Abstract
Human malignant pleural mesothelioma (MPM) is considered a rare tumor, but recent estimations indicate that one-quarter million people will die of this neoplasm in Europe in the next three decades. The mineral asbestos is considered the main causative agent of this neoplasm. MPM is largely unresponsive to conventional chemotherapy/radiotherapy. In addition to asbestos exposure, genetic predisposition to asbestos carcinogenesis and to simian virus (SV)40 infection has also been suggested. SV40 is a DNA tumor virus found in some studies to be associated at high prevalence with MPM. SV40 sequences have also been detected, although at a lower prevalence than in MPM, in blood specimens from healthy donors. However, some studies have failed to reveal SV40 footprints in MPM and its association with this neoplasm. These conflicting results indicate the need for further investigations with new approaches. We report on the presence of antibodies in serum samples from patients affected by MPM that specifically react with two different SV40 mimotopes. The two SV40 peptides used in indirect ELISAs correspond to viral capsid proteins. ELISA with the two SV40 mimotopes gave overlapping results. Our data indicate that in serum samples from MPM-affected patients (n = 97), the prevalence of antibodies against SV40 viral capsid protein antigens is significantly higher (26%, P = 0.043) than in the control group (15%) represented by healthy subjects (n = 168) with the same median age (66 y) and sex. Our results suggest that SV40 is associated with a subset of MPM and circulates in humans.
Keywords: antibody, neoplasia
Human malignant pleural mesothelioma (MPM) is considered a rare tumor, but recent statistical data estimate that one-quarter million people will die of this neoplasm in Europe in the next three decades. MPM is a highly aggressive neoplasm that leads to death in patients in a few months/years from the time of diagnosis (1). The mineral asbestos is the main etiological agent of this neoplasm. Because MPM arises most frequently in workers exposed to asbestos fibers, this neoplasm is considered an occupational disease. The latency period of MPM after exposure to asbestos may reach 40 y or more. In Europe, the MPM peak is expected to occur around 2020–2025 (2). There is no standard curative therapy for MPM, which is largely unresponsive to conventional chemotherapy/radiotherapy. It has been shown that 2–10% of asbestos-exposed individuals may develop MPM (3). Because MPM onset occurs decades after asbestos exposure, subjects potentially at risk of developing this malignancy may benefit from early diagnosis (4). At present there is no recognized specific marker for the diagnosis of MPM or for screening of at-risk asbestos-exposed individuals (5). Recent investigations have allowed for the selection of putative markers such as osteopontin (6) and the soluble mesothelin-related protein (7). However, additional studies found that osteopontin is not specific for MPM (8), whereas mesothelin, once considered specific for the MPM epithelial subtype, has been found to be present in serum samples of asbestos workers, too (9). So far, due to a lack of accurate tools and a curative treatment, screening for MPM is currently not recommended. Therefore, the detection of specific markers that can be useful for MPM screening and diagnosis is of paramount importance (10, 11).
In addition to asbestos exposure, many investigations have found an association of MPM with oncogenic simian virus (SV) 40 (12, 13). Indeed, many reports have detected SV40 sequences at high prevalence and the expression of its large tumor antigen (Tag) oncoprotein in MPM. A transforming synergistic action between asbestos fibers and SV40 has been proved in human, hamster, and murine mesothelial cells and in animal models (14–16).
In MPM patients, genetic predisposition and specific susceptibility to asbestos carcinogenesis and to SV40 infection were also suggested (3, 17–19). Contrasting reports have appeared in the literature on the presence of SV40 footprints in humans and its association with human tumors (20–22). SV40 antibody detection had been attempted in several studies using serologic methods with SV40 virus-like particles (VLPs), but because of high protein homology among the three main polyomaviruses SV40, BKV, and JCV, the results were always affected by some cross-reactivity (23–30).
Considerable debate has developed in the scientific community (31). Problems relating to SV40 infection in the human population and its contribution to human cancer were summarized in an evaluation by the Immunization Safety Review Committee established by the Institute of Medicine of the National Academies (32). The committee concluded that “the biological evidence is strong that SV40 is a transforming virus, but it is of moderate strength that SV40 exposure from polio vaccine is related to SV40 infection in humans and that SV40 exposure could lead to cancer in humans under natural conditions.” The committee also recommended the development of specific and sensitive serologic tests for SV40 and the use of standardized techniques that should be accepted and shared by all laboratories involved in SV40 detection. A recent investigation reported that an indirect ELISA with SV40-specific synthetic peptides from its viral proteins was successfully used in detecting SV40 antibodies in serum samples from normal individuals, without cross-reactivity with other closely related polyomaviruses such as BKV and JCV (33).
In this investigation, serum samples from MPM-affected patients and healthy subjects, together with other controls, were analyzed for exposure to SV40 infection by an indirect ELISA using specific mimotopes from SV40 viral capsid proteins (33).
Results
Serum samples from oncologic patients affected by MPM, together with controls, were analyzed by indirect ELISA for the presence of IgG-class antibodies against SV40 viral capsid protein (VP) mimotopes designated VP1 B and VP2/3 C (33) (Fig. 1). The ELISA indicated that seropositive samples for SV40 VP1 B were also found positive for SV40 VP2/3 C. Conversely, seronegative samples for SV40 VP1 peptide B failed to react with SV40 VP2/3 peptide C. The exceptions were negligible, represented by a few serum samples that were found negative for VP1 B whereas testing positive for VP2/3 C peptide, and vice versa. This difference, between the number of samples found positive for VP1 B and positive for VP2/3 C in the distinct cohorts analyzed, was not statistically significant (P > 0.05) (Tables 1 and 2). In our study, sera were considered SV40-positive when reacting with both VP1 B and VP2/3 C peptides.
Fig. 1.
Computer-assisted representation of the structure of the SV40 capsid virion refined at 3.1-Å resolution (60), which is publicly available at the Protein Data Bank (PDB) online archive (PDB ID code 1SVA). The macromolecular assembly of the functional form of the molecule is available from the same source. Crystallographic and noncrystallographic transformations, both rotational and translational, are needed to generate the biological assembly. The biological assembly was generated with the RasMol Molecular Graphics Visualization Tool (61, 62) available at www.rasmol.org. The VP1 peptide B (red) was mapped on VP1 capsid proteins, which are present 360 times in the virion. The lack of VP2 and VP3 crystallographic forms did not allow mapping peptide C on these capsid proteins.
Table 1.
Prevalence of serum IgG antibodies reacting with SV40 VP mimotopes in serum samples from MPM-affected patients, healthy subjects, workers exposed to asbestos, and pregnant women
| No. of positive samples (%) |
||||||
| Human sera | No. of subjects/patients | Median age (y) | Male (%) | VP B | VP C | VPs (B-C) |
| Mesothelioma | 97 | 66 | 68 | 31 (32) | 31 (32) | 25 (26) |
| Healthy subjects | 168 | 66 | 62.5 | 31 (18) | 28 (17) | 25 (15) |
| Workers exposed to asbestos fibers | 90 | 54.5 | 100 | 9 (10) | 8 (9) | 8 (9) |
| Pregnant women | 94 | 36 | — | 15 (16) | 13 (14) | 12 (13) |
Statistical analysis was performed by the χ2 test with Yates’ correction. The different prevalence of SV40 antibodies for the cohort of mesothelioma-affected patients was statistically significant compared with the cohort of healthy individuals (P = 0.043), the cohort of worker exposed to asbestos fibers (P = 0.0046), and the cohort of pregnant women (P = 0.036).
Table 2.
Prevalence of serum IgG antibodies reacting with SV40 VP mimotopes in serum samples from pregnant women, workers exposed to asbestos, and blood donors
| No. of positive samples (%) |
|||||||
| Human sera | No. of subjects/patients | Median age (y) | Male (%) | VP B | VP C | VPs (B-C) | P value |
| Pregnant women | 94 | 36 | — | 15 (16) | 13 (14) | 12 (13) | 0.43 |
| Healthy blood donors | 125 | 36 | 80 | 26 (21) | 27 (22) | 22 (18) | |
| Workers exposed to asbestos fibers | 90 | 54.5 | 100 | 9 (10) | 8 (9) | 8 (9) | 0.19 |
| Healthy blood donors | 174 | 54.5 | 100 | 34 (19) | 36 (21) | 27 (15) | |
Statistical analysis was performed by the χ2 test with Yates’ correction. No statistically significant differences of SV40 seroprevalence were found between the cohorts of pregnant women and healthy blood donors (P = 0.43) and between the cohorts of subjects exposed to asbestos fibers and healthy blood donors (P = 0.19).
SV40-positive sera tested by indirect ELISA diluted at 1/20 had a general cutoff, by spectrophotometric reading, in the range of 0.17–0.19 OD (33). Specifically, in serum samples from MPM patients (n = 97), the prevalence of specific SV40 VP antibodies was 26%, whereas in healthy subjects, the control group (n = 168), it was 15%. This difference is statistically significant (P = 0.043). MPM patient and control groups had the same median age (66 y) and sex (they were all males) (Fig. 2 and Table 1). Serologic profiles of serum antibody reactivity to SV40 mimotopes are presented in Fig. 3. The difference in OD mean value of sera from MPM and healthy subjects is not statistically significant (P > 0.05) (Fig. 3).
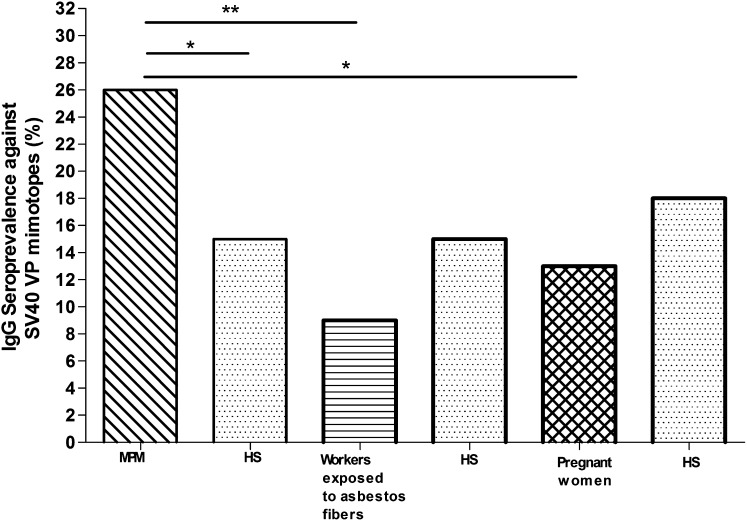
Fig. 2.
Prevalence of SV40-positive serum samples in patients with malignant pleural mesothelioma, workers exposed to asbestos fibers, and pregnant women. To compare the prevalence of MPM, workers exposed to asbestos fibers, and pregnant women, three different groups of healthy subjects (HS) were chosen with the same median age and sex. Statistical analysis was performed by the χ2 test with Yates’ correction. Statistical analyses revealed significant differences in SV40 prevalence between MPM and the relative cohort of healthy subjects (P = 0.043) with the subjects exposed to asbestos fibers (P = 0.0046) and with pregnant women (P = 0.036). No statistically significant differences in SV40 seroprevalence were found between the cohorts of pregnant women and subjects exposed to asbestos fibers with their control groups of healthy subjects. *P < 0.05; **P < 0.01.
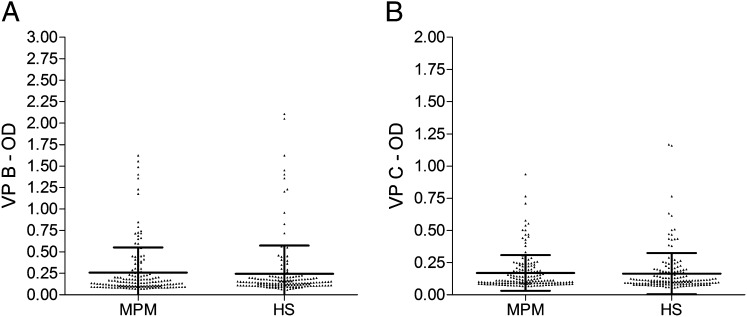
Fig. 3.
Serologic profile of serum antibody reactivity to SV40 mimotopes VP1 B (A) and VP2/3 C (B). Immunologic data are from patients affected by MPM and from healthy subjects. Results are presented as values of OD readings at 405 nm of serum samples diluted at 1:20 detected in an indirect ELISA. In scatter dot plotting, each plot represents the dispersion of OD values to a mean level indicated by the line inside the scatter with SEM for each group of subjects analyzed. The difference in OD mean value was not statistically significant (P > 0.05).
In a second step of our investigation, we verified whether the prevalence of SV40 antibodies determined in serum samples from MPM patients differed from that of workers who had been exposed to asbestos, which is a natural oncogenic mineral with immunosuppressive activity (34, 35). Our study indicated that the prevalence of SV40 VP antibodies detected in workers who had been exposed to asbestos (n = 90) is 9% (Fig. 2 and Table 1). The comparative analysis indicates that the prevalence of SV40-positive sera is higher in MPM patients (26%) compared with that of workers who had been exposed to asbestos. This difference is statistically significant (P = 0.0046) (Fig. 2 and Table 1). Interestingly, the reduced prevalence of SV40-positive sera in workers previously exposed to asbestos is very likely due to the immune-suppressive activity of asbestos (34, 35). This prevalence was also analyzed in comparison with that of the control group represented by healthy blood donors (n = 174) with the same median age (54.5 y). The difference in prevalence is not statistically significant (15% vs. 9%, P = 0.19) (Fig. 2 and Table 2).
In a third step of our investigation, we analyzed by indirect ELISA the prevalence of SV40 VP mimotopes in a cohort of pregnant women (n = 94). This control group was introduced in this study because pregnancy induces a temporary state of immune depression/immune tolerance (36), and thus would mimic the group of healthy workers exposed to asbestos. The prevalence of SV40 antibodies determined in serum samples from pregnant women was 13%. The comparative analysis indicated that the prevalence of SV40-positive sera is higher in malignant pleural mesothelioma patients (26%) compared with that of pregnant women. This difference is statistically significant (P = 0.036) (Fig. 2 and Table 1). It is worth mentioning that the difference in prevalence of SV40 antibodies between pregnant woman (n = 94, 13%) and healthy blood donors (n = 125, 18%) with the same median age (36 y) is not statistically significant (P = 0.43) (Fig. 2 and Table 2).
Discussion
In our investigation, serum samples from MPM-affected patients and normal individuals were analyzed for their reactivity to SV40 VP mimotopes using indirect ELISA. Sera from MPM patients reacted against SV40 VP antigens with a higher prevalence (26%) compared with the cohort of controls represented by healthy individuals (15%). The difference is statistically significant. These results indicate that SV40 infection is associated with a subset of MPM and that SV40 antibodies are also present in the adult population, although at a lower prevalence.
The high prevalence of SV40 antibodies in serum samples from MPM patients seems to confirm earlier data obtained in investigations carried out with PCR techniques, which indicated a high prevalence of SV40 sequences in MPM DNA (13, 20). Data obtained in normal individuals suggest that natural SV40 infection occurs in human populations with a prevalence lower than that of the ubiquitous JCV and BKV (60–90%) (25). It is possible that SV40 is transmitted through contact in the human environment. Indeed, it has been shown that SV40 is present in urine, stool, tonsil, and blood specimens of carriers, suggesting that different routes of transmission are responsible for SV40 infection (37–45).
Our data seem to confirm results obtained by earlier studies in subjects administered SV40-contaminated vaccines by different routes. In these individuals, SV40 was detected/isolated after a number of weeks or days, either in their stool or from their throat depending on oral or nasal spray administration of contaminated vaccines (46, 47). At present, SV40 infection seems to occur independently from early contaminated vaccines. Indeed, many studies have reported SV40 sequences, serum antibodies against SV40, and SV40 isolation/rescue from subjects too young or too old to have been vaccinated with SV40-contaminated vaccines. Altogether, these investigations suggest that SV40 may be contagiously transmitted in the human population either directly by person-to-person contact or indirectly by orofecal and other routes (reviewed in refs. 20 and 31).
In healthy individuals (median age 66 y), the overall prevalence of the IgG class of SV40 antibodies is around 15%. It is important to note that SV40 prevalence in human sera from healthy subjects detected by our immunologic study does not differ substantially from that reported by a previous study carried out in the United States using neutralization testing against SV40 infectivity, which is considered the gold standard for measuring the presence of the SV40 antibody (40). In addition, SV40 sequences were detected in earlier studies with a similar prevalence (16%) by PCR assays in healthy blood donors (48).
Interestingly, SV40-positive MPM sera had a higher prevalence (26%) than that of workers exposed to asbestos fibers (9%), an immunosuppressive mineral (34, 35), suggesting that asbestos may depress the immune system of these workers. A lower prevalence of SV40-positive sera was also detected in pregnant women (13%). This result is probably due to pregnancy, which induces immune tolerance in women. Serum samples from MPM-affected patients had a prevalence of SV40 antibody that was higher than that of these cohorts of controls, that is, pregnant women and workers exposed to asbestos. The difference was statistically significant.
In should be noted that the prevalence of SV40 antibodies determined in asbestos-exposed workers and pregnant women reflects the seroprevalence of the general population. Altogether, this result remarks on the significance of the higher seroprevalence detected in the MPM patient cohort.
The onset and progression of MPM, as for other cancers, are associated with specific gene mutations (18, 19). However, the agents responsible for the occurrence of mutations/chromosome alterations are poorly understood. Although asbestos is considered the main cause, SV40 may be an additional candidate for causing mutations and chromosome alterations in tumors. SV40 was found to be mutagenic in human cells (20, 31). The viral oncoprotein Tag induces mutations and chromosomal damage characterized by numerical and structural chromosomal alterations such as gaps, breaks, dicentric and ring chromosomes, chromatid exchanges, deletions, duplications, and translocations (20). Alternatively, SV40, although able with its Tag oncogene to bind and inactivate p53- and pRB-family proteins, could only be a passenger virus, multiplying better in some transformed cells than in normal cells. Indeed, it has been proved that normal human mesothelial cells as well as other normal human cells are only semipermissive to SV40 multiplication (49–52). The high prevalence of SV40 antibodies in sera from MPM-affected patients is not proof of cause and effect in inducing human tumors by SV40. However, one may postulate that after infecting the host, SV40 may exert its tumorigenic potential when the immune system is impaired. If SV40 is proven to act as a tumor agent in humans, new therapeutic/preventive strategies could be applied with antiviral drugs or vaccines.
Materials and Methods
Human Samples.
Serum samples were collected in different Italian institutions during 2006–2012. Anonymously collected sera were coded with indications of age and sex. Informed written consent was obtained from patients and healthy subjects. The project was approved by the County Ethical Committee, Ferrara, Italy. Different cohorts were homogeneously clustered by age, sex, and pathology, if any. Sera from MPM-affected patients (n = 97) were from the universities/hospitals of Brescia, Casale Monferrato, Pisa, and Trieste, whereas sera from asbestos-exposed workers (n = 90) were from the University of Pisa. Sera from pregnant women (n = 94) were from the Istituto Superiore di Sanità, Rome, whereas those from normal individuals (n = 168) and healthy blood donors (n = 299) were from the universities/hospitals of Alessandria, Ferrara, Novara, Republic of San Marino, Rome, and Trieste. Human sera from three different groups of healthy subjects were analyzed: (i) Healthy individuals (n = 168, 66 y median age) were analyzed to compare SV40 seroprevalence with that detected in mesothelioma patients. (ii) Healthy blood donors (n = 125, 36 y median age) was the control group analyzed in comparison with pregnant women. (iii) Healthy blood donors (n = 174, 54.5 y) was the control group analyzed in comparison with the group of workers exposed to asbestos fibers.
SV40 Mimotopes.
Computer-assisted analyses allowed us to select two specific SV40 peptides from the late viral region by comparing the three capsid proteins VP 1–3 from SV40 with the amino acids of the human BK (BKV) and JC (JCV) polyomaviruses, which are highly homolog to SV40, as well as with other, less homologous, polyomaviruses (33). Previous ELISA results indicated that the two SV40 peptides did not cross-react with the BKV and JCV hyperimmune sera used as controls (33). The two peptides belong to the viral capsid proteins VP1/VP2/VP3 (www.ncbi.nlm.nih.gov/nuccore). The amino acid sequences of the two peptides, named VP1 B and VP2/3 C, are as follows:
VP1 B: NH2-NPDEHQKGLSKSLAAEKQFTDDSP-COOH
VP2/3 C: NH2-IQNDIPRLTSQELERRTQRYLRD-COOH
VP1 B and VP2/3 C mimotopes were selected because they react specifically in indirect ELISA with the rabbit hyperimmune serum that had been experimentally immunized with SV40 (positive control serum). BKV and JCV hyperimmune sera did not react with VP1 B and VP2/3 C peptides (negative control sera). The amino acid residues of the two specific SV40 VP peptides show low homology to the VPs of BKV and JCV (33). The human peptide hNPS, amino acid sequence SFRNGVGTGMKKTSFQRAKS, was used as a negative control peptide (53). The synthetic peptides were synthesized by standard procedures and purchased from UFPeptides.
Control Immune Sera.
Hyperimmune sera against SV40 and BKV were obtained in rabbits inoculated with purified viral stocks as previously reported (54–56). The serum against JCV was kindly provided by E.O. Major (Laboratory of Molecular Medicine and Neuroscience, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD) (57). The immune serum anti-BKV was titered by the hemagglutination inhibition test using human erythrocytes from the 0, Rh+ group (55, 58). Anti-SV40 serum was titered by neutralization assay (54, 59).
Indirect ELISA.
Indirect ELISA was developed and standardized to detect specific antibodies against SV40 in human sera using synthetic peptides (33).
Peptide coating.
Plates were coated with 5 μg of the selected peptide for each well, diluted in 100 μL of coating buffer (Candor Bioscience).
Peptide blocking.
Blocking was done with 200 μL per well of blocking solution (Candor Bioscience) at 37 °C for 90 min.
Primary antibody addition.
Different wells were covered with 100 μL containing the following sera: positive control, represented by immune rabbit serum containing anti-SV40 antibodies; negative controls, represented by immune sera anti-BKV and anti-JCV and human serum samples under analysis diluted at 1:20 in low cross-buffer (Candor Bioscience). Each sample was analyzed three times.
Secondary antibody addition.
The solution contained a goat anti-human IgG heavy chain- and light chain-specific peroxidase conjugate (Calbiochem-Merck) or peroxidase-labeled affinity-purified antibody to human IgM ν (KPL) diluted 1:10,000 in low cross-buffer.
Dye treatment and spectrophotometric reading.
Samples were treated with 100 μL of 2,2′-azino-bis-3-ethylbenzthiazoline-6-sulfonic acid solution (Sigma-Aldrich) and then read by spectrophotometer (Thermo Electron; model Multiskan EX) at a wavelength of 405 nm. This approach detects color intensity in wells by OD where immunocomplexes are formed.
Automatic ELISA.
Indirect ELISA was transferred and repeated on the automatic processing system with a DSX instrument (Dynex Technologies).
Cutoff determination.
The cutoff was determined in each assay by an OD reading of two negative controls added to the SD and multiplied three times (+3 SD). Sera with antibodies against SV40 were considered VP-positive upon reaction with both peptides from the late region. The reproducibility of the results was assessed with three duplicated experiments carried out by independent operators with no data variability.
Statistical Analysis.
Statistical analyses were performed using Prism software (GraphPad). Data are presented as a percentage of positive samples. Differences among proportions of percentages were calculated by χ2 with Yates’ correction testing in contingency tables. Human sera from three different groups of healthy subjects were analyzed: (i) healthy individuals (n = 168, 66 y median age) were analyzed to compare SV40 seroprevalence with that detected in mesothelioma patients; (ii) healthy blood donors (n = 125, 36 y median age) was the control group analyzed in comparison with pregnant women; and (iii) healthy blood donors (n = 174, 54.5 y) was the control group analyzed in comparison with the group of workers exposed to asbestos fibers. The serologic profile of serum antibody reactivity to SV40 mimotopes was statistically analyzed using a Student’s unpaired t test. P values less than 0.05 were considered statistically significant.
Acknowledgments
We thank Dr. Eugene O. Major (Laboratory of Molecular Medicine and Neuroscience, National Institute of Neurological Disorders and Stroke) for the hyperimmmune serum against JCV. E.M. is a postdoctoral fellow of the Fondazione Umberto Veronesi, Milan. This work was supported, in part, by grants from Fondazione Buzzi UNICEM (Unione Cementieri), Casale Monferrato; ASLEM (Associazione Sanmarinese Leucemie Emopatie Maligne), Repubblica di San Marino; Regione Autonoma Friuli-Venezia Giulia, Assessorato alla Salute e Protezione Sociale, LR 22/2001, 969/APREV, Trieste; ISS (Istituto Superiore di Sanità), Roma; ISPESL (Istituto superiore per la prevenzione e la sicurezza del lavoro), Roma; Fondazione Cassa di Risparmio di Cento, Cento; Regione Emilia-Romagna, Bologna; and Università di Ferrara, FAR (Fondo di Ateneo per la Ricerca) Projects, Ferrara, Italy.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
References
- 1.Robinson BW, Musk AW, Lake RA. Malignant mesothelioma. Lancet. 2005;366(9483):397–408. doi: 10.1016/S0140-6736(05)67025-0. [DOI] [PubMed] [Google Scholar]
- 2.Peto J, Decarli A, La Vecchia C, Levi F, Negri E. The European mesothelioma epidemic. Br J Cancer. 1999;79(3-4):666–672. doi: 10.1038/sj.bjc.6690105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ismail-Khan R, et al. Malignant pleural mesothelioma: A comprehensive review. Cancer Contr. 2006;13(4):255–263. doi: 10.1177/107327480601300402. [DOI] [PubMed] [Google Scholar]
- 4.Zucali PA, Giaccone G. Biology and management of malignant pleural mesothelioma. Eur J Cancer. 2006;42(16):2706–2714. doi: 10.1016/j.ejca.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 5.Zucali PA, et al. New tricks for old biomarkers: Thymidylate synthase expression as a predictor of pemetrexed activity in malignant mesothelioma. Ann Oncol. 2010;21(7):1560–1561. doi: 10.1093/annonc/mdq253. [DOI] [PubMed] [Google Scholar]
- 6.Pass HI, et al. Asbestos exposure, pleural mesothelioma, and serum osteopontin levels. N Engl J Med. 2005;353(15):1564–1573. doi: 10.1056/NEJMoa051185. [DOI] [PubMed] [Google Scholar]
- 7.Robinson BW, et al. Mesothelin-family proteins and diagnosis of mesothelioma. Lancet. 2003;362(9396):1612–1616. doi: 10.1016/S0140-6736(03)14794-0. [DOI] [PubMed] [Google Scholar]
- 8.Paleari L, et al. Osteopontin is not a specific marker in malignant pleural mesothelioma. Int J Biol Markers. 2009;24(2):112–117. doi: 10.1177/172460080902400208. [DOI] [PubMed] [Google Scholar]
- 9.Rodríguez Portal JA, et al. Serum levels of soluble mesothelin-related peptides in malignant and nonmalignant asbestos-related pleural disease: Relation with past asbestos exposure. Cancer Epidemiol Biomarkers Prev. 2009;18(2):646–650. doi: 10.1158/1055-9965.EPI-08-0422. [DOI] [PubMed] [Google Scholar]
- 10.Varani K, et al. A3 receptors are overexpressed in pleura from patients with mesothelioma and reduce cell growth via Akt/nuclear factor-κB pathway. Am J Respir Crit Care Med. 2011;183(4):522–530. doi: 10.1164/rccm.201006-0980OC. [DOI] [PubMed] [Google Scholar]
- 11.Balatti V, et al. MicroRNAs dysregulation in human malignant pleural mesothelioma. J Thorac Oncol. 2011;6(5):844–851. doi: 10.1097/JTO.0b013e31820db125. [DOI] [PubMed] [Google Scholar]
- 12.Klein G, Powers A, Croce C. Association of SV40 with human tumors. Oncogene. 2002;21(8):1141–1149. doi: 10.1038/sj.onc.1205173. [DOI] [PubMed] [Google Scholar]
- 13.Cristaudo A, et al. SV40 enhances the risk of malignant mesothelioma among people exposed to asbestos: A molecular epidemiologic case-control study. Cancer Res. 2005;65(8):3049–3052. doi: 10.1158/0008-5472.CAN-04-2219. [DOI] [PubMed] [Google Scholar]
- 14.Pietruska JR, Kane AB. SV40 oncoproteins enhance asbestos-induced DNA double-strand breaks and abrogate senescence in murine mesothelial cells. Cancer Res. 2007;67(8):3637–3645. doi: 10.1158/0008-5472.CAN-05-3727. [DOI] [PubMed] [Google Scholar]
- 15.Robinson C, et al. A novel SV40 TAg transgenic model of asbestos-induced mesothelioma: Malignant transformation is dose dependent. Cancer Res. 2006;66(22):10786–10794. doi: 10.1158/0008-5472.CAN-05-4668. [DOI] [PubMed] [Google Scholar]
- 16.Kroczynska B, et al. Crocidolite asbestos and SV40 are cocarcinogens in human mesothelial cells and in causing mesothelioma in hamsters. Proc Natl Acad Sci USA. 2006;103(38):14128–14133. doi: 10.1073/pnas.0604544103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zucali PA, et al. Advances in the biology of malignant pleural mesothelioma. Cancer Treat Rev. 2011;37(7):543–558. doi: 10.1016/j.ctrv.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 18.Bott M, et al. The nuclear deubiquitinase BAP1 is commonly inactivated by somatic mutations and 3p21.1 losses in malignant pleural mesothelioma. Nat Genet. 2011;43(7):668–672. doi: 10.1038/ng.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Testa JR, et al. Germline BAP1 mutations predispose to malignant mesothelioma. Nat Genet. 2011;43(10):1022–1025. doi: 10.1038/ng.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barbanti-Brodano G, et al. Simian virus 40 infection in humans and association with human diseases: Results and hypotheses. Virology. 2004;318(1):1–9. doi: 10.1016/j.virol.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 21.López-Ríos F, Illei PB, Rusch V, Ladanyi M. Evidence against a role for SV40 infection in human mesotheliomas and high risk of false-positive PCR results owing to presence of SV40 sequences in common laboratory plasmids. Lancet. 2004;364(9440):1157–1166. doi: 10.1016/S0140-6736(04)17102-X. [DOI] [PubMed] [Google Scholar]
- 22.Henzi T, et al. SV40-induced expression of calretinin protects mesothelial cells from asbestos cytotoxicity and may be a key factor contributing to mesothelioma pathogenesis. Am J Pathol. 2009;174(6):2324–2336. doi: 10.2353/ajpath.2009.080352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ribeiro T, et al. Investigation of the prevalence of antibodies against neurotropic polyomaviruses BK, JC and SV40 in sera from patients affected by multiple sclerosis. Neurol Sci. 2010;31(4):517–521. doi: 10.1007/s10072-010-0353-y. [DOI] [PubMed] [Google Scholar]
- 24.Lundstig A, et al. Prevalence and stability of human serum antibodies to simian virus 40 VP1 virus-like particles. J Gen Virol. 2005;86(Pt 6):1703–1708. doi: 10.1099/vir.0.80783-0. [DOI] [PubMed] [Google Scholar]
- 25.Viscidi RP, et al. Serological cross-reactivities between antibodies to simian virus 40, BK virus, and JC virus assessed by virus-like-particle-based enzyme immunoassays. Clin Diagn Lab Immunol. 2003;10(2):278–285. doi: 10.1128/CDLI.10.2.278-285.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barbanti-Brodano G, Corallini A, Accolla RS, Martini F, Tognon M. Re: Lack of serologic evidence for prevalent simian virus 40 infection in humans. J Natl Cancer Inst. 2004;96(10):803–804. doi: 10.1093/jnci/djh151. [DOI] [PubMed] [Google Scholar]
- 27.Carter JJ, et al. Lack of serologic evidence for prevalent simian virus 40 infection in humans. J Natl Cancer Inst. 2003;95(20):1522–1530. doi: 10.1093/jnci/djg074. [DOI] [PubMed] [Google Scholar]
- 28.Kjaerheim K, et al. Absence of SV40 antibodies or DNA fragments in prediagnostic mesothelioma serum samples. Int J Cancer. 2007;120(11):2459–2465. doi: 10.1002/ijc.22592. [DOI] [PubMed] [Google Scholar]
- 29.Kean JM, Rao S, Wang M, Garcea RL. Seroepidemiology of human polyomaviruses. PLoS Pathog. 2009;5(3):e1000363. doi: 10.1371/journal.ppat.1000363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bouvard V, et al. WHO International Agency for Research on Cancer Monograph Working Group Carcinogenicity of malaria and of some polyomaviruses. Lancet Oncol. 2012;13(4):339–340. doi: 10.1016/s1470-2045(12)70125-0. [DOI] [PubMed] [Google Scholar]
- 31.Martini F, et al. Simian virus 40 in humans. Infect Agent Cancer. 2007;2:13. doi: 10.1186/1750-9378-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stratton K, Almario DA, McCormick MC, editors. Immunization Safety Review: SV40 Contamination of Polio Vaccine and Cancer. Washington, DC: Natl Acad Press; 2003. [PubMed] [Google Scholar]
- 33.Corallini A, et al. Specific antibodies reacting with simian virus 40 capsid protein mimotopes in serum samples from healthy blood donors. Hum Immunol. 2012;73(5):502–510. doi: 10.1016/j.humimm.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 34.Maeda M, et al. Dysregulation of the immune system caused by silica and asbestos. J Immunotoxicol. 2010;7(4):268–278. doi: 10.3109/1547691X.2010.512579. [DOI] [PubMed] [Google Scholar]
- 35.Tulinska J, et al. Immunomodulatory effects of mineral fibres in occupationally exposed workers. Mutat Res. 2004;553(1-2):111–124. doi: 10.1016/j.mrfmmm.2004.06.030. [DOI] [PubMed] [Google Scholar]
- 36.Chang D, et al. Genotypes of human polyomaviruses in urine samples of pregnant women in Taiwan. J Med Virol. 1996;48(1):95–101. doi: 10.1002/(SICI)1096-9071(199601)48:1<95::AID-JMV15>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 37.Butel JS, Arrington AS, Wong C, Lednicky JA, Finegold MJ. Molecular evidence of simian virus 40 infections in children. J Infect Dis. 1999;180(3):884–887. doi: 10.1086/314915. [DOI] [PubMed] [Google Scholar]
- 38.Butel JS, et al. Evidence of SV40 infections in hospitalized children. Hum Pathol. 1999;30(12):1496–1502. doi: 10.1016/s0046-8177(99)90173-9. [DOI] [PubMed] [Google Scholar]
- 39.Comar M, Zanotta N, Bovenzi M, Campello C. JCV/BKV and SV40 viral load in lymphoid tissues of young immunocompetent children from an area of north-east Italy. J Med Virol. 2010;82(7):1236–1240. doi: 10.1002/jmv.21786. [DOI] [PubMed] [Google Scholar]
- 40.Jafar S, Rodriguez-Barradas M, Graham DY, Butel JS. Serological evidence of SV40 infections in HIV-infected and HIV-negative adults. J Med Virol. 1998;54(4):276–284. doi: 10.1002/(sici)1096-9071(199804)54:4<276::aid-jmv7>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 41.Li RM, et al. Molecular identification of SV40 infection in human subjects and possible association with kidney disease. J Am Soc Nephrol. 2002;13(9):2320–2330. doi: 10.1097/01.asn.0000028249.06596.cf. [DOI] [PubMed] [Google Scholar]
- 42.Li RM, et al. BK virus and SV40 co-infection in polyomavirus nephropathy. Transplantation. 2002;74(11):1497–1504. doi: 10.1097/00007890-200212150-00004. [DOI] [PubMed] [Google Scholar]
- 43.Patel NC, et al. Detection of polyomavirus SV40 in tonsils from immunocompetent children. J Clin Virol. 2008;43(1):66–72. doi: 10.1016/j.jcv.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vanchiere JA, White ZS, Butel JS. Detection of BK virus and simian virus 40 in the urine of healthy children. J Med Virol. 2005;75(3):447–454. doi: 10.1002/jmv.20287. [DOI] [PubMed] [Google Scholar]
- 45.Vanchiere JA, et al. Polyomavirus shedding in the stool of healthy adults. J Clin Microbiol. 2009;47(8):2388–2391. doi: 10.1128/JCM.02472-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Melnick JL, Stinebaugh S. Excretion of vacuolating SV-40 virus (papova virus group) after ingestion as a contaminant of oral poliovaccine. Proc Soc Exp Biol Med. 1962;109:965–968. doi: 10.3181/00379727-109-27392. [DOI] [PubMed] [Google Scholar]
- 47.Morris JA, Johnson KM, Aulisio CG, Chanock RM, Knight V. Clinical and serologic responses in volunteers given vacuolating virus (SV-40) by respiratory route. Proc Soc Exp Biol Med. 1961;108:56–59. doi: 10.3181/00379727-108-26843. [DOI] [PubMed] [Google Scholar]
- 48.Pancaldi C, et al. Simian virus 40 sequences in blood specimens from healthy individuals of Casale Monferrato, an industrial town with a history of asbestos pollution. J Infect. 2009;58(1):53–60. doi: 10.1016/j.jinf.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 49.Bocchetta M, et al. Human mesothelial cells are unusually susceptible to simian virus 40-mediated transformation and asbestos cocarcinogenicity. Proc Natl Acad Sci USA. 2000;97(18):10214–10219. doi: 10.1073/pnas.170207097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cacciotti P, et al. SV40 replication in human mesothelial cells induces HGF/Met receptor activation: A model for viral-related carcinogenesis of human malignant mesothelioma. Proc Natl Acad Sci USA. 2001;98(21):12032–12037. doi: 10.1073/pnas.211026798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Morelli C, Barbisan F, Iaccheri L, Tognon M. Simian virus 40 persistent infection in long-term immortalized human fibroblast cell lines. J Neurovirol. 2004;10(4):250–254. doi: 10.1080/13550280490441185. [DOI] [PubMed] [Google Scholar]
- 52.Mazzoni E, et al. Simian virus 40 efficiently infects human T lymphocytes and extends their lifespan. Exp Hematol. 2012;40(6):466–476. doi: 10.1016/j.exphem.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 53.Guerrini R, Salvadori S, Rizzi A, Regoli D, Calo’ G. Neurobiology, pharmacology, and medicinal chemistry of neuropeptide S and its receptor. Med Res Rev. 2010;30(5):751–777. doi: 10.1002/med.20180. [DOI] [PubMed] [Google Scholar]
- 54.Swetly P, Brodano GB, Knowles B, Koprowski H. Response of simian virus 40-transformed cell lines and cell hybrids to superinfection with simian virus 40 and its deoxyribonucleic acid. J Virol. 1969;4(4):348–355. doi: 10.1128/jvi.4.4.348-355.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Portolani M, Marzocchi A, Barbanti-Brodano G, La Placa M. Prevalence in Italy of antibodies to a new human papovavirus (BK virus) J Med Microbiol. 1974;7(4):543–546. doi: 10.1099/00222615-7-4-543. [DOI] [PubMed] [Google Scholar]
- 56.Maraldi NM, Barbanti-Brodano G, Portolani M, La Placa M. Ultrastructural aspects of BK virus uptake and replication in human fibroblasts. J Gen Virol. 1975;27(1):71–80. doi: 10.1099/0022-1317-27-1-71. [DOI] [PubMed] [Google Scholar]
- 57.Major EO, et al. Establishment of a line of human fetal glial cells that supports JC virus multiplication. Proc Natl Acad Sci USA. 1985;82(4):1257–1261. doi: 10.1073/pnas.82.4.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Neel JV, et al. Hypothesis: “Rogue cell”-type chromosomal damage in lymphocytes is associated with infection with the JC human polyoma virus and has implications for oncopenesis. Proc Natl Acad Sci USA. 1996;93(7):2690–2695. doi: 10.1073/pnas.93.7.2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Barbanti-Brodano G, et al. Reactivation of infectious simian virus 40 from normal human tissues. J Neurovirol. 2004;10(3):199–205. doi: 10.1080/13550280490441112. [DOI] [PubMed] [Google Scholar]
- 60.Stehle T, Gamblin SJ, Yan Y, Harrison SC. The structure of simian virus 40 refined at 3.1 Å resolution. Structure. 1996;4(2):165–182. doi: 10.1016/s0969-2126(96)00020-2. [DOI] [PubMed] [Google Scholar]
- 61.Bernstein HJ. Recent changes to RasMol, recombining the variants. Trends Biochem Sci. 2000;25(9):453–455. doi: 10.1016/s0968-0004(00)01606-6. [DOI] [PubMed] [Google Scholar]
- 62.Sayle RA, Milner-White EJ. RASMOL: Biomolecular graphics for all. Trends Biochem Sci. 1995;20(9):374–376. doi: 10.1016/s0968-0004(00)89080-5. [DOI] [PubMed] [Google Scholar]