Abstract
The wingless/int1 (WNT)/Frizzled (FZD) signalling pathway controls numerous cellular processes such as proliferation, differentiation, cell-fate decisions, migration and plays a crucial role during embryonic development. Nineteen mammalian WNTs can bind to 10 FZDs thereby activating different downstream pathways such as WNT/β-catenin, WNT/planar cell polarity and WNT/Ca2+. However, the mechanisms of signalling specification and the involvement of heterotrimeric G proteins are still unclear. Disturbances in the pathways can lead to various diseases ranging from cancer, inflammatory diseases to metabolic and neurological disorders. Due to the presence of seven-transmembrane segments, evidence for coupling between FZDs and G proteins and substantial structural differences in class A, B or C GPCRs, FZDs were grouped separately in the IUPHAR GPCR database as the class FZD within the superfamily of GPCRs. Recently, important progress has been made pointing to a direct activation of G proteins after WNT stimulation. WNT/FZD and G protein coupling remain to be fully explored, although the basic observation supporting the nature of FZDs as GPCRs is compelling. Because the involvement of different (i) WNTs; (ii) FZDs; and (iii) intracellular binding partners could selectively affect signalling specification, in this review we present the current understanding of receptor/ligand selectivity of FZDs and WNTs. We pinpoint what is known about signalling specification and the physiological relevance of these interactions with special emphasis on FZD–G protein interactions.
LINKED ARTICLESThis article is part of a themed section on Molecular Pharmacology of GPCRs. To view the other articles in this section visit http://dx.doi.org/10.1111/bph.2014.171.issue-5
Keywords: Frizzled, WNT, heterotrimeric G proteins, GPCR, Dishevelled
Links to online information in IUPHAR-DB and the BPS Concise Guide to PHARMACOLOGY
| Targets | Ligands | ||
|---|---|---|---|
| 5-HT2c | GSK-3β | β-catenin | WNT-1 |
| Akt | iNOS | cAMP | WNT-2 |
| Alzheimer's%20Disease | JNK | cGMP | WNT-2B |
| COX-2 | mTOR | DKK1 | WNT-3 |
| ERK1 | nemo-like%20kinase | GDP | WNT-3A |
| ERK2 | PDE6 | GTP | WNT-4 |
| FZDs | PI3K | IFNγ | WNT-5A |
| FZD1 | PKA | IL-6 | WNT-5B |
| FZD2 | PKC | IL-8 | WNT-6 |
| FZD3 | PKCδ | IL-15 | WNT-7A |
| FZD4 | ROR1 | Norrin | WNT-7B |
| FZD5 | ROR2 | Rac1 | WNT-8B |
| FZD6 | RYK | R-Spondin | WNT-9B |
| FZD7 | SMO | WNT-10B | |
| FZD8 | |||
| FZD9 | |||
| FZD10 | |||
| GPCRs |
This table lists chemical names, words and phrases that are hyperlinked to relevant entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Pawson et al., 2014; PMID: 24234439) and the Concise Guide to PHARMACOLOGY 2013/14 (http://onlinelibrary.wiley.com/doi/10.1111/bph.2013.170.issue-8/issuetoc, Alexander et al., 2013).
Introduction
Frizzleds (FZDs) are seven-transmembrane (7TM) domain-spanning cell surface receptors, very much like the classical GPCRs. They are activated by the wingless/int1 (WNT) family of lipoglycoproteins (Schulte, 2010; Willert and Nusse, 2012). Cellular communication mediated by WNTs and FZDs is crucial for proper embryonic development, stem cell differentiation, organogenesis and patterning. Also in the adult organism, stem cells are regulated by WNTs and it is also emerging that WNTs have an important role in the regulation of basic physiology. Furthermore, WNT/FZD signalling plays a crucial role in the adult in the maintenance of tissue homeostasis, regeneration, plasticity and repair (Logan and Nusse, 2004; van Amerongen and Nusse, 2009; Chien et al., 2009). Even though many cellular processes are regulated by WNTs, surprisingly little is known about the underlying mechanisms involving the 10 different mammalian FZDs, their 19 WNT ligands and signal initiation at the level of the cell membrane.
From a pharmacological and mechanistic point of view, class FZD receptors as 7TM receptors are poorly understood. Hardly any quantitative information is available about ligand selectivity or efficacy and the most proximal signalling events induced by activated receptors have not been fully elucidated. The field is hampered by a complete lack of pharmacological compounds that can directly target FZDs, which originates in the long-standing difficulties to obtain recombinant pure and biologically active WNTs. Even though WNT/β-catenin pathway activation can be studied through the T-cell factor/lymphoid enhancer-binding factor (TCF/LEF) reporter assay called TOPflash assay (Molenaar et al., 1996; Van de Wetering et al., 1996), there is a lack of suitable tools for studying the β-catenin-independent pathways. This methodological imbalance has resulted in a strong bias towards WNT/β-catenin signalling.
The possibility of targeting WNT signalling therapeutically is widely regarded as attractive and molecules such as glycogen synthase kinase 3 (GSK3) inhibitors, tankyrase inhibitors, secreted FZD-related proteins, R-spondin, Dickkopf1 (DKK1), Norrin and molecules targeting the co-receptors of the WNT/FZD pathway are being considered as putative drugs for diseases such as different types of cancer, neurodegenerative diseases and osteoporosis (Logan and Nusse, 2004; Moon et al., 2004; Chien and Moon, 2007). Drug screening employing, for example, TOPflash reporter assays has identified novel and apparently promising compounds but has never identified FZD-targeting molecules. A recent screen with FZD internalization as readout has identified the antihelminthic drug niclosamide, even though the molecular mechanism and direct action through FZD remains elusive (Chen et al., 2009). Thus, more appropriate screening technologies could aid the development of FZD targeting molecules, so providing useful research tools and therapeutics (Koval and Katanaev, 2012).
In this review we aim to summarize the status quo of our knowledge about WNT/FZD selectivity, signalling trafficking and the functionality of class FZDs as GPCRs in vitro and in vivo. The nomenclature used in this review conforms to the BJP's Concise Guide to PHARMACOLOGY (Alexander et al., 2013).
WNT/FZD signalling pathways
So far, the underlying mechanisms and associated components that determine the outcome of WNT-induced FZD activation are poorly understood. First of all, the degree of WNT/FZD binding specificity in mammals is generally unknown and the question of whether certain WNT/FZD combinations can selectively activate certain signalling routes over others has not been systematically addressed. Secondly, coupling selectivity of activated FZDs to downstream signalling pathways remains obscure. There is emerging evidence that FZDs serve to induce complex signalling networks integrating multiple pathways rather than activating a single, linear signalling pathway. Moreover, different WNT signalling branches crosstalk as exemplified by the inhibition of WNT-3A-induced signalling by WNT-5A (Topol et al., 2003; Mikels and Nusse, 2006). Further, the selective recruitment and participation of co-receptors, such as single transmembrane-spanning receptors of the low-density lipoprotein receptor-related protein family (LRP5/6) and receptor TK-like orphan receptor (ROR1/2) and receptor-like TK, can influence the signalling outcome (van Amerongen et al., 2008; Hendrickx and Leyns, 2008). WNTs are also known to bind to transmembrane heparin sulphate proteoglycans such as glypican and syndecan, resulting in the accumulation of WNT molecules at the cell surface and the prevention of aggregate formation in the extracellular environment (Fuerer et al., 2010). The importance of this phenomenon for the WNT/FZD interaction is unclear, and the role of these cofactors and other extracellular matrix components create an additional level of complexity for ligand/receptor binding studies.
To date, several distinct FZD signalling pathways have been identified and can be divided into β-catenin-dependent and β-catenin-independent branches (Macdonald et al., 2007; Semenov et al., 2007; Schulte, 2010). In the β-catenin-dependent pathway, WNT binding to FZD induces FZD to interact with the co-receptor LRP6, or the similar LRP5 (van Amerongen et al., 2008; MacDonald et al., 2009; MacDonald and He, 2012). The formation of a WNT-FZD-LRP5/6 complex and the recruitment of the scaffolding protein Dishevelled (DVL) leads to the co-clustering of these proteins in signalosomes, which in turn triggers LRP6 phosphorylation (Bilic et al., 2007). Subsequently, the activated co-receptor causes a redistribution of axin to the cell membrane and thus an incomplete and dysfunctional destruction complex – composed of GSK3β, axin, adenomatous polyposis coli (APC) and other proteins – in the cytosol. This, in turn, causes β-catenin to accumulate within the cytosol and enter the nucleus, where it regulates TCF/LEF-dependent transcription. Classical WNT/β-catenin target genes include cyclin D1, c-myc, COX-2 and inducible NOS (Ramsay et al., 2003; Barker, 2008; Du et al., 2009; MacDonald et al., 2009). β-Catenin regulation of TCF/LEF transcription factors can be assessed with the TCF luciferase reporter/TOPflash assay (Molenaar et al., 1996; Van de Wetering et al., 1996), a method that is extensively used. The components of the WNT/β-catenin pathway have been and are being mapped in detail (MacDonald et al., 2009). However, the signal initiation events involving FZD-DVL-LRP5/6 complex formation, kinase activation and recruitment, phosphorylation of DVL and inhibition of the destruction complex are not understood at a molecular level. Generally, this pathway is considered to be independent of heterotrimeric G proteins, although accumulating evidence suggests the opposite (Liu et al., 2001; Katanaev et al., 2005; Halleskog and Schulte, 2013a). For example, Gαq and Gαo have been shown to dissociate the axin-GSK3β and axin2-GSK3β complex, respectively (Liu et al., 2005), and Gα12 and Gαs act on axin, which is the key negative regulator of the β-catenin-dependent WNT/FZD signalling pathway (Castellone et al., 2005; Stemmle et al., 2006).
The β-catenin-independent pathways comprise a steadily increasing number of complex signalling routes: FZD/planar cell polarity (PCP), WNT/Ca2+, WNT/cAMP, WNT/ROR, WNT/RAP and WNT/RAC and WNT/RHO pathways (Semenov et al., 2007; Schulte, 2010). In fact, these pathways are so diverse that it is a strong oversimplification to name them collectively as ‘non-canonical’ or ‘β-catenin-independent’.
Because detailed information about the individual signalling pathways is available in recent, detailed review articles, we will focus here on what is relevant for the discussion about FZDs as unconventional GPCRs (Macdonald et al., 2007; Semenov et al., 2007; Chien et al., 2009; Schulte, 2010).
The first indications of a G protein-dependent WNT/FZD/Ca2+ pathway were obtained using injections of RNA coding for different WNTs and FZDs into Danio rerio and Xenopus laevis embryos. The induction of intracellular calcium release upon XWNT-5A and rat FZD2 could be mimicked by 5-HT2C receptor overexpression and inhibited by using agents that inhibit specific G protein subunits such as pertussis toxin (PTX) or overexpression of Gα sequestering βγ subunits (Slusarski et al., 1997a,b1997b). These studies were followed up by experiments addressing WNT-induced PKC membrane recruitment in X. leavis embryos, a pertussis-sensitive response to intracellular Ca2+ mobilization (Sheldahl et al., 1999). After having named this WNT-induced and G protein-dependent pathway WNT/Ca2+ pathway (Kuhl et al., 2000), PTX-sensitive and rapid Ca2+ release upon WNT stimulation has been shown to be functional in several cellular systems (Sheldahl et al., 2003; Kremenevskaja et al., 2005; Dejmek et al., 2006; Ma and Wang, 2006; Halleskog et al., 2012; Halleskog and Schulte, 2013a). In addition to the WNT/Ca2+ pathway, other G protein-dependent signalling routes involving classical GPCR-associated second messengers and signalling pathways emerge, such as the WNT/cAMP, WNT/ERK1/2 and inositol phosphate signalling (Saneyoshi et al., 2002; Semenov et al., 2007; Bikkavilli and Malbon, 2009; Hansen et al., 2009; Cervenka et al., 2011; Halleskog et al., 2012; Halleskog and Schulte, 2013a).
In accordance with the ternary complex model known from classical GPCRs (De Lean et al., 1980), which describes the allosteric interaction between heterotrimeric G proteins and agonists mediated by the GPCR, it is natural to assume that FZDs directly interact with heterotrimeric G protein to regulate distinct signalling routes, such as WNT/Ca2+ and WNT/cAMP signalling. As a side note, it should be mentioned that the same could be assumed for FZD-β-arrestin interaction, which, however, appeared to be indirectly bridged by the phosphoprotein DVL to mediate agonist-induced internalization (Chen et al., 2003). So far, a growing body of evidence indicates that WNT binding to FZDs induces G protein activation, clearly suggesting that FZDs can interact with heterotrimeric G-proteins. For example, WNTs induce G protein activation by inducing the guanine-nucleotide exchange on Gαi/o proteins (Katanaev and Buestorf, 2009; Kilander et al., 2011a,b2011b; Koval and Katanaev, 2011; Halleskog et al., 2012). Although these studies provide a direct measure of WNT-induced activation of heterotrimeric G proteins in reconstituted systems, membrane preparations from primary cells and brain tissue, the underlying mechanisms of FZD-G protein coupling remain obscure.
Historically, the WNT/β-catenin, PCP, WNT/RHO and WNT/RAC signalling are seen as heterotrimeric G protein-independent, whereas the WNT/Ca2+ branch is recognized as the G protein-dependent WNT pathway. However, over the years several important studies have shown that heterotrimeric G proteins play a more global role in the WNT signalling network including the WNT/β-catenin pathway (Liu et al., 2001; Katanaev et al., 2005; Egger-Adam and Katanaev, 2010). In a recent study from our laboratory using primary microglia cells, we have, for example, shown that PTX does not only block the WNT/Ca2+-mediated ERK1/2 phosphorylation but also the parallel WNT/β-catenin signalling, including the WNT-3A-induced phosphorylation of LRP6, the formation of PS-DVL3 and the stabilization of β-catenin (Halleskog and Schulte, 2013a). In addition, when comparing the signalling capacities of several recombinant WNTs in the N13 microglia-like cell line, we observed that only WNT-3A induced WNT/β-catenin signalling, whereas all the WNTs (WNT-3A,-4,-5A,-5B,-7A,-9B) led to the activation of PTX-sensitive heterotrimeric G proteins (Kilander et al., 2011b). These findings show that heterotrimeric G proteins probably have a more central role in the field of WNT signalling than previously appreciated.
Intracellular binding partners for FZDs: heterotrimeric G proteins and DVL
The two most prominent and widely discussed intracellular binding partners for class FZD receptors are the scaffold protein DVL and heterotrimeric G proteins.
In humans, there are three variants of DVL, which consist of 670 (DVL1), 736 (DVL2) or 716 (DVL3) amino acids. The protein has three main functional domains: the N-terminal end of the protein consists of a so-called DIX (Dishevelled and Axin) polymerization domain, at the C-terminal region, there is a DEP (Dishevelled, Egl-10 and Pleckstrin) domain, functionally involved in the regulation of small GTPases. In addition, the protein has a centrally located PDZ (Psd-95/Disc large/ZO-1 homologous) binding domain, which enables interactions with other proteins. DVL is a central scaffold protein localized at the crossroads of several FZD-induced pathways and plays not only an important role in the β-catenin-dependent but also in the independent signalling pathway, such as the WNT/β-catenin, the PCP pathway, the WNT/RAC, the WNT/RHO and possibly also the WNT/Ca2+ pathway (Gao and Chen, 2010). DVL exists in multiprotein complexes with punctuate appearance in the cytosol (Schwarz-Romond et al., 2005). These multiprotein complexes can be dissolved, for example, by casein kinase overexpression, by WNT-5A stimulation and by expression of FZD (Cong et al., 2004a; Strutt et al., 2006; Bryja et al., 2007a,b2007b). Co-expression with FZDs recruits DVL to the membrane and so far, it is generally unknown if this interaction is static or dynamic, for example, upon agonist treatment (Cong et al., 2004b).
In addition to DVL, the solid evidence for G protein involvement in WNT/FZD signalling shows that heterotrimeric G proteins – according to the ternary complex model valid for GPCRs – interact directly with FZDs. Agonist-bound GPCRs act as a guanine nucleotide exchange factor for the G proteins inducing the GTP-bound active state (Pfeuffer and Helmreich, 1988; Chung et al., 2011). The G protein heterotrimer consists of three subunits, Gα, Gβ, Gγ. Currently, it is known that there are 16 Gα, 5 Gβ genes and 12 genes encoding Gγ (Downes and Gautam, 1999). Commonly, the G proteins can be divided according to their Gα subunit into four groups: Gαi, Gαs, Gαq and Gα12/13 (Neubig and Siderovski, 2002). See also Figure 1.
Figure 1.

Heterotrimeric G protein α-subunit subfamilies and their effects in WNT/FZD signalling. This figure provides an overview of all 16 heterotrimeric G protein α-subunits, which are categorized according to their Gα subunit (Gαi, Gαs, Gαq and Gα12/13). In addition, information about specific G α-subunits in direct relation to certain FZDs and WNT proteins is listed.
Despite the fact that there is experimental evidence indicating that both DVL and heterotrimeric G proteins bind and signal through class FZD receptors, the exact binding sites have not yet been clearly defined. Thus, it is still unclear whether these two proteins compete for FZD binding or if they actually cooperate. While it is known that DVL-FZD interaction is mainly based on three mechanisms involving (i) the KTxxxW–DVL interaction; (ii) DEP–domain interaction with a bipartite motif in the i3 of FZD; and (iii) DEP-domain-mediated electrostatic interactions with the negatively charged head groups of membrane lipids, we can only extrapolate from what is known for other GPCRs and speculate about FZD-G protein contact surfaces. Because classical GPCRs engage mainly the i3 and the C terminus for G protein interaction (Wess, 1998), it appears that the G protein and DVL contact surfaces on FZDs show substantial overlap.
Mutagenesis has revealed several residues in the intracellular loops and in the C-terminal domain of FZDs that are crucial for FZD signalling. Specifically, the mutation of the highly conserved internal KTxxxW motif after the seventh hydrophobic domain, or single amino acid exchanges in the first (R340A) or the third (L524A) intracellular loops of human FZD5, completely abolished FZD signalling (Cong et al., 2004b; Schulte and Bryja, 2007). The KTxxxW motif, which is supposedly located two amino acids after the seventh transmembrane domain on the intracellular HELIX 8 (Wang et al., 2013), is essential for the activation of the WNT/β-catenin signalling pathway (Umbhauer et al., 2000). Flanking sequences of the KTxxxW motif significantly influence the affinity of peptides derived from FZD7 for the PDZ domain of DVL (Punchihewa et al., 2009) suggesting a role in FZD–DVL interaction. Mutation in the KTxxxW motif of Drosophila melanogaster FZD, which blocks DVL binding, results in a protein that is deficient in cell-autonomous planar polarity activity (Wu and Mlodzik, 2008; Wu et al., 2008). Apart from the KTxxxW motif, the C-terminal tail is not well conserved among FZDs, suggesting differences in the pattern of downstream signalling pathways.
Structural features of FZDs and WNTs
Class FZD receptors were separated from the classical class A, B, C family GPCRs due to particular structural and functional features (Foord et al., 2005). Class FZD receptors contain conserved cysteines in the extracellular loops 1 and 2, as is common for GPCRs. These cysteines could engage in stabilizing disulfide bonds. Also, a series of charged residues at the C-and N-terminal ends of intracellular loop 3, which are known to be essential for receptor–G protein coupling, are present in FZD. But class FZD receptors also lack other domains, which are important in many GPCRs. For example, they lack the DRY motif at the C-terminal end of the third transmembrane domain known to be important for G protein coupling and specificity (Schulte, 2010). The recent X-ray structure of the related Smoothened (SMO) indicates clearly that domains common to class A GPCRs are not present in class FZD receptors (Wang et al., 2013). Even though the SMO structure provides an interesting insight into possible mechanisms, more detailed structural and functional analysis is required to understand the functional features of this receptor family. Nevertheless, SMO does signal through G proteins and can couple to them, as demonstrated in biochemical and genetic models (Riobo and Manning, 2007; Philipp and Caron, 2009; Ayers and Therond, 2010). There is strong experimental evidence that the constitutive active state of SMO results in GDP/GTP exchange in heterotrimeric G proteins (Riobo et al., 2006), and genetic experiments indicate that SMO recruits Gαi/o proteins to modulate cAMP levels (Ogden et al., 2008). Furthermore, in a study using micelles, an eighth helix – similar to other GPCRs including SMO – was found in the c-terminal region of FZD1 (Gayen et al., 2013).
Taken together, these findings indicate that so far very little is known about the exact FZD structure. Nevertheless, it has been possible to crystallize and analyse the mouse FZD8-cysteine-rich domain (CRD) domain together with the secreted CRD antagonist FZD-related protein 3. Thereby, it was shown that the CRDs are ∼160 residue and primarily consist of α-helical proteins (Dann et al., 2001; Janda et al., 2012). In addition, lipid modification of WNTs complicates the expression, purification and crystallization of active WNTs (Hausmann and Basler, 2006). However, recently, Janda et al. were able to crystallize a Xenopus WNT-8 (XWNT8) in complex with mouse FZD8-CRD. This study revealed an unusual two-domain WNT structure, not obviously related to known protein folds, and suggests that binding to the FZD8-CRD shields the WNT lipid from aqueous solvent (Janda et al., 2012). These results highlight the presumed selectivity of WNT/FZD.
WNT binding to FZD and physiological relevance
Very few studies provide direct evidence and quantitative measurements of the WNT/FZD interaction. The conventional idea of a ligand-binding assay between hydrophilic small molecule ligands or peptides with their receptors cannot be applied to assess the WNT/FZD interaction quantitatively due to the lipophilic characteristics of the WNT proteins (Willert et al., 2003). Several studies have used isolated FZD-CRDs to measure WNT/FZD selectivity and affinity and have quantified WNT-CRD affinities in the lower nanomolar range (Kawasaki et al., 2007; Carmon and Loose, 2008; Sato et al., 2010). In the following paragraphs we attempt to provide a WNT/FZD binding profile based on what is known about the interactions between the ten mammalian FZD isoforms and the 19 WNTs in the context of their physiological relevance (mammalian vs. more primitive organisms). Figure 2 lists putative WNT/FZD combinations based either on direct binding experiments determined by immunoprecipitation or similar experiments that allow conclusions on protein–protein interaction, such as mass spectrometry, functional testing and signalling outcome. However, immunoprecipitation in this case is the most powerful and direct evidence for a receptor–ligand interaction, depending on the experimental conditions.
Figure 2.
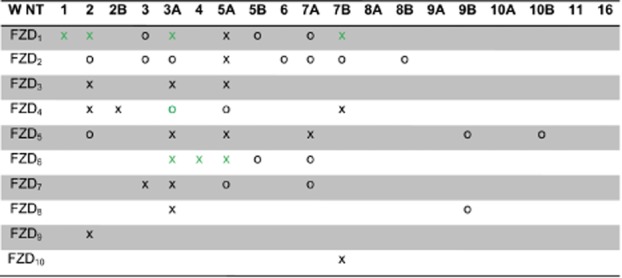
WNT/FZD interaction. This figure provides an overview of reported WNT/FZD combinations and their possible interaction. Crosses (x) indicate evidence for direct binding based on immunoprecipitation, whereas circles (o) indicate colocalization, signalling outcomes such as TCF reporter activity, TOPflash or internalization. Green indicates data already present in the IUPHAR Database (http://www.iuphar-db.org/DATABASE/FamilyIntroductionForward?familyId=25). All references can be found within the text or at the IUPHAR Database homepage. Today, all WNTs are available from different companies such as R&D systems, Abcam, ABMGood or Genemed. However, activity and purity of these WNTs should be carefully controlled.
FZD1
FZD1 has been associated with neuroprotective effects in combination with for example WNT-3A (Chacon et al., 2008). To date, WNT-1,-2,-3,-3A,-5A and-7B are known to serve as binding partners for FZD1 (Gazit et al., 1999; Wang et al., 2005; Koval and Katanaev, 2011). In a study, where TOPflash reporter activity was used as an outcome for WNT activity, only WNT-1,-3,-3A and to a lesser extent WNT-2 were revealed as activators of this FZD1-dependent pathway. In addition, the authors identify WNT-5A as a binding partner for FZD1 by co-immunoprecipitation experiments, even though this particular WNT did not enhance TOPflash reporter activity (Gazit et al., 1999). WNT-7B can bind to overexpressed FZD1 on the cell surface of HEK293 cells. Furthermore, it cooperatively activates the TOPflash reporter in the presence of LRP5 in HEK293 and PAC-1 cells (Wang et al., 2005). L'Episcopo et al. demonstrated, by use of in vitro experiments in neuronal cultures, that WNT-1/FZD1 binding, via β-catenin stabilization, has neuroprotective effects. Exogenous WNT-1 increased the survival of dopaminergic neurons after treatment with 6-hydroxydopamine and MPP+ (L'Episcopo et al., 2011) – neurotoxic components that are known to mimic the biochemical characteristics of Parkinson's disease (Blum et al., 2001). Further, FZD1 and FZD5 were significantly up-regulated in Mycobacterium tuberculosis-infected mice. The authors suggested that WNT-3A is a possible ligand for FZD1 during this process, based on the soluble FZD1/Fc fusion protein-mediated inhibition of WNT-3A-induced Axin2 mRNA expression levels after addition to macrophages (Neumann et al., 2010). Additionally, WNT-3A stimulation initiates DVL3 mobilization to mouse F9 cell membranes through FZD1 upon which TCF/LEF-sensitive transcription is activated. This activation depends on inositol polyphosphate multikinase (Wang and Wang, 2012). In a study with FZD1-expressing L-cells, WNT-3A increased G protein activation substantially during which members of the Go/i subtype (Gαo, Gαi1 and Gαi2) were responsive, whereas Gαz, Gαs, Gαq and Gα12 were not (Koval and Katanaev, 2011).
Additionally, formation of primitive endoderm from F9 stem cells in response to WNT-8 can be blocked by treatment with PTX and by the depletion of the G proteins Gαo and Gαq (Liu et al., 1999).
In membranes of E. coli overexpressing FZD1, WNT-3A, WNT-5A, WNT-5B and WNT-7B specifically activated the trimeric Gαo protein. WNT-3A and WNT-5A were the most active, whereas WNT-5B and WNT-7A demonstrated a more modest stimulation (Katanaev and Buestorf, 2009).
FZD2
FZD2 triggers both β-catenin-dependent signalling as well as β-catenin-independent signalling, among which the Ca2+ pathway has been studied in more depth for this receptor (Sato et al., 2010). For example, after FZD2/WNT-5A binding, PDE6 is activated after which calcium mobilization occurs in melanoma cell lines. Pretreatment with an antibody against FZD2 suppressed this effect as well as pretreatment with PTX, indicating the specific binding of WNT-5A to FZD2 and the role of G proteins in this pathway (Bazhin et al., 2010). Additionally, Ahumada et al. showed that WNT-5A/FZD2 binding in F9 cells induced a PTX-sensitive and PDE6-dependent decrease in cGMP and increase in Ca2+ further confirming a role for heterotrimeric G proteins (Ahumada et al., 2002). Furthermore, WNT-5A and FZD2 binding simultaneously expressed in HeLaS3 cells co-localize in the membrane of the leading edge of the cell and the border of the cell-to-cell adhesion, revealing a function for this binding pair in cell motility (Matsumoto and Kikuchi, 2012). Further, this ligand/receptor pair seems to play a role in WNT/RAC signalling, yet another pathway in the β-catenin-independent signalling network. Knockdown of FZD2 in the cervical cancer cell line HeLaS3 suppressed the WNT-5A-dependent activation of RAC (Sato et al., 2010). However, in FZD2-transfected human pulmonary carcinoma H441 cells, a 20-fold increase in the TOPflash response was observed after WNT-3A stimulation. This response was even stronger after co-transfection of FZD2 and ROR2, depicting β-catenin-dependent signalling as a possible downstream event of FZD2 activation (Li et al., 2008). These data are supported by a study from Yu et al., where the authors created a heat map of TOPflash luciferase activity following transient transfection of different WNTs and FZDs in HEK293 cells. WNT-2,-3A and-3 increased β-catenin-dependent TOPflash luciferase activity through FZD2 in overexpressing cells, similar to WNT-6,-7A,-7B and-8B, but to a lesser extent (Yu et al., 2012).
The signal specification present downstream of FZD2 is likely to be WNT isoform-dependent. For example, WNT-5A competed with WNT-3A for binding to the CRD domain of FZD2, suggesting competition at the receptor level as a possible mechanism for the WNT-5A-mediated inhibition of WNT-3A-dependent accumulation of β-catenin and phosphorylation of the co-receptor LRP6 (Sato et al., 2010).
FZD3
FZD3 is of particular interest for chronic lymphocytic leukaemia (CLL) because expression levels are significantly higher than in normal B cells (Lu et al., 2004; Kaucka et al., 2013). WNT-3A is also noted to be a ‘signature gene’ in CLL (Rosenwald et al., 2001), suggesting that this ligand/receptor pair are significantly involved with this disease. WNT-3A activates the TOPflash reporter in HEK293 cells overexpressing WNT-3A/FZD3/LRP6 (Lu et al., 2004). However, it also mediates neurite outgrowth through FZD3 in Ewing sarcoma cells, seemingly independent of β-catenin, but instead mediated by NK1 receptors (Endo et al., 2008).
WNT-5A has also been shown to be a binding partner for FZD3, triggering downstream pathways independent of β-catenin. For example, it was found to activate the Gαs/cAMP/PKA pathway, resulting in the phosphorylation of Thr34 of DARPP-32 (dopamine-and cAMP-regulated neuronal phosphoprotein-32), which in turn leads to anti-migratory effects in breast cancer cells. The authors observed a strong inhibition of the phosphorylation of DARPP-32 after the use of siRNA for Gαs in MCF-7 cells, even after stimulation of WNT-5A (Hansen et al., 2009). Furthermore, WNT-5A can activate the PI3K/Akt signalling pathway through FZD3 and, thereby, promotes adhesion of human dermal fibroblasts in vitro. This effect could be blocked with recombinant FZD3-CRD, but not by FZD6-CRD, suggesting that WNT-5A is a binding partner for FZD3 but not FZD6 (Kawasaki et al., 2007). Co-immunoprecipitation studies have shown that WNT-2 can interact with FZD3 in human cumulus cells, but it is not known which downstream signalling pathways are activated after this binding interaction (Wang et al., 2009).
FZD4
Investigations of FZD4 have focused on the relationship between receptor mutations and familial exudative vitreoretinopathy, an impairment of retinal vascularization that leads to blindness (Robitaille et al., 2002). In this context, Norrin, structurally not related to WNTs, was found to serve as a ligand of FZD4, capable of activating β-catenin signalling in the retina. The CRD of FZD4 is essential in this binding interaction (Zhang et al., 2011). FZD4-deficient mice have defects in their intraretinal capillary bed (Xu et al., 2004). Similar effects can be seen in LRP5 and Norrin knockout models, suggesting a ligand–receptor connection between the three (Luhmann et al., 2005; Xia et al., 2008).
WNT-5A induces the endocytosis of FZD4-GFP in HEK293 cells, shown using the adaptor protein β-arrestin2 and the phosphorylation of DVL2 (Chen et al., 2003). Further, WNT-5A triggers β-catenin signalling in HEK294/FZD4 cells, a response that can be inhibited when it is co-transfected with the receptor ROR2 (Mikels and Nusse, 2006). Further, inhibition of clathrin-mediated endocytosis of FZD4 by monodansylcadaverine inhibited the WNT-5A-dependent accumulation of β-catenin in the nucleus (Despeaux et al., 2012). Additionally, WNT-5A-mediated endothelial cell proliferation could be blocked by the addition of FZD4-CRD. However, these authors suggested that this response is independent of β-catenin signalling but rather mediated through DVL and ERK1/2 phosphorylation (Masckauchan et al., 2006). Furthermore, WNT-2B (Ohta et al., 2011), WNT-2 (Klein et al., 2008) and WNT-7B (Wang et al., 2005) have been identified as FZD4-binding partners in immunoprecipitation experiments and WNT-3A, through increased accumulation of phosphatidylinositol 4,5-bisphosphate (Pan et al., 2008).
FZD5
There is abundant evidence that WNT-5A and FZD5 are associated with different disease models. For example, both proteins are up-regulated in mouse models of Alzheimer's disease (AD), and WNT-5A enhances Aβ-evoked neurotoxicity by the induction of TNF-α and IL-1 (Li et al., 2011). Aβ by itself in turn stimulates WNT-5A and FZD5 expression in primary cortical cultures in vitro. Also in the synovium tissue rheumatoid arthritis (RA), the WNT-5A/FZD5 pair is up-regulated compared with other human adult tissue. Also transfection of normal synovial fibroblasts with WNT-5A results in RA fibroblast-like synoviocytes, as revealed by enhanced IL-6, IL-8 and IL-15 mRNA levels (Sen et al., 2000). More direct proof of the identification of FZD5 as a receptor for WNT-5A comes from He et al.; they showed that co-injection of hFZD5 and XWNT-5A RNA induced the formation of dorsal axis duplication in X. laevis embryos, although structural and putative functional differences between XWNT-5A and mammalian WNT-5A should not be disregarded. The axis duplication was suppressed after co-injection of RNA for human GSK-3β, suggesting the involvement β-catenin-dependent signalling in this receptor–ligand combination (He et al., 1997). Furthermore, in additional axis induction assay experiments in Xenopus embryos, it was shown that mouse FZD5 specifically has a synergistic effect with mouse WNT-2, WNT-5A and WNT-10B (Ishikawa et al., 2001). TOPflash assay experiments revealed WNT-9B as a binding partner for FZD5/LRP-6 in HEK293 cells (Liu et al., 2008). Finally, WNT-7A and WNT-3A bind to FZD5/CRD, as shown by an elisa-based protein binding assay (Carmon and Loose, 2008; 2010). Knockdown of FZD5 or the addition of CRD-FZD5 blocked the ability of WNT-7A to stimulate synaptogenesis in cultured hippocampal neurons (Sahores et al., 2010).
FZD6
Historically, FZD6 was thought to be involved in the PCP pathway, where it plays an important role in the orientation of hair follicles (Wang et al., 2006a), auditory sensory cells and neural tube closure (Wang et al., 2006b). Recently, it was shown that FZD6 mutations can result in defects in nail and claw formation. The mutated form of FZD6 (R511C) alters the subcellular distribution of the receptor and this results in dysfunctional WNT/FZD signalling. The WNT-3A-induced stabilization of β-catenin and WNT-5A-dependent increase in the WNT inhibitor DKK1 shown in primary human fibroblasts was completely abolished in fibroblasts from patients with the FZD6 mutant (Frojmark et al., 2011; Naz et al., 2012).
Most studies have demonstrated that FZD6 is associated with β-catenin-independent signalling. In fact, some studies have identified FZD6 as a negative regulator of the β-catenin-dependent signalling cascade (Golan et al., 2004). Combining FZD6, TCF/LEF luciferase reporters and WNT-1,-3,-3A,-4 or-5A in HEK293T cells, did not induce increased reporter activity with any of the chosen WNTs. On the contrary, FZD6 inhibited the reporter activity induced by FZD1 and WNT-3A. This inhibitory effect of FZD6 results from the activation of different pathways, for example, Nemo-like kinase, which leads to the phosphorylation of TCF/LEF reducing its binding to target DNA (Golan et al., 2004). This crosstalk could be highly relevant in colon cancer with constitutively active APC mutations (Rubinfeld et al., 1996). Furthermore, WNT-3A stimulated G protein activation in L-cells expressing FZD6 (Koval and Katanaev, 2011).
Heinonen et al. identified FZD6 as a receptor for WNT-4 in immature haematopoietic progenitor cells, based on a decrease in WNT-4-mediated expansion of the cell pool in the absence of FZD6 (Heinonen et al., 2011). Furthermore, WNT-4 binds the CRD of FZD6, but this receptor–ligand pair did not induce TOPflash activation in Madin–Darby Canine Kidney cells (Lyons et al., 2004). Despite the strong link between FZD6 and β-catenin-independent signal transduction, FZD6 was also shown to be associated with WNT/β-catenin signalling. A positive correlation between FZD6 and WNT-3A was reported for β-catenin activation in human mesenchymal stem cells (Kolben et al., 2012).
Lastly, WNT-7A, and to a lesser extent WNT-5A and WNT-5B but not WNT-3A, were shown to induce human FZD6-mediated Gαo activation in an E. coli reconstitution system (Katanaev and Buestorf, 2009).
FZD7
So far, FZD7 has mostly been studied in combination with PCP signalling in vertebrates. The combination of WNT-7A-FZD7 activates the PCP pathway by a mechanism involving Vangl2, demonstrated by the expansion of satellite stem cells resulting in the repair of mouse muscle (Le Grand et al., 2009). In addition, the interaction between WNT-3 and FZD7 leads to increased stabilization of β-catenin in human hepatocellular carcinoma cells and may therefore play a role in hepatocarcinogenesis (Kim et al., 2008).
Interestingly, in myotubes it was shown that FZD7, WNT-7A, Gα(s) and PI3K interact to induce the subsequent activation of the Akt/mammalian target of rapamycin growth pathway (von Maltzahn et al., 2012). The broad signalling network induced by FZD7 means the receptor is involved in many pathological conditions. Up-regulation of FZD7 is denoted in different types of cancer such as hepatocellular carcinoma, colorectal-and breast-cancer – to name but a few (King et al., 2012). Also an increased expression of FZD7 has been found to be associated with a less favourable clinical prognosis in CLL patients (Kaucka et al., 2013). Furthermore, tumour growth could be reduced by the use of FZD7shRNA in mice with xenografts of triple negative breast cancer cells (Yang et al., 2009). Interestingly, calcium-dependent PKC signalling is also closely linked to FZD7. In X. laevis embryo experiments, tissue separation was restored by co-expression of PKCα after blocking G protein-mediated signalling with the inhibitor PTX (Winklbauer et al., 2001). In this context, in vitro and in vivo disruption of the FZD7-DVL protein–protein interaction with the small peptide RHPD-P1 shows anticancer properties, probably through the involvement of β-catenin and PKCδ (Nambotin et al., 2011). Lastly, the CRD of FZD7 binds WNT-3A, as shown by the inhibition of WNT-3A-induced β-catenin stabilization in L-cells after addition of FZD7-CRD (Kemp et al., 2007).
In E. coli experiments, WNT-5A induced FZD7-dependent Gαo activation, whereas WNT-3A and WNT-7A were less effective (Katanaev and Buestorf, 2009).
FZD8
So far, FZD8 is mostly thought to be a WNT receptor mediating β-catenin-independent signalling. Initially, evidence that FZD8 is important for β-catenin-independent signalling was obtained in gastrulating X. laevis embryos. FZD8 led to activation of JNK and rapid apoptotic cell death (Lisovsky et al., 2002). In addition, purified, secreted forms of FZD5, FZD7 and FZD8 CRDs can antagonize WNT-3A-induced β-catenin accumulation in L-cells, whereas in mouse embryonic stem cells, the same CRDs can inhibit spontaneous mesoderm formation and promote neural differentiation, indicating that FZDs interpret WNT signals during embryonic mesoderm and neural induction (Kemp et al., 2007). FZD8 together with Flamingo – a central component of WNT/PCP signalling – maintains haematopoietic stem cells in a long-term quiescent state through their cooperation in the osteoblast niche. The mechanism is based on the suppression of a Ca2+-NFAT-IFNγ (calcium–nuclear factor of activated T-cells–IFNγ) pathway. Furthermore β-catenin-dependent WNT signalling can also be antagonized by this receptor in this situation (Sugimura et al., 2012).
Recently, the structural complex of XWNT8 and FZD8-CRD has been clarified to a resolution of 3.25 Å. Guided by the structure, the authors were able to generate a biochemically tractable version of XWNT8, the so-called ‘mini-WNTs’, and discovered that they discriminate between binding to the CRD of FZD4,5,8 (Janda et al., 2012).
Further, the soluble CRD of FZD8 was used as a WNT inhibitor in many ways and studies. For example, this WNT inhibitor can block endogenous WNTs and the effects of WNT-1 and-5A on proliferation and the acquisition of a dopaminergic phenotype in precursor cultures (Castelo-Branco et al., 2003). In addition, the soluble CRD of FZD8 binds the CRDs of FZD1 and FZD8 receptors, reducing cell sensitivity to WNT-3A (Hendaoui et al., 2012). Additionally, WNT-9B can activate TOPflash activity through FZD8 in HEK293 cells (Liu et al., 2008).
FZD9
The FZD9 gene was identified in 1997 and is located in the chromosomal region 7q11.23, which is the same location at which a 2 Mb deletion occurs in the developmental disorder called Williams–Beuren syndrome (Wang et al., 1997). To determine whether specific symptoms of the syndrome can be explained by the deletion of FZD9, 2 years later, murine FZD9 was characterized. Expression analysis revealed that FZD9 is expressed in the mouse in many tissues, including heart, brain and skeletal muscle (Wang et al., 1999; Zhao and Pleasure, 2005). To analyse the physiological relevance of FZD9, two FZD9-deficient mouse models were generated and analysed independently (Ranheim et al., 2005; Zhao et al., 2005). In both studies, the mice developed normally, were fertile and showed no external abnormalities. However, in one study, differences in the hippocampus and learning defects were diagnosed in FZD9-/- mice (Zhao et al., 2005), whereas in the second study, a defect in the development of B cells, as well as reduced life expectancy were observed (Ranheim et al., 2005). Nevertheless, no symptoms similar to the human Williams–Beuren syndrome were found.
FZD9 could also be involved in β-catenin stabilization and recruitment of DVL1 from the cytoplasm to the cell membrane. In addition, it was shown that WNT-2-activated FZD9 results in TOPflash activity. Deletion mutant analysis demonstrated that there is a difference in FZD9 C-terminal residues required for the modifications of DVL1 and those required for the inductions of β-catenin stabilization and TCF transactivation. It was also shown that most of the C-terminal tail domain of FZD9 is not required for the induction of DVL1 hyperphosphorylation and relocalization. However, all DVL-C mutants used in this study except for one failed to induce β-catenin accumulation (Karasawa et al., 2002). This highlights the role of the C terminus for the stabilization of β-catenin and suggests a separate interaction/binding site for DVL1. FZD9 shows a rapidly increasing expression pattern during early differentiation of osteoblasts, in parallel with established osteoblast marker genes such as Runx2, bone sialoprotein and osteocalcin (Karsenty, 2008). In an Affymetrix GeneChip hybridization study, the FZD9 gene, as the only gene out of the class FZD, was induced during osteoblast differentiation, in parallel with known osteoblast markers. In addition, the expression of FZD9 in osteoblasts has been detected by in situ hybridization. Thus, it was concluded that FZD9 has an important role in the differentiation and function of osteoblasts and, therefore, in bone formation (Albers et al., 2011). This indicates that FZD9 is important physiologically for the differentiation or function of osteoblasts.
FZD10
FZD10 is involved in the formation of colorectal cancer, mainly through the WNT/β-catenin pathway (Terasaki et al., 2002). In addition, FZD10 can be co-immunoprecipitated together with WNT-7B. This WNT/FZD combination also activates β-catenin-dependent signalling in PAC-1 cells (Wang et al., 2005). However, in synovial sarcomas, the transactivation of FZD10, which is highly up-regulated in synovial sarcomas, can be linked to the β-catenin-independent DVL-Rac1-JNK pathway and plays a critical role in the development/progression of synovial sarcomas (Fukukawa et al., 2009). Furthermore, an enhanced expression of FZD10 occurs during embryonic development of the CNS (Yan et al., 2009).
In situ hybridization experiments have been used to visualize FZD10 mRNA expression during mouse development and it was found that FZD10 mRNA showed a similar expression pattern to WNT-7A in the neural tube, limb buds and Müllerian duct in mouse embryos suggesting that these two interact (Nunnally and Parr, 2004).
Also, WNT-3A is known to stimulated G protein activation in L-cells expressing FZD10 (Koval and Katanaev, 2011).
Evidence for the physiological relevance of FZD-G protein signalling
As pointed out in the previous sections, evidence exists on WNT/FZD selectivity but this has obviously not been systematically investigated, partly due to the difficulties in obtaining recombinant, pure and active WNTs (Willert et al., 2003; Schulte et al., 2005; Willert, 2008; Willert and Nusse, 2012). With increasing knowledge about the underlying signalling mechanisms and the refinement of methodologies to quantify pathway activation, the structure–function relationship between the 19 WNTs and 10 FZDs can be investigated in more detail and questions regarding β-catenin-dependent and-independent signalling and its association with FZD-induced activation of heterotrimeric G proteins can be studied in more depth.
As early as 1999, the first proof was obtained that WNT/β-catenin signalling is inhibited in F9 teratocarcinoma cells after inhibition of Gα1 and Gαo via PTX (Liu et al., 1999). In parallel, biochemical evidence emerged supporting the hypothesis that FZDs that mediate β-catenin stabilization are pre-coupled to Gαo (Liu et al., 2005). As mentioned earlier, studies of β-catenin-independent WNT pathways have also provided evidence for G protein signalling linked with FZDs. Studies with zebrafish embryos showed that inhibition of G proteins blocked the changes in Ca2+ influx, which occur in response to WNT-5A and FZD2 (Slusarski et al., 1997a,b1997b). Further, Katanaev et al. showed in vivo that FZDs act as guanine nucleotide exchange factors of PTX-sensitive G proteins mediating both WNT/β-catenin and PCP signals in Drosophila melanogaster (Katanaev et al., 2005).
For example, loss of WNT-7A resulted in a reduction in satellite cells in skeletal muscle 3 weeks after injury, demonstrating its physiological role in regulating the homeostatic level of satellite cells (Le Grand et al., 2009; von Maltzahn et al., 2012). In the same study, mass spectrometry and co-immunoprecipitation studies revealed an association between FZD7 and Gαs and possibly Gαo.
Physiologically, the effect of XWNT-11/Xfz7 signalling was shown to regulate convergent extension movements. These movements affected the dorsal marginal zone of gastrulating Xenopus embryos. Interestingly, FZD7 and the βγ-subunits of heterotrimeric G proteins were required for this process (Penzo-Mendez et al., 2003).
WNT-3A signals through the Gαq/11 subunits of G proteins to activate phosphatidylinositol signalling and PKCδ in murine ST2 cells. However, direct evidence for any regulation of FZD was not obtained (Tu et al., 2007). Furthermore, PKA/CREB signalling may contribute to other WNT-regulated processes, such as stem cell renewal, cancer and embryonic patterning. The authors also suggested that Gα proteins modulate adenylyl cyclase activity, which may be a mechanism by which WNT proteins, mainly WNT-1 and WNT-7A, signal possibly through class FZD receptors (Chen et al., 2005). Lately, there has been an emerging interest in WNTs and their role in inflammation and immune response including neuroinflammatory diseases (Gattinoni et al., 2010; Miao et al., 2013). For example, it has been suggested that genetic variations within WNT signalling components contribute to the initiation and maturation of prevalent neurological disorders (De Ferrari and Moon, 2006). Studies from our lab characterized WNTs as modulators of the CNS immune response and showed that β-catenin levels are elevated in pro-inflammatory microglia of AD patients (Halleskog et al., 2011). WNT-3A and WNT-5A induce a pro-inflammatory response in primary mouse microglia through an ERK1/2-dependent pathway (Halleskog et al., 2012; Halleskog and Schulte, 2013a). In contrast, after LPS induced pro-inflammatory transformation of microglia, WNT-3A and WNT-5A were found to induce a dose-dependent decrease in the pro-inflammatory marker COX2.
Most interestingly, both the WNT-3A-induced ERK1/2 pathway and the β-catenin signalling require heterotrimeric G proteins (Halleskog and Schulte, 2013b).
When investigating physiological responses of WNT signalling, the possibility of heterotrimeric G protein binding to class FZD receptors is often being overlooked or simply not addressed, possibly due to lack of direct and straightforward methodological readouts.
Today, the TOPflash assay, which is a powerful tool for measuring transcriptional activity of β-catenin levels, has been preferentially used to study activation of the WNT signalling. This assay is also frequently used in the industry to find molecules that will interact with the WNT/FZD signalling pathway.
A direct and less pathway-biased readout system for class FZD receptors would be of great value. Recent data pinpoint the activation of heterotrimeric G proteins as a more general downstream event activated by WNTs (Katanaev and Buestorf, 2009; Kilander et al., 2011a). Using, for example, a [35S]-GTPγS or the non-radioactive alternative europium-labelled GTP analogue (Eu-GTP) assay – frequently used for other GPCRs – as a readout system would potentially improve screening efforts for FZD-targeting drugs (Koval et al., 2010).
Conclusions
The signalling network induced by WNTs acting at class FZD receptors is complex. In order to understand signalling specification, it is crucial to systematically address receptor–ligand interactions and quantify which WNT/FZD combinations activate which downstream events.
The role of FZDs in many types of cancer and other diseases is diverse, rendering class FZD receptors an obvious target for therapeutic intervention (Logan and Nusse, 2004; Janssens et al., 2006; van Amerongen and Nusse, 2009; Chien et al., 2009; King et al., 2012). Small molecular libraries are waiting to be screened for specific interaction with FZDs. Agonists and antagonists targeting FZD as well as positive and negative allosteric modulators would be of great value and could serve as pharmacophores for the development of new drugs (Chien and Moon, 2007; Anastas and Moon, 2013). Nevertheless, suitable readout assays are the key to successful high throughput screening (Koval and Katanaev, 2012). TOPflash assays reporting the transcriptional activity of β-catenin have been of great importance in the understanding of the biological importance of the WNT/β-catenin pathway and have proven partially successful in drug screening as well (Huang et al., 2009). However, additional responses, such as activation of heterotrimeric G proteins or second messengers, which have been used for GPCR screening for decades, could complement the assay repertoire very well (Koval et al., 2011).
The discussion about signal specification also opens up possibilities to employ pathway-selective ligands for putative therapeutic intervention. It might be possible to develop biased ligands that, for example, can block the WNT-β-catenin pathway at the same time as they maintain β-catenin-independent pathways. So far, this is purely hypothetical, but the proof-of-concept for biased drugs with class A GPCRs might be transferrable to class FZD receptors (Whalen et al., 2011).
Acknowledgments
We apologize to authors whose original work has not been cited because of space restrictions. The work in the Schulte laboratory is supported by the Swedish Medical Research Council K2008-68P-20810-01-4, K2008-68X-20805-01-4, Swedish Cancer Society CAN 2008/539 and 2011/690, Karolinska Institutet, The KI-NIH Joint PhD program (JPD), The Board of Doctoral Education at Karolinska Institutet (JP) Knut & Alice Wallenberg Foundation KAW2008.0149, Signhild Engkvists Foundation and the Foundations of the National Board of Health and Welfare of Sweden.
Abbreviations
- 7TM
seven transmembrane
- APC
adenomatous polyposis coli
- CLL
chronic lymphocytic leukaemia
- CRD
cysteine-rich domain
- DARPP-32
dopamine-and cAMP-regulated neuronal phosphoprotein-32
- DKK
Dickkopf
- DVL
Dishevelled
- FZD
Frizzled
- GSK
glycogen synthase kinase
- LEF
lymphoid enhancer-binding factor
- LRP
lipoprotein receptor-related protein
- PCP
planar cell polarity
- PTX
pertussis toxin
- ROR
receptor TK-like orphan receptor
- SMO
Smoothened
- TCF
T-cell factor
- WNT
wingless/int1
Conflict of interest
None.
References
- Ahumada A, Slusarski DC, Liu X, Moon RT, Malbon CC, Wang HY. Signaling of rat Frizzled-2 through phosphodiesterase and cyclic GMP. Science. 2002;298:2006–2010. doi: 10.1126/science.1073776. [DOI] [PubMed] [Google Scholar]
- Albers J, Schulze J, Beil FT, Gebauer M, Baranowsky A, Keller J, et al. Control of bone formation by the serpentine receptor Frizzled-9. J Cell Biol. 2011;192:1057–1072. doi: 10.1083/jcb.201008012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Catterall WA, Spedding M, Peters JA, Harmar AJ CGTP Collaborators. The Concise Guide to PHARMACOLOGY 2013/14: G Protein-Coupled Receptors. Br J Pharmacol. 2013;170:1459–1581. doi: 10.1111/bph.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Amerongen R, Nusse R. Towards an integrated view of Wnt signaling in development. Development. 2009;136:3205–3214. doi: 10.1242/dev.033910. [DOI] [PubMed] [Google Scholar]
- van Amerongen R, Mikels A, Nusse R. Alternative wnt signaling is initiated by distinct receptors. Sci Signal. 2008;1:re9. doi: 10.1126/scisignal.135re9. [DOI] [PubMed] [Google Scholar]
- Anastas JN, Moon RT. WNT signalling pathways as therapeutic targets in cancer. Nat Rev Cancer. 2013;13:11–26. doi: 10.1038/nrc3419. [DOI] [PubMed] [Google Scholar]
- Ayers KL, Therond PP. Evaluating Smoothened as a G-protein-coupled receptor for Hedgehog signalling. Trends Cell Biol. 2010;20:287–298. doi: 10.1016/j.tcb.2010.02.002. [DOI] [PubMed] [Google Scholar]
- Barker N. The canonical Wnt/beta-catenin signalling pathway. Methods Mol Biol. 2008;468:5–15. doi: 10.1007/978-1-59745-249-6_1. [DOI] [PubMed] [Google Scholar]
- Bazhin AV, Tambor V, Dikov B, Philippov PP, Schadendorf D, Eichmuller SB. cGMP-phosphodiesterase 6, transducin and Wnt5a/Frizzled-2-signaling control cGMP and Ca(2+) homeostasis in melanoma cells. Cell Mol Life Sci. 2010;67:817–828. doi: 10.1007/s00018-009-0214-0. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Bikkavilli RK, Malbon CC. Mitogen-activated protein kinases and Wnt/beta-catenin signaling: molecular conversations among signaling pathways. Commun Integr Biol. 2009;2:46–49. doi: 10.4161/cib.2.1.7503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilic J, Huang YL, Davidson G, Zimmermann T, Cruciat CM, Bienz M, et al. Wnt induces LRP6 signalosomes and promotes dishevelled-dependent LRP6 phosphorylation. Science. 2007;316:1619–1622. doi: 10.1126/science.1137065. [DOI] [PubMed] [Google Scholar]
- Blum D, Torch S, Lambeng N, Nissou M, Benabid AL, Sadoul R, et al. Molecular pathways involved in the neurotoxicity of 6-OHDA, dopamine and MPTP: contribution to the apoptotic theory in Parkinson's disease. Prog Neurobiol. 2001;65:135–172. doi: 10.1016/s0301-0082(01)00003-x. [DOI] [PubMed] [Google Scholar]
- Bryja V, Gradl D, Schambony A, Arenas E, Schulte G. Beta-arrestin is a necessary component of Wnt/beta-catenin signaling in vitro and in vivo. Proc Natl Acad Sci U S A. 2007a;104:6690–6695. doi: 10.1073/pnas.0611356104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryja V, Schulte G, Rawal N, Grahn A, Arenas E. Wnt-5a induces Dishevelled phosphorylation and dopaminergic differentiation via a CK1-dependent mechanism. J Cell Sci. 2007b;120(Pt 4):586–595. doi: 10.1242/jcs.03368. [DOI] [PubMed] [Google Scholar]
- Carmon KS, Loose DS. Wnt7a interaction with Fzd5 and detection of signaling activation using a split eGFP. Biochem Biophys Res Commun. 2008;368:285–291. doi: 10.1016/j.bbrc.2008.01.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmon KS, Loose DS. Development of a bioassay for detection of Wnt-binding affinities for individual frizzled receptors. Anal Biochem. 2010;401:288–294. doi: 10.1016/j.ab.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellone MD, Teramoto H, Williams BO, Druey KM, Gutkind JS. Prostaglandin E2 promotes colon cancer cell growth through a Gs-axin-beta-catenin signaling axis. Science. 2005;310:1504–1510. doi: 10.1126/science.1116221. [DOI] [PubMed] [Google Scholar]
- Castelo-Branco G, Wagner J, Rodriguez FJ, Kele J, Sousa K, Rawal N, et al. Differential regulation of midbrain dopaminergic neuron development by Wnt-1, Wnt-3a, and Wnt-5a. Proc Natl Acad Sci U S A. 2003;100:12747–12752. doi: 10.1073/pnas.1534900100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervenka I, Wolf J, Masek J, Krejci P, Wilcox WR, Kozubik A, et al. Mitogen-activated protein kinases promote WNT/beta-catenin signaling via phosphorylation of LRP6. Mol Cell Biol. 2011;31:179–189. doi: 10.1128/MCB.00550-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chacon MA, Varela-Nallar L, Inestrosa NC. Frizzled-1 is involved in the neuroprotective effect of Wnt3a against Abeta oligomers. J Cell Physiol. 2008;217:215–227. doi: 10.1002/jcp.21497. [DOI] [PubMed] [Google Scholar]
- Chen AE, Ginty DD, Fan CM. Protein kinase A signalling via CREB controls myogenesis induced by Wnt proteins. Nature. 2005;433:317–322. doi: 10.1038/nature03126. [DOI] [PubMed] [Google Scholar]
- Chen M, Wang J, Lu J, Bond MC, Ren XR, Lyerly HK, et al. The anti-helminthic niclosamide inhibits Wnt/Frizzled1 signaling. Biochemistry. 2009;48:10267–10274. doi: 10.1021/bi9009677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, ten Berge D, Brown J, Ahn S, Hu LA, Miller WE, et al. Dishevelled 2 recruits beta-arrestin 2 to mediate Wnt5A-stimulated endocytosis of Frizzled 4. Science. 2003;301:1391–1394. doi: 10.1126/science.1082808. [DOI] [PubMed] [Google Scholar]
- Chien AJ, Moon RT. WNTS and WNT receptors as therapeutic tools and targets in human disease processes. Front Biosci. 2007;12:448–457. doi: 10.2741/2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien AJ, Conrad WH, Moon RT. A Wnt survival guide: from flies to human disease. J Invest Dermatol. 2009;129:1614–1627. doi: 10.1038/jid.2008.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung KY, Rasmussen SG, Liu T, Li S, DeVree BT, Chae PS, et al. Conformational changes in the G protein Gs induced by the beta2 adrenergic receptor. Nature. 2011;477:611–615. doi: 10.1038/nature10488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cong F, Schweizer L, Varmus H. Casein kinase Iepsilon modulates the signaling specificities of dishevelled. Mol Cell Biol. 2004a;24:2000–2011. doi: 10.1128/MCB.24.5.2000-2011.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cong F, Schweizer L, Varmus H. Wnt signals across the plasma membrane to activate the beta-catenin pathway by forming oligomers containing its receptors, Frizzled and LRP. Development. 2004b;131:5103–5115. doi: 10.1242/dev.01318. [DOI] [PubMed] [Google Scholar]
- Dann CE, Hsieh JC, Rattner A, Sharma D, Nathans J, Leahy DJ. Insights into Wnt binding and signalling from the structures of two Frizzled cysteine-rich domains. Nature. 2001;412:86–90. doi: 10.1038/35083601. [DOI] [PubMed] [Google Scholar]
- De Ferrari GV, Moon RT. The ups and downs of Wnt signaling in prevalent neurological disorders. Oncogene. 2006;25:7545–7553. doi: 10.1038/sj.onc.1210064. [DOI] [PubMed] [Google Scholar]
- De Lean A, Stadel JM, Lefkowitz RJ. A ternary complex model explains the agonist-specific binding properties of the adenylate cyclase-coupled beta-adrenergic receptor. J Biol Chem. 1980;255:7108–7117. [PubMed] [Google Scholar]
- Dejmek J, Safholm A, Kamp Nielsen C, Andersson T, Leandersson K. Wnt-5a/Ca2+-induced NFAT activity is counteracted by Wnt-5a/Yes-Cdc42-casein kinase 1alpha signaling in human mammary epithelial cells. Mol Cell Biol. 2006;26:6024–6036. doi: 10.1128/MCB.02354-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Despeaux M, Chicanne G, Rouer E, De Toni-Costes F, Bertrand J, Mansat-De Mas V, et al. Focal adhesion kinase splice variants maintain primitive acute myeloid leukemia cells through altered Wnt signaling. Stem Cells. 2012;30:1597–1610. doi: 10.1002/stem.1157. [DOI] [PubMed] [Google Scholar]
- Downes GB, Gautam N. The G protein subunit gene families. Genomics. 1999;62:544–552. doi: 10.1006/geno.1999.5992. [DOI] [PubMed] [Google Scholar]
- Du Q, Zhang X, Cardinal J, Cao Z, Guo Z, Shao L, et al. Wnt/beta-catenin signaling regulates cytokine-induced human inducible nitric oxide synthase expression by inhibiting nuclear factor-kappaB activation in cancer cells. Cancer Res. 2009;69:3764–3771. doi: 10.1158/0008-5472.CAN-09-0014. [DOI] [PubMed] [Google Scholar]
- Egger-Adam D, Katanaev VL. The trimeric G protein Go inflicts a double impact on axin in the Wnt/frizzled signaling pathway. Dev Dyn. 2010;239:168–183. doi: 10.1002/dvdy.22060. [DOI] [PubMed] [Google Scholar]
- Endo Y, Beauchamp E, Woods D, Taylor WG, Toretsky JA, Uren A, et al. Wnt-3a and Dickkopf-1 stimulate neurite outgrowth in Ewing tumor cells via a Frizzled3-and c-Jun N-terminal kinase-dependent mechanism. Mol Cell Biol. 2008;28:2368–2379. doi: 10.1128/MCB.01780-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foord SM, Bonner TI, Neubig RR, Rosser EM, Pin JP, Davenport AP, et al. International Union of Pharmacology. XLVI. G protein-coupled receptor list. Pharmacol Rev. 2005;57:279–288. doi: 10.1124/pr.57.2.5. [DOI] [PubMed] [Google Scholar]
- Frojmark AS, Schuster J, Sobol M, Entesarian M, Kilander MB, Gabrikova D, et al. Mutations in Frizzled 6 cause isolated autosomal-recessive nail dysplasia. Am J Hum Genet. 2011;88:852–860. doi: 10.1016/j.ajhg.2011.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuerer C, Habib SJ, Nusse R. A study on the interactions between heparan sulfate proteoglycans and Wnt proteins. Dev Dyn. 2010;239:184–190. doi: 10.1002/dvdy.22067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukukawa C, Nagayama S, Tsunoda T, Toguchida J, Nakamura Y, Katagiri T. Activation of the non-canonical Dvl-Rac1-JNK pathway by Frizzled homologue 10 in human synovial sarcoma. Oncogene. 2009;28:1110–1120. doi: 10.1038/onc.2008.467. [DOI] [PubMed] [Google Scholar]
- Gao C, Chen YG. Dishevelled: the hub of Wnt signaling. Cell Signal. 2010;22:717–727. doi: 10.1016/j.cellsig.2009.11.021. [DOI] [PubMed] [Google Scholar]
- Gattinoni L, Ji Y, Restifo NP. Wnt/beta-catenin signaling in T-cell immunity and cancer immunotherapy. Clin Cancer Res. 2010;16:4695–4701. doi: 10.1158/1078-0432.CCR-10-0356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gayen S, Li Q, Kim YM, Kang C. Structure of the C-terminal region of the frizzled receptor 1 in detergent micelles. Molecules. 2013;18:8579–8590. doi: 10.3390/molecules18078579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazit A, Yaniv A, Bafico A, Pramila T, Igarashi M, Kitajewski J, et al. Human frizzled 1 interacts with transforming Wnts to transduce a TCF dependent transcriptional response. Oncogene. 1999;18:5959–5966. doi: 10.1038/sj.onc.1202985. [DOI] [PubMed] [Google Scholar]
- Golan T, Yaniv A, Bafico A, Liu G, Gazit A. The human Frizzled 6 (HFz6) acts as a negative regulator of the canonical Wnt. beta-catenin signaling cascade. J Biol Chem. 2004;279:14879–14888. doi: 10.1074/jbc.M306421200. [DOI] [PubMed] [Google Scholar]
- Halleskog C, Schulte G. Pertussis toxin-sensitive heterotrimeric G(alphai/o) proteins mediate WNT/beta-catenin and WNT/ERK1/2 signaling in mouse primary microglia stimulated with purified WNT-3A. Cell Signal. 2013a;25:822–828. doi: 10.1016/j.cellsig.2012.12.006. [DOI] [PubMed] [Google Scholar]
- Halleskog C, Schulte G. WNT-3A and WNT-5A counteract lipopolysaccharide-induced pro-inflammatory changes in mouse primary microglia. J Neurochem. 2013b;125:803–808. doi: 10.1111/jnc.12250. [DOI] [PubMed] [Google Scholar]
- Halleskog C, Mulder J, Dahlstrom J, Mackie K, Hortobagyi T, Tanila H, et al. WNT signaling in activated microglia is proinflammatory. Glia. 2011;59:119–131. doi: 10.1002/glia.21081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halleskog C, Dijksterhuis JP, Kilander MB, Becerril-Ortega J, Villaescusa JC, Lindgren E, et al. Heterotrimeric G protein-dependent WNT-5A signaling to ERK1/2 mediates distinct aspects of microglia proinflammatory transformation. J Neuroinflammation. 2012;9:111. doi: 10.1186/1742-2094-9-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen C, Howlin J, Tengholm A, Dyachok O, Vogel WF, Nairn AC, et al. Wnt-5a-induced phosphorylation of DARPP-32 inhibits breast cancer cell migration in a CREB-dependent manner. J Biol Chem. 2009;284:27533–27543. doi: 10.1074/jbc.M109.048884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausmann G, Basler K. Wnt lipid modifications: not as saturated as we thought. Dev Cell. 2006;11:751–752. doi: 10.1016/j.devcel.2006.11.007. [DOI] [PubMed] [Google Scholar]
- He X, Saint-Jeannet JP, Wang Y, Nathans J, Dawid I, Varmus H. A member of the Frizzled protein family mediating axis induction by Wnt-5A. Science. 1997;275:1652–1654. doi: 10.1126/science.275.5306.1652. [DOI] [PubMed] [Google Scholar]
- Heinonen KM, Vanegas JR, Lew D, Krosl J, Perreault C. Wnt4 enhances murine hematopoietic progenitor cell expansion through a planar cell polarity-like pathway. PLoS ONE. 2011;6:e19279. doi: 10.1371/journal.pone.0019279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendaoui I, Lavergne E, Lee HS, Hong SH, Kim HZ, Parent C, et al. Inhibition of Wnt/beta-catenin signaling by a soluble collagen-derived frizzled domain interacting with Wnt3a and the receptors frizzled 1 and 8. PLoS ONE. 2012;7:e30601. doi: 10.1371/journal.pone.0030601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrickx M, Leyns L. Non-conventional Frizzled ligands and Wnt receptors. Dev Growth Differ. 2008;50:229–243. doi: 10.1111/j.1440-169X.2008.01016.x. [DOI] [PubMed] [Google Scholar]
- Huang SM, Mishina YM, Liu S, Cheung A, Stegmeier F, Michaud GA, et al. Tankyrase inhibition stabilizes axin and antagonizes Wnt signalling. Nature. 2009;461:614–620. doi: 10.1038/nature08356. [DOI] [PubMed] [Google Scholar]
- Ishikawa T, Tamai Y, Zorn AM, Yoshida H, Seldin MF, Nishikawa S, et al. Mouse Wnt receptor gene Fzd5 is essential for yolk sac and placental angiogenesis. Development. 2001;128:25–33. doi: 10.1242/dev.128.1.25. [DOI] [PubMed] [Google Scholar]
- Janda CY, Waghray D, Levin AM, Thomas C, Garcia KC. Structural basis of Wnt recognition by Frizzled. Science. 2012;337:59–64. doi: 10.1126/science.1222879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssens N, Janicot M, Perera T. The Wnt-dependent signaling pathways as target in oncology drug discovery. Invest New Drugs. 2006;24:263–280. doi: 10.1007/s10637-005-5199-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karasawa T, Yokokura H, Kitajewski J, Lombroso PJ. Frizzled-9 is activated by Wnt-2 and functions in Wnt/beta-catenin signaling. J Biol Chem. 2002;277:37479–37486. doi: 10.1074/jbc.M205658200. [DOI] [PubMed] [Google Scholar]
- Karsenty G. Transcriptional control of skeletogenesis. Annu Rev Genomics Hum Genet. 2008;9:183–196. doi: 10.1146/annurev.genom.9.081307.164437. [DOI] [PubMed] [Google Scholar]
- Katanaev VL, Buestorf S. Frizzled proteins are bona fide G protein-coupled receptors. Nat Proc. 2009 hdl:10101/npre.2009.2765.1. [Google Scholar]
- Katanaev VL, Ponzielli R, Semeriva M, Tomlinson A. Trimeric G protein-dependent frizzled signaling in Drosophila. Cell. 2005;120:111–122. doi: 10.1016/j.cell.2004.11.014. [DOI] [PubMed] [Google Scholar]
- Kaucka M, Plevova K, Pavlova S, Janovska P, Mishra A, Verner J, et al. The planar cell polarity pathway drives pathogenesis of chronic lymphocytic leukemia by the regulation of B-lymphocyte migration. Cancer Res. 2013;73:1491–1501. doi: 10.1158/0008-5472.CAN-12-1752. [DOI] [PubMed] [Google Scholar]
- Kawasaki A, Torii K, Yamashita Y, Nishizawa K, Kanekura K, Katada M, et al. Wnt5a promotes adhesion of human dermal fibroblasts by triggering a phosphatidylinositol-3 kinase/Akt signal. Cell Signal. 2007;19:2498–2506. doi: 10.1016/j.cellsig.2007.07.023. [DOI] [PubMed] [Google Scholar]
- Kemp CR, Willems E, Wawrzak D, Hendrickx M, Agbor Agbor T, Leyns L. Expression of Frizzled5, Frizzled7, and Frizzled10 during early mouse development and interactions with canonical Wnt signaling. Dev Dyn. 2007;236:2011–2019. doi: 10.1002/dvdy.21198. [DOI] [PubMed] [Google Scholar]
- Kilander MB, Dijksterhuis JP, Ganji RS, Bryja V, Schulte G. WNT-5A stimulates the GDP/GTP exchange at pertussis toxin-sensitive heterotrimeric G proteins. Cell Signal. 2011a;23:550–554. doi: 10.1016/j.cellsig.2010.11.004. [DOI] [PubMed] [Google Scholar]
- Kilander MB, Halleskog C, Schulte G. Recombinant WNTs differentially activate beta-catenin-dependent and-independent signalling in mouse microglia-like cells. Acta Physiol (Oxf) 2011b;203:363–372. doi: 10.1111/j.1748-1716.2011.02324.x. [DOI] [PubMed] [Google Scholar]
- Kim M, Lee HC, Tsedensodnom O, Hartley R, Lim YS, Yu E, et al. Functional interaction between Wnt3 and Frizzled-7 leads to activation of the Wnt/beta-catenin signaling pathway in hepatocellular carcinoma cells. J Hepatol. 2008;48:780–791. doi: 10.1016/j.jhep.2007.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King TD, Zhang W, Suto MJ, Li Y. Frizzled7 as an emerging target for cancer therapy. Cell Signal. 2012;24:846–851. doi: 10.1016/j.cellsig.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein D, Demory A, Peyre F, Kroll J, Augustin HG, Helfrich W, et al. Wnt2 acts as a cell type-specific, autocrine growth factor in rat hepatic sinusoidal endothelial cells cross-stimulating the VEGF pathway. Hepatology. 2008;47:1018–1031. doi: 10.1002/hep.22084. [DOI] [PubMed] [Google Scholar]
- Kolben T, Perobner I, Fernsebner K, Lechner F, Geissler C, Ruiz-Heinrich L, et al. Dissecting the impact of Frizzled receptors in Wnt/beta-catenin signaling of human mesenchymal stem cells. Biol Chem. 2012;393:1433–1447. doi: 10.1515/hsz-2012-0186. [DOI] [PubMed] [Google Scholar]
- Koval A, Katanaev VL. Wnt3a stimulation elicits G-protein-coupled receptor properties of mammalian Frizzled proteins. Biochem J. 2011;433:435–440. doi: 10.1042/BJ20101878. [DOI] [PubMed] [Google Scholar]
- Koval A, Katanaev VL. Platforms for high-throughput screening of Wnt/Frizzled antagonists. Drug Discov Today. 2012;17:1316–1322. doi: 10.1016/j.drudis.2012.07.007. [DOI] [PubMed] [Google Scholar]
- Koval A, Kopein D, Purvanov V, Katanaev VL. Europium-labeled GTP as a general nonradioactive substitute for [(35)S]GTPgammaS in high-throughput G protein studies. Anal Biochem. 2010;397:202–207. doi: 10.1016/j.ab.2009.10.028. [DOI] [PubMed] [Google Scholar]
- Koval A, Purvanov V, Egger-Adam D, Katanaev VL. Yellow submarine of the Wnt/Frizzled signaling: submerging from the G protein harbor to the targets. Biochem Pharmacol. 2011;82:1311–1319. doi: 10.1016/j.bcp.2011.06.005. [DOI] [PubMed] [Google Scholar]
- Kremenevskaja N, von Wasielewski R, Rao AS, Schofl C, Andersson T, Brabant G. Wnt-5a has tumor suppressor activity in thyroid carcinoma. Oncogene. 2005;24:2144–2154. doi: 10.1038/sj.onc.1208370. [DOI] [PubMed] [Google Scholar]
- Kuhl M, Sheldahl LC, Park M, Miller JR, Moon RT. The Wnt/Ca2+ pathway: a new vertebrate Wnt signaling pathway takes shape. Trends Genet. 2000;16:279–283. doi: 10.1016/s0168-9525(00)02028-x. [DOI] [PubMed] [Google Scholar]
- L'Episcopo F, Serapide MF, Tirolo C, Testa N, Caniglia S, Morale MC, et al. A Wnt1 regulated Frizzled-1/beta-Catenin signaling pathway as a candidate regulatory circuit controlling mesencephalic dopaminergic neuron-astrocyte crosstalk: therapeutical relevance for neuron survival and neuroprotection. Mol Neurodegener. 2011;6:49. doi: 10.1186/1750-1326-6-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Grand F, Jones AE, Seale V, Scime A, Rudnicki MA. Wnt7a activates the planar cell polarity pathway to drive the symmetric expansion of satellite stem cells. Cell Stem Cell. 2009;4:535–547. doi: 10.1016/j.stem.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Zhong L, Yang X, Andersson T, Huang M, Tang SJ. WNT5A signaling contributes to Abeta-induced neuroinflammation and neurotoxicity. PLoS ONE. 2011;6:e22920. doi: 10.1371/journal.pone.0022920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Chen H, Hu L, Xing Y, Sasaki T, Villosis MF, et al. Ror2 modulates the canonical Wnt signaling in lung epithelial cells through cooperation with Fzd2. BMC Mol Biol. 2008;9:11. doi: 10.1186/1471-2199-9-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisovsky M, Itoh K, Sokol SY. Frizzled receptors activate a novel JNK-dependent pathway that may lead to apoptosis. Curr Biol. 2002;12:53–58. doi: 10.1016/s0960-9822(01)00628-5. [DOI] [PubMed] [Google Scholar]
- Liu C, Wang Y, Smallwood PM, Nathans J. An essential role for Frizzled5 in neuronal survival in the parafascicular nucleus of the thalamus. J Neurosci. 2008;28:5641–5653. doi: 10.1523/JNEUROSCI.1056-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Liu X, Wang H, Moon RT, Malbon CC. Activation of rat frizzled-1 promotes Wnt signaling and differentiation of mouse F9 teratocarcinoma cells via pathways that require Galpha(q) and Galpha(o) function. J Biol Chem. 1999;274:33539–33544. doi: 10.1074/jbc.274.47.33539. [DOI] [PubMed] [Google Scholar]
- Liu T, DeCostanzo AJ, Liu X, Wang H, Hallagan S, Moon RT, et al. G protein signaling from activated rat frizzled-1 to the beta-catenin-Lef-Tcf pathway. Science. 2001;292:1718–1722. doi: 10.1126/science.1060100. [DOI] [PubMed] [Google Scholar]
- Liu X, Rubin JS, Kimmel AR. Rapid, Wnt-induced changes in GSK3beta associations that regulate beta-catenin stabilization are mediated by Galpha proteins. Curr Biol. 2005;15:1989–1997. doi: 10.1016/j.cub.2005.10.050. [DOI] [PubMed] [Google Scholar]
- Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- Lu D, Zhao Y, Tawatao R, Cottam HB, Sen M, Leoni LM, et al. Activation of the Wnt signaling pathway in chronic lymphocytic leukemia. Proc Natl Acad Sci U S A. 2004;101:3118–3123. doi: 10.1073/pnas.0308648100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luhmann UF, Lin J, Acar N, Lammel S, Feil S, Grimm C, et al. Role of the Norrie disease pseudoglioma gene in sprouting angiogenesis during development of the retinal vasculature. Invest Ophthalmol Vis Sci. 2005;46:3372–3382. doi: 10.1167/iovs.05-0174. [DOI] [PubMed] [Google Scholar]
- Lyons JP, Mueller UW, Ji H, Everett C, Fang X, Hsieh JC, et al. Wnt-4 activates the canonical beta-catenin-mediated Wnt pathway and binds Frizzled-6 CRD: functional implications of Wnt/beta-catenin activity in kidney epithelial cells. Exp Cell Res. 2004;298:369–387. doi: 10.1016/j.yexcr.2004.04.036. [DOI] [PubMed] [Google Scholar]
- Ma L, Wang HY. Suppression of cyclic GMP-dependent protein kinase is essential to the Wnt/cGMP/Ca2+ pathway. J Biol Chem. 2006;281:30990–31001. doi: 10.1074/jbc.M603603200. [DOI] [PubMed] [Google Scholar]
- MacDonald BT, He X. Frizzled and LRP5/6 receptors for Wnt/beta-catenin signaling. Cold Spring Harb Perspect Biol. 2012;4 doi: 10.1101/cshperspect.a007880. a007880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald BT, Semenov MV, He X. SnapShot: Wnt/beta-catenin signaling. Cell. 2007;131:1204. doi: 10.1016/j.cell.2007.11.036. [DOI] [PubMed] [Google Scholar]
- MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell. 2009;17:9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Maltzahn J, Bentzinger CF, Rudnicki MA. Wnt7a-Fzd7 signalling directly activates the Akt/mTOR anabolic growth pathway in skeletal muscle. Nat Cell Biol. 2012;14:186–191. doi: 10.1038/ncb2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masckauchan TN, Agalliu D, Vorontchikhina M, Ahn A, Parmalee NL, Li CM, et al. Wnt5a signaling induces proliferation and survival of endothelial cells in vitro and expression of MMP-1 and Tie-2. Mol Biol Cell. 2006;17:5163–5172. doi: 10.1091/mbc.E06-04-0320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto S, Kikuchi A. Regulation of focal adhesion dynamics by Wnt5a signaling. Methods Mol Biol. 2012;839:215–227. doi: 10.1007/978-1-61779-510-7_17. [DOI] [PubMed] [Google Scholar]
- Miao CG, Yang YY, He X, Li XF, Huang C, Huang Y, et al. Wnt signaling pathway in rheumatoid arthritis, with special emphasis on the different roles in synovial inflammation and bone remodeling. Cell Signal. 2013;25:2069–2078. doi: 10.1016/j.cellsig.2013.04.002. [DOI] [PubMed] [Google Scholar]
- Mikels AJ, Nusse R. Purified Wnt5a protein activates or inhibits beta-catenin-TCF signaling depending on receptor context. PLoS Biol. 2006;4:e115. doi: 10.1371/journal.pbio.0040115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molenaar M, van de Wetering M, Oosterwegel M, Peterson-Maduro J, Godsave S, Korinek V, et al. XTcf-3 transcription factor mediates beta-catenin-induced axis formation in Xenopus embryos. Cell. 1996;86:391–399. doi: 10.1016/s0092-8674(00)80112-9. [DOI] [PubMed] [Google Scholar]
- Moon RT, Kohn AD, De Ferrari GV, Kaykas A. WNT and beta-catenin signalling: diseases and therapies. Nat Rev Genet. 2004;5:691–701. doi: 10.1038/nrg1427. [DOI] [PubMed] [Google Scholar]
- Nambotin SB, Lefrancois L, Sainsily X, Berthillon P, Kim M, Wands JR, et al. Pharmacological inhibition of Frizzled-7 displays anti-tumor properties in hepatocellular carcinoma. J Hepatol. 2011;54:288–299. doi: 10.1016/j.jhep.2010.06.033. [DOI] [PubMed] [Google Scholar]
- Naz G, Pasternack SM, Perrin C, Mattheisen M, Refke M, Khan S, et al. FZD6 encoding the Wnt receptor frizzled 6 is mutated in autosomal-recessive nail dysplasia. Br J Dermatol. 2012;166:1088–1094. doi: 10.1111/j.1365-2133.2011.10800.x. [DOI] [PubMed] [Google Scholar]
- Neubig RR, Siderovski DP. Regulators of G-protein signalling as new central nervous system drug targets. Nat Rev Drug Discov. 2002;1:187–197. doi: 10.1038/nrd747. [DOI] [PubMed] [Google Scholar]
- Neumann J, Schaale K, Farhat K, Endermann T, Ulmer AJ, Ehlers S, et al. Frizzled1 is a marker of inflammatory macrophages, and its ligand Wnt3a is involved in reprogramming Mycobacterium tuberculosis-infected macrophages. FASEB J. 2010;24:4599–4612. doi: 10.1096/fj.10-160994. [DOI] [PubMed] [Google Scholar]
- Nunnally AP, Parr BA. Analysis of Fz10 expression in mouse embryos. Dev Genes Evol. 2004;214:144–148. doi: 10.1007/s00427-004-0386-4. [DOI] [PubMed] [Google Scholar]
- Ogden SK, Fei DL, Schilling NS, Ahmed YF, Hwa J, Robbins DJ. G protein Galphai functions immediately downstream of Smoothened in Hedgehog signalling. Nature. 2008;456:967–970. doi: 10.1038/nature07459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta K, Ito A, Kuriyama S, Lupo G, Kosaka M, Ohnuma S, et al. Tsukushi functions as a Wnt signaling inhibitor by competing with Wnt2b for binding to transmembrane protein Frizzled4. Proc Natl Acad Sci U S A. 2011;108:14962–14967. doi: 10.1073/pnas.1100513108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan W, Choi SC, Wang H, Qin Y, Volpicelli-Daley L, Swan L, et al. Wnt3a-mediated formation of phosphatidylinositol 4,5-bisphosphate regulates LRP6 phosphorylation. Science. 2008;321:1350–1353. doi: 10.1126/science.1160741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penzo-Mendez A, Umbhauer M, Djiane A, Boucaut JC, Riou JF. Activation of Gbetagamma signaling downstream of Wnt-11/Xfz7 regulates Cdc42 activity during Xenopus gastrulation. Dev Biol. 2003;257:302–314. doi: 10.1016/s0012-1606(03)00067-8. [DOI] [PubMed] [Google Scholar]
- Pfeuffer T, Helmreich EJ. Structural and functional relationships of guanosine triphosphate binding proteins. Curr Top Cell Regul. 1988;29:129–216. doi: 10.1016/b978-0-12-152829-4.50006-9. [DOI] [PubMed] [Google Scholar]
- Philipp M, Caron MG. Hedgehog signaling: is Smo a G protein-coupled receptor? Curr Biol. 2009;19:R125–R127. doi: 10.1016/j.cub.2008.12.010. [DOI] [PubMed] [Google Scholar]
- Punchihewa C, Ferreira AM, Cassell R, Rodrigues P, Fujii N. Sequence requirement and subtype specificity in the high-affinity interaction between human frizzled and dishevelled proteins. Protein Sci. 2009;18:994–1002. doi: 10.1002/pro.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsay RG, Ciznadija D, Vanevski M, Mantamadiotis T. Transcriptional regulation of cyclo-oxygenase expression: three pillars of control. Int J Immunopathol Pharmacol. 2003;16(2 Suppl):59–67. [PubMed] [Google Scholar]
- Ranheim EA, Kwan HC, Reya T, Wang YK, Weissman IL, Francke U. Frizzled 9 knock-out mice have abnormal B-cell development. Blood. 2005;105:2487–2494. doi: 10.1182/blood-2004-06-2334. [DOI] [PubMed] [Google Scholar]
- Riobo NA, Manning DR. Pathways of signal transduction employed by vertebrate Hedgehogs. Biochem J. 2007;403:369–379. doi: 10.1042/BJ20061723. [DOI] [PubMed] [Google Scholar]
- Riobo NA, Saucy B, Dilizio C, Manning DR. Activation of heterotrimeric G proteins by Smoothened. Proc Natl Acad Sci U S A. 2006;103:12607–12612. doi: 10.1073/pnas.0600880103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robitaille J, MacDonald ML, Kaykas A, Sheldahl LC, Zeisler J, Dube MP, et al. Mutant frizzled-4 disrupts retinal angiogenesis in familial exudative vitreoretinopathy. Nat Genet. 2002;32:326–330. doi: 10.1038/ng957. [DOI] [PubMed] [Google Scholar]
- Rosenwald A, Alizadeh AA, Widhopf G, Simon R, Davis RE, Yu X, et al. Relation of gene expression phenotype to immunoglobulin mutation genotype in B cell chronic lymphocytic leukemia. J Exp Med. 2001;194:1639–1647. doi: 10.1084/jem.194.11.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinfeld B, Albert I, Porfiri E, Fiol C, Munemitsu S, Polakis P. Binding of GSK3beta to the APC-beta-catenin complex and regulation of complex assembly. Science. 1996;272:1023–1026. doi: 10.1126/science.272.5264.1023. [DOI] [PubMed] [Google Scholar]
- Sahores M, Gibb A, Salinas PC. Frizzled-5, a receptor for the synaptic organizer Wnt7a, regulates activity-mediated synaptogenesis. Development. 2010;137:2215–2225. doi: 10.1242/dev.046722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saneyoshi T, Kume S, Amasaki Y, Mikoshiba K. The Wnt/calcium pathway activates NF-AT and promotes ventral cell fate in Xenopus embryos. Nature. 2002;417:295–299. doi: 10.1038/417295a. [DOI] [PubMed] [Google Scholar]
- Sato A, Yamamoto H, Sakane H, Koyama H, Kikuchi A. Wnt5a regulates distinct signalling pathways by binding to Frizzled2. EMBO J. 2010;29:41–54. doi: 10.1038/emboj.2009.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte G. International Union of Basic and Clinical Pharmacology. LXXX. The class Frizzled receptors. Pharmacol Rev. 2010;62:632–667. doi: 10.1124/pr.110.002931. [DOI] [PubMed] [Google Scholar]
- Schulte G, Bryja V. The Frizzled family of unconventional G-protein-coupled receptors. Trends Pharmacol Sci. 2007;28:518–525. doi: 10.1016/j.tips.2007.09.001. [DOI] [PubMed] [Google Scholar]
- Schulte G, Bryja V, Rawal N, Castelo-Branco G, Sousa KM, Arenas E. Purified Wnt-5a increases differentiation of midbrain dopaminergic cells and dishevelled phosphorylation. J Neurochem. 2005;92:1550–1553. doi: 10.1111/j.1471-4159.2004.03022.x. [DOI] [PubMed] [Google Scholar]
- Schwarz-Romond T, Merrifield C, Nichols BJ, Bienz M. The Wnt signalling effector Dishevelled forms dynamic protein assemblies rather than stable associations with cytoplasmic vesicles. J Cell Sci. 2005;118:5269–5277. doi: 10.1242/jcs.02646. [DOI] [PubMed] [Google Scholar]
- Semenov MV, Habas R, Macdonald BT, He X. SnapShot: noncanonical Wnt signaling pathways. Cell. 2007;131:1378. doi: 10.1016/j.cell.2007.12.011. [DOI] [PubMed] [Google Scholar]
- Sen M, Lauterbach K, El-Gabalawy H, Firestein GS, Corr M, Carson DA. Expression and function of wingless and frizzled homologs in rheumatoid arthritis. Proc Natl Acad Sci U S A. 2000;97:2791–2796. doi: 10.1073/pnas.050574297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldahl LC, Park M, Malbon CC, Moon RT. Protein kinase C is differentially stimulated by Wnt and Frizzled homologs in a G-protein-dependent manner. Curr Biol. 1999;9:695–698. doi: 10.1016/s0960-9822(99)80310-8. [DOI] [PubMed] [Google Scholar]
- Sheldahl LC, Slusarski DC, Pandur P, Miller JR, Kuhl M, Moon RT. Dishevelled activates Ca2+ flux, PKC, and CamKII in vertebrate embryos. J Cell Biol. 2003;161:769–777. doi: 10.1083/jcb.200211094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slusarski DC, Corces VG, Moon RT. Interaction of Wnt and a Frizzled homologue triggers G-protein-linked phosphatidylinositol signalling. Nature. 1997a;390:410–413. doi: 10.1038/37138. [DOI] [PubMed] [Google Scholar]
- Slusarski DC, Yang-Snyder J, Busa WB, Moon RT. Modulation of embryonic intracellular Ca2+ signaling by Wnt-5A. Dev Biol. 1997b;182:114–120. doi: 10.1006/dbio.1996.8463. [DOI] [PubMed] [Google Scholar]
- Stemmle LN, Fields TA, Casey PJ. The regulator of G protein signaling domain of axin selectively interacts with Galpha12 but not Galpha13. Mol Pharmacol. 2006;70:1461–1468. doi: 10.1124/mol.106.023705. [DOI] [PubMed] [Google Scholar]
- Strutt H, Price MA, Strutt D. Planar polarity is positively regulated by casein kinase Iepsilon in Drosophila. Curr Biol. 2006;16:1329–1336. doi: 10.1016/j.cub.2006.04.041. [DOI] [PubMed] [Google Scholar]
- Sugimura R, He XC, Venkatraman A, Arai F, Box A, Semerad C, et al. Noncanonical Wnt signaling maintains hematopoietic stem cells in the niche. Cell. 2012;150:351–365. doi: 10.1016/j.cell.2012.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terasaki H, Saitoh T, Shiokawa K, Katoh M. Frizzled-10, up-regulated in primary colorectal cancer, is a positive regulator of the WNT-beta-catenin-TCF signaling pathway. Int J Mol Med. 2002;9:107–112. [PubMed] [Google Scholar]
- Topol L, Jiang X, Choi H, Garrett-Beal L, Carolan PJ, Yang Y. Wnt-5a inhibits the canonical Wnt pathway by promoting GSK-3-independent beta-catenin degradation. J Cell Biol. 2003;162:899–908. doi: 10.1083/jcb.200303158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu X, Joeng KS, Nakayama KI, Nakayama K, Rajagopal J, Carroll TJ, et al. Noncanonical Wnt signaling through G protein-linked PKCdelta activation promotes bone formation. Dev Cell. 2007;12:113–127. doi: 10.1016/j.devcel.2006.11.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umbhauer M, Djiane A, Goisset C, Penzo-Mendez A, Riou JF, Boucaut JC, et al. The C-terminal cytoplasmic Lys-thr-X-X-X-Trp motif in frizzled receptors mediates Wnt/beta-catenin signalling. EMBO J. 2000;19:4944–4954. doi: 10.1093/emboj/19.18.4944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Wetering M, Castrop J, Korinek V, Clevers H. Extensive alternative splicing and dual promoter usage generate Tcf-1 protein isoforms with differential transcription control properties. Mol Cell Biol. 1996;16:745–752. doi: 10.1128/mcb.16.3.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Wu H, Katritch V, Han GW, Huang XP, Liu W, et al. Structure of the human smoothened receptor bound to an antitumour agent. Nature. 2013;497:338–343. doi: 10.1038/nature12167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HX, Tekpetey FR, Kidder GM. Identification of WNT/beta-CATENIN signaling pathway components in human cumulus cells. Mol Hum Reprod. 2009;15:11–17. doi: 10.1093/molehr/gan070. [DOI] [PubMed] [Google Scholar]
- Wang Y, Wang HY. Dvl3 translocates IPMK to the cell membrane in response to Wnt. Cell Signal. 2012;24:2389–2395. doi: 10.1016/j.cellsig.2012.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Badea T, Nathans J. Order from disorder: self-organization in mammalian hair patterning. Proc Natl Acad Sci U S A. 2006a;103:19800–19805. doi: 10.1073/pnas.0609712104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Guo N, Nathans J. The role of Frizzled3 and Frizzled6 in neural tube closure and in the planar polarity of inner-ear sensory hair cells. J Neurosci. 2006b;26:2147–2156. doi: 10.1523/JNEUROSCI.4698-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YK, Samos CH, Peoples R, Perez-Jurado LA, Nusse R, Francke U. A novel human homologue of the Drosophila frizzled wnt receptor gene binds wingless protein and is in the Williams syndrome deletion at 7q11.23. Hum Mol Genet. 1997;6:465–472. doi: 10.1093/hmg/6.3.465. [DOI] [PubMed] [Google Scholar]
- Wang YK, Sporle R, Paperna T, Schughart K, Francke U. Characterization and expression pattern of the frizzled gene Fzd9, the mouse homolog of FZD9 which is deleted in Williams–Beuren syndrome. Genomics. 1999;57:235–248. doi: 10.1006/geno.1999.5773. [DOI] [PubMed] [Google Scholar]
- Wang Z, Shu W, Lu MM, Morrisey EE. Wnt7b activates canonical signaling in epithelial and vascular smooth muscle cells through interactions with Fzd1, Fzd10, and LRP5. Mol Cell Biol. 2005;25:5022–5030. doi: 10.1128/MCB.25.12.5022-5030.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wess J. Molecular basis of receptor/G-protein-coupling selectivity. Pharmacol Ther. 1998;80:231–264. doi: 10.1016/s0163-7258(98)00030-8. [DOI] [PubMed] [Google Scholar]
- Whalen EJ, Rajagopal S, Lefkowitz RJ. Therapeutic potential of beta-arrestin-and G protein-biased agonists. Trends Mol Med. 2011;17:126–139. doi: 10.1016/j.molmed.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willert K, Nusse R. Wnt proteins. Cold Spring Harb Perspect Biol. 2012;4:a007864. doi: 10.1101/cshperspect.a007864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willert K, Brown JD, Danenberg E, Duncan AW, Weissman IL, Reya T, et al. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature. 2003;423:448–452. doi: 10.1038/nature01611. [DOI] [PubMed] [Google Scholar]
- Willert KH. Isolation and application of bioactive Wnt proteins. Methods Mol Biol. 2008;468:17–29. doi: 10.1007/978-1-59745-249-6_2. [DOI] [PubMed] [Google Scholar]
- Winklbauer R, Medina A, Swain RK, Steinbeisser H. Frizzled-7 signalling controls tissue separation during Xenopus gastrulation. Nature. 2001;413:856–860. doi: 10.1038/35101621. [DOI] [PubMed] [Google Scholar]
- Wu J, Mlodzik M. The frizzled extracellular domain is a ligand for Van Gogh/Stbm during nonautonomous planar cell polarity signaling. Dev Cell. 2008;15:462–469. doi: 10.1016/j.devcel.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Jenny A, Mirkovic I, Mlodzik M. Frizzled-Dishevelled signaling specificity outcome can be modulated by Diego in Drosophila. Mech Dev. 2008;125:30–42. doi: 10.1016/j.mod.2007.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia CH, Liu H, Cheung D, Wang M, Cheng C, Du X, et al. A model for familial exudative vitreoretinopathy caused by LPR5 mutations. Hum Mol Genet. 2008;17:1605–1612. doi: 10.1093/hmg/ddn047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Wang Y, Dabdoub A, Smallwood PM, Williams J, Woods C, et al. Vascular development in the retina and inner ear: control by Norrin and Frizzled-4, a high-affinity ligand-receptor pair. Cell. 2004;116:883–895. doi: 10.1016/s0092-8674(04)00216-8. [DOI] [PubMed] [Google Scholar]
- Yan Y, Li Y, Hu C, Gu X, Liu J, Hu YA, et al. Expression of Frizzled10 in mouse central nervous system. Gene Expr Patterns. 9:173–177. doi: 10.1016/j.gep.2008.11.001. [DOI] [PubMed] [Google Scholar]
- Yang L, Wu X, Wang Y, Zhang K, Wu J, Yuan YC, et al. FZD7 has a critical role in cell proliferation in triple negative breast cancer. Oncogene. 2009;30:4437–4446. doi: 10.1038/onc.2011.145. 2011. [DOI] [PubMed] [Google Scholar]
- Yu H, Ye X, Guo N, Nathans J. Frizzled 2 and frizzled 7 function redundantly in convergent extension and closure of the ventricular septum and palate: evidence for a network of interacting genes. Development. 2012;139:4383–4394. doi: 10.1242/dev.083352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Harada Y, Wei X, Shukla D, Rajendran A, Tawansy K, et al. An essential role of the cysteine-rich domain of FZD4 in Norrin/Wnt signaling and familial exudative vitreoretinopathy. J Biol Chem. 2011;286:10210–10215. doi: 10.1074/jbc.M110.194399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Pleasure SJ. Frizzled9 protein is regionally expressed in the developing medial cortical wall and the cells derived from this region. Brain Res Dev Brain Res. 2005;157:93–97. doi: 10.1016/j.devbrainres.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Zhao C, Aviles C, Abel RA, Almli CR, McQuillen P, Pleasure SJ. Hippocampal and visuospatial learning defects in mice with a deletion of frizzled 9, a gene in the Williams syndrome deletion interval. Development. 2005;132:2917–2927. doi: 10.1242/dev.01871. [DOI] [PubMed] [Google Scholar]


