Abstract
Saccharomyces cerevisiae provides a well-studied model system for heritable silent chromatin, in which a nonhistone protein complex—the SIR complex—represses genes by spreading in a sequence-independent manner, much like heterochromatin in higher eukaryotes. The ability to study mutations in histones and to screen genome-wide for mutations that impair silencing has yielded an unparalleled depth of detail about this system. Recent advances in the biochemistry and structural biology of the SIR-chromatin complex bring us much closer to a molecular understanding of how Sir3 selectively recognizes the deacetylated histone H4 tail and demethylated histone H3 core. The existence of appropriate mutants has also shown how components of the silencing machinery affect physiological processes beyond transcriptional repression.
Studies in budding yeast are uncovering basic mechanisms of heterochromatin-mediated gene silencing. For example, studies are revealing how Sir3 recognizes specific nucleosomes to induce SIR-mediated repression.
OVERVIEW
The fraction of chromatin in a eukaryotic nucleus that bears active genes is termed euchromatin. This chromatin condenses in mitosis to allow chromosomal segregation and decondenses in interphase of the cell cycle to allow transcription to occur. However, some chromosomal domains were observed by cytological criteria to remain condensed in interphase, and this constitutively compacted chromatin was called heterochromatin. With the development of new techniques, molecular rather than cytological features have been used to define this portion of the genome, and heterochromatin, which is often found at centromeres and telomeres, was shown to contain many thousands of simple repeat sequences, particularly in higher eukaryotic organisms. The repeat-rich genomic DNA tends to replicate late in S phase of the cell cycle, is found clustered at the nuclear periphery or near the nucleolus, and is resistant to nuclease attack. Importantly, the characteristic chromatin structure that is formed on repeat DNA tends to spread and repress nearby genes. In the case of the fruit fly locus white, a gene that determines red eye color, epigenetic repression yields a red and white sectored eye through a phenomenon called position effect variegation (PEV). Mechanistically, PEV in flies reflects the recognition of methylated histone H3K9 by heterochromatin protein 1 (HP1), which can spread along the chromosomal arm. In Saccharomyces cerevisiae, also known as budding yeast, a distinct mechanism of heterochromatin formation has evolved, yet it achieves a very similar result.
S. cerevisiae is a microorganism commonly used for making beer and baking bread. However, unlike bacteria, it is a eukaryote. The chromosomes of budding yeast, like those of more complex eukaryotes, are bound by histones, enclosed in a nucleus and replicated from multiple origins during S phase. Still, the yeast genome is tiny with only 14 megabase pairs of genomic DNA divided among its 16 chromosomes, some not much larger than a bacteriophage genome. There are approximately 6000 genes in the yeast genome, closely packed along chromosomal arms, generally with less than 2 kb spacing between them. The vast majority of yeast genes are in an open chromatin state, meaning that they are either actively transcribed or can be rapidly induced. This, coupled with a very limited amount of simple repeat DNA, makes the detection of heterochromatin by cytological techniques very difficult in yeast.
Nonetheless, budding yeast has distinct heterochromatin-like regions adjacent to all 32 telomeres and at two silent mating loci on chromosome III (Chr III), shown using molecular tools. Transcriptional repression at telomeres and the silent mating loci can spread into adjacent DNA and repression of the silent mating loci is essential for maintaining a mating-competent haploid state. Both the subtelomeric regions and the silent mating type loci repress integrated reporter genes in a position-dependent, epigenetic manner; they replicate late in S phase and are present at the nuclear periphery. Thus, these loci bear most of the functional characteristics of heterochromatin, without having cytologically visible condensation in interphase. By exploiting the advantages afforded by the small genome of yeast and its powerful genetic and biochemical tools, many basic principles of chromatin-mediated repression that are relevant to heterochromatin in more complex organisms have been discovered. Nonetheless, silent chromatin in budding yeast is dependent on a unique set of nonhistone proteins that do not deposit nor recognize histone H3 lysine 9 methylation.
1. THE GENETIC AND MOLECULAR TOOLS OF YEAST
Yeast provides a flexible and rapid genetic system for studying cellular events. With an approximate generation time of 90 min, colonies containing millions of cells are produced after just 2 d of growth. In addition, yeast can propagate in both haploid and diploid forms, greatly facilitating genetic analysis. Like bacteria, haploid yeast cells can be mutated to produce specific nutritional requirements or auxotrophic genetic phenotypes, and recessive lethal mutations can either be maintained in haploids as conditional lethal alleles (e.g., temperature-sensitive mutants), or in heterozygotic diploids, which carry both wild-type and mutant alleles.
Extremely useful is the efficient homologous recombination system of budding yeast, which allows the alteration of any chosen chromosomal sequence at will. In addition, portions of chromosomes can be manipulated and reintroduced on plasmids that are stably maintained through cell division, thanks to short sequences that provide centromere and replication origin function. Large linear plasmids, or minichromosomes, which carry telomeric repeats to cap their ends, also propagate stably in yeast.
Yeast also has a unique advantage in the genetic analysis of histones and their roles in gene regulation: Unlike mammalian cells, which have as many as 60–70 copies of the core histone coding genes (H2A, H2B, H3, and H4), yeast contains only two copies of each of these genes. Because these two copies are functionally redundant, this has enabled the production of cells containing single histone gene copies. Deletion analysis or mutation of defined histone amino acids in these genes has uncovered specific roles for histone residues in heterochromatin and other cellular functions. By searching for suppressors of these mutant phenotypes it has also been possible to identify heterochromatin proteins that interact with the histone sites in question. The ease of generating histone mutants in yeast has also led to the systematic mutagenesis of most amino acid residues in each of the core histones (http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2666297/) and analysis of their effects on genome function (Huang et al. 2009).
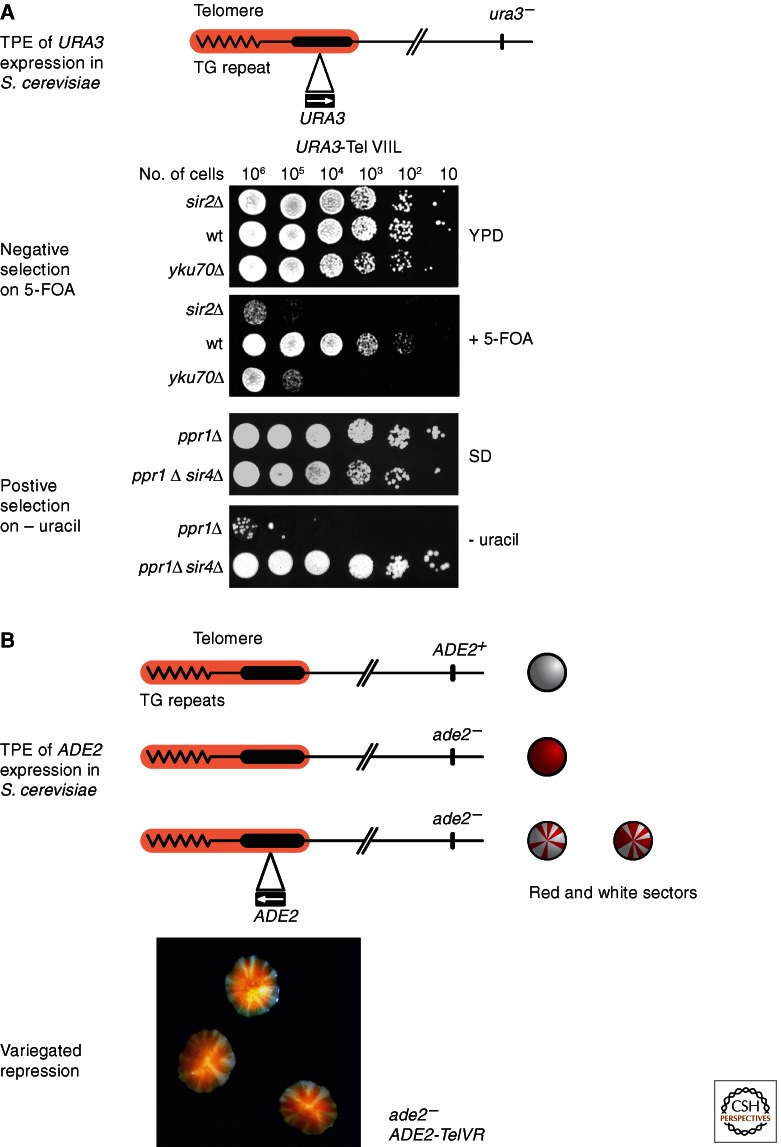
Budding yeast also provides powerful cellular readouts for epigenetic gene regulation, conceptually similar to PEV in flies, in which the white gene provides a visible screen for variegated gene expression (see Elgin and Reuter 2013 for more detail). A parallel phenomenon called telomere position effect (TPE) occurs near yeast telomeres. The study of TPE has been aided analogously by the use of the URA3 and ADE2 reporter genes (Fig. 1). The URA3 gene product is necessary for pyrimidine biosynthesis, and cells that do not express the Ura3 protein cannot grow on synthetic media lacking uracil. In addition, URA3 allows for a counter-selection against expression in the presence of 5-fluoroorotic acid (5-FOA), because the Ura3 gene product converts 5-FOA to 5-fluorouracil, an inhibitor of DNA synthesis that causes cell death. Thus, when URA3 is integrated near heterochromatin and the gene is repressed in some but not all cells, only the cells that silence URA3 are able to grow in the presence of 5-FOA. Conversely, if the strain lacks the strong activator of URA3, Ppr1, positive selection is possible; only URA3-expressing cells can grow on uracil-deficient plates. Thus the efficiency of URA3 repression/expression can be scored accurately in serial dilution assays (over a 1–106-fold range) on plates that either contain 5-FOA, or lack uracil (Fig. 1A). Because 5-FOA is mutagenic and puts strong pressure on cells to repress the reporter gene, some conditions or yeast backgrounds favor the use of uracil-drop out plates over counter-selection on 5-FOA.
Figure 1.
Silencing and TPE in yeast. (A) The URA3 gene, inserted near the telomeric simple TG-rich repeat at the left arm of Chr VII, is silenced by telomeric heterochromatin in this yeast strain. In normal rich media (YPD) no growth difference can be detected between wild-type (wt) cells that repress the subtelomeric URA3 gene, and silencing mutants that lose telomeric heterochromatin and express URA3. In media containing 5-FOA (middle panel), on the other hand, cells that repress URA3 (e.g., wt cells) can grow, whereas cells that express it (sir2Δ and yku70Δ) cannot, because the URA3 gene product converts 5-FOA to the toxic intermediate 5-flourouracil. The serial dilution/drop assay allows detection of silencing in as few as 1 in 106 cells. In cells deleted for the URA3 activator, Ppr1 (ppr1Δ), one can screen for repression by plating on synthetic dextrose (SD) medium, lacking uracil. In this case, silencing the gene inhibits colony growth. (B) Cells containing the wild-type ADE2 gene produce a colony that is white, whereas those containing mutant ade2 appear red, because of the accumulation of a reddish intermediate in adenine biosynthesis. When the ADE2 gene is inserted near the telomere at the right arm of Chr V it is silenced in an epigenetic manner. The silent ADE2 state and the active ADE2 state in genetically identical cells are both inherited creating red and white sectors in a colony.
Another useful assay for epigenetic repression is based on the insertion of the reporter gene ADE2 near heterochromatin. When ADE2 is repressed, a precursor in adenine biosynthesis accumulates, turning the cell red. When ADE2 is expressed, cells are white (Fig. 1B). The epigenetic nature of repression of a subtelomeric ADE2 gene is visible within a single colony of genetically identical cells because the variegated expression of ADE2 generates white sectors in a red colony background, attributable to ADE2 repression (Fig. 1B). This reporter avoids the stress of selective pressure against cells that fail to repress ADE2, and thus the sectored phenotype illustrates both the switching rate and inheritance of the epigenetic state through mitotic division, much like the sectored white phenotype in the Drosophila melanogaster eye.
Combined with these genetic approaches, biochemical techniques for mapping epigenetic modifiers are readily applied to yeast. Large yeast cultures can be grown either synchronously or asynchronously. A battery of molecular tools, which includes transcriptome analysis and chromatin immunoprecipitation (ChIP), can be combined with multiplexed next generation sequencing for efficient whole-genome coverage. Combining this with proteomic approaches that map protein networks enables a comprehensive and quantitative comparison of gene expression, transcription factor binding, histone modification, and protein–protein interactions. Finally, a technology developed in yeast, called chromosome conformation capture, scores protein-mediated contacts between unlinked chromosomal domains to monitor long-range chromatin interactions (for more detail, see Dekker and Misteli 2014). With this broad range of tools, scientists have explored the mechanisms that regulate both the establishment of heterochromatin and its physiological roles in budding yeast for more than 30 years. Before describing these discoveries, however, we will first review the life cycle of yeast in more detail.
2. THE LIFE CYCLE OF YEAST
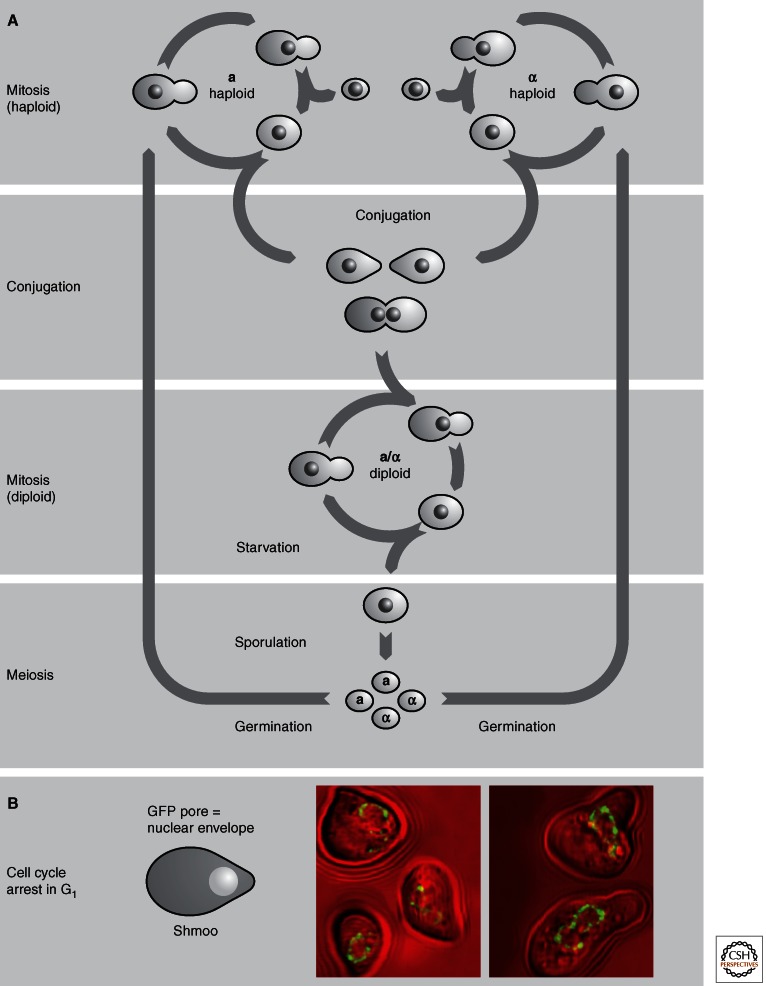
S. cerevisiae multiplies through mitotic division in either a haploid or a diploid state by producing a bud that enlarges and eventually separates from the mother cell (Fig. 2A). This is why it is called budding yeast. Haploid yeast cells can mate with each other (i.e., conjugate) because they exist in one of two mating types, termed a or α, reminiscent of the two sexes in mammals. Yeast cells of each mating type produce a distinct pheromone that attracts the cells of the opposite mating type: a cells produce a peptide of 12 amino acids (aa) called a factor, which binds to a membrane spanning a-factor receptor on the surface of an α cell. Conversely, α cells produce a 13 aa peptide (α-factor) that binds to the α-factor-receptor on the surface of a cells. These interactions result in the arrest of the two cell types in mid-to-late G1 phase of the cell cycle. The arrested cells assume “shmoo”-like shapes (named after the pear-shaped Al Capp cartoon character; Fig. 2B). Shmoos of opposite mating type fuse at their tips to produce an a/α diploid cell.
Figure 2.
The life cycle of budding yeast. (A) Yeast cells divide mitotically in both haploid and diploid forms. Sporulation is induced in a diploid by starvation, whereas mating occurs spontaneously when haploids of opposite mating type are in the vicinity of each other. This occurs by pheromone secretion, which arrests the cell cycle in G1 of a cell of the opposite mating type, and after sufficient exposure to pheromone the mating pathway is induced. The diploid state represses the mating pathway. (B) In response to pheromone, haploid cells distort toward cells of the opposite mating type. These are called shmoos. The nuclear envelope is shown in green fluorescence, showing distortions of the nucleus.
In diploid cells the mating response is repressed, and cells propagate vegetatively (i.e., by mitotic division) unless they are exposed to starvation conditions. Nitrogen starvation induces a meiotic program in diploids that provokes the formation of an ascus containing four spores, two of each mating type. When nutrient levels are restored, these haploid spores grow into a or α cells that are again capable of mating to form a diploid, starting the life cycle over again.
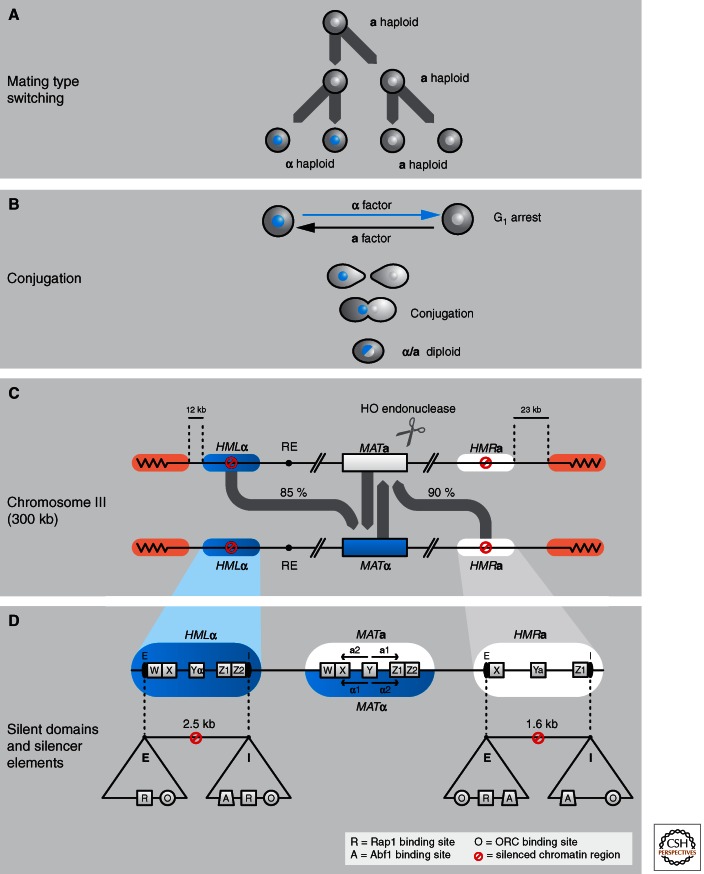
Although haploid yeast cells in the laboratory are usually genetically constructed to be stable α or a cells, yeast in the wild switch their mating type nearly every cell cycle (Fig. 3A). Mating type switching is provoked by an endogenous cell-cycle-regulated endonuclease activity (HO) that induces a site-specific double-strand break at the MAT locus. A gene conversion event (in which the donor DNA sequence remains unchanged, but the recipient DNA is altered) transposes the opposite mating type information from one of two constitutively silent donor loci, HMLα or HMRa, to the MAT locus, in which the mating type–determining genes, a1 and a2, or α1 and α2, are expressed. Strains capable of mating type switching are called homothallic. This name reflects the fact that a single vegetative MATa cell can produce MATα progeny, and vice versa, allowing offspring to mate with each other.
Figure 3.
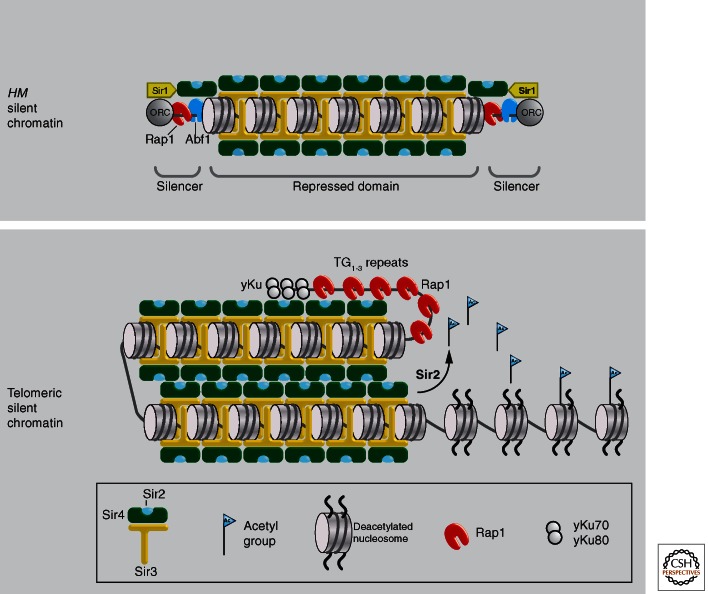
Mating type switching in yeast. (A) Homothallic yeast strains are able to switch mating type after one division cycle. The switch occurs before DNA replication so that both mother and daughter cells assume the new mating type. (B) In a wild-type population of yeast, this allows rapid conjugation between daughter cells to form a diploid. (C) The position of the silent and expressed mating type loci on Chr III are shown here. The active MAT locus is able to switch through gene conversion roughly once per cell cycle because of cleavage by the HO endonuclease. The percentages indicated show the frequency with which the gene conversion event replaced the MAT locus with the opposite mating type information. The directionality of switching is guaranteed by the recombination enhancer on the left arm of Chr III. (D) Repression at the silent mating type loci HMR and HML is mediated by two silencer DNA elements that flank the silent genes. These silencers are termed E (for essential) or I (for important) (Brand et al. 1987) and provide binding sites for Rap1 (R), Abf1 (A), and origin recognition complex (O). Artificial silencers can be created using various combinations of the redundant binding sites, although their efficiency is less than that of the native silencers. HMLα and HMRa are 12 kb and 23 kb, respectively, from the telomeres of Chr III. Telomeric heterochromatin domains at Chr III are silenced independently from the HM loci in a process that is initiated at the telomeres through binding sites for Rap1.
In the laboratory it is useful to have strains with stable mating types, and thus laboratory yeast generally contain a mutant HO endonuclease gene (ho–). These cells fail to induce a double-strand cleavage at the MAT locus, and therefore cannot switch mating type. These heterothallic cells are stable haploid strains of either a or α mating type, unless placed in proximity of cells of the opposite mating type, in which case the two haploid cell types will mate to form a diploid.
Importantly, the two stable haploid cell types do not require silencing for viability, yet they must repress the genes at the homothallic mating type loci, HML and HMR, to retain their ability to mate. If repression fails and cells express both sets of mating type genes at once, then a haploid will behave as if it were diploid, suppressing mating competence and generating a sterile haploid strain (Fig. 3B). The mechanisms that repress the two homothallic mating type loci, HML and HMR, have become a classic system for the study of heterochromatin-mediated repression.
3. YEAST HETEROCHROMATIN IS PRESENT AT THE SILENT HM MATING LOCI AND AT TELOMERES
The three mating type loci, HMLα, MAT, and HMRa, are located on one small chromosome, Chr III, and contain the information that determines α or a mating type in yeast. The silent loci, HMLα (∼12 kb from the left telomere) and HMRa (∼23 kb from the right telomere), are situated between short DNA elements called E and I silencers (Fig. 3B,C). In a wild-type cell, the silent cassettes are active only once copied and integrated into the MAT locus, which lacks silencer elements. The transfer of HMLα information into MATa results in an α mating type (MATα) cell, whereas the transfer of HMRa information into MAT results in the a mating type (MATa) (Fig. 3B). This shows that the promoters and genes at the HM loci are completely intact, and remain repressed because of their position between the E and I silencers. Deletion of the flanking silencer sequences indeed allows expression of the silent information, generating a nonmating state.
By scoring for haploid sterility, mutations that impair silencing at the HM loci were isolated (Rine et al. 1979). This allowed identification of the silent information regulatory proteins, Sir1, Sir2, Sir3, and Sir4, as being essential for the full repression of HM loci (Rine and Herskowitz 1987). Mutations in sir2, sir3, or sir4 caused a complete loss of mating, which is attributable to a loss of HM repression. In sir1 mutants, only a fraction of MATa cells were unable to mate. Taking advantage of the partial phenotype of sir1 deficient cells, it could be shown that the two alternative states (mating and nonmating) are heritable through successive cell divisions in genetically identical cells (Pillus and Rine 1989). This provided a clear demonstration that mating type repression displays the hallmark characteristic of epigenetically controlled repression. Later genetic studies showed that the amino termini of histones H3 and H4, repressor activator protein 1 (Rap1), and the origin recognition complex (ORC) are also components of silent mating locus heterochromatin (reviewed by Rusche et al. 2003). These latter two DNA binding factors have other essential functions in the nucleus, namely, the regulation of ribosomal protein gene expression or the initiation of DNA replication, and, thus, only “moonlight” as corepressors. Although less well studied, the same appears true for Abf1, a third silencer binding factor.
A similar position-dependent repression occurs immediately adjacent to the yeast telomeric repeat DNA (C1–3A/TG1-3) found at the ends of all yeast chromosomes. As mentioned above, the variegated but heritable repression of subtelomeric reporter genes such as URA3 and ADE2 is called TPE (Gottschling et al. 1990). TPE shares the HM requirement for Rap1, Sir2, Sir3, Sir4, and the histone amino termini (Aparicio et al. 1991; Thompson et al. 1994a), and the repression mechanisms have proven to be closely related. However, given that subtelomeric reporters can switch at detectable rates between silent and expressed states, unlike HM loci, telomere-proximal gene repression appears to be more similar to fly PEV (see Elgin and Reuter 2013).
4. Sir PROTEIN STRUCTURE AND EVOLUTIONARY CONSERVATION
The known chromatin binding factors that are essential for Sir mediated silencing are Sir2, Sir3, and Sir4, whereas Sir1 enhances the efficiency of repression at HM loci, but is not found at telomeres. The Sir2-3-4 proteins work as a trimeric complex with 1:1:1 stoichiometry (Cubizolles et al. 2006). Both Sir3 and Sir4 are able to bind nucleosomes and DNA independently, yet the Sir holo-complex remains a trimer when bound to nucleosomes. Moreover, Sir3 and Sir4 each have homo- and heterodimerization motifs in their carboxyl termini. Mutation or deletion of their interaction domains disrupts silencing in vivo (Murphy et al. 2003; Rudner et al. 2005; Ehrentraut et al. 2011; Oppikofer et al. 2013).
Sir protein expression levels are tightly regulated and a single extra copy of the SIR4 gene impairs repression, as does strong induction of SIR2 (Cockell et al. 1998). On the other hand, increasing levels of Sir3 protein alone extends the spreading of Sir3 along nucleosomes, and with it, transcriptional repression (Renauld et al. 1993; Hecht et al. 1996). Balanced overexpression of all three proteins greatly improves silencing efficiency at telomeres, and allows repression of reporters located in euchromatic regions that are flanked by silencer elements, which at normal Sir protein levels are not repressed (Maillet et al. 1996).
Although Sir2, Sir3, and Sir4 are equally essential for the structural integrity of the Sir complex and therefore, for both the establishment and maintenance of silent chromatin, each has a different function. Sir2 provides a nicotinamide dinucleotide (NAD)-dependent histone deacetylase activity that is essential for repression in a wild-type background (Imai et al. 2000), whereas Sir3 and Sir4 fulfill structural roles without obvious enzymatic activities. Sir3 is a member of the AAA+ ATPase family, which lacks ATPase activity. It is largely responsible for the specificity of Sir complex binding to nucleosomes because of its selective affinity for nucleosomes with unacetylated histone H4 lysine 16 (H4K16) and unmethylated histone H3 lysine 79 (H3K79) (Johnson et al. 1990; Altaf et al. 2007; Oppikofer et al. 2011). The sensitivity of Sir3 to these histone modifications helps restrict the binding of the Sir complex to appropriate sites.
Sir4 is the largest (152 kDa) and the least conserved of the Sir proteins, yet it forms a stable heterodimer with Sir2 (Moazed et al. 1997; Strahl-Bolsinger et al. 1997) and enhances Sir2 deacetylase activity (Tanny et al. 1999; Cubizolles et al. 2006). Structural information on the Sir4 interface indicates that most of the Sir2-interaction domain of Sir4 (aa 737–839) is buried in a pocket formed by the poorly conserved amino terminus and the carboxy-terminal catalytic domain of Sir2 (Hsu et al. 2013). The Sir3-binding domain within Sir4 is contained in the parallel coiled-coil structure at its extreme carboxyl terminus, which also serves as a binding site for other proteins (Chang et al. 2003; Rudner et al. 2005).
4.1. The “Scaffold” Role of Sir4
The affinity of Sir4 for both Sir2 and Sir3 already suggested that it might act as a scaffold for the assembly of the silencing complex. Much of this scaffolding role is achieved by the carboxy-terminal half of Sir4, which is sufficient for repression at HM loci (Kueng et al. 2012). Best studied is the extreme carboxy-terminal coiled-coil domain of Sir4 (aa 1257–1358), that forms a continuous parallel homodimer with two Sir3 binding sites on its outer surface (Chang et al. 2003). Mutations within the dimerization motif disrupt both Sir3 binding and silencing (Murphy et al. 2003). However, this same carboxy-terminal coiled-coil domain also binds yKu70 and Rap1, which recruit Sir4 to telomeric repeats or HM silencers (Moretti et al. 1994; Tsukamoto et al. 1997; Mishra and Shore 1999; Luo et al. 2002). Finally, Sir4 binds the second yKu subunit, called yKu80, through both yKu80's amino and carboxyl termini, and interacts with Esc1 (establishes silent chromatin 1) through its partitioning and anchoring domain (PAD; Sir4 aa 950–1262; Ansari and Gartenberg 1997; Andrulis et al. 2002). The yKu80 and Esc1 interactions serve to tether Sir4, and the silent chromatin it binds, to the nuclear periphery (Gartenberg et al. 2004; Taddei et al. 2004). The Sir4 PAD domain also binds ubiquitin binding protein 10 (Ubp10), a histone H2B deubiquitylase that reduces levels of H2B K123ub at telomeres (Gardner et al. 2005). Loss of H2B K123ub in turn reduces histone H3K79 methylation, which directly interferes in Sir3-nucleosome binding (Armache et al. 2011; Oppikofer et al. 2011).
The Sir4 amino terminus is necessary for silencing in subtelomeric domains, but not at HM loci. It appears to both regulate the efficiency of recruitment by binding yKu80 and provide linker DNA protection when bound to reconstituted nucleosomes (Kueng et al. 2012). The amino terminus is also heavily phosphorylated in vivo in a cell-cycle-dependent manner, allowing modification of repression by the cyclin-dependent kinase Cdc28 (Kueng et al. 2012). These observations highlight the role of Sir4 as a multifaceted scaffold that recruits, binds, and regulates the binding of various factors that impinge on Sir-mediated repression.
4.2. Evolutionary Conservation of Sir2 and Sir3
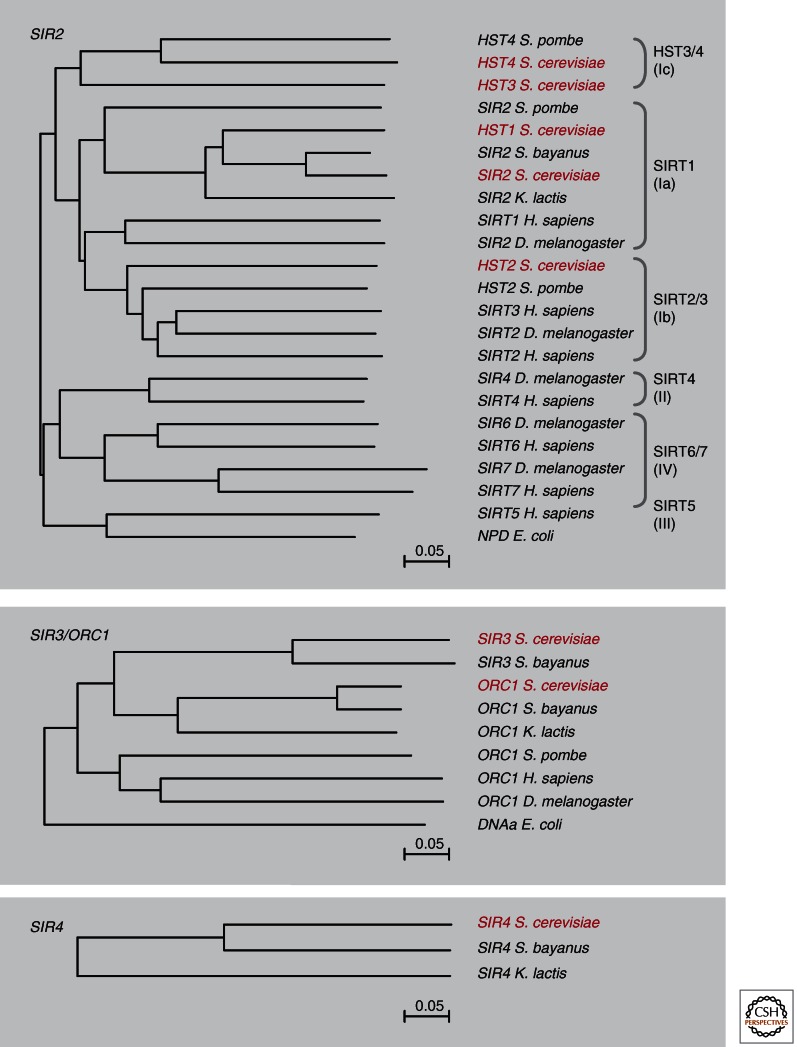
As mentioned above, the Sir2 deacetylase is well conserved, with homologs in all species extending from eubacteria and archaea to man (Fig. 4). Many species have multiple Sir2 family members, although some members are cytoplasmic and serve primarily to deacetylate nonhistone proteins (detailed in Seto and Yoshida 2014). S. cerevisiae has five Sir2-related deacetylases (SIR2 and HST1–4), but only Sir2 functions together with Sir3 and Sir4 in silent chromatin, in which it targets the amino-terminal tails of histones H3 and H4.
Figure 4.
Sir protein family trees. Sir2 is the founding member of a large family of NAD-dependent deacetylases. The Sir2 family of proteins is highly conserved, found in multiple isoforms in organisms that range from bacteria to man. In the latter, there are both nuclear and cytoplasmic isoforms. Homologs of Sir2, Sir3, and Sir4 from Saccharomyces bayanus, Kluyveromyces lactis, Schizosaccharomyces pombe, D. melanogaster, and Homo sapiens were collected from UniProt and were aligned using ClustalW2 alignment. The phylogenetic tree was created using neighbor joining. Sir2 classification is according to Frye (2000). K. lactis has 4 SIR2 orthologs (homologs to S. cerevisiae Sir2, Hst2, Hst3, and Hst4), but the HST homologs were omitted in the tree for clarity. For S. bayanus, only the Sir2 homolog is annotated to date. S. cerevisiae homologs are in red. Sir3 arose through a gene duplication of a gene encoding an ancient Orc1, and Sir4 is a rapidly evolving protein that is only found in related budding yeasts. The related proteins shown are not exhaustive, particularly for Sir2.
The Sir2 family is defined by a conserved catalytic domain in which deacetylation is coupled to the breakdown of NAD+. The coupling of NAD hydrolysis with deacetylation produces O-acetyl-ADP-ribose, an intermediate that may have a function of its own (Tanner et al. 2000). The Sir2-like NAD-dependent histone deacetylases (HDACs) are implicated in transcriptional repression in many distant species such as fission yeast and flies, although they lack the other Sir proteins (reviewed in Chopra and Mishra 2005). Therefore, it is thought that an ancient Sir2 deacetylase evolved to acquire unique interaction interface with the species-specific factor Sir4, in budding yeast.
S. cerevisiae Sir2 plays an important role beyond TPE and HM locus silencing in that it suppresses nonreciprocal recombination in the highly repetitive rDNA locus (Gottlieb and Esposito, 1989). In this context Sir2 does not function as part of the Sir2-3-4 complex, but associates with an alternative group of factors that regulate exit from mitosis (the regulator of nucleolar silencing and telophase exit or RENT complex containing the phosphatase Cdc14, Net1/Cfi1, and the mitotic monopolin proteins, Lrs4 and Csm1; Mekhail et al. 2008; Chan et al. 2011). These proteins are also involved in the maintenance of rDNA repeat stability.
Sir3 contains several conserved domains, as the gene itself arose from an ancient version of ORC1, a subunit of the ORC that is found in all eukaryotes. The carboxy-terminal half of Sir3 contains a large AAA+ ATPase domain, much like all ORC subunits and their loading protein, Cdc6 (Norris and Boeke 2010). AAA+ domain proteins generally hydrolyze ATP to drive the assembly and disassembly of macromolecular complexes. Sir3, however, has an altered nucleotide binding pocket that precludes nucleoside binding (Ehrentraut et al. 2011). Sir3 and Orc1 further share a conserved amino-terminal BAH (bromo-adjacent homology) domain that binds nucleosomes (Armache et al. 2011), and a carboxy-terminal winged helix domain that mediates dimerization (Oppikofer et al. 2013). Interestingly, whereas the BAH domain of yeast Sir3 recognizes histone H4 deacetylated at K16, the BAH domain of evolutionarily related HsORC1 recognizes histone H4 dimethylated at K20 (H4K20me2), linking heterochromatin with origin function in vertebrates (Beck et al. 2012).
Whereas Orc1 is found in all eukaryotic species, Sir3 is only found in budding yeast species that underwent whole-genome duplication approximately 100 million years ago (Hickman et al. 2011). In very closely related budding yeast species that have both Sir3 and Orc1 orthologs, Sir3 mediates TPE and mating type repression. However, in Kluyveromyces lactis, which lacks Sir3, Orc1 appears to assume Sir3’s role in repression. Indeed, the carboxy-terminal winged helix-turn-helix domain of Sir3 and Orc1 in these two yeasts has a similar dimerization function, which is lacking in Orc1 proteins from other species.
Sir1 and Sir4, in contrast to Sir2 and the Sir3/Orc1 family, are present only in species closely related to S. cerevisiae (i.e., the Saccharomycetaceae family). Intriguingly, despite the restricted evolutionary distribution of Sir1, it contains a functionally defined OIR (ORC-interacting region) domain that associates both with the Orc1 BAH domain and with Sir4 (Hickman et al. 2011).
5. SILENT CHROMATIN IS DISTINGUISHED BY A REPRESSIVE STRUCTURE THAT SPREADS THROUGH THE ENTIRE DOMAIN
Repression of gene activity in euchromatin often requires the presence of a repressive protein or complex that recognizes a specific sequence in the promoter of a gene, thus preventing the active engagement of the transcription machinery. In contrast, heterochromatic repression occurs through a different mechanism that is not promoter specific: Repression initiates at specific nucleation sites, yet spreads continuously throughout a domain, silencing any and all promoters in the region (Fig. 5) (Brand et al. 1985; Renauld et al. 1993). The correlation of transcriptional repression with Sir binding was confirmed by the use of ChIP, which showed that Sir2, Sir3, and Sir4 proteins interact physically with chromatin throughout the subtelomeric domain of silent chromatin and spread continuously inward from the chromosomal end (Strahl-Bolsinger et al. 1997). Evidence that this induces a repressive, less accessible chromatin structure in vivo comes from other approaches. For instance, it was shown that the DNA of silenced chromatin was not methylated efficiently in yeast cells that express a bacterial dam methylase, although the enzyme readily methylated sequences outside the silent region. This suggests that heterochromatin restricts access to macromolecules like dam methyltransferase (Gottschling, 1992). Similarly, the ∼3 kb HMR locus in isolated nuclei is preferentially resistant to certain restriction endonucleases (Loo and Rine 1994), and nucleosomes were shown to be tightly positioned between two silencer elements creating nuclease resistant domains at silent, but not active, HM loci (Weiss and Simpson, 1998). The reduced accessibility of yeast silent chromatin to nucleolytic attack is also observed in vitro when Sir-nucleosome complexes are reconstituted from recombinant proteins (Martino et al. 2009).
Figure 5.
Model for yeast heterochromatin at telomeres and the HM loci. The telomere and HM silencer mechanisms for nucleating Sir complex spreading both use Rap1, Sir2, Sir3, and Sir4. Yet they differ in that telomeres also rely on yKu, whereas the HM silencer elements use the factors ORC, Abf1 and Sir1. Telomeric heterochromatin is thought to fold back onto itself to form a cap that protects the telomere from degradation and whose condensation and folding silences genes. In the case of HM heterochromatin, the repressed domain between the silencer elements consists of closely spaced nucleosomes that form a condensed structure. Both the telomeric and HM silent regions are inaccessible to a number of transcription factors and degradative enzymes.
The extent to which either yeast or metazoan heterochromatin is hypercondensed to hinder access to transcription factors sterically is less certain. Surprisingly, the repressive complex formed by the interaction of Sir proteins and histones appears to be dynamic because Sir proteins can be incorporated into HM silent chromatin even when cells are arrested at a stage in the cell cycle when heterochromatin assembly generally does not occur (Cheng and Gartenberg 2000). This may explain why Sir-bound heterochromatin can serve as a binding site for certain transcription factors (e.g., the heat shock transcriptional activator, HSF1) even in its repressed state (Sekinger and Gross 1999). Although such studies argue that heterochromatin does not simply hinder access for all nonhistone proteins, no obvious transcription occurs, and engaged RNA polymerases cannot be detected experimentally. Experiments by Chen and Widom (2005) argue that the step that is specifically prevented by yeast heterochromatin is formation of a complex of RNA polymerase II (RNA Pol II) with the promoter-binding transcription factors TFIIB and TFIIE. Consistently, a drop in RNA Pol II binding was seen in a system in which silencing was induced by the controlled expression of Sir3. Thus, silent yeast chromatin may allow turnover of Sir factors and some transcription factors, yet it selectively impedes the binding of specific elements of the basal transcription machinery, thereby blocking mRNA production.
6. DISTINCT STEPS IN HETEROCHROMATIN ASSEMBLY
The assembly of heterochromatin in budding yeast involves a series of molecular steps, starting with a site-specific nucleation step. This requires DNA recognition by a sequence-specific DNA binding factor. Next, heterochromatin spreads from the initiation site, limited by specific boundary mechanisms. A change in higher organization of the repressed chromatin then occurs, which is distinct from the simple binding of Sir factors. Finally, yeast silent chromatin is sequestered near the nuclear envelope, generating a subnuclear compartment that favors heterochromatin-mediated repression by promoting its duplication. Although the assembly of heterochromatin at telomeres varies in some aspects from its assembly at HM loci, both embody a very similar principle: the presence of specific DNA binding factors that nucleate the spread of general repressors. In both cases, the spreading requires active deacetylation by Sir2. These mechanisms are described in the following paragraphs.
6.1. HM Heterochromatin
The silent mating loci HML and HMR are bracketed by short DNA elements termed silencers (Fig. 3), which provide binding sites for at least two, and in most cases, all three multifunctional nuclear factors, namely Rap1, Abf1, and the ORC complex (Brand et al. 1987). The deletion of HMR-E, which has three recognition sites, has a much stronger effect on silencing than deletion of HMR-I, which has only two, whereas at HML, the two silencers have more equal roles. The factors bound to silencers are able to cooperate with a distant silencer through Sir proteins to promote repression, possibly by forming a looped domain (Hofmann et al. 1989). This would explain the cooperative effects of the E and I silencers on the initiation of repression (Valenzuela et al. 2008), and the effects of the silencers on nucleosome spacing throughout the locus (Weiss and Simpson 1998).
Redundancy of silencer element function is a hallmark of heterochromatic repression, and redundancy is also found within silencer elements: DNA binding sites for any two of the three silencer binding factors allow repression (Brand et al. 1987). This redundancy likely stems from the factors that they recruit. For example, Rap1 is able to recruit either Sir4 or Sir3 (Moretti et al. 1994; Luo et al. 2002; Chen et al. 2011), Abf1 interacts with Sir3, and ORC has high affinity for Sir1, which in turn binds Sir4 (Triolo and Sternglanz 1996). Thus, each of the silencer-binding factors leads to the recruitment of Sir4 and/or Sir3, and in turn, the Sir2-3-4 complex. Although targeting Sir2 artificially can also nucleate repression, none of the HM silencer elements nucleates repression by first recruiting Sir2. The redundancy among Rap1, Abf1, and ORC (at silencers) or the Ku heterodimer (at telomeres), thus reflects the ability of each nucleator to bind Sir3 or Sir4 and, in turn, to recruit the entire Sir complex to the silencer or telomeric repeat element. Thus, sequence-specific recognition is at the heart of position-dependent repression.
Sir1, which bridges from ORC to Sir4, is unique among the Sir factors. Unlike the others, Sir1 does not spread with the Sir complex beyond the silencers (Fig. 5) (Rusche et al. 2002). Moreover, once it helps establish silencing, Sir1 is no longer needed for the stable maintenance of the repressed state (Pillus and Rine 1989). This argues that Sir1 primarily serves in the establishment step of repression, most likely through its ability to bind the DNA-bound ORC and Sir4 (Triolo and Sternglanz, 1996). Its role in nucleation was shown by tethering the protein artificially through a Gal4 DNA binding domain to Gal4 binding sites, which replaced the HMR-E silencer. In this context, GBD-Sir1 could efficiently nucleate repression, rendering the silencer and its binding factors unnecessary (Chien et al. 1993), although the other Sir proteins and intact histone tails were still required for transcriptional repression.
6.2. Telomeric Heterochromatin
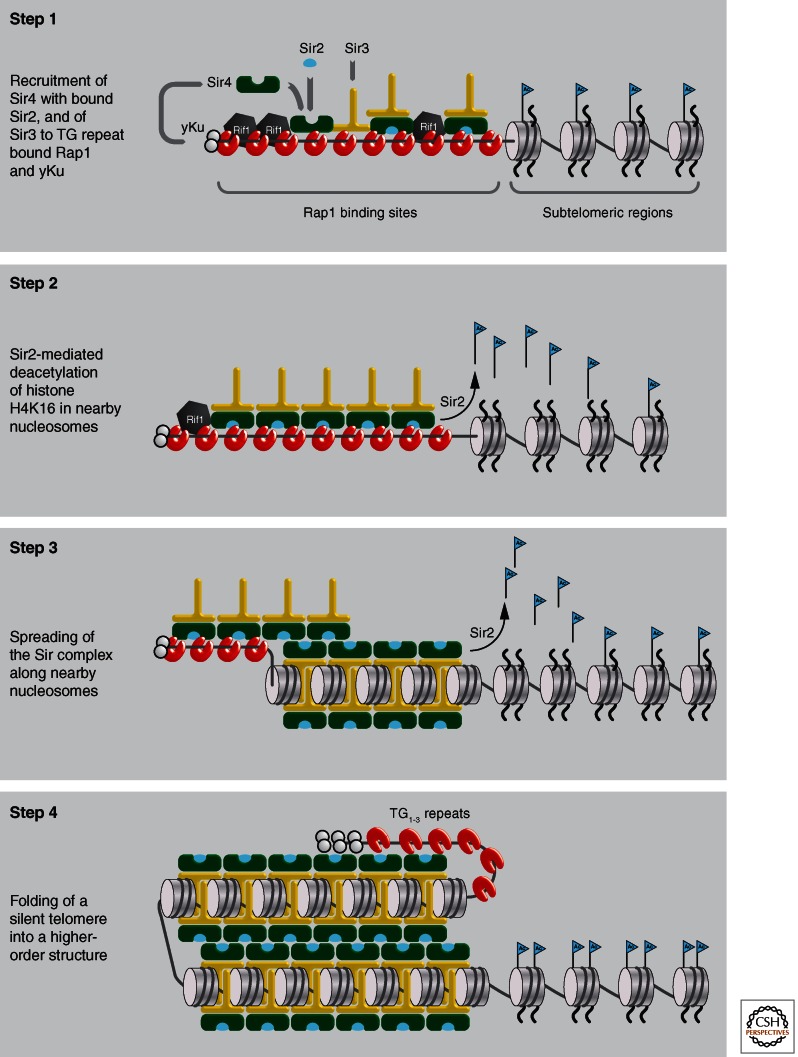
At telomeres, an RNA-based enzyme called telomerase maintains a simple but irregular TG-rich repeat of 300–350 bp in length, which provides 16 to 20 consensus sites for Rap1 binding. This array of Rap1 binding sites forms a nonnucleosomal cap on the chromosomal end, and plays a critical role in telomere length maintenance (Kyrion et al. 1992; Marcand et al. 1997). Along the telomeric repeat, Rap1 binds its consensus through a core DNA binding domain and Sir4 through its carboxy-terminal domain, even in the absence of the other Sir proteins or the H4 amino terminus. Point mutations that disrupt the Rap1-Sir4 interaction disrupt TPE, although the effect on HM repression is very slight (Buck and Shore 1995). Rap1 also binds Sir3 through its carboxy-terminal domain, and mutation of this interface has similar effects on silencing (Chen et al. 2011). However, because the loss of Sir4 prevents other Sir proteins from binding to telomeric chromatin (Luo et al. 2002), Sir4 is apparently the crucial link between nucleation factors and the ensuing silent chromatin structure (Fig. 6).
Figure 6.
Model for stepwise assembly of heterochromatin in yeast. (Step 1) At telomeres, Rap1 and yKu recruit Sir4 even in the absence of Sir2 or Sir3. Only Sir4 can be recruited in the absence of the other Sir proteins, and its binding is antagonized by Rif1 and Rif2 (Mishra and Shore 1999). (Step 2) Sir4-Sir2 and Sir4-Sir3 interact strongly creating Sir complexes along the TG repeats. Sir2 NAD-dependent histone deacetylase activity is stimulated by complex formation and Sir2 deacetylates the acetylated histone H4K16 residue in nearby nucleosomes. (Step 3) Sir complexes spread along the nucleosomes, perhaps making use of the O-acetyl-ADP-ribose intermediate produced by NAD hydrolysis (Liou et al. 2005). Sir3 and Sir4 bind the deacetylated histone H4 tails. Although the deacetylated histone H3 amino-terminal tail also binds Sir3 and Sir4 proteins, it is not shown here. (Step 4) The silent chromatin “matures” at the end of M phase to create an inaccessible structure. This may entail higher-order folding and sequestering at the nuclear envelope.
Equally potent for the nucleation of repression at telomeres is the DNA end-binding complex yKu70/yKu80. The yKu heterodimer also recruits Sir4, and loss of yKu strongly derepresses TPE. Conversely, a targeted GBD-yKu fusion efficiently nucleates repression at silencer-compromised reporter genes. The requirement for yKu at telomeres can be bypassed by eliminating the Rap1-interacting factor, Rif1, which competes for the interaction of Sir4 with the Rap1 carboxy-terminal domain (Fig. 6) (Mishra and Shore 1999). This illustrates the redundancy between the two telomeric nucleation factors, yKu and Rap1, based on their affinity for Sir4.
There is a clear correlation between the amount of Rap1 bound at telomeres that is Rif1-free and the efficiency of silencing. Deletion of RIF1 gene or lesions in Rap1 that block Rif1 binding leads to relatively stable increases in telomere length. Wild-type cells inheriting these longer telomeres show increased frequency of repression of reporter genes such as URA3 or ADE2 integrated at the telomeres on Chr VIIL or Chr VR (Kyrion et al. 1993). Moreover, in a diploid strain containing both elongated and wild-type telomeres on Chr VIIL, the elongated telomere with increased repression did not affect repression at a wild-type length telomere (Park and Lustig 2000). Thus, the effect of telomere length on the frequency of silencing occurs in cis. Moreover, the frequency of switching from a derepressed to a repressed state (white colonies to red in the case of subtelomeric ADE2 gene) also depends on the length of its adjacent telomere; a longer telomeric repeat imposed a lower frequency of switching from derepressed to repressed states (Park and Lustig 2000). Thus, both Rap1 and yKu, which provide recruitment sites for Sir3 and Sir4, can be limiting for the nucleation of TPE.
7. THE CRUCIAL ROLE OF HISTONE H4K16 ACETYLATION AND ITS DEACETYLATION BY Sir2
The molecular interactions between the Sir proteins have been well characterized. Sir4 interacts strongly with Sir2 and separately with Sir3 in vivo and in vitro (Moazed et al. 1997; Strahl-Bolsinger et al. 1997; Hoppe et al. 2002). When coordinately expressed in insect cells, Sir2, Sir3, and Sir4 proteins can be isolated as a stable complex with a 1:1:1 stoichiometry (Cubizolles et al. 2006). Nonetheless, Sir3 has a special role in this process because Sir3 can form a stable extended multimer in vitro (Liou et al. 2005) and its overexpression extends the subtelomeric silent domain from its normal ∼3 kb to ∼15 kb from the telomeric end, coincident with the binding of Sir3 (Renauld et al. 1993; Hecht et al. 1996).
The platform on which the Sir complex spreads consists of nucleosomes with deacetylated histone H3 and H4 amino termini (Braunstein et al. 1996; Suka et al. 2001), and the manner in which Sir3 interacts with histones helps explain how spreading occurs (Fig. 7). Sir3 binds the deacetylated histone H4 amino terminus in a highly selective manner both in vitro and in vivo (Johnson et al. 1990; Johnson et al. 1992; Carmen et al. 2002; Yang et al. 2008a). In this regard, the most important histone region is contained in residues 16–29 of histone H4, and lysine 16 must be positively charged (unmodified or substituted by arginine) for Sir3 to bind (Johnson et al. 1990, 1992).
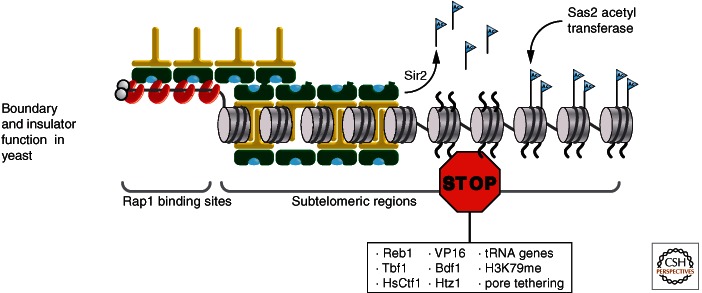
Figure 7.
Heterochromatin boundary function in budding yeast. Spreading of heterochromatin through deacetylation of histone H4K16 by Sir2 is limited by the competing activity of Sas2 histone acetyltransferase, which acetylates H4K16 in adjacent euchromatin, thus preventing Sir3 binding. Methylation of K79 in histone H3 in adjacent euchromatin also affects the spreading of heterochromatin. In addition, factors such as Reb1, Tbf1, and mammalian or viral factors Ctf1 or VP16; nuclear pore tethering; and the presence of tRNA genes may also mediate boundary function. It is conceivable that several of these factors function through the recruitment of histone acetyltransferases, like Sas2.
A cocrystal structure of the Sir3 amino-terminal BAH domain explains this specificity quite well (Armache et al. 2011). Sixteen residues in the Sir3 BAH domain interact with H4 tail residues 13 to 23 which are held in a rigid conformation, primarily through electrostatic interactions with amino acid side chains. A negatively charged binding pocket of BAH Sir3 accommodates the side chains of unmodified H4K16 and H4H18. Indeed, acetylation of H4K16 could potentially disrupt most of the electrostatic contacts in this pocket. In contrast, the Sir2-Sir4 subcomplex binds with slight preference to nucleosomes bearing an acetylated H4K16 residue, at least in the absence of NAD+ (Oppikofer et al. 2011). This is consistent with acetylated histone H4K16 being a preferred and crucial target for the Sir2 enzyme (Imai et al. 2000; Suka et al. 2002; Cubizolles et al. 2006). The AAA+ domain of Sir3 also binds unmodified nucleosomes in vitro (Ehrentraut et al. 2011) and requires that all four H4 acetylation sites (K5, K8, K12, and K16) are deacetylated for optimal binding (Carmen et al. 2002) and for telomeric silencing (Thompson et al. 1994a). Because histones are naturally deacetylated throughout telomeric heterochromatin, the Sir3 carboxyl terminus may contribute to the stability of the Sir3 complex through its interaction with the fully deacetylated histone tail.
On addition of NAD+, the Sir2-catalyzed deacetylation of H4K16ac generates a by-product called O-acetyl-ADP-ribose, as well as a deacetylated histone H4 tail (illustrated in Fig. 5 of Seto and Yoshida 2014). Intriguingly, not only the generation of the high-affinity binding site for Sir3, but apparently also production of the intermediate metabolite O-acetyl-ADP-ribose, enhances the affinity of Sir2-3-4 complexes for chromatin (Johnson et al. 2009; Martino et al. 2009). The generation of O-acetyl-ADP-ribose enhances the interaction of Sir3 with Sir4-Sir2 in vitro (Liou et al. 2005), favors the oligomerization of Sir proteins on nucleosomal arrays (Onishi et al. 2007), and enhances protection of the linker DNA from micrococcal nuclease digestion (Oppikofer et al. 2011). This is consistent with genetic studies showing that any mutation of histone H4K16 disrupts telomere repression, even substitution of lysine by the similarly charged amino acid arginine, or glutamine, which mimics the uncharged nature of acetylated lysine.
Interestingly, the basic region of aa 16–24 within the H4 amino terminus also promotes nucleosomal array compaction in vitro, suggesting that the acetylation state of H4K16 may regulate the higher-order folding of the nucleosomal fiber (Shogren-Knaak et al. 2006). Thus, histone H4K16 deacetylation by Sir2 may actively promote silent chromatin formation in several ways: First, a conformational change may be triggered in the Sir complex by the by-product O-acetyl-ADP-ribose; second, the affinity of the Sir complex for chromatin increases thanks to generation of a high-affinity Sir3 site; and third, even when Sir complexes are not bound, nucleosomal arrays may compact because of contact between the H4 tail and the adjacent nucleosomal face. From this it was clear that control over Sir-mediated silencing would lie in the acetylation/deacetylation cycle of histone H4.
We summarize here the different steps for the initiation and spreading of heterochromatin in an environment enriched for acetylated histone H4, which is likely to be deposited immediately after replication (Fig. 6). At telomeres, Rap1 and yKu recruit Sir4, and Sir4 forms a dimer with Sir2 to deacetylate histone H4 and H3 amino-terminal tails of nearby nucleosomes. Sir3 is recruited by its affinity for Sir4, but also for Abf1 and Rap1. The deacetylation of the histone H4 tail produces a high affinity Sir3 binding site on the nucleosome, which favors assembly of a Sir2-3-4 complex. The interactions of Sir3 with Sir4, Sir3 with the H4 tail and the nucleosomal core, and Sir4 nonspecifically with linker DNA, all seem to contribute to the stable binding of the Sir complex to the nucleosomal fiber. The action of Sir2 on adjacent acetylated nucleosomes appears to trigger the spread of the complex along adjacent histone tails. Finally, the long-range folding of the chromatin fiber may stabilize the repressed state. Most of these events are likely to be very similar at HM loci, although there the initial recruitment of Sir4 needs Rap1, Abf1, or ORC and Sir1. The question then arises, what causes Sir complex spreading to stop?
8. BARRIER FUNCTIONS: HISTONE MODIFICATIONS RESTRICT Sir COMPLEX SPREADING
Because the acetylated histone H4K16 binds Sir2-4 tightly, whereas its deacetylation by Sir2 is crucial for the spreading of heterochromatin, it is not surprising that interfering with the cycle of H4K16 acetylation/deacetylation impedes heterochromatin propagation (Kimura et al. 2002; Suka et al. 2002). The yeast histone acetyltransferase (HAT) Sas2, a member of the highly conserved MYST class of HATs, modifies K16 in bulk euchromatin. Therefore at the boundaries of silent chromatin, one expects to find acetylated histone H4K16. Accordingly, if the SAS2 gene is deleted, or if H4K16 is changed to arginine to simulate the deacetylated state, Sir3, Sir4, and Sir2 spread at low levels inward from the telomeric repeats, approximately fivefold further than in a wild-type cell. This suggests that the spreading of subtelomeric heterochromatin is controlled, at least in part, by the opposing activities of Sir2 and Sas2 on lysine 16 of H4 (Fig. 7). Limiting the global amount of Sir2 (or of the Sir2-Sir4 complex) naturally limits spread by limiting deacetylation.
At the HM loci, restricting the spread of silent chromatin is perhaps even more critical than at telomeres because genes important for growth are found on Chr III, and it is known that silencing can spread bidirectionally from silencers into flanking DNA sequence. One boundary that prevents further spreading of silencing from HMR, is a tRNA gene (Donze and Kamakaka 2001). This boundary function is likely to require the HAT activity that is associated either with transcription or the transcriptional potential of this locus. It is significant that one of these HATs is Sas2, although the H3 HAT, Gcn5, can also affect boundary function of the tRNA gene. This suggests that transcriptional activators, in general, can restrict Sir complex propagation by recruiting HATs. Consistently, in subtelomeric euchromatin regions, boundary activity has been attributed to the general transcription factors, Reb1, Tbf1, and to the acidic trans-activating domain of VP16 (Fourel et al. 1999, 2001). These factors are likely to promote the hyperacetylation of histones (Fig. 7).
Another modification that antagonizes the spread of telomeric heterochromatin is histone H3K79 methylation, which is deposited by the lysine methyltransferase Dot1 (Van Leeuwen et al. 2002; Ng et al. 2002). This histone lysine methyltransferase (KMT) was discovered in a screen for factors whose overexpression caused loss of telomeric silencing (Singer et al. 1998). However, Dot1 does not methylate H3K79 in heterochromatin itself, but instead in adjacent euchromatin and at active genes. Indeed, the artificial targeting of Dot1 to telomeric heterochromatin derepresses silencing by Sir proteins (Stulemeijer et al. 2011), most likely by reducing the affinity of Sir3 for nucleosomes.
Surprisingly, the H4 tail is required for the bulk methylation of H3K79 by Dot1. An elegant set of experiments (Altaf et al. 2007; Fingerman et al. 2007) has generated a model that helps explain the interplay between the H4 tail and the demethylated state of K79 in heterochromatin. Namely, when H4K16 is deacetylated by Sir2, a high affinity binding site for Sir3 is generated by a charged patch in the H4 tail (K16, R17, H18, R19, K20). Interestingly, Dot1 and Sir3 compete for this charged patch, although Sir3 is sensitive to H4K16 acetylation, and Dot1 is not. Thus, in deacetylated heterochromatin, Sir3 is a potent inhibitor of Dot1 binding. However, in adjacent euchromatin, in which K16 is acetylated, Dot1 preferentially binds the charge patch and methylates histone H3K79. The Sir3N-nucleosome crystal structure confirmed genetic evidence, suggesting that Sir3 interacts with H3K79 and that it is in close proximity to H4K16 (Armache et al. 2011). Both the binding of Sir3 and that of the holo-SIR complex are, in turn, weakened by histone H3K79 methylation. Again, this could be reconstituted in vitro, in binding assays between Sir3 and reconstituted nucleosomes that bear either H4K16ac or H3K79me (Oppikofer et al. 2011). Thus, the weak binding of Sir3 to H4K16ac in euchromatin favors Dot1 binding to the H4 tail and subsequent H3K79 methylation, which, in turn, weakens interaction with Sir3. This provides a good example of interhistone interactions as discussed and illustrated in Figure 12 of Allis et al. 2014.
In addition to the mechanism regulating H3K79 methylation and H4K16 acetylation near boundaries, it was reported that in euchromatin the presence of the variant histone H2A.Z and the RNA polymerase associated factor Bdf1 (Meneghini et al. 2003), as well as the tethering of DNA to nuclear pores (Ishii et al. 2002), generate boundaries that limit the spread of silent chromatin. Although the mechanisms by which these factors affect heterochromatin spreading are unknown, it is interesting to note that some inducible genes with H2A.Z-containing promoters, associate with nuclear pores on activation, and remain associated there in a manner that facilitates their re-induction (Ishii et al. 2002; Brickner and Walter 2004). Thus, boundary function may reflect a chromatin state that allows rapid recruitment of transcriptional activators, including HATs, KMTs like Dot1, or nucleosome remodelers that directly or indirectly disrupt histone interactions with heterochromatin proteins (Fig. 7).
9. A ROLE FOR THE H3 AMINO-TERMINAL TAIL IN HIGHER-ORDER CHROMATIN STRUCTURES
There is increasing evidence that the formation of heterochromatin involves a series of steps that include, but go beyond, the binding of Sir proteins. When Sir protein expression is induced artificially in G1, Sir proteins spread from their initiation site by interacting with deacetylated H4 amino termini, but silencing is still defective (Kirchmaier and Rine 2006). Also, when the histone H3K56 acetylation site is mutated, Sir protein spreading occurs, but silencing is disrupted. Similarly, the presence of H3K56 acetylation leads to increased accessibility of Sir-bound nucleosomes to micrococcal nuclease in vitro without impairing Sir protein association (Oppikofer et al. 2011). Thus, the establishment of silencing requires not only Sir protein spreading, but also the deacetylation of H3K56, an event that enables nucleosomes to bind DNA more tightly to form a structure resistant to ectopically expressed bacterial dam methylase (Maas et al. 2006; Xu et al. 2007; Celic et al. 2008; Yang et al. 2008b).
Repression also involves the replacement of histone H3 methylated at K4 and K79 with unmethylated histone H3 (Katan-Khayakovich and Struhl 2005; Osborne et al. 2009). Whereas H3K56 deacetylation by Hst3 and Hst4, two homologs of Sir2, takes place every S phase, demethylation of H3K79 requires dilution by replication, and can take up to four cell divisions. These changes may parallel the formation of a compact, higher-order chromatin structure.
At first, the role of the H3 amino terminus in silencing appeared to be similar to that of the H4 amino terminus because the domain in each tail involved in silencing in vivo was shown to bind Sir3 and Sir4 in vitro (Hecht et al. 1995). However, although the H4 amino-terminal residues are required for the recruitment and spreading of Sir3 and the remaining Sir complex, the H3 tail was required neither for recruitment nor for spreading of the Sir proteins. Nonetheless, deletion of the H3 amino terminus or mutation of critical residues 11–15 (T–G–G–K–A) led to altered topology, increased accessibility to bacterial dam methylase expressed in yeast, and less tightly folded chromatin (Sperling and Grunstein 2009; Yu et al. 2011). Thus, although Sir proteins are clearly recruited by the H4 tail, they may subsequently interact with the H3 amino terminus to form compacted chromatin.
10. TRANS-INTERACTION OF TELOMERES, AND PERINUCLEAR ATTACHMENT OF HETEROCHROMATIN
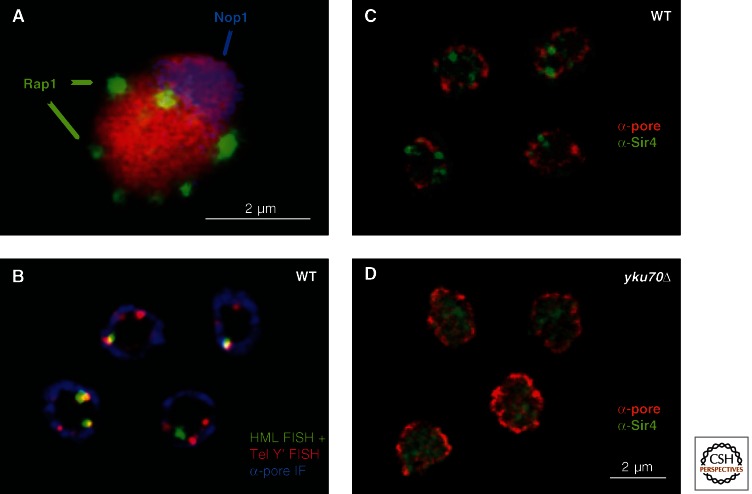
In budding yeast, as in many lower eukaryotes, telomeres cluster together during interphase, in close association with the nuclear envelope. This clustering was initially observed as prominent foci of Rap1 and Sir proteins, which were detected above a diffuse nuclear background by immunostaining (Fig. 8). Disruption of silencing by histone H4K16 mutation, or interference in Rap1 or yKu function, led to the dispersion of the Sir proteins from these clusters (Hecht et al. 1995; Laroche et al. 1998). Later it was shown that not only telomeres, but also the silent HML and HMR loci are closely associated with telomeres at the nuclear envelope. Binding to the nuclear envelope is mediated by redundant pathways that depend either on the telomere-bound yKu factor, or on components of silent chromatin itself. Interestingly, the interactions that lead to telomere clustering can be genetically separated from telomere anchoring to the nuclear envelope, although both pathways involve Sir proteins (Ruault et al. 2011).
Figure 8.
Sir proteins and Rap1 are found in foci at the nuclear periphery. (A) Rap1 (anti-Rap1, green) identifies seven clusters representing all 64 telomeres in this diploid yeast cell nucleus, in which DNA is stained red. Telomeres are either perinuclear or adjacent to the nucleolus (blue, anti-Nop1). (B) Telomeric repeat DNA (red) and HML (green) is identified by fluorescent in situ hybridization. The two colocalize in ∼70% of the cases and both are adjacent to the nuclear envelope (anti-pore staining, blue). (C) The focal distribution of Sir4 (green) adjacent to the nuclear envelope (Mab414, red). (D) This pattern is lost in a yKu70 deletion strain, coincident with the loss of telomeric silencing (Laroche et al. 1998).
Within silent chromatin, the anchoring function has been assigned to the PAD of Sir4 (aa 950–1262) and its interaction with the nuclear envelope associated protein, Esc1 (Andrulis et al. 2002; Taddei et al. 2004). Sir4-Esc1 interactions tether the Sir-repressed chromatin domain at perinuclear sites distinct from pores. Even in the absence of a yKu anchoring pathway, the association of telomeres with the nuclear periphery can be achieved by Sir4-Esc1 interactions, as long as silent chromatin is formed (Hediger et al. 2002). Thus, rings of silent chromatin excised from their chromosomal context by recombination and lacking TG repeats, remain associated with perinuclear foci in a Sir-dependent manner (Gartenberg et al. 2004).
Initially, telomeres are recruited to the nuclear envelope by yKu, given that yKu-dependent tethering occurs even in the absence of silencing. This interaction with the nuclear envelope, together with interactions in trans between telomeres, generates a nuclear subcompartment that appears to sequester Sir proteins from the rest of the nucleoplasm (Fig. 9). yKu mediated anchoring is achieved either through yKu-Sir4 interaction, or through the interaction of yKu with telomerase (Schober et al. 2009). The Est1 subunit of telomerase binds specifically to an inner nuclear membrane-spanning protein, Mps3, which is a member of the conserved SUN domain family of inner nuclear envelope proteins. Intriguingly, this interaction is cell-cycle specific and mediates the link between yKu and the nuclear envelope only in S phase, possibly to maintain anchorage while subtelomeric chromatin is disrupted by the replication fork. In G1 phase, there appears to be a secondary yKu anchoring pathway, and Sir4 may bind Mps3 through an intermediary to help tether telomeres (Bupp et al. 2007).
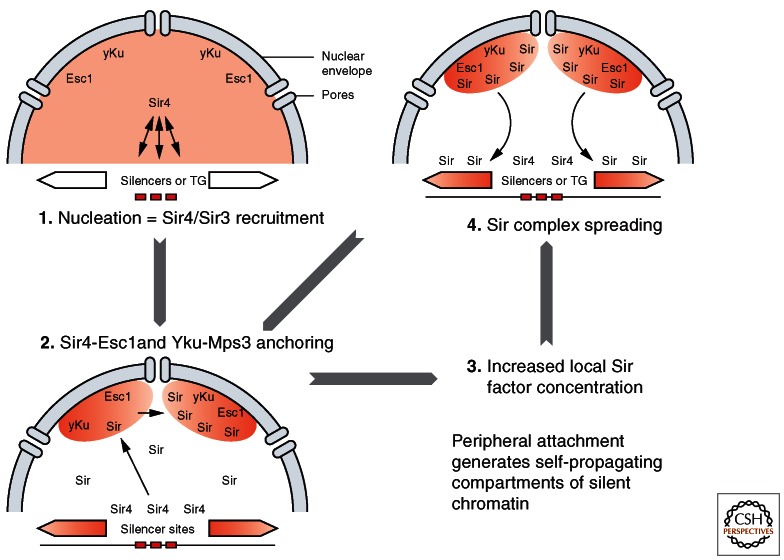
Figure 9.
Spontaneous formation of silencing subcompartments. A simple model for the formation of subnuclear compartments is shown. (1) Sir4 is first recruited at the nucleation center by DNA binding proteins that can bind Sir4. These include Rap1, ORC, Abf1, and yKu. (2) The presence of Sir4 at the locus will then bring it to the nuclear periphery through one of the two Sir4 anchoring pathways (yKu or Esc1). (3) At the nuclear envelope, the high local concentrations of Sir proteins will help silencing complexes assemble and spread. (4) The ability of silent loci to remain attached at the periphery increases the local concentration of Sir proteins and reinforces the silencing of other loci within this region. Importantly, telomere-bound yKu can independently recruit telomeres to the nuclear envelope just as Sir4 recruits silencer sequences.
Both pathways of telomere anchoring, through Sir4-Esc1 and S-phase yKu-Est1-Mps3 pathway, are controlled by post-translational modifications. Sir4 and both subunits of yKu are modified by SUMO, a ubiquitin-like moiety, deposited specifically by the E3 SUMO ligase Siz2. Remarkably, loss of Siz2 led to the displacement of telomeres from the nuclear envelope (Ferreira et al. 2011). Silencing was reduced only slightly in siz2 mutants, possibly because telomeres became abnormally long due to telomerase deregulation (see Section 12). Thus, the perinuclear compartment created by telomere anchoring and silent chromatin appears to regulate telomere functions beyond silencing.
The perinuclear clustering generates a subnuclear compartment that favors silencing (Fig. 9). Evidence supporting this conclusion includes the fact that silencer-flanked HM constructs repress less efficiently when they are integrated far from telomeres (Thompson et al. 1994b; Maillet et al. 1996), and this can be reversed by artificially tethering the domain at the nuclear envelope through a targeted transmembrane factor (Andrulis et al. 1998). Importantly, the ability to improve repression by peripheral tethering (or by being placed in telomere proximity) is lost when Sir3 and Sir4 are no longer sequestered in foci (Taddei et al. 2009). Similarly, coordinate overexpression of Sir3 and Sir4 proteins ablates the positive effects of tethering. Thus, an uneven distribution of Sir proteins is the relevant feature of telomere sequestration at the nuclear envelope for chromatin-mediated repression. Given that displaced Sir proteins can repress promiscuously (Taddei et al. 2009), the sequestration of Sir foci also positively reinforces active gene expression. It is proposed that the assembly of newly replicated DNA into heterochromatin is likely to be favored when DNA is replicated in a zone rich in silencing factors.
The crucial interaction that mediates telomere–telomere clustering, even in the absence of silencing, appears to be Sir3. Whether this is mediated by Sir3 itself or ligands of Sir3 remains to be determined, yet some form of clustering can occur even in the absence of Sir2 and Sir4 (Ruault et al. 2011). Other factors also affect telomere–telomere interaction, namely, the other Sir proteins, the Ku heterodimer, Asf1, Rtt109, Esc2, the Cohibin complex, and two factors involved in ribosome biogenesis, Ebp2 and Rrs1. However, because these factors also affect heterochromatin formation, one cannot rule out that they promote clustering by promoting Sir3 recruitment to telomeres.
11. TELOMERE LOOPING
A further long-range interaction may stem from the folding back of a single telomere on itself, which may allow silent chromatin to bypass subtelomeric boundary elements and stabilize repressed chromatin at subtelomeric genes (Figs. 5 and 6). Although Rap1 binding sites are found only within the first ∼300 bp of TG repeat DNA on the end of a telomere, ChIP showed that Rap1 is associated with nucleosomes as far as ∼3 kb away from the TG repeat (Strahl-Bolsinger et al. 1997). Similarly, yKu is recovered for ∼3 kb from the chromosomal end to which it binds (Martin et al. 1999). When silencing is disrupted by mutation of SIR genes, both Rap1 and yKu are lost exclusively from the more internal subtelomeric sequences and not from the terminal TG repeats (Hecht et al. 1996; Martin et al. 1999). This was interpreted as showing that the truncated telomere folds back, enabling TG-bound Rap1 and yKu to bind Sir proteins in trans (Figs. 5 and 6).
Evidence for telomere looping comes from the work of de Bruin et al. (2001), who have exploited the inability of yeast transcriptional activators, such as Gal4, to function from a site downstream of the targeted gene. Strains were constructed in which the Gal4 upstream activating sequence (UAS) element was placed beyond the 3′ termination site of a reporter gene, and the construct was inserted either at an internal chromosomal location or near a telomere. At an internal site, this construct could not be induced by activating Gal4, but in a subtelomeric context, the Gal4 UAS could activate the promoter from a site 1.9 kb downstream of the promoter. This was Sir3-dependent, arguing that the telomeric end can fold back in the presence of Sir3, but not in its absence, to allow the Gal4 UAS to position itself proximal to the transcription start site. In this way, silent chromatin appears to promote at least a transient folding of the chromosome end.
12. VARIABLE REPRESSION AT NATURAL SUBTELOMERIC DOMAINS
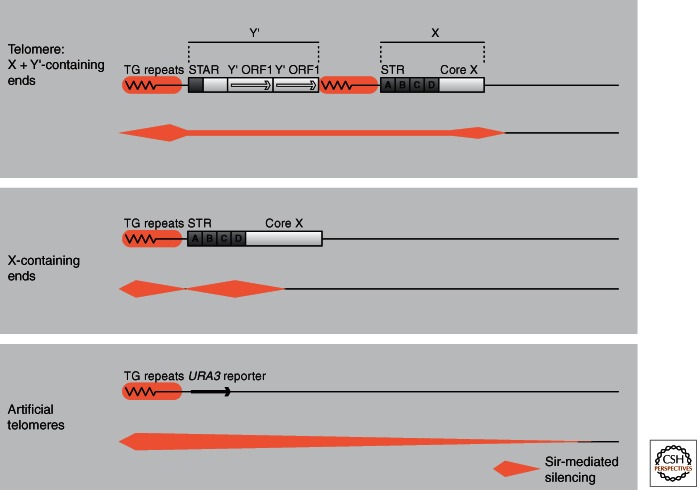
We have set forth here a simplistic view of continuous silent chromatin emanating from the telomeric Rap1 binding sites, yet the situation at native telomeres is significantly more complex, largely because of the presence of natural boundary elements found in subtelomeric repeat sequences. Generally, when reporter constructs for telomeric repression are integrated, the subtelomeric repeat elements called X and Y′ at telomeres are deleted, placing the reporter gene and unique sequence immediately adjacent to TG repeats. All native telomeres, on the other hand, contain a core subtelomeric repeat element, X, which is positioned between the TG repeat and the most telomere-proximal gene, and 50%–70% of native telomeres also contain at least one copy of a larger subtelomeric element called Y′ (Fig. 10). Both X and Y′ elements contain binding sites for the transcriptional regulators Tbf1 and Reb1, which have been shown to reduce the spread of silent chromatin (Fourel et al. 1999). However, X elements also contain autonomously replicating sequence (ARS) consensus sequences, and binding sites for Abf1 and other transcription factors, which have the opposite effect: These re-initiate or boost the repression of reporters placed on the centromere proximal side of these elements. The result is one of discontinuity in silencing at many native telomeres. This adds a level of complexity to the model of continuous spreading outlined in Figure 6. Pryde and Louis (1999) have proposed that the unrepressed Y′ element loops out when it is found between two repressed domains, leading to discontinuity in silent domains without eliminating the need for nucleation and spreading from the TG repeats.
Figure 10.
The organization of native telomeres and their silencing patterns. Subtelomeric elements are shown with their major protein binding sites. Telomeres fall into the two general classes: X-containing or X+Y′-containing ends. The STAR and STR elements block the propagation of repression and leave a region of reduced repression within the Y′ or X element. This is not the case at artificially truncated telomeres in which there is a gradient of repression that extends 3–4 kb from the TG repeat. Looping similar to that in Figures 5 and 6 is proposed for native telomeres, so that repressed regions contact each other leaving unrepressed chromatin in between areas of contact. (Adapted from Pryde and Louis 1999.)
There is large variation in the efficiency of TPE at different native telomeres in budding yeast. If one inserts a reporter gene near a telomere without deleting the subtelomeric repeats, only about half of the telomeres appear to be subject to TPE (Pryde and Louis 1999). Empirically, it was shown that telomeres containing only X subtelomeric elements (rather than XY′ telomeres) are more likely to silence, possibly because the Y′ long terminal repeats bind factors that prevent Sir spreading, whereas various transcription factors in the X element (e.g., Reb1, Tbf1, and Abf1) contribute to Sir factor nucleation (Mak et al. 2009). These subtelomeric elements are particularly enriched for factors that regulate stress genes, whose effects on TPE may differ from their effects on transcription at nontelomeric loci. For example, Reb1 has boundary activity at telomeres (Fourel et al. 1999), but is a gene activator at internal sites. The binding of such factors can explain the discontinuity of Sir-mediated repression at native telomeres, yet they do not appear to affect the repression of classical reporters for TPE, which are integrated without X or Y′ elements (Renauld et al. 1993).
Interestingly, many of the genes found in subtelomeric domains are repressed by the HDAC Hda1 and the repressor Tup1 (Robyr et al. 2002), and are induced only on stress conditions (Ai et al. 2002). In general, the 267 genes that are within 20 kb of budding yeast telomeres produce roughly five times fewer mRNA molecules (average of 0.5/cell) than nontelomeric genes. Importantly, only 20 of these genes show derepression upon loss of Sir3. This leads to a logic of genome organization in which genes that are rarely or conditionally induced are found near telomeres, in which they are repressed by a non-Sir mechanism, but are adjacent to domains repressed by Sir proteins and, therefore, also tethered near the nuclear envelope.
13. INHERITANCE OF EPIGENETIC STATES
A universal characteristic of heterochromatin is that its silent state is passed from one generation to the next. This requires the reassembly of a repressive chromatin structure on daughter strands soon after replication of the DNA template. Pioneering work on the role of the cell cycle in the establishment or inheritance of the silent state was performed by Miller and Nasmyth, who studied the onset and loss of silencing with a temperature-sensitive sir3ts mutant (Miller and Nasmyth 1984). A shift from permissive temperature to nonpermissive temperature caused silencing to be lost immediately, indicating that SIR3 was required for maintenance of the repressed state. However, in the reciprocal experiment, shifting from nonpermissive temperature to permissive temperature (SIR3+) did not lead to immediate restoration of repression; passage through the cell cycle was required. They concluded that an event in S phase was required for establishment of heritably repressed chromatin. This requirement was later shown to involve events in both S and G2/M phases (Lau et al. 2002).
Initially it was thought that origin firing from the silencer linked ARS elements might be a critical event in the establishment or inheritance of silent chromatin, but because there was no detectable initiation from the origins flanking the HML locus, this seemed an unlikely explanation. Indeed, an experiment showing that ORC can be efficiently replaced by a targeted GDB-Sir1 fusion protein put to rest the notion that origin firing is essential for the inheritance of silent chromatin. In addition, recent experiments have shown that establishment of repression can occur on DNA that does not replicate (Kirchmaier and Rine 2001; Li et al. 2001). Nonetheless, on rings of silent chromatin that are excised from the genome either with or without silencers, Sir complex association is in continual flux, arguing that nucleation and/or stabilization provided by silencer elements at HM loci is needed to actively suppress rapid decay (Cheng and Gartenberg 2000).
What occurs in S phase to enable the propagation of silent chromatin? One candidate event could be the deacetylation of H4K16ac or H3K56ac, or else the suppression of the enzymes that deposit these modifications (Xu et al. 2007; Neumann et al. 2009). Alternatively, chaperones necessary for histone deposition (CAF1) may be required for generating repressed chromatin after replication. The deacetylation of histone H3K56 can be achieved in vitro by Sir2 family members (Xu et al. 2007; Oppikofer et al. 2011) and in vivo during late S phase by Hst3 and Hst4 (Maas et al. 2006; Celic et al. 2008; Yang et al. 2008b). These two Sir2 paralogs are nuclear enzymes whose activities are required in S phase when newly synthesized DNA must be assembled into repressed chromatin, yet they are not structural components of heterochromatin. Importantly, disruption of the two genes weakens, but does not eliminate, Sir mediated silencing (Yang et al. 2008b).
Other studies have shown that robust silencing is not achieved until telophase, well beyond the S-phase window of nucleosome assembly. It appears that prevention of the metaphase degradation of the cohesin subunit, Scc1, inhibits stable repression (Lau et al. 2002). Propagation of repression thus depends both on a critical S-phase component, and a further event that entails continual recruitment and loading of Sir proteins.
Intriguingly, when the proteins of telomeric heterochromatin were examined by ChIP in the transcriptionally OFF and ON states, the major difference found between them was the presence of H3K79 methylation at telomeric chromatin in the ON state (Kitada et al. 2012). Because Dot1 is recruited during transcription (Shahbazian et al. 2005), this suggests a positive feedback loop in which the ON state is triggered, possibly by decreased telomere length, to initiate transcription and K79 methylation. Dot1 would then be responsible for maintaining the ON state through its promotion of K79 methylation.
14. OTHER FUNCTIONS OF Sir PROTEINS AND SILENT CHROMATIN
Although the standard function of heterochromatin is the silencing of adjacent genes, a closer examination of silencing factors has uncovered a plethora of new functions that correlate with silencing or require silencing factors. Particularly in organisms in which repetitive centromeric DNA plays a crucial role in centromere function, it is clear that heterochromatin contributes to centromere and kinetochores function. Budding yeast, on the other hand, does not depend on silent chromatin for centromere function. Nonetheless, a number of other roles have been identified for silent chromatin or silent chromatin factors, and these are described in this section.
14.1. Suppression of Recombination
In Drosophila highly active rDNA repeats are adjacent to centromeric heterochromatin, and in many higher eukaryotic species, nucleoli and condensed heterochromatin are spatially juxtaposed. It is significant, therefore, that yeast Sir2, independent of the other Sir proteins, is genetically and physically associated with rDNA repeats (Gotta et al. 1997). Importantly, the loss of Sir2 in budding yeast leads to a dramatic increase in rDNA recombination and reduction of the contiguous integrated array (Gottlieb and Esposito, 1989). Instability of the rDNA has been also correlated with an accumulation of extrachromosomal rDNA circles, which arise from unequal crossing over between sister chromatids (Kobayashi et al. 2004). These events that are normally suppressed by a complex called Cohibin, a V-shaped complex of two Lrs4 proteins and two Csm1 homodimers (Mekhail et al. 2008; Chan et al. 2011), which also mediates the association of rDNA repeats with the two nuclear envelope proteins, Heh1 (a human Man1 paralog) and Nur1. Loss of either Sir2 or the cohibin-Heh1 anchoring pathway leads to instability of the rDNA repeat, followed by cell-cycle arrest or premature senescence (Fig. 11A) (Sinclair and Guarente 1997; Kaeberlein et al. 1999). Although Sir2 can also silence RNA Pol II genes integrated in the rDNA (Smith and Boeke 1997), recent evidence separates this effect on transcription from the role of Sir2 in preventing rDNA recombination.
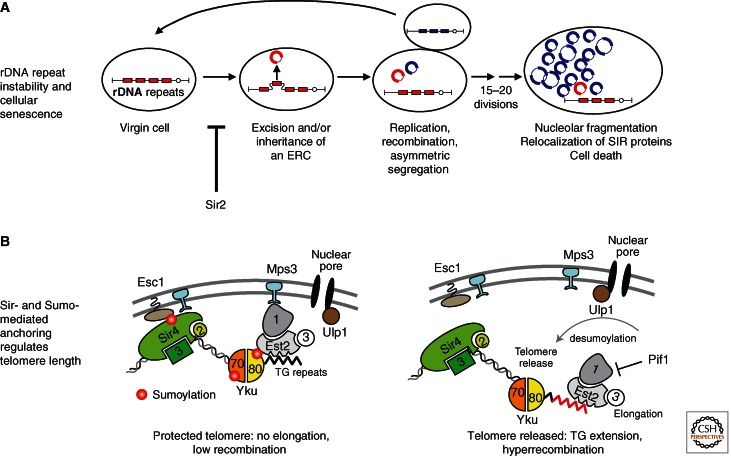
Figure 11.
Secondary functions of Sir proteins and silent chromatin. (A) rDNA recombination leads to cellular senescence in yeast. The rDNA is organized in an array of 140–200 direct repeats of a 9.1 kb unit (red block). These encode the 18S, 5.8S, 25S, and 5S rRNAs, and contain two Sir2 responsive elements downstream of the 5S gene and within the 18S gene. The rDNA repeats tend to be excised in aging yeast cells, and the circles accumulate in the mother cell (Kaeberlein et al. 1999). This correlates with premature senescence and can be antagonized by Sir2, which helps suppress unequal recombination and ring excision. (B) Telomere anchoring and silent chromatin contribute to telomere homeostasis. Redundant pathways that tether yeast telomeres to the nuclear envelope include sumoylation targets, Sir4, yKu70, and yKu80 (Ferreira et al. 2011). The relevant Sumo E3 ligase is Siz2. Loss of Siz2, ablation of the Mps3 amino terminus, or deletion of Sir4 all lead to release of telomeres from the nuclear envelope and longer steady state telomere length. Loss of Mps3 amino terminus or yKu also increases telomere recombination. This suggests that sequestration at the nuclear envelope may limit access for both recombination and telomerase activation mechanisms, and that loss of anchoring increases both pathways. Regulated desumoylation by Ulp1 may play a role in releasing telomeres from the periphery allowing efficient elongation in late S phase. Siz2-mediated sumoylation is indicated by red circles.
Exactly how the tethering of rDNA repeats to the nuclear envelope reduces recombination is still unknown. The simplest explanation may be that it imposes a steric hindrance on the binding of Rad52, a protein essential for homologous exchange in yeast. Indeed, Rad52 is excluded from the nucleolus and when rDNA requires repair by recombination, the damage is extruded from the nucleolus. Intriguingly, other mutations that reduce the efficiency of rDNA excision, such as the elimination of the replication fork barrier protein Fob1, extend replicative lifespan of yeast cells, confirming that rDNA instability is indeed a key culprit for limiting cellular lifespan.
Because Sir2 is a NAD-dependent deacetylase, and because NAD levels act as a metabolic thermostat, it was proposed that the effect of yeast Sir2 on lifespan might be related to the extension of lifespan by calorie restriction, a conserved pathway that functions in many species. However, Sir2 and caloric restriction increase lifespan through independent pathways (Kaeberlein et al. 2004). It is also relevant to note that an accumulation of extrachromosomal rDNA rings has not been detected during aging in any other species. This is probably because of the unique budding mechanism through which yeast divides; the budding geometry and rapid kinetics of mitosis in yeast leads to an inevitable retention of noncentromere-bearing DNA elements in the mother cell (Gehlen et al. 2011).
14.2. Preventing Homologous Recombination on Chr III
Switching mating type involves the generation of a double strand break induced by the HO endonuclease at the MAT locus. That break is then repaired with homologous sequences that recombine from the heterochromatic silent HM loci. In vitro studies have shown that the holo-Sir complex and histone sequences involved in silencing prevent cleavage by the HO endonuclease at HM loci, and impair the early steps in strand invasion by the break induced at MAT. The nucleosome remodeling SWI/SNF complex, which displaces Sir3 from nucleosomes (Sinha et al. 2009), is needed to counteract the repression of strand invasion conferred by silent chromatin. This enables recombinational repair of MAT through an appropriate HM donor.
The deletion of SIR3 enhanced general recombination rates throughout the genome (Palladino et al. 1993). More specifically the loss of telomeric anchoring correlates with increased rates of recombination between a telomeric sequence and internal sequences (Marvin et al. 2009). The latter involves the integrity of yKu, but probably does not simply reflect the loss of anchoring alone.
14.3. Chromosomal Cohesion
Cohesion of sister chromatids is made possible by a complex of proteins known as cohesin. Yeast heterochromatin, like that of more complex eukaryotes, is enriched in cohesin which holds these silent regions together in sister chromatids. When Sir proteins were tethered to chromosomal sites, in which pairing of sister chromatids could be seen by fluorescence microscopy, it was evident that Sir2 alone could mediate cohesion. Although this required the cohesin complex, it did not require the deacetylase activity of Sir2 indicating yet another function for Sir2 in chromosomal mechanics (Wu et al. 2011). It remains to be seen if this involves components of the Cohibin complex (Chan et al. 2011).
14.4. Telomere Length Regulation
In yeast, modification of histone H2A serine 129 by phosphorylation generates a modified form of H2A, called γH2A. In other species, this serine acceptor site is only present in an H2A variant called H2AX. Histone H2A phosphorylation is mediated by the checkpoint kinases, Tel1 or Mec1, and occurs when these central checkpoint kinases are recruited to DNA damage by a complex of replication protein A with ssDNA or by the Mre11 complex. Intriguingly, even without exogenously induced damage, γH2A was found by ChIP to be coextensive with and dependent on silent subtelomeric chromatin (Szilard et al. 2010; Kitada et al. 2011). The phosphorylation of H2A in telomeres is mediated by Tel1 kinase with weaker contributions from Mec1 kinase. This suggests that subtelomeric domains trigger a low-level checkpoint response, possibly during telomere replication. The persistence of γH2A in silent domains may stem from reduced histone turnover, given that the enzyme that dephosphorylates γH2A acts only on nonnucleosomal H2A (Keogh et al. 2006). Point mutations that remove the H2A phosphoacceptor residue render telomeres slightly shorter in certain genetic backgrounds, again linking telomeric chromatin structure to aspects of telomerase control (Kitada et al. 2011).
As discussed above, telomerase activity is increased when telomeres are released from the nuclear envelope because of the absence of Sir4 or the SUMO ligase Siz2 (Palladino et al. 1993; Ferreira et al. 2011). This further links telomere length homeostasis to subtelomeric chromatin status, implicating both the Sir proteins and the binding of the yKu complex to Mps3 (Fig. 11). Interestingly, activation of Mec1 kinase by double-strand breaks leads to a partial release of Sir proteins and displacement of telomeres (Martin et al. 1999), although it is unclear what Sir protein release achieves in this situation.
14.5. A Link with Replication Factors
Whereas passage through an event between early S and G2/M is required for silencing, this critical event is not replication itself. Nevertheless, a number of replication factors are involved in silencing, most likely through structural roles that are independent of their roles in replication. ORC binding sites are found at each of the silencer E and I elements, and ORC directly recruits Sir1 in the absence of replication. Moreover, ORC binds not only at the silencer elements, but also in the region between E and I at HMR in a manner that is dependent on Sir proteins.
Like ORC, the helicase complex Mcm2-7 is part of the prereplicative complex (pre-RC) that assembles on replication origins before the initiation of replication in S phase. However, MCM proteins are found in abundance in yeast cells and may have functions in processes distinct from DNA replication, such as silencing. Indeed, Sir2 interacts indirectly with proteins of the Mcm2-7 complex outside of S phase through a carboxy-terminal protein bridge (53aa) found in another pre-RC component, Mcm10. Mutations in this bridge disrupt the binding of Mcm2-7 with Sir2 and weaken silencing efficiency, but they do not disrupt replication nor the association of Sir2 with chromatin (Liachko and Tye, 2009). In a speculative model, it is suggested that Mcm10, which itself forms a ring-shaped hexamer, binds Sir2 away from chromatin, ensuring that Sir2 undergoes a modification that renders it more competent for silencing. In the absence of Mcm10 and the MCM complex, a less competent Sir2 would be incorporated into chromatin, reducing repression. What this modification might be remains unknown, but it is noteworthy that in fruit flies, Mcm10 also interacts with Hp1, and that in yeast, Mcm10’s silencing function can be genetically separated from its replication function.
Independent of Mcm10, there is a strong evolutionary relationship between silencing and certain replication factors, revealed by a phylogenetic analysis of Orc1, the largest subunit of the ORC complex and Sir3. K. lactis contains Orc1 that is found at replication origins and HMLα. As mentioned above, K. lactis contains the Orc1 paralog of Sir3, but not Sir3. The BAH domain of Orc1, however, interacts with the deacetylated H4K16 just like S. cerevisiae Sir3, allowing Orc1 to spread along telomeric and HMLα heterochromatin in a manner that is dependent on Sir2 and Sir4 (Hickman and Rusche 2010). Surprisingly, however, HMR in K. lactis is silenced by a different mechanism that involves neither Orc1 nor Sir4.
14.6. Regulating Replication Origin Choice
Replication origins are primed in G1 phase of the cell cycle by the formation of a pre-RC that recognizes DNA replication origins. Interestingly, Sir2 inhibits pre-RC assembly at certain origins, including one that is found at HMR-E. This origin is sensitive to the presence of Sir2 and Sir3, arguing that the silencing mechanism itself influences origin choice. In contrast, certain origins that are not in heterochromatin are sensitive to the presence of Sir2 only, indicating that Sir2 has a unique function in origin function that is independent of transcriptional silencing. This function may be in nucleosome placement near the origins that prevents pre-RC assembly and is controlled by the deacetylation of histone H4K16 by Sir2 (Fox and Weinreich 2008). This is but one among many aspects of chromatin structure that affect origin function, particularly in higher eukaryotic species.
15. SUMMARY
Combined genetic, biochemical, and cytological techniques have been exploited in budding yeast to show fundamental principles at work during heterochromatin-mediated gene silencing. These principles include (1) the mechanism of initiation, spreading, and barriers to spreading of heterochromatin; (2) the balance of heterochromatin factors and their distribution within a subnuclear environment; (3) higher-order folding of heterochromatin; and (4) the effects of cell-cycle involvement in its formation. The in vivo and in vitro studies to date provide a strong mechanistic basis for our understanding of the assembly of heterochromatin from chromatin fibers in all eukaryotes. The in vitro systems currently being developed for the reconstitution of yeast heterochromatin promise to yield a structural reconstruction of Sir complexes bound to chromatin, enabling a first glimpse at higher-order chromatin folding.
Footnotes
Editors: C. David Allis, Marie-Laure Caparros, Thomas Jenuwein, and Danny Reinberg
Additional Perspectives on Epigenetics available at www.cshperspectives.org
REFERENCES
* Reference is also in this collection.
- Ai W, Bertram PC, Tsang CK, Chan TF, Zheng XF 2002. Regulation of subtelomeric silencing during stress response. Mol Cell 10: 1295–1305 [DOI] [PubMed] [Google Scholar]
- *.Allis CD, Jenuwein T, Reinberg D 2014. Overview and concepts. Cold Spring Harb Perspect Biol 10.1101/cshperspect.a018739 [DOI] [Google Scholar]
- Altaf M, Utley RT, Lacoste N, Tan S, Briggs SD, Côté J 2007. Interplay of chromatin modifiers on a short basic patch of histone H4 tail defines the boundary of telomeric heterochromatin. Mol Cell 28: 1002–1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrulis ED, Neiman AM, Zappulla DC, Sternglanz R 1998. Perinuclear localization of chromatin facilitates transcriptional silencing. Nature 394: 592–595 [DOI] [PubMed] [Google Scholar]
- Andrulis ED, Zappulla DC, Ansari A, Perrod S, Laiosa CV, Gartenberg MR, Sternglanz R 2002. Esc1, a nuclear periphery protein required for Sir4-based plasmid anchoring and partitioning. Mol Cell Biol 22: 8292–8301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansari A, Gartenberg M 1997. The yeast silent information regulator Sir4p anchors and partitions plasmids. Mol Cell Biol 17: 7061–7068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aparicio OM, Billington BL, Gottschling DE 1991. Modifiers of position effect are shared between telomeric and silent mating-type loci in S. cerevisiae. Cell 66: 1279–1287 [DOI] [PubMed] [Google Scholar]
- Armache KJ, Garlick JD, Canzio D, Narlikar GJ, Kingston RE 2011. Structural basis of silencing, Sir3 BAH domain in complex with a nucleosome at 3.0 Å resolution. Science 334: 977–982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck DB, Burton A, Oda H, Ziegler-Birling C, Torres-Padilla ME, Reinberg D 2012. The role of PR-Set7 in replication licensing depends on Suv4-20h. Genes Dev 26: 2580–2589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand AH, Breeden L, Abraham J, Sternglanz R, Nasmyth K 1985. Characterization of a “silencer” in yeast: A DNA sequence with properties opposite to those of a transcriptional enhancer. Cell 41: 41–48 [DOI] [PubMed] [Google Scholar]
- Brand AH, Micklem G, Nasmyth K 1987. A yeast silencer contains sequences that can promote autonomous plasmid replication and transcriptional activation. Cell 51: 709–719 [DOI] [PubMed] [Google Scholar]
- Braunstein M, Sobel RE, Allis CD, Turner BM, Broach JR 1996. Efficient transcriptional silencing in Saccharomyces cerevisiae requires a heterochromatin histone acetylation pattern. Mol Cell Biol 16: 4349–4356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickner JH, Walter P 2004. Gene recruitment of the activated INO1 locus to the nuclear membrane. PLoS Biol 2: e342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck SW, Shore D 1995. Action of a RAP1 carboxy-terminal silencing domain reveals an underlying competition between HMR and telomeres in yeast. Genes Dev 9: 370–384 [DOI] [PubMed] [Google Scholar]
- Bupp JM, Martin AE, Stensrud ES, Jaspersen SJ 2007. Telomere anchoring at the nuclear periphery requires the budding yeast Sad1-UNC-84 domain protein Mps3. J Cell Biol 179: 845–854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmen AA, Milne L, Grunstein M 2002. Acetylation of the yeast histone H4 N terminus regulates its binding to heterochromatin protein SIR3. J Biol Chem 277: 4778–4781 [DOI] [PubMed] [Google Scholar]
- Celic I, Verreault A, Boeke JD 2008. Histone H3K56 hyperacetylation perturbs replisomes, causes DNA damage. Genetics 179: 1769–1784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan JN, Poon BP, Salvi J, Olsen JB, Emili A, Mekhail K 2011. Perinuclear cohibin complexes maintain replicative life span via roles at distinct silent chromatin domains. Dev Cell 20: 867–879 [DOI] [PubMed] [Google Scholar]
- Chang JF, Hall BE, Tanny JC, Moazed D, Filman D, Ellenberger T 2003. Structure of the coiled-coil dimerization motif of Sir4 and its interaction with Sir3. Structure 11: 637–649 [DOI] [PubMed] [Google Scholar]
- Chen Y, Rai R, Zhou ZR, Kanoh J, Ribeyre C, Yang Y, Zheng H, Damay P, Wang F, Tsujii H, et al. 2011. A conserved motif within RAP1 has diversified roles in telomere protection, regulation in different organisms. Nat Struct Mol Biol 18: 213–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Widom J 2005. Mechanism of transcriptional silencing in yeast. Cell 120: 37–48 [DOI] [PubMed] [Google Scholar]
- Cheng TH, Gartenberg MR 2000. Yeast heterochromatin is a dynamic structure that requires silencers continuously. Genes Dev 14: 452–463 [PMC free article] [PubMed] [Google Scholar]
- Chien CT, Buck S, Sternglanz R, Shore D 1993. Targeting of SIR1 protein establishes transcriptional silencing at HM loci and telomeres in yeast. Cell 75: 531–541 [DOI] [PubMed] [Google Scholar]
- Chopra VS, Mishra RK 2005. To SIR with Polycomb: Linking silencing mechanisms. Bioessays 27: 119–121 [DOI] [PubMed] [Google Scholar]
- Cockell M, Gotta M, Palladino F, Martin SG, Gasser SM 1998. Targeting Sir proteins to sites of action: A general mechanism for regulated repression. Cold Spring Harb Symp Quant Biol 63: 401–412 [DOI] [PubMed] [Google Scholar]
- Cubizolles F, Martino F, Perrod S, Gasser SM 2006. A homotrimer-heterotrimer switch in Sir2 structure differentiates rDNA and telomeric silencing. Mol Cell 21: 825–836 [DOI] [PubMed] [Google Scholar]
- de Bruin D, Zaman Z, Liberatore RA, Ptashne M 2001. Telomere looping permits gene activation by a downstream UAS in yeast. Nature 409: 109–113 [DOI] [PubMed] [Google Scholar]
- *.Dekker J, Misteli T 2014. Long-range chromatin interactions. Cold Spring Harb Perspect Biol 10.1101/cshperspect.a019356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donze D, Kamakaka RT 2001. RNA polymerase III and RNA polymerase II promoter complexes are heterochromatin barriers in S. cerevisiae. EMBO J 20: 520–531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrentraut S, Hassler M, Oppikofer M, Kueng S, Weber JM, Mueller JW, Gasser SM, Ladurner AG, Ehrenhofer-Murray AE 2011. Structural basis for the role of the Sir3 AAA+ domain in silencing, interaction with Sir4 and unmethylated histone H3K79. Genes Dev 25: 1835–1846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Elgin SCR, Reuter G 2013. Position-effect variegation, heterochromatin formation, and gene silencing in Drosophila. Cold Spring Harb Perspect Biol 10.1101/cshperspect.a017780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira HC, Luke B, Schober H, Kalck V, Lingner J, Gasser SM 2011. The PIAS homologue Siz2 regulates perinuclear telomere position and telomerase activity in budding yeast. Nat Cell Biol 13: 867–874 [DOI] [PubMed] [Google Scholar]
- Fingerman IM, Li HC, Briggs SD 2007. A charge-based interaction between histone H4 and Dot1 is required for H3K79 methylation and telomere silencing: Identification of a new trans-histone pathway. Genes Dev 21: 2018–2029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fourel G, Revardel E, Koering CE, Gilson E 1999. Cohabitation of insulators, silencing elements in yeast subtelomeric regions. EMBO J 18: 2522–2537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fourel G, Boscheron C, Revardel E, Lebrun E, Hu YF, Simmen KC, Muller K, Li R, Mermod N, Gilson E 2001. An activation-independent role of transcription factors in insulator function. EMBO Rep 2: 124–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox CA, Weinreich M 2008. Beyond heterochromatin: SIR2 inhibits the initiation of DNA replication. Cell Cycle 7: 3330–3334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye RA 2000. Phylogenetic classification of prokaryotic and eukaryotic Sir2-like proteins. Biochem Biophys Res Commun 273: 793–798 [DOI] [PubMed] [Google Scholar]
- Gardner RG, Nelson ZW, Gottschling DE 2005. Ubp10/Dot4p regulates the persistence of ubiquitinated histone H2B: Distinct roles in telomeric silencing and general chromatin. Mol Cell Biol 25: 6123–6139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gartenberg MR, Neumann FR, Laroche T, Blaszczyk M, Gasser SM 2004. Sir-mediated repression can occur independently of chromosomal and subnuclear contexts. Cell 119: 955–967 [DOI] [PubMed] [Google Scholar]
- Gehlen LR, Nagai S, Shimada K, Meister P, Taddei A, Gasser SM 2011. Nuclear geometry and rapid mitosis ensure asymmetric episome segregation in yeast. Curr Biol 21: 25–33 [DOI] [PubMed] [Google Scholar]
- Gotta M, Strahl-Bolsinger S, Renauld H, Laroche T, Kennedy BK, Grunstein M, Gasser SM 1997. Localization of Sir2p: The nucleolus as a compartment for silent information regulators. EMBO J 16: 3243–3255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb S, Esposito RE 1989. A new role for a yeast transcriptional silencer gene, SIR2, in regulation of recombination in ribosomal DNA. Cell 56: 771–776 [DOI] [PubMed] [Google Scholar]
- Gottschling DE 1992. Telomere-proximal DNA in Saccharomyces cerevisiae is refractory to methyltransferase activity in vivo. Proc Natl Acad Sci 89: 4062–4065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottschling DE, Aparicio OM, Billington BL, Zakian VA 1990. Position effect at S. cerevisiae telomeres: Reversible repression of Pol II transcription. Cell 63: 751–762 [DOI] [PubMed] [Google Scholar]
- Hecht A, Laroche T, Strahl-Bolsinger S, Gasser SM, Grunstein M 1995. Histone H3, H4 N-termini interact with SIR3 and SIR4 proteins: A molecular model for the formation of heterochromatin in yeast. Cell 80: 583–592 [DOI] [PubMed] [Google Scholar]
- Hecht A, Strahl-Bolsinger S, Grunstein M 1996. Spreading of transcriptional repressor SIR3 from telomeric heterochromatin. Nature 383: 92–96 [DOI] [PubMed] [Google Scholar]
- Hediger F, Neumann FR, Van Houwe G, Dubrana K, Gasser SM 2002. Live imaging of telomeres. yKu and Sir proteins define redundant telomere-anchoring pathways in yeast. Curr Biol 12: 2076–2089 [DOI] [PubMed] [Google Scholar]
- Hickman MA, Rusche LN 2010. Transcriptional silencing functions of the yeast protein Orc1/Sir3 subfunctionalized after gene duplication. Proc Natl Acad Sci 107: 19384–19389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickman MA, Froyd CA, Rusche LN 2011. Reinventing heterochromatin in budding yeasts, Sir2, the origin recognition complex take center stage. Eukaryot Cell 10: 1183–1192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann JF, Laroche T, Brand AH, Gasser SM 1989. RAP-1 factor is necessary for DNA loop formation in vitro at the silent mating type locus HML. Cell 57: 725–737 [DOI] [PubMed] [Google Scholar]
- Hoppe GJ, Tanny JC, Rudner AD, Gerber SA, Danaie S, Gygi SP, Moazed D 2002. Steps in assembly of silent chromatin in yeast: Sir3-independent binding of a Sir2/Sir4 complex to silencers and role for Sir2-dependent deacetylation. Mol Cell Biol 12: 4167–4180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu HC, Wang CL, Wang M, Yang N, Chen Z, Sternglanz R, Xu R-M 2013. Structural basis for allosteric stimulation of Sir2 activity by Sir4 binding. Genes Dev 27: 64–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Maertens AM, Hyland EM, Dai J, Norris A, Boeke JD, Bader JS 2009. HistoneHits: A database for histone mutations and their phenotypes. Genome Res 19: 674–681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai S, Armstrong CM, Kaeberlein M, Guarente L 2000. Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature 403: 795–800 [DOI] [PubMed] [Google Scholar]
- Ishii K, Arib G, Lin C, Van Houwe G, Laemmli UK 2002. Chromatin boundaries in budding yeast: The nuclear pore connection. Cell 109: 551–562 [DOI] [PubMed] [Google Scholar]
- Johnson LM, Kayne PS, Kahn ES, Grunstein M 1990. Genetic evidence for an interaction between SIR3 and histone H4 in the repression of the silent mating loci in S. cerevisiae. Proc Natl Acad Sci 87: 6286–6290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson LM, Fisher-Adams G, Grunstein M 1992. Identification of a non-basic domain in the histone H4 N-terminus required for repression of the yeast silent mating loci. EMBO J 11: 2201–2209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson A, Li G, Sikorski TW, Buratowski S, Woodcock CL, Moazed D 2009. Reconstitution of heterochromatin-dependent transcriptional gene silencing. Mol Cell 35: 769–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaeberlein M, McVey M, Guarente L 1999. The SIR2/3/4 complex, SIR2 alone promote longevity in S. cerevisiae by two different mechanisms. Genes Dev 13: 2570–2580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaeberlein M, Kirkland KT, Fields S, Kennedy BK 2004. Sir2-independent life span extension by calorie restriction in yeast. PLoS Biol 2: 296–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katan-Khaykovich Y, Struhl K 2005. Heterochromatin formation involves changes in histone modifications over multiple cell generations. EMBO J 24: 2138–2149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keogh MC, Kim JA, Downey M, Fillingham J, Chowdhury D, Harrison JC, Onishi M, Datta N, Galicia S, Emili A, et al. 2006. A phosphatase complex that dephosphorylates γH2AX regulates DNA damage checkpoint recovery. Nature 439: 497–501 [DOI] [PubMed] [Google Scholar]
- Kimura A, Umehara T, Horikoshi M 2002. Chromosomal gradient of histone acetylation established by Sas2p and Sir2p functions as a shield against gene silencing. Nat Genet 3: 370–377 [DOI] [PubMed] [Google Scholar]
- Kirchmaier AL, Rine J 2001. DNA replication-independent silencing in S. cerevisiae. Science 291: 646–650 [DOI] [PubMed] [Google Scholar]
- Kirchmaier AL, Rine J 2006. Cell cycle requirements in assembling silent chromatin in Saccharomyces cerevisiae. Mol Cell Biol 26: 852–862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitada T, Schleker T, Sperling AS, Xie W, Gasser SM, Grunstein M 2011. γH2A is a component of yeast heterochromatin required for telomere elongation. Cell Cycle 10: 293–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitada T, Kuryan BG, Tran NN, Song C, Xue Y, Carey M, Grunstein M 2012. Mechanism for epigenetic variegation of gene expression at yeast telomeric heterochromatin. Genes Dev 26: 2443–2455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T, Horiuchi T, Tongaonkar P, Vu L, Nomura M 2004. Sir2 regulates recombination between different rDNA repeats, but not recombination within individual rRNA genes in yeast. Cell 117: 441–453 [DOI] [PubMed] [Google Scholar]
- Kueng S, Tsai-Pflugfelder M, Oppikofer M, Ferreira HC, Roberts E, Tsai C, Roloff TC, Sack R, Gasser SM 2012. Regulating repression: Roles for the sir4 N-terminus in linker DNA protection and stabilization of epigenetic states. PLoS Genet 8: e1002727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyrion G, Boakye KA, Lustig AJ 1992. C-terminal truncation of RAP1 results in the deregulation of telomere size, stability, and function in Saccharomyces cerevisiae. Mol Cell Biol 12: 5159–5173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyrion G, Liu K, Liu C, Lustig AJ 1993. RAP1 and telomere structure regulate telomere position effects in Saccharomyces cerevisiae. Genes Dev 7: 1146–1159 [DOI] [PubMed] [Google Scholar]
- Laroche T, Martin SG, Gotta M, Gorham HC, Pryde FE, Louis EJ, Gasser SM 1998. Mutation of yeast Ku genes disrupts the subnuclear organization of telomeres. Curr Biol 8: 653–656 [DOI] [PubMed] [Google Scholar]
- Lau A, Blitzblau H, Bell SP 2002. Cell-cycle control of the establishment of mating-type silencing in S. cerevisiae. Genes Dev 16: 2935–2945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YC, Cheng TH, Gartenberg MR 2001. Establishment of transcriptional silencing in the absence of DNA replication. Science 291: 650–653 [DOI] [PubMed] [Google Scholar]
- Liachko I, Tye BK 2009. Mcm10 mediates the interaction between DNA replication and silencing machineries. Genetics 181: 379–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liou GG, Tanny JC, Kruger RG, Walz T, Moazed D 2005. Assembly of the SIR complex and its regulation by O-acetyl-ADP-ribose, a product of NAD-dependent histone deacetylation. Cell 121: 515–527 [DOI] [PubMed] [Google Scholar]
- Loo S, Rine J 1994. Silencers and domains of generalized repression. Science 264: 1768–1771 [DOI] [PubMed] [Google Scholar]
- Luo K, Vega-Palas MA, Grunstein M 2002. Rap1-Sir4 binding independent of other Sir, yKu, or histone interactions initiates the assembly of telomeric heterochromatin in yeast. Genes Dev 12: 1528–1539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas NL, Miller KM, DeFazio LG, Toczyski DP 2006. Cell cycle and checkpoint regulation of histone H3K56 acetylation by Hst3 and Hst4. Mol Cell 23: 109–119 [DOI] [PubMed] [Google Scholar]
- Maillet L, Boscheron C, Gotta M, Marcand S, Gilson E, Gasser SM 1996. Evidence for silencing compartments within the yeast nucleus: A role for telomere proximity and Sir protein concentration in silencer-mediated repression. Genes Dev 10: 1796–1811 [DOI] [PubMed] [Google Scholar]
- Mak HC, Pillus L, Ideker T 2009. Dynamic reprogramming of transcription factors to and from the subtelomere. Genome Res 19: 1014–1025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcand S, Gilson E, Shore D 1997. A protein-counting mechanism for telomere length regulation in yeast. Science 275: 986–990 [DOI] [PubMed] [Google Scholar]
- Martin SG, Laroche T, Suka N, Grunstein M, Gasser SM 1999. Relocalization of telomeric Ku and SIR proteins in response to DNA strand breaks in yeast. Cell 97: 621–633 [DOI] [PubMed] [Google Scholar]
- Martino F, Kueng S, Robinson P, Tsai-Pflugfelder M, van Leeuwen F, Ziegler M, Cubizolles F, Cockell MM, Rhodes D, Gasser SM 2009. Reconstitution of yeast silent chromatin, multiple contact sites and O-AADPR binding load SIR complexes onto nucleosomes in vitro. Mol Cell 33: 323–334 [DOI] [PubMed] [Google Scholar]
- Marvin ME, Becker MM, Noel P, Hardy S, Bertuch AA, Louis EJ 2009. The association of yKu with subtelomeric core X sequences prevents recombination involving telomeric sequences. Genetics 183: 453–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mekhail K, Seebacher J, Gygi SP, Moazed D 2008. Role for perinuclear chromosome tethering in maintenance of genome stability. Nature 456: 667–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meneghini MD, Wu M, Madhani HD 2003. Conserved histone variant H2A.Z protects euchromatin from the ectopic spread of silent heterochromatin. Cell 112: 725–736 [DOI] [PubMed] [Google Scholar]
- Miller AM, Nasmyth KA 1984. Role of DNA replication in the repression of silent mating type loci in yeast. Nature 312: 247–251 [DOI] [PubMed] [Google Scholar]
- Mishra K, Shore D 1999. Yeast Ku protein plays a direct role in telomeric silencing, counteracts inhibition by Rif proteins. Curr Biol 9: 1123–1126 [DOI] [PubMed] [Google Scholar]
- Moazed D, Kistler A, Axelrod A, Rine J, Johnson AD 1997. Silent information regulator protein complexes in S. cerevisiae: A SIR2/SIR4 complex and evidence for a regulatory domain in SIR4 that inhibits its interaction with SIR3. Proc Natl Acad Sci 94: 2186–2191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretti P, Freeman K, Coodly L, Shore D 1994. Evidence that a complex of SIR proteins interacts with the silencer and telomere-binding protein RAP1. Genes Dev 8: 2257–2269 [DOI] [PubMed] [Google Scholar]
- Murphy GA, Spedale EJ, Powell ST, Pillus L, Schultz SC, Chen L 2003. The Sir4 C-terminal coiled coil is required for telomeric and mating type silencing in Saccharomyces cerevisiae. J Mol Biol 334: 769–780 [DOI] [PubMed] [Google Scholar]
- Neumann H, Hancock SM, Buning R, Routh A, Chapman L, Somers J, Owen-Hughes T, van Noort J, Rhodes D, Chin JW 2009. A method for genetically installing site-specific acetylation in recombinant histones defines the effects of H3K56 acetylation. Mol Cell 36: 153–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng HH, Feng Q, Wang H, Erdjument-Bromage H, Tempst P, Zhang Y, Struhl K 2002. Lysine methylation within the globular domain of histone H3 by Dot1 is important for telomeric silencing and Sir protein association. Genes Dev 16: 1518–1527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris A, Boeke JD 2010. Silent information regulator 3: The Goldilocks of the silencing complex. Genes Dev 24: 115–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onishi M, Liou GG, Buchberger JR, Walz T, Moazed D 2007. Role of the conserved Sir3-BAH domain in nucleosome binding and silent chromatin assembly. Mol Cell 28: 1015–1028 [DOI] [PubMed] [Google Scholar]
- Oppikofer M, Kueng S, Martino F, Soeroes S, Hancock SM, Chin JW, Fischle W, Gasser SM 2011. A dual role of H4K16 acetylation in the establishment of yeast silent chromatin. EMBO J 30: 2610–2621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppikofer M, Kueng S, Keusch JJ, Hassler M, Ladurner AG, Gut H, Gasser SM 2013. Dimerization through the Sir3 C-terminal winged helix domain is essential for yeast heterochromatin formation. EMBO J 32: 437–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne EA, Dudoit S, Rine J 2009. The establishment of gene silencing at single-cell resolution. Nat Genet 41: 800–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palladino F, Laroche T, Gilson E, Axelrod A, Pillus L, Gasser SM 1993. SIR3 and SIR4 proteins are required for the positioning and integrity of yeast telomeres. Cell 75: 543–555 [DOI] [PubMed] [Google Scholar]
- Park Y, Lustig AL 2000. Telomere structure regulates the heritability of repressed subtelomeric chromatin in S. cerevisiae. Genetics 154: 587–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillus L, Rine J 1989. Epigenetic inheritance of transcriptional states in S. cerevisiae. Cell 59: 637–647 [DOI] [PubMed] [Google Scholar]
- Pryde FE, Louis EJ 1999. Limitations of silencing at native yeast telomeres. EMBO J 18: 2538–2550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renauld H, Aparicio OM, Zierath PD, Billington BL, Chhablani SK, Gottschling DE 1993. Silent domains are assembled continuously from the telomere and are defined by promoter distance and strength, and by SIR3 dosage. Genes Dev 7: 1133–1145 [DOI] [PubMed] [Google Scholar]
- Rine J, Herskowitz I 1987. Four genes responsible for a position effect on expression from HML and HMR in Saccharomyces cerevisiae. Genetics 116: 9–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rine J, Strathern JN, Hicks JB, Herskowitz I 1979. A suppressor of mating-type locus mutations in S. cerevisiae: Evidence for and identification of cryptic mating-type loci. Genetics 93: 877–901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robyr D, Suka Y, Xenarios I, Kurdistani SK, Wang A, Suka N, Grunstein M 2002. Microarray deacetylation maps determine genome-wide functions for yeast histone deacetylases. Cell 109: 437–446 [DOI] [PubMed] [Google Scholar]
- Ruault M, De Meyer A, Loïodice I, Taddei A 2011. Clustering heterochromatin: Sir3 promotes telomere clustering independently of silencing in yeast. J Cell Biol 192: 417–431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudner AD, Hall BE, Ellenberger T, Moazed D 2005. A nonhistone protein-protein interaction required for assembly of the SIR complex and silent chromatin. Mol Cell Biol 25: 4514–4528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusche LN, Kirchmaier AL, Rine J 2002. Ordered nucleation and spreading of silenced chromatin in Saccharomyces cerevisiae. Mol Biol Cell 7: 2207–2222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusche LN, Kirchmaier AL, Rine J 2003. The establishment, inheritance, and function of silenced chromatin in Saccharomyces cerevisiae. Annu Rev Biochem 72: 481–516 [DOI] [PubMed] [Google Scholar]
- Schober H, Ferreira H, Kalck V, Gehlen LR, Gasser SM 2009. Yeast telomerase and the SUN domain protein Mps3 anchor telomeres and repress subtelomeric recombination. Genes Dev 23: 928–938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekinger EA, Gross DS 1999. SIR repression of a yeast heat shock gene: UAS and TATA footprints persist within heterochromatin. EMBO J 18: 7041–7055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Seto E, Yoshida M 2014. Erasers of histone acetylation: The histone deacetylase enzymes. Cold Spring Harb Perspect Biol 10.1101/cshperspect.a018713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahbazian MD, Zhang K, Grunstein M 2005. Histone H2B ubiquitylation controls processive methylation but not monomethylation by Dot1 and Set1. Mol Cell 19: 271–277 [DOI] [PubMed] [Google Scholar]
- Shogren-Knaak M, Ishii H, Sun J-M, Pazin MJ, Davie JR, Peterson CL 2006. Histone H4K16 acetylation controls chromatin structure and protein interactions. Science 311: 844–847 [DOI] [PubMed] [Google Scholar]
- Sinclair DA, Guarente L 1997. Extrachromosomal rDNA circles—A cause of aging in yeast. Cell 91: 1033–1042 [DOI] [PubMed] [Google Scholar]
- Singer MS, Kahana A, Wolf AJ, Meisinger LL, Peterson SE, Goggin C, Mahowald M, Gottschling DE 1998. Identification of high-copy disruptors of telomeric silencing in Saccharomyces cerevisiae. Genetics 150: 613–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha M, Watanabe S, Johnson A, Moazed D, Peterson CL 2009. Recombinational repair within heterochromatin requires ATP-dependent chromatin remodeling. Cell 138: 1109–1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JS, Boeke JD 1997. An unusual form of transcriptional silencing in yeast ribosomal DNA. Genes Dev 11: 241–254 [DOI] [PubMed] [Google Scholar]
- Sperling AS, Grunstein M 2009. Histone H3 N-terminus regulates higher order structure of yeast heterochromatin. Proc Natl Acad Sci 106: 13153–13159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strahl-Bolsinger S, Hecht A, Luo K, Grunstein M 1997. SIR2 and SIR4 interactions differ in core and extended telomeric heterochromatin in yeast. Genes Dev 11: 83–93 [DOI] [PubMed] [Google Scholar]
- Stulemeijer IJ, Pike BL, Faber AW, Verzijlbergen KF, van Welsem T, Frederiks F, Lenstra TL, Holstege FC, Gasser SM, van Leeuwen F 2011. Dot1 binding induces chromatin rearrangements by histone methylation-dependent, and -independent mechanisms. Epigenetics Chromatin 4: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suka N, Suka Y, Carmen AA, Wu J, Grunstein M 2001. Highly specific antibodies determine histone acetylation site usage in yeast heterochromatin and euchromatin. Mol Cell 8: 473–479 [DOI] [PubMed] [Google Scholar]
- Suka N, Luo K, Grunstein M 2002. Sir2p and Sas2p opposingly regulate acetylation of yeast histone H4 lysine16 and spreading of heterochromatin. Nat Genet 3: 378–383 [DOI] [PubMed] [Google Scholar]
- Szilard RK, Jacques PE, Laramée L, Cheng B, Galicia S, Bataille AR, Yeung M, Mendez M, Bergeron M, Robert F, et al. 2010. Systematic identification of fragile sites via genome-wide location analysis of γ-H2AX. Nat Struct Mol Biol 17: 299–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taddei A, Hediger F, Neumann FR, Bauer C, Gasser SM 2004. Separation of silencing from perinuclear anchoring functions in yeast Ku80 Sir4 and Esc1 proteins. EMBO J 23: 1301–1312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taddei A, Van Houwe G, Nagai S, Erb I, van Nimwegen E, Gasser SM 2009. The functional importance of telomere clustering: Global changes in gene expression result from SIR factor dispersion. Genome Res 19: 611–625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner KG, Landry J, Sternglanz R, Denu JM 2000. Silent information regulator 2 family of NAD-dependent histone/protein deacetylases generates a unique product, 1-O-acetyl-ADP-ribose. Proc Natl Acad Sci 97: 14178–14182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanny JC, Dowd GJ, Huang J, Hilz H, Moazed D 1999. An enzymatic activity in the yeast Sir2 protein that is essential for gene silencing. Cell 99: 735–745 [DOI] [PubMed] [Google Scholar]
- Thompson JS, Ling X, Grunstein M 1994a. Histone H3 amino terminus is required for telomeric and silent mating locus repression in yeast. Nature 369: 245–247 [DOI] [PubMed] [Google Scholar]
- Thompson JS, Johnson LM, Grunstein M 1994b. Specific repression of the yeast silent mating locus HMR by an adjacent telomere. Mol Cell Biol 14: 446–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triolo T, Sternglanz R 1996. Role of interactions between the origin recognition complex and SIR1 in transcriptional silencing. Nature 381: 251–253 [DOI] [PubMed] [Google Scholar]
- Tsukamoto Y, Kato J, Ikeda H 1997. Silencing factors participate in DNA repair and recombination in Saccharomyces cerevisiae. Nature 388: 900–903 [DOI] [PubMed] [Google Scholar]
- Valenzuela L, Dhillon N, Dubey RN, Gartenberg MR, Kamakaka RT 2008. Long-range communication between the silencers of HMR. Mol Cell Biol 28: 1924–1935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Leeuwen F, Gafken PR, Gottschling DE 2002. Dot1p modulates silencing in yeast by methylation of the nucleosome core. Cell 109: 745–756 [DOI] [PubMed] [Google Scholar]
- Weiss K, Simpson RT 1998. High-resolution structural analysis of chromatin at specific loci: Saccharomyces cerevisiae silent mating type locus HMLα. Mol Cell Biol 18: 5392–5403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CS, Chen YF, Gartenberg MR 2011. Targeted sister chromatid cohesion by Sir2. PLoS Genet 7: e1002000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu F, Zhang Q, Zhang K, Xie W, Grunstein M 2007. Sir2 deacetylates histone H3 lysine 56 to regulate telomeric heterochromatin structure in yeast. Mol Cell 27: 890–900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B, Britton J, Kirchmaier AL 2008a. Insights into the impact of histone acetylation and methylation on Sir protein recruitment, spreading, and silencing in Saccharomyces cerevisiae. J Mol Biol 381: 826–844 [DOI] [PubMed] [Google Scholar]
- Yang B, Miller A, Kirchmaier AL 2008b. HST3/HST4-dependent deacetylation of lysine 56 of histone H3 in silent chromatin. Mol Biol Cell 19: 4993–5005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Q, Olsen L, Zhang X, Boeke JD, Bi X 2011. Differential contributions of histone H3 and H4 residues to heterochromatin structure. Genetics 188: 291–308 [DOI] [PMC free article] [PubMed] [Google Scholar]