Abstract
Background & Aims
Necrotizing enterocolitis (NEC) is the most common gastrointestinal emergency of premature infants. While the effect of bile acids (BAs) on intestinal mucosal injury is known, we investigated the contribution of BAs during the development of NEC in neonatal rats.
Methods
Premature rats were fed with cow’s milk–based formula and subjected to asphyxia and cold stress to develop NEC. Jejunal and ileal luminal BAs, portal blood BAs, and messenger RNA and protein for the apical sodium-dependent bile acid transporter, the ileal bile acid binding protein, and the heteromeric organic solute transporter (Ostα/Ostβ)were evaluated.
Results
Ileal luminal BAs levels were increased significantly during disease development and the removal of ileal BAs significantly decreased the incidence and severity of disease. Furthermore, when NEC was reduced via treatment with epidermal growth factor (EGF), BA levels were reduced significantly. Jejunal luminal BA levels were similar between animals with NEC and controls, but portal/ileal luminal BA ratios were decreased significantly in animals with NEC. The apical sodium-dependent bile acid transporter was up-regulated at the site of injury in animals with NEC and decreased after EGF treatment; however, the ileal bile acid binding protein was up-regulated only in the NEC and EGF group. Ostα/Ostβ expression was low in all groups, and only slightly increased in the NEC group.
Conclusions
These data strongly suggest that BAs play a role in the development of ileal damage in experimental NEC and that alterations in BA transport in the neonatal ileum may contribute to disease development.
Necrotizing enterocolitis (NEC) is a common and devastating gastrointestinal disease predominantly occurring in prematurely born infants. Severe NEC is characterized by an extensive hemorrhagic inflammatory necrosis of the distal ileum and proximal colon.1 The pathophysiology of this disease remains poorly understood; however, prematurity, enteral feeding, intestinal hypoxia-ischemia, and bacterial colonization are considered major risk factors.2,3
Currently, one of the best animal models used to study NEC is the neonatal rat model.4–7 In this model, NEC is developed in prematurely born rats by feeding milk-based formula coupled with exposure to hypoxia and cold stress. The major advantage of the neonatal rat NEC model is that many clinical and pathologic changes are similar to those found in human beings; the abdomen is distended, blood is detected in stool, and the ileum and proximal colon are the most affected parts of the intestine. 8 In addition, the major risk factors for human NEC (intestinal immaturity, enteral formula feeding, and hypoxia/ ischemia) are essential factors to develop disease in the rat model.3,9,10
Recently, we showed that overproduction of proinflammatory mediators in the liver are correlated with the progression of intestinal damage in an experimental rat model of NEC.11 In addition, when Kupffer cells, the resident hepatic macrophage, were inhibited in animals with NEC, the incidence and severity of NEC was decreased significantly.11 These results clearly suggest the importance of the liver in the pathophysiology of NEC through the release of inflammatory mediators into the biliary system, which can exacerbate injury in the intestine.
Enterohepatic circulation of bile acids (BAs) is an essential process involving coordinated regulation of BA synthesis in the liver, transport of BAs from the liver to the intestine, and transport of BAs via portal blood back to the liver. BA homeostasis is regulated in the distal ileum through transport from the apical surface of enterocytes into the cell by the apical sodium-dependent bile acid transporter (ASBT).12 BAs then can be bound to the ileal bile acid binding protein (IBABP) for transport across the enterocyte. The recently described heteromeric organic solute transporter (Ostα/Ostβ) may be a mechanism by which BAs then are removed from the cell and transported into portal circulation.13 In the intestine, ASBT, IBABP, and Ostα/Ostβ are expressed predominantly in the terminal ileum,13–16 the site of injury in NEC. In normal rat ileum, ASBT and IBABP undergo biphasic regulation; they are expressed in the fetus, down-regulated in the neonate, then up-regulated again at weaning.17–22 The developmental expression patterns of Ostα/Ostβ have not yet been reported.
When enterohepatic circulation is altered, accumulation of BAs in the intestine can result in damage to the intestinal epithelium.23–25 The effect of cytotoxic BAs on the development of intestinal mucosal injury is known. Because the ileal damage observed during development of experimental NEC is exacerbated by hepatic proinflammatory mediators11 and occurs at the site of BA absorption,14–16 we investigated if BAs play a role in intestinal damage during NEC using a neonatal rat model.
Materials and Methods
Development of Experimental NEC
This protocol was approved by the Animal Care and Use Committee of the University of Arizona (A-324801-95081). Sprague–Dawley rats (Charles River Labs, Pontage, MI) were collected by caesarian section 1 day before scheduled birth. Pups were hand fed with cow’s milk– based rat milk substitute formula (NEC, n = 20),26 and stressed twice daily with asphyxia (breathing 100% nitrogen gas for 60 seconds) followed by cold (4°C for 10 minutes) to induce experimental NEC. For comparison, dam-fed (DF, n = 25) littermates also were asphyxia and cold stressed. By using this method, 70%–80% of the formula-fed rats developed NEC vs 0% of the DF rats.8,27 After 96 hours, all surviving animals were killed via decapitation.8,27,28
Epidermal Growth Factor Treatment
Rats collected via caesarian section 1 day before scheduled birth were hand fed with cow’s milk– based rat milk substitute formula supplemented with 500 ng/mL epidermal growth factor (EGF) (NEC + EGF, n = 20). In previous publications, we have found that the incidence of NEC was reduced by approximately 45%–55% in pups supplemented with EGF.8,28
BA Gavage
Two separate protocols for introducing exogenous BA were used. In the first, bile acid gavage– high (BAG-high), 2-day-old rats were fasted for 18 hours before a single gavaged dose of 50 mmol/L sodium deoxycholic acid (NaDOC). Two hours after gavage, ileal tissue was removed and processed for histology and RNA extraction. To induce full necrosis, a second bolus of 50 mmol/L NaDOC was gavaged 1 hour after the first in a separate group of animals. In the second protocol, newborn rats were given 20 mmol/L NaDOC twice per day for 2 days (BAG-low). Control rats were gavaged with vehicle (phosphate-buffered saline). Pups were dam fed during the 2-day study, but fasted for 8 hours before NaDOC gavage.
BA Sequestration
To examine the effects of BA sequestration on the development of NEC, animals were hand fed with cow’s milk–based rat milk substitute formula, asphyxia and cold stressed twice per day, and gavaged with either 120 mg/kg/day cholestyramine (Eon Labs, Laurelton, NY) or vehicle (phosphate-buffered saline) for 4 days. These groups were designated NEC + Chol (n = 15) and NEC (n = 16), respectively.
NEC Evaluation
Pathologic changes in intestinal architecture were evaluated using our previously published NEC scoring system.8,11,27–29 Histologic changes in the ileum were scored by a blinded evaluator and graded as follows: 0 (normal), no damage; 1 (mild), slight submucosal and/or lamina propria separation; 2 (moderate), moderate separation of submucosa and/or lamina propria, and/or edema in submucosal and muscular layers; 3 (severe), severe separation of submucosa and/or lamina propria, and/or severe edema in submucosa and muscular layers, region villous sloughing; 4 (necrosis), loss of villi and necrosis. Intermediate scores of .5, 1.5, 2.5, and 3.5 also were used to assess more accurately the levels of ileal damage when necessary.8,11,27–29 To determine the incidence of NEC, animals with histologic scores of less than 2 have not developed NEC and animals with histologic scores of 2 or greater have developed NEC.8,11,27–29
Total BA Levels
Intestinal luminal BA levels were determined by flushing a 3-cm section of either distal ileum or proximal jejunum with .4 mL sterile cold phosphate-buffered saline.11 Samples were frozen at −70°C until assayed. Portal blood was removed from the portal vein of anesthetized animals before death. Because of the technical difficulty, usable quantities of portal blood could not be obtained from all animals. Trunk (systemic) blood was collected after decapitation. Serum from portal and systemic blood was collected and frozen at −70°C until assayed. Total BA levels were determined from ileal flushes and from portal and systemic blood using the Total Bile Acids Assay Kit (Diazyme, San Diego, CA) according to the manufacturer’s protocol.
BA Composition
BA composition from ileal luminal contents was determined by the Arizona Cancer Center Analytical Core Shared Service (Tucson, AZ) using a TSQ Quantum triple quadruple LC/MS/MS system with a Surveyor MS pump and autosampler (Thermo Finnigan, San Jose, CA). A mass spectrometer was run in negative ion mode using electrospray ionization. Detection was through selective ion monitoring. Chromatographic separation was obtained using a Beckman Ultrasphere-XL C-18 column (Beckman, Palo Alto, CA). Isocratic solvent flow was used, consisting of 75% methanol and 25% ammonium acetate at a flow rate of .3 mL/min. Cholic acid, deoxycholic acid, lithocholic acid, chenodeoxycholic acid, and ursodeoxycholic acid levels were monitored.
RNA Preparation
Total RNA was isolated from ileal tissue using the RNeasy Mini Kit (Qiagen, Santa Clarita, CA) as described in the manufacturer’s protocol. All samples were incubated with RNase-free DNase (20 U per reaction) for 10 minutes at 37°C to eliminate DNA contamination. RNA concentration was quantified by ultraviolet spectrophotometry at 260 nm and the purity was determined by the A260/A280 ratio (SPECTRAmax PLUS; Molecular Devices, Sunnyvale, CA). The integrity of RNA was verified by electrophoresis on a 1.2% agarose gel containing formaldehyde (2.2 mol/L) and ethidium bromide in 1 × 3-(N-morpholino)propanesulfonic acid buffer (40 mmol/L 3-(N-morpholino)propanesulfonic acid, pH 7.0, 10 mmol/L sodium acetate, and 1 mmol/L ethylenediaminetetraacetic acid, pH 8.0). Only tissue from animals without full necrosis were extracted and analyzed.
Reverse-Transcription and Real-Time Polymerase Chain Reaction
Real-time polymerase chain reaction assays were performed specifically to quantify steady-state messenger RNA (mRNA) levels. Single-stranded complementary DNA was reverse-transcribed from 1 µg of total RNA in a 10 µL reaction mixture, as previously described in detail.30 The amounts of total RNA used in the reverse-transcribed reactions were calculated from the absorbency at 260 nm, and verified by densitometry of the 28S ribosomal RNA band separated on denaturing agarose gels (by Gel Doc 1000 Documentation System with Molecular Analyst/PC software; Bio-Rad, Hercules, CA). Real-time polymerase chain reaction amplification31,32 was performed using Assay By Design (Applied Biosystems, Foster City, CA) sequenced primers and probes for rat ASBT, IBABP, and OstαOstβ. ASBT primer, forward probe, and reverse probe sequences: TGGACCTCAGTGTTAGC, GGCTCCAATATCCTGGCCTAT, and AGCAAGCAGTGTGGAGCAAGT. IBABAP primer, forward probe, and reverse probe sequences: CACGGTTGCCTTGAA, CAAAGAATGTGAAATGCAGACCAT, and ACCTTGCCACCCTCCATCTT. Ostα primer, forward probe, and reverse probe sequences: CTGCCCACCCCTCATAC, CTGCTGCTGCTGTCCCT, and GCAACTGTAGCTTCTTCCTGGTAA. Ostβ primer, forward probe, and reverse probe sequences: TGGCTCCAATATCCTGGCCTAT, TATTCCATCCTGGTT, and AAGAAGCAGCCACAAGACAACG. Predeveloped TaqMan primers and probe were used for detection of farnesoid X receptor (FXR) (Applied Biosystems). Samples were subjected to 40 cycles of amplification at 95° for 15 seconds followed by 1 minute at 60° using a GeneAmp 5700 Sequence Detection System (Applied Biosystems) according to the manufacturer’s instructions. Water controls were included to ensure specificity. All mRNA levels were normalized according to the level of 18S within each sample, which was used as an endogenous control to ensure the accuracy and integrity of the tissue samples. Cleavage of the sequence-specific probe by Taq polymerase created an increase in fluorescent signal, which was observed during the exponential phase of amplification and allowed the determination of a threshold value for all samples. This threshold value, once normalized, was expressed as a fold change of gene expression relative to control samples.11,27
Immunohistology
A portion of ileal tissue was removed and fixed overnight in 70% ethanol, paraffin embedded, and serial sectioned at 4–6 µm. Briefly, after deparaffinizing, sections were blocked with 1.5% appropriate serum (Vector Laboratories, Burlingame, CA), incubated with either anti-rat ASBT (kindly provided by Dr Stelzner, University of California at Los Angeles, Los Angeles, CA), anti-rat IBABP (kindly provided by Dr Crossman, Children’s Hospital Medical Center, Cincinnati, OH), anti-rat Ostα or anti-rat Ostβ followed by biotinylated secondary antibody (Vector Laboratories), Vectastain Elite ABC reagent (Vector Laboratories), diaminobenzidine, and counterstained with hematoxylin. Ost antibodies were raised in rabbits using synthetic peptides corresponding to amino acids 315–329 of rat Ostα (MYYRKKDNKVGYEAC), and 90–103 of rat Ostβ (ILRETLISEKADLA). Peptides were coupled to keyhole limpet hemocyanin and used to immunize New Zealand white rabbits (AnaSpec, San Jose, CA). Each of the antibodies was affinity purified using their respective peptide antigen (AnaSpec). No immunostaining was observed in the controls. Sections from experimental groups were stained for a specific primary antibody at the same time so that comparisons between groups could be assessed.
Western Blot
Individual frozen ileum samples were homogenized with a hand-held homogenizer (Pellet Pestle; Kimble/Kontes, Vineland, NJ) in a 5× volume of ice-cold homogenization buffer (50 mmol/L Tris HCl, pH 7.4, 150 mmol/L NaCl, 1 mmol/L ethylenediaminetetraacetic acid, .1% sodium dodecyl sulfate, 1% Na-deoxycholic acid, 1% Triton X-100, 50 mmol/L dithiothreitol, 50 µg/mL aprotinin, 50 µg/mL leupeptin, and 5 mmol/L phenylmethylsulfonyl fluoride). The homogenates were centrifuged at 10,000 rpm for 5 minutes at 4°C and the supernatant was collected. The total protein concentration was quantified using the Bradford protein assay. 4 For protein analysis, 50 g of protein was added to an equal volume of 2× Laemmli sample buffer and boiled for 5 minutes. The samples were run on a 10%–20% gradient polyacrylamide gel (Bio-Rad) at 95 V for 1 hour. Protein was transferred to Immuno-Blot polyvinylidene difluoride membranes (Bio-Rad) at 15 V for 1 hour. Membranes were blocked with 5% nonfat milk in Tris-buffered saline with .1% Tween 20 (Sigma, St. Louis, MO) for 1 hour at room temperature and then incubated with anti-ASBT, anti-IBABP, anti-Ostα, or anti-Ostβ antibody overnight at 4°C. After being washed, the membranes were incubated for 1 hour at room temperature with the appropriate horseradish-peroxidase–conjugated secondary antibody. Proteins were visualized with a chemiluminescent system (Pierce, Rockford, IL) and exposed to radiographic film. Membranes were stripped and probed with anti–β-actin to determine equal loading of protein. Densitometry was performed to compare protein expression between groups using QuantityOne software (Bio-Rad).
Statistics
Statistical analyses between groups were performed using analysis of variance followed by Fisher protected least significant difference. Correlation analyses were performed using Spearman rank correlation. Analysis of NEC score was performed using the Mann–Whitney test for nonparametric values. The χ2 test was used to analyze differences in disease incidence. All statistical analyses were determined using the statistical program StatView (Abacus Concepts, Berkeley, CA). All numeric data are expressed as mean ± SEM.
Results
Increased BAs in the Luminal Contents of Rats With NEC
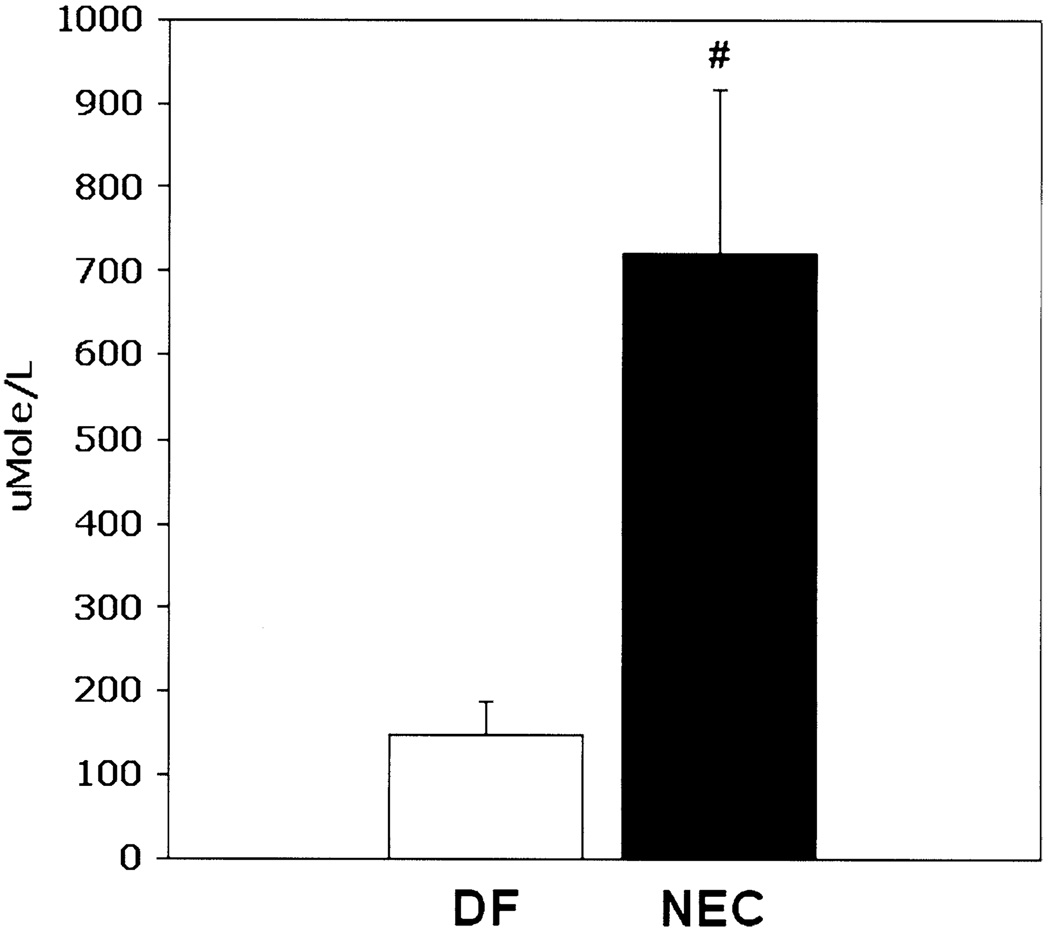
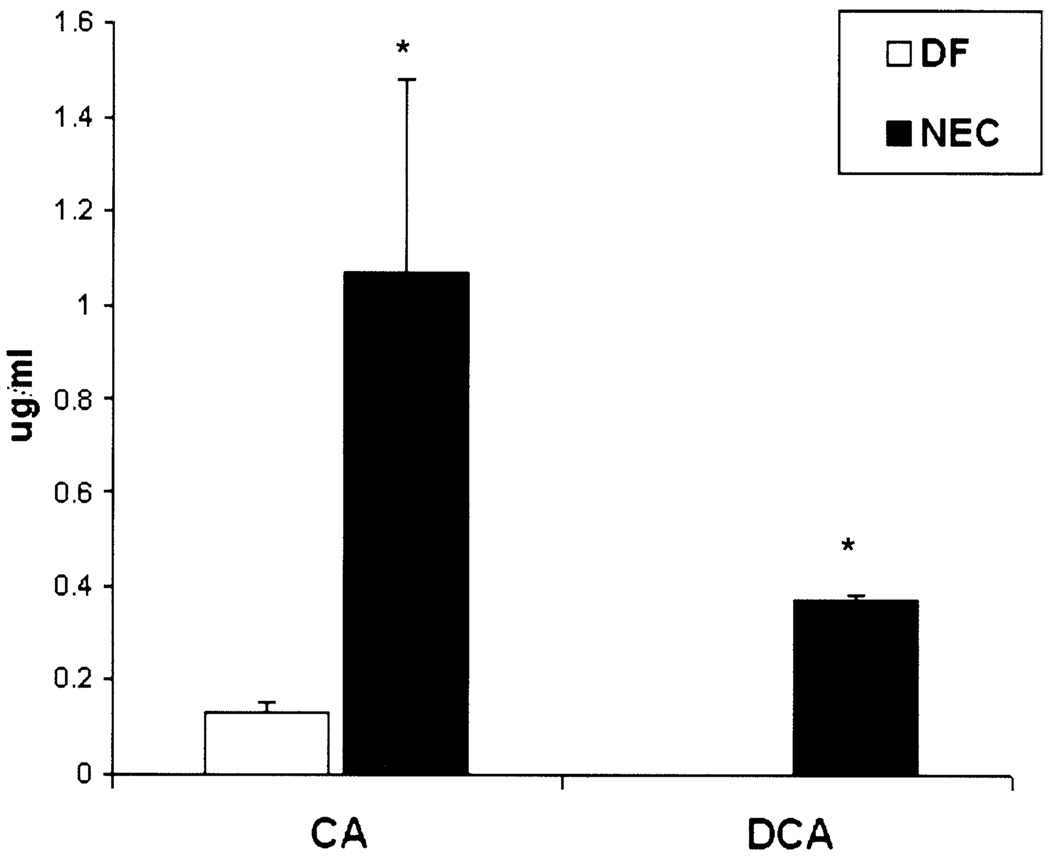
To determine if BAs play a role in NEC pathogenesis, we evaluated total BA levels in ileal luminal flushes from DF and NEC groups. Total BA levels were increased significantly in the ileal luminal contents of neonatal rats with NEC (Figure 1). Serum BA levels were similar between groups (data not shown). Animals with NEC had significantly higher levels of cholic acid compared with control and DF animals, deoxycholic acid was detected only in the NEC group (Figure 2). No other toxic BAs were detected in either group.
Figure 1.
Total BA levels are increased during NEC. Total BA levels from ileal luminal flushes from NEC and DF groups. #P ≤ .01.
Figure 2.
BA composition of ileal luminal contents. Ileal luminal contents were examined using LC/MS/MS for specific BA composition. Three separate samples from each group were tested; each sample consisted of pooled ileal luminal contents from 4 different animals. *P ≤ .05. □, DF; ■, NEC.
Ileal/Jejunal and Portal/Ileal BA Ratios in NEC
Levels of ileal BAs can be affected by both the amount of BA produced by the liver and the transport of ileal luminal BAs out of the ileal lumen via enterohepatic circulation. Because direct cannulation of the bile duct is not possible in 4-day-old rats, we evaluated total BA levels in the proximal jejunal contents. The overall levels of jejunal BAs were similar between DF animals and those with NEC. However, the ileal/jejunal total BA ratio from the NEC group was significantly higher than the DF group (Table 1). Under normal circumstances, increased ileal BA levels should correspond to increased portal blood BA levels in an effort to clear excess BA from the ileum. However, animals with NEC had significantly decreased portal/luminal BA ratios compared with DF controls despite their increased ileal luminal BA levels (Table 1).
Table 1.
Jejunal, Ileal, and Portal BA Ratios in Experimental NEC
| Group | Jejunal luminal BA (n) |
Ileal luminal/jejunal luminal BA (n) |
Portal/ileal luminal BA (n) |
|---|---|---|---|
| DF | 215 ± 38 (12) | 0.22 ± 0.08 (12) | 0.52 ± 0.21 (11) |
| NEC | 254 ± 46 (20) | 1.05 ± 0.26a (20) | 0.20 ± 0.10a (9) |
NOTE. Data are expressed as mean ± SEM, BA units are measured as µg/mL. Portal/luminal BA ratios were determined only from animals in which both portal blood and luminal flushes were obtained.
P ≤ .01 vs DF.
Sequestration of Ileal BAs Decreases the Incidence and Severity of NEC
If intestinal BAs are required for the development of NEC injury, then reduction of BAs should result in decreased incidence and severity of NEC. Chol binds BAs in the intestine and the Chol–BA complex passes out of the body without being absorbed.33–35 In a separate set of experiments, neonatal rats artificially were fed cow’s milk formula with or without Chol (NEC + Chol and NEC, respectively) and asphyxia/cold stressed for 4 days. Results are summarized in Table 2. Pharmacologic sequestration of intestinal BAs significantly decreased NEC injury in the terminal ileum and increased survival in the neonatal rat model of NEC.
Table 2.
Sequestration of Ileal BAs Reduces NEC
| Group | Total ileal BAs (µg/L) |
Median NEC score |
Survival |
|---|---|---|---|
| NEC | 562 ± 139 | 2.5 | 7/16 |
| NEC + Chol | 79 ± 18a | 1.5b | 11/15b |
NOTE. Total BA are expressed as mean ± SEM.
P ≤ .01;
P ≤ .05.
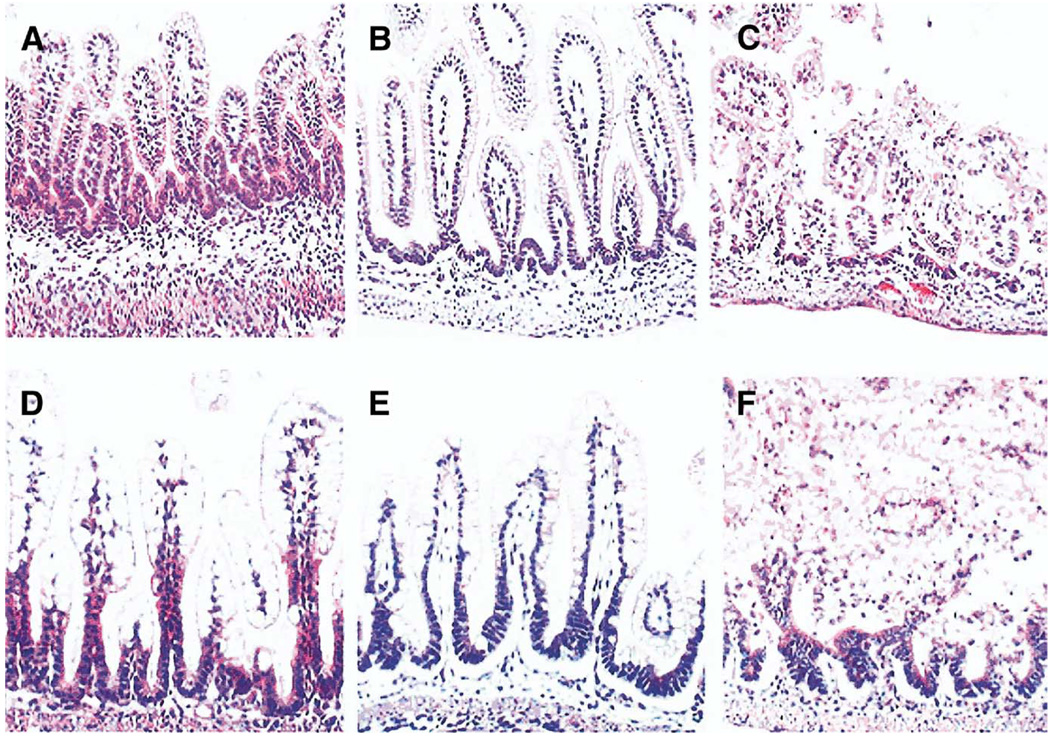
The accumulation of BAs is known to produce intestinal damage in adult systems. We introduced exogenous BAs to neonatal rats via intragastric gavage to determine if increased levels of ileal BAs would result in ileal damage similar to what is seen in the neonatal rat model of NEC. Neonatal rats given high-dose NaDOC (BAG-high) showed histologic damage to the ileal architecture similar to what is observed in animals with NEC (Figure 3). Animals given lower doses of NaDOC over a 2-day period (BAG-low) did not show significant ileal damage (data not shown).
Figure 3.
Ileal damage in BA-gavaged rats resembles damage in experimental NEC. (A–C) Representative slides from neonatal rats gavaged with (A) phosphate-buffered saline, (B) 1 dose of 50 mmol/L sodium deoxycholic acid, and (C) 2 doses of 50 mmol/L sodium deoxycholic acid. (D–F) Representative slides from (D) DF, histologic score=0; (E) NEC, histologic score +2; (F) NEC animals with full necrosis, histologic score +4. In contrast to the (A) ileal tissue, the large enterocytes visible in the (D) DF ileum are seen because these animals were not fasted before removal of ileal tissue. Original magnification, 100×.
To determine further if BA levels or composition are reduced when disease is reduced, we examined neonatal rats treated for 4 days with EGF. We previously showed that enteral administration of EGF significantly decreased the incidence and severity of NEC in the neonatal rat model.8 Total BA levels and the levels of both cholic acid and deoxycholic acid were reduced significantly after treatment with EGF (Table 3).
Table 3.
Reduction of NEC with EGF Decreases BA Levels
| Group | NEC incidence | Total ileal BA (µM/L) | CA (µg/mL) | DCA (µg/mL) |
|---|---|---|---|---|
| DF | 0/12 | 148 ± 40 | 0.14 ± 0.02 | ND |
| NEC | 9/12a | 722 ± 186b | 1.10 ± 0.41c | 0.40 ± 0.02b |
| NEC + EGF | 4/12d | 204 ± 52 | 0.4 ± 0.05e | 0.02 ± 0.008 |
NOTE. Data are expressed as mean ± SEM.
P ≤ .01 vs DF.
P ≤ .01 vs DF and NEC + EGF.
P ≤ .05 vs DF and NEC + EGF.
P ≤ .05 vs NEC.
P ≤ .05 vs DF.
ND, none detected.
Reduction of NEC and BAs
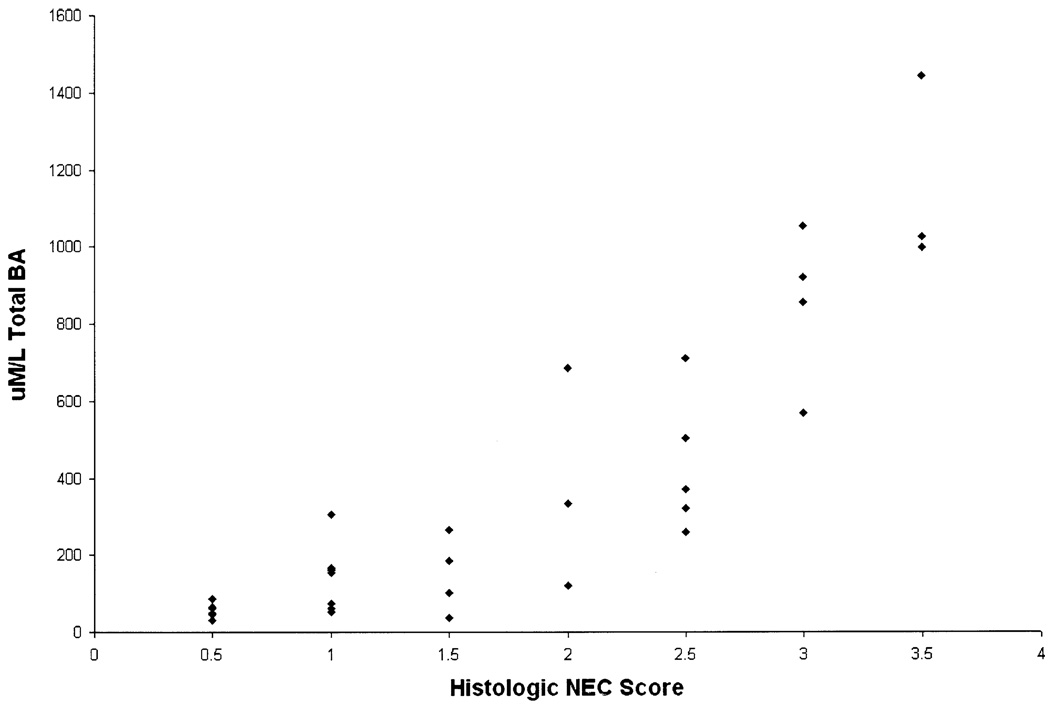
Luminal total BA levels were correlated positively (r = .744, P ≤ .001) with progression of ileal injury, as determined via histologic NEC score (Figure 4).
Figure 4.
BA levels are correlated positively with the NEC score. Total BA levels from ileal luminal flushes from animals in NEC, NEC + Chol, and NEC + EGF groups were correlated with the degree of ileal injury evaluated by histologic scoring of ileal tissue.
Ileal BA Transporter mRNA Expression in NEC
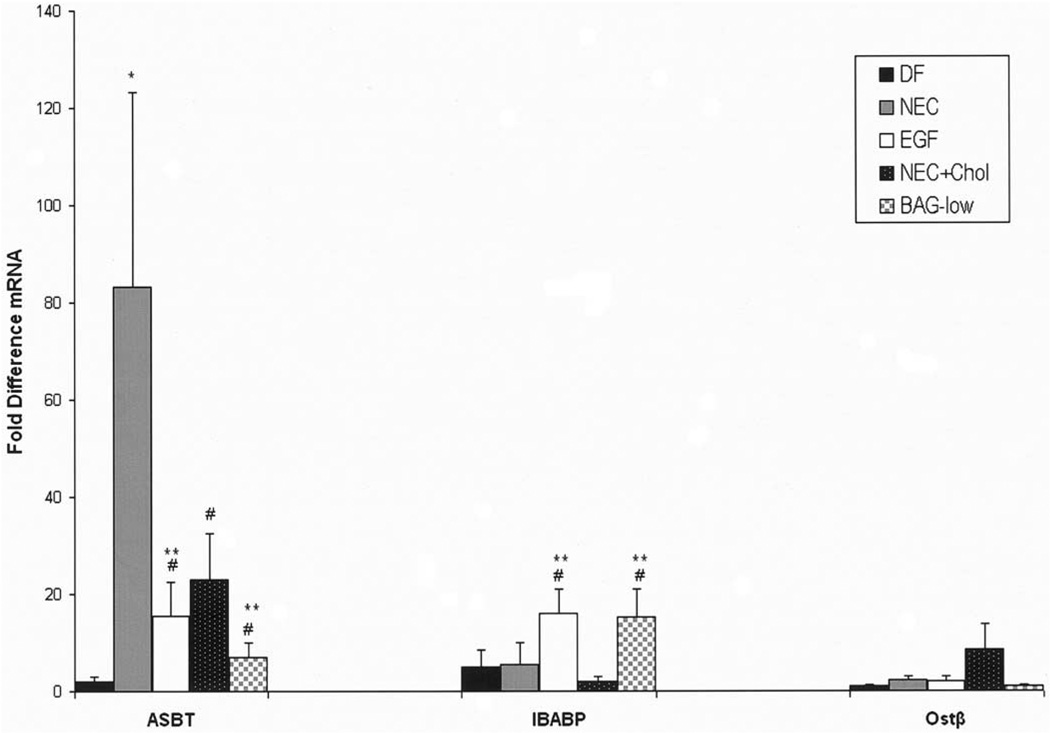
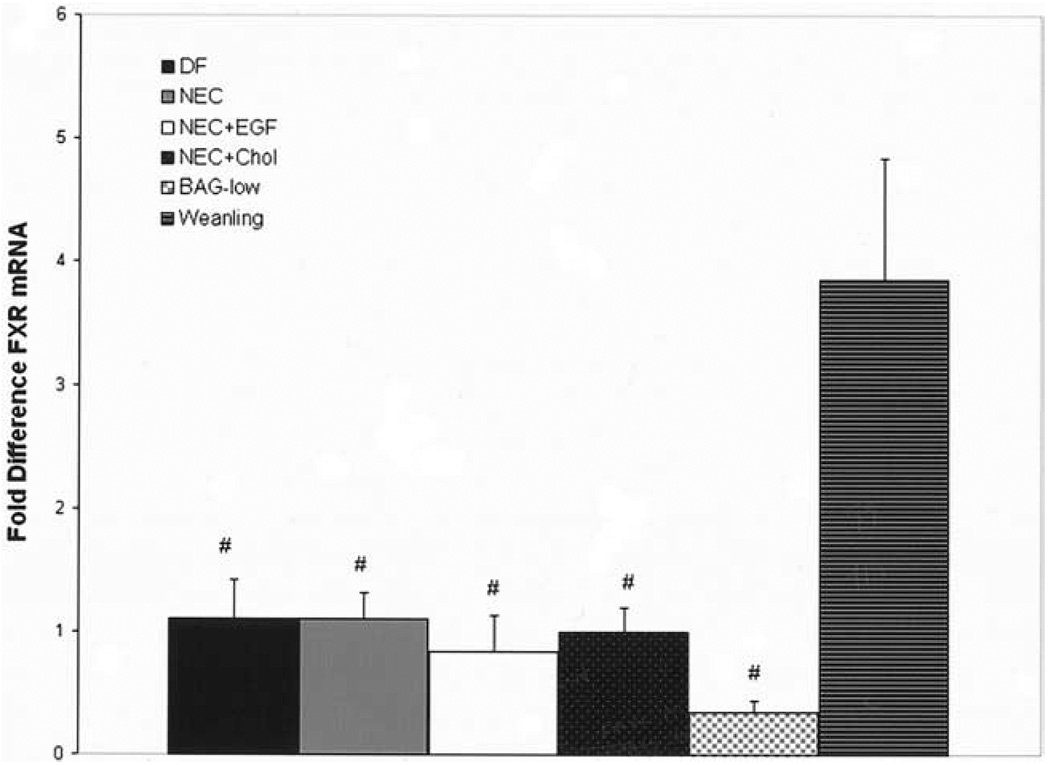
The transport of BAs from the ileal lumen to portal circulation is crucial for BA homeostasis. We first evaluated mRNA levels from the BA transporters ASBT, IBABP, Ostα, and Ostβ in the distal ileum during the development of experimental NEC. ASBT mRNA was up-regulated in NEC ileal tissue compared with DF controls. In contrast, there was no change in IBABP mRNA expression between the DF and NEC groups. To determine if ileal BA sequestration would decrease the expression of ASBT, we found ASBT mRNA was decreased in the NEC + Chol group after BA sequestration. In contrast, BAG-low increased the expression of IBABP, but not ASBT. Further, ASBT mRNA was decreased significantly and IBABP mRNA was increased significantly in the NEC + EGF group compared with the NEC group. The mRNA levels for Ostβ were low and without statistical differences in all groups (Figure 5). No significant changes were observed for Ostα between groups (data not shown).
Figure 5.
Relative mRNA levels for ileal transporters. Fold differences for ASBT, IBABP, and Ostβ mRNA from DF controls (n = 12), NEC (n = 12), NEC + EGF (n = 12), NEC + Chol (n = 9), and BAG-low (n = 5) groups. *P ≤ .01 vs DF control; #P ≤ .05 vs NEC; **P ≤ .05 vs DF control. The 28-day-old weaned rat ileal tissues were assayed for ASBT, IBABP, and Ostβ mRNA as a positive control: ASBT = 1451 ± 312; IBABP = 19,508 ± 624; Ostβ = 35 ± 9. ■, DF;  , NEC; □, EGF; ■, NEC + Chol;
, NEC; □, EGF; ■, NEC + Chol;  , BAG-low.
, BAG-low.
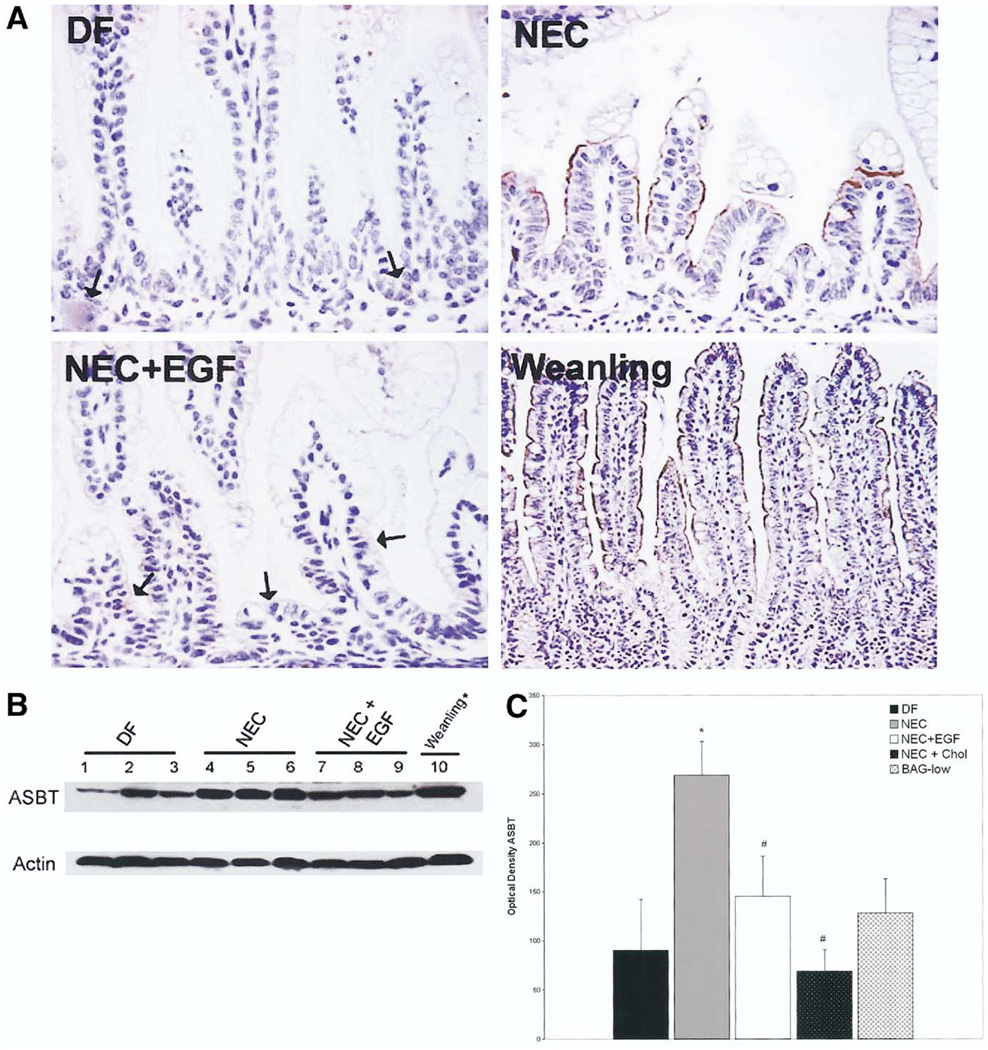
Ileal BA Transporter Protein in NEC
We evaluated ASBT, IBABP, Ostα, and Ostβ protein levels from the distal ileum during the development of experimental NEC. ASBT was not present in most 4-day-old DF neonates. In the NEC group, ASBT was localized on the apical membrane, and EGF treatment significantly reduced the increased ASBT seen in the NEC group (Figure 6A). To quantify changes in ASBT further, we evaluated ileal protein levels using Western blot analysis. Protein extracted from ileal tissue from a 28-day-old weanling rat, diluted 1:10, was run on the same gel for comparison. ASBT was detected in all groups, however, levels in the NEC group were higher than in the DF or NEC + EGF groups (Figure 6B). In addition, ASBT protein levels were decreased significantly in the NEC + Chol group compared with the NEC group (Figure 6C).
Figure 6.
(A) ASBT is increased in animals with NEC. Representative sections from DF, NEC, NEC + EGF, and 28-day-old weanling ileal tissue stained with anti-rat ASBT. Arrows in DF and NEC + EGF groups indicate isolated areas of positive staining. Original magnification, 400×. (B) ASBT protein levels in rat ileum. Representative ileal protein samples from DF, NEC, and NEC + EGF subjected to Western blotting to detect ASBT. Weanling protein diluted 1:10. (C) Mean densitometry values from ASBT Western blots. DF (n = 6), NEC (n = 6), NEC + EGF (n = 6), NEC + Chol (n = 3), and BAG-low (n = 3). ■, DF;  , NEC; □, EGF; ■, NEC + Chol;
, NEC; □, EGF; ■, NEC + Chol;  , BAG-low. *P ≤ .05 vs DF; #P ≤ .05 vs NEC.
, BAG-low. *P ≤ .05 vs DF; #P ≤ .05 vs NEC.
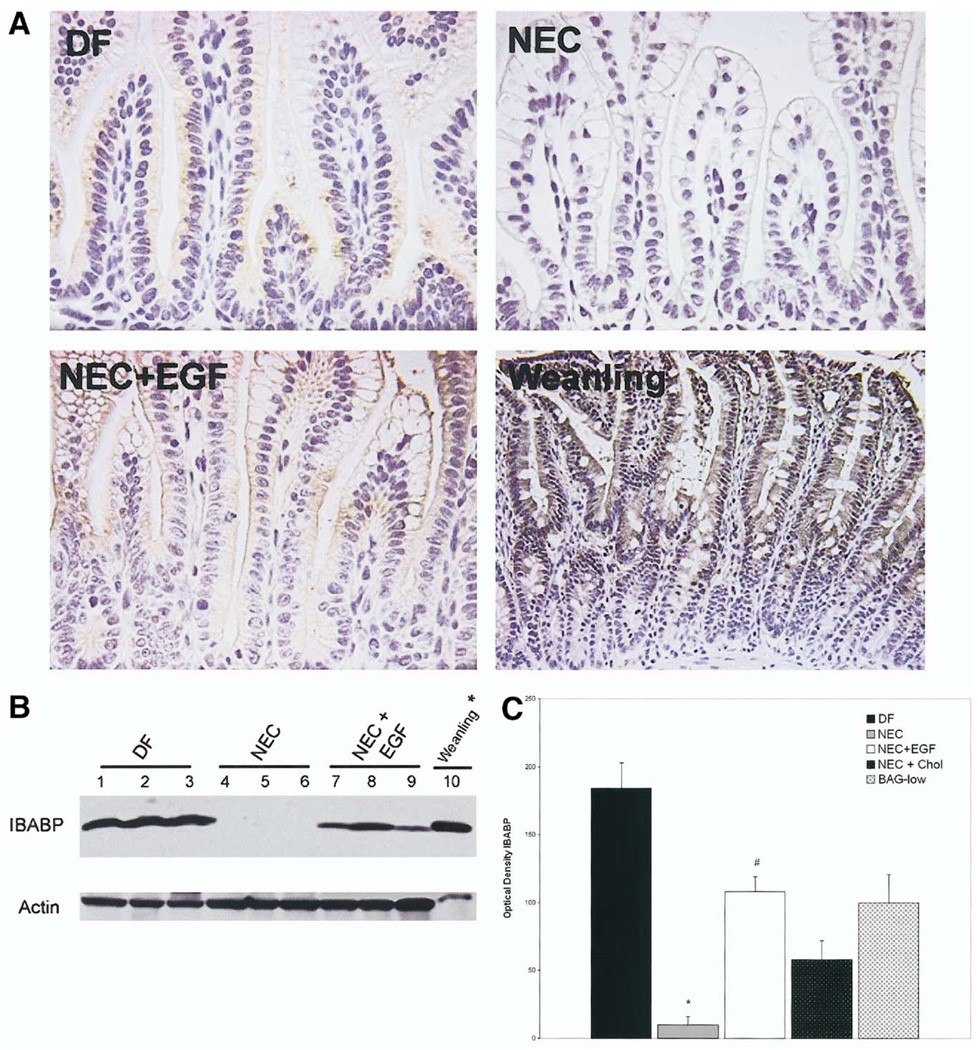
In contrast to ASBT, immunohistologic evaluation showed that IBABP is localized in the cytoplasm of ileal enterocytes in the DF and NEC + EGF groups only. IBABP is observed rarely in ileal tissue from the NEC group (Figure 7A). To confirm that IBABP is produced only minimally in animals with NEC, we evaluated ileal protein levels of IBABP using Western blot (Figure 7B). Protein extracted from ileal tissue from a 28-day-old weanling rat, diluted 1:100, was run on the same gel for comparison. IBABP is produced in both DF and NEC + EGF animals, albeit at significantly lower levels than in weanling rats, but is not detectable in most of the animals in the NEC group. IBABP protein levels were decreased in the NEC + Chol group compared with the DF group and increased in the BAG group compared with the NEC group, but not significantly different from the DF animals (Figure 7C).
Figure 7.
(A) IBABP localization is diminished during NEC. Representative sections from DF, NEC, NEC + EGF, and 28-day-old weanling ileal tissue stained with anti-rat IBABP. Original magnification, 400×. (B) IBABP protein levels in rat ileum. Representative ileal protein samples from DF, NEC, and NEC + EGF were subjected to Western blotting to detect IBABP. *Weanling protein diluted 1:100. (C) Mean densitometry values from IBABP Western blots. DF (n = 6), NEC (n = 6), NEC + EGF (n = 6), NEC + Chol (n = 3), and BAG-low (n = 3). ■, DF;  , NEC; □, EGF; ■, NEC + Chol;
, NEC; □, EGF; ■, NEC + Chol;  , BAG-low. *P ≤ .05 vs DF; #P ≤ .05 vs NEC.
, BAG-low. *P ≤ .05 vs DF; #P ≤ .05 vs NEC.
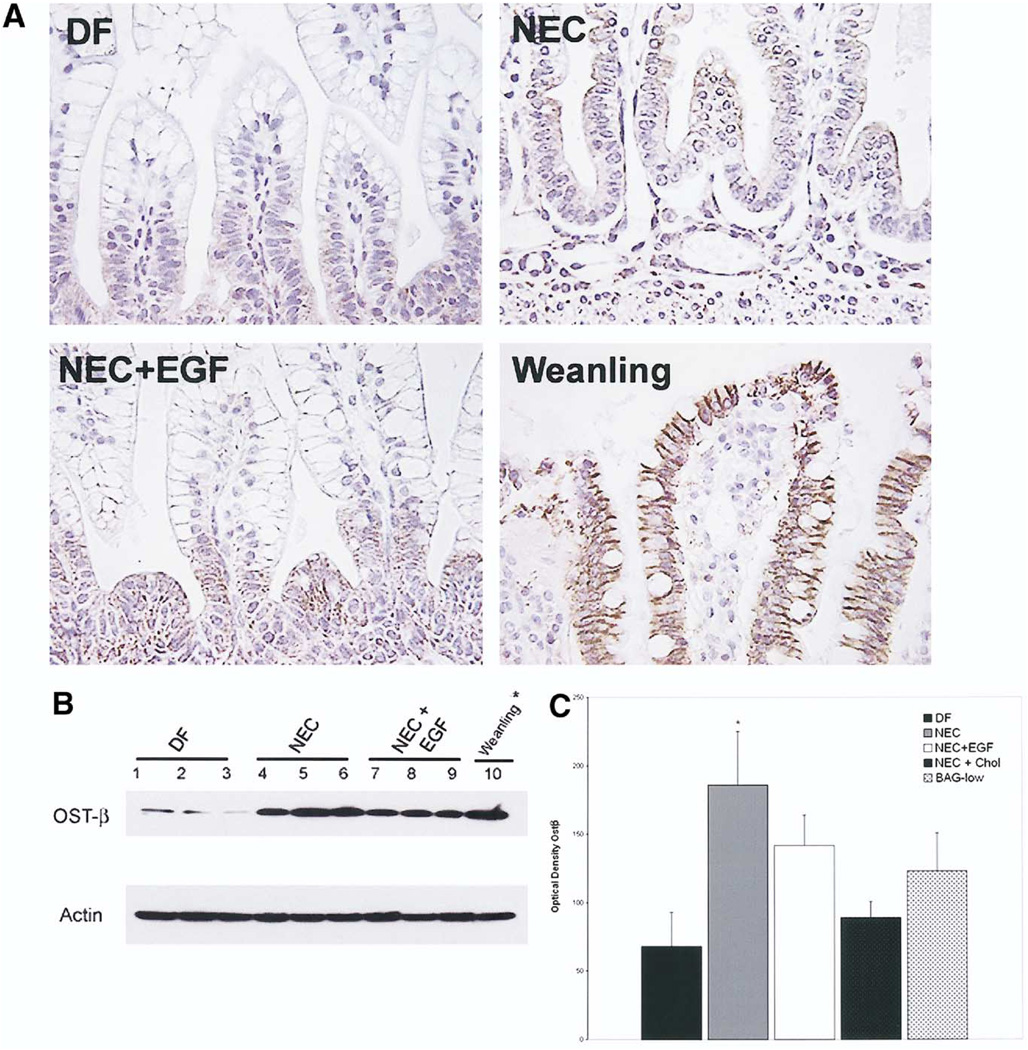
Ostα and Ostβ protein localization and quantification also were examined. Immunohistologic staining and Western blot showed very low levels of Ostα in all neonatal rat groups (data not shown). For Ostβ, similar localization and intensity of staining was observed between DF, NEC, and NEC + EGF groups. However, in neonatal rats, the localization of Ostβ was found primarily in the crypts and not in the upper villi as seen in weanling animals (Figure 8A). Western blotting showed variability, particularly among DF animals, but confirmed that there are no significant differences in Ostβ protein between the NEC and NEC + EGF groups (Figure 8B). Ostβ protein levels were decreased in the NEC + Chol group compared with the NEC group, and increased in the BAG group compared with DF animals (Figure 8C).
Figure 8.
(A) Immunohistologic staining of Ostβ. Representative sections from DF, NEC, NEC + EGF, and 28-day-old weanling ileal tissue stained with anti-rat Ostβ. Original magnification, 400×. (B) Ostβ protein levels in rat ileum. Representative ileal protein samples from DF, NEC, and NEC + EGF were subjected to Western blotting to detect Ostβ. *Weanling protein diluted 1:10. (C) Mean densitometry values from Ostβ Western blots. DF (n = 6), NEC (n = 6), NEC + EGF (n = 6), NEC + Chol (n = 3), and BAG-low (n = 3). ■, DF;  , NEC; □, EGF; ■, NEC + Chol;
, NEC; □, EGF; ■, NEC + Chol;  , BAG-low. *P ≤ .05 vs DF.
, BAG-low. *P ≤ .05 vs DF.
BA responsiveness can be regulated by positive and negative feedback mechanisms via interactions between BAs and the FXR.36,37 Because BA transporters can be regulated by the FXR, we examined the FXR mRNA levels in all experimental groups. There were no differences between the neonatal groups, however, the FXR expression in DF, NEC, NEC + EGF, NEC + Chol, and BAG-low groups was significantly lower than what was observed in weanling rats (Figure 9).
Figure 9.
Relative mRNA levels for FXR. Fold differences for FXR mRNA from DF controls (n = 24), NEC (n = 24), NEC + EGF (n = 24), NEC + Chol (n = 9), BAG-low (n = 5), and weanling (n = 3) groups. #P ≤ .01 vs weanling. ■, DF;  , NEC; □, EGF; ■, NEC + Chol;
, NEC; □, EGF; ■, NEC + Chol;  , BAG-low.
, BAG-low.
Discussion
BAs are synthesized by the liver, secreted into the duodenum, reabsorbed in the distal ileum, and returned to the liver via enterohepatic circulation.12 The effect of cytotoxic BA on the development of intestinal mucosal injury is well established,23–25 but has not been examined previously in NEC. The data presented herein show that BAs may play a crucial role in NEC pathogenesis and suggest a novel mechanism in the development of experimental NEC. Further, the inability of neonatal rats to regulate BA transporters adequately may be a mechanism by which this damage occurs.
Previous studies have reported significant pathologic changes in hepatic morphology and hepatobiliary functions in patients with NEC.38–41 This type of remote organ injury to the liver and other organs is well documented after intestinal ischemia/reperfusion,42–44 and also has been implicated in the development of NEC.45–48 We recently showed that the liver plays an important role in experimental NEC.11 Intestinal damage observed during NEC occurs in the distal ileum, the site where most bile reclamation occurs. Taken together, these data suggested that BA may be important in NEC. The marked decrease in the incidence and severity of NEC after pharmacologic sequestration of BAs coupled with decreased BA levels after reduction of disease using EGF strongly suggests an important role of BAs in NEC pathogenesis.
BA transporters are essential components of bile acid metabolism in the ileum. Both ASBT and IBABP are expressed at high levels in fetal rat ileum but are down-regulated significantly in neonates. After weaning, ASBT and IBABP levels increase again to facilitate BA transport from the ileal lumen.17–22 This biphasic expression may reflect the changing levels of BA from fetal to neonatal to weanling intestine. In the fetus, ASBT and IBABP may be required to transport BAs found in fetal meconium. In the normal neonate, BA levels remain low during the suckling period, and BA transport may not be as important. At weaning, bile levels associated with solid diet would necessitate increased BA transport. Previous research has shown that unlike rabbits, mice, and human beings, the rat ASBT gene is not regulated via a negative feedback mechanism by BAs.49–53 BA responsiveness of ASBT is mediated by FXR-dependent activation of small heterodimer partner and subsequent inhibition of liver receptor homologue-1.52,53 The rat ASBT promoter lacks a functional liver receptor homologue-1 cis-acting element, and rat ileum does not express the liver receptor homologue-1 protein.54 Our finding that ASBT protein levels are not increased in neonatal rats gavaged with BAs is consistent with these previous studies. It is compelling, therefore, that in our studies neonatal rats with NEC have statistically significantly increased ileal ASBT in conjunction with increased levels of ileal BA. FXR mRNA levels were extremely low in all of our neonatal experimental groups, regardless of luminal BA levels. These data suggest that up-regulation of ASBT during the development of NEC is a result of non-FXR cascade-mediated mechanisms. Although ASBT may be increased in animals with NEC compared with DF control littermates, the overall levels still may be insufficient to transport BAs across the apical surface of the villi adequately, resulting in accumulation of ileal luminal BAs.
In the NEC group, IBABP protein was decreased significantly compared with all other groups studied. Previously we showed that EGF treatment in experimental NEC significantly reduces disease incidence and severity. 8,28,55 IBABP mRNA and protein expression were up-regulated after EGF treatment despite the reduced levels of intraluminal BAs. Thus, the inability to increase the expression of IBABP may indicate a potential mechanism by which ileal damage occurs during the development of experimental NEC. In contrast to ASBT, FXR contributes to positive regulation of IBABP in the presence of luminal BAs.19 The low levels of FXR mRNA observed are consistent with the low levels of IBABP protein in all neonatal groups relative to weanling levels. However, although the role of IBABP in BA transport has not been determined conclusively, this study suggests that the ability to up-regulate IBABP in EGF-treated animals may contribute to the reduction of NEC in this group. The lack of IBABP in NEC pathogenesis may contribute to BA accumulation within the epithelial cells, leading to damage to intestinal architecture that is observed commonly in NEC.
These results show that the expression of Ostα/Ostβ in the early neonatal period is similar to ASBT and IBABP with relatively low levels compared with older animals. Although the Ostβ protein level was increased slightly in the NEC group, mRNA expression and protein levels did not always correspond. This may represent regulation of expression that differs from ASBT or IBABP, and/or the ability of neonatal rats to up-regulate Ostβ mRNA in general because levels were low in all groups. Our observation that there was no difference in Ostβ protein between the NEC and NEC + EGF groups suggest that down-regulation of Ostβ is not required to facilitate EGF-mediated reduction of disease.
In this study, we observed no difference in jejunal BA levels between groups, but significantly higher ileal luminal/jejunal luminal BA ratios coupled with decreased ratios of portal/luminal BA levels in animals with NEC. These data suggest that the increased levels of ileal luminal BA in experimental NEC are not a result of increased production of BAs, but rather an accumulation of BAs in the ileum. Although increased levels of ASBT in the NEC group should facilitate the transport of more BAs into portal circulation, thus increasing the portal/luminal BA ratio, the increased expression of ASBT during NEC pathogenesis (which is 10% of that seen in adults) may not be sufficient to deal with the increased levels of BAs. This may account for the diminished portal/luminal BA ratios seen in animals with NEC. Further, the apparent inability of animals with NEC to transport BAs effectively, as measured by the increased ileal/jejunal and decreased portal/luminal BA ratios, may be related to the immaturity of many components of BA homeostasis. Based on the studies presented herein, we cannot conclusively rule out the possibility that diminished intestinal motility contributes to the excess ileal BA levels seen in this experimental model. Diminished motility has been shown in NEC,56–58 however, there is no evidence that motility of the proximal intestine is affected differently from that of the distal intestine. Thus, the accumulation of BAs as a result of changes in motility should result in an accumulation of high levels of BAs throughout the intestine. Our finding that BA levels in the proximal intestine are similar between animals with NEC and DF controls, even when the levels in the distal intestine are significantly greater in the animals with NEC, suggests that changes in motility do not significantly affect the accumulation of BAs. However, BAs can inhibit ileal motility.59 Therefore, increased BA levels during NEC development could lead to exacerbation of BA accumulation in the ileum.
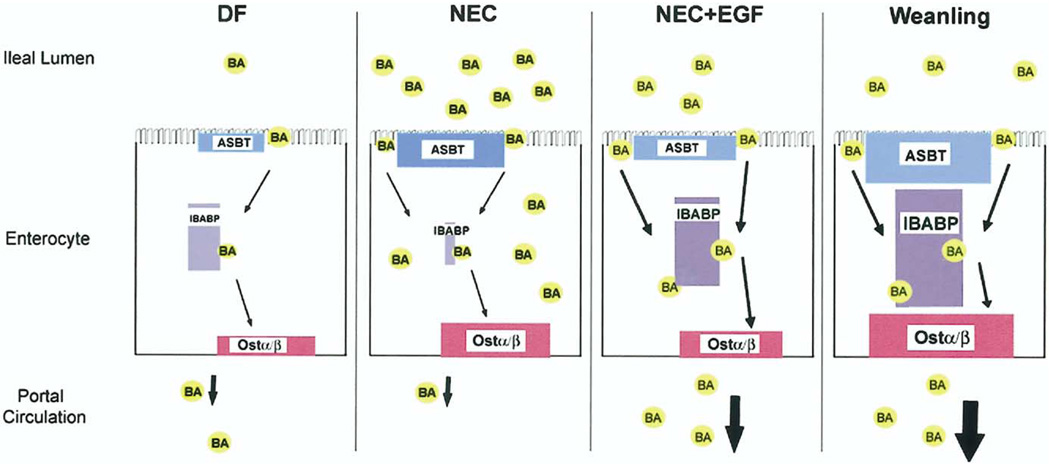
Our current data suggest the following paradigm: during development of experimental NEC, BAs are secreted at normal levels by the liver into the proximal intestine. However, BAs in the terminal ileum of animals with NEC begin to accumulate, perhaps as a result of inflammatory changes in the terminal ileum, which can effect motility, barrier functions, and cellular turnover. The accumulation of BAs leads to precocious expression of ASBT, which may allow some of the increased levels of BA to be transported across the apical surface of enterocytes, but transport is compromised because IBABP is not induced sufficiently in the neonatal intestine. The unsuccessful transport of BAs is reflected in the diminished ileal luminal/portal blood BA ratio, and the further accumulation of toxic BAs promotes accelerated cellular damage to the terminal ileum. The addition of EGF promotes up-regulation of IBABP, allowing more efficient transport of BAs across the cell, less accumulation of luminal BAs, and less ileal damage (Figure 10).
Figure 10.
Working paradigm for BAs in NEC. In DF 4-day-old neonatal rats, low levels of ileal BA transporters can transport low levels of luminal BA effectively through the enterocyte and out into portal circulation. During NEC, ileal luminal BA levels are increased significantly compared with DF. ASBT levels precociously are up-regulated, perhaps in an attempt to deal with the increased luminal BA levels. However, IBABP protein is decreased significantly. Our data suggest that without adequate levels of IBABP, BAs are not transported efficiently into portal circulation and may accumulate in the enterocytes where they contribute to ileal damage. When supplemented with EGF, animals with NEC are able to up-regulate IBABP, albeit at levels much lower than in weanling rats. Weanling animals express high levels of ASBT, IBABP, and Ostα/Ostβ when compared with neonatal animals, and efficiently transfer bile acids from the lumen into portal blood. Our data suggest that the ability to produce sufficient levels of IBABP allows the increased ileal luminal BAs to be transported into portal circulation, keeping toxic BAs from accumulating and contributing to tissue damage in the ileum.
The mechanisms by which BA levels are increased during experimental NEC and their relation to human disease require further elucidation. The results of this study suggest a novel mechanism for the development of intestinal damage in NEC and may facilitate the identification of future therapeutic approaches for this disease.
Acknowledgments
Supported by a National Institute of Child Health and Human Development grant (HD-39657 to B.D.). The Arizona Cancer Center Analytical Core Shared Service is supported by grant no. 5 P30 CA23074.
Abbreviations used in this paper
- ASBT
apical sodium-dependent bile acid transporter
- BA
bile acid
- Chol
cholestyramine
- DF
dam fed
- DOC
deoxycholic acid
- EGF
epidermal growth factor
- FXR
farnesoid X receptor
- IBABP
ileal bile acid binding protein
- NEC
necrotizing enterocolitis
- Ostα/Ostβ
heteromeric organic solute transporter
References
- 1.Israel EJ. Neonatal necrotizing enterocolitis, a disease of the immature intestinal mucosal barrier. Acta Paediatr Suppl. 1994;396:27–32. doi: 10.1111/j.1651-2227.1994.tb13238.x. [DOI] [PubMed] [Google Scholar]
- 2.Schanler RJ, Hurst NM, Lau C. The use of human milk and breastfeeding in premature infants. Clin Perinatol. 1999;26:379–398. vii. [PubMed] [Google Scholar]
- 3.Caplan MS, MacKendrick W. Necrotizing enterocolitis: a review of pathogenetic mechanisms and implications for prevention. Pediatr Pathol. 1993;13:357–369. doi: 10.3109/15513819309048223. [DOI] [PubMed] [Google Scholar]
- 4.Barlow B, Santulli TV, Heird WC, Pitt J, Blanc WA, Schullinger JN. An experimental study of acute neonatal enterocolitis—the importance of breast milk. J Pediatr Surg. 1974;9:587–595. doi: 10.1016/0022-3468(74)90093-1. [DOI] [PubMed] [Google Scholar]
- 5.Caplan MS, Hedlund E, Adler L, Hsueh W. Role of asphyxia and feeding in a neonatal rat model of necrotizing enterocolitis. Pediatr Pathol. 1994;14:1017–1028. doi: 10.3109/15513819409037698. [DOI] [PubMed] [Google Scholar]
- 6.Crissinger KD. Animal models of necrotizing enterocolitis. J Pediatr Gastroenterol Nutr. 1995;20:17–22. [PubMed] [Google Scholar]
- 7.Barlow B, Santulli TV. Importance of multiple episodes of hypoxia or cold stress on the development of enterocolitis in an animal model. Surgery. 1975;77:687–690. [PubMed] [Google Scholar]
- 8.Dvorak B, Halpern MD, Holubec H, Williams CS, McWilliam DL, Dominguez JA, Stepankova R, Payne CM, McCuskey RS. Epidermal growth factor reduces the development of necrotizing enterocolitis in a neonatal rat model. Am J Physiol. 2002;282:G156–G164. doi: 10.1152/ajpgi.00196.2001. [DOI] [PubMed] [Google Scholar]
- 9.Caplan MS, Miller-Catchpole R, Kaup S, Russell T, Lickerman M, Amer M, Xiao Y, Thomson R., Jr Bifidobacterial supplementation reduces the incidence of necrotizing enterocolitis in a neonatal rat model. Gastroenterology. 1999;117:577–583. doi: 10.1016/s0016-5085(99)70450-6. [DOI] [PubMed] [Google Scholar]
- 10.Caplan MS, Russel T, Xiao Y, Amer M, Kaup S, Jilling T. Effect of polyunsaturated fatty acid (PUFA) supplementation on intestinal inflammation and necrotizing enterocolitis (NEC) in a neonatal rat model. Pediatr Res. 2001;49:647–652. doi: 10.1203/00006450-200105000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Halpern MD, Holubec H, Dominguez JA, Meza YG, Williams CS, Ruth MC, McCuskey RS, Dvorak B. Hepatic inflammatory mediators contribute to intestinal damage in necrotizing enterocolitis. Am J Physiol. 2003;284:G695–G702. doi: 10.1152/ajpgi.00353.2002. [DOI] [PubMed] [Google Scholar]
- 12.Shneider BL. Intestinal bile acid transport: biology, physiology, and pathophysiology. J Pediatr Gastroenterol Nutr. 2001;32:407–417. doi: 10.1097/00005176-200104000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Dawson PA, Hubbert M, Haywood J, Craddock AL, Zerangue N, Christian WV, Ballatori N. The heteromeric organic solute transporter alpha-beta, Ostalpha-Ostbeta, is an ileal basolateral bile acid transporter. J Biol Chem. 2005;280:6960–6968. doi: 10.1074/jbc.M412752200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crossman MW, Hauft SM, Gordon JI. The mouse ileal lipid-binding protein gene: a model for studying axial patterning during gut morphogenesis. J Cell Biol. 1994;126:1547–1564. doi: 10.1083/jcb.126.6.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coppola CP, Gosche JR, Arrese M, Ancowitz B, Madsen J, Vanderhoof J, Shneider BL. Molecular analysis of the adaptive response of intestinal bile acid transport after ileal resection in the rat. Gastroenterology. 1998;115:1172–1178. doi: 10.1016/s0016-5085(98)70088-5. [DOI] [PubMed] [Google Scholar]
- 16.Stelzner M, Hoagland V, Somasundaram S. Distribution of bile acid absorption and bile acid transporter gene message in the hamster ileum. Pflugers Arch. 2000;440:157–162. doi: 10.1007/s004240000281. [DOI] [PubMed] [Google Scholar]
- 17.Christie DM, Dawson PA, Thevananther S, Shneider BL. Comparative analysis of the ontogeny of a sodium-dependent bile acid transporter in rat kidney and ileum. Am J Physiol. 1996;271:G377–G385. doi: 10.1152/ajpgi.1996.271.2.G377. [DOI] [PubMed] [Google Scholar]
- 18.Hwang ST, Henning SJ. Hormonal regulation of expression of ileal bile acid binding protein in suckling rats. Am J Physiol. 2000;278:R1555–R1563. doi: 10.1152/ajpregu.2000.278.6.R1555. [DOI] [PubMed] [Google Scholar]
- 19.Hwang ST, Urizar NL, Moore DD, Henning SJ. Bile acids regulate the ontogenic expression of ileal bile acid binding protein in the rat via the farnesoid X receptor. Gastroenterology. 2002;122:1483–1492. doi: 10.1053/gast.2002.32982. [DOI] [PubMed] [Google Scholar]
- 20.Shneider BL, Dawson PA, Christie DM, Hardikar W, Wong MH, Suchy FJ. Cloning and molecular characterization of the ontogeny of a rat ileal sodium-dependent bile acid transporter. J Clin Invest. 1995;95:745–754. doi: 10.1172/JCI117722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shneider BL, Setchell KD, Crossman MW. Fetal and neonatal expression of the apical sodium-dependent bile acid transporter in the rat ileum and kidney. Pediatr Res. 1997;42:189–194. doi: 10.1203/00006450-199708000-00010. [DOI] [PubMed] [Google Scholar]
- 22.Sacchettini JC, Hauft SM, Van Camp SL, Cistola DP, Gordon JI. Developmental and structural studies of an intracellular lipid binding protein expressed in the ileal epithelium. J Biol Chem. 1990;265:19199–19207. [PubMed] [Google Scholar]
- 23.Craven PA, DeRubertis FR. Role of activation of protein kinase C in the stimulation of colonic epithelial proliferation by unsaturated fatty acids. Gastroenterology. 1988;95:676–685. doi: 10.1016/s0016-5085(88)80014-3. [DOI] [PubMed] [Google Scholar]
- 24.Craven PA, Pfanstiel J, Saito R, DeRubertis FR. Relationship between loss of rat colonic surface epithelium induced by deoxycholate and initiation of the subsequent proliferative response. Cancer Res. 1986;46:5754–5759. [PubMed] [Google Scholar]
- 25.Milovic V, Teller IC, Faust D, Caspary WF, Stein J. Effects of deoxycholate on human colon cancer cells: apoptosis or proliferation. Eur J Clin Invest. 2002;32:29–34. doi: 10.1046/j.0014-2972.2001.00938.x. [DOI] [PubMed] [Google Scholar]
- 26.Dvorak B, McWilliam DL, Williams CS, Dominguez JA, Machen NW, McCuskey RS, Philipps AF. Artificial formula induces precocious maturation of the small intestine of artificially reared suckling rats. J Pediatr Gastroenterol Nutr. 2000;31:162–169. doi: 10.1097/00005176-200008000-00013. [DOI] [PubMed] [Google Scholar]
- 27.Halpern MD, Holubec H, Dominguez JA, Williams CS, Meza YG, McWilliam DL, Payne CM, McCuskey RS, Besselsen DG, Dvorak B. Up-regulation of IL-18 and IL-12 in the ileum of neonatal rats with necrotizing enterocolitis. Pediatr Res. 2002;51:733–739. doi: 10.1203/00006450-200206000-00012. [DOI] [PubMed] [Google Scholar]
- 28.Halpern MD, Dominguez JA, Dvorakova K, Holubec H, Williams CS, Meza YG, Ruth MC, Dvorak B. Ileal cytokine dysregulation in experimental necrotizing enterocolitis is reduced by epidermal growth factor. J Pediatr Gastroenterol Nutr. 2003;36:126–133. doi: 10.1097/00005176-200301000-00024. [DOI] [PubMed] [Google Scholar]
- 29.Dvorak B, Halpern MD, Holubec H, Dvorakova K, Dominguez JA, Williams CS, Meza YG, Kozakova H, McCuskey RS. Maternal milk reduces severity of necrotizing enterocolitis and increases intestinal IL-10 in a neonatal rat model. Pediatr Res. 2003;53:426–433. doi: 10.1203/01.PDR.0000050657.56817.E0. [DOI] [PubMed] [Google Scholar]
- 30.Dvorak B, Kolinska J, McWilliam DL, Williams CS, Higdon T, Zakostelecka M, Koldovsky O. The expression of epidermal growth factor and transforming growth factor-alpha mRNA in the small intestine of suckling rats: organ culture study. FEBS Lett. 1998;435:119–124. doi: 10.1016/s0014-5793(98)01050-3. [DOI] [PubMed] [Google Scholar]
- 31.Brieland JK, Jackson C, Menzel F, Loebenberg D, Cacciapuoti A, Halpern J, Hurst S, Muchamuel T, Debets R, Kastelein R, Churakova T, Abrams J, Hare R, O’arra A. Cytokine networking in lungs of immunocompetent mice in response to inhaled Aspergillus fumigatus. Infect Immun. 2001;69:1554–1560. doi: 10.1128/IAI.69.3.1554-1560.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gibson UE, Heid CA, Williams PM. A novel method for real time quantitative RT-PCR. Genome Res. 1996;6:995–1001. doi: 10.1101/gr.6.10.995. [DOI] [PubMed] [Google Scholar]
- 33.Grundy SM, Ahrens EH, Jr, Salen G. Interruption of the enterohepatic circulation of bile acids in man: comparative effects of cholestyramine and ileal exclusion on cholesterol metabolism. J Lab Clin Med. 1971;78:94–121. [PubMed] [Google Scholar]
- 34.Huff JW, Gilfillan JL, Hunt VM. Effect of cholestyramine, a bile acid-binding polymer on plasma cholesterol and fecal bile acid excretion in the rat. Proc Soc Exp Biol Med. 1963;114:352–355. doi: 10.3181/00379727-114-28674. [DOI] [PubMed] [Google Scholar]
- 35.Shepherd J, Packard CJ, Bicker S, Lawrie TD, Morgan HG. Effect of cholestyramine on low-density lipoproteins. N Engl J Med. 1980;303:943–944. doi: 10.1056/nejm198010163031620. [DOI] [PubMed] [Google Scholar]
- 36.Wang H, Chen J, Hollister K, Sowers LC, Forman BM. Endogenous bile acids are ligands for the nuclear receptor FXR/BAR. Mol Cell. 1999;3:543–553. doi: 10.1016/s1097-2765(00)80348-2. [DOI] [PubMed] [Google Scholar]
- 37.Parks DJ, Blanchard SG, Bledsoe RK, Chandra G, Consler TG, Kliewer SA, Stimmel JB, Willson TM, Zavacki AM, Moore DD, Lehmann JM. Bile acids: natural ligands for an orphan nuclear receptor. Science. 1999;284:1365–1368. doi: 10.1126/science.284.5418.1365. [DOI] [PubMed] [Google Scholar]
- 38.Andorsky DJ, Lund DP, Lillehei CW, Jaksic T, Dicanzio J, Richardson DS, Collier SB, Lo C, Duggan C. Nutritional and other post-operative management of neonates with short bowel syndrome correlates with clinical outcomes. J Pediatr. 2001;139:27–33. doi: 10.1067/mpd.2001.114481. [DOI] [PubMed] [Google Scholar]
- 39.Gambarara M, Ferretti F, Bagolan P, Papadatou B, Rivosecchi M, Lucchetti MC, Nahom A, Castro M. Ultra-short-bowel syndrome is not an absolute indication to small-bowel transplantation in childhood. Eur J Pediatr Surg. 1999;9:267–270. doi: 10.1055/s-2008-1072261. [DOI] [PubMed] [Google Scholar]
- 40.Morecroft JA, Spitz L, Hamilton PA, Holmes SJ. Necrotizing enterocolitis—multisystem organ failure of the newborn? Acta Paediatr Suppl. 1994;396:21–23. [PubMed] [Google Scholar]
- 41.Moss RL, Das JB, Raffensperger JG. Necrotizing enterocolitis and total parenteral nutrition-associated cholestasis. Nutrition. 1996;12:340–343. doi: 10.1016/s0899-9007(96)00062-7. [DOI] [PubMed] [Google Scholar]
- 42.Habib AA, Chatterjee S, Park SK, Ratan RR, Lefebvre S, Vartanian T. The epidermal growth factor receptor engages receptor interacting protein and nuclear factor-kappa B (NF-kappa B)-inducing kinase to activate NF-kappa B. Identification of a novel receptor-tyrosine kinase signalosome. J Biol Chem. 2001;276:8865–8874. doi: 10.1074/jbc.M008458200. [DOI] [PubMed] [Google Scholar]
- 43.Horie Y, Wolf R, Chervenak RP, Jennings SR, Granger DN. T-lymphocytes contribute to hepatic leukostasis and hypoxic stress induced by gut ischemia-reperfusion. Microcirculation. 1999;6:267–280. [PubMed] [Google Scholar]
- 44.Horie Y, Wolf R, Anderson DC, Granger DN. Nitric oxide modulates gut ischemia-reperfusion-induced P-selectin expression in murine liver. Am J Physiol. 1998;275:H520–H526. doi: 10.1152/ajpheart.1998.275.2.H520. [DOI] [PubMed] [Google Scholar]
- 45.Beeby PJ, Jeffery H. Risk factors for necrotising enterocolitis: the influence of gestational age. Arch Dis Child. 1992;67:432–435. doi: 10.1136/adc.67.4_spec_no.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stevenson JK, Oliver TK, Jr, Graham CB, Bell RS, Gould VE. Aggressive treatment of neonatal necrotizing enterocolitis: 38 patients with 25 survivors. J Pediatr Surg. 1971;6:28–35. doi: 10.1016/0022-3468(71)90664-6. [DOI] [PubMed] [Google Scholar]
- 47.Stevenson JK, Graham CB, Oliver TK, Jr, Goldenberg VE. Neonatal necrotizing enterocolitis. A report of twenty-one cases with fourteen survivors. Am J Surg. 1969;118:260–272. doi: 10.1016/0002-9610(69)90129-9. [DOI] [PubMed] [Google Scholar]
- 48.Touloukian RJ, Posch JN, Spencer R. The pathogenesis of ischemic gastroenterocolitis of the neonate: selective gut mucosal ischemia in asphyxiated neonatal piglets. J Pediatr Surg. 1972;7:194–205. doi: 10.1016/0022-3468(72)90496-4. [DOI] [PubMed] [Google Scholar]
- 49.Neimark E, Chen F, Li X, Shneider BL. Bile acid-induced negative feedback regulation of the human ileal bile acid transporter. Hepatology. 2004;40:149–156. doi: 10.1002/hep.20295. [DOI] [PubMed] [Google Scholar]
- 50.Li H, Chen F, Shang Q, Pan L, Shneider BL, Chiang JY, Forman BM, Ananthanarayanan M, Tint GS, Salen G, Xu G. FXR-activating ligands inhibit rabbit ASBT expression via FXR-SHP-FTF cascade. Am J Physiol. 2005;288:G60–G66. doi: 10.1152/ajpgi.00170.2004. [DOI] [PubMed] [Google Scholar]
- 51.Arrese M, Trauner M, Sacchiero RJ, Crossman MW, Shneider BL. Neither intestinal sequestration of bile acids nor common bile duct ligation modulate the expression and function of the rat ileal bile acid transporter. Hepatology. 1998;28:1081–1087. doi: 10.1002/hep.510280424. [DOI] [PubMed] [Google Scholar]
- 52.Lu TT, Makishima M, Repa JJ, Schoonjans K, Kerr TA, Auwerx J, Mangelsdorf DJ. Molecular basis for feedback regulation of bile acid synthesis by nuclear receptors. Mol Cell. 2000;6:507–515. doi: 10.1016/s1097-2765(00)00050-2. [DOI] [PubMed] [Google Scholar]
- 53.Goodwin B, Jones SA, Price RR, Watson MA, McKee DD, Moore LB, Galardi C, Wilson JG, Lewis MC, Roth ME, Maloney PR, Willson TM, Kliewer SA. A regulatory cascade of the nuclear receptors FXR, SHP-1, and LRH-1 represses bile acid biosynthesis. Mol Cell. 2000;6:517–526. doi: 10.1016/s1097-2765(00)00051-4. [DOI] [PubMed] [Google Scholar]
- 54.Chen F, Ma L, Dawson PA, Sinal CJ, Sehayek E, Gonzalez FJ, Breslow J, Ananthanarayanan M, Shneider BL. Liver receptor homologue-1 mediates species- and cell line-specific bile acid-dependent negative feedback regulation of the apical sodium-dependent bile acid transporter. J Biol Chem. 2003;278:19909–19916. doi: 10.1074/jbc.M207903200. [DOI] [PubMed] [Google Scholar]
- 55.Clark JA, Lane RH, Maclennan NK, Holubec H, Dvorakova K, Halpern MD, Williams CS, Payne CM, Dvorak B. Epidermal growth factor reduces intestinal apoptosis in an experimental model of necrotizing enterocolitis. Am J Physiol. 2005;288:G755–G762. doi: 10.1152/ajpgi.00172.2004. [DOI] [PubMed] [Google Scholar]
- 56.Berseth CL. Gut motility and the pathogenesis of necrotizing enterocolitis. Clin Perinatol. 1994;21:263–270. [PubMed] [Google Scholar]
- 57.Berseth CL. Gastrointestinal motility in the neonate. Clin Perinatol. 1996;23:179–190. [PubMed] [Google Scholar]
- 58.Rudmann DG, VanderEide SL. Necrotizing enterotyphlocolitis in dogs treated with a potent antimuscarinic. Vet Pathol. 2003;40:710–713. doi: 10.1354/vp.40-6-710. [DOI] [PubMed] [Google Scholar]
- 59.Smout AJ. Small intestinal motility. Curr Opin Gastroenterol. 2004;20:77–81. doi: 10.1097/00001574-200403000-00005. [DOI] [PubMed] [Google Scholar]