Abstract
Cytomegalovirus (CMV) transmission via stem cells or marrow in CMV donor seropositive/recipient seronegative (D+/R−) hematopoietic cell transplantation (HCT) is surprisingly inefficient, and factors associated with transmission in these high-risk HCT recipients are unknown. In a retrospective cohort of D+/R− HCT recipients, cumulative incidence curve estimates were used to determine posttransplantation rates of CMV and multivariable Cox proportional models to assess risk factors associated with transmission. A total of 447 patients from 1995 to 2007 were eligible for enrollment. Overall, 85 of 447 (19.0%) acquired CMV at a median of 49 days (IQR 41–60) posttransplantation. CMV disease before day 100 occurred in 6 of 447 (1.3%) patients and in 7 of 447 (1.6%) after day 100. The donor graft, specifically the total nucleated cell count (adjusted hazard ratio [HR] 2.7; 95% confidence interval [CI], 1.4–4.7, P = .0002), was the only factor associated with CMV transmission in multivariable analyses. Notably, the source stem cells (marrow versus peripheral blood stem cell [PBSC]), screening method, and graft-versus-host disease (GVHD) were not associated with transmission. Thus, a highly cellular graft was the only identifiable risk factor associated with CMV transmission, suggesting that viral genomic content of the donor graft determines transmission efficiency in D+/R− HCT recipients.
Keywords: Cytomegalovirus, Transmission, Donor, Graft
INTRODUCTION
The β herpesvirus cytomegalovirus (CMV) is the most frequent viral complication following hematopoietic cell transplantation (HCT). CMV seropositive recipients (R+) have consistently been shown to be at highest risk for developing CMV infection and disease in HCT [1]. Seronegative patients who receive seropositive grafts (D+/R−) are also at risk for primary CMV transmission, and prior studies suggest that approximately 9% to 21% will develop CMV posttransplantation [2–6]. D+/R− recipients have also been shown to have a higher incidence of non-CMV infections and an overall mortality disadvantage when compared with seronegative recipients who receive seropositive grafts (D−/R−) [2].
With improvements in strategies to prevent transfusion-associated CMV [7–9], the seropositive allograft is likely responsible for most if not all CMV transmission during the first 100 days after transplantation in D+/R− HCT recipients. Not only are specific cellular reservoirs for CMV latency in donor grafts still debated [10], but donor and recipient factors associated with CMV transmission during transplantation are also poorly understood [1]. In order to better understand primary CMV, we set out to determine risk factors associated with CMV transmission in a large single-center cohort of D+/R− HCT recipients. Our primary goal was to assess the association between CMV transmission in D+/R− transplantations and the cellular components of the seropositive donor graft. Additionally, we assessed other pre- and posttransplantation factors thought to potentially alter risk for CMV transmission in this population.
MATERIALS AND METHODS
Subjects
We evaluated a cohort of CMV seronegative HCT recipients who underwent their first allogeneic HCT from a seropositive donor at the Fred Hutchinson Cancer Research Center (FHCRC) in Seattle, WA, between January 1, 1995, and December 31, 2007. Subjects who had been tested weekly for CMV, had complete screening data, and survived long enough to undergo center-based preemptive screening were eligible for inclusion in this study. Patients were excluded if they either died before initiating standard CMV testing (around day 10 after HCT) and did not develop CMV disease, or if they had no record of either antigenemia or polymerase chain reaction (PCR) testing for CMV during their posttransplantation period. Subjects who had undergone tandem transplant but were receiving their first allogeneic transplant were eligible for inclusion in this cohort.
All study activities were approved by the FHCRC institutional review board, and all participants provided written informed consent according the principles of the Declaration of Helsinki.
Data Source
Retrospective data were retrieved from a prospectively collected database of patients undergoing HCT at the FHCRC. In this dataset, pre- and posttransplantation demographic and outcome data were available for the first 100 days after HCT from clinical databases and medical records. Clinical and laboratory records after discharge are maintained in a long-term follow-up database and were also available for review.
Transplant Standards, Viral Prevention, and Surveillance
All patients and were followed using a preemptive strategy for CMV prevention. Testing consisted of either the pp65 antigenemia assay and or dual primer PCR testing of the plasma [11–13]. Subjects tested for antigenemia underwent weekly monitoring beginning around day 20, when sufficient neutrophil counts were present to allow for testing. Subjects tested by PCR underwent weekly testing of plasma for CMV DNA beginning the first week after transplant. All patients were tested until day 100 after HCT for primary CMV infection [14].
The institutional cutoff for initiation of preemptive antiviral therapy during the first 100 days after HCT was antigenemia at any level or any PCR level >500 copies/mL, unless patients were receiving >1 mg/kg of steroids at the time of diagnosis or had undergone a T cell–depleted transplantation procedure, where the cutoff was set at >100 copies/mL. Upon detection of CMV infection, patients were started on induction-dose ganciclovir or foscarnet followed by maintenance therapy [15]. “Noninterpretable” or an insufficient number of cells for antigenemia results were not treated, but patients were retested approximately 2 to 4 days later. All patients received seronegative or leukoreduced blood products per institutional protocol.
Antigenemia testing was the primary screening method for preemptive therapy from 1995 through 2005, at which point CMV PCR testing became routinely available. During the period between 2005 and February 2007, the use of PCR testing was driven by the discretion of the primary attending physician and some primary oncology protocols; PCR testing became the primary method beginning in February 2007. In order to assess the diagnostic effect of PCR testing, a subset of patients who were tested initially by antigenemia methods and who had stored frozen plasma available were retested by PCR methods. These samples, which had been frozen at −20°C at the time of collection, were thawed and retested for CMV DNA using a standardized dual-primer PCR-based assay [12].
All subjects received low-dose acyclovir or valacyclovir prophylaxis for herpes simplex virus 1 and 2 and varicellazoster virus as has previously been described [16], but none received high-dose acyclovir. All subjects received daily fluconazole as antifungal prophylaxis and standard Pneumocystis jirovercii antimicrobial therapy after engraftment. Patients who were neutropenic received prophylactic antibacterial therapy. Patients in our system have baseline IgG levels and then are tested routinely at +30, +60, and +90 posttransplantation. Patients with IgG levels<400 receive 400 mg/kg/month with postinfusion trough levels obtained monthly and redosed if levels remain low. Conditioning for HCT, as well as prophylaxis and treatment of graft-versus-host disease (GVHD), were performed according to current protocols standardized within the center as previously described elsewhere [15].
Definitions
For the purposes of this study, seronegative recipients who received a seropositive graft and developed any detectable CMV during the posttransplantation period were considered to have developed primary “CMV transmission.” Transmission detected in blood was defined as detection of any antigenemia or detection of CMV DNA by PCR in the plasma. As has been previously described, patients who developed CMV detectable by PCR but who cleared their DNAemia without therapeutic intervention or the development of disease were considered to have abortive infections [17]. CMV disease was defined by standardized criteria [18].
Subjects were followed to day +100 for the development of any CMV in blood and until 365 days after transplantation if they developed evidence of CMV transmission in the first 100 days; all were followed until day 365 for evidence of CMV disease. For the purposes of analysis, patients were classified by the screening method used posttransplantation (antigenemia only versus PCR only), except patients who underwent a mix of testing strategies or had retrospective PCR testing, who were classified as combination antigenemia/PCR. Fungal infections were defined by standard criteria [19] and patients with 1 or more positive blood cultures for Gram negative (GNR) bacteria during the first 100 days were considered to have GNR bacteremia. Acute GVHD (aGVHD) and chronic GVHD were defined according to established criteria [20] and subjects were divided into low and high-risk groups based on their primary malignancy at transplantation [21].
Statistical Analysis
Probabilities of CMV transmission were summarized using cumulative incidence estimates [22], where death or retransplantation were considered competing risks. To assess the utility of different preemptive screening methods, a subanalysis of all patients who had both antigenemia and PCR testing was completed, and cumulative incidence curves were used to summarize the rates of CMV acquisition detected. Univariable Cox proportional hazard regression models were used to examine the effect of host-, donor-, and transplant-associated factors on the development of CMV transmission at any site posttransplantation in CMV D+/R− HCT recipients. The following demographic variables were assessed: recipient and donor age, race, sex, stem cell source, underlying disease risk, conditioning regimen, and GVHD prophylaxis. The development of aGVHD (grade 0–2 versus 3–4) was evaluated in the model as a time-dependent risk factor. Additionally, hematologic and graft-associated risk factors were included: cellular components of the graft (CD14, CD34, total nucleated cells [TNC], and mononuclear cell count), donor and recipient ABO types, and number of platelet and red blood cell (RBC) units transfused. Because of the large variance in values for graft components and number of platelet and RBC units, these continuous variables were categorized in quartiles for the purposes of analyses.
A multivariable Cox proportional hazards model was built using a priori factors drawn from prior studies, including donor age, number of RBC units, and aGVHD. Other factors associated with CMV in univariable analyses (P<.20) were also included in multivariable analyses. Because CD34, TNC, and graft cell type are not independent variables, quartiles of CD34 were assessed in multivariate analysis in place of TNC to determine the hazard ratios specifically associated with this variable; graft cell type was not included in multivariate models. A receiver-operating curve was used to determine a specific threshold for TNC of the graft and CMV acquisition.
Additional assessment of risk of bacterial and fungal infections related to development of CMV transmission was assessed in a Cox proportional multivariable model that included age (>40 or ≤40), HLA mismatching, unrelated donor status, myeloablative versus nonmyeloablative transplant, CMV detected in blood, and aGVHD (grade 2–4). Patients with preexisting fungal infections were excluded from analyses of fungal incidence. CMV transmission and GVHD were considered time-dependent variables in these analyses. Finally, overall mortality and nonrelapse mortality were assessed in a Cox proportional multivariate model, which included disease risk and any CMV detection and GVHD (grade 3–4) as time-dependent variables. All statistical analyses were performed using SAS 9.1 (SAS Institute Inc., Cary, NC).
RESULTS
Study Population
From January 1995 to December 2007, 471 patients underwent a CMV D+/R− allogeneic transplant procedure at the FHCRC in Seattle, WA. Of these eligible patients, 24 were excluded because they died before the institution of CMV testing or they had incomplete CMV screening data, leaving 447 patients eligible for inclusion in this cohort. Patient demographic and transplant characteristics are shown in Table 1. The majority of patients underwent HCT for leukemia (acute myelogenous leukemia, acute lymphoblastic leukemia, and chronic myeloid leukemia) and received non-T cell–depleted myeloablative conditioning; only a small portion received antithymocyte globulin during conditioning (27 of 477 [5.7%]). More than one-half had primary malignancies that were considered to be high risk at time of transplantation.
Table 1.
Population Demographic Characteristics (n = 447)
| Characteristics | n (%)* |
|---|---|
| Age | |
| Median (IQR) | 42.3 (31.2–51.6) |
| Sex | |
| Male | 286 (64) |
| Female | 161 (36) |
| Race/Ethnicity | |
| Caucasian | 356 (80) |
| Hispanic | 21 (5) |
| Asian Pacific-Islander | 18 (4) |
| Native American | 12 (3) |
| African American | 9 (2) |
| Other | 31 (7) |
| Disease | |
| AML | 133 (30) |
| CML | 93 (21) |
| MDS | 68 (15) |
| ALL | 65 (15) |
| NHL | 29 (6) |
| MM | 19 (4) |
| HD | 12 (3) |
| Other | 28 (6) |
| Risk category† | |
| High | 238 (53) |
| Low | 209 (47) |
| Graft | |
| Bone marrow | 218 (49) |
| PBSC | 229 (51) |
| Donor relationship | |
| Sibling | 222 (50) |
| Unrelated | 194 (43) |
| Haploidentical | 27 (6) |
| Other | 4 (<1) |
| HLA disparity | |
| Matched | 326 (73) |
| Mismatched | 121 (27) |
| ABO disparity | |
| Matched | 223 (50) |
| Mismatched | 224 (50) |
| Conditioning regimen | |
| Myeloablative | |
| CY/TBI | 172 (38) |
| BU/CY | 126 (28) |
| T cell–depleted | 27 (6) |
| Other | 42 (9) |
| Nonmyeloablative | |
| Flu/TBI | 54 (12) |
| Other | 26 (6) |
| GVHD prophylaxis | |
| CSP/FK-506 ± MTX | 325 (73) |
| MMF | 94 (21) |
| Others | 28 (6) |
IQR indicates interquartile range; AML, acute myelogenous leukemia; CML, chronic myelogenous leukemia; MDS, myelodysplastic syndrome; ALL, acute lymphocytic leukemia; NHL, non-Hodgkin lymphoma; MM, multiple myeloma; HD, Hodgkin’s lymphoma; PBSC, peripheral blood stem cell; HLA, human leukocyte antigen; CY, cytoxan; TBI, total body irradiation; BU, busulfan; Flu, fludarabine; CSP, cyclosporine; MTX, methotrexate; MMF, mycophenolate mofetil.
Data are no. (%) of patients, unless otherwise indicated. Values may not equal 100% because of rounding.
High risk: accelerated phase, blast crisis, or relapse; low risk: remission or chronic phase.
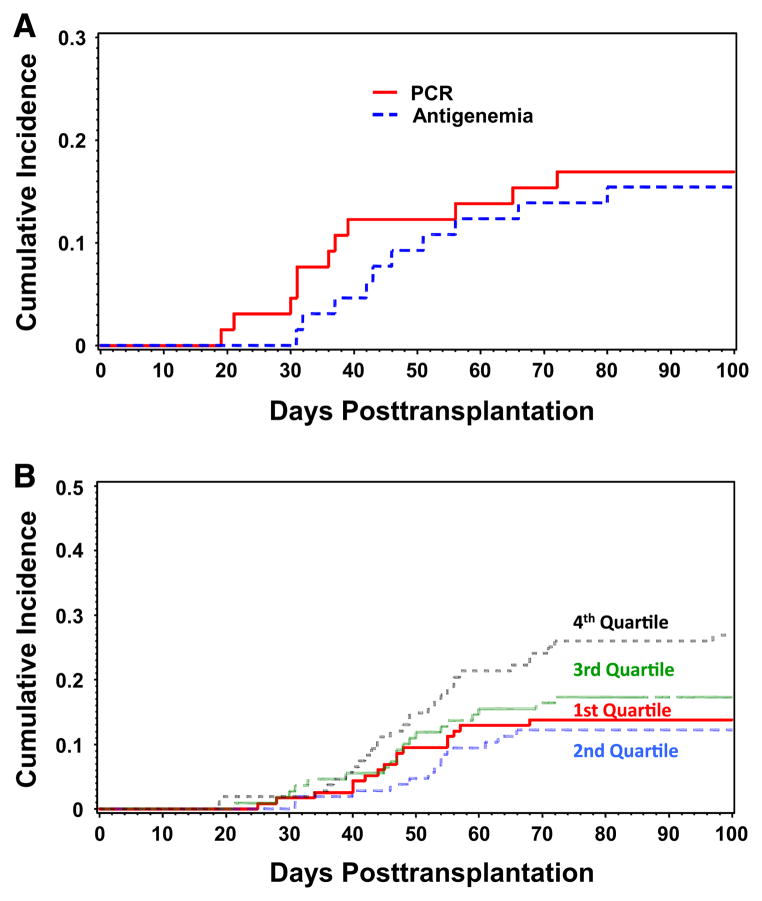
All patients underwent surveillance for CMV posttransplantation during the first 100 days (Figure 1). The majority of patients underwent testing by antigenemia only (393 of 447 [88%], compared with antigenemia/PCR (28 of 447 [6%]) and PCR only (26 of 447 [6%]). A total of 65 (14.5%) patients underwent additional testing by CMV PCR on stored frozen samples. A subset of those were tested initially by antigenemia only (n = 49), whereas the others had a mix of antigenemia/PCR (n = 16) during the immediate posttransplantation period. All patients in this subset had repeat PCR testing on their stored blood. Patients who had retrospective testing by PCR were included in the PCR group for purposes of analyses.
Figure 1.
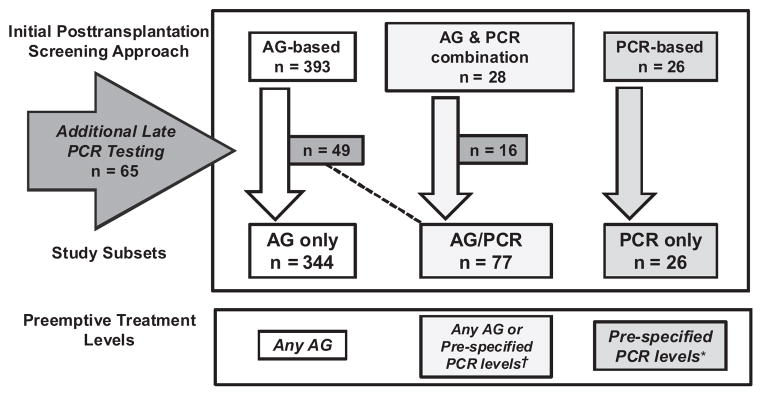
Schema of CMV screening and development of study subsets (n = 447). Abbreviations: AG, antigenemia; PCR, polymerase chain reaction; † treated for any AG, except a few cases that had both AG testing and PCR where patients were given preemptive therapy for positive PCR at varying levels, *patients given preemptive therapy at CMV PCR levels ≥500 copies/mL, except patients on ≥1 mg/kg of steroids who are started on treatment at a level of ≥100 copies/mL.
CMV Outcome
CMV acquisition
CMV transmission was detected in 85 of 447 (19%) patients during the first year posttransplantation. Of these, 76 of 447 (17%) CMV transmission was detected before day 100 (Figure 2), and an additional 9 of 447 (2%) had CMV transmission detected between day 100 and 1 year. The median time to development of CMV transmission in the first year posttransplantation was 49 days (interquartile ratio [IQR] 41–60).
Figure 2.
Cumulative incidence of CMV transmission by day 100 in a cohort of CMV D+/R− hematopoietic cell transplant recipients (n = 447). Abbreviations: CMV, cytomegalovirus; D+, donor positive; R−, recipient negative. Patients censored at date or last contact, and death and retransplantation were considered competing risks.
CMV detection in blood
A total of 81 of 447 (18.1%) patients had evidence of CMV transmission detected in blood through a positive antigenemia or plasma PCR during the first year posttransplantation. The majority (73 of 447 [16.3%]) of patients were positive during the first 100 days, the period of routine preemptive screening; 8 of 447 (2%) were positive beyond 100 days. The overall incidence of CMV in the first year posttransplantation was higher in subjects tested by PCR during the preemptive period (25 of 103 [24.3%]) than in those tested by antigenemia only (56 of 344 [16.3%]) (P = .065). Because a small number of patients were tested by PCR only (6 of 26 [23%]), and because rates were comparable to those tested by a combination of antigenemia/PCR (29 of 77 [25%], P = .87), these 2 groups were combined for the purposes of analyses.
The median level of first detection in patients who were antigenemia positive by day 100 was 2 cells/slide (IQR 1,6), and the median maximum detected level was 6 cells/slide (IQR 1,18). The median level of first detection in patients who were PCR positive by day 100 was 64 copies/mL (IQR 44, 315), and the median of the maximum viral load detected was 1467 copies/mL (IQR 167, 7401). Patients who were tested by PCR at some point within the first 100 days had CMV detected earlier posttransplantation (median 41 days, IQR 31, 56) when compared with those tested by antigenemia only (median 49.5, IQR 45, 55) (P = .02).
A total of 49 patients who were initially tested by antigenemia only and 16 patients in the antigenemia/PCR only group underwent retrospective PCR testing to supplement data and to assure testing for the entire posttransplantation period for both modalities (n=65) (Figure 1). Of this subgroup, 11 of 65 were positive by antigenemia (17%) and 13 of 65 by PCR (20%) (Figure 3A). Nine patients were positive by both methods: 2 only by antigenemia and 4 only by PCR testing. Of the 9 total patients detected by both methods, 7 of 9 (80%) were found earlier by PCR at a mean of 10.6 days earlier (range: 7–15) than by antigenemia and all had a low viral load detected (mean 53 copies/mL [range: 43–421]); 2 patients were positive by PCR and antigenemia on the same day.
Figure 3.
Cumulative incidence of CMV transmission detected in blood by testing method* and total nucleated count in graft (in quartiles) in a cohort of CMV D+/R− hematopoietic cell transplant recipients (n = 447). (A) CMV outcomes by testing method. Abbreviations: CMV, cytomegalovirus; D+, donor positive; R−, recipient negative; antigenemia = pp65 antigenemia testing; PCR, polymerase chain reaction testing. Patients censored at date or last contact, and death and retransplantation were considered competing risks. *This figure represents patients who had supplemental retrospective PCR testing to assure complete testing by both modalities. (B) CMV incidence by total nucleated count quartiles. Patients censored at date of last contact, and death and retransplantation were considered competing risks.
CMV disease
A total of 6 of 447 (1.3%) patients developed CMV disease during the first 100 days posttransplantation at a mean of 50.3 days (range: 19–78). Site of CMV disease included 3 gastrointestinal (GI) disease, 1 pneumonia, and 1 each with disseminated disease and biopsy-proven invasive sinus disease. A similar percentage presented with disease (7 of 337 [1.5%]) from day 101 to 1 year posttransplantation. Pneumonia was the most common cause of late disease (n = 4), followed by GI disease (n = 3); late disease occurred at a mean of 150.3 days (range: 101–314). There was no difference in rates of disease based on type of primary surveillance in the immediate posttransplantation period (antigenemia/PCR 2 of 103 [1.9%] versus antigenemia only (4 of 344 [1.1%], P = .62) or the late period (antigenemia/PCR 1 of 103 [0.97%] versus antigenemia only 6 of 344[1.7%]), P = .69); none of those tested by PCR only developed disease. Three patients presented with disease as their only manifestation of CMV transmission:, 2 with early GI disease (days 19 and 47) and 1 with late pneumonia (day 314). One additional patient developed invasive sinus disease, proven by histopathology, before developing antigenemia (day 55 versus 71).
Abortive Infections
Because all patients who had any positive level of antigenemia were given preemptive ganciclovir per institutional standard practice, only patients tested by PCR could have potentially developed a level of detection below which therapy was not administered. A total of 4 of 102 (3.9%) patients tested by PCR were noted to have CMV DNAemia detected but did not receive antiviral therapy. Initial detectable CMV DNAemia levels (mean 61.8 copies/mL [range: 27–140]), and maximum values (mean 101.5 copies/mL [range: 39–167]) were low. Two had more than 1 positive weekly sample (2 and 4 weeks after the first), including a patient who had retrospective sampling on frozen samples. Of these 4 patients, all survived >3 months posttransplantation, and by definition, no patient with an abortive infection developed CMV disease or required CMV therapy [17].
CMV shedding
Only 1 patient was noted to be positive by shedding only; our center does not routinely assess for CMV shedding. This patient was found to have CMV by culture from nasal wash at day 69 posttransplant but did not develop other CMV related complications.
RISK FACTOR ANALYSES
In univariable analyses, patients transplanted with a higher number of TNC in their graft (upper fourth quartile) were at significantly higher risk for CMV transmission (hazard ratio [HR] 2.0; 95% confidence interval [CI], 1.2–3.1) (Table 2). Additionally there was a trend toward an association with a higher risk for CMV transmission with a higher quartile of CD34 cells in the graft (HR 1.5; 95% CI, 0.9–2.6). Other cellular components, such as mononuclear cell count and CD14 components of the graft, did not appear to alter risk of CMV acquisition.
Table 2.
Cox Regression of Risk Factors for Any CMV Transmission (n = 77) during the First 100 Days after Transplantation in D+/R− Patients (n = 447)
| Risk Factor | Univariable HR (95% CI) | Multivariable† HR (95% CI) | P Value |
|---|---|---|---|
| Demographic factors | |||
| Recipient age (per 10 years) | 1.0 (0.9–1.2) | — | |
| Donor age (per 10 years) | 0.9 (0.8–1.1) | 0.9 (0.7–1.1) | .15 |
| Gender | |||
| Recipient | |||
| Male | 1 | — | |
| Female | 1.2 (0.8–2.0) | ||
| Donor | |||
| Male | 1 | — | |
| Female | 1.0 (0.6–1.6) | ||
| Risk group | |||
| High | 1 | — | |
| Low | 1.0 (0.7–1.6) | ||
| Transplant characteristics | |||
| Conditioning regimen | |||
| Myeloablative | 1 | 1 | .26 |
| Nonmyeloablative | 1.6 (0.9–2.6) | 1.4 (0.8–2.6) | |
| GVHD prophylaxis | |||
| CSP/FK-506 ± MTX | 1 | ||
| MMF | 1.4 (0.9–2.4) | — | |
| Others | 0.7 (0.2–2.3) | ||
| aGVHD | |||
| Grade 0–2 | 1 | — | |
| Grade 3–4 | 0.7 (0.2–2.8) | ||
| CMV test method | |||
| AG only | 1 | 1 | .12 |
| PCR or AG + PCR | 1.7 (1.1–2.8) | 1.5 (0.9–2.5) | |
| Graft characteristics | |||
| Cell type | |||
| BM | 1 | — | |
| PBSC | 1.7 (1.1–2.7) | ||
| HLA matching | |||
| Matched | 1 | 1 | |
| Mismatched | 1.2 (0.6–2.6) | 1.9 (0.9–4.3) | .11 |
| Unrelated | 0.7 (0.4–1.1) | 1.2 (0.7–2.2) | .54 |
| ABO recipient | |||
| A | 1 | ||
| B | 0.8 (0.4–1.9) | — | |
| AB | 1.1 (0.7–1.8) | ||
| O | NA | ||
| Donor | 1 | ||
| A | 1 | ||
| B | 0.2 (0.05–0.9) | 0.3 (0.1–1.1) | .06 |
| AB | 1.1 (0.7–1.8) | 1.0 (0.6–1.8) | .83 |
| O | 1.1 (0.3–3.4) | 1.5 (0.4–5.1) | .51 |
| ABO mismatch | |||
| Matched | 1 | — | |
| Mismatched | 0.9 (0.6–1.4) | ||
| Cellular components | |||
| TNC‡ | |||
| Below Q3 | 1 | 1 | |
| Above Q3 | 2.0 (1.2–3.1) | 2.7 (1.6–4.7) | .0002 |
| CD-14 | |||
| Below Q3 | 1 | — | |
| Above Q3 | 0.9 (0.5–1.8) | ||
| MNC | |||
| Below Q3 | 1 | — | |
| Above Q3 | 1.0 (0.5–2.3) | ||
| Transfusion risks | |||
| Total RBC units | 1 | ||
| Below Q3 | 1 | 0.7 (0.4–1.3) | .25 |
| Above Q3 | 0.99 (0.6–1.7) | ||
| Total PLT units | — | ||
| Below Q3 | 1 | ||
| Above Q3 | 0.97 (0.6–1.7) | ||
D+/R− indicates donor positive/recipient negative; CSP, cyclosporine; FK, FK-506 (tacrolimus); MMF, mycophenolate mofetil; AG, antigenemia; BM, bone marrow; PBSC, peripheral blood stem cell; HLA, human leukocyte antigen; Q3, third quartile; MNC, mononuclear count; PLT, platelets.
Because of rounding not all values equal 100.
Multivariate model adjusted a priori risk factors and risk factors associated with P < .20 in univariate analyses. Factors in multivariate model included, donor age, HLA matching, CMV test method, TNC of graft, donor ABO, and total RBC units transfused.
All results run with TNC, except CD-34, which replaced TNC in the multivariate model for this result.
The only other transplant factors that appeared to potentially alter the risk for CMV transmission were donor blood type B when compared with blood type A and donor graft type (peripheral blood stem cell versus bone marrow). Other donor blood types (AB and O) were similar to blood type A, and there was no association with recipient blood type or blood type mismatch (Table 2). The total number of RBC units transfused (highest quartile) (HR 0.99; 95% CI 0.6–1.7) and number of platelet transfusions (highest quartile [HR 0.97; 95% CI 0.6–1.7]) were not associated. All other potential risk factors were not associated with CMV transmission, including the use of antithymocyte globulin during conditioning (data not shown).
In multivariable analyses, a high TNC in the donor graft (HR 2.7; 95% CI, 1.4–4.7, P = .0002) was significantly associated with CMV transmission. There were trends toward increased rates of transmission detected in those tested by antigenemia/PCR during follow-up (HR 1.5; 95% CI, 0.9–2.5), and toward protection from transmission by donor ABO type (others versus type B) (HR 0.3; 95% CI 0.1–1.1, P = .06). When CD34 was replaced in the model for TNC, it demonstrated a trend toward an association (HR 1.6; 95% CI, 0.9–2.9) that did not reach statistical significance. Cumulative incidence curves for the quartiles of TNC are also shown in Figure 3B. In order to assess if there was a threshold for CMV transmission based on the TNC of the graft, a receiver-operating curve was performed (Figure 4). All other transplant and hematologic factors, including conditioning regimen, RBC units transfused, donor age, and HLA matching were not associated with CMV transmission in multivariable analyses.
Figure 4.
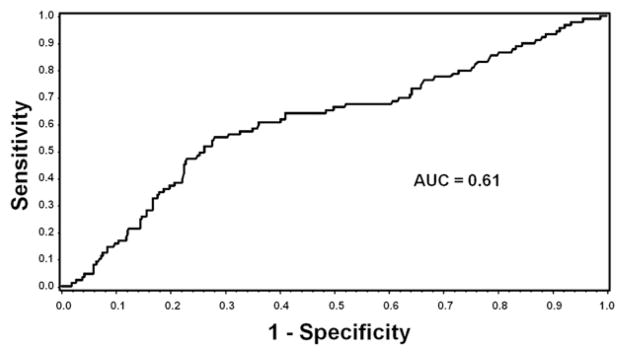
ROC analysis of total nucleated count and any CMV reactivation.* Abbreviations: ROC, receiver-operating curve; CMV, cytomegalovirus; AUC, area under the curve. *Any CMV reactivation includes both patients with shedding and disease only. Best sensitivity (64%) and specificity (59%) at TNC of 7.84 × 108.
OTHER COMPLICATIONS
A total of 50 (11%) patients developed GNR infections during the first 100 days following transplantation, and 68 of 447 (15%, 50 invasive molds, 18 candidemia) developed fungal infections in the first year posttransplantation. In univariable models, neither GNR bacteremia (HR 1.06, 95% CI 0.53–2.12) nor fungal infections (HR 1.00, 95% CI 0.46–2.14) were associated with CMV infection in the blood in this cohort. Multivariable analyses demonstrated no association between CMV transmission detected in blood and GNR bacteremia at day 100 (HR 0.90, 95% CI 0.10–8.40) or fungal infections at 1 year (HR 1.01, 95% CI 0.47–2.18). Detection of CMV was also not associated with a risk of overall mortality (HR 0.96, 95% CI 0.60–1.53) or nonrelapse mortality at 1 year (HR 1.18, 95% CI 0.68–2.05) in multivariable analyses.
DISCUSSION
CMV seronegative HCT recipients who receive a seropositive graft are at significant risk for CMV complications following hematopoietic cell transplantation. In our cohort, 20% of D+/R− patients developed CMV transmission during the first year of follow-up, and 3% developed invasive disease. The only risk factor associated with CMV transmission was a high graft TNC count. Other cellular components of the donor graft, including monocytes (CD14), did not appear to affect CMV transmission. No other pre- or posttransplant risk factors appeared to alter efficiency of CMV transmission, although the addition of CMV PCR testing did appear to detect CMV transmission earlier and identified several abortive infections.
Epidemiologic data have demonstrated that CMV seropositive recipients are at highest risk for the development of CMV complications. The majority of epidemiologic and intervention trials focus on this high risk population or combine D+/R− with R+ patients. Even studies focusing on risk related to donor CMV serostatus have centered on seropositive recipients [23,24]. This distinction is important because CMV outcomes seen in seropositive recipients are because of different pathologic mechanisms than those that occur in D+/R− transplant recipients. In seropositive recipients, CMV complications primarily stem from reactivation of latent virus [25,26], whereas in seronegative patients these events indicate transmission of virus. Risk factors for development of CMV in D+/R− patients may therefore be very different than those seen in seropositive recipients, depending less on posttransplant immunosuppression and more on total exposure to the virus [27]. Furthermore, there are no studies that have focused on the smaller population of D+/R− patients and assessed risk factors for CMV acquisition that are specific to this population.
These data help to define the importance of the donor graft as the most frequent source of CMV transmission in D+/R− recipients. Previous studies have suggested that in infected individuals, bone marrow-derived hematopoietic cells, granulocyte-macrophage progenitors, and peripheral blood monocytes serve as reservoirs of latent virus from which reactivation occurs during immunosuppression or immunodeficiency [28–34]. These cells can support active CMV replication and the allogeneic transplant process itself may stimulate reactivation [35]. Still, the challenge of successfully reactivating CMV from naturally infected samples in the laboratory has limited the ability to translate these findings into clinically relevant predictors for transmission [27,35]. Although determining a cell-specific reservoir of CMV latency has continued to be a source of ongoing research [10,35], the lack of such models has hampered the development of prevention strategies in this population.
Donor PBSC as a graft source was also associated with CMV transmission in univariable analyses and is consistent with data from a recent randomized trial that demonstrated a trend toward higher rates of CMV complications in PBSC graft recipients [36]. The association did not remain statistically significant in multivariable analyses, suggesting that the higher cellular content found in PBSC grafts when compared with bone marrow plays a more important role than the type of graft [37–39]. We also analyzed other pretransplant risk factors considered to be associated with CMV transmission, including total-body irridiation dose, donor age, HLA matching, as well as posttransplant factors such as aGVHD [24,40–43]. In our study, aGVHD itself, as well as other factors associated with the development of GVHD such as immunosuppressive prophylaxis [42,43], were not associated with CMV transmission. HLA matching and conditioning with TBI also had no clear association with transmission. Similarly, although previously reported in the context of blood transfusion-associated CMV transmission [8], we found no associations between transmission and number of RBC or platelet units transfused. Perhaps it is the allogeneic process itself, as has been suggested, that is the most important factor leading to the lytic phase of replication and subsequent detection [35].
The close association with donor blood type B seen in univariate analyses is unclear. There are no known associations between ABO blood type and CMV, and this association did not appear to be linked to ABO mismatch between the donor and recipient. Risk of viral infections has been associated with ABO blood type. Interestingly blood type B has been associated with a decreased risk of Norovirus infection [44] but an increased risk for HHV-8 infection [45]. Although there continued to be a trend in multivariate analyses, this association did not remain significant. Additionally, the percentage of donors with blood type B (10.5%) was consistent with those reported in multicenter blood donor registry data, suggesting no baseline protection from CMV infection [46]. Although it is possible that donor blood type B is more resistant to primary CMV infection, this needs further evaluation in larger cohort studies.
CMV transmission was more frequently detected and at earlier time points by PCR testing during the posttransplant period than with standard antigenemia testing; however, the effect of this improved detection was not statistically significant when controlling for other factors such as TNC. Also, in a subset of patients who were tested by both methods, PCR identified CMV transmission earlier than antigenemia but the rates of detection were similar (Figure 3A). The risk of CMV transmission in seronegative recipients in prior studies is reported to be between 9% and 21% [2–5,15,47], although population differences (eg, pediatric versus adult) and variable prevention and screening strategies make comparisons between studies difficult. We found that approximately 19% of patients acquired CMV during the first year posttransplantation. Furthermore, the higher rates seen in those tested by PCR indicate that rates of CMV transmission in D+/R− transplant recipients may actually be higher than previously reported. However, some of those detected by PCR are likely abortive infections [17]. The cases of abortive infection described in this study are plausible because viral load was low and patients did not progress to antigenemia or disease. Earlier detection through PCR screening did not appear to translate into lower CMV disease rates. This is not surprising, considering that there were only a small number of patients who were missed by antigenemia and relatively few that developed preengraftment CMV, a time period where PCR has advantages over antigenemia. Finally, CMV transmission was not associated with bacterial or fungal complications.
The strengths of our study include the size of our cohort, the uniformity of prevention strategies, and consistency of short and long-term clinical data available for review. As with all retrospective studies, these analyses are not without limitations. We included all patients with evidence of CMV infection from plasma or disease locations, but it is possible that we missed evidence of transmission through viral shedding at other sites. Urine and oral screening may have little relevance for disease outcomes [48–50], suggesting that additional screening from nonblood sites would not have altered our results. Although not all patients were tested with both PCR and the antigenemia assay, we do not think that this affected that transmission rate significantly as both multivariate model and the head-to-head comparison of PCR versus antigenemia did not suggest a statistically significant increase in detecting CMV transmission (although PCR detected it earlier).
Although it is possible that seropositive recipients who were falsely seronegative on screening may have been included in our cohort, it is unlikely to have affected our results because misclassification of serostatus is a very uncommon event. Although we did not find an association with blood transfusions, the most likely alternate source during transplantation, we could not account for other possible sources of CMV acquisition not included in our risk factor analyses. The amount of intravenous immunoglobulin use is not available in this dataset and may have affected rates of CMV transmission in this cohort; however, the modest benefit seen with weekly CMV-specific intravenous immunoglobulin [51] suggests that this would have had little impact on these analyses. Finally, the limited data on the specific cellular makeup of the seropositive graft and T cell reconstitution following transplantation do not allow for a complete in-depth analyses of other graft components and immune reconstitution on CMV outcomes.
In summary, we demonstrate a critical role for the cellularity of the donor graft in CMV transmission in D+/R− HCT recipients. Our improved preemptive strategies have helped to decrease the rate of CMV disease in HCT [52], but it remains an important yet elusive goal to prevent primary transmission in the D+/R− population. These data make a strong case for the importance of the donor graft in primary CMV transmission to D+/R− patients, but suggest that until we understand primary CMV biology there may be limitations in our ability to intervene to prevent CMV transmission. As such, future studies aimed at understanding primary reservoirs for latent CMV within the donor graft and the role of viral load in transmission are needed. Assessments of transplant or immunologic factors, such as the role of concomitant transfer of donor-derived cellular and humoral immunity associated with conversion from latent to lytic replication, are also needed. Future studies addressing recipient target cell receptors and host genetics, or those evaluating donor immunity may provide additional predictors for viral transmission that could lead to important changes in donor selection or assist in risk stratification.
Acknowledgments
The authors would like to acknowledge Craig Silva and Chris Davis for their assistance with computerized database support.
Footnotes
AUTHORSHIP STATEMENT
S.P. and R.S. participated in research design, data collection, performed statistical analyses, and wrote the manuscript. H.X. performed statistical analyses and contributed to the research design, writing, and review of the manuscript. M.P., J.S., V.I., and L.K. participated in data collection and contributed to the writing and review of the manuscript. T.S., M.H., and T.H. organized CMV plasma samples, performed the retrospective CMV PCR tests, and contributed to the writing and review of the manuscript. M.B. and L.C. participated in research design and contributed to the writing and review of the manuscript.
Financial disclosure: This research was supported by NIH Grants CA-18029 and CA-15704. Dr. Pergam is supported by NIH Grant K23HL096831 and an ASBMT/Viropharma New Investigator Award. Dr. Pollack is supported by the Joel Meyers Endowment and an IDSA/Astellas Postdoctoral Fellowship Award in Transplant Infectious Diseases. Dr. Boeckh is supported by NIH Grant K24HL093294. Data from this manuscript have been presented in part at the ASBMT/CIBMTR Tandem Meeting in San Diego, CA, February 2008.
AUTHOR CONFLICT OF INTEREST STATEMENT
S.A.P. has received research support from Chimerix, Merck, Viropharma, and has been a consultant from Chimerix and Optimer Pharmaceuticals. M.J.B. has received research funding from Chimerix, Roche, Viropharma, and Vical and has been a consultant for Chimerix, Genentech, Roche, Vical, Novartis, Astellas, Boehringer Ingelheim, and Viropharma.
References
- 1.Boeckh M, Nichols WG. The impact of cytomegalovirus serostatus of donor and recipient before hematopoietic stem cell transplantation in the era of antiviral prophylaxis and preemptive therapy. Blood. 2004;103:2003–2008. doi: 10.1182/blood-2003-10-3616. [DOI] [PubMed] [Google Scholar]
- 2.Nichols WG, Corey L, Gooley T, Davis C, Boeckh M. High risk of death due to bacterial and fungal infection among cytomegalovirus (CMV)-seronegative recipients of stem cell transplants from seropositive donors: evidence for indirect effects of primary CMV infection. J Infect Dis. 2002;185:273–282. doi: 10.1086/338624. [DOI] [PubMed] [Google Scholar]
- 3.George B, Pati N, Gilroy N, et al. Pre-transplant cytomegalovirus (CMV) serostatus remains the most important determinant of CMV reactivation after allogeneic hematopoietic stem cell transplantation in the era of surveillance and preemptive therapy. Transpl Infect Dis. 2010;12:322–329. doi: 10.1111/j.1399-3062.2010.00504.x. [DOI] [PubMed] [Google Scholar]
- 4.Walker CM, van Burik JA, De For TE, Weisdorf DJ. Cytomegalovirus infection after allogeneic transplantation: comparison of cord blood with peripheral blood and marrow graft sources. Biol Blood Marrow Transplant. 2007;13:1106–1115. doi: 10.1016/j.bbmt.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 5.Matthes-Martin S, Lion T, Aberle SW, et al. Pre-emptive treatment of CMV DNAemia in paediatric stem cell transplantation: the impact of recipient and donor CMV serostatus on the incidence of CMV disease and CMV-related mortality. Bone Marrow Transplant. 2003;31:803–808. doi: 10.1038/sj.bmt.1703927. [DOI] [PubMed] [Google Scholar]
- 6.Trenschel R, Ross S, Husing J, et al. Reduced risk of persisting cytomegalovirus pp65 antigenemia and cytomegalovirus interstitial pneumonia following allogeneic PBSCT. Bone Marrow Transplant. 2000;25:665–672. doi: 10.1038/sj.bmt.1702216. [DOI] [PubMed] [Google Scholar]
- 7.Bowden RA, Slichter SJ, Sayers M, et al. A comparison of filtered leukocyte-reduced and cytomegalovirus (CMV) seronegative blood products for the prevention of transfusion-associated CMV infection after marrow transplant. Blood. 1995;86:3598–3603. [PubMed] [Google Scholar]
- 8.Nichols WG, Price TH, Gooley T, Corey L, Boeckh M. Transfusion-transmitted cytomegalovirus infection after receipt of leukoreduced blood products. Blood. 2003;101:4195–4200. doi: 10.1182/blood-2002-10-3143. [DOI] [PubMed] [Google Scholar]
- 9.Boeckh M, Fries B, Nichols WG. Recent advances in the prevention of CMV infection and disease after hematopoietic stem cell transplantation. Pediatr Transplant. 2004;8(Suppl 5):19–27. doi: 10.1111/j.1398-2265.2004.00183.x. [DOI] [PubMed] [Google Scholar]
- 10.Mocarski ES, Shenk T, Pass R. Cytomegaloviruses. In: Knipe D, Howley P, Griffin D, et al., editors. Field’s Virology. Philadelphia, PA: Lippincott Williams & Wilkins; 2007. pp. 2701–2772. [Google Scholar]
- 11.Boeckh M, Ljungman P. How we treat cytomegalovirus in hematopoietic cell transplant recipients. Blood. 2009;113:5711–5719. doi: 10.1182/blood-2008-10-143560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boeckh M, Huang M, Ferrenberg J, et al. Optimization of quantitative detection of cytomegalovirus DNA in plasma by realtime PCR. J Clin Microbiol. 2004;42:1142–1148. doi: 10.1128/JCM.42.3.1142-1148.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boeckh M, Gallez-Hawkins GM, Myerson D, Zaia JA, Bowden RA. Plasma polymerase chain reaction for cytomegalovirus DNA after allogeneic marrow transplantation: comparison with polymerase chain reaction using peripheral blood leukocytes, pp65 antigenemia, and viral culture. Transplantation. 1997;64:108–113. doi: 10.1097/00007890-199707150-00020. [DOI] [PubMed] [Google Scholar]
- 14.Boeckh M, Gooley TA, Myerson D, Cunningham T, Schoch G, Bowden RA. Cytomegalovirus pp65 antigenemia-guided early treatment with ganciclovir versus ganciclovir at engraftment after allogeneic marrow transplantation: a randomized double-blind study. Blood. 1996;88:4063–4071. [PubMed] [Google Scholar]
- 15.Nakamae H, Kirby KA, Sandmaier BM, et al. Effect of conditioning regimen intensity on CMV infection in allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2009;15:694–703. doi: 10.1016/j.bbmt.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Erard V, Guthrie KA, Varley C, et al. One-year acyclovir prophylaxis for preventing varicellazoster virus disease after hematopoietic cell transplantation: no evidence of rebound varicellazoster virus disease after drug discontinuation. Blood. 2007;110:3071–3077. doi: 10.1182/blood-2007-03-077644. [DOI] [PubMed] [Google Scholar]
- 17.de Gast GC, Boland GJ, Vlieger AM, et al. Abortive human cytomegalovirus infection in patients after allogeneic bone marrow transplantation. Bone Marrow Transplant. 1992;9:221–225. [PubMed] [Google Scholar]
- 18.Ljungman P, Griffiths P, Paya C. Definitions of cytomegalovirus infection and disease in transplant recipients. Clin Infect Dis. 2002;34:1094–1097. doi: 10.1086/339329. [DOI] [PubMed] [Google Scholar]
- 19.De Pauw B, Walsh TJ, Donnelly JP, et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis. 2008;46:1813–1821. doi: 10.1086/588660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus conference on acute GVHD grading. Bone Marrow Transplant. 1995;15:825–828. [PubMed] [Google Scholar]
- 21.Junghanss C, Boeckh M, Carter RA, et al. Incidence and outcome of cytomegalovirus infections following nonmyeloablative compared with myeloablative allogeneic stem cell transplantation, a matched control study. Blood. 2002;99:1978–1985. doi: 10.1182/blood.v99.6.1978. [DOI] [PubMed] [Google Scholar]
- 22.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18:695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 23.Ljungman P, Brand R, Einsele H, Frassoni F, Niederwieser D, Cordonnier C. Donor CMV serologic status and outcome of CMV-seropositive recipients after unrelated donor stem cell transplantation: an EBMT megafile analysis. Blood. 2003;102:4255–4260. doi: 10.1182/blood-2002-10-3263. [DOI] [PubMed] [Google Scholar]
- 24.Kollman C, Howe CW, Anasetti C, et al. Donor characteristics as risk factors in recipients after transplantation of bone marrow from unrelated donors: the effect of donor age. Blood. 2001;98:2043–2051. doi: 10.1182/blood.v98.7.2043. [DOI] [PubMed] [Google Scholar]
- 25.Meyers JD, Flournoy N, Thomas ED. Risk factors for cytomegalovirus infection after human marrow transplantation. J Infect Dis. 1986;153:478–488. doi: 10.1093/infdis/153.3.478. [DOI] [PubMed] [Google Scholar]
- 26.Boeckh M, Ljungman P. Cytomegalovirus infection after hemopoietic stem cell transplantation. In: Bowden RA, Ljungman P, Paya C, editors. Transplant Infections. Philadelphia, PA: Lippincott Williams & Wilkins; 2003. pp. 277–297. [Google Scholar]
- 27.Slobedman B, Mocarski ES. Quantitative analysis of latent human cytomegalovirus. J Virol. 1999;73:4806–4812. doi: 10.1128/jvi.73.6.4806-4812.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kondo K, Xu J, Mocarski ES. Human cytomegalovirus latent gene expression in granulocyte-macrophage progenitors in culture and in seropositive individuals. Proc Natl Acad Sci USA. 1996;93:11137–11142. doi: 10.1073/pnas.93.20.11137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kondo K, Kaneshima H, Mocarski ES. Human cytomegalovirus latent infection of granulocyte-macrophage progenitors. Proc Natl Acad Sci USA. 1994;91:11879–11883. doi: 10.1073/pnas.91.25.11879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kondo K, Mocarski ES. Cytomegalovirus latency and latency-specific transcription in hematopoietic progenitors. Scand J Infect Dis Suppl. 1995;99:63–67. [PubMed] [Google Scholar]
- 31.Hahn G, Jores R, Mocarski ES. Cytomegalovirus remains latent in a common precursor of dendritic and myeloid cells. Proc Natl Acad Sci USA. 1998;95:3937–3942. doi: 10.1073/pnas.95.7.3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taylor-Wiedeman J, Sissons JG, Borysiewicz LK, Sinclair JH. Monocytes are a major site of persistence of human cytomegalovirus in peripheral blood mononuclear cells. J Gen Virol. 1991;72(Pt 9):2059–2064. doi: 10.1099/0022-1317-72-9-2059. [DOI] [PubMed] [Google Scholar]
- 33.Stanier P, Taylor DL, Kitchen AD, Wales N, Tryhorn Y, Tyms AS. Persistence of cytomegalovirus in mononuclear cells in peripheral blood from blood donors. BMJ. 1989;299:897–898. doi: 10.1136/bmj.299.6704.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Champlin RE, Schmitz N, Horowitz MM, et al. Blood stem cells compared with bone marrow as a source of hematopoietic cells for allogeneic transplantation. IBMTR Histocompatibility and Stem Cell Sources Working Committee and the European Group for Blood and Marrow Transplantation (EBMT) Blood. 2000;95:3702–3709. [PubMed] [Google Scholar]
- 35.Soderberg-Naucler C, Fish KN, Nelson JA. Reactivation of latent human cytomegalovirus by allogeneic stimulation of blood cells from healthy donors. Cell. 1997;91:119–126. doi: 10.1016/s0092-8674(01)80014-3. [DOI] [PubMed] [Google Scholar]
- 36.Manteiga R, Martino R, Sureda A, et al. Cytomegalovirus pp65 antigenemia-guided pre-emptive treatment with ganciclovir after allogeneic stem transplantation: a single-center experience. Bone Marrow Transplant. 1998;22:899–904. doi: 10.1038/sj.bmt.1701439. [DOI] [PubMed] [Google Scholar]
- 37.Bensinger WI, Martin PJ, Storer B, et al. Transplantation of bone marrow as compared with peripheral-blood cells from HLA-identical relatives in patients with hematologic cancers. N Engl J Med. 2001;344:175–181. doi: 10.1056/NEJM200101183440303. [DOI] [PubMed] [Google Scholar]
- 38.Korbling M, Huh YO, Durett A, et al. Allogeneic blood stem cell transplantation: peripheralization and yield of donor-derived primitive hematopoietic progenitor cells (CD34+ Thy-1dim) and lymphoid subsets, and possible predictors of engraftment and graft-versus-host disease. Blood. 1995;86:2842–2848. [PubMed] [Google Scholar]
- 39.Ottinger HD, Beelen DW, Scheulen B, Schaefer UW, Grosse-Wilde H. Improved immune reconstitution after allotransplantation of peripheral blood stem cells instead of bone marrow. Blood. 1996;88:2775–2779. [PubMed] [Google Scholar]
- 40.Junghanss C, Storb R, Maris MB, et al. Impact of unrelated donor status on the incidence and outcome of cytomegalovirus infections after non-myeloablative allogeneic stem cell transplantation. Br J Haematol. 2003;123:662–670. doi: 10.1046/j.1365-2141.2003.04671.x. [DOI] [PubMed] [Google Scholar]
- 41.Martino R, Caballero MD, Canals C, et al. Reduced-intensity conditioning reduces the risk of severe infections after allogeneic peripheral blood stem cell transplantation. Bone Marrow Transplant. 2001;28:341–347. doi: 10.1038/sj.bmt.1703150. [DOI] [PubMed] [Google Scholar]
- 42.Ljungman P, Aschan J, Lewensohn-Fuchs I, et al. Results of different strategies for reducing cytomegalovirus-associated mortality in allogeneic stem cell transplant recipients. Transplantation. 1998;66:1330–1334. doi: 10.1097/00007890-199811270-00012. [DOI] [PubMed] [Google Scholar]
- 43.Miller W, Flynn P, McCullough J, et al. Cytomegalovirus infection after bone marrow transplantation: an association with acute graft-v-host disease. Blood. 1986;67:1162–1167. [PubMed] [Google Scholar]
- 44.Hutson AM, Atmar RL, Graham DY, Estes MK. Norwalk virus infection and disease is associated with ABO histo-blood group type. J Infect Dis. 2002;185:1335–1337. doi: 10.1086/339883. [DOI] [PubMed] [Google Scholar]
- 45.Hudnall SD, Chen T, Rady P, Tyring S, Allison P. Human herpesvirus 8 seroprevalence and viral load in healthy adult blood donors. Transfusion. 2003;43:85–90. doi: 10.1046/j.1537-2995.2003.00272.x. [DOI] [PubMed] [Google Scholar]
- 46.Garratty G, Glynn SA, McEntire R. ABO and Rh(D) phenotype frequencies of different racial/ethnic groups in the United States. Transfusion. 2004;44:703–706. doi: 10.1111/j.1537-2995.2004.03338.x. [DOI] [PubMed] [Google Scholar]
- 47.Buyck HC, Prentice HG, Griffiths PD, Emery VC. The risk of early and late CMV DNAemia associated with Campath use in stem cell transplant recipients. Bone Marrow Transplant. 2010;45:1212–1219. doi: 10.1038/bmt.2009.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.de Correia-Silva JF, Victoria JM, Guimaraes AL, et al. Cytomegalovirus shedding in the oral cavity of allogeneic haematopoietic stem cell transplant patients. Oral Dis. 2007;13:163–169. doi: 10.1111/j.1601-0825.2006.01240.x. [DOI] [PubMed] [Google Scholar]
- 49.Preiser W, Brauninger S, Schwerdtfeger R, et al. Evaluation of diagnostic methods for the detection of cytomegalovirus in recipients of allogeneic stem cell transplants. J Clin Virol. 2001;20:59–70. doi: 10.1016/s1386-6532(00)00156-6. [DOI] [PubMed] [Google Scholar]
- 50.Sola R, Rabella N, Guirado LL, Diaz JM, Facundo C, Garcia R. Relation between pp65 antigenemia, RT-PCR and viruria for cytomegalovirus detection in kidney transplant recipients. Transplant Proc. 2005;37:3768–3769. doi: 10.1016/j.transproceed.2005.09.107. [DOI] [PubMed] [Google Scholar]
- 51.Bowden RA, Fisher LD, Rogers K, Cays M, Meyers JD. Cytomegalovirus (CMV)-specific intravenous immunoglobulin for the prevention of primary CMV infection and disease after marrow transplant. J Infect Dis. 1991;164:483–487. doi: 10.1093/infdis/164.3.483. [DOI] [PubMed] [Google Scholar]
- 52.Gooley TA, Chien JW, Pergam SA, et al. Reduced mortality after allogeneic hematopoietic-cell transplantation. N Engl J Med. 2010;363:2091–2101. doi: 10.1056/NEJMoa1004383. [DOI] [PMC free article] [PubMed] [Google Scholar]