Abstract
The author focused on the functional decline of synapses in the brain with aging to understand the underlying mechanisms and to ameliorate the deficits. The first attempt was to unravel the neuronal functions of gangliosides so that gangliosides could be used for enhancing synaptic activity. The second attempt was to elicit the neuronal plasticity in aged animals through enriched environmental stimulation and nutritional intervention. Environmental stimuli were revealed neurochemically and morphologically to develop synapses leading to enhanced cognitive function. Dietary restriction as a nutritional intervention restored the altered metabolism of neuronal membranes with aging, providing a possible explanation for the longevity effect of dietary restriction. These results obtained with aging and dementia models of animals would benefit aged people.
Keywords: synaptic aging, synaptic plasticity, ganglioside, carnitine, enriched environment, dietary restriction
Introduction
The senescence of organisms is characterized by a gradual functional decline of all organ systems. What is responsible for maintaining the metabolism and function of every organ is the central nervous system (CNS). Age-related changes of the CNS may affect the regulation of the other extraneural systems and their aging processes. As synapses are functional units of the neuronal networks in the brain, the first objective was focused on a study to examine the functional changes of synapses with aging. Acetylcholine release from synapses (see the scheme of cholinergic transmission in Fig. 1) was found to be decreased in the aging brain, and the possible underlying mechanisms were explored,1,2) leading to the concept of synaptic membrane aging.3)
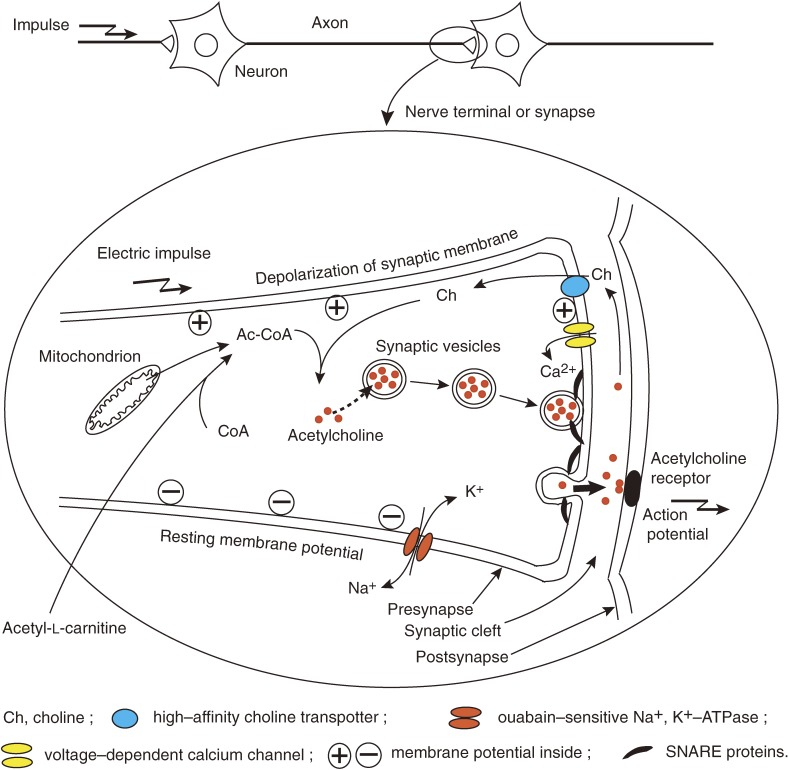
Figure 1.
Synaptic mechanisms in relation to cholinergic neurotransmission.Acetylcholine is synthesized from choline and acetyl-CoA in synapses and stored in synaptic vesicles. Synaptic vesicles move and attach to the inside of synaptic membranes to be ready for the release of acetylcholine. Synaptic membranes are polarized by the action of ouabain-sensitive Na+,K+-ATPase to keep resting membrane potential negative inside. Electric impulses come down to nerve terminals or synapses to depolarize the synaptic membranes. The membrane depolarization very shortly open voltage-dependent calcium channels to allow calcium ions to enter into presynapses. The calcium ion influx triggers membrane fusion between synaptic vesicles and synaptic membranes by means of SNARE proteins to form pores, leading to the release of acetylcholine into synaptic cleft.22) Acetylcholine released binds to acetylcholine receptors on postsynaptic membranes to induce new action potentials. Most of acetylcholine molecules released are degraded, and free choline is taken up by presynapses through high-affinity choline transporters and reused for acetylcholine synthesis.
To correct the synaptic dysfunction associated with aging became the second objective in my study. In order to directly ameliorate the decreased function of synapses, two ways were employed. The first was to enhance the acetylcholine production in synapses. Dietary carnitine was shown to increase the acetylcholine synthesis in synapses and to enhance the acetylcholine release from synapses.4) The second way was to stimulate the excitability of synaptic membranes to enhance synaptic transmission. Gangliosides, sialic acid-containing glycolipids which are localized in neuronal membranes, have been speculated to be involved in the synaptic function. Especially, ganglioside GQ1b was found to stimulate synaptic activity as revealed by the enhancement of long-term potentiation in synaptic transmission.5) While GQ1b widely distributes to neuronal membranes, Chol-1α gangliosides are known to be cholinergic-specific. Chol-1α gangliosides were isolated and identified by our group.6,7) The gangliosides were found to stimulate the high-affinity choline uptake by synapses followed by enhanced acetylcholine synthesis. An experiment using anti-Chol-1α antibody showed that memory and learning ability were remarkably suppressed in rats infused with the antibody into the cholinergic septal area. These in vitro and in vivo studies suggest that Chol-1α gangliosides play a pivotal role in cholinergic synaptic transmission and participate in cognitive function.8)
Although intelligence is thought to be decreasing on the whole with aging, some intelligence called crystalline intelligence is believed to continue to develop even during aging processes.9) This suggests that aged brains might still have some potency to develop, that is called brain plasticity. This potency could be used to restore the impaired brain function and possibly to mitigate the cognitive deficits that come with aging. As an experimental model for enhancing brain plasticity, an enriched environment has been employed as a rearing condition for aging rats. Rats that were reared in enriched environments were found to have enhanced synaptic plasticity,10,11) and increased learning and memory ability.12)
Dietary restriction is known to extend the life spans of organisms.13) This suggests that dietary or calorie restriction may delay the aging processes. In order to verify this idea, the metabolic turnover of the neuronal membrane components was measured using an in vivo D2O-labeling technique.14) Dietary restriction was found to reduce the turnover rates of lipidic components to various extents depending upon the lipid species, while the turnover of proteinic components was shown to be accelerated by the same dietary manipulation.15) These long-term experiments revealed that dietary restriction delayed the age-related changes in the neuronal membrane metabolism.
It would be reasonable to extrapolate from experiments using animals that provided beneficial anti-aging effects, to adequate interventions in humans. Effects of the modulation of environments, including nutritional states on humans are thought to be rather easily observed with minimal ethical problems, compared with effects derived from genetic manipulation. For instance, benefits of supplementation with micronutrients such as carnitine and folic acid were observed with geriatric patients.16,17) In relation to aging and anti-aging with animals and humans, perspectives will be stated.
1. Synaptic aging
To elucidate age-related functional changes in cholinergic neurotransmission (Fig. 1), acetylcholine release mechanisms were examined in terms of acetylcholine release activity and synaptic membrane excitability.
(1). Failure of acetylcholine release mechanism.
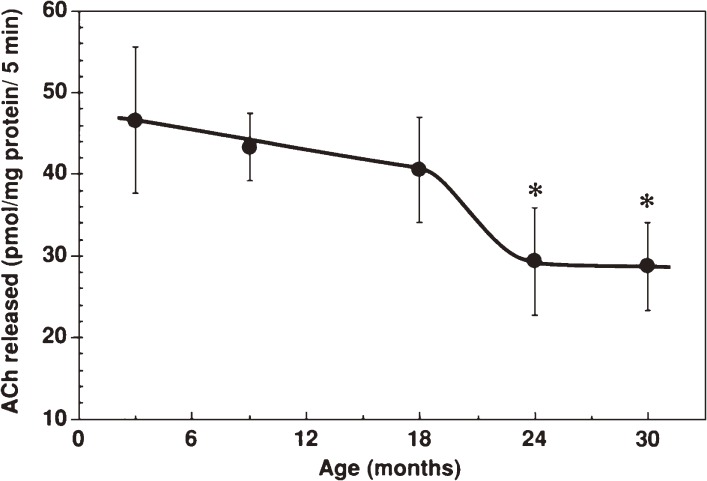
Age-related alterations of presynaptic functions were studied in terms of acetylcholine synthesis and release using synaptosomes isolated from mouse brain cortices.1) Depolarization-induced release of acetylcholine from synaptosomes significantly decreased in the senescent stage (Fig. 2). The decreased release of acetylcholine might be explained by decreased production of acetylcholine. Gibson and Peterson18) reported decreased synthesis of acetylcholine in tissue slices from aged rat brains using radio-labeled precursors. Their technique that focused only on labeled tracers might have a pitfall, that is, to miss the unlabeled fraction of acetylcholine derived from non-labeled acetyl-CoA from mitochondria. On the contrary, our experiments in which total amounts of acetylcholine produced were determined by an electrochemical detector showed that acetylcholine production rates remained constant throughout all ages tested and the acetylcholine contents in synaptosomes did not change with aging.1) Supporting this, choline acetyltransferase activity in synaptosomes remained unchanged from young to old ages. The decreases in acetylcholine release from aged synapses were thought to be ascribed to dysfunction of releasing mechanisms rather than decreased acetylcholine synthesis.
Figure 2.
Age-related decrease of acetylcholine release from synaptosomes isolated from different age-groups of C57BL/6 mice.1) Synaptosomes were incubated with 50 mM KCL-containing Krebs-Ringer solution for 5 min at 37 ℃. Acetylcholine released was quantified and expressed as the means ± SD. *, p < 0.01 as compared to 3-month-old mice (two-tailed Student’s t-test).
Diminished calcium influx via voltage-dependent calcium channels is thought to reduce the acetylcholine release in aged synapses.1) Age-related changes in the contribution of each voltage-dependent calcium channel to depolarization-induced calcium influx into synapses and the densities of the calcium channels in synaptic plasma membranes were investigated.19) The relative contribution of different types of calcium channels to calcium ion influx evoked by membrane depolarization was determined by measuring the inhibition of calcium influx with their specific blockers for L-, N-, P- and Q-type channels. Brain aging significantly reduced the contributions of N- and P-type channels. The densities of the calcium channels were found to be significantly decreased in aged synaptic plasma membranes as determined by binding experiments using radiolabeled specific blockers. These changes in voltage-dependent calcium channels may lead to age-related hypofunction of synaptic transmission.19)
(2). Synaptic membranes being less excitable with aging.
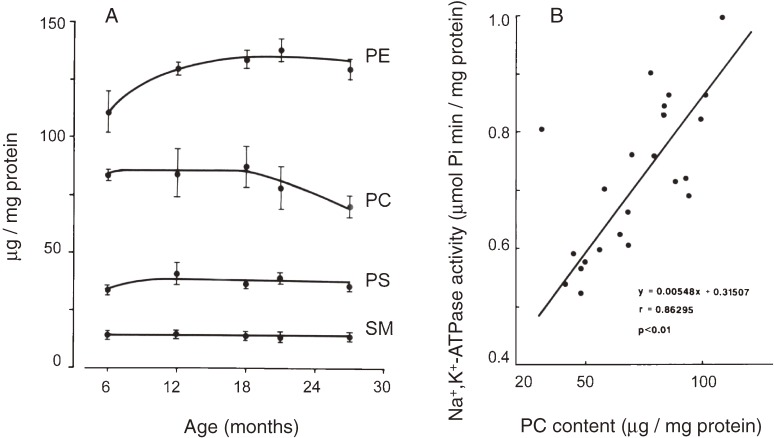
The electric excitability of synaptic plasma membranes would be another regulatory factor for synaptic transmission. As neuronal membrane depolarization activates voltage-dependent calcium channels leading to the release of transmitters, resting membrane potential prior to depolarization has to be set at adequate levels. The resting membrane potential as measured using tritium-labeled triphenylmethylphosphonium ion in mouse cortical synaptosomes was found to be less negative by 10% at advancing age.2) The diminished membrane potential was consistent with the changes in intrasynaptosomal potassium concentrations that were decreased by 13% in aged mice as compared to adult mice. The activity of an electrogenic membrane enzyme, ouabain-sensitive Na+,K+-ATPase was shown to decrease by 18% in the aged as compared to other adults. As the enzyme activity is known to be regulated in part by its lipid microenvironment,20) the correlation of age-related changes in the enzyme activity with the lipid compositional changes in synaptic plasma membranes was examined. Among the membrane phospholipids, only the content of phosphatidylcholine decreased (Fig. 3) and its changes were shown to be closely correlated with the changes in the activity of the Na+,K+-ATPase (correlation factor r = 0.86, p < 0.01).2)
Figure 3.
Compositional changes in the lipids of synaptic membranes with aging (A) and correlation between Na+,K+-ATPase activity and phosphatidylcholine contents (B).2) PE, phosphatidylethanolamine; PC, phosphatidylcholine; PS, phosphatidylserine; and SM, sphingomyelin.
Another possibility for causing the decreased activity of the enzyme was examined. To test whether decreases in the number of the enzyme molecules occur during aging, binding experiments with [3H]ouabain were carried out. Scatchard analysis of the binding revealed that the maximum number of binding sites was lower in the aged, while the binding affinity for ouabain receptor remained unchanged.21) The results indicate that the density of the Na+,K+-ATPase decreases in the late senescent stage. However, the activity of the Na+,K+-ATPase was found to start declining at a much earlier stage than that at which the decreased density of the enzyme was manifested. This discrepancy may be explained by speculating that two different mechanisms responsible for the decrease of the enzyme activity are proceeding in aging, that is, the lipid microenvironment which regulates the enzyme activity starts to change at the early stage of senescence, followed by the decrease in the enzyme density in the later stage.21)
Aging processes of cholinergic synapses have been delineated in Section 1 as follows: acetylcholine release from synapses decreases due to diminished calcium influx via voltage-dependent calcium channels, and the electric excitability of synaptic membranes is reduced due to the decreased activity of the electrogenic enzyme ouabain-sensitive Na+,K+-ATPase. The decrease of the enzyme activity was speculated to be caused by the changes in membrane lipid microenvironment and decreased production of the enzyme. In addition to these two causes, other potential mechanisms are considered to be involved in the decreased acetylcholine release. As SNARE (N-ethylmaleimide-sensitive fusion factor attachment receptor) proteins are known to participate in the process of exocytosis of neurotransmitters22) (Fig. 1), the age-related changes in the protein complex should be explored in relation to the deterioration of synaptic function. The age-related decline of synaptic transmission will additionally be implicated by the decreased density of synaptic vesicles as discussed in Section 3(2).
2. Amelioration of impaired synaptic function
(1). Cholinergic enhancement by carnitine.
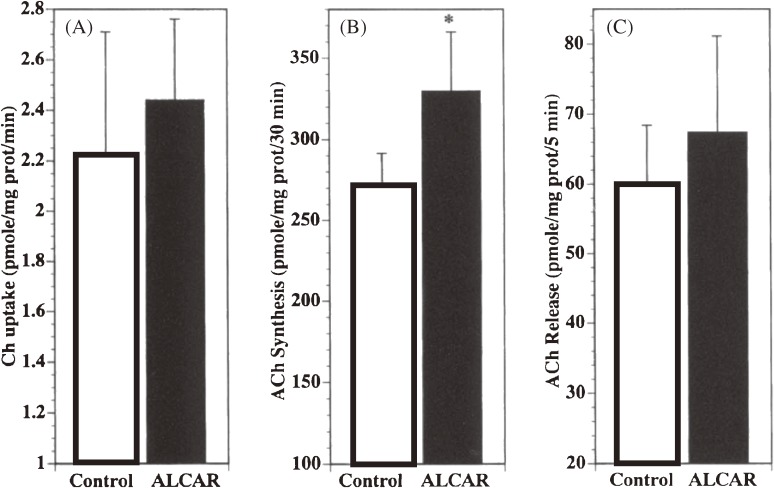
It would be highly desirable for impaired synaptic function with aging to be ameliorated and much more desirable for it to be prevented. Acetyl-l-carnitine is known to be a potential precursor for acetylcholine synthesis (see Fig. 1).23) Carnitine is acetylated by the action of mitochondrial carnitine acetyltransferase that regulates the availability of acetyl-CoA, a substrate of choline acetyltransferase. The effects of acetyl-l-carnitine on the cholinergic activity in synapses and on the cognitive function were examined with aged rats.4) The high-affinity choline uptake by synaptosomes, acetylcholine synthesis in synaptosomes and acetylcholine release from synaptosomes on membrane depolarization were all enhanced in the rats that were chronically administered acetyl-l-carnitine (Fig. 4).
Figure 4.
Effects of acetyl-l-carnitine administration on cholinergic parameters in rat cerebral cortical synaptosomes.4) High-affinity choline uptake (A), acetylcholine synthesis (B) and high-K+ (50 mM)-evoked acetylcholine release (C) are shown in comparison between the acetyl-l-carnitine group and control. *, p < 0.05 (vs. control, Student’s t-test).
Normal aging is generally thought to impair cognitive function in small animals24,25) and in humans.26,27) The cognitive deficit has been ascribed to cholinergic dysfunction. This is well known as the “cholinergic hypothesis”, as proposed by Bartus et al. in 1982.28) Based on this hypothesis, we attempted to answer the question whether acetyl-l-carnitine could ameliorate the cognitive deficit occurring with aging.4) Presenile Fischer rats (19 month-old male) were given acetyl-l-carnitine (100 mg/kg) per os for three months and were subjected to maze tests (see an example of Hebb-Williams maze test in Fig. 6) to assess their learning ability. The learning ability or learning speed of the acetyl-l-carnitine-treated group was found to be superior to that of the non-treated control. This study indicates that chronic administration of acetyl-l-carnitine increases cholinergic synaptic transmission and consequently enhances learning ability as a cognitive function in aged rats.
Figure 6.
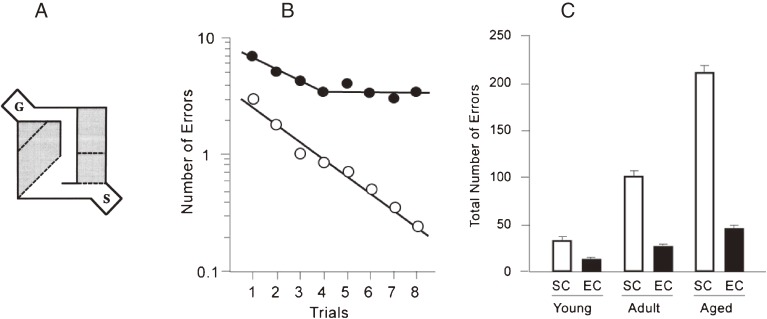
Cognitive function is deteriorated with aging in rats reared in standard social condition (SC), and the age related cognitive dysfunction is largely ameliorated by an enriched environment (EC).12) To assess the cognitive function, Hebb-Williams maze problems were employed. A: The maze pattern of one problem out of Hebb-Williams maze problems is shown. Shadowed areas indicate error zones and broken lines indicate the borders for counting errors when rats cross the lines. S and G indicate start and goal boxes, respectively. B: Changes of the number of errors in the Hebb-Williams maze test with training (Trials 1–8) are drawn in logarithmic plot. Two fitted lines show the learning rates in the case of aged rats that were reared in SC or EC. Other plots for the cases of young and adult rats12) are not shown. C: Effects of different ages and environmental conditions on cognitive function are shown by the total number of errors in the Hebb-Williams maze test. Error bars indicate standard error of means.
(2). Restoration of synaptic function in lesioned brains through administration of carnitine.
Disease-related synaptic damage is observed in the brains of patients with Alzheimer’s disease. The synaptic pathology is thought to be the major correlate of cognitive impairment or dementia.29) Enhancement of the activity of residual still-intact synapses could be a strategy with a therapy for dementia. An animal dementia model was established and the effect of acetyl-l-carnitine on cognitive deficit was examined with the dementia model.30) A rat dementia model was generated by synapse-specific lesions using botulinum neurotoxin type B in the entorhinal cortex. Cognitive deficit in lesioned aged rats was detected by continuous alteration and delayed non-matching-to-sample tasks in a T-maze. Chronic administration of acetyl-l-carnitine over time during pre- and post-lesion periods appeared to partially ameliorate the cognitive deficit caused by the synaptic lesion. The recovery from synaptic damage was observed by electron microscopy. Large synapses full of synaptic vesicles were seen as well as many perforated synaptic contacts in the toxin-injected and carnitine-treated group, while heterogeneous synapses with sparse synaptic vesicles were observed in the toxin-injected and non-carnitine-treated group. This study demonstrates that the botulinum toxin-lesioned rat can be used as a model for dementia and that cognitive deficit can be alleviated in part by acetyl-l-carnitine administration.30)
(3). Enhancement of synaptic plasticity by ganglioside GQ1b.
Long-term potentiation (LTP) that is defined as persistent enhancement of synaptic transmission induced by a brief period of electrical stimulation of afferents is assumed to be involved in the cellular basis of learning and memory.31) Wieraszko and Seifert first demonstrated an enhancement of LTP in hippocampal slices in which the content of ganglioside GM1 was increased.32) We attempted to enhance LTP in hippocampal neurons by the treatment with gangliosides GM1 and GQ1b that had been known to localize in the synaptic membranes.5,33) Tetrasialoganglioside GQ1b was shown to be more potent in the action of enhancing LTP than monosialoganglioside GM1. In addition, the application of GQ1b reversed the blocking effect of an NMDA-receptor antagonist, APP-5, on the induction of LTP, resulting in forming LTP.5) In another experimental setting, GQ1b was shown to enhance ATP-induced LTP, suggesting a cooperative effect of extracellular ATP and GQ1b in the formation of LTP.33) These results may indicate that ganglioside GQ1b enhances the LTP formation through the modulation of NMDA receptors and calcium ion channels. The mice transfected with the β1,4-N-acetylgalactosaminyl-transferase gene were tested for the efficacy of LTP formation by Igarashi et al.34) The hippocampal CA1 neurons from the transgenic mice that expressed low levels of B-pathway gangliosides, including GQ1b in the brain, showed a smaller magnitude of LTP than those from wild-type mice. The transgenic mice had learning deficits as shown using a 4-pellet-taking test. Thus, it is suggested that ganglioside GQ1b is involved in the mechanism of synaptic plasticity and the improvement of cognitive function.
(4). Synaptic function of cholinergic ganglioside Chol-1α.
Possible involvement of gangliosides in neurotransmission has been the subject of speculation based on studies with Guillain-Barré syndrome. Roberts et al.35) reported that muscle weakness in the Miller-Fisher variant of Guillain-Barré syndrome might be caused by the anti-GQ1b antibody that led to failure of acetylcholine release from motor nerve terminals. Although many studies using antibodies suggest the possible involvement of endogenous gangliosides in synaptic function, it has not yet been determined what specific gangliosides participate in any particular functions. Whittaker and his colleagues36) found that cholinergic-specific antigens were of a ganglioside nature, and named them Chol-1 that was composed of three groups, Chol-1α, Chol-1β and Chol-1γ. Our group isolated and characterized two molecular species corresponding to Chol-1α, termed GT1aα and GQ1bα, from bovine brains.6,7) The structure was finally confirmed by chemical synthesis.37) The unique branching structure of a sialic acid residue linked at the 6th position of N-acetylgalactosamine in the ganglioside backbone was assigned to the α-structure, based on our previous report.38) Chol-1α was localized in cholinergic nuclei such as the septal nucleus by immunostaining.39) The finding of Chol-1α as cholinergic specific gangliosides encouraged us to examine the physiological function of the endogenous Chol-1α.
To examine the involvement of various gangliosides in the cholinergic function of synapses, a series of monoclonal antibodies against gangliosides was employed in order to test their potency to suppress the acetylcholine release. None of the antibodies except anti-Chol-1α antibody, GGR-41, affected acetylcholine release.8) To know the reason for the effect of GGR-41, choline uptake and acetylcholine synthesis were measured with synaptosomes in the presence of GGR-41. Choline uptake and acetylcholine synthesis both were inhibited dose-dependently by GGR-41. When one molecular species of Chol-1α, GT1aα, was added to a synaptosomal fraction, the choline uptake was accelerated, resulting in the enhancement of acetylcholine synthesis.8) In a control experiment, GT1a, a stereoisomer of GT1aα, had no effect on choline transport and acetylcholine synthesis, suggesting the specificity of GT1aα.
An interesting question is raised whether the Chol-1α gangliosides physiologically participate in the cognitive function of the brain. To answer this question, a monoclonal antibody against Chol-1α, GGR-41, was continuously infused into the septal area of rat brains by an osmotic pump to disrupt the septohippocampal cholinergic pathway (Fig. 5).8) The learning ability of the rats infused with GGR-41 was remarkably reduced to the same extent as seen in the rats given mecamylamine, a nicotinic cholinergic receptor antagonist. The memory retention also was severely impaired in the rats infused with GGR-41. Chol-1α is thought to be of a nicotinic character, because it was originally found in torpedo electric organs that are composed of pure nicotinic nerve terminals. These results indicate that Chol-1α accelerates the choline uptake and acetylcholine synthesis at the synapses, and Chol-1α is involved in the cognitive function of the brain.
Figure 5.
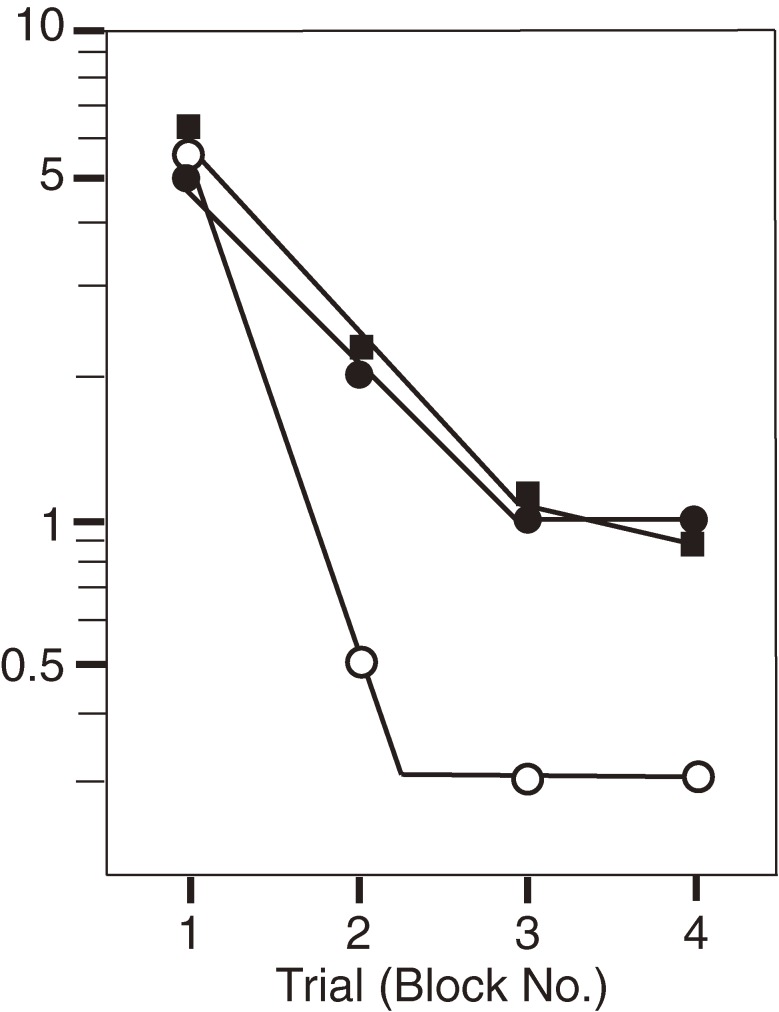
Effect of anti-Chol-1α antibody, GGR-41, on learning activity as assessed by Hebb-Williams maze #8.8) Learning rates were severely retarded in the rats given GGR-41 or mecamylamine, a nicotinic cholinergic receptor antagonist, compared with sham-operated rats. Open circles indicate controls that received only the vehicle; closed circles, rats given GGR-41 and closed squares, rats given mecamylamine.
(5). Enhancement of synaptic function by ganglioside analogues.
Since sialic acid-containing glycolipids, gangliosides, had been shown to activate cholinergic function as mentioned above, chemically synthesized sialic acid-containing compounds were examined for their effects on cholinergic synapses. Synthetic α- and β-stereoisomers of sialylcholesterol were employed as ganglioside analogues in our study.40) Two stereoisomers of sialylcholesterol were separately added to synaptosomes, and were both shown to stimulate high K+-evoked acetylcholine release from synaptosomes. To elucidate the underlying mechanisms by which these sialyl compounds enhanced the acetylcholine release, their effects on calcium ion influx and choline uptake were examined using a fluorescence calcium indicator and [3H]choline, respectively. Alpha-sialylcholesterol increased the depolarization-induced influx of calcium ions resulting in an increment of acetylcholine release, but did not affect the choline uptake. On the other hand, β-sialylcholesterol activated high-affinity choline uptake, resulting in the enhancement of acetylcholine synthesis followed by the augmentation of acetylcholine release. The β-isomer had no effects on calcium ion influx. These results imply that the two isomers of sialylcholesterol modulate the synaptic membrane mechanisms in different ways and that both mechanisms may synergistically act on synapses to enhance acetylcholine release.
The results obtained by these experiments indicate that synthetic sialylcholesterols mimic naturally occurring gangliosides in their action on synapses. Alpha-sialylcholesterol seemed to activate the calcium channels in a similar manner as ganglioside GQ1b.5,41) The β-isomer, on the other hand, was revealed to activate the choline transporter just like Chol-1α gangliosides.8,40) The mimicry of the synaptic action exerted by gangliosides and sialylcholesterols is summarized in Table 1.42)
Table 1.
Effects of sialyl compounds on presynaptic functions42)
| Acetylcholine release | Ca ion influx | Choline uptake | Acetylcholine synthesis | |
|---|---|---|---|---|
| Gangliosides | ||||
| GQ1b | ⇑ | ⇑ | → | → |
| Chol-1α | ⇑ | → | ⇑ | ⇑ |
| Sialylcholesterol | ||||
| α-isomer | ⇑ | ⇑ | → | → |
| β-isomer | ⇑ | → | ⇑ | ⇑ |
Note: ⇑, increase; →, no change.
3. Enhancement of brain plasticity by an enriched environment
Neuronal plasticity, that is a crucial requirement for adapting to environmental stimuli to improve cognitive potency, is a central theme of neuroscience through basic research with animals to rehabilitation for humans. The stimuli required to elicit plasticity are thought to be experience-dependent and to enhance synaptic efficacy. Hebb43) first noted that rats reared as pets in his house performed better on a maze task compared with rats housed in standard laboratory cages. Based on the observation, he proposed the ‘enriched environment’ as a concept for plasticity in small animals. Rosenzweig and colleagues44) later introduced enriched environments as a testable scientific concept. They made reports in many papers giving evidence of the effects of environmental stimuli on the adult brain such as brain weight gain and developed dendritic arborization. To elucidate the effects of an enriched environment on aged brains, its effects on cognitive functions such as learning and memory were examined first in our study. In the second attempt, the changes in the densities of synapses in the brain were investigated with aged rats which received enriched environmental stimuli.
(1). Amelioration of cognitive function in aged rats by an enriched environment.
Although some groups showed improvements in the cognitive function of aged animals when reared in enriched environments,45) others failed to show significant improvements.46–48) This inconsistency may indicate some difficulty in detecting small changes in cognitive improvement between two animal groups that were housed in enriched environments and standard laboratory cages, respectively. On the contrary, it seems much easier to find differences in cognitive capacity achieved between animals reared in an enriched environment and those housed in an impoverished condition.47) The latter condition, that is living alone in a small cage, is thought to be stressful because of no social contacts. Considering animal welfare, therefore, impoverished conditions should not be used for animal experiments. Even though the differences to be detected would not be large, the comparison of cognitive improvements should be done between animal groups reared in enriched environmental conditions (EC) and those housed in standard laboratory cages as a control that may provide minimal social contacts (standard social conditions, SC). EC were created in a large cage (120 × 50 × 40 cm) containing various toys and small constructions as environmental stimuli where 12 rats were reared together, while SC were set up in a small cage where three rats lived.10)
It might be due to the variety of experimental procedures that the outcomes were not always consistent among studies of the effects of EC on animals reported so far. Controlling factors to be considered for this kind of behavioral experiment are as follows: timing and duration of exposure to EC and methods sensitive enough for detecting the changes in cognitive capacity. Many papers were published on the effect of EC on cognitive function, but no studies have examined whether the timing and duration of exposure to EC affect the outcomes. In our study,12) rats were exposed to EC from weaning until the age of 2.5, 15, or 25 months to see the effects of the duration of EC. Short-term (3 months) exposure to EC was carried out with adult rats (11 months old) and aged (22 months old), respectively, to see the effects of different timing of EC.
A sensitive method for detecting the changes in cognitive function was developed.12) To assess the intelligence of small animals, the Hebb-Williams maze task49) has been employed most frequently for examining problem-solving ability. The Hebb-Williams maze is composed of a series of 12 maze problems (Fig. 6A shows one of those). Those problems seemed to be heterogeneous regarding effective cognitive assessment. Eight maze problems out of the 12 were selected for measuring the learning ability of rats, because they were proved to be sensitive for detecting the changes in cognitive function due to aging and environmental manipulation.12)
Cognitive function as measured by the selected problems of the Hebb-Williams maze was examined with rats that had been exposed to EC for periods of variable duration and at different ages. It was revealed that rats reared in EC from weaning to the senescent stage were superior in the maze performance to ones reared in SC (Fig. 6). Our long-term rearing experiments definitely showed the positive effects of EC on learning capacity,12) whereas previous studies45,47) had failed to show any improvements in the cognitive function of rats reared in EC for long periods. Also it was shown that a short-term (3 months) exposure to EC enhanced the cognitive function of both adult and aged rats.12)
(2). Synaptic plasticity in response to an enriched environment.
Synaptic failures with aging are supposed to underlie deteriorating brain function. Age-related changes in the functional aspects of synapses are described in Sections 1 and 2. These changes include decreased acetylcholine release and reduced induction of long-term potentiation. On the other hand, synaptic dysfunction may be interpreted by observing aspects of morphological change in synapses. Age-related changes in synaptic density have not been understood in detail. Morphometrical studies have showed decreases in synaptic density to various degrees. Each study was focused on particular regions, such as the human frontal cortex,50) the cat caudate nucleus,51) and the rat hippocampal dentate gyrus.52) However, regional variations and age-related changes in synaptic density have not been covered in the literature. We attempted first to delineate the region-specific synaptic densities in the rat brain and their region-characteristic changes with aging. The next attempt was to answer the question of what plastic changes of synapses would occur in the brain of aged rats that were exposed to EC.
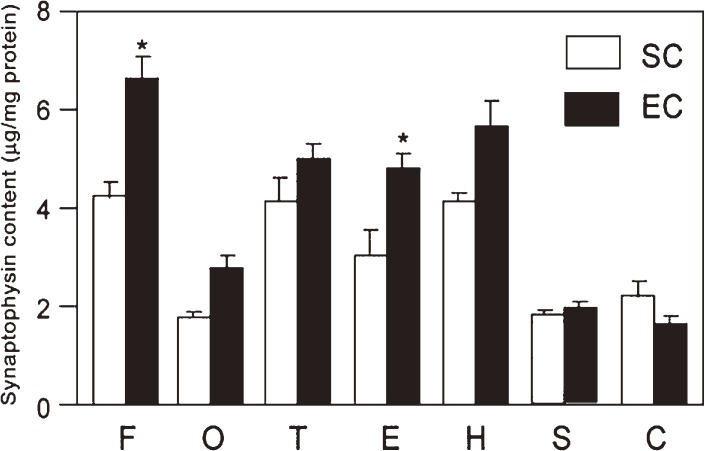
To measure the synaptic density, synaptophysin (glycoprotein p38) that was known to be localized at synaptic vesicles was determined using an enzyme-linked immunosorbent assay. Synaptophysin contents were determined in seven brain regions of rats reared in standard laboratory cages (SC group) at their ages of 3, 12, 24 and 33 months (Fig. 7).10) The synaptophysin contents decreased in every region in the aged brain, and the largest decrement occurred in the occipital cortex. In another experiment, synaptophysin contents were determined in the same brain regions of rats reared in enriched environmental conditions (EC) from weaning until 30 months of age. The synaptophysin contents in EC rats were revealed to be greatly increased compared to those in SC rats of the same advanced age (Fig. 7).
Figure 7.
Effects of different environmental conditions on synaptophysin contents.10) Two groups of rats (30 months) maintained in standard social condition (SC) or enriched environmental condition (EC) were examined for the synaptophysin contents in various brain regions. F, O, T and E are frontal, occipital, temporal and entorhinal cortices; H, S and C are hippocampus, striatum and cerebellum, respectively. The data represent mean values ± SEM. Statistically significant differences are observed for frontal and entorhinal cortices.
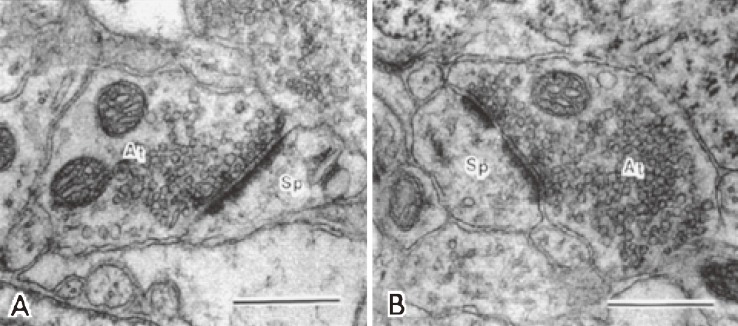
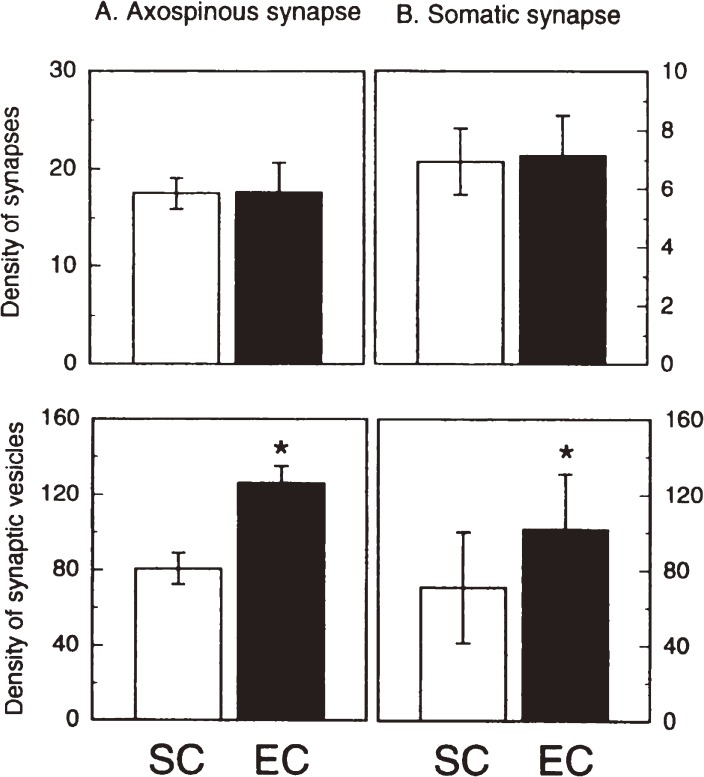
As synaptic plasticity was shown to be elicited in aged rats exposed to EC using a synaptic marker synaptophysin, we attempted to observe the morphological changes of synapses by electron microscopy. A critical question was which of the results might occur in response to EC, increase in the numerical density of synapses or increase in the packing density of synaptic vesicles. The former corresponds to synaptogenesis and the latter to synaptic enlargement or synaptic strengthening. Numerical synaptic density and synaptic vesicle density in the fifth layer of the neocortex of rats were measured as a function of age. The fifth layer was reported to be vulnerable to aging.53) Electron micrographs of synapses appeared different in the shape of synapses and the density of synaptic vesicles between two groups of rats that were housed in SC and EC (Fig. 8). The quantitative data in Fig. 9 turned to be unexpected results.11) Synaptic density remained the same in both groups, but the density of synaptic vesicles in either axospinous and somatic synapses increased in the EC group compared to the SC group. This may indicate that not synaptogenesis but synaptic strengthening occurs in the brains of rats exposed to EC. In contrast, studies reported so far suggest that synaptogenesis might occur in adult brains.54,55) Based on our observations, we present a new concept that activity-dependent plasticity of synapses in mature and aged brains is elicited as enlargement or strengthening of existing synapses, but not as formation of new synapses or synaptogenesis.
Figure 8.
Electron micrographs of axospinous synapses in the fifth layer of rat cortices at 30 months of age.11) At, axon; and Sp, spine. Scale bars = 0.5 µm. A: An axospinous synapse in the rat reared in standard social condition. An asymmetric and nonperforated synapse contains spherical vesicles. B: An axospinous synapse in the rat reared in enriched environment. An asymmetric and perforated synapse is filled with more abundant vesicles.
Figure 9.
Effects of different environmental conditions on synaptic morphological parameters in the fifth layer of the neocortex of rats aged 30 months.11) A: Synaptic parameters of axospinous synapses. B: Synaptic parameters of somatic synapses. Top: Density of synapses (number per 100 µm2 for axospinous synapses, and number per 100 µm for somatic synapses). Bottom: Density of synaptic vesicles (number per µm2). SC and EC represent standard and enriched condition groups, respectively. Error bars show standard deviations. Asterisks indicate significant differences (P < 0.01).
4. Age-related alterations in neuronal membrane metabolism and its modulation by dietary restriction
Enriched environmental stimuli as external environments are revealed to elicit brain plasticity as described in the previous section. Nutrition as an internal environment also has been known to affect brain development.56) Starvation or malnutrition is an issue of concern in early brain development. On the other hand, dietary restriction or calorie restriction with sufficient nutrients in adult stages is recognized to extend life spans57) and to attenuate age-related disease states in animals.58) Dietary restriction has been found to ameliorate cognitive aging too.59) Synaptic plasticity was shown to be enhanced by dietary restriction as evidenced by increased long-term potentiation.60) It is speculated that dietary restriction modulates the neuronal metabolism so that the neuronal membranes altered with aging can be juvenilized. One question is whether the metabolic turnover of neuronal membranes could be changed by dietary restriction in a favorable manner in order to reduce age-associated deficits. To address the question, we have developed a method using stable isotope-labeling and mass spectrometry to measure the metabolic turnover of neuronal membranes.61) In the method, animals are given heavy water (D2O) that passes through the blood-brain barrier. D2O molecules distribute throughout the whole brain and deuterium is incorporated into the lipids, proteins and carbohydrates in membranes via physiological metabolic reactions. Some disadvantages experienced in usual tracer experiments using labeled biomolecules with limited diffusibility are overcome by our D2O-labeling method. Synthesis rates of neuronal membrane components can be measured by the rates of labeling with deuterium and turnover rates of each component can later be measured by disappearance rates of the endogenously labeled molecules from neuronal membranes (Fig. 10).14)
Figure 10.
Incorporation into and disappearance from myelin of deuterated cholesterol in mice given 30% D2O. The turnover rate of cholesterol as expressed by Fraction I (closed circles) was obtained by subtracting the contribution of recycling components, Fraction II (open triangles), from the total deuterated cholesterol (open circles).14)
Mice were divided into two groups, an ad libitum-fed control group and a dietary-restricted group that received 70% of the ad libitum-fed calories. Both groups were given diluted D2O as drinking water for 10–20 days, and thereafter every five mice were killed at various intervals from day zero (the third day after the cessation of D2O administration) to day 70. Lipids were separated from synaptic plasma membranes. Synaptophysin, a major membrane protein specific to synaptic vesicles, was isolated from synaptosomes and degraded to produce amino acids. Deuterated lipids and amino acids were determined by mass spectrometry. To delineate the molar distribution of deuterated molecules, the deuterated and non-deuterated compounds in the derivatives were determined in a selected-ion mode by gas chromatography-mass spectrometry. Disappearance curves of the deuterated compounds from synaptic membranes were drawn using our method.14)
The disappearance curves for each fatty acid constituent of phosphatidylethanolamine appeared to be distinct from each other. Palmitic acid had the shortest half life-time, and half-lives increased in the order of stearic acid, palmitoaldehyde, arachidonic acid and docosatetraenoic acid. The recycling or reuse of precursors would result in apparently longer half-lives than the true values. Other studies using radio-isotopic precursors reported that the half-life of phosphatidylethanolamine in the synaptic membranes was 11.8 days in an adult mouse.62) In contrast, our study revealed the half-life to be much shorter, 5.7 days as shown in Table 2.15) Our method eliminates the contribution due to recycling of labeled molecules so that nearly true disappearance curves of initially incorporated membrane constituents can be drawn. The half-lives of two major phospholipids in synaptic membranes are listed in Table 2. Phosphatidylethanolamine appeared to be more rapidly metabolized than phosphatidylcholine. The turnover rates of both phospholipids were faster in young mice (2 months old) than in adult (6 months old) and aged mice (22 months old) reared ad libitum. Under the dietary restricted conditions, the turnover rates of both phospholipids became slow.
Table 2.
Half-lives (days) of synaptic membrane components in the mouse brain15)
| Age (months) | PE (16:0) | PC (16:0) | Cholesterol | Synaptophysin |
|---|---|---|---|---|
| 2 | 5.7 | 8.7 | 74 | n.d. |
| 6 | 7.6 | 10.8 | 158 | 12.7 |
| 22 (ad libitum) | 7.2 | 11.0 | 87 | 15.1 |
| 22 (dietary restricted) | 9.4 | 14.2 | 120 | 10.4 |
PE, phosphatidylethanolamine; PC, Phosphatidylcholine; 16:0, palmitic acid-containing species; n.d., not determined.
To our knowledge, the turnover of cholesterol in synaptic membranes has not been reported on. The turnover rate of cholesterol was rapid in young brains (half-life time T1/2, 74 days) and slowed down in adult brains (T1/2, 158 days) as shown in Table 2.15) To our surprise, the turnover of cholesterol appeared to be accelerated in aged brains, unlike the turnover of phospholipids. When animals were reared under dietary-restricted conditions, the turnover rate of cholesterol in aged brains was found to remain at a level near that seen in adult brains.
With respect to the turnover of proteins in the brain, most previous studies did not specify protein species but presented average metabolic rates for total proteins. We focused on synaptophysin because it is known as a major constituent of synapses, and determined its turnover in adult and aged mouse brains (Table 2).15) The turnover rate of synaptophysin appeared to be slower in senescence than in the adult stage. The decreased turnover rate of synaptophysin in senescence was accelerated by dietary restriction. This metabolic response to dietary restriction is quite the reverse observed in the metabolism of phospholipids and cholesterol. The accelerated turnover of synaptophysin seemed to support the observation by Takahashi and Goto63) in that the removal of damaged proteins was enhanced in the brains of dietary restricted mice. This may raise the idea that dietary restriction increases the turnover of proteins to promote the replacement of damaged constituents and decreases the turnover of lipids to slow the age-associated alterations in the lipid composition so as to maintain the membranes in a youthful condition.
5. Extrapolation of animals to humans with perspectives
Age-related declines in physiological functions, especially of the central nervous system, are key issues in the challenge to delay the onset of frailty and promote healthy aging. Synaptic hypofunction associated with normal aging and synaptic failure caused by degenerative diseases could be ameliorated by environmental manipulation. Enriched rearing conditions as external environments and nutritional interventions as internal environments were shown to exert beneficial effects on the brain in terms of cognitive capacity and underlying metabolism in animal aging models as mentioned in Sections 3 and 4. Some of the findings with animals will be worthwhile to be discussed in relation to their extrapolation to human cases.
(1). Expression of unique gangliosides in diseases and its diagnostic and therapeutic significance.
We isolated a new series of gangliosides named C-series from fish brains.64) The C-series of gangliosides was found to be expressed in embryonic chicken brains and assigned as fetal antigens.65) An immunohistological study on Alzheimer’s brains was performed using monoclonal antibodies against C-series gangliosides. Senile plaques were stained with the anti-C-series ganglioside antibodies,66) indicating that expression of glyco-genes characteristic for the fetal brain may occur in Alzheimer’s brains. This may imply some neuronal regeneration occurring in degenerative brains.67,68)
Guillain-Barré syndrome is an autoimmune disorder affecting the peripheral nervous system causing neuromuscular paralysis, usually triggered by acute microbial infections. Antibodies against a wide variety of gangliosides including O-acetylated species have been detected in the patient sera.69) The anti-ganglioside antibodies are likely to damage peripheral nerves through the impairment of voltage-dependent sodium channels. To eliminate the pathogenic antibodies, anti-ganglioside anti-idiotype monoclonal antibody (BEC 2)70) and a ganglioside-like peptide (P(GD3)-4)71) were administered using an animal model of Guillain-Barré syndrome. The treated animals had a remarkable reduction of the anti-ganglioside antibody titers and improvement of motor nerve functions. These successful experiments to neutralize pathogenic anti-ganglioside antibodies will open a new window to a therapy for Guillain-Barré syndrome.
(2). Therapeutic trials of carnitine.
We observed that carnitine enhances acetylcholine synthesis in synapses and restores the cognitive deficits in normal aging animals and with dementia animal models.4,30) While clinical trials of carnitine on dementia patients have not been performed in Japan yet, many trials were reported in other countries. An outstanding work by Montgomery et al.72) on the meta-analysis with the reported data from 22 papers provided definite evidence for the beneficial effect of carnitine on Alzheimer’s disease patients at early disease stages. Based on these favorable results obtained from the basic and clinical research, we would like to propose that it should benefit aged people to enhance the cognitive reserve through the supplementation of carnitine starting from presenile periods, possibly leading to a delayed onset of dementia.
In the course of the study to see the effect of carnitine on acetylcholine synthesis (Section 2(1)), aged animals given carnitine were shown to lose weight.73) This was supposed to be caused by increased fatty acid oxidation to reduce fat deposits. Long-term carnitine supplementation was revealed to decrease the cholesterol content and cholesterol-phospholipid ratio in the cerebral cortex of aged rats as well as the levels of triacylglycerol and cholesterol esters in plasma.73) These lipid-lowering effects of carnitine were not observed in plasma and brain tissues of young rats. In a clinical trial, a woman aged 84 years who originally suffered from obesity (89 kg; BMI, 41), type 2 diabetes, dermatitis, decubitus and constipation was given carnitine for 9 months.16) She lost 23 kg (BMI, 31) and recovered from most of her disease states, resulting in much improvement in daily life activity. Carnitine may benefit aged people with severe obesity.
(3). Environmental interventions with elderly people.
Enriched environmental conditions were revealed to enhance the cognitive function in aging animals (Section 3). Most promisingly, interventions affecting cognitive activity might improve the cognitive function and delay the onset of dementia in humans. The Nun Study74) (longitudinal cohort study that enrolled 801 older Catholic nuns with mean follow-up of 4.5 years) obviously indicates that frequent participation in cognitively stimulating activities is associated with reduced risk of Alzheimer’s disease. On the other hand, physical activity is known to enhance cognitive function. The Hisayama Study,75) an epidemiological study performed in the town of Hisayama in Kyushu, Japan, reported for the first time that moderate physical activity has a preventive effect on Alzheimer’s disease. Following this, several papers on interventional studies reported that physical activity reduced the risk of cognitive impairment and dementia in the elderly.76–78) The question has been raised, which really has an effect on cognition, cognitive stimulation or physical activity? In a well-controlled animal experiment in which the effects of learning and physical activity components were separately observed, it was shown that the learning component was related to increased expression of neurotrophic factors underlying neuroplasticity, while physical activity was connected with only a minor increase in the expression.79) The Nun Study74) also indicated that risk of Alzheimer’s disease was not significantly reduced by physical activity. Physical activity in exercise training that was reported to reduce the risk of cognitive impairment might include a learning component exerting cognitive stimulation. This may provide us with the realization that active lifestyles, including cognitive stimulation, improve cognitive function which leads to successful aging.
Intervention with nutritional conditions is considered to be another key factor to modulate internal environments of the body for enhancing neural and physical activities. Dietary restriction without malnutrition at adult stages is known to extend life spans and delay the onset of age-related diseases in animals.57) Dietary restriction was shown to delays age-related diseases, brain atrophy and mortality in a primate species, rhesus monkeys.80) Although it is thought to be not feasible to conduct studies on the effects of dietary restriction on longevity of humans, a challenging project has been started in the U.S.A., that is, the CALERIE (Comprehensive Assessment of Long-Term Effects of Reducing Calorie Intake) research program.81) Non-obese healthy people were enrolled as participants in Phase 2 of the project. Two years of sustained dietary restriction, involving a 25% reduction of ad libitum energy intake, resulted in beneficial effects similar to those observed in animal studies.82) In another study on humans, dietary restriction was shown to increase verbal memory scores.83) These studies indicate that restricted calorie intake benefits elderly humans in terms of cognitive function.
Poor nutrition is an issue of concern in the health management of old people. We found an unexpectedly high prevalence of folate deficiency (38%) among elderly patients in a long-term care health facility.17) Folate deficiency has been implicated in various adverse outcomes such as neural-tube defects as inborn errors and pernicious anemia. For elderly people, low folate status is reported to be correlated with senile dementia84) and fractures.85) In order to correct the folate deficient states, we employed a method of nutritional supplementation to meals using folic acid-fortified rice. The folate-deficient states were ameliorated within 6 months and the elevated levels of serum folate improved macrocytic anemia.17) A unique project of folic acid-supplementation for community-dwelling people has been carried out in Sakado City, Saitama since 2006. This project has been promoted by Yasuo Kagawa’s group in Kagawa Nutrition University and successfully resulted in tremendous reduction of medical expenses by preventing cardiovascular diseases, fractures and so on.86)
Concluding remarks
Aging processes of cholinergic synapses have been delineated as follows: ① acetylcholine release from synapses decreases due to diminished calcium influx via voltage-dependent calcium channels, and ② the electric excitability of synaptic plasma membranes is reduced due to the decreased activity of the electrogenic enzyme ouabain-sensitive Na+,K+-ATPase. Gangliosides were found to enhance the synaptic function. Ganglioside GQ1b stimulates the depolarization-evoked calcium influx and ganglioside Chol-1α activates the high-affinity choline uptake transporter, indicating that these functional gangliosides and their chemical analogues could potentially be used for ameliorating synaptic functional failure. A cholinergic enhancer, carnitine was revealed to restore the cognitive deficits in aged animals.
Enriched environmental stimuli enhanced the cognitive function in rats throughout their life spans. The cognitive enhancement was speculated to be elicited by synaptic development. The findings lead to a new concept for synaptic plasticity, that is, enlargement of existing synapses does occur in mature and aged brains rather than synaptic proliferation. Dietary restriction, a nutritional intervention, appeared to prevent the age-related alterations in neuronal membrane metabolism, supporting a mechanism for elongating life spans. Some of these findings with animals will be worthwhile considering for their value in extrapolation to human cases.
Acknowledgements
I would like to thank Prof. Tamio Yamakawa, M.J.A. for developing my ganglioside research career starting from a study on the blood-group substance glycolipids in his laboratory and for encouraging me in performing succeeding research. I am grateful to Dr. Kunihiko Suzuki, M.J.A. (Communicator of this article) for his generous support to my research in the U.S.A. My collaborators and colleagues are greatly appreciated for their excellent achievements in this study.
Profile
Susumu Ando graduated from Tokyo Medical and Dental University in 1966 to obtain M.D. He received Ph.D. from The University of Tokyo in 1971 for the study on blood-group A substance in erythrocyte membranes under the direction of Prof. Tamio Yamakawa. Dr. Ando held a research position at Tokyo Metropolitan Institute of Gerontology (1971–2004). He stayed at Yale University, U.S.A., as a senior research scientist of the Neurology Department (1976–1978). After coming back to Japan, he was promoted to the Vice-Director of Tokyo Metropolitan Institute of Gerontology. He has established the chemical structures of C-series and α-series of brain gangliosides. His ganglioside research story is published in a review article (Ando, S. (1983) Gangliosides in the nervous system. Neurochem. Int. 5, 507–537). Another research interest of Dr. Ando was directed to the metabolic changes in the brain with aging. To perform the study, a new methodology using stable isotopes was developed early in 1981, and it is recognized to be a prototype of present metabolomics as described in a review article (Ref. 61). He was awarded Japanese Biochemistry Society Award for his glycolipid study and Sandoz Research Award for his work on brain aging. He was given the Lands Award from Japan Society for Lipid Nutrition for his study on lipolytic and anti-brain aging effects of carnitine in 2010. He served as the Vice-President of Japan Society for Lipid Nutrition (1992–2007). He is now working in a geriatric health care facility as the director, and his recent research interest is focused on the amelioration of malnutritional states in elderly patients (some data seen in Refs. 16 and 17).
References
- 1).Tanaka Y., Hasegawa A., Ando S. (1996) Impaired synaptic functions with aging as characterized by decreased calcium influx and acetylcholine release. J. Neurosci. Res. 43, 63–70 [DOI] [PubMed] [Google Scholar]
- 2).Tanaka Y., Ando S. (1990) Synaptic aging as revealed by changes in membrane potential and decreased activity of Na+,K+-ATPase. Brain Res. 506, 46–52 [DOI] [PubMed] [Google Scholar]
- 3).Ando S., Tanaka Y. (1990) Synaptic membrane aging in the central nervous system. Gerontology 36 (Supple. 1), 10–14 [DOI] [PubMed] [Google Scholar]
- 4).Ando S., Tadenuma T., Tanaka Y., Fukui F., Kobayashi S., Ohashi Y., Kawabata T. (2001) Enhancement of learning activity and cholinergic synaptic function by carnitine in aging rats. J. Neurosci. Res. 66, 266–271 [DOI] [PubMed] [Google Scholar]
- 5).Furuse H., Waki H., Kaneko K., Fujii S., Miura M., Sasaki H., Ito K.-I., Kato H., Ando S. (1998) Effect of the mono- and tetra-sialogangliosides, GM1 and GQ1b, on long-term potentiation in the CA1 hippocampal neurons of the guinea pig. Exp. Brain Res. 123, 307–314 [DOI] [PubMed] [Google Scholar]
- 6).Ando S., Hirabayashi Y., Kon K., Inagaki F., Tate S., Whittaker V.P. (1992) A trisialoganglioside containing a sialyl-α2,6-N-acetylgalactosamine residue is a cholinergic-specific antigen, Chol-1α. J. Biochem. 111, 287–290 [DOI] [PubMed] [Google Scholar]
- 7).Hirabayashi Y., Nakao T., Irie F., Whittaker V.P., Kon K., Ando S. (1992) Structural characterization of a novel cholinergic neuron-specific ganglioside in bovine brain. J. Biol. Chem. 267, 12973–12978 [PubMed] [Google Scholar]
- 8).Ando S., Tanaka Y., Kobayashi S., Fukui F., Iwamoto M., Waki H., Tai T., Hirabayashi Y. (2004) Synaptic function of cholinergic-specific Chol-1α ganglioside. Neurochem. Res. 29, 857–867 [DOI] [PubMed] [Google Scholar]
- 9).Cattell R.B. (1963) Theory of fluid and crystallized intelligence: A critical experiment. J. Educ. Psychol. 54, 1–22 [DOI] [PubMed] [Google Scholar]
- 10).Saito S., Kobayashi S., Ohashi Y., Igarashi M., Komiya Y., Ando S. (1994) Decreased synaptic density in aged brains and its prevention by rearing under enriched environment as revealed by synaptophysin content. J. Neurosci. Res. 39, 57–62 [DOI] [PubMed] [Google Scholar]
- 11).Nakamura H., Kobayashi S., Ohashi Y., Ando S. (1999) Age-changes of brain synapses and synaptic plasticity in response to an enriched environment. J. Neurosci. Res. 56, 307–315 [DOI] [PubMed] [Google Scholar]
- 12).Kobayashi S., Ohashi Y., Ando S. (2002) Effects of enriched environments with different durations and starting times on learning capacity during aging in rats assessed by a refined procedure of the Hebb-Williams maze task. J. Neurosci. Res. 70, 340–346 [DOI] [PubMed] [Google Scholar]
- 13).Yu B.P., Masaro E.J., Murata I., Bertrand H.A., Lynd F.T. (1982) Life span study of SPF Fisher 344 male rats fed ad libitum or restricted diets: longevity, growth, lean body mass and disease. J. Gerontol. 37, 130–141 [DOI] [PubMed] [Google Scholar]
- 14).Ando S., Tanaka Y., Toyoda Y., Kon K. (2003) Turnover of myelin lipids in aged brain. Neurochem. Res. 28, 5–13 [DOI] [PubMed] [Google Scholar]
- 15).Ando S., Tanaka Y., Toyoda Y., Kon K., Kawashima S. (2002) Turnover of synaptic membranes: age-related changes and modulation by dietary restriction. J. Neurosci. Res. 70, 290–297 [DOI] [PubMed] [Google Scholar]
- 16).Ando S. (2010) Studies on lipolytic and anti-brain aging effects of carnitine. J. Lipid Nutr. 19, 19–23 [Google Scholar]
- 17).Ando S., Sekine S., Murosaki S. (2011) Folate deficiency among aged patients and its amelioration through consumption of folic acid-fortified rice. J. Faculty Human Stud. Bunkyo Gakuin Univ. 13, 123–137 [Google Scholar]
- 18).Gibson G.E., Peterson C. (1981) Aging decreases oxidative metabolism and the release and synthesis of acetylcholine. J. Neurochem. 37, 978–984 [DOI] [PubMed] [Google Scholar]
- 19).Tanaka Y., Ando S. (2001) Age-related changes in the subtypes of voltage-dependent calcium channels in rat brain cortical synapses. Neurosci. Res. 39, 213–220 [DOI] [PubMed] [Google Scholar]
- 20).Marcus M.M., Apell H.-J., Roudna M., Schwendener R.A., Wecler H.-G., Lauger P. (1986) (Na++K+)-ATPase in artificial lipid vesicles: influence of lipid structures on pumping rate. Biochim. Biophys. Acta 854, 270–278 [DOI] [PubMed] [Google Scholar]
- 21).Tanaka Y., Ando S. (1992) Age-related changes in [3H]ouabain binding to synaptic plasma membranes isolated from mouse brains. J. Biochem. 112, 117–121 [DOI] [PubMed] [Google Scholar]
- 22).Südhof T.C. (2004) The synaptic vesicle cycle. Annu. Rev. Neurosci. 27, 509–547 [DOI] [PubMed] [Google Scholar]
- 23).White H.L., Scates P.W. (1990) Acetyl-l-carnitine as a precursor of acetylcholine. Neurochem. Res. 15, 597–601 [DOI] [PubMed] [Google Scholar]
- 24).Ando S., Ohashi Y. (1991) Longitudinal study on age-related changes of working and reference memory in the rats. Neurosci. Lett. 128, 17–20 [DOI] [PubMed] [Google Scholar]
- 25).Ingram D.K. (1995) Analysis of age-related impairments in learning and memory in rodent models. Ann. N.Y. Acad. Sci. 444, 321–331 [DOI] [PubMed] [Google Scholar]
- 26).Daum I., Gräber S., Schugens M.M., Mayes A.R. (1996) Memory dysfunction of the frontal type in normal aging. Neuroreport 7, 2625–2628 [DOI] [PubMed] [Google Scholar]
- 27).Gabrieli J.D.E. (1996) Memory system analyses of mnemonic disorders in aging and age-related diseases. Proc. Natl. Acad. Sci. U.S.A. 93, 13534–13540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28).Bartus R.T., Dean R.L., Beer B., Lippa A.S. (1982) The cholinergic hypothesis of geriatric memory dysfunction. Science 217, 408–417 [DOI] [PubMed] [Google Scholar]
- 29).Masliah E., Terry R.D. (1993) Role of synaptic pathology in the mechanisms of dementia in Alzheimer’s disease. Clin. Neurosci. 1, 192–198 [Google Scholar]
- 30).Ando S., Kobayashi S., Waki H., Kon K., Fukui F., Tadenuma T., Iwamoto M., Tanaka Y., Izumiyama N., Watanabe K., Nakamura H. (2002) Animal model of dementia induced by entorhinal synaptic damage and partial restoration of cognitive deficits by BDNF and carnitine. J. Neurosci. Res. 70, 519–527 [DOI] [PubMed] [Google Scholar]
- 31).Bliss T.V.P., Collingridge G.L. (1993) A synaptic model of memory: long-term potentiation in the hippocampus. Nature 361, 31–39 [DOI] [PubMed] [Google Scholar]
- 32).Wieraszko A., Seifert W. (1985) The role of monosialoganglioside GM1 in the synaptic plasticity: in vitro study on rat hippocampal slices. Brain Res. 345, 159–164 [DOI] [PubMed] [Google Scholar]
- 33).Fujii S., Igarashi K., Sasaki H., Furuse H., Ito K., Kaneko K., Kato H., Inokuti J., Waki H., Ando S. (2002) Effects of mono- and tetra-sialogangliosides GM1 and GQ1b on ATP-induced long-term potentiation in hippocampal CA1 neurons. Glycobiology 12, 339–344 [DOI] [PubMed] [Google Scholar]
- 34).Igarashi K., Fujiwara H., Yamazaki Y., Goto J.-I., Kaneko K., Kato H., Fujii S., Sasaki H., Fukumoto S., Furukawa K., Waki H., Furukawa K. (2011) Impaired hippocampal long-term potentiation and failure of learning in β1,4-N-acetylgalactosaminyltransferase gene transgenic mice. Glycobiology 21, 1373–1381 [DOI] [PubMed] [Google Scholar]
- 35).Roberts M., Willison H., Viencent A., Newsom-Davis J. (1994) Serum factor in Miller-Fisher variant of Guillain-Barré syndrome and neurotransmitter release. Lancet 343, 454–455 [DOI] [PubMed] [Google Scholar]
- 36).Richardson P.J., Walker J.H., Jones R.T., Whittaker V.P. (1982) Identification of cholinergic-specific antigen Chol-1α as a ganglioside. J. Neurochem. 38, 1605–1614 [DOI] [PubMed] [Google Scholar]
- 37).Ito H., Ishida H., Waki H., Ando S., Kiso M. (1999) Total synthesis of a cholinergic neuron-specific ganglioside GT1aα: a high affinity ligand for myelin-associated glycoprotein (MAG). Glycoconj. J. 16, 585–598 [DOI] [PubMed] [Google Scholar]
- 38).Taki T., Hirabayashi Y., Ichikawa H., Ando S., Kon K., Tanaka Y., Matsumoto M. (1986) A ganglioside of rat ascites hepatoma AH 7974F cells: occurrence of a novel disialoganglioside (GD1aα) with a unique N-acetylneuraminosyl-α2,6-N-acetylgalactosamine structure. J. Biol. Chem. 261, 3075–3078 [PubMed] [Google Scholar]
- 39).Irie F., Hashikura T., Tai T., Seyama Y., Hirabayashi Y. (1994) Distribution of cholinergic neuron-specific gangliosides (GT1aα and GQ1bα) in the rat central nervous system. Brain Res. 665, 161–166 [DOI] [PubMed] [Google Scholar]
- 40).Tanaka Y., Ando S. (1996) Modulation of cholinergic synaptic functions by sialylcholesterol. Glycoconj. J. 13, 321–326 [DOI] [PubMed] [Google Scholar]
- 41).Tanaka Y., Waki H., Kon K., Ando S. (1997) Gangliosides enhance KCl-induced Ca2+ influx and acetylcholine release in brain synaptosomes. Neuroreport 8, 2203–2207 [DOI] [PubMed] [Google Scholar]
- 42).Ando S., Tanaka Y., Waki H., Kon K., Iwamoto M., Fukui F. (1998) Gangliosides and sialylcholesterol as modulators of synaptic functions. Ann. N.Y. Acad. Sci. 845, 232–239 [DOI] [PubMed] [Google Scholar]
- 43).Hebb, D.O. (1949) The Organization of Behavior: a neuropsychological theory. Wiley. New York, p. 298. [Google Scholar]
- 44).Rosenzweig, M.R. (2006) Mark R. Rosenzweig In The History of Neuroscience in Autobiography (ed. Squire, L.R.). Elsevier, Amserdam. vol. 5, pp. 613–654. [Google Scholar]
- 45).Soffié M., Hahn K., Terao E., Eclancher F. (1999) Behavioural and glial changes in old rats following environmental enrichment. Behav. Brain Res. 101, 37–49 [DOI] [PubMed] [Google Scholar]
- 46).van Gool W.A., Mirmiran M., van Haaren F. (1985) Spatial memory and visual evoked potentials in young and old rats after housing in an enriched environment. Behav. Neural Biol. 44, 454–469 [DOI] [PubMed] [Google Scholar]
- 47).van Waas M., Soffié M. (1996) Differential environmental modulations on locomotor activity, exploration and spatial behaviour in young and old rats. Physiol. Behav. 59, 265–271 [DOI] [PubMed] [Google Scholar]
- 48).Winocur G. (1998) Environmental influences on cognitive decline in aged rats. Neurobiol. Aging 19, 589–597 [DOI] [PubMed] [Google Scholar]
- 49).Rabinovitch M.S., Rosvolt H.E. (1951) A closed-field intelligence test for rats. Can. J. Psychol. 5, 122–128 [DOI] [PubMed] [Google Scholar]
- 50).Masliah E., Mallory M., Hansen L., De Teresa R., Terry R.D. (1993) Quantitative synaptic alterations in the human neocortex during normal aging. Neurobiology 43, 192–197 [DOI] [PubMed] [Google Scholar]
- 51).Levine M.S., Adinolfi A.M., Fisher R.S., Hull C.D., Guthrie D., Buchwald N.A. (1988) Ultrastructural alterations in caudate nucleus in aged cats. Brain Res. 440, 267–279 [DOI] [PubMed] [Google Scholar]
- 52).de Jong G.I., Buwalda B., Schuurman T., Luiten P.G.M. (1992) Synaptic plasticity in the dentate gyrus of aged rats is altered after chronic nimodipine application. Brain Res. 596, 345–348 [DOI] [PubMed] [Google Scholar]
- 53).Peinado, M.A., Ramirez, M.J., Pedrosa, J.A., Martinez, M., Quesada, A., del Moral, M.L., Estevan, F.J., Rodrigo, J. and Peinado, J. M. (1998) Effects of contralateral lesions and aging on the neuronal and glial population of the cerebral cortex of the rat. In Understanding Glial Cells (eds. Castellano, B., Gonzalez, B. and Nieto-Sampedro, M.). Kluwer Academic Publications. Dordrecht, The Netherlands, pp. 297–317. [Google Scholar]
- 54).Turner A.M., Greenough W.T. (1985) Differential rearing effects on rat visual cortex synapses. I. Synaptic and neuronal density and synapses per neuron. Brain Res. 329, 195–203 [DOI] [PubMed] [Google Scholar]
- 55).Zito K., Svoboda K. (2002) Activity-dependent synaptogenesis in the adult mammalian cortex. Neuron 35, 1015–1017 [DOI] [PubMed] [Google Scholar]
- 56).Patel A.J. (1983) Undernutrition and brain development. Trends Neurosci. 6, 151–154 [Google Scholar]
- 57).Masoro E.J. (1992) Potential role of the modulation of fuel use in the antiaging action of dietary restriction. Ann. N.Y. Acad. Sci. 663, 403–411 [DOI] [PubMed] [Google Scholar]
- 58).Roth G.S., Ingram D.K., Joseph J.A. (2007) Nutritional interventions in aging and age-associated diseases. Ann. N.Y. Acad. Sci. 1114, 369–371 [DOI] [PubMed] [Google Scholar]
- 59).Greenwood P.M., Parasuraman R. (2010) Neuronal and cognitive plasticity: a neurocognitive framework for ameliorating cognitive aging. Frontiers Aging Neurosci. 2, Article 150., pp. 1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60).Hori N., Hirotsu I., Davis P.J., Carpenter D.O. (1992) Long-term potentiation is lost in aged rats but preserved by calorie restriction. Neuroreport 3, 1085–1088 [DOI] [PubMed] [Google Scholar]
- 61).Ando S., Tanaka Y. (2005) Mass spectrometric studies on brain metabolism, using stable isotopes. Mass Spectrom. Rev. 24, 865–886 [DOI] [PubMed] [Google Scholar]
- 62).Sun G.Y., Su K.L. (1979) Metabolism of arachidonoyl phosphoglycerides in mouse brain subcellular fractions. J. Neurochem. 32, 1053–1059 [DOI] [PubMed] [Google Scholar]
- 63).Takahashi R., Goto S. (1987) Influence of dietary restriction on accumulation of heat-labile enzyme molecules in the liver and brain of mice. Arch. Biochem. Biophys. 257, 200–206 [DOI] [PubMed] [Google Scholar]
- 64).Ando S., Yu R.K. (1979) Isolation and characterization of two isomers of brain tetrasialogangliosides. J. Biol. Chem. 254, 12224–12229 [PubMed] [Google Scholar]
- 65).Hirabayashi Y., Hirota M., Matsumoto M., Tanaka H., Obata K., Ando S. (1988) Developmental changes of C-series polysialogangliosides in chick brains revealed by mouse monoclonal antibodies M6704 and M7103 with different epitope specificities. J. Biochem. 104, 973–979 [DOI] [PubMed] [Google Scholar]
- 66).Takahashi H., Hirokawa K., Ando S., Obata K. (1991) Immunohistological study on brains of Alzheimer’s disease using antibodies to fetal antigens, C-series gangliosides and microtubule-associated protein 5. Acta Neuropathol. 81, 626–631 [DOI] [PubMed] [Google Scholar]
- 67).Uchida Y. (2010) Molecular mechanism of regeneration in Alzheimer’s disease brain. Geriatr. Gerontol. Int. 10, S158–S168 [DOI] [PubMed] [Google Scholar]
- 68).Lopez-Toledano M.A., Shelanski M.L. (2004) Neurogenic effect of beta-amyloid peptide in the development of neural stem cells. J. Neurosci. 24, 5439–5444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69).Hitoshi S., Kusunoki S., Kon K., Chiba A., Waki H., Ando S., Kanazawa I. (1996) A novel ganglioside, 9-O-acetyl GD1b, is recognized by serum antibodies in Guillain-Barré syndrome. J. Neuroimmunol. 66, 95–101 [DOI] [PubMed] [Google Scholar]
- 70).Usuki S., Taguchi K., Thompson S.A., Chapman P.B., Yu R.K. (2010) Novel anti-idiotype antibody therapy for lipooligosaccharide-induced experimental autoimmune neuritis: use relevant to Guillain-Barré syndrome. J. Neurosci. Res. 88, 1651–1663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71).Usuki S., Taguchi K., Gu Y.H., Thompson S.A., Yu R.K. (2010) Development of a novel therapy for lipooligosaccharide-induced experimental autoimmune neuritis: use of peptide glycomimics. J. Neurochem. 113, 351–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72).Montgomery S.A., Thal L.J., Amrein R. (2003) Meta-analysis of double blind randomized controlled clinical trials of acetyl-l-carnitine versus placebo in the treatment of mild cognitive and mild Alzheimer’s disease. Int. Clin. Psychopharmacol. 18, 61–71 [DOI] [PubMed] [Google Scholar]
- 73).Tanaka Y., Sasaki R., Fukui F., Waki H., Kawabata T., Okazaki M., Hasegawa K., Ando S. (2004) Acetyl-l-carnitine supplementation restores decreased tissue carnitine levels and impaired lipid metabolism in aged rats. J. Lipid Res. 45, 729–735 [DOI] [PubMed] [Google Scholar]
- 74).Wilson R.S., Mendes de Leon C.F., Barnes L.L., Schneider J.A., Bienias J.L., Evans D.A., Benett D.A. (2002) Participation in cognitively stimulating activities and risk of incident Alzheimer disease. JAMA 287, 742–748 [DOI] [PubMed] [Google Scholar]
- 75).Yoshitake T., Kiyohara Y., Kato I., Ohmura T., Iwamoto H., Nakayama K., Ohmori S., Nomiyama K., Kawamoto H., Ueda K., Sueishi K., Tsuneyoshi M., Fujishima M. (1995) Incidence and risk factors of vascular dementia and Alzheimer’s disease in a defined elderly Japanese population: The Hisayama Study. Neurology 45, 1161–1168 [DOI] [PubMed] [Google Scholar]
- 76).Laurin D., Verreault R., Lindsay J., MacPherson K., Rockwood K. (2001) Physical activity and risk of cognitive impairment and dementia in elderly persons. Arch. Neurol. 58, 498–504 [DOI] [PubMed] [Google Scholar]
- 77).Larson E.B., Wang L., Bowen J.D., McCormick W.C., Teri L., Crane P., Kukull W. (2006) Exercise is associated with reduced risk for incident dementia among persons 65 years of age and older. Ann. Intern. Med. 144, 73–81 [DOI] [PubMed] [Google Scholar]
- 78).Geda Y.E., Roberts R.O., Knopman D.S., Christianson T.J.H., Pankretz V.S., Ivnik R.J., Boeve B.F., Tangalos E.G., Petersen R.C., Rocca W.A. (2010) Physical exercise, aging, and mild cognitive impairment: a population-based study. Arch. Neurol. 67, 80–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79).Gómez-Pinilla F., So V., Kesslak J.P. (1998) Spatial learning and physical activity contribute to the induction of fibroblast growth factor: neural substrates for increased cognition associated with exercise. Neuroscience 85, 53–61 [DOI] [PubMed] [Google Scholar]
- 80).Colman R.J., Anderson R.M., Johnson S.C., Kastman E.K., Kosmatka K.J., Beasley T.M., Allison D.B., Cruzen C., Simmons H.A., Kemnitz J.W., Weindruch R. (2009) Caloric restriction delays disease onset and mortality in rhesus monkeys. Science 325, 201–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81).Holloszy J.O., Fontana L. (2007) Caloric restriction in humans. Exp. Gerontol. 42, 709–712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82).Rochon J., Bales C.W., Ravussin E., Redman L.M., Holloszy J.O., Racette S.B., Roberts S.B., Das S.K., Romashkan S., Galan K.M., Hadley E.C., Kraus W.E., CALERIE Study group (2011) Design and conduct of the CALERIE study: Comprehensive assessment of the long-term effects of reducing intake of energy. J. Gerontol. A Biol. Sci. Med. Sci. 66, 97–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83).Witte A.V., Fobker M., Gellner R., Knecht S., Flöel A. (2009) Caloric restriction improves memory in elderly humans. Proc. Natl. Acad. Sci. U.S.A. 106, 1255–1260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84).Kageyama M., Hiraoka M., Kagawa Y. (2008) Relationship between genetic polymorphism, serum folate and homocysteine in Alzheimer’s disease. Asia Pac. J. Public Health 20 (Supple.), 111–117 [PubMed] [Google Scholar]
- 85).Sato Y., Honda Y., Iwamoto J., Kanoko T., Satoh K. (2005) Effect of folate and mecobalamin on hip fracture in patients with stroke. JAMA 293, 1082–1088 [DOI] [PubMed] [Google Scholar]
- 86).Hiraoka M., Kageyama M., Yurimoto M., Kontai Y., Yatomi Y., Ohkawa R., Kunieda H., Horie S., Kagawa Y. (2009) Tailor-made nutrition based on polymorphism of folate metabolism: Sakado Folate Projects. Vitamin 83, 264–274 [Google Scholar]