Abstract
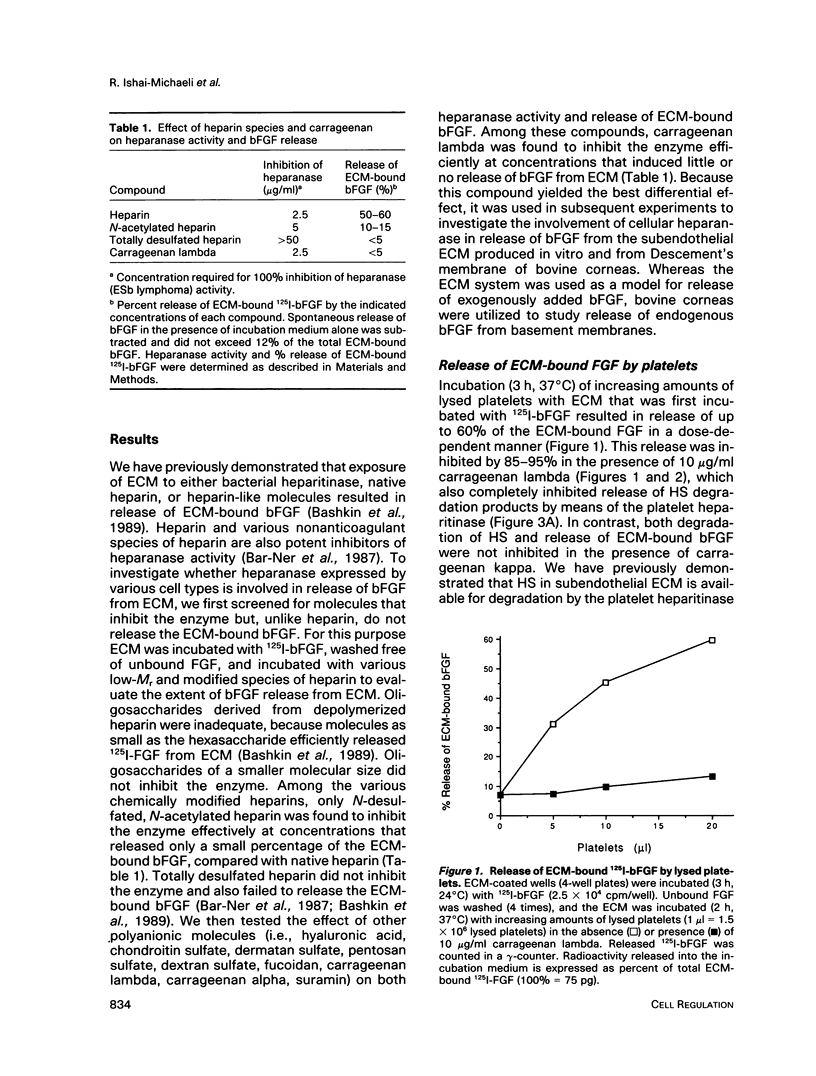
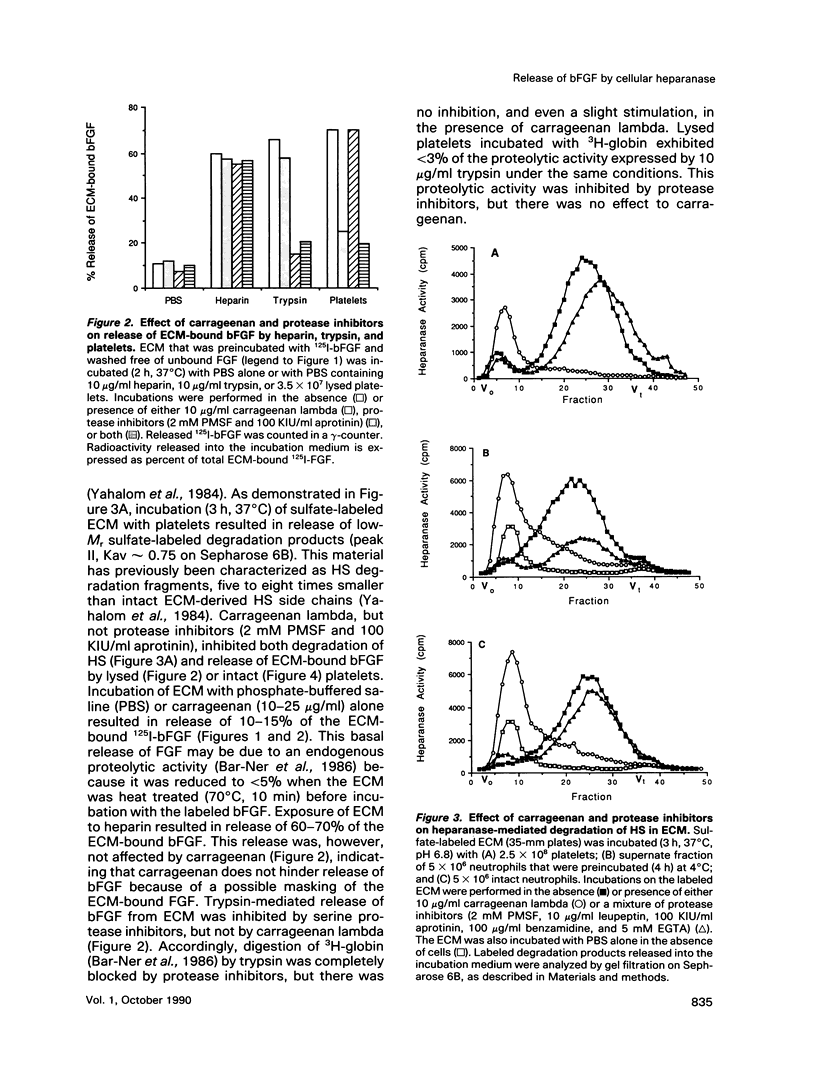
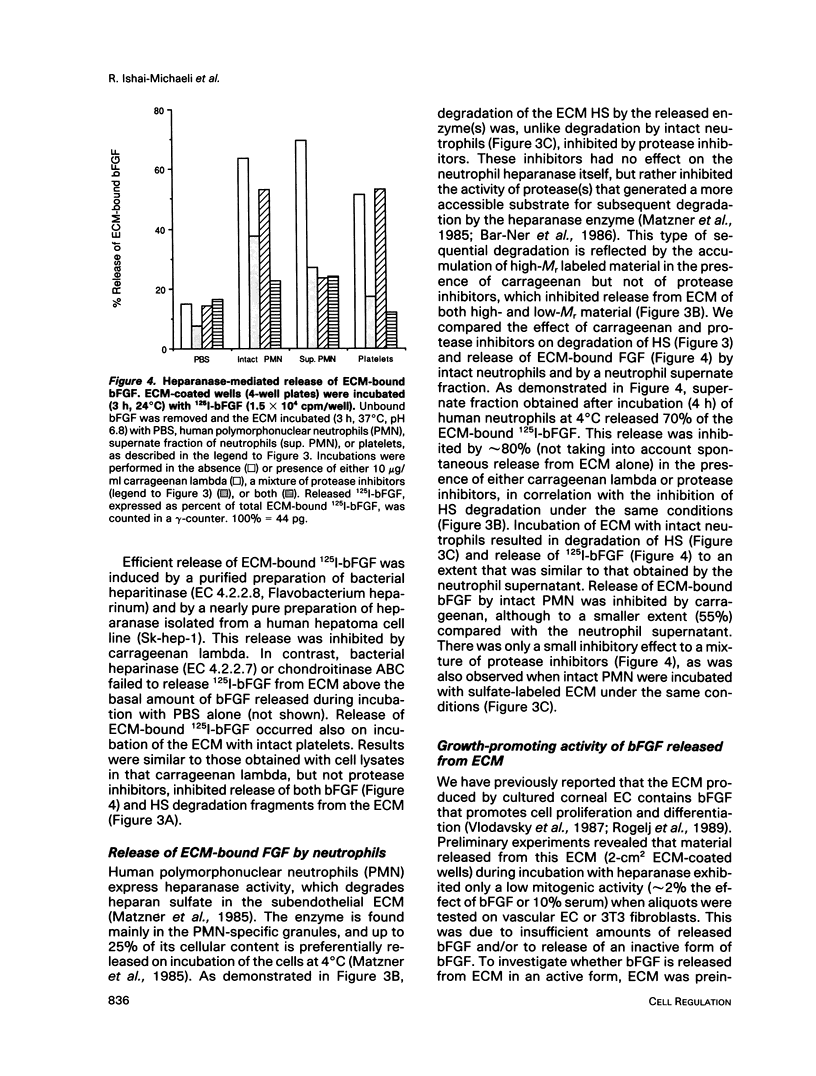
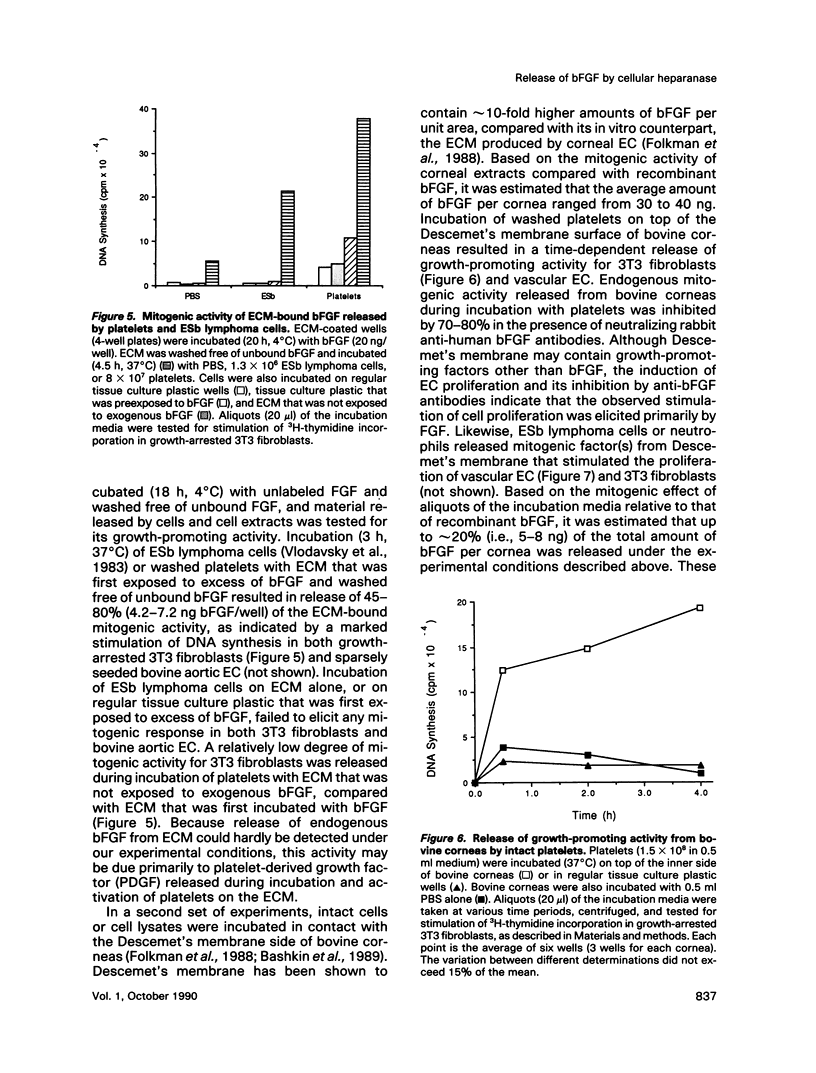
Incubation of platelets, neutrophils, and lymphoma cells with Descemet's membranes of bovine corneas and with the extracellular matrix (ECM) produced by cultured corneal endothelial cells resulted in release of basic fibroblast growth factor (bFGF), which stimulated the proliferation of 3T3 fibroblasts and vascular endothelial cells. Similar requirements were observed for release of endogenous bFGF stored in Descemet's membrane and of exogenous bFGF sequestered by the subendothelial ECM. Release of ECM-resident bFGF by platelets, neutrophils, and lymphoma cells was inhibited by carrageenan lambda, but not by protease inhibitors, in correlation with the inhibition of heparanase activity expressed by these cells. Degradation of the ECM-heparan sulfate side chains by this endo-beta-D-glucuronidase is thought to play an important role in cell invasion, particularly in the extravasation of blood-borne tumor cells and activated cells of the immune system. We propose that both heparanase and ECM-resident bFGF may modulate the cell response to contact with its local environment. Heparanase-mediated release of active bFGF from storage in basement membranes provides a novel mechanism for a localized induction of neovascularization in various normal and pathological processes, such as wound healing, inflammation, and tumor development.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham J. A., Mergia A., Whang J. L., Tumolo A., Friedman J., Hjerrild K. A., Gospodarowicz D., Fiddes J. C. Nucleotide sequence of a bovine clone encoding the angiogenic protein, basic fibroblast growth factor. Science. 1986 Aug 1;233(4763):545–548. doi: 10.1126/science.2425435. [DOI] [PubMed] [Google Scholar]
- Andres J. L., Stanley K., Cheifetz S., Massagué J. Membrane-anchored and soluble forms of betaglycan, a polymorphic proteoglycan that binds transforming growth factor-beta. J Cell Biol. 1989 Dec;109(6 Pt 1):3137–3145. doi: 10.1083/jcb.109.6.3137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird A., Ling N. Fibroblast growth factors are present in the extracellular matrix produced by endothelial cells in vitro: implications for a role of heparinase-like enzymes in the neovascular response. Biochem Biophys Res Commun. 1987 Jan 30;142(2):428–435. doi: 10.1016/0006-291x(87)90292-0. [DOI] [PubMed] [Google Scholar]
- Baird A., Walicke P. A. Fibroblast growth factors. Br Med Bull. 1989 Apr;45(2):438–452. doi: 10.1093/oxfordjournals.bmb.a072333. [DOI] [PubMed] [Google Scholar]
- Bar-Ner M., Eldor A., Wasserman L., Matzner Y., Cohen I. R., Fuks Z., Vlodavsky I. Inhibition of heparanase-mediated degradation of extracellular matrix heparan sulfate by non-anticoagulant heparin species. Blood. 1987 Aug;70(2):551–557. [PubMed] [Google Scholar]
- Bar-Ner M., Mayer M., Schirrmacher V., Vlodavsky I. Involvement of both heparanase and plasminogen activator in lymphoma cell-mediated degradation of heparan sulfate in the subendothelial extracellular matrix. J Cell Physiol. 1986 Aug;128(2):299–306. doi: 10.1002/jcp.1041280223. [DOI] [PubMed] [Google Scholar]
- Bashkin P., Doctrow S., Klagsbrun M., Svahn C. M., Folkman J., Vlodavsky I. Basic fibroblast growth factor binds to subendothelial extracellular matrix and is released by heparitinase and heparin-like molecules. Biochemistry. 1989 Feb 21;28(4):1737–1743. doi: 10.1021/bi00430a047. [DOI] [PubMed] [Google Scholar]
- Bashkin P., Razin E., Eldor A., Vlodavsky I. Degranulating mast cells secrete an endoglycosidase that degrades heparan sulfate in subendothelial extracellular matrix. Blood. 1990 Jun 1;75(11):2204–2212. [PubMed] [Google Scholar]
- Burgess W. H., Maciag T. The heparin-binding (fibroblast) growth factor family of proteins. Annu Rev Biochem. 1989;58:575–606. doi: 10.1146/annurev.bi.58.070189.003043. [DOI] [PubMed] [Google Scholar]
- Folkman J., Klagsbrun M. Angiogenic factors. Science. 1987 Jan 23;235(4787):442–447. doi: 10.1126/science.2432664. [DOI] [PubMed] [Google Scholar]
- Folkman J., Klagsbrun M., Sasse J., Wadzinski M., Ingber D., Vlodavsky I. A heparin-binding angiogenic protein--basic fibroblast growth factor--is stored within basement membrane. Am J Pathol. 1988 Feb;130(2):393–400. [PMC free article] [PubMed] [Google Scholar]
- Fridman R., Fuks Z., Ovadia H., Vlodavsky I. Differential structural requirements for the induction of cell attachment, proliferation and differentiation by the extracellular matrix. Exp Cell Res. 1985 Mar;157(1):181–194. doi: 10.1016/0014-4827(85)90161-2. [DOI] [PubMed] [Google Scholar]
- Goldman R., Bar-Shavit Z. Dual effect of normal and stimulated macrophages and their conditioned media on target cell proliferation. J Natl Cancer Inst. 1979 Oct;63(4):1009–1016. [PubMed] [Google Scholar]
- Gonzalez A. M., Buscaglia M., Ong M., Baird A. Distribution of basic fibroblast growth factor in the 18-day rat fetus: localization in the basement membranes of diverse tissues. J Cell Biol. 1990 Mar;110(3):753–765. doi: 10.1083/jcb.110.3.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gospodarowicz D., Bialecki H., Greenburg G. Purification of the fibroblast growth factor activity from bovine brain. J Biol Chem. 1978 May 25;253(10):3736–3743. [PubMed] [Google Scholar]
- Gospodarowicz D., Cheng J. Heparin protects basic and acidic FGF from inactivation. J Cell Physiol. 1986 Sep;128(3):475–484. doi: 10.1002/jcp.1041280317. [DOI] [PubMed] [Google Scholar]
- Gospodarowicz D., Ferrara N., Schweigerer L., Neufeld G. Structural characterization and biological functions of fibroblast growth factor. Endocr Rev. 1987 May;8(2):95–114. doi: 10.1210/edrv-8-2-95. [DOI] [PubMed] [Google Scholar]
- Gospodarowicz D., Gonzalez R., Fujii D. K. Are factors originating from serum, plasma, or cultured cells involved in the growth-promoting effect of the extracellular matrix produced by cultured bovine corneal endothelial cells? J Cell Physiol. 1983 Feb;114(2):191–202. doi: 10.1002/jcp.1041140208. [DOI] [PubMed] [Google Scholar]
- Gospodarowicz D., Mescher A. L., Birdwell C. R. Stimulation of corneal endothelial cell proliferations in vitro by fibroblast and epidermal growth factors. Exp Eye Res. 1977 Jul;25(1):75–89. doi: 10.1016/0014-4835(77)90248-2. [DOI] [PubMed] [Google Scholar]
- Kardami E., Fandrich R. R. Basic fibroblast growth factor in atria and ventricles of the vertebrate heart. J Cell Biol. 1989 Oct;109(4 Pt 1):1865–1875. doi: 10.1083/jcb.109.4.1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler D. A., Langer R. S., Pless N. A., Folkman J. Mast cells and tumor angiogenesis. Int J Cancer. 1976 Nov 15;18(5):703–709. doi: 10.1002/ijc.2910180520. [DOI] [PubMed] [Google Scholar]
- Kimelman D., Kirschner M. Synergistic induction of mesoderm by FGF and TGF-beta and the identification of an mRNA coding for FGF in the early Xenopus embryo. Cell. 1987 Dec 4;51(5):869–877. doi: 10.1016/0092-8674(87)90110-3. [DOI] [PubMed] [Google Scholar]
- Kramer R. H., Vogel K. G., Nicolson G. L. Solubilization and degradation of subendothelial matrix glycoproteins and proteoglycans by metastatic tumor cells. J Biol Chem. 1982 Mar 10;257(5):2678–2686. [PubMed] [Google Scholar]
- Lider O., Mekori Y. A., Miller T., Bar-Tana R., Vlodavsky I., Baharav E., Cohen I. R., Naparstek Y. Inhibition of T lymphocyte heparanase by heparin prevents T cell migration and T cell-mediated immunity. Eur J Immunol. 1990 Mar;20(3):493–499. doi: 10.1002/eji.1830200306. [DOI] [PubMed] [Google Scholar]
- Matzner Y., Bar-Ner M., Yahalom J., Ishai-Michaeli R., Fuks Z., Vlodavsky I. Degradation of heparan sulfate in the subendothelial extracellular matrix by a readily released heparanase from human neutrophils. Possible role in invasion through basement membranes. J Clin Invest. 1985 Oct;76(4):1306–1313. doi: 10.1172/JCI112104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima M., Irimura T., Nicolson G. L. Heparanases and tumor metastasis. J Cell Biochem. 1988 Feb;36(2):157–167. doi: 10.1002/jcb.240360207. [DOI] [PubMed] [Google Scholar]
- Naparstek Y., Cohen I. R., Fuks Z., Vlodavsky I. Activated T lymphocytes produce a matrix-degrading heparan sulphate endoglycosidase. Nature. 1984 Jul 19;310(5974):241–244. doi: 10.1038/310241a0. [DOI] [PubMed] [Google Scholar]
- Parish C. R., Coombe D. R., Jakobsen K. B., Bennett F. A., Underwood P. A. Evidence that sulphated polysaccharides inhibit tumour metastasis by blocking tumour-cell-derived heparanases. Int J Cancer. 1987 Oct 15;40(4):511–518. doi: 10.1002/ijc.2910400414. [DOI] [PubMed] [Google Scholar]
- Rifkin D. B., Moscatelli D. Recent developments in the cell biology of basic fibroblast growth factor. J Cell Biol. 1989 Jul;109(1):1–6. doi: 10.1083/jcb.109.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts R., Gallagher J., Spooncer E., Allen T. D., Bloomfield F., Dexter T. M. Heparan sulphate bound growth factors: a mechanism for stromal cell mediated haemopoiesis. Nature. 1988 Mar 24;332(6162):376–378. doi: 10.1038/332376a0. [DOI] [PubMed] [Google Scholar]
- Rogelj S., Klagsbrun M., Atzmon R., Kurokawa M., Haimovitz A., Fuks Z., Vlodavsky I. Basic fibroblast growth factor is an extracellular matrix component required for supporting the proliferation of vascular endothelial cells and the differentiation of PC12 cells. J Cell Biol. 1989 Aug;109(2):823–831. doi: 10.1083/jcb.109.2.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saksela O., Moscatelli D., Sommer A., Rifkin D. B. Endothelial cell-derived heparan sulfate binds basic fibroblast growth factor and protects it from proteolytic degradation. J Cell Biol. 1988 Aug;107(2):743–751. doi: 10.1083/jcb.107.2.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saksela O., Rifkin D. B. Release of basic fibroblast growth factor-heparan sulfate complexes from endothelial cells by plasminogen activator-mediated proteolytic activity. J Cell Biol. 1990 Mar;110(3):767–775. doi: 10.1083/jcb.110.3.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampath T. K., Muthukumaran N., Reddi A. H. Isolation of osteogenin, an extracellular matrix-associated, bone-inductive protein, by heparin affinity chromatography. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7109–7113. doi: 10.1073/pnas.84.20.7109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlodavsky I., Folkman J., Sullivan R., Fridman R., Ishai-Michaeli R., Sasse J., Klagsbrun M. Endothelial cell-derived basic fibroblast growth factor: synthesis and deposition into subendothelial extracellular matrix. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2292–2296. doi: 10.1073/pnas.84.8.2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlodavsky I., Fuks Z., Bar-Ner M., Ariav Y., Schirrmacher V. Lymphoma cell-mediated degradation of sulfated proteoglycans in the subendothelial extracellular matrix: relationship to tumor cell metastasis. Cancer Res. 1983 Jun;43(6):2704–2711. [PubMed] [Google Scholar]
- Weiner H. L., Swain J. L. Acidic fibroblast growth factor mRNA is expressed by cardiac myocytes in culture and the protein is localized to the extracellular matrix. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2683–2687. doi: 10.1073/pnas.86.8.2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yahalom J., Eldor A., Fuks Z., Vlodavsky I. Degradation of sulfated proteoglycans in the subendothelial extracellular matrix by human platelet heparitinase. J Clin Invest. 1984 Nov;74(5):1842–1849. doi: 10.1172/JCI111603. [DOI] [PMC free article] [PubMed] [Google Scholar]


