Abstract
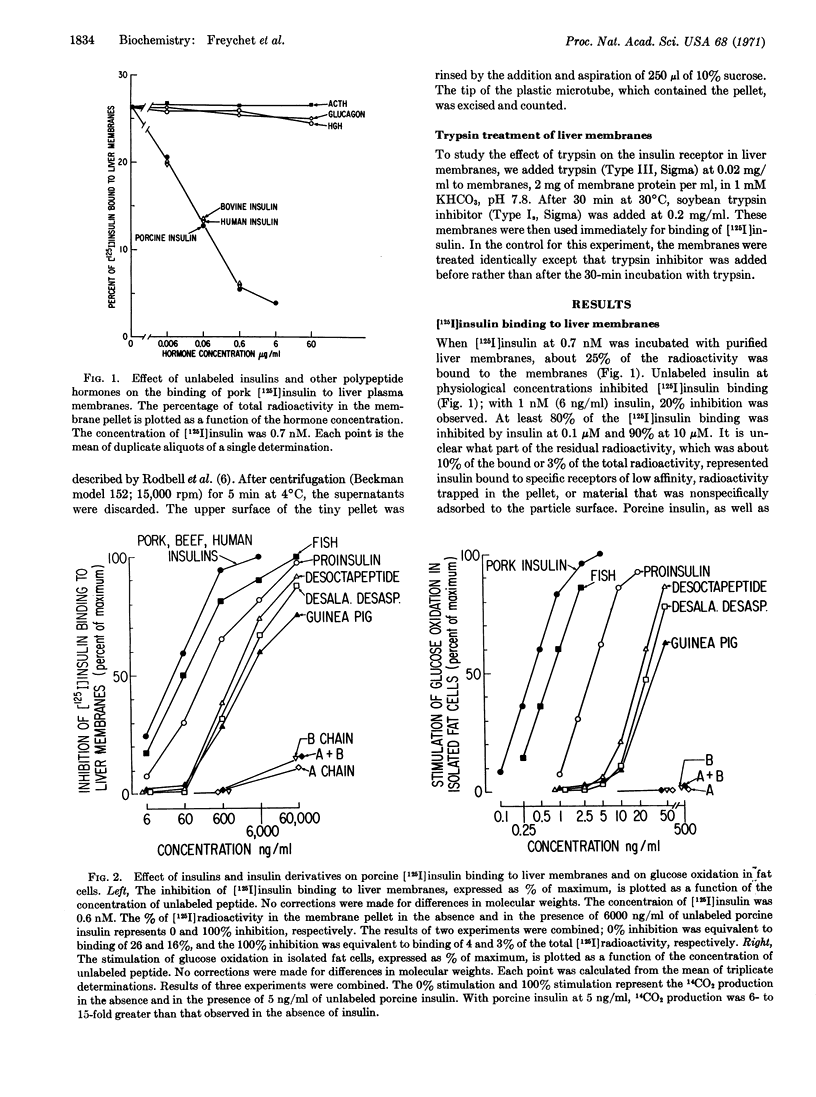
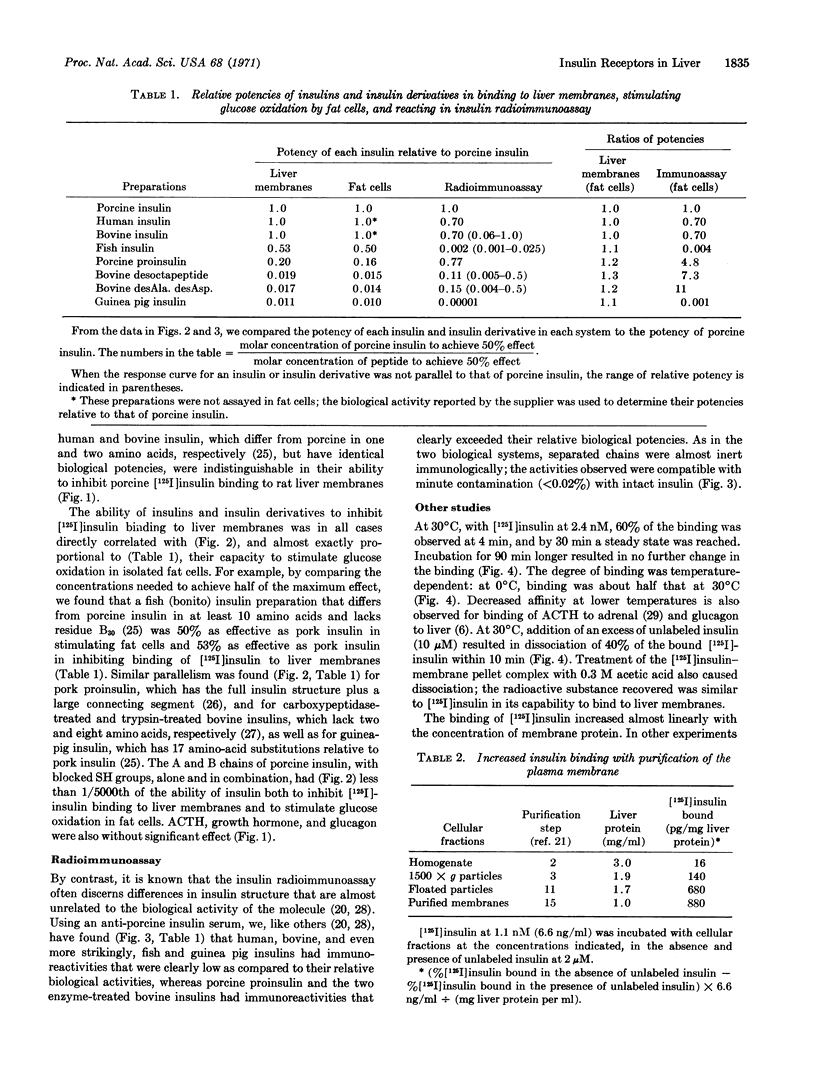
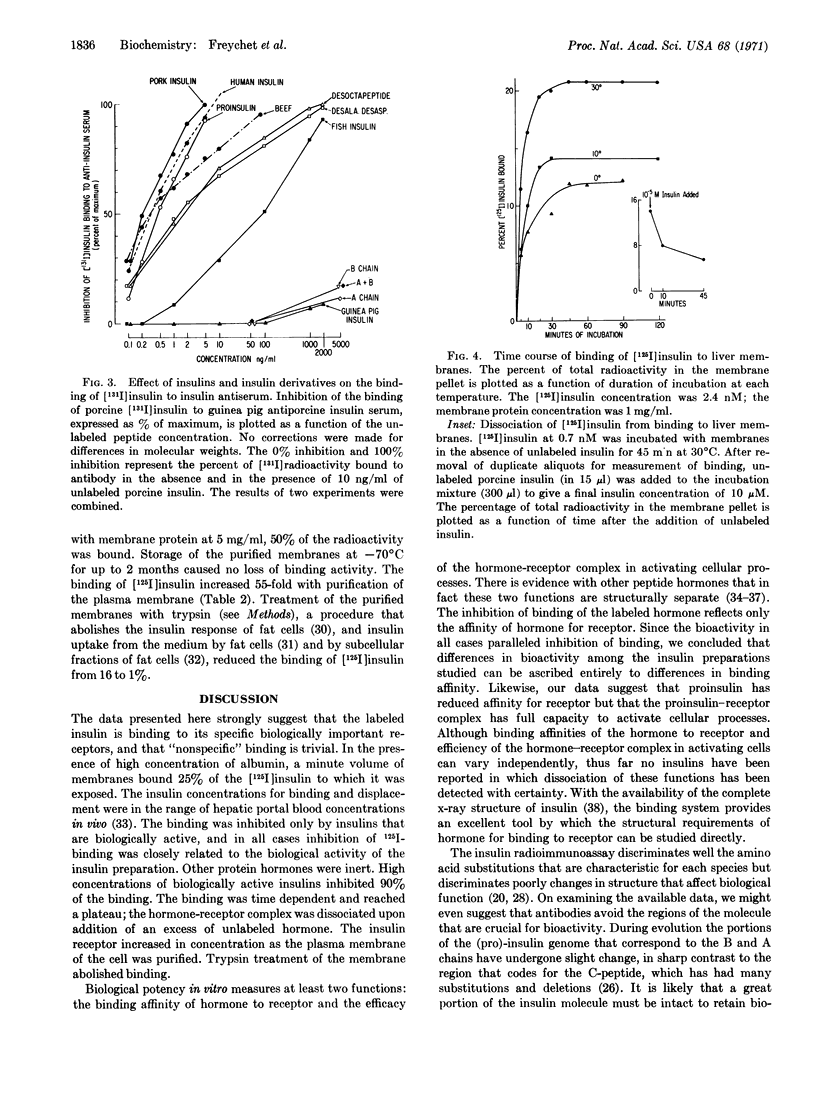
With [125I]insulin at 7 × 10-10 M, 25% of the radioactivity was bound to plasma membranes purified from rat liver. 20% of the [125I]insulin binding was inhibited by unlabeled insulin at 10-9 M (6 ng/ml), equivalent to insulin concentrations in hepatic portal blood; inhibition of [125I]insulin binding was 80% at 10-7 M and 90% at 10-5 M. Eight insulins and derivatives with biological potencies that differed over a 100-fold range inhibited the binding of [125I]insulin to liver membranes in direct proportion to their ability to stimulate glucose oxidation in isolated fat cells. Inactive insulin chains, as well as glucagon, ACTH, and human growth harmone were without effect. The binding of [125I]insulin increased 55-fold as plasma membrane was purified from crude homogenate. Binding was time- and temperature-dependent, and addition of excess insulin produced rapid dissociation of [125I]insulin. This study demonstrates directly the binding of insulin to its biologically important receptors.
Keywords: insulins and derivatives
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berson S. A., Yalow R. S. Insulin in blood and insulin antibodies. Am J Med. 1966 May;40(5):676–690. doi: 10.1016/0002-9343(66)90148-3. [DOI] [PubMed] [Google Scholar]
- Bewsher P. D., Hillman C. C., Ashmore J. Effectsof nethalide on insulin activity and binding by rat muscle and adipose tissue. Mol Pharmacol. 1966 May;2(3):227–236. [PubMed] [Google Scholar]
- Birnbaumer L., Rodbell M. Adenyl cyclase in fat cells. II. Hormone receptors. J Biol Chem. 1969 Jul 10;244(13):3477–3482. [PubMed] [Google Scholar]
- Blackard W. G., Nelson N. C. Portal and peripheral vein immunoreactive insulin concentrations before and after glucose infusion. Diabetes. 1970 May;19(5):302–306. doi: 10.2337/diab.19.5.302. [DOI] [PubMed] [Google Scholar]
- Carpenter F. H. Relationship of structure to biological activity of insulin as revealed by degradative studies. Am J Med. 1966 May;40(5):750–758. doi: 10.1016/0002-9343(66)90156-2. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P. Insulin--receptor interactions in adipose tissue cells: direct measurement and properties. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1264–1268. doi: 10.1073/pnas.68.6.1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuatrecasas P. Interaction of insulin with the cell membrane: the primary action of insulin. Proc Natl Acad Sci U S A. 1969 Jun;63(2):450–457. doi: 10.1073/pnas.63.2.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freychet P., Roth J., Neville D. M., Jr Monoiodoinsulin: demonstration of its biological activity and binding to fat cells and liver membranes. Biochem Biophys Res Commun. 1971 Apr 16;43(2):400–408. doi: 10.1016/0006-291x(71)90767-4. [DOI] [PubMed] [Google Scholar]
- Garratt C. J., Jarrett R. J., Keen H. The relationship between insulin association with tissues and insulin action. Biochim Biophys Acta. 1966 May 26;121(1):143–150. doi: 10.1016/0304-4165(66)90357-6. [DOI] [PubMed] [Google Scholar]
- HILLS A. G., STADIE W. C. The effect of combined insulin upon the metabolism of the lactating mammary glands of the rat. J Biol Chem. 1952 Jan;194(1):25–31. [PubMed] [Google Scholar]
- Hofmann K., Wingender W., Finn F. M. Correlation of adrenocorticotropic activity of ACTH analogs with degree of binding to an adrenal cortical particulate preparation. Proc Natl Acad Sci U S A. 1970 Oct;67(2):829–836. doi: 10.1073/pnas.67.2.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- House P. D., Weidemann M. J. Characterization of an [125 I]-insulin binding plasma membrane fraction from rat liver. Biochem Biophys Res Commun. 1970 Nov 9;41(3):541–548. doi: 10.1016/0006-291x(70)90046-x. [DOI] [PubMed] [Google Scholar]
- Kono T. Destruction and restoration of the insulin effector system of isolated fat cells. J Biol Chem. 1969 Nov 10;244(21):5777–5784. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lefkowitz R. J., Roth J., Pricer W., Pastan I. ACTH receptors in the adrenal: specific binding of ACTH-125I and its relation to adenyl cyclase. Proc Natl Acad Sci U S A. 1970 Mar;65(3):745–752. doi: 10.1073/pnas.65.3.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S. Y., Goodfriend T. L. Angiotensin receptors. Am J Physiol. 1970 May;218(5):1319–1328. doi: 10.1152/ajplegacy.1970.218.5.1319. [DOI] [PubMed] [Google Scholar]
- Malaisse W., Franckson J. R. Application des radioisotopes à l'étude de la consommation de glucose par le diaphragme de rat normal. IV. Relation entre la fixation tissulaire et l'effet métabolique de l'insuline. Arch Int Pharmacodyn Ther. 1965 Jun;155(2):484–494. [PubMed] [Google Scholar]
- Melani F., Rubenstein A. H., Oyer P. E., Steiner D. F. Identification of proinsulin and C-peptide in human serum by a specific immunoassay. Proc Natl Acad Sci U S A. 1970 Sep;67(1):148–155. doi: 10.1073/pnas.67.1.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEVILLE D. M., Jr The isolation of a cell membrane fraction from rat liver. J Biophys Biochem Cytol. 1960 Oct;8:413–422. doi: 10.1083/jcb.8.2.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEWERLY K., BERSON S. A. Lack of specificity of insulin-I 131-binding by isolated rat diaphragm. Proc Soc Exp Biol Med. 1957 Apr;94(4):751–755. doi: 10.3181/00379727-94-23075. [DOI] [PubMed] [Google Scholar]
- Neville D. M., Jr Isolation of an organ specific protein antigen from cell-surface membrane of rat liver. Biochim Biophys Acta. 1968 Apr 9;154(3):540–552. doi: 10.1016/0005-2795(68)90014-7. [DOI] [PubMed] [Google Scholar]
- Pastan I., Roth J., Macchia V. Binding of hormone to tissue: the first step in polypeptide hormone action. Proc Natl Acad Sci U S A. 1966 Dec;56(6):1802–1809. doi: 10.1073/pnas.56.6.1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohl S. L., Birnbaumer L., Rodbell M. The glucagon-sensitive adenyl cyclase system in plasma membranes of rat liver. I. Properties. J Biol Chem. 1971 Mar 25;246(6):1849–1856. [PubMed] [Google Scholar]
- RODBELL M. METABOLISM OF ISOLATED FAT CELLS. I. EFFECTS OF HORMONES ON GLUCOSE METABOLISM AND LIPOLYSIS. J Biol Chem. 1964 Feb;239:375–380. [PubMed] [Google Scholar]
- Rodbell M., Birnbaumer L., Pohl S. L., Sundby F. The reaction of glucagon with its receptor: evidence for discrete regions of activity and binding in the glucagon molecule. Proc Natl Acad Sci U S A. 1971 May;68(5):909–913. doi: 10.1073/pnas.68.5.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodbell M., Krans H. M., Pohl S. L., Birnbaumer L. The glucagon-sensitive adenyl cyclase system in plasma membranes of rat liver. 3. Binding of glucagon: method of assay and specificity. J Biol Chem. 1971 Mar 25;246(6):1861–1871. [PubMed] [Google Scholar]
- STADIE W. C., HAUGAARD N., VAUGHAN M. Studies of insulin binding with isotopically labeled insulin. J Biol Chem. 1952 Dec;199(2):729–739. [PubMed] [Google Scholar]
- Sherman B. M., Gorden P., Roth J., Freychet P. Circulating insulin: th proinsulin-like properties of "big" insulin in patients withou islet cell tumors. J Clin Invest. 1971 Apr;50(4):849–858. doi: 10.1172/JCI106556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith L. F. Species variation in the amino acid sequence of insulin. Am J Med. 1966 May;40(5):662–666. doi: 10.1016/0002-9343(66)90145-8. [DOI] [PubMed] [Google Scholar]
- Tomasi V., Koretz S., Ray T. K., Dunnick J., Marinetti G. V. Hormone action at the membrane level. II. The binding of epinephrine and glucagon to the rat liver plasma membrane. Biochim Biophys Acta. 1970 Jul 7;211(1):31–42. doi: 10.1016/0005-2736(70)90120-3. [DOI] [PubMed] [Google Scholar]
- Wohltmann H. J., Narahara H. T. Binding of insulin-131-I by isolated frog sartorius muscles. Relationship to changes in permeability to sugar caused by insulin. J Biol Chem. 1966 Nov 10;241(21):4931–4939. [PubMed] [Google Scholar]
- YALOW R. S., BERSON S. A. REACTION OF FISH INSULINS WITH HUMAN INSULIN ANTISERUMS. POTENTIAL VALUE IN THE TREATMENT OF INSULIN RESISTANCE. N Engl J Med. 1964 May 28;270:1171–1178. doi: 10.1056/NEJM196405282702207. [DOI] [PubMed] [Google Scholar]


