Abstract
DNA methylation is the most extensively studied mechanism of epigenetic gene regulation. Increasing evidence indicates DNA methylation is labile in response to nutritional and environmental influences. Alternations in DNA methylation profiles can lead to changes in gene expression, resulting in diverse phenotypes with the potential for increased disease risk. The primary methyl donor for DNA methylation is S-adenosylmethionine (SAM), a species generated in the cyclical cellular process called one-carbon metabolism. One-carbon metabolism is catalyzed by several enzymes in the presence of dietary micronutrients, including folate, choline, betaine, and other B vitamins. For this reason, nutrition status, particularly micronutrient intake, has been a focal point when investigating epigenetic mechanisms. Though animal evidence linking nutrition and DNA methylation is fairly extensive, epidemiological evidence is less comprehensive. This review serves to integrate studies of the animal in vivo with human epidemiological data pertaining to nutritional regulation of DNA methylation, and to further identify areas in which current knowledge is limited.
Keywords: Epigenetics, nutri-epigenomics, one-carbon metabolism, dietary methyl donors, DNA methylation
Examining DNA Methylation in the Context of Diet
Epigenetics is the study of mitotically heritable yet potentially reversible, molecular modifications to DNA and chromatin without alteration to the underlying DNA sequence [1–2]. Increasingly, it is recognized that epigenetic marks provide a mechanistic link between environment, nutrition, and disease. Though DNA sequence is fairly permanent, epigenetic modifications are dynamic throughout the life course and can be heavily influenced by external factors [2]. Thus, external effects on the epigenome may alter gene expression, potentially giving rise to phenotypic disparity including disease formation. Epigenetic modifications include chromatin remodeling, histone tail modifications, DNA methylation, and more recently have expanded to include non-coding RNA and microRNA gene regulation [3].
DNA methylation is the most widely studied form of epigenetic modification and occurs within the one-carbon metabolism pathway, which is dependent upon several enzymes in the presence of dietary micronutrients as cofactors, including the availability of folate, choline, and betaine through the diet (Figure 1). Through an ATP-driven reaction methionine is converted into S-adenosylmethionine (SAM), the universal cellular methyl donor [4]. DNA methyltransferases (DNMTs) covalently attach methyl groups from SAM to the carbon-5 position of cytosine bases, generating 5-methylcytosine thus methylating DNA.
Figure 1. Involvement of dietary micronutrients in one-carbon metabolism.
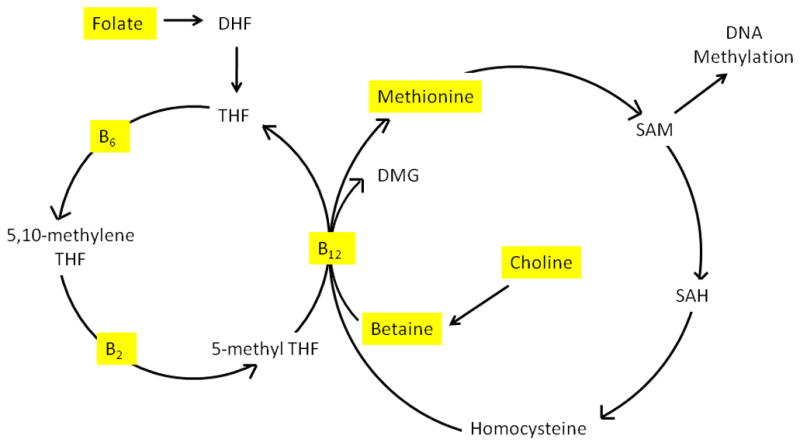
Substrates obtained via diet are highlighted in yellow. (1) Vitamin B6 is a cofactor to serine hydroxymethyltransferase in the conversion of tetrahydrofolate (THF) to 5,10-methylene THF. (2) Vitamin B2 is a precursor to FAD, which is a cofactor to methylenetetrahydrofolate reductase (MTHFR) in the conversion of 5,10-methylene THF to 5-methyl THF. (3) Vitamin B12 is a precursor to methionine synthase, involved in the production of methionine from homocysteine and betaine. Acronyms: dihydrofolate (DHF), flavin adenine dinucleotide (FAD), dimethylglycine (DMG), methylenetetrahydrofolate reductase (MTHFR), S-adenosylhomocysteine (SAH), tetrahydrofolate (THF).
In mammals, DNA methylation is primarily a stable repressive mark found at cytosines in CpG dinucleotides, however its regulation is more dynamic than previously believed [5]. These regulatory events may occur in a gene-specific and global-manner. Passive demethylation of DNA from tissues is widely accepted, though evidence for active demethylation is mostly limited to various stages of development [6]. Passive demethylation typically occurs when DNMT activity is reduced or inhibited at the time that DNA replication occurs. Simply, there is no addition of methyl groups to newly synthesized strands of DNA during cell division. Active demethylation, or the enzymatic conversion of 5-methylcytosine to cytosine, is fairly controversial, and the mechanisms by which it occurs still remain to be elucidated. Enzymatic conversion of 5-methylcytosine has produced three additional bases, 5-hydroxylmethylcytosine, 5-formylcytosine, and 5-carboxylcytosine, yet their potential roles in epigenetic regulation have yet to be characterized [7]. In addition, Lister et al. [8] provided evidence for methylation of non-CpG cytosines in human embryonic stem cells, suggesting that methylation at non-CpG sites may be important to developmental homeostasis. The distribution of CpG sequences in mammalian genomes is non-random [9]. CpG dinucleotides are greatly underrepresented in the mammalian genome due to evolutionary spontaneous deamination of 5-methylcytosine to thymine [10]. The majority of unmethylated CpG sites occur within CpG islands, defined as discreet regions containing a preponderance of CpG content [11]. Normally, CpG islands are located within or near gene promoters or in the first exons of housekeeping genes. In contrast, the promoter and regulatory regions of repetitive DNA sequences, such as transposable elements, are methylated, consequently inhibiting the parasitic transposable and repetitive elements from replicating.
DNA methylation patterns are prone to change throughout the life course especially during reprogramming events associated with normal development and aging [12–14]. For example, the epigenome is particularly dynamic during embryogenesis because of extensive DNA synthesis, and the elaborate DNA methylation patterning required for normal tissue development is established during early development [15]. As individuals age, gradual DNA hypomethylation occurs at the genome-wide level, concurrent with locus-specific promoter hypermethylation at normally unmethylated CpG islands, leading for example, to genome instability or gene-specific suppression, respectively [16]. Additionally, cancer is often associated with hypomethylated DNA and notable hypermethylation of tumor suppressor genes, as compared to normal tissue [17]. These reprogramming events throughout the life course result in tissue-specific DNA methylation patterning [2, 13]. Differences in these epigenetic patterns are important to cellular differentiation and tissue homeostasis.
In the context of nutritional biochemistry, it is significant that the one-carbon metabolism pathway is cyclical, and is regenerated via dietary micronutrients. Nutri-epigenomics is an emerging discipline examining the role of dietary influences on gene expression. Ultimately, DNA methylation events, and dietary practices, particularly micronutrient intake, may influence disease phenotypes. The study of nutri-epigenomics is particularly timely in the context of the developmental origins of health and disease (DOHaD) hypothesis [18], which posits that increased susceptibility to disease following early life experiences is shaped by epigenetic modifications such as DNA methylation and chromatin modifications [19]. In this review, we take an interspecies approach to synthesize the existing nutri-epigenomic literature in order to identify sensitive periods throughout the life course where diet may substantially alter DNA methylation. By investigating varying levels of nutrient exposure during vulnerable time points, researchers can grasp the magnitude and degree of impact that each nutrient has on one-carbon metabolism, and subsequently, DNA methylation. Throughout the review, careful attention is given to areas in which further research is needed to understand the link between dietary micronutrients and DNA methylation, and life-course health and disease.
Nutri-epigenomics studies have utilized a combination of global, candidate gene, and to a lesser extent, genome-wide approaches to examine the influence of methyl donors on DNA methylation. Until recently, most attempts to elucidate the effects nutritional status on the epigenome were either 1) candidate gene driven or based on epigenetic techniques with limited genome coverage/sensitivity, 2) restricted in dose-response assessment, or 3) confined to animal models. Emerging advances in epigenomic and high-throughput quantitative epigenetic technologies now allow for the identification of the constellation of genomic loci with altered epigenetic status following dose-dependent exposures. Epigenetic epidemiology approaches facilitate the identification of epigenetically modified regions of the genome in human cells. Thus, identifying epigenetic biomarkers will enable clinicians to identify at-risk individuals prior to disease onset. Furthermore, unlike genetic mutations, epigenetic marks are potentially reversible. Therefore, epigenetic approaches for prevention and treatment, such as nutritional supplementation and/or pharmaceutical therapies, may be developed to counteract negative epigenomic profiles.
Studies of Combined Methyl Donor Supplementation
Novel experiments investigating maternal methyl donor supplementation in utero clearly demonstrate the impact early nutrition has in shaping the epigenome. The axin fused (AxinFu) and viable yellow agouti (Avy) murine models contain metastable epialleles, loci variably expressed in isogenic individuals due to the establishment of stochastic epigenetic modifications early in development [20]. Variable DNA methylation of an intracisternal A particle (IAP) retroelement in intron 6 of the Axin gene results in distinct kinky tail phenotypes, from very kinky (hypomethylated) to no tail kink (hypermethylated) among genetically identical AxinFu mice [21]. Waterland et al. [22] demonstrated that maternal diet supplemented with methyl donors including folic acid, vitamin B12, betaine, and choline resulted in offspring displaying less kinky tail phenotype and accordingly hypermethylation of the AxinFu metastable epiallele. Similarly, the coat color of isogenic Avy mice correlates to stochastic methylation of an IAP upstream of the Agouti gene promoter, resulting in phenotypes ranging from yellow (hypomethylated) to brown (hypermethylated) fur [23]. A methyl donor supplemented maternal diet shifted the distribution of coat color phenotype towards brown in comparison to unsupplemented mothers [24]. The shift towards the brown phenotype was concomitant to hypermethylation at Avy IAP. Furthermore, using the Avy model, we have demonstrated that maternal diet supplemented with methyl donors negated a shift towards hypomethylation due to bisphenol A (BPA) exposure, restoring normal stochastic methylation in offspring [25].
The AxinFuand Avy models serve as informative visual epigenetic biosensors for maternal nutritional status, particularly methyl donors, needed for the maintenance of one-carbon metabolism and DNA methylation. Additional in vivo rodent studies have relied upon wild-type strains of rodents to investigate the effects of maternal methyl donor supplementation in altering phenotypes, especially in the exacerbation of disease. For example, modulation of allergic airway disease risk was explored using C57BL/6 mice [26]. Hypermethylation of a gene crucial in lymphocyte regulation, Runx3, arose in offspring exposed to methyl donors in utero resulting in increased allergic airway disease development in conjunction with amplified symptom severity. Furthermore, C57BL/6 mice exposed to methyl donor supplementation in utero exhibited enhanced colitis susceptibility, which was also associated with aberrant DNA methylation among genes associated with immunologic processes [27].
Methylenetetrahydrofolate reductase (MTHFR) is crucial to one-carbon metabolism, catalyzing the conversion of homocysteine to methionine and generating 5-methyltetrahydrofolate [28]. Because of this relationship, the role of MTHFR genotype in DNA methylation has been investigated. An extensive study looking at MTHFR variants was conducted in healthy women [29]. Over fourteen weeks participants were randomly assigned to four study diets consisting of established concentrations of folate, betaine, choline phosphotidylcholine, sphingomyelin, and various other choline sources within the cell. A cytosine extension assay was used to determine global percent methylation, but neither diet nor MTHFR covariate showed a significant effect on leukocyte DNA methylation. In another study, reduced dietary choline and folate intake by women ages 20–30 years was associated with decreased global methylation of leukocyte DNA [30]. Moreover, dietary re-supplementation with folate resulted in DNA re-methylation on the global scale. This strength of the association was more robust in individuals with MTHFR mutations, suggesting that folate supplementation may be crucial for methylation maintenance in individuals with MTHFR polymorphisms.
Studies of Individual Methyl Donors
Folate
Dietary folate is the most extensively studied micronutrient in animal and epidemiological DNA methylation research (Table 1). Folate is reduced to dihydrofolate (DHF) and subsequently to tetrahydrofolate (THF), serving as a single carbon donor in the form of 5-methyl THF (Figure 1). Consequently, 5-methyl THF feeds into the one-carbon metabolism cycle by donating its methyl group to homocysteine converting it to methionine. Cofactor B vitamins provide the enzymatic support necessary for these transformations, making it possible for dietary folate to feed into the one-carbon metabolism cycle to replenish cellular SAM. For this reason, folate supplementation has generally been associated with increased DNA methylation and vice versa for folate restriction. As underscored below, however, conflicting evidence has emerged, suggesting the mechanisms associated with micronutrient influence on DNA methylation are more complex than previously understood.
Table 1.
Studies Providing for Folate Impacts on DNA Methylation
| Authors | Study Population/Tissue | Methylation Measure |
|---|---|---|
| Human | ||
| Ba et al. (2011) [47] | Pregnant women, maternal blood and cord blood | Gene-specific |
| Christensen et al. (2011) [50] | Primary breast tumors | Epigenome-wide |
| Hoyo et al. (2011) [49] | Pregnant women, cord blood leukocytes | Gene-specific |
| Vineis et al. (2011) [46] | Lung cancer cases and controls, leukocytes | Gene-specific |
| Stidley et al. (2010) [45] | Smokers, sputum | Gene-specific |
| Hervouet et al. (2009) [37] | Cells cultured from glioblastomas | Global, Gene-specific |
| van den Donk et al. (2007) [43] | Colon tumors from MTHFR variants | Gene-specific |
| Pufulete et al. (2005) [35] | Healthy adult colonic mucosa | Global |
| Pufulete et al. (2005) [34] | Adults with CRC, leukocytes and colonic mucosa | Global |
| Shelnutt et al. (2004) [30] | Adult women, leukocytes | Global |
| van Engeland et al. (2003) [44] | Colon tumors | Gene-specific |
| Rampersaud et al. (2000) [36] | Postmenopausal women, leukocytes | Global |
| Mouse | ||
| McKay et al. (2011) [42] | Various adult tissues | Gene-specific |
| McKay et al. (2011) [31] | Adult small intestine | Global |
| Wakefield et al. (2010) [38] | Various adult tissues | Gene-specific |
| Rat | ||
| Ly et al. (2011) [32] | Adult mammary tissue | Global |
| McKay et al. (2011) [41] | Adult small intestine | Gene-specific |
| Sie et al. (2011) [33] | Adult colonic mucosa | Global |
| Burdge et al. (2009) [40] | Adult liver and adipose tissue | Gene-specific |
| Kim et al. (1997) [39] | Adult liver and blood | Global, Gene-specific |
Three notable animal studies have evaluated the global methylation effects of maternal folate status. First, murine offspring exposed to a low folate diet during gestation and lactation exhibited decreased global methylation in small intestinal tissue as adults [31], as measured by a cytosine extension technique quantifying unmethylated cytosines within CpG islands. In contrast, the measurement of global methylation by methyl acceptance through the incorporation of 3H-methyl SAM following in utero folate supplementation was associated with CpG hypomethylation of mammary tissue among rat offspring 28 weeks of age [32]. Finally, post weaning folate supplementation equivalent to recommended intake levels for U.S. women of child-bearing age resulted in decreased global methylation levels in murine colorectal tissue, as measured by liquid chromatography-mass spectrometry [33]. Thus, these in vivo animal data support a role for global DNA hypomethylation in both folate restriction and folate supplementation.
A number of human epidemiological studies have also measured global DNA methylation indirectly through the incorporation of 3H-methyl SAM, looking at the correlation between folate and DNA methylation in colorectal cancer (CRC) and in postmenopausal women [30, 34–36]. First, two studies were conducted on patients admitted for colonoscopies. In patients that tested negative for CRC, colonic mucosa and lymphocyte DNA methylation were found to be weakly associated with serum folate concentrations and dietary folate as measured using a food frequency questionnaire [35]. In patients diagnosed with CRC, folate supplementation after diagnosis was correlated with an increase in global DNA methylation [34]. Second, among postmenopausal women, dietary folate restriction and corresponding decreased serum folate concentration were associated with decreased DNA methylation in leukocytes in a dose-dependent manner [36]. Moreover, folate supplementation to cells cultured from human glioblastomas methylates DNA that is globally hypomethylated in glioblastoma [37]. Therefore, human evidence suggests that folate intake and global DNA methylation are positively correlated.
Numerous studies utilize a candidate gene-specific approach to investigate the effects of folate on DNA methylation. Genomic loci selected are often associated with a disease outcome of interest, such as cancer or diabetes, for the prospect of future preventative or therapeutic strategies through diet targeting the epigenome. For example, N-acetyltransferase 2 (Nat2), a gene whose human homologue NAT1is associated with cancer development including breast carcinogenesis when overexpressed, displayed increased methylation after adult rats were exposed to a high folate diet [38]. Additionally, the effect of folate deficiency on the methylation of p53, an important tumor suppressor gene was evaluated [39]. Rats fed low folate diets exhibited hepatic hypomethylation within two exon regions of p53, further suggesting that methylation status can play a role in the molecular underpinnings of carcinogenesis. Folic acid supplementation during the rat juvenile-pubertal period increased methylation in the promoter regions of insulin receptor, PPAR-α and glucocorticoid receptor, genes involved in metabolic homeostasis, compared to offspring exposed to the same in utero environment with the exception of a folate adequate diet during puberty [40]. Wild type (WT) and Apc+/Min rats exhibited distinct alterations in DNA methylation measured at several loci involved in CRC at weaning and as adults after exposure to folate depletion during gestation and lactation [41]. Importantly, the results of this experiment indicate that the same folate exposure alters DNA methylation in a gene and tissue specific manner at different life stages. Additionally, differences in methylation were dependent on sex and genotype.
The aforementioned in vivo candidate-gene driven studies explored folate’s role in methylation of target tissue types. Using blood as a surrogate biomarker for methylation in targeted tissues will permit thorough human epigenetic research, and the development of non-invasive methylation measurements for epigenetic epidemiology studies. Mice deprived of folate in utero showed hypomethylation at the differentially methylated region (DMR) 1 of the imprinted locus insulin-like growth factor 2 (Igf2) and at Slc389a4CGl1 across blood, liver, and kidney tissue. Conversely, Igf2 DMR2 showed significant methylation differences in blood versus kidney and liver [42], providing further evidence that methylation is gene and tissue specific.
There is growing investigation of folate’s role in methylation status of select genes known to alter disease susceptibility in humans. Evidence obtained via food frequency questionnaire suggests that folate intake did not have any significant affect on CRC-associated gene-specific methylation of white blood cells [43]. Likewise, low folate intake combined with high alcohol consumption demonstrated non-significant changes in gene promoter methylation in blood from CRC patients [44]. Another study examined serum folate concentrations among smokers to detect methylation in eight genes that have previously been hypermethylated in lung cancer [45]. Interestingly, increased serum folate was associated with decreased methylation among smokers in 5 of the 8 genes examined. Alternatively, a positive correlation between serum folate concentrations and promoter methylation was identified in RASSF1A, a gene whose increased expression is implicated in multiple diseases, such as breast and lung cancer [46]. Although gene expression was not examined in this study, increased promoter methylation may repress RASSF1A expression subsequently decreasing cancer risk. Together, these data suggest that folate may be associated with protection against some cancers.
Human studies have also investigated the role of maternal folate status and offspring methylation. A cross-sectional study of pregnant women at the time of parturition looked at the imprinted gene IGF2 [47]. Imprinted genes are monoallelically expressed due to allelic repression from epigenetic modifications such as DNA methylation. Because imprinted genes are functionally haploid, they are more prone to deregulating events and have been investigated in methylation based studies [48]. Cord blood and maternal blood were collected at time of delivery, and serum folate concentrations were determined for both specimens [47]. Methylation-specific PCR determined that maternal and cord blood folate concentrations were not associated with methylation outcomes in the P2 and P3 promoters of IGF2. Additionally, hypomethylation within the IGF2 promoter of umbilical cord blood leukocytes was inversely associated with maternal report of folate supplementation during pregnancy [49].
One study has looked for correlations between diet and methylation on an epigenome-wide scale. The Illumina GoldenGate bead array was used to conduct an epigenome-wide study examining connections between human folate intake and breast cancer [50]. Dietary folate, as determined by food frequency questionnaires, was strongly associated on a genome-wide scale with hypermethylation of CpG sites in cancer related gene promoters. More epigenome-wide scale studies are necessary in discovering candidate loci with variable methylation that have the potential to increase individual or population susceptibility to disease.
Choline and Betaine
Choline is an indirect methyl group donor for one-carbon metabolism. Within this pathway, dietary choline is oxidized to betaine. Betaine then contributes to methionine homeostasis through the donation of a methyl group to homocysteine, resulting in homocysteine’s conversion to methionine (Figure 1) [51]. Thus, a number of animal in vivo and to a lesser extent human studies have investigated the role of dietary choline and/or betaine and their impact on global and candidate gene DNA methylation (Table 2).
Table 2.
Studies Providing for Choline, Betaine, Vitamin B, and Methionine Impacts on DNA Methylation
| Authors | Study Population/Tissue | Methylation Measure |
|---|---|---|
| Choline and Betaine | ||
| Xing et al. (2011) [58] | Chicken adipocytes | Gene-specific |
| Mehedint et al. (2010) [53] | Mouse fetal brain | Gene-specific |
| Mehedint et al. (2010) [55] | Mouse fetal brain | Gene-specific |
| Du et al. (2009) [57] | Rat adult liver | Gene-specific |
| Kovacheva et al. (2007) [56] | Rat fetal liver and brain | Global, Gene-specific |
| Niculescu et al. (2006) [54] | Mouse fetal brain | Global, Gene-specific |
| Vitamin B | ||
| Ba et al. (2011) [47] | Pregnant women, maternal blood and cord blood | Gene-specific |
| Kulkarni et al. (2011) [61] | Rat placenta | Global |
| Vineis et al. (2011) [46] | Lung cancer cases and controls, leukocytes | Gene-specific |
| Methionine | ||
| Amaral et al. (2011) [62] | Rat adult kidney | Gene-specific |
| Vineis et al. (2011) [46] | Lung cancer cases and controls, leukocytes | Gene-specific |
Choline deficiency is associated with altered neurogenesis followed by declined memory function [52–53]. Since choline is crucial for maintenance of one-carbon metabolism, several studies have investigated choline deficiency and its influence on global and candidate gene methylation of fetal brain as well as other tissues. Global methylation analysis of embryonic day (ED) 17 mouse fetal brain displayed a significant shift towards hypomethylation [54]. Three candidate genes were also analyzed for DNA methylation status including Cdkn3, Cdkn2b, and Calb2. Of these candidate genes Cdkn3 showed hypomethylation in its promoter region in offspring exposed to a maternal choline-deficient diet. Additionally, choline deficiency during gestational days 12–17 decreased methylation within a CpG site located in the Calb1 promoter of the fetal hippocampus [55]. Further examination of early choline exposure on fetal brain gene methylation was performed on Vegfc and Angpt2 [53]. Both candidate loci displayed hypomethylation when exposed to a maternal choline-deficient diet with correlating increased expression of their encoded proteins, VEGF-C and angiopoietin 2, respectively.
Global methylation of rat brain and liver tissue was analyzed following exposure to a choline-deficient diet from embryonic days 11–17 [56]. Interestingly, global hypomethylation occurred in the brain while global hypermethylation was observed in liver tissue. Furthermore, hypermethylation at the DMR 2 of Igf2 within liver was correlated with hypomethylation of CpG sites within Dnmt1, the gene encoding DNA methyltransferase. Thus, these data suggest early choline-deficiency has the ability to deprive DNA methylation machinery of proper substrates of one-carbon metabolism leading to increased expression of methyltransferases, and subsequently increased methylation at specific loci, in this case the imprinted gene Igf2. This is a complex concept that must be explored further to understand wholly the mechanisms involved in gene-specific methylation.
Betaine supplementation has also been explored for its role in carcinogenesis. Adult rats were exposed to three varying levels of betaine through the diet [57]. Hypermethylation of the tumor suppressor gene p16 was displayed in liver tissue of the rats exposed to all three doses when compared to the control group. Hypermethylation of p16 was concomitantly correlated to decreased mRNA expression of p16. In chickens, betaine supplementation was associated with modest positional effects in the lipoprotein lipase (LPL) gene promoter, as measured in adipocytes [58]. While most of the promoter was highly hypomethylated, several CpG sites were found to be hypermethylated with betaine supplementation. Interestingly, LPL gene expression was significantly decreased with betaine supplementation, but the influence of DNA methylation on this change is unknown.
Vitamins B2, B6, and B12
The water-soluble vitamins B2, B6, and B12 have an important catalytic role in folate and one-carbon metabolism (Figure 1). Vitamin B6 serves as a coenzyme to serine hydroxymethyltransferase, the key enzyme in the folate cycle converting THF to 5,10-methylene THF [59]. Riboflavin, or vitamin B2, is a precursor for flavin adenine dinucleotides (FAD), which is a cofactor to MTHFR, the enzyme responsible in the reduction of 5,10-methylene THF to 5-methyl THF [60]. Vitamin B12 is the coenzyme of methionine synthase, which catalyzes the reaction of homocysteine, the by-product of SAM, to methionine. Thus, dietary consumption of these water-soluble B vitamins has the potential to affect the efficiency of the one-carbon metabolism pathway.
Both animal and human studies have evaluated vitamin B12’s role in DNA methylation profiles (Table 2). An in vivo rat model showed that deprivation of vitamin B12 in addition to a diet supplemented with folate enhanced placental global hypomethylation compared to a diet exclusively supplemented with folate, suggesting that the interaction between micronutrients can alter methylation patterns more profoundly than excess or deprivation of just one micronutrient [61]. Two human epidemiological studies have examined the role of vitamin B12 in the regulation of DNA methylation. Using a cross-sectional study design, Ba et al.[47] assessed vitamin B12 and folate status in pregnant women at the time of parturition. Cord blood IGF2 methylation at the P3 promoter was inversely correlated with maternal blood serum B12. Additionally, maternal blood methylation in the P2 promoter was inversely correlated with maternal serum B12. A second study examined the relationship among plasma vitamin B2, B6, and B12 concentrations, DNA methylation, and smoking status [46]. Among former smokers, an increase in serum vitamin B12 concentration was associated with a decrease in methylation for several multi-disease-related gene promoters. A similar decrease in methylation was associated with increased serum vitamin B6 concentration for one of the disease-linked genes.
Methionine
Methionine is an essential amino acid that is continuously regenerated from homocysteine in one-carbon metabolism to serve as the precursor to SAM (Figure 1). Thus, fluctuation of methionine in the diet has potential effects on DNA methylation. Although methionine has an immense role as a methyl donor, there is limited evidence when evaluating methionine and DNA methylation (Table 2). Rats fed a methionine supplemented diet had no change in p53 promoter region methylation [62]. Additionally, plasma methionine concentrations were inversely correlated to DNA methylation in the promoters of several multi-disease related genes among former and current smokers [46].
Discussion
The role of nutrition in one-carbon metabolism and DNA methylation has been more extensively studied in animal models than in humans; however this review reveals that the epidemiologic data is becoming increasingly robust. Herein, we have highlighted data in which altered consumption of folate, choline, betaine, B vitamins, and methionine acts to modify methylation both globally and in the promoters of disease-related genes in animal and humans. Thus, nutri-epigenetics approaches provide a molecular foundation for understanding the role of diet throughout the life course and its prospective role in disease prevention and/or therapy.
There are a limited number of human studies on micronutrient intake and methylation; nonetheless multiple laboratories have established a solid foundation using animal in vivo models for future studies in this discipline. This body of literature indicates the importance of exposure timing, genotype, and tissue and gene specific DNA methylation and the interpretation of results.. Because methylation is cell type dependent, a comprehensive epigenetic analysis remains an optimal approach. Future studies should continue to focus on tissue specificity in DNA methylation investigation, as this will likely most significantly influence varying disease states. In addition to examining tissue specific methylation, data surrounding methyl donor exposure during windows of susceptibility throughout the life course such as embryogenesis, fertilization, neonatal, puberty, and aging should be thoroughly investigated. Vulnerable epigenetic states combined with tissue-specific outcomes can shape the knowledge of disease etiology, and possibly preventive and therapeutic approaches to disease.
As reviewed above a vast array of methodologies have been used to detect DNA methylation in nutri-epigenetics studies. Even over the past decade, epigenetic technologies have evolved from once traditional methods using restriction enzymes or focusing on candidate genes to modern technologies allowing for unbiased epigenome-wide investigation across tissues and species. Since a main goal of nutri-epigenetics is to better understand the role of diet in disease, epigenome-wide investigations involving deep sequencing and tiling array technologies will be valuable in future studies, as they apply an integrative approach and can identify key regulatory pathways and interactions to which diet is a modifier. Other epigenetic mechanisms such as histone modifications and chromatin remodeling complexes should also be considered [63]. Additionally, investigating the interactions among micronutrients required in one-carbon metabolism and those that may indirectly affect their supply to maintain cycle efficiency can be implicit by integrating a genome-wide application.
As the field of nutri-epigenetics continues to emerge, it will enable clinical and public health practices to apply epigenetically-driven therapeutic and preventative strategies when evaluating a population or individuals in a certain disease state. For example, registered dietitians can make nutritional recommendations based upon individual epigenetic profiles. Because the epigenome is particularly sensitive at various time points throughout the life course, targeted nutri-epigenomic population interventions may be efficient and cost-effective. Continued animal nutri-epigenomic models translated into human research will strengthen our understanding in the biological pathways associated with diet and human health.
Acknowledgments
This work was supported by NIH grant ES017524 and the University of Michigan NIEHS P30 Core Center P30 ES017885as well as NIH/EPA P20 grant ES018171/RD 83480001. Support for KES was provided by NIEHS Institutional Training Grant T32 ES007062.
Footnotes
Conflict of Interest Statement:
The authors have no conflicts of interest and declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Li E. Chromatin modification and epigenetic reprogramming in mammalian development. Nat Rev Genet. 2002;3(9):662–673. doi: 10.1038/nrg887. [DOI] [PubMed] [Google Scholar]
- 2.Reik W, Dean W, Walter J. Epigenetic Reprogramming in Mammalian Development. Science. 2001;293(5532):1089–1093. doi: 10.1126/science.1063443. [DOI] [PubMed] [Google Scholar]
- 3.Matzke MA, Birchler JA. RNAi-mediated pathways in the nucleus. Nat Rev Genet. 2005;6(1):24–35. doi: 10.1038/nrg1500. [DOI] [PubMed] [Google Scholar]
- 4.Selhub J. Homocysteine Metabolism. Annual Review of Nutrition. 1999;19(1):217–246. doi: 10.1146/annurev.nutr.19.1.217. [DOI] [PubMed] [Google Scholar]
- 5.Maunakea AK, et al. Conserved role of intragenic DNA methylation in regulating alternative promoters. Nature. 2010;466(7303):253–257. doi: 10.1038/nature09165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu SC, Zhang Y. Active DNA demethylation: many roads lead to Rome. Nat Rev Mol Cell Biol. 2010;11(9):607–620. doi: 10.1038/nrm2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ito S, et al. Tet Proteins Can Convert 5-Methylcytosine to 5-Formylcytosine and 5-Carboxylcytosine. Science. 2011;333(6047):1300–1303. doi: 10.1126/science.1210597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lister R, et al. Hotspots of aberrant epigenomic reprogramming in human induced pluripotent stem cells. Nature. 2011;471(7336):68–73. doi: 10.1038/nature09798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cooper DN, Krawczak M. Cytosine methylation and the fate of CpG dinucleotides in vertebrate genomes. Human Genetics. 1989;83(2):181–188. doi: 10.1007/BF00286715. [DOI] [PubMed] [Google Scholar]
- 10.Holliday R, Grigg GW. DNA methylation and mutation. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis. 1993;285(1):61–67. doi: 10.1016/0027-5107(93)90052-h. [DOI] [PubMed] [Google Scholar]
- 11.Deaton AM, Bird A. CpG islands and the regulation of transcription. Genes & Development. 2011;25(10):1010–1022. doi: 10.1101/gad.2037511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fraga MF, et al. Epigenetic differences arise during the lifetime of monozygotic twins. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(30):10604–10609. doi: 10.1073/pnas.0500398102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hajkova P, et al. Epigenetic reprogramming in mouse primordial germ cells. Mechanisms of Development. 2002;117(1–2):15–23. doi: 10.1016/s0925-4773(02)00181-8. [DOI] [PubMed] [Google Scholar]
- 14.Martin GM. Epigenetic drift in aging identical twins. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(30):10413–10414. doi: 10.1073/pnas.0504743102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Faulk C, Dolinoy DC. Timing is everything: The when and how of environmentally induced changes in the epigenome of animals. Epigenetics. 2011;6(7):791–797. doi: 10.4161/epi.6.7.16209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mugatroyd C, et al. The Janus face of DNA methylation in aging. Aging. 2010;2(2):107–110. doi: 10.18632/aging.100124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feinberg AP. Phenotypic plasticity and the epigenetics of human disease. Nature. 2007;447(7143):433–440. doi: 10.1038/nature05919. [DOI] [PubMed] [Google Scholar]
- 18.Barker DJP. The Developmental Origins of Adult Disease. Journal of the American College of Nutrition. 2004;23(suppl 6):588S–595S. doi: 10.1080/07315724.2004.10719428. [DOI] [PubMed] [Google Scholar]
- 19.Gabory A, Attig L, Junien C. Developmental programming and epigenetics. The American Journal of Clinical Nutrition. 2011;94(6 Suppl):1943S–1952S. doi: 10.3945/ajcn.110.000927. [DOI] [PubMed] [Google Scholar]
- 20.Rakyan VK, et al. Metastable epialleles in mammals. Trends in Genetics. 2002;18(7):348–351. doi: 10.1016/s0168-9525(02)02709-9. [DOI] [PubMed] [Google Scholar]
- 21.Vasicek TJ, et al. Two Dominant Mutations in the Mouse Fused Gene Are the Result of Transposon Insertions. Genetics. 1997;147(2):777–786. doi: 10.1093/genetics/147.2.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Waterland RA, et al. Maternal methyl supplements increase offspring DNA methylation at Axin fused. Genesis. 2006;44(9):401–406. doi: 10.1002/dvg.20230. [DOI] [PubMed] [Google Scholar]
- 23.Duhl DMJ, et al. Neomorphic agouti mutations in obese yellow mice. Nat Genet. 1994;8(1):59–65. doi: 10.1038/ng0994-59. [DOI] [PubMed] [Google Scholar]
- 24.Waterland RA, Jirtle RL. Transposable Elements: Targets for Early Nutritional Effects on Epigenetic Gene Regulation. Molecular and Cellular Biology. 2003;23(15):5293–5300. doi: 10.1128/MCB.23.15.5293-5300.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dolinoy DC, Huang D, Jirtle RL. Maternal nutrient supplementation counteracts bisphenol A-induced DNA hypomethylation in early development. Proceedings of the National Academy of Sciences. 2007;104(32):13056–13061. doi: 10.1073/pnas.0703739104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hollingsworth JW, et al. In utero supplementation with methyl donors enhances allergic airway disease in mice. The Journal of Clinical Investigation. 2008;118(10):3462–3469. doi: 10.1172/JCI34378. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 27.Schaible TD, et al. Maternal methyl-donor supplementation induces prolonged murine offspring colitis susceptibility in association with mucosal epigenetic and microbiomic changes. Human Molecular Genetics. 2011;20(9):1687–1696. doi: 10.1093/hmg/ddr044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bailey LB, Gregory JF. Polymorphisms of Methylenetetrahydrofolate Reductase and Other Enzymes: Metabolic Significance, Risks and Impact on Folate Requirement. The Journal of Nutrition. 1999;129(5):919–922. doi: 10.1093/jn/129.5.919. [DOI] [PubMed] [Google Scholar]
- 29.Abratte CM, et al. Choline status is not a reliable indicator of moderate changes in dietary choline consumption in premenopausal women. The Journal of Nutritional Biochemistry. 2009;20(1):62–69. doi: 10.1016/j.jnutbio.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 30.Shelnutt KP, et al. Methylenetetrahydrofolate reductase 677C→T polymorphism affects DNA methylation in response to controlled folate intake in young women. The Journal of Nutritional Biochemistry. 2004;15(9):554–560. doi: 10.1016/j.jnutbio.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 31.McKay J, et al. Folate depletion during pregnancy and lactation reduces genomic DNA methylation in murine adult offspring. Genes & Nutrition. 2011;6(2):189–196. doi: 10.1007/s12263-010-0199-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ly A, et al. Effect of Maternal and Postweaning Folic Acid Supplementation on Mammary Tumor Risk in the Offspring. Cancer Research. 2011;71(3):988–997. doi: 10.1158/0008-5472.CAN-10-2379. [DOI] [PubMed] [Google Scholar]
- 33.Sie KKY, et al. Effect of maternal and postweaning folic acid supplementation on colorectal cancer risk in the offspring. Gut. 2011;60(12):1687–1694. doi: 10.1136/gut.2011.238782. [DOI] [PubMed] [Google Scholar]
- 34.Pufulete M, et al. Effect of folic acid supplementation on genomic DNA methylation in patients with colorectal adenoma. Gut. 2005;54(5):648–653. doi: 10.1136/gut.2004.054718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pufulete M, et al. Influence of folate status on genomic DNA methylation in colonic mucosa of subjects without colorectal adenoma or cancer. Br J Cancer. 2005;92(5):838–842. doi: 10.1038/sj.bjc.6602439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rampersaud GC, et al. Genomic DNA methylation decreases in response to moderate folate depletion in elderly women. The American Journal of Clinical Nutrition. 2000;72(4):998–1003. doi: 10.1093/ajcn/72.4.998. [DOI] [PubMed] [Google Scholar]
- 37.Hervouet E, et al. Folate Supplementation Limits the Aggressiveness of Glioma via the Remethylation of DNA Repeats Element and Genes Governing Apoptosis and Proliferation. Clinical Cancer Research. 2009;15(10):3519–3529. doi: 10.1158/1078-0432.CCR-08-2062. [DOI] [PubMed] [Google Scholar]
- 38.Wakefield L, Boukouvala S, Sim E. Characterisation of CpG methylation in the upstream control region of mouse Nat2: Evidence for a gene–environment interaction in a polymorphic gene implicated in folate metabolism. Gene. 2010;452(1):16–21. doi: 10.1016/j.gene.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 39.Kim Y, et al. Folate deficiency in rats induces DNA strand breaks and hypomethylation within the p53 tumor suppressor gene. The American Journal of Clinical Nutrition. 1997;65(1):46–52. doi: 10.1093/ajcn/65.1.46. [DOI] [PubMed] [Google Scholar]
- 40.Burdge GC, et al. Folic Acid Supplementation during the Juvenile-Pubertal Period in Rats Modifies the Phenotype and Epigenotype Induced by Prenatal Nutrition. The Journal of Nutrition. 2009;139(6):1054–1060. doi: 10.3945/jn.109.104653. [DOI] [PubMed] [Google Scholar]
- 41.McKay JA, Williams EA, Mathers JC. Effect of maternal and post weaning folate supply on gene-specific DNA methylation in the small intestine of weaning and adult Apc+/Min and wild type mice. Frontiers in Genetics. 2011:2. doi: 10.3389/fgene.2011.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McKay JA, et al. Blood as a surrogate marker for tissue-specific DNA methylation and changes due to folate depletion in post-partum female mice. Molecular Nutrition & Food Research. 2011;55(7):1026–1035. doi: 10.1002/mnfr.201100008. [DOI] [PubMed] [Google Scholar]
- 43.van den Donk M, et al. Dietary Folate Intake in Combination with MTHFR C677T Genotype and Promoter Methylation of Tumor Suppressor and DNA Repair Genes in Sporadic Colorectal Adenomas. Cancer Epidemiology Biomarkers & Prevention. 2007;16(2):327–333. doi: 10.1158/1055-9965.EPI-06-0810. [DOI] [PubMed] [Google Scholar]
- 44.van Engeland M, et al. Effects of Dietary Folate and Alcohol Intake on Promoter Methylation in Sporadic Colorectal Cancer: The Netherlands Cohort Study on Diet and Cancer. Cancer Research. 2003;63(12):3133–3137. [PubMed] [Google Scholar]
- 45.Stidley CA, et al. Multivitamins, Folate, and Green Vegetables Protect against Gene Promoter Methylation in the Aerodigestive Tract of Smokers. Cancer Research. 2010;70(2):568–574. doi: 10.1158/0008-5472.CAN-09-3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vineis P, et al. DNA methylation changes associated with cancer risk factors and blood levels of vitamin metabolites in a prospective study. Epigenetics. 2011;6(2):195–201. doi: 10.4161/epi.6.2.13573. [DOI] [PubMed] [Google Scholar]
- 47.Ba Y, et al. Relationship of folate, vitamin B12 and methylation of insulin-like growth factor-II in maternal and cord blood. Eur J Clin Nutr. 2011;65(4):480–485. doi: 10.1038/ejcn.2010.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dolinoy DC, Jirtle RL. Environmental epigenomics in human health and disease. Environmental and Molecular Mutagenesis. 2008;49(1):4–8. doi: 10.1002/em.20366. [DOI] [PubMed] [Google Scholar]
- 49.Hoyo C, et al. Methylation variation at IGF2 differentially methylated regions and maternal folic acid use before and during pregnancy. Epigenetics. 2011;6(7):928–936. doi: 10.4161/epi.6.7.16263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Christensen BC, et al. Breast Cancer DNA Methylation Profiles Are Associated with Tumor Size and Alcohol and Folate Intake. PLoS Genet. 2010;6(7):e1001043. doi: 10.1371/journal.pgen.1001043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mostowska A, et al. Folate and choline metabolism gene variants and development of uterine cervical carcinoma. Clinical Biochemistry. 2011;44(8–9):596–600. doi: 10.1016/j.clinbiochem.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 52.Craciunescu CN, Johnson AR, Zeisel SH. Dietary Choline Reverses Some, but Not All, Effects of Folate Deficiency on Neurogenesis and Apoptosis in Fetal Mouse Brain. The Journal of Nutrition. 2010;140(6):1162–1166. doi: 10.3945/jn.110.122044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mehedint MG, Craciunescu CN, Zeisel SH. Maternal dietary choline deficiency alters angiogenesis in fetal mouse hippocampus. Proceedings of the National Academy of Sciences. 2010;107(29):12834–12839. doi: 10.1073/pnas.0914328107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Niculescu MD, Craciunescu CN, Zeisel SH. Dietary choline deficiency alters global and gene-specific DNA methylation in the developing hippocampus of mouse fetal brains. The FASEB Journal. 2006;20(1):43–49. doi: 10.1096/fj.05-4707com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mehedint MG, et al. Choline deficiency alters global histone methylation and epigenetic marking at the Re1 site of the calbindin 1 gene. The FASEB Journal. 2010;24(1):184–195. doi: 10.1096/fj.09-140145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kovacheva VP, et al. Gestational Choline Deficiency Causes Global and Igf2 Gene DNA Hypermethylation by Up-regulation of Dnmt1 Expression. Journal of Biological Chemistry. 2007;282(43):31777–31788. doi: 10.1074/jbc.M705539200. [DOI] [PubMed] [Google Scholar]
- 57.Du Y-p, et al. Assessment of the effect of betaine on p16 and c-myc DNA methylation and mRNA expression in a chemical induced rat liver cancer model. BMC Cancer. 2009;9(1):261. doi: 10.1186/1471-2407-9-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xing J, Kang L, Jiang Y. Effect of dietary betaine supplementation on lipogenesis gene expression and CpG methylation of lipoprotein lipase gene in broilers. Molecular Biology Reports. 2011;38(3):1975–1981. doi: 10.1007/s11033-010-0319-4. [DOI] [PubMed] [Google Scholar]
- 59.Perry C, et al. Effect of vitamin B6 availability on serine hydroxymethyltransferase in MCF-7 cells. Archives of Biochemistry and Biophysics. 2007;462(1):21–27. doi: 10.1016/j.abb.2007.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chan J, et al. Low dietary choline and low dietary riboflavin during pregnancy influence reproductive outcomes and heart development in mice. The American Journal of Clinical Nutrition. 2010;91(4):1035–1043. doi: 10.3945/ajcn.2009.28754. [DOI] [PubMed] [Google Scholar]
- 61.Kulkarni A, et al. Effects of Altered Maternal Folic Acid, Vitamin B12 and Docosahexaenoic Acid on Placental Global DNA Methylation Patterns in Wistar Rats. PLoS ONE. 2011;6(3):e17706. doi: 10.1371/journal.pone.0017706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Amaral CLD, et al. The effects of dietary supplementation of methionine on genomic stability and p53 gene promoter methylation in rats. Mutation Research/Genetic Toxicology and Environmental Mutagenesis. 2011;722(1):78–83. doi: 10.1016/j.mrgentox.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 63.Dolinoy DC, et al. Variable histone modifications at the Avy metastable epiallele. Epigenetics. 2010;5(7):637–644. doi: 10.4161/epi.5.7.12892. [DOI] [PMC free article] [PubMed] [Google Scholar]


