Abstract
Increased attention is paid to the structural components of tissues. These components are mostly collagens and various proteoglycans. Emerging evidence suggests that altered components and noncoded modifications of the matrix may be both initiators and drivers of disease, exemplified by excessive tissue remodeling leading to tissue stiffness, as well as by changes in the signaling potential of both intact matrix and fragments thereof. Although tissue structure until recently was viewed as a simple architecture anchoring cells and proteins, this complex grid may contain essential information enabling the maintenance of the structure and normal functioning of tissue. The aims of this review are to (1) discuss the structural components of the matrix and the relevance of their mutations to the pathology of diseases such as fibrosis and cancer, (2) introduce the possibility that post-translational modifications (PTMs), such as protease cleavage, citrullination, cross-linking, nitrosylation, glycosylation, and isomerization, generated during pathology, may be unique, disease-specific biochemical markers, (3) list and review the range of simple enzyme-linked immunosorbent assays (ELISAs) that have been developed for assessing the extracellular matrix (ECM) and detecting abnormal ECM remodeling, and (4) discuss whether some PTMs are the cause or consequence of disease. New evidence clearly suggests that the ECM at some point in the pathogenesis becomes a driver of disease. These pathological modified ECM proteins may allow insights into complicated pathologies in which the end stage is excessive tissue remodeling, and provide unique and more pathology-specific biochemical markers.
Introduction
The extracellular matrix (ECM) is of paramount importance for tissue function, and controls cell phenotype and function. That was initially illustrated by Mintz and colleagues who showed that the normal mouse embryonic tissue microenvironment could repress expression of the tumor phenotype;1,2 thus, the ECM was able to control genotype/phenotype relationships. These interactions between cells and the ECM components are mediated through receptors, such as integrins and the discoidin receptors.3 To maintain healthy tissue, the ECM must regenerate itself by normal remodeling, in which old or damaged proteins are broken down in a specific sequence of proteolytic events and replaced by new proteins. However, during pathological conditions, such as cancer, fibrosis, and inflammation, the delicate repair–response balance is disturbed.4,5 The original proteins of the ECM are replaced by different matrix constituents, and consequently, the composition and quality of the matrix are altered. During cancer and fibrosis propagation, the ECM may be stiffened, and this can actually enhance tumor cell migration, myofibroblast activation, and collagen deposition,6–14 thereby linking the actual matrix quality to disease progression.
During this pathological remodeling of the ECM, excessive levels of tissue- and pathology-specific turnover products are released into the circulation. Turnover products holding post-translational modifications (PTMs) are defined as modifications made secondary to translation of the protein into the peptide sequence from mRNA. Thus, most PTMs are not directly DNA coded, and are a consequence of tissue physiology and pathophysiology.15,16 PTMs may be derived from processes, such as aging (in which amino acid isomerization occurs), citrullination (during inflammation), protease degradation (fibrosis and inflammation), and glycosylation (diabetes),15,16 as will be carefully discussed. Protease-generated neoepitopes have, to date, received more attention than other PTMs. However, potentially important PTMs that are believed to be specific for cancer as well as fibrotic and other pathological conditions have recently been identified.15,17–19 The PTMs made to proteins result in unique protein fingerprints.20 These modified structures are prime candidates for biochemical marker development, as they may be more related to the pathogenesis than unmodified proteins. Several lines of independent evidence suggest that PTMs to specific proteins contribute to abnormal cellular proliferation, adhesion characteristics, and morphology,21 and may cause many of the differences in cancer tissue compared to normal tissue.21–26 Furthermore, the generation of PTMs of key structural proteins, generated by protease cleavage, citrullination, nitrosylation, glycosylation, and isomerization, is emerging as a critical factor in tissue homeostasis and remodeling. Thus, PTM profiles may be used as biochemical fingerprints for detecting and verifying the function and activity of key cellular signaling pathways21–26 involved in tissue homeostasis and integrity. Additional lines of evidence highlight that the structural components of the matrix, after PTM, are central part of the pathogenesis itself,15 thus highlighting the matrix structural proteins as central and active participants rather than passive bystanders in disease pathogenesis.
The aims of this review are to discuss the structural components of the matrix, the potential applicability to pathology, and the measurement of structural molecules in serum. We review the PTMs, which may be both a consequence of disease and a part of the pathogenesis, as exemplified by the role of tissue stiffness in cancer and fibrosis. Lastly, we list the current methods for measuring post-translational modified matrix proteins in serum. These PTMs may serve as disease-specific biochemical markers and assist in the identification of key molecular pathways leading to enhanced connective tissue remodeling.
Discussion
Function of the ECM
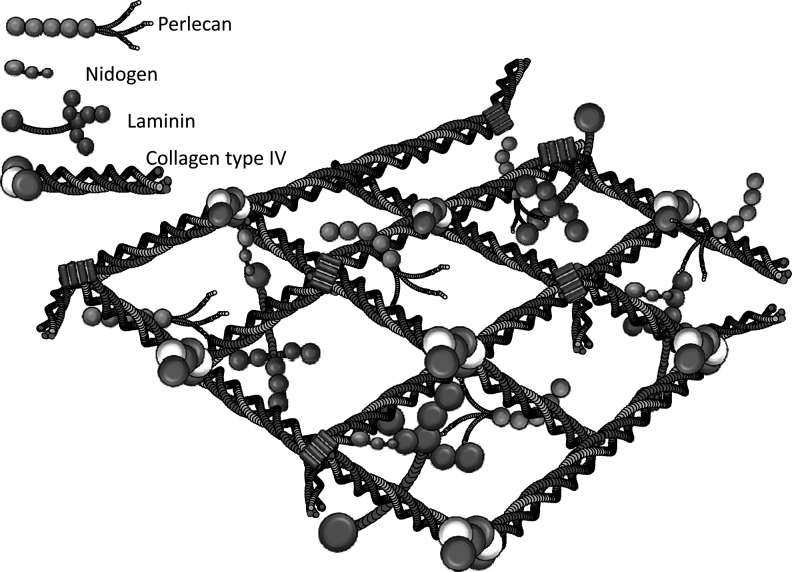
The ECM is a three-dimensional (3-D) structure that encapsulates cells and defines their microenvironment.27 It consists of a meshwork of proteins to which soluble factors, such as growth factors and cytokines, can bind. There are two main types of ECM. The first is the basement membrane (BM), which interacts directly with the epithelium and endothelium, and it is composed of primarily of type IV collagen, laminins, entactin/nidogen, and heparan sulfate proteoglycans (e.g., perlecan) (Fig. 1).28
Fig. 1.
The molecular structure of a typical basal lamina. The basal lamina is formed by specific interactions between the proteins type IV collagen, laminin, and entactin plus the proteoglycan Perlecan. Adapted by S.H. Madsen, from Yurchenco and Schittny.28
The second type is the interstitial matrix, which makes up the bulk of the ECM in the body. The interstitial matrix consists of many types of collagens, including types I and III, together with fibronectin. The interstitial matrix additionally consists of tenascin and proteoglycans that provide tissue hydration, enable binding of growth factors and cytokines to the tissue, and cross-link the matrix to enhance its integrity.29
Although originally considered as merely a support system for the cells within the tissue, the ECM is now recognized as a central regulator of cell and tissue behavior via transmembrane signaling.1,30–33 While the basic characteristics and composition of the BM and interstitial matrix are constant across tissues, variations in ECM components, such as protein isoform expression, ratio between individual matrix components, and PTMs, contribute to differences in ECM organization and structure and ensure tissue specificity.15 PTMs, such as glycosylation and cross-linking, significantly affect the mechanical properties of the ECM, including its viscoelasticity or stiffness. Both the stiffness and topology (3-D appearance) of the ECM regulate the growth, remodeling, differentiation, migration, and phenotype of a wide variety of cell and tissue types.8–14,34
Matrix Composition Affects Cell Phenotype
The importance of matrix stiffness in tissue-specific differentiation is exemplified by the fact that cells grown as monolayers (two-dimensional: 2-D) on top of either a plastic substrate or a glass coverslip, with or without ECM ligand, fail to assemble the same tissue-like structures as those growing in the normal ECM (3-D). Cells growing on plastic or glass are less likely to express differentiated proteins upon stimulation,34 or respond to growth factors or protease inhibitors in the same way as cells growing in a 3-D setting.35 These phenotypic disparities can be explained, in part, by the fact that living tissues in 3-D emit biological signals that may be read by specific integrins, but this signaling is nonexistent in 2-D substrata such as tissue culture on plastic. The role of cell polarity versus non-polarity in cultures is receiving increased attention. Another illustration of this phenomenon is that when epithelial cells and melanocytes are grown in a 3-D ECM microenvironment, they assemble into tissue-like structures and express differentiated proteins when given the correct soluble stimuli.36 Neither behavior is seen when the same cells are cultured on 2-D plastic substrata.
The architecture of the interstitial matrix in vivo also differs substantially from that found typically in tissues cultured on plastic, and this too can have dramatic effects on cell behavior.35 For instance, osteoblasts grown on plastic in 2-D do not rely on matrix metalloproteinases (MMPs) for survival, whereas osteoblasts embedded in an interstitial matrix, such as 3-D type I collagen, are critically dependent for their survival on MMP activation of latent transforming growth factor (TGF)-β.35 Thus, the matrix architecture is crucial to the phenotype and survival of cells. Interestingly, the orientation of collagen fibers can critically regulate cell and tissue behavior.37–39 This 3-D contextual information is lost when cells are grown in 2-D.
Varying components of the ECM also influence the ability of the matrix to regulate cell and tissue behavior. The ECM transmits signals through various specialized cell membrane receptors, including integrins, discoidin domain receptors (DDRs), and syndecans.40–44 Integrins provide an excellent model of how an altered ECM could promote tumor progression. Integrins consist of 24 distinct transmembrane heterodimers that relay cues from the surrounding ECM to regulate cell growth, survival, motility, invasion, and differentiation.40–44 They are able to interact with the ECM externally, and with cytoplasmic adhesion plaque proteins and the cytoskeleton intracellularly to influence cell behavior. Integrin–ECM interactions regulate the cell fate by activating multiple biochemical signaling circuits and altering the cell shape.45,46 This occurs either through direct interactions between ECM receptors and actin-linked proteins or cytoskeletal reorganization induced by activating cytoskeletal-remodeling enzymes, such as RhoGTPases.45,46
This section highlights that the composition of the ECM affects the phenotype of cells through specific receptor-mediated interactions. Certain ECM compositions and structures result in a context-dependent response to a given stimulus, which is absent in other experimental settings.
ECM Proteins
The ECM mainly consists of collagens and proteoglycans, each with their unique function. In the following section, the most important and well-investigated collagens and proteoglycans are discussed, together with other important structural components of the ECM.
Collagens
Collagens are a family of proteins made up of three α-chains supercoiled around each other completely or partially in a triple helix with a characteristic Gly-X-Y repeat. Intra- and intermolecular cross-links bring stability to the collagen molecules, contributing to the characteristically high tensile strength and minimal extensibility of collagen. Type I, II, III, and V collagens belong to the group of fibrillar collagens, which are the most abundant collagen group in the body. In addition to the triple-helical domain, they also contain N- and C-terminal propeptide domains that are cleaved off by N- and C-procollagenases, respectively, before fibril assembly.47 Type I–VI collagens are the most well described at present and are the focus of this section.
Type I collagen is composed of the heterotrimer α1α1α2(I) and is the most abundant type of collagen that is ubiquitously expressed. It provides tensile stiffness in bone and has important load-bearing, tensile strength, and stress-carrying properties in other tissues as well. In tendons, type I collagen fibrils are arranged in parallel to form bundles, whereas in skin, the arrangement is more random, forming a complex network of interlaced fibrils. These different arrangements contribute to the different properties of the tissues. Type I collagen is often incorporated into fibrils with either type III48 or type V collagen.49 The synthesis, concentration, and circulating levels (serum concentration) of degradation products of type I collagen have been proven to be increased during breast, bone, lung, ovarian, prostate, and skin malignancy.50–55
Type II collagen is the major component of hyaline cartilage, but is also found in the vitreous body of the eye, the corneal epithelium, the notochord, the nucleus pulposus of invertebral discs, and embryonic epithelial-to-mesenchymal transitions (EMTs).47 Type II collagen is a homotrimer consisting of three α1(II) chains, and the primary sequence has a high content of hydroxylysine and glycosyl residues, which mediate interactions with proteoglycans, another important component of hyaline cartilage. Type II collagen degradation is mainly associated with rheulatological diseases such as osteoarthritis and rheumatoid artiritis.56
Type III collagen is mainly present in association with type I collagen and is an important component of the interstitial tissues of the lung, liver, dermis, spleen, and vessels. Type III collagen is a homotrimer consisting of three α1(III) chains. A characteristic feature of type III collagen is that it is correlated to extensibility of tissues, and that it may contribute to elasticity, a property that is uniquely connected to this type of collagen.57 Type III collagen has been mostly assiociated with various fibrotic diseases.58–61
Type IV collagen is the main component of the BM, a specialized type of ECM that separates the epithelium from the stroma in all tissues in the body. It consists of three domains: N-terminal 7S domain, a central triple helix, and a large C-terminal NC1 globular domain. Its triple helix is ∼25% longer than those seen in the fibrillar collagens, and the Gly-X-Y repeat is frequently interrupted, accounting for the relatively high flexibility of this type of collagen.28 Instead of fibrils, type IV collagen molecules assemble into a flexible 3-D network. The most abundant isoform of type IV collagen is α1α1α2(IV), but tissue-specific isoforms also exist: α3α4α5(IV) heterotrimers are found in the lung, glomeruli of the kidney, cochlea, eyes, and testis, whereas α5α5α6(IV) is found in skin, Bowman's capsule of the kidney, the esophagus, and the knee joint.62 Turnover of the basement membrane is associated with a range of diseases.
Type V collagen is expressed in tissues containing type I collagen, but is a quantitatively minor component.63 The most common structure of type V collagen is α1α1α2(V), although homotrimers of three α1(V) chains and heterotrimers of the α1α2α3(V) isoforms have also been detected.64 It typically forms heterofibrils with type I collagen,49,63 where it makes up the core structure of these heterotypic fibrils. Type V collagen is of special importance for the structure of tissues. It has been shown to be essential for the correct assembly of collagen fibrils and to regulate their size and organization.65 This characteristic makes type V collagen especially unique and interesting to study. The N-terminal domain contains a high level of tyrosine sulfated residues that contribute to the strong interactions that type V collagen has with triple-helical domains of other collagen types. This enhances the stability of fibrils.66
Type VI collagen is a heterotrimeric molecule with the isoform α1α2α3(VI) and consists of a short triple helix flanked by two extended globular domains, and it is expressed, albeit variably, in virtually all tissues. The primary fibrils are arranged in overlapping dimers in an antiparallel manner and form parallel tetramers that are stabilized by intermolecular disulfide bonds. They aggregate to form filaments and an independent microfibrillar network. Type VI collagen molecules have a uniquely beaded appearance and interact with several ECM components such as type I collagen and fibronectin.67
Proteoglycans
Proteoglycans are ECM macromolecules formed by a protein core with one or more glycosaminoglycans (GAGs) bound covalently. Due to the negative charge and structural conformation of GAGs, proteoglycans can interact with a large variety of macromolecules.68 Proteoglycans can be divided into five families according to the structural properties of their core protein.69,70
The small leucine-rich proteoglycan (SLRP) family is formed by proteoglycans that bind specifically to other ECM constituents and contribute to the structural framework of connective tissues. SLRPs are small molecules, with core proteins of 40 kDa, and possess characteristic 6–10 leucine residuces at conserved locations between the flanking cystein-rich disulfide-bonded domains at the N- and C-terminus that participate in protein–protein interactions with collagens, matrix glycoproteins, and cell membrane components.70,71 Based on several parameters, including gene organization and amino acid homologies, SLRPs are further divided into five classes: class I includes decorin, biglycan, and asporin; class II includes fibromodulin, lumican, keratocan, proline arginine-rich end leucine-rich repeat protein (PRELP), and osteoadherin; class III includes epiphycan, mimecan, and opticin; class IV includes chondroadherin and nyctalopin; and class V includes podocan.69,72
Decorin, fibromodulin, asporin, lumican, PRELP, and chondroadherin can interact with collagen and influence collagen fibril formation and interaction.69 In addition to their ECM functions in tissue hydration and collagen fibrillogenesis, proteoglycans are able to influence tissue repair and tumor growth, to facilitate cellular adhesion, proliferation, and migration, and to modulate growth factors and cytokine activities. For this reason, they are referred to as matricellular protein, with the ability to modulate cell–matrix interactions and cell functions.72 In particular, decorin, biglycan, and lumican exert many modulation roles in different biological processes. These functions highlight the important effect of ECM components in the cellular phenotype by influencing cell communication through, that is, signal transduction, cytokine modulation, adhesion, and migration.72 All the different matricellular functions exerted by these three important SLRPs are detailed in Table 1.3,73–118
Table 1.
The Matricellular Effects of Extracellular Matrix Components
| Protein or PTM | Cellular phenotype | Responsible receptor | Reference |
|---|---|---|---|
| Elastin-derived peptides | Chemotaxis of monocytes, fibroblasts, and endothelial cells | Elastin-binding protein in complex with protective protein/cathepsin A and neuraminidase-1 | 73,74 |
| Proliferation of fibroblasts and smooth muscle cells | |||
| Protease release from fibroblasts and leukocytes | |||
| Thrombospondin | Inhibition of angiogenesis | CD36 and CD47 | 75–77 |
| Type I collagen | Fibroblast migration | DDR2; integrins α1, α2, α10, α11, and β1 | 3,78 |
| Acetylated Proline–Glycine–Proline (acPGP; fragment of type I collagen) | Neutrophil chemotaxis | CXCR1 and CXCR2 | 79,80 |
| Arresten, canstatin, and tumstatin (fragments of type IV collagen) | Inhibition of angiogenesis, tumor growth, and endothelial cell proliferation and migration. | Various integrins | 81,82 |
| Induction of apoptosis | |||
| Endostatin (fragment of collagen type XVIII) | Inhibition of endothelial proliferation, angiogenesis, and tumor growth | Glypicans, nucleolin | 83–86 |
| Induction of endothelial cell apoptosis | |||
| RGD motif (present in collagens, laminin, and fibronectin) | Cell adhesion, angiogenesis, and apoptosis | Various integrins | 87,88 |
| Fibromodulin | Proliferation, migration, and chemotaxis of HSCs | Unknown | 89 |
| Laminin-332 (elastase-generated fragment of γ2) | Neutrophil chemotaxis | Unknown | 90 |
| SIKVAV and ASKVKV (sequences in linker regions between coiled-coil and globular domains of laminin α1 and α5 chains) | Neutrophil and macrophage chemotaxis | Unknown receptors; SIKVAV interacts with integrins α1, α6, and β1 in the salivary gland carcinoma cell line | 91,92 |
| Laminin | Chemotactic migration of malignant cells toward laminin | 67LR (LamR) | 93,94 |
| Lumican | Regulation of inflammation and innate immunity | CD14, FasL, CXCL1 | 95–98 |
| Apoptosis induction | Fas | ||
| Biglycan | Regulation of inflammation and innate immunity | TLR2, TLR4, P2X4/P2X7, selectin L/CD44, C1q | 99–110 |
| Cytokine modulation (PDGF, TGF-β, TNF-α, WISP-1, BMP-4) | |||
| Adhesion and migration | RhoA, Rac1 | ||
| Decorin | Signal transduction | LRP-1, c-MET | 102–106,110–118 |
| Cytokine modulation (PDGF, TGF-β, TNF-α, VWF, and WISP-1) | |||
| Regulation of inflammation and innate immunity | TGB-β, C1q | ||
| Antiapoptotic effect. | IGF-IR | ||
| Antioncogenic effect. | EGF-R, VEGF-R2, | ||
| Adhesion and migration | IGF-IR, integrin α2β1, RhoA, Rac1 |
PTMs, post-translational modifications; HSC, hepatic stellate cell; PDGF, platelet-derived growth factor; TGF, transforming growth factor; TNF, tumor necrosis factor; VEGF, vascular endothelial growth factor.
Some of the most important proteoglycans expressed in the ECM are briefly described in the following paragraphs.
Aggrecan is the major proteoglycan of the cartilage, and it is the most highly glycosylated, with 150 chondroitin sulfate and keratan sulfate GAGs bound to a large central core protein. Through its specific binding with hyaluronan and link protein, it forms a supramolecular structure whose characteristics make it able to retain water molecules in the cartilage, providing the tissue with the property of resisting compressional forces with minimal deformation.69
Versican is a large interstitial chondroitin sulfate proteoglycan. It is present in many tissues, and it is one of the principal ECM components of normal blood vessels where it influences the assembly of ECM and controls elastic fiber fibrillogenesis.119 It is present in the intima and adventitia of most arteries and veins, and it is synthesized by vascular smooth muscle cells as well as endothelial cells, myofibroblasts, and macrophages.120 Versican interacts with hyaluronan and link protein to form high molecular weight stable aggregates. These complexes create a reversibly compressive compartment and provide a swelling pressure within the ECM that is compensated by collagen and elastic fibers. Dramatically increased levels of versican have been observed in atherosclerosis and restenosis, implying that this proteoglycan is a specific component of developing lesions and contributes to their progression in atherosclerosis and restenosis.119
Perlecan is a heparan sulfate proteoglycan widely distributed in BMs, and it has the largest core protein found in proteoglycans. It is able to self-associate or interact with several other BM macromolecules, including laminin and type IV collagen.68
Decorin is the most abundant SLRP in cartilage. It contains one GAG chain, often dermatan sulfate, which can adopt complex secondary structures and form specific interactions with matrix molecules. Its level increases with age. Its main function is to regulate collagen fibrillogenesis and to maintain tissue integrity by its binding with fibronectin and thrombospondin.69 However, decorin also exerts important matricellular functions, favoring the cell–matrix interactions and influencing cell phenotype (see Table 1). Decorin is an important antifibrotic agent: it influences fibrogenesis in different organs by inhibiting TGF-β; it regulates ECM synthesis and turnover, and it is involved in regulation of cell death, adhesion, and migration.72
Biglycan is a small SLRP. It is found in many connective tissues, such as skin, bones, and blood vessels. Within the hyaline cartilage tissue, biglycan is localized mainly pericellularly.70 Together with decorin, biglycan is a key regulator of the lateral assembly of collagen fibres, and it interacts primarily with type VI collagen.69 Biglycan is thought to have a role also in fibrogenesis and in assembly of elastin fibers.121 Moreover, this proteoglycan is able to bind to the membrane-bound proteoglycan, dystroglycan, and to a wide variety of proteins. It is involved, for instance, in cell signal transduction during cell growth and differentiation and in regulating cytokine activity through its capacity to bind TGF-β and tumor necrosis factor (TNF) α (see Table 1).99
Mimecan is a keratan sulfate proteoglycan belonging to the SLRP family, and it is the product of the gene that encodes for osteoglycin.122 Its main role consists in regulating the collagen fibril diameter.123 Apart from corneal tissue, where it has been first identified, mimecan is expressed also in other tissues, such as medullary bone,124 amniotic membrane,125 cartilage126 and pituitary.127 In the lung, mimecan mRNA expression is correlated to nonsmall-cell lung cancers,128 and in arteries, there is an indication that it can be involved in arterial remodeling during atherosclerosis.129
Fibromodulin is one predominant SLRP in cartilage. It contains up to four keratan sulfate chains, and it is able to influence collagen fibril formation and maintain a sustained interaction with the formed fibrils.69,130
Lumican is a highly biologically active SLRP. It can exist as proteoglycan (with GAG chains) and as glycoprotein (with mono- or polysaccharide chains). In the human adult cornea, it is present in the first form, whereas it is in a glycoprotein form in embryonic cornea and in skin. Lumican expression in cornea has been widely studied. In this tissue, it exerts its main role in controlling the polymerization of collagen into small-diameter fibrils.131 Lumican is also highly present in skeletal muscle, kidneys, placenta, heart, intervertebral discs, blood vessels, intestine, uterus, and pancreas,71 and it has a widespread distribution in connective tissues, including cartilage, where it modulates collagen fibrillogenesis and regulates the assembly and diameter of collagen fibers and interfibrillar spacing, enhancing collagen fibril stability.132 Together with decorin and biglycan, it is an important component of the ECM exerting matricellular functions (see Table 1).
Other proteins: The glycoproteins fibronectin and tenascin C modulate the integrin-mediated adhesion of cells to other ECM proteins, such as collagens, and as such play a key role in cancer invasion. A single gene encodes fibronectin, but alternative splicing allows formation of multiple isoforms from which some are tumor specific.133 The fibulins, Galectin-1 and Fibulin-1, function as intramolecular bridges in the organization of ECM supramolecular structures, such as elastic fibres and BMs.134 Galectin-1 and Fibulin-1 can bind ECM components, that is, laminin and fibronectin, and therefore modifies the adhesive properties of cancer cells.134–136
Effect of structural proteins on cellular phenotype: selected examples
There is growing evidence that ECM molecules have functions other than structural roles, but as integrated players in the structure and functional homeostasis of tissue. A nonexhaustive list of these proteins is given in Table 1. This highlights that ECM proteins are beginning to be recognized as paracrine-signaling molecules, with profound effects on cellular phenotypes that until recently was restricted to cytokines, growth factors, and hormones.137 Of particular relevance, which will be discussed later in this review, some proteins do not change cellular phenotypes in their native conformation, whereas subsequent to a specific PTM, a highly potent and novel function of that protein is revealed. A well-thought example of such cryptic sites is RGD sequences that are either exposed by protease digestion in most collagen species87 or even more scholarly exemplified by endostatin, which is a fragment of collagen type XVIII that by cleavage becomes possibly the most powerful anti-angiogenic molecule to date.83
Table 1 lists examples from outstanding research groups which serve to highlight that the matrix encompasses strong signaling motifs that may be revealed during the pathological process. Consequently, the matrix molecules themselves, in addition to cytokines, growth factors, and hormones, become essential players in tissue homeostasis. As the ECM molecules both anchor cells in the right spatial distribution and cell orientation, these structural components may have a dual effect due to their emerging signaling roles.
ECM Remodeling in Cancer and Fibrosis
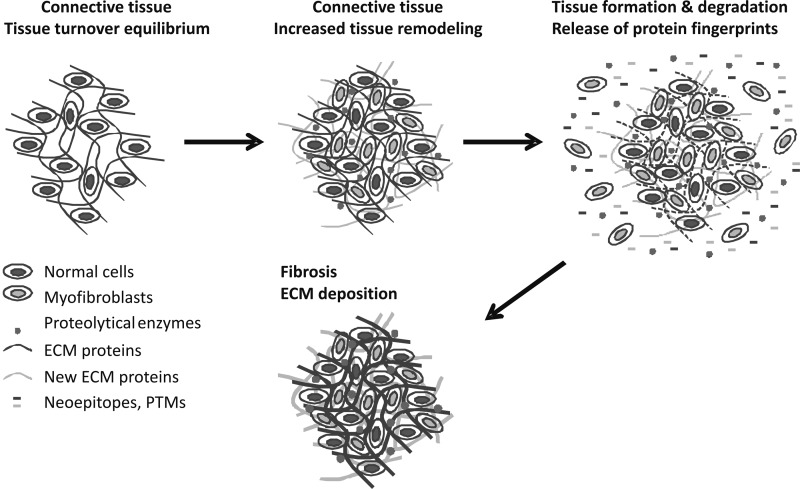
Cancer and fibrosis share a number of abnormal characteristics of the ECM structure and function, including constitutively high matrix degradation, formation, and turnover. Interestingly, both diseases involve aspects of inflammation and matrix assembly, destruction, and disorganization.24,138,139 As illustrated in Figure 2, cancer cell metastasis results in extensive ECM remodeling (ECMR), resulting in the release of matrix components, including neoepitopes, into the circulation. ECM components and remodeling enzymes are known to be elevated in the circulation of cancer patients.140,141
Fig. 2.
Schematic representation of the high extracellular matrix remodeling in fibrosis. All steps involve extracellular matrix (ECM) remodeling that generates unique protein degradation fingerprints. These enzymes degrade the ECM, releasing smaller fragments of protein from the ECM into the circulation. Interestingly, many of the same processes occur in both fibrosis and cancer.
The architecture of the tumor-associated ECM is fundamentally different from that of the normal tissue stroma.142 As an example, type I collagen is situated parallel to the epithelial cells in healthy tissue, but is less organized in the stroma surrounding metastases.143 These stromal changes to the ECM promote transformation, tumor growth, motility, and invasion; enhance cancer cell survival; enable metastatic dissemination; and facilitate the establishment of tumor cells at distant sites.143 Cancer is caused when the essential rules governing how cells should be organized in a stable manner within all living tissue are disregarded. Uncontrolled cell growth is necessary for cancer formation. Such growth becomes self-directed, leading to a disorganization of the normal tissue architecture, which is known as neoplastic transformation. More than 90% of malignant tumors are epithelial tumors,4 occurring where there is a collapse in the boundary between the epithelial and connective tissues that encompass a given organ. Interruption of these tissue boundaries promotes cancer cell migration to nearby blood vessels or the lymph node system, enabling the cells to metastasize to remote organs resulting in multiorgan failure and death.
Fibrosis is an end-stage representation of a repair–response process after an injury. Like cancer, it may lead to serious organ damage. The development of liver fibrosis resembles the process of wound healing, including the three essential phases after tissue injury: inflammation, synthesis of collagenous and noncollagenous ECM components, and tissue remodeling. Fibrosis may begin in response to various acute or chronic stimuli, including infections, autoimmune reactions, toxins, radiation, and mechanical injury.144 The pathogenic process driving fibrogenesis is believed to be a dynamic series of events involving complex cellular and molecular mechanisms evolving from the acute or chronic activation of tissue repair that follows repeated tissue injury,5 In the case of liver fibrosis, these stimuli give rise to a series of events that involve several cell types working in synergy toward irreversible damage of the liver.145
Identification and characterization of the cell types and the different mediators involved in liver fibrogenesis have expanded significantly during recent years.146–148 Hepatic stellate cells (HSCs) have been identified as the driving force of liver fibrosis. When HSCs are activated by inflammatory mediators,149 they differentiate into hepatic myofibroblast-like cells (hMFB) capable of expression and secretion of several connective tissue components (for example, collagens, elastin, proteoglycans, and hyaluronan).149,150 HSCs are believed to be the main source of ECM proteins accumulated in the liver during chronic liver disease. Recent research has clearly demonstrated that other cell types contribute to the hMFB-pool.151–153 These cells can come from local sources such as portal myofibroblasts154 or, may be, newly formed HSCs that originate from a process called EMT, in which biliary epithelial cells or hepatocytes transform into fibroblasts.155 In addition, contributions to the hMFB-pool come from outside the liver from cells like bone marrow156 and circulating fibrocytes.157 The bone marrow-derived myofibroblasts have been shown to be of a surprisingly large importance, as they can transdifferentiate into epithelial cells.158–161 The accumulation of fibrous tissue and myofibroblast contraction in the liver leads to mechanical increase of hepatic vascular resistance to portal vein blood.159,162 This in turn leads to loss of oxygen to the surrounding tissue, facilitating neoangiogenesis as HSCs and Kupffer cells begin overexpressing proangiogenic growth factors and cytokines.163
The activation of HSCs involves multiple intracellular pathways and gene regulation. Regulation of growth factors plays an important role in HSC activation, with platelet-derived growth factor (PDGF) signaling is the best-characterized pathway leading to HSC activation. Binding of PDGF results in dimerization and phosphorylation of the tyrosine residues in the intracellular domain of the receptor. This activates the Ras/MAPK and the PI3K-AKT/PKB pathways, leading to cellular proliferation.164 The increased matrix production by HSCs is controlled by TGF-β, which is the most potent fibrogenic cytokine in the liver, by signaling via Smad proteins.165 Chemokines induce the NFκB signaling pathway, leading to further migration and proliferation of HSCs. Continued deposition of matrix proteins is controlled by a positive feedback loop that sustains the inflammatory response and proliferation and migration of HSCs as chemokines interact with immune cells.166,167
Disregulation of ECM homeostasis is also central in the development of fibrosis of the lung, although the origin of fibrogenic precursors remains a subject of debate, and is potentially multifactorial in nature. Activation of resident fibroblasts, recruitment of circulating progenitors such as fibrocytes or other candidate progenitors, and EMT of alveolar epithelia have all been implicated in the formation of activated myofibroblasts168–172 in the lung. Consistent with findings in fibrotic liver disease, these activated myofibroblasts produce fibrillar collagens such as type I collagen and other matrix proteins, which apart from promoting remodeling and ultimately scarring the lung parenchyma, drive a sustained cycle of ongoing fibrogenesis, even in the absence of ongoing inflammatory insult. As with liver fibrosis, studies in disease models indicate that TGF-β is a key fibrogenic cytokine. Together with other cytokines, signaling pathways, and matrix proteins, TGF-β contributes to the ongoing disease cascade and destructive remodeling of the lung.172–178
As a biomechanically sensitive organ, the lung could be considered as particularly dependent on the composition and architectural organization of ECM components, including BM collagens such as type IV collagen, structural fibrillar collagens (type I and III), and elastin.179 The importance of collagen remodeling in resolution of fibrosis has been demonstrated in models in which inhibition of the lysyl oxidase (LOX) family member, LOXL2, which catalyzes the cross-linking of fibrillar collagen, and thereby increases tissue tension, was sufficient to reverse established fibroblast activation and reduce TGF-β signaling, cytokine production, inflammation, and other markers of profibrogenic imbalance.14 These findings are consistent with previous data showing that ongoing myofibroblast activation and TGF-β signaling from the latency-associated complex can be driven by altered mechanical tension in a feed-forward loop.180,181 Selective inhibition of LOXL2, which is overexpressed in both human fibrotic disease and disease models, may also constitute a therapeutic target. Inhibition of aberrant fibrogenesis, while avoiding inhibition of other LOX family members, such as LOX and LOXL1, may play a critical role in elastin homeostasis in the lung.182,183
Studies in humans and in animal models have suggested that some elements of fibrosis are reversible, and in specific circumstances, restoration to near-normal organ architecture can be achieved.184–189 Consequent to these findings is an emerging interest in the fibrosis field with focus on the ECM components. Measurement of the individual molecules gives a deeper understanding of fibrosis and attenuates pathological processes.
Noninvasive biomarkers of liver fibrosis have been sought for decades, and the FibroTest multimarker panel is approved for clinical usage in Europe. However, all of the current markers and panels have limitations,190 and none have been recommended by the American Association for the Study of Liver Disease (AASLD) to replace liver biopsy.191 Clearly, novel markers are still needed, and measuring neoepitopes of ECM proteins composes a snapshot of matrix dynamics that may be of diagnostic and prognostic value.192 Examples of well-studied liver ECM markers include collagen propeptides, notably PIIINP,193 and caspase fragments of cytokeratin 18.194 A recent mass spectrometry study of the ECM in two rodent models identified 16 different collagens in the liver, and profiled changes in the abundance of collagens and integrins in tumors compared with healthy livers and precancerous fibrotic livers.195 Neoepitopes of these proteins may serve as valuable markers of liver ECMR. Promising candidates have been reported, including those derived from type IV collagen,196,197 type I collagen,198 type V collagen,199 and type VI collagen.200 Systemic approaches, such as global profiling of serum glycoproteins, have also been utilized,201 and this technique is now being validated in rodent models (e.g., Fang et al.,202 Blomme et al.,203) and in additional cohorts of liver disease patients.204,205
A range of diseases involve excessive matrix remodeling in specific matrices. For example, in rheumatoid arthritis the turnover of type I, II, and III collagens are highly upregulated in the cartilage and synovium.206 The high turnover of ECM proteins are also found in other diseases, such as:
• osteoarthritis affecting the articular cartilage (type II collagen and aggrecan)56
• atherosclerosis (type I and III collagens, titin and versican)217
• various fibrotic diseases including liver (type I, III, IV, V, VI collagens and biglycan),59–61,144,196,197,199,200,218 lung (elastin, type I, III, and V collagen),74,220–232 and kidney.233
Key lessons on the importance of the structural components of the matrix may be harvested from the genetic mutations that lead to pathologies. Table 2 contains a summary of key structural proteins and their known mutations leading to matrix and tissue failure.224,233–271 These disease phenotypes provide pivotal information on proteins important for tissue function, and thus how they are involved in some pathologies of nongenomic disorders, and subsequently how treatments that affect these proteins may counter disease progression.
Table 2.
Genetic Mutations in Structural Proteins Leading to Distinct Pathologies
| Protein | Disease | Reference |
|---|---|---|
| Type I collagen | Osteogenesis imperfecta, Ehlers-Danlos syndrome type VII | 234,235 |
| Type II collagen | Several chondrodysplasias, osteoarthritis | 236–239 |
| Type III collagen | Ehlers-Danlos syndrome type IV, aortic aneurysms | 240,241 |
| Type IV collagen | Kidney fibrosis, Alport syndrome | 233,242–244 |
| Type V collagen | Ehlers-Danlos syndrome type I and II | 245,246 |
| Type VI collagen | Bethlem myopathy, Ullrich congenital muscular dystrophy | 247 |
| Type VII collagen | Epidermolysis bullosa dystrophica | 248 |
| Type IX collagen | MED | 249 |
| Type X collagen | SMCD and Japanese-type SMD | 250,251 |
| Type XV collagen | Cardiac and muscle phenotypes | 243 |
| Yype XVII collagen | Growth retardation | 243 |
| Type XVIII collagen | Renal filtration defects | 243 |
| Elastin | Lung, skin and arterial defects, SVAS, WBS, CL | 224,252,253 |
| Laminin | Alport syndrome | 233 |
| Biglycan | Cardiovascular disease, osteoporosis | 254–256 |
| Biglycan/decorin | Osteopenia and skin fragility | 257 |
| Biglycan/fibromodulin | Osteoarthritis | 258 |
| Perlecan | Multiple developmental defects and myotonia. Schwartz-Jampel syndrome | 243 |
| Nidogen 1 and 2 | Lung and kidney development | 243 |
| Fibromodulin | Osteoarthritis | 259 |
| Lumican/fibromodulin | Joint laxity and impaired tendon integrity | 260,261 |
| Lumican | Reduced corneal transparency and skin fragility | 262 |
| Decorin | Intestinal tumor; skin fragility; Ehlers-Danlos syndrome-like. | 263–265 |
| Mimecan | Colorectal cancer early formation | 266 |
| Fibrillin | Marfan syndrome | 267 |
| COMP | PSACH and MED | 268–270 |
| Matrillin-3 | MED | 271 |
MED, multiple epiphyseal dysplasia; SMCD, Schmid-type metaphyseal chondrodysplasia; SMD, spondylometaphyseal dysplasia; SVAS, supravalvular aortic stenosis; WBS, William-Beuren syndrome; CL, cutis laxa; COMP, Cartilage oligomeric matrix protein; PSACH, pseudoachondroplasia.
PTMs in the ECM
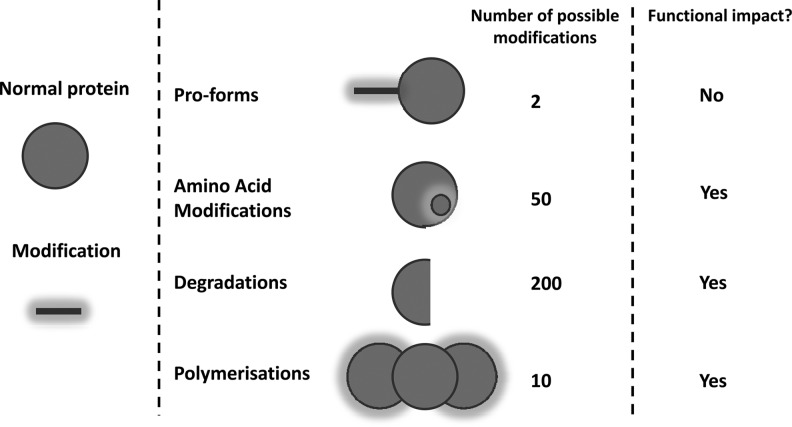
PTMs are non-DNA-coded modifications to the composition or structure of proteins, which generate unique parts of a molecule known as neoepitopes.17 Pathologically relevant protein modifications are not restricted to protease activity, although the subpopulation of neoepitopes generated through this mechanism may be of paramount importance. Figure 3 depicts a handful of different types of PTMs. Some have been identified and used as biochemical markers as a measure of the disease activity,272 but also as contributions to disease process,17 as they change the functionality of the proteins.
Fig. 3.
Schematic figure of the modifications made to a protein that causes unique subpools to be generated, which each may entail specific pathological or physiological information.
One gene, 1,000 protein subtypes
The importance of PTMs is best described by the fact that one gene may result in 1,000 different and unique proteins with different functional implications. This is illustrated in Figure 3. Here, modifications to amino acids by specific PTMs or degradation of the protein result in both immunologically different as well as functionally different proteins. Measurement of the same protein may provide highly different information, such as either protein formation or protein degradation, which obviously entails opposite information. Some pathologies may further modify the protein specifically, and thus give a specific protein fingerprint of pathology such as glycosylation of hemoglobin resulting in HbA1c type I diabetes.273 A long line of evidence suggests that measurement of the intact protein provides some information, and measurement of one of these subforms of a protein provides different and more pathologically-relevant information. These have been carefully described for C-reactive protein (CRP),274 type I and XVI collagens,275,276 osteocalcin,277 and a selection of other analytes. Thus, it is becoming increasingly clear that measurement of a given pathologically-modified protein enables refinement of clinical chemistry and diagnostic procedures. Most likely the best example is hemoglobin, in which the modification of glycosylation is the gold standard marker of diabetes; thus, the intact protein is a necessity of life, whereas the PTM-modified protein is a pathology-specific marker.
As will later be described in larger molecular detail, many amino acids are amenable to specific modifications (citrullinations, phosphorylations, acetylations, methylations, nitrosylations, and glycosylations17). These modifications can have both positive and negative impacts on the function of the protein, and even target the protein for degradation. In addition, many proteins are born with a propeptide that needs to be cleaved before the protein is in the active configuration that being enzymatic activity or structural enablement. Both N- and C-terminal propeptides are present, which may be further modified, and thus is a waste underestimation of the complexity of these peptides. The degradation products of proteins may be the specific action of pathological-specific enzymes, and there is an accumulating amount of evidence suggesting that different fragments of the same protein may have different physiological and pathophysiological meanings.278 Lastly, polymerization may both be understood as aggregates of the same protein such as hyperphosphorylated Tau or cross-linked collagens, but also pentameric CRP. Each of these subpools obviously holds unique information.
Cross-linking
Cross-linking plays an important role in the ECM meshwork and thereby in tissue integrity. Cross-linking between different ECM components or between different protein chains can result from enzymatic and nonenzymatic pathways. Enzymatic cross-linking is often processed by members of the LOX enzyme family, whose members have been shown to promote the linearization of interstitial collagens which stiffen the tissues, thus leading to neoplastic progression of tumor cells.280–283 Interestingly, this matrix stiffness was associated with different phenotypes and enhanced mechanoresponsiveness of the epithelium.280,281 Therefore, cross-linking plays an important part in both the initiation and progression of metastasis. Similarly, in fibrotic disease, increases in tissue tension mediated by cross-linking can lead to activation of TGF-β signaling from the latency-associated complex and other signaling changes, driving a fibrogenic feed-forward loop.
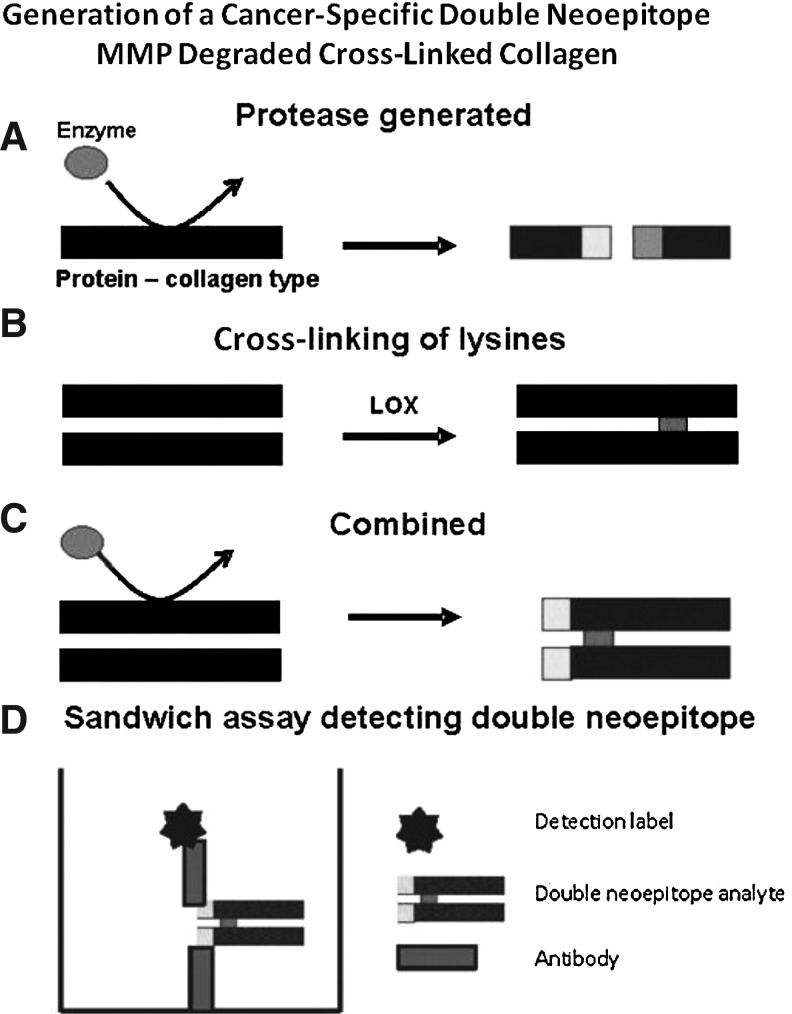
Valuable assays for evaluation of bone- and cartilage-related diseases have been developed using antibodies highly specific for protease-cleaved sites in type I collagen276 and type II collagen,284 respectively. The antibodies in these assays also assess the cross-linking between the lysines in the epitopes. C-terminal telopeptide of type I collagen (CTX-I) is an 8-amino acid fragment from the C-telopeptide of type I collagen generated by cathepsin K activity, and the rate of its release from bone is a useful reflection of the resorbing activity of osteoclasts.285 Measuring this fragment is useful for the evaluation of treatment efficacy in bone diseases such as osteoporosis.286 The CTX epitope contains an aspartylglycine motif (DG) that is prone to spontaneous isomerization. In other words, EKAHD(α)GGR epitopes are released during degradation of newly synthesized type I collagen, whereas EKAHD(β)GGR epitopes are released from matured type I collagen. It has been established that the α/β ratio is a useful measure of the age of bone tissue;287–289 the lower the ratio, the older the bone tissue.290 Further, the lysine residue of the CTX residue is cross-linked. Figure 4 outlines schematically how assessment of both a cross-linked and cathepsin K-degraded epitope may be undertaken through the use of sandwich enzyme-linked immunosorbent assay (ELISA) technology. Additional ECM assays may be constructed by a similar approach, to include as much possible information of protein subtype as possible. Resorption rates of newly synthesized collagen type I can be assessed by specific immunoassays targeting the detection of αCTX in urine samples.291 Degradation rate of matured, isomerized collagen can be estimated by another specific assay targeting βCTX in both urine and serum samples.276
Fig. 4.
Development of an assay to detect a cancer-specific double neoepitope. (A) An enzyme, most likely an MMP, cleaves collagen molecules. This produces a cut in the peptide sequence, exposing an N- and C-terminal-truncated molecule. (B) Lysil oxidase family members are highly upregulated in many cancers. This family of enzymes enzymatically cross-links the lysines in the collagen chains, resulting in stiffened tissue. In the local area of cancer metastasis and growth, these processes are occurring at a more rapid pace than in other parts of the body, resulting in increased expression of a range of collagen, proteases, and other enzymes. (C) The processes of protease generation and lysine cross-linking are combined. (D) Design and generation of a sandwich assay to detect both the lysine cross-link and the protease-generated degradation product. Thus, this type of ELISA contains more information than traditional assays (i.e., both degradation and cross-link information).
Another way of cross-linking is through the actions of tissue transglutaminases (TGs). They play a fundamental role in tissue stabilization by transamidation of glutamine residues of one protein chain to the amino group of a lysine residue in a second protein chain. This results in the formation of the covalent N-γ-glutaminyl-ɛ-lysyl-isopeptide bond, which is resistant to proteolytic degradation.292 As several ECM proteins, such as collagens, fibronectin, laminin, and vimentin, act as substrates for TGs, they are involved in physiologic tissue integrity while being associated with various pathologies, including neurodegenerative diseases, cancer, inflammation, and fibrosis. In fibrosis, TGF-β promotes activation of TG cross-linking, thereby reducing the ECM turnover, leading to deposition and accumulation of ECM proteins, and thus stabilizing the ECM network and facilitating proteolytic resistance. In cancer, intracellular cross-linking by TG2 has been shown to be both pro- and antiapoptotic, and favoring cell survival, invasion, and motility by the close association with surface integrins.293,294
Oxidations and hydroxylations
Oxidative damage to proteins is often caused by the action of free radicals, reactive oxygen species (ROS) and reactive nitrogen species such as hydrogen peroxide and nitric oxide, generated in cells by the mitochondrial respiratory chain.295 Oxidizing PTMs have been implicated in several pathological and healthy tissue turnover processes. Although many amino acids can be attacked by ROS, some seem more likely to undergo oxidation than others. For example, lysine and proline are readily oxidized to aldehydes; methionine is sulfoxidized; and tyrosines are nitrosylated.296 Under normal conditions, these ROS are strictly regulated by antioxidants, such as peroxidases and dimutases among others.297 However, under pathological conditions, oxidation may be implicated in tissue destruction. The role of ROS in almost all aspects of cancer initiation and development139,295,298–303 is still debated. Measurement of specific components of the ECM that hold these oxidized PTMs may be useful for both early diagnosis and prognosis of cancer.
Protease-generated neoepitopes
Matrix remodeling at specific disease stages results in both elevated levels of, and uniquely modified, proteins. Endopeptidases, such as MMPs and cysteine proteases, play major roles in the degradation of extracellular macromolecules such as collagens and proteoglycans. Specific proteolytic activities are a prerequisite for a range of cellular functions and interactions with the ECM, resulting in the generation of specific cleavage fragments. Even though many components of the ECM, as well as enzymes responsible for remodeling, are present in different tissues, the combination of a specific peptidase and specific ECM protein may provide a unique combination that elucidates activity in a particular tissue or a specific disease mechanism.
One often-taught example of protease degradation of a given tissue is that of joint degenerative diseases. Joint degenerative diseases lead to alterations in the metabolism of the articular cartilage and subchondral bone.278,304–309 Cartilage is for the most part composed of collagen type II, which accounts for 60%–70% of the dry weight of cartilage, and proteoglycans accounting for 10% of the dry weight, of which aggrecan is the most abundant.310 Since type II collagen is the most abundant protein in cartilage, several different degradation fragments of collagen type II have been indicated as useful for monitoring degenerative diseases of the cartilage.272,311 C-terminal telopeptide of type II collagen (CTX-II) is an MMP-generated neoepitope derived from the C-terminal part of type II collagen,310 and measurement of CTX-II is highly useful for monitoring degradation of type II collagen in experimental setups assessing cartilage degradation.278,312 Examples of a range of protease-generated neoepitopes have already been described in the literature, but they have not been utilized by applied science to produce quantifiable methods of disease assessment. Assays detecting a few neoepitopes that have been developed and that are used in both clinical and preclinical studies were reviewed recently.313
To some extent, C-terminal telopeptide of type I collagen (ICTP) and MMP-derived fragments of type I collagen assays53,54,314–316 as an indicator of cancer progression have been developed and used in prognosis of lung and ovarian cancers. A range of biochemical markers based on degradation products of the ECM, particularly collagen, may be identified and used in cancer. The collagen composition of the BM and interstitial matrix may be relevant for the development of the given marker for the ECMR associated with soft tissue metastasis.
Isomerization: Advanced glycation end product of ECM proteins
Proteins containing aspartate (D), asparagine (N), glutamate (E), or glutamine (Q) residues linked to a low–molecular-weight amino acid, such as glycine (G), can undergo spontaneous nonenzymatic isomerization.15 This isomerization introduces a kink in the conformation of the molecule, as the peptide backbone is redirected from the γ-carboxyl group in the native newly synthesized form to the side chain γ-carboxyl.290 Peptides that contain amino acid isomerizations are often resistant to proteolysis.317,318 This feature affects the processing of antigens for presentation on the major histocompatibility complex II during the immune response signaling for the production of T-cells and antibodies.15 In preclinical studies, it has been shown that various known autoantigens contain sites prone to deamidation and isomerization. These autoantigens are involved in type I diabetes, rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), and experimental autoimmune encephalomyelitis.317,319–322
The C-telopeptide of type I collagen marker CTX-I is a marker of bone resorption. It has been shown that assessment of the nonisomerized epitope (αCTX-I) is more sensitive as a marker for bone metastases secondary to breast and prostate cancer than the isomerized epitope (βCTX-I).207 This is due to the high ECMR of type I collagen in the bone area invaded by cancer cells, and thus a high amount of newly formed nonisomerized collagen type I undergoes resorption by osteoclasts.
Nonenzymatic glycosylation
Nonenzymatic glycosylation is also called the Maillard reaction, and leads to PTMs of proteins, nucleic acids, and lipids.273 A common cause of nonenzymatic glycosylation is increased blood glucose levels, and accordingly, most knowledge about nonenzymatic glycosylation arises from studies performed in diabetics.273 The marker HbA1c is an established PTM marker in type II diabetes. Recently, advanced glycation end products (AGEs) have been implicated in cancers. The nicotine-induced accumulation of AGEs is a cause of cancer.323 The receptor for AGEs, called RAGE, is currently under intense investigation as both a marker and an inducer of cancer324 and to assess whether there is a link between chronic inflammation and cancer, since inflammatory mediators can both be pro- and antitumorigenic.139,216,324,325
Citrullination
Citrullination or deimination is the term used for the PTM of the amino acid arginine, which can transform into the amino acid citrulline. The change is facilitated by peptidylarginine deiminases (PADs).326,327 The conversion of arginine into citrulline can have important consequences for the structure and function of proteins, since arginine is positively charged at a neutral pH, whereas citrulline is uncharged. The positive charge increases the hydrophobicity of the protein, leading to changes in protein folding.
Histone deacetylase 1 (HDAC1) inhibitors are currently under development for the treatment of certain cancers, particularly breast cancer.21 Histone lysine and arginine residues contain a wide array of PTM-producing processes, including methylation, citrullination, acetylation, ubquitination, and sumoylation. The combined action of these modifications regulates critical DNA processes, including replication, repair, and transcription. In addition, enzymes that modify histone lysine and arginine residues have been correlated with not only cancer but also arthritis, heart disease, diabetes, and neurodegenerative disorders.328,329
Histone methylation plays a key role in regulating the chromatin structure and function. The recent identification of enzymes that antagonize or remove histone methylation offers new insights into histone methylation plasticity in the regulation of epigenetic pathways. Peptidylarginine deiminase 4 (PADI4; also known as PAD4) was the first enzyme shown to antagonize histone methylation. PADI4 functions as a histone deiminase, converting a methylarginine residue to citrulline at specific sites on the tails of histones H3 and H4. PADI4 associates with HDAC1.328–330
Novel Techniques Currently Available for Assessing the Structure of the ECM
Clinical biochemistry provides a battery of assessments for profiling tissue turnover profiles. A range of serological assessments have been developed to investigate some of the key structural proteins of the ECM (Table 3).56,60,129,144,196–200,217,274,276,278,311,314,331–347 Measurement of these proteins may provide key information in clinical settings on the tissue turnover profile, and thereby assists in patient diagnosis, in identification of those patients in most need of treatment, and finally, in monitoring of clinical efficacy of interventions. These technologies may also be used in preclinical settings, in ex vivo and in vitro cultures, to determine the molecular mode of action in the assembly and maintenance of the matrix.311
Table 3.
Currently Available Serological Markers Assessing the Structure of the Extracellular Matrix
| Name of protein fragment | ECM component | Reference |
|---|---|---|
| C1M | MMP-mediated type I collagen degradation | 198 |
| C2M | MMP-mediated type II collagen degradation | 56 |
| C3M | MMP-mediated type III collagen degradation | 60 |
| C4M | MMP-mediated type IV collagen degradation | 197 |
| C5M | MMP-mediated type V collagen degradation | 331 |
| C6M | MMP-mediated type VI collagen degradation | 200 |
| P1NP | Type I collagen formation in tissues other than bone | 144 |
| P4NP 7S | Type IV collagen formation | 196 |
| P5CP | Type V collagen formation | 199 |
| PIIANP | Type II collagen formation | 332,333 |
| PIIINP | Type III collagen formation | 334 |
| VICM | MMP-mediated citrullinated vimentin degradation | 335 |
| CRPM | MMP-mediated CRP degradation | 274 |
| ELM | MMP-mediated elastin degradation | 217 |
| BGM | MMP-mediated biglycan degradation | 336 |
| MIM | MMP-mediated mimican degradation | 129 |
| VCANM | MMP-mediated versican degradation | 337 |
| TIM | MMP-mediated titin degradation | 338 |
| Aggrecan | MMP- and aggrenase-cleaved aggrecan | 278 |
| COMP | Intact COMP | 339 |
| Osteocalcin | Intact osteocalcin bone formation | 340 |
| HA | Hyalonic acid | 334 |
| ICTP | MMP-mediated type I collagen destruction | 314 |
| CTX-I | Cathepsin K degraded type I collagen | 276,311,341,342 |
| CTX-II | MMP-degraded type II collagen | 343,344 |
| C2C | MMP-degraded type II collagen | 345 |
| TELO-I | Citrullinated carboxyterminal telopeptides of type I collagen | 346 |
| TELO-II | Citrullinated carboxyterminal telopeptides of type II collagen | 346 |
| MCV | Mutated citrullinated vimentin | 347 |
ECM, extracellular matrix; MMP, matrix metalloproteinase; CRP, C-reactive protein.
An example of a combined aged, cross-linked, and cleaved neoepitope for the evaluation of bone metastases
The relationship between skeletal tumor load and elevations in serum or urine levels of αCTX and seven other biomarkers related to bone turnover has been investigated in a pooled group of breast and prostate cancer patients.207 Patients were stratified according to the Soloway score:
• Score 0: 0 bone metastases
• Score 1: <6 bone metastases
• Score 2: 6–20 bone metastases
• Score 3: >20 bone metastases
• Score 4: superscan showing that >75% ribs, vertebrae, and pelvic bone are affected.
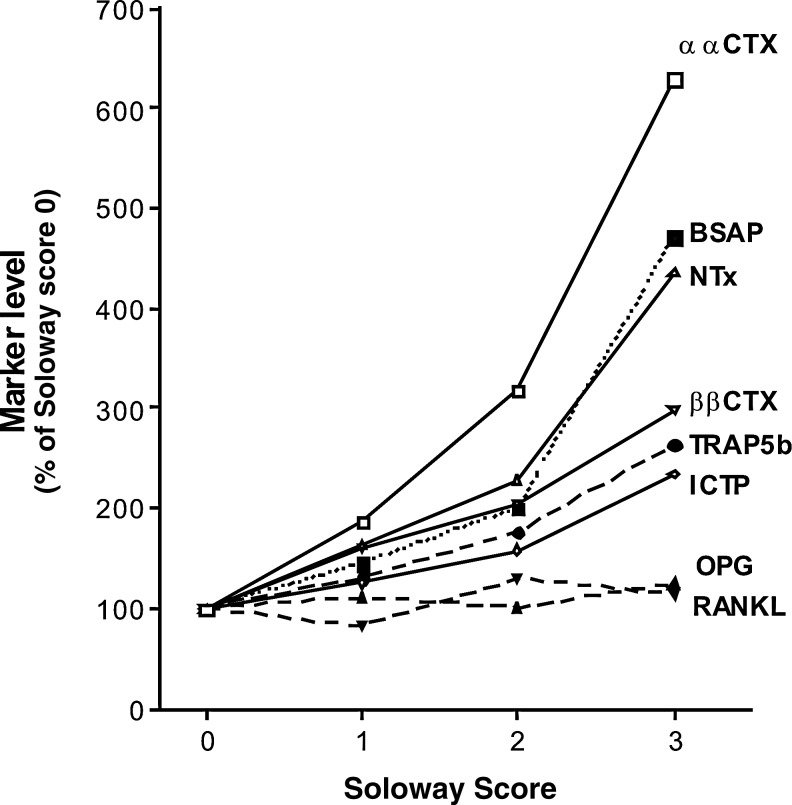
In breast cancer patients, a strong linear association was observed between bone metastases and all biomarkers, except osteoprotegerin and receptor activator of nuclear factor κB ligand (Figure 5). All six remaining markers were significantly elevated in patients with a Soloway score of 1. The relative percent increases in biomarker levels in the presence of bone metastases were most pronounced for αCTX-I, which was elevated by more than 600% in patients with Soloway score of 3. The next highest increases were in bone-specific alkaline phosphatase and N-telopeptide of type I collagen (NTX), which were elevated by 470% and 440% at Soloway score 3, respectively. These findings were supported by observations in prostate cancer patients, which showed that of the seven biomarkers, αCTX-I was the most sensitive for bone metastases.348 The higher sensitivity of αCTX-I could be explained by the fact that this epitope is released from sites of high bone remodeling, where collagen fibrils do not have time to mature and undergo β-isomerization. The αCTX-1 epitope was located by immunostaining adjacent sections of bones invaded by breast cancer or prostate cancer,208 and at the sites of high bone remodeling.
Fig. 5.
Relative increases in bone resorption, bone formation, and osteoclastogenesis marker levels as a function of the extent of skeletal metastasis, assessed in 132 patients with breast or prostate cancer. Relative increases are expressed as a percentage of levels in patients with a Soloway score 0.207
These data support that careful selection of matrix constituents and, in particular, those that carry one or more PTMs such as isomerization in a type I collagen fragment generated by cathepsin K as described in this example may be superior markers reflecting pathological, including malignant, events in the ECM.
An example of MMP-degraded type III collagen for the assessment of liver fibrosis
The central pathological change in fibrosis is uncontrolled ECMR.349,350 During fibrogenesis, the quantity and composition of matrix proteins in the liver change, resulting in excessive accumulation of fibrous (scar) tissue and an overall increase in the ECM density.351 ECM matrix proteins in a normal liver are distributed mainly in the portal tracts, whereas a BM-like matrix is located in the perisinusoidal space of Disse. The most abundant collagens in the liver are type I and III collagens, which by immunohistochemistry are found predominantly in the perisinusoidal spaces, in portal tracts, and in subcapsular areas.5,352 The ECM of the cirrhotic liver contains approximately six times as much matrix as the normal liver,353 which is a result of increased levels of type I, III, and IV collagens.354 However, levels of MMPs such as MMP-9 also increase in cirrhosis.349,355 The combination of active and overexpressed MMP-9 with the accumulation of type III collagen poses the interesting hypothesis that an MMP-9-generated fragment of type III collagen could be used as a biochemical marker of liver fibrosis.
Type III collagen degradation by MMPs, and even MMP-9 exclusively, may result in many unique fragments, such as those derived from type II collagen and previously published.81 The CO3-610 (C3M) fragment (KNGETGPQGP) is one of those, and is exclusively derived from MMP-9. When this C3M fragment was assessed in two separate animal models of liver fibrosis, the BDL and CCl4 animal models, a >200% fold upregulation was observed, as well as a highly significant correlation to portal pressure.60,356,357 These data strongly suggest that liver fibrosis is not merely an accumulation of ECM proteins, but a dynamic condition with accelerated ECM turnover, in which both tissue formation and tissue degradation are highly upregulated. In the case of liver fibrosis, ECM tissue formation outstrips tissue degradation, leading to a net accumulation of scar tissue over time. This example also suggests that PTMs released by protease degradation of proteins may in some cases be more sensitive markers for pathology than intact proteins. This idea is receiving increased attention.17 This approach has been recently been described as the protein fingerprint technology, in which the different subpools of the total pool of information about one protein during formation or degradation provide distinct and important data.20
PTMs: The Cause or Consequence of the Disease?
Proteins are complex molecules susceptible to numerous PTMs occurring spontaneously during aging or as a consequence of physiologic or pathologic processes. Today, it is well established that PTMs can uncover cryptic epitopes and/or create novel epitopes, to which no tolerance exists.15 Antigenicity and interactions of proteins with components of the immune system may be profoundly affected by PTMs. Thus, modified self-antigens may be absent (indicating they are not tolerated) during early T-cell selection, and trigger reactions by the immune system as they arise later in life. In turn, this may play a role in the initiation and pathogenesis of autoimmune diseases.15 Several studies have shown that various types of PTMs are hallmarks of aging and are associated with autoimmune diseases, such as RA, SLE, and type I diabetes.15,16,358–375
The presence of PTMs in several known autoantigens has been reported. Many of these modifications have been implicated in the antigenicity of the proteins, as outlined in Table 4 (modified from Cloos and Christgau15).296,311,319–322,346,347,376–389 These observations have sparked a growing interest in the role and assessment of PTMs in autoimmune diseases as well as other pathological conditions associated with aging. Whether the presence of PTMs is merely a secondary phenomenon accompanying the disease or a primary event in disease initiation remains to be resolved.
Table 4.
List of Post-Translational Modifications Involved in Immune Responses in Different Autoimmune Diseases
| Autoantigen | Relevant disease/animal model | Modification | Reference |
|---|---|---|---|
| MBP | MS/EAE | Acetylation | 376 |
| Citrullination | 377 | ||
| Isomerization | 296 | ||
| Phosphorylation | 378 | ||
| aB-crystallin | MS/EAE | Citrullination | 379,380 |
| Isomerization | 321 | ||
| Phosphorylation | 381 | ||
| Type I collagen | RA | Citrullination | 346 |
| Type II collagen | RA/CIA | Glycosylation | 382 |
| Protease degradation | 311 | ||
| Hydroxylation | 382 | ||
| Citrullination | 346 | ||
| Fibrin | RA | Citrullination | 383 |
| Fillagrin | RA | Citrullination | 384 |
| Vimentin | RA | Citrullination | 347,385 |
| IgG | RA | Isomerization | 296 |
| Glycation | 386 | ||
| Insulin | Type I diabetes | Deamidation | 319 |
| Isomerization | 319 | ||
| GAD | Type I diabetes | Oxidative damage | 387 |
| Histone H2B | SLE | Isomerization | 322 |
| Deamidation | 388 | ||
| Transglutamination | 388 | ||
| SnRNP D | SLE | Isomerization | 320 |
| SnRNP 70k | SLE | Phosphorylation | 389 |
MS, multiple sclerosis; EAE, experimental autoimmune ecephalomyelitis; RA, rheumatoid arthritis; CIA, collagen-induced arthritis; SLE, systemic lupus erythematosus.
It is noteworthy that T-cell responses to modified antigens in general are very specific.390,391 In contrast, autoantibodies recognizing modified proteins tend to be more nonspecific and often cross-react with the native antigen. This B-cell promiscuity may play an important role in the phenomenon of epitope-spreading characteristics of many autoimmune diseases,392 which in part may be the disease driver in illnesses such as RA. These examples serve to highlight that in the immune system, PTMs, in various ways, may initiate, play parts in the pathogenesis, or even constitute the central events in some diseases. Regardless of whether PTMs are the chicken or the egg, these examples further emphasize that PTMs are relevant markers of diseases. Tools developed to measure specific monoclononal antibodies may aid the understanding of the temporal events leading to PTMs, and their role in disease mechanisms.
Future Directions
In this review, we have described the key components of the ECM and highlighted recent developments in the identification and measurement of PTMs. There is a growing body of evidence that modifications made to the structural proteins of the matrix may both be a consequence of the disease as well as drivers of disease progression. Thus, PTMs to specific ECM proteins may be more integrated in pathogenesis than previously thought. Indeed, the matrix serves as much more than just a structural framework for tissues.
Fibrosis and cancer involve signature proteins and enzymes. These enzymes degrade the ECM and create a range of other PTMs, releasing smaller fragments of ECM proteins into the circulation. An optimal biochemical marker may be designed by identifying the common denominator of specific pathophysiological processes to determine the marker's tissue specificity and sensitivity. Different cells of a particular tissue predominately express given proteases that in combination with different signature proteins from different host tissues, which may provide optimal selective markers for connective tissue diseases. Biochemical markers based on the advanced disease/tissue neoepitope approach could become an important tool to be used in combination with others for diagnosing and staging disease as well as assessing efficacy and safety of new therapeutic interventions.
Abbreviations
- 2-D
two-dimensional
- 3-D
three-dimensional
- AASLD
American Association for the Study of Liver Disease
- AGE
advanced glycation/glycosylation end product
- BM
basement membrane
- BSAP
bone-specific alkaline phosphatase
- CIA
collagen-induced arthritis
- CL
cutis laxa
- COMP
cartilage oligomeric matrix protein
- CRP
C-reactive protein
- CTX-I
cross-linked C-terminal telopeptide of type I collagen
- CTX-II
cross-linked C-terminal telopeptide of type II collagen
- DDR
discoidin domain receptors
- EAE
experimental autoimmune encephalomyelitis
- ECM
extracellular matrix
- ECMR
ECM remodeling
- ELISA
enzyme-linked immunosorbent assay
- EMT
epithelial-to-mesenchymal transition
- GAG
glycosaminoglycan
- hMFB
hepatic myofibroblast-like cells
- HDAC1
histone deacetylase 1
- HSCs
hepatic stellate cells
- ICTP
C-terminal telopeptide of type I collagen
- LN
laminin N-terminal
- LOX
lysyl oxidase
- MED
multiple epiphyseal dysplasia
- MHC
major histocompatibility complex
- MMP
matrix metalloproteinase
- MS
multiple sclerosis
- NO
nitrogen oxide
- NTX
N-telopeptide of type I collagen
- PADs
peptidylarginine deiminases
- PDGF
platelet-derived growth factor
- PIIANP
type IIA procollagen N-terminal peptide
- PRELP
proline arginine-rich end leucine-rich repeat protein
- PSACH
pseudoachondroplasia
- PTM
post-translational modifications
- RA
rheumatoid arthritis
- RAGE
receptor for AGEs
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- SLE
systemic lupus erythematosus
- SLRPs
small leucine-rich proteoglycans
- SMCD
Schmid type metaphyseal chondrodysplasia
- SMD
spondylometaphyseal dysplasia
- SVAS
supravalvular aortic stenosis
- TG
transglutaminase
- TGF
transforming growth factor
- TNF
tumor necrosis factor
- WBS
William-Beuren syndrome
Acknowledgments
We acknowledge funding from the Danish Ministry of Science, Technology, and Science, and the Danish Science Foundation (Den Danske Forskningsfond).
Disclosure Statement
No competing financial interests exist.
References
- 1.Dolberg DS. Bissell MJ. Inability of Rous sarcoma virus to cause sarcomas in the avian embryo. Nature. 1984;309:552–556. doi: 10.1038/309552a0. [DOI] [PubMed] [Google Scholar]
- 2.Mintz B. Illmensee K. Normal genetically mosaic mice produced from malignant teratocarcinoma cells. Proc Natl Acad Sci USA. 1975;72:3585–3589. doi: 10.1073/pnas.72.9.3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ruiz PA. Jarai G. Discoidin domain receptors regulate the migration of primary human lung fibroblasts through collagen matrices. Fibrogenesis Tissue Repair. 2012;5:3. doi: 10.1186/1755-1536-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ingber DE. Can cancer be reversed by engineering the tumor microenvironment? Semin Cancer Biol. 2008;18:356–364. doi: 10.1016/j.semcancer.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schuppan D. Ruehl M. Somasundaram R. Hahn EG. Matrix as a modulator of hepatic fibrogenesis. Semin Liver Dis. 2001;21:351–372. doi: 10.1055/s-2001-17556. [DOI] [PubMed] [Google Scholar]
- 6.Condeelis J. Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, metastasis. Cell. 2006;124:263–266. doi: 10.1016/j.cell.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 7.Ingman WV. Wyckoff J. Gouon-Evans V. Condeelis J. Pollard JW. Macrophages promote collagen fibrillogenesis around terminal end buds of the developing mammary gland. Dev Dyn. 2006;235:3222–3229. doi: 10.1002/dvdy.20972. [DOI] [PubMed] [Google Scholar]
- 8.Barker HE. Erler JT. The potential for LOXL2 as a target for future cancer treatment. Future Oncol. 2011;7:707–710. doi: 10.2217/fon.11.46. [DOI] [PubMed] [Google Scholar]
- 9.Moreno-Bueno G. Salvador F. Martin A, et al. Lysyl oxidase-like 2 (LOXL2), a new regulator of cell polarity required for metastatic dissemination of basal-like breast carcinomas. EMBO Mol Med. 2011;3:528–544. doi: 10.1002/emmm.201100156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bignon M. Pichol-Thievend C. Hardouin J, et al. Lysyl oxidase-like protein-2 regulates sprouting angiogenesis and type IV collagen assembly in the endothelial basement membrane. Blood. 2011;118:3979–3989. doi: 10.1182/blood-2010-10-313296. [DOI] [PubMed] [Google Scholar]
- 11.Rodriguez HM. Vaysberg M. Mikels A, et al. Modulation of lysyl oxidase-like 2 enzymatic activity by an allosteric antibody inhibitor. J Biol Chem. 2010;285:20964–20974. doi: 10.1074/jbc.M109.094136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim YM. Kim EC. Kim Y. The human lysyl oxidase-like 2 protein functions as an amine oxidase toward collagen and elastin. Mol Biol Rep. 2011;38:145–149. doi: 10.1007/s11033-010-0088-0. [DOI] [PubMed] [Google Scholar]
- 13.Peng L. Ran YL. Hu H, et al. Secreted LOXL2 is a novel therapeutic target that promotes gastric cancer metastasis via the Src/FAK pathway. Carcinogenesis. 2009;30:1660–1669. doi: 10.1093/carcin/bgp178. [DOI] [PubMed] [Google Scholar]
- 14.Barry-Hamilton V. Spangler R. Marshall D, et al. Allosteric inhibition of lysyl oxidase-like-2 impedes the development of a pathologic microenvironment. Nat Med. 2010;16:1009–1017. doi: 10.1038/nm.2208. [DOI] [PubMed] [Google Scholar]
- 15.Cloos PA. Christgau S. Post-translational modifications of proteins: implications for aging, antigen recognition, and autoimmunity. Biogerontology. 2004;5:139–158. doi: 10.1023/B:BGEN.0000031152.31352.8b. [DOI] [PubMed] [Google Scholar]
- 16.Cloos PA. Jensen AL. Age-related de-phosphorylation of proteins in dentin: a biological tool for assessment of protein age. Biogerontology. 2000;1:341–356. doi: 10.1023/a:1026534400435. [DOI] [PubMed] [Google Scholar]
- 17.Karsdal MA. Henriksen K. Leeming DJ, et al. Novel combinations of Post-Translational Modification (PTM) neo-epitopes provide tissue-specific biochemical markers—are they the cause or the consequence of the disease? Clin Biochem. 2010;43:793–804. doi: 10.1016/j.clinbiochem.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 18.Colado MI. Ormazabal MJ. Goicoechea C, et al. Involvement of central serotonergic pathways in analgesia elicited by salmon calcitonin in the mouse. Eur J Pharmacol. 1994;252:291–297. doi: 10.1016/0014-2999(94)90175-9. [DOI] [PubMed] [Google Scholar]
- 19.Skjot-Arkil H. Barascuk N. Larsen L, et al. Tumor necrosis factor-alpha and receptor activator of nuclear factor-kappaB ligand augment human macrophage foam-cell destruction of extracellular matrix through protease-mediated processes. Assay Drug Dev Technol. 2012;10:69–77. doi: 10.1089/adt.2010.0366. [DOI] [PubMed] [Google Scholar]
- 20.Karsdal MA. Delvin E. Christiansen C. Protein fingerprints—relying on and understanding the information of serological protein measurements. Clin Biochem. 2011;44:1278–1279. doi: 10.1016/j.clinbiochem.2011.08.1135. [DOI] [PubMed] [Google Scholar]
- 21.Krueger KE. Srivastava S. Posttranslational protein modifications: current implications for cancer detection, prevention, and therapeutics. Mol Cell Proteomics. 2006;5:1799–1810. doi: 10.1074/mcp.R600009-MCP200. [DOI] [PubMed] [Google Scholar]
- 22.Bosques CJ. Raguram S. Sasisekharan R. The sweet side of biomarker discovery. Nat Biotechnol. 2006;24:1100–1101. doi: 10.1038/nbt0906-1100. [DOI] [PubMed] [Google Scholar]
- 23.Hanash SM. Pitteri SJ. Faca VM. Mining the plasma proteome for cancer biomarkers. Nature. 2008;452:571–579. doi: 10.1038/nature06916. [DOI] [PubMed] [Google Scholar]
- 24.Marx J. Cancer research. Inflammation and cancer: the link grows stronger. Science. 2004;306:966–968. doi: 10.1126/science.306.5698.966. [DOI] [PubMed] [Google Scholar]
- 25.Sawyers CL. The cancer biomarker problem. Nature. 2008;452:548–552. doi: 10.1038/nature06913. [DOI] [PubMed] [Google Scholar]
- 26.Spickett CM. Pitt AR. Morrice N. Kolch W. Proteomic analysis of phosphorylation, oxidation and nitrosylation in signal transduction. Biochim Biophys Acta. 2006;1764:1823–1841. doi: 10.1016/j.bbapap.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 27.Aumailley M. Gayraud B. Structure and biological activity of the extracellular matrix. J Mol Med. 1998;76:253–265. doi: 10.1007/s001090050215. [DOI] [PubMed] [Google Scholar]
- 28.Yurchenco PD. Schittny JC. Molecular architecture of basement membranes. FASEB J. 1990;4:1577–1590. doi: 10.1096/fasebj.4.6.2180767. [DOI] [PubMed] [Google Scholar]
- 29.Bosman FT. Stamenkovic I. Functional structure and composition of the extracellular matrix. J Pathol. 2003;200:423–428. doi: 10.1002/path.1437. [DOI] [PubMed] [Google Scholar]
- 30.Bissell MJ. Aggeler J. Dynamic reciprocity: how do extracellular matrix and hormones direct gene expression? Prog Clin Biol Res. 1987;249:251–262. [PubMed] [Google Scholar]
- 31.Bissell MJ. Radisky D. Putting tumours in context. Nat Rev Cancer. 2001;1:46–54. doi: 10.1038/35094059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lochter A. Bissell MJ. An odyssey from breast to bone: multi-step control of mammary metastases and osteolysis by matrix metalloproteinases. APMIS. 1999;107:128–136. doi: 10.1111/j.1699-0463.1999.tb01535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Radisky DC. Bissell MJ. Cancer. Respect thy neighbor! Science. 2004;303:775–777. doi: 10.1126/science.1094412. [DOI] [PubMed] [Google Scholar]
- 34.Paszek MJ. Zahir N. Johnson KR, et al. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8:241–254. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 35.Karsdal MA. Larsen L. Engsig MT, et al. Matrix metalloproteinase-dependent activation of latent transforming growth factor-beta controls the conversion of osteoblasts into osteocytes by blocking osteoblast apoptosis. J Biol Chem. 2002;277:44061–44067. doi: 10.1074/jbc.M207205200. [DOI] [PubMed] [Google Scholar]
- 36.Haass NK. Smalley KS. Herlyn M. The role of altered cell-cell communication in melanoma progression. J Mol Histol. 2004;35:309–318. doi: 10.1023/b:hijo.0000032362.35354.bb. [DOI] [PubMed] [Google Scholar]
- 37.O'Brien LE. Jou TS. Pollack AL, et al. Rac1 orientates epithelial apical polarity through effects on basolateral laminin assembly. Nat Cell Biol. 2001;3:831–838. doi: 10.1038/ncb0901-831. [DOI] [PubMed] [Google Scholar]
- 38.Pedersen JA. Lichter S. Swartz MA. Cells in 3D matrices under interstitial flow: effects of extracellular matrix alignment on cell shear stress and drag forces. J Biomech. 2010;43:900–905. doi: 10.1016/j.jbiomech.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 39.Pedersen JA. Swartz MA. Mechanobiology in the third dimension. Ann Biomed Eng. 2005;33:1469–1490. doi: 10.1007/s10439-005-8159-4. [DOI] [PubMed] [Google Scholar]
- 40.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 41.Hynes RO. Structural biology. Changing partners. Science. 2003;300:755–756. doi: 10.1126/science.1084854. [DOI] [PubMed] [Google Scholar]
- 42.Hynes RO. The emergence of integrins: a personal and historical perspective. Matrix Biol. 2004;23:333–340. doi: 10.1016/j.matbio.2004.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hynes RO. Cell-matrix adhesion in vascular development. J Thromb Haemost. 2007;5(Suppl 1):32–40. doi: 10.1111/j.1538-7836.2007.02569.x. [DOI] [PubMed] [Google Scholar]
- 44.Hynes RO. The extracellular matrix: not just pretty fibrils. Science. 2009;326:1216–1219. doi: 10.1126/science.1176009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Clark EA. King WG. Brugge JS. Symons M. Hynes RO. Integrin-mediated signals regulated by members of the rho family of GTPases. J Cell Biol. 1998;142:573–586. doi: 10.1083/jcb.142.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Clark EA. Brugge JS. Integrins and signal transduction pathways: the road taken. Science. 1995;268:233–239. doi: 10.1126/science.7716514. [DOI] [PubMed] [Google Scholar]
- 47.Gelse K. Poschl E. Aigner T. Collagens—structure, function, and biosynthesis. Adv Drug Deliv Rev. 2003;55:1531–1546. doi: 10.1016/j.addr.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 48.Fleischmajer R. MacDonald ED. Perlish JS. Burgeson RE. Fisher LW. Dermal collagen fibrils are hybrids of type I and type III collagen molecules. J Struct Biol. 1990;105:162–169. doi: 10.1016/1047-8477(90)90110-x. [DOI] [PubMed] [Google Scholar]
- 49.Birk DE. Fitch JM. Babiarz JP. Linsenmayer TF. Collagen type I and type V are present in the same fibril in the avian corneal stroma. J Cell Biol. 1988;106:999–1008. doi: 10.1083/jcb.106.3.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jussila T. Kauppila S. Bode M, et al. Synthesis and maturation of type I and type III collagens in endometrial adenocarcinoma. Eur J Obstet Gynecol Reprod Biol. 2004;115:66–74. doi: 10.1016/S0301-2115(02)00406-2. [DOI] [PubMed] [Google Scholar]
- 51.Parikka V. Vaananen A. Risteli J, et al. Human mesenchymal stem cell derived osteoblasts degrade organic bone matrix in vitro by matrix metalloproteinases. Matrix Biol. 2005;24:438–447. doi: 10.1016/j.matbio.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 52.Santala M. Simojoki M. Risteli J. Risteli L. Kauppila A. Type I and III collagen metabolites as predictors of clinical outcome in epithelial ovarian cancer. Clin Cancer Res. 1999;5:4091–4096. [PubMed] [Google Scholar]
- 53.Ylisirnio S. Sassi ML. Risteli J. Turpeenniemi-Hujanen T. Jukkola A. Serum type I collagen degradation markers, ICTP and CrossLaps, are factors for poor survival in lung cancer. Anticancer Res. 1999;19:5577–5581. [PubMed] [Google Scholar]
- 54.Ylisirnio S. Hoyhtya M. Makitaro R. Paaakko P. Risteli J. Kinnula VL. Turpeenniemi-Hujanen T. Jukkola A. Elevated serum levels of type I collagen degradation marker ICTP and tissue inhibitor of metalloproteinase (TIMP) 1 are associated with poor prognosis in lung cancer. Clin Cancer Res. 2001;7:1633–1637. [PubMed] [Google Scholar]
- 55.Zhu GG. Risteli L. Makinen M. Risteli J. Kauppila A. Stenback F. Immunohistochemical study of type I collagen and type I pN-collagen in benign and malignant ovarian neoplasms. Cancer. 1995;75:1010–1017. doi: 10.1002/1097-0142(19950215)75:4<1010::aid-cncr2820750417>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 56.Bay-Jensen AC. Liu Q. Byrjalsen I. Li Y. Wang J. Pedersen C. Leeming DJ. Dam EB. Zheng Q. Qvist P, et al. Enzyme-linked immunosorbent assay (ELISAs) for metalloproteinase derived type II collagen neoepitope, CIIM—increased serum CIIM in subjects with severe radiographic osteoarthritis. Clin Biochem. 2011;44:423–429. doi: 10.1016/j.clinbiochem.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 57.Kadler KE. Holmes DF. Trotter JA. Chapman JA. Collagen fibril formation. Biochem J. 1996;316(Pt 1):1–11. doi: 10.1042/bj3160001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Barascuk N. Vassiliadis E. Larsen L, et al. Development and validation of an enzyme-linked immunosorbent assay for the quantification of a specific MMP-9 mediated degradation fragment of type III collagen—a novel biomarker of atherosclerotic plaque remodeling. Clin Biochem. 2011;44:900–906. doi: 10.1016/j.clinbiochem.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 59.Vassiliadis E. Larsen DV. Clausen RE, et al. Measurement of CO3-610, a potential liver biomarker derived from matrix metalloproteinase-9 degradation of collagen type III, in a rat model of reversible carbon-tetrachloride-induced fibrosis. Biomark Insights. 2011;6:49–58. doi: 10.4137/BMI.S6347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Barascuk N. Veidal SS. Larsen L, et al. A novel assay for extracellular matrix remodeling associated with liver fibrosis: an enzyme-linked immunosorbent assay (ELISA) for a MMP-9 proteolytically revealed neo-epitope of type III collagen. Clin Biochem. 2010;43:899–904. doi: 10.1016/j.clinbiochem.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 61.Attallah AM. Mosa TE. Omran MM, et al. Immunodetection of collagen types I, II, III, and IV for differentiation of liver fibrosis stages in patients with chronic HCV. J Immunoassay Immunochem. 2007;28:155–168. doi: 10.1080/15321810701212088. [DOI] [PubMed] [Google Scholar]
- 62.Kruegel J. Miosge N. Basement membrane components are key players in specialized extracellular matrices. Cell Mol Life Sci. 2010;67:2879–2895. doi: 10.1007/s00018-010-0367-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Birk DE. Type V collagen: heterotypic type I/V collagen interactions in the regulation of fibril assembly. Micron. 2001;32:223–237. doi: 10.1016/s0968-4328(00)00043-3. [DOI] [PubMed] [Google Scholar]
- 64.Patino MG. Neiders ME. Andreana S. Noble B. Cohen RE. Collagen: an overview. Implant Dent. 2002;11:280–285. doi: 10.1097/00008505-200207000-00014. [DOI] [PubMed] [Google Scholar]
- 65.Wenstrup RJ. Florer JB. Brunskill EW, et al. Type V collagen controls the initiation of collagen fibril assembly. J Biol Chem. 2004;279:53331–53337. doi: 10.1074/jbc.M409622200. [DOI] [PubMed] [Google Scholar]
- 66.Fessler LI. Brosh S. Chapin S. Fessler JH. Tyrosine sulfation in precursors of collagen V. J Biol Chem. 1986;261:5034–5040. [PubMed] [Google Scholar]
- 67.Engvall E. Hessle H. Klier G. Molecular assembly, secretion, and matrix deposition of type VI collagen. J Cell Biol. 1986;102:703–710. doi: 10.1083/jcb.102.3.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yanagishita M. Function of proteoglycans in the extracellular matrix. Acta Pathol Jpn. 1993;43:283–293. doi: 10.1111/j.1440-1827.1993.tb02569.x. [DOI] [PubMed] [Google Scholar]
- 69.Monfort J. Tardif G. Reboul P, et al. Degradation of small leucine-rich repeat proteoglycans by matrix metalloprotease-13: identification of a new biglycan cleavage site. Arthritis Res Ther. 2006;8:R26. doi: 10.1186/ar1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bock HC. Michaeli P. Bode C, et al. The small proteoglycans decorin and biglycan in human articular cartilage of late-stage osteoarthritis. Osteoarthritis Cartilage. 2001;9:654–663. doi: 10.1053/joca.2001.0420. [DOI] [PubMed] [Google Scholar]
- 71.Lu YP. Ishiwata T. Kawahara K, et al. Expression of lumican in human colorectal cancer cells. Pathol Int. 2002;52:519–526. doi: 10.1046/j.1440-1827.2002.01384.x. [DOI] [PubMed] [Google Scholar]
- 72.Merline R. Schaefer RM. Schaefer L. The matricellular functions of small leucine-rich proteoglycans (SLRPs) J Cell Commun Signal. 2009;3:323–335. doi: 10.1007/s12079-009-0066-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Duca L. Floquet N. Alix AJ. Haye B. Debelle L. Elastin as a matrikine. Crit Rev Oncol Hematol. 2004;49:235–244. doi: 10.1016/j.critrevonc.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 74.Senior RM. Griffin GL. Mecham RP, et al. Val-Gly-Val-Ala-Pro-Gly, a repeating peptide in elastin, is chemotactic for fibroblasts and monocytes. J Cell Biol. 1984;99:870–874. doi: 10.1083/jcb.99.3.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Good DJ. Polverini PJ. Rastinejad F, et al. A tumor suppressor-dependent inhibitor of angiogenesis is immunologically and functionally indistinguishable from a fragment of thrombospondin. Proc Natl Acad Sci USA. 1990;87:6624–6628. doi: 10.1073/pnas.87.17.6624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lowin T. Straub RH. Integrins and their ligands in rheumatoid arthritis. Arthritis Res Ther. 2011;13:244. doi: 10.1186/ar3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Asch AS. Barnwell J. Silverstein RL. Nachman RL. Isolation of the thrombospondin membrane receptor. J Clin Invest. 1987;79:1054–1061. doi: 10.1172/JCI112918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang XQ. Frazier WA. The thrombospondin receptor CD47 (IAP) modulates and associates with alpha2 beta1 integrin in vascular smooth muscle cells. Mol Biol Cell. 1998;9:865–874. doi: 10.1091/mbc.9.4.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pfister RR. Haddox JL. Sommers CI. Lam KW. Identification and synthesis of chemotactic tripeptides from alkali-degraded whole cornea. A study of N-acetyl-proline-glycine-proline and N-methyl-proline-glycine-proline. Invest Ophthalmol Vis Sci. 1995;36:1306–1316. [PubMed] [Google Scholar]
- 80.Weathington NM. van Houwelingen AH. Noerager BD, et al. A novel peptide CXCR ligand derived from extracellular matrix degradation during airway inflammation. Nat Med. 2006;12:317–323. doi: 10.1038/nm1361. [DOI] [PubMed] [Google Scholar]
- 81.Mundel TM. Kalluri R. Type IV collagen-derived angiogenesis inhibitors. Microvasc Res. 2007;74:85–89. doi: 10.1016/j.mvr.2007.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hamano Y. Zeisberg M. Sugimoto H, et al. Physiological levels of tumstatin, a fragment of collagen IV alpha3 chain, are generated by MMP-9 proteolysis and suppress angiogenesis via alphaV beta3 integrin. Cancer Cell. 2003;3:589–601. doi: 10.1016/s1535-6108(03)00133-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.O'Reilly MS. Boehm T. Shing Y, et al. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell. 1997;88:277–285. doi: 10.1016/s0092-8674(00)81848-6. [DOI] [PubMed] [Google Scholar]
- 84.Dhanabal M. Ramchandran R. Waterman MJ, et al. Endostatin induces endothelial cell apoptosis. J Biol Chem. 1999;274:11721–11726. doi: 10.1074/jbc.274.17.11721. [DOI] [PubMed] [Google Scholar]
- 85.Karumanchi SA. Jha V. Ramchandran R, et al. Cell surface glypicans are low-affinity endostatin receptors. Mol Cell. 2001;7:811–822. doi: 10.1016/s1097-2765(01)00225-8. [DOI] [PubMed] [Google Scholar]
- 86.Shi H. Huang Y. Zhou H, et al. Nucleolin is a receptor that mediates antiangiogenic and antitumor activity of endostatin. Blood. 2007;110:2899–2906. doi: 10.1182/blood-2007-01-064428. [DOI] [PubMed] [Google Scholar]
- 87.Ruoslahti E. RGD and other recognition sequences for integrins. Annu Rev Cell Dev Biol. 1996;12:697–715. doi: 10.1146/annurev.cellbio.12.1.697. [DOI] [PubMed] [Google Scholar]
- 88.Buckley CD. Pilling D. Henriquez NV, et al. RGD peptides induce apoptosis by direct caspase-3 activation. Nature. 1999;397:534–539. doi: 10.1038/17409. [DOI] [PubMed] [Google Scholar]
- 89.Mormone E. Lu Y. Ge X. Fiel MI. Nieto N. Fibromodulin, an oxidative stress-sensitive proteoglycan, regulates the fibrogenic response to liver injury in mice. Gastroenterology. 2012;142:612–621. doi: 10.1053/j.gastro.2011.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mydel P. Shipley JM. ir-Kirk TL, et al. Neutrophil elastase cleaves laminin-332 (laminin-5) generating peptides that are chemotactic for neutrophils. J Biol Chem. 2008;283:9513–9522. doi: 10.1074/jbc.M706239200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.ir-Kirk TL. Atkinson JJ. Broekelmann TJ, et al. A site on laminin alpha 5, AQARSAASKVKVSMKF, induces inflammatory cell production of matrix metalloproteinase-9 and chemotaxis. J Immunol. 2003;171:398–406. doi: 10.4049/jimmunol.171.1.398. [DOI] [PubMed] [Google Scholar]
- 92.Freitas VM. Vilas-Boas VF. Pimenta DC, et al. SIKVAV, a laminin alpha1-derived peptide, interacts with integrins and increases protease activity of a human salivary gland adenoid cystic carcinoma cell line through the ERK 1/2 signaling pathway. Am J Pathol. 2007;171:124–138. doi: 10.2353/ajpath.2007.051264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Song T. Choi CH. Cho YJ, et al. Expression of 67-kDa laminin receptor was associated with tumor progression and poor prognosis in epithelial ovarian cancer. Gynecol Oncol. 2012;125:427–432. doi: 10.1016/j.ygyno.2012.01.030. [DOI] [PubMed] [Google Scholar]
- 94.Di GC. Grottesi A. Lavecchia A. Conformational switch of a flexible loop in human laminin receptor determines laminin-1 interaction. Eur Biophys J. 2012;41:353–358. doi: 10.1007/s00249-012-0793-9. [DOI] [PubMed] [Google Scholar]
- 95.Wu F. Vij N. Roberts L, et al. A novel role of the lumican core protein in bacterial lipopolysaccharide-induced innate immune response. J Biol Chem. 2007;282:26409–26417. doi: 10.1074/jbc.M702402200. [DOI] [PubMed] [Google Scholar]
- 96.Funderburgh JL. Mitschler RR. Funderburgh ML, et al. Macrophage receptors for lumican. A corneal keratan sulfate proteoglycan. Invest Ophthalmol Vis Sci. 1997;38:1159–1167. [PubMed] [Google Scholar]
- 97.Carlson EC. Lin M. Liu CY, et al. Keratocan and lumican regulate neutrophil infiltration and corneal clarity in lipopolysaccharide-induced keratitis by direct interaction with CXCL1. J Biol Chem. 2007;282:35502–35509. doi: 10.1074/jbc.M705823200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Vij N. Roberts L. Joyce S. Chakravarti S. Lumican suppresses cell proliferation and aids Fas-Fas ligand mediated apoptosis: implications in the cornea. Exp Eye Res. 2004;78:957–971. doi: 10.1016/j.exer.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 99.Schaefer L. Babelova A. Kiss E, et al. The matrix component biglycan is proinflammatory and signals through Toll-like receptors 4 and 2 in macrophages. J Clin Invest. 2005;115:2223–2233. doi: 10.1172/JCI23755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Babelova A. Moreth K. Tsalastra-Greul W, et al. Biglycan, a danger signal that activates the NLRP3 inflammasome via toll-like and P2× receptors. J Biol Chem. 2009;284:24035–24048. doi: 10.1074/jbc.M109.014266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kitaya K. Yasuo T. Dermatan sulfate proteoglycan biglycan as a potential selectin L/CD44 ligand involved in selective recruitment of peripheral blood CD16(-) natural killer cells into human endometrium. J Leukoc Biol. 2009;85:391–400. doi: 10.1189/jlb.0908535. [DOI] [PubMed] [Google Scholar]
- 102.Sjoberg AP. Manderson GA. Morgelin M, et al. Short leucine-rich glycoproteins of the extracellular matrix display diverse patterns of complement interaction and activation. Mol Immunol. 2009;46:830–839. doi: 10.1016/j.molimm.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nili N. Cheema AN. Giordano FJ, et al. Decorin inhibition of PDGF-stimulated vascular smooth muscle cell function: potential mechanism for inhibition of intimal hyperplasia after balloon angioplasty. Am J Pathol. 2003;163:869–878. doi: 10.1016/S0002-9440(10)63447-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kolb M. Margetts PJ. Sime PJ. Gauldie J. Proteoglycans decorin and biglycan differentially modulate TGF-beta-mediated fibrotic responses in the lung. Am J Physiol Lung Cell Mol Physiol. 2001;280:L1327–L1334. doi: 10.1152/ajplung.2001.280.6.L1327. [DOI] [PubMed] [Google Scholar]
- 105.Tufvesson E. Westergren-Thorsson G. Tumour necrosis factor-alpha interacts with biglycan and decorin. FEBS Lett. 2002;530:124–128. doi: 10.1016/s0014-5793(02)03439-7. [DOI] [PubMed] [Google Scholar]
- 106.Guidetti GF. Bartolini B. Bernardi B, et al. Binding of von Willebrand factor to the small proteoglycan decorin. FEBS Lett. 2004;574:95–100. doi: 10.1016/j.febslet.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 107.Inkson CA. Ono M. Bi Y, et al. The potential functional interaction of biglycan and WISP-1 in controlling differentiation and proliferation of osteogenic cells. Cells Tissues Organs. 2009;189:153–157. doi: 10.1159/000151377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Moreno M. Munoz R. Aroca F, et al. Biglycan is a new extracellular component of the Chordin-BMP4 signaling pathway. EMBO J. 2005;24:1397–1405. doi: 10.1038/sj.emboj.7600615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chen XD. Fisher LW. Robey PG. Young MF. The small leucine-rich proteoglycan biglycan modulates BMP-4-induced osteoblast differentiation. FASEB J. 2004;18:948–958. doi: 10.1096/fj.03-0899com. [DOI] [PubMed] [Google Scholar]
- 110.Tufvesson E. Westergren-Thorsson G. Biglycan and decorin induce morphological and cytoskeletal changes involving signalling by the small GTPases RhoA and Rac1 resulting in lung fibroblast migration. J Cell Sci. 2003;116:4857–4864. doi: 10.1242/jcs.00808. [DOI] [PubMed] [Google Scholar]
- 111.Brandan E. Retamal C. Cabello-Verrugio C. Marzolo MP. The low density lipoprotein receptor-related protein functions as an endocytic receptor for decorin. J Biol Chem. 2006;281:31562–31571. doi: 10.1074/jbc.M602919200. [DOI] [PubMed] [Google Scholar]
- 112.Goldoni S. Humphries A. Nystrom A, et al. Decorin is a novel antagonistic ligand of the Met receptor. J Cell Biol. 2009;185:743–754. doi: 10.1083/jcb.200901129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Desnoyers L. Arnott D. Pennica D. WISP-1 binds to decorin and biglycan. J Biol Chem. 2001;276:47599–47607. doi: 10.1074/jbc.M108339200. [DOI] [PubMed] [Google Scholar]
- 114.Comalada M. Cardo M. Xaus J, et al. Decorin reverses the repressive effect of autocrine-produced TGF-beta on mouse macrophage activation. J Immunol. 2003;170:4450–4456. doi: 10.4049/jimmunol.170.9.4450. [DOI] [PubMed] [Google Scholar]
- 115.Schaefer L. Tsalastra W. Babelova A, et al. Decorin-mediated regulation of fibrillin-1 in the kidney involves the insulin-like growth factor-I receptor and Mammalian target of rapamycin. Am J Pathol. 2007;170:301–315. doi: 10.2353/ajpath.2007.060497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Seidler DG. Goldoni S. Agnew C, et al. Decorin protein core inhibits in vivo cancer growth and metabolism by hindering epidermal growth factor receptor function and triggering apoptosis via caspase-3 activation. J Biol Chem. 2006;281:26408–26418. doi: 10.1074/jbc.M602853200. [DOI] [PubMed] [Google Scholar]
- 117.Iacob D. Cai J. Tsonis M, et al. Decorin-mediated inhibition of proliferation and migration of the human trophoblast via different tyrosine kinase receptors. Endocrinology. 2008;149:6187–6197. doi: 10.1210/en.2008-0780. [DOI] [PubMed] [Google Scholar]
- 118.Fiedler LR. Schonherr E. Waddington R, et al. Decorin regulates endothelial cell motility on collagen I through activation of insulin-like growth factor I receptor and modulation of alpha2beta1 integrin activity. J Biol Chem. 2008;283:17406–17415. doi: 10.1074/jbc.M710025200. [DOI] [PubMed] [Google Scholar]
- 119.Wight TN. Merrilees MJ. Proteoglycans in atherosclerosis and restenosis: key roles for versican. Circ Res. 2004;94:1158–1167. doi: 10.1161/01.RES.0000126921.29919.51. [DOI] [PubMed] [Google Scholar]
- 120.Asplund A. Friden V. Stillemark-Billton P. Camejo G. Bondjers G. Macrophages exposed to hypoxia secrete proteoglycans for which LDL has higher affinity. Atherosclerosis. 2011;215:77–81. doi: 10.1016/j.atherosclerosis.2010.12.017. [DOI] [PubMed] [Google Scholar]
- 121.Melchior-Becker A. Dai G. Ding Z, et al. Deficiency of biglycan causes cardiac fibroblasts to differentiate into a myofibroblast phenotype. J Biol Chem. 2011;286:17365–17375. doi: 10.1074/jbc.M110.192682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Funderburgh JL. Corpuz LM. Roth MR, et al. Mimecan, the 25-kDa corneal keratan sulfate proteoglycan, is a product of the gene producing osteoglycin. J Biol Chem. 1997;272:28089–28095. doi: 10.1074/jbc.272.44.28089. [DOI] [PubMed] [Google Scholar]
- 123.Michelacci YM. Collagens and proteoglycans of the corneal extracellular matrix. Braz J Med Biol Res. 2003;36:1037–1046. doi: 10.1590/s0100-879x2003000800009. [DOI] [PubMed] [Google Scholar]
- 124.Wang X. Ford BC. Praul CA. Leach RM., Jr Characterization of the non-collagenous proteins in avian cortical and medullary bone. Comp Biochem Physiol B Biochem Mol Biol. 2005;140:665–672. doi: 10.1016/j.cbpc.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 125.Hopkinson A. McIntosh RS. Shanmuganathan V. Tighe PJ. Dua HS. Proteomic analysis of amniotic membrane prepared for human transplantation: characterization of proteins and clinical implications. J Proteome Res. 2006;5:2226–2235. doi: 10.1021/pr050425q. [DOI] [PubMed] [Google Scholar]
- 126.Zhen EY. Brittain IJ. Laska DA, et al. Characterization of metalloprotease cleavage products of human articular cartilage. Arthritis Rheum. 2008;58:2420–2431. doi: 10.1002/art.23654. [DOI] [PubMed] [Google Scholar]
- 127.Ma QY. Zuo CL. Ma JH, et al. Glucocorticoid up-regulates mimecan expression in corticotroph cells. Mol Cell Endocrinol. 2010;321:239–244. doi: 10.1016/j.mce.2010.02.021. [DOI] [PubMed] [Google Scholar]
- 128.Zheng CX. Zhao SX. Wang P, et al. Different expression of mimecan as a marker for differential diagnosis between NSCLC and SCLC. Oncol Rep. 2009;22:1057–1061. doi: 10.3892/or_00000536. [DOI] [PubMed] [Google Scholar]
- 129.Barascuk N. Vassiliadis E. Zheng Q, et al. Levels of circulating MMCN-151, a degradation product of mimecan, reflect pathological extracellular matrix remodeling in apolipoprotein E knockout mice. Biomark Insights. 2011;6:97–106. doi: 10.4137/BMI.S7777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Heathfield TF. Onnerfjord P. Dahlberg L. Heinegard D. Cleavage of fibromodulin in cartilage explants involves removal of the N-terminal tyrosine sulfate-rich region by proteolysis at a site that is sensitive to matrix metalloproteinase-13. J Biol Chem. 2004;279:6286–6295. doi: 10.1074/jbc.M307765200. [DOI] [PubMed] [Google Scholar]
- 131.Chakravarti S. Magnuson T. Lass JH, et al. Lumican regulates collagen fibril assembly: skin fragility and corneal opacity in the absence of lumican. J Cell Biol. 1998;141:1277–1286. doi: 10.1083/jcb.141.5.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Naito Z. Role of the small leucine-rich proteoglycan (SLRP) family in pathological lesions and cancer cell growth. J Nihon Med Sch. 2005;72:137–145. doi: 10.1272/jnms.72.137. [DOI] [PubMed] [Google Scholar]
- 133.Kaspar M. Zardi L. Neri D. Fibronectin as target for tumor therapy. Int J Cancer. 2006;118:1331–1339. doi: 10.1002/ijc.21677. [DOI] [PubMed] [Google Scholar]
- 134.Gallagher WM. Currid CA. Whelan LC. Fibulins and cancer: friend or foe? Trends Mol Med. 2005;11:336–340. doi: 10.1016/j.molmed.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 135.Camby I. Le MM. Lefranc F. Kiss R. Galectin-1: a small protein with major functions. Glycobiology. 2006;16:137R–157R. doi: 10.1093/glycob/cwl025. [DOI] [PubMed] [Google Scholar]
- 136.Rabinovich GA. Galectin-1 as a potential cancer target. Br J Cancer. 2005;92:1188–1192. doi: 10.1038/sj.bjc.6602493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.ir-Kirk TL. Senior RM. Fragments of extracellular matrix as mediators of inflammation. Int J Biochem Cell Biol. 2008;40:1101–1110. doi: 10.1016/j.biocel.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Leeming DJ. Bay-Jensen AC. Vassiliadis E, et al. Post-translational modifications of the extracellular matrix are key events in cancer progression: opportunities for biochemical marker development. Biomarkers. 2011;16:193–205. doi: 10.3109/1354750X.2011.557440. [DOI] [PubMed] [Google Scholar]
- 139.Schetter AJ. Heegaard NH. Harris CC. Inflammation and cancer: interweaving microRNA, free radical, cytokine and p53 pathways. Carcinogenesis. 2010;31:37–49. doi: 10.1093/carcin/bgp272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Winding B. NicAmhlaoibh R. Misander H, et al. Synthetic matrix metalloproteinase inhibitors inhibit growth of established breast cancer osteolytic lesions and prolong survival in mice. Clin Cancer Res. 2002;8:1932–1939. [PubMed] [Google Scholar]
- 141.Yu AE. Hewitt RE. Connor EW. Stetler-Stevenson WG. Matrix metalloproteinases. Novel targets for directed cancer therapy. Drugs Aging. 1997;11:229–244. doi: 10.2165/00002512-199711030-00006. [DOI] [PubMed] [Google Scholar]
- 142.Clarijs R. Ruiter DJ. De Waal RM. Pathophysiological implications of stroma pattern formation in uveal melanoma. J Cell Physiol. 2003;194:267–271. doi: 10.1002/jcp.10214. [DOI] [PubMed] [Google Scholar]
- 143.Ruiter D. Bogenrieder T. Elder D. Herlyn M. Melanoma-stroma interactions: structural and functional aspects. Lancet Oncol. 2002;3:35–43. doi: 10.1016/s1470-2045(01)00620-9. [DOI] [PubMed] [Google Scholar]
- 144.Veidal SS. Vassiliadis E. Bay-Jensen AC, et al. Procollagen type I N-terminal propeptide (PINP) is a marker for fibrogenesis in bile duct ligation-induced fibrosis in rats. Fibrogenesis Tissue Repair. 2010;3:5. doi: 10.1186/1755-1536-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Copple BL. Allen K. Welch TP. Mechanisms of Liver Fibrosis. Compr Toxicol. 2010;9:263–274. [Google Scholar]
- 146.Borkham-Kamphorst E. van Roeyen CR. Ostendorf T, et al. Pro-fibrogenic potential of PDGF-D in liver fibrosis. J Hepatol. 2007;46:1064–1074. doi: 10.1016/j.jhep.2007.01.029. [DOI] [PubMed] [Google Scholar]
- 147.Guyot C. Lepreux S. Combe C, et al. Hepatic fibrosis and cirrhosis: the (myo)fibroblastic cell subpopulations involved. Int J Biochem Cell Biol. 2006;38:135–151. doi: 10.1016/j.biocel.2005.08.021. [DOI] [PubMed] [Google Scholar]
- 148.Moreira RK. Hepatic stellate cells and liver fibrosis. Arch Pathol Lab Med. 2007;131:1728–1734. doi: 10.5858/2007-131-1728-HSCALF. [DOI] [PubMed] [Google Scholar]
- 149.Gressner OA. Weiskirchen R. Gressner AM. Biomarkers of hepatic fibrosis, fibrogenesis and genetic pre-disposition pending between fiction and reality. J Cell Mol Med. 2007;11:1031–1051. doi: 10.1111/j.1582-4934.2007.00092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Lin WR. Brittan M. Alison MR. The role of bone marrow-derived cells in fibrosis. Cells Tissues Organs. 2008;188:178–188. doi: 10.1159/000113530. [DOI] [PubMed] [Google Scholar]
- 151.Wells RG. Cellular sources of extracellular matrix in hepatic fibrosis. Clin Liver Dis. 2008;12:759–768. doi: 10.1016/j.cld.2008.07.008. viii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wallace K. Burt AD. Wright MC. Liver fibrosis. Biochem J. 2008;411:1–18. doi: 10.1042/BJ20071570. [DOI] [PubMed] [Google Scholar]
- 153.Friedman SL. Hepatic fibrosis—overview. Toxicology. 2008;254:120–129. doi: 10.1016/j.tox.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 154.Kinnman N. Francoz C. Barbu V, et al. The myofibroblastic conversion of peribiliary fibrogenic cells distinct from hepatic stellate cells is stimulated by platelet-derived growth factor during liver fibrogenesis. Lab Invest. 2003;83:163–173. doi: 10.1097/01.lab.0000054178.01162.e4. [DOI] [PubMed] [Google Scholar]
- 155.Kalluri R. Neilson EG. Epithelial-mesenchymal transition and its implications for fibrosis. J Clin Invest. 2003;112:1776–1784. doi: 10.1172/JCI20530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Forbes SJ. Russo FP. Rey V, et al. A significant proportion of myofibroblasts are of bone marrow origin in human liver fibrosis. Gastroenterology. 2004;126:955–963. doi: 10.1053/j.gastro.2004.02.025. [DOI] [PubMed] [Google Scholar]
- 157.Quan TE. Cowper S. Wu SP. Bockenstedt LK. Bucala R. Circulating fibrocytes: collagen-secreting cells of the peripheral blood. Int J Biochem Cell Biol. 2004;36:598–606. doi: 10.1016/j.biocel.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 158.Wynn TA. Common and unique mechanisms regulate fibrosis in various fibroproliferative diseases. J Clin Invest. 2007;117:524–529. doi: 10.1172/JCI31487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol. 2008;214:199–210. doi: 10.1002/path.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Wynn TA. Integrating mechanisms of pulmonary fibrosis. J Exp Med. 2011;208:1339–1350. doi: 10.1084/jem.20110551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Wynn TA. Barron L. Macrophages: master regulators of inflammation and fibrosis. Semin Liver Dis. 2010;30:245–257. doi: 10.1055/s-0030-1255354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Saile B. Ramadori G. Inflammation, damage repair and liver fibrosis—role of cytokines and different cell types. Z Gastroenterol. 2007;45:77–86. doi: 10.1055/s-2006-927395. [DOI] [PubMed] [Google Scholar]
- 163.Fernandez M. Semela D. Bruix J, et al. Angiogenesis in liver disease. J Hepatol. 2009;50:604–620. doi: 10.1016/j.jhep.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 164.Choi JH. Hwang YP. Park BH, et al. Anthocyanins isolated from the purple-fleshed sweet potato attenuate the proliferation of hepatic stellate cells by blocking the PDGF receptor. Environ Toxicol Pharmacol. 2011;31:212–219. doi: 10.1016/j.etap.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 165.Inagaki Y. Okazaki I. Emerging insights into Transforming growth factor beta Smad signal in hepatic fibrogenesis. Gut. 2007;56:284–292. doi: 10.1136/gut.2005.088690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Schwabe RF. Bataller R. Brenner DA. Human hepatic stellate cells express CCR5 and RANTES to induce proliferation and migration. Am J Physiol Gastrointest Liver Physiol. 2003;285:G949–G958. doi: 10.1152/ajpgi.00215.2003. [DOI] [PubMed] [Google Scholar]
- 167.Friedman SL. Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver. Physiol Rev. 2008;88:125–172. doi: 10.1152/physrev.00013.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Maharaj SS. Baroke E. Gauldie J. Kolb MR. Fibrocytes in chronic lung disease—facts and controversies. Pulm Pharmacol Ther. 2012;25:263–267. doi: 10.1016/j.pupt.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 169.Kim KK. Wei Y. Szekeres C, et al. Epithelial cell alpha3beta1 integrin links beta-catenin and Smad signaling to promote myofibroblast formation and pulmonary fibrosis. J Clin Invest. 2009;119:213–224. doi: 10.1172/JCI36940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Collard HR. Calfee CS. Wolters PJ, et al. Plasma biomarker profiles in acute exacerbation of idiopathic pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2010;299:L3–L7. doi: 10.1152/ajplung.90637.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Rock JR. Barkauskas CE. Cronce MJ, et al. Multiple stromal populations contribute to pulmonary fibrosis without evidence for epithelial to mesenchymal transition. Proc Natl Acad Sci USA. 2011;108:E1475–E1483. doi: 10.1073/pnas.1117988108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Li Y. Jiang D. Liang J, et al. Severe lung fibrosis requires an invasive fibroblast phenotype regulated by hyaluronan and CD44. J Exp Med. 2011;208:1459–1471. doi: 10.1084/jem.20102510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Gauldie J. Bonniaud P. Sime P. Ask K. Kolb M. TGF-beta, Smad3 and the process of progressive fibrosis. Biochem Soc Trans. 2007;35:661–664. doi: 10.1042/BST0350661. [DOI] [PubMed] [Google Scholar]
- 174.Moeller A. Gilpin SE. Ask K, et al. Circulating fibrocytes are an indicator of poor prognosis in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2009;179:588–594. doi: 10.1164/rccm.200810-1534OC. [DOI] [PubMed] [Google Scholar]
- 175.Park SH. Saleh D. Giaid A. Michel RP. Increased endothelin-1 in bleomycin-induced pulmonary fibrosis and the effect of an endothelin receptor antagonist. Am J Respir Crit Care Med. 1997;156:600–608. doi: 10.1164/ajrccm.156.2.9607123. [DOI] [PubMed] [Google Scholar]
- 176.Xu J. Mora A. Shim H, et al. Role of the SDF-1/CXCR4 axis in the pathogenesis of lung injury and fibrosis. Am J Respir Cell Mol Biol. 2007;37:291–299. doi: 10.1165/rcmb.2006-0187OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Pulichino AM. Wang IM. Caron A, et al. Identification of transforming growth factor beta1-driven genetic programs of acute lung fibrosis. Am J Respir Cell Mol Biol. 2008;39:324–336. doi: 10.1165/rcmb.2007-0186OC. [DOI] [PubMed] [Google Scholar]
- 178.Tager AM. LaCamera P. Shea BS, et al. The lysophosphatidic acid receptor LPA1 links pulmonary fibrosis to lung injury by mediating fibroblast recruitment and vascular leak. Nat Med. 2008;14:45–54. doi: 10.1038/nm1685. [DOI] [PubMed] [Google Scholar]
- 179.Suki B. Ito S. Stamenovic D. Lutchen KR. Ingenito EP. Biomechanics of the lung parenchyma: critical roles of collagen and mechanical forces. J Appl Physiol. 2005;98:1892–1899. doi: 10.1152/japplphysiol.01087.2004. [DOI] [PubMed] [Google Scholar]
- 180.Hinz B. Phan SH. Thannickal VJ, et al. The myofibroblast: one function, multiple origins. Am J Pathol. 2007;170:1807–1816. doi: 10.2353/ajpath.2007.070112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Wipff PJ. Rifkin DB. Meister JJ. Hinz B. Myofibroblast contraction activates latent TGF-beta1 from the extracellular matrix. J Cell Biol. 2007;179:1311–1323. doi: 10.1083/jcb.200704042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Liu X. Zhao Y. Gao J, et al. Elastic fiber homeostasis requires lysyl oxidase-like 1 protein. Nat Genet. 2004;36:178–182. doi: 10.1038/ng1297. [DOI] [PubMed] [Google Scholar]
- 183.Maki JM. Sormunen R. Lippo S, et al. Lysyl oxidase is essential for normal development and function of the respiratory system and for the integrity of elastic and collagen fibers in various tissues. Am J Pathol. 2005;167:927–936. doi: 10.1016/S0002-9440(10)61183-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Iredale JP. Models of liver fibrosis: exploring the dynamic nature of inflammation and repair in a solid organ. J Clin Invest. 2007;117:539–548. doi: 10.1172/JCI30542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Desmet VJ. Roskams T. Cirrhosis reversal: a duel between dogma and myth. J Hepatol. 2004;40:860–867. doi: 10.1016/j.jhep.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 186.Dienstag JL. Goldin RD. Heathcote EJ, et al. Histological outcome during long-term lamivudine therapy. Gastroenterology. 2003;124:105–117. doi: 10.1053/gast.2003.50013. [DOI] [PubMed] [Google Scholar]
- 187.Hammel P. Couvelard A. O'Toole D, et al. Regression of liver fibrosis after biliary drainage in patients with chronic pancreatitis and stenosis of the common bile duct. N Engl J Med. 2001;344:418–423. doi: 10.1056/NEJM200102083440604. [DOI] [PubMed] [Google Scholar]
- 188.Issa R. Zhou X. Constandinou CM, et al. Spontaneous recovery from micronodular cirrhosis: evidence for incomplete resolution associated with matrix cross-linking. Gastroenterology. 2004;126:1795–1808. doi: 10.1053/j.gastro.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 189.Poynard T. McHutchison J. Manns M. Impact of pegylated interferon alfa-2b and ribavirin on liver fibrosis in patients with chronic hepatitis C. Gastroenterology. 2002;122:1303–1313. doi: 10.1053/gast.2002.33023. [DOI] [PubMed] [Google Scholar]
- 190.Castera L. Invasive and non-invasive methods for the assessment of fibrosis and disease progression in chronic liver disease. Best Pract Res Clin Gastroenterol. 2011;25:291–303. doi: 10.1016/j.bpg.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 191.Ghany MG. Strader DB. Thomas DL. Seeff LB. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009;49:1335–1374. doi: 10.1002/hep.22759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Schuppan D. Connective tissue polypeptides in serum as parameters to monitor antifibrotic treatment in hepatic fibrogenesis. J Hepatol. 1991;13(Suppl 3):S17–S25. doi: 10.1016/0168-8278(91)90004-u. [DOI] [PubMed] [Google Scholar]
- 193.Jensen LT. The aminoterminal propeptide of type III procollagen. Studies on physiology and pathophysiology. Dan Med Bull. 1997;44:70–78. [PubMed] [Google Scholar]
- 194.Yilmaz Y. Systematic review: caspase-cleaved fragments of cytokeratin 18- the promises and challenges of a biomarker for chronic liver disease. Aliment Pharmacol Ther. 2009;30:1103–1109. doi: 10.1111/j.1365-2036.2009.04148.x. [DOI] [PubMed] [Google Scholar]
- 195.Lai KK. Shang S. Lohia N, et al. Extracellular matrix dynamics in hepatocarcinogenesis: a comparative proteomics study of PDGFC transgenic and Pten null mouse models. PLoS Genet. 2011;7:e1002147. doi: 10.1371/journal.pgen.1002147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Leeming DJ. Nielsen MJ. Dai Y, et al. An Enzyme-linked Immunosorbent Serum Assay ELISA specific for the 7S domain of Collagen Type IV (P4NP 7S)—a marker related to the extracellular matrix remodeling during liver fibrogenesis. Hepatol Res. 2012;42:482–493. doi: 10.1111/j.1872-034X.2011.00946.x. [DOI] [PubMed] [Google Scholar]
- 197.Veidal SS. Karsdal MA. Nawrocki A, et al. Assessment of proteolytic degradation of the basement membrane: a fragment of type IV collagen as a biochemical marker for liver fibrosis. Fibrogenesis Tissue Repair. 2011;4:22. doi: 10.1186/1755-1536-4-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Leeming D. He Y. Veidal S, et al. A novel marker for assessment of liver matrix remodeling: an enzyme-linked immunosorbent assay (ELISA) detecting a MMP generated type I collagen neo-epitope (C1M) Biomarkers. 2011;16:616–628. doi: 10.3109/1354750X.2011.620628. [DOI] [PubMed] [Google Scholar]
- 199.Vassiliadis E. Veidal SS. Simonsen H, et al. Immunological detection of the type V collagen propeptide fragment, PVCP-1230, in connective tissue remodeling associated with liver fibrosis. Biomarkers. 2011;16:426–433. doi: 10.3109/1354750X.2011.584131. [DOI] [PubMed] [Google Scholar]
- 200.Veidal SS. Karsdal MA. Vassiliadis E, et al. MMP mediated degradation of type VI collagen is highly associated with liver fibrosis—identification and validation of a novel biochemical marker assay. PLoS One. 2011;6:e24753. doi: 10.1371/journal.pone.0024753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Callewaert N. Van VH. Van HA, et al. Noninvasive diagnosis of liver cirrhosis using DNA sequencer-based total serum protein glycomics. Nat Med. 2004;10:429–434. doi: 10.1038/nm1006. [DOI] [PubMed] [Google Scholar]
- 202.Fang M. Dewaele S. Zhao YP, et al. Serum N-glycome biomarker for monitoring development of DENA-induced hepatocellular carcinoma in rat. Mol Cancer. 2010;9:215. doi: 10.1186/1476-4598-9-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Blomme B. Van SC. Grassi P, et al. Alterations of serum protein N-glycosylation in two mouse models of chronic liver disease are hepatocyte and not B cell driven. Am J Physiol Gastrointest Liver Physiol. 2011;300:G833–G842. doi: 10.1152/ajpgi.00228.2010. [DOI] [PubMed] [Google Scholar]
- 204.Vanderschaeghe D. Laroy W. Sablon E, et al. GlycoFibroTest is a highly performant liver fibrosis biomarker derived from DNA sequencer-based serum protein glycomics. Mol Cell Proteomics. 2009;8:986–994. doi: 10.1074/mcp.M800470-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Klein A. Carre Y. Louvet A. Michalski JC. Morelle W. Immunoglobulins are the major glycoproteins involved in the modifications of total serum N-glycome in cirrhotic patients. Proteomics Clin Appl. 2010;4:379–393. doi: 10.1002/prca.200900133. [DOI] [PubMed] [Google Scholar]
- 206.Karsdal MA. Woodworth T. Henriksen K, et al. Biochemical markers of ongoing joint damage in rheumatoid arthritis—current and future applications, limitations and opportunities. Arthritis Res Ther. 2011;13:215. doi: 10.1186/ar3280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Leeming DJ. Koizumi M. Byrjalsen I, et al. The relative use of eight collagenous and noncollagenous markers for diagnosis of skeletal metastases in breast, prostate, or lung cancer patients. Cancer Epidemiol Biomarkers Prev. 2006;15:32–38. doi: 10.1158/1055-9965.EPI-05-0492. [DOI] [PubMed] [Google Scholar]
- 208.Leeming DJ. Delling G. Koizumi M, et al. Alpha CTX as a biomarker of skeletal invasion of breast cancer: immunolocalization and the load dependency of urinary excretion. Cancer Epidemiol Biomarkers Prev. 2006;15:1392–1395. doi: 10.1158/1055-9965.EPI-05-0909. [DOI] [PubMed] [Google Scholar]
- 209.Leeming DJ. Byrjalsen I. Qvist P, et al. Does increased local bone resorption secondary to breast and prostate cancer result in increased cartilage degradation? BMC Cancer. 2008;8:180. doi: 10.1186/1471-2407-8-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Jensen VK. Nosjean O. Dziegiel MH, et al. A quantitative assay for lysosomal acidification rates in human osteoclasts. Assay Drug Dev Technol. 2011;9:157–164. doi: 10.1089/adt.2010.0272. [DOI] [PubMed] [Google Scholar]
- 211.O'Connell K. Posthumus M. Collins M. COL6A1 gene and Ironman triathlon performance. Int J Sports Med. 2011;32:896–901. doi: 10.1055/s-0031-1277181. [DOI] [PubMed] [Google Scholar]
- 212.Allamand V. Brinas L. Richard P, et al. ColVI myopathies: where do we stand, where do we go? Skelet Muscle. 2011;1:30. doi: 10.1186/2044-5040-1-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Bonnemann CG. The collagen VI-related myopathies: muscle meets its matrix. Nat Rev Neurol. 2011;7:379–390. doi: 10.1038/nrneurol.2011.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Egeblad M. Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2:161–174. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- 215.Kerkela E. Saarialho-Kere U. Matrix metalloproteinases in tumor progression: focus on basal and squamous cell skin cancer. Exp Dermatol. 2003;12:109–125. doi: 10.1034/j.1600-0625.2003.120201.x. [DOI] [PubMed] [Google Scholar]
- 216.Tlsty TD. Coussens LM. Tumor stroma and regulation of cancer development. Annu Rev Pathol. 2006;1:119–150. doi: 10.1146/annurev.pathol.1.110304.100224. [DOI] [PubMed] [Google Scholar]
- 217.Skjot-Arkil H. Barascuk N. Register T. Karsdal MA. Macrophage-mediated proteolytic remodeling of the extracellular matrix in atherosclerosis results in neoepitopes: a potential new class of biochemical markers. Assay Drug Dev Technol. 2010;8:542–552. doi: 10.1089/adt.2009.0258. [DOI] [PubMed] [Google Scholar]
- 218.Veidal SS. Bay-Jensen AC. Tougas G. Karsdal MA. Vainer B. Serum markers of liver fibrosis: combining the BIPED classification and the neo-epitope approach in the development of new biomarkers. Dis Markers. 2010;28:15–28. doi: 10.3233/DMA-2010-0678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Walsh KM. Fletcher A. MacSween RN. Morris AJ. Basement membrane peptides as markers of liver disease in chronic hepatitis C. J Hepatol. 2000;32:325–330. doi: 10.1016/s0168-8278(00)80079-3. [DOI] [PubMed] [Google Scholar]
- 220.Dillon TJ. Walsh RL. Scicchitano R, et al. Plasma elastin-derived peptide levels in normal adults, children, and emphysematous subjects. Physiologic and computed tomographic scan correlates. Am Rev Respir Dis. 1992;146:1143–1148. doi: 10.1164/ajrccm/146.5_Pt_1.1143. [DOI] [PubMed] [Google Scholar]
- 221.Heinz A. Jung MC. Jahreis G, et al. The action of neutrophil serine proteases on elastin, its precursor. Biochimie. 2011 doi: 10.1016/j.biochi.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 222.Heinz A. Jung MC. Duca L, et al. Degradation of tropoelastin by matrix metalloproteinases—cleavage site specificities and release of matrikines. FEBS J. 2010;277:1939–1956. doi: 10.1111/j.1742-4658.2010.07616.x. [DOI] [PubMed] [Google Scholar]
- 223.Houghton AM. Quintero PA. Perkins DL, et al. Elastin fragments drive disease progression in a murine model of emphysema. J Clin Invest. 2006;116:753–759. doi: 10.1172/JCI25617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Jakob A. Unger S. Arnold R, et al. A family with a new elastin gene mutation: broad clinical spectrum, including sudden cardiac death. Cardiol Young. 2011;21:62–65. doi: 10.1017/S1047951110001563. [DOI] [PubMed] [Google Scholar]
- 225.Kielty CM. Sherratt MJ. Shuttleworth CA. Elastic fibres. J Cell Sci. 2002;115:2817–2828. doi: 10.1242/jcs.115.14.2817. [DOI] [PubMed] [Google Scholar]
- 226.Luisetti M. Ma S. Iadarola P, et al. Desmosine as a biomarker of elastin degradation in COPD: current status and future directions. Eur Respir J. 2008;32:1146–1157. doi: 10.1183/09031936.00174807. [DOI] [PubMed] [Google Scholar]
- 227.Ma S. Lieberman S. Turino GM. Lin YY. The detection and quantitation of free desmosine and isodesmosine in human urine and their peptide-bound forms in sputum. Proc Natl Acad Sci USA. 2003;100:12941–12943. doi: 10.1073/pnas.2235344100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Marciniak SJ. Lomas DA. What can naturally occurring mutations tell us about the pathogenesis of COPD? Thorax. 2009;64:359–364. doi: 10.1136/thx.2008.099408. [DOI] [PubMed] [Google Scholar]
- 229.Mecham RP. Broekelmann TJ. Fliszar CJ, et al. Elastin degradation by matrix metalloproteinases. Cleavage site specificity and mechanisms of elastolysis. J Biol Chem. 1997;272:18071–18076. doi: 10.1074/jbc.272.29.18071. [DOI] [PubMed] [Google Scholar]
- 230.Petersen E. Gineitis A. Wagberg F. Angquist KA. Serum levels of elastin-derived peptides in patients with ruptured and asymptomatic abdominal aortic aneurysms. Eur J Vasc Endovasc Surg. 2001;22:48–52. doi: 10.1053/ejvs.2001.1404. [DOI] [PubMed] [Google Scholar]
- 231.Taddese S. Weiss AS. Jahreis G. Neubert RH. Schmelzer CE. In vitro degradation of human tropoelastin by MMP-12 and the generation of matrikines from domain 24. Matrix Biol. 2009;28:84–91. doi: 10.1016/j.matbio.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 232.Wendel DP. Taylor DG. Albertine KH. Keating MT. Li DY. Impaired distal airway development in mice lacking elastin. Am J Respir Cell Mol Biol. 2000;23:320–326. doi: 10.1165/ajrcmb.23.3.3906. [DOI] [PubMed] [Google Scholar]
- 233.Barker DF. Hostikka SL. Zhou J, et al. Identification of mutations in the COL4A5 collagen gene in Alport syndrome. Science. 1990;248:1224–1227. doi: 10.1126/science.2349482. [DOI] [PubMed] [Google Scholar]
- 234.Sykes B. Ogilvie D. Wordsworth P, et al. Consistent linkage of dominantly inherited osteogenesis imperfecta to the type I collagen loci: COL1A1 and COL1A2. Am J Hum Genet. 1990;46:293–307. [PMC free article] [PubMed] [Google Scholar]
- 235.Weil D. D'Alessio M. Ramirez F. Eyre DR. Structural and functional characterization of a splicing mutation in the pro-alpha 2(I) collagen gene of an Ehlers-Danlos type VII patient. J Biol Chem. 1990;265:16007–16011. [PubMed] [Google Scholar]
- 236.Fernandes RJ. Wilkin DJ. Weis MA, et al. Incorporation of structurally defective type II collagen into cartilage matrix in kniest chondrodysplasia. Arch Biochem Biophys. 1998;355:282–290. doi: 10.1006/abbi.1998.0745. [DOI] [PubMed] [Google Scholar]
- 237.Ahmad NN. Donald-McGinn DM. Zackai EH, et al. A second mutation in the type II procollagen gene (COL2AI) causing stickler syndrome (arthro-ophthalmopathy) is also a premature termination codon. Am J Hum Genet. 1993;52:39–45. [PMC free article] [PubMed] [Google Scholar]
- 238.Palotie A. Vaisanen P. Ott J, et al. Predisposition to familial osteoarthrosis linked to type II collagen gene. Lancet. 1989;1:924–927. doi: 10.1016/s0140-6736(89)92507-5. [DOI] [PubMed] [Google Scholar]
- 239.Ala-Kokko L. Baldwin CT. Moskowitz RW. Prockop DJ. Single base mutation in the type II procollagen gene (COL2A1) as a cause of primary osteoarthritis associated with a mild chondrodysplasia. Proc Natl Acad Sci USA. 1990;87:6565–6568. doi: 10.1073/pnas.87.17.6565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Tromp G. Kuivaniemi H. Stolle C. Pope FM. Prockop DJ. Single base mutation in the type III procollagen gene that converts the codon for glycine 883 to aspartate in a mild variant of Ehlers-Danlos syndrome IV. J Biol Chem. 1989;264:19313–19317. [PubMed] [Google Scholar]
- 241.Kontusaari S. Tromp G. Kuivaniemi H. Romanic AM. Prockop DJ. A mutation in the gene for type III procollagen (COL3A1) in a family with aortic aneurysms. J Clin Invest. 1990;86:1465–1473. doi: 10.1172/JCI114863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Van AT. Bailey MA. Schlotzer-Schrehardt U, et al. Col4a1 mutation in mice causes defects in vascular function and low blood pressure associated with reduced red blood cell volume. Hum Mol Genet. 2010;19:1119–1128. doi: 10.1093/hmg/ddp584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Van AT. Bruckner-Tuderman L. Basement membranes and human disease. Cell Tissue Res. 2010;339:167–188. doi: 10.1007/s00441-009-0866-y. [DOI] [PubMed] [Google Scholar]
- 244.Taylor SH. Al-Youha S. Van AT, et al. Tendon is covered by a basement membrane epithelium that is required for cell retention and the prevention of adhesion formation. PLoS One. 2011;6:e16337. doi: 10.1371/journal.pone.0016337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Wenstrup RJ. Florer JB. Willing MC, et al. COL5A1 haploinsufficiency is a common molecular mechanism underlying the classical form of EDS. Am J Hum Genet. 2000;66:1766–1776. doi: 10.1086/302930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Richards AJ. Martin S. Nicholls AC, et al. A single base mutation in COL5A2 causes Ehlers-Danlos syndrome type II. J Med Genet. 1998;35:846–848. doi: 10.1136/jmg.35.10.846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Lampe AK. Bushby KM. Collagen VI related muscle disorders. J Med Genet. 2005;42:673–685. doi: 10.1136/jmg.2002.002311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Dang N. Murrell DF. Mutation analysis and characterization of COL7A1 mutations in dystrophic epidermolysis bullosa. Exp Dermatol. 2008;17:553–568. doi: 10.1111/j.1600-0625.2008.00723.x. [DOI] [PubMed] [Google Scholar]
- 249.Czarny-Ratajczak M. Lohiniva J. Rogala P, et al. A mutation in COL9A1 causes multiple epiphyseal dysplasia: further evidence for locus heterogeneity. Am J Hum Genet. 2001;69:969–980. doi: 10.1086/324023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Woelfle JV. Brenner RE. Zabel B. Reichel H. Nelitz M. Schmid-type metaphyseal chondrodysplasia as the result of a collagen type X defect due to a novel COL10A1 nonsense mutation: a case report of a novel COL10A1 mutation. J Orthop Sci. 2011;16:245–249. doi: 10.1007/s00776-011-0021-y. [DOI] [PubMed] [Google Scholar]
- 251.Makitie O. Susic M. Cole WG. Early-onset metaphyseal chondrodysplasia type Schmid associated with a COL10A1 frame-shift mutation and impaired trimerization of wild-type alpha1(X) protein chains. J Orthop Res. 2010;28:1497–1501. doi: 10.1002/jor.21161. [DOI] [PubMed] [Google Scholar]
- 252.Milewicz DM. Urban Z. Boyd C. Genetic disorders of the elastic fiber system. Matrix Biol. 2000;19:471–480. doi: 10.1016/s0945-053x(00)00099-8. [DOI] [PubMed] [Google Scholar]
- 253.Kielty CM. Elastic fibres in health and disease. Expert Rev Mol Med. 2006;8:1–23. doi: 10.1017/S146239940600007X. [DOI] [PubMed] [Google Scholar]
- 254.Heegaard AM. Corsi A. Danielsen CC, et al. Biglycan deficiency causes spontaneous aortic dissection and rupture in mice. Circulation. 2007;115:2731–2738. doi: 10.1161/CIRCULATIONAHA.106.653980. [DOI] [PubMed] [Google Scholar]
- 255.Chen XD. Shi S. Xu T. Robey PG. Young MF. Age-related osteoporosis in biglycan-deficient mice is related to defects in bone marrow stromal cells. J Bone Miner Res. 2002;17:331–340. doi: 10.1359/jbmr.2002.17.2.331. [DOI] [PubMed] [Google Scholar]
- 256.Xu T. Bianco P. Fisher LW, et al. Targeted disruption of the biglycan gene leads to an osteoporosis-like phenotype in mice. Nat Genet. 1998;20:78–82. doi: 10.1038/1746. [DOI] [PubMed] [Google Scholar]
- 257.Young MF. Bi Y. Ameye L. Chen XD. Biglycan knockout mice: new models for musculoskeletal diseases. Glycoconj J. 2002;19:257–262. doi: 10.1023/A:1025336114352. [DOI] [PubMed] [Google Scholar]
- 258.Ameye L. Aria D. Jepsen K, et al. Abnormal collagen fibrils in tendons of biglycan/fibromodulin-deficient mice lead to gait impairment, ectopic ossification, and osteoarthritis. FASEB J. 2002;16:673–680. doi: 10.1096/fj.01-0848com. [DOI] [PubMed] [Google Scholar]
- 259.Gill MR. Oldberg A. Reinholt FP. Fibromodulin-null murine knee joints display increased incidences of osteoarthritis and alterations in tissue biochemistry. Osteoarthritis Cartilage. 2002;10:751–757. doi: 10.1053/joca.2002.0527. [DOI] [PubMed] [Google Scholar]
- 260.Jepsen KJ. Wu F. Peragallo JH, et al. A syndrome of joint laxity and impaired tendon integrity in lumican- and fibromodulin-deficient mice. J Biol Chem. 2002;277:35532–35540. doi: 10.1074/jbc.M205398200. [DOI] [PubMed] [Google Scholar]
- 261.Svensson L. Aszodi A. Reinholt FP, et al. Fibromodulin-null mice have abnormal collagen fibrils, tissue organization, and altered lumican deposition in tendon. J Biol Chem. 1999;274:9636–9647. doi: 10.1074/jbc.274.14.9636. [DOI] [PubMed] [Google Scholar]
- 262.Chakravarti S. Functions of lumican and fibromodulin: lessons from knockout mice. Glycoconj J. 2002;19:287–293. doi: 10.1023/A:1025348417078. [DOI] [PubMed] [Google Scholar]
- 263.Bi X. Tong C. Dockendorff A, et al. Genetic deficiency of decorin causes intestinal tumor formation through disruption of intestinal cell maturation. Carcinogenesis. 2008;29:1435–1440. doi: 10.1093/carcin/bgn141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Danielson KG. Baribault H. Holmes DF, et al. Targeted disruption of decorin leads to abnormal collagen fibril morphology and skin fragility. J Cell Biol. 1997;136:729–743. doi: 10.1083/jcb.136.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Corsi A. Xu T. Chen XD. Boyde A, et al. Phenotypic effects of biglycan deficiency are linked to collagen fibril abnormalities, are synergized by decorin deficiency, and mimic Ehlers-Danlos-like changes in bone and other connective tissues. J Bone Miner Res. 2002;17:1180–1189. doi: 10.1359/jbmr.2002.17.7.1180. [DOI] [PubMed] [Google Scholar]
- 266.Wang Y. Ma Y. Lu B, et al. Differential expression of mimecan and thioredoxin domain-containing protein 5 in colorectal adenoma and cancer: a proteomic study. Exp Biol Med (Maywood) 2007;232:1152–1159. doi: 10.3181/0701-RM-8. [DOI] [PubMed] [Google Scholar]
- 267.Hayward C. Brock DJ. Fibrillin-1 mutations in Marfan syndrome and other type-1 fibrillinopathies. Hum Mutat. 1997;10:415–423. doi: 10.1002/(SICI)1098-1004(1997)10:6<415::AID-HUMU1>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 268.Paulsson M. Heinegard D. Purification and structural characterization of a cartilage matrix protein. Biochem J. 1981;197:367–375. doi: 10.1042/bj1970367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Briggs MD. Rasmussen IM. Weber JL, et al. Genetic linkage of mild pseudoachondroplasia (PSACH) to markers in the pericentromeric region of chromosome 19. Genomics. 1993;18:656–660. doi: 10.1016/s0888-7543(05)80369-6. [DOI] [PubMed] [Google Scholar]
- 270.Jackson GC. Mittaz-Crettol L. Taylor JA, et al. Pseudoachondroplasia and multiple epiphyseal dysplasia: a 7-year comprehensive analysis of the known disease genes identify novel and recurrent mutations and provides an accurate assessment of their relative contribution. Hum Mutat. 2012;33:144–157. doi: 10.1002/humu.21611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Briggs MD. Wright MJ. Mortier GR. Multiple epiphyseal dysplasia, dominant. In: Pagon RA, editor; Bird TD, editor; Dolan CR, editor; Stephens K, editor; Adam MP, editor. GeneReviews™. University of Washington; Seattle, WA: 2003. Jan 8, [Internet]. [updated 2011 Feb 1]. [PubMed] [Google Scholar]
- 272.Karsdal MA. Henriksen K. Leeming DJ, et al. Biochemical markers and the FDA Critical Path: how biomarkers may contribute to the understanding of pathophysiology and provide unique and necessary tools for drug development. Biomarkers. 2009;14:181–202. doi: 10.1080/13547500902777608. [DOI] [PubMed] [Google Scholar]
- 273.Lapolla A. Traldi P. Fedele D. Importance of measuring products of non-enzymatic glycation of proteins. Clin Biochem. 2005;38:103–115. doi: 10.1016/j.clinbiochem.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 274.Skjot-Arkil H. Schett G. Zhang C, et al. Investigation of two novel biochemical markers of inflammation, matrix metalloproteinase and cathepsin generated fragments of C-reactive protein, in patients with ankylosing spondylitis. Clin Exp Rheumatol. 2012;30:371–379. [PubMed] [Google Scholar]
- 275.Lin HC. Chang JH. Jain S, et al. Matrilysin cleavage of corneal collagen type XVIII NC1 domain and generation of a 28-kDa fragment. Invest Ophthalmol Vis Sci. 2001;42:2517–2524. [PubMed] [Google Scholar]
- 276.Rosenquist C. Fledelius C. Christgau S, et al. Serum CrossLaps one step ELISA. First application of monoclonal antibodies for measurement in serum of bone-related degradation products from C-terminal telopeptides of type I collagen. Clin Chem. 1998;44:2281–2289. [PubMed] [Google Scholar]
- 277.Cloos PA. Christgau S. Characterization of aged osteocalcin fragments derived from bone resorption. Clin Lab. 2004;50:585–598. [PubMed] [Google Scholar]
- 278.Karsdal MA. Madsen SH. Christiansen C, et al. Cartilage degradation is fully reversible in the presence of aggrecanase but not matrix metalloproteinase activity. Arthritis Res Ther. 2008;10:R63. doi: 10.1186/ar2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279. Reference deleted.
- 280.Erler JT. Bennewith KL. Nicolau M, et al. Lysyl oxidase is essential for hypoxia-induced metastasis. Nature. 2006;440:1222–1226. doi: 10.1038/nature04695. [DOI] [PubMed] [Google Scholar]
- 281.Erler JT. Weaver VM. Three-dimensional context regulation of metastasis. Clin Exp Metastasis. 2009;26:35–49. doi: 10.1007/s10585-008-9209-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Kass L. Erler JT. Dembo M. Weaver VM. Mammary epithelial cell: influence of extracellular matrix composition and organization during development and tumorigenesis. Int J Biochem Cell Biol. 2007;39:1987–1994. doi: 10.1016/j.biocel.2007.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Levental KR. Yu H. Kass L, et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 2009;139:891–906. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Oestergaard S. Chouinard L. Doyle N, et al. The utility of measuring C-terminal telopeptides of collagen type II (CTX-II) in serum and synovial fluid samples for estimation of articular cartilage status in experimental models of destructive joint diseases. Osteoarthritis Cartilage. 2006;14:670–679. doi: 10.1016/j.joca.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 285.Bone HG. The future of osteoporosis diagnosis and therapy. Ann Ital Med Int. 1992;7:166S–170S. [PubMed] [Google Scholar]
- 286.Leeming DJ. Alexandersen P. Karsdal MA, et al. An update on biomarkers of bone turnover and their utility in biomedical research and clinical practice. Eur J Clin Pharmacol. 2006;62:781–792. doi: 10.1007/s00228-006-0174-3. [DOI] [PubMed] [Google Scholar]
- 287.Byrjalsen I. Leeming DJ. Qvist P. Christiansen C. Karsdal MA. Bone turnover and bone collagen maturation in osteoporosis: effects of antiresorptive therapies. Osteoporos Int. 2008;19:339–348. doi: 10.1007/s00198-007-0462-5. [DOI] [PubMed] [Google Scholar]
- 288.Karsdal MA. Byrjalsen I. Leeming DJ. Delmas PD. Christiansen C. The effects of oral calcitonin on bone collagen maturation: implications for bone turnover and quality. Osteoporos Int. 2008;19:1355–1361. doi: 10.1007/s00198-008-0603-5. [DOI] [PubMed] [Google Scholar]
- 289.Leeming DJ. Henriksen K. Byrjalsen I, et al. Is bone quality associated with collagen age? Osteoporos Int. 2009;20:1461–1470. doi: 10.1007/s00198-009-0904-3. [DOI] [PubMed] [Google Scholar]
- 290.Fledelius C. Johnsen AH. Cloos PA. Bonde M. Qvist P. Characterization of urinary degradation products derived from type I collagen. Identification of a beta-isomerized Asp-Gly sequence within the C-terminal telopeptide (alpha1) region. J Biol Chem. 1997;272:9755–9763. doi: 10.1074/jbc.272.15.9755. [DOI] [PubMed] [Google Scholar]
- 291.Cloos PA. Lyubimova N. Solberg H, et al. An immunoassay for measuring fragments of newly synthesized collagen type I produced during metastatic invasion of bone. Clin Lab. 2004;50:279–289. [PubMed] [Google Scholar]
- 292.Grenard P. Bresson-Hadni S. El AS, et al. Transglutaminase-mediated cross-linking is involved in the stabilization of extracellular matrix in human liver fibrosis. J Hepatol. 2001;35:367–375. doi: 10.1016/s0168-8278(01)00135-0. [DOI] [PubMed] [Google Scholar]
- 293.Elli L. Bergamini CM. Bardella MT. Schuppan D. Transglutaminases in inflammation and fibrosis of the gastrointestinal tract and the liver. Dig Liver Dis. 2009;41:541–550. doi: 10.1016/j.dld.2008.12.095. [DOI] [PubMed] [Google Scholar]
- 294.Collighan RJ. Griffin M. Transglutaminase 2 cross-linking of matrix proteins: biological significance and medical applications. Amino Acids. 2009;36:659–670. doi: 10.1007/s00726-008-0190-y. [DOI] [PubMed] [Google Scholar]
- 295.Poyton RO. Ball KA. Castello PR. Mitochondrial generation of free radicals and hypoxic signaling. Trends Endocrinol Metab. 2009;20:332–340. doi: 10.1016/j.tem.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 296.Cloos PA. Christgau S. Non-enzymatic covalent modifications of proteins: mechanisms, physiological consequences and clinical applications. Matrix Biol. 2002;21:39–52. doi: 10.1016/s0945-053x(01)00188-3. [DOI] [PubMed] [Google Scholar]
- 297.Kowluru RA. Atasi L. Ho YS. Role of Mitochondrial Superoxide Dismutase in the Development of Diabetic Retinopathy. Invest Ophthalmol Vis Sci. 2006;47:1594–1599. doi: 10.1167/iovs.05-1276. [DOI] [PubMed] [Google Scholar]
- 298.Chaiswing L. Oberley TD. Extracellular/microenvironmental redox state. Antioxid Redox Signal. 2010;13:449–465. doi: 10.1089/ars.2009.3020. [DOI] [PubMed] [Google Scholar]
- 299.Colell A. Green DR. Ricci JE. Novel roles for GAPDH in cell death and carcinogenesis. Cell Death Differ. 2009;16:1573–1581. doi: 10.1038/cdd.2009.137. [DOI] [PubMed] [Google Scholar]
- 300.DeNicola GM. Tuveson DA. RAS in cellular transformation and senescence. Eur J Cancer. 2009;45(Suppl 1):211–216. doi: 10.1016/S0959-8049(09)70036-X. [DOI] [PubMed] [Google Scholar]
- 301.Faux SP. Tai T. Thorne D, et al. The role of oxidative stress in the biological responses of lung epithelial cells to cigarette smoke. Biomarkers. 2009;14(Suppl 1):90–96. doi: 10.1080/13547500902965047. [DOI] [PubMed] [Google Scholar]
- 302.Lu JM. Lin PH. Yao Q. Chen C. Chemical and molecular mechanisms of antioxidants: experimental approaches and model systems. J Cell Mol Med. 2010;14:840–860. doi: 10.1111/j.1582-4934.2009.00897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Weinberg F. Chandel NS. Mitochondrial metabolism and cancer. Ann NY Acad Sci. 2009;1177:66–73. doi: 10.1111/j.1749-6632.2009.05039.x. [DOI] [PubMed] [Google Scholar]
- 304.Karsdal MA. Leeming DJ. Dam EB, et al. Should subchondral bone turnover be targeted when treating osteoarthritis? Osteoarthritis Cartilage. 2008;16:638–646. doi: 10.1016/j.joca.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 305.Anderson-MacKenzie JM. Quasnichka HL. Starr RL, et al. Fundamental subchondral bone changes in spontaneous knee osteoarthritis. Int J Biochem Cell Biol. 2005;37:224–236. doi: 10.1016/j.biocel.2004.06.016. [DOI] [PubMed] [Google Scholar]
- 306.Bailey AJ. Mansell JP. Do subchondral bone changes exacerbate or precede articular cartilage destruction in osteoarthritis of the elderly? Gerontology. 1997;43:296–304. doi: 10.1159/000213866. [DOI] [PubMed] [Google Scholar]
- 307.Dieppe P. Subchondral bone should be the main target for the treatment of pain and disease progression in osteoarthritis. Osteoarthritis Cartilage. 1999;7:325–326. doi: 10.1053/joca.1998.0182. [DOI] [PubMed] [Google Scholar]
- 308.Felson DT. Neogi T. Osteoarthritis: is it a disease of cartilage or of bone? Arthritis Rheum. 2004;50:341–344. doi: 10.1002/art.20051. [DOI] [PubMed] [Google Scholar]
- 309.Bay-Jensen AC. Sondergaard BC. Christiansen C, et al. Biochemical markers of joint tissue turnover. Assay Drug Dev Technol. 2010;8:118–124. doi: 10.1089/adt.2009.0199. [DOI] [PubMed] [Google Scholar]
- 310.Sondergaard BC. Henriksen K. Wulf H, et al. Relative contribution of matrix metalloprotease and cysteine protease activities to cytokine-stimulated articular cartilage degradation. Osteoarthritis Cartilage. 2006;14:738–748. doi: 10.1016/j.joca.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 311.Schaller S. Henriksen K. Hoegh-Andersen P, et al. In vitro, ex vivo, and in vivo methodological approaches for studying therapeutic targets of osteoporosis and degenerative joint diseases: how biomarkers can assist? Assay Drug Dev Technol. 2005;3:553–580. doi: 10.1089/adt.2005.3.553. [DOI] [PubMed] [Google Scholar]
- 312.Karsdal MA. Sumer EU. Wulf H, et al. Induction of increased cAMP levels in articular chondrocytes blocks matrix metalloproteinase-mediated cartilage degradation, but not aggrecanase-mediated cartilage degradation. Arthritis Rheum. 2007;56:1549–1558. doi: 10.1002/art.22599. [DOI] [PubMed] [Google Scholar]
- 313.Qvist P. Christiansen C. Karsdal MA, et al. Application of biochemical markers in development of drugs for treatment of osteoarthritis. Biomarkers. 2010;15:1–19. doi: 10.3109/13547500903295873. [DOI] [PubMed] [Google Scholar]
- 314.Garnero P. Ferreras M. Karsdal MA, et al. The type I collagen fragments ICTP and CTX reveal distinct enzymatic pathways of bone collagen degradation. J Bone Miner Res. 2003;18:859–867. doi: 10.1359/jbmr.2003.18.5.859. [DOI] [PubMed] [Google Scholar]
- 315.Santala M. Risteli J. Kauppila A. Comparison of carboxyterminal telopeptide of type I collagen (ICTP) and CA 125 as predictors of prognosis in ovarian cancer. Anticancer Res. 2004;24:1057–1062. [PubMed] [Google Scholar]
- 316.Simojoki M. Santala M. Risteli J. Kauppila A. Carboxyterminal telopeptide of type I collagen (ICTP) in predicting prognosis in epithelial ovarian cancer. Gynecol Oncol. 2001;82:110–115. doi: 10.1006/gyno.2001.6212. [DOI] [PubMed] [Google Scholar]
- 317.Cloos PA. Fledelius C. Collagen fragments in urine derived from bone resorption are highly racemized and isomerized: a biological clock of protein aging with clinical potential. Biochem J. 2000;345(Pt 3):473–480. [PMC free article] [PubMed] [Google Scholar]
- 318.Johnson BA. Aswad DW. Fragmentation of isoaspartyl peptides and proteins by carboxypeptidase Y: release of isoaspartyl dipeptides as a result of internal and external cleavage. Biochemistry. 1990;29:4373–4380. doi: 10.1021/bi00470a017. [DOI] [PubMed] [Google Scholar]
- 319.Brange J. Langkjaer L. Havelund S. Volund A. Chemical stability of insulin. 1. Hydrolytic degradation during storage of pharmaceutical preparations. Pharm Res. 1992;9:715–726. doi: 10.1023/a:1015835017916. [DOI] [PubMed] [Google Scholar]
- 320.Mamula MJ. Gee RJ. Elliott JI, et al. Isoaspartyl post-translational modification triggers autoimmune responses to self-proteins. J Biol Chem. 1999;274:22321–22327. doi: 10.1074/jbc.274.32.22321. [DOI] [PubMed] [Google Scholar]
- 321.Voorter CE. de Haard-Hoekman WA. van den Oetelaar PJ. Bloemendal H. de Jong WW. Spontaneous peptide bond cleavage in aging alpha-crystallin through a succinimide intermediate. J Biol Chem. 1988;263:19020–19023. [PubMed] [Google Scholar]
- 322.Young AL. Carter WG. Doyle HA. Mamula MJ. Aswad DW. Structural integrity of histone H2B in vivo requires the activity of protein L-isoaspartate O-methyltransferase, a putative protein repair enzyme. J Biol Chem. 2001;276:37161–37165. doi: 10.1074/jbc.M106682200. [DOI] [PubMed] [Google Scholar]
- 323.Treweek JB. Dickerson TJ. Janda KD. Drugs of abuse that mediate advanced glycation end product formation: a chemical link to disease pathology. Acc Chem Res. 2009;42:659–669. doi: 10.1021/ar800247d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Riehl A. Nemeth J. Angel P. Hess J. The receptor RAGE: Bridging inflammation and cancer. Cell Commun Signal. 2009;7:12. doi: 10.1186/1478-811X-7-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Johansen JS. Studies on serum YKL-40 as a biomarker in diseases with inflammation, tissue remodelling, fibroses and cancer. Dan Med Bull. 2006;53:172–209. [PubMed] [Google Scholar]
- 326.Anzilotti C. Pratesi F. Tommasi C. Migliorini P. Peptidylarginine deiminase 4 and citrullination in health and disease. Autoimmun Rev. 2010;9:158–160. doi: 10.1016/j.autrev.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 327.Gyorgy B. Toth E. Tarcsa E. Falus A. Buzas EI. Citrullination: a posttranslational modification in health and disease. Int J Biochem Cell Biol. 2006;38:1662–1677. doi: 10.1016/j.biocel.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 328.Chang X. Han J. Pang L, et al. Increased PADI4 expression in blood and tissues of patients with malignant tumors. BMC Cancer. 2009;9:40. doi: 10.1186/1471-2407-9-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Chang X. Han J. Expression of peptidylarginine deiminase type 4 (PAD4) in various tumors. Mol Carcinog. 2006;45:183–196. doi: 10.1002/mc.20169. [DOI] [PubMed] [Google Scholar]
- 330.Smith BC. Denu JM. Chemical mechanisms of histone lysine and arginine modifications. Biochim Biophys Acta. 2009;1789:45–57. doi: 10.1016/j.bbagrm.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Veidal SS. Larsen DV. Chen X, et al. MMP mediated type V collagen degradation (C5M) is elevated in ankylosing spondylitis. Clin Biochem. 45:541–546. doi: 10.1016/j.clinbiochem.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 332.Quintana DJ. Garnero P. Huebner JL. Charni-Ben TN. Kraus VB. PIIANP and HELIXII diurnal variation. Osteoarthritis Cartilage. 2008;16:1192–1195. doi: 10.1016/j.joca.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Rousseau JC. Zhu Y. Miossec P, et al. Serum levels of type IIA procollagen amino terminal propeptide (PIIANP) are decreased in patients with knee osteoarthritis and rheumatoid arthritis. Osteoarthritis Cartilage. 2004;12:440–447. doi: 10.1016/j.joca.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 334.Trocme C. Leroy V. Sturm N, et al. Longitudinal evaluation of a fibrosis index combining MMP-1 and PIIINP compared with MMP-9, TIMP-1 and hyaluronic acid in patients with chronic hepatitis C treated by interferon-alpha and ribavirin. J Viral Hepat. 2006;13:643–651. doi: 10.1111/j.1365-2893.2006.00730.x. [DOI] [PubMed] [Google Scholar]
- 335.Vassiliadis E. Oliveira CP. Alvares-da-Silva MR, et al. Circulating levels of citrullinated and MMP-degraded vimentin (VICM) in liver fibrosis related pathology. Am J Transl Res. 2012;4:403–414. [PMC free article] [PubMed] [Google Scholar]
- 336.Barascuk N. Genovese F. Larsen L, et al. Quantification of a specific matrix metalloproteinase-mediated degradation product of biglycan during extracellular matrix remodeling in liver fibrosis. Hepatol Res. 2012 (In press) [Google Scholar]
- 337.Barascuk N. Larsen L. Byrjalsen I, et al. A MMP derived versican neoepitope is elevated in plasma from patients with atherosclerotic heart disease. Atherosclerosis. 2012 (In press) [PMC free article] [PubMed] [Google Scholar]
- 338.Vassiliadis E. Rasmussen LM. Byrjalsen I, et al. Clinical evaluation of a matrix metalloproteinase-12 cleaved fragment of titin as a cardiovascular-specific serological biomarker. J Translational Med. 2012;10:140. doi: 10.1186/1479-5876-10-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Crnkic M. Mansson B. Larsson L, et al. Serum cartilage oligomeric matrix protein (COMP) decreases in rheumatoid arthritis patients treated with infliximab or etanercept. Arthritis Res Ther. 2003;5:R181–R185. doi: 10.1186/ar760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Christiansen C. Tanko LB. Warming L, et al. Dose dependent effects on bone resorption and formation of intermittently administered intravenous ibandronate. Osteoporos Int. 2003;14:609–613. doi: 10.1007/s00198-003-1409-0. [DOI] [PubMed] [Google Scholar]
- 341.Christgau S. Bitsch-Jensen O. Hanover BN, et al. Serum CrossLaps for monitoring the response in individuals undergoing antiresorptive therapy. Bone. 2000;26:505–511. doi: 10.1016/S8756-3282(00)00248-9. [DOI] [PubMed] [Google Scholar]
- 342.Qvist P. Christgau S. Pedersen BJ. Schlemmer A. Christiansen C. Circadian variation in the serum concentration of C-terminal telopeptide of type I collagen (serum CTx): effects of gender, age, menopausal status, posture, daylight, serum cortisol, and fasting. Bone. 2002;31:57–61. doi: 10.1016/s8756-3282(02)00791-3. [DOI] [PubMed] [Google Scholar]
- 343.Alexandersen P. Karsdal MA. Qvist P. Reginster JY. Christiansen C. Strontium ranelate reduces the urinary level of cartilage degradation biomarker CTX-II in postmenopausal women. Bone. 2007;40:218–222. doi: 10.1016/j.bone.2006.07.028. [DOI] [PubMed] [Google Scholar]
- 344.Dam EB. Byrjalsen I. Karsdal MA. Qvist P. Christiansen C. Increased urinary excretion of C-telopeptides of type II collagen (CTX-II) predicts cartilage loss over 21 months by MRI. Osteoarthritis Cartilage. 2009;17:384–389. doi: 10.1016/j.joca.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 345.Conrozier T. Poole AR. Ferrand F, et al. Serum concentrations of type II collagen biomarkers (C2C, C1, 2C and CPII) suggest different pathophysiologies in patients with hip osteoarthritis. Clin Exp Rheumatol. 2008;26:430–435. [PubMed] [Google Scholar]
- 346.Koivula MK. Heliovaara M. Rissanen H, et al. Antibodies binding to citrullinated telopeptides of type I and type II collagens and to mutated citrullinated vimentin synergistically predict the development of seropositive rheumatoid arthritis. Ann Rheum Dis. 2012;71:1666–1670. doi: 10.1136/annrheumdis-2011-200848. [DOI] [PubMed] [Google Scholar]
- 347.Wagner E. Skoumal M. Bayer PM. Klaushofer K. Antibody against mutated citrullinated vimentin: a new sensitive marker in the diagnosis of rheumatoid arthritis. Rheumatol Int. 2009;29:1315–1321. doi: 10.1007/s00296-009-0854-2. [DOI] [PubMed] [Google Scholar]
- 348.Garnero P. Buchs N. Zekri J, et al. Markers of bone turnover for the management of patients with bone metastases from prostate cancer. Br J Cancer. 2000;82:858–864. doi: 10.1054/bjoc.1999.1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Arthur MJ. Fibrogenesis II. Metalloproteinases and their inhibitors in liver fibrosis. Am. J Physiol Gastrointest Liver Physiol. 2000;279:G245–G249. doi: 10.1152/ajpgi.2000.279.2.G245. [DOI] [PubMed] [Google Scholar]
- 350.Friedman SL. Liver fibrosis—from bench to bedside. J Hepatol. 2003;38(Suppl 1):S38–S53. doi: 10.1016/s0168-8278(02)00429-4. [DOI] [PubMed] [Google Scholar]
- 351.Martinez-Hernandez A. Amenta PS. The hepatic extracellular matrix. II. Ontogenesis, regeneration and cirrhosis. Virchows Arch A Pathol Anat Histopathol. 1993;423:77–84. doi: 10.1007/BF01606580. [DOI] [PubMed] [Google Scholar]
- 352.Martinez-Hernandez A. Amenta PS. The hepatic extracellular matrix. I. Components and distribution in normal liver. Virchows Arch A Pathol Anat Histopathol. 1993;423:1–11. doi: 10.1007/BF01606425. [DOI] [PubMed] [Google Scholar]
- 353.Schuppan D. Structure of the extracellular matrix in normal and fibrotic liver: collagens and glycoproteins. Semin Liver Dis. 1990;10:1–10. doi: 10.1055/s-2008-1040452. [DOI] [PubMed] [Google Scholar]
- 354.Rojkind M. Giambrone MA. Biempica L. Collagen types in normal and cirrhotic liver. Gastroenterology. 1979;76:710–719. [PubMed] [Google Scholar]
- 355.Hemmann S. Graf J. Roderfeld M. Roeb E. Expression of MMPs and TIMPs in liver fibrosis—a systematic review with special emphasis on anti-fibrotic strategies. J Hepatol. 2007;46:955–975. doi: 10.1016/j.jhep.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 356.Segovia-Silvestre T. Reichenbach V. Fernandez-Varo G, et al. Circulating CO3-610, a degradation product of collagen III, closely reflects liver collagen and portal pressure in rats with fibrosis. Fibrogenesis Tissue Repair. 2011;4:19. doi: 10.1186/1755-1536-4-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Veidal SS. Vassiliadis E. Barascuk N, et al. Matrix metalloproteinase-9-mediated type III collagen degradation as a novel serological biochemical marker for liver fibrogenesis. Liver Int. 2010;30:1293–1304. doi: 10.1111/j.1478-3231.2010.02309.x. [DOI] [PubMed] [Google Scholar]
- 358.Takahashi M. Suzuki M. Kushida K. Miyamoto S. Inoue T. Relationship between pentosidine levels in serum and urine and activity in rheumatoid arthritis. Br J Rheumatol. 1997;36:637–642. doi: 10.1093/rheumatology/36.6.637. [DOI] [PubMed] [Google Scholar]
- 359.Senolt L. Braun M. Olejarova M, et al. Increased pentosidine, an advanced glycation end product, in serum, synovial fluid from patients with knee osteoarthritis, its relation with cartilage oligomeric matrix protein. Ann Rheum Dis. 2005;64:886–890. doi: 10.1136/ard.2004.029140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Saudek DM. Kay J. Advanced glycation endproducts and osteoarthritis. Curr Rheumatol Rep. 2003;5:33–40. doi: 10.1007/s11926-003-0081-x. [DOI] [PubMed] [Google Scholar]
- 361.DeGroot J. Verzijl N. Wenting-Van Wijk MJ, et al. Age-related decrease in susceptibility of human articular cartilage to matrix metalloproteinase-mediated degradation: the role of advanced glycation end products. Arthritis Rheum. 2001;44:2562–2571. doi: 10.1002/1529-0131(200111)44:11<2562::aid-art437>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 362.Schwartz AV. Garnero P. Hillier TA, et al. Pentosidine, increased fracture risk in older adults with type 2 diabetes. J Clin Endocrinol Metab. 2009;94:2380–2386. doi: 10.1210/jc.2008-2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Chen JR. Takahashi M. Suzuki M, et al. Pentosidine in synovial fluid in osteoarthritis and rheumatoid arthritis: relationship with disease activity in rheumatoid arthritis. J Rheumatol. 1998;25:2440–2444. [PubMed] [Google Scholar]
- 364.Yamamoto M. Yamaguchi T. Yamauchi M. Yano S. Sugimoto T. Serum pentosidine levels are positively associated with the presence of vertebral fractures in postmenopausal women with type 2 diabetes. J Clin Endocrinol Metab. 2008;93:1013–1019. doi: 10.1210/jc.2007-1270. [DOI] [PubMed] [Google Scholar]
- 365.Miyata T. Ishiguro N. Yasuda Y, et al. Increased pentosidine, an advanced glycation end product, in plasma and synovial fluid from patients with rheumatoid arthritis and its relation with inflammatory markers. Biochem Biophys Res Commun. 1998;244:45–49. doi: 10.1006/bbrc.1998.8203. [DOI] [PubMed] [Google Scholar]
- 366.Yamamoto M. Yamaguchi T. Yamauchi M. Sugimoto T. Low serum level of the endogenous secretory receptor for advanced glycation end products (esRAGE) is a risk factor for prevalent vertebral fractures independent of bone mineral density in patients with type 2 diabetes. Diabetes Care. 2009;32:2263–2268. doi: 10.2337/dc09-0901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Kurien BT. Scofield RH. Autoimmunity and oxidatively modified autoantigens. Autoimmun Rev. 2008;7:567–573. doi: 10.1016/j.autrev.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Yoshida N. Okumura K. Aso Y. High serum pentosidine concentrations are associated with increased arterial stiffness and thickness in patients with type 2 diabetes. Metabolism. 2005;54:345–350. doi: 10.1016/j.metabol.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 369.Choi YG. Lim S. Characterization of anti-advanced glycation end product antibodies to nonenzymatically lysine-derived and arginine-derived glycated products. J Immunoassay Immunochem. 2009;30:386–399. doi: 10.1080/15321810903188136. [DOI] [PubMed] [Google Scholar]
- 370.Griffiths HR. Is the generation of neo-antigenic determinants by free radicals central to the development of autoimmune rheumatoid disease? Autoimmun Rev. 2008;7:544–549. doi: 10.1016/j.autrev.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 371.Sheikh Z. Ahmad R. Sheikh N. Ali R. Enhanced recognition of reactive oxygen species damaged human serum albumin by circulating systemic lupus erythematosus autoantibodies. Autoimmunity. 2007;40:512–520. doi: 10.1080/08916930701574331. [DOI] [PubMed] [Google Scholar]
- 372.Kralev S. Zimmerer E. Brueckmann M, et al. Elevation of the glycoxidation product N(epsilon)-(carboxymethyl)lysine in patients presenting with acute myocardial infarction. Clin Chem Lab Med. 2009;47:446–451. doi: 10.1515/CCLM.2009.100. [DOI] [PubMed] [Google Scholar]
- 373.Kurien BT. Scofield RH. Lipid peroxidation in systemic lupus erythematosus. Indian J Exp Biol. 2006;44:349–356. [PubMed] [Google Scholar]
- 374.Ahsan H. Ali A. Ali R. Oxygen free radicals and systemic autoimmunity. Clin Exp Immunol. 2003;131:398–404. doi: 10.1046/j.1365-2249.2003.02104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Sjoberg JS. Bulterijs S. Characteristics, formation, and pathophysiology of glucosepane: a major protein cross-link. Rejuvenation Res. 2009;12:137–148. doi: 10.1089/rej.2009.0846. [DOI] [PubMed] [Google Scholar]
- 376.Zamvil SS. Mitchell DJ. Moore AC, et al. T-cell epitope of the autoantigen myelin basic protein that induces encephalomyelitis. Nature. 1986;324:258–260. doi: 10.1038/324258a0. [DOI] [PubMed] [Google Scholar]
- 377.Moscarello MA. Wood DD. Ackerley C. Boulias C. Myelin in multiple sclerosis is developmentally immature. J Clin Invest. 1994;94:146–154. doi: 10.1172/JCI117300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Pritzker LB. Joshi S. Harauz G. Moscarello MA. Deimination of myelin basic protein. 2. Effect of methylation of MBP on its deimination by peptidylarginine deiminase. Biochemistry. 2000;39:5382–5388. doi: 10.1021/bi9925571. [DOI] [PubMed] [Google Scholar]
- 379.Fujii N. Momose Y. Ishii N, et al. The mechanisms of simultaneous stereoinversion, racemization, and isomerization at specific aspartyl residues of aged lens proteins. Mech Ageing Dev. 1999;107:347–358. doi: 10.1016/s0047-6374(98)00129-8. [DOI] [PubMed] [Google Scholar]
- 380.Fujii N. Momose Y. Ishibashi Y, et al. Specific racemization and isomerization of the aspartyl residue of alphaA-crystallin due to UV-B irradiation. Exp Eye Res. 1997;65:99–104. doi: 10.1006/exer.1997.0315. [DOI] [PubMed] [Google Scholar]
- 381.van Stipdonk MJ. Willems AA. Amor S, et al. T cells discriminate between differentially phosphorylated forms of alphaB-crystallin, a major central nervous system myelin antigen. Int Immunol. 1998;10:943–950. doi: 10.1093/intimm/10.7.943. [DOI] [PubMed] [Google Scholar]
- 382.Corthay A. Backlund J. Broddefalk J, et al. Epitope glycosylation plays a critical role for T cell recognition of type II collagen in collagen-induced arthritis. Eur J Immunol. 1998;28:2580–2590. doi: 10.1002/(SICI)1521-4141(199808)28:08<2580::AID-IMMU2580>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 383.Masson-Bessiere C. Sebbag M. Girbal-Neuhauser E, et al. The major synovial targets of the rheumatoid arthritis-specific antifilaggrin autoantibodies are deiminated forms of the alpha- and beta-chains of fibrin. J Immunol. 2001;166:4177–4184. doi: 10.4049/jimmunol.166.6.4177. [DOI] [PubMed] [Google Scholar]
- 384.Girbal-Neuhauser E. Durieux JJ. Arnaud M, et al. The epitopes targeted by the rheumatoid arthritis-associated antifilaggrin autoantibodies are posttranslationally generated on various sites of (pro)filaggrin by deimination of arginine residues. J Immunol. 1999;162:585–594. [PubMed] [Google Scholar]
- 385.Asaga H. Yamada M. Senshu T. Selective deimination of vimentin in calcium ionophore-induced apoptosis of mouse peritoneal macrophages. Biochem Biophys Res Commun. 1998;243:641–646. doi: 10.1006/bbrc.1998.8148. [DOI] [PubMed] [Google Scholar]
- 386.Newkirk MM. Goldbach-Mansky R. Lee J, et al. Advanced glycation end-product (AGE)-damaged IgG and IgM autoantibodies to IgG-AGE in patients with early synovitis. Arthritis Res Ther. 2003;5:R82–R90. doi: 10.1186/ar622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Trigwell SM. Radford PM. Page SR, et al. Islet glutamic acid decarboxylase modified by reactive oxygen species is recognized by antibodies from patients with type 1 diabetes mellitus. Clin Exp Immunol. 2001;126:242–249. doi: 10.1046/j.1365-2249.2001.01653.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Kim JH. Nam KH. Kwon OS, et al. Histone cross-linking by transglutaminase. Biochem Biophys Res Commun. 2002;293:1453–1457. doi: 10.1016/S0006-291X(02)00393-5. [DOI] [PubMed] [Google Scholar]
- 389.Monneaux F. Lozano JM. Patarroyo ME. Briand JP. Muller S. T cell recognition and therapeutic effect of a phosphorylated synthetic peptide of the 70K snRNP protein administered in MR/lpr mice. Eur J Immunol. 2003;33:287–296. doi: 10.1002/immu.200310002. [DOI] [PubMed] [Google Scholar]
- 390.Doyle HA. Mamula MJ. Post-translational protein modifications in antigen recognition and autoimmunity. Trends Immunol. 2001;22:443–449. doi: 10.1016/s1471-4906(01)01976-7. [DOI] [PubMed] [Google Scholar]
- 391.Doyle HA. Yan J. Liang B. Mamula MJ. Lupus autoantigens: their origins, forms, and presentation. Immunol Res. 2001;24:131–147. doi: 10.1385/IR:24:2:131. [DOI] [PubMed] [Google Scholar]
- 392.Mamula MJ. Epitope spreading: the role of self peptides and autoantigen processing by B lymphocytes. Immunol Rev. 1998;164:231–239. doi: 10.1111/j.1600-065x.1998.tb01223.x. [DOI] [PubMed] [Google Scholar]