Abstract
Early science fiction envisioned the future of drug delivery as targeted micron-scale submarines and ‘Cyborg’ body parts. Here we describe the progression of the field toward technologies that are now beginning to capture aspects of this early vision. Specifically, we focus on the two most prominent types of systems in drug delivery – the intravascular micro/nano drug carriers for delivery to the site of pathology and drug-loaded implantable devices that facilitate release with the pre-defined kinetics or in response to a specific cue. We discuss the unmet clinical needs that inspire these designs, the physiological factors that pose difficult challenges for their realization, and viable technologies that promise robust solutions. We also offer a perspective on where drug delivery may be in the next 50 years based on expected advances in material engineering and in the context of future diagnostics.
Keywords: drug delivery, drug carriers, nanotechnology, controlled release implants, physiological barriers, pharmacokinetics, translational medicine
1. Introduction
A submarine struggles to maneuver through turbulent waters while trying to evade attack in a hostile environment. This vessel must reach its destination; mission failure assures death. An interdisciplinary team joins forces to bring the vessel to its target; the mission succeeds– a life is saved. What might appear to be the synopsis of a typical war film also describes the plot of “Fantastic Voyage”, a science fiction film from 1966. Rather than a deep-sea battlefield, the mission for this particular vessel occurs within the human body. A 100-meter-long submarine is reduced to the size of a red blood cell and deployed within human vasculature on a mission to deliver a cure to its target– a diseased region of the brain (Figure 1).
Figure 1.
An illustration depicting the vision for drug delivery from “Fantastic voyage”, a science fiction movie describing the journey of a miniaturized submarine through circulation to its vascular target within the brain. Image used with permission courtesy of David Morgan-Mar, © 1999 David Morgan-Mar.
Over a century ago, research had begun to link the pharmacological effect of drugs to their concentrations at the site of disease. In 1847, a manuscript on ether anesthesia correlated the depth of narcosis with the content of anesthetic drugs in the brain1. Furthermore, studies conducted in the 1950s and 60s alluded to a relationship between drug distribution in non-target tissues and toxicity. The concept of a micron-scale submarine traversing the circulation to carry drug to its target illustrates an elegant solution that could assure pharmacologically-relevant drug concentrations reach their target site without off-site toxicity.
Early literature also noted that the kinetics of drug presentation in tissue was an important factor in determining the pharmacological effect of the drug. In 1950, publications by De Jong and van Gemert discussed the importance of controlling drug concentrations over time and the challenges in achieving such control through repeated drug administrations2, 3. One early “automated” device that emerged to address this challenge was a continuous long-term injector, invented by Rose in 19554, that used osmotic actuation for continuous and autonomous delivery of a drug. In 1960, Clyne and Kline, inspired by this device, conceived the concept of a “Cyborg”– a human with body parts that are machines5. In their article titled “Cyborgs and Space” they proposed coupling Rose's device with sensing and controlling mechanisms to form an organism-integrated unit. They envisioned this unit to operate autonomously and “leave the man free to explore, to create, to think, and to feel”.
Since the early science fiction visions of Cyborg body parts and micron-scale submarines, the field of drug delivery has expanded tremendously. In 2012, over 45,000 research manuscripts were published on topics related to drug delivery, a publication rate of over 120 papers per day. The field, driven by unmet clinical needs, continues to progress toward clinically viable technological solutions. Here, we aim to describe the progression in the field of drug delivery over the past 50 years with a specific focus on the unmet clinical needs that inspire science fiction ideas, the physiological challenges that introduce technology constraints, and the engineering advances that promise robust solutions. We also offer a perspective on where drug delivery may be in the next 50 years, based on the emerging technologies being developed today.
2. Clinical Needs
Though curiosity and imagination spark science fiction ideas, clinical needs are the inspiration for researchers to develop new therapies to combat disease. The increased understanding of the intricate pathological processes underlying disease reveals the inadequacy of current therapeutic strategies. This understanding also informs a more rational approach to develop the next-generation of drug delivery therapeutics, expand the delivery arsenal, and bring some of the “science fiction” vision into practice. In this section, we will highlight key clinical nuances relevant for drug delivery using several illustrative disease examples that demonstrate unmet clinical needs.
2.1 The “Journey” is Just Beginning
Achieving drug bioavailability at therapeutic concentrations within the target tissues is a pre-requisite for therapeutic efficacy. However, the journey of a drug to the target is rife with challenges and detours, and is constrained by dose-limiting toxicity. The distribution of a drug into diseased target tissue and non-target healthy tissues, along with excretion and metabolism, are governed by its physicochemical properties including molecular weight, pKa, and hydrophilic/lipophilic balance. Many pharmacologically potent compounds are unfortunately insoluble and have unfavorable pharmacokinetics6. One of the most pressing clinical situations where drug performance must be improved is cancer therapy. In the United States, each year over 1.6 million people are diagnosed with malignant solid tumors, accounting for over 580,000 deaths annually7.
The therapeutic efficacy of conventional chemotherapies is limited. These compounds often undergo rapid metabolism and renal clearance, resulting in short plasma residence time and inadequate distribution to the target site. Additionally, a high volume of distribution across healthy tissues increases the likelihood of side-effects resulting from non-specific exposure. Limited mechanistic selectivity of these agents further compounds these issues by narrowing their therapeutic index, thereby preventing dose escalation and reducing efficacy. Common chemotherapeutics such as doxorubicin, which is used for AIDS-related Kaposi's sarcoma, and carmustine (BCNU), which is used as an adjunct therapy for malignant gliomas, illustrate these issues. Doxorubicin is effective in vitro against the Kaposi's sarcoma cells but its effectiveness as an anti-neoplastic in vivo is severely limited by its pharmacokinetics. Following intravenous administration, doxorubicin undergoes a rapid triphasic plasma clearance, each phase with relatively short half-lives (12 minutes, 3.3 hours and ∼30-40 hours)8. This limits the amount of drug passing through the tumor vasculature over time, and thereby the tumor exposure to the drug. In addition, the high volume of distribution, in excess of 20-30 L/kg, indicates a wide distribution of doxorubicin in non-target tissues, which when combined with poor selectivity for neoplastic tissue, results in dose-limiting toxicities that include cardiotoxicity, neutropenia, and myelosupression9, 10. Similarly, systemic administration of carmustine to treat gliomas, the most common brain tumor in adults, has demonstrated limited efficacy due to high toxicity, short half-life, and poor brain tissue penetration11, 12.
Complications in dosing and poor site-specific accumulation are just some of the issues faced in treating cancer patients. Comorbid conditions, such as impaired liver or renal function, can further limit dose tolerance, and thus the efficacy, for chemotherapeutics. Therefore, effective treatment entails a balance between optimizing therapeutic effect while also reducing dose to achieve tolerability and limit side-effects that interfere with patient compliance. Thus, in the battle with cancer there is an urgent clinical need for more effective therapies that can provide longer plasma residence times, improved biodistribution, and broadened therapeutic indices.
2.2 The Dynamics of Disease
The effective treatment of many diseases requires an understanding of specific nuances in dynamics of the disease and the ability to address these dynamics with the appropriate drug presentation kinetics. The temporal profiles of plasma drug concentrations generated with traditional drug administration schedules fail to adequately meet the dynamic therapeutic needs, limiting the efficacy of otherwise potent drugs. Strategies that alter the temporal profile of drug presentation, either autonomously or in response to physiological stimuli, can offer opportunities to mirror physiological dynamics and improve drug efficacy by ensuring a drug is available only when it is needed.
Diabetes, a common disease that affects 8% of Americans13, illustrates the complexities of matching a therapy to disease dynamics. In healthy individuals, an increase in blood glucose levels from a meal triggers insulin release from pancreatic beta cells, which signals glucose uptake by cells. The process is short and defined: the half-life for endogenous insulin is 4-6 minutes, with the liver primarily responsible for insulin clearance14. Along with glucagon signaling, this mechanism provides dynamic regulation of blood glucose levels. In a diabetic state, however, this homeostatic mechanism is impaired by inadequate insulin secretion and/or a diminished cellular response to insulin, which can result in erratic blood glucose levels. This has led to the common therapeutic strategy of treating diabetic patients with regular injections of exogenous insulin. Yet, this strategy ignores the natural fluctuations of glucose levels in the blood, which may cause periods of hypo- and hyper-glycemia, the complications of which can be coma or death. Additionally, in Type 2 Diabetes, chronic variability in fasting glucose levels is an independent predictor of total, cardiovascular, and cancer mortality15. This standard of care also relies on a predefined patient dosing schedule in combination with manual glucose measurements in attempt to recreate the dynamic nature of glucose levels, but even with perfect compliance, this approach is inadequate. To attempt to address these issues with dynamics, exogenous insulins with varying pharmacokinetics have been developed for use alone or in combination. These can include combining regular Insulin (intermediate acting) with special formulations for ultrarapid-acting insulin (Lispro: onset 0.5min, peak 0.5-2min and duration of 3-4 min) which offers the convenience of injecting only minutes before a meal16. Long-acting formulations, such as Glargine and Ultralente, have a 4-6 hour onset and last for 18-24 hours, thus offering the ability to stabilize basal levels over a longer time16. However, as each patient is unique with different nutritional requirements and dietary intake, complicated combined regimens consisting of formulations of different modified insulins are often necessary. There exists a need to develop integrated, dynamic drug systems capable of stabilizing glucose levels and adapting to changes in the immediate environment, all while minimizing stringent patient compliance constraints.
Other diseases, such as osteoporosis, may also benefit from temporal modulation of drug therapy. Osteoporosis, affecting over 200 million people worldwide, is characterized by low bone mass and structural deterioration of the normal bony architecture17. Parathyroid hormone (PTH) has shown efficacy for the treatment of osteoporosis in humans, demonstrating improvements in vertebral bone-mineral density, reduction in vertebral and non-vertebral bone fractures, and increase in total-body bone density18, 19. Interestingly, the anabolic effects of PTH (human analogues marketed as Preotact and Teriparatide) only persist if given intermittently and at a low dose, whereas continuous administration reduces bone density20. This is thought to be due to an “anabolic window”, a period of time when PTH is able to act maximally as an anabolic agent, prior to initiating bone resorption21, 22. Intermittent, low dose administration of PTH can leverage this anabolic window without causing excessive resorption, thus promoting net increases in bone mass23. Therefore, drug dosing kinetics and precise administration schedules are crucial to the effective use of PTH analogues in osteoporosis. Though treatment with the PTH analogue Teriparatide has shown safety and efficacy in reducing fractures24, the daily subcutaneous injections required for efficacy is a patient-reported factor for early discontinuation of therapy25. Thus, there exists a desperate need for low-dose pulsatile delivery to improve patient compliance and assure the appropriate kinetics for optimal therapeutic effectiveness.
3. Physiological Constraints
The unmet clinical needs that arise from inadequacies in standard drug therapies inspire efforts toward biomaterial-based ‘submarine’ and ‘cyborg’ drug delivery carriers. Such carriers are expected to encapsulate drug cargo, prevent rapid elimination and degradation, deliver the drug to the site of pathology, and facilitate the desired release kinetics, thereby overcoming limitations in standard drug administration. However, success in these tasks is at odds with normal human physiology which, through millennia of evolution, has developed efficient defense mechanisms against pathogens and harmful xenobiotics. These mechanisms collectively create a hostile environment that can be antithetical to the goals of drug delivery. In this section, we will highlight the physiological and pathophysiological factors that present challenges and opportunities in realizing the science fiction vision for functional drug carriers.
3.1 Physiological Barriers on the Journey
Following intravascular administration, a ‘submarine’-type drug carrier distributes through systemic circulation, encountering a number of obstacles that threaten its ability to complete its mission. Mechanical forces in the blood are the first such obstacle, as flow-induced shear stresses can deform or fragment the material26, thereby limiting its ability to encapsulate therapeutic cargo and altering its interaction with the physiological milieu. Flow though narrow microvascular capillaries, such as pulmonary capillaries, can impose up to 100% strain on micron-scale drug carriers as they pass through conduits with diameter narrower than their size27. Some cardiovascular pathologies, such as severe arterial stenosis and aortic valve disease28, 29 can induce turbulent flow that promotes particle collisions and agglomeration30, 31 and further elevates destabilizing local shear stresses32.
A myriad of blood components, such as plasma proteins, lipoproteins, and cells, initiate an attack on the circulating particle. The interaction of lipid-based carriers such as liposomes with endogenous lipid-transport vesicles including chylomicrons and high density lipoproteins, can promote destabilizing lipid exchange and pre-mature release of the encapsulated material33. The “foreign” surface of a carrier triggers adsorption of highly abundant plasma proteins and protein-opsonins, including albumin, immunoglobulins, and complement activation products34. The adsorption of these proteins can cause carrier destabilization and aggregation, serve to flag the particle for recognition and sequestration by phagocytic cells like macrophages, and bias its distribution to off-target locations as a result of uptake in the liver and spleen35.
The role played by the mononuclear phagocyte system, which has evolved to engulf invading pathogens and dying cells, is especially important to the design of particle carriers. The major components of this system– circulating macrophages and tissue-resident phagocytic cells (e.g Kupfer cells of the liver and fixed spleen macrophages) – efficiently phagocytose these “foreign” particles, thereby reducing their circulation time and creating a barrier for the vehicle to access the target tissue. Macrophages, nevertheless, efficiently discriminate between “foreign” and “self”, allowing long-term circulation of intact endogenous cells. Recent studies suggest that surface decoration with self-identifiers, such as CD47 and its analogs, can inhibit macrophage uptake of circulating particles, a finding which may open new avenues for engineering phagocytosis-evading drug carriers36.
In addition to phagocytosis, a drug carrier can also be removed from circulation by eliminating organs through filtration or mechanical entrapment. The microvasculature in the kidney, liver, and spleen is tailored for this purpose. In the kidney, nanoparticles smaller than 5-6 nm in diameter can pass through the fenestrated glomerular endothelium for excretion in urine37. In the liver, nanoparticles up to 100 nm in diameter, including endogenous particles like chylomicrons and lipoproteins, can extravasate and escape into the liver parenchyma through the fenestrated sinusoid endothelium38. The vasculature of the red pulp cords in the spleen is efficient in removing aging stiff erythrocytes, and can also mechanically capture large (>200 nm) and rigid drug vehicles39, 40.
Although many physiological processes can prevent a drug carrier from having a successful mission in the body, some physiological and pathological situations could actually present therapeutic opportunities. One such opportunity is in leveraging the differential selectivity of the microvascular barrier. The blood-tissue interface for most tissues that do not specialize in elimination, including muscle, lung, fat, brain, and connective tissue, is lined with a continuous endothelium that creates a stringent barrier to paracellular transport. While such permselectivity could exclude a drug vehicle from access to target parenchyma, it also limits the off-site toxicity for carrier-based delivery approaches compared to a free drug that can cross this barrier. For example, in contrast to the free doxorubicin, a liposomal formulation is unable to sieve through the endothelial wall of healthy cardiac microvasculature, alleviating the dose-limiting cardiotoxicity that limits use of the free drug41, 42. In addition, some pathological conditions can alter the local microvascular permselectivity, which presents an opportunity for disease-selective drug delivery. For example, the neo-vasculature in solid tumors exhibits fenestrations ranging from 200 to 12,000 nm in size43, and this hyperpermeability in combination with other tumor-associated fluid transport abnormalities, such as impaired lymphatic drainage, gives rise to the enhanced permeability and retention (EPR) phenomenon44. Through EPR, drug carriers that would normally be excluded from tissue parenchyma can selectively accumulate at the tumor site, thereby improving target drug delivery while shielding healthy tissues from drug toxicity.
The range of the aforementioned deleterious and beneficial interactions of drug carriers with the physiological milieu is largely dependent on the vehicle structural (e.g. size, shape, surface curvature, modulus) and surface (e.g. charge, hydrophobicity) properties45. Variation of these parameters creates a vast design space that can be leveraged to engineer physiologically-robust drug carriers in the overarching pursuit of more efficacious and less toxic drug therapies.
3.2 Challenges in Mirroring Physiological Dynamics
Engineering drug presentation kinetics using implantable drug-loaded constructs introduces new physiological challenges that are not encountered for intravascular drug delivery. Once a drug is released from an implant, it must traverse a number of physiological barriers in order to access the systemic circulation compartment or its local site of action. While devices can be engineered to control the drug release rate, the design of implantable strategies should be based on an understanding that bioavailability of a drug released from an implant is governed by its transport through physiological tissue barriers specific to the implant site. Among the factors to consider are the permeability of the membrane, interstitial fluid dynamics, charge of extracellular matrix proteins, and the presence of extracellular enzymes that could degrade the drug.
Drug delivering constructs are commonly implanted in subcutaneous, intramuscular, and intraperitoneal sites. Once the drug is delivered within the interstitial space at these sites, it can reach systemic circulation via local vasculature or lymphatics. Small drugs (<1kDa) are preferentially absorbed by blood capillaries as they are typically unrestricted by vascular permeability, and the rate of filtration and reabsorption of vascular membranes is roughly tenfold higher than that for the lymphatic system46. However, larger drugs or macromolecules are restricted by the limited permeability of the vascular endothelium, but can leverage the net fluid flux from capillaries through the interstitium and into the draining lymphatics; thus initial absorption of larger drugs and macromolecules by lymphatics is the precursor to their entrance into vascular circulation47. Due to the low fluid flow rates for lymphatic circulation, this route results in delayed systemic bioavailability. Electrostatic interactions with negatively charged glycosaminoglycans or interactions with interstitial proteins can also slow the rate of lymphatic absorption. These barriers must be considered when developing materials for drug delivery, and in order for release kinetics from a material in vitro to be predictive of systemic input kinetics in vivo these specific challenges at the implantation site must be overcome. In some circumstances, such as localized chemotherapy at the site of tumor resection or growth factor delivery to promote wound healing, it may be desirable for the drug to be primarily active near the site of implantation instead of becoming systemically available. In this case, slow absorption could be leveraged in order to achieve higher drug concentration near the site of implant.
Other devices have been designed to deliver drugs to tissue sites with mucosal membrane barriers, such as intestinal, nasal, oral, buccal, vaginal, or rectal routes. These membranes present different obstacles, as they are formed by a monolayer of epithelial cells held together through tight junctions and there is no active vascular or lymphatic exchange in tissue sites. The permeability of a mucosal membrane varies by tissue site, but generally compounds in excess of 700 Da cannot passively move through these membranes48. Most often, compounds including small molecules, peptides, and antibiotics traverse the mucosal membrane through carrier-mediated mechanisms. The negative charge of these mucosal membranes also introduces permselectivity based on charge, with the transport ratios for positive molecules compared to negative in the range of 1.33 to 1.78, depending on tissue site49.
Insulin therapy is a good example that illustrates the tissue site-specific differences in absorption. It is known that insulin serum levels peak at 2 minutes following intravenous administration, while for intraperitoneal, intramuscular, and subcutaneous delivery this occurs at 50, 60, and 90 minutes following administration, respectively50. Subcutaneous delivery is the preferred route of insulin administration, but delayed absorption via this approach results in a depot effect, meaning that insulin remains concentrated at the injection site for several hours following administration. This prolonged local retention results in enzymatic degradation of approximately 20% of the insulin at the injection site, thus reducing the amount of total dose that becomes bioavailable51. Therefore, degradation at the implantation site, specifically for protein drugs such as insulin, introduces additional dosing considerations for device design.
Implanted devices that are intended to function long-term must also contend with physiological constraints that arise at the material-tissue interface52. Termed the biocompatibility of the implant, the natural foreign body response of the host immune system to a material can be of great detriment to proper device function. The response to implanted materials usually begins with protein adsorption on the material surface, while cytokine cascades resulting from injury during implantation recruit neutrophils and macrophages to the material. These immune cells participate in degradation of the material through secreted enzymes and phagocytosis. This response can promote the formation of a thick fibrotic capsule at the surface of the implant, which can impose additional transport limitations to the diffusion and absorption of released drugs or macromolecules.
4. Engineered Drug Delivery Solutions
A broad arsenal of materials has been engineered in order to overcome physiological barriers and ensure that drugs reach their specific tissue targets and are presented with appropriate kinetics. These efforts point to a number of design criteria that could be exploited in the development of new solutions to address urgent clinical needs. In addition, the clinical successes exhibited by many of these early strategies serve as both validation and motivation for further development of material-based approaches to improve drug therapy.
4.1 Engineering Vehicles for the Journey to the Target
The broad design space for new materials, especially in emerging areas of nanotechnology, has been exploited to develop carriers for systemic administration and preferential accumulation at the disease site45. As discussed, the process of traversing the circulatory system and reaching a target is rife with challenges. A vast number of design strategies have been developed in an attempt to navigate the physiologic terrain and deliver a drug to its target. A particle-based approach enables multifaceted therapies, as drugs can be combined within a particle that is passively or actively targeted. Particles with dimensions ranging across three orders of magnitude have been used, with a lower end of 6-7 nm necessary to escape glomerular filtration in the kidney and an upper end of 3-5 μm restricted by the diameter of a microcapillary. The use of a particle carrier for a drug should ideally extend its circulation half-life and prevent off-target accumulation to maximize the effective dose and avoid clinical side-effects. Microparticles engineered with mechanical properties and deformability matching those of a red blood cell have demonstrated enhanced circulation times27.
On the nanoscale, deformability and mechanical properties are less crucial, but it is critical that particles do not aggregate, are able to resist protein adsorption, and avoid phagocytic uptake. Thus, the surface chemistry of a nanoscale drug carrier is especially important to its success, an understanding highlighted by work in the area of cationic liposomes for gene delivery. Though these materials proved to be extremely efficient gene delivery vectors in vitro, they were found to aggregate and cause embolism in the lung microvasculature when injected intravenously, indicating a need to control the surface chemistry to prevent aggregation53. Hydrophilic chemistries, especially those with zero or net-zero charge, have demonstrated benefits in preparing particles with a high level of surface hydration, a scenario that is entropically favorable to avoid protein adsorption, opsonization, and aggregation which are precursors to particle uptake and removal by macrophages54. Polyethylene glycol (PEG) is the most frequently used of these so-called “stealth” coatings. The chemical structure of repeat ethylene glycol monomers enables formation of hydrogen bonds between the polymer and the nearby water molecules in a way that resembles the normal tetrahedral coordination between water molecules55. This interaction with the bulk solvent is entropically favorable to protein adsorption. Recent work has also demonstrated the utility of cloaking a nanoparticle in self-peptide antigens to evade uptake by the immune system36. Nanoparticles also demonstrated benefits in passive targeting, as particles of 70-200 nm in diameter readily accumulate in tumors through the enhanced permeation and retention effect56. Nanoparticle shape and design is another feature that can be used to increase its circulation half-life and facilitate improved accumulation at the target57. These particles can also be engineered to interact with target cells once they have escaped circulation, with designs that can promote cell uptake, disrupting the structure of the cell membrane, or escape the endosome58. Here we will highlight examples of nanoparticles that begin to bring the science fiction vision of a miniature submarine to life, and have been engineered to traverse the difficult physiologic landscape and reach their therapeutic target.
One of the first examples of a nanoparticle drug carrier to be clinically implemented was DOXIL®, a PEGylated liposomal carrier of crystalline doxorubicin59, 60. Doxil has been applied primarily for the treatment of solid tumors. The size of the carrier, approximately 100 nm in diameter, is appropriate for preferential accumulation in tumors by EPR, and its functionalization with PEG improves particle stability and protects against aggregation, protein adsorption and cell uptake. This particle's design resulted in a dramatic reduction in clearance of over 250-fold for liposome-encapsulated doxorubicin compared to that of the free drug61, enabling a 5-11 fold increase in drug concentration in the tumor tissue of a Kaposi's Sarcoma (KS) in Phase I trials62. Additionally, the sequestration of the doxorubicin within the PEGylated particle resulted in a 60-fold reduction in the volume of distribution61, including reduced drug distribution in cardiac tissue, and consequently decreased the rate of cardiovascular toxicity by over 3 fold compared to the free drug63. When compared to conventional treatment (ABV; doxorubicin, bleomycin and vincristine), the response rate for Doxil was significantly improved, as were the number of adverse side-effects, which in turn resulted in improved patient compliance and quality of life. With the many demonstrated improvements over the free drug, DOXIL® has had a huge clinical impact and has become the standard-bearer for nanoparticle cancer therapeutics.
Nanoparticle therapies that are sensitive to changes in blood flow as a result of thrombosis or embolisms have also been developed32. Biodegradable poly(lactic-co-glycolic acid) (PLGA, 50:50, 17 kDa) nanoparticles were engineered to adhere together through hydrophobic interactions to form platelet-sized clusters and then disaggregate in response to elevated shear stress as a result of blood vessel narrowing by thrombotic blockage. The disaggregated nanoparticles then stick to the endothelial lining of the vessel due to their hydrophobic character. These particles were used to deliver plasminogen to the site of a blood clot in a mouse mesenteric injury model. This shear-activated delivery was found to significantly prolong survival following injury and promote clot dissolution. Additionally this strategy minimizes dose as well as off-target effects common for systemic anticoagulant delivery.
An emerging area in therapeutic nanotechnology involves the development of platforms that can respond to external stimuli in order to deliver a localized function. Gold nanoparticles have demonstrated potential in photo-thermal applications, as they undergo a plasmon resonance when irradiated, which could be used for localized ablation of tumor tissue64, 65. Magnetically-responsive nanoparticles have been engineered to carry functional proteins into brain tumors that were subjected to magnetic flux gradients66. Polymeric perfluorocarbon micellar nanoparticles and microbubbles have been used along with ultrasound stimulation to facilitate the transport of drugs and macromolecules through vascular and cellular membranes in tumors67, 68, and the release of localized thrombolytic agents at the site of blood clots69. In all cases, the responsiveness of these drug carriers to external stimuli (light, ultrasound, magnetic field, etc.) allows for site-specific control over their localization and activity. Moreover, the visibility of these carriers to clinical imaging modalities (e.g. CT, MRI, and ultrasound) offers the attractive potential of image-guided drug delivery.
4.2 Engineering Dynamic Cyborg Materials
A half-century of research has established that implanted “cyborg” biomaterials could be used to provide controlled, long-term release of drugs and proteins70-72. Both the chemical and material properties of these systems can be engineered to control parameters such as degradability, cross-linking, and swelling in order to provide fine control over the kinetics, duration, and location of drug and protein release73. New “smart” biomaterials have also incorporated biological sensing, demonstrating the ability to respond to cues in the physiologic environment such as temperature, pH, or the presence of enzymes or biomarkers74, 75. As such, a broad engineering toolbox has been established over the past 5+ decades that has enabled the creation of materials that can release drugs at a predetermined rate or in response to a specific cue. Here, we will highlight some promising examples of “cyborg-like” biomaterials that couple science fiction vision with innovative engineering to overcome physiological challenges and meet demonstrated clinical needs.
Materials could be used to localize the release of chemotherapeutic agents in order to reduce the dose of a drug, decrease systemic side-effects, and increase its site-specific tissue absorption. One important example is the use of polymeric drug depots to localize the release of chemotherapeutics for malignant gliomas76. Due to the location in the brain and the invasiveness of primary gliomas, complete resection is often not possible. As an alternative to systemic chemotherapy, millimeter-scale disks prepared from biodegradable polyanhydrides formulated with BCNU (carmustine), the Gliadel© wafer, have been developed for localized chemotherapy release at the site of primary tumor resection11, 77, 78. These wafers degrade slowly via surface erosion, allowing them to release encapsulated BCNU at a near-constant rate for approximately 3 weeks directly to the site of primary tumor resection. In a multi-center phase III clinical trial with 240 patients, the Gliadel® wafer significantly prolonged life for treated patients by over 2 months compared to a placebo wafer, with both groups receiving standard radiation therapy.
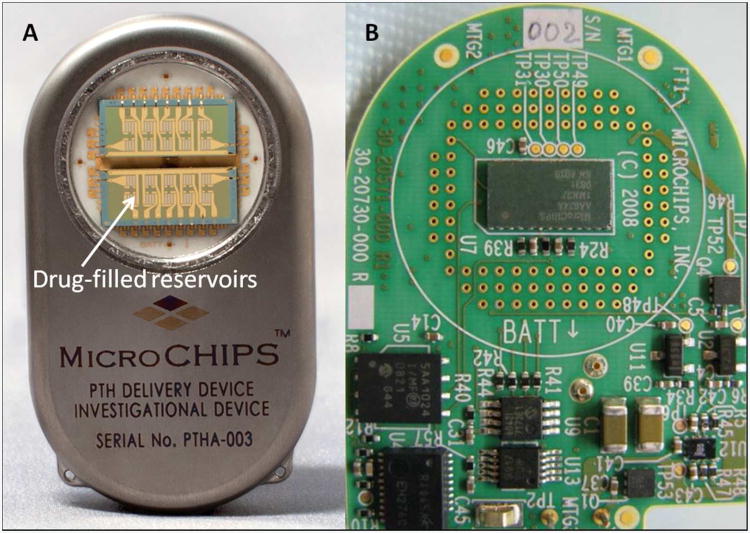
The controlled delivery of low-dose hormones has broad implications in the treatment of many diseases and disorders, including osteoporosis, infertility, or growth hormone deficiency. Best outcomes for many of these therapies are obtained from microgram quantities of hormones being regularly delivered with pulsatile pharmacokinetics. A creative and science fiction themed materials approach to address these challenges has examined microchip-based tunable and intermittent burst release of therapeutic compounds. Early generations of these devices used a microwell array sealed by membranes of different molecular weight PLGA polymers79. As PLGA degradation rate is dictated by molecular weight, the use of polymers with four different molecular weights to seal wells of a single device enabled four separate intermittent burst release events of therapeutic compounds contained within the microwells over the course of several weeks. A next-generation approach evaluated an implantable wirelessly controlled microchip comprised of an array of individual drug-filled reservoirs sealed with a thin metallic membrane of either gold or layers of titanium and platinum80, 81. These seals could be individually disintegrated by electrothermal ablation in response to a threshold level of an applied electric current. The electrothermal activation allowed an individual reservoir to be opened within microseconds, resulting in controlled pulsatile drug release. The fully assembled device included a drug filled array, microprocessor, implantable battery, and wireless communication components in a titanium housing (Figure 2). This device demonstrated utility in the pulsatile delivery of PTH (specifically the recombinant variant teriparatide) for 20 consecutive days when implanted subcutaneously in a clinical trial of eight osteoporotic post-menopausal women. This type of finely controlled drug delivery could combat issues with patient compliance and enable therapy to be delivered with optimal kinetics. Additionally, the wireless control makes possible post-implant modifications to dosage and dosing schedule in response to clinical indications.
Figure 2.
An example demonstrating technological realization of science fiction vision. The wirelessly controlled microchip, comprised of drug reservoirs side-by-side with control and communication electronics, can be used for pulsatile drug delivery. (A) The fully assembled implantable device containing an array of electrothermally activated drug-filled reservoirs, and (B) The printed electronic circuit board within the device with control and wireless communication components. Images courtesy of Dr. Robert Farra, MicroCHIPS. Inc.
Efforts to make a “synthetic pancreas” for the treatment of diabetes highlight work in the area of physiologically responsive drug delivery82. This concept seeks to use glucose as a trigger for insulin release from a material depot, thus replacing or supplementing the endocrine function of pancreatic beta cells. Early efforts in this field lacked a glucose-responsive element, relying on prolonged low-dose release of insulin from polymer scaffolds. Though this basic approach was successful in maintaining prolonged normoglycemia in a diabetic rat model for at least 100 days83, glucose-triggered insulin release is a more natural strategy to address the complex challenges of mirroring endogenous insulin dynamics in order to achieve glycemic management in diabetic patients. To incorporate glucose sensing in material design, one strategy has relied on inclusion of the glucose oxidase (GO×) enzyme within the material, which catalyzes the conversion of glucose to gluconic acid accompanied by a concomitant drop in pH. Early strategies for passive release of insulin from polymer matrices were improved by GO× incorporation, as the glucose-triggered drop in pH increased insulin solubility and release from the material84. This strategy could alternatively be used to prompt a structural or conformational change in the materials by inducing swelling or degradation85. Recently, a nanoparticle network prepared from acid-degradable dextran encapsulating insulin and GO× was shown to allow glucose-mediated insulin release and demonstrated the ability to maintain normoglycemia in a diabetic mouse model for 10 days following subcutaneous administration86. In spite of extensive efforts to design a “synthetic pancreas” using the cyborg-like approach of polymeric materials, the complicated task of mirroring the body's natural insulin dynamics for a prolonged time remains a considerable challenge.
5. Prospects for a New Age in Drug Delivery
Although progress has been made in translating drug delivery to the clinic, the battle against disease remains in need of technologies with improved sophistication. In this section, we highlight developing technologies, and project how these advances may shape the future of drug delivery and contribute to increased realization of science fiction ideas in therapy. Through examples of promising new developments, we hope to provide a vision for the future technological implementation of the “submarine” and the “cyborg” concepts in drug delivery over the next 50 years.
5.1 The Miniature Submarine– The Next Generation
The idea of shrinking a 100-meter-long submarine to the micron scale remains as exciting today as it was in movies a half-a-century ago. Unfortunately, the poor scalability of such a process would severely limit its clinical translation. Moreover, inter-subject variability in pharmacokinetics, pharmacodynamics, physiology, and pathology would necessitate submarine personalization to address both the patient and disease-specific needs. This entails a customizable approach that ensures the submarine is personalized for chemical properties, morphological details, shape, and mechanical properties. Also, the time required to build an actual 8,000-ton Navy submarine is approximately 5 years, and disease progression often follows much more rapid kinetics. For example, patients diagnosed with non-resectable brain tumors live an average of 1-2 years following diagnosis, an insufficient timeframe to construct the personalized submarine that may save their life. Thus, the future of the “submarine” in drug delivery would certainly require dramatically accelerated production technologies.
One potential approach to address production issues could be to print tiny patient-specific submarines of pre-defined shape and surface. Technologies that can generate biocompatible micron and sub-micron particles with precise control over shape and some control over surface topography are already in use. One example is a refined soft-lithography process known as PRINT87, which utilizes fluorinated molds and non-wetting substrates to polymerize a liquid precursor within the mold, producing particles with precise control over features and sub-50nm resolution. Micron and sub-micron particles shaped as trapezoids, hexnuts, boomerangs and arrows have been PRINT-fabricated from a range of biocompatible materials including poly(D-lactic) acid (PLA), poly(ethylene glycol) (PEG) and proteins87, 88.
Another emerging technology that could allow 3D printing of free-standing “submarines” is two-photon lithography89, 90. This technology is capable of voxel-by-voxel additive construction of arbitrary 3D microstructures without requiring pre-manufactured molds. The technique is performed using ultrafast lasers with photosensitive laser-transparent materials. Using the two-photon excitation mechanism, the energy transfer from the laser to the material is spatially confined to the vicinity of the laser focus. Thus, a photo-reaction in the material, often polymerization, can be confined to a very small volume (voxel) using laser focusing. Therefore, it is possible to control material properties voxel-by-voxel by moving the laser focus in a pre-defined pattern throughout the volume of the material. Yang et al. successfully pushed the limits of this technology in fabricating a 20 μm replica of Rodin's famous sculpture, “The Thinker”91, a remarkable 93,000-fold reduction in size compared to the 186 cm tall original. The impressive detail in the micro-replica includes nanoscale resolution of features such as muscles, feet and toes.
In the future, “submarines” could also be 3D-printed using computer-assisted design (CAD) models of patient-specific cells, creating a personalized high fidelity replica of cell shape and surface morphology. This could enable techniques to disguise the submarine in circulation, thereby protecting it from rapid reticulo-endothelial clearance. Camouflaging PLGA nanoparticles with the membranes of red blood cells (RBC) has been reported to extend the elimination half-life of the vehicles in mice 2.5-fold compared to state-of-the-art PEG-coatings92. The printing process could also allow flexibility in material selection to fine-tune the mechanical properties of the submarine. For example, controlling deformability to match that of a native RBC was shown to significantly extend the circulation time of cross-linked polymeric hydrogel microparticles fabricated in the characteristic RBC shape of biconcave disks27.
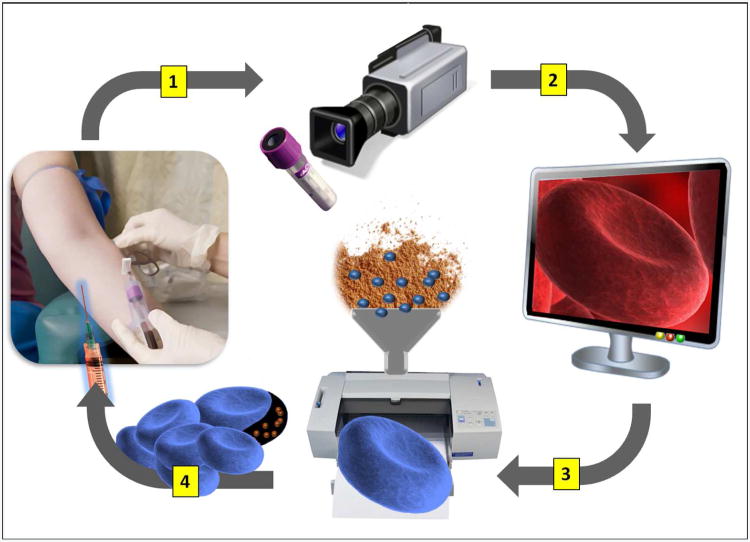
To develop materials that resemble patient-specific cells, high-resolution 3D imaging modalities will be required. Several emerging techniques demonstrate potential for high-fidelity 3D mapping of human cells93,94,95. For example, the 3D structure of a human RBC has been reconstructed using scanning transmission electron tomography at a 5-10 nm spatial resolution from a series of four consecutive 1 μm cell sections95. In addition, the 3D reconstruction of an intact mouse cell in its physiologically hydrated state has been achieved using soft X-ray tomography93. In the future, these advances in imaging techniques could enable 3D-scanning of a patient's cells following a routine diagnostic blood draw (Figure 3). The 3D-models from these cells could be reconstructed and then stored as part of a patient's personal medical records. Therefore, should a need arise for a personalized drug-loaded submarine, the 3D structural model of the patient's cells could literally just be sent to a printer.
Figure 3.
A schematic depicting the possible fabrication process for the next-generation ‘submarine’ drug-delivery vehicles that mimic the structure of patient-specific cells. (1) A blood sample collected as part of a routine diagnostic blood draw is analyzed by a high-resolution 3D imaging modality. (2) A 3D-model of the patient's specific cells are reconstructed from the imaging data and stored electronically as a part of patient's personal medical records. (3) If needed, the 3D computer model can be used to print high-fidelity biocompatible drug-loaded polymer replicas of a patient's cells that preserve cell shape, morphology, and modulus. (4) The ‘submarine particle, disguised to resemble ‘self’ can be administered to treat the patient
In the future, personalized “submarine” drug delivery could be designed to address a wide range of clinical needs. One exciting future area can be envisioned in the context of advanced diagnostics and patient profiling. Rapid progress in molecular profiling to determine disease susceptibility promises to shift the emphasis of future clinical care from the treatment of existing disease to preventative medicine. Significant efforts have been made to characterize the normal human genome, epigenome, transcriptome, proteome, and metabolome, as well as the accompanying disease variants96-98. This work is expected to allow for a predictive assessment of an individual's disease risk and also to help identify reliable pre-pathological biomarkers of the early processes that initiate a transformation from normal physiological functions to disease. For example, a sudden overexpression of an oncogene known to be responsible for a hereditary cancer could be potentially reflected by a specific metabolite - a biomarker of an early shift toward pathology. Databases containing this biomarker information as well as the options for pharmacological intervention to reverse the biomarker-mapped progression to pathology will become readily available to clinicians.
In this context, patient-specific “submarines” could be designed as latent preventative therapies that passively reside in circulation for prolonged periods of time and initiate drug release upon specific biomarker recognition. This “sleeping beauty” paradigm, combining long-term surveillance in circulation with on-demand activated drug delivery, could be realized in a biomaterial format based on combination of shape-shifting polymers, molecular switches and bio-recognition elements. Polymeric microparticles capable of stimuli-responsive shape reconfiguration have already been described99. Specific patterns of shape reconfiguration could be designed to initiate drug release and tune release kinetics to levels of the bioanalyte. Patients at high risk for disease will be able to periodically self-administer the latent submarines. A submarine engineered to be a high-fidelity replica of the patient RBCs could follow normal RBC clearance kinetics and survive in circulation for months, thereby offering a reasonable re-administration schedule.
5.2 The Cyborg– The Next Generation
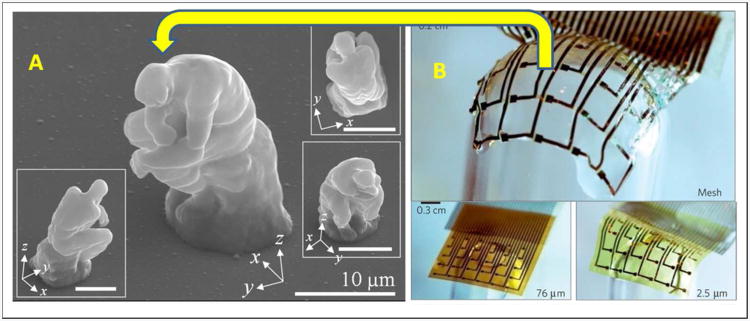
The Cyborg, a man with body parts that are machines, is a long-held vision that has provided inspiration for the development of new technologies that could be broadly useful in drug delivery to address a variety of unmet clinical needs. The applications could range from improving diabetes management with an artificial pancreas100 to manipulating the human mind and treating mental illnesses using voltage-sensitive and neurotransmitter-releasing artificial synapses101. The tremendous progress made in engineering materials for drug-delivering implants supports the vision that soon we could develop free-standing micro-devices with precise nanoscale features and defined 3D architecture, morphology, and mechanical properties. The first step in this direction is illustrated by the micro-replica of the Thinker91. The next creative challenge is outfitting this micro-Thinker with the ability to “think” (Figure 4).
Figure 4.
Biocompliant and bioresorbable electronics could be incorporated into micro-scale drug delivery devices to create future ‘Cyborg’ devices for drug delivery, outfitting the micro-Thinker with the ability to ‘think’. (A) SEM images from the microscale replica of the Rodin's sculpture, ‘The Thinker’, fabricated by two-photon lithography. Image courtesy of Dr. Dong-Yol Yang, KAIST and reprinted with permission from Dong-Yol Yang, Sang Hu Park, Tae Woo Lim, Hong-Jin Kong, et al. Applied Physics Letters, 90(1), 013113 (2007), © 2007, AIP Publishing LLC. (B) A flexible ultra-thin electrode array conformally deposited onto a glass hemisphere using a bioresorbable silk fibroin substrate. The image is reprinted by permission from Macmillan Publishers Ltd.: Dae-Hyeong Kim and Jonathan Viventi et al, Nature Materials, 9, 511-517, 2010, © 2010.
The process of “thinking” entails a sequence of actions that includes signal detection, signal processing, decision making, and response actuation, ideally in real-time. This sequence of functions is realistically attainable using modern System-on-Chip integrated circuits, which presently constitute the “thinking mechanism” for a wide variety of consumer electronics such as smart phones and digital cameras102, 103. Transformation of drug delivery devices into “thinking” units could, therefore, rely on incorporation of integrated circuits– a vision already prototyped in the remotely-controlled drug-releasing microchip by combining drug-containing reservoirs with control and communication components104.
The next generation of “thinking” microchip-based drug delivery devices could be constructed from bioresorbable and biocompliant electronic components. The field of biomaterials-based electronics is rapidly expanding105, 106, and bio-derived and bioresorbable materials have shown promise to capture the essential functionalities of a traditional integrated circuit in a biomaterial format. For example, silk, a naturally occurring biodegradable polypeptide, has been used as a substitute for silicon substrates to fabricate an electronic sensor array107. DNA was used to replace silicon dioxide as a gate dielectric in field effect transistors108. Chitosan, a biopolymer derived from shrimp, was shown to function as a proton conductor in transistors, a role traditionally performed by ceramic materials109. Therefore, it could be envisioned that soon a fully functional microelectronic “Cyborg” unit could be fabricated entirely from biological components.
Perhaps the biggest challenge in realizing the “Cyborg” vision lies in engineering the biotic-abiotic interface. Preserving rapid material-tissue communication at the interface is a key to achieving autonomous device function. Yet, at present device implantation elicits a foreign body response that often include macrophage-mediated formation of a fibrotic capsule isolating the device110, 111, an outcome antithetical to the concept of interfacial integration. Future work to mitigate this response could be assisted by efforts to miniaturize drug delivery devices106. Less invasive tissue incorporation of smaller devices could minimize tissue trauma. In addition, reduction in the implant dimensions has been shown to dramatically reduce both the extent of macrophage immunoreactivity and the thickness of the fibrotic capsule112,113. One could envision that in the future, large all-in-one devices would be replaced with an ensemble of small modular components communicating to perform a task. Each device module could be equipped with biomaterial-based electronics to enable a pre-defined set of functionalities and the tissue-material interface would be engineered to evade immune defenses with a combination of small size, appropriate surface chemistry, low modulus, and optimized architecture.
In the future, tissue-invisible communicating micro-modules could be designed to function as diagnostic sensors and drug releasing actuators. A key challenge to developing autonomously-functioning ‘Cyborg’ materials is to establish a module-to-module communication that would be able to mirror the endogenous dynamics of endocrine communication between tissues. This design is compounded by high inter-individual variability and would require personalization. Personalized communication could potentially be achieved by incorporating mechanisms of machine learning, analogous to those currently pursued in the fields of advanced neuro-prosthetics, rehabilitation robotics and modular neuronal assemblies114,115,116. As one possible scenario, an array of sensing micro-modules would collect patient-specific physiological details such as endocrine dynamics, biomarker-reflected tissue responsiveness, metabolic kinetics, and even relevant behavioral traits. This information would be wirelessly transmitted and analyzed by an external CPU, and the generated outputs fed back to the micro-actuating implant, allowing the actuator biomaterial to “learn” and “adapt” to the patient through adjustments in the many device variables such as shape, morphology and surface attributes. These adjustments would in turn serve to “interpret” inputs from the sensors in a patient-specific manner and tailor drug delivery to the individualized needs.
The potential value of this paradigm can be illustrated in the context of glycemic management in diabetes. In the current standard of glycemic management, schedules of insulin dosing are designed based on a single parameter –blood glucose readings. The ability of this approach to mirror physiological glucose dynamics is, however, limited. Recent advances in continuous glucose monitoring have made it possible to alert a patient to peaks or troughs in glucose levels in-real-time. Nevertheless, even with the continuous glucose monitoring, hypo-and hyper-glycemia episodes cannot be foreseen and prevented, leading to potentially dangerous side-effects117. In this context, systems that could predict and anticipate the magnitude and trend of change in blood glucose would be a major benefit to diabetes patients. The human body has evolved mechanisms for anticipating abrupt changes in glucose levels and adequately preparing for them. For example, in a healthy individual, insulin secretion by the pancreas begins prior to a meal, and therefore, well ahead of the meal-reflecting glucose peak in plasma. This secretion is mediated by a Pavlovian response to cues such as the sight and smell of food as well as a preconditioned eating schedule118. Future “Cyborg” biomaterial systems for glycemic management could be designed to similarly anticipate and prepare for glucose spikes and troughs by “learning” and “adapting” to the patient. To realize this concept, sensor micro-modules could be placed in different locations of the body, including the gastrointestinal tract, and engineered to acquire over time not only the measurements of blood glucose but also a range of other patient-specific metrics such as the composition and the glycemic index of meals, the timing of routine meals, levels of plasma insulin, and other relevant plasma and GI factors. This information could be used to characterize the patient-specific ‘impulse response’ of blood glucose-insulin dynamics in the context of other physiological and dietary variables and identify early predictors for the upcoming abrupt changes in blood glucose. These predicting sensors would then wirelessly alert the drug-releasing actuator of the impending rise or decline in blood glucose, allowing sufficient time for the material to self-tune to provide the required levels and kinetics of drug release.
6. Conclusions
Over the last half-century, ideas that had only existed in science fiction have been transformed through extensive research into practical strategies for sophisticated therapies. Many exciting drug delivery technologies are now beginning to realize the concepts of targeted miniature submarines or autonomous cyborg implants. However, the battle against disease is far from won, and the continued technological development of these strategies remains an urgent priority to further improve the efficacy and outcomes for drug therapy. Though it has long been understood that spatiotemporal drug presentation is a critical determinant of efficacy, achieving optimal spatiotemporal presentation within the hostile physiological milieu remains a challenge in need of new and innovative solutions. This challenge is unlikely to be conquered without collaboration and integration between several scientific disciplines. Fifty years ago, ‘Fantastic Voyage’ envisioned a miniature submarine on a mission to deliver a therapy to a vascular target within the brain. This mission was successful through the joint efforts of an interdisciplinary team of scientists, engineers, and clinicians. This interdisciplinary approach remains a crucial component for success in the field of drug delivery today. Close collaboration between scientists in the fields of materials, pharmaceutics, engineering, biology, and medicine is necessary to form a deeper appreciation for the many complicated interfaces between drugs, materials, and the human body in health and disease. This crucial insight will serve as the blueprint in order to transform science fiction therapies into technological realities.
Acknowledgments
Robert Langer thanks the NIH, the NCI and the NSF for their continuing support. Beata Chertok thanks the NIH National Institute of Biomedical Imaging and Bioengineering (grant 1F32EB015835-01) and the MIT-Harvard Cancer Center for Nanotechnology Excellence for postdoctoral support.
References
- 1.Wagner JG. History of pharmacokinetics. Pharmacol Ther. 1981;12(3):537–62. doi: 10.1016/0163-7258(81)90097-8. [DOI] [PubMed] [Google Scholar]
- 2.De Jong SE, Wijans M. The influence of divided doses of drugs on the duration of effect and integral of effect. Acta Physiol Pharmacol Neerl. 1950;1:237–258. [PubMed] [Google Scholar]
- 3.Van Gemert AGM, Duyff JW. Optimal dosage of drugs. Acta Physiol Pharmacol Neerl. 1950;(1):256–278. [PubMed] [Google Scholar]
- 4.Rose S, Nelson JF. A continuous long-term injector. Aust J Exp Biol Med Sci. 1955;33(4):415–9. doi: 10.1038/icb.1955.44. [DOI] [PubMed] [Google Scholar]
- 5.Clynes ME, Kline NS. Cyborgs and Space. Astronautics. 1960:26–27. [Google Scholar]
- 6.Panchagnula R, Thomas NS. Biopharmaceutics and pharmacokinetics in drug research. Int J Pharm. 2000;201(2):131–50. doi: 10.1016/s0378-5173(00)00344-6. [DOI] [PubMed] [Google Scholar]
- 7.Society AC. Cancer Facts and Figures 2013. In: C A, editor. Society. Atlanta, GA,: 2013. [Google Scholar]
- 8.Johnson J. amp. Background Information Regarding Accelerated Approval of DOXIL® in AIDS-Related Kaposi's Sarcoma. Oncologic Drugs Advisory Committee (ODAC) Meeting. 2005 [Google Scholar]
- 9.Speth PA, van Hoesel QG, Haanen C. Clinical pharmacokinetics of doxorubicin. Clin Pharmacokinet. 1988;15(1):15–31. doi: 10.2165/00003088-198815010-00002. [DOI] [PubMed] [Google Scholar]
- 10.McEvoy GK. American Society of Health-System Pharmacists. AHFS Drug Information: Doxorubicin Hydrochloride. 2013 [Google Scholar]
- 11.Westphal M, Hilt DC, Bortey E, Delavault P, Olivares R, Warnke PC, Whittle IR, Jaaskelainen J, Ram Z. A phase 3 trial of local chemotherapy with biodegradable carmustine (BCNU) wafers (Gliadel wafers) in patients with primary malignant glioma. Neuro Oncol. 2003;5(2):79–88. doi: 10.1215/S1522-8517-02-00023-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perry J, Chambers A, Spithoff K, Laperriere N. Gliadel wafers in the treatment of malignant glioma: a systematic review. Current oncology (Toronto, Ont) 2007;14(5):189–94. doi: 10.3747/co.2007.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prevention CfDCa. National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States. In: P CfDCa., editor. Department of Health and Human Services. Atlanta, GA: U.S; p. 2011. [Google Scholar]
- 14.Duckworth WC, Bennett RG, Hamel FG. Insulin Degradation: Progress and Potential. 1998 doi: 10.1210/edrv.19.5.0349. [DOI] [PubMed] [Google Scholar]
- 15.Muggeo M, Zoppini G, Bonora E, Brun E, Bonadonna RC, Moghetti P, Verlato G. Fasting plasma glucose variability predicts 10-year survival of type 2 diabetic patients: the Verona Diabetes Study. Diabetes care. 2000;23(1):45–50. doi: 10.2337/diacare.23.1.45. [DOI] [PubMed] [Google Scholar]
- 16.Golan DE, Tashjian AmernH, Jr, Armstrong EhrinJ, Armstrong AprilW. Principles of Pharmacology. Lippincott Williams & Wilkins; 2008. Principles of Pharmacology. [Google Scholar]
- 17.Reginster JY, Burlet N. Osteoporosis: a still increasing prevalence. Bone. 2006;38(2 Suppl 1):S4–9. doi: 10.1016/j.bone.2005.11.024. [DOI] [PubMed] [Google Scholar]
- 18.Lindsay R, Nieves J, Formica C, Henneman E, Woelfert L, Shen V, Dempster D, Cosman F. Randomised controlled study of effect of parathyroid hormone on vertebral-bone mass and fracture incidence among postmenopausal women on oestrogen with osteoporosis. Lancet. 1997;350(9077):550–5. doi: 10.1016/S0140-6736(97)02342-8. [DOI] [PubMed] [Google Scholar]
- 19.Greenspan SL, Bone HG, Ettinger MP, Hanley DA, Lindsay R, Zanchetta JR, Blosch CM, Mathisen AL, Morris SA, Marriott TB. In Ann Intern Med. Vol. 146. United States: 2007. Effect of recombinant human parathyroid hormone (1-84) on vertebral fracture and bone mineral density in postmenopausal women with osteoporosis: a randomized trial; pp. 326–39. [DOI] [PubMed] [Google Scholar]
- 20.Hock JM, Gera I. Effects of continuous and intermittent administration and inhibition of resorption on the anabolic response of bone to parathyroid hormone. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 1992;7(1):65–72. doi: 10.1002/jbmr.5650070110. [DOI] [PubMed] [Google Scholar]
- 21.Pleiner-Duxneuner J, Zwettler E, Paschalis E, Roschger P, Nell-Duxneuner V, Klaushofer K. Treatment of osteoporosis with parathyroid hormone and teriparatide. Calcified tissue international. 2009;84(3):159–70. doi: 10.1007/s00223-009-9218-x. [DOI] [PubMed] [Google Scholar]
- 22.Rubin MR, Bilezikian JP. New anabolic therapies in osteoporosis. Current opinion in rheumatology. 2002;14(4):433–40. doi: 10.1097/00002281-200207000-00018. [DOI] [PubMed] [Google Scholar]
- 23.Aslan D, Andersen MD, Gede LB, de Franca TK, Jorgensen SR, Schwarz P, Jorgensen NR. Mechanisms for the bone anabolic effect of parathyroid hormone treatment in humans. Scandinavian journal of clinical and laboratory investigation. 2012;72(1):14–22. doi: 10.3109/00365513.2011.624631. [DOI] [PubMed] [Google Scholar]
- 24.Han SL, Wan SL. Effect of teriparatide on bone mineral density and fracture in postmenopausal osteoporosis: meta-analysis of randomised controlled trials. International journal of clinical practice. 2012;66(2):199–209. doi: 10.1111/j.1742-1241.2011.02837.x. [DOI] [PubMed] [Google Scholar]
- 25.Gold DT, Weinstein DL, Pohl G, Krohn KD, Chen Y, Meadows ES. Factors Associated with Persistence with Teriparatide Therapy: Results from the DANCE Observational Study. Journal of osteoporosis. 2011;2011:314970. doi: 10.4061/2011/314970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marhefka JN, Velankar SS, Chapman TM, Kameneva MV. Mechanical degradation of drag reducing polymers in suspensions of blood cells and rigid particles. Biorheology. 2008;45(5):599–609. [PubMed] [Google Scholar]
- 27.Merkel TJ, Jones SW, Herlihy KP, Kersey FR, Shields AR, Napier M, Luft JC, Wu H, Zamboni WC, Wang AZ, Bear JE, DeSimone JM. Using mechanobiological mimicry of red blood cells to extend circulation times of hydrogel microparticles. Proc Natl Acad Sci U S A. 2011;108(2):586–91. doi: 10.1073/pnas.1010013108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stein PD, Sabbah HN. Turbulent blood flow in the ascending aorta of humans with normal and diseased aortic valves. Circ Res. 1976;39(1):58–65. doi: 10.1161/01.res.39.1.58. [DOI] [PubMed] [Google Scholar]
- 29.Ku DN. Blood flow in arteries. Annual Reviews of Fluid Mechanics. 1997;29:399–434. [Google Scholar]
- 30.Gualtieri P, Picano F, Sardina G, Casciola CM. Clustering and turbulence modulation in particle laden shear flows. Journal of Physics: Conference Series. 2011;333:1–15. [Google Scholar]
- 31.Lin J, Liu S, Chan T. Nanoparticle migration in a fully developed turbulent pipe flow considering the particle coagulation. Chinese Journal of Chemical Engineering. 2012;20(4):679–685. [Google Scholar]
- 32.Korin N, Kanapathipillai M, Matthews BD, Crescente M, Brill A, Mammoto T, Ghosh K, Jurek S, Bencherif SA, Bhatta D, Coskun AU, Feldman CL, Wagner DD, Ingber DE. Shear-activated nanotherapeutics for drug targeting to obstructed blood vessels. Science. 2012;337(6095):738–42. doi: 10.1126/science.1217815. [DOI] [PubMed] [Google Scholar]
- 33.Williams KJ, Phillips MC, Rodrigueza WV. Structural and metabolic consequences of liposome-lipoprotein interactions. Adv Drug Deliv Rev. 1998;32(1-2):31–43. doi: 10.1016/s0169-409x(97)00130-0. [DOI] [PubMed] [Google Scholar]
- 34.Moghimi SM, Hunter AC, Andresen TL. Factors controlling nanoparticle pharmacokinetics: an integrated analysis and perspective. Annu Rev Pharmacol Toxicol. 2012;52:481–503. doi: 10.1146/annurev-pharmtox-010611-134623. [DOI] [PubMed] [Google Scholar]
- 35.Aggarwal P, Hall JB, McLeland CB, Dobrovolskaia MA, McNeil SE. Nanoparticle interaction with plasma proteins as it relates to particle biodistribution, biocompatibility and therapeutic efficacy. Adv Drug Deliv Rev. 2009;61(6):428–37. doi: 10.1016/j.addr.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rodriguez PL, Harada T, Christian DA, Pantano DA, Tsai RK, Discher DE. Minimal “Self” peptides that inhibit phagocytic clearance and enhance delivery of nanoparticles. Science. 2013;339(6122):971–5. doi: 10.1126/science.1229568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Choi HS, Liu W, Misra P, Tanaka E, Zimmer JP, Itty Ipe B, Bawendi MG, Frangioni JV. Renal clearance of quantum dots. Nat Biotechnol. 2007;25(10):1165–70. doi: 10.1038/nbt1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Protzer U, Maini MK, Knolle PA. Living in the liver: hepatic infections. Nat Rev Immunol. 2012;12(3):201–13. doi: 10.1038/nri3169. [DOI] [PubMed] [Google Scholar]
- 39.Mebius RE, Kraal G. Structure and function of the spleen. Nat Rev Immunol. 2005;5(8):606–16. doi: 10.1038/nri1669. [DOI] [PubMed] [Google Scholar]
- 40.Moghimi SM, Porter CJ, Muir IS, Illum L, Davis SS. Non-phagocytic uptake of intravenously injected microspheres in rat spleen: influence of particle size and hydrophilic coating. Biochem Biophys Res Commun. 1991;177(2):861–6. doi: 10.1016/0006-291x(91)91869-e. [DOI] [PubMed] [Google Scholar]
- 41.Menna P, Salvatorelli E, Gianni L, Minotti G. Anthracycline cardiotoxicity. Top Curr Chem. 2008;283:21–44. doi: 10.1007/128_2007_11. [DOI] [PubMed] [Google Scholar]
- 42.Lukyanenko V. Delivery of nano-objects to functional sub-domains of healthy and failing cardiac myocytes. Nanomedicine (Lond) 2007;2(6):831–46. doi: 10.2217/17435889.2.6.831. [DOI] [PubMed] [Google Scholar]
- 43.Hobbs SK, Monsky WL, Yuan F, Roberts WG, Griffith L, Torchilin VP, Jain RK. Regulation of transport pathways in tumor vessels: role of tumor type and microenvironment. Proc Natl Acad Sci U S A. 1998;95(8):4607–12. doi: 10.1073/pnas.95.8.4607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maeda H. The enhanced permeability and retention (EPR) effect in tumor vasculature: the key role of tumor-selective macromolecular drug targeting. Adv Enzyme Regul. 2001;41:189–207. doi: 10.1016/s0065-2571(00)00013-3. [DOI] [PubMed] [Google Scholar]
- 45.Wang J, Byrne JD, Napier ME, DeSimone JM. More effective nanomedicines through particle design. Small. 2011;7(14):1919–31. doi: 10.1002/smll.201100442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McLennan DN, Porter CJH, Charman SA. Subcutaneous drug delivery and the role of the lymphatics. Drug Discovery Today: Technologies. 2005;2(1):89–96. doi: 10.1016/j.ddtec.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 47.Swartz MA. The physiology of the lymphatic system. Adv Drug Deliv Rev. 2001;50(1-2):3–20. doi: 10.1016/s0169-409x(01)00150-8. [DOI] [PubMed] [Google Scholar]
- 48.Amidon GL, Lee HJ. Absorption of peptide and peptidomimetic drugs. Annu Rev Pharmacol Toxicol. 1994;34:321–41. doi: 10.1146/annurev.pa.34.040194.001541. [DOI] [PubMed] [Google Scholar]
- 49.Rojanasakul Y, Wang LY, Bhat M, Glover DD, Malanga CJ, Ma JK. The transport barrier of epithelia: a comparative study on membrane permeability and charge selectivity in the rabbit. Pharm Res. 1992;9(8):1029–34. doi: 10.1023/a:1015802427428. [DOI] [PubMed] [Google Scholar]
- 50.Owens DR, Jones MK, Hayes TM, Heding LG, Alberti KG, Home PD, Burrin JM. Comparative study of subcutaneous, intramuscular, and intravenous administration of human insulin. Lancet. 1981;2(8238):118–22. doi: 10.1016/s0140-6736(81)90300-7. [DOI] [PubMed] [Google Scholar]
- 51.Lauritzen T, Pramming S, Deckert T, Binder C. Pharmacokinetics of continuous subcutaneous insulin infusion. Diabetologia. 1983;24(5):326–9. doi: 10.1007/BF00251817. [DOI] [PubMed] [Google Scholar]
- 52.Williams DF. On the mechanisms of biocompatibility. Biomaterials. 2008;29(20):2941–53. doi: 10.1016/j.biomaterials.2008.04.023. [DOI] [PubMed] [Google Scholar]
- 53.Liu Y, Mounkes LC, Liggitt HD, Brown CS, Solodin I, Heath TD, Debs RJ. Factors influencing the efficiency of cationic liposome-mediated intravenous gene delivery. Nat Biotechnol. 1997;15(2):167–73. doi: 10.1038/nbt0297-167. [DOI] [PubMed] [Google Scholar]
- 54.Muller RH, Mader K, Gohla S. Solid lipid nanoparticles (SLN) for controlled drug delivery - a review of the state of the art. Eur J Pharm Biopharm. 2000;50(1):161–77. doi: 10.1016/s0939-6411(00)00087-4. [DOI] [PubMed] [Google Scholar]
- 55.Wattendorf U, Merkle HP. PEGylation as a tool for the biomedical engineering of surface modified microparticles. J Pharm Sci. 2008;97(11):4655–69. doi: 10.1002/jps.21350. [DOI] [PubMed] [Google Scholar]
- 56.Farokhzad OC, Karp JM, Langer R. Nanoparticle-aptamer bioconjugates for cancer targeting. Expert Opin Drug Deliv. 2006;3(3):311–24. doi: 10.1517/17425247.3.3.311. [DOI] [PubMed] [Google Scholar]
- 57.Geng Y, Dalhaimer P, Cai S, Tsai R, Tewari M, Minko T, Discher DE. Shape effects of filaments versus spherical particles in flow and drug delivery. Nat Nanotechnol. 2007;2(4):249–55. doi: 10.1038/nnano.2007.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Panyam J, Labhasetwar V. Biodegradable nanoparticles for drug and gene delivery to cells and tissue. Adv Drug Deliv Rev. 2003;55(3):329–47. doi: 10.1016/s0169-409x(02)00228-4. [DOI] [PubMed] [Google Scholar]
- 59.Udhrain A, Skubitz KM, Northfelt DW. Pegylated liposomal doxorubicin in the treatment of AIDS-related Kaposi's sarcoma. Int J Nanomedicine. 2007;2(3):345–52. [PMC free article] [PubMed] [Google Scholar]
- 60.Gabizon A, Catane R, Uziely B, Kaufman B, Safra T, Cohen R, Martin F, Huang A, Barenholz Y. Prolonged circulation time and enhanced accumulation in malignant exudates of doxorubicin encapsulated in polyethylene-glycol coated liposomes. Cancer Res. 1994;54(4):987–92. [PubMed] [Google Scholar]
- 61.Gabizon AF, Shmeeda H, Barenholz Y. Pharmacokinetics of pegylated liposomal Doxorubicin: review of animal and human studies. Clin Pharmacokinet. 2003;42(5):419–36. doi: 10.2165/00003088-200342050-00002. [DOI] [PubMed] [Google Scholar]
- 62.Northfelt DW, Martin FJ, Working P, Volberding PA, Russell J, Newman M, Amantea MA, Kaplan LD. Doxorubicin encapsulated in liposomes containing surface-bound polyethylene glycol: pharmacokinetics, tumor localization, and safety in patients with AIDS-related Kaposi's sarcoma. J Clin Pharmacol. 1996;36(1):55–63. doi: 10.1002/j.1552-4604.1996.tb04152.x. [DOI] [PubMed] [Google Scholar]
- 63.O'Brien ME, Wigler N, Inbar M, Rosso R, Grischke E, Santoro A, Catane R, Kieback DG, Tomczak P, Ackland SP, Orlandi F, Mellars L, Alland L, Tendler C. Reduced cardiotoxicity and comparable efficacy in a phase III trial of pegylated liposomal doxorubicin HCl (CAELYX/Doxil) versus conventional doxorubicin for first-line treatment of metastatic breast cancer. Ann Oncol. 2004;15(3):440–9. doi: 10.1093/annonc/mdh097. [DOI] [PubMed] [Google Scholar]
- 64.Pissuwan D, Valenzuela SM, Cortie MB. Therapeutic possibilities of plasmonically heated gold nanoparticles. Trends Biotechnol. 2006;24(2):62–7. doi: 10.1016/j.tibtech.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 65.O'Neal DP, Hirsch LR, Halas NJ, Payne JD, West JL. Photo-thermal tumor ablation in mice using near infrared-absorbing nanoparticles. Cancer Lett. 2004;209(2):171–6. doi: 10.1016/j.canlet.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 66.Chertok B, David AE, Yang VC. Magnetically-enabled and MR-monitored selective brain tumor protein delivery in rats via magnetic nanocarriers. Biomaterials. 2011;32(26):6245–53. doi: 10.1016/j.biomaterials.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dromi S, Frenkel V, Luk A, Traughber B, Angstadt M, Bur M, Poff J, Xie J, Libutti SK, Li KC, Wood BJ. Pulsed-high intensity focused ultrasound and low temperature-sensitive liposomes for enhanced targeted drug delivery and antitumor effect. Clin Cancer Res. 2007;13(9):2722–7. doi: 10.1158/1078-0432.CCR-06-2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Frenkel V, Etherington A, Greene M, Quijano J, Xie J, Hunter F, Dromi S, Li KC. Delivery of liposomal doxorubicin (Doxil) in a breast cancer tumor model: investigation of potential enhancement by pulsed-high intensity focused ultrasound exposure. Acad Radiol. 2006;13(4):469–79. doi: 10.1016/j.acra.2005.08.024. [DOI] [PubMed] [Google Scholar]
- 69.Stone MJ, Frenkel V, Dromi S, Thomas P, Lewis RP, Li KC, Horne M, 3rd, Wood BJ. Pulsed-high intensity focused ultrasound enhanced tPA mediated thrombolysis in a novel in vivo clot model, a pilot study. Thromb Res. 2007;121(2):193–202. doi: 10.1016/j.thromres.2007.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Folkman J, Long DM, Jr, Rosenbaum R. Silicone rubber: a new diffusion property useful for general anesthesia. Science. 1966;154(3745):148–9. doi: 10.1126/science.154.3745.148. [DOI] [PubMed] [Google Scholar]
- 71.Folkman J, Long DM. The Use of Silicone Rubber as a Carrier for Prolonged Drug Therapy. J Surg Res. 1964;4:139–42. doi: 10.1016/s0022-4804(64)80040-8. [DOI] [PubMed] [Google Scholar]
- 72.Langer R, Folkman J. Polymers for the sustained release of proteins and other macromolecules. Nature. 1976;263(5580):797–800. doi: 10.1038/263797a0. [DOI] [PubMed] [Google Scholar]
- 73.Langer R, Tirrell DA. Designing materials for biology and medicine. Nature. 2004;428(6982):487–92. doi: 10.1038/nature02388. [DOI] [PubMed] [Google Scholar]
- 74.Anderson DG, Burdick JA, Langer R. Materials science. Smart biomaterials. Science. 2004;305(5692):1923–4. doi: 10.1126/science.1099987. [DOI] [PubMed] [Google Scholar]
- 75.Ulijn RV. Enzyme-responsive materials: a new class of smart biomaterials. J Mater Chem. 2006;16:2217–2225. [Google Scholar]
- 76.Langer R. Polymer implants for drug delivery in the brain. journal of Controlled Release. 1991;16:53–60. [Google Scholar]
- 77.Attenello FJ, Mukherjee D, Datoo G, McGirt MJ, Bohan E, Weingart JD, Olivi A, Quinones-Hinojosa A, Brem H. Use of Gliadel (BCNU) wafer in the surgical treatment of malignant glioma: a 10-year institutional experience. Ann Surg Oncol. 2008;15(10):2887–93. doi: 10.1245/s10434-008-0048-2. [DOI] [PubMed] [Google Scholar]
- 78.Westphal M, Ram Z, Riddle V, Hilt D, Bortey E. Gliadel wafer in initial surgery for malignant glioma: long-term follow-up of a multicenter controlled trial. Acta Neurochir (Wien) 2006;148(3):269–75. doi: 10.1007/s00701-005-0707-z. discussion 275. [DOI] [PubMed] [Google Scholar]
- 79.Richards Grayson AC, Choi IS, Tyler BM, Wang PP, Brem H, Cima MJ, Langer R. Multi-pulse drug delivery from a resorbable polymeric microchip device. Nat Mater. 2003;2(11):767–72. doi: 10.1038/nmat998. [DOI] [PubMed] [Google Scholar]
- 80.Prescott JH, Lipka S, Baldwin S, Sheppard NF, Jr, Maloney JM, Coppeta J, Yomtov B, Staples MA, Santini JT., Jr Chronic, programmed polypeptide delivery from an implanted, multireservoir microchip device. Nat Biotechnol. 2006;24(4):437–8. doi: 10.1038/nbt1199. [DOI] [PubMed] [Google Scholar]
- 81.Maloney JM, Uhland SA, Polito BF, Sheppard NF, Jr, Pelta CM, Santini JT., Jr Electrothermally activated microchips for implantable drug delivery and biosensing. J Control Release. 2005;109(1-3):244–55. doi: 10.1016/j.jconrel.2005.09.035. [DOI] [PubMed] [Google Scholar]
- 82.Bratlie KM, York RL, Invernale MA, Langer R, Anderson DG. Materials for diabetes therapeutics. Adv Healthc Mater. 2012;1(3):267–84. doi: 10.1002/adhm.201200037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Brown L, Munoz C, Siemer L, Edelman E, Langer R. Controlled release of insulin from polymer matrices Control of diabetes in rats. Diabetes. 1986;35(6):692–7. doi: 10.2337/diab.35.6.692. [DOI] [PubMed] [Google Scholar]
- 84.Brown LR, Edelman ER, Fischel-Ghodsian F, Langer R. Characterization of glucose-mediated insulin release from implantable polymers. J Pharm Sci. 1996;85(12):1341–5. doi: 10.1021/js9600686. [DOI] [PubMed] [Google Scholar]
- 85.Kost J, Langer R. Responsive polymer systems for controlled delivery of therapeutics. Trends Biotechnol. 1992;10(4):127–31. doi: 10.1016/0167-7799(92)90194-z. [DOI] [PubMed] [Google Scholar]
- 86.Gu Z, Aimetti AA, Wang Q, Dang TT, Zhang Y, Veiseh O, Cheng H, Langer RS, Anderson DG. Injectable Nano-Network for Glucose-Mediated Insulin Delivery. ACS Nano. 2013 doi: 10.1021/nn400630x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rolland JP, Maynor BW, Euliss LE, Exner AE, Denison GM, DeSimone JM. Direct fabrication and harvesting of monodisperse, shape-specific nanobiomaterials. J Am Chem Soc. 2005;127(28):10096–100. doi: 10.1021/ja051977c. [DOI] [PubMed] [Google Scholar]
- 88.Kelly JY, DeSimone JM. Shape-specific, monodisperse nano-molding of protein particles. J Am Chem Soc. 2008;130(16):5438–9. doi: 10.1021/ja8014428. [DOI] [PubMed] [Google Scholar]
- 89.Schmidt V, Kuna L, Satzinger V, Jakopic G, Leising G. Two-photon 3D lithography: A Versatile Fabrication Method for Complex 3D Shapes and Optical Interconnects within the Scope of Innovative Industrial Applications. J Laser Micro Nanoen. 2007;2(3):170–177. [Google Scholar]
- 90.Lee K, Kim R, Yang D, Park S. Advances in 3D nano/microfabrication using two-photon initiated polymerization. Prog Polym Sci. 2008;33(6):631–681. [Google Scholar]
- 91.Yang D, Park S, Lim T, Kong H, Yi S, Yang H, Lee K. Ultraprecise microreproduction of a three-dimensional artistic sculpture by multipath scanning method in two-photon photopolymerization. Appl Phys Lett. 2007;90(1) [Google Scholar]
- 92.Hu CM, Zhang L, Aryal S, Cheung C, Fang RH, Zhang L. Erythrocyte membrane-camouflaged polymeric nanoparticles as a biomimetic delivery platform. Proc Natl Acad Sci U S A. 2011;108(27):10980–5. doi: 10.1073/pnas.1106634108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Schneider G, Guttmann P, Heim S, Rehbein S, Mueller F, Nagashima K, Heymann J, Muller W, McNally J. Three-dimensional cellular ultrastructure resolved by X-ray microscopy. Nat Methods. 2010;7(12):985–U116. doi: 10.1038/nmeth.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Leis A, Rockel B, Andrees L, Baumeister W. Visualizing cells at the nanoscale. Trends Biochem Sci. 2009;34(2):60–70. doi: 10.1016/j.tibs.2008.10.011. [DOI] [PubMed] [Google Scholar]
- 95.Hohmann-Marriott M, Sousa A, Azari A, Glushakova S, Zhang G, Zimmerberg J, Leapman R. Nanoscale 3D cellular imaging by axial scanning transmission electron tomography. Nat Methods. 2009;6(10):729–732. doi: 10.1038/nmeth.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.McKillop AM, Flatt PR. Emerging applications of metabolomic and genomic profiling in diabetic clinical medicine. Diabetes Care. 2011;34(12):2624–30. doi: 10.2337/dc11-0837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Manolio TA, Collins FS. The HapMap and genome-wide association studies in diagnosis and therapy. Annu Rev Med. 2009;60:443–56. doi: 10.1146/annurev.med.60.061907.093117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Honda K, Ono M, Shitashige M, Masuda M, Kamita M, Miura N, Yamada T. Proteomic approaches to the discovery of cancer biomarkers for early detection and personalized medicine. Jpn J Clin Oncol. 2013;43(2):103–9. doi: 10.1093/jjco/hys200. [DOI] [PubMed] [Google Scholar]
- 99.Newcomb CJ, Moyer TJ, Lee SS, Stupp SI. Advances in cryogenic transmission electron microscopy for the characterization of dynamic self-assembling nanostructures. Curr Opin Colloid Interface Sci. 2012;17(6):350–359. doi: 10.1016/j.cocis.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ricotti L, Assaf T, Dario P, Menciassi A. Wearable and implantable pancreas substitutes. J Artif Organs. 2013;16(1):9–22. doi: 10.1007/s10047-012-0660-6. [DOI] [PubMed] [Google Scholar]
- 101.Simon D, Kurup S, Larsson K, Hori R, Tybrandt K, Goiny M, Jager E, Berggren M, Canlon B, Richter-Dahlfors A. Organic electronics for precise delivery of neurotransmitters to modulate mammalian sensory function. Nature Materials. 2009;8(9):742–746. doi: 10.1038/nmat2494. [DOI] [PubMed] [Google Scholar]
- 102.De Micheli G. An outlook on design technologies for future integrated systems. IEEE Transaction on computer-aided design of integrated circuits and systems. 2009;28(6):777–790. [Google Scholar]
- 103.Disney D, Shen Z. Review of Silicon Power Semiconductor Technologies for Power Supply on Chip and Power Supply in Package Applications. IEEE T Power Electr. 2013;28(9):4168–4181. [Google Scholar]
- 104.Farra R, Sheppard N, McCabe L, Neer R, Anderson J, Santini J, Cima M, Langer R. First-in-Human Testing of a Wirelessly Controlled Drug Delivery Microchip. Sci Transl Med. 2012;4(122) doi: 10.1126/scitranslmed.3003276. [DOI] [PubMed] [Google Scholar]
- 105.Irimia-Vladu M, Glowacki E, Voss G, Bauer S, Sariciftci N. Green and biodegradable electronics. Mater Today. 2012;15(7-8):340–346. [Google Scholar]
- 106.Muskovich M, Bettinger C. Biomaterials-Based Electronics: Polymers and Interfaces for Biology and Medicine. Advanced Healthcare Materials. 2012;1(3):248–266. doi: 10.1002/adhm.201200071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kim D, Viventi J, Amsden J, Xiao J, Vigeland L, Kim Y, Blanco J, Panilaitis B, Frechette E, Contreras D, Kaplan D, Omenetto F, Huang Y, Hwang K, Zakin M, Litt B, Rogers J. Dissolvable films of silk fibroin for ultrathin conformal bio-integrated electronics. Nature Materials. 2010;9(6):511–517. doi: 10.1038/nmat2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Stadler P, Oppelt K, Singh T, Grote J, Schwodiauer R, Bauer S, Piglmayer-Brezina H, Bauerle D, Sariciftci N. Organic field-effect transistors and memory elements using deoxyribonucleic acid (DNA) gate dielectric. Org Electron. 2007;8(6):648–654. [Google Scholar]
- 109.Zhong C, Deng Y, Roudsari A, Kapetanovic A, Anantram M, Rolandi M. A polysaccharide bioprotonic field-effect transistor. Nat Commun. 2011;2 doi: 10.1038/ncomms1489. [DOI] [PubMed] [Google Scholar]
- 110.Brown B, Badylak S. Expanded applications, shifting paradigms and an improved understanding of host-biomaterial interactions. Acta Biomater. 2013;9(2):4948–4955. doi: 10.1016/j.actbio.2012.10.025. [DOI] [PubMed] [Google Scholar]
- 111.Anderson J, Rodriguez A, Chang D. Foreign body reaction to biomaterials. Semin Immunol. 2008;20(2):86–100. doi: 10.1016/j.smim.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Skousen J, Merriam M, Srivannavit O, Perlin G, Wise K, Tresco P. Reducing surface area while maintaining implant penetrating profile lowers the brain foreign body response to chronically implanted planar silicon microelectrode arrays. Prog Brain Res. 2011;194:167–180. doi: 10.1016/B978-0-444-53815-4.00009-1. [DOI] [PubMed] [Google Scholar]
- 113.Sanders J, Stiles C, Hayes C. Tissue response to single-polymer fibers of varying diameters: Evaluation of fibrous encapsulation and macrophage density. J Biomed Mater Res. 2000;52(1):231–237. doi: 10.1002/1097-4636(200010)52:1<231::aid-jbm29>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 114.Casadio M, Ranganathan R, Mussa-Ivaldi FA. The body-machine interface: a new perspective on an old theme. J Mot Behav. 2012;44(6):419–33. doi: 10.1080/00222895.2012.700968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tessadori J, Bisio M, Martinoia S, Chiappalone M. Modular neuronal assemblies embodied in a closed-loop environment: toward future integration of brains and machines. Front Neural Circuits. 2012:6, 99. doi: 10.3389/fncir.2012.00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Carmena JM. Advances in neuroprosthetic learning and control. PLoS Biol. 2013;11(5):e1001561. doi: 10.1371/journal.pbio.1001561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hovorka R. Continuous glucose monitoring and closed-loop systems. Diabet Med. 2006;23(1):1–12. doi: 10.1111/j.1464-5491.2005.01672.x. [DOI] [PubMed] [Google Scholar]
- 118.Bellisle F, Louis-Sylvestre J, Demozay F, Blazy D, Le Magnen J. Cephalic phase of insulin secretion and food stimulation in humans: a new perspective. Am J Physiol. 1985;249(6 Pt 1):E639–45. doi: 10.1152/ajpendo.1985.249.6.E639. [DOI] [PubMed] [Google Scholar]