Abstract
Marburg and Ebola viruses cause severe hemorrhagic fever in humans and nonhuman primates. Currently, there are no effective treatments and no licensed vaccines; although a number of vaccine platforms have proven successful in animal models. The ideal filovirus vaccine candidate should be able to provide rapid protection following a single immunization, have the potential to work postexposure and be cross-reactive or multivalent against all Marburg virus strains and all relevant Ebola virus species and strains. Currently, there are multiple platforms that have provided prophylactic protection in nonhuman primates, including DNA, recombinant adenovirus serotype 5, recombinant human parainfluenza virus 3 and virus-like particles. In addition, a single platform, recombinant vesicular stomatitis virus, has demonstrated both prophylactic and postexposure protection in nonhuman primates. These results demonstrate that achieving a vaccine that is protective against filoviruses is possible; the challenge now is to prove its safety and efficacy in order to obtain a vaccine that is ready for human use.
Keywords: Ebola virus, filovirus, Marburg virus, postexposure, prophylactic, vaccine
Ebola virus (EBOV) and Marburg virus (MARV) are enveloped, nonsegmented, negative-sense RNA viruses that belong to the family Filoviridae [1]. Both EBOV and MARV cause geographically and temporally unpredictable outbreaks of severe hemorrhagic fever in equatorial Africa [2], with case–fatality rates in humans as high as 90% [3,4]. The disease syndrome is characterized by systemic viral replication, high levels of inflammatory cytokines, generalized immunosuppression, coagulation abnormalities and fluid distribution problems [5–9]. The end result of these processes is hemorrhage and vascular leakage, finally culminating in shock and multiorgan failure [3,9]. An absence of a vigorous immune response and lymphopenia are characteristics of individuals who do not survive infection [10–16].
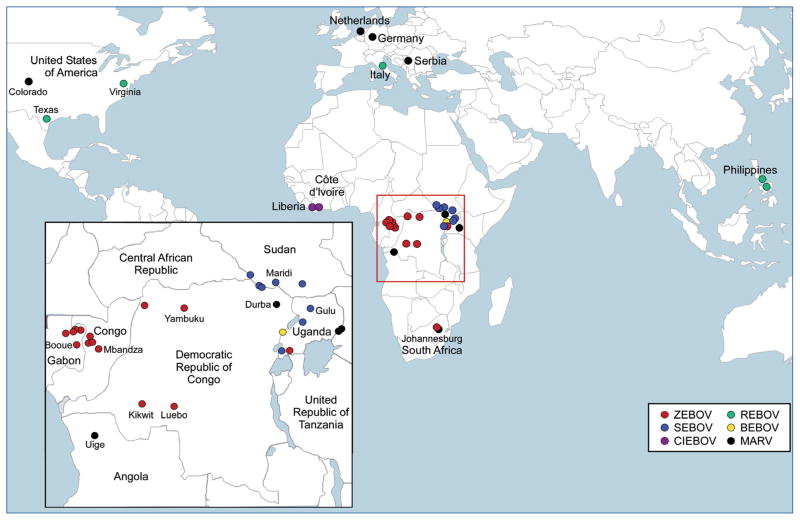
Based on serological cross-reactivity and genetic analyses, there are five species of EBOV: Zaire ebolavirus (ZEBOV), Sudan ebolavirus (SEBOV), Cote d’Ivoire ebolavirus (CIEBOV), Reston ebolavirus (REBOV) and Bundibugyo ebolavirus (BEBOV) – a recently identified, proposed separate species, isolated in Uganda in 2007 [17]. By contrast, MARV contains a single species, Lake Victoria marburgvirus. See Figure 1 for the geographic locations of filovirus outbreaks. REBOV is the only species of EBOV that has not appeared in tropical Africa. It has been identified multiple times in cynomolgus macaques imported from the Philippines [18,19] and in 2008, it was identified during a high incidence of pig mortality in the Philippines [20–23]. While virulent in monkeys, REBOV has no apparent pathogenicity in humans, despite at least 25 cases of infection (0% case–fatality rate) [24,25]. To date, there have only been two reported cases of CIEBOV (with no fatalities); therefore, REBOV and CIEBOV are of less concern regarding vaccine development. Thus, the minimal filovirus vaccine would be protective against all MARV strains and at least the three African EBOV species: ZEBOV, SEBOV and BEBOV.
Figure 1. Map of filovirus occurrences worldwide.
Zaire ebolavirus (ZEBOV): red; Sudan ebolavirus (SEBOV): blue; Cote d’Ivoire ebolavirus (CIEBOV): purple; Reston ebolavirus (REBOV): green; Bundibugyo ebolavirus (BEBOV): yellow; Lake Victoria marburgvirus (MARV): black. The inset shows an enlarged view of equatorial Africa where the majority of filovirus infections occur.
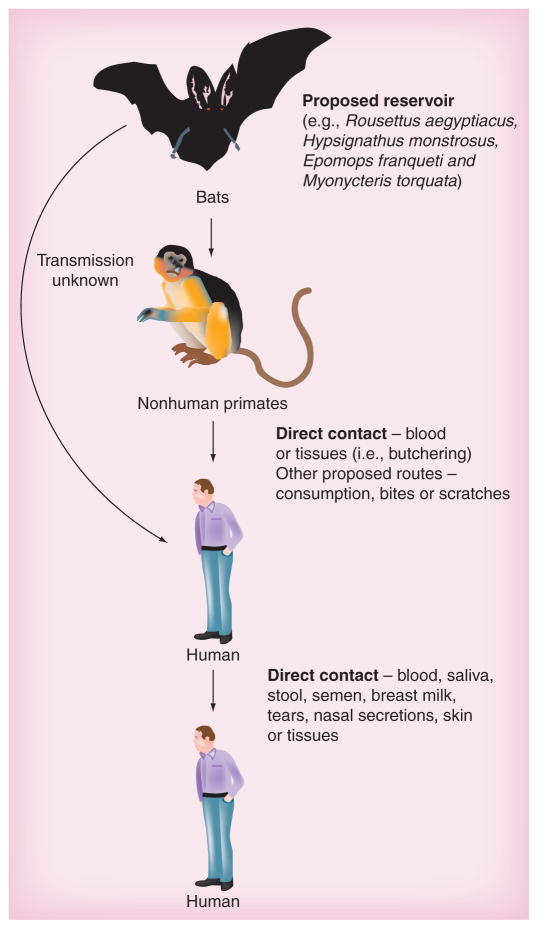
Filovirus transmission is typically due to direct contact with blood [26], secretions or tissues from infected patients or animals (e.g., gorillas and chimpanzees) (Figure 2) [3]. Analysis of clinical specimens has found evidence of the virus in saliva, stool, semen, breast milk, tears and blood, as well as nasal and skin swabs [27]. Mucosal exposure has also been demonstrated as a route of infection in experimentally exposed nonhuman primates [28,29]. In addition, there are unconfirmed data that suggest an airborne route as the mode of transmission in a limited number of human cases [30]. Current data point toward a number of fruit bat species as the reservoir for both EBOV and MARV (Figure 2) [31–34], suggesting that sporadic outbreaks of these viruses will continue as vector control is not a viable option.
Figure 2. Transmission of filoviruses.
The proposed reservoirs for both Ebola and Marburg viruses are a number of species of African fruit bats. Multiple outbreaks have been associated with locations where contact with or the consumption of bats has occurred. Contact with infected nonhuman primates, typically as bush meat, has also been a suspected source of infection. While the mechanism of transmission from bats to humans and nonhuman primates is not known, transmission from nonhuman primates to humans is likely through contact with blood and tissues during butchering or from bites and scratches. Transmission between humans probably occurs via direct contact with blood or secretions; however, there is limited evidence of aerosol transmission. In the event of an act of bioterrorism the most likely route of infection would be by the aerosol route.
A number of laboratory-acquired infections have been documented since the first filovirus incident in 1967, where 32 people (seven of whom died) developed what would be named Marburg hemorrhagic fever following handling of African green monkey tissues in Germany and the former Yugoslavia. Two laboratory-acquired MARV infections (one fatal) have been reported in Russia (1988 and 1990). Four known high-risk laboratory exposures to EBOV have been reported. The first occurred in the UK in 1976 (SEBOV), where following treatment with human interferon (IFN) and human convalescent plasma, the individual survived [35–37]. In two separate incidents in 2004, accidental needle sticks occurred while working with animals; one in the USA (mouse-adapted ZEBOV), where the person did not appear to be infected [38], and one in Russia (guinea pig-adapted ZEBOV), where the infected individual did not survive [39]. Most recently (2009), a needle stick incident occurred while infecting animals with ZEBOV in Germany. It is uncertain whether this individual was actually infected; however, following postexposure vaccination with a live-attenuated recombinant vesicular stomatitis virus (rVSV)-based vaccine expressing ZEBOV glycoprotein (GP) [40,41], the individual did not exhibit any signs of Ebola hemorrhagic fever (EHF) [42]. In the absence of an effective treatment, vaccination remains the best option to protect laboratory staff.
The sporadic and geographically diverse occurrence of filovirus outbreaks makes it difficult to select a target population for vaccination. It also causes considerable difficulty in establishing traditional clinical trials. Considering the fact that the endemic zones of MARV and EBOV overlap, a single vaccination against both would be ideal. Multiple approaches have yielded promising results for prophylactic vaccinations that have proven highly efficacious in nonhuman primate studies, while rVSV has proven to work both as a prophylactic vaccine and as a postexposure treatment. As the logistics of distributing vaccinations that require multiple immunizations in central Africa are difficult, it would be preferable for a prophylactic vaccine to be efficacious following a single immunization.
Filovirus vaccine platforms are almost always initially tested in the mouse model (for EBOV) that, if successful, progresses to the guinea pig model (the starting model for MARV as a suitable mouse model is not available) and then finally into nonhuman primates [43]. To date, small animal models have not served as good predictors of success in the nonhuman primate models. Thus, only platforms that exhibit extremely high success in the small animal models are progressed into nonhuman primates. Currently, the rVSV and recombinant adenovirus serotype 5 (rAd5; with and without DNA priming) vaccine platforms have undergone the most extensive and varied (multiple antigens, multivalent vaccines, cross-protection studies, and so on) testing in nonhuman primates and consistently demonstrate protection. A number of other platforms do exist (e.g., DNA, other recombinant adenovirus [rAd] platforms, recombinant human para-influenza virus 3 [rHPIV3] and virus-like particles [VLPs]) and are reviewed here, but have either not proven 100% efficacious or have not been tested as extensively in nonhuman primates.
Immune correlates of protection
When circumstances prevent traditional drug and/or vaccine testing from being performed, the US FDA now allows the use of the ‘animal rule’, whereby laboratory animal data can be used to demonstrate vaccine efficacy in place of human trials [44]. For vaccines against MARV and EBOV viruses, the ability to use this regulation would greatly speed up the possibility of obtaining an available vaccine. However, this requires the immune correlates of protection to be defined in nonhuman primates, as the other filovirus animal models (mouse, guinea pig and hamster) either do not fully recapitulate disease or require adapted viruses to produce similar disease characteristics.
Unfortunately, the immune correlates of protection for filoviruses are not well defined. All vaccine platforms discussed here (DNA/rAd [45], rAd alone, VLP [46], rHPIV3 [47] and rVSV-based [41]) have all reported that the production of virus-specific IgG antibodies correlates with protection in survivors. Furthermore, all vaccines that have resulted in 100% survival following challenge successfully induced antibody responses. As the pathophysiology and clinical symptoms of EBOV and MARV infection are accurately represented in the nonhuman primate model [7,48], this model meets the requirement of the animal rule [44]. Near-complete survival can be predicted if a certain IgG titer is produced in the rAd5 vaccine platform. This suggests that a specific threshold of IgG titer may be a useful correlate of protection [44]. Currently, it is unclear whether antibodies alone are sufficient for protection, as passive transfer of immune serum to naive nonhuman primates was not protective [49,50]; however, it has recently been reported that purified convalescent IgG successfully protected 100% of rhesus macaques from MARV challenge [51]. In the rodent models, where passive transfer is sufficient for protection [52–56], adoptive transfer and knockout models have suggested that both B and T cells are mediators of protective immunity [57]. This may also be likely in the nonhuman primate model.
Depletion of T-cell subsets indicates that these cells are required for virus clearance [44]. Despite the apparent requirement for T cells, the CD4+ and CD8+ responses are either low or inconsistent. Limited data suggest that the IgG titer also correlates with survival during infection in humans [58], in addition to specific HLA class B alleles [59] and T-cell activation (as measured by the production of specific cytokines) [60,61]. Thus, GP-specific IgG titers correlate with survival following experimental infection in nonhuman primates; however, it is clear that antigen-specific T-cell responses are also elicited and are probably involved in viral clearance and protection, although the mechanism of protection remains undefined [44]. Clarification of the immune correlates of protection from the leading vaccine platforms would open the door to more rapid development of vaccines, as recipients could then be tested to ensure that the vaccine elicits the desirable (and predictive for protection) response following vaccination.
Replication-competent vaccines
Replication-competent vaccines have a number of advantages over nonreplicating vaccines, including the induction of a more durable, longer-lasting immunity that typically requires fewer immunizations to confer protection. While the effectiveness of many vector-based vaccines may be compromised by pre-existing immunity (e.g., seropositivity to Ad5 or HPIV3), in many cases there are methods to overcome this, such as by altering the vector background (Ad, HPIV3) [62–64] or mucosal delivery (Ad5, HPIV3) [65–68]. For vesicular stomatitis virus (VSV), the general population has a low rate of exposure. Moreover, individuals that are seropositive generally have an immune response that is directed towards neutralizing VSV G, which is not present in these vaccine vectors [69,70]. A modified HPIV3 vector, whereby its major surface proteins (fusion protein [F] and hemagglutinin [HN]) have been replaced exclusively with ZEBOV GP, also exploits this method of avoiding pre-existing immunity. In addition, the ability to replicate postvaccination results in a smaller number of particles being required for vaccination.
Recombinant VSV
Vesicular stomatitis virus, a member of the Rhabdoviridae family, has been developed into an attenuated replication-competent recombinant vaccine platform (rVSV) for a number of pathogens including HIV [71], hepatitis B virus [72], influenza [73], SARS coronavirus [74], respiratory syncytial virus [75], human papillomavirus (HPV) [76] and HPV-associated cancer [77]. Reverse genetics was used to replace the native VSV G with the glycoprotein of either ZEBOV, SEBOV or MARV to generate virus particles that appear morphologically similar to VSV particles, but contain either ZEBOV GP, SEBOV GP or MARV GP on their surface.
In the initial study, both rVSV-MARV GP and rVSV-ZEBOV GP demonstrated 100% protection against homologous challenge in cynomolgus macaques following a single intramuscular immunization [41]. Animals vaccinated with rVSV-ZEBOV GP that survived challenge were back-challenged with SEBOV. All developed clinical symptoms and only one out of four survived. The survivor may be attributed to the SEBOV model not resulting in 100% lethality [78,79]. Animals vaccinated with rVSV-MARV GP (strain Musoke) back-challenged with a different MARV strain (Popp) survived without apparent illness [41]. Animals vaccinated with rVSV-MARV GP and subsequently challenged with ZEBOV were not protected. Similarly, animals vaccinated with rVSV-ZEBOV GP and subsequently challenged with MARV were not protected. Together, these data suggest that within a filovirus species (e.g., MARV) there is cross-protection against different strains, but no cross-protection between species and genera (e.g., between MARV and ZEBOV or ZEBOV and SEBOV). Cross-protection between heterologous MARV strains has also been demonstrated in cynomolgus macaques that were protected from challenge strains Ravn (the most divergent MARV isolate) and Angola (the most virulent MARV isolate to date) when vaccinated 28 days prior with rVSV-MARV GP (strain Musoke) [69]. Both the rVSV-ZEBOV GP and rVSV-MARV GP vaccines were able to protect cynomolgus macaques from homologous aerosol challenge with ZEBOV and MARV, respectively [80].
Multiple routes of immunization with rVSV have also been demonstrated to be effective. Delivering the rVSV-ZEBOV GP vaccine by the oral, intranasal or intramuscular route 28 days prior to challenge protected all cynomolgus macaques from subsequent ZEBOV challenge [81]. The intranasal route appeared to elicit the highest antibody titers, followed by the oral and then intramuscular routes [81]. Similar to other studies, the level of neutralizing antibodies was low by all routes of immunization, supporting the hypothesis that they are not solely critical for protection. In contrast to control animals, which undergo a reduction in lymphocytes during infection, vaccinated animals either showed a stable or small increase in CD3+, CD4+ and CD8+ cells, typical of a secondary immune response and suggestive that a strong Th1 cell-mediated immune response is induced [81].
A proof-of-concept study has also been carried out that demonstrated that a single-injection blended vaccine (containing equal amounts of rVSV-ZEBOV GP, rVSV-SEBOV GP and rVSV-MARV GP) could protect cynomolgus macaques against challenge with ZEBOV, SEBOV and MARV [82]. This illustrates that, in principle, it should be possible to generate a pan-filovirus rVSV-based blended vaccine. The addition of rVSV-BEBOV GP to the blended vaccine would have the potential to protect against all current filovirus species and strains of current importance to human health. The reusability of the rVSV platform has also been demonstrated, whereby rhesus macaques were vaccinated with rVSV-SEBOV GP and then vaccinated 2 weeks later with rVSV-ZEBOV GP and rVSV-MARV GP. The animals were protected from subsequent challenge with SEBOV and MARV, thus demonstrating the reusability of the rVSV vector [82]. As the exposed glycoproteins differ from each other and VSV G is not included in the construct, any neutralizing antibodies that are present should not interfere with the different vector constructs; however, the time frame between vaccinations may not have been sufficient for the development of antivector immunity. Regardless, these data suggest that separate immunizations could be used to generate immunity to multiple filoviruses, despite the convenience of a one-shot blended vaccine, which would be logistically superior.
In rVSV-vaccinated macaques that are protected from challenge, there is no evidence of EBOV or MARV viremia by virus isolation and/or reverse transcriptase-PCR, nor were there changes in blood chemistry or hematology. This indicates the robustness of protection, demonstrating that animals are completely protected from disease and is highly suggestive of sterile immunity. Following a single dose of rVSV, vaccinated animals typically have high anti-MARV or -EBOV IgG prior to challenge. There are also low levels of neutralizing antibodies in some animals; however, approximately half of the animals do not have neutralizing antibodies. As observed in the rAd5 platform, this suggests that neutralizing antibodies are not a correlate of protection; however, following challenge, most animals have a low level of neutralizing antibodies.
Currently, the main criticism of the rVSV platform has been concerns over its safety, especially in immunocompromised individuals, as it is a replicating vaccine. One of the characteristics of the rVSV vaccine that reinforces these concerns is the consistent detection of transient low-level VSV viremia, typically noted on day 3 postvaccination. While this is not unexpected (as it is a replication-competent vaccine), it does suggest that the potential for vaccine-induced morbidity exists. Animal studies have suggested that rVSV vectors are safe, as rVSV-ZEBOV GP did not induce any signs of clinical illness in severely immunocompromised (nonobese diabetic/severe combined immunodeficient) mice, even when given ten-times the normal dose [52]. Moreover, no adverse effects have been reported in a variety of immunocompetent mice, outbred guinea pigs (strain Hartley), goats or hamsters [52,70] [Feldmann H, Unpublished Data]. However, vaccination of STAT1−/− mice with rVSV vectors is lethal [Feldmann H, Unpublished Data]. Assessment of the toxicity of rVSV-HIV vaccines has demonstrated that rVSV is attenuated in nonhuman primates, which suggests that they would be safe to advance into Phase I clinical trials [83]. In addition, a major virulence factor in VSV is thought to be G, which is not included in the vaccine constructs. To date, over 90 macaques have been administered replication-competent rVSV vectors with no adverse effects (38 cynomolgus and three rhesus macaques with VSV-ZEBOV GP; 12 cynomolgus and three rhesus macaques with rVSV-SEBOV GP; 29 cynomolgus and three rhesus macaques with rVSV-MARV GP; and six cynomolgus macaques with rVSV-Lassa GPC) [40,41,69,80–82,84–88].
Recently, the safety of the rVSV vaccine has been further demonstrated following immunization of simian–human immunodeficiency virus (SHIV)-infected rhesus macaques with rVSV-ZEBOV GP [85]. Not only did these animals show no evidence of illness from vaccination, but four out of six were also protected from subsequent challenge with ZEBOV, demonstrating that this vaccine may also be effective in immunocompromised individuals. In these animals, there was a correlation between low CD4+ counts and a lethal outcome, suggesting that these cells may play a role in mediating protection. Low-level VSV viremia was detected on day 2 postvaccination in four out of six animals. Interestingly, the two animals with the most significant reduction in CD4+ cells following SHIV infection, the highest levels of SHIV viremia and that showed no evidence of VSV viremia did not survive challenge [85]. Two of the surviving animals showed no evidence of illness while the other two developed fever but few other clinical symptoms. None of the vaccinated animals developed IgG titers against ZEBOV GP by the time of ZEBOV challenge; however, IgG was detectable following infection in three animals. This suggests that the short-lived low-level VSV viremia may be important for protection, CD4+ cells play a role in protection and/or IgG antibodies may not be as important for protection as previously thought.
rVSV postexposure
Populations at risk for EBOV and MARV infection are largely limited to accidental laboratory exposures and regions where natural filovirus outbreaks occur. However, as filoviruses occur over a wide geographic area and are unpredictable, it is difficult to determine a critical target population for vaccination campaigns. In the event that a filovirus is used as a biological weapon, any population could be at risk. Therefore, in the absence of an effective treatment, a postexposure vaccination would be an ideal treatment option. The utility of the rVSV platform as a postexposure treatment was determined in a study by Feldmann and colleagues [40]. rVSV-ZEBOV GP administered to guinea pigs and mice 24 h prior to challenge, 1 h postchallenge and 24 h postchallenge resulted in 67, 83 and 50% survival in guinea pigs, respectively, while all treated mice survived [40]. All guinea pigs showed weight loss and clinical symptoms; however, in those that did not survive, time to death was delayed. More importantly, rVSV-ZEBOV GP also protected 50% of rhesus macaques when administered 30 min following challenge [40]. While all macaques developed fever and lymphopenia, 50% of the animals did not progress to more severe disease, including alterations in clinical chemistry and macular rash. As noted previously, rVSV viremia was detected on day 3 postimmunization in most immunized animals [41]; however, this did not correlate with survival [40]. ZEBOV viremia in survivors was 2–4 logs lower than in non-survivors and survivors developed low-titer IgM and moderate IgG antibody responses, as well as neutralizing antibody titers, while antibody responses were not detected in nonsurvivors [40]. Of note, animals treated with rVSV did not show a decrease in natural killer (NK) cells following challenge but instead showed a substantial increase. A postexposure treatment for SEBOV was also investigated. rVSV-SEBOV GP protected 100% of rhesus macaques when administered postexposure, following a lethal challenge with SEBOV [86]. This demonstrates that 100% postexposure protection is possible.
Postexposure treatment with rVSV-MARV GP at 30 min, 24 h and 48 h following infection of rhesus macaques with MARV (strain Musoke) resulted in 100, 83 and 33% survival, respectively [87,88]. All treated animals generated IgM, IgG and neutralizing antibodies [87]. Transient MARV viremia was detectable by reverse transcriptase-PCR in all groups. Animals that did not survive showed symptoms consistent with MARV infection, while some survivors showed no clinical symptoms. Some survivors exhibited alterations in their hematology and/or blood parameters, but all surviving animals cleared viremia by day 14 with little evidence of illness. Together, this indicates that postexposure vaccination is possible.
In its first use in humans, 5 × 107 plaque-forming units (pfu; consistent with the dose give to nonhuman primates) of rVSV-ZEBOV GP was given to a laboratory worker in Germany approximately 40 h following a needle stick that potentially exposed the person to ZEBOV [42]. This individual developed fever, headache and myalgia following vaccination; however, these symptoms were managed with analgesics and antipyretics. No additional adverse effects were reported and the individual did not become ill, either with EHF or with any further VSV-associated illness. The effectiveness of rVSV-ZEBOV GP in this case remains unclear, as it has never been confirmed that the individual was actually infected with ZEBOV.
Currently, postexposure treatment of nonhuman primates has been more successful for MARV and SEBOV than for ZEBOV. Apart from the differences between the viruses, the development of symptoms and death is delayed in MARV- and SEBOV-infected monkeys when compared with ZEBOV, which may be one of the reasons behind the greater postexposure efficacy of the rVSV-MARV GP and -SEBOV GP vaccines. It will be interesting to determine whether rVSV is also as efficacious postexposure against heterologous MARV strains, most notably Angola, which results in a time to death that is similar to that of ZEBOV. As both EBOV and MARV infections appear to progress somewhat slower in humans than in nonhuman primates, this would suggest that the therapeutic window for postexposure treatment in humans may be longer. Furthermore, the challenge dose used in rVSV trials represents approximately 1000 lethal dose 50 (LD50) [89], which under almost all circumstances is higher than what would be expected from laboratory or direct contact exposures. In future studies, it will be interesting to see if a blended vaccine (similar to that used for prophylactic protection) has the ability to also work postexposure.
Similar to prophylactic vaccination, the correlates of protection in postexposure treatment are also unclear. An indicator of survival included the ability to control viremia by day 6 postinfection. Current speculation suggests the rapid development of non-neutralizing antibodies and an increase in NK cells correlates with survival following postexposure treatment. NK cells have also been associated with protection in the VLP platform [90]. Another hypothesis is that rVSV vaccine vectors infect the same target cells as filoviruses and outcompete the wild-type infection, thereby inhibiting filovirus replication through viral interference [40]. Alternatively, VSV may induce an innate immune response that is sufficient to overcome challenge virus infection.
Recombinant human parainfluenza virus 3
Human parainfluenza virus 3, a member of the Paramyxoviridae family, is a common pediatric respiratory virus that was modified to express ZEBOV GP in addition to its other proteins (rHPIV3-ZEBOV GP). Live-attenuated HPIV3 has also been investigated as a pediatric vaccine [91] and as a dual vaccine vector against HPIV3 and measles [92]. rHPIV3-ZEBOV GP did not cause any obvious disease in guinea pigs or rhesus macaques when administered by the intranasal and intratracheal route [47,93]. A single intranasal/intratracheal dose of either rHPIV3-ZEBOV GP or rHPIV3-ZEBOV GP/nucleoprotein (NP) was sufficient to protect guinea pigs from ZEBOV challenge [93]. In rhesus macaques, a single intranasal dose induced a moderate immune response and protected 88% of animals from ZEBOV challenge, although 55% showed signs of disease. This was improved to 100% survival with two intranasal immunizations, which induced a stronger immune response and prevented any signs of disease [47].
Since nearly all adults have immunity to HPIV3, pre-existing immunity may interfere with vaccination. In HPIV3-immune guinea pigs, despite restriction of rHPIV3-ZEBOV GP replication by HPIV3 neutralizing antibodies, these animals produced ZEBOV GP-specific antibodies at nearly the same level as HPIV3-naive animals [94]. In HPIV3-immune rhesus macaques, the antibody response to ZEBOV GP was substantially lower when compared with HPIV3-naive animals following the first immunization. However, the antibody response was comparable between HPIV3-naive and -immune animals following a second immunization [65], achieving a level that has been previously characterized as being required for 100% protection in this model [47]. Unfortunately, there is no report of these animals being challenged to demonstrate that pre-existing immunity does not interfere with protection from challenge. In order to circumvent pre-existing vector immunity, a construct that does not contain the F or HN gene of HPIV3 was developed that solely expresses ZEBOV GP on its surface. While its replication in guinea pig lungs appeared to be even more restricted, a single immunization was sufficient to protect guinea pigs from lethal ZEBOV challenge and induced an immune response comparable to previous constructs [62]. This construct was able to incorporate twofold more ZEBOV GP into virus particles and also appears to be even more attenuated than the complete rHPIV3 vector used in previous experiments. To date, there are no reports of the utility of this construct in a macaque model.
While these initial results are encouraging, further development of the HPIV3 platform is required to demonstrate that pre-existing immunity can be overcome in the macaque model, to determinine whether it is cross-protective against other EBOV species and to develop a MARV GP-expressing construct. The requirement for intranasal delivery eliminates the need for needles and the restriction of vector replication to the respiratory tract is thought to add to its safety [65]. Similar to the other vaccines, the IgG response appears to correlate with immunity; however, the cellular immune response to these vectors has not been described. The rHPIV3 platform appears to induce both systemic and local immune responses. It is possible that the IgA response in the lungs may be advantageous against the aerosol infection route. Production is straightforward and can be performed in approved cell lines.
Nonreplicating vaccines
Vaccine candidates that are nonreplicating are generally considered to be safer than live vector vaccines, as they do not have the ability to replicate under any conditions (e.g., in a compromised immune system or in the case of concurrent infection with another pathogen). The trade-off for potential enhanced safety can be a higher dose of vaccine, multiple immunizations and less robust, shorter-duration immunity. In some cases (e.g., adenovirus), pre-existing immunity against the vector is an additional obstacle. For filoviruses, a number of effective nonreplicating vaccines have been developed including Venezuelan equine encephalitis virus replicons (VEEr), DNA, rAd5 and other adenovirus (rAd) serotypes, combined DNA/rAd5, VLPs and an EBOV construct with a critical gene deletion (ΔVP30 ZEBOV).
Venezuelan equine encephalitis virus replicon
Venezuelan equine encephalitis virus (VEEV) is an alphavirus that can be genetically manipulated such that in place of the open-reading frame that encodes the VEEV structural proteins, genes of interest (e.g., ZEBOV GP, NP, and so on) can be added, resulting in a ‘replicon’ (VEEr). When the structural genes of VEEV are supplied in trans, VEEV-like particles are produced that are capable of carrying out a single-cycle infection, thus expressing the immunogen(s) of interest. VEEr expressing MARV GP-Musoke (with or without NP) protected 100% of cynomolgus macaques from homologous challenge following three vaccinations [95]; however, subsequent experiments showed this platform provided no protection from heterologous MARV-Ravn challenge [96]. VEEr expressing ZEBOV GP (with or without NP) was shown to be completely protective against homologous challenge in BALB/c mice (where CD8+ cells were shown to be important for protection [97]) and guinea pigs [98] but not cynomolgus macaques [99]. While development of the VEEr platform has progressed for biodefense purposes (e.g., the development of a multi-agent platform [100]), further utility of this platform in nonhumans primates has not been reported.
DNA
DNA vaccines are a novel vaccine platform currently being developed for multiple pathogens, such as hepatitis B virus, influenza virus, HIV, SARS-coronavirus and West Nile virus [101]. DNA vaccines are attractive as they are easy to produce and readily scalable, noninfectious, can be reused as pre-existing immunity is not relevant, and have the ability to induce both humoral and cellular immunity. Genetic-based vaccines can also be rapidly altered as pathogens evolve, cross species barriers or are intentionally modified [102]. In clinical trials, DNA vaccines appear to have a very good safety profile; however, their immunogenicity has been less than desirable [101,103,104]. Currently, it appears that DNA-based platforms will not be used as standalone vaccines but will instead be part of a prime–boost strategy (e.g., with Ad5 vector).
Regardless of their limited immunogenicity, the effectiveness of DNA vaccines alone against filoviruses has been investigated. In cynomolgus macaques, 67% of animals were protected from homologous challenge with MARV-Musoke after receiving three doses of a DNA vaccine containing MARV-Musoke GP [105]. Surprisingly, there was no correlation between antibody production and protection, suggesting that cell-mediated immunity may be important; however, this was not investigated. A single immunization of an optimized (codon-optimized and promoter modification) MARV-Angola GP DNA vaccine was immunogenic, but evoked an IgG response that was order of magnitude less than that induced by a single immunization with an rAd5 construct [102]. Following four vaccinations, this DNA vaccine was able to induce an IgG titer comparable to a single immunization of rAd5-MARV-Angola GP. This regimen was able to protect 100% of cynomolgus macaques from homologous challenge; however, 75% of the animals showed clinical signs of illness and lymphocyte depletion, suggesting that while they survived the challenge, DNA vaccines do not optimally control MARV infection [102].
In the first clinical trial of a filovirus vaccine, the three-plasmid DNA vaccine (used in the combined DNA/rAd5 experiments described below [45,106]), that encodes ZEBOV GP, SEBOV GP and ZEBOV NP was found to be safe and immunogenic during a Phase I trial in humans [107]. Three injections of either 2, 4 or 8 mg of the vector were given. No significant adverse reaction or coagulation abnormalities were reported and all 20 individuals in the study were observed to have specific antibodies and/or CD4+ T-cell responses to at least one of the antigens. Antibody titers were reported to be comparable to that of nonhuman primates immunized with a similar regimen. This suggests these individuals may survive EBOV infection but are likely to suffer illness.
Combined DNA/rAd5
The first vaccine to offer 100% protection from EBOV in non-human primates was a combination DNA/rAd5 vaccination that protected animals from ZEBOV challenge [45]. DNA prime and rAd5 boost strategies result in broad immune responses that include both T- and B-cell responses [45] and the combination strategy results in responses that are at least 1-log higher than DNA or rAd5 alone [108]. Three DNA vaccinations against ZEBOV GP, SEBOV GP, CIEBOV GP and ZEBOV NP were followed with a single rAd5 ZEBOV GP boost. Upon challenge, animals did not develop disease and only one out of four animals developed viremia.
A DNA/rAd5 strategy was also carried out using MARV-Angola GP. In comparison to either DNA or rAd5 alone, by far the highest titers were obtained in the combined immunization schedule whereby DNA-primed animals were boosted with rAd5. Similar to IgG titers, the highest cellular responses were also observed in the DNA/rAd5-immunized animals. Animals receiving rAd5 (either alone or in combination) generally had more predominant CD8+ responses, while those receiving DNA alone tended to favor CD4+. All DNA/rAd5-vaccinated animals were protected from viremia [102], indicating that the most successful method of vaccination would include both DNA and rAd5 vectors.
As there are multiple species of EBOV, it has proven difficult to generate a single vaccine that is protective against all species. A multivalent or a blended vaccine can provide protection against multiple species; however, this adds additional complexity and would not necessarily provide protection against newly emerging species. While four species of EBOV have been definitively established, a new species, BEBOV, emerged in 2007 [17]. Based on previous data from the blended vaccine (rVSV) or the multivalent DNA/rAd5 vaccine, neither platform would be expected to be protective against a new species. However, it was recently reported that the DNA/rAd5 vaccine that expresses the GPs of both ZEBOV and SEBOV was able to provide 100% protection against challenge with BEBOV, despite a sequence difference of 38–47% at the amino acid level [109]. While the mechanism providing cross-protection is not clear, it is speculated that the prime–boost regimen elicits a stronger immune response (with one magnitude increase in antibody titers). In addition, BEBOV appears to be less pathogenic than either ZEBOV or SEBOV (based on time to death in nonhuman primates, the case–fatality rates in the human outbreak and the observance of one survivor out of four in the control group) [109]. There was no evidence of IgG cross-reactivity with BEBOV; therefore, despite extremely high titers (1/40,000), antibodies do not seem to be the mechanism of protection. CD4+ and CD8+ cells were stimulated with BEBOV peptides but to a lesser extent than ZEBOV peptides. The animal with the lowest CD8+ stimulation showed the greatest symptoms of disease, including elevated liver enzymes and detectable viremia on day 6. This suggests that a cellular response may be involved in cross-protection. While this is the first description of cross-protection between EBOV species in this system, the vaccination regimen included four DNA immunizations and one rAd5 boost over the course of nearly 1.5 years. Unfortunately, this vaccination schedule is not practical for use in filovirus-endemic regions where vaccine delivery is logistically challenging.
Recombinant adenovirus 5
While DNA prime–rAd5 boost vaccine regimens have proven to be effective in providing prophylactic protection, the vaccine dosage schedule requires multiple immunizations over 6 months to 1 year, a time frame that is too long to be effective as an intervention during an outbreak or bioterrorism event. A two-dose rAd5 ZEBOV GP schedule provided 100% survival and viremia was not detected; however, the booster did not increase the antibody response, presumably due to vector immunity from the primary immunization, so a single immunization was also tried. A single vaccination with 1012 particles of rAd5 ZEBOV GP and ZEBOV NP also provided 100% survival just 28 days postvaccination [106]. Further studies determined that at least 1010 particles of rAd5 ZEBOV GP were required to prevent viremia and maintain survival, in addition to showing that the inclusion of NP was not required [110].
A pan-filovirus complex adenovirus (CAdVax) has been developed that, from four different constructs, delivers two copies of ZEBOV NP and a single copy of SEBOV GP, ZEBOV GP, MARV-Ci67 GP, MARV-Ravn GP, MARV-Musoke GP and MARV-Musoke NP. Following two immunizations, this blended vaccine was able to protect cynomolgus macaques from challenge with ZEBOV and MARV-Musoke and subsequent back-challenge with MARV-Ci67 and SEBOV respectively [111]. Animals showed no signs of illness or alterations in liver enzymes. Animals showed comparable IgG titers against all five filoviruses. This demonstrates that a single blended/complex vaccine can provide protection against multiple filoviruses. Similarly, a multivalent vaccine candidate, EBO7, which expresses the ZEBOV GP and SEBOV GP in a single CAdVax, was able to provide protection against parenteral and aerosol challenge with SEBOV (this required two vaccinations) and ZEBOV [112].
Pre-existing immunity to Ad5 (present in up to 60% of the general population and as high as 85% in Africa) is a serious problem for vaccine efficacy, as macaques with immunity to Ad5 were not protected from ZEBOV challenge when immunized with rAd5-ZEBOV GP [113]. To overcome this, different serotypes of adenovirus have been used. A chimpanzee-based adenovirus vaccine (AdC7) expressing ZEBOV GP stimulated robust T- and B-cell responses against ZEBOV in naive mice, inducing complete protection from homologous lethal challenge [64]. This platform was also able to protect 100% of guinea pigs from challenge, with a relatively low vaccine dose of 5 × 109 particles/kg. Pre-existing immunity to Ad5 in mice did not affect the efficacy of the vaccine, suggesting it has potential to be used in order to circumvent pre-existing immunity. Similarly, a chimeric simian adenovirus AdC5/C1 expressing ZEBOV GP was generated (AdC5/C1 ZEBOV GP). As described for other Ad constructs, it induced ZEBOV GP-specific T- and B-cell responses. These were of a lower magnitude compared with rAd5 constructs; however, the total IgG response to ZEBOV GP was comparable. A single administration of AdC5/C1 ZEBOV protected mice from lethal challenge [63]. Rhesus macaques vaccinated with AdC5/C1 ZEBOV GP also mounted T-cell and antibody responses to ZEBOV GP. While this appears to be a successful strategy to avoid pre-existing immunity, the utility of these vectors has yet to be demonstrated in a nonhuman primate challenge model.
As an alternative means of avoiding pre-existing immunity, Ad-based vectors can be delivered by oral or nasal vaccination, which protects against a lethal challenge in the ZEBOV mouse model. Both T- and B-cell responses observed in mice receiving oral or nasal vaccination indicate a qualitative improvement of the immune response after mucosal immunization compared with intramuscular vaccination. Thus, mucosal immunization results in better stimulation of CD8+ T cells and effector memory T cells than intramuscular administration of the vaccine [68]. However, in the presence of pre-existing immunity, only mice vaccinated by the intranasal route were protected [67].
A next-generation rAd5 vector that contains an improved expression cassette (Ad-CAGoptZGP) demonstrated higher expression of ZEBOV GP and significantly improved T- and B-cell responses at doses that were ten to 100-fold lower than that required with Ad-CMVZGP (on which previous rAd5 vaccines were based). In addition, this vector provided 100% protection against lethal challenge in mice at a dose 100-times lower than the previous rAd5 vector. Surprisingly, it was able to protect mice when given 30 min postchallenge, suggesting that rAd-based vectors may also have the ability to provide protection postexposure [114]. While these data are interesting, it must be demonstrated in the nonhuman primate model, where it has proven more difficult to protect postexposure when compared with either the mouse or guinea pig models.
Virus-like particles
Virus-like particles have been successfully developed into widely available commercial vaccines for hepatitis B virus and HPV. Both of these vaccines have proven to be highly efficacious and have excellent safety records [115–117]. As VLPs lack viral genetic material, they are considered noninfectious and thus safer than replicating vaccines. It is also likely that VLPs interact with the same target cells as the actual virus, they can be administered multiple times to increase the immune response and they present the virus glycoprotein in its native state. For EBOV and MARV, expression of both structural proteins, VP40 (matrix protein) and GP, results in the formation of VLPs that are similar in morphology to virions [118,119]. While VP40 alone is sufficient to drive VLP formation and budding, this process is enhanced in the presence of GP and NP [120]; moreover, protection is mediated by GP and not VP40.
Zaire ebolavirus and MARV VLPs were demonstrated to provide 100% protection from homologous ZEBOV or MARV challenge in mice and guinea pigs, respectively [119,121]. As seen with other vaccine platforms, there was no cross-protection between filovirus species [121]. In an effort to develop a pan-filovirus vaccine, hybrid VLPs that incorporated both ZEBOV and MARV GP with either MARV or ZEBOV VP40 were generated. These hybrid VLPs were not effective at protecting against heterologous challenge; however, a blended vaccine with Ribi adjuvant containing both ZEBOV and MARV GP/VP40 VLPs provided a high level of protection from challenge with either ZEBOV (90% survival) or MARV (100% survival) in guinea pigs [122]. The efficacy of VLPs in nonhuman primates has been demonstrated for both MARV and ZEBOV. Cynomolgus macaques immunized with three doses of ZEBOV VLPs containing GP, NP and VP40 and Ribi adjuvant resulted in survival of all animals without signs of illness or viremia [46]. While MARV-Musoke GP, NP and VP40 VLPs with QS-21 adjuvant were shown to provide protection against challenge by other MARV strains including Angola, Ravn and Ci67 in cynomolgus macaques [123]; however, animals challenged with Ravn showed sign of illness. Production of VLPs in mammalian cells is expensive; therefore, alternative production methods have been investigated. ZEBOV VLPs produced in insect cells via baculovirus expression were shown to have comparable immunogenicity to mammalian-produced VLPs [124]. Following two immunizations, insect cell-derived VLPs protected mice from ZEBOV challenge [125].
ΔVP30 ZEBOV
Reverse genetics was used to replace the essential transcription factor VP30 in the ZEBOV genome with a reporter gene (neomycin) generating Ebola ΔVP30-neo [126]. Replication of this virus was restricted to cells that express VP30 in trans (i.e., a stable cell line: VeroVP30). Ebola ΔVP30-neo produced in the VeroVP30 cells appeared identical in morphology to wild-type virus, had similar growth kinetics and was demonstrated to be genetically stable over multiple (seven) passages. This suggests that recombination whereby VP30 could be reintroduced into the EBOV genome is unlikely [126]. Infection of cells that do not express VP30 in trans demonstrated that replication of Ebola ΔVP30-neo was restricted to VeroVP30 cells [126].
To evaluate safety, delivery of Ebola ΔVP30-neo alone and with VeroVP30 cells into STAT1−/− mice was performed, showing that neither caused any signs of illness or disease in this highly susceptible animal model, nor is recombination likely [127]. Mice vaccinated with this virus developed a detectable IgG antibody response following the first vaccination that was subsequently increased with a second and third boost. An increase in the CD8+ T-cell response was also noted following two immunizations. All mice immunized twice with 106 Ebola ΔVP30-neo survived subsequent challenge with mouse-adapted ZEBOV and showed no evidence of illness [127]. Viremia in vaccinated mice was significantly lower than nonvaccinated mice. Guinea pigs vaccinated twice (107) with Ebola ΔVP30-neo also survived challenge with guinea pig-adapted ZEBOV and also did not show signs of illness [127]. While this vaccine platform has yet to be evaluated in nonhuman primates, current data would suggest that it has proven safe and effective in the vaccination of mice and guinea pigs. Its benefit of delivering all viral proteins may prove useful in cross-protection against other EBOV species, although this remains to be determined. Regardless, its perceived safety risk (as six out of seven genes remain in the construct) may limit its potential for further development.
Applications for filovirus vaccines
There is a lack of a well-defined population to target a filovirus vaccination campaign. Only high-risk groups, such as specific populations in equatorial Africa, would probably be targeted for vaccination as there is currently no demonstrated need for wider coverage. As MARV outbreaks have been both temporally and geographically sporadic, determining regions for prophylactic vaccination is extremely difficult. In EBOV endemic areas (i.e., eastern Gabon, western Republic of Congo, a large portion of the Democratic Republic of the Congo and part of Uganda) a widespread vaccination campaign could be considered. Including protection against MARV in these populations is not without merit as there is overlap between the viruses in these regions. As human MARV infections have been associated with visits to caves around Mount Elgon, near the Kenya/Sudan border, appropriate education and precautions should be considered at this time, rather than vaccination. International aid workers who work in endemic regions or respond to disease outbreaks in equatorial Africa would also benefit from prophylactic vaccination. The other priority population would be laboratory workers who handle these agents. Additional populations that may benefit from prophylactic vaccination would include first responders, front-line hospital staff and military personnel. While a prophylactic vaccine is desirable, the logistics of ensuring good vaccine coverage in equatorial Africa are difficult. This makes a rapid-acting and/or postexposure vaccine desirable as it would have the potential to decrease the case–fatality rates and prevent further spread of disease during an outbreak. This would also be the most useful strategy to minimize fatalities following a bioterrorism event.
Expert commentary
Multiple vaccine platforms (summarized in Table 1) have proven to be efficacious as preventative vaccines in nonhuman primate models of filovirus infections. The most detailed progress is currently available for the rVSV and rAd5 vaccine platforms. While the DNA vaccine alone has been tested in a Phase I clinical trial, its immunogenicity is less than desirable and it requires multiple immunizations over a long time period. The requirement for multiple immunizations makes this platform difficult to implement in filovirus-endemic regions, where vaccination of the entire population is probably logistically and financially impossible. However, it may be useful for vaccinating individuals with high potential exposure risks (e.g. biosafety level-4 workers, front-line hospital staff/first responders and members of agencies who work in endemic areas). While these groups are useful targets for vaccination, the most at-risk population would be unlikely to benefit from such a vaccine. The addition of rAd5 in a prime–boost regimen or rAd5 alone is probably of more utility; however, a method to overcome pre-existing Ad5 immunity has not been definitely proven in the nonhuman primate model. Multiple options to avoid or circumvent pre-existing immunity while still using an Ad vector have been presented, although none of these methods has been thoroughly tested in a nonhuman primate challenge model. Owing to the amount of data available from nonhuman primate studies and limited clinical trials, the combined DNA/rAd5 vaccine platform is likely to progress the fastest to further clinical trials.
Table 1.
Comparison of the currently available filovirus vaccine platforms.
| Platform | Protection | Cross-protection | Benefits | Limitations |
|---|---|---|---|---|
| DNA | Prophylactic | No | Nonreplicating Production |
Multiple immunizations Limited immunogenicity |
| DNA/rAd5 | Prophylactic | Blended vaccine† | Nonreplicating Cross-protection‡ |
Large dose (Ad) Multiple immunizations Two components |
| rAd | Prophylactic | Blended vaccine† | Nonreplicating Multiroute delivery§ |
Pre-existing immunity Large dose (>1010 particles) |
| VLPs | Prophylactic | Blended vaccine†§ | Nonreplicating Successful platform (HBV, HPV vaccines) |
Multiple immunizations Production |
| ΔVP30 ZEBOV§ | Prophylactic | Unknown | Nonreplicating All viral antigens (potential for cross-protection) |
Reintegration of VP30 Limited to ZEBOV |
| rVSV | Prophylactic Postexposure | Blended vaccine† | Single immunization Multiroute delivery |
Replication-competent (attenuated) |
| rHPIV3 | Prophylactic | Unknown | Single immunization potential Mucosal delivery |
Replication-competent (attenuated) Pre-existing immunity¶ Limited to ZEBOV |
Only a multivalent vaccine formulation elicits cross-protection.
Cross-protection has been observed using a rigorous ZEBOV-based vaccination schedule followed by Bundibugyo ebolavirus challenge.
Not tested in nonhuman primates.
ΔF/HN circumvents pre-existing immunity§.
Ad: Adenovirus; F: Fusion protein; HBV: Hepatitis B virus; HN: Hemagglutinin; HPV: Human papillomavirus; rAd: Recombinant adenovirus; rHPIV3: Recombinant human parainfluenza virus 3; rVSV: Recombinant vesicular stomatitis virus; VLP: Virus-like particle; ZEBOV: Zaire ebolavirus.
Concerns over safety continue to delay the use of rVSV as a vaccine platform, despite convincing evidence of its safety in nonhuman primate models, including an immunocompromised SHIV model. rVSV continues to be the only vaccine platform with the potential to prevent disease and death postexposure and was the chosen treatment following a recent laboratory exposure. Its demonstrated effectiveness following a single dose should be a significant advantage for providing vaccinations to target populations in central Africa. Furthermore, a vaccine that works both pre- and postexposure with a relatively short time to immunity could be stored until required (i.e., laboratory exposure, bioterrorism or outbreaks) and then be rapidly implemented as needed. This probably represents the most cost-effective approach and could possibly circumvent the need for full regulatory approval, as such a vaccine could be added to the new investigational drug list for rapid use in the event of an emergency.
Other vaccine platforms appear promising but have not accumulated as great a quantity of data as the two leading platforms. rHPIV3 appears to have potential as a preventative vaccine; however, cross-protection studies and the development of vaccines against anything other than ZEBOV have not been reported. Nonhuman primate studies using the ΔF/HN construct to completely avoid pre-existing immunity should dictate the future success of this platform. VLPs are also a promising preventative vaccine; however, their requirement for multiple immunizations and expected high production costs may limit their usefulness in target populations (e.g., central Africa). Furthermore, the generation of a VLP vaccine against all EBOV species has yet to be demonstrated and the ability to combine all VLPs (while technically possible) may further increase the cost of this platform. Finally, the ΔVP30 ZEBOV vaccine has yet to be proven in nonhuman primates and is currently only available for ZEBOV, thus the limited amount of data and the uncertainty over safety of this vaccine precludes a detailed evaluation of its potential for future clinical use.
Five-year view
Multiple options are currently available that could probably be developed into successful vaccines for both EBOV and MARV. Unfortunately, lessons learned from the past are discouraging and, in the absence of a precipitating factor such as a laboratory exposure or disease outbreak in the western world, development of a preventative and/or postexposure vaccine are likely to be slow. The limited interest by industry, given a lack of a profit for such vaccines, is another inhibiting factor. Thus, government agencies need to provide the funding for vaccine development in this field. An imminent use is obvious in the high containment field, which is anxiously awaiting treatment and/or vaccine options for its workers. Therefore, pre-investigational new drug (IND) approval should be considered for the most promising platforms. This, however, would trigger further requests from aid agencies and the military who have already indicated interest in protecting their staff. The availability of pre-IND approved vaccines would also probably create an ethical dilemma with governments, healthcare providers and populations in the affected endemic central African countries who are most in need of vaccines and treatment options. In the absence of well-defined correlates of protection, establishing clinical trials that meet current standards will be difficult in these areas and probably controversial. Overall, this means that the availability of a filovirus vaccine will probably remain unrealistic within the next 5 years. A step in the right direction might be pre-IND approval of candidate vaccines for emergency use, which will then hopefully pave the way for the development of future licensed vaccines.
Key issues.
Filoviruses cause unpredictable, sporadic outbreaks with high case–fatality rates in equatorial Africa, have the potential to be used for bioterrorism, and have resulted in and continue to have the potential to cause laboratory fatalities following accidental exposure.
There are no approved options for intervention, making the development of prophylactic and therapeutic vaccines desirable.
A number of platforms have proven successful as prophylactic vaccines (recombinant vesicular stomatitis virus, DNA/recombinant adenovirus 5, virus-like particles and recombinant human parainfluenza virus 3) but only the DNA platform has proceeded to Phase I clinical trials.
Clinical trials for Ebola and Marburg viruses are complicated by the low frequency and geographic unpredictability of outbreaks, a lack of correlates of protection and the inability to test vaccine efficacy with a challenge.
Several vaccine approaches should be considered for distinct applications to best address the contraindications associated with certain platforms.
Postexposure treatment or vaccination appears to be the most efficient and economical means of controlling outbreaks and responding to bioterrorism events.
Acknowledgments
The authors would like to thank the many people in the field for their contributions and helpful discussions. They would also like to thank Anita Mora (Rocky Mountain Laboratories, NIAID) for assistance with production of Figure 1. Research on filoviruses is supported in part by the Division of Intramural Research, National Institute of Allergy and Infectious Disease (NIAID), National Institutes of Health (NIH).
Footnotes
For reprint orders, please contact reprints@expert-reviews.com
Financial & competing interests disclosure
Heinz Feldmann claims intellectual property regarding vesicular stomatitis virus-based filovirus vaccines. Thomas W Geisbert claims intellectual property regarding vesicular stomatitis virus-based filovirus vaccines and adenovirus-based filovirus vaccines. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest
- 1.Feldmann H, Geisbert T, Jahrling P, et al. Filoviridae. In: Fauquet CM, Mayo MA, Maniloff J, Desselberger U, Ball LA, editors. Virus Taxonomy: Eighth Report of the International Committee on the Taxonomy of Viruses. Elsevier; San Diego, CA, USA: 2005. pp. 645–653. [Google Scholar]
- 2.Hoenen T, Groseth A, Falzarano D, Feldmann H. Ebola virus: unravelling pathogenesis to combat a deadly disease. Trends Mol Med. 2006;12(5):206–215. doi: 10.1016/j.molmed.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 3.Feldmann H, Jones S, Klenk HD, Schnittler HJ. Ebola virus: from discovery to vaccine. Nature Rev. 2003;3(8):677–685. doi: 10.1038/nri1154. [DOI] [PubMed] [Google Scholar]
- 4.Sanchez A, Geisbert TW, Feldmann H. Filoviridae: Marburg and Ebola viruses. In: Knipe DM, Howley PM, Griffin DE, et al., editors. Fields’ Virology. Lippincott Williams and Wilkins; PA, USA: 2007. pp. 1409–1448. [Google Scholar]
- 5.Bray M, Mahanty S. Ebola hemorrhagic fever and septic shock. J Infect Dis. 2003;188(11):1613–1617. doi: 10.1086/379727. [DOI] [PubMed] [Google Scholar]
- 6.Geisbert TW, Young HA, Jahrling PB, Davis KJ, Kagan E, Hensley LE. Mechanisms underlying coagulation abnormalities in ebola hemorrhagic fever: overexpression of tissue factor in primate monocytes/macrophages is a key event. J Infect Dis. 2003;188(11):1618–1629. doi: 10.1086/379724. [DOI] [PubMed] [Google Scholar]
- 7.Geisbert TW, Young HA, Jahrling PB, et al. Pathogenesis of Ebola hemorrhagic fever in primate models: evidence that hemorrhage is not a direct effect of virus-induced cytolysis of endothelial cells. Am J Pathol. 2003;163(6):2371–2382. doi: 10.1016/S0002-9440(10)63592-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hensley LE, Young HA, Jahrling PB, Geisbert TW. Proinflammatory response during Ebola virus infection of primate models: possible involvement of the tumor necrosis factor receptor superfamily. Immunol Lett. 2002;80(3):169–179. doi: 10.1016/s0165-2478(01)00327-3. [DOI] [PubMed] [Google Scholar]
- 9.Geisbert TW, Jahrling PB. Exotic emerging viral diseases: progress and challenges. Nat Med. 2004;10(12 Suppl):S110–S121. doi: 10.1038/nm1142. [DOI] [PubMed] [Google Scholar]
- 10.Baize S, Leroy EM, Georges-Courbot MC, et al. Defective humoral responses and extensive intravascular apoptosis are associated with fatal outcome in Ebola virus-infected patients. Nat Med. 1999;5(4):423–426. doi: 10.1038/7422. [DOI] [PubMed] [Google Scholar]
- 11.Geisbert TW, Hensley LE, Gibb TR, Steele KE, Jaax NK, Jahrling PB. Apoptosis induced in vitro and in vivo during infection by Ebola and Marburg viruses. Lab Invest. 2000;80(2):171–186. doi: 10.1038/labinvest.3780021. [DOI] [PubMed] [Google Scholar]
- 12.Ksiazek TG, Rollin PE, Williams AJ, et al. Clinical virology of Ebola hemorrhagic fever (EHF): virus, virus antigen, and IgG and IgM antibody findings among EHF patients in Kikwit, Democratic Republic of the Congo, 1995. J Infect Dis. 1999;179(Suppl 1):S177–S187. doi: 10.1086/514321. [DOI] [PubMed] [Google Scholar]
- 13.Sanchez A, Lukwiya M, Bausch D, et al. Analysis of human peripheral blood samples from fatal and nonfatal cases of Ebola (Sudan) hemorrhagic fever: cellular responses, virus load, and nitric oxide levels. J Virol. 2004;78(19):10370–10377. doi: 10.1128/JVI.78.19.10370-10377.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mohamadzadeh M, Chen L, Olinger GG, Pratt WD, Schmaljohn AL. Filoviruses and the balance of innate, adaptive, and inflammatory responses. Viral Immunol. 2006;19(4):602–612. doi: 10.1089/vim.2006.19.602. [DOI] [PubMed] [Google Scholar]
- 15.Mohamadzadeh M. Potential factors induced by filoviruses that lead to immune supression. Curr Mol Med. 2009;9(2):174–185. doi: 10.2174/156652409787581628. [DOI] [PubMed] [Google Scholar]
- 16.Mohamadzadeh M, Chen L, Schmaljohn AL. How Ebola and Marburg viruses battle the immune system. Nat Rev. 2007;7(7):556–567. doi: 10.1038/nri2098. [DOI] [PubMed] [Google Scholar]
- 17.Towner JS, Sealy TK, Khristova ML, et al. Newly discovered Ebola virus associated with hemorrhagic fever outbreak in Uganda. PLoS Pathog. 2008;4(11):e1000212. doi: 10.1371/journal.ppat.1000212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dalgard DW, Hardy RJ, Pearson SL, et al. Combined simian hemorrhagic fever and Ebola virus infection in cynomolgus monkeys. Lab Anim Sci. 1992;42(2):152–157. [PubMed] [Google Scholar]
- 19.Jahrling PB, Geisbert TW, Dalgard DW, et al. Preliminary report: isolation of Ebola virus from monkeys imported to USA. Lancet. 1990;335(8688):502–505. doi: 10.1016/0140-6736(90)90737-p. [DOI] [PubMed] [Google Scholar]
- 20.WHO. Outbreak news. Ebola Reston in pigs and humans. Philippines Wkly Epidemiol Rec. 2009;84(7):49–50. [PubMed] [Google Scholar]
- 21.Cyranoski D. Ebola outbreak has experts rooting for answers. Nature. 2009;457(7228):364–365. doi: 10.1038/457364b. [DOI] [PubMed] [Google Scholar]
- 22.Normile D. Emerging infectious diseases. Scientists puzzle over Ebola–Reston virus in pigs. Science. 2009;323(5913):451. doi: 10.1126/science.323.5913.451a. [DOI] [PubMed] [Google Scholar]
- 23.Barrette RW, Metwally SA, Rowland JM, et al. Discovery of swine as a host for the Reston ebolavirus. Science. 2009;325(5937):204–206. doi: 10.1126/science.1172705. [DOI] [PubMed] [Google Scholar]
- 24.Miranda ME, Ksiazek TG, Retuya TJ, et al. Epidemiology of Ebola (subtype Reston) virus in the Philippines, 1996. J Infect Dis. 1999;179(Suppl 1):S115–S119. doi: 10.1086/514314. [DOI] [PubMed] [Google Scholar]
- 25.Hayes CG, Burans JP, Ksiazek TG, et al. Outbreak of fatal illness among captive macaques in the Philippines caused by an Ebola-related filovirus. Am J Trop Med Hyg. 1992;46(6):664–671. doi: 10.4269/ajtmh.1992.46.664. [DOI] [PubMed] [Google Scholar]
- 26.Ndambi R, Akamituna P, Bonnet MJ, Tukadila AM, Muyembe-Tamfum JJ, Colebunders R. Epidemiologic and clinical aspects of the Ebola virus epidemic in Mosango, Democratic Republic of the Congo, 1995. J Infect Dis. 1999;179(Suppl 1):S8–S10. doi: 10.1086/514297. [DOI] [PubMed] [Google Scholar]
- 27.Bausch DG, Towner JS, Dowell SF, et al. Assessment of the risk of Ebola virus transmission from bodily fluids and fomites. J Infect Dis. 2007;196(Suppl 2):S142–S147. doi: 10.1086/520545. [DOI] [PubMed] [Google Scholar]
- 28.Johnson E, Jaax N, White J, Jahrling P. Lethal experimental infections of rhesus monkeys by aerosolized Ebola virus. Int J Exp Pathol. 1995;76(4):227–236. [PMC free article] [PubMed] [Google Scholar]
- 29.Jaax N, Jahrling P, Geisbert T, et al. Transmission of Ebola virus (Zaire strain) to uninfected control monkeys in a biocontainment laboratory. Lancet. 1995;346(8991–8992):1669–1671. doi: 10.1016/s0140-6736(95)92841-3. [DOI] [PubMed] [Google Scholar]
- 30.Roels TH, Bloom AS, Buffington J, et al. Ebola hemorrhagic fever, Kikwit, Democratic Republic of the Congo, 1995: risk factors for patients without a reported exposure. J Infect Dis. 1999;179(Suppl 1):S92–S97. doi: 10.1086/514286. [DOI] [PubMed] [Google Scholar]
- 31.Swanepoel R, Smit SB, Rollin PE, et al. Studies of reservoir hosts for Marburg virus. Emerg Infect Dis. 2007;13(12):1847–1851. doi: 10.3201/eid1312.071115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Towner JS, Pourrut X, Albarino CG, et al. Marburg virus infection detected in a common African bat. PLoS ONE. 2007;2(1):e764. doi: 10.1371/journal.pone.0000764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leroy EM, Kumulungui B, Pourrut X, et al. Fruit bats as reservoirs of Ebola virus. Nature. 2005;438(7068):575–576. doi: 10.1038/438575a. [DOI] [PubMed] [Google Scholar]
- 34.Leroy EM, Epelboin A, Mondonge V, et al. Human ebola outbreak resulting from direct exposure to fruit bats in Luebo, Democratic Republic of Congo, 2007. Vector Borne Zoonotic Dis. 2009;9(6):723–728. doi: 10.1089/vbz.2008.0167. [DOI] [PubMed] [Google Scholar]
- 35.WHO. Viral haemorrhagic fever. Wkly Epidemiol Rec. 1977;52(21):177–180. [Google Scholar]
- 36.Emond RT, Evans B, Bowen ET, Lloyd G. A case of Ebola virus infection. Br Med J. 1977;2(6086):541–544. doi: 10.1136/bmj.2.6086.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.CDC. Follow-up on viral hemorrhagic fever – Zaire, United Kingdom. MMWR Morb Mortal Wkly Rep. 1976;25(47):383. [Google Scholar]
- 38.Kortepeter MG, Martin JW, Rusnak JM, et al. Managing potential laboratory exposure to Ebola virus by using a patient biocontainment care unit. Emerg Infect Dis. 2008;14(6):881–887. doi: 10.3201/eid1406.071489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fatal Ebola laboratory accident, Siberia. Int J Infect Dis. 2004;8(4):199–200. [Google Scholar]
- 40.Feldmann H, Jones SM, Daddario-DiCaprio KM, et al. Effective post-exposure treatment of Ebola infection. PLoS Pathog. 2007;3(1):e2. doi: 10.1371/journal.ppat.0030002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jones SM, Feldmann H, Stroher U, et al. Live attenuated recombinant vaccine protects nonhuman primates against Ebola and Marburg viruses. Nat Med. 2005;11(7):786–790. doi: 10.1038/nm1258. [DOI] [PubMed] [Google Scholar]
- 42•.Tuffs A. Experimental vaccine may have saved Hamburg scientist from Ebola fever. Br Med J Clin Res Ed. 2009;338:b1223. doi: 10.1136/bmj.b1223. Describes the first postexposure treatment for Ebola virus (EBOV) in humans. [DOI] [PubMed] [Google Scholar]
- 43•.Bente D, Gren J, Strong JE, Feldmann H. Disease modeling for Ebola and Marburg viruses. Dis Model Mech. 2009;2(1–2):12–17. doi: 10.1242/dmm.000471. Provides an overview of the animal models used for filovirus infection and summarizes why it is necessary to carry out vaccine efficacy testing in nonhuman primatemodels. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sullivan NJ, Martin JE, Graham BS, Nabel GJ. Correlates of protective immunity for Ebola vaccines: implications for regulatory approval by the animal rule. Nat Rev Microbiol. 2009;7(5):393–400. doi: 10.1038/nrmicro2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sullivan NJ, Sanchez A, Rollin PE, Yang ZY, Nabel GJ. Development of a preventive vaccine for Ebola virus infection in primates. Nature. 2000;408(6812):605–609. doi: 10.1038/35046108. [DOI] [PubMed] [Google Scholar]
- 46.Warfield KL, Swenson DL, Olinger GG, Kalina WV, Aman MJ, Bavari S. Ebola virus-like particle-based vaccine protects nonhuman primates against lethal Ebola virus challenge. J Infect Dis. 2007;196(Suppl 2):S430–S437. doi: 10.1086/520583. [DOI] [PubMed] [Google Scholar]
- 47.Bukreyev A, Rollin PE, Tate MK, et al. Successful topical respiratory tract immunization of primates against Ebola virus. J Virol. 2007;81(12):6379–6388. doi: 10.1128/JVI.00105-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Geisbert TW, Hensley LE, Larsen T, et al. Pathogenesis of Ebola hemorrhagic fever in cynomolgus macaques: evidence that dendritic cells are early and sustained targets of infection. Am J Pathol. 2003;163(6):2347–2370. doi: 10.1016/S0002-9440(10)63591-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Oswald WB, Geisbert TW, Davis KJ, et al. Neutralizing antibody fails to impact the course of Ebola virus infection in monkeys. PLoS Pathog. 2007;3(1):e9. doi: 10.1371/journal.ppat.0030009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jahrling PB, Geisbert J, Swearengen JR, et al. Passive immunization of Ebola virus-infected cynomolgus monkeys with immunoglobulin from hyperimmune horses. Arch Virol. 1996;11:135–140. doi: 10.1007/978-3-7091-7482-1_12. [DOI] [PubMed] [Google Scholar]
- 51.Dye JM, Barth J, Kuehne A, et al. Post-exposure transfer of immunoglobulin G from convalescent survivors protects rhesus macaques from Marburg virus. Presented at: 5th International Symposium on Filoviruses; Tokyo, Japan. 18–21 April 2010. [Google Scholar]
- 52.Jones SM, Stroher U, Fernando L, et al. Assessment of a vesicular stomatitis virus-based vaccine by use of the mouse model of Ebola virus hemorrhagic fever. J Infect Dis. 2007;196(Suppl 2):S404–S412. doi: 10.1086/520591. [DOI] [PubMed] [Google Scholar]
- 53.Gupta M, Mahanty S, Bray M, Ahmed R, Rollin PE. Passive transfer of antibodies protects immunocompetent and imunodeficient mice against lethal Ebola virus infection without complete inhibition of viral replication. J Virol. 2001;75(10):4649–4654. doi: 10.1128/JVI.75.10.4649-4654.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Takada A, Ebihara H, Jones S, Feldmann H, Kawaoka Y. Protective efficacy of neutralizing antibodies against Ebola virus infection. Vaccine. 2007;25(6):993–999. doi: 10.1016/j.vaccine.2006.09.076. [DOI] [PubMed] [Google Scholar]
- 55.Parren PW, Geisbert TW, Maruyama T, Jahrling PB, Burton DR. Pre- and postexposure prophylaxis of Ebola virus infection in an animal model by passive transfer of a neutralizing human antibody. J Virol. 2002;76(12):6408–6412. doi: 10.1128/JVI.76.12.6408-6412.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wilson JA, Hevey M, Bakken R, et al. Epitopes involved in antibody-mediated protection from Ebola virus. Science. 2000;287(5458):1664–1666. doi: 10.1126/science.287.5458.1664. [DOI] [PubMed] [Google Scholar]
- 57.Gupta M, Mahanty S, Greer P, et al. Persistent infection with Ebola virus under conditions of partial immunity. J Virol. 2004;78(2):958–967. doi: 10.1128/JVI.78.2.958-967.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Baize S. A single shot against Ebola and Marburg virus. Nat Med. 2005;11(7):720–721. doi: 10.1038/nm0705-720. [DOI] [PubMed] [Google Scholar]
- 59.Sanchez A, Wagoner KE, Rollin PE. Sequence-based human leukocyte antigen-B typing of patients infected with Ebola virus in Uganda in 2000: identification of alleles associated with fatal and nonfatal disease outcomes. J Infect Dis. 2007;196(Suppl 2):S329–S336. doi: 10.1086/520588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Leroy EM, Baize S, Debre P, Lansoud-Soukate J, Mavoungou E. Early immune responses accompanying human asymptomatic Ebola infections. Clin Exp Immunol. 2001;124(3):453–460. doi: 10.1046/j.1365-2249.2001.01517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Leroy EM, Baize S, Volchkov VE, et al. Human asymptomatic Ebola infection and strong inflammatory response. Lancet. 2000;355(9222):2210–2215. doi: 10.1016/s0140-6736(00)02405-3. [DOI] [PubMed] [Google Scholar]
- 62.Bukreyev A, Marzi A, Feldmann F, et al. Chimeric human parainfluenza virus bearing the Ebola virus glycoprotein as the sole surface protein is immunogenic and highly protective against Ebola virus challenge. Virology. 2009;383(2):348–361. doi: 10.1016/j.virol.2008.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Roy S, Zhi Y, Kobinger GP, et al. Generation of an adenoviral vaccine vector based on simian adenovirus 21. J Gen Virol. 2006;87(Pt 9):2477–2485. doi: 10.1099/vir.0.81989-0. [DOI] [PubMed] [Google Scholar]
- 64.Kobinger GP, Feldmann H, Zhi Y, et al. Chimpanzee adenovirus vaccine protects against Zaire Ebola virus. Virology. 2006;346(2):394–401. doi: 10.1016/j.virol.2005.10.042. [DOI] [PubMed] [Google Scholar]
- 65.Bukreyev AA, Dinapoli JM, Yang L, Murphy BR, Collins PL. Mucosal parainfluenza virus-vectored vaccine against Ebola virus replicates in the respiratory tract of vector-immune monkeys and is immunogenic. Virology. 2010;399(2):290–298. doi: 10.1016/j.virol.2010.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bangari DS, Mittal SK. Curent strategies and future directions for eluding adenoviral vector immunity. Curr Gene Ther. 2006;6(2):215–226. doi: 10.2174/156652306776359478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Croyle MA, Patel A, Tran KN, et al. Nasal delivery of an adenovirus-based vaccine bypasses pre-existing immunity to the vaccine carrier and improves the immune response in mice. PLoS ONE. 2008;3(10):e3548. doi: 10.1371/journal.pone.0003548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Patel A, Zhang Y, Croyle M, et al. Mucosal delivery of adenovirus-based vaccine protects against Ebola virus infection in mice. J Infect Dis. 2007;196(Suppl 2):S413–S420. doi: 10.1086/520603. [DOI] [PubMed] [Google Scholar]
- 69.Daddario-DiCaprio KM, Geisbert TW, Geisbert JB, et al. Cross-protection against Marburg virus strains by using a live, attenuated recombinant vaccine. J Virol. 2006;80(19):9659–9666. doi: 10.1128/JVI.00959-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Garbutt M, Liebscher R, Wahl-Jensen V, et al. Properties of replication-competent vesicular stomatitis virus vectors expressing glycoproteins of filoviruses and arenaviruses. J Virol. 2004;78(10):5458–5465. doi: 10.1128/JVI.78.10.5458-5465.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rose NF, Marx PA, Luckay A, et al. An effective AIDS vaccine based on live attenuated vesicular stomatitis virus recombinants. Cell. 2001;106(5):539–549. doi: 10.1016/s0092-8674(01)00482-2. [DOI] [PubMed] [Google Scholar]
- 72.Cobleigh MA, Buonocore L, Uprichard SL, Rose JK, Robek MD. A vesicular stomatitis virus-based hepatitis B virus vaccine vector provides protection against challenge in a single dose. J Virol. 2010;84(15):7513–7522. doi: 10.1128/JVI.00200-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schwartz JA, Buonocore L, Suguitan AL, Jr, et al. Potent vesicular stomatitis virus-based avian influenza vaccines provide long-term sterilizing immunity against heterologous challenge. J Virol. 2010;84(9):4611–4618. doi: 10.1128/JVI.02637-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kapadia SU, Simon ID, Rose JK. SARS vaccine based on a replication-defective recombinant vesicular stomatitis virus is more potent than one based on a replication-competent vector. Virology. 2008;376(1):165–172. doi: 10.1016/j.virol.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kahn JS, Roberts A, Weibel C, Buonocore L, Rose JK. Replication-competent or attenuated, nonpropagating vesicular stomatitis viruses expressing respiratory syncytial virus (RSV) antigens protect mice against RSV challenge. J Virol. 2001;75(22):11079–11087. doi: 10.1128/JVI.75.22.11079-11087.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Roberts A, Reuter JD, Wilson JH, Baldwin S, Rose JK. Complete protection from papillomavirus challenge after a single vaccination with a vesicular stomatitis virus vector expressing high levels of L1 protein. J Virol. 2004;78(6):3196–3199. doi: 10.1128/JVI.78.6.3196-3199.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liao JB, Publicover J, Rose JK, DiMaio D. Single-dose, therapeutic vaccination of mice with vesicular stomatitis virus expressing human papillomavirus type 16 E7 protein. Clin Vaccine Immunol. 2008;15(5):817–824. doi: 10.1128/CVI.00343-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bowen ET, Platt GS, Lloyd G, Raymond RT, Simpson D. A comparative study of strains of Ebola virus isolated from southern Sudan and northern Zaire in 1976. J Med Virol. 1980;6(2):129–138. doi: 10.1002/jmv.1890060205. [DOI] [PubMed] [Google Scholar]
- 79.Reed DS, Mohamadzadeh M. Status and challenges of filovirus vaccines. Vaccine. 2007;25(11):1923–1934. doi: 10.1016/j.vaccine.2006.11.037. [DOI] [PubMed] [Google Scholar]
- 80.Geisbert TW, Daddario-Dicaprio KM, Geisbert JB, et al. Vesicular stomatitis virus-based vaccines protect nonhuman primates against aerosol challenge with Ebola and Marburg viruses. Vaccine. 2008;26(52):6894–6900. doi: 10.1016/j.vaccine.2008.09.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Qiu X, Fernando L, Alimonti JB, et al. Mucosal immunization of cynomolgus macaques with the VSVΔG/ZEBOVGP vaccine stimulates strong ebola GP-specific immune responses. PLoS One. 2009;4(5):e5547. doi: 10.1371/journal.pone.0005547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82•.Geisbert TW, Geisbert JB, Leung A, et al. Single-injection vaccine protects nonhuman primates against infection with marburg virus and three species of ebola virus. J Virol. 2009;83(14):7296–7304. doi: 10.1128/JVI.00561-09. First study to demonstrate that a blended recombinant vesicular stomatitis virus-based vaccine can provide protection with a single immunization against multiple EBOV species and Marburg virus strains. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Johnson JE, Nasar F, Coleman JW, et al. Neurovirulence properties of recombinant vesicular stomatitis virus vectors in non-human primates. Virology. 2007;360(1):36–49. doi: 10.1016/j.virol.2006.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Geisbert TW, Jones S, Fritz EA, et al. Development of a new vaccine for the prevention of Lassa fever. PLoS Med. 2005;2(6):e183. doi: 10.1371/journal.pmed.0020183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85•.Geisbert TW, Daddario-Dicaprio KM, Lewis MG, et al. Vesicular stomatitis virus-based Ebola vaccine is well-tolerated and protects immunocompromised nonhuman primates. PLoS Pathog. 2008;4(11):e1000225. doi: 10.1371/journal.ppat.1000225. Demonstrates the safety of the recombinant vesicular stomatitis virus-based vaccine in immunocompromised nonhuman primates and provides further directions for pursuing correlates of protection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Geisbert TW, Daddario-DiCaprio KM, Williams KJ, et al. Recombinant vesicular stomatitis virus vector mediates postexposure protection against Sudan Ebola hemorrhagic fever in nonhuman primates. J Virol. 2008;82(11):5664–5668. doi: 10.1128/JVI.00456-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87•.Daddario-DiCaprio KM, Geisbert TW, Stroher U, et al. Postexposure protection against Marburg haemorrhagic fever with recombinant vesicular stomatitis virus vectors in non-human primates: an efficacy assessment. Lancet. 2006;367(9520):1399–1404. doi: 10.1016/S0140-6736(06)68546-2. First demonstration of the potential for postexposure protection from infection. [DOI] [PubMed] [Google Scholar]
- 88.Geisbert TW, Hensley LE, Geisbert JB, et al. Postexposure treatment of Marburg virus infection. Emerg Infect Dis. 2010;16(7):1119–1122. doi: 10.3201/eid1607.100159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gonchar NI, Pshenichnov VA, Pokhodiaev VA, Lopatov KL, Firsova IV. The sensitivity of different experimental animals to the Marburg virus. Vopr Virusol. 1991;36(5):435–437. [PubMed] [Google Scholar]
- 90.Warfield KL, Perkins JG, Swenson DL, et al. Role of natural killer cells in innate protection against lethal Ebola virus infection. J Exp Med. 2004;200(2):169–179. doi: 10.1084/jem.20032141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Karron RA, Belshe RB, Wright PF, et al. A live human parainfluenza type 3 virus vaccine is attenuated and immunogenic in young infants. Pediatr Infect Dis J. 2003;22(5):394–405. doi: 10.1097/01.inf.0000066244.31769.83. [DOI] [PubMed] [Google Scholar]
- 92.Durbin AP, Skiadopoulos MH, McAuliffe JM, et al. Human parainfluenza virus type 3 (PIV3) expressing the hemagglutinin protein of measles virus provides a potential method for immunization against measles virus and PIV3 in early infancy. J Virol. 2000;74(15):6821–6831. doi: 10.1128/jvi.74.15.6821-6831.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bukreyev A, Yang L, Zaki SR, et al. A single intranasal inoculation with a paramyxovirus-vectored vaccine protects guinea pigs against a lethal-dose Ebola virus challenge. J Virol. 2006;80(5):2267–2279. doi: 10.1128/JVI.80.5.2267-2279.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yang L, Sanchez A, Ward JM, Murphy BR, Collins PL, Bukreyev A. A paramyxovirus-vectored intranasal vaccine against Ebola virus is immunogenic in vector-immune animals. Virology. 2008;377(2):255–264. doi: 10.1016/j.virol.2008.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hevey M, Negley D, Pushko P, Smith J, Schmaljohn A. Marburg virus vaccines based upon alphavirus replicons protect guinea pigs and nonhuman primates. Virology. 1998;251(1):28–37. doi: 10.1006/viro.1998.9367. [DOI] [PubMed] [Google Scholar]
- 96.Hevey M, Negley D, Staley A, Schmaljohn A. Determination of vaccine components required for protecting cynomolgus macaques against genotypically divergent isolates of Marburg virus. Presented at: 20th Annual Meeting of the American Society for Virology; Madison, WI, USA. 21–25 July 2001. [Google Scholar]
- 97.Olinger GG, Bailey MA, Dye JM, et al. Protective cytotoxic T-cell responses induced by venezuelan equine encephalitis virus replicons expressing Ebola virus proteins. J Virol. 2005;79(22):14189–14196. doi: 10.1128/JVI.79.22.14189-14196.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pushko P, Bray M, Ludwig GV, et al. Recombinant RNA replicons derived from attenuated Venezuelan equine encephalitis virus protect guinea pigs and mice from Ebola hemorrhagic fever virus. Vaccine. 2000;19(1):142–153. doi: 10.1016/s0264-410x(00)00113-4. [DOI] [PubMed] [Google Scholar]
- 99.Geisbert TW, Pushko P, Anderson K, Smith J, Davis KJ, Jahrling PB. Evaluation in nonhuman primates of vaccines against Ebola virus. Emerg Infect Dis. 2002;8(5):503–507. doi: 10.3201/eid0805.010284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lee JS, Groebner JL, Hadjipanayis AG, et al. Multiagent vaccines vectored by Venezuelan equine encephalitis virus replicon elicits immune responses to Marburg virus and protection against anthrax and botulinum neurotoxin in mice. Vaccine. 2006;24(47–48):6886–6892. doi: 10.1016/j.vaccine.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 101.Lu S, Wang S, Grimes-Serrano JM. Current progress of DNA vaccine studies in humans. Expert Rev Vaccines. 2008;7(2):175–191. doi: 10.1586/14760584.7.2.175. [DOI] [PubMed] [Google Scholar]
- 102.Geisbert T, Bailey M, Geisbert J, et al. Vector choice determines immunogenicity and potency of genetic vaccines against Angola Marburg virus in nonhuman primates. J Virol. 2010;84(19):10386–10394. doi: 10.1128/JVI.00594-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Monie A, Tsen SW, Hung CF, Wu TC. Theraoeutic HPV DNA vaccines. Expert Rev Vaccines. 2009;8(9):1221–1235. doi: 10.1586/erv.09.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rosenberg ES, Graham BS, Chan ES, et al. Safety and immunogenicity of therapeutic DNA vaccination in individuals treated with antiretroviral therapy during acute/early HIV-1 infection. PLoS ONE. 2010;5(5):e10555. doi: 10.1371/journal.pone.0010555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Riemenschneider J, Garrison A, Geisbert J, et al. Comparison of individual and combination DNA vaccines for B. anthracis, Ebola virus, Marburg virus and Venezuelan equine encephalitis virus. Vaccine. 2003;21(25–26):4071–4080. doi: 10.1016/s0264-410x(03)00362-1. [DOI] [PubMed] [Google Scholar]
- 106•.Sullivan NJ, Geisbert TW, Geisbert JB, et al. Accelerated vaccination for Ebola virus haemorrhagic fever in non-human primates. Nature. 2003;424(6949):681–684. doi: 10.1038/nature01876. Presents the first vaccine against EBOV that has the potential to be practical and efficacious in nonhuman primates. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Martin JE, Sullivan NJ, Enama ME, et al. A DNA vaccine for Ebola virus is safe and immunogenic in a Phase I clinical trial. Clin Vaccine Immunol. 2006;13(11):1267–1277. doi: 10.1128/CVI.00162-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Santra S, Seaman MS, Xu L, et al. Replication-defective adenovirus serotype 5 vectors elicit durable cellular and humoral immune responses in nonhuman primates. J Virol. 2005;79(10):6516–6522. doi: 10.1128/JVI.79.10.6516-6522.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109•.Hensley LE, Mulangu S, Asiedu C, et al. Demonstration of cross-protective vaccine immunity against an emerging pathogenic Ebolavirus species. PLoS Pathog. 2010;6(5):e1000904. doi: 10.1371/journal.ppat.1000904. Demonstration of cross-protection between EBOV species. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sullivan NJ, Geisbert TW, Geisbert JB, et al. Immune protection of nonhuman primates against Ebola virus with single low-dose adenovirus vectors encoding modified GPs. PLoS Med. 2006;3(6):e177. doi: 10.1371/journal.pmed.0030177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Swenson DL, Wang D, Luo M, et al. Vaccine to confer to nonhuman primates complete protection against multistrain Ebola and Marburg virus infections. Clin Vaccine Immunol. 2008;15(3):460–467. doi: 10.1128/CVI.00431-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Pratt WD, Wang D, Nichols DK, et al. Protection of nonhuman primates against two species of Ebola virus infection with a single complex adenovirus vector. Clin Vaccine Immunol. 2010;17(4):572–581. doi: 10.1128/CVI.00467-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Geisbert TW, Bausch DG, Feldmann H. Prospects for immunisation against Marburg and Ebola viruses. Rev Med Virol. 2010;20(6):344–357. doi: 10.1002/rmv.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Richardson JS, Yao MK, Tran KN, et al. Enhanced protection against Ebola virus mediated by an improved adenovirus-based vaccine. PLoS ONE. 2009;4(4):e5308. doi: 10.1371/journal.pone.0005308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Berry JM, Palefsky JM. A review of human papillomavirus vaccines: from basic science to clinical trials. Front Biosci. 2003;8:s333–s345. doi: 10.2741/1003. [DOI] [PubMed] [Google Scholar]
- 116.Stanley MA. Human papillomavirus vaccines. Rev Med Virol. 2006;16(3):139–149. doi: 10.1002/rmv.498. [DOI] [PubMed] [Google Scholar]
- 117.Zanetti AR, Van Damme P, Shouval D. The global impact of vaccination against hepatitis B: a historical overview. Vaccine. 2008;26(49):6266–6273. doi: 10.1016/j.vaccine.2008.09.056. [DOI] [PubMed] [Google Scholar]
- 118.Watanabe S, Watanabe T, Noda T, et al. Production of novel ebola virus-like particles from cDNAs: an alternative to ebola virus generation by reverse genetics. J Virol. 2004;78(2):999–1005. doi: 10.1128/JVI.78.2.999-1005.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Warfield KL, Bosio CM, Welcher BC, et al. Ebola virus-like particles protect from lethal Ebola virus infection. Proc Natl Acad Sci USA. 2003;100(26):15889–15894. doi: 10.1073/pnas.2237038100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Licata JM, Johnson RF, Han Z, Harty RN. Contribution of ebola virus glycoprotein, nucleoprotein, and VP24 to budding of VP40 virus-like particles. J Virol. 2001;78(14):7344–7351. doi: 10.1128/JVI.78.14.7344-7351.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Warfield KL, Swenson DL, Negley DL, Schmaljohn AL, Aman MJ, Bavari S. Marburg virus-like particles protect guinea pigs from lethal Marburg virus infection. Vaccine. 2004;22(25–26):3495–3502. doi: 10.1016/j.vaccine.2004.01.063. [DOI] [PubMed] [Google Scholar]
- 122•.Swenson DL, Warfield KL, Negley DL, Schmaljohn A, Aman MJ, Bavari S. Virus-like particles exhibit potential as a pan-filovirus vaccine for both Ebola and Marburg viral infections. Vaccine. 2005;23(23):3033–3042. doi: 10.1016/j.vaccine.2004.11.070. First demonstration that a blended virus-like particle vaccine can protect against EBOV and Marburg virus. [DOI] [PubMed] [Google Scholar]
- 123.Swenson DL, Warfield KL, Larsen T, Alves DA, Coberley SS, Bavari S. Monovalent virus-like particle vaccine protects guinea pigs and nonhuman primates against infection with multiple Marburg viruses. Expert Rev Vaccines. 2008;7(4):417–429. doi: 10.1586/14760584.7.4.417. [DOI] [PubMed] [Google Scholar]
- 124.Ye L, Lin J, Sun Y, et al. Ebola virus-like particles produced in insect cells exhibit dendritic cell stimulating activity and induce neutralizing antibodies. Virology. 2006;351(2):260–270. doi: 10.1016/j.virol.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 125.Sun Y, Carrion R, Jr, Ye L, et al. Protection against lethal challenge by Ebola virus-like particles produced in insect cells. Virology. 2009;383(1):12–21. doi: 10.1016/j.virol.2008.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Halfmann P, Kim JH, Ebihara H, et al. Generation of biologically contained Ebola viruses. Proc Natl Acad Sci USA. 2008;105(4):1129–1133. doi: 10.1073/pnas.0708057105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Halfmann P, Ebihara H, Marzi A, et al. Replication-deficient ebolavirus as a vaccine candidate. J Virol. 2009;83(8):3810–3815. doi: 10.1128/JVI.00074-09. [DOI] [PMC free article] [PubMed] [Google Scholar]