Abstract
Background/Objective
Several studies have described the epidemiological distribution of bla OXA-58-harboring Acinetobacter baumannii in China. However, there is limited data concerning the replicon types of bla OXA-58-carrying plasmids and the genetic context surrounding bla OXA-58 in Acinetobacter spp. in China.
Methodology/Principal Findings
Twelve non-duplicated bla OXA-58-harboring Acinetobacter spp. isolates were collected from six hospitals in five different cities between 2005 and 2010. The molecular epidemiology of the isolates was carried out using PFGE and multilocus sequence typing. Carbapenemase-encoding genes and plasmid replicase genes were identified by PCR. The genetic location of bla OXA-58 was analyzed using S1-nuclease method. Plasmid conjugation and electrotransformation were performed to evaluate the transferability of bla OXA-58-harboring plasmids. The genetic structure surrounding bla OXA-58 was determined by cloning experiments. The twelve isolates included two Acinetobacter pittii isolates (belong to one pulsotype), three Acinetobacter nosocomialis isolates (belong to two pulsotypes) and seven Acinetobacter baumannii isolates (belong to two pulsotypes/sequence types). A. baumannii ST91 was found to be a potential multidrug resistant risk clone carrying both bla OXA-58 and bla OXA-23. bla OXA-58 located on plasmids varied from ca. 52 kb to ca. 143 kb. All plasmids can be electrotransformed to A. baumannii recipient, but were untypeable by the current replicon typing scheme. A novel plasmid replicase named repAci10 was identified in bla OXA-58-harboring plasmids of two A. pittii isolates, three A. nosocomialis isolates and two A. baumannii isolates. Four kinds of genetic contexts of bla OXA-58 were identified. The transformants of plasmids with structure of IS6 family insertion sequence (ISOur1, IS1008 or IS15)-ΔISAba3-like element-bla OXA-58 displayed carbapenem nonsusceptible, while others with structure of intact ISAba3-like element-bla OXA-58 were carbapenem susceptible.
Conclusion
The study revealed the unique features of bla OXA-58-carrying plasmids in Acinetobacter spp. in China, which were different from that of Acinetobacter spp. found in European countries. The diversity of the genetic contexts of bla OXA-58 contributed to various antibiotics resistance profiles.
Introduction
Members of the genus Acinetobacter are significant nosocomial pathogens. Acinetobacter baumannii and its two close relatives, Acinetobacter pittii and Acinetobacter nosocomialis account for the majority of Acinetobacter infections [1]. A number of reports have detailed the significant increase in resistance of Acinetobacter spp. to conventional antibiotics, including carbapenems, the main therapeutic alternative against multidrug resistant Acinetobacter infections [2].
The worldwide emergence of carbapenem resistant Acinetobacter may be attributed to the spread of some risk resistant clones and the horizontal transmission of carbapenemase genes [1], [3]. Carbapenem-hydrolyzing class D β-lactamases (CHDLs) are the most concerning carbapenem resistant determinants in Acinetobacter spp [1]. OXA-58 is a widely spread CHDL that has been reported in Acinetobacter spp. from Europe [4], Argentina [5], Australia [6], the United States [7] and many Asian countries [8]. Though OXA-58 shows only low carbapenem-hydrolyzing activity in vitro, the insertion sequence upstream of bla OXA-58 enhances its transcription greatly and mediates resistance to carbapenems [9]–[11].
bla OXA-58 exists not only in A. baumannii, but also in A. pittii [12], A. nosocomialis [11], Acinetobacter radioresistens [13], Acinetobacter junii [6], and Acinetobacter phenon 6/ct13TU [14]. bla OXA-58 is usually plasmid-borne, which may explain its wide dissemination. It has been reported that OXA-58 producing A. baumannii from European countries are associated with carriage of plasmid replicase gene repAci1 [15]. However, little is known about the replicon types of bla OXA-58-carrying plasmids in A. baumannii and non-baumannii Acinetobacter spp. outside of Europe.
bla OXA-58 is the second most frequently identified CHDL in A. baumannii in China. However, the current data is limited to simple epidemiological distribution [16], [17]. In this study, we detailed characterized the genetic contexts surrounding bla OXA-58 and the replicon typing of the bla OXA-58-carrying plasmids in Acinetobacter spp. isolates from multiple cities in China.
Materials and Methods
Bacterial Strains and Antimicrobial Susceptibility Testing
Twelve non-duplicated bla OXA-58-harboring Acinetobacter spp. isolates collected from six hospitals in five different cities in China between 2005 and 2010 were analyzed in this study (Table 1). The genomic species identification was performed by sequence analysis of the 16S-23S rRNA intergenic spacer region [18].
Table 1. Basic information, epidemiological features and resistant genes of Acinetobacter spp. included in this studya.
| Strain | Species | Hospital (Cities) | Year | PFGEtype | ST | allelic profilesb | bla OXA genes | ESBLgenes |
| AP04 | A. pittii | HZ (Hangzhou) | 2009 | A | ND | – | bla OXA-58 | Neg |
| AP25 | A. pittii | TZ (Taizhou) | 2009 | A | ND | – | bla OXA-58 | Neg |
| AN113 | A. nosocomialis | WZ (Wenzhou) | 2009 | B | ND | – | bla OXA-58 | Neg |
| AN116 | A. nosocomialis | WZ (Wenzhou) | 2009 | B | ND | – | bla OXA-58 | Neg |
| AN119 | A. nosocomialis | WZ (Wenzhou) | 2009 | C | ND | – | bla OXA-58 | Neg |
| WA3 | A. baumannii | WHC (Wuhan) | 2008 | E | 363 | 51-54-49-11-48-25-4 | bla OXA-58, bla OXA-51 | bla PER-1 |
| WA8 | A. baumannii | WHC (Wuhan) | 2008 | E | 363 | 51-54-49-11-48-25-4 | bla OXA-58, bla OXA-51 | bla PER-1 |
| WH8144 | A. baumannii | WH (Wuhan) | 2010 | D | 91 | 22-15-13-12-4-62-2 | bla OXA-58, bla OXA-23, bla OXA-51 | Neg |
| JH01 | A. baumannii | JH (Jinhua) | 2005 | D | 91 | 22-15-13-12-4-62-2 | bla OXA-58, bla OXA-23, bla OXA-51 | Neg |
| JH02 | A. baumannii | JH (Jinhua) | 2005 | D | 91 | 22-15-13-12-4-62-2 | bla OXA-58, bla OXA-23, bla OXA-51 | Neg |
| AB212 | A. baumannii | JH (Jinhua) | 2009 | D | 91 | 22-15-13-12-4-62-2 | bla OXA-58, bla OXA-23, bla OXA-51 | Neg |
| AB222 | A. baumannii | JH (Jinhua) | 2009 | D | 91 | 22-15-13-12-4-62-2 | bla OXA-58, bla OXA-23, bla OXA-51 | Neg |
| LS0148 | A. baumannii | LS (Lishui) | 2005 | ND | 20 | 1-15-13-12-4-12-2 | bla OXA-51 | Neg |
a Abbreviations: HZ, Hangzhou First hospital; TZ, Taizhou Hospital; WZ, The First Affiliated Hospital of Wenzhou Medical College; WHC, Wuhan Children Hospital; WH, Wuhan Tongji Hospital; JH, Jinhua Center Hospital; LS, Lishui People Hospital; ND: not defined; Neg: negative; Pos: positive.
b Seven loci in the order of gltA-gyrB-gdhB-recA-cpn60-gpi-rpoD.
Imipenem and ticarcillin-susceptible clinical A. baumannii strain LS0148 (imipenem MIC, 0.5 mg/L; ticarcillin MIC, 16 mg/L), deposited in our laboratory, was used as the recipient for plasmid electrotransformation (Table 1). A colistin-resistant mutant strain of A. baumannii LS0148 (colistin MIC, 64 mg/L) was used as the recipient for plasmid conjugation.
MICs were determined by the agar dilution method. Interpretation of the results was in accordance with the CLSI 2013 criteria.
All isolates present in this study were stored in the Department of Microbiology, the First Affiliated Hospital, College of Medicine, Zhejiang University. We obtained an exempt status from the Institutional Review Board of the First Affiliated Hospital, College of Medicine, Zhejiang University to use these strains to perform all experiments in this study.
PCR Experiments for the Resistance Genes
PCR assays for the presence of carbapenemase encoding genes (bla OXA-51-like, bla OXA-58-like, bla OXA-23-like, bla OXA-40-like, bla OXA-143, bla IMP, bla VIM, bla SIM and bla NDM) and ESBL genes (bla PER and bla SHV) were performed as previously reported [19]–[21].
Pulsed-field Gel Electrophoresis and Multilocus Sequence Typing Analysis
The genetic relationship of the isolates was evaluated by pulsed-field gel electrophoresis (PFGE) and multilocus sequence typing (MLST). The results of PFGE were interpreted as Tenover et al. recommended [22]. MLST was carried out using the scheme developed by Bartual et al. with some modifications to the primers of the alleles of gyrB and rpoD as we previously reported [23], [24].
Plasmid Conjugation and Electrotransformation
Plasmid conjugations were performed between OXA-58 producing Acinetobacter spp. as donors and a colistin-resistant mutant strain of A. baumannii LS0148 as the recipient. The transconjugants were selected on MH agar plates containing ticarcillin (100 mg/L) and colistin (10 mg/L).
The electrical pulse setting of plasmid electrotransformation was 1.8 kV, 25 µF, 200 Ω with Bio-Rad GenePulser Xcell system (Bio-Rad, Shanghai, China). A. baumannii strain LS0148 was used as the recipient. The transformants were selected on MH agar plates containing ticarcillin (100 mg/L).
S1 Nuclease-based Plasmid Analysis
The plasmid size and the location of bla OXA-58 were analyzed using the S1 nuclease-PFGE method as previously reported [25]. The bacterial-imbedded gel slices were incubated with 10 U S1 nuclease (Takara, Dalian, China) for 40 minutes in 37°C water bath. The digestion products were separated by PFGE using Bio-Rad CHEF Mapper XA system (Bio-Rad, Shanghai, China) with switch times of 2.16S to 63.8S for 18 hours.
The separated DNA was transferred to a positive charged Nylon membrane (Millipore, Shanghai, China) and hybridized with a digoxigenin-labled bla OXA-58 probe. The detection of hybrids was performed using enzyme immunoassay and NBT/BCIP coloration according to the manufacturer’s instruction (Roche, Shanghai, China).
PCR-based Plasmid Replicon Typing
The plasmid replicase genes were detected by multiplex PCR scheme developed by Bertini et al [26]. The novel replicase gene repAci10 was detected by a single PCR with primers designed in this study (Forward primer: 5′-TAGGACGTCAAGCATCTTA-3′; backward primer: 5′-TCGCTATCAAGAAGATCAC-3′).
Cloning Experiments
The genetic contexts of bla OXA-58 were determined by cloning and sequencing experiments. The plasmids or total DNA were digested by EcoRI or SacI. The digested fragments were inserted into corresponding sites of pET28a, and the ligation mixture was used for transformation. Transformants were selected on MH agar containing ampicillin 50 mg/L and kanamycin 50 mg/L. The bla OXA-58-containing inserts were fully or partially sequenced to obtain the context of bla OXA-58.
Nucleotide Sequence Accession Numbers
The novel insertion sequence ISAba20 has been submitted to the IS Finder Database (http://www-is.biotoul.fr/). The nucleotide sequences surrounding bla OXA-58 of AN119, AP04, WA3 and WH8144 are deposited in the GenBank database under accession no. JQ241789 to JQ241792 respectively.
Results
Species Identification and Antimicrobial Susceptibility Profiles
The 12 OXA-58-producing Acinetobacter spp. isolates were assigned to three genomic species: A. baumannii (seven isolates), A. nosocomialis (three isolates) and A. pittii (two isolates), and showed various resistance profiles (Table 1 and 2). The five non-baumanii Acinetobacter displayed imipenem and meropenem susceptible. On the contrary, all of the A. baumannii isolates were imipenem and meropenem resistance. In general, the A. baumannii isolates were more frequently resistant to broad-spectrum cephalosporins, ampicillin/sulbactam, aminoglycosides, ciprofloxacin and minocycline than the five non-baumanii Acinetobacter.
Table 2. The sizes and replicon types of bla OXA-58-harboring plasmids, genetic contexts of bla OXA-58, and MICs (mg/L) of represented strainsa.
| Isolatesb | Plasmidsize (kb) | rep genegroupc | Geneticcontextsof bla OXA-58 d | IPM | MEM | FEP | CAZ | CTX | SAM | TZP | TIC | GEN | AMK | MIN | CIP |
| AP04 | 93 | aci10 | A | 0.5 | 0.5 | 4 | 4 | 16 | 4 | 32 | >256 | 64 | 2 | <0.125 | <0.125 |
| AP25 | 52 | aci10 | A | 1 | 0.5 | 2 | 4 | 16 | 2 | 16 | >256 | 2 | 2 | <0.125 | <0.125 |
| AN113 | 143 | aci10 | A | 0.5 | 1 | 4 | 4 | 16 | 1 | 16 | >256 | >256 | 4 | 4 | 0.5 |
| AN116 | 143 | aci10 | A | 1 | 2 | 16 | 8 | 32 | 16 | 128 | >256 | >256 | 4 | 4 | 0.25 |
| AN119 | 76 | aci10 | D | 4 | 2 | 4 | 8 | 16 | 4 | 64 | >256 | 128 | 4 | 0.5 | 0.25 |
| WA3 | 101 | aci10 | C | >32 | 8 | 256 | >256 | >256 | 64 | 256 | >256 | 64 | 16 | <0.125 | <0.125 |
| WH8144 | 55 | – | B | >32 | >32 | 128 | 32 | 64 | 128 | >256 | >256 | >256 | 256 | 64 | 64 |
| JH01 | 55 | GR8 | B | >32 | >32 | 256 | 128 | 256 | 128 | >256 | >256 | >256 | >256 | 64 | 16 |
| AB212 | 55 | – | B | >32 | >32 | 32 | 64 | 128 | 32 | >256 | >256 | >256 | >256 | 32 | 16 |
| LS0148 | – | [GR3, GR7] | – | 0.5 | 0.5 | 2 | 4 | 16 | 2 | 16 | 16 | 1 | 2 | 8 | 16 |
| TAP04 | 93 | aci10, [GR3, GR7] | A | 2 | 2 | 4 | 8 | 16 | 4 | 64 | >256 | 16 | 2 | 8 | 32 |
| TAP25 | 52 | aci10, [GR3, GR7] | A | 2 | 1 | 4 | 8 | 16 | 4 | 64 | >256 | 4 | 4 | 8 | 32 |
| TAN113 | 143 | aci10, [GR3, GR7] | A | 1 | 1 | 4 | 8 | 16 | 2 | 32 | >256 | >256 | 4 | 4 | 32 |
| TAN116 | 143 | aci10, [GR3, GR7] | A | 1 | 2 | 4 | 4 | 16 | 2 | 16 | >256 | >256 | 4 | 4 | 32 |
| TAN119 | 76 | aci10, [GR3, GR7] | D | 16 | 16 | 4 | 8 | 16 | 16 | 256 | >256 | 64 | 4 | 8 | 32 |
| TWA3 | 101 | aci10, [GR3, GR7] | C | 16 | 8 | 4 | 8 | 16 | 16 | 256 | >256 | 1 | 2 | 8 | 16 |
| TWH8144 | 55 | [GR3, GR7] | B | 8 | 4 | 4 | 8 | 16 | 8 | 128 | >256 | 256 | 128 | 8 | 16 |
| TJH01 | 55 | [GR3, GR7] | B | 8 | 4 | 4 | 8 | 16 | 8 | 128 | >256 | 256 | 128 | 8 | 16 |
| TAB212 | 55 | [GR3, GR7] | B | 16 | 4 | 4 | 8 | 16 | 8 | 256 | >256 | 256 | 128 | 8 | 16 |
a IPM, imipenem; MEM, meropenem; FEP, cefepime; CAZ, ceftazidime; CTX, cefotaxime; SAM, ampicillin/sulbactam; TZP, piperacillin/tazobactam; TIC, ticarcillin; GEN, gentamicin; AMK, amikacin; MIN, minocycline; CIP, ciprofloxacin.
b The isolates with names starting with alphabet T- were transformants;
c Brackets indicate that GR3 and GR7 replicases are present in the recipient strain LS0148.
d The alphabet corresponded to four kinds of genetic structure displayed in Figure 1.
Molecular Epidemiology of the OXA-58-producing Acinetobacter spp.
PFGE identified five pulsotypes among the 12 OXA-58-producing Acinetobacter spp. isolates (Table. 1). Two A. pittii isolates from different hospitals showed a same pulsotype. Three A. nosocomialis isolates from a single hospital belonged to two pulsotypes. Seven A. baumannii isolates were divided into two pulsotypes, corresponding to two sequence types (ST91 and ST363). A. baumannii ST91 were identified from two hospitals in different cities (Wuhan and Jinhua). Moreover, ST91 were detected in A. baumannii collected from Jinhua Center Hospital in 2005 and 2009, implying probable endemic in this hospital.
Distribution of Resistance Genes
The A. pittii and A. nosocomialis were negative for other carbapenemase genes and ESBLs. Intrinsic bla OXA-51 was detected in all A. baumannii isolates. All A. baumannii ST91 isolates carried another CHDL gene bla OXA-23. bla PER-1 was detected in WA3 and WA8 (Table 1).
The Plasmid Localization of bla OXA-58
The bla OXA-58-probe hybridized with plasmid bands of different sizes, from ca. 52 kb to 143 kb. Isolates with same pulsotypes generally possessed same plasmid location of bla OXA-58, except AP04 and AP25 (Table 2).
The Transferability of bla OXA-58-carrying Plasmids
While plasmid conjugation ultimately failed, bla OXA-58-carrying plasmids were successfully electro-transferred from all Acinetobacter spp. isolates to the recipient strain.
PCR detection of transformants found bla PER-1 and bla OXA-23 were not co-transferred with bla OXA-58, suggesting these genes are not colocalized on a single plasmid.
The results of antimicrobial susceptibility testing are presented in Table 2. Electrotransformation of bla OXA-58-harboring plasmids into recipient strain LS0148 resulted in high resistance to ticarcillin (>256 mg/L) and increased MICs of imipenem and meropenem (2 to 32 folds), but transformants retained similar MICs of cefepime, ceftazidime and cefotaxime when compared with that of the original LS0148 strain. The transformants of A. nosocomialis AN119 and all A. baumannii displayed higher MICs of imipenem and meropenem than transformants of A. pittii isolates and remaining A. nosocomialis isolates. Transformants TWH8144, TJH01, TAB212 also showed gentamicin and amikacin resistance, implying potential aminoglycosides resistant determinants are colocalized with bla OXA-58 on the same plasmid.
Identification a Novel Plasmid Replicase Gene
Further investigation of the bla OXA-58-containing clone fragment of strain WA3 identified a novel plasmid replication protein gene (Figure. 1). This replication protein belonged to Rep-3 superfamily group. It shared similarity with two replication proteins deposited in GenBank database: Acinetobacter sp. RUH2624 (ZP_05826577; 100% amino acid identity) and A. radioresistens SH164 (ZP_06073941; 73% amino acid identity). We have designated this novel replicase gene as repAci10 herein. Of the available A. baumannii replicase genes in the current replicon typing scheme [26], repAci5 was most similar to repAci10 (66% nucleotide identity). Therefore, repAci10 should be assigned as a novel homolog group (GR20). No iteron was identified upstream of repAci10.
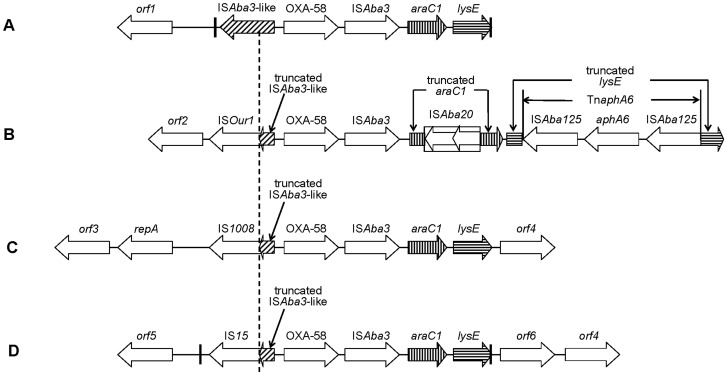
Figure 1. Schematic map of the genetic contexts of bla OXA-58.
Structure A (AP04, AP25, AN113 and AN116); Structure B (WH8144, JH01, JH02, AB212 and AB222); Structure C (WA3 and WA8); Structure D (AN119). The dash line indicates the truncated positions of ISAba3-like element. The thick vertical lines indicate the Re27 recombination points. The location and orientation of primers are indicated by arrows and numbers, being consistent with Table 2. orf1, DNA-binding response regulator gene; orf2, putative exodeoxyribonuclease VII large subunit gene; orf3, ParA family protein gene; orf4, putative inner membrane protein gene; orf5, putative chromate transporter gene; orf6, putative cytoplasmic protein gene. GenBank accession No.: A, JQ241790; B, JQ241792; C, JQ241791; D, JQ241789. The figure is not to scale.
Using the current PCR-based replicon typing scheme of A. baumannii [26], only GR8 was detected in strain JH01 and JH02 from the 12 OXA-58 producing Acinetobacter spp. (Table 2). GR3 and GR7 are the intrinsic plasmid rep genes of recipient strain LS0148. No other replicase genes were detected in the transformants except for the intrinsic plasmid replicase genes of LS0148 (GR3 and GR7), suggesting the bla OXA-58-carrying plasmids do not belong to any previously known replicon group. The novel replicase gene repAci10 was detected in A. pittii (AP04, AP25), A. nosocomialis (AN113, AN116 and AN119), A. baumannii (WA3, WA8) and their transformants (Table 2).
Genetic Contexts of bla OXA-58
Four kinds of genetic contexts of bla OXA-58 were identified among 12 Acinetobacter spp. (Figure. 1). Structure A included two A. pittii isolates (AP04, AP25) and two A. nosocomialis isolates (AN113 and AN116). Structure B encompassed all A. baumannii ST91 isolates of WH8144, JH01, JH02, AB212 and AB222. Structure C encompassed A. baumannii isolates WA3 and WA8. Structure D included A. nosocomialis isolate AN119. The most notable difference was the IS elements located upstream of bla OXA-58. In structure A, an intact ISAba3-like element was exclusively present upstream of bla OXA-58. However, in structure B, C and D, the ISAba3-like element upstream of bla OXA-58 was truncated at a same position (58 bp downstream of the start codon of the transposase gene of ISAba3-like element) by ISOur1, IS1008 and IS15 respectively. All of the latter three IS elements belong to the IS6 family. The transformants of the plasmids with the structure of IS6 family-ΔISAba3-like-bla OXA58 displayed a much higher increase in imipenem MICs (16–32 folds) than those with intact ISAba3-like-bla OXA58 (2–4 folds) (Table 2).
ISAba3, araC1 (putative transcriptional regulator gene) and lysE (putative threonine efflux protein gene) were identified downstream of bla OXA-58 in all isolates. However, the araC1 and lysE in structure B were disrupted by ISAba20 and TnaphA6 respectively. ISAba20 is a novel insertion sequence of IS3 family, and is 1199 bp long with two ORFs. The insertion of ISAba20 into araC1 generated two 4-bp direct repeats (CTTA). TnaphA6 was a composite transposon, comprising an aminoglycoside O-phosphotransferase gene, aphA6, and two flanked ISAba125 of same orientation. TnaphA6 was inserted into lysE and generated two 3-bp target site duplications (CTG).
It has been reported that the acquisition of bla OXA-58 is usually associated with recombination events characterized by the presence of two 27-bp sequences named Re27-1 and Re27-2 [9]. In structure A, we identified a similar Re27-1 sequence located 8 bp downstream of the intact ISAba3-like element (5′-ATTTAACATAATGGCTGTTATACGAAA-3′), and an imperfect Re27-2 sequence (5′-ATTTAACATAATGGTGGTTATACGCAA-3′) was just adjacent to the downstream of lysE. In structure D, a pair of 29-bp imperfect probable recombination points were identified 748 bp downstream of IS15 element (5′-ATTTAACATAATGGTGGTTATGCGAAGTC-3′) and adjacent to lysE (5′-ATTTAACATAATGGGCGTTATGCGAAGTC-3′). In structure B and C, we failed to find pairs of Re27-like regions.
Discussion
Previous studies reported that European clone II lineage OXA-23-producing A. baumannii CC92 was the most popular carbapenem-resistant clone in China [24], [27]. The OXA-58 producing A. baumannii of European clone II has been reported in Italy [28], Greece [29] and China [30]. However, only A. baumannii ST91 and ST363 were identified in this study without any European clone II lineage isolates. We have showed ST91 strains contain both bla OXA-23 and bla OXA-58, and possess multidrug resistance to carbapenems, broad-spectrum cephalosporins, aminoglycosides, ampicillin/sulbactam, minocycline, and ciprofloxacin. Moreover, ST91 was detected in two cities. Therefore, we speculate ST91 is a potential risk multidrug resistant clone that is widely present in China. A larger scale epidemiological investigation would be necessary to fully elucidate the true distribution of ST91 in China.
Gentamicin and amikacin resistance were observed in transformants of A. baumannii ST91. The analysis of nucleotide sequence around bla OXA-58 identified an aminoglycoside O-phosphotransferase gene, aphA6. The gentamicin and amikacin resistance gene aphA6 was first reported in A. baumannii in 1988 [31]. Nigro et al. recently reported aphA6 located in a potential transposon TnaphA6, flanked by two copies of ISAba125 [32]. An identical transposon was identified in our study and TnaphA6 was inserted into a putative threonine efflux protein gene lysE. It should be noticed that the susceptible A. baumannii could develop carbapenem and amikacin resistance simultaneously via the bla OXA-58 and aphA6 co-harboring plasmid.
Bertini et al. reported the bla OXA-58 harboring plasmids could be classified into various groups, including GR2 (Aci1), GR3 (Aci3 and Aci7), GR4 (Aci4) and GR5 (Aci5) [26]. Using the same typing scheme, Towner reported that OXA-58 producing A. baumannii from European countries were commonly associated with Aci1, Aci3, Aci4, and AciX [15]. However, the bla OXA-58-carrying plasmids in this study did not belong to any known replicon groups, and a novel replicase gene repAci10 was identified This suggests that the spread of bla OXA-58 in China may be mediated by unique plasmids being different from those of Europe. Meanwhile, the plasmids of repAci10 are viable in different genomic species of Acinetobacter and may contribute to horizontal transmission of resistance genes. However, the replicase genes of the plasmids of A. baumannii ST91 remain unknown and further complete plasmid sequencing is in process.
The acquisition of bla OXA-58 is associated with a recombination event at site of Re27 sequence [9]. However, the pairs of Re27 sequence around bla OXA-58 were absent in partial isolates in this study, suggesting it may have been lost during plasmid evolution.
It is speculated that the insertion of other IS element into ISAba3-like could generate a hybrid promoter to enhance the transcription of bla OXA-58 and mediate greater carbapenem resistance than the intact ISAba3-like element as previously reported [9]–[11], [33]. In this study, for plasmids that the ISAba3-like element was disrupted by ISOur1, IS1008 or IS15, their corresponding transformants showed a high increase in imipenem MICs (16–32 folds), while for plasmids that the ISAba3-like element was intact, the imipenem MICs of their corresponding transformants were only slightly increasing (2–4 folds). The special structure of IS6 family-ΔISAba3-like-bla OXA-58 is different from ISAba2, IS18, ISAba125, ISAba1 and ISAba825 that is usually inserted into ISAba3-like in Acinetobacter spp. from Europe [9], [34], [35].
In conclusion, the genetic background of OXA-58-producing Acinetobacter spp. in China was diverse, and the multidrug resistant A. baumannii ST91 is a potential risk clone. The STs of A. baumannii, replicon typing of bla OXA-58-harboring plasmids and genetic contexts of bla OXA-58 were distinct from those of Europe, implying the unique evolution and transmission pattern of bla OXA-58 in Acinetobacter spp. in China.
Acknowledgments
We are grateful to Dr Christopher Weier (Johns Hopkins University School of Medicine) for his comments and revision on the manuscript.
Funding Statement
This work was funded by research grants from National Natural Science Foundation of China (nos. 81230039 and 81000757) and Science Technology Department of Zhejiang Province (no. 2008C13029-1). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Peleg AY, Seifert H, Paterson DL (2008) Acinetobacter baumannii: emergence of a successful pathogen. Clin Microbiol Rev 21: 538–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Karageorgopoulos DE, Falagas ME (2008) Current control and treatment of multidrug-resistant Acinetobacter baumannii infections. Lancet Infect Dis 8: 751–762. [DOI] [PubMed] [Google Scholar]
- 3. Woodford N, Turton JF, Livermore DM (2011) Multiresistant Gram-negative bacteria: the role of high-risk clones in the dissemination of antibiotic resistance. FEMS Microbiol Rev 35: 736–755. [DOI] [PubMed] [Google Scholar]
- 4. Poirel L, Marque S, Heritier C, Segonds C, Chabanon G, et al. (2005) OXA-58, a novel class D β-lactamase involved in resistance to carbapenems in Acinetobacter baumannii . Antimicrob Agents Chemother 49: 202–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Coelho J, Woodford N, Afzal-Shah M, Livermore D (2006) Occurrence of OXA-58-like carbapenemases in Acinetobacter spp. collected over 10 years in three continents. Antimicrob Agents Chemother 50: 756–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Peleg AY, Franklin C, Walters LJ, Bell JM, Spelman DW (2006) OXA-58 and IMP-4 carbapenem-hydrolyzing β-lactamases in an Acinetobacter junii blood culture isolate from Australia. Antimicrob Agents Chemother 50: 399–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Castanheira M, Wanger A, Kruzel M, Deshpande LM, Jones RN (2008) Emergence and clonal dissemination of OXA-24- and OXA-58-producing Acinetobacter baumannii strains in Houston, Texas: report from the SENTRY Antimicrobial Surveillance Program. J Clin Microbiol 46: 3179–3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mendes RE, Bell JM, Turnidge JD, Castanheira M, Jones RN (2009) Emergence and widespread dissemination of OXA-23, −24/40 and −58 carbapenemases among Acinetobacter spp. in Asia-Pacific nations: report from the SENTRY Surveillance Program. J Antimicrob Chemother 63: 55–59. [DOI] [PubMed] [Google Scholar]
- 9. Poirel L, Nordmann P (2006) Genetic structures at the origin of acquisition and expression of the carbapenem-hydrolyzing oxacillinase gene bla OXA-58 in Acinetobacter baumannii . Antimicrob Agents Chemother 50: 1442–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen TL, Wu RC, Shaio MF, Fung CP, Cho WL (2008) Acquisition of a plasmid-borne bla OXA-58 gene with an upstream IS1008 insertion conferring a high level of carbapenem resistance to Acinetobacter baumannii . Antimicrob Agents Chemother 52: 2573–2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chen TL, Chang WC, Kuo SC, Lee YT, Chen CP, et al. (2010) Contribution of a plasmid-borne bla OXA-58 gene with its hybrid promoter provided by IS1006 and an ISAba3-like element to beta-lactam resistance in acinetobacter genomic species 13TU. Antimicrob Agents Chemother 54: 3107–3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Marti S, Sanchez-Cespedes J, Blasco MD, Ruiz M, Espinal P, et al. (2008) Characterization of the carbapenem-hydrolyzing oxacillinase oxa-58 in an Acinetobacter genospecies 3 clinical isolate. Antimicrob Agents Chemother 52: 2955–2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mendes RE, Bell JM, Turnidge JD, Castanheira M, Deshpande LM, et al. (2009) Codetection of bla OXA-23-like gene (bla OXA-133) and bla OXA-58 in Acinetobacter radioresistens: report from the SENTRY antimicrobial surveillance program. Antimicrob Agents Chemother 53: 843–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Marti S, Sanchez-Cespedes J, Blasco MD, Espinal P, Ruiz M, et al. (2008) Characterization of the carbapenem-hydrolyzing oxacillinase OXA-58 in an Acinetobacter phenon 6/ct13TU clinical isolate. Diagn Microbiol Infect Dis 61: 468–470. [DOI] [PubMed] [Google Scholar]
- 15. Towner KJ, Evans B, Villa L, Levi K, Hamouda A, et al. (2011) Distribution of intrinsic plasmid replicase genes and their association with carbapenem-hydrolyzing class D β-lactamase genes in European clinical isolates of Acinetobacter baumannii . Antimicrob Agents Chemother 55: 2154–2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang H, Guo P, Sun H, Yang Q, Chen M, et al. (2007) Molecular epidemiology of clinical isolates of carbapenem-resistant Acinetobacter spp. from Chinese hospitals. Antimicrob Agents Chemother 51: 4022–4028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhou H, Yang Q, Yu YS, Wei ZQ, Li LJ (2007) Clonal spread of imipenem-resistant Acinetobacter baumannii among different cities of China. J Clin Microbiol 45: 4054–4057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chang HC, Wei YF, Dijkshoorn L, Vaneechoutte M, Tang CT, et al. (2005) Species-level identification of isolates of the Acinetobacter calcoaceticus-Acinetobacter baumannii complex by sequence analysis of the 16S-23S rRNA gene spacer region. J Clin Microbiol 43: 1632–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Adams-Haduch JM, Paterson DL, Sidjabat HE, Pasculle AW, Potoski BA, et al. (2008) Genetic basis of multidrug resistance in Acinetobacter baumannii clinical isolates at a tertiary medical center in Pennsylvania. Antimicrob Agents Chemother 52: 3837–3843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Higgins PG, Poirel L, Lehmann M, Nordmann P, Seifert H (2009) OXA-143, a novel carbapenem-hydrolyzing class D β-lactamase in Acinetobacter baumannii . Antimicrob Agents Chemother 53: 5035–5038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chen Y, Zhou Z, Jiang Y, Yu Y (2011) Emergence of NDM-1-producing Acinetobacter baumannii in China. J Antimicrob Chemother 66: 1255–1259. [DOI] [PubMed] [Google Scholar]
- 22. Tenover FC, Arbeit RD, Goering RV, Mickelsen PA, Murray BE, et al. (1995) Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J Clin Microbiol 33: 2233–2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bartual SG, Seifert H, Hippler C, Luzon MA, Wisplinghoff H, et al. (2005) Development of a multilocus sequence typing scheme for characterization of clinical isolates of Acinetobacter baumannii . J Clin Microbiol 43: 4382–4390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fu Y, Zhou J, Zhou H, Yang Q, Wei Z, et al. (2010) Wide dissemination of OXA-23-producing carbapenem-resistant Acinetobacter baumannii clonal complex 22 in multiple cities of China. J Antimicrob Chemother 65: 644–650. [DOI] [PubMed] [Google Scholar]
- 25. Barton BM, Harding GP, Zuccarelli AJ (1995) A general method for detecting and sizing large plasmids. Anal Biochem 226: 235–240. [DOI] [PubMed] [Google Scholar]
- 26. Bertini A, Poirel L, Mugnier PD, Villa L, Nordmann P, et al. (2010) Characterization and PCR-based replicon typing of resistance plasmids in Acinetobacter baumannii . Antimicrob Agents Chemother 54: 4168–4177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. He C, Xie Y, Fan H, Kang M, Tao C, et al. (2011) Spread of imipenem-resistant Acinetobacter baumannii of European clone II in Western China. Int J Antimicrob Agents 38: 257–260. [DOI] [PubMed] [Google Scholar]
- 28. D’Arezzo S, Capone A, Petrosillo N, Visca P, Ballardini M, et al. (2009) Epidemic multidrug-resistant Acinetobacter baumannii related to European clonal types I and II in Rome (Italy). Clin Microbiol Infect 15: 347–357. [DOI] [PubMed] [Google Scholar]
- 29. Gogou V, Pournaras S, Giannouli M, Voulgari E, Piperaki ET, et al. (2011) Evolution of multidrug-resistant Acinetobacter baumannii clonal lineages: a 10 year study in Greece (2000–09). J Antimicrob Chemother 66: 2767–2772. [DOI] [PubMed] [Google Scholar]
- 30. Zhang JP, Zhu W, Tian SF, Chu YZ, Chen BY (2010) Molecular characteristics and resistant mechanisms of imipenem-resistant Acinetobacter baumannii isolates in Shenyang, China. J Microbiol 48: 689–694. [DOI] [PubMed] [Google Scholar]
- 31. Martin P, Jullien E, Courvalin P (1988) Nucleotide sequence of Acinetobacter baumannii aphA-6 gene: evolutionary and functional implications of sequence homologies with nucleotide-binding proteins, kinases and other aminoglycoside-modifying enzymes. Mol Microbiol 2: 615–625. [DOI] [PubMed] [Google Scholar]
- 32. Nigro SJ, Post V, Hall RM (2011) Aminoglycoside resistance in multiply antibiotic-resistant Acinetobacter baumannii belonging to global clone 2 from Australian hospitals. J Antimicrob Chemother 66: 1504–1509. [DOI] [PubMed] [Google Scholar]
- 33. Boo TW, Crowley B (2009) Detection of bla OXA-58 and bla OXA-23-like genes in carbapenem-susceptible Acinetobacter clinical isolates: should we be concerned? J Med Microbiol 58: 839–841. [DOI] [PubMed] [Google Scholar]
- 34. Ravasi P, Limansky AS, Rodriguez RE, Viale AM, Mussi MA (2011) ISAba825, a functional insertion sequence modulating genomic plasticity and bla OXA-58 expression in Acinetobacter baumannii . Antimicrob Agents Chemother 55: 917–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Evans BA, Hamouda A, Towner KJ, Amyes SG (2010) Novel genetic context of multiple bla OXA-58 genes in Acinetobacter genospecies 3. J Antimicrob Chemother 65: 1586–1588. [DOI] [PubMed] [Google Scholar]