Summary
The CD8+ memory T cell population is heterogeneous and it is unclear which subset(s) optimally mediate the central goal of the immune system-protection against infection. Here we investigate the protective capacities of CD8+ T cell subsets present at the memory stage of the immune response. We show that a population of CD8+ T cells bearing markers associated with effector cells (KLRG1hi, CD27lo, T-bethi, Eomeslo) persisted to the memory phase and provided optimal control of Listeria monocytogenes and vaccinia virus, despite weak recall proliferative responses. After antigen specific boosting, this population formed the predominant secondary memory subset, and maintained superior pathogen control. The effector-like memory subset displayed a distinct pattern of tissue distribution and localization within the spleen, and their enhanced capacity to eliminate Listeria involved specialized utilization of cytolysis. Together these data suggest “long-lived effector” CD8+ T cells are optimal for protective immunity against certain pathogens.
Introduction
During a typical immune response, antigen specific CD8+ T cells undergo three characteristic phases: massive clonal expansion, contraction of effector cells and establishment of memory. Considerable efforts have been made to define the factors that control generation of short-lived effector cells and establishment of long-lived memory (Jameson and Masopust, 2009; Kaech and Wherry, 2007; Masopust et al., 2007; Rutishauser and Kaech, 2010; Williams and Bevan, 2007). However, the memory pool that is formed as the result of an immune response is not homogenous but rather contains distinct subsets of cells that differ in their functional, proliferative, trafficking, and survival characteristics (Jameson and Masopust, 2009; Seder et al., 2008). Some phenotypic features of memory cells define their trafficking characteristics (e.g. CCR7 and CD62L) or survival potential (e.g. the cytokine receptor chains CD127 and CD122), while others are used as correlative markers (such as the expression of KLRG1 on cells that are typically thought to be senescent) (Hikono et al., 2007; Joshi et al., 2007; Masopust et al., 2006a; Nolz et al., 2012; Sallusto, 1999; Sallusto et al., 2004; Sarkar et al., 2008). The best characterized division scheme for CD8+ memory T cells is the paradigm of central and effector memory cells, based on CD62L and CCR7 expression. Central memory T cells (Tcm), which express CD62L and CCR7, tend to localize to lymphoid tissues and are capable of robust recall proliferation and IL-2 production, whereas effector-memory T cells (Tem), characterized by lack of CD62L and CCR7 expression, are prevalent at peripheral sites and can quickly become cytoytic, yet exhibit more limited recall proliferation function (Bachmann et al., 2005a; Bachmann et al., 2005b; Seder et al., 2008; Wherry, 2003; Wolint et al., 2004). Another division scheme for CD8+ memory T cells was proposed by Hikono, et. al., who used expression of CXCR3, CD27 and a glyco-form of CD43 as a basis for subset identification (Hikono et al., 2006). These markers subdivide the Tcm and Tem pools, offering refinement of functional properties within the memory-stage pool: for example the CD27hiCD43lo subset becomes dominant over time and shows optimal recall proliferation - and hence were presumed to be functionally superior (Hikono et al., 2007).
These studies support the concept that the fully mature memory pool consists of long-lived CD8+ T cells with a CD62Lhi CD27hi CD43lo KLRG1lo CD127hi phenotype, characterized by efficient recall proliferation. However, such findings do not necessarily mean that this population is optimal for immediate protective immunity - the intended goal of vaccination. Indeed, there is considerable controversy about which subset(s) of memory CD8+ T cells are most potent for pathogen control. For example, in studies on CD8+ T cell control of vaccinia virus, some groups proposed that Tcm are optimal for protection (Laouar et al., 2008; Wherry, 2003) while others proposed that Tem are the more potent subset (Bachmann et al., 2005a; Bachmann et al., 2005b). There is better consensus that Tcm, with their superior recall proliferative characteristics, are best suited for control of LCMV (Bachmann et al., 2005a; Bachmann et al., 2005b; Wherry, 2003) - but the mechanisms involved in control of this non-cytopathic virus may not correspond to the responses needed to eliminate a pathogen that causes direct tissue damage.
Implicit in these studies is the idea that cells with effector-like properties - for example, cells with the KLRG1hi CD62Llo CD27lo phenotype - play no role in protective immunity at the memory stage. Initially this conclusion seems reasonable since effector cells are notable for their lack of recall proliferation and susceptibility to death, leading to loss of most effector-phenotype cells prior to the memory phase (Joshi et al., 2007; Kaech et al., 2003; Prlic and Bevan, 2008; Sarkar et al., 2008). Yet it is also notable that such studies find a small subset of cells with effector-like traits that persists for many months after a primary immune response. Furthermore, the size and durability of CD8+ T cells with effector-like properties is greatly enhanced following antigen-specific boosting of the CD8+ T cell pool (Jabbari and Harty, 2006; Masopust et al., 2006a; Sandau et al., 2010; Wirth et al., 2010b) - however, it remains controversial whether production of such cells by boosting enhances or diminishes the protective function of CD8+ T cells (Hansen et al., 2011; Hansen et al., 2009; Huster et al., 2009; Jabbari and Harty, 2006; Nolz and Harty, 2011; Vezys et al., 2009; Wirth et al., 2010b).
In the current report we examine the functional significance of cells with effector-like traits that persist to the memory phase. By cell sorting, we show that memory-stage cells, with a KLRG1hi, CD127int, CD27lo, CD62Llo phenotype, mediate rapid protective immunity against infection with vaccinia virus and Listeria monocytogenes, in spite of suboptimal recall proliferative capacity. This population is expanded and maintained long term following boosting, without losing their protective superiority. This “long-lived effector” subset shows distinct tissue localization (that can enhance encounter with pathogens), and uniquely takes advantage of cytolytic capacity in its control of Listeria. Together, these data suggest that immediate protective immunity against acute pathogen infection is best provided by cells with effector-like traits that persist to the memory phase, suggesting a different focus for vaccination strategies.
Results
Three subsets of memory cells defined by CD27 and CD43 expression are maintained long term after systemic infection
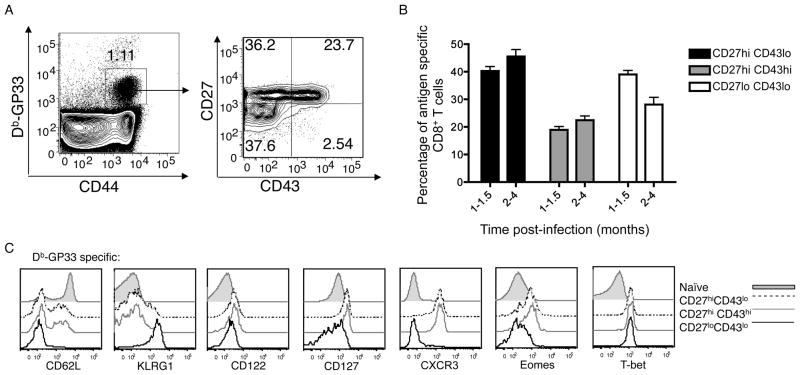
Studies testing whether protective immunity is best mediated by central or effector memory CD8+ T cells have led to contradictory findings (Bachmann et al., 2005a; Bachmann et al., 2005b; Jameson and Masopust, 2009; Laouar et al., 2008; Seder et al., 2008; Wherry, 2003). Hence we investigated an alternative subsetting system (based on expression of CD27 and an alternative glycoform of CD43), which was shown to accurately predict the memory CD8+ T cell recall response (Hikono et al., 2007). We first examined expression of these markers in antigen-specific CD8+ T cell populations persisting to the memory phase following a primary immune response against Listeria monocytogenes (LM) or LCMV. In keeping with previous studies (Hikono et al., 2007; Sandau et al., 2010) three main populations were observed: CD27hi CD43lo, CD27hi CD43hi, and CD27lo CD43lo subsets (Figure 1A). These three populations arose following systemic infection with LCMV or LM recombinants (Fig 1B and Supp. Fig. 1A) and also developed after VV infection or LM delivered by the intranasal route (data not shown), suggesting the type and route of acute infection does not influence the formation of such subsets. Likewise, similar subsets were generated with priming of T cell receptor transgenic CD8+ T cells (Supp. Fig. 1B)(Sandau et al., 2010), indicating that TCR affinity for antigen does not dictate differentiation into these different memory stage subsets. As reported previously (Hikono et al., 2007), the CD27hi CD43lo subset came to dominate the memory pool at later time points, but all three populations were well represented for many months following a primary immune response (Fig 1B).
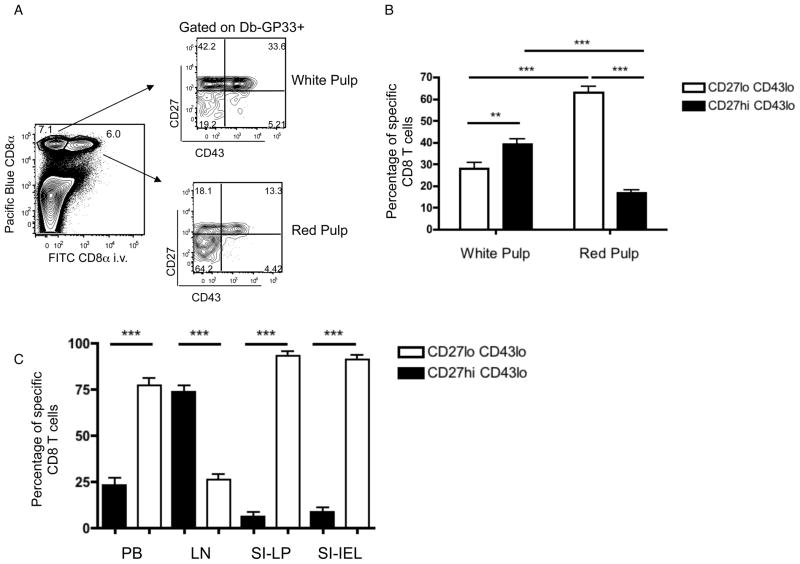
Figure 1. CD27lo CD43lo CD8+ memory T cells display a unique phenotype.
(A) Mice were infected with LCMV. One month post-infection, splenocytes were stained for CD8+, gp33-Db tetramer, and the indicated markers. Data is representative of at least 4 experiments (n=12). (B) shows compiled data from experiments in which mice were infected with LCMV, LM-OVA or LM-B8R. At the indicated time periods, antigen specific cells were identified (using gp33-Db, Ova-Kb or B8R-Kb tetramers, respectively) and the frequency of indicated subsets was determined. Data is compiled from at least 4 experiments (n=14 for 1–1.5 month period, n=25 for 2–4 month period), presented as mean +/− SEM. (C) gp33-Db tetramer-binding CD8+ T cells from spleen and lymph nodes were stained with the indicated markers one month post-infection with LCMV. Data is representative of 4 experiments (n=12). See also Figure S1.
Examination of other cell surface markers revealed striking differences between these subsets. CD27hi populations (whether CD43hi or CD43lo) contained CD62Lhi and CD62lo subsets and displayed “memory CD8+ T cell” markers, including high expression of the cytokine receptor chains CD122 and CD127, with low expression of the “effector cell” marker, KLRG1 (Figure 1C). Hence, prototypical Tcm and Tem populations are both contained in the CD27hi subset. In contrast, the CD27lo CD43lo pool showed a substantially different phenotype, being CD127int and KLRG1hi. Furthermore, CD27lo CD43lo cells exhibited high expression of the transcription factor T-bet but minimal expression of the related factor Eomes, features which are also associated with effector cells (Banerjee et al., 2010; Intlekofer et al., 2005; Joshi et al., 2007). At first glance, this phenotype suggests similarity between the CD27lo CD43lo subset and short-lived effector cells (SLEC)(Joshi et al., 2007). However, in contrast to the drastic downregulation of CD127 reported for short-lived effector cells (Joshi et al., 2007; Kaech et al., 2003; Obar and Lefrancois, 2010), the memory stage CD27lo CD43lo population expressed CD127 at levels similar to naïve CD8+ T cells (albeit at lower levels than either CD27hi population) (Fig 1C and Supp. Fig. 1C,D). Hence this population is more similar to the KLRG-1, CD127 “double positive” population reported by others (Obar and Lefrancois, 2010).
The CD27lo CD43lo subset shows optimal protection against Listeria and vaccinia infection
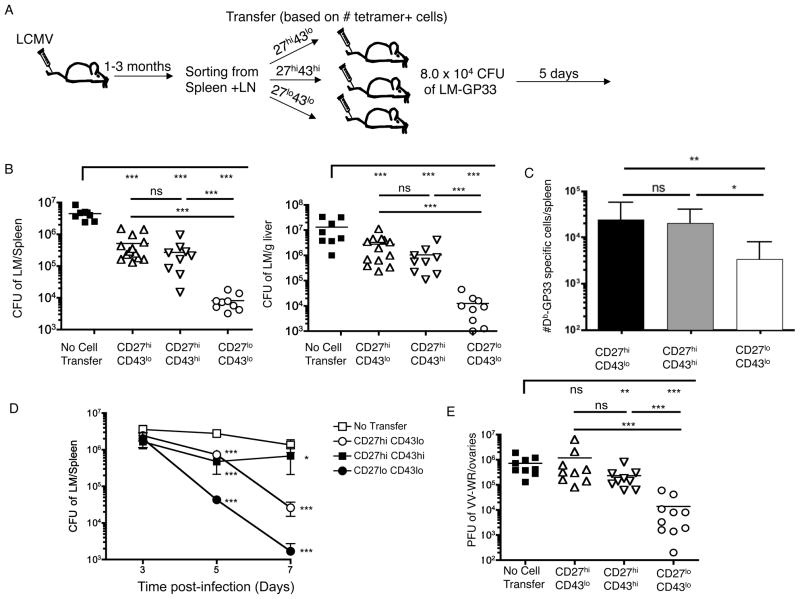
Previous data suggest that the CD27lo CD43lo pool was poor at recall proliferation (Hikono et al., 2007), and several phenotypic features of this pool (high expression of KLRG1, low expression of CD27 and CD127) have been correlated with senescent CD8+ T cells (Baars et al., 2005; Heffner and Fearon, 2007; Hikono et al., 2007). Furthermore, this population (in contrast to the CD27hi memory subsets) lacked expression of CXCR3 (Fig 1C), which might be expected to limit their ability to promptly traffic to sites of inflammation. Hence, one might anticipate that CD27lo CD43lo cells would show inferior protection compared to the prototypical Tem and Tcm populations present in the CD27hi pool. Yet this has not been directly tested. Thus, we explored how CD27, CD43 defined subsets performed in protection assays using a heterologous protection model (Fig. 2A). Mice were primed with LCMV, memory populations were sorted and equivalent numbers of gp33-Db specific CD8+ T cells from each memory subset adoptively transferred into normal recipients. Host animals were infected with LM-gp33, and 5 days later the donor cell expansion and LM clearance was determined.
Figure 2. Robust protection by CD27lo CD43lo memory cells.
(A) Experimental schematic. CD8+ CD44hi LCMV memory cells were sort purified 1–3 months post-infection according to CD27 and CD43 expression and each population injected into naïve recipients, such that an equal number of gp33-Db tetramer-binding cells were adoptively transferred. (B and C) Mice were challenged with LM-gp33. Five days after challenge the number of CFU of LM in the spleen and liver was determined (B) and the number of gp33-Db specific CD8+ T cells in the spleen quantified (C). Data are from compiled from 3 independent experiments. Data in (B) shows individual CFU, with mean values indicated by a line, while the data in (C) shows mean +/− SD. P-values are represented as follows: ***, p<0.001; **, p<0.01; *, p<0.05. (D) Mice were primed with ActA LM-OVA, and at least one month later memory subsets were sorted and 3 × 104 cells of each subset adoptively transferred per recipient. Host animals (and “No-transfer” controls) were infected with virulent LM-OVA and protection determined at 3, 5 or 7 days post-infection. Data are compiled from 3 experiments, and shown as mean +/− SEM. Statistical analysis is represented by asterisks, indicating significant changes in comparison with the no-transfer group for each time point. (E) A similar experimental plan to (A) was used, except that mice were infected with LM-B8R to induce a B8R-Kb specific response and, following transfer of sorted memory CD8+ T cells recipient mice were challenged with VV-WR. Three days after VV-WR infection, the number of PFU of VV in the ovaries was determined. Individual mice compiled from 3 experiments are shown. See also Figure S2
Surprisingly, the CD27lo CD43lo subset provided significantly greater protective immunity than either of the CD27hi memory CD8+ T cell populations (Fig. 2B). Interestingly, the enhanced protection provided by the CD27lo CD43lo subset did not correspond to the magnitude of the donor cell response during challenge - indeed, fewer donor cells were isolated from animals receiving the CD27lo CD43lo pool (Fig. 2C). This likely results from less efficient adoptive transfer “take” of CD27lo CD43lo cells initially (Supp. Fig. 2A), together with reduced expansion of this subsets after antigen encounter (in keeping with previous studies (Hikono et al., 2007)). Regardless of mechanism, our findings indicate that the size of the donor cell response did not accurately predict LM elimination.
Similar results were observed when OT-I memory T cell subsets were tested for protection against LM-OVA (Supp. Fig. 2B), ruling out potential differences in TCR affinity within the memory subsets as a basis for disintct protective capacity. Also, studies using titrated donor cell transfers indicated that low numbers of the CD27lo CD43lo subset were more effective at protection than an excess of CD27hi CD43lo or CD27hi CD43hi cells (Supp. Fig. 2C).
Kinetic studies (using a homologous LM-OVA protection assay) indicated that the CD27lo subset continued to reduce the pathogen burden at day 7, outperforming both CD27 subsets (Fig 2D). Whereas the CD27hiCD43lo population also offered substantial immunity by day 7, CD27hiCD43hi cells were much more variable in mediating LM clearance. None of the transferred subsets were protective by the day 3 time point - indicating that subsequent differences in donor cell expansion did not relate to differential early bacterial clearance. Together, these data suggest that the CD27lo CD43lo subset offers the most potent and efficient protective immunity. In contrast, the CD27hi subsets (regardless of CD43 expression), offered considerably weaker protective immunity, despite the fact that these subsets include prototypical Tcm and Tem populations.
It was possible that optimal protection by the CD27lo CD43lo memory pool was unique to assays involving LM control. Therefore, we also investigated protective immunity against vaccinia virus (VV), after priming with LM-B8R (a recombinant LM strain bearing an immunodominant VV epitope). Furthermore, we analyzed viral control in a non-lymphoid organ (the ovary, which forms a site for intense VV replication (Karupiah et al., 1990)) rather than the spleen and liver analyzed for LM control. Again, the CD27lo CD43lo population offered superior pathogen elimination, compared to the other memory subsets (Figure 2E). Therefore, in diverse models of acute pathogen infection, CD27lo CD43lo KLRG-1hi phenotype cells provide optimal protection, compared to the classic memory phenotype CD8+ T cell pools.
Boosting increases the frequency of protective CD27lo CD43loKLRG1hi CD8+ cells
Although the CD27lo CD43lo subset persists for many months following priming (Fig 1), these cells eventually decline (Hikono et al., 2007). Comparison between P14 memory CD8+ T cells at 1 month versus ~1 year after priming demonstrated almost complete loss of this subset in the “old” memory pool (Supplementary figure 3A). Preliminary studies indicated that challenge of these immunized mice resulted in efficient LM-gp33 control, regardless of the time after priming (data not shown) - however, since such animals have a large population of memory P14 CD8+ T cells (>5% of the CD8+ T cell pool), we further assayed the protective function of “old” versus “young” P14 memory cells using adoptive transfer assays. Interestingly, aged memory P14 CD8+ T cells showed considerably weaker per-cell protection against LM-gp33 (Supplementary figure 3B). While there may be diverse reasons for this outcome, such results are consistent with the CD27lo CD43lo subset offering superior control against Listeria.
These findings raise the question of whether the size and persistence of the CD27lo CD43lo subset can be extended. Interestingly, previous work showed that antigen specific boosting increases the number and duration of CD27lo KLRG1+ cells within the antigen specific CD8+ memory T cell pool (Masopust et al., 2006a; Sandau et al., 2010; Wirth et al., 2010a; Wirth et al., 2010b). However, it was not clear whether these cells would show efficient protective functions, since some studies suggest boosting decreases the fitness of CD8+ T cells and impairs their ability to respond toward certain pathogens (Nolz and Harty, 2011; Wirth et al., 2010b).
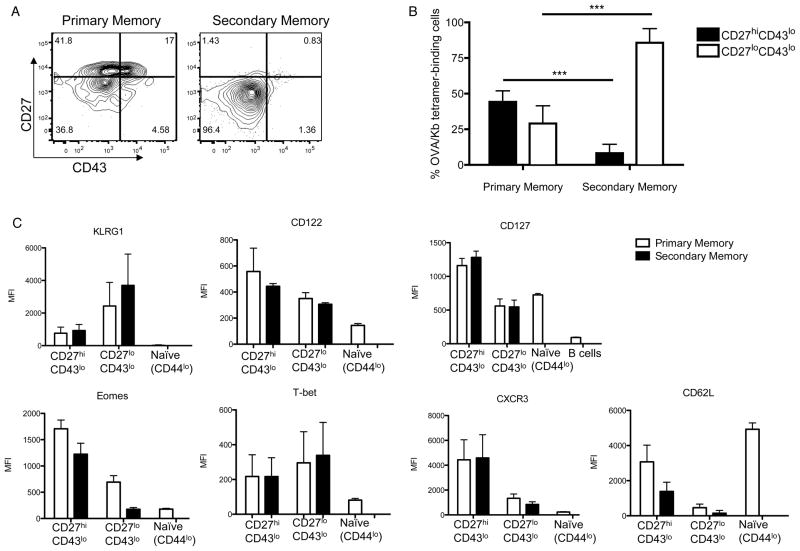
To investigate this issue, we used an efficient prime-boost strategy involving immunization with OVA peptide-coated cells, followed by boosting with LM-OVA (Badovinac et al., 2005; Pham et al., 2010). In such approaches, very few effector cells are produced in the primary (1°) response, with the vast majority of antigen specific cells rapidly adopting a central memory phenotype (Badovinac et al., 2005; Pham et al., 2010) (data not shown), and these memory cells quickly become competent to undergo an efficient secondary (2°) response (Badovinac et al., 2005; Pham et al., 2010). We selected this rapid prime-boost system since it has been shown to induce potent 2° CD8+ T cell memory, capable of protecting against viral, bacterial and parasitic infections (Pham et al., 2010). At least 3 months after the last immunization, OVA-Kb specific cells were analyzed. Secondary memory cells showed a greatly expanded population of CD27lo CD43lo cells, with few CD27hi CD43lo cells (and virtually no CD27hi CD43hi cells) (Fig. 3A,B), in contrast to the diverse populations present in the 1° memory CD8+ pool (which were analyzed in parallel, following immunization with LM-OVA alone). The 2° CD27lo CD43lo pool was even more markedly reduced for expression of Eomes, but was similar to its counterpart in the 1° memory pool in being KLRG1hi T-bethi, CD62lo and CXCR3lo, and CD127int (Fig. 3C). Therefore, boosting greatly enriches the frequency of the CD27lo CD43lo population surviving to the 2° memory stage, without changing their major phenotypic features.
Figure 3. CD27lo CD43lo cells are strongly increased with boosting.
(A) Example staining of the frequency of Kb-OVA+ cells expressing CD27 and CD43, 3–4 months post-infection with LM-OVA Att (primary memory) or 3–4 months after boosting with LM-OVA (secondary memory). Data is representative of 3 experiments (n=9). (B) Compiled data of the frequency of CD27hi CD43lo and CD27lo CD43lo within Kb-OVA+ cells. Bars show the average +/− SD from 3 experiments (n=9). (C) MFI of phenotypic markers on primary and secondary memory populations. Naïve (CD44lo) CD8+ T cells and/or B cells (CD19+) are also included for comparison. Bars show the mean +/− SD from 3 experiments (n=9). See also Figure S3
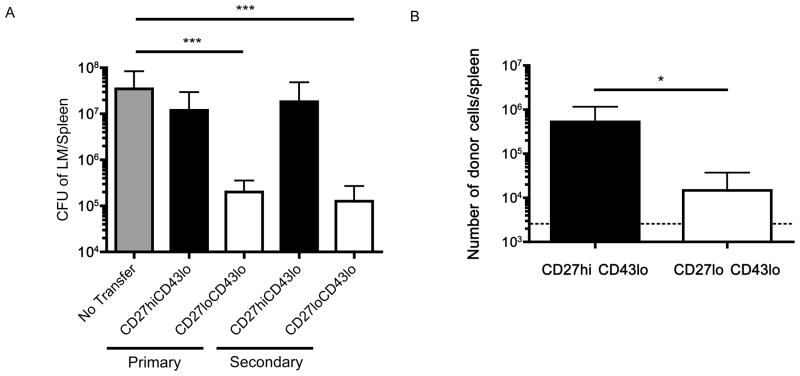
Next, we assessed the protective capacity of 2° memory subsets, in comparison with cells from the 1° memory pool. The lack of CD27hi CD43hi cells precluded assessment of this subset in the 2° memory pool, and our studies on the 1° memory population suggested similar protection by CD27hi CD43hi and CD27hi CD43lo populations (Fig. 2): Hence, we sorted CD27hi CD43lo and CD27lo CD43lo subsets from 1° or 2° memory pools, adoptively transferred these cells into normal recipients, which were then challenged with virulent LM-OVA.
As expected the 1° memory CD27lo CD43lo population displayed superior protective capacity compared to prototypical memory cells (CD27hi CD43lo), and similar outcomes were observed for the 2° memory subsets (Fig 4A). Once again, efficient protection by the secondary memory CD27lo CD43lo subset did not correlate with enhanced expansion of this population (Fig 4B). Hence, these data suggest boosting expands the number of CD27lo CD43lo memory CD8+ T cells without eroding their protective capacity.
Figure 4. Boosting increases the number of protective memory cells.
CD27hi CD43lo and CD27lo CD43lo phenotype memory CD8+ T cells were sorted from primary and secondary memory mice 2.5 – 3.5 months after the last antigen stimulation. Equal numbers of OVA-Kb tetramer staining cells were transferred into normal recipients, which were challenged with LM-OVA one day later. Five days following LM-OVA challenge, animals were sacrificed and (A) CFU of LM was determined in the spleen. (B) The expansion of donor CD27hi CD43lo and CD27lo CD43lo secondary memory cells was determined in the spleen 5 days post LM-OVA challenge. The dotted line indicates the limit of detection. Data are shown as mean +/− SD and are compiled from two independent experiments.
The CD27lo memory CD8+ T cell subset shows differential tissue localization and compartmentalization
Previous studies have shown that blood-borne LM initially infects macrophages in the splenic red pulp and marginal zone (Aoshi et al., 2009), and have proposed that initial memory CD8+ T cell encounter with LM-infected cells takes place in the red pulp (Bajenoff et al., 2010). Furthermore, short-lived effector cells selectively occupy the red pulp (Jung et al., 2010). Hence, we considered whether CD27lo CD43lo KLRG-1hi memory cells showed preferential localization within the spleen. To analyze this, we intravenously injected mice with fluorescent anti-CD8+ antibody and isolated tissues after a brief delay: This technique labels CD8+ T cells exposed to the blood (including the splenic red pulp), but does not label cells in tissue parenchyma (including the white pulp) (Anderson et al., 2012; Galkina et al., 2005; Teijaro et al., 2011). Interestingly, the CD27lo CD43lo pool was selectively enriched in the red pulp, while the CD27hi CD43lo population showed preferential localization to the white pulp (Fig. 5A,B). This asymmetry in red and white pulp distribution was also observed for memory subsets after adoptive transfer (Supplementary Fig 4), and hence might contribute to the differences in protective immunity documented above. We also examined the frequency of the CD27lo CD43lo population in other tissues. While this subset was 30–40% of the splenic memory pool (Fig. 1B), it constituted around 75% of circulating memory cells, yet was largely excluded from lymph nodes (as expected from the lack of CD62L expression) (Fig. 5C). CD27lo memory cells constituted the vast majority of antigen specific cells in the small intestine IEL and LP compartments (Fig 5C), as expected from earlier work (Masopust et al., 2006b). These cells express CD43 (Masopust et al., 2006b)(data not shown) and the extent of their similarity to CD27lo cells in lymphoid tissues is not clear, but these findings support the conclusion that localization of CD27lo memory CD8+ T cell pool is optimal for prompt engagement with invading pathogens.
Figure 5. CD27lo CD43lo memory cells localize to the red pulp and non-lymphoid sites.
(A) Discrimination of splenic red and white pulp by injection of anti-CD8+α antibody. CD27 and CD43 expression on gp33-Db tetramer-binding CD8+ T cells within each compartment is shown, from mice infected 1–2 months earlier with LCMV. A representative example is displayed. (B) Compiled data showing the localization of CD27hi CD43lo and CD27lo CD43lo memory CD8+ T cells, 1–2 months after LM-OVA or LCMV infection (gating on OVA-Kb and gp33-Db tetramer binding cells, respectively) (n=13). The data represent mean +/− SEM. (C) The frequency of CD27hi or CD27lo antigen-specific memory CD8+ T cells in the blood, LN, IEL and LPL compartments was determined, 1–2 months after LCMV or LM-OVA infection. Bars show compiled data from 4 experiments (n=9) and are shown as mean +/− SEM. See also Figure S4.
Superior protection against LM depends on cytolytic potential of the CD27lo CD43lo subset
Recall expansion is frequently used as a parameter of memory CD8+ T cell function, and is often assumed to correspond to potency in protective immunity. However, our data shows that recall proliferation of primary and secondary memory CD8+ T cell subsets did not predict pathogen control (Figs 2, 4). What then accounts for the enhanced protection mediated by the CD27lo CD43lo pool? To address this, we first assessed the ability of primary memory subsets to produce inflammatory cytokines. Antigen induced production of IFNγ (Supp. Fig. 5A) and TNF (data not shown) was similar for all three CD27, CD43 defined subsets, assessed after cell sorting and in vitro activation with specific peptide-MHC. This finding contrasts with a recent study suggesting CD27lo memory CD8+ T cells exhibit impaired induction of IFN-γ compared to CD27hi cells (Nolz et al., 2012), but supports conclusions from earlier studies (Hikono et al., 2007; Sandau et al., 2010). Memory CD8+ T cells can release IFN-γ in response to IL-12 and IL-18, and this may contribute to pathogen clearance in a non-antigen specific manner (Berg et al., 2003) (Kambayashi et al., 2003). However, that function was also similar for all three subsets defined using CD27 and CD43 (Supp. Fig. 5B). We also considered that the CD27lo CD43lo KLRG1hi subset may exhibit greater functional avidity (i.e. sensitivity to low dose peptide-MHC ligand)(Slifka and Whitton, 2001). However, when tested for upregulation of CD69, IFN-γ and CD107a (a marker of degranulation), we observed similar dose responses for KLRG1hi and KLRG1lo memory subsets (Supp. Fig 5C).
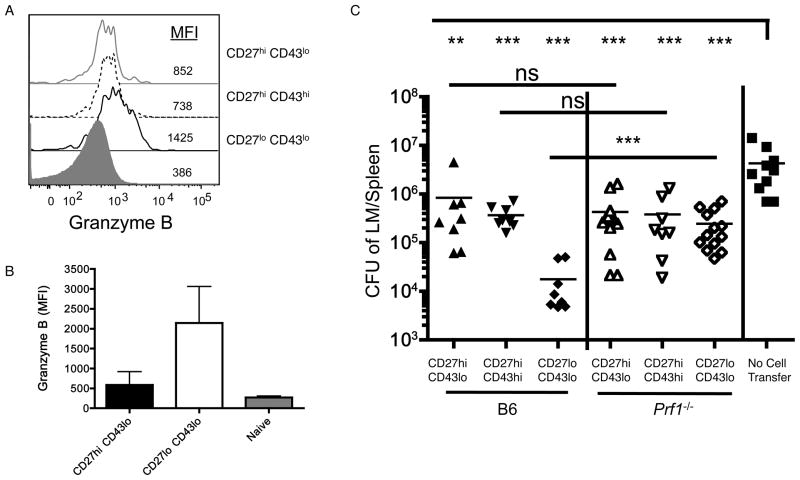
Cytolytic ability is important for function of CD8+ T cells during many infections - for example, Prf1−/− memory CD8+ T cells (lacking Perforin expression) are approximately 5-times less active on a per cell basis in the clearance of LM (Messingham et al., 2003). Previous studies reported that CD27lo CD43lo cells (but not other memory subsets) express Granzyme B at steady state (Hikono et al., 2007; Sandau et al., 2010), and we confirm this finding (Figures 6A and 6B). However, the functional significance of cytolysis has not been assessed in these memory subsets. To evaluate this, we infected wild type or Prf1−/− mice with attenuated LM-OVA and, after memory differentiation, sorted CD27, CD43 subsets (which developed similarly in both types of mice - data not shown). Each subset was transferred at equal number into wild type mice followed by homologous challenge with virulent LM-OVA and protection was monitored 5 days post infection. As expected, the CD27lo CD43lo memory subset isolated from normal mice offered significantly better protection than the other memory subsets (Fig 6C, left). However, this protective advantage was completely lost when the CD27lo CD43lo subset from Prf1−/− animals was analyzed. Surprisingly, this requirement for cytolytic potential was uniquely invested in the CD27lo CD43lo population - Perforin-deficiency did not compromise the ability of either CD27hi subset to mediate partial protection against LM infection (Fig 6C). This finding suggests that cytolysis is critical for the ability of CD27lo CD43lo cells to mediate rapid protection against LM, but also reveals that this effector function is uniquely invested in the CD27lo CD43lo pool, with the protective function of other memory subsets being Perforin independent. Such findings suggest specialized distribution of key effector functions to certain memory CD8+ T cell subsets.
Figure 6. Cytolytic function is required for enhanced protective immunity by CD27lo CD43lo cells.
(A) Intracellular staining of granzyme B by indicated memory subsets of gp33-Db tetramer-binding cells, 30 days post LCMV infection. (B) Granzyme B expression in OVA-Kb tetramer staining memory CD8+ T cell subsets isolated from animals immunized 3–4 months earlier with ActA LM-OVA. Naïve (CD44lo) CD8+ T cells are included as a control. Data is shown as mean +/− SD (n=9). (C) Memory subsets were sorted from ActA LM-OVA infected B6 or Perforin-deficient (Prf1−/−) mice, 1–2 months post infection. Cells were transferred in equal number into B6.SJL recipients, which were then challenged with LM-OVA. Five days after infection, the number of CFU in the spleen was calculated. Symbols represent individual mice compiled from 3 different experiments. See also Figure S5.
Discussion
The goal of successful vaccination is rapid control of infection - the timing of which could make the difference between asymptomatic clearance or severe tissue damage and death. Yet despite decades of work on memory T cell responses to pathogens, surprisingly little is known about which memory CD8+ T subsets mediate optimal protective immunity. In this report, we focus on a memory CD8+ T cell subset that develops during varied immune responses. The phenotypic traits of this Tem subset (CD62Llo, CD27lo, KLRG-1hi, T-bethi, Eomeslo and low CD127 expression) resemble those of short lived effector cells, and this feature (together with the reduced long-term survival and weaker recall proliferation of this subset), has lead to the conclusion that such cells may be senescent (Baars et al., 2005; Heffner and Fearon, 2007; Hikono et al., 2007; Nolz et al., 2012). Yet we found that this CD27lo CD43lo memory subset to provide the most robust resistance against certain acute bacterial (Listeria) and viral (vaccinia) infections, and demonstrated that this protective population is expanded and maintained in the secondary memory pool (following rapid prime-boost immunization).
Many studies use recall proliferation as an index for a “useful” memory T cell population, with the tacit assumption that rapid production of numerous effector cells is a key element in control of a rapidly replicating pathogen. However, our finding that the CD27lo CD43lo memory CD8+ T cell pool excels at protection despite displaying modest recall proliferation brings this correlation into question. Rather than superior expansion, cytolytic potential of the CD27lo CD43lo pool was critical for their enhanced control of LM while, surprisingly, this function played little part in the more moderate protection mediated by CD27hi memory subsets. Studies from Harty’s group showed that bulk Perforin-deficient memory CD8+ T cells displayed a reduced per-cell capacity to control LM (Messingham et al., 2003): Our data suggest that reliance on killing does not apply equally to all memory cell subsets, but rather reflects a selective utilization of cytolysis by the highly protective CD27lo CD43lo population. Furthermore, the CD27lo CD43lo memory pool showed preferential localization to the red pulp of the spleen, which forms a major site for encounter with blood-borne pathogens, including Listeria (Aoshi et al., 2009) and is implicated as a key site for the initial encounter of memory CD8+ T cells with LM infected macrophages (Bajenoff et al., 2010). Previous studies found preferentially localization of effector cells and (at later time points) effector-memory cells to the red pulp (Jung et al., 2010): Our data extend those observations, demonstrating that the highly protective CD27lo CD43lo memory CD8+ T cell subset is optimally placed to encounter microbes in the blood. Such findings fit with the concept of poly-functionality - in which optimally protective memory T cells display multiple specialized traits (Seder et al., 2008) - but do not exclude a model in which distinct pools of memory T cells cooperate for protection. For example, in our studies rapid pathogen control was mediated by CD27lo CD43lo cells, yet the poor expansion of this subset suggests that progeny of the CD27hi population will dominate in the recall effector pool - the protective capacity of the CD27hi pool, although delayed, may be essential for elimination of residual infection.
Our data tested protection against LM and VV - does the CD27lo CD43lo “long-lived effector” pool mediate protection against other pathogens? Using similar rapid prime-boost approaches, Harty and colleagues showed that resistance to Listeria, vaccinia, influenza and malaria were all enhanced (Badovinac et al., 2005; Pham et al., 2010): Those studies did not define the full phenotype of the secondary memory population, nor test protection by distinct memory subsets, but the predominance of CD27lo, CD43lo, KLRG-1hi, CD127int cells that we detect in prime-boosted animals (Fig. 3) suggests these cells are a likely source for protection in those studies. Alternatively, it is likely that responses to some infectious agents will involve distinct protection mechanisms, mediated by different memory subsets. For example, several studies indicate central memory CD8+ T cells are best suited for control of LCMV (Bachmann et al., 2005a; Bachmann et al., 2005b; Nolz and Harty, 2011; Wherry, 2003), and the superlative recall expansion of Tcm pool may be critical for keeping pace with rapidly spreading virus. In our hands, the CD62Lhi central-memory phenotype population was exclusively found in the CD27hi memory CD8+ T cell subset, and hence this pool may be key for responses that require efficient lymph node entry (Nolz and Harty, 2011). Preliminary data indicate that all three CD27, CD43 defined memory subsets offer similar, modest control of LCMV clone 13 infection (data not shown), but further studies will be required to fully assess their protective capacity compared to “classic” central memory CD8+ T cells. Hence, our data should not be taken to mean that CD27lo CD43lo are superior for control of all pathogen infections. Prior studies testing protection by bulk secondary memory CD8+ T cells showed improved protection against LM and vaccinia virus (in keeping with our findings on the elevated frequency of the CD27lo CD43lo subset), but reduced control of MHV and LCMV Clone 13 (Nolz and Harty, 2011). Hence, control of different microbes may rely on the diversity of subsets within the memory pool (especially following a primary immune response). It will also be important to determine whether distinct memory CD8+ T cell populations are optimal at control of systemic versus local (e.g. mucosal) infections.
Nevertheless, our studies suggest production and preservation of CD27lo CD43lo cells will be useful as a vaccine goal for various pathogens. Based primarily on studies of short-lived effector cells, it has been proposed that CD27lo, KLRG1hi phenotype cells are a transient, senescent pool (Baars et al., 2005; Heffner and Fearon, 2007; Hikono et al., 2007; Nolz et al., 2012), although some studies reported that memory populations enriched in cells of this phenotype survive and undergo recall responses similarly to the CD27hi KLRG1lo pool (Prlic et al., 2012). It is important to stress that, in contrast to short-lived effectors, the CD27lo CD43lo KLRG1hi memory pool studied here expresses CD127 (although at reduced levels compared to CD27hi subsets) and CD122, cytokine receptor chains critical for response to IL-7 and IL-15 respectively, and that this population persists >4 months after primary and secondary infections (Figs. 1 and 3). However, we did confirm that the CD27lo CD43lo population declines considerably by 1 year post-priming, and that this accompanies a loss in per-cell protective capacity by the “aged” memory pool (Supp Fig 3). Interestingly, recent studies suggest that cells with an overlapping phenotype (CD27lo CD62lo) constitute a death-intermediate pool of memory cells, being an apoptosis-prone, non-functional population derived from homeostasis of the central memory CD27hi subset (Nolz et al., 2012). Also, some studies suggest “unhelped” memory CD8+ T cells (those primed in the absence of CD4 help) display a related phenotype, and have impaired functional traits (Edwards et al., 2013; Intlekofer et al., 2007). In contrast, our studies indicate that the CD27lo population exhibits cytokine production similar to CD27hi cells, undergoes recall proliferation (albeit less efficiently than other memory subsets) and mediates excellent protective function. Furthermore, the high frequency of CD27lo CD43lo CD8+ T cells in the secondary memory pool makes it unlikely that they are derived from rare central memory cells in boosted animals. It will be important to determine whether immunization strategies such as the rapid prime boost approach employed here, affects the nature of CD27lo CD43lo memory CD8+ T cell homeostasis.
In summary, our studies indicate that cells with effector-like traits persist to the memory phase and constitute the most protective pool for control of certain infections. This subset shows unique tissue localization and utilization of cytolysis in pathogen control, and the fact that this subset is substantially expanded by boosting suggests such cells should be a desired focus of vaccination approaches.
Experimental Procedures
Mice
6–12 week old female C57BL/6 and B6.SJL mice were purchased from the National Cancer Institute. TCR transgenic mice (P14 (Pircher et al., 1991) - a kind gift of Dr. David Masopust, University of Minnesota, and OT-I (Hogquist et al., 1994)) were maintained on a B6.PL (Thy-1.1) background. Perforin deficient mice were obtained from Jackson labs, and provided by Dr. Stephen McSorely, UC Davis). All mice were maintained in SPF conditions and all mouse protocols were approved by the University of Minnesota Institutional Animal Care and Use Committee.
Bacterial and Viral Infections
Recombinant LM strains LM-OVA (virulent and ActA attenuated) and LM-gp33 were provided by Hao Shen (University of Pennsylvania School of Medicine, Philadelphia, PA) and John Harty (University of Iowa) and have been described (Pope, 2001). In addition, a novel strain of virulent LM-gp33 was kindly provided by Dr. Dietmar Zehn (University of Lausanne, Switzerland). Attenuated strain LM-B8R (expressing the B8R CD8+ epitope from vaccinia virus)(Hamilton et al., 2010) was provided by Dr. Ross Kedl (National Jewish Medical Research Center, University of Colorado, Denver, CO). LCMV (Armstrong) was provided by Dr. David Masopust (University of Minnesota).
LM was grown in tryptic soy broth with 50 μg/ml streptomycin to an OD600 of ~0.1. For LM-OVA challenges 8 × 104 CFU were injected i.v. For primary infection with attenuated LM-OVA or LM-B8R, 3–6 ×106 CFU were injected i.v. Primary infection with LCMV Armstrong was 2 × 105 PFU i.p. For challenge with vaccinia virus, 2×106 PFU of VV-WR was diluted in PBS and injected i.v. Secondary memory cells were generated by immunization with OVA protein coated splenocytes followed by boosting with LM-OVA one week later (Pham et al., 2010).
Flow Cytometry and Cell Sorting
For surface phenotype experiments, cells were stained with the following antibodies from eBioscience or BD Biosciences unless otherwise noted: CD8+ (53–6.7), CD44 (IM7), CD43 (glycoform sensitive antibody, 1B11; Biolegend), CD27 (LG.7F9), KLRG1 (2F1), CD62L (MEL-14), CD122 (TMb1), CD127 (A7R34), and CXCR3 (CXCR3–173; Biolegend). The gp33-Db, OVA-Kb, and B8R-Kb tetramers were generated as previously described (Daniels and Jameson, 2000), or were provided by the NIH Tetramer Facility. For intracellular staining, cells were fixed and permeabilized with Foxp3 Fixation and Permeabilization Buffers (eBioscience), and stained with antibodies to T-bet (4B10), Eomesodermin (Dan11mag), and Granzyme B (MHGB04; Invitrogen) for 1 hour at 4°C in Permeabilization Solution. Flow cytometry data was analyzed using FlowJo analysis software. Intraepithelial and Lamina Propria cells of the small intestine were isolated as previously described (Casey et al., 2012; Masopust et al., 2010).
For cell sorting, spleen or spleen and lymph nodes were harvested from mice >30 days post-infection with LCMV or LM. Collagenase digestion was performed on tissues followed by negative enrichment for CD8+ T cells using Miltenyi enrichment antibody cocktail and beads. Cells were then stained with CD8+, CD44, CD27, and CD43 and sorted into populations using a FACSAria. Purity was confirmed, and where applicable, the number of antigen specific cells within each subset was determined using tetramer staining or analysis with relevant congenic markers. Next, 2–3 × 104 cells of the indicated phenotype were injected (i.v.) into naïve, congenically distinct hosts, which were infected (as indicated) one day later.
Flow cytometric discrimination of cells in splenic white and red pulp
As described (Anderson et al., 2012; Galkina et al., 2005; Teijaro et al., 2011), mice were first injected i.v. with fluorescently labeled anti-CD8+α antibody. The animals were sacrificed 3 minutes later, a splenocytes single-cell suspension prepared and the cells stained again for CD8+α, using a distinct fluorochrome conjugate, and simultaneously stained for other cell surface markers. In some experiments, mice were perfused immediately after sacrifice, with similar findings. As shown by others (Anderson et al., 2012), cells in contact with the blood circulation (including the splenic red pulp) are labeled by the intravenously injected anti-CD8+ antibody, while cells in parenchymal locations (including the splenic white pulp) are not.
In vitro stimulations
Sorted memory CD8+ T cells (P14 or OT-1) were incubated with peptide (KAVYNFATM or SIINFEKL), and Brefeldin A (BD Biosciences) for 5–6 hours. Following surface staining, cells were fixed and permeabilized with BD Cytofix and Cytoperm (Becton Dickinson) followed by intracellular staining for IFNγ, TNF, and IL-2 in BD Perm Wash Buffer (Becton Dickinson) (Hamilton, 2006). Cytokine stimulations with IL-2, IL-12, and IL-18 were performed as described previously (Haluszczak et al., 2009).
Determination of Colony Forming Units (CFU) and cell expansion
This was performed as previously described (Hamilton et al., 2010; Hamilton, 2006). Briefly, on day 5 after infection, the spleen and liver were removed and placed in a 0.2% IGEPAL solution (Sigma-Aldrich). Organs were homogenized and serial dilutions were plated onto TSB plates containing 50μg/ml streptomycin. Bacterial colonies were counted following plate incubation for ~24 hours at 37°C. The limit of detection (approximately 100 organisms) is indicated on graphs by a dashed line. To determine the number of transferred cells in recipient mice, splenic single cell suspensions were counted and stained with antibodies to CD8+, CD44 and CD45.2 (104) or Thy1.1 (HIS51) (to identify donor cells) and, in some cases, relevant peptide-MHC tetramer. In some experiments, no-transfer control groups were used to determine the limit of donor cell detection.
Plaque Assay
This was performed as previously described (Hamilton et al., 2010). Briefly, on day 3 after infection with VV-WR, ovaries were harvested in PBS and frozen as a single cell suspension. After two freeze-thaw cycles, the ovary homogenate was incubated at 37°C for 45 minutes with 0.25mg/ml trypsin (Sigma). 143B cells (ATCC) were grown to confluence. Dilutions of the ovary homogenate were added in duplicate to the cellular monolayer and left for two days. Staining with 1% crystal violet was then performed. Plaques were counted and total viral load per ovaries was calculated.
Statistics
A two-tailed, unpaired, Student’s t-test was performed on log transformed data, using Prism (GraphPad Software Inc.). In some figures, P-values are represented as follows: ***, p<0.001; **, p<0.01; *, p<0.05.
Supplementary Material
Highlights.
Effector-like, CD27lo CD8+ T cells persist into primary and secondary memory pools
Despite weak expansion “long-lived effectors” optimally control Listeria & vaccinia
CD27lo memory CD8+ cells show tissue localization favoring rapid pathogen encounter
Cytolysis is selectively utilized by CD27lo memory cells for Listeria control
Acknowledgments
We thank the Masopust lab (especially Kristin Anderson) for help establishing splenic localization assays and timely provision of aged memory P14 CD8+ T cells, Josiah Zacharias for valuable experimental input, and the University of Minnesota Flow Core personnel for cell sorting. We are grateful to Kris Hogquist, Matt Mescher, Dave Masopust, Stephen McSorley and members of the Jamequist lab for mouse strains and helpful discussions. This work was supported by the National Institutes of Health (grants R01AI75168 and R37AI38903 to S.C.J., and Cancer Biology Postdoctoral Training Grant 5T32CA009138-38 to J.A.O) and a Leukemia and Lymphoma Career Development Award (to S.E.H.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson KG, Sung H, Skon CN, Lefrancois L, Deisinger A, Vezys V, Masopust D. Intravascular Staining Redefines Lung CD8+ T Cell Responses. J Immunol. 2012 doi: 10.4049/jimmunol.1201682. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoshi T, Carrero JA, Konjufca V, Koide Y, Unanue ER, Miller MJ. The cellular niche of Listeria monocytogenes infection changes rapidly in the spleen. Eur J Immunol. 2009;39:417–425. doi: 10.1002/eji.200838718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baars PA, Sierro S, Arens R, Tesselaar K, Hooibrink B, Klenerman P, van Lier RA. Properties of murine (CD8+)CD27- T cells. Eur J Immunol. 2005;35:3131–3141. doi: 10.1002/eji.200425770. [DOI] [PubMed] [Google Scholar]
- Bachmann MF, Wolint P, Schwarz K, Jager P, Oxenius A. Functional properties and lineage relationship of CD8+ T cell subsets identified by expression of IL-7 receptor alpha and CD62L. J Immunol. 2005a;175:4686–4696. doi: 10.4049/jimmunol.175.7.4686. [DOI] [PubMed] [Google Scholar]
- Bachmann MF, Wolint P, Schwarz K, Oxenius A. Recall proliferation potential of memory CD8+ T cells and antiviral protection. J Immunol. 2005b;175:4677–4685. doi: 10.4049/jimmunol.175.7.4677. [DOI] [PubMed] [Google Scholar]
- Badovinac VP, Messingham KA, Jabbari A, Haring JS, Harty JT. Accelerated CD8+ T-cell memory and prime-boost response after dendritic-cell vaccination. Nat Med. 2005;11:748–756. doi: 10.1038/nm1257. [DOI] [PubMed] [Google Scholar]
- Bajenoff M, Narni-Mancinelli E, Brau F, Lauvau G. Visualizing early splenic memory CD8+ T cells reactivation against intracellular bacteria in the mouse. PLoS One. 2010;5:e11524. doi: 10.1371/journal.pone.0011524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee A, Gordon SM, Intlekofer AM, Paley MA, Mooney EC, Lindsten T, Wherry EJ, Reiner SL. Cutting edge: The transcription factor eomesodermin enables CD8+ T cells to compete for the memory cell niche. Journal of immunology. 2010;185:4988–4992. doi: 10.4049/jimmunol.1002042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg RE, Crossley E, Murray S, Forman J. Memory CD8+ T cells provide innate immune protection against Listeria monocytogenes in the absence of cognate antigen. J Exp Med. 2003;198:1583–1593. doi: 10.1084/jem.20031051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey KA, Fraser KA, Schenkel JM, Moran A, Abt MC, Beura LK, Lucas PJ, Artis D, Wherry EJ, Hogquist K, et al. Antigen-independent differentiation and maintenance of effector-like resident memory T cells in tissues. Journal of immunology. 2012;188:4866–4875. doi: 10.4049/jimmunol.1200402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels MA, Jameson SC. Critical role for CD8+ in T cell receptor binding and activation by peptide/major histocompatibility complex multimers. J Exp Med. 2000;191:335–346. doi: 10.1084/jem.191.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards LE, Haluszczak C, Kedl RM. Phenotype and function of protective, CD4-independent CD8+ T cell memory. Immunologic research. 2013;55:135–145. doi: 10.1007/s12026-012-8356-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galkina E, Thatte J, Dabak V, Williams MB, Ley K, Braciale TJ. Preferential migration of effector CD8+ T cells into the interstitium of the normal lung. J Clin Invest. 2005;115:3473–3483. doi: 10.1172/JCI24482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haluszczak C, Akue AD, Hamilton SE, Johnson LD, Pujanauski L, Teodorovic L, Jameson SC, Kedl RM. The antigen-specific CD8+ T cell repertoire in unimmunized mice includes memory phenotype cells bearing markers of homeostatic expansion. J Exp Med. 2009;206:435–448. doi: 10.1084/jem.20081829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton SE, Schenkel JM, Akue AD, Jameson SC. IL-2 complex treatment can protect naive mice from bacterial and viral infection. J Immunol. 2010;185:6584–6590. doi: 10.4049/jimmunol.1001215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton SE, Wolkers MC, Schoenberger SP, Jameson SC. The generation of protective memory-like CD8+ T cells during homeostatic proliferation requires CD4+ T cells. Nat Immunol. 2006;7:475–481. doi: 10.1038/ni1326. [DOI] [PubMed] [Google Scholar]
- Hansen SG, Ford JC, Lewis MS, Ventura AB, Hughes CM, Coyne-Johnson L, Whizin N, Oswald K, Shoemaker R, Swanson T, et al. Profound early control of highly pathogenic SIV by an effector memory T-cell vaccine. Nature. 2011;473:523–527. doi: 10.1038/nature10003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen SG, Vieville C, Whizin N, Coyne-Johnson L, Siess DC, Drummond DD, Legasse AW, Axthelm MK, Oswald K, Trubey CM, et al. Effector memory T cell responses are associated with protection of rhesus monkeys from mucosal simian immunodeficiency virus challenge. Nature medicine. 2009;15:293–299. doi: 10.1038/nm.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heffner M, Fearon DT. Loss of T cell receptor-induced Bmi-1 in the KLRG1(+) senescent CD8+(+) T lymphocyte. Proc Natl Acad Sci U S A. 2007;104:13414–13419. doi: 10.1073/pnas.0706040104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikono H, Kohlmeier JE, Ely KH, Scott I, Roberts AD, Blackman MA, Woodland DL. T-cell memory and recall responses to respiratory virus infections. Immunol Rev. 2006;211:119–132. doi: 10.1111/j.0105-2896.2006.00385.x. [DOI] [PubMed] [Google Scholar]
- Hikono H, Kohlmeier JE, Takamura S, Wittmer ST, Roberts AD, Woodland DL. Activation phenotype, rather than central- or effector-memory phenotype, predicts the recall efficacy of memory CD8+ T cells. J Exp Med. 2007;204:1625–1636. doi: 10.1084/jem.20070322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogquist KA, Jameson SC, Heath WR, Howard JL, Bevan MJ, Carbone FR. T cell receptor antagonist peptides induce positive selection. Cell. 1994;76:17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- Huster KM, Stemberger C, Gasteiger G, Kastenmuller W, Drexler I, Busch DH. Cutting edge: memory CD8+ T cell compartment grows in size with immunological experience but nevertheless can lose function. Journal of immunology. 2009;183:6898–6902. doi: 10.4049/jimmunol.0902454. [DOI] [PubMed] [Google Scholar]
- Intlekofer AM, Takemoto N, Kao C, Banerjee A, Schambach F, Northrop JK, Shen H, Wherry EJ, Reiner SL. Requirement for T-bet in the aberrant differentiation of unhelped memory CD8+ T cells. J Exp Med. 2007;204:2015–2021. doi: 10.1084/jem.20070841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Intlekofer AM, Takemoto N, Wherry EJ, Longworth SA, Northrup JT, Palanivel VR, Mullen AC, Gasink CR, Kaech SM, Miller JD, et al. Effector and memory CD8+ T cell fate coupled by T-bet and eomesodermin. Nature immunology. 2005;6:1236–1244. doi: 10.1038/ni1268. [DOI] [PubMed] [Google Scholar]
- Jabbari A, Harty JT. Secondary memory CD8+ T cells are more protective but slower to acquire a central-memory phenotype. J Exp Med. 2006;203:919–932. doi: 10.1084/jem.20052237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jameson SC, Masopust D. Diversity in T cell memory: an embarrassment of riches. Immunity. 2009;31:859–871. doi: 10.1016/j.immuni.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi NS, Cui W, Chandele A, Lee HK, Urso DR, Hagman J, Gapin L, Kaech SM. Inflammation directs memory precursor and short-lived effector CD8+(+) T cell fates via the graded expression of T-bet transcription factor. Immunity. 2007;27:281–295. doi: 10.1016/j.immuni.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung YW, Rutishauser RL, Joshi NS, Haberman AM, Kaech SM. Differential localization of effector and memory CD8+ T cell subsets in lymphoid organs during acute viral infection. Journal of immunology. 2010;185:5315–5325. doi: 10.4049/jimmunol.1001948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaech SM, Tan JT, Wherry EJ, Konieczny BT, Surh CD, Ahmed R. Selective expression of the interleukin 7 receptor identifies effector CD8+ T cells that give rise to long-lived memory cells. Nature immunology. 2003;4:1191–1198. doi: 10.1038/ni1009. [DOI] [PubMed] [Google Scholar]
- Kaech SM, Wherry EJ. Heterogeneity and cell-fate decisions in effector and memory CD8+ T cell differentiation during viral infection. Immunity. 2007;27:393–405. doi: 10.1016/j.immuni.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kambayashi T, Assarsson E, Lukacher AE, Ljunggren HG, Jensen PE. Memory CD8+ T cells provide an early source of IFN-gamma. J Immunol. 2003;170:2399–2408. doi: 10.4049/jimmunol.170.5.2399. [DOI] [PubMed] [Google Scholar]
- Karupiah G, Coupar B, Ramshaw I, Boyle D, Blanden R, Andrew M. Vaccinia virus-mediated damage of murine ovaries and protection by virus-expressed interleukin-2. Immunol Cell Biol. 1990;68(Pt 5):325–333. doi: 10.1038/icb.1990.44. [DOI] [PubMed] [Google Scholar]
- Laouar A, Manocha M, Haridas V, Manjunath N. Concurrent generation of effector and central memory CD8+ T cells during vaccinia virus infection. PLoS One. 2008;3:e4089. doi: 10.1371/journal.pone.0004089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masopust D, Choo D, Vezys V, Wherry EJ, Duraiswamy J, Akondy R, Wang J, Casey KA, Barber DL, Kawamura KS, et al. Dynamic T cell migration program provides resident memory within intestinal epithelium. The Journal of experimental medicine. 2010;207:553–564. doi: 10.1084/jem.20090858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masopust D, Ha SJ, Vezys V, Ahmed R. Stimulation history dictates memory CD8+ T cell phenotype: implications for prime-boost vaccination. J Immunol. 2006a;177:831–839. doi: 10.4049/jimmunol.177.2.831. [DOI] [PubMed] [Google Scholar]
- Masopust D, Vezys V, Wherry EJ, Ahmed R. A brief history of CD8+ T cells. Eur J Immunol. 2007;37(Suppl 1):S103–110. doi: 10.1002/eji.200737584. [DOI] [PubMed] [Google Scholar]
- Masopust D, Vezys V, Wherry EJ, Barber DL, Ahmed R. Cutting edge: gut microenvironment promotes differentiation of a unique memory CD8+ T cell population. J Immunol. 2006b;176:2079–2083. doi: 10.4049/jimmunol.176.4.2079. [DOI] [PubMed] [Google Scholar]
- Messingham KA, Badovinac VP, Harty JT. Deficient anti-listerial immunity in the absence of perforin can be restored by increasing memory CD8+ T cell numbers. J Immunol. 2003;171:4254–4262. doi: 10.4049/jimmunol.171.8.4254. [DOI] [PubMed] [Google Scholar]
- Nolz JC, Harty JT. Protective capacity of memory CD8+ T cells is dictated by antigen exposure history and nature of the infection. Immunity. 2011;34:781–793. doi: 10.1016/j.immuni.2011.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolz JC, Rai D, Badovinac VP, Harty JT. Division-linked generation of death-intermediates regulates the numerical stability of memory CD8+ T cells. Proc Natl Acad Sci U S A. 2012;109:6199–6204. doi: 10.1073/pnas.1118868109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obar JJ, Lefrancois L. Early signals during CD8+ T cell priming regulate the generation of central memory cells. Journal of immunology. 2010;185:263–272. doi: 10.4049/jimmunol.1000492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham NL, Pewe LL, Fleenor CJ, Langlois RA, Legge KL, Badovinac VP, Harty JT. Exploiting cross-priming to generate protective CD8+ T-cell immunity rapidly. Proc Natl Acad Sci U S A. 2010;107:12198–12203. doi: 10.1073/pnas.1004661107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pircher H, Rohrer UH, Moskophidis D, Zinkernagel RM, Hengartner H. Lower receptor avidity required for thymic clonal deletion than for effector T-cell function. Nature. 1991;351:482–485. doi: 10.1038/351482a0. [DOI] [PubMed] [Google Scholar]
- Pope C, Kim S, Marzo A, Williams K, Jiang J, Shen H, Lefrancois L. Organ-specific regulation of the CD8+ T cell response to Listeria monocytogenes infection. J Immunol. 2001;166:3402–3409. doi: 10.4049/jimmunol.166.5.3402. [DOI] [PubMed] [Google Scholar]
- Prlic M, Bevan MJ. Exploring regulatory mechanisms of CD8+ T cell contraction. Proc Natl Acad Sci U S A. 2008;105:16689–16694. doi: 10.1073/pnas.0808997105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prlic M, Sacks JA, Bevan MJ. Dissociating markers of senescence and protective ability in memory T cells. PloS one. 2012;7:e32576. doi: 10.1371/journal.pone.0032576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutishauser RL, Kaech SM. Generating diversity: transcriptional regulation of effector and memory CD8+ T-cell differentiation. Immunol Rev. 2010;235:219–233. doi: 10.1111/j.0105-2896.2010.00901.x. [DOI] [PubMed] [Google Scholar]
- Sallusto E, Lenig D, Farster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol. 2004;22:745–763. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- Sandau MM, Kohlmeier JE, Woodland DL, Jameson SC. IL-15 regulates both quantitative and qualitative features of the memory CD8+ T cell pool. J Immunol. 2010;184:35–44. doi: 10.4049/jimmunol.0803355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar S, Kalia V, Haining WN, Konieczny BT, Subramaniam S, Ahmed R. Functional and genomic profiling of effector CD8+ T cell subsets with distinct memory fates. J Exp Med. 2008;205:625–640. doi: 10.1084/jem.20071641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seder RA, Darrah PA, Roederer M. T-cell quality in memory and protection: implications for vaccine design. Nat Rev Immunol. 2008;8:247–258. doi: 10.1038/nri2274. [DOI] [PubMed] [Google Scholar]
- Slifka MK, Whitton JL. Functional avidity maturation of CD8+(+) T cells without selection of higher affinity TCR. Nature immunology. 2001;2:711–717. doi: 10.1038/90650. [DOI] [PubMed] [Google Scholar]
- Teijaro JR, Turner D, Pham Q, Wherry EJ, Lefrancois L, Farber DL. Cutting edge: Tissue-retentive lung memory CD4 T cells mediate optimal protection to respiratory virus infection. Journal of immunology. 2011;187:5510–5514. doi: 10.4049/jimmunol.1102243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezys V, Yates A, Casey KA, Lanier G, Ahmed R, Antia R, Masopust D. Memory CD8+ T-cell compartment grows in size with immunological experience. Nature. 2009;457:196–199. doi: 10.1038/nature07486. [DOI] [PubMed] [Google Scholar]
- Wherry EJ, Teichgraber V, Becker TC, Masopust D, Kaech SM, Antia R, von Andrian UH, Ahmed R. Lineage relationship and protective immunity of memory CD8+ T cell subsets. Nat Immunol. 2003;4:225–234. doi: 10.1038/ni889. [DOI] [PubMed] [Google Scholar]
- Williams MA, Bevan MJ. Effector and memory CTL differentiation. Annu Rev Immunol. 2007;25:171–192. doi: 10.1146/annurev.immunol.25.022106.141548. [DOI] [PubMed] [Google Scholar]
- Wirth TC, Harty JT, Badovinac VP. Modulating numbers and phenotype of CD8+ T cells in secondary immune responses. Eur J Immunol. 2010a;40:1916–1926. doi: 10.1002/eji.201040310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirth TC, Xue HH, Rai D, Sabel JT, Bair T, Harty JT, Badovinac VP. Repetitive antigen stimulation induces stepwise transcriptome diversification but preserves a core signature of memory CD8+(+) T cell differentiation. Immunity. 2010b;33:128–140. doi: 10.1016/j.immuni.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolint P, Betts MR, Koup RA, Oxenius A. Immediate cytotoxicity but not degranulation distinguishes effector and memory subsets of CD8+ T cells. J Exp Med. 2004;199:925–936. doi: 10.1084/jem.20031799. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.