Abstract
Introduction:
Nicotine is a major psychoactive ingredient in tobacco yet very few individuals quit smoking with the aid of nicotine replacement therapy. Targeted therapies with more selective action at nicotinic acetylcholine receptors (nAChRs) that contain a β2 subunit (β2*nAChRs; *denotes assembly with other subunits) have enjoyed significantly greater success, but exhibit potential for unwanted cardiac, gastrointestinal, and emotive side effects.
Discussion:
This literature review focuses on the preclinical evidence that suggests that subclasses of β2*nAChRs that assemble with the α6 subunit may provide an effective target for tobacco cessation. α6β2*nAChRs have a highly selective pattern of neuroanatomical expression in catecholaminergic nuclei including the ventral tegmental area and its projection regions. α6β2*nAChRs promote dopamine (DA) neuron activity and DA release in the mesolimbic dopamine system, a brain circuitry that is well-studied for its contributions to addiction behavior. A combination of genetic and pharmacological studies indicates that activation of α6β2*nAChRs is necessary and sufficient for nicotine psychostimulant effects and nicotine self-administration. α6β2*nAChRs support maintenance of nicotine use, support the conditioned reinforcing effects of drug-associated cues, and regulate nicotine withdrawal.
Conclusions:
These data suggest that α6β2*nAChRs represent a critical pool of high affinity β2*nAChRs that regulates nicotine dependence phenotype and suggest that inhibition of these receptors may provide an effective strategy for tobacco cessation therapy.
Introduction
There is a growing need for identification of novel targets for tobacco cessation. Cigarette smoking remains the leading preventable cause of death in developed countries and continues to be a growing health problem worldwide (World Health Organization, 2008). Nicotine, a major psychoactive ingredient in tobacco, binds to a variety of subclasses of nicotinic receptors in the brain as well as in the periphery. All of these receptors are the molecular target of nicotine replacement therapies (NRT). NRT aids approximately 10% of smokers in quitting smoking. Quit rates are increased approximately threefold with varenicline, a more selective drug that acts as a partial agonist at the high affinity β2 subunit containing nicotinic acetylcholine receptors (β2*nAChR; *denotes assembly with other subunits e.g., α3, α4, α5, α6, β3; Coe et al., 2005; Gonzales et al., 2006), suggesting that smoking cessation can be improved using more targeted strategies. This review will summarize a growing literature that suggests that a subtype of β2*nAChRs, which contains the α6 nAChR subunit (α6β2*nAChR) is a strong candidate for the next wave of tobacco cessation therapeutics. The nicotine addiction field has benefited greatly from the isolation and development of peptides that selectively target nicotinic receptors that contain the α6 subunit (Azam & McIntosh, 2005; Cartier et al., 1996; Dowell et al., 2003; McIntosh et al., 2004), the development of molecular chimeras and concatamers that can test drug efficacy at α6β2*nAChRs in vitro (Kuryatov & Lindstrom, 2011; Kuryatov, Olale, Cooper, Choi, & Lindstrom, 2000; for detailed review see Letchworth & Whiteaker, 2011), and from transgenic knockout and knockin technologies and viral-mediated rescue of nAChR subunit genes that enable observation of subunit contributions to neurochemistry and behaviors that are relevant to nicotine and tobacco addiction (Cui et al., 2003; Drenan et al., 2008; Marubio et al., 2003; Maskos et al., 2005; McGranahan, Patzlaff, Grady, Heinemann, & Booker, 2011; Mineur et al., 2009; Picciotto et al., 1998; Pons et al., 2008; Tapper et al., 2004). An accumulation of findings in the preclinical literature show that activation of α6β2*nAChRs promotes phenotypic behaviors that support tobacco addiction.
Nicotine-Stimulated Mesocorticolimbic DA Release
A preponderance of evidence suggests that nicotine addiction behavior is regulated in part by the mesocorticolimbic dopamine (DA) pathway, which projects from the ventral tegmental area (VTA) to the nucleus accumbens (NAc), anterior cingulate cortex, hippocampus, and amygdala. Human and animal studies show that nicotine and other drugs of abuse result in elevated DA release in the NAc (Barrett, Boileau, Okker, Pihl, & Dagher, 2004; Benwell & Balfour, 1992; Brody et al., 2004; Di Chiara et al., 2004). Imaging studies in human smokers show that these areas are not only activated by nicotine and tobacco smoke, but are associated with feelings of “high” and “liking” the drug (Barrett et al., 2004; Brody et al., 2004; Stein et al., 1998). It is interesting that cigarette-associated cues also activate mesolimbic projection regions of smokers, demonstrating that cues associated with tobacco ingestion can become conditioned reinforcers that are capable of activating the DA circuitry in the absence of nicotine (Due, Huettel, Hall, & Rubin, 2002; Franklin et al., 2007). Rodent studies show that lesions of DA projections to the NAc attenuate nicotine self-administration (Corrigall, Franklin, Coen, & Clarke, 1992), suggesting that these DA inputs are critical for nicotine reinforcement. Nicotine-associated mesolimbic DA release is regulated in part by nAChRs within the VTA as well as by nicotinic receptors on DA terminals in the dorsal and ventral striatum where α6β2*nAChRs are enriched (Champtiaux et al., 2003; Grady et al., 2007; Gotti et al., 2010; Mansvelder, Keath, & McGehee, 2002; Pidoplichko et al., 2004; Salminen et al., 2004, 2005, 2007). Studies have begun to identify mesolimbic α6β2*nAChRs for their contributions to nicotine-associated mesolimbic DA release as well as to the behavioral and neurochemical effects of nicotine. This paper will review the evidence which suggests that activation of α6β2*nAChRs is critical for nicotine reinforcement and reward, conditioned reinforcement, nicotine withdrawal, and psychoactive effects of nicotine.
How Nicotine Affects DA Release: Building a Nicotinic Receptor Profile
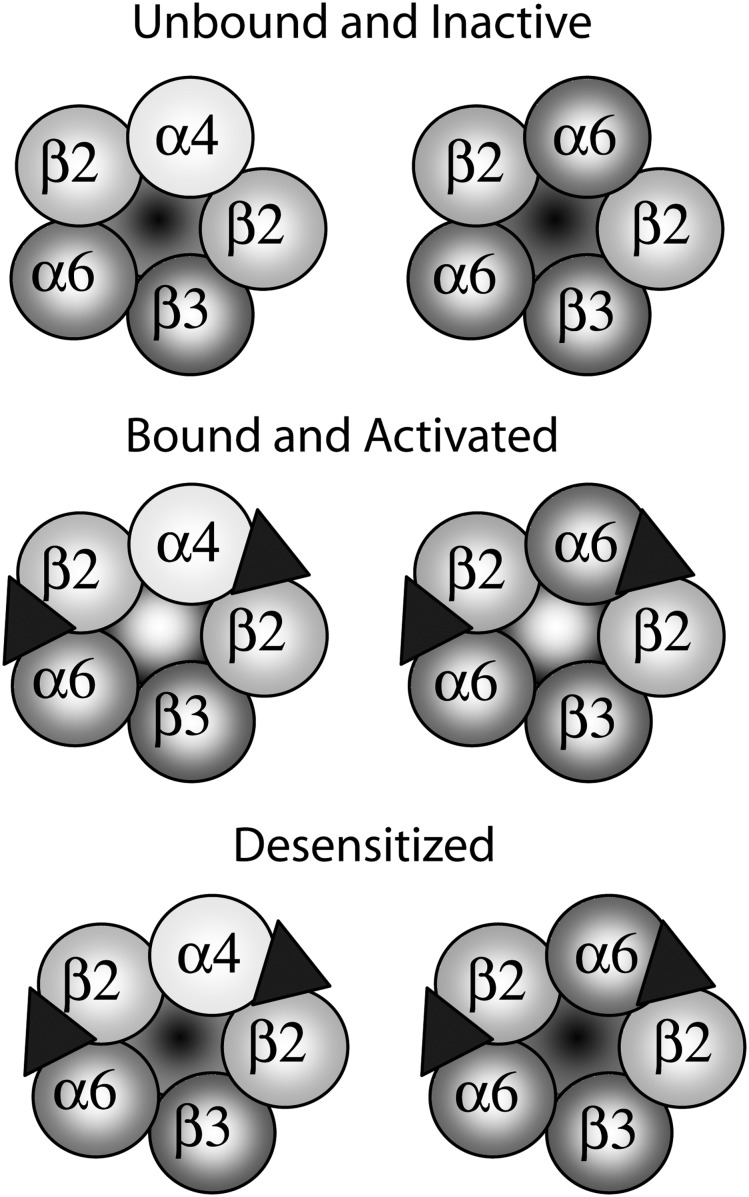
The nAChRs are ion channels made of a combination of five subunits that exist in a variety of functional states including a resting state, an activated state, and a desensitized, unresponsive state (Changeux, Devillers-Thiery, & Chemouilli, 1984; Figure 1). nAChRs reside on neurons that release various types of neurotransmitters including acetylcholine, gamma-aminobutyric acid (GABA), glutamate, serotonin, norepinephrine, and DA. nAChRs reside on the soma and axon terminals of neurons where they promote neurotransmitter release. Binding of nicotine or acetylcholine leads to a conformational change in the nAChR that renders the ion pore permeable to cations that excite the cell (Arias, 2000; Karlin & Akabas, 1995; Leonard, Labarca, Charnet, Davidson, & Lester, 1988). The predominant low affinity nAChRs in brain are homomers made up of five α7 nAChR subunits. The heteromeric nAChR in brain is made of a combination of α and β subunits (α3–α7; β2–β4) but the assembly and neuroanatomical expression of central nervous system nAChRs are tightly regulated. α6β2*nAChRs almost exclusively assemble with a β3 subunit (Cui et al., 2003; Gotti et al., 2005) and when assembled with α4 have the highest affinity for nicotine and ACh (Salminen et al., 2007). Assembly with α4 also increases the sensitivity of α6β2*nAChR to compounds such as varenicline and dihydro-beta-erythroidine (DHβE) which are selective for α4β2*nAChRs (Grady et al., 2010; Kuryatov & Lindstrom, 2011). Although the current evidence suggests that two major α6β2*nAChRs are expressed in brain (α4α6β2β3nAChRs and α6β2β3nAChRs), we reserve the use of the more conservative nomenclature, α6β2*nAChRs, to indicate that other receptor conformations may contribute to the observations presented in this paper.
Figure 1.
The putative composition of mesolimblic α6β2* nicotinic acetylcholine receptors (nAChRs). nAChRs are pentameric in structure, made up of a combination of five subunits that assemble around an ion pore. The ACh binding site is believed to be at the interface of the α6/α4 and β2 subunits. The accessory subunit, β3, is highly expressed in α6β2*nAChRs. Agonist binding results in opening of the ion pore and activation of the receptor. Following activation, the bound nAChRs desensitize and become impermeable to cation penetration through the ion pore. The α4α6β2*nAChRs have a higher affinity for agonist binding and are slower to desensitize.
Pharmacological and genetic studies suggest that nicotine-associated DA release and nicotine reinforcement are regulated by nAChRs that contain the β2 subunit (Champtiaux et al., 2003; Corrigall, Coen, & Adamson, 1994; Drenan et al., 2008; Maskos et al., 2005; Picciotto et al., 1998; Pons et al., 2008; Salminen et al., 2004, 2007; Tapper et al., 2004), making these high affinity receptors an attractive therapeutic target for tobacco cessation (Gonzales et al., 2006; Rollema et al., 2007). The β2*nAChRs are the primary target of varenicline, the first “receptor-selective” therapeutic for tobacco dependence (Coe et al., 2005; Gonzales et al., 2006) but see (Mihalak, Carroll, & Luetje, 2006). β2*nAChRs, however, are not only expressed in brain areas that regulate motivation to use cigarettes but also expressed in brain areas that contribute more generally to motivation, mood, and cognition (for review see Brunzell & Picciotto, 2009; Levin, McClernon, & Rezvani, 2006). Varenicline is highly effective in some smokers, but for others may result in unfavorable emotional, cardiovascular, and gastrointestinal side effects (Hays & Ebbert, 2010; Leung, Patafio, & Rosser, 2011; Moore, Furberg, Glenmullen, Maltsberger, & Singh, 2011; Singh, Loke, Spangler, & Furberg, 2011). Fortunately, β2*nAChRs are also very diverse in their composition so that identification of receptor subunits that assemble with β2 but that have a more selective neuroanatomical expression pattern may identify targets for tobacco cessation therapies that have less potential for side effects. One such candidate is the α6 nAChR subunit. Unlike other β2*nAChRs that do not assemble with α6 (e.g., α4β2nAChRs and α4α5β2nAChRs), the α6β2*nAChRs are not expressed in the periphery and show a selective neuroanatomical pattern of expression within catecholaminergic nuclei, retinal ganglion cells, and catacholaminergic and retinal projection regions of the brain (Champtiaux et al., 2002; Cui et al., 2003; Klink, de Kerchove d’Exaerde, Zoli, & Changeux, 2001; Whiteaker, McIntosh, Luo, Collins, & Marks, 2000). Recent in vitro data suggest that α6β2*nAChRs, like α4β2*nAChRs, are pharmacologically inhibited by the partial agonist properties of varenicline (Grady et al., 2010; Kuryatov, Berrettini, & Lindstrom, 2011). Their enrichment in the mesolimbic dopamine (DA) system, a brain pathway long known to contribute to motivational valence for drug reward (Volkow, Wang, Fowler, & Tomasi, 2011), makes these α6β2*nAChRs a promising novel target for tobacco cessation therapies.
Mesolimbic α6β2*nAChRs on Soma and Terminals Support Nicotine-Associated DA Release
α6 assembles with β2 with a selective neuroanatomical pattern of expression in catecholaminergic nuclei in the brain and on DA neuron axon terminals in catecholaminergic projection areas (Champtiaux et al., 2002; Klink et al., 2001; Le Novere, Zoli, & Changeux, 1996; Marks et al., 2010; Mineur et al., 2009; Whiteaker et al., 2000). Although the focus of this review is on the mesolimbic DA system, α6β2*nAChRs are also highly expressed within the nigrostriatal system, the locus coeruleus, and in retinal ganglion cells and the visual system (Champtiaux et al., 2002; Guo, Liu, Sorenson, & Chiappinelli, 2005; Lecchi, McIntosh, Bertrand, Safran, & Bertrand, 2005; Whiteaker et al., 2000). α6β2*nAChRs, like α3β2*nAChRs are classified according to their sensitivity to the cone snail peptide, α-conotoxin MII (α-CTX MII; Cartier et al., 1996; Champtiaux et al., 2002; Whiteaker et al., 2000). Studies of α-CTX MII binding in α6 and α3 subunit knockout mice reveal that with the exception of the interpeduncular nucleus, the α6β2*nAChRs and not α3β2*nAChRs predominate in these regions (Champtiaux et al., 2002; Whiteaker et al., 2002). α-CTX MII binding studies also show that α6β2*nAChRs on catecholaminergic terminals are most highly expressed in the dorsal striatum and NAc regions with sparse binding apparent in other DA projection regions including the anterior cingulate cortex, hippocampus, and amygdala (Cartier et al., 1996; Champtiaux et al., 2002; Marks et al., 2010; Mineur et al., 2009; Whiteaker et al., 2000). Nicotinic receptor knockout strategies used in combination with α-CTX MII have enabled researchers to identify that nicotine-stimulated DA release on terminals in the NAc/striatum involves activation of several combinations of β2*nAChRs including α-CTX MII sensitive (α6β2β3, α4α6β2β3) and insensitive (α4α5β2, α4β2) nAChRs (Champtiaux et al., 2003; Salminen et al., 2004, 2007; Figure 2). Cyclic voltammetry studies suggest that α6β2*nAChRs support 80% of the nicotine-stimulated DA release in the NAc (Exley, Clements, Hartung, McIntosh, & Cragg, 2008). The α6β2*nAChRs are also localized on DA cell bodies in the VTA and activation of these receptors by nicotine is sufficient to stimulate firing of VTA DA neurons (Drenan et al., 2008; Zhao-Shea et al., 2011).
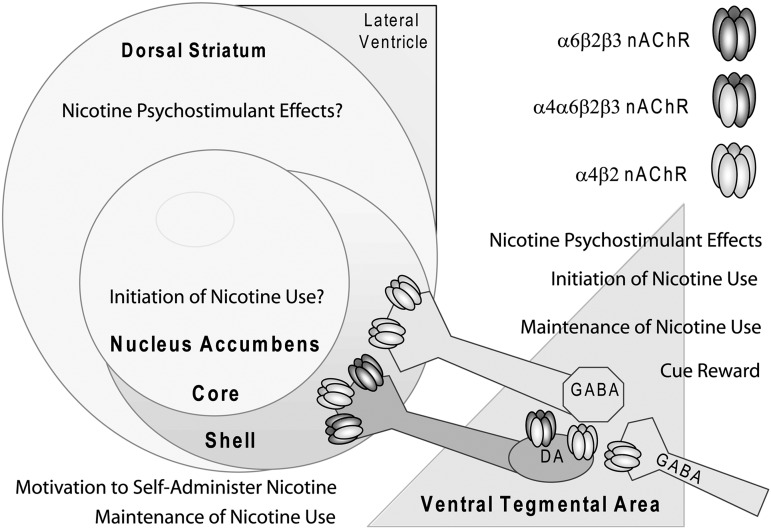
Figure 2.
A neuroanatomical summary of α6β2*nicotinic acetylcholine receptor (nAChR) contributions to behaviors associated with nicotine and tobacco addiction. A preponderance of the evidence suggests that activation of α6β2*nAChRs in the ventral tegmental area (VTA) and on dopamine (DA) terminals in the dorsal and ventral striatum (aka NAc core and shell) supports nicotine-stimulated DA release. Antagonism of α6β2*nAChRs in the NAc shell or VTA greatly attenuates nicotine self-administration. α6β2*nAChRs in the NAc shell regulate motivation to self-administer nicotine. Mesolimbic rescue of α4, α6, or β2 subunits on a null mutant background is sufficient to rescue self-administration phenotype, suggesting that α4α6β2*nAChRs regulate this behavior. IntraVTA infusion of α-conotoxin MII also blocks the rewarding effects of cues paired with a drug reinforcer. Studies in transgenic mice with highly sensitive α4 or α6 subunit containing nAChRs show that nicotine-associated stimulation of VTA DA neurons is at the α4α6β2*nAChRs and the α6-sensitive mice bred on an α4 knockout background show that α4α6β2*nAChRs regulate the locomotor stimulating effects of nicotine, likely at the level of the VTA and perhaps within the nigrostriatal circuitry that includes projections to the dorsal striatum. Affective but not somatic withdrawal is attenuated by injection of an α6β2*nAChR antagonist into the lateral ventricle; the locus of this effect is not clear. Several studies show that antagonism of α6β2*nAChRs and α4β2*nAChR does not have the same effect as selective antagonism of α6β2*nAChRs, perhaps due to expression of α4β2*nAChRs on gamma-aminobutyric acid terminals in mesolimblic DA areas. These studies suggest that α6β2*nAChRs may be an effective target for tobacco cessation. Increasing evidence suggests that α4α6β2β3nAChRs represent a critical pool of high affinity β2*nAChRs that support nicotine addiction behavior.
Psychostimulant Effects of Nicotine
The psychostimulant effects of nicotine are thought to be regulated in large part via DA release and an accumulation of evidence suggests that these effects are regulated by α6β2*nAChRs (Drenan et al., 2008, 2010). Mice lacking either the α6 or β2 subunit fail to show locomotor activating effects of nicotine (King, Caldarone, & Picciotto, 2004; Le Novere et al., 1996; Mineur, Somenzi, & Picciotto, 2007), a phenotype that is rescued by partial reinsertion of β2 nAChR subunit messenger RNA (mRNA) into the VTA-nigra region (Mineur et al., 2007). Studies in mice with a single point mutation that renders their α6β2*nAChRs hypersensitive to nicotine reveal that subthreshold doses for activation of other nAChRs are sufficient for locomotor activating effects of nicotine in this strain (Drenan et al., 2008, 2010). This phenotype is blocked by systemic DA receptor antagonism and abolished in mice crossed to an α4 knockout background; these studies suggest that α4α6β2*nAChR-stimulated DA release supports the locomotor activating effects of nicotine (Drenan et al., 2008, 2010). These mice also fail to show locomotor suppressant effects of acute nicotine at low doses, unmasking the fact that locomotor suppressant effects of nicotine are regulated by a class of nAChRs that does not contain an α6 subunit. Studies comparing mice with a selective null mutation of the α4 nAChR subunit in DA neurons with global α4 knockouts suggest that locomotor suppressant effects of nicotine appear to be regulated by α4 subunit containing nicotinic receptors that are not expressed on mesolimblic DA neurons (McGranahan et al., 2011). The locomotor stimulant effects of nicotine do not appear to be due to activation of α6β2*nAChRs on DA terminals in the NAc shell since local infusion of α-CTX MII in this region does not affect locomotor stimulating effects of nicotine in rats (Brunzell, Boschen, Hendrick, Beardsley, & McIntosh, 2010). This behavior is more likely mediated in the VTA where, unlike the NAc, local administration of nicotine results in hyperactivity (Ferrari, Le Novere, Picciotto, Changeux, & Zoli, 2002). The dorsal striatum receives inputs from the substantia nigra that have α6β2*nAChRs on their terminals (Meyer, Yoshikami, & McIntosh, 2008; Perez, Bordia, McIntosh, & Quik, 2010). Lesions of the VTA and substantia nigra greatly attenuate nicotine locomotor activation (Louis & Clarke, 1998). It remains to be determined if α6β2*nAChRs on DA terminals in the dorsal striatum, NAc core, or elsewhere contribute to nicotine’s psychostimulant effects. It is important to note that although nicotine reinforcement and psychostimulant effects of nicotine both appear to depend on DA release (Boye, Grant, & Clarke, 2001; Cadoni & Di Chiara, 2000; Corrigall & Coen, 1991; Corrigall et al., 1992; Di Chiara et al., 2004; Iyaniwura, Wright, & Balfour, 2001; Kelsey, Gerety, & Guerriero, 2009; Louis & Clarke, 1998), a series of studies have shown a dissociation between the neuroanatomical networks that support nicotine psychostimulant effects versus nicotine reinforcement and reward (e.g., Brunzell et al., 2010; Corrigall et al., 1994).
Nicotine Reinforcement and Reward
Nicotine Reinforcement is measured in rodents using intravenous nicotine self-administration, a model with good face validity and predictive validity for tobacco smoking. Nicotine reward is generally measured using nicotine conditioned place preference (CPP), a Pavlovian paradigm where preference for a nicotine-paired chamber is compared with preference for a neutral chamber after several exposures to nicotine. Both nicotine self-administration and nicotine CPP are absent in null mutant mice lacking their α4, α6, or β2 subunits (Maskos et al., 2005; McGranahan et al., 2011; Picciotto et al., 1998; Pons et al., 2008; Walters, Brown, Changeux, Martin, & Damaj, 2006). Nicotine self-administration is recovered in mice that have a reintroduction of the α4, α6, or β2 subunit mRNA in the VTA region (Pons et al., 2008). These 3-day place conditioning studies and acute, single-day tail-vein procedures suggest that α4α6β2*nAChRs may be critical for initiation of tobacco use. Intraventricular injection of a selective α6β2*nAChR antagonist also blocks expression of nicotine CPP in adult, wild-type mice that have grown up with their nAChRs intact (Jackson, McIntosh, Brunzell, Sanjakdar, & Damaj, 2009). The possibility that α6β2*nAChRs may affect initiation of tobacco use is supported by genetic research in humans that have implicated CHRNA6 and CHRNB3 in sensitivity to initial tobacco exposure (Zeiger et al., 2008; Hoft et al., 2009). Youth with polymorphisms in the genes that encode the α6 or β3 nAChR subunits had an elevated risk for tobacco dependence (Hoft et al., 2009) and increased dizziness in response to nicotine (Zeiger et al., 2008). More research is necessary to discover how these genes might impact the development of habitual tobacco use.
It is not clear from genetic rescue and intracerebral ventricular infusion studies in mice if α6β2*nAChRs within the VTA or on terminals in VTA projection regions may regulate self-administration of nicotine or if α6β2*nAChRs continue to contribute to nicotine ingestion following chronic exposure. Pharmacological studies which target specific neuroanatomical structures suggest that α6β2*nAChRs exert their effects on DA release and self-administration at DA terminals in the NAc shell (Brunzell et al., 2010; Champtiaux et al., 2003; Exley et al., 2008; Kulak, Nguyen, Olivera, & McIntosh, 1997; Salminen et al., 2004, 2007). Studies in naïve rats chronically trained to self-administer intravenous nicotine show that NAc shell infusions of concentrations of α-CTX MII that are capable of blocking nicotine-stimulated DA release (Kulak et al., 1997; Salminen et al., 2004) greatly reduce how hard rats are willing to work for nicotine using a progressive ratio (PR) schedule of reinforcement (Brunzell et al., 2010). The PR schedule requires rats to give an increasing number of responses for a single i.v. infusion of nicotine (Brunzell et al., 2010). Since there are virtually no α3β2*nAChRs in the NAc shell, these behavioral data and in vitro studies suggest that activation of α6β2*nAChRs on DA terminals in the NAc shell support motivation to self-administer nicotine (Brunzell et al., 2010; Champtiaux et al., 2003; Exley et al., 2008; Kulak et al., 1997; Salminen et al., 2004, 2007). Of import from a therapeutic standpoint, these data further suggest that α6β2*nAChRs continue to support self-administration of nicotine following a more chronic dosing paradigm. It is interesting that self-administration of nicotine was not affected following NAc administration of DHβE, a drug that antagonizes both α4β2nAChRs and α4α6β2*nAChRs (Corrigall et al., 1994; and Brunzell, unpublished findings), raising the possibility that these distinct receptor populations have opposing effects on self-administration behavior in this region, perhaps due to the prevalence of α4β2nAChRs but not α6β2*nAChRs on GABA terminals in the NAc (Drenan et al., 2008; Gotti et al., 2010; Tapper et al., 2004) but see (Yang et al., 2011). This is in contrast to the VTA where infusion of DHβE greatly attenuates nicotine self-administration (Corrigall et al., 1994). Other studies show that local infusion of an α6β2*nAChR antagonist, α-conotoxin PIA, (Dowell et al., 2003) into the VTA also attenuates systemic administration of nicotine in rats that were previously trained for food (Gotti et al., 2010). Immunoprecipitation studies suggest that these receptors are α4α6β2β3nAChRs (Gotti et al., 2010). Studies that use local self-administration of low-dose nicotine in the VTA of nicotinic subunit knockout mice reveal similar behavioral effects although intraVTA infusion of nicotine is attenuated to a greater extent in α4 subunit knockout mice than in α6 subunit knockouts (Exley et al., 2011), suggesting that within the VTA α4β2*nAChRs without an α6 subunit regulate intraVTA administration of nicotine (Exley et al., 2011). These studies also showed that VTA DA neuron activity using this regimen was specifically abolished in α4KO mice (Exley et al., 2011) and not α6KO subjects, suggesting that α6β2*nAChRs exert their DA-releasing activity (Drenan et al., 2008) elsewhere in the mesolimbic circuitry, namely in the NAc or on terminals in the dorsal striatum (Champtiaux et al., 2003; Drenan et al., 2008; Exley et al., 2008; Gotti et al., 2010; Kulak et al., 1997; Meyer et al., 2008; Salminen et al., 2004, 2007). Evidence suggests that the nM concentrations of nicotine used in the intraVTA study, however, may preferentially desensitize rather than activate nAChRs (Fenster et al., 1999; Grady, Marks, & Collins, 1994; Grady, Wageman, Patzlaff, & Marks, 2012; Lester & Dani, 1995; Lu, Marks, & Collins, 1999; Marks, Grady, Yang, Lippiello, & Collins, 1994; Paradiso & Steinbach, 2003). Recent data suggest that α6β2*nAChRs are persistently activated by 300 nM nicotine and in comparison with α4β2*nAChRs, α6β2*nAChRs are resistant to the desensitizing effects of low-dose nicotine (Grady et al., 2012; Liu, Zhao-Shea, McIntosh, Gardner, & Tapper, 2012). Whereas inhibition of α6β2*nAChRs would be expected to reduce VTA DA activity, previous data using slice electrophysiology have shown that desensitization of α4β2*nAChRs on GABA terminals in the VTA results in a disinhibition of DA receptors that may increase their sensitivity to further excitatory input (Mansvelder, Mertz, & Role, 2009; Mansvelder et al., 2002; Pidoplichko, DeBiasi, Williams, & Dani, 1997). Studies using systemic administration of independent compounds with antagonist activity at α6β2*nAChRs have reported reductions of DA release and nicotine self-administration in rats (Markou & Paterson, 2001; Mogg et al., 2002; Neugebauer, Zhang, Crooks, Dwoskin, & Bardo, 2006; Rahman et al., 2007). Together these findings suggest that ligands that reduce α6β2*nAChR-mediated DA release may promote tobacco cessation.
Conditioned Reinforcement
The human and animal literature suggest that tobacco- and nicotine-associated cues play a critical role in tobacco use (Besheer, Palmatier, Metschke, & Bevins, 2004; Caggiula et al., 2002; Donny, Houtsmuller, & Stitzer, 2007). Cigarette cues can elicit craving and precipitate relapse (Carter & Tiffany, 1999). Sensory cues such as taste and the feeling of smoke traveling down one’s throat play a large role in how pleasurable an individual reports that it is to smoke a cigarette (Perkins et al., 2001). By virtue of their association with smoking behavior, cigarette-associated cues become capable of activating the same mesolimbic brain structures that are stimulated by nicotine (Due et al., 2002; Schroeder, Binzak, & Kelley, 2001; Stein et al., 1998; Volkow, Fowler, Wang, Swanson, & Telang, 2007). Perhaps, this explains in part why individuals will smoke denicotinized cigarettes, albeit at a reduced rate (Donny et al., 2007). Animal studies support the premise that these cues become conditioned reinforcers capable of regulating behavior on their own, and evidence suggests that conditioned reinforcement is supported by α6β2*nAChRs (Brunzell et al., 2006; Olausson, Jentsch, & Taylor, 2004a, 2004b; Lof et al., 2007). Cues greatly increase the level at which animals self-administer nicotine (Caggiula et al., 2002) and nicotine-associated cues can maintain self-administration behavior for weeks after removal of nicotine in rats (Cohen, Perrault, Griebel, & Soubrie, 2005). Human laboratory studies suggest that cue-induced craving increases following protracted abstinence (Bedi et al., 2011), although it is not clear if this translates to nicotine seeking (Perkins, 2011). Other studies show that nicotine also enhances conditioned reinforcement (Brunzell et al., 2006; Olausson et al., 2004a, 2004b) via regulation of the β2*nAChRs (Brunzell et al., 2006). Recent data using ethanol as a reinforcer suggest that α6β2*nAChRs in the VTA may contribute to conditioned reinforcement that supports tobacco addiction. In this study, an ethanol-paired tone but not an unpaired tone, led to elevated DA release in the NAc and supported acquisition of responding reinforced by a previously ethanol-paired cue (Lof et al., 2007). These behavioral effects were blocked by local infusion of α-CTX MII into the VTA, suggesting that acetylcholinergic tone at α6β2*nAChRs or perhaps α3β2*nAChRs mediates this effect (Lof et al., 2007). Self-administration of nicotine and cues, but not cues alone, was greatly attenuated by intraaccumbens shell infusion of α-CTX MII (Brunzell et al., 2010); it remains to be determined if this α6β2*nAChR-associated behavior is mediated in part by the conditioned reinforcing properties of the cues by virtue of their association with nicotine. In further support of α6β2*nAChRs mediating conditioned reinforcement, systemic administration of methylcaconitine, an antagonist of α7 and α6β2*nAChRs (Mogg et al., 2002) is also sufficient to block responding for cues associated with sucrose reward (Lof, Olausson, Stomberg, Taylor, & Soderpalm, 2010). Although intraVTA infusion of DHβE blocks nicotine self-administration (Corrigall et al., 1994), this antagonist of α6β2*nAChRs and α4β2*nAChRs had no effect on responding for a conditioned reinforcer or on cue-associated DA release when infused into the VTA (Lof et al., 2007), suggesting that α6β2*nAChRs and not α4β2nAChRs contribute to conditioned reinforcement in this region.
Nicotine Withdrawal
Nicotine withdrawal is characterized by a combination of somatic and affective symptoms that are observable in humans and rodents (Damaj et al., 2004; Donny et al., 2007; Jackson, Martin, Changeux, & Damaj, 2008; Jackson et al., 2009; Kenford et al., 2002; Salas, Pieri, & De Biasi, 2004; Salas, Sturm, Boulter, & De Biasi, 2009; Vann, Balster, & Beardsley, 2006). Intracerebral infusion of a selective α6β2*nAChR antagonist (McIntosh et al., 2004) dose dependently blocks conditioned place aversion and withdrawal-precipitated anxiety-like behavior in and elevated plus maze (Jackson et al., 2009) while leaving somatic withdrawal symptoms intact. This is consistent with studies showing that β2KO mice show a selective reduction in affective and not somatic withdrawal (Jackson et al., 2008), however it is likely that other subpopulations of nicotinic receptors regulate withdrawal behavior as well (e.g., α4α5β2nAChRs, α4β2nAChRs, α3β4nAChRs, and α7nAChRs; Jackson et al., 2008, 2009; Salas et al., 2004, 2009).
Does Activation or Inhibition of Mesolimblic α6β2*nAChRs Support Nicotine Addiction Behavior?
The traditional view is that DA release in the NAc produces rewarding effects of nicotine and to the extent that we can measure DA activity in human subjects this premise has generally held true (Barrett et al., 2004; Brody et al., 2004; Stein et al., 1998). Nicotine-associated DA release is abolished in β2nAChR subunit knockout mice that also fail to self-administer nicotine (Picciotto et al., 1998). Nicotine-mediated elevations in DA release are also inhibited by intraVTA infusion of α-CTX MII (Gotti et al., 2010), suggesting that α6β2*nAChRs regulate this behavior. In vitro electrophysiology, synaptosome release assays, and cyclic voltammetry studies in tonic-firing neurons show that nicotine results in elevated DA release that is blocked by antagonism of α6β2*nAChRs (Champtiaux et al., 2003; Drenan et al., 2008; Perez, O’Leary, Parameswaran, McIntosh, & Quik, 2009; Perez et al., 2010; Salminen et al., 2004, 2007; Zhang & Sulzer, 2004; Zhao-Shea et al., 2011). In contrast, cyclic voltammetry studies assessing neurons after phasic stimulation show that nicotine, DHβE, or α-CTX MII have similar effects to increase DA release in neurons that are phasically stimulated (Exley et al., 2008; Rice & Cragg, 2004; Perez et al., 2009, 2010; Zhang & Sulzer, 2004). This preparation using bath application of nicotine and nicotinic antagonists could model the brains of smokers after they have had their first cigarette of the day when most of the receptors would likely be desensitized (Brody et al., 2006; Changeux et al., 1984). These data suggest that under conditions when most of the high affinity β2*nAChRs are in the desensitized state, behavior that supports phasic activity of DA neurons could be further reinforced by desensitization, rather than excitation, of α6β2*nAChRs. It is important to note, however, that following repeated in vivo nicotine exposure, α-CTX MII-sensitive nAChRs in the dorsal striatum no longer have a stimulatory effect on DA release in phasically active DA neurons (Perez, Bordia, McIntosh, Grady, & Quik, 2008). It is unknown how the α6β2*nAChRs adapt to chronic nicotine exposure in the NAc.
Relevance to Tobacco Dependence
Regardless of the mechanism, animal models with good predictive validity for tobacco use suggest that antagonism of α6β2*nAChRs would be an effective strategy for tobacco cessation. The preponderance of the behavioral data show that nicotine self-administration, nicotine CPP, conditioned reinforcement, and nicotine withdrawal are significantly attenuated by selective antagonism of α6β2*nAChRs. From a therapeutic standpoint, it is encouraging that global knockdown or blockade of α6β2*nAChRs have thus far had similar effects on these behaviors that are relevant to tobacco addiction. The more selective expression profile of α6β2*nAChRs versus other nAChRs makes them an attractive target for tobacco cessation, but consideration should be given to the expression of these receptors in the retinal ganglion cells and the visual system. Development of selective antagonists or partial agonists of α6β2*nAChRs that cross the blood brain barrier may lead to effective treatment of tobacco dependence in smokers.
Funding
The author is supported by grants 8520667 from the Virginia Foundation for Healthy Youth and DA031289 from the National Institutes of Health.
Declaration of Interests
None declared.
References
- Arias HR. Localization of agonist and competitive antagonist binding sites on nicotinic acetylcholine receptors. Neurochemistry International. 2000;36:595–645. doi: 10.1016/s0197-0186(99)00154-0. doi:10.1016/S0197-0186(99)00154-0. [DOI] [PubMed] [Google Scholar]
- Azam L, McIntosh JM. Effect of novel alpha-conotoxins on nicotine-stimulated [3H]dopamine release from rat striatal synaptosomes. Journal of Pharmacology and Experimental Therapeutics. 2005;312:231–237. doi: 10.1124/jpet.104.071456. doi:10.1124/jpet.104.071456. [DOI] [PubMed] [Google Scholar]
- Barrett SP, Boileau I, Okker J, Pihl RO, Dagher A. The hedonic response to cigarette smoking is proportional to dopamine release in the human striatum as measured by positron emission tomography and [11C]raclopride. Synapse. 2004;54:65–71. doi: 10.1002/syn.20066. doi:10.1002/syn.20066. [DOI] [PubMed] [Google Scholar]
- Bedi G, Preston KL, Epstein DH, Heishman SJ, Marrone GF, Shaham Y, et al. Incubation of cue-induced cigarette craving during abstinence in human smokers. Biological Psychiatry. 2011;69:708–711. doi: 10.1016/j.biopsych.2010.07.014. doi:10.1016/j.biopsych.2010.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benwell ME, Balfour DJ. The effects of acute and repeated nicotine treatment on nucleus accumbens dopamine and locomotor activity. British Journal of Pharmacology. 1992;105:849–856. doi: 10.1111/j.1476-5381.1992.tb09067.x. Retrieved from http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1908718/?tool=pubmed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besheer J, Palmatier MI, Metschke DM, Bevins RA. Nicotine as a signal for the presence or absence of sucrose reward: A Pavlovian drug appetitive conditioning preparation in rats. Psychopharmacology (Berlin) 2004;172:108–117. doi: 10.1007/s00213-003-1621-9. doi:10.1007/s00213-003-1621-9. [DOI] [PubMed] [Google Scholar]
- Boye SM, Grant RJ, Clarke PB. Disruption of dopaminergic neurotransmission in nucleus accumbens core inhibits the locomotor stimulant effects of nicotine and D-amphetamine in rats. Neuropharmacology. 2001;40:792–805. doi: 10.1016/s0028-3908(01)00003-x. doi:10.1016/S0028-3908(01)00003-X. [DOI] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, London ED, Olmstead RE, Farahi J, Scheibal D, et al. Cigarette smoking saturates brain alpha 4 beta 2 nicotinic acetylcholine receptors. Archives of General Psychiatry. 2006;63:907–915. doi: 10.1001/archpsyc.63.8.907. Retrieved from http://archpsyc.ama-assn.org/cgi/content/full/63/8/907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody AL, Olmstead RE, London ED, Farahi J, Meyer JH, Grossman P, et al. Smoking-induced ventral striatum dopamine release. American Journal of Psychiatry. 2004;161:1211–1218. doi: 10.1176/appi.ajp.161.7.1211. Retrieved from http://ajp.psychiatryonline.org/article.aspx?volume=161&page=1211. [DOI] [PubMed] [Google Scholar]
- Brunzell DH, Boschen KE, Hendrick ES, Beardsley PM, McIntosh JM. Alpha-conotoxin MII-sensitive nicotinic acetylcholine receptors in the nucleus accumbens shell regulate progressive ratio responding maintained by nicotine. Neuropsychopharmacology. 2010;35:665–673. doi: 10.1038/npp.2009.171. doi:10.1038/npp.2009.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunzell DH, Chang JR, Schneider B, Olausson P, Taylor JR, Picciotto MR. beta2-Subunit-containing nicotinic acetylcholine receptors are involved in nicotine-induced increases in conditioned reinforcement but not progressive ratio responding for food in C57BL/6 mice. Psychopharmacology (Berlin) 2006;184:328–338. doi: 10.1007/s00213-005-0099-z. doi:10.1007/s00213-005-0099-z. [DOI] [PubMed] [Google Scholar]
- Brunzell DH, Picciotto MR. Molecular mechanisms underlying the motivational effects of nicotine. Nebraska Symposium on Motivation. 2009;55:17–30. doi: 10.1007/978-0-387-78748-0_3. doi:10.1007/978-0-387-78748-0_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadoni C, Di Chiara G. Differential changes in accumbens shell and core dopamine in behavioral sensitization to nicotine. European Journal of Pharmacology. 2000;387:R23–R25. doi: 10.1016/s0014-2999(99)00843-2. doi:10.1016/S0014-2999(99)00843-2. [DOI] [PubMed] [Google Scholar]
- Caggiula AR, Donny EC, Chaudhri N, Perkins KA, Evans-Martin FF, Sved AF. Importance of nonpharmacological factors in nicotine self-administration. Physiology & Behavior. 2002;77:683–687. doi: 10.1016/s0031-9384(02)00918-6. doi:10.1016/S0031-9384(02)00918-6. [DOI] [PubMed] [Google Scholar]
- Carter BL, Tiffany ST. Meta-analysis of cue-reactivity in addiction research. Addiction. 1999;94:327–340. doi:10.1046/j.1360-0443.1999.9433273.x. [PubMed] [Google Scholar]
- Cartier GE, Yoshikami D, Gray WR, Luo S, Olivera BM, McIntosh JM. A new alpha-conotoxin which targets alpha3beta2 nicotinic acetylcholine receptors. Journal of Biological Chemistry. 1996;271:7522–7528. doi: 10.1074/jbc.271.13.7522. doi:10.1074/jbc.271.13.7522. [DOI] [PubMed] [Google Scholar]
- Champtiaux N, Gotti C, Cordero-Erausquin M, David DJ, Przybylski C, Lena C, et al. Subunit composition of functional nicotinic receptors in dopaminergic neurons investigated with knock-out mice. Journal of Neuroscience. 2003;23:7820–7829. doi: 10.1523/JNEUROSCI.23-21-07820.2003. Retrieved from http://www.jneurosci.org/content/23/21/7820.long. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champtiaux N, Han ZY, Bessis A, Rossi FM, Zoli M, Marubio L, et al. Distribution and pharmacology of alpha 6-containing nicotinic acetylcholine receptors analyzed with mutant mice. Journal of Neuroscience. 2002;22:1208–1217. doi: 10.1523/JNEUROSCI.22-04-01208.2002. Retrieved from http://www.jneurosci.org/content/22/4/1208.long. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Changeux JP, Devillers-Thiery A, Chemouilli P. Acetylcholine receptor: An allosteric protein. Science. 1984;225:1335–1345. doi: 10.1126/science.6382611. doi:10.1126/science.6382611. [DOI] [PubMed] [Google Scholar]
- Coe JW, Brooks PR, Vetelino MG, Wirtz MC, Arnold EP, Huang J, et al. Varenicline: An alpha4beta2 nicotinic receptor partial agonist for smoking cessation. Journal of Medicinal Chemistry. 2005;48:3474–3477. doi: 10.1021/jm050069n. doi:10.1021/jm050069n. [DOI] [PubMed] [Google Scholar]
- Cohen C, Perrault G, Griebel G, Soubrie P. Nicotine-associated cues maintain nicotine-seeking behavior in rats several weeks after nicotine withdrawal: Reversal by the cannabinoid (CB1) receptor antagonist, rimonabant (SR141716) Neuropsychopharmacology. 2005;30:145–155. doi: 10.1038/sj.npp.1300541. doi:10.1038/sj.npp.1300541. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM. Selective dopamine antagonists reduce nicotine self-administration. Psychopharmacology (Berlin) 1991;104:171–176. doi: 10.1007/BF02244174. doi:10.1007/BF02244174. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM, Adamson KL. Self-administered nicotine activates the mesolimbic dopamine system through the ventral tegmental area. Brain Research. 1994;653:278–284. doi: 10.1016/0006-8993(94)90401-4. doi:10.1016/0006-8993(94)90401-4. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Franklin KB, Coen KM, Clarke PB. The mesolimbic dopaminergic system is implicated in the reinforcing effects of nicotine. Psychopharmacology (Berlin) 1992;107:285–289. doi: 10.1007/BF02245149. doi:10.1007/BF02245149. [DOI] [PubMed] [Google Scholar]
- Cui C, Booker TK, Allen RS, Grady SR, Whiteaker P, Marks MJ, et al. The beta3 nicotinic receptor subunit: A component of alpha-conotoxin MII-binding nicotinic acetylcholine receptors that modulate dopamine release and related behaviors. Journal of Neuroscience. 2003;23:11045–11053. doi: 10.1523/JNEUROSCI.23-35-11045.2003. Retrieved from http://www.jneurosci.org/content/23/35/11045.long. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damaj MI, Carroll FI, Eaton JB, Navarro HA, Blough BE, Mirza S, et al. Enantioselective effects of hydroxy metabolites of bupropion on behavior and on function of monoamine transporters and nicotinic receptors. Molecular Pharmacology. 2004;66:675–682. doi: 10.1124/mol.104.001313. doi:10.1124/mol.104.001313. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Bassareo V, Fenu S, De Luca MA, Spina L, Cadoni C, et al. Dopamine and drug addiction: The nucleus accumbens shell connection. Neuropharmacology. 2004;47(Suppl. 1):227–241. doi: 10.1016/j.neuropharm.2004.06.032. doi:10.1016/j.neuropharm.2004.06.032. [DOI] [PubMed] [Google Scholar]
- Donny EC, Houtsmuller E, Stitzer ML. Smoking in the absence of nicotine: Behavioral, subjective and physiological effects over 11 days. Addiction. 2007;102:324–334. doi: 10.1111/j.1360-0443.2006.01670.x. doi:10.1111/j.1360-0443.2006.01670.x. [DOI] [PubMed] [Google Scholar]
- Dowell C, Olivera BM, Garrett JE, Staheli S, Watkins M, Kuryatov A, et al. Alpha-conotoxin PIA is selective for alpha6 subunit-containing nicotinic acetylcholine receptors. Journal of Neuroscience. 2003;23:8445–8452. doi: 10.1523/JNEUROSCI.23-24-08445.2003. Retrieved from http://www.jneurosci.org/content/23/24/8445.long. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drenan RM, Grady SR, Steele AD, McKinney S, Patzlaff NE, McIntosh JM, et al. Cholinergic modulation of locomotion and striatal dopamine release is mediated by alpha6alpha4* nicotinic acetylcholine receptors. Journal of Neuroscience. 2010;30:9877–9889. doi: 10.1523/JNEUROSCI.2056-10.2010. Retrieved from http://www.jneurosci.org/content/30/29/9877.long. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drenan RM, Grady SR, Whiteaker P, McClure-Begley T, McKinney S, Miwa JM, et al. In vivo activation of midbrain dopamine neurons via sensitized, high-affinity alpha 6 nicotinic acetylcholine receptors. Neuron. 2008;60:123–136. doi: 10.1016/j.neuron.2008.09.009. doi:10.1016/j.neuron.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Due DL, Huettel SA, Hall WG, Rubin DC. Activation in mesolimbic and visuospatial neural circuits elicited by smoking cues: Evidence from functional magnetic resonance imaging. American Journal of Psychiatry. 2002;159:954–960. doi: 10.1176/appi.ajp.159.6.954. Retrieved from http://ajp.psychiatryonline.org/article.aspx?volume=159&page=954. [DOI] [PubMed] [Google Scholar]
- Exley R, Clements MA, Hartung H, McIntosh JM, Cragg SJ. Alpha6-containing nicotinic acetylcholine receptors dominate the nicotine control of dopamine neurotransmission in nucleus accumbens. Neuropsychopharmacology. 2008;33:2158–2166. doi: 10.1038/sj.npp.1301617. doi:10.1038/sj.npp.1301617. [DOI] [PubMed] [Google Scholar]
- Exley R, Maubourguet N, David V, Eddine R, Evrard A, Pons S, et al. Distinct contributions of nicotinic acetylcholine receptor subunit alpha4 and subunit alpha6 to the reinforcing effects of nicotine. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:7577–7582. doi: 10.1073/pnas.1103000108. doi:10.1073/pnas.1103000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenster CP, Hicks JH, Beckman ML, Covernton PJ, Quick MW, Lester RA. Desensitization of nicotinic receptors in the central nervous system. Annals of the New York Academy of Sciences. 1999;868:620–623. doi: 10.1111/j.1749-6632.1999.tb11335.x. doi:10.1111/j.1749-6632.1999.tb11335.x. [DOI] [PubMed] [Google Scholar]
- Ferrari R, Le Novere N, Picciotto MR, Changeux JP, Zoli M. Acute and long-term changes in the mesolimbic dopamine pathway after systemic or local single nicotine injections. European Journal of Neuroscience. 2002;15:1810–1818. doi: 10.1046/j.1460-9568.2001.02009.x. doi:10.1046/j.1460-9568.2001.02009.x. [DOI] [PubMed] [Google Scholar]
- Franklin TR, Wang Z, Wang J, Sciortino N, Harper D, Li Y, et al. Limbic activation to cigarette smoking cues independent of nicotine withdrawal: A perfusion fMRI study. Neuropsychopharmacology. 2007;32:2301–2309. doi: 10.1038/sj.npp.1301371. doi:10.1038/sj.npp.1301371. [DOI] [PubMed] [Google Scholar]
- Gonzales D, Rennard SI, Nides M, Oncken C, Azoulay S, Billing CB, et al. Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: A randomized controlled trial. Journal of the American Medical Association. 2006;296:47–55. doi: 10.1001/jama.296.1.47. doi:10.1001/jama.296.1.47. [DOI] [PubMed] [Google Scholar]
- Gotti C, Guiducci S, Tedesco V, Corbioli S, Zanetti L, Moretti M, et al. Nicotinic acetylcholine receptors in the mesolimbic pathway: Primary role of ventral tegmental area alpha6beta2* receptors in mediating systemic nicotine effects on dopamine release, locomotion, and reinforcement. Journal of Neuroscience. 2010;30:5311–5325. doi: 10.1523/JNEUROSCI.5095-09.2010. doi:10.1523/JNEUROSCI.5095-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotti C, Moretti M, Clementi F, Riganti L, McIntosh JM, Collins AC, et al. Expression of nigrostriatal alpha 6-containing nicotinic acetylcholine receptors is selectively reduced, but not eliminated, by beta 3 subunit gene deletion. Molecular Pharmacology. 2005;67:2007–2015. doi: 10.1124/mol.105.011940. doi:10.1124/mol.105.011940. [DOI] [PubMed] [Google Scholar]
- Grady SR, Drenan RM, Breining SR, Yohannes D, Wageman CR, Fedorov NB, et al. Structural differences determine the relative selectivity of nicotinic compounds for native alpha 4 beta 2*-, alpha 6 beta 2*-, alpha 3 beta 4*- and alpha 7-nicotine acetylcholine receptors. Neuropharmacology. 2010;58:1054–1066. doi: 10.1016/j.neuropharm.2010.01.013. doi:10.1016/j.neuropharm.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady SR, Marks MJ, Collins AC. Desensitization of nicotine-stimulated [3H]dopamine release from mouse striatal synaptosomes. Journal of Neurochemistry. 1994;62:1390–1398. doi: 10.1046/j.1471-4159.1994.62041390.x. doi:10.1046/j.1471-4159.1994.62041390.x. [DOI] [PubMed] [Google Scholar]
- Grady SR, Salminen O, Laverty DC, Whiteaker P, McIntosh JM, Collins AC, et al. The subtypes of nicotinic acetylcholine receptors on dopaminergic terminals of mouse striatum. Biochemical Pharmacology. 2007;74:1235–1246. doi: 10.1016/j.bcp.2007.07.032. doi:10.1016/j.bcp.2007.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady SR, Wageman CR, Patzlaff NE, Marks MJ. Low concentrations of nicotine differentially desensitize nicotinic acetylcholine receptors that include alpha5 or alpha6 subunits and that mediate synaptosomal neurotransmitter release. Neuropharmacology. 2012;62:1935, ––1943. doi: 10.1016/j.neuropharm.2011.12.026. doi:10.1016/j.neuropharm.2011.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo JZ, Liu Y, Sorenson EM, Chiappinelli VA. Synaptically released and exogenous ACh activates different nicotinic receptors to enhance evoked glutamatergic transmission in the lateral geniculate nucleus. Journal of Neurophysiology. 2005;94:2549–2560. doi: 10.1152/jn.00339.2005. doi:10.1152/jn.00339.2005. [DOI] [PubMed] [Google Scholar]
- Hays JT, Ebbert JO. Adverse effects and tolerability of medications for the treatment of tobacco use and dependence. Drugs. 2010;70:2357–2372. doi: 10.2165/11538190-000000000-00000. doi:10.2165/11538190-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Hoft NR, Corley RP, McQueen MB, Schlaepfer IR, Huizinga D, Ehringer MA. Genetic association of the CHRNA6 and CHRNB3 genes with tobacco dependence in a nationally representative sample. Neuropsychopharmacology. 2009;34:698–706. doi: 10.1038/npp.2008.122. doi:10.1038/npp.2008.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyaniwura TT, Wright AE, Balfour DJ. Evidence that mesoaccumbens dopamine and locomotor responses to nicotine in the rat are influenced by pretreatment dose and strain. Psychopharmacology (Berlin) 2001;158:73–79. doi: 10.1007/s002130100852. doi:10.1007/s002130100852. [DOI] [PubMed] [Google Scholar]
- Jackson KJ, Martin BR, Changeux JP, Damaj MI. Differential role of nicotinic acetylcholine receptor subunits in physical and affective nicotine withdrawal signs. Journal of Pharmacology and Experimental Therapeutics. 2008;325:302–312. doi: 10.1124/jpet.107.132977. doi:10.1124/jpet.107.132977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson KJ, McIntosh JM, Brunzell DH, Sanjakdar SS, Damaj MI. The role of alpha6-containing nicotinic acetylcholine receptors in nicotine reward and withdrawal. Journal of Pharmacology and Experimental Therapeutics. 2009;331:547–554. doi: 10.1124/jpet.109.155457. doi:10.1124/jpet.109.155457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlin A, Akabas MH. Toward a structural basis for the function of nicotinic acetylcholine receptors and their cousins. Neuron. 1995;15:1231–1244. doi: 10.1016/0896-6273(95)90004-7. doi:10.1016/0896-6273(95)90004-7. [DOI] [PubMed] [Google Scholar]
- Kelsey JE, Gerety LP, Guerriero RM. Electrolytic lesions of the nucleus accumbens core (but not the medial shell) and the basolateral amygdala enhance context-specific locomotor sensitization to nicotine in rats. Behavioral Neuroscience. 2009;123:577–588. doi: 10.1037/a0015573. doi:10.1037/a0015573. [DOI] [PubMed] [Google Scholar]
- Kenford SL, Smith SS, Wetter DW, Jorenby DE, Fiore MC, Baker TB. Predicting relapse back to smoking: Contrasting affective and physical models of dependence. Journal of Consulting and Clinical Psychology. 2002;70:216–227. doi:10.1037/0022-006X.70.1.216. [PubMed] [Google Scholar]
- King SL, Caldarone BJ, Picciotto MR. Beta2-subunit-containing nicotinic acetylcholine receptors are critical for dopamine-dependent locomotor activation following repeated nicotine administration. Neuropharmacology. 2004;47(Suppl. 1):132–139. doi: 10.1016/j.neuropharm.2004.06.024. doi:10.1016/j.neuropharm.2004.06.024. [DOI] [PubMed] [Google Scholar]
- Klink R, de Kerchove d’Exaerde A, Zoli M, Changeux JP. Molecular and physiological diversity of nicotinic acetylcholine receptors in the midbrain dopaminergic nuclei. Journal of Neuroscience. 2001;21:1452–1463. doi: 10.1523/JNEUROSCI.21-05-01452.2001. Retrieved from http://www.jneurosci.org/content/21/5/1452.long. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulak JM, Nguyen TA, Olivera BM, McIntosh JM. Alpha-conotoxin MII blocks nicotine-stimulated dopamine release in rat striatal synaptosomes. Journal of Neuroscience. 1997;17:5263–5270. doi: 10.1523/JNEUROSCI.17-14-05263.1997. Retrieved from http://www.jneurosci.org/content/17/14/5263.long. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuryatov A, Berrettini W, Lindstrom J. Acetylcholine receptor (AChR) alpha5 subunit variant associated with risk for nicotine dependence and lung cancer reduces (alpha4beta2)alpha5 AChR function. Molecular Pharmacology. 2011;79:119–125. doi: 10.1124/mol.110.066357. doi:10.1124/mol.110.066357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuryatov A, Lindstrom J. Expression of functional human alpha6beta2beta3* acetylcholine receptors in Xenopus laevis oocytes achieved through subunit chimeras and concatamers. Molecular Pharmacology. 2011;79:126–140. doi: 10.1124/mol.110.066159. doi:10.1124/mol.110.066159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuryatov A, Olale F, Cooper J, Choi C, Lindstrom J. Human alpha6 AChR subtypes: Subunit composition, assembly, and pharmacological responses. Neuropharmacology. 2000;39:2570–2590. doi: 10.1016/s0028-3908(00)00144-1. doi:10.1016/S0028-3908(00)00144-1. [DOI] [PubMed] [Google Scholar]
- Le Novere N, Zoli M, Changeux JP. Neuronal nicotinic receptor alpha 6 subunit mRNA is selectively concentrated in catecholaminergic nuclei of the rat brain. European Journal of Neuroscience. 1996;8:2428–2439. doi: 10.1111/j.1460-9568.1996.tb01206.x. doi:10.1111/j.1460-9568.1996.tb01206.x. [DOI] [PubMed] [Google Scholar]
- Lecchi M, McIntosh JM, Bertrand S, Safran AB, Bertrand D. Functional properties of neuronal nicotinic acetylcholine receptors in the chick retina during development. European Journal of Neuroscience. 2005;21:3182–3188. doi: 10.1111/j.1460-9568.2005.04150.x. doi:10.1111/j.1460-9568.2005.041.50.x. [DOI] [PubMed] [Google Scholar]
- Leonard RJ, Labarca CG, Charnet P, Davidson N, Lester HA. Evidence that the M2 membrane-spanning region lines the ion channel pore of the nicotinic receptor. Science. 1988;242:1578–1581. doi: 10.1126/science.2462281. doi:10.1126/science.2462281. [DOI] [PubMed] [Google Scholar]
- Lester RA, Dani JA. Acetylcholine receptor desensitization induced by nicotine in rat medial habenula neurons. Journal of Neurophysiology. 1995;74:195–206. doi: 10.1152/jn.1995.74.1.195. Retrieved from http://jn.physiology.org/content/74/1/195.long. [DOI] [PubMed] [Google Scholar]
- Letchworth SR, Whiteaker P. Progress and challenges in the study of alpha6-containing nicotinic acetylcholine receptors. Biochemical Pharmacology. 2011;82:862–872. doi: 10.1016/j.bcp.2011.06.022. doi:10.1016/j.bcp.2011.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung LK, Patafio FM, Rosser WW. Gastrointestinal adverse effects of varenicline at maintenance dose: A meta-analysis. BMC Clinical Pharmacology. 2011;11:15. doi: 10.1186/1472-6904-11-15. doi:10.1186/1472-6904-11-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ED, McClernon FJ, Rezvani AH. Nicotinic effects on cognitive function: Behavioral characterization, pharmacological specification, and anatomic localization. Psychopharmacology (Berlin) 2006;184:523–539. doi: 10.1007/s00213-005-0164-7. doi:10.1007/s00213-005-0164-7. [DOI] [PubMed] [Google Scholar]
- Lof E, Olausson P, deBejczy A, Stomberg R, McIntosh JM, Taylor JR, et al. Nicotinic acetylcholine receptors in the ventral tegmental area mediate the dopamine activating and reinforcing properties of ethanol cues. Psychopharmacology (Berlin) 2007;195:333–343. doi: 10.1007/s00213-007-0899-4. doi:10.1007/s00213-007-0899-4. [DOI] [PubMed] [Google Scholar]
- Lof E, Olausson P, Stomberg R, Taylor JR, Soderpalm B. Nicotinic acetylcholine receptors are required for the conditioned reinforcing properties of sucrose-associated cues. Psychopharmacology (Berlin) 2010;212:321–328. doi: 10.1007/s00213-010-1957-x. doi:10.1007/s00213-010-1957-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis M, Clarke PB. Effect of ventral tegmental 6-hydroxydopamine lesions on the locomotor stimulant action of nicotine in rats. Neuropharmacology. 1998;37:1503–1513. doi: 10.1016/s0028-3908(98)00151-8. doi:10.1016/S0028-3908(98)00151-8. [DOI] [PubMed] [Google Scholar]
- Liu L, Zhao-Shea R, McIntosh JM, Gardner P, Tapper A. Nicotine persistently activates ventral tegmental area dopaminergic neurons via nicotinic acetylcholine receptors containing alpha4 and alpha6 subunits. Molecular Pharmacology. 2012 doi: 10.1124/mol.111.076661. Advance online publication. doi:10.1124/mol.111.076661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Marks MJ, Collins AC. Desensitization of nicotinic agonist-induced [3H]gamma-aminobutyric acid release from mouse brain synaptosomes is produced by subactivating concentrations of agonists. Journal of Pharmacology and Experimental Therapeutics. 1999;291:1127–1134. Retrieved from http://jpet.aspetjournals.org/content/291/3/1127.long. [PubMed] [Google Scholar]
- Mansvelder HD, Keath JR, McGehee DS. Synaptic mechanisms underlie nicotine-induced excitability of brain reward areas. Neuron. 2002;33:905–919. doi: 10.1016/s0896-6273(02)00625-6. doi:10.1016/S0896-6273(02)00625-6. [DOI] [PubMed] [Google Scholar]
- Mansvelder HD, Mertz M, Role LW. Nicotinic modulation of synaptic transmission and plasticity in cortico-limbic circuits. Seminars in Cell and Developmental Biology. 2009;20:432–440. doi: 10.1016/j.semcdb.2009.01.007. doi:10.1016/j.semcdb.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markou A, Paterson NE. The nicotinic antagonist methyllycaconitine has differential effects on nicotine self-administration and nicotine withdrawal in the rat. Nicotine & Tobacco Research. 2001;3:361–373. doi: 10.1080/14622200110073380. doi:10.1080/14622200110073380. [DOI] [PubMed] [Google Scholar]
- Marks MJ, Grady SR, Yang JM, Lippiello PM, Collins AC. Desensitization of nicotine-stimulated 86Rb+ efflux from mouse brain synaptosomes. Journal of Neurochemistry. 1994;63:2125–2135. doi: 10.1046/j.1471-4159.1994.63062125.x. doi:10.1046/j.1471-4159.1994.63062125.x. [DOI] [PubMed] [Google Scholar]
- Marks MJ, Laverty DS, Whiteaker P, Salminen O, Grady SR, McIntosh JM, et al. John Daly's compound, epibatidine, facilitates identification of nicotinic receptor subtypes. Journal of Molecular Neuroscience. 2010;40:96–104. doi: 10.1007/s12031-009-9264-x. doi:10.1007/s12031-009-9264-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marubio LM, Gardier AM, Durier S, David D, Klink R, Arroyo-Jimenez MM, et al. Effects of nicotine in the dopaminergic system of mice lacking the alpha4 subunit of neuronal nicotinic acetylcholine receptors. European Journal of Neuroscience. 2003;17:1329–1337. doi: 10.1046/j.1460-9568.2003.02564.x. doi:10.1046/j.1460-9568.2003.02564.x. [DOI] [PubMed] [Google Scholar]
- Maskos U, Molles BE, Pons S, Besson M, Guiard BP, Guilloux JP, et al. Nicotine reinforcement and cognition restored by targeted expression of nicotinic receptors. Nature. 2005;436:103–107. doi: 10.1038/nature03694. doi:10.1038/nature03694. [DOI] [PubMed] [Google Scholar]
- McGranahan TM, Patzlaff NE, Grady SR, Heinemann SF, Booker TK. alpha4beta2 nicotinic acetylcholine receptors on dopaminergic neurons mediate nicotine reward and anxiety relief. Journal of Neuroscience. 2011;31:10891–10902. doi: 10.1523/JNEUROSCI.0937-11.2011. doi:10.1523/JNEUROSCI.0937-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh JM, Azam L, Staheli S, Dowell C, Lindstrom JM, Kuryatov A, et al. Analogs of alpha-conotoxin MII are selective for alpha6-containing nicotinic acetylcholine receptors. Molecular Pharmacology. 2004;65:944–952. doi: 10.1124/mol.65.4.944. doi:10.1124/mol.65.4.944. [DOI] [PubMed] [Google Scholar]
- Meyer EL, Yoshikami D, McIntosh JM. The neuronal nicotinic acetylcholine receptors alpha 4* and alpha 6* differentially modulate dopamine release in mouse striatal slices. Journal of Neurochemistry. 2008;105:1761–1769. doi: 10.1111/j.1471-4159.2008.05266.x. doi:10.1111/j.1471-4159.2008.05266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihalak KB, Carroll FI, Luetje CW. Varenicline is a partial agonist at alpha4beta2 and a full agonist at alpha7 neuronal nicotinic receptors. Molecular Pharmacology. 2006;70:801–805. doi: 10.1124/mol.106.025130. doi:10.1124/mol.106.025130. [DOI] [PubMed] [Google Scholar]
- Mineur YS, Brunzell DH, Grady SR, Lindstrom JM, McIntosh JM, Marks MJ, et al. Localized low-level re-expression of high-affinity mesolimbic nicotinic acetylcholine receptors restores nicotine-induced locomotion but not place conditioning. Genes, Brain and Behavior. 2009;8:257–266. doi: 10.1111/j.1601-183X.2008.00468.x. doi:10.1111/j.1601-183X.2008.00468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mineur YS, Somenzi O, Picciotto MR. Cytisine, a partial agonist of high-affinity nicotinic acetylcholine receptors, has antidepressant-like properties in male C57BL/6J mice. Neuropharmacology. 2007;52:1256–1262. doi: 10.1016/j.neuropharm.2007.01.006. doi:10.1016/j.neuropharm.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogg AJ, Whiteaker P, McIntosh JM, Marks M, Collins AC, Wonnacott S. Methyllycaconitine is a potent antagonist of alpha-conotoxin-MII-sensitive presynaptic nicotinic acetylcholine receptors in rat striatum. Journal of Pharmacology and Experimental Therapeutics. 2002;302:197–204. doi: 10.1124/jpet.302.1.197. doi:10.1124/jpet.302.1.197. [DOI] [PubMed] [Google Scholar]
- Moore TJ, Furberg CD, Glenmullen J, Maltsberger JT, Singh S. Suicidal behavior and depression in smoking cessation treatments. PLoS One. 2011;6:e27016. doi: 10.1371/journal.pone.0027016. doi:10.1371/journal.pone.0027016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neugebauer NM, Zhang Z, Crooks PA, Dwoskin LP, Bardo MT. Effect of a novel nicotinic receptor antagonist, N, N’-dodecane-1,12-diyl-bis-3-picolinium dibromide, on nicotine self-administration and hyperactivity in rats. Psychopharmacology (Berlin) 2006;184:426–434. doi: 10.1007/s00213-005-0163-8. doi:10.1007/s00213-005-0163-8. [DOI] [PubMed] [Google Scholar]
- Olausson P, Jentsch JD, Taylor JR. Nicotine enhances responding with conditioned reinforcement. Psychopharmacology (Berlin) 2004a;171:173–178. doi: 10.1007/s00213-003-1575-y. doi:10.1007/s00213-003-1575-y. [DOI] [PubMed] [Google Scholar]
- Olausson P, Jentsch JD, Taylor JR. Repeated nicotine exposure enhances responding with conditioned reinforcement. Psychopharmacology (Berlin) 2004b;173:98–104. doi: 10.1007/s00213-003-1702-9. doi:10.1007/s00213-003-1702-9. [DOI] [PubMed] [Google Scholar]
- Paradiso KG, Steinbach JH. Nicotine is highly effective at producing desensitization of rat alpha4beta2 neuronal nicotinic receptors. Journal of Physiology. 2003;553:857–871. doi: 10.1113/jphysiol.2003.053447. doi:10.1113/jphysiol.2003.053447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez XA, Bordia T, McIntosh JM, Grady SR, Quik M. Long-term nicotine treatment differentially regulates striatal alpha6alpha4beta2* and alpha6(nonalpha4)beta2* nAChR expression and function. Molecular Pharmacology. 2008;74:844–853. doi: 10.1124/mol.108.048843. doi:10.1124/mol.108.048843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez XA, Bordia T, McIntosh JM, Quik M. alpha6ss2* and alpha4ss2* nicotinic receptors both regulate dopamine signaling with increased nigrostriatal damage: Relevance to Parkinson's disease. Molecular Pharmacology. 2010;78:971–980. doi: 10.1124/mol.110.067561. doi:10.1124/mol.110.067561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez XA, O’Leary KT, Parameswaran N, McIntosh JM, Quik M. Prominent role of alpha3/alpha6beta2* nAChRs in regulating evoked dopamine release in primate putamen: Effect of long-term nicotine treatment. Molecular Pharmacology. 2009;75:938–946. doi: 10.1124/mol.108.053801. doi:10.1124/mol.108.053801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA. Subjective reactivity to smoking cues as a predictor of quitting success. Nicotine & Tobacco Research. 2011;14:383–387. doi: 10.1093/ntr/ntr229. doi:10.1093/ntr/ntr229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA, Gerlach D, Vender J, Grobe J, Meeker J, Hutchison S. Sex differences in the subjective and reinforcing effects of visual and olfactory cigarette smoke stimuli. Nicotine & Tobacco Research. 2001;3:141–150. doi: 10.1080/14622200110043059. doi:10.1080/14622200110043059. [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Zoli M, Rimondini R, Lena C, Marubio LM, Pich EM, et al. Acetylcholine receptors containing the beta2 subunit are involved in the reinforcing properties of nicotine. Nature. 1998;391:173–177. doi: 10.1038/34413. doi:10.1038/34413. [DOI] [PubMed] [Google Scholar]
- Pidoplichko VI, DeBiasi M, Williams JT, Dani JA. Nicotine activates and desensitizes midbrain dopamine neurons. Nature. 1997;390:401–404. doi: 10.1038/37120. doi:10.1038/37120. [DOI] [PubMed] [Google Scholar]
- Pidoplichko VI, Noguchi J, Areola OO, Liang Y, Peterson J, Zhang T, et al. Nicotinic cholinergic synaptic mechanisms in the ventral tegmental area contribute to nicotine addiction. Learning & Memory. 2004;11:60–69. doi: 10.1101/lm.70004. doi:10.1101/lm.70004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pons S, Fattore L, Cossu G, Tolu S, Porcu E, McIntosh JM, et al. Crucial role of alpha4 and alpha6 nicotinic acetylcholine receptor subunits from ventral tegmental area in systemic nicotine self-administration. Journal of Neuroscience. 2008;28:12318–12327. doi: 10.1523/JNEUROSCI.3918-08.2008. doi:10.1523/JNEUROSCI.3918-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman S, Neugebauer NM, Zhang Z, Crooks PA, Dwoskin LP, Bardo MT. The effects of a novel nicotinic receptor antagonist N, N-dodecane-1,12-diyl-bis-3-picolinium dibromide (bPiDDB) on acute and repeated nicotine-induced increases in extracellular dopamine in rat nucleus accumbens. Neuropharmacology. 2007;52:755–763. doi: 10.1016/j.neuropharm.2006.09.012. doi:10.1016/j.neuropharm.2006.09.012. [DOI] [PubMed] [Google Scholar]
- Rice ME, Cragg SJ. Nicotine amplifies reward-related dopamine signals in striatum. Nature Neuroscience. 2004;7:583–584. doi: 10.1038/nn1244. doi:10.1038/nn1244. [DOI] [PubMed] [Google Scholar]
- Rollema H, Chambers LK, Coe JW, Glowa J, Hurst RS, Lebel LA, et al. Pharmacological profile of the alpha4beta2 nicotinic acetylcholine receptor partial agonist varenicline, an effective smoking cessation aid. Neuropharmacology. 2007;52:985–994. doi: 10.1016/j.neuropharm.2006.10.016. doi:10.1016/j.neuropharm.2006.10.016. [DOI] [PubMed] [Google Scholar]
- Salas R, Pieri F, De Biasi M. Decreased signs of nicotine withdrawal in mice null for the beta4 nicotinic acetylcholine receptor subunit. Journal of Neuroscience. 2004;24:10035–10039. doi: 10.1523/JNEUROSCI.1939-04.2004. doi:10.1523/JNEUROSCI.1939-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas R, Sturm R, Boulter J, De Biasi M. Nicotinic receptors in the habenulo-interpeduncular system are necessary for nicotine withdrawal in mice. Journal of Neuroscience. 2009;29:3014–3018. doi: 10.1523/JNEUROSCI.4934-08.2009. doi:10.1523/JNEUROSCI.4934-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen O, Drapeau JA, McIntosh JM, Collins AC, Marks MJ, Grady SR. Pharmacology of alpha-conotoxin MII-sensitive subtypes of nicotinic acetylcholine receptors isolated by breeding of null mutant mice. Molecular Pharmacology. 2007;71:1563–1571. doi: 10.1124/mol.106.031492. doi:10.1124/mol.106.031492. [DOI] [PubMed] [Google Scholar]
- Salminen O, Murphy KL, McIntosh JM, Drago J, Marks MJ, Collins AC, et al. Subunit composition and pharmacology of two classes of striatal presynaptic nicotinic acetylcholine receptors mediating dopamine release in mice. Molecular Pharmacology. 2004;65:1526–1535. doi: 10.1124/mol.65.6.1526. doi:10.1124/mol.65.6.1526. [DOI] [PubMed] [Google Scholar]
- Salminen O, Whiteaker P, Grady SR, Collins AC, McIntosh JM, Marks MJ. The subunit composition and pharmacology of alpha-conotoxin MII-binding nicotinic acetylcholine receptors studied by a novel membrane-binding assay. Neuropharmacology. 2005;48:696–705. doi: 10.1016/j.neuropharm.2004.12.011. doi:10.1016/j.neuropharm.2004.12.011. [DOI] [PubMed] [Google Scholar]
- Schroeder BE, Binzak JM, Kelley AE. A common profile of prefrontal cortical activation following exposure to nicotine- or chocolate-associated contextual cues. Neuroscience. 2001;105:535–545. doi: 10.1016/s0306-4522(01)00221-4. doi:10.1016/S0306-4522(01)00221-4. [DOI] [PubMed] [Google Scholar]
- Singh S, Loke YK, Spangler JG, Furberg CD. Risk of serious adverse cardiovascular events associated with varenicline: A systematic review and meta-analysis. Canadian Medical Association Journal. 2011;183:1359–1366. doi: 10.1503/cmaj.110218. doi:10.1503/cmaj.110218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein EA, Pankiewicz J, Harsch HH, Cho JK, Fuller SA, Hoffmann RG, et al. Nicotine-induced limbic cortical activation in the human brain: A functional MRI study. American Journal of Psychiatry. 1998;155:1009–1015. doi: 10.1176/ajp.155.8.1009. Retrieved from http://ajp.psychiatryonline.org/article.aspx?volume=155&page=1009. [DOI] [PubMed] [Google Scholar]
- Tapper AR, McKinney SL, Nashmi R, Schwarz J, Deshpande P, Labarca C, et al. Nicotine activation of alpha4* receptors: Sufficient for reward, tolerance, and sensitization. Science. 2004;306:1029–1032. doi: 10.1126/science.1099420. doi:10.1126/science.1099420. [DOI] [PubMed] [Google Scholar]
- Vann RE, Balster RL, Beardsley PM. Dose, duration, and pattern of nicotine administration as determinants of behavioral dependence in rats. Psychopharmacology (Berlin) 2006;184:482–493. doi: 10.1007/s00213-005-0037-0. doi:10.1007/s00213-005-0037-0. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Swanson JM, Telang F. Dopamine in drug abuse and addiction: Results of imaging studies and treatment implications. Archives of Neurology. 2007;64:1575–1579. doi: 10.1001/archneur.64.11.1575. Retrieved from http://archneur.ama-assn.org/cgi/content/full/64/11/1575. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Tomasi D. Addiction circuitry in the human brain. Annual Reviews in Pharmacology and Toxicology. 2011;52:321–336. doi: 10.1146/annurev-pharmtox-010611-134625. doi:10.1146/annurev-pharmtox-010611-134625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters CL, Brown S, Changeux JP, Martin B, Damaj MI. The beta2 but not alpha7 subunit of the nicotinic acetylcholine receptor is required for nicotine-conditioned place preference in mice. Psychopharmacology (Berlin) 2006;184:339–344. doi: 10.1007/s00213-005-0295-x. doi:10.1007/s00213-005-0295-x. [DOI] [PubMed] [Google Scholar]
- Whiteaker P, McIntosh JM, Luo S, Collins AC, Marks MJ. 125I-alpha-conotoxin MII identifies a novel nicotinic acetylcholine receptor population in mouse brain. Molecular Pharmacology. 2000;57:913–925. Retrieved from http://molpharm.aspetjournals.org/content/57/5/913.long. [PubMed] [Google Scholar]
- Whiteaker P, Peterson CG, Xu W, McIntosh JM, Paylor R, Beaudet AL, et al. Involvement of the alpha3 subunit in central nicotinic binding populations. Journal of Neuroscience. 2002;22:2522–2529. doi: 10.1523/JNEUROSCI.22-07-02522.2002. Retrieved from http://www.jneurosci.org/content/22/7/2522.long. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. WHO report on the global tobacco epidemic, 2008: The MPOWER package. 2008 Geneva, Switzerland: Author. ISBN 978 92 4 159628 2. [Google Scholar]
- Yang K, Buhlman L, Khan GM, Nichols RA, Jin G, McIntosh JM, et al. Functional nicotinic acetylcholine receptors containing alpha6 subunits are on GABAergic neuronal boutons adherent to ventral tegmental area dopamine neurons. Journal of Neuroscience. 2011;31:2537–2548. doi: 10.1523/JNEUROSCI.3003-10.2011. doi:10.1523/JNEUROSCI.3003-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeiger JS, Haberstick BC, Schlaepfer I, Collins AC, Corley RP, Crowley TJ, et al. The neuronal nicotinic receptor subunit genes (CHRNA6 and CHRNB3) are associated with subjective responses to tobacco. Human Molecular Genetics. 2008;17:724–734. doi: 10.1093/hmg/ddm344. doi:10.1093/hmg/ddm344. [DOI] [PubMed] [Google Scholar]
- Zhang H, Sulzer D. Frequency-dependent modulation of dopamine release by nicotine. Nature Neuroscience. 2004;7:581–582. doi: 10.1038/nn1243. doi:10.1038/nn1243. [DOI] [PubMed] [Google Scholar]
- Zhao-Shea R, Liu L, Soll LG, Improgo MR, Meyers EE, McIntosh JM, et al. Nicotine-mediated activation of dopaminergic neurons in distinct regions of the ventral tegmental area. Neuropsychopharmacology. 2011;36:1021–1032. doi: 10.1038/npp.2010.240. doi:10.1038/npp.2010.240. [DOI] [PMC free article] [PubMed] [Google Scholar]