Abstract
Background
The prognostic significance of p53 aberration in hepatocellular carcinoma (HCC) remains inconclusive. This review aimed to provide comprehensive evidence on the association of p53 alterations with recurrence-free survival (RFS) and overall survival (OS) in HCC patients.
Methods
Systematic literature searches were conducted until July 2010. Meta-analysis was performed to estimate prognostic effects of p53 alterations on patient outcomes in HCC. Sensitivity and subgroup analyses were also conducted in the meta-analysis.
Results
Thirty-seven studies (7 tumour p53 mutation, 23 tumour p53 expression and 7 serum anti-p53 antibodies) were included in the systematic review. The average percentages of p53 mutation, p53 expression upregulation and anti-p53 antibody level elevation in HCC patients were 31.5%, 35.0% and 23.8%, respectively. Tumour p53 alterations were associated significantly with poor patient outcomes in HCC: the summary hazard ratio (HR) of mutant p53 versus wild type p53 phenotype was 2.58 [95% confidence interval (CI): 1.96–3.41] for OS and 3.19 [95% CI: 2.21–4.60] for RFS, respectively; and the summary HR of upregulated p53 expression versus low/undetectable p53 expression was 1.68 [95% CI: 1.49–1.90] for OS and 1.89 [95% CI: 1.34–2.66] for RFS, respectively. However, elevated serum anti-p53 antibody was only associated with poor OS in HCC group with high propotion (≥50%) of hepatitis C virus (HCV) infection [HR: 1.92; 95% CI: 1.30–2.85]. Moreover, sensitivity analyses showed that the results of meta-analyses were not altered.
Conclusion
HCC patients with p53 mutation and upregulated expression in tumour tissue have a shorter OS and RFS than patients with wild type p53 and low/undetectable p53 expression. However, the prognostic value of serum anti-p53 antibody is required to be further examined.
Keywords: systematic review, meta-analysis, TP53, hepatocellular carcinoma, tumour marker
1. Introduction
Hepatocellular carcinoma (HCC) is one of the most prevalent cancers and the third common cause of cancer-related deaths worldwide.1–3 Although improved diagnostic techniques have contributed more HCC patients to undergo curative surgery at early stages, the tumour recurrence and mortality rates are still high due to its aggressive behaviours and limited response to adjunctive therapies in advanced stages.1,2,4 Therefore, understanding precisely the biological behaviours of the tumour is critical for outcome prediction in HCC patients. The most well-known prognostic factors are related to clinicopathological characteristics of HCC. Recently, two systematic reviews indicated that the most robust predictors of overall survival (OS) in HCC patients with cirrhosis and untreated HCC patients were bad performance status, portal vein tumour thrombus (PVTT), tumour size, α-fetoprotein (AFP) and Child-Pugh class.5,6
Tumour suppressor gene p53, its wild-type protein is responsible for cell-cycle regulation and apoptosis after DNA damage. If p53 is mutated, however, the cell with DNA damage can escape from apoptosis and turn into cancer cells.7 Furthermore, the mutant p53 protein, which lost the function of wild-type protein, can accumulate in cell nuclei and is regarded as a highly specific indicator of malignancy.8 To date, some studies have documented that p53 alterations are correlated with tumour differentiation, vascular invasion, tumour stage, Child-Pugh class and serum AFP in HCC.9–12 However, the prognostic significance of p53 alterations in HCC has not been concluded as clinical evidence. Some prognostic studies suggested that tumour p53 upregulation and serum anti-p53 antibody elevation were associated with recurrence-free survival (RFS) and OS in HCC patients12–16 while the similar results were not confirmed in other studies.17–21 Furthermore, most of studies supported that HCC patients with mutant p53 phenotype had poor survival, but the prognostic effect fluctuated with a wide range of hazard ratios (HRs) (1.98 to 13.88) due to small and heterogeneous studies.11,22–24
The association between p53 alterations and patient outcomes in HCC had been represented by a systematic review, but it has still not been reached a comprehensive conclusion due in part to not including serum p53 alteration and adopting a quantitative analysis in this review.25 To obtain precise clinical evidence on the prognostic significance of p53 alterations in HCC patients, we conducted a systematic review and meta-analysis of published studies on the association of tumour p53 mutation, p53 expression and serum anti-p53 antibody with RFS and OS in HCC patients.
2. Materials and Methods
2.1. Literature search and eligibility criteria
A computer-aided literature search was conducted in the Cochrane Library, MEDLINE and Science Citation Index Expanded databases up to July 2010 using the random combination of following search terms: ‘liver neoplasm or hepatocellular carcinoma’, ‘tumour suppressor protein p53, anti-p53 antibody, or p53’ and ‘prognosis, survival, or recurrence’. Additionally, we manually searched the reference lists of identified articles for missing papers.
Eligible studies were required to match the following criteria: (1) proven diagnosis of HCC in humans; (2) reported explicit methods for the detection of p53 alterations; (3) the endpoints were RFS and OS; (4) provided HR/logHR and 95% confidence interval (CI)/standard error (SE) or crude data; and (5) articles written in English, French, German, or Chinese.
The two researchers (J.L. and M.Z.) read independently the title and abstract of identified studies, and subsequently excluded the irrelevant ones. Then, the full-texts of preserved studies were scrutinized. After comprehensive evaluation according to the inclusion criteria, the two researchers determined whether the studies were included. If disagreements occurred in the eligibility of studies, the two researchers would conduct a discussion or resort to the third researcher (Q.M.) until reach a consensus. Simultaneously, careful attention was paid to studies stemmed from overlapping populations. If they were found, usually the one with the largest/newest was included.
2.2. Data extraction and quality assessment
The eligible studies were divided into three groups: tumour p53 gene (mutation versus wild type), tumour p53 expression (upregulation versus low/undetectable) and serum anti-p53 antibodies (elevation versus low/undetectable). Two researchers (J.L. and M.Z.) extracted independently raw data using a predefined form including the characteristics of eligible studies (Table S1) and statistical material. Thereafter, the quality of each study was assessed independently by the two researchers according to Newcastle-Ottawa Quality Assessment Scale (NOS) for cohort studies.26 A total of 0 and 9 scores were respectively designated as lowest and highest quality, and the studies with 6 scores or more were graded as the high quality ones in the scale (Table S2).
The primary goal of this systematic review was to explore the prognostic effect of p53 alterations on RFS and OS among HCC patients. In this review, the RFS was defined as the interval of time from post-surgery to HCC recurrence, while patients’ OS was designated as the period of time from patients receiving different therapeutics or natural history after diagnosis of the disease to death. To exactly evaluate the value of p53 alterations in predicting patient outcomes from different treatments, we divided eligible studies into three groups: curative treatment group in which all HCC patients received curative hepatic resection and/or liver transplantation, comprehensive treatment group in which patients were treated with surgery or nonoperative management, and support/no treatment group.
2.3. Statistical analysis
Review Manager (RevMan) 5.0 (Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2008) and Stata version 11 (StataCorp LP, TX) were used in the meta-analysis. All p-values were two sided.
The logHR and SE of every study were extracted or calculated and then meta-analysis was performed using generic inverse variance method in RevMan. Because most of studies did not directly report logHR and SE, we obtained the statistics according to previous methods.27 In meta-analyses, a fixed-effects model was used if between-study heterogeneity was absent, while a random-effects model28 would be conducted if heterogeneity was substantial (the determination of heterogeneity among studies described below). This meta-analysis combined primarily the results of univariate survival analysis. However, when the study only reported the results of multivariable Cox hazard regression, the available statistics were also extracted. Finally, a combined HR of more than 1 favoured that HCC with p53 abnormalities have poor survival, and statistical significance could be considered if the 95% CI did not overlap with 1 (p-value < 0.05).
The heterogeneity among studies was evaluated by use of the I2 statistic. An I2 statistic index greater than 50% and a χ2 p-value less than 0.10 indicated presence of substantial heterogeneity.29 If significant heterogeneity existed, we took subgroup analysis to investigate potential sources of heterogeneity. Meta-regression was not conducted in this review because of insufficient clinicopathological data in eligible studies. Egger’s test was used to estimate publication bias of eligible studies, where if a p-value less than 0.05, publication bias was considered.30
Sensitivity analyses were performed by excluding each study individually (the results shown as the summary HR of eligible studies after excluding the largest effect one) and omitting the eligible studies with small sample (< 100) or high quality (NOS score < 6). Simultaneosly, for verifying the reliability of this systematic review, we further analyzed the studies with no less than three of the clinicopathological factors (we designated the studies as complete report) including tumour stage, PVTT, liver cirrhosis and hepatitis B/C virus infection (HBV/HCV), which were considered to be related to clinical outcomes in HCC patients. Finally, subgroup analyses were performed to investigate the prognostic impact of p53 alterations on HCC patients in different geographical locations, treatments, HBV/HCV infection and liver cirrhosis.
3. Results
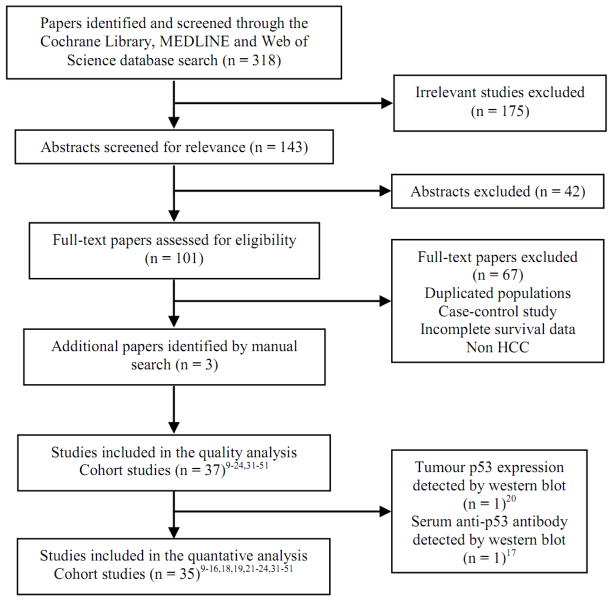
Initial search retrieved 318 published studies. After the careful screening process, thirty-seven cohort studies (36 in English9–24,31–49,51 and 1 in French50) were included into the systematic review, which yielded level 2b prognostic evidence on the Oxford Level of Evidence scale. Fig. 1 shows the literature selection strategy for the systematic review.
Fig. 1.
Flow chart of literature selection for the systematic review and meta-analysis.
3.1. Description of eligible studies
The characteristics of eligible studies are listed in Table S1. Of 37 studies, 25 were launched in high-incidence area (Asia and Africa)9,11,12,13,14,16,21–24,32,34,35,37,39–44,47–49,51 and 12 in low-incidence area (Europe and USA).10,15,18,31,33,36,38,45,46,50 The eligible studies had NOS scores of 3 to 8 (median = 5).
Seven studies on tumour p53 mutation and patient outcomes reported 11 analyses, including 543 HCC patients.9,11,22–24,31,32 The mean percentage of p53 mutation was 31.5% (range 19.3% to 45.0%) and the most frequently mutable sites were exon 5 and 7, accounting for 36.0% and 34.0%, respectively. Twenty-three studies including 29 analyses of tumour p53 expression and patient outcomes met this systematic review, which was the most frequently p53 alteration studies in HCC, including 2 464 patients.10,13–15,20,21,33–49 Of twenty-three studies, 22 studies10,13–15,21,33–49 detecting p53 expression by immunohistochemistry (IHC) were included the final quantative meta-analysis. The most common cut-off values designating p53 upregulation were more than 10% (n = 12) and 5% (n = 4) nuclear staining. Of 22 IHC studies, the median percentage of p53 upregulation was 35.0% (range 19.0% to 71.0%). With respect to a possible association between serum anti-p53 antibodies and patient outcomes in HCC, seven studies including 687 patients was eligible for the systematic review.12,16–19,50,51 Most of the included patients received non-surgical treatments, and therefore the diagnosis of HCC in these patients was mainly made by abdominal ultrasonography, computerized tomography, magnetic resonance imaging and serum AFP. The median percentage of serum anti-p53 antibodies elevation was 23.8% (range 7.0% to 68.3%).
3.2. The results of meta-analyses
3.2.1. Impact of tumour p53 mutation on patient survival
Fig. 2 shows the forest plot for the association between p53 mutations and patient outcomes in HCC. Table 1 lists the summary HR of OS and RFS in patients with mutant p53 phenotype compared with patients with wild type p53 phenotype. Egger’s test showed publication bias was present in studies on p53 mutation associated with OS (p = 0.047) but absent in RFS studies (p = 0.191). The summary HRs of the studies with complete report were same as the statistics of all eligible studies but all with no evidence of publication bias (p for Egger’s test of studies on OS = 0.093 and on RFS = 0.191), supporting p53 mutation was a prognostic factor for HCC patietns (Table 1). Sensitivity analyses were performed by excluding the largest effect size study and including only large sample size or high quality studies, where the summary HRs of the eligible studies were not altered, which were similar to the overall effect of the meta-analysis (Table 1).
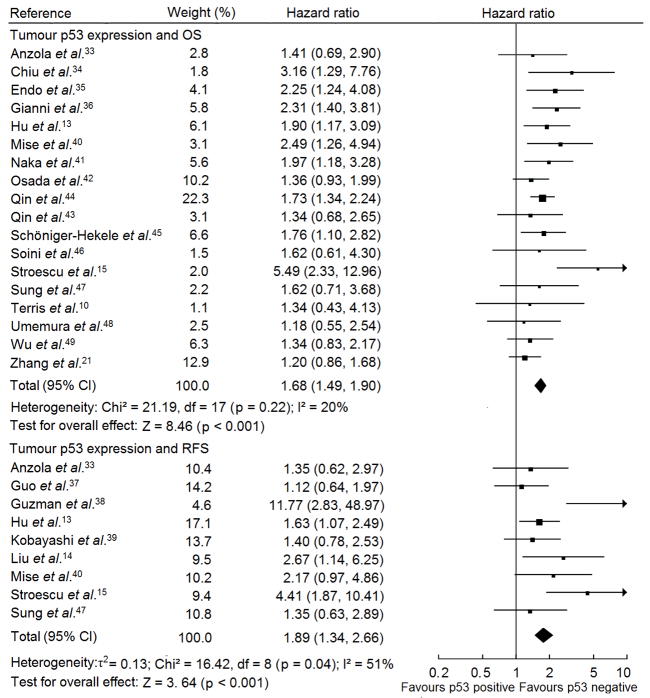
Fig. 2.
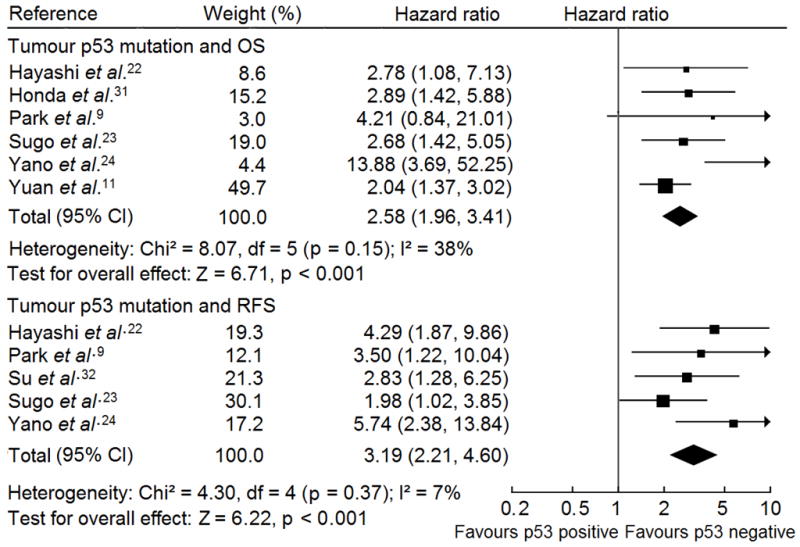
Forest plot of comparison between p53 mutation and wild type p53 phenotype on OS and RFS in HCC patients. Hazard ratio and associated 95% confidence interval were calculated using the fixed-effects model. OS, overall survival; RFS, recurrence-free survival.
Table 1.
The results of meta-analyses and sensitivity analyses.
| Analysis | No. of studies | Pooled hazard ratio (95% CI) | I2 statistic, % | χ2 p-value for heterogeneity | Analytical model | p-value for overall effect |
|---|---|---|---|---|---|---|
| Primary analyses | ||||||
| OS | ||||||
| Tumour p53 mutation | 6 | 2.58 (1.96–3.41) | 38 | 0.15 | FEM | < 0.001 |
| Tumour p53 upregulation | 18 | 1.68 (1.49–1.90) | 20 | 0.22 | FEM | < 0.001 |
| Serum anti-p53 antibody elevation | 5 | 1.39 (0.96–2.03) | 52 | 0.08 | REM | 0.08 |
| RFS | ||||||
| Tumour p53 mutation | 5 | 3.19 (2.21–4.60) | 7 | 0.37 | FEM | < 0.001 |
| Tumour p53 upregulation | 9 | 1.89 (1.34–2.66) | 51 | 0.04 | REM | < 0.001 |
| Serum anti-p53 antibody elevation | 1 | 3.00 (1.00–8.99) | Not applicable | – | – | 0.05 |
| Sensitivity analyses | ||||||
| Tumour p53 mutation and OS | ||||||
| Exclusion of study with the largest effect size24 | 5 | 2.39 (1.80–3.18) | 0 | 0.80 | FEM | < 0.001 |
| Complete report9,11,22–24 | 5 | 3.07 (1.82–2.62) | 50 | 50 | FEM | < 0.001 |
| Sample size ≥ 10011 | 1 | 2.04 (1.37–3.02) | Not applicable | – | – | < 0.001 |
| NOS scoring ≥ 611,23,24 | 3 | 3.31 (1.52–7.20) | 73 | 0.02 | REM | 0.002 |
| Tumour p53 mutation and RFS | ||||||
| Exclusion of study with the largest effect size24 | 4 | 2.82 (1.89–4.22) | 0 | 0.53 | FEM | < 0.001 |
| Complete report9,22–24,32 | 5 | 3.19 (2.21–4.60) | 7 | 0.37 | FEM | < 0.001 |
| NOS scoring ≥ 623,24,32 | 3 | 2.89 (1.86–4.49) | 44 | 0.17 | FEM | < 0.001 |
| Tumour p53 upregulation and OS | ||||||
| Exclusion of study with the largest effect size15 | 17 | 1.64 (1.45–1.85) | 0 | 0.62 | FEM | < 0.001 |
| Complete report10,13,21,33,35,41,43,45,47,48 | 10 | 1.56 (1.31–1.86) | 0 | 0.71 | FEM | < 0.001 |
| Sample size ≥ 10010,13,21,35,40–42,44,47 | 9 | 1.63 (1.41–1.89) | 0 | 0.45 | FEM | < 0.001 |
| NOS scoring ≥ 613,21,36,40,47,49 | 6 | 1.59 (1.30–1.94) | 33 | 0.19 | FEM | < 0.001 |
| IHC protocol with DO-7 antibody10,15,33–35,40,42,48 | 8 | 1.96 (1.39–2.76) | 48 | 0.06 | REM | < 0.001 |
| Tumour p53 upregulation and RFS | ||||||
| Exclusion of study with the largest effect size38 | 8 | 1.65 (1.31–2.07) | 25 | 0.23 | FEM | < 0.001 |
| Complete report13,14,33,39,47 | 5 | 1.58 (1.20–2.09) | 0 | 0.74 | FEM | 0.001 |
| Sample size ≥ 10013,14,47 | 3 | 1.70 (1.21–2.38) | 0 | 0.48 | FEM | 0.002 |
| NOS scoring ≥ 613,14,39,40,47 | 5 | 1.68 (1.27–2.21) | 0 | 0.69 | FEM | < 0.001 |
| IHC protocol with DO-7 antibody14,15,33,37–40 | 7 | 2.15 (1.33–3.47) | 62 | 0.02 | REM | 0.002 |
| Serum anti-p53 antibody elevation and OS | ||||||
| Complete report12,18,50,51 | 4 | 1.26 (0.84–1.91) | 56 | 0.08 | REM | 0.280 |
| Sample size ≥ 10050,51 | 2 | 0.90 (0.60–1.35) | 0 | 0.63 | FEM | 0.610 |
| NOS scoring ≥ 616,18,50,51 | 4 | 1.29 (0.99–1.69) | 47 | 0.13 | FEM | 0.060 |
CI: confidence interval; REM: Random-effects model; FEM: Fixed-effects model.
3.2.2. Impact of tumour p53 expression on patient survival
Fig. 3 shows the forest plot for the eligible studies on p53 expression and patient outcomes in HCC. Table 1 demonstrates the summary HR of the eligible IHC studies in regard to RFS and OS in patients with p53 upregulation compared with patients with low/undetectable p53 expression. There was no heterogeneity between studies on OS (I2 = 20; p = 0.22). However, with respect to RFS studies, there was marked between-study heterogeneity (I2 = 51; p = 0.04). Investigation of heterogeneity showed that the study38 with the smallest sample size (n for participants = 20) and all patients received liver transplantation, resulting the largest effect size [HR: 11.77; 95% CI: 2.83–48.97], was recognized as the main contributor of heterogeneity. Egger’s test suggested that publication bias was absent in the studies on p53 expression and OS (p = 0.201) but present in the studies on p53 expression and RFS (p = 0.039). The summary HR of eligible studies with complete report was similar to the statistics of all eligible studies but all with no evidence of publication bias (p for Egger’s test of studies on OS = 0.711 and on RFS = 0.812), supporting p53 upregulation was a prognostic factor for HCC patietns (Table 1). We conducted a sensitivity analysis by excluding the largest effect size study and restricting the eligible studies to large sample size, high quality or IHC assay with DO-7 antibody ones, where the summary HRs still provided significantly the prognostic information of p53 expression alteration for HCC patients (Table 1).
Fig. 3.
Forest plot of comparison between p53 upregulation and low/undetectable p53 expression on OS and RFS in HCC patients. Hazard ratio and associated 95% confidence interval were calculated using the fixed-effects model for studies with OS and the random-effects model for RFS. OS, overall survival; RFS, recurrence-free survival.
3.2.3. Prognostic significance of serum anti-p53 antibody in HCC
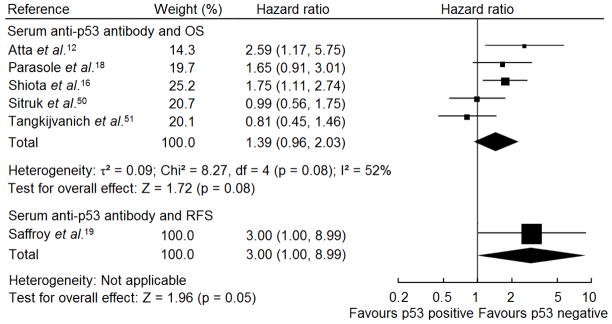
Atta et al.12 and Shiota et al.16 suggested elevated serum anti-p53 antibodies level correlated with poor OS in HCC patients. However, four studies17,18,50,51 on p53 antibody associated with OS and one19 in relation with RFS did not find its prognostic value in HCC patients. We conducted a meta-analysis of six studies where serum anti-p53 antibodies were detected by enzyme-linked immunosorbent assay (ELISA).12,16,18,19,50,51 Fig. 4 shows the forest plot for the included studies. Table 1 shows the summary HR for RFS and OS in patients with elevated serum anti-p53 antibodiy versus patients with low/undetectable antibody level. There was no evidence of publication bias (p = 0.837). The summary HR of the studies with complete report on OS was similar to the statistics of all eligible studies and without publication bias (p for Egger’s test = 0.199), supporting serum anti-p53 antibody elevation was not a prognosticator for HCC patietns (Table 1). Because the systematic review suggested the prognostic value of serum anti-p53 antibody was indeterminate, sensitivity analyses were only performed by restricting the eligible criteria. The summary HRs of the large sample and high quality studies showed similar estimation to the meta-analysis of all eligible studies (Table 1).
Fig. 4.
Forest plot of comparison between elevated and low/undetectable serum anti-p53 antibody on OS and RFS in HCC patients. Hazard ratio and associated 95% confidence interval were calculated using the fixed-effects model. OS, overall survival; RFS, recurrence-free survival.
3.3 Subgroup analyses
Tables 2 and 3 show the detailed results of subgroup analyses. Subgroup analysis revealed the prognostic effects of p53 mutation on OS and RFS in HCC patients were similar between high-incidence and low-incidence area, between HBV infection and HCV infection, and between high liver cirrhosis and low liver cirrhosis (χ2 p-value for subgroup differences > 0.05) (Table 2, 3). Similar to the results of p53 mutation, the summary HRs of OS from the eligible IHC p53 expression studies were not influenced by study type, country, hepatitis and treatments (Table 2, 3). However, the summary HR from eligible studies on population with low propotion (< 50%) of liver cirrhosis which was distinct from the estimation in high liver cirrhosis population, supporting the predictive value of p53 upregulation in the mortality risk of the former was fluctuative and inconclusive (p for overall effect = 0.06) (Table 2). In addition, the summary HR of two prospective studies37,39 was distinct from two retrospective studies15,38 in the association between p53 upregulation and RFS (χ2 p-value for subgroup differences < 0.001) (Table 3). Notably, the summary HR of two prospective studies37,39 suggested no association between p53 upregulation and RFS in HCC patients (p = 0.29) (Table 3). The prognostic value of p53 upregulation for RFS was more evident in populations with low liver cirrhosis rate than high propotion of cirrhotic populations (χ2 p-value for subgroup differences = 0.001) (Table 3). Next, subgroup analyses of five eligible ELISA studies showed that there existed an association between serum anti-p53 antibody and OS in high propotion (≥50%) of HCV infection and liver cirrhosis populations (Table 2).
Table 2.
Subgroup analysis of the eligible studies on p53 alterations associated with overall survival in HCC.
| Analysis | No. of studies | Pooled hazard ratio (95% CI) | I2 statistic, % | χ2 p-value for heterogeneity | Analytical model | χ2 p-value for subgroup differences |
|---|---|---|---|---|---|---|
| 1. Tumour p53 mutation | ||||||
| Prospective design31 | 1 | 2.89 (1.42–5.88) | Not applicable | – | – | – |
| Curative resection ≥ 90%22–24 | 3 | 4.02 (1.70–9.47) | 60 | 0.08 | REM | – |
| Viral infection | ||||||
| HBV infection ≥ 50%9,11 | 2 | 2.12 (1.45–3.11) | 0 | 0.39 | FEM | |
| HCV infection ≥ 50%22–24 | 3 | 4.02 (1.70–9.47) | 60 | 0.08 | REM | 0.18 |
| Liver cirrhosis | ||||||
| Liver cirrhosis ≥ 50%9,22–24 | 4 | 3.45 (2.16–5.51) | 41 | 0.16 | FEM | |
| Liver cirrhosis < 50%31 | 1 | 2.89 (1.42–5.88) | Not applicable | – | – | 0.68 |
| Country | ||||||
| Asia9,11,22–24 | 5 | 3.07 (1.82–2.62) | 50 | 50 | 50 | |
| Europe31 | 1 | 2.89 (1.42–5.88) | Not applicable | – | – | 0.74 |
| 2. Tumor p53 expression | ||||||
| Study type | ||||||
| Prospective design36,49 | 1 | 1.74 (1.23–2.46) | 58 | 0.12 | FEM | |
| Retrospective design10,15,41,44,45, | 5 | 1.87 (1.53–2.27) | 42 | 0.14 | FEM | 0.73 |
| Treatments | ||||||
| Curative treatments a ≥ 90%15,33,40–42,46–48 | 8 | 1.73 (1.38–2.15) | 37 | 0.13 | FEM | |
| No or support treatments45,49 | 2 | 1.54 (1.10–2.16) | 0 | 0.43 | FEM | |
| Comprehensive treatment36 | 1 | 2.31 (1.40–3.81) | Not applicable | – | – | 0.42 |
| Viral infection | ||||||
| HBV infection ≥ 50%13,21,34,43,47 | 5 | 1.49 (1.17–1.88) | 25 | 0.25 | FEM | |
| HCV infection ≥ 50%33,36,40,42,48 | 5 | 1.65 (1.29–2.11) | 21 | 0.28 | FEM | 0.54 |
| Liver cirrhosis | ||||||
| Liver cirrhosis ≥ 50%13,33–35,42,44,45,47,48 | 9 | 1.70 (1.45–1.98) | 0 | 0.71 | FEM | |
| Liver cirrhosis < 50%10,15,46 | 3 | 2.40 (0.97–5.96) | 61 | 0.08 | REM | 0.15 |
| Country | ||||||
| Asia13,21,34,35,40–44,47–49 | 12 | 1.61 (1.40–1.84) | 4 | 0.41 | FEM | |
| Europe10,15,33,36,45,46, | 6 | 2.02 (1.55–2.65) | 33 | 0.19 | FEM | 0.13 |
| 3. Serum anti-p53 antibody | ||||||
| Viral infection | ||||||
| HBV infection ≥ 50%51 | 1 | 0.81 (0.45–1.46) | Not applicable | – | – | |
| HCV infection ≥ 50%12,16 | 2 | 1.92 (1.30–2.85) | 0 | 0.40 | FEM | 0.02 |
| Liver cirrhosis ≥ 50% | ||||||
| Liver cirrhosis ≥ 50%12,16,50 | 3 | 1.55 (1.12–2.15) | 53 | 0.12 | FEM | |
| Liver cirrhosis < 50%18,51 | 2 | 1.15 (0.76–1.75) | 64 | 0.10 | FEM | 0.27 |
| Country | ||||||
| Asia and Africa12,16,51 | 3 | 1.50 (0.80–2.79) | 69 | 0.04 | REM | |
| Europe/Prospective design18,50 | 2 | 1.26 (0.84–1.91) | 32 | 0.22 | FEM | 0.68 |
HBV: hepatitis B virus infection; HCV: hepatitis C virus infection; CI: confidence interval; REM: Random-effects model; FEM: Fixed-effects model.
Included curative hepatic resection and liver transplantation.
Table 3.
Subgroup analysis of the eligible studies on p53 alterations associated with recurrence-free survival in HCC.
| Analysis | No. of studies | Pooled hazard ratio (95% CI) | I2 statistic, % | χ2 p-value for heterogeneity | Analytical model | χ2 p-value for subgroup differences |
|---|---|---|---|---|---|---|
| 1. Tumor p53 mutation | ||||||
| Curative resection ≥ 90%22–24 | 3 | 3.26 (2.08–5.10) | 52 | 0.12 | FEM | – |
| Liver cirrhosis ≥ 50%/Asia9,22–24,32 | 5 | 3.19 (2.21–4.60) | 7 | 0.37 | FEM | – |
| Viral infection | ||||||
| HBV infection ≥ 50%9,32 | 2 | 3.06 (1.62–5.76) | 0 | 0.75 | FEM | |
| HCV infection ≥ 50%22–24 | 3 | 3.26 (2.08–5.10) | 52 | 0.12 | FEM | 0.87 |
| 2. Tumor p53 expression | ||||||
| Curative treatments a ≥ 90%14,15,33,37,38,40,47 | 7 | 2.17 (1.32–3.57) | 61 | 0.02 | REM | – |
| Study type | ||||||
| Prospective design37,39 | 2 | 1.25 (0.83–1.88) | 0 | 0.59 | FEM | |
| Retrospective design15,38 | 2 | 5.73 (2.75–11.95) | 25 | 0.25 | FEM | < 0.001 |
| Liver cirrhosis | ||||||
| Liver cirrhosis ≥ 50%13,14,33,39,47 | 5 | 1.58 (1.20–2.09) | 0 | 0.74 | FEM | |
| Liver cirrhosis < 50%15,38 | 2 | 5.73 (2.75–11.95) | 25 | 0.25 | FEM | 0.001 |
| Viral infection | ||||||
| HBV infection ≥ 50%13,14,47 | 3 | 1.70 (1.21–2.38) | 0 | 0.48 | FEM | |
| HCV infection ≥ 50%33,38–40 | 4 | 2.14 (1.08–4.25) | 63 | 0.04 | REM | 0.81 |
| Country | ||||||
| Asia13,14,37,39,40,47 | 6 | 1.55 (1.21–1.99) | 0 | 0.58 | FEM | |
| Europe and USA15,33,38 | 3 | 3.69 (1.16–11.70) | 76 | 0.02 | REM | 0.15 |
HBV: hepatitis B virus infection; HCV: hepatitis C virus infection; CI: confidence interval; REM: Random-effects model; FEM: Fixed-effects model.
Curative treatments included curative hepatic resection and liver transplantation.
4. Discussion
Identification of the risk of disease recurrence and mortality in HCC patients is critical to guide surveillance and select adjunctive therapies. However, to identify accurately the risk of individual patients match the difficult.52 The systematic review and meta-analysis firstly collected comprehensive evidence for prognostic significance of p53 alterations in HCC patients received surgical or non-surgical treatments. We found that HCC patients with mutant p53 phenotype had a shorter RFS and OS than those with wild-type p53 status. Similar to p53 mutation, p53 expression upregulation also increased significantly the risk of mortality and tumour recurrence in HCC, resulting in poor patient outcomes. However, the systematic review did not suggest there was an association between serum anti-p53 antibody and patient survival in HCC patients based current studies. According to the systematic review results, we believe that our review will provide useful information for decision-making in HCC clinically.
In HCC, p53 mutation is related to carcinogenesis of common etiological factors, and its mutant phenotypes differ due to mutagen and other factors prior to tumour development.53,54 In the systematic review, when considering simultaneously p53 mutation and hepatitis virus infection in HCC patients, we found that the mortality risk associating p53 mutation in high HCV infection population was higher than high HBV infection population despite of no statistical significance (Table 2), which could be interpreted by different p53 mutation.54 Usually, wild type p53 protein can be degraded rapidly in MDM-dependent manner and keep undetectable.55 However, mutant p53 protein can accumulate to a detectable level in cell nuclei due to escaping from the degradation,55 and was associated with tumour progression.56 In the systematic review, our results revealed that p53 upregulation conformed nearly to p53 mutation in associating with patient survival in HCC. Recently, a well-designed prognostic study showed the combined detection of p53 mutation and p53 upregulation could predict effectively recurrence in bladder cancer.57 However, there were almost no studies to make it in HCC. Therefore, it is promising to perform further similar study. In addition, mutant p53 protein can be released into blood from HCC cell and subsequently induces the production of serum anti-p53 antibodies, and the latter can be detected by ELISA.58 Serum anti-p53 antibody level in theory can reflect p53 protein acumulation in cancer cell after p53 mutation. Therefore, its prognostic significance in HCC should be similar to the upregulation and mutation of p53. However, interestingly, evidence from the systematic review did not suggest that serum anti-p53 antibody was a predictor of clinical outcomes in HCC patients. Some reasons for the result merited consideration. In the eligible studies, firstly, the most common cut-off value to designate elevated serum anti-p53 antibody level was the ratio of the marker in HCC patient versus health control, and it was widely distributed and difficult to be uniform due to different populations. Moreover, the only study on serum anti-p53 antibody and RFS in HCC only included less than a quarter patients into survival analysis due to lacking relevant pathological data, and therefore its result must had marked deviation.19 Therefore, we believe that the prognostic value of serum anti-p53 antibody in HCC should be further examined.
We performed sensitivity analyses by excluding each study individually and omitting small or low quality studies. The large sample or high quality studies always speak louder than small or low quality ones. Sensitivity analysis results verified further the prognostic value of p53 alterations in HCC patients. To avoid the influence of insufficient report on the reliability of the systematic review, we further analyzed the studies with complete report including tumour stage, PVTT, cirrhosis and HBV/HCV infection information. The reanalysis of the complete report studies showed similar estimation to the meta-analyses of all eligible studies. It demonstrated that our systematic review produced reliable clinical evidences for HCC patients. Next, we conducted subgroup analyses stratify by study design and geographical locations. The combined analysis of two prospective studies37,39 suggested that there was no association between p53 expression and RFS in HCC patients despite of the retrospective studies shown significant result. Because RFS can reflect directly the predictive value of prosticators, we believe it is required that the well-designed prospective studies on p53 expression and RFS. Notably, the prognostic effects of p53 alterations on HCC patients were not different between high-incidence area and low-incidence area, supporting our evidence could be used in different populations. Subsequently, because the eligible studies did not report patient survival associated with p53 alterations according to tumour stage and PVTT, we only stratified studies according to treatment, HBV/HCV infection and liver cirrhosis. Patients received no or palliative treatments seem to reveal natural history of HCC. In the systematic review, p53 upregulation is also supported as a prognosticator for untreated HCC patients. Additionally, subgroup anslyses showed that there was a significant association between serum anti-p53 antibody and OS in populations with high propotion of liver cirrhosis and HCV infection. However, notably, there existed substantial between-study heterogeneity (I2 = 53; p = 0.12) in eligible studies with high liver cirrhosis population (though not statistically significant). When we altered fixed-effects to random-effects model, the significant summary HR and 95% CI disappeared (p = 0.07).
The NOS scale was chosen to assess the quality of eligible studies. According to this scale, we found that high quality (> 6 scores) studies coincided essentially with larger sample (≥ 100) ones, which could make reporting more convincing. Furthermore, the combined analyses of the high quality studies also yielded similar results to the large sample ones. Of eligible studies, nevertheless, most lacked sufficient report about prognostic information of HCC patients, which is not propitious for the production and application of clinical evidence. Therefore, for improving the reporting of prognsotic tumour marker studies, we suggest that new studies in this topic should comply with the REMARK guidelines.59
Though we evaluated comrehensively the association between p53 alterations and patient outcomes in HCC, there were some limitations in our systematic review and meta-analysis. Firstly, cohort studies are difficult to control confounders, which could influence authentic prognostic value of p53 alterations in HCC. In addition, our review could not clarify the association between the phenotype of p53 mutation and patient survival because of lacking individual patient data. We believe there could be potential language bias in this review, because we only sought studies writen in English, French, German and Chinese. Thus, we suggest that the results of the systematic review should be interpreted cautiously.
In conclusion, our review suggested tumour p53 mutation and protein upregulation shortened markedly RFS and OS in HCC patients. Combinated with other prognostic factors, tumour p53 alterations could identify much better patient outcomes and then be used to inform the choice of adjunctive therapy in HCC. However, serum anti-p53 antibody could not be recommended for outcome prediction in HCC based on current studies, and further well-designed studies could define its authentic prognostic value. In the design of primary research projects, if multilevel p53 parameters could be simultaneously taken into account, it will present compelling evidence regarding the prognostic importance of p53 alterations among HCC patients.
Supplementary Material
Acknowledgments
Funding: This study was supported by a project grant (EW) from the National Center for Research Resources (NCRR; P20 RR020151) and the National Institute of General Medical Sciences (NIGMS; P20 GM103505) from the National Institutes of Health (NIH). The contents of this report are solely the responsibility of the authors and do not necessarily reflect the official views of the NIH, NCRR, or NIGMS.
The authors would like to thank Prof. Guihua Zhuang and Prof. Quanqing Zheng from Medical College of Xi’an Jiaotong University for providing the statistical assistance in this study. The authors would also like to thank Qinfen Xue from Xi’an Jiaotong University Library for helping get full-text for paper in literature retrieval.
Footnotes
Conflict of interest statement
The authors of this manuscript have no potential conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127(12):2893–917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel RWE, Hao YP, Xu JQ, Thun MJ. Cancer Statistics, 2009. CA Cancer J Clin. 2009;59(4):225–49. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 3.McGlynn KA, Tsao L, Hsing AW, Devesa SS, Fraumeni JF., Jr International trends and patterns of primary liver cancer. Int J Cancer. 2001;94(2):290–96. doi: 10.1002/ijc.1456. [DOI] [PubMed] [Google Scholar]
- 4.La Vecchia C, Lucchini F, Franceschi S, Negri E, Levi F. Trends in mortality from primary liver cancer in Europe. Eur J Cancer. 2000;36(7):909–15. doi: 10.1016/s0959-8049(00)00052-6. [DOI] [PubMed] [Google Scholar]
- 5.Tandon P, Garcia-Tsao G. Prognostic indicators in hepatocellular carcinoma: a systematic review of 72 studies. Liver Int. 2009;29(4):502–10. doi: 10.1111/j.1478-3231.2008.01957.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cabibbo G, Enea M, Attanasio M, Bruix J, Craxi A, Camma C. A meta-analysis of survival rates of untreated patients in randomized clinical trials of hepatocellular carcinoma. Hepatology. 2010;51(4):1274–83. doi: 10.1002/hep.23485. [DOI] [PubMed] [Google Scholar]
- 7.Lai PB, Chi TY, Chen GG. Different levels of p53 induced either apoptosis or cell cycle arrest in a doxycycline-regulated hepatocellular carcinoma cell line in vitro. Apoptosis. 2007;12(2):387–93. doi: 10.1007/s10495-006-0571-1. [DOI] [PubMed] [Google Scholar]
- 8.Dowell SP, Wilson PO, Derias NW, Lane DP, Hall PA. Clinical utility of the immunocytochemical detection of p53 protein in cytological specimens. Cancer Res. 1994;54(11):2914–18. [PubMed] [Google Scholar]
- 9.Park NH, Chung YH, Youn KH, et al. Close correlation of p53 mutation to microvascular invasion in hepatocellular carcinoma. J Clin Gastroenterol. 2001;33(5):397–401. doi: 10.1097/00004836-200111000-00011. [DOI] [PubMed] [Google Scholar]
- 10.Terris B, Laurent-Puig P, Belghitti J, Degott C, Hénin D, Fléjou JF. Prognostic influence of clinicopathologic features, DNA-ploidy, CD44H and p53 expression in a large series of resected hepatocellular carcinoma in France. Int J Cancer. 1997;74(6):614–9. doi: 10.1002/(sici)1097-0215(19971219)74:6<614::aid-ijc10>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 11.Yuan RH, Jeng YM, Chen HL, et al. Stathmin overexpression cooperates with p53 mutation and osteopontin overexpression, and is associated with tumour progression, early recurrence, and poor prognosis in hepatocellular carcinoma. J Pathol. 2006;209(4):549–58. doi: 10.1002/path.2011. [DOI] [PubMed] [Google Scholar]
- 12.Atta MM, El-Masry SA, Abdel-Hameed M, Baiomy HA, Ramadan NE. Value of serum anti-p53 antibodies as a prognostic factor in Egyptian patients with hepatocellular carcinoma. Clin Biochem. 2008;41(14–15):1131–9. doi: 10.1016/j.clinbiochem.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 13.Hu TH, Wang CC, Huang CC, et al. Down-regulation of tumour suppressor gene PTEN, overexpression of p53, plus high proliferating cell nuclear antigen indexpredict poor patient outcome of hepatocellular carcinoma after resection. Oncol Rep. 2007;18(6):1417–26. [PubMed] [Google Scholar]
- 14.Liu AW, Cai J, Zhao XL, et al. The clinicopathological significance of BUBR1 overexpression in hepatocellular carcinoma. J Clin Pathol. 2009;62(11):1003–8. doi: 10.1136/jcp.2009.066944. [DOI] [PubMed] [Google Scholar]
- 15.Stroescu C, Dragnea A, Ivanov B, et al. Expression of p53, Bcl-2, VEGF, Ki67 and PCNA and prognostic significance in hepatocellular carcinoma. J Gastrointestin Liver Dis. 2008;17(4):411–7. [PubMed] [Google Scholar]
- 16.Shiota G, Kishimoto Y, Suyama A, et al. Prognostic significance of serum anti-p53 antibody in patients with hepatocellular carcinoma. J Hepatol. 1997;27(4):661–8. doi: 10.1016/s0168-8278(97)80083-9. [DOI] [PubMed] [Google Scholar]
- 17.Müller M, Meyer M, Schilling T, et al. Testing for anti-p53 antibodies increases the diagnostic sensitivity of conventional tumour markers. Int J Oncol. 2006;29(4):973–80. [PubMed] [Google Scholar]
- 18.Parasole R, Izzo F, Perrone F, et al. Prognostic value of serum biological markers in patients with hepatocellular carcinoma. Clin Cancer Res. 2001;7(11):3504–9. [PubMed] [Google Scholar]
- 19.Saffroy R, Lelong JC, Azoulay D, et al. Clinical significance of circulating anti-p53 antibodies in European patients with hepatocellular carcinoma. Br J Cancer. 1999;79(3–4):604–10. doi: 10.1038/sj.bjc.6690095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yeh CT, Kuo CJ, Lai MW, et al. CD133-positive hepatocellular carcinoma in an area endemic for hepatitis B virus infection. Bmc Cancer. 2009;11(9):324. doi: 10.1186/1471-2407-9-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang MF, Zhang ZY, Fu J, Yang YF, Yun JP. Correlation between expression of p53, p21/WAF1, and MDM2 proteins and their prognostic significance in primary hepatocellular carcinoma. J Transl Med. 2009;7:110. doi: 10.1186/1479-5876-7-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hayashi H, Sugio K, Matsumata T, Adachi E, Takenaka K, Sugimachi K. The clinical significance of p53 gene mutation in hepatocellular carcinomas from Japan. Hepatology. 1995;22(6):1702–7. [PubMed] [Google Scholar]
- 23.Sugo H, Takamori S, Kojima K, Beppu T, Futagawa S. The significance of p53 mutations as an indicator of the biological behavior of recurrent hepatocellular carcinomas. Surg Today. 1999;29(9):849–55. doi: 10.1007/BF02482774. [DOI] [PubMed] [Google Scholar]
- 24.Yano M, Hamatani K, Eguchi H, et al. Prognosis in patients with hepatocellular carcinoma correlates to mutations of p53 and/or hMSH2 genes. Eur J Cancer. 2007;43(6):1092–100. doi: 10.1016/j.ejca.2007.01.032. [DOI] [PubMed] [Google Scholar]
- 25.Mann CD, Neal CP, Garcea G, Manson MM, Dennison AR, Berry DP. Prognostic molecular markers in hepatocellular carcinoma: a systematic review. Eur J Cancer. 2007;43(6):979–92. doi: 10.1016/j.ejca.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 26.Wells GA, Brodsky L, O’Connell D, et al. [accessed 7 July 2010];The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm.
- 27.Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. doi: 10.1186/1745-6215-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 29.Thompson SG, Higgins JPT. How should meta-regression analyses be undertaken and interpreted? Stat Med. 2002;21(11):1559–73. doi: 10.1002/sim.1187. [DOI] [PubMed] [Google Scholar]
- 30.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Honda K, Sbisà E, Tullo A, et al. p53 mutation is a poor prognostic indicator for survival in patients with hepatocellular carcinoma undergoing surgical tumour ablation. Br J Cancer. 1998;77(5):776–82. doi: 10.1038/bjc.1998.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Su H, Zhao J, Xiong Y, et al. Large-scale analysis of the genetic and epigenetic alterations in hepatocellular carcinoma from Southeast China. Mutat Res. 2008;641(1–2):27–35. doi: 10.1016/j.mrfmmm.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 33.Anzola M, Saiz A, Cuevas N, Lopez-Martinez M, Martinez De Pancorbo MA, Burgos JJ. High levels of p53 protein expression do not correlate with p53 mutations in hepatocellular carcinoma. J Viral Hepat. 2004;11(6):502–10. doi: 10.1111/j.1365-2893.2004.00541.x. [DOI] [PubMed] [Google Scholar]
- 34.Chiu CT, Yeh TS, Hsu JC, Chen MF. Expression of Bcl-2 family modulated through p53-dependent pathway in human hepatocellular carcinoma. Dig Dis Sci. 2003;48(4):670–6. doi: 10.1023/a:1022816204831. [DOI] [PubMed] [Google Scholar]
- 35.Endo K, Ueda T, Ohta T, Terada T. Protein expression of MDM2 and its clinicopathological relationships in human hepatocellular carcinoma. Liver. 2000;20(3):209–15. doi: 10.1034/j.1600-0676.2000.020003209.x. [DOI] [PubMed] [Google Scholar]
- 36.Gianni S, Cecchetto A, Altavilla G, et al. Tumour staging, morphology and p53 overexpression concur in predicting survival in hepatocellular carcinoma. J Intern Med. 2005;257(4):367–73. doi: 10.1111/j.1365-2796.2005.01460.x. [DOI] [PubMed] [Google Scholar]
- 37.Guo RP, Zhong C, Shi M, et al. Clinical value of apoptosis and angiogenesis factors in estimating the prognosis of hepatocellular carcinoma. J Cancer Res Clin Oncol. 2006;132(9):547–55. doi: 10.1007/s00432-006-0097-5. [DOI] [PubMed] [Google Scholar]
- 38.Guzman G, Alagiozian-Angelova V, Layden-Almer JE, et al. p53, Ki-67, and serum alpha feto-protein as predictors of hepatocellular carcinoma recurrence in liver transplant patients. Modern Pathol. 2005;18(11):1498–503. doi: 10.1038/modpathol.3800458. [DOI] [PubMed] [Google Scholar]
- 39.Kobayashi T, Sugawara Y, Shi YZ, Makuuchi M. Telomerase expression and p53 status in hepatocellular carcinoma. Am J Gastroenterol. 2002;97(12):3166–71. doi: 10.1111/j.1572-0241.2002.07125.x. [DOI] [PubMed] [Google Scholar]
- 40.Mise K, Tashiro S, Yogita S, et al. Assessment of the biological malignancy of hepatocellular carcinoma: relationship to clinicopathological factors and prognosis. Clin Cancer Res. 1998;4(6):1475–82. [PubMed] [Google Scholar]
- 41.Naka T, Toyota N, Kaneko T, Kaibara N. Protein expression of p53, p21WAF1, and Rb as prognostic indicators in patients with surgically treated hepatocellular carcinoma. Anticancer Res. 1998;18(1B):555–64. [PubMed] [Google Scholar]
- 42.Osada S, Saji S, Kuno T. Clinical significance of combination study of apoptotic factors and proliferating cell nuclear antigen in estimating the prognosis of hepatocellular carcinoma. J Surg Oncol. 2004;85(1):48–54. doi: 10.1002/jso.20006. [DOI] [PubMed] [Google Scholar]
- 43.Qin HX, Nan KJ, Yang G, et al. Expression and clinical significance of TAp73α, p53, PCNA and apoptosis in hepatocellular carcinoma. World J Gastroenterol. 2005;11(18):2709–13. doi: 10.3748/wjg.v11.i18.2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Qin LX, Tang ZY, Ma ZC, et al. p53 immunohistochemical scoring: an independent prognostic marker for patients after hepatocellular carcinoma resection. World J Gastroenterol. 2002;8(3):459–63. doi: 10.3748/wjg.v8.i3.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schöniger-Hekele M, Hänel S, Wrba F, Müller C. Hepatocellular carcinoma-survival and clinical characteristics in relation to various histologic molecular markers in Western patients. Liver Int. 2005;25(1):62–9. doi: 10.1111/j.1478-3231.2004.0997.x. [DOI] [PubMed] [Google Scholar]
- 46.Soini Y, Virkajärvi N, Lehto VP, Pääkkö P. Hepatocellular carcinomas with a high proliferation index and a low degree of apoptosis and necrosis are associated with a shortened survival. Br J Cancer. 1996;73(9):1025–30. doi: 10.1038/bjc.1996.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sung CO, Yoo BC, Koh KC, Cho JW, Park CK. Prognostic significance of p53 overexpression after hepatic resection of hepatocellular carcinoma. Korean J Gastroenterol. 2005;45(6):425–30. [PubMed] [Google Scholar]
- 48.Umemura A, Itoh Y, Itoh K, et al. Association of gankyrin protein expression with early clinical stages and insulin-like growth factor-binding protein 5 expression in human hepatocellular carcinoma. Hepatology. 2008;47(2):493–502. doi: 10.1002/hep.22027. [DOI] [PubMed] [Google Scholar]
- 49.Wu PC, Lau VK, Fang JW, Lai VC, Lai CL, Lau JY. Imbalance between cell proliferation and cellular DNA fragmentation in hepatocellular carcinoma. Liver. 1999;19(5):444–51. doi: 10.1111/j.1478-3231.1999.tb00076.x. [DOI] [PubMed] [Google Scholar]
- 50.Sitruk V, Vaysse J, Chevret S, et al. Prevalence and prognostic value of serum anti-p53 antibodies in hepatocellular carcinoma. A study of 159 patients. Gastroenterol Clin Biol. 2000;24(12):1159–63. [PubMed] [Google Scholar]
- 51.Tangkijvanich P, Janchai A, Charuruks N, et al. Clinical associations and prognostic significance of serum anti-p53 antibodies in Thai patients with hepatocellular carcinoma. Asian Pac J Allergy Immunol. 2000;18(4):237–43. [PubMed] [Google Scholar]
- 52.Bruix J, Sherman M, Llovet JM, et al. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL Conference. J Hepatol. 2001;35(3):421–30. doi: 10.1016/s0168-8278(01)00130-1. [DOI] [PubMed] [Google Scholar]
- 53.Bressac B, Kew M, Wands J, Ozturk M. Selective G to T mutations of p53 gene in hepatocellular carcinoma from southern Africa. Nature. 1991;350(6317):429–31. doi: 10.1038/350429a0. [DOI] [PubMed] [Google Scholar]
- 54.Teramoto T, Satonaka K, Kitazawa S, Fujimori T, Hayashi K, Maeda S. p53 gene abnormalities are closely-related to hepatoviral infections and occur at a late-stage of hepatocarcinogenesis. Cancer Res. 1994;54(1):231–5. [PubMed] [Google Scholar]
- 55.Midgley CA, Lane DP. p53 protein stability in tumour cells is not determined by mutation but is dependent on Mdm2 binding. Oncogene. 1997;15(10):1179–89. doi: 10.1038/sj.onc.1201459. [DOI] [PubMed] [Google Scholar]
- 56.Su Q, Schroder CH, Otto G, Bannasch P. Overexpression of p53 protein is not directly related to hepatitis B x protein expression and is associated with neoplastic progression in hepatocellular carcinomas rather than hepatic preneoplasia. Mutat Res-Rev Mutat. 2000;462(2–3):365–80. doi: 10.1016/s1383-5742(00)00026-0. [DOI] [PubMed] [Google Scholar]
- 57.George B, Datar RH, Wu L, et al. p53 gene and protein status: the role of p53 alterations in predicting outcome in patients with bladder cancer. J Clin Oncol. 2007;25(34):5352–8. doi: 10.1200/JCO.2006.10.4125. [DOI] [PubMed] [Google Scholar]
- 58.Volkmann M, Müller M, Hofmann WJ, et al. The humoral immune response to p53 in patients with hepatocellular carcinoma is specific for malignancy and independent of the alpha-fetoprotein status. Hepatology. 1993;18(3):559–65. [PubMed] [Google Scholar]
- 59.McShane LM, Altman DG, Sauerbrei W, et al. REporting recommendations for tumor Marker prognostic studies (REMARK) Eur J Cancer. 2005;41(12):1690–6. doi: 10.1016/j.ejca.2005.03.032. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.