inhibitor. Aliment Pharmacol Ther. 2012;36:644–649. doi: 10.1111/apt.12012. [DOI] [PubMed] [Google Scholar]
- 15.Jianu CS, Lange OJ, Viset T, et al. Gastric neuroendocrine carcinoma after long-term use of proton pump inhibitor. Scand J Gastroenterol. 2012;47:64–67. doi: 10.3109/00365521.2011.627444. [DOI] [PubMed] [Google Scholar]
- 16.Fossmark R, Jianu CS, Martinsen TC, et al. Serum gastrin and chromogranin A levels in patients with fundic gland polyps caused by long-term proton-pump inhibition. Scand J Gastroenterol. 2008;43:20–24. doi: 10.1080/00365520701561959. [DOI] [PubMed] [Google Scholar]
- 17.Abraham NS. Proton pump inhibitors: potential adverse effects. Curr Opin Gastroenterol. 2012;28:615–620. doi: 10.1097/MOG.0b013e328358d5b9. [DOI] [PubMed] [Google Scholar]
- 18.Bajaj JS, Ratliff SM, Heuman DM, et al. Proton pump inhibitors are associated with a high rate of serious infections in veterans with decompensated cirrhosis. Aliment Pharmacol Ther. 2012;36:866–874. doi: 10.1111/apt.12045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Juel J, Pareek M, Jensen SE. The clopidogrel-PPI interaction: an updated mini-review. Curr Vasc Pharmacol. 2012 doi: 10.2174/157016111205140926161509. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 20.Rotramel A, Poritz LS, Messaris E, et al. PPI therapy and albumin are better predictors of recurrent Clostridium difficile colitis than choice of antibiotics. J Gastrointest Surg. 2012;16:2267–2273. doi: 10.1007/s11605-012-2037-9. [DOI] [PubMed] [Google Scholar]
- 21.Vakil N. Prescribing proton pump inhibitors: is it time to pause and rethink? Drugs. 2012;72:437–445. doi: 10.2165/11599320-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 22.Lau YT, Ahmed NN. Fracture risk and bone mineral density reduction associated with proton pump inhibitors. Pharmacotherapy. 2012;32:67–79. doi: 10.1002/PHAR.1007. [DOI] [PubMed] [Google Scholar]
- 23.Dirschmid K, Platz-Baudin C, Stolte M. Why is the hyperplastic polyp a marker for the precancerous condition of the gastric mucosa? Virchows Arch. 2006;448:80–84. doi: 10.1007/s00428-005-0068-2. [DOI] [PubMed] [Google Scholar]
- 24.Archimandritis A, Spiliadis C, Tzivras M, et al. Gastric epithelial polyps: a retrospective endoscopic study of 12974 symptomatic patients. Ital J Gastroenterol. 1996;28:387–390. [PubMed] [Google Scholar]
- 25.Abraham SC, Singh VK, Yardley JH, et al. Hyperplastic polyps of the stomach: associations with histologic patterns of gastritis and gastric atrophy. Am J Surg Pathol. 2001;25:500–507. doi: 10.1097/00000478-200104000-00010. [DOI] [PubMed] [Google Scholar]
- 26.Cerwenka H, Bacher H, Mischinger HJ. Pyloric obstruction caused by prolapse of a hyperplastic gastric polyp. Hepatogastroenterology. 2002;49:958–960. [PubMed] [Google Scholar]
- 27.Chen HW, Lu CH, Shun CT, et al. Gastric outlet obstruction due to giant hyperplastic gastric polyps. J Formos Med Assoc. 2005;104:852–855. [PubMed] [Google Scholar]
- 28.Dixon MF, O’Connor HJ, Axon AT, et al. Reflux gastritis: distinct histopathological entity? J Clin Pathol. 1986;39:524–530. doi: 10.1136/jcp.39.5.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maguilnik I, Neumann WL, Sonnenberg A, et al. Reactive gastropathy is associated with inflammatory conditions throughout the gastrointestinal tract. Aliment Pharmacol Ther. 2012;36:736–743. doi: 10.1111/apt.12031. [DOI] [PubMed] [Google Scholar]
- 30.Lauwers GY, Wahl SJ, Melamed J, et al. p53 expression in precancerous gastric lesions: an immunohistochemical study of PAb 1801 monoclonal antibody on adenomatous and hyperplastic gastric polyps. Am J Gastroenterol. 1993;88:1916–1919. [PubMed] [Google Scholar]
- 31.Nogueira AM, Carneiro F, Seruca R, et al. Microsatellite instability in hyperplastic and adenomatous polyps of the stomach. Cancer. 1999;86:1649–1656. [PubMed] [Google Scholar]
- 32.Murakami K, Mitomi H, Yamashita K, et al. p53, but not c-Ki-ras, mutation and down-regulation of p21WAF1/CIP1 and cyclin D1 are associated with malignant transformation in gastric hyperplastic polyps. Am J Clin Pathol. 2001;115:224–234. doi: 10.1309/VLF5-UCNH-XQM2-X410. [DOI] [PubMed] [Google Scholar]
- 33.Yao T, Kajiwara M, Kuroiwa S, et al. Malignant transformation of gastric hyperplastic polyps: alteration of phenotypes, proliferative activity, and p53 expression. Hum Pathol. 2002;33:1016–1022. doi: 10.1053/hupa.2002.126874. [DOI] [PubMed] [Google Scholar]
- 34.Hattori T. Morphological range of hyperplastic polyps and carcinomas arising in hyperplastic polyps of the stomach. J Clin Pathol. 1985;38:622–630. doi: 10.1136/jcp.38.6.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zea-Iriarte WL, Sekine I, Itsuno M, et al. Carcinoma in gastric hyperplastic polyps. A phenotypic study. Dig Dis Sci. 1996;41:377–386. doi: 10.1007/BF02093832. [DOI] [PubMed] [Google Scholar]
- 36.Rugge M, Leandro G, Farinati F, et al. Gastric epithelial dysplasia. How clinicopathologic background relates to management. Cancer. 1995;76:376–382. doi: 10.1002/1097-0142(19950801)76:3<376::aid-cncr2820760305>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 37.Capelle LG, de Vries AC, Haringsma J, et al. The staging of gastritis with the OLGA system by using intestinal metaplasia as an accurate alternative for atrophic gastritis. Gastrointest Endosc. 2010;71:1150–1158. doi: 10.1016/j.gie.2009.12.029. [DOI] [PubMed] [Google Scholar]
- 38.Rugge M, Meggio A, Pennelli G, et al. Gastritis staging in clinical practice: the OLGA staging system. Gut. 2007;56:631–636. doi: 10.1136/gut.2006.106666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dixon MF, Genta RM, Yardley JH, et al. Classification and grading of gastritis. The updated Sydney system. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996;20:1161–1181. doi: 10.1097/00000478-199610000-00001. [DOI] [PubMed] [Google Scholar]
- 40.Graham DY, Nurgalieva ZZ, el-Zimaity HM, et al. Noninvasive versus histologic detection of gastric atrophy in a Hispanic population in North America. Clin Gastroenterol Hepatol. 2006;4:306–314. doi: 10.1016/j.cgh.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 41.Vilaichone RK, Mahachai V, Graham DY. Helicobacter pylori diagnosis and management. Gastroenterol Clin North Am. 2006;35:229–247. doi: 10.1016/j.gtc.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 42.Suzuki S, Ohkusa T, Shimoi K, et al. Disappearance of multiple hyperplastic polyps after the eradication of Helicobacter pylori. Gastrointest Endosc. 1997;46:566–568. doi: 10.1016/s0016-5107(97)70020-8. [DOI] [PubMed] [Google Scholar]
- 43.Ohkusa T, Takashimizu I, Fujiki K, et al. Disappearance of hyperplastic polyps in the stomach after eradication of Helicobacter pylori. A randomized, clinical trial. Ann Intern Med. 1998;129:712–715. doi: 10.7326/0003-4819-129-9-199811010-00006. [DOI] [PubMed] [Google Scholar]
- 44.Lauwers GY, Carneiro F, Graham DY, et al. Gastric carcinoma. In: Bosman FT, Carneiro F, Hruban RH, editors. WHO classification of tumors of the digestive system. 4th ed. Lyon: International Agency for Research Against Cancer; 2010. pp. 48–58. [Google Scholar]
- 45.Lauwers GY, Srivastava A. Gastric preneoplastic lesions and epithelial dysplasia. Gastroenterol Clin North Am. 2007;36:813–829. vi. doi: 10.1016/j.gtc.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 46.Stolte M, Sticht T, Eidt S, et al. Frequency, location, and age and sex distribution of various types of gastric polyp. Endoscopy. 1994;26:659–665. doi: 10.1055/s-2007-1009061. [DOI] [PubMed] [Google Scholar]
- 47.Nakamura T, Nakano G. Histopathological classification and malignant change in gastric polyps. J Clin Pathol. 1985;38:754–764. doi: 10.1136/jcp.38.7.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Laxén F, Sipponen P, Ihamäki T, et al. Gastric polyps; their morphological and endoscopical characteristics and relation to gastric carcinoma. Acta Pathol Microbiol Immunol Scand A. 1982;90:221–228. doi: 10.1111/j.1699-0463.1982.tb00085_90a.x. [DOI] [PubMed] [Google Scholar]
- 49.Abraham SC, Park SJ, Lee JH, et al. Genetic alterations in gastric adenomas of intestinal and foveolar phenotypes. Mod Pathol. 2003;16:786–795. doi: 10.1097/01.MP.0000080349.37658.5E. [DOI] [PubMed] [Google Scholar]
- 50.Rugge M, Farinati F, Baffa R, et al. Gastric epithelial dysplasia in the natural history of gastric cancer: a multicenter prospective follow-up study. Interdisciplinary Group on Gastric Epithelial Dysplasia. Gastroenterology. 1994;107:1288–1296. doi: 10.1016/0016-5085(94)90529-0. [DOI] [PubMed] [Google Scholar]
- 51.Tan CB, Zhi W, Shahzad G, et al. Gastrointestinal stromal tumors: a review of case reports, diagnosis, treatment, and future directions. ISRN Gastroenterol. 2012;2012:595968. doi: 10.5402/2012/595968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.DeMatteo RP, Lewis JJ, Leung D, et al. Two hundred gastrointestinal stromal tumors: recurrence patterns and prognostic factors for survival. Ann Surg. 2000;231:51–58. doi: 10.1097/00000658-200001000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.DeMatteo RP, Ballman KV, Antonescu CR, et al. Adjuvant imatinib mesylate after resection of localised, primary gastrointestinal stromal tumour: a randomised, double-blind, placebo-controlled trial. Lancet. 2009;373:1097–1104. doi: 10.1016/S0140-6736(09)60500-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.DeMatteo RP. Nanoneoadjuvant therapy of gastrointestinal stromal tumor (GIST) Ann Surg Oncol. 2009;16:799–800. doi: 10.1245/s10434-009-0316-9. [DOI] [PubMed] [Google Scholar]
- 55.Demetri GD, Benjamin RS, Blanke CD, et al. NCCN Task Force report: management of patients with gastrointestinal stromal tumor (GIST)– update of the NCCN clinical practice guidelines. J Natl Compr Canc Netw. 2007;5(Suppl 2):S1–S29. [PubMed] [Google Scholar]
- 56.Miettinen M, Lasota J. Gastrointestinal stromal tumors–definition, clinical, histological, immunohistochemical, and molecular genetic features and differential diagnosis. Virchows Arch. 2001;438:1–12. doi: 10.1007/s004280000338. [DOI] [PubMed] [Google Scholar]
- 57.Miettinen M, Lasota J. Histopathology of gastrointestinal stromal tumor. J Surg Oncol. 2011;104:865–873. doi: 10.1002/jso.21945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kawanowa K, Sakuma Y, Sakurai S, et al. High incidence of microscopic gastrointestinal stromal tumors in the stomach. Hum Pathol. 2006;37:1527–1535. doi: 10.1016/j.humpath.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 59.Hueman MT, Schulick RD. Management of gastrointestinal stromal tumors. Surg Clin North Am. 2008;88:599–614. vii. doi: 10.1016/j.suc.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 60.Nilsson B, Sjölund K, Kindblom LG, et al. Adjuvant imatinib treatment improves recurrence-free survival in patients with high-risk gastrointestinal stromal tumours (GIST) Br J Cancer. 2007;96:1656–1658. doi: 10.1038/sj.bjc.6603797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Miettinen M, Lasota J. Gastrointestinal stromal tumors: pathology and prognosis at different sites. Semin Diagn Pathol. 2006;23:70–83. doi: 10.1053/j.semdp.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 62.Hasegawa T, Yang P, Kagawa N, et al. CD34 expression by inflammatory fibroid polyps of the stomach. Mod Pathol. 1997;10:451–456. [PubMed] [Google Scholar]
- 63.Allibone RO, Nanson JK, Anthony PP. Multiple and recurrent inflammatory fibroid polyps in a Devon family (“Devon polyposis syndrome”): an update. Gut. 1992;33:1004–1005. doi: 10.1136/gut.33.7.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pantanowitz L, Antonioli DA, Pinkus GS, et al. Inflammatory fibroid polyps of the gastrointestinal tract: evidence for a dendritic cell origin. Am J Surg Pathol. 2004;28:107–114. doi: 10.1097/00000478-200401000-00013. [DOI] [PubMed] [Google Scholar]
- 65.Schildhaus HU, Cavlar T, Binot E, et al. Inflammatory fibroid polyps harbour mutations in the platelet-derived growth factor receptor alpha (PDGFRA) gene. J Pathol. 2008;216:176–182. doi: 10.1002/path.2393. [DOI] [PubMed] [Google Scholar]
- 66.Rossi P, Montuori M, Balassone V, et al. Inflammatory fibroid polyp. A case report and review of the literature. Ann Ital Chir. 2012;83:347–351. [PubMed] [Google Scholar]
- 67.Matsushita M, Okazaki K. Atypical EUS features of gastric inflammatory fibroid polyps. Gastrointest Endosc. 2005;61:637–638. doi: 10.1016/s0016-5107(05)00137-9. [DOI] [PubMed] [Google Scholar]
- 68.Matsushita M, Takakuwa H, Nishio A. Characteristic endosonographic features of gastric inflammatory fibroid polyps. Endoscopy. 2001;33:729–730. [PubMed] [Google Scholar]
- 69.Matsushita M, Hajiro K, Okazaki K, et al. Endoscopic features of gastric inflammatory fibroid polyps. Am J Gastroenterol. 1996;91:1595–1598. [PubMed] [Google Scholar]
- 70.Klöppel G, Perren A, Heitz PU. The gastroenteropancreatic neuroendocrine cell system and its tumors: the WHO classification. Ann N Y Acad Sci. 2004;1014:13–27. doi: 10.1196/annals.1294.002. [DOI] [PubMed] [Google Scholar]
- 71.Solcia E, Arnold R, Capella C, et al. Neuroendocrine neoplasms of the stomach. In: Bosman FT, Carneiro F, Hruban RH, editors. WHO classification of tumors of the digestive system. 4th ed. Lyon: International Agency for Research Against Cancer; 2010. pp. 64–68. [Google Scholar]
- 72.Solcia E, Rindi G, Paolotti D, et al. Natural history, clinicopathologic classification and prognosis of gastric ECL cell tumors. Yale J Biol Med. 1998;71:285–290. [PMC free article] [PubMed] [Google Scholar]
- 73.Rindi G, Bordi C, Rappel S, et al. Gastric carcinoids and neuroendocrine carcinomas: pathogenesis, pathology, and behavior. World J Surg. 1996;20:168–172. doi: 10.1007/s002689900026. [DOI] [PubMed] [Google Scholar]
- 74.Solcia E, Fiocca R, Villani L, et al. Morphology and pathogenesis of endocrine hyperplasias, precarcinoid lesions, and carcinoids arising in chronic atrophic gastritis. Scand J Gastroenterol Suppl. 1991;180:146–159. doi: 10.3109/00365529109093193. [DOI] [PubMed] [Google Scholar]
- 75.von Rosenvinge EC, Wank SA, Lim RM. Gastric masses in multiple endocrine neoplasia type I-associated Zollinger-Ellison syndrome. Gastroenterology. 2009;137:1222, 537. doi: 10.1053/j.gastro.2009.03.050. [DOI] [PubMed] [Google Scholar]
- 76.Kulke MH, Anthony LB, Bushnell DL, et al. NANETS treatment guidelines: well-differentiated neuroendocrine tumors of the stomach and pancreas. Pancreas. 2010;39:735–752. doi: 10.1097/MPA.0b013e3181ebb168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kulke MH, Chan JA, Meyerhardt JA, et al. A prospective phase II study of 2-methoxyestradiol administered in combination with bevacizumab in patients with metastatic carcinoid tumors. Cancer Chemother Pharmacol. 2011;68:293–300. doi: 10.1007/s00280-010-1478-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rindi G, Azzoni C, La Rosa S, et al. ECL cell tumor and poorly differentiated endocrine carcinoma of the stomach: prognostic evaluation by pathological analysis. Gastroenterology. 1999;116:532–542. doi: 10.1016/s0016-5085(99)70174-5. [DOI] [PubMed] [Google Scholar]
- 79.Borch K, Ahrén B, Ahlman H, et al. Gastric carcinoids: biologic behavior and prognosis after differentiated treatment in relation to type. Ann Surg. 2005;242:64–73. doi: 10.1097/01.sla.0000167862.52309.7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fossmark R, Sørdal O, Jianu CS, et al. Treatment of gastric carcinoids type 1 with the gastrin receptor antagonist netazepide (YF476) results in regression of tumours and normalisation of serum chromogranin A. Aliment Pharmacol Ther. 2012;36:1067–1075. doi: 10.1111/apt.12090. [DOI] [PubMed] [Google Scholar]
- 81.Muehldorfer SM, Stolte M, Martus P, et al. Diagnostic accuracy of forceps biopsy versus polypectomy for gastric polyps: a prospective multicentre study. Gut. 2002;50:465–470. doi: 10.1136/gut.50.4.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dinis-Ribeiro M, Areia M, de Vries AC, et al. Management of precancerous conditions and lesions in the stomach (MAPS): guideline from the European Society of Gastrointestinal Endoscopy (ESGE), European Helicobacter Study Group (EHSG), European Society of Pathology (ESP), and the Sociedade Portuguesa de Endoscopia Digestiva (SPED) Endoscopy. 2012;44:74–94. doi: 10.1055/s-0031-1291491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rugge M, Cassaro M, Di Mario F, et al. The long term outcome of gastric non-invasive neoplasia. Gut. 2003;52:1111–1116. doi: 10.1136/gut.52.8.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]