A. Overview
Mesenchymal stem cells (MSC) are a promising tool for cell therapy, either through direct contribution to the repair of bone, tendon and cartilage, or as adjunct therapy through protein production and immune mediation. They are currently being tested in clinical trials for such diverse applications as myocardial infarction, stroke, meniscus injury, limb ischemia, graft-vs.-host disease and autoimmune disorders. To date, MSC have been extensively tested and proven effective in pre-clinical studies for these and many other disorders including neurodegenerative diseases. 1–5
MSC are an attractive vehicle for cellular therapies due to a variety of cell intrinsic and environmentally-responsive properties. Molecularly-equivalent MSC have been successfully harvested from several locations, including the marrow space and various fat pads, with minimal patient discomfort. Once acquired, MSC are easily isolated and characterized in vitro, and exhibit rapid proliferation with minimal senescence through multiple passages.6,7 In contrast to hematopoietic progenitors, this rapid expansion does not dilute the capacity for self-renewal, and provides the unique opportunity to easily introduce a target gene of interest through a variety of methods.8 Also in contrast to many other potent progenitor populations, MSC require minimal culture conditions and media, as they produce many of their own essential growth factors to self-sustain through autocrine and paracrine mechanisms. Following transplantation, MSC are capable of systemic migration,8–13 are not prone to tumor formation,14 and in fact appear to tolerize the immune response across donor mismatch.5,15. These attributes combine to allow MSC to reside in many different tissue types without disrupting the local microenvironment, and in some cases, responding to the local environment with appropriate protein secretion.16–18
Due to their ability to reside within many tissue environments, MSC have been pursued as a therapeutic intervention in several models of chronic and acute injury. The factors governing the duration of their residence in the damaged tissue are not yet fully understood, and seem to differ between injury types. In cases of acute injury or inflammation, MSC respond to the injury robustly but only transiently, and do not become a stable part of the repaired tissue or vasculature to a significant degree. The same data has been obtained in large animal models, and appears to be independent of immune rejection. In the transient engraftment/acute injury models, data suggest that their efficacy relies upon paracrine actions rather than differentiation and direct contribution to the damaged tissue. MSC home to the injured area, in particular to hypoxic, apoptotic, or inflamed areas, and release trophic factors that hasten endogenous repair. These secreted bioactive products can suppress the local immune system, enhance angiogenesis, inhibit fibrosis and apoptosis, and stimulate recruitment, retention, proliferation and differentiation of tissue-residing stem cells. Paracrine effects exerted by MSC are distinct from the classical model of direct differentiation of stem cells into the tissue to be regenerated. Some current studies aim to enhance these paracrine effects through forced over-production of various paracrine elements, to further hasten the endogenous repair processes.5,19–22
In contrast to the short-term survival patterns in the acute injury setting, when MSC are infused intravenously into immune deficient mice that have low level systemic damage from irradiation, or a chronic disease, the cells migrate through all tissues in a relatively evenly dispersed and long-lasting manner. 8,12,13,23 In our laboratory, we have recovered genetically engineered human MSC from numerous organs of mice at timepoints from 6–18 months post-transplantation, with continued expression of transgene products for the duration of the experiments. 8,12,13,23 In situ examination revealed a lack of scarring or inflammation around these cells, and explant cultures have demonstrated that these MSC retained the ability to proliferate once released from the extracellular matrix constraints of their resident tissues. 12,23 Current work in our laboratory is focused on determining the molecular adaptations that occur in these tissue-resident MSC, and how this microenvironmental responsiveness can be efficiently exploited in chronic injury therapeutics.
These specialized characteristics of MSC have made them a promising vehicle for cell-based therapeutic intervention of acute and chronic injury, through direct integration into damaged tissue as well as trophic support and immune mediation during endogenous regeneration and repair mechanisms. They are easily harvested and expanded, amenable to genetic manipulations, and have a decade-long record of biosafety data in vivo 14,21 Therefore while the factors that govern the duration of residence of MSC in different tissues during acute vs. chronic disorders must be further delineated, the potential for these cells to secrete endogenous or transgene products in a sustained and long-term manner is highly promising, and is discussed in the current review.
B. Characterization and Utility of Mesenchymal Stem Cells
Mesenchymal stem cells (MSC) were originally identified by Friedenstein and his colleagues as the primary transplantable component of the bone marrow microenvironment necessary for maintenance of definitive hematopoiesis24. This original description established several characteristics of this cell type which are still the primary hallmarks of MSC today, in defiance of 3 decades of technological advances in scientific research and an expanding knowledge base on the nature of stromal cell influence in the development of blood systems. Specifically, Friedenstein defined mesenchymal stem cells as a fibroblastic mesodermally-derived cell, adherent, and clonogenic 24–26. This clonogenic expansion was measured in an assay termed the CFU-F, or colony forming unit-fibroblast, and presumed to be the mechanism through which the marrow microenvironment could survive through myeloablative conditioning and facilitate hematopoietic reconstitution. Subsequent experiments by Friedenstein and his collaborators established in vivo evidence using a rat transplant model to demonstrate the importance of the stromal component in hematopoiesis24,27. These early findings led to increased examination of the marrow microenvironment, its role in the propagation of hematopoiesis, and the mechanisms by which these cells were able to elude cell death throughout regimens of myeloablation.
The rapidly dividing adherent myofibroblastic cells from the human bone marrow microenvironment were previously referred to as “stroma”, but the better term “Mesenchymal Stem Cells” reflects their capacity to differentiate into multiple tissues; bone, cartilage, tendon, fibroblast, fat, and muscle 28–30. The term “MSC” can also be used to denote “marrow stromal cells” and the terms are often used interchangeably, although true mesenchymal stem cells, the most primitive subset, are likely rare in the marrow stromal cell myofibroblastic layer. Unfortunately, the phenotype of the most primitive MSC compartment has not yet been clearly defined, and the phenotype may vary with culture or expansion conditions, as can be seen with hematopoietic stem cells, making it difficult to standardize. We and others have grown the cells out of bone marrow spicules8 and marrow samples based on their ability to adhere to plastic and to rapidly expand in minimal medium. Description of some of the markers that are found on MSC has been done. However, these markers may or may not be on overlapping subsets, and there has been no systematic analysis of differentially sorted populations, as has been done with human hematopoietic stem cells. Therefore, a major goal in the field is to characterize the most primitive subsets of human MSC and to define their functions and differentiative capacity in vitro and in vivo.
Phenotypic characterization of the most primitive subset of MSC is therefore still elusive, and prospective identification of a homogenous, self-renewing and pluripotent population has been hampered by the diversity of cell populations referred to in the literature as MSC. Conventional assays such as the CFU-F do not sufficiently define the cell type responsible for observed multi-lineage differentiation, and in fact, cannot adequately rule out the possibility that multiple cell types within the marrow space are contributing. To address this inconsistency, The International Society for Cellular Therapy recently established minimal criteria for defining multipotent mesenchymal stromal cells 31. These basal attributes include the ability to adhere to plastic under normal cell culture conditions, to express a distinct set of 3 cell surface antigens (CD105, CD73, and CD90) while not expressing antigens indicative of other cell lineages, and to differentiate into adipocytes, osteoblasts, and chondroblasts under specific conditions. This set of minimal guidelines has served to allow a basis of comparison between the results of different investigators, and has allowed a more focused investigation into the clinical utility of stromal stem cells.
Investigation into the physiological relevance of mesenchymal stem cells has revealed several characteristics that make them an appealing target for use in cell or gene therapy - based interventions. Kuznetsov and colleagues described several morphologically-distinct cell populations within their isolations which were found to have varying clonogenic potential when transplanted into animal recipients 32. These differences have since been attributed to a surprising amount of lineage differentiation diversity from a cell population resident in the marrow space. When transplanted under the kidney capsule of rats, for example, individual MSC clones were found capable of forming a complete marrow ossicle 32,33. These de novo ossicles contained osteogenic cells interfacing with traditional marrow sinusoidal cells and adipocytes, and were capable of supporting hematopoiesis. Upon closer examination, they discovered that these functional marrow sinusoids were not generating their own hematopoietic progenitors, but rather had recruited host origin HSC to the transplant site. At once, these experiments implied that not only were marrow stromal stem cells able to completely recapitulate an environment for development of hematopoiesis, but they were immune-privileged and compatible to interact with many diverse cell types without rejection. Further, these experiments imply a mechanism for the active recruitment of hematopoietic progenitors to areas hospitable for expansion, a mechanism indispensable to post-myeloablative transplant repopulation and hematopoietic reconstitution.
In the marrow cavity, it seems unlikely that all MSC lining the marrow sinusoidal space are involved equally in the processes of self-renewal, hematopoietic support, and secretion of autocrine and paracrine factors, 34 but rather, that distinct subsets of MSC function in tandem to maintain the microenvironmental niche. Generation of the type of diverse microenvironment seen by Kuznetsov et al from a single MSC clone 32,33 equires a massive cell proliferation, and upon examination in vitro, mesenchymal stem cells have been shown to undergo as many as 25 self-renewing replications without detectable morphological change or loss of lineage potentiality. This potential for self-renewing proliferation and generation of multilineage progeny, coupled with the immunological tolerance initially suggested by the kidney capsule transplant model and later confirmed by many other studies, 15 have presented MSC as a highly attractive target for clinical therapeutic intervention.
C. Systemic cytokine production from genetically engineered human MSC
The first use of MSC in the gene therapy field was as a supportive monolayer for hematopoietic stem and progenitor cells 35. During gene transduction of hematopoietic stem cells, the stromal layer was found to improve survival through a “co-culture” method. 36 As this practice was improved, it was discovered that in addition to providing ex vivo support during the gene transfer or expansion phase of HSC-directed therapy, the MSC themselves could be engineered to provide increased human cytokine support when co-transplanted with the HSC 23,37. As more becomes known about the biological characterization of bone marrow derived MSC, more interest is generated in using these cells for cell- or gene-directed therapy. Special interest in particular has evolved for using MSC as a protein delivery vehicle following gene therapy modification. From a very small marrow aspirate, human mesenchymal stem cells are easily isolated and will rapidly begin clonogenic expansion in vitro. When compared to hematopoietic stem cells, which can be harvested in similar fashion, MSC cultures do not have the expensive cytokine and growth factor requirements since they produce many autocrine factors on their own 38. Also unlike traditionally used HSC, there is no defined hierarchy of MSC development, either in vitro or in vivo, that describes specific conditions for maintenance of the most potent cell type. Following transduction of MSC monolayers, relatively equivalent expression of the transgene product can be achieved throughout the culture.
In contrast to HSC, retroviral engineering did not seem to impair the ability of MSC to proliferate, self-renew, migrate post-transplantation, or differentiate appropriately 39–41. Further, these studies suggested that MSC have fewer complications regarding the insertion of virally-delivered transgenes. Whereas HSC seem to maintain a highly quiescent pool of true stem cells 42–47 that are resistant to retroviral transduction, MSC seem to have no comparable metabolic barrier. Several studies have verified this finding, demonstrating that MSC can be efficiently and durably transduced without intensive labor, and that this transgene expression is maintained throughout lineage differentiation and without compromising the proliferation rate or quality of progeny 48–51. Clonal analysis of the resultant cell populations showed wide variation; however, some clones contained several thousand copies of transgene RNA per cell and were able to maintain this expression for up to 6 months post-transduction 50. Examination of the starting cell population further showed that nearly 90% of all cells capable of producing colony-forming unit-fibroblast (CFU-F) colonies were transduced using standard transduction procedures 50. In comparison to HSC transduction, this represented an astounding and intriguing finding for cellular therapy and genetic engineering.
Interestingly, after transplantation human MSC do not migrate effectively to the bone marrow stromal compartment. However, Bubnic et al demonstrated that W/Wv mouse - derived marrow stromal cells engrafted into the marrow compartment and enhanced early erythropoiesis in unconditioned Sl/Sld murine recipients. Engraftment of donor stromal cells reached levels of up to 1.0% of total marrow cells 4 months post transplantation 52. This report was interesting because transplanted MSC do not usually replace the endogenous microenvironment, even after conditioning. 53,54. This report therefore suggests that the defect in the stromal compartment of the Sl/Sld mice played a role in permitting MSC engraftment in the marrow compartment. Almeida-Porada et al demonstrated that co-transplantation of both autologous and allogeneic human bone marrow-derived HSC and MSC in a fetal sheep model resulted in higher levels of long-term engraftment of human hematopoietic cells in the bone marrow of the chimeric animals during gestation and after birth 55,56. By using marked MSC, they also demonstrated that injected stromal cells engrafted and retained function within the sheep marrow. This study suggests that the fetal microenvironment could also be more permissive to allowing MSC engraftment. These reports could provide clues as to how to better replace at least a portion of the microenvironment for in vivo studies or clinical indications.
In the majority of the MSC transplantation studies reported to date, the transplanted cells lodge in multiple organs and continue to secrete their transgene products from those locations, throughout numerous tissues 8–13. Noort et al identified a population of MSC derived from human fetal lung which promoted engraftment of co-transplanted umbilical cord blood CD34(+) cells in bone marrow, spleen, and blood. Again, no MSC were found in the marrow compartment suggesting that the mechanisms by which the engraftment was enhanced did not require homing of MSC to the bone marrow 57.
Angelopoulou et al showed that co-transplantation of human mesenchymal stem cells with HSC enhances human myelopoiesis and megakaryocytopoiesis in NOD/SCID mice 58. Co-transplantation of genetically engineered human MSC solved a problem that had hampered the field’s use of human-to-murine xenotransplantation prior to 1988: the lack of cytokines cross-reactive to human stem cells in the mice. In that year, Kamel-Reid et al described the bnx/hu model, in which the immune deficient mice were injected with human interleukin 3 (IL-3), and human progenitors could survive for several months, with a primarily myeloid differentiative capacity 59. To avoid expensive and time-consuming injections of cytokines every 48 hours, as Kamel-Reid et al had described 59, our group co-transplanted human marrow stromal cells (MSC) engineered to secrete human IL-3, in addition to their endogenous human growth factors 49. The species specificity of IL-3 allowed support of human hematopoietic cells in the mice without perturbing murine hematopoiesis. Jiang et al later confirmed that Co-transplantation of stromal cells engineered to secrete IL-3 with T cell depleted marrow grafts improved hematopoiesis following allogeneic transplantation in mice.60
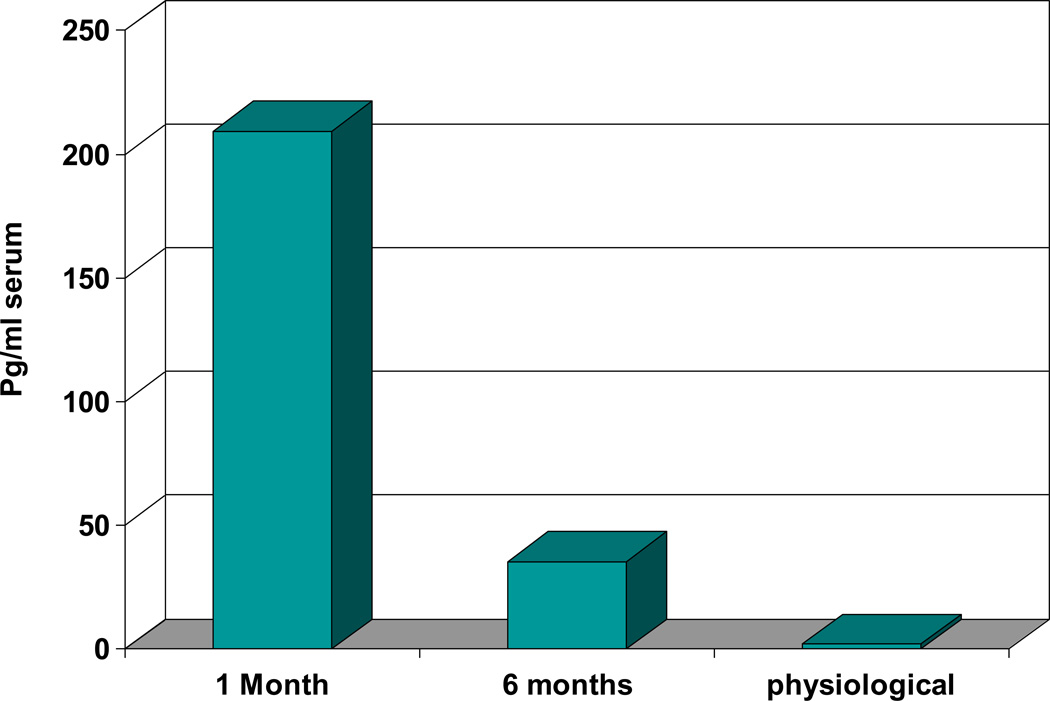
In our group’s co-transplantation system, gene- transduced human CD34+ progenitor cells were transplanted into immunodeficient mice after in vitro binding to primary human bone marrow (BM) stromal cells engineered to produce human interleukin-3 (IL-3). The IL- 3-secreting stroma produced sustained supraphysiological circulating levels of human IL-3 for at least 4 months in the mice. HuIL-3 levels were 209.4±15.5 pg/ml at 1 month and 35.5±5 at 6 months (N=7) (Figure 1). Levels at six months remain higher than normal human physiological levels of IL-3 (2pg/ml).61 There were significant influences from the IL-3 expressing MSC on the development of co-transplanted human hematopoietic stem cells (HSC). HuIL-3 allowed huHSC survival in beige/nude/xid (bnx) mice, with little engraftment of hematopoietic cells observed in control animals.
Figure 1. IL-3 levels were assessed in the serum of immune deficient mice one and six months after co-transplantation with engineered human mesenchymal stem cells.
At one and six months post-transplantation, blood was collected from the tail vein of the mice and the level of HuIL-3 in each serum sample was assessed (N=7). Serum levels were calculated by linear regression analysis in comparison to a standard curve generated from dilutions of recombinant HuIL-3. Levels at six months remained higher than normal human physiological levels of IL-3 (2pg/ml).
In related studies, we co-injected human bone marrow- derived hematopoietic CD34+ cells with human marrow stromal cells engineered to secrete human IL-2, IL-7, Stem Cell Factor (SCF) or FLT3 ligand (FL), with and without IL-3. No single factor other than IL-3 supported sustained human hematopoiesis in the mice. The use of IL-2 was discontinued due to cross-reaction and adverse effects on the murine hematopoietic system, and vascular leaking. Co-expression of SCF or FL with IL-3 had no overt effect on human hematopoiesis. Production of both human IL-3 and IL-7 in the mice supported extrathymic development of human T lymphocytes for 6–8 months,62 but no B cells, myeloid cells, or colony-forming progenitors were detected in those mice, whereas they developed in mice transplanted with IL-3 producing MSC alone. This demonstrated the capacity of the engineered MSC to skew hematopoietic development from the hematopoietic stem and progenitor cells in the mice 49.
Li et al later showed that cytokine - transduced murine bone marrow stromal cell lines promoted immunohematopoietic reconstitution in mice after co-transplantation with HSC. Murine MSC were transfected with the murine IL-3 and/ or IL-2 genes, then injected into lethally irradiated C57BL/6 mice with allogeneic T cell depleted bone marrow. The cytokine-transduced stromal cells significantly increased the numbers of hematopoietic progenitors and lymphocytes derived from the co-transplanted HSC 63 .
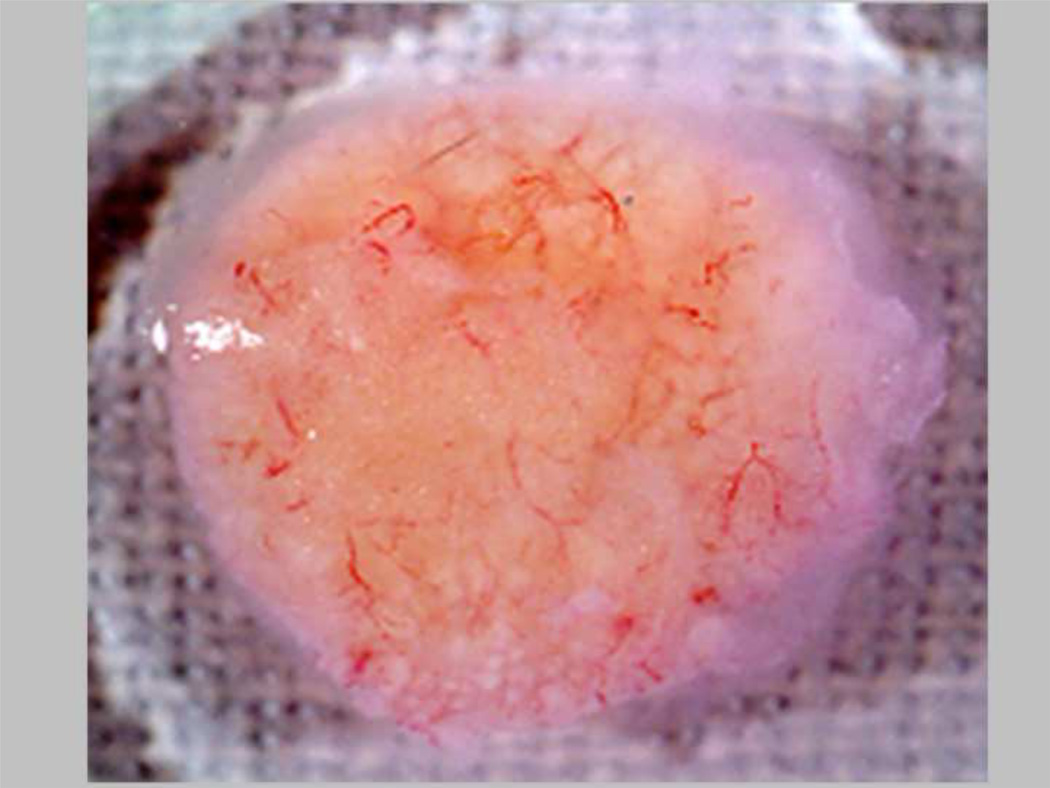
A limitation to the study of human hematopoietic development in immune deficient mouse models is a lack of maturation of human red blood cells, although BFU-E are maintained. Mature red cell development in the mice could provide a useful model to study gene therapy for globin disorders in vivo. To try to obtain maturation from the BFU-E stage, we co-transplanted human marrow stromal cells secreting human erythropoietin (Epo) and IL-3 into mice. An increase in hematocrit from 40–45% to 80–85% resulted, with production of human and murine red blood cells. Unfortunately the production of Epo was too effective using this model, since all mice (N=9) suffered strokes, displayed paralysis and died within three weeks 49. We were able to refine this system by reducing the level of human Epo expression through the use of alternate MSC delivery methods, such as a biochamber or neo-organoid which limited the systemic Epo levels by restricting the number of Epo-producing MSC 64. (Figure 2) These studies are illustrative of the effects that can be achieved from supraphysiological expression of cytokines from MSC, which can produce and secrete transgene products at relatively high levels.
Figure 2.
Epo-expressing human BM-MSC organoid implanted into nude/NOD/SCID mice on a biodegradable matrix. The MSC-based tissue is highly vascularized at the time of harvest, one month after implantation.
MSC genetically engineered to secrete cytokines and other growth factors have been used recently in animal models of various tissue repair studies. Kucic et al showed that Epo-producing MSC were useful in the long-term correction of renal failure-induced anemia.65,66 They next demonstrated that co-transplantation of insulin-like growth factor I (IGF-I)-overexpressing MSC improved the MSC-based gene therapy of anemia by providing paracrine support to Epo-secreting MSC. 67 Mice were rendered anemic by right kidney electrocoagulation and left nephrectomy. MSC engineered to express Epo were subsequently implanted subcutaneously in a bovine collagen matrix, in combination with MSC expressing IGF-1 or MSC alone in mice with renal failure. In mice that had received MSC-EPO coimplanted with MSC-IGF, the hematocrit elevation was enhanced as compared with control mice, and heart function was also improved. Their strategy of coimplantation of MSC-IGF with MSC-Epo therefore represents a promising strategy for improving cell-based gene therapy of renal anemia.67
Xu et al examined the efficacy of murine MSC-based angiopoietin-1 gene delivery for acute lung injury. 68 Angiopoietin-1 (Ang1) is a critical factor for endothelial survival and vascular stabilization. In their in vivo mouse model, LPS-induced lung injury was markedly alleviated in the group treated with MSCs engineered to express Ang1, as compared to groups treated with MSCs or Ang1 alone. Their results indicated that MSC-based Ang1 gene therapy could potentially be developed as a novel strategy for the treatment of acute lung injury.68
In addition to these specific examples of growth factor-expressing MSC in tissue repair, other fields of tissue injury repair have been explored, including but not limited to: revascularization after myocardial infarction (reviewed in 69,70), regeneration of intervertebral disc defects and spine therapy (reviewed in 71,72), repair of stroke (reviewed in 73), therapy for epilepsy (reviewed in 74), skeletal tissue repair (reviewed in 19), Chondrogenesis/ knee and joint repair (reviewed in 75) and neurodegenerative diseases (reviewed in 1,76), among many others. Genetically engineered MSC have thus proven safe and efficacious in numerous animal models of tissue repair, and are poised to be tested in human clinical trials.
D. Systemic production of enzyme from human MSC
While there is now ample evidence for the utility of MSC as trophic support for other cell types, both in vitro and in vivo, a related area of research has emerged to examine the use of MSC as a stand-alone therapeutic in vivo. The ability of MSC to traffic systemically, coupled with their ease of transduction, present a favorable platform for delivery of a therapeutic protein in models of genetic insufficiency. Of particular interest is the clinical intervention of so-called orphan diseases, which affect so few individuals nationwide that robust protein replacement regimens have not yet been developed through pharmaceutical pipelines. These treatments are further complicated by their rare incidence, as appropriate donor matches for bone marrow transplant may be familial carriers.
Lysosomal storage disorders (LSDs) present an appealing model for studying the efficacy of MSC in cell- and gene-therapy based treatment. This group of disorders encompasses greater than 45 distinctly characterized diseases, most of which result from enzyme deficiencies in the cellular breakdown of waste products through lysosomes. Mucopolysaccharidosis type VII (MPSVII) in particular results from the lack of beta-glucuronidase (GUSB) production caused by a variety of genetic mutations within chromosome 7. The loss of enzyme function in these disorders leads to the progressive accumulation of waste material within cells throughout the body, causing multi-system failure and profound developmental defects. Therapeutic intervention of MPSVII, and other LSDs, can be achieved by taking advantage of cross-correction through an intracellular transport system of these enzymes via mannose/mannose-6-phosphate receptors. In many cases, cross-correction can be achieved with introduction of single-digit percentage-of-normal amounts of enzyme.77
An alternative strategy to allogeneic bone marrow transplantation for lysososmal storage disorders is an autologous transplant using gene therapy to correct the deficiency in the patient’s own cells and then to re-introduce them to the patient. This approach bypasses the need for a matched allogeneic donor, as the therapy uses the patient’s own corrected cells. Previous work with the MPS diseases has reported good success with gene therapy approaches in a variety of models and transplant schematics. Initial investigations proved the efficacy of human GUSB in correction of animal models 78,79, then continued to examine alternative cellular targets for therapy, including hematopoietic progenitors 80,81 and skin fibroblasts 82,83. Hematopoietic progenitors were primarily re-introduced via traditional BMT procedures, whereas therapies involving fibroblasts met with greater success as implantable neo-organs 82,83 within the peritoneal cavity. While both approaches achieved moderate success and global distribution of enzyme, effective correction was not seen in all organs, thus prompting inquiry into alternative methods to effectively target specific organ systems.
Translation of these preliminary data into clinically-relevant information is a current area of focus in the field of gene therapy in general, and an active area of research for MPSVII disease. The most promising translational data to date has come from lentiviral engineering of human hematopoietic progenitors followed by transplantation into an immune-deficient mouse model of MPSVII 84. This study by Hofling et al. showed efficient cross-correction of disease systemically, and was followed up by an elegant study utilizing the lentiviral gene-replacement therapy on HSC from a human MPSVII patient, followed by transplantation into the murine MPSVII model 85. Similar to the prior study, the human donor cells were able to efficiently repopulate the murine hematopoietic system, showing correction of disease with no adverse effects of lentiviral transduction 85. In spite of these promising results, gene-replacement therapy in the hematopoietic system is not without drawbacks. The transduction efficiency of pseudotyped lentiviral vectors is not 100% which, when coupled with the colony-forming efficiency of the best CD34+ progenitor population, leaves much room for improvement.
While this limited success through various methods was encouraging, each paradigm came with a unique set of limitations. Given the multi-system defects and need for systemic delivery of a protein, MSC transplantation seemed a promising area of research. In regard to MSC engraftment within the model, while there are no assays complementary to the marrow replacement seen in an HSC transplant, transplanted MSC have been shown to disperse to a wide range of tissues following a variety of routes of administration 41. Following this differential repopulation into multiple tissue beds, the transplanted MSC were found to persist and maintain viability at least 75 days post-transplantation. Explant cultures also demonstrated that the MSC retained a limited ability to proliferate again once the restrictions of extracellular matrix contact within the tissues were removed 41.
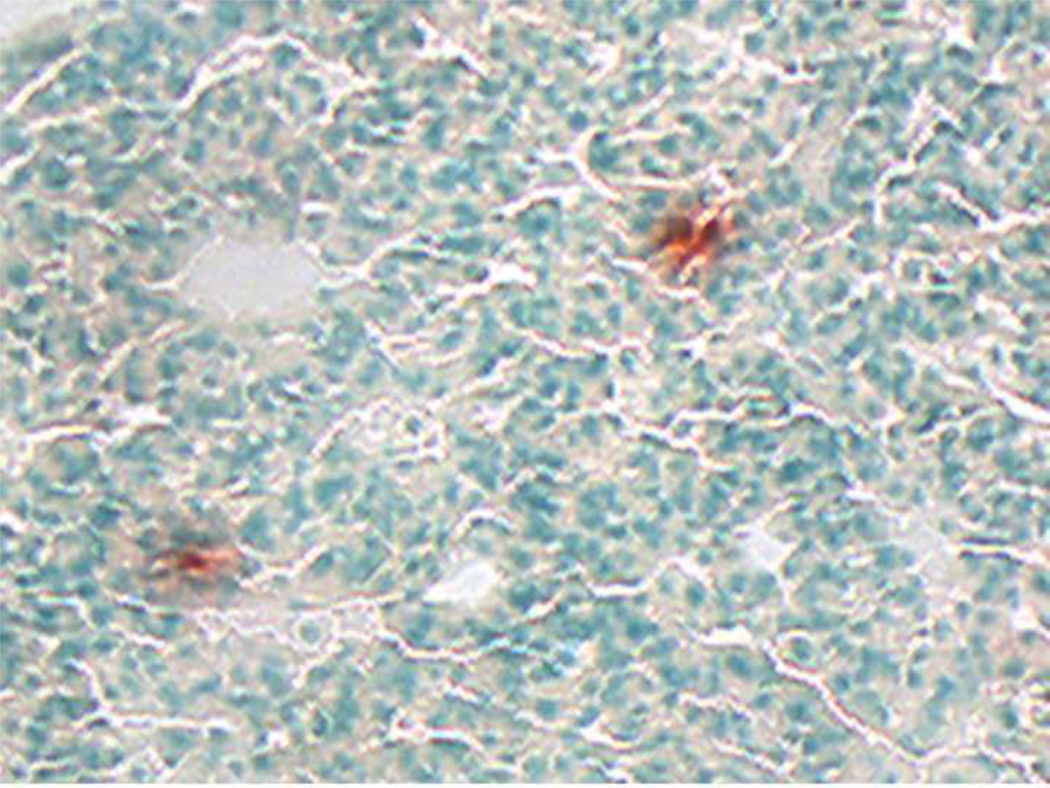
Our group further evaluated the potential for human adipose-derived mesenchymal stem cells to traffic into various tissue compartments in the NOD/SCID/MPSVII mice.12 For up to 75 days post-transplantation, donor-derived cells were observed in multiple tissues, with similar levels across the various administration routes and independent of transduction parameters. Tissue localization studies showed that the primary MSC did not proliferate extensively at the sites of lodgment (Figure 3).12 Human adipose MSC therefore represent a population of stem cells with a ubiquitous pattern of tissue distribution after administration, similar to the traditional bone marrow resident cells. However, a cellular therapy solution for MPSVII, and other enzyme deficiency disorders, will require not only widespread distribution of cells,86 but also sufficient enzyme production and secretion by the transplanted donor cells.
Figure 3.
Tissue section from nonobese diabetic/severe combined immunodeficient/MPSVII immunodeficient mouse transplanted intravenously with human adipose-derived mesenchymal stem cells. This strain is null for the enzyme beta-glucuronidase (GUSB). Tissue sections were stained for enzyme activity using a substrate (red) to identify normal GUSB levels in transplanted human MSC, and then were counterstained with methyl green. Example of MSC localization in lung is shown.
In order to evaluate the potential for MSC in treating these diseases, we performed extensive assays in vitro to quantify the GUSB enzyme production of MSC in comparison to other cell types, as well as the increased production from lentiviral transduction for constitutive expression.13 A corollary goal of this study was to examine the amount of enzyme secreted from MSC to be available for cellular re-uptake and cross-correction. While previous reports had used a variety of cell types to deliver GUSB, direct measurements of these parameters had not been performed and compared between cell types. Our results demonstrated that MSC produce and secrete minimal amounts of GUSB natively, in a comparable range with other fibroblastic cell types. Conversely, following lentiviral transduction, primary human MSC produced nearly 100-fold more GUSB per cell. In comparison with primary human hematopoietic progenitors (total lineage depleted umbilical cord blood-derived HPC) this represents a 200-fold greater production, and approximately 158% more GUSB than parallel cohorts of normal human skin fibroblasts. Consequently, this increased total GUSB production results in a similarly increased amount of secreted enzyme.13 The observed in vitro cross correction in those studies demonstrated that the engineered human MSC were producing and secreting conformationally-correct enzyme with proper phosphorylation. Therefore we demonstrated that constitutive over-expression of GUSB did not lead to faulty protein processing or excretion.13
Having demonstrated the ability of MSC to both produce and secrete supraphysiologic levels of enzyme in vitro, the next step was to ensure the fidelity and maintenance of this process in vivo. Histologic examination of multiple murine tissues post-transplant confirmed the presence of GUSB and successful cross-correction. 13 Histopathologic scoring of these tissues showed significant reduction in the amount of accumulated GAG from cellular cross-correction, with biochemical staining in many organs nearly as intense as enzymatically normal NOD-SCID animals. 13 This finding was confirmed through direct measurement of enzyme activity using tissue homogenates and was further validated using measurements of secondary enzyme elevations.13 Further validation for the efficacy of MSC to deliver enzymes in this model of MPS was provided from the pathological examination of tissues from mice that had received GUSB-expressing MSC. The tissues from those mice, in comparison to untreated controls, had a marked reduction in lysosomal storage in nearly every organ system evaluated, including previously refractory targets such as kidney and brain.13
Quantitative PCR analysis of biochemically-corrected organ lysates showed that the correction was not due to a significantly different repopulation by donor cells in the virally-engineered cohorts, and that the biochemical correction observed was due to cross-correction of murine cells. Calculations to estimate the total number of human cells present at the 4 month timepoint indicated no significant difference in engraftment levels between engineered and unmanipulated cells. Further investigation revealed that the increased production of lentiviral-expressed GUSB had allowed serum levels in treated animals to approach nearly 40% that of wild type MPSVII mice. This level of circulating GUSB represents a significant improvement over the levels attained in any other cellular therapy to date, and is the driving force behind the correction observed in these animals.13
The results of these experiments indicate that MSC may have utility in therapies involving systemic correction of protein insufficiency, even gaining access to immune-privileged organs through elevation of serum levels of therapeutic protein. In this series of experiments, the use of a clinically-relevant lentiviral vector to constitutively express GUSB in the donor cell population produced exceptional correction of disease in vivo with no evidence of negative effects on the pluripotency or proliferative capacity of mesenchymal stem cells.13 Considering the ease and efficiency of MSC transduction combined with their dispersal to multiple tissues following transplantation, human MSC have potential as a successful therapy option for MPSVII disease and other enzyme deficiencies.
E. Biosafety of genetically engineered human MSC
Numerous studies have now suggested that MSC have fewer complications regarding the insertion of virally-delivered transgenes. Whereas HSC seem to maintain a highly quiescent pool of true stem cells 42–47 that are resistant to retroviral transduction, MSC seem to have no comparable metabolic barrier. Several studies have verified this finding, demonstrating that MSC can be efficiently and durably transduced without intensive labor, and that this transgene expression is maintained throughout lineage differentiation and without compromising the proliferation rate or quality of progeny 48–51. Clonal analysis of the resultant cell populations showed wide variation; however, some clones contained several thousand copies of transgene RNA per cell and were able to maintain this expression for up to 6 months post-transduction 50. Examination of the starting cell population further showed that nearly 90% of all cells capable of producing CFU-F colonies were transduced using the standard procedures of the time 50. In comparison to HSC transduction, this represented an astounding and intriguing finding for cellular therapy and genetic engineering.
However, a fear for MSC-based tissue therapy is that ectopic bone formation, or even tumor formation could occur if the cells are not induced into the correct tissue at sites of damage. This fear is fostered by the fact that human and murine embryonic cells can form teratoma in vivo, but should not apply to primary human cells that are subject to proper contact inhibition-mediated cessation of cell division. We have specifically searched, throughout two decades of using genetically engineered human MSC in highly sensitive transplant models, for any adverse events such as ectopic aberrant tissue differentiation or tumor formation occurring from human MSC in vivo. Our groups’ immune deficient mouse studies with human marrow and adipose derived MSC are conducted under GLP (Good Laboratory Practice) conditions as mandated by the FDA, so that they can be directly translational for MSC-based tissue repair therapies. We recommend similar methods of study and record-keeping for other teams who plan to take MSC- or other cell-based therapies into the clinic. All experimentation that could be used as supporting evidence for an Investigational New Drug (IND) application to the FDA must be conducted under GLP, with proper retention of source documents and experimental write-ups kept in a separate binder with tracking to the primary data storage site.
We have had many years of experience at the level of GLP, in determining that tumors and ectopic, unwanted tissues are not formed from human bone marrow – derived MSC in immune deficient mice, when conducting careful biosafety studies for retroviral and lentiviral vector trials. Human hematopoietic stem cells and human mesenchymal stem cells carrying two different Moloney-based vectors were co-transplanted together into immune deficient mice. A total of 481 mice were monitored for adverse events for 7–18 months post-transplantation. Following co-transplantation of the engineered HSC/MSC inoculums, mice were assessed twice a day for signs of ill health, as defined by any of the following: weight loss, hunching, lethargy, rapid breathing, skin discoloration or irregularities, bloating, hemi-paresis, visibly enlarged lymph nodes, or visible solid tumors under the skin. If any type of irregularity was observed, the mouse was immediately killed by 75% CO2 / 25%O2 narcosis, autopsied, and subjected to the full range of tissue and serum banking and biosafety analyses described below. If no signs of ill health were observed, the engraftment with transduced cells was allowed to continue until 7–18 months after transplantation. No evidence for insertional mutagenesis causing human leukemias or solid tumors in any of the mice was detected. No evidence for RCR in 117 serum samples analyzed by vector rescue assay could be seen.
In addition, 149 mice were transplanted with human hematopoietic progenitor cells transduced with HIV-1–based lentiviral vectors, and were followed for 2–6 months. No adverse events caused by the vectors could be observed, and none of the mice had detectable HIV p24 antigen in their serum. Our in vivo system therefore proves to be a valuable assay for potential risk assessment when retroviral or lentiviral vectors are considered to be used in human clinical gene therapy applications. We have published a manuscript that shows the safety of retroviral and lentiviral vectors (when they are not bearing a growth factor receptor gene, such as the common gamma chain), following up to 18 months of analyses of transduced human mesenchymal and hematopoietic cells co- transplanted into over 600 mice with no immune system.14
In summary, MSC-based cellular therapy, when combined with genetic engineering, can provide a safe and effective means by which to systemically produce factors that are needed by other cells in the organs of a recipient that has enzymatic or other defects. Numerous MSC-based therapies conducted by the companies Osiris, Athersys, and others, have demonstrated the safety of systemic infusion in phase I – II trials. 5 Biosafety data from our group and others have further shown that genetic engineering of MSC can provide a safe and effective cell-based therapy for different disorders where a single protein or enzyme is lacking. Genetically engineered MSC should be considered as a cell-based therapy for some disorders, especially orphan diseases, when the risk to benefit ratio has been carefully considered.
Supplementary Material
Acknowledgements
This work was supported by the National Institutes of Health (NIH), National Institutes of Diabetes and Digestive and Kidney Diseases (NIDDK #2R01DK61848 and 2R01DK53041 (JN)), and National Heart, Lung and Blood Institute (NHLBI #RO1HL073256 (JN). The California Institute for regenerative Medicine (CIRM) provided funding for batches of human bone marrow-derived MSC that were used in comparisons when in excess from directly funded studies (CIRM TR1-01257 (JN)). Funding bodies supported salaries, equipment, mice and supplies needed for the collection and analysis of the data.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions:
Todd Meyerrose: Conception and design, Collection and/or assembly of data, Data analysis and interpretation, Manuscript writing, Final approval of manuscript
Mo Dao: Conception and design, Collection and/or assembly of data, Data analysis and interpretation, Final approval of manuscript
Scott Olson: Collection and/or assembly of data, Manuscript writing, Final approval of manuscript
Stefanos Kalomoiris: Collection and/or assembly of data, Manuscript writing, Final approval of manuscript
Yunjoon Jung: Collection and/or assembly of data, Manuscript writing, Final approval of manuscript
Geralyn Annett: Data analysis and interpretation, Manuscript writing, Final approval of manuscript
Gerhard Bauer: Manuscript writing, Final approval of manuscript
Jan A. Nolta: Conception and design, Collection and/or assembly of data, Data analysis and interpretation, Manuscript writing, Final approval of manuscript
Bibliography
- 1.Annett G, Olson S, Wirthlin L, Bauer G, Nolta JA. Mesenchymal Stem Cells for the Treatment of Neurodegenerative Diseases. Future Medicine. 2010 doi: 10.2217/rme.10.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98:1076–1084. doi: 10.1002/jcb.20886. [DOI] [PubMed] [Google Scholar]
- 3.Salem HK, Thiemermann C. Mesenchymal stromal cells: current understanding and clinical status. Stem Cells. 28:585–596. doi: 10.1002/stem.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Noort WA, Feye D, Van Den Akker F, et al. Mesenchymal stromal cells to treat cardiovascular disease: strategies to improve survival and therapeutic results. Panminerva Med. 52:27–40. [PubMed] [Google Scholar]
- 5.Tolar J, Le Blanc K, Keating A, Blazar BR. Concise review: hitting the right spot with mesenchymal stromal cells. Stem Cells. 28:1446–1455. doi: 10.1002/stem.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Ugarte DA, Morizono K, Elbarbary A, et al. Comparison of multi-lineage cells from human adipose tissue and bone marrow. Cells Tissues Organs. 2003;174:101–109. doi: 10.1159/000071150. [DOI] [PubMed] [Google Scholar]
- 7.Zuk PA, Zhu M, Mizuno H, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 8.Meyerrose T, Rosova I, Dao M, Herrbrich P, Bauer G, Nolta J. Establishment and transduction of primary human stromal/ mesenchymal stem cell monolayers. Vol. Chapter 2. Dordrecht, the Netherlands: Kluwer Academic Publishers; 2006. [Google Scholar]
- 9.Gao J, Dennis JE, Muzic RF, Lundberg M, Caplan AI. The dynamic in vivo distribution of bone marrow-derived mesenchymal stem cells after infusion. Cells Tissues Organs. 2001;169:12–20. doi: 10.1159/000047856. [DOI] [PubMed] [Google Scholar]
- 10.Karp JM, Leng Teo GS. Mesenchymal stem cell homing: the devil is in the details. Cell Stem Cell. 2009;4:206–216. doi: 10.1016/j.stem.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 11.Sordi V. Mesenchymal stem cell homing capacity. Transplantation. 2009;87:S42–S45. doi: 10.1097/TP.0b013e3181a28533. [DOI] [PubMed] [Google Scholar]
- 12.Meyerrose T, De Ugarte D, Hofling A, et al. In vivo distribution of human adipose-derived mesenchymal stem cells in novel xenotransplantation models. Stem Cells. 2007;25:220–227. doi: 10.1634/stemcells.2006-0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meyerrose TE, Roberts M, Ohlemiller KK, et al. Lentiviral-transduced human mesenchymal stem cells persistently express therapeutic levels of enzyme in a xenotransplantation model of human disease. Stem Cells. 2008;26:1713–1722. doi: 10.1634/stemcells.2008-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bauer G, Dao MA, Case SS, et al. In vivo biosafety model to assess the risk of adverse events from retroviral and lentiviral vectors. Mol Ther. 2008;16:1308–1315. doi: 10.1038/mt.2008.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Le Blanc K. Mesenchymal stromal cells: Tissue repair and immune modulation. Cytotherapy. 2006;8:559–561. doi: 10.1080/14653240601045399. [DOI] [PubMed] [Google Scholar]
- 16.Gregory CA, Ylostalo J, Prockop DJ. Adult bone marrow stem/progenitor cells (MSCs) are preconditioned by microenvironmental “niches” in culture: a two-stage hypothesis for regulation of MSC fate. Sci STKE. 2005;2005:e37. doi: 10.1126/stke.2942005pe37. [DOI] [PubMed] [Google Scholar]
- 17.Huang NF, Li S. Mesenchymal stem cells for vascular regeneration. Regen Med. 2008;3:877–892. doi: 10.2217/17460751.3.6.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kurpinski K, Chu J, Wang D, Li S. Proteomic Profiling of Mesenchymal Stem Cell Responses to Mechanical Strain and TGF-beta1. Cell Mol Bioeng. 2009;2:606–614. doi: 10.1007/s12195-009-0090-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arthur A, Zannettino A, Gronthos S. The therapeutic applications of multipotential mesenchymal/stromal stem cells in skeletal tissue repair. J Cell Physiol. 2009;218:237–245. doi: 10.1002/jcp.21592. [DOI] [PubMed] [Google Scholar]
- 20.Gnecchi M, Melo LG. Bone marrow-derived mesenchymal stem cells: isolation, expansion, characterization, viral transduction, and production of conditioned medium. Methods Mol Biol. 2009;482:281–294. doi: 10.1007/978-1-59745-060-7_18. [DOI] [PubMed] [Google Scholar]
- 21.Nolta J. Genetic Engineering of Mesenchymal Stem Cells. Kluwer; 2006. [Google Scholar]
- 22.Satija NK, Singh VK, Verma YK, et al. Mesenchymal stem cell-based therapy: a new paradigm in regenerative medicine. J Cell Mol Med. 2009;13:4385–4402. doi: 10.1111/j.1582-4934.2009.00857.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dao MA, Pepper KA, Nolta JA. Long-term cytokine production from engineered primary human stromal cells influences human hematopoiesis in an in vivo xenograft model. Stem Cells. 1997;15:443–454. doi: 10.1002/stem.150443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Friedenstein AJ, Chailakhyan RK, Latsinik NV, Panasyuk AF. Keiliss-Borok IV Stromal cells responsible for transferring the microenvironment of the hemopoietic tissues Cloning in vitro and retransplantation in vivo. Transplantation. 1974;17:331–340. doi: 10.1097/00007890-197404000-00001. [DOI] [PubMed] [Google Scholar]
- 25.Friedenstein AJ, Piatetzky S, II, Petrakova KV. Osteogenesis in transplants of bone marrow cells. J Embryol Exp Morphol. 1966;16:381–390. [PubMed] [Google Scholar]
- 26.Friedenstein AJ, Chailakhjan RK, Lalykina KS. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 1970;3:393–403. doi: 10.1111/j.1365-2184.1970.tb00347.x. [DOI] [PubMed] [Google Scholar]
- 27.Friedenstein AJ, Latzinik NW, Grosheva AG, Gorskaya UF. Marrow microenvironment transfer by heterotopic transplantation of freshly isolated and cultured cells in porous sponges. Exp Hematol. 1982;10:217–227. [PubMed] [Google Scholar]
- 28.Pereira RF, Halford KW, O'Hara MD, et al. Cultured adherent cells from marrow can serve as long-lasting precursor cells for bone, cartilage, and lung in irradiated mice. Proc Natl Acad Sci U S A. 1995;92:4857–4861. doi: 10.1073/pnas.92.11.4857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276:71–74. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- 30.Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 31.Horwitz EM, Le Blanc K, Dominici M, et al. Clarification of the nomenclature for MSC: The International Society for Cellular Therapy position statement. Cytotherapy. 2005;7:393–395. doi: 10.1080/14653240500319234. [DOI] [PubMed] [Google Scholar]
- 32.Kuznetsov SA, Krebsbach PH, Satomura K, et al. Single-colony derived strains of human marrow stromal fibroblasts form bone after transplantation in vivo. J Bone Miner Res. 1997;12:1335–1347. doi: 10.1359/jbmr.1997.12.9.1335. [DOI] [PubMed] [Google Scholar]
- 33.Bianco P, Gehron Robey P. Marrow stromal stem cells. J Clin Invest. 2000;105:1663–1668. doi: 10.1172/JCI10413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Owen M. Marrow stromal stem cells. J Cell Sci Suppl. 1988;10:63–76. doi: 10.1242/jcs.1988.supplement_10.5. [DOI] [PubMed] [Google Scholar]
- 35.Dexter TM. Stromal cell associated haemopoiesis. J Cell Physiol Suppl. 1982;1:87–94. doi: 10.1002/jcp.1041130414. [DOI] [PubMed] [Google Scholar]
- 36.Moore KA, Deisseroth AB, Reading CL, Williams DE, Belmont JW. Stromal support enhances cell-free retroviral vector transduction of human bone marrow long-term culture-initiating cells. Blood. 1992;79:1393–1399. [PubMed] [Google Scholar]
- 37.Dao MA, Nolta JA. Use of the bnx/hu xenograft model of human hematopoiesis to optimize methods for retroviral-mediated stem cell transduction (Review) Int J Mol Med. 1998;1:257–264. doi: 10.3892/ijmm.1.1.257. [DOI] [PubMed] [Google Scholar]
- 38.Wineman J, Moore K, Lemischka I, Muller-Sieburg C. Functional heterogeneity of the hematopoietic microenvironment: rare stromal elements maintain long-term repopulating stem cells. Blood. 1996;87:4082–4090. [PubMed] [Google Scholar]
- 39.Brouard N, Chapel A, Thierry D, Charbord P, Peault B. Transplantation of gene-modified human bone marrow stromal cells into mouse-human bone chimeras. J Hematother Stem Cell Res. 2000;9:175–181. doi: 10.1089/152581600319388. [DOI] [PubMed] [Google Scholar]
- 40.Ding L, Lu S, Batchu R, Iii RS, Munshi N. Bone marrow stromal cells as a vehicle for gene transfer. Gene Ther. 1999;6:1611–1616. doi: 10.1038/sj.gt.3300973. [DOI] [PubMed] [Google Scholar]
- 41.Meyerrose TE, De Ugarte DA, Hofling AA, et al. In vivo Distribution of Human Adipose-Derived MSC. Stem Cells. 2006 doi: 10.1634/stemcells.2006-0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Haas DL, Case SS, Crooks GM, Kohn DB. Critical factors influencing stable transduction of human CD34(+) cells with HIV-1-derived lentiviral vectors. Mol Ther. 2000;2:71–80. doi: 10.1006/mthe.2000.0094. [DOI] [PubMed] [Google Scholar]
- 43.Case SS, Price MA, Jordan CT, et al. Stable transduction of quiescent CD34(+)CD38(−) human hematopoietic cells by HIV-1-based lentiviral vectors. Proc Natl Acad Sci U S A. 1999;96:2988–2993. doi: 10.1073/pnas.96.6.2988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dao MA, Nolta JA. Cytokine and integrin stimulation synergize to promote higher levels of GATA-2, c-myb, and CD34 protein in primary human hematopoietic progenitors from bone marrow. Blood. 2006 doi: 10.1182/blood-2006-05-026039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schmidt M, Carbonaro DA, Speckmann C, et al. Clonality analysis after retroviral-mediated gene transfer to CD34+ cells from the cord blood of ADA-deficient SCID neonates. Nat Med. 2003;9:463–468. doi: 10.1038/nm844. [DOI] [PubMed] [Google Scholar]
- 46.Dao MA, Arevalo J, Nolta JA. Reversibility of CD34 expression on human hematopoietic stem cells that retain the capacity for secondary reconstitution. Blood. 2003;101:112–118. doi: 10.1182/blood-2002-01-0025. [DOI] [PubMed] [Google Scholar]
- 47.Dao MA, Nolta JA. CD34: to select or not to select? That is the question. Leukemia. 2000;14:773–776. doi: 10.1038/sj.leu.2401781. [DOI] [PubMed] [Google Scholar]
- 48.Li KJ, Dilber MS, Abedi MR, et al. Retroviral-mediated gene transfer into human bone marrow stromal cells: studies of efficiency and in vivo survival in SCID mice. Eur J Haematol. 1995;55:302–306. doi: 10.1111/j.1600-0609.1995.tb00701.x. [DOI] [PubMed] [Google Scholar]
- 49.Nolta JA, Hanley MB, Kohn DB. Sustained human hematopoiesis in immunodeficient mice by cotransplantation of marrow stroma expressing human interleukin-3: analysis of gene transduction of long-lived progenitors. Blood. 1994;83:3041–3051. [PubMed] [Google Scholar]
- 50.Mosca JD, Hendricks JK, Buyaner D, et al. Mesenchymal stem cells as vehicles for gene delivery. Clin Orthop Relat Res. 2000:S71–S90. doi: 10.1097/00003086-200010001-00011. [DOI] [PubMed] [Google Scholar]
- 51.Allay JA, Dennis JE, Haynesworth SE, et al. LacZ and interleukin-3 expression in vivo after retroviral transduction of marrow-derived human osteogenic mesenchymal progenitors. Hum Gene Ther. 1997;8:1417–1427. doi: 10.1089/hum.1997.8.12-1417. [DOI] [PubMed] [Google Scholar]
- 52.Bubnic SJ, Wang XH, Clark BR, Keating A. W/Wv marrow stromal cells engraft and enhance early erythropoietic progenitors in unconditioned Sl/Sld murine recipients. Bone Marrow Transplant. 2002;30:867–872. doi: 10.1038/sj.bmt.1703761. [DOI] [PubMed] [Google Scholar]
- 53.Awaya N, Rupert K, Bryant E, Torok-Storb B. Failure of adult marrow-derived stem cells to generate marrow stroma after successful hematopoietic stem cell transplantation. Exp Hematol. 2002;30:937–942. doi: 10.1016/s0301-472x(02)00821-4. [DOI] [PubMed] [Google Scholar]
- 54.Simmons PJ, Przepiorka D, Thomas ED, Torok-Storb B. Host origin of marrow stromal cells following allogeneic bone marrow transplantation. Nature. 1987;328:429–432. doi: 10.1038/328429a0. [DOI] [PubMed] [Google Scholar]
- 55.Almeida-Porada G, Flake AW, Glimp HA, Zanjani ED. Cotransplantation of stroma results in enhancement of engraftment and early expression of donor hematopoietic stem cells in utero. Exp Hematol. 1999;27:1569–1575. doi: 10.1016/s0301-472x(99)00090-9. [DOI] [PubMed] [Google Scholar]
- 56.Almeida-Porada G, Porada CD, Tran N, Zanjani ED. Cotransplantation of human stromal cell progenitors into preimmune fetal sheep results in early appearance of human donor cells in circulation and boosts cell levels in bone marrow at later time points after transplantation. Blood. 2000;95:3620–3627. [PubMed] [Google Scholar]
- 57.Noort WA, Kruisselbrink AB, in't Anker PS, et al. Mesenchymal stem cells promote engraftment of human umbilical cord blood-derived CD34(+) cells in NOD/SCID mice. Exp Hematol. 2002;30:870–878. doi: 10.1016/s0301-472x(02)00820-2. [DOI] [PubMed] [Google Scholar]
- 58.Angelopoulou M, Novelli E, Grove JE, et al. Cotransplantation of human mesenchymal stem cells enhances human myelopoiesis and megakaryocytopoiesis in NOD/SCID mice. Exp Hematol. 2003;31:413–420. doi: 10.1016/s0301-472x(03)00042-0. [DOI] [PubMed] [Google Scholar]
- 59.Kamel-Reid S, Dick JE. Engraftment of immune-deficient mice with human hematopoietic stem cells. Science. 1988;242:1706–1709. doi: 10.1126/science.2904703. [DOI] [PubMed] [Google Scholar]
- 60.Jiang J, Zhang J, Hao J, Xie S. [Promotion of post BMT hematopoiesis reconstitution by cotransplantation of IL-3 transfected marrow stromal cells in mice] Zhonghua Xue Ye Xue Za Zhi. 2002;23:407–410. [PubMed] [Google Scholar]
- 61.Jorgensen HG, Allan EK, Jiang X, et al. Stage-specific alterations in serum levels of G-CSF in chronic myeloid leukaemia. Leukemia. 2003;17:1430–1432. doi: 10.1038/sj.leu.2402967. [DOI] [PubMed] [Google Scholar]
- 62.Tsark E, Dao M, Wang X, Weinberg K, Nolta J. IL-7 enhances the responsiveness of human T cells that develop in the bone marrow of athymic mice. J Immunol. 2001;166:170–181. doi: 10.4049/jimmunol.166.1.170. [DOI] [PubMed] [Google Scholar]
- 63.Li A, Jiang J, Zhang Q, Hao J, Xie S. Cytokines transduced bone marrow stromal cell lines promote immunohematopoietic reconstitution in mice after allogeneic bone marrow transplantation. Immunol Lett. 2005;98:216–224. doi: 10.1016/j.imlet.2004.11.018. [DOI] [PubMed] [Google Scholar]
- 64.DC Ertl MD, Pepper K, Kohn DB, Nolta JA. Primary human stromal cells engineered to secrete human erythropoietin (hEpo) promote terminal human red blood cell maturation in bnx/hu mice. Blood. 1998;92:718a. [Google Scholar]
- 65.Eliopoulos N, Al-Khaldi A, Crosato M, Lachapelle K, Galipeau J. A neovascularized organoid derived from retrovirally engineered bone marrow stroma leads to prolonged in vivo systemic delivery of erythropoietin in nonmyeloablated, immunocompetent mice. Gene Ther. 2003;10:478–489. doi: 10.1038/sj.gt.3301919. [DOI] [PubMed] [Google Scholar]
- 66.Eliopoulos N, Gagnon RF, Francois M, Galipeau J. Erythropoietin delivery by genetically engineered bone marrow stromal cells for correction of anemia in mice with chronic renal failure. J Am Soc Nephrol. 2006;17:1576–1584. doi: 10.1681/ASN.2005101035. [DOI] [PubMed] [Google Scholar]
- 67.Kucic T, Copland IB, Cuerquis J, et al. Mesenchymal stromal cells genetically engineered to overexpress IGF-I enhance cell-based gene therapy of renal failure-induced anemia. Am J Physiol Renal Physiol. 2008;295:F488–F496. doi: 10.1152/ajprenal.00044.2008. [DOI] [PubMed] [Google Scholar]
- 68.Xu J, Qu J, Cao L, et al. Mesenchymal stem cell-based angiopoietin-1 gene therapy for acute lung injury induced by ]lipopolysaccharide in mice. J Pathol. 2008;214:472–481. doi: 10.1002/path.2302. [DOI] [PubMed] [Google Scholar]
- 69.Griffin M, Greiser U, Barry F, O'Brien T, Ritter T. Genetically modified mesenchymal stem cells and their clinical potential in acute cardiovascular disease. Discov Med. 9:219–223. [PubMed] [Google Scholar]
- 70.Nesselmann C, Ma N, Bieback K, et al. Mesenchymal stem cells and cardiac repair. J Cell Mol Med. 2008;12:1795–1810. doi: 10.1111/j.1582-4934.2008.00457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Freimark D, Czermak P. Cell-based regeneration of intervertebral disc defects: review and concepts. Int J Artif Organs. 2009;32:197–203. doi: 10.1177/039139880903200403. [DOI] [PubMed] [Google Scholar]
- 72.Aslan H, Sheyn D, Gazit D. Genetically engineered mesenchymal stem cells: applications in spine therapy. Regen Med. 2009;4:99–108. doi: 10.2217/17460751.4.1.99. [DOI] [PubMed] [Google Scholar]
- 73.van Velthoven CT, Kavelaars A, van Bel F, Heijnen CJ. Regeneration of the ischemic brain by engineered stem cells: fuelling endogenous repair processes. Brain Res Rev. 2009;61:1–13. doi: 10.1016/j.brainresrev.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 74.Boison D. Engineered adenosine-releasing cells for epilepsy therapy: human mesenchymal stem cells and human embryonic stem cells. Neurotherapeutics. 2009;6:278–283. doi: 10.1016/j.nurt.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Steinert AF, Noth U, Tuan RS. Concepts in gene therapy for cartilage repair. Injury. 2008;39(Suppl 1):S97–S113. doi: 10.1016/j.injury.2008.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Suzuki M, McHugh J, Tork C, et al. Direct muscle delivery of GDNF with human mesenchymal stem cells improves motor neuron survival and function in a rat model of familial ALS. Mol Ther. 2008;16:2002–2010. doi: 10.1038/mt.2008.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Donsante A, Levy B, Vogler C, Sands MS. Clinical response to persistent, low-level beta-glucuronidase expression in the murine model of mucopolysaccharidosis type VII. J Inherit Metab Dis. 2007;30:227–238. doi: 10.1007/s10545-007-0483-4. [DOI] [PubMed] [Google Scholar]
- 78.Kyle JW, Birkenmeier EH, Gwynn B, et al. Correction of murine mucopolysaccharidosis VII by a human beta-glucuronidase transgene. Proc Natl Acad Sci U S A. 1990;87:3914–3918. doi: 10.1073/pnas.87.10.3914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Simonaro CM, Haskins ME, Abkowitz JL, et al. Autologous transplantation of retrovirally transduced bone marrow or neonatal blood cells into cats can lead to long-term engraftment in the absence of myeloablation. Gene Ther. 1999;6:107–113. doi: 10.1038/sj.gt.3300797. [DOI] [PubMed] [Google Scholar]
- 80.Wolfe JH, Sands MS, Barker JE, et al. Reversal of pathology in murine mucopolysaccharidosis type VII by somatic cell gene transfer. Nature. 1992;360:749–753. doi: 10.1038/360749a0. [DOI] [PubMed] [Google Scholar]
- 81.Marechal V, Naffakh N, Danos O, Heard JM. Disappearance of lysosomal storage in spleen and liver of mucopolysaccharidosis VII mice after transplantation of genetically modified bone marrow cells. Blood. 1993;82:1358–1365. [PubMed] [Google Scholar]
- 82.Moullier P, Marechal V, Danos O, Heard JM. Continuous systemic secretion of a lysosomal enzyme by genetically modified mouse skin fibroblasts. Transplantation. 1993;56:427–432. doi: 10.1097/00007890-199308000-00034. [DOI] [PubMed] [Google Scholar]
- 83.Moullier P, Bohl D, Cardoso J, Heard JM, Danos O. Long-term delivery of a lysosomal enzyme by genetically modified fibroblasts in dogs. Nat Med. 1995;1:353–357. doi: 10.1038/nm0495-353. [DOI] [PubMed] [Google Scholar]
- 84.Hofling AA, Vogler C, Creer MH, Sands MS. Engraftment of human CD34+ cells leads to widespread distribution of donor-derived cells and correction of tissue pathology in a novel murine xenotransplantation model of lysosomal storage disease. Blood. 2003;101:2054–2063. doi: 10.1182/blood-2002-08-2597. [DOI] [PubMed] [Google Scholar]
- 85.Hofling AA, Devine S, Vogler C, Sands MS. Human CD34+ hematopoietic progenitor cell-directed lentiviral-mediated gene therapy in a xenotransplantation model of lysosomal storage disease. Mol Ther. 2004;9:856–865. doi: 10.1016/j.ymthe.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 86.Hess DA, Craft TP, Wirthlin L, et al. Widespread tissue distribution by transplanted human progenitor cells with high aldehyde dehydrogenase activity. Stem Cells. 2008;26:611–620. doi: 10.1634/stemcells.2007-0429. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.