Abstract
The Drosophila larval neuromuscular system is relatively simple, containing only 32 motor neurons in each abdominal hemisegment, and its neuromuscular junctions (NMJs) are large, individually specified, and easy to visualize and record from. NMJ synapses exhibit developmental and functional plasticity while displaying stereotyped connectivity. Drosophila Type I NMJ synapses are glutamatergic, while the vertebrate NMJ uses acetylcholine as its primary neurotransmitter. The larval NMJ synapses use ionotropic glutamate receptors (GluRs) that are homologous to AMPA-type glutamate receptors in the mammalian brain, and they have postsynaptic scaffolds that resemble those found in mammalian postsynaptic densities. These features make the Drosophila neuromuscular system an excellent genetic model for the study of excitatory synapses in the mammalian central nervous system.
The first section of the review presents an overview of NMJ development. The second section describes genes that regulate NMJ development, including: 1) genes that positively and negatively regulate growth of the NMJ; 2) genes required for maintenance of NMJ bouton structure; 3) genes that modulate neuronal activity and alter NMJ growth; 4) genes involved in trans-synaptic signaling at the NMJ. The third section describes genes that regulate acute plasticity, focusing on translational regulatory mechanisms. Since this review is intended for a developmental biology audience, it does not cover NMJ electrophysiology in detail, and does not review genes for which mutations produce only electrophysiological but no structural phenotypes.
I. Introduction
Chemical synapses are specialized junctions between cells that mediate transmission of information via small molecule and/or peptide neurotransmitters. The presynaptic terminals of these synapses contain neurotransmitter-filled vesicles and the machinery necessary for neurotransmitter release. The postsynaptic partners, which can be other neurons or non-neuronal cells, have specialized postsynaptic structures containing receptors that bind to the neurotransmitter(s) released by the presynaptic cell and transduce electrical and/or chemical signals.
Excitatory synapses in the vertebrate nervous system that use glutamate as their primary neurotransmitter are characterized by postsynaptic densities (PSDs), which are very large protein complexes that contain ionotropic glutamate receptors (GluRs) and numerous scaffolding and signaling proteins. These types of synapses exhibit plasticity, which is a process whereby the connections between the neuron and its partner are modified in response to neuronal activity. Synaptic plasticity usually involves both structural and functional changes, and it is thought to be the foundation of learning and memory. Plastic changes are also observed during synaptic development and maturation. Many of the molecules and mechanisms used for synaptic plasticity during development are re-used later for plasticity linked to learning and memory in mature neurons.1 Thus, the study of synaptic plasticity during development can provide important insights into learning and memory mechanisms. Studies performed in invertebrate genetic model organisms such as Drosophila melanogaster and Caenorhabditis elegans have provided important insights into the molecular mechanisms involved in synaptic development and function.2 These organisms have nervous systems with fewer cells than those in vertebrates, and are amenable to gene discovery through forward genetic screening. Many genes involved in nervous system development and function that are conserved between invertebrates and vertebrates have been identified in such screens.
In this review, we focus on neuromuscular junction (NMJ) synapses in Drosophila larvae. These synapses are glutamatergic and similar to those in the vertebrate CNS. Larval NMJ synapses use ionotropic GluRs that are homologous to AMPA-type GluRs in the mammalian brain, and they have postsynaptic scaffolds that resemble those found in mammalian PSDs. Many of the vertebrate synaptic components also have Drosophila orthologs, including Neurexin,3 Neuroligin,4,5 PSD-956 and Phosphodiesterase 4 (PDE-4)7,8. The Drosophila larval neuromuscular system is relatively simple, containing only 32 motor neurons in each abdominal hemisegment, and its NMJs are large, individually specified, and easy to visualize and record from. As discussed below, fly NMJ synapses also exhibit developmental and functional plasticity while displaying stereotyped connectivity. Because of these features, the Drosophila larval NMJ is an excellent genetic model for glutamatergic synapses in the mammalian brain (CNS).9-12
II. NMJ development
A. A brief overview of Drosophila NMJ development
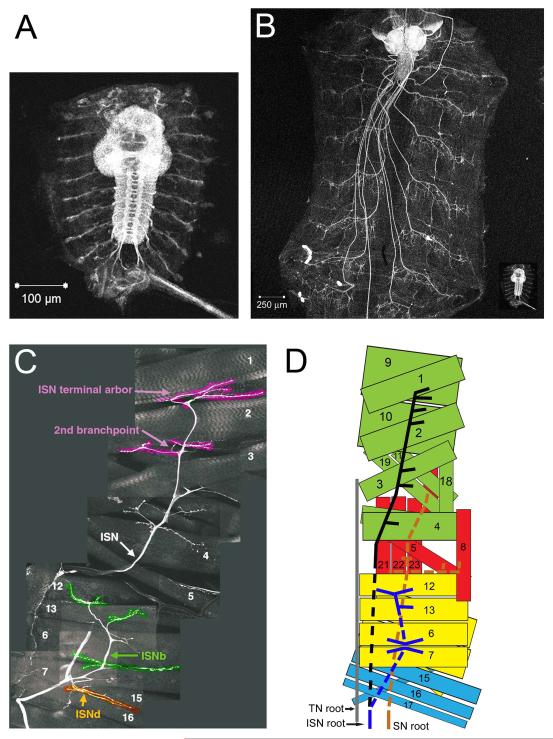
Motor neurons are individually specified, and are generated in lineages deriving from at least 10 different neuroblasts.13,14 Their muscle targets, which are also individually specified, are produced by cell fusion events. During stages 13-15 of embryonic development, motor neurons extend their axons into the musculature. Motor axons leave the CNS in three pathways: the segmental (SN) and intersegmental (ISN) nerve roots and the transverse nerve (TN). In the periphery, the SN and ISN split into five nerve pathways, designated as the SNa (innervates lateral muscles), SNc (innervates ventral muscles), ISN (innervates dorsal muscles), ISNb (innervates ventrolateral muscles (VLMs)), and ISNd (innervates other ventral muscles).15 Each motor axon follows a genetically determined pathway to a specific muscle fiber or group of fibers.16 These are shown in both an immunohistological composite (ISN root-derived branches only, Figure 1C) and as a schematic in Figure 1D.
Figure 1. Growth of the larva and its neuromuscular system.
(A) A dissected late stage embryo ‘fillet’ stained with anti-horseradish peroxidase (anti-HRP), which stains neuronal membranes. The bright structure in the center is the ventral nerve cord (VNC), with the brain at the top and the ladder-like axon array extending downward from the brain. Extending outwards from each segment of the VNC are the motor and sensory axon tracts. (B) A third instar larval fillet expressing green fluorescent protein (GFP) in all neurons. The brain and VNC are now located at the anterior end, and the motor/sensory nerves run posteriorly from the VNC to reach each body segment. Note the stereotyped array of nerve endings in each segment. The inset in B shows the embryo from A at the same scale as the larva, illustrating the dramatic growth of the animal during larval life (the embryo is about the same size as a newly hatched first instar larva). (C) Innervation pattern of the intersegmental nerve (ISN) and its ISNb and ISNd branches in a third instar larva. This is a composite of many confocal images of GFP-labeled neurons in an abdominal hemisegment. Numbers indicate muscles innervated by the different branches of the ISN. (D) A schematic representing the three nerve roots: ISN, the segmental nerve (SN), and the transverse nerve (TN), and their respective innervation patterns. For clarity, not all muscles and nerve branches are shown. For the SN root, we show only the SNa nerve; SNc is not depicted. The ISNd branch of the ISN root, visible in C, has also been omitted from the diagram. The dashed lines are used to indicate the sections of the nerves that lie under (ventral to) the muscle(s). Scale bars in A and B are 100 μm and 250 μm, respectively.
After an axonal growth cone makes contact with its target muscle, postsynaptic GluRs and Discs large (Dlg), the Drosophila ortholog of the mammalian PSD-95 postsynaptic scaffolding protein, begin to cluster at the contact site.17,18 The growth cone then differentiates into a presynaptic terminal. By the end of embryonic development, functional NMJs, each containing a few synaptic boutons, have formed on each muscle fiber (Figure 3C, D). Boutons are oval-shaped structures that house synapses. Boutons contain multiple active zones (neurotransmitter release sites), and each of these is apposed to a GluR cluster. The presynaptic bouton at larval NMJs eventually becomes surrounded by an infolded membranous structure called the subsynaptic reticulum (SSR), which contains neurotransmitter receptors, scaffolding proteins, and postsynaptic signaling complexes.
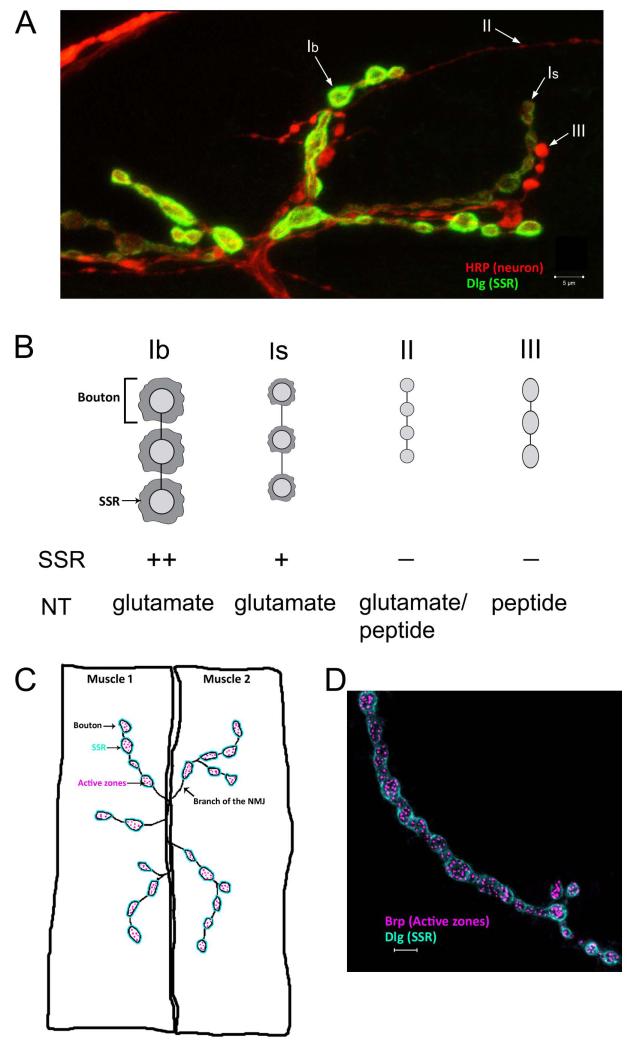
Figure 3. Types and structure of boutons at the Drosophila larval NMJ.
(A) Type Ib, Is, II, and III boutons on muscle 12 are indicated by arrows. Type Ib and type 1s boutons differ in size, morphology, physiology, and the amount of subsynaptic reticulum (SSR) that surrounds them. The SSR is stained by Discs-Large (Dlg) antibody, which labels both Type Ib boutons and type Is boutons. Type Ib boutons are surrounded by more SSR membrane as compared to the Type Is boutons, resulting in the differential staining of the two types. Dlg is absent from type II and III boutons. Anti-HRP labels the presynaptic neuronal membrane and allows visualization of all bouton types. (B) A cartoon depicting the differences in the bouton types seen in (A). Other than the size and morphological differences, the boutons also differ in the neurotransmitter utilized. (C) A schematic showing a neuromuscular junction on arbitrary muscles labeled 1 and 2. One of the branches of the neuromuscular junction (NMJ) is indicated. Active zones localized inside each bouton are represented as pink dots. The subsynaptic reticulum (SSR), which consists of postsynaptic muscle membrane surrounding each bouton, is shown in blue. (D) Immunohistological staining showing type Ib boutons on muscle 4 stained with a Bruchpilot (Brp) antibody that labels the presynaptic active zones, which are visualized as punctate structures. Also stained with a Dlg antibody to show the SSR. Scale bars are 5 μm.
Early neural development is often characterized by an initial overproduction of synaptic connections, followed by a period of selective elimination of improper processes. This phenomenon was first observed in vertebrates19 and has been studied extensively at the visual system and NMJ. In the visual system, the refinement of the connections is necessary for the formation of the retinotopic maps in the mammalian brain. Relay of visual information from the retina to the primary visual cortex in the brain occurs through the lateral geniculate nucleus (LGN) located in the thalamus. Initially, axon terminals of retinal ganglion cells (RGCs) from the two eyes form ectopic connections and overlap within the different layers of the LGN. Later on, these connections are refined to form specific eye layers. This segregation of RGC inputs involves retraction from incorrect target layers and synapse formation in the correct layer.20,21 At the vertebrate NMJ, multiple motor neurons initially innervate the same muscle fiber. As development progresses, all but one of the motor neurons are eliminated.22 Activity is critical for this refinement: altering the activity of the motor neurons results in the more active neuron being maintained and stabilized.23
Synaptic refinement also occurs at the Drosophila NMJ. However, this refinement is most similar to the process that happens in the vertebrate visual system as opposed to the vertebrate NMJ. In early embryonic development, motor neurons form ectopic contacts on non-target muscles. These misplaced synapses are then eliminated in late-stage embryos by an activity-dependent process.24-27 An additional form of refinement occurs after embryogenesis at the level of synaptic gain control once the motor neurons have reached their appropriate muscle targets. Here, the NMJ arbor must grow in order to maintain the proper synaptic drive that is needed due to the dramatic increase in muscle fiber size. From hatching of the embryo to the late third instar, the surface area of each muscle fiber increases by up to 100-fold (Figures 1A, B and 2A). During this growth period, boutons are continuously being added (and some are eliminated), and these processes result in the number of boutons and the number of active zones per bouton both increasing by up to 10-fold.28,29 The final increase in the number of active zones by up to 100-fold matches the increase in muscle surface area (Figure 1B). Another round of synapse elimination occurs during metamorphosis.30
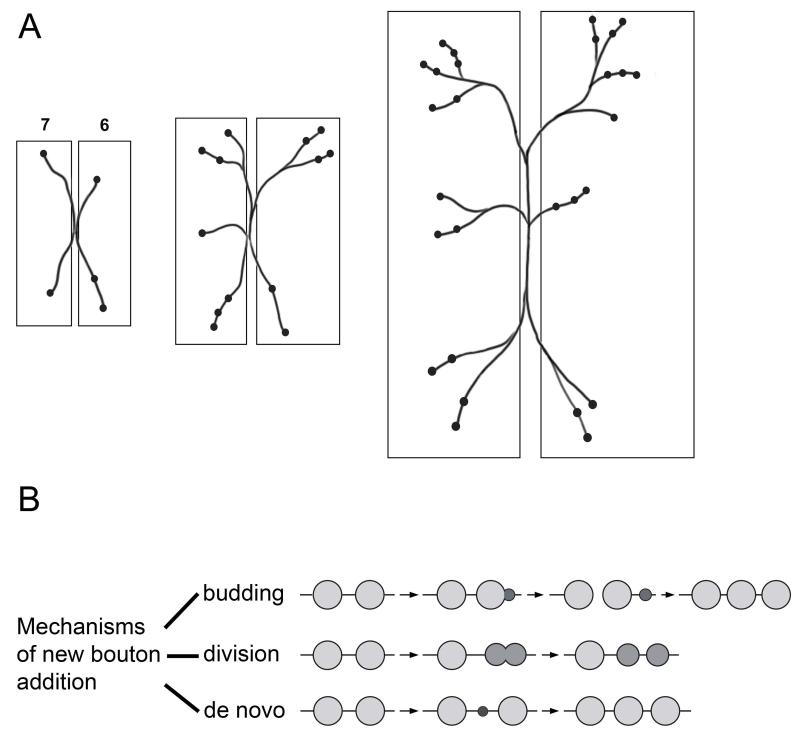
Figure 2. NMJ expansion and synaptic growth.
(A) A cartoon depicting patterned growth of the type 1b boutons on muscles 6 and 7 NMJ during larval development from the first instar (left panel) to the third instar (right panel). As the muscles increase in size, the NMJs add more branches and boutons. (B) During NMJ growth, new boutons are added by any of the these mechanisms: 1) asymmetric budding of a pre-existing bouton, similar to cell division in yeast, 2) symmetric division of a bouton, 3) de novo formation of a bouton from the axonal membrane (adapted from Zito et al.31).
In addition to the structural changes that result because of expansion in muscle size, Drosophila NMJs also undergo plastic changes in response to short-term perturbations of neuronal and muscle activity. Some of these involve structural alterations in the NMJ, and will be reviewed here. In others, such as facilitation and homeostatic compensation, electrophysiological changes that alter transmitter release and/or postsynaptic responses are observed. In many of these cases, these changes are not associated with major alterations in NMJ structure, and thus are not discussed in this review.
B. Patterned growth of the larval neuromuscular junction
The region of contact between the motor neuron and the muscle is the NMJ. The presynaptic terminals of Drosophila larval NMJs are organized into branched arbors that are composed of chains of synaptic boutons. There are 3 types of boutons: Types I, II and III (Figure 3A). These differ in size and shape, the neurotransmitter that is released, the amount of SSR that surrounds them, and the subunit composition of the glutamate receptors with which they are associated (Figure 3B). In this review, we consider only Type I boutons, which are glutamatergic and can be divided into two classes: 1b (large) and 1s (small). Type II and type III boutons are modulatory and use other neurotransmitters. In a third instar larva, a typical NMJ has approximately 20-50 Type I boutons on each muscle, with each individual bouton housing about 10 active zones (Figures 3C, D). Most studies examine NMJs on muscles 6/7 (this NMJ has twice as many boutons because it innervates two muscles), muscle 4, or muscle 12, in segments A2-A5.16
The structure of a larval NMJ is stereotypic and shows a similar arborization pattern for a specific muscle in different abdominal segments. Numerous studies have examined development of this complex structure from a 1st larval instar to a 3rd instar using fixed larval preparations. However, live imaging of NMJ development has allowed the study of the mechanisms involved in bouton addition and branch formation.31 Live imaging was first done by using a chimeric transmembrane green fluorescent protein that was targeted to the SSR. This construct allowed visualization of postsynaptic structures that outline Type Ib synaptic boutons, through the cuticle of live larvae. The same NMJ could be viewed multiple times during development, from first instar through third instar. These studies showed that location of new bouton formation was either between pre-existing boutons or at the end of a branch of the NMJ arbor. The new boutons arose by asymmetric budding of a mature (parent) bouton (similar to cell division in yeast), by symmetric division of a pre-exisiting bouton, or by de novo formation of a bouton from the axonal membrane (Figure 2B).
During NMJ development, many transient structures are either stabilized or retracted during formation of the complex terminal arbor. Since the above study examined synaptic boutons indirectly through visualization of a postsynaptic marker protein that surrounds these boutons, nascent presynaptic structures could not be observed. These include synaptopods, presynaptic debris, and ghost boutons. These transient structures are seen at normal NMJs during development and seem to be remnants of the synaptic refinement process. However, under various conditions, such as acute stimulation of motor neurons, these structures are stabilized and not properly eliminated.32 Synaptopods are highly dynamic presynaptic filopodial extensions that can only be visualized in live larval preparations.33-36
C. Molecular mechanisms involved in NMJ growth
The mechanisms that regulate the different stages of bouton formation, development and maintenance are not fully understood. Our current knowledge of the molecules involved is based on genetic and biochemical analyses of mutants that have morphological NMJ phenotypes. The purpose of this section is to review a few of the genes involved in bouton growth and classify them based on their mutant phenotypes. The ultimate goal of investigators would be to understand the molecular mechanisms involved in the life history of a bouton from birth through maturity, as well as those that are required for branch formation during development of the terminal arbor. The genes discussed below function either cell autonomously on the presynaptic side (in motor neurons) or in trans-synaptic pathways involved in signaling from muscles to the neurons. Trans-synaptic signaling pathways are also discussed in a separate section below. Table 1 is a partial list of genes that are implicated in NMJ growth, which includes many genes in addition to those explicitly discussed in the text.
Table 1.
| Effects in mutant → Gene↓ |
Bouton number |
Bouton size | Ghost boutons |
Presynaptic debris |
Presynaptic retractions |
Satellite boutons | References |
|---|---|---|---|---|---|---|---|
| Cytoskeletal proteins and adaptors | |||||||
| nervous wreck (nwk) | incr | decr | incr | 64, 70 | |||
| wsp | incr | incr | 70 | ||||
| Adducin/Hu-li tai shao (hts) | incr | yes | 184 | ||||
| Spastin | incr | decr | 42 | ||||
| PP2A | decr | incr | 187 | ||||
| aPKC | decr | 40 | |||||
| Ankyrin2 | decr | incr | yes | 62, 63 | |||
| Spectrin - pre- and post-synaptic RNAi | decr (post) | 61, 185 | |||||
| Dynactin complex - centractin and Glued/P150 | yes | 60 | |||||
| Dliprin-alpha | decr | 86 | |||||
| LIMkinase | incr | yes | 188, 191 | ||||
| Stathmin | decr | yes | 84 | ||||
| Diaphanous (Formin) Rho GTPase | decr | incr | 87 | ||||
| Futsch | decr | incr | 38 | ||||
| Cell adhesion | |||||||
| Neurexin | decr | 99 | |||||
| Neuroligin | decr | 99 | |||||
| Fasciclin II | incr | 29, 72 | |||||
| Fasciclin II pre- and post-synaptic overexpression (OE) | incr | incr | 72 | ||||
| Fasciclin II pre- or post-synaptic overexpression (OE) | decr | 72 | |||||
| Teneurin-a and Teneurin-m | decr | incr | 181 | ||||
| Syndecan | decr | 85 | |||||
| Dally-like protein (Dlp) | 85 | ||||||
| Dlp OE | decr | 85 | |||||
| Endocytic proteins | |||||||
| Cdc42 | incr (very slightly) | 68 | |||||
| Rab11 | incr | 180 | |||||
| dynamin (shi ts1 at non-permissive temp) | incr | 65 | |||||
| Dap160 (dynamin associated protein 160) | incr | 189, 190 | |||||
| endophilin | incr | 65 | |||||
| synaptojanin | incr | 65 | |||||
| Spinster | incr | 186 | |||||
| VAP 33A | decr | incr | 183 | ||||
| VAP-33A OE | incr | decr | 183 | ||||
| Exocytosis | |||||||
| Complexin | incr | 179 | |||||
| Receptors | |||||||
| Draper | decr | incr | incr | incr | 33 | ||
| APPL OE | incr | 67 | |||||
| Arrow | decr | 41 | |||||
| Dlar | decr | 85 | |||||
| Arrow OE | incr | incr | 41 | ||||
| Transcription factor | |||||||
| Dad | incr | 69 | |||||
| Kinases | |||||||
| shaggy | incr | 78 | |||||
| Protein synthesis | |||||||
| Pumilio | decr | incr | 155 | ||||
| Nanos | incr | 152 | |||||
| FMRP | incr | incr | 140, 141 | ||||
| miR-8 sponge in muscles | decr | 122 | |||||
| Protein degradation | |||||||
| hiw | incr | decr | 49, 50 | ||||
| fat facets OE | incr | 45 | |||||
| Anaphase promoting complex/Cyclosome (APC/C) | incr | 48 | |||||
| wnd (wallenda MAPKKK) OE | incr | decr | 52 | ||||
| cAMP | |||||||
| dunce (cAMP phosphodiesterase) | incr | 55 | |||||
| rutabaga (adenylate cyclase) | wt | 55 | |||||
| dunce rut | wt | 55 | |||||
| eag Sh | incr | 53 | |||||
| dnc eag more | incr | 53 | |||||
| dnc Sh more | incr | 53 | |||||
| Channels | |||||||
| K+ channels sei- and slo- | incr | 71 | |||||
| cac (Calcium channel) | decr | 56, 57 | |||||
| K+ channels eag and Sh combined | incr | 53 | |||||
| Ligand | |||||||
| wg | decr | incr | 182 | ||||
| wg OE | incr | incr | 41, 182 |
For the purposes of organization, we group the genes that play a role in the development of the larval NMJ into four categories. Each of these categories includes genes that encode proteins that function in a variety of different pathways. The first category consists of genes whose products promote NMJ growth. These are defined as those for which loss-of-function (LOF) mutants have smaller terminal arbors. In the second category are genes whose products inhibit NMJ growth, and LOF mutants for these genes have expanded terminal arbors. The third category discusses genes involved in neuronal activity that alter NMJ growth. The fourth category encompasses genes that regulate the formation and maintenance of boutons and do not fall into the other 3 groups. Disruption of these genes produces boutons that are arrested at various stages of development. For each of these categories, only a few genes that fall into these groups are discussed. In the last part of this section we describe how many of these genes may work in parallel to affect the same downstream effectors that regulate NMJ growth.
C.1. Genes that promote synaptic growth
LOF mutations for genes in this category produce phenotypes that are characterized by decreases in the number of boutons and are sometimes associated with an increase in their sizes (Figures 4C, D). A large subset of genes in this category alter the microtubule (MT) cytoskeleton. The cytoskeleton of NMJ presynaptic terminals can be divided into core and membrane-associated components. MTs and MT binding proteins are part of the core cytoskeleton. The membrane-associated cytoskeleton is a filamentous network of spectrin molecules linked together by actin and attached to cell adhesion proteins in the plasma membrane.37 The presynaptic MT cytoskeleton is most easily visualized using antibodies against Futsch, which encodes the Drosophila MT-associated protein (MAP) 1b ortholog.38 Fragmentation of the MT network is correlated with decreased bouton numbers in futsch mutants.38-40 Similar phenotypes are seen in mutants lacking atypical protein kinase C (aPKC).40 aPKC activity is thought to stabilize MTs during bouton maturation and Futsch is required for the aPKC-mediated MT stabilization.
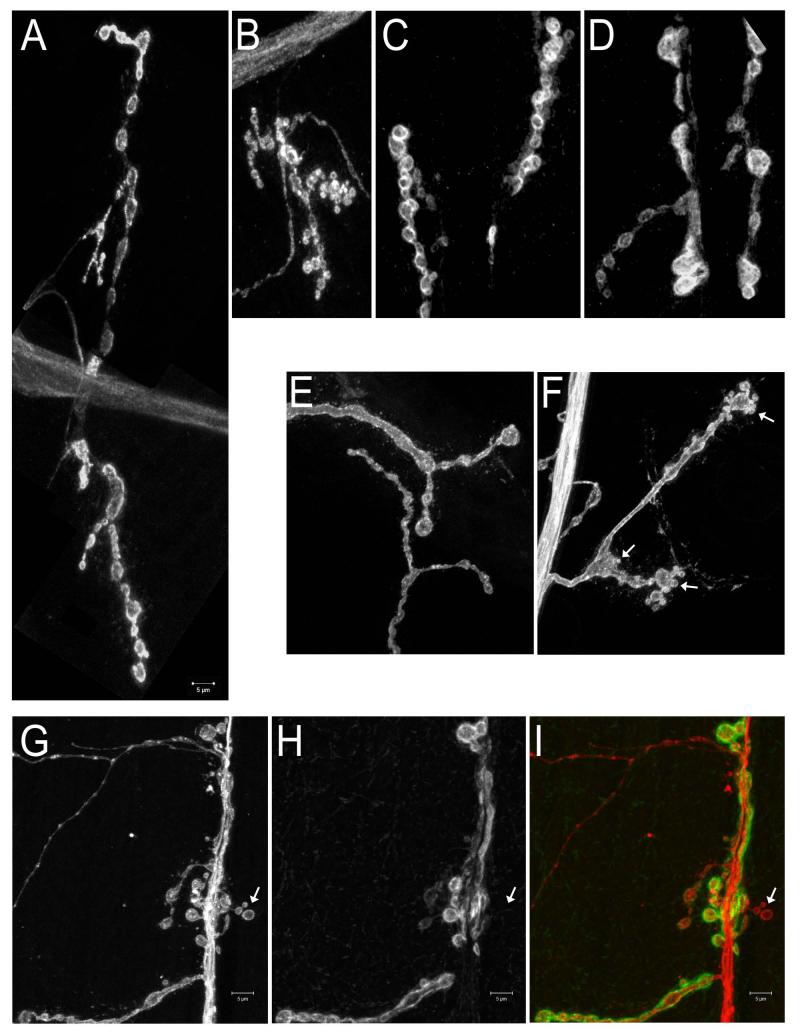
Figure 4. Examples of NMJ phenotypes.
(A,B,E,F) Muscle 4 NMJs; (C,D,G,H,I) Muscle 6-7 NMJs. (A-F) are labeled with anti-HRP; (G-I) are double-labeled with anti-HRP and anti-Dlg. (A) Wild-type; the entire NMJ is shown. (B) A mutant NMJ with boutons that are greater in number but smaller in size than in wild-type. Entire muscle 4 NMJs are shown. (C) Wild-type. (D) A mutant NMJ with boutons that are fewer in number but larger in size than in wild-type. Partial muscle 6-7 NMJs are shown. (E) Wild-type. (F) An NMJ with a satellite bouton phenotype. Satellite boutons (arrows) resemble ‘budding’ structures, and are seen here on the terminal parent boutons and on the branching bouton. (G-I) An NMJ with ghost boutons. Ghost boutons (arrow in I) appear as boutons that have the presynaptic marker, anti-HRP (G; red in I), but lack postsynaptic markers, such as Dlg (H, green in I). Scale bar is 5 μm.
Some genes that function within the Wnt signaling pathway also promote NMJ growth by altering the MT cytoskeleton. Two of the Wnt pathway genes for which mutations affect the MT cytoskeleton are arrow (arr) and dishevelled (dsh). arr encodes a co-receptor of Frizzled 2 (Fz2), the receptor for the secreted Wnt protein Wingless (Wg). Dsh is a cytoplasmic phosphoprotein that is downstream of Fz2. Presynaptic loss of either of these molecules results in a decrease in bouton number, accompanied by abnormal enlargement of some of the boutons. Loss of presynaptic Arr or Dsh also causes disorganization of the MT cytoskeleton.41
C.2. Genes that negatively regulate synaptic growth
The second category of genes includes those for which LOF mutants have increased numbers of boutons, sometimes accompanied by a decrease in their sizes (Figures 4A, B). These genes may normally function as negative regulators of NMJ growth. It is important to note, however, that investigators often only report bouton numbers and do not quantitate bouton size, so some mutations that increase bouton number may not increase the total number of NMJ active zones because there is a corresponding decrease in bouton size and/or active zones per bouton. We also distinguish phenotypes with increases in the number of normal boutons from those that are characterized by the presence of ‘satellite’ boutons. Satellite bouton phenotypes are described another section below. Mutant NMJs with larger numbers of normal boutons may have longer synaptic arbors with unaltered branching patterns, or may have increased numbers of secondary and tertiary arbor branches. Genes within this category encode proteins involved in a variety of signaling pathways, and only a few of these are highlighted here.
Another cytoskeletal modulator, Spastin, appears to be a negative regulator of bouton growth. spastin mutant NMJs show a slight increase in the number of boutons along with a decrease in bouton size. Spastin is an MT-severing protein belonging to the family of AAA ATPases. One might have expected that a decrease in Spastin activity would results in less severing of MTs and consequently should result in an increase in Futsch labeling. Instead, Futsch and tubulin staining in NMJs are reduced in spastin mutant NMJs, particularly at terminal boutons. The data suggest that severing of MTs into smaller segments may facilitate transport of MTs from the axon into the NMJ.42,43
NMJ growth is also regulated by protein degradation pathways. The ubiquitin-proteasome system (UPS) has many ubiquitinating and deubiquitinating enzymes that function in almost all developmental decisions.44 Two E3 ubiquitin ligase complexes known to function in the cell cycle are SCF (Skp/Cullin/F-box) and the Anaphase promoting complex/Cyclosome (APC/C). The APC/C complex is composed of core and catalytic subunits. The catalytic subunits are APC2 and APC11. Cdc27 is one of the core subunits and Cdh1 is an activator subunit that regulates activity of the APC/C complex.45-47 APC2, Cdc27 and Cdh1 localize to the Drosophila larval NMJ. APC2 is a negative regulator of bouton growth. Lack of APC2 (morula) in neurons results in an increase in bouton number, although bouton size does not change. One of the substrates of the APC/C complex is DLiprin-alpha, a scaffolding protein that promotes bouton growth. In an apc2 mutant, DLiprin-alpha is not ubiquitinated and the protein accumulates at the NMJ. This lack of degradation results in an increase in bouton number.48
Highwire (Hiw) is a ubiquitin ligase that is part of the SCF complex, and Fat facets (Faf) is a deubiquitinating protease. Both are required presynaptically to control bouton growth.45,49,50 hiw and faf mutants have greatly expanded presynaptic NMJ arbors. Bouton number and NMJ span are increased, but bouton size is decreased. Hiw controls NMJ growth by regulating the MAP kinase signaling pathway through Wallenda (Wnd), a dual leucine zipper kinase (DLK) that is orthologous to MAPKKK.51 When overexpressed, wnd displays an overgrowth phenotype similar to that of a hiw LOF mutant.52
C.3. Neuronal activity and synaptic growth
Neuronal activity plays a critical role in synaptic growth at the Drosophila NMJ. Double LOF mutants that have reductions in the levels of two voltage-gated K+ channels, Ether-a-go-go (Eag) and Shaker (Sh), or eag mutants expressing a Shaker dominant-negative subunit, have hyperexcitable neurons. These mutants have increased numbers of boutons, suggesting that neuronal activity can positively regulate NMJ growth.53,54 However, the increase in bouton number seen in such mutants might also be due to satellite bouton formation (see below). cAMP plays a role in the activity-dependent effect of Eag and Sh on synaptic growth, as shown by analysis of the phenotypes of double mutant combinations involving genes that regulate cAMP levels (dunce, rutabaga, and others) and those that alter electrical activity. It has been suggested that neuronal activity increases the amount of intracellular calcium, which subsequently affects signaling through the cAMP pathway.53,55
The importance of calcium regulation in bouton growth is further evident when investigating calcium channels. The voltage-gated N-type Ca+ channel Cacophony (Cac) is expressed presynaptically and functions in neurotransmitter release. An independent role of Cac is in regulation of bouton formation, since cac mutants have reduced numbers of boutons and terminal arbor branches. The data suggest that calcium entry through Cac channels has dual roles: it triggers synaptic vesicle fusion and also promotes bouton formation.56,57
C.4. Genes that affect maintenance of bouton structure
Based on LOF phenotypes, we suggest that another category of genes includes those involved in maintaining the integrity of bouton structure during development of the NMJ. Mutants for genes within this group have NMJs that display increased numbers of boutons that are arrested (or captured by fixation) at various stages of development. These include ‘ghost boutons’, presynaptic retractions (also known as ‘footprints’), and satellite boutons. Synaptopods are not included in this list, although they might also be increased in number in mutant NMJs, because they can only be observed in live preparations, and most studies of mutant phenotypes are of fixed samples.32
Ghost boutons are newly formed, ‘immature’ boutons that contain synaptic vesicles, but no active zones, and have not recruited postsynaptic elements such as Discs-Large (Dlg)58 (Figures 4G-I). They express the neuronal membrane marker recognized by anti-HRP antibody, the cell surface protein Fas2, and the synaptic vesicle markers cysteine string protein (CSP) and Synapsin. However, they lack the postsynaptic Dlg protein and GluRs. In addition, they rarely contain any Bruchpilot (Brp), which is an active zone component. Ghost boutons are transitional structures that are in the process of being stabilized into mature boutons and are not a result of degeneration of mature boutons. This was shown by live imaging of wild-type NMJs where ghost boutons, although rare, do exist.32 Stimulation of motor neurons results in an increase in ghost bouton numbers. Ghost boutons are also prominent in draper mutants. draper encodes an engulfment receptor.33 A more detailed description of Draper function is provided in the trans-synaptic section below. The process of ghost bouton formation at the Drosophila NMJ is comparable to synapse elimination in vertebrates.59
Presynaptic retractions, in which previously formed boutons disappear, are marked by footprints, which are postsynaptic relics that mark the spots that had been occupied by the boutons. Presynaptic retractions have been observed during normal growth for both Type 1b and 1s boutons, with a moderate frequency (18% of NMJs) during early larval development and a much lower frequency in 3rd instar (6% of NMJs).60 These structures are characterized by a simultaneous lack of Synapsin and Bruchpilot staining (presynaptic) and positive Dlg and glutamate receptor immunoreactivity (postsynaptic). Presynaptic retractions differ from ghost boutons not only by the pre- and postsynaptic molecules that are retained (see above), but also by the morphological changes that each encompasses: the former involves an entire arbor (with many boutons) whereas the latter involves a single bouton. In addition, the presynaptic retraction seems to occur later in the time course of bouton growth as indicated by the markers retained.
The genes implicated in presynaptic retraction are those regulating the cytoskeletal architecture. Increased retraction of synaptic boutons occurs when components of the core or the membrane cytoskeleton are disrupted. The presynaptic Dynactin complex, which includes the Arp-2 (centractin) subunit and P150/Glued, binds to the microtubule component of the core cytoskeleton. Mutants for Arp-2 or P150/Glued show disorganization of the MT network. These mutants also display a high frequency of presynaptic retractions.60
Proteins within the membrane cytoskeleton that affect synaptic retraction include Spectrin and Hu-li tai shao (Hts), the Adducin ortholog, which binds spectrin and caps actin filaments to stabilize synapses. hts mutants show an elevated number of retractions. In addition, Hts is a negative regulator of NMJ growth. When Hts is knocked down presynaptically via RNAi, the number of boutons increases and small-diameter membrane protrusions are seen at the ends of type 1b synaptic terminals. Spectrin also affects presynaptic stability. In its absence, the cell adhesion molecules Fas2 and Neuroglian (Nrg) disappear, and this is followed by synaptic retraction.61 A final player is Ankyrin2-L (the long isoform of Ankyrin2), which is thought to link the core MT cytoskeleton to the spectrin-actin membrane cytoskeleton. In its absence, the MT skeleton becomes disorganized, and this results in an increase in synaptic retractions.62,63
Satellite boutons are small boutons that bud from a parent bouton present in a branch of the terminal arbor. Since satellite boutons contain Synapsin and Brp, and are apposed to postsynaptic Dlg and GluRs, they presumably contain functional synapses. Satellite boutons are more prevalent in mutants that display NMJ overgrowth. In wild-type larval NMJs, a branching parent bouton normally has no more than two new branches.64 Mutants that exhibit the satellite bouton phenotype have parent boutons with 3-5 small boutons budding from the parent bouton64-66 (Figures 4E, F). Satellite boutons are also seen budding off from the axonal segment that connects two adjacent boutons.67 The satellite bouton phenotype is distinct from that of mutant NMJs that have many small-sized boutons (e.g., hiw, see above), since it is characterized by parent boutons of normal size with many small boutons attached to them.
Genes for which LOF mutants have satellite bouton phenotypes encode molecules that are implicated in endocytosis, Wnt signaling, and control of neuronal activity. Loss of endocytic molecules, including Dynamin, Dap-160, Endophilin, Synaptotagmin, and Synaptojanin, produces NMJs with large numbers of satellite boutons.65 Nervous wreck (Nwk) is an adaptor protein that localizes to the periactive zone.64,68 It has been shown to interact with components of the endocytic machinery and negatively regulates BMP signaling by direct interaction with the BMP receptor Thickveins (Tkv).69,70 Mutations in nwk also produce large increases in the numbers of satellite boutons. Altering the levels of some of the components of the trans-synaptic Wnt signaling pathway, including Wg, Arr, and Dsh, produces satellite bouton phenotypes Neuronal activity changes resulting from K+ channel mutants seizure (sei) and slowpoke (slo) also result in satellite bouton formation. Lee and Wu carried out a detailed study on molecules important for the formation of satellite boutons in these mutants.71 They found that these satellites were suppressed by pre- or postsynaptic cAMP signaling and that Dlg was required. Their data suggested that there is postsynaptic involvement for the early steps in satellite formation, but that the later steps are regulated presynaptically.
Other proteins that regulate satellite bouton formation include the fly homolog of the Amyloid precursor protein (APPL) and Fasciclin 2 (Fas2), the Drosophila NCAM ortholog.67 APPL is a transmembrane glycoprotein that might function as a Go-coupled receptor. Satellite boutons form when APPL is overexpressed and is not internalized, resulting in excess APPL protein on the plasma membrane. When Fas2 is selectively overexpressed on either side of the synapse, bouton number is decreased. However, overexpressing Fas2 simultaneously on both sides of the NMJ results in the formation of satellite boutons.72
C.5. Mechanisms involved in development and maintenance of NMJ arbors
The genes described in the above four sections ultimately converge to control the growth of boutons and the arborization pattern of the larval NMJ. Positive and negative regulators of bouton growth (1st and 2nd categories, respectively), modulators of neuronal activity (3rd category), and genes with structural bouton phenotypes (4th category) affect NMJ development through a variety of molecular pathways. Signaling through each of these pathways is likely to be continuously modulated by antagonistic and cooperative pathways whose input is dependent on the physiological states of the muscles and neurons. Here we describe some of the systems within which the proteins described above function in order to control the pattern of NMJ development.
Numerous studies indicate that the cytoskeleton is the primary driver in forming the presynaptic structures during development of the Drosophila neuromuscular system.73 In both vertebrate and invertebrate systems, the presynaptic terminal can be compartmentalized into the core and membrane-associated cortical cytoskeletons, as described above. Downstream cytoplasmic molecules that affect polymerization of actin or tubulin structures play roles in bouton formation and growth. Many of the MT severing proteins, extracellular matrix molecules and cell adhesion molecules (detailed in the next few paragraphs), converge through indirect pathways to these downstream effector molecules to alter the presynaptic cytoskeleton. Some of the known downstream proteins are ADF/Cofilin, LIM kinase, and Futsch. Cofilin depolymerizes actin,74 whereas LIM kinase promotes actin polymerization by inactivating Cofilin.75 LIM kinase is activated by p21-activated kinase (PAK), which is in turn stimulated by Cdc 42 and Rac.76,77 These small Rho-like GTPases control the formation of polymerized actin structures. Some of the genes listed in Table 1 seem to affect the MT-associated neuronal protein Futsch directly or indirectly. Futsch colocalizes with microtubules in boutons and may increase their stability.38 Some of the actions of the Wnt signaling pathway target Futsch presynaptically (via receptors present on the neuronal membrane and cytoplasmic proteins located intracellularly).41,78 This is a form of autocrine signaling, since the Wnt ligand, Wg, is released by the motor neuron.
NMJ development is affected by a variety of proteins that alter MT dynamics. Katanin, Spastin, and Fidgetin are enzymes that sever MTs in vitro.79,80 Spastin is important for NMJ development, but it remains to be seen if the other proteins have roles in the neuromuscular system43,81,82 (see Category 2). Atlastin, an integral membrane protein GTPase that affects microtubule stability in muscles, has been shown to bind to Spastin in vitro.83 It is not known if this interaction is relevant to bouton growth. Spastin function, like LIM kinase function, seems to be regulated by PAK.42 In mammalian cells, PAK induces phosphorylation of Stathmin, a MT binding protein, resulting in changes in actin polymerization. At Drosophila NMJs, Stathmin acts presynaptically in neurons to affect NMJ development.84
The extracellular matrix molecules Syndecan and Dally-like (Dlp) are cell surface heparan sulfate proteoglycans (HSPGs). These affect NMJ growth by interacting with leukocyte-antigen-related-like (Lar), a transmembrane receptor protein expressed in neurons.85,86 Lar is a receptor tyrosine phosphatase (RPTP) whose cytoplasmic domain interacts with several downstream signaling proteins. For the growth of boutons, Lar signals via Trio, a Rho-GEF (GEF, guanine nucleotide exchange factor) and Diaphanous, a Rho GTPase, to control the actin and microtubule cytoskeleton.87 Lar also interacts with Dliprin-alpha (Syd-2 ortholog) which is involved in the organization of active zones.86,88 Syd-1, a Rho-GTPase activating protein (RhoGAP) is required for the correct localization of Dliprin-alpha to active zones.89 To assemble both pre- and postsynaptic proteins across the synaptic cleft, Owald et al.90 showed that Syd-1 recruits the cell adhesion molecule Neurexin and its postsynaptic partner, Neuroligin. These proteins assemble earlier than the localization of Bruchpilot (active zone protein) and the postsynaptic glutamate receptors.
Many synaptically localized cell adhesion molecules (CAMs) affect morphology and growth of boutons at the NMJ.91,92 Fas2 can be a positive or a negative regulator of bouton formation, depending on whether it is expressed on both sides of the synaptic cleft or on one side, respectively. Fas2 stimulates growth by signaling through APPL and a cytosolic APPL-binding protein protein, Mint.72 Fas2 homophilic interactions across the cleft may trigger the phosphorylation of APPL. The latter molecule could relay a signal to microtubules by binding to the heterotrimeric GTP binding protein G 72o and thus stimulate bouton growth by affecting MT dynamics.
Ubiquitylation and sumoylation are two processes that affect diverse cellular processes. SUMO (Small Ubiquitin like Modifier) proteins are small protein tags that are covalently attached to other proteins to modify their function. Sumoylation is similar to ubiquitylation, but has different functions. The latter is used to tag proteins for degradation whereas the former is used mainly for modification of proteins. Although there is extensive evidence (see section C.2. above) for roles of ubiquitylating proteins in NMJ development in Drosophila, sumoylation has not been shown to play a role in bouton growth.44 Recently, Berdnik et al. have shown the involvement of a SUMO protease participating in the Drosophila olfactory system.93 It will be of interest to examine the roles of the sumoylation machinery in the growth and development of boutons in the Drosophila NMJ.
D. Trans-synaptic signaling pathways
Two of the most extensively studied trans-synaptic pathways that regulate development of synaptic arbors at Drosophila NMJs are the Wnt pathway and the BMP pathway. Excellent recent reviews exist for these pathways, so we do not discuss them here.35,94-98 The Neurexin-Neuroligin trans-synaptic pathway has also recently been reviewed.99 Below we discuss two less well-known trans-synaptic pathways.
D.1. The Draper/Ced-6 signaling pathway
Draper is an engulfment receptor molecule that is involved in removal of neuronal cell fragments during programmed cell death in the Drosophila brain.100 At the larval NMJ, the Draper/Ced-6 pathway functions to clear presynaptic neuronal debris and ghost boutons that have not stabilized. This pathway operates in the muscles and in the glial cells that surround the synaptic boutons. In draper mutants, the number of boutons decreases and the number of ghost boutons increases. The latter is due to inability of the muscle cells to phagocytose immature ghost boutons. The fact that synaptic growth is decreased in draper mutants suggests that accumulated presynaptic debris not cleared by Draper inhibits growth at the NMJ.33 We classify this pathway as trans-synaptic in this review because the results suggest that presynaptic neuronal debris contains signaling molecules that might activate the Draper/Ced-6 pathway in muscles and glia to clear the debris and thus allow synaptic growth.
D.2. Synaptotagmin-4 (Syt 4) retrograde signaling pathway
Syt 4 localizes to vesicles in the postsynaptic muscles. Syt 4 mRNA and protein expression are modulated by neuronal activity. In wild type NMJs, increasing neuronal activity (by increasing temperature or in hyperexcitability mutants) results in increased numbers of boutons.53,101 In syt 4 mutants, there is no synaptic overgrowth when neuronal activity is increased.102 Thus Syt 4 seems to control the postsynaptic signal that promotes bouton growth when induced by activity.
III. Translational Regulatory Mechanisms and Acute Plasticity at the NMJ
Translational regulation is used to modulate protein expression and localization in a variety of biological contexts, including early embryonic development, cell differentiation, and neuronal plasticity. In both Drosophila and vertebrates, translation of many specific mRNAs is regulated during early embryonic patterning. Translationally regulated Drosophila maternal mRNAs that are essential for development include hunchback, oskar, gurken, and nanos (nos).103-105 In many cases, translational regulation involves protein-RNA and/or RNA-RNA interactions with the 3′ untranslated region (UTR) sequences of the regulated transcripts.
Long-lasting changes in the structure and function of synapses are required for the storage and processing of information. Regulated ‘local’ postsynaptic protein synthesis is an attractive long-term plasticity mechanism because it provides a way to maintain synaptic states beyond the lifetime of any individual protein in the synapse. Newly synthesized proteins could in principle be selectively directed only to those synapses that have undergone modification. It is known that components necessary for translation are present in mammalian dendrites, including polyribosomes,106,107 mRNAs, and miRNA machinery.108,109 Dendritic protein synthesis is required for long-term maintenance of changes in synaptic efficacy. However, it has not been demonstrated that newly synthesized proteins are actually selectively routed to dendritic spines containing potentiated synapses.
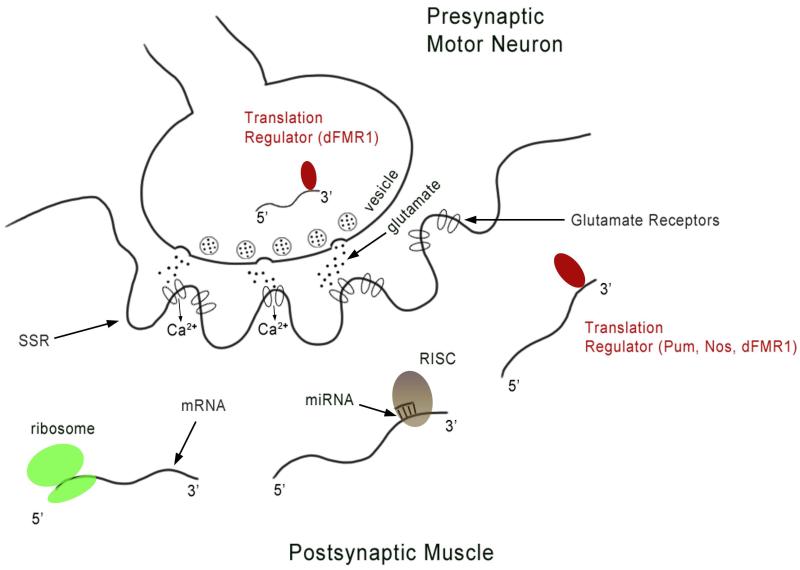
Although Ib and Is synapses, which derive from different neurons, can be separately regulated,110 there is no evidence that single boutons of the same type within a single Drosophila NMJ are independently controlled. However, the Drosophila NMJ is still a useful system in which to study translational regulation, because it exhibits both developmental and short-term plasticity, and control of postsynaptic mRNA translation is essential for these events. Some translational control mechanisms may operate throughout the entire postsynaptic muscle fiber, while others may be specific to the postsynaptic SSR, which has been shown to contain polyribosomes.111,112 In this section of the review, we will consider some translational regulatory mechanisms that function at the Drosophila NMJ to regulate synaptic growth and plasticity, focusing on translational repression by microRNAs (miRNAs) and the RNA-binding proteins fragile X mental retardation protein (FMRP), Pumilio (Pum), and Nanos (Nos) (Figure 5).
Figure 5. A schematic diagram of the NMJ depicting some of the translational regulatory mechanisms that function in the postsynaptic muscle or in the motor neuron, possibly in its presynaptic terminal.
In the presynaptic motor neuron, dFMR1 (indicated by the dark oval) binds to futsch mRNA and inhibits its translation. In the postsynaptic muscle, mRNAs are shown that are being actively translated (indicated by the ribosome), regulated by either microRNA and the RISC complex (indicated by base-paired complementary strand and light oval) or by translational regulatory proteins (indicated by dark oval). miR-8 regulates translation of enabled and other target mRNAs. The translational repressors Pum and Nos regulate expression of GluRIIA and GluRIIB, respectively, and Pum binds directly to GluRIIA mRNA. dFMR1 represses expression of both GluRII subunits. RISC: RNAi-silencing complex, SSR: subsynaptic reticulum.
A. miRNAs
miRNAs are 21-25 nucleotide non-coding RNAs that regulate gene expression by binding to target mRNAs and recruiting a repressor complex known as the RNA-induced silencing complex (RISC), which includes Dicer, Argonaute proteins, and other components.113,114 RISC not only functions in the biogenesis of miRNAs and siRNAs (silencing RNAs), but also is required for their activity. The degree of complementarity between the miRNA and its target mRNA determines the mode of regulation: near-perfect complementarity results in cleavage of the duplex, whereas partial complementarity leads to translational repression.
miRNAs are known to be important regulators of neural development and function in a variety of systems.115-117 However, the only miRNA whose individual function at the NMJ has been characterized thus far is miR-8. This miRNA is also implicated in neurodegeneration,118 Wnt signaling,119 and innate immune homeostasis.120 miR-8 was found to be a regulator of NMJ growth in a forward genetic screen. miR-8 activity at the NMJ was examined using a deletion of miR-8 and a modified ‘microRNA sponge’ system, which can inactivate specific miRNAs.121 Knocking out miR-8 function presynaptically had no effect. However, postsynaptic knockout resulted in a decrease in the number of synaptic boutons and branches. The 3′UTR of the enabled (ena) mRNA has one predicted miR-8 binding site, and if miR-8 indeed binds to and represses ena mRNA translation, Ena protein levels should increase when miR-8 activity is inhibited. This was in fact observed: postsynaptic expression of the miR-8 sponge resulted in elevated levels of Ena. It was also shown that postsynaptic overexpression of Ena can phenocopy the loss of miR-8.122 These results provide evidence for a role of miRNA-mediated translational repression in regulating synaptic growth at the NMJ. Some important questions remain, however. First, are ena mRNA and/or miR-8 localized to the SSR? Second, the genetic interaction between miR-8 and ena does not necessarily indicate that miR-8 directly controls Ena expression. It will be important to determine whether miR-8 binds to the ena 3′UTR.
Although miR-8 is the only miRNA that has been shown to regulate NMJ growth thus far, Dicer and miR-284a control GluRIIA and GluRIIB protein levels at the NMJ34 (Figure 5). GluR mutations can affect bouton numbers,123-125 so miRNA-mediated effects on translation of GluR mRNAs may have an impact on NMJ growth during development.
The roles of miRNAs at the NMJ have also been explored using mutations that affect the RISC complex. Argonaute 2 (Ago2) is expressed presynaptically at the NMJ and has been shown to be a positive regulator of bouton growth. Ago 2 mutants have decreases in bouton number and in the number of arbor branches.126 Ago2 functions predominantly in the siRNA pathway rather than in miRNA processing, although alternate miRNA biogenesis pathways may require Ago2.127
Two other Drosophila miRNAs have been demonstrated to regulate dendritic outgrowth and to repress translation of proteins that are important for NMJ growth. It would be interesting to examine whether these miRNAs also have functions at the NMJ. In Drosophila embryos and larvae, GFP driven by the miR-124a promoter is expressed at high levels in the ventral nerve cord and motor neurons and at lower levels in dendritic arborization (DA) neurons in the peripheral nervous system (PNS).128 Overexpression of miR-124a in DA neurons caused a reduction in the number of dendritic ends. miR-184 functions in ovaries to allow differentiation of the germline stem cells by reducing expression of the Decapentaplegic receptor, Saxophone (Sax).129 sax mutants also have an NMJ phenotype characterized by reduced numbers of boutons and decreased synaptic strength.130 It is not known whether miR-184 contributes to this sax phenotype.
B. Fragile X mental retardation protein
Fragile X syndrome is one of the most common forms of mental retardation in humans, affecting an estimated 1 in 4000 males and 1 in 8000 females. The disease is caused by mutations in a single gene, FMR1 (for fragile X mental retardation 1).131-133 FMR1 protein (FMRP) is widely expressed in fetal and adult tissues with enhanced expression in the brain and testes where major symptoms are manifested.134 Loss of FMRP results in delayed dendritic spine maturation in both FXS patients and Fmr1 knockout mice.135-137 FMRP is a translational repressor 138,139 that interacts with sequences in the 3′UTRs of its target mRNAs. Flies have only one ortholog of FMRP, called dFMR1, which regulates synaptic structure and physiology.140
At the Drosophila NMJ, dFMR1 is expressed presynaptically in the motor neurons and postsynaptically in the muscles. Analysis of dfmr1 mutants revealed synaptic overgrowth characterized by an increase in the number of boutons, an increase in branching, and increased synaptic strength. Satellite boutons were also present in greater numbers than in wild-type.141 Overexpression of dFMR1 presynaptically produces a phenotype with fewer boutons that are significantly larger than normal. One of the neuronal targets of FMRP is Futsch, the MAP-1b ortholog.142,143 Futsch mRNA levels are upregulated in dfmr1 mutants. dFMR1 repression of Futsch expression is important for NMJ development, because futsch mutations suppress the dfmr1 NMJ phenotype.140
Like miRNAs, dFMR1 controls expression of the subunits of the postsynaptic ionotropic glutamate receptor.144 NMJ GluRs can be divided into two classes: the larger amplitude, slower acting A class and the smaller amplitude, faster-acting B class. Each functional GluR consists of four subunits, three of which are shared among all receptors (GluRIIC, GluRIID, GluRIIE). The fourth subunit is either GluRIIA (A class) or GluRIIB (B class).110,145 In dfmr1 mutants, GluRIIA is increased and GluRIIB is decreased (Figure 6). Postsynaptic overexpression of dFMR1 caused a decrease in both GluRIIA and GluRIIB levels. Because GluRIIA mRNA has been localized to the postsynaptic region of the muscle fiber, it is possible that this repression of the GluRIIA subunit by dFmr1 occurs locally at synapses.111 The contribution of dFMR1-regulated GluR expression to bouton growth has not been examined, although it is well documented that GluR levels affect NMJ growth during development.123-125
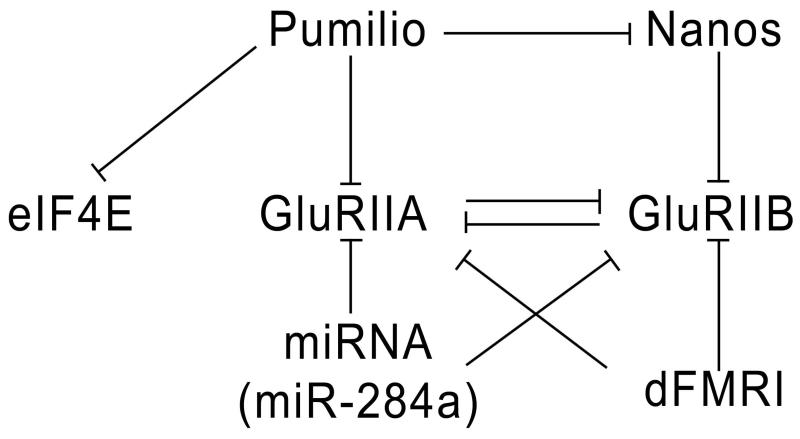
Figure 6. A diagram depicting the actions of translational regulatory proteins on GluRIIA, GluRIIB, and eIF-4E mRNAs.
GluRIIA is repressed by Pum, miR-284a, and dFMR1. However, only Pum has been shown to directly bind to the 3′UTR of GluRIIA mRNA. Pum also binds to eIF-4E and nos mRNAs. GluRIIB expression is repressed by Nos, miR-284a and dFMR1. GluRIIA and GluRIIB compete for synaptic occupancy, so that GluRIIA represses GluRIIB expression and vice versa.
As described above, miR-124a may be likely to have roles at the NMJ, due to its high expression in motor neurons. miR-124a levels are regulated by dFmr1.128 Thus, other than direct repression of target mRNAs, dFmr1 may also affect neuronal development at the NMJ by controlling levels of miR-124a. Another potential target of dFMR1 is Dlg, the PSD-95 ortholog. In mice, FMRP interacts with the 3′UTR of PSD-95 mRNA to regulate its stability.146 Since Dlg plays a major role in synaptic structure and function at the larval NMJ,6,18 dFMR1 could also regulate bouton growth by repressing translation of dlg mRNA. Finally, interactions between FMRP and the miRNA pathway may further contribute to the roles that both of these systems play in synaptic growth. These interactions are discussed in more detail below.
C. Translational repression by Pumilio and Nanos
The Drosophila Pumilio (Pum) protein is a member of the PUF RNA-binding protein family. Maternal Pum functions primarily as a translational repressor in embryonic patterning and germ cell proliferation and migration.147,148 Pum recognizes sites called Nanos response elements (NREs) in its target mRNAs, including those encoding Hunchback, Cyclin B, and Para.149-151 Most NREs are in 3′ UTRs. In most cases, Nanos (Nos) functions as a corepressor necessary for Pum-mediated translational repression. However, Pum and Nos also have functions that are separate from those of their partners.152-154
Zygotic Pum also functions later in development at the larval NMJ. Pum is postsynaptically localized at the NMJ, and is also present in neuronal cell bodies.155 Pum has distinct roles on the two sides of the synapse. In pum mutants, type 1b boutons are much larger and their numbers are decreased, whereas the number of 1s boutons is increased. The 1b bouton phenotype can be fully rescued by neuronal expression of full-length Pum in the pum mutant background. Postsynaptic Pum expression in pum mutants has no effect on the 1b bouton phenotype, but it rescues the increase in 1s bouton numbers.155
The idea that local postsynaptic translation occurs at the NMJ emerged from the findings that puncta (‘aggregates’) of the translation factors eIF-4E and PABP appear at NMJ boutons after larval motor activity is induced by moving larvae from slurry (liquid) to solid food for a period of a few hours.111 Larvae can remain relatively stationary while ingesting liquid food, but must actively burrow through solid food. GluRIIA levels are also increased by this protocol. It was speculated that the purpose of local postsynaptic translation at the NMJ is to allow rapid changes in synaptic strength and facilitate the growth of new boutons in response to increases in larval motor activity. These synaptic alterations might be able to occur more quickly if they are implemented through translation of mRNAs that are already localized to the postsynaptic SSR.
Since Pum is a translational repressor156 and is postsynaptically localized, Menon et al.155 reasoned that it might control the levels of postsynaptic eIF-4E, which is limiting for translation in many systems. Indeed, it was observed that eIF-4E levels at the NMJ are very high in pum mutants (up to 12-fold higher than in wild-type), and that these levels are unchanged by increases in larval motor activity. eIF-4E is encoded by a single essential gene, so eIF-4E protein is also present within the cytoplasm of the muscle fiber. However, cytoplasmic eIF-4E levels are unchanged in pum mutants, indicating that translational repression by Pum only occurs at the synaptic sites where Pum Is localized. Pum binds selectively to the 3′ UTR of eIF-4E mRNA, suggesting that it is a direct target. GluRIIA levels are also greatly increased in pum mutants, and Pum binds selectively to the 3′ UTR of GluRIIA mRNA as well.152 These results are consistent with a model in which Pum normally represses translation of synaptically localized eIF-4E and GluRIIA mRNAs in larvae that are not moving vigorously. When larval motor activity increases, Pum (or a Pum cofactor) is partially inactivated. This would cause the levels of eIF-4E, GluRIIA, and other direct Pum targets to increase rapidly, since these proteins would be translated from pre-existing mRNAs that had been translationally repressed by Pum. When larvae are forced to move they require more transmission at the NMJ, and this could be facilitated by eIF-4E induction, since eIF-4E might be limiting for translation of all postsynaptically localized mRNAs. The induction of GluRIIA, which produces receptor complexes that conduct more current, would also increase the ability of the NMJs to effectively depolarize the muscles. The mechanisms by which Pum might be inactivated after induction of larval motor activity are unknown, but there is evidence that Pum’s postsynaptic functions are regulated by an aggregation-prone sequence within its unstructured N-terminal region.157
The regulation of eIF-4E and GluRIIA by Pum is part of a more complex circuit of translational regulation that operates at the NMJ. During early development, Pum and Nos work together to repress Hb and other targets. However, they work in opposition to each other at the NMJ. Pum binds to the 3′ UTR of nos mRNA, and Nos levels are increased in pum mutants. Nos represses expression of the alternate GluR subunit GluRIIB. Also, GluRIIA and GluRIIB compete with each other for occupancy in synaptic receptor clusters. Thus, when Pum levels are reduced, Nos is increased, leading to downregulation of GluRIIB, which amplifies the elevation of GluRIIA produced by loss of repression of its mRNA by Pum. Conversely, if Pum is increased, GluRIIA and Nos are both repressed, leading to increased expression of GluRIIB at the expense of GluRIIA152 (Figure 6). The mechanisms by which Nos represses GluRIIB without the involvement of Pum are unknown.
Pum is also involved in synaptic growth and plasticity in other types of neurons, both in Drosophila and in vertebrate systems. Pum can bind to the 3′UTR of dlg mRNA. In the mushroom bodies of adult Drosophila, overexpression of Pum reduces the levels of Dlg and causes a defect in the elaboration of axonal projections.158 However, Dlg levels at the NMJ are not affected by Pum. Hypomorphic pum mutants have been reported to have learning and memory defects.159 Pum and Nos also affect dendritic arborization in Drosophila sensory neurons.160 In dissociated mammalian hippocampal neurons, Pum is localized to granules in dendrites.161 Knockdown of Pum by siRNA causes increases in dendritic arborization, while Pum overexpression reduces the size of the dendritic arbor.162 Finally, a novel function of Pum in controlling translational repression by miRNAs was recently reported163 and this is discussed below.
D. Orb2/CPEB2
CPEB proteins can be divided into two subfamilies. The first CPEB subfamily functions mainly during oogenesis and early embryonic development 164 and includes the Drosophila CPEB ortholog Orb. CPEBs recognize specific sequences in the 3′UTRs of their target mRNAs and control their translation. Orb is required for establishing anteroposterior and dorsoventral axes in early development by translationally activating oskar and gurken mRNAs, respectively.165-169 The second CPEB subfamily includes vertebrate CPEB2-4 and Drosophila Orb2. This subfamily is more broadly expressed and has roles outside of the germline.170,171
Although no direct evidence yet links Orb2 to synaptic growth at the NMJ, it is reasonable to think that it may have a role there. Drosphila Orb2 is widely expressed in the nervous system from embryonic to adult stages. Specific localization of Orb2 at synaptic sites was observed in the CNS, suggesting that it might be involved in synaptic translation.172 A study aimed at identifying mRNA targets of Orb2 identified a variety of genes involved in synaptic growth and stability at the NMJ, including neuroligin, still life, and aPKC.173 It is unknown, however, if Orb2 regulates translation of these mRNAs in the neuromuscular system. Finally, Orb2 was identified in a screen for proteins likely to function in the dFmr1 pathway, suggesting that that Orb2 might regulate dFmr1-mediated synaptic growth.174
E. Interplay among translational regulation pathways
In addition to the separate action of each of these translational regulatory mechanisms on its respective targets, evidence suggests that these systems can regulate one another and/or work in tandem to control the same target mRNAs. The miRNA pathway and mammalian FMRP are intimately linked.175,176 In Drosophila, orb mRNA translation is activated by Orb protein, forming a positive feedback loop. dFMR1 also binds orb mRNA and inhibits its translation, thus keeping the positive feedback loop in check.177 The 3′UTR of the mammalian tumor suppressor p27 mRNA has binding sites for Pum and for two miRNAs, miR-221 and miR-222. However, in order for the miRNAs to efficiently repress p27 translation, Pum must first bind and induce a conformational change in the mRNA.163 A similar observation was made for translational regulation of the E2F3 oncogene.178
Collectively, these interactions suggest that a complex series of interconnected regulatory mechanisms control the translation of mRNAs that encode key regulators of synaptic growth and function at the NMJ. However, we still lack an overall understanding of how these mechanisms work together to ensure that mRNAs encoding synaptic regulators are translated at the correct times and places. We need to determine which mechanisms function in the postsynaptic SSR, and which act in the muscle and neuronal cytoplasm. Are all of the necessary components for translation present in the SSR? This is not yet known. It is also unknown whether all of these mechanisms function during the same developmental stages. Finally, it has not yet been directly demonstrated that local synaptic translation actually occurs at the NMJ. It would be valuable to develop an optical method to detect and localize synaptic translational events in wild-type and mutant larvae.
The genes described in this review encode proteins that control synaptic bouton growth through many different mechanisms. These include cytoskeletal dynamics, protein degradation, cell adhesion, and neuronal activity. NMJ growth and development is further fine-tuned at the level of protein synthesis by translational regulators that include miRNAs, FMRP and Pum. The study of the larval NMJ is likely to continue to generate exciting new findings. It will also be of interest to examine the development and maintenance of the adult neuromuscular system, which is still poorly understood. We have highlighted molecular mechanisms employed at the Drosophila NMJ that are similar to those used at glutamatergic synapses in the vertebrate nervous system. Many vertebrate synaptic proteins have orthologs that are used for the development and function of the fly NMJ. Because of this, researchers can productively use forward genetic screens in Drosophila to find new synaptic components that are likely to be important for development and/or function of mammalian excitatory synapses. Insights gained from studies of the fly NMJ should provide information relevant to the development and function of synapses in many other systems
Acknowledgments
We would like to thank Violana Nesterova for help with the figures. This work was supported by NIH RO1 grants NS62821 and MH65537 to K.Z.
Footnotes
Additional online resources:
The interactive fly: http://www.sdbonline.org/fly/aimain/1aahome.htm
Flybase: http://flybase.org/
iHOP (information hyperlinked over proteins): http://www.ihop-net.org/UniPub/iHOP/
Zinn lab motor axon development primer: http://www.its.caltech.edu/~zinnlab/motoraxons/fmaHomePage3.html
References
- 1.Shen K, Cowan CW. Guidance molecules in synapse formation and plasticity. Cold Spring Harb Perspect Biol. 2010 Apr;2(4):a001842. doi: 10.1101/cshperspect.a001842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Griffith LC, Budnik V. Plasticity and second messengers during synapse development. Int Rev Neurobiol. 2006;75:237–265. doi: 10.1016/S0074-7742(06)75011-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tabuchi K, Sudhof TC. Structure and evolution of neurexin genes: insight into the mechanism of alternative splicing. Genomics. 2002 Jun;79(6):849–859. doi: 10.1006/geno.2002.6780. [DOI] [PubMed] [Google Scholar]
- 4.Banovic D, Khorramshahi O, Owald D, et al. Drosophila neuroligin 1 promotes growth and postsynaptic differentiation at glutamatergic neuromuscular junctions. Neuron. 2010 Jun 10;66(5):724–738. doi: 10.1016/j.neuron.2010.05.020. [DOI] [PubMed] [Google Scholar]
- 5.Sun M, Xing G, Yuan L, et al. Neuroligin 2 is required for synapse development and function at the Drosophila neuromuscular junction. J Neurosci. 2011 Jan 12;31(2):687–699. doi: 10.1523/JNEUROSCI.3854-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lahey T, Gorczyca M, Jia XX, Budnik V. The Drosophila tumor suppressor gene dlg is required for normal synaptic bouton structure. Neuron. 1994 Oct;13(4):823–835. doi: 10.1016/0896-6273(94)90249-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen CN, Denome S, Davis RL. Molecular analysis of cDNA clones and the corresponding genomic coding sequences of the Drosophila dunce+ gene, the structural gene for cAMP phosphodiesterase. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9313–9317. doi: 10.1073/pnas.83.24.9313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davis RL, Takayasu H, Eberwine M, Myres J. Cloning and characterization of mammalian homologs of the Drosophila dunce+ gene. Proc Natl Acad Sci U S A. 1989 May;86(10):3604–3608. doi: 10.1073/pnas.86.10.3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johansen J, Halpern ME, Johansen KM, Keshishian H. Stereotypic morphology of glutamatergic synapses on identified muscle cells of Drosophila larvae. J Neurosci. 1989 Feb;9(2):710–725. doi: 10.1523/JNEUROSCI.09-02-00710.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Budnik V. Synapse maturation and structural plasticity at Drosophila neuromuscular junctions. Curr Opin Neurobiol. 1996 Dec;6(6):858–867. doi: 10.1016/s0959-4388(96)80038-9. [DOI] [PubMed] [Google Scholar]
- 11.Chiba A, Keshishian H. Neuronal pathfinding and recognition: roles of cell adhesion molecules. Dev Biol. 1996 Dec 15;180(2):424–432. doi: 10.1006/dbio.1996.0316. [DOI] [PubMed] [Google Scholar]
- 12.Keshishian H, Broadie K, Chiba A, Bate M. The drosophila neuromuscular junction: a model system for studying synaptic development and function. Annu Rev Neurosci. 1996;19:545–575. doi: 10.1146/annurev.ne.19.030196.002553. [DOI] [PubMed] [Google Scholar]
- 13.Landgraf M, Bossing T, Technau GM, Bate M. The origin, location, and projections of the embryonic abdominal motorneurons of Drosophila. J Neurosci. 1997 Dec 15;17(24):9642–9655. doi: 10.1523/JNEUROSCI.17-24-09642.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schmid A, Chiba A, Doe CQ. Clonal analysis of Drosophila embryonic neuroblasts: neural cell types, axon projections and muscle targets. Development. 1999 Nov;126(21):4653–4689. doi: 10.1242/dev.126.21.4653. [DOI] [PubMed] [Google Scholar]
- 15.Hoang B, Chiba A. Single-cell analysis of Drosophila larval neuromuscular synapses. Dev Biol. 2001 Jan 1;229(1):55–70. doi: 10.1006/dbio.2000.9983. [DOI] [PubMed] [Google Scholar]
- 16.Ruiz-Canada C, Budnik V. Introduction on the use of the Drosophila embryonic/larval neuromuscular junction as a model system to study synapse development and function, and a brief summary of pathfinding and target recognition. Int Rev Neurobiol. 2006;75:1–31. doi: 10.1016/S0074-7742(06)75001-2. [DOI] [PubMed] [Google Scholar]
- 17.Broadie K, Bate M. Innervation directs receptor synthesis and localization in Drosophila embryo synaptogenesis. Nature. 1993 Jan 28;361(6410):350–353. doi: 10.1038/361350a0. [DOI] [PubMed] [Google Scholar]
- 18.Chen K, Featherstone DE. Discs-large (DLG) is clustered by presynaptic innervation and regulates postsynaptic glutamate receptor subunit composition in Drosophila. BMC Biol. 2005;3:1. doi: 10.1186/1741-7007-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang LI, Poo MM. Electrical activity and development of neural circuits. Nat Neurosci. 2001 Nov;4(Suppl):1207–1214. doi: 10.1038/nn753. [DOI] [PubMed] [Google Scholar]
- 20.Shatz CJ. Competitive interactions between retinal ganglion cells during prenatal development. J Neurobiol. 1990 Jan;21(1):197–211. doi: 10.1002/neu.480210113. [DOI] [PubMed] [Google Scholar]
- 21.Wong RO. Retinal waves and visual system development. Annu Rev Neurosci. 1999;22:29–47. doi: 10.1146/annurev.neuro.22.1.29. [DOI] [PubMed] [Google Scholar]
- 22.Sanes JR, Lichtman JW. Development of the vertebrate neuromuscular junction. Annu Rev Neurosci. 1999;22:389–442. doi: 10.1146/annurev.neuro.22.1.389. [DOI] [PubMed] [Google Scholar]
- 23.Buffelli M, Burgess RW, Feng G, Lobe CG, Lichtman JW, Sanes JR. Genetic evidence that relative synaptic efficacy biases the outcome of synaptic competition. Nature. 2003 Jul 24;424(6947):430–434. doi: 10.1038/nature01844. [DOI] [PubMed] [Google Scholar]
- 24.Carrillo RA, Olsen DP, Yoon KS, Keshishian H. Presynaptic activity and CaMKII modulate retrograde semaphorin signaling and synaptic refinement. Neuron. 2010 Oct 6;68(1):32–44. doi: 10.1016/j.neuron.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jarecki J, Keshishian H. Role of neural activity during synaptogenesis in Drosophila. J Neurosci. 1995 Dec;15(12):8177–8190. doi: 10.1523/JNEUROSCI.15-12-08177.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Keshishian H, Chang TN, Jarecki J. Precision and plasticity during Drosophila neuromuscular development. FASEB J. 1994 Jul;8(10):731–737. doi: 10.1096/fasebj.8.10.8050672. [DOI] [PubMed] [Google Scholar]
- 27.Keshishian H, Chiba A, Chang TN, et al. Cellular mechanisms governing synaptic development in Drosophila melanogaster. J Neurobiol. 1993 Jun;24(6):757–787. doi: 10.1002/neu.480240606. [DOI] [PubMed] [Google Scholar]
- 28.Atwood HL, Govind CK, Wu CF. Differential ultrastructure of synaptic terminals on ventral longitudinal abdominal muscles in Drosophila larvae. J Neurobiol. 1993 Aug;24(8):1008–1024. doi: 10.1002/neu.480240803. [DOI] [PubMed] [Google Scholar]
- 29.Schuster CM, Davis GW, Fetter RD, Goodman CS. Genetic dissection of structural and functional components of synaptic plasticity. I. Fasciclin II controls synaptic stabilization and growth. Neuron. 1996 Oct;17(4):641–654. doi: 10.1016/s0896-6273(00)80197-x. [DOI] [PubMed] [Google Scholar]
- 30.Liu Z, Chen Y, Wang D, Wang S, Zhang YQ. Distinct presynaptic and postsynaptic dismantling processes of Drosophila neuromuscular junctions during metamorphosis. J Neurosci. 2010 Sep 1;30(35):11624–11634. doi: 10.1523/JNEUROSCI.0410-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zito K, Parnas D, Fetter RD, Isacoff EY, Goodman CS. Watching a synapse grow: noninvasive confocal imaging of synaptic growth in Drosophila. Neuron. 1999 Apr;22(4):719–729. doi: 10.1016/s0896-6273(00)80731-x. [DOI] [PubMed] [Google Scholar]
- 32.Ataman B, Ashley J, Gorczyca M, et al. Rapid activity-dependent modifications in synaptic structure and function require bidirectional Wnt signaling. Neuron. 2008 Mar;57(5):705–718. doi: 10.1016/j.neuron.2008.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fuentes-Medel Y, Logan MA, Ashley J, Ataman B, Budnik V, Freeman MR. Glia and muscle sculpt neuromuscular arbors by engulfing destabilized synaptic boutons and shed presynaptic debris. PLoS Biol. 2009 Aug;7(8):e1000184. doi: 10.1371/journal.pbio.1000184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Karr J, Vagin V, Chen K, et al. Regulation of glutamate receptor subunit availability by microRNAs. J Cell Biol. 2009 May 18;185(4):685–697. doi: 10.1083/jcb.200902062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koles K, Budnik V. Wnt signaling in neuromuscular junction development. Cold Spring Harb Perspect Biol. 2012 Jun;4(6) doi: 10.1101/cshperspect.a008045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koon AC, Ashley J, Barria R, et al. Autoregulatory and paracrine control of synaptic and behavioral plasticity by octopaminergic signaling. Nat Neurosci. 2011 Feb;14(2):190–199. doi: 10.1038/nn.2716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goellner B, Aberle H. The synaptic cytoskeleton in development and disease. Dev Neurobiol. 2012 Jan;72(1):111–125. doi: 10.1002/dneu.20892. [DOI] [PubMed] [Google Scholar]
- 38.Roos J, Hummel T, Ng N, Klambt C, Davis GW. Drosophila Futsch regulates synaptic microtubule organization and is necessary for synaptic growth. Neuron. 2000 May;26(2):371–382. doi: 10.1016/s0896-6273(00)81170-8. [DOI] [PubMed] [Google Scholar]
- 39.Hummel T, Krukkert K, Roos J, Davis G, Klambt C. Drosophila Futsch/22C10 is a MAP1B-like protein required for dendritic and axonal development. Neuron. 2000 May;26(2):357–370. doi: 10.1016/s0896-6273(00)81169-1. [DOI] [PubMed] [Google Scholar]
- 40.Ruiz-Canada C, Ashley J, Moeckel-Cole S, Drier E, Yin J, Budnik V. New synaptic bouton formation is disrupted by misregulation of microtubule stability in aPKC mutants. Neuron. 2004 May 27;42(4):567–580. doi: 10.1016/s0896-6273(04)00255-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miech C, Pauer HU, He X, Schwarz TL. Presynaptic local signaling by a canonical wingless pathway regulates development of the Drosophila neuromuscular junction. J Neurosci. 2008 Oct 22;28(43):10875–10884. doi: 10.1523/JNEUROSCI.0164-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ozdowski EF, Gayle S, Bao H, Zhang B, Sherwood NT. Loss of Drosophila melanogaster p21-activated kinase 3 suppresses defects in synapse structure and function caused by spastin mutations. Genetics. 2011 Sep;189(1):123–135. doi: 10.1534/genetics.111.130831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sherwood NT, Sun Q, Xue M, Zhang B, Zinn K. Drosophila spastin regulates synaptic microtubule networks and is required for normal motor function. PLoS Biol. 2004 Dec;2(12):e429. doi: 10.1371/journal.pbio.0020429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.DiAntonio A, Hicke L. Ubiquitin-dependent regulation of the synapse. Annu Rev Neurosci. 2004;27:223–246. doi: 10.1146/annurev.neuro.27.070203.144317. [DOI] [PubMed] [Google Scholar]
- 45.DiAntonio A, Haghighi AP, Portman SL, Lee JD, Amaranto AM, Goodman CS. Ubiquitination-dependent mechanisms regulate synaptic growth and function. Nature. 2001 Jul 26;412(6845):449–452. doi: 10.1038/35086595. [DOI] [PubMed] [Google Scholar]
- 46.Peters JM. The anaphase promoting complex/cyclosome: a machine designed to destroy. Nature reviews. Molecular cell biology. 2006 Sep;7(9):644–656. doi: 10.1038/nrm1988. [DOI] [PubMed] [Google Scholar]
- 47.Tyers M, Jorgensen P. Proteolysis and the cell cycle: with this RING I do thee destroy. Curr Opin Genet Dev. 2000 Feb;10(1):54–64. doi: 10.1016/s0959-437x(99)00049-0. [DOI] [PubMed] [Google Scholar]
- 48.van Roessel P, Elliott DA, Robinson IM, Prokop A, Brand AH. Independent regulation of synaptic size and activity by the anaphase-promoting complex. Cell. 2004 Nov 24;119(5):707–718. doi: 10.1016/j.cell.2004.11.028. [DOI] [PubMed] [Google Scholar]
- 49.Wan HI, DiAntonio A, Fetter RD, Bergstrom K, Strauss R, Goodman CS. Highwire regulates synaptic growth in Drosophila. Neuron. 2000 May;26(2):313–329. doi: 10.1016/s0896-6273(00)81166-6. [DOI] [PubMed] [Google Scholar]
- 50.Wu C, Wairkar YP, Collins CA, DiAntonio A. Highwire function at the Drosophila neuromuscular junction: spatial, structural, and temporal requirements. J Neurosci. 2005 Oct 19;25(42):9557–9566. doi: 10.1523/JNEUROSCI.2532-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fulga TA, Van Vactor D. Synapses and growth cones on two sides of a highwire. Neuron. 2008 Feb 7;57(3):339–344. doi: 10.1016/j.neuron.2008.01.016. [DOI] [PubMed] [Google Scholar]
- 52.Collins CA, Wairkar YP, Johnson SL, DiAntonio A. Highwire restrains synaptic growth by attenuating a MAP kinase signal. Neuron. 2006 Jul 6;51(1):57–69. doi: 10.1016/j.neuron.2006.05.026. [DOI] [PubMed] [Google Scholar]
- 53.Budnik V, Zhong Y, Wu CF. Morphological plasticity of motor axons in Drosophila mutants with altered excitability. J Neurosci. 1990 Nov;10(11):3754–3768. doi: 10.1523/JNEUROSCI.10-11-03754.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mosca TJ, Carrillo RA, White BH, Keshishian H. Dissection of synaptic excitability phenotypes by using a dominant-negative Shaker K+ channel subunit. Proc Natl Acad Sci U S A. 2005 Mar 1;102(9):3477–3482. doi: 10.1073/pnas.0406164102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhong Y, Budnik V, Wu CF. Synaptic plasticity in Drosophila memory and hyperexcitable mutants: role of cAMP cascade. J Neurosci. 1992 Feb;12(2):644–651. doi: 10.1523/JNEUROSCI.12-02-00644.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rieckhof GE, Yoshihara M, Guan Z, Littleton JT. Presynaptic N-type calcium channels regulate synaptic growth. J Biol Chem. 2003 Oct 17;278(42):41099–41108. doi: 10.1074/jbc.M306417200. [DOI] [PubMed] [Google Scholar]
- 57.Xing B, Ashleigh Long A, Harrison DA, Cooper RL. Developmental consequences of neuromuscular junctions with reduced presynaptic calcium channel function. Synapse. 2005 Sep 1;57(3):132–147. doi: 10.1002/syn.20165. [DOI] [PubMed] [Google Scholar]
- 58.Ataman B, Budnik V, Thomas U. Scaffolding proteins at the Drosophila neuromuscular junction. Int Rev Neurobiol. 2006;75:181–216. doi: 10.1016/S0074-7742(06)75009-7. [DOI] [PubMed] [Google Scholar]
- 59.Chung WS, Barres BA. The role of glial cells in synapse elimination. Curr Opin Neurobiol. 2012 Jun;22(3):438–445. doi: 10.1016/j.conb.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Eaton BA, Fetter RD, Davis GW. Dynactin is necessary for synapse stabilization. Neuron. 2002 May 30;34(5):729–741. doi: 10.1016/s0896-6273(02)00721-3. [DOI] [PubMed] [Google Scholar]
- 61.Pielage J, Fetter RD, Davis GW. Presynaptic spectrin is essential for synapse stabilization. Curr Biol. 2005 May 24;15(10):918–928. doi: 10.1016/j.cub.2005.04.030. [DOI] [PubMed] [Google Scholar]
- 62.Koch I, Schwarz H, Beuchle D, Goellner B, Langegger M, Aberle H. Drosophila ankyrin 2 is required for synaptic stability. Neuron. 2008 Apr 24;58(2):210–222. doi: 10.1016/j.neuron.2008.03.019. [DOI] [PubMed] [Google Scholar]
- 63.Pielage J, Cheng L, Fetter RD, Carlton PM, Sedat JW, Davis GW. A presynaptic giant ankyrin stabilizes the NMJ through regulation of presynaptic microtubules and transsynaptic cell adhesion. Neuron. 2008 Apr 24;58(2):195–209. doi: 10.1016/j.neuron.2008.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Coyle IP, Koh YH, Lee WC, et al. Nervous wreck, an SH3 adaptor protein that interacts with Wsp, regulates synaptic growth in Drosophila. Neuron. 2004 Feb 19;41(4):521–534. doi: 10.1016/s0896-6273(04)00016-9. [DOI] [PubMed] [Google Scholar]
- 65.Dickman DK, Lu Z, Meinertzhagen IA, Schwarz TL. Altered synaptic development and active zone spacing in endocytosis mutants. Curr Biol. 2006 Mar 21;16(6):591–598. doi: 10.1016/j.cub.2006.02.058. [DOI] [PubMed] [Google Scholar]
- 66.Oh E, Robinson I. Barfly: sculpting membranes at the Drosophila neuromuscular junction. Dev Neurobiol. 2012 Jan;72(1):33–56. doi: 10.1002/dneu.20923. [DOI] [PubMed] [Google Scholar]
- 67.Torroja L, Packard M, Gorczyca M, White K, Budnik V. The Drosophila beta-amyloid precursor protein homolog promotes synapse differentiation at the neuromuscular junction. J Neurosci. 1999 Sep 15;19(18):7793–7803. doi: 10.1523/JNEUROSCI.19-18-07793.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rodal AA, Motola-Barnes RN, Littleton JT. Nervous wreck and Cdc42 cooperate to regulate endocytic actin assembly during synaptic growth. J Neurosci. 2008 Aug 13;28(33):8316–8325. doi: 10.1523/JNEUROSCI.2304-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.O’Connor-Giles KM, Ho LL, Ganetzky B. Nervous wreck interacts with thickveins and the endocytic machinery to attenuate retrograde BMP signaling during synaptic growth. Neuron. 2008 May 22;58(4):507–518. doi: 10.1016/j.neuron.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rodal AA, Blunk AD, Akbergenova Y, Jorquera RA, Buhl LK, Littleton JT. A presynaptic endosomal trafficking pathway controls synaptic growth signaling. J Cell Biol. 2011 Apr 4;193(1):201–217. doi: 10.1083/jcb.201009052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lee J, Wu CF. Orchestration of stepwise synaptic growth by K+ and Ca2+ channels in Drosophila. J Neurosci. 2010 Nov 24;30(47):15821–15833. doi: 10.1523/JNEUROSCI.3448-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ashley J, Packard M, Ataman B, Budnik V. Fasciclin II signals new synapse formation through amyloid precursor protein and the scaffolding protein dX11/Mint. J Neurosci. 2005 Jun 22;25(25):5943–5955. doi: 10.1523/JNEUROSCI.1144-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ruiz-Canada C, Budnik V. Synaptic cytoskeleton at the neuromuscular junction. Int Rev Neurobiol. 2006;75:217–236. doi: 10.1016/S0074-7742(06)75010-3. [DOI] [PubMed] [Google Scholar]
- 74.Theriot JA. Accelerating on a treadmill: ADF/cofilin promotes rapid actin filament turnover in the dynamic cytoskeleton. J Cell Biol. 1997 Mar 24;136(6):1165–1168. doi: 10.1083/jcb.136.6.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ohashi K, Hosoya T, Takahashi K, Hing H, Mizuno K. A Drosophila homolog of LIM-kinase phosphorylates cofilin and induces actin cytoskeletal reorganization. Biochemical and biophysical research communications. 2000 Oct 5;276(3):1178–1185. doi: 10.1006/bbrc.2000.3599. [DOI] [PubMed] [Google Scholar]
- 76.Edwards DC, Sanders LC, Bokoch GM, Gill GN. Activation of LIM-kinase by Pak1 couples Rac/Cdc42 GTPase signalling to actin cytoskeletal dynamics. Nat Cell Biol. 1999 Sep;1(5):253–259. doi: 10.1038/12963. [DOI] [PubMed] [Google Scholar]
- 77.Maekawa M, Ishizaki T, Boku S, et al. Signaling from Rho to the actin cytoskeleton through protein kinases ROCK and LIM-kinase. Science. 1999 Aug 6;285(5429):895–898. doi: 10.1126/science.285.5429.895. [DOI] [PubMed] [Google Scholar]
- 78.Franco B, Bogdanik L, Bobinnec Y, et al. Shaggy, the homolog of glycogen synthase kinase 3, controls neuromuscular junction growth in Drosophila. J Neurosci. 2004 Jul 21;24(29):6573–6577. doi: 10.1523/JNEUROSCI.1580-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Roll-Mecak A, McNally FJ. Microtubule-severing enzymes. Curr Opin Cell Biol. 2010 Feb;22(1):96–103. doi: 10.1016/j.ceb.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang D, Rogers GC, Buster DW, Sharp DJ. Three microtubule severing enzymes contribute to the “Pacman-flux” machinery that moves chromosomes. J Cell Biol. 2007 Apr 23;177(2):231–242. doi: 10.1083/jcb.200612011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Stewart A, Tsubouchi A, Rolls MM, Tracey WD, Sherwood NT. Katanin p60-like1 Promotes Microtubule Growth and Terminal Dendrite Stability in the Larval Class IV Sensory Neurons of Drosophila. J Neurosci. 2012 Aug 22;32(34):11631–11642. doi: 10.1523/JNEUROSCI.0729-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Trotta N, Orso G, Rossetto MG, Daga A, Broadie K. The hereditary spastic paraplegia gene, spastin, regulates microtubule stability to modulate synaptic structure and function. Curr Biol. 2004 Jul 13;14(13):1135–1147. doi: 10.1016/j.cub.2004.06.058. [DOI] [PubMed] [Google Scholar]
- 83.Lee M, Paik SK, Lee MJ, et al. Drosophila Atlastin regulates the stability of muscle microtubules and is required for synapse development. Dev Biol. 2009 Jun 15;330(2):250–262. doi: 10.1016/j.ydbio.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 84.Graf ER, Heerssen HM, Wright CM, Davis GW, DiAntonio A. Stathmin is required for stability of the Drosophila neuromuscular junction. J Neurosci. 2011 Oct 19;31(42):15026–15034. doi: 10.1523/JNEUROSCI.2024-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Johnson KG, Tenney AP, Ghose A, et al. The HSPGs Syndecan and Dallylike bind the receptor phosphatase LAR and exert distinct effects on synaptic development. Neuron. 2006 Feb 16;49(4):517–531. doi: 10.1016/j.neuron.2006.01.026. [DOI] [PubMed] [Google Scholar]
- 86.Kaufmann N, DeProto J, Ranjan R, Wan H, Van Vactor D. Drosophila liprin-alpha and the receptor phosphatase Dlar control synapse morphogenesis. Neuron. 2002 Mar 28;34(1):27–38. doi: 10.1016/s0896-6273(02)00643-8. [DOI] [PubMed] [Google Scholar]
- 87.Pawson C, Eaton BA, Davis GW. Formin-dependent synaptic growth: evidence that Dlar signals via Diaphanous to modulate synaptic actin and dynamic pioneer microtubules. J Neurosci. 2008 Oct 29;28(44):11111–11123. doi: 10.1523/JNEUROSCI.0833-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Stryker E, Johnson KG. LAR, liprin alpha and the regulation of active zone morphogenesis. J Cell Sci. 2007 Nov 1;120(Pt 21):3723–3728. doi: 10.1242/jcs.03491. [DOI] [PubMed] [Google Scholar]
- 89.Owald D, Fouquet W, Schmidt M, et al. A Syd-1 homologue regulates pre- and postsynaptic maturation in Drosophila. J Cell Biol. 2010 Feb 22;188(4):565–579. doi: 10.1083/jcb.200908055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Owald D, Khorramshahi O, Gupta VK, et al. Cooperation of Syd-1 with Neurexin synchronizes pre- with postsynaptic assembly. Nat Neurosci. 2012 Aug 5;15(9):1219–1226. doi: 10.1038/nn.3183. [DOI] [PubMed] [Google Scholar]
- 91.Carrero-Martínez FAaC A. Cell Adhesion Molecules at the Drosophila Neuromuscular Junction. In: Umemori MHaH., editor. The Sticky Synapse. Springer Publishing; 2009. pp. 11–37. [Google Scholar]
- 92.Dalva MB, McClelland AC, Kayser MS. Cell adhesion molecules: signalling functions at the synapse. Nat Rev Neurosci. 2007 Mar;8(3):206–220. doi: 10.1038/nrn2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Berdnik D, Favaloro V, Luo L. The SUMO protease Verloren regulates dendrite and axon targeting in olfactory projection neurons. J Neurosci. 2012 Jun 13;32(24):8331–8340. doi: 10.1523/JNEUROSCI.6574-10.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Collins CA, DiAntonio A. Synaptic development: insights from Drosophila. Curr Opin Neurobiol. 2007 Feb;17(1):35, 42. doi: 10.1016/j.conb.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 95.Keshishian H, Kim YS. Orchestrating development and function: retrograde BMP signaling in the Drosophila nervous system. Trends Neurosci. 2004 Mar;27(3):143–147. doi: 10.1016/j.tins.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 96.Marques G. Morphogens and synaptogenesis in Drosophila. J Neurobiol. 2005 Sep 15;64(4):417–434. doi: 10.1002/neu.20165. [DOI] [PubMed] [Google Scholar]
- 97.Marques G, Zhang B. Retrograde signaling that regulates synaptic development and function at the Drosophila neuromuscular junction. Int Rev Neurobiol. 2006;75:267–285. doi: 10.1016/S0074-7742(06)75012-7. [DOI] [PubMed] [Google Scholar]
- 98.Speese SD, Budnik V. Wnts: up-and-coming at the synapse. Trends Neurosci. 2007 Jun;30(6):268, 275. doi: 10.1016/j.tins.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Knight D, Xie W, Boulianne GL. Neurexins and neuroligins: recent insights from invertebrates. Mol Neurobiol. 2011 Dec;44(3):426–440. doi: 10.1007/s12035-011-8213-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Logan MA, Freeman MR. The scoop on the fly brain: glial engulfment functions in Drosophila. Neuron Glia Biol. 2007 Feb;3(1):74. doi: 10.1017/S1740925X07000646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sigrist SJ, Reiff DF, Thiel PR, Steinert JR, Schuster CM. Experience-dependent strengthening of Drosophila neuromuscular junctions. J Neurosci. 2003 Jul 23;23(16):6546–6556. doi: 10.1523/JNEUROSCI.23-16-06546.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Barber CF, Jorquera RA, Melom JE, Littleton JT. Postsynaptic regulation of synaptic plasticity by synaptotagmin 4 requires both C2 domains. J Cell Biol. 2009 Oct 19;187(2):295–310. doi: 10.1083/jcb.200903098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kugler JM, Lasko P. Localization, anchoring and translational control of oskar, gurken, bicoid and nanos mRNA during Drosophila oogenesis. Fly (Austin) 2009 Jan-Mar;3(1):15–28. doi: 10.4161/fly.3.1.7751. [DOI] [PubMed] [Google Scholar]
- 104.Lasko P. Posttranscriptional regulation in Drosophila oocytes and early embryos. Wiley Interdiscip Rev RNA. 2011 May;2(3):408–416. doi: 10.1002/wrna.70. [DOI] [PubMed] [Google Scholar]
- 105.Roth S, Lynch JA. Symmetry breaking during Drosophila oogenesis. Cold Spring Harb Perspect Biol. 2009 Aug;1(2):a001891. doi: 10.1101/cshperspect.a001891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Martin KC, Zukin RS. RNA trafficking and local protein synthesis in dendrites: an overview. J Neurosci. 2006 Jul 5;26(27):7131–7134. doi: 10.1523/JNEUROSCI.1801-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Steward O, Levy WB. Preferential localization of polyribosomes under the base of dendritic spines in granule cells of the dentate gyrus. J Neurosci. 1982 Mar;2(3):284–291. doi: 10.1523/JNEUROSCI.02-03-00284.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Caudy AA, Myers M, Hannon GJ, Hammond SM. Fragile X-related protein and VIG associate with the RNA interference machinery. Genes Dev. 2002 Oct 1;16(19):2491–2496. doi: 10.1101/gad.1025202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ishizuka A, Siomi MC, Siomi H. A Drosophila fragile X protein interacts with components of RNAi and ribosomal proteins. Genes Dev. 2002 Oct 1;16(19):2497–2508. doi: 10.1101/gad.1022002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Marrus SB, Portman SL, Allen MJ, Moffat KG, DiAntonio A. Differential localization of glutamate receptor subunits at the Drosophila neuromuscular junction. J Neurosci. 2004 Feb 11;24(6):1406–1415. doi: 10.1523/JNEUROSCI.1575-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sigrist SJ, Thiel PR, Reiff DF, Lachance PE, Lasko P, Schuster CM. Postsynaptic translation affects the efficacy and morphology of neuromuscular junctions. Nature. 2000 Jun 29;405(6790):1062–1065. doi: 10.1038/35016598. [DOI] [PubMed] [Google Scholar]
- 112.Speese SD, Ashley J, Jokhi V, et al. Nuclear envelope budding enables large ribonucleoprotein particle export during synaptic Wnt signaling. Cell. 2012 May 11;149(4):832–846. doi: 10.1016/j.cell.2012.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hammond SM. Dicing and slicing: the core machinery of the RNA interference pathway. FEBS Lett. 2005 Oct 31;579(26):5822–5829. doi: 10.1016/j.febslet.2005.08.079. [DOI] [PubMed] [Google Scholar]
- 114.Murchison EP, Hannon GJ. miRNAs on the move: miRNA biogenesis and the RNAi machinery. Curr Opin Cell Biol. 2004 Jun;16(3):223–229. doi: 10.1016/j.ceb.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 115.Gao FB. Posttranscriptional control of neuronal development by microRNA networks. Trends Neurosci. 2008 Jan;31(1):20–26. doi: 10.1016/j.tins.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kosik KS. The neuronal microRNA system. Nat Rev Neurosci. 2006 Dec;7(12):911–920. doi: 10.1038/nrn2037. [DOI] [PubMed] [Google Scholar]
- 117.McNeill E, Van Vactor D. MicroRNAs Shape the Neuronal Landscape. Neuron. 2012 Aug 9;75(3):363–379. doi: 10.1016/j.neuron.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Karres JS, Hilgers V, Carrera I, Treisman J, Cohen SM. The conserved microRNA miR-8 tunes atrophin levels to prevent neurodegeneration in Drosophila. Cell. 2007 Oct 5;131(1):136–145. doi: 10.1016/j.cell.2007.09.020. [DOI] [PubMed] [Google Scholar]
- 119.Kennell JA, Gerin I, MacDougald OA, Cadigan KM. The microRNA miR-8 is a conserved negative regulator of Wnt signaling. Proc Natl Acad Sci U S A. 2008 Oct 7;105(40):15417–15422. doi: 10.1073/pnas.0807763105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Choi IK, Hyun S. Conserved microRNA miR-8 in fat body regulates innate immune homeostasis in Drosophila. Dev Comp Immunol. 2011 Dec 22; doi: 10.1016/j.dci.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 121.Ebert MS, Neilson JR, Sharp PA. MicroRNA sponges: competitive inhibitors of small RNAs in mammalian cells. Nat Methods. 2007 Sep;4(9):721–726. doi: 10.1038/nmeth1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Loya CM, Lu CS, Van Vactor D, Fulga TA. Transgenic microRNA inhibition with spatiotemporal specificity in intact organisms. Nat Methods. 2009 Dec;6(12):897, 903. doi: 10.1038/nmeth.1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bogdanik L, Mohrmann R, Ramaekers A, et al. The Drosophila metabotropic glutamate receptor DmGluRA regulates activity-dependent synaptic facilitation and fine synaptic morphology. J Neurosci. 2004 Oct 13;24(41):9105–9116. doi: 10.1523/JNEUROSCI.2724-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Featherstone DE, Rushton E, Rohrbough J, et al. An essential Drosophila glutamate receptor subunit that functions in both central neuropil and neuromuscular junction. J Neurosci. 2005 Mar 23;25(12):3199–3208. doi: 10.1523/JNEUROSCI.4201-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sigrist SJ, Thiel PR, Reiff DF, Schuster CM. The postsynaptic glutamate receptor subunit DGluR-IIA mediates long-term plasticity in Drosophila. J Neurosci. 2002 Sep 1;22(17):7362–7372. doi: 10.1523/JNEUROSCI.22-17-07362.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Pepper AS, Beerman RW, Bhogal B, Jongens TA. Argonaute2 suppresses Drosophila fragile X expression preventing neurogenesis and oogenesis defects. PLoS One. 2009;4(10):e7618. doi: 10.1371/journal.pone.0007618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yang JS, Lai EC. Alternative miRNA biogenesis pathways and the interpretation of core miRNA pathway mutants. Mol Cell. 2011 Sep 16;43(6):892–903. doi: 10.1016/j.molcel.2011.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Xu XL, Li Y, Wang F, Gao FB. The steady-state level of the nervous-system-specific microRNA-124 a is regulated by dFMR1 in Drosophila. J Neurosci. 2008 Nov 12;28(46):11883–11889. doi: 10.1523/JNEUROSCI.4114-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Iovino N, Pane A, Gaul U. miR-184 has multiple roles in Drosophila female germline development. Dev Cell. 2009 Jul;17(1):123–133. doi: 10.1016/j.devcel.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 130.Rawson JM, Lee M, Kennedy EL, Selleck SB. Drosophila neuromuscular synapse assembly and function require the TGF-beta type I receptor saxophone and the transcription factor Mad. J Neurobiol. 2003 May;55(2):134–150. doi: 10.1002/neu.10189. [DOI] [PubMed] [Google Scholar]
- 131.Kremer EJ, Yu S, Pritchard M, et al. Isolation of a human DNA sequence which spans the fragile X. Am J Hum Genet. 1991 Sep;49(3):656–661. [PMC free article] [PubMed] [Google Scholar]
- 132.Oberle I, Rousseau F, Heitz D, et al. Instability of a 550-base pair DNA segment and abnormal methylation in fragile X syndrome. Science. 1991 May 24;252(5010):1097–1102. doi: 10.1126/science.252.5009.1097. [DOI] [PubMed] [Google Scholar]
- 133.Verkerk AJ, Pieretti M, Sutcliffe JS, et al. Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell. 1991 May 31;65(5):905–914. doi: 10.1016/0092-8674(91)90397-h. [DOI] [PubMed] [Google Scholar]
- 134.Devys D, Lutz Y, Rouyer N, Bellocq JP, Mandel JL. The FMR-1 protein is cytoplasmic, most abundant in neurons and appears normal in carriers of a fragile X premutation. Nat Genet. 1993 Aug;4(4):335–340. doi: 10.1038/ng0893-335. [DOI] [PubMed] [Google Scholar]
- 135.Comery TA, Harris JB, Willems PJ, et al. Abnormal dendritic spines in fragile X knockout mice: maturation and pruning deficits. Proc Natl Acad Sci U S A. 1997 May 13;94(10):5401–5404. doi: 10.1073/pnas.94.10.5401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Irwin SA, Galvez R. Greenough WT. Dendritic spine structural anomalies in fragile-X mental retardation syndrome. Cereb Cortex. 2000 Oct;10(10):1038–1044. doi: 10.1093/cercor/10.10.1038. [DOI] [PubMed] [Google Scholar]
- 137.Nimchinsky EA, Oberlander AM, Svoboda K. Abnormal development of dendritic spines in FMR1 knock-out mice. J Neurosci. 2001 Jul 15;21(14):5139–5146. doi: 10.1523/JNEUROSCI.21-14-05139.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Laggerbauer B, Ostareck D, Keidel EM, Ostareck-Lederer A, Fischer U. Evidence that fragile X mental retardation protein is a negative regulator of translation. Hum Mol Genet. 2001 Feb 15;10(4):329–338. doi: 10.1093/hmg/10.4.329. [DOI] [PubMed] [Google Scholar]
- 139.Li Z, Zhang Y, Ku L, Wilkinson KD, Warren ST, Feng Y. The fragile X mental retardation protein inhibits translation via interacting with mRNA. Nucleic Acids Res. 2001 Jun 1;29(11):2276–2283. doi: 10.1093/nar/29.11.2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zhang YQ, Bailey AM, Matthies HJ, et al. Drosophila fragile X-related gene regulates the MAP1B homolog Futsch to control synaptic structure and function. Cell. 2001 Nov 30;107(5):591–603. doi: 10.1016/s0092-8674(01)00589-x. [DOI] [PubMed] [Google Scholar]
- 141.Gatto CL, Broadie K. Temporal requirements of the fragile X mental retardation protein in the regulation of synaptic structure. Development. 2008 Aug;135(15):2637–2648. doi: 10.1242/dev.022244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Brown V, Jin P, Ceman S, et al. Microarray identification of FMRP-associated brain mRNAs and altered mRNA translational profiles in fragile X syndrome. Cell. 2001 Nov 16;107(4):477–487. doi: 10.1016/s0092-8674(01)00568-2. [DOI] [PubMed] [Google Scholar]
- 143.Darnell JC, Jensen KB, Jin P, Brown V, Warren ST, Darnell RB. Fragile X mental retardation protein targets G quartet mRNAs important for neuronal function. Cell. 2001 Nov 16;107(4):489–499. doi: 10.1016/s0092-8674(01)00566-9. [DOI] [PubMed] [Google Scholar]
- 144.Pan L, Broadie KS. Drosophila fragile X mental retardation protein and metabotropic glutamate receptor A convergently regulate the synaptic ratio of ionotropic glutamate receptor subclasses. J Neurosci. 2007 Nov 7;27(45):12378–12389. doi: 10.1523/JNEUROSCI.2970-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Qin G, Schwarz T, Kittel RJ, et al. Four different subunits are essential for expressing the synaptic glutamate receptor at neuromuscular junctions of Drosophila. J Neurosci. 2005 Mar 23;25(12):3209–3218. doi: 10.1523/JNEUROSCI.4194-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zalfa F, Eleuteri B, Dickson KS, et al. A new function for the fragile X mental retardation protein in regulation of PSD-95 mRNA stability. Nat Neurosci. 2007 May;10(5):578, 587. doi: 10.1038/nn1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Gamberi C, Peterson DS, He L, Gottlieb E. An anterior function for the Drosophila posterior determinant Pumilio. Development. 2002 Jun;129(11):2699–2710. doi: 10.1242/dev.129.11.2699. [DOI] [PubMed] [Google Scholar]
- 148.Parisi M, Lin H. The Drosophila pumilio gene encodes two functional protein isoforms that play multiple roles in germline development, gonadogenesis, oogenesis and embryogenesis. Genetics. 1999 Sep;153(1):235–250. doi: 10.1093/genetics/153.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Mee CJ, Pym EC, Moffat KG, Baines RA. Regulation of neuronal excitability through pumilio-dependent control of a sodium channel gene. J Neurosci. 2004 Oct 6;24(40):8695–8703. doi: 10.1523/JNEUROSCI.2282-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Nakahata S, Kotani T, Mita K, et al. Involvement of Xenopus Pumilio in the translational regulation that is specific to cyclin B1 mRNA during oocyte maturation. Mech Dev. 2003 Aug;120(8):865–880. doi: 10.1016/s0925-4773(03)00160-6. [DOI] [PubMed] [Google Scholar]
- 151.Sonoda J, Wharton RP. Recruitment of Nanos to hunchback mRNA by Pumilio. Genes Dev. 1999 Oct 15;13(20):2704–2712. doi: 10.1101/gad.13.20.2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Menon KP, Andrews S, Murthy M, Gavis ER, Zinn K. The translational repressors Nanos and Pumilio have divergent effects on presynaptic terminal growth and postsynaptic glutamate receptor subunit composition. J Neurosci. 2009 Apr 29;29(17):5558–5572. doi: 10.1523/JNEUROSCI.0520-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Weidmann CA, Goldstrohm AC. Drosophila Pumilio Protein Contains Multiple Autonomous Repression Domains That Regulate mRNAs Independently of Nanos and Brain Tumor. Mol Cell Biol. 2012 Jan;32(2):527, 540. doi: 10.1128/MCB.06052-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Forbes A, Lehmann R. Nanos and Pumilio have critical roles in the development and function of Drosophila germline stem cells. Development. 1998 Feb;125(4):679, 690. doi: 10.1242/dev.125.4.679. [DOI] [PubMed] [Google Scholar]
- 155.Menon KP, Sanyal S, Habara Y, et al. The translational repressor Pumilio regulates presynaptic morphology and controls postsynaptic accumulation of translation factor eIF-4E. Neuron. 2004 Nov 18;44(4):663–676. doi: 10.1016/j.neuron.2004.10.028. [DOI] [PubMed] [Google Scholar]
- 156.Baines RA. Neuronal homeostasis through translational control. Mol Neurobiol. 2005 Oct;32(2):113–121. doi: 10.1385/MN:32:2:113. [DOI] [PubMed] [Google Scholar]
- 157.Salazar AM, Silverman EJ, Menon KP, Zinn K. Regulation of synaptic Pumilio function by an aggregation-prone domain. J Neurosci. 2010 Jan 13;30(2):515–522. doi: 10.1523/JNEUROSCI.2523-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Chen G, Li W, Zhang QS, et al. Identification of synaptic targets of Drosophila pumilio. PLoS Comput Biol. 2008 Feb;4(2):e1000026. doi: 10.1371/journal.pcbi.1000026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Dubnau J, Chiang AS, Grady L, et al. The staufen/pumilio pathway is involved in Drosophila long-term memory. Curr Biol. 2003 Feb 18;13(4):286–296. doi: 10.1016/s0960-9822(03)00064-2. [DOI] [PubMed] [Google Scholar]
- 160.Ye B, Petritsch C, Clark IE, Gavis ER, Jan LY, Jan YN. Nanos and Pumilio are essential for dendrite morphogenesis in Drosophila peripheral neurons. Curr Biol. 2004 Feb 17;14(4):314–321. doi: 10.1016/j.cub.2004.01.052. [DOI] [PubMed] [Google Scholar]
- 161.Vessey JP, Vaccani A, Xie Y, et al. Dendritic localization of the translational repressor Pumilio 2 and its contribution to dendritic stress granules. J Neurosci. 2006 Jun 14;26(24):6496–6508. doi: 10.1523/JNEUROSCI.0649-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Vessey JP, Schoderboeck L, Gingl E, et al. Mammalian Pumilio 2 regulates dendrite morphogenesis and synaptic function. Proc Natl Acad Sci U S A. 2010 Feb 16;107(7):3222–3227. doi: 10.1073/pnas.0907128107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kedde M, van Kouwenhove M, Zwart W, Oude Vrielink JA, Elkon R, Agami R. A Pumilio-induced RNA structure switch in p27-3′ UTR controls miR-221 and miR-222 accessibility. Nat Cell Biol. 2010 Oct;12(10):1014–1020. doi: 10.1038/ncb2105. [DOI] [PubMed] [Google Scholar]
- 164.Richter JD. CPEB: a life in translation. Trends Biochem Sci. 2007 Jun;32(6):279–285. doi: 10.1016/j.tibs.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 165.Castagnetti S, Ephrussi A. Orb and a long poly(A) tail are required for efficient oskar translation at the posterior pole of the Drosophila oocyte. Development. 2003 Mar;130(5):835–843. doi: 10.1242/dev.00309. [DOI] [PubMed] [Google Scholar]
- 166.Chang JS, Tan L, Schedl P. The Drosophila CPEB homolog, orb, is required for oskar protein expression in oocytes. Dev Biol. 1999 Nov 1;215(1):91–106. doi: 10.1006/dbio.1999.9444. [DOI] [PubMed] [Google Scholar]
- 167.Chang JS, Tan L, Wolf MR, Schedl P. Functioning of the Drosophila orb gene in gurken mRNA localization and translation. Development. 2001 Aug;128(16):3169–3177. doi: 10.1242/dev.128.16.3169. [DOI] [PubMed] [Google Scholar]
- 168.Christerson LB, McKearin DM. orb is required for anteroposterior and dorsoventral patterning during Drosophila oogenesis. Genes Dev. 1994 Mar 1;8(5):614–628. doi: 10.1101/gad.8.5.614. [DOI] [PubMed] [Google Scholar]
- 169.Lantz V, Chang JS, Horabin JI, Bopp D, Schedl P. The Drosophila orb RNA-binding protein is required for the formation of the egg chamber and establishment of polarity. Genes Dev. 1994 Mar 1;8(5):598–613. doi: 10.1101/gad.8.5.598. [DOI] [PubMed] [Google Scholar]
- 170.Kurihara Y, Tokuriki M, Myojin R, et al. CPEB2, a novel putative translational regulator in mouse haploid germ cells. Biol Reprod. 2003 Jul;69(1):261–268. doi: 10.1095/biolreprod.103.015677. [DOI] [PubMed] [Google Scholar]
- 171.Theis M, Si K, Kandel ER. Two previously undescribed members of the mouse CPEB family of genes and their inducible expression in the principal cell layers of the hippocampus. Proc Natl Acad Sci U S A. 2003 Aug 5;100(16):9602–9607. doi: 10.1073/pnas.1133424100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Hafer N, Xu S, Bhat KM, Schedl P. The Drosophila CPEB protein Orb2 has a novel expression pattern and is important for asymmetric cell division and nervous system function. Genetics. 2011 Nov;189(3):907–921. doi: 10.1534/genetics.110.123646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Mastushita-Sakai T, White-Grindley E, Samuelson J, Seidel C, Si K. Drosophila Orb2 targets genes involved in neuronal growth, synapse formation, and protein turnover. Proc Natl Acad Sci U S A. 2010 Jun 29;107(26):11987–11992. doi: 10.1073/pnas.1004433107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Cziko AM, McCann CT, Howlett IC, et al. Genetic modifiers of dFMR1 encode RNA granule components in Drosophila. Genetics. 2009 Aug;182(4):1051–1060. doi: 10.1534/genetics.109.103234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Cheever A, Ceman S. Translation regulation of mRNAs by the fragile X family of proteins through the microRNA pathway. RNA Biol. 2009 Apr-Jun;6(2):175–178. doi: 10.4161/rna.6.2.8196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Li Y, Lin L, Jin P. The microRNA pathway and fragile X mental retardation protein. Biochim Biophys Acta. 2008 Nov;1779(11):702–705. doi: 10.1016/j.bbagrm.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Costa A, Wang Y, Dockendorff TC, et al. The Drosophila fragile X protein functions as a negative regulator in the orb autoregulatory pathway. Dev Cell. 2005 Mar;8(3):331–342. doi: 10.1016/j.devcel.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 178.Miles WO, Tschop K, Herr A, Ji JY, Dyson NJ. Pumilio facilitates miRNA regulation of the E2F3 oncogene. Genes Dev. 2012 Feb 15;26(4):356–368. doi: 10.1101/gad.182568.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Huntwork S, Littleton JT. A complexin fusion clamp regulates spontaneous neurotransmitter release and synaptic growth. Nat Neurosci. 2007 Oct;10(10):1235–1237. doi: 10.1038/nn1980. [DOI] [PubMed] [Google Scholar]
- 180.Khodosh R, Augsburger A, Schwarz TL, Garrity PA. Bchs, a BEACH domain protein, antagonizes Rab11 in synapse morphogenesis and other developmental events. Development. 2006 Dec;133(23):4655–4665. doi: 10.1242/dev.02650. [DOI] [PubMed] [Google Scholar]
- 181.Mosca TJ, Hong W, Dani VS, Favaloro V, Luo L. Trans-synaptic Teneurin signalling in neuromuscular synapse organization and target choice. Nature. 2012 Apr 12;484(7393):237–241. doi: 10.1038/nature10923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Packard M, Koo ES, Gorczyca M, Sharpe J, Cumberledge S, Budnik V. The Drosophila Wnt, wingless, provides an essential signal for pre- and postsynaptic differentiation. Cell. 2002 Nov 1;111(3):319–330. doi: 10.1016/s0092-8674(02)01047-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Pennetta G, Hiesinger PR, Fabian-Fine R, Meinertzhagen IA, Bellen HJ. Drosophila VAP-33A directs bouton formation at neuromuscular junctions in a dosage-dependent manner. Neuron. 2002 Jul 18;35(2):291–306. doi: 10.1016/s0896-6273(02)00769-9. [DOI] [PubMed] [Google Scholar]
- 184.Pielage J, Bulat V, Zuchero JB, Fetter RD, Davis GW. Hts/Adducin controls synaptic elaboration and elimination. Neuron. 2011 Mar 24;69(6):1114–1131. doi: 10.1016/j.neuron.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Pielage J, Fetter RD, Davis GW. A postsynaptic spectrin scaffold defines active zone size, spacing, and efficacy at the Drosophila neuromuscular junction. J Cell Biol. 2006 Nov 6;175(3):491–503. doi: 10.1083/jcb.200607036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Sweeney ST, Davis GW. Unrestricted synaptic growth in spinster-a late endosomal protein implicated in TGF-beta-mediated synaptic growth regulation. Neuron. 2002 Oct 24;36(3):403–416. doi: 10.1016/s0896-6273(02)01014-0. [DOI] [PubMed] [Google Scholar]
- 187.Viquez NM, Li CR, Wairkar YP, DiAntonio A. The B’ protein phosphatase 2A regulatory subunit well-rounded regulates synaptic growth and cytoskeletal stability at the Drosophila neuromuscular junction. J Neurosci. 2006 Sep 6;26(36):9293–9303. doi: 10.1523/JNEUROSCI.1740-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Eaton BA, Davis GW. LIM Kinase1 controls synaptic stability downstream of the type II BMP receptor. Neuron. 2005 Sep 1;47(5):695–708. doi: 10.1016/j.neuron.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 189.Koh TW, Verstreken P, Bellen HJ. Dap160/intersectin acts as a stabilizing scaffold required for synaptic development and vesicle endocytosis. Neuron. 2004 Jul 22;43(2):193–205. doi: 10.1016/j.neuron.2004.06.029. [DOI] [PubMed] [Google Scholar]
- 190.Marie B, Sweeney ST, Poskanzer KE, Roos J, Kelly RB, Davis GW. Dap160/intersectin scaffolds the periactive zone to achieve high-fidelity endocytosis and normal synaptic growth. Neuron. 2004 Jul 22;43(2):207–219. doi: 10.1016/j.neuron.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 191.Ang LH, Chen W, Yao Y, et al. Lim kinase regulates the development of olfactory and neuromuscular synapses. Dev Biol. 2006 May 1;293(1):178–190. doi: 10.1016/j.ydbio.2006.01.030. [DOI] [PubMed] [Google Scholar]