Abstract
Controlled generation of reactive oxygen species orchestrates numerous physiological signaling events (Finkel, T. (2011) Signal transduction by reactive oxygen species. J. Cell Biol. 194, 7–15). A major cellular target of reactive oxygen species is the thiol side chain (RSH) of Cys, which may assume a wide range of oxidation states (i.e. −2 to +4). Within this context, Cys sulfenic (Cys-SOH) and sulfinic (Cys-SO2H) acids have emerged as important mechanisms for regulation of protein function. Although this area has been under investigation for over a decade, the scope and biological role of sulfenic/sulfinic acid modifications have been recently expanded with the introduction of new tools for monitoring cysteine oxidation in vitro and directly in cells. This minireview discusses selected recent examples of protein sulfenylation and sulfinylation from the literature, highlighting the role of these post-translational modifications in cell signaling.
Keywords: Hydrogen Peroxide, Post-translational Modification, Redox Regulation, Redox Signaling, Thiol, Cysteine Oxidation
Sulfenic Acid Formation and Reactivity
RSOH is directly generated by the oxidation of RSH with two-electron oxidants (Fig. 1A). Hydrogen peroxide (H2O2) reacts with small-molecule thiols at a constant rate of ∼20 m−1 s−1, but this reaction can take place up to 8 orders of magnitude faster (105–108 m−1 s−1) with specific Cys residues within proteins (2). The propensity of Cys residues to undergo oxidation is influenced mainly by three general factors: thiol nucleophilicity, surrounding protein microenvironment, and proximity of the target thiol to the reactive oxygen species (ROS)2 source. Peroxide-mediated thiol oxidation is an SN2 reaction (Fig. 1B) whereby the actual reactive species is the much more nucleophilic thiolate (RS−). Accordingly, susceptibility to oxidation is usually correlated with pKa, although for cysteines with pKa < 7, the RS− becomes less nucleophilic with the decrease in pKa (3). In proteins, microenvironments can influence Cys acidity through the presence of polar amino acids or specific hydrogen bonds, which contribute to a decrease in pKa by balancing the negative charge on the sulfur atom (4). The same interactions, which affect the pKa of Cys thiol, also influence the stability of the related sulfenic acid. The microenvironment can also help to stabilize the leaving group by lowering the transition state energy barrier (2). However, these parameters are not sufficient to rationalize the selective oxidation of specific proteins. Increasing evidence shows that ROS signaling responses are compartmentalized, and the proximity of the target protein to the ROS source is a key aspect of spatial regulation of Cys oxidation (5, 6).
FIGURE 1.
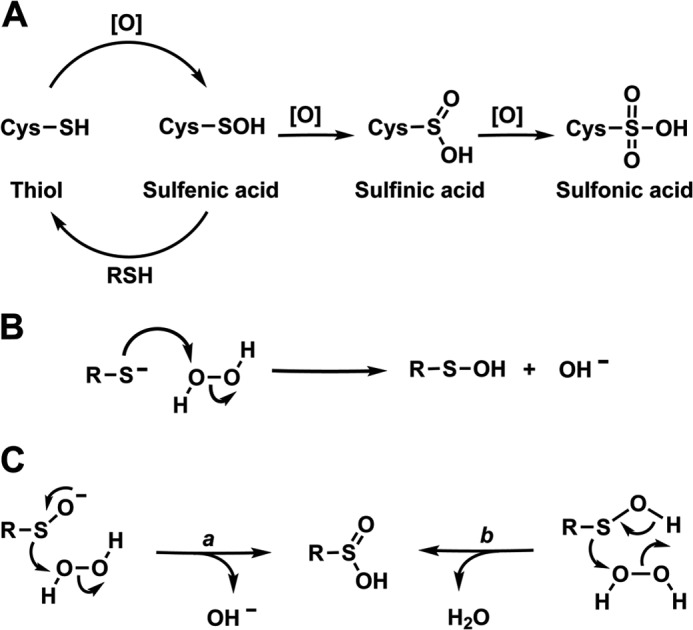
Main oxidative modifications of protein cysteine residues. A, the diagram shows the main oxidative modifications of protein cysteine residues. The initial reaction of cysteine with oxidants ([O] = ROS/RNS) yields a sulfenic acid (SOH). Once formed, the SOH can be reduced to thiol or further oxidized to generate Cys-SO2H and Cys-SO3H. B, thiolate anion is much more nucleophilic than the corresponding protonated form and can be readily oxidized to sulfenic acid. The protein microenvironment can help to stabilize the poor hydroxide leaving group and thus accelerate the reaction rate. C, two possible mechanisms have been proposed for the H2O2-mediated oxidation of RSOH to RSO2H: the first pathway, which involves the direct participation of a sulfenate anion, or the second concerted mechanism, which is mediated by a hydrogen bond.
By virtue of the transient nature of RSOH, the study of its chemical-physical properties has been rendered extremely challenging. The pKa of RSOH has been determined in only a few proteins (7, 8). The experimentally determined pKa of some small-molecule sulfenic acids is 1–2 orders of magnitude lower than that of the corresponding thiols (9, 10); however, it is not clear whether such compounds are appropriate models of cysteine sulfenic acid in proteins. From the chemical point of view, RSOH exhibits both electrophilic and nucleophilic behavior. Thiosulfinate formation clearly exemplifies this dual nature (11), although this self-condensation has little biological relevance due to high abundant thiols and steric hindrance, which make this reaction negligible in cells. Therefore, oxidation to Cys-SO2H appears to be the only significant reaction in which RSOH exhibits its nucleophilic nature. In contrast, this species shows high reactivity toward nucleophiles. Intramolecular or low-molecular-weight thiols may react with RSOH to generate a mixed disulfide, which constitutes the principal mechanism for disulfide bond formation in proteins (12). In the absence of adjacent thiols, RSOH can also react with nitrogen nucleophiles to form a sulfenamide, although this species has been identified in only a few proteins (13, 14).
Sulfenic Acid as a Post-translational Modification
Disulfide and sulfenamide formations protect Cys-SOH from further oxidation and lay the foundation for redox signaling. In fact, these post-translational modifications (PTMs) can generate conformational changes in protein structure and subsequent modulation of protein activity. In addition, as a result of its intrinsic nucleophilicity, Cys is present in the active site of many enzymes. Transient oxidation of these Cys residues is a well established process through which proteins can be spatially and temporally inhibited (1).
From the first evidence of its existence reported in 1976 (15) to the present, RSOH has been identified in a relatively small number of proteins. In fact, the identification of this elusive modification remains difficult. In 2008, Fetrow and co-workers (16) published a review that included a list of 47 proteins in which Cys-SOH was identified by crystal structure analysis. Because identification of the crystal structure of protein-SOH is problematic, their list represents only the tip of the iceberg. Direct mass analysis shows similar issues, making the use of chemical probes the only suitable technique to monitor RSOH formation (17).
Table 1 provides a list of the principal proteins in which Cys-SOH has been identified using chemical-trapping reagents. In addition to 4-chloro-7-nitrobenzofurazan, which can be employed only in vitro, dimedone-based probes (DBPs) are emerging as the most promising tool for RSOH trapping. These reagents are capable of crossing the cell membrane, capturing RSOHs directly in the cell (17). We concentrated our attention exclusively on those proteins in which the formation of Cys-SOH has been experimentally substantiated and shown to play a regulatory role. Proteomic studies based on the employment of DBPs have identified many other proteins that are able to generate an RSOH transient species in cells (18, 19), although the biological relevance of these oxidations remains unclear. Table 1 clearly demonstrates that many redox-regulated proteins are directly involved in cell signaling.
TABLE 1.
Protein sulfenic acid modification
IKK-β, IκB kinase β; PGKase, phosphoglycerate kinase; L-PYK, liver pyruvate kinase; MTAP, methylthioadenosine phosphorylase.
| Function/protein | Organism | Cys-SOH | Ref. |
|---|---|---|---|
| Peroxidase | |||
| AhpC | M. tuberculosis | Cys-165a | 80 |
| Prx | H. sapiens | Cys-51 (PrxI)a,b | 60, 81 |
| Orp1/GPx3 | S. cerevisiae | Cys-36a | 38 |
| Phosphatase | |||
| PTP1B | H. sapiens | Cys-215a | 6, 82 |
| YopH | Y. enterocolitica | Cys-403a | 82 |
| PTEN | H. sapiens | Cys-124a | 6, 21 |
| Cdc25a | H. sapiens | Cys-431a | 83 |
| SHP-1 | H. sapiens, M. musculus | Cys-455a | 22, 84 |
| SHP-2 | H. sapiens, M. musculus | Cys-459a | 6, 22 |
| Kinase | |||
| EGFR | H. sapiens | Cys-797a | 6 |
| JAK2 | M. musculus | Cys-866/Cys-917a | 27 |
| Akt2 | M. musculus | Cys-124a | 28 |
| IKK-β | H. sapiens | Cys-179a | 41 |
| RegB | R. capsulatus | Cys-265a | 85 |
| PGKase | P. tricornutum | Cys-77c | 86 |
| L-PYK | H. sapiens | Cys-436b,c | 87 |
| Transcription Factor | |||
| AphB | V. cholerae | Cys-235c | 35 |
| MgrA | S. aureus | Cys-12c | 32 |
| SarZ | S. aureus | Cys-13b,c | 36 |
| OhrR | B. subtilis | Cys-15c | 34 |
| OxyR | E. coli | Cys-199c | 88 |
| CrtJ | R. capsulatus | Cys-420a | 33 |
| p50 (NF-κB ) | H. sapiens | Cys-62a | 40 |
| Cysteine Protease | |||
| USP1 | H. sapiens | Cys-90a | 43 |
| USP7 | H. sapiens | Cys-223a | 43 |
| A20 | H. sapiens | Cys-103a | 44 |
| Cathepsin K | H. sapiens | Cys-25a,a,c | 89 |
| Papain | P. latex | Cys-25a | 80 |
| Channel | |||
| Kv1.5 | H. sapiens | Cys-581a | 50 |
| Oxidoreductase | |||
| GAPDH | O. cuniculus | Cys-298a | 81 |
| Aldose reductase | H. sapiens | Cys-298a | 90 |
| MsrA | S. cerevisiae | Cys-72a,b | 91 |
| Transferase | |||
| MTAP | H. sapiens | Cys-136/Cys-223c | 92 |
| Integrin | |||
| α7β1 | R. rattus | Cys-923/Cys-928a | 93 |
| Chaperone | |||
| Hsp70 | H. sapiens | Cys-306a | 94 |
| Serum protein | |||
| HSA | H. sapiens | Cys-34a,c | 51 |
| Oxygen carrier | |||
| Hemoglobin | H. sapiens | Cys- 93a | 95 |
| Apoptotic Regulator | |||
| Bcl-2 | H. sapiens | Cys-158/Cys-229a | 96 |
| Cytoskeleton Protein | |||
| β-Actin | H. sapiens | Cys-272a | 97 |
a Identified using dimedone or DBPs.
b Identified by crystal structure.
c Identified using 4-chloro-7-nitrobenzofurazan.
Protein-tyrosine Phosphatases
Tyrosine phosphorylation levels are maintained by the balanced action of protein-tyrosine kinases (PTKs) and phosphatases (PTPs). Sulfenylation of the catalytic Cys residue (pKa = 4–6) of PTPs has emerged as a dynamic mechanism for inactivation of this protein family (20). The half-life of RSOH is generally quite low in PTPs. In fact, a neighboring cysteine residue (e.g. in PTEN) or the backbone amide nitrogen (PTP1B) readily reacts with RSOH to yield an intramolecular disulfide (21) or a cyclic sulfenamide species (14), respectively.
Recently, an alternative mechanism of inactivation emerged in SH2 domain-containing PTPs (SHP-1 and SHP-2) (22), which possess two highly conserved distal cysteines, both of which can generate a disulfide with the oxidized catalytic Cys residue. This intermediate disulfide typically rearranges into the more stable disulfide formed by the two backdoor cysteines to regenerate the free catalytic Cys residue. Surprisingly, the conformational change produced by the backdoor disulfide leads to an increased catalytic Cys pKa value (∼9) with resultant inhibition.
Although SHP-1 and SHP-2 have structural similarities, they are regulated by different cell signaling pathways. For example, SHP-2 exhibits selective oxidation in response to PDGF in association with a PDGF receptor (23). However, these regulatory differences can be influenced by the method used to analyze their oxidation state. Employing an indirect RSOH detection method, T-cell activation, which induces H2O2 production, has shown transient oxidation of SHP-2 but not of SHP-1 (24). In a later work in which a DBP was used, both SHP-1 and SHP-2 showed Cys oxidation after T-cell activation, although with a different response time (25). These results could be rationalized by the greater sensitivity of direct RSOH analysis.
In vitro experiments have demonstrated that H2O2 deactivates SHP-1 and SHP-2 with second-order rate constants of 2.0 and 2.4 m−1 s−1, respectively (22). These values are similar to those observed with other PTPs and are apparently too low to justify their oxidation within the cellular context. Recently, our group has observed that, following EGF stimulation, SHP-2 forms a complex with the EGF receptor (EGFR) and Nox2 (6), which could provide an explanation for its high propensity to oxidize. In a similar manner, PTP1B, which is localized exclusively on the cytoplasmic face of the endoplasmic reticulum, appears to be oxidized through the H2O2 generated by Nox4, an NADPH oxidase highly abundant in the endoplasmic reticulum (5). These two examples highlight the importance of the proximity of the target protein to the ROS source in explaining PTP oxidation.
Kinases
Increasing research has highlighted the key role of H2O2 in the modulation of PTK activity. In comparison with PTPs, which are always inhibited by ROS, the oxidation of PTKs can lead to both enhancement and inhibition of kinase activity (26, 27).
The central role of Cys oxidation in PTK activity is exemplified by the redox control of EGFR signaling. EGFR is a receptor tyrosine kinase, the activation of which is involved in the regulation of cell proliferation, differentiation, and survival. In addition to promoting the tyrosine phosphorylation of protein targets, EGFR stimulation triggers the production of endogenous H2O2 by Nox activation. This localized increase in H2O2 concentration leads to the sulfenylation of a conserved Cys residue located within the intracellular kinase domain of EGFR (Cys-797), which enhances its tyrosine kinase activity (6). The redox regulation of EGFR could represent a more general mechanism for the modulation of other receptor tyrosine kinase activity. In fact, nine additional members of this family show a Cys residue structurally analogous to EGFR Cys-797, although further studies are needed in this direction.
Recently, Akt (a serine/threonine protein kinase) was also identified as a redox target. PDGF stimulation of fibroblasts induced H2O2 production, which led to isoform-specific regulation of Akt2 (28). A cysteine (Cys-124) positioned in the linker region connecting the PH (pleckstrin homology) domain to the kinase domain was found to be susceptible to sulfenylation. Cys-124-SOH can generate a disulfide with two distinct Cys residues (Cys-297 and Cys-311) located in the kinase domain. This modification negatively modulates Akt2, although in vitro experiments showed that disulfide formation has no direct effect on kinase activity. The inhibition mechanism remains unclear, but a previous work showed that Akt oxidation enhances its association with PP2A (protein phosphatase 2A), which could promote dephosphorylation of Akt (29).
Transcription Factors
In addition to the redox switch of PTK and PTP activities, which indirectly regulate transcription factors (TFs), H2O2 can directly modulate several TFs through the formation of intra- and intermolecular disulfide bonds (30). The first evidence of a redox-sensitive TF was identified in the bacterial TF OxyR, in which Cys-SOH mediates disulfide bond formation between Cys-199 and Cys-208 (31). Many other TFs are redox-regulated in prokaryotes (32–36), but relatively few cases have been identified in eukaryotes. In yeast, the activation of Yap1 represents an interesting case of TF redox regulation in which Gpx3-SOH mediates the oxidation of Yap1 through the formation of a Gpx3-Yap1 intermolecular disulfide (37, 38).
The anti-apoptotic NF-κB remains the only mammalian TF in which formation of Cys-SOH has been verified experimentally; however, this modification may also have a role in other peroxide-sensitive pathways of gene activation, such as the Nrf2/Keap1 system (39). H2O2 negatively switches the DNA affinity of NF-κB: directly through the oxidation of its p50 subunit at Cys-62 (40) and indirectly via Cys-179 sulfenylation of the β-subunit of the IκB kinase complex (IκB kinase β), the kinase that is responsible for the NF-κB activation along the canonical pathway (41).
Cysteine Proteases
Protein ubiquitination has emerged as a central PTM whereby lysine residues are conjugated to ubiquitin (Ub), a 76-amino acid polypeptide (42). Deubiquitinating enzymes (DUBs) cleave Ub or Ub-like proteins from the target, contributing to the balance of the Ub system. Four of the five different families of DUBs are cysteine proteases, which share in common a low-pKa Cys residue essential for the catalytic mechanism.
Recently, three distinct works have shown that Cys oxidation can modulate DUB activity. Cotto-Rios et al. (43) reported transient sulfenylation of catalytic Cys for several members of the Ub-specific protease (USP) family and for UCH-L1. In particular, the authors established that USP1, a DUB involved in DNA damage response pathways, is reversibly inactivated following the induction of oxidative stress in cells. Additionally, Komander, in collaboration with our group (44), demonstrated that many members of the ovarian tumor DUBs also undergo Cys oxidation upon H2O2 treatment, including the tumor suppressor A20. Crystal structure analysis of oxidized A20 showed that transient RSOH can be stabilized by the formation of hydrogen bonds with the highly conserved residues located in the loop preceding catalytic Cys. Both works noted that each DUB family member exhibits a distinct level of sensitivity to oxidation. Differences in behavior can reflect various ranges of catalytic activation in which the conformational inactive enzyme could be less susceptible to oxidation. Lee et al. (45) confirmed this hypothesis by showing that preincubation of USP7 with Ub, which behaves as an allosteric activator, increases USP7 sensitivity to ROS.
An analogous inhibition has been found in small Ub-like modifier (SUMO) proteases. H2O2 treatment induces RSOH-mediated formation of an intermolecular disulfide in the yeast SUMO protease Ulp1 and in its human equivalent, SENP1 (46). Interestingly, SUMOylation also appears to be redox-regulated by reversible oxidation of the catalytic Cys residue of SUMO-conjugating enzymes (47), although no clear evidence of Cys-SOH formation has been provided.
Ion Channels
It is well established that ROS plays a regulatory role for some ion channels (48), but little is known about the molecular mechanism through which this modulation is explicated. For example, human T-helper lymphocyte ORAI1 channels, a member of the CRAC (Ca2+ release-activated Ca2+) channel family, are inhibited by oxidation of the extracellular Cys-195 residue (49), although the nature of this Cys oxidation remains unknown.
One exception is represented by the redox regulation of Kv1.5, a potassium voltage-gated channel expressed in the heart and pulmonary vasculature. Several studies have highlighted the fact that increased ROS concentration in cells is correlated with a reduction in Kv1.5 expression but have not provided a clear relationship between the two events. In collaboration with the Martens laboratory, we were recently able to elucidate the specific mechanism for Kv1.5 channel redox regulation (50). Labeling studies with DBPs have shown that a single Cys residue located in the extracellular C-terminal domain of Kv1.5 (Cys-581) forms Cys-SOH after H2O2 exposure. This modification triggers channel internalization, blocking Kv1.5 recycling to the cell membrane, and promotes its degradation.
Cellular Lifetime of Sulfenic Acid
Although limited solvent access and nearby hydrogen bond acceptors would contribute to RSOH stabilization, the absence of proximal thiols capable of generating an intramolecular disulfide is considered a major stabilizing factor. In the absence of neighboring Cys residues, RSOH can be directly reduced to RSH by thioredoxin (Trx) (Fig. 2A, Cycle 1) or may react with GSH to generate a mixed disulfide, which is later reduced by glutaredoxin (Fig. 2A, Cycle 2). For example, human serum albumin (HSA) has only one free cysteine residue (Cys-34), which is susceptible to H2O2 oxidation (rate constant of 2.5 m−1 s−1). We can estimate the half-life of HSA-SOH based on its reaction with GSH. Using the known second-order rate constant for this reaction (∼3 m−1 s−1) (51) and estimating the GSH concentration at 1 mm, the first-order rate constant would be 0.003 s−1. Substituting this value in the equation t½ = ln 2/k, the estimated half-life of HSA-SOH would be ∼4 min.
FIGURE 2.
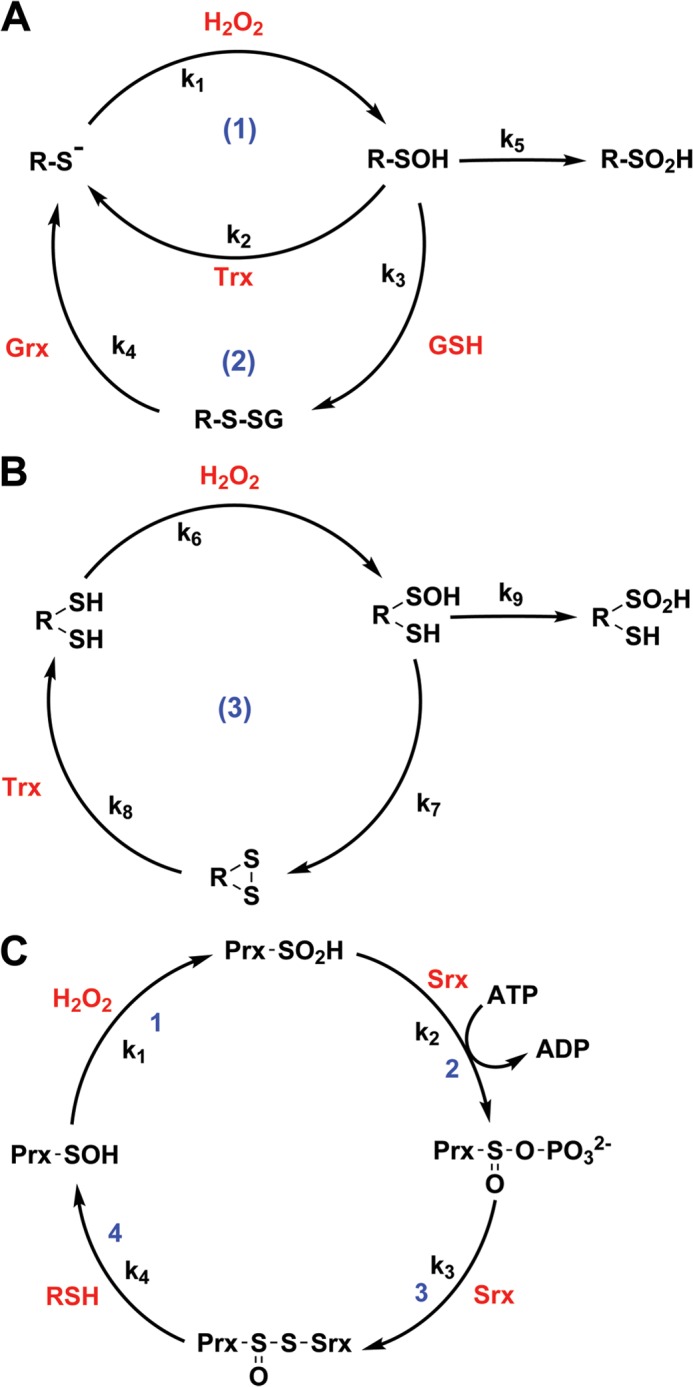
Sulfenic and sulfinic acid redox cycles. A, RSOH can be directly reduced to free thiol by Trx, although the importance of this pathway in cells is still debated (Cycle 1). RSOH can also react with GSH to generate a mixed disulfide (although not all protein-SOHs are accessible to GSH), which is subsequently reduced by glutaredoxin (Grx; Cycle 2). B, in the presence of a neighboring Cys residue, RSOH forms an internal disulfide that is later reduced by Trx (Cycle 3). C, typical eukaryotic 2-Cys Prxs are inactivated by overoxidation to sulfinic acid (Step 1). Srx restores the sulfenic acid group using an ATP-dependent mechanism in which an activated sulfinic phosphoryl ester is generated (Step 2). This intermediate collapses to form a thiosulfinate moiety with Cys-99 of Srx (Step 3). It has been proposed that this intramolecular thiosulfinate is finally resolved by a common cellular reductant, such as GSH or Trx, with consequent release of Prx-SOH (Step 4).
In contrast, many redox-regulated proteins have a second proximal Cys residue that can form an internal disulfide with RSOH (Fig. 2B). In Cdc25c, for example, Cys-377-SOH reacts with the “backdoor” Cys-330 residue at a rate constant of 0.012 s−1 (52). Applying the same calculations as above, the half-life of Cdc25c-SOH would be ∼1 min. Taken together, these estimated protein-SOH half-lives correlate well with the sulfenylation studies published by our group, and they appear similar to the cellular lifetimes of many other PTMs, such as phosphorylation. In A431 cells, we observed a peak of protein sulfenylation at ∼5 min after EGF stimulation, with a subsequent decay over 30 min (6).
Sulfinic Acid Formation and Reactivity
RSOH may be overoxidized to RSO2H by two-electron oxidants (Fig. 1A). This reaction requires nucleophilic attack by RSOH on the peroxide species. Although the H2O2-mediated oxidation of RSOH can proceed through two possible pathways (Fig. 1C), the pH profile indicates that sulfenate anion (RSO−) is the reacting species. Therefore, the pKa of RSOH should influence this reaction (7). As we emphasized above, the formation of a more stable disulfide (or sulfenamide) should prevent RSOH oxidation. Taking Cdc25c as an example, the oxidation of Cys-377-SOH to RSO2H has a rate constant of 110 m−1 s−1 (52); this value is on par with the general tendency of protein-SOHs to oxidize, which is generally in the range of 10–102 m−1 s−1 (7, 51, 52). Because internal disulfide formation has a rate constant of 0.012 s−1, the oxidation of Cys-377-SOH has significance only over 100 μm H2O2.
With a pKa of ∼2, RSO2H exists exclusively in a deprotonated form at physiological pH. The sulfinate group (RSO2−), which behaves primarily as a soft nucleophile (53), shows low spontaneous reactivity in cells, and because it is not reducible by typical cellular reductants, its oxidation to sulfonic acid (RSO3H) (Fig. 1A) appears to be the only relevant reaction in cells. The considerations adduced above for RSOH stability can also be applied to RSO2H; therefore, the formation of hydrogen bonds and steric hindrance may stabilize Cys-SO2H within proteins, reducing its propensity to oxidize (54).
Sulfinic Acid as a PTM
Cys-SO2H was long considered merely an artifact of protein purification. However, increasing evidence indicates that hyperoxidation to RSO2H is not a rare event. Indeed, quantitative analysis of soluble proteins from rat liver has shown that ∼5% of Cys residues exist as Cys-SO2H (55). Finally, the discovery of sulfiredoxin (Srx), an ATP-dependent protein that specifically reduces Cys-SO2H in the peroxiredoxin (Prx) family, has opened the door to an additional layer of redox regulation and increased interest in this specific modification (56).
Table 2 provides a list of proteins in which a biological functional role has emerged for RSO2H. In comparison with Table 1, the number of reported proteins is decidedly exiguous. This does not necessarily indicate that RSO2H plays a negligible role in protein redox regulation but rather reflects the lack of robust methods for monitoring the formation of such modifications within proteins. Although RSO2H shows higher stability in comparison with RSOH, mass and crystal structure analyses can introduce a high percentage of artifacts. In addition, the emerging relevance of persulfide modification (RSSH), which has the same nominal mass shift of 32 Da, makes the use of high-resolution mass spectroscopy essential (57). We believe that the development of chemical probes capable of specifically trapping RSO2H will push this Cys modification from the minor role to which it has been relegated. In this connection, we recently proposed the use of aryl-nitroso compounds as chemoselective probes for RSO2H (58).
TABLE 2.
Protein sulfinic acid modification
L-PGDS, lipocalin-type prostaglandin D synthase.
| Function/protein | Organism | Cys-SOH | Ref. |
|---|---|---|---|
| Peroxidase | |||
| Prx | H. sapiens | Cys-51 (PrxI)a | 60 |
| Chaperone (?) | |||
| DJ-1 | H. sapiens | Cys-106 | 67–69 |
| YaiL | E. coli | Cys-106b | 98 |
| Oxidoreductase | |||
| d-Amino acid oxidase | T. variabilis | Cys-108a | 99 |
| Protease | |||
| MMP-7 | H. sapiens | Prodomain Cysb | 72 |
| Hydratase | |||
| NHase | P. thermophila | Cys-131b | 74 |
| SCNase | R. erythropolis | Cys-133b | 77 |
| S-Transferase | |||
| L-PGDS | H. sapiens | Cys-65a | 100 |
a Identified by mass.
b Identified by crystal structure.
Prxs and Srx
Prxs are a family of cysteine-based peroxidases that remove H2O2 and other peroxides from cells. Being highly abundant and exceptionally efficient (second-order rate constant of 105–107 m−1 s−1), Prxs maintain the cytosolic concentration of H2O2 under 100 nm (59). Therefore, regulation of Prx activity is required to trigger H2O2-mediated intracellular signaling.
Typical Prxs exist in antiparallel dimeric or decameric forms and possess two Cys residues: “peroxidatic” Cys, which reacts directly with H2O2 to generate Cys-SOH, and “resolving” Cys, which forms an intramolecular disulfide with transient sulfenic acid. Finally, Trx reduces disulfide, restoring the catalytic cycle. Eukaryotic 2-Cys Prxs possess two sequence motifs (GGLG and YF) in their C termini that reduce the ability of resolving Cys to approach peroxidatic Cys-SOH (60). The resulting decrease in the disulfide formation rate allows a second molecule of H2O2 to react with peroxidatic Cys-SOH (Fig. 2C), generating a Cys-SO2H. Such overoxidation leads to the deactivation of peroxidase activity and the formation of high-molecular-weight aggregates, which exhibit molecular chaperone activity (61). Although just 0.1% of peroxidatic Cys in human PrxI is oxidized to Cys-SO2H during each turnover (62) at low concentrations of H2O2, recent kinetic studies demonstrate that Prx2 and Prx3 can undergo appreciable hyperoxidation without requiring recycling of the disulfide (63).
The peroxidase activity of 2-Cys Prxs is restored by Srx (64). The first step in the proposed catalytic mechanism involves the oxygen attack of RSO2− on the γ-phosphate of ATP and the resulting generation of a sulfinic phosphoryl ester (Fig. 2C). This species represents a sort of activated SO2H, which collapses to a thiosulfinate intermediate (Prx-S(O)-S-Srx) after attack by a conserved Cys residue in Srx (65). Thiosulfinate is subsequently resolved by a third reducing species. Kinetic studies show that Srx is an inefficient enzyme. The rate of Prx-SO2H reduction is indeed rather low (k2 > 120 s−1, k3 ∼ 85 s−1), suggesting that Prx requires a slow reparation process to allow H2O2 transient accumulation in response to extracellular signals.
Parkinson Disease Protein DJ-1
DJ-1 is a homodimeric small protein that has been associated with early-onset Parkinson disease (66). Many studies demonstrate that DJ-1 protects cells against oxidative stress-mediated apoptosis; however, the mechanism of its protective function remains largely unknown (54).
A conserved Cys residue, Cys-106, is extremely sensitive to oxidation and tends to form a Cys-SO2H species, the generation of which appears to be critical for DJ-1 function. The highly conserved Glu-18 residue facilitates the ionization of Cys-106, reduces its pKa, and helps to stabilize Cys-106-SO2H through the formation of an unusually short and consequently strong hydrogen bond (67). Wilson and co-workers (68) have shown that small changes in this position can drastically influence the oxidation propensity of Cys-106. For example, in the DJ-1 E18D mutant, the distance between the thiolate and the protonated carboxylic side chain is increased, and Cys-106 is oxidized predominantly to sulfenic acid (68). In fact, Asp-18 tends to stabilize Cys-106-SOH, hampering further oxidation. In contrast, the structurally similar E18N mutant shows an increased propensity to oxidize even in the absence of H2O2. More important, E18D mutants fail to protect cells from ROS, whereas E18N mutant show similar levels of cell viability in comparison with the wild type, demonstrating that Cys-106 oxidation to RSO2H is essential for maintaining protective functions (69).
Considering the high propensity of Cys-106 to oxidize, it has been proposed that DJ-1 acts merely as a direct ROS scavenger. However, an elegant new study reported that the DJ-1 C106DD mutant is still able to protect cells against oxidative stress (70), excluding direct scavenger action by Cys oxidation.
Cysteine Oxidation and Metal Binding Properties
Cys residues are very common in metal-binding motifs and can form coordinative bonds with several metal ions, including zinc, copper, and iron. Many proteins contain a Cys-zinc-Cys complex, for example, which furnishes structural rigidity. Oxidation of these cysteines causes Zn2+ release and a subsequent conformational change, which can switch protein function. Although oxidation is usually transient, through the formation of a disulfide bond, in some cases, it can lead to irreversible Cys-SO2H (71).
Redox zinc switching is also involved in the activation of matrix metalloproteinases (MMPs). Matrilysin (MMP-7) contains a highly conserved cysteine switch sequence, PRCGVPDVA, in its prodomain. The thiolate side chain coordinates the catalytic Zn2+, contributing to the maintenance of enzyme inactivity. Fu et al. (72) showed that hypochlorous acid (HOCl), but not H2O2, can activate the enzyme through the conversion of Cys to RSO2H, which disrupts zinc coordination. An analogous redox mechanism also appears to be involved in the activation of other MMPs (73).
The unique active site of nitrile hydratase (NHase) offers a sort of compendium of thiol oxidation states and metal coordinations. Structural analysis reveals that NHase contains an FeIII or CoIII active site, in which three Cys residues, with three different oxidation states (RSH, RSOH, and RSO2H), contribute to the coordination of the metal ion (74, 75). The fully reduced enzyme appears inactive, suggesting that Cys sulfenylation and sulfinylation are critical in maintaining the catalytic activity of NHase (76), probably by increasing the Lewis acidity of the metal ion. An analogous motif was more recently found in the catalytic site of thiocyanate hydrolase (SCNase), which incorporates CoIII only after Cys oxidation (77).
The active site of NHase and SCNase suggests that the oxidation state may influence Cys binding properties, switching the affinity from zinc (for RSH) to iron and cobalt (for oxygenated sulfur species). This change could provide additional redox control of protein functions (78).
Conclusions and Perspectives
Protein sulfenylation influences a wide range of PTMs both directly and especially indirectly (through the switching of protein function). We have seen how the oxidation of specific Cys residues in PTPs, PTKs, and cysteine proteases may regulate levels of phosphorylation, ubiquitination, and SUMOylation in cells. The modulation of TFs and channel activity by Cys-SOH adds another level to the redox signaling cascade.
The role of protein sulfinylation in cell signaling appears mainly confined in the Prx/Srx pair. We believe that the development of specific chemical probes for RSO2H may help to find new Srx substrates or alternative reducing systems. Generally speaking, there is an urgent need for new protocols to analyze the full proteome and identify new targets. A deeper exploration of Cys oxidation in relation to metal binding properties could open up new vistas on redox signaling. Finally, the development of drugs that specifically target different oxidation states of proteins would appear to be a worthwhile goal (79).
This work was supported, in whole or in part, by National Institutes of Health Grant R01GM102187 (to K. S. C.). This is the first third article in the Thematic Minireview Series on Redox-active Protein Modifications and Signaling.
- ROS
- reactive oxygen species
- PTM
- post-translational modification
- DBP
- dimedone-based probe
- PTK
- protein-tyrosine kinase
- PTP
- protein-tyrosine phosphatase
- EGFR
- EGF receptor
- TF
- transcription factor
- Ub
- ubiquitin
- DUB
- deubiquitinating enzyme
- USP
- Ub-specific protease
- SUMO
- small Ub-like modifier
- Trx
- thioredoxin
- HSA
- human serum albumin
- Srx
- sulfiredoxin
- Prx
- peroxiredoxin
- MMP
- matrix metalloproteinase
- NHase
- nitrile hydratase
- SCNase
- thiocyanate hydrolase.
REFERENCES
- 1. Finkel T. (2011) Signal transduction by reactive oxygen species. J. Cell Biol. 194, 7–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hall A., Parsonage D., Poole L. B., Karplus P. A. (2010) Structural evidence that peroxiredoxin catalytic power is based on transition-state stabilization. J. Mol. Biol. 402, 194–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ferrer-Sueta G., Manta B., Botti H., Radi R., Trujillo M., Denicola A. (2011) Factors affecting protein thiol reactivity and specificity in peroxide reduction. Chem. Res. Toxicol. 24, 434–450 [DOI] [PubMed] [Google Scholar]
- 4. Roos G., Foloppe N., Messens J. (2013) Understanding the pKa of redox cysteines: the key role of hydrogen bonding. Antioxid. Redox Signal. 18, 94–127 [DOI] [PubMed] [Google Scholar]
- 5. Chen K., Kirber M. T., Xiao H., Yang Y., Keaney J. F. (2008) Regulation of ROS signal transduction by NADPH oxidase 4 localization. J. Cell Biol. 181, 1129–1139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Paulsen C. E., Truong T. H., Garcia F. J., Homann A., Gupta V., Leonard S. E., Carroll K. S. (2012) Peroxide-dependent sulfenylation of the EGFR catalytic site enhances kinase activity. Nat. Chem. Biol. 8, 57–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hugo M., Turell L., Manta B., Botti H., Monteiro G., Netto L. E. S., Alvarez B., Radi R., Trujillo M. (2009) Thiol and sulfenic acid oxidation of AhpE, the one-cysteine peroxiredoxin from Mycobacterium tuberculosis: kinetics, acidity constants, and conformational dynamics. Biochemistry 48, 9416–9426 [DOI] [PubMed] [Google Scholar]
- 8. Nelson K. J., Parsonage D., Hall A., Karplus P. A., Poole L. B. (2008) Cysteine pKa values for the bacterial peroxiredoxin AhpC. Biochemistry 47, 12860–12868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Enami S., Hoffmann M. R., Colussi A. J. (2009) Simultaneous detection of cysteine sulfenate, sulfinate, and sulfonate during cysteine interfacial ozonolysis. J. Phys. Chem. B 113, 9356–9358 [DOI] [PubMed] [Google Scholar]
- 10. McGrath A. J., Garrett G. E., Valgimigli L., Pratt D. A. (2010) The redox chemistry of sulfenic acids. J. Am. Chem. Soc. 132, 16759–16761 [DOI] [PubMed] [Google Scholar]
- 11. Davis F. A., Jenkins L. A., Billmers R. L. (1986) Chemistry of sulfenic acids. 7. Reason for the high reactivity of sulfenic acids. Stabilization by intramolecular hydrogen-bonding and electronegativity effects. J. Org. Chem. 51, 1033–1040 [Google Scholar]
- 12. Rehder D. S., Borges C. R. (2010) Cysteine sulfenic acid as an intermediate in disulfide bond formation and nonenzymatic protein folding. Biochemistry 49, 7748–7755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lee J. W., Soonsanga S., Helmann J. D. (2007) A complex thiolate switch regulates the Bacillus subtilis organic peroxide sensor OhrR. Proc. Natl. Acad. Sci. U.S.A. 104, 8743–8748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Salmeen A., Andersen J. N., Myers M. P., Meng T. C., Hinks J. A., Tonks N. K., Barford D. (2003) Redox regulation of protein tyrosine phosphatase 1B involves a sulphenyl-amide intermediate. Nature 423, 769–773 [DOI] [PubMed] [Google Scholar]
- 15. Allison W. S. (1976) Formation and reactions of sulfenic acids in proteins. Acc. Chem. Res. 9, 293–299 [Google Scholar]
- 16. Salsbury F. R., Jr., Knutson S. T., Poole L. B., Fetrow J. S. (2008) Functional site profiling and electrostatic analysis of cysteines modifiable to cysteine sulfenic acid. Protein Sci. 17, 299–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Leonard S. E., Carroll K. S. (2011) Chemical “omics” approaches for understanding protein cysteine oxidation in biology. Curr. Opin. Chem. Biol. 15, 88–102 [DOI] [PubMed] [Google Scholar]
- 18. Charles R. L., Schröder E., May G., Free P., Gaffney P. R., Wait R., Begum S., Heads R. J., Eaton P. (2007) Protein sulfenation as a redox sensor: proteomics studies using a novel biotinylated dimedone analogue. Mol. Cell. Proteomics 6, 1473–1484 [DOI] [PubMed] [Google Scholar]
- 19. Leonard S. E., Reddie K. G., Carroll K. S. (2009) Mining the thiol proteome for sulfenic acid modifications reveals new targets for oxidation in cells. ACS Chem. Biol. 4, 783–799 [DOI] [PubMed] [Google Scholar]
- 20. Tanner J. J., Parsons Z. D., Cummings A. H., Zhou H., Gates K. S. (2011) Redox regulation of protein tyrosine phosphatases: structural and chemical aspects. Antioxid. Redox Signal. 15, 77–97 [DOI] [PubMed] [Google Scholar]
- 21. Lee S. R., Yang K. S., Kwon J., Lee C., Jeong W., Rhee S. G. (2002) Reversible inactivation of the tumor suppressor PTEN by H2O2. J. Biol. Chem. 277, 20336–20342 [DOI] [PubMed] [Google Scholar]
- 22. Chen C. Y., Willard D., Rudolph J. (2009) Redox regulation of SH2-domain-containing protein tyrosine phosphatases by two backdoor cysteines. Biochemistry 48, 1399–1409 [DOI] [PubMed] [Google Scholar]
- 23. Meng T. C., Fukada T., Tonks N. K. (2002) Reversible oxidation and inactivation of protein tyrosine phosphatases in vivo. Mol. Cell 9, 387–399 [DOI] [PubMed] [Google Scholar]
- 24. Kwon J., Qu C. K., Maeng J. S., Falahati R., Lee C., Williams M. S. (2005) Receptor-stimulated oxidation of SHP-2 promotes T-cell adhesion through SLP-76-ADAP. EMBO J. 24, 2331–2341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Michalek R. D., Nelson K. J., Holbrook B. C., Yi J. S., Stridiron D., Daniel L. W., Fetrow J. S., King S. B., Poole L. B., Grayson J. M. (2007) The requirement of reversible cysteine sulfenic acid formation for T cell activation and function. J. Immunol. 179, 6456–6467 [DOI] [PubMed] [Google Scholar]
- 26. Giannoni E., Buricchi F., Raugei G., Ramponi G., Chiarugi P. (2005) Intracellular reactive oxygen species activate Src tyrosine kinase during cell adhesion and anchorage-dependent cell growth. Mol. Cell. Biol. 25, 6391–6403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Smith J. K., Patil C. N., Patlolla S., Gunter B. W., Booz G. W., Duhé R. J. (2012) Identification of a redox-sensitive switch within the JAK2 catalytic domain. Free Radic. Biol. Med. 52, 1101–1110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wani R., Qian J., Yin L., Bechtold E., King S. B., Poole L. B., Paek E., Tsang A. W., Furdui C. M. (2011) Isoform-specific regulation of Akt by PDGF-induced reactive oxygen species. Proc. Natl. Acad. Sci. U.S.A. 108, 10550–10555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Murata H., Ihara Y., Nakamura H., Yodoi J., Sumikawa K., Kondo T. (2003) Glutaredoxin exerts an antiapoptotic effect by regulating the redox state of Akt. J. Biol. Chem. 278, 50226–50233 [DOI] [PubMed] [Google Scholar]
- 30. Brigelius-Flohé R., Flohé L. (2011) Basic principles and emerging concepts in the redox control of transcription factors. Antioxid. Redox Signal. 15, 2335–2381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lee C., Lee S. M., Mukhopadhyay P., Kim S. J., Lee S. C., Ahn W. S., Yu M. H., Storz G., Ryu S. E. (2004) Redox regulation of OxyR requires specific disulfide bond formation involving a rapid kinetic reaction path. Nat. Struct. Mol. Biol. 11, 1179–1185 [DOI] [PubMed] [Google Scholar]
- 32. Chen P. R., Bae T., Williams W. A., Duguid E. M., Rice P. A., Schneewind O., He C. (2006) An oxidation-sensing mechanism is used by the global regulator MgrA in Staphylococcus aureus. Nat. Chem. Biol. 2, 591–595 [DOI] [PubMed] [Google Scholar]
- 33. Cheng Z., Wu J., Setterdahl A., Reddie K., Carroll K., Hammad L. A., Karty J. A., Bauer C. E. (2012) Activity of the tetrapyrrole regulator CrtJ is controlled by oxidation of a redox active cysteine located in the DNA binding domain. Mol. Microbiol. 85, 734–746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fuangthong M., Helmann J. D. (2002) The OhrR repressor senses organic hydroperoxides by reversible formation of a cysteine-sulfenic acid derivative. Proc. Natl. Acad. Sci. U.S.A. 99, 6690–6695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Liu Z., Yang M., Peterfreund G. L., Tsou A. M., Selamoglu N., Daldal F., Zhong Z., Kan B., Zhu J. (2011) Vibrio cholerae anaerobic induction of virulence gene expression is controlled by thiol-based switches of virulence regulator AphB. Proc. Natl. Acad. Sci. U.S.A. 108, 810–815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Poor C. B., Chen P. R., Duguid E., Rice P. A., He C. (2009) Crystal structures of the reduced, sulfenic acid, and mixed disulfide forms of SarZ, a redox active global regulator in Staphylococcus aureus. J. Biol. Chem. 284, 23517–23524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Delaunay A., Pflieger D., Barrault M. B., Vinh J., Toledano M. B. (2002) A thiol peroxidase is an H2O2 receptor and redox-transducer in gene activation. Cell 111, 471–481 [DOI] [PubMed] [Google Scholar]
- 38. Paulsen C. E., Carroll K. S. (2009) Chemical dissection of an essential redox switch in yeast. Chem. Biol. 16, 217–225 [DOI] [PubMed] [Google Scholar]
- 39. Fourquet S., Guerois R., Biard D., Toledano M. B. (2010) Activation of NRF2 by nitrosative agents and H2O2 involves KEAP1 disulfide formation. J. Biol. Chem. 285, 8463–8471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pineda-Molina E., Klatt P., Vázquez J., Marina A., García de Lacoba M., Pérez-Sala D., Lamas S. (2001) Glutathionylation of the p50 subunit of NF-κB: a mechanism for redox-induced inhibition of DNA binding. Biochemistry 40, 14134–14142 [DOI] [PubMed] [Google Scholar]
- 41. Reynaert N. L., van der Vliet A., Guala A. S., McGovern T., Hristova M., Pantano C., Heintz N. H., Heim J., Ho Y. S., Matthews D. E., Wouters E. F., Janssen-Heininger Y. M. (2006) Dynamic redox control of NF-κB through glutaredoxin-regulated S-glutathionylation of inhibitory κB kinase β. Proc. Natl. Acad. Sci. U.S.A. 103, 13086–13091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Komander D., Rape M. (2012) The ubiquitin code. Annu. Rev. Biochem. 81, 203–229 [DOI] [PubMed] [Google Scholar]
- 43. Cotto-Rios X. M., Békés M., Chapman J., Ueberheide B., Huang T. T. (2012) Deubiquitinases as a signaling target of oxidative stress. Cell Rep. 2, 1475–1484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kulathu Y., Garcia F. J., Mevissen T. E., Busch M., Arnaudo N., Carroll K. S., Barford D., Komander D. (2013) Regulation of A20 and other OTU deubiquitinases by reversible oxidation. Nat. Commun. 4, 1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lee J. G., Baek K., Soetandyo N., Ye Y. (2013) Reversible inactivation of deubiquitinases by reactive oxygen species in vitro and in cells. Nat. Commun. 4, 1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Xu Z., Lam L. S., Lam L. H., Chau S. F., Ng T. B., Au S. W. (2008) Molecular basis of the redox regulation of SUMO proteases: a protective mechanism of intermolecular disulfide linkage against irreversible sulfhydryl oxidation. FASEB J. 22, 127–137 [DOI] [PubMed] [Google Scholar]
- 47. Bossis G., Melchior F. (2006) Regulation of SUMOylation by reversible oxidation of SUMO conjugating enzymes. Mol. Cell 21, 349–357 [DOI] [PubMed] [Google Scholar]
- 48. Song M. Y., Makino A., Yuan J. X. (2011) Role of reactive oxygen species and redox in regulating the function of transient receptor potential channels. Antioxid. Redox Signal. 15, 1549–1565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bogeski I., Kummerow C., Al-Ansary D., Schwarz E. C., Koehler R., Kozai D., Takahashi N., Peinelt C., Griesemer D., Bozem M., Mori Y., Hoth M., Niemeyer B. A. (2010) Differential redox regulation of ORAI ion channels: a mechanism to tune cellular calcium signaling. Sci. Signal. 3, ra24. [DOI] [PubMed] [Google Scholar]
- 50. Svoboda L. K., Reddie K. G., Zhang L., Vesely E. D., Williams E. S., Schumacher S. M., O'Connell R. P., Shaw R., Day S. M., Anumonwo J. M., Carroll K. S., Martens J. R. (2012) Redox-sensitive sulfenic acid modification regulates surface expression of the cardiovascular voltage-gated potassium channel Kv1.5. Circ. Res. 111, 842–853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Turell L., Botti H., Carballal S., Ferrer-Sueta G., Souza J. M., Durán R., Freeman B. A., Radi R., Alvarez B. (2008) Reactivity of sulfenic acid in human serum albumin. Biochemistry 47, 358–367 [DOI] [PubMed] [Google Scholar]
- 52. Sohn J., Rudolph J. (2003) Catalytic and chemical competence of regulation of Cdc25 phosphatase by oxidation/reduction. Biochemistry 42, 10060–10070 [DOI] [PubMed] [Google Scholar]
- 53. Reddie K. G., Carroll K. S. (2008) Expanding the functional diversity of proteins through cysteine oxidation. Curr. Opin. Chem. Biol. 12, 746–754 [DOI] [PubMed] [Google Scholar]
- 54. Wilson M. A. (2011) The role of cysteine oxidation in DJ-1 function and dysfunction. Antioxid. Redox Signal. 15, 111–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hamann M., Zhang T., Hendrich S., Thomas J. A. (2002) Quantitation of protein sulfinic and sulfonic acid, irreversibly oxidized protein cysteine sites in cellular proteins. Methods Enzymol. 348, 146–156 [DOI] [PubMed] [Google Scholar]
- 56. Jacob C., Holme A. L., Fry F. H. (2004) The sulfinic acid switch in proteins. Org. Biomol. Chem. 2, 1953–1956 [DOI] [PubMed] [Google Scholar]
- 57. Mustafa A. K., Gadalla M. M., Sen N., Kim S., Mu W., Gazi S. K., Barrow R. K., Yang G., Wang R., Snyder S. H. (2009) H2S signals through protein S-sulfhydration. Sci. Signal. 2, ra72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Lo Conte M., Carroll K. S. (2012) Chemoselective ligation of sulfinic acids with aryl-nitroso compounds. Angew. Chem. Int. Ed. Engl. 51, 6502–6505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Rhee S. G., Yang K. S., Kang S. W., Woo H. A., Chang T. S. (2005) Controlled elimination of intracellular H2O2: regulation of peroxiredoxin, catalase, and glutathione peroxidase via post-translational modification. Antioxid. Redox Signal. 7, 619–626 [DOI] [PubMed] [Google Scholar]
- 60. Wood Z. A., Poole L. B., Karplus P. A. (2003) Peroxiredoxin evolution and the regulation of hydrogen peroxide signaling. Science 300, 650–653 [DOI] [PubMed] [Google Scholar]
- 61. Jang H. H., Lee K. O., Chi Y. H., Jung B. G., Park S. K., Park J. H., Lee J. R., Lee S. S., Moon J. C., Yun J. W., Choi Y. O., Kim W. Y., Kang J. S., Cheong G. W., Yun D. J., Rhee S. G., Cho M. J., Lee S. Y. (2004) Two enzymes in one: two yeast peroxiredoxins display oxidative stress-dependent switching from a peroxidase to a molecular chaperone function. Cell 117, 625–635 [DOI] [PubMed] [Google Scholar]
- 62. Yang K. S., Kang S. W., Woo H. A., Hwang S. C., Chae H. Z., Kim K., Rhee S. G. (2002) Inactivation of human peroxiredoxin I during catalysis as the result of the oxidation of the catalytic site cysteine to cysteine-sulfinic acid. J. Biol. Chem. 277, 38029–38036 [DOI] [PubMed] [Google Scholar]
- 63. Peskin A. V., Dickerhof N., Poynton R. A., Paton L. N., Pace P. E., Hampton M. B., Winterbourn C. C. (2013) Hyperoxidation of peroxiredoxins 2 and 3. Rate constants for the reactions of the sulfenic acid of the peroxidatic cysteine. J. Biol. Chem. 288, 14170–14177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Lowther W. T., Haynes A. C. (2011) Reduction of cysteine sulfinic acid in eukaryotic, typical 2-Cys peroxiredoxins by sulfiredoxin. Antioxid. Redox Signal. 15, 99–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Biteau B., Labarre J., Toledano M. B. (2003) ATP-dependent reduction of cysteine-sulphinic acid by S. cerevisiae sulphiredoxin. Nature 425, 980–984 [DOI] [PubMed] [Google Scholar]
- 66. Bonifati V., Rizzu P., van Baren M. J., Schaap O., Breedveld G. J., Krieger E., Dekker M. C. J., Squitieri F., Ibanez P., Joosse M., van Dongen J. W., Vanacore N., van Swieten J. C., Brice A., Meco G., van Duijn C. M., Oostra B. A., Heutink P. (2003) Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism. Science 299, 256–259 [DOI] [PubMed] [Google Scholar]
- 67. Canet-Avilés R. M., Wilson M. A., Miller D. W., Ahmad R., McLendon C., Bandyopadhyay S., Baptista M. J., Ringe D., Petsko G. A., Cookson M. R. (2004) The Parkinson's disease protein DJ-1 is neuroprotective due to cysteine-sulfinic acid-driven mitochondrial localization. Proc. Natl. Acad. Sci. U.S.A. 101, 9103–9108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Witt A. C., Lakshminarasimhan M., Remington B. C., Hasim S., Pozharski E., Wilson M. A. (2008) Cysteine pKa depression by a protonated glutamic acid in human DJ-1. Biochemistry 47, 7430–7440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Blackinton J., Lakshminarasimhan M., Thomas K. J., Ahmad R., Greggio E., Raza A. S., Cookson M. R., Wilson M. A. (2009) Formation of a stabilized cysteine sulfinic acid is critical for the mitochondrial function of the parkinsonism protein DJ-1. J. Biol. Chem. 284, 6476–6485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Waak J., Weber S. S., Görner K., Schall C., Ichijo H., Stehle T., Kahle P. J. (2009) Oxidizable residues mediating protein stability and cytoprotective interaction of DJ-1 with apoptosis signal-regulating kinase 1. J. Biol. Chem. 284, 14245–14257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Maret W. (2006) Zinc coordination environments in proteins as redox sensors and signal transducers. Antioxid. Redox Signal. 8, 1419–1441 [DOI] [PubMed] [Google Scholar]
- 72. Fu X., Kassim S. Y., Parks W. C., Heinecke J. W. (2001) Hypochlorous acid oxygenates the cysteine switch domain of pro-matrilysin (MMP-7). A mechanism for matrix metalloproteinase activation and atherosclerotic plaque rupture by myeloperoxidase. J. Biol. Chem. 276, 41279–41287 [DOI] [PubMed] [Google Scholar]
- 73. Visse R., Nagase H. (2003) Matrix metalloproteinases and tissue inhibitors of metalloproteinases–structure, function, and biochemistry. Circ. Res. 92, 827–839 [DOI] [PubMed] [Google Scholar]
- 74. Miyanaga A., Fushinobu S., Ito K., Wakagi T. (2001) Crystal structure of cobalt-containing nitrile hydratase. Biochem. Biophys. Res. Commun. 288, 1169–1174 [DOI] [PubMed] [Google Scholar]
- 75. Yano T., Ozawa T., Masuda H. (2008) Structural and functional model systems for analysis of the active center of nitrile hydratase. Chem. Lett. 37, 672–677 [Google Scholar]
- 76. Murakami T., Nojiri M., Nakayama H., Odaka M., Yohda M., Dohmae N., Takio K., Nagamune T., Endo I. (2000) Post-translational modification is essential for catalytic activity of nitrile hydratase. Protein Sci. 9, 1024–1030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Arakawa T., Kawano Y., Katayama Y., Nakayama H., Dohmae N., Yohda M., Odaka M. (2009) Structural basis for catalytic activation of thiocyanate hydrolase involving metal-ligated cysteine modification. J. Am. Chem. Soc. 131, 14838–14843 [DOI] [PubMed] [Google Scholar]
- 78. Giles N. M., Giles G. I., Jacob C. (2003) Multiple roles of cysteine in biocatalysis. Biochem. Biophys. Res. Commun. 300, 1–4 [DOI] [PubMed] [Google Scholar]
- 79. Truong T. H., Carroll K. S. (2012) Redox regulation of epidermal growth factor receptor signaling through cysteine oxidation. Biochemistry 51, 9954–9965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Poole L. B., Klomsiri C., Knaggs S. A., Furdui C. M., Nelson K. J., Thomas M. J., Fetrow J. S., Daniel L. W., King S. B. (2007) Fluorescent and affinity-based tools to detect cysteine sulfenic acid formation in proteins. Bioconjug. Chem. 18, 2004–2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Seo Y. H., Carroll K. S. (2009) Facile synthesis and biological evaluation of a cell-permeable probe to detect redox-regulated proteins. Bioorg. Med. Chem. Lett. 19, 356–359 [DOI] [PubMed] [Google Scholar]
- 82. Leonard S. E., Garcia F. J., Goodsell D. S., Carroll K. S. (2011) Redox-based probes for protein tyrosine phosphatases. Angew. Chem. Int. Ed. Engl. 50, 4423–4427 [DOI] [PubMed] [Google Scholar]
- 83. Seo Y. H., Carroll K. S. (2009) Profiling protein thiol oxidation in tumor cells using sulfenic acid-specific antibodies. Proc. Natl. Acad. Sci. U.S.A. 106, 16163–16168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Crump K. E., Juneau D. G., Poole L. B., Haas K. M., Grayson J. M. (2012) The reversible formation of cysteine sulfenic acid promotes B-cell activation and proliferation. Eur. J. Immunol. 42, 2152–2164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Wu J., Cheng Z., Reddie K., Carroll K., Hammad L. A., Karty J. A., Bauer C. E. (2013) RegB kinase activity is repressed by oxidative formation of cysteine sulfenic acid. J. Biol. Chem. 288, 4755–4762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Bosco M. B., Aleanzi M. C., Iglesias A. Á. (2012) Plastidic phosphoglycerate kinase from Phaeodactylum tricornutum: on the critical role of cysteine residues for the enzyme function. Protist 163, 188–203 [DOI] [PubMed] [Google Scholar]
- 87. Holyoak T., Zhang B., Deng J., Tang Q., Prasannan C. B., Fenton A. W. (2013) Energetic coupling between an oxidizable cysteine and the phosphorylatable N-terminus of human liver pyruvate kinase. Biochemistry 52, 466–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Saurin A. T., Neubert H., Brennan J. P., Eaton P. (2004) Widespread sulfenic acid formation in tissues in response to hydrogen peroxide. Proc. Natl. Acad. Sci. U.S.A. 101, 17982–17987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Godat E., Hervé-Grvépinet V., Veillard F., Lecaille F., Belghazi M., Brömme D., Lalmanach G. (2008) Regulation of cathepsin K activity by hydrogen peroxide. Biol. Chem. 389, 1123–1126 [DOI] [PubMed] [Google Scholar]
- 90. Wetzelberger K., Baba S. P., Thirunavukkarasu M., Ho Y. S., Maulik N., Barski O. A., Conklin D. J., Bhatnagar A. (2010) Postischemic deactivation of cardiac aldose reductase. Role of glutathione S-transferase P and glutaredoxin in regeneration of reduced thiols from sulfenic acids. J. Biol. Chem. 285, 26135–26148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Lim J. C., You Z., Kim G., Levine R. L. (2011) Methionine sulfoxide reductase A is a stereospecific methionine oxidase. Proc. Natl. Acad. Sci. U.S.A. 108, 10472–10477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Fernández-Irigoyen J., Santamaría M., Sánchez-Quiles V., Latasa M. U., Santamaría E., Muñoz J., Sánchez Del Pino M. M., Valero M. L., Prieto J., Avila M. A., Corrales F. J. (2008) Redox regulation of methylthioadenosine phosphorylase in liver cells: molecular mechanism and functional implications. Biochem. J. 411, 457–465 [DOI] [PubMed] [Google Scholar]
- 93. de Rezende F. F., Martins Lima A., Niland S., Wittig I., Heide H., Schröder K., Eble J. A. (2012) Integrin α7β1 is a redox-regulated target of hydrogen peroxide in vascular smooth muscle cell adhesion. Free Radic. Biol. Med. 53, 521–531 [DOI] [PubMed] [Google Scholar]
- 94. Miyata Y., Rauch J. N., Jinwal U. K., Thompson A. D., Srinivasan S., Dickey C. A., Gestwicki J. E. (2012) Cysteine reactivity distinguishes redox sensing by the heat-inducible and constitutive forms of heat shock protein 70. Chem. Biol. 19, 1391–1399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Regazzoni L., Panusa A., Yeum K. J., Carini M., Aldini G. (2009) Hemoglobin glutathionylation can occur through cysteine sulfenic acid intermediate: electrospray ionization LTQ-Orbitrap hybrid mass spectrometry studies. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 877, 3456–3461 [DOI] [PubMed] [Google Scholar]
- 96. Luanpitpong S., Chanvorachote P., Stehlik C., Tse W., Callery P. S., Wang L., Rojanasakul Y. (2013) Regulation of apoptosis by Bcl-2 cysteine oxidation in human lung epithelial cells. Mol. Biol. Cell 24, 858–869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Johansson M., Lundberg M. (2007) Glutathionylation of β-actin via a cysteinyl sulfenic acid intermediary. BMC Biochem. 8, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Wilson M. A., Ringe D., Petsko G. A. (2005) The atomic resolution crystal structure of the YajL (ThiJ) protein from Escherichia coli: a close prokaryotic homologue of the Parkinsonism-associated protein DJ-1. J. Mol. Biol. 353, 678–691 [DOI] [PubMed] [Google Scholar]
- 99. Slavica A., Dib I., Nidetzky B. (2005) Single-site oxidation, cysteine 108 to cysteine sulfinic acid, in d-amino acid oxidase from Trigonopsis variabilis and its structural and functional consequences. Appl. Environ. Microbiol. 71, 8061–8068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Fukuhara A., Yamada M., Fujimori K., Miyamoto Y., Kusumoto T., Nakajima H., Inui T. (2012) Lipocalin-type prostaglandin D synthase protects against oxidative stress-induced neuronal cell death. Biochem. J. 443, 75–84 [DOI] [PubMed] [Google Scholar]


