Abstract
Background & Aims
Patients with asymptomatic or poorly managed celiac disease can experience bone loss, placing them at risk for hip and vertebral fractures. We analyzed the cost-effectiveness of universal serologic screening (USS) vs symptomatic at-risk screening (SAS) strategies for celiac disease, given the risk of non-traumatic hip and vertebral fractures if untreated or undiagnosed.
Method
We developed a lifetime Markov model of the screening strategies, each with male or female cohorts of 1000 patients, 12 years old when screening began. We screened serum samples for levels of immunoglobulin A (IgA), compared with tissue transglutaminase and total IgA, and findings were confirmed by mucosal biopsy. Transition probabilities and quality of life estimates were obtained from the literature. We used generalizable cost estimates and Medicare reimbursement rates, and ran deterministic and probabilistic sensitivity analyses.
Result
For men, the average life-time costs were $8532 and $8472 for USS and SAS strategies, respectively, corresponding to average quality adjusted life years (QALY) gains of 25.511 and 25.515. Similarly, for women, costs were $11,383 and $11,328 for USS and SAS strategies, corresponding to QALY gains of 25.74 and 25.75. Compared to the current standard of care (SAS), USS produced higher average lifetime costs and lower quality of life for each sex. Deterministic and probabilistic sensitivity analyses showed that the model was robust to realistic changes in all the variables, making USS cost ineffective, based on these outcomes.
Conclusion
USS and SAS are similar in lifetime costs and quality of life, although the current SAS strategy was overall more cost effective in preventing bone loss and fractures among patients with undiagnosed or subclinical disease. Based on best available supportive evidence, it is more cost effective to maintain the standard celiac screening practices, although future robust population-based evidence in other health outcomes could be leveraged to re-evaluate current screening guidelines.
Keywords: tTG IgA, cost-benefit, cost-utility, bone health, detection, diagnosis
INTRODUCTION
Celiac disease (CD) is an autoimmune enteropathy triggered by the ingestion of gluten containing products, including wheat, barley, rye, and possibly oats. Ingestion of gluten can cause inflammation of the small bowel, leading to intestinal and extraintestinal symptoms.1,2 Classic CD is characterized by gastrointestinal symptoms such as abdominal pain, diarrhea, bloating, and failure to thrive in children. However, manifestations of celiac disease CD are diverse, and can present in various ways from an asymptomatic presentation (silent CD)3 to exclusively extra-intestinal manifestations.4,5 The prevalence of CD has been increasing over time,6 and has been shown to be as high as 0.8-1.5% in the North American and European populations.7,8 The latest consensus among gastroenterologists is that CD is currently underdiagnosed, due to the frequency of silent or latent disease.9 The prevalence in at-risk groups have been shown to be as high 1:56 in symptomatic patients, 1:22 in first degree relative, and significantly increased in various autoimmune conditions.10
Undiagnosed and untreated CD can lead to significant complications, including poor intestinal absorption of macro- and micro-nutrients, potentially leading to poor growth in children and chronic nutritional deficiencies. In particular, evidence supports that untreated CD universally leads to progressive bone loss and derangements,6,11 increasing the risk for early osteoporosis and non-traumatic fractures. Although the etiology of bone derangements in CD is thought to be multifactorial,12,13 almost all longstanding disability from non-traumatic fractures occurs from 2 primary sites: hip and vertebrae.14 Furthermore, these two sites are used as the standard of care locations to measure osteoporosis based on bone mineral density criteria.15,16
Standard practice screening for CD involves screening symptomatic individuals as well as some high-risk groups.4,17 Diagnosis of CD involves serologic screening followed by confirmation of characteristic biopsy findings from upper endoscopy. Although serologic screening tests are relatively inexpensive and have excellent sensitivity and specificity,18,19 the role of universal screening for CD continues to be a difficult decision due to multiple factors including the utility of serologic screening, low adherence to GFD when CD is accurately diagnosed, and unclear long-term patient benefits in reducing potential morbidity and mortality among treated CD patients, especially if asymptomatic.20,21
Based on a comprehensive review of relevant literature supporting the various rationales behind universal screening, we find that bone disease – specifically non-traumatic fractures at the hip and vertebrae – is currently the most quantifiable and analyzable outcome measure validated by robust literature findings. We hypothesize that universal serologic screening during early adolescence may represent an optimal clinical strategy to detect subclinical CD patients and prevent future health consequences from bone disease. The aim of our investigation is to determine the cost-effectiveness of universal serologic screening with serum tTG IgA and total IgA compared to the standard diagnostic screening which is limited to at-risk and symptomatic patients – given the increased risk of non-traumatic fractures among undiagnosed or untreated CD patients.
METHODS
Decision-analytic Model
We constructed a decision-analytic Markov model of 12 year old cohorts with population-based prevalence of CD in North America. A natural history and progression toward hip bone and vertebral fractures were used as clinical endpoints to assess the cost-effectiveness of providing universal CD serologic screening. A base case age of 12 years was determined clinically relevant for serologic CD testing since dietary habits are more likely shaped by peers, and primary physicians are screening for baseline anemia and dyslipidemia as per standard of care during preadolescence.22,23 Because the natural age progression to osteoporosis and bone loss is different for male and female, our model is categorized in two groups based on male or female gender. We considered 2 strategies for comparison, as shown in Figure 1: universal serologic screening versus standard of care screening.
Figure 1.
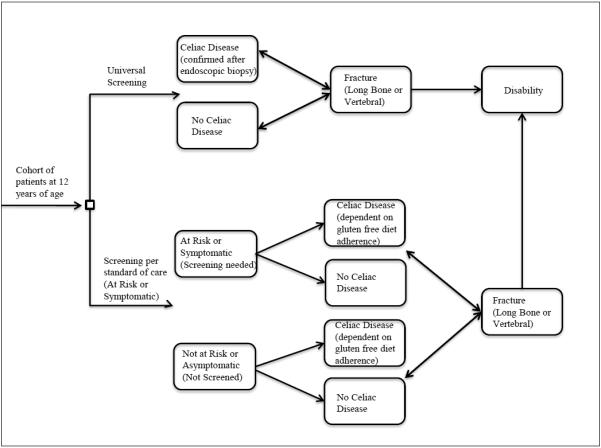
Simplified schematic of Markov model with major health states.
Our model followed the recommendations of the US Panel on Cost-Effectiveness in Health and Medicine in the development and the analysis of results, using a societal perspective, considering costs and benefits over a lifetime horizon, and discounting at 3% annually.24 Base case parameter estimates and ranges and distributions used in the sensitivity analysis are presented in Table 1. We constructed and implemented the model in TreeAge Pro 2012 Suite (TreeAge Sofware Inc, Williamstown, MA) and Microsoft Excel 2007 (Microsoft Corportation, Redmond, WA). The two cohorts progressed through the model in 1-year time steps until death or 100 years of age. Within each health state, patients could die at a rate based on the average age-specific mortality tables, as estimated by the Centers for Disease Control and Prevention.25 The model analyzed the differences in lifetime discounted costs and benefits (measured in life-years and quality-adjusted life years) between the 2 competing strategies. The primary outcome measure was the incremental cost-effectiveness ratio (ICER), which is defined as the difference in costs in dollars divided by the difference in effectiveness in quality-adjusted life-years (QALYs) between the 2 competing strategies.
Table 1.
Model Assumptions
| Variables | Base case |
Sensitivity range |
Monte Carlo Distribution (base case, 95% CI) |
Sources |
|---|---|---|---|---|
| Health State Probabilities | ||||
|
| ||||
| Prevalence of CD in the US | 0.01 | 0.005 – 0.03 | Beta (0.01, 0.005 – 0.02) | Fasano48 |
| Prevalence with clinical signs and symptoms needing CD screening in the US | 0.15 | 0.05 – 0.3 | Beta (0.15, 0.08 – 0.22) | Hyams,34 Saito,35 Hahn,36 Talley37 |
| Prevalence of CD among IBS patients in the US | 0.04 | 0.02 – 0.1 | Beta (0.04, 0.02 – 0.07) | Ford38 |
| Specificity of serologic screening for CD | 0.955 | 0.9 – 0.99 | Beta (0.955, 0.92 – 0.97) | NIH Consensus17 |
| Sensitivity of serologic screening for CD | 0.92 | 0.85 – 0.98 | Beta (0.92, 0.88 – 0.94) | NIH Consensus17 |
| Proportion of CD patients with fracture as initial presentation | 0.035 | 0.01 – 0.2 | Beta (0.035, 0.01 – 0.07) | Stenson,39 Thomason,40 |
| Proportion needing serologic screening due to risk factors for CD | 0.027 | 0.01 – 0.05 | Beta (0.027, 0.01 – 0.04) | Ludvigsson41 |
| Proportion with CD among asymptomatic and at-risk group | 0.067 | 0.01 – 0.1 | Beta (0.067, 0.04 – 0.09) | Multiple42, 43, 44, 45, 46, 47 |
| Proportion with CD adherent to strict gluten free diet (GFD) at age 12 | 0.67 | 0.4 – 0.9 | Beta (0.67, 0.49 – 0.82) | Multiple 48, 49, 50, 51, 52, 53 |
| Annual probability of becoming non-adherent to GFD in CD | 0.25 | 0.1 – 0.6 | Beta (0.25, 0.11 – 0.41) | Mearin54 |
| Annual probability of becoming adherent to GFD in CD | 0.1 | 0.05 – 0.2 | Beta (0.1, 0.03 – 0.19) | Expert opinion |
| Annual probability of vertebral fracture on strict GFD in CD (women) | 0.003 | 0.001 -0.01 | Beta (0.003, 0.001 – 0.01) | Expert opinion |
| Annual probability of vertebral fracture on strict GFD in CD (men) | 0.001 | 0.0001 – 0.01 | Beta (0.001, 0.0001 -0.01) | Ioannidis55 |
| Annual probability of vertebral fracture if non-adherent to GFD in CD (women) | 0.017 | 0.001 – 0.1 | Beta (0.017, 0.01 – 0.04) | Ioannidis55 |
| Annual probability of vertebral fracture if non-adherent to GFD in CD (men) | 0.007 | 0.001 – 0.1 | Beta (0.007, 0.001 – 0.03) | West56 |
| Annual probability of long bone fracture on strict GFD in CD (women) | 0.006 | 0.001 – 0.1 | Beta (0.006, 0.001 – 0.02) | West56 |
| Annual probability of long bone fracture on strict GFD in CD (men) | 0.018 | 0.001 – 0.1 | Beta (0.018, 0.001 – 0.05) | Sanchez57 |
| Annual probability of long bone fracture if non-adherent to GFD in CD (women) | 0.007 | 0.001 – 0.1 | Beta (0.007, 0.001 – 0.02) | Sanchez57 |
| Annual probability of long bone fracture if non-adherent to GFD in CD (men) | 0.03 | 0.001 – 0.1 | Beta (0.03, 0.001 – 0.07) | Sanchez57 |
| Probability of success after vertebral fracture surgery / treatment | 0.4 | 0.2 – 0.6 | Beta (0.4, 0.3 – 0.5) | Sanchez57 |
| Probability of success after hip fracture surgery / treatment | 0.9 | 0.75 – 0.99 | Beta (0.9, 0.83 – 0.95) | Wardlaw58 |
| Mancuso59 | ||||
|
| ||||
| Costs (dollars) | ||||
|
| ||||
| Cost of serologic screening for CD (Anti-tTG IgA + Total IgA) | 26 | 10 – 100 | Lognormal (26, 17 – 41) | LPCH/SUMC, Shamir60 |
| Cost of CD biopsy confirmation by endoscopy | 737 | 500 – 1500 | Lognormal (737, 558 – 950) | LPCH/SUMC |
| Additional cost of being on strict GFD per month | 72 | 15 – 150 | Lognormal (72, 55 – 92) | Lee,61 USDA62 |
| Annual additional cost of physician follow up visits for CD | 317 | 150 – 600 | Lognormal (317, 192 – 493) | LPCH |
| Cost of clinical dietician for GFD education | 45 | 20 – 80 | Lognormal (45, 30 – 65) | LPCH/SUMC |
| Cost of surgical intervention for hip fracture | 1646 | 800 – 4000 | Lognormal (1646, 1249 – 2124) | CMS63 |
| Cost of surgical intervention for vertebral fracture | 3018 | 1500 – 6000 | Lognormal (3018, 1865 – 4000) | CMS63 |
| Annual cost of treating functional disability after morbidity from fracture | 5200 | 2500 – 10000 | Lognormal (5200, 3511 – 7416) | CMS63 |
|
| ||||
| Utilities | ||||
|
| ||||
| Utility of symptomatic CD | 0.71 | 0.6 – 0.9 | Beta (0.71, 0.53 – 0.85) | Norström,64 Tengs,65 Hershcovici66 |
| Utility of hip fracture (disabled) | 0.9 | 0.6 – 0.99 | Beta (0.9, 0.77 – 0.97) | Jönsson67 |
| Utility of vertebral fracture (disabled) | 0.9 | 0.6 – 0.99 | Beta (0.9, 0.77 – 0.97) | Oleksik68 |
|
| ||||
| Discount Rate | ||||
|
| ||||
| Annual discount rate | 0.03 | 0.0 – 0.05 | Beta (0.03, 0 – 0.1) | Gold et al24 |
Symptomatic and At-Risk Screening (SAS)
The current standard of care guidelines for serologic screening captures at-risk populations and patients with symptomatic gastrointestinal complaints for CD. Populations at-risk for CD, as defined by literature, include Type 1 diabetes mellitus, Down, Turner, and Williams syndromes, immunoglobulin A deficiency, systemic lupus, autoimmune thyroiditis, and having a first and/or second degree relative with CD (Table 1).422,43,44,45,46,47,48,49,50,51,52,53 Under this strategy, patients without a genetic or hereditary risk factor for CD were normally screened due to associated gastrointestinal complaints, such as diarrhea, abdominal pain, bloating, and other irritable-bowel-like symptoms.34,35,36,37 In children, poor growth, wasting, failure-to-thrive, and anemia also warranted CD screening (Table 1).4,17,26 Any positive serologic screens required diagnostic confirmation via endoscopic duodenal mucosal biopsies. Once a CD patient started life-long therapy by maintaining a strict gluten-free diet (GFD), patients were subject to natural adherence and non-adherence rates reported in literature.54,55,56,57
Universal Serologic Screening (USS)
Under this strategy, all patients received a one-time serologic test for immunoglobulin A antibodies to tissue transglutaminase (tTG IgA) and total immunoglobulin A (IgA). The combination of high levels of tTG IgA with normal IgA is validated as a screening modality of choice, conferring high sensitivity and specificity in subjects >2 years of age (Table 1).17 As in the SAS strategy, all positive screening tests were then confirmed via duodenal mucosal biopsies. The natural probabilities of adherence and non-adherence to strict GFD were also applied to this cohort after diagnosis of CD (Table 1).
Hip & Vertebral Fractures
Current literature supports the development of deteriorating bone disease among undiagnosed CD patients and CD patients who were poorly managed or non-adherent to strict GFD (Table 1). We assumed that CD patients with progressive bone disease developed non-traumatic fractures at the same rate as the non-CD population who had comparable bone demineralization. The model aims specifically to evaluate the health and economic consequence of long bone (i.e., hip) and vertebral fractures among CD patients who are undiagnosed or untreated with GFD. We chose to focus on the development of these two types of fractures in particular in order to ensure that – whenever possible – the model captures the specific costs and quality-of-life trade-offs between the competing screening strategies based on clearly defined clinical end-points. Hip and vertebral fractures also carry the highest morbidity rate in terms of progressing to long-term disability.32,33
Disability
Patients in the model who developed hip or vertebral fractures were at risk for developing long-term disability. The surgical success rates of total hip arthroplasty and kyphoplasty were included in the model.58,59 Among patients who require surgery, if they did not achieve full functional capacity after a 1 year cycle, patients incurred decrements in quality of life and medical disability costs as reported by literature in a disabled state, where they remained until death.
Costs
Cost estimates for total hip arthroplasty, kyphoplasty, and annual disability after fractures were derived from the Centers for Medicare and Medicaid Services (CMS) for 2012.63 Additional costs required in CD for a strict GFD were approximated by considering the report by Lee et al61 and the baseline average monthly costs for food among males and females according to the USDA.62 We assumed bi-annual physician follow up clinic visits and one initial educational visit with a dietician for CD. These added healthcare provider costs were estimated by querying de-identified patient billing records in 2011 from Lucile Packard Children’s Hospital (LPCH) and Stanford University Medical Center (SUMC). The cost of serologic screening using tTG IgA and total IgA was reported by Shamir et al60 and validated by the clinical laboratory at LPCH and SUMC.
Utilities
Utilities estimate patient quality of life (QOL), ranging from 0 (death) to 1 (perfect health). Previous literature suggested a difference in QOL between symptomatic and asymptomatic CD. The utility of 0.71 for symptomatic CD (i.e., abdominal pain, bloating, diarrhea, etc) in Table 1 was a conservative estimate based on two utility estimates. We averaged 0.66 based on a report from Norström et al64 surveying CD patients 1 year prior to CD diagnosis and 0.76 based on QOL living with dyspepsia and diarrhea in Tengs and Wallace.65 Our estimation of 0.71 was validated in a cost-effectiveness analysis performed by Hershcovici et al.66 Patients who recovered completely after a fracture did not progress to the disabled state and were subject to same pre-fracture transition probabilities. For disabled patients after a fracture, we incorporated the reports from Jönsson et al67 and Oleksik et al68 that suggest patients who had either a hip or vertebral fracture exhibit a QOL that is approximately 90% of the previous non-fracture or healthy state. The QOL in disability varies considerably, and our approximation may not adequately represent poor QOL in severely disabled states. However, we critically evaluated this variable under a wide sensitivity range to ensure that a substantially lower QOL in disability does not impact the results.
Sensitivity Analyses
We performed a deterministic and a probabilistic sensitivity analyses on all health state probabilities, costs, and utilities in the model (Table 1). One-way deterministic sensitivity analyses were performed to identify variables that had important effects on the decision for either USS or SAS over the specified range of values reported in literature or expert opinion. Each variable was tested separately to determine whether varying the particular variable over a broad range alters the ICER. For probabilistic sensitivity analysis and for calculating the 95% confidence intervals around the base case results, we performed 1,000 independent Monte Carlo simulations. Beta distributions were used for probabilities and utilities. Lognormal distributions were used for costs. We assumed a willingness-to-pay (WTP) threshold of <$50,000 per QALY as being cost-effective, but generated acceptability curves for each strategy over a $0 to $100,000 WTP thresholds to estimate the net benefit.
RESULTS
Symptomatic and At-Risk Screening (SAS) vs. Universal Serologic Screening (USS)
Our model shows complete dominance of the USS strategy by the SAS strategy, which is the current standard of care in most clinical practices. The USS strategy for either gender is not cost-effective. Table 2 shows a summary of the cost-effectiveness of the 2 strategies. For males, USS accrues a life-time average cost of $8,532 with an associated QALY-gained of 25.511, but SAS had lower costs of $8,472 and minimally higher QALY-gained of 25.515. Similarly, for females, USS accrues a life-time average cost of $11,383 with an associated QALY-gained of 25.74, while SAS had lower costs of $11,328 and minimally higher QALY-gained of 25.75. Figure 2 shows the cost-effectiveness planes of the two strategies stratified by gender.
Table 2.
Average lifetime discounted costs and quality-adjusted life years (QALYs) and incremental cost-effectiveness ratios of Symptomatic and At-Risk Screening (SAS) Strategy versus Universal Serologic Screening (USS) Strategy per patient with empirically estimated 95% confidence intervals from 1,000 simulations
| Strategy | Cost | QALY | Incremental Cost |
Incremental QALYs |
ICER |
|---|---|---|---|---|---|
| MALE | |||||
| Universal Serologic | 8,532 | 25.511 | 59.66 | −0.005 | Dominated† |
| Screening | (683 – 36,162) | (23.00 – 26.19) | |||
| Symptomatic and | 8,472 | 25.515 | -- | -- | |
| At-Risk Screening | (651 – 36,093) | (23.00 – 26.18) | |||
| FEMALE | |||||
| Universal Serologic | 11,383 | 25.74 | 54.99 | −0.01 | Dominated† |
| Screening | (1,602 – 96,413) | (22.79 – 26.57) | |||
| Symptomatic and | 11,328 | 25.75 | -- | -- | |
| At-Risk Screening | (1,557 – 43,840) | (22.79 – 26.58) |
“Dominated” – refers to complete dominance in decision science when one strategy offers both more effectiveness at lower costs.
Figure 2.
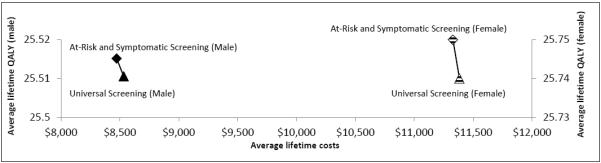
Cost-effectiveness plane comparing the average (per patient) lifetime costs and QALYs of Universal Serologic Screening (USS) versus Symptomatic and At-Risk Screening (SAS)
Deterministic Sensitivity Analyses
We tested the robustness of our model by varying each of the variables over a wide range of values as listed in Table 1. This method identifies the key parameters that impact the cost-effectiveness of the competing strategies. One-way sensitivity analysis identifies potential variables that may change the dominance of one strategy to a competing strategy, if base case values are different than what is assumed in the model. No variable was found to be significant, and potential changes in the individual variables did not change the ICER for either males or females. In other words, we did not identify any specific threshold value for any of the variables whereby changing the base value within sensitivity range limits would alter the result of the model from a cost-ineffective to a cost-effective USS strategy. From an epidemiologic perspective, we hypothesized that a high prevalence of celiac disease in the general population could conceivably make the USS strategy more cost-effective. However, our model indicates that with any CD prevalence > 1.03%, the average QALY gained in the SAS strategy was greater than in the more expensive USS strategy. The implication is that false positives by serology screening would drive unnecessary costs and invasive endoscopy, which carries a separate and non-negligible risk.27
Probabilistic Sensitivity Analysis
Probabilistic sensitivity analysis using multivariable Monte Carlo simulation of the model supports the dominance SAS as the more optimal strategy in detecting CD. We performed 1,000 independent simulations of the model (Figure 3). SAS strategy is more cost-effective in 67-80% of the 1,000 iterations of the model for men and women within all WTP thresholds < $100,000 per QALY-gained. Even at higher WTP thresholds between $50,000 to $100,000 per QALY-gained, USS strategy accrues more expense and yields less lifetime QALYs in approximately 70% of the time for either men or women. In every clinically realistic scenario when each variable is allowed to fluctuate between individual 95% confidence intervals, the current SAS strategy remains a more cost-effective approach to serologic screening for CD. For either gender, the likely clinical outcome under SAS strategy is increased QALYs and decreased costs (Figure 4), as indicated on an incremental cost versus incremental QALY plot.
Figure 3. Cost-effectiveness Acceptability Curves (Males and Females).
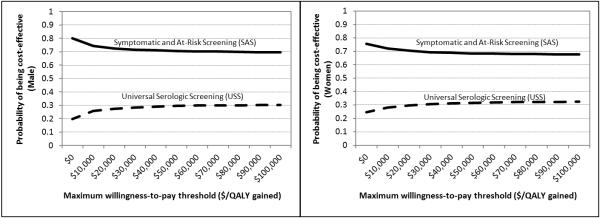
At every willingness-to-pay, SAS strategy was more cost-effective than USS stategy.
Figure 4. Incremental Cost-effectiveness Scatter Plots from 1,000 Simulations for SAS Strategy (Males and Females).
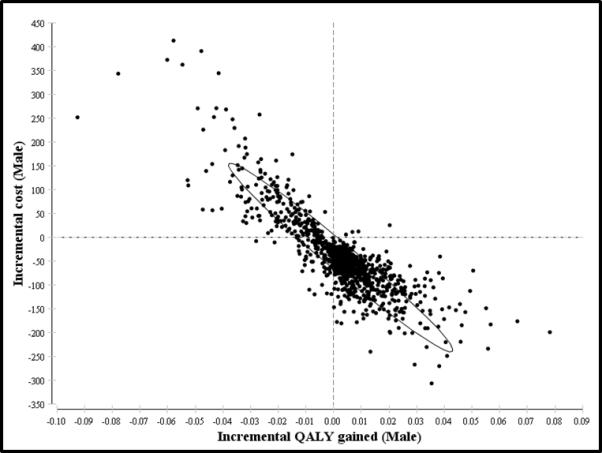
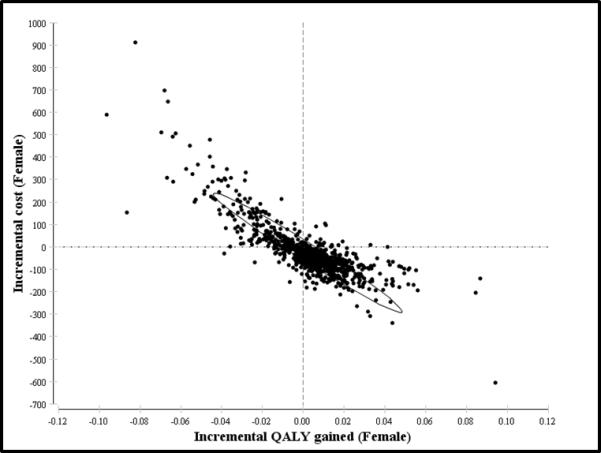
95% Confidence ellipses showing increased cluster for incremental QALY gains and incremental cost-savings
DISCUSSION
Our investigation is a cost-effectiveness analysis to determine whether universal screening for CD is a reasonable alternative to the current practice of screening patients who are either symptomatic or have inherited risk factors, since there is a measurable increased risk of non-traumatic fractures in undiagnosed or untreated CD. The findings from our analysis show that the current SAS strategy is more optimal than the USS strategy. Adopting the USS strategy – where virtually every preadolescent child would be screening for CD as part of his/her routine blood work in the primary care setting – would be more expensive and fails to increase the long-term quality of life of the population as a whole. The USS strategy introduces potential harm from unnecessary endoscopic evaluations of healthy individuals if serologic screening is falsely positive.
The rationale for modeling the clinical endpoints of hip and vertebral fractures is supported by literature in 3 ways. First, sub-optimal bone health in the form of non-traumatic fractures is an established risk factor for CD patients who are non-adherent to GFD or have undiagnosed subclinical disease. Among the several other potential health risks – including lymphoma and early mortality – considered as possible endpoints, bone disease is arguably the leading, quantifiable outcome measure without conflicting reports. Secondly, bone mass testing at the hip and vertebrae remains the “gold-standard” in assessing bone fragility (i.e., osteoporosis). As stated, the occurrence of fractures in these two sites represents the measurable risk of long-term disability. Lastly, narrowing the outcome measures to fractures at hip and vertebrae minimized the introduction of model uncertainties. Our model represents a focused policy evaluation based on evidence to potentially change CD screening practices. Whenever possible, we adopt a conservative modeling approach where literature-supported input values trump clinical assumptions or theories. This analysis attempts to address the question of expanding current screening recommendations for CD through the use of best-available evidence for mass screening.
It is evident from gastrointestinal society meetings and forums that there is ongoing clinical concern that current practice of CD screening misses a considerable proportion of asymptomatic CD patients. Unfortunately, there is no formal consensus on the precise long-term health benefits if expanded screening effectively decreases the proportion of undiagnosed or untreated CD patients. The position of universal serologic screening considers the potential morbidity and mortality when CD patients are non-adherent to the strict GFD. A recent AGA Perspectives lead article showcases national experts in CD discussing the issues around universal screening.28 We quote three direct statements in this printed discussion to summarize the dialog between national CD experts: 1) “There is insufficient data to recommend universal screening;” 2) “The possible benefits of screening for subclinical disease are theoretical rather than evidence-based;” 3) “A formal cost-effectiveness analysis based on outcome data is required.” In sum, our investigation is an analytical attempt to consolidate the data and produce the latest cost-effectiveness analysis to determine whether universal screening may be a feasible alternative to standard screening.
Due to recently validated evidence of the benefits of strict GFD on bone health, our analysis uses CD-relevant endpoint of non-traumatic fractures – which was not used in previously published cost-effectiveness analyses.60,66 Previous literature substantiates the need to evaluate fractures as a separate clinical outcome measure in untreated CD. The prevalence of decreased bone mineral density in untreated celiac patients has been shown to be as high as 70%, in both symptomatic and asymptomatic individuals.29,30,31 West et al56 reports that decreased bone mineral density is responsible for 30% increased risk for osteoporosis and overall fractures in CD. Among patients with osteoporosis, there is significant increased mortality risk after hip and compression fractures.32,33
As robust as the evidence may be for increased fracture risk, less is known regarding the timing and natural progression of bone disease – from normal bone density to osteoporosis – in untreated CD, although some literature is available in cohorts > 50 years of age without CD.55 We conjecture that this is due to the insidious nature of bone disease in asymptomatic and undiagnosed CD, where patients are often not aware of progressive osteopenia and osteoporosis until fracture occurrence. Therefore, a limitation of our analysis is that the osteoporosis state could not be modeled due to insufficient data. Another limitation of our model results is that literature is not entirely clear about differentiating hip from non-hip fractures within the category of long bone fractures in CD. As such, our model may overestimate the rate of hip fractures, although the potentially higher base case value did not make USS strategy more cost-effective.
In conclusion, in an effort to provide a substantive basis for the consideration of mass serologic CD screening, the current SAS strategy remains more cost-effective than the USS strategy. Initiating universal screening to prevent bone disease and subsequent non-traumatic fractures alone in undiagnosed or untreated CD patients does not appear to be a viable health policy alternative to the standard of care. Further analysis of risk and cost of other potential consequences of undiagnosed and untreated CD, such as anemia, infertility, and malignancy, could change the cost-effectiveness of universal screening for CD.
Acknowledgements
Authors thank Jeremy Goldhaber-Fiebert, PhD and Douglas Owens, MD, MS for their expertise in decision science. KTP is supported by NIH DK094868. The manuscript contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions: KT Park, MD, MS (co-first author) – obtaining funding source, concept for the study, supporting construction and editing of the model, collecting and interpreting data, and drafting/editing the manuscript
Raymond Tsai, MS (co-first author) – planning and constructing the model, collecting and interpreting data, and editing the manuscript
Louise Wang, BS – collecting data, planning the study
Nasim Khavari, MD, MPH – planning the study, clinical expertise in celiac disease, editing the manuscript
Laura Bachrach, MD – planning the study, clinical expertise in bone disease, editing the manuscript
Dorsey Bass, MD – concept for the study, planning the study, editing the manuscript
Potential competing interests: None
Disclosures Authors report no conflicts of interest as described by Clinical Gastroenterology and Hepatology
REFERENCES
- 1.Haas SV. Celiac disease, its specific treatment and cure without nutritional relapse. JAMA. 1932;99:448. [Google Scholar]
- 2.Booth CC. History of celiac disease. BMJ. 1989:298–527. [Google Scholar]
- 3.Bottaro G, Cataldo F, Rotolo N, Spina M, Corazza GR. The clinical pattern of subclinical/silent celiac disease: an analysis on 1026 consecutive cases. Am J Gastroenterol. 1999 Mar;94(3):691–6. doi: 10.1111/j.1572-0241.1999.00938.x. [DOI] [PubMed] [Google Scholar]
- 4.AGA Institute AGA Institute Medical Position Statement on the Diagnosis and Management of Celiac Disease. Gastroenterology. 2006 Dec;131(6):1977–80. doi: 10.1053/j.gastro.2006.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holmes GK. Non-malignant complications of coeliac disease. Acta Paediatr Suppl. 1996 May;412:68–75. doi: 10.1111/j.1651-2227.1996.tb14257.x. [DOI] [PubMed] [Google Scholar]
- 6.Rubio-Tapia A, Kyle RA, Kaplan EL, Johnson DR, Page W, Erdtmann F, Brantner TL, Kim WR, Phelps TK, Lahr BD, Zinsmeister AR, Melton LJ, 3rd, Murray JA. Increased prevalence and mortality in undiagnosed celiac disease. Gastroenterology. 2009 Jul;137(1):88–93. doi: 10.1053/j.gastro.2009.03.059. Epub 2009 Apr 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mäki M, Mustalahti K, Kokkonen J, Kulmala P, Haapalahti M, Karttunen T, Ilonen J, Laurila K, Dahlbom I, Hansson T, Höpfl P, Knip M. Prevalence of Celiac disease among children in Finland. N Engl J Med. 2003 Jun 19;348(25):2517–24. doi: 10.1056/NEJMoa021687. [DOI] [PubMed] [Google Scholar]
- 8.Katz KD, Rashtak S, Lahr BD, Melton LJ, 3rd, Krause PK, Maggi K, Talley NJ, Murray JA. Screening for celiac disease in a North American population: sequential serology and gastrointestinal symptoms. Am J Gastroenterol. 2011 Jul;106(7):1333–9. doi: 10.1038/ajg.2011.21. doi: 10.1038/ajg.2011.21. Epub 2011 Mar 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rubio-Tapia A, Ludvigsson JF, Brantner TL, Murray JA, Everhart JE. The Prevalence of Celiac Disease in the United States. Am J Gastroenterol. 2012 Jul 31; doi: 10.1038/ajg.2012.219. doi: 10.1038/ajg.2012.219. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 10.Fasano A, Berti I, Gerarduzzi T, Not T, Colletti RB, Drago S, Elitsur Y, Green PH, Guandalini S, Hill ID, Pietzak M, Ventura A, Thorpe M, Kryszak D, Fornaroli F, Wasserman SS, Murray JA, Horvath K. Prevalence of celiac disease in at-risk and not-at-risk groups in the United States: a large multicenter study. Arch Intern Med. 2003 Feb 10;163(3):286–92. doi: 10.1001/archinte.163.3.286. [DOI] [PubMed] [Google Scholar]
- 11.Godfrey JD, Brantner TL, Brinjikji W, Christensen KN, Brogan DL, Van Dyke CT, Lahr BD, Larson JJ, Rubio-Tapia A, Melton LJ, 3rd, Zinsmeister AR, Kyle RA, Murray JA. Morbidity and mortality among older individuals with undiagnosed celiac disease. Gastroenterology. 2010 Sep;139(3):763–9. doi: 10.1053/j.gastro.2010.05.041. Epub 2010 Jun 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bianchi ML, Bardella MT. Bone in celiac disease. Osteoporos Int. 2008 Dec;19(12):1705–16. doi: 10.1007/s00198-008-0624-0. Epub 2008 Apr 17. [DOI] [PubMed] [Google Scholar]
- 13.McFarlane XA, Bhalla AK, Reeves DE, Morgan LM, Robertson DA. Osteoporosis in treated adult coeliac disease. Gut. 1995 May;36(5):710–4. doi: 10.1136/gut.36.5.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Silverman SL, Cummings SR, Watts NB, Consensus Panel of the ASBMR, ISCD, and NOF Recommendations for the clinical evaluation of agents for treatment of osteoporosis: consensus of an expert panel representing the American Society for Bone and Mineral Research (ASBMR), the International Society for Clinical Densitometry (ISCD), and the National Osteoporosis Foundation (NOF) J Bone Miner Res. 2008 Jan;23(1):159–65. doi: 10.1359/jbmr.070905. [DOI] [PubMed] [Google Scholar]
- 15.Orimo H, Hayashi Y, Fukunaga M, Sone T, Fujiwara S, Shiraki M, Kushida K, Miyamoto S, Soen S, Nishimura J, Oh-Hashi Y, Hosoi T, Gorai I, Tanaka H, Igai T, Kishimoto H, Osteoporosis Diagnostic Criteria Review Committee: Japanese Society for Bone and Mineral Research Diagnostic criteria for primary osteoporosis: year 2000 revision. J Bone Miner Metab. 2001;19(6):331–7. doi: 10.1007/s007740170001. [DOI] [PubMed] [Google Scholar]
- 16.Kanis JA, Glüer CC. An update on the diagnosis and assessment of osteoporosis with densitometry. Committee of Scientific Advisors, International Osteoporosis Foundation. Osteoporos Int. 2000;11(3):192–202. doi: 10.1007/s001980050281. [DOI] [PubMed] [Google Scholar]
- 17.NIH Consensus Development Conference on Celiac Disease. NIH Consens State Sci Statements. 2004 Jun 28-30;21(1):1–23. [PubMed] [Google Scholar]
- 18.Lurz E, Scheidegger U, Spalinger J, Schöni M, Schibli S. Clinical presentation of celiac disease and the diagnostic accuracy of serologic markers in children. Eur J Pediatr. 2009 Jul;168(7):839–45. doi: 10.1007/s00431-008-0845-4. Epub 2008 Oct 16. [DOI] [PubMed] [Google Scholar]
- 19.Maglio M, Tosco A, Paparo F, Auricchio R, Granata V, Colicchio B, Indolfi V, Miele E, Troncone R. Serum and intestinal celiac disease-associated antibodies in children with celiac disease younger than 2 years of age. J Pediatr Gastroenterol Nutr. 2010 Jan;50(1):43–8. doi: 10.1097/MPG.0b013e3181b99c8f. [DOI] [PubMed] [Google Scholar]
- 20.Aggarwal S, Lebwohl B, Green PH. Screening for celiac disease in average-risk and high-risk populations. Therap Adv Gastroenterol. 2012 Jan;5(1):37–47. doi: 10.1177/1756283X11417038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kochhar R, Sachdev S, Kochhar R, Aggarwal A, Sharma V, Prasad KK, Singh G, Nain CK, Singh K, Marwaha N. Prevalence of coeliac disease in healthy blood donors: a study from north India. Dig Liver Dis. 2012 Jun;44(6):530–2. doi: 10.1016/j.dld.2012.01.004. Epub 2012 Apr 10. [DOI] [PubMed] [Google Scholar]
- 22.See AAP Pediatric Nutrition Handbook. 5th Edition 2003. for a discussion of universal and selective screening options. See also Recommendations to prevent and control iron deficiency in the United States. MMWR Recomm Rep. 1998;47(RR-3):1–36.
- 23.National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002 Dec 17;106(25):3143–421. [PubMed] [Google Scholar]
- 24.Gold MR, Siegel JE, Russell LB, Weinstein MC, editors. Cost-effectiveness in Health and Medicine. Oxford University Press; New York: 1996. [Google Scholar]
- 25. http://www.cdc.gov/nchs/data/nvsr/nvsr52/nvsr52_14.pdf.
- 26.Fasano A, Araya M, Bhatnagar S, Cameron D, Catassi C, Dirks M, Mearin ML, Ortigosa L, Phillips A, Celiac Disease Working Group, FISPGHAN Federation of International Societies of Pediatric Gastroenterology, Hepatology, and Nutrition consensus report on celiac disease. J Pediatr Gastroenterol Nutr. 2008 Aug;47(2):214–9. doi: 10.1097/MPG.0b013e318181afed. [DOI] [PubMed] [Google Scholar]
- 27.Li G, Warner M, Lang BH, Huang L, Sun LS. Epidemiology of anesthesia-related mortality in the United States, 1999-2005. Anesthesiology. 2009 Apr;110(4):759–65. doi: 10.1097/aln.0b013e31819b5bdc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.AGA Perspectives. 2012 Apr-May;Vol.8(No. 2) [Google Scholar]
- 29.Meyer D, Stavropolous S, Diamond B, Shane E, Green PH. Osteoporosis in a north american adult population with celiac disease. Am J Gastroenterol. 2001 Jan;96(1):112–9. doi: 10.1111/j.1572-0241.2001.03507.x. [DOI] [PubMed] [Google Scholar]
- 30.Cellier C, Flobert C, Cormier C, Roux C, Schmitz J. Severe osteopenia in symptom-free adults with a childhood diagnosis of coeliac disease. Lancet. 2000 Mar 4;355(9206):806. doi: 10.1016/S0140-6736(99)04855-2. [DOI] [PubMed] [Google Scholar]
- 31.Mustalahti K, Collin P, Sievänen H, Salmi J, Mäki M. Osteopenia in patients with clinically silent coeliac disease warrants screening. Lancet. 1999 Aug 28;354(9180):744–5. doi: 10.1016/S0140-6736(99)01990-X. [DOI] [PubMed] [Google Scholar]
- 32.Hannan EL, Magaziner J, Wang JJ, Eastwood EA, Silberzweig SB, Gilbert M, Morrison RS, McLaughlin MA, Orosz GM, Siu AL. Mortality and locomotion 6 months after hospitalization for hip fracture: risk factors and risk-adjusted hospital outcomes. JAMA. 2001 Jun 6;285(21):2736–42. doi: 10.1001/jama.285.21.2736. [DOI] [PubMed] [Google Scholar]
- 33.Kanis JA, Borgstrom F, Johnell O, Jonsson B. Cost-effectiveness of risedronate for the treatment of osteoporosis and prevention of fractures in postmenopausal women. Osteoporos Int. 2004 Nov;15(11):862–71. doi: 10.1007/s00198-004-1643-0. Epub 2004 Jun 3. [DOI] [PubMed] [Google Scholar]
- 34.Hyams JS, Burke G, Davis PM, Rzepski B, Andrulonis PA. Abdominal pain and irritable bowel syndrome in adolescents: a community-based study. J Pediatr. 1996 Aug;129(2):220–6. doi: 10.1016/s0022-3476(96)70246-9. [DOI] [PubMed] [Google Scholar]
- 35.Saito YA, Locke GR, Talley NJ, Zinsmeister AR, Fett SL, Melton LJ., 3rd A comparison of the Rome and Manning criteria for case identification in epidemiological investigations of irritable bowel syndrome. Am J Gastroenterol. 2000 Oct;95(10):2816–24. doi: 10.1111/j.1572-0241.2000.03192.x. [DOI] [PubMed] [Google Scholar]
- 36.Hahn BA, Saunders WB, Maier WC. Differences between individuals with self-reported irritable bowel syndrome (IBS) and IBS-like symptoms. Dig Dis Sci. 1997 Dec;42(12):2585–90. doi: 10.1023/a:1018889318063. [DOI] [PubMed] [Google Scholar]
- 37.Talley NJ, Zinsmeister AR, Van Dyke C, Melton LJ., 3rd Epidemiology of colonic symptoms and the irritable bowel syndrome. Gastroenterology. 1991 Oct;101(4):927–34. doi: 10.1016/0016-5085(91)90717-y. [DOI] [PubMed] [Google Scholar]
- 38.Ford AC, Chey WD, Talley NJ, Malhotra A, Spiegel BM, Moayyedi P. Yield of diagnostic tests for celiac disease in individuals with symptoms suggestive of irritable bowel syndrome: systematic review and meta-analysis. Arch Intern Med. 2009 Apr 13;169(7):651–8. doi: 10.1001/archinternmed.2009.22. [DOI] [PubMed] [Google Scholar]
- 39.Stenson WF, Newberry R, Lorenz R, Baldus C, Civitelli R. Increased prevalence of celiac disease and need for routine screening among patients with osteoporosis. Arch Intern Med. 2005 Feb 28;165(4):393–9. doi: 10.1001/archinte.165.4.393. [DOI] [PubMed] [Google Scholar]
- 40.Thomason K, West J, Logan RF, Coupland C, Holmes GK. Fracture experience of patients with coeliac disease: a population based survey. Gut. 2003 Apr;52(4):518–22. doi: 10.1136/gut.52.4.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ludvigsson JF, Michaelsson K, Ekbom A, Montgomery SM. Coeliac disease and the risk of fractures - a general population-based cohort study. Aliment Pharmacol Ther. 2007 Feb 1;25(3):273–85. doi: 10.1111/j.1365-2036.2006.03203.x. [DOI] [PubMed] [Google Scholar]
- 42.Bell RA, Mayer-Davis EJ, Beyer JW, D’Agostino RB, Jr, Lawrence JM, Linder B, Liu LL, Marcovina SM, Rodriguez BL, Williams D, Dabelea D, SEARCH for Diabetes in Youth Study Group Diabetes in non-Hispanic white youth: prevalence, incidence, and clinical characteristics: the SEARCH for Diabetes in Youth Study. Diabetes Care. 2009 Mar;32(Suppl 2):S102–11. doi: 10.2337/dc09-S202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shin M, Besser LM, Kucik JE, Lu C, Siffel C, Correa A, Congenital Anomaly Multistate Prevalence and Survival Collaborative Prevalence of Down syndrome among children and adolescents in 10 regions of the United States. Pediatrics. 2009 Dec;124(6):1565–71. doi: 10.1542/peds.2009-0745. [DOI] [PubMed] [Google Scholar]
- 44.Gunther DF, Eugster E, Zagar AJ, Bryant CG, Davenport ML, Quigley CA. Ascertainment bias in Turner syndrome: new insights from girls who were diagnosed incidentally in prenatal life. Pediatrics. 2004 Sep;114(3):640–4. doi: 10.1542/peds.2003-1122-L. [DOI] [PubMed] [Google Scholar]
- 45.Strømme P, Bjørnstad PG, Ramstad K. Prevalence estimation of Williams syndrome. J Child Neurol. 2002 Apr;17(4):269–71. doi: 10.1177/088307380201700406. [DOI] [PubMed] [Google Scholar]
- 46.Rankin EC, Isenberg DA. IgA deficiency and SLE: prevalence in a clinic population and a review of the literature. Lupus. 1997;6(4):390–4. doi: 10.1177/096120339700600408. [DOI] [PubMed] [Google Scholar]
- 47.McGrogan A, Seaman HE, Wright JW, de Vries CS. The incidence of autoimmune thyroid disease: a systematic review of the literature. Clin Endocrinol (Oxf) 2008 Nov;69(5):687–96. doi: 10.1111/j.1365-2265.2008.03338.x. Epub 2008 Jul 31. [DOI] [PubMed] [Google Scholar]
- 48.Fasano A, Berti I, Gerarduzzi T, Not T, Colletti RB, Drago S, Elitsur Y, Green PH, Guandalini S, Hill ID, Pietzak M, Ventura A, Thorpe M, Kryszak D, Fornaroli F, Wasserman SS, Murray JA, Horvath K. Prevalence of celiac disease in at-risk and not-at-risk groups in the United States: a large multicenter study. Arch Intern Med. 2003 Feb 10;163(3):286–92. doi: 10.1001/archinte.163.3.286. [DOI] [PubMed] [Google Scholar]
- 49.Cronin CC, Feighery A, Ferriss JB, Liddy C, Shanahan F, Feighery C. High prevalence of celiac disease among patients with insulin-dependent (type I) diabetes mellitus. Am J Gastroenterol. 1997 Dec;92(12):2210–2. [PubMed] [Google Scholar]
- 50.Meini A, Pillan NM, Villanacci V, Monafo V, Ugazio AG, Plebani A. Prevalence and diagnosis of celiac disease in IgA-deficient children. Ann Allergy Asthma Immunol. 1996 Oct;77(4):333–6. doi: 10.1016/S1081-1206(10)63329-7. [DOI] [PubMed] [Google Scholar]
- 51.Carlsson A, Axelsson I, Borulf S, Bredberg A, Forslund M, Lindberg B, Sjöberg K, Ivarsson SA. Prevalence of IgA-antigliadin antibodies and IgA-antiendomysium antibodies related to celiac disease in children with Down syndrome. Pediatrics. 1998 Feb;101(2):272–5. doi: 10.1542/peds.101.2.272. [DOI] [PubMed] [Google Scholar]
- 52.Badenhoop K, Dieterich W, Segni M, Hofmann S, Hüfner M, Usadel KH, Hahn EG, Schuppan D. HLA DQ2 and/or DQ8 is associated with celiac disease-specific autoantibodies to tissue transglutaminase in families with thyroid autoimmunity. Am J Gastroenterol. 2001 May;96(5):1648–9. doi: 10.1111/j.1572-0241.2001.03821.x. [DOI] [PubMed] [Google Scholar]
- 53.Doğan Y, Yldrmaz S, Ozercan IH. Prevalence of Celiac Disease Among First-degree Relatives of Patients With Celiac Disease. J Pediatr Gastroenterol Nutr. 2012 Aug;55(2):205–8. doi: 10.1097/MPG.0b013e318249378c. [DOI] [PubMed] [Google Scholar]
- 54.Mearin ML. Celiac disease among children and adolescents. Curr Probl Pediatr Adolesc Health Care. 2007 Mar;37(3):86–105. doi: 10.1016/j.cppeds.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 55.Ioannidis G, Papaioannou A, Hopman WM, Akhtar-Danesh N, Anastassiades T, Pickard L, Kennedy CC, Prior JC, Olszynski WP, Davison KS, Goltzman D, Thabane L, Gafni A, Papadimitropoulos EA, Brown JP, Josse RG, Hanley DA, Adachi JD. Relation between fractures and mortality: results from the Canadian Multicentre Osteoporosis Study. CMAJ. 2009 Sep 1;181(5):265–71. doi: 10.1503/cmaj.081720. Epub 2009 Aug 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.West J, Logan RF, Card TR, Smith C, Hubbard R. Fracture risk in people with celiac disease: a population-based cohort study. Gastroenterology. 2003 Aug;125(2):429–36. doi: 10.1016/s0016-5085(03)00891-6. [DOI] [PubMed] [Google Scholar]
- 57.Sánchez MI, Mohaidle A, Baistrocchi A, Matoso D, Vázquez H, González A, Mazure R, Maffei E, Ferrari G, Smecuol E, Crivelli A, de Paula JA, Gómez JC, Pedreira S, Mauriño E, Bai JC. Risk of fracture in celiac disease: gender, dietary compliance, or both? World J Gastroenterol. 2011 Jul 7;17(25):3035–42. doi: 10.3748/wjg.v17.i25.3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wardlaw D, Cummings SR, Van Meirhaeghe J, Bastian L, Tillman JB, Ranstam J, Eastell R, Shabe P, Talmadge K, Boonen S. Efficacy and safety of balloon kyphoplasty compared with non-surgical care for vertebral compression fracture (FREE): a randomised controlled trial. Lancet. 2009 Mar 21;373(9668):1016–24. doi: 10.1016/S0140-6736(09)60010-6. Epub 2009 Feb 24. [DOI] [PubMed] [Google Scholar]
- 59.Mancuso CA, Salvati EA. Patients’ satisfaction with the process of total hip arthroplasty. J Healthc Qual. 2003 Mar-Apr;25(2):12–8. doi: 10.1111/j.1945-1474.2003.tb01039.x. quiz 18-9. [DOI] [PubMed] [Google Scholar]
- 60.Shamir R, Hernell O, Leshno M. Cost-effectiveness analysis of screening for celiac disease in the adult population. Med Decis Making. 2006 May-Jun;26(3):282–93. doi: 10.1177/0272989X06289012. [DOI] [PubMed] [Google Scholar]
- 61.Lee AR, Ng DL, Zivin J, Green PH. Economic burden of a gluten-free diet. J Hum Nutr Diet. 2007 Oct;20(5):423–30. doi: 10.1111/j.1365-277X.2007.00763.x. [DOI] [PubMed] [Google Scholar]
- 62. http://www.cnpp.usda.gov/Publications/FoodPlans/2011/CostofFoodMay2011.pdf.
- 63. http://www.cms.gov/apps/physician-fee-schedule/overview.aspx.
- 64.Norström F, Lindholm L, Sandström O, Nordyke K, Ivarsson A. Delay to celiac disease diagnosis and its implications for health-related quality of life. BMC Gastroenterol. 2011 Nov 7;11:118. doi: 10.1186/1471-230X-11-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tengs TO, Wallace A. One thousand health-related quality-of-life estimates. Med Care. 2000 Jun;38(6):583–637. doi: 10.1097/00005650-200006000-00004. [DOI] [PubMed] [Google Scholar]
- 66.Hershcovici T, Leshno M, Goldin E, Shamir R, Israeli E. Cost effectiveness of mass screening for coeliac disease is determined by time-delay to diagnosis and quality of life on a gluten-freediet. Aliment Pharmacol Ther. 2010 Apr;31(8):901–10. doi: 10.1111/j.1365-2036.2010.04242.x. Epub 2010 Jan 19. [DOI] [PubMed] [Google Scholar]
- 67.Jönsson B, Christiansen C, Johnell O, Hedbrandt J. Cost-effectiveness of fracture prevention in established osteoporosis. Osteoporos Int. 1995 Mar;5(2):136–42. [PubMed] [Google Scholar]
- 68.Oleksik A, Lips P, Dawson A, Minshall ME, Shen W, Cooper C, Kanis J. Health-related quality of life in postmenopausal women with low BMD with or without prevalent vertebral fractures. J Bone Miner Res. 2000 Jul;15(7):1384–92. doi: 10.1359/jbmr.2000.15.7.1384. [DOI] [PubMed] [Google Scholar]


