Abstract
Objectives
Patients with chronic noncancer pain frequently report symptoms of depression and anxiety (negative affect), which are associated with higher ratings of pain intensity and a greater likelihood of being prescribed chronic opioid therapy. The purpose of this secondary analysis was to test the hypothesis that initial levels of negative affect can predict treatment-related outcomes in a double-blind, placebo-controlled study of extended-release (ER) hydromorphone among opioid-tolerant patients with chronic low back pain.
Methods
Four hundred fifty-nine (N = 459) patients participated in the titration/conversion phase of a multicenter study, of which 268 were randomized to receive once-daily hydromorphone or placebo. All patients completed the Hospital Anxiety and Depression Scale (HADS) at baseline and were divided evenly into Low (N = 157), Moderate (N = 155), and High (N = 147) negative affect groups based on their scores. Group differences in numerical pain intensity measures at home and in the clinic, Roland–Morris Disability ratings, and measures of symptoms from the Subjective Opiate Withdrawal Scale (SOWS) throughout the trial were analyzed.
Results
Two hundred sixty-eight of the initial 459 subjects who entered the 2 to 4-week titration/conversion phase (pretreatment) were successfully randomized to either placebo or ER hydromorphone; a total of 110 patients then completed this double-blind phase of the study. Those in the Moderate and High negative affect groups tended to drop out more often during the titration/conversion phase because of the adverse effects or lack of efficacy of their prescribed opioid than those in the Low negative mood group (P < 0.05). Overall, those patients in the Moderate and High groups reported significantly higher pain intensity scores in at-home and in-clinic pain intensity ratings (P < 0.05), greater disability on the Roland–Morris Scale (P < 0.01), and more withdrawal symptoms on the SOWS (P < 0.05) than those in the Low group. Higher negative affect scores also predicted less favorable ratings of the study drug during the titration phase (P < 0.05). Interestingly, the High negative affect group showed the most improvement in pain in the placebo condition (P < 0.05).
Conclusions
Negative affect is associated with diminished benefit during a trial of opioid therapy and is predictive of dropout in a controlled clinical trial.
Keywords: chronic pain, negative affect, mood, opioids, clinical trial, randomized controlled trial, RCT, double-blind, placebo-controlled, opioid tolerance
INTRODUCTION
Chronic pain is a widespread, costly condition that influences every aspect of normal functioning.1–4 Collectively, pain affects as many as 116 million adults in the United States and imposes a greater economic burden than any other disease, with estimates of annual costs between $560 and $630 billion.5 Among the most prevalent and disabling of chronic pain syndromes is low back pain (LBP),6,7 which affects approximately 50 million Americans,8 and which accounts for a significant portion of pain-related disability in the United States.6,7
A number of factors that correlate with greater risk for poor outcomes from treatment for chronic pain have been identified in the literature. These include pain chronicity, psychological distress, a history of abuse or trauma, poor social support, and significant cognitive deficits.9–12 In particular, extreme emotionality has been seen as a contraindication for certain therapies.13–15 Outcome studies highlight the poor response to many treatments of patients with psychiatric comorbidity.16–19 Specifically, spinal pain patients with both anxiety and depression have been found to have a 62% worse return-to-work rate, and poorer functional outcomes following surgery, an implantable device, or nerve block procedures.10,20–22 Based on this literature, one would predict that those with greater depression and anxiety would also be prone to the worst outcomes in a clinical trial of opioids.
This study involved secondary analyses of data collected on subjects who participated in a multicenter, double-blind, placebo-controlled study of hydromorphone extended-release (ER).23 The aim of the study was to determine the role of negative affect in predicting treatment outcomes among opioid-tolerant patients with LBP receiving either once-daily hydromorphone ER or placebo. The results of the Hospital Anxiety and Depression Scale (HADS) were not included in the original report. We hypothesized that patients with moderate to high levels of negative affect (depression and anxiety) would have a diminished response to an opioid trial and have a greater placebo response compared with those with lower levels of self-reported negative affect.
PATIENTS AND METHODS
Data were analyzed from a large multicenter, double-blind, parallel-group, placebo-controlled study using a flexible dosing regimen in an open-label enrichment phase (conversion and titration), followed by a randomized withdrawal phase conducted in opioid-tolerant patients with chronic LBP at 66 centers in the United States. The ClinicalTrials.gov identifier for this trial is NCT00549042. The study protocol was approved by the institutional review boards at all centers. All patients gave written informed consent before undergoing any study procedure. The full details of this study are available, including the CONSORT flow diagram.23
Subjects were eligible to participate in this study if they were: (1) between the ages of 18 to 75, (2) had a documented diagnosis of moderate to severe LBP for more than 6 months, and (3) were taking opioids for pain. Patients were excluded if they: (1) had back surgery within 6 months, (2) were diagnosed with fibromyalgia or complex regional pain syndrome, (3) had other medical conditions that might interfere with the study (eg, infection or tumor), or (4) had a major psychiatric condition (eg, dementia, schizophrenia, or bipolar disorder).
All eligible subjects participated in a screening visit, a 2 to 4-week open-label conversion/titration phase of opioid therapy, and a 12-week double-blind treatment phase (Figure 1). Patients completed a series of self-report measures during the screening visit and periodically throughout the trial.
Figure 1.
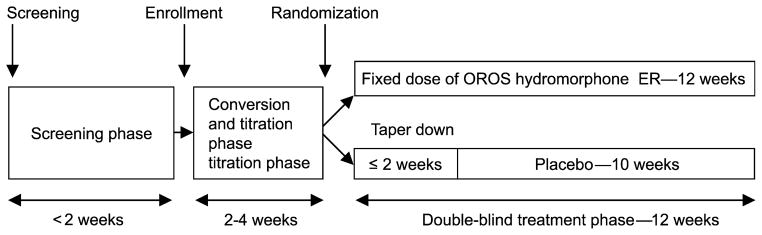
Schema of the study design.
During the conversion and titration phase of the original study, patients were converted to hydromorphone ER using a standard morphine conversion table with a hydromorphone to morphine ratio of 1:5. The conversion and titration took place over a 2 to 4-week period, during which patients were allowed up to 2 dose increases per week. Patients were started at 75% of their total daily dose of morphine equivalent. Only doses of 12, 16, 24, 32, 40, 48, or 64 mg per day were allowed. After conversion and titration, those patients who were stable on doses between 12 and 64 mg per day for at least 7 days, on average took no more than 2 tablets of rescue medication per day, had an average pain intensity NRS score of 4 or less, had no intolerable side effects, and said “yes” when asked “has this medication helped your pain enough so that you would continue to take the medication,” continued onto the double-blind withdrawal phase of the study.
During the 12-week randomized, double-blind phase of the study, patients were randomized to either the active drug group, where they continued receiving the dose of hydromorphone ER they were stabilized on during the conversion and titration phase, or the placebo group, where they were given a tapering dose of hydromorphone ER. In the placebo group, the tapering of hydromorphone ER occurred over a maximum 14-day period, so that all patients in this group were on placebo after day 14. During this phase of the study, rescue medication of hydromorphone IR was allowed in both groups, with a maximum of 6 tablets per day during the first week, 4 tablets per day during the second week, and 2 tablets per day for the remainder of the study.
Study Measures
A number of instruments were employed in the study to assess patient characteristics and to test the effectiveness of the study drug. These included numeric pain intensity ratings obtained during the clinic visits and on paper diaries used at home, the Roland–Morris Disability Questionnaire (RMDQ), the Subjective Opioid Withdrawal Scale (SOWS), the Patient Global Assessment (PGA) scale, and the HADS. Subjects repeatedly rated their pain intensity on an 11-point Likert numeric rating scale (NRS; 0 to 10) during every clinic visit and 14 separate times throughout the study on paper diaries. A similar scale is used as part of the Brief Pain Inventory,24 that is a well-known measure of clinical pain and has shown sufficient reliability and validity (r = 0.77 to 0.91).25,26 Test–retest reliability of the NRS reveals correlations of 0.93 for worst pain, 0.78 for usual pain, and 0.59 for pain now.24 Data were collected each week over the 12-week treatment period.
The RMDQ27 is a well-known 24-item self-administered disability questionnaire used to evaluate a patient’s ability to perform routine tasks. The RMDQ has been shown to yield reliable measures of disability with greater levels reflected by higher numbers. It has been found to be sensitive to change over time for groups of patients with LBP.27 The SOWS28 is a 10-item self-report measure of common physical symptoms associated with withdrawal effects from opioids, which is a short version of the original 32-item Opiate Withdrawal Scale.29 The items are rated as none = 0, mild = 1, moderate = 2, and severe = 3. This scale has been frequently used in opioid therapy trials with evidence of adequate criterion validity and discriminative reliability.30 The SOWS was repeated during the conversion/titration and double-blind treatment phases of the study.
The PGA31 is a rating scale to assess how the subject felt overall about the study drug from 1 = excellent, 2 = very good, 3 = good, 4 = fair, and 5 = poor. This scale has been used in other clinical trials to assess response to a study drug and efficacy of new medication32 and has demonstrated good test–retest reliability (ICC = 0.70).31
The HADS33 is a 14-item self-report questionnaire designed to assess symptoms of anxiety and depression in those with medical illness. It does not include somatic symptoms, such as fatigue and sleeplessness, which may otherwise be attributable to physical illness, and it has been standardized among large community samples. It has also been validated in several medical illness populations with a sensitivity and specificity of 0.66 to 0.97 for a DSM-IV major depression or generalized anxiety disorder diagnosis.34 Scores between 0 and 7 are considered normal, and scores above 11 are abnormal.33 In this study, those with total HADS scores of 7 or less were classified as Low, those with combined scores > 12 were classified as High, and subjects with scores between these points were placed in the Moderate group. This method has been used in similar studies.35,36
Statistical Analyses
The original study demonstrated that there was adequate power (99%) to detect a 2-point difference on a 0 to 10 visual analog scale between placebo and treatment groups at a significance level of P < 0.05. The last observation in the NRS, RWD, and SOWS was carried forward for those who dropped out except for patients who discontinued because of the withdrawal symptoms or adverse effects, in which case the baseline scores were carried forward. This method was used in the original report and was employed to minimize potential bias toward a positive outcome.23 Parametric and nonparametric analyses were used to compare differences between groups. An analysis of variance (ANOVA) was run to assess differences among groups on pain intensity, disability, and withdrawal symptoms combined and separately based on treatment group. We also examined differences among the HADS groups on baseline demographic variables, dropout, adverse events, physical examination results, and diagnoses, as covariates. Analyses were run using SAS version v.9.3 (SAS Institute Inc., Cary, NC, U.S.A.) and SPSS v.17 (Chicago, IL, U.S.A.). The key outcome parameter was the significance of the differences between the HADS groups.
RESULTS
Four hundred fifty-nine patients with LBP (N = 459) were entered into this study from a total of 806 subjects who were screened. Two hundred sixty-eight (N = 268) of the subjects who entered the 2 to 4 week titration/conversion phase were successfully randomized to either placebo or ER hydromorphone. One hundred and ten (N = 110) patients completed the 12-week double-blind phase, while 158 (59.0%) withdrew from the study primarily for lack of efficacy of the drug (35.4%), mostly among the placebo group. Other reasons to withdraw included noncompliance (13.9%), unacceptable rescue medication (12.7%), protocol violation (10.1%), adverse events (8.2%), opioid withdrawal symptoms (6.3%), lost to follow-up (3.2%), and other reasons (3.2%). The subjects ranged in age from 18 to 75 (mean 48.9, SD = 10.45), 51.3% were men, 85.5% were Caucasian, and 64.3% were judged to have non-neuropathic LBP.
The subjects were divided into groups based on their total scores from the HADS, which ranged from 0 to 29 (Mean = 10.01; SD = 5.15). The HADS anxiety subscale scores averaged 5.24 (SD = 2.88), and the HADS depression subscale scores averaged 4.76 (SD = 2.94), and both were highly correlated (r = 0.57). It was decided that the combined HADS scores would be used throughout the analyses. One hundred fifty-four were classified as Low (HADS < 8), 155 Moderate (HADS 8 to 12), and 147 were High (HADS > 12) based on their baseline scores. No differences were found between groups at baseline on age, gender, race, weight, height, body mass index, physical examination findings, number of prescription medications, rescue doses, or the amount of starting dose of the study drug. Those with neuropathic LBP (N = 282) were found to have lower HADS scores (mean = 9.66 ± 5.35) than those judged to have non-neuropathic LBP (N = 171; mean = 10.66 ± 4.76; t = 2.01; P = 0.05). Table 1 shows baseline comparisons of groups on demographic, pain intensity, function (RMDQ), and HADS scores. Significant differences were found among the 3 groups on employment status, disability, neuropathic pain, and, as predicted, HADS anxiety and depression scores.
Table 1.
Baseline comparisons among Low, Moderate, and High HADS groups on demographic variables, pain intensity, function, medication use, and mood (mean, SD)
| Variable | Low (N = 157) | Moderate (N = 155) | High (N = 147) | P | Sig. |
|---|---|---|---|---|---|
| Age | 48.80 ± 11.49 | 47.79 ± 10.27 | 50.12 ± 9.38 | F = 1.90 | 0.151 |
| Gender (% male) | 48.1 | 51.6 | 54.4 | χ2 = 1.23 | 0.541 |
| Race (% White) | 86.4 | 83.9 | 86.4 | χ2 = 6.89 | 0.736 |
| Employed (% yes) | 48.6 | 55.3 | 40.4 | χ2 = 6.48 | 0.039 |
| Neuropathic (% yes) | 70.9 | 60.0 | 58.8 | χ2 = 7.72 | 0.021 |
| Avg. pain | 6.47 ± 1.94 | 6.62 ± 1.72 | 6.85 ± 1.66 | F = 1.76 | 0.174 |
| Body mass index | 30.17 ± 6.98 | 30.88 ± 6.77 | 31.60 ± 8.26 | F = 1.43 | 0.240 |
| Height (cm) | 170.21 ± 11.35 | 170.96 ± 10.97 | 171.99 ± 9.89 | F = 1.04 | 0.354 |
| Weight (kg) | 87.65 ± 22.92 | 90.41 ± 21.55 | 93.50 ± 25.23 | F = 2.38 | 0.093 |
| R-M disability | 13.09 ± 5.16 | 13.41 ± 4.67 | 15.39 ± 4.39 | F = 10.22 | 0.000 |
| Stable dose hydromorph. (mg) | 35.63 ± 17.63 | 38.19 ± 16.91 | 39.23 ± 18.17 | F = 0.49 | 0.615 |
| HADS anxiety | 2.55 ± 1.44 | 5.23 ± 1.70 | 8.07 ± 2.23 | F = 348.75 | 0.000 |
| HADS depression | 2.07 ± 1.44 | 4.37 ± 1.73 | 8.00 ± 1.79 | F = 487.11 | 0.000 |
HADS, Hospital Anxiety and Depression Scale.
P-values < 0.05 are in bold.
Overall, 189 subjects discontinued the study during the conversion/titration phase, while 133 were successfully randomized to the 12-week placebo treatment, and 134 were assigned to the 12-week hydromorphone ER group. Those who discontinued the study during the conversion and titration phase reported significantly greater negative affect (HADS = 10.74, SD = 5.34) compared with those who were randomized to treatment (HADS = 9.49, SD = 4.49; P = 0.01). Altogether 53 subjects (34.4%) discontinued the conversion/titration phase from the Low HADS group compared with 62 subjects (40.0%) from the Moderate HADS groups and 74 subjects (50.3%) from the High HADS group (χ2 = 11.82; P = 0.019). By the end of the trial, among the placebo group, 13 and 14 subjects successfully completed the trial from the Moderate and High HADS groups compared with the 17 subjects from the Low HADS group, and among those randomized to the hydromorphone ER group, 24 and 20 subjects completed the trial in the Moderate and High HADS groups compared with 22 subjects in the Low HADS group. Group differences at the end of study were not significant.
Of the subjects who discontinued during the titration/conversion phase, those in the Moderate and High groups rated their opioid medication as least favorable on the PGA (Low = 3.17 ± 1.02; Mod = 3.68 ± 0.74; High = 3.93 ± 0.71; F = 7.74; P < 0.001). Those subjects randomized to the hydromorphone ER group demonstrated twice as much improvement than the placebo group in pain intensity ratings based on the in-clinic pain ratings (9.0% vs. 24.6%) and at-home diary ratings (17.9% vs. 35.1%; P < 0.001).
Figure 2 presents differences in numerical pain intensity ratings obtained during the clinic visits and the at-home paper diary pain ratings among patients classified as Low, Moderate, and High on negative affect. Those in the Low group were found to have lower pain intensity scores than subjects with moderate and high HADS scores on the mean in-clinic rating (Low = 4.67 ± 2.42, Moderate = 5.13 ± 2.17; High = 5.40 ± 2.36; P = 0.03) and on the mean at-home diary ratings (Low = 4.58 ± 2.17, Moderate = 5.18 ± 2.14, High = 5.19 ± 2.20; P = 0.02). Among subjects in the placebo treatment arm, the Low negative affect group showed the greatest increase in average pain scores based on in-clinic pain intensity (mean 1.96 ± 1.69) over the whole trial compared with the High negative affect group (mean 1.05 ± 1.27; F = 3.84; P = 0.02), and there was a similar trend found with the diary data (Low = 1.39 ± 1.38 vs. High = 1.00 ± 1.29; F = 0.90; NS). Generally, the High negative affect group showed the greatest improvement with placebo based on change scores. Although change scores were similar among groups assigned to the hydromorphone ER group, surprisingly, the Moderate negative affect group responded least well with hydromorphone ER based on the diary data (Low = 0.39, Mod = 0.72, High = 0.10; F = 3.02; P = 0.05).
Figure 2.
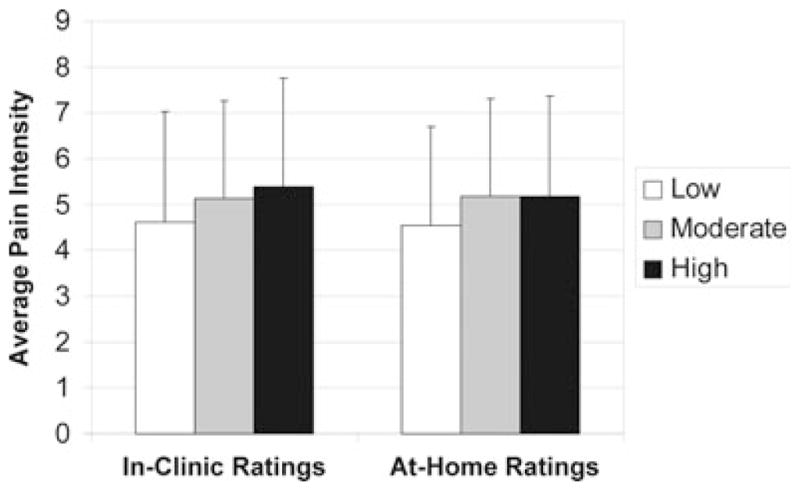
Low, Moderate, and High Hospital Anxiety and Depression Scale (HADS) groups and average pain intensity ratings based on in-clinic (P = 0.03) and at-home (P = 0.03) diary data (N = 415).
Figure 3 presents the differences in disability ratings on the Roland–Morris Disability Scale among patients classified as Low, Moderate, and High negative affect. Similar to the pain intensity ratings, those in the Low group reported significantly less disability and pain interference during the pretreatment and randomized treatment periods compared with the Moderate and High groups (Low = 10.68 ± 6.25, Moderate = 12.04 ± 6.40, High = 13.65 ± 6.33; P < 0.001). Subjects in both the placebo and hydromorphone ER treatment arms reported consistently lower disability scores in the Low negative affect group compared with the Moderate and High groups, but none of the individual difference scores reached significance.
Figure 3.
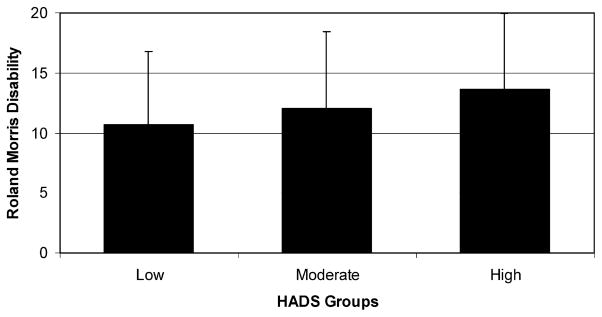
Low, Moderate, and High groups on the Hospital Anxiety and Depression Scale (HADS) and mean ratings on the Roland–Morris Disability Scale (N = 410; P = 0.001).
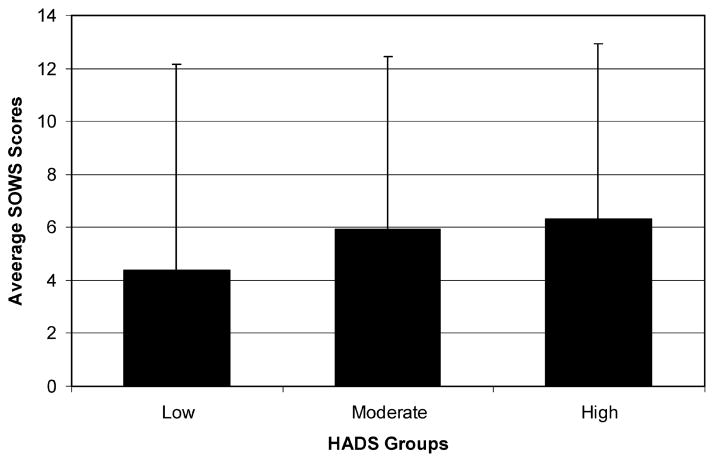
Figure 4 shows the differences in symptoms ratings on the SOWS among patients classified as Low, Moderate, and High negative affect. Similar to the pain intensity ratings, those in the Low group reported less disability and pain interference during the pretreatment and randomized treatment periods compared with the Moderate and High groups (Low = 4.39 ± 7.78, Moderate = 5.92 ± 6.38, High = 6.32 ± 6.63, P = 0.05). Subjects in the placebo group reported higher SOWS scores in the Moderate and High negative affect groups compared with the Low group with 2 of the 6 ratings scores during the randomization phase reaching significance (P < 0.05). Likewise, among those assigned to the hydromorphone ER group, patients in the Low group reported lower SOWS scores compared with those in the Moderate or High groups with significant differences noted in 1 of the 6 comparisons (P < 0.05).
Figure 4.
Low, Moderate, High groups on the Hospital Anxiety and Depression Scale (HADS) and symptom ratings on the Subjective Opiate Withdrawal Scale (SOWS) (N = 414; P = 0.05).
DISCUSSION
These results demonstrate that patients with elevated levels of depression and anxiety are less likely to have a positive outcome during an opioid titration period and after a double-blind trial of either ER opioid or placebo. Patients in the High and Moderate negative affect groups reported more pain and withdrawal symptoms, greater disability, greater dislike of the opioid during the dose-conversion/titration phase and were prone to discontinue the trial more often compared with those in the Low negative affect (lower depression and anxiety) group.
Depression and anxiety are associated with greater somatic complaints and higher pain intensity, and this leads to an increased likelihood of being prescribed opioids for pain.37,38 These results suggest that the assessment of negative mood is important in identifying patients who may benefit most from opioid therapy. Similar to past studies, these data also support the notion that identifying and treating a comorbid mood disorder prior to or in conjunction with a trial of opioid therapy may improve the response rate.39,40 Conducting a mental health assessment is important in any comprehensive chronic pain evaluation,20,41 and evidence suggests that emotional comorbidity can be easily identifiable and treatable.42 Patients with moderate negative affect can be readily identified with the use of standardized measures administered by primary care and pain physicians, and treatment for a mood disorder has been shown to improve pain treatment outcomes.39,40,42,43 Ideally, a multidisciplinary approach in managing chronic pain patients offers the greatest efficacy for treating chronic back pain patients with a mood disorder.44
While higher levels of depression and anxiety predicted a negative response to opioid therapy, just as interesting is that other variables assessed in this study were unrelated to negative affect levels. For instance, no differences were found between HADS groups on variables such as gender, age, race, objective physical exam findings, and the amount of medication that the subjects were taking. This is contrary to other studies that have shown demographic differences on response to the HADS.45,46 Negative affect did not predict whether the subjects liked their study drug or not once they entered the double-blind phase of the study. Also, it is uncertain whether less negative affect in patients identified as having neuropathic pain compared with those with non-neuropathic pain is a chance result. This finding would warrant further investigation. A strength of this study is that many patients were enrolled, and multiple ratings were obtained over the course of the study. Even though our results compare favorably with the findings of other studies associating psychological variables to results from chronic spinal pain treatments,47,48 a replication of these findings is needed.
The rates of negative affect compared with other studies may have been lowered by the participants’ use of antidepressant and anxiolytic medications. There are many non-opioid medications prescribed to improve pain and sleep that are also beneficial in improving mood among chronic pain patients. No differences were found among the groups in the number of other prescription medications that were prescribed, but it is likely that those patients with increased levels of anxiety and depression were taking psychotropic medications for their mood. Thus, the HADS scores may have been artificially lowered. It is also uncertain how many were receiving psychotherapy for their mood and pain problems. Future studies in which these factors are assessed and controlled would be valuable.
Several other limitations of this study deserve to be mentioned. This study is an exploratory, secondary analysis, and the design was not suited to infer causal relationships. As with any correlational finding, no direction can be assumed, and more research is needed to help establish the relationships between negative affect and outcome from a clinical drug trial. There is also no consensus on the demarcation of high and low negative affect, and potentially clinically important contrasts may have been overlooked. We also only had access to data that was collected as part of the initial trial on the efficacy of hydromorphone ER, and some potentially relevant information may not have been collected (eg, other psychiatric comorbidities, number of past surgeries, length of time on opioid therapy, perceived social support, etc.). Relatedly, while negative affective processes may influence pain-related outcomes via numerous pathways (eg, reduced compliance, neuroendocrine effects, etc.);49 the present study does not include data on these potential mechanisms that may underlie the observed effects. Further, by the nature of any controlled trial, selection bias and drop out may have influenced the results.
Subjects were excluded if they had evidence of significant psychopathology. Although the exact number excluded because the significant psychopathology is unknown, this exclusion criteria would suggest that the study included functioning individuals who had self-reported negative mood without severe psychiatric symptoms and that these individuals were not incapacitated because of a severe mood disorder commonly found among patients treated in specialty pain center. Thus, the data may not generalize to all chronic back pain populations. Another limitation is that the study population included heterogeneous back pain patients. This is inherent to many studies on chronic LBP, and back pain can represent a mixed condition. Although we did not discover differences among groups on diagnosis or physical exam findings, future studies could focus on specific diagnostic categories (eg, degenerative disk disease, failed back surgery, rheumatoid arthritis, stenosis, etc.). The measures reported in this study were predominantly self-report measures, and it is possible that the scales were measuring a generalized complaining factor (problems with pain, mood, function, and symptoms) similar to those who present with somatization. Nonetheless, the results of this study suggest that persons who tend to report problems with symptoms and negative mood tend to benefit less from a trial of opioids. Lastly, HADS scores were obtained only at baseline, and it is uncertain whether negative mood remained stable over the course of the study. Also, we elected to examine the combined HADS scores, and subtle differences may have been missed between those predominantly presenting with either severe depression or anxiety alone.
Despite these shortcomings, the results suggest that high levels of negative affect have a significant impact on a trial of opioid therapy. Importantly, no other medical covariates affected this result other than whether they had neuropathic pain or not. Future studies should examine whether these findings extend to those in a general clinical practice. It will also be fruitful to examine whether appropriate treatment of anxiety and depression in a group of patients with chronic LBP improves the results of opioid therapy and other pain medicine treatments.
Acknowledgments
This research was supported in part with an investigator-initiated grant provided by Mallinckrodt, a Covidien company.
Footnotes
Disclosure: All authors read and agreed to the contents of this paper. Dr. Warnick is an employee of Covidien. None of the other authors have any conflicts of interest to declare.
References
- 1.Garofalo JP, Polatin P. Low back pain: an epidemic in industrialized countries. In: Gatchel RJ, Turk DC, editors. Psychosocial Factors in Pain: Clinical Perspectives. New York: The Guilford Press; 1999. pp. 164–174. [Google Scholar]
- 2.Fordyce WE. Back Pain in the Workplace: Management of Disability in Nonspecific conditions. Seattle: IASP Press; 1995. [DOI] [PubMed] [Google Scholar]
- 3.Ehrlich GE. Back pain. J Rheumatol Suppl. 2003;67:26–31. [PubMed] [Google Scholar]
- 4.Frymoyer JW, Cats-Baril WL. An overview of the incidences and costs of low back pain. Orthop Clin North Am. 1991;22:263–271. [PubMed] [Google Scholar]
- 5.Institute of Medicine. Consensus Report. Washington DC: Jun 29, 2011. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. [Google Scholar]
- 6.Devereaux MW. Low back pain. Prim Care. 2004;31:33–51. doi: 10.1016/S0095-4543(03)00114-3. [DOI] [PubMed] [Google Scholar]
- 7.Boswell MV, Trescot AM, Datta S, et al. Interventional techniques: evidence-based practice guidelines in the management of chronic spinal pain. Pain Physician. 2007;10:7–111. [PubMed] [Google Scholar]
- 8.Lawrence RC, Felson DT, Helmick CG, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58:26–35. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mannion AF, Elfering A. Predictors of surgical outcome and their assessment. Eur Spine J. 2006;15:S93–S108. doi: 10.1007/s00586-005-1045-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boersma K, Linton S. Screening to identify patients at risk: profiles of psychological risk factors for early intervention. Clin J Pain. 2005;21:38–43. doi: 10.1097/00002508-200501000-00005. [DOI] [PubMed] [Google Scholar]
- 11.Rubin DI. Epidemiology and risk factors for spine pain. Neurol Clin. 2007;25:353–371. doi: 10.1016/j.ncl.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 12.Tunks ER, Crook J, Weir R. Epidemiology of chronic pain with psychological comorbidity: prevalence, risk, course, and prognosis. Can J Psychiatry. 2008;53:224–234. doi: 10.1177/070674370805300403. [DOI] [PubMed] [Google Scholar]
- 13.Block AR. Presurgical Psychological Screening in Chronic Pain Syndromes: A Guide for the Behavioral Health Practitioner. Mahwah: Lawrence Erlbaum Associates; 1996. [Google Scholar]
- 14.Main CJ, Spanswick CC. Pain Management: An Interdisciplinary Approach. New York: Churchill Livingstone; 2000. [Google Scholar]
- 15.Savage SR. Addiction in the treatment of pain: significance, recognition, and management. J Pain Symptom Manage. 1993;8:265–278. doi: 10.1016/0885-3924(93)90155-o. [DOI] [PubMed] [Google Scholar]
- 16.Holroyd KA. Recurrent headache disorders. In: Dworkin RH, Breitbart WS, editors. Psychosocial Aspects of Pain: A Handbook for Health Care Providers. Seattle: IASP Press; 2004. pp. 370–403. [Google Scholar]
- 17.Fishbain D. Approaches to treatment decisions for psychiatric comorbidity in the management of the chronic pain patient. Med Clin North Am. 1999;83:737–759. doi: 10.1016/s0025-7125(05)70132-2. [DOI] [PubMed] [Google Scholar]
- 18.Nelson D, Novy D. Self-report differentiation of anxiety and depression in chronic pain. J Pers Assess. 1997;69:392–407. doi: 10.1207/s15327752jpa6902_10. [DOI] [PubMed] [Google Scholar]
- 19.Evers A, Kraaimaat F, Van Reil P, Bijlsma J. Cognitive, behavioral and physiological reactivity to pain as a predictor of long-term pain in rheumatoid arthritis patients. Pain. 2001;9:139–146. doi: 10.1016/S0304-3959(01)00303-7. [DOI] [PubMed] [Google Scholar]
- 20.Celestin J, Edwards RR, Jamison RN. Pretreatment psychosocial variables as predictors of outcomes following lumbar surgery and spinal cord stimulation: a systematic review and literature synthesis. Pain Med. 2009;10:639–653. doi: 10.1111/j.1526-4637.2009.00632.x. [DOI] [PubMed] [Google Scholar]
- 21.Manchikanti L, Pampati VS, Fellows B. Influence of psychological factors on the ability to diagnose chronic low back pain of facet joint origin. Pain Physician. 2001;4:349–357. [PubMed] [Google Scholar]
- 22.Edwards RR, Klick B, Buenaver L, et al. Symptoms of distress as prospective predictors of pain-related sciatica treatment outcomes. Pain. 2007;130:47–55. doi: 10.1016/j.pain.2006.10.026. [DOI] [PubMed] [Google Scholar]
- 23.Hale A, Khan A, Kutch M, Li S. Once-daily OROS hydromorphone ER compared with placebo in opioid-tolerant patients with chronic low back pain. Curr Med Res Opin. 2010;26:1505–1518. doi: 10.1185/03007995.2010.484723. [DOI] [PubMed] [Google Scholar]
- 24.Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23:129–138. [PubMed] [Google Scholar]
- 25.Keller S, Bann CM, Dodd SL. Validity of the Brief Pain Inventory for use in documenting the outcomes of patients with noncancer pain. Clin J Pain. 2004;20:309–318. doi: 10.1097/00002508-200409000-00005. [DOI] [PubMed] [Google Scholar]
- 26.Mendoza TR, Mayne T, Rublee D, Cleeland CS. Reliability and validity of a modified Brief Pain Inventory short form in patients with osteoarthritis. Eur J Pain. 2006;10:353–361. doi: 10.1016/j.ejpain.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 27.Stratford PW, Binkley J, Solomon P. Defining the minimum level of detectable change for the Roland–Morris Questionnaire. Phys Ther. 1996;76:359–365. doi: 10.1093/ptj/76.4.359. [DOI] [PubMed] [Google Scholar]
- 28.Gossop M. The development of a short opiate withdrawal scale (SOWS) Addict Behav. 1990;15:487–490. doi: 10.1016/0306-4603(90)90036-w. [DOI] [PubMed] [Google Scholar]
- 29.Handelsman L, Cochrane KJ, Aronson M, Ness R, Rubinstein KJ, Kanof PD. Two new rating scales for opiate withdrawal. Am J Drug Alcohol Abuse. 1987;13:293–308. doi: 10.3109/00952998709001515. [DOI] [PubMed] [Google Scholar]
- 30.Bradley B, Gossop M, Phillips G, Legardy J. The development of an opiate withdrawal score (OWS) Br J Addict. 1987;82:1139–1142. doi: 10.1111/j.1360-0443.1987.tb03294.x. [DOI] [PubMed] [Google Scholar]
- 31.Rohekar G, Pope J. Test–retest reliability of patient global assessment and physician global assessment in rheumatoid arthritis. J Rheumatol. 2009;36:2178–2182. doi: 10.3899/jrheum.090084. [DOI] [PubMed] [Google Scholar]
- 32.Pincus T, Sokka T. Quantitative measures for assessing rheumatoid arthritis in clinical trials and clinical care. Best Pract Res Clin Rheumatol. 2003;17:753–781. doi: 10.1016/s1521-6942(03)00077-9. [DOI] [PubMed] [Google Scholar]
- 33.Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand. 1983;37:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 34.Bjelland I, Dahl AA, Huag TT, Neckelmann D. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J Psychosom Res. 2002;52:69–77. doi: 10.1016/s0022-3999(01)00296-3. [DOI] [PubMed] [Google Scholar]
- 35.Wasan AD, Davar G, Jamison R. The association between negative affect and opioid analgesia in patients with discogenic low back pain. Pain. 2005;117:450–461. doi: 10.1016/j.pain.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 36.Wasan A, Fernandez E, Jamison RN, Bhattacharyya N. Association of anxiety and depression with reported disease severity in patients undergoing evaluation for chronic rhinosinusitis. Ann Otol Rhinol Laryngol. 2007;116:491–497. doi: 10.1177/000348940711600703. [DOI] [PubMed] [Google Scholar]
- 37.Sullivan MD, Edlund MJ, Fan MY, Devries A, Braden JB, Martin BC. Trends in use of opioids for non-cancer pain conditions 2000–2005 in commercial and Medicaid insurance plans: the TROUP study. Pain. 2008;138:440–449. doi: 10.1016/j.pain.2008.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Braden JB, Fan MY, Edlund MJ, Martin BC, DeVries A, Sullivan MD. Trends in use of opioids by noncancer pain type 2000–2005 among Arkansas Medicaid and HealthCore enrollees: results from the TROUP study. J Pain. 2008;11:1026–1035. doi: 10.1016/j.jpain.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wells K, Sherbourne C, Duan N. Quality improvement for depression in primary care: do patients with subthreshold depression benefit in the long run? Am J Psychiatry. 2005;162:1149–1157. doi: 10.1176/appi.ajp.162.6.1149. [DOI] [PubMed] [Google Scholar]
- 40.Rickels K, Schweizer E. The spectrum of generalized anxiety in clinical practice: the role of short-term, intermittent treatment. Br J Psychiatry Suppl. 1998;1:49–54. [PubMed] [Google Scholar]
- 41.Pincus T, Burton K, Vogel S. A systematic review of psychological risk factors for chronicity/disability in prospective cohorts of low back pain. Spine. 2002;27:109–120. doi: 10.1097/00007632-200203010-00017. [DOI] [PubMed] [Google Scholar]
- 42.Weisberg RB, Dyck I, Culpepper L. Psychiatric treatment in primary care patients with anxiety disorders: a comparison of care received from primary care providers and psychiatrists. Am J Psychiatry. 2007;164:276–282. doi: 10.1176/appi.ajp.164.2.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wasan AD, Jamison RN, Pham L, Tipirneni N, Nedeljkovic SS, Katz JN. Psychopathology predicts the outcome of medial branch blocks with corticosteroid for chronic axial low back or cervical pain: a prospective cohort study. BMC Musculoskelet Disord. 2009;10:22. doi: 10.1186/1471-2474-10-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Flor H, Turk DC. Chronic Pain: An Integrated Biobehavioral Approach. Seattle: IASP Press; 2011. [Google Scholar]
- 45.Svenningsson I, Bjorkelund C, Marklund B, Gedda B. Anxiety and depression in obese and normal-weight individuals with diabetes type 2: a gender perspective. Scand J Caring Sci. 2012;26:349–354. doi: 10.1111/j.1471-6712.2011.00940.x. [DOI] [PubMed] [Google Scholar]
- 46.Tamam MO, Bagcioglu E, Mulazimoglu M, Tamam L, Ozpacaci T. Evaluation of anxiety and depression in patients prior to myocardial perfussion scintigraphy. Int J Psychiatry Clin Pract. 2012;16:93–97. doi: 10.3109/13651501.2011.631017. [DOI] [PubMed] [Google Scholar]
- 47.Andersson G. Epidemiological features of low back pain. Lancet. 1999;354:581–585. doi: 10.1016/S0140-6736(99)01312-4. [DOI] [PubMed] [Google Scholar]
- 48.Linton S. A review of psychological risk factors in back and neck pain. Spine. 2000;25:1148–1156. doi: 10.1097/00007632-200005010-00017. [DOI] [PubMed] [Google Scholar]
- 49.Edwards RR, Calahan C, Mensing G, Smith M, Haythornthwaite JA. Pain, catastrophizing, and depression in the rheumatic diseases. Nat Rev. 2011;7:216–224. doi: 10.1038/nrrheum.2011.2. [DOI] [PubMed] [Google Scholar]