Abstract
Diabetes mellitus (DM) portends a higher risk of coronary heart disease mortality in women compared to men. This relationship appears to be independent of traditional cardiac risk factors and the role of reproductive hormones has been postulated. We assessed the relationship between DM, Hypothalamic hypoestrogenemia (HHE), angiographic coronary artery disease (CAD) and major adverse cardiovascular events (MACE) over a median 5.9 years in premenopausal women enrolled in the Women’s Ischemia Syndrome Evaluation (WISE) study. We evaluated 95 premenopausal WISE women who underwent coronary angiography for suspected ischemia and were not taking exogenous reproductive hormones. Results showed that there was no difference in age between women with (n=30) and without (n=65) DM (43±6 yrs). DM was associated with hypertension, HHE, angiographic CAD and coronary artery severity score (CSS) (all p<0.05). Women with DM were twice as likely to have HHE (50% vs. 26% p=0.02) compared to women without DM. Presence of both DM and HHE was associated with increased prevalence (40% vs. 12% or 13%, p=0.006) and severity of angiographic coronary artery disease (CSS 19.9 (19.2) vs. 7.7 (4.6) or 12.3 (18.8), p=0.008) as compared to either HHE or DM alone, respectively. DM was moderately predictive of MACE. In conclusion, among premenopausal women undergoing coronary angiography for suspected myocardial ischemia, DM is associated with HHE. Presence of both DM and HHE predicts greater burden of angiographic CAD. Prospective research is warranted to better understand causal relationships between DM, endogenous hormones, and MACE in premenopausal women.
Several biological mechanisms have been proposed to explain the increased risk of ischemic heart disease in diabetics and include increased platelet reactivity with decreased fibrinolysis (1–3), coagulopathy, promotion of endothelial dysfunction (4), and decreased nitric oxide release resulting in decreased vascular tone, further increases in platelet reactivity and increased vascular smooth muscle cell proliferation (5, 6). However, the excess relative mortality in women compared to men with DM still remains unexplained (7–10). Because of the postulated interaction between estrogen deficiency and DM, and in light of the recent finding that HHE is a potent independent risk factor for angiographic CAD in premenopausal women, (11) we sought to assess the relationship between DM, HHE, and CAD in premenopausal women enrolled in the WISE study.
METHODS
The WISE study is a NHLBI-sponsored cross-sectional, observational 4 center study that aims to improve diagnostic testing and advance new hypotheses relative to the pathophysiology of ischemic heart disease in women. A total of 936 women undergoing clinically-indicated coronary angiography for suspected ischemia were recruited into WISE. Our analysis is limited to 95 women out of the entire cohort who were premenopausal, not taking hormone therapy or oral contraceptives, and had no prior diagnosis of CAD. All women received annual follow-up for a median of 5.9 years. Coronary risk factors were defined according to the National Cholesterol Education Program, Adult Panel III (12). DM was defined as a self reported history of DM or serum fasting blood glucose >126 mg/dl. The complete design and methodology of the WISE study are described elsewhere (13). Premenopausal status was determined using a previously published reproductive status algorithm developed for the WISE. This algorithm combined menstrual cycling history with age and with endogenous reproductive hormone profiles (14). Current menstrual cycling was not considered the only determinative variable because 17/95 (18%) of the premenopausal women had a prior hysterectomy. Reproductive hormone assays were performed at an established reproductive hormone core laboratory using stored serum samples (15). This methodology was maintained for the duration of the WISE study. For the purpose of these analyses, HHE was defined as blood estradiol level <50 pg/ml, follicular stimulating hormone level <10 mIU/ml, and luteinizing hormone level <10 mIU/ml. This definition was prospectively derived from examination of hypothalamic amenorrhea primate animal (16) and human data (17, 18). Lipoprotein determinations were performed at a lipid core laboratory enrolled in the Centers for Disease Control and Prevention lipid standardization program and previously used in multiple NHLBI-sponsored lipid-lowering intervention trials using the Friedewald formula, as previously published (19). An experienced core laboratory assessed coronary angiography. Details about the methods used have been published previously (20). For these analyses, angiographic CAD was defined as ≥70 % luminal diameter stenosis in ≥one epicardial coronary artery. Follow-up was conducted by telephone and/or mail contact at 6 weeks and then yearly thereafter. Each woman was queried about symptoms, cardiovascular events since last contact, hospitalizations, and diagnostic or revascularization procedures. A MACE was defined as all cause mortality, non-fatal myocardial infarction, congestive heart failure, stroke, or other vascular event (e.g. peripheral thrombosis, carotid endarterectomy, transient ischemic attack.). When a MACE was identified, the referring physician was contacted, if possible, for confirmation, dates, and documentation of the occurrence. In the event of death, a death certificate was obtained.
Baseline data are reported as means (standard deviations) or frequencies (percentages). Because many of the continuous variables were non-normally distributed, we used the nonparametric Kruskal-Wallis statistic to compare these variables among subgroups of women. For frequencies we used Chi Squares or Fisher exact methods, where appropriate. We used a one-way analysis of covariance to adjust for confounders when comparing CAD prevalence and severity among 4 subgroups. The Cox proportional hazards method was used to model event rates and to adjust for confounders. The Kaplan-Meier method was used for graphic representation comparing the 6-year event-free survival rate in women with vs. without DM and with vs. without HHE. For the event-rate analyses, women not experiencing an adverse event were censored at either 6 years or the last date of follow-up before 6 years. P-values ≤ 0.05 were considered statistically significant. All analyses were performed using the SAS 9.1 software (Cary, NC).
RESULTS
Among the 936 WISE participants, 515 were not currently taking exogenous hormones, had no prior diagnosis of CAD, and had complete demographic and reproductive status data. Of these, 95 (18%) were premenopausal. Their ages ranged from 21 to 54 years, 23 (24%) were non-white, mostly African American, and the mean (SD) BMI was 31.2 (6.9), with a range of 17.7 to 52.2. Despite a high prevalence of coronary risk factors, only a minority (13%) of these premenopausal women had angiographic CAD. A total of 30 women reported either a history of DM (19 women) or had a fasting serum glucose >126 mg/dl (11 women). Among the 19 women with self-reported DM, 12 (63%) used insulin.
Coronary risk factor assessment among women with (n=30) and without (n=65) DM are shown in Table 1. There were no group differences in historical reproductive variables and current menstrual cycle phase was similarly distributed between the two groups (Table 2). However, women with DM were more likely to have anovulatory cycles compared to women without DM. Importantly, comparison of reproductive hormone levels showed that 32/95 (34%) women had HHE, and women with DM had a significantly higher prevalence of HHE compared to women without DM (Table 3). Women with DM also had lower overall estradiol levels compared to women without DM.
Table 1.
Demographic and Coronary Risk Factor Variables by Diabetes Status
| Variable | No DM (n=65) | DM (N=30) | p* |
|---|---|---|---|
| Age / Years Mean (SD) | 43 (6) | 43 (5) | 0.77 |
| Non-white | 15 (23%) | 8 (28%) | 0.64 |
| Current Smoking | 20 (31%) | 3 (10%) | 0.03 |
| Hypertension | 25 (38%) | 20 (67%) | 0.01 |
| Hyperlipidemia | 14 (24%) | 11 (39%) | 0.15 |
| Body Mass Index Mean (SD) (kg/m2) | 30.9 (7.6) | 31.8 (5.3) | 0.35 |
| Waist Hip Ratio Mean (SD) | 0.83 (0.08) | 0.86 (0.05) | 0.052 |
| Adult Treatment Panel 10-Year Risk >3% | 6 (9%) | 4 (13%) | 0.72 |
| Angiographic coronary artery disease | 4 6%) | 8 (27%) | 0.02 |
| Coronary artery disease severity score Mean (SD) | 6.8 (4.3) | 16.1 (19.1) | 0.01 |
| Low density lipid cholesterol Mean (SD) mg/dl | 105 (37) | 115 (38) | 0.21 |
| High density lipid cholesterol Mean (SD) mg/dl | 50 (10) | 50 (12) | 0.89 |
| Triglycerides Mean (SD) mg/dl | 118 (80) | 137 (97) | 0.64 |
| Total Cholesterol Mean (SD) mg/dl | 177 (43) | 196 (51) | 0.15 |
P-values derived by the Kruskal-Wallis test for continuous variables and Chi Square (or Fisher exact, where appropriate) tests for percentages. Hypertension defined as systolic blood pressure >140 mm Hg. Angiographic coronary artery disease defined as ≥70% luminal diameter stenosis in ≥ one epicardial coronary artery.
Table 2.
Historical Reproductive Variables by Diabetes Status
| Variable | No DM (n=65) | DM (n=30) | p* |
|---|---|---|---|
| History of Irregular Menstrual Periods | 14 (22%) | 2 (7%) | 0.07 |
| Current Menstrual Phase | |||
| Follicular | 22 (34%) | 6 (20%) | 0.17 |
| Luteal | 21 (32%) | 10 (33%) | 0.92 |
| Menstrual | 12 (18%) | 4 (13%) | 0.53 |
| Anovulatory | 10 (15%) | 10 (33%) | 0.046 |
| Overall | 0.19 | ||
| Hysterectomy | 12 (18%) | 6 (20%) | 0.86 |
| Polycystic Ovary Disease | 5 (8%) | 1 (3% | 0.66 |
| Number of Pregnancies Mean (SD) | 3.0 (1.7) | 2.7 (1.8) | 0.57 |
| Number of Live Births Mean (SD) | 2.6 (1.3) | 2.2 (1.5) | 0.15 |
P-values derived by the Kruskal-Wallis test for continuous variables and Chi Square (or Fisher exact, where appropriate) tests for percentages.
Table 3.
Reproductive Hormones and Hypothalamic Hypoestrogenemia by Diabetes Status
| Hormone | No DM (n=65) | DM (n=30) | p* |
|---|---|---|---|
| Estradiol (pg/mL) (mean)(SD) | 91(60) | 65(69) | 0.01 |
| Bioavailable Estradiol (pg/mL) (mean)(SD) | 56 (40) | 38 (26) | 0.04 |
| Estrone (pg/mL) (mean)(SD) | 105(67) | 99(60) | 0.83 |
| Follicle stimulating hormone (mlU/mL) (mean)(SD) | 5.1(2.8) | 4.8(3.5) | 0.21 |
| Luteinizing hormone (mlU/mL) (mean)(SD) | 4.8(4.7) | 4.0(5.0) | 0.14 |
| Progesterone (ng/mL) (mean)(SD) | 2.5(4.1) | 2.1(2.8) | 0.57 |
| Hypothalamic hypoestrogenemia | 17 (26%) | 15 (50%) | 0.02 |
| N=51 | n=27 | ||
| Androsteindione (pg/mL) (mean)(SD) | 945(429) | 1085(1007) | 0.61 |
| Testosterone (ng/dL) (mean)(SD) | 26(12) | 27 (26) | 0.66 |
| Free Testosterone (pg/mL) (mean)(SD) | 4.3 (2.0) | 4.8 (3.3) | 0.74 |
| Active Testosterone (ng/dL) (mean)(SD) | 9.4(4.4) | 10.4(7.1) | 0.74 |
| Sex hormone binding globulin (nmol/L) (mean)(SD) | 42 (20) | 37 (23) | 0.20 |
P-values derived by the Kruskal-Wallis test for continuous variables and Chi Square (or Fisher exact, where appropriate) tests for percentages.
When stratified by DM and HHE, both DM alone and HHE alone were associated with a higher prevalence and severity of angiographic CAD. Presence of both DM and HHE predicted increased prevalence and severity of angiographic CAD (Figure 1) compared to the other subgroups. Adjusting this analysis by age, a major predictor of CAD in WISE, did not change the relationship between the 4 groups and CAD prevalence or severity (p=0.004 and p=0.001, respectively).
Figure 1.
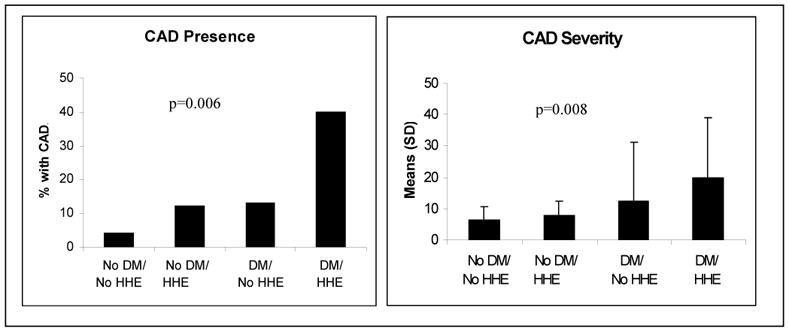
Prevalence of Angiographic Coronary Artery Disease Stratified by Diabetes and Hypothalamic Hypoestrogenemia Status. (CAD=coronary artery disease; DM= diabetes mellitus; HHE= hypothalamic hypoestrogenemia)
Among the 95 women in this analysis, 11 (12%) experienced a MACE over a median 5.9 years. A total of 5 (8%) of the 65 women without DM experienced an event while 6 (20%) of the 30 women with DM experienced an event. Figure 2 suggests that DM conferred a higher risk for MACE; however this difference was not statistically significant. The presence of HHE was not associated with a significant difference in MACE (Figure 2). Adjustments for CAD risk factors did not have an appreciable effect on the relationship of HHE or DM with MACE.
Figure 2.
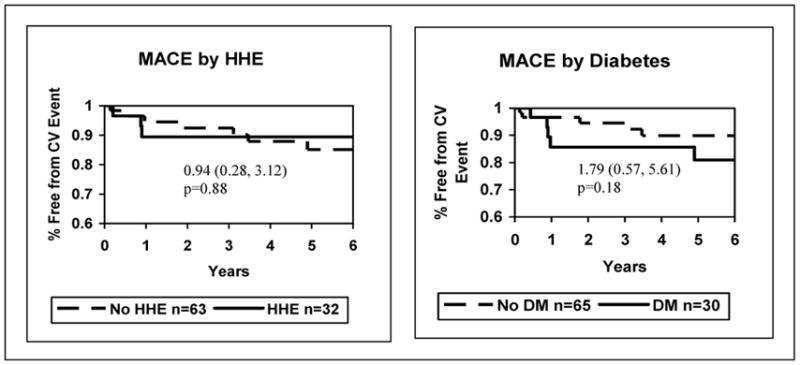
Diabetes, Hypothalamic Hypoestrogenemia and Major Adverse Cardiac Events Hazard Ratios, 95% Confidence Intervals, and p-values. (DM= diabetes mellitus; HHE= hypothalamic hypoestrogenemia; MACE= major adverse cardiac event)
DISCUSSION
Our findings demonstrate that DM is associated with HHE in premenopausal women undergoing coronary angiography for suspected myocardial ischemia. We also demonstrate increased prevalence and extent of angiographic coronary artery disease in women with DM and HHE when compared to women without DM or with DM or HHE alone.
The exact mechanism by which DM ameliorates the female protection against CHD, especially in young pre-menopausal women is not well understood. Endogenous estrogens in the pre-menopause have known protective effects on the vasculature and exert both genomic and non-genomic effects on vascular cells. Estrogen-mediated nitric oxide release diminishes vascular constriction, attenuates platelet aggregation, and impairs vascular growth resulting in anti-atherogenic effects (21–23). It is hypothesized that hyperglycemia may counter the protective cardiovascular effects of estrogen by decreasing estrogen-mediated nitric oxide production and providing a link between DM and altered gender specific pathophysiology and CAD risk (10). In addition, there are human and genetic mouse models supporting the anti-diabetic actions of endogenous estrogens and the role of estradiol in maintenance of glucose homeostasis (24). We further hypothesize that the premature loss of endogenous estrogens due to central suppression may worsen hyperglycemia which could further perpetuate the loss of protective physiologic anti-atherogenic effects of estrogens (Figure 3).
Figure 3.
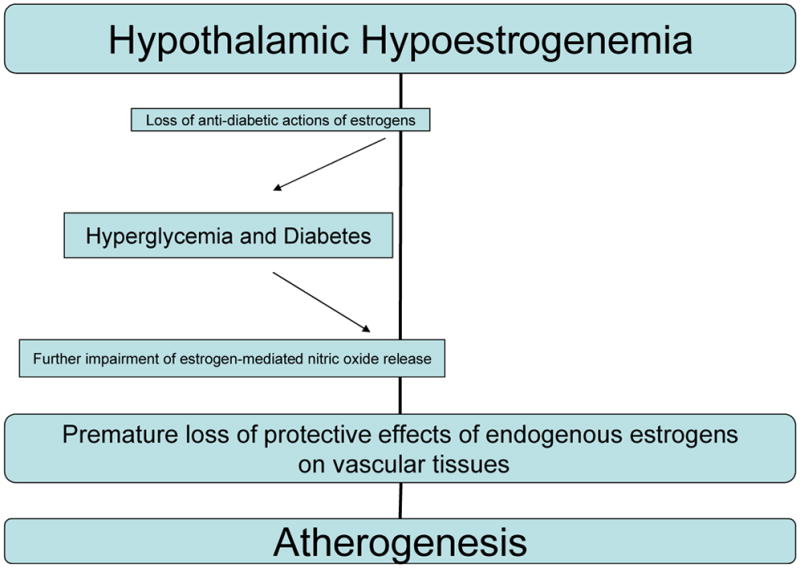
Proposed mechanistic interplay between premature estrogen deficiency, diabetes mellitus and atherogenesis.
Prior primate work has demonstrated atherosclerosis is significantly accelerated in pre-menopausal monkeys with estrogen deficiency secondary to stress-induced disrupted ovarian function of hypothalamic origin (25). In support, the WISE study has previously reported that HHE is a potent and independent predictor of angiographic coronary artery disease in our premenopausal women undergoing coronary angiography for suspected myocardial ischemia (11). Our current findings extend these results to the high-risk subgroup of women with DM. They suggest that estrogen deficiency is relatively more prevalent in premenopausal women with DM and is associated with an increased prevalence and severity of angiographic coronary artery disease in this patient population.
We also assessed for a potential confounding role of polycystic ovary syndrome and the associated increase risk of DM in this patient population (26). Women with PCOS are considered at higher risk for DM (27) and endogenous hormones, specifically androgens, have been hypothesized to play a role (28). In our premenopausal women, a history of DM was not associated with serological markers of polycystic ovary syndrome such as higher luteinizing hormone, testosterone or dehydroepiandrosterone sulphate (29). These results need to be confirmed in prospective studies; however, they do suggest that estrogen deficiency due to central (hypothalamic) origin such as HHE rather than androgen excess due to polycystic ovary syndrome may be responsible for the relatively greater elevated risk of DM in premenopausal women.
It is important to note here that although DM moderately (but non-significantly) predicted MACE, the presence of HHE did not independently predict MACE in diabetic women, despite adding predictive value for the presence and severity of angiographic CAD. A possible explanation for this is the overall low number of events and even smaller number of events when the data were stratified by subgroups. This did not allow for sufficient power to test for significant differences in outcomes among the different groups.
The current study results are limited by several factors. First, the WISE study is an observational, cross-sectional study design that precludes inferring causal relationships. Our hypothesized mechanisms to explain our results need prospective study. Secondly, our small sample size reduces the confidence of our MACE findings, particularly with respect to the relationship between DM and MACE. Specifically, calculations using a two-tailed alpha of 0.05 with 80% power indicate that group differences had to be at least 26% to be detectable in the present sample. Thirdly, since this was a highly specialized population of women undergoing coronary angiography for signs and symptoms suggestive of CAD, the results may not be generalized to the population of premenopausal women. The relationship between HHE and DM and HHE and CAD needs to be further studied in the general population.
Acknowledgments
This work was supported by contracts from the National Heart, Lung and Blood Institutes, nos. N01-HV-68161, N01-HV-68162, N01-HV-68163, N01-HV-68164, a GCRC grant MO1-RR00425 from the National Center for Research Resources, and grants from the Gustavus and Louis Pfeiffer Research Foundation, Denville, New Jersey, The Women’s Guild of Cedars-Sinai Medical Center, Los Angeles, California, and The Ladies Hospital Aid Society of Western Pennsylvania, Pittsburgh, Pennsylvania.
Footnotes
None of the work represents a conflict of interest and there are no financial relationships to disclose among the coauthors.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Davi G, Catalano I, Averna M, Notarbartolo A, Strano A, Ciabattoni G, Patrono C. Thromboxane biosynthesis and platelet function in type II DM. N Engl J Med. 1990;322:1769–1774. doi: 10.1056/NEJM199006213222503. [DOI] [PubMed] [Google Scholar]
- 2.Standley PR, Ali S, Bapna C, Sowers JR. Increased platelet cytosolic calcium responses to low density lipoprotein in type II DM with and without hypertension. Am J Hypertens. 1993;6:938–943. doi: 10.1093/ajh/6.11.938. [DOI] [PubMed] [Google Scholar]
- 3.Carmassi F, Morale M, Puccetti R, De Negri F, Monzani F, Navalesi R, Mariani G. Coagulation and fibrinolytic system impairment in insulin dependent diabetes. Thromb Res. 1992;67:643–654. doi: 10.1016/0049-3848(92)90068-l. [DOI] [PubMed] [Google Scholar]
- 4.Goodfellow J, Ramsey MW, Luddington LA, Jones CJH. Flow-related endothelial function is impaired in non–insulin dependent diabetes. Circulation. 1994;90(Suppl I):513. [Google Scholar]
- 5.Donahue RP, Barrett-Connor E, Orchard TJ, Gutai JP. Endogenous insulin and sex hormones in atherosclerosis and coronary heart disease. Arteriosclerosis. 1988;8:544–548. doi: 10.1161/01.atv.8.5.544. [DOI] [PubMed] [Google Scholar]
- 6.Sowers JR. Insulin and insulin-like growth factor in normal and pathological cardiovascular physiology. Hypertension. 1997;29:691–699. doi: 10.1161/01.hyp.29.3.691. [DOI] [PubMed] [Google Scholar]
- 7.Barrett-Connor E, Wingard DL. Sex differential in ischemic heart disease mortality in diabetics: a prospective population-based study. Am J Epidemiol. 1983;118:489–496. doi: 10.1093/oxfordjournals.aje.a113654. [DOI] [PubMed] [Google Scholar]
- 8.Barrett-Connor EL, Cohn BA, Wingard DL, Edelstein SL. Why is DM a stronger risk factor for fatal ischemic heart disease in women than in men; The Rancho Bernardo Study. JAMA. 1991;265:627–631. [PubMed] [Google Scholar]
- 9.Kannel WB. Metabolic risk factors for CHD in women: perspective from the Framingham Study. An Heart J. 1987;114(2):413–419. doi: 10.1016/0002-8703(87)90511-4. [DOI] [PubMed] [Google Scholar]
- 10.Garcia MJ, McNamara PM, Gordon T, Kannel WB. Morbidity and mortality in diabetics in the Framingham population: sixteen year follow-up study. Diabetes Care. 1990;12:631–654. doi: 10.2337/diab.23.2.105. [DOI] [PubMed] [Google Scholar]
- 11.Bairey Merz CN, Johnson BD, Sharaf BL, Bittner V, Berga SL, Braunstein GD, Hodgson TK, Matthews KA, Pepine CJ, Reis SE, Reichek N, Rogers WJ, Pohost GM, Kelsey SF, Sopko G. Hypoestrogenemia of hypothalamic origin and coronary artery disease in premenopausal women: a report from the NHLBI-sponsored WISE Study. J Am Coll Cardiol. 2003;41(3):413–419. doi: 10.1016/s0735-1097(02)02763-8. [DOI] [PubMed] [Google Scholar]
- 12.Grundy SM, Pasternak R, Greenland Assessment of Cardiovascular Risk by Use of Multiple-Risk-Factor Assessment Equations A Statement for Healthcare Professionals From the American Heart Association and the American College of Cardiology. Circulation. 1999;100:1481–1492. doi: 10.1161/01.cir.100.13.1481. [DOI] [PubMed] [Google Scholar]
- 13.Bairey Merz CN, Kelsey SF, Pepine CJ, Reichek N, Reis SE, Rogers BJ, Sharaf BL, Sopko G. The Women’s Ischemia Syndrome (WISE) Study: Protocol Design, Methodology and Feasibility Report. J Am Coll Cardiol. 1999;33:1453–61. doi: 10.1016/s0735-1097(99)00082-0. [DOI] [PubMed] [Google Scholar]
- 14.Johnson BD, Bairey Merz CN, Braunstein GD. Determination of menopausal status in women: the NHLBI-sponsored Women’s Ischemia Syndrome Evaluation (WISE) Study. J Womens Health. 2004 Oct;13(8):872–887. doi: 10.1089/jwh.2004.13.872. [DOI] [PubMed] [Google Scholar]
- 15.Anderson DC, Hopper BR, Lasley BL, Yen SSC. A simple method for the assay of eight steroids in small volumes of plasma. Steroids. 1976;28:179–184. doi: 10.1016/0039-128x(76)90108-2. [DOI] [PubMed] [Google Scholar]
- 16.Kaplan JR, Adams MR, Clarkson TB, Koritnik DR. Psychosocial influences on female “protection” among cynomolgus macaques. Atherosclerosis. 1984;53:283–295. doi: 10.1016/0021-9150(84)90129-1. [DOI] [PubMed] [Google Scholar]
- 17.Berga SL, Mortola JF, Suh GB, Laughlin G, Pham P, Yen SSC. Neuroendocrine aberrations in women with functional hypothalamic amenorrhea. J Clin Endocrinol Metab. 1989;68:301–308. doi: 10.1210/jcem-68-2-301. [DOI] [PubMed] [Google Scholar]
- 18.Berga SL. Behaviorally induced reproductive compromise in women and men. Semin Reprod Endocrino. 1997 Feb;15(1):47–53. doi: 10.1055/s-2008-1067967. [DOI] [PubMed] [Google Scholar]
- 19.Lipid Research Clinics Program. The Manual of Laboratory Operations: Lipid and Lipoprotein Analysis. 75. Bethesda, MD: National Institutes of Health DHEW Publication; 1974. pp. 628–678. [Google Scholar]
- 20.Sharaf BL, Pepine CJ, Kerensky RA, Reis SE, Reicheck N, Rogers WJ, Sopko G, Kelsey SF, Holubkov R, Olson M, Miele NJ, Williams DO, Bairey Merz CN. Detailed angiographic analysis of women presenting with suspected ischemic chest pain: pilot phase data from the NHLBI-sponsored Women’s Ischemia Syndrome Evaluation (WISE) study angiographic core laboratory. Am J Cardiol. 2001;87:937–941. doi: 10.1016/s0002-9149(01)01424-2. [DOI] [PubMed] [Google Scholar]
- 21.Zhang F, Ram JL, Standley PR, Sowers JR. 17 beta-estradiol attenuates voltage-dependant currents in A75 vascular smooth muscle cell line. Am J Physio. 1994;193:C975–C980. doi: 10.1152/ajpcell.1994.266.4.C975. [DOI] [PubMed] [Google Scholar]
- 22.Williams JK, Adams MR, Klopfenstein HS. Estrogens modulated responses to atherosclerotic coronary arteries. Circulation. 1990;81:1680–1687. doi: 10.1161/01.cir.81.5.1680. [DOI] [PubMed] [Google Scholar]
- 23.Horwitz JB, Horwitz LD. Canine vascular tissues are targets for androgens, estrogens, progesterones and glucocorticoids. J Clin Invest. 1982;69:750–758. doi: 10.1172/JCI110513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Louel JF, LeMay C, Mauvais-Jarvis F. Antidiabetic actions of estrogen: insight from human and genetic mouse models. Curr Atheroscl Rep. 2004;6(3):180–184. doi: 10.1007/s11883-004-0030-9. [DOI] [PubMed] [Google Scholar]
- 25.Kaplan J, Manuck SB, Anthony MS, Clarkson TB. Premenopausal social status and hormone exposure predict premenopausal atherosclerosis in female monkeys. Obstet Gynecol. 2002;99:381–388. doi: 10.1016/s0029-7844(01)01659-3. [DOI] [PubMed] [Google Scholar]
- 26.Ehrmann DA, Barnes RB, Rosenfield RL, Cavaghan MK, Imperial J. Prevalence of impaired glucose tolerance and DM in women with polycystic ovary syndrome. Diab Care. 1999;22:141–146. doi: 10.2337/diacare.22.1.141. [DOI] [PubMed] [Google Scholar]
- 27.Dunaif A, Segal KR, Futterweit W, Dobrjansky A. Profound peripheral insulin resistance, independent of obesity, in polycystic ovary syndrome. Diabetes. 1989;38:1165–1174. doi: 10.2337/diab.38.9.1165. [DOI] [PubMed] [Google Scholar]
- 28.Burghen GA, Givens JR, Kitabchi AE. Correlation of hyperandrogenism with hyperinsulinism in polycystic ovarian disease. J Clin Endocrinol Metab. 1980;50:113–116. doi: 10.1210/jcem-50-1-113. [DOI] [PubMed] [Google Scholar]
- 29.Polycystic Ovarian Syndrome: Diagnosis and Management. Clin Med Res. 2004;2(1):13– 27. doi: 10.3121/cmr.2.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]


