Abstract
Background and Purpose
Albuminuria is an important biomarker of renal dysfunction and is a major mediator of renal damage and fibrosis during kidney disease. The mechanisms underlying albumin-induced renal fibrosis remain unclear. There has been significant interest in γ-secretase activity in tubular epithelial cells in recent times; however, its potential role in albumin-induced fibrosis has not been investigated.
Experimental Approach
The primary aim of this study was to examine the role of γ-secretase in albumin-induced fibrotic effects in proximal tubular cells. The effects of increasing albumin concentrations on fibrosis indicators and mediators in the human HK-2 cell line were examined in the presence and absence of a γ-secretase inhibitor, compound E.
Key Results
Treatment with albumin resulted in a number of pro-fibrotic effects, including up-regulation of fibronectin, TGF-β1 and the EGF-R. Interestingly, similar effects were observed in response to treatment with the γ-secretase inhibitor, compound E. Co-treatment of cells with albumin and an EGF-R inhibitor, AG-1478, resulted in significant inhibition of the observed pro-fibrotic effects, suggesting a major role for the EGF-R in albumin-induced fibrotic events. Albumin-induced effects on the EGF-R appeared to be mediated through inhibition of γ-secretase activity and were dependent on ERK-MAPK signalling.
Conclusions and Implications
These results provide novel insights into the mechanisms of albumin-induced fibrotic effects in tubular epithelial cells, suggesting important roles for the γ-secretase and the EGF-R. These results suggest that the proposed use of γ-secretase inhibitors as anti-fibrotic agents requires further investigation.
Keywords: albuminuria, γ-secretase, epidermal growth factor receptor, renal epithelial cell, renal fibrosis
Introduction
End-stage renal disease is a progressive disorder characterized by declining renal function eventually progressing to end-stage renal failure. Severity of proteinuria predicts the rate of decline of renal function across a range of renal diseases and is therefore widely recognized as an important prognostic indicator (Rossing et al., 1994a, b). However, several studies have demonstrated the direct pathogenic effects of proteinuria and its potential role in the progression of renal disease (Abbate et al., 2006; Schieppati and Remuzzi, 2003). As the principal component of proteinuria, the pro-fibrotic effects of serum albumin are of particular interest (Brunskill, 2004; Diwakar et al., 2007; Baines and Brunskill, 2008; Chana et al., 2008). Exposure of proximal tubular epithelial cells (PTECs) to albumin has been shown to result in enhanced production of pro-inflammatory cytokines (e.g. RANTES and monocyte chemoattractant protein) (Zoja et al., 1998), extracellular matrix proteins such as fibronectin, and pro-fibrotic cytokines (e.g. TGF-β1) (Arici et al., 2002; Diwakar et al., 2007). All of these factors contribute significantly to the progression of renal interstitial fibrosis, with TGF-β1 of particular importance (Hills and Squires, 2011). While some cellular effects of albumin have been shown to be dependent on megalin-mediated endocytosis in PTECs (Slattery et al. 2011; Wang et al., 1999; Wohlfarth et al., 2003), the induction of TGF-β1 is not (Diwakar et al., 2007). Diwakar et al. (2007) demonstrated that in a cell line incapable of endocytosing albumin, TGF-β1 production was still induced by exposure to increasing albumin concentrations. The molecular mechanisms underlying albumin-induced TGF-β1 production and other pro-fibrotic effects remain undefined.
Although albumin-induced pro-fibrotic effects occur independently of megalin-mediated endocytosis, megalin may play still play a role in mediating these effects by alternative mechanisms (Baines and Brunskill, 2008). Several cell surface receptors, including megalin, have been shown to undergo regulated intramembrane proteolysis (RIP) (Landman and Kim, 2004). RIP is an evolutionarily conserved process that links proteolysis of membrane proteins with regulation of gene expression (Biemesderfer, 2006). RIP is a multi-step process that includes PKC-regulated ectodomain shedding of specific receptors by metalloproteases to produce a membrane-associated C-terminal fragment. The C-terminal fragment is then released from the membrane by γ-secretase activity at a cleavage site within the target protein's membrane spanning domain. The C-terminal fragment is then free to participate in the regulation of target gene transcription. γ-Secretase-mediated RIP was initially characterized in the intramembraneous cleavage of the amyloid-β precursor protein C-terminal fragment (Wong et al., 2005). More recently, RIP of megalin has been shown to influence the expression of the Na+/H+ exchanger, NHE3, in PTECs (Li et al., 2008). In the context of proteinuria, RIP has been proposed as a potential link between albumin and growth factor receptor signalling in epithelial cells (Baines and Brunskill, 2008; Biemesderfer, 2006; Li et al., 2008). However, more recent experimental data suggest that overexpression of the megalin C-terminal fragment (MCTF) is not detrimental to normal proximal tubule cell function in vivo (Christ et al., 2010). γ-Secretase activity is also central to Notch signalling, a developmentally critical cell–cell communication mechanism. Emerging evidence suggests that Notch signalling may play a role in promoting renal fibrosis during the development of diabetic nephropathy and focal segmental glomerulosclerosis (Murea et al., 2010) and may mediate TGF-β1-induced fibrogenic effects including epithelial mesenchymal transition (EMT) (Nyhan et al., 2010). In light of these observations, it has been hypothesized that RIP may be a therapeutic target in renal fibrosis and γ-secretase inhibition has been proposed as a potential mechanism to achieve this. Therefore, in this study, we investigated the effects of γ-secretase inhibition on albumin-induced pro-fibrotic effects in PTECs.
Methods
Reagents
Tissue culture grade BSA (fatty-acid free and low endotoxin), latrunculin A, DAPT and the MTT [3-(4,5-dimethylthiazol-2-yi)-2,5-diphenyl-tetrazolium bromide] assay kit were obtained from Sigma. Antibodies were obtained from Cell Signaling (EGF-R, phospho-EGF-R, ERK, phospho-ERK; Danvers, MA, USA), BD Pharmigen (fibronectin; Oxford, UK) and Santa Cruz Biotechnology (HES1; Santa Cruz, CA, USA). The TGF-β1 elisa kit was obtained from R&D Systems (Minneapolis, MN, USA). Compound E (CpdE), DAPT, AG-1478 and the fluorogenic γ-secretase substrate were obtained from Calbiochem (Darmstadt, Germany). U0126 was obtained from Cell Signaling. The LDH assay kit was obtained from Merck (Hertfordshire, UK). Texas red (TR)-albumin and DQ-albumin was obtained from Invitrogen (Paisley, UK). All other reagents were of the highest available purity from commercial sources.
Cell culture and treatment
The human renal proximal tubular cell line, HK-2, was a kind gift from Professor Carol Pollock (University of Sydney) and maintained under serum-free conditions in DMEM containing 100 U·mL−1 penicillin, 100 g·mL−1 streptomycin, 5 μg·mL−1 insulin, 10 ng·mL−1 EGF and 2 mM l-glutamine, as previously described (Slattery et al., 2008b). Cell culture medium was changed every 48 h. Cells maintained in 75 cm2 Costar flasks at 37°C in a humidified atmosphere containing 95% air and 5% CO2. In all experiments, cells were cultured to confluence and allowed to differentiate for at least 3 days. Cells were deprived of EGF for 24 h prior to treatments. AG-1478 and U0126 were prepared as stock solutions of 10 mmol·L−1 in dimethyl sulfoxide (DMSO) and stored in aliquots at −20°C in the dark. Cells were pre-incubated for 1 h with the indicated concentration of inhibitor, prior to other treatments. In experiments involving inhibitors, control cells were also exposed to an appropriate concentration of DMSO.
Albumin uptake/degradation assay
Albumin endocytosis and proteolysis in OK and HK-2 cells was quantified using established techniques (Hryciw et al., 2003, 2004; Slattery et al., 2008a). Albumin endocytosis in HK-2 cells was visualized by confocal microscopy (Zeiss LSM 510 Meta confocal microscope, Carl Zeiss, Ltd., Cambridge, UK). Z-scan optical sections were collected at 0.2 μM intervals. TR-albumin was excited at 543 nm and emission was measured at 570 nm.
Cell viability assays
Cytotoxicity of CpdE in HK-2 cells was assessed using an MTT-reduction assay (Mosmann, 1983) and an LDH release assay. Both assays were performed according to the manufacturer's instructions. Data were expressed as a percentage of vehicle control (0.1% DMSO).
γ-Secretase activity
γ-Secretase activity was assessed using the previously described methods (Yu et al., 2004; Kim et al., 2006). Briefly, 1 μg of cell lysate [prepared in CHAPSO buffer (50 mM HEPES, pH 7.4, 150 mM NaCl, 2 mM EDTA, 1% 3-[(3-choloamydopropyl)dimethylammonio]-2-hydroxy-1propanesulfonate (CHAPSO)] and protease inhibitor cocktail (Roche Applied Sciences, Indianapolis, IN, USA) was diluted to 150 μL using assay buffer containing 50 mM Tris–HCl, (pH 6.8), 2 mM EDTA. 0.25% w/v CHAPSO was plated onto an opaque 96-well microplate. A fluorogenic γ-secretase substrate containing the amyloid β, γ-secretase target sequence [NMA-GGVVIATVK(DNP)-DRDRDR-NH2] was added for a 10 μM final concentration. The peptide is internally quenched and contains an amino acid sequence that is cleaved by γ-secretase, resulting in enhanced fluorescence (excitation max.: ∼355 nm; emission max.: ∼440 nm). Fluorescence was measured after 1 h in a BMG Fluostar Optima fluorescent plate reader (BMG Lab Technologies, Offenburg, Germany). γ-Secretase activity was measured as an increase in fluorescence, which is achieved only when the substrate is cleaved (Farmery et al., 2003).
TGF-β1 elisa
TGF-β1 in HK-2 cell supernatants was quantitatively measured by the specific elisa method according to the manufacturer's instructions.
TGF-β1-Smad reporter assay
Transient transfections and Luciferase reporter assays were performed as previously described (Martin-Martin et al., 2011). Briefly, 90% confluent HK-2 cells were co-transfected with 1 μg of Smad3-responsive reporter plasmid (CAGA – courtesy of Dr. Roel Goldschmeding) and 50 ng of Renilla luciferase plasmid (pCMV-hRL, internal control). Transfectants were grown to confluency before exposure to TGF-β1 (5 ng·mL−1, R&D Systems) for 12, 24 or 48 h. Cells were lysed in reporter lysis buffer (Promega, Southampton, UK). Firefly and Renilla luciferase content was quantified using the Dual-Glo assay (Promega).
Western analysis
After treatment, cells were lysed with ice-cold lysis buffer (20 mM Tris–HCl, 150 mM NaCl, 1% Triton X-100, 0.5% sodium deoxycholate, 0.1% SDS, 200 μM PMSF, 2 mM EDTA, 1 mM sodium orthovanadate, protease inhibitor cocktail and 50 mM sodium fluoride). The lysate was placed on ice for 20 min and then spun at 14 000 rpm for 10 min at 4°C to precipitate any cell debris. Protein concentration of the supernatant was measured with a bicinchoninic acid protein assay kit (Pierce Thermo Scientific, Rockford, IL, USA). Equal total protein amounts of cell lysates or concentrated supernatants were electrophoresed using the procedure of Laemmli (1970). For detection of EGF-R, ERK-MAPK and fibronectin, membranes were probed with the respective antibodies. The results shown are representative of at least three experiments with similar results.
Results
Albumin endocytosis in HK-2 tubular epithelial cells
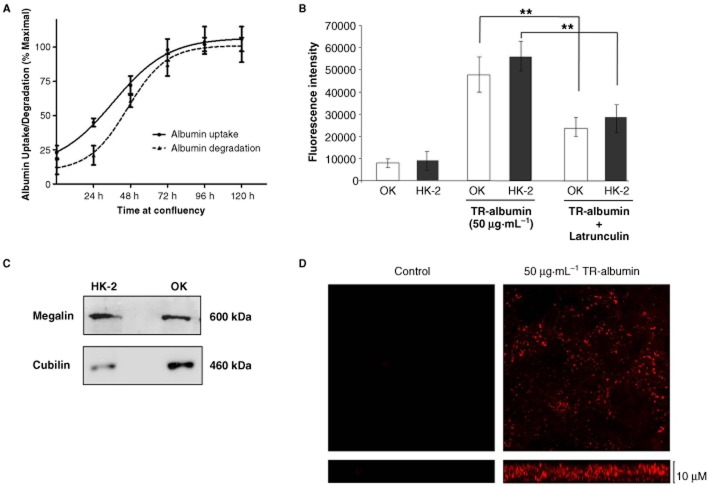
In all experiments, HK-2 cells were cultured in EGF-free medium for 24 h prior to treatment. To determine whether the HK-2 cell model system was a suitable model for receptor-mediated albumin endocytosis, we investigated both albumin uptake and degradation in these cells. The opossum kidney (OK) proximal tubule cell line is the standard model for receptor-mediated albumin uptake in vitro and is used as a comparison. We first performed time-course experiments for the uptake of TR-albumin and degradation of DQ-albumin (50 μg mL−1) in HK-2 cells (Figure 1a). The results at each time-point were expressed as percentage of maximal albumin uptake in HK-2 cells. Both albumin uptake and degradation in HK-2 s increased until day 3, after which no further significant increases in albumin processing capacity were observed. Based on these observations, all subsequent experiments were performed after a minimum of 3 days at full confluency to ensure the albumin endocytic pathway was well established. We have previously reported that albumin uptake in OK cells was significantly reduced by disruption of the actin cytoskeleton by latrunculin A treatment (Hryciw et al., 2005; Slattery et al., 2008a). Similarly, we found that treatment of HK-2 tubular epithelial cells with latrunculin A (2 μM) resulted in a comparable degree of inhibition of albumin uptake as shown by the reduction in TR-albumin fluorescence in cell lysates (38 ± 9.1%; n = 3; P < 0.01) (Figure 1b). This suggested that albumin uptake in HK-2 tubular epithelial cells was dependent on cytoskeletal dynamics and was likely receptor-mediated. Expression of the albumin co-receptors megalin and cubilin was assessed by Western blotting in HK-2 tubular epithelial cells and OK cells (Figure 1c). Both megalin and cubilin were abundantly expressed in HK-2 cells. Albumin uptake in HK-2 cells was further characterized by confocal microscopy to visualize the distribution of TR-albumin in HK-2 tubular epithelial cells (Figure 1d). Cells were seeded on glass coverslips, allowed to differentiate and exposed to TR-albumin (50 μg·mL−1) for 1 h at 37°C. Cells were fixed and then imaged (Zeiss LSM 510 Meta confocal microscope – 40× objective). TR-albumin was distributed throughout the cells from apical surface towards the basolateral. These results demonstrate HK-2 cell's endocytose albumin in a similar manner to the OK cells and thus represent a model of receptor-mediated albumin uptake.
Figure 1.
Albumin endocytosis in HK-2 tubular epithelial cells. (A) HK-2 cells were grown to confluence on 48-well plates. Cells were exposed to TR-albumin (50 μg·mL−1) at 24, 48, 72 and 96 h after confluence, for 2 h at which time TR-albumin fluorescence in cell lysates was measured. Data are expressed as % of maximal albumin uptake observed over the time-course. (B) OK cells and HK-2 cells cultured on 48-well plates were exposed to TR-albumin (50 μg·mL−1) in the absence and presence of latrunculin A (2 μM) for 2 h at which time TR-albumin fluorescence in cell lysates was measured. Error bars represent the mean ± SEM of at least three independent experiments. **P < 0.01 and ***P < 0.001 indicate statistically significant difference compared with the control. (C) Equal amounts of whole cell lysates (OK cells and HK-2 cells) were subjected to SDS-PAGE and probed with antibodies for megalin and cubilin. Blots shown are representative of at least three independent experiments. (D) HK-2 cells cultured on coverslip cells were incubated with TR-albumin (50 μg·mL−1) for 2 h at 37°C and then fixed. Fluorescent albumin distribution was assessed by confocal microscopy.
Determination of appropriate working concentration of CpdE in HK-2 cells
The γ-secretase inhibitor, CpdE, has been used previously in proximal tubule epithelial cells (Zou et al., 2004; Biemesderfer, 2006; Li et al., 2008; Nyhan et al., 2010). The IC50 of CpdE in the HK-2 cell model was determined using a fluorescence-based γ-secretase activity assay as described in the Methods section (Figure 2a). The IC50 of DAPT was also determined for comparison purposes. HK-2 cells were incubated with increasing amounts of CpdE or DAPT for 6 h and the IC50 for γ-secretase inhibition determined as 1.5 nM for CpdE and 142.3 nM for DAPT. Yang et al. (2007) reported dose-selective inhibition of amyloid β cleavage over Notch cleavage in the region of two orders of magnitude and demonstrated that optimal inhibition of γ-secretase-mediated notch cleavage required 100 nM CpdE. Therefore, we elected to use 100 nM CpdE in all subsequent studies. At this concentration, CpdE did not affect HK-2 cellular viability as assessed by MTT reduction and LDH release (Figure 2b).
Figure 2.
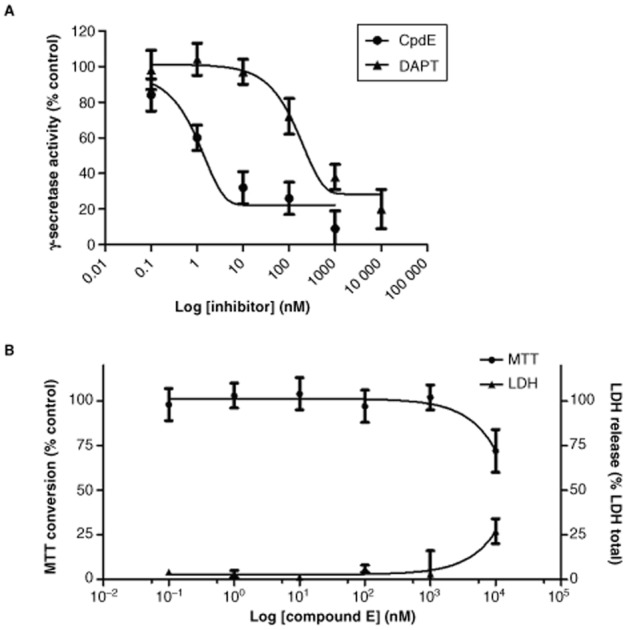
Determination of CpdE working concentration in HK-2 tubular epithelial cells. (A) HK-2 cells grown on 6-well culture plates were exposed to increasing concentrations of CpdE or DAPT for 6 h. Whole cell lysates were prepared in CHAPSO and γ-secretase activity in whole cell lysates was determined using fluorescence-based assays as described in the Methods section. (B) HK-2 cells grown on 96-well culture plates were exposed to increasing concentrations of CpdE for 72 h. Cell viability was assessed using the MTT assay and LDH assays.
Effects of albumin and CpdE on TGF-β1 and fibronectin secretion from HK-2 tubular epithelial cells
To assess the pro-fibrotic effects of albumin in the HK-2 cell model, TGF-β1 secretion and signalling and fibronectin secretion were assessed in HK-2 cells by specific elisa, reporter assay and Western analysis respectively, after treatment for 72 h (Figure 3). Exposure to 0.1 mg·mL−1 albumin did not significantly alter TGF-β1 or fibronectin secretion compared with the control levels. In contrast, treatment with 1 mg·mL−1 albumin resulted in a significant increase in fibronectin levels. Exposure to 5 mg·mL−1 resulted in a significant increase in both TGF-β1 and fibronectin secretion, and significant activation of Smad-dependent transcription. Importantly, treatment with the γ-secretase inhibitor CpdE (100 nM) alone resulted in similar increases in TGF-β1 and fibronectin release as well as Smad-dependent transcription in PTECs. Treatment with a combination of 5 mg·mL−1 albumin and 100 nM CpdE resulted in significantly elevated TGF-β1 (Figure 3a) release, Smad-dependent transcription (Figure 3b) and fibronectin secretion (Figure 3c) compared with the controls. However, when CpdE was added together with albumin, no additive effect was observed, suggesting that both treatments were working through similar pathways. This pro-fibrotic effect of γ-secretase was unexpected, since in other reports (Bielesz et al., 2010; Nyhan et al., 2010; Leask, 2011), γ-secretase inhibition has been proposed as a potential anti-fibrotic strategy. The molecular mechanisms underlying these observations were further investigated.
Figure 3.
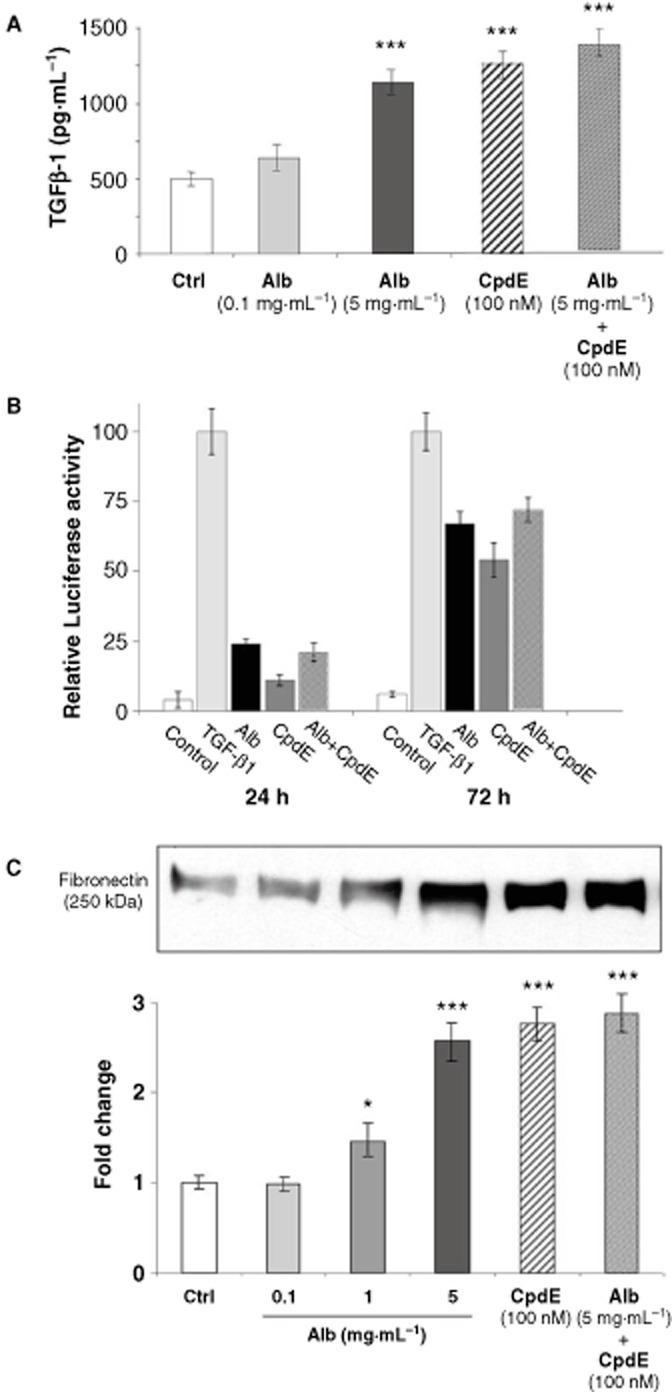
Effects of albumin on TGF-β and fibronectin in HK-2 tubular epithelial cells. HK-2 cells grown on 6-well culture plates were treated with control medium, albumin (0.1, 1 or 5 mg·mL−1) or CpdE (100 nM) for 72 h. Cell supernatants were collected and stored at −20°C. (A) The levels of TGF-β1 in cell supernatants obtained were determined by specific elisa. Each value represents the mean ± SEM of three independent experiments performed in duplicate. (B) HK-2 cells on 24 well plates were co-transfected with CAGA-luciferase and pCMV-hRL for 18 h. Cells were treated with control medium, TGF-β1 (5 ng·mL−1), albumin (5 mg·mL−1) or CpdE (100 nM) for 24 or 72 h, and harvested for assay of luciferase activity. Each value represents the mean ± SEM of three independent experiments performed in triplicate after normalization to Renilla luciferase activity. (C) After concentration, cell supernatants were subjected to SDS-PAGE and probed with an antibody for fibronectin. Band intensities were quantified densitometrically and are shown as mean ± SEM of three independent experiments. *Indicates statistically different to control: *P < 0.05, **P < 0.01, ***P < 0.001.
Effects of albumin and CpdE on Notch signalling in HK-2 tubular epithelial cells
To assess if activation of Notch signalling in this model, expression of HES1, a Notch responsive gene in tubular epithelial cells (Nyhan et al., 2010; Sumual et al., 2010), was examined (Figure 4). Exposure to albumin (5 mg·mL−1) or CpdE (100 nM) did not result in significant up-regulation of HES1 after 72 h. In contrast, exposure to TGF-β1 (5 ng·mL−1) for 72 h resulted in a significant increase in HES1 protein. This effect has been reported previously (Nyhan et al., 2010). EDTA induces ligand-independent Notch activation (Rand et al., 2000) and was utilized as a positive control. HK-2 cells were exposed to EDTA for 3 h, resulting in significant up-regulation of HES1. This effect was inhibited in the presence of CpdE (100 nM). These results indicated that neither high albumin nor CpdE exerted their pro-fibrotic effects via the Notch pathway.
Figure 4.
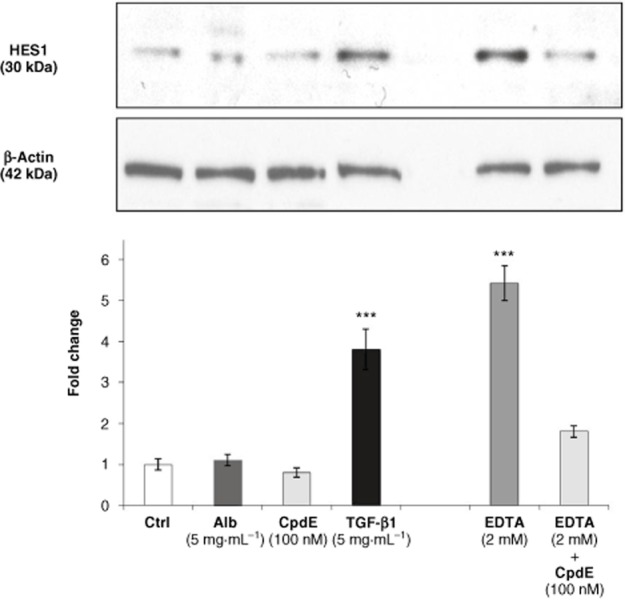
Effects of albumin on HES1 expression in HK-2 tubular epithelial cells. HK-2 were grown on 6-well culture plates. Cells were treated with control medium, albumin (5 mg·mL−1), CpdE (100 nM) or TGF-β1 (5 ng·mL−1) for 72 h, and to EDTA or EDTA + CpdE for 1 h. Whole cell lysates were subjected to SDS-PAGE and probed with an antibody for HES1. β-Actin levels were determined for normalization. Band intensities were quantified densitometrically and are shown as mean ± SEM of three independent experiments. *Indicates statistically different to control: ***P < 0.001.
Effects of albumin on EGF-R and ERK-MAPK signalling in HK-2 tubular epithelial cells
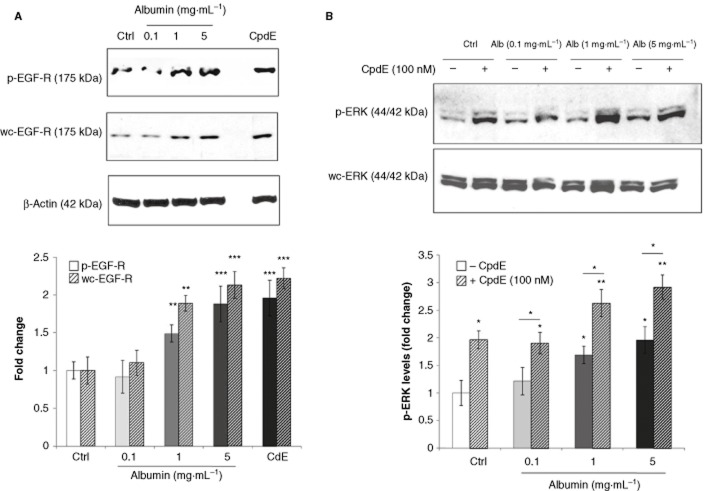
The EGF-R is recognized to play a central role in renal fibrosis (Melenhorst et al., 2008) and has been shown to be regulated by the activity of γ-secretase in an ERK-dependent manner (Kim et al., 2006). Therefore, the effects of albumin on EGF-R and ERK-MAPK signalling were examined at time-points up to 1 and at 72 h using Western analysis (Figures 5 and 6). EGF-R phosphorylation was significantly increased within 5 min of albumin exposure and increased up to 60 min (Figure 5a). Similarly rapid, significant activation of ERK-MAPK was also observed within 5 min of albumin treatment (Figure 5b). Whole cell levels EGF-R and ERK-MAPK proteins were not significantly affected over this time-course. These results demonstrate rapid, potent activation of EGF-R and ERK-MAPK proteins by albumin in PTECs, an observation in agreement with those of Reich et al. (2005) in primary human renal epithelial cells.
Figure 5.
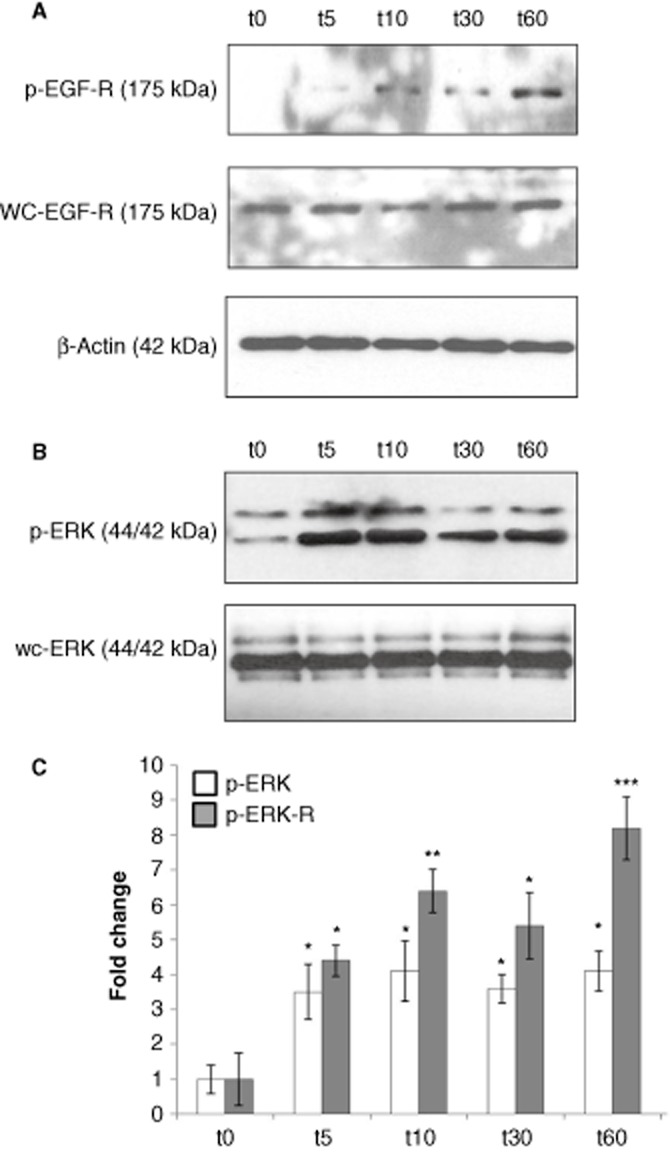
Immediate effects of albumin on EGF-R and ERK-MAPK in HK-2 tubular epithelial cells. HK-2 were grown on 6-well culture plates. Cells were treated with control medium or albumin (5 mg·mL−1) for 3 h. Whole cell lysates prepared at 5, 10, 30 and 60 min were subjected to SDS-PAGE and probed with an antibody for (A) p-EGF-R, wc-EGF-R and (B) p-ERK, wc-ERK. β-Actin levels were determined for normalization. Band intensities were quantified densitometrically and are shown as mean ± SEM of three independent experiments (phospho levels were normalized against appropriate whole cell protein levels). *Indicates statistically different to control: *P < 0.05, **P < 0.01, ***P < 0.001.
Figure 6.
Sustained effects of albumin on EGF-R and ERK-MAPK in HK-2 tubular epithelial cells. (A) HK-2 were grown on 6-well culture plates. Cells were treated with control medium, albumin (0.1, 1 or 5 mg·mL−1) or CpdE (100 nM) for 72 h. Whole cell lysates were subjected to SDS-PAGE and probed with an antibody for (A) p-EGF-R, wc-EGF-R and (B) p-ERK, wc-ERK. β-Actin levels were determined for normalization. Band intensities were quantified densitometrically and are shown as mean ± SEM of three independent experiments (phospho levels were normalized against appropriate whole cell protein levels). *Indicates statistically different from the control: *P < 0.05, **P < 0.01, ***P < 0.001.
In PTECs exposed to albumin for 72 h, both phospho- and whole cell-EGF-R levels were increased in a dose-dependent manner (Figure 6a). Treatment with 100 nM CpdE for 72 h also resulted in significant increases in EGF-R expression and phosphorylation. These results suggest that after prolonged exposure to high concentrations of albumin or CpdE, basal levels of EGF-R expression and activity were significantly elevated. The effects of albumin and CpdE treatment on ERK-MAPK signalling were also examined after 72 h (Figure 6b). HK-2 cells were exposed to albumin (0.1, 1 and 5 mg·mL−1) in the presence or absence of CpdE (100 nM) for 72 h. ERK-MAPK phosphorylation was significantly increased by exposure to 1 and 5 mg·mL−1 albumin. Treatment with CpdE also induced ERK-MAPK phosphorylation, and combined treatment with albumin and CpdE resulted in a further significant increase in ERK-MAPK phosphorylation.
Effects of EGF-R inhibition on albumin-induced TGF-β1 and fibronectin secretion from HK-2 tubular epithelial cells
In light of the observed increase in EGF-R signalling after 72 h of albumin treatment, the role of the EGF-R in albumin-induced effects in HK-2 tubular cells was examined using the EGF-R inhibitor, AG-1478 (250 nM). As previously observed, treatment with 5 mg·mL−1 albumin and 100 nM CpdE for 72 h resulted in significant increases in TGF-β1 and fibronectin release from HK-2 cells (Figure 7). Co-treatment with AG-1478 (250 nM) resulted in complete inhibition of these effects in both cases.
Figure 7.
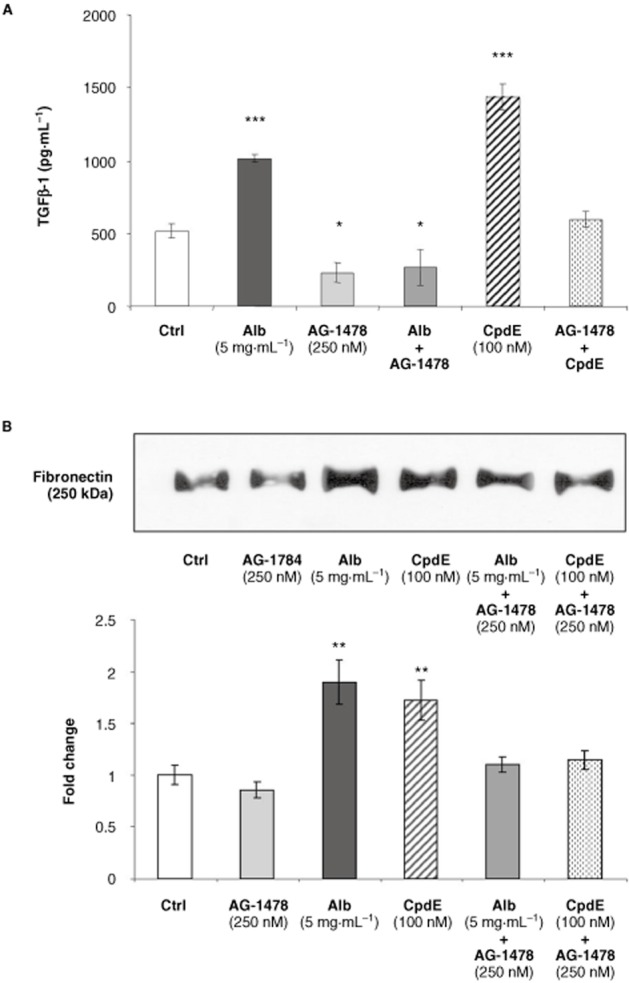
Effects of EGF-R inhibition on albumin-induced TGF-β1 and fibronectin secretion from HK-2 tubular epithelial cells. HK-2 cells grown on 6-well culture plates were treated with control medium, albumin (0.1, 1 or 5 mg·mL−1) or CpdE (100 nM), in the presence or absence of the EGF-R inhibitor, AG-1784 (250 nM), for 72 h. Cell supernatants were collected and stored at −20°C. (A) The levels of TGF-β1 in cell supernatants obtained were determined by specific elisa. Each value represents the mean ± SEM of three independent experiments performed in duplicate. (B) After concentration, cell supernatants were subjected to SDS-PAGE and probed with an antibody for fibronectin. Band intensities were quantified densitometrically and are shown as mean ± SEM of three independent experiments. *Indicates statistically different from the control: *P < 0.05, **P < 0.01, ***P < 0.001.
Role of ERK-MAPK signalling in albumin-induced effects in HK-2 tubular epithelial cells
Considering the observed albumin-induced increase in ERK-MAPK phosphorylation, the role that ERK-MAPK signalling played in the long-term pro-fibrotic effects of albumin in this model was investigated using the ERK-MAPK inhibitor, U0126. HK-2 cells were treated with control medium or albumin (5 mg·mL−1) in the presence or absence of U0126 (10 μM) for 72 h. Whole cell lysates were analysed by Western blotting for whole cell-EGF-R (Figure 8a). As previously observed, albumin treatment (5 mg·mL−1) significantly increased the EGF-R protein levels. This effect was significantly inhibited by co-treatment with U0126 (10 μM). Interestingly, treatment with 10 μM U0126 alone significantly decreased EGF-R levels compared with the control, suggesting that basal EGF-R protein levels are regulated by an ERK-sensitive pathway in HK-2 cells. As the γ-secretase inhibitor, CpdE, induced similar effects on EGF-R protein levels to albumin, the effect of albumin on γ-secretase activity was investigated (Figure 8b). HK-2 cells were treated with control medium, albumin (5 mg·mL−1), in the presence or absence of U0126 (10 μM), or CpdE, for 72 h. Whole cell lysates were prepared in CHAPSO, and γ-secretase activity in whole cell lysates was determined using a fluorescence-based assay as described in the Methods section. In the presence of CpdE (100 nM), γ-secretase substrate cleavage was significantly reduced compared with the control cells. Importantly, treatment with 5 mg·mL−1 albumin for 72 h resulted in significant reductions in γ-secretase activity. This effect was reversed in the presence of U0126 (10 μM). While treatment with U0126 alone did not have a significant effect on γ-secretase activity, a trend towards increased γ-secretase activity was observed.
Figure 8.
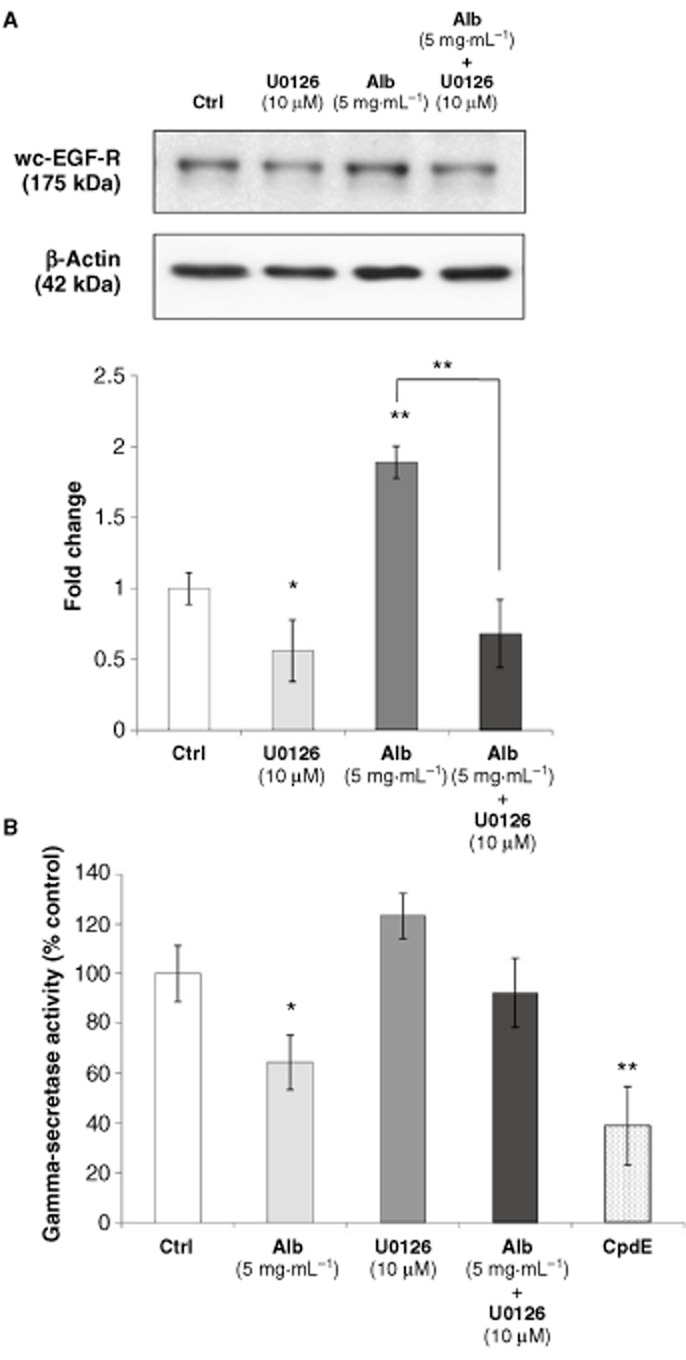
Effects of ERK-MAPK inhibition on albumin-induced effects on EGF-R expression and γ-secretase activity. (A) HK-2 cells grown on 6-well culture plates were treated with control medium or albumin (5 mg·mL−1) in the presence or absence of an ERK-MAPK inhibitor, U0126 (10 μM), for 72 h. Whole cell lysates were subjected to SDS-PAGE and probed with an antibody for wc-EGF-R. β-Actin levels were determined for normalization. Band intensities were quantified densitometrically and are shown as mean ± SEM of three independent experiments (normalized for β-actin levels). (B) HK-2 cells grown on 6-well culture plates were treated with control medium, albumin (5 mg·mL−1) in the presence or absence of U0126 (10 μM), or CpdE, for 72 h. Whole cell lysates were prepared in CHAPSO and γ-secretase activity in whole cell lysates was determined using a fluorescence-based assays as described in the Methods section. *Indicates statistically different from the control: *P < 0.05, **P < 0.01, ***P < 0.001.
Discussion
The pro-fibrotic effects of albumin have been described in a number of in vitro models. Diwakar et al. (2007) observed increased TGF-β1 production from OK and HKC-8 PTEC lines after albumin exposure. A number of studies have also demonstrated increased fibronectin secretion in response to albumin treatment (Stephan et al., 2004; Yang et al., 2008). However, the precise molecular events leading to these important, disease-relevant outcomes remain to be fully defined. In the current study, our aim was to elucidate the mechanisms underlying the pro-fibrotic effects of albumin on PTECs in vitro, which are likely relevant to in vivo proteinuric conditions and the development of a fibrotic environment. In the current study, we showed that the albumin-induced increases in TGF-β1 and fibronectin secretion from human PTECs are mediated by the EGF-R signalling pathway. We present novel data to show that inhibition of γ-secretase activity also activates EGF-R activated ERK-MAPK pathway, leading to the enhanced pro-fibrotic response in PTECs.
Our interest in γ-secretase arose from previous reports that γ-secretase-mediated RIP may be important in tubular uptake mechanisms (Baines and Brunskill, 2008; Biemesderfer, 2006; Li et al., 2008). We hypothesized that γ-secretase activity may be involved in the albumin-induced fibrotic effects observed in the proximal tubule. We found that the γ-secretase inhibitor, CpdE, did not inhibit albumin-induced effects in PTECs. This surprising finding suggested that constitutive γ-secretase activity could play an important maintenance role in differentiated PTECs, a role that is intrinsically anti-fibrotic. Christ et al. (2010) reported recently that overexpression of the MCTF was not detrimental to PTECs in vivo, lending support to the hypothesis that γ-secretase activity is not inherently damaging to the proximal tubule. These observations led us to investigate potential mechanisms of these effects. The EGF-R, a receptor tyrosine kinase of the ErbB/HER family, is of increasing interest in the pathogenesis of renal fibrosis (Melenhorst et al., 2008). Mice overexpressing a dominant-negative form of the EGF-R exhibited reduced tubulointerstitial fibrosis in an angiotensin II-induced model of renal disease suggesting a role for the EGF-R in disease progression (Lautrette et al., 2005). It has been suggested that EGF itself may not be responsible for these effects as a number of other factors, including angiotensin II (Flannery and Spurney, 2006) and albumin (Lee and Han, 2008), are capable of trans-activating and modulating the expression of the EGF-R in the absence of direct EGF stimulation. This is in keeping with the observations of the current study where we observed EGF-independent activation of the EGF-R after albumin exposure. The mechanisms underlying this effect and indeed the significance of the effect in a pathophysiological setting have not been previously examined.
Previous studies have demonstrated activation of several cellular signalling pathways in PTECs by albumin over short time courses (less than 12 h), including ERK-MAPK, EGF-R and, to a lesser degree, PKC (Reich et al., 2005; Baines and Brunskill, 2008; Lee and Han, 2008). In light of this, we investigated ERK-MAPK and the EGF-R. We observed that exposure to 5 mg·mL−1 albumin over a 1 h period resulted in a rapid and significant increases in both ERK-MAPK and EGF-R phosphorylation without a change in whole cell levels of the respective proteins. These observations are similar to the findings of Lee and Han (2008) and Reich et al. (2005). Rapid albumin-induced activation of ERK-MAPK and the EGF-R in PTECs has been previously characterized and has been shown to be the result of albumin-induced PKC activation, leading to EGF-R trans-activation, which, in turn, mediates downstream ERK-MAPK activation (Lee and Han, 2008). However, this signalling schema was time-restricted and was not sustained over a longer time period (Lee and Han, 2008). In the current study, we examined ERK-MAPK and EGF-R phosphorylation and expression after 72 h of albumin treatment to investigate any correlation with TGF-β1 and fibronectin release. At the earlier time-points, both ERK-MAPK and EGF-R phosphorylation were significantly increased. Interestingly, however, in contrast to earlier time-points, after 72 h, the enhancement of EGF-R activation in the presence of albumin appeared to be largely due to elevated amounts of total EGF-R protein in the cells, suggesting that prolonged albumin treatment resulted in enhancement of the EGF-R/ERK signalling axis. We examined the role of the EGF-R in albumin-induced pro-fibrotic effects in PTECs, specifically albumin-induced elevations in TGF-β1 and fibronectin levels. Inhibition of the EGF-R using AG-1478 completely inhibited these albumin-induced increases, further supporting a central role for the EGF-R in this model.
In our model, we found that the pro-fibrotic effects were EGF-R-dependent; however, other studies show inverse correlations between γ-secretase activity and the expression of EGF-R (Kim et al., 2006; Li et al., 2007). The mechanism underlying this negative regulation of the EGF-R by γ-secretase appears to be largely transcriptional, although some evidence suggests that the EGF-R protein may also be a direct target of γ-secretase-mediated proteolysis (Ni et al., 2001; Landman and Kim, 2004). We therefore investigated the effects of γ-secretase inhibition on EGF-R activity and expression. γ-Secretase inhibition in PTECs resulted in a significant increase in the level of EGF-R phosphorylation after 72 h. As was the case with albumin treatment, this increase appeared to be largely due to increased levels of total EGF-R protein. We also investigated the effects of γ-secretase inhibition on ERK-MAPK activity. γ-Secretase inhibition resulted in increased ERK-MAPK phosphorylation but did not affect the total ERK-MAPK protein. These results suggest significant overlap between the mechanisms by which albumin and CpdE were mediating their effects via ERK-MAPK and the EGF-R. Kim et al. (2006) demonstrated that ERK-MAPK activity was an endogenous negative regulator of γ-secretase activity and that, in fact, ERK-MAPK inhibition stimulated γ-secretase activity. Consequently, the relationship between albumin, ERK-MAPK signalling and γ-secretase activity was examined in this model. After 72 h of albumin exposure, γ-secretase activity was significantly reduced in albumin-treated PTECs compared with the controls. This effect was abolished in the presence of the ERK-MAPK inhibitor U0126, suggesting that albumin-induced γ-secretase activity in this model was ERK-MAPK dependent. The presence of U0126 also inhibited the albumin-induced up-regulation of EGF-R.
Our findings clearly show that inhibition of constitutive γ-secretase activity in PTECs produces a pro-fibrotic response via EGF-R/ERK signalling axis. This, in turn, suggests that in the normal polarized tubular epithelium, constitutive γ-secretase activity has a protective role in the proximal tubule as a negatively regulator of the EGF-R pathway. In the context of renal fibrosis, this finding is relevant recent as studies have reported that inhibition of Notch signalling by inhibition of γ-secretase could be beneficial as an anti-fibrotic therapy (Bielesz et al., 2010; Nyhan et al., 2010; Leask, 2011). These studies focused mainly on the TGF-β1/Jag1/Notch-induced renal EMT. It is clear that TGF-β1 is a potent activator of the Notch pathway in PTECs both in vivo and in vitro. Indeed, the data in our current study show that although TGF-β1 is a potent inducer of HES-1, the increases in EGF-R levels produced by inhibition of γ-secretase activity (either by CpdE or high albumin) did not act through the Notch pathway. Taken together, these data show that constitutive γ-secretase activity plays a regulatory role under normal conditions and that inhibition of its activity may shift the cells towards a more pro-fibrotic condition. During EMT, the TGF-β1/Notch pathway becomes the predominant pathway driving the pro-fibrotic changes such that γ-secretase inhibition can ameliorate the progression of EMT.
The premature termination of recent phase III clinical trials of a γ-secretase inhibitor (semagacestat) for Alzheimer's disease highlights the complexities of action of γ-secretase. Aberrant cleavage of the β-amyloid peptide by γ-secretase had been proposed as a mechanism of progression in Alzheimer's disease (Shen and Kelleher, 2007). However, data from the trials showed no effect on, or even slight worsening of, cognition in patients with Alzheimer's disease (Samson, 2010). These findings have since been interpreted as suggesting that inhibition of γ-secretase actually enhanced β-amyloid formation (Schor, 2011). A number of other unforeseen side effects were also observed (Samson, 2010). In the context of renal fibrosis, such unexpected outcomes, together with our demonstration of a ‘maintenance’ role, indicate that γ-secretase may play a more complex role in renal tubule function and disease progression.
Acknowledgments
This work was supported by project grant funding from the National Health and Medical Research Council of Australia (P. P.). C. S. is a Government of Ireland Research Fellow funded by the Irish Research Council for Science, Engineering and Technology.
Glossary
- CpdE
compound E
- HK-2
human kidney 2
- MCTF
megalin C-terminal fragment
- PTECs
proximal tubular epithelial cells
- RIP
regulated intramembrane proteolysis
Conflict of interest
On behalf of all authors, we declare no conflict of interest.
References
- Abbate M, Zoja C, Remuzzi G. How does proteinuria cause progressive renal damage? J Am Soc Nephrol. 2006;17:2974–2984. doi: 10.1681/ASN.2006040377. [DOI] [PubMed] [Google Scholar]
- Arici M, Brown J, Williams M, Harris KP, Walls J, Brunskill NJ. Fatty acids carried on albumin modulate proximal tubular cell fibronectin production: a role for protein kinase C. Nephrol Dial Transplant. 2002;17:1751–1757. doi: 10.1093/ndt/17.10.1751. [DOI] [PubMed] [Google Scholar]
- Baines RJ, Brunskill NJ. The molecular interactions between filtered proteins and proximal tubular cells in proteinuria. Nephron Exp Nephrol. 2008;110:e67–e71. doi: 10.1159/000161982. [DOI] [PubMed] [Google Scholar]
- Bielesz B, Sirin Y, Si H, Niranjan T, Gruenwald A, Ahn S, et al. Epithelial Notch signaling regulates interstitial fibrosis development in the kidneys of mice and humans. J Clin Invest. 2010;120:4040–4054. doi: 10.1172/JCI43025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biemesderfer D. Regulated intramembrane proteolysis of megalin: linking urinary protein and gene regulation in proximal tubule? Kidney Int. 2006;69:1717–1721. doi: 10.1038/sj.ki.5000298. [DOI] [PubMed] [Google Scholar]
- Brunskill NJ. Albumin signals the coming of age of proteinuric nephropathy. J Am Soc Nephrol. 2004;15:504–505. doi: 10.1097/01.asn.0000112912.40303.81. [DOI] [PubMed] [Google Scholar]
- Chana RS, Sidaway JE, Brunskill NJ. Statins but not thiazolidinediones attenuate albumin-mediated chemokine production by proximal tubular cells independently of endocytosis. Am J Nephrol. 2008;28:823–830. doi: 10.1159/000137682. [DOI] [PubMed] [Google Scholar]
- Christ A, Terryn S, Schmidt V, Christensen EI, Huska MR, Andrade-Navarro MA, et al. The soluble intracellular domain of megalin does not affect renal proximal tubular function in vivo. Kidney Int. 2010;78:473–477. doi: 10.1038/ki.2010.169. [DOI] [PubMed] [Google Scholar]
- Diwakar R, Pearson AL, Colville-Nash P, Brunskill NJ, Dockrell ME. The role played by endocytosis in albumin-induced secretion of TGF-beta1 by proximal tubular epithelial cells. Am J Physiol Renal Physiol. 2007;292:F1464–F1470. doi: 10.1152/ajprenal.00069.2006. [DOI] [PubMed] [Google Scholar]
- Farmery MR, Tjernberg LO, Pursglove SE, Bergman A, Winblad B, Naslund J. Partial purification and characterization of gamma-secretase from post-mortem human brain. J Biol Chem. 2003;278:24277–24284. doi: 10.1074/jbc.M211992200. [DOI] [PubMed] [Google Scholar]
- Flannery PJ, Spurney RF. Transactivation of the epidermal growth factor receptor by angiotensin II in glomerular podocytes. Nephron Exp Nephrol. 2006;103:e109–e118. doi: 10.1159/000092196. [DOI] [PubMed] [Google Scholar]
- Hills CE, Squires PE. The role of TGF-β and epithelial-to mesenchymal transition in diabetic nephropathy. Cytokine Growth Factor Rev. 2011;22:131–139. doi: 10.1016/j.cytogfr.2011.06.002. [DOI] [PubMed] [Google Scholar]
- Hryciw DH, Wang Y, Devuyst O, Pollock CA, Poronnik P, Guggino WB. Cofilin interacts with ClC-5 and regulates albumin uptake in proximal tubule cell lines. J Biol Chem. 2003;278:40169–40176. doi: 10.1074/jbc.M307890200. [DOI] [PubMed] [Google Scholar]
- Hryciw DH, Ekberg J, Lee A, Lensink IL, Kumar S, Guggino WB, et al. Nedd4-2 functionally interacts with ClC-5: involvement in constitutive albumin endocytosis in proximal tubule cells. J Biol Chem. 2004;279:54996–55007. doi: 10.1074/jbc.M411491200. [DOI] [PubMed] [Google Scholar]
- Hryciw DH, Pollock CA, Poronnik P. PKC-alpha-mediated remodeling of the actin cytoskeleton is involved in constitutive albumin uptake by proximal tubule cells. Am J Physiol Renal Physiol. 2005;288:F1227–F1235. doi: 10.1152/ajprenal.00428.2003. [DOI] [PubMed] [Google Scholar]
- Kim SK, Park HJ, Hong HS, Baik EJ, Jung MW, Mook-Jung I. ERK1/2 is an endogenous negative regulator of the gamma-secretase activity. FASEB J. 2006;20:157–159. doi: 10.1096/fj.05-4055fje. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Landman N, Kim TW. Got RIP? Presenilin-dependent intramembrane proteolysis in growth factor receptor signaling. Cytokine Growth Factor Rev. 2004;15:337–351. doi: 10.1016/j.cytogfr.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Lautrette A, Li S, Alili R, Sunnarborg SW, Burtin M, Lee DC, et al. Angiotensin II and EGF receptor cross-talk in chronic kidney diseases: a new therapeutic approach. Nat Med. 2005;11:867–874. doi: 10.1038/nm1275. [DOI] [PubMed] [Google Scholar]
- Leask A. Targeting the jagged/notch pathway: a new treatment for fibrosis? J Cell Commun Signal. 2011;4:197–198. doi: 10.1007/s12079-010-0101-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YJ, Han HJ. Albumin-stimulated DNA synthesis is mediated by Ca2+/PKC as well as EGF receptor-dependent p44/42 MAPK and NF-kappaB signal pathways in renal proximal tubule cells. Am J Physiol Renal Physiol. 2008;294:F534–F541. doi: 10.1152/ajprenal.00408.2007. [DOI] [PubMed] [Google Scholar]
- Li T, Wen H, Brayton C, Das P, Smithson LA, Fauq A, et al. Epidermal growth factor receptor and notch pathways participate in the tumor suppressor function of gamma-secretase. J Biol Chem. 2007;282:32264–32273. doi: 10.1074/jbc.M703649200. [DOI] [PubMed] [Google Scholar]
- Li Y, Cong R, Biemesderfer D. The COOH terminus of megalin regulates gene expression in opossum kidney proximal tubule cells. Am J Physiol Cell Physiol. 2008;295:C529–C537. doi: 10.1152/ajpcell.00037.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Martin N, Slattery C, McMorrow T, Ryan MP. TGF-βi mediates sirolimus and cyclosporine A-induced alterations of barrier function in renal epithelial cells via a non-cannonical ERK1/2 signaling pathway. Am J Physiol Renal Physiol. 2011;301:F1281–F1292. doi: 10.1152/ajprenal.00188.2010. [DOI] [PubMed] [Google Scholar]
- Melenhorst WB, Mulder GM, Xi Q, Hoenderop JG, Kimura K, Eguchi S, et al. Epidermal growth factor receptor signaling in the kidney: key roles in physiology and disease. Hypertension. 2008;52:987–993. doi: 10.1161/HYPERTENSIONAHA.108.113860. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assaus. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Murea M, Park JK, Sharma S, Kato H, Gruenwald A, Niranjan T, et al. Expression of Notch pathway proteins correlates with albuminuria, glomerulosclerosis, and renal function. Kidney Int. 2010;78:514–522. doi: 10.1038/ki.2010.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni CY, Murphy MP, Golde TE, Carpenter G. gamma -Secretase cleavage and nuclear localization of ErbB-4 receptor tyrosine kinase. Science. 2001;294:2179–2181. doi: 10.1126/science.1065412. [DOI] [PubMed] [Google Scholar]
- Nyhan KC, Faherty N, Murray G, Cooey LB, Godson C, Crean JK, et al. Jagged/Notch signalling is required for a subset of TGFbeta1 responses in human kidney epithelial cells. Biochim Biophys Acta. 2010;1803:1386–1395. doi: 10.1016/j.bbamcr.2010.09.001. [DOI] [PubMed] [Google Scholar]
- Rand MD, Grimm LM, Artavanis-Tsakonas S, Patriub V, Blacklow SC, Sklar J, et al. Calcium depletion dissociates and activates heterodimeric notch receptors. Mol Cell Biol. 2000;20:1825–1835. doi: 10.1128/mcb.20.5.1825-1835.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich H, Tritchler D, Herzenberg AM, Kassiri Z, Zhou X, Gao W, et al. Albumin activates ERK via EGF receptor in human renal epithelial cells. J Am Soc Nephrol. 2005;16:1266–1278. doi: 10.1681/ASN.2004030222. [DOI] [PubMed] [Google Scholar]
- Rossing P, Hommel E, Smidt UM, Parving HH. Reduction in albuminuria predicts a beneficial effect on diminishing the progression of human diabetic nephropathy during antihypertensive treatment. Diabetologia. 1994a;37:511–516. doi: 10.1007/s001250050140. [DOI] [PubMed] [Google Scholar]
- Rossing P, Hommel E, Smidt UM, Parving HH. Reduction in albuminuria predicts diminished progression in diabetic nephropathy. Kidney Int Suppl. 1994b;45:S145–S149. [PubMed] [Google Scholar]
- Samson K. NerveCenter: phase III Alzheimer trial halted: search for therapeutic biomarkers continues. Ann Neurol. 2010;68:A9–A12. doi: 10.1002/ana.22249. [DOI] [PubMed] [Google Scholar]
- Schieppati A, Remuzzi G. Proteinuria and its consequences in renal disease. Acta Paediatr Suppl. 2003;92:9–13. doi: 10.1111/j.1651-2227.2003.tb00213.x. discussion 15. [DOI] [PubMed] [Google Scholar]
- Schor NF. What the halted phase III gamma-secretase inhibitor trial may (or may not) be telling us. Ann Neurol. 2011;69:237–239. doi: 10.1002/ana.22365. [DOI] [PubMed] [Google Scholar]
- Shen J, Kelleher RJ., 3rd The presenilin hypothesis of Alzheimer's disease: evidence for a loss-of-function pathogenic mechanism. Proc Natl Acad Sci U S A. 2007;104:403–409. doi: 10.1073/pnas.0608332104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slattery C, Lee A, Zhang Y, Kelly DJ, Thorn P, Nikolic-Paterson DJ, et al. In vivo visualization of albumin degradation in the proximal tubule. Kidney Int. 2008a;74:1480–1486. doi: 10.1038/ki.2008.463. [DOI] [PubMed] [Google Scholar]
- Slattery C, Ryan MP, McMorrow T. Protein kinase C beta overexpression induces fibrotic effects in human proximal tubular epithelial cells. Int J Biochem Cell Biol. 2008b;40:2218–2229. doi: 10.1016/j.biocel.2008.03.005. [DOI] [PubMed] [Google Scholar]
- Slattery C, Jenkin KA, Lee A, Simcocks AC, McAinch AJ, Poronnik P, et al. Na+-H+ exchanger regulatory factor 1 (NHERF1) PDZ scaffold binds an internal binding site in the scavenger receptor megalin. Cell Physiol Biochem. 2011;27:171–178. doi: 10.1159/000325219. [DOI] [PubMed] [Google Scholar]
- Stephan JP, Mao W, Filvaroff E, Cai L, Rabkin R, Pan G. Albumin stimulates the accumulation of extracellular matrix in renal tubular epithelial cells. Am J Nephrol. 2004;24:14–19. doi: 10.1159/000075347. [DOI] [PubMed] [Google Scholar]
- Sumual S, Saad S, Tang O, Yong R, McGinn S, Chen XM, et al. Differential regulation of snail by hypoxia and hyperglycemia in human proximal tubule cells. Int J Biochem Cell Biol. 2010;42:1689–1697. doi: 10.1016/j.biocel.2010.06.023. [DOI] [PubMed] [Google Scholar]
- Wang Y, Rangan GK, Tay YC, Harris DC. Induction of monocyte chemoattractant protein-1 by albumin is mediated by nuclear factor kappaB in proximal tubule cells. J Am Soc Nephrol. 1999;10:1204–1213. doi: 10.1681/ASN.V1061204. [DOI] [PubMed] [Google Scholar]
- Wohlfarth V, Drumm K, Mildenberger S, Freudinger R, Gekle M. Protein uptake disturbs collagen homeostasis in proximal tubule-derived cells. Kidney Int Suppl. 2003;63:S103–S109. doi: 10.1046/j.1523-1755.63.s84.13.x. [DOI] [PubMed] [Google Scholar]
- Wong HK, Sakurai T, Oyama F, Kaneko K, Wada K, Miyazaki H, et al. beta Subunits of voltage-gated sodium channels are novel substrates of beta-site amyloid precursor protein-cleaving enzyme (BACE1) and gamma-secretase. J Biol Chem. 2005;280:23009–23017. doi: 10.1074/jbc.M414648200. [DOI] [PubMed] [Google Scholar]
- Yang T, Arslanova D, Gu Y, Augelli-Szafran C, Xia W. Quantification of gamma-secretase modulation differentiates inhibitor compound selectivity between two substrates Notch and amyloid precursor protein. Mol Brain. 2007;1:15. doi: 10.1186/1756-6606-1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YL, Lin SH, Chuang LY, Guh JY, Liao TN, Lee TC, et al. CD36 is a novel and potential anti-fibrogenic target in albumin-induced renal proximal tubule fibrosis. J Cell Biochem. 2008;101:735–744. doi: 10.1002/jcb.21236. [DOI] [PubMed] [Google Scholar]
- Yu WH, Kumar A, Peterhoff C, Shapiro Kulnane L, Uchiyama Y, Lamb BT, et al. Autophagic vacuoles are enriched in amyloid precursor protein-secretase activities: implications for beta-amyloid peptide over-production and localization in Alzheimer's disease. Int J Biochem Cell Biol. 2004;36:2531–2540. doi: 10.1016/j.biocel.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Zoja C, Donadelli R, Colleoni S, Figliuzzi M, Bonazzola S, Morigi M, et al. Protein overload stimulates RANTES production by proximal tubular cells depending on NF-kappa B activation. Kidney Int. 1998;53:1608–1615. doi: 10.1046/j.1523-1755.1998.00905.x. [DOI] [PubMed] [Google Scholar]
- Zou Z, Chung B, Nguyen T, Mentone S, Thomson B, Biemesderfer D. Linking receptor-mediated endocytosis and cell signaling: evidence for regulated intramembrane proteolysis of megalin in proximal tubule. J Biol Chem. 2004;279:34302–34310. doi: 10.1074/jbc.M405608200. [DOI] [PubMed] [Google Scholar]