Abstract
Influenza H3N2 A viruses continue to circulate in swine and occasionally infect humans, resulting in outbreaks of variant influenza H3N2 [A(H3N2)v] virus. It has been previously demonstrated in ferrets that A(H3N2)v viruses transmit as efficiently as seasonal influenza viruses, raising concern over the pandemic potential of these viruses. However, A(H3N2)v viruses have not acquired the ability to transmit efficiently among humans, which may be due in part to existing cross-reactive immunity to A(H3N2)v viruses. Although current seasonal H3N2 and A(H3N2)v viruses are antigenically distinct from one another, historical H3N2 viruses have some antigenic similarity to A(H3N2)v viruses and previous exposure to these viruses may provide a measure of immune protection sufficient to dampen A(H3N2)v virus transmission. Here, we evaluated whether prior seasonal H3N2 influenza virus vaccination or infection affects virus replication and transmission of A(H3N2)v virus in the ferret animal model. We found that the seasonal trivalent inactivated influenza virus vaccine (TIV) or a monovalent vaccine prepared from an antigenically related 1992 seasonal influenza H3N2 (A/Beijing/32/1992) virus failed to substantially reduce A(H3N2)v (A/Indiana/08/2011) virus shedding and subsequent transmission to naive hosts. Conversely, ferrets primed by seasonal H3N2 virus infection displayed reduced A(H3N2)v virus shedding following challenge, which blunted transmission to naive ferrets. A higher level of specific IgG and IgA antibody titers detected among infected versus vaccinated ferrets was associated with the degree of protection offered by seasonal H3N2 virus infection. The data demonstrate in ferrets that the efficiency of A(H3N2)v transmission is disrupted by preexisting immunity induced by seasonal H3N2 virus infection.
INTRODUCTION
Swine origin influenza A H3N2 variant [A(H3N2)v] viruses have been responsible for numerous transmissions from pigs to humans since 2011. To date, there have been over 330 laboratory-confirmed human cases of A(H3N2)v virus infection, and the first A(H3N2)v cases of 2013 were reported on June 28, 2013 (1). Although A(H3N2)v viruses generally induce mild symptoms similar to those normally seen with seasonal influenza, there have been 16 hospitalizations and 1 death reported in the U.S. since July 2012 (1). An investigation of one of the first A(H3N2)v influenza cases detected at a U.S. state agricultural fair in 2011 identified 3 virologically confirmed cases, 4 seropositive probable cases, and a further 82 suspected cases of respiratory illness associated with a fair visit, suggesting that only a minority of A(H3N2)v influenza cases are laboratory confirmed (37). In a retrospective cohort study conducted among children of an agricultural club who attended a fair, the risk for suspected case status increased with increasing exposure to swine (37).
The A(H3N2)v viruses that have infected humans originated in pigs following the introduction of hemagglutinin (HA) H3 and neuraminidase N2 genes from human seasonal H3N2 influenza viruses that circulated globally in the mid-1990s (2). The introduction of seasonal H3N2 virus into pigs also contributed to multiple reassortment events resulting in the emergence of a swine H3N2 virus with a triple-reassortant internal gene (TRIG) cassette containing a combination of avian, swine, and human influenza virus genes (2, 3). The H3 HA of human and swine influenza viruses followed divergent evolutionary pathways resulting in antigenically distinct influenza H3N2 viruses (4–6). Experimentally, the seasonal 2011-2012 trivalent inactivated influenza virus vaccine (TIV) failed to generate a cross-reactive antibody response to A(H3N2)v virus in ferrets and offered no protection from A(H3N2)v virus challenge (7). However, A(H3N2)v viruses retained a low degree of serologic cross-reactivity with human H3N2 viruses that circulated in the early 1990s (4, 6). This is supported by human serology studies showing that young adults aged 18 to 39 years possess substantial levels of preexisting cross-reactive antibodies to A(H3N2)v viruses (8–10). In contrast, children younger than 12 years of age have little to no preexisting cross-reactive antibodies to A(H3N2)v viruses (10). The observed immunity may exist in this population due to exposure to H3N2 influenza virus antigens through natural infection and/or vaccination during the 1990s.
The ferret model recapitulates the efficient transmission of seasonal influenza viruses and the poor transmission of avian influenza viruses in humans (11). Using this model, previous studies have shown that A(H3N2)v virus transmitted efficiently to naive ferrets by respiratory droplets (12). However, there has been no evidence of sustained human-to-human transmission of A(H3N2)v virus and most of the rare, limited human-to-human transmission has occurred between children within familial clusters or within day-care settings (13, 14). It is conceivable that the lack of efficient A(H3N2)v virus transmission in humans is partly due to the presence of preexisting cross-reactive immunity to this virus in the human population.
The impact of prior virus infection or vaccination on influenza virus transmission has been evaluated in only a limited number of studies. The ferret and guinea pig models have been used to evaluate the protection against the 2009 H1N1 pandemic [A(H1N1)pdm] virus transmission conferred by previous exposure to a seasonal influenza virus (15–17). Our laboratory showed that prior immunization with seasonal (2008-2009) live attenuated influenza virus vaccine (LAIV) or infection with a former seasonal H1N1 virus provides some cross-protection against A(H1N1)pdm virus challenge but has no significant effect on the transmission efficiency of A(H1N1)pdm virus in ferrets (18). To date, animal studies have not been performed to assess the effect of seasonal H3N2 influenza vaccination or infection on the transmission of A(H3N2)v virus. Here, we used the ferret model to determine if the degree of protection offered by previous exposure to seasonal human viruses (historical or contemporary) could account for the limited capacity of these viruses to transmit among the human population. We demonstrated that prior H3N2 virus infection, but not immunization with seasonal TIV, substantially reduced A(H3N2)v virus shedding and subsequent transmission to naive ferrets. Specific IgA and IgG antibody responses in serum and mucosal secretions were also measured to help explain the observed differences in protection induced by prior H3N2 virus infection versus seasonal influenza virus vaccination.
MATERIALS AND METHODS
Viruses.
The seasonal H3N2 viruses A/Beijing/32/1992 (Beijing/92) and A/Perth/16/2009 (Perth/09) were propagated in the allantoic cavity of 10-day-old embryonated eggs at 34°C for 48 h. The A(H3N2)v virus, A/Indiana/08/2011 (IN/11), was grown in Madin-Darby kidney (MDCK) cells at 37°C for 48 h. IN/11 is a representative H3N2v virus containing the M gene from A(H1N1)pdm09 virus (12). All virus stocks were clarified by centrifugation after collection and stored at −80°C. Stocks were titrated in a standard plaque assay on MDCK cells and expressed as PFU (19).
Whole-virus vaccine preparation.
Virus was concentrated from large volumes of allantoic fluid by ultracentrifugation at 17,000 rpm for 3 h at 4°C. Concentrated viruses were purified over a 30- to 60%-sucrose cushion as previously described (20). The HA gene of each vaccine stock was confirmed by sequencing to ensure no inadvertent mutations were introduced during propagation of new vaccine stocks. Concentrated viruses were inactivated with 0.025% formalin at 4°C for 3 days. The treatment resulted in the complete loss of infectivity of the virus, as determined by titration of vaccine preparations in eggs. The vaccine doses used are expressed as amounts of total protein measured by Bradford assay (Bio-Rad Laboratories, Hercules, CA). Evaluation of the HA protein content of purified vaccines was done using a Coomassie gel system as previously described (21). The HA protein was estimated to make up 40 to 55% of the total protein of purified vaccines.
Ferret immunizations.
All animal experiments were performed in biosafety level 3 laboratories with enhancements (BSL3+) as outlined in Biosafety in Microbiological and Biomedical Laboratories (22).
Animal research was conducted under the guidance of the Centers for Disease Control and Prevention's Institutional Animal Care and Use Committee in an Association for Assessment and Accreditation of Laboratory Animal Care International-accredited animal facility. Adult male Fitch ferrets, 5 to 8 months of age (Triple F Farms, Sayre, PA) and serologically negative for currently circulating influenza viruses as determined by hemagglutination inhibition (HI) assay (described below), were used in this study. Ferrets were vaccinated twice (with 3 to 5 weeks between vaccinations) intramuscularly (i.m.) with an adult human dose (in a 0.5-ml prefilled syringe) of the seasonal (2011-2012) commercially available split-product TIV. In some experiments, ferrets were injected i.m. with 15 μg of inactivated whole-virus vaccine prepared from Beijing/92, Perth/09, or IN/11 virus. Prior to initial vaccination, vaccine boost, and viral challenge, all ferrets were bled for collection of serum and analyzed by HI assay. For determination of prechallenge antibody titers, sera were collected 5 weeks following the final vaccination. For primary Perth/09 or Beijing/92 virus infections, ferrets were first anesthetized with a ketamine-xylazine-atropine cocktail given i.m. followed by a 1-ml intranasal (i.n.) inoculation (500 μl per nostril) of 106 PFU of infectious virus diluted in phosphate-buffered saline (PBS). Nasal wash titers were collected (described below) on day 3 postinoculation (p.i.), and sera were collected at 6 weeks p.i. to confirm viral replication and seroconversion.
Virus challenge and transmission experiments.
Baseline serum, temperature, and weight measurements were obtained before virus inoculations. Temperatures were measured with a subcutaneous (s.c.) implantable temperature transponder (BioMedic Data Systems, Seaford, DE). For A(H3N2)v virus challenges, ferrets were inoculated with 106 PFU of virus as described above. Virus challenges occurred 5 weeks after the final vaccine boost and 6 weeks after prior infection. For respiratory droplet transmission experiments, naive-contact animals were placed in adjacent cages 24 h after challenge using a previously established model (11, 23). Following inoculation, animals were monitored daily for changes in body weight and temperature as well as clinical signs of illness. Nasal washes were collected on alternate days postchallenge with 1 ml of PBS containing bovine serum albumin (BSA) and antibiotics (11, 23). Viral titers of the nasal washes were determined by plaque assay, and sera were collected from the contact ferrets approximately 21 days postcontact (p.c.) to check for seroconversion via HI.
Hemagglutination inhibition.
HI assays were performed using infectious virus stocks or a World Health Organization (WHO) influenza reagent kit (according to instructions) obtained through the Influenza Reagent Resource, Influenza Division, WHO Collaborating Center for Surveillance, Epidemiology and Control of Influenza, Centers for Disease Control and Prevention (Atlanta, GA). Sera were initially treated with receptor-destroying enzyme (RDE) from Vibrio cholerae (Denka Seiken, Tokyo, Japan) overnight at 37°C. The enzyme was then inactivated at 56°C for 30 min and PBS was added to the sera for a final dilution of 1:10. The sera were then absorbed to turkey RBCs (tRBCs) for 30 min at 4°C to remove any nonspecific agglutinins. The HI assay was performed in V-bottom 96-well plates using 4 hemagglutinating units (HAU) of virus and 0.5% tRBCs. Ferret sera were tested for HI antibody titers against a selection of the following antigens: Beijing/92, Perth/09, Ind/11, A/California/07/2009 (pH1N1), and B/Brisbane/60/2008 (influenza B).
Ferret mucosal wash collection.
Both upper respiratory tract (URT; sampled by tracheal/nasal wash) and lower respiratory tract (LRT; bronchoaveolar [lung] wash samples) were collected 5 weeks after prior infection or final vaccination using a novel protocol adapted from protocols established for mice (24). Ferrets were exsanguinated by cardiac puncture under ketamine anesthesia to best avoid blood contamination of the mucosal samples. The chest cavity was opened to expose the lungs and an incision was made to expose the trachea. The trachea was cut just below the larynx and clamped before the entire respiratory tract was carefully removed with the heart. For LRT washes, a volume of 10 ml of PBS containing antibiotics was infused slowly down the trachea into the lung using a pipette. After filling, the lungs were gently massaged and inverted, and the fluid was allowed to drip back through the trachea and collected in 35-mm petri dishes. Approximately 6 to 8 ml of fluid was retrieved and immediately kept on ice. The URT washes from the same animals were collected by flushing 5 ml of PBS with antibiotics through the open tracheal incision and forward into the nasal passages 3 times. The supernatant from lavage fluid was collected following centrifugation at 200 × g at 4°C for 10 min and frozen at −20°C until analyzed by enzyme-linked immunosorbent assay (ELISA). Any sample with blood contamination was excluded from analysis.
ELISA.
Ferret IgG and IgA antibodies were detected using a modified ELISA procedure as previously described (20, 25). Flat-bottom 96-well microtiter plates (Immulon II; Dynatech Laboratories, Chantilly, VA) were coated with 100 HAU per well of purified Perth/09 inactivated virus overnight at 4°C. Serum samples were added to the plates in 4-fold serial dilutions starting at 1:100 for IgG and 1:20 for IgA. LRT/URT samples were added at starting dilutions of 1:10 for IgG and 1:2 for IgA. Bound antibody was detected by IgG horseradish peroxidase (HRP)-conjugated antibody (Bethyl Laboratories, Montgomery, TX) or by a goat anti-ferret IgA followed by a rabbit anti-goat HRP-conjugated antibody (Bethyl). The absorbance was measured at 490 nm following the addition of o-phenylenediamine(dihydrochloride) (OPD) in citrate buffer (Sigma). Titers are expressed as the highest dilution that yielded an optical density greater than the mean plus two standard deviations of similarly diluted PBS control sera.
Statistics.
Statistical significance was determined by one of the following methods: Student's t test, 2-way analysis of variance (ANOVA) with a Bonferroni's posttest, or area under the curve (AUC) with a Mann-Whitney posttest.
RESULTS
Transmission of seasonal H3N2 and A(H3N2)v viruses.
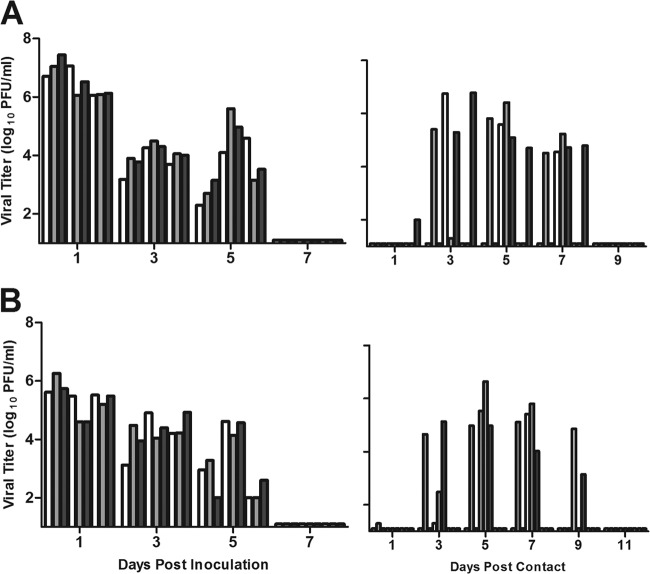
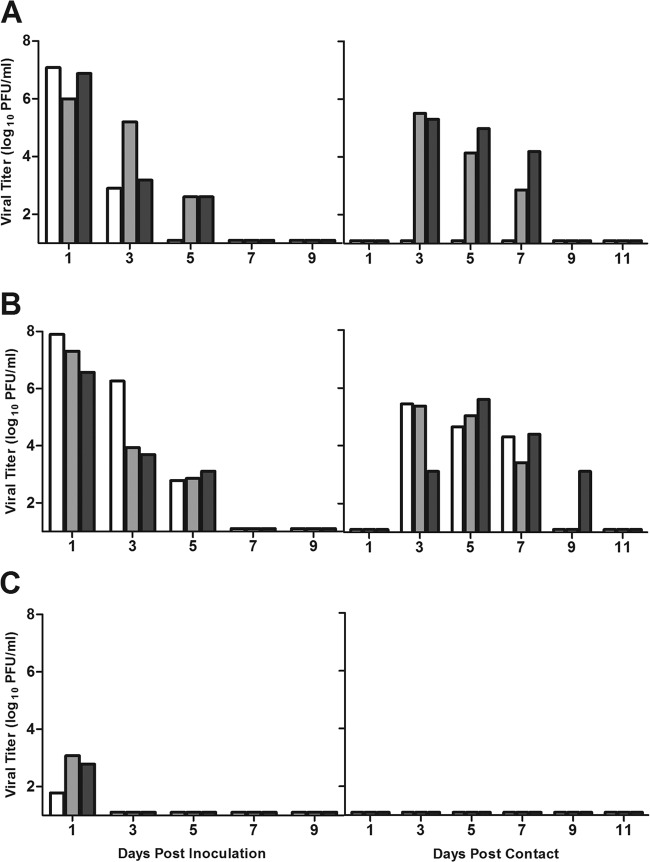
To examine whether seasonal H3N2 vaccination or prior infection affects the transmission efficiency of A(H3N2)v or homologous seasonal H3N2 viruses, we first determined the transmission efficiencies of these viruses in naive ferrets. For respiratory droplet transmission, ferrets were housed in adjacent cages with a perforated side wall, allowing air exchange between ferrets in the absence of direct or indirect contact (11, 23). Ferrets were inoculated with 106 PFU of Perth/09, representative of H3N2 viruses circulating in the 2011-2012 Northern Hemisphere season. Nasal washes were collected every other day, and clinical signs were monitored daily for both inoculated and contact ferrets. All Perth/09 virus-inoculated ferrets exhibited a mean temperature increase of 1.0°C (Table 1) and shed high titers of infectious virus as early as day 1 that were sustained at titers of ≥104.0 PFU/ml for 5 days p.i. (Fig. 1A). Although viral titers were detected in the nasal washes of only 2 of the 3 contact ferrets, all 3 animals displayed body temperature elevation and seroconverted to Perth/09 virus (Table 1). Thus, consistent with the experimental transmission data obtained with other seasonal human influenza viruses (11, 23), Perth/09 H3N2 virus exhibited efficient respiratory droplet transmission.
Table 1.
Clinical symptoms of inoculated and contact ferrets
| Treatment | Challenge virus | Mean max wt lossa (%) |
Mean max temp increaseb (°C) |
No. of seroconverted contact ferrets/total no. of contact ferretsc | ||
|---|---|---|---|---|---|---|
| Inoculated | Contacts | Inoculated | Contacts | |||
| None | 6/6 | |||||
| Perth/09 | 4.3 | 2.0 | 1.0 | 0.9 | 3/3 (640) | |
| IN/11 | 5.8 | 2.8 | 2.2 | 1.6 | 3/3 (1,015) | |
| TIV | 10/18 | |||||
| Perth/09 | 6.3 | 3.0 | 1.3 | 1.0 | 4/9 (1,076) | |
| IN/11 | 7.3 | 6.0 | 1.1 | 1.5 | 6/9 (640) | |
| Monovalent vaccine | 5/9 | |||||
| Perth/09 vaccine | IN/11 | 8.1 | 6.3 | 1.3 | 1.1 | 2/3 (453) |
| Beijing/92 vaccine | IN/11 | 7.5 | 6.0 | 1.3 | 1.2 | 3/3 (640) |
| IN/11 vaccine | IN/11 | 1.6 | 0.8 | 0/3 (<10) | ||
| Perth/09 infection | 1/12 | |||||
| Perth/09 | 2.1 | 0.7 | 0/6 (<10) | |||
| IN/11 | 3.8 | 3.5 | 1.1 | 1.7 | 1/6 (1,280) | |
Percentage of mean maximum (max) weight loss shown for each ferret group.
Mean maximum temperature increase calculated as rise over ferret baseline of 38.3 ± 0.5°C. All maximum temperatures were observed on days 1 to 2 postinoculation.
Seroconversion was measured by HI from sera collected on day 21 p.c. Geometric mean titers for seroconverted contact ferrets are shown in parentheses. Values of <10 were below the limit of detection in this assay.
Fig 1.
Transmissibility of seasonal H3N2 and A(H3N2)v influenza viruses among naive ferrets in the respiratory droplet model. Ferrets were inoculated i.n. with 106 PFU of virus with 3 transmission pairs per experiment. Contact animals were placed in adjacent cages with perforated side walls 24 h postinoculation. (A) Transmission of seasonal H3N2 virus, Perth/09. (B) Transmission of A(H3N2)v virus, IN/11. Each bar represents the virus titer detected in nasal washes of an inoculated ferret (left half of figure) and its corresponding contact animal (right half). Limit of virus detection, 1 log10 PFU/ml.
Ferrets inoculated with A(H3N2)v (IN/11) virus exhibited modest weight loss and a maximum temperature increase of 2.2°C on day 2 p.i. (Table 1). Similar to the results described for the seasonal Perth/09 virus, IN/11 virus replicated efficiently in the upper respiratory tract of inoculated ferrets and spread efficiently to contact ferrets (Fig. 1B). The results are consistent with earlier ferret studies which found that A(H3N2)v viruses isolated since 2010 exhibited efficient respiratory droplet transmission (12).
Transmission of A(H3N2)v virus following seasonal TIV immunization.
To test whether seasonal influenza virus vaccination reduces the transmission of A(H3N2)v virus to naive ferrets, we set up experiments in which TIV-immune ferrets were challenged with IN/11 virus and subsequent transmission to naive animals was analyzed. Prior to virus challenge, all nine vaccinated ferrets displayed HI titers of ≥40 to each of the three influenza virus HA antigens present in the 2011-2012 TIV but lacked detectable cross-reactive HI antibodies to IN/11 virus antigen (Table 2). Seasonal TIV immunization did not protect ferrets from weight loss, fever (Table 1), or virus replication (Fig. 2A) following IN/11 virus challenge. As late as 5 days p.i., virus titers of ≥102.6 PFU/ml were still present in nasal washes of all vaccinated ferrets, and A(H3N2)v virus spread to six of nine naive-contact ferrets (Fig. 2A and Table 1). Virus transmission occurred with kinetics similar to that observed in naive ferrets, with the majority of contacts displaying viral titers in their nasal washes by day 3 p.c.
Table 2.
Prechallenge hemagglutination inhibition antibody responses to immunization in ferrets
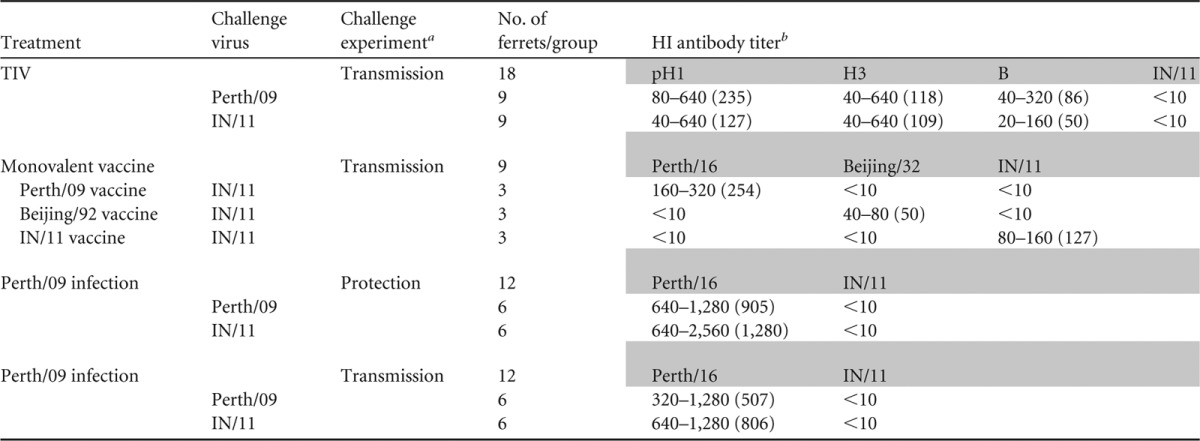
Ferrets for each experiment were divided into groups based on the vaccine tested and the challenge virus.
HI titers per virus separated by experiment. Antibody titer ranges within each group of ferrets are shown. Geometric means are included in parentheses. Values of <10 were below the limit of detection in this assay. pH1, A/California/7/2009; H3, A/Perth/16/2009; B, B/Brisbane/60/2008.
Fig 2.
Transmission of seasonal H3N2 and A(H3N2)v viruses following vaccination with the seasonal TIV. Five weeks after the final vaccine boost, TIV-immune ferrets were challenged with heterologous IN/11 virus (A) or homologous seasonal Perth/09 virus (B). Each bar represents the viral titer detected in nasal washes of inoculated ferrets (n = 6) and their corresponding contact animals (n = 6). Limit of virus detection, 1 log10 PFU/ml.
A separate group of TIV-immune ferrets were challenged with homologous Perth/09 virus for comparison. Despite prechallenge HI titers of ≥40 to homologous virus (Table 2), TIV did not provide sterilizing immunity against Perth/09 virus challenge. All ferrets displayed viral titers (≥102.1 PFU/ml) in their nasal washes for 5 days p.i., and respiratory droplet transmission of Perth/09 virus still occurred; virus spread to four of nine naive-contact ferrets (Fig. 2B), resulting in seroconversion to Perth/09 virus (Table 1).
Impact of vaccination with a historical seasonal H3N2 virus on A(H3N2)v virus transmission.
Serologic studies have previously demonstrated that the young adult population has the highest percentage of individuals with preexisting cross-reactive antibodies to A(H3N2)v virus (8–10). The individuals within this age range would have encountered antigenically related H3N2 viruses through virus infection or vaccination in the 1990s. We first performed antigenic analyses of 6 representative seasonal H3N2 viruses (spanning the years 1990 to 2013) by HI assay and the use of reference ferret antisera. The only seasonal H3N2 virus that displayed limited cross-reactivity with antisera to A(H3N2)v virus was A/Beijing/32/1992 (Beijing/92) virus, a component in the 1993-1994 seasonal TIV. Ferret antisera to Beijing/92 virus infection reacted to low titers (HI titer = 20) of IN/11 virus. These results are consistent with previous antigenic characterization which demonstrated that A(H3N2)v virus retains antigenic properties more similar to human seasonal viruses from the early 1990s than to currently circulating human A(H3N2) viruses (4).
We next assessed the ability of vaccination with inactivated whole-virus monovalent vaccines prepared from Beijing/92, Perth/09, and IN/11 viruses to limit infection and transmission of A(H3N2)v virus. Groups of ferrets were vaccinated i.m. with 15 μg of formalin-inactivated vaccine and two boosts in order to obtain HI titers of ≥40 against the homologous viral strain. HI antibody titers of ≥40 have been correlated with reduction in influenza-like illness in adult populations (26–28). The vaccines failed to elicit cross-reactive HI antibodies against heterologous H3N2 or A(H3N2)v viruses using the standard starting dilution of 1:10 serum (Table 2). However, a repeat of the HI test using a starting dilution of 1:4 revealed that Beijing/92-immune sera gave HI titers of 4 to 8 to IN/11 virus, whereas Perth/09-immune sera had undetectable (<4) HI titers to IN/11 virus (data not shown). Five weeks after the final vaccine boost, ferrets were set up in transmission cages and vaccinated ferrets were challenged with 106 PFU of IN/11 virus. As indicated in Fig. 3A, relatively high viral titers were detected in the nasal washes of Perth/09-vaccinated ferrets and transmission of IN/11 virus occurred in 2 of the 3 contact animals. Furthermore, Perth/09-vaccinated ferrets displayed weight loss and fever consistent with that observed in unimmunized PBS controls (Table 1). Despite the low HI cross-reactivity to IN/11 virus, Beijing/92-vaccinated ferrets also shed high titers of infectious virus and full transmission of IN/11 virus to naive contacts was observed (Fig. 3B). All three of the contacts began to shed virus by day 3 p.c. and seroconverted to A(H3N2)v virus (Table 1). Consistent with unimmunized controls, Beijing/92-vaccinated ferrets displayed a mean maximum weight loss of 7.5% and a fever spike of 1.3°C (Table 1). For comparison, a transmission efficiency experiment with IN/11 virus was performed among homologous IN/11-vaccinated ferrets. We found that in contrast to the lack of protection against IN/11 virus transmission conferred by heterologous vaccination, homologous-virus challenge of IN/11-vaccinated ferrets blunted transmission to the naive-contact ferrets (Fig. 3C). Viral titers were detected only in the nasal washes of the inoculated ferrets on day 1 p.i., and vaccinated animals displayed marginal weight loss (1.6%) and reduced fever (0.8°C) (Table 1). Taken together, our results show that monovalent vaccines prepared from Perth/09 virus or an antigenically related seasonal influenza H3N2 (Beijing/92) virus failed to substantially reduce A(H3N2)v virus shedding and subsequent transmission to naive hosts. Furthermore, the vaccine prepared from the 1992 seasonal H3N2 virus (Beijing/92) offered no added benefit over a contemporary seasonal H3N2 vaccine.
Fig 3.
Impact of vaccination of a historical seasonal H3N2 virus on A(H3N2)v virus transmission. Five weeks after the final vaccine boost, monovalent-vaccinated ferrets were placed into transmission cages and challenged with IN/11 virus. Groups of ferrets were vaccinated with inactivated vaccines prepared from Perth/09 (A), Beijing/92 (B), or IN/11 (C) virus. Transmission from inoculated (vaccinated) ferrets (n = 3) to naive contacts (n = 3) was determined. Limit of virus detection, 1 log10 PFU/ml.
Effect of prior seasonal H3N2 virus infection on cross-protection and transmission of A(H3N2)v virus.
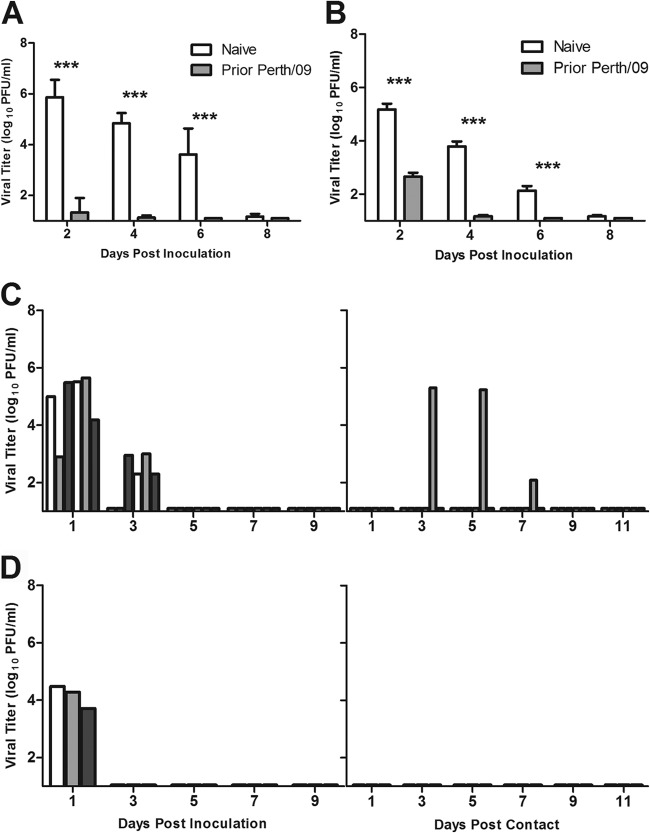
Next, we evaluated the level of cross-protection against A(H3N2)v virus that develops from seasonal H3N2 virus infection. Although seasonal H3N2 viruses are antigenically distinct from the A(H3N2)v viruses, prior influenza virus infection generally induces an effective mucosal immune response and may provide greater cross-protection than that induced by inactivated influenza virus vaccines given parenterally (16, 29). Ferrets were inoculated i.n. with 106 PFU of Perth/09 virus or PBS for the controls and challenged 6 weeks later with either Perth/09 or IN/11 virus. Following homologous-virus challenge with Perth/09, prior infection resulted in significantly lower viral titers in nasal washes than in those of the PBS controls on all days postchallenge (P = 0.004, P = 0.004, and P = 0.003 for days 2, 4, and 6, respectively) (Fig. 4A). Of the 6 ferrets that received primary virus infections, 5 displayed a complete lack of detectable virus in nasal washes. Primary infection with Perth/09 virus also significantly lowered viral titers in nasal washes following heterologous IN/11 virus infection (P = 0.002, P = 0.004, and P = 0.003 for days 2, 4, and 6, respectively) (Fig. 4B). Moreover, prior infection lowered the mean maximum weight loss by more than half (5.9% in PBS controls compared to 2.3% for Perth/09-immune ferrets) (Table 3).
Fig 4.
Effect of prior seasonal H3N2 virus infection on cross-protection and transmission of A(H3N2)v virus. Six weeks after primary seasonal H3N2 virus infection, naive and Perth/09-immune ferrets were challenged with homologous Perth/09 (A) or heterologous IN/11 (B) virus. Protection against challenge is represented by virus titers in nasal wash samples of naive ferrets (white bars) and Perth/09-immune ferrets (gray bars). Error bars correspond to standard deviations. ***, P ≤ 0.005 by Mann-Whitney nonparametric t test. Perth/09-immune (C) or Beijing/92-immune (D) ferrets were placed into transmission cages and challenged with heterologous IN/11 virus. Transmission from inoculated ferrets to naive contacts was determined. Limit of virus detection, 1 log10 PFU/ml.
Table 3.
Effect of prior seasonal H3N2 virus infection on protection against homologous and heterologous virus challenge
| Treatment group | Challenge virus | Mean max wt lossa (%) | Mean max temp increaseb (°C) | Mean peak viral titerc |
|---|---|---|---|---|
| Prior Perth/09 infection | Perth/09 | 1.7 | 1.1 | 1.2 ± 0.6 |
| PBS controls | Perth/09 | 1.9 | 1.9 | 5.9 ± 0.7 |
| Prior Perth/09 infection | IN/11 | 2.3 | 2.0 | 2.7 ± 0.4 |
| PBS controls | IN/11 | 5.9 | 1.8 | 5.2 ± 0.5 |
Percentage of mean maximum (max) weight loss shown for each ferret group.
Mean maximum temperature increases calculated as rise over ferret baseline of 38.3 ± 0.5°C. All maximum temperatures were observed on days 1 to 2 postchallenge.
Mean peak titer shown as log10 PFU/ml including the standard deviation. All peak titers are from day 2 postchallenge
Next we assessed the effect of primary seasonal H3N2 virus infection on the transmission of A(H3N2)v (IN/11) virus to naive animals. For comparison, a group of H3N2-immune ferrets received a homologous Perth/09 virus challenge. No virus shedding was detected from any of the six Perth/09-challenged animals on any day p.i. (data not shown), and immune ferrets displayed minimal weight loss (2.1%) and fever (maximum temperature increase = 0.7°C) (Table 1). Moreover, none of the contact ferrets shed virus (data not shown) or showed a serological response to Perth/09 virus (Table 1). Following heterologous virus challenge, IN/11 virus titers were substantially reduced in Perth/09-immune ferrets and were detected only through day 3 p.i. (Fig. 4C). Only one of six contact ferrets shed infectious virus and seroconverted to IN/11 virus. We also assessed whether primary infection with the antigenically related Beijing/92 virus would provide equivalent or greater protection against A(H3N2)v virus infection. Nasal wash viral titers were markedly reduced in the Beijing/92-immune ferrets and no transmission of IN/11 virus was observed (Fig. 4D). Taken together, the results show that prior seasonal virus infection effectively limited virus growth and blunted the transmission of A(H3N2)v virus.
IgG and IgA in upper and lower respiratory tracts of ferrets following primary virus infection or TIV immunization.
To elucidate the differences observed in the transmission efficiency of A(H3N2)v virus following Perth/09 virus infection versus vaccination, prechallenge sera and mucosal washes were collected and tested for antiviral IgG and IgA antibodies. Immune and control ferrets were necropsied for collection of mucosal washes from the upper respiratory tract (URT) and lower respiratory tract (LRT; bronchoaveolar-lung). In general, IgG and IgA responses among vaccinated and previously infected ferrets were increased compared to those of naive animals. As shown in Table 4, the highest antibody titers were IgG titers observed in the sera and LRTs of ferrets that received prior infections. Lung IgG titers were approximately 10,000-fold higher than those induced by TIV immunization. Serum IgA titers induced by either mode of immunization were similar; however, prior infection induced IgA responses in the URT that were 16-fold higher than those elicited by vaccination. Taken together, the results show that Perth/09 virus infection resulted in consistently higher IgG and URT IgA titers than TIV immunization.
Table 4.
Antiviral IgG and IgA antibody responses among vaccinated and infected ferretsa
| Antibody | Titers for group |
|||||
|---|---|---|---|---|---|---|
| Serum |
URT wash fluid |
LRT wash fluid |
||||
| Infected | Vaccinated | Infected | Vaccinated | Infected | Vaccinated | |
| IgG | 409,600 | 102,400 | 160 | ND | 409,600 | 40 |
| IgA | 1,280 | 1,280 | 128 | 8 | 8 | 2 |
Ferrets were inoculated i.n. with 106 PFU of Perth/09 virus or vaccinated with TIV. ELISA plates were coated with purified Perth/09 inactivated virus. Titers from 4 ferrets per group are for the largest dilution of the sample having a mean optical density greater than the mean plus two standard deviations of the value for the similarly diluted control (unimmunized) sample. ND, not detected; no dilution read above the mean plus 2 standard deviations of the control sample.
DISCUSSION
New reports of human infections with A(H3N2)v in 2013 continue to raise public health concerns and highlight the need to better understand the potential of these viruses to transmit efficiently among humans (1). A paradox exists with regard to the transmission efficiency of A(H3N2)v virus in humans versus that observed in the ferret model. Sustained or community-wide transmission of A(H3N2)v has not occurred, whereas these viruses transmit efficiently between immunologically naive ferrets (12). We hypothesized that preexisting immunity to seasonal H3N2 viruses present in the general population accounts for the contrasting results. Vaccination is currently the best public health measure to prevent influenza virus infection, and human epidemiological studies have clearly established that immunization of those who are most likely to acquire and transmit influenza (usually children) can reduce the risk of secondary transmission of influenza virus within families (26, 27). However, the impact of prior seasonal influenza vaccination on influenza virus transmission has not been extensively modeled in animals (16). In this study, ferrets were used to model the transmission of A(H3N2)v virus following preexposure by seasonal H3N2 virus infection or vaccination. The data presented suggest that vaccination with inactivated seasonal influenza virus vaccines would not provide any significant protection against A(H3N2)v virus infection and subsequent transmission of the virus. Conversely, prior infection with a contemporary seasonal H3N2 virus effectively limited virus shedding and blunted subsequent transmission of A(H3N2)v virus. The cross-protective response induced by influenza virus infection was associated with higher levels of virus-specific IgG and IgA antibodies than in ferrets that received inactivated influenza virus vaccines.
We previously demonstrated that in comparison to unimmunized controls, TIV-immunized ferrets displayed a significant reduction in viral titers following homologous H3N2 virus challenge (7). However, even with an identical antigenic match between the challenge and vaccine virus, TIV does not provide sterilizing immunity against homologous virus challenge in ferrets, as observed here (Fig. 2B) and previously (7). The inability of seasonal TIV immunization to block transmission of A(H3N2)v virus to naive-contact animals prompted us to prepare a monovalent vaccine to a seasonal influenza H3N2 virus, Beijing/92, that showed some, albeit low, antigenic similarity to A(H3N2)v virus using postinfection ferret antisera (4, 6). For comparison, a monovalent Perth/09 vaccine, the H3N2 virus component of the seasonal (2011-2012) TIV, was similarly tested. Although Beijing/92 virus is more closely related antigenically to A(H3N2)v (IN/11) virus, as an inactivated vaccine it failed to provide greater protection than that observed in ferrets vaccinated with monovalent Perth/09 vaccine. Thus, in our hands, a distinction could not be made between the historical and contemporary H3N2 inactivated vaccines administered parenterally in ferrets. Although beyond the scope of this study, a vaccine dose response experiment may have revealed efficacy differences between the historical and contemporary seasonal H3N2 vaccines. It is conceivable that the H3N2 whole-virus vaccines induced non-HA immune responses that masked any differences that would have been observed from vaccinating with HA only. A different vaccine formulation, such as one using recombinant HA proteins in which the HA proteins are the primary antigens, may yield differences in protection against A(H3N2)v virus challenge in ferrets.
We found that in contrast to results obtained with inactivated vaccines, ferrets previously exposed to seasonal influenza H3N2 virus exhibited stronger immune protection and that transmission was blocked following subsequent challenge with homologous or heterologous virus. Prior infection with Perth/09 virus dramatically reduced transmission rates from 100% in naive ferrets to 0% following homologous-virus challenge and to 17% for heterologous challenge. Moreover, primary infection with Beijing/92 virus blunted transmission of A(H3N2)v virus. However, the impact of prior influenza virus infection on limiting transmission appears to be dependent on the transmission model and/or the mode of viral transmission. Using a direct-contact transmission (cohoused) model, Ellebedy et al. reported that prior H1N1 virus infection was unable to alter transmission in ferrets following challenge with A(H1N1)pdm virus (16). Similarly, Laurie et al. showed that prior infection with A(H1N1)pdm virus did not significantly alter A(H1N1)pdm virus transmission in a direct-contact ferret model (30). Interestingly, the latter study demonstrated that multiple preinfections with seasonal H1N1 virus were required to reduce A(H1N1)pdm virus transmission. Using guinea pigs in a direct-contact model, two studies found that transmission of influenza virus could be disrupted when guinea pigs had prior exposure to seasonal virus (16). However, unlike our study, these studies did not assess the impact of prior influenza virus infection on respiratory droplet (or airborne) transmission.
To understand the immunological basis of conferring protection by virus infection, we compared the IgG and IgA antibody levels present in sera and respiratory tract washings. Both routes (vaccination or infection) of immunization induced high and similar levels of antibodies in the serum; however, virus infection induced consistently higher specific IgG and IgA antibody titers in the respiratory tract than in the respiratory tract of TIV-immunized ferrets. The differences in IgA and IgG antibodies between the immunization groups were not evident using the standard HI assay. These results are consistent with the ability of a live attenuated influenza vaccine to elicit higher levels of nasal wash IgA and greater reduction of virus shedding than an inactivated influenza virus vaccine (20, 25). Although the precise immunological mechanism(s) involved in viral clearance was not addressed in the current study, it is reasonable to speculate that raised antibody levels in the ferret respiratory tract of virus-infected ferrets would have a greater capacity to neutralize virus infectivity and blunt transmission.
Because of the paucity of immunological reagents for ferrets, the role of T cell immunity and cytokines involved in conferring protection by infection was not addressed in the current study. Parenterally administered (unadjuvanted) vaccines such as TIV do not effectively induce influenza-specific CD8+ T cells or mucosal immune responses, two immune effectors capable of protecting the host (31, 32). CD8+ T cell responses are important in heterologous protection, and children have been shown to have weaker heterologous responses following vaccination than adults (33). Although non-HA components in the TIV have been shown previously to contribute to the immune response and these components may contain conserved T cell epitopes (34, 35), such cross-reactive immunity was not sufficient to provide protection against A(H3N2)v virus transmission. The immune responses induced by H3N2 virus infection and its effect on subsequent viral transmission need further investigation.
The potential of A(H3N2)v virus to mutate or undergo additional reassortment events and thus improve its transmissibility is of concern. Determining the level of immunity required to blunt transmission of novel influenza viruses such as A(H3N2)v could deepen our understanding of how pandemic influenza virus subtypes arise and spread in humans. It is apparent from this study and others (discussed above) that influenza virus transmission is influenced by the infection history of the host. The current data suggest that inactivated vaccines would be effective at reducing A(H3N2)v virus shedding and subsequent transmission only if the vaccine were A(H3N2)v specific, whereas prior infection with infectious virus capable of inducing protective mucosal immunity can generate heterologous protection in the absence of specific A(H3N2)v antibody. One caveat to our studies is that the immune response to vaccination was studied only in naive ferrets, while TIV has been shown to boost preexisting immunity in humans and result in limited mucosal antibody production (36). There are certainly additional unknown factors contributing to the poor transmission of A(H3N2)v virus in humans, especially in light of the fact that the largest concentration of confirmed A(H3N2)v infections have been in children that have the least previous exposure to influenza virus and the most limited preexisting serological and heterologous CD8+ T cell responses against these viruses (8, 9).
ACKNOWLEDGMENTS
We thank Nedzad Music for helpful discussions regarding ferret mucosal-wash collections and Xiuhua Lu for ELISA assistance.
K.V.H. received financial support for this work from the Oak Ridge Institute for Science and Education, Oak Ridge, TN. The findings and conclusions in this report are those of the authors and do not necessarily reflect the views of the funding agency.
Footnotes
Published ahead of print 2 October 2013
REFERENCES
- 1.CDC 5 July 2013, posting date. Case count: detected U.S. human infections with H3N2v by state since August 2011. Centers for Disease Control and Prevention, Atlanta, GA: http://www.cdc.gov/flu/swineflu/h3n2v-case-count.htm [Google Scholar]
- 2.Vincent AL, Ma W, Lager KM, Janke BH, Richt JA. 2008. Swine influenza viruses a North American perspective. Adv. Virus Res. 72:127–154 [DOI] [PubMed] [Google Scholar]
- 3.Zhou NN, Senne DA, Landgraf JS, Swenson SL, Erickson G, Rossow K, Liu L, Yoon K, Krauss S, Webster RG. 1999. Genetic reassortment of avian, swine, and human influenza A viruses in American pigs. J. Virol. 73:8851–8856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shu B, Garten R, Emery S, Balish A, Cooper L, Sessions W, Deyde V, Smith C, Berman L, Klimov A, Lindstrom S, Xu X. 2012. Genetic analysis and antigenic characterization of swine origin influenza viruses isolated from humans in the United States, 1990-2010. Virology 422:151–160 [DOI] [PubMed] [Google Scholar]
- 5.Richt JA, Lager KM, Janke BH, Woods RD, Webster RG, Webby RJ. 2003. Pathogenic and antigenic properties of phylogenetically distinct reassortant H3N2 swine influenza viruses cocirculating in the United States. J. Clin. Microbiol. 41:3198–3205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lindstrom S, Garten R, Balish A, Shu B, Emery S, Berman L, Barnes N, Sleeman K, Gubareva L, Villanueva J, Klimov A. 2012. Human infections with novel reassortant influenza A(H3N2)v viruses, United States, 2011. Emerg. Infect. Dis. 18:834–837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Houser KV, Katz JM, Tumpey TM. 2013. Seasonal trivalent inactivated influenza vaccine does not protect against newly emerging variants of influenza A (H3N2v) virus in ferrets. J. Virol. 87:1261–1263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Skowronski DM, Janjua NZ, De Serres G, Purych D, Gilca V, Scheifele DW, Dionne M, Sabaiduc S, Gardy JL, Li G, Bastien N, Petric M, Boivin G, Li Y. 2012. Cross-reactive and vaccine-induced antibody to an emerging swine-origin variant of influenza A virus subtype H3N2 (H3N2v). J. Infect. Dis. 206:1852–1861 [DOI] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention 2012. Antibodies cross-reactive to influenza A (H3N2) variant virus and impact of 2010-11 seasonal influenza vaccine on cross-reactive antibodies—United States. MMWR Morb. Mortal. Wkly. Rep. 61:237–241 [PubMed] [Google Scholar]
- 10.Waalen K, Kilander A, Dudman SG, Ramos-Ocao R, Hungnes O. 2012. Age-dependent prevalence of antibodies cross-reactive to the influenza A(H3N2) variant virus in sera collected in Norway in 2011. Euro Surveill. 17(19):pii=20170 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=20170 [PubMed] [Google Scholar]
- 11.Maines TR, Chen LM, Matsuoka Y, Chen H, Rowe T, Ortin J, Falcon A, Nguyen TH, Mai LQ, Sedyaningsih QER, Harun S, Tumpey TM, Donis RO, Cox NJ, Subbarao K, Katz JM. 2006. Lack of transmission of H5N1 avian-human reassortant influenza viruses in a ferret model. Proc. Natl. Acad. Sci. U. S. A. 103:12121–12126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pearce MB, Jayaraman A, Pappas C, Belser JA, Zeng H, Gustin KM, Maines TR, Sun X, Raman R, Cox NJ, Sasisekharan R, Katz JM, Tumpey TM. 2012. Pathogenesis and transmission of swine origin A(H3N2)v influenza viruses in ferrets. Proc. Natl. Acad. Sci. U. S. A. 109:3944–3949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.CDC 2011. Limited human-to-human transmission of novel influenza A (H3N2) virus—Iowa, November 2011. MMWR Morb. Mortal. Wkly. Rep. 60:1615–1617 [PubMed] [Google Scholar]
- 14.Epperson S, Jhung M, Richards S, Quinlisk P, Ball L, Moll M, Boulton R, Haddy L, Biggerstaff M, Brammer L, Trock S, Burns E, Gomez T, Wong KK, Katz J, Lindstrom S, Klimov A, Bresee JS, Jernigan DB, Cox N, Finelli L, Influenza A (H3N2)v Virus Investigation Team. 2013. Human infections with influenza A(H3N2) variant virus in the United States, 2011-2012. Clin. Infect. Dis. 57(Suppl. 1):S4–S11 [DOI] [PubMed] [Google Scholar]
- 15.Pascua PN, Song MS, Lee JH, Park KJ, Kwon HI, Baek YH, Hong SP, Rho JB, Kim CJ, Poo H, Ryoo TS, Sung MH, Choi YK. 2009. Evaluation of the efficacy and cross-protectivity of recent human and swine vaccines against the pandemic (H1N1) 2009 virus infection. PLoS One 4:e8431. 10.1371/journal.pone.0008431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ellebedy AH, Ducatez MF, Duan S, Stigger-Rosser E, Rubrum AM, Govorkova EA, Webster RG, Webby RJ. 2011. Impact of prior seasonal influenza vaccination and infection on pandemic A (H1N1) influenza virus replication in ferrets. Vaccine 29:3335–3339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Steel J, Staeheli P, Mubareka S, Garcia-Sastre A, Palese P, Lowen AC. 2010. Transmission of pandemic H1N1 influenza virus and impact of prior exposure to seasonal strains or interferon treatment. J. Virol. 84:21–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pearce MB, Belser JA, Houser KV, Katz JM, Tumpey TM. 2011. Efficacy of seasonal live attenuated influenza vaccine against virus replication and transmission of a pandemic 2009 H1N1 virus in ferrets. Vaccine 29:2887–2894 [DOI] [PubMed] [Google Scholar]
- 19.Zeng H, Goldsmith C, Thawatsupha P, Chittaganpitch M, Waicharoen S, Zaki S, Tumpey TM, Katz JM. 2007. Highly pathogenic avian influenza H5N1 viruses elicit an attenuated type I interferon response in polarized human bronchial epithelial cells. J. Virol. 81:12439–12449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tumpey TM, Renshaw M, Clements JD, Katz JM. 2001. Mucosal delivery of inactivated influenza vaccine induces B-cell-dependent heterosubtypic cross-protection against lethal influenza A H5N1 virus infection. J. Virol. 75:5141–5150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harvey R, Wheeler JX, Wallis CL, Robertson JS, Engelhardt OG. 2008. Quantitation of haemagglutinin in H5N1 influenza viruses reveals low haemagglutinin content of vaccine virus NIBRG-14 (H5N1). Vaccine 26:6550–6554 [DOI] [PubMed] [Google Scholar]
- 22.Health and Human Services 2009. Biosafety in microbiological and biomedical laboratories, 5th ed. HHS publication no. (CDC) 21-1112. Department of Health and Human Services, Washington, DC [Google Scholar]
- 23.Van Hoeven N, Pappas C, Belser JA, Maines TR, Zeng H, Garcia-Sastre A, Sasisekharan R, Katz JM, Tumpey TM. 2009. Human HA and polymerase subunit PB2 proteins confer transmission of an avian influenza virus through the air. Proc. Natl. Acad. Sci. U. S. A. 106:3366–3371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Katz JM, Lu X, Young SA, Galphin JC. 1997. Adjuvant activity of the heat-labile enterotoxin from enterotoxigenic Escherichia coli for oral administration of inactivated influenza virus vaccine. J. Infect. Dis. 175:352–363 [DOI] [PubMed] [Google Scholar]
- 25.Gustin KM, Maines TR, Belser JA, van Hoeven N, Lu X, Dong L, Isakova-Sivak I, Chen LM, Voeten JT, Heldens JG, van den Bosch H, Cox NJ, Tumpey TM, Klimov AI, Rudenko L, Donis RO, Katz JM. 2011. Comparative immunogenicity and cross-clade protective efficacy of mammalian cell-grown inactivated and live attenuated H5N1 reassortant vaccines in ferrets. J. Infect. Dis. 204:1491–1499 [DOI] [PubMed] [Google Scholar]
- 26.Cox NJ, Subbarao K. 1999. Influenza. Lancet 354:1277–1282 [DOI] [PubMed] [Google Scholar]
- 27.Ghendon YZ, Kaira AN, Elshina GA. 2006. The effect of mass influenza immunization in children on the morbidity of the unvaccinated elderly. Epidemiol. Infect. 134:71–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hannoun C, Megas F, Piercy J. 2004. Immunogenicity and protective efficacy of influenza vaccination. Virus Res. 103:133–138 [DOI] [PubMed] [Google Scholar]
- 29.Lowen AC, Mubareka S, Tumpey TM, Garcia-Sastre A, Palese P. 2006. The guinea pig as a transmission model for human influenza viruses. Proc. Natl. Acad. Sci. U. S. A. 103:9988–9992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Laurie KL, Carolan LA, Middleton D, Lowther S, Kelso A, Barr IG. 2010. Multiple infections with seasonal influenza A virus induce cross-protective immunity against A(H1N1) pandemic influenza virus in a ferret model. J. Infect. Dis. 202:1011–1020 [DOI] [PubMed] [Google Scholar]
- 31.Forrest BD, Pride MW, Dunning AJ, Capeding MR, Chotpitayasunondh T, Tam JS, Rappaport R, Eldridge JH, Gruber WC. 2008. Correlation of cellular immune responses with protection against culture-confirmed influenza virus in young children. Clin. Vaccine Immunol. 15:1042–1053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gorse GJ, Otto EE, Powers DC, Chambers GW, Eickhoff CS, Newman FK. 1996. Induction of mucosal antibodies by live attenuated and inactivated influenza virus vaccines in the chronically ill elderly. J. Infect. Dis. 173:285–290 [DOI] [PubMed] [Google Scholar]
- 33.He XS, Holmes TH, Mahmood K, Kemble GW, Dekker CL, Arvin AM, Greenberg HB. 2008. Phenotypic changes in influenza-specific CD8+ T cells after immunization of children and adults with influenza vaccines. J. Infect. Dis. 197:803–811 [DOI] [PubMed] [Google Scholar]
- 34.Richards KA, Chaves FA, Alam S, Sant AJ. 2012. Trivalent inactivated influenza vaccines induce broad immunological reactivity to both internal virion components and influenza surface proteins. Vaccine 31:219–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jeisy-Scott V, Kim JH, Davis WG, Cao W, Katz JM, Sambhara S. 2012. TLR7 Recognition is dispensable for influenza virus A infection but important for the induction of hemagglutinin-specific antibodies in response to the 2009 pandemic split vaccine in mice. J. Virol. 86:10988–10998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Clements ML, Murphy BR. 1986. Development and persistence of local and systemic antibody responses in adults given live attenuated or inactivated influenza A virus vaccine. J. Clin. Microbiol. 23:66–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wong KK, Greenbaum A, Moll ME, Lando J, Moore EL, Ganatra R, Biggerstaff M, Lam E, Smith EE, Storms AD, Miller JR, Dato V, Nalluswami K, Nambiar A, Silvestri SA, Lute JR, Ostroff S, Hancock K, Branch A, Trock SC, Klimov A, Shu B, Brammer L, Epperson S, Finelli L, Jhung MA. 2012. Outbreak of influenza A (H3N2) variant virus infection among attendees of an agricultural fair, Pennsylvania, USA, 2011. Emerg. Infect. Dis. 18:1937–1944 [DOI] [PMC free article] [PubMed] [Google Scholar]