Abstract
Islet1 (Isl1) is a transcription factor transiently expressed in a subset of heart and limb progenitors. During studies of limb development, conditional Isl1 deletion produced unexpected kidney abnormalities. Here, we studied the renal expression of Isl1 and whether it has a role in kidney development. In situ hybridization revealed Isl1 expression in the mesenchymal cells surrounding the base of the ureteric bud in mice. Conditional deletion of Isl1 caused kidney agenesis or hypoplasia and hydroureter, a phenotype resembling human congenital anomalies of the kidney and urinary tract (CAKUT). The absence of Isl1 led to ectopic branching of the ureteric bud out from the nephric duct or to the formation of accessory buds, both of which could lead to obstruction of the ureter-bladder junction and consequent hydroureter. The abnormal elongation and poor branching of the ureteric buds were the likely causes of the kidney agenesis or hypoplasia. Furthermore, the lack of Isl1 reduced the expression of Bmp4, a gene implicated in the CAKUT-like phenotype, in the metanephric region before ureteric budding. In conclusion, Isl1 is essential for proper development of the kidney and ureter by repressing the aberrant formation of the ureteric bud. These observations call for further studies to investigate whether Isl1 may be a causative gene for human CAKUT.
The mammalian kidney, the metanephros, is formed by reciprocally inductive interactions between two precursor tissues: the metanephric mesenchyme and the ureteric bud. The metanephric mesenchyme attracts the ureteric bud tips toward the mesenchyme and subsequently induces branching of the ureteric buds. The attracted ureteric bud tips in turn induce the mesenchymal cells to differentiate into the epithelia of the glomeruli and renal tubules. Impairment of these processes can lead to a variety of abnormal developmental disorders of the kidney. Meanwhile, the stalks of the ureteric buds elongate and differentiate into collecting ducts and the ureter. The ureteric epithelium also interacts with the surrounding ureteral mesenchyme, which differentiates into the smooth muscle layer of the ureter that pushes the urine downward by peristaltic movements. Therefore, mechanical obstruction of the ureter or malformation of the smooth muscle layer causes dilatation of the ureter (hydroureter) and, in more severe cases, of the renal pelvis (hydronephrosis). The combination of kidney and ureter defects caused by gene mutations is termed congenital anomalies of the kidney and urinary tract (CAKUT) and constitutes a major cause of renal failure in the perinatal period in humans.1
Bmp4 is expressed in the mesenchyme surrounding the ureteric stalk, and Bmp4 heterozygous mutant mice display abnormalities that mimic human CAKUT, including hypoplastic kidneys and hydroureter.2 These mice exhibit ectopic or accessory ureteric bud formation, which results in misconnection of the ureter to the urinary bladder or duplicated ureters. It has been proposed that Bmp4 has dual functions, namely inhibition of ectopic budding of the ureteric tips and promotion of ureteric stalk elongation,2 although the precise mechanisms remain unknown. Shh is expressed in the ureteric epithelium, and its deletion results in reduced expression of Bmp4 in the surrounding mesenchyme and eventually hydroureter.3 The transcription factors Tbx18 and Sox9 are expressed in the ureteric mesenchyme, and their deletion also leads to hydroureter resulting from impaired development of the smooth muscle layer of the ureter.4,5
In this study, we identify Islet1 (Isl1; Mouse Genome Informatics), which encodes an LIM-homeodomain protein, as a key regulator of kidney and ureter development. Mice deficient for Isl1 die by embryonic day (E) 11.5 and exhibit developmental arrest of motor neurons.6 Isl1 is transiently expressed in a subset of heart and limb progenitors and is required for the normal development of these tissues.7,8 In the course of conditional Isl1 deletion in the lateral mesoderm using Hoxb6Cre for analysis of limb development,9 kidney abnormalities were observed. This finding was unexpected because no previous literature has reported Isl1 expression in the developing kidney to the best of our knowledge. Therefore, we examined the expression and roles of Isl1 in kidney development in detail.
Results
Isl1 Is Transiently Expressed in the Mesenchyme Surrounding the Ureteric Bud Stalk
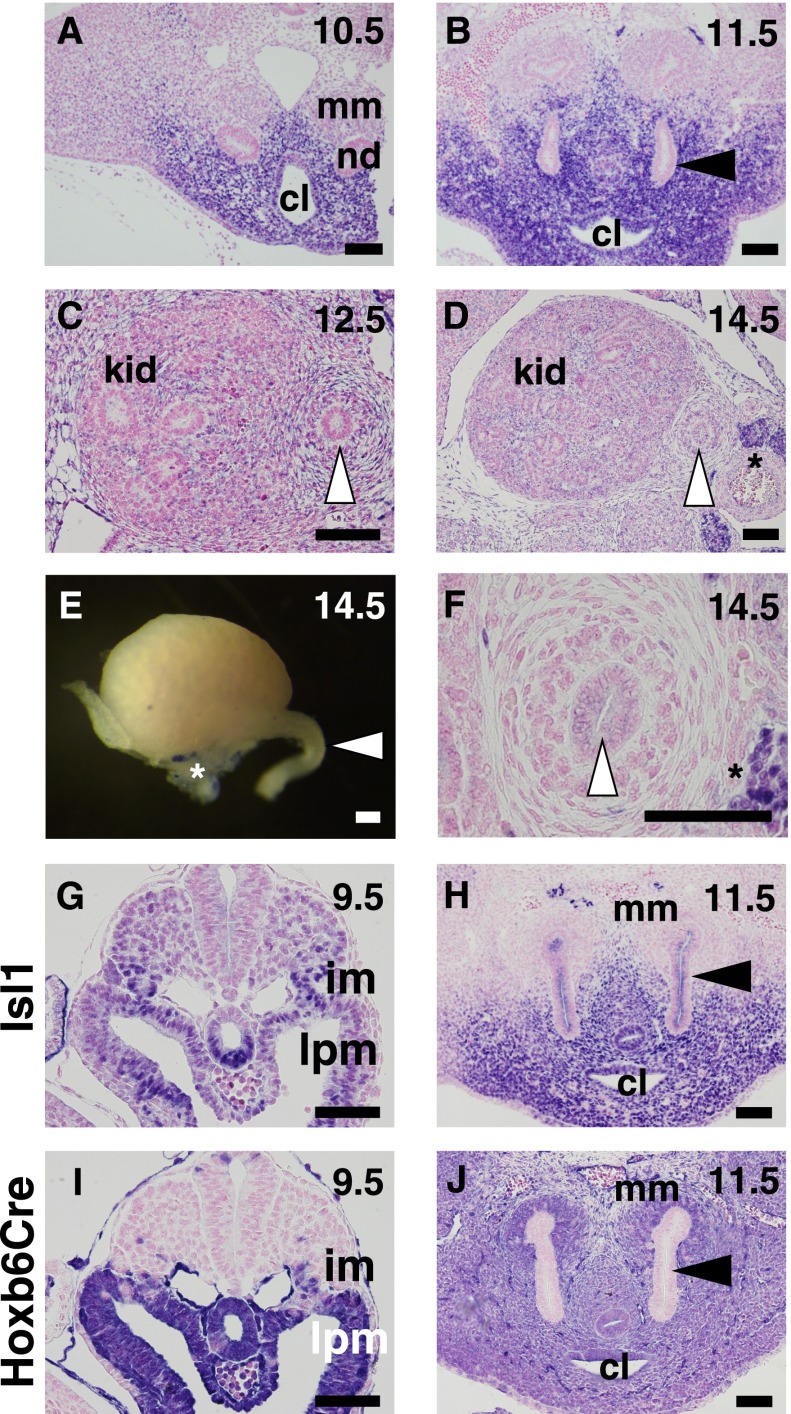
First, we examined the expression patterns of Isl1 during kidney development by in situ hybridization. At E10.5, Isl1 was expressed in tissues surrounding the cloaca but was excluded from the kidney primordia, comprising the metanephric mesenchyme and nephric duct (Figure 1A). At E11.5, a similar expression pattern was observed, in that Isl1 expression was detected in the ureteric mesenchyme surrounding the ureteric bud stalk and cloaca but was not expressed in the metanephric mesenchyme or ureteric bud epithelia (Figure 1B). Subsequently, Isl1 expression was rapidly downregulated at E12.5 (Figure 1C), and no Isl1 expression was observed in the kidney proper or the ureter at E14.5 (Figure 1D). These observations were confirmed by whole-mount in situ hybridization, showing no Isl1 signals in the kidney or ureter at E14.5 (Figure 1E). Staining with an anti-Isl1 antibody confirmed these observations, including the negative expression in the ureter at E14.5 (Figure 1F). Nuclear Isl1 staining was observed weakly in the lateral and intermediate mesoderm at E9.5 and robustly in the ureteric mesenchyme at E11.5. (Figure 1, G and H).
Figure 1.
Isl1 is transiently expressed in the mesenchyme surrounding the kidney primordia. (A–D) In situ hybridization of Isl1 during kidney development. Note that Isl1 is expressed in the mesenchyme surrounding the ureteric buds until E11.5 but rapidly decreases thereafter. The signal at E14.5 is absent except for the preaortic sympathetic ganglia (*). (E) Whole-mount in situ hybridization of Isl1 in the E14.5 kidney. (F–H) Immunostaining for Isl1 using an anti-Isl1 antibody. (I and J) Cre activity in Hoxb6Cre mice. Sections of Hoxb6Cre;tdTomato reporter mice were stained with an anti-RFP antibody. Note the significant overlap of Isl1 and Hobx6Cre expression. Black arrowheads, ureteric bud stalk; white arrowheads, ureter; cl, cloaca; im, intermediate mesoderm; kid, kidney; lpm, lateral plate mesoderm; mm, metanephric mesenchyme; nd, nephric duct. Scale bars: 100 μm.
Next, the Cre recombinase activity of Hoxb6Cre mice was examined. This mouse strain is often used for deletion of genes in lateral mesoderm-derived tissues, such as the limb buds.10 To examine the Cre activity in the developing kidney, we crossed Hoxb6Cre mice with a reporter strain, in which the CAG promoter, stop sequences flanked by loxP sites, and the tandem dimer Tomato (tdTomato) are inserted into the Rosa26 locus.11 Owing to the presence of the potent CAG promoter, this mouse strain serves as a very sensitive reporter for Cre recombinase activity. When these mice were crossed with Hoxb6Cre mice, the tdTomato signals were detected mainly in the lateral mesoderm and weakly in the intermediate mesoderm at E9.5 (Figure 1I), which is consistent with the reported Cre activity.10 At E11.5, Cre activity was broadly detected, except for the ureteric bud epithelia (Figure 1J). Although the activity in the metanephric mesenchyme was mosaic, the regions with Cre activity cover all the Isl1-positive populations described above. Thus Hoxb6Cre mice are useful for efficient deletion of Isl1 during kidney development. The non-nuclear signal in the ureteric epithelium in Figure 1H, which is not consistent with the data obtained by in situ hybridization, could result from background staining of the antibody. In addition, the cryptic Isl1 expression in the ureteric epithelium does not affect our analysis because Hobx6Cre does not delete genes in this population.
Isl1 Deletion Causes Kidney Agenesis or Hydroureter: A CAKUT-Like Phenotype
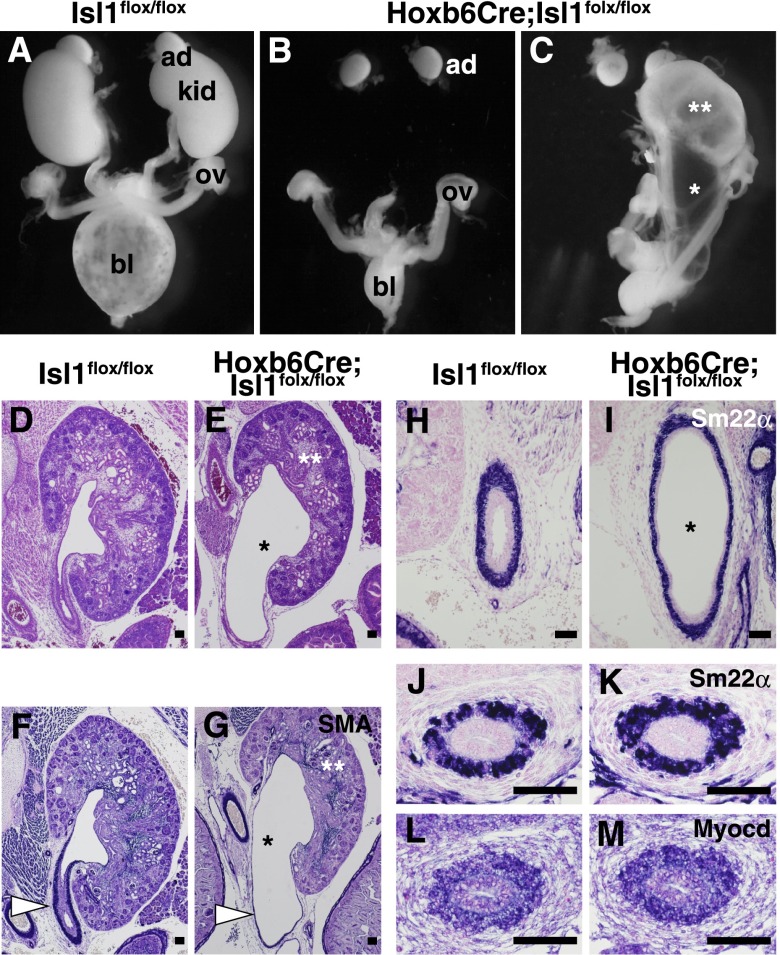
All the Hoxb6Cre;Isl1flox/flox mice died shortly after birth (n=11), while Isl1flox/flox, Isl1flox/+, and Hoxb6Cre;Isl1flox/+ mice were apparently normal (n=9, n=13, and n=21, respectively). Regarding the 22 presumptive urogenital systems in the 11 Hoxb6Cre;Isl1flox/flox mutant mice, 11 (50%) showed complete kidney agenesis (Figure 2, A and B), 10 (45.5%) exhibited hydronephrosis and hydroureter (Figure 2C), and one (4.5%) had normal-sized kidneys. At E16.5, 50% of the kidneys examined (three of six) were absent in Hoxb6Cre;Isl1flox/flox mice, and dilatation of the pelvis and ureter all along the tract was observed in the remaining three kidneys (Figure 2, D and E). The mutant ureter had a thinner wall but retained the smooth muscle layer, which was confirmed by immunostaining for smooth muscle actin (Figure 2, F and G). The mutant smooth muscle layer was also positive for Sm22α, a maturation marker for this ureteric mesenchyme-derived lineage (Figure 2, H–K). Myocardin, another differentiation marker expressed earlier than Sm22α, was also detected in the Isl1 mutants (Figure 2, L and M), indicating that maturation of the ureteral mesenchyme was unaffected in the absence of Isl1. Therefore, the hydroureter is unlikely to result from developmental impairment of the ureteric mesenchyme and could be caused by other mechanisms, such as mechanical obstruction in the lower urinary tract.
Figure 2.
Isl1 deletion causes kidney agenesis or hydroureter: a CAKUT-like phenotype. (A) Kidneys in a newborn Isl1flox/flox mouse (P0). ad, adrenal gland; bl, bladder; kid, kidney; ov, ovary. (B) Bilateral kidney agenesis in a Hoxb6Cre;Isl1flox/flox mouse (P0). (C) Unilateral kidney agenesis accompanied by hydroureter (*) and hydronephrosis (**) in a Hoxb6Cre;Isl1flox/flox mouse (P0). (D and E) Hematoxylin-eosin staining of E16.5 kidneys. Severe ureter dilatation (*) is observed in the Hoxb6Cre;Isl1flox/flox mouse. The mutant kidney (**) is squashed. (F and G) Immunostaining for smooth muscle actin (SMA) in the ureter. Note that the mutant ureteric wall is thinner but expresses SMA (white arrowheads). (H and I) Immunostaining for Sm22α in the ureter at E16.5. (J and K) Immunostaining for Sm22α in the ureteral mesenchyme at E14.5. (L and M) In situ hybridization of Myocardin in the ureteral mesenchyme at E14.5. Scale bars: 100 μm.
The Ureter Is Not Properly Connected to the Urinary Bladder in the Absence of Isl1
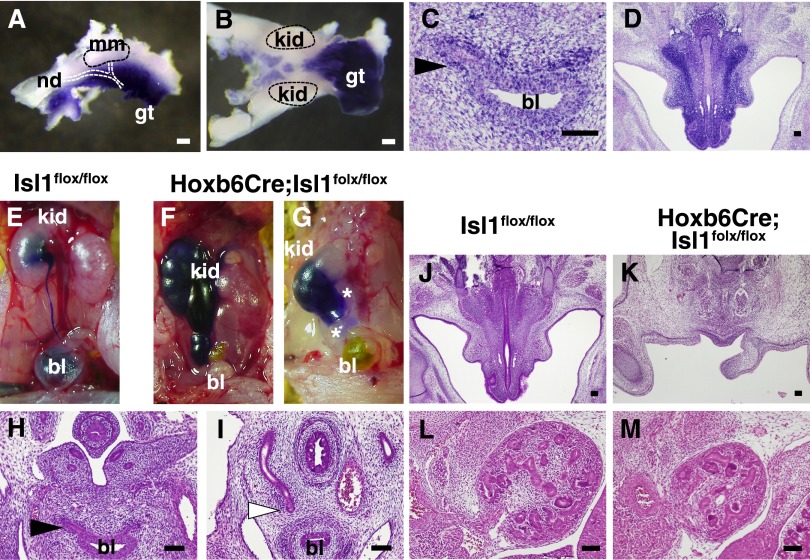
We then focused on Isl1 expression in the lower urinary tract. Isl1 was robustly expressed along the nephric duct, at the base of the ureteric stalk, and in the genital tubercle at E11.5 (Figure 3A). This expression was retained at E12.5 when the expression in the kidney proper was significantly reduced (Figure 3B). At E14.5, Isl1 was still expressed, albeit weakly, at the ureterovesical junction (Figure 3C), as well as in the region along the urethra (Figure 3D).
Figure 3.
The ureter is not properly connected to the urinary bladder in the absence of Isl1. (A and B) Whole-mount in situ hybridization of Isl1 at E11.5 (A) and 12.5 (B). (C and D) Section in situ hybridization of Isl1 at the E14.5 ureterovesical junction (C) and along the urethra (D). (E–G) Antegrade ink injection into the pelvis. Note that the ink in the mutant does not reach the bladder. Duplicated ureters are shown in G. *Second ureter is not filled with ink. (H and I) Hematoxylin-eosin (HE) staining of the ureterovesical junctions at E14.5. The mutant ureter (white arrowhead) fails to connect to the bladder. (J and K) HE staining of male urethral regions at E14.5. (L and M) HE staining of the E14.5 kidneys. The mutant kidney is reduced in size. Black arrowheads, ureterovesical junctions; white arrowheads, blunt-ended ureter; bl, bladder; gt, genital tubercle; kid, kidney; mm, metanephric mesenchyme; nd, nephric duct. Scale bars: 100 μm.
Ink injected into the pelvis filled up the dilated pelvis and ureters, but did not reach the urinary bladder in any of the newborn mutants (n=4), suggesting the existence of urinary tract obstruction (Figure 3, E–G). We also observed duplicated ureters in one mutant, as shown by negative ink flow in the second ureter (Figure 3G). Serial sectioning of the ureter-bladder (ureterovesical) junctions of these samples, as well as those at E14.5, revealed that the ureters failed to reach the urinary bladder and ended blindly in nine of 11 Isl1 mutant mice examined (Figure 3, H and I). In addition, we found impaired formation of the urethra in a subset of the mutants (three of nine), all of which had the abnormal ureterovesical junctions (Figure 3, J and K). Therefore, the hydroureter in the Isl1 mutants is mainly caused by impaired connection of the ureter to the bladder.
However, at E14.5, when no hydronephrosis became apparent because of the negligible urine production, the kidney size ranged from normal to complete agenesis among 10 kidneys examined (two agenesis, four hypoplasia, four normal size; see Figure 3, L and M, for hypoplasia), suggesting that the development of the kidney proper was also impaired independently of the urinary tract obstruction. Because we did not detect any defects in metanephric mesenchymal genes, such as Six2 or Cited1, in the mutant kidneys, we searched for additional abnormalities.
Ectopic or Accessory Ureteric Buds Are Formed in the Absence of Isl1
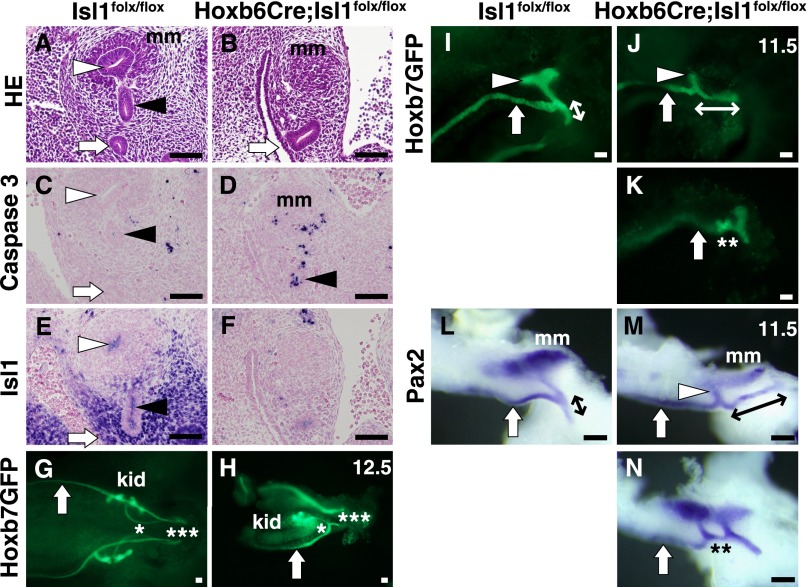
At E11.5, the ureteric buds became elongated and invaded into the metanephric mesenchyme in the wild-type kidneys (Figure 4A). In contrast, the mutant kidneys showed variable phenotypes at this step. Overall, 50% of the kidneys examined (6 of 12) showed impaired elongation of the ureteric buds (Figure 4B), while the remaining buds reached the metanephric mesenchyme. In the former, apoptosis was observed in the ureteric mesenchyme and the ureteric bud epithelia (Figure 4, C and D). Isl1 was expressed in the ureteric mesenchyme surrounding the base of the ureteric bud stalk and the nephric duct in the wild-type kidneys (Figure 4E; see also Figures 1 and 3A), which was abolished in the mutant kidneys (Figure 4F). However, some mutant kidneys showed residual Isl1 expression, as evaluated by immunostaining, probably owing to the mosaic deletion of Hoxb6Cre as reported previously,9 which could partly explain the variable kidney sizes at E14.5. If the kidney is formed to some extent, hydronephrosis and/or hydroureter could be a prominent phenotype owing to the obstruction at the ureterovesical junction.
Figure 4.
Ectopic or accessory ureteric buds are formed in the absence of Isl1. (A and B) Hematoxylin-eosin staining of E11.5 kidneys. The ureteric bud tips invade into the metanephric mesenchyme in the wild-type kidney, but not in the mutant kidney. (C and D) Immunostaining for cleaved caspase 3. Positive signals are detected in the ureteric mesenchyme and also in the ureteric bud stalk (black arrowhead). (E and F) Immunostaining for Isl1. Isl1 expression around the ureteric stalk and the nephric duct is absent in the mutant kidney. (G and H) Visualization of ureteric branching at E12.5 using Hoxb7GFP. The mutant ureter (*) is still connected to the nephric duct, and not to the urogenital sinus (***). No ureteric bud exists on the other side. (I–K) Ureteric buds at E11.5. Note the poor branching of the ureteric bud and longer common nephric duct in the mutant (bidirectional arrows). An accessory ureteric bud (**) is observed in K. (L–N) Whole-mount in situ hybridization of Pax2, showing staining of both the ureteric buds and the metanephric mesenchyme. Ectopic and accessory (**) ureteric buds are observed in M and K, respectively. Black arrowhead, ureteric bud stalk; white arrowhead, ureteric bud tip; white arrow, nephric duct; kid, kidney; mm, metanephric mesenchyme. Scale bars: 100 μm.
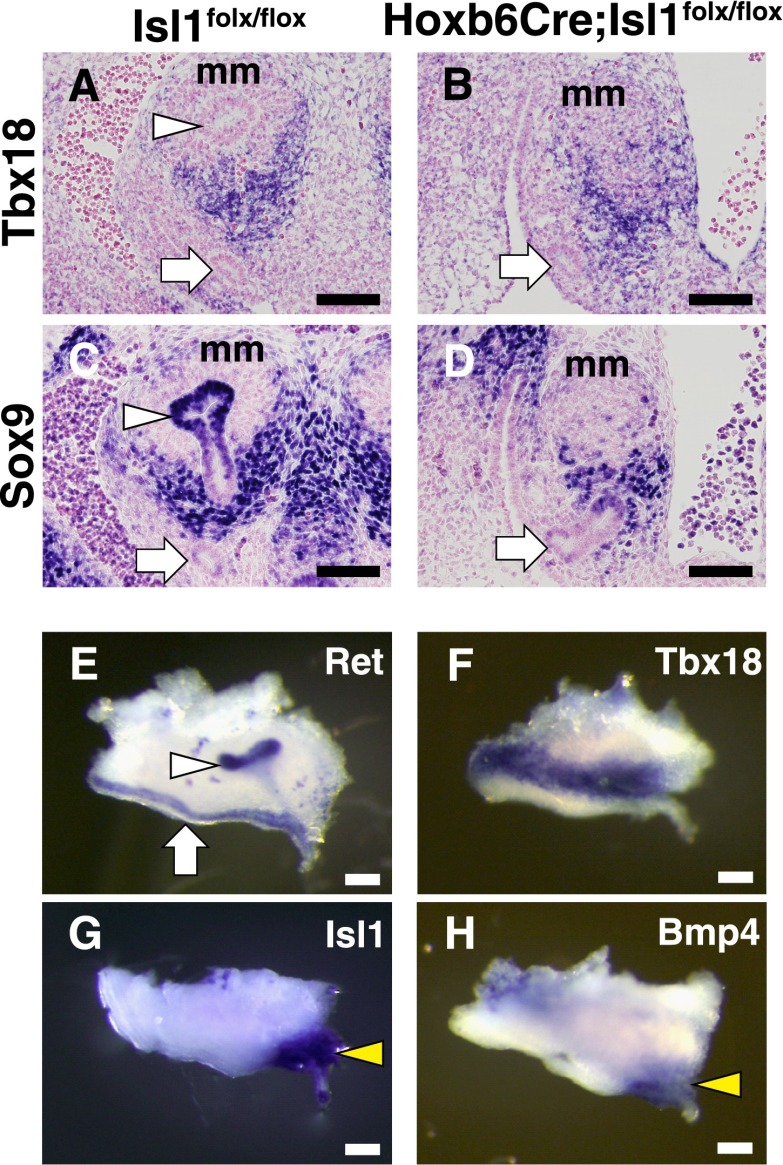
Tbx18 and Sox9 were expressed in the ureteric mesenchyme at this stage, and their expression levels were unaffected in the mutant kidneys, although the expression domains were slightly narrower (Figure 5, A–D). We also found that these two genes were expressed in the ureteric mesenchyme close (or proximal) to the metanephric mesenchyme, while Isl1 was distributed in the distant (or distal) domains at the base of the ureteric stalks and around the nephric duct, although these two domains overlapped to some extent. We confirmed these observations by whole-mount in situ hybridization (Figure 5, E–H).
Figure 5.
Tbx18 and Sox9 expression in the ureteric mesenchyme is unaffected in the absence of Isl1. (A and B) In situ hybridization of Tbx18. Tbx18 is expressed in the ureteric mesenchyme close to the metanephric mesenchyme, but not around the nephric duct. (C and D) Immunostaining for Sox9. Sox9 is expressed in the ureteric mesenchyme close to the metanephric mesenchyme. Sox9 is also expressed in the ureteric bud tips at this stage. (E–H) Whole-mount in situ hybridization of Ret, Tbx18, Isl1, and Bmp4 in the wild-type kidneys at E11.5. Ret is expressed along the nephric duct and in the ureteric bud, and is most abundant in the ureteric bud tips. Tbx18 is broadly expressed in the ureteric mesenchyme close to the ureteric bud tip. In contrast, Isl1 and Bmp4 are expressed around the base of the ureteric bud stalk (yellow arrowheads). White arrowhead, ureteric bud tip; white arrow, nephric duct; mm, metanephric mesenchyme. Scale bars: 100 μm.
Serial sectioning of the mutant ureteric buds suggested that the direction of the buds might be altered (see Figures 4B and 5D). Therefore, we visualized the ureter branching by crossing the Isl1 mutants with Hoxb7GFP mice. At E12.5, while the control ureteric stalks were connected close to the urogenital sinus, the mutant ureteric bud branched poorly and was still connected to the common nephric duct (Figure 4, G and H). The mutant ureter on the other side failed to bud. At E11.5, the ureteric bud elongated from the nephric duct and branched into the metanephric mesenchyme in the control mice (Figure 4I). In the absence of Isl1, however, the ureteric budding occurred ectopically, as shown by a longer common nephric duct, in three kidneys examined (Figure 4J). We also found an accessory ureteric bud in one mutant kidney (Figure 4K). Whole-mount in situ hybridization of Pax2 using additional samples confirmed these observations (Figure 4, L–N). Seven of nine kidneys examined at E11.5 showed cranially positioned and poorly branched ureteric buds (Figure 4M), while two exhibited accessory buds (Figure 4N). These defects (i.e., ectopically positioned or accessory ureteric buds) could lead to misconnection of the ureterovesical junction or duplicated ureters and eventually hydroureter. The subsequent poor branching of the ureteric buds is likely to cause the kidney agenesis/hypoplasia observed in the Isl1 mutants.
Bmp4 Expression Is Reduced in the Absence of Isl1
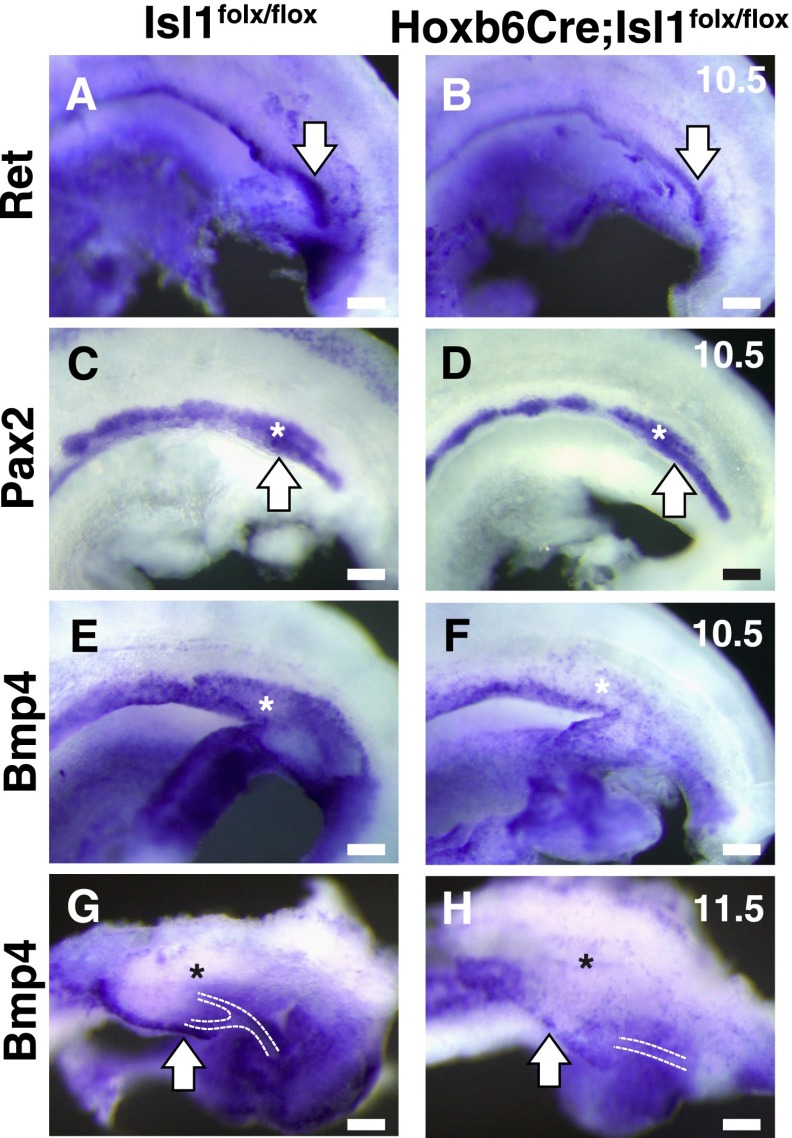
At E10.5, a stage before the ureteric bud is formed, a bulge was observed in the nephric duct in the presumptive metanephric region of the control mice (Figure 6A), which was not apparent in the Isl1 mutants (Figure 6B). The size of the Pax2-positive metanephric mesenchyme in the Isl1 mutants was similar to that in the control mice (Figure 6, C and D).
Figure 6.
Bmp4 expression is reduced in the absence of Isl1. Whole-mount in situ hybridization is shown. (A and B) Ret expression at E10.5 shows the absence of the bulge in the nephric duct in the Isl1 mutant. (C and D) Pax2 is expressed not only in the nephric duct but also in the metanephric mesenchyme at E10.5. (E and F) Bmp4 expression is reduced, especially in the metanephric region, in the Isl1 mutant at E10.5. (G and H) Bmp4 expression is also reduced at E11.5. White arrows, nephric duct; *metanephric mesenchyme. Scale bars: 100 μm.
Mice heterozygous for Bmp4 showed variable kidney sizes and hydroureter resulting from ectopic or accessory ureteric buds,2 similar to the phenotypes of the Isl1 mutant mice. At E10.5, Bmp4 was expressed along the nephric duct and around the metanephric mesenchyme (Figure 6E). Bmp4 expression, especially in the presumptive metanephric region, was significantly reduced in all of the Isl1 mutant kidneys examined (n=4) (Figure 6F). Similarly, we observed Bmp4 reduction in all of the Isl1 mutants examined at E11.5 (n=8), while Bmp4 was expressed along the nephric duct and around the ureteric bud stalk in the control mice (Figure 1, G and H). This reduction is consistent with the similar CAKUT-like phenotypes in both Isl1 and Bmp4 mutant mice.
However, BMP4 supplementation to the organ cultures starting at E10.5 did not significantly rescue the Isl1 mutant phenotypes, which are variable in nature (Supplementary Figure 1). BMP4 addition inhibited ureteric budding and metanephros development even in control kidneys as reported previously,2 while lower concentrations had minimal effects on the Isl1 mutants. It is possible that strict spatiotemporal regulation of BMP activity is necessary for proper ureteric budding, and this cannot be mimicked faithfully by exogenous BMP4 addition. Indeed, there are no reports of successful rescue of the kidney phenotypes even in Bmp4 heterozygotes. Although Bmp4-independent mechanisms could also be involved, the phenotypic similarities between the Isl1 and Bmp4 mutants, and Bmp4 reduction in the Isl1 mutants, suggest that Isl1 is essential for urogenital development, at least partly by regulating Bmp4.
Discussion
We have shown that Isl1 deletion results in kidney agenesis/hypoplasia and hydroureter that resembles CAKUT. Isl1 is expressed in the ureteric mesenchyme at the base of the ureteric buds and is subsequently maintained in the lower urinary tract. In the absence of Isl1, the ureteric budding occurs ectopically along the nephric duct or duplicated ureters are formed, both of which could lead to the impaired connection at the ureterovesical junction and subsequent hydroureter. Because Isl1 is not expressed in the ureteric bud epithelia or the metanephric mesenchyme, its action should be non–cell-autonomous. It is well established that Bmp4 reduction causes CAKUT-like phenotypes. Bmp4 heterozygous mice show a variety of kidney abnormalities, including kidney hypoplasia, and hydroureter.2 Bmp4 inhibits ureteric budding, and its deletion leads to ectopic or accessory ureteric bud formation. Our data indicate that Isl1 deletion results in reduced Bmp4 expression, thereby mimicking the Bmp4 heterozygous mutant phenotypes.
Although Isl1 is weakly expressed in the intermediate mesoderm at E9.5, we did not detect apparent abnormalities in the metanephric mesenchyme before ureteric budding. Therefore, kidney agenesis or hypoplasia caused by the absence of Isl1 could mainly be explained by impaired elongation and poor branching of the ureteric buds. Indeed, ureteric bud-specific deletion of the BMP receptor ALK3 (Bmpr1a) also leads to kidney hypoplasia.12
A link between Isl1 and Bmp4 has also been reported in the development of other organs. In tooth development, Isl1 and Bmp4 are coexpressed in the incisor epithelium, and their expression is interdependent.13 Inhibition of Isl1 expression causes a reduction in Bmp4 and a corresponding loss of Msx1 in the underlying mesenchyme. Isl1 also marks a subset of cardiac progenitors, and Bmp4 and Bmp7 are expressed in regions that overlap with or are adjacent to Isl1-expressing cells.8 Isl1Cre-dependent deletion of Bmpr1a leads to abnormal heart and limb development. Thus, the interaction of Isl1 and Bmp4 is conserved, yet distinct, in the development of different organs.
However, there should also be Isl1-independent regulation of Bmp4 because Bmp4 expression is not completely abolished in the absence of Isl1. In addition, the expression of these two genes does not completely overlap. For example, Isl1 is not expressed in the ureteric mesenchyme at E14.5, while Bmp4 is expressed and plays a major role in the development of this tissue.3 It was recently reported that Bmp4 deletion in mice using Isl1Cre causes lethality during mid-gestation with more severe phenotypes than the Isl1 mutants, including abnormal development of the lower limbs and pelvis, as well as kidney and bladder agenesis.14 Isl1Cre-mediated gene deletion occurs in all cells that express Isl1 at least once. Indeed, when Isl1Cre was bred into the indicator strain, Isl1Cre activity at E14.5 was found in most of the urogenital tissues except for the ureteric epithelia, including the metanephric and ureteral mesenchyme, bladder, and urethra (Supplementary Figure 2). Therefore, Isl1-independent Bmp4 expression in these tissues should also play important roles in urogenital development.
Isl1 has not been considered as a candidate gene for the CAKUT phenotype, partly because its expression is excluded from the major part of the kidney primordia, such as the metanephric mesenchyme and ureteric buds. To the best of our knowledge, mutations in human ISL1 have been reported in a family with type 2 diabetes,15 but not in CAKUT. Because the present data clearly indicate that mutations in Isl1 can cause a CAKUT-like phenotype, human ISL1 mutations should be examined in a subset of patients who have CAKUT, with or without diabetes.
Concise Methods
Generation of Mutant Mice
Isl1flox/flox and Isl1Cre mice were described previously.16,17 Hoxb6Cre and BMPflox/flox mice were kindly provided by Drs. M. Kuehn (National Cancer Institute, National Institutes of Health, Bethesda, MD).10 Hoxb7GFP mice, which were generated by Dr. F. Costantini (Columbia University, New York, NY),18 were obtained from the Jackson Laboratory. All animal experiments were performed in accordance with our institutional guidelines and ethical review committees. Mice carrying the Cre allele were genotyped with forward primer Cre 1 (5′-AGG TTC GTT CAC TCA TGG A-3′) and reverse primer Cre 2 (5′-TCG ACC AGT TTA GTT ACC C-3′). PCR amplifications were performed under identical conditions using GoTaq DNA polymerase (Promega), with denaturation at 95°C for 5 minutes, 35 cycles of 95°C for 30 seconds, 58°C for 60 seconds, and 72°C for 30 seconds, and a final extension at 72°C for 7 minutes. The PCR products were analyzed by electrophoresis in a 1.2% agarose gel and visualized by ethidium bromide staining.
In situ Hybridization and Immunostaining
Histologic examinations were performed as described previously.19,20 Mice were fixed in 10% formalin, embedded in paraffin, and cut into 6-μm sections. In situ hybridization was performed using an automated Discovery System (Roche) according to the manufacturer’s protocols. The probe for Bmp4 was a kind gift from Dr. N. Ueno (National Institute for Basic Biology, Japan). Templates for other probes were generated by RT-PCR and sequenced. Immunostaining was carried out automatically using a BlueMap Kit (Roche) and the Discovery System. The following primary antibodies were used: anti-Isl1 (Abcam); anti–smooth muscle actin (Abcam); anti-Sm22α (Abcam); anti-pSmad1/5/8 (Cell Signaling Technology); anti-Sox9 (Millipore); anti–cleaved caspase 3 (Cell Signaling Technology); anti-RFP (Rockland); and anti–cytokeratin 8 (Developmental Studies Hybridoma Bank). For whole-mount in situ hybridization, samples were fixed with 4% paraformaldehyde and processed using an automated InsituPro VS (Intavis AG) according to the manufacturer’s protocol.
Disclosures
None.
Supplementary Material
Acknowledgments
We thank G. Yamada for helpful discussions and S. Usui for technical assistance.
This study was supported in part by Grants-in-Aid from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) and by the Global COE Program (Cell Fate Regulation Research and Education Unit), MEXT, Japan.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2012050528/-/DCSupplemental.
References
- 1.Pope JC, 4th, Brock JW, 3rd, Adams MC, Stephens FD, Ichikawa I: How they begin and how they end: classic and new theories for the development and deterioration of congenital anomalies of the kidney and urinary tract, CAKUT. J Am Soc Nephrol 10: 2018–2028, 1999 [DOI] [PubMed] [Google Scholar]
- 2.Miyazaki Y, Oshima K, Fogo A, Hogan BL, Ichikawa I: Bone morphogenetic protein 4 regulates the budding site and elongation of the mouse ureter. J Clin Invest 105: 863–873, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yu J, Carroll TJ, McMahon AP: Sonic hedgehog regulates proliferation and differentiation of mesenchymal cells in the mouse metanephric kidney. Development 129: 5301–5312, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Airik R, Bussen M, Singh MK, Petry M, Kispert A: Tbx18 regulates the development of the ureteral mesenchyme. J Clin Invest 116: 663–674, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Airik R, Trowe M-O, Foik A, Farin HF, Petry M, Schuster-Gossler K, Schweizer M, Scherer G, Kist R, Kispert A: Hydroureternephrosis due to loss of Sox9-regulated smooth muscle cell differentiation of the ureteric mesenchyme. Hum Mol Genet 19: 4918–4929, 2010 [DOI] [PubMed] [Google Scholar]
- 6.Pfaff SL, Mendelsohn M, Stewart CL, Edlund T, Jessell TM: Requirement for LIM homeobox gene Isl1 in motor neuron generation reveals a motor neuron-dependent step in interneuron differentiation. Cell 84: 309–320, 1996 [DOI] [PubMed] [Google Scholar]
- 7.Cai CL, Liang X, Shi Y, Chu PH, Pfaff SL, Chen J, Evans S: Isl1 identifies a cardiac progenitor population that proliferates prior to differentiation and contributes a majority of cells to the heart. Dev Cell 5: 877–889, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang L, Cai C-L, Lin L, Qyang Y, Chung C, Monteiro RM, Mummery CL, Fishman GI, Cogen A, Evans S: Isl1Cre reveals a common Bmp pathway in heart and limb development. Development 133: 1575–1585, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Itou J, Kawakami H, Quach T, Osterwalder M, Evans SM, Zeller R, Kawakami Y: Islet1 regulates establishment of the posterior hindlimb field upstream of the Hand2-Shh morphoregulatory gene network in mouse embryos. Development 139: 1620–1629, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lowe LA, Yamada S, Kuehn MR: HoxB6-Cre transgenic mice express Cre recombinase in extra-embryonic mesoderm, in lateral plate and limb mesoderm and at the midbrain/hindbrain junction. Genesis 26: 118–120, 2000 [DOI] [PubMed] [Google Scholar]
- 11.Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, Ng LL, Palmiter RD, Hawrylycz MJ, Jones AR, Lein ES, Zeng H: A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci 13: 133–140, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Di Giovanni V, Alday A, Chi L, Mishina Y, Rosenblum ND: Alk3 controls nephron number and androgen production via lineage-specific effects in intermediate mesoderm. Development 138: 2717–2727, 2011 [DOI] [PubMed] [Google Scholar]
- 13.Mitsiadis TA, Angeli I, James C, Lendahl U, Sharpe PT: Role of Islet1 in the patterning of murine dentition. Development 130: 4451–4460, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Suzuki K, Adachi Y, Numata T, Nakada S, Yanagita M, Nakagata N, Evans SM, Graf D, Economides A, Haraguchi R, Moon AM, Yamada G: Reduced BMP signaling results in hindlimb fusion with lethal pelvic/urogenital organ aplasia: a new mouse model of sirenomelia. PLoS ONE 7: e43453, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shimomura H, Sanke T, Hanabusa T, Tsunoda K, Furuta H, Nanjo K: Nonsense mutation of islet-1 gene (Q310X) found in a type 2 diabetic patient with a strong family history. Diabetes 49: 1597–1600, 2000 [DOI] [PubMed] [Google Scholar]
- 16.Sun Y, Dykes IM, Liang X, Eng SR, Evans SM, Turner EE: A central role for Islet1 in sensory neuron development linking sensory and spinal gene regulatory programs. Nat Neurosci 11: 1283–1293, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Song MR, Sun Y, Bryson A, Gill GN, Evans SM, Pfaff SL: Islet-to-LMO stoichiometries control the function of transcription complexes that specify motor neuron and V2a interneuron identity. Development 136: 2923–2932, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Srinivas S, Goldberg MR, Watanabe T, D’Agati V, al-Awqati Q, Costantini F: Expression of green fluorescent protein in the ureteric bud of transgenic mice: A new tool for the analysis of ureteric bud morphogenesis. Dev Genet 24: 241–251, 1999 [DOI] [PubMed] [Google Scholar]
- 19.Fujimura S, Jiang Q, Kobayashi C, Nishinakamura R: Notch2 activation in the embryonic kidney depletes nephron progenitors. J Am Soc Nephrol 21: 803–810, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Uchiyama Y, Sakaguchi M, Terabayashi T, Inenaga T, Inoue S, Kobayashi C, Oshima N, Kiyonari H, Nakagata N, Sato Y, Sekiguchi K, Miki H, Araki E, Fujimura S, Tanaka SS, Nishinakamura R: Kif26b, a kinesin family gene, regulates adhesion of the embryonic kidney mesenchyme. Proc Natl Acad Sci U S A 107: 9240–9245, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.