Summary
Multiple sclerosis (MS) is a chronic progressive inflammatory demyelinating disease affecting the central nervous system. The most common clinical type of MS tends to follow a relapsing course, affecting the vast majority of patients living with this disease. Relapses are a hallmark of MS, and are often associated with significant functional impairment and decreased quality of life. Although usually followed by a period of remission, residual symptoms after MS relapses may persist and lead to sustained disability. Adequate management of MS relapses is important, as it may help to shorten and lessen the disability associated with their course. Historically, treatment of MS relapse was the first approach (and for a period of time, the only approach) to MS treatment in general. Systemic corticosteroids and adrenocorticotropic hormone (ACTH) have broad regulatory approval and remain the most established and validated treatment options for MS relapse. Therapeutic mechanisms of ACTH were previously associated (perhaps mistakenly) with only corticotropic actions; however, recently the direct anti-inflammatory effects and immunomodulatory activity of ACTH gel acting through melanocortin pathways have been shown. Second-line treatments of steroid-unresponsive MS relapses and a possible algorithm for MS relapse management are also reviewed in this article.
Electronic supplementary material
The online version of this article (doi:10.1007/s13311-012-0160-7) contains supplementary material, which is available to authorized users.
Keywords: Relapse, ACTH, Corticosteroids, Plasmapheresis, Algorithm.
MS Relapse Definition and Diagnosis
Multiple sclerosis (MS) is a chronic progressive inflammatory demyelinating disease; the relapsing-remitting MS course is the most common clinical type of MS affecting the vast majority of patients living with this disease.
MS relapses are typically defined as a new or worsening neurological deficit lasting 24 h or more in the absence of fever or infection. Relapses are a hallmark of MS, and are often associated with significant functional impairment and decreased health-related quality of life [1]. For the vast majority of MS patients, relapses are the biggest concern associated with the disease and the unpredictability of MS exacerbations further compounds the potential impact on quality of life.
MS relapses could represent formation of the new demyelinating activity or inflammation of any previously existing demyelinating lesion or lesions located in any segment of the central nervous system (CNS) [2, 3]. In general, the most commonly seen symptom complexes are related to new or worsening inflammatory processes involving the optic nerve, spinal cord, cerebellum, and/or cerebrum. Thus, presenting symptoms may vary or may be a combination of visual disturbances, motor and sensory impairments, balance issues, and cognitive deficits [2, 3].
It is important to rule out certain conditions that may lead to so-called “pseudo-exacerbations,” which include fever and infections (most commonly seen as urinary tract and upper respiratory infections), as well as stress and heat exposure [1–3].
The natural course of most of MS exacerbations is usually completed with a period of repair leading to clinical remission and sometimes (especially early on in the disease course) leading to a complete recovery; however, the residual deficit after MS relapse may persist and contribute to the stepwise progression of disability [1]. Therefore, treatment of MS relapses is important as it may help to shorten and lessen the disability associated with their course.
In the author’s opinion, successful treatment of MS relapse has another and rather important, if psychological, aspect in that it helps to establish good physician-patient relationships while contributing to the ability of MS patients to gain a vital sense of being capable of taking control of the disease.
History and Practice of MS Relapse Treatments: From Adrenocorticotropic Hormone to Corticosteroids and Back
In the early 20th century, the treatment of choice in acute relapse of MS was usually bed rest, which was accepted as an effective measure to facilitate recovery and shorten the duration of the attack. Several publications written in the German language from the 1930s and 1940s supported this approach [4–6].
The Early Years of MS Relapse Treatment: Adrenocorticotropic Hormone as the Gold Standard
One of the first peer-reviewed publications written in English on the subject reported results of a controlled study by Miller et al. [7], in which 40 MS patients with acute exacerbations were treated with either corticotrophin (ACTH) or saline. This work is arguably the very first controlled clinical trial in MS. The article provides a clear definition of an MS patient experiencing a relapse as an individual with “unequivocal multiple sclerosis, who presented with an assessable new symptom or sign of less than 14 days’ duration and showing no spontaneous improvement” [7]. The 40 patients with such presentation “were randomly allocated to two treatment groups, one receiving corticotrophin gel (ACTH gel) and the other comparable injections of saline” [7]. The patients treated with ACTH gel were given 60 units of gel twice daily during the first week of treatment; 40 units twice daily during the second week; and 60, 40, and 20 units on the second, fourth, and sixth days of the third week. In the words of the authors, the study “has confirmed the clinical impression that the hormone exercises a favorable effect on the outcome of some of such episodes.” The article references earlier publications by Fog [8] and Jӧnsson et al. [9], who previously described similar outcomes of ACTH treatment based on their clinical observations.
These and other similar data provide evidence that ACTH gel is beneficial in the treatment of acute MS [10, 11].
Another controlled study in 1967, reported by Rinne et al. [12], suggested that 30 units of corticotrophin per day (but not 90 units) given for 35 days were effective in the treatment of “acute MS.” Interestingly, this clinical study also evaluated so-called “chronic cases of MS,” although no clear definition for that subgroup was provided.
The results of a true milestone (i.e., a well-designed large, controlled, double-blind, multicenter clinical study) were published by Rose et al. [13, 14]. Patients were randomly assigned to either ACTH gel or a placebo. The 197 patients were enrolled from 10 centers throughout the United States. This study was probably the first multicenter trial in MS, and it was also the first study to introduce the Disability Status Scale (DSS), which was later modified to the Expanded DSS (EDSS). In this study, 40 units of ACTH gel was given as intramuscular injections twice daily for 7 days, then 20 units twice daily for 4 days, and then 20 units twice daily for 3 days, which demonstrated beneficial effects in comparison to the similarly administered placebo gel [13, 14]. This data eventually lead to the acceptance of ACTH as a treatment for MS relapse and eventually to the Food and Drug Administration (FDA) approval of the Questcor Pharmaceuticals, Inc. H.P. Acthar® Gel (repository corticotrophin injection), (Questcor Pharmaceuticals, Inc., Hayward, CA) for this indication in 1978 [15].
The old idea of bed rest for MS relapse treatment was visited 1 more time, as it was used as a comparator to ACTH in the trial by Hoogstraten et al. [16], and was found to be as effective when bed rest was combined with ACTH. However, this treatment approach did not seem to acquire many followers, and another study by the same author confirmed efficacy of ACTH in MS relapse treatment, although “those effects did not outlast 6 months” [17].
It is worth mentioning that at the that time, mechanisms of ACTH action were attributed to the steroidogenic potential of the compound [18].
Systemic Steroids for MS Relapse Treatment
Second, and so far the last medication approved by the FDA for the treatment of MS relapse was intravenous methylprednisolone (IV-MP).
Several studies were done comparing IV-MP to the “gold standard” then for MS exacerbation treatment: ACTH [19–22] and to the placebo [18, 23–25], which are reviewed as follows.
Mechanisms of action of systemic corticosteroids in the treatment of acute relapse were thought to be attributed to immunologic alterations they cause and are “likely that the main, if not the sole, mechanism is the resolution of edema” [22]. Furthermore, it is known that acute corticosteroid administration “brings about a lymphocytopenia” [23], including the reduction of B-lymphocyte counts and their availability at the inflammatory sites, which could result in a decreased number of immunoglobulin (Ig) G synthesizing cells in the CNS [23]. This may lead to reduction of the blood–brain barrier abnormally increased permeability (and decrease of active lesions on magnetic resonance imaging) [2, 23, 28].
In the study by Abbruzzese et al. [19], which was published in 1983, there were 60 patients randomly assigned to 1 of 2 groups; the first group was receiving synthetic ACTH at the dose of 1 mg/day in 2 of the 250 ml saline infusions for 15 days. The second group was receiving “bolus” IV-MP in dosages of 20 mg/kg/day for the first 3 days, followed by 10 mg/kg/day on days 4 through 7, then 5 mg/kg/day on days 8 through 10, and finally 1 mg/kg/day on days 11 through 15. If we suppose the average weight of a patient to be 70 kg, then the IV-MP dosages can be translated into 1400 mg/day, 700 mg/day, 350 mg/day, and 70 mg/day, respectively. No significant difference was observed between patients treated with ACTH and methylprednisolone (MP). The authors came to the conclusion that “these two treatments therefore seem to have the same effectiveness.” It was noticed that MP “showed prompt effectiveness” and “high dose intravenous steroids may, therefore, be regarded as a useful alternative in the management of acute demyelinating disease.”
Chronologically the next study indicated “intravenous methylprednisolone for multiple sclerosis in relapse” by Barnes et al. [20] was published in 1985. There were 25 patients randomized to either IV-MP given 1 g daily for 7 days, or intramuscular ACTH at 80 units, then 60 units, then 40 units, and then 20 units daily, each for 7 days (for a total of 28 days). It has been observed that “the methylprednisolone group improved faster than the ACTH group over the first 3 days and this difference was maintained at day 28. However, at 3 months there was no longer any significant difference between the two groups.”
A double-blind randomized trial of ACTH versus dexamethasone versus MP was published by Milanese et al. [21] a few years later in 1989. There were 30 patients randomized to 1 of 3 groups: 1) ACTH, 50 units/day for 7 days, then 25 units/day for 4 days, and then 12.5 units/day for 3 days; 2) dexamethasone, 8 mg/day for 7 days, then 4 mg/day for 4 days, and then 2 mg/day for 3 days; 3) MP, 40 mg/day for 7 days, then 20 mg/day for 4 days, and then 10 mg/day for 3 days. Each dose was administered by intravenous infusion in 250 ml saline. It was observed that “dexamethasone and ACTH-treated patients showed clinical improvement.” However, it was remarked that “dexamethasone seems to be the most effective of the three drugs studied,” and “methylprednisolone — at equivalent dosages — was not effective as dexamethasone and ACTH”. Thus, the stronger effects of high-dose versus low-dose corticosteroids have been shown.
In the double-blind, randomized, controlled study by Thompson et al. [22], relative efficacy of IV-MP and ACTH in the treatment of acute relapse in MS was evaluated. There were 61 patients randomly allocated to 2 groups: 1) received IV-MP 1 gram daily for 3 days and intramuscular placebo injections for 14 days, and 2) received an intravenous placebo daily for 3 days and a reducing course of intramuscular ACTH for the course of 14 days, consisting of 80 units for 7 days, then 40 units for 4 days, and then 20 units for 3 days. “A clear improvement in both groups over the course of the study” was indicated, but “no significant difference between the 2 groups in either rate of recovery or final outcome at 3 months” was found. Still, it was remarked that a “3-day course of [intravenous] IV treatments rather than 14 days of [intramuscular] IM injections has obvious advantages.”
The placebo-controlled trial of IV-MP versus placebo was published by Durelli et al. in 1986 [23]. There were 23 patients randomly allocated to 2 groups: group A was the MP group. The IV-MP group was given 15 mg/kg/day on days 1 to 3, then 10 mg/kg/day on days 4 to 6, then 5 mg/kg/day on days 7 to 9, then 2.5 mg/kg/day on days 10 to 12, and 1 mg/kg/day on days 13 to 15. Group B was the placebo group whose patients received 500 ml of physiologic solution for 15 days. At the end of the controlled phase of the study, the patients in group B were administered IV-MP according to the schedule of group A. After the IV-MP treatment period, both groups received oral prednisone starting with 100 mg/day, which was slowly tapered for 120 days. There were 6 of 10 placebo patients who reported subjective improvement 4 to 8 days after starting treatment, with clinically significant improvement being measured in only 4 patients. When passed to MP treatment after the 15th day of the placebo, all but 1 patient significantly improved. All patients of the MP group reported subjective improvement at 1 to 3 days after starting treatment, and 12 of the 13 patients improved significantly. During the whole double-blind part of the study, the ratio of improved and unimproved patients was significantly higher in the MP group than in the placebo group.
Another double-blind, placebo-controlled trial of high-dose IV-MP was accomplished by Milligan et al. [24], which was published in 1987. There were 50 patients randomized to IV-MP (500 mg daily) for 5 days or to an equivalent volume of saline given intravenously for 5 days. MP-treated patients showed decreased disability scores at 4 weeks in 19 of 26 patients; none had increased disability, and 6 of 7 nonresponders were patients with progressive MS. In “the control group, 7/24 cases showed a decrease in disability scores at 4 weeks, 6/24 had increased disability and 10/24 were unchanged. These results show a significant effect in favor of methylprednisolone treatment.”
The Optic Neuritis Treatment Trial published in 1992, although not done on MS patients, certainly had “a bearing on the treatment of multiple sclerosis with corticosteroids” [25]. The study compared oral prednisone (1 mg/kg/day for 14 days; n = 156), IV-MP (1 g/day for 3 days followed by oral prednisone 1 mg/kg/day for 11 days; n = 151), and oral placebo (n = 150) for 14 days in the treatment of 457 patients with optic neuritis [25]. The study used sensitive outcome measures, such as contrast sensitivity, visual fields, color vision, and visual acuity. It was found that visual function recovered faster in the group receiving IV-MP than in the placebo group; although the differences between the groups decreased with time, at 6 months the IV-MP group had better visual fields, contrast sensitivity, and color vision, although not better visual acuity. The outcome in the oral prednisone group did not differ from that in the placebo group. In addition, the rate of new episodes of optic neuritis was higher in the group receiving oral prednisone, but not the group receiving IV-MP. It was concluded that the IV-MP followed by oral prednisone speeds the recovery of visual loss due to optic neuritis, but the oral prednisone alone is an ineffective treatment and increases the risk of new episodes of optic neuritis [25].
As we see, the dosages of IV-MP used in these studies differ: from as low as 40 mg/day [21] to 500 mg [24], and to 15 mg/kg/day IV [23], and finally to 1 g a day [22, 25]. The low dosages were found to be ineffective [21] and the dosages from 500 mg to 1 g of IV-MP per day became a widely accepted and preferred regiment.
The length of treatment also varied in the studies, and the general consensus on “for how long the MS relapse should be treated” had undergone a remarkable transformation during these years. Although back in the 1960s to the 1980s, it was a common practice to treat MS exacerbation for 4 weeks and even for as long as 35 days [12, 14, 21]. In the later and more recent years, significantly shorter courses of 3 to 7 days were found to be quite adequate [2, 3, 18, 21, 22, 25].
Since the 1990s, the concept of oral high-dose MP has been studied, which in most trials was found to be comparable with the effects of IV-MP administration [26–28]. Financial and other practical advantages associated with the oral route of administration are apparent [2]. Several clinical trials addressing this concept were done.
A double-blind, randomized, placebo-controlled study by Sellebjerg et al. was published in 1998 [26]. There were 51 patients randomized to either the group of oral MP, given as 500 mg once a day for 5 days followed by a tapering period, or to the placebo group. There were significantly more responders in the MP group than in the placebo group. The study concluded that oral high-dose MP is efficacious in managing attacks of MS. Also, although adverse effects were common, no serious side effects were observed.
In the study by Barnes et al. [27], 42 patients with MS relapse were assigned to receive oral MP, and 38 to IV-MP. The primary outcome was a difference between the 2 treatment groups of 1 or more EDSS grades at 4 weeks; there were no significant differences found between the 2 groups. The authors concluded that it is preferable to prescribe oral rather than intravenous steroids for acute relapses of MS for reasons of patient convenience, safety, and cost.
The short-term randomized magnetic resonance imaging study of high-dose oral versus IV-MP by Martinelli et al. [28] was published more recently, in 2009. There were 40 patients randomized to receive either 1 g/day of oral MP for 5 days, or 1 g/day of IV-MP for the same 5 days. It was concluded that the oral MP was as effective as IV-MP in reducing gadolinium-enhancing lesions with similar clinical, safety, and tolerability profiles [28].
Adverse Events during Steroid and ACTH Therapy
The use of either corticosteroids or ACTH has been reported to be associated with a variety of potential adverse effects. The susceptibility to steroid- or ACTH- induced adverse effects and their relative frequencies vary from patient to patient and depend on several factors, including the individual patient’s comorbidities and the dose, duration, and possibly the type and route of administration [3]. In general, although not without risks, short-term use of either steroids or ACTH (even in high doses) has been reported in randomized clinical trials to be associated with only relatively minor, if frequent, side effects [7, 13, 18, 20, 22–28]. The adverse effects that may occur with ACTH are thought to be related primarily to its steroidogenic effects and are similar to corticosteroids. There may be increased susceptibility to new infection and increased risk of reactivation of latent infections; adrenal insufficiency may occur after abrupt withdrawal of the drug after prolonged therapy; Cushing’s syndrome, elevated blood pressure, salt and water retention, and hypokalemia may be seen; masking of symptoms of other underlying disease/disorders may occur; there is a risk of gastrointestinal perforation and bleeding with increased risk of perforation in patients with certain gastrointestinal disorders; onset or worsening of euphoria, insomnia, irritability, mood swings, personality changes, depression, and psychosis may occur [15]. Caution should be used when prescribing ACTH to patients with diabetes or myasthenia gravis; prolonged use may produce cataracts, ocular infections, or glaucoma; use in patients with hypothyroidism or liver cirrhosis may result in an enhanced effect; there may be negative effects on growth and physical development and decreases in bone density [15]. Cass et al. [29] catalogued adverse events in 47 patients with MS undergoing ACTH treatment. The treatment consisted of an initial “intensive” phase followed by a “maintenance” treatment phase. In the intensive phase, nonrepository ACTH (40 IU/day) was administered as a daily intravenous infusion for a duration of 8 hours each for 10 days. In the maintenance phase, repository ACTH was administered (50–60 IU IM b.i.d. for 4–11 days and then tapered by 20 IU every 3–5 days until a maintenance dose of 20–40 IU q.o.d. was achieved). There were 41 patients who received maintenance therapy, which had been ongoing for 1 to 4 years. During initial intensive ACTH therapy, moderate hyperglycemia (fasting blood glucose of 120 to 150 mg/dL) was observed in 19 patients. One patient developed steroid diabetes (fasting blood sugar of 185 mg/dL) in conjunction with severe adrenal exhaustion and required temporary discontinuation of therapy; ACTH was later reinstated and for a 4-year duration of maintenance therapy the blood sugar levels remained normal. During maintenance therapy, 1 patient who had had no complications during intensive treatment developed a high fasting blood sugar level, went into a diabetic coma, and died. One (2 %) patient developed osteoporosis during the intensive ACTH treatment phase. During the maintenance treatment, 2 (5 %) patients developed osteoporosis. All 3 patients that developed osteoporosis were female [29].
Overall, the most frequently reported corticosteroid side effects were gastrointestinal symptoms (most commonly heartburn), weight gain, edema, mood changes, dysphoria, or anxiety, insomnia, musculoskeletal pain, palpitations, edema, acne, weight gain, headache, and unpleasant (metallic) taste during or after intravenous infusion. Hyperglycemia, hypertension, moon face, and hirsutism were less frequently reported [2, 3, 18–25].
Among adverse events involving the musculoskeletal system, osteoporosis has been estimated to develop in at least 50 % of individuals requiring long-term corticosteroid therapy [30]. According to Schwid et al. [31], “single corticosteroid pulse did not reduce bone density in fully ambulatory patients with MS, and multiple pulses did not have a cumulative effect on bone density in retrospective analysis; the change in femoral density in poorly ambulatory patients may have been related to inactivity rather than the steroid pulse.” Also, although there are data indicating that repeat courses of pulse steroid therapy may increase risk of complications [24], the study evaluating long-term effects of intravenous high-dose MP pulses on bone mineral density in patients with MS by Zorzon et al. [32], found that treatment with repeated IV-MP pulses was not associated with osteoporosis. However, osteopenia was observed more frequently in MS patients than healthy controls; found only in patients treated for relapses who had a significantly higher EDSS score, it is suggested that decreased mobility may contribute to bone loss more than corticosteroid use [32].
Aseptic necrosis has been reported to be a “not infrequent complication of long-term steroid use” [33]; the highest numbers for avascular (aseptic) necrosis were reported for patients with systemic lupus erythematosus, also using chronic steroid treatment [33]. Its development is unpredictable and may occur within the first few weeks of therapy [33, 34]. The direct association between steroid use and pathogenesis of aseptic necrosis appears to be less clear than with other adverse effects.
Psychiatric side effects are also frequently reported in steroid-treated patients. Severe psychiatric disorders, such as psychosis, depression, or manic episodes are reported in as much as a third of steroid-treated patients. Insomnia has been reported by approximately 50 % of patients after pulse or oral corticosteroid treatment. The risk of psychosis appears to be highest within the first days or weeks of starting therapy and may be higher in women [34].
Other frequently reported side effects are infections; the most common infections include pneumonia, complicated urinary tract infections, and septic arthritis/bursitis [1–3, 22–28, 34].
The development of posterior and subcapsular cataracts is a well-known adverse event associated with corticosteroid treatment and has been reported to occur mostly with low steroid dosage [34].
Side effects, such as hypertension, dyslipidemia, and hyperglycemia/diabetes, have been reported with varying frequency in the literature. The frequency of hypertension seems to vary depending on the duration, daily dosage of steroid, and the population under investigation [34]. Effects of steroid treatment on carbohydrate metabolism and glucose tolerance appear to be proportional to the preexisting status of glucose tolerance, and the abnormalities induced by corticosteroids fit the pattern of an insulin-resistant state. A case control study of more than 20,000 patients reported that the relative risk for development of hyperglycemia requiring treatment was significantly increased in patients taking corticosteroids compared to nonusers [35]. Feldman-Billard et al. [36] assessed short-term tolerance of 3-day pulse IV-MP therapy in 80 patients with type-2 diabetes treated for eye disorders, and reported that each MP pulse induced a mean 2-fold increase of peak blood glucose levels 10 hours later. Rapid insulin was required in 100 and 45 % of patients with glycosylated hemoglobin >8 % or as much as 8 %, respectively. Patients >70 years of age with glycosylated hemoglobin as much as 8 % had a 3-fold increased risk of requiring insulin [36]. The double-blind, randomized, controlled study comparing the efficacy of ACTH and IV-MP in the treatment of acute exacerbation of MS by Thompson et al. [22] reported that 1 patient in the IV-MP group had the treatment discontinued due to a rise in blood glucose to very high levels, whereas 2 patients in the ACTH group developed glycosuria, which did not require discontinuation of the study.
Relatively low rate and severity of the side effects of ACTH and systemic steroids observed in short-term treatment studies may be due in part to the exclusion of patients with conditions or comorbidities that may preclude their use, which may thus lead to an underestimation of the frequency of more serious adverse effects [11–14, 22, 24, 26, 28].
The Renaissance of ACTH for MS Relapses
It used to be generally understood and accepted as a fact that the efficacy of ACTH in MS relapses is determined only by its corticotrophic effects. However, more recent data in other disease states like nephrotic syndrome [37], opsoclonus-myoclonus [38], and infantile spasms [39] provide clinical evidence that steroidogenic actions fail to explain the efficacy of ACTH in these conditions. For example, in infantile spasms (a disease confined to the CNS), ACTH has been shown to alter brain function after systemic administration; this alteration cannot be explained by glucocorticoid actions because corticosteroid treatment has doubtful efficacy in infantile spasms and doses of ACTH that surpass those required to elicit maximal glucocorticoid release are required to obtain optimal efficacy [39].
Recently it has been shown that ACTH has direct anti-inflammatory and immunomodulatory effects via activation of central and peripheral melanocortin (MC) receptors, in addition to the effects achieved by systems originating in the adrenal gland [40]. ACTH is a melanocortin agonist; it binds to all 5 known classes of MC receptors, of which only one (the second type or MC 2 receptor) is implicated in adrenal steroidogenesis [40]. MC 1 receptor is expressed in the skin in melanocytes, epithelial cells, in monocytes, neutrophils, lymphocytes, in podocytes of the kidney, periaqueductal gray matter in the CNS, and in microvascular endothelial cells, astrocytes, and Schwann cells. MC 2 receptor is the receptor in the adrenal glands underlying the steroidogenic actions of ACTH, and it has also been localized to osteoblasts and skin. MC 3 receptor and MC 4 receptor have been identified in the CNS; MC 3 receptor occurs primarily in the hypothalamus and limbic system, whereas MC 4 receptor is the prevalent receptor in the CNS, with wide expression in the cortex, thalamus, hypothalamus, brain stem, spinal cord, and astrocytes. MC 5 receptor is widely distributed and occurs in exocrine glands and lymphocytes [40, 41].
Thus, research into MC peptides and their receptors argue against the longstanding belief that the beneficial effect of ACTH depends solely on its ability to stimulate the release of endogenous corticosteroids. Evidently, the MC system has many diverse regulatory functions in the human body, including melanogenesis, glucocorticoid production, control of food intake and energy expenditure, control of sexual function, behavioral effects, attention, memory, learning, and, especially important for MS, neuroprotection, immune modulation, and anti-inflammatory effects [40–42].
These new data may explain the reason of increased interest in the “old” ACTH we see these days. Quite clearly, back in 1970s the ACTH was left rather understudied. In addition, successful arrival of robustly effective and relatively inexpensive corticosteroids helped deviate professional attentions from the ACTH for decades. The new research may contain a perspective on the potential value of MC system agonists (such as ACTH) for the treatment of MS, and suggests that further exploration of how best to use ACTH in MS should be considered [42].
Although data from clinical trials have not demonstrated a clear difference in the efficacy of ACTH gel and corticosteroids in the treatment of MS relapse [19, 22], there have been anecdotal reports of patients who do not respond to steroids, but do respond to ACTH gel [43, 44]. Likewise, some patients who cannot tolerate steroids may tolerate ACTH gel [44]. Still, more data are needed before firm recommendations can be made to support the use of ACTH versus IV-MP or oral steroids, considering the high price of ACTH gel.
The important question, which at this point remains unanswered, is a question of potential differences in safety of ACTH gel relative to the corticosteroids. For example, risk for bone loss is particularly important in the context of high-dose or protracted use of corticosteroids; such treatment leads to reduced estrogen, testosterone, and renal androgens, reduced gastrointestinal calcium absorption, and increased calcium excretion and increased parathyroid hormone lead to excessive osteoclastic bone removal [45, 46]; corticosteroids also induce osteonecrosis supposedly via increased apoptosis of osteoblasts [46]. In contrast, potential osteoprotective properties of ACTH were shown in experimental studies by Zaidi et al. [45]. If supported by well-designed clinical trials, the data may prove to be feasible in the setting of comorbid osteoporosis. However, the existing data favoring ACTH in this respect are purely experimental, and further clinical studies are necessary. Furthermore, ACTH may be a reasonable option for MS patients with comorbid autoimmune conditions [47], and again, this hypothesis requires clinical evidence. Thus, despite the confounding amount of preclinical data currently available on ACTH and MCs, many clinical questions remain to be answered. Clinical studies to further examine immunological aspects of ACTH are needed to determine its mechanisms of action in treating MS. In addition, investigations of pulse therapy with ACTH gel in relapsing-remitting MS or secondary progressive MS may help to clarify possible disease-modifying effects. Exploration of how the effects of ACTH on secretion of neurotransmitters (e.g., noradrenalin, acetylcholine, and dopamine) might improve treatment for and possibly affect the course of progressive forms of MS is an intriguing possibility [42]. Additional basic science studies are needed to more definitively establish the mechanism of effects of ACTH on bone, as well as clinical trials to evaluate the effects of ACTH gel on bone in frequent steroid users, such as patients being repeatedly treated for MS relapses.
The presumption that the efficacy of ACTH gel results solely from its corticotrophic effects, as we now see, may not be absolutely accurate; however, back in the early 1980s, this presumption played a positive role as it led to increased interest and later to acceptance of high-dose corticosteroids for MS exacerbations treatment [18]. Since that time, the focus shifted to IV-MP as the preferred treatment option for MS relapse.
Second Line of Treatment for MS Relapse Cases Unresponsive to Corticosteroids or ACTH
It is clear that there are patients who respond to neither corticosteroids nor ACTH for relapse treatment. Several alternatives, including plasmapheresis [48–51], cyclophosphamide [48, 52, 53], intravenous immunoglobulin G (IV-IG) [54–58], and natalizumab [59] have been studied with plasmapheresis as the only option supported by strong clinical evidence. The recent American Academy of Neurology guideline published in January 2011 [60] recommends considering using plasma exchange as a secondary treatment for severe flares in remitting-relapsing MS.
A large, multicenter, randomized, double-blind controlled trial of an 8-week course of 11 plasma exchange treatments was examined for MS relapse treatment by Weiner et al. [48]. In this study, 116 subjects were randomized to either sham or true plasmapheresis, and both groups received identical treatment with intraumuscular ACTH and oral cyclophosphamide. Serum IgG decreased with plasmapheresis treatment by 76 % versus 22 % by treatment 5, and by 64 % versus 14 % by treatment 11. Plasmapheresis produced significant reductions in IgA, IgM, C3, and fibrinogen. Subjects undergoing plasmapheresis had moderately enhanced improvement at 2 weeks relative to the sham group. The results suggested that plasma exchange given with ACTH, plus cyclophosphamide enhances recovery from an exacerbation of disease in relapsing-remitting MS patients [48].
The results of a randomized, double-blind, sham-controlled study of either plasmapheresis or sham treatment in patients who did not respond to IV-MP were published in 1999 by Weinshenker et al. [49], which showed significant efficacy of plasma exchange in this category of MS patients. In the study, 12 subjects with MS and 10 with other acute inflammatory demyelinating conditions were randomized to either plasmapheresis or sham treatment, which were given as 7 exchanges every other day for 14 days. There were 19 courses of plasmapheresis performed, resulting in 8 moderate or marked improvements, and only 1 moderate improvement was noted across 17 courses of sham treatment.
In a subsequent retrospective review of 59 steroid-unresponsive demyelinating events treated by plasmapheresis, Keegan et al. [50] found that the male gender, preserved or brisk reflexes, and early initiation of treatment (i.e., less than 60 days) were associated with improvement.
More recently, Keegan et al. [51] correlated the pathological features of demyelination with responsiveness to plasma exchange. Subjects who responded to plasma exchange in their series (10 of 19) had a particular pattern of demyelination, characterized by the presence of antibodies and complement, whereas nonresponders had pathological characteristics of T-cell/macrophage-associated demyelination or distal oligodendrogliopathy [51].
The role of IV-IG in MS relapse treatment remains to be defined. Although there are many anecdotal observations of beneficial effects of IV-IG in the treatment of MS relapses, most published clinical studies did not provide any clear evidence to support this. Part of the controversy is a result of the fact that IV-IG is usually being attempted after the IV-MP administration; thus, possible delayed effects of IV-MP may overlap and obscure the pure IV-IG effects.
Results of a large, double-blind, placebo-controlled trial by Noseworthy at al. [54] suggest that delayed IV-IG administration had no effect on recovery from optic neuritis. However, an open-label study by Tselis et al. [55] showed that some cases of steroid-unresponsive optic neuritis may respond to IV-IG administered 0.4 g/kg/day for 5 days, followed by monthly 0.4 g/kg infusion for 5 months. Visual acuity response (as defined by a change to 20/30 or better 1 year later) was significantly better in subjects with IV-IG than in those that received only IV-MP. Other studies did not show IV-IG effects when administered concomitantly with, or immediately subsequent, to IV-MP [56–58]. A possible role of IV-IG as a therapeutic option for treating MS relapse will require further study.
Summary and Practical Considerations
There is a general consensus that while mild exacerbations may not require immediate treatment, the moderate-to-severe MS relapses with disabling symptoms should be treated using systemic steroids or ACTH [2, 3].
However, there is no consensus in regard to what point that treatment should be initiated to be effective. In general, starting the treatment as early as possible (within a week) of the MS-relapse symptom onset is considered best, although there is no direct evidence to support this approach. Is has been observed that relapse treatment can be successfully initiated as late as 1 to 2 months into a relapse [2].
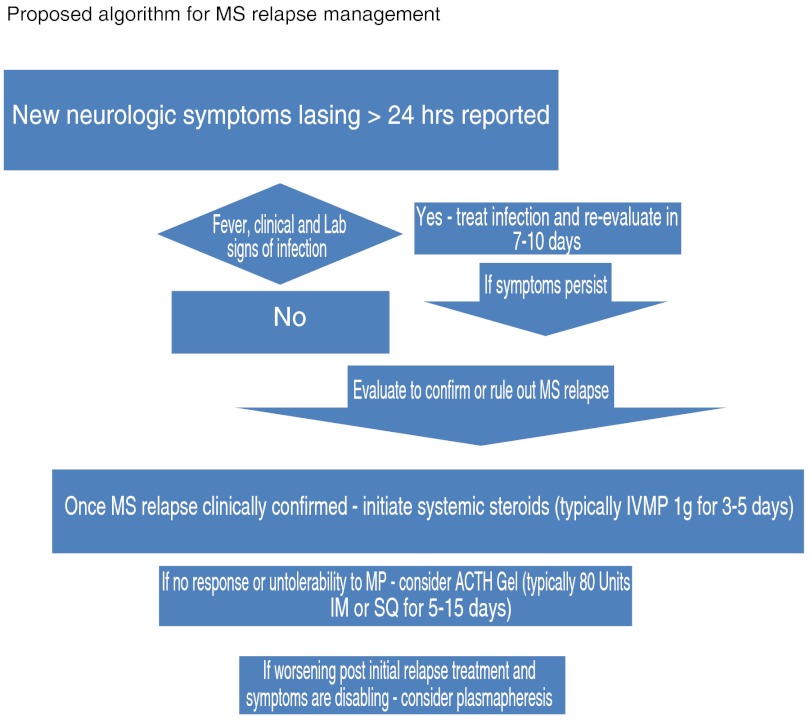
Similarly, there is no consensus regarding the minimal effective dosage, length of treatment, and route of administration for systemic corticosteroid treatment of relapse. In practice, varied treatment regimens for MS relapses have been adopted in large part based on the many clinical trials in MS performed in the last 20 years. We propose building on these generally accepted treatment guidelines to expand options for those who fail steroids (Fig. 1).
Evaluate patients with possible MS relapse within a week (or 5 working days) of the new or worsened symptoms onset
- If MS relapse is confirmed, start the treatment as soon as possible
- First line of treatment: IV-MP 1 g/day for 3 to 5 days, which is generally recommended as a first choice
- The need for oral prednisone tapering after the IV-MP should be considered on an individual basis (although there are data [61], suggesting no additional benefit for oral taper).
- Although not FDA approved, oral administration of high-dose MP instead of IV-MP may be suggested [2].
In respect to the patients with MS relapse, who did not respond to the high-dose MP, the consideration of clinical status (no improvement versus possible clinical worsening of symptoms after initial treatment) should be seen as different clinical scenarios of acute demyelinating events that can be encountered.
Patients who did not improve or could not tolerate the MP may be offered another FDA-approved option: ACTH. It should be noted that the effects of IV-MP or oral high-dose MP may be delayed, therefore, in general, as a rule in our practice, we wait 2 to 3 weeks after the last dosage of high-dose corticosteroids before initiating ACTH gel therapy administered either intramuscularly or subcutaneously [44, 62, 63] at a dose of 80 units/day for at least 5 days and for as many as 15 days [2, 13, 63]. The experience in our clinic indicates that the majority of MS patients in acute exacerbation, who previously failed (did not improve) or could not tolerate the MP treatment, experienced positive clinical outcomes and fewer adverse events with ACTH gel treatment [44].
For the patients with disabling MS relapse symptoms that do not respond to the initial treatment, especially those patients who experience clinical worsening of the symptoms after first-line treatment, the plasmapheresis options should be considered on an individual basis; it should be administered as every other day procedures for as many as 5 to 10 exchanges on average [49, 60].
Fig. 1.
Proposed algorithm for multiple sclerosis (MS) relapse management
Electronic supplementary material
(PDF 510 kb)
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
References
- 1.Lublin FD, Baier M, Cutter G. Effect of relapses on development of residual deficit in multiple sclerosis. Neurology. 2003;61:1528–1532. doi: 10.1212/01.WNL.0000096175.39831.21. [DOI] [PubMed] [Google Scholar]
- 2.Frohman EM, Shah A, Eggenberger E, et al. Corticosteroids for multiple sclerosis: I. Application for treating exacerbations. Neurotherapeutics. 2007;4:618–626. doi: 10.1016/j.nurt.2007.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Repovic P, Lublin FD. Treatment of multiple sclerosis exacerbations. Neurol Clin. 2011;29:389–400. doi: 10.1016/j.ncl.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 4.Hoch E. Ergebnisse verschiedener Behandlungsversuche der multiplen Sklerose an der neurologischen Abteilung der Medizinische und Nervenklinik der Universitat Wiirzburg wahrend der Jahre 1935/ 1936. Wiirzburg: Inaugural-Dissertation. Leipzig: Schneider and Mischkewitz, Naunhof, 1937.
- 5.Pette H. Die akut entziindlichen Erkrankungen des Nervensystems. Leipzig: Thieme 1942:516–517.
- 6.Schaltenbrand G. Die multiple Sklerose des Menschen. Leipzig: Thieme 1943:238–246.
- 7.Miller H, Newell DJ, Ridley A. Multiple sclerosis. Treatment of acute exacerbations with corticotrophin (ACTH) Lancet. 1961;11:1120–1122. doi: 10.1016/S0140-6736(61)91030-3. [DOI] [PubMed] [Google Scholar]
- 8.Fog T. Nord. Med. 1951;46:1742. [PubMed] [Google Scholar]
- 9.Jӧnsson B, von Reis G, Sahlgren E. Acta Psychiat. 1951;74:60. [PubMed] [Google Scholar]
- 10.Alexander L, Cass LJ. The present status of ACTH therapy in multiple sclerosis. Ann Intern Med. 1963;58:454–471. doi: 10.7326/0003-4819-58-3-454. [DOI] [PubMed] [Google Scholar]
- 11.Millar JHD, Belf MD, Vas CJ, et al. Long-term treatment of multiple sclerosis with corticotrophin. The Lancet. 1967;7513:429–431. doi: 10.1016/S0140-6736(67)90850-1. [DOI] [PubMed] [Google Scholar]
- 12.Rinne UK, Sonninen V, Tuovinen T. Corticotrophin treatment in multiple sclerosis. Acta Neurologica Scandinavica. 1967;31:185–186. doi: 10.1111/j.1600-0404.1967.tb02112.x. [DOI] [PubMed] [Google Scholar]
- 13.Rose AS, Kuzma JW, Kurtzke JF, Sibley WA, Tourtellotte WW. Cooperative study in the evaluation of therapy in multiple sclerosis: ACTH vs placebo in acute exacerbation. Trans Am Neurol Assoc. 1969;94:126–133. [PubMed] [Google Scholar]
- 14.Rose AS, Kuzma JW, Kurtzke JF, Namerow NS, Sibley WA, Tourtellotte WW. Cooperative study in the evaluation of therapy in multiple sclerosis: ACTH vs. placebo — final report. Neurology. 1970;20:1–59. doi: 10.1212/wnl.20.5_part_2.1. [DOI] [PubMed] [Google Scholar]
- 15.Questcor Pharmaceuticals, Inc. H.P. Acthar® Gel (repository corticotrophin injection) [prescribing information]. Hayward, CA: Questcor Pharmaceuticals, Inc.; June 2011.
- 16.Hoogstraten MC, Cats A, Minderhoud JM. Bed rest and ACTH in the treatment of exacerbations in multiple sclerosis patients. Acta Neurol Scand. 1987;76:346–350. doi: 10.1111/j.1600-0404.1987.tb03592.x. [DOI] [PubMed] [Google Scholar]
- 17.Hoogstraten MC, Minderhoud JM. Long-term effect of ACTH treatment of relapse in multiple sclerosis. Acta Neurol Scand. 1990;82:74–77. doi: 10.1111/j.1600-0404.1990.tb01590.x. [DOI] [PubMed] [Google Scholar]
- 18.Miller DM, Weinstock-Guttman B, Bethoux F, et al. A meta-analysis of methylprednisolone in recovery from multiple sclerosis exacerbations. Mult Scler. 2000;6:267–273. doi: 10.1177/135245850000600408. [DOI] [PubMed] [Google Scholar]
- 19.Abbruzzese G, Gandolfo C, Loeb C. "Bolus" methylprednisolone versus ACTH in the treatment of multiple sclerosis. Ital J Neurol Sci. 1983;2:169–172. doi: 10.1007/BF02043900. [DOI] [PubMed] [Google Scholar]
- 20.Barnes M, Bateman D, Cleland P, et al. Intravenous methylprednisolone for multiple sclerosis in relapse. J Neurol Neurosurg Psychiatry. 1985;48:157–159. doi: 10.1136/jnnp.48.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Milanese C, La Mantia L, Salmaggi A, et al. Double-blind randomized trial of ACTH versus dexamethasone versus methylprednisolone in multiple sclerosis bouts. Clinical, cerebrospinal fluid and neurophysiological results. Eur Neurol. 1989;29:10–14. doi: 10.1159/000116368. [DOI] [PubMed] [Google Scholar]
- 22.Thompson AJ, Kennard C, Swash M, et al. Relative efficacy of intravenous methylprednisolone and ACTH in the treatment of acute relapse in MS. Neurology. 1989;39:969–971. doi: 10.1212/WNL.39.7.969. [DOI] [PubMed] [Google Scholar]
- 23.Durelli L, Cocito D, Riccio A, et al. High-dose intravenous methylprednisolone in the treatment of multiple sclerosis: clinical-immunologic correlations. Neurology. 1986;36:238–243. doi: 10.1212/WNL.36.2.238. [DOI] [PubMed] [Google Scholar]
- 24.Milligan NM, Newcombe R, Compston DA. A double-blind controlled trial of high dose methylprednisolone in patients with multiple sclerosis: 1. clinical effects. J Neurol Neurosurg Psychiatry. 1987;50:511–516. doi: 10.1136/jnnp.50.5.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beck RW, Cleary PA, Anderson MM, et al. A randomized, controlled trial of corticosteroids in the treatment of acute optic neuritis. N Engl J Med. 1992;9:581–588. doi: 10.1056/NEJM199202273260901. [DOI] [PubMed] [Google Scholar]
- 26.Sellebjerg F, Frederiksen JL, Nielsen PM, Olesen J. Double-blind, randomized, placebo-controlled study of oral, high-dose methylprednisolone in attacks of MS. Neurology. 1998;51:529–534. doi: 10.1212/WNL.51.2.529. [DOI] [PubMed] [Google Scholar]
- 27.Barnes D, Hughes RAC, Morris RW, et al. Randomised trial of oral and intravenous methylprednisolone in acute relapses of multiple sclerosis. Lancet. 1997;349:902–906. doi: 10.1016/S0140-6736(96)06453-7. [DOI] [PubMed] [Google Scholar]
- 28.Martinelli V, Rocca MA, Annovazzi P, et al. A short-term randomized MRI study of high-dose oral vs intravenous methylprednisolone in MS. Neurology. 2009;73:1842–1848. doi: 10.1212/WNL.0b013e3181c3fd5b. [DOI] [PubMed] [Google Scholar]
- 29.Cass LJ, Alexander L, Enders M. Complications of corticotropin therapy in multiple sclerosis. JAMA. 1966;197:173–178. doi: 10.1001/jama.1966.03110030067027. [DOI] [PubMed] [Google Scholar]
- 30.Lukert BP, Raisz LG. Glucocorticoid-induced osteoporosis: pathogenesis and management. Ann Intern Med. 1990;112:352–364. doi: 10.7326/0003-4819-112-5-352. [DOI] [PubMed] [Google Scholar]
- 31.Schwid SR, Goodman AD, Puzas JE, et al. Sporadic corticosteroid pulses and osteoporosis in multiple sclerosis. Arch Neurol. 1996;8:753–757. doi: 10.1001/archneur.1996.00550080071014. [DOI] [PubMed] [Google Scholar]
- 32.Zorzon M, Zivadinov R, Locatelli L, et al. Long-term effects of intravenous high dose methylprednisolone pulses on bone mineral density in patients with multiple sclerosis. Eur J Neurol. 2005;7:550–556. doi: 10.1111/j.1468-1331.2005.00988.x. [DOI] [PubMed] [Google Scholar]
- 33.Weiner ES, Abeles M. Aseptic necrosis and glucocorticois in systemic lupus erythematosus: a reevaluation. J Rheumatol. 1989;16:604–608. [PubMed] [Google Scholar]
- 34.Fardet L, Kassar A, Cabane J, et al. Corticosteroid-induced adverse events in adults: frequency, screening and prevention. Drug Saf. 2007;30:861–881. doi: 10.2165/00002018-200730100-00005. [DOI] [PubMed] [Google Scholar]
- 35.Gurwitz JH, Bohn RL, Glynn RJ, et al. Glucocorticoids and the risk for initiation of hypoglycemic therapy. Arch Intern Med. 1994;154:97–101. doi: 10.1001/archinte.1994.00420010131015. [DOI] [PubMed] [Google Scholar]
- 36.Feldman-Billard S, Lissak B, Kassaei R, et al. Short-term tolerance of pulse methylprednisolone therapy in patients with diabeles mellitus. Ophthalmology. 2005;112:511–515. doi: 10.1016/j.ophtha.2004.10.032. [DOI] [PubMed] [Google Scholar]
- 37.Bomback AS, Tumlin JA, Baranski J, et al. Treatment of nephrotic syndrome with adrenocorticotropic horone (ACTH) gel. Drug Des Devel Ther. 2011;5:147–153. doi: 10.2147/DDDT.S17521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pranzatelli MR, Chun KY, Moxness M, Tate ED, Allison TJ. Cerebrospinal fluid ACTH and cortisol in opsoclonus-myoclonus: effect of therapy. Pediatr Neurol. 2005;33:121–126. doi: 10.1016/j.pediatrneurol.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 39.Stafstrom CE, Arnason BG, Baram TZ, et al. Treatment of infantile spasms: emerging insights from clinical and basic science perspectives. J Child Neurol. 2011;11:1411–1421. doi: 10.1177/0883073811413129. [DOI] [PubMed] [Google Scholar]
- 40.Catania A, Gatti S, Colombo G, Lipton JM. Targeting melanocortin receptors as a novel strategy to control inflammation. Pharmacol Rev. 2004;56:1–29. doi: 10.1124/pr.56.1.1. [DOI] [PubMed] [Google Scholar]
- 41.Catania A. Neuroprotective actions of melanocortins: a therapeutic opportunity. Trends Neurosci. 2008;31:353–360. doi: 10.1016/j.tins.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 42.Arnason B, Berkovich R, Catania A., et al. Therapeutic mechanisms of action of adrenocorticotropic hormone (ACTH) and other melanocortin peptides for the clinical management of patients with MS. 2012;(in press). [DOI] [PMC free article] [PubMed]
- 43.Poser CM. Corticotropin is superior to corticosteroids in the treatment of MS. Arch Neurol. 1989;46:946. doi: 10.1001/archneur.1989.00520450016008. [DOI] [PubMed] [Google Scholar]
- 44.Berkovich R, Fernandez M, Subhani D. Adrenocorticotropic hormone treatment of multiple sclerosis exacerbations. Presented at the Consortium of Multiple Sclerosis Centers; June 2, 2012; San Diego.
- 45.Zaidi M, Sun L, Robinson LJ, et al. ACTH protects against glucocorticoid-induced osteonecrosis of bone. Proc Natl Acad Sci USA. 2010;107:8782–8787. doi: 10.1073/pnas.0912176107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weinstein RS. Clinical practice. Glucocorticoid-induced bone disease. N Engl J Med. 2011;365:62–70. doi: 10.1056/NEJMcp1012926. [DOI] [PubMed] [Google Scholar]
- 47.Berkovich R, Subhani D, Steinman L. Autoimmune Comorbid Conditions in Multiple Sclerosis. US Neurol. 2011;7:132–138. [Google Scholar]
- 48.Weiner HL, Dau PC, Khatri BO, et al. Double-blind study of true vs. sham plasma exchange in patients treated with immunosupression for acute attacks of multiple sclerosis. Neurology. 1989;38:1143–1149. doi: 10.1212/WNL.39.9.1143. [DOI] [PubMed] [Google Scholar]
- 49.Weinshenker BG, O’Brien PC, Petterson TM, et al. A randomized trial of plasma exchange in acute central nervous system inflammatory demyelinating disease. Ann Neurol. 1999;46:878–886. doi: 10.1002/1531-8249(199912)46:6<878::AID-ANA10>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 50.Keegan M, Pineda AA, McClelland RL, et al. Plasma exchange for severe attacks of CNS demyelination: predictors of response. Neurology. 2002;58:143–146. doi: 10.1212/WNL.58.1.143. [DOI] [PubMed] [Google Scholar]
- 51.Keegan M, Konig F, Mcclelland R, et al. Relation between humoral pathological changes in multiple sclerosis and response to therapeutic plasma exchange. Lancet. 2005;366:579–582. doi: 10.1016/S0140-6736(05)67102-4. [DOI] [PubMed] [Google Scholar]
- 52.Barile-Fabris L, Ariza-Andraca R, Olguín-Ortega L, et al. Controlled clinical trial of IV cyclophosphamide versus IV methylprednisolone in severe neurological manifestations in systemic lupus erythematosus. Ann Rheum Dis. 2005;64:620–625. doi: 10.1136/ard.2004.025528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Greenberg BM, Thomas KP, Krishnan C, et al. Idiopathic transverse myelitis: corticosteroids, plasma exchange, or cyclophosphamide. Neurology. 2007;68:1614–1617. doi: 10.1212/01.wnl.0000260970.63493.c8. [DOI] [PubMed] [Google Scholar]
- 54.Noseworthy JH, O’Brien PC, Petterson TM, et al. A randomized trial of intravenous immunoglobulin in inflammatory demyelinating optic neuritis. Neurology. 2001;56:1514–1522. doi: 10.1212/WNL.56.11.1514. [DOI] [PubMed] [Google Scholar]
- 55.Tselis A, Perumal J, Caon C, et al. Treatment of corticosteroid refractory optic neuritis in multiple sclerosis patients with intravenous immunoglobulin. Eur J Neurol. 2008;15:1163–1167. doi: 10.1111/j.1468-1331.2008.02258.x. [DOI] [PubMed] [Google Scholar]
- 56.Visser LH, Beekman R, Tijssen CC, et al. A randomized, double-blind, placebocontrolled pilot study of i.v. immune globulins in combination with i.v. methylprednisolone in the treatment of relapses in patients with MS. Mult Scler. 2004;10:89–91. doi: 10.1191/1352458504ms978sr. [DOI] [PubMed] [Google Scholar]
- 57.Sorensen PS, Haas J, Sellebjerg F, et al. IV immunoglobulins as add-on treatment to methylprednisolone for acute relapses in MS. Neurology. 2004;63:2028–2033. doi: 10.1212/01.WNL.0000145798.61383.39. [DOI] [PubMed] [Google Scholar]
- 58.Roed HG, Langkilde A, Sellebjerg F, et al. A double-blind, randomized trial of IV immunoglobulin treatment in acute optic neuritis. Neurology. 2005;64:804–810. doi: 10.1212/01.WNL.0000152873.82631.B3. [DOI] [PubMed] [Google Scholar]
- 59.O’Connor PW, Goodman A, Willmer-Hulme AJ, et al. Randomized multicenter trial of natalizumab in acute MS relapses: clinical and MRI effects. Neurology. 2004;62:2038–2043. doi: 10.1212/01.WNL.0000128136.79044.D6. [DOI] [PubMed] [Google Scholar]
- 60.Cortese V, Chaudhry YT, So F, et al. Evidence-based guideline update: plasmapheresis in neurologis disorders: report of the therapeutics and technology assessment subcommittee of the Americal Academy of Neurology. Neurology 2011;76:294–300. [DOI] [PMC free article] [PubMed]
- 61.Perumal JS, Caon C, Hreha S, et al. Oral prednisone taper following intravenous steroids fails to improve disability or recovery from relapses in multiple sclerosis. Eur J Neurol. 2008;7:677–680. doi: 10.1111/j.1468-1331.2008.02146.x. [DOI] [PubMed] [Google Scholar]
- 62.Brod SA, Morales MM. Bio-equivalence of intramuscular and subcutaneous H.P. Acthar Gel. Biomed Pharmacother. 2009;63:251–253. doi: 10.1016/j.biopha.2008.08.022. [DOI] [PubMed] [Google Scholar]
- 63.Simsarian JP, Saunders C, Smith DM. Five-day regimen of intramuscular or subcutaneous self-administered adrenocorticotropic hormone gel for acute exacerbations of multiple sclerosis: a prospective, randomized, open-label pilot trial. Drug Des Devel Ther. 2011;5:381–389. doi: 10.2147/DDDT.S19331. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 510 kb)