Abstract
Sorting nexins are a large family of evolutionarily conserved phosphoinositide-binding proteins that play fundamental roles in orchestrating the process of cargo sorting through the membranous maze that is the endosomal network. One ancient group of sorting nexin-containing complexes is the retromers. Here we discuss how retromer complexes regulate endosomal sorting, and describe how this is generating exciting new insight into the central role played by endosomal sorting in development, and normal tissue homeostasis.
Introduction: the endosomal network
The endosomal network comprises a series of interconnected membranous compartments that begins at the plasma membrane with internalisation of cargo through endocytosis1. Internalised cargo enters the early endosome, an endomembrane compartment enriched in phosphatidylinositol 3-monophosphate (PtdIns(3)P) and morphologically characterised by vacuolar and tubular sub-domains2. Early endosomes initiate cargo sorting, principally into two distinct pathways: a central endo-lysosomal degradative route, one major function of which is to down-regulate signalling receptors; and a collection of distinct retrieval pathways that recycle cargo, such as nutrient sensing receptors, away from the degradative axis (Figure 1A). While a great deal is known about the mechanistic details of cargo sorting into the endo-lysosomal degradative pathway, and the central role played by PtdIns(3)P3, a mechanistic understanding of how cargo is retrieved from lysosomal degradation is only now beginning to emerge4, 5.
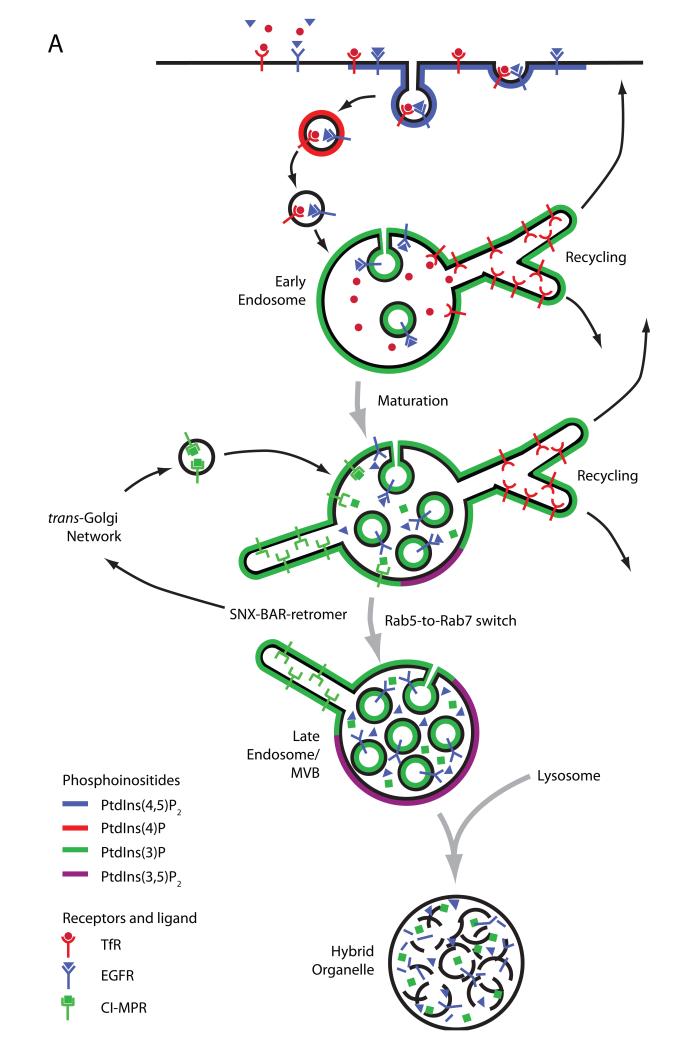
Figure 1. Sorting itineraries in the mammalian endosomal network and the distinct SNX-BAR-retromer and SNX3-retromer complexes.
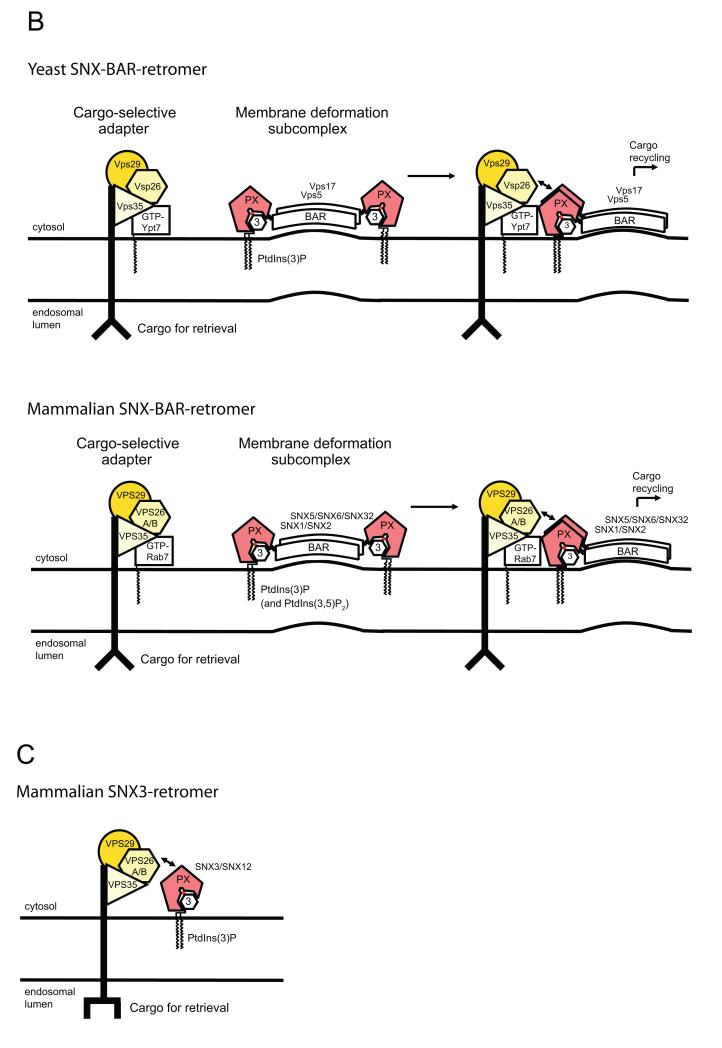
(A). Internalised cargo destined for degradation, such as the epidermal growth factor receptor (EGFR), are first sorted into intraluminal vesicles (ILVs) and by a process of endosomal maturation arrive in late endosomes/multivesicular bodies (MVBs)3. Fusion of MVBs with the lysosomal compartment brings about degradation of cargo associated with ILVs96. In contrast, retrieval from the degradative pathway of, for example the nutrient sensing Transferrin receptor (TfR), occurs back to the plasma membrane via fast or slow recycling routes, while retrieval of the cation-independent mannose 6-phosphate receptor (CI-MPR) occurs back to the trans-Golgi network (TGN). The later route, often referred to as retrograde transport, comprises a number of distinct pathways, which include those dependent on Rab9- and SNX-BAR-retromer4. Enrichment of different phosphoinositides within compartments that make up the endosomal network is shown, including the early endosomal PtdIns(3)P. (B). The distinct cargo-selective and membrane deformation sub-complexes that together form the yeast and mammalian SNX-BAR-retromers. The mammalian genome contains two VPS26’s. (C). The evolutionarily conserved mammalian SNX3-retromer complex. Gene duplication has resulted in two orthologs of C. elegans snx-3, SNX3 and SNX12.
As with a number of membrane trafficking events, retrieval is orchestrated through the assembly of coat complexes, which function to recognise and concentrate specific cargo, drive membrane remodelling and elicit scission to form a cargo-enriched carrier6. One family of PtdIns(3)P-binding proteins that have emerging roles as coat complexes in multiple endosomal retrieval pathways are the evolutionarily conserved sorting nexins (SNXs)2, 7. In particular, much of our insight into how these proteins regulate retrieval, and how this basic aspect of cell biology impinges on organism development, has stemmed from the study of the retromer complex.
Retromer
Retromer’s primary role is to select cargo proteins for retrograde endosome-to-Golgi transport (Table 1). In yeast, retromer is classically considered a multi-protein complex formed by association of two sub-complexes: a stable Vps26, Vps29, and Vps35 trimer and a membrane-bound heterodimer of the SNXs, Vps5 and Vps178 (Figure 1B). The Vps26-Vps29-Vps35 trimer is termed the cargo selective adaptor, on account of Vps35 directly binding to sorting motifs present in the cytoplasmic domains of cargo proteins, while the SNX heterodimer is called the membrane deforming sub-complex to acknowledge its ability to induce and/or stabilise the formation of membrane tubules. Interwoven with these events, retromer recruits additional proteins that aid further cargo capture and packaging9, 10, as well as accessory proteins that regulate maturation and scission of the retromer tubules11-15. Retromer therefore shares a number of similarities with archetypal coat complexes such as COPI, COPII and clathrin coats6: it is an evolutionarily conserved, multi-protein assembly designed to select cargo, drive membrane remodelling and elicit carrier scission.
Table 1. Identified retromer cargo proteins.
SNX-BAR-retromer and SNX3-retromer regulate a wide variety of cellular functions based primarily, but perhaps not solely, on their ability to regulate retrograde endosome-to-TGN transport. Of great interest is a growing appreciation of how retromer complexes regulate organism development and homeostasis, and equally, how defects in retromer function lead to human disease. Adapted from120.
| Process | Mechanism |
|---|---|
| Cargo sorting of intracellular receptors including Vps10, MPRs, DMT1-II, sortilin, SorLA, AtVSR1/AtELP, in various organisms. |
Retrieval of the receptors from endosomes-to-TGN. Described tripeptide sorting motifs through which VPS35 associates with cargo being: WLM – CI-MPR; FLV – sortilin; YLL – DMT1-II. |
| Transcytosis of polymeric immunoglobulin receptor (pIgR) and its ligand, polymeric IgA |
Retrieval of internalised pIgR-pIgA, thus allowing for its transport to the opposite plasma membrane. |
| Maintenance at the plasma membrane of the reductive iron transporter, Fet3-Ftr1, under low iron conditions in S. cerevisiae. |
Retrieval of internalised transporter to the TGN mediated in conjunction with the adaptor Grd19/Snx3. |
| Termination of cAMP by internalized parathyroid hormone receptor (PTHR). |
Retrieval of PTHR from endosomes-to-TGN. |
| Recycling of β2-adrenoreceptor. | Retrieval of internalised receptor directly to the plasma membrane in conjunction with the adaptor SNX27. |
| Cell polarity and organ initiation mediated by the phytohormone auxin, and plant growth and senescence in vegetative organs in A. thaliana. |
Retrieval and polarised delivery of the auxin efflux carrier, PIN. Transport of proteins to protein storage vacuoles (PSVs). |
| Secretion of Wnt morphogens and generation of Wnt gradients during development. |
SNX3-retromer regulated retrieval of the Wnt receptor/chaperone, Wntless, from endosomes-to- TGN to allow for further rounds of Wnt secretion. |
| Endocytic trafficking, signaling and tumor suppression in D. melanogaster. |
Inhibition of Rac1-dependent actin polymerisation. |
| Apoptotic cell clearance in C. elegans. | Retrieval of internalized phagocytic receptor, CED-1, back to cell surface. |
| Maintaining apico-basal polarity and integrity of epithelial tissues in D. melanogaster. |
Retrieval of the apical determinant Crumbs from endosomes. Neurodegenerative disease Retrieval of the amyloid precursor protein (APP), its sorting receptor (SorLA) or its processing peptidase (β- and γ-secretase). Link with late on-set Alzheimer’s disease. VPS35 mutation, Asp620Asn, associated with late on-set Parkinson disease. |
| Trafficking of pathogens (Salmonella, Herpesvirus saimiri), and toxins (e.g. Shiga toxin). |
Retrieval of membrane and cargo from Salmonella-containing vacuole. Endosomes-to-TGN transport of internalized toxins. VPS35 target of HVS Tip protein, which by inhibiting retromer contributes to efficient T cell transformation. |
| Type 2 Diabetes | Genome-wide association study identified variants in VPS26A in South Asians. |
| Mitochondria to peroxisome transport | Association with mitochondrial-anchored protein ligase, MAPL. |
Membrane remodelling – the SNX-BAR sub-complex
In metazoans, gene duplication at the vertebrate ancestor has resulted in two Vps5 orthologs SNX1 and SNX2, whereas duplication of Vps17 in the metazoan ancestor has resulted in SNX5, SNX6 and possibly SNX3216,17 (Figure 1B). These SNXs are members of the SNX-BAR sub-family as they contain two membrane-binding modules: a membrane curvature sensing BAR domain (Bin/amphiphysin/Rvs), and a phosphoinositide-binding PX domain (phox homology). The PX domain in SNX1 and SNX2 associates with the endosomal phosphoinositides, PtdIns(3)P and phosphatidylinositol 3,5-bisphosphate (PtdIns(3,5)P2)18. For BAR domains the ability to sense membrane curvature arises upon dimerisation, which forms a positively charged crescent-shaped surface that through electrostatic interactions binds to membranes of positive curvature19. Dimerisation of retromer SNX-BARs follows a simple rule: one Vps5 equivalent dimerises with one Vps17 equivalent20; eukaryotic lineages between Amoebozoa and Excavata, which lack a Vps17 equivalent, appear to rely upon Vps5 homodimerisation17. In combining the membrane binding affinities of the PX and BAR domains, retromer SNX-BARs utilise an avidity-based system to sense high curvature sub-domains of the PtdIns(3)P/PtdIns(3,5)P2-enriched endosomal network21, 22.
Besides sensing membrane curvature, certain BAR domains are capable of inducing membrane remodelling leading to membrane tubulation23. Both SNX1 and SNX2 are predicted to contain amphipathic helixes amino-terminal to their BAR domains24. For N-BARs (N-terminal amphipathic helix-BARs), the amphipathic helix forms in the cytosol-lipid interface leading to the formation of surface tension between leaflets of the bilayer19. The membrane accommodates this tension by generating positive curvature (i.e. bends into the cytosol) which is stabilised by the curvature sensing properties of the BAR domain. As the effective membrane-bound concentration of the BAR domain increases25, it is argued that local membrane remodelling is translated into a global re-sculpturing by self-assembly of the BAR domain-containing protein into a higher-ordered helical array23, 26, 27. A model therefore emerges in which retromer SNX-BARs switch between curvature-sensing and curvature-inducing modes and in doing so drive the formation of endosomal tubules28. That retromer SNX-BARs can induce vesicle-to-tubule transitions in vitro and in vivo is entirely consistent with this model21, 29, as is data that tubules generated in an in vitro reconstitution have a diameter comparable with that observed for SNX-BAR-retromer tubules from 3D electron tomography (30-55 nm versus 20-50 nm respectively)21, 30. It should be stressed however, that it remains to be determined if the physiological concentration of retromer SNX-BARs corresponds to the specific concentration range required for switching between curvature-sensing and curvature-inducing modes.
Cargo selection – the VPS26-VPS29-VPS35 sub-complex
The VPS26-VPS29-VPS35 sub-complex is a highly conserved protein assembly that originated before the last eukaryotic common ancestor17. The core component is VPS35, which forms an extended horseshoe-shaped α-helical solenoid onto which VPS26 and VPS29 independently associate at either distal end31, 32. Interaction with the cytoplasmic domains of cargo proteins is of low affinity and, for the majority of cargoes no strong consensus-binding motif has been described33-43 (Table 1). The VPS26-VPS29-VPS35 adaptor itself lacks any recognisable membrane binding motifs, being associated with endosomes through interaction with the GTP-loaded form of Rab714, 44, 45 (see below for discussion of an additional mechanism for membrane targeting of VPS26-VPS29-VPS35). Binding to Rab7 presumably restricts the diffusion of the VPS26-VPS29-VPS35 adaptor allowing the on-rate of cargo binding to increase favouring a more stable cargo-bound nucleation complex, while the GTP-dependency incorporates timing into the nucleation event. Moreover, as the VPS26-VPS29-VPS35 adaptor also interacts with the Rab GAP TBC1D514, fine-tuning of the Rab7 GTPase cycle may regulate the assembly and turnover of the nucleation complex. Indeed, a similar role for Arf1-GTP and Sar1-GTP, and their respective GAP proteins ARFGAP1 and Sec23, in controlling the dynamics of COPI and COPII coats is a well-established concept6.
For SNX-BAR-retromer little is know about the regulation of cargo capture: does it rely on chance encounters governed by random diffusion and low-affinity interactions (i.e. a stochastic event) or does it incorporate a ‘pre-nucleation’ priming of cargo that may enhance avidity and generate co-operativity in cargo recognition47? Indeed, it has been proposed that clathrin and a variety of clathrin adaptors and binding proteins, e.g. Hrs, epsinR and RME-8, function to cluster retromer cargo on the endosomal surface prior to SNX-BAR-retromer-mediated sorting (see 47 for an extensive review of the interface between clathrin and SNX-BAR-retromer). Finally, it is important to consider how non-SNX-BAR-retromer cargoes are excluded from packaging into the forming tubular profiles. Here, the interwoven aspects of cargo capture/packaging and membrane re-modelling may combine to exclude unwanted proteins: efficient packing of captured cargo limiting the membrane environment available for non-specific integral membrane proteins, while the geometrical shape of the tubular profile has the effect of reducing lateral diffusion into and out of the forming carrier.
Additional cargo-specific adaptors
The repertoire of retromer cargoes is expanding with the identification of cargo-specific adaptors that mediate recycling by piggy-backing onto the SNX-BAR-retromer pathway. In yeast, recycling of the Fet3p-Ftr1p reductive iron transporter requires recognition of the cytoplasmic domain of Ftr1p by the sorting nexin Grd19/Snx3p9. Grd19/Snx3p physically associates with the SNX-BAR-retromer thereby allowing recycling of the Fet3p-Ftr1p back to the plasma membrane via the Golgi apparatus9 (Figure 3B). Moreover, two novel WD-40 domain proteins, Ere1 and Ere2 (Endosomal Recycling proteins) also appear to function as adaptors for SNX-BAR-retromer-mediated sorting: Ere1 interacts with the arginine transporter Can1 regulating its recycling via the SNX-BAR-retromer pathway48. In mammalian cells SNX27, a PDZ domain-containing SNX, interacts with the carboxy-terminal PDZ ligand of the β2-adrenergic receptor (β2AR), and by doing so acts as an adaptor for recycling of this receptor through SNX-BAR-retromer decorated tubules10. Interestingly, SNX27 associates with the SNX-BAR-retromer through binding to the Wiskott-Aldrich syndrome protein and SCAR homologue (WASH) complex10 and, furthermore, mediates recycling of β2AR through a direct Rab4-dependent endosome-to-plasma membrane pathway rather than via the TGN10, 49. SNX27 also associates with, and regulates the endosomal sorting of other PDZ ligand-containing transmembrane spanning proteins: 5-hydroxytryptamine type 4 receptor (5-HT4R)50; the G protein-gated inward rectifying potassium (GIRK, or Kir3) channels51, 52, and the NMDA receptor subunit NR2C53. Whether recycling is mediated through the SNX-BAR-retromer pathway, and if this is via the TGN or directly back to the plasma membrane, remains to be established. SNX27 also contains a FERM-like domain and hence may function as an adaptor for NPxY motif-containing cargoes. Indeed, the FERM-like domain of a closely related protein SNX17 (this lacks a PDZ domain) has been shown to regulate the endosomal recycling of a number of NPxY-containing proteins54-56. Whether SNX17 also functions as an adaptor for the SNX-BAR-retromer pathway remains to be determined. Based on these examples, and the precedent set by other coat complexes, it would appear that a multitude of cargo-specific adaptors, many of which may be SNXs, have evolved to utilize the core machinery of the SNX-BAR-retromer pathway to allow for recycling of specific cargo.
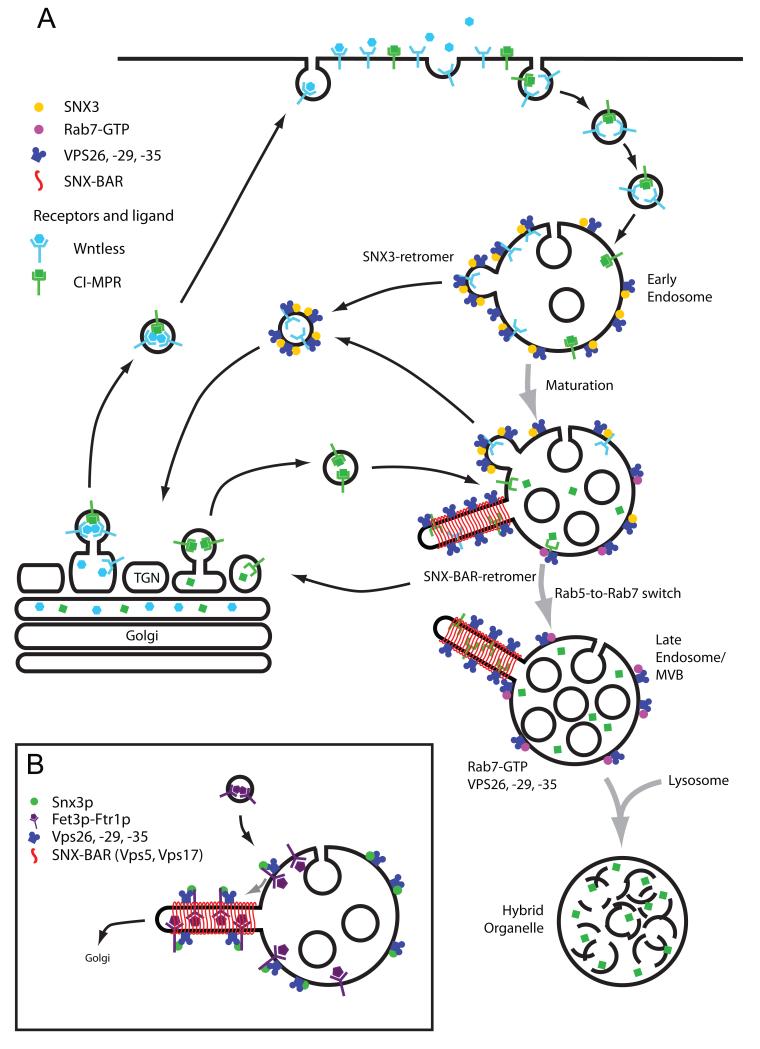
Figure 3. Spatial segregation model to account for differential cargo sorting through SNX3-retromer and SNX-BAR-retromer pathways.
(A). A model based on spatial segregation to describe differential sorting of Wntless and CI-MPR by SNX3-retromer and SNX-BAR-retromer complexes respectively. Briefly, Wntless bound to Wnt morphogens is secreted to the cell surface where release of Wnt establishes short and long-range morphogenic gradients. Internalised Wntless initially enters the early endosome where it engages the SNX3-retromer for retrieval back to the TGN. In contrast, the transport of CI-MPR bound to newly synthesized hydrolase enzymes exits the TGN and is principally trafficked directly to the endosomal network, entering at a stage of maturation downstream of SNX3-retromer. The acid environment of this compartment leads to dissociation of the hydrolases, allowing retrieval of the CI-MPR via the tubular SNX-BAR-retromer. It is important to note that this model does not suggest exclusive transport of Wntless and CI-MPR through the SNX3-retromer and SNX-BAR-retromer pathway respectively. It simply reflects that steady-state transport of these cargoes appears to be dependent upon specific retromer complexes. (B). As discussed in the text, in yeast recycling of Fet3p-Ftr1p requires recognition of the cytoplasmic domain of Ftr1p by Grd19/Snx3p9. Grd19/Snx3p physically associates with the SNX-BAR-retromer thereby allowing recycling of the Fet3p-Ftr1p9 The function of SNX3 in Wntless retrieval and Wnt secretion is therefore. fundamentally different to this role of Grd19/Snx3p.
Accessory proteins for SNX-BAR-retromer
In addition to cargo-specific adaptors the area of concentrated VPS26-VPS29-VPS35 and SNX-BAR binding sites act to recruit a number of accessory proteins that stabilise further tubule growth and ultimately drive the scission of retromer tubules. Among these is EHD1, a member of the carboxy-terminal Eps15 homology domain (EHD) family11. These proteins have similarities to dynamin GTPases57, being capable of assembling into oligomeric structures, inducing liposome tubulation in vitro, and hydrolysing ATP (not GTP)58. Binding of EHD1 to SNX-BAR-retromer is sub-stoichiometric and is required to stabilise tubule formation possibly by assisting further sculpturing of the maturing tubule11. In addition, given the similarity to dynamin, it is tempting to speculate that ATP hydrolysis may induce conformation changes that lead to scission of SNX-BAR-retromer tubules57. EHD proteins also associate with proteins containing the tripeptide NPF motif and so EHD1 may recruit additional proteins required for further cargo capture and/or processing of the SNX-BAR-retromer tubules58.
For a number of coat complexes, further membrane re-modelling, including the efficiency of membrane scission, are intricately linked with the underlying actin and microtubule cytoskeleton59, 60. This is also the case with the SNX-BAR-retromer where the Vps17 orthologs SNX5 and SNX6 associate with the p150glued component of dynactin and hence couple to the minus-end directed microtubule motor dynein15, 61. Interestingly, uncoupling to the dynein motor leads to extended SNX-BAR-retromer tubules through an apparent decrease in the efficiency of tubule scission15. More recently, the association of VPS26-VPS29-VPS35 to the WASH complex has established a link with actin polymerisation12, 13. WASH is a member of the WASP family that regulates the actin nucleating properties of the Arp2/3 complex59. WASH is found associated with a regulatory complex composed of FAM21, SWIP, strumpellin and CCDC5312, 62, and interacts with VPS26-VPS29-VPS35 sub-complex through binding of VPS35 to FAM21, with additional interactions between VPS35 and SNX1/SNX2 to WASH and FAM21 further stabilising the association12, 13. Knock down of WASH leads to the formation of elongated SNX-BAR-retromer tubules that align along microtubules, presumably due to association with the dynactin-dynein motor complex12. Again this phenotype appears to arise from a decrease in the efficiency of tubule scission12, 62. Importantly, the WASH complex associates with the scission factor dynamin-II, and CAPZ, a capping protein for the barbed ends of actin filaments which, by promoting branching leads to the generation of longitudinal force12, 62, 63. The SNX-BAR-retromer therefore assembles two opposing force-generating systems: a motor–dependent pulling force on the tubule and a pushing force on the endosomal vacuole generated by a localised burst of actin polymerisation15, 62. These forces appear to combine to increase membrane tension thereby enhancing the efficiency of membrane scission elicited by either line tension formed by lipid phase separation or by constriction/twisting mediated by dynamin-II and possibly EHD164.
Once the coated tubular carrier has been generated, for fusion with the recipient compartment the carrier must undergo uncoating. For COPII this requires the GAP activity of Sec23, with an analogous function being achieved by recruitment of ARFGAP1 during biogenesis of COPI transport carriers65. Binding of VPS26-VPS29-VPS35 to TBC1D5 may therefore perform a similar function in the SNX-BAR-retromer pathway14. In addition, one cannot exclude a role for phosphoinositide turnover, and the need for ATP hydrolysis to destabilise the high avidity nature of the coat complex and reprime components for further rounds of coat assembly.
Pathway progression
The preceding discussion has focused on individual processes that are classic elements of coat complexes (Figure 2). How are these separate events co-ordinated to achieve pathway progression? By their very nature coat complexes are characterised by low affinity interactions and the SNX-BAR-retromer is no exception66, 67. In a pathway defined by low affinity interactions, many individual steps are dependent on the integration of the affinities from a series of interactions to generate the required avidity for progression from one step to another68. This builds into the pathway dynamic instability, as progression is dependent upon the required number of nucleating cargo-bound VPS26-VPS29-VPS35 adaptors, and the correct level of membrane re-modelling SNX-BAR sub-complexes and other accessory proteins. In turn this leads to proof reading, where pathway progression can be checked at various steps and aborted if the necessary affinities (i.e. interactions) are not in place69. While we currently lack a great deal of basic information, for example quantified affinities and a true detailed network view of protein:protein and protein:lipid interactions required for SNX-BAR-retromer function, it would be very surprising if this pathway did not conform to an avidity-based system for pathway progression that includes a series of defined checkpoints.
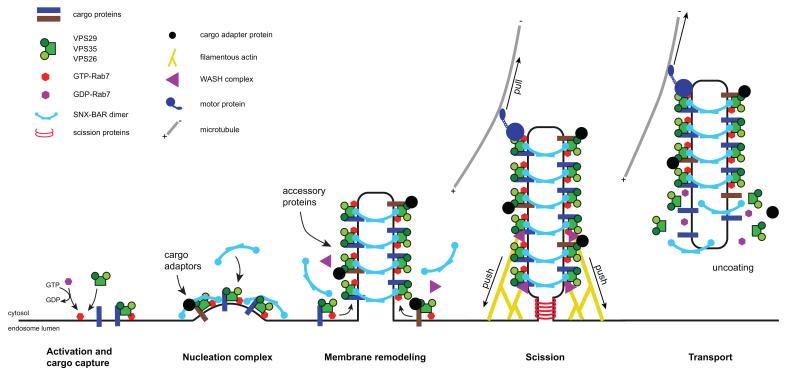
Figure 2. A proposed pathway for SNX-BAR-retromer-mediated sorting.
Activation and cargo capture is initiated by association of the VPS26-VPS29-VPS35 sub-complex to early-to-late endosomes through interaction with the GTP-loaded form of Rab7, thus forming the nucleation complex. Not depicted are the possible roles of clathrin and various clathrin-adaptors and binding proteins in cargo clustering prior to formation of the nucleation complex47. Incorporation of other cargo into the nucleation complex is achieved through specific cargo adaptors, and the SNX-BAR dimer enters the nucleation complex either by direct binding from the cytosol and/or lateral movement on the early-to-late endosomal surface. This increases the effective concentration of retromer SNX-BARs resulting in their switch to curvature inducing mode and re-model the membrane into tubular profiles. An area of concentrated VPS26-VPS29-VPS35 and SNX-BAR-binding sites is therefore formed, onto which accessory proteins are sequestered, leading to further re-modelling of the maturing tubular profile. Generation of opposing forces through actin polymerisation and motor activity aids carrier scission through either line tension and/or mechano-enzymatic fission possibly catalysed by dynamin-II and/or EHD1. Uncoating of the isolated carrier, possibly requiring ATP hydrolysis and/or Rab7-GTP hydrolysis, occurs either immediately after scission or upon tethering to the recipient compartment. See text for more details.
SNX-BAR-retromer does not however conform to all aspects of a classic coat complex. For example, unlike conventional coats SNX-BAR-retromer does not form an electron dense layer on membranes21. Moreover, for a number of coats the subunits that stabilise membrane deformation, such as clathrin and Sec13/Sec31, do not themselves interact directly with the membrane. For example, clathrin coats are formed by sequential layering, with first the adaptors binding the membrane and forming the cargo-bound nucleation complex, followed by the binding and assembly of clathrin70. This ensures co-ordination of membrane deformation with cargo sorting, and avoids membrane remodelling in the absence of cargo. In contrast, retromer SNX-BARs have their own mode of membrane association entirely independent of the VPS26-VPS29-VPS35 adaptor17. How does the SNX-BAR-retromer avoid the formation of unwanted endosomal tubules devoid of enriched cargo? One potential answer lies with the low affinity of the interaction between the SNX-BAR sub-complex and the VPS26-VPS29-VPS35 adaptor67. If the SNX-BAR dimer preferentially associates with the cargo-bound VPS26-VPS29-VPS35 adaptor, then the elevation in the effective concentration of the membrane re-modelling complex would be co-ordinated with cargo selection, consistent with the low affinity interactions required for pathway progression. Indeed, SNX-BAR-retromer tubulation occurs with the greatest frequency at the Rab5-to-Rab7 early-to-late endosomal switch30, 45. What advantages arise from having a membrane re-modelling coat component directly binding to membranes? One possibility is an increased level of regulation which may stem from the mutually exclusive binding to PtdIns(3)P versus PtdIns(3,5)P2. Hence, PtdIns(3)P-to-PtdIns(3,5)P2 switching may constitute an additional point of regulation for the SNX-BAR-retromer pathway71, 72.
Unexpected surprises: retromer-mediated sorting of Wntless
An interesting aspect of defining the mechanistic details of the SNX-BAR-retromer has been the insight obtained into the interface between endosomal retrieval and developmental biology (Table 1). This is particularly evident with the regulation of Wnt signalling. The seven-transmembrane-domain protein Wntless41-43,73-77, which binds to Wnts (lipid-modified glycoproteins with roles in development, adult tissue homeostasis and disease78), is a cargo for the VPS26-VPS29-VPS35 adaptor (Table 1). Wntless is required for the transport of Wnts from the Golgi apparatus to the cell surface, where their release generates short and long-range morphogenic gradients79-81. Continuous Wnt secretion is needed to maintain these gradients and is achieved through Wntless undergoing endocytosis prior to VPS26-VPS29-VPS35-mediated retrieval back to the TGN73-77,82,83. Disruption of VPS26-VPS29-VPS35 results in inefficient retrieval of Wntless and missorting into the lysosomal degradation pathway73-77. The resultant loss of Wntless manifests as a strong defect in Wnt secretion, failure of gradient formation and a loss of signalling to Wnt receiving cells73-77.
Unexpectedly, in C. elegans snx-1 (orthologue of mammalian SNX1/SNX2) and snx-6 (orthologue of mammalian SNX5/SNX6/SNX32) are not required for VPS26-VPS29-VPS35-dependent retrieval of Wntless: identical data has also been obtained in Drosophila16. Importantly, C. elegans express a functional SNX-BAR-retromer as sorting of the CED-1 phagocytic receptor is dependent on the SNX-BAR-retromer complex16, 37. Intriguingly, a separate C. elegans SNX, encoded by the snx-3 gene, is required for several Wnt specific processes16. SNX3 lacks a carboxy-terminal BAR domain and only contains one recognised domain, the SNX PX domain2. In a predicted snx-3 null the range and penetrance of Wnt-dependent phenotypes are similar to those observed in VPS26-VPS29-VPS35 mutants16. Moreover, the formation of Wnt morphogenic gradients in both C. elegans (EGL-20) and Drosophila (Wg) are strongly reduced in mutant snx-3 strains16. As with the loss of the VPS26-VPS29-VPS35 adaptors, the Wnt phenotypes observed upon loss of snx-3 arise from increased lysosomal-mediated degradation of Wntless and decreased secretion of Wnt morphogens16. Indeed, the Wnt signalling phenotype of the C. elegans snx-3 mutant are fully rescued by overexpression of Wntless16. Further establishing the evolutionarily conserved function of SNX3 in the regulation of Wnt secretion, knock down of SNX3 expression in mammalian cells also leads to a decrease in steady-state levels of Wntless16.
The SNX3-retromer complex
SNX3 is an established PtdIns(3)P-binding protein84. As the VPS26-VPS29-VPS35-dependent sorting of Wntless is dependent on this phosphoinositide, SNX3 is an attractive candidate for linking PtdIns(3)P to the VPS26-VPS29-VPS35-dependent sorting of Wntless. Indeed, the VPS26-VPS29-VPS35 sub-complex is found associated with immunoprecipitates of SNX3, and moreover, in in vitro assays recombinant SNX3 directly binds to the recombinant VPS26-VPS29-VPS35 adaptor16. These data therefore establish that besides the SNX1/SNX2 and SNX5/SNX6/SNX32-containing SNX-BAR-retromer (Figure 1B), there is a second evolutionarily conserved retromer complex formed by the direct association of the VPS26-VPS29-VPS35-adaptor with the non-BAR domain-containing SNX316 (Figure 1C). It is the latter, SNX3-retromer, that regulates endosome-to-TGN retrieval of Wntless. In addition, as SNX3 is important in targeting the VPS26-VPS29-VPS35 sub-complex to an early endosomal compartment16, at least two independent mechanisms have evolved for the endosomal association of the VPS26-VPS29-VPS35 adaptor: binding to Rab7-GTP for association to endosomes undergoing early-to-late endosomal transition14, 45, and association to SNX3 for targeting to early endosomes16.
The identification of SNX3 and SNX-BAR-retromers raises a number of interesting questions of which perhaps the most intriguing is: In sharing a common cargo adaptor how do two distinct retromers function to differentially sort endosomal cargo? As discussed above, in mammalian cells SNX3 and the SNX-BAR-retromer SNXs appear spatially separated along the endosomal maturation pathway16. Although there is some overlap between their distributions, SNX3 is principally associated with early endosomes whereas the SNX-BAR-retromer functions at endosomes undergoing early-to-late endosome transition16,84,85. One can argue therefore that endocytosed Wntless initially enters the SNX3-retromer labelled early endosome, where binding to the VPS26-VPS29-VPS35 adaptor initiates endosome-to-TGN retrieval (Figure 3A). In the absence of SNX3, internalised Wntless follows one of two routes: missorting into intraluminal vesicles and hence lysosomal degradation, or retrieval back to the TGN via the SNX-BAR-retromer. In the absence of SNX3 the steady-state level of Wntless is defined by the relative flux through these pathways. As Wntless levels are greatly reduced upon loss of SNX3, the lysosomal degradative pathway appears to dominate16. Thus although some internalised Wntless may be retrieved by the SNX-BAR-retromer pathway86, in the absence of SNX3 this is insufficient to maintain the necessary level of Wnt secretion required to establish and maintain the morphogenic gradient for normal development.
Clearly another important issue relates to how SNX3-retromer co-ordinates cargo selection with membrane re-modelling in order to form a cargo-enriched carrier. As SNX3 lacks a BAR domain, tubular-based sorting may not be a characteristic of this pathway. Indeed, Wntless appears to exit the SNX3-labeled early endosome via small SNX3-decorated vesicular carriers that are labelled with clathrin16,87. This would be consistent with the proposed association of clathrin to SNX3, although the direct versus indirect nature of this interaction remains unclear16,87. SNX3-retromer and SNX-BAR-retromer therefore sort their respective cargoes through two morphologically distinct carriers.
Returning to issues relating to pathway progression, precise control of the level of PtdIns(3)P is vital for efficient SNX3-retromer mediated retrieval of Wntless in C. elegans and Drosophila. MTM-6 and MTM-9 are two PtdIns(3)P 3-phosphatases of the myotubularin family that form a complex and regulate Wntless trafficking88,89. Mutation of mtm-6 leads to a defect in Wntless recycling through an excess of PtdIns(3)P that results in a more pronounced association of SNX3 with endosomes and a reduction of Wntless levels through lysosomal degradation88. These data suggest that with elevated levels of PtdIns(3)P the dynamic exchange of SNX3 between cytosolic and membrane-bound forms is perturbed to an extent that adversely affects pathway progression. Indeed, partially knocking down expression of the PtdIns 3-kinase vps-34 in mtm-6 mutants, and hence lowering the excess level of PtdIns(3)P, partially restores the Wntless trafficking phenotype (i.e. pathway progression)88. Alongside emerging evidence that VPS34 may reside in complexes with specific myotubularins90,91, this suggests that tight regulation of PtdIns-to-Ptdins(3)P switching may be an important aspect of SNX3-retromer function.
Finally, in identifying the SNX3-retromer it becomes important to reassess those cargoes whose sorting has been described to be VPS26-VPS29-VPS35-dependent (Table 1), in order to determine whether their retrieval is mediated via SNX3-retromer, SNX-BAR-retromer or a combination of both pathways (i.e. like the Grd19p/Snx3p SNX-BAR retromer in yeast (Figure 3B)). In so doing the validity of the spatial segregation model will be tested, as will issues relating to the influence cargo binding has on the SNX complex to which the VPS26-VPS29-VPS35 adaptor associates.
Looking ahead: the possibility of other SNX coat complexes
The precedent set by the function of SNXs in SNX-BAR-retromer and SNX3-retromer has placed greater emphasis on the importance of the SNX components in defining separate endosomal retrieval pathways that function through morphologically distinct carriers. With emerging evidence that specific SNXs also function as adaptors for cargo recruitment into the SNX-BAR-retromer pathway (e.g. SNX27 and possibly SNX179, 10, and one should not exclude evidence that retromer SNX-BARs can themselves interact with cargo e.g. 92), the central role of this evolutionarily conserved protein family in endosomal sorting appears even more complex than first envisaged7. Establishing the credentials of other SNX-BAR and SNX-PX proteins as potential coat components will certainly lead to new insights into the mechanistic basis and complexities of endosomal sorting93-95. In turn, by enhancing the molecular understanding of this fundamental aspect of cell biology we will undoubtedly obtain greater insight into the exciting link between endosomal sorting, development and human disease.
Acknowledgements
We are indebted to Jan van Weering for Figures, and George Banting, Dan Billadeau, Martin Harterink, Wanjin Hong, Jim Hurley, Magdalena Lorenowicz, Ian McGough, Sean Munro, Matthew Seaman, Florian Steinberg, David Stephens, Jan van Weering and Lois Weismann for their comments and thought provoking discussions. Work in the authors laboratories are supported by the Wellcome Trust (089928/Z/09/Z and 085743) (P.J.C.) and the Dutch Cancer Society (HUBR 2008-4114) and a NWO VIDI fellowship (016.076.317) (H.C.K.).
References
- 1.Mayor S, Pagano RE. Pathways of clathrin-independent endocytosis. Nat Rev Mol Cell Biol. 2007;8:603–612. doi: 10.1038/nrm2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cullen PJ. Endosomal sorting and signalling: an emerging role for sorting nexins. Nat Rev Mol Cell Biol. 2008;9:574–582. doi: 10.1038/nrm2427. [DOI] [PubMed] [Google Scholar]
- 3.Hurley JH, Hanson PI. Membrane budding and scission by the ESCRT machinery: it’s all in the neck. Nat Rev Mol Cell Biol. 2010;11:556–566. doi: 10.1038/nrm2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johannes L, Popoff V. Tracing the retrograde route in protein trafficking. Cell. 2008;135:1175–1187. doi: 10.1016/j.cell.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 5.Grant BD, Donaldson JG. Pathways and mechanisms of endocytic recycling. Nat Rev Mol Cell Biol. 2009;10:597–608. doi: 10.1038/nrm2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pucadyil TJ, Schmid SL. Conserved functions of membrane active GTPases in coated vesicle formation. Science. 2009;325:1217–1220. doi: 10.1126/science.1171004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carlton J, Bujny M, Rutherford A, Cullen P. Sorting nexins--unifying trends and new perspectives. Traffic. 2005;6:75–82. doi: 10.1111/j.1600-0854.2005.00260.x. [DOI] [PubMed] [Google Scholar]
- 8.Attar N, Cullen PJ. The retromer complex. Adv Enzyme Regul. 2009;50:216–236. doi: 10.1016/j.advenzreg.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 9.Strochlic TI, Setty TG, Sitaram A, Burd CG. Grd19/Snx3p functions as a cargo-specific adapter for retromer-dependent endocytic recycling. J Cell Biol. 2007;177:115–125. doi: 10.1083/jcb.200609161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Temkin P, et al. SNX27 mediates retromer tubule entry and endosome-to-plasma membrane trafficking of signalling receptors. Nat Cell Biol. 2011;13:717–723. doi: 10.1038/ncb2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gokool S, Tattersall D, Seaman MN. EHD1 interacts with retromer to stabilize SNX1 tubules and facilitate endosome-to-Golgi retrieval. Traffic. 2007;8:1873–1886. doi: 10.1111/j.1600-0854.2007.00652.x. [DOI] [PubMed] [Google Scholar]
- 12.Gomez TS, Billadeau DD. A FAM21-containing WASH complex regulates retromer-dependent sorting. Dev Cell. 2009;17:699–711. doi: 10.1016/j.devcel.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harbour ME, et al. The cargo-selective retromer complex is a recruiting hub for protein complexes that regulate endosomal tubule dynamics. J Cell Sci. 2010;123:3703–3717. doi: 10.1242/jcs.071472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seaman MN, Harbour ME, Tattersall D, Read E, Bright N. Membrane recruitment of the cargo-selective retromer subcomplex is catalysed by the small GTPase Rab7 and inhibited by the Rab-GAP TBC1D5. J Cell Sci. 2009;122:2371–2382. doi: 10.1242/jcs.048686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wassmer T, et al. The retromer coat complex coordinates endosomal sorting and dynein-mediated transport, with carrier recognition by the trans-Golgi network. Dev Cell. 2009;17:110–122. doi: 10.1016/j.devcel.2009.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harterink M, et al. A SNX3-dependent retromer pathway mediates retrograde transport of the Wnt sorting receptor Wntless and is required for Wnt secretion. Nat Cell Biol. 2011;13:914–923. doi: 10.1038/ncb2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koumandou VL, et al. Evolutionary reconstruction of the retromer complex and its function in Trypanosoma brucei. J Cell Sci. 2011;124:1496–1509. doi: 10.1242/jcs.081596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cozier GE, et al. The phox homology (PX) domain-dependent, 3-phosphoinositide-mediated association of sorting nexin-1 with an early sorting endosomal compartment is required for its ability to regulate epidermal growth factor receptor degradation. J Biol Chem. 2002;277:48730–48736. doi: 10.1074/jbc.M206986200. [DOI] [PubMed] [Google Scholar]
- 19.Peter BJ, et al. BAR domains as sensors of membrane curvature: the amphiphysin BAR structure. Science. 2004;303:495–499. doi: 10.1126/science.1092586. [DOI] [PubMed] [Google Scholar]
- 20.van Weering JR, Verkade P, Cullen PJ. SNX-BAR proteins in phosphoinositide-mediated, tubular-based endosomal sorting. Semin Cell Dev Biol. 2009;21:371–380. doi: 10.1016/j.semcdb.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carlton J, et al. Sorting nexin-1 mediates tubular endosome-to-TGN transport through coincidence sensing of high-curvature membranes and 3-phosphoinositides. Curr Biol. 2004;14:1791–1800. doi: 10.1016/j.cub.2004.09.077. [DOI] [PubMed] [Google Scholar]
- 22.Carlton JG, et al. Sorting nexin-2 is associated with tubular elements of the early endosome, but is not essential for retromer-mediated endosome-to-TGN transport. J Cell Sci. 2005;118:4527–4539. doi: 10.1242/jcs.02568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frost A, Unger VM, De Camilli P. The BAR domain superfamily: membrane-molding macromolecules. Cell. 2009;137:191–196. doi: 10.1016/j.cell.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bhatia VK, et al. Amphipathic motifs in BAR domains are essential for membrane curvature sensing. EMBO J. 2009;28:3303–3314. doi: 10.1038/emboj.2009.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roux A, et al. Membrane curvature controls dynamin polymerization. Proc Natl Acad Sci U S A. 2010;107:4141–4146. doi: 10.1073/pnas.0913734107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pylypenko O, Lundmark R, Rasmuson E, Carlsson SR, Rak A. The PX-BAR membrane-remodeling unit of sorting nexin 9. EMBO J. 2007;26:4788–4800. doi: 10.1038/sj.emboj.7601889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shimada A, et al. Curved EFC/F-BAR-domain dimers are joined end to end into a filament for membrane invagination in endocytosis. Cell. 2007;129:761–772. doi: 10.1016/j.cell.2007.03.040. [DOI] [PubMed] [Google Scholar]
- 28.van Weering JR, et al. Intracellular membrane traffic at high resolution. Methods Cell Biol. 2010;96:619–648. doi: 10.1016/S0091-679X(10)96026-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kurten RC, et al. Self-assembly and binding of a sorting nexin to sorting endosomes. J Cell Sci. 2001;114:1743–1756. doi: 10.1242/jcs.114.9.1743. [DOI] [PubMed] [Google Scholar]
- 30.Mari M, et al. SNX1 defines an early endosomal recycling exit for sortilin and mannose 6-phosphate receptors. Traffic. 2008;9:380–393. doi: 10.1111/j.1600-0854.2007.00686.x. [DOI] [PubMed] [Google Scholar]
- 31.Norwood SJ, et al. Assembly and solution structure of the core retromer protein complex. Traffic. 2011;12:56–71. doi: 10.1111/j.1600-0854.2010.01124.x. [DOI] [PubMed] [Google Scholar]
- 32.Hierro A, et al. Functional architecture of the retromer cargo-recognition complex. Nature. 2007;449:1063–1067. doi: 10.1038/nature06216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nothwehr SF, Ha SA, Bruinsma P. Sorting of yeast membrane proteins into an endosome-to-Golgi pathway involves direct interaction of their cytosolic domains with Vps35p. J Cell Biol. 2000;151:297–310. doi: 10.1083/jcb.151.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arighi CN, Hartnell LM, Aguilar RC, Haft CR, Bonifacino JS. Role of the mammalian retromer in sorting of the cation-independent mannose 6-phosphate receptor. J Cell Biol. 2004;165:123–133. doi: 10.1083/jcb.200312055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seaman MN. Identification of a novel conserved sorting motif required for retromer-mediated endosome-to-TGN retrieval. J Cell Sci. 2007;120:2378–2389. doi: 10.1242/jcs.009654. [DOI] [PubMed] [Google Scholar]
- 36.Tabuchi M, Yanatori I, Kawai Y, Kishi F. Retromer-mediated direct sorting is required for proper endosomal recycling of the mammalian iron transporter DMT1. J Cell Sci. 2010;123:756–766. doi: 10.1242/jcs.060574. [DOI] [PubMed] [Google Scholar]
- 37.Chen D, et al. Retromer is required for apoptotic cell clearance by phagocytic receptor recycling. Science. 2010;327:1261–1264. doi: 10.1126/science.1184840. [DOI] [PubMed] [Google Scholar]
- 38.Feinstein TN, et al. Retromer terminates the generation of cAMP by internalized PTH receptors. Nat Chem Biol. 2011;7:278–284. doi: 10.1038/nchembio.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pocha SM, Wassmer T, Niehage C, Hoflack B, Knust E. Retromer Controls Epithelial Cell Polarity by Trafficking the Apical Determinant Crumbs. Curr Biol. 2011;21:1111–1117. doi: 10.1016/j.cub.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 40.Shi H, Rojas R, Bonifacino JS, Hurley JH. The retromer subunit Vps26 has an arrestin fold and binds Vps35 through its C-terminal domain. Nat Struct Mol Biol. 2006;13:540–548. doi: 10.1038/nsmb1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Banziger C, et al. Wntless, a conserved membrane protein dedicated to the secretion of Wnt proteins from signaling cells. Cell. 2006;125:509–522. doi: 10.1016/j.cell.2006.02.049. [DOI] [PubMed] [Google Scholar]
- 42.Bartscherer K, Pelte N, Ingelfinger D, Boutros M. Secretion of Wnt ligands requires Evi, a conserved transmembrane protein. Cell. 2006;125:523–533. doi: 10.1016/j.cell.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 43.Goodman RM, et al. Sprinter: a novel transmembrane protein required for Wg secretion and signaling. Development. 2006;133:4901–4911. doi: 10.1242/dev.02674. [DOI] [PubMed] [Google Scholar]
- 44.Balderhaar HJ, et al. The Rab GTPase Ypt7 is linked to retromer-mediated receptor recycling and fusion at the yeast late endosome. J Cell Sci. 2010;123:4085–4094. doi: 10.1242/jcs.071977. [DOI] [PubMed] [Google Scholar]
- 45.Rojas R, et al. Regulation of retromer recruitment to endosomes by sequential action of Rab5 and Rab7. J Cell Biol. 2008;183:513–526. doi: 10.1083/jcb.200804048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mukhopadhyay A, Pan X, Lambright DG, Tissenbaum HA. An endocytic pathway as a target of tubby for regulation of fat storage. EMBO Rep. 2007;8:931–938. doi: 10.1038/sj.embor.7401055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McGough IJ, Cullen PJ. Recent Advances in Retromer Biology. Traffic. 2011;12:963–971. doi: 10.1111/j.1600-0854.2011.01201.x. [DOI] [PubMed] [Google Scholar]
- 48.Shi Y, Stefan CJ, Rue SM, Teis D, Emr SD. Tow novel WD40 domain-containing proteins, Ere1 and Ere2, function in the retromer-mediated endosomal recycling pathway. Mol Biol Cell. 2011 doi: 10.1091/mbc.E11-05-0440. PMID: 21880895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lauffer BE, et al. SNX27 mediates PDZ-directed sorting from endosomes to the plasma membrane. J Cell Biol. 2010;190:565–574. doi: 10.1083/jcb.201004060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Joubert L, et al. New sorting nexin (SNX27) and NHERF specifically interact with the 5-HT4a receptor splice variant: roles in receptor targeting. J Cell Sci. 2004;117:5367–5379. doi: 10.1242/jcs.01379. [DOI] [PubMed] [Google Scholar]
- 51.Balana B, et al. Mechanism underlying selective regulation of G protein-gated inwardly rectifying potassium channels by the psychostimulant-sensitive sorting nexin 27. Proc Natl Acad Sci U S A. 2011;108:5831–5836. doi: 10.1073/pnas.1018645108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lunn ML, et al. A unique sorting nexin regulates trafficking of potassium channels via a PDZ domain interaction. Nat Neurosci. 2007;10:1249–1259. doi: 10.1038/nn1953. [DOI] [PubMed] [Google Scholar]
- 53.Cai L, Loo LS, Atlashkin V, Hanson BJ, Hong W. Deficiency of sorting nexin 27 (SNX27) leads to growth retardation and elevated levels of N-methyl-D-aspartate receptor 2C (NR2C) Mol Cell Biol. 2011;31:1734–1747. doi: 10.1128/MCB.01044-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee J, et al. Adaptor protein sorting nexin 17 regulates amyloid precursor protein trafficking and processing in the early endosomes. J Biol Chem. 2008;283:11501–11508. doi: 10.1074/jbc.M800642200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stockinger W, et al. The PX-domain protein SNX17 interacts with members of the LDL receptor family and modulates endocytosis of the LDL receptor. EMBO J. 2002;21:4259–4267. doi: 10.1093/emboj/cdf435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.van Kerkhof P, et al. Sorting nexin 17 facilitates LRP recycling in the early endosome. EMBO J. 2005;24:2851–2861. doi: 10.1038/sj.emboj.7600756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Daumke O, et al. Architectural and mechanistic insights into an EHD ATPase involved in membrane remodelling. Nature. 2007;449:923–927. doi: 10.1038/nature06173. [DOI] [PubMed] [Google Scholar]
- 58.Naslavsky N, Caplan S. EHD proteins: key conductors of endocytic transport. Trends Cell Biol. 2011;21:122–131. doi: 10.1016/j.tcb.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Campellone KG, Welch MD. A nucleator arms race: cellular control of actin assembly. Nat Rev Mol Cell Biol. 2010;11:237–251. doi: 10.1038/nrm2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Soldati T, Schliwa M. Powering membrane traffic in endocytosis and recycling. Nat Rev Mol Cell Biol. 2006;7:897–908. doi: 10.1038/nrm2060. [DOI] [PubMed] [Google Scholar]
- 61.Hong Z, et al. The retromer component SNX6 interacts with dynactin p150(Glued) and mediates endosome-to-TGN transport. Cell Res. 2009;19:1334–1349. doi: 10.1038/cr.2009.130. [DOI] [PubMed] [Google Scholar]
- 62.Derivery E, et al. The Arp2/3 activator WASH controls the fission of endosomes through a large multiprotein complex. Dev Cell. 2009;17:712–723. doi: 10.1016/j.devcel.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 63.Jia D, et al. WASH and WAVE actin regulators of the Wiskott-Aldrich syndrome protein (WASP) family are controlled by analogous structurally related complexes. Proc Natl Acad Sci U S A. 2010;107:10442–10447. doi: 10.1073/pnas.0913293107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lenz M, Morlot S, Roux A. Mechanical requirements for membrane fission: common facts from various examples. FEBS Lett. 2009;583:3839–3846. doi: 10.1016/j.febslet.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 65.McMahon HT, Mills IG. COP and clathrin-coated vesicle budding: different pathways, common approaches. Curr Opin Cell Biol. 2004;16:379–391. doi: 10.1016/j.ceb.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 66.Collins BM, et al. Structure of Vps26B and mapping of its interaction with the retromer protein complex. Traffic. 2008;9:366–379. doi: 10.1111/j.1600-0854.2007.00688.x. [DOI] [PubMed] [Google Scholar]
- 67.Swarbrick JD, et al. VPS29 Is Not an Active Metallo-Phosphatase but Is a Rigid Scaffold Required for Retromer Interaction with Accessory Proteins. PLoS One. 2011;6:e20420. doi: 10.1371/journal.pone.0020420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schmid EM, McMahon HT. Integrating molecular and network biology to decode endocytosis. Nature. 2007;448:883–888. doi: 10.1038/nature06031. [DOI] [PubMed] [Google Scholar]
- 69.Loerke D, et al. Cargo and dynamin regulate clathrin-coated pit maturation. PLoS biology. 2009;7:e57. doi: 10.1371/journal.pbio.1000057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Traub LM. Regarding the amazing choreography of clathrin coats. PLoS biology. 2011;9:e1001037. doi: 10.1371/journal.pbio.1001037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dove SK, Dong K, Kobayashi T, Williams FK, Michell RH. Phosphatidylinositol 3,5-bisphosphate and Fab1p/PIKfyve underPPIn endo-lysosome function. Biochem J. 2009;419:1–13. doi: 10.1042/BJ20081950. [DOI] [PubMed] [Google Scholar]
- 72.Rutherford AC, et al. The mammalian phosphatidylinositol 3-phosphate 5-kinase (PIKfyve) regulates endosome-to-TGN retrograde transport. J Cell Sci. 2006;119:3944–3957. doi: 10.1242/jcs.03153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Belenkaya TY, et al. The retromer complex influences Wnt secretion by recycling wntless from endosomes to the trans-Golgi network. Dev Cell. 2008;14:120–131. doi: 10.1016/j.devcel.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 74.Franch-Marro X, et al. Wingless secretion requires endosome-to-Golgi retrieval of Wntless/Evi/Sprinter by the retromer complex. Nat Cell Biol. 2008;10:170–177. doi: 10.1038/ncb1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pan CL, et al. C. elegans AP-2 and retromer control Wnt signaling by regulating mig-14/Wntless. Dev Cell. 2008;14:132–139. doi: 10.1016/j.devcel.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Port F, et al. Wingless secretion promotes and requires retromer-dependent cycling of Wntless. Nat Cell Biol. 2008;10:178–185. doi: 10.1038/ncb1687. [DOI] [PubMed] [Google Scholar]
- 77.Yang PT, et al. Wnt signaling requires retromer-dependent recycling of MIG-14/Wntless in Wnt-producing cells. Dev Cell. 2008;14:140–147. doi: 10.1016/j.devcel.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 78.Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 79.Hausmann G, Banziger C, Basler K. Helping Wingless take flight: how WNT proteins are secreted. Nat Rev Mol Cell Biol. 2007;8:331–336. doi: 10.1038/nrm2141. [DOI] [PubMed] [Google Scholar]
- 80.Lorenowicz MJ, Korswagen HC. Sailing with the Wnt: charting the Wnt processing and secretion route. Exp Cell Res. 2009;315:2683–2689. doi: 10.1016/j.yexcr.2009.06.015. [DOI] [PubMed] [Google Scholar]
- 81.Port F, Basler K. Wnt trafficking: new insights into Wnt maturation, secretion and spreading. Traffic. 2010;11:1265–1271. doi: 10.1111/j.1600-0854.2010.01076.x. [DOI] [PubMed] [Google Scholar]
- 82.Coudreuse DY, Roel G, Betist MC, Destree O, Korswagen HC. Wnt gradient formation requires retromer function in Wnt-producing cells. Science. 2006;312:921–924. doi: 10.1126/science.1124856. [DOI] [PubMed] [Google Scholar]
- 83.Prasad BC, Clark SG. Wnt signaling establishes anteroposterior neuronal polarity and requires retromer in C. elegans. Development. 2006;133:1757–1766. doi: 10.1242/dev.02357. [DOI] [PubMed] [Google Scholar]
- 84.Xu Y, Hortsman H, Seet L, Wong SH, Hong W. SNX3 regulates endosomal function through its PX-domain-mediated interaction with PtdIns(3)P. Nat Cell Biol. 2001;3:658–666. doi: 10.1038/35083051. [DOI] [PubMed] [Google Scholar]
- 85.van Weering JR, Verkade P, Cullen PJ. SNX-BAR-mediated endosome tubulation is coordinated with endosome maturation. Traffic. 2011 doi: 10.1111/j.1600-0854.2011.01297.x. in press. [DOI] [PubMed] [Google Scholar]
- 86.Shi A, et al. Regulation of endosomal clathrin and retromer-mediated endosome to Golgi retrograde transport by the J-domain protein RME-8. EMBO J. 2009;28:3290–3302. doi: 10.1038/emboj.2009.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Skanland SS, Walchli S, Brech A, Sandvig K. SNX4 in complex with clathrin and dynein: implications for endosome movement. PLoS One. 2009;4:e5935. doi: 10.1371/journal.pone.0005935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Silhankova M, Port F, Harterink M, Basler K, Korswagen HC. Wnt signalling requires MTM-6 and MTM-9 myotubularin lipid-phosphatase function in Wnt-producing cells. EMBO J. 2010;29:4094–4105. doi: 10.1038/emboj.2010.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Xue Y, et al. Genetic analysis of the myotubularin family of phosphatases in Caenorhabditis elegans. J Biol Chem. 2003;278:34380–34386. doi: 10.1074/jbc.M303259200. [DOI] [PubMed] [Google Scholar]
- 90.Cao C, Backer JM, Laporte J, Bedrick EJ, Wandinger-Ness A. Sequential actions of myotubularin lipid phosphatases regulate endosomal PI(3)P and growth factor receptor trafficking. Mol Biol Cell. 2008;19:3334–3346. doi: 10.1091/mbc.E08-04-0367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cao C, Laporte J, Backer JM, Wandinger-Ness A, Stein MP. Myotubularin lipid phosphatase binds the hVPS15/hVPS34 lipid kinase complex on endosomes. Traffic. 2007;8:1052–1067. doi: 10.1111/j.1600-0854.2007.00586.x. [DOI] [PubMed] [Google Scholar]
- 92.Heydorn A, Sondergaard BP, Ersboll B, Holst B, Nielsen FC, Haft CR, Whistler J, Schwartz TW. A library of 7TM receptor C-terminal tails. Interactions with the proposed post-endocytic sorting proteins ERM-binidng phosphoprotein 50 (EBP50), N-ethylmaleimide-sensitive factor (NSF), sorting nexin-1 (SNX1), and G protein-coupled receptor-associated sorting protein (GASP) J Biol Chem. 2004;279:54291–54303. doi: 10.1074/jbc.M406169200. [DOI] [PubMed] [Google Scholar]
- 93.Dyve AB, Bergan J, Utskarpen A, Sandvig K. Sorting nexin 8 regulates endosome-to-Golgi transport. Biochem Biophys Res Comm. 2009;390:109–114. doi: 10.1016/j.bbrc.2009.09.076. [DOI] [PubMed] [Google Scholar]
- 94.Traer CJ, et al. SNX4 coordinates endosomal sorting of TfnR with dynein-mediated transport into the endocytic recycling compartment. Nat Cell Biol. 2007;9:1370–1380. doi: 10.1038/ncb1656. [DOI] [PubMed] [Google Scholar]
- 95.Wassmer T, et al. A loss-of-function screen reveals SNX5 and SNX6 as potential components of the mammalian retromer. J Cell Sci. 2007;120:45–54. doi: 10.1242/jcs.03302. [DOI] [PubMed] [Google Scholar]
- 96.Saftig P, Klumperman J. Lysosome biogenesis and lysosomal membrane proteins: trafficking meets function. Nat Rev Mol Cell Biol. 2009;10:623–635. doi: 10.1038/nrm2745. [DOI] [PubMed] [Google Scholar]
- 97.Seaman MN. Cargo-selective endosomal sorting for retrieval to the Golgi requires retromer. J Cell Biol. 2004;165:111–122. doi: 10.1083/jcb.200312034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Seaman MN, McCaffery JM, Emr SD. A membrane coat complex essential for endosome-to-Golgi retrograde transport in yeast. J Cell Biol. 1998;142:665–681. doi: 10.1083/jcb.142.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Oliviusson P, et al. Plant retromer, localized to the prevacuolar compartment and microvesicles in Arabidopsis, may interact with vacuolar sorting receptors. Plant Cell. 2006;18:1239–1252. doi: 10.1105/tpc.105.035907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Verges M, et al. The mammalian retromer regulates transcytosis of the polymeric immunoglobulin receptor. Nat Cell Biol. 2004;6:763–769. doi: 10.1038/ncb1153. [DOI] [PubMed] [Google Scholar]
- 101.Jaillais Y, et al. The retromer protein VPS29 links cell polarity and organ initiation in plants. Cell. 2007;130:1057–1070. doi: 10.1016/j.cell.2007.08.040. [DOI] [PubMed] [Google Scholar]
- 102.Bujny MV, Popoff V, Johannes L, Cullen PJ. The retromer component sorting nexin-1 is required for efficient retrograde transport of Shiga toxin from early endosome to the trans Golgi network. J Cell Sci. 2007;120:2010–2021. doi: 10.1242/jcs.003111. [DOI] [PubMed] [Google Scholar]
- 103.Popoff V, et al. The retromer complex and clathrin define an early endosomal retrograde exit site. J Cell Sci. 2007;120:2022–2031. doi: 10.1242/jcs.003020. [DOI] [PubMed] [Google Scholar]
- 104.Bujny MV, et al. Sorting nexin-1 defines an early phase of Salmonella-containing vacuole-remodeling during Salmonella infection. J Cell Sci. 2008;121:2027–2036. doi: 10.1242/jcs.018432. [DOI] [PubMed] [Google Scholar]
- 105.Nielsen MS, et al. Sorting by the cytoplasmic domain of the amyloid precursor protein binding receptor SorLA. Mol Cell Biol. 2007;27:6842–6851. doi: 10.1128/MCB.00815-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rogaeva E, et al. The neuronal sortilin-related receptor SORL1 is genetically associated with Alzheimer disease. Nat Genet. 2007;39:168–177. doi: 10.1038/ng1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kleine-Vehn J, Leitner J, Zwiewka M, Sauer M, Abas L, Luschnig C, Friml J. Differential degradation of PIN2 auxin efflux carrier by retromer-dependent vacuolar targeting. Proc Natl Acad Sci USA. 2008;105:17812–17817. doi: 10.1073/pnas.0808073105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yamazaki M, et al. Arabidopsis VPS35, a retromer component, is required for vacuolar protein sorting and involved in plant growth and leaf senescence. Plant Cell Physiol. 2008;49:142–156. doi: 10.1093/pcp/pcn006. [DOI] [PubMed] [Google Scholar]
- 109.Korolchuk VI, et al. Drosophila Vps35 function is necessary for normal endocytic trafficking and actin cytoskeleton organisation. J Cell Sci. 2007;120:4367–4376. doi: 10.1242/jcs.012336. [DOI] [PubMed] [Google Scholar]
- 110.Lu N, Shen Q, Mahoney TR, Liu X, Zhou Z. Three sorting nexins drive the degradation of apoptotic cells in response to PtdIns(3)P signaling. Mol Biol Cell. 2011;22:354–374. doi: 10.1091/mbc.e10-09-0756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Muhammad A, et al. Retromer deficiency observed in Alzheimer’s disease causes hippocampal dysfunction, neurodegeneration, and A accumulation. Proc Natl Acad Sci USA. 2008;105:7327–7332. doi: 10.1073/pnas.0802545105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Vieira SI, et al. Retrieval of the Alzheimer’s amyloid precursor protein from the endosome to the TGN is S655 phosphorylation state-dependent and retromer-mediated. Mol Neurodegener. 2010;5:40. doi: 10.1186/1750-1326-5-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sullivan CP, et al. Retromer disruption promotes amyloidogenic APP processing. Neurobiol Dis. 2011;43:338–345. doi: 10.1016/j.nbd.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zimprich A, et al. A mutation in VPS35, encoding a subunit of the retromer complex, causes late-onset Parkinson disease. Am J Hum Genet. 2011;89:168–175. doi: 10.1016/j.ajhg.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Vilarino-Guell C, et al. VPS35 mutations in Parkinson disease. Am J Hum Genet. 2011;89:162–167. doi: 10.1016/j.ajhg.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Braschi E, et al. Vps35 mediates vesicle transport between the mitochondria and peroxisomes. Curr Biol. 2010;20:1310–1315. doi: 10.1016/j.cub.2010.05.066. [DOI] [PubMed] [Google Scholar]
- 117.Kingston D, et al. Inhibition of retromer activity by Herpesvirus saimiri Tip leads to CD4 downregulation and efficient T cell transformation. J Virol. 2011 doi: 10.1128/JVI.00757-11. PMID: 21849449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kooner SJ, et al. Genome-wide association study in individuals of South Asian ancestry identifies six new type 2 diabetes susceptibility loci. Nat Genet. 2011;43:984–989. doi: 10.1038/ng.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhou B, Wu Y, Lin X. Retromer regulates apical-basal polarity through recycling crumbs. Dev Biol. 2011 doi: 10.1016/j.ydbio.2011.09.009. PMID: 21958744. [DOI] [PubMed] [Google Scholar]
- 120.Bonifacino JS, Hurley JH. Retromer. Curr Opin Cell Biol. 2008;20:427–436. doi: 10.1016/j.ceb.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]