Summary
Vaccines work by eliciting an immune response and consequent immunological memory that mediates protection from infection or disease. Recently new methods have been developed to dissect the immune response in experimental animals and humans which have led to increased understanding of the molecular mechanisms that control differentiation and maintenance of memory T and B cells. In this review we will provide an overview of the cellular organization of immune memory and underline some of the outstanding questions on immunological memory and how they pertain to vaccination strategies. Finally we will discuss how we can learn about antigen design from the interrogation of our memory T and B cells – a journey from vaccines to memory and back.
Introduction
Immunological memory is the ability of the immune system to respond with greater vigor upon re-encounter with the same pathogen and constitutes the basis for vaccination (Ahmed and Gray, 1996). In fact, the concept of vaccination originated several hundred years ago from historical observations, dating as far back as 400 B.C., that individuals that survived a disease rarely got the same disease a second time (Finley Jr, 1951; Plotkin and Plotkin, 2008). The first recorded attempts at immunization occurred in the 16th century when the process of variolation was used to prevent smallpox (Plotkin and Plotkin, 2008). This involved injecting smallpox pustules from an infected patient into healthy individuals. It is remarkable that these first attempts at immunization pre-date any knowledge about microbiology and immunology (Janeway et al., 2005). The major breakthrough in vaccination came in 1796 when Jenner used cowpox as a vaccine against smallpox. It is worth noting that this landmark work of Jenner was also rooted in the concept of memory since he had astutely observed that milkmaids who had gotten cowpox were spared the ravages of smallpox (Janeway et al., 2005; Plotkin and Plotkin, 2008).
Vaccination remains the most effective method of preventing infectious diseases and represents the most relevant contribution of immunology to human health (Plotkin and Plotkin, 2008; Siegrist, 2008). The phenomenal success of vaccines against polio, smallpox, measles, diphtheria, tetanus, rabies, etc., demonstrates the potential of this approach in reducing the global burden of infectious diseases and, in the case of smallpox, of completely eradicating a scourge that used to kill and disfigure a significant population of the world (Breman and Arita, 1980; WHO, 1980). However, despite these remarkable successes there are major challenges that still remain and there is an urgent need to develop vaccines against important human pathogens such as HIV, Mycobacterium tuberculosis, Plasmodium falciparum, HCV, RSV and dengue virus (Hall, 2001; Hill, 2006; Houghton and Abrignani, 2005; Johnston and Fauci, 2007; Langhorne et al., 2008; McMichael et al., 2010; Skeiky and Sadoff, 2006; Walker and Burton, 2008; Whitehead et al., 2007). In addition, vaccines to fight cancer and autoimmune diseases are now a highly sought after goal and represent an exciting new area of vaccine research and development (Larche and Wraith, 2005; Lollini et al., 2006; Melief and van der Burg, 2008).
In this review we will discuss how studies on immunological memory, by defining the metrics, quality and specificity of the immune response, may provide a rational approach to vaccine development. Accordingly, our article is divided into three sections: in the first part we will provide an overview of the cellular organization of immune memory; in the second part we will consider some of the outstanding questions on immunological memory and protective immunity and how they pertain to vaccination strategies; and in the last section we will discuss how we can learn from our memory T and B cells – a process of going back from memory to vaccine design.
Cellular Organization of Immunological Memory
Division of labor among memory cells
Memory T and B cells are the progeny of antigen specific naïve cells that have been clonally expanded in the course of an immune response and survive once antigen has been eliminated. Memory cells confer immediate protection and generate secondary responses which are more rapid and of higher magnitude as compared to primary responses (Table 1). In the B cell system immediate protection is mediated by long-lived plasma cells that are present in the bone marrow and secrete antibodies in an antigen-independent fashion thus maintaining constant levels in serum and body fluids (Radbruch et al., 2006); recall responses are mediated by memory B cells that rapidly proliferate and differentiate in response to antigenic stimulation generating a burst of plasma cells and a marked but transient elevation in serum antibodies. A similar division of labor applies to the T cell system (Sallusto et al., 2004). Immediate protection is conferred by circulating or tissue resident effector memory T cells (TEM) that survey frontline barriers and diseased tissues for incoming pathogens and display immediate effector function upon antigen recognition; recall responses are mediated by central memory T cells (TCM) that patrol the T cell areas of secondary lymphoid tissues where they can rapidly proliferate in response to antigens presented by dendritic cells (DC). This division of labor has implications for vaccination strategies since protection from infection or disease may rely primarily on either mechanism depending on the nature of the pathogen and the size and route of challenge. For instance preformed antibodies and long lived plasma cells are required to neutralize toxins or prevent infection by an incoming virus, while recall responses mediated by TCM and memory B cells may be sufficient to protect against viruses with long incubation time (Plotkin et al., 2008).
Table 1.
First and second lines of adaptive immunity against pathogens
| First line | Second line | |
|---|---|---|
| Central players | Antibody (IgA and IgG) TEM (tissue resident memory T cells) |
Memory B cells TCM |
| Localization of the central players | Sites of pathogen entry (gut, genitaltract, respiratory tract, blood etc.) | Lymphoid tissues |
| Rapid protection | Yes, immediately. | Delayed. Memory cells in lympoid tissues respond rapidly compared to naïve cells. However, their capacity for immediate protection is limited because of the delay in reaching sites of infection. |
| Recall response | Minimal to moderate. | Substantial clonal expansion. Differentiation into effector and plasma cells. Migration into multiple tissues including sites of infection. |
From naïve to memory T cells
The quality and amount of memory T cells is set during antigen-driven primary immune responses which are initiated in the T cell areas of secondary lymphoid organs where rare antigen-specific naïve T cells are stimulated by antigen presented by activated DC. Pathogens can activate DC by triggering multiple innate receptors either directly via pathogen associated molecular patterns (PAMPS) or indirectly via danger associated molecular patterns (DAMPS) leading to enhanced antigen presentation, costimulation and production of polarizing cytokines (Iwasaki and Medzhitov, 2010). It is the nature and combination of the innate receptors triggered on DC that determine their capacity to imprint different fates on proliferating T cells (Reiner et al., 2007). In response to certain viruses and intracellular pathogens DC produce IL-12, which promotes differentiation to Th1 cells capable of producing IFN-γ which is effective against such pathogens (Macatonia et al., 1995). Likewise, in response to fungi or certain bacteria, DC and monocytes produce IL-1β, IL-6 and IL-23 that drive differentiation of Th17 cells that through secretion of IL-17 and recruitment of neutrophils mediate protection against extracellular pathogens (Acosta-Rodriguez et al., 2007). The protective nature of these polarized responses is underlined by studies of human immunodeficiencies, where patients with defective Th1 or Th17 responses suffer from mycobacterial or fungal infections, respectively (Ma et al., 2008; Milner et al., 2008). A more complex pathway triggered by helminths or allergens and involving epithelial cells and IL-4-producing innate or natural helper cells appears to control the induction of Th2 cells that produce IL-4, IL-5 and IL-13 and mediate protection or allergy (Coffman, 2010). Other effector (and memory) T helper cell subsets have been recently characterized in mice and humans, such as Th9 (Veldhoen et al., 2008) and Th22 (Duhen et al., 2009; Trifari et al., 2009) which may be involved in allergy and skin defense respectively. CD4 T cells not only act directly to promote different types of inflammatory responses in tissues, but also play an essential role in B cell and CD8 T cell responses. A dedicated subset of follicular helper T cells (TFH) is required for induction of germinal center reactions that leads to differentiation of memory B cells and long lived plasma cells secreting high affinity antibodies of switched isotypes (Vinuesa et al., 2005). TFH cells produce IL-21 and their differentiation is dependent on Bcl-6 (Kassiotis and O’Garra, 2009) and a high avidity interaction with antigen specific B cells (Fazilleau et al., 2009; Qi et al., 2008). T helper cells can also promote DC maturation via CD40L–CD40 interaction and in this way help the generation of effector and memory CD8 T cells against poorly immunogenic antigens such as protein antigens (Lanzavecchia, 1998). In the context of vaccine design, more detailed studies should aim at defining on the one hand the pattern of expression of innate receptors in mice and humans, on the other the cytokines and cues that drive differentiation of different effector T cell subsets.
The quality of the T cell response is profoundly influenced not only by the PAMPs and DAMPs but also by the nature of the DC that present antigen and by the tissue microenvironment in which the T cell response takes place. A distinct subset of mouse CD8α DC can process and present exogenous antigens on MHC class I molecules and crossprime CTL responses (Heath and Carbone, 2009) and DC with similar properties have been recently described in humans (Villadangos and Shortman, 2010). Gut and skin derived DC can imprint the expression of skin and gut homing receptors, such as CCR9 or CCR10, in differentiating T cells (Sigmundsdottir and Butcher, 2008). Monocytes represent an abundant and heterogeneous population of circulating cells that can be rapidly recruited to sites of inflammation and immune response where they differentiate into DC (Auffray et al., 2009). The importance of studies addressing the role of different DC and monocytes subsets in T cell responses cannot be overemphasized in view of the possibility of targeting antigens and vaccines to different cell types in vivo (Bonifaz et al., 2004; Kamphorst et al., 2010).
B cells and antibody responses
The B cell response to protein antigens is a highly orchestrated process that is initiated at the boundary between T and B cell areas where T cells primed by antigen-presenting DC encounter specific B cells that have captured and processed native antigen relayed by macrophages lining the subcapsular sinus (Phan et al., 2009). The antigen-specific T-B interaction leads to a rapid expansion and differentiation of B cells into short lived plasma cells producing unmutated IgM antibody. This extrafollicular response is followed by the formation of germinal center reaction where TFH cells and antigen trapped on follicular dendritic cell (FDC) networks drive proliferation, isotype switch and affinity maturation of antigen-specific B cells leading to the generation of memory B cells and long-lived plasma cells that produce high affinity somatically mutated antibodies of switched isotypes (Allen et al., 2007). The duration of the germinal center reaction may vary depending on the nature of the antigen and may last for several weeks or months, implying that high affinity antibodies and memory cells can be generated well after elimination of the pathogen (Dogan et al., 2009b).
Unlike protein antigens that elicit T-dependent B cell responses, bacterial polysaccharides trigger T-independent B cell responses that result in the generation of short lived plasma cells without memory cells. Repeated stimulations with polysaccharides can lead to progressively decreased responses and eventually exhaust specific B cells (Pollard et al., 2009). In contrast the same polysaccharides conjugated to protein antigens elicit a T-dependent B cell response and memory B cells that can be effectively boosted by the same glycoconjugate. This approach has been successfully used to develop highly effective conjugated vaccines against pneumococcus and meningococcus (Plotkin and Plotkin, 2008).
In humans a large fraction of circulating B cells are IgM+ and carry somatically mutated Ig genes. It is currently unclear whether these cells represent a circulating equivalent of mouse marginal zone B cells that respond to blood born pathogens or a population of bona fide memory cells (Seifert and Kuppers, 2009; Weill et al., 2009). Recently, using a mouse model of AID-dependent memory B cell labeling it was shown that B cell memory appears in IgM+ and IgG1+ B cells that are found both in germinal centers as well as outside of B cell follicles. After challenge, the IgG subset differentiated into plasma cells, whereas the IgM subset reinitiated a germinal center reaction suggesting (Dogan et al., 2009a). These findings, when translated into the human system will have important implication for monitoring vaccination by dissecting immediate antibody responses from germinal center dependent secondary responses.
Maintenance of memory cells
Only a small fraction of the expanded cells present at the peak of the immune response survive as memory cells. In mice the precursors of memory CD8 T cells could be identified as IL-7Rhi cells, while the more abundant KLRG1hi cells were shown to represent short lived effector cells (Joshi et al., 2007; Kaech et al., 2003; Kaech and Wherry, 2007; Sarkar et al., 2008). It appears that the ratio between effectors and memory precursors is set by differential expression of transcription factors, such as T-bet and Eomesodermin, which in turn is dependent on the strength of stimulation by antigen, cytokines and Wnt signaling (Gattinoni et al., 2009; Intlekofer et al., 2005). Using methods for analyzing the progeny of single cells it has been recently shown that within a single clone T cells can adopt multiple fates, including different types of effector and memory cells, and that individual naive T cells expressing high or low avidity TCR yield both effector and memory T cells (Gerlach et al., 2010; Stemberger et al., 2007).
Memory T and B cells as well as long lived plasma cells can be maintained at relatively constant numbers in the absence of the eliciting antigen for virtually a lifetime. Their survival is dependent on exogenous cytokines that are available in distinct niches and determine the size of the memory pool. For CD4 and CD8 memory T cells the survival cytokines are IL-7 and IL-15 which maintain these cells in a state of slow but continuous proliferation (Surh and Sprent, 2008). Memory B cells also divide at low rate but a survival cytokine has not yet been defined although it has been shown that an intact BCR and phospholipase Cγ2 are required for their long term maintenance and function (Hikida et al., 2009). In contrast, long lived plasma cells survive without dividing in bone marrow niches organized by stromal cells that provide, in association with other cells, survival cytokines such as IL-6 and APRIL (Radbruch et al., 2006). Unlike memory T cells that elaborate their cytokines only in response to antigenic stimulation, long lived plasma cells continually produce antibodies thus maintaining serum levels constant. The bone marrow also contains memory CD4 and CD8 T cells which may recirculate (Di Rosa and Pabst, 2005), as well as a recently described population of sessile resting memory T cells that occupies a distinct niche in contact with IL-7 producing stromal cells (Tokoyoda et al., 2009).
The fact that terminally differentiated effector memory cells such as TEM and plasma cells have reduced or no proliferative and reconstitution capacity led to the hypothesis that long term maintenance of memory cells would be dependent on cells which retain proliferative capacity, such as TCM and memory B cells or even on a specialized subset of “memory stem cells” (Lanzavecchia and Sallusto, 2002). In the case of T cells this model is supported by the findings that long-term reconstitution capacity is characteristic of TCM rather than TEM cells (Wherry et al., 2003) and by the prospective isolation of a putative memory stem cell (Gattinoni et al., 2009; Turtle et al., 2009). Recent studies identified signaling pathways that appear to be involved in the differentiation of memory stem cells as well as drugs that favor the generation of TCM cells (Araki et al., 2009; Gattinoni et al., 2009; Pearce et al., 2009).
There has been much debate on the role of antigen in maintaining memory but it is now clear from carefully done cell transfer experiments employing a variety of model systems that both memory T and B cells can persist in the absence of antigen (Gray, 2002; Hou et al., 1994; Lau et al., 1994; Zinkernagel, 2003). These findings were further extended by showing that memory T cells can persist even in the absence of the restricting MHC molecules (Murali-Krishna et al., 1999; Swain et al., 1999). However, antigen persistence in GC plays an important role in selection and maturation of the B cell response and there are data suggesting that antigen-antibody complexes can persist on follicular dendritic cells for extended periods of time and this antigen may play a role in shaping the B cell repertoire (Chen et al., 1978; Tew et al., 1980). Interestingly, sustained high levels of soluble antigens can often lead to induction of tolerance or exhaustion both in T and B cells. In the case of certain chronic viral infections antigen specific T and B cells express a variety of inhibitory receptors that result in functional exhaustion of these cells (Barber et al., 2006; Virgin et al., 2009; Wherry et al., 2007).
Genomics of memory T and B cell differentiation
During the past decade there has been considerable interest in defining the global gene expression profiles of memory T and B cells (Haining and Wherry, 2010; Kaech and Wherry, 2007; Staal and Clevers, 2005). This approach has been highly informative since it provides an unbiased look at all the genes that are up or down regulated during memory differentiation. Particularly valuable have been studies that have performed an integrative genomic analysis of antigen specific T cells as they progress from the naïve to effector to memory transition (Kaech et al., 2002). Such longitudinal studies have not only identified key genes (transcription factors, survival molecules, cytokine and chemokine receptors, homing and signaling molecules, etc) that are differentially expressed between naïve and memory T cells but have also provided insights into mechanisms of memory formation and in defining the signatures of effector and memory T cells. Gene profiling studies also provided insights into the mechanisms of T cell dysfunction during chronic infections and identified key inhibitory receptors that are expressed by the exhausted virus-specific T cells (Barber et al., 2006; Wherry et al., 2007).
Naïve B cells may differentiate directly into short-lived plasma cells, or may pass through the germinal center reaction to become either memory B cells or long-lived plasma cells. The gene expression patterns associated with these transitions have been analyzed by several groups using DNA microarrays (Alizadeh et al., 2000; Bhattacharya et al., 2007; Good et al., 2009; Good and Tangye, 2007; Jourdan et al., 2009; Klein et al., 2003; Tarte et al., 2003; Tomayko et al., 2008; Underhill et al., 2003; Vinuesa et al., 2002). The differentiation of a naïve B cell to either a germinal center B cell or a plasma cell is accompanied by major changes in gene regulation, driven by the master transcription factors Bcl-6 and blimp-1. Less is known about the gene expression differences that distinguish short- from long-lived plasma cells. The gene expression signatures of long-lived plasma cells isolated from human bone marrow has recently been compared with that of short-lived plasma cells derived from in vitro stimulation of memory B cells (Jourdan et al., 2009). This study showed that bone marrow plasma cells display a reduced expression of the pro-apoptic molecule Fas as well as downregulation of molecules involved in lymphocyte trafficking such as CD62L and S1PR1. Thus, it appears that bone marrow plasma cells display resistance to apoptosis and a reduced ability to traffic to other anatomic sites. More detailed studies defining the signatures of long-lived versus short-lived plasma cells will be useful in developing vaccines that induce long-term humoral immunity.
Outstanding Questions on Immunological Memory and Vaccination
In the previous section we have given an overview of the cellular organization of immunological memory. We will now consider some key questions about memory and protective immunity and how they pertain to developing the next generation of vaccines. Our remarkable successes in vaccines have come mostly against acute infections caused by invariant pathogens (Plotkin et al., 2008). However, we lack effective vaccines against pathogens that are highly variable and/or cause persistent and latent infections such as HIV, HCV, and M. tuberculosis (Houghton and Abrignani, 2005; Johnston and Fauci, 2007; McMichael et al., 2010; Skeiky and Sadoff, 2006; Walker and Burton, 2008). It has also proven difficult to develop successful vaccines against acute infections such as RSV and malaria where the natural infection itself does not result in complete protection against re-infection – in this case the vaccine has to trump Nature (Hall, 2001; Hill, 2006; Langhorne et al., 2008). Developing effective vaccines against these important human diseases presents a unique set of challenges for each pathogen and there are articles in this issue of Immunity that deal specifically with HIV, TB and malaria vaccines ( ). However, there are also fundamental concepts of immune memory and vaccination that cut across different pathogens and we will now consider a few of these key issues starting with a brief general discussion on protective immunity and correlates of vaccine efficacy.
Protective immunity and correlates of vaccine efficacy
There has been considerable interest and debate in determining the relative importance of T and B cell responses in protective immunity (Ahmed and Gray, 1996; Ahmed et al., 2007; Appay et al., 2008). When examining this issue, one must remember that antibodies and T cells have evolved to perform distinct functions. The business of antibodies is to deal with the microbe itself (i.e., free virus particles, bacteria, and parasites), and that of T cells is to deal with infected cells. Since T cells can recognize microbial antigens only in association with host MHC molecules, the free virus particles or bacteria are invisible to them. Thus, antibody provides our only specific defense against free microbial organisms, and the importance of preexisting antibody in protective immunity against infectious diseases cannot be overemphasized. In fact, antibody is likely to be the sole mechanism of protective immunity against bacteria and parasites that have an exclusively extracellular lifestyle. In these situations, it is relatively easy to determine the correlates of vaccine efficacy based on the levels of serum antibody against the pathogen or the toxin (Siegrist, 2008). However, the equation begins to change for viruses and for bacteria and parasites that can survive or replicate intracellularly. Although antibody again provides the first line of defense against such infections and antibody levels are used as the correlates of vaccine efficacy for several viral vaccines such as influenza virus and yellow fever virus (Siegrist, 2008), there are often situations where not all of the inoculum is neutralized or opsonized by the preexisting antibody. This is where the T cells come into play by either killing the infected cell and/or releasing cytokines that inhibit growth of the microbe or impair the ability of the pathogen to survive inside the cell. For example, T cell immunity is a more accurate correlate for efficacy of the zoster vaccine (Levin, 2008).
Defining correlates of vaccine efficacy against pathogens that vary is going to be much more challenging since the mechanism of protective immunity may vary depending upon the viral strain that the individual is exposed to. It is likely that newer approaches will be needed to define correlates and the traditional method of using a single parameter to determine vaccine efficacy is simply not going to work with our more difficult vaccines. There is now considerable excitement at the prospect of using systems biology approaches to define correlates of vaccine efficacy (see review by Pulendran et al. in this issue). However, even this by itself may not be sufficient and perhaps the solution lies in combining the systems biology approaches with more novel methods of assessing not only the quantity but also the quality of the memory T and B cell responses.
How many memory cells can we accommodate - issues of competition and space
One of the more fascinating things about the immune system is the ability of lymphocytes to sense their numbers (Takada and Jameson, 2009). This homeostatic regulation is quite cell specific; B cells sense each other and not T cells and there is also specificity in the counting of CD4 versus CD8 T cells. For example, if CD4 T cells are depleted then there is a selective expansion of CD4 T cells and not CD8 T cells to fill up the compartment, and vice versa upon reduction of CD8 T cells. The mechanism of this sensing is not known but the ability of the immune system to count has important implications for vaccination. The conventional wisdom is that the size of the immune system including the number of memory cells is fixed and that as new memory cells are generated upon infection or vaccination there must be an erosion of pre-existing memory cells to make room for the new ones (Freitas and Rocha, 2000; Goldrath, 2002; Welsh et al., 2010). In principle this is correct but it is not known how fixed this ceiling is and how much flexibility exists in accommodating new memory cells. A recent study has addressed this critical issue and shown that the size of the total memory CD8 T cell pool in mice can almost double to accommodate new cells with minimal attrition of pre-existing memory cells (Vezys et al., 2009). It is interesting that this increase occurred almost exclusively in effector memory T cells suggesting that this memory T cell subset adapts in the host according to immunological experience. In this context it is worth noting that large numbers of HCMV and EBV specific effector memory CD8 T cells accumulate in humans over time (Klenerman and Hill, 2005). In some instances this increase is so striking that T cells specific to a single HCMV epitope can account for up to 20% of the total CD8 T cell response.
It is well documented from studies in both mice and humans that the percentage of naïve T cells declines with age whereas the percentage of memory T cells increases (Linton and Dorshkind, 2004; Nikolich-Zugich, 2008). It is assumed that this reversal in the ratio of naïve versus memory T cells is entirely due to loss of naïve T cells reflecting decreased thymic output as a function of age. However, it has not been rigorously ruled out that this observed change in the ratio is also due to an increase in the number of memory T cells as a result of increased immunological experience over time. This issue needs to be addressed in detail by quantitating memory T cell numbers in lymphoid and non-lymphoid tissues using murine as well as non-human primate models. Another important question that deserves more attention is to determine if there is any sensing of numbers between naïve and memory T cells. In other words, does our immune system count total numbers of T cells irrespective of whether they are naïve or memory or are naïve and memory T cell numbers regulated independently. An understanding of these issues has significant implications for designing vaccination approaches.
The B cell system is also under homeostatic control and there are mechanisms for regulating naïve and memory B cell numbers as well as total immunoglobulin levels in the serum (Nimmerjahn and Ravetch, 2008; Sanz et al., 2008). A better understanding of the underlying regulatory mechanisms is needed. In particular, the issue of plasma cell homeostasis is critical for vaccine induced protective immunity. Vaccine induced antibodies can be maintained in the serum for extended periods by long-lived plasma cells that reside in the bone marrow and constitutively produce antibodies in the absence of antigen (Amanna et al., 2007; Manz et al., 1997; Slifka et al., 1998). It is believed that the bone marrow environment is particularly conducive for providing the necessary survival signals for plasma cells (Manz et al., 1997; O’Connor et al., 2004). It is important to determine how competition for these survival niches in the bone marrow impacts vaccine induced immunity. A critical question is how many plasma cells can the bone marrow accommodate? Do certain types of plasma cells compete better for these survival niches? If so, what are the defining characteristics of these cells? How can such plasma cells be generated? Answers to these questions will provide a rational approach to developing vaccines that induce long-term antibody responses.
Importance of mucosal versus systemic immunity
The immune system has evolved two lines of defense against pathogens (Table 1). The first line of defense consists of pre-formed mucosal antibody (IgA and IgG) and effector memory T cells that are present at the portals of pathogen entry. In addition, serum antibody also provides an important first line of defense against pathogens that initiate infection through the blood. The second line of defense consists of memory T and B cells present in lymphoid tissues; these cells proliferate and differentiate into effector cells and migrate to sites of infection. It should be noted that memory cells in lymphoid tissues also elaborate effector functions rapidly (much faster than naïve cells) but their importance in immediate protection is limited because of the delay in reaching sites of infection. Consequently, the most critical difference between TCM and TEM is not so much the speed at which they become effectors but their actual anatomic location. The inability of TCM to provide immediate protection at mucosal sites is compensated by their ability to proliferate and generate a large pool of effector cells that are critical for limiting the spread of infection and for eventual clearance of the pathogen. Thus, an ideal vaccine should induce both mucosal as well as systemic immunity to provide both the first and second line of defense against infection and disease. However, for “hit and run” pathogens that infect mucosal sites and cause clinical symptoms within 24–48hrs (ex., rotavirus, norovirus, enteric bacteria, etc) it is essential to have effective mucosal immunity. This first line of defense is also important for viruses like HIV that can establish a latent infection if initial acquisition is not blocked.
Considerable progress has been made in identifying homing molecules on T and B cells that are utilized for tissue entry (Table 2). Of particular interest for mucosal immunity are homing receptors that lymphocytes use for migration to the gut, genital tract and respiratory tract – three major sites of pathogen entry. An important question that is not well understood is how these receptors are imprinted on activated T and B cells and how this knowledge can be harnessed to develop vaccines that will induce effective mucosal immunity. Several studies have shown that expression of specific homing receptors is coupled to the location of activation; for example, priming of T cells in mesenteric lymph nodes induces expression of a4b7 that is needed for migration to the gut whereas priming within inguinal lymph nodes induces expression of skin homing molecules (Sigmundsdottir and Butcher, 2008). These data are consistent with the observation that mucosal vaccines or infections are best for inducing T and B cell responses at the mucosa (Plotkin et al., 2008).
Table 2.
Lymphocyte homing molecules utilized for tissue entry
| Tissue | Homing receptors expressed by lymphocytes |
|---|---|
| LN T and B cell zones(Bromley et al., 2008; von Andrian and Mackay, 2000) | L-selectin, CCR7, CXCR4, CXCR5 |
| PP(von Andrian and Mackay, 2000) | L-selectin, a4b7, CCR7, CXCR5, CCR6 |
| Intestinal non-lymphoid tissue(Schon et al., 1999; Sigmundsdottir and Butcher, 2008) | a4b7, aielb7?, CCR9 |
| Female reproductive tract(Nakanishi et al., 2009) | a4b1, CXCR3 |
| Skin(Sigmundsdottir and Butcher, 2008) | CLA, CCR4, CCR10 |
| Bone Marrow(Denucci et al., 2009; Kunkel and Butcher, 2003) | a4b1, CXCR4 |
| Lung airways(Kohlmeier and Woodland, 2009) | a1b1, CXCR3, CCR4, CCR5 |
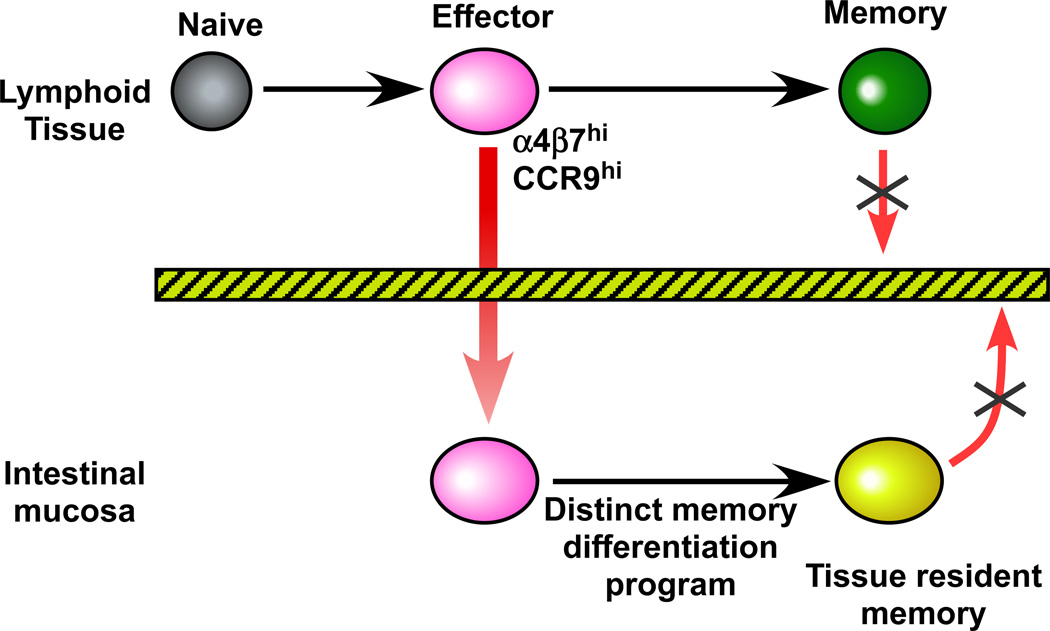
However, there are many examples of systemic infections inducing good mucosal immunity and also of some parenterally given vaccines eliciting mucosal responses (Plotkin et al., 2008). A recent study has provided a potential explanation for these observations by showing that priming of CD8 T cells in the spleen results in a promiscuous homing program that can drive cells to the intestinal mucosa (Masopust et al., 2010). The spleen is a central lymphoid organ and is not directly associated with any particular body surface. Thus, it makes teleological sense that T cells activated in the spleen have a promiscuous homing program so they can sample multiple tissues for infection. Interestingly expression of the gut homing receptors was very transient and only the early effector T cells in the spleen could migrate to the gut showing that there is only a small window of opportunity for seeding the mucosa (Fig. 1). What happens to the T cells after they migrate to the gut? Several studies have now shown that these T cells don’t recirculate with memory cells in the blood or in other tissues and also end up undergoing a unique differentiation program that is driven by the environment of the tissue (Fig. 1) (Jameson and Masopust, 2009).
Figure 1. T cell migration to the gut.
Effector CD8 T cells activated in lymphoid tissues transiently express gut homing receptors ( a4b7 , CCR9 ) and migrate to the intestinal mucosa. These T cells then undergo a distinct memory differentiation program within the gut and do not recirculate with memory T cells in other tissues. These tissue resident memory cells provide a first line of defense against pathogens that initiate infection through the intestinal mucosa.
An important difference between systemic and mucosal immunity is the duration of memory. As described in the earlier sections of this review, it is well documented that systemic memory persists for extended periods in both experimental animals and in humans; this is true not only for memory T and B cells but also for antibody levels in the serum that are maintained by long-lived plasma cells in the bone marrow. In contrast, the duration of mucosal immunity is relatively short lived. The underlying mechanisms of this waning memory at mucosal sites are not well understood and this remains one of the most challenging questions in vaccinology. Many critical issues need to be addressed: Do effector memory T cells in the mucosa turn over and replenish their numbers? If so, is this mediated by cytokines or by periodic re-exposure to antigen? What is the inter-mitotic life-span of these tissue resident memory T cells and what are the molecules necessary for their survival? What is the life-span of memory B cells and plasma cells at mucosal sites? Do mucosal plasma cells get the necessary survival signals and niches that are present in the bone marrow? A recent study has suggested that there is attrition of previously induced immune responses in the gut due to competition by ongoing responses being continuously generated by the commensal flora (Hapfelmeier et al., 2010). Answers to these above questions and others are needed to understand how memory is regulated at the mucosa and to design effective vaccine strategies for eliciting long-term mucosal immunity (Jameson and Masopust, 2009).
Optimizing prime-boost regimens
A multitude of expression vectors (bacterial, viral, DNA) have been developed during the past two decades and considerable effort has gone into optimizing their use as vaccine vectors (see article in this issue by Liu). Several studies have shown that boosting with a different vector carrying the same antigen is better for enhancing immune responses compared to boosting with the homologous vector. Such heterologous prime-boost approaches are now widely used in efforts to develop vaccines against HIV, HCV, malaria and TB (see articles in this issue). Although promising results have been obtained with various heterologous prime-boost vaccines some fundamental questions remain unanswered. Here we will briefly address two key questions; first, what is the optimal time for boosting and second, what is the effect of repeated booster shots on the quality of the immune response?
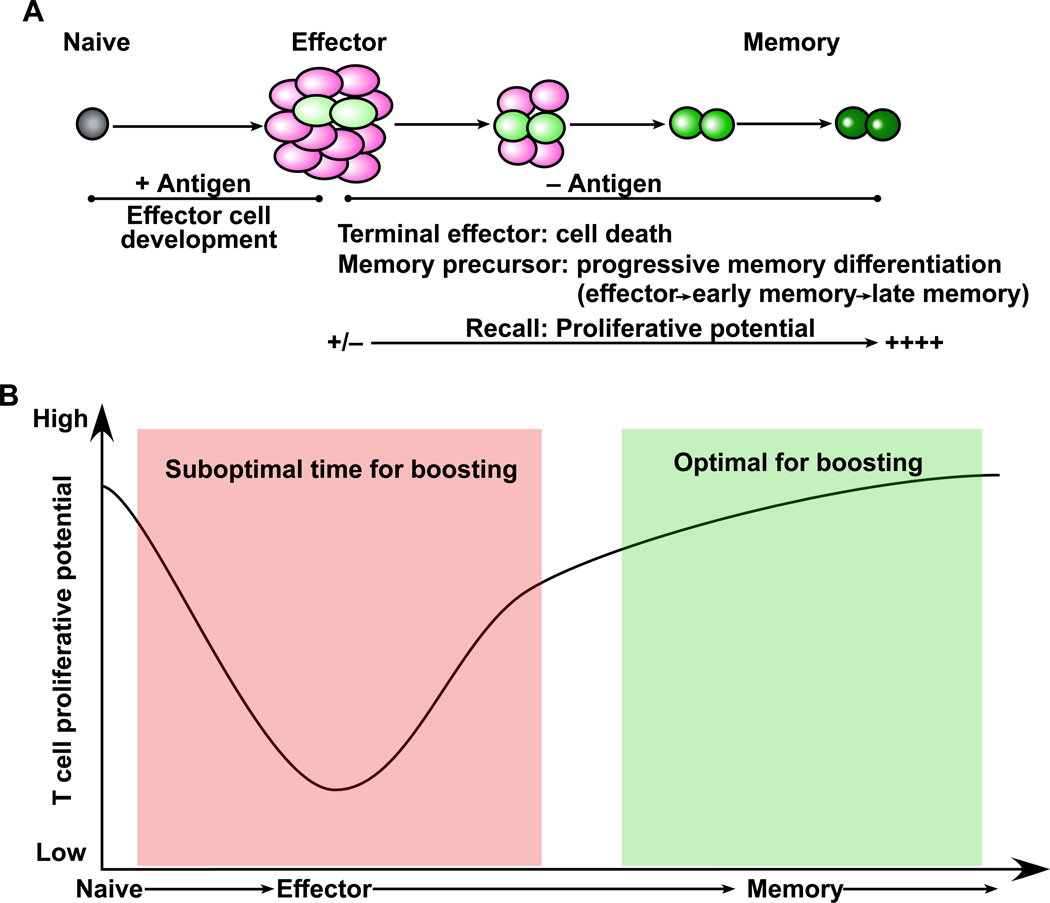
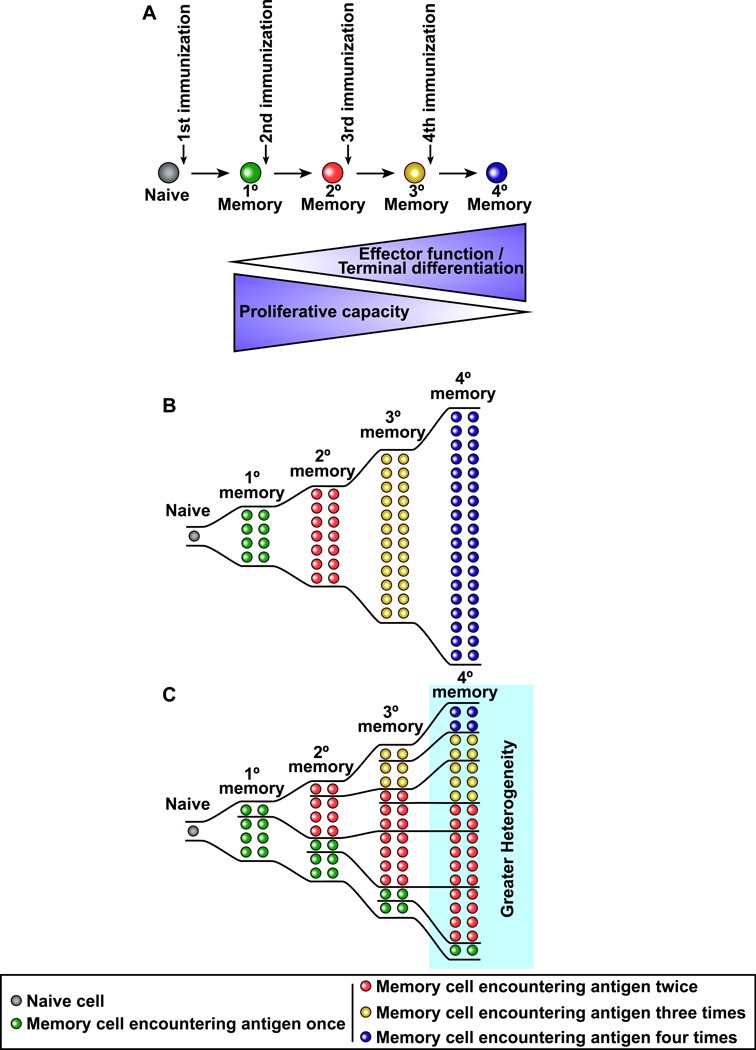
In determining the interval between the first immunization and the booster shot it is important to consider the differentiation pathways of memory B and T cells. Memory T cells with high proliferative potential do not form until several weeks after the first immunization so as a general rule it is better to have an interval of at least 2–3 months between the prime and the boost (Fig. 2). Boosting too early will give sub-optimal responses. Similar rules apply for boosting antibody responses since memory B cells have to go through the germinal center reaction and take several months to develop (Crotty et al., 2010). Recent studies have shown that repeated boosting can drive memory T cells towards terminal differentiation (Masopust et al., 2006; Wirth et al., 2010). This is good for generating effector memory T cells and also for driving cells to the mucosa but runs the risk of depleting the population of central memory cells (Fig. 3). However, if the repeated booster shots only recruit a subset of the previously generated memory cells then this will result in a heterogenous population of memory T cells at various stages of differentiation (Fig. 3). This could end up providing the right balance of TCM and TEM cells. In summary, by using our understanding of memory T and B cell differentiation it should be possible to optimize prime-boost strategies.
Figure 2. Using the principles of memory T cell differentiation to determine the optimal time for boosting.
(A) Following primary vaccination naïve T cells proliferate and differentiate into effector cells. The majority of these effectors undergo apoptosis but a subset further differentiates to form the pool of long-lived memory cells. This model of progressive memory T cell differentiation is characterized by a gradual acquisition of memory T cell properties such as the ability to make effective proliferative responses upon re-encounter with antigen. (B) Based on this memory differentiation program the optimal time for boosting is during the late stages of the effector to memory transition and therefore an interval of 2–3 months is recommended between the prime and the boost.
Figure 3. Repeated immunizations drive memory T cells towards terminal differentiation: Implications for memory cell heterogeneity and protective immunity.
(A) Repetitive antigen encounter results in memory T cells with more effector-like properties and with preferential location in non-lymphoid tissues but with reduced proliferative potential. (B) If all memory T cells are recruited into the response following each booster shot then the entire pool of memory T cells would be driven towards terminal differentiation. (C) If only some of the memory T cells are activated upon subsequent booster immunizations then one would end up with a heterogenous pool of memory T cells with both effectorlike cells at mucosal sites and also memory cells with proliferative capacity.
Modulating the quality of memory T cells
In order to develop successful vaccines against pathogens such as HIV, HCV, malaria, etc., it will be essential for the vaccine to induce not only neutralizing antibody responses but also to generate highly effective T cell immunity. Considerable effort has gone into developing vaccine regimens that will induce high frequencies of memory T cells, but there has been minimal emphasis on developing strategies to improve the functional qualities of the vaccine-induced memory T cells. However, there is now an appreciation that by targeting appropriate innate receptors on DC it is possible to modulate the functional qualities of memory cells. See the article in this issue by Coffman et al. on innate receptors and adjuvants for more information on this approach ( ).
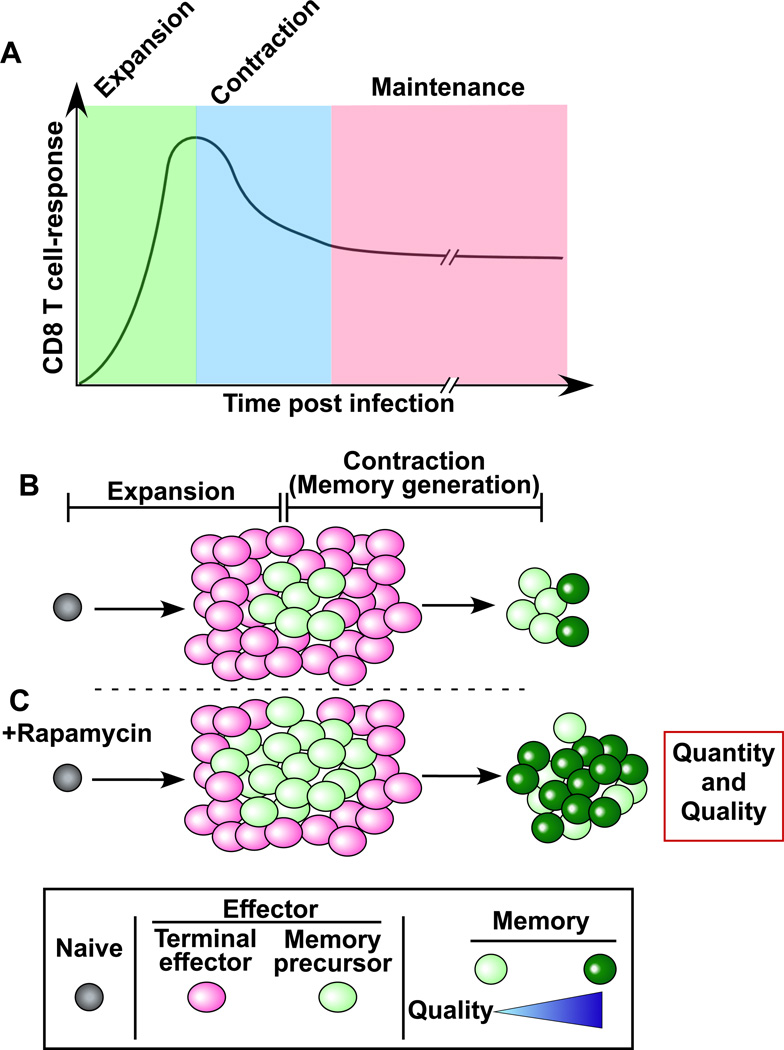
Recent studies have identified an additional approach using drugs that directly act on T cells to regulate memory differentiation (Araki et al., 2009; Pearce et al., 2009; Rao et al., 2010). These studies showed that mammalian target of rapamycin (mTOR) is a major regulator of memory CD8 T cell differentiation and that memory T cell qualities can be manipulated by regulating the mTOR pathway. In vivo administration of rapamycin, a specific inhibitor of mTOR, into vaccinated or infected mice significantly changed the fate of antigen-specific CD8 T cells. Rapamycin treatment during the T cell expansion phase increased the quantity of memory CD8 T cells by promoting the generation of memory precursors that survive and differentiate into long-lived memory cells. This treatment resulted in a similar number of antigen-specific effector CD8 T cells at the peak of the clonal expansion compared to untreated mice but reduced the apoptotic cell death during contraction phase. On the other hand, inhibiting mTOR with rapamycin during the contraction phase accelerated the effector to memory cell transition and improved the quality of memory CD8 T cells. Thus, targeting the mTOR pathway can enhance not only the magnitude but also the quality of memory CD8 T cells (Fig. 4).
Figure 4. Using drugs to modulate memory CD8 T cell differentiation.
(A) Antigen specific CD8 T cell responses after an acute infection or vaccination. During the expansion phase, naïve CD8 T cells proliferate and then become effector cells. After clearance of the pathogen, 90 to 95% of the effector T cells die during a contraction phase. The surviving 5–10% of the antigen-specific T cells become the memory population. (B) Without rapamycin treatment. (C) Rapamycin improves both quality and quantity of memory CD8 T cells. Rapamycin treatment increases memory precursor effector cells that survive during the contraction phase, and also improves quality of memory T cells by accelerating effector to memory T cell formation.
Using drugs that modulate memory differentiation is an interesting new approach to enhance vaccine-mediated immunity. Since rapamycin has historically been used as an “immunosuppressive” drug, there will be obvious safety concerns about using this drug as a vaccine adjuvant. However, it should be possible to identify safer drugs that target the mTOR pathway as potential adjuvants to enhance the functional qualities of memory T cells.
From Immunological Memory to Vaccine Design: Analytic Vaccinology
As mentioned in the previous section, our successes in vaccines have mostly come against invariant pathogens that cause acute infections followed by long-term protective immunity. However, there are several cases where the primary infection does not lead to protective immunity. This is the case of variable pathogens such as dengue or influenza viruses where memory elicited by the natural infection protects from the homologous virus but not from viruses of a different serotypes (Green and Rothman, 2006; Halstead, 2007; Subbarao and Joseph, 2007), while in the case of HIV-1 a rapid diversification of the transmitted virus makes the immune response ineffective (Johnston and Fauci, 2007; McMichael et al., 2010; Moir and Fauci, 2009). For other pathogens such as RSV the clearance of infection is not followed by protective memory since re-infection with the same virus can occur multiple times, although with decreased severity of illness (Hall, 2001). Finally other pathogens such as HCMV, EBV or M. tuberculosis are not eliminated by the immune response and establish a life long carrier state where an equilibrium is reached between the host immune response and the pathogen that persists in a latent or dormant state (Reddehase, 2002; Sacchettini et al., 2008). The life long carrier state poses a risk of widespread infections when the individual becomes immunosuppressed or immunodeficient. In addition HCMV can superinfect an already immune donor and does so by evading the CD8 T cells response, a process that complicates the development of preventive vaccines (Hansen et al., 2010).
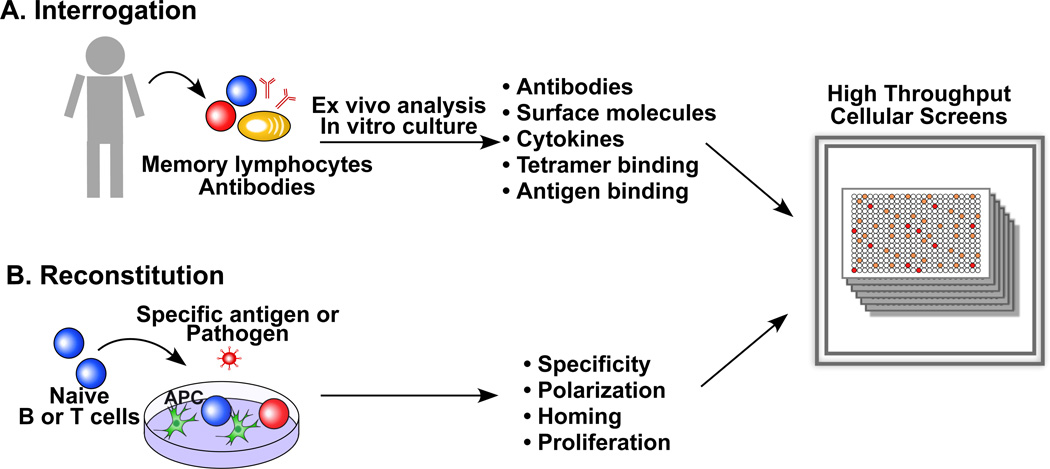
Can the analysis of human memory cells provide novel insights into developing vaccines against pathogens that induce partially protective, non-protective or non-sterilizing immunity? Recent studies suggest that it is possible to leverage on the analysis of the specificity and class of human memory B and T cells to identify conserved epitopes and protective effector mechanisms that represent a rational basis for vaccine design. Below we discuss some methodological and technical approaches that facilitate this process that we define as “analytic vaccinology” (Fig. 5).
Figure 5. The use of high throughput cellular screens to inform vaccine design.
(A) Memory B and T cells can be isolated according to the expression of surface markers and cultured in limiting dilution conditions to assess specificity, phenotype and function of individual cells. Specific antibodies can be retrieved by memory B cell immortalization or by amplification of the V region genes from single cells. T cells libraries can be screened in an iterative fashion to determine fine specificity and to isolate T cell clones. (B) Naïve T cells stimulated in vitro with monocytes or DC and whole pathogens recapitulate the phenotype of ex vivo isolated memory T cells.
Interrogation of memory B cells and isolation of monoclonal antibodies
The concept that cross-reactive protective antigens could be identified by studying the antibody response was initially proposed in the context of HIV vaccine design (Burton, 2002). The idea is to identify through the isolation of broadly neutralizing antibodies conserved epitopes and then produce immunogens that contain such epitopes in an immunodominant form. This approach offers the advantage of focusing the immune response to the most conserved epitopes while avoiding a response to the most variable sites.
For many years the isolation of monoclonal antibodies represented the rate limiting step in the analysis of the human antibody response and the identification of target antigens and conserved epitopes. In the last 5 years several methods have become available to isolate with high efficiency human monoclonal antibodies from memory B cells and plasma cells. These approaches are based on immortalization of total memory B cells followed by selection with functional assays (Traggiai et al., 2004), or by isolation of antigen-binding memory B cells or of total plasma cells followed by rescue of the specific antibodies by single cell PCR and expression in heterologous systems (Scheid et al., 2009; Wrammert et al., 2008). Using these methods it has become possible to interrogate the whole repertoire of antibodies made during an immune response or maintained in the memory compartment. These approaches have already provided exciting results with the identification of potent and broadly neutralizing antibodies against a variety of human pathogens. A common theme emerging from these novel studies is that broadly neutralizing antibodies do exist, but may be extremely rare. An extensive analysis of thousands of sera from HIV-1 infected individuals has led to the identification of two donors from whom novel potent and broadly neutralizing monoclonal antibodies were isolated. These antibodies identify highly conserved epitopes in the CD4-binding site (Wu et al., 2010) as well as conformational epitopes present in the gp160 trimer (Walker et al., 2009). Another relevant example is the selection in some individuals of memory B cells that make antibodies that broadly neutralize influenza viruses (Corti et al., 2010; Ekiert et al., 2009). These antibodies are directed against conserved epitopes in the stem region of the influenza virus hemagglutinin protein and, although less potent than classical neutralizing antibodies directed against the globular head, can neutralize in vitro and in vivo viruses belonging to different subtypes. Based on this notion, a headless hemagglutinin vaccine has been produced and has been shown to confer broad protection in an animal model (Steel et al., 2010). Human monoclonal antibodies with very high neutralizing activity against HCMV have been isolated from selected donors and used to map conformational epitopes on a pentameric complex which represents a candidate subunit vaccine (Macagno et al., 2009). The human antibody response to dengue virus has been dissected through the analysis of monoclonal antibodies revealing basic mechanisms of neutralization and infection enhancement (Beltramello et al., 2010; Dejnirattisai et al., 2010). Finally, the analysis of a large number of human monoclonal antibodies to HIV revealed a role for polyreactivity in increasing the apparent affinity of anti-HIV antibodies through heteroligation, revealing a new mechanism for the production of antibodies against pathogens displaying low density epitopes (Mouquet et al., 2010)
Besides their use for vaccine design, human monoclonal antibodies can find a direct application for passive vaccination, a prophylactic and therapeutic approach that was developed more than one hundred years ago and which has fallen short because of the limited availability of immune sera (Casadevall et al., 2004). To date only one humanized monoclonal antibody has been approved for prevention of RSV infection in newborns but several fully human antibodies are at different stages of development for prevention and treatment of various pathogens and toxins. Once identified, potent and broadly neutralizing antibodies could be used either as single agent or as a cocktail in order to minimize the selection of escape mutants. One should consider that in vitro neutralization assays do not fully reflect the in vivo usefulness of antibodies since this may critically depend on effector mechanisms such as complement- or Fc receptor-mediated killing (Hessell et al., 2007; Mozdzanowska et al., 2006). Experimental infection of mice carrying human Fc receptors may therefore be used to address the in vivo activity of human monoclonal antibodies (Nimmerjahn and Ravetch, 2008).
Another potential use of human monoclonal antibodies is their administration together with a vaccine to enhance or modulate the immune response. Antibodies can increase antigen uptake by targeting immune-complexes to Fc receptors expressed by DC leading to enhanced antigen presentation on MHC class I and II molecules and modulation of the epitopes presented (Simitsek et al., 1995). Furthermore, antibodies can suppress or enhance B cell responses to their target antigens by several hundredfold depending on isotype and stoichiometry (Heyman, 2000). Thus, by combining antigens and antibodies we can foresee a convergence of active and passive vaccination.
Interrogation of memory T cells and reconstruction of the immune response
Dissecting memory T cell subsets rather than a trivial pursuit, has revealed fundamental aspects of the immune response relevant for vaccine design. As discussed above intracellular pathogens, extracellular pathogens and parasites elicit effector and memory T cells with different functional properties. Thus it is important that vaccines are designed to exploit the full range of effector functions that are characteristic of protective responses (Sallusto and Lanzavecchia, 2009). While vaccines made by attenuated pathogens may carry the same PAMPS or elicit the same DAMPS as the virulent pathogens and thus may be able to induce the same type of response, individual antigens taken out of their context and used as recombinant proteins or inserted in heterologous vectors will critically require adjuvants capable of eliciting the appropriate type of immune response. Two experimental approaches can help to address these issues: i) the high throughput interrogation of memory T cells and ii) the reconstruction of the immune response in vitro (Fig. 5).
Several methodologies are available to characterize at the single cell level the antigenic specificity, cytokine profile, homing receptors and functional properties of human memory T cells including polychromatic flow cytometry using peptide-HLA class I tetramers, antibodies to surface molecules, cytokines and phospho-epitopes (Bolton and Roederer, 2009; Newell et al., 2009; Perez and Nolan, 2006). This analysis can be combined with global gene profiling that provide specific signatures of immune responses (Berry et al., 2010; Querec et al., 2009). An alternative approach that is suitable for the analysis of HLA class II restricted T cell responses to complex antigens, even whole pathogens and is not limited to a particular HLA molecule, consists in the screening of T cell libraries prepared from effector and memory T cell subsets (Fig. 5) (Geiger et al., 2009). This approach is revealing a remarkable compartmentalization of pathogen-specific memory T cells in individual memory subsets (FS, unpublished). In addition to the analysis of the memory pool, tetramers and T cell libraries can be used also to interrogate the naïve CD8 and CD4 repertoires (Geiger et al., 2009; Newell et al., 2009). Taken together, the high throughput interrogations of human naïve and memory T cell repertoires offer on the one hand the possibility of predicting antigenicity and identifying T cell epitopes in complex pathogens and on the other to gain information on the class of T cells elicited by a given pathogens thus providing two critical pieces of information that should guide T cell vaccine design.
An ambitious goal is currently to reconstruct in vitro the T and B cell response to a pathogen (Fig. 5). Although this may eventually require the development of organotypic 3D cultures (Ma et al., 2010), the priming of CD4 naïve T cells can be effectively achieved by coculture with APC and pathogens (FS and Christina Zielinsky, unpublished). These in vitro priming experiments can be used to identify polarizing cues in complex pathogens and to dissect the signals and mechanisms of T cell polarization, which may help defining the right type of adjuvant to be used in T cell vaccines.
Concluding Remarks
The many recent advances in our understanding of the immune system and the parallel development of various vectors and adjuvants has now set the stage where the principles of immunological memory can be used to rationally design the next generation of vaccines against infectious diseases of global importance. In addition it should also be possible to learn from our increasing understanding of T cell dysfunction during chronic viral infections and tumors to develop therapeutic vaccines against chronic diseases and cancers. There is growing consensus in the immunological community that three is an urgent need for new initiatives to strengthen research in the human system (Davis, 2008; Steinman and Mellman, 2004) and to bridge the fields of basic immunology and vaccine research. Among them the establishment of the Society for Dendritic Cell and Vaccine Science (www.dc-vaccine.org) which has been founded with the aim of promoting cross-disciplinary approaches that harness the properties of the innate immune system to design new vaccines.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F. Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nat Immunol. 2007;8:942–949. doi: 10.1038/ni1496. [DOI] [PubMed] [Google Scholar]
- Ahmed R, Gray D. Immunological memory and protective immunity: understanding their relation. Science. 1996;272:54–60. doi: 10.1126/science.272.5258.54. [DOI] [PubMed] [Google Scholar]
- Ahmed R, Oldstone MB, Palese P. Protective immunity and susceptibility to infectious diseases: lessons from the 1918 influenza pandemic. Nat Immunol. 2007;8:1188–1193. doi: 10.1038/ni1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alizadeh AA, Eisen MB, Davis RE, Ma C, Lossos IS, Rosenwald A, Boldrick JC, Sabet H, Tran T, Yu X, et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature. 2000;403:503–511. doi: 10.1038/35000501. [DOI] [PubMed] [Google Scholar]
- Allen CD, Okada T, Cyster JG. Germinal-center organization and cellular dynamics. Immunity. 2007;27:190–202. doi: 10.1016/j.immuni.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amanna IJ, Carlson NE, Slifka MK. Duration of humoral immunity to common viral and vaccine antigens. N Engl J Med. 2007;357:1903–1915. doi: 10.1056/NEJMoa066092. [DOI] [PubMed] [Google Scholar]
- Appay V, Douek DC, Price DA. CD8+ T cell efficacy in vaccination and disease. Nat Med. 2008;14:623–628. doi: 10.1038/nm.f.1774. [DOI] [PubMed] [Google Scholar]
- Araki K, Turner AP, Shaffer VO, Gangappa S, Keller SA, Bachmann MF, Larsen CP, Ahmed R. mTOR regulates memory CD8 T-cell differentiation. Nature. 2009;460:108–112. doi: 10.1038/nature08155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auffray C, Sieweke MH, Geissmann F. Blood Monocytes: Development, Heterogeneity, and Relationship with Dendritic Cells. Annu Rev Immunol. 2009 doi: 10.1146/annurev.immunol.021908.132557. [DOI] [PubMed] [Google Scholar]
- Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- Beltramello M, Williams KL, Simmons CP, Macagno A, Simonelli L, Quyen NT, Sukupolvi-Petty S, Navarro-Sanchez E, Young PR, de Silva AM, et al. The human immune response to dengue virus is dominated by highly cross-reactive antibodies endowed with neutralizing and enhancing activity. Cell Host Microbe. 2010;8:271–283. doi: 10.1016/j.chom.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry MP, Graham CM, McNab FW, Xu Z, Bloch SA, Oni T, Wilkinson KA, Banchereau R, Skinner J, Wilkinson RJ, et al. An interferon-inducible neutrophil-driven blood transcriptional signature in human tuberculosis. Nature. 2010;466:973–977. doi: 10.1038/nature09247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya D, Cheah MT, Franco CB, Hosen N, Pin CL, Sha WC, Weissman IL. Transcriptional profiling of antigen-dependent murine B cell differentiation and memory formation. J Immunol. 2007;179:6808–6819. doi: 10.4049/jimmunol.179.10.6808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton DL, Roederer M. Flow cytometry and the future of vaccine development. Expert Rev Vaccines. 2009;8:779–789. doi: 10.1586/erv.09.41. [DOI] [PubMed] [Google Scholar]
- Bonifaz LC, Bonnyay DP, Charalambous A, Darguste DI, Fujii S, Soares H, Brimnes MK, Moltedo B, Moran TM, Steinman RM. In vivo targeting of antigens to maturing dendritic cells via the DEC-205 receptor improves T cell vaccination. J Exp Med. 2004;199:815–824. doi: 10.1084/jem.20032220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breman JG, Arita I. The confirmation and maintenance of smallpox eradication. N Engl J Med. 1980;303:1263–1273. doi: 10.1056/NEJM198011273032204. [DOI] [PubMed] [Google Scholar]
- Bromley SK, Mempel TR, Luster AD. Orchestrating the orchestrators: chemokines in control of T cell traffic. Nat Immunol. 2008;9:970–980. doi: 10.1038/ni.f.213. [DOI] [PubMed] [Google Scholar]
- Burton DR. Antibodies, viruses and vaccines. Nat Rev Immunol. 2002;2:706–713. doi: 10.1038/nri891. [DOI] [PubMed] [Google Scholar]
- Casadevall A, Dadachova E, Pirofski L-a. Passive antibody therapy for infectious diseases. Nature Reviews Microbiology. 2004;2:695–703. doi: 10.1038/nrmicro974. [DOI] [PubMed] [Google Scholar]
- Chen LL, Frank AM, Adams JC, Steinman RM. Distribution of horseradish peroxidase (HRP)-anti-HRP immune complexes in mouse spleen with special reference to follicular dendritic cells. J Cell Biol. 1978;79:184–199. doi: 10.1083/jcb.79.1.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffman RL. Immunology. The origin of TH2 responses. Science. 2010;328:1116–1117. doi: 10.1126/science.1192009. [DOI] [PubMed] [Google Scholar]
- Corti D, Suguitan AL, Jr, Pinna D, Silacci C, Fernandez-Rodriguez BM, Vanzetta F, Santos C, Luke CJ, Torres-Velez FJ, Temperton NJ, et al. Heterosubtypic neutralizing antibodies are produced by individuals immunized with a seasonal influenza vaccine. J Clin Invest. 2010;120:1663–1673. doi: 10.1172/JCI41902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crotty S, Johnston RJ, Schoenberger SP. Effectors and memories: Bcl-6 and Blimp-1 in T and B lymphocyte differentiation. Nat Immunol. 2010;11:114–120. doi: 10.1038/ni.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MM. A prescription for human immunology. Immunity. 2008;29:835–838. doi: 10.1016/j.immuni.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejnirattisai W, Jumnainsong A, Onsirisakul N, Fitton P, Vasanawathana S, Limpitikul W, Puttikhunt C, Edwards C, Duangchinda T, Supasa S, et al. Cross-reacting antibodies enhance dengue virus infection in humans. Science. 2010;328:745–748. doi: 10.1126/science.1185181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denucci CC, Mitchell JS, Shimizu Y. Integrin function in T-cell homing to lymphoid and nonlymphoid sites: getting there and staying there. Crit Rev Immunol. 2009;29:87–109. doi: 10.1615/critrevimmunol.v29.i2.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Rosa F, Pabst R. The bone marrow: a nest for migratory memory T cells. Trends Immunol. 2005;26:360–366. doi: 10.1016/j.it.2005.04.011. [DOI] [PubMed] [Google Scholar]
- Dogan I, Bertocci B, Vilmont V, Delbos F, Mégret J, Storck S, Reynaud C-A, Weill J-C. Multiple layers of B cell memory with different effector functions. Nature Immunology. 2009a;10:1292–1299. doi: 10.1038/ni.1814. [DOI] [PubMed] [Google Scholar]
- Dogan I, Bertocci B, Vilmont V, Delbos F, Megret J, Storck S, Reynaud CA, Weill JC. Multiple layers of B cell memory with different effector functions. Nat Immunol. 2009b doi: 10.1038/ni.1814. [DOI] [PubMed] [Google Scholar]
- Duhen T, Geiger R, Jarrossay D, Lanzavecchia A, Sallusto F. Production of interleukin 22 but not interleukin 17 by a subset of human skin-homing memory T cells. Nat Immunol. 2009;10:857–863. doi: 10.1038/ni.1767. [DOI] [PubMed] [Google Scholar]
- Ekiert DC, Bhabha G, Elsliger MA, Friesen RH, Jongeneelen M, Throsby M, Goudsmit J, Wilson IA. Antibody recognition of a highly conserved influenza virus epitope. Science. 2009;324:246–251. doi: 10.1126/science.1171491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazilleau N, McHeyzer-Williams LJ, Rosen H, McHeyzer-Williams MG. The function of follicular helper T cells is regulated by the strength of T cell antigen receptor binding. Nat Immunol. 2009;10:375–384. doi: 10.1038/ni.1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finley JH., Jr . The Complete Writings of Thucydides: The Peloponnesian War. New York: Modern Library; 1951. [Google Scholar]
- Freitas AA, Rocha B. Population biology of lymphocytes: the flight for survival. Annu Rev Immunol. 2000;18:83–111. doi: 10.1146/annurev.immunol.18.1.83. [DOI] [PubMed] [Google Scholar]
- Gattinoni L, Zhong XS, Palmer DC, Ji Y, Hinrichs CS, Yu Z, Wrzesinski C, Boni A, Cassard L, Garvin LM, et al. Wnt signaling arrests effector T cell differentiation and generates CD8+ memory stem cells. Nat Med. 2009;15:808–813. doi: 10.1038/nm.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger R, Duhen T, Lanzavecchia A, Sallusto F. Human naive and memory CD4+ T cell repertoires specific for naturally processed antigens analyzed using libraries of amplified T cells. J Exp Med. 2009;206:1525–1534. doi: 10.1084/jem.20090504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlach C, van Heijst JW, Swart E, Sie D, Armstrong N, Kerkhoven RM, Zehn D, Bevan MJ, Schepers K, Schumacher TN. One naive T cell, multiple fates in CD8+ T cell differentiation. J Exp Med. 2010;207:1235–1246. doi: 10.1084/jem.20091175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldrath AW. Maintaining the status quo: T-cell homeostasis. Microbes Infect. 2002;4:539–545. doi: 10.1016/s1286-4579(02)01570-8. [DOI] [PubMed] [Google Scholar]
- Good KL, Avery DT, Tangye SG. Resting human memory B cells are intrinsically programmed for enhanced survival and responsiveness to diverse stimuli compared to naive B cells. J Immunol. 2009;182:890–901. doi: 10.4049/jimmunol.182.2.890. [DOI] [PubMed] [Google Scholar]
- Good KL, Tangye SG. Decreased expression of Kruppel-like factors in memory B cells induces the rapid response typical of secondary antibody responses. Proc Natl Acad Sci U S A. 2007;104:13420–13425. doi: 10.1073/pnas.0703872104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray D. A role for antigen in the maintenance of immunological memory. Nat Rev Immunol. 2002;2:60–65. doi: 10.1038/nri706. [DOI] [PubMed] [Google Scholar]
- Green S, Rothman A. Immunopathological mechanisms in dengue and dengue hemorrhagic fever. Curr Opin Infect Dis. 2006;19:429–436. doi: 10.1097/01.qco.0000244047.31135.fa. [DOI] [PubMed] [Google Scholar]
- Haining WN, Wherry EJ. Integrating genomic signatures for immunologic discovery. Immunity. 2010;32:152–161. doi: 10.1016/j.immuni.2010.02.001. [DOI] [PubMed] [Google Scholar]
- Hall CB. Respiratory syncytial virus and parainfluenza virus. N Engl J Med. 2001;344:1917–1928. doi: 10.1056/NEJM200106213442507. [DOI] [PubMed] [Google Scholar]
- Halstead SB. Dengue. Lancet. 2007;370:1644–1652. doi: 10.1016/S0140-6736(07)61687-0. [DOI] [PubMed] [Google Scholar]
- Hansen SG, Powers CJ, Richards R, Ventura AB, Ford JC, Siess D, Axthelm MK, Nelson JA, Jarvis MA, Picker LJ, Fruh K. Evasion of CD8+ T cells is critical for superinfection by cytomegalovirus. Science. 2010;328:102–106. doi: 10.1126/science.1185350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hapfelmeier S, Lawson MA, Slack E, Kirundi JK, Stoel M, Heikenwalder M, Cahenzli J, Velykoredko Y, Balmer ML, Endt K, et al. Reversible microbial colonization of germ-free mice reveals the dynamics of IgA immune responses. Science. 2010;328:1705–1709. doi: 10.1126/science.1188454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath WR, Carbone FR. Dendritic cell subsets in primary and secondary T cell responses at body surfaces. Nat Immunol. 2009;10:1237–1244. doi: 10.1038/ni.1822. [DOI] [PubMed] [Google Scholar]
- Hessell AJ, Hangartner L, Hunter M, Havenith CE, Beurskens FJ, Bakker JM, Lanigan CM, Landucci G, Forthal DN, Parren PW, et al. Fc receptor but not complement binding is important in antibody protection against HIV. Nature. 2007;449:101–104. doi: 10.1038/nature06106. [DOI] [PubMed] [Google Scholar]
- Heyman B. Regulation of antibody responses via antibodies, complement, and Fc receptors. Annu Rev Immunol. 2000;18:709–737. doi: 10.1146/annurev.immunol.18.1.709. [DOI] [PubMed] [Google Scholar]
- Hikida M, Casola S, Takahashi N, Kaji T, Takemori T, Rajewsky K, Kurosaki T. PLC-gamma2 is essential for formation and maintenance of memory B cells. J Exp Med. 2009;206:681–689. doi: 10.1084/jem.20082100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill AV. Pre-erythrocytic malaria vaccines: towards greater efficacy. Nat Rev Immunol. 2006;6:21–32. doi: 10.1038/nri1746. [DOI] [PubMed] [Google Scholar]
- Hou S, Hyland L, Ryan KW, Portner A, Doherty PC. Virus-specific CD8+ T-cell memory determined by clonal burst size. Nature. 1994;369:652–654. doi: 10.1038/369652a0. [DOI] [PubMed] [Google Scholar]
- Houghton M, Abrignani S. Prospects for a vaccine against the hepatitis C virus. Nature. 2005;436:961–966. doi: 10.1038/nature04081. [DOI] [PubMed] [Google Scholar]
- Intlekofer AM, Takemoto N, Wherry EJ, Longworth SA, Northrup JT, Palanivel VR, Mullen AC, Gasink CR, Kaech SM, Miller JD, et al. Effector and memory CD8+ T cell fate coupled by T-bet and eomesodermin. Nat Immunol. 2005;6:1236–1244. doi: 10.1038/ni1268. [DOI] [PubMed] [Google Scholar]
- Iwasaki A, Medzhitov R. Regulation of adaptive immunity by the innate immune system. Science. 2010;327:291–295. doi: 10.1126/science.1183021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jameson SC, Masopust D. Diversity in T cell memory: an embarrassment of riches. Immunity. 2009;31:859–871. doi: 10.1016/j.immuni.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janeway CA, Jr, Travers P, Walport M, Shlomchik MJ. Immunobiology: the immune system in health and disease. 6th edn. New York: Garland Science; 2005. [Google Scholar]
- Johnston MI, Fauci AS. An HIV vaccine--evolving concepts. N Engl J Med. 2007;356:2073–2081. doi: 10.1056/NEJMra066267. [DOI] [PubMed] [Google Scholar]
- Joshi NS, Cui W, Chandele A, Lee HK, Urso DR, Hagman J, Gapin L, Kaech SM. Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity. 2007;27:281–295. doi: 10.1016/j.immuni.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jourdan M, Caraux A, De Vos J, Fiol G, Larroque M, Cognot C, Bret C, Duperray C, Hose D, Klein B. An in vitro model of differentiation of memory B cells into plasmablasts and plasma cells including detailed phenotypic and molecular characterization. Blood. 2009;114:5173–5181. doi: 10.1182/blood-2009-07-235960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaech SM, Hemby S, Kersh E, Ahmed R. Molecular and functional profiling of memory CD8 T cell differentiation. Cell. 2002;111:837–851. doi: 10.1016/s0092-8674(02)01139-x. [DOI] [PubMed] [Google Scholar]
- Kaech SM, Tan JT, Wherry EJ, Konieczny BT, Surh CD, Ahmed R. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat Immunol. 2003;4:1191–1198. doi: 10.1038/ni1009. [DOI] [PubMed] [Google Scholar]
- Kaech SM, Wherry EJ. Heterogeneity and cell-fate decisions in effector and memory CD8+ T cell differentiation during viral infection. Immunity. 2007;27:393–405. doi: 10.1016/j.immuni.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamphorst AO, Guermonprez P, Dudziak D, Nussenzweig MC. Route of antigen uptake differentially impacts presentation by dendritic cells and activated monocytes. J Immunol. 2010;185:3426–3435. doi: 10.4049/jimmunol.1001205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassiotis G, O’Garra A. Establishing the follicular helper identity. Immunity. 2009;31:450–452. doi: 10.1016/j.immuni.2009.08.017. [DOI] [PubMed] [Google Scholar]
- Klein U, Tu Y, Stolovitzky GA, Keller JL, Haddad J, Jr, Miljkovic V, Cattoretti G, Califano A, Dalla-Favera R. Transcriptional analysis of the B cell germinal center reaction. Proc Natl Acad Sci U S A. 2003;100:2639–2644. doi: 10.1073/pnas.0437996100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klenerman P, Hill A. T cells and viral persistence: lessons from diverse infections. Nat Immunol. 2005;6:873–879. doi: 10.1038/ni1241. [DOI] [PubMed] [Google Scholar]
- Kohlmeier JE, Woodland DL. Immunity to respiratory viruses. Annu Rev Immunol. 2009;27:61–82. doi: 10.1146/annurev.immunol.021908.132625. [DOI] [PubMed] [Google Scholar]
- Kunkel EJ, Butcher EC. Plasma-cell homing. Nat Rev Immunol. 2003;3:822–829. doi: 10.1038/nri1203. [DOI] [PubMed] [Google Scholar]
- Langhorne J, Ndungu FM, Sponaas AM, Marsh K. Immunity to malaria: more questions than answers. Nat Immunol. 2008;9:725–732. doi: 10.1038/ni.f.205. [DOI] [PubMed] [Google Scholar]
- Lanzavecchia A. Immunology. Licence to kill. Nature. 1998;393:413–414. doi: 10.1038/30845. [DOI] [PubMed] [Google Scholar]
- Lanzavecchia A, Sallusto F. Progressive differentiation and selection of the fittest in the immune response. Nat Rev Immunol. 2002;2:982–987. doi: 10.1038/nri959. [DOI] [PubMed] [Google Scholar]
- Larche M, Wraith DC. Peptide-based therapeutic vaccines for allergic and autoimmune diseases. Nat Med. 2005;11:S69–76. doi: 10.1038/nm1226. [DOI] [PubMed] [Google Scholar]
- Lau LL, Jamieson BD, Somasundaram T, Ahmed R. Cytotoxic T-cell memory without antigen. Nature. 1994;369:648–652. [PubMed] [Google Scholar]
- Levin MJ. Zoster vaccine. In: Plotkin SA, Orenstein WA, Offit PA, editors. Vaccines. Elsevier Inc; 2008. pp. 1057–1068. [Google Scholar]
- Linton PJ, Dorshkind K. Age-related changes in lymphocyte development and function. Nat Immunol. 2004;5:133–139. doi: 10.1038/ni1033. [DOI] [PubMed] [Google Scholar]
- Lollini PL, Cavallo F, Nanni P, Forni G. Vaccines for tumour prevention. Nat Rev Cancer. 2006;6:204–216. doi: 10.1038/nrc1815. [DOI] [PubMed] [Google Scholar]
- Ma CS, Chew GY, Simpson N, Priyadarshi A, Wong M, Grimbacher B, Fulcher DA, Tangye SG, Cook MC. Deficiency of Th17 cells in hyper IgE syndrome due to mutations in STAT3. J Exp Med. 2008;205:1551–1557. doi: 10.1084/jem.20080218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Poisson L, Sanchez-Schmitz G, Pawar S, Qu C, Randolph GJ, Warren WL, Mishkin EM, Higbee RG. Assessing the immunopotency of Tolllike receptor agonists in an in vitro tissue-engineered immunological model. Immunology. 2010;130:374–387. doi: 10.1111/j.1365-2567.2009.03237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macagno A, Bernasconi N, Vanzetta F, Dander E, Sarasini A, Revello MG, Gerna G, Sallusto F, Lanzavecchia A. Isolation of human monoclonal antibodies that potently neutralize HCMV infection by targeting different epitopes on the gH/gL/UL128-131A complex. J Virol. 2009 doi: 10.1128/JVI.01809-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macatonia SE, Hosken NA, Litton M, Vieira P, Hsieh CS, Culpepper JA, Wysocka M, Trinchieri G, Murphy KM, O’Garra A. Dendritic cells produce IL-12 and direct the development of Th1 cells from naive CD4+ T cells. J Immunol. 1995;154:5071–5079. [PubMed] [Google Scholar]
- Manz RA, Thiel A, Radbruch A. Lifetime of plasma cells in the bone marrow. Nature. 1997;388:133–134. doi: 10.1038/40540. [DOI] [PubMed] [Google Scholar]
- Masopust D, Choo D, Vezys V, Wherry EJ, Duraiswamy J, Akondy R, Wang J, Casey KA, Barber DL, Kawamura KS, et al. Dynamic T cell migration program provides resident memory within intestinal epithelium. J Exp Med. 2010;207:553–564. doi: 10.1084/jem.20090858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masopust D, Ha SJ, Vezys V, Ahmed R. Stimulation history dictates memory CD8 T cell phenotype: implications for prime-boost vaccination. J Immunol. 2006;177:831–839. doi: 10.4049/jimmunol.177.2.831. [DOI] [PubMed] [Google Scholar]
- McMichael AJ, Borrow P, Tomaras GD, Goonetilleke N, Haynes BF. The immune response during acute HIV-1 infection: clues for vaccine development. Nat Rev Immunol. 2010;10:11–23. doi: 10.1038/nri2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melief CJ, van der Burg SH. Immunotherapy of established (pre)malignant disease by synthetic long peptide vaccines. Nat Rev Cancer. 2008;8:351–360. doi: 10.1038/nrc2373. [DOI] [PubMed] [Google Scholar]
- Milner JD, Brenchley JM, Laurence A, Freeman AF, Hill BJ, Elias KM, Kanno Y, Spalding C, Elloumi HZ, Paulson ML, et al. Impaired T(H)17 cell differentiation in subjects with autosomal dominant hyper-IgE syndrome. Nature. 2008 doi: 10.1038/nature06764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moir S, Fauci AS. B cells in HIV infection and disease. Nat Rev Immunol. 2009;9:235–245. doi: 10.1038/nri2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouquet H, Scheid JF, Zoller MJ, Krogsgaard M, Ott RG, Shukair S, Artyomov MN, Pietzsch J, Connors M, Pereyra F, et al. Polyreactivity increases the apparent affinity of anti-HIV antibodies by heteroligation. Nature. 2010;467:591–595. doi: 10.1038/nature09385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozdzanowska K, Feng J, Eid M, Zharikova D, Gerhard W. Enhancement of neutralizing activity of influenza virus-specific antibodies by serum components. Virology. 2006;352:418–426. doi: 10.1016/j.virol.2006.05.008. [DOI] [PubMed] [Google Scholar]
- Murali-Krishna K, Lau LL, Sambhara S, Lemonnier F, Altman J, Ahmed R. Persistence of memory CD8 T cells in MHC class I-deficient mice. Science. 1999;286:1377–1381. doi: 10.1126/science.286.5443.1377. [DOI] [PubMed] [Google Scholar]
- Nakanishi Y, Lu B, Gerard C, Iwasaki A. CD8(+) T lymphocyte mobilization to virus-infected tissue requires CD4(+) T-cell help. Nature. 2009;462:510–513. doi: 10.1038/nature08511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newell EW, Klein LO, Yu W, Davis MM. Simultaneous detection of many T-cell specificities using combinatorial tetramer staining. Nat Methods. 2009;6:497–499. doi: 10.1038/nmeth.1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolich-Zugich J. Ageing and life-long maintenance of T-cell subsets in the face of latent persistent infections. Nat Rev Immunol. 2008;8:512–522. doi: 10.1038/nri2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmerjahn F, Ravetch JV. Fcgamma receptors as regulators of immune responses. Nat Rev Immunol. 2008;8:34–47. doi: 10.1038/nri2206. [DOI] [PubMed] [Google Scholar]
- O’Connor BP, Raman VS, Erickson LD, Cook WJ, Weaver LK, Ahonen C, Lin LL, Mantchev GT, Bram RJ, Noelle RJ. BCMA is essential for the survival of long-lived bone marrow plasma cells. J Exp Med. 2004;199:91–98. doi: 10.1084/jem.20031330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce EL, Walsh MC, Cejas PJ, Harms GM, Shen H, Wang LS, Jones RG, Choi Y. Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature. 2009;460:103–107. doi: 10.1038/nature08097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez OD, Nolan GP. Phospho-proteomic immune analysis by flow cytometry: from mechanism to translational medicine at the single-cell level. Immunol Rev. 2006;210:208–228. doi: 10.1111/j.0105-2896.2006.00364.x. [DOI] [PubMed] [Google Scholar]
- Phan TG, Gray EE, Cyster JG. The microanatomy of B cell activation. Curr Opin Immunol. 2009;21:258–265. doi: 10.1016/j.coi.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotkin SA, Orenstein WA, Offit PA. Vaccines. 5th edn. Philadelphia, Pa: Saunders/Elsevier; 2008. [Google Scholar]
- Plotkin SL, Plotkin SA. A short history of vaccination. In: Plotkin SA, Orenstein WA, Offit PA, editors. Vaccines. Elsevier Inc; 2008. pp. 1–16. [Google Scholar]
- Pollard AJ, Perrett KP, Beverley PC. Maintaining protection against invasive bacteria with protein-polysaccharide conjugate vaccines. Nat Rev Immunol. 2009;9:213–220. doi: 10.1038/nri2494. [DOI] [PubMed] [Google Scholar]
- Qi H, Cannons JL, Klauschen F, Schwartzberg PL, Germain RN. SAP-controlled T-B cell interactions underlie germinal centre formation. Nature. 2008;455:764–769. doi: 10.1038/nature07345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Querec TD, Akondy RS, Lee EK, Cao W, Nakaya HI, Teuwen D, Pirani A, Gernert K, Deng J, Marzolf B, et al. Systems biology approach predicts immunogenicity of the yellow fever vaccine in humans. Nat Immunol. 2009;10:116–125. doi: 10.1038/ni.1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radbruch A, Muehlinghaus G, Luger EO, Inamine A, Smith KG, Dorner T, Hiepe F. Competence and competition: the challenge of becoming a long-lived plasma cell. Nat Rev Immunol. 2006;6:741–750. doi: 10.1038/nri1886. [DOI] [PubMed] [Google Scholar]
- Rao RR, Li Q, Odunsi K, Shrikant PA. The mTOR kinase determines effector versus memory CD8+ T cell fate by regulating the expression of transcription factors T-bet and Eomesodermin. Immunity. 2010;32:67–78. doi: 10.1016/j.immuni.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddehase MJ. Antigens and immunoevasins: opponents in cytomegalovirus immune surveillance. Nat Rev Immunol. 2002;2:831–844. doi: 10.1038/nri932. [DOI] [PubMed] [Google Scholar]
- Reiner SL, Sallusto F, Lanzavecchia A. Division of labor with a workforce of one: challenges in specifying effector and memory T cell fate. Science. 2007;317:622–625. doi: 10.1126/science.1143775. [DOI] [PubMed] [Google Scholar]
- Sacchettini JC, Rubin EJ, Freundlich JS. Drugs versus bugs: in pursuit of the persistent predator Mycobacterium tuberculosis. Nat Rev Microbiol. 2008;6:41–52. doi: 10.1038/nrmicro1816. [DOI] [PubMed] [Google Scholar]
- Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol. 2004;22:745–763. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- Sallusto F, Lanzavecchia A. Heterogeneity of CD4+ memory T cells: functional modules for tailored immunity. Eur J Immunol. 2009;39:2076–2082. doi: 10.1002/eji.200939722. [DOI] [PubMed] [Google Scholar]
- Sanz I, Wei C, Lee FE, Anolik J. Phenotypic and functional heterogeneity of human memory B cells. Semin Immunol. 2008;20:67–82. doi: 10.1016/j.smim.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar S, Kalia V, Haining WN, Konieczny BT, Subramaniam S, Ahmed R. Functional and genomic profiling of effector CD8 T cell subsets with distinct memory fates. J Exp Med. 2008;205:625–640. doi: 10.1084/jem.20071641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheid JF, Mouquet H, Feldhahn N, Seaman MS, Velinzon K, Pietzsch J, Ott RG, Anthony RM, Zebroski H, Hurley A, et al. Broad diversity of neutralizing antibodies isolated from memory B cells in HIV-infected individuals. Nature. 2009;458:636–640. doi: 10.1038/nature07930. [DOI] [PubMed] [Google Scholar]
- Schon MP, Arya A, Murphy EA, Adams CM, Strauch UG, Agace WW, Marsal J, Donohue JP, Her H, Beier DR, et al. Mucosal T lymphocyte numbers are selectively reduced in integrin alpha E (CD103)-deficient mice. J Immunol. 1999;162:6641–6649. [PubMed] [Google Scholar]
- Seifert M, Kuppers R. Molecular footprints of a germinal center derivation of human IgM+(IgD+)CD27+ B cells and the dynamics of memory B cell generation. J Exp Med. 2009;206:2659–2669. doi: 10.1084/jem.20091087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegrist CA. Vaccine immunology. In: Plotkin SA, Orenstein WA, Offit PA, editors. Vaccines. Elsevier Inc; 2008. pp. 17–36. [Google Scholar]
- Sigmundsdottir H, Butcher EC. Environmental cues, dendritic cells and the programming of tissue-selective lymphocyte trafficking. Nat Immunol. 2008;9:981–987. doi: 10.1038/ni.f.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simitsek PD, Campbell DG, Lanzavecchia A, Fairweather N, Watts C. Modulation of antigen processing by bound antibodies can boost or suppress class II major histocompatibility complex presentation of different T cell determinants. J Exp Med. 1995;181:1957–1963. doi: 10.1084/jem.181.6.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skeiky YA, Sadoff JC. Advances in tuberculosis vaccine strategies. Nat Rev Microbiol. 2006;4:469–476. doi: 10.1038/nrmicro1419. [DOI] [PubMed] [Google Scholar]
- Slifka MK, Antia R, Whitmire JK, Ahmed R. Humoral immunity due to long-lived plasma cells. Immunity. 1998;8:363–372. doi: 10.1016/s1074-7613(00)80541-5. [DOI] [PubMed] [Google Scholar]
- Staal FJ, Clevers HC. WNT signalling and haematopoiesis: a WNT-WNT situation. Nat Rev Immunol. 2005;5:21–30. doi: 10.1038/nri1529. [DOI] [PubMed] [Google Scholar]
- Steel J, Lowen AC, Wang T, Yondola M, Gao Q, Haye K, Garcia-Sastre A, Palese P. Influenza virus vaccine based on the conserved hemagglutinin stalk domain. MBio. 2010;1 doi: 10.1128/mBio.00018-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman RM, Mellman I. Immunotherapy: bewitched, bothered, and bewildered no more. Science. 2004;305:197–200. doi: 10.1126/science.1099688. [DOI] [PubMed] [Google Scholar]
- Stemberger C, Huster KM, Koffler M, Anderl F, Schiemann M, Wagner H, Busch DH. A single naive CD8+ T cell precursor can develop into diverse effector and memory subsets. Immunity. 2007;27:985–997. doi: 10.1016/j.immuni.2007.10.012. [DOI] [PubMed] [Google Scholar]
- Subbarao K, Joseph T. Scientific barriers to developing vaccines against avian influenza viruses. Nat Rev Immunol. 2007;7:267–278. doi: 10.1038/nri2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surh CD, Sprent J. Homeostasis of naive and memory T cells. Immunity. 2008;29:848–862. doi: 10.1016/j.immuni.2008.11.002. [DOI] [PubMed] [Google Scholar]
- Swain SL, Hu H, Huston G. Class II-independent generation of CD4 memory T cells from effectors. Science. 1999;286:1381–1383. doi: 10.1126/science.286.5443.1381. [DOI] [PubMed] [Google Scholar]
- Takada K, Jameson SC. Naive T cell homeostasis: from awareness of space to a sense of place. Nat Rev Immunol. 2009;9:823–832. doi: 10.1038/nri2657. [DOI] [PubMed] [Google Scholar]
- Tarte K, Zhan F, De Vos J, Klein B, Shaughnessy J., Jr Gene expression profiling of plasma cells and plasmablasts: toward a better understanding of the late stages of B-cell differentiation. Blood. 2003;102:592–600. doi: 10.1182/blood-2002-10-3161. [DOI] [PubMed] [Google Scholar]
- Tew JG, Phipps RP, Mandel TE. The maintenance and regulation of the humoral immune response: persisting antigen and the role of follicular antigen-binding dendritic cells as accessory cells. Immunol Rev. 1980;53:175–201. doi: 10.1111/j.1600-065x.1980.tb01044.x. [DOI] [PubMed] [Google Scholar]
- Tokoyoda K, Zehentmeier S, Hegazy AN, Albrecht I, Grun JR, Lohning M, Radbruch A. Professional memory CD4+ T lymphocytes preferentially reside and rest in the bone marrow. Immunity. 2009;30:721–730. doi: 10.1016/j.immuni.2009.03.015. [DOI] [PubMed] [Google Scholar]
- Tomayko MM, Anderson SM, Brayton CE, Sadanand S, Steinel NC, Behrens TW, Shlomchik MJ. Systematic comparison of gene expression between murine memory and naive B cells demonstrates that memory B cells have unique signaling capabilities. J Immunol. 2008;181:27–38. doi: 10.4049/jimmunol.181.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traggiai E, Becker S, Subbarao K, Kolesnikova L, Uematsu Y, Gismondo MR, Murphy BR, Rappuoli R, Lanzavecchia A. An efficient method to make human monoclonal antibodies from memory B cells: potent neutralization of SARS coronavirus. Nat Med. 2004;10:871–875. doi: 10.1038/nm1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trifari S, Kaplan CD, Tran EH, Crellin NK, Spits H. Identification of a human helper T cell population that has abundant production of interleukin 22 and is distinct from T(H)-17, T(H)1 and T(H)2 cells. Nat Immunol. 2009;10:864–871. doi: 10.1038/ni.1770. [DOI] [PubMed] [Google Scholar]
- Turtle CJ, Swanson HM, Fujii N, Estey EH, Riddell SR. A distinct subset of self-renewing human memory CD8+ T cells survives cytotoxic chemotherapy. Immunity. 2009;31:834–844. doi: 10.1016/j.immuni.2009.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Underhill GH, George D, Bremer EG, Kansas GS. Gene expression profiling reveals a highly specialized genetic program of plasma cells. Blood. 2003;101:4013–4021. doi: 10.1182/blood-2002-08-2673. [DOI] [PubMed] [Google Scholar]
- Veldhoen M, Uyttenhove C, van Snick J, Helmby H, Westendorf A, Buer J, Martin B, Wilhelm C, Stockinger B. Transforming growth factor-beta ‘reprograms’ the differentiation of T helper 2 cells and promotes an interleukin 9-producing subset. Nat Immunol. 2008;9:1341–1346. doi: 10.1038/ni.1659. [DOI] [PubMed] [Google Scholar]
- Vezys V, Yates A, Casey KA, Lanier G, Ahmed R, Antia R, Masopust D. Memory CD8 T-cell compartment grows in size with immunological experience. Nature. 2009;457:196–199. doi: 10.1038/nature07486. [DOI] [PubMed] [Google Scholar]
- Villadangos JA, Shortman K. Found in translation: the human equivalent of mouse CD8+ dendritic cells. J Exp Med. 2010;207:1131–1134. doi: 10.1084/jem.20100985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinuesa CG, Cook MC, Cooke MP, Maclennan IC, Goodnow CC. Analysis of B cell memory formation using DNA microarrays. Ann N Y Acad Sci. 2002;975:33–45. doi: 10.1111/j.1749-6632.2002.tb05939.x. [DOI] [PubMed] [Google Scholar]
- Vinuesa CG, Tangye SG, Moser B, Mackay CR. Follicular B helper T cells in antibody responses and autoimmunity. Nat Rev Immunol. 2005;5:853–865. doi: 10.1038/nri1714. [DOI] [PubMed] [Google Scholar]
- Virgin HW, Wherry EJ, Ahmed R. Redefining chronic viral infection. Cell. 2009;138:30–50. doi: 10.1016/j.cell.2009.06.036. [DOI] [PubMed] [Google Scholar]
- von Andrian UH, Mackay CR. T-cell function and migration. Two sides of the same coin. N Engl J Med. 2000;343:1020–1034. doi: 10.1056/NEJM200010053431407. [DOI] [PubMed] [Google Scholar]
- Walker BD, Burton DR. Toward an AIDS vaccine. Science. 2008;320:760–764. doi: 10.1126/science.1152622. [DOI] [PubMed] [Google Scholar]
- Walker LM, Phogat SK, Chan-Hui PY, Wagner D, Phung P, Goss JL, Wrin T, Simek MD, Fling S, Mitcham JL, et al. Broad and potent neutralizing antibodies from an African donor reveal a new HIV-1 vaccine target. Science. 2009;326:285–289. doi: 10.1126/science.1178746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weill JC, Weller S, Reynaud CA. Human marginal zone B cells. Annu Rev Immunol. 2009;27:267–285. doi: 10.1146/annurev.immunol.021908.132607. [DOI] [PubMed] [Google Scholar]
- Welsh RM, Che JW, Brehm MA, Selin LK. Heterologous immunity between viruses. Immunol Rev. 2010;235:244–266. doi: 10.1111/j.0105-2896.2010.00897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wherry EJ, Ha SJ, Kaech SM, Haining WN, Sarkar S, Kalia V, Subramaniam S, Blattman JN, Barber DL, Ahmed R. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity. 2007;27:670–684. doi: 10.1016/j.immuni.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Wherry EJ, Teichgraber V, Becker TC, Masopust D, Kaech SM, Antia R, von Andrian UH, Ahmed R. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat Immunol. 2003;4:225–234. doi: 10.1038/ni889. [DOI] [PubMed] [Google Scholar]
- Whitehead SS, Blaney JE, Durbin AP, Murphy BR. Prospects for a dengue virus vaccine. Nat Rev Microbiol. 2007;5:518–528. doi: 10.1038/nrmicro1690. [DOI] [PubMed] [Google Scholar]
- WHO. Weekly Epidemiological Record. 1980:148. [Google Scholar]
- Wirth TC, Xue HH, Rai D, Sabel JT, Bair T, Harty JT, Badovinac VP. Repetitive antigen stimulation induces stepwise transcriptome diversification but preserves a core signature of memory CD8(+) T cell differentiation. Immunity. 2010;33:128–140. doi: 10.1016/j.immuni.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrammert J, Smith K, Miller J, Langley WA, Kokko K, Larsen C, Zheng NY, Mays I, Garman L, Helms C, et al. Rapid cloning of high-affinity human monoclonal antibodies against influenza virus. Nature. 2008;453:667–671. doi: 10.1038/nature06890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Yang ZY, Li Y, Hogerkorp CM, Schief WR, Seaman MS, Zhou T, Schmidt SD, Wu L, Xu L, et al. Rational design of envelope identifies broadly neutralizing human monoclonal antibodies to HIV-1. Science. 2010;329:856–861. doi: 10.1126/science.1187659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinkernagel RM. On natural and artificial vaccinations. Annu Rev Immunol. 2003;21:515–546. doi: 10.1146/annurev.immunol.21.120601.141045. [DOI] [PubMed] [Google Scholar]