Abstract
Kaposi's sarcoma-associated herpesvirus (KSHV) is the etiologic agent associated with Kaposi's sarcoma (KS), primary effusion lymphoma (PEL), and multicentric Castleman disease (MCD). Similar to other herpesviruses, KSHV has two life cycles, latency and lytic replication. In latency, the KSHV genome persists as a circular episome in the nucleus of the host cell and only a few viral genes are expressed. In this review, we focus on oncogenic, antiapoptotic, and immunomodulating properties of KSHV-encoded homologues of cellular interferon regulatory factors (IRFs)—viral IRF1 (vIRF1) to vIRF4—and their possible role in the KSHV-mediated antiviral response, apoptosis, and oncogenicity.
INTRODUCTION
Kaposi's sarcoma-associated herpesvirus (KSHV), also known as human herpesvirus 8 (HHV8), is a DNA tumor virus of the gammaherpesvirus subfamily that has been associated with all epidemiological forms of Kaposi's sarcoma (KS) (1) and two lymphoproliferative disorders, primary effusion lymphoma (PEL) and multicentric Castleman disease (MCD) (2, 3, 4). KSHV is a double-stranded DNA (dsDNA) herpesvirus, with an estimated size of around 170 kb. Sequence analysis of the KSHV genome revealed the presence of about 80 open reading frames (ORFs), some of which show homology to cellular genes that regulate cell growth, the immune response, inflammation, and apoptosis (5–11). The incidence of KSHV-associated disease is increased by immunosuppression in recipients of organ transplants and AIDS patients (12). Like other herpesviruses, KSHV is maintained in a latent state, where the viral genome persists as a circular episome in the nucleus of the host cell. During latency, only a few viral genes are expressed, namely, those encoding latency-associated nuclear antigen 1 (LANA1), Kaposin, vFLIP (viral FLICE inhibitory protein), vCyclin, and viral interferon (IFN) regulatory factor 3 (vIRF3)/LANA2. These viral genes are responsible for regulation of host cell proliferation, prevention of apoptosis, facilitation of immune evasion, and maintenance of the extrachromosomal viral genome during cell divisions (13–18). In order to establish infection, KSHV needs to overcome the immune antiviral response of the host cell. To this effect, KSHV encodes homologues of cellular proteins that play an essential role in the early antiviral response (19). Among these are proteins resembling the cellular interferon regulatory factors (IRFs) that play a critical role in type I IFN gene induction and IFN action.
The cellular IRFs were first shown to play a role in virus-mediated induction of type I IFN, but later their importance was also demonstrated in the induction of chemokines (RANTES and MIP-1) and proinflammatory cytokines (interleukin-12 [IL-12] and IL-15) as well as in direct antiviral (dsRNA-activated protein kinase [PKR]), antibacterial (inducible nitric oxide synthase [iNOS]), and inflammatory (cox) responses (20, 21).
All cellular IRFs share homology in the N-terminal part of the polypeptide that represents the DNA-binding domain (DBD), which is characterized by the presence of five tryptophan repeats. The C-terminal part of these proteins is distinct and may contain the IRF-association domain (IAD) that facilitates the formation of IRF homo- or heterodimers and interaction with other transcription factors. IRFs can function as transcriptional activators or repressors. Three of these IRFs (IRF-3, -5, and -7) play a critical role in the innate antiviral response (20–22). Interferon regulatory factor 3 (IRF-3) acts as a direct transducer of virus-mediated signaling, and together with IRF-7 controls transcriptional activation of alpha/beta IFN (IFN-α/β) genes (23–26). All characterized IRFs contain nuclear localization signals that allow their translocation to the nucleus. However, during homeostasis certain IRFs reside in the cytoplasm of the cell and translocate to the nucleus in response to viral infection, where they participate in the transcription of type I IFN genes, inflammatory cytokines and chemokines, and interferon-stimulated genes (ISGs). The function of IRFs is not limited to the innate immune response, as they also play a role in the modulation of cell growth, differentiation, and apoptosis. Thus, deregulation of these functions may lead to tumorigenesis (20, 27–30). Indeed, KSHV-encoded viral homologues of IRFs (designated vIRFs) (Fig. 1A and B) have been identified as effective inhibitors of interferon signaling and modulators of cellular oncogenic pathways (Fig. 2 and Fig. 3). These functions of vIRFs may contribute to KSHV-associated pathogenesis.
Fig 1.
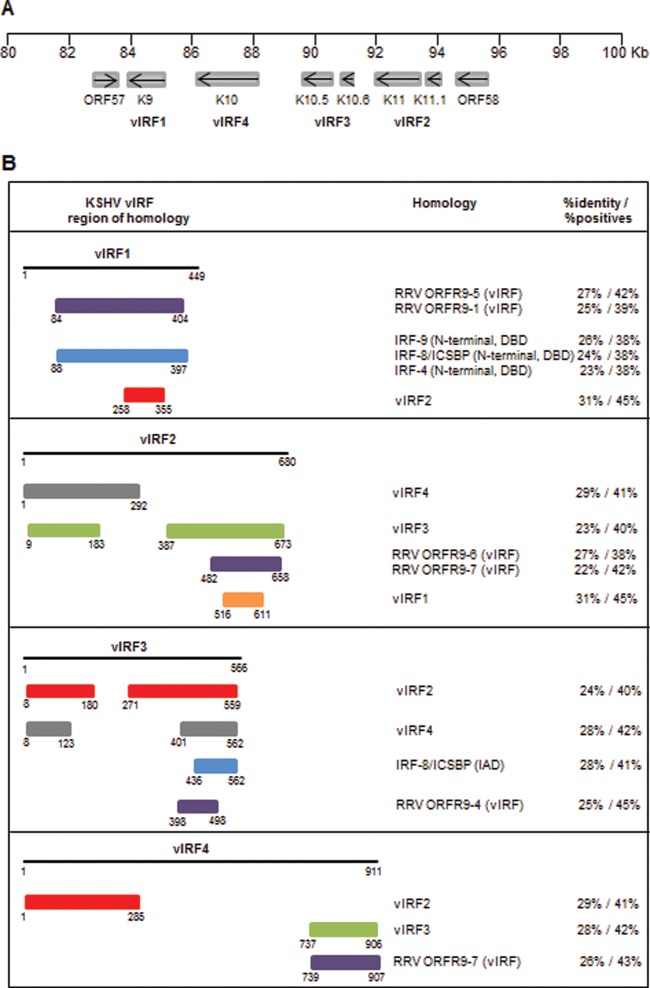
Homology and gene arrangement of KSHV-encoded vIRFs. (A) Gene arrangement of the vIRF locus in the KSHV genome. Open reading frames encoding vIRFs are located between ORF57 and ORF58 (83 to 95 kb). (B) Homology of vIRFs with cellular IRFs and rhesus macaque rhadinovirus (RRV)-encoded vIRFs. The degree of homology/identity was determined by BLASTp analysis using the UniProtKB database (http://uniprot.org).
Fig 2.
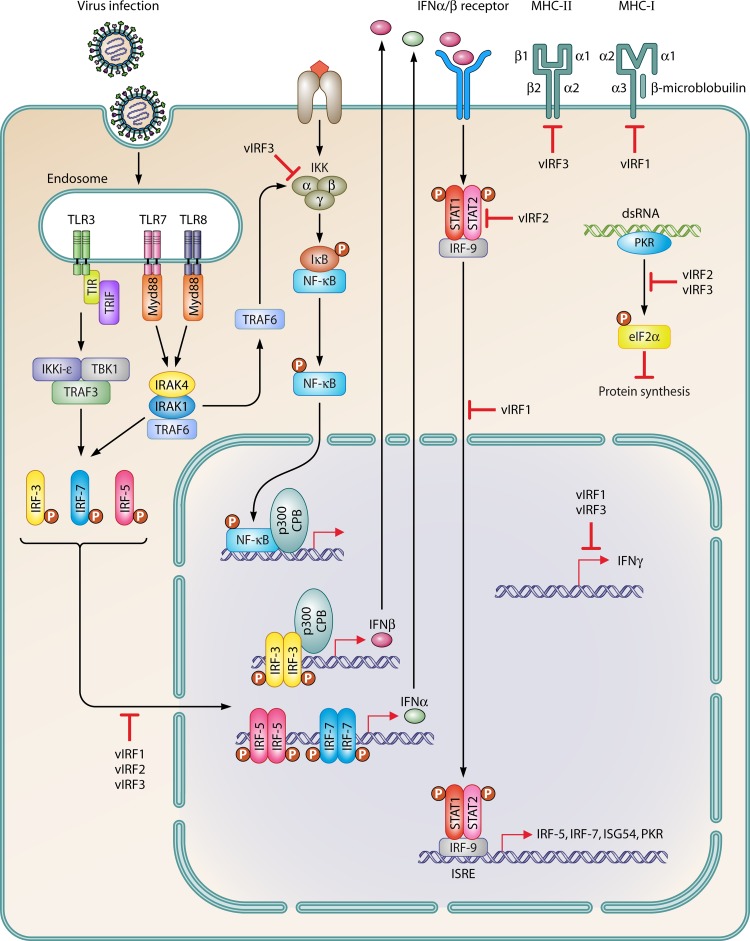
Schematic illustration of multiple immunomodulatory functions of KSHV within infected cells. Viral infection leads to activation of type I and II interferon signaling. Viral IRFs inhibit alpha, beta, and gamma interferon transcription, NF-κB activation, and MHC-I and II gene expression. P, phosphorus; TBK1, TANK-binding kinase 1.
Fig 3.
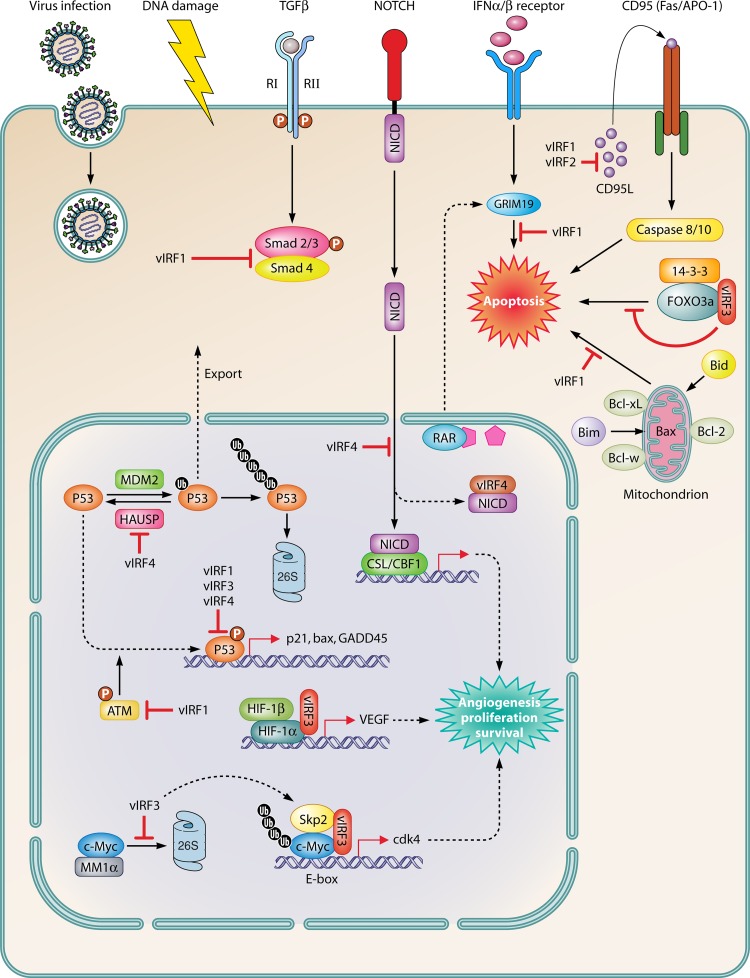
Schematic illustration of oncogenic and antiapoptotic functions of KSHV. Virus infection and DNA damage activate p53 and proapoptotic signaling. Viral IRFs effectively inhibit p53-mediated transcription and protein stability and function. vIRFs also regulate the proapoptotic pathway via inhibition of effector proteins, including GRIM19, 14-3-3, and FOXO3a, as well as Bim and Bid function. In addition, vIRF-3 activates c-Myc- and HIF-1α-mediated transcription. Other cellular pathways regulated by vIRFs include TGF-β, Notch, and CD95 signaling. Ub, ubiquitin.
KSHV-ENCODED vIRFs
KSHV interferon regulatory factors (vIRF1 to vIRF4) are encoded by a cluster of open reading frames (ORFs K9, K11/11.1, K10.5/10.6, and K10), which are inserted between ORF57 and ORF58 but transcribed in the opposite orientation (31) (Fig. 1A). vIRFs exert a certain degree of protein homology to cellular IRFs (Fig. 1B) (32); however, they do not contain five tryptophan residues in the DNA-binding region and are not able to bind to the promoters of type I IFN or ISGs.
vIRF1 (ORF K9)
Transcriptional regulation.
vIRF1 (449 amino acids) is a lytic gene (10, 33), expression of which can be induced by treatment with 12-O-tetradecanoyl-phorbol-13-acetate (TPA) of KSHV-positive primary effusion lymphoma (PEL) cells (14, 34, 35). However, low levels of vIRF1 were detected in latently infected KS and PEL cells, where it colocalizes to promyelocytic leukemia (PML) bodies (35, 36). The vIRF1 protein has a short half-life and does not remain at high levels throughout the lytic cascade (35). Transcription from the vIRF1 promoter can be upregulated by the KSHV-encoded replication and transcription activator (RTA) (37). However, the direct interaction of RTA with the vIRF1 promoter region has not been observed (38). Thus, it is likely that RTA transactivates the vIRF1 promoter through interaction with other DNA-binding factors.
Protein structure and DNA-binding properties of vIRF1.
The vIRF1 protein is comprised of 449 amino acids. Out of all four KSHV vIRFs, vIRF1 has the highest degree of homology with cellular IRFs (Fig. 1B). The amino-terminal region of vIRF1 displays significant amino acid identity with the N-terminal DNA-binding domain (DBD) of IRF-9, IFN consensus sequence-binding protein (ICSBP/IRF-8), and IRF-4 (Fig. 1B) (32); however, the putative DBD of vIRF1 contains only two out of five conserved tryptophan residues. Using gel shift and chromatin immunoprecipitation assays, Park and colleagues (39) identified the vIRF1-binding consensus sequence located in the promoter region of K3 (viral E3 ubiquitin ligase), viral dihydrofolate reductase (vDHFR; ORF2) and viral IL-6 (vIL6). Consistent with this finding, vIRF1 overexpression resulted in the activation of K3:vDHFR:vIL6 promoter-reporter, and knockdown of vIRF1 expression in KSHV-positive BCBL-1 cells inhibited vIL6 transcription (39, 40), suggesting that vIRF1 may also play a role in the induction of certain KSHV genes. In agreement with these observations, recent studies of the crystal structure of the vIRF1 DBD revealed that vIRF1 is in fact a DNA-binding protein that might act directly on different operator sequences, as proposed for IRF-3 (41).
vIRF1 AND IMMUNE RESPONSE
vIRF1 and interferon signaling.
vIRF1 represses expression of interferon-inducible genes and blocks IRF-1- and IRF-3-mediated transcription (Fig. 2). Several reporter studies have shown that in a transient expression assay, vIRF1 has the ability to block the IFN-α/β-, IFN-γ-, or IRF-1-induced activation of interferon-stimulated gene (ISG) promoters, such as ISG-54 and ISG-15, gamma interferon-activated sequence (GAS)-element activation, and IFN-β-induced p21WAF1/CIP1 transcription (33, 40, 42). Furthermore, vIRF1 efficiently inhibited Newcastle disease virus (NDV) activation of IFN-α4 promoter (10) and Sendai virus-induced expression of endogenous IFN-β, RANTES, and IP10 genes in human embryonic kidney (HEK293) cells (43).
The negative effect of vIRF1 on IFN-mediated signaling occurs through multiple protein-protein interactions between vIRF1 and cellular proteins. vIRF1 was reported to bind to IRF-1 and ICSBP/IRF-8 in vitro using a glutathione S-transferase (GST) pulldown assay (10). However, in another study, interaction between vIRF1 and IRF-1 could not be detected (33). Nevertheless, vIRF1 was shown to reduce both the DNA-binding affinity and transcription activity of IRF-1 (10, 33). Additionally, vIRF1 appears to interact with IRF-3 but does not affect IRF-3 dimerization, nuclear translocation, or DNA-binding activity (43).
In response to virus infection, cellular IRFs interact with and recruit the CBP/p300 transcriptional coactivator to type I IFN promoters. vIRF1 was shown to associate with the CBP/p300 coactivator and inhibit the formation of transcriptionally competent IRF-3-CBP/p300 complexes and consequently interfere with the recruitment of the basal transcription machinery (10, 43).
vIRF1 and MHC-I.
vIRF1 is capable of downregulation of basal and IFN-induced transcription of major histocompatibility complex class I (MHC-I) through its interaction with the transcriptional coactivator p300 (44). Thus, KSHV has evolved an efficient mechanism to evade antigen presentation, which results in the establishment of latency and host-pathogen equilibrium.
vIRF1 AND CANCER
vIRF1 and p53.
vIRF1 function is not limited to the antiviral response. It also inhibits the function of p53 and modulates apoptotic signaling (Fig. 3). p53 is a transcription factor which responds to stress signals, such as DNA damage, or viral infections, by inducing cell cycle arrest or apoptosis (45). p53 activity can be regulated through specific posttranslational modifications, e.g., phosphorylation, acetylation, ubiquitination, and SUMOylation (46, 47). Upon DNA damage, p53 is phosphorylated by ataxia telangiectasia-mutated (ATM) kinase and acetylated by CBP/p300, which leads to p53 activation and protein stabilization (48). On the other hand, polyubiquitination of p53 by murine double minute 2 (MDM2) E3 ubiquitin-protein ligase targets p53 for 26S proteasome-mediated degradation (49). vIRF1 negatively regulates p53 function by engaging two mechanisms. (i) vIRF1 interacts with ATM and blocks its kinase activity that is induced by DNA damage stress signals. As a consequence, vIRF1 greatly reduces the levels of p53 phosphorylation on serine residue 15, resulting in an increase of p53 ubiquitination by MDM2, leading to p53 degradation (50, 51). (ii) vIRF1 directly interacts with the DNA-binding domain (DBD) of p53 and suppresses p53 acetylation, resulting in the inhibition of p53 activity and p53-mediated apoptosis (52). Expression of vIRF1 in Saos-2 (human osteosarcoma with null p53) cells significantly reduced p53-mediated apoptosis (reduction of apoptotic cells from 75% to 32%), with an increased accumulation of cells in the G2/M phase of the cell cycle (51).
vIRF1 and TGF-β.
Inactivation of the transforming growth factor β (TGF-β) signaling pathway is important in the genesis of human malignancies (53, 54). Members of the TGF-β family regulate a variety of biological processes, including cell growth, differentiation, matrix production, and apoptosis (55, 56). TGF-β signaling results in the activation of members of the Smad family of tumor suppressors, which include Smad 2 and Smad 3. The activated Smads form complexes with a common mediator, Smad 4, and translocate to the nucleus, where they are involved in regulating the transcription of target genes (57, 58). TGF-β inhibits cell proliferation by regulating two classes of genes. First, TGF-β-activated Smad complexes target the promoter of the c-myc gene, leading to transcriptional inhibition of c-Myc. Second, activated Smad complexes are involved in the induction of two cyclin-dependent kinase inhibitors, p15 and p21 (59–61). vIRF1 was shown to suppress the TGF-β/Smad signaling pathway (62). vIRF-1 inhibited TGF-β-stimulated expression of a synthetic reporter containing four Smad elements. Direct interaction of vIRF1 with both Smad 3 and Smad 4 resulted in the inhibition of their transactivational activity. In addition, vIRF1 interfered with Smad 3-Smad 4 complex formation and DNA binding (62).
vIRF1 AND APOPTOSIS
vIRF-1 and the proapoptotic BOPs, Bim and Bid.
Both Bim and Bid are induced during KSHV lytic replication and are very powerful negative regulators of viral replication (63). Bim and Bid, like other BH3-only proteins (BOPs), function by virtue of their BH3 domains to target antiapoptotic members of the Bcl-2 family and to disrupt their interactions with apoptotic executioner proteins Bax and Bak. This liberates Bax and Bak for oligomerization and mitochondrial permeabilization (64, 65). However, Bim and Bid can also interact with and activate Bax and Bak directly, via induced conformational changes (66, 67). Direct interaction of vIRF1 with proapoptotic proteins Bim (68) and Bid (69) leads to inhibition of cellular proapoptotic signals (Fig. 3). The vIRF1-mediated inhibition of Bim utilizes a unique mechanism of Bim regulation, via nuclear sequestration of Bim away from mitochondria (68). vIRF-1-mediated relocalization of Bim was identified in transfected cells by both an immunofluorescence assay and Western blot analysis of fractionated cell extracts. Coimmunoprecipitation assays and GST pulldown assays utilizing various vIRF1 deletion mutants identified the minimal region of vIRF1 (residues 174 to 181) sufficient for direct interaction with Bim. Blocking of the vIRF1-Bim interaction with a cell-permeable peptide corresponding to the Bim-binding region of vIRF1 led to reduced virus titers in KSHV-infected telomerase-immortalized endothelial (TIME) cells (68). Thus, vIRF1-induced nuclear localization and inactivation of Bim represents a novel mechanism of viral evasion from antiviral defenses of the host. Recently, the Bim-binding region (BBD) of vIRF1 was shown to interact also with the BH-3 domain of other BOPs, e.g., Bid, Bik, Bmf, Hrk, and Noxa (69). In contrast to Bim, vIRF1 was unable to mediate nuclear sequestration of Bid protein. Direct functional inhibition of Bid by vIRF1 was demonstrated by the ability of vIRF1 to block Bid-induced mitochondrial permeabilization in vitro (69). Furthermore, Western blot analysis of mitochondrial preparations from KSHV-positive BCBL-1 cells, or TIME cells, revealed mitochondrial association of endogenous vIRF1 in latent and lytic cultures. Thus, Bid and Bim are contributors to negative regulation of KSHV infection, and their targeting by vIRF1 may be important for productive virus replication (69).
vIRF1 and GRIM19.
In PEL cells, vIRF1 was also shown to associate with the interferon/retinoid acid (IFN/RA)-inducible cell death regulator, GRIM19. GRIM19 encodes a 144-amino-acid protein that localizes predominantly to the nucleus and enhances caspase 9 activity and apoptotic cell death in response to IFN/RA (70). In the presence of vIRF1, GRIM19 was unable to induce apoptosis (71). The inhibition of apoptosis may be related to the ability of vIRF1 to induce cellular transformation. Overexpression of vIRF1 in NIH 3T3 cells resulted in the attenuation of growth regulation and contact inhibition. These cells were also able to grow as tumors in nude mice (42). However, coexpression of vIRF1 and GRIM19 in NIH 3T3 cells led to significant suppression of transformed colonies, suggesting that vIRF1-mediated inhibition of GRIM19 contributes to the cell-transforming properties of KSHV (72).
vIRF1 and CD95L.
vIRF1 was shown to be able to inhibit activation-induced cell death (AICD) mediated by CD95 death receptor signaling. The CD95 (also called Fas/APO-1) pathway plays a major role in the induction of apoptosis in lymphoid and nonlymphoid tissues. CD95 (Fas/APO-1), a type I transmembrane protein, is a member of the tumor necrosis factor (TNF) receptor superfamily, which is expressed in various tissues (73, 74). The CD95 ligand (CD95L) is induced in response to a variety of signals, including IFN-γ and T cell receptor (TCR)/CD3 stimulation. CD95L induces apoptosis via formation of a death-inducing signaling complex and initiation of a signaling cascade of caspases (73, 75). vIRF1 effectively prevents CD95L expression via inhibition of IRF-1 binding to regulatory IRF-1-dependent domains (PRIDDs) present at the CD95L promoter DNA (76). Consequently, vIRF1 is able to strongly inhibit TCR/CD3-mediated cell death.
Collectively, these data indicate that vIRF1 can affect two important cellular defense mechanisms: (i) innate antiviral response and (ii) apoptosis-mediated antiviral defense. Since vIRF1 is expressed predominantly in acutely infected KS cells, these data suggest that KSHV needs to overcome the antiviral response in order to establish infection.
vIRF2 (ORF K11 AND K11.1)
Transcriptional regulation.
The initial studies carried out by Burysek et al. identified vIRF-2 as a constitutively expressed protein encoded by a single ORF (K11.1) consisting of 163 amino acids (77) (Fig. 1A). More recently, other groups have found that the vIRF-2 gene encodes a 2.2-kb spliced transcript representing two exons of ORFs K11.1 and K11, from which the full-length vIRF-2 protein is translated (680 amino acids). Moreover, this spliced form of vIRF-2 was characterized as an inducible gene from microarray and quantitative PCR studies (16, 78). vIRF2 is present in the cytoplasm and the nucleus of infected cells, and its expression can be induced by IFN treatment (79).
vIRF2 AND IMMUNE RESPONSE
vIRF2 and cellular IRFs.
Studies with the short form of vIRF2 (K11.1; 20 kDa) demonstrated the binding of vIRF2 to a consensus NF-κB-binding site but not to an interferon-stimulated response element (ISRE) (77) (Fig. 2). Additionally, vIRF2 was shown to suppress IRF-1- and IRF-3-driven activation of an IFN-α reporter promoter in cells infected with NDV. In GST pulldown assays, this short form of vIRF2 interacted with cellular IRF-1, p300/CBP, p65, IRF-2, and ICSBP/IRF-8; however, it did not bind to IRF-3 (77).
Studies including full-length vIRF2 showed that vIRF2 downregulated both IFN-α- and IFN-λ-driven transactivation of reporter promoter containing ISRE (80). Furthermore, vIRF2 negatively regulated the transactivation of the ISRE promoter by IRF-1 as well as activation of IFN-β reporter promoter by either IRF-3 or IRF-1, but not by IRF-7 (80). Although interaction between the short form of vIRF-2 and IRF-3 was not observed (77), full-length vIRF-2 was found to associate with IRF-3 (79). vIRF2 was shown to recruit caspase 3 to IRF-3 and thus accelerate the caspase-dependent process of IRF-3 turnover, leading to an inefficient antiviral response (79). Recently, it was also shown that vIRF2 inhibits type I IFN signaling by targeting components of the interferon-stimulated gene factor 3 (ISGF3) complex, STAT1 and IRF-9, which results in the inhibition of ISG expression (81). Thus, vIRF2 appears to be able to inhibit both early and later steps of the antiviral signaling pathway.
vIRF2 and PKR.
The short form of vIRF2 (K11.1) also binds to the dsRNA-activated protein kinase (PKR) and blocks the autophosphorylation and phosphorylation of PKR substrates (82). PKR acts as a serine/threonine kinase that phosphorylates and activates downstream targets, including eukaryotic translation initiation factor 2 alpha (eIF-2α), histone 2A, and NF-κB, events that affect cell growth, cell differentiation, viral clearance, and induction of apoptosis (83–86). By inhibiting the kinase activity of PKR through vIRF2 and the consequent down-modulation of protein synthesis, KSHV has evolved a mechanism by which it can overcome the interferon-mediated antiviral effect (82). Thus, the anti-interferon functions of vIRF2 may contribute to the establishment of chronic or latent infection.
vIRF2 AND APOPTOSIS
vIRF2 and CD95L.
Similarly to vIRF1, vIRF2 was able to inhibit CD95L expression (76) (Fig. 3). However, vIRF2 did not modulate binding of IRF-1 to CD95L promoter DNA. The possible mechanism of vIRF2-mediated repression of CD95L induction is through NF-κB inhibition (77). These data suggest that both vIRF1 and vIRF2 act as modulators of the immune system by repressing activation-induced cell death (AICD) via modulation of TCR/CD3-mediated induction of CD95L (87).
vIRF3 (ORF K10.5 AND K10.6)
Transcriptional regulation.
vIRF3 (also called LANA2) is a spliced product of two ORFs, K10.5 and K10.6 (11) (Fig. 1A). vIRF3 (566 amino acids) is constitutively expressed in the nuclei of KSHV-infected hematopoietic tissues, including those from body cavity-based primary effusion lymphoma (PEL) and Castleman disease (CD), but not in Kaposi's sarcoma (KS) lesions (88). Unlike other vIRFs, vIRF3 is not induced during lytic reactivation of TPA-treated PEL cells (89). vIRF3 shows a significant degree of homology with the IRF-association domain (IAD) region of cellular IRF-8/ICSBP (Fig. 1B) (11, 88, 90). IAD is a conserved region shared by all IRFs, excluding IRF-1 and IRF-2. IAD mediates homo- and heteromeric interactions that occur between IRFs or IRFs and other cellular factors, e.g., STAT1/2 and PU.1. (91).
vIRF3 AND IMMUNE RESPONSE
vIRF3 and cellular IRFs.
Similarly to vIRF1 and vIRF2, vIRF3 is able to associate with activated nuclear IRF-3 and IRF-7 and consequently modulate the innate antiviral response by interfering with their functions (92) (Fig. 2). As shown recently, vIRF3 can also interact with IRF-5 (93, 94). The mapping of the vIRF3-binding domain revealed that vIRF3 associates with IRF-3 and IRF-7 through its C-terminal region (amino acids 254 to 566). Although vIRF3 is not a DNA-binding protein, it is recruited to IFN-α gene promoters via its interaction with IRF-3, IRF-7, and p300. We have shown that the presence of vIRF3 in the enhanceosome and its interaction with the IFN-α gene promoter increases the binding of IRF-3, IRF-7, and acetylated histone H3 to the promoters of IFN-α genes (90). In contrast, Joo et al. (92) showed that vIRF3 blocks cellular IRF-7-mediated innate immunity by interacting with the DNA-binding domain or the IRF-association domain of IRF-7. This interaction specifically suppresses IRF-7 DNA binding and consequently inhibits IFN-α1, -α4, and -α6 gene expression, as estimated by real-time quantitative reverse transcription-PCR (qRT-PCR) in transfected HEK293T cells infected with Sendai virus (92). The vIRF3-mediated modulation of IRF-7 function may also be important in the context of Epstein-Barr virus (EBV) transformation, where IRF-7 is activated in EBV-transformed cells (95) and induces the expression of LMP-1 (96), which has a critical role in EBV-induced lymphomagenesis.
vIRF-3 and IRF-5.
Interferon regulatory factor 5 (IRF-5) is a transcription factor that has a key role in the induction of antiviral and inflammatory responses and autoimmunity (97, 98). It is induced by viral infection and type I IFN and activated by TLR7- and TLR9/MyD88-dependent pathways. Although IRF-5 is a direct target of p53, its cell cycle regulatory and proapoptotic effects are p53 independent (27). IRF-5 is expressed in B cells, dendritic cells, and macrophages and remains in the cytoplasm as an inactive protein. Upon DNA damage signals or activation of the IFN, TLR7, or TLR9 pathways, IRF-5 is phosphorylated, K63 ubiquitinated, and subsequently translocated to the nucleus. In addition to its antiviral effect, its tumor suppressor properties and ability to regulate the cell cycle have also been shown (27, 99). Interestingly, in the nuclei of PEL cells, IRF-5 is present constitutively. The interaction of IRF-5 with vIRF3, which was demonstrated in transfected HEK293T cells and PEL cells, leads to the inhibition of IRF-5 binding to the promoters of IFN-β and interferon-responsive genes (ISGs) (94). Thus, vIRF3 blocks IRF-5-mediated activation of ISRE and IFN-β promoter reporter activity. In addition, vIRF3 antagonizes IRF-5-mediated activation of p21 promoter reporter and prevents IRF-5-mediated growth inhibition and G2/M cell cycle arrest (93, 94). Thus, the expression of vIRF3 in PEL cells may contribute to their tumorigenicity.
vIRF3 and NF-κB.
Association with cellular IRFs is not the only mechanism by which vIRF3 downregulates the innate inflammatory response. Activation of NF-κB pathway is also part of the inflammatory response and can be induced by viral infection. NF-κB is a transcription factor that plays a role in innate and adaptive immune responses through its ability to regulate the production of cytokines, receptors necessary for immune recognition, and proteins participating in antigen presentation (100, 101). In unstimulated cells, NF-κB is localized in the cytoplasm in an inactive form, where it associates with the inhibitory protein IκB. Upon viral infection, IκB is phosphorylated, which leads to the ubiquitination and subsequent degradation of the IκB inhibitor. Phosphorylation of IκB is mediated by the IκB kinase (IKK) complex that is composed of three subunits: IKKα, IKKβ, and IKKγ. Liberated NF-κB can then translocate to the nucleus and activate transcription of target genes (102). Phosphorylation of IκB is thus the key step in regulation of NF-κB activation. The IKKβ subunit of IKK was shown to be targeted by vIRF3 (103). Direct interaction between vIRF3 and IKKβ, which was demonstrated in transfected HEK293T cells, led to hypophosphorylation of IκB, impaired translocation of NF-κB to the nucleus, and reduced NF-κB-mediated transcription (103). However, coimmunoprecipitation experiments failed to demonstrate IKKβ-vIRF3 interaction in KSHV-positive BCBL-1 cells (103). In agreement with previous reports demonstrating that suppression of NF-κB results in enhanced TNF-α-induced apoptosis, vIRF3 expression significantly increased TNF-α-induced apoptosis (estimated by terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling [TUNEL]) in HEK293T cells (103).
vIRF3 and MHC-II
Escape from antigen presentation is an effective strategy of KSHV, and vIRF3 plays an important part by interfering with the adaptive immune response by inhibition of major histocompatibility complex class II (MHC-II) and IFN-γ expression. MHC-II molecules are found on only a few specialized cell types, including macrophages, dendritic cells, and B cells, all of which are professional antigen-presenting cells (APCs). In humans, MHC-II expression is inducible by gamma interferon (IFN-γ) in almost every cell type (104). KSHV-positive PEL cells exhibit a lower level of MHC-II expression on the cell surface than that exhibited by KSHV-negative B cell lymphomas. It was shown that the small interfering RNA (siRNA)-mediated knockdown of vIRF3 in KSHV-infected PEL cell lines resulted in increased MHC-II levels (105). Conversely, overexpression of vIRF3 in KSHV-negative B cells (BJAB) led to down-modulation of MHC-II. The observed suppression of MHC-II is due to the vIRF3-mediated inhibition of the class II trans-activator (CIITA) which is a major regulator of MHC-II transcription. The expression of CIITA is under the control of the B cell type-specific promoter PIII or the IFN-γ-inducible promoter PIV. The presence of vIRF3 in PEL cells reduced the activity of PIII and PIV promoters. In addition, vIRF3 also downregulates IFN-γ promoter activity. A vIRF3 knockdown in PEL cells resulted in increased levels of IFN-γ protein (105). Suppression of IFN-γ signaling leads to down-modulation of MHC-II. Thus, inhibition of MHC-I and MHC-II by vIRF1 and vIRF3, respectively, contributes to viral immunoevasion and the effective escape from antigen presentation in KSHV-infected cells.
vIRF3 AND CANCER
Oncogenesis is represented by multiple events which are characterized by the progression of cytological, genetic, and cellular changes that ultimately culminate in uncontrolled cell division and tumor growth. Oncogenesis requires inactivation of tumor suppressor genes and activation of cellular proto-oncogenes. In the case of virus-induced oncogenesis, attenuation of the antiviral response is also important. KSHV-encoded vIRF3 can promote tumorigenesis by at least two mechanisms: (i) association with a key tumor suppressor, p53, and downregulation of its transcription activity (88) and (ii) activation of the c-Myc proto-oncogene (106) (Fig. 3). vIRF3 is also involved in the regulation of apoptosis and the cell cycle and in the modulation of microtubule dynamics (107). Interestingly, vIRF3 is required for the survival of KSHV-infected PEL cells in vitro. Silencing of vIRF-3 by approximately 40% resulted in a 3-fold increase of caspase 3/7 activity and the subsequent induction of apoptosis (89). vIRF-3 can thus be considered a bona fide oncogene of KSHV.
vIRF-3 and p53.
The p53 gene was the first identified tumor suppressor gene. Attenuation of p53 function is among the most frequent molecular events occurring in human cancers (108). vIRF3 was shown to interact with p53 protein in vitro. The interaction domain is located in the region consisting of amino acids 290 and 393 of p53. This region encompasses the tetramerization and regulatory domains of p53. Cells expressing vIRF3 show inhibition of p53-mediated apoptosis and lower levels of caspase 8 activation (88). However, the direct interaction between endogenous vIRF3 and p53 in KSHV-infected PEL cells has not been demonstrated, and thus the molecular mechanism by which vIRF3 modulates p53 function is yet to be determined.
vIRF3 and c-Myc.
vIRF3 also stimulates the transcriptional activity of the c-Myc proto-oncogene. c-Myc is a transcription factor that activates expression of a majority of the transcriptionally active cellular genes (109) including those involved in the control of cellular growth, proliferation, and cell survival. Thus, c-Myc activation reduces growth regulation, drives cell proliferation, and blocks cellular differentiation. It also plays an important role in stem cell self-renewal (110, 111). Although c-Myc was originally thought to stimulate promoters of genes containing E-box consensus sequences (112), recent data indicate that c-Myc does not target a specific transcriptional program(s) but is a universal amplifier of gene expression, which increases the output of all active promoters (113). The E-box-dependent transcriptional activity of c-Myc can be repressed by Myc modulator 1 (MM-1) (114, 115). This repression is disturbed in the presence of vIRF3. The direct association of vIRF3 with MM-1α results in the inhibition of MM-1α-c-Myc complex formation. Released c-Myc stimulates the transcriptional activity of the cdk4 promoter and promoters of other c-Myc regulated genes. Moreover, vIRF3 is recruited to the cdk4 promoter in PEL cell lines via its interaction with c-Myc. The association of vIRF3 with c-Myc increases the DNA-binding capacity of c-Myc and c-Myc-mediated transcription (106). In addition, vIRF3 can increase c-Myc protein stability by extending its half-life approximately 4-fold. The siRNA-mediated knockdown of vIRF3 in KSHV-positive PEL cells led to a significant decrease in endogenous c-Myc protein levels (116). The enhanced c-Myc stability and transcriptional activity in vIRF3-expressing cells is a consequence of the direct association of vIRF3 with Skp2. Skp2 is a key component of the SCFSkp2 ubiquitin ligase complex that acts as a c-Myc transcriptional cofactor. vIRF3 binds to the F-box of Skp2, resulting in the activation of c-Myc-dependent transcription. The fact that cells overexpressing vIRF3 exhibit higher levels of c-Myc ubiquitination supports the evidence for the necessity of ubiquitination for c-Myc function (116). It would be interesting to see if the increase of c-Myc stability and transcriptional activity leads to the enhancement of the total transcriptional profile in PEL cells, as seen in other tumor cells (109).
vIRF3 and PODs.
The multiple effects of vIRF3 on cellular transformation and carcinogenesis are strongly supported by its contribution to the disruption of PML oncogenic domains (PODs) and interaction with 14-3-3 regulatory proteins. The promyelocytic leukemia (PML) protein is a multifunctional protein that has a role in tumor suppression and host defense against virus infection. It is a major component of PODs, also known as ND10 domains, which form nuclear depots for a number of proteins that are involved in gene transcription, genomic stability, apoptosis, and cell cycle regulation (117–119). In addition to PML, PODs contain other proteins, including Sp100, Daxx, pRb, CBP, and p53 (120). vIRF3 expression induces the displacement of PML or Sp100 from PODs and the degradation of PML by a proteasome-mediated mechanism (121). Moreover, silencing of vIRF3 expression in PEL cells by RNA interference led to an increase in the PML levels. vIRF3 also interferes with the PML-mediated transcriptional repression of survivin, a protein that contributes to the malignant progression of PEL cells (121). Recently, vIRF3 was shown to covalently conjugate to small ubiquitin-like modifier 1 (SUMO1) and SUMO2 both in vitro and in latently KSHV-infected PEL cells (122). SUMO is an 11.5-kDa protein that has the ability to conjugate to multiple proteins and modulate protein stability, subcellular localization, and protein activity and function (123). It is believed that covalent conjugation of SUMO to vIRF3 may serve as a bridge between vIRF3 and other SUMO-interacting proteins that are required for vIRF3-mediated disruption of PODs (122). Importantly, vIRF3 was also shown to inhibit SUMOylation of three pocket proteins, pRb, p107, and p130, which are key tumor suppressors frequently targeted by oncoproteins expressed by DNA tumor viruses (124). vIRF3 contains an LXCXE motif that mediates the interaction with these cellular proteins and is required for inhibition of their conjugation to SUMO (124).
vIRF3 and HIF-1.
Hypoxia-inducible factor 1 (HIF-1), a key regulator of cellular responses in low-oxygen concentrations, is involved in developmental and pathological angiogenesis. Under normal conditions, HIF-1α, an oxygen-sensitive subunit of HIF-1, undergoes rapid ubiquitination and proteasomal degradation (125). In contrast, under hypoxic conditions, HIF-1α is stabilized, accumulated, and translocated into the nucleus, where it forms a heterodimeric complex with HIF-1β to activate transcription of its target genes, including vascular endothelial growth factor (VEGF), which plays an important role in angiogenesis and tumor growth (126). By direct binding to HIF-1α, vIRF3 robustly induces HIF-1α transcriptional activity and its stability and thereby increases VEGF production, which may affect the proliferation of neighboring cells by a paracrine mechanism and may facilitate endothelial tube formation (127). However, the expression of vIRF3 has not been detected in Kaposi's sarcoma lesions; thus, its biological relevance may be limited to KSHV-positive B cells.
vIRF3 and 14-3-3.
The 14-3-3 proteins are a family of highly conserved dimeric regulatory proteins which are involved in the regulation of the cell cycle, apoptosis, and oncogenesis (128). 14-3-3 proteins promote the cytoplasmic localization of members of the forkhead box O (FOXO) family of transcription factors, FOXO1, FOXO3a, and FOXO4, resulting in the inhibition of their transcription activity (129). Activation of genes by FOXO members leads to G1 cell cycle arrest and apoptosis in many tumor cell lines (130, 131). Phosphorylation of FOXO3a by AKT generates binding sites for 14-3-3 proteins, which leads to translocation of FOXO3a from the nucleus to the cytoplasm, where it remains in an inactive form (132, 133). Phosphorylated vIRF-3 can interact with 14-3-3 proteins and FOXO3a. Importantly, vIRF3 is able to bind also to the nonphosphorylated form of FOXO3a. Thus, this interaction facilitates the binding between 14-3-3 and nonphosphorylated FOXO3a and inhibits the transactivation of FOXO3a targets, such as proapoptotic Bim. In addition, vIRF3 also blocks G2/M cell cycle arrest, which is induced by 14-3-3 protein overexpression (134).
vIRF3 AND APOPTOSIS
vIRF3 and PKR.
Activation of IFN-responsive genes (ISGs) is an important mechanism for the establishment of an effective antiviral immune response. vIRF3 was shown to target the function of IFN-induced dsRNA-activated protein kinase (PKR). The expression of vIRF3 in BSC-40 cells resulted in reduced levels of PKR-mediated apoptosis as measured by DNA ladder formation (135). Furthermore, the presence of vIRF3 leads to decreased levels of phosphorylated eIF-2α by PKR (135). Deregulation of the eIF-2α checkpoint and consequent permissiveness to virus infection may be a common occurrence in tumorigenic mammalian cell lines (136). The direct interaction of vIRF-3 and PKR was not detected, and vIRF3 was not able to inhibit NF-κB activation in response to PKR. However, vIRF3 inhibited PKR-induced activation of caspase 3, but not that of caspase 9, suggesting that only the Fas-associated protein with death domain (FADD)/caspase 8 pathway is affected by vIRF3 (135).
Likewise, the work of several other groups has shown the association of vIRF3 with the regulation of apoptosis. Rivas et al. showed that vIRF3 is able to inhibit p53- and doxorubicin-induced apoptosis in Saos-2 (human osteosarcoma with null p53) and U2OS (human osteosarcoma with wild-type p53) cells (88). In contrast to these findings, Seo et al. reported that vIRF3 is able to induce apoptosis in TNF-α-treated HEK293T cells via inhibition of NF-κB activity (103). Based on these contradictory findings, it is not clear whether vIRF3 is pro- or antiapoptotic. The discrepancy may be due to heterologous cell systems studied, overexpression of exogenous vIRF3, and/or the use of different apoptosis-inducible agents. However, recent data from Wies et al. showed that the knockdown of vIRF3 in KSHV-infected PEL (BC-3, JSC-1) cells led to reduced cell proliferation and increased caspase 3/7 activity, demonstrating the antiapoptotic and tumorigenic properties of vIRF3 (89).
vIRF4 (ORF K10)
Transcriptional regulation.
vIRF4 (911 amino acids) is expressed during virus reactivation and serves as a positive coregulator for RTA, the master regulator of the switch from latency to lytic reactivation in KSHV (137) (Fig. 1A and B). Depletion of vIRF4 during TPA-mediated reactivation from latency resulted in a reduced yield of infectious KSHV virions, underlying its role in efficient KSHV reactivation.
vIRF4 AND CANCER
vIRF4 and p53.
Unlike other vIRFs, vIRF4 does not target and antagonize the host IFN-mediated antiviral response. However, it was shown that vIRF-4 interacts with the murine double minute 2 (MDM2) E3 ubiquitin ligase, leading to the reduction of p53 via proteasome-mediated degradation (138) (Fig. 3). The central region of vIRF4 (amino acids 606 to 758) is required for its interaction with MDM2, which leads to the suppression of MDM2 autoubiquitination, resulting in a dramatic increase in MDM2 stability. Consequently, vIRF4 expression markedly enhances p53 ubiquitination and degradation, which effectively suppresses p53-mediated apoptosis. Recently, vIRF4 was also shown to specifically inhibit the herpesvirus-associated ubiquitin-specific protease (HAUSP) that regulates the stability of p53 and MDM2. vIRF4 protein binds both the HAUSP TNF receptor-associated factor (TRAF) and the catalytic domains, resulting in the inhibition of substrate binding and HAUSP deubiquitination activity (139). This study showed that two vIRF-4-derived peptides, vif1 and vif2, are able to suppress HAUSP activity and restore p53-dependent apoptosis in PEL cells, as well as suppress tumor growth, in a mouse xenograft model (139). Thus, the virus has developed a unique strategy to target the HAUSP-MDM2-p53 pathway.
vIRF4 and Notch.
Activated Notch CSL/CBF1 signaling was shown to promote the survival of KSHV-infected cells (140, 141, 142). Notch signaling is an evolutionarily conserved signal transduction pathway which regulates multiple developmental processes. Its deregulation is directly linked to many human disorders, including cancer (143). Notch receptors are transmembrane proteins that upon ligand binding are proteolytically cleaved to generate the intracellular Notch fragment, NICD, which translocates into the cell nucleus and binds to the CSL/CBF1 protein. This DNA-binding factor then recruits corepressor and coactivator complexes to modulate the expression of target genes (143). vIRF4 was shown to act as a potential antagonist of the Notch/CBF1 signal transduction pathway. Interaction of vIRF4 with CBF1 results in inhibition of CBF1-NICD complex formation. These observations were further supported in reporter gene assays, in which vIRF4 interfered with CBF1-dependent Notch transactivation. Thus, it appears that vIRF4 and NICD binding to CBF1 is mutually exclusive and vIRF4 can interfere with NICD-mediated promoter activation via direct competition for CBF1 binding (144).
CONCLUSION AND PERSPECTIVES
The critical role of interferon in the innate and adaptive antiviral response has been clearly established. It has also become clear that viruses have evolved mechanisms by which they can attenuate antiviral responses. KSHV has developed multiple redundancies of antiviral proteins to control cellular mechanisms that are involved in immune responses. With the ability to attenuate both arms of the antiviral response, KSHV also hijacked several cellular genes to act as regulators of their cellular homologues. Among these are homologues of the transcription factors of the IRF family, vIRFs (Table 1) (Fig. 1, 2, and 3). The present data indicate that vIRFs function generally as negative regulators of the antiviral response and apoptosis mediated by cellular IRFs.
Table 1.
Cellular pathways modulated by KSHV-encoded vIRFs
| vIRF | Transcriptional regulation | Genomic locus (protein size [aa]) | Immunomodulation | Reference(s) | Cellular pathways/oncogenesis/apoptosis | Reference(s) |
|---|---|---|---|---|---|---|
| vIRF1 | Lytic | K9 (449) | Interferon signaling (IRF-1, -3, -7, and -8; p300) | 10, 33, 40, 42, 43 | p53 signaling (p53, ATM, MDM2) | 50–52 |
| MHC-I | 44 | TGF-β signaling (Smad 3, Smad 4) | 62 | |||
| Apoptosis (Bim, Bid, GRIM19) | 68, 69, 71, 72 | |||||
| CD95/Fas/APO-1 (CD95L, IRF-1) | 76 | |||||
| vIRF2 | Lytic/latent | K11/K11.1 (680/163) | Interferon signaling (IRF-1, IRF-3, p300, ISGF3, PKR, caspase 3) | 77, 79, 82 | CD95/Fas/APO-1 (CD95L) | 76 |
| vIRF3 | Latent | K10.5/K10.6 (566) | Interferon signaling (IRF-3, -5, and -7; p300, PKR) | 90, 92, 94 | p53 signaling | 88 |
| NF-κB (IKKβ) | 103 | c-Myc pathway (c-Myc, MM-1α, Skp2) | 106, 116 | |||
| MHC-II (CIITA) | 105 | Disruption of PODs (PML, Sp100) | 121 | |||
| SUMOylation of pocket proteins (pRB, p107, p130) | 122, 124 | |||||
| Angiogenesis (HIF-1α, VEGF) | 127 | |||||
| G2/M arrest (14-3-3, FOXO3a) | 134 | |||||
| Apoptosis (PKR, NFκB, p53, caspase 3/7) | 88, 89, 103, 135 | |||||
| vIRF4 | Lytic | K10 (911) | ND | p53 signaling (p53, MDM2, HAUSP) | 138, 139 | |
| Notch signaling (CSL/CBF1) | 144 |
aND, not determined.
KSHV and rhesus macaque rhadinovirus (RRV), the two highly related gammaherpesviruses, are the only viruses known to encode gene products with significant homology to cellular IRFs. Characterization of an RRV recombinant clone lacking all eight vIRFs (vIRF-knockout [vIRF-KO] RRV) demonstrated that RRV-encoded vIRFs inhibit type I and II IFN gene induction (145). Moreover, infection of vIRF-KO RRV resulted in decreased viral loads and diminished B cell hyperplasia, a characteristic pathology of RRV infection (146). Collectively, these findings demonstrate that both KSHV and RRV vIRFs have a broad impact on herpesvirus pathogenesis and host immune responses.
The role of vIRFs in KSHV pathogenesis may extend beyond their immunomodulatory functions. In addition to the previously reported upregulation of the Toll-like receptor 3 (TLR3) pathway in human monocytes during KSHV primary infection, activation of TLR7/8 signaling is important for reactivation of KSHV from latency (147–149). Agonists specific for TLR7/8 reactivated latent KSHV and induced viral lytic gene transcription and replication. Thus, the vIRF-mediated downmodulation of TLR signaling and its effector molecules, IRFs and NF-κB, may also prevent KSHV reactivation and serve as an important control mechanism for the latent-to-lytic switch.
In addition to their immunomodulating effects, KSHV-encoded viral IRFs were also shown to modulate cell growth by targeting the function of the tumor suppressor p53 and enhancing the activity of the c-Myc proto-oncogene. Since p53 is rarely mutated in KSHV-associated tumors (150), the inhibition of p53-mediated signaling appears to be a key regulatory pathway through which KSHV is able to establish malignancy. Supporting this finding, chemical activation of p53 by an MDM2 antagonist, Nutlin-3, in PEL cells led to unimpaired induction of p53 target genes as well as growth inhibition and apoptosis (151).
Although different vIRFs often target the same cellular pathways, their specificity and redundancy is yet to be determined. The recent work of Jacobs and colleagues attempted to shed light on the differences in the mechanisms through which vIRF1, -2, and -3 could inhibit TLR3-mediated activation of IFN-β. Although all three vIRFs were able to inhibit TLR3-mediated activation of IFN transcription reporters, only vIRF1 and -2 inhibited IFN-β message and protein levels (152). Furthermore, the expression of vIRF1 seemed to reduce IRF-3 phosphorylation and nuclear localization compared to results for vIRF2, suggesting that while both vIRF1 and vIRF2 inhibit TLR3-mediated induction of IFN-β, they may accomplish this via distinct mechanisms. The advantage of the redundancy of the vIRFs in the antiviral response is easy to understand. However, it is not clear why KSHV evolved these vIRFs to also control so many growth regulatory and antiapoptotic functions. One has to wonder if the multiple roles of vIRFs in the modulation of cellular growth and apoptosis mirror the multiple ways in which KSHV uses cellular machinery for its own replication and the establishment and maintenance of latency. Future studies will undoubtedly answer some of these questions.
ACKNOWLEDGMENTS
We thank Jasper Manning for critical readings of the manuscript.
Barbora Lubyova is supported by the Grant Agency of the Czech Republic (project 204/09/0773) and Charles University in Prague (project PRVOUK-P24/LF1/3). Petra Baresova is supported by Charles University training grants (projects GA UK 436711 and SVV-2013-266508).
We apologize to authors whose work we were unable to reference due to space limitations.
Footnotes
Published ahead of print 19 June 2013
REFERENCES
- 1.Chang Y, Cesarman E, Pessin MS, Lee F, Culpepper J, Knowles DM, Moore PS. 1994. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science 266:1865–1869 [DOI] [PubMed] [Google Scholar]
- 2.Cesarman E, Chang Y, Moore PS, Said JW, Knowles DM. 1995. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N. Engl. J. Med. 332:1186–1191 [DOI] [PubMed] [Google Scholar]
- 3.Soulier J, Grollet L, Oksenhendler E, Cacoub P, Cazals-Hatem D, Babinet P, d'Agay M-F, Clauvel J-P, Raphael M, Degos L, Sigaux F. 1995. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in multicentric Castleman's disease. Blood 86:1276–1280 [PubMed] [Google Scholar]
- 4.Wen KW, Damania B. 2010. Kaposi sarcoma-associated herpesvirus (KSHV): molecular biology and oncogenesis. Cancer Lett. 289:140–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boshoff C, Endo Y, Collins PD, Takeuchi Y, Reeves JD, Schweickart VL, Siani MA, Sasaki T, Williams TJ, Gray PW, Moore PS, Chang Y, Weiss RA. 1997. Angiogenic and HIV-inhibitory functions of KSHV-encoded chemokines. Science 278:290–294 [DOI] [PubMed] [Google Scholar]
- 6.Molden J, Chang Y, You Y, Moore PS, Goldsmith MA. 1997. A Kaposi's sarcoma-associated herpesvirus-encoded cytokine homolog (vIL-6) activates signaling through the shared gp130 receptor subunit. J. Biol. Chem. 272:19625–19631 [DOI] [PubMed] [Google Scholar]
- 7.Thome M, Schneider P, Hofmann K, Fickenscher H, Meinl E, Neipel F, Mattmann C, Burns K, Bodmer JL, Schroter M, Scaffidi C, Krammer PH, Peter ME, Tschopp J. 1997. Viral FLICE-inhibitory proteins (FLIPs) prevent apoptosis induced by death receptors. Nature 386:517–521 [DOI] [PubMed] [Google Scholar]
- 8.Bais C, Santomasso B, Coso O, Arvanitakis L, Raaka EG, Gutkind JS, Asch AS, Cesarman E, Gershengorn MC, Mesri EA. 1998. G-protein-coupled receptor of Kaposi's sarcoma-associated herpesvirus is a viral oncogene and angiogenesis activator. Nature 391:86–89 [DOI] [PubMed] [Google Scholar]
- 9.Moore PS, Chang Y. 1998. Kaposi's sarcoma-associated herpesvirus-encoded oncogenes and oncogenesis. J. Natl. Cancer Inst. Monogr. 1998:65–71 [DOI] [PubMed] [Google Scholar]
- 10.Burýsek L, Yeow WS, Lubyová B, Kellum M, Schafer SL, Huang YQ, Pitha PM. 1999. Functional analysis of human herpesvirus 8-encoded viral interferon regulatory factor 1 and its association with cellular interferon regulatory factors and p300. J. Virol. 73:7334–7342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lubyova B, Pitha PM. 2000. Characterization of a novel human herpesvirus 8-encoded protein, vIRF-3, that shows homology to viral and cellular interferon regulatory factors. J. Virol. 74:8194–8201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hayward GS. 1999. KSHV strains: the origins and global spread of the virus. Semin. Cancer Biol. 9:187–199 [DOI] [PubMed] [Google Scholar]
- 13.Dittmer D, Lagunoff M, Renne R, Staskus K, Haase A, Ganem D. 1998. A cluster of latently expressed genes in Kaposi's sarcoma-associated herpesvirus. J. Virol. 72:8309–8315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sarid R, Flore O, Bohenzky RA, Chang Y, Moore PS. 1998. Transcription mapping of the Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) genome in a body cavity-based lymphoma cell line (BC-1). J. Virol. 72:1005–1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Talbot SJ, Weiss RA, Kellam P, Boshoff C. 1999. Transcriptional analysis of human herpesvirus-8 open reading frames 71, 72, 73, K14, and 74 in a primary effusion lymphoma cell line. Virology 257:84–94 [DOI] [PubMed] [Google Scholar]
- 16.Fakhari FD, Dittmer DP. 2002. Charting latency transcripts in Kaposi's sarcoma-associated herpesvirus by whole-genome real-time quantitative PCR. J. Virol. 76:6213–6223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu M, Suen J, Frias C, Pfeiffer R, Tsai MH, Chuang E, Zeichner SL. 2004. Dissection of the Kaposi's sarcoma-associated herpesvirus gene expression program by using the viral DNA replication inhibitor cidofovir. J. Virol. 78:13637–13652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chandriani S, Ganem D. 2010. Array-based transcript profiling and limiting-dilution reverse transcription-PCR analysis identify additional latent genes in Kaposi's sarcoma-associated herpesvirus. J. Virol. 84:5565–5573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.West JA, Damania B. 2010. Kaposi's sarcoma-associated herpesvirus and innate immunity. Future Virol. 5:185–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tamura T, Yanai H, Savitsky D, Taniguchi T. 2008. The IRF family transcription factors in immunity and oncogenesis. Annu. Rev. Immunol. 26:535–584 [DOI] [PubMed] [Google Scholar]
- 21.Paun A, Pitha PM. 2007. The IRF family, revisited. Biochimie 89:744–753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takeuchi O, Hemmi H, Akira S. 2004. Interferon response induced by Toll-like receptor signaling. J. Endotoxin Res. 10:252–256 [DOI] [PubMed] [Google Scholar]
- 23.Au WC, Moore PA, Lowther W, Juang YT, Pitha PM. 1995. Identification of a member of the interferon regulatory factor family that binds to the interferon-stimulated response element and activates expression of interferon-induced genes. Proc. Natl. Acad. Sci. U. S. A. 92:11657–11661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Au WC, Yeow WS, Pitha PM. 2001. Analysis of functional domains of interferon regulatory factor 7 and its association with IRF-3. Virology 280:273–282 [DOI] [PubMed] [Google Scholar]
- 25.Zhang L, Pagano JS. 2002. Structure and function of IRF-7. J. Interferon Cytokine Res. 22:95–101 [DOI] [PubMed] [Google Scholar]
- 26.Solis M, Goubau D, Romieu-Mourez R, Genin P, Civas A, Hiscott J. 2006. Distinct functions of IRF-3 and IRF-7 in IFN-alpha gene regulation and control of anti-tumor activity in primary macrophages. Biochem. Pharmacol. 72:1469–1476 [DOI] [PubMed] [Google Scholar]
- 27.Barnes BJ, Kellum MJ, Pinder KE, Frisancho JA, Pitha PM. 2003. Interferon regulatory factor 5, a novel mediator of cell cycle arrest and cell death. Cancer Res. 63:6424–6431 [PubMed] [Google Scholar]
- 28.Savitsky D, Tamura T, Yanai H, Taniguchi T. 2010. Regulation of immunity and oncogenesis by the IRF transcription factor family. Cancer Immunol. Immunother. 59:489–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harada H, Kitagawa M, Tanaka N, Yamamoto H, Harada K, Ishihara M, Taniguchi T. 1993. Anti-oncogenic and oncogenic potentials of interferon regulatory factors-1 and -2. Science 259:971–974 [DOI] [PubMed] [Google Scholar]
- 30.Takaoka A, Tamura T, Taniguchi T. 2008. Interferon regulatory factor family of transcription factors and regulation of oncogenesis. Cancer Sci. 99:467–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Offermann MK. 2007. Kaposi sarcoma herpesvirus-encoded interferon regulator factors. Curr. Top. Microbiol. Immunol. 312:185–209 [DOI] [PubMed] [Google Scholar]
- 32.Russo JJ, Bohenzky RA, Chien MC, Chen J, Yan M, Maddalena D, Parry JP, Peruzzi D, Edelman IS, Chang Y, Moore PS. 1996. Nucleotide sequence of the Kaposi sarcoma-associated herpesvirus (HHV8). Proc. Natl. Acad. Sci. U. S. A. 93:14862–14867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zimring JC, Goodbourn S, Offermann MK. 1998. Human herpesvirus 8 encodes an interferon regulatory factor (IRF) homolog that represses IRF-1-mediated transcription. J. Virol. 72:701–707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Inagi R, Okuno T, Ito M, Chen J, Mori Y, Haque M, Zou P, Yagi H, Kiniwa S, Saida T, Ueyama Y, Hayashi K, Yamanishi K. 1999. Identification and characterization of human herpesvirus 8 open reading frame K9 viral interferon regulatory factor by a monoclonal antibody. J. Hum. Virol. 2:63–71 [PubMed] [Google Scholar]
- 35.Pozharskaya VP, Weakland LL, Zimring JC, Krug LT, Unger ER, Neisch A, Joshi H, Inoue N, Offermann MK. 2004. Short duration of elevated vIRF-1 expression during lytic replication of human herpesvirus 8 limits its ability to block antiviral responses induced by alpha interferon in BCBL-1 cells. J. Virol. 78:6621–6635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dittmer DP. 2003. Transcription profile of Kaposi's sarcoma-associated herpesvirus in primary Kaposi's sarcoma lesions as determined by real-time PCR arrays. Cancer Res. 63:2010–2015 [PubMed] [Google Scholar]
- 37.Chen J, Ueda K, Sakakibara S, Okuno T, Yamanishi K. 2000. Transcriptional regulation of the Kaposi's sarcoma-associated herpesvirus viral interferon regulatory factor gene. J. Virol. 74:8623–8634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ueda K, Ishikawa K, Nishimura K, Sakakibara S, Do E, Yamanishi K. 2002. Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) replication and transcription factor activates the K9 (vIRF) gene through two distinct cis elements by a non-DNA-binding mechanism. J. Virol. 76:12044–12054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Park J, Lee MS, Yoo SM, Jeong KW, Lee D, Choe J, Seo T. 2007. Identification of the DNA sequence interacting with Kaposi's sarcoma-associated herpesvirus viral interferon regulatory factor 1. J. Virol. 81:12680–12684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li M, Lee H, Guo J, Neipel F, Fleckenstein B, Ozato K, Jung JU. 1998. Kaposi's sarcoma-associated herpesvirus viral interferon regulatory factor. J. Virol. 72:5433–5440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hew K, Dahlroth SL, Venkatachalam R, Nasertorabi F, Lim BT, Cornvik T, Nordlund P. 2013. The crystal structure of the DNA-binding domain of vIRF-1 from the oncogenic KSHV reveals a conserved fold for DNA binding and reinforces its role as a transcription factor. Nucleic Acids Res. 41:4295–4306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gao SJ, Boshoff C, Jayachandra S, Weiss RA, Chang Y, Moore PS. 1997. KSHV ORF K9 (vIRF) is an oncogene which inhibits the interferon signaling pathway. Oncogene 15:1979–1985 [DOI] [PubMed] [Google Scholar]
- 43.Lin R, Genin P, Mamane Y, Sgarbanti M, Battistini A, Harrington WJ, Jr, Barber GN, Hiscott J. 2001. HHV-8 encoded vIRF-1 represses the interferon antiviral response by blocking IRF-3 recruitment of the CBP/p300 coactivators. Oncogene 20:800–811 [DOI] [PubMed] [Google Scholar]
- 44.Lagos D, Trotter MW, Vart RJ, Wang HW, Matthews NC, Hansen A, Flore O, Gotch F, Boshoff C. 2007. Kaposi sarcoma herpesvirus-encoded vFLIP and vIRF1 regulate antigen presentation in lymphatic endothelial cells. Blood 109:1550–1558 [DOI] [PubMed] [Google Scholar]
- 45.Vogelstein B, Lane D, Levine AJ. 2000. Surfing the p53 network. Nature 408:307–310 [DOI] [PubMed] [Google Scholar]
- 46.Gu B, Zhu WG. 2012. Surf the post-translational modification network of p53 regulation. Int. J. Biol. Sci. 8:672–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dai C, Gu W. 2010. p53 post-translational modification: deregulated in tumorigenesis. Trends Mol. Med. 16:528–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Caspari T. 2000. How to activate p53. Curr. Biol. 10:R315–R317 [DOI] [PubMed] [Google Scholar]
- 49.Brooks CL, Gu W. 2006. p53 ubiquitination: Mdm2 and beyond. Mol. Cell 21:307–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shin YC, Nakamura H, Liang X, Feng P, Chang H, Kowalik TF, Jung JU. 2006. Inhibition of the ATM/p53 signal transduction pathway by Kaposi's sarcoma-associated herpesvirus interferon regulatory factor 1. J. Virol. 80:2257–2266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nakamura H, Li M, Zarycki J, Jung JU. 2001. Inhibition of p53 tumor suppressor by viral interferon regulatory factor. J. Virol. 75:7572–7582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Seo T, Park J, Lee D, Hwang SG, Choe J. 2001. Viral interferon regulatory factor 1 of Kaposi's sarcoma-associated herpesvirus binds to p53 and represses p53-dependent transcription and apoptosis. J. Virol. 75:6193–6198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Massagué J, Blain SW, Lo RS. 2000. TGFbeta signaling in growth control, cancer, and heritable disorders. Cell 103:295–309 [DOI] [PubMed] [Google Scholar]
- 54.Kim SJ, Im YH, Markowitz SD, Bang YJ. 2000. Molecular mechanisms of inactivation of TGF-beta receptors during carcinogenesis. Cytokine Growth Factor Rev. 11:159–168 [DOI] [PubMed] [Google Scholar]
- 55.Moustakas A, Pardali K, Gaal A, Heldin CH. 2002. Mechanisms of TGF-beta signaling in regulation of cell growth and differentiation. Immunol. Lett. 82:85–91 [DOI] [PubMed] [Google Scholar]
- 56.Heldin CH, Miyazono K, ten Dijke P. 1997. TGF-beta signalling from cell membrane to nucleus through SMAD proteins. Nature 390:465–471 [DOI] [PubMed] [Google Scholar]
- 57.Moustakas A, Souchelnytskyi S, Heldin CH. 2001. Smad regulation in TGF-beta signal transduction. J. Cell Sci. 114:4359–4369 [DOI] [PubMed] [Google Scholar]
- 58.Moustakas A. 2002. Smad signalling network. J. Cell Sci. 115:3355–3356 [DOI] [PubMed] [Google Scholar]
- 59.Datto MB, Li Y, Panus JF, Howe DJ, Xiong Y, Wang XF. 1995. Transforming growth factor beta induces the cyclin-dependent kinase inhibitor p21 through a p53-independent mechanism. Proc. Natl. Acad. Sci. U. S. A. 92:5545–5549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hannon GJ, Beach D. 1994. p15INK4B is a potential effector of TGF-beta-induced cell cycle arrest. Nature 371:257–261 [DOI] [PubMed] [Google Scholar]
- 61.Iavarone A, Massague J. 1997. Repression of the CDK activator Cdc25A and cell-cycle arrest by cytokine TGF-beta in cells lacking the CDK inhibitor p15. Nature 387:417–422 [DOI] [PubMed] [Google Scholar]
- 62.Seo T, Park J, Choe J. 2005. Kaposi's sarcoma-associated herpesvirus viral IFN regulatory factor 1 inhibits transforming growth factor-beta signaling. Cancer Res. 65:1738–1747 [DOI] [PubMed] [Google Scholar]
- 63.Choi YB, Nicholas J. 2008. Autocrine and paracrine promotion of cell survival and virus replication by human herpesvirus 8 chemokines. J. Virol. 82:6501–6513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Adams JM, Cory S. 2007. The Bcl-2 apoptotic switch in cancer development and therapy. Oncogene 26:1324–1337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ewings KE, Wiggins CM, Cook SJ. 2007. Bim and the pro-survival Bcl-2 proteins: opposites attract, ERK repels. Cell Cycle 6:2236–2240 [DOI] [PubMed] [Google Scholar]
- 66.Kim H, Tu HC, Ren D, Takeuchi O, Jeffers JR, Zambetti GP, Hsieh JJ, Cheng EH. 2009. Stepwise activation of BAX and BAK by tBID, BIM, and PUMA initiates mitochondrial apoptosis. Mol. Cell 36:487–499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kuwana T, Bouchier-Hayes L, Chipuk JE, Bonzon C, Sullivan BA, Green DR, Newmeyer DD. 2005. BH3 domains of BH3-only proteins differentially regulate Bax-mediated mitochondrial membrane permeabilization both directly and indirectly. Mol. Cell 17:525–535 [DOI] [PubMed] [Google Scholar]
- 68.Choi YB, Nicholas J. 2010. Bim nuclear translocation and inactivation by viral interferon regulatory factor. PLoS Pathog. 6:e1001031. 10.1371/journal.ppat.1001031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Choi YB, Sandford G, Nicholas J. 2012. Human herpesvirus 8 interferon regulatory factor-mediated BH3-only protein inhibition via Bid BH3-B mimicry. PLoS Pathog. 8:e1002748. 10.1371/journal.ppat.1002748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Angell JE, Lindner DJ, Shapiro PS, Hofmann ER, Kalvakolanu DV. 2000. Identification of GRIM-19, a novel cell death-regulatory gene induced by the interferon-beta and retinoic acid combination, using a genetic approach. J. Biol. Chem. 275:33416–33426 [DOI] [PubMed] [Google Scholar]
- 71.Seo T, Lee D, Shim YS, Angell JE, Chidambaram NV, Kalvakolanu DV, Choe J. 2002. Viral interferon regulatory factor 1 of Kaposi's sarcoma-associated herpesvirus interacts with a cell death regulator, GRIM19, and inhibits interferon/retinoic acid-induced cell death. J. Virol. 76:8797–8807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hu J, Angell JE, Zhang J, Ma X, Seo T, Raha A, Hayashi J, Choe J, Kalvakolanu DV. 2002. Characterization of monoclonal antibodies against GRIM-19, a novel IFN-beta and retinoic acid-activated regulator of cell death. J. Interferon Cytokine Res. 22:1017–1026 [DOI] [PubMed] [Google Scholar]
- 73.Krammer PH. 1999. CD95(APO-1/Fas)-mediated apoptosis: live and let die. Adv. Immunol. 71:163–210 [DOI] [PubMed] [Google Scholar]
- 74.Peter ME, Hellbardt S, Schwartz-Albiez R, Westendorp MO, Walczak H, Moldenhauer G, Grell M, Krammer PH. 1995. Cell surface sialylation plays a role in modulating sensitivity towards APO-1-mediated apoptotic cell death. Cell Death Differ. 2:163–171 [PubMed] [Google Scholar]
- 75.Krammer PH, Galle PR, Moller P, Debatin KM. 1998. CD95(APO-1/Fas)-mediated apoptosis in normal and malignant liver, colon, and hematopoietic cells. Adv. Cancer Res. 75:251–273 [DOI] [PubMed] [Google Scholar]
- 76.Kirchhoff S, Sebens T, Baumann S, Krueger A, Zawatzky R, Li-Weber M, Meinl E, Neipel F, Fleckenstein B, Krammer PH. 2002. Viral IFN-regulatory factors inhibit activation-induced cell death via two positive regulatory IFN-regulatory factor 1-dependent domains in the CD95 ligand promoter. J. Immunol. 168:1226–1234 [DOI] [PubMed] [Google Scholar]
- 77.Burysek L, Yeow WS, Pitha PM. 1999. Unique properties of a second human herpesvirus 8-encoded interferon regulatory factor (vIRF-2). J. Hum. Virol. 2:19–32 [PubMed] [Google Scholar]
- 78.Jenner RG, Alba MM, Boshoff C, Kellam P. 2001. Kaposi's sarcoma-associated herpesvirus latent and lytic gene expression as revealed by DNA arrays. J. Virol. 75:891–902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Aresté C, Mutocheluh M, Blackbourn DJ. 2009. Identification of caspase-mediated decay of interferon regulatory factor-3, exploited by a Kaposi sarcoma-associated herpesvirus immunoregulatory protein. J. Biol. Chem. 284:23272–23285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fuld S, Cunningham C, Klucher K, Davison AJ, Blackbourn DJ. 2006. Inhibition of interferon signaling by the Kaposi's sarcoma-associated herpesvirus full-length viral interferon regulatory factor 2 protein. J. Virol. 80:3092–3097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mutocheluh M, Hindle L, Areste C, Chanas SA, Butler LM, Lowry K, Shah K, Evans DJ, Blackbourn DJ. 2011. Kaposi's sarcoma-associated herpesvirus viral interferon regulatory factor-2 inhibits type 1 interferon signalling by targeting interferon-stimulated gene factor-3. J. Gen. Virol. 92:2394–2398 [DOI] [PubMed] [Google Scholar]
- 82.Burýsek L, Pitha PM. 2001. Latently expressed human herpesvirus 8-encoded interferon regulatory factor 2 inhibits double-stranded RNA-activated protein kinase. J. Virol. 75:2345–2352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gil J, Alcami J, Esteban M. 1999. Induction of apoptosis by double-stranded-RNA-dependent protein kinase (PKR) involves the alpha subunit of eukaryotic translation initiation factor 2 and NF-kappaB. Mol. Cell. Biol. 19:4653–4663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nakayama Y, Plisch EH, Sullivan J, Thomas C, Czuprynski CJ, Williams BR, Suresh M. 2010. Role of PKR and type I IFNs in viral control during primary and secondary infection. PLoS Pathog. 6:e1000966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sadler AJ, Williams BR. 2007. Structure and function of the protein kinase R. Curr. Top. Microbiol. Immunol. 316:253–292 [DOI] [PubMed] [Google Scholar]
- 86.Williams BR. 1997. Role of the double-stranded RNA-activated protein kinase (PKR) in cell regulation. Biochem. Soc. Trans. 25:509–513 [DOI] [PubMed] [Google Scholar]
- 87.Chow WA, Fang JJ, Yee JK. 2000. The IFN regulatory factor family participates in regulation of Fas ligand gene expression in T cells. J. Immunol. 164:3512–3518 [DOI] [PubMed] [Google Scholar]
- 88.Rivas C, Thlick AE, Parravicini C, Moore PS, Chang Y. 2001. Kaposi's sarcoma-associated herpesvirus LANA2 is a B-cell-specific latent viral protein that inhibits p53. J. Virol. 75:429–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wies E, Mori Y, Hahn A, Kremmer E, Sturzl M, Fleckenstein B, Neipel F. 2008. The viral interferon-regulatory factor-3 is required for the survival of KSHV-infected primary effusion lymphoma cells. Blood 111:320–327 [DOI] [PubMed] [Google Scholar]
- 90.Lubyova B, Kellum MJ, Frisancho AJ, Pitha PM. 2004. Kaposi's sarcoma-associated herpesvirus-encoded vIRF-3 stimulates the transcriptional activity of cellular IRF-3 and IRF-7. J. Biol. Chem. 279:7643–7654 [DOI] [PubMed] [Google Scholar]
- 91.Ozato K, Tailor P, Kubota T. 2007. The interferon regulatory factor family in host defense: mechanism of action. J. Biol. Chem. 282:20065–20069 [DOI] [PubMed] [Google Scholar]
- 92.Joo CH, Shin YC, Gack M, Wu L, Levy D, Jung JU. 2007. Inhibition of interferon regulatory factor 7 (IRF7)-mediated interferon signal transduction by the Kaposi's sarcoma-associated herpesvirus viral IRF homolog vIRF3. J. Virol. 81:8282–8292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bi X, Yang L, Mancl ME, Barnes BJ. 2011. Modulation of interferon regulatory factor 5 activities by the Kaposi sarcoma-associated herpesvirus-encoded viral interferon regulatory factor 3 contributes to immune evasion and lytic induction. J. Interferon Cytokine Res. 31:373–382 [DOI] [PubMed] [Google Scholar]
- 94.Wies E, Hahn AS, Schmidt K, Viebahn C, Rohland N, Lux A, Schellhorn T, Holzer A, Jung JU, Neipel F. 2009. The Kaposi's sarcoma-associated herpesvirus-encoded vIRF-3 inhibits cellular IRF-5. J. Biol. Chem. 284:8525–8538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Martin HJ, Lee JM, Walls D, Hayward SD. 2007. Manipulation of the Toll-like receptor 7 signaling pathway by Epstein-Barr virus. J. Virol. 81:9748–9758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhang L, Pagano JS. 2001. Interferon regulatory factor 7: a key cellular mediator of LMP-1 in EBV latency and transformation. Semin. Cancer Biol. 11:445–453 [DOI] [PubMed] [Google Scholar]
- 97.Paun A, Reinert JT, Jiang Z, Medin C, Balkhi MY, Fitzgerald KA, Pitha PM. 2008. Functional characterization of murine interferon regulatory factor 5 (IRF-5) and its role in the innate antiviral response. J. Biol. Chem. 283:14295–14308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Takaoka A, Yanai H, Kondo S, Duncan G, Negishi H, Mizutani T, Kano S, Honda K, Ohba Y, Mak TW, Taniguchi T. 2005. Integral role of IRF-5 in the gene induction programme activated by Toll-like receptors. Nature 434:243–249 [DOI] [PubMed] [Google Scholar]
- 99.Hu G, Barnes BJ. 2009. IRF-5 is a mediator of the death receptor-induced apoptotic signaling pathway. J. Biol. Chem. 284:2767–2777 [DOI] [PubMed] [Google Scholar]
- 100.Silverman N, Maniatis T. 2001. NF-kappaB signaling pathways in mammalian and insect innate immunity. Genes Dev. 15:2321–2342 [DOI] [PubMed] [Google Scholar]
- 101.Karin M, Lin A. 2002. NF-kappaB at the crossroads of life and death. Nat. Immunol. 3:221–227 [DOI] [PubMed] [Google Scholar]
- 102.Ghosh S, Karin M. 2002. Missing pieces in the NF-kappaB puzzle. Cell 109(Suppl.):S81–S96 [DOI] [PubMed] [Google Scholar]
- 103.Seo T, Park J, Lim C, Choe J. 2004. Inhibition of nuclear factor kappaB activity by viral interferon regulatory factor 3 of Kaposi's sarcoma-associated herpesvirus. Oncogene 23:6146–6155 [DOI] [PubMed] [Google Scholar]
- 104.Reith W, LeibundGut-Landmann S, Waldburger JM. 2005. Regulation of MHC class II gene expression by the class II transactivator. Nat. Rev. Immunol. 5:793–806 [DOI] [PubMed] [Google Scholar]
- 105.Schmidt K, Wies E, Neipel F. 2011. Kaposi's sarcoma-associated herpesvirus viral interferon regulatory factor 3 inhibits gamma interferon and major histocompatibility complex class II expression. J. Virol. 85:4530–4537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lubyova B, Kellum MJ, Frisancho JA, Pitha PM. 2007. Stimulation of c-Myc transcriptional activity by vIRF-3 of Kaposi sarcoma-associated herpesvirus. J. Biol. Chem. 282:31944–31953 [DOI] [PubMed] [Google Scholar]
- 107.Muñoz-Fontela C, Marcos-Villar L, Hernandez F, Gallego P, Rodriguez E, Arroyo J, Gao SJ, Avila J, Rivas C. 2008. Induction of paclitaxel resistance by the Kaposi's sarcoma-associated herpesvirus latent protein LANA2. J. Virol. 82:1518–1525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hussain SP, Harris CC. 1999. p53 mutation spectrum and load: the generation of hypotheses linking the exposure of endogenous or exogenous carcinogens to human cancer. Mutat. Res. 428:23–32 [DOI] [PubMed] [Google Scholar]
- 109.Lin CY, Loven J, Rahl PB, Paranal RM, Burge CB, Bradner JE, Lee TI, Young RA. 2012. Transcriptional amplification in tumor cells with elevated c-Myc. Cell 151:56–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hermeking H, Funk JO, Reichert M, Ellwart JW, Eick D. 1995. Abrogation of p53-induced cell cycle arrest by c-Myc: evidence for an inhibitor of p21WAF1/CIP1/SDI1. Oncogene 11:1409–1415 [PubMed] [Google Scholar]
- 111.Knudsen KJ, Nelander Holm GM, Krabbe JS, Listov-Saabye N, Kiehr B, Dufva M, Svendsen JE, Oleksiewicz MB. 2009. Driving gradual endogenous c-myc overexpression by flow-sorting: intracellular signaling and tumor cell phenotype correlate with oncogene expression. Arch. Toxicol. 83:1061–1074 [DOI] [PubMed] [Google Scholar]
- 112.Grandori C, Cowley SM, James LP, Eisenman RN. 2000. The Myc/Max/Mad network and the transcriptional control of cell behavior. Annu. Rev. Cell Dev. Biol. 16:653–699 [DOI] [PubMed] [Google Scholar]
- 113.Nie Z, Hu G, Wei G, Cui K, Yamane A, Resch W, Wang R, Green DR, Tessarollo L, Casellas R, Zhao K, Levens D. 2012. c-Myc is a universal amplifier of expressed genes in lymphocytes and embryonic stem cells. Cell 151:68–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mori K, Maeda Y, Kitaura H, Taira T, Iguchi-Ariga SM, Ariga H. 1998. MM-1, a novel c-Myc-associating protein that represses transcriptional activity of c-Myc. J. Biol. Chem. 273:29794–29800 [DOI] [PubMed] [Google Scholar]
- 115.Kimura Y, Nagao A, Fujioka Y, Satou A, Taira T, Iguchi-Ariga SM, Ariga H. 2007. MM-1 facilitates degradation of c-Myc by recruiting proteasome and a novel ubiquitin E3 ligase. Int. J. Oncol. 31:829–836 [PubMed] [Google Scholar]
- 116.Baresova P, Pitha PM, Lubyova B. 2012. Kaposi sarcoma-associated herpesvirus vIRF-3 protein binds to F-box of Skp2 protein and acts as a regulator of c-Myc protein function and stability. J. Biol. Chem. 287:16199–16208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ferbeyre G, de Stanchina E, Querido E, Baptiste N, Prives C, Lowe SW. 2000. PML is induced by oncogenic ras and promotes premature senescence. Genes Dev. 14:2015–2027 [PMC free article] [PubMed] [Google Scholar]
- 118.Ishov AM, Sotnikov AG, Negorev D, Vladimirova OV, Neff N, Kamitani T, Yeh ET, Strauss JF, III, Maul GG. 1999. PML is critical for ND10 formation and recruits the PML-interacting protein Daxx to this nuclear structure when modified by SUMO-1. J. Cell Biol. 147:221–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yip KW, Cuddy M, Pinilla C, Giulanotti M, Heynen-Genel S, Matsuzawa S, Reed JC. 2011. A high-content screening (HCS) assay for the identification of chemical inducers of PML oncogenic domains (PODs). J. Biomol. Screen. 16:251–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Maul GG, Negorev D, Bell P, Ishov AM. 2000. Review: properties and assembly mechanisms of ND10, PML bodies, or PODs. J. Struct. Biol. 129:278–287 [DOI] [PubMed] [Google Scholar]
- 121.Marcos-Villar L, Lopitz-Otsoa F, Gallego P, Munoz-Fontela C, Gonzalez-Santamaria J, Campagna M, Shou-Jiang G, Rodriguez MS, Rivas C. 2009. Kaposi's sarcoma-associated herpesvirus protein LANA2 disrupts PML oncogenic domains and inhibits PML-mediated transcriptional repression of the survivin gene. J. Virol. 83:8849–8858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Marcos-Villar L, Campagna M, Lopitz-Otsoa F, Gallego P, Gonzalez-Santamaria J, Gonzalez D, Rodriguez MS, Rivas C. 2011. Covalent modification by SUMO is required for efficient disruption of PML oncogenic domains by Kaposi's sarcoma-associated herpesvirus latent protein LANA2. J. Gen. Virol. 92:188–194 [DOI] [PubMed] [Google Scholar]
- 123.Zhao J. 2007. Sumoylation regulates diverse biological processes. Cell. Mol. Life Sci. 64:3017–3033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Marcos-Villar L, Gallego P, Munoz-Fontela C, de la Cruz-Herrera CF, Campagna M, Gonzalez D, Lopitz-Otsoa F, Rodriguez MS, Rivas C. 14 January 2013. Kaposi's sarcoma-associated herpesvirus LANA2 protein interacts with the pocket proteins and inhibits their sumoylation. Oncogene. 10.1038/onc.2012.603 [DOI] [PubMed] [Google Scholar]
- 125.Tanimoto K, Makino Y, Pereira T, Poellinger L. 2000. Mechanism of regulation of the hypoxia-inducible factor-1 alpha by the von Hippel-Lindau tumor suppressor protein. EMBO J. 19:4298–4309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kallio PJ, Okamoto K, O'Brien S, Carrero P, Makino Y, Tanaka H, Poellinger L. 1998. Signal transduction in hypoxic cells: inducible nuclear translocation and recruitment of the CBP/p300 coactivator by the hypoxia-inducible factor-1alpha. EMBO J. 17:6573–6586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Shin YC, Joo CH, Gack MU, Lee HR, Jung JU. 2008. Kaposi's sarcoma-associated herpesvirus viral IFN regulatory factor 3 stabilizes hypoxia-inducible factor-1 alpha to induce vascular endothelial growth factor expression. Cancer Res. 68:1751–1759 [DOI] [PubMed] [Google Scholar]
- 128.Fu H, Subramanian RR, Masters SC. 2000. 14-3-3 proteins: structure, function, and regulation. Annu. Rev. Pharmacol. Toxicol. 40:617–647 [DOI] [PubMed] [Google Scholar]
- 129.Biggs WH, III, Meisenhelder J, Hunter T, Cavenee WK, Arden KC. 1999. Protein kinase B/Akt-mediated phosphorylation promotes nuclear exclusion of the winged helix transcription factor FKHR1. Proc. Natl. Acad. Sci. U. S. A. 96:7421–7426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME. 1999. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell 96:857–868 [DOI] [PubMed] [Google Scholar]
- 131.Gilley J, Coffer PJ, Ham J. 2003. FOXO transcription factors directly activate bim gene expression and promote apoptosis in sympathetic neurons. J. Cell Biol. 162:613–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Dobson M, Ramakrishnan G, Ma S, Kaplun L, Balan V, Fridman R, Tzivion G. 2011. Bimodal regulation of FoxO3 by AKT and 14-3-3. Biochim. Biophys. Acta 1813:1453–1464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Tzivion G, Dobson M, Ramakrishnan G. 2011. FoxO transcription factors; regulation by AKT and 14-3-3 proteins. Biochim. Biophys. Acta 1813:1938–1945 [DOI] [PubMed] [Google Scholar]
- 134.Muñoz-Fontela C, Marcos-Villar L, Gallego P, Arroyo J, Da Costa M, Pomeranz KM, Lam EW, Rivas C. 2007. Latent protein LANA2 from Kaposi's sarcoma-associated herpesvirus interacts with 14-3-3 proteins and inhibits FOXO3a transcription factor. J. Virol. 81:1511–1516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Esteban M, Garcia MA, Domingo-Gil E, Arroyo J, Nombela C, Rivas C. 2003. The latency protein LANA2 from Kaposi's sarcoma-associated herpesvirus inhibits apoptosis induced by dsRNA-activated protein kinase but not RNase L activation. J. Gen. Virol. 84:1463–1470 [DOI] [PubMed] [Google Scholar]
- 136.Balachandran S, Barber GN. 2007. PKR in innate immunity, cancer, and viral oncolysis. Methods Mol. Biol. 383:277–301 [DOI] [PubMed] [Google Scholar]
- 137.Xi X, Persson LM, O'Brien MW, Mohr I, Wilson AC. 2012. Cooperation between viral interferon regulatory factor 4 and RTA to activate a subset of Kaposi's sarcoma-associated herpesvirus lytic promoters. J. Virol. 86:1021–1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lee HR, Toth Z, Shin YC, Lee JS, Chang H, Gu W, Oh TK, Kim MH, Jung JU. 2009. Kaposi's sarcoma-associated herpesvirus viral interferon regulatory factor 4 targets MDM2 to deregulate the p53 tumor suppressor pathway. J. Virol. 83:6739–6747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lee HR, Choi WC, Lee S, Hwang J, Hwang E, Guchhait K, Haas J, Toth Z, Jeon YH, Oh TK, Kim MH, Jung JU. 2011. Bilateral inhibition of HAUSP deubiquitinase by a viral interferon regulatory factor protein. Nat. Struct. Mol. Biol. 18:1336–1344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hayward SD. 2004. Viral interactions with the Notch pathway. Semin. Cancer Biol. 14:387–396 [DOI] [PubMed] [Google Scholar]
- 141.Curry CL, Reed LL, Golde TE, Miele L, Nickoloff BJ, Foreman KE. 2005. Gamma secretase inhibitor blocks Notch activation and induces apoptosis in Kaposi's sarcoma tumor cells. Oncogene 24:6333–6344 [DOI] [PubMed] [Google Scholar]
- 142.Lan K, Murakami M, Bajaj B, Kaul R, He Z, Gan R, Feldman M, Robertson ES. 2009. Inhibition of KSHV-infected primary effusion lymphomas in NOD/SCID mice by gamma-secretase inhibitor. Cancer Biol. Ther. 8:2136–2143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kopan R, Ilagan MX. 2009. The canonical Notch signaling pathway: unfolding the activation mechanism. Cell 137:216–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Heinzelmann K, Scholz BA, Nowak A, Fossum E, Kremmer E, Haas J, Frank R, Kempkes B. 2010. Kaposi's sarcoma-associated herpesvirus viral interferon regulatory factor 4 (vIRF4/K10) is a novel interaction partner of CSL/CBF1, the major downstream effector of Notch signaling. J. Virol. 84:12255–12264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Robinson BA, Estep RD, Messaoudi I, Rogers KS, Wong SW. 2012. Viral interferon regulatory factors decrease the induction of type I and type II interferon during rhesus macaque rhadinovirus infection. J. Virol. 86:2197–2211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Robinson BA, O'Connor MA, Li H, Engelmann F, Poland B, Grant R, DeFilippis V, Estep RD, Axthelm MK, Messaoudi I, Wong SW. 2012. Viral interferon regulatory factors are critical for delay of the host immune response against rhesus macaque rhadinovirus infection. J. Virol. 86:2769–2779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.West J, Damania B. 2008. Upregulation of the TLR3 pathway by Kaposi's sarcoma-associated herpesvirus during primary infection. J. Virol. 82:5440–5449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Gregory SM, West JA, Dillon PJ, Hilscher C, Dittmer DP, Damania B. 2009. Toll-like receptor signaling controls reactivation of KSHV from latency. Proc. Natl. Acad. Sci. U. S. A. 106:11725–11730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Gregory SM, Damania B. 2009. KSHV and the toll of innate immune activation. Cell Cycle 8:3246–3247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Katano H, Sato Y, Itoh H, Sata T. 2001. Expression of human herpesvirus 8 (HHV-8)-encoded immediate early protein, open reading frame 50, in HHV-8-associated diseases. J. Hum. Virol. 4:96–102 [PubMed] [Google Scholar]
- 151.Petre CE, Sin SH, Dittmer DP. 2007. Functional p53 signaling in Kaposi's sarcoma-associated herpesvirus lymphomas: implications for therapy. J. Virol. 81:1912–1922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Jacobs SR, Gregory SM, West JA, Wollish AC, Blackbourn DJ, Heise MT, Damania B. 2013. The viral interferon regulatory factors of Kaposi's sarcoma-associated herpesvirus differ in their inhibition of interferon activation mediated by Toll-like receptor 3. J. Virol. 87:798–806 [DOI] [PMC free article] [PubMed] [Google Scholar]