Abstract
Viral and pharmacologic inducers of PKR-like ER kinase (PERK) were shown to accelerate the phosphorylation-dependent degradation of the IFNAR1 chain of the type 1 interferon (IFN) receptor and to limit cell sensitivity to IFN. Here we report that hypoxia can elicit these effects in a PERK-dependent manner. The altered fate of IFNAR1 affected by signaling downstream of PERK depends on phosphorylation of eIF2α and ensuing activation of p38α kinase. Activators of other eIF2α kinases such as PKR or GCN2 are also capable of eliminating IFNAR1 and blunting IFN responses. Modulation of constitutive PKR activity in human breast cancer cells stabilizes IFNAR1 and sensitizes these cells to IFNAR1-dependent anti-tumorigenic effects. Whereas downregulation of IFNAR1 and impaired IFNAR1 signaling can be elicited in response to amino acid deficit, the knockdown of GCN2 in melanoma cells reverses these phenotypes. We propose that, in cancer cells and the tumor microenvironment, activation of diverse eIF2α kinases followed by IFNAR1 downregulation enables multiple cellular components of tumor tissue to evade the direct and indirect anti-tumorigenic effects of Type 1 IFN.
Keywords: interferon, tumor microenvironment, integrated stress response, PERK, PKR, GCN2, IFNAR1
Introduction
Tumors are sculpted toward a more malignant phenotype while selecting for highly aggressive and resilient clones under limitations of nutrients and oxygen (1–3) and attack by the host immune system (4). The harsh conditions of the tumor microenvironment may activate mechanisms that enable tumor cells to increase their proliferative, survival and invasive potential (5). Given that successful execution of these mechanisms encourage the cells to outgrow/outlive these conditions or move away from the primary tumor site (i.e., metastasize), delineation of these mechanisms is paramount to understanding tumor progression and devising the means for cancer treatment.
Insufficient availability of essential amino acids triggers the activation of GCN2 (General Control Nonrepressed-2, GCN2) protein kinase, which phosphorylates the translational regulator eIF2α on Ser51. This phosphorylation can be also mediated by PKR-like ER kinase (PERK, induced by an unfolded protein response to an insufficient supply of oxygen or/and glucose), or by protein kinase RNA-activated (PKR). These signaling pathways, known under an overarching term of integrative signaling response (ISR; reviewed in Ref (6)), converge on eIF2α phosphorylation resulting in temporal stalling of global translation while activating ATF4-mediated transcription of genes that replenish amino acids, deal with accumulated unfolded proteins and restore the oxidative-reductive balance (5, 7).
Intriguingly, various branches of ISR have been implicated in the mechanisms of tumor development and progression. For example, despite well documented pro-apoptotic activity of PKR under conditions of viral infection, this kinase is overexpressed and constitutively activated in some hematologic malignancies (8) as well as in breast cancers (9–11). Furthermore, previous reports demonstrated that the functions of GCN2 (2) and PERK (12–14) are essential for efficient tumorigenesis and tumor progression. We have also found that activation of PERK within the unfolded protein response triggered either by viral infection or by the Ca2+ channel inhibitor thapsigargin (TG), stimulated phosphorylation-dependent ubiquitination and degradation of the IFNAR1 chain of the Type 1 interferon (IFN) receptor rendering cells insensitive to the effects of IFNα/β (15, 16). These cytokines signal through the IFNAR1/IFNAR2c chains of the Type 1 IFN receptor to activate Janus kinases and signal transducers and activators of transcription (STAT) to elicit the anti-tumorigenic effects that include inhibition of tumor cell motility, growth, and survival (17–19). Here we report that activation of diverse branches of ISR downregulates IFNAR1 and desensitizes cells to the anti-tumorigenic effects of IFN.
Results
Integrated stress signals converging on phosphorylation of eIF2α mediate phosphorylation and downregulation of IFNAR1
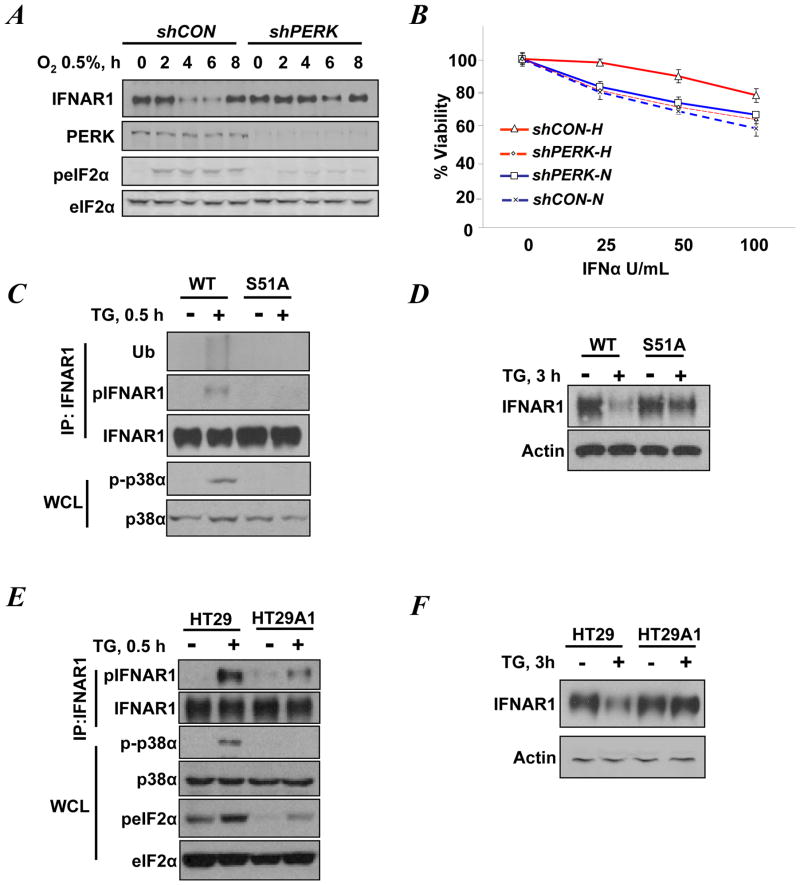
PERK-dependent acceleration of IFNAR1 proteolysis in cells undergoing unfolded protein response evoked by viral infection or TG treatment prompted us to investigate the effects of tumor microenvironment-relevant inducers of PERK such as hypoxia (13). As expected, the exposure of WM266-4 human melanoma cells to hypoxia (0.5% oxygen) induced phosphorylation of eIF2α (Figure 1A). Knockdown of PERK attenuated this phosphorylation but not the accumulation of HIF1α protein (Figure S1). Intriguingly, hypoxia triggered a robust IFNAR1 downregulation that could be partially reversed upon the knockdown of PERK (Figure 1A). We next tested sensitivity of cells to Type 1 IFN using a cell viability assay that combines assessment of anti-proliferative and anti-survival effects of these cytokines. Consistent with the importance of IFNAR1 levels for responsiveness of cells to its ligands (15, 16, 20–26), hypoxic conditions also attenuated the inhibition of cell viability by various doses of IFNα (Figure 1B). Importantly, this attenuation could be reversed by knocking down PERK (Figure 1B). Under hypoxic conditions, the IC50 of IFNα for cells that received PERK shRNA was 125 U/mL (versus that of 207 U/mL for control shRNA-treated cells, Figure S2). Collectively these data suggest that activation of PERK by hypoxia triggers downregulation of IFNAR1 and ensuing desensitization of cells to IFNα.
Figure 1. PERK inducers trigger phosphorylation and downregulation of IFNAR1 in a manner that depends on phosphorylation of eIF2α.
A. WM266-4 cells that received shRNA against PERK or GFP (control, shCON) were exposed to 0.5% O2 for the indicated times. Levels of IFNAR1, PERK, eIF2α, and the phosphorylation of eIF2α on S51 were detected by immunoblotting.
B. WM266-4 cells transduced with the indicated shRNA and incubated under normoxic (N) or hypoxic (H) conditions for 4h were treated with the indicated doses of IFNα. The extent of PERK knockdown in these cells is shown in panel A. Cell viability was assessed 24 h later.
C. Phosphorylation, ubiquitination, and levels of IFNAR1 immunopurified from untreated or TG-treated (0.5 h) MEFs were analyzed by immunoblotting using the indicated antibodies. Levels and activation of p38α in the whole cell lysates were also analyzed.
D. Immunoblotting analyses of the levels of IFNAR1 and β-actin (used as a loading control) in untreated or TG-treated (3 h) MEFs.
E. Immunoblotting analysis of phosphorylation and levels of IFNAR1, p38α, and eIF2α in untreated or TG-treated (0.5 h) HT29-derived cells treated.
F. Immunoblotting analyses of the levels of IFNAR1 and β-actin (used as a loading control) in untreated or TG-treated (3 h) HT29-derived cells.
Previous studies suggested that activation of PERK mediates IFNAR1 degradation thorough stimulation of the stress activated p38α protein kinase (27) that mediates phosphorylation of a priming site (Ser532 within human IFNAR1 or Ser523 within the mouse receptor (15)). In turn, this priming phosphorylation increases the efficacy of IFNAR1 degron phosphorylation by casein kinase 1α (28) leading to the recruitment of the β-Trcp E3 ubiquitin ligase, IFNAR1 ubiquitination, internalization and lysosomal degradation (reviewed in (29, 30)). Besides phosphorylating eIF2α, PERK has been also implicated in directly activating other phosphorylation cascades (31, 32). Given that, we sought to investigate how PERK signals towards IFNAR1 phosphorylation. A short term treatment of mouse embryo fibroblasts (MEF) from wild type mice with thapsigargin (TG) led to a robust activation of p38 kinase as well as priming phosphorylation and ubiquitination of IFNAR1 (Figure 1C). Consistent with earlier reports (15, 16, 27), a longer exposure to TG decreased the levels of IFNAR1 in these cells (Figure 1D). Intriguingly, the effects of either short term or prolonged treatment with TG were not evident in MEFs from eIF2αS51A knock-in mice (Figures 1C–1D). These results suggest that inducers of PERK require phosphorylation of eIF2α for the ubiquitination and downregulation of IFNAR1.
To further test this hypothesis, we used isogenic human HT29-derived cells that were stably transduced with empty retrovirus or made to express the constitutively active fragment of eIF2α phosphatase, GADD34 (33). Consistent with previous reports, basal or TG-induced phosphorylation of eIF2α on Ser51 in these HT29A1 cells was attenuated (Figure 1E). In response to TG these cells neither activated p38 kinase nor phosphorylated IFNAR1 at its priming site (Figure 1E). Furthermore, a prolonged TG treatment did not efficiently downregulate IFNAR1 in cells expressing active GADD34 (Figure 1F). These results collectively suggest that phosphorylation of eIF2α downstream of PERK is required for IFNAR1 phosphorylation, ubiquitination and downregulation in response to PERK inducers.
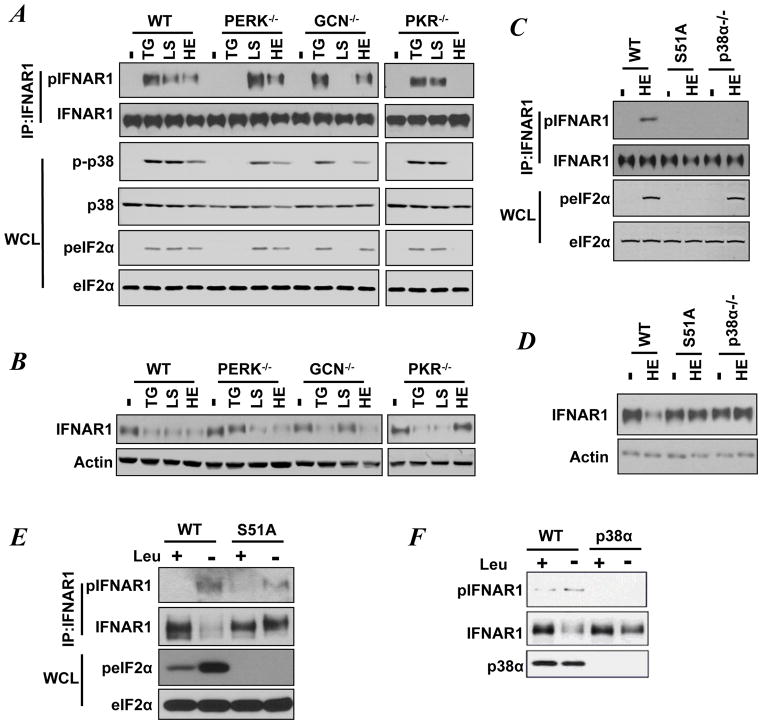
Phosphorylation of eIF2α on Ser51 can also be stimulated by inducers of the ISR such as amino acid deficit acting via GCN2 or activators of PKR (7, 30). Thus we sought to investigate whether these pathways converge and affect phosphorylation and levels of IFNAR1. To this end, we used a panel of MEFs derived from wild type mice or mice lacking PERK, GCN2, or PKR. This panel was subjected to either TG treatment to induce PERK, or heparin (HE) treatment to induce PKR, or leucine starvation (LS) to activate GCN2. All of these inducers stimulated phosphorylation of Ser51 of eIF2α, activation of p38 kinase and priming phosphorylation of IFNAR1 in wild type MEFs (Figure 2A). Furthermore, a prolonged exposure of wild type MEFs to these inducers noticeably downregulated IFNAR1 (Figure 2B). As expected the phosphorylation of eIF2α and activation of p38α kinase in response to TG was impaired in PERK-null cells. Similarly, knockout of GCN2 impaired eIF2α/p38α phosphorylation in response to leucine starvation, and knockout of PKR had the same effect in cells treated with heparin (Figure 2A). Remarkably, the patterns of either priming phosphorylation of IFNAR1 (Figure 2A) or its downregulation (Figure 2B) followed an analogous trend.
Figure 2. Diverse stress stimuli induce eIF2α phosphorylation to mediate phosphorylation and downregulation of IFNAR1.
A. Immunoblotting analyses of phosphorylation and levels of IFNAR1, p38α, and eIF2α in MEFs exposed to TG (for 30 min) or heparin (HE, for 40 min), or leucine starvation (LS, for 24 h).
B. Immunoblotting analyses of the levels of IFNAR1 and β-actin in MEFs treated with TG (for 3 h), or HE (for 7 h), or incubated with media without leucine (LS, 48 h).
C. Immunoblotting analyses of phosphorylation and levels of IFNAR1 and eIF2α in MEFs exposed to heparin (HE) for 40 min.
D. Immunoblotting analyses of phosphorylation and levels of IFNAR1 and eIF2α in MEFs exposed to HE for 7 h.
E. Immunoblotting analyses of phosphorylation and levels of IFNAR1 and eIF2α in MEFs cultured in the presence or absence of leucine for 48 h.
F. Immunoblotting analyses of phosphorylation and levels of IFNAR1 and p38α kinase in MEFs cultured in the presence or absence of leucine for 48 h.
Furthermore, the results of additional experiments using MEFs from eIF2αS51A knock-in and p38α knockout mice suggested that the integrity of eIF2α Ser51 phosphorylation and the availability of p38α protein kinase were required for IFNAR1 priming phosphorylation and downregulation elicited in response to either heparin (Figure 2C–2D) or leucine starvation (Figure 2E–2F). In all, these results suggest that, in addition to established PERK-dependent regulation, the inducers of the ISR can utilize activation of either GCN2 or PKR to trigger eIF2α/p38α-dependent phosphorylation and ensuing downregulation of IFNAR1. A key role of the stress-activated p38α protein kinase in regulating IFNAR1 levels is consistent with a previous report that activation of this kinase was inversely correlated with levels of endogenous IFNAR1 in clinical malignant melanoma samples (34).
Constitutive PKR activity downregulates IFNAR1 levels and signaling
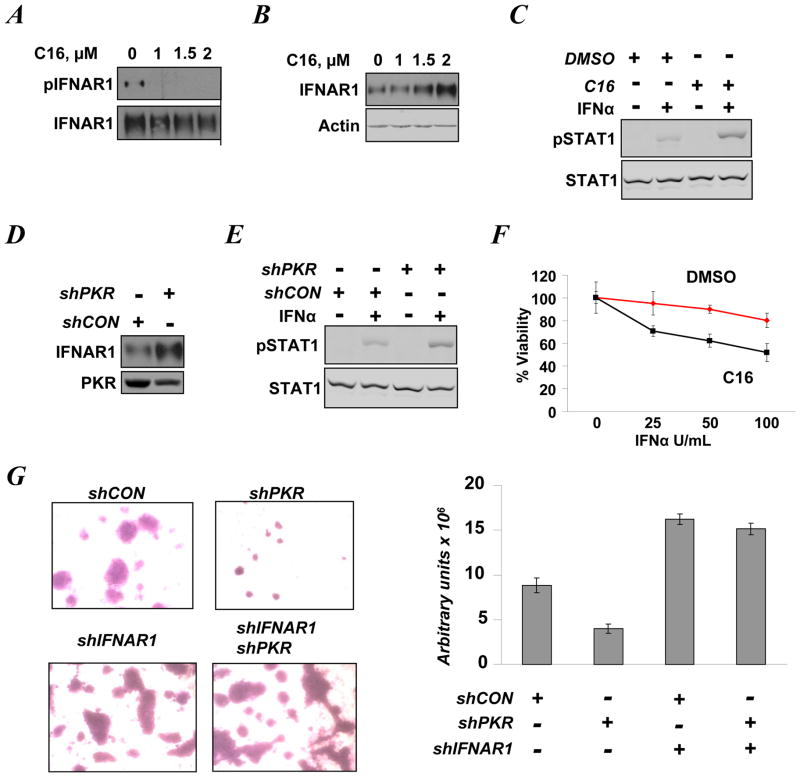
We further sought to corroborate the role of PKR in regulating IFNAR1 levels and activities. Although linked to induction of apoptosis (35, 36), a high level of constitutively active PKR has been reported in clinical human breast cancer tissues and cell lines including T47D cells (9–11). Whereas priming phosphorylation of endogenous IFNAR1 in these cells could be detected upon treatment with a lysosomal inhibitor (to prevent IFNAR1 degradation), further addition of PKR inhibitor C16 readily decreased this phosphorylation (Figure 3A). Furthermore, merely adding C16 to T47D cells led to an increase in IFNAR1 levels in a dose-dependent manner (Figure 3B). Pre-treatment of T47D cells with this PKR inhibitor also increased the IFNα-induced phosphorylation of STAT1 (Figure 3C).
Figure 3. Constitutively active PKR suppresses IFNAR1 levels and signaling in T47D human breast cancer cells.
A. T47D cells treated with indicated doses of C16 (for 24 h) were incubated with the lysosomal inhibitor methyl amine chloride (40 mM) for 4 h prior to harvesting. Phosphorylation and levels of IFNAR1 were analyzed by immunoblotting.
B. Immoblotting analysis of IFNAR1 and β-actin levels in T47D cells treated with C16 for 24 h.
C. Immunoblotting analyses of levels and phosphorylation of STAT1 in T47D cells treated with vehicle (DMSO) or C16 (2 μM) for 24 h prior to treatment with 250 U/mL of IFNα (30 min).
D. Levels of IFNAR1and PKR in T47D cells that received control or PKR shRNA.
E. Immunoblot analysis of levels and phosphorylation of STAT1on Tyr701 in T47D cells that received control or PKR shRNA 48 h prior to treatment with of IFNα (250 U/mL for 30 min). The extent of PKR knockdown in the cells before treatment is shown in panel D.
F. Viability of T47D cells treated with vehicle (DMSO) or C16 (2 μM) for 24 h prior to treatment with the indicated amounts of IFNα (for 48 h) was analyzed using a MTT assay.
G. Images and quantification of T47D cells that received indicated shRNA and migrated through Matrigel/membrane in Boyden chambers. Density of migrating cells was assessed by densitometry and presented in arbitrary units of absorbance. The extent of PKR and IFNAR1 knockdown is shown in Figure S4.
This pharmacologic analysis was supplemented with genetic experiments using a RNAi approach. Knockdown of PKR in T47D cells increased the levels of endogenous IFNAR1 (Figure 3D) and augmented STAT1 activation assessed by its phosphorylation on Tyr701 (Figure 3E) or Ser727 (Figure S3). Importantly, while viability of T47D cells did not robustly change in response to IFNα, these cells could be significantly sensitized to this treatment by adding PKR inhibitor C16 (Figure 3F). Knockdown of PKR noticeably decreased the ability of T47D cells to invade matrigel and/or to migrate through a membrane in a Boyden chamber invasion/motility assay (Figure 3G). In line with the well-established role of Type 1 IFN in restricting cell motility (37), knockdown of IFNAR1 (Figure S4) noticeably stimulated the breast cancer cells movement through the Matrigel/membrane (Figure 3G). Remarkably, in cells that received shRNA targeting IFNAR1, knockdown of PKR did not significantly decrease cell migration/invasion (Figure 3G) indicating that inhibition of invasive phenotype can at least in part be attributed to IFNAR1 stabilization. These results collectively suggest that high levels of constitutive PKR activity in breast cancer cells may restrict IFNAR1 expression and signaling as well as IFNAR1-mediated anti-tumorigenic effects.
Induction of GCN2-dependent pathways promotes IFNAR1 downregulation to attenuate IFN signaling
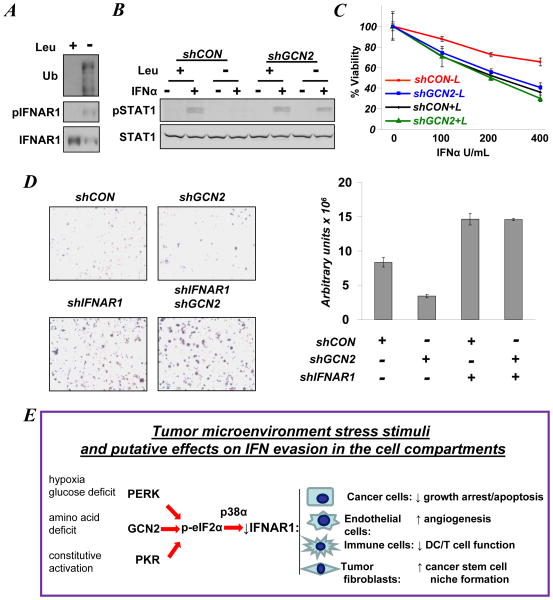
Culturing MEFs in medium deficient in the essential amino acid leucine triggered IFNAR1 phosphorylation and downregulation that was dependent on GCN2, eIF2α phosphorylation and p38α kinase (Figures 2A, E, F). Leucine starvation also stimulated priming phosphorylation, ubiquitination and downregulation of endogenous IFNAR1 in WM266-4 melanoma cells (Figure 4A). Similar effects were seen in 1205Lu human melanoma cells (Figure S5). We next investigated the role of activated GCN2 in cellular responses to Type 1 IFN. When WM266-4 cells were cultured in the absence of leucine, the activation of STAT1 in response to IFNα was attenuated (Figure 4B and S6). A similar attenuation was seen in 1205Lu cells treated with IFNα but not with IFN (Figure S7) that signals through a different receptor, IFNGR (19). Furthermore, leucine deficit noticeably blunted the sensitivity of WM266-4 cells to the anti-proliferative effects of IFNα (Figure 4C). Importantly, knockdown of GCN2 in these cells at least partially restored their responsiveness to IFNα as evident from analyses of STAT1 phosphorylation (Figures 4B, S6) and cell viability (Figure 4C). In addition, migration and/or invasion of WM266-4 cells grown without leucine was markedly inhibited by knockdown of GCN2 unless IFNAR1 also was downregulated (Figures 4D and S8). These results collectively suggest that activation of GCN2 triggered by amino acid deficit leads to downregulation of IFNAR1 and attenuation of its signaling and of ensuing anti-tumorigenic effects.
Figure 4. Activation of GCN2 by amino acid deficit promotes downregulation of IFNAR1 and inhibits its signaling.
A. Levels, phosphorylation, and ubiquitination of IFNAR1 immunopurified from WM266-4 cells grown in the presence or absence of leucine for 48 h were analyzed by immunoblotting using the indicated antibodies.
B. STAT1 phosphorylation and levels in WM266-4 cells that received the indicated shRNAs and were incubated in medium in the presence or absence of leucine for 48 h prior to treatment with IFNα (250 U/mL for 30 min). Extent of protein knockdown is shown in Figure S6.
C. Viability of WM266-4 cells that received the indicated shRNAs and incubated in the presence (+L) or absence (−L) of leucine for 48 h prior to treatment with the indicated doses of IFNα (for 24 h) and subsequent analysis using a MTT assay.
D. Images and quantification of WM266-4 cells (that received indicated shRNA and were incubated in media lacking leucine) and migrated through Matrigel/membrane in Boyden chambers. Density of migrating cells was assessed by densitometry and presented in arbitrary units of absorbance. Extent of protein knockdown in shown in Figure S8.
E. Hypothetical model in which various types of ISR signaling converge on phosphorylation of eIF2α and activation of p38α kinase to downregulate IFNAR1 and inhibit IFNα responses in various compartments of the tumor. This leads to alleviation of growth inhibition, stimulation of angiogenesis, and niche formation and impairment of anti-tumorigenic immune responses.
Discussion
Numerous studies define the robust anti-tumorigenic effects of IFN in vitro (38). Perplexingly, human malignancy manifestation occurs despite the presence of endogenous Type 1 IFN. Furthermore, while recombinant IFNα/β has been used extensively as anti-cancer agent, the efficacy of this treatment is limited (38–40). Why are these cytokines ineffective in protecting the hosts from developing tumors and in treating already developed malignancies? A plausible answer to this paradox is that tumors may evolve to develop adaptive mechanisms that help them to evade the effects of endogenous Type 1 IFN. Our current work contributes to the delineation of such mechanisms.
Previous findings suggested that induction of PERK by pharmacological agents or viruses attenuates the cellular responses to Type 1 IFN via phosphorylation-dependent ubiquitination and degradation of IFNAR1. Here we report that hypoxia, a stress stimulus that can induce PERK within the tumor microenvironment, is capable of a similar effect. Delineation of a signaling pathway downstream of PERK identified phosphorylation of eIF2α as a requisite mediator of p38α protein kinase activation and subsequent phosphorylation and downregulation of IFNAR1 in response to PERK activation (Figure 1). Remarkably, activation of other eIF2α kinases (GCN2 and PKR) that function within the complex signaling pathway termed the ISR are also capable of phosphorylation and downregulation of IFNAR1 in an eIF2α/p38α-dependent manner (Figure 2). Furthermore, human breast cancer cells harboring constitutively active PKR display low IFNAR1 levels and attenuated signaling as well as limited sensitivity to the anti-tumorigenic effects of IFNα. These phenotypical characteristics can be ameliorated by either pharmacologic or genetic inhibition of PKR (Figure 3). Conversely, human melanoma cells that are intrinsically sensitive to IFNα partially forfeit their ability to respond to this cytokine upon essential amino acid starvation-induced GCN2 activation and ensuing IFNAR1 downregulation. Knockdown of GCN2 restores the sensitivity of these cells to IFNα signaling and IFNAR1-dependent anti-tumorigenic effects (Figure 4).
Constitutive activity of PKR in human breast cancer cells is likely to suppress the effects of IFN in a tumor cell autonomous manner. Activities of GCN2 or PERK in tumor cells were implicated in supporting tumor growth and progression (2, 12, 13). Furthermore, it is also plausible that downregulation of IFNAR1 and ensuing deficiency in Type 1 IFN signaling may also occur in non-tumor cells within the tumor microenvironment. Accordingly, we propose a model wherein activation of the pathway encompassed into the ISR in the tumor microenvironment may summarily downregulate IFNAR1 and reduce overall Type 1 IFN responses in all cell types constituting the tumor (Figure 4E). Given that IFNα/β can act via numerous direct and indirect mechanisms, the overall decrease in sensitivity of tumor tissue to these cytokines may encourage tumor development and progression in a number of ways.
Within this model (Figure 4E), activation of various branches of the ISR can alleviate the direct anti-proliferative, anti-migratory, or pro-apoptotic effects of Type 1 IFN on tumor cells. In addition, accelerated degradation of IFNAR1 in the endothelial cells and their precursors may alleviate the suppressive effects of these cytokines on angiogenesis (41). Lack of IFNAR1-mediated suppression of growth and proliferation of tumor-associated fibroblasts may not only change tumor architecture but also hypothetically stimulate and optimize the ability of these fibroblasts to provide a niche for putative cancer stem cells (42).Finally, downregulation of IFNAR1 in infiltrating immune cells is expected to dramatically impair anti-tumor immunity.
It has been suggested that the immune system follows the “elimination-equilibrium-escape” mode to suppresses viability/growth of cancer cells while also selecting for resistant sub-clones that subsequently invade and metastasize (43). Given a paramount role for Type 1 IFN in the tumor-specific dendritic cell-mediated recruitment and priming of infiltrating T cells and subsequent tumor elimination (44, 45), it is plausible that downregulation of IFNAR1 in the tumor microenvironment represents an essential mechanism that enables the switch from the elimination to the equilibrium to the escape phases of cancer immunoediting. It has been indeed suggested that hypoxic conditions favor tumor immunosuppression (46). Future studies will determine the role of IFNAR1 degradation in tumor stroma and immune compartments of tumors in the processes of tumor development, progression and metastases.
Furthermore, given the limited efficacy of IFN therapy, investigation of potential role of mechanisms uncovered here in the innate or acquired resistance of tumors to exogenously administered Type 1 IFN is also of substantial merit. As diverse branches of ISR signaling converge on the activation of the stress-activated p38 protein kinase, it is worth noting that activation of this kinase was inversely correlated with IFNAR1 levels in clinical malignant melanoma samples and that inhibition of this kinase sensitized human melanoma cells to the anti-tumorigenic effects of IFN in vitro and in a xenograft tumor model (34). Further delineation of these pathways may lead to identification of important therapeutic targets, the inhibitors of which may enable stabilization of IFNAR1 and could be combined with pharmacologic IFNα/β modalities. Conversely, it is hypothetically possible to exploit accelerated IFNAR1 degradation in hypoxic tumors to target these regions with oncolytic viruses that would be otherwise counteracted by Type 1 IFN pathway.
Materials and Methods
Plasmids, Chemicals and Cells
Short hairpin RNA (shRNA) against GCN2 (2), IFNAR1 (21), or PERK (16) have been described previously. PKR inducer heparin sodium salt (HE, used at 1μg/mL), thapsigargin (TG, used at 1μg/mL) PKR inhibitor Imidazolo-oxindole (C16), and MCDB 153 medium without leucine were purchased from Sigma. ShRNA for knockdown of PKR were purchased from Santa Cruz Biotechnology (sc-36263-SH). Dulbecco’s Modification of Eagle’s medium without leucine was obtained from MP Biomedicals. Human IFNα2 was purchased from Bio-Sidius S.A. Murine IFNβ was expressed in Chinese Hamster Ovary cells, and purified to homogeneity from conditioned culture medium using successive Blue Sepharose, copper chelating Sepharose, lentil lectin Sepharose, and Uno S (Biorad) cationic ion exchange chromatographies. The specific activity of the protein was 2 × 108 units/mg in an in vitro antiviral cytopathic effect assay using murine L929 cells challenged with encephalomyocarditis virus. The purified protein had an endotoxin level of <0.05 EU/mg.
Human breast cancer T47D cells (from Ze’ev Ronai, Burnham Institute, San Diego, CA) and melanoma WM266-4 and 1205Lu cells (from Meenhard Herlyn, Wistar Institute, Philadelphia, PA) were cultured as described previously (47, 48). WM266-4 cells were maintained in MCDB 153 medium supplemented with bovine insulin (5μg/mL). Other human cells as well as mouse embryo fibroblasts (MEFs) from knockout mice lacking p38α (from Angelo Nebreda, Centro Nacional de Investigaciones Oncológicas, Madrid, Spain), PKR (from Antonis Koromilas, McGill University, Montreal, Quebec), PERK or GCN2 (from David Ron, New York University, New York, NY) or knock-in mice harboring the eIF2αS51A allele (from Randal Kaufman, University of Michigan, Ann Arbor, MI) were maintained in Dulbecco’s modified Eagle’s medium supplemented with 10% (v/v) fetal bovine serum (Hyclone), non-essential amino acids and β-mercaptoethanol (55 μM). For knockdown experiments in human cells, T47D or WM266-4 cells were transduced with lentiviral particles encoding shRNA against GFP, PKR or GCN2 and selected in 2 μg/mL of puromycin for 2 weeks. The human colon adenocarcinoma HT-29 cell line and its derivative stably expressing the constitutively active GADD34 C-terminal fragment (HT29-A1) were cultured in the presence of puromycin (1 μg/mL) as described previously (33).Cells were exposed to the hypoxia condition of 0.5% oxygen, 5% carbon dioxide and 94.5% nitrogen for the indicated times through the use of the Invivo2–400 Hypoxia Workstation (Ruskinn Technology Ltd).
Antibodies and Immunotechniques
Analyses of phosphorylated (on Ser532 in human IFNAR1 or Ser523 on mouse IFNAR1) and total levels of human and mouse IFNAR1 were carried out as described elsewhere (22, 49). Commercially available antibodies against pSTAT1, p-eIF2α, p-p38 kinase, STAT1, PKR, (Cell Signaling), eIF2α (Biosources), β-actin (Sigma), HIF1α (BD Biosciences), p38α, and ubiquitin (Santa Cruz Biotechnology) were purchased. Immunoprecipitations, immunoblotting, and in vivo ubiquitination assay using denaturing immunoprecipitation were carried out as described previously (23).
Cell invasion assays
BD Biocoat chambers coated with Matrigel matrix (BD Matrigel, 354234) were overlaid with 0.5 mL of cell suspension (2.5 × 104 cells) to each 24-well invasion chamber in media without serum. Medium with 10% serum (0.75 mL) was then added to the outside chamber and cells were incubated at 37°C with 5% CO2 for either 24 hours (WM266-4 cells) or for 5 days (T47D cells). Cells were stained with crystal violet and images were obtained using a BX-51 Olympus microscope. The images were quantified using Image J.
Viability assays
Cells transfected with the indicated plasmids were selected with puromycin for 3 days and then seeded at 5 × 104 cells per well in a 96 well plate. The cells were treated with indicated doses of IFNα for 24–48 h and were analyzed using a cell proliferation colorimetric assay kit, CellTiter 96 (Promega). The Student t test was used for analysis of statistical significance.
Supplementary Material
Acknowledgments
We thank M. Herlyn, R. Kaufman, A. Koromilas, A. Nebreda, D. Ron, and Z. Ronai for reagents, Drs. Yong Zhang for technical help, and the members of Fuchs, Diehl and Koumenis labs for discussion. The support to S.B. from the “Training in Tumor Virology” grant 2-T32-CA-557726-06 is greatly appreciated. This work was supported by the NIH grants CA92900 and CA142425 (to S.Y.F.), CA94214 (to C.K.), CA104838 (to J.A.D.) and by a grant with the Pennsylvania Department of Health (to J.A.D., C.K., and S.Y.F.). The Department specifically disclaims responsibility for any analyses, interpretation, or conclusions.
ABBREVIATIONS
- GCN2
general control nonrepressed 2
- eIF2α
eukaryotic translation initiation factor 2-alpha
- HE
heparin
- IFN
interferon
- IFNAR1
IFNα/β receptor chain 1
- ISR
integrated stress response
- LS
leucine starvation
- MEF
mouse embryo fibroblasts
- PERK
pancreatic ER kinase
- PKR
Protein kinase RNA-activated
- STAT
Signal Transducer and Activator of Transcription
- TG
thapsigargin
Footnotes
CONFLICT OF INTEREST:
The authors declare no conflict of interest except for Dr. D.P. Baker who is an employee of BiogenIDEC, Inc and owns stock of this company.
References
- 1.Ye J, Koumenis C. ATF4, an ER stress and hypoxia-inducible transcription factor and its potential role in hypoxia tolerance and tumorigenesis. Curr Mol Med. 2009;9(4):411–6. doi: 10.2174/156652409788167096. [DOI] [PubMed] [Google Scholar]
- 2.Ye J, Kumanova M, Hart LS, Sloane K, Zhang H, De Panis DN, et al. The GCN2-ATF4 pathway is critical for tumour cell survival and proliferation in response to nutrient deprivation. EMBO J. 2010;29(12):2082–96. doi: 10.1038/emboj.2010.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu Y, Laszlo C, Liu W, Chen X, Evans SC, Wu S. Regulation of G(1) arrest and apoptosis in hypoxia by PERK and GCN2-mediated eIF2alpha phosphorylation. Neoplasia. 2010;12(1):61–8. doi: 10.1593/neo.91354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331(6024):1565–70. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 5.Moenner M, Pluquet O, Bouchecareilh M, Chevet E. Integrated endoplasmic reticulum stress responses in cancer. Cancer Res. 2007;67(22):10631–4. doi: 10.1158/0008-5472.CAN-07-1705. [DOI] [PubMed] [Google Scholar]
- 6.Wek RC, Jiang HY, Anthony TG. Coping with stress: eIF2 kinases and translational control. Biochem Soc Trans. 2006;34(Pt 1):7–11. doi: 10.1042/BST20060007. [DOI] [PubMed] [Google Scholar]
- 7.Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8(7):519–29. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 8.Blalock WL, Bavelloni A, Piazzi M, Faenza I, Cocco L. A role for PKR in hematologic malignancies. J Cell Physiol. 2010;223(3):572–91. doi: 10.1002/jcp.22092. [DOI] [PubMed] [Google Scholar]
- 9.Nussbaum JM, Major M, Gunnery S. Transcriptional upregulation of interferon-induced protein kinase, PKR, in breast cancer. Cancer Lett. 2003;196(2):207–16. doi: 10.1016/s0304-3835(03)00276-3. [DOI] [PubMed] [Google Scholar]
- 10.Kim SH, Forman AP, Mathews MB, Gunnery S. Human breast cancer cells contain elevated levels and activity of the protein kinase, PKR. Oncogene. 2000;19(27):3086–94. doi: 10.1038/sj.onc.1203632. [DOI] [PubMed] [Google Scholar]
- 11.Savinova O, Joshi B, Jagus R. Abnormal levels and minimal activity of the dsRNA-activated protein kinase, PKR, in breast carcinoma cells. Int J Biochem Cell Biol. 1999;31(1):175–89. doi: 10.1016/s1357-2725(98)00140-x. [DOI] [PubMed] [Google Scholar]
- 12.Bobrovnikova-Marjon E, Grigoriadou C, Pytel D, Zhang F, Ye J, Koumenis C, et al. PERK promotes cancer cell proliferation and tumor growth by limiting oxidative DNA damage. Oncogene. 2010;29(27):3881–95. doi: 10.1038/onc.2010.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koumenis C, Naczki C, Koritzinsky M, Rastani S, Diehl A, Sonenberg N, et al. Regulation of protein synthesis by hypoxia via activation of the endoplasmic reticulum kinase PERK and phosphorylation of the translation initiation factor eIF2alpha. Mol Cell Biol. 2002;22(21):7405–16. doi: 10.1128/MCB.22.21.7405-7416.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fels DR, Koumenis C. The PERK/eIF2alpha/ATF4 module of the UPR in hypoxia resistance and tumor growth. Cancer Biol Ther. 2006;5(7):723–8. doi: 10.4161/cbt.5.7.2967. [DOI] [PubMed] [Google Scholar]
- 15.Bhattacharya S, HuangFu WC, Liu J, Veeranki S, Baker DP, Koumenis C, et al. Inducible priming phosphorylation promotes ligand-independent degradation of the IFNAR1 chain of type I interferon receptor. J Biol Chem. 2010;285(4):2318–25. doi: 10.1074/jbc.M109.071498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu J, HuangFu WC, Kumar KG, Qian J, Casey JP, Hamanaka RB, et al. Virus-induced unfolded protein response attenuates antiviral defenses via phosphorylation-dependent degradation of the type I interferon receptor. Cell Host Microbe. 2009;5(1):72–83. doi: 10.1016/j.chom.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eggermont AM. The role interferon-alpha in malignant melanoma remains to be defined. Eur J Cancer. 2001;37(17):2147–53. doi: 10.1016/s0959-8049(01)00272-6. [DOI] [PubMed] [Google Scholar]
- 18.Fidler IJ. The organ microenvironment and cancer metastasis. Differentiation. 2002;70(9–10):498–505. doi: 10.1046/j.1432-0436.2002.700904.x. [DOI] [PubMed] [Google Scholar]
- 19.Platanias LC. Mechanisms of type-I- and type-II-interferon-mediated signalling. Nat Rev Immunol. 2005;5(5):375–86. doi: 10.1038/nri1604. [DOI] [PubMed] [Google Scholar]
- 20.Hwang SY, Hertzog PJ, Holland KA, Sumarsono SH, Tymms MJ, Hamilton JA, et al. A null mutation in the gene encoding a type I interferon receptor component eliminates antiproliferative and antiviral responses to interferons alpha and beta and alters macrophage responses. Proc Natl Acad Sci U S A. 1995;92(24):11284–8. doi: 10.1073/pnas.92.24.11284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bhattacharya S, Zheng H, Tzimas C, Carroll M, Baker DP, Fuchs SY. Bcr-abl signals to desensitize chronic myeloid leukemia cells to IFNalpha via accelerating the degradation of its receptor. Blood. 2011;118(15):4179–87. doi: 10.1182/blood-2010-12-325373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kumar KG, Tang W, Ravindranath AK, Clark WA, Croze E, Fuchs SY. SCF(HOS) ubiquitin ligase mediates the ligand-induced down-regulation of the interferon-alpha receptor. EMBO J. 2003;22(20):5480–90. doi: 10.1093/emboj/cdg524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu J, Plotnikov A, Banerjee A, Suresh Kumar KG, Ragimbeau J, Marijanovic Z, et al. Ligand-independent pathway that controls stability of interferon alpha receptor. Biochem Biophys Res Commun. 2008;367(2):388–93. doi: 10.1016/j.bbrc.2007.12.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zheng H, Qian J, Carbone CJ, Leu NA, Baker DP, Fuchs SY. Vascular endothelial growth factor-induced elimination of the type 1 interferon receptor is required for efficient angiogenesis. Blood. 2011;118(14):4003–6. doi: 10.1182/blood-2011-06-359745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zheng H, Qian J, Varghese B, Baker DP, Fuchs S. Ligand-stimulated downregulation of the alpha interferon receptor: role of protein kinase D2. Mol Cell Biol. 2011;31(4):710–20. doi: 10.1128/MCB.01154-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qian J, Zheng H, Huangfu WC, Liu J, Carbone CJ, Leu NA, et al. Pathogen recognition receptor signaling accelerates phosphorylation-dependent degradation of IFNAR1. PLoS Pathog. 2011;7(6):e1002065. doi: 10.1371/journal.ppat.1002065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bhattacharya S, Qian J, Tzimas C, Baker DP, Koumenis C, Diehl JA, et al. Role of p38 protein kinase in the ligand-independent ubiquitination and down-regulation of the IFNAR1 chain of type I interferon receptor. J Biol Chem. 2011;286(25):22069–76. doi: 10.1074/jbc.M111.238766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu J, Carvalho LP, Bhattacharya S, Carbone CJ, Kumar KG, Leu NA, et al. Mammalian casein kinase 1alpha and its leishmanial ortholog regulate stability of IFNAR1 and type I interferon signaling. Mol Cell Biol. 2009;29(24):6401–12. doi: 10.1128/MCB.00478-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huangfu WC, Fuchs SY. Ubiquitination-dependent regulation of signaling receptors in cancer. Genes Cancer. 2010;1(7):725–34. doi: 10.1177/1947601910382901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Diehl JA, Fuchs SY, Koumenis C. The cell biology of the unfolded protein response. Gastroenterology. 2011;141(1):38–41. 41, e1–2. doi: 10.1053/j.gastro.2011.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bobrovnikova-Marjon E, Pytel D, Riese MJ, Vaites LP, Singh N, Koretzky GA, et al. PERK utilizes intrinsic lipid kinase activity to generate phosphatidic acid, mediate AKT activation and promote adipocyte differentiation. Mol Cell Biol. 2012 doi: 10.1128/MCB.00063-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hamanaka RB, Bobrovnikova-Marjon E, Ji X, Liebhaber SA, Diehl JA. PERK-dependent regulation of IAP translation during ER stress. Oncogene. 2009;28(6):910–20. doi: 10.1038/onc.2008.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blais JD, Filipenko V, Bi M, Harding HP, Ron D, Koumenis C, et al. Activating transcription factor 4 is translationally regulated by hypoxic stress. Mol Cell Biol. 2004;24(17):7469–82. doi: 10.1128/MCB.24.17.7469-7482.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huangfu WC, Qian J, Liu C, Liu J, Lokshin AE, Baker DP, et al. Inflammatory signaling compromises cell responses to interferon alpha. Oncogene. 2012;31(2):161–72. doi: 10.1038/onc.2011.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pindel A, Sadler A. The role of protein kinase R in the interferon response. J Interferon Cytokine Res. 2011;31(1):59–70. doi: 10.1089/jir.2010.0099. [DOI] [PubMed] [Google Scholar]
- 36.Williams BR. PKR; a sentinel kinase for cellular stress. Oncogene. 1999;18(45):6112–20. doi: 10.1038/sj.onc.1203127. [DOI] [PubMed] [Google Scholar]
- 37.Pfeffer LM, Wang E, Tamm I. Interferon effects on microfilament organization, cellular fibronectin distribution, and cell motility in human fibroblasts. J Cell Biol. 1980;85(1):9–17. doi: 10.1083/jcb.85.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brassard DL, Grace MJ, Bordens RW. Interferon-alpha as an immunotherapeutic protein. J Leukoc Biol. 2002;71(4):565–81. [PubMed] [Google Scholar]
- 39.Parmar S, Platanias LC. Interferons: mechanisms of action and clinical applications. Curr Opin Oncol. 2003;15(6):431–9. doi: 10.1097/00001622-200311000-00005. [DOI] [PubMed] [Google Scholar]
- 40.Li Y, Srivastava KK, Platanias LC. Mechanisms of type I interferon signaling in normal and malignant cells. Arch Immunol Ther Exp (Warsz) 2004;52(3):156–63. [PubMed] [Google Scholar]
- 41.Folkman J, Ingber D. Inhibition of angiogenesis. Semin Cancer Biol. 1992;3(2):89–96. [PubMed] [Google Scholar]
- 42.Borovski T, De Sousa EMF, Vermeulen L, Medema JP. Cancer stem cell niche: the place to be. Cancer Res. 2011;71(3):634–9. doi: 10.1158/0008-5472.CAN-10-3220. [DOI] [PubMed] [Google Scholar]
- 43.Dunn GP, Koebel CM, Schreiber RD. Interferons, immunity and cancer immunoediting. Nat Rev Immunol. 2006;6(11):836–48. doi: 10.1038/nri1961. [DOI] [PubMed] [Google Scholar]
- 44.Diamond MS, Kinder M, Matsushita H, Mashayekhi M, Dunn GP, Archambault JM, et al. Type I interferon is selectively required by dendritic cells for immune rejection of tumors. J Exp Med. 2011;208(10):1989–2003. doi: 10.1084/jem.20101158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fuertes MB, Kacha AK, Kline J, Woo SR, Kranz DM, Murphy KM, et al. Host type I IFN signals are required for antitumor CD8+ T cell responses through CD8{alpha}+ dendritic cells. J Exp Med. 2011;208(10):2005–16. doi: 10.1084/jem.20101159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Noman MZ, Messai Y, Carre T, Akalay I, Meron M, Janji B, et al. Microenvironmental hypoxia orchestrating the cell stroma cross talk, tumor progression and antitumor response. Crit Rev Immunol. 2011;31(5):357–77. doi: 10.1615/critrevimmunol.v31.i5.10. [DOI] [PubMed] [Google Scholar]
- 47.Tang W, Li Y, Yu D, Thomas-Tikhonenko A, Spiegelman VS, Fuchs SY. Targeting beta-transducin repeat-containing protein E3 ubiquitin ligase augments the effects of antitumor drugs on breast cancer cells. Cancer Res. 2005;65(5):1904–8. doi: 10.1158/0008-5472.CAN-04-2597. [DOI] [PubMed] [Google Scholar]
- 48.Soldatenkov VA, Dritschilo A, Ronai Z, Fuchs SY. Inhibition of homologue of Slimb (HOS) function sensitizes human melanoma cells for apoptosis. Cancer Res. 1999;59(20):5085–8. [PubMed] [Google Scholar]
- 49.Kumar KG, Krolewski JJ, Fuchs SY. Phosphorylation and specific ubiquitin acceptor sites are required for ubiquitination and degradation of the IFNAR1 subunit of type I interferon receptor. J Biol Chem. 2004;279(45):46614–20. doi: 10.1074/jbc.M407082200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.