Abstract
Diacylglycerol (DAG), a second messenger generated by phospholipase Cγ1 activity upon T cell receptor (TCR) engagement, triggers several signaling cascades that play important roles in T cell development and function. A family of enzymes called diacylglycerol kinases (DGKs) catalyzes the phosphorylation of DAG to phosphatidic acid, acting as a braking mechanism that terminates DAG-mediated signals. Two DGK isoforms, α and ζ, are predominantly expressed in T cells and synergistically regulate the development of both conventional αβ T cells and invariant NKT cells in the thymus. In mature T cells, the activity of these DGK isoforms aids in the maintenance of self-tolerance by preventing T cell hyper-activation upon TCR stimulation and by promoting T cell anergy. In CD8 cells, reduced DGK activity is associated with enhanced primary responses against viruses and tumors. Recent work has also established an important role for DGK activity at the immune synapse and identified partners that modulate DGK function. In addition, emerging evidence points to previously unappreciated roles for DGK function in directional secretion and T cell adhesion. In this review, we discuss the multitude of roles played by DGKs in T cell development and function, while emphasizing recent advances in the field.
Keywords: Diacylglycerol kinase, phosphatidic acid, signal transduction, T cell receptor
I. INTRODUCTION
Diacylglycerol kinases (DGKs) are a family of enzymes that catalyze the conversion of lipid second messenger diacylglycerol (DAG) to phosphatidic acid (PA). Work from several groups, including ours, has shown that DGKs serve as a braking mechanism in immune cell signaling, dampening DAG levels after receptor stimulation and preventing hyper-activation of immune cells.1–4 Ten isoforms of DGK have been identified in mammals, many of which are expressed in cells of the immune system.
Notably, both the substrate and product of the DGK-catalyzed reaction, DAG and PA, are bioactive lipids that can act as second messengers.5–8 DGK activity therefore serves as a switch to simultaneously dampen DAG-mediated signals and boost PA-mediated signals. In T cells, DAG recruits RasGRP1 and PKCθ to the cell membrane, leading to signaling via the RasGRP1/Ras/ERK and PKCθ/IKK/NF-κB pathways.9,10 Previous studies have shown that PA, on the other hand, can bind to signaling molecules such as mammalian target of rapamycin (mTOR), SHP-1, RasGAP, Sos, PI5Kα, and p47(phox).8,11–17
All mammalian DGKs contain a catalytic kinase domain, consisting of a conserved motif and an accessory domain, and at least two cysteine-rich DAG-binding C1 domains. However, DGKs also possess other distinct structural domains, based on which they are classified into five types (Figure 1). Two DGK isoforms, the type-I α isoform and the type-IV ζ isoform, are highly expressed in T cells.18,19 The type-I DGK isoforms α, β and γ possess an N-terminal recoverin homology domain and two Ca2+-binding EF hand motifs. While the recoverin homology domain is related to the N terminal region of the recoverin family of neuronal calcium sensors, the EF hands are involved in auto-inhibition.20 Type-IV DGK isoforms, ζ and ι, contain a myristoylated alanine rich C-kinase substrate (MARCKS) motif, four ankyrin repeats, and a C-terminal PDZ binding domain. As its name suggests, the MARCKS domain can be phosphorylated by PKC isoforms. Studies in cell lines have shown that PKCα can phosphorylate the DGKζ MARCKS motif to negatively regulate both DGKζ’s catalytic activity and its ability to interact with other proteins.21,22 In addition, the MARCKS motif contains a nuclear localization sequence.23 The ankyrin repeats and PDZ binding motif are thought to play a role in protein-protein interactions, with the latter binding to PDZ domains on proteins such as syntrophins.24,25 Similar to some other DGKp isoforms such as β, δ, and η, DGKζ contains several alternative splicing isoforms.26,27 The functional differences among these DGKζ isoforms in the immune system remain to be clearly defined.
Figure 1. Structure-based classification of mammalian DGK isoforms.
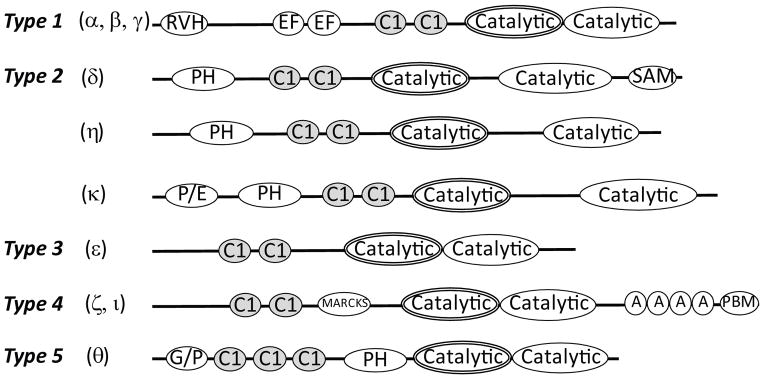
Based on the presence of certain structural features, mammalian DGK isoforms are classified into five types. The DGK catalytic domain consists of a conserved motif (shown with double lines) and an accessory domain (shown with a single line). RVH – recoverin homology domain, EF – EF hand, C1- cysteine-rich DAG-binding domain, PH – plextrin homology domain, SAM – sterile alpha motif, P/E- proline/glutamate-rich region, MARCKS – myristoylated alanine-rich C kinase substrate domain, A – ankyrin repeat motif, PBM – PDZ binding motif, G/P – glycine/proline-rich region.
Members of the DGK family show substantial diversity in the cell types they are expressed in and their localization within those cells. Notably, DGK isoforms are highly expressed in cells of the hematopoietic and nervous systems. Multiple DGK isoforms are often expressed simultaneously in a given cell type. Though DGK α and ζ isoforms predominate in T cells, we can be detected multiple DGK isoforms by reverse transcriptase-PCR in T cells (α, γ, δ, ζ, θ), macrophages (α, β, γ, δ, ζ, ι) and mast cells (α, γ, δ, ε, ζ, ι)(References 28, 29 and our unpublished observations). Due to their distinct structural domains, different types of DGKs tend to localize to specific subcellular compartments and are regulated by unique cues in the intracellular milieu.30 Six DGK isoforms – α, γ, δ, ζ, ι, and θ - have been observed to reside in or move to the nucleus upon stimulation in different cell types.31 For instance, stimulation via the TCR leads to the nuclear translocation of DGKα and its binding to the nuclear matrix in primary rat T cells.32 Immunohistochemical analyses have also revealed that DGKζ localizes to the nucleus in neurons in various parts of the rat brain, and that this subcellular distribution is specifically disrupted in hippocampal pyramidal neurons in a model of forebrain ischemia.33 Nuclear localization of DGKζ has also been demonstrated in other cell types.23,24 Nuclear translocation of DGKs could control nuclear DAG and PA concentrations and/or prevent DGKs from terminating DAG in the cytoplasm membrane. Whether DGKζ localizes to the nucleus and the functional importance of nuclear localization of DGK isoforms in T cells remain to be defined.
From an organismal standpoint, it is interesting to note that DGKα protein expression appears to be restricted to certain cell types such as T-lineage cells and oligodendrocytes,34,35 while DGKζ is expressed more ubiquitously in the brain, lungs, heart, hematopoietic system and skeletal muscles.27,36,37 However, as stated previously, our unpublished data suggests that DGKα, at least at the mRNA level, may also be expressed in other hematopoietic cells including mast cells and macrophages. In this review, we discuss the varied roles played by DGKs in T cell development and function, while emphasizing recent advances that have helped move the field forward.
II. ROLE AND REGULATION OF DAG IN TCR SIGNALING
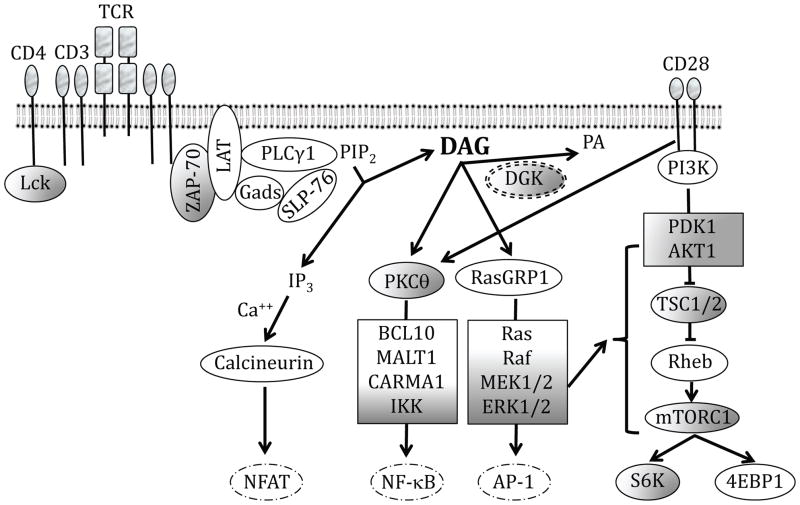
Engagement of the TCR by a cognate peptide-MHC complex triggers a multitude of signaling pathways that, in the presence of additional signals, cooperate to turn on a transcriptional program of T cell activation (Figure 2). Following TCR engagement, Lck, a Src family tyrosine kinase that associates with the cytoplasmic tails of CD4 and CD8, is activated by CD45-mediated dephosphorylation.38, 39 Active Lck, in turn, phosphorylates immunoreceptor tyrosine-based activation motifs (ITAMs) on CD3, leading to the recruitment of the kinase ZAP-70.40, 41 Lck phosphorylates and activates ZAP-70, which then phosphorylates the adaptor protein LAT.42, 43 Phosphorylated LAT recruits a number of signaling molecules, including adaptor SLP-76 and phospholipase PLCγ1, to the cell membrane.44,45
Figure 2. DAG-mediated pathways in T cell receptor signaling.
Schematic representation of various signaling pathways activated upon engagement of the T cell receptor and the CD28 co-stimulatory receptor, with an emphasis on DAG-mediated pathways. Please see the text for further details.
Active PLCγ1 hydrolyzes membrane phospholipid PIP2 to produce two second messengers - DAG and inositol triphosphate (IP3). IP3 binds to its receptors on the endoplasmic reticulum (ER) to trigger the release of intracellular ER calcium stores. Depletion of calcium in the ER lumen causes a conformational change in the ER-associated calcium sensors STIM1 and STIM2.46 This conformational change activates the calcium-release activated calcium (CRAC) channel Orai1 on the cell membrane, leading to an influx of calcium from the extracellular milieu.47 Increasing calcium levels in the cytosol activate the phosphatase calcineurin, which dephosphorylates the transcription factor NFAT to trigger its nuclear translocation.48 Signaling via the NFAT pathway is important for T cell development, activation, anergy, and in the function of Treg, TFH, TH17, and CD8 T cells.49,50
Membrane-associated DAG, on the other hand, recruits PKCθ, PKD1, and Ras family guanine nucleotide exchange factor RasGRP1 to the cell membrane through their C1 domains.10,51–53 Activated RasGRP1, along with Sos, helps convert the small GTPase Ras from its GDP-bound inactive form to a GTP-bound active form, and active Ras then activates the kinase Raf.54, 55 In turn, Raf activates MEK1/2, which subsequently activate MAP kinases ERK1/2. ERK activity increases the expression of transcription factor c-Fos and also phosphorylates c-Fos, leading to its dimerization with c-Jun to form the transcription factor AP-1.56, 57 NFAT and AP-1 interact with each other and bind cooperatively to composite binding elements on the promoters of several genes, including IL-2.58–60 The RasGRP1-Ras-ERK1/2 pathway has been shown to play a critical role in positive selection during intrathymic T cell development and in the activation of peripheral T cells.61–64
Active PKCθ phosphorylates the adaptor CARD11, leading to the formation of a signalosome with Bcl10 and MALT1.65 This signalosome activates the three-subunit IKK complex, which phosphorylates IκB. Phosphorylation of IκB eventually leads to its degradation, allowing active NF-κB dimers to translocate to the nucleus.66, 67 The PKCθ-IKK-NFκB pathway is not essential for conventional T cell maturation, but is critical for iNKT cell and regulatory T cell development and for peripheral T cell activation,68–71 particularly for effective TH2 immune responses.72–74 It is important to note here that DAG-independent mechanisms may also play important roles in PKCθ activation. For instance, CD28 can directly recruits PKCθ to the immunological synapse to promote its activation.75,76
DAG-activated PKD1 phosphorylates the transcriptional repressor HDAC7, leading to its export from the nucleus and the de-repression of its target genes, such as Nur77.77,78 Membrane-localized and cytosolic forms of PKD1 may have distinct functions during thymocyte development.79 The individual DAG-mediated pathways outlined above have also been shown to cooperate with each other. For example, PKCθ phosphorylates RasGRP1 and PKD1 to enhance their activation.80 Thus, by recruiting PKCθ, RasGRP1, and PKD1, DAG controls signaling via a number of interconnected pathways in response to TCR engagement.
DAG-mediated activation of the Ras-ERK1/2 and NFκB pathways also indirectly activates signaling via the PI3K-mTOR pathway. The mTOR pathway and its tight regulation play an important role in T cell development, homeostasis, activation, and differentiation,81–88 while PI3K isoforms function redundantly to promote T cell development, activation, survival, and self-tolerance.89, 90 Studies in cell lines have shown that active Ras can bind to and activate PI3K,91, 92 which catalyzes the conversion of PIP2 to PIP3. While it was known that PI3K activity can recruit the kinases PDK1 and Akt to activate the Akt-mTOR complex 1 pathway,93 recent studies using primary mouse thymocytes and T cells have provided direct evidence that DAG-mediated activation of the Ras-MEK1/2-ERK1/2 pathway can also initiate signaling through mTOR complex 1 and mTOR complex 2.94,95
Given the nature and number of signaling pathways activated by DAG, it stands to reason that DAG levels must be tightly regulated to prevent T cell hyper-activation. This hypothesis is supported by findings that dysregulation of individual DAG-mediated signaling pathways can have profound effects on T cell function and iNKT cell development.71,96, 97 DGKs play a critical role in regulating intracellular DAG levels, removing DAG through phosphorylation to produce PA. DGKα and DGKζ are the predominant isoforms expressed in T cells, as mentioned previously. Two splice variants of DGKζ, DGKζ1 (130 kDa) and DGKζ2 (115 kDa), are expressed in thymocytes and matureT cells.19 Splicing of the first coding exon directly to the third generates the smaller splice variant, while transcription from an alternative promoter at the second exon and subsequent splicing to the third generates the longer variant.26,98 Interestingly, the splicing isoforms are expressed in a complementary fashion, with higher expression of DGKζ1 in CD4− CD8− and CD4+ CD8+ thymocytes, and higher expression of DGKζ2 in mature (CD4SP and CD8SP) thymocytes and peripheral T cells. The mechanisms that control the differential expression of DGKζ1/ζ2 during T cell development are currently unclear, as are the functional differences between these two isoforms. Experiments with deletion mutants in Jurkat cells showed that the N-terminal end of DGKζ, but not the C-terminal end, is essential for optimal inhibition of TCR signaling.19 Other studies have shown that DGKα is expressed in thymocytes and peripheral T cells,34, 99,100 but its expression levels at different stages of T cell development remain to be examined. As discussed below, synergistic regulation of DAG signaling by DGKα and DGKζ is essential for normal T cell development and function.
III. ROLE OF DGKα AND DGKζ IN T CELL DEVELOPMENT
Lymphoid progenitor cells generated in the bone marrow migrate to the thymus, where they travel through the cortex and medulla, developing into mature T cells.101, 102 Successive developmental stages of a thymocyte can be distinguished by the combination of CD4 and CD8 co-receptors expressed on its surface. Early committed T cells do not express TCR, CD4, or CD8 on the cell surface and are called CD4− CD8− double-negative (DN) cells. DN cells rearrange V, D, and J gene segments at the TCRβ locus, leading to the expression of a pre-TCR. Cells that express a functional pre-TCR pass through the so-called “β-selection” checkpoint, while others undergo apoptosis. The β-selected DN cells undergo several rounds of proliferation, maturing into CD4+ CD8+ double-positive (DP) cells that constitute about 90 percent of all thymocytes. DP cells rearrange V and J gene segments at the TCRa locus, leading to the expression of a unique TCR on the cell surface.
Following TCR expression, DP thymocytes are subjected to processes called positive and negative selection,103,104 that ensure the generation of a functional, non self-reactive T cell repertoire. In order to be positively selected, DP cells must express a TCR that is able to recognize self-peptide-MHC complexes expressed by thymic epithelial cells or bone-marrow-derived dendritic cells in the thymus. In general, DP cells with TCRs that fail to recognize self-peptide-MHC complexes are eliminated at this stage, as they fail to receive survival signals. On the other hand, DP cells with TCRs that recognize self-peptide-MHC with high affinity also undergo apoptosis, a process referred to as negative selection. Thus, only DP cells with TCRs that recognize self-peptide-MHC molecules with low affinity survive positive and negative selection, developing further into mature CD4+ CD8− single positive (CD4SP) or CD4− CD8+ single positive (CD8SP) cells.
Several signaling pathways, including MAP kinase, NF-κB, and NFAT pathways, are known to play critical roles in thymocyte selection. PLCγ1 deficiency in thymocytes impairs both positive and negative selection processes, suggesting a potential role for DAG-mediated signals in T cell development.105 Numerous studies have shown that defects in DAG-effector pathways profoundly impact thymocyte development, lending further credence to this notion. For instance, thymocytes deficient in RasGRP1 display severely impaired positive selection, with a marked paucity of mature single positive cells106 Expression of a dominant negative form of Ras or MEK1 inhibits positive but not negative selection,107, 108 leading to a block in thymocyte development at the DP stage. While thymocytes lacking ERK1 experience a partial developmental block at the DP stage with a concomitant reduction of mature thymocytes,109 combined deficiency of ERK1 and ERK2 has been shown to impair positive but not negative selection.110,111 The Ras-MEK1/2-ERK1/2 pathway is thus thought to play a critical role in the positive selection of thymocytes. On the other hand, signaling via the p38 and JNK MAP kinase pathways is thought to play an essential role in negative selection.112 Though deficiency of PKCθ or IKKβ does not appear to affect conventional αβ T cell maturation,70, 113 a recent study has revealed a differential role for NF-κB in the selection and survival of CD4 and CD8 thymocytes.114 Moreover, PKCθ-mediated signaling is pivotal for natural regulatory T cell and iNKT cell development.115 The importance of DAG-triggered Ras-ERK and PKCθ-NF-κB pathways in thymocyte selection processes thus suggests that tight regulation of DAG levels by DGKs may be critical for normal T cell development.
Previous studies have shown that signaling via the pre-TCR increases DGKα expression in thymocytes.116 Although pharmacological inhibition of DGKα activity (with the inhibitor R59949) suggested that DGKα could promote DP thymocyte survival via a Bcl-xL mediated pathway,116 other studies have revealed that genetic deficiency of either DGKα or ζ does not obviously alter thymocyte populations.117,118 Additional studies should determine whether this type-I DGKα inhibitor may possess off-target activities that are yet to be identified or if other type-I DGKs are expressed in developing thymocytes that may compensate for DGKα deficiency. More recent work from our group has provided genetic evidence that DGKα and DGKζ synergistically regulate T cell development.119 Combined deficiency of the DGKα and ζ isoforms led to a severe block in murine thymocyte development at the DP stage, with a dramatic reduction in the number of mature CD4SP and CD8SP cells. Crossing with HY TCR transgenic mice revealed that combined DGKαζ deficiency was associated with impaired positive selection, but not negative selection. Reduced DGK activity in the DGKαζ double knockout (DKO) thymocytes was associated with increased levels of intracellular DAG after TCR stimulation, and enhanced signaling via the Ras-ERK pathway. However, the developmental blockade was partially overcome by PA treatment, suggesting that DGKs play a critical role in thymocyte development not only by terminating DAG-mediated signaling but also by initiating PA-mediated signals. How PA promotes T cell maturation is a critical question that remains to be addressed.
A novel role for DGKs in regulating mTOR activity in thymocytes has also emerged recently.94 Upon TCR stimulation, DGKαζ DKO thymocytes showed elevated levels of S6K1 and 4E-BP1 phosphorylation, suggestive of increased mTOR complex 1 activity. This was correlated with increased ERK1/2 and Rsk1 activation. Phosphorylation of Akt at S473 was also increased in DKO thymocytes, indicating enhanced mTOR complex 2 activity. Inhibition of MEK1/2 dramatically reduced TCR-induced mTOR activation in both wild-type (WT) and DGKαζ DKO thymocytes, indicating that DGK activity inhibits TCR-induced mTOR activation by attenuating signaling via the RasGRP1-Ras-ERK1/2 pathway. In non-T cell lines, ERK1/2 phosphorylate and inactivate TSC2, a negative regulator of mTOR complex 1 activation, to promote signaling via mTOR complex 1.120 Whether the RasGRP1-Ras-ERK1/2 pathway activates mTOR complex 1 signaling via similar mechanisms in T cells needs to be confirmed. In addition, the mechanisms by which DGK (α and ζ) activity inhibits and RasGRP1-Ras-ERK1/2 signaling promotes mTORC2 signaling remain to be explored.
IV. ROLE OF DGKα AND DGKζ IN iNKT CELL DEVELOPMENT
Natural killer T (NKT) cells are a rare subset of lymphocytes that express both NK family receptors (such as NK1.1 in mice) and a semi-invariant TCR.121–123 Unlike conventional αβ T cells, the TCR on NKT cells recognizes glycolipids presented on MHC-like CD1d molecules. Capable of producing an array of cytokines within minutes to hours of stimulation, NKT cells have been shown to modulate several important immune phenomena including responses to infection and cancer, allergy, and autoimmunity. A majority of NKT cells in humans and mice are characterized by their unique usage of TCR Vα24 (human) or Vα14 (murine) and Jα18 segments and a limited TCR Vβ repertoire. Such NKT cells are called type I or invariant NKT (iNKT) cells. Developing iNKT cells are classified into successive developmental stages from 0 to 3, based on the surface expression of CD24, NK1.1, and CD44124 Invariant NKT cell development and function remain actively investigated.
In the thymus, DP cells that express appropriate TCRs to enter the NKT lineage are thought to undergo selection processes analogous to those of conventional αβ T cells, which require signaling from the iVα14TCR.125, 126 However, unlike conventional αβ thymocytes that are selected on thymic epithelial cells, NKT thymocytes are selected on fellow CD1d-expressing DP thymocytes.127–129 Results from previous studies have also suggested that developing iNKT cells may differ from αβ thymocytes in certain signaling requirements for proper development. For example, a SLAM/SAP/Fyn/PKCθsignaling pathway is critical for iNKT cell ontogeny but exerts minimal impact on conventional αβT cell development.130–134 Several recent reports have demonstrated that DAG-mediated signaling and its proper regulation are pivotal for normal iNKT cell development and homeostasis. Absence of RasGRP1 or expression of a dominant negative Ras impairs iNKT cell development at the earliest stage.97,135 In contrast, hyper-activation of this pathway by the expression of constitutively active K-Ras in thymocytes causes defective iNKT cell terminal maturation, correlating with decreased T-bet expression.71 Similarly, absence of PKCθ impairs iNKT cell development and overactive IKKβ severely reduces iNKT cell numbers.69, 71 Recent studies have also provided genetic evidence that individual deficiency of DGKα or DGKζ does not significantly affect iNKT development. However, simultaneous deficiency of both isoforms led to a severe iNKT cell-intrinsic developmental blockade/homeostasis defect and a concomitant paucity of iNKT cells in the thymus, spleen and liver. In DGKαζDKO thymocytes, both Ras-ERK1/2 and PKCθ-IKK signaling are elevated. These observations have not only revealed the importance of DAG-effector signaling pathways in iNKT cell development but also elucidated the requirement of DGKαζ activity for normal iNKT cell ontogeny via tight control of these pathways. It remains unclear whether DGKα and ζ promote iNKT cell development solely by terminating DAG signaling or also by initiating PA-mediated signaling. Further studies are required to determine how dysregulation of DAG-mediated signaling might affect iNKT cell function. The generation of mice that allow for conditional deletion of DGKα or ζ isoforms is likely to prove instrumental in defining the role of DAG-mediated signaling in mature iNKT cell homeostasis and function.
V. ROLE OF DGKα AND DGKζ IN T CELL FUNCTION
A. DGK activity in T cell activation and anergy
Mice deficient in DGKζ have slightly fewer T cells in the periphery than WT counterparts.118 DGK ζ−/− T cells show selective perturbations in DAG-mediated signaling including enhanced Ras-ERK activation and reduced PA production upon TCR stimulation. However, DAG-independent events including TCR-induced calcium mobilization remain unaffected. Upon TCR cross-linking with anti-CD3 antibodies, a greater proportion of DGKζ−/− T cells upregulate surface markers of activation, such as CD69 and CD25, as compared to DGKζ-sufficient counterparts. In addition, T cells deficient in DGKζ proliferate more readily and rapidly than WT T cells upon ex vivo stimulation with anti-CD3 or transfer to lymphopenic hosts. Thus, deficiency of DGKζ enhances T cell activation and proliferation.
T cell numbers in the spleens and lymph nodes of DGKα−/− mice are comparable to those of WT littermates.117 DGKα−/− T cells resemble DGKζ−/− counterparts in showing enhanced activation of the Ras-ERK pathway and increased proliferation in response to TCR stimulation. However, unlike DGKζ−/− T cells, DGKα−/− T cells show normal PA production upon TCR stimulation, suggesting that these isoforms may somehow differ in activity or substrate specificity. Taken together, studies with DGKα−/− and DGKζ−/− mice establish important and non-redundant roles for these isoforms in regulating T cell activation and proliferation in response to TCR stimulation.
Proper immune function is critically dependent on the ability of the immune system to distinguish between self and non-self antigens. While mounting effective immune responses to foreign pathogens is important for host defense, retaining tolerance to self-antigens is necessary to prevent autoimmunity. Rendering auto-reactive T cells functionally inactive (a state termed anergy) is an important means of generating peripheral tolerance.136, 137 Anergized T cells are refractory to subsequent stimulation and fail to proliferate or produce IL-2, even in the presence of co-stimulation. E3 ubiquitin ligases such as Cbl-b, Itch and GRAIL are upregulated in response to anergizing stimuli, and act as anergy effectors by mechanisms that include preventing PI3K recruitment by CD28 and promoting lysosomal trafficking of endocytosed signaling molecules.138–142
In keeping with the two-signal model,143 binding of TCR to cognate peptide-MHC must be accompanied by co-stimulation (for instance via the CD28 receptor) to fully trigger all TCR-coupled signaling pathways and result in T cell activation. In the absence of co-stimulation, TCR engagement selectively activates the Ca2+/calcineurin/NFAT pathway (downstream of IP3) to trigger the transcription of anergy-inducing genes.144, 145 Treatment of T cells with the Ca2+ ionophore ionomycin is sufficient to induce anergy. Given these observations and the equimolar production of DAG and IP3 following TCR engagement, it stands to reason that DGKs may play a role in anergy induction by selectively dampening DAG-mediated signals in the absence of co-stimulation.
Studies have revealed a critical role for DGK isoforms, particularly DGKα, in the induction and enforcement of T cell anergy. In primary T cells, both DGKα and ζ are expressed at higher levels in the anergic state than in the activated state.117 Similarly, anergic CD4 (TH1 clone) cells express five-fold to ten-fold more DGKα and two-fold more DGKζ than control CD4 cells 100 Overexpression of DGKα in TH1 cells resulted in an anergy-like state, characterized by suppressed Ras-ERK activation and reduced IL-2 transcription in response to stimulation with anti-CD3 and anti-CD28. DGKα overexpression also produced an anergy-like state in 2C TCR transgenic CD8 cells, as seen by impaired recruitment of RasGRP1 to the plasma membrane. Pharmacological inhibition of DGK activity led to a dose-dependent recovery of IL-2 production by anergic TH1 cells ex vivo, and anergic 2C cells in vivo. In an in vivo model of anergy induction with staphylococcal enterotoxin B (SEB), T cells from DGKα;−/− mice (in contrast to WT counterparts) were resistant to the induction of anergy and retained the ability to produce IL-2 and proliferate when re-stimulated with SEB ex vivo, providing direct genetic evidence of the role of DGKα in enforcing T cell anergy.117 When CD8-depleted splenocytes were stimulated under anergy-inducing conditions (anti-CD3 and CTLA4-Ig) ex vivo, very few surviving WT cells divided in 48 hours. In contrast, DGKα−/− and DGKζ−/− T cells were relatively resistant to anergy induction and underwent two to three rounds of cell division. When DGKζ−/− cells were stimulated in a similar fashion, but in the presence of a DGKα inhibitor, they showed growth and division comparable to WT cells receiving anti-CD3 and anti-CD28 stimulation. Taken together, results from these studies reveal a key role for DGKs in regulating whether a T cell gets activated or anergized in response to signals via the TCR. They also lend credence to a model of T cell anergy in which DGKα and DGKζ (both of which are expressed at high levels in naïve T cells and down-regulated upon productive activation) selectively dampen DAG-mediated signals in the absence of co-stimulation to promote the induction and enforcement of anergy.
B. DGK localization and regulation at the immune synapse
The immunological synapse is an interface formed between a T cell and an antigen-presenting cell by membrane apposition when a TCR on the former recognizes a cognate peptide-MHC complex on the latter.146 Previous studies using Jurkat and other cell lines have demonstrated the accumulation of DAG at the immunological synapse,147 and the translocation of DGKα and DGKζ to the cell membrane upon TCR crosslinking.18,148 A recent study has revealed a critical role for DGKζ in regulating DAG metabolism at the immune synapse.149 In this study, examination of TCR complexes isolated from Jurkat cells directly demonstrated the recruitment of endogenous DGKα and DGKζ isoforms to TCR engagement. RNA interference experiments revealed complexes upon TCR and CD28 a critical role for DGKζ, but not DGKα, in PA production in these complexes. The use of GFP fusion proteins also showed rapid translocation of DGKζ, but not DGKα, to the cell membrane at early stages of immunological synapse formation. Future studies are required to dissect the relative contributions of DAG-binding and protein-protein interactions towards the recruitment of DGKs to the immune synapse. The functional consequences of DGK recruitment to the synapse also remain to be determined.
Experiments with HeLa cell lines have shown that DGKη may act as an adaptor protein during EGF-mediated ERK1/2 activation,150 raising the possibility that DGK isoforms may serve in a similar capacity in T cells. A proteomics-based approach revealed that sorting nexin 27 (SNX27), a PDZ-domain containing protein that participates in vesicular and protein trafficking, could interact with DGKζ in a PDZ-dependent manner.151 While more recent studies have suggested that SNX27 localizes to the immune synapse after TCR engagement, results from co-localization experiments with tagged SNX27 and DGKζ overexpression argue against a role for DGKζ in recruiting SNX27 to the immune synapse.152 Further studies are needed to thoroughly examine a possible role for DGKα andζ isoforms as scaffolding proteins at the T cell synapse.
Emerging evidence points to the existence of multiple positive and negative regulators of DGK activity. Lck-dependent phosphorylation at Y335 was recently shown to be critical for membrane association and enzymatic function of DGKα, in studies with Jurkat cell lines.153 The Y335 residue is located at a hinge region between the catalytic domain and C1 domains of DGKα. Results from cell fractionation experiments indicated that Y335-phosphorylated DGKα localized specifically to membranes. Unlike its WT counterpart, the Y335F mutant form failed to translocate to the cell membrane in response to TCR stimulation, when transfected into Jurkat cells. In addition, while expression of WT DGKα in HEK293 cells reduced ERK phosphorylation in response to PMA stimulation, expression of the Y335F mutant did not. Together, these findings suggest an important role for Lck-mediated phosphorylation of DGKα at Y335 in membrane translocation and function of the enzyme.
In addition to the Y335 residue, Y218 on DGKα can be phosphorylated by tyrosine kinase c-Abl in NIH 3T3 cells following serum stimulation. Y218 phosphorylation contributes to the spatio-temporal regulation of DGKα in NIH 3T3 cell lines.154 Results from this study showed that GFP-tagged DGKα moves from the cytoplasm to the nucleus in response to serum starvation, and in the opposite direction in response to serum restoration. Knockdown of c-Abl impaired DGKα export from the nucleus after serum restoration, and Y218 on DGKα was identified as the site of c-Abl mediated phosphorylation. At present, it is unclear whether Y218 is similarly phosphorylated in T cells and what the functional significance of such phosphorylation in T cells might be. Similar to DGKα, the distribution of DGKζ between the nucleus and cytoplasm is regulated by phosphorylation events. Studies with COS-7 cells have shown that PKCα or PKCγ can phosphorylate DGKζ, and that this phosphorylation promotes the nuclear export of DGKζ.23
Other work has demonstrated a positive effect of DAG itself (and its analog PMA) on DGKζ activity in Jurkat cells,149 suggesting the existence of a feedback loop by which DAG can activate DGKζ to promote its own consumption. On the other hand, recent studies have implicated the adaptor SLAM-associated protein (SAP) as a negative regulator of DGKα activity during T cell activation.155 SAP is essential for SLAM-mediated signaling, and mutations in SAP are associated with X-linked lympho-proliferative disease (XLP) in humans.156 Experiments using primary blood lymphocytes and Jurkat cell lines demonstrated a loss of DGKα activity (without changes in its protein levels), following stimulation via the TCR and CD28/SLAM. Inhibition of DGKα activity was dependent on SAP expression, and overexpression of SAP was sufficient to impair DGKα activity. SAP-dependent blunting of DGK activity was isoform-specific, and not seen with DGKζ. In addition, pharmacological inhibition or siRNA knockdown of DGKα activity was able to rescue TCR-mediated signaling in SAP-deficient Jurkat cells and T cells from XLP patients.
C. DGK activity in CD8 cell function
When CD8 cells recognize a cognate antigen in an appropriate milieu of co-stimulatory molecules and cytokines, the ensuing immune response consists of three distinct phases. First, the CD8 cells undergo exponential clonal expansion, reaching peak numbers at around seven days after infection. Once the infection is cleared, a majority of the CD8 cells undergo apoptosis in the contraction phase, leaving behind a small pool of memory cells in the maintenance phase.157,158 In an early study, DGKζ−/− mice showed a greater increase in CD8+ splenocyte numbers upon infection with lymphocytic choriomeningitis virus (LCMV), as compared to WT counterparts.118 In addition, a higher percentage of CD8 cells in DGKζ−/− mice showed an activated phenotype, as evidenced by up-regulation of CD44 and down-regulation of CD62L markers on the cell surface. A recent study investigated in further detail the effect of DGK deficiency on CD8 T cell responses to LCMV.159 DGKα−/− and DGKζ−/− mice showed increased CD8 T cell expansion upon infection with LCMV, and more DGK-deficient CD8 cells produced IFNγ than WT counterparts. These changes were determined to be CD8 cell intrinsic in DGKζ−/−, but not DGKα−/− mice, by adoptive transfer experiments. Fewer memory cells were generated/maintained in the absence of either DGK isoform. When equal numbers of WT or DGK-deficient LCMV-specific CD8 memory cells were transferred into WT recipients and re-challenged with LCMV, DGK-deficient memory cells showed impaired expansion but normal cytokine production. Of note, impaired recall response of DGK-deficient memory T cells is correlated with increased S6 phosphorylation, an event that is usually dependent on mTOR activity. Since mTOR signaling promotes primary but inhibits memory CD8 T cell responses, it would be interesting to determine if DGK activity controls CD8 T cell responses in part via modulating mTOR signaling. Taken together, studies with the LCMV model have revealed that DGK activity may differentially regulate primary and memory CD8 immune responses.
Apart from their role in responding to pathogens, CD8 cells play a critical role in defending against tumors.160 Recent experiments have shown that DGKζ−/− mice develop smaller tumors than WT mice upon implantation with EL4 lymphoma cells expressing ovalbumin.161 An increased proportion of CD44hi CD62Llo “effector memory” type CD8 cells was found in the spleens of DGKζ−/− mice, and a greater proportion of tumor-infiltrating CD8 cells was proliferating (as shown by Ki-67 staining) in DGKζ−/− mice than WT counterparts. Adoptive transfer of congenically marked WT OT1 or DGKζ−/− OT1 cells into WT mice that were subsequently injected with EL4-Ova cells produced similar results, arguing for a CD8 cell-intrinsic role of DGKζ deficiency in enhancing anti-tumor responses. While WT and DGKζ−/− CD8 cells lysed target cells comparably ex vivo, DGKζ−/− cells showed enhanced IL-2 production and proliferation.
A higher expression of DGKα was found in tumor-infiltrating CD8 cells from renal cell carcinoma patients, as compared to non-tumor kidney-infiltrating cells, in another recently published study.162 While the tumor-infiltrating cells showed normal TCR proximal signaling, distal events such as phosphorylation of ERK, JNK, Akt, and IκB were impaired. No such defects were observed in CD8 cells residing outside tumors. The signaling defects in tumor-infiltrating cells also correlated with functional impairment in lytic activity and cytokine production. Treatment of tumor-infiltrating CD8 cells with a DGK inhibitor or with low-dose IL-2 was found to enhance ERK phosphorylation and lytic granule exocytosis, suggesting that enhancement of DGK expression/activity may be a possible mechanism by which infiltrating T cells are rendered less potent by the tumor micro-environment. Interestingly, a recent study has found that FoxO1 and FoxO3 can bind to the DGKα promoter to activate its transcription.163 It is known that FoxO proteins are sequestered in the cytosol after Akt-mediated phosphorylation,164 and that ERK1/2 can promote PI3K/Akt signaling in T cells.94 Decreased ERK1/2 and Akt activity in the tumor-infiltrating CD8 T cells may therefore cause enhanced FoxO function and DGKα transcription.
Taken together, the findings from these studies argue that restraining DGK activity in T cells may prove valuable in generating more vigorous immune responses against pathogens and tumors. However, decreased DGK activity was found to promote thymic lymphomagenesis in mice bearing the HY transgenic TCR, suggesting that the development of therapeutic solutions involving DGK inhibition may not be entirely straightforward.119 Perhaps the increased incidence of thymic lymphomas in these mice should not be surprising, considering that the highly oncogenic Ras-ERK1/2 and PI3K pathways are hyper-activated by TCR stimulation in the presence of reduced DGK activity. Future work should attempt to delineate strategies that manipulate DGK activity to enhance CD8 cell function while minimizing the risk of triggering oncogenesis.
D. DGK activity in directional secretion and T cell adhesion
Single-cell photo-activation experiments have recently revealed that polarization of the T cell microtubule-organizing center (MTOC) toward the immune synapse (with an antigen-presenting cell or target cell) is driven by localized DAG accumulation in the cell membrane.165 Polarization of the MTOC is thought to play an important role in the directional secretion of cytokines, cytolytic molecules, and other soluble factors by T cells.166 While previous studies have shown that the MTOC aligns itself with the immune synapse within minutes of TCR stimulation, the exact mechanisms linking TCR stimulation to MTOC re-alignment were previously unknown. Recent work has demonstrated that DAG-mediated recruitment of three distinct PKC isoforms (θ, ε, and η) to the immune synapse promotes MTOC reorientation.167 MTOC polarization was blocked by PLCγ inhibition (but not by Ca2+ blockade), suggesting that DAG may play a critical role in this process. Photo-activation in the presence of a DGK inhibitor was associated with failure to establish a stable DAG gradient (as reported by C1 domain-GFP fusion proteins) and defective MTOC polarization. MTOC recruitment toward the synapse was spatially correlated with and temporally preceded by DAG accumulation. Experiments with a photo-activated form of DAG also showed that localized DAG signaling was sufficient to drive transient MTOC polarization. Treatment with agents such as PMA and DGK inhibitors that perturb MTOC polarization impaired the ability of cytotoxic T cells to kill target cells. Taken together, these observations suggest the hypothesis that DGK isoforms may play a critical role in T cell function by regulating MTOC-directing DAG gradients.
In addition to secreting cytokines and other soluble effectors, studies have shown that T cells can induce apoptosis of target cells by secreting exosomes that bear membrane-bound FasL.168 A possible role for DGK activity in negatively regulating the secretion of these exosomes was revealed when inhibition of DGKα activity in human primary T cell blasts was shown to increase the secretion of FasL-bearing exosomes and subsequent activation induced cell death.169 However, the mechanisms by which DGKα inhibits exosome secretion have remained unclear. Results from a recent study suggest that DGKα may inhibit the formation of FasL-bearing exosomes and multi-vesicular bodies, but aid in their polarization towards the immune synapse, in T cells.170 Multi-vesicular bodies (MVBs) are late endosomes that contain smaller vesicles inside their lumen. In this study, the activation of T cell lines was found to increase the formation of FasL-containing MVBs, and pharmacological inhibition of DGKα activity increased the number of mature MVBs. In addition, siRNA mediated inhibition of DGKα expression hindered the polarization of MVBs towards the immune synapse. Taken together, these results suggest that DGKα may play a role in regulating both the formation and polarization of FasL-bearing exosomes and vesicles in T cells.
Interaction of T cells with vascular endothelial cells followed by T cell arrest in the microvasculature is an essential step in the process of lymphocyte extravasation. A recent study has identified DGKζ as a critical negative regulator of CXCR4-stimulated T cell firm arrest on surfaces presenting ICAM1 under conditions of shear flow.171 Binding of CXCR4 to its ligand CXCL12 on the microvasculature converts integrin LFA1 on the vasculature to its active form, in a process called inside-out signaling. The active form of LFA1 can then be bound by ICAM1 on the T cell surface, resulting in firm arrest even in the presence of shear forces. Since DAG-mediated signals play an important role in inside-out activation of LFA1, the authors hypothesized that deficiency of DGKζ would lead to enhanced activation of LFA1 and increased cell arrest under shear flow. Results from flow chamber experiments showed that DGKζ deficiency in T cells indeed increased firm arrest to ICAM1-coated surfaces and shortened the time to stop without affecting the rolling velocity.
VI. SUMMARY
Recent studies have revealed a host of new functions for DGK isoforms in T cell development and function (Figure 3). Apart from their role in the development of conventional αβ T cells, newer work has unveiled a previously unappreciated requirement for synergistic DGKα and ζ activity during invariant NKT cell development. Several modulators of DGK activity, including Lck, SAP, and c-Abl, have been identified. In addition, the importance of DGK activity in promoting MTOC polarization and directional secretion, and in restraining CD8 T cell responses against LCMV infection and tumors, has come to the fore. However, a number of fundamental questions about DGKs remain unanswered. Transcriptional control and regulation of DGK activity via post-translational modifications and protein-protein interactions during T cell development and immune responses are poorly understood. The spatial and temporal regulation of DAG by individual DGK isoforms in T cells remains to be defined. The importance of DGK-generated PA and the downstream effector pathways controlled by PA in T cells need to be explored. A better understanding of the role and regulation of DGK activity and DAG signaling in T cells can enable us to modulate immune responses, producing better outcomes during vaccination, tumor responses, and autoimmunity.
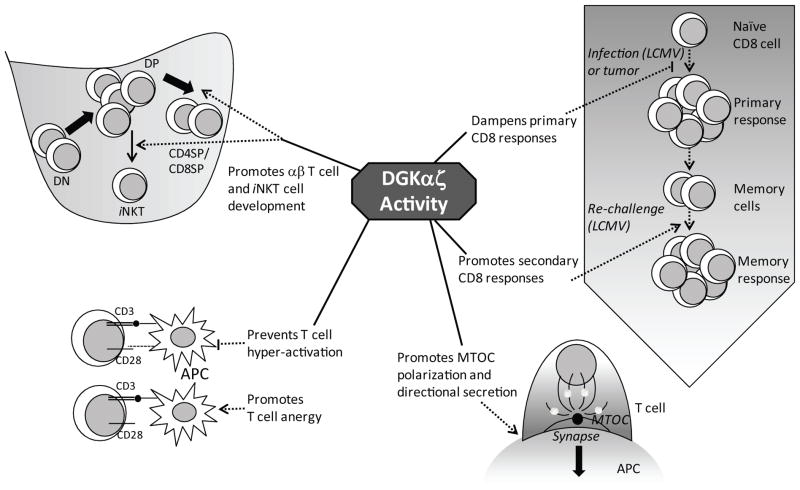
Figure 3. DGKs in T cell development and function.
Schematic summary of the numerous roles played by DGK α and ζ in T cell development and function. In the thymus, these two DGK isoforms synergistically promote the development of conventional αβT cells and invariant NKT cells. DGK activity in mature peripheral T cells prevents their hyper-activation upon TCR engagement in the presence of co-stimulatory signals. On the other hand, DGK isoforms are highly expressed in anergic T cells and studies have revealed a critical role for DGK isoforms, particularly DGKα, in promoting T cell anergy. In CD8 cells, DGK α and ζ serve to dampen primary responses against tumor antigens and viral infection (LCMV), while promoting memory responses in the LCMV model. DGKs also play a role in establishing a stable DAG gradient that enables T cells to directionally secrete cytolytic granules and other soluble factors across the immunological synapse.
Acknowledgments
This work is supported by the National Institutes of Health (AI076357, AI079088, and AI101206) and the American Cancer Society (RSG-08–186–01-LIB).
LIST OF ABBREVIATIONS
- CD4SP
CD4 single positive
- CD8SP
CD8 single positive
- DAG
diacylglycerol
- DKO
double knock out
- DGK
diacylglycerol kinase
- DN
CD4 CD8 double negative
- DP
CD4 CD8 double positive
- iNKT
invariant NKT cell
- LCMV
lymphocytic choriomeningitis virus
- MTOC
microtubule organizing center
- MVB
multi-vesicular body
- PA
phosphatidic acid
- TCR
T cell receptor
Footnotes
The authors declare no conflict of interest.
This article has not been published elsewhere and has not been submitted simultaneously for publication elsewhere.
Contributor Information
Sruti Krishna, Email: ss195@duke.edu, Department of Pediatrics-Allergy and Immunology and Department of Immunology, Duke University Medical Center.
Xiao-Ping Zhong, Email: zhong001@mc.duke.edu, Department of Pediatrics-Allergy and Immunology, Rm. 133 MSRB-I, Research Drive, Box 2644, Duke University Medical Center, Durham, NC 27710. Phone: 919-681-9450; Fax: 919-668-3750.
References
- 1.Wattenberg BW, Raben DM. Diacylglycerol kinases put the brakes on immune function. Science’s STKE: signal transduction knowledge environment. 2007 Aug 25;2007(398):pe43. doi: 10.1126/stke.3982007pe43. [DOI] [PubMed] [Google Scholar]
- 2.Zhong XP, Guo R, Zhou H, Liu C, Wan CK. Diacylglycerol kinases in immune cell function and self-tolerance. Immunol Rev. 2008 Aug;224:249–64. doi: 10.1111/j.1600-065X.2008.00647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhong XP, Shin J, Gorentla BK, O’Brien T, Srivatsan S, Xu L, Chen Y, Xie D, Pan H. Receptor signaling in immune cell development and function. Immunol Res. 2011 Apr;49(1–3):109–23. doi: 10.1007/s12026-010-8175-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rincon E, Gharbi SI, Santos-Mendoza T, Merida I. Diacylglycerol kinase zeta: at the crossroads of lipid signaling and protein complex organization. Prog Lipid Res. 2012 Jan;51(1):1–10. doi: 10.1016/j.plipres.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 5.Huang YH, Sauer K. Lipid signaling in T-cell development and function. Cold Spring Harbor perspectives in biology. 2010 Nov;2(11):a002428. doi: 10.1101/cshperspect.a002428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang X, Devaiah SP, Zhang W, Welti R. Signaling functions of phosphatidic acid. Prog Lipid Res. 2006 May;45(3):250–78. doi: 10.1016/j.plipres.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 7.Stace CL, Ktistakis NT. Phosphatidic acid- and phosphatidylserine-binding proteins. Biochim Biophys Acta. 2006 Aug;1761(8):913–26. doi: 10.1016/j.bbalip.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 8.Cai J, Abramovici H, Gee SH, Topham MK. Diacylglycerol kinases as sources of phosphatidic acid. Biochim Biophys Acta. 2009 Sep;1791(9):942–8. doi: 10.1016/j.bbalip.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roose JP, Mollenauer M, Gupta VA, Stone J, Weiss A. A diacylglycerol-protein kinase C-RasGRP1 pathway directs Ras activation upon antigen receptor stimulation of T cells. Mol Cell Biol. 2005 Jun;25(11):4426–41. doi: 10.1128/MCB.25.11.4426-4441.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carrasco S, Merida I. Diacylglycerol-dependent binding recruits PKCtheta and RasGRP1 C1 domains to specific subcellular localizations in living T lymphocytes. Molecular biology of the cell. 2004 Jun;15(6):2932–42. doi: 10.1091/mbc.E03-11-0844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fang Y, Vilella-Bach M, Bachmann R, Flanigan A, Chen J. Phosphatidic acid-mediated mitogenic activation of mTOR signaling. Science. 2001 Nov 30;294(5548):1942–5. doi: 10.1126/science.1066015. [DOI] [PubMed] [Google Scholar]
- 12.Frank C, Keilhack H, Opitz F, Zschornig O, Bohmer FD. Binding of phosphatidic acid to the protein-tyrosine phosphatase SHP-1 as a basis for activity modulation. Biochemistry. 1999 Sep 14;38(37):11993–2002. doi: 10.1021/bi982586w. [DOI] [PubMed] [Google Scholar]
- 13.Tsai MH, Roudebush M, Dobrowolski S, Yu CL, Gibbs JB, Stacey DW. Ras GTPase-activating protein physically associates with mitogenically active phospholipids. Mol Cell Biol. 1991 May;11(5):2785–93. doi: 10.1128/mcb.11.5.2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao C, Du G, Skowronek K, Frohman MA, Bar-Sagi D. Phospholipase D2-generated phosphatidic acid couples EGFR stimulation to Ras activation by Sos. Nat Cell Biol. 2007 Jun;9(6):706–12. doi: 10.1038/ncb1594. [DOI] [PubMed] [Google Scholar]
- 15.Waite KA, Wallin R, Qualliotine-Mann D, McPhail LC. Phosphatidic acid-mediated phosphorylation of the NADPH oxidase component p47-phox. Evidence that phosphatidic acid may activate a novel protein kinase. J Biol Chem. 1997 Jun 13;272(24):15569–78. doi: 10.1074/jbc.272.24.15569. [DOI] [PubMed] [Google Scholar]
- 16.Shin JJ, Loewen CJ. Putting the pH into phosphatidic acid signaling. BMC Biol. 2011;9:85. doi: 10.1186/1741-7007-9-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karathanassis D, Stahelin RV, Bravo J, Perisic O, Pacold CM, Cho W, Williams RL. Binding of the PX domain of p47(phox) to phosphatidylinositol 3,4-bisphosphate and phosphatidic acid is masked by an intramolecular interaction. The EMBO journal. 2002 Oct 1;21(19):5057–68. doi: 10.1093/emboj/cdf519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sanjuan MA, Jones DR, Izquierdo M, Merida I. Role of diacylglycerol kinase alpha in the attenuation of receptor signaling. The Journal of cell biology. 2001 Apr 2;153(1):207–20. doi: 10.1083/jcb.153.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhong XP, Hainey EA, Olenchock BA, Zhao H, Topham MK, Koretzky GA. Regulation of T cell receptor-induced activation of the Ras-ERK pathway by diacylglycerol kinase zeta. The Journal of biological chemistry. 2002 Aug 23;277(34):31089–98. doi: 10.1074/jbc.M203818200. [DOI] [PubMed] [Google Scholar]
- 20.Jiang Y, Qian W, Hawes JW, Walsh JP. A domain with homology to neuronal calcium sensors is required for calcium-dependent activation of diacylglycerol kinase alpha. The Journal of biological chemistry. 2000 Nov 3;275(44):34092–9. doi: 10.1074/jbc.M004914200. [DOI] [PubMed] [Google Scholar]
- 21.Luo B, Prescott SM, Topham MK. Association of diacylglycerol kinase zeta with protein kinase C alpha: spatial regulation of diacylglycerol signaling. The Journal of cell biology. 2003 Mar 17;160(6):929–37. doi: 10.1083/jcb.200208120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luo B, Prescott SM, Topham MK. Protein kinase C alpha phosphorylates and negatively regulates diacylglycerol kinase zeta. The Journal of biological chemistry. 2003 Oct 10;278(41):39542–7. doi: 10.1074/jbc.M307153200. [DOI] [PubMed] [Google Scholar]
- 23.Topham MK, Bunting M, Zimmerman GA, McIntyre TM, Blackshear PJ, Prescott SM. Protein kinase C regulates the nuclear localization of diacylglycerol kinase-zeta. Nature. 1998 Aug 13;394(6694):697–700. doi: 10.1038/29337. [DOI] [PubMed] [Google Scholar]
- 24.Hogan A, Shepherd L, Chabot J, Quenneville S, Prescott SM, Topham MK, Gee SH. Interaction of gamma 1-syntrophin with diacylglycerol kinase-zeta. Regulation of nuclear localization by PDZ interactions. The Journal of biological chemistry. 2001 Jul 13;276(28):26526–33. doi: 10.1074/jbc.M104156200. [DOI] [PubMed] [Google Scholar]
- 25.Abramovici H, Hogan AB, Obagi C, Topham MK, Gee SH. Diacylglycerol kinase-zeta localization in skeletal muscle is regulated by phosphorylation and interaction with syntrophins. Mol Biol Cell. 2003 Nov;14(11):4499–511. doi: 10.1091/mbc.E03-03-0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ding L, Bunting M, Topham MK, McIntyre TM, Zimmerman GA, Prescott SM. Alternative splicing of the human diacylglycerol kinase zeta gene in muscle. Proc Natl Acad Sci U S A. 1997 May 27;94(11):5519–24. doi: 10.1073/pnas.94.11.5519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sakane F, Imai S, Kai M, Yasuda S, Kanoh H. Diacylglycerol kinases: why so many of them? Biochim Biophys Acta. 2007 Jul;1771(7):793–806. doi: 10.1016/j.bbalip.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 28.Liu CH, Machado FS, Guo R, Nichols KE, Burks AW, Aliberti JC, Zhong XP. Diacylglycerol kinase zeta regulates microbial recognition and host resistance to Toxoplasma gondii. J Exp Med. 2007 Apr 16;204(4):781–92. doi: 10.1084/jem.20061856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Olenchock BA, Guo R, Silverman MA, Wu JN, Carpenter JH, Koretzky GA, Zhong XP. Impaired degranulation but enhanced cytokine production after Fc epsilonRI stimulation of diacylglycerol kinase zeta-deficient mast cells. J Exp Med. 2006 Jun 12;203(6):1471–80. doi: 10.1084/jem.20052424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kobayashi N, Hozumi Y, Ito T, Hosoya T, Kondo H, Goto K. Differential subcellular targeting and activity-dependent subcellular localization of diacylglycerol kinase isozymes in transfected cells. Eur J Cell Biol. 2007 Aug;86(8):433–44. doi: 10.1016/j.ejcb.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 31.Raben DM, Tu-Sekine B. Nuclear diacylglycerol kinases: regulation and roles. Frontiers in bioscience: a journal and virtual library. 2008;13:590–7. doi: 10.2741/2704. [DOI] [PubMed] [Google Scholar]
- 32.Wada I, Kai M, Imai S, Sakane F, Kanoh H. Translocation of diacylglycerol kinase alpha to the nuclear matrix of rat thymocytes and peripheral T-lymphocytes. FEBS letters. 1996 Sep 9;393(1):48–52. doi: 10.1016/0014-5793(96)00857-5. [DOI] [PubMed] [Google Scholar]
- 33.Ali H, Nakano T, Saino-Saito S, Hozumi Y, Katagiri Y, Kamii H, Sato S, Kayama T, Kondo H, Goto K. Selective Translocation Of Diacylglycerol Kinase Zeta In Hippocampal Neurons Under Transient Forebrain Ischemia. Neurosci Lett. 2004 Dec 6;372(3):190–5. doi: 10.1016/j.neulet.2004.09.052. [DOI] [PubMed] [Google Scholar]
- 34.Sanjuan MA, Pradet-Balade B, Jones DR, Martinez AC, Stone JC, Garcia-Sanz JA, Merida I. T cell activation in vivo targets diacylglycerol kinase alpha to the membrane: a novel mechanism for Ras attenuation. Journal of Immunology. 2003 Mar 15;170(6):2877–83. doi: 10.4049/jimmunol.170.6.2877. [DOI] [PubMed] [Google Scholar]
- 35.Goto K, Watanabe M, Kondo H, Yuasa H, Sakane F, Kanoh H. Gene cloning, sequence, expression and in situ localization of 80 kDa diacylglycerol kinase specific to oligodendrocyte of rat brain. Brain Res Mol Brain Res. 1992 Nov;16(1–2):75–87. doi: 10.1016/0169-328x(92)90196-i. [DOI] [PubMed] [Google Scholar]
- 36.Topham MK, Prescott SM. Mammalian diacylglycerol kinases, a family of lipid kinases with signaling functions. The Journal of biological chemistry. 1999 Apr 23;274(17):11447–50. doi: 10.1074/jbc.274.17.11447. [DOI] [PubMed] [Google Scholar]
- 37.Merida I, Avila-Flores A, Merino E. Diacylglycerol kinases: at the hub of cell signalling. Biochem J. 2008 Jan 1;409(1):1–18. doi: 10.1042/BJ20071040. [DOI] [PubMed] [Google Scholar]
- 38.Ostergaard HL, Trowbridge IS. Coclustering CD45 with CD4 or CD8 alters the phosphorylation and kinase activity of p56lck. J Exp Med. 1990 Jul 1;172(1):347–50. doi: 10.1084/jem.172.1.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ostergaard HL, Shackelford DA, Hurley TR, Johnson P, Hyman R, Sefton BM, Trowbridge IS. Expression of CD45 alters phosphorylation of the lck-encoded tyrosine protein kinase in murine lymphoma T-cell lines. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8959–63. doi: 10.1073/pnas.86.22.8959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Oers NS, Killeen N, Weiss A. Lck regulates the tyrosine phosphorylation of the T cell receptor subunits and ZAP-70 in murine thymocytes. J Exp Med. 1996 Mar 1;183(3):1053–62. doi: 10.1084/jem.183.3.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Iwashima M, Irving BA, van Oers NS, Chan AC, Weiss A. Sequential interactions of the TCR with two distinct cytoplasmic tyrosine kinases. Science. 1994 Feb 25;263(5150):1136–9. doi: 10.1126/science.7509083. [DOI] [PubMed] [Google Scholar]
- 42.Chan AC, Iwashima M, Turck CW, Weiss A. ZAP-70: a 70 kd protein-tyrosine kinase that associates with the TCR zeta chain. Cell. 1992 Nov 13;71(4):649–62. doi: 10.1016/0092-8674(92)90598-7. [DOI] [PubMed] [Google Scholar]
- 43.Zhang W, Sloan-Lancaster J, Kitchen J, Trible RP, Samelson LE. LAT: the ZAP-70 tyrosine kinase substrate that links T cell receptor to cellular activation. Cell. 1998 Jan 9;92(1):83–92. doi: 10.1016/s0092-8674(00)80901-0. [DOI] [PubMed] [Google Scholar]
- 44.Bubeck Wardenburg J, Fu C, Jackman JK, Flotow H, Wilkinson SE, Williams DH, Johnson R, Kong G, Chan AC, Findell PR. Phosphorylation of SLP-76 by the ZAP-70 protein-tyrosine kinase is required for T-cell receptor function. The Journal of biological chemistry. 1996 Aug 16;271(33):19641–4. doi: 10.1074/jbc.271.33.19641. [DOI] [PubMed] [Google Scholar]
- 45.Wange RL. LAT, the linker for activation of T cells: a bridge between T cell-specific and general signaling pathways. Science’s STKE: signal transduction knowledge environment. 2000 Dec 19;2000(63):re1. doi: 10.1126/stke.2000.63.re1. [DOI] [PubMed] [Google Scholar]
- 46.Oh-Hora M, Yamashita M, Hogan PG, Sharma S, Lamperti E, Chung W, Prakriya M, Feske S, Rao A. Dual functions for the endoplasmic reticulum calcium sensors STIM1 and STIM2 in T cell activation and tolerance. Nat Immunol. 2008 Apr;9(4):432–43. doi: 10.1038/ni1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Feske S, Gwack Y, Prakriya M, Srikanth S, Puppel SH, Tanasa B, Hogan PG, Lewis RS, Daly M, Rao A. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature. 2006 May 11;441(7090):179–85. doi: 10.1038/nature04702. [DOI] [PubMed] [Google Scholar]
- 48.Oh-hora M, Rao A. Calcium signaling in lymphocytes. Current opinion in immunology. 2008 Jun;20(3):250–8. doi: 10.1016/j.coi.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Muller MR, Rao A. NFAT, immunity and cancer: a transcription factor comes of age. Nat Rev Immunol. 2010 Sep;10(9):645–56. doi: 10.1038/nri2818. [DOI] [PubMed] [Google Scholar]
- 50.Oh-hora M. Calcium signaling in the development and function of T-lineage cells. Immunol Rev. 2009 Sep;231(1):210–24. doi: 10.1111/j.1600-065X.2009.00819.x. [DOI] [PubMed] [Google Scholar]
- 51.Dries DR, Gallegos LL, Newton AC. A single residue in the C1 domain sensitizes novel protein kinase C isoforms to cellular diacylglycerol production. J Biol Chem. 2007 Jan 12;282(2):826–30. doi: 10.1074/jbc.C600268200. [DOI] [PubMed] [Google Scholar]
- 52.Oancea E, Bezzerides VJ, Greka A, Clapham DE. Mechanism of persistent protein kinase D1 translocation and activation. Dev Cell. 2003 Apr;4(4):561–74. doi: 10.1016/s1534-5807(03)00087-x. [DOI] [PubMed] [Google Scholar]
- 53.Colon-Gonzalez F, Kazanietz MG. C1 domains exposed: from diacylglycerol binding to protein-protein interactions. Biochim Biophys Acta. 2006 Aug;1761(8):827–37. doi: 10.1016/j.bbalip.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 54.Ebinu JO, Bottorff DA, Chan EY, Stang SL, Dunn RJ, Stone JC. RasGRP, a Ras guanyl nucleotide- releasing protein with calcium- and diacylglycerol-binding motifs. Science. 1998 May 15;280(5366):1082–6. doi: 10.1126/science.280.5366.1082. [DOI] [PubMed] [Google Scholar]
- 55.Roose JP, Mollenauer M, Ho M, Kurosaki T, Weiss A. Unusual interplay of two types of Ras activators, RasGRP and SOS, establishes sensitive and robust Ras activation in lymphocytes. Mol Cell Biol. 2007 Apr;27(7):2732–45. doi: 10.1128/MCB.01882-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Karin M, Liu Z, Zandi E. AP-1 function and regulation. Curr Opin Cell Biol. 1997 Apr;9(2):240–6. doi: 10.1016/s0955-0674(97)80068-3. [DOI] [PubMed] [Google Scholar]
- 57.Murphy LO, Smith S, Chen RH, Fingar DC, Blenis J. Molecular interpretation of ERK signal duration by immediate early gene products. Nat Cell Biol. 2002 Aug;4(8):556–64. doi: 10.1038/ncb822. [DOI] [PubMed] [Google Scholar]
- 58.Jain J, McCaffrey PG, Valge-Archer VE, Rao A. Nuclear factor of activated T cells contains Fos and Jun. Nature. 1992 Apr 30;356(6372):801–4. doi: 10.1038/356801a0. [DOI] [PubMed] [Google Scholar]
- 59.Macian F, Lopez-Rodriguez C, Rao A. Partners in transcription: NFAT and AP-1. Oncogene. 2001 Apr 30;20(19):2476–89. doi: 10.1038/sj.onc.1204386. [DOI] [PubMed] [Google Scholar]
- 60.Macian F. NFAT proteins: key regulators of T-cell development and function. Nat Rev Immunol. 2005 Jun;5(6):472–84. doi: 10.1038/nri1632. [DOI] [PubMed] [Google Scholar]
- 61.Mariathasan S, Zakarian A, Bouchard D, Michie AM, Zuniga-Pflucker JC, Ohashi PS. Duration and strength of extracellular signal-regulated kinase signals are altered during positive versus negative thymocyte selection. Journal of Immunology. 2001 Nov 1;167(9):4966–73. doi: 10.4049/jimmunol.167.9.4966. [DOI] [PubMed] [Google Scholar]
- 62.Alberola-Ila J, Hernandez-Hoyos G. The Ras/MAPK cascade and the control of positive selection. Immunol Rev. 2003 Feb;191:79–96. doi: 10.1034/j.1600-065x.2003.00012.x. [DOI] [PubMed] [Google Scholar]
- 63.Goplen N, Karim Z, Guo L, Zhuang Y, Huang H, Gorska MM, Gelfand E, Pages G, Pouyssegur J, Alam R. ERK1 is important for Th2 differentiation and development of experimental asthma. Faseb J. 2012 May;26(5):1934–45. doi: 10.1096/fj.11-196477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nekrasova T, Shive C, Gao Y, Kawamura K, Guardia R, Landreth G, Forsthuber TG. ERK1-deficient mice show normal T cell effector function and are highly susceptible to experimental autoimmune encephalomyelitis. Journal of Immunology. 2005 Aug 15;175(4):2374–80. doi: 10.4049/jimmunol.175.4.2374. [DOI] [PubMed] [Google Scholar]
- 65.Rosebeck S, Rehman AO, Lucas PC, McAllister-Lucas LM. From MALT lymphoma to the CBM signalosome: three decades of discovery. Cell Cycle. 2011 Aug 1;10(15):2485–96. doi: 10.4161/cc.10.15.16923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schulze-Luehrmann J, Ghosh S. Antigen-receptor signaling to nuclear factor kappa B. Immunity. 2006 Nov;25(5):701–15. doi: 10.1016/j.immuni.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 67.Ruland J, Mak TW. Transducing signals from antigen receptors to nuclear factor kappaB. Immunol Rev. 2003 Jun;193:93–100. doi: 10.1034/j.1600-065x.2003.00049.x. [DOI] [PubMed] [Google Scholar]
- 68.Gerondakis S, Siebenlist U. Roles of the NF-kappaB pathway in lymphocyte development and function. Cold Spring Harbor perspectives in biology. 2010 May;2(5):a000182. doi: 10.1101/cshperspect.a000182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schmidt-Supprian M, Tian J, Grant EP, Pasparakis M, Maehr R, Ovaa H, Ploegh HL, Coyle AJ, Rajewsky K. Differential dependence of CD4+CD25+ regulatory and natural killer-like T cells on signals leading to NF-kappaB activation. Proc Natl Acad Sci U S A. 2004 Mar 30;101(13):4566–71. doi: 10.1073/pnas.0400885101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schmidt-Supprian M, Courtois G, Tian J, Coyle AJ, Israel A, Rajewsky K, Pasparakis M. Mature T cells depend on signaling through the IKK complex. Immunity. 2003 Sep;19(3):377–89. doi: 10.1016/s1074-7613(03)00237-1. [DOI] [PubMed] [Google Scholar]
- 71.Shen S, Wu J, Srivatsan S, Gorentla BK, Shin J, Xu L, Zhong XP. Tight regulation of diacylglycerol-mediated signaling is critical for proper invariant NKT cell development. Journal of Immunology. 2011 Sep 1;187(5):2122–9. doi: 10.4049/jimmunol.1100495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Marsland BJ, Soos TJ, Spath G, Littman DR, Kopf M. Protein kinase C theta is critical for the development of in vivo T helper (Th)2 cell but not Th1 cell responses. J Exp Med. 2004 Jul 19;200(2):181–9. doi: 10.1084/jem.20032229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Salek-Ardakani S, So T, Halteman BS, Altman A, Croft M. Differential regulation of Th2 and Th1 lung inflammatory responses by protein kinase C theta. Journal of immunology. 2004 Nov 15;173(10):6440–7. doi: 10.4049/jimmunol.173.10.6440. [DOI] [PubMed] [Google Scholar]
- 74.Cannons JL, Wu JZ, Gomez-Rodriguez J, Zhang J, Dong B, Liu Y, Shaw S, Siminovitch KA, Schwartzberg PL. Biochemical and genetic evidence for a SAP-PKC-theta interaction contributing to IL-4 regulation. Journal of immunology. 2010 Sep 1;185(5):2819–27. doi: 10.4049/jimmunol.0902182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kong KF, Yokosuka T, Canonigo-Balancio AJ, Isakov N, Saito T, Altman A. A motif in the V3 domain of the kinase PKC-theta determines its localization in the immunological synapse and functions in T cells via association with CD28. Nat Immunol. 2011 Nov;12(11):1105–12. doi: 10.1038/ni.2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Isakov N, Altman A. Pkc-Theta-Mediated Signal Delivery From The Tcr/Cd28 Surface Receptors. Front Immunol. 2012;3:273. doi: 10.3389/fimmu.2012.00273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dequiedt F, Kasler H, Fischle W, Kiermer V, Weinstein M, Herndier BG, Verdin E. HDAC7, a thymus-specific class II histone deacetylase, regulates Nur 77 transcription and TCR-mediated apoptosis. Immunity. 2003 May;18(5):687–98. doi: 10.1016/s1074-7613(03)00109-2. [DOI] [PubMed] [Google Scholar]
- 78.Dequiedt F, Van Lint J, Lecomte E, Van Duppen V, Seufferlein T, Vandenheede JR, Wattiez R, Kettmann R. Phosphorylation of histone deacetylase 7 by protein kinase D mediates T cell receptor-induced Nur77 expression and apoptosis. J Exp Med. 2005 Mar 7;201(5):793–804. doi: 10.1084/jem.20042034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Marklund U, Lightfoot K, Cantrell D. Intracellular location and cell context-dependent function of protein kinase D. Immunity. 2003 Oct;19(4):491–501. doi: 10.1016/s1074-7613(03)00260-7. [DOI] [PubMed] [Google Scholar]
- 80.Yuan J, Bae D, Cantrell D, Nel AE, Rozengurt E. Protein kinase D is a downstream target of protein kinase Ctheta. Biochemical and biophysical research communications. 2002 Mar 1;291(3):444–52. doi: 10.1006/bbrc.2002.6469. [DOI] [PubMed] [Google Scholar]
- 81.Powell JD, Pollizzi KN, Heikamp EB, Horton MR. Regulation of immune responses by mTOR. Annual review of immunology. 2012;30:39–68. doi: 10.1146/annurev-immunol-020711-075024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.O’Brien TF, Zhong XP. The role and regulation of mTOR in T-lymphocyte function. Arch Immunol Ther Exp (Warsz) 2012 Jun;60(3):173–81. doi: 10.1007/s00005-012-0171-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lee K, Gudapati P, Dragovic S, Spencer C, Joyce S, Killeen N, Magnuson MA, Boothby M. Mammalian target of rapamycin protein complex 2 regulates differentiation of Th1 and Th2 cell subsets via distinct signaling pathways. Immunity. 2010 Jun 25;32(6):743–53. doi: 10.1016/j.immuni.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yang K, Neale G, Green DR, He W, Chi H. The tumor suppressor Tsc1 enforces quiescence of naive T cells to promote immune homeostasis and function. Nat Immunol. 2011 Sep;12(9):888–97. doi: 10.1038/ni.2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.O’Brien TF, Gorentla BK, Xie D, Srivatsan S, McLeod IX, He YW, Zhong XP. Regulation of T-cell survival and mitochondrial homeostasis by TSC1. Eur J Immunol. 2011 Nov;41(11):3361–70. doi: 10.1002/eji.201141411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhang L, Zhang H, Li L, Xiao Y, Rao E, Miao Z, Chen H, Sun L, Li H, Liu G, Zhao Y. TSC1/2 signaling complex is essential for peripheral naive CD8+ T cell survival and homeostasis in mice. PLoS One. 2012;7(2):e30592. doi: 10.1371/journal.pone.0030592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wu Q, Liu Y, Chen C, Ikenoue T, Qiao Y, Li CS, Li W, Guan KL, Zheng P. The tuberous sclerosis complex-mammalian target of rapamycin pathway maintains the quiescence and survival of naive T cells. Journal of immunology. 2011 Aug 1;187(3):1106–12. doi: 10.4049/jimmunol.1003968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chi H. Regulation and function of mTOR signalling in T cell fate decisions. Nat Rev Immunol. 2012 May;12(5):325–38. doi: 10.1038/nri3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fruman DA. The role of class I phosphoinositide 3-kinase in T-cell function and autoimmunity. Biochem Soc Trans. 2007 Apr;35(Pt 2):177–80. doi: 10.1042/BST0350177. [DOI] [PubMed] [Google Scholar]
- 90.Koyasu S. The role of PI3K in immune cells. Nat Immunol. 2003 Apr;4(4):313–9. doi: 10.1038/ni0403-313. [DOI] [PubMed] [Google Scholar]
- 91.Rodriguez-Viciana P, Warne PH, Khwaja A, Marte BM, Pappin D, Das P, Waterfield MD, Ridley A, Downward J. Role of phosphoinositide 3-OH kinase in cell transformation and control of the actin cytoskeleton by Ras. Cell. 1997 May 2;89(3):457–67. doi: 10.1016/s0092-8674(00)80226-3. [DOI] [PubMed] [Google Scholar]
- 92.Poon HY, Stone JC. Functional links between diacylglycerol and phosphatidylinositol signaling systems in human leukocyte-derived cell lines. Biochemical and biophysical research communications. 2009 Dec 25;390(4):1395–401. doi: 10.1016/j.bbrc.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 93.Finlay D, Cantrell D. Phosphoinositide 3-kinase and the mammalian target of rapamycin pathways control T cell migration. Annals of the New York Academy of Sciences. 2010 Jan;1183:149–57. doi: 10.1111/j.1749-6632.2009.05134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gorentla BK, Wan CK, Zhong XP. Negative regulation of mTOR activation by diacylglycerol kinases. Blood. 2011 Apr 14;117(15):4022–31. doi: 10.1182/blood-2010-08-300731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Salmond RJ, Emery J, Okkenhaug K, Zamoyska R. MAPK, phosphatidylinositol 3-kinase, and mammalian target of rapamycin pathways converge at the level of ribosomal protein S6 phosphorylation to control metabolic signaling in CD8 T cells. Journal of Immunology. 2009 Dec 1;183(11):7388–97. doi: 10.4049/jimmunol.0902294. [DOI] [PubMed] [Google Scholar]
- 96.Krishna S, Xie D, Gorentla B, Shin J, Gao J, Zhong XP. Chronic activation of the kinase IKKbeta impairs T cell function and survival. Journal of Immunology. 2012 Aug 1;189(3):1209–19. doi: 10.4049/jimmunol.1102429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hu T, Gimferrer I, Simmons A, Wiest D, Alberola-Ila J. The Ras/MAPK pathway is required for generation of iNKT cells. PLoS One. 2011;6(5):e19890. doi: 10.1371/journal.pone.0019890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bunting M, Tang W, Zimmerman GA, McIntyre TM, Prescott SM. Molecular cloning and characterization of a novel human diacylglycerol kinase zeta. The Journal of biological chemistry. 1996 Apr 26;271(17):10230–6. [PubMed] [Google Scholar]
- 99.Sakane F, Yamada K, Imai S, Kanoh H. Porcine 80-kDa diacylglycerol kinase is a calcium-binding and calcium/phospholipid-dependent enzyme and undergoes calcium-dependent translocation. The Journal of biological chemistry. 1991 Apr 15;266(11):7096–100. [PubMed] [Google Scholar]
- 100.Zha Y, Marks R, Ho AW, Peterson AC, Janardhan S, Brown I, Praveen K, Stang S, Stone JC, Gajewski TF. T cell anergy is reversed by active Ras and is regulated by diacylglycerol kinase-alpha. Nat Immunol. 2006 Nov;7(11):1166–73. doi: 10.1038/ni1394. [DOI] [PubMed] [Google Scholar]
- 101.Weerkamp F, Pike-Overzet K, Staal FJ. T-sing progenitors to commit. Trends Immunol. 2006 Mar;27(3):125–31. doi: 10.1016/j.it.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 102.Takahama Y. Journey through the thymus: stromal guides for T-cell development and selection. Nature reviews Immunology. 2006 Feb;6(2):127–35. doi: 10.1038/nri1781. [DOI] [PubMed] [Google Scholar]
- 103.Starr TK, Jameson SC, Hogquist KA. Positive and negative selection of T cells. Annual review of immunology. 2003;21:139–76. doi: 10.1146/annurev.immunol.21.120601.141107. [DOI] [PubMed] [Google Scholar]
- 104.Wiegers GJ, Kaufmann M, Tischner D, Villunger A. Shaping the T-cell repertoire: a matter of life and death. Immunol Cell Biol. 2011 Jan;89(1):33–9. doi: 10.1038/icb.2010.127. [DOI] [PubMed] [Google Scholar]
- 105.Fu G, Chen Y, Yu M, Podd A, Schuman J, He Y, Di L, Yassai M, Haribhai D, North PE, Gorski J, Williams CB, Wang D, Wen R. Phospholipase C{gamma}1 is essential for T cell development, activation, and tolerance. J Exp Med. 2010 Feb 15;207(2):309–18. doi: 10.1084/jem.20090880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Dower NA, Stang SL, Bottorff DA, Ebinu JO, Dickie P, Ostergaard HL, Stone JC. RasGRP is essential for mouse thymocyte differentiation and TCR signaling. Nat Immunol. 2000 Oct;1(4):317–21. doi: 10.1038/79766. [DOI] [PubMed] [Google Scholar]
- 107.Alberola-Ila J, Forbush KA, Seger R, Krebs EG, Perlmutter RM. Selective Requirement For Map Kinase Activation In Thymocyte Differentiation. Nature. 1995 Feb 16;373(6515):620–3. doi: 10.1038/373620a0. [DOI] [PubMed] [Google Scholar]
- 108.Alberola-Ila J, Hogquist KA, Swan KA, Bevan MJ, Perlmutter RM. Positive And Negative Selection Invoke Distinct Signaling Pathways. J Exp Med. 1996 Jul 1;184(1):9–18. doi: 10.1084/jem.184.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Pages G, Guerin S, Grall D, Bonino F, Smith A, Anjuere F, Auberger P, Pouyssegur J. Defective thymocyte maturation in p44 MAP kinase (Erk 1) knockout mice. Science. 1999 Nov 12;286(5443):1374–7. doi: 10.1126/science.286.5443.1374. [DOI] [PubMed] [Google Scholar]
- 110.McGargill MA, Ch’en IL, Katayama CD, Pages G, Pouyssegur J, Hedrick SM. Cutting edge: Extracellular signal-related kinase is not required for negative selection of developing T cells. Journal of Immunology. 2009 Oct 15;183(8):4838–42. doi: 10.4049/jimmunol.0902208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Fischer AM, Katayama CD, Pages G, Pouyssegur J, Hedrick SM. The role of erk1 and erk2 in multiple stages of T cell development. Immunity. 2005 Oct;23(4):431–43. doi: 10.1016/j.immuni.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 112.Rincon M, Flavell RA, Davis RA. The JNK and P38 MAP kinase signaling pathways in T cell-mediated immune responses. Free radical biology & medicine. 2000 May 1;28(9):1328–37. doi: 10.1016/s0891-5849(00)00219-7. [DOI] [PubMed] [Google Scholar]
- 113.Sun Z, Arendt CW, Ellmeier W, Schaeffer EM, Sunshine MJ, Gandhi L, Annes J, Petrzilka D, Kupfer A, Schwartzberg PL, Littman DR. PKC-theta is required for TCR-induced NF-kappaB activation in mature but not immature T lymphocytes. Nature. 2000 Mar 23;404(6776):402–7. doi: 10.1038/35006090. [DOI] [PubMed] [Google Scholar]
- 114.Jimi E, Strickland I, Voll RE, Long M, Ghosh S. Differential role of the transcription factor NF-kappaB in selection and survival of CD4+ and CD8+ thymocytes. Immunity. 2008 Oct 17;29(4):523–37. doi: 10.1016/j.immuni.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gupta S, Manicassamy S, Vasu C, Kumar A, Shang W, Sun Z. Differential requirement of PKC-theta in the development and function of natural regulatory T cells. Mol Immunol. 2008 Dec;46(2):213–24. doi: 10.1016/j.molimm.2008.08.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Outram SV, Crompton T, Merida I, Varas A, Martinez AC. Diacylglycerol kinase alpha activity promotes survival of CD4+ 8+ double positive cells during thymocyte development. Immunology. 2002 Apr;105(4):391–8. doi: 10.1046/j.1365-2567.2002.01385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Olenchock BA, Guo R, Carpenter JH, Jordan M, Topham MK, Koretzky GA, Zhong XP. Disruption of diacylglycerol metabolism impairs the induction of T cell anergy. Nat Immunol. 2006 Nov;7(11):1174–81. doi: 10.1038/ni1400. [DOI] [PubMed] [Google Scholar]
- 118.Zhong XP, Hainey EA, Olenchock BA, Jordan MS, Maltzman JS, Nichols KE, Shen H, Koretzky GA. Enhanced T cell responses due to diacylglycerol kinase zeta deficiency. Nat Immunol. 2003 Sep;4(9):882–90. doi: 10.1038/ni958. [DOI] [PubMed] [Google Scholar]
- 119.Guo R, Wan CK, Carpenter JH, Mousallem T, Boustany RM, Kuan CT, Burks AW, Zhong XP. Synergistic control of T cell development and tumor suppression by diacylglycerol kinase alpha and zeta. Proc Natl Acad Sci U S A. 2008 Aug 19;105(33):11909–14. doi: 10.1073/pnas.0711856105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ma L, Chen Z, Erdjument-Bromage H, Tempst P, Pandolfi PP. Phosphorylation and functional inactivation of TSC2 by Erk implications for tuberous sclerosis and cancer pathogenesis. Cell. 2005 Apr 22;121(2):179–93. doi: 10.1016/j.cell.2005.02.031. [DOI] [PubMed] [Google Scholar]
- 121.Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annual review of immunology. 2007;25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- 122.Van Kaer L. NKT cells: T lymphocytes with innate effector functions. Current opinion in immunology. 2007 Jun;19(3):354–64. doi: 10.1016/j.coi.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 123.Van Kaer L. Regulation of immune responses by CD1d-restricted natural killer T cells. Immunol Res. 2004;30(2):139–53. doi: 10.1385/IR:30:2:139. [DOI] [PubMed] [Google Scholar]
- 124.Matsuda JL, Gapin L. Developmental program of mouse Valpha14i NKT cells. Current opinion in immunology. 2005 Apr;17(2):122–30. doi: 10.1016/j.coi.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 125.Cui J, Shin T, Kawano T, Sato H, Kondo E, Toura I, Kaneko Y, Koseki H, Kanno M, Taniguchi M. Requirement for Valpha14 NKT cells in IL-12-mediated rejection of tumors. Science. 1997 Nov 28;278(5343):1623–6. doi: 10.1126/science.278.5343.1623. [DOI] [PubMed] [Google Scholar]
- 126.Godfrey DI, Berzins SP. Control points in NKT-cell development. Nature reviews Immunology. 2007 Jul;7(7):505–18. doi: 10.1038/nri2116. [DOI] [PubMed] [Google Scholar]
- 127.Bendelac A. Positive selection of mouse NK1+ T cells by CD1-expressing cortical thymocytes. J Exp Med. 1995 Dec 1;182(6):2091–6. doi: 10.1084/jem.182.6.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wei DG, Lee H, Park SH, Beaudoin L, Teyton L, Lehuen A, Bendelac A. Expansion and long-range differentiation of the NKT cell lineage in mice expressing CD1d exclusively on cortical thymocytes. J Exp Med. 2005 Jul 18;202(2):239–48. doi: 10.1084/jem.20050413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hu T, Gimferrer I, Alberola-Ila J. Control of early stages in invariant natural killer T-cell development. Immunology. 2011 Sep;134(1):1–7. doi: 10.1111/j.1365-2567.2011.03463.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Griewank K, Borowski C, Rietdijk S, Wang N, Julien A, Wei DG, Mamchak AA, Terhorst C, Bendelac A. Homotypic interactions mediated by Slamf1 and Slamf6 receptors control NKT cell lineage development. Immunity. 2007 Nov;27(5):751–62. doi: 10.1016/j.immuni.2007.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Eberl G, Lowin-Kropf B, MacDonald HR. Cutting edge: NKT cell development is selectively impaired in Fyn- deficient mice. Journal of Immunology. 1999 Oct 15;163(8):4091–4. [PubMed] [Google Scholar]
- 132.Gadue P, Morton N, Stein PL. The Src family tyrosine kinase Fyn regulates natural killer T cell development. J Exp Med. 1999 Oct 18;190(8):1189–96. doi: 10.1084/jem.190.8.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Nichols KE, Hom J, Gong SY, Ganguly A, Ma CS, Cannons JL, Tangye SG, Schwartzberg PL, Koretzky GA, Stein PL. Regulation of NKT cell development by SAP, the protein defective in XLP. Nat Med. 2005 Mar;11(3):340–5. doi: 10.1038/nm1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Pasquier B, Yin L, Fondaneche MC, Relouzat F, Bloch-Queyrat C, Lambert N, Fischer A, de Saint-Basile G, Latour S. Defective NKT cell development in mice and humans lacking the adapter SAP, the X-linked lymphoproliferative syndrome gene product. J Exp Med. 2005 Mar 7;201(5):695–701. doi: 10.1084/jem.20042432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Shen S, Chen Y, Gorentla BK, Lu J, Stone JC, Zhong XP. Critical roles of RasGRP1 for invariant NKT cell development. Journal of Immunology. 2011 Nov 1;187(9):4467–73. doi: 10.4049/jimmunol.1003798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Schwartz RH. T cell anergy. Annu Rev Immunol. 2003;21:305–34. doi: 10.1146/annurev.immunol.21.120601.141110. [DOI] [PubMed] [Google Scholar]
- 137.Chappert P, Schwartz RH. Induction of T cell anergy: integration of environmental cues and infectious tolerance. Curr Opin Immunol. 2010 Oct;22(5):552–9. doi: 10.1016/j.coi.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Fang D, Liu YC. Proteolysis-independent regulation of PI3K by Cbl-b-mediated ubiquitination in T cells. Nat Immunol. 2001 Sep;2(9):870–5. doi: 10.1038/ni0901-870. [DOI] [PubMed] [Google Scholar]
- 139.Heissmeyer V, Macian F, Im SH, Varma R, Feske S, Venuprasad K, Gu H, Liu YC, Dustin ML, Rao A. Calcineurin imposes T cell unresponsiveness through targeted proteolysis of signaling proteins. Nat Immunol. 2004 Mar;5(3):255–65. doi: 10.1038/ni1047. [DOI] [PubMed] [Google Scholar]
- 140.Mueller DL. E3 ubiquitin ligases as T cell anergy factors. Nat Immunol. 2004 Sep;5(9):883–90. doi: 10.1038/ni1106. [DOI] [PubMed] [Google Scholar]
- 141.Schartner JM, Fathman CG, Seroogy CM. Preservation of self: an overview of E3 ubiquitin ligases and T cell tolerance. Semin Immunol. 2007 Jun;19(3):188–96. doi: 10.1016/j.smim.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 142.Anandasabapathy N, Ford GS, Bloom D, Holness C, Paragas V, Seroogy C, Skrenta H, Hollenhorst M, Fathman CG, Soares L. GRAIL: an E3 ubiquitin ligase that inhibits cytokine gene transcription is expressed in anergic CD4+ T cells. Immunity. 2003 Apr;18(4):535–47. doi: 10.1016/s1074-7613(03)00084-0. [DOI] [PubMed] [Google Scholar]
- 143.Baxter AG, Hodgkin PD. Activation rules: the two-signal theories of immune activation. Nat Rev Immunol. 2002 Jun;2(6):439–46. doi: 10.1038/nri823. [DOI] [PubMed] [Google Scholar]
- 144.Macian F, Garcia-Cozar F, Im SH, Horton HF, Byrne MC, Rao A. Transcriptional mechanisms underlying lymphocyte tolerance. Cell. 2002 Jun 14;109(6):719–31. doi: 10.1016/s0092-8674(02)00767-5. [DOI] [PubMed] [Google Scholar]
- 145.Zheng Y, Zha Y, Gajewski TF. Molecular regulation of T-cell anergy. EMBO Rep. 2008 Jan;9(1):50–5. doi: 10.1038/sj.embor.7401138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Alarcon B, Mestre D, Martinez-Martin N. The immunological synapse: a cause or consequence of T-cell receptor triggering? Immunology. 2011 Aug;133(4):420–5. doi: 10.1111/j.1365-2567.2011.03458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Spitaler M, Emslie E, Wood CD, Cantrell D. Diacylglycerol and protein kinase D localization during T lymphocyte activation. Immunity. 2006 May;24(5):535–46. doi: 10.1016/j.immuni.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 148.Santos T, Carrasco S, Jones DR, Merida I, Eguinoa A. Dynamics of diacylglycerol kinase zeta translocation in living T-cells. Study of the structural domain requirements for translocation and activity. J Biol Chem. 2002 Aug 16;277(33):30300–9. doi: 10.1074/jbc.M200999200. [DOI] [PubMed] [Google Scholar]
- 149.Gharbi SI, Rincon E, Avila-Flores A, Torres-Ayuso P, Almena M, Cobos MA, Albar JP, Merida I. Diacylglycerol kinase zeta controls diacylglycerol metabolism at the immunological synapse. Mol Biol Cell. 2011 Nov;22(22):4406–14. doi: 10.1091/mbc.E11-03-0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Yasuda S, Kai M, Imai S, Takeishi K, Taketomi A, Toyota M, Kanoh H, Sakane F. Diacylglycerol kinase eta augments C-Raf activity and B-Raf/C-Raf heterodimerization. J Biol Chem. 2009 Oct 23;284(43):29559–70. doi: 10.1074/jbc.M109.043604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Rincon E, Santos T, Avila-Flores A, Albar JP, Lalioti V, Lei C, Hong W, Merida I. Proteomics identification of sorting nexin 27 as a diacylglycerol kinase zeta-associated protein: new diacylglycerol kinase roles in endocytic recycling. Mol Cell Proteomics. 2007 Jun;6(6):1073–87. doi: 10.1074/mcp.M700047-MCP200. [DOI] [PubMed] [Google Scholar]
- 152.Rincon E, Saez de Guinoa J, Gharbi SI, Sorzano CO, Carrasco YR, Merida I. Translocation dynamics of sorting nexin 27 in activated T cells. J Cell Sci. 2011 Mar 1;124(Pt 5):776–88. doi: 10.1242/jcs.072447. [DOI] [PubMed] [Google Scholar]
- 153.Merino E, Avila-Flores A, Shirai Y, Moraga I, Saito N, Merida I. Lck-dependent tyrosine phosphorylation of diacylglycerol kinase alpha regulates its membrane association in T cells. Journal of immunology. 2008 May 1;180(9):5805–15. doi: 10.4049/jimmunol.180.9.5805. [DOI] [PubMed] [Google Scholar]
- 154.Matsubara T, Ikeda M, Kiso Y, Sakuma M, Yoshino K, Sakane F, Merida I, Saito N, Shirai Y. c-Abl tyrosine kinase regulates serum-induced nuclear export of diacylglycerol kinase alpha by phosphorylation at Tyr-218. The Journal of biological chemistry. 2012 Feb 17;287(8):5507–17. doi: 10.1074/jbc.M111.296897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Baldanzi G, Pighini A, Bettio V, Rainero E, Traini S, Chianale F, Porporato PE, Filigheddu N, Mesturini R, Song S, Schweighoffer T, Patrussi L, Baldari CT, Zhong XP, van Blitterswijk WJ, Sinigaglia F, Nichols KE, Rubio I, Parolini O, Graziani A. SAP-mediated inhibition of diacylglycerol kinase alpha regulates TCR-induced diacylglycerol signaling. Journal of Immunology. 2011 Dec 1;187(11):5941–51. doi: 10.4049/jimmunol.1002476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Cannons JL, Tangye SG, Schwartzberg PL. SLAM family receptors and SAP adaptors in immunity. Annual review of immunology. 2011;29:665–705. doi: 10.1146/annurev-immunol-030409-101302. [DOI] [PubMed] [Google Scholar]
- 157.Harty JT, Badovinac VP. Shaping and reshaping CD8+ T-cell memory. Nat Rev Immunol. 2008 Feb;8(2):107–19. doi: 10.1038/nri2251. [DOI] [PubMed] [Google Scholar]
- 158.Obar JJ, Lefrancois L. Memory CD8+ T cell differentiation. Annals of the New York Academy of Sciences. 2010 Jan;1183:251–66. doi: 10.1111/j.1749-6632.2009.05126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Shin J, O’Brien TF, Grayson JM, Zhong XP. Differential regulation of primary and memory CD8 T cell immune responses by diacylglycerol kinases. Journal of Immunology. 2012 Mar 1;188(5):2111–7. doi: 10.4049/jimmunol.1102265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ochsenbein AF. Principles of tumor immunosurveillance and implications for immunotherapy. Cancer Gene Ther. 2002 Dec;9(12):1043–55. doi: 10.1038/sj.cgt.7700540. [DOI] [PubMed] [Google Scholar]
- 161.Riese MJ, Grewal J, Das J, Zou T, Patil V, Chakraborty AK, Koretzky GA. Decreased diacylglycerol metabolism enhances ERK activation and augments CD8+ T cell functional responses. The Journal of biological chemistry. 2011 Feb 18;286(7):5254–65. doi: 10.1074/jbc.M110.171884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Prinz PU, Mendler AN, Masouris I, Durner L, Oberneder R, Noessner E. High DGK-alpha and disabled MAPK pathways cause dysfunction of human tumor-infiltrating CD8+ T cells that is reversible by pharmacologic intervention. Journal of Immunology. 2012 Jun 15;188(12):5990–6000. doi: 10.4049/jimmunol.1103028. [DOI] [PubMed] [Google Scholar]
- 163.Martinez-Moreno M, Garcia-Lievana J, Soutar D, Torres-Ayuso P, Andrada E, Zhong XP, Koretzky GA, Merida I, Avila-Flores A. FoxO-Dependent Regulation of Diacylglycerol Kinase alpha Gene Expression. Mol Cell Biol. 2012 Oct;32(20):4168–80. doi: 10.1128/MCB.00654-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Tzivion G, Dobson M, Ramakrishnan G. FoxO transcription factors; Regulation by AKT and 14–3–3 proteins. Biochim Biophys Acta. 2011 Nov;1813(11):1938–45. doi: 10.1016/j.bbamcr.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 165.Quann EJ, Merino E, Furuta T, Huse M. Localized diacylglycerol drives the polarization of the microtubule-organizing center in T cells. Nat Immunol. 2009 Jun;10(6):627–35. doi: 10.1038/ni.1734. [DOI] [PubMed] [Google Scholar]
- 166.Huse M, Quann EJ, Davis MM. Shouts, whispers and the kiss of death: directional secretion in T cells. Nat Immunol. 2008 Oct;9(10):1105–11. doi: 10.1038/ni.f.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Quann EJ, Liu X, Altan-Bonnet G, Huse M. A cascade of protein kinase C isozymes promotes cytoskeletal polarization in T cells. Nat Immunol. 2011 Jul;12(7):647–54. doi: 10.1038/ni.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Martinez-Lorenzo MJ, Anel A, Gamen S, Monle n I, Lasierra P, Larrad L, Pineiro A, Alava MA, Naval J. Activated human T cells release bioactive Fas ligand and APO2 ligand in microvesicles. Journal of immunology. 1999 Aug 1;163(3):1274–81. [PubMed] [Google Scholar]
- 169.Alonso R, Rodriguez MC, Pindado J, Merino E, Merida I, Izquierdo M. Diacylglycerol kinase alpha regulates the secretion of lethal exosomes bearing Fas ligand during activation-induced cell death of T lymphocytes. J Biol Chem. 2005 Aug 5;280(31):28439–50. doi: 10.1074/jbc.M501112200. [DOI] [PubMed] [Google Scholar]
- 170.Alonso R, Mazzeo C, Rodriguez MC, Marsh M, Fraile-Ramos A, Calvo V, Avila-Flores A, Merida I, Izquierdo M. Diacylglycerol kinase alpha regulates the formation and polarisation of mature multivesicular bodies involved in the secretion of Fas ligand-containing exosomes in T lymphocytes. Cell Death Differ. 2011 Jul;18(7):1161–73. doi: 10.1038/cdd.2010.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Lee D, Kim J, Beste MT, Koretzky GA, Hammer DA. Diacylglycerol kinase zeta negatively regulates CXCR4-stimulated T lymphocyte firm arrest to ICAM-1 under shear flow. Integr Biol (Camb) 2012 Jun;4(6):606–14. doi: 10.1039/c2ib00002d. [DOI] [PubMed] [Google Scholar]