Summary
For acutely lethal influenza infections, the relative pathogenic contributions of direct viral damage to lung epithelium vs. dysregulated immunity remain unresolved. Here we take a top-down systems approach to this question. Multigene transcriptional signatures from infected lungs suggested that elevated activation of inflammatory signaling networks distinguished lethal from sublethal infections. Flow cytometry and gene expression analysis involving isolated cell subpopulations from infected lungs showed that neutrophil influx largely accounted for the predictive transcriptional signature. Automated imaging analysis together with these gene expression and flow data identified a chemokine-driven feed-forward circuit involving pro-inflammatory neutrophils potently driven by poorly contained lethal viruses. Consistent with these data, attenuation but not ablation of the neutrophil-driven response increased survival without changing viral spread. These findings establish the primacy of damaging innate inflammation in at least some forms of influenza-induced lethality and provide a roadmap for the systematic dissection of infection-associated pathology.
Introduction
Influenza causes widespread infection with serious consequences despite available anti-viral drugs and vaccines against seasonal strains. While many deaths from influenza involve the elderly and very young, some influenza A strains cause elevated fatalities among young healthy adults with the extreme example being the 1918 pandemic virus (Taubenberger and Morens, 2006). A central question in such cases is the relative contribution of direct pathogen cytopathicity versus immune or inflammatory damage to disruption of host homeostasis.
During infections with highly pathogenic H1N1 or H5N1, high virus titers and severe illness correlate with a robust, host immune response (Bautista et al., 2010; Beigel et al., 2005; de Jong et al., 2006; Kash et al., 2006; Perrone et al., 2008). Indeed, “immune and cell death responses” was the major gene ontology association distinguishing mild from highly pathogenic infections, based on transcriptome analysis of whole lung tissue samples from infected mice (Kash et al., 2006). One interpretation of these findings was that the innate immune system reached a high level of activation but was unable to contain the pathogen before viral cytopathicity caused loss of lung homeostasis (Boon et al., 2011; Sanders et al., 2011). The inflammatory response is in this view a correlate of the damaging infection, not a major contributor to pathogenesis. Indeed, the depletion of multiple immune cell types attenuated inflammatory cytokine levels in mouse lung homogenates but resulted in elevated pulmonary viral titers, virus spread to remote tissues, and decreased survival (Tumpey et al., 2005). Furthermore, mice with decreased myeloid infiltrates and reduced chemokine and cytokine production due to lack of NLRP3 inflammasome activation show increased susceptibility to influenza A-associated morbidity (Allen et al., 2009; Thomas et al., 2009). Selective neutrophil targeting in infected mice also enhanced disease and mortality (Tate et al., 2009), suggesting that innate inflammatory cells have host beneficial functions rather than a primary causal role in pathology (Brincks et al., 2008; Tate et al., 2012; Tate et al., 2009; Tate et al., 2011b).
An alternative view is that lethality is linked to an excessive innate immune response, especially strong in young healthy adults. This model postulates that lung function is largely dysregulated through the damaging effects of leukocytes on epithelial and endothelial cells (Aldridge et al., 2009; Le Goffic et al., 2006; Lin et al., 2008). Support for this idea came from the discovery that inflammatory monocytes, or monocyte-derived inflammatory macrophages and dendritic cells contributed to fatality (Lin et al., 2008).
To more clearly differentiate host protective from damaging immunity, larger, comprehensive datasets at both the organ and the cell level acquired under realistic infection conditions are needed. Here we analyze influenza-associated lethality using an unbiased, top-down systems approach in mice and show that a virus strain- and dose-dependent early engagement of neutrophils instigates a damaging feed-forward innate inflammatory circuit responsible for acute early death.
Results
Multiplex Perturbation and Modular Transcriptome Analysis Strategy to Identify Influenza-induced, Lethality-associated Biological Processes on the Organ Level
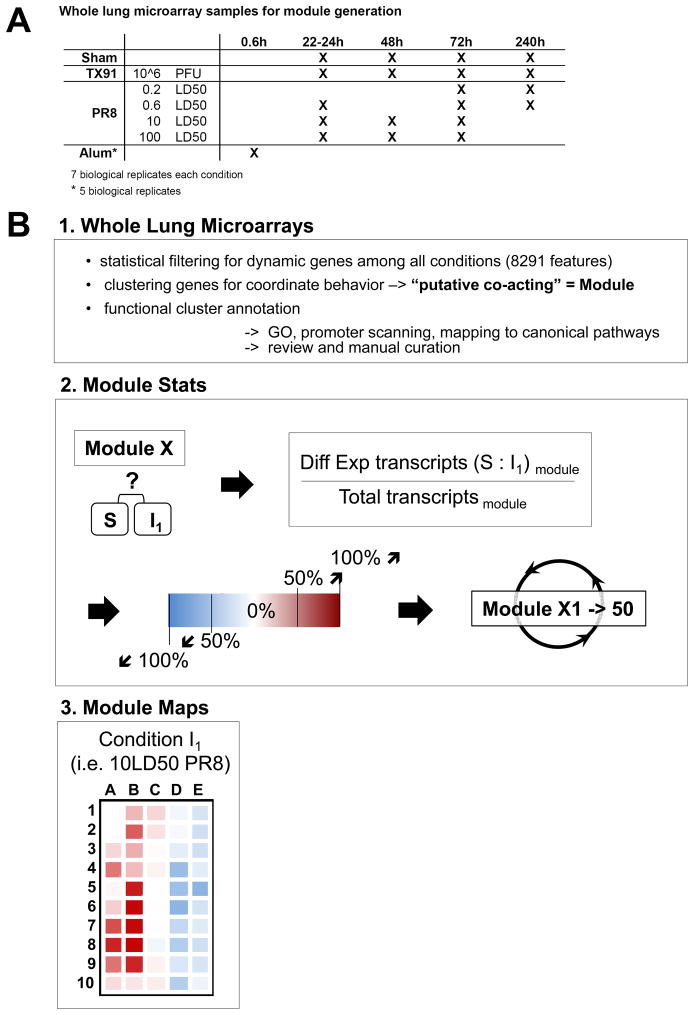
Our top-down system approach combined an extensive matrix of viral strains, infectious doses, time points, and measurements of the host response, including transcriptional studies of infected lungs, flow cytometry, automated image analysis of infected tissue, classical virologic studies, and measurements of physiologic status. We compared infection with the H1N1 virus A/Texas/36/91 (Tx91), which is non-lethal in C57Bl/6 mice at 106 infectious particles and causes transient morbidity seen as minor weight loss (Figure S1A) with infections involving sublethal and lethal doses of the highly pathogenic H1N1 virus A/Puerto Rico/8/34 (PR8), which causes death in less than a week at 600 infectious particles (Figure S1B). PR8 infection in mice is a widely used model system (Allen et al., 2009; Ichinohe et al., 2009; Tate et al., 2011a; Thomas et al., 2009) that mimics pathological features seen with the 1918 virus, (Taubenberger and Morens, 2008; Watanabe and Kawaoka, 2011), including efficient alveolar spread, fatal viral pneumonia, acute pulmonary hemorrhage, and extraordinarily strong host responses.
To obtain a broad view of the biological processes associated with lethal as opposed to non-lethal influenza infection, we first generated gene expression microarrays using RNA extracted from lung samples under 19 experimental conditions. Multiple mice were either sham infected, infected with TX91, or infected with various sublethal or lethal doses of PR8. Lung tissues were collected at various time points (Figure 1A) and whole lung RNA from individual animals used for analysis. We aimed to identify the biological processes that distinguished lethal from non-lethal influenza infection. Because transcripts with a coordinate expression pattern are likely enriched for genes that act together to drive biological processes, we used a modular approach to data analysis. Dynamic genes were defined based on statistical filtering of the microarray data for features that change in at least one of all possible pair-wise comparisons across the 19 experimental conditions (Table S1, Methods). To define putative co-acting biological processes among the 8291 dynamic features identified in this manner, we clustered the features using an optimization strategy that generated 50 modules consisting of gene sets with co-ordinate patterns of regulation when considered over the entire data set. Each module was annotated based on GO-term enrichment and manually curated (Table S2, Methods). Next, we calculated the fraction of genes within each module with differential expression between infectious condition (Ii) and sham condition (S) (Figure 1B), similar to (Chaussabel et al., 2008) and displayed these data as color-coded maps.
Figure 1. Experimental Scheme and Generation of Modular Co-regulated Gene Sets.
(A) Transcript expression data were derived using microarrays from RNA of whole mouse lungs representing 19 experimental conditions each with 7 biological replicates and these data used to define putative co-acting biological processes (modules). (B) Generation of modular gene expression maps. (B1) One-way ANOVA testing assuming unequal variance was used to define dynamic features with a corrected p-value <0.001 based on multiple testing correction. K-means clustering (k=50) was applied to group the 8291 dynamic features across all tested conditions (including an additional group consisting of intranasal alum administration) into 50 modules based on shared expression pattern. The modules were functionally annotated based on highly enriched GO terms. (B2) The fraction of differential expressed features per module was calculated based on lung samples from sham-treated (S) and infected animals (I) and transformed into a color-code. (B3) Matrix maps show the status of all modules for each infectious condition. The statistics for all the 50 modules (X1 to X50) for one representative infectious condition (10LD50 PR8) are shown in a map in which each square from A-1 to E-10 represents one of the 50 modules. The direction of the change (transcript decrease= blue, no change = white, increase = red), and the fractions of genes within each module contributing to the change (intensity of red or blue) were computed for each of the 15 different infectious settings (I1 to I15) to produce a modular map of this type for all tested conditions. See also Figure S1 and Table S1.
Broadly Shared Anti-viral but Condition-specific Innate Inflammatory Signatures Characterize the Response to Influenza Infection
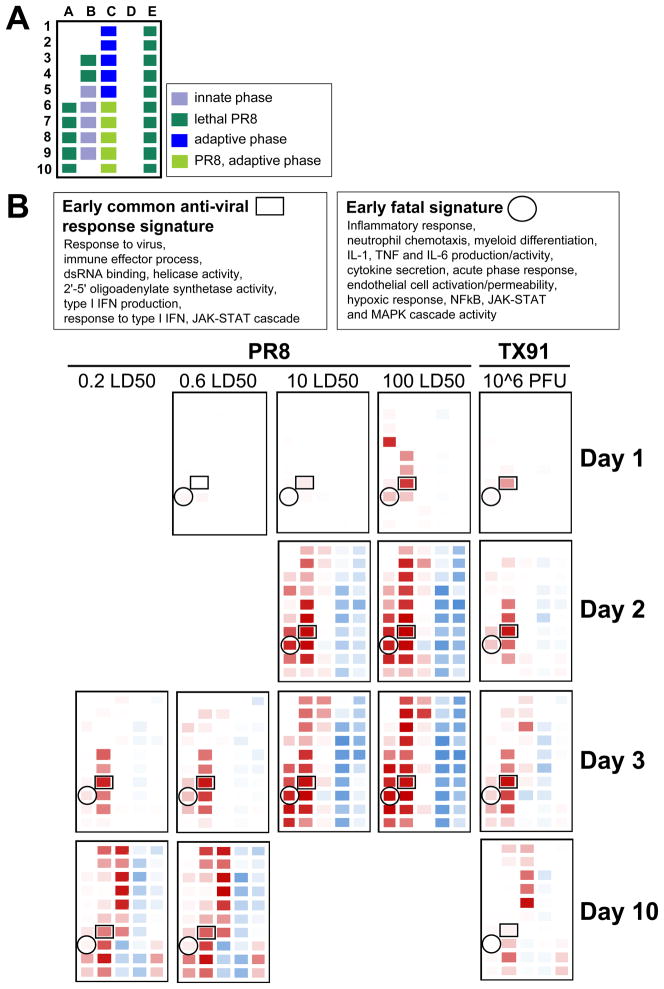
GO-annotated modules (Table S2) were categorized based on changes associated with the temporal phase of the host response and the pathophysiologic status of the infected animals (Figure 2A). Cross-correlation analysis of changing gene expression at early times of infection (days 1 to 3) identified two distinct patterns associated with innate immunity, each involving several modules. One pattern was associated with viral infection per se and did not distinguish influenza types or infecting doses (Figure S2A), whereas the other was closely associated with fatal disease (Figure S2B). The first signature represented gene sets involved in anti-viral responses, such as type 1 interferons and interferon γ, and genes induced downstream of these cytokines or downstream of viral pathogen-associated molecular pattern (PAMP)-driven Irf activity (Figure S2A). The earliest changes within this signature were detected in Module B-7 and reflected increases in both regulators and targets of anti-viral and interferon signaling networks (Table S2, S3).
Figure 2. Modular Map Analysis Reveals Early Shared Anti-viral Signatures and Condition-specific Inflammatory Signatures.
(A) Module assignments to different phases of the host response and infectious conditions. (B) Identification of key modules associated with evolving anti-viral responses shared across infectious conditions vs. lethality-associated inflammatory processes. Change in module B-7 transcript frequency preceded the emergence of the common anti-viral pattern, whereas change in A-8 was the leading indicator of the development of the inflammatory pattern. Text boxes to the left display a summary of the GO term enrichment attributed to the modules B-7 and A-8. See also Figure S2 and Table S2 and S3.
In contrast, the gene sets uniquely associated with lethal infection (Figure S2B) were distinctly characterized by inflammatory signaling cascades. There were changes in pathways associated with TLR4 and pro-inflammatory cytokine signaling, such as NF-kB, MAPK, and JAK-STAT cascades (Figure S2B). This signature was visible well before the onset of severe morbidity or mortality in mice given lethal doses of PR8 virus (Figure S1B). The signature did not include all gene products previously reported to be connected to pathogenic influenza infection such as mRNAs for ligands binding to CCR2, CCR5, and CXCR3 (Chakrabarti et al., 2010; Chang et al., 2011; Kash et al., 2006) (Figure S2B).
Besides the increase in inflammatory and anti-viral transcripts, transcript decreases were also observed. Decreases in modules D-1 to D-10 and E-1 to E-10 were delayed as compared to the two innate modular patterns and were not detected before day 2 p.i. with lethal doses of PR8 or after day 3 p.i. with sublethal but pathogenic doses of PR8. These genes correspond to proteins identified in a recent meta-analysis of lung transcriptome data to decrease during highly pathogenic viral infections of the respiratory system, including genes with key roles in maintaining pulmonary homeostasis (Chang et al., 2011). We also observed decreased transcripts for metabolic and tissue repair genes, suggesting that the lower respiratory tract was broadly affected in its capacity to cope with noxious substances and regenerate from injury (Table S2). The delay between the appearance of the two innate signatures (A-8 and B-7) and the modular states involving down-regulated transcripts (D-1 to D-10 and E-1 to E-10) indicated that the onset of the innate response preceded and was not dependent on this loss of pulmonary homeostasis.
Deconstruction of the Organ-level Transcriptome Analysis Using mRNA from Sorted Cells
We next focused on the earliest processes that distinguished lethal from sublethal infections (Figure 2B). The first changes within the global “fatal signature” were observed in module A-8. Transcripts encoding major components of the inflammatory signaling cascade (regulators) and mRNAs of prototype inflammatory response genes (targets) populated this module (Figure S2C, Table S2, Table S3). Based on module A-8 composition, increased activation of inflammatory signaling pathways, neutrophil recruitment, myeloid cell differentiation, production of pro-inflammatory cytokines, and endothelial cell activation were highly correlated with death of the infected animals (Figure S2C). The clear segregation of inflammatory features in lethality-associated module A-8 from the anti-viral infection-associated features in module B-7 shared in all infectious conditions suggested that the two innate pathways were activated in distinct manners, with the inflammatory gene set uniquely linked to infections with lethal viral doses.
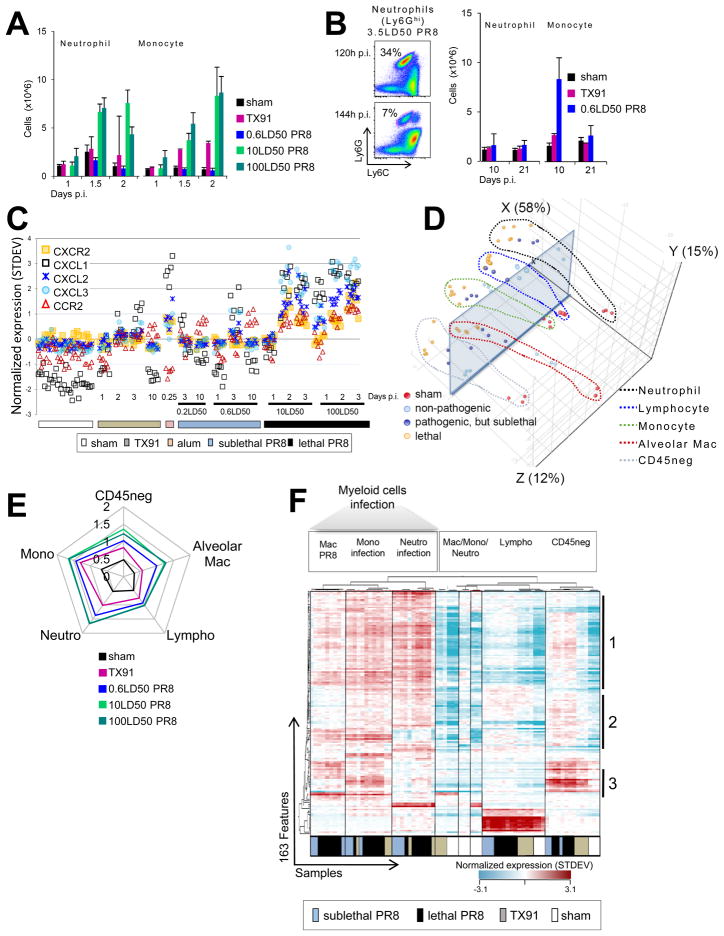
The influx of leukocytes into infected lungs complicates the interpretation of organ level changes in transcript representation, which may result from changes in cell composition, transcriptional induction within a cell population, or both. Consistent with previous studies (Lin et al., 2008; Perrone et al., 2008), flow cytometric analyses of cells from infected lungs revealed a large increase in neutrophils, inflammatory Ly6Chigh monocytes, and related myeloid cells (Ly6Chigh macrophages and DCs) early after infection (Figure 3A). In accord with the data associating genes in module A-8 with lethal infection, notably those encoding pro-IL-1b and neutrophil chemokines, a robust neutrophil infiltrate was detected before 2 days p.i. in animals given lethal doses of PR8 virus. This neutrophil influx preceded the onset of a moribund state and death by several days (Figure 3A, S1B). The rapid decline in pulmonary neutrophils after 5 days during PR8 infections as compared to the Ly6Chi mononuclear myeloid infiltrate (Figure 3B) agrees with other reports (de Bruin et al., 2012; Nandi and Behar, 2011). High numbers of inflammatory monocytes and related mononuclear cells were also observed for up to 6 days in lethal conditions. In contrast, only modest increases in Ly6Chi mononuclear myeloid cell infiltrates were seen with TX91 infection (Figure 3B). An increased signal for the constitutively-expressed neutrophil-specific chemokine receptor CXCR2 in lungs of lethally infected animals (Figure 3C) agreed with the dynamics of the neutrophil infiltrate and exemplified changes in transcript frequencies as a consequence of changes in cellular composition.
Figure 3. Microarrays from Flow-sorted Cells Reveal the Activation of Anti-viral and Interferon Pathways in Multiple Cell Types.
(A) Early changes in the cellular composition of lungs from infected animals involving inflammatory monocytes and neutrophils. (B) Decline in the neutrophil but not monocyte infiltrate with the rising adaptive response. (C) Changes in CXCR2 mRNA in whole lung samples reflect changes in the neutrophil infiltrate size. (D) PCA analysis of the transcriptomic response to influenza infection represented in module B-7. Visualization of the major difference between samples is supported by a transparent plane. (E) Quantitative ‘spider-plot’ representation of condition-associated changes in transcripts defining module B-7. (F) Unsupervised hierarchical clustering of the 163 B-7 features and the 74 “SortedCell” microarray samples. Sub-grouped gene lists [1–3] are reported in Table S6. See also Figure S3 and Tables S4 and S5. Error bars represent SD.
Given these dramatic cell changes, we repeated our transcriptional analysis using RNA extracted 48h post infection from 5 different populations (Figure S3A) purified by flow sorting from individual lungs (Figure S3B). One population consisted of non-hematopoietic cells (CD45neg), 45–55% of which were pulmonary epithelial cells, the primary target cell for viral replication. The others were alveolar macrophages, lymphocytes (T cells, B cells, and NK cells), neutrophils, and inflammatory monocytes together with related mononuclear myeloid cells.
Individual samples of the same cell population from different animals showed a high correlation across the transcriptome (45,281 features) but a substantial divergence in the level of expressed genes was seen among the distinct cell types (Figure S3C). Within the “SortedCell” microarray samples, 3746 dynamic features were defined based on statistical filtering of the data from the 5 sorted cell populations across 5 experimental conditions (Table S4, Figure S3D, S3E, S3F, Methods). A summary of top-ranking annotations attributed to shared or condition-specific transcript increases for CD45neg cells and for each myeloid cell type is provided in the supplemental information (Table S5).
Anti-viral and Interferon Pathways Are Robustly Activated in Multiple Cell Types
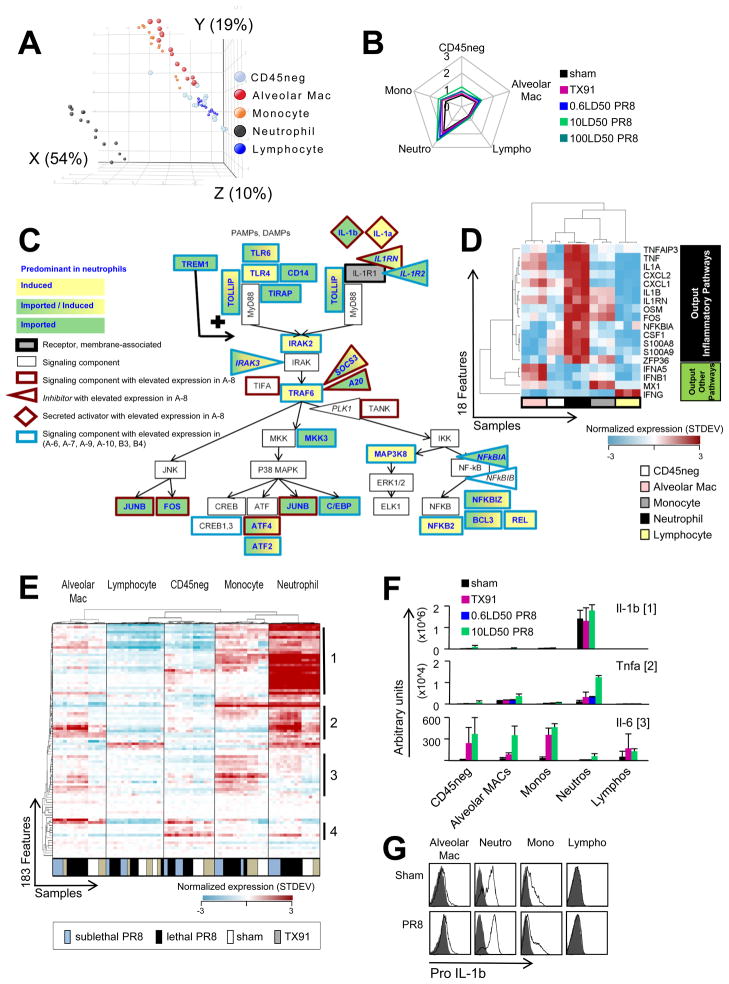
We next performed a principal component analysis (PCA) for infection-associated module B-7 (anti-viral) features derived from the “SortedCell” sample data (Figure 3D) to determine if the increase in these transcripts in the lungs resulted from preferential transcriptional induction in specific cell types or transcript import with infiltrating cells. The major distinction between samples on the x-axis accounted for 58% of the differences detected for this module and depended on whether samples were obtained from infected vs. sham infected animals, indicating that the transcriptional response to infection per se, as opposed to sample cellular composition, accounted for the principal changes. This implies that on the organ-level, the status of module B-7 largely reflected induced transcriptional changes contributed by all cell types. The finding that these major differences in transcriptional activity were not uniquely associated with cells hosting viral replication (CD45neg cells) indicated that this anti-viral response also depended on paracrine interferon activity.
We next visualized for B-7 features the normalized mean expression levels derived from “SortedCell” samples in a quantitative spider-plot (Figure 3E). Elevated average expression of B-7 transcripts was observed for all cell types from infected as opposed to sham animals, in agreement with the PCA analysis. Unsupervised hierarchical clustering of both “SortedCell” samples and module B-7 features further suggested that phagocytic cells shared a more similar anti-viral response pattern compared to CD45neg cells and lymphocytes (Figure 3F, Table S6). Overall, the analysis of these data by three different methods (Eigenvalues [PCA], average expression levels [Spider plot representation], and unsupervised hierarchical clustering [heat map visualization]) revealed that the activation of anti-viral and interferon pathways was not dominated by the cell type hosting viral replication but shared by all cell types.
Neutrophil Accumulation in Infected Lungs as a Major Driver of the Lethality-associated Gene Signature
We likewise conducted PCA on the “SortedCell” data for the features of lethality-associated module A-8. Here amplitude in X largely depended on whether samples were obtained from neutrophils or not (X: 54%; Figure 4A, S4A), revealing that on the organ level the distinctive signature monitored in the A-8 gene-set was highly sensitive to changes in neutrophil numbers infiltrating the lung. This agreed with the high mean expression level of A-8 transcripts in neutrophils (Figure 4B), whereas anti-viral module B-7 transcripts were largely independent of the cellular composition in lungs (Figure 3D, Figure S4B). Thus, the mRNAs related to inflammatory signaling components appeared to largely originate from neutrophils (Figure S2C, Figure 4C). Elevated expression of these signaling components influences signaling output. High expression of the known target genes of these pathways was observed in microarray samples and β-actin normalized qPCR samples of neutrophils (Figure 4D and not shown), indicating the relevance of the signaling component mRNA levels.
Figure 4. Neutrophil Infiltrates Largely Account for the Lethality-associated Signature Involving Inflammatory Signaling Networks.
(A) PCA for the 74 “SortedCell” microarray samples based on module A-8 features. (B) Quantitative ‘spider-plot’ representation of condition-associated changes in transcripts defining module A-8. (C) Inflammatory pathway signaling components elevated in module A-8 (outline red) and other modules associated with fatal disease (outline blue) and predominant expression in neutrophils (constitutive [fill green, import], activation-dependent [fill yellow, induction] or further induced but constitutive [fill green/yellow]). (D) Heat map shows unsupervised hierarchical clustering of downstream genes from inflammatory signaling cascades and reveals highest expression in neutrophil samples (microarrays 10LD50 PR8). (E) Unsupervised hierarchical clustering of the 183 A-8 features and the 74 “SortedCell” microarray samples showing cell-type segregation and a major fraction of transcripts (subgroup 1 and 2) largely associating with neutrophils. (F) qPCR validation of microarray data for inflammatory cytokine transcripts from different subgroups of genes within A-8 (subgroup membership = numbers to the right of the heat map). Error bars represent SD. (G) Pro-IL-1β protein levels in various hematopoietic cells from infected lungs assessed by flow cytometry (pro-IL-1β staining = open histograms; isotype-control = grey filled histograms). See also Figure S4 and Table S7.
Many A-8 features were abundantly expressed in neutrophils from sham-infected animals (Figure 4E, Table S7). Therefore, both import of transcripts by neutrophils (Figure 4E subgroup 1) and additional transcriptional induction in these cells (Figure 4E subgroup 2) contributed to signals in the key lethality-associated module A-8. Importantly, transcriptional induction of gene products co-clustering in subgroup 2 was most prominent in neutrophils from lethal infections and suggested that enhanced activation of infiltrating neutrophils distinguished lethal from sublethal infections.
Constitutive pro-IL-1b expression in neutrophils was detected on both the mRNA and protein level (Figure 4F, 4G), with flow cytometry revealing high pro-IL-1b protein levels in all lung-derived neutrophils. Constitutive pro-IL-1b expression was readily detected in mature bone marrow neutrophils (Ly6Ghigh, c-kit−,CD11bhigh) but absent in immature neutrophils (Ly6Ghigh, c-kit+,CD11bmed) (Figure S4C) in both germ-free and non-germ-free animals (Figure S4D). In contrast, pro-IL-1b protein was largely absent in Ly6Chigh monocytes of the bone marrow. Pro-IL-1b protein was also expressed more highly in neutrophils upon in vitro stimulation with ultrapure LPS compared to inflammatory monocytes (Figure S4E), challenging the view that IL-1b activity derives predominantly from myelomonocytic cells and macrophages (Goldbach-Mansky and Kastner, 2009). The constitutive neutrophil pro-IL-1b expression we observed was unexpected, as pro-IL-1b expression is generally though to be controlled by transcriptional induction dependent on pro-inflammatory signaling.
Intriguingly, neutrophil pro-IL-1b expression was associated with expression of transcripts encoding major negative regulators of inflammatory signaling cascades (i.e. A20, NFKBIA, and ZFP36), all previously reported to act in transcriptionally-controlled, NFkB-driven negative feedback loops to control inflammatory responses in PAMP or cytokine-activated mononuclear myeloid cells (Boone et al., 2004; Hayden and Ghosh, 2008; Qiu et al., 2012; Shembade et al., 2010; Sun et al., 1993; Turer et al., 2008; Werner et al., 2008; Wertz et al., 2004). Such heightened expression of key negative regulators in neutrophils suggested that transcription-dependent inflammatory output from this cell type might require a stronger input signal as compared to mononuclear myeloid cells. In support of this hypothesis, induction of TNFa protein was readily detected in inflammatory monocytes at 10ng/ml LPS, whereas neutrophils required 100ng/ml (Figure S4F). A similar difference in the response initiation threshold between inflammatory monocytes and neutrophils was observed in germ-free or IL-1R1−/− animals (Figure S4G), implying a pre-programmed elevated response initiation threshold in neutrophils.
In addition to very high levels of pro-IL-1b mRNA (Figure 4E, among subgroup 1 features), neutrophils also expressed Tnfa and Il1a transcripts when activated (Figure 4E, among subgroup 2 features), but not high levels of Il6 transcripts (Figure 4E, among subgroup 3 features). IL-6 was instead produced by resident lung cells, both CD45neg non-hematopoietic cells and CD45pos alveolar macrophages, as well as by infiltrating inflammatory monocytes and their differentiated progeny. qPCR assays (Figure 4F) confirmed the microarray data. Thus, a full type 1 inflammatory response in the lung requires the conjoint action of diverse cells types producing different cytokines.
The constitutive expression level of the anti-viral B-7 features was substantially lower than the steady state level of inflammatory A-8 features in the whole lung samples (Figure S4H). The differential basal expression level and the accumulation of prototype TLR4-responsive immediate early response genes in A-8 agree with recent findings suggesting that inflammatory responses often reflect quantitative modulation of basal levels of gene expression rather than qualitative transitions from “off” to “on”” (Escoubet-Lozach et al., 2011). In terms of the inflammatory features in A-8, only very large increases in transcript frequency may thus be detectable because of the very high baseline expression, whereas the low basal level of anti-viral or interferon-driven transcripts in B-7 permits detection of even modest transcriptional increases.
Two Independent Chemokine Feed-Forward Circuits Regulate the Magnitude of Myeloid Cell Infiltration
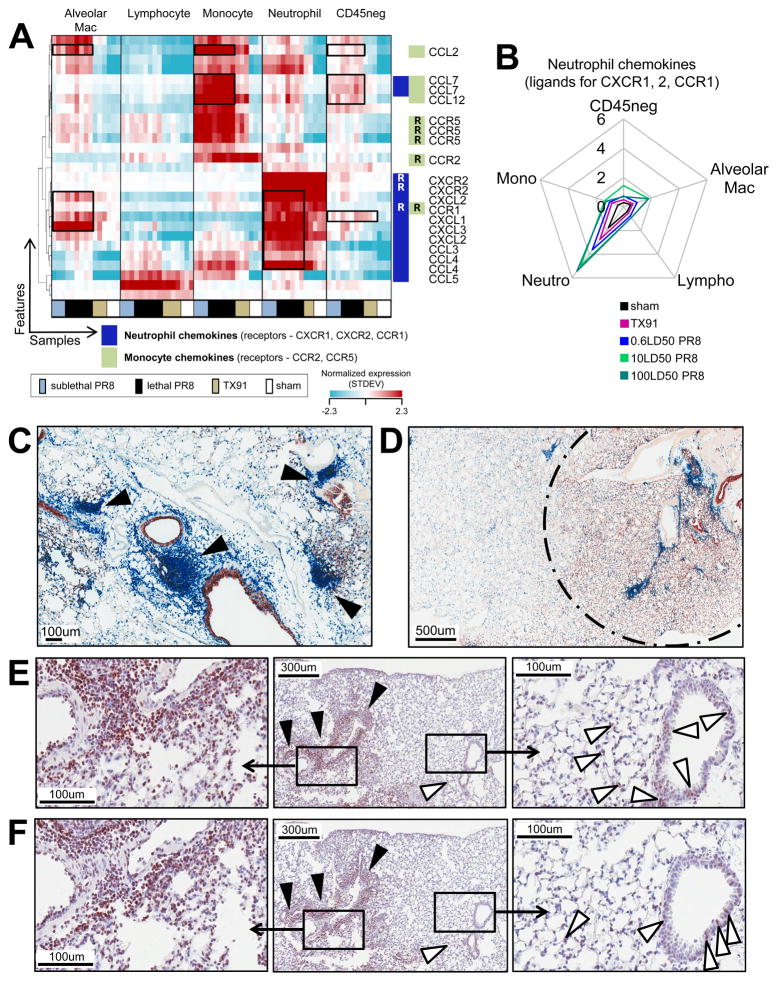
The disproportionate contribution of neutrophils to the early elevated inflammatory response, and the early neutrophil infiltrate characteristic of lethal but not sublethal PR8 infections prompted us to explore how the infiltrate was controlled. Chemokines are responsible for the accumulation of high neutrophil numbers in inflamed lungs of influenza A infected mice. To identify the origin of chemokines involved in neutrophil and monocyte recruitment, we performed a hierarchical clustering on all features representing chemokines or chemokine receptors in the “SortedCell” microarray samples (Figure 5A). This revealed that most neutrophil-attractive chemokines are produced at the highest levels by neutrophils themselves (Figure 5B), whereas monocyte-attractive chemokines are predominantly produced by monocytes (Figure 5A, S5B). Neutrophils from sham-infected animals expressed high levels of transcripts for a single neutrophil chemokine CXCL2 (Figure S5A). Low amounts of mRNA for the monocyte chemokine CCL2 were found in steady-state inflammatory monocytes without detectable CCL2 protein (Figure S5B, S5C). Strong induction of additional chemokine transcripts was seen with cells obtained from infected lungs, again mainly in a cell-type reflexive manner (Figure S5A, S5B). In infected mice a high level of CCL2 mRNA expression in inflammatory monocytes was reflected in CCL2 protein readily detectable by flow cytometry in only these cells (Figure S5C).
Figure 5. Two Self-reflexive Chemokine Feed-forward Loops Involving Myeloid Cells from Infected Lungs.
(A) Hierarchical clustering of chemokine and chemokine receptor mRNA levels for “SortedCell” samples. (B) Quantitative ’spider-plot’ representation of neutrophil chemokine expression data. (C, D) Virus protein expression (red-brown) and leukocyte distribution (CD45, blue) in the lung tissue of animals infected with a lethal dose of PR8. Focal leukocyte distribution pattern in the interstitial tissue at (C) 36h p.i. with 10LD50PR8 or (D) 24h p.i. with 100LD50 PR8 is shown; infected lung tissue = area within dashed circle. (E, F) (E) CCL2 and (F) TNFα protein expression (red-brown) at 36h p.i. in the lung tissue of animals infected with 10LD50PR8. Interstitial leukocytic infiltrate denoted by black arrow heads. Cells in the broncho-alveolar lining are denoted by white arrowheads. See also Figure S5.
If neutrophils and monocytes amplify their own recruitment in a feed-forward manner, one would expect this to be evidenced in situ by focal leukocyte accumulations. We therefore stained sections from lungs collected 24 or 36h p.i. for virus proteins and leukocytes (Figure 5C, 5D). Regardless of widespread infection in the lower respiratory tract, accumulations of leukocytes appeared to be tightly clustered and restricted to select localizations (Figure 5D). Lethal infections were characterized by very early dense neutrophilic foci (Figure S5D). Consistent with the predominant interstitial localization of these infiltrates, neutrophil depletion or CCR2 deficiency did not result in major changes of bronchoalveolar lavage (BAL) cytokine levels (Figure S5E; (Teijaro et al., 2011). This suggests that early activation of infiltrating myeloid cells and subsequent release of chemokines (and other inflammatory mediators) by these cells was largely confined to the pulmonary interstitial tissue. Indeed, TNFa and CCL2 were detected in association with both the interstitial, peri- and intravascular infiltrates, and with cells in the lining of the broncho-alveolar space, with the latter cells presumably producing the cytokines detected in the BAL fluid (Figure 5E, 5F, S5F).
Overall, these data provide strong evidence for the operation of a feed-forward circuit in which recruited myeloid cells, especially neutrophils but also inflammatory monocytes, are triggered in the infected site to immediately release pre-formed protein (CXCL2) in the case of neutrophils and to produce even more attractants that amplify the interstitial cellular inflammatory process.
Poorly Controlled Infectious Spread During the Innate Phase Sets the Stage for Lethality-associated Neutrophil Activation
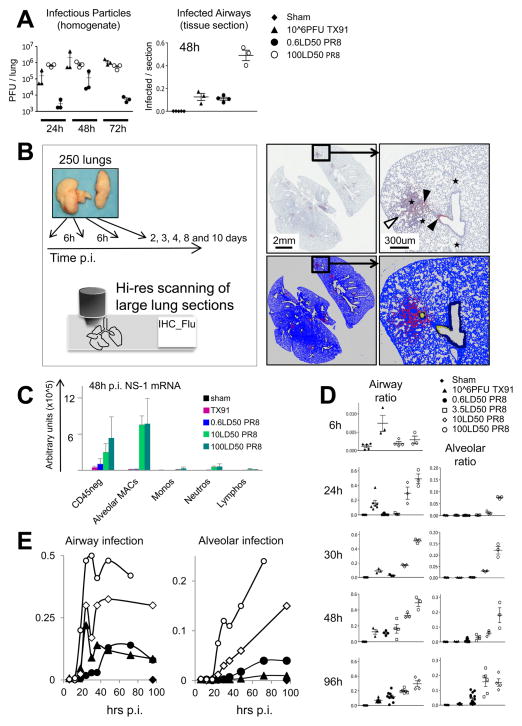
Innate inflammatory responses are initiated by exposure to pathogen or damage associated conserved molecular cues and by pro-inflammatory cytokines. To further understand how highly pathogenic PR8 virus promotes the lethality-associated early recruitment and activation of a large neutrophil population, we investigated whether virus abundance itself or associated tissue damage correlated with neutrophil recruitment and physiological dysregulation. Virus titers from whole lung homogenates were compared to infectious spread in situ (Figure 6A), quantified using newly developed automated image analysis software (Figure 6B and Methods).
Figure 6. Early Poorly Controlled Infectious Spread of Pathogenic Virus Revealed by Automated Image Analysis.
(A) Pulmonary infectious particle loads after infection with influenza virus, assessed using lung homogenates and standard TCID50 assay (left panel). Infection load assessed by immunohistochemistry for viral proteins at 48h p.i., expressed as the ratio of infected versus total lung tissue per section based on the area quantification (right panel). Each mark represents the section statistics for an individual animal and bars indicate mean and SD. (B) Scheme for time-resolved analysis of influenza infection using automated image analysis. Sections stained for virus proteins (counterstained with hematoxylin) were scanned and quantified, distinguishing between infected airways and infected lung parenchyma (alveoli). Scan of an entire section (3.5LD50 PR8, 48h p.i.) (upper left) and detailed view (upper right) with virus-stain (red-brown) and counterstain (blue-grey). Infected airways (black arrow heads) and infected alveoli (white arrow heads) were distinguished by the software. Large and middle size vessels (black star) were also distinguished from airways. The software-generated transformation of the imaging data (lower panels) shows “lung tissue” (blue, dark blue), airways (dark blue), and alveoli/vessels (blue) Alveoli-associated virus signals (red) are distinguished from airway-related viral signals (yellow). (C) Validation of the in situ image analysis data using qPCR for NS-1. Error bars represent SD. (D) Automated in situ analysis at 6, 24, 30, 48, and 96h p.i. Each mark represents an individual animal as indicated in A. (E) In situ dynamics of the infection load based on mean values for the entire innate phase involving all time points between 6 and 96h p.i. Symbols are the same as indicated in D. See also Figure S6.
Conventional TCID50 lung virus titers did not discriminate between pathogenic (PR8) and non-pathogenic (TX91) infections (Figure 6A), in line with recent observations (Qi et al., 2011). Image analysis of infectious spread, however, very clearly distinguished between sublethal and lethal infections (Figure 6A). These imaging data were further supported by β-actin normalized qPCR assays on pulmonary CD45neg cells for viral NS-1 mRNA, which is expressed in infected cells but not in virions (Figure 6C). Viral NS-1 mRNA in CD45neg cells (Figure 6C) and the proportion of infected lung tissue (Figure 6D) both measure the infectious spread in the lung, in the contrast to measurements of infectious particles using TCID50 assays. These analyses reveal that at the same infectious particle load PR8 has spread much more through the tissue as compared with Tx91, indicating that PR8 has a higher capacity to infect host cells but a lower capacity to generate new infectious particles per infected cell.
We extended our analysis of the relationship between viral PAMPs and fatal disease course by collecting, staining, scanning, and analyzing lung sections collected from approx. 250 animals, every 6h up to 36h p.i. and at broader time intervals thereafter (Figure 6B). For infections with 10 and 100LD50PR8, the in situ dynamics showed an initial peak in the airways around 24h p.i., similar to the timing after infections with 106 PFU TX91 but at higher levels (Figure 6D, 6E). The time course of airway infection ratios further indicated that infections with 10 and 100LD50 PR8 are more difficult to contain during the innate phase as compared to TX91. Similar to lethal doses, infection loads at sublethal doses of PR8 did not substantially decrease by 72h p.i. (Figure 6E) despite lack of the early initial infectious peak at 24h p.i.
These data could reflect lethal doses overwhelming innate host defense mechanisms, whereas due to the low infection load during the initial 36h p.i., sublethal doses do not produce enough immunogenic signals to show the decline typical of TX91. However, poorly contained infection in the course of co-infections with 0.6LD50PR8 and 106 PFU TX91 indicate that irrespective of an early infectious peak and associated immune stimulus, infections with inocula as low as 40 PFU PR8 are difficult to contain during the innate phase (Figure S6A). The dose-independent limitation in containment of PR8 thus pointed to a strain inherent property related to either increased infectivity and/or efficient evasion of innate defense mechanisms.
Together with the preceding data on chemokine expression, these findings on viral spread indicate that continuous high infectious loads with PR8 during the innate phase are associated with a feed-forward circuit in which neutrophils are triggered in the infected site to release or/and produce more attractants that then guide more neutrophils to where previously arriving neutrophils have been effectively activated. The poorly constrained spread of the highly pathogenic PR8 virus provides a clue to how neutrophils, with a higher threshold for activation but the potential for greater toxicity if triggered, could be primarily associated with the lethal signature.
Viral NS-1 mRNA expressed in infected host cells correlated with the infectious spread in situ (Figure 6C, 6D) but not with the infectious particle load (Figure 6A). We compared NS-1 mRNA levels during PR8 versus TX91 infections (Figure S6B) in individual cell types purified from lungs of infected animals to screen for PAMP interactions. This revealed markedly greater amounts of NS-1 mRNA in infiltrating myeloid cells purified from animals infected with lethal as compared to sublethal doses of PR8. These data were consistent with the idea that other, immunogenic viral RNA species (potent PAMPs) were also available to myeloid cells and correlated with enhanced neutrophil activation and high levels of early infiltration. The discrepancy between NS-1mRNA levels measured in alveolar macrophages vs. neutrophils most likely relates to the different compartments to which the two cell types are localized and accordingly, to differences in exposure to pathogen-derived material (Figure 6C).
We then measured viral proteins by flow cytometry to obtain single cell level statistics (Figure S6C). Histograms showed a homogenous staining shift of the entire population for influenza A proteins in neutrophils and alveolar macrophages at lethal but not sublethal doses, revealing that abundant interactions between pathogen-derived material and the majority of neutrophils distinguished lethal from sublethal conditions (Figure 6C). Signals for viral proteins in immune cells were only obtained by intracellular staining and contrasted with CD45neg cells where viral proteins were readily detected by cell surface staining (Figure S6D), indicating that macrophages and neutrophils did not contribute substantially to virus production. Phagocytosis of infected dead cells and debris (Hashimoto et al., 2007) containing a wealth of viral proteins (Figure S6E) and viral RNA most likely accounts for the pathogen-derived material within neutrophils and macrophages (Figure 6C, S6B, S6C). Together with observations showing that detection of influenza virus-associated PAMPs by endosomal TLR7 triggers activation of neutrophils (Wang et al., 2008), these findings support the hypothesis that poor innate immune containment of PR8 virus contributes to infectious dose-dependent triggering of invading neutrophils through signals derived from excess phagocytosis-associated PAMP encounter and possibly also DAMPs from damaged cells.
Deleterious Neutrophil Responses Promote Fatal Outcomes at Low Lethal Doses
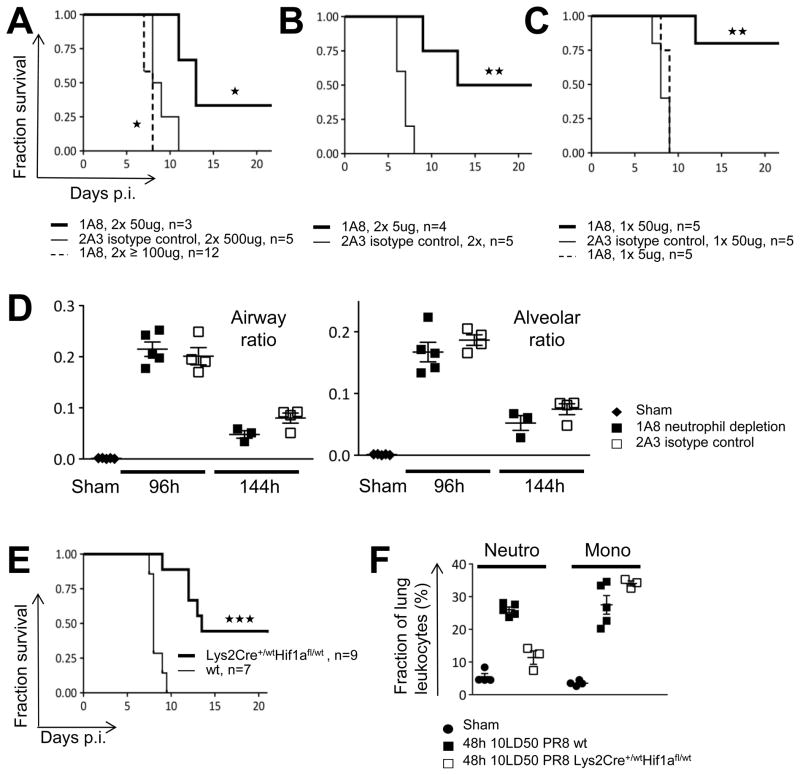
Lung-recruited, highly pro-inflammatory neutrophils are a major correlate of lethal infection and when activated by viral PAMPS, readily release pre-formed chemokines while making additional chemokines that bring more neutrophils into the infected tissue, a sequence of events driven most strongly by pathogenic virus PR8. The question still remained, however, whether this potent inflammatory response was a consequence or a cause of lethal disease. Previous data clearly showed that total ablation of neutrophils promoted mortality among infected animals, but the feed-forward nature of the recruitment and activation of these cells as revealed in our data suggested that attenuation of this explosive process might provide protection against lethal effects caused by excess inflammatory responses while preserving essential innate host defense activities. We tested whether this excessively amplified innate inflammatory response was causal in PR8 lethality by reducing pulmonary neutrophil numbers in animals infected with low lethal doses of PR8 (3.5LD50 PR8; Figure S7A, B) using limiting amounts of a neutrophil-depleting antibody (clone 1A8). Infected animals treated with two doses of 5ug or 50ug of 1A8 (Figure 7A, 7B, S7E), or a single dose of 50ug (Figure 7C, S7E) showed increased survival as compared to animals treated with the isotype control reagent (clone 2A3). A single injection of 5ug (Figure 7C, S7E) did not improve or prolong survival. This contrasted with two doses of 5ug, which produce a more sustained partial depletion and yielded only 2–8% neutrophils among lung leukocytes at 120h p.i. (Figure S7C). Depletion treatments with doses of 100ug or above (Figure 7A) resulted in premature death, consistent with a recent report (Tate et al., 2011b). The narrow therapeutic window of neutrophil depletion regimens raised the possibility that high dose regimens depleted other cell types with non-redundant beneficial functions and cells expressing low levels of the depletion epitope Ly6G were indeed readily detected in the CD11bhigh fraction (Figure S7D).
Figure 7. Attenuation of Lethality-preceding Neutrophil Influx Improves Survival without Changing Infection Loads.
(A–C) Survival plots for animals treated with varying neutrophil depletion regimens. Stars indicate significance level. (D) Infection loads in neutrophil-depleted animals. Each mark represents the section-based infection load for an individual animal. (E) Survival curves for animals with diminished myeloid cell recruitment in Hif-1a (Lys2Cre+/−Hif1afl/wt) animals. (F) Myeloid cells in infected WT and Hif-1a (Lys2Cre+/−Hif1afl/wt) animals. The cellular composition in lung homogenates was determined by flow cytometry and each mark represents an individual animal. Bars indicate mean and SD. See also Figure S7.
Beneficial effects through neutrophil depletion could arise through a reduction in immunopathologic effects or from unexpectedly lower infection loads and a reduction of direct virus-related epithelial damage. We therefore compared infectious spread in animals receiving a single dose 50ug depletion regimen or isotype control treatment. No statistically significant differences were observed at 96h and 144h p.i. in terms of airway or alveolar infection ratios (Figure 7D). Thus, low dose neutrophil depletion regimens increased survival through reduction of the damaging effects of neutrophil activation and not by limiting direct viral epithelial cytopathicity.
To further test the role of myeloid cell recruitment and activation as a causative factor in fatal influenza infection, we took advantage of observations showing that myeloid cell-intrinsic Hif-1a activity is essential for early myeloid infiltrate-dependent inflammation (Cramer et al., 2003; Jacobs et al., 2010). Lys2Cre+/wtHif-1afl/wt and wt animals were infected with a low lethal dose of 3.5LD50 PR8 (Figure 7E, S7E). In accord with our model and the results of the antibody depletion studies, animals with reduced levels of myeloid cell Hif-1a indeed showed prolonged survival as compared to wt animals. Surprisingly, myeloid Hif-1a reduction in Lys2Cre+/wtHif-1afl/wt mice did not have major effects on the inflammatory myelomonocytic infiltrate while still resulting in a dramatic reduction of neutrophilic accumulations (Figure 7F).
Discussion
Dissecting the contributions to infection-associated lethality of direct pathogen damage versus that caused by innate and adaptive immune responses has proved difficult. Here we have used a top-down systems analysis to unravel the basis of lethal influenza infection in a mouse model. Using organ-level transcriptional profiling with data-driven gene set (modular) analysis to highlight biological processes rather than specific genes, we uncovered a dramatic difference in the early innate response between lethal and non-lethal infections. Modular changes in anti-viral gene expression were similar across the various infectious conditions, whereas a module of genes annotated as highly pro-inflammatory was uniquely associated with lethal conditions. Our data pointed to import of transcripts from pre-programmed inflammatory neutrophils, as well as PAMP-induced transcription in these myeloid cells of genes encoding additional potentially damaging products, as the main origin of the lethal signature. We uncovered a potent chemokine circuit in which myeloid cells reaching the infected lung readily released pre-formed and newly synthesized chemokines that attracted additional members of the same cell subset in a potentially explosive feed-forward pathway. More robust and accelerated operation of this loop with lethal PR8 infection correlated with extensive viral tissue spread and elevated PAMP encounters by myeloid cells. Together, these findings all pointed to a possible primary role for excess neutrophil-mediated inflammatory damage in fatal infection and in accord with this notion, experimental reduction of neutrophil numbers effectively led to rescue of animals from death without changing the extent of tissue infection by the PR8 virus. These findings provided clear evidence that lethality in this mouse model of acute influenza-induced death primarily does not arise directly from the cytopathic effects of the virus on non-hematopoietic cells but rather from damage due to inadequately constrained innate inflammation primarily involving neutrophils co-acting with monocytes (Figure S7E).
Other studies have also sought to clarify the relative contributions of direct viral cytopathicity versus inflammatory damage to morbidity and mortality following infection with highly pathogenic influenza (Boon et al., 2011; Kash et al., 2004; Kash et al., 2006; Kobasa et al., 2007; Salomon et al., 2007; Tate et al., 2009). In aggregate they have used many but not all of the methods we employed, yet failed to clearly separate the contributions of these two components of infection-associated damage. Several experimental design choices and technical developments played key roles in our ability to dissect the origin of infectious lethality in this model. First, we utilized a dense matrix of virus types and doses that included a range close to the minimum inducing lethality, to best mimic natural disease versus the very high levels of virus employed in past studies (Kash et al., 2006; Kobasa et al., 2007). Second, we did not seek to identify individual gene products that caused tissue damage; rather we employed a data-driven modular approach that more broadly assesses biological processes at an integrated level. This led to the clear identification of a dichotomy between early anti-viral vs. early inflammatory innate immune responses, with only the latter associated with lethal disease. Third, we did not rely solely on tissue-level transcriptome analysis, but isolated multiple relevant cell populations by flow sorting and repeated much of the original matrixed analysis with these isolated cells. Finally, we also included a new method for quantitative cell and tissue level assessment of viral infection across large volumes of the infected lungs, revealing strikingly greater infectious spread by PR8 as compared to Tx91, at doses where infections with the two viruses showed similar TCID50 titers.
Our feed-forward model suggested that a very non-linear process was involved in distinguishing between lethality and mild disease. Under such conditions, global cell depletion, gene knockout, or cytokine neutralization can obscure the system behavior and indeed, previous studies using total neutrophil depletion showed that this was deleterious in infected animals (Tate et al., 2009; Tate et al., 2011b). Our analysis predicted that attenuation of this self-amplifying process to constrain its explosive nature might preserve a positive contribution of neutrophils to innate host defense, while ameliorating damaging excess inflammation, an expectation consistent with our findings of increased post-infection survival using limited Ly6G-based neutrophil depletion or animals with reduced HiF1a levels in myeloid cells.
The detailed basis for terminal loss of pulmonary function has not yet been fully elucidated. Possible mechanism may relate to the preferential localization of the early infiltrate in the interstitial tissue as perivascular sheets or as intravascular clumps associated with mid- and large size blood vessels. The lungs of moribund or recently deceased animals are also typically grossly hemorrhagic, suggesting that the excessive interstitial inflammatory response to lethal influenza infection may affect vascular homeostasis, the loss of which contributes to acute death (London et al., 2010; Zhu et al., 2012). Neutrophils have a high potential to promote vascular dysregulation through many mediators (OSM, pro-IL1b, pro-IL1b activating PR3, IL1a, TNFa, reactive oxygen species, cationic peptides, and toxic enzymes). Although capillary leak and pulmonary edema can precipitate adult respiratory distress syndrome (ARDS), our observations suggest that hemorrhagic events resulting from inflammation-associated vascular fragility and possibly impaired thrombus formation may make a particular contribution to PR8 lethality. The declining expression of endothelial von Willebrand factor (vWF) in the CD45neg fraction (Table S4) may explain the unexpected lack of thrombus formation at sites of large intravascular leukocyte aggregates (data not shown), potentially amplifying the bleeding diathesis caused through vascular fragility. Our finding of contitutive pro-IL-1b expression in neutrophils may be very relevant in this regard, as IL1R1-dependent VE-cadherin internalization disrupts endothelial integrity and stabilization of VE-cadherin surface expression improves survival during influenza infections (Zhu et al., 2012) (London et al., 2010). These data on constitutive pro-IL-1b expression by neutrophils may also have broader significance for understanding sterile inflammatory diseases in which poorly controlled IL-1b activity leads to damaging inflammation (Kastner et al., 2010).
Therapeutic opportunities identified by the present study include agents that attenuate the chemokine-driven feed-forward loop so as to reduce the noxious effects of some myeloid cells while still maintaining sufficient innate host-protective functions or that block the pathophysiologic effect of major toxic mediators derived from neutrophils and monocytes. In line with these concepts, recent data show increased survival for PR8 infections using an inhibitor of TLR4 (Shirey et al., 2013), a receptor activated through a variety of DAMPs and whose signaling components and target genes are over-represented in the lethality-associated gene sets and in neutrophils. However, there may be no universal mechanism of pathogenicity among different human influenza strains (Garigliany et al., 2010; Otte et al., 2011; Shinya et al., 2011) and conclusions drawn from an individual model system (i.e., PR8) may therefore not generally apply to all aspects of influenza-associated disease in humans. Further experimental as well as clinical studies will be needed to determine if the contribution of neutrophil-related innate inflammation has the central role revealed in the present model system.
EXPERIMENTAL PROCEDURES
Animals, Infections, and Lung Harvest
5 to 8-week-old male C57BL/6, CCR2 deficient, or Lys2Cre+/−Hif1afl/wt (Jackson Laboratories) mice were intranasally infected with different doses of H1N1 influenza A viruses. Bone marrow cells of wt and IL1R1−/− (Glaccum et al., 1997) (Jackson Laboratories), or SFP and germ-free mice (kind gifts of Y. Belkaid, NIAID, NIH) were used for detection of intracellular cytokines. Further information is detailed in Supplemental Material. All animal procedures were performed under IACUC guidelines and approved NIAID, NIH Animal Care and Use Committee study protocols.
Antibodies, and Viruses
Antibodies are listed in the corresponding table in Extended Experimental Procedures. Influenza H1N1 A/PR/8/34 and A/Tx/91 seeds (a kind gift from J. R. Bennink, NIAID, NIH) were propagated in low-passage MDCK cultures.
RNA Isolation, Microarrays, qPCR
Methods for RNA preparation, amplification, hybridization and use in qPCR assays are detailed in Supplemental Material.
Bioinformatics
Detailed information on microarray analysis is provided in Extended Experimental Procedures.
Virus Detection and Quantification
Viral titers from lung homogenates, measurement of NS-1 viral RNA, staining for viral proteins, and automated image analysis are described in detail in Supplemental Material.
Supplementary Material
Highlights.
Early neutrophil infiltration largely accounts for fatal influenza gene signatures.
Self-reflexive chemokine feed-forward circuits underlie dysregulated inflammation.
Fatal interstitial neutrophil activation correlates with unconstrained PAMP encounters.
Neutrophil reduction prevents death from self-amplifying damaging inflammation.
Acknowledgments
We thank J.R. Bennink and S.E. Hensley for virus seeds and initial advice, T.A. Torrey, C.L. Thomas, and the animal facility team for support and animal health observations, K.L. Holmes and C.L Eigsti for FACS Aria operation, T.G. Myers, G. Owens, A. Godinez for microarray hybridization and QC analysis, R. Kastenmayer, M.S. Orandle, O.M. Schwartz, and L. Koo for slide scanner access and advice, Y. Belkaid for bones from germ-free mice, B. Dutta, J.R. Bennink, W. Resch, and I. Fraser for critical reading of the manuscript and valuable comments. This work was supported by the Intramural Research Program of NIAID, NIH, a fellowship from the Swiss Science Foundation PA00A-1114666 (M.B.), and a Human Frontier Science Program Young Investigator Award RGY0077/2011 (F.K.).
Footnotes
ACCESSION NUMBERS
Complete microarray datasets are available in the NCBI Gene Expression Omnibus (accession number GSE42641).
Supplemental Information includes Extended Experimental Procedures, seven figures, and eight tables.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aldridge JR, Jr, Moseley CE, Boltz DA, Negovetich NJ, Reynolds C, Franks J, Brown SA, Doherty PC, Webster RG, Thomas PG. TNF/iNOS-producing dendritic cells are the necessary evil of lethal influenza virus infection. Proc Natl Acad Sci U S A. 2009;106:5306–5311. doi: 10.1073/pnas.0900655106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen IC, Scull MA, Moore CB, Holl EK, McElvania-TeKippe E, Taxman DJ, Guthrie EH, Pickles RJ, Ting JP. The NLRP3 inflammasome mediates in vivo innate immunity to influenza A virus through recognition of viral RNA. Immunity. 2009;30:556–565. doi: 10.1016/j.immuni.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bautista E, Chotpitayasunondh T, Gao Z, Harper SA, Shaw M, Uyeki TM, Zaki SR, Hayden FG, Hui DS, Kettner JD, et al. Clinical aspects of pandemic 2009 influenza A (H1N1) virus infection. N Engl J Med. 2010;362:1708–1719. doi: 10.1056/NEJMra1000449. [DOI] [PubMed] [Google Scholar]
- Beigel JH, Farrar J, Han AM, Hayden FG, Hyer R, de Jong MD, Lochindarat S, Nguyen TK, Nguyen TH, Tran TH, et al. Avian influenza A (H5N1) infection in humans. N Engl J Med. 2005;353:1374–1385. doi: 10.1056/NEJMra052211. [DOI] [PubMed] [Google Scholar]
- Boon AC, Finkelstein D, Zheng M, Liao G, Allard J, Klumpp K, Webster R, Peltz G, Webby RJ. H5N1 influenza virus pathogenesis in genetically diverse mice is mediated at the level of viral load. MBio. 2011:2. doi: 10.1128/mBio.00171-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boone DL, Turer EE, Lee EG, Ahmad RC, Wheeler MT, Tsui C, Hurley P, Chien M, Chai S, Hitotsumatsu O, et al. The ubiquitin-modifying enzyme A20 is required for termination of Toll-like receptor responses. Nat Immunol. 2004;5:1052–1060. doi: 10.1038/ni1110. [DOI] [PubMed] [Google Scholar]
- Brincks EL, Katewa A, Kucaba TA, Griffith TS, Legge KL. CD8 T cells utilize TRAIL to control influenza virus infection. J Immunol. 2008;181:4918–4925. doi: 10.4049/jimmunol.181.7.4918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti AK, Vipat VC, Mukherjee S, Singh R, Pawar SD, Mishra AC. Host gene expression profiling in influenza A virus-infected lung epithelial (A549) cells: a comparative analysis between highly pathogenic and modified H5N1 viruses. Virol J. 2010;7:219. doi: 10.1186/1743-422X-7-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang ST, Tchitchek N, Ghosh D, Benecke A, Katze MG. A chemokine gene expression signature derived from meta-analysis predicts the pathogenicity of viral respiratory infections. BMC Syst Biol. 2011;5:202. doi: 10.1186/1752-0509-5-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaussabel D, Quinn C, Shen J, Patel P, Glaser C, Baldwin N, Stichweh D, Blankenship D, Li L, Munagala I, et al. A modular analysis framework for blood genomics studies: application to systemic lupus erythematosus. Immunity. 2008;29:150–164. doi: 10.1016/j.immuni.2008.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer T, Yamanishi Y, Clausen BE, Forster I, Pawlinski R, Mackman N, Haase VH, Jaenisch R, Corr M, Nizet V, et al. HIF-1alpha is essential for myeloid cell-mediated inflammation. Cell. 2003;112:645–657. doi: 10.1016/s0092-8674(03)00154-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bruin AM, Libregts SF, Valkhof M, Boon L, Touw IP, Nolte MA. Interferon-gamma induces monopoiesis and inhibits neutrophil development during inflammation. Blood. 2012 doi: 10.1182/blood-2011-07-367706. [DOI] [PubMed] [Google Scholar]
- de Jong MD, Simmons CP, Thanh TT, Hien VM, Smith GJ, Chau TN, Hoang DM, Chau NV, Khanh TH, Dong VC, et al. Fatal outcome of human influenza A (H5N1) is associated with high viral load and hypercytokinemia. Nat Med. 2006;12:1203–1207. doi: 10.1038/nm1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escoubet-Lozach L, Benner C, Kaikkonen MU, Lozach J, Heinz S, Spann NJ, Crotti A, Stender J, Ghisletti S, Reichart D, et al. Mechanisms establishing TLR4-responsive activation states of inflammatory response genes. PLoS Genet. 2011;7:e1002401. doi: 10.1371/journal.pgen.1002401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garigliany MM, Habyarimana A, Lambrecht B, Van de Paar E, Cornet A, van den Berg T, Desmecht D. Influenza A strain-dependent pathogenesis in fatal H1N1 and H5N1 subtype infections of mice. Emerg Infect Dis. 2010;16:595–603. doi: 10.3201/eid1604.091061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaccum MB, Stocking KL, Charrier K, Smith JL, Willis CR, Maliszewski C, Livingston DJ, Peschon JJ, Morrissey PJ. Phenotypic and functional characterization of mice that lack the type I receptor for IL-1. J Immunol. 1997;159:3364–3371. [PubMed] [Google Scholar]
- Goldbach-Mansky R, Kastner DL. Autoinflammation: the prominent role of IL-1 in monogenic autoinflammatory diseases and implications for common illnesses. J Allergy Clin Immunol. 2009;124:1141–1149. doi: 10.1016/j.jaci.2009.11.016. quiz 1150-1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto Y, Moki T, Takizawa T, Shiratsuchi A, Nakanishi Y. Evidence for phagocytosis of influenza virus-infected, apoptotic cells by neutrophils and macrophages in mice. J Immunol. 2007;178:2448–2457. doi: 10.4049/jimmunol.178.4.2448. [DOI] [PubMed] [Google Scholar]
- Hayden MS, Ghosh S. Shared principles in NF-kappaB signaling. Cell. 2008;132:344–362. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- Ichinohe T, Lee HK, Ogura Y, Flavell R, Iwasaki A. Inflammasome recognition of influenza virus is essential for adaptive immune responses. J Exp Med. 2009;206:79–87. doi: 10.1084/jem.20081667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs JP, Ortiz-Lopez A, Campbell JJ, Gerard CJ, Mathis D, Benoist C. Deficiency of CXCR2, but not other chemokine receptors, attenuates autoantibody-mediated arthritis in a murine model. Arthritis Rheum. 2010;62:1921–1932. doi: 10.1002/art.27470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kash JC, Basler CF, Garcia-Sastre A, Carter V, Billharz R, Swayne DE, Przygodzki RM, Taubenberger JK, Katze MG, Tumpey TM. Global host immune response: pathogenesis and transcriptional profiling of type A influenza viruses expressing the hemagglutinin and neuraminidase genes from the 1918 pandemic virus. J Virol. 2004;78:9499–9511. doi: 10.1128/JVI.78.17.9499-9511.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kash JC, Tumpey TM, Proll SC, Carter V, Perwitasari O, Thomas MJ, Basler CF, Palese P, Taubenberger JK, Garcia-Sastre A, et al. Genomic analysis of increased host immune and cell death responses induced by 1918 influenza virus. Nature. 2006;443:578–581. doi: 10.1038/nature05181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastner DL, Aksentijevich I, Goldbach-Mansky R. Autoinflammatory disease reloaded: a clinical perspective. Cell. 2010;140:784–790. doi: 10.1016/j.cell.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobasa D, Jones SM, Shinya K, Kash JC, Copps J, Ebihara H, Hatta Y, Kim JH, Halfmann P, Hatta M, et al. Aberrant innate immune response in lethal infection of macaques with the 1918 influenza virus. Nature. 2007;445:319–323. doi: 10.1038/nature05495. [DOI] [PubMed] [Google Scholar]
- Le Goffic R, Balloy V, Lagranderie M, Alexopoulou L, Escriou N, Flavell R, Chignard M, Si-Tahar M. Detrimental contribution of the Toll-like receptor (TLR)3 to influenza A virus-induced acute pneumonia. PLoS Pathog. 2006;2:e53. doi: 10.1371/journal.ppat.0020053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin KL, Suzuki Y, Nakano H, Ramsburg E, Gunn MD. CCR2+ monocyte-derived dendritic cells and exudate macrophages produce influenza-induced pulmonary immune pathology and mortality. J Immunol. 2008;180:2562–2572. doi: 10.4049/jimmunol.180.4.2562. [DOI] [PubMed] [Google Scholar]
- London NR, Zhu W, Bozza FA, Smith MC, Greif DM, Sorensen LK, Chen L, Kaminoh Y, Chan AC, Passi SF, et al. Targeting Robo4-dependent Slit signaling to survive the cytokine storm in sepsis and influenza. Sci Transl Med. 2010;2:23ra19. doi: 10.1126/scitranslmed.3000678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandi B, Behar SM. Regulation of neutrophils by interferon-gamma limits lung inflammation during tuberculosis infection. J Exp Med. 2011;208:2251–2262. doi: 10.1084/jem.20110919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otte A, Sauter M, Alleva L, Baumgarte S, Klingel K, Gabriel G. Differential host determinants contribute to the pathogenesis of 2009 pandemic H1N1 and human H5N1 influenza A viruses in experimental mouse models. Am J Pathol. 2011;179:230–239. doi: 10.1016/j.ajpath.2011.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrone LA, Plowden JK, Garcia-Sastre A, Katz JM, Tumpey TM. H5N1 and 1918 pandemic influenza virus infection results in early and excessive infiltration of macrophages and neutrophils in the lungs of mice. PLoS Pathog. 2008;4:e1000115. doi: 10.1371/journal.ppat.1000115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi L, Kash JC, Dugan VG, Jagger BW, Lau YF, Sheng ZM, Crouch EC, Hartshorn KL, Taubenberger JK. The ability of pandemic influenza virus hemagglutinins to induce lower respiratory pathology is associated with decreased surfactant protein D binding. Virology. 2011;412:426–434. doi: 10.1016/j.virol.2011.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu LQ, Stumpo DJ, Blackshear PJ. Myeloid-specific tristetraprolin deficiency in mice results in extreme lipopolysaccharide sensitivity in an otherwise minimal phenotype. J Immunol. 2012;188:5150–5159. doi: 10.4049/jimmunol.1103700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon R, Hoffmann E, Webster RG. Inhibition of the cytokine response does not protect against lethal H5N1 influenza infection. Proc Natl Acad Sci U S A. 2007;104:12479–12481. doi: 10.1073/pnas.0705289104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders CJ, Doherty PC, Thomas PG. Respiratory epithelial cells in innate immunity to influenza virus infection. Cell Tissue Res. 2011;343:13–21. doi: 10.1007/s00441-010-1043-z. [DOI] [PubMed] [Google Scholar]
- Shembade N, Ma A, Harhaj EW. Inhibition of NF-kappaB signaling by A20 through disruption of ubiquitin enzyme complexes. Science. 2010;327:1135–1139. doi: 10.1126/science.1182364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinya K, Okamura T, Sueta S, Kasai N, Tanaka M, Ginting TE, Makino A, Eisfeld AJ, Kawaoka Y. Toll-like receptor pre-stimulation protects mice against lethal infection with highly pathogenic influenza viruses. Virol J. 2011;8:97. doi: 10.1186/1743-422X-8-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun SC, Ganchi PA, Ballard DW, Greene WC. NF-kappa B controls expression of inhibitor I kappa B alpha: evidence for an inducible autoregulatory pathway. Science. 1993;259:1912–1915. doi: 10.1126/science.8096091. [DOI] [PubMed] [Google Scholar]
- Tate MD, Brooks AG, Reading PC. Specific sites of N-linked glycosylation on the hemagglutinin of H1N1 subtype influenza A virus determine sensitivity to inhibitors of the innate immune system and virulence in mice. J Immunol. 2011a;187:1884–1894. doi: 10.4049/jimmunol.1100295. [DOI] [PubMed] [Google Scholar]
- Tate MD, Brooks AG, Reading PC, Mintern JD. Neutrophils sustain effective CD8(+) T-cell responses in the respiratory tract following influenza infection. Immunol Cell Biol. 2012;90:197–205. doi: 10.1038/icb.2011.26. [DOI] [PubMed] [Google Scholar]
- Tate MD, Deng YM, Jones JE, Anderson GP, Brooks AG, Reading PC. Neutrophils ameliorate lung injury and the development of severe disease during influenza infection. J Immunol. 2009;183:7441–7450. doi: 10.4049/jimmunol.0902497. [DOI] [PubMed] [Google Scholar]
- Tate MD, Ioannidis LJ, Croker B, Brown LE, Brooks AG, Reading PC. The role of neutrophils during mild and severe influenza virus infections of mice. PLoS One. 2011b;6:e17618. doi: 10.1371/journal.pone.0017618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taubenberger JK, Morens DM. 1918 Influenza: the mother of all pandemics. Emerg Infect Dis. 2006;12:15–22. doi: 10.3201/eid1201.050979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taubenberger JK, Morens DM. The pathology of influenza virus infections. Annu Rev Pathol. 2008;3:499–522. doi: 10.1146/annurev.pathmechdis.3.121806.154316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teijaro JR, Walsh KB, Cahalan S, Fremgen DM, Roberts E, Scott F, Martinborough E, Peach R, Oldstone MB, Rosen H. Endothelial cells are central orchestrators of cytokine amplification during influenza virus infection. Cell. 2011;146:980–991. doi: 10.1016/j.cell.2011.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas PG, Dash P, Aldridge JR, Jr, Ellebedy AH, Reynolds C, Funk AJ, Martin WJ, Lamkanfi M, Webby RJ, Boyd KL, et al. The intracellular sensor NLRP3 mediates key innate and healing responses to influenza A virus via the regulation of caspase-1. Immunity. 2009;30:566–575. doi: 10.1016/j.immuni.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumpey TM, Garcia-Sastre A, Taubenberger JK, Palese P, Swayne DE, Pantin-Jackwood MJ, Schultz-Cherry S, Solorzano A, Van Rooijen N, Katz JM, et al. Pathogenicity of influenza viruses with genes from the 1918 pandemic virus: functional roles of alveolar macrophages and neutrophils in limiting virus replication and mortality in mice. J Virol. 2005;79:14933–14944. doi: 10.1128/JVI.79.23.14933-14944.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turer EE, Tavares RM, Mortier E, Hitotsumatsu O, Advincula R, Lee B, Shifrin N, Malynn BA, Ma A. Homeostatic MyD88-dependent signals cause lethal inflamMation in the absence of A20. J Exp Med. 2008;205:451–464. doi: 10.1084/jem.20071108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JP, Bowen GN, Padden C, Cerny A, Finberg RW, Newburger PE, Kurt-Jones EA. Toll-like receptor-mediated activation of neutrophils by influenza A virus. Blood. 2008;112:2028–2034. doi: 10.1182/blood-2008-01-132860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T, Kawaoka Y. Pathogenesis of the 1918 pandemic influenza virus. PLoS Pathog. 2011;7:e1001218. doi: 10.1371/journal.ppat.1001218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner SL, Kearns JD, Zadorozhnaya V, Lynch C, O’Dea E, Boldin MP, Ma A, Baltimore D, Hoffmann A. Encoding NF-kappaB temporal control in response to TNF: distinct roles for the negative regulators IkappaBalpha and A20. Genes Dev. 2008;22:2093–2101. doi: 10.1101/gad.1680708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wertz IE, O’Rourke KM, Zhou H, Eby M, Aravind L, Seshagiri S, Wu P, Wiesmann C, Baker R, Boone DL, et al. De-ubiquitination and ubiquitin ligase domains of A20 downregulate NF-kappaB signalling. Nature. 2004;430:694–699. doi: 10.1038/nature02794. [DOI] [PubMed] [Google Scholar]
- Zhu W, London NR, Gibson CC, Davis CT, Tong Z, Sorensen LK, Shi DS, Guo J, Smith MC, Grossmann AH, et al. Interleukin receptor activates a MYD88-ARNO-ARF6 cascade to disrupt vascular stability. Nature. 2012;492:252–255. doi: 10.1038/nature11603. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.