Abstract
Changes that occur in the skeletal muscle environment with the progress of muscular dystrophies may affect stem cell function and result in impaired muscle regeneration. It has previously been suggested that the success of stem cell transplantation could therefore be dependent both on the properties of the cell itself and on the host muscle environment. Here we engrafted young and mature adult mdx-nude mice, which are the genetic homolog of Duchenne muscular dystrophy, with a small number of satellite cells freshly isolated from young, normal donor mice. We found that the donor satellite cells contributed to muscle regeneration and self-renewal as efficiently within mature adult, as in young, dystrophic host muscle. Donor-derived satellite cells also contributed to robust regeneration after further injury, showing that they were functional despite the more advanced dystrophic muscle environment. These findings provide evidence that muscle tissue in a later stage of dystrophy may be effectively treated by stem cells.
Keywords: Satellite cell, Stem cell, Self-renewal, Aged, Skeletal muscle regeneration, Muscular dystrophy
Introduction
The ability of resident stem cells to self-renew and differentiate in response to either normal growth stimuli or tissue damage guarantees body maintenance throughout life. However, it has been suggested that the processes of aging or muscular dystrophy may affect the efficient function of stem cells that occurs in young adults, implying that modifications in the microniche and in the macro tissue environment could impair stem cell regenerative ability [1, 2].
Skeletal muscle tissue, which constitutes most of our body mass, is formed of highly specialized contractile cells called myofibers. The resident muscle stem cells are satellite cells, located between the plasmalemma and the basal lamina of the myofiber; this niche microenvironment provides signals for both satellite cell quiescence and myogenic potential [3, 4]. That the satellite cell is indeed a muscle stem cell has been shown by grafting them in their niche on a muscle fiber: a small proportion of these satellite cells were able to robustly regenerate skeletal muscle and reconstitute the satellite cell compartment with functional satellite cells [3]. However, the microniche is not necessary for satellite cells to regenerate in vivo, because grafted satellite cells that had been mechanically removed from their parent fibers also efficiently regenerated skeletal muscle [3].
Although regeneration of whole muscle grafts derived from either young or old donors is efficient in young rodents, it is compromised in aged rats [5]. On the other hand, although the satellite cell number in aged muscles is reduced and the aged niche microenvironment is in part compromised [6, 7], we have shown that satellite cells from aged mice can regenerate skeletal muscle as efficiently as satellite cells from young mice, after grafting into irradiated young dystrophic host mice [6]. This shows that, despite the reduction in satellite cell number [6], regeneration-competent satellite cells are retained with age.
Factors that regulate muscle homeostasis, including systemic factors, components of the microvasculature, and cells of the immune system [1, 8] are altered with increasing age [9]. By altering environmental components, aged skeletal muscle tissue can be rejuvenated. This has been shown in parabiotic pairs of young and aged mice, where the sharing of the circulatory system allows “young” factors to act on the aged environment and improve skeletal muscle regeneration in aged mice [10]. Other factors that may influence the efficiency of regeneration are the changes in the Wnt signaling pathway with age, leading to conversion of myogenic cells to fibroblasts [11]. The increase of muscle fibrosis [12] has been implicated as a major factor responsible for the reduction in muscle regenerative capacity with age. Changes in the aged muscle environment may affect either early [8] (e.g., activation, proliferation, and fusion of satellite cells into muscle fibers and inflammation vascularization of regenerating muscles) or late [5] events (e.g., innervation) in skeletal muscle regeneration.
In pathological muscle conditions such as muscle dystrophies, particularly Duchenne muscular dystrophy (DMD), the serial cycles of skeletal muscle regeneration that occur in response to the ongoing necrosis of dystrophic muscle fibers become less efficient with time; this leads to a net loss of muscle fibers, which are replaced by fibrotic and adipose tissue [10, 13–15]. Furthermore, changes in the inflammatory response and extracellular matrix remodeling in dystrophic human [16, 17] and mouse [18–21] muscles may inhibit endogenous stem cells from efficiently regenerating skeletal muscle and reconstituting the satellite cell pool. In addition, there seems to be a loss of the more “stem cell-like” satellite cells in dystrophic mdx mouse muscle [19]. The inflammatory process and changes in connective tissue that occur in muscular dystrophies seem to hasten the muscle aging process.
Here we studied whether satellite cells derived from young mice are able to regenerate and self-renew effectively after their grafting into muscles of older mdx-nude mice, in which the mature phenotype has been attained, with most myofibers having gone through at least one round of regeneration. We found that, within an irradiated muscle environment, donor satellite cells are capable of restoring dystrophin expression and functionally repopulating the satellite cell pool as efficiently as they do in younger muscle. This suggests that, by appropriately modifying the skeletal muscle environment, stem cell therapy may be an effective treatment also in older dystrophic patients.
Materials and Methods
Muscle DNA Extraction and Real-Time Polymerase Chain Reaction Assay
Total genomic DNA used for this assay was extracted after phenol-chloroform extraction (Sigma, St. Louis, MO, http://www.sigmaaldrich.com) with isopropanol-ethanol precipitation (Sigma) from tibialis and diaphragm muscle tissues of 2-month-old mdx mice (n = 3), 12-month-old mdx mice (n = 2), and 11-month-old mdx-nude mice (n = 2). Relative quantification using real-time analysis was performed by modifying a previously published protocol [22]. Housekeeping and telomere sequences were amplified using primers previously published, and real-time polymerase chain reaction (PCR) reactions were optimized (supporting information Fig. 1) using an automated thermocycler (Abi Prism 7700 Sequence Detection System; Applied BioSystems, Foster City, CA, http://www.appliedbiosystems.com) as follows: 95°C for 10 minutes, followed by 40 cycles of data collection at 95°C for 15 seconds, with 30 seconds at temperature annealing, and a final step of extension at 72°C for 30 seconds. The annealing temperature was set at 51°C for the housekeeping acidic ribosomal phosphoprotein PO (36B4) gene and at 58°C for the telomere portion. Each reaction volume contained 12.5 μl Syber Green PCR Master Mix (Applied BioSystems), 300 nM each of the forward and reverse primers, and double-distilled water to give a final volume of 25 μl. The assay was performed on three 5-ng samples of DNA from each animal sample, placed in adjacent wells in 96-well plates. For calculating the standard curve, an individual sample of DNA was serially diluted from 10 to 0.01 ng both for 36B4 and the telomere product. Real-time PCR results were exported and analyzed with Excel program (Microsoft, Redmond, WA, http://www.microsoft.com), and standard curves were generated according to the relative standard curve protocol reported in the Applied Biosystems Prism 7700 Sequence Detection System user’s manual. Input amount of amplified DNA were calculated for each reaction. The relative amount of telomere PCR was divided by the relative amount of the 36B4 obtained in each reaction (three repeated reactions for each considered sample). The average of the ratios of telomere:36B4 per sample was reported as “relative telomere length,” and the average telomere lengths in both tibialis anterior (TA) and diaphragms in young mdx mice were set as references.
Single Myofiber Isolation
Single myofibers were isolated from muscles of 3F-nLacZ-2E mice [23] and of Myf5nLacZ/+ mice [24] as previously described [3]. For characterization of Myf5nLacZ/+ marker expression on aged fibers, extensor digitorum longus (EDL), TA, soleus, and gastrocnemius (GA) muscles were dissected out from 1.5- to 3-year-old Myf5nLacZ/+ mice, digested in 2% (wt/vol) collagenase type I (Sigma) in Dulbecco’s modified Eagle’s medium (DMEM; Gibco, Grand Island, NY, http://www.invitrogen.com) for up to 1.5-2 hours in a 35°C water bath, and after serial washes to discard cell contaminants, they were fixed for X-gal staining and immunostaining.
For satellite cell grafting, EDL myofibers were similarly isolated from 5- to 8-week-old mice. All fibers were collected in one plate in 8 ml of plating medium (DMEM supplemented with 10% horse serum; Gibco), 005% chick embryo extract, 4 mM l-glutamine (Sigma), and 1% penicillin and streptomycin antibiotics (Sigma).
Immunohistochemistry of Single Myofibers
An aliquot of fixed single myofibers isolated from aged Myf5nLacZ/+ mice were X-gal stained as previously described [25]. Another group of fibers from the same preparation was permeabilized with 0.5% Triton X-100 (Sigma), blocked with 10% goat and 10% swine serum, and incubated overnight with primary antibodies mouse anti-Pax7 (Developmental Studies Hybridoma Bank, Iowa City, IA, http://www.uiowa.edu/~dshbwww.com) and rabbit anti laminin (Sigma). Secondary antibodies used were Alexa Fluor 488-conjugated goat anti-mouse Ig (Molecular Probes, Eugene, OR, http://www.probes.invitrogen.com) and Alexa Fluor 594-conjugated goat anti-rabbit Ig (Molecular Probes).
Satellite Cell Preparation by Physical Titration for Grafting
Fibers collected in one plate in plating medium were triturated using a 1-ml syringe with a 19-gauge needle for 5 minutes to release from single fibers satellite cells. Myofibers were removed by passing the solution through a 40-μm cell sieve. Cells were centrifuged for 20 minutes, the pelleted cells were resuspended, and trypan blue-excluding cells were counted. For grafting, cells were resuspended in medium and kept on ice until injection.
Cell Grafting and Muscle Damage
Mice were bred, and experimental procedures were carried out in the Biological Services Unit, of both Imperial College Faculty of Medicine, Hammersmith Hospital, and of University College London, Institute of Child Health, in accordance with the Animals (Scientific Procedures) Act 1986. Experiments were carried out under Home Office license. Aged mice were given Septrin (Glaxo-SmithKline, UK, http://www.gsk.com) in their drinking water (sulfamethoxazole 1.2 mg/ml and trimethoprim 0.24 mg/ml) for 3 days a week, followed by no antibiotics for the remaining 4 days a week.
Three- to 4-week-old or 9-month-old mdx-nude mice were anesthetized, and both hindlimbs were irradiated with 18 Gy, as described previously [26]. Controls were age-matched mice that were nonirradiated. Three days later, both irradiated and nonirradiated host mice were anesthetized with isoflurane, and 400 satellite cells were grafted in 4 μl of medium into the TA muscles using pulled PCR pipettes under microscopic observation.
In some experiments, 3 weeks after grafting, both TA muscles of anesthetized host mice were injected with 10 μl Notechis scutatus scutatus (notexin; Latoxan Latoxan, France, http://www.latoxan.com) 10 μg/ml) using a Hamilton syringe [27].
In experiments aimed to study the regenerative capability of TA muscles in both 9-month-old and 3-week-old mdx-nude mice, 15 μl notexin (10 μg/ml) was injected per leg.
Vetergesic (Buprenorphine (Reckitt Benckiser Healthcare, UK, http://www.reckittbenckiser.com); 0.05 mg/kg) was administered for postoperative analgesia.
Muscles Harvesting and Immunohistochemistry
Engrafted muscles were removed 4 weeks after cell injection, and when notexin was used, 1 week after myotoxin injection. Muscles that had been grafted with 3F-nLacZ-2E satellite cells were frozen in isopentane cooled in liquid nitrogen. Seven-micrometer transverse cryosections were collected at 100-μm intervals from the entire muscle. Sections were X-gal stained as previously described [27] and, only if positive signal was detected (thus under-reporting the amount of donor muscle, but avoiding inclusion of host, revertant fibers in our quantification of donor-derived muscle fibers), serial sections were immunostained using primary antibodies rabbit anti-dystrophin (P7) and, in some experiments, with mouse anti-neonatal myosin (BF34; Developmental Studies Hybridoma Bank) after blocking with 10% goat serum.
Collagen VI (Abcam, Cambridge, U.K., http://www.abcam.com) and laminin (Sigma) antibodies were used in representative sections of TA muscle from 3-week-old (n = 4) and 9-month-old (n = 3) mdx-nude mice and of 12- (n = 3) and 24-month-old (n = 2) mdx mice for detection of increase in connective tissue indicative of fibrosis Secondary antibodies used were as follows: Alexa Fluor 488-conjugated goat anti-mouse Ig (Molecular Probes) and Alexa Fluor 594-conjugated goat anti-rabbit Ig (Molecular Probes).
Muscles that had been grafted with Myf5nLacZ/+ satellite cells were fixed in paraformaldehyde and X-gal stained as described elsewhere [3, 27].
Microscopy
Fluorescence and bright-field microscopy and image were captured using a Zeiss Axiophot (Carl Zeiss, UK, http://www.zeiss.co.uk) microscope and Metamorph (Metamorph Productions, UK, http://metamorph-productions.co.uk.) software. Minor adjustments to the quality of images were done using Adobe Photoshop CS2 (Adobe Photoshop UK, http://www.Adobe.com.)
Data Analysis
Counts were made of the maximum number of donor (dystrophin positive) fibers in transverse sections of grafted muscles serial to those where donor, X-gal-positive myonuclei were detected.
INSTAT was used for statistical analysis. For quantification of fibrosis, the total area of four randomly encountered fields of one representative section of each muscle that was collagen VI positive was measured using SigmaScan software. The fibrotic index (%) was defined as the percentage of the total area that was collagen VI positive.
Results
Dystrophin-Deficient Mdx-Nude Mouse Muscles Age Prematurely
The immunodeficient mdx-nude mouse is a good model for testing the capacity of donor-derived cells to regenerate skeletal muscle [3, 6, 21]. We wished to compare the function of grafted satellite cells in young mice at a time when the host muscles are undergoing extensive degeneration and regeneration [28] with mice whose muscles are at a later stage of the pathological process, when the dystrophic phenotype has evolved to more resemble that found in DMD [29]. However, the immunodeficiency of these mice, which has the advantage of preventing immunological rejection of donor cells, reduces their lifespan [20]. To have enough healthy old mice for our experiments, the mice were treated with septrin and were kept until they were 9 months of age, after which mice began to die despite the antibiotic regimen. We therefore performed our comparison on 3- to 4-week-old and 9-month-old mice (that we defined as “mature adult” mice), to have a statistically significant number of recipient mice able to survive the anesthesia needed for hind limb irradiation and cell injection and live long enough for analysis 4 weeks later.
Although satellite cells turnover throughout life, for homeostasis and to contribute to muscle growth or repair, this process becomes less efficient with age as cells reach the end of their proliferative lifespan and the reserve satellite cell pool therefore decreases [6, 17]. This phenomenon is particularly accentuated in aged and in dystrophic muscles, whose satellite cells have undergone the most divisions [17]. Telomere length has been suggested as a biomarker for somatic cell turnover, because telomeres shorten after cell replication [30], and it has been shown that the telomere length is significantly shorter in dystrophic [2] and in older adult muscle tissue [31].
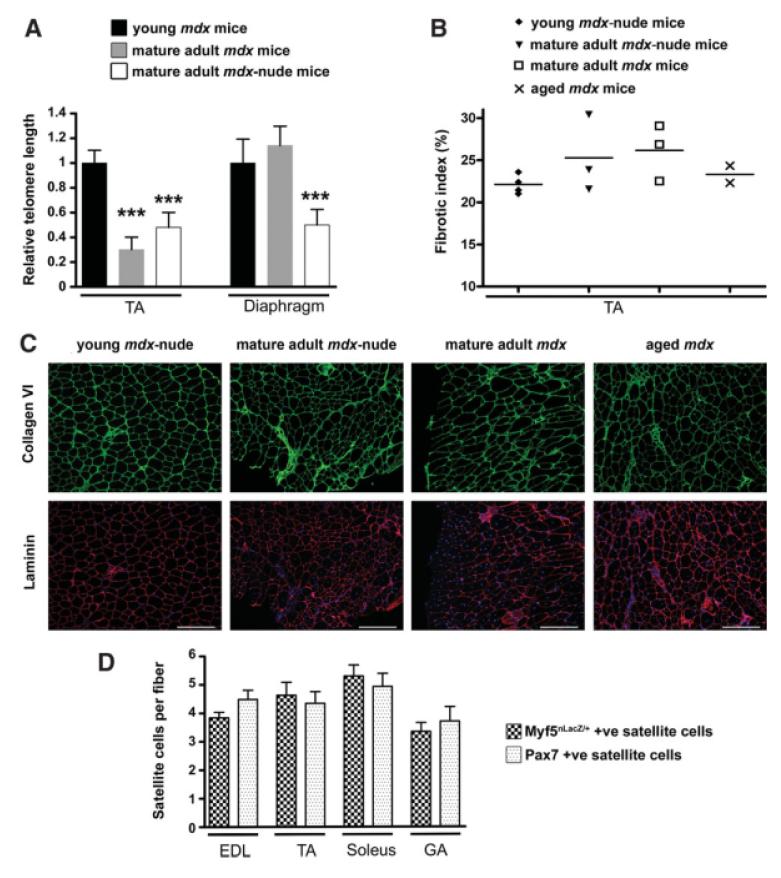
To determine whether older mdx-nude mouse muscles exhibit telomere shortening, we compared relative telomere length in young 2-month-old mdx mice with 12-month-old mdx mice and 11-month-old mdx-nude mice. Interestingly, in TA muscles of both 12-month-old mdx mice and 11-month-old mdx-nude mice, relative telomere length was greatly and similarly reduced in comparison to the reference young mouse group (Fig. 1A; one-way analysis of variance test, ***, p < .001). Intriguingly, when we studied the diaphragm muscles of these mice, there was a significant reduction in relative telomere length in the 11-month-old mdx-nude mice (Fig. 1A; one-way analysis of variance test, ***, p < .001), in comparison with both the young 2-month-old mdx mice and the 12-month-old mdx mice. However, diaphragm telomere length was not significantly different between young 2-month-old and the mature adult 12-month-old mdx mice.
Figure 1.
Validation of host and donor mice as an appropriate experimental model. (A): Relative telomere length was significantly lower in TA of 12- and 11-month-old, respectively, mdx and mdx-nude mice compared with young/adult 2-month mdx mice; diaphragm telomere length was significantly reduced only in mature adult mdx-nude mice compared with the young/adult mdx (n = 7, three mice were used in the young/adult mdx mouse group, and two mice were used in each of the other two groups; one-way analysis of variance test, ***, p < .001). (B): The collagen VI-positive area was measured in representative sections of TA muscle from 3-week-old (n = 4) and 9-month-old (n = 3) mdx-nude mice and of 12-month-old (n = 3) and 24-month-old (n = 2) mdx mice. Scale bars: 100 μm. (C): Expression of the Myf5nLacZ/+ locus and Pax7 expression are retained in satellite cells on myofibers from aged 1.5- to 3-year-old Myf5nLacZ/+ mice (n = 4). Abbreviations: EDL, extensor digitorum longus; GA, gastrocnemius; TA, tibialis anterior.
We also assessed the degree of fibrosis in the TA muscles of mature adult mdx-nude mice in comparison with mdx mice, because this is an important hallmark of the more advanced dystrophic muscle phenotype. We found no obvious difference in collagen VI content of mdx-nude (young and mature adult) compared with mdx (mature adult and aged) TA muscles (Fig. 1B and 1C), showing that the nude genotype does not significantly affect fibrosis in this particular muscle.
Furthermore, skeletal muscle regeneration persists, albeit at a lower level, with age in mdx-nude (supporting information Fig. 2), as has been shown for mdx mice [28, 29, 32]. Nevertheless, regeneration induced by an applied injury, notexin injection, elicited a similar regenerative response in TA muscles of both young and mature adult mdx-nude mice (supporting information Fig. 2).
The reduction in telomere length and features of advanced pathology—increased central nucleation and numbers of revertant fibers (supporting information Fig. 2) and fibrosis that we found in mature adult mdx-nude TA muscles is in keeping with previous literature on mdx mice [29, 32, 33]. These features of advanced muscle pathology, combined with reduced lifespan of mdx-nude mice, made us confident that our 9-month-old mdx-nude mice are a suitable model for late-stage muscular dystrophy.
Expression of Markers of Satellite Cells and Myonuclei of Donor Origin Is Retained in Mature Adult Muscle
We used myosin light chain as a marker of regenerated myofibers in mature adult host mice, because transgene 3F-nLacZ-2E expression has been shown to be retained in myofibers of older mice [6]. However, we did not know whether Myf5nLacZ/+ expression is retained within the aged muscle environment and whether it is therefore a good marker for detecting donor satellite cells within older host muscles. To determine whether Myf5nLacZ/+ does mark all satellite cells in aged muscles, we stained isolated fibers from different muscles (EDL, TA, soleus, GA) from 1.5- to 3-year-old Myf5nLacZ/+ mice with either Pax7 antibody or X-gal, for detecting the two main markers of satellite cells [3]. We found comparable numbers of Pax7-positive and Myf5nLacZ/+-expressing satellite cells, confirming that Myf5nLacZ/+ gene expression is retained, as is Pax7, within aged muscle and that the targeted Myf5 allele of the donor mouse strain is a good model to assay satellite cells of donor origin within older host mice (Fig. 1D).
Donor Satellite Cells Regenerate and Self-Renew Within Mature Adult Dystrophic Muscle
To study the behavior of a known number of freshly isolated, pure satellite cells, we mechanically dissociated satellite cells from donor myofibers [6, 7]. To first investigate the regenerative potential of satellite cells in a mature adult dystrophic environment, satellite cells were isolated from 3F-nLacZ-2E adult myofibers to allow detection of donor-derived muscle fibers (expressing dystrophin- and containing β-gal-positive nuclei).
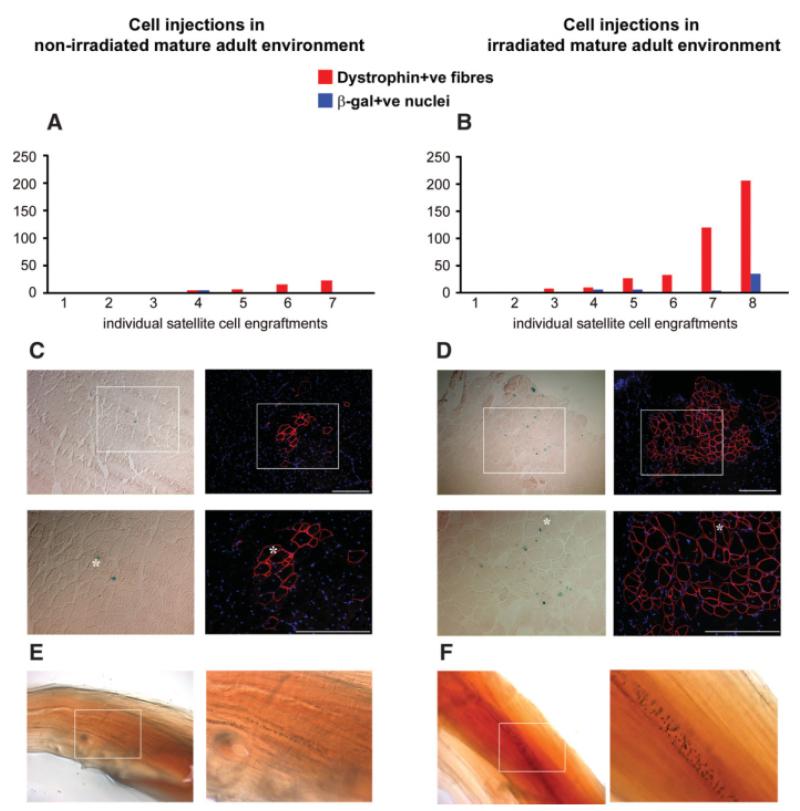
Irradiation is known to modify the muscle environment of young immunodeficient host mouse muscle, allowing donor-derived satellite cells to regenerate and self-renew [3, 6, 21, 34]. To test whether it also has the same effect on mature adult muscle, the right TA muscle of seven recipient mice that had not been irradiated and of eight mice that had both hind limbs irradiated with 18 Gy 3 days before grafting [19, 27] were transplanted with 400 freshly isolated 3F-nLacZ-2E satellite cells. Four weeks after cell grafting, cryosections were analyzed both for the presence of X-gal-positive nuclei and the restoration of dystrophin protein [3].
Satellite cells of donor origin contributed to regenerated muscle fibers within 9-month-old mdx-nude host muscles. However, as shown in Figure 2A and 2B, the amount of donor-derived muscle formed within irradiated host muscles was variable. It is interesting to note that, as in previous experiments in which either satellite cells [3, 6] or muscle precursor cells [21] were grafted into young mouse hosts, there is a marked variability between grafted muscles within an experimental group. Nevertheless, it is clear that large amounts of donor-derived muscle were only found within irradiated host muscles most likely close to the injection site (Fig. 2A–2D; 51 ± 26 dystrophin-positive fibers, 7 ± 4 X-gal-positive nuclei in irradiated, 7 ± 3 dystrophin-positive fibers, 1 ± 1 X-gal-positive nuclei in nonirradiated host mouse muscles).
Figure 2.
Fresh satellite cells stripped from single fibers of young mice efficiently regenerate and self-renew when injected in irradiated mature adult mdx-nude muscles. Four hundred satellite cells physically dissociated from extensor digitorum longus myofibers of 3F-nLacZ-2E and Myf5nLacZ/+ mice were injected into the tibialis anterior (TA) of mdx-nude recipient mice. Hindlimbs of one group of mice were exposed to 18-Gy γ-irradiation 3 days before grafting. In the irradiated TA host muscles, the amount of muscle regenerated by donor cells—quantified as dystrophin-positive fiber numbers corresponding in serial sections to the same fibers incorporating X-gal positive nucl—was generally higher (B) in comparison to the nonirradiated engrafted muscles (A). (C, D): Amount of donor muscle obtained from grafts, respectively in nonirradiated and irradiated mice (dystrophin immunostaining and serial section X-gal staining showing β-gal expressing myonuclei). However, two of eight individual engraftments in the irradiated mouse group did not show any sign of donor muscle regeneration (B), and rare β-gal-positive cells were detectable only in three of seven engraftments in nontreated mdx-nude mice, giving rise to limited groups of dystrophin-positive fibers (C). Interestingly, three of eight engraftments of 400 satellite cells mechanically isolated from Myf5nLacZ/+ fibers adopted the satellite cell position expressing the Myf5nLacZ/+ marker on single fibers, as seen from X-gal staining on the whole harvested muscles (F). A similar proportion (two of seven) of nonirradiated muscles grafted with the same donor cells showed the presence of myofibers carrying X-gal-positive satellite cells, as shown in E. Asterisks mark the same myofiber in each serial section. White squares show the area reported in the higher-magnification pictures. Scale bars: 100 μm.
To examine whether transplanted satellite cells also self-renewed in the mature adult dystrophic environment, donor satellite cells were prepared in the same way from Myf5nLacZ/+ donor mice and grafted into the contralateral (left TA) of the mature adult mdx-nude host mice used above. Four weeks later, X-gal staining of whole grafted TA muscles showed cells of donor origin, presumed to be mostly satellite cells [3], expressing Myf5nLacZ/+ in three of eight irradiated host muscles (Fig. 2F). In nonirradiated host muscles, the amount of satellite cells of donor origin (found in only two of seven grafted muscles; Fig. 2E) was appreciably reduced. It seems therefore that donor satellite cell self-renewal capability is correlated with their regenerative potential. We conclude that satellite cells can self-renew in a mature adult dystrophic environment and that preirradiation of host muscle enhances repopulation of the satellite cell pool.
Donor Satellite Cells Regenerate Skeletal Muscle and Self-Renew as Efficiently in Mature Adult as in Young Dystrophic Muscle
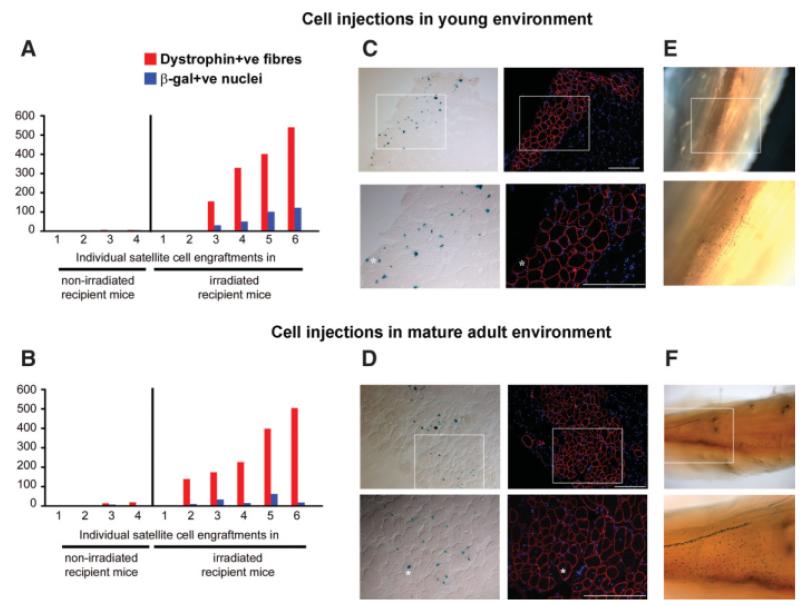
Four hundred freshly isolated 3F-nLacZ-2E satellite cells were injected into the right and left TA muscles, respectively, of both young (n = 10) and mature adult (n = 10) host mdx-nude mice, some of which (n = 6 in both cases) had both hindlimbs irradiated with 18 Gy 3 days before grafting. Muscles were removed for analysis 4 weeks after grafting, as described above. In irradiated host muscles, muscle of donor origin was found in five of six young and six of six mature adult host muscles, and the numbers of muscle fibers of donor origin were not significantly different (one-way analysis of variance test) between young (238 ± 90 dystrophin-positive fibers, 51 ± 21 X-gal-positive nuclei) and mature adult recipient mice (241 ± 70 dystrophin-positive fibers, 23 ± 9 X-gal-positive nuclei (Fig. 3A, 3B), as can be seen by dystrophin immunostaining and parallel X-gal staining on serial sections of grafted muscles (Fig. 3C, 3D).
Figure 3.
Adult satellite cells regenerate and self-renew equally well in the young and mature adult muscle environment. Four hundred satellite cells isolated from 3F-nLacZ-2E single fibers were injected in young (21 day old) and mature adult (250 day old) tibialis anterior of mdx-nude mice. (A, B): Four weeks after cell injections, a comparable amount of muscle of donor origin (number of dystrophin-positive fibers colocalized with β-gal-expressing myonuclei) was found in mature adult and young host mice. A negligible amount of muscle was formed when cells were injected in nonirradiated muscles both in the young and mature adult environments. (C, D): Two comparable grafts into irradiated host muscles, in which similar amounts of muscle were formed by donor satellite cells. Injections of 400 satellite cells isolated in the same way from Myf5nLacZ/+ donor mice gave rise to comparable numbers of self-renewed satellite cells when injected both in (E) young (seven of seven successful engraftments; magnification, ×4 and ×10) and in (F) mature adult (six of six successful engraftments; magnification, ×4 and ×10) irradiated muscles. Asterisks indicate the same myofiber in serial sections. White squares indicate the area shown in the higher-magnification pictures. Scale bars: 100 μm.
The extent of donor-derived repopulation of the satellite cell pool was similar within irradiated muscles of host mice of both ages. A similar incidence and amount of donor-derived Myf5nLacZ/+ satellite cells was obtained 4 weeks after injection of freshly isolated Myf5nLacZ/+ satellite cells into both young (six of six positive grafts) and mature adult host mice (six of six positive grafts; Fig. 3E, 3F).
In nonirradiated host muscles, muscle fibers of donor origin were found in only two of four host muscles at both ages. The number of muscle fibers of donor origin was similarly low both in young and mature adult recipient mice (3 ± 2 dystrophin-positive fibers and 2 ± 1 X-gal-positive nuclei in young hosts and 8 ± 5 dystrophin-positive fibers, 3 ± 2 X-gal-positive nuclei in mature adults; no significant difference by one-way analysis of variance test; Fig. 3A, 3B). The incidence and number of donor-derived satellite cells was similarly low, with only sporadic X-gal-positive nuclei present in two of four host mouse muscles of both ages (data not shown).
Satellite Cells of Donor Origin Are Functional Within Mature Adult Dystrophic Host Muscle
To be true stem cells, satellite cells of donor origin must, in the presence of appropriate stimuli, be able to regenerate the muscle tissue and again repopulate the satellite cell compartment weeks after transplantation, as they are able to do in young dystrophic host muscle [3]. To study the functional ability of donor-derived satellite cells within the mature adult dystrophic host muscle environment, we injected notexin into muscles that had been grafted 3 weeks previously with either 3F-nLacZ-2E (right TA) or Myf5nLacZ/+ (left TA) satellite cells. Notexin is a snake venom that causes myofiber destruction but spares satellite cells, leaving undamaged basal lamina and microcirculation [35]. One week later, muscles were removed for analysis.
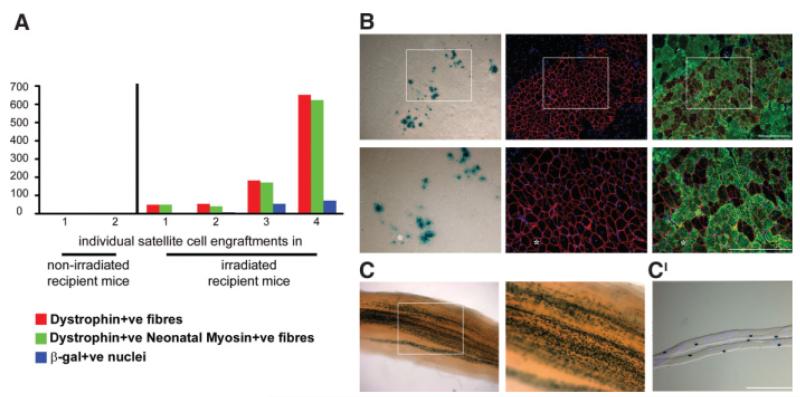
Newly regenerated muscle fibers (expressing neonatal myosin) of donor origin (expressing dystrophin) were present within the mature adult dystrophic host muscles, showing that satellite cells of donor origin remained functional after several weeks within this environment. The mean number of newly regenerated muscle fibers of donor origin after notexin injury in our mature adult mdx-nude mice was comparable to that previously obtained in young host mice [3, 6]: (six of six successful grafts, containing a mean of 221 ± 117 dystrophin and neonatal myosin-positive fibers, 35 ± 17 X-gal-positive nuclei; Fig. 4A and 4B). In one host muscle, more than 600 newly regenerated muscle fibers of donor origin were found (Fig. 4A).
Figure 4.
Donor satellite cells injected in mature adult and irradiated muscle tissue are functional. Four hundred satellite cells isolated both from 3F-nLacZ-2E and Myf5nLacZ/+ single fibers were injected into tibialis anterior (TA) muscles of mature adult mice; recipient muscles were notexin injured 3 weeks after cell injection and removed 1 week later. (A, B): Satellite cells of donor origin were able to generate new myofibers after the notexin damage, producing groups of new myofibers coexpressing dystrophin and neonatal myosin proteins and incorporating 3F-nLacZ-2E donor myonuclei shown by X-gal staining in serial sections. (C): Myf5nLacZ/+ donor satellite cells were detected as several β-gal-positive nuclei in X-gal-stained whole TA muscles (magnification, ×4 and ×10). (C’): Single fibers were dissected out from the X-gal-stained muscles and 4′6-diamidino-2-phenylindole stained, showing in particular the localization of the detected Myf5nLacZ/+ satellite cells on single myofibers. Asterisks indicate the same myofiber in each serial section. White squares highlight areas shown in the pictures at higher magnification. Scale bars: 100 μm.
In the two grafts in nonirradiated mature adult mdx-nude mouse muscles, a negligible number of newly regenerated, donor-derived muscle fibers was obtained (4 ± 0 dystrophin- and neonatal myosin-positive fibers, 3 ± 1 X-gal-positive nuclei).
Within irradiated mature adult dystrophic host muscles that had been injected with My5nLacZ/+ satellite cells, allowed to regenerate, and challenged with notexin, the number of X-gal-positive nuclei, found in all four grafted muscles (Fig. 4C), was appreciably greater than in nonirradiated muscles that had been grafted and injected with notexin, in which very few β-gal-expressing satellite cells were detected (data not shown). Because β-gal translocates between myonuclei of donor origin [3, 36], only cells on single fibers that expressed X-gal and were surrounded by 4′6-diamidino-2-phenylindole-positive/X-gal-negative nuclei, implying lack of cytoplasmic continuity of that cell with the myofiber [36], are confirmed as satellite cells (Fig. 4C′). Such analysis of single myofibers dissected out from the whole X-gal-stained, irradiated and notexin treated host muscles, confirmed that the X-gal activity was indeed in satellite cell nuclei.
We therefore provide clear evidence that satellite cells of donor origin had expanded and repopulated the satellite cell pool in irradiated mature adult host muscles and that they remained functional within this more advanced dystrophic environment.
Discussion
The stem cell mainly, if not totally, responsible for skeletal muscle regeneration is the satellite cell [37, 38], and the decrease in satellite cell number with time has been associated with the progressive loss of muscle regenerative capacity in sarcopenia as well as in a number of pathological muscle conditions [17, 39]. Despite the reduction of satellite cell number in aged mice, the myogenic potential of a subset of them is conserved, as shown by their ability to regenerate and self-renew to a similar extent as young satellite cells, within a young muscle environment [6]. This, in addition to our data presented here showing that mature adult mdx-nude host muscles regenerate as well as young mdx-nude host muscle in response to notexin injury, implies that an aged muscle environment retains the potential to be regenerated by its own endogenous satellite cells. However, changes in the host muscle environment that occur as a consequence of age or pathology seem to have a major impact on normal satellite cell function [9, 10, 40].
For grafting experiments, the mdx mouse has been bred onto the nu/nu background to prevent immunological rejection of donor cells [41]. However, the impaired immune system in mdx nude and mdx SCID mice has been associated with reduced fibrosis in some, but not all, skeletal muscles [42, 43]. Because this could have influenced the outcome of our studies, we studied the extent of fibrosis in TA muscles (the muscles into which we graft donor cells) of young and mature adult mdx-nude and mature adult and aged mdx mice. We quantified fibrosis by measuring the area of collagen VI in representative transverse sections, because expression of collagen in the extracellular matrix has been previously used to quantify the extent of fibrosis in several organs [11, 44–46]. Our finding that there was fibrosis in TA muscles of mdx-nude mice that was comparable to the level observed in mdx mice (Fig. 1B, 1C) is consistent with previous studies that used hydroxyproline content to quantify fibrosis in TA muscles of mdx nude compared with age-matched (24 week old) mdx mice [42]. This seems to be different from previous findings in heart and diaphragm in mdx-nude mice, which indeed showed reduced fibrosis and hydroxyproline levels [42]. Farini et al. [43] found reduced total transforming growth factor β1 (TGF-β1) expression in TA and quadriceps, but less active TGF-β1 only in the diaphragm of 12-month-old mdx SCID compared with age-matched mdx mice. The results from both these papers therefore conclude that fibrosis is not significantly reduced in the TA of immunodeficient mdx mice, and our data confirm this.
In contrast to DMD patients, the muscles of the biochemical and genetic homolog of DMD, the mdx mouse, as we have also shown in mdx-nude mice, retains the ability to regenerate throughout life. Muscle fiber degeneration/regeneration peaks at 3 weeks of age but continues throughout life, albeit less obviously [28, 32, 47]. In parallel, satellite cell activity diminishes with age in mdx mice [8], and old mdx mouse muscles exhibit muscle fiber loss [48] and more severe pathological features [49, 50]. To better reproduce the scenario found in DMD muscle, radiation has been applied to muscles at the peak of their degenerative/regenerative phase, between 2 and 4 weeks of age, to incapacitate host satellite cells and prevent muscle regeneration [51]. Radiation indeed kills the majority of satellite cells when they attempt their next mitosis, thereby partially emptying the satellite cell niche. However, radiation may also have other effects on the muscle that potentiate donor myoblast proliferation and regeneration [21, 52]. Although radiation is used clinically for hematopoietic stem cell transplantation [53], one would not envisage using high doses of radiation in all muscles of muscular dystrophy patients. Work is therefore ongoing to determine whether particular growth factors or cytokines are modulated within irradiated muscles. Such factors could be used in a therapeutic context.
Previous work has shown that satellite cells removed from their niche can robustly regenerate skeletal muscle [3, 34] and give rise to long-lived muscle precursor cells [34] within young, irradiated host muscles. However, it was not clear whether efficient satellite cell self-renewal [3] is dependent on the satellite cell niche. Here, we clearly show that satellite cells removed from their niche can both regenerate muscle fibers and reconstitute the satellite cell pool with functional satellite cells within mdx nu/nu host muscles.
Although little has been published on the subject of donor-derived muscle regeneration in aged hosts, there are reports of limited muscle regeneration in aged rodents after grafting of whole muscles explants [5, 8]. It is a matter of debate whether, as is sometimes reported [54], 9-month-old dystrophic mice can be termed “aged,” but by this age, nearly all muscle fibers in mdx mice had regenerated at least once [28]. By analyzing the biological marker of telomere length [2, 31, 33], we showed that mdx muscles of this age were becoming replicatively senescent, suggesting early aging as a result of the ongoing dystrophic pathology. Furthermore, the presence of fibrosis, the increase of centrally nucleated fibers and of revertant fibers, characterize the aging of the TA muscles of our mature adult mdx-nude recipient mice. Indeed, the amount of fibrosis in these muscles is similar to that in older mdx mice. This, in combination with literature describing shortened life span in immunodeficient mice [55, 56], adds support to the theory that mdx-nude mouse muscles undergo premature aging compared with mdx mice [57]. The additive effects of the dystrophic and immunodeficient phenotypes may accelerate the skeletal muscle aging process.
Using 3-week-old and 9-month-old host mice, we showed that donor-derived satellite cells performed equally well when grafted into dystrophic host muscles either at the height of their degeneration/regeneration, as at a later stage in the pathological process. Preirradiation significantly augmented the amount of donor-derived muscle fibers in young hosts, and large amounts of donor-derived muscle were only found in irradiated muscles in older hosts.
Contribution to functional satellite cells was augmented in host muscles of both ages by preirradiation. As in previous experiments using isolated fibers [3] grafted into young hosts, notexin injury of mature adult mdx-nude muscles that had been grafted with stripped satellite cells evoked extensive donor-derived muscle regeneration. The fact that satellite cells of donor origin remained robustly functional within older dystrophic muscles, both being able to regenerate muscle fibers and give rise to more satellite cells, is extremely encouraging. However, it must be borne in mind that in our model—irradiated mdx-nude mouse muscle—growth factors and inflammatory processes are attenuated compared with wild-type mdx, and this may have a positive effect on satellite cell function.
Conclusions
Our findings imply that the skeletal muscles of older dystrophic patients may be treated as effectively as those of younger patients by stem cell therapy. Further studies into factors that characterize the dystrophic environment at different ages and how these may be modified to augment stem cell function are therefore warranted.
Supplementary Material
Acknowledgments
L.B. was funded by the Muscular Dystrophy Campaign (grants held by J.M. and P.Z.). This work was also funded by the Association Francaise contre les Myopathies (grant held by J.M). J.M. was funded by an MRC collaborative career development fellowship in stem cell research and currently holds a Wellcome Trust University award. P.S.Z. is supported by The Medical Research Council and acknowledges the support of the MYORES Network of Excellence, Contract 511978, from the European Commission Sixth Framework Programme. J.M. and F.M. are principal investigators of the MRC Centre for Neuromuscular Diseases that supports the biobank of DMD cells. The Pax7 antibody developed by A. Kawakami was obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the National Institute of Child Health and Human Development and maintained by the University of Iowa, Department of Biological Sciences, Iowa City, IA.
Footnotes
Disclosure of Potential Conflicts of Interest The authors indicate no potential conflicts of interest.
See www.StemCells.com for supporting information available online.
References
- 1.Gopinath SD, Rando TA. Stem cell review series: aging of the skeletal muscle stem cell niche. Aging Cell. 2008;7:590–598. doi: 10.1111/j.1474-9726.2008.00399.x. [DOI] [PubMed] [Google Scholar]
- 2.Decary S, Hamida CB, Mouly V, et al. Shorter telomeres in dystrophic muscle consistent with extensive regeneration in young children. Neuromuscul Disord. 2000;10:113–120. doi: 10.1016/s0960-8966(99)00093-0. [DOI] [PubMed] [Google Scholar]
- 3.Collins CA, Olsen I, Zammit PS, et al. Stem cell function, self-renewal, and behavioral heterogeneity of cells from the adult muscle satellite cell niche. Cell. 2005;122:289–301. doi: 10.1016/j.cell.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 4.Mauro A. Satellite cell of skeletal muscle fibers. J Biophys Biochem Cytol. 1961;9:493–495. doi: 10.1083/jcb.9.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carlson BM, Faulkner JA. Muscle transplantation between young and old rats: age of host determines recovery. Am J Physiol. 1989;256:C1262–C1266. doi: 10.1152/ajpcell.1989.256.6.C1262. [DOI] [PubMed] [Google Scholar]
- 6.Collins CA, Zammit PS, Ruiz AP, et al. A population of myogenic stem cells that survives skeletal muscle aging. Stem Cells. 2007;25:885–894. doi: 10.1634/stemcells.2006-0372. [DOI] [PubMed] [Google Scholar]
- 7.Shefer G, Van de Mark DP, Richardson JB, et al. Satellite-cell pool size does matter: Defining the myogenic potency of aging skeletal muscle. Dev Biol. 2006;294:50–66. doi: 10.1016/j.ydbio.2006.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smythe GM, Shavlakadze T, Roberts P, et al. Age influences the early events of skeletal muscle regeneration: Studies of whole muscle grafts transplanted between young (8 weeks) and old (13–21 months) mice. Exp Gerontol. 2008;43:550–562. doi: 10.1016/j.exger.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 9.Lee CE, McArdle A, Griffiths RD. The role of hormones, cytokines and heat shock proteins during age-related muscle loss. Clin Nutr. 2007;26:524–534. doi: 10.1016/j.clnu.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 10.Conboy IM, Conboy MJ, Wagers AJ, et al. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 2005;433:760–764. doi: 10.1038/nature03260. [DOI] [PubMed] [Google Scholar]
- 11.Brack AS, Conboy MJ, Roy S, et al. Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science. 2007;317:807–810. doi: 10.1126/science.1144090. [DOI] [PubMed] [Google Scholar]
- 12.Hidestrand M, Richards-Malcolm S, Gurley CM, et al. Sca-1-expressing nonmyogenic cells contribute to fibrosis in aged skeletal muscle. J Gerontol A Biol Sci Med Sci. 2008;63:566–579. doi: 10.1093/gerona/63.6.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goldspink G, Fernandes K, Williams PE, et al. Age-related changes in collagen gene expression in the muscles of mdx dystrophic and normal mice. Neuromuscul Disord. 1994;4:183–191. doi: 10.1016/0960-8966(94)90019-1. [DOI] [PubMed] [Google Scholar]
- 14.Wallace K, Burt AD, Wright MC. Liver fibrosis. Biochem J. 2008;411:1–18. doi: 10.1042/BJ20071570. [DOI] [PubMed] [Google Scholar]
- 15.Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol. 2008;214:199–210. doi: 10.1002/path.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brunelli S, Rovere-Querini P. The immune system and the repair of skeletal muscle. Pharmacol Res. 2008;58:117–121. doi: 10.1016/j.phrs.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 17.Renault V, Thornell LE, Eriksson PO, et al. Regenerative potential of human skeletal muscle during aging. Aging Cell. 2002;1:132–139. doi: 10.1046/j.1474-9728.2002.00017.x. [DOI] [PubMed] [Google Scholar]
- 18.Gargioli C, Coletta M, De Grandis F, et al. PlGF-MMP-9-expressing cells restore microcirculation and efficacy of cell therapy in aged dystrophic muscle. Nat Med. 2008;14:973–978. doi: 10.1038/nm.1852. [DOI] [PubMed] [Google Scholar]
- 19.Heslop L, Morgan JE, Partridge TA. Evidence for a myogenic stem cell that is exhausted in dystrophic muscle. J Cell Sci. 2000;113:2299–2308. doi: 10.1242/jcs.113.12.2299. [DOI] [PubMed] [Google Scholar]
- 20.Morgan JE, Fletcher RM, Partridge TA. Yields of muscle from myogenic cells implanted into young and old mdx hosts. Muscle Nerve. 1996;19:132–139. doi: 10.1002/(SICI)1097-4598(199602)19:2<132::AID-MUS2>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 21.Morgan JE, Gross JG, Pagel CN, et al. Myogenic cell proliferation and generation of a reversible tumorigenic phenotype are triggered by preirradiation of the recipient site. J Cell Biol. 2002;157:693–702. doi: 10.1083/jcb.200108047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Callicott RJ, Womack JE. Real-time PCR assay for measurement of mouse telomeres. Comp Med. 2006;56:17–22. [PubMed] [Google Scholar]
- 23.Kelly R, Alonso S, Tajbakhsh S, et al. Myosin light chain 3F regulatory sequences confer regionalized cardiac and skeletal muscle expression in transgenic mice. J Cell Biol. 1995;129:383–396. doi: 10.1083/jcb.129.2.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tajbakhsh S, Bober E, Babinet C, et al. Gene targeting the myf-5 locus with nlacZ reveals expression of this myogenic factor in mature skeletal muscle fibres as well as early embryonic muscle. Dev Dyn. 1996;206:291–300. doi: 10.1002/(SICI)1097-0177(199607)206:3<291::AID-AJA6>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 25.Beauchamp JR, Heslop L, Yu DS, et al. Expression of CD34 and Myf5 defines the majority of quiescent adult skeletal muscle satellite cells. J Cell Biol. 2000;151:1221–1234. doi: 10.1083/jcb.151.6.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gross JG, Bou-Gharios G, Morgan JE. Potentiation of myoblast transplantation by host muscle irradiation is dependent on the rate of radiation delivery. Cell Tissue Res. 1999;298:371–375. doi: 10.1007/s004419900062. [DOI] [PubMed] [Google Scholar]
- 27.Gross JG, Morgan JE. Muscle precursor cells injected into irradiated mdx mouse muscle persist after serial injury. Muscle Nerve. 1999;22:174–185. doi: 10.1002/(sici)1097-4598(199902)22:2<174::aid-mus5>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 28.Muntoni F, Mateddu A, Marchei F, et al. Muscular weakness in the mdx mouse. J Neurol Sci. 1993;120:71–77. doi: 10.1016/0022-510x(93)90027-v. [DOI] [PubMed] [Google Scholar]
- 29.Pastoret C, Sebille A. mdx mice show progressive weakness and muscle deterioration with age. J Neurol Sci. 1995;129:97–105. doi: 10.1016/0022-510x(94)00276-t. [DOI] [PubMed] [Google Scholar]
- 30.Goyns MH, Lavery WL. Telomerase and mammalian ageing: a critical appraisal. Mech Ageing Dev. 2000;114:69–77. doi: 10.1016/s0047-6374(00)00095-6. [DOI] [PubMed] [Google Scholar]
- 31.Decary S, Mouly V, Hamida CB, et al. Replicative potential and telomere length in human skeletal muscle: Implications for satellite cell-mediated gene therapy. Hum Gene Ther. 1997;8:1429–1438. doi: 10.1089/hum.1997.8.12-1429. [DOI] [PubMed] [Google Scholar]
- 32.Pastoret C, Sebille A. Age-related differences in regeneration of dystrophic (mdx) and normal muscle in the mouse. Muscle Nerve. 1995;18:1147–1154. doi: 10.1002/mus.880181011. [DOI] [PubMed] [Google Scholar]
- 33.Lund TC, Grange RW, Lowe DA. Telomere shortening in diaphragm and tibialis anterior muscles of aged mdx mice. Muscle Nerve. 2007;36:387–390. doi: 10.1002/mus.20824. [DOI] [PubMed] [Google Scholar]
- 34.Sacco A, Doyonnas R, Kraft P, et al. Self-renewal and expansion of single transplanted muscle stem cells. Nature. 2008;456:502–506. doi: 10.1038/nature07384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harris JB. Myotoxic phospholipases A2 and the regeneration of skeletal muscles. Toxicon. 2003;42:933–945. doi: 10.1016/j.toxicon.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 36.Blaveri K, Heslop L, Yu DS, et al. Patterns of repair of dystrophic mouse muscle: studies on isolated fibers. Dev Dyn. 1999;216:244–256. doi: 10.1002/(SICI)1097-0177(199911)216:3<244::AID-DVDY3>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 37.Boldrin L, Morgan JE. Activating muscle stem cells: Therapeutic potential in muscle diseases. Curr Opin Neurol. 2007;20:577–582. doi: 10.1097/WCO.0b013e3282ef5919. [DOI] [PubMed] [Google Scholar]
- 38.Zammit PS. All muscle satellite cells are equal, but are some more equal than others? J Cell Sci. 2008;121:2975–2982. doi: 10.1242/jcs.019661. [DOI] [PubMed] [Google Scholar]
- 39.Reimann J, Irintchev A, Wernig A. Regenerative capacity and the number of satellite cells in soleus muscles of normal and mdx mice. Neuromuscul Disord. 2000;10:276–282. doi: 10.1016/s0960-8966(99)00118-2. [DOI] [PubMed] [Google Scholar]
- 40.Degens H. Age-related skeletal muscle dysfunction: Causes and mechanisms. J Musculoskelet Neuronal Interact. 2007;7:246–252. [PubMed] [Google Scholar]
- 41.Partridge TA, Morgan JE, Coulton GR, et al. Conversion of mdx myofibres from dystrophin-negative to -positive by injection of normal myoblasts. Nature. 1989;337:176–179. doi: 10.1038/337176a0. [DOI] [PubMed] [Google Scholar]
- 42.Morrison J, Lu QL, Pastoret C, et al. T-cell-dependent fibrosis in the mdx dystrophic mouse. Lab Invest. 2000;80:881–891. doi: 10.1038/labinvest.3780092. [DOI] [PubMed] [Google Scholar]
- 43.Farini A, Meregalli M, Belicchi M, et al. T and B lymphocyte depletion has a marked effect on the fibrosis of dystrophic skeletal muscles in the scid/mdx mouse. J Pathol. 2007;213:229–238. doi: 10.1002/path.2213. [DOI] [PubMed] [Google Scholar]
- 44.Groma V. Demonstration of collagen type VI and alpha-smooth muscle actin in renal fibrotic injury in man. Nephrol Dial Transplant. 1998;13:305–312. doi: 10.1093/oxfordjournals.ndt.a027823. [DOI] [PubMed] [Google Scholar]
- 45.Specks U, Nerlich A, Colby TV, et al. Increased expression of type VI collagen in lung fibrosis. Am J Respir Crit Care Med. 1995;151:1956–1964. doi: 10.1164/ajrccm.151.6.7767545. [DOI] [PubMed] [Google Scholar]
- 46.Naugle JE, Olson ER, Zhang X, et al. Type VI collagen induces cardiac myofibroblast differentiation: implications for postinfarction remodeling. Am J Physiol Heart Circ Physiol. 2006;290:H323–H330. doi: 10.1152/ajpheart.00321.2005. [DOI] [PubMed] [Google Scholar]
- 47.Pagel CN, Partridge TA. Covert persistence of mdx mouse myopathy is revealed by acute and chronic effects of irradiation. J Neurol Sci. 1999;164:103–116. doi: 10.1016/s0022-510x(99)00061-1. [DOI] [PubMed] [Google Scholar]
- 48.Lefaucheur JP, Pastoret C, Sebille A. Phenotype of dystrophinopathy in old mdx mice. Anat Rec. 1995;242:70–76. doi: 10.1002/ar.1092420109. [DOI] [PubMed] [Google Scholar]
- 49.Tseng BS, Zhao P, Pattison JS, et al. Regenerated mdx mouse skeletal muscle shows differential mRNA expression. J Appl Physiol. 2002;93:537–545. doi: 10.1152/japplphysiol.00202.2002. [DOI] [PubMed] [Google Scholar]
- 50.Wineinger MA, Abresch RT, Walsh SA, et al. Effects of aging and voluntary exercise on the function of dystrophic muscle from mdx mice. Am J Phys Med Rehabil. 1998;77:20–27. doi: 10.1097/00002060-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 51.Wakeford S, Watt DJ, Partridge TA. X-irradiation improves mdx mouse muscle as a model of myofiber loss in DMD. Muscle Nerve. 1991;14:42–50. doi: 10.1002/mus.880140108. [DOI] [PubMed] [Google Scholar]
- 52.Beauchamp JR, Morgan JE, Pagel CN, et al. Dynamics of myoblast transplantation reveal a discrete minority of precursors with stem cell-like properties as the myogenic source. J Cell Biol. 1999;144:1113–1122. doi: 10.1083/jcb.144.6.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang MM, Gopal AK. Radioimmunotherapy-based conditioning regimens for stem cell transplantation. Semin Hematol. 2008;45:118–125. doi: 10.1053/j.seminhematol.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wallace GQ, Lapidos KA, Kenik JS, et al. Long-term survival of transplanted stem cells in immunocompetent mice with muscular dystrophy. Am J Pathol. 2008;173:792–802. doi: 10.2353/ajpath.2008.080259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Prochazka M, Gaskins HR, Shultz LD, et al. The nonobese diabetic scid mouse: model for spontaneous thymomagenesis associated with immunodeficiency. Proc Natl Acad Sci U S A. 1992;89:3290–3294. doi: 10.1073/pnas.89.8.3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Serreze DV, Leiter EH, Hanson MS, et al. Emv30null NOD-scid mice. An improved host for adoptive transfer of autoimmune diabetes and growth of human lymphohematopoietic cells. Diabetes. 1995;44:1392–1398. doi: 10.2337/diab.44.12.1392. [DOI] [PubMed] [Google Scholar]
- 57.Chamberlain JS, Metzger J, Reyes M, et al. Dystrophin-deficient mdx mice display a reduced life span and are susceptible to spontaneous rhabdomyosarcoma. FASEB J. 2007;21:2195–2204. doi: 10.1096/fj.06-7353com. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.