Abstract
Polymers have played an integral role in the advancement of drug delivery technology by providing controlled release of therapeutic agents in constant doses over long periods, cyclic dosage, and tunable release of both hydrophilic and hydrophobic drugs. From early beginnings using off-the-shelf materials, the field has grown tremendously, driven in part by the innovations of chemical engineers. Modern advances in drug delivery are now predicated upon the rational design of polymers tailored for specific cargo and engineered to exert distinct biological functions. In this review, we highlight the fundamental drug delivery systems and their mathematical foundations and discuss the physiological barriers to drug delivery. We review the origins and applications of stimuli-responsive polymer systems and polymer therapeutics such as polymer-protein and polymer-drug conjugates. The latest developments in polymers capable of molecular recognition or directing intracellular delivery are surveyed to illustrate areas of research advancing the frontiers of drug delivery.
Keywords: controlled release, stimuli-responsive, responsive polymers, recognitive polymers, polymer therapeutics, intracellular delivery
INTRODUCTION
Hierarchical progress in modern drug delivery begins with the use of polymer carriers to elicit spatiotemporal release of therapeutics in both pulsatile dose delivery products and implanted reservoir systems. Although conventional drug delivery formulations have contributed greatly to the treatment of disease, the emergence of potent and specific biological therapeutics has escalated the impetus for intelligent delivery systems. Heller (1) and Langer & Peppas (2) pointed out the importance of chemical engineering innovation in the development of new drug delivery systems and suggested that feedback control should be a standard component of such systems. These systems must overcome many hurdles before clinical implementation is realized; a truly intelligent delivery system must address the need for specific targeting, intracellular transport, and biocompatibility while integrating elements of responsive behavior to physiological environments and recognitive feedback control.
Tremendous progress has been made as a result of the exploration of diffusion-controlled and solvent-activated formulations in drug delivery. Hydrogels and other polymer-based carriers have been developed to provide safe passage for pharmaceuticals through inhospitable physiological regions. Polymers of controlled molecular architecture can be engineered to give a well-defined response to external conditions as a result of a solid understanding of the underlying mechanisms and the nature of behavioral transitions. Polymers incorporated with therapeutics can be bioactive to provide their own therapeutic benefit or can be biodegradable to improve release kinetics and prevent carrier accumulation. Pharmaceutical agents have been conjugated to polymers to modify transport or circulation half-life characteristics as well as to allow for passive and active targeting. And finally, the latest drug delivery research using polymeric materials has produced recognitive systems and polymer carriers that facilitate cytoplasmic delivery of novel therapeutics.
This review aims to provide a unique coverage of the field of polymers in drug delivery, addressing the foundations of drug delivery in a conceptual and mathematical context and critically reviewing the recent developments in responsive polymers, polymer therapeutics, and advanced systems designed for molecular recognition or engineered for intracellular delivery of novel therapeutics.
CONVENTIONAL APPLICATIONS OF POLYMERS IN DRUG DELIVERY
For more than 50 years, techniques such as compression, spray and dip coating, and encapsulation have been used in the pharmaceutical industry to incorporate bioactive agents with polymers. Such polymers have largely included cellulose derivatives, poly(ethylene glycol) PEG, and poly(N-vinyl pyrrolidone) (3). From a drug delivery perspective, polymer devices can be categorized as diffusion-controlled (monolithic devices), solvent-activated (swelling- or osmotically-controlled devices (5)), chemically controlled (biodegradable), or externally-triggered systems (e.g., pH, temperature) (4).
Diffusion-Controlled Systems
Most diffusion-controlled carriers are simple and monolithic in nature. In these systems, a drug is dissolved (or dispersed if the concentration exceeds the polymer's solubility limit) in a nonswellable or fully swollen matrix that does not degrade during its therapeutic life. In dissolved systems (C0 < CS), C0 is the initial loading concentration and CS is the saturation concentration. Fick's second law, for slab geometry,
| (1) |
can be solved under the appropriate boundary conditions to obtain an expression for concentration Ci(x,t). Di is the diffusivity of the solute in the polymer matrix, and Ci is the concentration of species i. Equations for calculating Di for porous, microporous, and nonporous hydrogels have been tabulated (6). Differentiating Ci(x,t) with respect to x allows for substitution of this result into Fick's first law:
| (2) |
This expression can then be integrated under the appropriate boundary conditions at the interface, x, to develop an equation for Mt, where Mt is the cumulative mass or moles released from the system (7):
| (3) |
With dispersed systems (C0 > CS), the situation is more complex as the precipitated regions are considered nondiffusing and disappear as a function of drug release to create a moving boundary problem. The well-known Higuchi equation (for planar geometry),
| (4) |
provides a simple model for release by treating the problem as a pseudosteady state (8). In this expression, S represents the surface area available for drug release. Expansions to this model have produced expressions for spherical geometries (9) and to account for drug concentrations near the solubility limit for the polymer (10).
Solvent-Activated Systems
In traditional swellable systems, drugs are loaded into dehydrated hydrophilic polymers or hydrogels by simply packing the two substances together. In the absence of a plasticizing aqueous solvent, these systems are usually well below their glass transition temperature, Tg, and have very low diffusivities. Once exposed to an aqueous environment, the hydrogels imbibe water and swell. If the polymer is not chemically crosslinked (or crystalline), then dissolution creates an erosion front. Drug delivery devices that operate as swelling-controlled systems undergo a transition from the glassy to rubbery state during solvent swelling, which relaxes polymer chains and dissolves dispersed drug deposits. This process creates two simultaneously moving fronts, diffusion and swelling, in addition to the erosion front, if present. This has been shown dramatically using cylindrical hydroxypropyl methylcellulose (HPMC) sections loaded with buflomedil pyridoxal phosphate (11). The diffusion front is created at the dissolved-dispersed drug boundary where the localized solvent volume fraction is higher than at the core of the polymer matrix. The swelling front is created as water is imbibed into the matrix, thus increasing chain motility. Starting in the center of a polymer matrix, a negative gradient in polymer volume fraction and entanglement exists relative to the outside surfaces. The dispersion-dissolution and erosion boundaries are continuously moving relative to each other, and the associated diffusion lengths are constantly changing. A popular empirical model that can be used to describe transport for swellable systems is based on the power-law expression (12):
| (5) |
In this equation, M∞ is the total mass loaded into the polymer, and k and n are the constants of the power-law expression.
This expression provides the fractional mass released from a polymer matrix as a function of time. The value for n is dependent on the type of transport, geometry, and polydispersity. Case I or Fickian diffusion describes the condition in which diffusion is slow compared with the rate of chain relaxation. This condition is correlated to n = 0.50 for thin film geometries. For cylindrical and spherical geometries, the characteristic n values are 0.45 and 0.43, respectively (13, 14). For Case II diffusion, the system is relaxation controlled because the chain relaxation rate is the kinetically-limiting component, thus n = 1. Systems with values of n (0.43 < n < 1) experience anomalous transport and indicate that diffusion and relaxation mechanisms are similar in rate. This model has been expanded to account for lag times in release (15) and burst effect (16) as well as for separating diffusion and Case II contributions into separate terms (17). For more in-depth reviews of several mathematical models of polymer drug release, the reader is referred to Arifin et al. (18) and Masaro & Zhu (19).
Biodegradable Systems
Biodegradable and bioerodible polymers represent an important class of materials for drug delivery. Although often used interchangeably, degradation and erosion differ in that covalent bond cleavage by chemical reactions occurs in degradation. Erosion occurs by the dissolution of chain fragments in noncrosslinked systems without chemical alterations to the molecular structure. For dissolution to occur, the polymer must absorb the surrounding aqueous solvent and must interact with water via charge interactions (such as with polyacids and polybases) or hydrogen bonding mechanisms.
Both degradation and erosion can occur as surface or bulk processes. In surface degradation, the polymer matrix is progressively removed from the surface, but the polymer volume fraction remains fairly unchanged. Conversely, in bulk degradation, no significant change occurs in the physical size of the polymer carrier until it is almost fully degraded or eroded, but the fraction of polymer remaining in the carrier decreases over time. The dominant process is determined by the relative rates of solvent penetration into the polymer, diffusion of the degradation product, and degradation or dissolution of the macromolecular structure (20). These rate considerations are especially important in designing biodegradable hydrogels because they are often polymerized in the presence of an aqueous solvent.
To be chemically degradable, polymers require hydrolytically or proteolytically labile bonds in their backbone or crosslinker. The majority of biodegradable synthetic polymers rely on hydrolytic cleavage of ester bonds or ester derivatives such as poly(lactic/glycolic acid) and poly(ε-caprolactone). In addition to ester derivatives, hydrolysis also acts on poly(anhydrides), poly(orthoesters), poly(phosphoesters), poly(phosphazenes), and poly(cyanoacrylate) derivatives (21, 22). Degradation and dissolution processes can auto-accelerate because degradation mechanisms may release an acid product that catalyzes further degradation or ionizes an initially hydrophobic structure that encourages the matrix to further imbibe water, for example by hydrolyzing pendant anhydrides on poly(methyl acrylate).
A well-known issue with biodegradable polymers is uncertainty with regard to the safety of degradation products. Because degradation often results in a distribution of fragment sizes, toxicity is challenging to determine experimentally. Ideally, parenterally-administered polymers would degrade into small, metabolic compounds that are known to be nontoxic and are small enough for natural clearance mechanisms.
Pharmacological Considerations in Drug Delivery
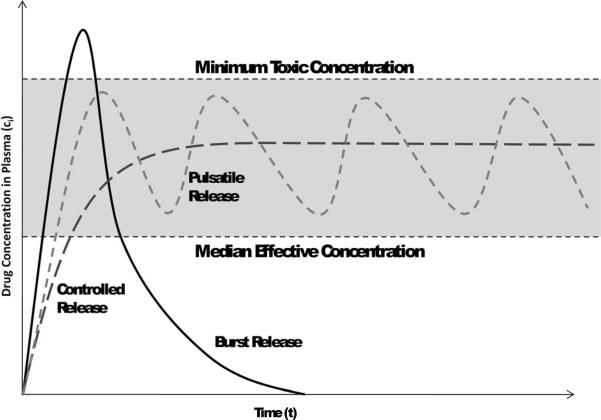
The central objective of a delivery system is to release therapeutics at the desired anatomical site and to maintain the drug concentration within a therapeutic band for a desired duration (Figure 1).
Figure 1.
Therapeutic band with showing impact of burst release, pulsatile release, and controlled release relative to effective concentration and toxic concentration.
Whether a drug is absorbed orally, parenterally, or by other means, such as inhalation or transdermal patches, bioavailability in the bloodstream allows for distribution to virtually all bodily tissues. Once in blood, drugs disseminate to all or most tissues by crossing endothelial barriers or by draining though endothelial gaps in tissues with “leaky” vasculature. Additionally, active targeting mechanisms may be employed by the polymer carrier, a polymer-drug conjugate, or the drug itself to disproportionally partition itself into the tissue of interest.
Physiology of oral delivery
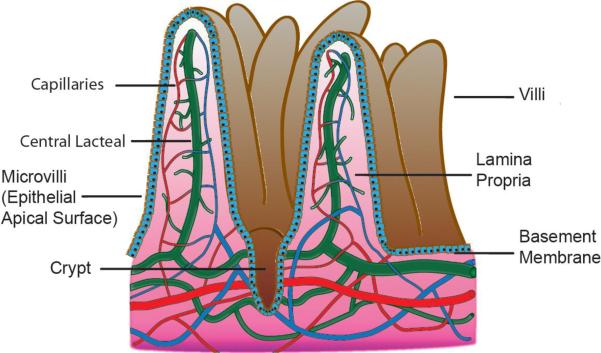
Oral formulations represent the most common platform for drug delivery. In conventional pharmaceutical formulations, such as those employing tablets and capsules, delivery of relatively small organic molecules via the gastrointestinal (GI) tract occurs by means of passive absorption down a concentration gradient on the intestinal surfaces as determined by three primary factors: extent of ionization, molecular weight (MW), and oil/water partition coefficient of the drug (23). Just as in the absorption of nutrients and ions, drugs generally pass through several physical barriers during transcytosis before entering intestinal capillaries or central lacteals, as shown in Figure 2 (see color insert). The boundaries, beginning with the lumen of the intestine, are the mucous layer, brush border (microvilli), epithelial apical membrane, cytoplasm, basal membrane, and basement membrane, before entering the lamina propria, where substances can either enter capillaries by diffusing through endothelial cells or pass into the central lacteal for passage into the lymphatic system, thereby avoiding first-pass metabolism. Except for extremely large molecules or molecules that partition heavily into chylomicrons, the vast majority of absorbed substances take the capillary exit from the intestine owing to the substantial perfusion of blood vessels.
Figure 2.
Schematic representation of the diffusional boundaries on a microvillus to draining capillary networks and lacteals
It is important to emphasize that effective release of a therapeutic agent in the vicinity of the mucous layer does not imply sufficient bioavailability. Significant fractions of a drug that diffuses into the mucous membranes may be effluxed back into the intestinal lumen, metabolized in the intestinal mucosa, or removed by the hepatic portal system during first-pass metabolism.
Physiology of parenteral delivery
Many therapeutic agents, such as proteins, lack the stability or absorption characteristics necessary for absorption in the GI tract. These and agents with very narrow therapeutic windows must be administered parenterally. Parenteral delivery bypasses the GI tract by direct injection, usually intravenously or interstitially, and is far more predictable and generally more rapid than oral delivery. Intravenous injection results in immediate drug availability, which is advantageous in many cases, but it also generally results in shorter drug circulation owing to rapid access to excretory mechanisms, and it can make overdoses nearly impossible to counteract. Drugs and polymer carriers for intravenous delivery must generally be soluble in aqueous environments. With subcutaneous and intramuscular routes, a drug bolus is temporarily implanted by injection into an interstitial environment and subsequently cleared from the site by absorption into the vasculature or drainage into the lymphatic system. This mechanism allows for slower absorption of the drug and may be used for oily substances. MW determines whether an injection site will be cleared by the tissue capillaries or lymphatics. As in the central lacteals of the intestine, substances with higher MWs (or greater hydrodynamic diameters) enter lymphatic capillaries and subsequently systemic circulation by drainage at the thoracic duct. Because tissue perfusion is substantial, absorption vastly dominates lymphatic draining with molecules of less than 5 kDa (25).
RESPONSIVE POLYMERS FOR DRUG DELIVERY
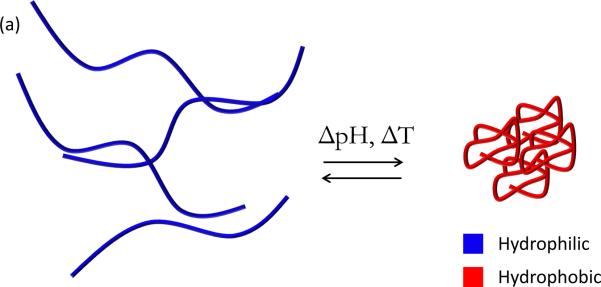
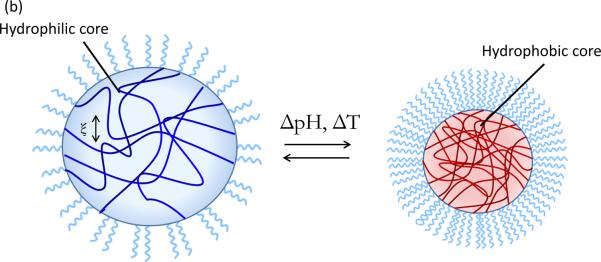
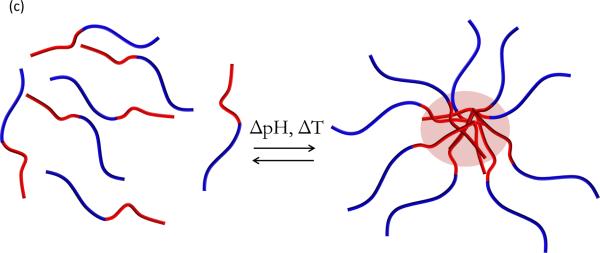
Environmentally-responsive polymers, or smart polymers, are a class of materials comprised of a large variety of linear and branched (co)polymers or crosslinked polymer networks. A hallmark of responsive polymers is their ability to undergo a dramatic physical or chemical change in response to an external stimulus. Temperature and pH changes are commonly used to trigger behavioral changes, but other stimuli, such as ultrasound, ionic strength, redox potential, electromagnetic radiation, and chemical or biochemical agents, can be used. These stimuli can be subsumed into discrete classifications of physical or chemical nature (26). Physical stimuli (i.e., temperature, ultrasound, light, and magnetic and electrical fields) directly modulate the energy level of the polymer/solvent system and induce a polymer response at some critical energy level. Chemical stimuli (i.e., pH, redox potential, ionic strength, and chemical agents) induce a response by altering molecular interactions between polymer and solvent (adjusting hydrophobic/hydrophilic balance) or between polymer chains (influencing crosslink or backbone integrity, proclivity for hydrophobic association, or electrostatic repulsion) (26). Types of behavioral change can include transitions in solubility, hydrophilic-hydrophobic balance, and conformation (27). These changes are manifested in many ways, such as the coil-globule transition of polymer chains (28), swelling/deswelling of covalently crosslinked hydrogels (29), sol-gel transition of physically crosslinked hydrogels (30), and self-assembly of amphiphilic polymers (31) (Figure 3, see color insert). The aim of this section is to review recent developments in temperature and pH-responsive polymers and highlight the emerging area of redox-responsive polymers for drug delivery systems. Additionally, a brief introduction to magnetically-triggered polymer nanocomposites is given below. Several excellent reviews that provide a comprehensive treatment of these topics are available (26, 27, 32–37).
Figure 3.
Illustrative examples of responsive behavior. (a) Coil-to-globule transition of linear polymer chain in solution (b) Responive swelling/deswelling of surface-grafted crosslinked hydrogel particle (c) Stimuli-responsive micellization of amphiphilic block copolymers
Applications and Examples
The majority of responsive polymers for drug delivery can be broadly categorized as hydrogels, micelles, polyplexes, or polymer-drug conjugates, which are covered in more detail below. Hydrogels are hydrophilic (co)polymeric networks capable of imbibing large amounts of water or biological fluids (38). Physical or covalent crosslinks render hydrogels insoluble in water. Hydrogels can be engineered to respond to various stimuli (32) and have demonstrated significant utility in the medical and pharmaceutical arenas. Peppas and coworkers have pioneered the use of pH-responsive complexation hydrogels of poly(methacrylic acid) grafted with PEG, referred to as P(MAA-g-EG), for oral protein delivery. Through interpolymer complexation in acidic conditions, this system has been shown to successfully entrap, protect, and mediate delivery of insulin (39), calcitonin (40), and interferon β (41). Micelle-forming polymers, such as block copolymers of poly(ethylene oxide) and poly(propylene oxide), or Pluronics®, have been thoroughly studied in drug delivery (33). These polymers exhibit temperature-responsive micellization (42), as do block copolymers of poly(N-isopropylacrylamide) (PNIPAAm) coupled with hydrophilic PEG (31). Polyplexes formed by cooperative electrostatic interactions between polyethyleneimine (PEI) and DNA are widely studied for gene delivery. Since the seminal paper by Boussif et al. (43), several facets of PEI-mediated gene delivery have been investigated, including the influence of crosslinking, MW, branching, and biodegradability (44--46).
Responsive Systems Based on Temperature
Temperature has been widely investigated as a stimulant for responsive polymer systems owing to its ease of modulation and applicability in drug delivery applications (26). Tanaka observed temperature-dependent swelling of polymer gels (47) following its theoretical prediction more than 40 years ago (48). One thermo-responsive polymer, PNIPAAm, has been thoroughly investigated for its ability to undergo a reversible, inverse (or negative) temperature-dependent phase transition (32). Below its lower critical solution temperature (LCST) near 32°C, PNIPAAm exists as a hydrophilic coil, whereas above the LCST, PNIPAAm chains collapse sharply into a hydrophobic globule (49). The nature of this volume phase transition stems from the hydrophilic/hydrophobic balance of polymer chains (50), which is modulated by continual establishment and disruption of intra- and intermolecular electrostatic and hydrophobic interactions. Below the LCST, water molecules exist in an ordered state in the local environment of polymer chains (51). As temperature rises above the LCST, polymer-polymer hydrophobic interactions dominate (49). Consequently, polymer chains collapse and water molecules are released to the bulk, resulting in a net entropic gain for the polymer/solvent system (52).
For drug delivery applications, it may be desirable to shift the critical temperature for volume phase transition to a specific temperature range. This can be accomplished through the inclusion of hydrophobic or hydrophilic moieties in the polymer chain. Polymers with a larger hydrophobic hydration area possess stronger hydrophobic interactive forces and undergo collapse at a lower temperature (28, 53). Conversely, increasing the hydrophilic content of the polymer chain will increase the LCST. Polymers that exhibit positive temperature-dependent swelling behavior, i.e., a globule-to-coil transition with increasing temperature, possess an upper critical solution temperature (UCST). These materials, such as poly(acrylic acid) and poly(acrylamide) interpenetrating networks (IPNs), are discussed in more detail elsewhere (54).
Physically crosslinked gels, such as methoxy-substituted cellulose derivatives, PNIPAAm copolymers, and various Pluronics can undergo a sol-gel phase transition near their LCST (33). These materials are attractive candidates for in situ implants in which thermoreversible gelation is exploited for the facile implantation of solid drug-depot preparations (55). In these systems, a liquid drug/polymer solution is injected into a target site at ambient temperature. As the solution temperature warms to body temperature, the polymer gels, which entraps the drug in the physically crosslinked matrix. Diffusion of the drug from the solid gel allows for sustained-release formulations. This approach was used in a study describing the release of model protein bovine serum albumin (BSA) from temperature-responsive chitosan grafted with PEG (PEG-g-chitosan) (56). PEG-g-chitosan containing 45 and 55 wt% grafted PEG were loaded with BSA and incubated at 37°C to evaluate release kinetics. Both gels demonstrated initial burst release of BSA during the first 5 hours followed by sustained, diffusion-driven release until approximately 70 hours. Crosslinking the gels with genipin resulted in prolonged release of BSA for up to 40 days.
Novel polymerization techniques, such as controlled radical polymerizations and click chemistry reactions, offer superior control over molecular architecture and present the opportunity to create novel materials highly tailored for specific responsive behavior. This approach was employed by Sumerlin and colleagues to produce folate-conjugated temperature-responsive block copolymers of N,N-dimethylacrylamide (DMAAm) and PNIPAAm with reversible addition fragmentation chain transfer RAFT polymerization (57). Above the LCST (34°C) of this polymer system, PNIPAAm blocks collapsed into hydrophobic globules whereas DMAAm blocks remained hydrophilic. Ensuing aggregation resulted in particles of approximately 46 nm postulated to be micelles with PNIPAAm cores and DMAAm shells. Surface decoration of the polymer chains with folate enables the system to be actively targeted to certain tissues via receptor-mediated endocytosis. Folate ligands are particularly applicable in cancer therapy and are discussed more thoroughly in the section on Other Areas of Polymer Therapeutics. Self assembled folate-conjugated polymers provided controlled release of a model hydrophobic drug, dipyridamole, over the course of 12 days.
Responsive Systems Based on pH
Physiological pH varies systematically in the body, particularly along the GI tract, where harsh pH and enzymatic conditions in the stomach (pH ~ 2) degrade macromolecules. The small intestine is substantially more alkaline, with pH ~ 6.2--7.5. Physiological pH profiles will also change among cellular compartments. For example, endosomes typically exhibit pH values of 5.0--6.8 and lysosomes 4.5--5.5 (58, 59). Also, it is well known that diseased or inflamed tissues and exhibit different pH profiles than normal tissue (35). Tumors have been widely reported to produce acidic conditions (pH ~ 6.5) in the extracellular milieu (60). Thus, it is no surprise that scientists and engineers have devoted considerable effort toward the rational design of polymers capable of exploiting these pH variations to selectively deliver valuable therapeutics to specific intracellular or extracellular sites of action. By judicious materials selection and careful engineering of molecular architecture, pH-responsive polymer delivery systems can be developed to give well-controlled pH response and drug release.
Recently we synthesized polycationic nanomatrices capable of well-defined hydrophilic-hydrophobic transitions near physiological pH (61, 62). Relatively uniform particles of poly[2-(diethylamino)ethyl methacrylate-co-t-butyl methacrylate-g-PEG] (PDBP) measuring 51 nm were synthesized using a novel photoemulsion polymerization technique (61). Relevant properties of the system, such as swelling ratio, critical swelling pH, surface charge, and biocompatibility, were tailored by tuning polymer composition, crosslinking density, and the incorporation of hydrophobic moieties into the hydrogel core. Ongoing work aims to optimize these systems for intracellular small interfering RNA (siRNA) delivery.
Promising work by Hu et al. (63) describes the development of pH-responsive core-shell hydrogels for intracellular delivery of ovalbumin to dendritic cells, a class of cells intimately involved with adaptive immunity. Emulsion polymerization was used to create crosslinked poly(2-(diethylamino)ethyl methacrylate) (PDEAEMA) core--poly(2-aminoethyl methacrylate) (PAEMA) shell nanoparticles measuring 205 nm in diameter. The authors hypothesized that PDEAEMA would exhibit pH-responsive behavior whereas PAEMA would remain constitutively ionized throughout the physiological pH range. Interestingly, the authors used the cationic PAEMA shell to adsorb and protect a model ovalbumin protein rather than the archetypal practice of loading therapeutics into the hydrogel core. Subsequent studies demonstrated the versatility of this approach through intracellular delivery of siRNA and influenza A particles (64). This strategy of using a charged, pH-insensitive shell distinct from the pH-responsive domain represents an intriguing departure from the current paradigm of using a neutral, hydrophilic shell, such as PEG, to shield surface charges. However, several drawbacks may limit the feasibility of this design in vivo. First, charged particles have a much higher opsonization rate than neutral particles (65), and the cationic PAEMA shell may attract opsonin proteins or promote adsorption of anionic serum proteins, resulting in rapid clearance by the reticuloendothelial system. Secondly, the slow dissociation of electrostatically bound cargo from a polymer shell may provide a kinetic barrier to therapeutic efficacy.
Bae and colleagues (66--68) have recently reported polymer micelles possessing dynamic, multifunctional behavior for drug delivery. Self-assembling amphoteric polyamine-based block copolymers were functionalized with folate (66), biotin (68), and HIV peptide trans-activating transcriptional activator (TAT) ligands (67), thus demonstrating robust applicability in targeted delivery. Folate or biotin ligands enhance cellular uptake via receptor-mediated endocytosis (69), and TAT is a well-known peptide transduction domain (70). By conjugating the cell-penetrating peptide TAT, particles of up to 200 nm gain direct access to the cell (71), effectively circumventing the intracellular trafficking pathway. The polymer system, a mixture of two block copolymers, poly(l-histidine)-b-PEG (polyHis-b-PEG) and poly(l-lactic acid)-b-PEG-b-polyHis-ligand (pLLA-b-PEG-b-polyHisligand), self-assembled into mixed micelles capable of ligand exposure, micelle destabilization, and endosomal disruption in response to pH (67, 68). A short polyHis block preceding the ligand serves to anchor the ligand at the core-shell interface, which effectively shields its presentation on the micelle surface at neutral pH. Upon exposure to a slightly acidic (6.5 < pH < 7.0) environment, the short polyHis anchor ionizes and PEG-b-polyHis arm unfurls, exposing the ligand on the micellar surface. This response is expected to confer tumor specificity to the micelle carrier, as the ligand will be unavailable to promote receptor-mediated endocytosis or cellular transduction in normal (pH 7.4) tissue. Further acidification (pH < 6.5) induced micelle dissociation by ionization of the His residues in the micelle core. Breast adenocarcinoma cells exposed to doxorubicin (Dox)-loaded mixed micelles displayed prominent intracellular distribution and nuclear localization of Dox after 30 minutes and experienced ~60% reduction in cell viability after 48 hours.
Responsive Systems Based on Redox Potential
Polymers containing labile linkages present an attractive opportunity to develop biodegradable or bioerodible delivery devices. Much of the early work in this field focused on acid labile linkages of polyanhydrides (72, 73), poly(lactic/glycolic acid) (74), and more recently poly(β-amino esters) (75, 76). However, intracellular cues are now being investigated as a means to trigger cytoplasmic degradation of polymer carriers incorporating advanced therapeutics such as siRNA and anticancer drugs. Disulfide linkages are well known to be unstable in a reductive environment as the disulfide bond is readily cleaved in favor of corresponding thiol groups. Polymers with disulfide cross-links have been synthesized as polymersomes (77), nanogels (78), and core-cross-linked polyplexes (79) and degrade when exposed to cysteine or glutathione, reductive amino-acid based molecules present at intracellular concentrations 50–1000 fold greater than those of the extracellular milieu (79).
The Hubbell group (77) has used amphiphilic copolymers of PEG and poly(propylene sulfide) (PPS) to form vesicular compartments. Rather than relying on hydrolytic linkages, which may respond too slowly to avoid nonproductive lysosomal accumulation of the polymer carrier, they have incorporated a disulfide linkage between the hydrophilic PEG and hydrophobic PPS portions of the polymer, which imparts a high degree of reductive sensitivity to the polymersomes.
In another study, glutathione-degradable nanogels were prepared by inverse emulsion atom transfer radical polymerization (ATRP) (78). Upon exposure to 10 wt% glutathione, half of the polymer degraded in nearly 6 hours. Exposing polymers to 20 wt% glutathione resulted in 85% degradation within 1 hour. Dox was efficiently incorporated into the polymer matrix at 16 wt% of the polymer with more than 50% loading efficiency, and the authors demonstrated in vitro release of fluorescent dye Rhodamine G6 and Dox. Dox-loaded nanogels displayed negligible toxicity toward HeLa cells in the absence of glutathione while causing approximately 40% reduction in cell viability following introduction of exogenous glutathione to the cellular media. It remains to be determined if this polymer system is capable of degrading and releasing drug upon exposure to intracellular glutathione concentrations or if the timescale for degradation in the presence of endogenous glutathione will allow efficient cytoplasmic delivery of incorporated therapeutics.
In a more recent investigation, Kataoka and colleagues (79) synthesized and thoroughly characterized a core-cross-linked polyplex composed of iminothiolane-modified PEG-block-poly(l-lysine), or [PEG-b-(PLL-IM)], for intracellular siRNA delivery. The use of a block copolymer affords modular functionality; the polycationic poly(l-lysine) segment serves to bind siRNA and provide endosomal buffering capacity (80) whereas the hydrophilic PEG segment prolongs circulation time, prevents aggregation, and reduces opsonization (65). Lysine groups of the PEG-b-PLL copolymer were reacted with 2-iminothiolane and subsequently oxidized to form disulfide cross-links. Introducing crosslinks to the micelle core confers stability to the system, as crosslinked polymers maintained micellar structure in physiological salt conditions whereas their noncrosslinked counterparts could not. The resulting polyion complex micelles were approximately 60 nm in diameter, a particle size well within the accepted limits (20–100 nm) for avoiding uptake by the RES and renal exclusion (81). Not surprisingly, micellar stability strongly influenced the ultimate siRNA transfection efficiency. The authors observed a narrowly defined N/P ratio at which stable micellization occurred. Interestingly, this optimum N/P ratio shifted to higher values with increased crosslinking. Highly efficient (more than 80%) knockdown of a reporter gene was detected at the optimum N/P ratio; however, a considerable decrease in transfection efficiency was observed upon slight departure from this critical value.
POLYMER THERAPEUTICS FOR DRUG DELIVERY
Polymer therapeutics is a term used to describe an increasingly important area of biopaharmaceutics in which a linear or branched polymer chain behaves either as the bioactive (a polymeric drug) or, more commonly, as the inert carrier to which a therapeutic is covalently linked, as in the case of polymer-drug conjugates, polymer-protein conjugates, polymeric micelles, and multicomponent polyplexes (82). Conjugation of the therapeutic to the polymer improves the pharmacokinetic and pharmacodynamic properties of biopharmaceuticals through a variety of measures, including increased plasma half-life (which improves patient compliance because less frequent doses are required), protection of the therapeutic from proteolytic enzymes, reduction in immmunogenicity, enhanced stability of proteins, enhanced solubility of low MW drugs, and the potential for targeted delivery (82–84).
The majority of polymer conjugates are designed as anticancer therapeutics, although other diseases have also been targeted, including rheumatoid arthritis, diabetes, hepatitis B and C, and ischemia (85). The popularity of conjugates for anticancer agents is a result of a passive tumor targeting phenomenon first coined by Matsumura & Maeda (86) as the enhanced permeation and retention (EPR) effect. It has been shown that the tumor concentration of anticancer therapeutics can increase up to 70-fold as a part of circulating macromolecular systems such as polymer conjugates (82). However, recent studies have shown that tumor targeting may not be able to be achieved exclusively by the EPR effect owing to difficulties in reaching cancer cells deep inside malignant tissues (87), which underscores the need for synergistic passive and active targeting strategies. Since the advent of controlled release polymer drug delivery systems (DDS), the polymer therapeutics field has exploded as the focus has shifted toward strategies that facilitate targeted release, especially for anticancer drugs, which often have severe negative side effects. For a polymer-drug conjugate to be both practical and effective, several features are desired: (a) nontoxic and nonimmunogenic polymer carrier, (b) MW high enough to ensure long circulation times, but <40 kDa for nonbiodegradable polymers to ensure renal elimination following drug release [N-(2-hydroxypropyl)methacrylamide (HPMA) has an optimal MW of ~30 kDa (82)], (c) adequate loading/carrying capacity in relation to the potency of the drug [PEG is not an ideal carrier as it has only two reactive groups, which leads to a low drug payload (82, 88)], (d) linker must be stable during transport but easily cleaved for optimum delivery upon arrival at target (frequently achieved using a Glycine-Phenylalinine-Leucine-Glycine, or GFLG, peptide linkage), and (e) the ability to target desired tissue by active and/or passive means (83).
The traditional approach to synthesizing polymer-protein conjugates involves the postpolymerization modification of the polymeric carrier, usually PEG, with protein-reactive end groups that facilitate binding between its own pendant groups and those of the amino acids in the protein. There are three general requirements for an effective polymer-protein conjugate system: a polymer with a single reactive group at only one terminal end (to prevent protein crosslinking), a nontoxic/immunogenic linker (including intermediate byproducts), and a method that will yield site-specific conjugation (82). The two main types of polymers are amine- and thiol-reactive polymers that target lysine and cysteine side chains, respectively. Thiol-reactive polymers have been used more recently in an effort to create site-specific conjugates because cysteines are not as common as lysine. Postpolymerization techniques typically employed to add thiol-reactive end groups include the use of vinyl sulfone, maleimide, iodoacetamide, and activated disulfide end groups (84, 89). In addition, several new approaches have been investigated to circumvent postpolymerization modifications and protein-polymer coupling reactions. There has been a strong impetus recently for these techniques, which enable the synthesis of the polymer directly from protein-reactive initiators, owing to the advent of living/controlled polymerization methodologies, such as RAFT and ATRP, as they are straightforward, less time intensive, and almost guarantee that each polymer chain contains only one reactive end-group (89–91).
Applications and Examples
The most common carriers for polymer therapeutics are HPMA (polymer-drug) and PEG (polymer-protein), but other systems studied include poly(glutamic acid), PEI, dextran, dextrin, chitosans, poly(l-lysine), and poly(aspartamides) as polymeric carriers (92). Polymer-drug and polymer-protein conjugates typically studied have a tripartite structure: polymer, linker, and therapeutic agent. However, more complex systems now exist that incorporate additional features for targeted delivery or combination therapies.
The large number of polymer conjugates either approved for clinical use or currently in clinical trials is a reflection of the fact that polymer therapeutics is no longer just an academic curiosity. The first-to-market products, with their brand name and year of approval in parentheses, include PEG-l-asparaginase (Oncaspar, 1994) for leukemia, styrene maleic anhydride-neocarzinostatin (Zinostatin Stimalmer, 1993) for hepatocellular carcinoma, PEG-granulocyte colony-stimulating factor (Neulasta, 2002) for neutropenia prevention associated with chemotherapy, PEG-interferon-α (PEGasys, 2002) for hepatitis C, and PEG-adenosine deaminase (Adagen, 1990) for severe combined immunodeficiency disease (83, 88, 93). Several excellent reviews have been published over the past several years that provide a more expansive overview of the field and conjugates currently in clinical testing (82, 83, 85, 93).
Polymer-Drug Conjugates
One of the most commonly studied areas of polymer therapeutics is polymer-drug conjugates in which the low MW therapeutic and polymeric carrier are most often an anticancer agent and HPMA copolymer, respectively. This area was born from a landmark study by Ringsdorf in 1975 (94) and then further pioneered in the 1980s by Duncan & Kopecek, who designed the first targeted synthetic polymer-anticancer conjugates to progress to clinical trials (95, 96). This work was comprehensively reviewed recently (97, 98). In contrast to free drugs, which usually distribute randomly throughout the body and thus exert deleterious side effects, attachment of the therapeutic to polymer carriers limits cellular uptake to endocytosis, extends circulation times to several hours, and facilitates passive targeting of tumors via the EPR effect (83).
Angiogenesis inhibitors, such as TNP-470 [O-(chloracetyl-carbomoyl) fumagillol] are currently receiving increased interest as anticancer drugs. In a landmark paper describing the first polymer-antiangiogenic conjugate, Satchi-Fainaro et al. (99) synthesized a conjugate of HPMA and TNP-470 that was covalently linked with GFLG via an enzymatically degradable bond, ethylenediamine. The tetrapeptide linker was designed to allow intralysosomal release of the therapeutic by cleaving the bond when in the presence of lysosomal cysteine proteases such as cathepsin B, levels of which are elevated in many tumor endothelial cells. In vivo studies not only demonstrated that the conjugate selectively accumulated in tumor vessels via the EPR effect, but also enhanced and prolonged the activity of TNP-470 without the neurotoxicity previously seen in animal studies conducted using only the antiangiogenic drug, likely because the size of the conjugate prevented it from crossing the blood-brain barrier. This HPMA copolymer-TNP-470 conjugate is currently in preclinical development under the name caplostatin by SynDevRx and has since been the focus of additional studies (100, 101).
Novel polymeric architectures, such as dendrimer, branched, grafted, and star polymers, are now being explored as conjugate carriers of the future owing to advances in polymer chemistry. In an elegant report, paclitaxil, a common chemotherapeutic with low solubility, was covalently conjugated with linear bis(PEG) and dendritic polyamidoamine (PAMAM) G4 to determine the influence of the architecture of the polymeric carrier on the efficacy of the anticancer DDS (102). Both PAMAM and PEG increased the solubility of paclitaxil in relation to the free drug (0.3 mg ml−1); however, solubility was improved further with the dendrimer (3.2 versus 2.5 mg ml−1). Confocal microscopy analysis of FITC (fluorescein isothiocyanate) labeled samples showed that both conjugates distributed in a more homogeneous and uniform manner than the free drug. In vitro cytotoxicity studies of A2780 human ovarian cancer cells demonstrated that although the PEG-based conjugate reduced the activity of the drug by 25-fold, the PAMAM-G4 dendrimer conjugate increased the efficacy of paclitaxil by more than 10 times compared with its free state. This study suggests that dendrimers are promising vehicles for intracellular delivery of poorly soluble drugs.
Polymer-Protein Conjugates
Pioneering studies published by Davis and colleagues in the late 1970s (103, 104) laid the foundation for the area now known as PEGylation in which peptides and proteins are covalently conjugated to PEG. This technique has become the method of choice over the past 20 years to improve the pharmacokinetic and pharmacodynamic properties of protein therapeutics (82, 83). PEG is commonly used as the preferred carrier in this application because of a lack of immunogenicity, antigenicity, and toxicity (PEG is approved by the FDA for injectable, topical, rectal, and nasal pharmaceutical formulations). Furthermore, it is extremely hydrophilic, which helps to protect the protein from an immune response, and it can be synthesized to facilitate specific conjugation without crosslinking the protein, which allows the therapeutic to be released (93). And although PEGylation can lead to a decrease in protein activity, the increased circulation time can compensate for this to still provide drug concentrations at relevant levels. The impact of PEGylation on pharmaceuticals has been extensively reviewed elsewhere (84, 93, 105).
Despite the successes and future promise of PEGylation in polymer-protein conjugates, PEG is limited due to a lack of biodegradability, and conjugation can reduce or alter protein activity. Duncan et al. (106) report a novel approach for polymer-protein conjugation called polymer-masking-unmasking protein therapy (PUMPT) in which a model enzyme, trypsin, was conjugated to dextrin, a natural polysaccharide that is biodegradable and has been clinically approved for a variety of uses. The researchers hypothesized that a conjugation of this nature would protect and mask the activity of the protein in transit while restoring the activity at the desired target site by triggered degradation of the polymer. Dextrin was functionalized via succinoylation as the resultant chemistry not only yields reactive groups necessary for covalent modification but also a nontoxic intermediate before the complete degradation to maltose and isomaltose occurs in the presence of α-amylase. Dextrin conjugation reduced the activity of the trypsin by 34–69% depending on the MW and level of succinoylation; subsequent incubation with α-amylase resulted in a return of activity to 92–115% of the original amount as analyzed using N-benzoyl-L-arginine-p-nitroanilide (l-BAPNA). PUMPT offers a novel strategy with great potential to improve the delivery of proteins that are toxic or typically inactivated in transit, and/or to allow targeted release of the protein. However, additional studies are required to optimize the conjugation chemistry on a system-by-system basis as well as to fully characterize the intermediate and final degradation products to ensure safe elimination.
Other Areas of Polymer Therapeutics
Polymeric micelles are a promising area of polymer therapeutics as a result of several advantages. These include easy conjugation for active targeting, high drug loading capacity in the hydrophobic core (especially for hydrophobic anticancer drugs), and rapid cellular uptake facilitated by their nanosize characteristics (107, 108). Cancerous tissues have been shown to exhibit a local pH decrease (pH 5–6) and local hyperthermia (T ~ 42°C) relative to surrounding normal tissues, and several studies have been conducted to exploit these characteristics (109––113). One particular type of micellar system combines pH-induced anticancer therapeutic release with a conjugated ligand to facilitate active tumor targeting (114, 115).
In a recent study, Bae et al. (115) functionalized PEG- poly(β-benzyl-L-aspartate) (PBLA) block copolymer micelles with adriamycin (an anticancer drug) via a pH-cleavable hydrazone bond at the hydrophobic core forming poly(aspartic acid) block and with various amounts of folate conjugated to the hydrophilic shell forming PEG block. Hydrazone is a popular acid-labile linker because this bond is stable at pH 7.4 but hydrolyzes under mild acidic conditions (pH 5–6) (108). Folate is commonly employed as a tumor targeting ligand because cancer cells have been shown to overexpress folate-binding proteins (FBP) (116). Previous studies from the same group showed intracellular pH-triggered release capability with the same micellar system synthesized without folate: no appreciable drug was released at physiological pH whereas release was observed once the pH decreased below pH= 6 (110). Surface plasmon resonance (SPR) analyses demonstrated that folate-conjugated micelles bound rapidly and strongly to FBP whereas the micelles prepared without folate functionalization did not show any interaction. Folate conjugation had a minimal effect on the pharmacokinetic profile compared to those systems without the targeting ligand and showed a fivefold increase in the safe dosage range compared with free adriamycin. More importantly, in vivo studies clearly showed that the folate-conjugated micelles had a higher antitumor activity (lower effective dose) than either the nonfolate-conjugated micelles or the free drug (7.5 mg kg−1 for the folate-conjugated micelles versus 20 mg kg−1 and 10 mg kg−1, respectively).
Due to the molecular complexity and variable nature of disease, especially cancer, researchers are starting to investigate combination therapies as these have the potential to improve long-term therapeutic prognosis. Over the past few years, interest has shifted toward using FDA-approved combinations of multiple drugs conjugated to the same polymer in an attempt to exploit potential synergy for a more robust therapeutic effect. However, careful consideration must be taken as molecules of different drugs can interact with each other to induce unfavorable side effects. Several studies have been conducted recently to further investigate these combination therapies (117–119). In the most recent report, Lammers et al. (119) synthesized a system of gemcitabine (Gem) and Dox conjugated to HPMA via the same tetrapeptide, GFLG, that is commonly used in polymer therapeutics. The circulatory, angiogenic, apoptotic, and tumor growth inhibition characteristics of a variety of doses of the novel conjugate, termed P-Gem-Dox, were compared with those of regimens consisting solely of HPMA, free Gem and free Dox, P-Gem, and P-Dox control samples. The in vivo analyses showed that P-Gem-Dox circulated for prolonged periods of time (21% and 9% of the injected dose was detected at 4 and 24 hours post IV injection, respectively) and selectively localized to the tumor, as higher levels of conjugate were present in the tumor than in 7 of 9 other healthy tissues. P-Gem-Dox significantly improved tumor growth inhibition (50–60% inhibition) compared with the free drugs (~30%) and P-Gem and P-Dox conjugates (~40%). In vivo studies also showed that P-Gem-Dox enhanced antiangiogenic and apoptotic induction relative to the free drugs without increased toxicity, although not compared with the individual conjugate regimen. Despite the promise of combination conjugates, further in vivo investigations are needed to confirm that such systems are a significant improvement over the well-established singular conjugates given individually or in parallel.
CURRENT THRUSTS IN DRUG DELIVERY
Molecularly Imprinted Polymers
Molecular imprinting is a promising field in which polymer networks are formed with specific recognition for a desired template molecule. Briefly, functional monomers are chosen that exhibit chemical structures designed to interact with the desired template molecule via covalent or noncovalent chemistry. The monomers are then polymerized in the presence of the desired template, the template is subsequently removed, and the product is a molecularly imprinted polymer (MIP) with binding sites specific to the template molecule. This technique was originally developed for separation applications; however, MIPs have recently been studied for biomedical applications, including drug delivery (120, 121).
MIPs demonstrate great potential as advanced drug delivery systems owing to the affinity between the drug template and polymer pendant groups. This can yield zero order release of the drug over longer periods of time, a distinct advantage over conventional drug delivery. The ideal MIP DDS will maintain a drug concentration in its therapeutic range, which eliminates the need for frequent high concentration doses. Additionally, a closed loop process is also possible in which the MIP can detect a biological event, such as elevated levels of an undesired biomolecule, and release the corresponding therapeutic while continuously monitoring the environment. When this biomarker is no longer prevalent, the MIP responds by terminating the drug release. Several excellent review papers provide a more detailed analysis of MIPs, especially for drug delivery applications s (120–122).
Drug delivery via the ocular route is a target application of MIPs owing to the drawbacks of conventional systems, including low bioavailability (~5%), frequent high concentration doses, and short term discomfort and blurred vision (123). MIPs can address some of these issues by providing enhanced bioavailability, extended retention time, and concentrations within the therapeutic range by exploiting the increased affinity between the drug template and polymer pendant groups to slow the rate of release. Several papers have investigated this application over the past few years (123–129).
In a recent study, Ali & Byrne (123) investigated the release of high MW hyaluronic acid (HA) from rationally designed imprinted soft contact lenses. Acrylamide (Aam), N-vinyl pyrrolidone (NVP), and DEAEMA were selected as monomers for this MIP system because of their respective structural similarity to Asn, Tyr, and Arg/Lys, amino acid residues important in the binding of HA to the CD44 receptor. Commercially available nelfilcon A was combined with the MW ~1.2 million HA and functional monomers and subsequently UV polymerized to prepare the imprinted hydrogels. The total amount of functional monomer was varied between 0.05 and 5%. Release studies in artificial lachrymal solution at 35°C showed that an increase in the amount of functional monomers decreased the rate and total amount of HA released over a 24 hour period and uncovered that increasing the diversity of monomers lowers the diffusion coefficient (up to 1.6 times lower than the nelfilcon A control polymer). The imprinted polymers were easily tailored to release HA at the therapeutic level of 6 μg hr−1 for 24 hours, which is superior to the control polymer profile. Structural studies confirmed that the addition of monomers did not result in a change in the mesh size, thus causing the decrease in diffusion in the MIPs.
In another study by the same group, Venkatesh et al. (130) performed structural and transport analyses for ketotifen fumarate imprinted hydrogels with multiple functional monomers. The formulation with the best results consisted of a total of 3 mol% of acrylic acid (AA), Aam, and NVP as the functional monomers, 32 mol% 2-hydroxyethylmethacrylate (HEMA) as the backbone monomer, and 5 mol% PEG(200)dimethacrylate as the cross-linker. This system displayed an affinity of ~4.5 (recognition over control) and a 2.5–9 times increase in capacity in comparison with the systems that contained one or two functional monomers. Additionally, the template diffusion coefficients were lower for all MIPs compared with their corresponding controls, the biggest decrease being ~18.8 times for the same system, despite comparable mesh sizes. These studies were conducted under conditions far from physiological, namely at room temperature (25°C) and in deionized water. However, this study was an extension of work published previously in which the MIP released therapeutically relevant concentrations of the antihistamine over five days under physiological conditions (129). The results of these three studies are extremely promising for providing ocular therapeutic delivery at a constant rate for an extended period of time.
Although MIPs have enormous potential to be used in feedback-controlled and targeted delivery devices, much of the literature to date has focused solely on extended release via interactions between the pendant groups along the polymer backbone and the drug template. Even though the practical applications of such systems is a long way off, these feedback-controlled systems will be able to provide personalized therapeutic properties because they have the ability to resolve any problems before undesirable symptoms appear.
Endosomolytic Polymers
The emergence of highly specific biological pharmaceutical agents, including proteins (monoclonal antibodies, hormones, enzymes) and nucleic acids (plasmid DNA, antisense oligonucleotides, siRNA) has highlighted the need for carrier systems to direct these fragile molecules to their specific site of action. Although these biopharmaceuticals hold immense promise in the treatment of disease, their clinical implementation is hampered by the lack of safe, effective delivery vectors (131, 132). Extracellular and intracellular trafficking barriers represent a significant limitation in the delivery of fragile therapeutics and must be overcome with innovative solutions (35, 131, 132). To this end, development of biomimetic polymers has gained traction with the aim of producing synthetic polymer systems capable of emulating the membrane-lytic abilities of toxins and viruses bearing fusogenic peptides. Increased understanding of disease pathology and interfacial phenomena between polymers and cell membranes as well as refined methodology for tailoring the responsive behavior of materials has furthered the development of polymer carriers with advanced functionality. Recent systematic studies have shed light on the factors influencing membrane interaction; polymer characteristics such as composition, hydrophobic/hydrophilic balance, surface charge, and distribution of functional groups (80, 133) have significant impact on cell membrane interactions and ultimate endosomolytic ability.
Polymers bearing weakly ionizable groups are attractive candidates for intracellular delivery because of their ability to undergo a pH-responsive conformational transition and destabilize endosomal membranes. The mechanism of endosomal disruption depends on the chemical nature of the ionizable group. Anionic polymers, such as those bearing carboxyl groups, undergo a conformational change from charged open chains to compact, hydrophobically-stabilized structures capable of disrupting endosomal membranes through pore formation and disruption of membrane integrity. The mechanism of membrane destabilization by anionic polymers is thought to be related to their pH-dependent conformational transition (134), and the extent of polymer association with the lipid bilayer and cellular uptake can be enhanced by increased polymer hydrophobicity (135).
Cationic polymers, such as those bearing amine groups, are thought to promote endosomal rupture through the “proton sponge” mechanism. These polymers absorb incoming protons during endosomal acidification. This action causes an accumulation of protons and counterions, such as Cl−, within the endosome. The high osmotic strength within the endosomal compartment subsequently leads to osmotic swelling and endosomal rupture (131).
Most biotherapeutics must localize in a particular subcellular site of action (e.g., nucleus, cytosol, mitochondria) to exert a therapeutic effect. Since the pioneering work of Hoffman and Stayton (136–138), much effort has been directed toward developing polymer carriers that can efficiently direct these molecules to their intended target site. Frequently, this involves escape from the endosomal trafficking pathway and translocation to the cytosol. Recently, hydrogel nanoparticles demonstrating pulsatile intracellular delivery of Dox have been described (139). These nanogels consist of a hydrophobic poly(His-co-phenylalanine) core surrounded by a telechelic PEG shell. An outer protein shell is formed by attaching BSA to the opposite end of the PEG chain, imitating the capsid of many viruses. BSA was further conjugated with folate moieties, creating a multilayer hydrogel particle. Although the hydrophobic core was not covalently crosslinked, the nanogels maintained structural integrity and demonstrated reversible swelling behavior in response to pH. Buffering capacity provided by His residues and considerable volumetric swelling (particle diameter increased from 55 nm at pH 7.4 to 355 nm at pH 6.4) contribute to destabilization of the endosomal membrane. Dox loaded into the initially hydrophobic core is released during endosomal trafficking as progressively protonated polyHis drives gel swelling and Dox efflux. Impressively, this polymer carrier has shown propensity for multiple “infection” cycles. After the loss of cell membrane integrity from Dox-induced apoptosis, the polymer carrier was able to diffuse from dead cells to previously untreated populations and mediate additional rounds of Dox delivery.
Convertine et al. (140) recently reported intracellular delivery of siRNA using ampholytic pH-responsive block copolymers. One block, composed of DMAEMA, provides cooperative electrostatic complexation with siRNA whereas the second block, a terpolymer of DMAEMA, t-butyl methacrylate, and propylacrylic acid, mediates endosomal disruption. The copolymer undergoes a transition from hydrophilic ampholyte to polycationic hydrophobe near endosomal pH. This transition can be tuned to specific values by adjusting the hydrophobic content of the polymer, a parameter that also modulates endosomolytic ability. Optimizing the N/P ratio, a critical design consideration for nucleic acid delivery (80), was imperative in achieving particle self-assembly. Polymers containing variable terpolymer block compositions were screened for hemolytic ability, cell internalization, cytotoxicity, and siRNA silencing efficiency. In general, polymers with increasing hydrophobic content possessed more desirable properties: higher hemolytic efficiency at endosomal pH, increased cellular uptake, and more efficient gene knockdown. Although increasing the hydrophobic content leads to greater carrier efficacy, this comes at the cost of solubility in aqueous media (140). These factors must therefore be carefully balanced when optimizing molecular architecture for drug delivery applications.
Recently, Chen et al. (141–144) have published a series of compelling studies describing the synthesis and in vitro characterization of a pH-responsive amphiphilic pseudopetide, poly(l-lysine iso-phthalamide). At low concentrations (≤0.1 mg ml−1), the polymer undergoes a pH-dependent conformational transition from an extended hydrophilic chain to a condensed hydrophobic globule with progressive protonation of its pendant carboxyl groups. These moieties provide an opportunity for functionalization with PEG (143, 144) or hydrophobic amino acids such as l-valine, l-leucine, and l-phenylalanine (141, 142). The pH-responsive conformational transition was examined as a function of pH, time, concentration, and degree of grafting. Polymers grafted with hydrophilic PEG side chains displayed modest hemolytic activity (~50%) in the endosomal pH range (144) whereas polymers grafted with hydrophobic l-phenylalanine exhibited near-complete erythrocyte membrane disruption (142). As expected, hemolytic efficiency increased with relative hydrophobicity of the amino acid graft, with l-valine being the least effective and l-phenylalanine being the most effective. The phenylalanine-grafted polymer, termed PP-75, displayed maximum hemolytic efficiency from pH 6.0–7.0 and was essentially nonhemolytic at pH 7.4. On this basis, PP-75 was selected as a promising candidate for drug delivery. After demonstrating endosomal release of the model drug calcein (142), Liechty et al. used PP-75 to successfully direct intracellular delivery of the novel anticancer protein apoptin (145).
CONCLUSIONS
Research in polymer therapeutics has enjoyed success over the past few decades in mediating safe and effective delivery of bioactive agents to treat an enormous variety of medical conditions. The research initiatives highlighted in this review show great promise in enhancing drug delivery so that drugs will be distributed only to locations where needed in therapeutically relevant quantities and will rely less on the dosing efforts of the patient. Looking ahead, research efforts should progress toward understanding more about how polymers and polymer products interface with biological systems. Many studies in recent years have reported on novel chemical roots for advanced drug delivery systems, but too often biocompatibility studies are overlooked until late in development. The result is that many new devices fail at a later stage of their development. Judicious cellular and animal studies early in device development will help to ensure that polymer-related breakthroughs and in vitro successes result in effective and safe drug delivery platforms.
FUTURE ISSUES
Endosomal and intracellular delivery: Advanced treatment of diseases will require vehicles that can deliver their payload in a highly regulated and site-specific manner to achieve therapeutically relevant concentrations in subcellular organelles.
Responding to highly specific biochemical cues: Future therapeutic systems will have the ability to recognize key bioanalytes responsible for or indicative of a particular disease. Through a unique triggering mechanism either physical or chemical in nature, this recognition process will lead to delivery of a therapeutic agent. Classical chemical engineering principles of control theory will undoubtedly have a major impact on the optimization of these systems.
Crossing the blood-brain barrier: The blood-brain barrier, a formation of tightly sealed endothelial cells, remains a major obstacle for effective delivery of many therapeutics used to treat neurological and psychiatric disorders. PEG-grafted polymer nanoparticles have shown promise as a means to facilitate transport into deep areas of the brain without damage to the blood-brain barrier or any other brain structures.
Tumor targeting: This is a major area for targeted therapeutic delivery of high potency drugs at relatively high payloads to specific sites. Advanced delivery systems will utilize a combination of chemical and biological means to achieve localization and therapy at specific sites
Nanocomposites for External In Situ Triggering
The emerging area of polymer-nanoparticle composites incorporates an inorganic nanoparticle responsive to externally-applied electromagnetic radiation. Two main strategies are employed in these composites. One technique is based on dielectric-core, metal shell nanoparticles that become excited by plasmon resonance heating in response to induced light. This characteristic allows them to be tuned for deep-penetrating, near-infrared light, which is useful when these nanoparticles are several centimeters below the skin. This strategy can be used to induce a positive (146) or negative (147) sigmoidal swelling response in hydrogels, both of which have potential utility in drug delivery applications. The alternative strategy relies on using magnetically responsive nanoparticles surrounded by a responsive polymer layer. This strategy develops heat by magnetic hysteresis in an electromagnetic field. Thermal energy can be used forcibly to swell or collapse a temperature-responsive hydrogel as with the core-shell metal nanoparticles (148), or it can be used to trigger degradation of a polymer shell, as demonstrated by poly(alkylcyanoacrylates) for delivery of 5-fluorouracil (149). Furthermore, drug carriers that incorporate inorganic cores may also serve as contrast agents for several imaging modalities such as magnetic resonance imaging (MRI) (150). This would potentially allow for image-guided triggering of drug release coupled to information regarding the anatomical location of the carrier system.
ACKNOWLEDGMENTS
A portion of this work was supported by NIH grant EB000426. W.B.L., D.R.K., and B.V.S. acknowledge the National Science Foundation for NSF Graduate Research Fellowships.
TERMS AND DEFINITIONS
- Transcytosis
the process of macromolecules traversing cells by entering and exiting their membranes and passing through cellular constituents
- N/P ratio
nitrogen equivalents of cationic polymer relative to phosphate equivalents of nucleic acid
- Micelles
supramolecular assemblies formed by self-assembly of amphiphilic block copolymers into spherical particles with a hydrophilic corona and hydrophobic core
- Endocytosis
the process by which cells internalize macromolecules Predominant mechanisms include passive (pinocytosis) or receptor-mediated internalization
- Hemolytic ability
ability of polymers to rupture red blood cell membranes; frequently correlated with their ability to disrupt endosomal membranes in vitro
- Small interfering RNA (siRNA)
21–23 nucleotide sequence of single stranded RNA capable of potent and specific gene silencing
- Enhanced permeation and retention (EPR)
phenomenon in which macromolecules accumulate passively in tumors owing to hyperpermeability into the leaky vasculature combined with insufficient lymphatic drainage.
- Angiogenesis
proliferation of new blood vessels from pre-existing vasculature, which provides nutrients and facilitates waste removal in normal and diseased tissues
- PDEAEMA
poly(2-(diethylamino)ethyl methacrylate)
- DMAEMA
2-(dimethylamino)ethyl methacrylate)
- PEG
poly(ethylene glycol)
- PAEMA
poly(2-aminoethyl methacrylate)
- RAFT
reversible addition-fragmentation chain transfer
- ATRP
atom transfer radical polymerization
- HPMA
N-(2-hydroxypropyl)methacrylamide
- PBLA
poly(β-benzyl-l-aspartate)
- DDS
drug delivery systems
- PNIPAAm
poly(N-isopropylacrylamide)
Footnotes
DISCLOSURE STATEMENT The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Heller A. Integrated medical feedback systems for drug delivery. AIChE J. 2005;51(4):1054–66. [Google Scholar]
- 2.Langer R, Peppas NA. Advances in biomaterials, drug delivery, and bionanotechnology. AIChE J. 2003;49(12):2990–3006. [Google Scholar]
- 3.Rowe RC, Sheskey PJ, Owen SC. Handbook of Pharmaceutical Excipients. 5th ed Pharmaceutical Press; American Pharmacists Association; Grayslake, IL: Washington, D.C.: 2005. p. 850. [Google Scholar]
- 4.Langer RS, Peppas NA. Present and future applications of biomaterials in controlled drug delivery systems. Biomaterials. 1981;2(4):201–14. doi: 10.1016/0142-9612(81)90059-4. [DOI] [PubMed] [Google Scholar]
- 5.Verma RK, Mishra B, Garg S. Osmotically controlled oral drug delivery. Drug Dev. Ind. Pharm. 2000;26(7):695–708. doi: 10.1081/ddc-100101287. [DOI] [PubMed] [Google Scholar]
- 6.Peppas NA. Drug delivery using smart polymers: recent advances. In: Galaev IM, Mattiasson B, editors. Smart Polymers: Applications in Biotechnology and Biomedicine. 2nd ed CRC Press; Boca Raton, FL: 2008. [Google Scholar]
- 7.Crank J. The Mathematics of Diffusion. 2nd ed Oxford Univ. Press; New York: 1975. p. 414. [Google Scholar]
- 8.Higuchi T. Mechanism of sustained-action medication. Theoretical analysis of rate of release of solid drugs dispersed in solid matrices. J. Pharm. Sci. 1963;52(12):114–49. doi: 10.1002/jps.2600521210. [DOI] [PubMed] [Google Scholar]; Pioneering paper in pharmaceutical delivery that established design conditions for new pharmaceutical formulations
- 9.Koizumi T, Panomsuk SP. Release of medicaments from spherical matrices containing drug in suspension: theoretical aspects. Int. J. Pharm. 1995;116(1):45–49. [Google Scholar]
- 10.Cohen DS, Erneux T. Controlled drug release asymptotics. Siam J. Appl. Math. 1998;58(4):1193–204. [Google Scholar]
- 11.Colombo P, Bettini R, Santi P, Peppas NA. Swellable matrices for controlled drug delivery: gel-layer behaviour, mechanisms and optimal performance. Pharm. Sci. Technol. Today. 2000;3(6):198–204. doi: 10.1016/s1461-5347(00)00269-8. [DOI] [PubMed] [Google Scholar]
- 12.Korsmeyer RW, Gurny R, Doelker EM, Buri P, Peppas NA. Mechanisms of solute release from porous hydrophilic polymers. Int. J. Pharm. 1983;15(1):25–35. doi: 10.1002/jps.2600721021. [DOI] [PubMed] [Google Scholar]
- 13.Ritger PL, Peppas NA. A simple equation for description of solute release. I. Fickian and non-fickian release from non-swellable devices in the form of slabs, spheres, cylinders or discs. J. Control. Release. 1987;5(1):23–36. [PubMed] [Google Scholar]
- 14.Ritger PL, Peppas NA. A simple equation for description of solute release. II. Fickian and anomalous release from swellable devices. J. Control. Release. 1987;5(1):37–42. [PubMed] [Google Scholar]
- 15.Kim H, Fassihi R. Application of binary polymer system in drug release rate modulation. 2. Influence of formulation variables and hydrodynamic conditions on release kinetics. J. Pharm. Sci. 1997;86(3):323–28. doi: 10.1021/js960307p. [DOI] [PubMed] [Google Scholar]
- 16.Lindner WD, Lippold BC. Drug-release from hydrocolloid embeddings with high or low susceptibility to hydrodynamic stress. Pharm. Res. 1995;12(11):1781–85. doi: 10.1023/a:1016238427313. [DOI] [PubMed] [Google Scholar]
- 17.Peppas NA, Sahlin JJ. A simple equation for the description of solute release. III. Coupling of diffusion and relaxation. Int. J. Pharm. 1989;57(2):169–72. [Google Scholar]
- 18.Arifin DY, Lee LY, Wang CH. Mathematical modeling and simulation of drug release from microspheres: implications to drug delivery systems. Adv. Drug Deliv. Rev. 2006;58(12--13):1274–325. doi: 10.1016/j.addr.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 19.Masaro L, Zhu XX. Physical models of diffusion for polymer solutions, gels and solids. Prog. Polym. Sci. 1999;24(5):731–75. [Google Scholar]
- 20.Tamada JA, Langer R. Erosion kinetics of hydrolytically degradable polymers. Proc. Natl. Acad. Sci. USA. 1993;90(2):552–56. doi: 10.1073/pnas.90.2.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nair LS, Laurencin CT. Biodegradable polymers as biomaterials. Prog. Polym. Sci. 2007;32(8--9):762–98. [Google Scholar]
- 22.Park K, Shalaby W, Paark H. Biodegradable Hydrogels for Drug Delivery. Technomic; Lancaster, PA: 1993. [Google Scholar]
- 23.Mayersohn M. Principles of drug absorption. In: Florence AT, Siepmann J, editors. Modern Pharmaceutics. 5th ed Informa Healthcare; New York: 2009. pp. 23–80. [Google Scholar]
- 24.— (Citation removed, new figure produced by authors)
- 25.Takakura Y, Hashida M, Sezaki H. Lymphatic transport after paraentersal drug administration. In: Charman W, Stella V, editors. Lymphatic Transport of Drugs. CRC Press; Boca Raton, FL: 1992. pp. 255–77. [Google Scholar]
- 26.Gil ES, Hudson SA. Stimuli-reponsive polymers and their bioconjugates. Prog. Polym. Sci. 2004;29(12):1173–222. [Google Scholar]
- 27.Schmaljohann D. Thermo- and pH-responsive polymers in drug delivery. Adv. Drug Deliv. Rev. 2006;58(15):1655–70. doi: 10.1016/j.addr.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 28.Schild HG. Poly (N-isopropylacrylamide): experiment, theory and application. Prog. Polym. Sci. 1992;17(2):163–249. [Google Scholar]
- 29.Khare AR, Peppas NA. Swelling/deswelling of anionic copolymer gels. Biomaterials. 1995;16(7):559–67. doi: 10.1016/0142-9612(95)91130-q. [DOI] [PubMed] [Google Scholar]
- 30.Malmsten M, Lindman B. Self-assembly in aqueous block copolymer solutions. Macromolecules. 1992;25(20):5440–45. [Google Scholar]
- 31.Topp MDC, Jijkstra PJ, Talsma H, Feijen J. Thermosensitive micelle-forming block copolymers of poly(ethylene glycol) and poly(N-isopropylacrylamide) Macromolecules. 1997;30(26):8518–20. [Google Scholar]
- 32.Qiu Y, Park K. Environment-sensitive hydrogels for drug delivery. Adv. Drug Deliv. Rev. 2001;53(3):321–39. doi: 10.1016/s0169-409x(01)00203-4. [DOI] [PubMed] [Google Scholar]
- 33.Ruel-Gariépy E, Leroux J-C. In situ-forming hydrogels---review of temperature-sensitive systems. Eur. J. Pharm. Biopharm. 2004;58(2):409–26. doi: 10.1016/j.ejpb.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 34.Alarcon CDH, Pennadam S, Alexander C. Stimuli responsive polymers for biomedical applications. Chem. Soc. Rev. 2005;34(3):276–85. doi: 10.1039/b406727d. [DOI] [PubMed] [Google Scholar]
- 35.Ganta S, Devalapally H, Shahiwala A, Amiji M. A review of stimuli-responsive nanocarriers for drug and gene delivery. J. Control. Release. 2008;126(3):187–204. doi: 10.1016/j.jconrel.2007.12.017. [DOI] [PubMed] [Google Scholar]
- 36.Hoare TR, Kohane DS. Hydrogels in drug delivery: progress and challenges. Polymer. 2008;49(8):1993–2007. [Google Scholar]
- 37.Kost J, Langer R. Responsive polymeric delivery systems. Adv. Drug Deliv. Rev. 2001;46(1--3):125–48. doi: 10.1016/s0169-409x(00)00136-8. [DOI] [PubMed] [Google Scholar]; One of the earliest and most cited contributions on drug delivery systems that respond to environmental conditions
- 38.Peppas NA, Bures P, Leobandung W, Ichikawa H. Hydrogels in pharmaceutical formulations. Eur. J. Pharm. Biopharm. 2000;50(1):27–46. doi: 10.1016/s0939-6411(00)00090-4. [DOI] [PubMed] [Google Scholar]; An important review on the use of hydrogels in pharmaceutical applications
- 39.Lowman AM, Morishita M, Kajita M, Nagai T, Peppas NA. Oral delivery of insulin using pH-responsive complexation gels. J. Pharm. Sci. 1999;88(9):933–37. doi: 10.1021/js980337n. [DOI] [PubMed] [Google Scholar]
- 40.Torres-Lugo M, Peppas NA. Molecular design and in vitro studies of novel pH-sensitive hydrogels for the oral delivery of calcitonin. Macromolecules. 1999;32(20):6646–51. [Google Scholar]
- 41.Kamei N, Morishita M, Chiba H, Kavimandan NJ, Peppas NA, Takayama K. Complexation hydrogels for intestinal delivery of interferon β and calcitonin. J. Control. Release. 2009;134(2):98–102. doi: 10.1016/j.jconrel.2008.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alexandridis P, Holzwarth JF, Hatton TA. Micellization of poly(ethylene oxide)-poly(propylene oxide)-poly(ethylene oxide) triblock copolymers in aqueous solutions: thermodynamics of copolymer association. Macromolecules. 1994;27(9):2414–25. [Google Scholar]
- 43.Boussif O, Lezoualc'h F, Zanta MA, Hergny MD, Scherman D, et al. A versatile vector for gene and oligonucleotide transfer into cells in culture and in-vivo--polyethyleneimine. Proc. Natl. Acad. Sci. USA. 1995;92(16):7297–301. doi: 10.1073/pnas.92.16.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mok H, Park TG. Functional polymers for targeted delivery of nucleic acid drugs. Macromol. Biosci. 2009;9(8):731–43. doi: 10.1002/mabi.200900044. [DOI] [PubMed] [Google Scholar]
- 45.Gosselin MA, Guo WJ, Lee RJ. Efficient gene transfer using reversibly cross-linked low molecular weight polyethylenimine. Bioconjug. Chem. 2001;12(6):989–94. doi: 10.1021/bc0100455. [DOI] [PubMed] [Google Scholar]
- 46.Godbey WT, Wu KK, Mikos AG. Size matters: molecular weight affects the efficiency of poly(ethylenimine) as a gene delivery vehicle. J. Biomed. Mater. Res. 1999;45(3):268–75. doi: 10.1002/(sici)1097-4636(19990605)45:3<268::aid-jbm15>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 47.Tanaka T. Collapse of gels and critical endpoint. Phys. Rev. Lett. 1978;40(12):820–23. [Google Scholar]; Important work on swelling and syneresis in gels; it laid the foundation for the development of novel temperature-sensitive drug delivery systems
- 48.Duscaronek K, Patterson D. Transition in swollen polymer networks induced by intramolecular condensation. J. Polym. Sci. Part A. 1968;6(7):1209–16. [Google Scholar]
- 49.Heskins M, Guillet JE. Solution properties of poly(N-isopropylacrylamide) J. Macromol. Sci. Pure Appl. Chem. 1968;2(8):1441–55. [Google Scholar]
- 50.Bae YH, Okano T, Kim SW. Temperature-dependence of swelling of cross-linked poly(N,N'-alkyl substituted acrylamides) in water. J. Polym. Sci. Part B. 1990;28(6):923–36. [Google Scholar]
- 51.Shibayama M, Tanaka T. Volume phase-transition and related phenomena of polymer gels. Adv. Polym. Sci. 1993;109:1–62. [Google Scholar]
- 52.Kopecek J. Smart and genetically engineered biomaterials and drug delivery systems. Eur. J. Pharm. Sci. 2003;20(1):1–16. doi: 10.1016/s0928-0987(03)00164-7. [DOI] [PubMed] [Google Scholar]
- 53.Inomata H, Goto S, Saito S. Phase-transition of N-substituted acrylamide gels. Macromolecules. 1990;23(22):4887–88. [Google Scholar]
- 54.Katono H, Maruyama A, Sanui K, Ogata N, Okano T, Sakurai Y. Thermoresponsive swelling and drug release switching of interpenetrating polymer networks composed pf poly(acrylamide-co-butyl methacrylate) and poly(acrylic-acid) J. Control. Release. 1991;16(1--2):215–27. [Google Scholar]
- 55.Klouda L, Mikos AG. Thermoresponsive hydrogels in biomedical applications. Eur. J. Pharm. Biopharm. 2008;68(1):34–45. doi: 10.1016/j.ejpb.2007.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bhattarai N, Ramay HR, Gunn J, Matsen FA, Zhang M. PEG-grafted chitosan as an injectable thermosensitive hydrogel for sustained protein release. J. Control. Release. 2005;103(3):609–24. doi: 10.1016/j.jconrel.2004.12.019. [DOI] [PubMed] [Google Scholar]
- 57.De P, Gondi SR, Sumerlin BS. Folate-conjugated thermoresponsive block copolymers: highly efficient conjugation and solution self-assembly. Biomacromolecules. 2008;9(3):1064–70. doi: 10.1021/bm701255v. [DOI] [PubMed] [Google Scholar]
- 58.Mukherjee S, Ghosh RN, Maxfield FR. Endocytosis. Physiol. Rev. 1997;77(3):759–803. doi: 10.1152/physrev.1997.77.3.759. [DOI] [PubMed] [Google Scholar]
- 59.Mellman I. Endocytosis and molecular sorting. Annu. Rev. Cell Dev. Biol. 1996;12:575–625. doi: 10.1146/annurev.cellbio.12.1.575. [DOI] [PubMed] [Google Scholar]
- 60.Vaupel P, Kallinowski F, Okunieff P. Blood-flow, oxygen and nutrient supply, and metabolic microenvironment of human-tumors: a review. Cancer Res. 1989;49(23):6449–65. [PubMed] [Google Scholar]
- 61.Fisher OZ, Peppas NA. Polybasic nanomatrices prepared by UV-initiated photopolymerization. Macromolecules. 2009;42(9):3391–98. doi: 10.1021/ma801966r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fisher O, Kim T, Dietz SR, Peppas NA. Enhanced core hydrophobicity, functionalization and cell penetration of polybasic nanomatrices. Pharm. Res. 2009;26(1):51–60. doi: 10.1007/s11095-008-9704-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hu Y, Litwin T, Nagaraja AR, Kwong B, Katz J, et al. Cytosolic delivery of membrane-impermeable molecules in dendritic cells using pH-responsive core-shell nanoparticles. Nano Lett. 2007;7(10):3056–64. doi: 10.1021/nl071542i. [DOI] [PubMed] [Google Scholar]
- 64.Hu Y, Atukorale PU, Lu JJ, Moon JJ, Um SH, et al. Cytosolic delivery mediated via electrostatic surface binding of protein, virus, or siRNA cargos to pH-responsive core-shell gel particles. Biomacromolecules. 2009;10(4):756–65. doi: 10.1021/bm801199z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Owens DE, Peppas NA. Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. Int. J. Pharm. 2006;307(1):93–102. doi: 10.1016/j.ijpharm.2005.10.010. [DOI] [PubMed] [Google Scholar]; Excellent review highlighting the opsonization and biodistribution of polymeric nanoparticles
- 66.Lee ES, Na K, Bae YH. Doxorubicin loaded pH-sensitive polymeric micelles for reversal of resistant MCF-7 tumor. J. Control. Release. 2005;103(2):405–18. doi: 10.1016/j.jconrel.2004.12.018. [DOI] [PubMed] [Google Scholar]
- 67.Lee ES, Gao Z, Kim D, Park K, Kwan IC, Bae YH. Super pH-sensitive multifunctional polymeric micelle for tumor pHe specific TAT exposure and multidrug resistance. J. Control. Release. 2008;129(3):228–36. doi: 10.1016/j.jconrel.2008.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lee ES, Na K, Bae YH. Super pH-sensitive multifunctional polymeric micelle. Nano Lett. 2005;5(2):325–29. doi: 10.1021/nl0479987. [DOI] [PubMed] [Google Scholar]
- 69.Byrne JD, Betancourt T, Brannon-Peppas L. Active targeting schemes for nanoparticle systems in cancer therapeutics. Adv. Drug Deliv. Rev. 2008;60(15):1615–26. doi: 10.1016/j.addr.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 70.Guelen L, Paterson H, Gäken J, Meyers M, Farzaneh F, Tavassoli M. TAT-apoptin is efficiently delivered and induces apoptosis in cancer cells. Oncogene. 2003;23(5):1153–65. doi: 10.1038/sj.onc.1207224. [DOI] [PubMed] [Google Scholar]
- 71.Sethuraman VA, Bae YH. TAT peptide-based micelle system for potential active targeting of anti-cancer agents to acidic solid tumors. J. Control. Release. 2007;118(2):216–24. doi: 10.1016/j.jconrel.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mathiowitz E, Jacob JS, Jong YS, Carino GP, Chickering DE, et al. Biologically erodable microspheres as potential oral drug delivery systems. Nature. 1997;386(6623):410–14. doi: 10.1038/386410a0. [DOI] [PubMed] [Google Scholar]; This contribution was the first serious effort to use biodegrable microparticles for oral delivery of proteins and DNA
- 73.Leong KW, Brott BC, Langer R. Bioerodible polyanhydrides as drug-carrier matrices. I: Characterization, degradation, and release characteristics. J. Biomed. Mater. Res. 1985;19(8):941–55. doi: 10.1002/jbm.820190806. [DOI] [PubMed] [Google Scholar]
- 74.Cohen S, Yoshioka T, Lucarelli M, Hwang LH, Langer R. Controlled delivery systems for proteins based on poly(lactic/glycolic acid) microspheres. Pharm. Res. 1991;8(6):713–20. doi: 10.1023/a:1015841715384. [DOI] [PubMed] [Google Scholar]
- 75.Shenoy D, Little S, Langer R, Amiji M. Poly(ethylene oxide)-modified poly(β-amino ester) nanoparticles as a pH-sensitive system for tumor-targeted delivery of hydrophobic drugs. 1. In vitro evaluations. Mol. Pharm. 2005;2(5):357–66. doi: 10.1021/mp0500420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shenoy D, Little S, Langer R, Amiji M. Poly(ethylene oxide)-modified poly(β-amino ester) nanoparticles as a pH-sensitive system for tumor-targeted delivery of hydrophobic drugs: Part 2. In vivo distribution and tumor localization studies. Pharm. Res. 2005;22(12):2107–14. doi: 10.1007/s11095-005-8343-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cerritelli S, Velluto D, Hubbell JA. PEG-SS-PPS: reduction-sensitive disulfide block copolymer vesicles for intracellular drug delivery. Biomacromolecules. 2007;8(6):1966–72. doi: 10.1021/bm070085x. [DOI] [PubMed] [Google Scholar]
- 78.Oh JK, Siegart DJ, Lee H, Sherwood G, Peteanu L, et al. Biodegradable nanogels prepared by atom transfer radical polymerization as potential drug delivery carriers: synthesis, biodegradation, in vitro release, and bioconjugation. J. Am. Chem. Soc. 2007;129(18):5939–45. doi: 10.1021/ja069150l. [DOI] [PubMed] [Google Scholar]
- 79.Matsumoto S, Christie RJ, Nishiyama N, Miyata K, Ishii A, et al. Environment-responsive block copolymer micelles with a disulfide cross-linked core for enhanced siRNA delivery. Biomacromolecules. 2009;10(1):119–27. doi: 10.1021/bm800985e. [DOI] [PubMed] [Google Scholar]
- 80.Dufresne MH, Elsabahy M, Leroux JC. Characterization of polyion complex micelles designed to address the challenges of oligonucleotide delivery. Pharm. Res. 2008;25(9):2083–93. doi: 10.1007/s11095-008-9591-6. [DOI] [PubMed] [Google Scholar]
- 81.Davis ME, Chen Z, Shin DM. Nanoparticle therapeutics: an emerging treatment modality for cancer. Nat. Rev. Drug Discov. 2008;7(9):771–82. doi: 10.1038/nrd2614. [DOI] [PubMed] [Google Scholar]
- 82.Duncan R. The dawning era of polymer therapeutics. Nat. Rev. Drug Discov. 2003;2(5):347–60. doi: 10.1038/nrd1088. [DOI] [PubMed] [Google Scholar]; An elegant and extensive overview of the area of polymer therapeutics
- 83.Duncan R. Polymer conjugates as anticancer nanomedicines. Nat. Rev. Cancer. 2006;6(9):688–701. doi: 10.1038/nrc1958. [DOI] [PubMed] [Google Scholar]
- 84.Roberts MJ, Bentley MD, Harris JM. Chemistry for peptide and protein PEGylation. Adv. Drug Deliv. Rev. 2002;54(4):459–76. doi: 10.1016/s0169-409x(02)00022-4. [DOI] [PubMed] [Google Scholar]
- 85.Vicent MJ, Dieudonné L, Carbajo RJ, Pineda-Lucena A. Polymer conjugates as therapeutics: future trends, challenges and opportunities. Expert Opin. Drug Deliv. 2008;5(5):593–614. doi: 10.1517/17425247.5.5.593. [DOI] [PubMed] [Google Scholar]
- 86.Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumortropic accumulation of proteins and the antitumor agent Smancs. Cancer Res. 1986;46(12):6387–92. [PubMed] [Google Scholar]; Landmark study reporting for the first time the significance of passive tumor targeting via the EPR effect by macromolecules
- 87.Minchinton AI, Tannock IF. Drug penetration in solid tumours. Nat. Rev. Cancer. 2006;6(8):583–92. doi: 10.1038/nrc1893. [DOI] [PubMed] [Google Scholar]
- 88.Pasut G, Veronese FM. Polymer-drug conjugation, recent achievements and general strategies. Prog. Polym. Sci. 2007;32:933–61. [Google Scholar]
- 89.Heredia KL, Maynard HD. Synthesis of protein-polymer conjugates. Org. Biomol. Chem. 2007;5(1):45–53. doi: 10.1039/b612355d. [DOI] [PubMed] [Google Scholar]
- 90.Bontempo D, Maynard HD. Streptavidin as a macroinitiator for polymerization: in situ protein-polymer conjugate formation. J. Am. Chem. Soc. 2005;127(18):6508–509. doi: 10.1021/ja042230+. [DOI] [PubMed] [Google Scholar]
- 91.Boyer C, Bulmus V, Liu J, Davis TP, Stenzel MH, Barner-Kowollik C. Well-defined protein-polymer conjugates via in situ RAFT polymerization. J. Am. Chem. Soc. 2007;129(22):7145–54. doi: 10.1021/ja070956a. [DOI] [PubMed] [Google Scholar]
- 92.Brocchini S, Duncan R. Pendant drugs, release from polymers. In: Mathiowitz E, editor. Encyclopedia of Controlled Drug Delivery. Wiley; New York: 1999. pp. 786–816. [Google Scholar]
- 93.Harris JM, Chess RB. Effect of pegylation on pharmaceuticals. Nat. Rev. Drug Discov. 2003;2(3):214–21. doi: 10.1038/nrd1033. [DOI] [PubMed] [Google Scholar]
- 94.Ringsdorf H. Structure and properties of pharmacologically active polymers. J. Polymer Sci. Part C. 1975;51(1):135–53. [Google Scholar]; A milestone paper that inspired the field of polymer-anticancer conjugates
- 95.Duncan R, Kopecek J. Soluble synthetic-polymers as potential drug carriers. Adv. Polym. Sci. 1984;57:51–101. [Google Scholar]
- 96.Duncan R, Lloyd JB, Kopecek J. Degradation of side-chains of N-(2-hydroxypropyl) methacrylamide copolymers by lysosomal enzymes. Biochem. Biophys. Res. Commun. 1980;94(1):284–90. doi: 10.1016/s0006-291x(80)80218-x. [DOI] [PubMed] [Google Scholar]
- 97.Khandare J, Minko T. Polymer-drug conjugates: progress in polymeric prodrugs. Prog. Polym. Sci. 2006;31(4):359–97. [Google Scholar]
- 98.Kopeček J, Kopečková P, Minko T, Lu Z-R. HPMA copolymer-anticancer drug conjugates: design, activity, and mechanism of action. Eur. J. Pharm. Biopharm. 2000;50(1):61–81. doi: 10.1016/s0939-6411(00)00075-8. [DOI] [PubMed] [Google Scholar]
- 99.Satchi-Fainaro R, Puder M, Davies JW, Tran HT, Sampson DA, et al. Targeting angiogenesis with a conjugate of HPMA copolymer and TNP-470. Nat. Med. 2004;10(3):255–61. doi: 10.1038/nm1002. [DOI] [PubMed] [Google Scholar]
- 100.Chesler L, Goldenberg DD, Seales IT, Satchi-Fainaro RS, Grimmer M, et al. Malignant progression and blockade of angiogenesis in a murine transgenic model of neuroblastoma. Cancer Res. 2007;67:9435–42. doi: 10.1158/0008-5472.CAN-07-1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Satchi-Fainaro R, Mamluk R, Wang L, Short SM, Nagy JA, et al. Inhibition of vessel permeability by TNP-470 and its polymer conjugate, caplostatin. Cancer Cell. 2005;7(3):251–61. doi: 10.1016/j.ccr.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 102.Khandare JJ, Jayant S, Singh A, Chandna P, Wang Y, et al. Dendrimer versus linear conjugate: influence of polymeric architecture on the delivery and anticancer effect of paclitaxel. Bioconjug. Chem. 2006;17(6):1464–72. doi: 10.1021/bc060240p. [DOI] [PubMed] [Google Scholar]
- 103.Abuchowski A, McCoy JR, Palczuk NC, van Es T, Davis FF. Effect of covalent attachment of polyethyleneglycol on immunogenicity and circulating life of bovine liver catalase. J. Biol. Chem. 1977;252(11):3582–86. [PubMed] [Google Scholar]
- 104.Abuchowski A, van Es T, Palczuk NC, Davis FF. Alteration of immunological properties of bovine serum albumin by covalent attachment of polyethylene glycol. J. Biol. Chem. 1977;252(11):3578–81. [PubMed] [Google Scholar]
- 105.Veronese FM, Mero A. The impact of PEGylation on biological therapies. Biodrugs. 2008;22(5):315–29. doi: 10.2165/00063030-200822050-00004. [DOI] [PubMed] [Google Scholar]
- 106.Duncan R, Gilbert HRP, Carbajo RJ, Vicent MJ. Polymer masked-unmasked protein therapy. 1. Bioresponsive dextrin-trypsin and -melanocyte stimulating hormone conjugates designed for α-amylase activation. Biomacromolecules. 9(4):1146–54. doi: 10.1021/bm701073n. [DOI] [PubMed] [Google Scholar]
- 107.Nishiyama N, Kataoka K. Current state, achievements, and future prospects of polymeric micelles as nanocarriers for drug and gene delivery. Pharmacol. Ther. 2006;112(3):630–48. doi: 10.1016/j.pharmthera.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 108.Wiradharma N, Zhang Y, Venkataraman S, Hedrick JL, Yang YY. Self-assembled polymer nanostructures for delivery of anticancer therapeutics. Nano Today. 2009;4(4):302–17. [Google Scholar]
- 109.Bae Y, Fukushima S, Harada A, Kataoka K. Design of environment-sensitive supramolecular assemblies for intracellular drug delivery: polymeric micelles that are responsive to intracellular pH change. Angew. Chem. Int. Ed. 2003;42(38):4640–43. doi: 10.1002/anie.200250653. [DOI] [PubMed] [Google Scholar]
- 110.Bae Y, Nishiyama N, Fukushima S, Koyama H, Yasuhiro M, Kataoka K. Preparation and biological characterization of polymeric micelle drug carriers with intracellular pH-triggered drug release property: tumor permeability, controlled subcellular drug distribution, and enhanced in vivo antitumor efficacy. Bioconjug. Chem. 2005;16(1):122–30. doi: 10.1021/bc0498166. [DOI] [PubMed] [Google Scholar]
- 111.Han M, Bae Y, Nishiyama N, Miyata K, Oba M, Kataoka K. Transfection study using multicellular tumor spheroids for screening non-viral polymeric gene vectors with low cytotoxicity and high transfection efficiencies. J. Control. Release. 2007;121(1--2):38–48. doi: 10.1016/j.jconrel.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 112.Kang SI, Na K, Bae YH. Physicochemical characteristics and doxorubicin-release behaviors of pH/temperature-sensitive polymeric nanoparticles. Colloids Surf. A. 2003;231(1--3):103–12. [Google Scholar]
- 113.Soppimath KS, Liu L-H, Seow WY, Liu S-Q, Powell R, et al. Multifunctional core/shell nanoparticles self-assembled from pH-induced thermosensitive polymers for targeted intracellular anticancer drug delivery. Adv. Funct. Mater. 2007;17(3):355–62. [Google Scholar]
- 114.Bae Y, Jang W-D, Nishiyama N, Fukushima S, Kataoka K. Multifunctional polymeric micelles with folate-mediated cancer cell targeting and pH-triggered drug releasing properties for active intracellular drug delivery. Mol. Biosyst. 2005;1(3):242–50. doi: 10.1039/b500266d. [DOI] [PubMed] [Google Scholar]
- 115.Bae Y, Nishiyama N, Kataoka K. In vivo antitumor activity of the folate-conjugated pH-sensitive polymeric micelle selectively releasing adriamycin in the intracellular acidic compartments. Bioconjug. Chem. 2007;18(4):1131–39. doi: 10.1021/bc060401p. [DOI] [PubMed] [Google Scholar]
- 116.Weitman SD, Lark RH, Coney LR, Fort DW, Frasca V, et al. Distribution of the folate receptor GP38 in normal and malignant-cell lines and tissues. Cancer Res. 1992;52(12):3396–401. [PubMed] [Google Scholar]
- 117.Greco F, Vicent MJ, Gee S, Jones AT, Gee J, et al. Investigating the mechanism of enhanced cytotoxicity of HPMA copolymer-Dox-AGM in breast cancer cells. J. Control. Release. 2007;117(1):28–39. doi: 10.1016/j.jconrel.2006.10.012. [DOI] [PubMed] [Google Scholar]
- 118.Greco F, Vicent MJ, Penning NA, Nicholson RI, Duncan R. HPMA copolymer-aminoglutethimide conjugates inhibit aromatase in MCF-7 cell lines. J. Drug Target. 2005;13(8--9):459–70. doi: 10.1080/10611860500383788. [DOI] [PubMed] [Google Scholar]
- 119.Lammers T, Subr V, Ulbrich K, Peschke P, Huber PE, et al. Simultaneous delivery of doxorubicin and gemcitabine to tumors in vivo using prototypic polymeric drug carriers. Biomaterials. 2009;30(20):3466–75. doi: 10.1016/j.biomaterials.2009.02.040. [DOI] [PubMed] [Google Scholar]
- 120.Hilt JZ, Byrne ME. Configurational biomimesis in drug delivery: molecular imprinting of biologically significant molecules. Adv. Drug Deliv. Rev. 2004;56(11):1599–620. doi: 10.1016/j.addr.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 121.Kryscio DR, Peppas NA. Mimicking biological delivery through feedback-controlled drug release systems based on molecular imprinting. AIChE J. 2009;55(6):1311–24. doi: 10.1002/aic.11779. [DOI] [PMC free article] [PubMed] [Google Scholar]; Excellent review of recent advances of feedback controlled release systems
- 122.Alvarez-Lorenzo C, Concheiro A. Molecularly imprinted polymers for drug delivery. J. Chromatogr. B. 2004;804(1):231–45. doi: 10.1016/j.jchromb.2003.12.032. [DOI] [PubMed] [Google Scholar]
- 123.Ali M, Byrne ME. Controlled release of high molecular weight hyaluronic acid from molecularly imprinted hydrogel contact lenses. Pharm. Res. 2009;26(3):714–26. doi: 10.1007/s11095-008-9818-6. [DOI] [PubMed] [Google Scholar]
- 124.Ali M, Horikawa S, Venkatesh S, Saha J, Hong JW, Byrne ME. Zero-order therapeutic release from imprinted hydrogel contact lenses within in vitro physiological ocular tear flow. J. Control. Release. 2007;124(3):154–62. doi: 10.1016/j.jconrel.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 125.Alvarez-Lorenzo C, Hiratani H, Gómez-Amoza JL, Martínez-Pacheco R, Souto C, Conchiero A. Soft contact lenses capable of sustained delivery of timolol. J. Pharm. Sci. 2002;91(10):2182–92. doi: 10.1002/jps.10209. [DOI] [PubMed] [Google Scholar]
- 126.Hiratani H, Alvarez-Lorenzo C. Timolol uptake and release by imprinted soft contact lenses made of N,N-diethylacrylamide and methacrylic acid. J. Control. Release. 2002;83(2):223–30. doi: 10.1016/s0168-3659(02)00213-4. [DOI] [PubMed] [Google Scholar]
- 127.Hiratani H, Alvarez-Lorenzo C. The nature of backbone monomers determines the performance of imprinted soft contact lenses as timolol drug delivery systems. Biomaterials. 2004;25(6):1105–13. doi: 10.1016/s0142-9612(03)00622-7. [DOI] [PubMed] [Google Scholar]
- 128.Hiratani H, Fujiwara A, Tamiya Y, Mizutani Y, Alvarez-Lorenzo C. Ocular release of timolol from molecularly imprinted soft contact lenses. Biomaterials. 2005;26(11):1293–98. doi: 10.1016/j.biomaterials.2004.04.030. [DOI] [PubMed] [Google Scholar]
- 129.Venkatesh S, Sizemore SP, Byrne ME. Biomimetic hydrogels for enhanced loading and extended release of ocular therapeutics. Biomaterials. 2007;28(4):717–24. doi: 10.1016/j.biomaterials.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 130.Venkatesh S, Saha J, Pass S, Byrne ME. Transport and structural analysis of molecular imprinted hydrogels for controlled drug delivery. Eur. J. Pharm. Biopharm. 2008;69(3):852–60. doi: 10.1016/j.ejpb.2008.01.036. [DOI] [PubMed] [Google Scholar]
- 131.Pack DW, Hoffman AS, Pun S, Stayton PS. Design and development of polymers for gene delivery. Nat. Rev. Drug Discov. 2005;4(7):581–93. doi: 10.1038/nrd1775. [DOI] [PubMed] [Google Scholar]
- 132.Whitehead KA, Langer R, Anderson DG. Knocking down barriers: advances in siRNA delivery. Nat. Rev. Drug Discov. 2009;8(2):129–38. doi: 10.1038/nrd2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Verma A, Uzun O, Hu Y, Hu Y, Han H-S, et al. Surface-structure-regulated cell-membrane penetration by monolayer-protected nanoparticles. Nat. Mater. 2008;7(7):588–95. doi: 10.1038/nmat2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Yessine M-A, Leroux J-C. Membrane-destabilizing polyanions: interaction with lipid bilayers and endosomal escape of biomacromolecules. Adv. Drug Deliv. Rev. 2004;56:999–1021. doi: 10.1016/j.addr.2003.10.039. [DOI] [PubMed] [Google Scholar]
- 135.Kurisawa M, Yokoyama M, Okano T. Transfection efficiency increases by incorporating hydrophobic monomer units into polymeric gene carriers. J. Control. Release. 2000;68(1):1–8. doi: 10.1016/s0168-3659(00)00246-7. [DOI] [PubMed] [Google Scholar]
- 136.Lackey CA, Press OW, Hoffman AS, Stayton PS. A biomimetic pH-responsive polymer directs endosomal release and intracellular delivery of an endocytosed antibody complex. Bioconjug. Chem. 2002;13(5):996–1001. doi: 10.1021/bc010053l. [DOI] [PubMed] [Google Scholar]
- 137.Murthy N, Robichaud JR, Tirrell DA, Stayton PS, Hoffman AS. The design and synthesis of polymers for eukaryotic membrane disruption. J. Control. Release. 1999;61(1--2):137–43. doi: 10.1016/s0168-3659(99)00114-5. [DOI] [PubMed] [Google Scholar]
- 138.Murthy N, Chang I, Stayton P, Hoffman A. pH-sensitive hemolysis by random copolymers of alkyl acrylates and acrylic acid. Macromol. Symp. 2001;172:49–55. [Google Scholar]
- 139.Lee ES, Kim D, Youn YS, Oh KT, Bae YH. A virus-mimetic nanogel vehicle. Angew. Chem. Int. Ed. 2008;47(13):2418–21. doi: 10.1002/anie.200704121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Convertine AJ, Benoit DSW, Duvall CL, Hoffman AS, Stayton PS. Development of a novel endosomolytic diblock copolymer for siRNA delivery. J. Control. Release. 2009;133(3):221–29. doi: 10.1016/j.jconrel.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Chen RJ, Khormaee S, Eccleston ME, Slater NKH. Effect of l-leucine graft content on aqueous solution behavior and membrane-lytic activity of a pH-responsive pseudopeptide. Biomacromolecules. 2009;10(9):2601–608. doi: 10.1021/bm900534j. [DOI] [PubMed] [Google Scholar]
- 142.Chen R, Khormaee S, Eccleston ME, Slater NKH. The role of hydrophobic amino acid grafts in the enhancement of membrane-disruptive activity of pH-responsive pseudo-peptides. Biomaterials. 2009;30(10):1954–61. doi: 10.1016/j.biomaterials.2008.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Chen RJ, Yue Z, Eccleston ME, Slater NKH. Aqueous solution behaviour and membrane disruptive activity of pH-responsive PEGylated pseudo-peptides and their intracellular distribution. Biomaterials. 2008;29(32):4333–40. doi: 10.1016/j.biomaterials.2008.07.040. [DOI] [PubMed] [Google Scholar]
- 144.Chen RJ, Yue Z, Eccleston ME, Williams S, Slater NKH. Modulation of cell membrane disruption by pH-responsive pseudo-peptides through grafting with hydrophilic side chains. J. Control. Release. 2005;108(1):63–72. doi: 10.1016/j.jconrel.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 145.Liechty WB, Chen R, Farzaneh F, Tavassoli M, Slater NKH. Synthetic pH-responsive polymers for protein transduction. Adv. Mater. 2009;21(38--39):3910–14. doi: 10.1002/adma.200901733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Owens DE, Eby JK, Jian Y, Peppas NA. Temperature-responsive polymer-gold nanocomposites as intelligent therapeutic systems. J. Biomed. Mater. Res. Part A. 2007;83A(3):692–95. doi: 10.1002/jbm.a.31284. [DOI] [PubMed] [Google Scholar]
- 147.Sershen SR, Westcott SL, West JL, Halas NJ. An opto-mechanical nanoshell-polymer composite. Appl. Phys. B. 2001;73(4):379–81. [Google Scholar]
- 148.Satarkar NS, Hilt JZ. Magnetic hydrogel nanocomposites for remote controlled pulsatile drug release. J. Control. Release. 2008;130(3):246–51. doi: 10.1016/j.jconrel.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 149.Arias JL, Gallardo V, Ruiz MA, Delgado AV. Magnetite/poly(alkylcyanoacrylate) (core/shell) nanoparticles as 5-fluorouracil delivery systems for active targeting. Eur. J. Pharm. Biopharm. 2008;69(1):54–63. doi: 10.1016/j.ejpb.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 150.Mornet S, Vasseur S, Grasset F, Duguet E. Magnetic nanoparticle design for medical diagnosis and therapy. J. Mater. Chem. 2004;14(14):2161–75. [Google Scholar]
RELATED RESOURCES
- Mikos AG, Murphy R, Bernstein H, Peppas NA, editors. Biomaterials for Drug and Cell Delivery. Mater. Res. Soc.; Pittsburgh, PA: 1994. p. 290. [Google Scholar]
- Peppas NA. Hydrogels in Medicine and Pharmacy, Vol. 3: Properties and Applications. CRC Press; Boca Raton, FL: 1987. p. 196. [Google Scholar]
- Peppas NA, Hilt JZ, Khademhosseini A, Langer R. Hydrogels in biology and medicine: from fundamentals to bionanotechnology. Adv. Mater. 2006;18:1345–60. [Google Scholar]
- Peppas NA, Hoffman AS, Kanamori T, Tojo K, editors. Advances in Medical Engineering, Drug Delivery Systems and Therapeutic Systems. AIChE; New York: 2006. p. 342. [Google Scholar]
- Peppas NA, Huang Y, Torres-Lugo M, Ward JH, Zhang J. Physicochemical foundations and structural design of hydrogels in medicine and biology. Annu. Rev. Biomed. Eng. 2000;2:9–29. doi: 10.1146/annurev.bioeng.2.1.9. [DOI] [PubMed] [Google Scholar]
- Slaughter B, Khurshid S, Fisher OZ, Khademhosseini A, Peppas NA. Hydrogels in regenerative medicine. Adv. Mater. 2009;21:3307–29. doi: 10.1002/adma.200802106. [DOI] [PMC free article] [PubMed] [Google Scholar]