Summary
Purpose
Antiepileptic drugs may cause congenital malformations. Less is known about the effect on development in infancy and childhood. The aim of this study was to examine whether exposure to antiepileptic drugs during pregnancy has an impact on early child development.
Methods
From mid-year 1999 through December 2008, children of mothers recruited at 13–17 weeks of pregnancy were studied in the ongoing prospective Norwegian Mother and Child Cohort Study. Information on birth outcomes were obtained from the Medical Birth Registry (108,264 –children), and mothers reported on their child’s motor development, language, social skills, and autistic traits using items from standardized screening tools at 18 months (61,351 children) and 36 months of age (44,147 children). The relative risk of adverse outcomes in children according to maternal or paternal epilepsy with and without prenatal exposure to antiepileptic drugs was estimated as odds ratios (ORs), using logistic regression with adjustment for maternal age, parity, education, smoking, depression/anxiety, folate-supplementation, and child congenital malformation or low birth weight.
Key findings
A total of 333 children were exposed to antiepileptic drugs in utero. At 18 months, the exposed children had increased risk of abnormal scores for gross motor skills (7.1 % vs. 2.9 %; OR, 2.0; 95 % Confidence Interval [CI], 1.1–3.7) and autistic traits (3.5 % vs. 0.9 %; OR, 2.7; CI, 1.1–6.7) compared to children of parents without epilepsy. At 36 months, the exposed children had increased risk of abnormal score for gross motor skills (7.5 % vs. 3.3 %; OR, 2.2; CI, 1.1–4.2), sentence skills (11.2 % vs. 4.8 %; OR, 2.1; CI, 1.2–3.6), and autistic traits (6.0 % vs. 1.5 %; OR, 3.4; CI, 1.6–7.0). The drug-exposed children also had increased risk of congenital malformations (6.1 % vs. 2.9 %; OR, 2.1; CI, 1.4–3.4), but exclusion of congenital malformations did not affect the risk of adverse development. Children born to women with epilepsy who did not use antiepileptic drugs had no increased risks. Children of fathers with epilepsy generally scored within the normal range.
Significance
Exposure to antiepileptic drugs during pregnancy is associated with adverse development at 18 and 36 months of age, measured as low scores within key developmental domains rated by mothers. Exposures to valproate, lamotrigine, carbamazepine, or multiple antiepileptic drugs were associated with adverse outcome within different developmental domains.
Keywords: Epilepsy, Pregnancy, Teratogenicity, MoBa-Study
Introduction
Epilepsy in women is relatively common during childbearing years and the reported prevalence of antiepileptic drug use during pregnancy ranges from 0.2 % to 0.5 % (Fairgrieve et al. 2000;Molgaard-Nielsen & Hviid 2011;Veiby et al. 2009;Wallace et al. 1998). Increased risk of congenital malformations in children born to mothers with epilepsy is well known, and mainly associated with use of older generation antiepileptic drugs (Harden et al. 2009;Holmes et al. 2011;Tomson et al. 2011). The effects of antiepileptic drugs on cognition is less clear, but intrauterine exposure has been associated with impaired psychomotor function, language, IQ scores, and behavioral difficulties (Banach et al. 2010;Forsberg et al. 2011;Meador et al. 2009;Nadebaum et al. 2011;Thomas et al. 2007;Vinten et al. 2009;Wide et al. 2002). People with epilepsy differ from the general population genetically and socio-economically, which also contributes to different developmental outcomes in the offspring (Titze et al. 2008). Studies on selected hospital-based populations do often not include representative controls, and also typically do not include women with non-active or untreated epilepsy. Register-based studies are generally larger but often lack information on type of epilepsy, antiepileptic drug dose, and seizure control during pregnancy (Tomson & Battino 2012).
There is a need for more knowledge concerning long-term outcome in children exposed to antiepileptic drugs in utero (Harden et al. 2009). Increased surveillance during childhood can lead to early identification of children at risk for developmental delay, and initiate appropriate follow-up and intervention (Dumont-Mathieu & Fein 2005;Rydz et al. 2005). In the Norwegian Mother and Child Cohort Study (MoBa), mothers have reported on their child’s motor, language, and social development at 18 and 36 months of age using standardized, validated screening instruments.
The primary aim of this study was to examine whether exposure to antiepileptic drugs during pregnancy has an impact on development at 18 and 36 months of age. As a second objective, development in children of untreated mothers with epilepsy and of fathers with epilepsy was examined, serving as comparative groups accounting for genetic and socioeconomic effects of parental epilepsy. Children of mothers and fathers with both treated and untreated epilepsy were compared to a large reference group of children of parents who did not have epilepsy.
Material and Methods
The Norwegian Mother and Child Cohort Study (MoBa)
MoBa was established by The Norwegian Institute of Public Health, aiming at discovering the causes of disease (Magnus et al. 2006). The target population was women who gave birth in Norway, recruited from hospitals and maternity units. Women attending free routine ultrasound scanning (> 98 % of pregnant women in Norway) were invited to participate. Provided mother’s consent, fathers were also invited. The pregnant women received a postal invitation prior to their scheduled ultrasound examination (pregnancy week 13–17), containing the first questionnaires, addressed separately to the mother and father. The hospitals provided a list of pregnant women and their identification, as well as the date of ultrasound appointment. Based on estimated date of birth, the timing of contacts with the participants was determined. The first questionnaire focused on medical history before pregnancy, medication, occupation, lifestyle habits, and mental health. A questionnaire 6 months after delivery focused on child health and nutrition, while questionnaires at 18 and 36 months explored the child’s developmental status. The questionnaires on child health and development were filled out by the mothers.
From mid-year 1999 to December 2008, 107,072 pregnancies were registered in the MoBa-cohort with a total of 108,976 children, constituting approximately 18 % of all births in Norway during this period (55,000–60,000 a year). The participation rate for all invited pregnancies was 38.5 % (Roth et al. 2011). For those included, the response rates for the 6, 18, and 36 months questionnaires were 85 %, 73 %, and 60 % respectively (Stoltenberg et al. 2010). Fathers were invited to participate in 87 % of the included pregnancies, with a positive response rate of 83 % (Magnus et al. 2006).
All MoBa-mothers with epilepsy residing in Western Norway, Hordaland County, were invited to participate in a sub-study. With the mothers’ consent, medical hospital-records were examined to validate the self-reported epilepsy diagnosis and use of antiepileptic drugs during pregnancy. Mothers of 78 children were invited, active consent was obtained for 40 (51 %). The invited mothers also provided additional information on drug-use and seizure activity prior to and during pregnancy.
This study and research protocol was approved by the Regional Committee for Medical Research Ethics in Western Norway.
Assessment of parental epilepsy and antiepileptic drug use
All 726 children of mothers and 653 children of fathers with self-reported epilepsy in the MoBa cohort formed the “epilepsy group”. The remaining 107,597 children of parents without epilepsy served as the reference base. Due to ongoing data collection in MoBa and loss to follow-up, the number of children of parents with and without epilepsy varied at the different assessment points (Tables 1, 2 and 3). Children of mothers with epilepsy were categorized according to antiepileptic drug-exposure, based on use of antiepileptic drugs during pregnancy as reported in the first MoBa-questionnaire and in the compulsory Medical Birth Registry. Antiepileptic drug-use during pregnancy in MoBa was reported by the mother at gestational week 13–17, whereas information in the Medical Birth Registry (available for 99.3 %) was collected at delivery by the attending physician or midwife, reporting drug-use throughout the pregnancy. In the drug-exposed group, 94 % had information on antiepileptic medication in MoBa and the remaining 6 % had additional information in the Medical Birth Registry. Discrepancy in recorded medication between the two registers could partly be a result of underreporting and partly due to changes in antiepileptic drug-treatment during the pregnancy. In pregnancies with drug-information from both MoBa and the Medical Birth Registry (74 %), there was 99.5 % agreement on recorded type of monotherapy. In the single case with disagreement, the self-reported antiepileptic drug in MoBa was selected. Antiepileptic drug monotherapy was classified into carbamazepine, lamotrigine, and valproate. Exposure to polytherapy included cases where more than one antiepileptic drug was reported during the pregnancy from at least one of the two sources.
Table 1.
Maternal characteristics for children of parents with epilepsy compared to a reference group of children of parents without epilepsy
| MATERNAL EPILEPSYa | PATERNAL EPILEPSYa | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| % (No),
P value |
% (No),
P value |
||||||||
| CHARACTERISTICS | Reference | AED exposureb | No AED exposureb | AED useb | No AED useb | ||||
| At baseline | n = 107,597 | n = 333 | n = 393 | n = 242 | n = 411 | ||||
| Age > 37 years c | 4.7 % | 5.1 % (17) | p=0.70 | 4.1 % (16) | p= 0.71 | 4.5 % (11) | p=1.00 | 5.6 % (23) | p=0.35 |
| Body mass index > 25 d | 26 % | 34 % (90) | p=0.004 | 34 % (120) | p=0.001 | 28 % (60) | p=0.65 | 34 % (121) | p=0.002 |
| Low income e | 18 % | 28 % (86) | p<0.001 | 21 % (77) | p=0.25 | 13 % (29) | p=0.03 | 21 % (81) | p=0.21 |
| High educational level f | 21 % | 13 % (44) | p<0.001 | 15 % (59) | p=0.002 | 22 % (52) | p=0.94 | 23 % (95) | p=0.40 |
| Folate pre-conceptiong | 30 % | 44 % (148) | p<0.001 | 31 % (123) | p=0.66 | 34 % (83) | p=0.18 | 31 % (128) | p=0.71 |
| Folate 1st trimester | 71 % | 77 % (256) | p=0.01 | 74 % (292) | p=0.12 | 77 % (186) | p=0.04 | 72 % (296) | p=0.59 |
| Smoking in pregnancy | 8.0 % | 11 % (36) | p=0.06 | 8.2 % (32) | p=0.86 | 8.3 % (20) | p=0.83 | 7.1 % (29) | p=0.58 |
| In vitro fertilization | 2.9 % | 2.7 % (9) | p=1.00 | 4.6 % (18) | p=0.05 | 3.3 % (8) | p=0.70 | 1.7 % (7) | p=0.18 |
| Unplanned pregnancy | 19 % | 24 % (75) | p=0.06 | 24 % (92) | p=0.03 | 21 % (49) | p=0.57 | 21 % (83) | p=0.41 |
| Previous late abortion h | 2.8 % | 4.9 % (14) | p=0.05 | 5.2 % (17) | p=0.02 | 1.4 % (3) | p=0.30 | 2.2 % (8) | p=0.63 |
| Nulliparous i | 50 % | 50 % (166) | p=0.96 | 53 % (209) | p=0.16 | 50 % (120) | p=1.00 | 49 % (203) | p=0.96 |
Numbers may not equal 100 % within groups due to variation of missing values.
Antiepileptic drug (AED) use by mother during pregnancy or by father within 6 months to conception.
Cut-off corresponding to the 95 percentile for maternal age at gestation.
Prior to pregnancy.
Annual income less than 25 000 USD.
More than 16 years of education (university or similar).
Folate supplementation last month prior to pregnancy.
Spontaneous abortion(s) in previous pregnancies during gestational week 12–23.
Not earlier completed a pregnancy beyond 21 weeks of gestation.
Table 2.
Risk for adverse development score at 18 months in children of parents with epilepsy a compared to a reference group of children of parents without epilepsy
| MATERNAL EPILEPSY :
Antiepileptic drug exposure in uterob |
PATERNAL EPILEPSYb |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ADVERSE SCORE | Reference | All exposures | Monotherapy | Lamotrigine | Valproate | Carbamazepine | Polytherapy | Unexposed | No treatmentd | Treatmentd |
| Age 18 months | n = 60,583 | n = 184 | n = 158 | n = 65 | n = 25 | n = 41 | n = 26 | n = 221 | n = 216 | n = 147 |
| Gross motor skills | 2.9 % | * 7.1 % (13) | 5.7 % (9) | 7.8 % (5) | * 16.0 % (4) | 0.0 % (0) | * 15.4 % (4) | 3.2 % (7) | 3.7 % (8) | 4.1 % (6) |
| OR (95 % CI) c | 2.0 (1.13.7) | 1.6 (0.8–3.4) | 1.7 (0.6–5.1) | 7.0 (2.4–21.0) | NA | 4.1 (1.3–13.3) | 1.2 (0.6–2.6) | 1.3 (0.7–2.7) | 1.6 (0.7–3.6) | |
| Fine motor skills | 3.1 % | * 6.1 % (11) | 4.5 % (7) | 3.1 % (2) | 4.0 % (1) | * 10.0 % (4) | * 15.4 % (4) | 5.1 % (11) | * 5.6 % (12) | 3.5 % (5) |
| OR (95 % CI) | 1.8 (1.0–3.4) | 1.4 (0.7–3.0) | 0.9 (0.2–3.7) | 1.3 (0.2–9.7) | 3.3 (1.1–9.2) | 4.3 (1.4–13.0) | 1.7 (0.9–3.1) | 1.9 (1.0–3.4) | 1.0 (0.4–2.6) | |
| Personal-Social skills | 4.2 % | * 9.4 % (17) | 6.5 % (10) | 3.1 % (2) | 0.0 % (0) | *12.2 % (5) | * 26.9 % (7) | 3.7 % (8) | 5.6 % (12) | * 10.3 % (15) |
| OR (95 % CI) | 2.2 (1.3–3.6) | 1.5 (0.8–2.9) | 0.6 (0,2–2.7) | NA | 3.2 (1.3–8.3) | 7.1 (2.9–17.8) | 0.9 (0.4–1.8) | 1.4 (0,8–2.5) | 2.3 (1.3–4.1) | |
| Autism checkliste | 7.8 % | * 14.0 % (24) | 10.9 % (16) | 15.6 % (10) | 8.3 % (2) | 8.8 % (3) | * 33.3 % (8) | 10.0 % (20) | 11.1 % (24) | 11.0 % (16) |
| OR (95 % CI) | 1.7 (1.1–2.6) | 1.3 (0.7–2.2) | 1.8 (0.9–3.8) | 1.0 (0.2–4.5) | 1.1 (0.3–3.6) | 4.5 (1.8–11.1) | 1.3 (0.8–2.0) | 1.4 (0.9–2.2) | 1.6 (1.0–2.7) | |
| Autistic traitse | 0.9 % | * 3.5 % (6) | 2.0 % (3) | 3.1 % (2) | 0.0 % (0) | 2.9 % (1) | * 12.5 % (3) | 0.5 % (1) | 1.4 % (3) | * 2.8 % (4) |
| OR (95 % CI) | 2.7 (1.1–6.7) | 1.4 (0.3–5.6) | 1.5 (0.2–11.0) | NA | 3.3 (0.5–24.8) | 8.3 (2.3–30.0) | 0.5 (0.1–3.7) | 1.6 (0.5–5.0) | 3.7 (1.4–10.1) | |
Each cell contains the percentage (No.) of adverse outcomes within groups and corresponding odds ratio (OR) with 95 % CI.
Numbers may not equal 100 % within groups due to variation of missing values. NA = Not applicable. * P value < 0.05.
ORs are adjusted for maternal age, parity, education, smoking, anxiety/depression, periconceptional folate use, and child low birth weight and malformation.
Antiepileptic drug use by father within 6 months to conception.
Assessable for 92 % of the 18 months cohort. Autism checklist: Modified Checklist for Autism in Toddlers (MCHAT). Autistic traits: Early Screening of Autistic Traits (ESAT).
Table 3.
Risk for adverse development score at 36 months in children of parents with epilepsy a compared to a reference group of children of parents without epilepsy
| MATERNAL EPILEPSY :
Antiepileptic drug exposure in uterob |
PATERNAL EPILEPSYb |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ADVERSE SCORE | Reference | All exposures | Monotherapy | Lamotrigine | Valproate | Carbamazepine | Polytherapy | Unexposed | No treatmentd | Treatmentd |
| Age 36 months | n = 43,571 | n = 139 | n = 114 | n = 44 | n = 19 | n = 31 | n = 25 | n = 154 | n = 173 | n = 110 |
| Gross motor skills | 3.3 % | * 7.5 % (10) | * 8.1 % (9) | 9.8 % (4) | 10.5 % (2) | 6.5 % (2) | 4.3 % (1) | 3.3 % (5) | 6.0 % (10) | 3.6 % (4) |
| OR (95 % CI) c | 2.2 (1.1–4.2) | 2.4 (1.2–4.9) | 2.4 (0.8–7.0) | 3.4 (0.8–14.9) | 2.3 (0.5–9.9) | 1.1 (0.1–8.5) | 0.9 (0.3–2.4) | 1.9 (1.0–3.5) | 1.2 (0.4–3.2) | |
| Fine motor skills | 3.3 % | 3.8 % (5) | 4.7 % (5) | 7.7 % (3) | 5.6 % (1) | 3.3 % (1) | 0.0 % (0) | 5.6 % (8) | 4.2 % (7) | 2.9 % (3) |
| OR (95 % CI) | 1.1 (0.5–2.8) | 1.4 (0.6–3.5) | 2.1 (0.7–7.0) | 1.7 (0.2–13.1) | 1.0 (0.1–7.5) | NA | 1.7 (0.8–3.6) | 1.3 (0.6–2.8) | 0.9 (0.3–2.9) | |
| Communication skills | 2.9 % | 5.9 % (8) | 4.5 % (5) | 7.1 % (3) | 10.5 % (2) | 0.0 % (0) | 12.5 % (3) | 1.3 % (2) | 5.2 % (9) | 2.7 % (3) |
| OR (95 % CI) | 1.6 (0.8–3.4) | 1.3 (0.5–3.3) | 2.0 (0.6–6.7) | 3.5 (0.8–15.4) | NA | 2.7 (0.8–9.5) | 0.4 (0.1–1.8) | 1.9 (1.0–3.7) | 1.0 (0.3–3.1) | |
| Sentence skills | 4.8 % | * 11.2 % (15) | * 9.9 % (11) | * 14.3 % (6) | * 15.8 % (3) | 6.5 % (2) | 17.4 % (4) | 3.9 % (6) | 3.5 % (6) | 6.4 % (7) |
| OR (95 % CI) | 2.1 (1.2–3.6) | 2.0 (1.0–3.7) | 2.8 (1.2–6.9) | 3.4 (1.0–12.0) | 1.2 (0.3–5.1) | 2.6 (0.8–7.9) | 0.8 (0.4–1.9) | 0.7 (0.3–1.7) | 1.4 (0.7–3.1) | |
| Autistic traits (SCQ) | 1.5 % | * 6.0 % (8) | * 5.6 % (6) | * 9.3 % (4) | 5.6 % (1) | 3.4 % (1) | 8.0 % (2) | 0.7 % (1) | 2.3 % (4) | 0.9 % (1) |
| OR (95 % CI) | 3.4 (1.6–7.0) | 3.3 (1.4–7.6) | 5.0 (1.7–14.4) | 3.7 (0.5–28.4) | 2.5 (0.3–19.1) | 3.6 (0.8–15.8) | 0.4 (0.1–2.9) | 1.5 (0.5–4.1) | 0.6 (0.1–4.2) | |
| ADHD symptoms | 4.0 % | 5.9 % (8) | 6.3 % (7) | 7.0 % (3) | 5.6 % (1) | 6.5 % (2) | 4.2 % (1) | 2.6 % (4) | 4.7 % (8) | 2.8 % (3) |
| OR (95 % CI) | 1.3 (0.6–2.7) | 1.4 (0.7–3.1) | 1.5 (0.4–4.8) | 1.3 (0.2–9.9) | 2.0 (0.5–8.6) | 0.8 (0.1–5.9) | 0.5 (0.2–1.5) | 1.0 (0.5–2.1) | 0.7 (0.2–2.1) | |
| Aggressive symptoms | 4.1 % | 8.1 % (11) | 8.0 % (9) | 7.0 % (3) | 5.6 % (1) | * 12.9 % (4) | 8.3 % (2) | 3.2 % (5) | 2.3 % (4) | 2.7 % (3) |
| OR (95 % CI) | 1.8 (1.0–3.4) | 1.8 (0.9–3.8) | 1.6 (0.5–5.2) | 1.2 (0.2–9.4) | 3.5 (1.2–10.2) | 1.6 (0.4–6.9) | 0.8 (0.3–1.9) | 0.4 (0.1–1.3) | 0.6 (0.2–2.0) | |
Each cell contains the percentage (No.) of adverse outcomes within groups and corresponding odds ratio (OR) with 95 % CI.
Numbers may not equal 100 % within groups due to variation of missing values. NA = Not applicable. * P value < 0.05.
ORs are adjusted for maternal age, parity, education, smoking, anxiety/depression, periconceptional folate use, and child low birth weight and malformation.
Antiepileptic drug use by father within 6 months to conception.
Children of fathers with epilepsy were included to evaluate potential genetic or socioeconomic effects of parental epilepsy on child outcome. Fathers with epilepsy were categorized according to antiepileptic drug-treatment within the last 6 months prior to conception. The distinction between fathers with and without antiepileptic treatment was used as a proxy for paternal disease severity. Fathers whose partners also had epilepsy (n=4) were excluded.
Measures of development
Mothers’ reports on motor development, language, and social behaviour provided the basis for the main outcome variables. The parent-reported screening tools in MoBa are based on standardized and validated scales constructed to identify difficulties within each developmental domain, and suited for research (Dale et al. 2003;Dumont-Mathieu & Fein 2005;Gollenberg et al. 2010;Novik 1999;Richter & Janson 2007;Robins et al. 2001;Snow & Lecavalier 2008;Swinkels et al. 2006). The instruments typically assess whether a child has reached critical developmental milestones (Snow & Lecavalier 2008). Due to space-limitations in the MoBa-questionnaires, some scales are represented with selected items from the original scales, aimed at items that are easily observable by parents and representative for each developmental domain. Total score on the scales were dichotomised by cut-off values into adverse or normal range.
The Ages and Stages Questionnaire (ASQ) assessed motor skills at 18 and 36 months, and communication skills at 36 months (Richter & Janson 2007;Squires et al. 1997). Social skills at 18 months were assessed by the ASQ personal-social subscale, measuring the child’s ability to play and interact with the caregiver. Sentence complexity at 36 months was measured based on Dale et al. (Dale et al. 2003), assessing the child’s typical level of sentence completeness. The 40-item Social Communication Questionnaire was applied to investigate autistic traits at 36 months, with a cut-off score at 15 or more positive items (Rutter 2006;Snow & Lecavalier 2008). To identify children with different abnormalities and onset-patterns, autistic traits at 18 months were measured by two separate checklists, focusing on different sets of symptoms and with different thresholds. A 23-item screening-instrument based on the Modified Checklist for Autism in Toddlers (MCHAT) was applied to detect children with a greater range of autistic traits, using a cut-off at two or more out of the six critical items or any three items of the total scale (Dumont-Mathieu & Fein 2005;Snow & Lecavalier 2008). To capture children with more severely affected behaviour at 18 months, the 14-item Early Screening of Autistic Traits questionnaire (ESAT) was applied, focusing on unusual features such as mannerisms and sensory abnormality, with cut-off value at three or more positive items (Swinkels et al. 2006). ADHD (Attention Deficit Hyperactivity Disorder) symptoms at 36 months were measured by a MoBa-specific checklist, including six items from the Child Behaviour Checklist (Achenbach et al. 1987;Novik 1999) and five items on inattention and hyperactivity from ADHD criteria in the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV). Aggressiveness at 36 months was assessed by seven items on externalising aggressive behaviour from the Child Behaviour Checklist. All included items are listed in the eAppendix (online supplemental material).
To recognize children with true impairment, adverse outcomes on scales without predefined cut-off values were chosen based on rarely observed scores in the reference group (95–98 percentile range), corresponding to a cut-off value ≥ three standard deviations of the mean for the Ages and Stages Questionnaires, and ≥ two standard deviations of the mean for the ADHD checklist and aggressive symptoms. Previous MoBa studies on the applied screening instruments have shown good reliability, especially for developmental scores that are distant from the mean (Brandlistuen et al. 2010;Brandlistuen et al. 2011;Schjolberg et al. 2011;Stene-Larsen et al. 2009).
Perinatal outcome
Data on birth outcome was obtained from the national Medical Birth Registry, including all pregnancies at 12 or more weeks of gestation. Medical information is collected during delivery by the attending physician and midwife, including compulsory notification of maternal epilepsy and drug-use. Selected adverse outcomes were preterm delivery (< 37 weeks), low birth weight (< 2500 grams), small for gestational age (SGA) birth weight (< 10th percentile) (Skjaerven et al. 2000), and low Apgar score (< 7 at 5 min.). Congenital malformations included major malformations diagnosed during the neonatal period or during pediatric follow-up within the first year. The Medical Birth Registry’s definition includes malformations causing functional impairment, leading to surgical intervention, or both.
Statistical analysis
In the primary statistical analyses, developmental outcomes in children of parents with epilepsy were compared to the reference base, using data from the full cohort of 108,976 cases and non-cases. The outcomes were analyzed according to maternal and paternal epilepsy, with and without prenatal exposure to antiepileptic drugs.
The analyses were performed using SPSS for Windows (SPSS Inc., Chicago, IL, USA). Baseline maternal characteristics collected in the first questionnaire were compared using Fisher’s exact Test. The relative risk of adverse developmental outcomes was estimated as odds ratios (ORs), using unconditional logistic regression and adjustment for potential confounders. Two-sided p-values ≤ 0.05 were considered statistically significant. Results are presented as crude frequencies and adjusted odds ratios (ORs) with the corresponding 95 % confidence interval (CI) and p-values. Categorical co-variates were: Maternal completed level of education (0–9 years, 10–12 years, 13–16 years, ≥ 17 years), maternal depression/anxiety (8-item short version of Hopkins Symptom Checklist) (Tambs & Moum 1993), smoking during pregnancy (yes/no), folate supplementation (preconception and/or during 1st trimester), child’s birth order (1st, 2nd, ≥ 3), low birth weight, and major congenital malformation. Maternal age was included as a continuous co-variate. Multiple births were similar in the epilepsy and reference group (2.1 % vs. 1.7 %), and were not adjusted for.
Explorative and sensitivity analyses
Choice of all included confounders in the logistic regression analyses was based on a priori considerations. Post-hoc, the effect of each included co-variate in the model was evaluated by comparing crude ORs to adjusted ORs. Stratified analysis and Breslow-Day homogeneity tests were also performed to assess potential effect-modification. Adverse perinatal outcomes in the epilepsy groups were examined to assess child comorbidity and potential related bias. To evaluate the effect of baseline differences between the exposed group and the reference group, propensity score matching was performed (D'Agostino, Jr. 1998), and risk-estimates for the matched groups were compared to the primary analysis (eAppendix: eTable 1 and eTable 2). Each child in the antiepileptic drug-exposed group was matched to four children in the reference group with identical or similar propensity scores. The predictors in the propensity score model included maternal age, parity, education, income, single parenting, unplanned pregnancy, folate supplementation, smoking, anxiety/depression, child congenital malformation and low birth weight.
Missing values
The various developmental scales had few missing values, on average 3.5 % (0.7–6.9 %) for children in the reference group, 3.7 % (0.6–8.4 %) for children of mothers with untreated epilepsy, 4.8 % (1.6–10.4 %) for antiepileptic drug-treated mothers, and 3.3 % (0.0–6.3 %) for paternal epilepsy. The exception was the 40-item SCQ, where 85 % had complete data, while 8.9 % was missing one value within the scale. To avoid potential sample distortions caused by missing data, a maximum likelihood estimation procedure was applied to impute missing values (Croy & Novins 2005). Similarly, imputation of missing values on maternal education (5.1%) was estimated using data on maternal and paternal income, and on paternal education. Less than 1 % had missing data on maternal smoking, parity, and age. Developmental scores with ≥ 20 % missing data were excluded.
Results
Epilepsy group
Overall, 726 children (0.7 %) in 711 pregnancies were born to 634 women with reported epilepsy (Table 1). Exposure to antiepileptic drugs during pregnancy was reported for 46 % of the children (n=333). 104 (31 %) had been exposed to monotherapy with lamotrigine, 69 (21 %) with carbamazepine, and 40 (12 %) with valproate. Other monotherapy-exposures in utero were levetiracetam (n=17), topiramate (n=10), oxcarbazepine (n=9), clonazepam (n=7), phenytoin (n=4), phenobarbital (n=4), gabapentin (n=3), primidone (n=1), clobazam (n=1), and unspecified (n=2). Polytherapy with antiepileptic drugs was recorded for 19 % (n=62), of which the majority was exposed to two antiepileptic drugs (n=52). A total of 653 children had a father with reported epilepsy, of which 37 % used antiepileptic drugs within 6 months prior to conception.
Developmental outcomes
Mothers of children exposed to antiepileptic drugs reported more concerns about their child’s development, and also more referrals to a specialist due to delayed motor development (5.5 % vs. 2.4 %; unadjusted OR, 2.7; CI, 1.3–4.4; p = 0.008), language delay (3.5 % vs. 1.2 %; unadjusted OR, 3.0; CI, 1.4–6.3; p = 0.01), or autistic traits (1.5 % vs. 0.1 %; unadjusted OR, 17.0; CI, 4.1–71.0; p = 0.007). Such referrals were not more frequent for children of mothers with epilepsy not treated with antiepileptic drugs, or for children of fathers with epilepsy.
Children exposed to antiepileptic drugs in utero more often scored outside the normal range for motor skills, autistic traits, personal-social skills, and language (Table 2 and Table 3). In affected children, the majority (on average 70 %) had adverse score in a single developmental domain at each assessment point. The exception was polytherapy-exposed children, where 55 % were impaired in more than one domain.
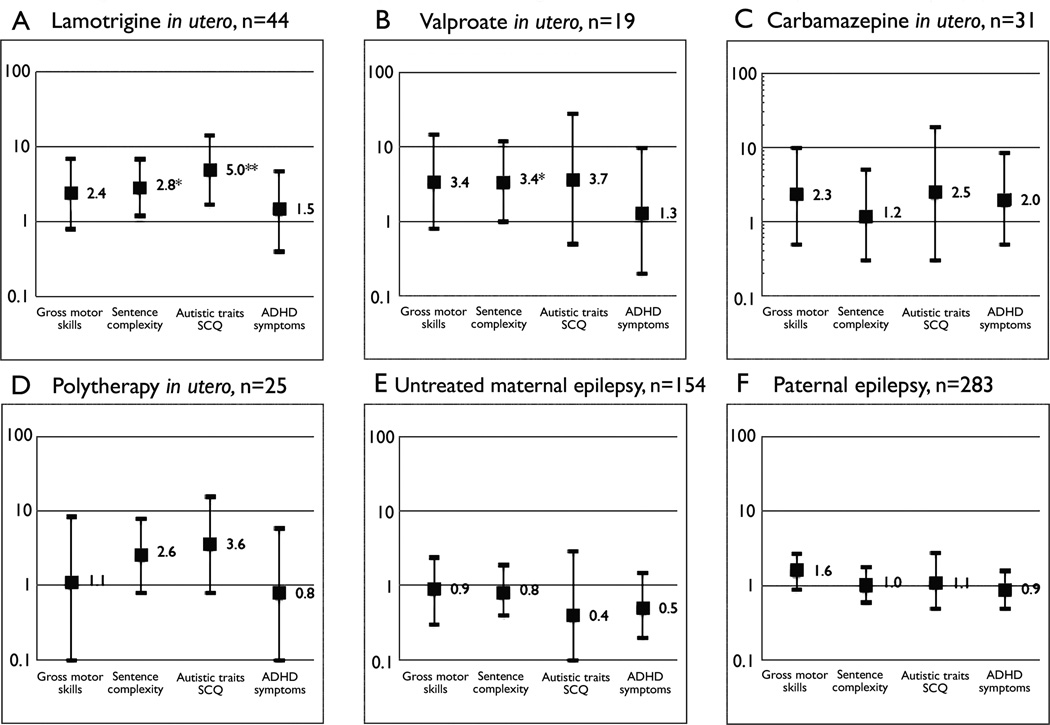
Children exposed to lamotrigine in utero had a higher risk for adverse score on autistic traits and language at 36 months (Table 3, Figure 1A). Carbamazepine-exposure was associated with impaired fine-motor and personal-social skills at 18 months (Table 2) and aggressive symptoms at 36 months (Table 3). Valproate-exposure was associated with adverse gross-motor skills at 18 months (Table 2) and language at 36 months (Table 3, Figure 1B). Children exposed to polytherapy with antiepileptic drugs more often scored outside the normal range for all developmental measures at 18 months (Table 2). Several risks were elevated but not significantly increased for the relatively small groups of valproate-exposed and polytherapy-exposed children at 36 months (Table 3, Figure 1B and 1D).
Figure 1.
Adjusted odds ratio with 95 % confidence interval (log scale) for adverse development in children of parents with epilepsy compared to the reference group. SCQ: Social Communication Questionnaire (Previously Autism Screening Questionnaire).
* P value < 0.05. ** P value < 0.01.
In the drug-exposed group, the positive predictive value for the various screening tools at 18 months ranged between 65 and 83 %, predicting developmental difficulties within one or several of the domains measured at 36 months.
Children of mothers with epilepsy not treated with antiepileptic drugs scored within the normal range for all developmental measures at 18 and 36 months (Table 2 and Table 3, Figure 1E).
Children of fathers with epilepsy were similar to the reference group at 36 months (Table 3, Figure 1F), but had a higher risk for positive screening on autistic traits and poor social skills at 18 months if the father used antiepileptic drugs (Table 2).
ADHD-symptoms were not increased in any group (Table 3, Figure 1A–F).
Validation-study
Mothers of 40 children in the epilepsy group were included in the validation-study. According to the hospital records, 52.5 % were classified as focal epilepsy, 25.0 % as primary generalized epilepsy, and 17.5 % as unspecified epilepsy with generalized seizures. In the subset of those with primary generalized epilepsy, 15.0 % had juvenile myoclonic epilepsy. The epilepsy-diagnosis could not be verified for two of the untreated mothers. There was 100 % agreement between the mothers’ reports of antiepileptic drug-use during pregnancy and drug-use registered in hospital records. 52.5 % of the mothers with validated epilepsy used antiepileptic drugs during pregnancy. Untreated mothers (47.5 %) generally had inactive epilepsy, as only one had experienced seizures within 2 years prior to the pregnancy. Six mothers (15 %) experienced seizures during pregnancy, five of them in the drug-treated group. There were no seizures during delivery.
Sensitivity analyses
Adverse perinatal outcomes in the epilepsy groups are presented in the eAppendix (eTable 3). The risk of major congenital malformation was increased in children exposed to antiepileptic drugs in utero, and highest for polytherapy. The risk of malformations in children exposed to lamotrigine was high compared to earlier reports (Tomson & Battino 2012). However, excluding children with major malformations had minimal effect on the risk-estimates in the antiepileptic drug-exposed groups (eTable 4). The risk of other adverse perinatal outcomes was as expected in the epilepsy group (Veiby et al. 2009).
In general, the risk-estimates in the drug-exposed group were negligibly affected by adjustment for potential confounders (less than 10 % change in crude ORs). In the stratified analyses, children with low birth weight and children of mothers with depression/anxiety had an increased risk of autistic traits at age 18 months. Otherwise, none of the co-variates acted as significant effect-modifiers within the drug-exposed group. The risk-estimates secondary to propensity score matching were generally comparable to the primary analysis (eTable 1 and eTable 2).
Maternal reports of specific child health problems other than impaired development (hyperactivity, asthma, allergy, heart defects, impaired vision or hearing, weight-problems, and febrile or epileptic seizures) were not increased in any of the epilepsy groups.
Discussion
Key findings
Children exposed to antiepileptic drugs in utero had an increased risk of adverse outcome in the most important developmental domains at 18 and 36 months of age, based on mothers rating of the child using validated screening tools. Scores on motor skills and language were poorer compared to children of parents without epilepsy, and they had more autistic traits. The relatively small groups of lamotrigine, valproate, carbamazepine, and polytherapy were all associated with adverse outcomes. The increased risks could not be ascribed to differences in malformations, low birth weight, or periconceptional folate supplementation. The affected developmental scores were supported by the mothers’ reporting more concerns about their child’s development and more referrals to a specialist; indicators shown to predict developmental difficulties (Rosenbaum 1998;Rydz et al. 2005).
Children of mothers with epilepsy not using antiepileptic drugs had risks similar to children of parents without epilepsy. Children of fathers with epilepsy scored within the normal range for nearly all developmental measures, and had malformation risk similar to the reference group.
ADHD symptoms were not increased in any group at this low age.
Interpretation
To our knowledge, this is the first large-scale population-based study on early developmental outcomes in offspring of parents with epilepsy, including also comparative analyses with children of fathers with epilepsy. Our findings indicate that antiepileptic drugs during pregnancy influence different developmental domains, with specific effects on motor development, language, behavior, and social interaction. Children exposed to antiepileptic drugs in fetal life had increased risk of major malformations and low birth weight, in line with previous reports (Harden et al. 2009). However, adverse development in the antiepileptic drug-exposed children could not be ascribed to malformations; supporting the hypothesis that behavioral and cognitive teratogenesis represents a distinct process. Antiepileptic drugs potentially affect fetal CNS-development throughout pregnancy and at multiple stages (Ikonomidou & Turski 2010;Kluger & Meador 2008). Several studies report lower IQ scores and psychomotor effects in antiepileptic drug-exposed children (Adab et al. 2004;Forsberg et al. 2011;Meador et al. 2009;Meador et al. 2012;Vinten et al. 2009;Wide et al. 2002), and associations with behavioral and autism spectrum disorders (ASD) have been suggested (Bromley et al. 2008;Rasalam et al. 2005). In our study, prenatal exposure to antiepileptic drugs was associated with more autistic traits at both 18 and 36 months. Autistic traits in very young children can predict general cognitive delay and behavioral problems as well as ASD (Moricke et al. 2010). Conversely, children with regressive autism develop normally during the first and second year of life, until they begin to lose previously acquired skills. Parental report of autistic traits in children older than two years is more specific and stable over time (Charman et al. 2004;Norris & Lecavalier 2010). Thus, the SCQ at 36 months provided the most reliable autism-screening tool in this study, capturing all the core features, and being highly suggestive of ASD. In the present study, all the antiepileptic drug-exposed groups were associated with more autistic traits at 36 months, including lamotrigine, which has not been demonstrated previously. Meador et al. found normal IQ scores in three-year old children exposed prenatally to lamotrigine (Meador et al. 2009), but follow-up studies reported lower verbal abilities in such children (Meador et al. 2011;Meador et al. 2012), consistent with our findings. Bromley et al. demonstrated possible specific deficits in two year-olds exposed to lamotrigine (nonverbal abilities, hand-eye coordination), but normal overall development (Bromley et al. 2010).
The higher frequency of autistic traits at 18 months in children of drug-treated fathers with epilepsy indicates developmental difficulties, but probably not specific for ASD as this was not observed at 36 months. Drug-treated fathers probably have similar epilepsy characteristics as drug-treated mothers. Thus, signs of adverse development in the offspring of both groups probably reflect a genetic disposition related to parental epilepsy itself. Alternatively, antiepileptic drugs might theoretically exert harmful effects on the male gamete. However, children of fathers treated with antiepileptic drugs generally contrasted markedly with the outcome for children of mothers treated with antiepileptic drugs, excluding genetic factors as a main cause. Epilepsy type and severity is probably different for mothers using antiepileptic drugs compared to untreated mothers. Still, the lack of effects on perinatal outcome and development in children of untreated mothers indicate that the risks are mainly related to the antiepileptic drug exposure, and not to seizures or other maternal characteristics.
Study strengths and limitations
This study included children of mothers with and without antiepileptic drug treatment, and of fathers with epilepsy. The epilepsy groups were followed prospectively in a large cohort, using validated screening tools to assess child development. This provided a very large reference group, as the study was not restricted by the resource-limitations that usually apply to methods involving professional assessment. Measures of development were based on mothers’ rating of their child. Such screening does not correspond directly to a diagnosis made by professionals, but still provide accurate information about early child behavior and development (Rydz et al. 2005;Sachse & Von 2008;Squires et al. 1997). Whereas child performance in test-situations can be unpredictable and vary at different times, screening tools measure the child’s typical behaviour in a manner that is easily accessible to the caregivers, and suited for research. However, potential information bias affecting maternal rating of child development is a concern. Women with epilepsy may be more prone to anxiety about their child’s health, given that the association between birth defects and antiepileptic drugs has been known for decades. Maternal report of medical problems other than impaired development was not increased for any of the epilepsy groups, indicating that mothers with epilepsy were not inclined to reinforce difficulties in their children. Furthermore, adjusting for maternal anxiety and depression should provide some control for potential reporter bias, as well as effects of maternal mood disorders on child development. Additional analyses with stratification and propensity-score matching suggest that the results were not due to differences in baseline variables. However, residual confounding due to unmeasured parameters, such as mothers’ cognitive status or severity and type of epilepsy, might perhaps explain some of the observed drug-exposure associations.
Limitations due to small sample size may have influenced the results, especially in the drug-exposed subgroups at 36 months. Several risk-estimates tended to be elevated, but the number of children may have been too low to capture problems within specific domains. Also, the cut-off values for adverse outcomes were defined very strictly. We cannot exclude that some of the children scoring within the normal-range had mild developmental problems.
Lack of information on antiepileptic drug-dose represents a limitation. Risk-estimates were especially high for polytherapy-exposure. The effect of total drug-load during pregnancy as opposed to the effect of different drug-combinations is an important aspect that could not be fully explored. The high number of tested associations might have resulted in some spurious associations.
Generalizability
Mothers and fathers in MoBa were recruited from a population-based national cohort. The participation rate of 38.5 % at first assessment is as expected for population-based studies (Nohr et al. 2006). Yet, potential systematic differences among participants compared to non-participants are a concern. A study investigating selection bias in MoBa (Nilsen et al. 2009) concluded that the selected exposure-outcome associations were not biased, but that prevalence estimates could be affected. However, the prevalence estimates for epilepsy did not differ from the total population. The frequency of antiepileptic drug-treated (0.3 %) and untreated (0.4 %) maternal epilepsy was similar to that of an unselected birth registry cohort including all Norwegian births during the same time period (Veiby et al. 2009). The relatively high frequency of malformations in children exposed to lamotrigine could indicate selection bias in this group, with potential influence on exposure-outcome associations. However, excluding children with malformations did not weaken the risk of adverse development in the lamotrigine-group, or in the other drug-exposed groups.
The decline in participation rate through the questionnaires was similar between the epilepsy and the reference group. Furthermore, there were no major differences comparing drug-exposed children that were lost to follow-up and those studied at 18 and 36 months (eAppendix: eTable 5).
Conclusion
Exposure to antiepileptic drugs in utero is associated with adverse development, measured as low scores within key developmental domains rated by mothers. Polytherapy, lamotrigine, valproate, and carbamazepine were associated with an increased rate of adverse development within several domains. Treatment that provides optimal seizure control is important during pregnancy, but should be balanced against potential effects on the fetal brain. Difficulties at an early age, i.e. minor motor delay, can be temporary, but other effects may continue or even worsen as the child develops. Further research should focus on in utero effects of specific antiepileptic drugs in larger cohorts, and whether such effects persist into school-age and adulthood.
Supplementary Material
Acknowledgements
This work was supported by the Norwegian Association for Epilepsy. We are grateful to all the participating families in Norway who take part in this ongoing cohort study.
The Norwegian Mother and Child Cohort Study is supported by the Norwegian Ministry of Health and the Ministry of Education and Research; the National Institutes of Health [contract no NO1-ES-75558, grants number 1 UO1 NS047537-01, 2 UO1 NS047537-06A1]; and the Norwegian Research Council [grant number 151918/S10].
Author G Veiby has received travel support from UCB Pharma and lecture fee from GlaxoSmithKline. Author B Engelsen has received travel support from GlaxoSmithKline and lecture fee from Lundbeck.
Footnotes
Disclosures
The remaining authors have no conflicts of interest.
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
References
- Achenbach TM, Edelbrock C, Howell CT. Empirically based assessment of the behavioral/emotional problems of 2- and 3- year-old children. J.Abnorm.Child Psychol. 1987;15:629–650. doi: 10.1007/BF00917246. [DOI] [PubMed] [Google Scholar]
- Adab N, Kini U, Vinten J, Ayres J, Baker G, Clayton-Smith J, Coyle H, Fryer A, Gorry J, Gregg J, Mawer G, Nicolaides P, Pickering L, Tunnicliffe L, Chadwick DW. The longer term outcome of children born to mothers with epilepsy. J.Neurol.Neurosurg.Psychiatry. 2004;75:1575–1583. doi: 10.1136/jnnp.2003.029132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banach R, Boskovic R, Einarson T, Koren G. Long-term developmental outcome of children of women with epilepsy, unexposed or exposed prenatally to antiepileptic drugs: a meta-analysis of cohort studies. Drug Saf. 2010;33:73–79. doi: 10.2165/11317640-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Brandlistuen RE, Stene-Larsen K, Holmstrom H, Landolt MA, Eskedal LT, Vollrath ME. Motor and social development in 6-month-old children with congenital heart defects. J.Pediatr. 2010;156:265–269. doi: 10.1016/j.jpeds.2009.08.035. [DOI] [PubMed] [Google Scholar]
- Brandlistuen RE, Stene-Larsen K, Holmstrom H, Landolt MA, Eskedal LT, Vollrath ME. Symptoms of communication and social impairment in toddlers with congenital heart defects. Child Care Health Dev. 2011;37:37–43. doi: 10.1111/j.1365-2214.2010.01148.x. [DOI] [PubMed] [Google Scholar]
- Bromley RL, Mawer G, Clayton-Smith J, Baker GA. Autism spectrum disorders following in utero exposure to antiepileptic drugs. Neurology. 2008;71:1923–1924. doi: 10.1212/01.wnl.0000339399.64213.1a. [DOI] [PubMed] [Google Scholar]
- Bromley RL, Mawer G, Love J, Kelly J, Purdy L, McEwan L, Briggs M, Clayton-Smith J, Sin X, Baker GA. Early cognitive development in children born to women with epilepsy: a prospective report. Epilepsia. 2010;51:2058–2065. doi: 10.1111/j.1528-1167.2010.02668.x. [DOI] [PubMed] [Google Scholar]
- Charman T, Howlin P, Berry B, Prince E. Measuring developmental progress of children with autism spectrum disorder on school entry using parent report. Autism. 2004;8:89–100. doi: 10.1177/1362361304040641. [DOI] [PubMed] [Google Scholar]
- Croy CD, Novins DK. Methods for addressing missing data in psychiatric and developmental research. J.Am.Acad.Child Adolesc.Psychiatry. 2005;44:1230–1240. doi: 10.1097/01.chi.0000181044.06337.6f. [DOI] [PubMed] [Google Scholar]
- D'Agostino RB., Jr. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat.Med. 1998;17:2265–2281. doi: 10.1002/(sici)1097-0258(19981015)17:19<2265::aid-sim918>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Dale PS, Price TS, Bishop DV, Plomin R. Outcomes of early language delay: I. Predicting persistent and transient language difficulties at 3 and 4 years. J.Speech Lang Hear.Res. 2003;46:544–560. doi: 10.1044/1092-4388(2003/044). [DOI] [PubMed] [Google Scholar]
- Dumont-Mathieu T, Fein D. Screening for autism in young children: The Modified Checklist for Autism in Toddlers (M-CHAT) and other measures. Ment.Retard.Dev.Disabil.Res.Rev. 2005;11:253–262. doi: 10.1002/mrdd.20072. [DOI] [PubMed] [Google Scholar]
- Fairgrieve SD, Jackson M, Jonas P, Walshaw D, White K, Montgomery TL, Burn J, Lynch SA. Population based, prospective study of the care of women with epilepsy in pregnancy. BMJ. 2000;321:674–675. doi: 10.1136/bmj.321.7262.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsberg L, Wide K, Kallen B. School performance at age 16 in children exposed to antiepileptic drugs in utero--a population-based study. Epilepsia. 2011;52(2):364–369. doi: 10.1111/j.1528-1167.2010.02778.x. available from: PM:21054354. [DOI] [PubMed] [Google Scholar]
- Gollenberg AL, Lynch CD, Jackson LW, McGuinness BM, Msall ME. Concurrent validity of the parent-completed Ages and Stages Questionnaires, 2nd Ed. with the Bayley Scales of Infant Development II in a low-risk sample. Child Care Health Dev. 2010;36:485–490. doi: 10.1111/j.1365-2214.2009.01041.x. [DOI] [PubMed] [Google Scholar]
- Harden CL, Meador KJ, Pennell PB, Hauser WA, Gronseth GS, French JA, Wiebe S, Thurman D, Koppel BS, Kaplan PW, Robinson JN, Hopp J, Ting TY, Gidal B, Hovinga CA, Wilner AN, Vazquez B, Holmes L, Krumholz A, Finnell R, Hirtz D, Le GC. Practice parameter update: management issues for women with epilepsy--focus on pregnancy (an evidence-based review): teratogenesis and perinatal outcomes: report of the Quality Standards Subcommittee and Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology and American Epilepsy Society. Neurology. 2009;73:133–141. doi: 10.1212/WNL.0b013e3181a6b312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes LB, Mittendorf R, Shen A, Smith CR, Hernandez-Diaz S. Fetal effects of anticonvulsant polytherapies: different risks from different drug combinations. Arch.Neurol. 2011;68:1275–1281. doi: 10.1001/archneurol.2011.133. [DOI] [PubMed] [Google Scholar]
- Ikonomidou C, Turski L. Antiepileptic drugs and brain development. Epilepsy Res. 2010;88:11–22. doi: 10.1016/j.eplepsyres.2009.09.019. [DOI] [PubMed] [Google Scholar]
- Kluger BM, Meador KJ. Teratogenicity of antiepileptic medications. Semin.Neurol. 2008;28:328–335. doi: 10.1055/s-2008-1079337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnus P, Irgens LM, Haug K, Nystad W, Skjaerven R, Stoltenberg C. Cohort profile: the Norwegian Mother and Child Cohort Study (MoBa) Int.J.Epidemiol. 2006;35:1146–1150. doi: 10.1093/ije/dyl170. [DOI] [PubMed] [Google Scholar]
- Meador KJ, Baker GA, Browning N, Clayton-Smith J, Combs-Cantrell DT, Cohen M, Kalayjian LA, Kanner A, Liporace JD, Pennell PB, Privitera M, Loring DW. Cognitive function at 3 years of age after fetal exposure to antiepileptic drugs. N.Engl.J.Med. 2009;360:1597–1605. doi: 10.1056/NEJMoa0803531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meador KJ, Baker GA, Browning N, Cohen MJ, Bromley RL, Clayton-Smith J, Kalayjian LA, Kanner A, Liporace JD, Pennell PB, Privitera M, Loring DW. Effects of fetal antiepileptic drug exposure: Outcomes at age 4.5 years. Neurology. 2012;78:1207–1214. doi: 10.1212/WNL.0b013e318250d824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meador KJ, Baker GA, Browning N, Cohen MJ, Clayton-Smith J, Kalayjian LA, Kanner A, Liporace JD, Pennell PB, Privitera M, Loring DW. Foetal antiepileptic drug exposure and verbal versus non-verbal abilities at three years of age. Brain. 2011:134396–404. doi: 10.1093/brain/awq352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molgaard-Nielsen D, Hviid A. Newer-generation antiepileptic drugs and the risk of major birth defects. JAMA. 2011;305(19):1996–2002. doi: 10.1001/jama.2011.624. [DOI] [PubMed] [Google Scholar]
- Moricke E, Swinkels SH, Beuker KT, Buitelaar JK. Predictive value of subclinical autistic traits at age 14–15 months for behavioural and cognitive problems at age 3–5 years. Eur.Child Adolesc.Psychiatry. 2010;19:659–668. doi: 10.1007/s00787-010-0103-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadebaum C, Anderson VA, Vajda F, Reutens DC, Barton S, Wood AG. Language skills of school-aged children prenatally exposed to antiepileptic drugs. Neurology. 2011;76:719–726. doi: 10.1212/WNL.0b013e31820d62c7. [DOI] [PubMed] [Google Scholar]
- Nilsen RM, Vollset SE, Gjessing HK, Skjaerven R, Melve KK, Schreuder P, Alsaker ER, Haug K, Daltveit AK, Magnus P. Self-selection and bias in a large prospective pregnancy cohort in Norway. Paediatr.Perinat.Epidemiol. 2009;23:597–608. doi: 10.1111/j.1365-3016.2009.01062.x. [DOI] [PubMed] [Google Scholar]
- Nohr EA, Frydenberg M, Henriksen TB, Olsen J. Does low participation in cohort studies induce bias? Epidemiology. 2006;17:413–418. doi: 10.1097/01.ede.0000220549.14177.60. [DOI] [PubMed] [Google Scholar]
- Norris M, Lecavalier L. Screening accuracy of Level 2 autism spectrum disorder rating scales. A review of selected instruments. Autism. 2010;14:263–284. doi: 10.1177/1362361309348071. [DOI] [PubMed] [Google Scholar]
- Novik TS. Validity of the Child Behaviour Checklist in a Norwegian sample. Eur.Child Adolesc.Psychiatry. 1999;8:247–254. doi: 10.1007/s007870050098. [DOI] [PubMed] [Google Scholar]
- Rasalam AD, Hailey H, Williams JH, Moore SJ, Turnpenny PD, Lloyd DJ, Dean JC. Characteristics of fetal anticonvulsant syndrome associated autistic disorder. Dev.Med.Child Neurol. 2005;47:551–555. doi: 10.1017/s0012162205001076. [DOI] [PubMed] [Google Scholar]
- Richter J, Janson H. A validation study of the Norwegian version of the Ages and Stages Questionnaires. Acta Paediatr. 2007;96:748–752. doi: 10.1111/j.1651-2227.2007.00246.x. [DOI] [PubMed] [Google Scholar]
- Robins DL, Fein D, Barton ML, Green JA. The Modified Checklist for Autism in Toddlers: an initial study investigating the early detection of autism and pervasive developmental disorders. J.Autism Dev.Disord. 2001;31:131–144. doi: 10.1023/a:1010738829569. [DOI] [PubMed] [Google Scholar]
- Rosenbaum P. Screening tests and standardized assessments used to identify and characterize developmental delays. Semin.Pediatr.Neurol. 1998;5:27–32. doi: 10.1016/s1071-9091(98)80015-6. [DOI] [PubMed] [Google Scholar]
- Roth C, Magnus P, Schjolberg S, Stoltenberg C, Suren P, McKeague IW, Davey SG, Reichborn-Kjennerud T, Susser E. Folic acid supplements in pregnancy and severe language delay in children. JAMA. 2011;306(14):1566–1573. doi: 10.1001/jama.2011.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter M. Autism: its recognition, early diagnosis, and service implications. J.Dev.Behav.Pediatr. 2006;27(2 Suppl):S54–S58. doi: 10.1097/00004703-200604002-00002. [DOI] [PubMed] [Google Scholar]
- Rydz D, Shevell MI, Majnemer A, Oskoui M. Developmental screening. J.Child Neurol. 2005;20:4–21. doi: 10.1177/08830738050200010201. [DOI] [PubMed] [Google Scholar]
- Sachse S, Von SW. Early identification of language delay by direct language assessment or parent report? J.Dev.Behav.Pediatr. 2008;29:34–41. doi: 10.1097/DBP.0b013e318146902a. [DOI] [PubMed] [Google Scholar]
- Schjolberg S, Eadie P, Zachrisson HD, Oyen AS, Prior M. Predicting language development at age 18 months: data from the norwegian mother and child cohort study. J.Dev.Behav.Pediatr. 2011;32:375–383. doi: 10.1097/DBP.0b013e31821bd1dd. [DOI] [PubMed] [Google Scholar]
- Skjaerven R, Gjessing HK, Bakketeig LS. Birthweight by gestational age in Norway. Acta Obstet.Gynecol.Scand. 2000;79(6):440–449. [PubMed] [Google Scholar]
- Snow AV, Lecavalier L. Sensitivity and specificity of the Modified Checklist for Autism in Toddlers and the Social Communication Questionnaire in preschoolers suspected of having pervasive developmental disorders. Autism. 2008;12:627–644. doi: 10.1177/1362361308097116. [DOI] [PubMed] [Google Scholar]
- Squires J, Bricker D, Potter L. Revision of a parent-completed development screening tool: Ages and Stages Questionnaires. J.Pediatr.Psychol. 1997;22:313–328. doi: 10.1093/jpepsy/22.3.313. [DOI] [PubMed] [Google Scholar]
- Stene-Larsen K, Borge AI, Vollrath ME. Maternal smoking in pregnancy and externalizing behavior in 18-month-old children: results from a population-based prospective study. J.Am.Acad.Child Adolesc.Psychiatry. 2009;48:283–289. doi: 10.1097/CHI.0b013e318195bcfb. [DOI] [PubMed] [Google Scholar]
- Stoltenberg C, Schjolberg S, Bresnahan M, Hornig M, Hirtz D, Dahl C, Lie KK, Reichborn-Kjennerud T, Schreuder P, Alsaker E, Oyen AS, Magnus P, Suren P, Susser E, Lipkin WI. The Autism Birth Cohort: a paradigm for gene-environment-timing research. Mol.Psychiatry. 2010;15:676–680. doi: 10.1038/mp.2009.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swinkels SH, Dietz C, van DE, Kerkhof IH, van EH, Buitelaar JK. Screening for autistic spectrum in children aged 14 to 15 months. I: the development of the Early Screening of Autistic Traits Questionnaire (ESAT) J.Autism Dev.Disord. 2006;36:723–732. doi: 10.1007/s10803-006-0115-0. [DOI] [PubMed] [Google Scholar]
- Tambs K, Moum T. How well can a few questionnaire items indicate anxiety and depression? Acta Psychiatr.Scand. 1993;87:364–367. doi: 10.1111/j.1600-0447.1993.tb03388.x. [DOI] [PubMed] [Google Scholar]
- Thomas SV, Sukumaran S, Lukose N, George A, Sarma PS. Intellectual and language functions in children of mothers with epilepsy. Epilepsia. 2007;48:2234–2240. doi: 10.1111/j.1528-1167.2007.01376.x. [DOI] [PubMed] [Google Scholar]
- Titze K, Koch S, Helge H, Lehmkuhl U, Rauh H, Steinhausen HC. Prenatal and family risks of children born to mothers with epilepsy: effects on cognitive development. Dev.Med.Child Neurol. 2008;50:117–122. doi: 10.1111/j.1469-8749.2007.02020.x. [DOI] [PubMed] [Google Scholar]
- Tomson T, Battino D. Teratogenic effects of antiepileptic drugs. Lancet Neurol. 2012;11:803–813. doi: 10.1016/S1474-4422(12)70103-5. [DOI] [PubMed] [Google Scholar]
- Tomson T, Battino D, Bonizzoni E, Craig J, Lindhout D, Sabers A, Perucca E, Vajda F. Dose-dependent risk of malformations with antiepileptic drugs: an analysis of data from the EURAP epilepsy and pregnancy registry. Lancet Neurol. 2011 doi: 10.1016/S1474-4422(11)70107-7. [DOI] [PubMed] [Google Scholar]
- Veiby G, Daltveit AK, Engelsen BA, Gilhus NE. Pregnancy, delivery, and outcome for the child in maternal epilepsy. Epilepsia. 2009;50:2130–2139. doi: 10.1111/j.1528-1167.2009.02147.x. [DOI] [PubMed] [Google Scholar]
- Vinten J, Bromley RL, Taylor J, Adab N, Kini U, Baker GA. The behavioral consequences of exposure to antiepileptic drugs in utero. Epilepsy Behav. 2009;14:197–201. doi: 10.1016/j.yebeh.2008.10.011. [DOI] [PubMed] [Google Scholar]
- Wallace H, Shorvon S, Tallis R. Age-specific incidence and prevalence rates of treated epilepsy in an unselected population of 2,052,922 and age-specific fertility rates of women with epilepsy. Lancet. 1998;352:1970–1973. doi: 10.1016/S0140-6736(98)04512-7. [DOI] [PubMed] [Google Scholar]
- Wide K, Henning E, Tomson T, Winbladh B. Psychomotor development in preschool children exposed to antiepileptic drugs in utero. Acta Paediatr. 2002;91(4):409–414. doi: 10.1080/080352502317371643. available from: PM:12061356. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.